Attached files
| file | filename |
|---|---|
| EX-31.2 - SECTION 302 CERTIFICATION - CHIEF FINANCIAL OFFICER - KENSEY NASH CORP | d230825dex312.htm |
| EX-32.1 - SECTION 906 CERTIFICATION - CHIEF EXECUTIVE OFFICER - KENSEY NASH CORP | d230825dex321.htm |
| EX-31.1 - SECTION 302 CERTIFICATION - CHIEF EXECUTIVE OFFICER - KENSEY NASH CORP | d230825dex311.htm |
| EX-32.2 - SECTION 906 CERTIFICATION - CHIEF FINANCIAL OFFICER - KENSEY NASH CORP | d230825dex322.htm |
| EX-21.1 - SUBSIDIARIES OF KENSEY NASH CORPORATION - KENSEY NASH CORP | d230825dex211.htm |
| EX-23.1 - CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM - KENSEY NASH CORP | d230825dex231.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended: June 30, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to .
Commission File Number: 001-34388
KENSEY NASH CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 36-3316412 | |
| (State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
735 Pennsylvania Drive, Exton, Pennsylvania 19341
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (484) 713-2100
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of exchange on which registered | |
| Common Stock, par value $.001 per share | NASDAQ Global Select Market | |
| Series A Junior Participating Preferred Stock Purchase Right | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant as of December 31, 2010 (the last business day of the registrant’s most recently completed second fiscal quarter) was $113,470,285, based on the closing price per share of Common Stock of $27.83 as of such date reported by the NASDAQ Global Select Market. Shares of the registrant’s Common Stock held by each executive officer and director and by each person who owns 10% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares outstanding of the registrant’s Common Stock, par value $.001 per share, as of August 31, 2011 was 8,636,090.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of registrant’s definitive Proxy Statement, to be filed pursuant to Regulation 14A under the Securities Exchange Act of 1934, in connection with the registrant’s 2011 Annual Meeting of Stockholders scheduled to be held on December 7, 2011, are incorporated by reference into Part III of this report.
Table of Contents
| Page | ||||||
| PART I |
||||||
| Item 1. |
4 | |||||
| Item 1A. |
19 | |||||
| Item 1B. |
30 | |||||
| Item 2. |
30 | |||||
| Item 3. |
30 | |||||
| PART II |
||||||
| Item 5. |
31 | |||||
| Item 6. |
34 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
35 | ||||
| Item 7A. |
54 | |||||
| Item 8. |
56 | |||||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
92 | ||||
| Item 9A. |
92 | |||||
| Item 9B. |
95 | |||||
| PART III |
||||||
| Item 10. |
96 | |||||
| Item 11. |
96 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
96 | ||||
| Item 13. |
Certain Relationships and Related Transactions and Director Independence |
96 | ||||
| Item 14. |
96 | |||||
| PART IV |
||||||
| Item 15. |
97 | |||||
| 101 | ||||||
2
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This report contains forward looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934. We have based these forward looking statements largely on our current expectations and projections about future events and trends affecting our business. In this report, the words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “forecast,” “expect,” “plan,” “should,” “is likely” and similar expressions, as they relate to Kensey Nash Corporation, our business or our management, are intended to identify forward looking statements, but they are not exclusive means of identifying them.
The forward looking statements in this report are only predictions and actual events or results may differ materially. In evaluating such statements, a number of risks, uncertainties and other factors could cause our actual results, performance, financial condition, cash flows, prospects and opportunities to differ materially from those expressed in, or implied by, the forward-looking statements. These risks, uncertainties and other factors include, among other things:
| • | general economic and business conditions, both nationally and in our markets; |
| • | the impact of competition; |
| • | anticipated trends in our business; |
| • | existing and future regulations affecting our business; |
| • | strategic alliance and acquisition opportunities; and |
| • | other risk factors set forth under “Item 1A. Risk Factors” below. |
Except as expressly required by the federal securities laws, we undertake no obligation to publicly update or revise any forward looking statements, whether as a result of new information or future events or otherwise after the date of this report. Our results of operations in any past period should not be considered indicative of the results to be expected for future periods. Fluctuations in operating results may also result in fluctuations in the trading price of our Common Stock.
References in this Form 10-K to “we,” “us,” “our” and “our company” refer to Kensey Nash Corporation and its subsidiaries.
3
Table of Contents
PART I
| ITEM 1. | BUSINESS |
OVERVIEW
Kensey Nash Corporation, incorporated in Delaware in 1984, is a medical device product development and manufacturing company with a history of innovation and success in bringing new products to the market. We focus on regenerative medicine by creating products and technologies that help the human body heal. We are recognized as a leader for innovative product development, as well as for our broad portfolio of resorbable biomaterials products. We have an extensive range of products, which are sold through strategic partners in multiple large medical markets, including cardiology, orthopaedic, sports medicine, spinal, cranio-maxillofacial (CMF), trauma and general surgery.
During the past 25 years, we have established ourselves as a leader in designing, developing, manufacturing and processing proprietary medical devices, which include resorbable biomaterials products such as collagen and synthetic polymers. We have expanded our base of technology, which has enabled us to develop multiple resorbable product platforms that can be used in a wide variety of applications. We have developed extensive expertise in tissue regeneration and tissue repair, and the ability to commercialize and produce products with these capabilities. In our fiscal year ended June 30, 2011 (fiscal 2011), we manufactured and sold approximately 3 million resorbable product units, representing approximately 270 commercialized products, to strategic partners and customers, many of which are leaders in their market segments. We continue to broaden our proprietary biomaterials technologies, as well as our development and manufacturing capabilities.
In fiscal 2011, sales to, and royalties from, our four largest customers, St. Jude Medical, Inc. (St. Jude Medical), Arthrex, Inc. (Arthrex), Orthovita, Inc. (Orthovita), which was recently acquired by Stryker Corporation (Stryker) on June 28, 2011, and Synthes, Inc. (Synthes) represented approximately 86% of our total revenues. Our other key customers include Johnson & Johnson, Inc. and its subsidiaries (Johnson & Johnson), Medtronic, Inc. (Medtronic), Stryker, Devicor Medical Products, Inc. (Devicor) and Zimmer, Inc. (Zimmer). In May 2011, Stryker signed a definitive agreement to acquire Orthovita and in April 2011, Synthes had entered into a definitive agreement to be acquired by Johnson & Johnson, which is expected to be completed in the first half of calendar 2012.
We are known as a pioneer in the field of arterial puncture closure, as the inventor and original developer of the Angio-Seal™ Vascular Closure Device (Angio-Seal), which is exclusively licensed to St. Jude Medical. Today, the Angio-Seal device (which is manufactured, marketed and sold by St. Jude Medical) is the leading arterial puncture closure product in the world with approximately 17 million Angio-Seal units having been sold since its market introduction approximately 15 years ago. We have manufactured key resorbable components for the Angio-Seal device and also receive an approximate 6% royalty on Angio-Seal end-user sales. See “St. Jude Medical Customer Relationship” for additional information.
In our fiscal 2011, we broadened our product and technology portfolio through investments in our internal research and development programs and strategic asset acquisitions of the net assets of Nerites Corporation, accounted for as an asset acquisition, and Norian Corporation (Norian), a subsidiary of Synthes, accounted for as a business combination, as well as our cost-method investment in and purchase of certain manufacturing rights from, Orteq Ltd (Orteq Sports Medicine or Orteq).
In January 2011, we acquired substantially all the assets and certain operational liabilities of Nerites Corporation (Nerites), a privately-held development stage company based in Wisconsin, which is the developer of medical adhesives and anti-fouling coatings, for approximately $20 million. Nerites’ technology and intellectual property primarily focus on the chemical formulation involved in creating a molecule for an adhesive material. We are applying this technology to expand our resorbable biomaterials product platform.
4
Table of Contents
In May 2011, we acquired certain operational assets and certain liabilities relating to the business and product lines of Norian Corporation (Norian), a wholly owned subsidiary of Synthes, Inc., for $26 million. We acquired the worldwide manufacturing rights to manufacture Norian’s product portfolio of orthobiologic products, which includes, bone void fillers and cements. In addition, we entered into an exclusive supply agreement and research and development agreement for related new products with Synthes. Our acquisition of the Norian product line immediately increased our product sales to Synthes (a total of $1.4 million in our fourth quarter of fiscal 2011), diversified our customer base, and added to our bone repair product technologies. We expect that total revenues from product sales to Synthes will substantially increase in future fiscal periods and that Synthes will become one of our larger customers in fiscal 2012, primarily due to our sales of the Norian products, which we expect to approximate $14 million in fiscal 2012.
In December 2010, we made a non-controlling minority investment in Orteq, a London-based company, and secured the rights to manufacture the Actifit® product line of Orteq, for a total of $4 million. The Actifit, a biocompatible synthetic meniscus scaffold offering sports medicine surgeons a new arthroscopic option for the treatment of irreparable partial meniscus repairs, is a CE marked approved, meniscus repair product and is currently available in Europe. Actifit received its CE Mark approval in 2008. Orteq is currently planning on initiating a US clinical trial in early calendar year 2012. Commercialization of Actifit in the U.S. is at least several years away. We expect transition of manufacturing from Orteq to our Exton manufacturing facility to be completed by mid- fiscal year 2012.
We are also building our pipeline of new proprietary products in the soft tissue and orthopaedic markets. Our expansion into the soft tissue repair market has been largely facilitated by our proprietary manufacturing Optrix™ process for extracellular matrix (ECM) products. Our first ECM product, Medeor™ Matrix, made from porcine dermis (pig’s skin), received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in October 2009 and CE Mark approval in April 2010. In May 2010, our second ECM product, the Meso BioMatrix™ product, which is made from porcine-mesothelium (Meso) tissue, derived from the abdominal wall of a pig, received 510(k) clearance from the FDA. We are currently evaluating partnering opportunities, as well as other fields of use, for our ECM products. We plan to continue to expand our relationships with our current customers and add new customers, by targeting new markets, including general, pelvic and urological surgery.
In fiscal 2011, we expanded our relationship with Synthes under the strategic porcine dermis ECM agreement that we announced in our prior fiscal year ended June 30, 2010 (fiscal 2010). Synthes launched the XCM Biologic™ Tissue Matrix, the first commercial product of our Medeor™ Matrix material, initially focused on ventral hernia repair, in the U.S. in May 2010, and in Europe in fiscal 2011.
Another product in our development pipeline is a cartilage repair device, which consists of a proprietary bi-phasic, resorbable biomaterials implant designed to repair focal (primary) defects of articular cartilage in joints. The device received CE Mark approval in February 2010. In November 2010, we entered into an agreement with Arthrex for the European distribution of our cartilage repair device and Arthrex recently initiated European commercialization activities. We received FDA approval to initiate our U.S. pilot trial for the cartilage repair device and enrolled our first patient in the second quarter of fiscal 2011. However, due to the significant cost and risk of obtaining U.S. regulatory approval with the uncertain U.S. regulatory climate, restrictive clinical requirements, protracted patient enrollment and an extended follow-up timeframe for our cartilage technology in the U.S., we shifted our clinical activities for the cartilage product to outside the U.S.
OUR BIOMATERIALS BUSINESS
Overview
Biomaterials can be generally defined as materials that treat, augment, or replace tissue, organs or body functions. We have specialized in resorbable biomaterials, meaning biomaterials that are absorbed over time and allow the body’s natural tissue to take their place. In the late 1980s and early 1990s, we became an innovator in expanding the use of biomaterials to other types of medical applications with our development of the Angio-Seal
5
Table of Contents
device, a vascular closure system that incorporates resorbable materials as the primary basis for the product. Our experience in developing the necessary materials for the biomaterial components and the scale-up of manufacturing capabilities for this product are the foundation of our biomaterials business.
Medical device companies, focusing on a wide variety of market segments, are now taking advantage of advances in materials technology and a better understanding of the biological processes involved in tissue formation and healing to introduce resorbable biomaterials-based products. This trend has been observed in many medical markets, including cardiology, orthopaedic, general, urological, cosmetic and dental segments. Companies and physicians are widely seeking new solutions to long-standing clinical problems. Resorbable biomaterials-based products have shown to be attractive solutions in these markets for a number of reasons. For example they:
| • | allow for the regeneration and remodeling of natural tissue as the resorbable biomaterial implant resorbs over time; |
| • | offer the ability to modify the rate of absorption of products to promote healing as the biomaterials-based products work with and assist the body’s natural healing response; |
| • | obviate the need for second surgeries, where traditionally needed, to remove non-resorbable implants; |
| • | improve the ability for physicians to image healing progress in patients with biomaterial implants compared to metallic implants, which is critical to the ability to visualize and assess healing; and |
| • | provide the potential for staged and sustained release of biologically active agents, including drugs, cells and growth factors and biologics specific for each clinical situation. |
Our Biomaterials Business Strategy
We intend to utilize our experience and expertise in biomaterials to continue to expand our business. Our strategy to accomplish this expansion is as follows:
| • | Develop New Proprietary Biomaterials Products. We continue to leverage our technology and expertise in materials and processes to develop new proprietary biomaterials products. By working with strategic partners or directly with surgeon clinicians we can identify desirable attributes of new resorbable biomaterials products and advance them toward commercialization. We will continue to invest in research and development to enable us to innovate and expand our range of biomaterials products. |
| • | Expand Our Biomaterials Products and Customer Base. We intend to broaden the product lines sold to our current customer base and to expand our number of customers by providing proprietary and technologically superior biomaterials products that improve patient outcomes and reduce the cost of healthcare. We also intend to continue to invest in new manufacturing technology and processes to meet our customers’ requirements, support product development, and expand our marketing efforts to increase the demand for our biomaterials products. |
| • | Manufacture. We are one the largest manufacturers of biomaterial products in the world. We intend to leverage our manufacturing expertise and capacity to scale up and mass produce regenerative medicine products for our partners and customers to meet the expected demands of these growing markets. |
| • | Pursue Strategic Acquisitions and Alliances. We continually seek strategic acquisitions and alliances that add new and complementary technologies and expertise, broaden our intellectual property portfolio and strengthen our competitive position in our biomaterials business. We believe that our expertise in biomaterials allows us to identify and attract these opportunities. |
6
Table of Contents
Our Biomaterials Technology
The technological challenges involved in developing biomaterials-based products can be substantial. As a result of our work with these types of materials and processes for approximately 25 years, we have built significant know-how and technology in the field.
| • | Materials Technology. Many of the products we have developed incorporate our highly proprietary bovine (cow) based collagen technology, which has an extensive safety profile and tailorable properties that make it uniquely suitable for multiple medical device applications. Our collagen can be fabricated in many forms, including powders, gels, pastes, sponges and other structural matrices. We often combine collagen with other materials, such as ceramics and other biomaterials, to further create new properties and characteristics. Our ability to utilize multiple process variables, such as fiber structure, cross-linking (a process for strengthening the collagen material) and chemistry, further increases the number of product configurations and properties that we can offer. |
We have more recently developed expertise in the processing of porcine (pig) based natural tissue matrices which are used in the development and manufacture of our ECM products. By selecting various tissues from a pig and processing them in a proprietary manner, we are able to produce a unique biomaterial, which may help the body’s ability to repair damaged or diseased tissues.
We also have extensive experience with processing a wide range of bioresorbable synthetic polymers, including polylactic acid (PLA), polyglycolic acid (PGA), poly-lactic-co-glycolic acid (PLGA) and many others. We have strong expertise in controlling architecture and processing variations of such polymers, which allows us to create new materials with unique properties and composites from those original materials.
| • | Processing Technology. Our Porous Tissue Matrix (PTM™) processing technology allows us to create porous implants from synthetic polymers that support cell growth and tissue regeneration and have the potential to deliver biologics, growth factors and drugs. The PTM technology allows us to create customized porous architectures and, in some applications, combine different materials in a way that optimizes the tissue engineering. The resulting implants can be designed to facilitate wound healing in both bone and soft tissue that resorb at controlled rates for specific functions and tissues. We have a series of products and development programs underway, including our cartilage repair device, utilizing intellectual property related to our porous biodegradable regeneration matrices. |
Our expansion into the soft tissue repair market has been largely facilitated by our proprietary Optrix™ ECM manufacturing process. The Optrix™ process gently disinfects tissues, inactivates viruses and removes cells while preserving extracellular matrix components. Our ability to apply this technology to multiple tissue sources enables us to create customized soft tissue regeneration products (e.g., biologic surgical scaffolds) for a variety of clinical applications. This process allows us to convert a wide variety of mammalian tissues into biological surgical scaffolds.
| • | Adhesive and Coating Technology. Our adhesive and coating technology, acquired in the acquisition of substantially all of the assets of Nerites, was inspired by marine mussels and the substance they secrete to bond to underwater surfaces. We are creating synthetic mimetics of these proteins and using them to build adhesive polymers that can be tailored to fit a broad variety of indications. We are working to develop products that can be delivered in the form of a hydrogel, liquid or spray, or can be pre-coated onto an adjunctive medical device. We believe that the flexibility of our chemistry will enable us to tailor performance characteristics of the material specifically for each application. |
| • | Delivery Technology. Our biomaterials technology has potential for use in the controlled release of biologically active agents, including drugs, cells and growth factors. In these applications, the agent could be deposited or incorporated into a biomaterials delivery matrix. As the matrix dissolves or is degraded by the body, the agent is gradually released. The use of a biomaterials matrix for the controlled release of these biologically active agents permits a locally targeted, optimal release profile, improving the delivery of the agents. |
7
Table of Contents
| • | Manufacturing/Scale-up Know-How. Resorbable biomaterials-based products tend to be particularly challenging to consistently manufacture on a large scale. There are variations in materials and material properties that must be continually controlled and monitored. We have core competencies in anticipating such variabilities and controlling them during our manufacturing processes. |
Biomaterials Product Lines and Product Applications
We have consistently focused on commercializing a broad array of products used in the orthopaedic markets, including the sports medicine and spinal markets, and supporting the Angio-Seal product line in the cardiovascular market. More recently, we have also commercialized our ECM products in the general surgery market, and our May 2011 acquisition of the Norian assets expanded our CMF and trauma orthopaedic product lines. In addition, to a lesser extent, we have commercialized biomaterials products for other market applications, such as diagnostic oncology and dentistry. While to date we have concentrated significant efforts on orthopaedic, general surgery related products and Angio-Seal components, we believe that there are also significant opportunities for our technologies in other markets.
Orthopaedic Market Products. We have commercialized a wide variety of products for the orthopaedic applications of biomaterials. Applications in the orthopaedic market for our biomaterials products include soft tissue and bone fixation, bone void fillers, bone cement and scaffolds.
| • | Soft Tissue and Bone Fixation. Many of our biomaterials products are designed as an alternative to the use of metallic and other permanent devices for the fixation and repair of musculoskeletal tissues. Among other advantages, use of resorbable biomaterials eliminates the need for a second surgery, which is frequently necessary to remove non-resorbable metallic implants, such as bone screws, plates and pins. The primary application for our biomaterials in the sports medicine arena is soft tissue fixation. Soft tissue fixation includes the repair of tendons and ligaments in the knee, such as the anterior cruciate ligament; in the shoulder, such as the rotator cuff; and in the extremities. We manufacture products such as suture anchors, interference screws and reinforcement materials for all of these applications from resorbable synthetic polymers and synthetic polymer/ceramic composites. We also manufacture pins, plates and screws of various designs for repair of the small bones of the extremities, for certain applications in the spine, and for other orthopaedic trauma applications. Key customers of these products include Arthrex and Stryker. |
| • | Bone Void Fillers and Scaffolds. Synthetic bone void fillers are increasingly being used as alternatives to autograft (bone harvested from another area of the patient’s body) or allograft (bone harvested from cadavers). We have developed or co-developed several different bone void fillers, essentially synthetic bone graft substitutes, for use in bone repair. We have fabricated these products from collagen, collagen/ceramic composites, synthetic polymers and synthetic/ceramic composite biomaterials. Key customers of these products include Orthovita (which was recently acquired by Stryker), Medtronic, Zimmer and Johnson & Johnson. |
The Norian product portfolio consists of several biomaterial-based surgical products including: the beta-TCP chronOS Preforms and chronOS Strip; the SynPOR Porous PE Implants; and the Norian family of calcium phosphate bone cements, which include reinforced and fast setting products as part of the Norian Skeletal Repair System and Norian Craniofacial Repair System product lines, primarily in the CMF and trauma areas for use in bone repair. Under terms of our supply agreement with Synthes, we will exclusively manufacture this family of products for Synthes.
Cardiovascular Market Products. We have developed the original Angio-Seal device, a vascular closure device, and manufactured two of the key resorbable components of the Angio-Seal device for St. Jude Medical. The collagen plug and the polymer anchor components form the basis for the resorbable implant that acts to seal a hole in the artery, created during a catheterization procedure, and are critical to the functionality of the Angio-Seal device. See “St. Jude Medical Customer Relationship” below for additional information. We also manufacture other products within the cardiovascular market, none of which materially contribute to our revenue at this time.
8
Table of Contents
General Surgery Market Products. We have recently commercialized with our partner, Synthes, our first ECM product, a porcine dermis surgical mesh used for abdominal wall reconstruction, breast reconstruction and select head and neck plastic surgery repair. See “Biomaterials Research and Development Programs-Extracellular Matrices” below for an expanded discussion of our ECM development activities. In addition, we manufacture resorbable carriers for the breast biopsy markers for Devicor, which in July 2010 acquired the Ethicon Endo-Surgery Breast Care unit, a former unit of Johnson & Johnson. Ethicon historically purchased these resorbable carriers from us.
Other Biomaterials Market Applications. While not yet a large part of our business, we develop, manufacture and sell a variety of biomaterials-based products designed for other market applications. These products include dental barrier membranes and resorbable materials used in ophthalmology applications.
Biomaterials Customer Relationships
We sell our biomaterials products to over 30 companies that ultimately sell them into the end-user marketplace. Our largest biomaterials customers include St. Jude Medical, Arthrex, Stryker/Orthovita, and Synthes. We also supply biomaterials products and development expertise to other orthopaedic companies including, BioMimetic Therapeutics, Johnson & Johnson, Medtronic, Stryker and Zimmer. In August 2009, we entered into a strategic agreement with Synthes to market and distribute our porcine dermis-based ECM products for select reconstructive surgical applications. In June 2010, we announced a second strategic distribution agreement for our ECM products with Arthrex for rotator cuff and other tendon repair procedures. In addition, in November 2010, we entered into an agreement with Arthrex for the European distribution of our cartilage repair device, and Arthrex recently initiated European commercialization activities. We continue to seek to expand relationships with companies targeting new markets.
Our customer relationships are both short-term and long-term and in most cases contractual in nature, with contracts specifying various terms which govern the relationship including, for example, product development, regulatory responsibilities and pricing. We often work with customers and potential customers at very early stages of feasibility and provide significant input in co-development types of programs. Once a product is approved for sale, we generally provide our customers fully packaged and sterilized products ready for sale or their further distribution. Our products often represent a key strategic source for these customers and partners. In many cases, our proprietary technology is incorporated in the product and cannot be replicated by other companies.
St. Jude Medical Customer Relationship
Our largest customer during our fiscal 2011 was, and prior to that had been, St. Jude Medical, to which we supply Angio-Seal device components. During fiscal 2011, 26% of our net sales and 44% of our total revenues, including royalty income, were derived from our relationship with St. Jude Medical. The Angio-Seal technology is licensed to St. Jude Medical, which manufactures, markets and distributes the Angio-Seal device worldwide.
Angio-Seal Vascular Closure Device Overview
We pioneered the concept of vascular closure, as the inventor and original developer of the Angio-Seal device marketed by St. Jude Medical. The Angio-Seal device is a biomaterials-based product which acts to close and seal femoral artery punctures made during diagnostic and therapeutic cardiovascular catheterizations. The device utilizes resorbable components to form a sandwich closure concept at the arterial puncture site to provide hemostasis (stoppage of bleeding) and speed the post-procedure recovery process.
The Angio-Seal device has been shown to have several advantages over traditional manual or mechanical compression procedures, including reduced time to hemostasis and ambulation, reduced staffing and hospital time, possible reduction in procedure costs, increased patient comfort, greater flexibility in post-procedure blood thinning therapy and increased blood flow to the leg.
9
Table of Contents
The Angio-Seal device is widely recognized as the leading product in the worldwide vascular puncture closure market (which consists of products developed for the purpose of closing and sealing of femoral artery punctures made during cardiovascular catheterizations). The Angio-Seal device has been sold in Europe since 1995, in the U.S. since 1996 and in Japan since 2003. There have been approximately 17 million Angio-Seal devices sold since its commercialization, making it what we believe is one of the most successful resorbable biomaterials-based medical products. The Angio-Seal device generated approximately $320 million in revenue for St. Jude Medical during our fiscal 2011.
Other treatment options include manual pressure and device-based therapy. Existing device-based treatment options include biomaterials, sutures, clips, and staples. There are also many topical patch devices which require adjunct manual pressure, and are used mostly for diagnostic catheterizations. There are alternative methods of diagnosis and treatments, such as cardiac catheterization procedures performed from other access sites, such as the radial artery, that are becoming more prevalent. These catheterization procedures through alternative access sites have adversely impacted the market use of vascular puncture closure devices designed for femoral artery puncture closure and could continue to do so.
St. Jude Medical Collagen Supply Agreement
In June 2010, we entered into a new two-year supply agreement with St. Jude Medical for the period from January 1, 2011 to December 31, 2012. Royalties under the Angio-Seal license agreements (See “St. Jude Medical Angio-Seal License Agreements”) between our Company and St. Jude Medical, which are based on total net end-user sales of the Angio-Seal device, are not affected by this new supply agreement. The new supply agreement provides for Kensey Nash to be the exclusive outside supplier of collagen plugs, one of the key resorbable components of the Angio-Seal device. The new supply agreement requires St. Jude Medical to purchase contractual minimum order levels of collagen plugs for calendar years 2011 and 2012. In fiscal year 2011, our collagen plug sales to St. Jude Medical were approximately $11.3 million and our polymer anchor sales to St. Jude Medical were approximately $0.8 million. We no longer supply any polymer anchors to St. Jude Medical. As of April 2011, St. Jude Medical had fulfilled their calendar year 2011 minimum order levels of $4.0 million, for products that were shipped in the second half of fiscal 2011. We currently do not expect St. Jude Medical to place additional orders for the remainder of calendar year 2011. As of June 30, 2011, St. Jude Medical placed a calendar 2012 order for approximately $6.4 million of collagen plugs, of which $4.0 million are to be shipped in the second half of fiscal 2012, and $2.4 million in the first half of fiscal 2013. The $6.4 million of orders for calendar year 2012 are $2.9 million higher than the contractual minimum.
St. Jude Medical Angio-Seal License Agreements
Under our license agreements, St. Jude Medical has exclusive worldwide rights to manufacture market and distribute the patented Angio-Seal device for hemostatic puncture closure. We retain the rights to use this technology for applications outside the cardiovascular system. We currently receive an approximate 6% royalty on Angio-Seal end-user sales by St. Jude Medical. The term of the license agreements continues until to the expiration of the last claim of any of the licensed patents.
St. Jude Medical may terminate the license agreement at any time, for any reason, upon 12 months notice. However, upon any such termination, all rights granted under the license, including sales, marketing, manufacturing and distribution rights, would revert back to our Company. The Angio-Seal trademark is owned, and would be retained, by St. Jude Medical.
Stryker/Orthovita Customer Relationship
Our second largest customer during fiscal 2011 was Orthovita. Since 2003, we have partnered with Orthovita to co-develop and commercialize a series of unique and proprietary bone void filler products used in the spinal market, branded Vitoss™ Foam and Vitoss™ Bioactive Foam technology. The first of these bone void filler products was
10
Table of Contents
launched in March 2004, the second, VitossTM Bioactive Foam technology, was launched during fiscal 2008, and the most recent, VitossTM BA2X Foam technology, was launched during fiscal 2011. On June 28, 2011, Stryker acquired Orthovita. We believe Stryker’s acquisition of Orthovita will lead to increased revenues in future years, driven by Stryker’s significantly larger worldwide sales force, relative to the sales force of Orthovita prior to such acquisition. Under our agreement with Orthovita, which was assumed by Stryker, we manufacture these bone void filler products and Orthovita markets and sells these products worldwide. Multiple versions of these co-developed products have been launched since March 2004. During fiscal 2011, 17% of our net sales and 19% of our total revenues, including royalty income, were derived from our relationship with Orthovita.
Arthrex Customer Relationship
Our third largest customer during fiscal 2011 was Arthrex, a privately held worldwide leader in sports medicine. We provide to Arthrex a number of soft tissue and bone fixation devices, including anchors, screws and pins, which are often required in sports medicine procedures, such as anterior cruciate ligament repairs and tendon repairs. During fiscal 2011, 24% of our net sales and 16% of our total revenues were derived from our relationship with Arthrex. In June 2010, we announced a strategic distribution agreement for our ECM products with Arthrex for rotator cuff and other tendon repair procedures. During the second quarter of fiscal 2011, we entered into a distribution agreement with Arthrex for the European distribution of our cartilage repair device, and Arthrex recently initiated European commercialization activities for that device.
Synthes Customer Relationship
Our fourth largest customer during fiscal 2011 was Synthes, a global medical device company, headquartered in Switzerland, that develops, produces and markets surgical instruments, implants and biomaterials for repairing bone and soft tissue. We provide to Synthes ECM products sold in the general surgery markets. Upon our acquisition of the operational assets of Norian, we also provide to Synthes the Norian products that are primarily sold in the trauma and CMF markets. During fiscal 2011, 11% of our net sales and 7% of our total revenues were derived from our relationship with Synthes. We anticipate in fiscal 2012, sales to Synthes will increase significantly due to net sales of the recently acquired Norian products, which are estimated to be approximately $14 million in fiscal 2012. In April 2011, Synthes entered into a definitive agreement to be acquired by Johnson & Johnson, which acquisition is expected to be completed in the first half of calendar 2012.
Additional Customer Relationships
BioMimetic Therapeutics, Johnson & Johnson, Medtronic, Stryker, Devicor, and Zimmer are other partners/customers of ours that have launched, or are in various stages of developing, their respective product lines that incorporate our product and technologies. See “Biomaterials Research and Development Programs” below.
Business development activities for our biomaterials business are primarily conducted by our senior management team. Technology and product evaluations and the related business agreements can take long periods of time to complete. Our marketing efforts are focused on the partnering of our technology and expertise to new potential customers, as well as seeking segment leading sales and marketing organizations for those specific products that we have developed.
Biomaterials Research and Development Programs
In addition to our relationships with partners and customers to commercialize near-term and long-term biomaterials product concepts, we have also been advancing development programs that are longer term in nature and are based on our proprietary technology in the following areas:
| • | Extracellular Matrices. We have developed a technology platform of porcine-derived extracellular matrix tissues to expand our presence in the soft tissue repair market. Our expansion into the soft tissue |
11
Table of Contents
| repair markets has been largely facilitated by our proprietary Optrix™ process, which allows us to manufacture ECM products. This process allows us to convert a wide variety of mammalian tissues into biological surgical scaffolds. Our first material, Medeor™ Matrix, is made from porcine dermis (pig’s skin) and received 510(k) clearance from the FDA in November 2009 and CE Mark approval in April 2010. It is indicated for use as a surgical mesh for abdominal wall reconstruction, plastic and reconstructive surgery, urologic and gynecologic surgeries and tendon repair (U.S. only). In May 2010, our second ECM product, the Meso BioMatrix™ material, which is made from porcine-mesothelium (Meso) tissue derived from the abdominal wall of a pig, received 510(k) clearance from the FDA. The clearance allows for the use of Meso BioMatrix™ in general surgery for the reinforcement and repair of soft tissue, including hernia repair, plastic and reconstructive surgery, and urologic, gynecologic and gastroenterological applications. Medeor™ Matrix and Meso BioMatrix™ materials are the first soft tissue products developed from our new Optrix™ ECM biologic surgical mesh platform. |
In August 2009, we signed a strategic distribution agreement with Synthes, which represented an important milestone in our plans to build upon our leadership position as a developer of innovative regenerative medicine products. Under the terms of the agreement with Synthes, we will develop and manufacture porcine dermis-based products, which Synthes will market and distribute for abdominal wall and chest reconstruction and for several applications in head and neck surgery. Synthes launched the XCM Biologic™ Tissue Matrix, the first commercial product based on our Medeor™ Matrix material, in the U.S. in May 2010 and outside the U.S. (OUS) in August 2010. In June 2010, we announced a strategic distribution agreement with Arthrex for rotator cuff and other tendon repair procedures, with the commercialization launch expected to begin in the first half of fiscal 2012.
We are currently evaluating partnering opportunities for our ECM products in the urogynecology, wound care, and orthopaedic fields. Our ability to apply the Optrix™ tissue processing technology to multiple tissue sources enables us to create customized soft tissue repair products for a variety of clinical applications. We expect these efforts to yield new collaborative partners and products in the rapidly growing biologic surgery market that will provide significant diversification and expansion of our product portfolio.
| • | Articular Cartilage Regeneration Matrix. We are developing a cartilage repair device, which consists of a proprietary bi-phasic, resorbable biomaterials implant designed to repair focal (primary) defects of articular cartilage in joints. The design has a collagen-based cartilage region and a ceramic/polymer subchondral bone region based on our PTM technology. These compartmentalized architectures are designed to allow for the regeneration of both cartilage and bone elements while maintaining their natural interface. We submitted an IDE application to the FDA to study the use of our cartilage repair device for treating articular cartilage defects of the knee. This device received CE mark approval in February 2010. In fiscal 2010, we received FDA approval to initiate our U.S. pilot trial for the cartilage repair device and enrolled our first patient in the trial in the second quarter of fiscal 2011. However, due to the significant cost and risk of obtaining U.S. regulatory approval with the uncertain U.S. regulatory climate, restrictive clinical requirements, protracted patient enrollment and an extended follow-up timeframe for our cartilage technology in the U.S., we shifted our clinical activities for the cartilage product to outside the U.S. As a result, any commercialization of our cartilage repair device in the U.S. is at least several years away. In November 2010, we entered into an agreement with Arthrex for the European distribution of our cartilage repair device, and Arthrex initiated European commercialization activities. We also initiated a European post market study in fiscal 2011 to collect safety and efficacy data on the cartilage repair device. |
| • | Adhesives and Coatings. Our new adhesive and coating technology (acquired from Nerites in January 2011), was inspired by marine mussels and the substances they secrete to bond to underwater surfaces. Our scientists create synthetic mimetics of these proteins to build adhesive polymers that can be tailored to fit a broad variety of clinical indications. Surgical sealants and adhesives have applications in most surgical procedures addressing a market that encompasses over 70 million surgical wounds in the U.S. alone. |
12
Table of Contents
Our Medhesive™ liquid and thin film adhesives are being developed to adhere to a wide variety of substrates and have applications for the repair of hernia, dura and gastrointestinal defects. Our technology is the subject of a Small Business Innovation Research (SBIR) grant related to the adhesive attachment of hernia repair meshes.
Bacterial infections complicate the outcome of millions of medical procedures each year. Catheters, orthopaedic implants, and other devices can become the locus of infection when pathogenic bacteria form biofilms. It is estimated that device-related biofilm infections increase hospital stays and add over $1 billion per year to U.S. hospitalization costs. We are developing anti-fouling coatings to prevent bacteria from attaching to surfaces, thereby disrupting the formation of biofilms and their harmful consequences. This technology is also the subject of a Phase II SBIR grant related to creating bacteria-resistant coatings for stents and catheters.
MAY 2008 SALE OF ENDOVASCULAR BUSINESS
In May 2008, we completed the sale of our Endovascular business to The Spectranetics Corporation (Spectranetics). This transaction included the sale of the ThromCat® (ThromCat), QuickCat™ (QuickCat) and Safe-Cross® (Safe-Cross) product lines in consideration for a $10.0 million cash payment at closing, with an opportunity for up to an additional $8.0 million in research and development milestone payments, a $6.0 million cumulative sales milestone payment and additional royalty payments based on future sales of the ThromCat and Safe-Cross products after the transition of manufacturing of the products from us to Spectranetics. Since the sale of our Endovascular business to Spectranetics, we have received $2.5 million of the research and development milestone payments. We continue to work with Spectranetics in the development of a next generation endovascular product. Endovascular net sales were less than 3% of our total revenue in fiscal 2011.
Spectranetics is exclusively responsible for sales and marketing of the endovascular product line. In early fiscal 2010, Spectranetics announced that they had discontinued sales of Safe-Cross products. In our second quarter ended December 31, 2009 of fiscal 2010, Spectranetics assumed manufacturing responsibility of the QuickCat products. In our fourth quarter ended June 30, 2011, Spectranetics assumed manufacturing responsibility of the ThromCat products. As a result, fiscal 2011 was the final fiscal year we expect to have endovascular product revenues from the Endovascular business. However, we expect to achieve a $6.0 million cumulative sales milestone in the second quarter of fiscal 2012. The achievement of the $6.0 million cumulative sales milestone and our receipt of any royalties, as well as the remaining research and development milestone payments, could be negatively affected by the performance of Spectranetics, and has been impacted by its discontinuance of sales of Safe-Cross products. See Note 21 to the Consolidated Financial Statements included in this Form 10-K.
See also “Item 1A. Risk Factors—Spectranetics is exclusively responsible for worldwide sales and marketing of the endovascular product line. If any or all of these products fail to gain market acceptance or if Spectranetics fails to successfully commercialize them, our business may suffer.”
