Attached files
| file | filename |
|---|---|
| EX-1.1 - FORM OF UNDERWRITING AGREEMENT - Aegerion Pharmaceuticals, Inc. | dex11.htm |
| EX-23.2 - CONSENT OF ERNST & YOUNG LLP - Aegerion Pharmaceuticals, Inc. | dex232.htm |
Table of Contents
As filed with the Securities and Exchange Commission on June 20, 2011
Registration No. 333-174944
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AEGERION PHARMACEUTICALS, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 2834 | 20-2960116 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
101 Main Street, Suite 1850,
Cambridge, Massachusetts 02142
(617) 500-7867
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Marc D. Beer
Chief Executive Officer
Aegerion Pharmaceuticals, Inc.
101 Main Street, Suite 1850,
Cambridge, Massachusetts 02142
(617) 500-7867
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Copies to:
| Jocelyn M. Arel, Esq. Goodwin Procter LLP 53 State Street Boston, Massachusetts 02109 (617) 570-1000 |
Christine A. Pellizzari, Esq. Executive Vice President, General Counsel and Secretary Aegerion Pharmaceuticals, Inc. 135 US Highway 202/206 South, Suite 15, Bedminster, New Jersey 07921 (908) 707-2100 |
Donald J. Murray, Esq. Dewey & LeBoeuf LLP 1301 Avenue of the Americas New York, New York 10019-6092 (212) 259-8000 |
Approximate date of commencement of proposed sale to public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ |
Accelerated filer ¨ | |||
| Non-accelerated filer x (Do not check if a smaller reporting company) | Smaller reporting company ¨ | |||
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered |
Proposed Maximum Aggregate Offering Price(1) (2) |
Amount of Registration Fee(3)(4) | ||
| Common Stock, par value $0.001 per share |
$86,606,500 | $10,055.01 | ||
| (1) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933. |
| (2) | Includes shares of common stock that the underwriters have an option to purchase to cover over-allotments, if any. |
| (3) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price. |
| (4) | A registration fee of $9,595.39 has been paid previously in connection with this Registration Statement based on an estimate of the aggregate offering price. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JUNE 20, 2011
PRELIMINARY PROSPECTUS
4,250,000 Shares

Common Stock
We are offering 3,250,000 shares of common stock, and the Selling Stockholders identified in this prospectus are offering an additional 1,000,000 shares of our common stock. We will not receive any of the proceeds from the sale of the shares being sold by the Selling Stockholders.
Our common stock is quoted on The NASDAQ Global Market under the symbol “AEGR.” On June 17, 2011, the last reported sale price of our common stock was $17.72 per share.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 9.
| Per Share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
| Proceeds, before expenses, to the Selling Stockholders |
$ | $ | ||||||
The underwriters may also purchase up to an additional 637,500 shares from us and certain of the Selling Stockholders, at the public offering price, less the underwriting discounts and commissions, within 30 days of the date of this prospectus to cover over-allotments, if any. If the underwriters exercise this option in full, the total underwriting discounts and commissions will be $ , total proceeds to the Selling Stockholders, before expenses, will be $ , and total proceeds to us, before expenses, will be $ .
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock on or about , 2011.
Joint Book-Running Managers
| Jefferies | Deutsche Bank Securities |
Co-Managers
| Leerink Swann | Needham & Company, LLC | Collins Stewart |
The date of this prospectus is , 2011.
Table of Contents
| Page | ||||
| 1 | ||||
| 9 | ||||
| 40 | ||||
| 42 | ||||
| 44 | ||||
| 44 | ||||
| 45 | ||||
| 47 | ||||
| 49 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
51 | |||
| 65 | ||||
| 91 | ||||
| 96 | ||||
| 113 | ||||
| 120 | ||||
| 123 | ||||
| 128 | ||||
| 132 | ||||
| 135 | ||||
| 135 | ||||
| 135 | ||||
| F-1 | ||||
You should rely only on the information contained in this prospectus and any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not authorized anyone to provide you with information that is different. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock.
For investors outside the United States: Neither we, the Selling Stockholders nor any of the underwriters have taken any action to permit a public offering of the shares of our common stock or the possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. Before you decide to invest in our common stock, you should read the entire prospectus carefully, including the “Risk Factors” section and the financial statements and related notes appearing at the end of this prospectus.
Our Company
We are an emerging biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat severe lipid disorders. Lipids are naturally occurring molecules, such as cholesterol and triglycerides that are transported in the blood. Our lead compound, lomitapide, is a microsomal triglyceride transfer protein inhibitor, or MTP-I, which limits secretion of cholesterol and triglycerides from the intestines and the liver, the main sources of circulating lipids in the body. We are initially developing lomitapide as an oral, once-a-day treatment for patients with a rare genetic lipid disorder called homozygous familial hypercholesterolemia, or HoFH. These patients are at very high risk of experiencing life threatening events at an early age as a result of extremely elevated cholesterol levels in the blood and, as a result have a substantially reduced life span relative to unaffected individuals. We believe that lomitapide, either on a stand-alone basis or in combination with other drugs, has the potential to help these patients achieve recommended target levels of low-density lipoprotein cholesterol, or LDL-C.
