Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Unilife Corp | c21975exv99w1.htm |
| EX-99.3 - EXHIBIT 99.3 - Unilife Corp | c21975exv99w3.htm |
| 8-K - FORM 8-K - Unilife Corp | c21975e8vk.htm |
Exhibit 99.2

| Fourth Quarter and Fiscal Year 2011 Earnings Conference Call August 30, 2011 |

| 2 Cautionary Note Regarding Forward-Looking Statements This presentation contains forward looking statements under the safe harbor provisions of the US securities laws. These forward- looking statements are based on management's beliefs and assumptions and on information currently available to our management. Our management believes that these forward- looking statements are reasonable as and when made. However you should not place undue reliance on any such forward looking statements as these are subject to risks and uncertainties. Please refer to our press release and our SEC filings for more information regarding the use of forward looking statements." |

| 3 Unilife's Opportunity A paradigm shift in the drug delivery devices marketMore than a thousand pipeline biologics under developmentCustomized devices needed to enhance / enable drug deliveryShift to patient self-injection of drugs outside of healthcare facilitiesIncreased therapeutic competition between brand-name, biosimilar and generics driving adoption of innovative, differentiated devices31 biologics approaching patent cliff seeking lifecycle extensionCompliance with needlestick safety laws in U.S. and Europe Incumbent manufacturers tethered to old business models |

| 4 Key Pillars of our Business Strategy 1. Diversified portfolio of advanced drug delivery systemsAddressing unmet needs of injectable drugs and vaccinesInnovative, differentiated devices (not 'me too' products)Devices can be customized to specific needs of target drugLead with Unifill, then expand into other high-value sectors:Patient Self-Injection Systems Drug Reconstitution SystemsTargeted Organ Drug Delivery Systems |
| 5 5 Key Pillars of our Business Strategy 2. Serve as Full Development and Commercial Supply PartnerPartnerships span early-stage drug pipeline to lifecycle extensionHigh-performance team with the expertise to fully serve partners Entrepreneurial structure with operational capacity to serve customers with unparalleled speed, agility and responsivenessState-of-the-art facilities in York, PA serving as integrated center for device innovation, production and quality assuranceDesign, rapid prototyping, pilot and commercial production, bio- analytical testing, quality control, supply chain, clinical supportBusiness systems including SAP and electronic QMS in place |

| Strategy Goals 6 Improve patient care and therapy complianceProtect healthcare staff, reduce healthcare costs, prevent diseaseBecome intertwined with $200 billion market for injectable drugsEnable and enhance clinical development of pipeline biologicsGenerate strong brand differentiation for drugs in competitive classesOptimize and extend drug lifecycles to build market share and revenuesLong-term commercial supply contracts for high-value devicesDevelopment funding by partners to develop customized device solutionsSolid recurring revenue streams with high margins over drug lifecycleOther potential revenues including exclusivity fees and royalties |

