Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Anacor Pharmaceuticals, Inc. | a11-16064_18k.htm |
| EX-99.1 - EX-99.1 - Anacor Pharmaceuticals, Inc. | a11-16064_1ex99d1.htm |
Exhibit 99.2
|
|
AN2728 Clinical Program Conference Call June 28, 2011 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements that relate to future events, including the development and commercialization of AN2728, and more specifically the outcome of an end of Phase 2 meeting with the FDA, the representative nature of the Phase 2b study plan and reported results as indicative of future clinical trials in support of regulatory approval, and the timing and potential for initiation, enrollment and conduct of AN2728 Phase 3 trials. These forward looking statements involve known and unknown risks, uncertainties and other factors that could cause timing, actual levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements, including risks related to enrollment and successful completion of our trials, risk of unforeseen side effects and risks related to regulatory approval of new drug candidates. These statements reflect the views of Anacor as of the date of this presentation with respect to future events and, except as required by law, it undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this presentation. |
|
|
David Perry Chief Executive Officer |
|
|
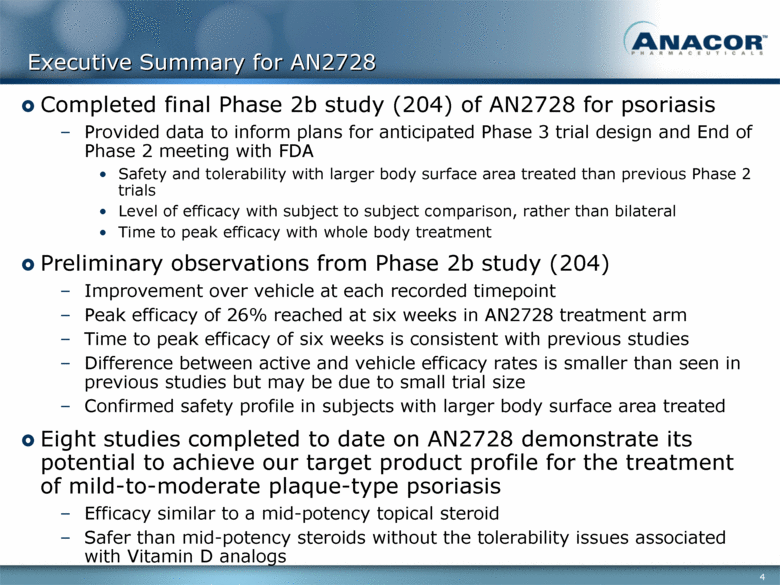
