Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - DELCATH SYSTEMS, INC. | form8-k.htm |
Investor Presentation
(NASDAQ: DCTH)
(NASDAQ: DCTH)
August 2011

2 DELCATH SYSTEMS, INC
Forward-looking Statements
This presentation contains forward-looking statements, within the meaning of federal securities laws,
related to future events and future financial performance which include statements about our
expectations, beliefs, plans, objectives, intentions, goals, strategies, assumptions and other statements
that are not historical facts. Forward-looking statements are subject to known and unknown risks and
uncertainties and are based on potentially inaccurate assumptions, which could cause actual results to
differ materially from expected results, performance or achievements expressed or implied by
statements made herein. Our actual results could differ materially from those anticipated in forward-
looking statements for many reasons, including; uncertainties relating to the time required to build
inventory and establish commercial operations in Europe, adoption, use and resulting sales, if any, for
the chemosaturation delivery system in the EEA, our ability to successfully commercialize the
chemosaturation system and the potential of the system as a treatment for patients with cancer in the
liver, availability of melphalan in the EEA, acceptability of the Phase III clinical trial data by the FDA, our
ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of
our re-submission of our NDA, re-submission and acceptance of the Company’s NDA by the FDA,
approval of the Company’s NDA for the treatment of metastatic melanoma to the liver, adoption, use and
resulting sales, if any, in the United States, approval of the current or future chemosaturation system for
other indications or the same indication in other foreign markets, actions by the FDA or other foreign
regulatory agencies, our ability to successfully enter into distribution and strategic partnership
agreements in foreign markets and the corresponding revenue associated with such foreign markets,
our ability to secure reimbursement for the chemosaturation system, progress of our research and
development programs and future clinical trials, uncertainties regarding our ability to obtain financial
and other resources for any research, development and commercialization activities, overall economic
conditions and other factors described in the section entitled ‘‘Risk Factors’’ in our most recent Annual
Report on Form 10-K and the Quarterly Reports on Form 10-Q that we file with the Securities and
Exchange Commission.
related to future events and future financial performance which include statements about our
expectations, beliefs, plans, objectives, intentions, goals, strategies, assumptions and other statements
that are not historical facts. Forward-looking statements are subject to known and unknown risks and
uncertainties and are based on potentially inaccurate assumptions, which could cause actual results to
differ materially from expected results, performance or achievements expressed or implied by
statements made herein. Our actual results could differ materially from those anticipated in forward-
looking statements for many reasons, including; uncertainties relating to the time required to build
inventory and establish commercial operations in Europe, adoption, use and resulting sales, if any, for
the chemosaturation delivery system in the EEA, our ability to successfully commercialize the
chemosaturation system and the potential of the system as a treatment for patients with cancer in the
liver, availability of melphalan in the EEA, acceptability of the Phase III clinical trial data by the FDA, our
ability to address the issues raised in the Refusal to File letter received from the FDA and the timing of
our re-submission of our NDA, re-submission and acceptance of the Company’s NDA by the FDA,
approval of the Company’s NDA for the treatment of metastatic melanoma to the liver, adoption, use and
resulting sales, if any, in the United States, approval of the current or future chemosaturation system for
other indications or the same indication in other foreign markets, actions by the FDA or other foreign
regulatory agencies, our ability to successfully enter into distribution and strategic partnership
agreements in foreign markets and the corresponding revenue associated with such foreign markets,
our ability to secure reimbursement for the chemosaturation system, progress of our research and
development programs and future clinical trials, uncertainties regarding our ability to obtain financial
and other resources for any research, development and commercialization activities, overall economic
conditions and other factors described in the section entitled ‘‘Risk Factors’’ in our most recent Annual
Report on Form 10-K and the Quarterly Reports on Form 10-Q that we file with the Securities and
Exchange Commission.

3 DELCATH SYSTEMS, INC
Company Highlights
• Making established chemotherapeutic drugs work better in target organs
• Initial focus is high dose chemotherapy for improved disease control in the liver
• Successful and highly statistically significant Phase III trial results reported
• Received CE Mark approval for Class III medical device on April 13, 2011
• Positioned to address potential $3.0 billion European labeled market opportunity
• Objective is to re-file 505(b)(2) NDA to FDA for orphan drug and delivery
apparatus by end of 2011
apparatus by end of 2011
• Potential $675 million US labeled market opportunity
• Issued patents and orphan drug designations create competitive barriers
• Deep and experienced management team
Concentrating the Power of Chemotherapy to Improve Disease Control in the Liver

4 DELCATH SYSTEMS, INC
Potential $3.75 Billion Labeled Market Opportunity*
Transparent Areas
Represent Potential
Additional Indications
Represent Potential
Additional Indications
13,505**
101,563
355,712
• CE Mark in EU for delivery of melphalan
to the liver permits physician use on a
broad range of liver cancers
to the liver permits physician use on a
broad range of liver cancers
• Potential $3 Billion Long term EU Market
Opportunity*
Opportunity*
• Leverage CE Mark to gain regulatory
approvals in Asia, America’s (EX US),
MEA, and Australia
approvals in Asia, America’s (EX US),
MEA, and Australia
• Potential $8 Billion Asia/Australia Market
Opportunity*
Opportunity*
• Seeking initial indication for melanoma
mets in U.S., a potential $670 million **
market opportunity
mets in U.S., a potential $670 million **
market opportunity
• Significant potential label expansion
possible is U.S. with additional studies
possible is U.S. with additional studies

