Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | d8k.htm |
| EX-99.1 - PRESS RELEASE - Ardea Biosciences, Inc./DE | dex991.htm |
 Long-term
Allopurinol
Safety
Trial
(LASSO)
–
open-label
interventional
studies
of
allopurinol
in gout patients otherwise eligible for the following Phase 3 studies:
Combination Study of Lesinurad in Allopurinol Standard of Care Inadequate Responders
(CLEAR #1)
–
randomized,
placebo-controlled
trial
of
lesinurad
added
to
allopurinol in
patients not reaching sUA target with allopurinol alone; North America
Combination Study of Lesinurad in Allopurinol Standard of Care Inadequate Responders
(CLEAR #2)
–
randomized,
placebo-controlled
trial
of
lesinurad
added
to
allopurinol in
patients not reaching sUA target with allopurinol alone; Global
Lesinurad Monotherapy in Gout Subjects Intolerant to Xanthine Oxidase Inhibitors (LIGHT) – randomized, placebo-controlled trial of
lesinurad monotherapy in patients where febuxostat and/or allopurinol
are
contraindicated
(due
to
intolerance,
drug
interactions,
co-morbidities,
etc); Global
Combination Treatment Study in Subjects with Tophaceous Gout Using Lesinurad and
Febuxostat (CRYSTAL)
–
randomized,
placebo-controlled
trial
of
lesinurad
in
combination
with febuxostat for the treatment of hyperuricemia in gout patients with tophi (resolution of
tophi is a key secondary endpoint); Global
Total patients planned for Phase
3
studies:
2000
–
2500
1
Exhibit 99.2 |
 Long-term
Allopurinol
Safety
Study
Evaluating
Outcomes
in
Gout
Patients
(LASSO
-
N.
America
&
Global)
Population:
–
Up
to
2500
pts
with
hyperuricemia
(sUA
>
8
mg/dL)
at
~300
centers
Design:
–
Provide therapy with allopurinol at the medically appropriate doses; monitor
patients for a minimum of 2 months prior to their being eligible for
enrollment into Phase 3 program (~3 months or greater on allopurinol prior
to Phase 3 baseline) Objectives:
–
Generate patients for the Phase 3 program with well-documented inadequate
efficacy or intolerance
to
allopurinol
(ultimately
will
speed
up
enrollment
of
Phase 3
program)
2
Allopurinol prescribed at
labeled dose
Phase 3 -
Allopurinol SoC Inadequate Responders NA and ROW
Phase 3 -
Allopurinol SoC Inadequate Responders North America
Phase
3
–
Febuxostat
and
Allopurinol
Intolerant
Monotherapy |
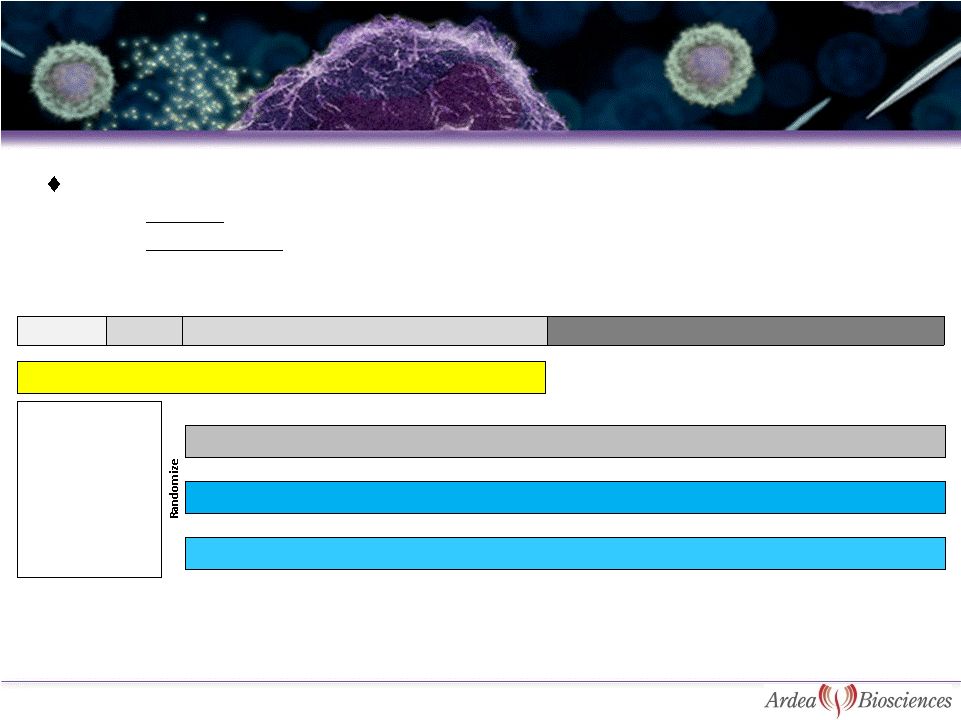 Combination Study
of
LEsinurad
in
Allopurinol
Standard
of
Care
Inadequate
Responders
(CLEAR
-
N.