OTHER INFORMATION CONCERNING OUR BUSINESSES
Patents and Proprietary Rights
Our intellectual property covers many fields and many areas of technology, including vascular puncture closure, blood vessel location detection, arterial revascularization, embolic protection systems, drug/biologics delivery, bone cement, bone repair/regeneration, wound care, periodontics and angiogenesis products, and various collagen and polymer processes which are used to manufacture these devices, as well as surgical instruments which may be used to implant such devices. We protect our technology by, among other things, filing patent applications for the patentable technologies that we consider material to our business.
We also rely heavily on trade secrets and non-patented proprietary know-how that we seek to protect through non-disclosure agreements with individuals, corporations, institutions and other entities exposed to proprietary
13
Table of Contents
information. As a condition of employment, we require that all full-time and part-time employees enter into an invention assignment and non-disclosure agreement. Additionally, non-compete agreements are utilized for certain employees who are exposed to our most sensitive trade secrets.
We intend to continue to aggressively protect new manufacturing processes, biomaterials products and technologies, and medical products and devices that we have invented or developed.
Manufacturing
We have developed various unique manufacturing and processing capabilities for resorbable biomaterials. We have our own capabilities in tool-and-die making, injection molding, extrusion, compounding, machining, model making, laser welding, and bovine and porcine collagen processing, which allow us to manufacture and engineer our developmental products on site. For our bovine collagen products, we have developed, and continue to expand, a closed-herd (i.e., closely monitored for disease and having no contact with other herds) sourcing infrastructure, which is helpful in allowing our bovine collagen-based products to be sold more broadly worldwide. Closed-herd sourcing of bovine materials provides an additional level of risk management that is recognized by many regulatory entities worldwide as an important means of documenting the safety of the animal-derived materials. Our methods and processes have allowed us to maintain EDQM (European Directorate and Quality of Medicines) certification, which documents the safety of our collagen products. We believe our biomaterials manufacturing capabilities and raw material sourcing infrastructure provide us with a competitive advantage.
We purchase raw materials, parts and peripheral components, which are used in our products. Although many of these supplies are off-the-shelf items readily available from several supply sources, others are custom-made to meet our specifications. We maintain safety stock levels of these custom materials to prevent any product downtime in the case of supply interruption. In addition, we believe that, in most of these cases, alternative sources of supply for custom-made materials are available or could be developed within a reasonable period of time. An exception to this is our raw material sourcing for collagen, which would be extremely time consuming and costly to duplicate.
We have an in-house quality assurance department that sets standards, monitors production, creates and reviews operating procedures and protocols, as well as performs in-process and final testing of sample devices and products manufactured by or for us.
Facility
Our corporate headquarters and substantially all of our operations are housed in a single 202,500 square foot facility in Exton, Pennsylvania, which maximizes efficiencies and facilitates our future growth. Our headquarters is subject to a Secured Commercial Mortgage (Mortgage), secured by the building and land, under which the outstanding principal balance was $30 million as of June 30, 2011. See Note 10 to our Consolidated Financial Statements included in this Form 10-K.
This facility has been optimized for our unique businesses and is equipped with state-of-the-art equipment for developing and manufacturing biomaterials-based products. We hold ISO 13485:2003 and CMDCAS certification. Certification is based on adherence to established standards of design, service, quality assurance and manufacturing process control. Our manufacturing facility is subject to regulatory requirements and periodic inspection by regulatory authorities.
In connection with the May 2011 acquisition of certain assets of Norian, we also acquired a manufacturing and development facility in West Chester, Pennsylvania, consisting of approximately 37,000 square feet. Additionally, we entered into a 10-year lease agreement with Synthes, under which we will lease that entire building back to Synthes, which will in turn sublease a portion of the building to us for approximately 18 months, while we are transitioning our manufacturing operations for the Norian products from the West Chester Facility to our Exton Facility.
14
Table of Contents
As a result of our January 2011 acquisition of certain assets of Nerites, we became party to a lease of approximately 3,500 square feet of office space in Madison, Wisconsin, for certain of our research and development activities.
Employees
As of August 31, 2011, we had 307 employees, including 183 employees in operations, 91 employees in research and development and clinical and regulatory affairs and 33 employees in general and administration. Substantially all of our employees are located at our headquarters in Exton, Pennsylvania and manufacturing facility in West Chester, Pennsylvania. Additionally, we have 8 employees in our Madison, Wisconsin development lab. We believe that our success depends in large part on our ability to attract and retain employees in all areas of our business.
Research and Development
Our research and development and regulatory and clinical staff consisted of 91 employees as of August 31, 2011. Our research and development efforts are focused on the continued development of our biomaterial capabilities. We incurred total research and development expenses of $17.5 million, $17.9 million and $18.1 million in fiscal 2011, 2010 and 2009, respectively. See Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations” below and the Notes to the Financial Statements included in this Form 10-K for further information.
In addition to the resources dedicated to the product development process, we have an internal regulatory affairs and clinical management staff responsible for managing our clinical trials and obtaining regulatory approvals for our products. Our regulatory department also works closely with several of our customers to obtain regulatory approvals for their products in the U.S., the European Union and several other countries.
Sales and Marketing
Our existing business model is to maintain and develop partnerships where partners’ sales and marketing teams sell and distribute our products to the end-user. Although, at this time, we do not have a sales force and we do not anticipate marketing and selling our biomaterials products directly to the end-user market, there may be opportunities in the future for us to distribute our products directly to the end-user market.
Competition
The markets for our current and proposed products are fragmented, intensely competitive, subject to rapid change and sensitive to new product introductions and enhancements. We expect that the competitive environment for our products will become more intense as additional companies enter the markets in which our products compete and as new techniques and technologies are adopted. Our biomaterials and medical devices compete directly and indirectly for customers with a range of products and technologies produced by a wide variety of companies, as well as other processes and procedures that do not require the use of our products or those of our competitors. Many of the competitors in these markets have longer operating histories in these markets, greater name recognition, larger customer bases and greater financial, technical and marketing resources. Generally, we believe that the principal competitive factors for our products include the following:
| • | regulatory approvals; |
| • | safety and effectiveness; |
| • | performance and quality; |
| • | ease of use; |
| • | marketing; |
15
Table of Contents
| • | distribution; |
| • | pricing; |
| • | cost effectiveness; |
| • | customer service; |
| • | improvements to existing technologies; |
| • | reimbursement; and |
| • | compliance with regulations. |
Our biomaterials products compete with products made by large companies, by small companies under OEM relationships and by start-up, new technology groups. However, we are not aware of competitors in the resorbable biomaterials medical device field that have the breadth of capabilities and size that we have, and that also make their technologies available to other companies. In many cases, our biomaterials-based products incorporate new technologies compared to what is in the marketplace, and therefore the competitive landscape is still developing.
The Angio-Seal device competes primarily with products sold by Abbott Laboratories, Inc. and Access Closure, Inc. There are also a few other smaller competitors, some of which recently gained approval for their devices, such as Arstasis, Inc. In addition, Cordis Corporation, a division of Johnson & Johnson, introduced a new competitive product, the EXOSEAL™ Vascular Closure Device. The mechanisms of action and designs of these products vary significantly and the products compete primarily on ease of use and clinical effectiveness. While we consider the use of an Angio-Seal device to be a preferred method of post-procedure care, there are alternative methods of diagnosis and treatments, such as cardiac catheterization procedures performed from other access sites, such as the radial artery, that are becoming more prevalent.
Our orthopaedic market products, which include soft tissue and bone fixation products, and bone void filler and scaffolds, face substantial competition from large and small companies. The orthopaedic market is very competitive.
The ECM products we have, or which we are developing, compete with human and animal-derived products from such companies as Kinetic Concepts, Inc., Johnson & Johnson, Covidien, C.R. Bard Medical, Cook Medical, Wright Medical and several other private and public companies that offer biologic scaffolds and meshes.
Our cartilage repair development program competes with existing surgical procedures, such as microfracture, osteoarticular transfer system and allograft procedures. Currently in the U.S., there are no approved competitive implantable products to repair focal articular cartilage defects. We believe there is only one U.S. approved cartilage repair product, which is commercialized by Genzyme Corporation, which is a cell-based product that requires two surgeries and is indicated to be used only after a failed microfracture procedure. We anticipate additional competition from several other companies that have cartilage products under various stages of development, including acellular cartilage repair products, as well as cell-based cartilage repair products.
Government Regulation
Medical devices are subject to extensive regulation by the FDA and by foreign governments. Current medical device regulation includes, but is not limited to, clinical testing, design, manufacture, labeling, distribution and promotion of medical devices. Noncompliance with applicable requirements can result in fines, injunctions, civil penalties, seizure of products, total or partial suspension of production, failure to grant premarket clearance or premarket approval for devices, withdrawal of marketing approvals and criminal prosecution. Regulatory agencies also have the authority to recall or request repair, replacement or refund of the cost of any device we manufacture or distribute.
16
Table of Contents
Authorization to commercially distribute a new medical device in the U.S. is generally received in one of two ways. The first, known as premarket notification or the 510(k) process, requires a company to demonstrate that a new medical device is substantially equivalent to a legally marketed medical device. In this process, a company must submit data that supports its equivalence claim. If human clinical data is required, it may need to be gathered in compliance with FDA investigational device exemption regulations. Before commercial distribution of the new medical device, the FDA must issue an order finding substantial equivalence of the medical device to another legally marketed medical device. The 510(k) process generally takes from three to 12 months from the time of submission, but may take longer. Under current FDA regulations, modifications to cleared medical devices can be made without using the 510(k) process if the changes do not significantly affect safety or effectiveness, or the intended use of the device. The FDA may, however, determine that a new or modified device is not substantially equivalent to a legally marketed medical device or may require additional information, including additional clinical data, prior to the FDA making a determination, which could significantly delay the introduction of new or modified products.
The second, more rigorous process, known as premarket approval (PMA), requires a company to independently demonstrate that a new medical device is safe and effective. This is done by collecting data regarding design, materials, bench and animal testing and human clinical data for the medical device. The FDA will authorize commercial release if it determines there is reasonable assurance that the medical device is safe and effective. This determination is based on the benefit outweighing the risk for the population intended to be treated with the device. As part of the PMA approval, the FDA may place restrictions on the device, such as requiring additional patient follow-up for an indefinite period of time. If the FDA’s evaluation of the PMA application is not favorable, the FDA may deny approval of the PMA application or issue a “not approvable” letter. This PMA process is more time-consuming and expensive than the 510(k) process, typically takes at least several years to complete, and presents a greater risk that the medical device will not obtain FDA approval.
International sales of medical devices are subject to the regulatory agency product registration requirements of each country in which they are sold. The regulatory review process varies from country to country. For example, the European Union has adopted numerous directives and standards relating to medical devices regulating their design, manufacture, clinical trials, labeling and adverse event reporting. Devices that comply with those requirements, as determined by a Notified Body authorized by the European Commission, are entitled to bear a CE Mark, indicating that the device conforms to the essential requirements of the applicable directives and can be commercially distributed in countries that are members of the European Union. As part of the CE marking process, the Notified Body may place restrictions on the device, such as requiring the company to perform a formal post-market clinical study to collect certain safety data. Many countries also impose product standards, packaging requirements, labeling requirements, price restraints and import restrictions on devices. Delays in receipt of, or a failure to receive, approvals or clearances, or the loss of any previously received approvals or clearances, could have a material adverse effect on our business, financial condition and results of operations. In addition, reimbursement coverage must be obtained in some countries.
In many cases, regulatory activities for our biomaterials products are the responsibility of our customers, since most of our sales in this area are on an OEM basis. As a result, our customers frequently are responsible for seeking FDA and other government approvals to market the products. For many of these customers, we provide significant regulatory support, which includes the ongoing development and maintenance of Master Files with the FDA. Master Files contain important information relating to the specifications, manufacturing, biochemical characterization, biocompatibility and viral safety of our biomaterials products. These confidential files, in addition to our technical expertise, help our clients in their regulatory approval process for products incorporating our biomaterials.
Medical device clinical trials are sometimes required as part of the 510(k) approval process, and are almost always required to support a PMA application. In some cases, a safety pilot trial may have to be performed prior to initiating a clinical trial to provide the FDA with preliminary safety data in a small population of human subjects for specific targeted indications. For example, we must conduct a pilot trial with respect to our cartilage repair device.
17
Table of Contents
When human clinical trials are required in connection with our new proprietary products, and the device is classified as a significant risk device, we must file for the appropriate regulatory approval(s) relative to the geographical region(s) where the trial(s) will be conducted. The clinical trial application must be supported by data, typically including the results of animal and laboratory testing. If the application is approved, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as set out in our test plan. The conduct of human clinical trials is also subject to regulation. In some countries, sponsors of clinical trials are permitted to sell those devices distributed during the course of the trial, provided the consideration received does not exceed recovery of the costs of manufacture, research, development and handling.
Any products we manufacture or distribute pursuant to regulatory agency clearance or approval are subject to pervasive and continuing regulation, including product validations, recordkeeping requirements, post-market surveillance and reporting of adverse experiences with the use of the device. As a device manufacturer, we are required to register our manufacturing facility and list our devices with relevant governmental authorities and/or sanctioned third parties and are subject to periodic inspections by those bodies, as well as other agencies. Relevant national laws in the regions where we sell our products require devices to be manufactured in accordance with formally implemented quality management systems, which impose certain procedural and documentation requirements with respect to design, manufacturing and quality assurance activities. Labeling and promotional activities are subject to regulatory scrutiny and, in certain instances, by the U.S. Federal Trade Commission. Regulations prohibiting marketing of products for unapproved uses are actively enforced.
Our manufacturing operations are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. We may be required to incur significant costs to comply with such laws and regulations in the future, and any failure to comply with such laws or regulations could have a material adverse effect upon our ability to do business.
Corporate Governance and Internet Address
We recognize that good corporate governance is an important means of protecting the interests of our stockholders, employees, customers and the community. We have closely monitored and implemented relevant legislative and regulatory corporate governance reforms, including provisions of the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley), the rules of the Securities and Exchange Commission (SEC) interpreting and implementing Sarbanes-Oxley, and the corporate governance listing standards of the NASDAQ Global Select Market (NASDAQ).
Our corporate governance information and materials, including our Code of Business Conduct and Ethics, are posted on the corporate governance section of our website at www.kenseynash.com and are available in print upon request to our Investor Relations department at our offices in Exton, Pennsylvania. Our Board of Directors regularly reviews corporate governance developments and modifies these materials and practices as warranted. We have also adopted a policy on monitoring and evaluating any interactions with, and payments to, healthcare professionals.
Our website also provides information on how to contact us and other items of interest to investors. We make available on our website, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to these reports, as soon as practical after we file these reports with the SEC.
Additional Information
For financial information, see the Consolidated Financial Statements and the related Notes as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” which are included in this Annual Report on Form 10-K. We have a single reportable segment for all our operations and business. Financial information about geographic areas can be found in Note 15 to the Consolidated Financial Statements included in this Form 10-K.
18
Table of Contents
| ITEM 1A. | RISK FACTORS |
You should carefully consider the risks, uncertainties and other factors described below, in addition to the other information set forth in this report, because they could materially and adversely affect our business, operating results, financial condition, cash flows and prospects as well as adversely affect the value of an investment in our Common Stock.
Risks Related to Our Business
We derive a substantial portion of our revenues from the Angio-Seal device, which is manufactured, marketed and distributed by St. Jude Medical. In June 2010, we have entered into a new component supply agreement with St. Jude Medical, under which their component purchase commitments are reduced. A termination of, or other adverse change in, our relationship with St. Jude Medical could have a material adverse impact on our business.
Under our license agreements with St. Jude Medical, the Angio-Seal device is manufactured, marketed and sold on a worldwide basis by St. Jude Medical. Two of our significant sources of revenue are the sale of components to St. Jude Medical for incorporation into the Angio-Seal device, and royalty income from the sale of the Angio-Seal device by St. Jude Medical to the end-user market. The amount of revenue we receive from the Angio-Seal device depends, in part, on the time, effort and attention that St. Jude Medical devotes to it, and on their success in manufacturing, marketing and selling the device worldwide. For fiscal 2011, royalty income from, and sales of components to, St. Jude Medical represented approximately 44% of our total revenue. Under the terms of our licenses with St. Jude Medical, we have no control over the pricing and marketing strategy for the Angio-Seal product line. In addition, we depend on St. Jude Medical to successfully maintain levels of manufacturing sufficient to meet anticipated demand, abide by applicable manufacturing regulations and seek reimbursement approvals. Royalty income under our license agreements with St. Jude Medical will end upon the earlier of (1) the termination of the license agreements, which may be terminated by St. Jude Medical for any reason upon 12 months notice, or (2) the expiration of the last claim of any of the licensed patents. St. Jude Medical may not adequately perform its manufacturing, marketing and selling duties. Any such failure by St. Jude Medical may negatively impact Angio-Seal unit sales and, therefore, reduce our royalties.
Pursuant to the prior component supply agreement with St. Jude Medical, which expired in December 2010, we manufactured two of the key resorbable components of the Angio-Seal device for St. Jude Medical; 100% of their requirements for the collagen plug and at least 30% of their requirements for the polymer anchors. In June 2010, we entered into a new two-year supply agreement with St. Jude Medical effective for the period from January 1, 2011 to December 31, 2012. Under this new supply agreement, we are the exclusive outside supplier of collagen plugs to St. Jude Medical. The new supply agreement provides for contractual minimum order levels of collagen plugs for calendar years of 2011 and 2012. During the Company’s third and fourth quarters of fiscal 2011, St. Jude Medical had fulfilled their calendar 2011 contractual minimum of $4.0 million under the new supply agreement. We do not expect any additional orders for calendar year 2011 shipment. In June 2011, St. Jude Medical placed its calendar 2012 order for $6.4 million of collagen plugs, of which $4.0 million are to be shipped in the second half of fiscal year 2012 and $2.4 million are to be shipped in the first half of fiscal 2013. St. Jude Medical may, but is not contractually bound to order any additional collagen plugs in calendar year 2012. In August 2008, St. Jude Medical acquired certain assets of Datascope Corporation providing St. Jude Medical with a potential alternative source for the collagen component that could reduce or eliminate the future sales of collagen supplied by us to St. Jude Medical. In February 2010, St. Jude Medical announced that they had received U.S. Food and Drug Administration approval as an alternative supplier for the collagen plug, and that they would be in a position to serve as a supplier of the collagen plug by December 2010. As a result, if St. Jude Medical is able to, and does in fact, rely on its internal manufacturing to fulfill requirements for collagen plugs for the Angio-Seal device, it may not, for calendar 2012, purchase in excess of the $6.4 million in collagen plugs that it has already ordered, and may not purchase any collagen plugs from us after calendar 2012. Royalties under the Angio-Seal license agreements between St. Jude Medical and us are not affected by the current supply agreement.
19
Table of Contents
We derive a substantial majority of our revenues from only four customers.
A substantial majority of our total revenues are derived from only four customers. For fiscal 2011, in addition to the approximate 44% of our total revenue derived from royalty income from and sales of components to St. Jude Medical, royalty income from and sales of biomaterials products to Orthovita (which was recently acquired by Stryker) represented approximately 19% of our total revenue, sales of biomaterials products to Arthrex represented approximately 16% of our total revenues and royalty income from and sales of biomaterials products to Synthes represented approximately 7% of our total revenue. We expect that total revenues from product sales to Synthes will substantially increase in future fiscal periods, primarily due to our sales of the Norian products to Synthes. On April 27, 2011, Johnson & Johnson and Synthes announced their entry into a definitive agreement, whereby Johnson & Johnson is to acquire Synthes, which this transaction is anticipated to close during the first half of 2012. Our business and operating results could suffer if the acquisition is consummated and the combined company diverts its focus and attention, and in particular, sales and marketing efforts, or shifts its business strategy, away from our ECM and Norian products. In June 2011, Stryker signed a definitive agreement to acquire Orthovita. Our business and operating results could similarly suffer if Orthovita, following its acquisition by Stryker, diverts resources, and in particular sales and marketing efforts, or shifts its business strategy, away from our co-developed bone void filler products, branded VitossTM Foam and VitossTM Bioactive Foam technology.
It is not possible for us to predict the future level of demand for our products that will be generated by these customers or the future demand for the products in the end-user marketplace. Our customer concentration exposes us to the risk of changes in the business condition of any of our major customers and to the risk that the loss of a major customer would materially adversely affect our results of operations. Our relationship with these customers is subject to change.
If our biomaterials products are not successful, our operating results and business may be substantially impaired.
The success of our existing and future biomaterials products depends on a number of factors, including our ability to manufacture, sell and competitively price these products and the acceptance of these products by the medical community. We cannot predict how quickly, if at all, the medical community will accept our future products, or the extent to which our future products will be used. In addition, regulatory approvals will be required before biomaterials products in development can be sold. The bovine materials we use in some of our products are not always accepted for use in medical devices in all international markets. If we encounter difficulties introducing future products into our targeted markets, our operating results and business may be substantially impaired. In addition, new technologies and techniques may be developed which may render obsolete our current products, along with those under development.
We will require substantial additional funds to develop and market our biomaterials products. We expect to fund the growth of our biomaterials business from cash flows from operations and cash and investments on-hand, but this operating income may not be sufficient to develop new biomaterials products. We rely on strategic partners or customers to market and sell our biomaterials products. We will need to continue to attract third parties, and retain our relationships with our current partners, to distribute our products to the end-user market.
We depend on our customers to market and, in some cases, obtain regulatory approvals for products that include our biomaterials components.
We depend on the efforts of our biomaterials customers in marketing their products that include our biomaterials components. There can be no assurance that our customers’ end-use products that include our biomaterials components will be commercialized successfully by our customers or that our customers will otherwise be able to compete effectively in their markets. While we take steps toward obtaining regulatory approval under certain of our partnerships and for some of our customers, in other cases, we rely on others to do these activities independently. There can be no assurance that our partners or customers will be successful in obtaining such regulatory approvals.
20
Table of Contents
The markets for our products are highly competitive and are likely to become more competitive, and our competitors may be able to respond more quickly to new or emerging technologies and changes in customer requirements.
The markets for our current and proposed products are fragmented, intensely competitive, subject to rapid change and sensitive to new product introductions and enhancements. We expect that the competitive environment for our products will become more intense as additional companies enter our markets, and as new techniques and technologies are adopted. Our biomaterials and medical devices compete directly and indirectly with a range of products and technologies produced by a wide variety of companies, as well as other processes and procedures which do not require the use of our products or those of our competitors. The bovine materials we use in some of our products are not always accepted for use in medical devices in all international markets. Many of our existing competitors, as well as a number of potential new competitors, have longer operating histories in these markets, greater name recognition, larger customer bases and greater financial, technical and marketing resources.
Our biomaterials products, including products under development, compete with the products of many companies in the industry, including some of the largest. In the vascular sealing device market, our products compete with products sold by Abbott Laboratories and Access Closure, Inc., along with other competitors and potential new entrants. The majority of vascular sealing is performed through manual compression, which represents our primary competition. Furthermore, there are alternative methods of diagnosis and treatments, such as cardiac catheterization procedures performed from other access sites, such as the radial artery, that are becoming more prevalent. These catheterization procedures through alternative access sites have adversely impacted the market use of vascular puncture closure devices designed for femoral artery puncture closure and could continue to do so.
The ECM products we are developing compete with human and animal-derived products from such companies as Kinetic Concepts, Johnson & Johnson, Covidien, C.R. Bard Medical, Cook Medical, Wright Medical, and several other private and public companies that offer biologic scaffolds and meshes. Our cartilage repair development program competes with existing surgical procedures, such as microfracture, osteoarticular transfer system and allograft procedures. Currently in the U.S., there are no approved competitive implantable products to address focal articular cartilage defects. We believe there is only one U.S. approved cartilage repair product which is commercialized by Genzyme, which is a cell-based product that requires two surgeries and indicated to be used only after a failed microfracture procedure. We anticipate additional competition from several other companies that have cartilage products under various stages of development.
Our competitors may have broader product lines, which allow them to negotiate exclusive, long-term supply contracts and offer comprehensive pricing for their products. Broader product lines may also provide our competitors with a significant advantage in marketing competing products to group purchasing organizations and other managed care organizations that are increasingly seeking to reduce costs through centralized purchasing. Greater financial resources and product development capabilities may allow our competitors to respond more quickly to new or emerging technologies and changes in customer requirements that may render our products obsolete.
Competition faced by our customers could also have an adverse effect on our business for a variety of reasons, including that our customers may compete directly with larger, dominant manufacturers with extensive product lines and greater sales, marketing and distribution capabilities. We are also unable to control other factors that may impact the commercialization of our components for end use products, such as marketing and sales efforts and competitive pricing pressures within particular markets.
Spectranetics is exclusively responsible for worldwide sales and marketing of the endovascular product line. If any or all of these products fail to gain market acceptance or if Spectranetics fails to successfully commercialize them, our business may suffer.
The QuickCat and ThromCat endovascular products, which are sold and marketed exclusively by Spectranetics, have limited product and brand recognition, and limited usage to date. Although we do not know if these
21
Table of Contents
products will be successful over the long term, or if they will be adopted by the physician community at rates that in turn generate enough revenue for us to sustain our ongoing investment in this business, we are required by contract to continue such investment. Market acceptance of these products may be hindered if physicians are not presented with compelling data from long-term studies of the safety and efficacy of the products compared to alternative procedures. Further, the success of these products depends, in large part, on the time, effort and attention that Spectranetics devotes to them, and on Spectranetics’ success in manufacturing and selling them.
We are entitled to various milestone payments under various agreements with Spectranetics. For these reasons discussed above, we cannot be certain if and when we will receive such milestone payments.
We recently entered into a manufacturing agreement with, and made a non-controlling minority cost—method investment in Orteq Ltd. Orteq could make business decisions that are not in our best interests or with which we do not agree, which could impair the value of our investment in Orteq.
Pursuant to our investment agreement with Orteq, as well as our additional cash advance to them, structured as convertible debt, in August 2011, we obtained a non-controlling minority cost-method investment in Orteq. Accordingly, we are not able to exert control or influence over Orteq or their business decisions. Our inability to control or influence Orteq could prevent us from liquidating our interests in them at a time or at a price that is favorable to us. Orteq may not act in ways that are consistent with our business strategy or that are in our best interests. These factors may limit our ability to maximize our return, and cause us to recognize losses, on our investment in Orteq.
The loss of, or interruption of supply from, key vendors could limit our ability to manufacture our products.
We purchase many materials and components for our products from various suppliers. Certain of these materials and components are custom made for us, and we have no ready alternative source. We also rely heavily on our closed-herd sourcing infrastructure for our collagen manufacturing, which cannot be readily replicated. Any loss of, problems with, or interruption of supply from, key vendors may require us to find new vendors. We could experience production or development delays while we attempt to seek new vendors, if we can find them.
We may have problems manufacturing and delivering our biomaterials products to our customers.
The biomaterials industry is an emerging area, using many materials which are untested or whose properties are still not known. In addition, many of our products are derived from natural materials, such as collagen, which can have varying properties, may be subject to international standards concerning their use in medical devices and require careful sourcing and handling controls. Consequently, from time to time we may experience unanticipated difficulties in manufacturing and delivering our biomaterials products to our customers. These difficulties may include an inability to meet customer supply demands, delays in delivering products, quality control problems or the need to react to changes in any standard that we adhere to. There can be no assurance that our closed-herd sourcing infrastructure will provide us with an adequate risk management protection mechanism for preventing animal derived diseases from entering the human medical device industry.
Our products may be the subject of recalls, which may result in other future expense or changes to product strategy.
The FDA and similar foreign governmental authorities have the authority to require the recall of our products in the event of any failure to comply with applicable laws and regulations or defects in design or manufacture. A government mandated or voluntary product recall by us could occur if any of our biomaterial products do not meet approved specifications or data demonstrates that any such products may be unsafe or ineffective. A product recall may have an adverse affect on our net sales and operating results. In addition to lost sales directly resulting from recalls, and the direct expenses associated with such resolution, a recall may have other materially
22
Table of Contents
adverse, and potentially longer-term effects, including a negative impact on our reputation in the marketplace with respect to these and other of our products, changes to future product marketing plans, changes to clinical trial plans, and discontinuation of the recalled products.
Our use of hazardous materials exposes us to the risk of material environmental liabilities.
We use hazardous substances in our research and development and manufacturing operations, and therefore, are potentially subject to material liabilities related to personal injuries or property damages that could be caused by hazardous substance releases or exposures at or from our facility. Decontamination costs, other clean-up costs and related damages or liabilities could substantially impair our business and operating results. We are required to comply with increasingly stringent laws and regulations governing environmental protection and workplace safety, including requirements governing the handling, storage and disposal of hazardous substances.
A substantial portion of our revenue is derived directly or indirectly from international markets.
Many of our biomaterials products are sold by our customers in international markets, including the recently acquired Norian products. St. Jude Medical, for example, derives a significant portion of its Angio-Seal revenue from international markets. Our sales and royalties from international sales of the Angio-Seal product line by St. Jude Medical and our revenues from other international sales are subject to several risks, including:
| • | the impact of recessions in economies outside the United States; |
| • | changes in regulatory requirements, reimbursement policies, tariffs or other trade barriers; |
| • | weaker intellectual property rights protection in some countries; |
| • | fluctuations in currency exchange rates; |
| • | potentially adverse tax consequences; and |
| • | political and economic instability. |
Our financial position, results of operations or cash flows may be negatively impacted by the current challenging economic conditions in the U.S. and the recent financial crisis.
The current challenging economic conditions in the U.S. could adversely affect our operating results and financial condition. Among other things, we believe this challenging economic climate, including high unemployment levels and increases in co-pays, has contributed to fewer patients electing to undergo elective procedures for which our products, or the products of our partners, are used. Further, the challenging economic environment and the decline in federal and state revenues resulting from these conditions may create additional pressures to reduce reimbursements for procedures performed by physicians, hospitals and other users of our products from third party insurance providers, as well as Medicare, Medicaid and other government sponsored programs. In particular, weakness in the orthopaedic market has been attributed to the ongoing high unemployment rate and increased insurance costs, which reduced doctor visits and negatively affected pricing, as well as, greater difficulty in obtaining insurance company procedure approvals.
The majority of our investments are in highly rated municipal bonds issued by states, cities, counties and other governmental entities. Due to the currently challenging economic conditions, bond issuers’ financial condition and the credit markets have continued to be highly volatile, and there can be no assurance that these conditions will not in the future adversely affect the liquidity or value of our investments in municipal bonds. If these economic conditions continue, or any investments in our portfolio experience any ratings downgrades, we may incur impairments to our investment portfolio, which could negatively affect our financial condition, cash flows and results of operation.
23
Table of Contents
The financial troubles affecting the banking system and financial markets and the on-going concerns and threats to investment banks and other financial institutions have resulted in a tightening in the credit markets, a low level of liquidity in many financial markets, and extreme volatility in fixed income, credit and equity markets. There could be a number of follow-on effects from the credit crisis on our business, including insolvency of key suppliers resulting in product delays; inability of customers to obtain credit to finance purchases of our products and/or customer insolvencies; and counterparty failures negatively impacting our debt and treasury activities.
Our business could be harmed if we lose the services of our key personnel.
Our business depends upon our ability to attract and retain highly qualified personnel, including managerial and technical personnel. The know-how and capabilities of many of our current employees is highly specialized and difficult to replicate or replace in short timeframes. We compete for key personnel with other medical device companies, healthcare institutions, and other organizations. Our ability to maintain and expand our business may be impaired if we are unable to retain our current key personnel or hire or retain other qualified personnel in the future.
Our recent acquisitions of assets from Nerites and Norian, and any other acquisitions that we undertake in the future could be difficult to integrate, disrupt our business, dilute stockholder value or harm our operating results.
In January 2011, we acquired substantially all of the assets and certain operational liabilities of Nerites, a developer of medical adhesives and anti-fouling coatings. In May 2011, we acquired certain operational assets and liabilities relating to the business and product lines of Norian, including bone void fillers and cements, as well as the manufacturing rights to these Norian products. These represented two significant transactions for our Company, in which the consideration paid by us used a substantial portion of our cash on hand. Additionally, we may in the future make other acquisitions or make investments in complementary businesses, technologies, services or products if appropriate opportunities arise. The process of integrating any acquired business, technology, service or product, including the assets recently acquired from Nerites and Norian, into our business and operations may result in unforeseen operating difficulties and expenditures, and integration of any acquired company also may consume much of our management’s time and attention that could otherwise be available for ongoing development of our business. In particular, we may be unable to successfully transition the manufacturing operations of the Norian products to our facility in Exton, Pennsylvania, which could increase expenses relating to the manufacturing of these Norian products and otherwise disrupt other areas of our ongoing business.
Furthermore, acquisitions involve a number of risks, which may cause the anticipated benefits of any acquisition not to be realized. Specifically, with respect to our acquisition of assets of Nerites, we did not acquire any commercial products, and the time and resources that we will need to devote to research and development efforts relating to commercializing the acquired adhesive-based biomaterials technology are uncertain, and could be greater than what we currently anticipate. Furthermore, such research and development efforts may not result in our successful development of marketable products that employ this technology. In addition, any products that we do develop that employ this technology, similar to our other products, will be required to receive regulatory approval and, accordingly, will be subject to the completion of clinical trials in both the U.S. and Europe. Any delays or other unexpected difficulties experienced in connection with this regulatory approval process may cause us to incur additional expenses and adversely affect our ability to distribute products using this acquired technology into the marketplace. Even if we are able to successfully develop and obtain regulatory approval for any products that employ the Nerites technology or any products that arise out of any other acquisitions we undertake, we cannot be certain that we, or our strategic partners, will be able to successfully sell any of these products into the marketplace. In the case of our acquisition of assets of Norian, our success will be dependent in large part upon Synthes in selling the Norian products and will otherwise be dependent upon market acceptance of new products and applications, our ability to manufacture to the product specifications and produce sufficient volumes to fulfill customer demand, future success of our research and development efforts with respect to the Norian products, and availability of vendor-sourced components at reasonable prices. See also the risks discussed under “We derive a substantial majority of our revenues from only four major customers.”
24
Table of Contents
In the future, we may be unable to identify, negotiate or finance future acquisitions successfully. Future acquisitions could result in potentially dilutive issuances of equity securities or the incurrence of debt, contingent liabilities or charges related to goodwill and other intangible assets.
Our future operating results are difficult to predict and may vary significantly from quarter to quarter.
Our operating results have varied significantly from quarter to quarter in the past and are likely to vary substantially in the future as a result of a number of factors, some of which are not in our control, including:
| • | ordering patterns by our customers/partners; |
| • | the focus and resources that our customers/partners place on developing/marketing the products that we develop and manufacture; |
| • | acceptance of the products we develop and manufacture in the end-user marketplace; |
| • | our ability to attract partners for our biomaterials products and technologies; |
| • | our efforts to gain European CE Mark and FDA approval for our devices; |
| • | the loss of significant orders; |
| • | changes in our relationship with St. Jude Medical and other major customers; |
| • | establishment of strategic alliances or acquisitions; |
| • | timely implementation of new and improved products; |
| • | delays in obtaining regulatory approvals; |
| • | reimbursement rates for our products; |
| • | fluctuations in exchange rates; and |
| • | changes in the competitive environment. |
You should not rely upon our results of operations for any particular quarter as an indication of our results for a full year or any other quarter.
Our products are subject to clinical trials, which may delay commercialization of our products and may impact our use of time and resources in a material manner.
A clinical trial is almost always required to support a PMA application, and is sometimes required for a 510(k) premarket notification. Accordingly, certain products we develop are subject to clinical trials, which must be conducted before our products can be marketed. These trials are for the purpose of confirming the safety and effectiveness of the product. We try to plan clinical trials prudently, but there is no guarantee that a balance between speed and testing can be made in each case. The length of time for each clinical trial may vary substantially according to the type, complexity and intended use of the product. Delays associated with products for which we are conducting clinical trials may cause us to incur additional expenses. Further, we may be affected by delays in clinical testing of certain products of our strategic partners, over which we may have no control. Unfavorable or inconsistent clinical data from existing or future clinical trials conducted by us, by our strategic partners, by our competitors or by other third parties, or the market’s perception of this clinical data, may adversely impact our ability to obtain product approvals, our position in, and share of, the markets in which we participate and our business, financial condition, results of operations or future prospects.
Risks Related to Our Intellectual Property
If we are unable to protect our patents and proprietary rights, our reputation and competitiveness in the marketplace may be materially damaged.
We regard our patents, trade secrets and other intellectual property as important to our success. We rely upon patent law, trade secret protection, license agreements and confidentiality agreements in protecting our
25
Table of Contents
proprietary rights. Although we have filed or secured certain of our patents with applicable international governmental authorities, effective patent protection may not be available in every country in which our products are made available, and we have not sought patent protection for our intellectual property in every country where our products may be sold. Furthermore, patents are of limited duration and may not provide protection for the entire useful life of a product or product line.
Our failure to obtain or maintain patent protection, or protect trade secrets, for any reason (or third-party claims against our patents, trade secrets, or proprietary rights, or our involvement in disputes over our patents, trade secrets, or proprietary rights, including involvement in litigation), could be costly and have a substantial negative effect on our results of operations and financial condition.
