Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AUXILIUM PHARMACEUTICALS INC | d8k.htm |
| EX-99.2 - PRESS RELEASE - AUXILIUM PHARMACEUTICALS INC | dex992.htm |
Exhibit 99.1

Auxilium Pharmaceuticals Announces Additional Data from Three-Year XIAFLEX® Recurrence Study in Dupuytren’s Contracture in E-poster at ASSH Meeting
– 71% of Patients Did Not Recur and Maintained Low Degrees of Contracture at Three Years
– Recurrent Patients Took Approximately Three Years to Return to Baseline Contracture Levels
MALVERN, PA, (September 8, 2011)- Auxilium Pharmaceuticals, Inc. (NASDAQ: AUXL), a specialty biopharmaceutical company, today announced additional three year recurrence data from the Collagenase Optimal Reduction of Dupuytren’s—Long-term Evaluation of Success Study (CORDLESS) for XIAFLEX® (collagenase clostridium histolyticum) in the treatment of adult Dupuytren’s contracture patients with a palpable cord. The information is available via e-poster at the American Society for Surgery of the Hand meeting in Las Vegas. In patients who achieved clinical success following XIAFLEX treatment and did not meet the criteria for recurrence through three years, the mean degree of contracture of successfully treated joints remained at approximately the same levels as seen at correction (~0 to 5 degrees). In patients who achieved clinical success following XIAFLEX treatment and had recurrence at three years, the mean degree of contracture of the recurrent joints had yet to return to pre-treatment levels.
“These data demonstrate the maintenance of success for the 71% of patients who did not recur over three years and a slow return to baseline degree of contracture in recurrent patients following XIAFLEX treatment,” said Dr. Philip Blazar, Assistant Professor of Orthopaedic Surgery, Harvard Medical School. “I believe treatment of Dupuytren’s patients with XIAFLEX can provide durable outcomes with a low rate of recurrence in the majority of patients.”
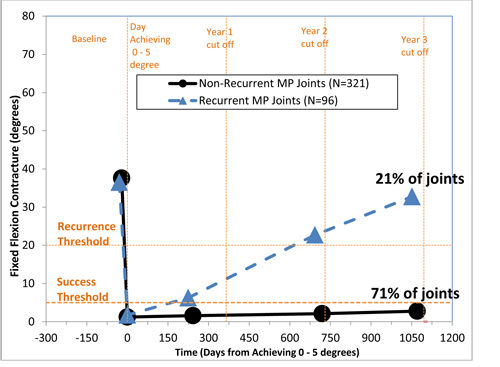
The CORDLESS study is one of the largest prospective long-term follow-up studies of Dupuytren’s contracture patients following an intervention. At three years, 451 patients with metacarpophalangeal (MP) joints that achieved clinical success (less than or equal to 5 degrees) were evaluable. In the 71% of patients with MP joints that achieved clinical success and did not recur through three years, the mean degree of contracture was 37.6 degrees at pre-treatment baseline and 2.8 degrees at three years. In the 21% of MP joints that achieved clinical success and did recur through three years, the mean degree of contracture was 40.1 degrees at pre-treatment baseline and 36.7 degrees at three years. Eight percent of patients with MP joints received medical or surgical intervention and were therefore not included in this analysis. Similar trends were seen through three years with evaluable patients with Proximal Interphalangeal (PIP) joints that achieved clinical success.
These data will be available starting September 8 via an e-poster from Dr. Thomas Kaplan et al. that is entitled “Year 3 recurrence data for the use of CCH in the treatment of Dupuytren’s Contracture with a palpable cord”. E-posters will be available during the entire ASSH meeting via the kiosks in the meeting hall or online at http://tinyurl.com/AM11eposters. A complete analysis of the three-year recurrence data is pending and the Company is seeking publication of full results in an appropriate medical journal.
