Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a11-20251_78k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a11-20251_7ex99d1.htm |
Exhibit 99.2
|
|
2Q 2011 Financial Results JULY 26, 2011 1 |
|
|
Forward-Looking Statements 2 Forward-looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, our statements regarding our belief that the addition of FOLOTYN® to our product portfolio will enable us to gain valuable synergies and create additional shareholder value, our beliefs in the strategic, commercial and financial rationale for the merger, our expectations regarding the future cash flow impact of the merger on the combined company, our expectations regarding the combined net product revenues and expenses of the combined company, our expectation to achieve cash flow neutrality by year-end 2013, our expectation regarding the combined company’s cash position, and the expanded timing of decisions with respect to European and Canadian review of our Feraheme marketing applications are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include: the failure of Allos or AMAG stockholders to approve the proposed transaction; the challenges and costs of closing the proposed transaction, integrating the two companies, restructuring the combined company; the possibility that the expected synergies and additional cost savings will not be realized, or will not be realized within the expected time period; the ability to retain key employees; and other economic, business, competitive, and/or regulatory factors affecting the businesses of Allos and AMAG generally, including those set forth in the filings of Allos and AMAG with the Securities and Exchange Commission, especially in the “Risk Factors” section of Allos’ Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 filed with the SEC on May 10, 2011, the “Risk Factors” section of AMAG’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011 filed with the SEC on May 9, 2011, and in Allos’ and AMAG’s other periodic reports and filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. |
|
|
Important Merger Information and Additional Information and Where to Find It This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. The proposed merger between AMAG and Allos will be submitted to the respective stockholders of AMAG and Allos for their consideration. AMAG will file a Registration Statement on Form S-4 containing a joint proxy statement/prospectus of Allos and AMAG and other documents concerning the proposed acquisition with the Securities and Exchange Commission (the “SEC”). Investors are urged to read the joint proxy statement/prospectus when it becomes available and other relevant documents filed with the SEC because they will contain important information. Security holders may obtain a free copy of the proxy statement/prospectus (when it is available) and other documents filed by Allos and AMAG with the SEC at the SEC’s website at www.sec.gov. The joint proxy statement/prospectus and other documents may also be obtained for free by contacting Allos’ Investor Relations by e-mail at investorrelations@allos.com, by telephone at (303) 426-6262 or by mail at Investor Relations, Allos Therapeutics, Inc., 11080 CirclePoint Road, Suite 200, Westminster, CO 80020 or by contacting AMAG’s Investor Relations by e-mail at cmiceli@amagpharma.com, by telephone at (617) 498-3361 or by mail at Investor Relations, AMAG Pharmaceuticals, Inc., 100 Hayden Avenue, Lexington, MA 02421. Allos, AMAG, certain of their respective directors, executive officers, members of management and employees may, under the rules of the SEC, be deemed to be participants in the solicitation of proxies in connection with the proposed merger. Information regarding Allos’ directors and executive officers and their beneficial ownership of Allos’ common stock is also set forth in Allos’ annual proxy statement on Schedule 14A filed with the SEC on April 29, 2011. This document is available free of charge at the SEC’s website at www.sec.gov or by going to Allos’ Investors page on its corporate website at www.allos.com. Information concerning AMAG’s directors and executive officers and their beneficial ownership of AMAG’s common stock is set forth in AMAG’s annual proxy statement on Schedule 14A filed with the SEC on April 18, 2011. This document is available free of charge at the SEC’s website at www.sec.gov or by going to AMAG’s Investors page on its corporate website at www.amagpharma.com. Additional information regarding the persons who may, under the rules of the SEC, be deemed “participants” in the solicitation of proxies in connection with the proposed merger, and a description of their direct and indirect interests in the proposed merger, which may differ from the interests of Allos’ investors or AMAG’s investors generally, will be set forth in the joint proxy statement/prospectus when it is filed with the SEC. 3 |
|
|
Agenda Opening Remarks – Brian Pereira, CEO Commercial – Gary Zieziula, Chief Commercial Officer Merger with Allos Therapeutics – Brian Pereira, CEO Q&A Session 4 |
|
|