Litigation, in general, and intellectual property litigation in particular, can be expensive, divert management time attention and otherwise be disruptive to normal business operations. Moreover, the results of legal proceedings cannot be predicted with any certainty and in the case of more complex legal proceedings, such as intellectual property litigation, the results are difficult to predict if at all. An adverse determination in any interference proceeding could prohibit us from selling our products, subject us to significant liabilities to third parties or require us to seek licenses from third parties. The costs associated with these license arrangements may be substantial and could include ongoing royalties. Furthermore, the necessary licenses may not be available to us, or may not be available on satisfactory terms. Failure to obtain necessary licenses could prevent us from manufacturing and selling our products.
The steps we take to protect our proprietary rights may not be adequate to ensure that third parties will not infringe or otherwise violate our patents or similar proprietary rights. We rely, in part, on confidentiality agreements with our partners, employees, advisors, vendors and consultants to protect our proprietary rights. There can be no guarantee that these agreements will not be breached, or that we will have adequate remedies for any such breach, or that our unpatented trade secrets and proprietary technological expertise will not otherwise become known or be independently discovered and developed by competitors.
We may be accused of infringing upon the proprietary rights of others and any related litigation could materially damage our operating results and business.
Third parties may claim that we have violated their intellectual property rights, any such claims, with or without merit, could subject us to costly litigation and divert the attention of key personnel and otherwise be disruptive to normal business operations. An adverse determination in any intellectual property litigation could prohibit us from selling our products, subject us to significant liabilities to third parties or require us to seek licenses from third parties. The costs associated with these license arrangements may be substantial and could include ongoing royalties. Furthermore, the necessary licenses may not be available to us, or may not be available on satisfactory terms. Failure to obtain necessary licenses could prevent us from manufacturing and selling our products.
The validity of our patents may be challenged by a competitor and our patents may not protect our business.
Third parties may claim that one of our patents is invalid based on their prior disclosure or that of another party, whether such disclosure is in a patent application or not. This type of challenge may be made in any country where our patents are issued. Any of these claims, with or without merit, could subject us to costly litigation and divert the attention of key personnel and otherwise be disruptive to normal business operations. If our competitors claim technology also claimed by us and prepare and file patent applications in the United States, we may have to participate in interference proceedings in the U.S. Patent and Trademark Office or a foreign patent office to determine priority of invention. While we undertake to search the prior art, there is no guarantee that we find all existing incidences of disclosure of devices or technologies related to our inventions. There is also no assurance that any of our patents will not become the subject of a re-examination proceeding following issuance, will not be involved in an interference prior to issuance, or will provide us with competitive advantages. Furthermore, there can be no guarantee that others will not design around our patents. Any of these instances could have a substantial, negative effect on our results of operations and financial condition.
26
Table of Contents
We do not own or control the use of the Angio-Seal device trademark.
The term “Angio-Seal” is a trademark of St. Jude Medical. All goodwill generated by the marketing and sales of devices bearing the Angio-Seal trademark belongs to St. Jude Medical and not to us. Should the St. Jude Medical license agreements terminate, we would not have the right to call any of our products “Angio-Seal” unless we purchase or license the trademark from St. Jude Medical.
Risks Related to Our Industry
We may face product liability claims that could result in costly litigation and significant liabilities.
The clinical testing, manufacture and sale of medical products involve an inherent risk that human subjects in clinical testing or consumers of the products may suffer serious bodily injury or death due to side effects or other unintended negative reactions to our products. Accordingly, the clinical testing, manufacture and sale of our products entail significant risk of product liability claims. The medical device industry in general has been subject to significant product liability litigation. Any product liability claims, with or without merit, could result in costly litigation, reduced sales, significant liabilities and diversion of our management’s time, attention and resources. We cannot be sure that our product liability insurance coverage is adequate or that it will continue to be available to us on acceptable terms, if at all.
We face uncertainty relating to third party reimbursement for our products.
We could be seriously harmed by changes in reimbursement policies of governmental or private healthcare payers, particularly to the extent any changes affect reimbursement for catheterization procedures in which Angio-Seal products are used. Physicians, hospitals and other users of our products, including our products under development such as our ECM and cartilage repair products, may fail to obtain sufficient reimbursement from healthcare payers for procedures in which our products are used, or are expected to be used, or adverse changes may occur in governmental and private third-party payers’ policies toward reimbursement for these procedures. For example, the decision by the Centers for Medicare and Medicaid Services (CMS) not to reimburse for treatment of asymptomatic patients suffering from carotid artery disease was a major factor in our fiscal 2007 decision not to continue to commercialize our embolic protection product line. Further, there have been, and continue to be, proposals by legislators, regulators and third-party payors to keep healthcare costs down. Certain proposals, if passed, would impose limitations on the prices we will be able to charge for our products, or the amounts of reimbursement available for our products from governmental agencies or third-party payors. These limitations, and other changes to reimbursement policy, could harm our business.
The adoption of healthcare reform in the United States may adversely affect our business, results of operations and/or financial condition.
In March 2010, the Patient Protection and Affordable Care Act, and the Health Care and Education Reconciliation Act of 2010 (collectively, being commonly referred to as, healthcare reform, and referred to below as, the Act), were enacted into law in the United States. The Act, which will affect major changes to the U.S. healthcare system, includes provisions that, among other things, will reduce and/or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions) and impose new and/or increased taxes. Specifically, the Act will require the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax on U.S. sales of most medical devices beginning in 2013. Under our current business model, we sell substantially all of our products through strategic partners; as a result, we are still evaluating the impact, if any, of this tax on our overall business. At a minimum, this excise tax will be an additional cost incurred by our customers. Various healthcare reform proposals have also emerged at the state level. The Act and these proposals, if passed into law, could reduce medical procedure volumes and impact the demand for our products or the prices at which we or our customers can sell our products. In addition, the excise tax may increase our cost of doing business. While we believe the Act may have the impact on our business as described above, it is too early to understand and predict the full impact of the Act on our business, as many of
27
Table of Contents
the details of the Act will be included in new and revised regulations, which have not yet been promulgated, and require additional guidance and specificity to be provided by the Department of Health and Human Services, the Department of Labor and the Department of Treasury.
Our products and manufacturing activities are subject to extensive governmental regulation that could make it more expensive and time consuming for us to introduce new and improved products or cause us to withdraw products from certain markets.
Our products and manufacturing activities are subject to extensive regulation by a number of governmental agencies, including the FDA and comparable international agencies. We are required to:
| • | obtain the approval of the FDA and international agencies before we can market and sell new products; |
| • | satisfy these agencies’ requirements for all of our labeling, sales and promotional materials in connection with our existing products; |
| • | comply with all applicable design and manufacturing regulations; and |
| • | undergo rigorous inspections by these agencies. |
Compliance with the regulations of these agencies may delay or prevent us from introducing any new or improved products. Furthermore, we may be subject to sanctions, including temporary or permanent suspension of operations, product recalls and marketing restrictions if we fail to comply with the laws and regulations pertaining to our business.
The FDA clearance and approval processes for a medical device, particularly for a device subject to PMA, can be expensive, uncertain and lengthy. There can be no assurance that we will be able to obtain necessary regulatory clearances or approvals for any product on a timely basis or at all. As an example, our cartilage repair device is subject to the PMA process. As with other medical devices subject to this rigorous process, our efforts to obtain FDA approval will be time consuming and expensive. The FDA ultimately may deny our PMA application for this device, and/or may require additional clinical trials, which could delay the PMA approval process by several years. The FDA and international regulatory agencies may also limit the indications for which our products are promoted.
The requirements governing the conduct of clinical trials and manufacturing and marketing of our products internationally vary from country to country. Foreign approvals may take longer to obtain than FDA approvals and can require, among other things, additional testing and different clinical trial designs. If we fail to comply with these regulatory requirements or obtain and maintain required approvals in any foreign country, we will not be able to sell our products (or our partners will not be able to sell their products which incorporate our devices) in that country.
We are also required to demonstrate compliance with the FDA’s Quality System Regulations. The FDA enforces its Quality System Regulations through pre-approval and periodic post-approval inspections of our manufacturing facility. These regulations relate to product testing, vendor qualification, manufacturing control, design control and quality assurance, as well as the maintenance of records and documentation. If we are found to be out of compliance with FDA regulations, it could be costly and difficult for us to correct the non-compliances and could seriously harm our business.
Regulatory agencies may restrict or withdraw approvals if information becomes available to support this action, which could include actions based on sourcing of animal derived tissue.
In addition, regulations regarding the development, manufacture and sale of medical devices are subject to future change, including the adoption of more rigorous regulation. We cannot predict what impact, if any, those changes might have on our business, the businesses of our strategic partners, or the businesses of our competitors.
28
Table of Contents
The adoption of reform to the FDA’s 510(k) process, as proposed by the FDA in its recently released preliminary recommendations, may adversely affect our business, results of operations and/or financial condition.
Our products are generally subject to the FDA’s 510(k) approval requirements, among other governmental regulations. In August 2010, the FDA issued its preliminary recommendations on reform of the 510(k) process for premarket clearance of medical devices. The FDA’s preliminary recommendations include, among other things, granting to the FDA of authority to rescind 510(k) clearance, revising existing guidance to clarify what types of modifications to existing 510(k) cleared devices warrant submission of a new 510(k), exploring the possibility of potentially requiring manufacturers to provide periodic updates to the FDA’s Center for Devices and Radiological Health listing modifications without submitting a new 510(k), adopting a framework for 510(k) submissions that requires formal validation of claims with supporting evidence and developing guidance requiring that the complete device description and intended use information be submitted and described in detail in a single section of a 510(k). While the FDA has indicated that these recommendations are preliminary in nature, if implemented, these recommendations could have the effect of making it more difficult and expensive for us, and other companies, to obtain 510(k) clearance and potentially jeopardizing the regulatory status of certain 510(k) cleared devices. It is too early to understand and predict the full impact that any modifications to the FDA’s 510(k) process may have on our business, as the FDA has emphasized that these recommendations are only preliminary in nature, and the FDA will solicit and evaluate public comments on these recommendations.
Risks Related To Our Securities
The trading price of our Common Stock is likely to fluctuate substantially in the future.
The trading price of our Common Stock may fluctuate widely as a result of a number of factors, some of which are not in our control, including:
| • | changes in our own forecasts or earnings estimates by analysts; |
| • | our customers and licensees’ ability to meet or exceed the forecasts or expectations of analysts or investors; |
| • | our ability to meet or exceed our own forecasts or expectations of analysts or investors; |
| • | quarter-to-quarter variations in our operating results; |
| • | announcements regarding clinical activities or new products by us or our competitors; |
| • | general conditions in the medical device industry; |
| • | price and volume fluctuations in the overall stock market, which have particularly affected the market prices of many medical device companies; |
| • | our stock repurchase programs; and |
| • | general economic conditions. |
In addition, the market for our stock has experienced, and may continue to experience, price and volume fluctuations unrelated or disproportionate to our operating performance. As a result, our stockholders may not be able to sell shares of our Common Stock at or above the price at which they purchase them. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If any securities litigation is initiated against us, with or without merit, we could incur substantial costs, and our management’s attention and resources could be diverted from our business.
Our second amended and restated certificate of incorporation, third amended and restated bylaws, stockholders rights plan and Delaware law may discourage an attempt to acquire our Company.
Provisions of our second amended and restated certificate of incorporation, third amended and restated bylaws, stockholders rights plan and Delaware law may render more difficult or discourage any attempt to acquire our
29
Table of Contents
Company, even if such acquisition may be favorable to the interests of our stockholders, discourage bids for our Common Stock at a premium over market price or adversely affect the market price of our Common Stock. In particular, our stockholders rights plan, adopted in June 2009, may cause substantial dilution to any person or group that attempts to acquire us without the approval of our Board of Directors.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
We own our corporate headquarters, which is a 202,500 square foot facility housing our executive offices, manufacturing and research and development activities, located at 735 Pennsylvania Drive, Exton, Pennsylvania 19341. We also own a 37,000 square foot manufacturing and research and development facility housing our manufacturing operations of Norian products and office space (which office space is leased to Synthes), located at 1230 Wilson Drive, West Chester, Pennsylvania 19380. We believe that our facilities are adequate to meet our needs for the foreseeable future. See Item 1—“Business—Facility” for additional information.
| ITEM 3. | LEGAL PROCEEDINGS |
From time to time, we are subject to various legal proceedings, including lawsuits, arbitrations or investigations. Litigation, in general, and intellectual property litigation in particular, can be expensive and disruptive to normal business operations. Moreover, the results of legal proceedings cannot be predicted with any certainty and in the case of more complex legal proceedings, such as intellectual property litigation, the results are difficult to predict at all. We do not expect that any currently pending legal proceedings will have a material adverse effect on our business, results of operations or financial condition.
30
Table of Contents
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our Common Stock is listed on the NASDAQ Global Select Market, under the symbol “KNSY” and has been traded publicly since our initial public offering in December 1995. The following table sets forth the high and low sale prices per share of our Common Stock as reported by NASDAQ for the periods indicated.
| Fiscal Year Ended June 30, 2010 |
||||||||
| First Quarter |
$ | 29.96 | $ | 24.75 | ||||
| Second Quarter |
29.43 | 22.18 | ||||||
| Third Quarter |
26.57 | 19.70 | ||||||
| Fourth Quarter |
25.50 | 20.54 | ||||||
| High | Low | |||||||
| Fiscal Year Ended June 30, 2011 |
||||||||
| First Quarter |
$ | 30.23 | $ | 21.91 | ||||
| Second Quarter |
30.13 | 26.31 | ||||||
| Third Quarter |
28.58 | 23.87 | ||||||
| Fourth Quarter |
26.56 | 23.38 | ||||||
On September 9, 2011 the last reported sale price of our Common Stock in the NASDAQ Global Select Market was $25.61 per share. As of August 30, 2011, there were 61 record owners of our Common Stock. There were also approximately 3,006 beneficial owners of the shares of our Common Stock at that date.
31
Table of Contents
Performance Graph
The following graph compares the cumulative 5-year total return that would have been attained by stockholders of Kensey Nash Corporation’s Common Stock relative to the cumulative total returns of the NASDAQ Composite index, the NASDAQ Medical Equipment index and a customized fiscal 2011 peer group of 12 companies, which are Anika Therapeutics, Inc., BioMimetic Therapeutics, Inc., Cryolife, Inc., Exactech, Inc., Osiris Therapeutics, Inc., RTI Biologics, Inc., Stereotaxis, Inc., Surmodics, Inc., Symmetry Medical, Inc., Synovis Life Technologies, Inc., The Spectranetics Corporation and Vascular Solutions, Inc. This customized fiscal 2011 peer group includes only publicly-traded, similar sized companies within the medical device industry (specifically cardiovascular or orthopaedics), with similar financial metrics, which include, but are not limited to, annual total revenue and market capitalization, and with similarities to our Company in terms of financial profile, development stage, product focus and growth potential. Our management uses this customized peer group for financial performance benchmarking, and the Compensation Committee of our Board of Directors uses this peer group for compensation practices benchmarking. Osiris Therapeutics, Inc. and Symmetry Medical, Inc. were added to our fiscal 2011 peer group because they operate in similar markets to us in the healthcare and medical device industries, and are considered to be of similar financial profile, investment in research and development and growth potential. Orthovita was removed from our fiscal 2011 peer group because it was acquired by Stryker and is no longer publicly traded. The deletion of Greatbatch, Inc. from our fiscal 2011 peer group was due to the fact that its financial metrics were no longer deemed consistent with that of our other peers. The graph tracks the performance of a $100 investment in our Common Stock, in each index and in the peer group (with the reinvestment of all dividends) from June 30, 2006 through June 30, 2011.
The total return of our fiscal 2010 customized peer group of 11 companies is also provided for transitional comparative purposes. That peer group consisted of Anika Therapeutics Inc, Biomimetic Therapeutics Inc, Cryolife Inc, Exactech Inc, Greatbatch Inc, RTI Biologics Inc, Stereotaxis Inc, Surmodics Inc, Synovis Life Technologies Inc, The Spectranetics Corp. and Vascular Solutions Inc. Orthovita is excluded from the fiscal 2010 peer group line in the graph below as there was no publically available historical data due to Orthovita’s acquisition prior to our fiscal 2011 year end.
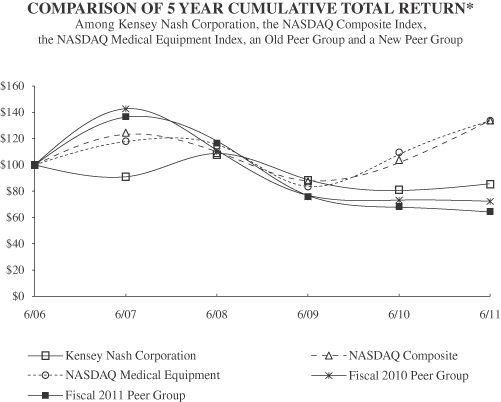
| * | Assumes $100 invested on 6/30/06 in KNSY shares or in a stock index, including reinvestment of dividends, if any. |
32
Table of Contents
| Cumulative Total Return | ||||||||||||||||||||||||
| 6/30/06 | 6/30/07 | 6/30/08 | 6/30/09 | 6/30/10 | 6/30/11 | |||||||||||||||||||
| Kensey Nash Corporation |
$ | 100.00 | $ | 90.88 | $ | 108.64 | $ | 88.85 | $ | 80.37 | $ | 85.53 | ||||||||||||
| NASDAQ Composite |
$ | 100.00 | $ | 123.31 | $ | 110.16 | $ | 87.92 | $ | 101.51 | $ | 133.69 | ||||||||||||
| NASDAQ Medical Equipment |
$ | 100.00 | $ | 117.77 | $ | 115.60 | $ | 83.54 | $ | 107.79 | $ | 133.53 | ||||||||||||
| Kensey Nash Corp. Peer Group—Fiscal 2011 |
$ | 100.00 | $ | 136.39 | $ | 118.23 | $ | 76.65 | $ | 67.68 | $ | 64.10 | ||||||||||||
| Kensey Nash Corp. Peer Group—Fiscal 2010 |
$ | 100.00 | $ | 142.87 | $ | 111.44 | $ | 76.80 | $ | 73.17 | $ | 72.22 | ||||||||||||
The above graph, including the related table, is not “soliciting material,” is not deemed to be “filed” with the SEC and is not incorporated by reference in any of our filings under the Securities Act or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
We have not declared or paid cash dividends and do not anticipate declaring or paying any dividends on our Common Stock in the near future. Any future determination as to the declaration and payment of dividends will be at the discretion of our Board of Directors and will depend on the then existing conditions, including our financial conditions, results of operations, contractual restrictions, capital requirements, business prospects and other relevant factors.
Our stock repurchases were executed under the various stock repurchase programs established by our Board of Directors. During the fiscal year ended June 30, 2011, we repurchased 1,175,738 shares of Common Stock, at a total cost of approximately $30.0 million, or an average market price of $25.52 per share, using available cash. As of June 30, 2011, no amount was remaining for repurchase under any Company stock repurchase programs.
During the fiscal year ended June 30, 2010, we repurchased and retired 1,794,705 shares of Common Stock at a cost of approximately $41,309,000, or an average market price of $23.02 per share, using available cash, executed under various stock repurchase programs. As of June 30, 2010, approximately $29,189,000 remained under our June 16, 2010 stock repurchase program to repurchase shares of our Common Stock.
33
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The following table sets forth selected Consolidated Statement of Income and Consolidated Balance Sheet data for the fiscal years ended June 30, 2011, 2010, 2009, 2008, and 2007. The selected financial data for each such fiscal year listed below has been derived from our Consolidated Financial Statements for those years, which have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, whose report for fiscal years 2011, 2010, and 2009 is included elsewhere herein. The following data for fiscal years 2011, 2010, and 2009 should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our Consolidated Financial Statements and the related notes and other financial information included herein.
| Fiscal Year Ended June 30, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands, except per share amounts) | ||||||||||||||||||||
| Statement of Income Data: |
||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Net sales |
||||||||||||||||||||
| Biomaterial sales |
$ | 44,410 | $ | 52,096 | $ | 51,046 | $ | 47,539 | $ | 41,116 | ||||||||||
| Endovascular sales |
1,589 | 2,180 | 3,858 | 6,222 | 3,786 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total net sales |
45,999 | 54,276 | 54,904 | 53,761 | 44,902 | |||||||||||||||
| Royalty income |
25,639 | 26,371 | 27,177 | 26,030 | 24,592 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
71,638 | 80,647 | 82,081 | 79,791 | 69,494 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating costs and expenses: |
||||||||||||||||||||
| Cost of products sold |
23,403 | 23,507 | 24,194 | 27,212 | 24,622 | |||||||||||||||
| Research and development |
17,550 | 17,877 | 18,134 | 17,201 | 20,265 | |||||||||||||||
| Selling, general and administrative |
8,974 | 9,009 | 9,219 | 27,575 | 20,824 | |||||||||||||||
| Loss on sale of endovascular assets |
— | — | — | 1,212 | — | |||||||||||||||
| Acquired in-process research and development |
18,233 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating costs and expenses |
68,160 | 50,393 | 51,547 | 73,200 | 65,711 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income from operations |
3,478 | 30,254 | 30,533 | 6,591 | 3,783 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest income |
422 | 704 | 1,180 | 1,775 | 1,087 | |||||||||||||||
| Interest expense |
(2,001 | ) | (2,087 | ) | (2,075 | ) | (1,540 | ) | (427 | ) | ||||||||||
| Other income (loss) |
101 | (19 | ) | 151 | (220 | ) | (23 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other (expense) income—net |
(1,478 | ) | (1,402 | ) | (744 | ) | 15 | 637 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income before income tax expense |
2,000 | 28,852 | 29,789 | 6,606 | 4,420 | |||||||||||||||
| Income tax benefit/(expense) |
78 | (9,388 | ) | (9,710 | ) | (1,816 | ) | (787 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income |
$ | 2,078 | $ | 19,464 | $ | 20,079 | $ | 4,790 | $ | 3,633 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic earnings per common share |
$ | 0.24 | $ | 1.83 | $ | 1.74 | $ | 0.40 | $ | 0.31 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted earnings per common share |
$ | 0.23 | $ | 1.78 | $ | 1.69 | $ | 0.38 | $ | 0.29 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average common shares outstanding |
8,658 | 10,624 | 11,547 | 11,891 | 11,773 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted weighted average common shares outstanding |
8,893 | 10,937 | 11,898 | 12,471 | 12,581 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| June 30, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and investments |
$ | 21,941 | $ | 65,674 | $ | 79,704 | $ | 63,496 | $ | 34,331 | ||||||||||
| Inventory |
16,629 | 8,886 | 10,585 | 9,271 | 7,392 | |||||||||||||||
| Working capital |
46,915 | 80,320 | 95,048 | 82,893 | 53,157 | |||||||||||||||
| Total assets |
146,920 | 154,121 | 171,090 | 162,429 | 140,525 | |||||||||||||||
| Long-term debt, net of current portion |
28,583 | 29,983 | 31,383 | 32,783 | 7,813 | |||||||||||||||
| Other long-term liabilities |
22,943 | 8,879 | 6,312 | 3,416 | 2,347 | |||||||||||||||
| Total stockholders’ equity |
86,837 | 104,823 | 123,275 | 114,570 | 123,650 | |||||||||||||||
34
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis should be read in conjunction with “Selected Consolidated Financial Data” and our financial statements and the related notes included in this report.
This discussion and analysis below contains forward-looking statements relating to future events or our future financial performance. These statements are only predictions and actual events or results may differ materially. In evaluating such statements, you should carefully consider the various factors identified in this report which could cause actual results to differ materially from those expressed in, or implied by, any forward-looking statements, including those set forth in “Risk Factors” in this Annual Report on Form 10-K. See “Cautionary Note Regarding Forward-Looking Statements.”
OVERVIEW
Kensey Nash Corporation is a medical device company primarily focused in the field of regenerative medicine, which is the application of devices, materials and other therapies to help repair damaged or diseased tissues. We are recognized as a leader for innovative product development, as well as for our broad portfolio of resorbable biomaterials products. We have an extensive range of products, which are sold through strategic partners in multiple large medical markets, including cardiology, orthopaedic, sports medicine, spinal cranio-maxillofacial (CMF), trauma and general surgery. We sell our products through strategic partners and do not sell direct to the end-user. Our revenues consist of two components: net sales, which include biomaterials sales and endovascular sales, and royalty income.
Net Sales
Biomaterials Sales
Sales of biomaterial orthopaedic products, including products with applications primarily in the sports medicine and spinal markets, and cardiovascular products, consisting primarily of Angio-Seal components sold to St. Jude Medical, continue to be our primary source of revenue. The table below shows the five-year trend in our biomaterials sales, including orthopaedic product sales, cardiovascular product sales and general surgery product sales:
| (in thousands) |
Fiscal Year Ended | %
change FY11 vs FY10 |
||||||||||||||||||||||
| Net Sales of | 6/30/07 | 6/30/08 | 6/30/09 | 6/30/10 | 6/30/11 | |||||||||||||||||||
| Orthopaedic Products |
$ | 21,804 | $ | 29,403 | $ | 29,790 | $ | 28,623 | $ | 25,617 | (11 | %) | ||||||||||||
| Cardiovascular Products |
17,632 | 15,861 | 18,334 | 19,326 | 12,690 | (34 | %) | |||||||||||||||||
| General Surgery Products |
1,102 | 1,304 | 2,102 | 3,650 | 4,412 | 21 | % | |||||||||||||||||
| Other Products |
578 | 971 | 820 | 497 | 1,691 | 240 | % | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Net Sales—Biomaterials |
$ | 41,116 | $ | 47,539 | $ | 51,046 | $ | 52,096 | $ | 44,410 | (15 | %) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Net sales of biomaterials products decreased 15% to $44.4 million in fiscal 2011 compared to $52.1 million in the prior fiscal year primarily due to a decrease in cardiovascular products due to the reduced requirements of the new supply agreement with St. Jude Medical, as well as, a decrease in our orthopaedic product sales. The negative economic climate, particularly during the first half of our fiscal year, has impacted the medical device industry. High unemployment and a challenging health insurance environment appear to have reduced procedures in our markets. In the first half of fiscal 2011, we experienced lower orthopaedic sales than originally anticipated; however, orthopaedic sales, primarily in sales of spine and sports medicine products, in the second half of fiscal 2011 increased, as customers re-balanced their inventory levels and we introduced new products into the market. We believe that in fiscal 2012, we will have continued stronger product sales of our orthopaedic products, primarily attributable to customers continuing to re-balancing their inventory levels, sales of our Norian products to Synthes as a result of the recent acquisition of the Norian product line and the impact of Stryker’s overall larger market presence and its marketing of Orthovita products, reflective of Stryker’s acquisition of Orthovita which occurred in the fourth quarter of fiscal 2011.
35
Table of Contents
In addition to the macroeconomic and health insurance environment factors discussed above, our net sales in the orthopaedic portion of our business are dependent on (1) our partners’ management of their inventory levels, (2) the success of our current partners in the orthopaedic markets of sports medicine, spine and extremities, (3) the continued acceptance of biomaterials-based products in these markets, as well as expanded future acceptance of such products, (4) competitive pricing, and (5) our ability to offer new products and technologies and to attract new partners in these markets. Due to these dependencies, and other factors, sales to our orthopaedic customers can vary significantly from quarter to quarter.
Our cardiovascular sales consist primarily of Angio-Seal components sold to St. Jude Medical. Sales to St. Jude Medical are highly dependent on ordering patterns of components used in the manufacturing of the Angio-Seal device by St. Jude Medical and can vary significantly from quarter to quarter. We previously supplied 100% of their requirements for the collagen plug and at least 30% of their requirements for the polymer anchors under a supply agreement that expired on December 31, 2010. In June 2010, we entered into a new two-year supply agreement with St. Jude Medical effective for the period from January 1, 2011 to December 31, 2012 to replace that previous supply agreement. The new supply agreement provides for us to be the exclusive outside supplier of collagen plugs, one of the key resorbable components of the Angio-Seal device. The new supply agreement requires St. Jude Medical to purchase contractual minimum order levels of collagen plugs for calendar years of 2011 and 2012. The new supply agreement does not call for us to supply any polymer anchors. In fiscal year 2011, collagen plug sales to St. Jude Medical were approximately $11.3 million and polymer anchor sales were approximately $0.8 million. As of April 2011, St. Jude Medical had filled their calendar year 2011 minimum order levels of $4.0 million in collagen plugs, which were shipped in the second half of fiscal 2011. We currently do not expect St. Jude Medical to place additional orders for the remainder of calendar year 2011. As of June 30, 2011, St. Jude Medical placed its calendar 2012 order for approximately $6.4 million of collagen plugs, of which $4.0 million are to be shipped in the second half of fiscal 2012, and $2.4 million in the first half of fiscal 2013. The $6.4 million of orders for calendar year 2012 are $2.9 million higher than the contractual minimum. Royalties under our license agreements with St. Jude Medical for the Angio-Seal device are not affected by this new supply agreement.
We also have developed and manufactured products used in the general surgery markets. These products primarily include products from our extracellular matrix (ECM) program, as well as a resorbable collagen product for use in breast biopsies. In our fiscal 2010, we announced two strategic agreements involving our Medeor™ Matrix porcine dermis ECM material, one with Synthes, and the other with Arthrex. Synthes launched the XCM Biologic™ Tissue Matrix, initially focused on ventral hernia repair and plastic and reconstructive procedures, in the U.S. in May 2010 and outside the U.S. (OUS) in August 2010. We expect to achieve further increases in general surgery product sales in fiscal 2012 as ECM product sales continue to expand in the U.S. and as the OUS launch gains momentum. Arthrex launched our rotator cuff repair product in our fourth quarter of fiscal 2011. We are currently evaluating additional partnering opportunities, as well as other fields of use, for our ECM products. We plan to continue to expand our relationships with our current customers and build relationships with new customers, by targeting new markets.
Other Biomaterials sales primarily include grant revenue generated from our research and development team acquired through the Nerites acquisition. Revenue under research and development contracts is recognized in the period that the related expenses are incurred. All grant revenues recorded are related to government programs under which the U.S. government funds the research of high risk, enabling technologies.
Royalty Income
We also derive a significant portion of our revenue and profitability from royalty income from proprietary products that we have developed or co-developed.
Angio-Seal™ Royalty Income. We are the inventor and original developer of the Angio-Seal device, a vascular closure device that reduces recovery time and enhances patient comfort following both diagnostic
36
Table of Contents
and therapeutic cardiovascular catheterizations. St. Jude Medical has the exclusive worldwide rights for the development, manufacturing and sales and marketing of the Angio-Seal device. We receive an approximate 6% royalty on all end-user product sales. Royalty income under the Angio-Seal device license agreements is not affected by our new supply agreement with St. Jude Medical. We believe this is a mature market and anticipate that sales of the Angio-Seal device by St. Jude Medical are expected to be relatively flat in the upcoming years.
Vitoss™ Foam, Vitoss™, and Vitoss™ Bioactive Foam Royalty Income. Since 2003, we have partnered with Orthovita, which was recently acquired by Stryker, to co-develop and commercialize a series of unique and proprietary bone void filler products, branded Vitoss™ Foam, the first of which was launched in March 2004, and the most recent of which, Vitoss™ Bioactive Foam technology, was launched during the fourth quarter of fiscal 2008. We receive a royalty on Orthovita’s end-user sales of Vitoss™ Foam and Vitoss™ Bioactive Foam products. In addition, in August 2004, we entered into an agreement to acquire the proprietary rights of a third party inventor of the Vitoss™ technology for $2.6 million (the Assignment Agreement). Under the Assignment Agreement, we receive an additional royalty from Orthovita on the end-user sales of all Orthovita products containing the Vitoss™ technology, up to a total royalty to be received of $4.0 million, with approximately $566,000 received in fiscal 2011 and $17,000 remaining to be received as of June 30, 2011. We believe the unique technology associated with the Vitoss™ Foam and Vitoss™ Bioactive Foam products, including product extensions, as well as Stryker’s worldwide marketing efforts, will result in the Orthovita component of our royalty income continuing to grow over the next fiscal year.
We have other royalty generating relationships, none of which materially contributes to revenue at this time, but which we expect to provide increased revenue as the related products gain market acceptance and additional products are commercialized.
Share-Based Compensation
The following table summarizes total share-based compensation expense within each operating expense category of our Consolidated Statements of Income for the fiscal years presented:
| Share-Based Compensation Fiscal Year Ended June 30, |
||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Total Share-Based Compensation |
Total Share-Based Compensation |
Total Share-Based Compensation |
||||||||||
| Cost of products sold |
$ | 973,326 | $ | 806,073 | $ | 504,552 | ||||||
| Research and development |
1,619,773 | 1,343,954 | 741,357 | |||||||||
| Selling, general and administrative |
1,519,244 | 1,234,996 | 816,548 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Share-Based Compensation Expense |
$ | 4,112,343 | $ | 3,385,023 | $ | 2,062,457 | ||||||
|
|
|
|
|
|
|
|||||||
Share-based compensation expense consists of (a) stock options granted to employees, non-employee members of our Board of Directors, executive officers and non-employee outside consultants, (b) nonvested stock awards (i.e., restricted stock) granted to non-employee members of our Board of Directors, an executive officer and a non-employee outside consultant, and (c) cash-settled stock appreciation rights (SARs) granted to executive officers and other non-executive employees of our Company. We cannot predict the market value of our Common Stock at the time of exercise for these grants, nor the magnitude of exercises at any particular time over the terms of these grants. Our cash-settled SARs, which are classified as liability awards, have been, and will continue to be, remeasured at each reporting period until all awards are settled. Fluctuations in the fair value of a liability award are recorded as increases or decreases in compensation cost, either immediately or over the remaining service period, depending on the vesting status of the award.
37
Table of Contents
Total share-based compensation expense for fiscal 2011 and 2010 increased in relation to fiscal year 2009, primarily due to the amortized expense related to the number of years of equity grants. Additionally, fiscal 2009 had higher favorable adjustments in the fair value of our cash-settled SARs than fiscal 2011 and 2010, which were remeasured based on, among other factors, our closing stock price on June 30, 2011, 2010 and 2009. See Note 14 to the Consolidated Financial Statements included in this Form 10-K for additional information concerning our share-based compensation.
The following table summarizes our share-based compensation expense, by fiscal year grant, for our fiscal years ended June 30, 2011, 2010 and 2009.
| Fiscal Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| SARS |
$ | (127,052 | ) | $ | (163,928 | ) | $ | (390,503 | ) | |||
|
|
|
|
|
|
|
|||||||
| Stock Options |
||||||||||||
| Fiscal Year 2008 Grant |
$ | 271,122 | $ | 787,462 | $ | 940,746 | ||||||
| Fiscal Year 2009 Grant |
1,164,600 | 1,214,237 | 998,584 | |||||||||
| Fiscal Year 2010 Grant |
1,184,242 | 948,092 | — | |||||||||
| Fiscal Year 2011 Grant |
1,088,662 | — | — | |||||||||
| Other Share-Based Compensation Adjustment |
— | 61,908 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 3,708,626 | $ | 3,011,699 | $ | 1,939,330 | |||||||
|
|
|
|
|
|
|
|||||||
| Nonvested Stock Awards |
||||||||||||
| Fiscal Year 2008 Grant |
$ | 64,538 | $ | 201,556 | $ | 336,963 | ||||||
| Fiscal Year 2009 Grant |
202,340 | 231,311 | 176,667 | |||||||||
| Fiscal Year 2010 Grant |
169,584 | 104,385 | — | |||||||||
| Fiscal Year 2011 Grant |
94,307 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 530,769 | $ | 537,252 | $ | 513,630 | |||||||
|
|
|
|
|
|
|
|||||||
| Total Share-Based Compensation Expense |
$ | 4,112,343 | $ | 3,385,023 | $ | 2,062,457 | ||||||
|
|
|
|
|
|
|
|||||||
Recent Strategic Acquisitions and Investments
Orteq Sports Medicine. On December 21, 2010, we entered into a manufacturing and supply agreement with, and, pursuant to an investment agreement, made a non-controlling minority cost-method investment in preferred shares of Orteq of approximately $2.5 million. Orteq is a privately-held medical device company headquartered in London, United Kingdom, specializing in the field of biodegradable polymer technology for meniscus repair. Pursuant to the manufacturing and supply agreement with Orteq, we acquired the exclusive worldwide manufacturing rights of Actifit® (Actifit) product line from Orteq, for a period of 10 years from the date of its first U.S. commercial sale, for approximately $1.6 million. Actifit is a biocompatible synthetic meniscal scaffold which received its CE Mark approval in 2008 for the treatment of irreparable partial meniscal tears and is currently being sold throughout Europe. As previously disclosed, under the investment agreement with Orteq, we had committed to making an additional minority cost-method investment of approximately 637,000 British Pounds, which was to be in preferred shares of Orteq, payable in U.S. Dollars, if Orteq receives approval or conditional approval from the FDA for an investigational device exemption for a clinical trial by the second anniversary of the our initial investment, which is December 21, 2012, or otherwise earlier, at our option, prior to December 21, 2012. On August 19, 2011 (an event subsequent to June 30, 2011), at our option, and pursuant to an ancillary agreement, we made a cash advance to Orteq of approximately 637,000 British Pounds, or $1,055,063, structured as convertible debt. Under the agreement, we have the option to convert our cash advance into preferred shares of Orteq as an additional cost-method investment under the investment agreement, or if we do not convert our cash advance to shares, the cash advance and accrued interest will be payable by Orteq to us prior to August 2016. See Note 8 to the Consolidated Financial Statements included in this Form 10-K for additional information regarding our relationship with Orteq and the accounting treatment of these transactions.