About Dupuytren’s contracture
Dupuytren’s contracture is a chronic condition that affects the connective tissue that lies beneath the skin in the palm. The disease is progressive in nature. Typically, skin pits then nodules develop in the palm as collagen deposits accumulate. As the disease progresses, the collagen deposits form a cord that stretches from the palm of the hand to the base of the finger. Once this cord develops, the patient’s fingers contract and the function of the hand is impaired. The incidence of Dupuytren’s disease, inclusive of pits, nodules and cords, is highest in Caucasians, historically those of Northern European descent, with a global prevalence of three to six percent of the Caucasian population1. Most cases of Dupuytren’s contracture occur in patients older than 50 years5.
The most frequently affected parts of the hand associated with Dupuytren’s contracture are the joints called the Metacarpophalangeal Joint, or MP joint, which is the joint closest to the palm of the hand and the Proximal Interphalangeal Joint, or the PIP joint, which is the middle joint in the finger. The little finger and ring finger are most frequently involved. XIAFLEX is the only drug approved by the U.S. Food and Drug Administration for treatment of Dupuytren’s contracture, which has historically been treated primarily by an open surgical procedure.
About the CORDLESS trial
The CORDLESS study is the largest prospective long term follow-up study of Dupuytren’s contracture patients following a corrective procedure. This five-year observational study was designed to assess the durability of response following treatment with XIAFLEX, as well as long-term safety and progression of disease in patients from earlier Auxilium studies. In order to qualify for the XIAFLEX long-term extension study, patients must have participated in the phase III XIAFLEX clinical trials (CORD I, CORD II, JOINT I, JOINT II, or pharmacokinetic study) and received at least one injection of XIAFLEX. Recurrence in the long-term extension study was standardized and prospectively defined as (a) a joint contracture that was successfully treated (had previously achieved a reduction in contracture to five degrees or less at the Day 30 evaluation after the last injection of XIAFLEX) and that subsequently increases by at least 20 degrees compared to the reference value with a palpable cord present or, (b) a joint which underwent medical or surgical correction to treat contracture in that joint.
At three years, the nominal recurrence rate for the 623 joints previously treated successfully with XIAFLEX was 34.8%. The study also tracks whether a joint successfully treated with XIAFLEX received any further medical intervention. Through year three of follow-up 93.1% of joints that were successfully treated with XIAFLEX did not receive any medical or surgical intervention. Of the 43 (6.9 %) successfully treated joints that received medical or surgical intervention through three years, 30 had surgery, seven received needle aponeurotomy (of which 2 subsequently received a third intervention), and six received XIAFLEX. XIAFLEX has been commercially available in the U.S. only since March 2010 with some interventions occurring in advance of its availability.
Through three years of follow-up, the adverse event profile of XIAFLEX treated joints demonstrated a local adverse event profile similar to previous first-line clinical studies and revealed no new long-term adverse events. One retreated patient reported a serious adverse event of a motor vehicle accident, which
was considered unrelated to drug. Of the 74 serious adverse events reported through three years of follow-up, none were considered related to XIAFLEX and none occurred in the treated finger.
Recurrence with XIAFLEX as defined by ³30 degrees standard used in surgical literature
Although there is no standard methodology to measure recurrence, some literature3,4 on surgical treatment does reference a definition of recurrence as a 30 degree worsening of contracture following an intervention. XIAFLEX recurrence was defined in the AUX-CC-860 clinical study as a 20 degree change in the presence of a palpable cord. In order to evaluate within the context of the existing surgical literature, the three year XIAFLEX data was examined using a criterion referenced in the literature. Specifically, for this post hoc analysis, the definition of recurrence was (a) a joint contracture that was successfully treated (had previously achieved a reduction in contracture to five degrees or less at the Day 30 evaluation after the last injection of XIAFLEX) that subsequently increased by at least 30 degrees compared with the reference value or, (b) a joint which underwent correction to treat contracture in that joint. By this definition of recurrence, only 22% of patients who had achieved clinical success had experienced recurrence at three years. The recurrence rate for MP joints was 16% and for PIP joints was 39%.
