Attached files
| file | filename |
|---|---|
| 8-K - Cytosorbents Corp | v227104_8k.htm |
| EX-99.3 - Cytosorbents Corp | v227104_ex99-3.htm |
| EX-99.1 - Cytosorbents Corp | v227104_ex99-1.htm |

CytoSorbents Investor Presentation
June 2011
OTCBB: CTSO
Cyto Sorbents Corporation
Working to Save Lives
Through
Blood Purification

2
Safe Harbor Statement
Statements in this presentation regarding CytoSorbents Corporation and its operating
subsidiary CytoSorbents, Inc that are not historical facts are forward-looking statements
and are subject
to risks and uncertainties that could cause actual future events or
results to differ materially from such statements. Any such forward-looking statements
are made pursuant to the safe harbor provisions of the Private Securities Litigation
Reform
Act of 1995. It is routine for our internal projections and expectations to change.
Although these expectations may change, we are under no obligation to inform you if
they do. Actual events or results may differ materially from those contained in the
projections or forward-looking statements. The following factors, among others, could
cause our actual results to differ materially from those described in a forward-looking
statement: our history of losses; potential fluctuations in our quarterly
and annual
results; competition, inability to achieve regulatory approval for our device, technology
systems beyond our control and technology-related defects that could affect the
companies’ products or reputation; risks related to adverse
business conditions; our
dependence on key employees; competition for qualified personnel; the possible
unavailability of financing as and if needed; and risks related to protecting our
intellectual property rights or potential infringement of the
intellectual property rights of
third parties. This list is intended to identify only certain of the principal factors that
could cause actual results to differ from those discussed
in the forward-looking
statements. Readers are referred to a discussion of important risk factors detailed in the
Company’s Form 10-K filed with the Securities and Exchange Commission
on May 31,
2011 and other reports and documents filed from time to time by us, which are available
online at www.sec.gov.

3
CytoSorbents Overview
CytoSorbents is a critical care-focused device company
using blood purification to treat disease, with its first
E.U. approved product, CytoSorb™
The heart of the technology is a biocompatible,
highly porous, polymer bead that can remove
a wide range of toxic substances such as
drugs, cytokines, and toxins
from blood and physiologic fluids
based on pore capture and surface adsorption

4
Obtain venous access with temporary dialysis catheter
Pump blood through the cartridge with standard pumps
The polymer beads remove toxic substances
“Purified” blood is pumped back into the patient
Can treat 20-30 total blood volumes per treatment
Each treatment uses a new cartridge
The polymer beads are packed into cartridges compatible
with standard hemodialysis machines or blood pumps
Concept is Simple But Elegant

5
Achieved European CE Mark approval for CytoSorb™ as an extracorporeal
cytokine filter, in clinical situations where cytokines are elevated
Completed our targeted 100 patient enrollment of our European Sepsis Trial
comparing CytoSorb hemoperfusion with SOC vs SOC alone
In an interim analysis, successfully achieved the primary endpoint of the trial,
demonstrating an average 49.1% IL-6 removal compared to control (p=0.01)
Currently ramping CytoSorb™ commercial production under ISO 13485 Full
Quality Systems certification of manufacturing and quality systems
Planning a controlled-market release of CytoSorb™ in Germany later this year
Responded to DARPA’s “Dialysis-Like Therapeutics” broad agency
announcement for new sepsis therapies as part of a multi-institutional
application and collaboration
Increased our IP portfolio to 29 issued US patents with multiple apps pending.
Broadened the applications for our technology
with an active R&D program
Recent Company Highlights

6
Management Team
Phillip Chan, MD, PhD – Chief Executive Officer and President
Board-certified internal medicine physician. MD/PhD from Yale School
of Medicine.
Internal Medicine residency at the Beth Israel Deaconess Medical Center at Harvard.
Former Partner at NJTC Venture Fund heading up healthcare and life science investments
for five years. Co-founder of the
venture-backed medical device firm, Andrew Technologies
Robert Bartlett, MD - Chief Medical Officer
World-renowned as the pioneer in extracorporeal membrane oxygenation
therapy (ECMO)
and the former Director of the Surgical Intensive Care Unit at University of Michigan, with
extensive experience in critical care medicine including the treatment of sepsis and
respiratory disease
Vincent Capponi, MS - Chief Operating Officer
More than 20 years experience in the medical device, pharmaceutical
and imaging fields at
Upjohn, Sims Deltec and Sabratek with strengths in operations and manufacturing
David Lamadrid, MBA - Chief Financial Officer
Over 18 years of business experience in finance and management, previously
at Chase
Manhattan Bank