OPENING REMARKS Brian Pereira, Chief Executive Officer 5 |
|
|
Business Update – 2Q 2011 Strong product revenue growth $15.4 million in total revenues, including $12.8 million in Feraheme net product revenues 24% increase in provider demand compared to 1Q11 Continued progress on market expansion for Feraheme European and Canadian regulatory review progressing well, expect decisions in 2011 Enrollment ongoing in global registrational IDA clinical program; currently 65% enrolled Strategic merger provides operating leverage and product diversification On July 19, 2011 we entered into a definitive merger agreement with Allos Therapeutics, subject to shareholder approval 6 |
|
|
COMMERCIAL Gary Zieziula, Chief Commercial Officer 7 |
|
|
2Q11 Commercial Update $15.4 million in total revenues, including $12.8 million in Feraheme net product revenues Provider demand increased 24% quarter-over-quarter Growth in all target segments Level playing field for non-dialysis CKD product labels Feraheme label update June 2011 Decreased observation period to 30 minutes Modifications to the Contraindications and Warnings and Precautions sections Venofer label update July 2011 Addition of 30 minute observation period Similar modifications to the Contraindications and Warning and Precautions sections 8 |
|
|
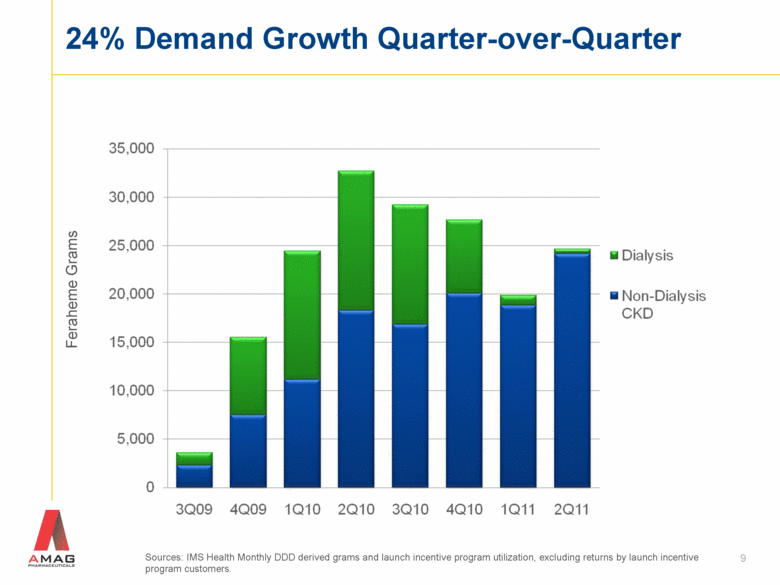
9 24% Demand Growth Quarter-over-Quarter Sources: IMS Health Monthly DDD derived grams and launch incentive program utilization, excluding returns by launch incentive program customers. Feraheme Grams |
|
|
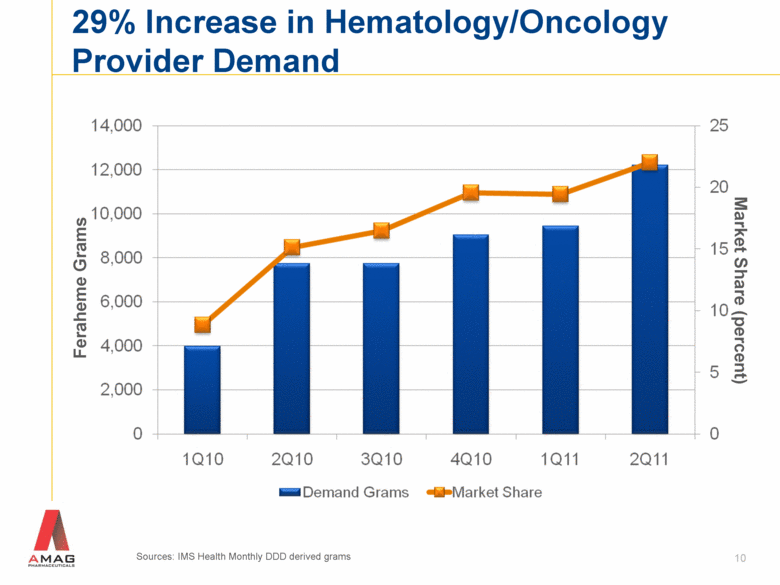
29% Increase in Hematology/Oncology Provider Demand Feraheme Grams 10 Market Share (percent) Sources: IMS Health Monthly DDD derived grams |
|
|
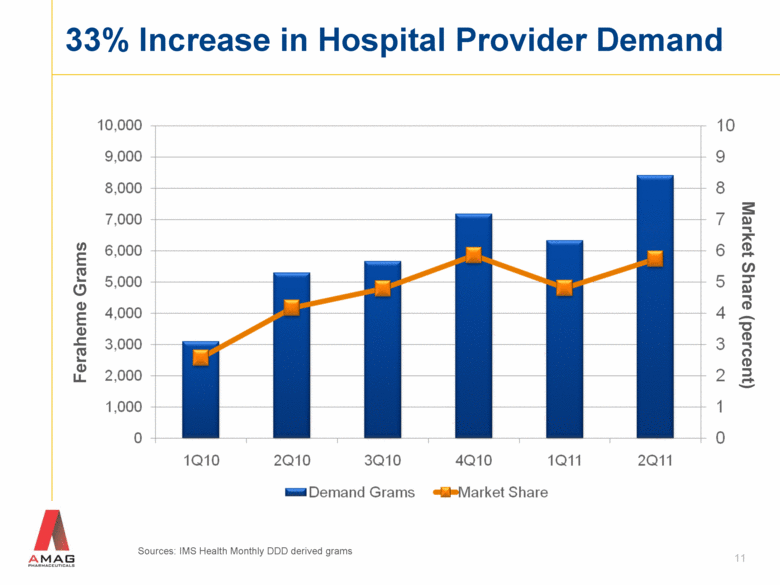
33% Increase in Hospital Provider Demand Feraheme Grams 11 Market Share (percent) Sources: IMS Health Monthly DDD derived grams |
|
|
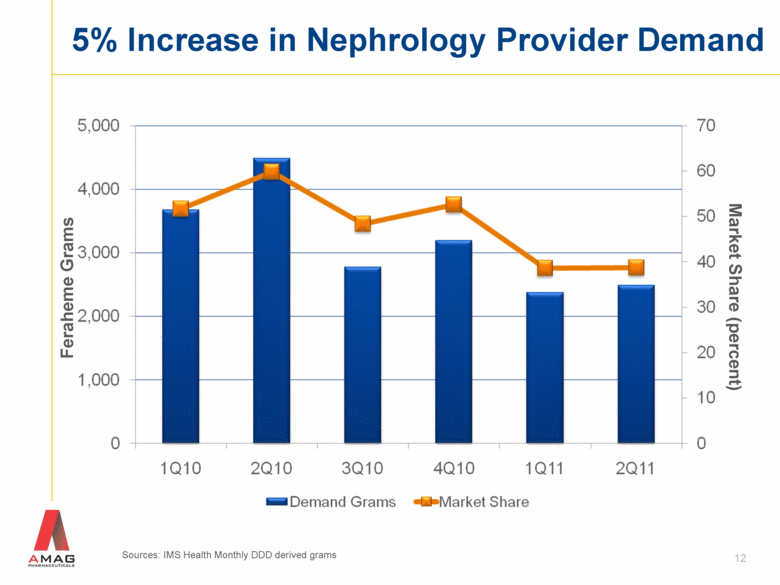
5% Increase in Nephrology Provider Demand 12 Feraheme Grams Market Share (percent) Sources: IMS Health Monthly DDD derived grams |
|
|
2Q 2011 Feraheme Commercial Summary Provider demand increased 24% quarter-over quarter Momentum building in hematology/oncology clinics and hospitals – high-volume sites of care where non-dialysis CKD patients receive IV iron Label changes to Feraheme and Venofer create a level playing field 13 |
|
|
COMMERCIAL RATIONALE of ALLOS MERGER Gary Zieziula, Chief Commercial Officer 14 |
|
|