| 7 Richard WielandChief Financial Officer Financial Data |
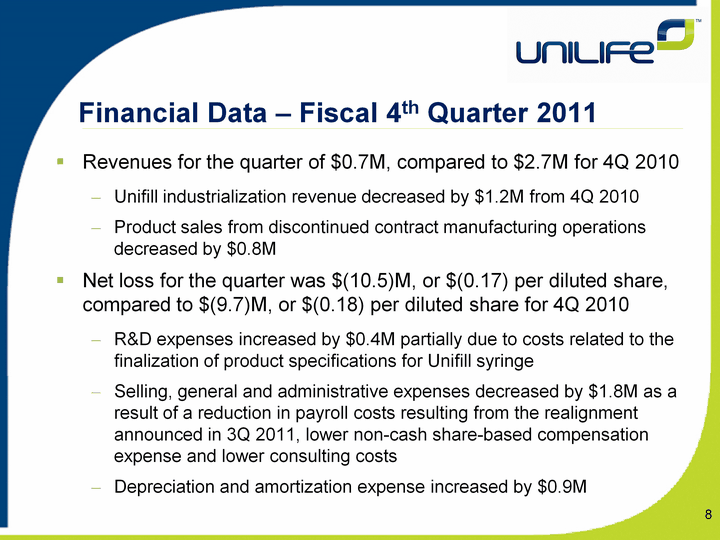
| 8 Financial Data - Fiscal 4th Quarter 2011 Revenues for the quarter of $0.7M, compared to $2.7M for 4Q 2010Unifill industrialization revenue decreased by $1.2M from 4Q 2010Product sales from discontinued contract manufacturing operations decreased by $0.8MNet loss for the quarter was $(10.5)M, or $(0.17) per diluted share, compared to $(9.7)M, or $(0.18) per diluted share for 4Q 2010R&D expenses increased by $0.4M partially due to costs related to the finalization of product specifications for Unifill syringe Selling, general and administrative expenses decreased by $1.8M as a result of a reduction in payroll costs resulting from the realignment announced in 3Q 2011, lower non-cash share-based compensation expense and lower consulting costsDepreciation and amortization expense increased by $0.9M |
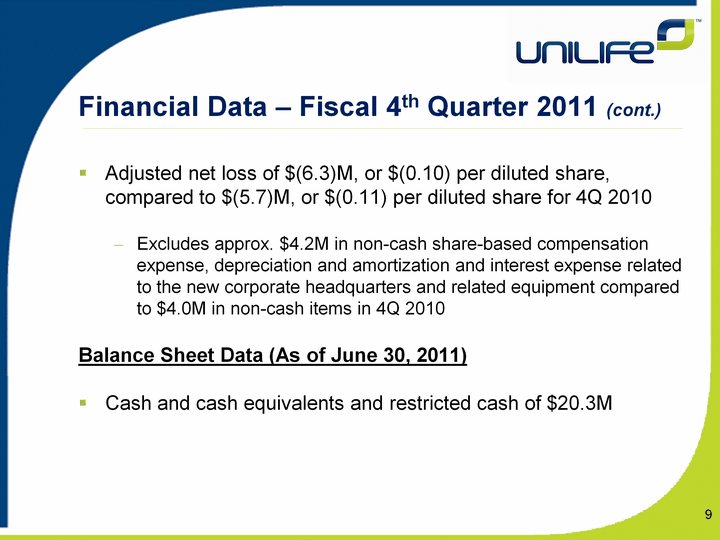
| Financial Data - Fiscal 4th Quarter 2011 (cont.) Adjusted net loss of $(6.3)M, or $(0.10) per diluted share, compared to $(5.7)M, or $(0.11) per diluted share for 4Q 2010Excludes approx. $4.2M in non-cash share-based compensation expense, depreciation and amortization and interest expense related to the new corporate headquarters and related equipment compared to $4.0M in non-cash items in 4Q 2010Balance Sheet Data (As of June 30, 2011)Cash and cash equivalents and restricted cash of $20.3M 9 |