7
Medical Advisory Board
John Kellum, MD
Emil Paganini, MD
Joseph Parrillo, MD
Claudio Ronco, MD
Thomas Stewart, MD
Professor of Critical Care Medicine & Anesthesiology
University of Pittsburgh Medical Center
Chair of the Sepsis Advisory Board
Former Section Head of Dialysis and Extracorporeal
Therapy with the Cleveland Clinic Foundation
Chief and Professor of Medicine
Director of the Cooper Heart Institute
University of Medicine and Dentistry of New Jersey
Editor-in-Chief of the journal Critical Care Medicine
Director, Dialysis and Renal Transplantation Programs of
St. Bartolo Hospital (Vicenza, Italy)
Associate Professor of Medicine and Anesthesiology
Director of Critical Care Medicine
Mount Sinai Hospital, University of Toronto

8
Critical Care Is a Lucrative Market
Critical care is a lucrative market for cost-effective therapies
Cost of treating US critical care patients was
$82 billion in 2005 or 0.7% of the US GDP *
In 2000, US hospitals lost $6 billion on Medicare
patients who spent at least 1 day in the ICU vs
$2 billion profit on those never in the ICU **
Reducing one day in the intensive care unit
saves ~$2,000 - 4,000 per patient per day
Approved products often achieve rapid market penetration due to
lack of competition
Sales and marketing can focus on a targeted group of hospital ICUs
* Halpern, NA, et al., Crit Care Med 2010, 38(1):65-71.
** Cooper, L, et al, Crit Care Med 2004, 32(11):2247-2253.

9
Critical Care Lacks “Active” Therapies
There are few “active” therapies available for serious diseases seen
in the intensive care unit
Severe sepsis and septic shock
Acute respiratory distress syndrome (ARDS)
Burn and smoke inhalation injury
Trauma
Influenza
Consequences of surgery
Severe acute pancreatitis
Many others

10
But Treatment Today is Mainly Supportive
Aside from antibiotics, current standard of care treatment is mainly
supportive care that helps keep the patient alive, but does not
necessarily hasten recovery or reverse the organ failure that can lead
to death
Patients have a very high risk of death due to multiple organ failure
and infection – two of the most common causes of death in the ICU
Better therapies are needed to prevent, mitigate or treat the root
causes of multiple organ failure and infection in order to change the
treatment paradigm of “watchful waiting and hoping for the best”
to “active treatment”
11
CytoSorbents is a Critical Care Pure Play
The cornerstone of our strategy to “actively treat”
critical care diseases is
CytoSorb TM
An efficient cytokine filter that can
potentially reduce deadly inflammation
caused by Cytokine Storm

CytoSorb TM - Approved For Sale in the E.U.
CytoSorb
TM has received European regulatory approval
under the CE Mark and can now be sold
throughout the European Union
Broad indication as an extracorporeal cytokine filter in situations
where cytokines are elevated. The only product, to our knowledge,
approved for this specific indication
Cytokine storm plays a critical damaging role in many
life-threatening illnesses commonly seen in the intensive care unit.
CytoSorb can be used “on-label” in these cases
CytoSorb has met the safety and efficacy label claims of the
European Medical Devices Directive
12

13
Cytokines: A Dual-Edged Sword
Cytokines are small proteins that, in moderation,
normally help stimulate and regulate the
immune system. They are required for proper
immune system function
However, in excess, cytokines can cause
disease
But in vast excess, often called “cytokine storm”,
cytokines can cause organ injury and failure,
and often death

14
Shock
Clotting
Cytokine Storm Leads to Organ Failure
Cytokines
Inflammation, Organ Failure and Infection
Lung Injury
Cell Death
Intestinal
Injury
Immune
Paralysis

15
Cytokine Storm in Action
TeGenero Anti-CD28 monoclonal antibody Phase I Trial
Designed to stimulate the immune system to treat cancer
Phase I safety trial (2006) – 6 healthy adult males
90 minutes: All had systemic inflammatory response
Headache, nausea, muscle pain, diarrhea, hypotension
12-16 hours: All became critically ill
Severe lung injury, renal failure, DIC
Cytokine storm persisted for 2-3 days
Two with the worst cytokine storm had prolonged shock and severe lung injury
Showed clearly that cytokine storm, and not infection or cancer, was the
cause of multiple organ failure
New England Journal of Medicine, 2006:355:1018-28