Executive Summary for AN2728 Completed final Phase 2b study (204) of AN2728 for psoriasis Provided data to inform plans for anticipated Phase 3 trial design and End of Phase 2 meeting with FDA Safety and tolerability with larger body surface area treated than previous Phase 2 trials Level of efficacy with subject to subject comparison, rather than bilateral Time to peak efficacy with whole body treatment Preliminary observations from Phase 2b study (204) Improvement over vehicle at each recorded timepoint Peak efficacy of 26% reached at six weeks in AN2728 treatment arm Time to peak efficacy of six weeks is consistent with previous studies Difference between active and vehicle efficacy rates is smaller than seen in previous studies but may be due to small trial size Confirmed safety profile in subjects with larger body surface area treated Eight studies completed to date on AN2728 demonstrate its potential to achieve our target product profile for the treatment of mild-to-moderate plaque-type psoriasis Efficacy similar to a mid-potency topical steroid Safer than mid-potency steroids without the tolerability issues associated with Vitamin D analogs |
|
|
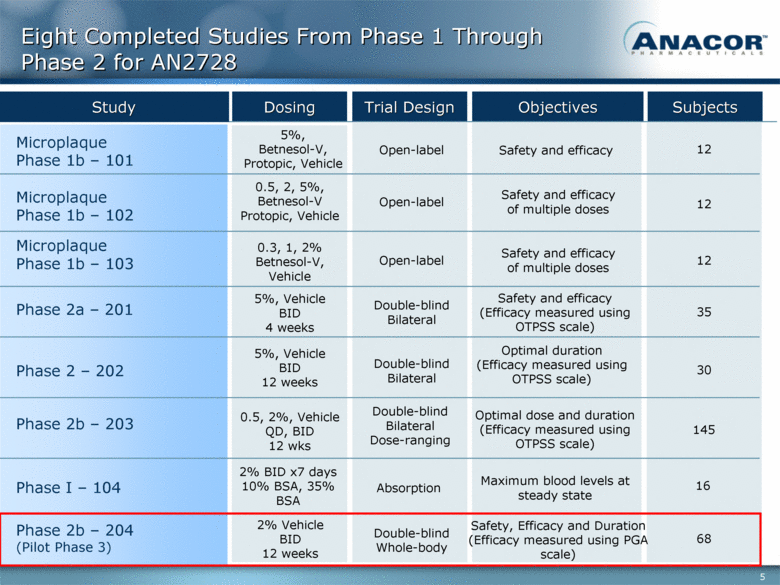
Phase I – 104 Phase 2a – 201 Microplaque Phase 1b – 103 Microplaque Phase 1b – 102 Microplaque Phase 1b – 101 Phase 2 – 202 Phase 2b – 203 Phase 2b – 204 (Pilot Phase 3) Eight Completed Studies From Phase 1 Through Phase 2 for AN2728 2% BID x7 days 10% BSA, 35% BSA 16 30 Dosing Trial Design Objectives Subjects 5%, Vehicle BID 4 weeks 0.3, 1, 2% Betnesol-V, Vehicle 0.5, 2, 5%, Betnesol-V Protopic, Vehicle 5%, Betnesol-V, Protopic, Vehicle 35 12 12 12 Study 5%, Vehicle BID 12 weeks 0.5, 2%, Vehicle QD, BID 12 wks 145 2% Vehicle BID 12 weeks 68 Open-label Open-label Open-label Double-blind Bilateral Double-blind Bilateral Double-blind Bilateral Dose-ranging Safety and