38
Table of Contents
We expect to begin generating revenues under the manufacturing and supply agreement with Orteq after the transition of manufacturing from Orteq is completed in fiscal 2012. Such revenues are not expected to be significant in fiscal 2012.
Nerites Corporation. In January 2011, we acquired substantially all of the assets and certain operational liabilities of privately-held Nerites Corporation (Nerites), a development stage company that was in the process of developing medical adhesives and anti-fouling coatings (i.e. coatings that passively inhibit bacterial attachment or prevent biofilm formation) based in Madison, Wisconsin, for approximately $19.7 million plus acquisition-related costs of approximately $300,000. Approximately $16.7 million of the purchase price was paid at the acquisition date, financed from our available cash and investments on hand. The remaining $3.0 million, which is also expected be financed with cash on hand, was held back by us as security for certain potential Nerites indemnification obligations; of such hold-back amount, $1.5 million will be released on each of the first and second anniversaries of the acquisition date to Nerites, to the extent that the hold-back amount is not applied toward any such indemnification obligations. We expect that the technology platform of adhesive-based biomaterials acquired from Nerites will enable us to further our penetration into the regenerative medicine markets.
The transaction was accounted for as an asset acquisition, with the total cost of the acquisition allocated to the net assets acquired ($22,000), other intangible assets ($1.8 million) and acquired in-process research and development (IPR&D) ($18.2 million).
As of the acquisition date, we planned to develop a portfolio of hybrid adhesive-based products that integrate our ECM, collagen and polymer technology platforms for applications in general, neurologic, plastic/reconstructive, orthopaedic, urologic, cardiovascular and thoracic surgical specialties. Initial product targets, for which the $18.2 million acquired IPR&D was assigned, included devices for (1) repairing defects in abdominal walls ($7.1 million), (2) meniscal tears ($4.7 million), (3) articular cartilage resurfacing ($0.2 million), (4) dural membranes ($5.3 million) and (5) gastrointestinal tracts ($0.9 million). As of the date of acquisition, each of these five projects was expected to utilize the adhesive technology and was 100% dependent on developing an adhesive raw material from the Nerites technology. As of the date of acquisition, we expected to spend approximately $25.0 million over the next seven years to bring the five projects under development to technological feasibility. We believe our current cash and investment balances and expected future cash generated from operations will be sufficient to develop these projects. As of the acquisition date, assuming the research and development program to create the adhesive raw material is successful, we expected to begin receiving the estimated revenues from the five in process projects between fiscal 2013 and 2017, depending on the project.
Substantial additional research and development will be required to finalize a raw material formulation, and the unique processes specific to that formulation must be developed to enable the formulation to have the ability to be manufactured into a commercially viable medical device. There is risk that a marketable material formulation may never be developed. We may have problems manufacturing materials which are untested or whose properties are still not known. Ultimately, in order to determine the technical feasibility and commercial viability of the initial product targets, the medical device would be subject to pre-clinical testing and we would potentially need to complete clinical trials and receive regulatory approvals prior to any potential commercialization. The length of time to develop a commercially viable medical device can vary substantially according to the type, complexity and intended use of the product. Delays associated with products that we expect to develop may cause us to incur additional expenses, affect our ability to attract customers to the market and sell the products and result in new or emerging technologies obsolescing the adhesive technology and products, and our competitors may introduce or gain market acceptance for comparable products prior to us, which would impact future revenues. There can be no assurance such efforts will be successful. If these projects are not successfully developed, our revenue and profitability may be adversely affected in future periods. We are continuously monitoring our development projects and believe that the assumptions used in the valuation of acquired IPR&D reasonably estimated the future benefits attributable to such acquired IPR&D. See Note 5 to the Consolidated Financial Statements included in this Form 10-K for additional information concerning the acquisition of the net assets of Nerites and the related IPR&D. No assurance can be given that actual results will not deviate from those
39
Table of Contents
assumptions in future periods. Additionally, the value of other acquired intangible assets may become impaired. See Item 1A. “Risk Factors” herein for additional risks, uncertainties and other factors that could impact the successful development of these projects, as these projects have similar risks and uncertainties as our other research and development efforts.
Norian Corporation. On May 24, 2011, we completed our acquisition of certain operational assets and certain liabilities relating to the business and product lines of Norian, a wholly owned subsidiary of Synthes, pursuant to an Asset Purchase Agreement and related Property Purchase Agreement, for a total purchase price of approximately $26 million. We have included the financial results of Norian in our Consolidated Financial Statements from the date of acquisition.
We have accounted for the asset acquisition using the purchase method of accounting under U.S. GAAP. Under the purchase method of accounting, the total purchase price is allocated to the tangible and intangible acquired assets and assumed liabilities of Norian, based on their respective fair values as of the date of the acquisition. In allocating the total purchase price for Norian based on estimated fair values, we recorded $14 million of identifiable intangible assets and $8 million of net tangible assets, and a gain on the bargain purchase after deferred taxes of $514,000.
On May 24, 2011, we also entered into a Supply Agreement with Synthes USA Sales, LLC, a subsidiary of Synthes, which provides for us to be the exclusive manufacturer and supplier of the Norian product lines acquired under the Asset Purchase Agreement, pursuant to which Synthes will exclusively purchase from us all of its requirements for such products exclusively, on the terms set forth in the Supply Agreement. Also, on May 24, 2011, we entered into a research and development agreement with Synthes to develop certain related future products.
See Item 1A. “Risk Factors” herein for additional risks, uncertainties and other factors that could impact the success of these products.
Cost Reduction Plan
In the second quarter of fiscal 2010, we implemented a cost reduction plan, primarily associated with our reduced endovascular activities and lower production volume. This cost reduction plan was comprised of headcount reductions, primarily through a voluntary retirement program, as well as reduced work schedules. In connection with this plan, we incurred $1.9 million in pre-tax charges in our second quarter of fiscal 2010 consisting of a $1.0 million pre-tax severance charge and a $944,000 pre-tax unabsorbed overhead expense charge.
The following table summarizes the pre-tax charges resulting from the cost reduction plan within each operating expense category of our Consolidated Statements of Income for fiscal 2010:
| Fiscal Year Ended June 30, 2010 | ||||||||||||
| Severance Charge |
Unabsorbed Overhead Expense Charge |
Total Cost Reduction Plan Charges |
||||||||||
| Cost of products sold |
$ | 404,474 | $ | 943,666 | $ | 1,348,140 | ||||||
| Research and development |
535,640 | — | 535,640 | |||||||||
| Selling, general and administrative |
70,944 | — | 70,944 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Cost Reduction Plan Charges |
$ | 1,011,058 | $ | 943,666 | $ | 1,954,724 | ||||||
|
|
|
|
|
|
|
|||||||
Property Insurance Claim
In fiscal 2010, a fire occurred which was contained to a small manufacturing area within our corporate headquarters building in Exton. Our property insurance policy covered all remediation efforts and fixed asset
40
Table of Contents
replacements, subject to a $25,000 deductible. We recognized a gain of $775,000 within Cost of products sold upon final settlement with the insurance carrier as remediation and recovery efforts were completed. We received $1.3 million in total insurance proceeds for remediation efforts, replacement value of fixed assets and other expenses. We have replaced damaged fixed assets with a total value of approximately $480,000. The remaining insurance proceeds were used by us to pay for other related expenses and added to our general funds. The total insurance proceeds, including amounts relating to the outside remediation services and fixed assets, have been classified within the investing activities section of the fiscal 2010 Consolidated Statement of Cash Flows, and the balance of the insurance proceeds, used by us to pay for other related expenses, has been classified within the operating activities section of the fiscal 2010 Consolidated Statement of Cash Flows.
CRITICAL ACCOUNTING POLICIES
Our critical accounting policies are those that require application of management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about matters that are inherently uncertain and may change in future periods. Our most critical accounting policies are identified and described below:
Revenue Recognition. We recognize revenue in accordance with FASB ASC Topic 605, “Revenue Recognition” (ASC 605-10-S99). We also follow FASB ASC Subtopic 605-25, “Multiple Element Arrangements” (ASC 605-25) for customer arrangements containing multiple revenue elements that were entered into, or materially amended, after June 30, 2003.
Sales Revenue. Sales revenue is recognized in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Subtopic 605-10-S99, “Revenue Recognition” (ASC 605-10-S99). Sales revenue is recognized when the products are shipped or the services are completed. Advance payments received for products or services are recorded as deferred revenue and are recognized when the product is shipped or services are performed. We reduce sales revenue for estimated customer returns and other allowances.
In addition, we account for customer arrangements containing multiple revenue elements in accordance with ASC 605-25. We consider a variety of factors in determining the appropriate method of accounting for our multiple elements agreements, including whether the various elements can be separated and accounted for individually as separate units of accounting. When our multiple element arrangements are combined into a single unit of accounting, revenues are deferred and recognized over the expected period of performance. The specific methodology for the recognition of the revenue is determined on a case-by-case basis based on the facts and circumstances applicable to each agreement.
Up-front, non-refundable payments that do not have stand-alone value are recorded as deferred revenue once received and recognized as revenues over the expected period of performance.
We evaluate milestone payments on an individual basis and recognize revenue from non-refundable milestone payments when the earnings process is complete and the payment is reasonably assured. Non-refundable milestone payments related to arrangements under which we have continuing performance obligations are recognized using a contingency-adjusted performance model over the period of performance, where revenue is recognized for the milestone proportionately to the extent of the performance period to date and the remainder is ratably spread over the remaining performance period of the arrangement.
We also recognize grant revenue under research and development related U.S. government programs in the periods that the related expenses are incurred.
Royalty Income. Royalty revenue is recognized at the end of each quarter, when the relevant net total end-user product sales dollars are reported by customers to us for the quarter. Royalty payments are typically received within 45 days after the end of each calendar quarter.
Accounting for Share-Based Compensation. We use various forms of equity compensation, including stock options and nonvested stock awards, as a major part of our compensation programs to retain and provide
41
Table of Contents
incentives to our top management team members and other employees. In the past, we have also issued cash-settled SARs to employees. We account for equity compensation in accordance with FASB ASC Topic 718, “Compensation—Stock Compensation.” Revisions to any of our estimates or methodologies could have a material impact on our financial statements.
| • | Fair value of option grants is estimated on the date of grant using the Black-Scholes option-pricing model, which uses weighted average assumptions. Expected volatilities are based on historical volatility of our Common Stock and other factors. We use historical data to estimate option exercise and employee termination behavior within the valuation model. We separate groups of employees that have similar historical exercise behavior and consider them separately for valuation purposes. The expected terms of options are derived from historical exercise behavior and represent the periods of time that options granted are expected to be outstanding. The risk-free rate for periods within the contractual life of an option is based on U.S. treasuries with constant maturities in effect at the time of grant. |
| • | Nonvested stock awards (i.e. restricted stock) granted to non-employee members of our Board of Directors and executive officers are accounted for using the fair value method. Fair value for nonvested stock awards is based upon the closing price of our Common Stock on the date of grant. |
| • | Cash-settled SARs awarded in share-based payment transactions are classified as liability awards; accordingly, we record these awards as a component of Other current liabilities on our Consolidated Balance Sheets. The fair value of each SAR is estimated using the Black-Scholes option-pricing model, which uses weighted average assumptions. Expected volatilities are based on the historical volatility of our Common Stock, as well as other factors. For liability awards, the fair value of the award, which determines the measurement of the liability on our Consolidated Balance Sheet, is remeasured at each reporting period until the award is settled. Fluctuations in the fair value of the liability award are recorded as increases or decreases in compensation cost, either immediately or over the remaining service period, depending on the vested status of the award. We use historical data to estimate employee termination within the valuation model; employees that have similar historical exercise behavior are considered separately for valuation purposes. The expected term of cash-settled SARs has been determined using the simplified method in accordance with ASC 718-10-S99. |
Accounting for Investments in Debt Securities. We account for our investment portfolio in accordance with FASB ASC Topic 320, “Investments—Debt and Equity Securities.” We have classified our entire investment portfolio as available-for-sale securities with secondary or resale markets and report the portfolio at fair value, with unrealized gains and losses included in Stockholders’ equity and realized gains and losses in Other income. We currently have various investment securities with fair values that are less than their amortized costs and, therefore, contain unrealized losses. The unrealized losses related to these investments were due to either changes in interest rates or investments obtained at a premium. We have evaluated these securities and have determined that the decline in value is not related to any Company or industry specific event. We have no intent to sell any of these investments until there is a recovery of the fair value, and there is no current requirement that we sell any of these investments. We anticipate full recovery of amortized costs with respect to these securities at maturity or sooner in the event of a more favorable market interest rate environment. Revisions to our classification of these investments, and/or a determination other than the anticipation of a full recovery of the amortized costs at maturity or sooner, could result in our realizing gains and losses on these investments and, therefore, have a material impact on our financial statements.
Business Combinations. In fiscal 2010, we adopted ASC 805, “Business Combinations.” Accounting for business combinations requires our management to make significant estimates and assumptions, especially at the acquisition date with respect to the fair values of certain intangible assets, inventory and fixed assets. Valuations are performed to assist in determining the fair values of assets acquired and liabilities assumed, which requires management to make significant estimates and assumptions, especially with respect to intangible assets. Management makes estimates of fair value based upon assumptions believed to be reasonable. These estimates are based in part on historical experience and information obtained from management of the acquired companies and expectations of future cash flows.
42
Table of Contents
In-process research and development (IPR&D) in a business combination is accounted for as an indefinite-lived asset on the balance sheet until the associated research and development efforts are completed or abandoned. While classified as an indefinite-lived asset, acquired IPR&D would be subject to impairment testing. We would determine the useful life of the intangible asset on completion or abandonment of the associated research and development efforts. Alternatively, in an asset acquisition, we account for IPR&D in accordance with FASB ASC Topic 730, “Research and Development,” whereby the costs of intangible assets that are purchased from others for a particular research and development project that have no alternative future uses (in other research and development projects or otherwise), and therefore no separate economic values, are research and development costs at the time the costs are incurred and would be expensed and not capitalized as they do not have an alternative future use.
Cost Method Investments. We account for our non-controlling minority cost-method investments using the cost method if we do not have the ability to exercise significant influence over the operating and financial policies of the investee. On a periodic basis, but no less frequently than quarterly, we assess such investments for impairment when there are events or changes in circumstances that may have a significant adverse effect on the fair value of the investment. If, and when, an event or change in circumstances that may have a significant adverse effect on the fair value of the investment is identified, we estimate the fair value of the investment, and, if the amount by which the carrying value of the cost method investment exceeds its fair value, and the reduction in value is determined to be other than temporary, we record an impairment loss on the investment.
Valuation of Financial Instruments. We adopted the provisions of FASB ASC Topic 820, “Fair Value Measurements and Disclosures,” (ASC 820) for financial assets and liabilities, which provides guidance for using fair value to measure financial assets and liabilities by defining fair value as the exit price, or the amount that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. Categorization is based on a three-tier valuation hierarchy, which prioritizes the inputs used in measuring fair value. Our assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the valuation of financial assets and financial liabilities and their placement within the fair value hierarchy. See Note 11 to the Consolidated Financial Statements included in this Form 10-K for further discussion regarding the fair value of financial assets and liabilities.
Inventory Valuation. Our inventory is stated at the lower of cost or market. Adjustments to inventory are made at the individual part level for estimated excess, obsolescence or impaired balances, to reflect inventory at the lower of cost or market. Factors influencing these adjustments include changes in demand, technological changes, product life cycle and development plans, component cost trends, product pricing, physical deterioration and quality concerns. Revisions to these adjustments would be required if any of these factors differ from our estimates.
Income Taxes. In the course of estimating our estimated annual effective tax rate and recording our quarterly income tax provisions, we consider many factors, including our expected earnings, state income tax apportionment, estimated research and development tax credits and manufacturing deductions, non-taxable interest income and other estimates. Material changes in, or differences from, these estimates, could have a significant impact on our effective tax rate. We follow the standards related to accounting for uncertainty in income taxes and record uncertainties and judgments in the application of complex tax regulations in a multitude of jurisdictions (see Note 18 to the Consolidated Financial Statements included in this Form 10-K for additional information).
43
Table of Contents
RESULTS OF OPERATIONS
Comparison of Fiscal Years 2011 and 2010
Total Revenues. Total revenues of $71.6 million in fiscal 2011 decreased 11% from total revenues of $80.6 million in fiscal 2010.
Total Net Sales. Net sales of products decreased 15% to $46.0 million for fiscal 2011, compared to net sales of $54.3 million for fiscal 2010. We had a $7.7 million, or 15%, decrease in our biomaterials sales and a $0.6 million, or 27%, decrease in our endovascular sales.
Biomaterials Sales. Biomaterials sales were $44.4 million in fiscal 2011, a 15% decrease compared to $52.1 million in fiscal 2010. Biomaterials sales include revenue recognized from products shipped, grant revenue, as well as revenue generated from product development programs with, and milestone revenue earned from, biomaterials customers.
Sports medicine product sales of $12.1 million in fiscal 2011 decreased 27% from $16.4 million in the prior fiscal year. Although sales of sports medicine products improved in the second half of fiscal 2011 as compared to the first half of fiscal 2011 by $1.4 million, inventory reductions by one of our major customers and overall weakness in this sector adversely impacted sales for the full year. Spine product sales of $11.8 million in the fiscal year ended June 30, 2011 increased 2% from $11.6 million in the prior fiscal year. The overall spine market was weak throughout the year, and one of our major customers reduced inventory levels in the first half of the fiscal year, but did improve significantly in the third and fourth quarters, as customer inventory levels were rebalanced. Trauma and CMF products, consisting primarily of one month of sales resulting from the Norian acquisition, increased to $1.7 million during fiscal 2011 from $0.6 million in the prior fiscal year.
Cardiovascular sales of $12.7 million, consisting primarily of sales of vascular closure product components to St. Jude Medical, decreased 34% from $19.3 million in the prior fiscal year, as St. Jude Medical purchased the minimum amount of collagen plugs called for under the new supply agreement.
General surgery sales, consisting primarily of the new extracellular matrix (ECM) product, the XCM Biologic, and breast biopsy products, of $4.4 million increased 21% in the fiscal year ended June 30, 2011 from $3.6 million in the prior fiscal year. Sales of ECM products in fiscal 2011 were $3.3 million compared to $1.1 million in the prior fiscal year. This increase in ECM product sales reflected the market launch of the new XCM products by Synthes, our strategic partner. Sales of breast biopsy products were $1.0 million compared to $2.5 million in the prior fiscal year comparable period due to inventory stocking orders placed in the first half of fiscal 2010, which negatively affected our sales throughout fiscal 2011.
Endovascular Sales. Endovascular sales were $1.6 million in fiscal 2011, a 27% decrease compared to sales of $2.2 million in fiscal 2010. Endovascular sales include revenue recognized from products shipped to, as well as milestone revenue recognized from product development programs with The Spectranetics Corporation (Spectranetics).
The endovascular sales decrease was primarily due to a decrease in QuickCat product sales. Our second quarter ended December 31, 2009 of fiscal 2010 was the last quarter reflecting our sales of the QuickCat device, as the QuickCat manufacturing was transferred to Spectranetics as of December 2009. In our fourth quarter ended June 30, 2011, Spectranetics assumed manufacturing responsibility of the ThromCat products. Therefore, fiscal 2011 was the final fiscal year in which we will have product revenues from the Endovascular business. In fiscal 2011 and fiscal 2010, we recognized revenue of $577,000 for two milestones achieved in fiscal 2009 under our Development and Regulatory Service Agreement with Spectranetics. The achievement of the future remaining research and development milestone payments pursuant to the agreements with Spectranetics could be negatively affected by the performance of Spectranetics, and may at a minimum be delayed and may not be received from Spectranetics at all. As disclosed, Spectranetics publicly announced the discontinuation of sales of the Safe-Cross product line
44
Table of Contents
during our second quarter of fiscal 2010. Spectranetics’ decision to discontinue the Safe-Cross product line negatively impacts our ability to receive future royalties based on sales of the Safe-Cross product, and at a minimum, delay our achievement of the $6 million cumulative sales milestone, which is based upon sales of the Safe-Cross, ThromCat and QuickCat products. The Company is currently expecting the achievement of a $6.0 million cumulative sales milestone payment from Spectranetics in the Company’s second quarter of fiscal 2012 of which a portion would be deferred and recognized over the expected period of performance.
Royalty Income. Royalty income decreased 3% to $25.6 million in fiscal 2011 from $26.4 million in fiscal 2010.
Royalty income of $19.4 million from St. Jude Medical’s Angio-Seal net end-user sales in fiscal 2011 decreased 5% from $20.3 million in fiscal 2010, which may be the result of increased competition and a reduction in the use of closure devices. End-user sales of the Angio-Seal device decreased 5% in fiscal 2011 over the prior fiscal year.
Royalty income of $5.8 million from Orthovita’s Vitoss™, Vitoss™ Foam and Vitoss™ Bioactive Foam products net end-user sales in fiscal 2011 were consistent with fiscal 2010. End-user sales of our co-developed Vitoss™ Foam and Vitoss™ Bioactive Foam products increased 3% in fiscal 2010 from the prior fiscal year.
Cost of Products Sold.
| Fiscal
Year Ended 6/30/11 |
Fiscal
Year Ended 6/30/10 |
% Change Prior Period to Current Period |
||||||||||
| Cost of products sold |
$ | 23,402,736 | $ | 23,507,351 | 0 | % | ||||||
| Gross Margin on Net Sales |
49 | % | 57 | % | ||||||||
Cost of products sold was $23.4 million in fiscal 2011, a $105,000 decrease from $23.5 million in fiscal 2010. Gross margin on net sales was 49% and 57% for fiscal 2011 and fiscal 2010, respectively. Negatively affecting our gross margin in fiscal 2011 were product mix and higher unabsorbed overhead period costs related to reduced production levels due to lower sales and the Norian inventory step-up charge, as described below. Included within cost of products sold during fiscal 2010, as a result of the previously disclosed cost reduction plan, was $944,000 in unabsorbed overhead charges due to reduced work schedules during the second quarter of fiscal 2010, as well as $404,000 in severance charges offset by our recognition of $775,000 in net gain from property insurance proceeds for a claim under our property insurance policy.
In connection with our asset acquisition of Norian in May 2011, we recorded the purchase accounting adjustment of the fair value of acquired inventory, commonly referred to as “stepped-up value,” of $1.3 million, representing the estimated capitalized manufacturing profit in acquired inventory. This non-recurring, non-cash charge to cost of products sold will be recognized over the expected inventory turn-over period, which approximates a 6 month period, as the capitalized manufacturing profit added to inventory under purchase accounting is expected to be sold within approximately 6 months after the acquisition date. Included within cost of products sold during the fourth quarter of fiscal 2011, was approximately $257,000 of an inventory step-up charge.
We believe gross margin on net sales for fiscal 2012 will decrease as compared to fiscal 2011 due to lower margins on our acquired Norian products (including inventory step-up charge), transition costs associated with the transition of the manufacturing operations of Norian products to our Exton facility and the expected reduction of sales of Angio-Seal components.
45
Table of Contents
Research and Development Expense.
| Fiscal
Year Ended 6/30/11 |
Fiscal
Year Ended 6/30/10 |
% Change Prior Period to Current Period |
||||||||||
| Research & Development |
$ | 17,549,881 | $ | 17,876,748 | (2 | %) | ||||||
| Research & Development as a % of Revenue |
24 | % | 22 | % | ||||||||
Research and development expense was $17.5 million in fiscal 2011, a decrease of $327,000, or 2%, from $17.9 million in fiscal 2010. During fiscal 2011, we refocused our research and development efforts towards our ECM and adhesives research and development efforts. This increase in expense year over year was offset by a reduction of our endovascular research and development. Further, research and development expense decreased due to $536,000 in severance costs related to the cost reduction plan that was implemented in the second quarter ended December 31, 2009 of fiscal 2010.
Research and development expense was 24% of our revenue in fiscal 2011 compared to 22% of our revenue in fiscal 2010. We believe research and development expense in fiscal 2012 will increase to $22 million, as we continue to our ECM and adhesive research and development efforts.
Selling, General and Administrative Expense.
| Fiscal
Year Ended 6/30/11 |
Fiscal
Year Ended 6/30/10 |
% Change Prior Period to Current Period |
||||||||||
| Selling, General and Administrative |
$ | 8,973,902 | $ | 9,009,329 | 0 | % | ||||||
| Selling, General and Administrative as a % of Revenue |
13 | % | 11 | % | ||||||||
Selling, general and administrative expense was $9.0 million in fiscal 2011 consistent with selling, general and administrative expense in fiscal 2010. Selling, general and administrative expense in fiscal 2011 included transaction costs of $506,000 related to the Norian acquisition and non-cash amortization of our intangible asset (customer relationship) related to our acquisition of Norian of $77,000, which were offset by a non-cash gain on bargain purchase of $514,000 recognized under the purchase method of accounting for the amount by which the aggregate fair value of the net assets acquired exceeded the Norian acquisition purchase price.
Selling, general and administrative expenses were 13% of our revenue for the fiscal year ended 2011 and 11% of our revenue for the fiscal year ended 2010. We believe selling, general and administrative expenditures in total as a percentage of revenue will be approximately 12% in fiscal 2012. The non-cash amortization of the intangible asset (customer relationship) related to our acquisition of Norian is expected to approximate $900,000 in fiscal 2012.
Acquired in-process research and development In connection with our asset acquisition of Nerites, we allocated approximately $18.2 million of the cost of the acquisition to in-process research and development projects. These costs were charged to expense in our third quarter of fiscal 2011 because, at the date of acquisition, the development of these projects had not yet reached technological feasibility, and the research and development in progress had no alternative future uses. At the acquisition date, Nerites had spent approximately $14.2 million on ongoing research initiatives targeted at developing three potential platform technologies that they had been researching: liquid adhesives/sealants, thin film adhesives and anti-fouling/anti-bacterial coating capabilities. Nerites’ research is in a very early concept phase, as they were still testing feasibility of the adhesive technology and were primarily focused on identifying the mechanical or physical properties and reaction attributes of an adhesive raw material. See “Recent Strategic Acquisitions and Investments” above and Note 5 to the Consolidated Financial Statements included in this Form 10-K for additional information concerning the Nerites asset acquisition and the related acquired IPR&D.
46
Table of Contents
Interest Income. Interest income decreased by 40% to $422,000 in fiscal 2011 from $704,000 in fiscal 2010. This decrease was due to significant decreases in on our average cash and investment balances during the fiscal year ended June 30, 2011 compared to the prior fiscal year, due to strategic acquisitions and stock repurchases during fiscal 2011.
Interest Expense. Interest expense on our Mortgage during fiscal 2011 was $2.0 million compared to $2.1 million in the prior year. We had borrowed $35 million under the Mortgage with Citibank, F.S.B., under which the outstanding principal balance was $30 million as of June 30, 2011. The Mortgage is hedged by a fixed interest rate Swap bearing interest of 6.44%. See Note 10 to the Consolidated Financial Statements included in this Form 10-K for additional information concerning the Mortgage and the Swap.
Other Income/Loss. Other non-operating income was $101,000 in fiscal 2011, an increase of $120,000, from non-operating loss of $19,000 in fiscal 2010. Other non-operating loss in fiscal 2010 primarily represents non-operating items, including loss on foreign currency exchange. Other non-operating income for fiscal 2011 primarily represents the gain on sale of securities during the period.
Income Tax Benefit. Our tax benefit in fiscal 2011 was $78,000, resulting in an effective tax rate of approximately (4%) primarily as a result of the acquired IPR&D charge in our third quarter ended March 31, 2011, compared to the fiscal 2010 effective tax rate of approximately 33%. See Note 18 to the Consolidated Financial Statements included in this Form 10-K for additional information concerning our effective tax rate.
RESULTS OF OPERATIONS
Comparison of Fiscal Years 2010 and 2009
Total Revenues. Total revenues of $80.6 million in fiscal 2010 decreased 2% from total revenues of $82.1 million in fiscal 2009.
Total Net Sales. Net sales of products decreased 1% to $54.3 million for fiscal 2010, compared to net sales of $54.9 million for fiscal 2009. We had a $1.1 million, or 2%, increase in our biomaterials sales and a $1.7 million, or 43%, decrease in our endovascular sales.
Biomaterials Sales. Biomaterials sales were $52.1 million in fiscal 2010, a 2% increase compared to $51.0 million in fiscal 2009. Biomaterials sales include revenue recognized from products shipped, as well as revenue generated from product development programs with, and milestone revenue earned from, biomaterials customers.
The biomaterials sales increase was primarily due to our cardiovascular and general surgery product lines. Cardiovascular product sales, primarily consisting of sales of Angio-Seal components to St. Jude Medical, of $19.3 million in fiscal 2010 increased $1.0 million, or 5%, from $18.3 million in fiscal 2009 due to favorable variations in ordering patterns of components used in the manufacture of the Angio-Seal device by St. Jude Medical. Cardiovascular product sales in fiscal 2009 included a cancellation fee charged to a customer of approximately $800,000 for cardiovascular product-related research and development work we performed. Also contributing to the increase in biomaterials sales was continued growth in general surgery product sales, which were $3.6 million for fiscal 2010, an increase of $1.5 million, or 74%, from $2.1 million in fiscal 2009. This increase was due to initial shipments to Synthes related to the U.S. launch of our new ECM product, the XCM Biologic™ Tissue Matrix, as well as due to the timing of orders of collagen product sales to a customer for use in breast biopsies.
These increases in product sales were offset in part by a decrease in orthopaedic product sales. Orthopaedic product sales, consisting primarily of sports medicine and spine products, were $28.6 million for fiscal 2010, a decrease of $1.2 million, or 4%, from $29.8 million in fiscal 2009. Sales of spine products decreased $2.1 million, or 16%, to $11.6 million in fiscal 2010 from $13.7 million in fiscal 2009. The decline in sales of spine products was due to the overall weakness in the spinal market,
47
Table of Contents
as well as Orthovita’s shift in sales focus during fiscal 2010 away from our co-developed products. Additionally, in fiscal 2009, spine product sales included a one-time cancellation fee of $825,000 charged to a customer for research and development work performed. This decrease in spine products was offset by an increase in our sports medicine product sales of $980,000, or 6%, to $16.4 million in fiscal 2010 from $15.5 million in fiscal 2009, which was due primarily to favorable variations in ordering patterns.
Endovascular Sales. Endovascular sales were $2.2 million in fiscal 2010, a 43% decrease compared to sales of $3.9 million in fiscal 2009. Endovascular sales include revenue recognized from products shipped to, as well as milestone revenue recognized from product development programs with, Spectranetics.
The endovascular sales decrease was primarily due to a decrease in Safe-Cross and QuickCat product sales. Our second quarter of fiscal 2010 ended December 31, 2009 was the last quarter reflecting our sales of the QuickCat device, as the QuickCat manufacturing was transferred to Spectranetics as of December 2009. Further, Spectranetics publicly announced the discontinuation of sales of the Safe-Cross product line in the second quarter of fiscal 2010. In fiscal 2010, we recognized revenue of $577,000 as compared to $625,000 in fiscal 2009 for two milestones achieved in fiscal 2009 under our Development and Regulatory Service Agreement with Spectranetics.
Royalty Income. Royalty income decreased 3% to $26.4 million in fiscal 2010 from $27.2 million in fiscal 2009.
Royalty income of $20.3 million from St. Jude Medical’s Angio-Seal net end-user sales in fiscal 2010 decreased 4% from $21.2 million in fiscal 2009, primarily due to fewer shipping days in fiscal 2010 compared to fiscal 2009, in combination with product mix changes and the effects of foreign currency exchange rate fluctuations in fiscal 2010. End-user sales of the Angio-Seal device decreased 4% in fiscal 2010 over the prior fiscal year.
Royalty income of $5.8 million from Orthovita’s Vitoss™, Vitoss™ Foam and Vitoss™ Bioactive Foam products net end-user sales in fiscal 2010 were consistent with fiscal 2009. End-user sales of our co-developed Vitoss™ Foam and Vitoss™ Bioactive Foam products increased 4% in fiscal 2010 from the prior fiscal year.
Cost of Products Sold.
| Fiscal
Year Ended 6/30/10 |
Fiscal
Year Ended 6/30/09 |
% Change Prior Period to Current Period |
||||||||||
| Cost of products sold |
$ | 23,507,351 | $ | 24,193,941 | (3 | %) | ||||||
| Gross Margin on Net Sales |
57 | % | 56 | % | ||||||||
Cost of products sold was $23.5 million in fiscal 2010, a $687,000, or 3%, decrease from $24.2 million in fiscal 2009. Gross margin on net sales was 57% and 56% for fiscal 2010 and fiscal 2009, respectively. The decrease in cost of products sold was a result of our recognition in fiscal 2010 of $775,000 in net gain from property insurance proceeds for a claim under our property insurance policy, as well as a $600,000 reduction in expense for a provision to upgrade the Safe-Cross product that was no longer necessary due to the cancellation of the Safe-Cross product line by Spectranetics. Further contributing to the reduction of cost of products sold was the $1.0 million decrease in net sales, excluding net milestone revenue recognized of $1.0 million in fiscal 2010 and $625,000 in fiscal 2009. Offsetting these decreases within cost of products sold for fiscal 2010, as a result of the fiscal 2010 second quarter cost reduction plan, was $944,000 in unabsorbed overhead charges due to reduced work schedules during that quarter and $404,000 in severance charges.
48
Table of Contents
Research and Development Expense.
| Fiscal
Year Ended 6/30/10 |
Fiscal
Year Ended 6/30/09 |
% Change Prior Period to Current Period |
||||||||||
| Research & Development |
$ | 17,876,748 | $ | 18,134,442 | (1 | %) | ||||||
| Research & Development as a % of Revenue |
22 | % | 22 | % | ||||||||
Research and development expense was $17.9 million in fiscal 2010, a $258,000, or 1%, decrease from $18.1 million in fiscal 2009. The decrease in research and development expense was due to a $352,000 decrease in animal studies and outside services costs, a $275,000 decrease in design, development and supply expenses, a $233,000 decrease in engineering consulting costs and $197,000 decrease in expenses due to a charge incurred in the prior fiscal year in connection with a customer cancellation of research and development projects. Offsetting these decreases within research and development expense for fiscal 2010, as a result of the previously disclosed fiscal 2010 second quarter cost reduction plan, was $536,000 in severance charges that increased research and development expense. Additionally in fiscal 2010, there was an increase in personnel costs, including salaries and equity compensation expense, of $239,000.
Research and development expense was 22% of our revenue in both fiscal 2010 and fiscal 2009.
Selling, General and Administrative Expense.
| Fiscal
Year Ended 6/30/10 |
Fiscal
Year Ended 6/30/09 |
% Change Prior Period to Current Period |
||||||||||
| Selling, General and Administrative |
$ | 9,009,329 | $ | 9,219,001 | (2 | %) | ||||||
| Selling, General and Administrative as a % of Revenue |
11 | % | 11 | % | ||||||||
Selling, general and administrative expense was $9.0 million in fiscal 2010, a $210,000, or 2%, decrease from $9.2 million in fiscal 2009. Selling, general and administrative expense decreased from the prior fiscal year primarily as a result of reduced professional fees of $360,000, partially offset by $71,000 in severance charges, as a result of the previously disclosed fiscal 2010 second quarter cost reduction plan.
Selling, general and administrative expenses were 11% of our revenue for each of the fiscal years ended 2010 and 2009, respectively.
Interest Income & Interest Expense. Interest income decreased by 40% to $704,000 in fiscal 2010 from $1.2 million in fiscal 2009. This decrease was due to significant decreases in interest rates earned on our average cash and investment balances during the fiscal year ended June 30, 2010 over the prior fiscal year.
Interest expense on our Mortgage during fiscal 2010 was essentially flat at $2.1 million compared to the prior year. We had borrowed $35 million under the Mortgage with Citibank, F.S.B., under which the outstanding principal balance was $31.4 million as of June 30, 2010. The Mortgage is hedged by a fixed interest rate Swap bearing interest of 6.44%.
Other Income/Loss. Other non-operating loss was $19,000 in fiscal 2010, a decrease of $170,000, from non-operating income of $151,000 in fiscal 2009. Other non-operating income for fiscal 2009 primarily represents non-operating items, including the $134,000 of deferred revenue recognized as a component of Other income, as a result of the August 2008 final settlement of outstanding items related to our Opportunity Grant Program of the Department of Community and Economic Development of the Commonwealth of Pennsylvania Governor’s Action Team and net gain/loss on foreign currency exchange. There was no remaining deferred revenue from the Opportunity Grant Program as of June 30, 2009.
49
Table of Contents
Income Tax Expense. Our tax expense in fiscal 2010 was $9.4 million, resulting in an effective tax rate of approximately 33%. We could only claim research and development tax credits through December 31, 2009 as a component of our 2010 tax provision related to our ongoing performance of qualified research and development under the previously approved October 2008 Congressional extension of the Research & Experimentation (R&E) Tax Credit. Our fiscal 2009 effective tax rate of 33% included a retroactive adjustment to our tax provision during our second fiscal quarter ended December 31, 2008 as a result of the October 2008 extension of the R&E Tax Credit.