Strong Strategic Commercial Fit Improved Access, Reach & Promotional Activity for Both Brands Common call points Hematology/oncology clinics, academic and community hospitals GPOs KOLs Increases account access & penetration 100% coverage of target accounts for both brands1 Increases share of voice for both brands Folotyn improves access for Feraheme 1. ZS Associates analysis, 2011. |
|
|
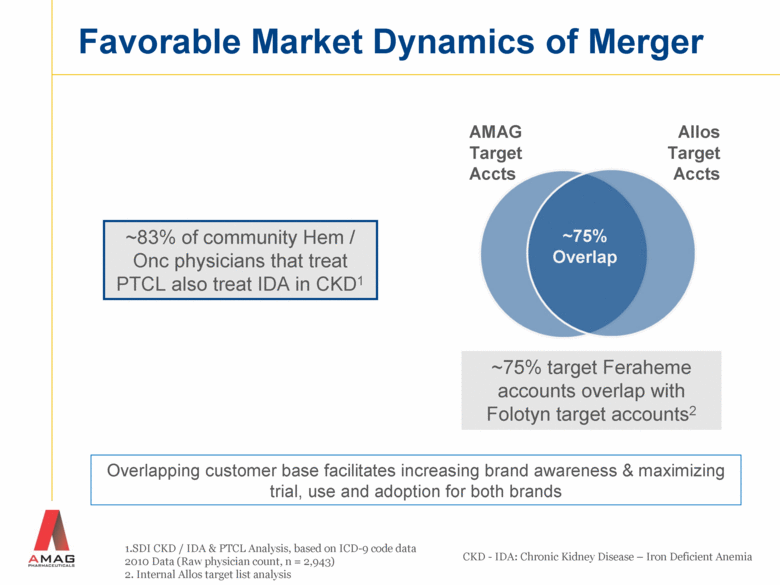
Favorable Market Dynamics of Merger 1.SDI CKD / IDA & PTCL Analysis, based on ICD-9 code data 2010 Data (Raw physician count, n = 2,943) 2. Internal Allos target list analysis ~83% of community Hem / Onc physicians that treat PTCL also treat IDA in CKD1 ~75% target Feraheme accounts overlap with Folotyn target accounts2 AMAG Target Accts Allos Target Accts ~75% Overlap CKD - IDA: Chronic Kidney Disease – Iron Deficient Anemia Overlapping customer base facilitates increasing brand awareness & maximizing trial, use and adoption for both brands |
|
|
ALLOS MERGER Brian Pereira, Chief Executive Officer 17 |
|
|
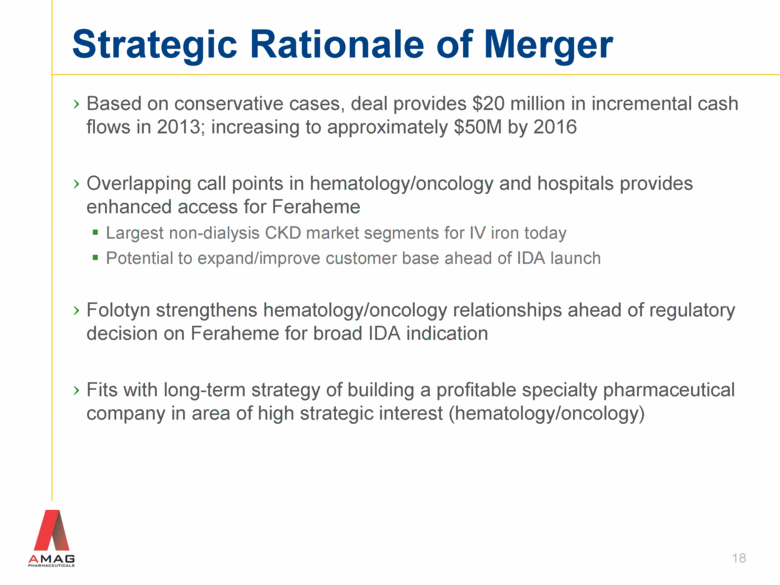
Strategic Rationale of Merger Based on conservative cases, deal provides $20 million in incremental cash flows in 2013; increasing to approximately $50M by 2016 Overlapping call points in hematology/oncology and hospitals provides enhanced access for Feraheme Largest non-dialysis CKD market segments for IV iron today Potential to expand/improve customer base ahead of IDA launch Folotyn strengthens hematology/oncology relationships ahead of regulatory decision on Feraheme for broad IDA indication Fits with long-term strategy of building a profitable specialty pharmaceutical company in area of high strategic interest (hematology/oncology) 18 |
|
|
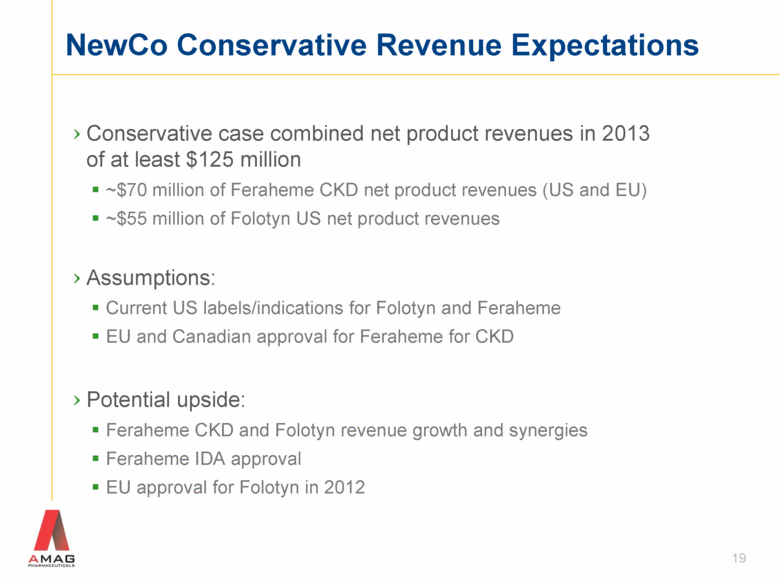
NewCo Conservative Revenue Expectations Conservative case combined net product revenues in 2013 of at least $125 million ~$70 million of Feraheme CKD net product revenues (US and EU) ~$55 million of Folotyn US net product revenues Assumptions: Current US labels/indications for Folotyn and Feraheme EU and Canadian approval for Feraheme for CKD Potential upside: Feraheme CKD and Folotyn revenue growth and synergies Feraheme IDA approval EU approval for Folotyn in 2012 19 |
|
|
NewCo Expense Expectations Companies had estimated $215 - $230 million in combined cash operating expenses for full year 2011 (excluding COGS) NewCo cash operating expenses in 2013 expected to be no greater than $120 million (excluding COGS) Synergies of $55 to $60 million annually, majority achieved in first fiscal year through elimination of redundant personnel, consolidation of locations, elimination