5 DELCATH SYSTEMS, INC
Spectrum of Liver Cancer Treatments
Existing Treatments Involve Significant Limitations
|
Type of Treatment
|
Advantages
|
Disadvantages
|
|
Systemic
|
o Non-invasive
o Repeatable
|
– Systemic toxicities
– Limited efficacy in liver
|
|
Regional (e.g., IHP)
|
o Therapeutic effect
o Targeted
|
– Invasive/limited repeatability
– Multiple treatments are
required |
|
Focal
|
o Isolated removal of tumor
|
– 90% unresectable
– Invasive and/or limited
repeatability |

6 DELCATH SYSTEMS, INC
Open Surgical IHP - Where It All Began
Isolated Hepatic Perfusion: Proof of Concept, but High Morbidity and Non-Repeatable
7 DELCATH SYSTEMS, INC
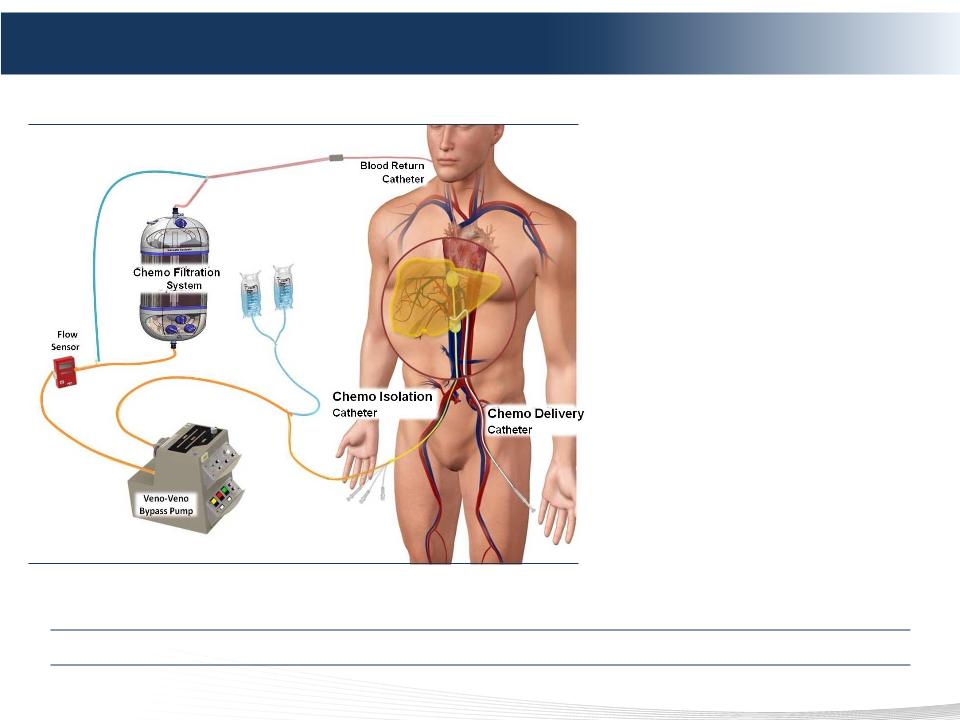
The Delcath Chemosaturation System
Three Steps of Chemosaturation
1) ISOLATION
2) SATURATION
3) FILTRATION
Advantages of Chemosaturation
• Improved disease control in the
liver
liver
• Treats entire liver
• Allows for ~ 100x effective dose
escalation of drug agents at
tumor site
escalation of drug agents at
tumor site
• Controls systemic toxicities
• Repeatable
• Complements systemic therapy
Minimally Invasive, Repeatable Liver Procedure That Could Complement Systemic Therapy
Note: Image not to scale.

8 DELCATH SYSTEMS, INC
Melphalan Dosing & Background
• Well understood, dose dependant, tumor preferential, alkylating cytotoxic agent that
demonstrates no hepatic toxicity
demonstrates no hepatic toxicity
• Manageable systemic toxicities associated with Neutropenia and Cytopenia
• Drug dosing over 10x higher than FDA-approved dose via systemic IV chemotherapy
• Dose delivered to tumor is approximately 100x higher than that of systemic IV
chemotherapy
chemotherapy
|
Type
|
Dosing (mg/kg)
|
|
Multiple Myeloma (label)
|
0.25
|
|
Chemoembolization
|
0.62
|
|
Surgical Isolated Hepatic Perfusion (IHP)
|
1.50
|
|
Myeloablation
|
2.50-3.50
|
|
Chemosaturation (PHP)
|
3.00
|
A Promising Drug For Liver Cancer Therapy