LIQUIDITY AND CAPITAL RESOURCES
Our cash, cash equivalents and investments were $21.9 million as of June 30, 2011, a decrease of $43.7 million from our balance of $65.7 million at June 30, 2010, the end of our prior fiscal year. Our working capital was $47.0 million as of June 30, 2011, a decrease of $33.3 million from our working capital of $80.3 million at June 30, 2010. The decrease in cash and working capital was primarily due to our stock repurchases of $30.0 million, principally under our stock repurchase programs and our acquisitions of the net assets of Nerites and Norian. See “Stock Repurchase Programs” below and Note 13 to the Consolidated Financial Statements included in this Form 10-K for additional information on our stock repurchases and Note 5 to the Consolidated Financial Statements included in this Form 10-K for additional information on our acquisitions.
Cash Flows
Operating Activities. Net cash provided by our operating activities was $20.4 million in the fiscal year ended June 30, 2011. For the fiscal year ended June 30, 2011, we had net income of $2.1 million, non-cash depreciation and amortization of $6.8 million, the net effect of non-cash employee share-based compensation and related tax events of $5.3 million and a non-cash acquired IPR&D charge of $18.2 million associated with the acquisition of the net assets of Nerites. The increases in cash were offset in part by a net change in deferred income taxes of $5.7 million, which was primarily the result of the increase in our non-current deferred tax assets, primarily due to the federal tax treatment of our acquired IPR&D, and a gain on bargain purchase after deferred taxes, related to the Norian acquisition of $514,000. Cash used by operations as a result of changes in asset and liability balances was $5.7 million. This decrease in cash was primarily a result of a decrease in accounts payable and accrued expenses of $2.6 million, primarily related to a federal tax payable balance and accrued bonuses in the prior fiscal year, which were paid out during fiscal 2011, an increase in prepaid expenses and other assets of $1.7 million, related to the Company prepaying its health insurance in fiscal 2011 to receive a discount and a decrease in deferred revenue of approximately $0.9 million related to recognition of deferred revenue from milestone payments from research and development and manufacturing agreements with customers. Also reducing cash provided by operations was an increase in our receivable balances of $0.3 million and an increase in our inventory balances of $0.2 million.
Investing Activities. Cash used in investing activities was $5.2 million for the fiscal year ended June 30, 2011. This use of cash was the result of $17.1 million of cash paid in the asset acquisition of the net assets of Nerites, $11.9 million of cash paid in the acquisition of the net assets of Norian and $4.1 million of cash paid in our cost method investment in Orteq and the purchase of the manufacturing rights of Actifit® products from Orteq, as well as from capital expenditures of $2.3 million to continue to expand our research and development and manufacturing capabilities and improve our information technology systems. These uses of cash were offset by $30.1 million of maturities within our investment portfolio.
Financing Activities. Cash used in financing activities was $28.1 million for the fiscal year ended June 30, 2011. This amount was primarily the result of $30.0 million in Common Stock repurchases and $1.4 million in repayments of long-term debt, offset in part by $4 million in net cash proceeds of approximately $8.3 million from the exercise of share-based awards, of which $4.4 million of the exercised shares were exchanged in a non-cash net share settlement.
50
Table of Contents
Stock Repurchase Program
From time to time, we have made repurchases of our Common Stock, executed under the various stock repurchase programs established by our Board of Directors, and through other miscellaneous transactions.
On December 10, 2009, we announced that our Board of Directors approved a stock repurchase program which allowed us to repurchase up to a total of 400,000 of our issued and outstanding shares of Common Stock. On February 9, 2010, we announced that our Board of Directors approved a new stock repurchase program allowing us to repurchase up to a total of $30 million of our issued and outstanding shares of Common Stock. On June 16, 2010, we announced that our Board of Directors approved an additional stock repurchase program allowing us to repurchase up to an additional $30 million of our issued and outstanding shares of Common Stock.
During fiscal 2010, we repurchased and retired 1,794,705 shares of Common Stock at a total cost of approximately $41.3 million, or an average price of $23.02 per share, using available cash. Commissions and other related costs associated with these stock repurchases totaled $55,788. An additional 34,400 shares were repurchased in June 2010, in trades that settled in July 2010, at a cost of $811,054, or an average market price per share of $23.58.
During the fiscal year ended June 30, 2011, we repurchased 1,175,738 shares of Common Stock, at a total cost of approximately $30.0 million, or an average market price of $25.52 per share, using available cash. This program was completed prior to the second quarter ended December 31, 2010, and accordingly, as of June 30, 2011, there were no amounts remaining to repurchase shares of Common Stock under that stock repurchase program. See Note 13 to the Consolidated Financial Statements included in this Form 10-K and Part II. Item 5 (Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities) of this Form 10-K.
General
We plan to continue to increase our research and development spending for our biomaterials products as we expand our clinical activities relating to our ECM programs both in the U.S. and outside the U.S., our cartilage repair program outside the U.S. and other new technologies, including those acquired in the acquisition of the net assets of Nerites. Furthermore, in fiscal 2012, we plan to build additional inventories related to the Norian product line as we prepare for the transfer of manufacturing of the Norian product line from the West Chester facility to the Exton facility over the next 18 to 24 months.
We continue to believe our current cash and investment balances and expected future cash generated from operations will be sufficient to meet our operating, financing and capital requirements for the next 12 months. Although we believe our cash and investment balances will also be sufficient on a longer term basis, that will depend on numerous factors, including the following: continuation of our existing customer relationships and royalty streams; market acceptance of our existing and future products; the successful commercialization of products in development; the costs associated with that commercialization; progress in our product development efforts; the magnitude and scope of such efforts; progress with pre-clinical studies, future clinical trials and product clearance by the FDA and other agencies; the cost and timing of our efforts to expand our manufacturing, sales, and marketing capabilities; the cost of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; competing technological and market developments; the development of strategic alliances for the marketing of certain of our products; and the cost of entering into any future acquisitions or other strategic transactions.
On May 26, 2011, we entered into a $35 million revolving credit facility with Wells Fargo. During fiscal 2011, we had no borrowings under the Revolving Credit Facility. As of June 30, 2011, the outstanding principal under the Mortgage was $30 million (See Note 8 to the Consolidated Financial Statements included in this Form 10-K).
The terms of any future equity financing we undertake may be dilutive to our stockholders and the terms of any debt financing may contain restrictive covenants that limit our ability to pursue desired courses of action. Our
51
Table of Contents
ability to obtain financing is dependent on the status of our future business prospects, as well as conditions prevailing in the relevant capital markets. No assurance can be given that any additional financing will be available to us, or will be available to us on acceptable terms, should such a need arise. As of June 30, 2011 we were in compliance with all of our affirmative, restrictive and financial maintenance covenants relating to our Mortgage and Revolving Credit Facility.
Contractual Obligations and Other Contingent Commitments
Presented below is a summary of our approximate aggregate contractual obligations and other contingent commitments at June 30, 2011, for future payments under contracts and other contingent commitments, for fiscal 2012 and beyond. These obligations are related to the Mortgage and other agreements that are legally binding and enforceable against us. Changes in our business needs, cancellation provisions, and other factors may result in actual payments differing from these estimates.
| Payments Due by Period | ||||||||||||||||||||
| Contractual Obligations |
Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years |
|||||||||||||||
| Long-Term Debt Obligations (1): |
||||||||||||||||||||
| Secured Commercial Mortgage |
$ | 29,983,330 | $ | 1,399,997 | $ | 2,800,000 | $ | 2,800,000 | $ | 22,983,333 | ||||||||||
| Interest on Mortgage |
21,055,698 | 1,910,515 | 3,557,876 | 3,200,555 | 12,386,752 | |||||||||||||||
| Operating Leases (2) |
1,224,797 | 894,911 | 329,886 | — | — | |||||||||||||||
| Purchase Obligations (3): |
1,903,345 | 1,903,345 | — | — | — | |||||||||||||||
| Other Obligations: |
||||||||||||||||||||
| Research and Development Contractual Obligations (4) |
2,720,157 | — | — | — | — | |||||||||||||||
| Cost Method Investment Cash Advance Obligation (5) |
1,055,063 | — | — | — | — | |||||||||||||||
| Employment Agreements (6) |
1,294,773 | 1,110,606 | 184,167 | — | — | |||||||||||||||
| Other Long-Term Liabilities Reflected on Consolidate Balance Sheet under U.S. GAAP |
||||||||||||||||||||
| Deferred Revenue Non-Current (7) |
2,465,943 | — | 804,784 | 636,312 | 1,024,847 | |||||||||||||||
| Other Non-Current Liabilities (8) |
4,919,657 | — | — | — | — | |||||||||||||||
| Nerites Corporation Purchase Price Obligation (9) |
3,000,000 | 1,500,000 | 1,500,000 | — | — | |||||||||||||||
| Norian Corporation Purchase Price Obligation (10) |
14,000,000 | — | 14,000,000 | — | — | |||||||||||||||
| FIN 48 Tax Obligations (11) |
— | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total Contractual Obligations |
$ | 83,622,763 | $ | 8,719,374 | $ | 23,176,713 | $ | 6,636,867 | $ | 36,394,932 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
These obligations are related to the Mortgage and other agreements that are legally binding and enforceable against us.
| (1) | The long-term debt obligations consist of principal and interest on the Mortgage outstanding principal balance of $29.9 million as of June 30, 2011. In accordance with accounting principles generally accepted in the United States of America (U.S. GAAP), the future interest obligations are not recorded on our Consolidated Balance Sheet. See Note 10 to the Consolidated Financial Statements included in this Form 10-K. |
| (2) | We have become party to certain operating leases, to which Nerites was previously a party for the leased space in Madison, Wisconsin, as well as for office equipment, for which the minimum lease payments of $119,797 are presented. Further, we entered into an agreement with Synthes to sublease from them manufacturing space in the Company’s West Chester, Pennsylvania facility (which facility we have purchased and leased back to Synthes), for which the minimum lease payments of $1,105,000 for the 18-month term lease are presented. |
52
Table of Contents
| (3) | These obligations consist of cancelable and non-cancelable purchase commitments related to inventory, capital expenditures and other goods or services. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. See Note 10 to the Consolidated Financial Statements included in this Form 10-K. |
| (4) | The amount reflects only payment obligations that are fixed and determinable. Under the Development and Regulatory Services Agreement with Spectranetics, as amended, our contributions are capped at a maximum amount of $2,750,000 toward the expenses associated with clinical studies to obtain approval from the FDA for certain next-generation endovascular products, reduced by the total cumulative expenses incurred through June 30, 2011 of approximately $30,000. We are unable to reliably estimate the amount and timing of these contributions because they are dependent on the type and complexity of the clinical studies and intended uses of the products, which have not been established. We entered into research and development service agreements with certain other customers which provide that we are to share certain regulatory and clinical costs associated with future research and development activities. The amounts and timing of any such future payment obligations cannot currently be determined, and therefore, are not included in the table above. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. |
| (5) | The amount reflects our additional committed investment in Orteq. On August 19, 2011 (a subsequent event to June 30, 2011), at our option, and pursuant to an ancillary agreement, we had made an additional investment in Orteq of approximately 637,000 British Pounds, or $1,055,063, in the form of a cash advance to Orteq, structured as convertible debt. As previously disclosed, under the investment agreement with Orteq, we had committed to making an additional minority cost-method investment of approximately 637,000 British Pounds, which was to be in preferred shares of Orteq, payable in U.S. Dollars, if Orteq receives approval or conditional approval from the FDA to conduct an investigational device exemption for a product pivotal trial by the second anniversary of the our initial investment, which is December 21, 2012, or otherwise earlier, at our option, prior to December 21, 2012. Under the ancillary agreement, we have the option to convert our cash advance into preferred shares of as an additional cost-method investment under the investment agreement,, or if we do not convert our cash advance to shares, the cash advance and accrued interest will be payable by Orteq to us prior to August 2016. This cash investment did not increase our ownership interest in Orteq. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. |
| (6) | We have entered into employment agreements with certain of our named executive officers. As of June 30, 2011, these employment agreements provided for, among other things, annual base salaries in an aggregate amount of not less than this amount, from that date through fiscal 2012. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. See Note 16 to the Consolidated Financial Statements included in this Form 10-K. |
| (7) | Non-current deferred revenue includes milestone payments received by us pursuant to customer agreements, as well as advance payments from customers for future services. Several of these deferred milestone revenues are non-refundable and may not require future performance from us. These liabilities are recorded in accordance with U.S. GAAP, and are recorded on our Consolidated Balance Sheets. |
| (8) | This value represents the estimated amount we would pay to terminate the Swap if we were to prepay the Mortgage. We currently do not intend to prepay the Mortgage and, therefore, are unable to reliably estimate the period of cash settlement of the Swap, if any. In accordance with U.S. GAAP, this liability is recorded on our Consolidated Balance Sheets. See Note 10 to the Consolidated Financial Statements included in this Form 10-K. |
| (9) | Under the Asset Purchase Agreement with Nerites, entered into on January 28, 2011, we acquired substantially all of the assets and certain operational liabilities of Nerites for approximately $20 million, of which approximately $16.7 million was paid at the acquisition date, with the remaining approximately $3 million held back under the terms of the acquisition as security for certain potential Nerites indemnification obligations; of such hold-back amount, $1.5 million will be released on each of the first and second anniversaries of the acquisition date to Nerites, to the extent that the hold-back amount is not applied toward such indemnification. |
| (10) | Under the Asset Purchase Agreement with Norian, entered into on May 24, 2011, we acquired certain operational assets and certain liabilities of Norian for approximately $26 million, of which approximately $12 million was paid at the acquisition date. We will pay the remaining $14 million on the earlier of the date |
53
Table of Contents
| on which the transfer of manufacturing operations from the purchased West Chester, Pennsylvania facility to the Company’s facility located in Exton, Pennsylvania has been completed or the 18-month anniversary of the acquisition date. |
| (11) | Liabilities for uncertain tax positions in the aggregate amount of approximately $104,000 have been omitted from the table above due to an inability to reliably estimate the period of cash settlement of these liabilities. See Note 18 to the Consolidated Financial Statements included in this Form 10-K. |
Our estimate of the time periods for which our cash and cash equivalents will be adequate to fund operations is a forward looking statement within the meaning of Private Securities Litigation Reform Act of 1995 and is subject to risks and uncertainties. Actual results may differ materially from those contemplated in such forward-looking statements. In addition to those described above, factors which may cause such a difference are set forth in Item 1A (Risk Factors) of this Annual Report on Form 10-K. See “Cautionary Note Regarding Forward-Looking Statements.”
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Our interest income and expense are sensitive to changes in the general level of interest rates. In this regard, changes in interest rates affect the interest earned on our cash, cash equivalents and investments, as well as the fair value of our investments and the Swap, and will also affect the borrowing rate available under our unsecured revolving credit facility.
Investment Portfolio
Our investment portfolio consists of high quality municipal securities. The majority of these investments have maturities ranging from less than one year to approximately three years. We mitigate default risk by investing in what we believe are safe and high credit quality securities and by monitoring the credit rating of investment issuers. Our portfolio includes only securities with secondary or resale markets. We have an audit committee-approved investment strategy, which currently limits the duration and types of our investments. These available-for-sale securities are subject to interest rate risk and decreases in market value if interest rates increase. As of June 30, 2011, our total investment portfolio consisted of approximately $11.7 million of investments. While our investments generally may be sold at any time because the portfolio includes available-for-sale securities with secondary or resale markets, we generally hold securities until the earlier of their call date or their maturity. We do not expect our results of operations or cash flows to be materially impacted due to a sudden change in interest rates. We review our investments to identify and evaluate investments that have an indication of possible impairment. We have no intent to sell any of these investments until a recovery of their respective fair values, which may be at maturity, and we have no current requirement to sell any of these investments. Additional information regarding our investments is located in Note 2 to the Consolidated Financial Statements included herein in this Form 10-K.
Debt
On May 25, 2006, we entered into a $35 million aggregate ten-year fixed interest rate Swap, with Citibank, N.A., to manage the market risk from changes in interest rates under the Mortgage. As of June 30, 2011, the outstanding principal under the Mortgage was $30 million (See Note 10 to the Consolidated Financial Statements included in this Form 10-K). Our objective and strategy for undertaking the Swap was to hedge our exposure to variability in cash flows and interest expense associated with the future interest rate payments under the Mortgage and to reduce our interest rate risk in the event of an unfavorable interest rate environment. We currently utilize the Hypothetical Derivative Method in determining the hedge effectiveness of the hedged item each period. We do not expect our results of operations or cash flows to be materially impacted due to a sudden change in interest rates. If the conditions underlying the Swap or the hedge item change, there is a risk that our hedged item would be deemed an ineffective hedge, and therefore, we would record changes in the fair value of the Swap within our Consolidated Statements of Income, as well as our Consolidated Statements of Cash Flows.
54
Table of Contents
Additional information regarding the Swap is located in Note 10—under the heading “Interest Rate Swap Agreement” to the Consolidated Financial Statements included in this Form 10-K.
On May 26, 2011, we entered into a Loan and Agency Agreement (the “Credit Agreement”) with Wells Fargo Bank, National Association (Wells Fargo) which provides for a three-year, unsecured revolving credit facility (Revolving Credit Facility) of $35,000,000 (See Note 10 to the Consolidated Financial Statements included in this Form 10-K). Under the terms of the Credit Agreement, we may borrow up to the aggregate amount of the unused commitment under the Revolving Credit Facility. The maturity date for the Revolving Credit Facility is May 25, 2014. Our objective and strategy for entering into the Credit Agreement was to have available resources that may be used for the working capital and general corporate purposes of the Company, including permitted acquisitions and capital expenditures. As of June 30, 2011, there were no borrowings under the Revolving Credit Facility. If interest rates were to fluctuate, there is a risk that any outstanding balance would be impacted by the prevailing rate, which may further impact our ability to repay the outstanding balance.
Foreign Currency Exchange Rate Risk
The Company’s business is not directly dependent on foreign operations, as the Company’s sales to customers outside the U.S. are not significant. However, a portion of the Company’s total revenues, including sales and royalties, are dependent on U.S. based customers selling to end-users outside the U.S. There is a risk related to the changes in foreign currency exchange rates as it relates to our royalties paid to us in U.S. dollars for which royalties are received on end-user sales within foreign countries. In addition, we have a cost method investment in Orteq, a United Kingdom-based company. We have also made a cash advance to Orteq, structured as convertible debt, in August 2011. We are currently not taking any affirmative steps to hedge the risk of fluctuations in foreign currency exchange rates. We do not expect our financial position, results of operations or cash flows to be materially impacted due to a sudden change in foreign currency exchange rates fluctuations relative to the U.S. Dollar.
55
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The following Consolidated Financial Statements, and the related Notes thereto, of Kensey Nash Corporation, and the Report of Independent Registered Public Accounting Firm are filed as a part of this Form 10-K.
All other schedules are omitted either because they are not applicable or because information required therein is shown in the Consolidated Financial Statements or Notes thereto.
56
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Kensey Nash Corporation
Exton, Pennsylvania
We have audited the accompanying consolidated balance sheets of Kensey Nash Corporation and subsidiaries (the “Company”) as of June 30, 2011 and 2010, and the related consolidated statements of income, stockholders’ equity, and cash flows for each of the three years in the period ended June 30, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of Kensey Nash Corporation and subsidiaries as of June 30, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended June 30, 2011, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of June 30, 2011, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated September 12, 2011 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ DELOITTE & TOUCHE LLP
Philadelphia, Pennsylvania
September 12, 2011
57
Table of Contents
CONSOLIDATED BALANCE SHEETS
| June 30, 2011 |
June 30, 2010 |
|||||||
| ASSETS |
||||||||
| CURRENT ASSETS: |
||||||||
| Cash and cash equivalents |
$ | 10,219,165 | $ | 23,102,362 | ||||
| Investments |
11,722,204 | 42,571,544 | ||||||
| Trade receivables, net of allowance for doubtful accounts of $5,867 and $13,513 at June 30, 2011 and 2010, respectively |
5,804,281 | 5,307,563 | ||||||
| Royalties receivable |
6,217,841 | 5,818,310 | ||||||
| Other receivables (including approximately $10,333 and $2,582 at June 30, 2011 and 2010, respectively, due from employees) |
508,517 | 1,119,703 | ||||||
| Inventory |
16,629,027 | 8,885,875 | ||||||
| Deferred tax asset, current portion |
1,564,086 | 2,857,262 | ||||||
| Prepaid expenses and other |
2,807,331 | 1,091,760 | ||||||
|
|
|
|
|
|||||
| Total current assets |
55,472,452 | 90,754,379 | ||||||
|
|
|
|
|
|||||
| PROPERTY, PLANT AND EQUIPMENT, AT COST: |
||||||||
| Land |
5,793,601 | 4,883,591 | ||||||
| Building |
50,165,848 | 46,489,239 | ||||||
| Machinery, furniture and equipment |
35,698,817 | 32,728,301 | ||||||
| Construction in progress |
232,145 | 63,322 | ||||||
|
|
|
|
|
|||||
| Total property, plant and equipment |
91,890,411 | 84,164,453 | ||||||
| Accumulated depreciation |
(33,941,952 | ) | (29,179,563 | ) | ||||
|
|
|
|
|
|||||
| Net property, plant and equipment |
57,948,459 | 54,984,890 | ||||||
|
|
|
|
|
|||||
| OTHER ASSETS: |
||||||||
| Deferred tax asset, non-current portion |
8,371,680 | 1,872,619 | ||||||
| Acquired patents and other intangibles, net of accumulated amortization of $7,730,462 and $6,628,605 at June 30, 2011 and 2010, respectively |
18,182,149 | 2,048,595 | ||||||
| Goodwill |
4,366,273 | 4,366,273 | ||||||
| Other non-current assets |
2,578,662 | 93,973 | ||||||
|
|
|
|
|
|||||
| Total other assets |
33,498,764 | 8,381,460 | ||||||
|
|
|
|
|
|||||
| TOTAL |
$ | 146,919,675 | $ | 154,120,729 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
| CURRENT LIABILITIES: |
||||||||
| Accounts payable |
$ | 2,455,607 | $ | 2,460,740 | ||||
| Accrued expenses |
2,250,113 | 5,494,910 | ||||||
| Other current liabilities |
1,504,784 | 131,836 | ||||||
| Current portion of debt |
1,399,997 | 1,399,997 | ||||||
| Deferred revenue |
946,568 | 947,378 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
8,557,069 | 10,434,861 | ||||||
|
|
|
|
|
|||||
| OTHER LIABILITIES: |
||||||||
| Long-term debt |
28,583,333 | 29,983,333 | ||||||
| Deferred revenue, non-current |
2,465,943 | 3,336,780 | ||||||
| Long-term deferred acquisition payments |
15,500,000 | — | ||||||
| Other non-current liabilities |
4,976,652 | 5,542,509 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
60,082,997 | 49,297,483 | ||||||
|
|
|
|
|
|||||
| COMMITMENTS AND CONTINGENCIES (Note 16) |
— | — | ||||||
| STOCKHOLDERS’ EQUITY: |
||||||||
| Preferred stock, $.001 par value, 100,000 shares authorized, of which 25,000 shares are designated as Series A junior participating preferred stock, $.001 par value, no shares issued or outstanding at June 30, 2011 and June 30, 2010 |
— | — | ||||||
| Common stock, $.001 par value, 25,000,000 shares authorized, 8,612,590 and 9,437,236 shares issued and outstanding at June 30, 2011 and 2010, respectively |
8,612 | 9,403 | ||||||
| Capital in excess of par value |
7,065,455 | 27,528,282 | ||||||
| Retained earnings |
82,639,873 | 80,561,830 | ||||||
| Accumulated other comprehensive loss |
(2,877,262 | ) | (3,276,269 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
86,836,678 | 104,823,246 | ||||||
|
|
|
|
|
|||||
| TOTAL |
$ | 146,919,675 | $ | 154,120,729 | ||||
|
|
|
|
|
|||||
See notes to Consolidated Financial Statements.
58
Table of Contents
CONSOLIDATED STATEMENTS OF INCOME
| Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| REVENUES: |
||||||||||||
| Net sales |
||||||||||||
| Biomaterial sales |
$ | 44,409,527 | $ | 52,096,283 | $ | 51,045,578 | ||||||
| Endovascular sales |
1,589,250 | 2,180,421 | 3,858,187 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total net sales |
45,998,777 | 54,276,704 | 54,903,765 | |||||||||
| Royalty income |
25,638,831 | 26,370,841 | 27,177,085 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
71,637,608 | 80,647,545 | 82,080,850 | |||||||||
|
|
|
|
|
|
|
|||||||
| OPERATING COSTS AND EXPENSES: |
||||||||||||
| Cost of products sold |
23,402,736 | 23,507,351 | 24,193,941 | |||||||||
| Research and development |
17,549,881 | 17,876,748 | 18,134,442 | |||||||||
| Selling, general and administrative |
8,973,902 | 9,009,329 | 9,219,001 | |||||||||
| Acquired in-process research and development |
18,233,006 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating costs and expenses |
68,159,525 | 50,393,428 | 51,547,384 | |||||||||
|
|
|
|
|
|
|
|||||||
| INCOME FROM OPERATIONS |
3,478,083 | 30,254,117 | 30,533,466 | |||||||||
|
|
|
|
|
|
|
|||||||
| OTHER INCOME (EXPENSE): |
||||||||||||
| Interest income |
421,920 | 704,435 | 1,179,851 | |||||||||
| Interest expense |
(2,000,549 | ) | (2,087,157 | ) | (2,075,486 | ) | ||||||
| Other income (loss) |
100,614 | (18,970 | ) | 151,435 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other expense—net |
(1,478,015 | ) | (1,401,692 | ) | (744,200 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| INCOME BEFORE INCOME TAX |
2,000,068 | 28,852,425 | 29,789,266 | |||||||||
| Income tax benefit/(expense) |
77,975 | (9,388,301 | ) | (9,710,156 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| NET INCOME |
$ | 2,078,043 | $ | 19,464,124 | $ | 20,079,110 | ||||||
|
|
|
|
|
|
|
|||||||
| BASIC EARNINGS PER SHARE |
$ | 0.24 | $ | 1.83 | $ | 1.74 | ||||||
|
|
|
|
|
|
|
|||||||
| DILUTED EARNINGS PER SHARE |
$ | 0.23 | $ | 1.78 | $ | 1.69 | ||||||
|
|
|
|
|
|
|
|||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING |
8,658,319 | 10,623,926 | 11,547,266 | |||||||||
|
|
|
|
|
|
|
|||||||
| DILUTED WEIGHTED AVERAGE COMMON SHARES OUTSTANDING |
8,893,402 | 10,936,590 | 11,897,835 | |||||||||
|
|
|
|
|
|
|
|||||||
See notes to Consolidated Financial Statements.
59
Table of Contents
KENSEY NASH CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| Capital | Accumulated | |||||||||||||||||||||||||||
| in Excess | Other | |||||||||||||||||||||||||||
| Common Stock | of Par | Retained | Comprehensive | Comprehensive | ||||||||||||||||||||||||
| Shares | Amount | Value | Earnings | (Loss)/Income | Income/(Loss) | Total | ||||||||||||||||||||||
| BALANCE, JUNE 30, 2008 |
11,640,221 | $ | 11,640 | $ | 75,242,265 | $ | 41,018,596 | $ | (1,702,845 | ) | $ | 114,569,656 | ||||||||||||||||
| Exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
301,930 | 302 | 5,148,912 | 5,149,214 | ||||||||||||||||||||||||
| Nonvested stock awards |
14,852 | 15 | (15 | ) | — | |||||||||||||||||||||||
| Stock repurchase |
(814,941 | ) | (815 | ) | (19,493,613 | ) | (19,494,428 | ) | ||||||||||||||||||||
| Tax benefit/(deficiency) from exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
1,756,244 | 1,756,244 | ||||||||||||||||||||||||||
| Nonvested stock awards |
(48,748 | ) | (48,748 | ) | ||||||||||||||||||||||||
| Employee stock-based compensation: |
||||||||||||||||||||||||||||
| Stock options |
1,990,956 | 1,990,956 | ||||||||||||||||||||||||||
| Nonvested stock awards |
439,607 | 439,607 | ||||||||||||||||||||||||||
| Net Income |
20,079,110 | $ | 20,079,110 | 20,079,110 | ||||||||||||||||||||||||
| Change in unrealized gain on investments (net of tax) |
123,869 | 123,869 | 123,869 | |||||||||||||||||||||||||
| Change in interest rate swap unrealized loss (net of tax) |
(1,290,901 | ) | (1,290,901 | ) | (1,290,901 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Comprehensive income |
$ | 18,912,078 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| BALANCE, JUNE 30, 2009 |
11,142,062 | $ | 11,142 | $ | 65,035,608 | $ | 61,097,706 | $ | (2,869,877 | ) | $ | 123,274,579 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
65,722 | 66 | 880,202 | 880,268 | ||||||||||||||||||||||||
| Nonvested stock awards |
21,577 | 22 | (22 | ) | — | |||||||||||||||||||||||
| Exchange of nonvested shares for taxes |
(920 | ) | (1 | ) | (20,976 | ) | (20,977 | ) | ||||||||||||||||||||
| Stock repurchase |
(1,825,605 | ) | (1,826 | ) | (42,088,971 | ) | (42,090,797 | ) | ||||||||||||||||||||
| Tax benefit from exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
192,407 | 192,407 | ||||||||||||||||||||||||||
| Nonvested stock awards |
5,015 | 5,015 | ||||||||||||||||||||||||||
| Employee stock-based compensation: |
||||||||||||||||||||||||||||
| Stock options |
3,011,699 | 3,011,699 | ||||||||||||||||||||||||||
| Nonvested stock awards |
513,320 | 513,320 | ||||||||||||||||||||||||||
| Net Income |
19,464,124 | $ | 19,464,124 | 19,464,124 | ||||||||||||||||||||||||
| Change in unrealized gain on investments (net of tax) |
269,354 | 269,354 | 269,354 | |||||||||||||||||||||||||
| Change in interest rate swap unrealized loss (net of tax) |
(675,746 | ) | (675,746 | ) | (675,746 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Comprehensive income |
$ | 19,057,732 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| BALANCE, JUNE 30, 2010 |
9,402,836 | $ | 9,403 | $ | 27,528,282 | $ | 80,561,830 | $ | (3,276,269 | ) | $ | 104,823,246 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
553,741 | 553 | 8,339,035 | 8,339,588 | ||||||||||||||||||||||||
| Nonvested stock awards |
25,523 | 25 | (25 | ) | — | |||||||||||||||||||||||
| Exchange of shares for taxes |
(50,365 | ) | (50 | ) | (1,239,935 | ) | (1,239,985 | ) | ||||||||||||||||||||
| Net share settlement of stock options |
(177,807 | ) | (178 | ) | (4,377,431 | ) | (4,377,609 | ) | ||||||||||||||||||||
| Stock repurchase |
(1,141,338 | ) | (1,141 | ) | (29,222,040 | ) | (29,223,181 | ) | ||||||||||||||||||||
| Tax benefit from exercise/issuance of: |
||||||||||||||||||||||||||||
| Stock options |
1,750,327 | 1,750,327 | ||||||||||||||||||||||||||
| Nonvested stock awards |
47,847 | 47,847 | ||||||||||||||||||||||||||
| Employee stock-based compensation: |
||||||||||||||||||||||||||||
| Stock options |
3,708,626 | 3,708,626 | ||||||||||||||||||||||||||
| Nonvested stock awards |
530,769 | 530,769 | ||||||||||||||||||||||||||
| Net Income |
2,078,043 | $ | 2,078,043 | 2,078,043 | ||||||||||||||||||||||||
| Change in unrealized gain on investments (net of tax) |
(194,354 | ) | (194,354 | ) | (194,354 | ) | ||||||||||||||||||||||
| Change in interest rate swap unrealized loss (net of tax) |
593,361 | 593,361 | 593,361 | |||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Comprehensive income |
$ | 2,477,050 | ||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| BALANCE, JUNE 30, 2011 |
8,612,590 | $ | 8,612 | $ | 7,065,455 | $ | 82,639,873 | $ | (2,877,262 | ) | $ | 86,836,678 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
See notes to Consolidated Financial Statements.
60
Table of Contents
KENSEY NASH CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| OPERATING ACTIVITIES: |
||||||||||||
| Net income |
$ | 2,078,043 | $ | 19,464,124 | $ | 20,079,110 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
| Depreciation and amortization |
6,753,597 | 7,276,947 | 6,723,345 | |||||||||
| Employee stock-based compensation: |
||||||||||||
| Stock options |
3,708,626 | 3,011,699 | 1,939,330 | |||||||||
| Nonvested stock awards |
530,769 | 537,252 | 513,630 | |||||||||
| Cash-settled stock appreciation rights |
(127,052 | ) | (163,928 | ) | (390,503 | ) | ||||||
| Tax benefit/(deficiency) from exercise of stock options |
||||||||||||
| Stock options |
1,750,327 | 192,407 | 1,756,244 | |||||||||
| Nonvested stock awards |
47,847 | 5,015 | (48,748 | ) | ||||||||
| Excess tax benefits from share-based payment arrangements |
(607,256 | ) | (150,480 | ) | (987,707 | ) | ||||||
| Deferred income taxes |
(5,702,589 | ) | (1,344,317 | ) | 1,218,097 | |||||||
| Loss on retirement of property, plant and equipment |
1,938 | 20,901 | 45,726 | |||||||||
| Gain on property insurance claim and related expenses |
— | (1,024,144 | ) | — | ||||||||
| Gain on bargain purchase of net assets acquired after deferred taxes |
(514,146 | ) | — | — | ||||||||
| Acquired in-process research & development charge |
18,233,006 | — | — | |||||||||
| Changes in assets and liabilities which provided (used) cash: |
||||||||||||
| Accounts receivable |
(269,314 | ) | (774,144 | ) | 2,827,150 | |||||||
| Prepaid expenses and other current assets |
(1,710,910 | ) | (190,755 | ) | 860,485 | |||||||
| Inventory |
(231,756 | ) | 1,699,190 | (1,314,200 | ) | |||||||
| Accounts payable and accrued expenses |
(2,608,435 | ) | (171,076 | ) | (1,474,752 | ) | ||||||
| Deferred revenue |
(20,092 | ) | 164,472 | 181,775 | ||||||||
| Deferred revenue, non-current |
(870,837 | ) | 1,527,878 | 1,503,963 | ||||||||
| Other non-current liabilities |
(11,400 | ) | — | (202,704 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by operating activities |
20,430,366 | 30,081,041 | 33,230,241 | |||||||||
|
|
|
|
|
|
|
|||||||
| INVESTING ACTIVITIES: |
||||||||||||
| Additions to property, plant and equipment |
(2,263,259 | ) | (2,668,175 | ) | (3,679,240 | ) | ||||||
| Acquisition of net assets of Nerites Corporation |
(17,068,434 | ) | — | — | ||||||||
| Acquisition of Norian Corporation |
(11,855,959 | ) | — | — | ||||||||
| Purchase of intangible assets |
(1,632,230 | ) | (330,833 | ) | — | |||||||
| Proceeds from maturity/redemption of investments |
30,065,000 | 27,095,000 | 7,430,000 | |||||||||
| Purchase of investments |
— | (40,106,459 | ) | (22,900,352 | ) | |||||||
| Proceeds from property insurance claim |
— | 1,313,892 | — | |||||||||
| Purchase of cost method investment |
(2,452,665 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) investing activities |
(5,207,547 | ) | (14,696,575 | ) | (19,149,592 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| FINANCING ACTIVITIES: |
||||||||||||
| Repayments of long term debt |
(1,400,000 | ) | (1,400,000 | ) | (1,400,000 | ) | ||||||
| Stock repurchases |
(30,035,266 | ) | (41,366,130 | ) | (19,423,525 | ) | ||||||
| Excess tax benefits from share-based payment arrangements |
607,256 | 150,480 | 987,707 | |||||||||
| Exchange of vested shares for taxes |
(1,239,985 | ) | (20,977 | ) | — | |||||||
| Proceeds from exercise of stock options |
3,961,979 | 880,268 | 6,523,192 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) financing activities |
(28,106,016 | ) | (41,756,359 | ) | (13,312,626 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| (DECREASE)/INCREASE IN CASH |
(12,883,197 | ) | (26,371,893 | ) | 768,023 | |||||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR |
23,102,362 | 49,474,255 | 48,706,232 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH AND CASH EQUIVALENTS, END OF YEAR |
$ | 10,219,165 | $ | 23,102,362 | $ | 49,474,255 | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
||||||||||||
| Cash paid for interest (net of interest capitalized of $7,195, $10,758, and $119,171, at June 30, 2011, 2010 and 2009, respectively) |
$ | 2,011,033 | $ | 2,098,891 | $ | 2,108,229 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for income taxes |
$ | 5,752,684 | $ | 9,745,800 | $ | 5,453,622 | ||||||
|
|
|
|
|
|
|
|||||||
| Retirement of fully depreciated property, plant and equipment |
$ | 275,238 | $ | 721,641 | $ | 817,341 | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL DISCLOSURE OF NONCASH INVESTING ACTIVITY: |
||||||||||||
| Accrual for deferred acquisition payments |
$ | 17,000,000 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL DISCLOSURE OF NONCASH FINANCING ACTIVITY: |
||||||||||||
| Net share settlement of stock options |
$ | (4,377,609 | ) | $ | — | $ | — | |||||
|
|
|
|
|
|
|
|||||||
See notes to Consolidated Financial Statements.