About Surgical Recurrence in Dupuytren’s contracture
The surgical literature has variable definitions as to what constitutes recurrence. Depending upon the publication, the type of surgery performed (fasciectomy, fasciotomy, or needle aponeurotomy) and the definition of recurrence, surgical recurrence rates of up to 34% have been reported within the first two years following fasciectomy6 and, at two years, rates range from 2% to 60% or more7. Three year surgical recurrence rates can vary from 10% to 65%2,3,4,8-15, depending upon definition of recurrence. When using a recurrence definition of 30 degrees of worsening, the recurrence rate for fasciectomy was 24% with a mean time to recurrence of 3.7 years4 and needle aponeurotomy demonstrated a recurrence rate of 65% at 32 months3,4. When using a recurrence definition of re-operative rate, fasciectomy was 10-15% at approximately 3 years10,14 and needle aponeurotomy was 19-42% at approximately 3 years2,3,15,16,17. Higher recurrence rates are seen with surgery in Proximal Interphalangeal (PIP) joints vs. Metacarpophalangeal (MP) joints7 and with needle aponeurotomy vs. fasciectomy2,3. Generally, the higher the initial stage of contracture before surgery, the more severe the recurrences18. The earliest reports of recurrence for fasciectomy were seen in 22% of female and 19% of male Dupuytren’s contracture patients at a mean of 12 months following fasciectomy19.
| (1) | Hurst, L. C. et al., Injectable Collagenase Clostridium Histolyticum for Dupuytren’s Contracture, New England Journal of Medicine, (2009; 361:968-979) |
| (2) | Foucher, Tech in Hand and Upper Ext Surgery 2001;5(3):161-164 |
| (3) | A.L. Van Rijssen, Journal of Hand Surgery (British and European Volume, 2006) 31B: 5: 498–501 |
| (4) | A.L. Van Rijssen, 2010 International Symposium on Dupuytren’s Disease |
| (5) | Badalamente, M. A., Hurst, L. C. et al., The Journal of Hand Surgery, (2002; 27A:788-798) |
| (6) | Leclercq C. Epidemiology. In: Tubiana R, Leclercq C, Hurst LC, Badalamente MA, Mackin EJ, eds, Dupuytren’s disease. Martin Dunitz Ltd, London, 2000; 239-249. |
| (7) | Rayan GM. J Bone Joint Surg Am. 2007; 89A(1):190-198. |
| (8) | Ullah, The Journal of Bone and Joint Surgery, vol. 91-B, No. 3, pgs. 374-378, 2009 |
| (9) | Citron, Journal of Hand Surgery, vol. 30B No. 6, pgs. 563-66, 2005 |
| (10) | Adam, Journal of Hand Surgery 1992;17A:312-7 |
| (11) | Tonkin MA, J Hand Surg [Br] 1985; 10:351-2. |
| (12) | Bulstrode, The Journal of Hand Surgery / Vol. 30A No. 5, pgs. 1021-1025, 2005 |
| (13) | Foucher, International Orthopaedics (SICOT) (1995) 19:285-288 |
| (14) | Skoff, Plastic and Reconstructive Surgery, Vol. 113, No. 2, pg. 540-544, 2004 |
| (15) | Foucher, et al. Journal of Hand Surgery (British and European Volume) 28B: 5: 427–431, 2003 |
| (16) | Rahr J et al. Hand Surg Eur 2011;May 19. [Epub ahead of print]PMID: 21593073 |
| (17) | Chen N et al. Hand, 2011; March 1. DOI:10.1007/s11552-011-9326-8 |
| (18) | Vigroux et al, Annals Hand Surgery, 1992, Vol. 11, No. 5; 367-374 |
| (19) | Anwar et al., Journal of Hand Surgery, Vol. 32A No. 9 November 2007 ; 1423-1428 |
About Auxilium
Auxilium Pharmaceuticals, Inc. is a specialty biopharmaceutical company with a focus on developing and marketing products to predominantly specialist audiences, such as urologists, endocrinologists, certain targeted primary care physicians, hand surgeons, subsets of orthopedic, general, and plastic surgeons who focus on the hand, and rheumatologists. Auxilium markets XIAFLEX® (collagenase clostridium histolyticum) for the treatment of adult Dupuytren’s contracture patients with a palpable cord and Testim® 1%, a testosterone gel, for the topical treatment of hypogonadism in the U.S. Pfizer has marketing rights for XIAPEX® (the EU trade name for collagenase clostridium histolyticum) in Europe and Asahi Kasei Pharma Corporation has development