| Financial Data - Fiscal Year 2011 Revenues of $6.7M, compared to $11.4M for FY 2010Unifill industrialization revenue decreased by $5.0m from FY 2010Net loss for the year was $(40.7)M, or $(0.70) per diluted share, compared to $(29.7)M, or $(0.64) per diluted share, for FY 2010R&D expenses decreased by $1.3M due to decreased non-cash share-based compensation costs relating to stock awards granted in the prior periodSelling, general and administrative expenses increased by $5.3M due to higher payroll and non-cash share-based compensation expense, partially offset by a decrease in legal and consulting fees due to significant costs incurred in the prior period in connection with the redomiciliation to the U.S. Depreciation and amortization expense increased by $1.7M 10 |
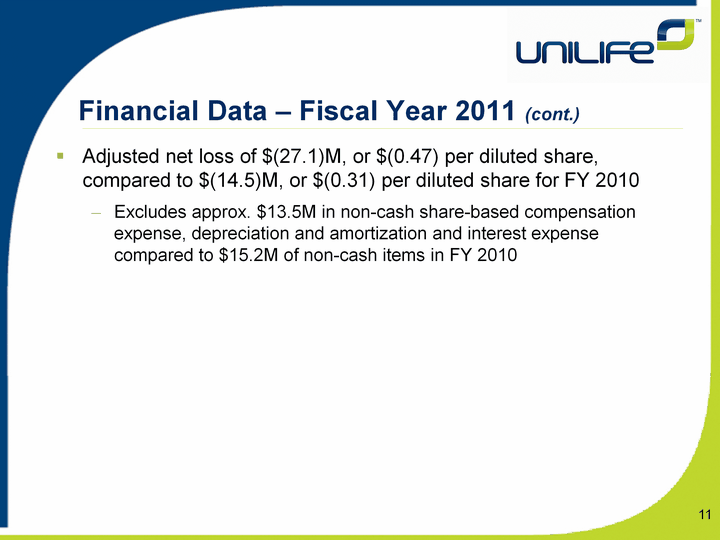
| Financial Data - Fiscal Year 2011 (cont.) Adjusted net loss of $(27.1)M, or $(0.47) per diluted share, compared to $(14.5)M, or $(0.31) per diluted share for FY 2010Excludes approx. $13.5M in non-cash share-based compensation expense, depreciation and amortization and interest expense compared to $15.2M of non-cash items in FY 2010 11 |
| Operational Realignment 12 In the fiscal 3rd quarter, we initiated a business realignment planImproved cash flow Extended cash reserves into calendar year 2012Enables Unilife to efficiently focus cash resources in key areas:Production and sale of the Unifill(r) prefilled syringe, The sale of the Unitract(r) 1mL syringe The commercialization of other pipeline products. |
| Equipment Financing Agreement Finalized previously disclosed Equipment Financing Agreement with Varilease Finance, Inc. for $10M in available fundingReplenishes cash Unilife has previously invested in the automated assembly line now manufacturing the Unifill(r) ready- to-fill (prefilled) syringe at its manufacturing facility in York, PAFinancing closed in Aug. 2011 (Benefits to be reflected in cash as of Sept. 30, 2011)Accelerates expansion of device portfolio 13 |

| 14 Fiscal 2011 Overview and Future Outlook Alan ShortallChief Executive Officer |

| 15 Operational Highlights Completed on-schedule construction of global headquarters and commercial production facility and relocated all staffRealigned business operations to support new initiatives and progression into a new phase of commercial expansionStrengthened balance sheet with $10 million equipment financing agreement through Varilease Finance, Inc.Expanded management team to support sales growth, and the development and commercialization of pipeline products |

| Expanded Management Team 16 Name Title Previous Employment Dr. Ramin Mojdeh COO BD (Head of Pharma Systems & Product Development) Rich Wieland CFO Cytochroma, Advanced Life Sci. Dr. Jack Kelley VP, Strategic Marketing BD, Medtronic Mike Ratigan VP, Commercial Development BD, Stryker Ian Hanson Director, Advanced Delivery Medtronic Diabetes Mark Hassett VP, Sales Safety Syringes Inc Dennis Pyers Financial Controller KPMG Chris Naftzger General Counsel Chesapeake Corporation Dr Masoud Samandi Unifill Senior Director BD Advanced Drug Delivery System Managers: Dr. Ashley Palmer, Dr. Jyoti Gupta, Dr. Gautam Shetty, and Dr. Molly Miller Advanced Drug Delivery System Managers: Dr. Ashley Palmer, Dr. Jyoti Gupta, Dr. Gautam Shetty, and Dr. Molly Miller Advanced Drug Delivery System Managers: Dr. Ashley Palmer, Dr. Jyoti Gupta, Dr. Gautam Shetty, and Dr. Molly Miller |