61
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED JUNE 30, 2011, 2010 AND 2009
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Description of the Company—Kensey Nash Corporation, incorporated in Delaware in 1984, is a medical device product development and manufacturing company with a history of innovation and success in bringing new products to the market. The Company focuses on regenerative medicine by creating products and technologies that help the human body heal. The Company is recognized as a leader for innovative product development, as well as for its broad portfolio of resorbable biomaterials products. The Company has an extensive range of products, which are sold through strategic partners in multiple large medical markets, including cardiology, orthopaedic, sports medicine, spinal, cranio-maxillofacial (CMF), trauma and general surgery.
Kensey Nash Corporation was incorporated in Delaware on August 6, 1984.
On January 28, 2011, the Company acquired substantially all of the assets and certain operational liabilities of Nerites Corporation (Nerites), and on May 24, 2011, the Company acquired certain operational assets and certain liabilities relating to the business and product lines of Norian Corporation (Norian). See Note 5 for additional information on these acquisitions. The Company’s acquisitions have added additional revenues and expenses to the Company’s results of operations in comparison to its historical operating results and the results of these acquisitions are included in the Company’s Consolidated Financial Statements beginning as of the respective acquisition date.
Principles of Consolidation and Basis of Presentation—The Consolidated Financial Statements include the accounts of Kensey Nash Corporation and its wholly owned subsidiaries (the Company). All intercompany transactions and balances have been eliminated.
The preparation of the financial statements in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP) and the notes to the financial statements requires management to make estimates and assumptions. These estimates and assumptions, which may differ from actual results, will affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the Consolidated Financial Statements, as well as the reported amounts of revenues, expenses and cash flows for the periods presented.
Subsequent Events—The Company has evaluated events and transactions subsequent to the balance sheet date. Based on this evaluation, with the exception of the Company’s August 2011 cash advance made to Orteq Ltd. (Orteq Sports Medicine or Orteq), as disclosed in Note 8, the Company is not aware of any events or transactions that occurred subsequent to the balance sheet date, but prior to the filing of this Form 10-K, which would require recognition or disclosure in its Consolidated Financial Statements.
Cash and Cash Equivalents—Cash and cash equivalents represent cash in banks and highly-liquid short-term investments that have an original maturity of less than three months. These highly-liquid short-term investments are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.
Fair Value of Financial Instruments—The carrying amounts of financial instruments, including cash and cash equivalents, investments, accounts receivable, accounts payable and debt approximated fair value as of June 30, 2011 and 2010. See Note 2 and Note 11 regarding the fair value determination of certain of these instruments.
Business Combinations—In accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 805, “Business Combinations” (ASC 805), the Company allocates the purchase price of acquired companies to the tangible and intangible assets acquired and liabilities assumed based
62
Table of Contents
on their estimated fair values. Valuations are performed to assist in determining the fair values of assets acquired and liabilities assumed, which requires management to make significant estimates and assumptions, in particular with respect to intangible assets. Management makes estimates of fair value based upon assumptions believed to be reasonable. These estimates are based in part on historical experience and information obtained from management of the acquired companies and expectations of future cash flows. Transaction costs and restructuring costs associated with a business combination transaction are expensed as incurred.
In-process research and development (IPR&D) in a business combination is accounted for as an indefinite-lived asset on the balance sheet until the associated research and development efforts are completed or abandoned. While classified as an indefinite-lived asset, acquired IPR&D would be subject to impairment testing. The Company would determine the useful life of the intangible asset on completion or abandonment of the associated research and development efforts. Alternatively, in an asset acquisition, the Company accounts for IPR&D in accordance with FASB ASC Topic 730, “Research and Development,” whereby the costs of intangible assets that are purchased from others for a particular research and development project that have no alternative future uses (in other research and development projects or otherwise), and therefore no separate economic values, are research and development costs at the time the costs are incurred and would be expensed and not capitalized as they do not have an alternative future use.
Comprehensive Income—The Company accounts for comprehensive income under the disclosure provisions of FASB ASC Topic 220, “Comprehensive Income.” Accordingly, accumulated other comprehensive (loss)/income is shown in the Consolidated Statements of Stockholders’ Equity at June 30, 2011, 2010 and 2009, and is comprised of net unrealized gains and losses on the Company’s available-for-sale securities, and change in interest rate swap value. The net tax effects/(benefit) for fiscal 2011, 2010 and 2009 of other comprehensive loss were $62,440, $(218,826) and $(658,775), respectively.
Property, Plant and Equipment—Property, plant and equipment consist primarily of land, building, machinery, furniture and equipment and construction in progress and are recorded at cost. Maintenance and repairs are typically expensed as incurred. Building, machinery, furniture and equipment are depreciated using the straight-line method over their useful lives ranging from two to 30 years. Depreciation expense on property, plant and equipment was $5,138,714, $5,212,039 and $5,367,713 for the fiscal years ended June 30, 2011, 2010 and 2009, respectively.
In fiscal 2011, in connection with the acquisition of the assets of Norian, the Company acquired a manufacturing and development facility in West Chester, Pennsylvania, consisting of approximately 37,000 square feet. See Note 5 for additional information.
In fiscal 2010, a fire occurred which was contained to a small manufacturing area within the Company’s corporate headquarters building located in Exton, Pennsylvania. The Company’s property insurance policy covered all remediation efforts and fixed asset replacements, subject to a $25,000 deductible. The Company recognized a gain of $775,000 within Cost of products sold upon final settlement with the insurance carrier, as remediation and recovery efforts were completed. A total of $1.3 million was received in insurance proceeds for remediation efforts, replacement value of fixed assets and other expenses. The Company replaced damaged fixed assets with a total value of approximately $480,000. The remaining insurance proceeds were used by the Company to pay for other related expenses and added to general Company funds. The total insurance proceeds, including amounts relating to the outside remediation services and fixed assets, have been classified within the investing activities section of the fiscal 2010 Consolidated Statement of Cash Flows, and the balance of the insurance proceeds, used by the Company to pay for other related expenses, has been classified within the operating activities section of the fiscal 2010 Consolidated Statement of Cash Flows.
Goodwill—Goodwill represents the excess of cost over the fair market value of the identifiable net assets of THM Biomedical, Inc. (THM), a company acquired in September 2000, and MacroPore Biosurgery Business Unit (MacroPore), acquired in May 2007. All of the acquisitions were accounted for under the provisions of ASC
63
Table of Contents
805 and FASB ASC Topic 350, “Intangibles—Goodwill and Other.” The net carrying amount of goodwill at June 30, 2011 was $4,366,273 and remained unchanged from the year ended June 30, 2010.
The accounting standards provide that goodwill and intangible assets with indefinite useful lives are not amortized, but are subject to impairment tests on an annual basis or at an interim date if certain events or circumstances indicate that an impairment loss may have been incurred. Intangible assets with definite useful lives will continue to be amortized over their respective useful lives. The annual assessments as of June 30, 2011, 2010 and 2009 indicated that goodwill was not impaired.
Impairment of Long-Lived Assets—Long-lived assets are reviewed for impairment whenever events or circumstances indicate that the carrying amount of an asset may not be recoverable. If the undiscounted expected future cash flows to be generated by the related asset are less than the carrying value of the asset, the Company measures the amount of the impairment by comparing the carrying amount of the asset to its fair value. The estimation of fair value is generally measured by discounting expected future cash flows.
Accounts Receivable Allowance—The Company had a trade receivable allowance balance of $5,867 at June 30, 2011 and $13,513 at June 30, 2010 and 2009. Included in Selling, general and administrative expense for the fiscal years ended June 30, 2011, 2010 and 2009 were write offs totaling $7,646, $0 and $1,200, respectively.
Revenue Recognition
Sales Revenue
The Company recognizes revenue in accordance with FASB ASC Subtopic 605-10-S99, “Revenue Recognition” (ASC 605-10-S99). Sales revenue is recognized when the products are shipped or the services are completed. Advance payments received for products or services are recorded as deferred revenue and are recognized when the product is shipped or services are performed. The Company reduces sales revenue for estimated customer returns and other allowances. The Company recorded net sales return provisions, credits and discounts of $30,064 for both the years ended June 30, 2011 and 2010 and $33,000 for the year ended June 30, 2009.
In addition, the Company accounts for customer arrangements containing multiple revenue elements in accordance with FASB ASC Subtopic 605-25, “Multiple Element Arrangements” (ASC 605-25). The Company considers a variety of factors in determining the appropriate method of accounting for its multiple elements agreements, including whether the various elements can be separated and accounted for individually as separate units of accounting. When the Company’s multiple element arrangements are combined into a single unit of accounting, revenues are deferred and recognized over the expected period of performance. The specific methodology for the recognition of revenue is determined on a case-by-case basis according to the facts and circumstances applicable to each agreement.
Up-front, non-refundable payments that do not have stand-alone value are recorded as deferred revenue once received and recognized as revenues over the expected period of performance.
The Company evaluates milestone payments on an individual basis and recognizes revenue from non-refundable milestone payments when the earnings process is complete and the payment is reasonably assured. Non-refundable milestone payments related to arrangements under which the Company has continuing performance obligations are recognized, using a contingency-adjusted performance model over the period of performance, where revenue is recognized for the milestone proportionately, to the extent of the performance period to date and the remainder ratably spread over the remaining performance period of the arrangement.
The Company also recognizes grant revenue under research and development related U.S. government programs in the period that the related expenses are incurred.
64
Table of Contents
Royalty Income
The Company recognizes its royalty revenue at the end of each quarter, when the relevant net total end-user product sales dollars are reported by customers to the Company for the quarter. Royalty payments are typically received within 45 days after the end of each calendar quarter.
Research and Development Costs—Research and development costs are charged to expense as they are incurred.
Income Taxes—The Company accounts for income taxes under the provisions of FASB ASC Subtopic 740, “Income Taxes” (See Note 18).
Earnings Per Share—Earnings per share (EPS) are calculated in accordance with FASB ASC Topic 260, “Earnings Per Share” (ASC 260). Basic and diluted EPS are computed using the weighted average number of shares of Common Stock outstanding, with common equivalent shares from options and nonvested stock awards included in the diluted computation when their effect is dilutive (See Note 19). There were no nonvested stock awards included for the years ended June 30, 2011, 2010 and 2009 that were antidilutive. Options to purchase shares of the Company’s Common Stock that were outstanding for the years ended June 30, 2011, 2010 and 2009, but were not included in the computation of diluted EPS because the options would have been antidilutive are shown in the table below:
| June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Number of options |
1,441,867 | 1,180,611 | 978,193 | |||||||||
|
|
|
|
|
|
|
|||||||
| Option exercise price range |
$23.45 - $35.71 | $23.45 - $35.71 | $25.55 - $35.71 | |||||||||
|
|
|
|
|
|
|
|||||||
Share-Based Compensation—The Company accounts for its share-based compensation plans in accordance with FASB ASC Topic 718, “Compensation—Stock Expense” (ASC 718), whereby all share-based compensation cost is measured at the grant date, based on the estimated fair value of the award, and is recognized as an expense in the income statement over the requisite service period.
The Company generally recognizes compensation expense for share-based awards granted over the relevant vesting period of the awards. Fair value for stock options and cash-settled stock appreciation rights (SARs) is determined using the Black-Scholes model, and the relevant expense is amortized over the applicable vesting period for stock options and marked to market for the SARs (See Note 14). Compensation expense related to share-based awards is classified on the Consolidated Statements of Income within the same line items as salary or consulting expense with respect to the award recipients, and is recorded over the awards’ relevant vesting periods. Compensation expense related to share-based awards granted to the members of the Board of Directors is recorded as a component of Selling, general and administrative expense on the Consolidated Statements of Income.
Derivative Instruments and Hedging Activities—The Company recognizes its only derivative as a cash flow hedge and measures this instrument at its fair value as either an asset or liability in the balance sheet, depending on the Company’s rights or obligations under the derivative contract (See Note 11). The change in the derivative’s fair value is recorded each period in Accumulated other comprehensive loss on the Consolidated Balance Sheets.
New Accounting Pronouncements—
ADOPTED: In October 2009, the FASB issued ASU 2009-13, “Revenue Recognition for Multiple-Deliverable Revenue Arrangements of FASB ASC Topic 605,” which amended ASC Subtopic 605-25, and was effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, which for the Company is its fiscal year ending June 30, 2011 (fiscal 2011). Alternatively,
65
Table of Contents
adoption could have been made on a retrospective basis, and early application was permitted. This statement provides principles for allocation of consideration among its multiple-elements based on an element’s estimated selling price if vendor-specific (VSOE) or other third-party evidence (TPE) of value is not available. This statement allows more flexibility in identifying and accounting for separate deliverables under an arrangement, introduces an estimated selling price method for valuing the elements of a bundled arrangement if VSOE or TPE of selling price is not available, and significantly expands related disclosure requirements. The Company’s prospective adoption of this new accounting update on July 1, 2010 did not have a significant impact on the Company’s consolidated financial position, results of operations or cash flows; however, the Company will continue to assess the impact of any future arrangements entered into or materially modified after the Company’s adoption.
In April 2010, the FASB issued ASU 2010-17, “Milestone Method of Revenue Recognition of FASB ASC Topic 605,” which was effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, which for the Company is its fiscal 2011. This statement provides criteria for recognizing the achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved, only if the milestone meets all criteria considered substantive made at the inception of the arrangement. The Company’s prospective adoption of this new accounting update on July 1, 2010 did not have a significant impact on the Company’s consolidated financial position, results of operations or cash flows; however, the Company will continue to assess the impact of any future arrangements entered into or materially modified after the Company’s adoption.
TO BE ADOPTED:
In June 2011, the FASB issued Accounting Standards Update (ASU) 2011-05, “Comprehensive Income (Topic 220)—Presentation of Comprehensive Income,” to require an entity to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. ASU 2011-05 eliminates the option to present the components of other comprehensive income as part of the statement of equity. ASU 2011-05 will be effective for the Company in its first quarter of fiscal 2013. The Company’s pending adoption of ASU 2011-05 is not expected to have a material effect on the Company’s financial position, results of operations or cash flows, other than the change to the required presentation format.
In May 2011, the FASB issued ASU No. 2011-04, “Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and International Financial Reporting Standards (IFRS) (Topic 820)—Fair Value Measurement” (ASU 2011-04), to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and IFRS. ASU 2011-04 will be effective for the Company in its third quarter of fiscal 2012. The Company is currently evaluating the impact of the pending adoption of ASU 2011-04 on its Consolidated Financial Statements.
In December 2010, the FASB issued ASU No. 2010-29, “Disclosure of Supplementary Pro Forma Information for Business Combinations (Topic 805)—Business Combinations” (ASU 2010-29), to improve consistency in how pro forma disclosures are calculated. Additionally, ASU 2010-29 enhances the disclosure requirements and requires description of the nature and amount of any material, nonrecurring pro forma adjustments directly attributable to a business combination. ASU 2010-29 will be effective for the Company in fiscal 2012 and should be applied prospectively to business combinations entered into in fiscal years beginning on or after December 15, 2010, which is the Company’s fiscal 2012. The Company will adopt ASU 2010-29 during fiscal 2012 and is not expected to have a material impact on its Consolidated Financial Statements.
| 2. | INVESTMENTS |
Investments as of June 30, 2011 consist of non-taxable and taxable high quality municipal obligations. In accordance with FASB ASC Topic 320, “Investments—Debt and Equity Securities,” the Company has classified
66
Table of Contents
its entire investment portfolio as available-for-sale securities with secondary or resale markets and, as such, its portfolio is reported at fair value with unrealized gains and losses included in Comprehensive Income in stockholders’ equity and realized gains and losses included in Other income/expense in Consolidated Statement of Operations. As of June 30, 2011, there were no investments with unrealized losses within the Company’s portfolio. The following is a summary of available-for-sale securities as of June 30, 2011 and June 30, 2010:
| June 30, 2011 | ||||||||||||||||
| Description |
Amortized Cost |
Gross Unrealized | Estimated Fair Value |
|||||||||||||
| Gain | Loss | |||||||||||||||
| Municipal Obligations |
$ | 11,506,392 | $ | 215,812 | $ | — | $ | 11,722,204 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Investments |
$ | 11,506,392 | $ | 215,812 | $ | — | $ | 11,722,204 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| June 30, 2010 | ||||||||||||||||
| Description |
Amortized Cost |
Gross Unrealized | Estimated Fair Value |
|||||||||||||
| Gain | Loss | |||||||||||||||
| Municipal Obligations |
$ | 33,693,441 | $ | 509,300 | $ | (9,683 | ) | $ | 34,193,058 | |||||||
| U.S. Government Securities |
8,376,007 | 2,479 | — | $ | 8,378,486 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Investments |
$ | 42,069,448 | $ | 511,779 | $ | (9,683 | ) | $ | 42,571,544 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of June 30, 2011, the Company’s investments had maturities ranging from less than one year to approximately two years. The Company’s U.S. government securities, all of which had matured as of October 2010, were comprised of U.S. government agency debt instruments. The fair values of the Company’s municipal obligations and U.S. government agency debt instruments are obtained from broker quotes using pricing matrices based on inputs that may include quoted prices for identical or similar assets in the municipal and U.S. government bond market and based on other various inputs that are directly or indirectly observable.
| 3. | INVENTORY |
Inventory is stated at the lower of cost (determined by the average cost method, which approximates first-in, first-out) or market value. Inventory primarily includes the cost of material utilized in the processing of the Company’s products and is as follows:
| June 30, | ||||||||
| 2011 | 2010 | |||||||
| Raw materials |
$ | 14,195,824 | $ | 7,550,043 | ||||
| Work in process |
2,150,144 | 1,425,174 | ||||||
| Finished goods |
2,057,054 | 2,018,512 | ||||||
|
|
|
|
|
|||||
| Gross inventory |
18,403,022 | 10,993,729 | ||||||
| Provision for inventory obsolescence |
(1,773,995 | ) | (2,107,854 | ) | ||||
|
|
|
|
|
|||||
| Inventory |
$ | 16,629,027 | $ | 8,885,875 | ||||
|
|
|
|
|
|||||
Adjustments to inventory are made at the individual part level for estimated excess, obsolescence or impaired balances, to reflect inventory at the lower of cost or market. Factors influencing these adjustments include: changes in demand, rapid technological changes, product life cycle and development plans, component cost trends, product pricing, physical deterioration and quality concerns. Revisions to these adjustments would be required if any of these factors differ from the Company’s estimates.
The June 30, 2011 gross inventory balance includes the inventory acquired as a result of the Company’s May 2011 acquisition of certain operational assets and certain liabilities relating to the business of Norian (See Note 5). The Company “stepped-up” the acquired inventory to its fair value as of the acquisition date, in accordance with ASC 805. The Company’s purchase accounting adjustment of the fair value of inventory, commonly
67
Table of Contents
referred to as “stepped-up value,” of $1,286,016, represented the estimated capitalized manufacturing profit in acquired inventory as of the date of acquisition, of which the Company has expensed $257,203 during the fourth quarter of fiscal 2011. This non-recurring, non-cash charge to Cost of products sold will be recognized over the expected inventory turn-over period as the related inventory is sold, which approximates a 6 month period, as the capitalized manufacturing profit added to inventory under purchase accounting is expected to be sold within approximately the 6 months after the date of acquisition through the Company’s second quarter of fiscal 2012.
The June 30, 2010 inventory balances also reflect the Company’s implemented cost reduction plan, which occurred in its second quarter of fiscal 2010, primarily associated with its reduced endovascular activities and lower production volume, which included reduced work schedules. In connection with this plan, the Company incurred a $944,000 pre-tax unabsorbed overhead expense charge in its second quarter of fiscal 2010.
| 4. | SELECT CUSTOMER AGREEMENTS |
These are select customer agreements not otherwise discussed elsewhere in the notes to the Consolidated Financial Statements.
St. Jude Medical, Inc.
The License Agreements—Under license agreements with St. Jude Medical, St. Jude Medical has exclusive worldwide rights to manufacture and market the Angio-Seal™ Vascular Closure Device (the Angio-Seal), for which the Company receives an approximate 6% royalty on end-user product sales by St. Jude Medical.
Prior Component Supply Contract—Under a supply agreement executed with St. Jude Medical in 2005, the Company was the exclusive supplier of 100% of the collagen plug and at least 30% of the bioresorbable polymer anchor components for the Angio-Seal over the term of the agreement, which expired on December 31, 2010. As part of that agreement, the Company received a $1.0 million origination fee upon execution, as consideration for the Company’s ongoing investments in collagen research and development. As of December 31, 2010, the Company had recognized the full $1.0 million of this origination fee. During the Company’s first and second quarters of fiscal 2011, St. Jude Medical had purchased approximately $8.1 million of Angio-Seal components under this prior component supply contract.
Current Collagen Supply Agreement—On June 23, 2010, the Company entered into a new two-year supply agreement with St. Jude Medical effective for the period from January 1, 2011 to December 31, 2012. Under this new supply agreement, the Company is the exclusive outside supplier of collagen plugs to St. Jude Medical. The new supply agreement provides for contractual minimum order levels of collagen plugs for calendar years of 2011 and 2012. During the Company’s third and fourth quarters of fiscal 2011, St. Jude Medical had fulfilled their calendar 2011 contractual minimum order levels under this new supply agreement of approximately $4 million of collagen plug sales for the Company. As of June 30, 2011, St. Jude Medical placed its calendar 2012 order for approximately $6.4 million of collagen plugs. This calendar 2012 order has exceeded the contractually minimum level and provides for collagen plug sales to be recognized when the Company ships the product during the Company’s second half of fiscal 2012 and first half of fiscal 2013. The new supply agreement does not call for the Company to supply polymer anchors to St. Jude Medical.
Orthovita, Inc.
The Company has a development, manufacturing and supply agreement with Orthovita under which the Company develops and commercializes products based on Orthovita’s proprietary Vitoss™ Bone Graft Substitute Material in combination with the Company’s proprietary biomaterials (the Orthovita Agreement). Under the Orthovita Agreement, the Company manufactures the products, while Orthovita markets and sells the products worldwide. Under the Orthovita Agreement, the Company receives royalty payments on co-developed Vitoss™, Vitoss™ Foam and Vitoss™ Bioactive Foam products based upon Orthovita’s net total end-user sales of such products.
68
Table of Contents
In a separate transaction in August 2004, the Company entered into an agreement (the Assignment Agreement) whereby the Company acquired the intellectual property rights of a third party having rights in the Vitoss™ technology, an inventor of the Vitoss™ technology (the Inventor), for $2,600,000. Under the Assignment Agreement, the Company received all intellectual property rights of the Inventor that had not previously been assigned to Orthovita. Also under the Assignment Agreement, the Company primarily receives a royalty on the sale of all Orthovita products containing the Vitoss™ technology, up to a total to be received of $4,035,782. The entire cost of these proprietary rights is expected to be amortized over an approximate 83 month period. As of June 30, 2011, the Company had recognized cumulative royalty income of $4,018,316 under the Assignment Agreement and $17,466 was yet to be received. The Company currently anticipates receiving the remaining economic benefit in relation to these proprietary rights during the first quarter of fiscal 2012.
| 5. | ACQUISITIONS |
Asset Acquisition of Nerites Corporation
In January 2011, the Company acquired substantially all of the assets and certain operational liabilities of Nerites, a privately-held development stage company based in Wisconsin, for $19,741,761 plus acquisition-related costs of $326,673. Approximately $16.7 million of the purchase price was paid at the acquisition date, financed from the Company’s available cash and investments on hand. The remaining $3.0 million, of which $1.5 million is reported as each a component of Other current liabilities and Other non-current liabilities on the Consolidated Balance Sheet as of June 30, 2011, was held back by the Company under the terms of the acquisition as security for certain potential Nerites indemnification obligations and is expected to be financed with cash on hand. Of such $3.0 million hold-back amount, $1.5 million will be released on each of the first and second anniversaries of the acquisition date to Nerites, to the extent that the hold-back amount is not applied toward any such indemnification obligations. Nerites is a surgical adhesive based biomaterials’ technology company founded in 2004 to design and develop products with a technology inspired by mussel adhesive proteins.
The Company accounted for the transaction as an asset acquisition based on an evaluation of the accounting guidance (ASC Topic 805) and considering the early research stage of Nerites’ technology. The Company concluded that the acquired net assets of Nerites did not constitute a business as defined under ASC 805 due to the incomplete nature of the inputs and the absence of processes from a market participant perspective. At the acquisition date, Nerites had spent approximately $14.2 million on ongoing research initiatives targeted at developing three potential platform technologies that they had been researching prior to the acquisition: liquid adhesives/sealants, thin film adhesives and anti-fouling/anti-bacterial coating capabilities. Nerites’ early research stage technology is based on a complex chemistry that produces an adhesive material that is novel and unproven and was previously unknown within the medical device community. Nerites’ technology and intellectual property primarily focus on the chemical formulation involved in creating a molecule for an adhesive material. At the time of the acquisition, Nerites was still testing feasibility of the adhesive technology and was primarily focused on identifying the mechanical or physical properties and reaction attributes of an adhesive raw material to serve as a starting point for a medical device. Substantial additional research and development will be required to finalize a raw material formulation, and the unique processes specific to that formulation needs to be developed to enable the formulation to have the ability to be manufactured into a commercially viable medical device. There is risk that a marketable material formulation may never be developed. Ultimately, the medical device would potentially need to complete clinical trials and receive regulatory approvals prior to any potential commercialization.
In accounting for the transaction as an asset acquisition, the Company initially assigned the value of the consideration transferred plus the acquisition related costs to the tangible assets and identifiable intangible assets acquired and operational liabilities assumed based on their fair values at the date of acquisition. Additionally, the Company allocated value for the identifiable intangible assets based on their relative fair values, which did not differ significantly from the fair values. The Company assessed the fair value of assets, including intangible assets such as IPR&D, using a variety of methods, including present-value
69
Table of Contents
models. Each asset was initially measured at fair value from the perspective of a market participant. Accounting for asset acquisitions requires extensive use of accounting estimates and judgments to allocate the total cost of the acquisition to the tangible and intangible assets acquired and liabilities assumed, including IPR&D. Management is responsible for the valuation and considered a number of factors, including internal and third party valuations and appraisals.
The following is a summary of the allocation of the total cost of the acquisition:
| Other receivables |
$ | 8,519 | ||
| Prepaid expenses and other |
6,504 | |||
| Machinery, furniture and equipment |
93,230 | |||
| Acquired other intangibles |
1,813,182 | |||
| Acquired in-process research and development |
18,233,006 | |||
|
|
|
|||
| Total assets acquired |
$ | 20,154,441 | ||
|
|
|
|||
| Accounts payable |
$ | (50,579 | ) | |
| Accrued expenses |
(16,146 | ) | ||
| Deferred revenue |
(19,282 | ) | ||
|
|
|
|||
| Total liabilities assumed |
$ | (86,007 | ) | |
|
|
|
|||
| Net assets acquired |
$ | 20,068,434 | ||
|
|
|
Of the approximate $1.8 million of Acquired other intangibles, $1,588,558 was assigned to know-how related to Nerites’ adhesive technology with an estimated useful life of 25 years, based on the estimated remaining period of economic benefit of the technology, $159,868 was assigned to assembled workforce with an estimated useful life of two years, and $64,756 was assigned to government grant applications with an estimated useful life of two years.
Acquired IPR&D in the asset acquisition was accounted for in accordance with FASB ASC Topic 730, “Research and Development” (ASC 730). At the date of acquisition, the Company determined that the development of the projects underway at Nerites had not yet reached technological feasibility and that the research in process had no alternative future uses. Accordingly, the acquired IPR&D was charged to expense in the Consolidated Statements of Income on the acquisition date. The acquired IPR&D charge is expected to be deductible over a 15-year period for income tax purposes.
The $18,233,006 value assigned to the acquired IPR&D was determined using the Multi-Period Excess Earnings Method (MPEEM). The MPEEM is an attribution model under the income approach, which incorporates estimating the costs to develop the acquired technology into commercially viable medical devices, estimating the resulting net cash flows from the projects, and discounting the estimated net cash flows to present value. The Company also considered other tangible and intangible assets required for successful exploitation of the technology resulting from the acquired IPR&D projects and adjusted estimated future cash flows for a charge reflecting the contribution to value of these assets. Such contributory tangible and intangible assets include working capital, fixed assets, know-how, assembled workforce and government grant applications. The resulting estimated net cash flows from such projects were based on management’s estimates of cost of products sold, operating cost and expenses, and income taxes associated with such projects. The market rate of return was utilized based on market participant data to discount the net cash flows to present value. Due to the nature of the forecast and the risk associated with the projected growth and profitability of the research projects, a discount rate of 36.5 percent was considered appropriate for the acquired IPR&D. This discount rate was commensurate with the project’s stage of development and, the risks relative to the technology’s feasibility and viability of commercial acceptance.
As of the acquisition date, the Company planned to develop a portfolio of hybrid adhesive-based products that integrate its extracellular matrix (ECM), collagen and polymer technology platforms for applications in
70
Table of Contents
general, neurologic, plastic/reconstructive, orthopaedic, urologic, cardiovascular and thoracic surgical specialties. Initial acquired IPR&D product targets included devices for (1) repairing defects in abdominal walls, (2) meniscal tears, (3) articular cartilage resurfacing, (4) dural membranes, and (5) gastrointestinal tracts. As of the date of acquisition, each of these five projects was expected to utilize the adhesive technology and was 100% dependent on developing the adhesive raw material as described above. As of the date of acquisition, the Company expected to spend approximately $25.0 million over the next seven years to bring the five projects under development to technological feasibility. As of the date of acquisition, assuming the research and development program to create the adhesive raw material is successful, the Company expected to begin receiving the estimated revenues from the five in-process projects between fiscal 2013 and 2017, depending on the project.
Determining the portion of the total cost of the acquisition to allocate to acquired IPR&D required the Company to make significant estimates and assumptions based on unobservable inputs, including, but not limited to, estimates of the timing of and expected costs to complete the acquired IPR&D projects; the ability to manufacture and commercialize the products; projections for marketing authorization and regulatory approvals; relevant market sizes, penetration and growth factors; current and expected trends in technology and product life cycles; the nature and expected timing of new product introductions by the Company and its competitors; estimates of future cash flows from product sales resulting from completed products and in-process projects; and appropriate discount rates and probability rates. The Company believed that the foregoing estimates and assumptions used in the acquired IPR&D analysis were reasonable based upon the Company’s best estimate of likely outcomes of its clinical development given available facts and circumstances at the time of the acquisition. No assurance can be given, however, that the underlying assumptions used to estimate expected project sales, development costs or profitability, or the events associated with such projects, will ultimately prove to have been appropriate. If the product development projects are not successful, the revenue and profitability of the Company may be adversely affected in future periods. Additionally, the value of other acquired intangible assets may become impaired.
Asset Acquisition of Norian
On May 24, 2011 (date of acquisition), the Company completed its acquisition of certain operational assets and certain liabilities relating to the business and product lines of Norian, a wholly owned subsidiary of Synthes, Inc. (Synthes), pursuant to an Asset Purchase Agreement and related Property Purchase Agreement, for a total purchase price of approximately $26 million ($22 million pursuant to the Asset Purchase Agreement and $4 million pursuant to the Property Purchase Agreement). On the date of acquisition, the Company paid to Synthes the total cash consideration of $11,855,959 from the Company’s available cash on hand. The Company is required to pay the remaining $14 million on the earlier of the date on which the transfer of the manufacturing operation from the purchased West Chester, Pennsylvania facility to the Company’s corporate headquarters facility located in Exton, Pennsylvania has been completed or the 18-month anniversary of the date of acquisition. The $14 million deferred payment has been classified by the Company as a long-term liability.
On May 24, 2011, the Company also entered into a Supply Agreement with Synthes USA Sales, LLC, a subsidiary of Synthes, which provides for the Company to be the exclusive manufacturer and supplier of the Norian product lines acquired by the Company under the Asset Purchase Agreement, pursuant to which Synthes will purchase all of its requirements for such products exclusively from the Company, on the terms set forth in the Supply Agreement. The Supply Agreement, which was effective on the date of acquisition, has a term of 10 years and will automatically renew for successive two-year terms. Also, on May 24, 2011, the Company entered into a research and development agreement with Synthes to develop certain related future products.
Norian was engaged in the development, manufacturing and marketing of bone repair cement, a proprietary cancellous bone replacement material designed for use in structurally compromised cancellous bone. Additionally, Norian also manufactured and sold related delivery systems including mixers, delivery devices and needles.
71
Table of Contents
The sale of Norian by Synthes is a result of the requirement for Synthes and Norian to divest the Norian Business under the agreements which were entered into in October 2010 with the U.S. Department of Justice and the Office of Inspector General of the Department of Health and Human Services (“OIG”). The sale was consummated to resolve the investigation related to the indictment against Synthes, Norian and four individuals who were executives of Synthes during the period in question, charging them with violations of the U.S. Food, Drug and Cosmetic Act in connection with certain marketing and promotional practices involving Norian Corporation products during the period from May 2002 to July 2004, as filed by the United States Attorney’s Office for the Eastern District of Pennsylvania. Under the agreements, Synthes agreed to pay fines and also agreed to divest the Norian Business.
In accordance with ASC 805, the Company has accounted for the asset acquisition using the purchase method of accounting under U.S. GAAP. Under the purchase method of accounting, the total purchase price is allocated to the tangible and intangible acquired assets and assumed liabilities of Norian, based on their respective estimated fair values as of the date of the acquisition.
The approximate $26 million purchase price has been allocated based upon estimates of the fair value of assets acquired and liabilities assumed. Independent valuation specialists conducted a valuation to assist management of the Company in determining the estimated fair values of property, machinery and equipment and intangibles. The Company’s management is responsible for these internal and third party valuations and appraisals. The work performed by the independent valuation specialists has been considered in management’s estimates of fair values reflected.
The following summarizes the estimated allocation of the fair values of the assets acquired and the liabilities assumed at the acquisition date:
| Cash paid for the Norian business |
$ | 11,855,959 | ||
| Long-term liability |
14,000,000 | |||
|
|
|
|||
| Total purchase price, net of assumed liabilities |
$ | 25,855,959 | ||
|
|
|
|||
| Accounts receivable |
$ | 7,230 | ||
| Inventory |
7,511,396 | |||
| Prepaid expenses |
45,160 | |||
| Land |
910,010 | |||
| Building |
3,390,000 | |||
| Machinery, furniture and equipment |
1,483,700 | |||
| Intangible asset (customer relationship) |
13,790,000 | |||
| Accrued expenses |
(196,431 | ) | ||
| Other current liabilities |
(136,789 | ) | ||
| Other non-current liabilities |
(68,394 | ) | ||
| Deferred tax liability |
(365,777 | ) | ||
|
|
|
|||
| Net assets acquired |
$ | 26,370,105 | ||
| Total purchase price |
$ | 25,855,959 | ||
|
|
|
|||
| Gain on bargain purchase of net assets acquired after deferred taxes |
$ | 514,146 | ||
|
|
|
|||
Based on the purchase price allocation, the fair value of the identifiable net assets acquired exceeded the fair value of the consideration paid, which was primarily a result of the fair values assigned to acquired building and land, as well as the additional stepped-up inventory fair value, which represents the estimated capitalized manufacturing profit in acquired inventory. The Company recognized a gain on bargain purchase of net assets acquired after deferred taxes of $514,146 in the fourth quarter of fiscal year 2011, as a result of the fair value of the net assets acquired exceeding the purchase price. The Company has reported $514,146 as the gain on bargain purchase after deferred taxes, which is included within Selling, general and administrative in the Consolidated Statements of Income for the year ended June 30, 2011.
72
Table of Contents
The Company has incurred through June 30, 2011 a total of $506,000 of acquisition related costs, including professional and legal fees, related to the acquisition, all of which were expensed as incurred in accordance with ASC 805 and reported as a component of Selling, general and administrative expense in the Consolidated Statements of Income for the year ended June 30, 2011.
Under the purchase method of accounting, the Company assigned $13,790,000 of the total purchase price to the intangible asset (customer relationship), which will be amortized over the remaining period of economic benefit of 15 years and reported as a component of Selling, general and administrative expense. The Company has reported the depreciation expense associated with the building, machinery, furniture and equipment as a component of Cost of products sold in the Consolidated Statements of Income for the year ended June 30, 2011.
The Company acquired inventory of $7,511,396, which included the Company’s purchase accounting adjustment of the fair value of inventory, commonly referred to as “stepped-up value”, of $1,286,016, representing the estimated capitalized manufacturing profit in acquired inventory. The Company will record a non-recurring, non-cash charge to Cost of products sold that will be recognized over the expected inventory turn-over period as the related inventory is sold, which will approximate a 6 month period after the date of acquisition through the Company’s second quarter of fiscal 2012. The Company has expensed $257,203 during the fourth quarter of fiscal 2011, which is included within Cost of products sold in the Consolidated Statements of Income for the year ended June 30, 2011.
On the date of acquisition, the Company did not assume any additional liabilities of Norian, other than the personnel related bonus and vacation accruals for certain Norian employees.
In connection with acquisition, pursuant to the Property Purchase Agreement, the Company acquired a manufacturing and development facility in West Chester, Pennsylvania, consisting of approximately 37,000 square feet. Additionally, the Company entered into a 10-year lease agreement with Synthes, under which the Company will lease the entire building back to Synthes, which will in turn sublease a portion of the building to the Company for approximately 18 months, while the Company is in the process of transitioning the manufacturing of the Norian product from the West Chester Facility to the Exton Facility.