9 DELCATH SYSTEMS, INC
What Chemosaturation Offers
Patients:
o Significant improvement in disease control in the liver compared to
standard of care in patients with unresectable hepatic melanoma mets
standard of care in patients with unresectable hepatic melanoma mets
o Manageable systemic toxicities
o Time, so that primary cancers can continue to be treated
Physicians:
o Novel, targeted liver directed treatment to complement other
cancer therapies
cancer therapies
o Repeatable, percutaneous procedure
o Ability to treat the entire liver, including both visible and micro tumors
o Ability to continue treating patients for extra-hepatic disease
Attractive Clinical and Economic Proposition For Patient and Providers

10 DELCATH SYSTEMS, INC
Interventional
Radiologist
Patient
Primary
Care
Medical
Oncologist
Offers systemic therapy to
treat Cancer
treat Cancer
Surgical
Oncologist
Offers resection or other focal therapy
to treat cancer in Liver
to treat cancer in Liver
Transferred for
chemosaturation
chemosaturation
Diagnosis
of Cancer
of Cancer
Identification of liver
involvement
involvement
with no improvement from
systemic therapy
systemic therapy
When liver disease is
controlled, patients return to the
Medical Oncologist for
additional systemic therapy
controlled, patients return to the
Medical Oncologist for
additional systemic therapy

11DELCATH SYSTEMS, INC
Summary of Phase III Results
• Primary endpoint exceeded, p value = 0.001, hazard ratio of .301
o Treatment arm shows 5x median hepatic progression free (hPFS) survival compared to control
arm
arm
o CS/PHP median hPFS of 245 days compared to 49 days for BAC
o 86% overall clinical benefit (CR + PR + SD)
• Secondary endpoints support results
o OS Secondary endpoint - No difference in Kaplan-Meier curves due to cross over treatment
response (298 days compared to 301 days)
response (298 days compared to 301 days)
• OS cohort analysis favorable
o Median survival of 298 days for treatment arm compared to 124 in non-crossover BAC patients
o 14 treatment patients (6 treatment, 8 crossover) and 3 BAC patients still alive at 12/31/2010
• Safety profile - expected and consistent with currently approved labeling
for melphalan
for melphalan
o Treatment related Deaths: 3/40 patients (7.5%) 3/116 procedures (2.6%)
o Neutropenic Sepsis (n=2) 5%, Hepatic Failure (n=1) 2.5% (95% tumor burden)
Trial Outcomes Favorable and Consistent with Special Protocol Assessment

12 DELCATH SYSTEMS, INC
Phase I/II NCI Trials - Neuroendocrine
Pre-CS
(Baseline)
Post-CS #2
(+4 Months)
Post-CS #1
(+6 Weeks)
Promising Initial Response Rate in Attractive Market
|
Neuroendocrine Tumor Trial Results (n=23)*
|
|
|
|
Number (n)
|
|
Primary Tumor Histology
|
|
|
Carcinoid
|
3
|
|
Pancreatic Islet Cell
|
17
|
|
Response
|
|
|
Not Evaluable (Toxicity, Incomplete Treatment, Orthotopic Liver
Transplantation) |
4
|
|
Progressive Disease
|
1
|
|
Minor Response / Stable Disease
|
3
|
|
Partial Response (30.0% - 99.0% Tumor Reduction)
|
13
|
|
Complete Response (No Evidence of Disease)
|
2
|
|
Objective Tumor Response
|
15
|
|
Objective Tumor Response Rate
|
79%
|
|
|
Duration (months)
|
|
Median Hepatic PFS
|
39
|
|
Overall Survival After CS
|
40
|
*Presentation at American Hepato-Pancreo-Biliary Association 2008 annual meeting

13 DELCATH SYSTEMS, INC
High Efficiency (HE) Filter Media Development
STATUS:
o Melphalan - Achieved consistent in-vitro first pass removal
efficiency of 98% or better
efficiency of 98% or better
o Internal development project
o Developed trade secret manufacturing process to create new filter
medium
medium
EXPECTED BENEFITS:
o Reduced systemic toxicity for improved safety profile
o Concomitant Therapy (complements systemic therapies)
o Increased utility in a wider range of patients
HE Filter Expected to Significantly Enhance Procedure and Market Opportunity

14 DELCATH SYSTEMS, INC
Product Development Pipeline
• Melanoma liver mets
• Proprietary drug-melphalan &
apparatus
apparatus
• EAP - melphalan
• All liver cancers - melphalan
• Class III device
• 3rd party melphalan
• Leverage strong data in
melanoma and NET liver mets
melanoma and NET liver mets
• Additional drugs
• Other organs
• HCC and CRC liver mets global
clinical trials - melphalan
clinical trials - melphalan
• Apparatus improvements
Initial Opportunity
Near Term (< 5 years)
Intermediate Term (> 5 years)
• HCC and CRC liver mets global
clinical trials - melphalan
clinical trials - melphalan
• Drug-melphalan & apparatus
• HCC and CRC livers met global
clinical trials
clinical trials
• Apparatus improvements
• Additional drugs
• Other organs
• Additional drugs
• Other organs
Robust Development Program Planned
• Leverage CE Mark approval
• Leverage strong data in
melanoma and NET liver mets
melanoma and NET liver mets
• 3rd party melphalan