16
Treating Cytokine Storm – The Holy Grail
Reduction in Cytokine Storm has been the
target of the industry for three decades
Unfortunately, most drugs and biologics can only reduce a single
cytokine or inflammatory mediator
Too much redundancy in the immune system for this strategy to work
A Broad Spectrum Solution is needed that can
remove many different cytokines and toxins
CytoSorb TM is a leader in this field

Blood Flow
Simulated for illustrative
purposes only
17

18
CytoSorb TM Active in Cytokine Sweet Spot
Hemodialysis
0
kDa
10
20
30
40
50
60
70
IL-1ßTNF-a monomer
IFN- monomer
IL-1Ra
IL-10
TNF-a trimer
IFN- dimer
HMGB1
TGF-ß
IL-6
sFas ligand
MCP-1
IL-13
MCP-1
glycosylated
Albumin
IL-8
MIP-1a
IL-18
sTNFR
IL-4
G-CSF

CytoSorb IL-6 Removal from Whole Blood
In vitro circulation system scaled for human treatment
19

20
CytoSorb is State of the Art Technology
CE Mark approved
Very efficient at removing cytokines from whole blood
Biocompatible and hemocompatible – ISO 10993
Good safety track record – 160 canine, >650 human treatments
Massive capacity
Easier to use than dialysis
Compatible with standard hospital dialysis equipment
Inherently high gross margins
3-year shelf life at room temperature
ISO 13485 Full Quality Systems certified re: manufacturing and
quality systems

The Sepsis Crisis
Severe sepsis and septic shock are often the result
of an “overwhelming infection”
Afflicts 18 million people worldwide every year and is growing
1 million in the U.S. and 1.5 million in Europe annually
Severe sepsis kills 1 in every 3 despite the best medical treatment.
Septic shock kills 1 in every 2
Kills more people in the U.S. than either heart attacks, strokes, or any
single type of cancer
Only Xigris has been approved to treat sepsis, but not widely adopted
with only $104M in worldwide sales in 2010 due to concerns of cost,
efficacy and safety
21

Time (days to weeks)
Sepsis has two facets but only one is treated
Pro-Inflammatory
Anti-inflammatory
Organ Failure
Death
Recovery
TOXIC
Cytokine Storm
The infectious insult can be treated with antibiotics/antivirals,
but there are currently no therapies to treat cytokine storm
22

CytoSorb Has Strong Animal Data
CytoSorb animal models simulating sepsis from a ruptured appendix
with no antibiotics and only a single 3-hour treatment…
Increased survival to 65% (treated) from 12.5% (sham)
Peng, ZY, Carter, MJ, Kellum, JA “Effects of hemoadsorption on cytokine
removal and short term survival in rats” Crit Care Med (2008) 36(5): 1573-7
23

…with prevention of shock
Peng, ZY, Carter, MJ, Kellum, JA “Effects of hemoadsorptionon cytokine removal and short term survival
in rats” Crit Care Med (2008) 36(5): 1573-7
Effects of hemoadsorption
on mean arterial pressure
(MAP) (Mean +/- SEM, mm
Hg) in the CLP rat sepsis
model. Hemoadsorption
is shown in the dashed
line while sham results
are
shown in the solid line.
MAP was significantly
different over time and
between groups (p<0.01).
For pair-wise
comparisons:
*hemoadsorption vs sham
(p<0.05) and T compared
with baseline
(p<0.05)
CytoSorb
Sham
24
Note the stabilizing effect on blood pressure persists 6 hours after the
3 hour CytoSorb treatment compared to the rapid decline in the
control animals
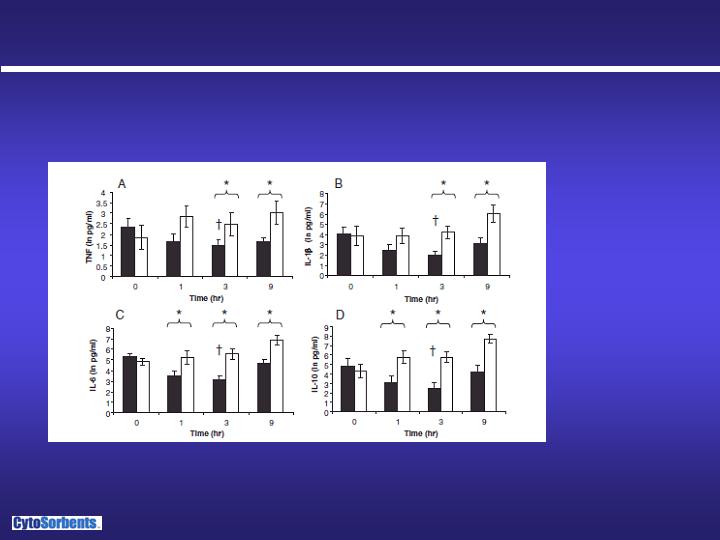
…and reduction of cytokines
20 hours after cecal ligation and puncture, rats were treated for 3 hours with
either CytoSorb or sham. Treatment was discontinued and cytokine values
were taken again 6 hours
after the end of treatment.
Peng, ZY, Carter, MJ,
Kellum, JA “Effects of
hemoadsorption on
cytokine removal and
short term survival in
rats” Crit Care Med
(2008) 36(5): 1573-7
25
Natural log scale demonstrates a 50-80% reduction of cytokines over
the 3 hour treatment and persistent reduction 6 hours after treatment
cessation, compared to sham, demonstrating control
of cytokine storm
Black bars = CytoSorb
White bars = Sham