efficacy Double-blind Whole-body Safety and efficacy of multiple doses Safety and efficacy of multiple doses Safety and efficacy (Efficacy measured using OTPSS scale) Optimal dose and duration (Efficacy measured using OTPSS scale) Optimal duration (Efficacy measured using OTPSS scale) Absorption Safety, Efficacy and Duration (Efficacy measured using PGA scale) Maximum blood levels at steady state |
|
|
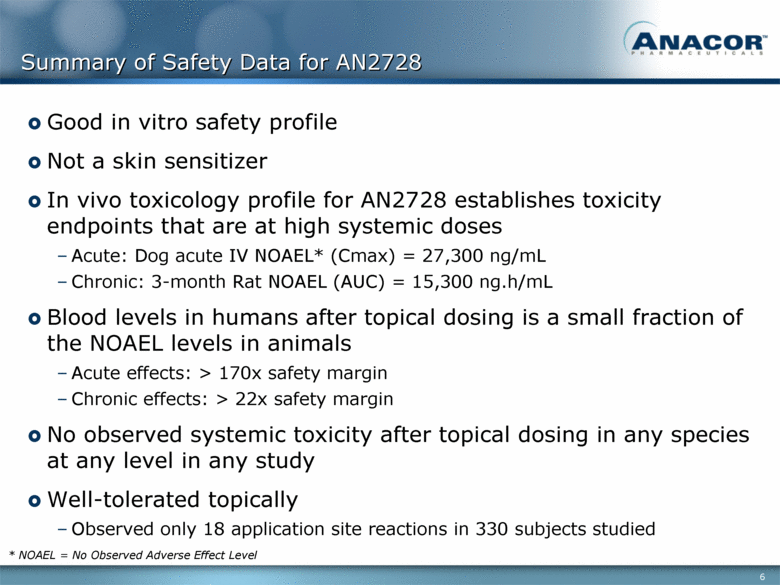
Summary of Safety Data for AN2728 Good in vitro safety profile Not a skin sensitizer In vivo toxicology profile for AN2728 establishes toxicity endpoints that are at high systemic doses Acute: Dog acute IV NOAEL* (Cmax) = 27,300 ng/mL Chronic: 3-month Rat NOAEL (AUC) = 15,300 ng.h/mL Blood levels in humans after topical dosing is a small fraction of the NOAEL levels in animals Acute effects: > 170x safety margin Chronic effects: > 22x safety margin No observed systemic toxicity after topical dosing in any species at any level in any study Well-tolerated topically Observed only 18 application site reactions in 330 subjects studied * NOAEL = No Observed Adverse Effect Level |
|
|
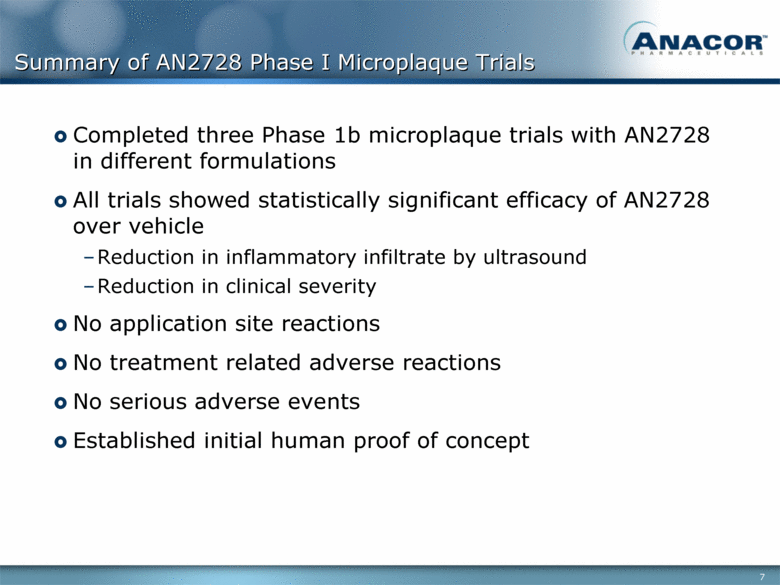
Summary of AN2728 Phase I Microplaque Trials Completed three Phase 1b microplaque trials with AN2728 in different formulations All trials showed statistically significant efficacy of AN2728 over vehicle Reduction in inflammatory infiltrate by ultrasound Reduction in clinical severity No application site reactions No treatment related adverse reactions No serious adverse events Established initial human proof of concept |
|
|
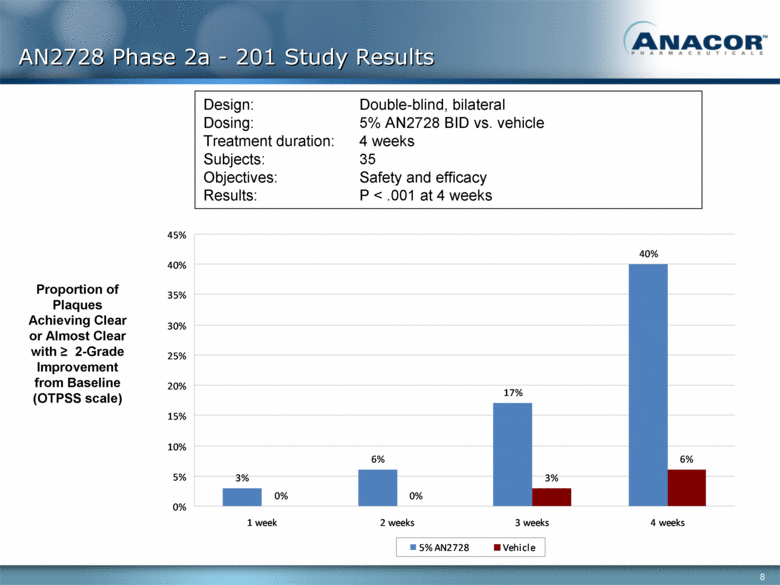