15 DELCATH SYSTEMS, INC
Clinical Data Development Plans
• Utilize High Efficiency (HE) Filter
o Concomitant therapy to complement standard of care treatments
o Increase safety (reduce systemic side effects) of procedure
o Small Pk study commencing in Q4 in Australia
• Enroll clinical trials in 2012 to expand data and enhance commercial
adoption
adoption
o Expanded Access Program (EAP) in U.S. for metastatic melanoma at 4-5 centers
o 2 L HCC: randomized, Global Phase 3 of Chemosaturation vs BSC for sorafenib
refractory patients (registration study for expanded labeling of melphalan)
refractory patients (registration study for expanded labeling of melphalan)
o 1L HCC: randomized, Global Phase 4 sorafenib vs Chemosaturation in first line setting
o Metastatic colorectal (mCRC): Phase 2, single arm for patients refractory to1L therapy
• Future possibilities may include: use in adjuvant setting in melanoma, combination
therapy with ipilimumab, combination with SOC in mCRC, combination with resection
therapy with ipilimumab, combination with SOC in mCRC, combination with resection
Goal Of Establishing Chemosaturation As Standard of Care For Disease Control In The Liver
16 DELCATH SYSTEMS, INC
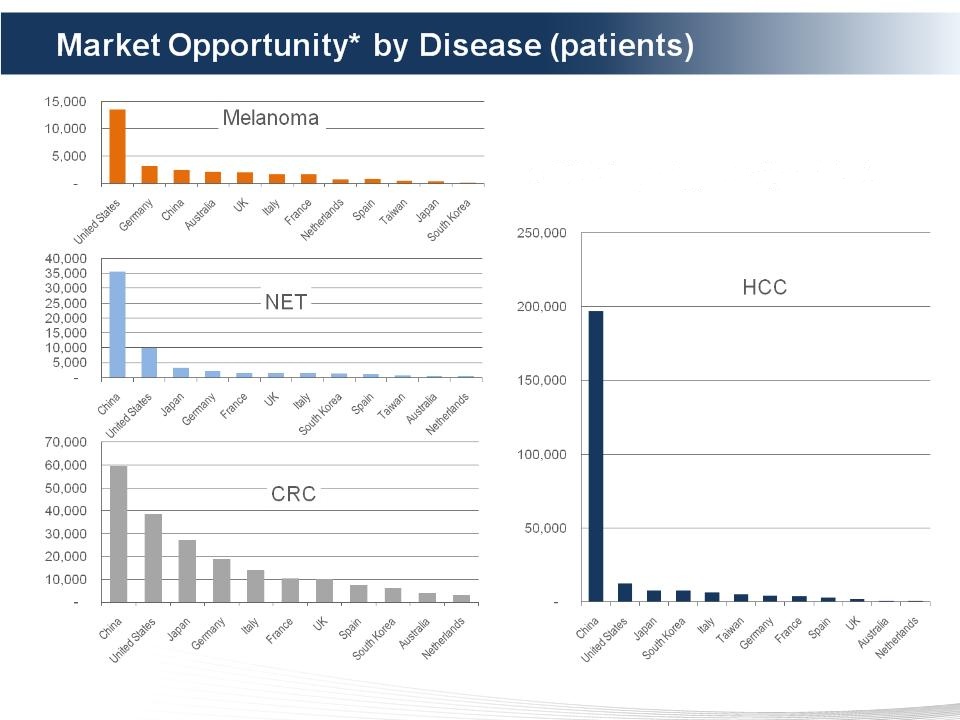
• US - largest opportunity for Melanoma
• China- largest opportunity for HCC
• CRC - largest opportunity worldwide
*TPM Total Potential Market

17 DELCATH SYSTEMS, INC
EEA Landscape
• CE Mark device approval covers 30 countries in the European
Economic Area (EEA)
Economic Area (EEA)
• Indication is for “intra-arterial delivery of chemotherapeutic agent
(melphalan hydrochloride) to the liver”
(melphalan hydrochloride) to the liver”
• Hospitals procure melphalan separately from existing sources
• Melphalan for injection approved in 14 countries, but
commercially available in remaining EEA countries
commercially available in remaining EEA countries
• Estimate potentially applicable to ~100,000 patients annually
• 6 top countries (DE, UK, FR, IT, SP, NL) represent ~70% of total
patient population
patient population
Large European Market Opportunity Concentrated in Six Countries

18 DELCATH SYSTEMS, INC
European Commercialization Plans
Objective: broad commercial adoption
Major Assumptions:
• HE filter available for full commercial launch ( Q3 2012)
• 6-8 Centers of Excellence for training
• Initiate test market in 2011 for 6 months to validate assumptions and finalize model
• Full commercialization in 2012
Tactics & Execution:
• Market to medical oncologists via contract sales organization (CSO) to create “Push”
• Sell to hospital-based interventional radiologists and surgeons with combination of direct sales and
distributors to create “Pull”
distributors to create “Pull”
• Establish European patient education & awareness programs (PR, website)
• Leverage existing new technology reimbursement channels, while pursuing permanent procedure
reimbursement via Health Technology Assessment (HTA)
reimbursement via Health Technology Assessment (HTA)
• New clinical trials to generate additional data for HCC & mCRC
Strategy and Tactics to Address All Key Constituents