26
Conducted a human sepsis pilot comparing standard of care
therapy versus standard of care plus Cyto
Sorb treatment in
patients with severe sepsis and respiratory failure
All data from a single major academic site (6 treated, 6 control)
demonstrated substantial improvements in
CytoSorb treated patients
compared to controls
28-day and 60-day all cause mortality
Ventilator free days
Pace of ventilator weaning
Organ failure scores
Vasopressor use
Days in the intensive care unit
No serious device related adverse events
Positive Human Proof-of-Concept

27
CytoSorbTM European Sepsis Trial
Completed a 100 patient randomized,
controlled clinical trial to treat patients with
severe sepsis or septic shock in the setting
of
acute respiratory distress syndrome/ALI
Compared standard of care therapy alone with
standard of care therapy plus CytoSorb treatment
Successfully achieved the primary endpoint of the trial, reducing IL-6 by
an average of 49.1% (p=0.01) during the CytoSorb treatment period
compared to controls
Data on secondary clinical endpoints is still pending data monitoring
Treatment was well-tolerated by patients
No serious device related adverse events in more than 650 human
treatments, of which more than 300 have been in septic patients

CytoSorb TM Controlled Market Release in 2011
Focus will initially be on Germany – the largest medical device market
in the E.U. and the third largest in the world
Will conduct a 3-6 month controlled market release of CytoSorb™ in
select areas in Germany later in 2011, before a broader launch in
Germany in 2012
Plan to sell direct, targeting the main 380 hospitals in Germany that
have more than 400 beds each
Currently ramping manufacturing of CytoSorb™ at our manufacturing
facility in New Jersey under CE Mark and ISO 13485 Certification
Building the infrastructure to ensure a smooth transition to a
commercialization stage company
28

Why Germany?
With a population of 82M, and € 250 Billion
spent annually on healthcare, Germany is the
largest medical device market in the E.U.
We have experience in Germany, working with
major thought leaders in sepsis research during
our European Sepsis Trial, many of whom are
part of the SepNet Sepsis Trial Network
Medical practice and technologies are advanced, with intensivists
routinely performing extracorporeal therapies, and ICUs are modern
We have a good understanding of the medical system and
reimbursement process
Sepsis is prevalent, with ~154,000 new cases per year, and remains
a major unmet medical need with mortality of ~40%
Many opportunities exist to use CytoSorb in critical care illnesses
with a cumulative total addressable market approaching €1 Billion
29

Future Commercialization Plans
CytoSorbents intends to expand its footprint in Europe
and in other countries that accept CE Mark approval
Currently working to establish relationships with independent
distributors in other key countries
Targeting strategic partnership or distributorships that could
help further drive market adoption and distribution
After successful commercialization in the E.U., the Company intends
to conduct clinical registration trials in the U.S. to pursue FDA
approval of its products
30