| 17 Recent Highlights:Commenced initial production in March 2011Industrialization completed in June 2011Initial supply to Sanofi in July 2011 Initiated sales to another undisclosed top-tier pharma company in July 2011Next Steps:Customers will enter drugs into stability studies with the Unifill syringeAccelerating discussions with multiple other companies - advancing quickly The Unifill(r) Syringe - Path to Market |
| 18 Transition of automated assembly line into York, PA facility Initial U.S. rollout to IMCO distributor network Now supplying multiple IMCO members across U.S.Strong traction in long-term care and correctional health facilitiesNew and repeat orders acceleratingSales to other distributors and U.S. facilities now acceleratingAdvancing discussions with Group Purchasing Organizations Unitract(r) 1mL syringes |
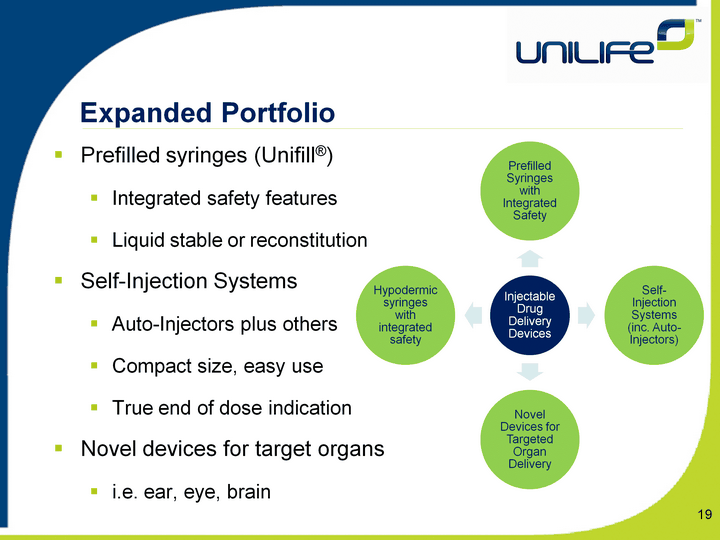
| Expanded Portfolio 19 Prefilled syringes (Unifill(r))Integrated safety featuresLiquid stable or reconstitutionSelf-Injection SystemsAuto-Injectors plus othersCompact size, easy useTrue end of dose indicationNovel devices for target organsi.e. ear, eye, brain |

| Unifill EZMix Multi-Chamber Prefilled Syringe 1/3rd all drugs FDA approved last three years are reconstitutedOnly prefilled safety syringe for reconstitution lyophilized drugsDeveloped in direct response to unmet market needsTwo or more primary drug containers within single glass barrelAvailable in either fixed needle for subcutaneous injections or with attachable needles of up to 1.5 inches in length Offers minimal steps of use by healthcare workers or patientsCommercialization strategy for EZMix underwayReceived strong interest in device since announcement 20 |

| Unifill Auto Injectors Self-injection device market $1.2 B in 2015 (14.5% CAGR)Unifill syringe serves as 'engine' for range of auto-injectorsDisposable and reusable variantsHighly compact size for portability and easy handlingAudible tactile click makes it only known auto-injector technology with a true end-of-dose indicatorDiscussions under way with multiple interested partiesAuto-injector platform further expands direct sales opportunities for Unifill syringe 21 |

| Milestones for FY 2012 Unifill SyringeContinued sales to current / additional pharmaceutical companies Initial sales for stability and compatibility tests with target drugsSupply contracts expected to commence during fiscal year Number of short and medium term revenue generating options including access feesAgreements to commercialize additional Unilife devicesSome devices expected to enter clinical trials with target drugsFunding from partners for development and industrialization programs, plus other revenue streams including exclusivity 22 |
| Summary Operational capabilities in place to serve customers with speed and reliability when developing innovative drug delivery systemsDiscussions accelerating with multiple customers for multiple Unilife devices across multiple therapeutic classesEmerging as global leader for innovative drug delivery systemsTargeting high-value injectable drug delivery device sectorsDeveloped diversified product portfolio in direct response to unmet needs, with all technologies designed for customizationAttractive model building to long-term, high margin sales 23 |

| 24 Questions |