19 DELCATH SYSTEMS, INC
European Marketing Considerations
Reimbursement:
o No centralized EEA device reimbursement body - regional and national systems
o Devices typically reimbursed under DRG as part of a procedure
o Immediate reimbursement plans:
• Utilize existing codes where permitted until permanent reimbursement established (e.g. Italy)
• Apply for funding under new technology programs (e.g. NUB in Germany and HAS in France)
• Other oncology therapies currently reimbursed, despite lacking randomized data
o Retained reimbursement experts to obtain new procedure specific coding and payment
o Developing Health Technology Assessment (HTA)
o Focused on highlighting clinical value proposition and demonstrating cost effectiveness
Melphalan:
o Delcath approved in the EEA for the intra-arterial administration of melphalan to the liver
o Physicians will continue to procure melphalan independently
o Clinical experience in EEA and publications support use of melphalan for disease control in the liver
Clinical Data:
o Delcath Phase 3 and Phase 2 data supplements extensive surgical IHP data with melphalan
o Expect to initiate additional studies with Standard of Care (SOC) in 2012 with availability of HE filter in
HCC, and metastatic CRC
HCC, and metastatic CRC
o Marketing to medical oncologists will be data driven
Required Elements In Place To Support Commercial Launch
20 DELCATH SYSTEMS, INC
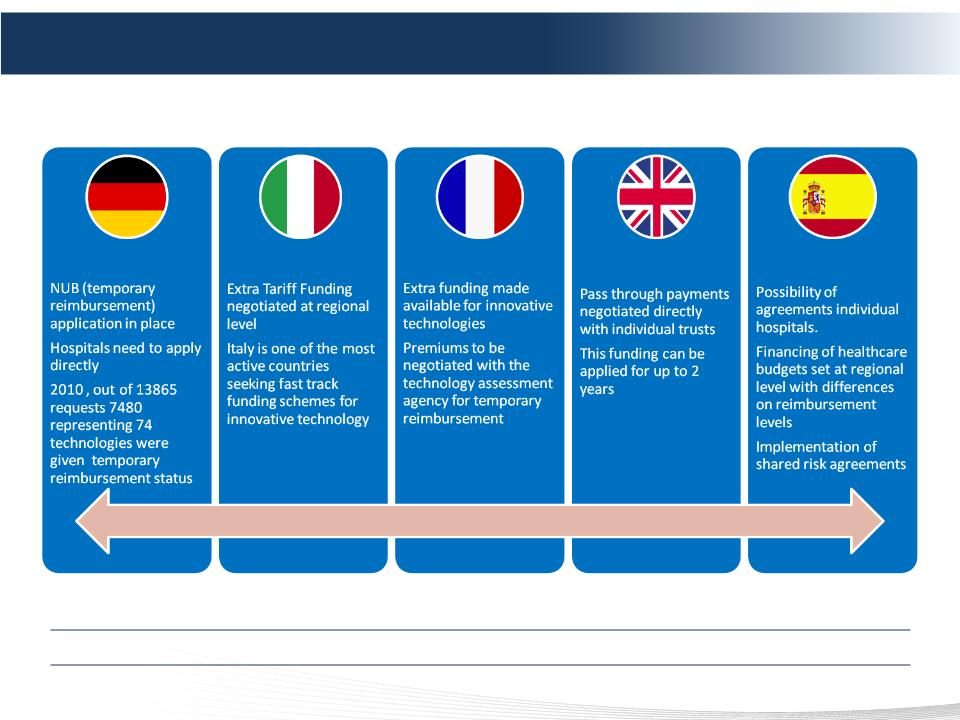
European Interim New Technology Reimbursement Programs
Interim New Technology Payment Programs Already Exist in Major European Markets

21 DELCATH SYSTEMS, INC
Market by Disease - EEA Device Only
|
|
Germany
(Direct)
|
UK
(Direct)
|
France
(Indirect)
|
Italy
(Indirect)
|
Spain
(Indirect)
|
Netherlands
(Direct)
|
Total
Potential (patients)
|
Potential
Market ($ millions)1,2,3
|
|
|
||||||||
|
Total Potential Market #Patients
|
||||||||
|
Ocular Melanoma
|
403
|
296
|
294
|
284
|
197
|
79
|
1,553
|
$46.6
|
|
Cutaneous
Melanoma |
2,834
|
1,735
|
1,314
|
1,398
|
628
|
662
|
8.571
|
$257.1
|
|
CRC
|
18,978
|
10,155
|
10,490
|
13,952
|
7,694
|
3,151
|
64,420
|
$1,932.6
|
|
HCC (Primary)
|
3,941
|
1,734
|
3,645
|
6,253
|
2,616
|
197
|
18,386
|
$551.6
|
|
NET
|
2,168
|
1,624
|
1,645
|
1,579
|
1,185
|
438
|
8,639
|
$259.2
|
|
TOTAL
|
25,087
|
13,513
|
15,780
|
21,784
|
11,495
|
3,786
|
91,445
|
$3,047.1
|
Europe is Potential $3.0 Billion Market Opportunity for Device Only
1. Assumes 2.5 treatments per patient
2. Assumes ASP of $12K (device only)
3. Assumes mix of direct sales and distributors