America)
Endpoints:
–
Primary: proportion of subjects with sUA level < 6.0 mg/dL after 6
months –
Key Secondary: Assessment of gout flare rate, tophi resolution and quality
of life measurements. Screening
Period
Gout flare prophylaxis
3
RDEA594
200 mg + ALLO
RDEA594
Placebo + ALLO
3 weeks
1 week
Month 1 to Month 6 primary endpoint
Month 12 secondary endpoints
RDEA594
400 mg + ALLO |
 Combination Study of LEsinurad in Allopurinol Standard of
Care
Inadequate
Responders
(CLEAR
-
Global)
Endpoints:
–
Primary:
proportion
of
subjects
with
sUA
level
<
6.0
mg/dL
after
6
months
–
Key
Secondary:
Assessment
of
gout
flare
rate,
tophi
resolution
and
quality
of
life
measurements.
Screening
Period
Gout flare prophylaxis
4
RDEA594
200 mg + ALLO
RDEA594
Placebo + ALLO
3 weeks
1 week
Month 1 to Month 6 primary endpoint
Month 12 secondary endpoints
RDEA594
400 mg + ALLO |
 LesInurad Monotherapy
in
Gout
Subjects
Intolerant
to
Xanthine
Oxidase
Inhibitors
(LIGHT
-
Global)
Endpoints:
–
Primary: Proportion of patients whose sUA levels are < 6.0 mg/dL after 6 months
5
Screening
Period
Gout flare prophylaxis
RDEA594
400 mg
RDEA594
Placebo
3 weeks
Month 1 to Month 6 primary endpoint
Placebo Patients Switched to Active at 6 months
RDEA594
400 mg |
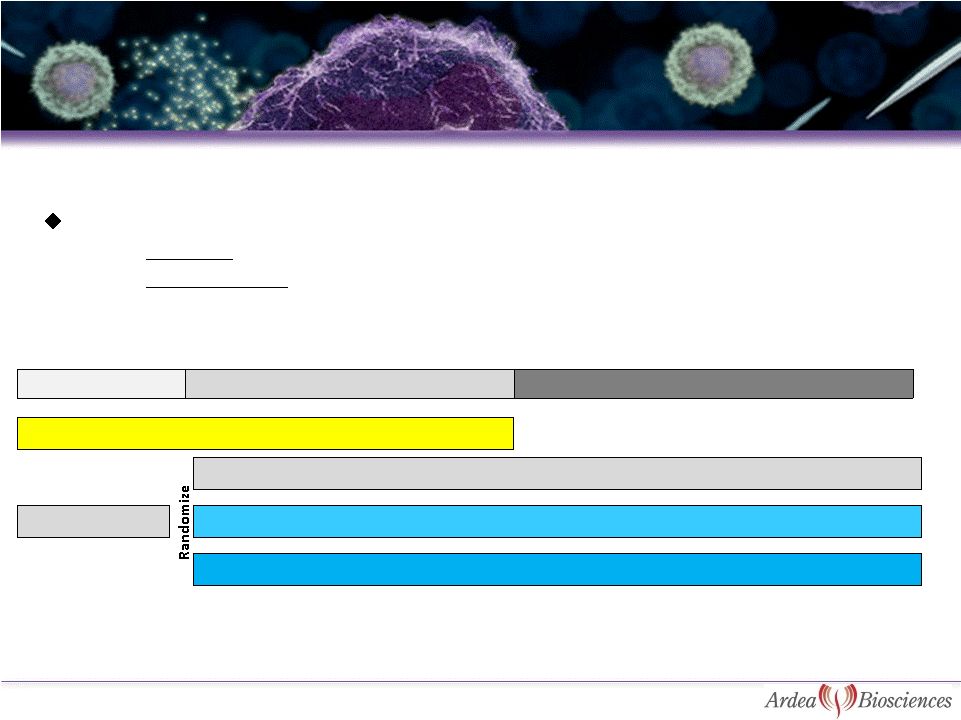 Combination TReatment StudY in
Subjects
with
TophAeous
Gout
Using
LesInurad
and
Febuxostat
(CRYSTAL
-
Global)
Endpoints:
–
Primary –
proportion of subjects with sUA level < 5.0 mg/dL after 6 months
–
Key Secondary -
proportion of subjects with resolution of tophi at 6, 9 and 12 mos, gout flare
rate and quality of life measurements
RDEA594 200 mg +FBX 80 mg
Gout flare prophylaxis
6
RDEA594
Placebo +FBX 80 mg
RDEA594 400 mg +FBX 80 mg
FBX 80 mg
3 weeks
Month 1 to Month 6 primary endpoint
Month 12 secondary endpoints |