AN2728 Phase 2a - 201 Study Results Proportion of Plaques Achieving Clear or Almost Clear with > 2-Grade Improvement from Baseline (OTPSS scale) Design: Double-blind, bilateral Dosing: 5% AN2728 BID vs. vehicle Treatment duration: 4 weeks Subjects: 35 Objectives: Safety and efficacy Results: P < .001 at 4 weeks 3% 6% 17% 40% 0% 0% 3% 6% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 1 week 2 weeks 3 weeks 4 weeks 5% AN2728 Vehicle |
|
|
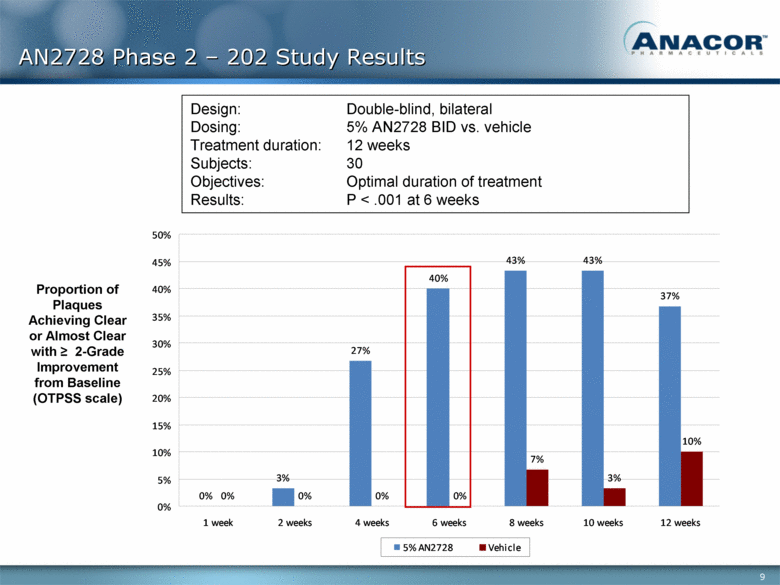
AN2728 Phase 2 – 202 Study Results Proportion of Plaques Achieving Clear or Almost Clear with > 2-Grade Improvement from Baseline (OTPSS scale) Design: Double-blind, bilateral Dosing: 5% AN2728 BID vs. vehicle Treatment duration: 12 weeks Subjects: 30 Objectives: Optimal duration of treatment Results: P < .001 at 6 weeks 0% 3% 27% 40% 43% 43% 37% 0% 0% 0% 0% 7% 3% 10% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% 1 week 2 weeks 4 weeks 6 weeks 8 weeks 10 weeks 12 weeks 5% AN2728 Vehicle |
|
|
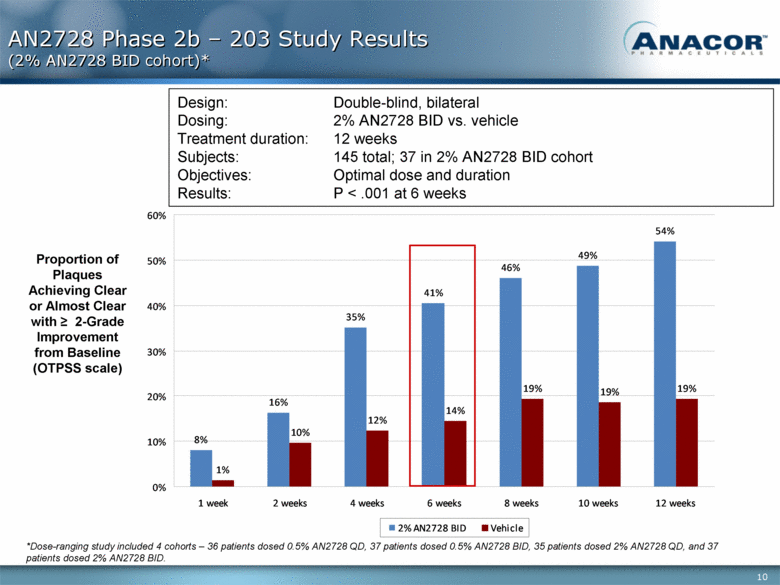
AN2728 Phase 2b – 203 Study Results (2% AN2728 BID cohort)* Proportion of Plaques Achieving Clear or Almost Clear with > 2-Grade Improvement from Baseline (OTPSS scale) Design: Double-blind, bilateral Dosing: 2% AN2728 BID vs. vehicle Treatment duration: 12 weeks Subjects: 145 total; 37 in 2% AN2728 BID cohort Objectives: Optimal dose and duration Results: P < .001 at 6 weeks *Dose-ranging study included 4 cohorts – 36 patients dosed 0.5% AN2728 QD, 37 patients dosed 0.5% AN2728 BID, 35 patients dosed 2% AN2728 QD, and 37 patients dosed 2% AN2728 BID. 8% 16% 35% 41% 46% 49% 54% 1% 10% 12% 14% 19% 19% 19% 0% 10% 20% 30% 40% 50% 60% 1 week 2 weeks 4 weeks 6 weeks 8 weeks 10 weeks 12 weeks 2% AN2728 BID Vehicle |