22 DELCATH SYSTEMS, INC
U.S. FDA Regulatory Status
• On February 22, 2011, received Refusal to File (RTF) letter from the FDA
§ Manufacturing plant inspection timing
§ Product and sterilization validation
§ Additional statistical analysis clarification
§ Additional safety data
o RTF stated that safety information provided was insufficient to allow FDA to
accept our application and review the overall risk/benefit profile
accept our application and review the overall risk/benefit profile
o FDA & SPA approved CRF’s did not collect all hospitalization data in the
patient records in an effective manner
patient records in an effective manner
• Follow-up meeting with FDA held in April 2011 to review proposed plan
of action which includes:
o Collection of all available safety information in new CRF for all 186 patients in
the Phase I, II and III clinical trials
the Phase I, II and III clinical trials
• No additional studies or generation of new data requested
Intend to Submit Revised NDA By End of 2011

23 DELCATH SYSTEMS, INC
Market by Disease* - USA
|
Liver Metastasis
|
Potential Market
# Patients
|
Potential Market
# Procedures
(Avg 2.5/patient)
|
Potential Market ($MM)
$20K ASP **
|
|
Ocular
Melanoma
|
1,622
|
4,055
|
$81.1
|
|
Cutaneous
Melanoma
|
11,883
|
29,708
|
$594.2
|
|
TOTAL MELANOMA
(Initial Expected Label)
|
13,505
|
33,763
|
$675.3
|
|
CRC
|
38,423
|
96,057
|
$1,921.1
|
|
HCC (Primary)
|
12,386
|
30,964
|
$619.3
|
|
NET
|
9,986
|
24,965
|
$499.3
|
|
TOTAL OTHER
(Potential Label Expansion)
|
60,794
|
151,985
|
$3,039.7
|
*TPM Total Potential Market
** Estimated ASP

24 DELCATH SYSTEMS, INC
U.S. Commercialization Strategy
• Initial focus on leading cancer centers and referring community hospitals
• Market to Medical Oncologists via CSO
• Direct Strategy to sell to Interventional Radiologists and Surgeons: 12
Sales & Medical Science Liaison territories ultimately expanding to as
many as 60 territories as revenues ramp
Sales & Medical Science Liaison territories ultimately expanding to as
many as 60 territories as revenues ramp
• 5 Clinical Specialists initially to support site initiation and training
• Utilize top centers from Phase III trial as Centers of Excellence for training
and support
and support
Direct Sales Model Supplemented With CSO Detailing Program

25 DELCATH SYSTEMS, INC
U.S. Reimbursement Strategy
Strategy: intend to seek chemosaturation specific codes based upon value
proposition relative to other cancer therapies
proposition relative to other cancer therapies
o Physician:
• Applied for CPT Category III code
• Convert the Category III code to Category I following FDA approval
o Hospital:
• Apply for new ICD-9/10 procedure code to capture full procedure of
hepatic isolation and chemosaturation
hepatic isolation and chemosaturation
• Request new DRG based on costs above those of existing DRGs and
clinical dissimilarity to other hepatic procedures in current DRGs
clinical dissimilarity to other hepatic procedures in current DRGs
Pursuing New Specific Codes For Chemosaturation Procedure

26 DELCATH SYSTEMS, INC
Strategy For Asia, Ex US America’s, MEA and Australia
• Intend to leverage CE Mark to obtain reciprocal regulatory approvals for our
Delcath Hepatic CHEMOSAT System
Delcath Hepatic CHEMOSAT System
• Utilize existing 3rd party melphalan available to physicians
• Seek to secure strategic partners and specialty distributors
• Intend to initiate melphalan HCC trial in Taiwan with partner Chi-Fu in 2012
Combination of Direct Sales, Strategic Partnerships & Specialty Distributors

27 DELCATH SYSTEMS, INC
Market by Disease - Australia/Asia
Initial Target Markets (China, Japan, S. Korea, Taiwan, Australia)
Initial Target Markets (China, Japan, S. Korea, Taiwan, Australia)
1. Assumes 2.5 treatments per patient
2. Assumes ASP of $9K
3. Assumes mix of systems with and without Delcath branded melphalan
4. Assumes sales by distributors
|
|
China
(Drug)
|
S. Korea
(Drug)
|
Japan
(Device)
|
Taiwan
(Drug)
|
Australia
(Device)
|
Total
Potential (patients)
|
Potential
Market 1,2,3,4 |
|
|
|||||||
|
Total Potential Market #Patients
|
|||||||
|
HCC (Primary)
|
197,082
|
7,486
|
7,625
|
4,945
|
604
|
217,742
|
$4,899.2
|
|
Other
|
|||||||
|
CRC
|
59,644
|
6,219
|
27,396
|
2,762
|
3,891
|
99,912
|
$2,248.0
|
|
NET
|
35,503
|
1,275
|
3,355
|
608
|
562
|
41,303
|
$929.3
|
|
Ocular Melanoma
|
1,760
|
66
|
175
|
31
|
96
|
2,128
|
$47.9
|
|
Cutaneous
Melanoma |
667
|
74
|
238
|
429
|
1,996
|
3,404
|
$76.6
|
|
OTHER TOTAL
|
292,229
|
14,980
|
38,376
|
8,315
|
5,057
|
358,957
|
$8,201.0
|
Asia Represents Potential $8.2 Billion Market Opportunity