31
CytoSorbents seeks partners for its growth technology portfolio
protected by 29 issued patents and multiple
applications pending
CytoSorb™ has blockbuster potential as a cytokine filter
US and EU total addressable market for these apps is ~ $10-15 billion
Limited competition
Differentiated, “best of breed” approach
Highly profitable “razor/razorblade” business model
Near-term commercialization
Favorable DRG reimbursement in US and Germany
Can impact top and bottom line growth of any company in this field
CytoSorbents Seeks Strategic Alliances
32
Cyto Sorbents has a robust, innovative pipeline with
strong development capabilities
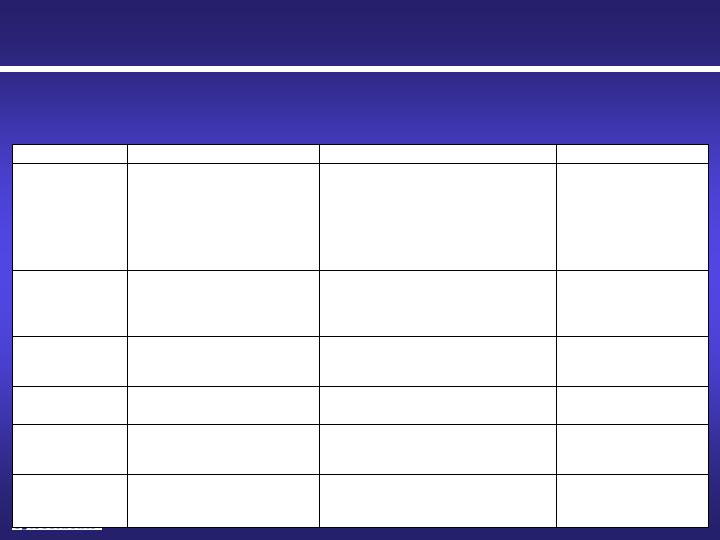
Cyto Sorbents Pipeline
Four human pilot studies
completed
Removal of mid-molecular weight toxins
that are inefficiently removed by standard
dialysis
Improvement of hemodialysis in
end-stage renal disease
BetaSorb™
NAME
INDICATION
DESCRIPTION
STATUS
CytoSorb™
Severe sepsis and septic shock
ARDS/ acute lung injury
Burn and smoke inhalation injury
Trauma
Severe acute pancreatitis
Complications of influenza
Autoimmune disease flares
Highly efficient cytokine filter that is
designed to treat cytokine storm and
inflammation
European CE Mark
approved as a cytokine
filter in cases where
cytokines are elevated
CytoSorb™
Cardiac Surgery
Protection of organ transplants
Highly efficient cytokine filter to reduce
cytokine-induced organ injury
Observational human
study completed
Human pilot study
completed
CST 101
Drug overdose, chemical
exposure or intoxication
Efficient single pass removal of a number
of drugs from blood
Pre-clinical proof of
concept completed
CST 201
Adjunct cancer treatment:
Chemotherapy removal during
high dose regional chemotherapy
Efficient single pass removal of certain
chemotherapy agents from blood
Pre-clinical proof of
concept completed
CST 301
Trauma
Removal of myoglobin from blood caused
by muscle breakdown and
rhabdomyolysis in trauma
Pre-clinical proof of
concept completed

33
CytoSorb
is uniquely designed to address the key
reasons why our wounded warfighters die
Many Applications for Military Use
Severe sepsis and septic shock from
complex, penetrating wound injuries
Blast burn and smoke inhalation injuries
from incendiary munitions, IED and
projectile explosions, and gasoline and
vehicle fires
Polytrauma and rhabdomyolysis from
hemorrhage, blunt trauma, crush injury,
percussive shock injuries, organ rupture
and amputations
CytoSorbents is pursuing both military and
government funding of its technologies

34
CytoSorb™ – A Powerful New Approach
CytoSorb has the potential to help save lives
by reducing the cytokine storm that leads to
multi-organ failure and death from many causes
CytoSorb™
Sepsis
ARDS/ALI
Burns
Smoke
Biowarfare
Trauma
Pancreatitis
Influenza

35
ISO 13485:2003 certification
Successful trial completion
CE Mark approval
Expansion of pipeline opportunities
European distribution and sales
Out-licensing of technologies
Commencement of a US trial
Government and/or military funding
Stock listing on a national exchange
CytoSorbents Goals and Milestones 2011-12
With success, Cyto Sorbents seeks to transition from a
development stage to a commercialization stage company
with revenue – a major inflection point for the company
CytoSorbents has made excellent progress towards
its stated goals and milestones
Phillip P. Chan, MD, PhD
CEO and President
7 Deer Park Drive, Suite K
Monmouth Junction, NJ 08852
(732) 329-8885
pchan@cytosorbents.com
CytoSorbents Corporation
Working to Save Lives
Through
Blood Purification
OTCBB: CTSO