|
|
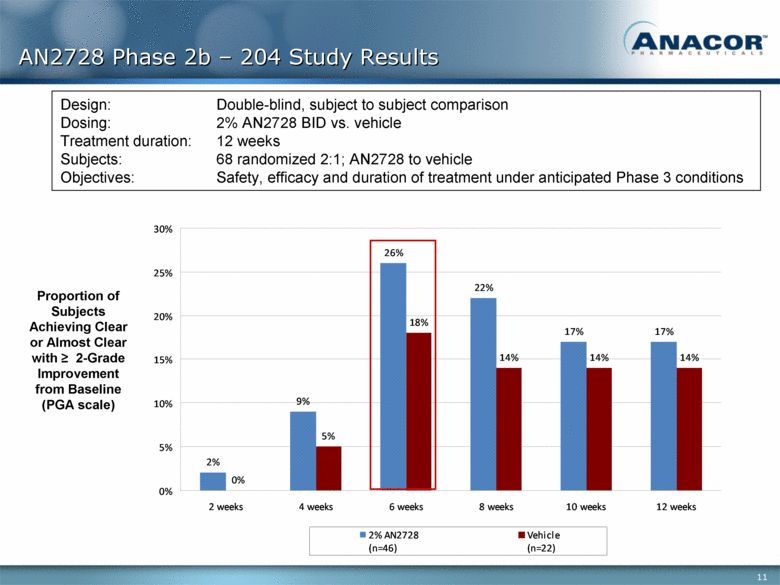
AN2728 Phase 2b – 204 Study Results Proportion of Subjects Achieving Clear or Almost Clear with > 2-Grade Improvement from Baseline (PGA scale) Design: Double-blind, subject to subject comparison Dosing: 2% AN2728 BID vs. vehicle Treatment duration: 12 weeks Subjects: 68 randomized 2:1; AN2728 to vehicle Objectives: Safety, efficacy and duration of treatment under anticipated Phase 3 conditions 2% 9% 26% 22% 17% 17% 0% 5% 18% 14% 14% 14% 0% 5% 10% 15% 20% 25% 30% 2 weeks 4 weeks 6 weeks 8 weeks 10 weeks 12 weeks 2% AN2728 (n=46) Vehicle (n=22) |
|
|
Geoff Parker Chief Financial Officer |
|
|
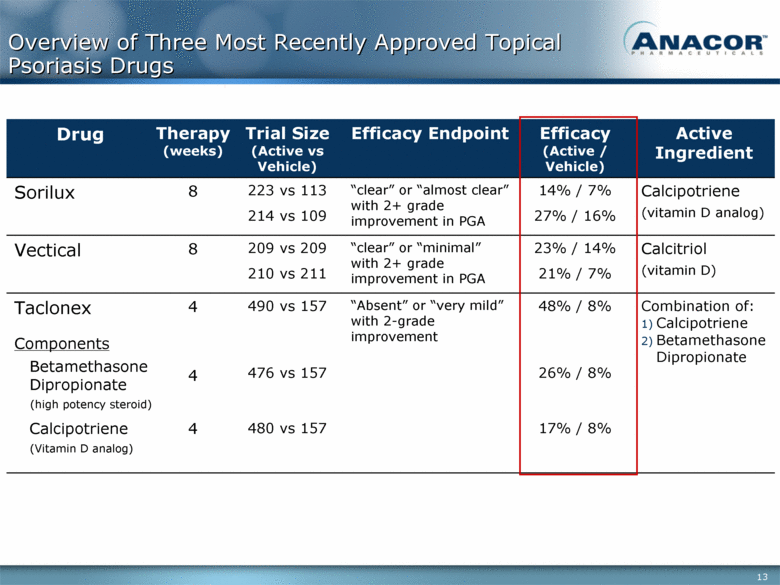
Overview of Three Most Recently Approved Topical Psoriasis Drugs Drug Therapy (weeks) Trial Size (Active vs Vehicle) Efficacy Endpoint Efficacy (Active / Vehicle) Active Ingredient Sorilux 8 223 vs 113 214 vs 109 “clear” or “almost clear” with 2+ grade improvement in PGA 14% / 7% 27% / 16% Calcipotriene (vitamin D analog) Vectical 8 209 vs 209 210 vs 211 “clear” or “minimal” with 2+ grade improvement in PGA 23% / 14% 21% / 7% Calcitriol (vitamin D) Taclonex 4 490 vs 157 “Absent” or “very mild” with 2-grade improvement 48% / 8% Combination of: 1) Calcipotriene 2) Betamethasone Dipropionate Components Betamethasone Dipropionate (high potency steroid) 4 476 vs 157 26% / 8% Calcipotriene (Vitamin D analog) 4 480 vs 157 17% / 8% |
|
|
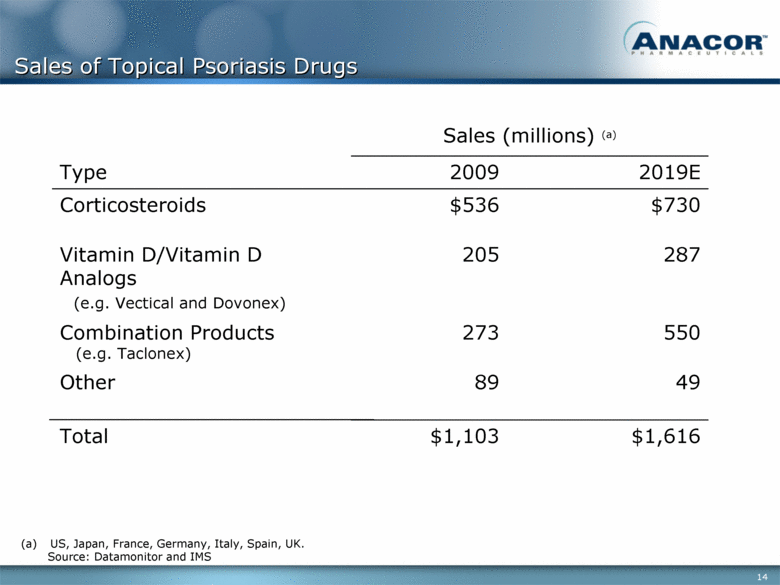