28 DELCATH SYSTEMS, INC
Intellectual Property
Patent Protection
• 7 issued U.S. patents, 10 foreign patents issued and 4 pending
• Primary device patent set to expire August 2016
• Up to 5 years of patent extension post FDA approval
Trade Secret Protection
• Developed High Efficiency (HE) filter media via new manufacturing processes
FDA Protection
• Orphan Drug Designation granted for melphalan in the treatment of ocular melanoma,
cutaneous melanoma and metastatic neuroendocrine tumors, as well as for doxorubicin
in the treatment of HCC
cutaneous melanoma and metastatic neuroendocrine tumors, as well as for doxorubicin
in the treatment of HCC
o Provides 7 years of marketing exclusivity post FDA approval
• Additional Orphan Drug applications to be filed for other drugs and indications, including melphalan
for HCC and CRC
for HCC and CRC
Multiple Levels of Protection

29DELCATH SYSTEMS, INC
Deep and Experienced Management Team
Significant Combination Product Approval and Commercialization Experience
|
Executive
|
Title
|
Prior Affiliation(s)
|
Years of
Experience |
|
Eamonn Hobbs
|
President and CEO
|
AngioDynamics, E-Z-EM
|
30
|
|
David McDonald
|
CFO
|
AngioDynamics, RBC Capital
Markets |
28
|
|
Krishna Kandarpa,
M.D., Ph.D. |
CMO and EVP, R&D
|
Harvard, MIT, Cornell, UMass
|
37
|
|
Agustin Gago
|
EVP, Global Sales & Marketing
|
AngioDynamics, E-Z-EM
|
29
|
|
Peter Graham, J.D.
|
EVP & General Counsel
|
Bracco, E-Z-EM
|
16
|
|
John Purpura
|
EVP, Regulatory Affairs &
Quality Assurance |
E-Z-EM, Sanofi-Aventis
|
27
|
|
Bill Appling
|
SVP Operations & Medical
Device R&D |
AngioDynamics
|
25
|
|
Harold Mapes
|
EVP, Global Operations
|
AngioDynamics, Mallinkrodt
|
25
|
|
Dan Johnston, Ph.D.
|
VP, Pharma R&D
|
Pfizer, Wyeth
|
10
|

FINANCIALS

31 DELCATH SYSTEMS, INC
Financial Summary
Financial & Operating Overview
• Follow On Offerings: Raised ~ $94 million since November 2009
• Burn Rate: Anticipate ~$3.0 million per month
• Cash: ~ $53 million at July 31, 2011
• Debt: None
• Shares Out: 48.0 million (54.8 million fully diluted*)
• Institutional Ownership: ~ 28% at March 31, 2011
• Market Capitalization: ~ $214 million as of July 31, 2011
• Avg. Daily Volume (3 months) ~ 815,000
Balance Sheet Strengthened Significantly in Past Two Years To Support Growth Activities
As of July 31st, 2011 fully diluted includes an additional 4.1 million options at $5.04, 2.5 million warrants at $3.51, and 174,682 unvested restricted shares.

32 DELCATH SYSTEMS, INC
Company Highlights
• Making established chemotherapeutic drugs work better in target organs
• Initial focus is high dose chemotherapy for improved disease control in the liver
• Successful and highly statistically significant Phase III trial results reported
• Received CE Mark approval for Class III medical device on April 13, 2011
• Positioned to address potential $3.0 billion European labeled market opportunity
• Objective is to re-file 505(b)(2) NDA to FDA for orphan drug and delivery
apparatus by end of 2011
apparatus by end of 2011
• Potential $675 million US labeled market opportunity
• Issued patents and orphan drug designations create competitive barriers
• Deep and experienced management team
Concentrating the Power of Chemotherapy to Improve Disease Control in the Liver