The results of operations associated with Norian have been consolidated with those of the Company since the date of acquisition. Total revenues of $1,378,152 and net income of $84,768 attributable to the Norian product sales were recognized in the Consolidated Statement of Income for the fiscal year ended June 30, 2011.
The following unaudited pro forma information sets forth the combined revenues and net income of the Company and the historical unaudited Norian financial information for the fiscal years ended June 30, 2011 and 2010, as if the acquisition had occurred at the beginning of each of the periods presented. Historically, Norian had not maintained certain distinct and separate accounts from Synthes. Therefore, certain corporate overhead expenses, including but not limited to, human resources, legal, finance, tax and treasury functions, and other expenses such as intangible amortization, and related income taxes, were not historically identified and accounted for in the separate historical unaudited financial records of Norian. In addition, the historical unaudited Norian financial information included in this pro forma includes total revenue of the Norian product sales sold at arm’s-length to Synthes, who then was responsible for subsequently selling these products to the end-user market. The historical unaudited Norian financial information reflected the manufacturing and selling of the product lines through inter-company arms-length transactions with Synthes at negotiated prices. Therefore, the unaudited pro forma information presented below is not necessarily indicative of that which would have been attained had the transaction occurred at an earlier date, nor are these results necessarily indicative of future consolidated results of operations of the Company.
| Fiscal Year Ended June 30, | ||||||||
| 2011 | 2010 | |||||||
| Total revenues |
$ | 87,294,370 | $ | 99,103,635 | ||||
| Net income |
$ | 2,708,859 | $ | 21,019,182 | ||||
| Basic earnings per share |
$ | 0.31 | $ | 1.98 | ||||
| Diluted earnings per share |
$ | 0.30 | $ | 1.92 | ||||
73
Table of Contents
| 6. | CONCENTRATION OF CREDIT RISK |
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents, investments, accounts receivable and debt (See Note 10). The Company manages its cash, cash equivalents and investments based on established guidelines relative to diversification and maturities to maintain safety and liquidity. No single component of the Company’s investment portfolio represented more than 10% or 11% of the total investments as of June 30, 2011 and 2010, respectively.
The Company’s trade receivables at June 30, 2011 are primarily due from Synthes, Orthovita and Arthrex and represented approximately 29%, 28% and 26%, respectively, of the Company’s total trade. The Company’s trade receivables at June 30, 2010 were due from Synthes, Orthovita, Arthrex, as well as St. Jude Medical, and represented approximately 1%, 13%, 29% and 40%, respectively, of the Company’s total trade receivables at June 30, 2010. The Company’s royalty receivables are primarily due from St. Jude Medical and Orthovita, and represented approximately 80% and 18%, respectively, of the Company’s total royalty receivable balance at June 30, 2011, and approximately 90% and 9%, respectively, of the Company’s total royalty receivable balance at June 30, 2010 (See Note 4). If any of these customers’ receivable balances should be deemed uncollectible, it would have a material adverse effect on the Company’s results of operations and financial condition. The Company performs ongoing credit evaluations on all of its customers’ financial conditions, but does not require collateral to support customer receivables. See Note 15 for additional information regarding significant customer revenue disclosure.
| 7. | ACQUIRED PATENTS AND OTHER INTANGIBLE ASSETS |
The costs of internally developed patents are expensed when incurred due to the long development cycle for products and the Company’s inability to measure the recoverability of these costs when incurred. From time to time, the Company has acquired portfolios of patents and other intangibles that it believes are beneficial and complementary to the Company’s existing intellectual property and material processing knowledge platform. These acquisitions have included a portfolio of puncture closure patents acquired in November 1997, patents acquired in the asset purchase of THM Biomedical, Inc. (THM) in 2000, certain intellectual property and other rights related to the Vitoss™ product line acquired from a third party inventor in 2004 (See Note 4), certain assets of MacroPore Biosurgery, Inc. (MacroPore) acquired in 2007, as well as other smaller purchases.
In fiscal 2011, the Company had acquired the following intangible assets:
On December 21, 2010, in addition to making a non-controlling minority cost-method investment in Orteq (see Note 8), the Company entered into a manufacturing and supply agreement with Orteq and acquired the exclusive worldwide manufacturing rights of the Actifit® (Actifit) product line for a period of ten years beginning with the date of its first U.S. commercial sale. The Company assigned $1,632,230 to the cost of the manufacturing rights and related costs associated with the transaction. Actifit is a biocompatible synthetic meniscal scaffold which received its CE Mark approval in 2008 for the treatment of irreparable partial meniscal tears, and is currently being sold throughout Europe. The acquired manufacturing rights are expected to be amortized over the period of economic benefit of approximately 13 years, and amortization is expected to begin in fiscal 2012, when the Company begins to manufacture the Actifit product line.
On January 28, 2011, the Company acquired certain intangible assets of Nerites through an asset acquisition transaction (see Note 5). The Company assigned $1,813,182 of the total acquisition costs to the intangible assets, which will be amortized over the remaining period of economic benefit ranging from 2 to 25 years, depending on the intangible asset.
On May 24, 2011, the Company acquired an intangible asset (customer relationship) of Norian through the Norian asset acquisition transaction (see Note 5). Under the purchase method of accounting, the Company assigned $13,790,000 of the total purchase price to the intangible asset (customer relationship), which will be amortized over the remaining period of economic benefit of 15 years.
74
Table of Contents
The Company amortizes the entire cost of acquired patents and intangible assets over their respective remaining periods of economic benefit, ranging from approximately 1 to 25 years as of June 30, 2011. The gross carrying amount of such patents and intangible assets as of June 30, 2011 was $25,912,611, with accumulated amortization of $7,730,462. The gross carrying amount of such patents and intangible assets as of June 30, 2010 was $8,677,200, with accumulated amortization of $6,628,605.
Total amortization expense on these patents and intangible assets was $1,101,858, $967,279 and $1,122,937 for the fiscal years ended June 30, 2011, 2010 and 2009, respectively. The table below details the estimated amortization expense as of June 30, 2011, for the next five fiscal years on the patents and intangible assets previously acquired by the Company:
| Fiscal year ending June 30, |
Amortization Expense |
|||
| 2012 |
$ | 1,828,435 | ||
| 2013 |
1,418,846 | |||
| 2014 |
1,309,391 | |||
| 2015 |
1,128,884 | |||
| 2016 |
1,111,324 | |||
| Thereafter |
11,385,269 | |||
| 8. | COST METHOD INVESTMENT |
On December 21, 2010, the Company made a non-controlling minority cost-method investment recorded at the value of $2,452,665 in preferred shares of Orteq. The Company accounted for the investment in Orteq under the cost method. Orteq is a privately-held medical device company headquartered in London, United Kingdom, specializing in the field of biodegradable polymer technology for meniscus repair. The Company has an approximate 8% ownership interest in Orteq and does not have the ability to exercise significant influence over Orteq’s financial and operating policies.
The cost method investment was assessed for impairment, and there were no indicators of any such impairment, as of June 30, 2011.
Subsequent to June 30, 2011, on August 19, 2011, at the Company’s option, and pursuant to an ancillary agreement, the Company made an additional investment in Orteq of approximately 637,000 British Pounds, or $1,055,063, in the form of a cash advance, structured as convertible debt, to Orteq. As previously disclosed, under the investment agreement with Orteq, the Company had committed to making an additional minority cost-method investment of approximately 637,000 British Pounds, which was to be in preferred shares of Orteq, payable in U.S. Dollars, if Orteq receives approval or conditional approval from the FDA to conduct an investigational device exemption for a product pivotal trial by the second anniversary of the Company’s initial investment, which is December 21, 2012, or otherwise earlier, at the Company’s option, prior to December 21, 2012. Under the ancillary agreement, the Company has the option to convert its cash advance into preferred shares of Orteq as an additional cost-method investment under the investment agreement, or if it does not convert its cash advance to shares, the cash advance and accrued interest will be payable by Orteq to the Company prior to August 2016. This additional cash investment in Orteq did not impact the Company’s ownership interest in Orteq. In the event the Company, pursuant to the ancillary agreement, agrees to convert its cash advance into preferred shares, the Company’s ownership interest in Orteq would continue to be less than 10%.
| 9. | ACCRUED EXPENSES |
As of June 30, 2011 and 2010, accrued expenses consisted of the following:
| June 30, 2011 | June 30, 2010 | |||||||
| Accrued payroll and related compensation |
$ | 1,134,312 | $ | 2,987,702 | ||||
| Other |
1,115,801 | 879,578 | ||||||
| Taxes Payable |
— | 1,627,630 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 2,250,113 | $ | 5,494,910 | ||||
|
|
|
|
|
|||||
75
Table of Contents
The Company’s payroll accrual for its prior fiscal year ended June 30, 2010, amongst other compensation related items, included a cash bonus of approximately $1.9 million, which the Company paid during its fiscal 2011, as well as the remaining severance amount of $82,277, primarily resulting from a voluntary retirement program, which occurred during the Company’s second quarter ended December 31, 2009 of fiscal 2010, in which the Company incurred a total severance charge of $1,011,058 that was included in the same line items as salary expense on the Consolidated Statements of Income. As of June 30, 2011, there was no accrual remaining related to the severance charge included in accrued payroll and related compensation.
| 10. | DEBT |
Secured Commercial Mortgage—On May 25, 2006, the Company entered into an agreement for a $35 million Secured Commercial Mortgage (the Mortgage) with Citibank, F.S.B. The Mortgage is secured by the Company’s facility and land in Exton, Pennsylvania and bears interest at one-month LIBOR plus an 0.82% Loan Credit Spread.
At June 30, 2011, the outstanding Mortgage balance was $30 million. The remaining principal payments due are detailed below:
| Fiscal Year ending June 30, |
Principal Payments |
|||
| 2012 |
$ | 1,399,997 | ||
| 2013 |
1,400,000 | |||
| 2014 |
1,400,000 | |||
| 2015 |
1,400,000 | |||
| 2016 |
1,400,000 | |||
| Thereafter |
22,983,333 | |||
The Mortgage contains various conditions to borrowing, including affirmative, restrictive and financial maintenance covenants. Certain of the more significant covenants require the Company to maintain a Minimum Fixed Charge Coverage Ratio of EBITDA (as defined in the Mortgage) to debt service equal to or greater than 1.50–to–1.0 and an interest rate hedge of at least 50 percent of the outstanding principal balance of the Mortgage through an interest rate protection product reasonably acceptable to Citibank, F.S.B.
Interest Rate Swap Agreement—In order to hedge its interest rate risk under the Mortgage, the Company entered into a $35 million aggregate 10-year fixed interest rate swap agreement (the Swap) with Citibank, N.A. in May 2006. The Swap is secured by the Company’s facility and land in Exton, Pennsylvania. The Company is using the Swap as a cash flow hedge of the Company’s interest payments under the Mortgage. The Swap converts the variable LIBOR portion of the Mortgage payments to a fixed rate of 6.44% (5.62% fixed interest rate plus a 0.82% Loan Credit Spread).
The Company follows the provisions of FASB ASC Topic 815, “Derivatives and Hedging,” to account for the Swap as a cash flow hedge due to the hedging of forecasted interest rate payments and to record the Swap at its fair value on the Consolidated Balance Sheets. This value represents the estimated amount the Company would receive or pay to terminate the Swap. As such, the Company records a mark-to-market adjustment at the end of each period. In establishing the fair value, the Company includes and evaluates dealer quotes, the counterparty’s ability to settle the asset or liability and the counterparty’s creditworthiness. Additionally, current interest rates, collateralization of the Mortgage and the Swap by the land and building and any adverse Company or industry specific events that would impact the fair value measurement are considered.
The Company utilizes the Hypothetical Derivative Method in determining hedge effectiveness each period. Transactions that would cause ineffectiveness would include the prepayment of the Mortgage or an adverse Company or industry specific event that would impact the fair value measurement, which would result in the
76
Table of Contents
Company reclassifying the ineffective portion into current earnings. If the conditions underlying the Swap or the hedge item do not change, the Swap will be considered to be highly effective. The effective portion of the Swap’s gain or loss, due to a change in the fair value, is reported as a component of Accumulated other comprehensive loss and has no impact on the Consolidated Statements of Income or Cash Flows.
As of June 30, 2011 and 2010, the fair value of the Swap was in an unrealized loss position of $4,919,658 ($3,009,270, net of tax) and $5,542,509 ($3,602,631, net of tax), respectively, with the gross unrealized loss position included in Other non-current liabilities and the net of tax position included in Accumulated other comprehensive loss on the Consolidated Balance Sheets. Interest expense under the Swap is recorded in earnings at the fixed rate set forth in the Swap.
For the fiscal years ended June 30, 2011, 2010 and 2009, no amounts were recognized in current earnings due to ineffectiveness or amounts excluded from the assessment of hedge effectiveness. The Company does not anticipate any material unrealized losses to be recognized within the next 12 months as the anticipated transactions under the Mortgage and Swap occur, unless the Mortgage or a portion thereof, is prepaid.
The following table summarizes the fair value of the Swap as of June 30, 2011 and June 30, 2010 on the Consolidated Balance Sheet:
| Derivative Designed as a |
Location in the Condensed |
Fair Value as of June 30, 2011 |
Fair Value as of June 30, 2010 |
|||||||
| Interest Rate Swap Contract |
Other non-current liabilities | $ | 4,919,658 | $ | 5,542,509 | |||||
|
|
|
|
|
|||||||
| Total Derivative |
$ | 4,919,658 | $ | 5,542,509 | ||||||
|
|
|
|
|
|||||||
The following tables summarize the Swap’s impact on Accumulated other comprehensive loss and earnings for the years ended June 30, 2011 and 2010:
| Amount of Gain/(Loss) Recognized in Other Comprehensive Loss on Derivative (Effective Portion) For the Twelve Months Ended June 30, |
||||||||||||
| Derivative in Cash Flow Hedging Relationship |
2011 | 2010 | 2009 | |||||||||
| Interest Rate Swap Contract |
$ | (1,056,266 | ) | $ | (2,786,046 | ) | $ | (1,290,901 | ) | |||
|
|
|
|
|
|
|
|||||||
| Total |
$ | (1,056,266 | ) | $ | (2,786,046 | ) | $ | (1,290,901 | ) | |||
|
|
|
|
|
|
|
|||||||
| Amount of Gain/(Loss) Reclassed
From Accumulated Other Comprehensive Loss Into Income (Effective Portion) For the Twelve Months Ended June 30, |
||||||||||||
| Location of Loss Reclassed From Accumulated Other | ||||||||||||
| Comprehensive Loss Into Income |
2011 | 2010 | 2009 | |||||||||
| Interest expense |
$ | (1,679,118 | ) | $ | (1,746,437 | ) | $ | (1,398,298 | ) | |||
|
|
|
|
|
|
|
|||||||
| Total |
$ | (1,679,118 | ) | $ | (1,746,437 | ) | $ | (1,398,298 | ) | |||
|
|
|
|
|
|
|
|||||||
| Amount of Gain/(Loss) Recognized in Income on Derivative (Ineffective Portion) For the Twelve Months Ended June 30, |
||||||||||||
| Location of Loss Reclassed From Accumulated Other | ||||||||||||
| Comprehensive Loss Into Income |
2011 | 2010 | 2009 | |||||||||
| Interest expense |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
77
Table of Contents
Credit Agreement—On May 26, 2011, the Company entered into a Loan and Agency Agreement (the Credit Agreement) with Wells Fargo Bank, National Association (Wells Fargo). The Credit Agreement provides for a three-year, unsecured revolving credit facility (Revolving Credit Facility) of $35,000,000, evidenced by a Revolving Credit Note, dated May 26, 2011, issued by the Company in favor of Wells Fargo.
Under the terms of the Credit Agreement, the Company may borrow up to the aggregate amount of the unused commitment under the Revolving Credit Facility. The Revolving Credit Facility may be used for the working capital and general corporate purposes of the Company, including permitted acquisitions and capital expenditures.
Borrowings under the Revolving Credit Facility will, at the Company’s option, bear interest at a rate based upon the London Inter Bank Offering Rate (LIBOR) of LIBOR as in effect for one, two, three or six-month periods, as elected by the Company, plus an applicable margin, or if no election is made, the one-month LIBOR plus an applicable margin. The Credit Agreement will terminate on May 25, 2014, unless the lenders elect to extend the Credit Agreement for up to two additional one-year periods upon the request of the Company, and which contains customary representations, covenants and events of default. Certain of the more significant covenants set forth in the Credit Agreement require the Company to maintain (i) a maximum funded debt to EBITDA calculation of 3.0; (ii) a minimum tangible net worth of $35 million; (iii) a minimum fixed charge coverage ratio of 1.5; and (iv) impose restrictions on indebtedness and liens against the Company’s assets. During fiscal 2011, and as of June 30, 2011, there were no borrowings on the Revolving Credit Facility.
| 11. | FAIR VALUE OF FINANCIAL ASSETS AND FINANCIAL LIABILITIES |
The Company follows the provisions of FASB ASC Topic 820 for fair value measurement recognition and disclosure purposes for its financial assets and financial liabilities that are remeasured and reported at fair value each reporting period. The Company measures certain financial assets and liabilities at fair value on a recurring basis, including cash equivalents, available-for-sale securities and the Swap. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the valuation of financial assets and financial liabilities and their placement within the fair value hierarchy. Categorization is based on a three-tier valuation hierarchy, which prioritizes the inputs used in measuring fair value, as follows:
| • | Level 1—Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| • | Level 2—Inputs that are other than quoted prices in active markets for identical assets and liabilities, inputs that are quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are either directly or indirectly observable; and |
| • | Level 3—Unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. |
78
Table of Contents
The following table presents the Company’s financial assets and liabilities measured at fair value on a recurring basis as of June 30, 2011 and June 30, 2010:
| Fair Value Measurements as of June 30, 2011 |
||||||||||||
| Level 1 | Level 2 | Level 3 | ||||||||||
| Assets |
||||||||||||
| Money market funds (a) |
$ | 9,594,034 | $ | — | $ | — | ||||||
| Available-for-sale marketable securities: |
||||||||||||
| Municipal Obligations (See Note 2) |
— | 11,722,204 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Assets |
$ | 9,594,034 | $ | 11,722,204 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities |
||||||||||||
| Interest rate swap (See Note 10) |
$ | — | $ | 4,919,658 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Total Liabilities |
$ | — | $ | 4,919,658 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Fair Value Measurements as of June 30, 2010 |
||||||||||||
| Level 1 | Level 2 | Level 3 | ||||||||||
| Assets |
||||||||||||
| Money market funds (a) |
$ | 21,813,659 | $ | — | $ | — | ||||||
| Available-for-sale marketable securities: |
||||||||||||
| Municipal Obligations and U.S. Government Securities (See Note 2) |
— | 42,571,544 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Assets |
$ | 21,813,659 | $ | 42,571,544 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Liabilities |
||||||||||||
| Interest rate swap (See Note 10) |
$ | — | $ | 5,542,509 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Total Liabilities |
$ | — | $ | 5,542,509 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| (a) | The Company’s money market funds are classified along with the Company’s cash balances as Cash and cash equivalents within the Consolidated Balance Sheets. Money market funds are valued at quoted prices in active markets. |
The Company follows the disclosure provisions of FASB ASC Topic 825, “Financial Instruments” (ASC 825), for disclosure purposes for financial assets and financial liabilities that are not measured at fair value. As of June 30, 2011, the financial assets and liabilities recorded on the Consolidated Balance Sheets that are not measured at fair value on a recurring basis include accounts receivable, net, accounts payable, deferred acquisition payments and debt obligations. The carrying value of accounts receivable, net, accounts payable and current debt obligations, approximate fair value due to the short-term nature of these instruments. The fair value of long-term debt, where a quoted market price was not available, is evaluated based on, among other factors, interest rates currently available to the Company for debt with similar terms, remaining payments and considerations of the Company’s creditworthiness. The Company determined that the recorded book value of long-term debt approximates fair value at June 30, 2011 due to the variable LIBOR portion of the Mortgage payments.
| 12. | PREFERRED STOCK |
The Company has authorized Preferred Stock consisting of 100,000 shares with a $.001 par value, 25,000 of which are designated as Series A Junior Participating Preferred Stock, as discussed below, and 75,000 of which are undesignated. The Board of Directors may authorize the issuance of Preferred Stock, which ranks senior to
79
Table of Contents
the Common Stock with respect to the payment of dividends and the distribution of assets on liquidation. In addition, the Board of Directors is authorized to fix the limitations and restrictions, if any, upon the payment of dividends on Common Stock to be effective while any shares of Preferred Stock are outstanding. The Board of Directors, without stockholder approval, can issue Preferred Stock with voting and conversion rights, which could adversely affect the voting power of the holders of Common Stock. At June 30, 2011 and 2010, no shares of Preferred Stock were outstanding. The Company has no present intention to issue shares of Preferred Stock (except that shares of Series A Preferred Stock (as defined below) may be issued pursuant to a stockholders’ rights plan, as described below).
Stockholders’ Rights Plan
On June 19, 2009, the Company announced that its Board of Directors adopted a stockholders’ rights plan (rights plan) that is designed to protect the Company and its stockholders from potentially coercive takeover practices or bids, and to prevent an acquiror from gaining control of the Company without offering a fair price to the stockholders. The Company adopted the plan in order to give its Board of Directors time to evaluate and respond to any unsolicited potential takeover attempts.
Under the rights plan, the Company distributed, as a dividend, one preferred stock purchase right for each share of Common Stock held of record as of the close of business on June 19, 2009. The rights plan has a term of 10 years and will expire on June 19, 2019, unless the Company earlier redeems the rights or the Board of Directors terminates the rights plan. Each right, if and when it becomes exercisable, entitles a holder to buy one one-thousandth of a share of Series A Junior Participating Preferred Stock (collectively, Preferred Shares) for $200.00, subject to certain conditions in the plan under which the exercise price may change.
Pursuant to the Certificate of Designations of Series A Junior Participating Preferred Stock (Series A Preferred Stock), there are 25,000 shares of Series A Preferred Stock authorized for issuance. Each share of Series A Preferred Stock will entitle the holder thereof to 1,000 votes on all matters submitted to a vote of the stockholders of the Company, and the holders of the shares of Series A Preferred Stock and the holders of shares of Common Stock of the Company and any other capital stock of the Company having general voting rights will vote together as one class on all matters submitted to a vote of the stockholders of the Company. Holders of the shares of Series A Preferred Stock will be entitled to receive, if declared by the Board of Directors, dividends payable when and as dividends are declared on the shares of Common Stock of the Company in an amount, subject to adjustment, equal to 1,000 times the aggregate per share amount of all cash dividends, and 1,000 times the aggregate per share amount of all non-cash dividends or other distributions, declared on the shares of the Company’s Common Stock. At June 30, 2011 and 2010, no shares of Series A Preferred Stock were outstanding.
| 13. | STOCK REPURCHASE PROGRAM |
From time to time, the Company has made repurchases of its stock, as authorized by the various stock repurchase programs established by the Company’s Board of Directors, and through other miscellaneous transactions. On June 16, 2010, the Company announced that its Board of Directors approved a stock repurchase program allowing the Company to repurchase up to a total of $30 million of its issued and outstanding shares of Common Stock.
During the year ended June 30, 2011, the Company repurchased and retired 1,175,738 shares of Common Stock at a total cost of approximately $29,999,994, or an average market price of $25.52 per share, using available cash. Included in these totals were 34,400 shares that were repurchased in June 2010, but settled in July 2010, at a cost of $811,054, or an average price per share of $23.58. As of June 30, 2011, no amount was remaining for repurchase under the Company’s current or prior stock repurchase programs.
During the year ended June 30, 2010, the Company repurchased and retired 1,794,705 shares of Common Stock at a cost of $41,309,153, or an average market price of $23.02 per share, using available cash. An additional 3,500 shares were repurchased in June 2009, and were settled in July 2009 at a cost of $87,279, or an average market price per share of $24.94.
80
Table of Contents
During the year ended June 30, 2009, the Company repurchased and retired 806,666 shares of Common Stock at a cost of $19,294,595, or an average market price of $23.92 per share, using available cash.
| 14. | SHARE-BASED COMPENSATION |
The Company accounts for its share-based compensation plans in accordance with ASC 718. Compensation expense related to share-based awards is classified on the Consolidated Statements of Income within the same line items as salary or consulting expense with respect to the award recipients, and is recorded over the awards’ relevant vesting periods. Compensation expense related to share-based awards granted to the members of the Board of Directors is recorded as a component of Selling, general and administrative expense on the Consolidated Statements of Income.
The following table provides a summary of the Company’s share-based compensation expense:
| Estimated Unrecognized Share-based Compensation as of |
Weighted Average as of |
|||||||||||||||||||
| Year Ending June 30, | June 30, | June 30, | ||||||||||||||||||
| 2011 | 2010 | 2009 | 2011 | 2011 | ||||||||||||||||
| Stock options |
$ | 3,708,626 | $ | 3,011,699 | $ | 1,939,330 | $ | 4,756,201 | 1.67 | |||||||||||
| Non-vested stock awards |
530,769 | 537,252 | 513,630 | 751,000 | 1.73 | |||||||||||||||
| SARs |
(127,052 | ) | (163,928 | ) | (390,503 | ) | — | 0.34 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total share-based compensation |
$ | 4,112,343 | $ | 3,385,023 | $ | 2,062,457 | $ | 5,507,201 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
Stock Options
Stock options have been granted to officers and other employees and members of the Board of Directors of the Company, as well as non-employee outside consultants under the Company’s Eighth Amended and Restated Kensey Nash Corporation Employee Incentive Compensation Plan, and prior versions of this incentive compensation plan (the Employee Plan). In addition, the Company also has a Non-employee Directors’ Stock Option Plan (the Directors’ Plan), under which no shares are available for new awards and any awards of the type granted previously under the Directors’ Plan are now granted under the Employee Plan. The fair value of each option grant under the Employee Plan was estimated on the date of grant using the Black-Scholes option-pricing model that uses the assumptions noted in the following table.
| Granted During Year Ended June 30, | ||||||
| 2011 | 2010 | 2009 | ||||
| Dividend yield |
0% | 0% | 0% | |||
| Expected volatility |
35% - 39% | 33% - 49% | 35% | |||
| Weighted-average volatility |
37.60% | 39.36% | 35% | |||
| Risk-free interest rate |
0.130% - 2.255% | 0.060% - 3.160% | 1.14% - 3.492% | |||
| Expected lives (in years): |
0.25 - 7.50 | 0.25 - 7.57 | 0.25 - 6.30 | |||
Options are exercisable over a maximum term of ten years from the date of grant and typically vest over periods of zero to four years from the grant date. Expected volatilities are based on historical volatility of the Company’s Common Stock, and other factors. The Company uses historical data to estimate option exercise and employee termination within the valuation model; separate groups of employees that have similar historical exercise behavior are considered separately for valuation purposes. The expected terms of options are derived from historical exercise behavior and represent the periods of time that options granted are expected to be outstanding. The total intrinsic value of options exercised during the years ended June 30, 2011, 2010 and 2009, was $5,505,908, $787,511 and $5,052,764, respectively.
81
Table of Contents
A summary of the stock option activity under both plans for the fiscal years ended June 30, 2011, 2010 and 2009, is as follows:
| Employee Plan | Directors’ Plan | |||||||||||||||||||||||||||||||
| Shares | Weighted Avg Exercise Price |
Aggregate Intrinsic Value |
Weighted Avg Remaining Contractual Term |
Shares | Weighted Avg Exercise Price |
Aggregate Intrinsic Value |
Weighted Avg Remaining Contractual Term |
|||||||||||||||||||||||||
| Balance at June 30, 2008 |
1,639,044 | $ | 21.62 | $ | 17,547,996 | 4.78 | 310,500 | $ | 20.43 | $ | 3,607,560 | 4.00 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Granted |
364,970 | $ | 30.86 | — | — | |||||||||||||||||||||||||||
| Cancelled |
(89,180 | ) | $ | 29.08 | (19,500 | ) | $ | 26.69 | ||||||||||||||||||||||||
| Exercised |
(243,930 | ) | $ | 17.43 | (58,000 | ) | $ | 15.47 | ||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance at June 30, 2009 |
1,670,904 | $ | 23.85 | $ | 8,222,304 | 5.04 | 233,000 | $ | 21.14 | $ | 1,441,568 | 3.53 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Granted |
364,590 | $ | 28.55 | — | — | |||||||||||||||||||||||||||
| Cancelled |
(152,066 | ) | $ | 30.65 | — | — | ||||||||||||||||||||||||||
| Exercised |
(42,944 | ) | $ | 13.56 | (22,500 | ) | $ | 13.25 | ||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance at June 30, 2010 |
1,840,484 | $ | 24.46 | $ | 5,966,212 | 4.93 | 210,500 | $ | 21.98 | $ | 736,218 | 2.52 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Granted |
385,825 | $ | 28.99 | — | — | |||||||||||||||||||||||||||
| Cancelled |
(67,131 | ) | $ | 30.46 | (9,000 | ) | $ | 32.00 | ||||||||||||||||||||||||
| Exercised |
(493,241 | ) | $ | 14.93 | (60,500 | ) | $ | 16.13 | ||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Balance at June 30, 2011 |
1,665,937 | $ | 28.09 | $ | 1,872,889 | 6.35 | 141,000 | $ | 23.86 | $ | 436,740 | 2.30 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Shares vested + expected to vest |
1,646,755 | $ | 28.08 | $ | 1,868,351 | 6.32 | 141,000 | $ | 23.86 | $ | 436,740 | 2.30 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Exercisable portion |
988,148 | $ | 27.48 | $ | 1,745,287 | 4.79 | 141,000 | $ | 23.86 | $ | 436,740 | 2.30 | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Available for future grant |
525,614 | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| Weighted-average fair value of options granted during the year ended June 30, |
||||||||||||||||||||||||||||||||
| 2009 |
$ | 11.08 | $ | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| 2010 |
$ | 10.51 | $ | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
| 2011 |
$ | 11.15 | $ | — | ||||||||||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||||||||||
Nonvested Stock Awards
Nonvested stock awards have been granted to the non-employee members of the Board of Directors, executive officers, certain other management of the Company and a non-employee outside consultant, pursuant to the Employee Plan, and generally vest in three equal annual installments based solely on continued employment or service, as applicable, with the Company. Nonvested stock awards granted to executive officers, management, non-employee members of the Board of Directors and non-employee consultants usually are referred to as restricted stock, but ASC 718 reserves that term for fully
82
Table of Contents
vested and outstanding shares, the sale of which is contractually or governmentally prohibited for a specified period of time. Fair value is based upon the closing price of the Company’s Common Stock on the date of grant.
The total fair value of shares vested during the years ended June 30, 2011, 2010 and 2009 was $712,180, $496,692 and $271,714, respectively.
The following table outlines nonvested stock award activity for the fiscal years ended June 30, 2011, 2010 and 2009:
| Employee Plan | ||||||||
| Shares | Weighted Average Price Per Share |
|||||||
| Balance June 30, 2008 |
31,199 | $ | 29.37 | |||||
| Granted: |
||||||||
| Non-employee Directors |
50,309 | 17.72 | ||||||
| Executive officers |
10,000 | 17.00 | ||||||
| Issued: |
||||||||
| Non-employee Directors |
(14,852 | ) | 27.67 | |||||
| Cancelled: |
||||||||
| Non-employee Directors |
(18,200 | ) | 21.06 | |||||
|
|
|
|
|
|||||
| Balance June 30, 2009 |
58,456 | $ | 18.75 | |||||
|
|
|
|
|
|||||
| Granted: |
||||||||
| Non-employee Directors |
25,186 | 22.86 | ||||||
| Issued: |
||||||||
| Non-employee Directors |
(19,077 | ) | 22.48 | |||||
| Executive officers & management |
(2,500 | ) | 17.00 | |||||
| Cancelled: |
||||||||
| Non-employee Directors |
(5,631 | ) | 20.42 | |||||
|
|
|
|
|
|||||
| Balance June 30, 2010 |
56,434 | $ | 21.05 | |||||
|
|
|
|
|
|||||
| Granted: |
||||||||
| Non-employee Directors |
17,818 | 27.22 | ||||||
| Issued: |
||||||||
| Non-employee Directors |
(23,023 | ) | 22.38 | |||||
| Executive officers & management |
(2,500 | ) | 17.00 | |||||
|
|
|
|
|
|||||
| Balance June 30, 2011 |
48,729 | $ | 22.89 | |||||
|
|
|
|
|
|||||
| Weighted-average fair value of nonvested stock awards granted during the year ended June 30, |
||||||||
| 2009 |
$ | 17.85 | ||||||
|
|
|
|||||||
| 2010 |
$ | 22.86 | ||||||
|
|
|
|||||||
| 2011 |
$ | 27.22 | ||||||
|
|
|
|||||||
Cash Settled Stock Appreciation Rights
Cash-settled stock appreciation rights (SARs) awards were granted to eligible employees during the fiscal year ended June 30, 2007, and provided each participant with the right to receive payment in cash, upon exercise, for the appreciation in market value of a specified number of shares of the Company’s Common Stock over the award’s exercise price. No SARs awards were granted during the fiscal years ended June 30, 2011, 2010 or 2009. The per-share exercise price of a SAR is equal to the closing market price of a share of the Company’s Common Stock on the date of grant.
83
Table of Contents
On November 14, 2007, the Company offered to buy back the SARs from all current eligible employees, excluding the executive officers, at the fair market value (Buyout Price) based on the closing price of the Company’s Common Stock on December 14, 2007. This offer resulted in the Company purchasing 173,940 SARs at a Buyout Price of $6.47 per SAR, for a total of $1,125,392 (excluding the Company’s portion of payroll taxes) paid to the participating eligible employees, which represented approximately 99% participation by eligible employees of the SARs outstanding.
As of June 30, 2011 the average fair market value of each remaining SAR was $.05 and the related liability for all remaining SARs was $4,784. These SARs will continue to be remeasured at each reporting period until all awards are settled. No cash settled SARs were exercised or repurchased during the Company’s fiscal years 2011 and 2010.
The following table outlines cash-settled SAR award activity for the fiscal years ended June 30, 2011, 2010 and 2009.
| Shares | Weighted Average Price Per Share |
|||||||
| Balance June 30, 2008 |
104,700 | $ | 31.35 | |||||
| Cancelled |
(10,000 | ) | 31.36 | |||||
|
|
|
|
|
|||||
| Balance June 30, 2009 |
94,700 | $ | 31.34 | |||||
| No activity |
— | — | ||||||
|
|
|
|
|
|||||
| Balance June 30, 2010 |
94,700 | $ | 31.34 | |||||
| No activity |
— | — | ||||||
|
|
|
|
|
|||||
| Balance June 30, 2011 |
94,700 | $ | 31.34 | |||||
|
|
|
|
|
|||||
The fair value of each SAR award is remeasured at each reporting period using the Black-Scholes option-pricing model that uses the assumptions noted in the following table.
| Year Ended June 30, | ||||||
| 2011 | 2010 | 2009 | ||||
| Dividend yield |
0% | 0% | 0% | |||
| Expected volatility |
15% – 35% | 35% | 35% | |||
| Risk-free interest rate |
0.124% –0.333 | 0.388% –0.820% | 0.566% –2.451% | |||
| Expected lives (in years) |
0.34 – 1.13 | 1.17 – 1.63 | 1.30 – 1.80 | |||
SARs are exercisable over a maximum term of five years from the date of grant and originally were to vest over a period of three years from the grant date. Expected volatilities are based on historical volatility of the Company’s Common Stock and other factors. The Company uses historical data to estimate SAR employee termination behavior within the valuation model; separate groups of employees that have similar historical exercise behavior are considered separately for valuation purposes. The risk-free rate for periods within the contractual life of the SAR is based on U.S. treasuries with constant maturities in effect at the time of grant.
| 15. | SEGMENT REPORTING |
The Company’s operations and products have been aggregated into a single reportable segment since they have similar economic characteristics, production processes, types of customers and distribution methods under the provisions of FASB ASC Topic 280, “Segment Reporting” (ASC 280). Operating segments are identified as components of an enterprise, about which separate discrete financial information is available for evaluation by the chief operating decision-making group in making decisions regarding how to allocate resources and assess performance.
84
Table of Contents
The Company generates revenue from the sale of products sold primarily in the cardiovascular, orthopaedic, sports medicine, spinal and general surgery markets. The largest customers include St. Jude Medical, Arthrex, Orthovita and Synthes. The Company also receives royalty income generated primarily from the net end-user sale of Angio-Seal units by St. Jude Medical and from the sales of Vitoss™ Foam and Vitoss™ Bioactive Foam products by Orthovita.