and commercial rights for XIAFLEX in Japan. Ferring International Center S.A. markets Testim in the EU and Paladin Labs Inc. markets Testim in Canada. Auxilium has three projects in clinical development. XIAFLEX is in phase III of development for the treatment of Peyronie’s disease, in phase IIa of development for the treatment of Frozen Shoulder syndrome (Adhesive Capsulitis) and is in phase Ib of development for the treatment of cellulite (edematous fibrosclerotic panniculopathy). Auxilium also has rights to pursue additional indications for XIAFLEX. For additional information, visit http://www.auxilium.com.
SAFE HARBOR STATEMENT UNDER THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995
This release contains “forward-looking-statements” within the meaning of The Private Securities Litigation Reform Act of 1995, including statements regarding the durability of XIAFLEX treatment outcomes; future rates of recurrence; the Company’s publication of the complete data in an appropriate medical journal; the number of patients with Dupuytren’s disease; and products in development for Peyronie’s disease, Frozen Shoulder syndrome and cellulite; and all other statements containing projections, statements of future performance or expectations, our beliefs or statements of plans or objectives for future operations (including statements of assumption underlying or relating to any of the foregoing). Forward-looking statements can generally be identified by words such as “believe,” “appears,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and other words and terms of similar meaning in connection with any discussion of projections, future performance or expectations, beliefs, plans or objectives for future operations (including statements of assumption underlying or relating to any of the foregoing). Actual results may differ materially from those reflected in these forward-looking statements due to various factors, including further evaluation of clinical data, results of clinical trials, decisions by regulatory authorities as to whether and when to approve drug applications, and general financial, economic, regulatory and political conditions affecting the biotechnology and pharmaceutical industries and those discussed in Auxilium’s Annual Report under the heading “Risk Factors” on Form 10-K for the year ended December 31, 2010 and Form 10-Q for the quarter ended June 30, 2011, which are on file with the Securities and Exchange Commission (the “SEC”) and may be accessed electronically by means of the SEC’s home page on the Internet at http://www.sec.gov or by means of Auxilium’s home page on the Internet at http://www.Auxilium.com under the heading “For Investors — SEC Filings.” There may be additional risks that Auxilium does not presently know or that Auxilium currently believes are immaterial which could also cause actual results to differ from those contained in the forward-looking statements. Given these risks and uncertainties, any or all of these forward-looking statements may prove to be incorrect. Therefore, you should not rely on any such factors or forward-looking statements.
In addition, forward-looking statements provide Auxilium’s expectations, plans or forecasts of future events and views as of the date of this release. Auxilium anticipates that subsequent events and developments will cause Auxilium’s assessments to change. However, while Auxilium may elect to update these forward-looking statements at some point in the future, Auxilium specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Auxilium’s assessments as of any date subsequent to the date of this release.
CONTACT:
James E. Fickenscher
Chief Financial Officer, Auxilium Pharmaceuticals, Inc.
+1-484-321-5900
jfickenscher@auxilium.com
or
William Q. Sargent Jr.
Vice-President, Investor Relations and Corporate Communications
+1-484-321-5900
wsargent@auxilium.com