33 DELCATH SYSTEMS, INC
Appendix I. - Delcath Sources for Market Estimates
American Cancer Society. Cancer Facts & Figures 2010. Atlanta: American Cancer Society; 2010.
Alexander, Richard H., David L. Bartlett, and Steven K. Libutti. "Current Status of Isolated Hepatic Perfusion With or
Without Tumor Necrosis Factor for the Treatment of Unresectable Cancers Confined to the Liver." The Oncologist 5
(2000): 416-24.
Without Tumor Necrosis Factor for the Treatment of Unresectable Cancers Confined to the Liver." The Oncologist 5
(2000): 416-24.
Blake, Simon P., Karen Weisinger, Michael B. Atkins, and Vassilios Raptopoulos. "Liver Metastases from Melanoma:
Detection with Multiphasic Contrast Enhanced CT." Radiology 213 (1999): 92-96. Print
Detection with Multiphasic Contrast Enhanced CT." Radiology 213 (1999): 92-96. Print
Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM.
GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet].
Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr
GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet].
Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr
Nawaz Khan, Ali, Sumaira MacDonald, Ajay Pankhania and David Sherlock. "Liver, Metastases: [Print] - EMedicine
Radiology." Liver, Metastases. EMedicine - Medical Reference, 10 Feb. 2009. Web.
<http://emedicine.medscape.com/article/369936-print>.
Radiology." Liver, Metastases. EMedicine - Medical Reference, 10 Feb. 2009. Web.
<http://emedicine.medscape.com/article/369936-print>.
Neuroendocrine Tumors. Practice Guidelines in Oncology- v.2.2009. National Comprehensive Cancer Network
(NCCN). 2009.
(NCCN). 2009.
Pawlik, Timothy M., Daria Zorzi, Eddie K. Abdalla, Bryan M. Clary, Jeffrey E. Gershenwald, Merrick I. Ross, Thomas A.
Aloia, Steven A. Curley, Luis H. Camacho, Lorenzo Capussotti, Dominique Elias, and Jean-Nicolas Vauthey. "Hepatic
Resection for Metastatic Melanoma: Distinct Patterns of Recurrence and Prognosis for Ocular Versus Cutaneous
Disease." Annals of Surgical Oncology 13.5 (2006): 712-20.
Aloia, Steven A. Curley, Luis H. Camacho, Lorenzo Capussotti, Dominique Elias, and Jean-Nicolas Vauthey. "Hepatic
Resection for Metastatic Melanoma: Distinct Patterns of Recurrence and Prognosis for Ocular Versus Cutaneous
Disease." Annals of Surgical Oncology 13.5 (2006): 712-20.

34 DELCATH SYSTEMS, INC
Appendix II. - Phase 3 Pivotal Trial Details

35 DELCATH SYSTEMS, INC
Phase III Clinical Trial Design
Randomized to CS
92 patients: ocular
or cutaneous melanoma
CS/Melphalan
Treat every 4 weeks x 4 rounds
(responders can receive up to 6 rounds)
Cross-over
Primary Trial Endpoint
• Statistically significant difference in Hepatic Progression
Free Survival (“hPFS”): p < 0.05
Free Survival (“hPFS”): p < 0.05
• Over 80% of Oncologic drugs approved by FDA between
2005 - 2007 on endpoints other than overall survival
2005 - 2007 on endpoints other than overall survival
Modeled hPFS for Trial Success:
7.73 months (CS)
vs.
4 months (BAC)
Secondary Trial Endpoints
• Hepatic response and duration of hepatic response
• Overall response and duration of overall response
• Overall Survival - Diluted by Cross Over
• SAP calls for analysis of various patient cohorts
Pre-CS (Baseline)
Post-CS (22+ Months)
Hepatic Response - Metastatic Melanoma
Fully Powered, 93 Patient, Randomized, Multi-Center NCI Led Study

36 DELCATH SYSTEMS, INC
ASCO 2010 Presentation of Phase 3 Clinical Trial Results
• Trial results exceed primary endpoint expectations; p value = 0.001
• Treatment arm shows 5x median hPFS compared to control arm
• CS/PHP median hPFS of 245 days compared to 49 days for BAC
• Hazard Ratio = .301
• Patients failed prior therapies (radiation, chemo, immuno, image guided local)
• 90% Ocular, 10% Cutaneous - No difference in response
• Overall PFS 186 vs. 46 days for BAC
• 34% response rate for CS/PHP compared to 2% for BAC
• 52% stable disease for CS/PHP compared to 27% for BAC
• 86% overall clinical benefit (CR + PR + SD)
Strong Clinical Trial Results

37 DELCATH SYSTEMS, INC
ASCO 2010 Presentation of Phase 3 Clinical Trial (cont.)
• Majority of BAC patients crossed over and obtained similar response from treatment
• Total 93 patient trial - 10 months median OS vs. 4 months expected1 (due to cross over
provision, most patients received PHP/CS treatment)
provision, most patients received PHP/CS treatment)
• OS cohort analysis - all positive trends
a) Median survival of 298 days for treatment arm compared to 124 in non-crossover BAC patients
b) Median survival of 398 days for BAC Cross Over patients vs. 124 non-cross over BAC patients
• OS Secondary endpoint - No difference in Kaplan-Meier curves(due to cross over
treatment response)
treatment response)
• Safety profile as expected - in line with current FDA approved labeling for IV
administration of Melphalan and Phase I CS/PHP study results
administration of Melphalan and Phase I CS/PHP study results
o Treatment related Deaths: 3/40 patients (7.5%) 3/116 procedures (2.6%)
o Neutropenic Sepsis (n=2) 5%, Hepatic Failure (n=1) 2.5% (95% tumor burden)
o Current approved labeling for Melphalan - 3% to 10% mortality rate.
Encouraging Survival Data With Expected Safety Profile
1. Source: Unger et. al. Cancer 2001;91: 1148

* DELCATH SYSTEMS, INC
© 2011 DELCATH SYSTEMS, INC. ALL RIGHTS RESERVED