of duplicative structures and expenses Additional $10 to $20 million of annual cost savings to be achieved starting in 2013 through additional commercial and clinical development cost reductions ~$30 million in reduction of clinical trial expenses associated with IDA program completion in 2012 Potential upside: EU approval of Folotyn in 2012 leading to 50/50 split in clinical trial costs with Mundipharma from 60/40 split today 20 |
|
|
NewCo Conservative Cash Expectations Management is committed to driving NewCo to cash flow breakeven by year-end 2013, earlier than with Feraheme alone Approximately $20 million in incremental annual cash flow generated by deal in 2013, increasing to at least $50 million by 2016 NewCo cash position not expected to fall below $220 million Includes $33 million in milestones associated with Feraheme EU and Canadian approvals and launches Potential to deliver more to NewCo cash position and bottom line: Approximately $500 million in additional partner milestones Feraheme IDA approval Folotyn label expansions Folotyn EU approval in 2012 21 |
|
|
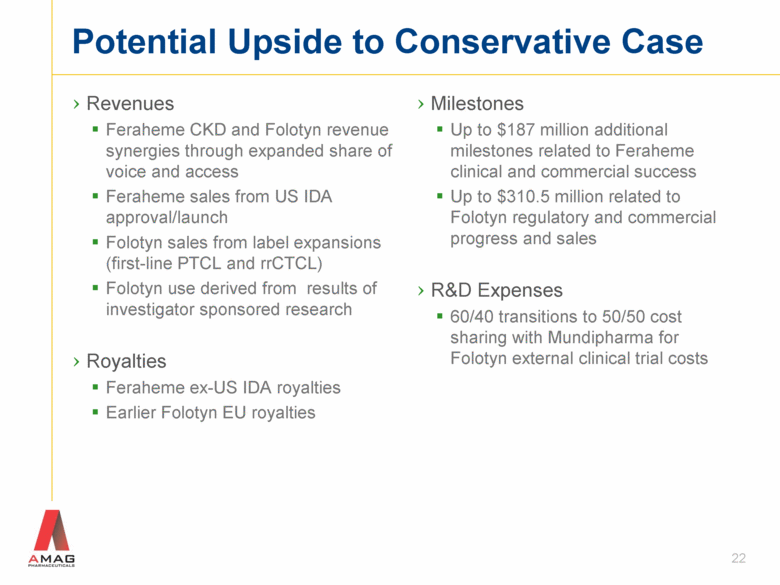
Potential Upside to Conservative Case Revenues Feraheme CKD and Folotyn revenue synergies through expanded share of voice and access Feraheme sales from US IDA approval/launch Folotyn sales from label expansions (first-line PTCL and rrCTCL) Folotyn use derived from results of investigator sponsored research Royalties Feraheme ex-US IDA royalties Earlier Folotyn EU royalties Milestones Up to $187 million additional milestones related to Feraheme clinical and commercial success Up to $310.5 million related to Folotyn regulatory and commercial progress and sales R&D Expenses 60/40 transitions to 50/50 cost sharing with Mundipharma for Folotyn external clinical trial costs 22 |
|
|
Focused on Building Long-term Value for Shareholders We believe the merger with Allos Therapeutics Expands access to hematology/oncology for Feraheme in CKD and broad IDA indications Leverages existing commercial infrastructure and cost-base in hematology/oncology space Spreads public company costs over larger revenue base Provides further revenue opportunities and diversification through new geographical markets and additional indications NewCo’s center of gravity is hematology/oncology Feraheme and Folotyn share this critical call point An area of high strategic interest (much greater than renal) Strategically positions NewCo for future growth and value generation through additional product acquisitions or licensing 23 |
|
|
Q&A 24 |