Net sales by product line and reconciliation to total revenue are as follows:
| Fiscal Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Biomaterials |
$ | 44,409,527 | $ | 52,096,283 | $ | 51,045,578 | ||||||
| Endovascular |
1,589,250 | 2,180,421 | 3,858,187 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net Sales |
45,998,777 | 54,276,704 | 54,903,765 | |||||||||
| Royalty income |
25,638,831 | 26,370,841 | 27,177,085 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Revenue |
$ | 71,637,608 | $ | 80,647,545 | $ | 82,080,850 | ||||||
|
|
|
|
|
|
|
|||||||
For the years ended June 30, 2011, 2010 and 2009, revenues from St. Jude Medical, Arthrex, Orthovita and Synthes represented the following percentages of total revenues to the Company:
| Percentage of Total Revenue for the Fiscal Year Ended June 30, |
||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| St. Jude Medical: |
||||||||||||
| Net sales |
17 | % | 24 | % | 22 | % | ||||||
| Royalty Income (see Note 4) |
27 | % | 25 | % | 26 | % | ||||||
| Arthrex: |
||||||||||||
| Net sales |
16 | % | 20 | % | 18 | % | ||||||
| Orthovita: |
||||||||||||
| Net sales |
11 | % | 10 | % | 10 | % | ||||||
| Royalty Income (see Note 4) |
8 | % | 7 | % | 7 | % | ||||||
| Synthes: |
||||||||||||
| Net sales |
7 | % | 2 | % | 1 | % | ||||||
| Royalty Income |
<1 | % | <1 | % | 0 | % | ||||||
Revenues are attributed to a country based on the location of the customer. The Company’s business is not directly dependent on foreign operations, as the Company’s sales to customers outside the U.S. are not significant. However, a portion of the Company’s revenues, including sales and royalties, are dependent on U.S. based customers selling to end-users outside the U.S. No one country where the Company sells its products, other than the U.S., represented more than 10% of the Company’s revenues in fiscal 2011. In addition, all of the Company’s long-lived assets are located in the U.S. The Company’s revenues are categorized geographically below:
| Revenues for the Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| United States |
$ | 70,103,387 | $ | 79,987,176 | $ | 81,518,199 | ||||||
| Other foreign countries |
1,534,221 | 660,369 | 562,651 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 71,637,608 | $ | 80,647,545 | $ | 82,080,850 | ||||||
|
|
|
|
|
|
|
|||||||
85
Table of Contents
| 16. | COMMITMENT AND CONTIGENCIES |
Certain Compensation and Employment Agreements
The Company has entered into employment agreements with certain of its named executive officers. As of June 30, 2011, these employment agreements provided for, among other things, annual base salaries in an aggregate amount of not less than $1,294,773, from that date through fiscal 2013.
Purchase Commitments
As of June 30, 2011, the Company had outstanding non-cancelable and cancelable purchase commitments in the amount of $1,903,345 related to inventory, capital expenditures and other goods and services.
Research and Development Contractual Obligations
Under the Company’s Development and Regulatory Services Agreement with Spectranetics, as amended, the Company’s future contributions are limited to a maximum amount of $2,750,000 toward the expenses associated with clinical studies to obtain approval from the FDA for certain next-generation endovascular products, which is reduced by the total cumulative expenses incurred through June 30, 2011 of approximately $30,000.
The Company has entered into other research and development service agreements with certain other customers, which provide that the Company is to share certain regulatory and clinical costs associated with future research and development activities. The amounts and timing of any such future payment obligations cannot currently be determined. Research and development costs, if any, under these agreements would be expensed as they are incurred.
Cost Method Investment Obligations
Subsequent to June 30, 2011, on August 19, 2011, at the Company’s option, and pursuant to an ancillary agreement, the Company made a cash advance to Orteq of approximately 637,000 British Pounds, or $1,055,063, in the form of a cash advance, structured as convertible debt to Orteq. See Note 8 for additional information.
Nerites Purchase Price Obligations
Pursuant to the terms of the asset purchase agreement entered into with Nerites, the Company held back $3.0 million of the purchase price as security for certain potential indemnification obligations of Nerites. The Company will release this $3.0 million of the acquisition purchase price to Nerites in increments of $1.5 million on each of the first and second anniversaries of the acquisition date, to the extent that the hold-back amount is not applied toward such indemnification obligations. See Note 5 for additional information.
Norian Purchase Price Obligation
Pursuant to the terms of the asset purchase agreement entered into with Norian, the Company is required to pay the remaining $14 million of the purchase price on the earlier of the date on which the transfer of the manufacturing operations from the purchased West Chester, Pennsylvania facility to the Company’s corporate headquarters facility located in Exton, Pennsylvania has been completed or the 18-month anniversary of the date of acquisition. See Note 5 for additional information.
Operating Lease Commitments
As a result of the Company’s asset acquisition of Nerites, the Company became party to leased space in Madison, Wisconsin, as well as a lease for office equipment. As of June 30, 2011, the Company’s minimum future annual rental commitment under non-cancelable operating leases related to Nerites was $119,797.
86
Table of Contents
Pursuant to the Company’s acquisition of certain assets of Norian, the Company entered into an 18-month sublease agreement with Synthes to rent the manufacturing space in the Company’s West Chester, Pennsylvania facility. As of June 30, 2011, the Company’s minimum future annual rental commitment under non-cancelable operating leases related to Norian was $1,105,000.
As of June 30, 2011, the Company’s total minimum future annual rental commitment under non-cancelable operating leases was $1,224,797. The following table is a schedule by year of the future minimum lease payments for operating leases with original terms in excess of one year:
| Fiscal year ending June 30, |
Rental Expense |
|||
| 2012 |
$ | 894,911 | ||
| 2013 |
329,886 | |||
| 2014 |
— | |||
| 2015 |
— | |||
| 2016 |
— | |||
| Thereafter |
— | |||
| 17. | RETIREMENT PLAN |
The Company has a voluntary 401(k) Savings Plan (the 401(k) Plan) in which all employees that are at least 21 years of age and have three months of service with the Company are eligible to participate. The Company provides a 50% discretionary matching contribution on the first 6% of an employee’s total salaried compensation, for all employee contributions. Total Company contributions to the 401(k) plan for fiscal years ended June 30, 2011, 2010 and 2009 were $384,503, $388,720 and $427,540, respectively.
| 18. | INCOME TAXES |
Income Tax Expense
Income before income taxes was earned within the United States and is shown in the table below:
| June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Income before income taxes |
$ | 2,000,068 | $ | 28,852,425 | $ | 29,789,266 | ||||||
|
|
|
|
|
|
|
|||||||
The provision for income taxes is composed of the following:
| Fiscal Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Taxes on U.S. earnings |
||||||||||||
| Federal |
||||||||||||
| Current |
$ | 5,577,404 | $ | 10,588,087 | $ | 7,685,165 | ||||||
| Deferred |
(5,663,337 | ) | (1,252,458 | ) | 1,404,428 | |||||||
| State |
||||||||||||
| Current |
47,210 | 144,531 | 806,422 | |||||||||
| Deferred |
(397,407 | ) | — | 8,946 | ||||||||
| Valuation allowance |
358,155 | (91,859 | ) | (194,805 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total income tax (benefit)/expense |
$ | (77,975 | ) | $ | 9,388,301 | $ | 9,710,156 | |||||
|
|
|
|
|
|
|
|||||||
87
Table of Contents
The differences between the Company’s income tax expense and the income tax expense (benefit) computed using the U.S. federal income tax rate were as follows:
| Fiscal Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net income before income taxes |
$ | 2,000,068 | $ | 28,852,425 | $ | 29,789,266 | ||||||
|
|
|
|
|
|
|
|||||||
| Tax provision at U.S. statutory rate |
700,023 | 10,098,349 | 10,426,243 | |||||||||
| State income tax provision, net of federal benefit |
9,840 | 93,945 | 524,174 | |||||||||
| Reconciliation to actual tax rate: |
||||||||||||
| Deferred tax expense on gain on bargain purchase of net assets acquired (See Note 5) |
(179,950 | ) | — | — | ||||||||
| Non-deductible meals and entertainment |
11,126 | 10,106 | 9,778 | |||||||||
| Capital loss valuation allowance |
(38,303 | ) | 7,093 | 25,901 | ||||||||
| Release of state valuation allowance |
— | (91,859 | ) | (194,805 | ) | |||||||
| Research and development credits |
(442,574 | ) | (265,249 | ) | (464,362 | ) | ||||||
| Domestic production deduction |
4,901 | (221,056 | ) | (148,317 | ) | |||||||
| Non-taxable municipal bond interest income |
(139,685 | ) | (236,243 | ) | (345,862 | ) | ||||||
| Other |
(3,353 | ) | (6,785 | ) | (122,594 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total income tax (benefit)/expense |
$ | (77,975 | ) | $ | 9,388,301 | $ | 9,710,156 | |||||
|
|
|
|
|
|
|
|||||||
| Current income tax expense |
$ | 5,624,614 | $ | 10,732,618 | $ | 8,296,782 | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred income tax (benefit)/expense |
$ | (5,702,589 | ) | $ | (1,344,317 | ) | $ | 1,413,374 | ||||
|
|
|
|
|
|
|
|||||||
Deferred Taxes
Significant components of the Company’s deferred tax assets and liabilities are as follows:
| Fiscal Year Ended June 30, | ||||||||||||||||
| 2011 | 2010 | |||||||||||||||
| Current | Noncurrent | Current | Noncurrent | |||||||||||||
| Deferred Tax Asset: |
||||||||||||||||
| Accrued expenses |
$ | 102,324 | $ | — | $ | 775,561 | $ | — | ||||||||
| Basis difference—intangibles |
— | 6,543,808 | — | 553,435 | ||||||||||||
| Inventory |
967,162 | — | 1,249,734 | — | ||||||||||||
| Goodwill |
— | — | 174,150 | |||||||||||||
| Stock options |
396,256 | 3,566,300 | 700,197 | 1,800,507 | ||||||||||||
| Nonvested stock awards |
101,910 | — | 107,510 | — | ||||||||||||
| Milestone payments |
285,807 | 1,133,007 | 286,682 | 1,278,557 | ||||||||||||
| Mortgage interest swap |
— | 1,910,387 | — | 1,939,878 | ||||||||||||
| Basis difference—Cost Method Investment |
— | 191,019 | — | — | ||||||||||||
| Other |
24,165 | 43,302 | (156,513 | ) | 65,044 | |||||||||||
| Net operating loss carryforwards |
— | 3,965,390 | — | 3,896,100 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 1,877,624 | 17,353,213 | 2,963,171 | 9,707,671 | |||||||||||||
| Less valuation allowance |
(147,925 | ) | (4,178,522 | ) | — | (3,968,238 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Deferred tax asset |
1,729,699 | 13,174,691 | 2,963,171 | 5,739,433 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Deferred Tax Liability: |
||||||||||||||||
| Basis difference—fixed assets |
— | (4,702,835 | ) | — | (3,866,814 | ) | ||||||||||
| Basis difference—intangibles |
— | (64,172 | ) | — | — | |||||||||||
| Prepaid insurance |
(114,557 | ) | (105,909 | ) | ||||||||||||
| Other |
(51,056 | ) | (36,004 | ) | — | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Deferred tax liability |
(165,613 | ) | (4,803,011 | ) | (105,909 | ) | (3,866,814 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net Deferred Tax Asset |
$ | 1,564,086 | $ | 8,371,680 | $ | 2,857,262 | $ | 1,872,619 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
88
Table of Contents
A portion of the Company’s deferred tax asset is offset by a valuation allowance relating to state net operating loss (NOL) carryforwards due to restrictions imposed and uncertainty surrounding its use. The valuation allowance reduces deferred tax assets to an amount that represents management’s best estimate of the amount of such deferred tax assets that more than likely will be realized. At June 30, 2011, the Company had state NOL carryforwards of approximately $61.9 million, which will expire through 2029, and no longer had a federal NOL carryforward. The Company has recorded a valuation allowance against the states’ net operating losses with uncertainty surrounding its use of the $61.9 million.
As a result of the December 2010 Congressional approval of an extension of the Research & Experimentation (R&E) Tax Credit, the Company recorded retroactive adjustments to its tax provision during the quarter ended December 31, 2010 of fiscal 2011. These adjustments reflect the fact that the legislation is retroactive to January 1, 2010 and therefore, reduced the Company’s effective tax rate in fiscal 2011. The Company recorded total R&D Tax Credits of $442,574, $265,249 and $464,362, for the fiscal years ended June 30, 2011, 2010 and 2009, respectively.
Uncertain Tax Positions
The Company adopted the provisions of FASB ASC Subtopic 740-10, “Income Taxes,” on July 1, 2007. The amount of unrecognized tax benefits at June 30, 2011, 2010 and 2009 were $103,694, $117,216 and $140,650, respectively. If these gross unrecognized tax benefits were recognized, $101,837, $115,359 and $139,183 would impact the Company’s tax rate at June 30, 2011, 2010 and 2009, respectively. The Company recorded interest and penalties of less than $2,000 for each of the fiscal years ended June 30, 2011, 2010 and 2009. Interest and penalties are included in Interest expense and Other income (loss) respectively on the Consolidated Statements of Income.
Changes in the Company’s uncertain tax positions for the years ended June 30, 2011, 2010 and 2009 were as follows:
| Fiscal Year Ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Balance at beginning of year |
$ | 117,216 | $ | 140,650 | $ | 193,132 | ||||||
| Increases related to current year tax positions |
— | 1,114 | — | |||||||||
| Increases related to prior year tax positions |
26,787 | 21,866 | 64,907 | |||||||||
| Settlements/payments |
— | — | (71,121 | ) | ||||||||
| Decreases related to prior year tax positions |
— | — | (2,098 | ) | ||||||||
| Reduction due to lapse in statute of limitations |
(40,309 | ) | (46,414 | ) | (44,170 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance at end of year |
$ | 103,694 | $ | 117,216 | $ | 140,650 | ||||||
|
|
|
|
|
|
|
|||||||
The Company and its subsidiaries file U.S. federal and various state income tax returns. The Company is no longer subject to U.S. federal income tax examination for years prior to fiscal 2005 due to the expiration of applicable statutes of limitation. The Company does not expect the total amount of unrecognized tax benefits to change significantly in the next 12 months.
89
Table of Contents
| 19. | EARNINGS PER SHARE |
The following table shows the reconciliation between the numerators and denominators for the basic and diluted EPS calculations, where income is the numerator and the weighted average number of shares is the denominator.
| Year Ended June 30, 2011 | ||||||||||||
| Income | Shares | Per Share Amount |
||||||||||
| Basic EPS |
||||||||||||
| Income available to common shareholders |
$ | 2,078,043 | 8,658,319 | $ | 0.24 | |||||||
|
|
|
|||||||||||
| Effect of Dilutive Securities |
||||||||||||
| Options & nonvested stock |
— | 235,083 | ||||||||||
|
|
|
|
|
|||||||||
| Diluted EPS |
||||||||||||
| Income available to common shareholders including assumed conversions |
$ | 2,078,043 | 8,893,402 | $ | 0.23 | |||||||
|
|
|
|
|
|
|
|||||||
| Year Ended June 30, 2010 | Year Ended June 30, 2009 | |||||||||||||||||||||||
| Income | Shares | Per Share Amount |
Income | Shares | Per Share Amount |
|||||||||||||||||||
| Basic EPS |
||||||||||||||||||||||||
| Income available to common shareholders |
$ | 19,464,124 | 10,623,926 | $ | 1.83 | $ | 20,079,110 | 11,547,266 | $ | 1.74 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Effect of Dilutive Securities |
||||||||||||||||||||||||
| Options & nonvested stock |
— | 312,664 | — | 350,569 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Diluted EPS |
||||||||||||||||||||||||
| Income available to common shareholders including assumed conversions |
$ | 19,464,124 | 10,936,590 | $ | 1.78 | $ | 20,079,110 | 11,897,835 | $ | 1.69 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 20. | QUARTERLY FINANCIAL DATA (UNAUDITED) |
The summarized quarterly results of operations of the Company for the years ended June 30, 2011 and 2010 are presented below:
| Year Ended June 30, 2011 | ||||||||||||||||
| 1st Quarter | 2nd Quarter | 3rd Quarter (a) | 4th Quarter | |||||||||||||
| Total revenues |
$ | 16,964,599 | $ | 17,354,923 | $ | 18,592,383 | $ | 18,725,703 | ||||||||
| Gross profit |
$ | 6,659,830 | $ | 4,559,965 | $ | 6,413,445 | $ | 4,962,802 | ||||||||
| Operating costs and expenses |
$ | 10,795,383 | $ | 12,375,115 | $ | 30,693,673 | $ | 14,295,354 | ||||||||
| Net income / (loss) |
$ | 3,844,890 | $ | 3,333,625 | $ | (8,049,314 | ) | $ | 2,948,841 | |||||||
| Basic earnings / (loss) per share |
$ | 0.43 | $ | 0.39 | $ | (0.94 | ) | $ | 0.34 | |||||||
| Diluted earnings / (loss) per share |
$ | 0.41 | $ | 0.38 | $ | (0.94 | ) | $ | 0.34 | |||||||
| Year Ended June 30, 2010 | ||||||||||||||||
| 1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | |||||||||||||
| Total revenues |
$ | 19,741,818 | $ | 19,070,632 | $ | 19,946,414 | $ | 21,888,681 | ||||||||
| Gross profit |
$ | 7,894,302 | $ | 5,977,706 | $ | 7,551,135 | $ | 9,346,210 | ||||||||
| Operating costs and expenses |
$ | 11,993,902 | $ | 13,282,807 | $ | 12,332,722 | $ | 12,783,997 | ||||||||
| Net income |
$ | 4,880,809 | $ | 3,659,529 | $ | 5,001,560 | $ | 5,922,226 | ||||||||
| Basic earnings per share |
$ | 0.44 | $ | 0.33 | $ | 0.47 | $ | 0.61 | ||||||||
| Diluted earnings per share |
$ | 0.43 | $ | 0.32 | $ | 0.46 | $ | 0.60 | ||||||||
| (a) | Total operating costs and expenses for the Company’s third quarter of fiscal 2011 included an IPR&D charge of $18,233,006 related to the Company’s asset acquisition of Nerites in January 2011 (See Note 5). |
90
Table of Contents
Quarterly and total year earnings per share are calculated independently based on the weighted average number of shares outstanding during each period.
| 21. | SALE OF ENDOVASCULAR BUSINESS |
On May 30, 2008, the Company completed the sale of its Endovascular business to Spectranetics.
Pursuant to an Asset Purchase Agreement, the Company sold to Spectranetics the assets related to the QuickCat™ (QuickCat), ThromCat® (ThromCat) and Safe-Cross® (Safe-Cross) product lines, including the stock of its European subsidiary Kensey Nash Europe GmbH, for a $10.0 million cash payment at closing, with an opportunity for up to an additional $8.0 million in research and development milestone payments, a $6.0 million cumulative sales milestone payment and additional royalty payments based on future sales of the ThromCat and Safe-Cross products after the transition of manufacturing of the products from the Company to Spectranetics. These payments and other related agreements are described below.
On May 30, 2008, the Company and Spectranetics also entered into a Manufacturing and Licensing Agreement pursuant to which the Company agreed to manufacture for Spectranetics the endovascular products acquired by Spectranetics under the Asset Purchase Agreement and for which Spectranetics will purchase such products for the specified time periods. Spectranetics further agreed that, during the manufacturing period, it would pay transfer prices for the products based on the Company’s cost to manufacture such products plus a percentage of the end-user sales price of the ThromCat and Safe-Cross products. Additionally, Spectranetics agreed that, after the Company’s manufacture of the ThromCat and Safe-Cross products was transferred to Spectranetics, Spectranetics will be obligated to pay the Company a royalty on the end-user sales of such products.
The Company’s second quarter ended December 31, 2009 of its prior fiscal 2010 was the last quarter reflecting product sales of the QuickCat device, as the Company transferred its QuickCat manufacturing to Spectranetics as of December 31, 2009. Spectranetics has no obligation to make royalty payments to the Company related to end-user sales of the QuickCat product.
As previously disclosed, Spectranetics publicly announced the discontinuation of sales of the Safe-Cross product line during the Company’s second quarter of fiscal 2010. Spectranetics’ decision to discontinue the Safe-Cross product line negatively impacts the Company’s ability to receive future royalties based on sales of the Safe-Cross product, and at a minimum, delays the Company’s achievement of the $6 million cumulative sales milestone, which is based upon sales of the Safe-Cross, ThromCat and QuickCat products. This milestone is due to the Company when Spectranetics reaches a cumulative $20 million in end user sales of the product lines purchased by SPNC from the Company in May 2008.
During fiscal 2011, the Company’s manufacturing of the endovascular product line was limited to the ThromCat product. The Company’s fourth quarter of fiscal 2011 ended June 30, 2011 is the last quarter reflecting its sales of the ThromCat product, as the ThromCat manufacturing was transferred to Spectranetics in May 2011, pursuant to the terms of the Manufacturing and Licensing Agreement with Spectranetics. Following the transition of manufacturing of the ThromCat product to Spectranetics, Spectranetics will pay to the Company a predetermined royalty rate on the end-user sales of the ThromCat product. The amount of this royalty will be based upon the timing and reason for the manufacturing transfer and, in certain cases, upon the amount of revenue generated by the ThromCat products during the applicable year. The royalty is subject to reduction depending upon the scope of the patent protection obtained for the ThromCat product.
On May 30, 2008, the Company and Spectranetics also entered into a Development and Regulatory Services Agreement, pursuant to which the Company agreed to conduct work to develop and obtain regulatory approval from the FDA for certain next-generation Safe-Cross and ThromCat products at the Company’s expense on behalf of Spectranetics. Spectranetics owns all intellectual property resulting from this development work. If clinical studies are required to obtain approval from the FDA for those next-generation products, the costs will be
91
Table of Contents
shared equally by the Company and Spectranetics; however, pursuant to an amendment to the Development and Regulatory Services Agreement the Company’s contributions are limited to a maximum amount of $2,750,000 toward these clinical studies. Spectranetics will pay the Company up to $8.0 million upon completion of such product development activities and regulatory approvals for certain of the next-generation products. These milestones will be recorded as revenue and recognized over the period of the agreement (including through the date of receipt of the latest expected regulatory approvals and/or completion of product development activities). In fiscal 2009, the Company announced the accomplishment of two milestones under this agreement, which resulted in a total payment of $2.5 million. The Company has recognized $577,000 in revenue during fiscal 2011, as well as fiscal 2010.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act)), as of the end of the period covered by this report. Our disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported accurately and within the time frames specified in the SEC’s rules and forms and that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of fiscal 2011, our disclosure controls and procedures are effective at the reasonable assurance level.
Changes In Internal Control Over Financial Reporting
There have not been any changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the fourth quarter of the fiscal year ended June 30, 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in the rules promulgated under the Exchange Act. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we have conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in “Internal Control-Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management has excluded from the scope of its assessment of internal control over financial reporting the operations and related net assets it acquired from Norian Corporation on May 24, 2011. At June 30, 2011, the total assets and total net income of Norian Corporation that were not subject to internal control over financial reporting represented less than 10% and 5%, respectively, of the Company’s consolidated total assets and net income, respectively, at June 30, 2011. Based on this evaluation, our management has concluded that, as of June 30, 2011, we did not have any material weaknesses in our internal control over financial reporting and our internal control over financial reporting was effective.
92
Table of Contents
Inherent Limitations on the Effectiveness of Controls
Our management does not expect that our disclosure controls and procedures or our internal controls will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within our Company have been detected.
These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies and procedures.
Our independent registered public accounting firm, Deloitte & Touche LLP, has issued an audit report on the effectiveness of our internal control over financial reporting. This audit report appears below.
93
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Kensey Nash Corporation
Exton, Pennsylvania
We have audited the internal control over financial reporting of Kensey Nash Corporation and subsidiaries (the “Company”) as of June 30, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. As described in “Item 9A. Controls and Procedures,” management has excluded from the scope of its assessment of internal control over financial reporting the operations and related net assets, which the Company acquired from Norian Corporation on May 24, 2011 (“Norian”). As of June 30, 2011, the total assets and total net income of Norian Corporation that were not subject to internal control over financial reporting represented less than 10% and 5%, respectively, of the Company’s consolidated total assets and net income, respectively, as of and for the year ended June 30, 2011. Accordingly, our audit did not include the internal control over financial reporting of Norian. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2011, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended June 30, 2011 of the Company and our report dated September 12, 2011 expressed an unqualified opinion on those financial statements.
| /s/ DELOITTE & TOUCHE LLP |
| Philadelphia, Pennsylvania |
| September 12, 2011 |
94
Table of Contents
| ITEM 9B. | OTHER INFORMATION |
Not applicable.
95
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
Information required by this item is incorporated by reference to the information under the captions “Proposal No. 1—Election of Directors,” “—Nominees,” “—Other Continuing Directors,” “Executive Officers,” “—Governance of the Company,” and “—Section 16(a) Beneficial Ownership Reporting Compliance” in our definitive Proxy Statement in connection with our 2011 Annual Meeting of Stockholders scheduled to be held on December 7, 2011 (the 2011 Proxy Statement), which will be filed with the Securities and Exchange Commission no later than 120 days after June 30, 2011, or October 28, 2011, pursuant to Regulation 14A.
| ITEM 11. | EXECUTIVE COMPENSATION |
Information in response to this item is incorporated by reference to the information under the captions “Executive Compensation” and “Board of Director Compensation” in the 2011 Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item is incorporated by reference to the information under the captions “Security Ownership of Management and Certain Stockholders,” and “Equity Compensation Plan Information” in the 2011 Proxy Statement.
| ITEM 13. | CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
Information required by this item is incorporated by reference to the information under the captions “Related Party Transactions and Approval Policy” and “Governance of the Company” in the 2011 Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Information required by this item is incorporated by reference to the information under the caption “Proposal No. 2—Ratification of Appointment of Independent Registered Public Accounting Firm—Independent Auditor Fees” in the 2011 Proxy Statement.
96
Table of Contents
PART IV
| ITEM 15. | EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
15 (a) CONSOLIDATED FINANCIAL STATEMENTS AND SCHEDULES
Incorporated by reference to Item 8 of this Report on Form 10-K.
15 (b) and 15 (c) EXHIBITS, FINANCIAL STATEMENT SCHEDULES
FINANCIAL STATEMENT SCHEDULES
All other schedules are omitted because they are not required, are not applicable or the information is scheduled in our financial statements or notes thereto.
EXHIBITS
| Exhibit |
Description |
Incorporation By Reference To | ||
| 2.1 | Asset Purchase Agreement dated September 1, 2000 by and among Kensey Nash Corporation, THM Acquisition Sub, Inc., THM Biomedical, Inc. and the stockholders of THM Biomedical, Inc. | Exhibit 2.1 to our Annual Report on Form 10-K for the annual period ended June 30, 2006. | ||
| 2.2 | Asset Purchase Agreement by and among KNC NER Acquisition Sub, Inc., Nerites Corporation, and Kensey Nash Corporation, dated January 28, 2011.* | Exhibit 2.1 to Amendment No. 1 to our Current Report on Form 8-K filed with the SEC on May 13, 2011. | ||
| 2.3 | Asset Purchase Agreement by and between Norian Corporation, KNC NOR Acquisition Sub, Inc. and Kensey Nash Corporation, a Delaware corporation, dated May 24, 2011.* | Exhibit 2.1 to our Current Report on Form 8-K filed with the SEC on May 27, 2011. | ||
| 2.4 | Agreement of Sale (for 1230 Wilson Drive, West Chester, Pennsylvania) by and between Norian Corporation and KNC NOR Acquisition Sub, Inc., dated May 24, 2011.* | Exhibit 2.2 to our Current Report on Form 8-K filed with the SEC on May 27, 2011. | ||
| 3.1 | Second Amended and Restated Certificate of Incorporation of Kensey Nash Corporation | Exhibit 3.1 to our Registration Statement on Form S-1, Registration No. 33-98722. | ||
| 3.2 | Third Amended and Restated Bylaws of Kensey Nash Corporation, as amended | Exhibit 3.1 to our Current Report on Form 8-K filed with the SEC on June 19, 2009. | ||
| 3.3 | Certificate of Designations of Series A Junior Participating Preferred Stock of Kensey Nash Corporation | Exhibit 3.2 to our Current Report on Form 8-K filed with the SEC on June 19, 2009. | ||
| 4.1 | Specimen stock certificate representing Kensey Nash Corporation common stock | Exhibit 3.1 to our Registration Statement on Form S-1, Registration No. 33-98722. | ||
| 4.2 | Rights Agreement, dated as of June 18, 2009, by and between Kensey Nash Corporation and Computershare Trust Company, N.A., as Rights Agent | Exhibit 4.1 to our Current Report on Form 8-K filed with the SEC on June 19, 2009. | ||
97
Table of Contents
| Exhibit |
Description |
Incorporation By Reference To | ||
| 10.1 | Kensey Nash Corporation Eighth Amended and Restated Kensey Nash Corporation Employee Incentive Compensation Plan, as amended† | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on December 7, 2010. | ||
| 10.2 | Kensey Nash Corporation Fourth Amended and Restated Nonemployee Directors’ Stock Option Plan and form of Stock Option Agreement† | Exhibit 4.5 to our Registration Statement on Form S-8, Registration No. 333-71050. | ||
| 10.3 | Form of Stock Option Agreement† | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on December 16, 2008. | ||
| 10.4 | Form of Restricted Stock Agreement† | Exhibit 10.3 to our Current Report on Form 8-K filed with the SEC on December 16, 2008. | ||
| 10.5 | Form of Cash Settled Stock Appreciation Right Agreement† | Exhibit 10.5 to our Current Report on Form 8-K filed with the SEC on December 6, 2006. | ||
| 10.6 | Form of Directors’ Indemnification Agreement | Exhibit 3.1 to our Registration Statement on Form S-1, Registration No. 33-98722. | ||
| 10.7 | License Agreement (United States) dated September 4, 1991, by and between Kensey Nash Corporation and American Home Products Corporation (assumed by St. Jude Medical, Inc.) | Exhibit 3.1 to our Registration Statement on Form S-1, Registration No. 33-98722. | ||
| 10.8 | License Agreement (Foreign) dated September 4, 1991, by and between Kensey Nash Corporation and American Home Products Corporation (assumed by St. Jude Medical, Inc.) | Exhibit 3.1 to our Registration Statement on Form S-1, Registration No. 33-98722. | ||
| 10.9 | Amendment to the Development and Distribution Agreement dated February 28, 2005 between Kensey Nash Corporation and Orthovita, Inc. | Exhibit 10.9 to our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2005. | ||
| 10.10 | Supply Agreement, dated June 15, 2005, by and between Kensey Nash Corporation and St. Jude Medical, Daig Division, Inc. | Exhibit Number 10 to our Current Report on Form 8-K filed with the SEC on June 21, 2005. | ||
| 10.11 | Secured Commercial Mortgage, dated May 25, 2006, between Kensey Nash Corporation and Citibank, F.S.B. | Exhibit 10.11 to our Current Report on Form 8-K filed with the SEC on June 1, 2006. | ||
| 10.12 | Swap Agreement, dated May 24, 2006, between Kensey Nash Corporation and Citibank, N.A. | Exhibit 10.12 to our Current Report on Form 8-K filed with the SEC on June 1, 2006. | ||
| 10.13 | Settlement Agreement with Ramius Group dated October 24, 2007 | Exhibit Number 10.1 to our Current Report on Form 8-K filed with the SEC on October 24, 2007. | ||
| 10.14 | Confidentiality Agreement with Ramius Group dated October 24, 2007 | Exhibit Number 10.2 to our Current Report on Form 8-K filed with the SEC on October 24, 2007. | ||
| 10.15 | Asset Purchase Agreement dated May 12, 2008 by and among Kensey Nash Corporation, ILT Acquisition Sub, Inc., Kensey Nash Holding Corporation and The Spectranetics Corporation | Exhibit Number 10.1 to our Current Report on Form 8-K filed with the SEC on May 16, 2008. | ||
98
Table of Contents
| Exhibit |
Description |
Incorporation By Reference To | ||
| 10.16 | Amended and Restated Employment Agreement for Joseph W. Kaufmann, Chief Executive Officer† | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on January 7, 2009. | ||
| 10.17 | Amended and Restated Employment Agreement for Douglas G. Evans, P.E., Chief Operating Officer† | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on January 7, 2009. | ||
| 10.18 | Amended and Restated Employment Agreement dated January 1, 2009, by and between Kensey Nash Corporation and Todd M. DeWitt† | Exhibit 10.2 to our Quarterly Report on Form 10-Q for the quarterly period ended December 31, 2008. | ||
| 10.19 | Amended and Restated Employment Agreement dated January 1, 2009, by and between Kensey Nash Corporation and James T. Rauth, P.E.† | Exhibit 10.4 to our Quarterly Report on Form 10-Q for the quarterly period ended December 31, 2008. | ||
| 10.20 | Employment Agreement between Kensey Nash Corporation and Michael Celano, Chief Financial Officer, dated as of March 10, 2011.† | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on March 15, 2011. | ||
| 10.21 | Mutual Consent to Extend Employment Term for Joseph W. Kaufmann, President and Chief Executive Officer, dated March 16, 2010.† | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on March 22, 2010. | ||
| 10.22 | Mutual Consent to Extend Employment Term for Douglas G. Evans, P.E., Chief Operating Officer, dated March 16, 2010.† | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on March 22, 2010. | ||
| 10.23 | Supply Agreement, dated June 23, 2010, by and between Kensey Nash Corporation and St. Jude Medical, Cardiology Division, Inc. | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on June 28, 2010. | ||
| 10.24 | Mutual Consent to Extend Employment Term for Todd M. DeWitt, Vice President of Biomaterials, dated October 15, 2010. | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on October 21, 2010. | ||
| 10.25 | Mutual Consent to Extend Employment Term for James T. Rauth, P.E., Vice President of Operations, dated October 15, 2010. | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on October 21, 2010. | ||
| 10.26 | Consulting Agreement, dated as of October 22, 2010 between Kensey Nash Corporation and Harold N. Chefitz. | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on October 25, 2010. | ||
| 10.27 | Loan and Agency Agreement, dated as of May 26, 2011, between Kensey Nash Corporation, the Lenders, and Wells Fargo Bank, National Association. | Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on May 26, 2011. | ||
| 10.28 | Revolving Credit Note, dated May 26, 2011, issued by Kensey Nash Corporation in favor of Wells Fargo Bank, National Association. | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on May 26, 2011 | ||
| 10.29 | Supply Agreement by and between Synthes USA Sales, LLC, and KNC NOR Acquisition Sub, Inc., dated May 24, 2011, as amended by the First Amendment thereto, dated July 2011.** | Exhibit 101 to Amendment No. 2 to our Current Report on Form 8-K filed with the SEC on September 9, 2011. | ||
99
Table of Contents
| Exhibit |
Description |
Incorporation By Reference To | ||
| 10.30 | Waiver and Amendment to Employment Agreement between Kensey Nash Corporation and Joseph W. Kaufmann, dated as of July 18, 2011.† |
Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on July 22, 2011 | ||
| 10.31 | Waiver and Amendment to Employment Agreement between Kensey Nash Corporation and Douglas G. Evans, P.E., dated as of July 18, 2011.† | Exhibit 10.2 to our Current Report on Form 8-K filed with the SEC on July 22, 2011 | ||
| 10.32 | Waiver and Amendment to Employment Agreement between Kensey Nash Corporation and Todd M. DeWitt, dated as of July 18, 2011.† |
Exhibit 10.3 to our Current Report on Form 8-K filed with the SEC on July 22, 2011 | ||
| 10.33 | Waiver and Amendment to Employment Agreement between Kensey Nash Corporation and James T. Rauth, P.E., dated as of July 18, 2011.† |
Exhibit 10.4 to our Current Report on Form 8-K filed with the SEC on July 22, 2011 | ||
| 21.1 | Subsidiaries of Kensey Nash Corporation | |||
| 23.1 | Consent of Independent Registered Public Accounting Firm | |||
| 31.1 | Certification of Chief Executive Officer pursuant to Exchange Act Rule 13a-14(a). | |||
| 31.2 | Certification of Chief Financial Officer pursuant to Exchange Act Rule 13a-14(a). | |||
| 32.1 | Certification of the Chief Executive Officer pursuant to Section 906 of the Sarbanes Oxley Act of 2002. | |||
| 32.2 | Certification of the Chief Financial Officer pursuant to Section 906 of the Sarbanes Oxley Act of 2002. | |||
| † | Management contract or compensatory plan or arrangement. |
| * | Exhibits and schedules omitted pursuant to Item 601(b)(2) of Regulation S-K. |
| ** | Portions of this exhibit were omitted and have been filed separately with the Securities and Exchange Commission pursuant to an application for confidential treatment under Rule 24b-2 under the Securities Exchange Act of 1934 and Rule 406 under Securities Act of 1933. |
100
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized, on the 12th day of September 2011.
| KENSEY NASH CORPORATION | ||
| By: | /S/ MICHAEL CELANO | |
| Michael Celano | ||
| Chief Financial Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated on the 12th day of September 2011.
| Signature | Titles | |
| /S/ JOSEPH W. KAUFMANN Joseph W. Kaufmann |
Chief Executive Officer (principal executive officer), President, Secretary and Director | |
| /S/ DOUGLAS G. EVANS, P.E. Douglas G. Evans, P.E. |
Chief Operating Officer, Assistant Secretary and Director | |
| /S/ MICHAEL CELANO Michael Celano |
Chief Financial Officer (principal financial and accounting officer) | |
| /S/ ROBERT J. BOBB Robert J. Bobb |
Director | |
| /S/ WALTER R. MAUPAY, JR. Walter R. Maupay, Jr. |
Director | |
| /S/ C. MCCOLLISTER EVARTS, M.D. C. McCollister Evarts, M.D. |
Director | |
| /S/ DONALD E. MOREL, JR., PH.D. Donald E. Morel, Jr., Ph.D. |
Director | |
101