Sales of Topical Psoriasis Drugs Sales (millions) (a) Type 2009 2019E Corticosteroids $536 $730 Vitamin D/Vitamin D Analogs (e.g. Vectical and Dovonex) 205 287 Combination Products (e.g. Taclonex) 273 550 Other 89 49 Total $1,103 $1,616 (a) US, Japan, France, Germany, Italy, Spain, UK. Source: Datamonitor and IMS |
|
|
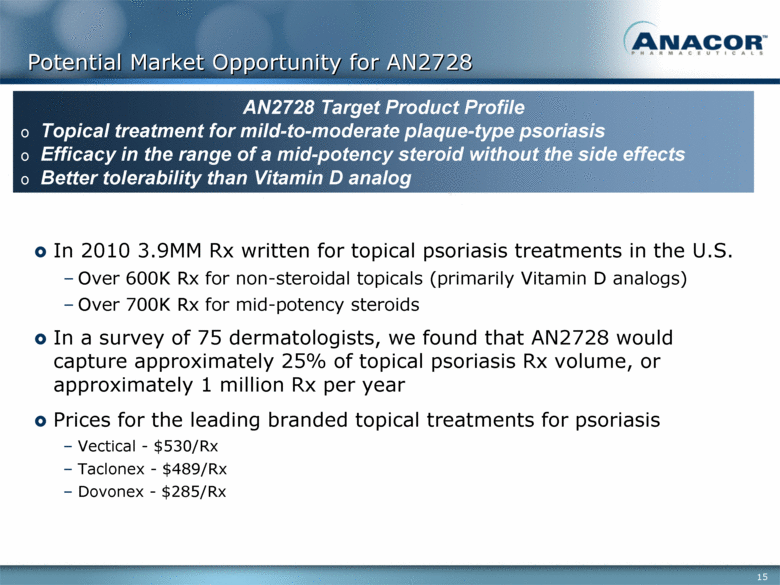
Potential Market Opportunity for AN2728 In 2010 3.9MM Rx written for topical psoriasis treatments in the U.S. Over 600K Rx for non-steroidal topicals (primarily Vitamin D analogs) Over 700K Rx for mid-potency steroids In a survey of 75 dermatologists, we found that AN2728 would capture approximately 25% of topical psoriasis Rx volume, or approximately 1 million Rx per year Prices for the leading branded topical treatments for psoriasis Vectical - $530/Rx Taclonex - $489/Rx Dovonex - $285/Rx AN2728 Target Product Profile Topical treatment for mild-to-moderate plaque-type psoriasis Efficacy in the range of a mid-potency steroid without the side effects Better tolerability than Vitamin D analog |
|
|
AN2728 Data to be Presented in 2011 Abstracts accepted for the European Society for Dermatological Research (ESDR) Annual Meeting September 7–10, 2011 (Barcelona, Spain) Safety and Efficacy of AN2728 Ointment in a 12-week, Randomized, Double-Blind, Vehicle-Controlled, Bilateral Trial for the Treatment of Psoriasis (Phase 2) Safety, Tolerability and Pharmacokinetics of AN2728 Ointment, a Potential Boron-based Treatment for Psoriasis (Phase 1) Dermal Tolerability and Preliminary Efficacy of AN2728 and AN2898, Novel Boron-Based Small Molecules, in the Treatment of Psoriasis (Phase 1) |
|
|
AN2728 Clinical Program Q&A June 28, 2011 |