We are currently evaluating lomitapide in a pivotal Phase III clinical trial for the treatment of patients with HoFH. On May 31, 2011, we announced the results of this trial, through 56 weeks of treatment. We believe based on our prior discussions with the U.S. Food and Drug Administration, or FDA, that these results demonstrate sufficient long-term safety and efficacy to support the submission of our New Drug Application, or NDA, for lomitapide. We refer to these week 56 results as our Filing Data, and we will later supplement the Filing Data with data reflecting the full 78-week trial duration. Before we can submit an NDA, we must complete additional clinical and non-clinical studies to assess various other aspects of lomitapide. On June 15, 2011, we met with the FDA, which informed us that it is not opposed to our submitting our NDA based on the Filing Data. We plan to submit our NDA to the FDA and a Marketing Authorization Application, or MAA, to the European Medicines Agency, or EMA, before the end of 2011.
Assuming we obtain approval, in anticipation of our commercial launch of lomitapide initially in the United States and European Union, we have begun to recruit a team of sales representatives and medical education specialists who are experienced in marketing drugs for the treatment of rare, often genetic, disorders. We initially plan to hire a medical education, marketing and sales force of approximately 15 people in the United States and approximately 18 people in the European Union. We also are evaluating other markets to determine other geographies where we will commercialize lomitapide, either alone or in partnership with others.
We are planning a Phase III pediatric clinical trial to evaluate lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH. In addition to HoFH, we are also in the process of developing a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with a severe genetic form of elevated triglycerides, or hypertriglyceridemia, called familial chylomicronemia, or FC. In October 2010, the EMA granted lomitapide orphan drug designation for the treatment of FC. In March 2011, the FDA granted lomitapide orphan drug designation for the same indication. In October 2007, the FDA granted lomitapide orphan drug designation for the treatment of HoFH. In the United States, orphan drug designation is given to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States. If our NDA for lomitapide receives the first FDA approval for the disease for which it has such designation, it is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. The EMA generally grants orphan drug designation to drugs that may offer therapeutic benefits for life-threatening or chronically debilitating conditions affecting not more than five in
1
Table of Contents
10,000 people in the European Union. In the European Union, orphan drug designation provides ten years of market exclusivity following drug approval, although the exclusivity period may be reduced to six years if the designation criteria are no longer met.
To date, we have not generated revenue from the sale of any product, and we do not expect to generate significant revenue unless and until we obtain marketing approval of, and commercialize, lomitapide. As of March 31, 2011, we had an accumulated deficit of $97.8 million.
Lomitapide
We are currently evaluating lomitapide in an ongoing pivotal Phase III clinical trial for the treatment of patients with HoFH. We completed enrollment for this single-arm, open-label trial in March 2010 with a total of 29 patients. Three of these patients withdrew their consent to participate in the trial and three patients discontinued treatment due to gastrointestinal adverse events. The 23 patients remaining in the trial have completed the 26 week period of therapy after which the primary efficacy endpoint was measured, and the 56 week period of therapy during which the Filing Data were collected. As of the date of this prospectus, 19 of these patients have completed the entire 78 weeks of the trial. We expect to complete this trial in the third quarter of 2011.
Researchers at the University of Pennsylvania, or UPenn, completed a Phase II clinical trial of lomitapide for the treatment of patients with HoFH in 2004. In addition to the UPenn trial, lomitapide has been evaluated in 13 Phase I and five Phase II clinical trials. A total of 915 patients were treated with lomitapide in these Phase I and Phase II trials, including the patients in the UPenn trial. Currently, there are no MTP-Is approved by the FDA for any indication.
Early clinical trials of lomitapide produced meaningful percent reductions in LDL-C levels, but patients discontinued use of lomitapide at a high rate due to gastrointestinal adverse events, such as diarrhea, nausea and vomiting. In addition, a small proportion of patients experienced elevations in liver enzymes and increased mean levels of fat in the liver, or hepatic fat, both of which have also been observed in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH. We believe the high rate of discontinuations due to gastrointestinal adverse events in the early clinical trials of lomitapide resulted in large part from the failure to employ dose titration, which is the gradual increase in dosing over time to allow the body to adapt to the impact of a higher dose coupled with a low fat diet. Patients in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, where dose titration has been employed, have also experienced adverse gastrointestinal events, but to a lesser extent than those experienced in the earlier clinical trials.
Patient Populations of Interest
We are initially developing lomitapide for the treatment of the small number of patients with the rare genetic disorder HoFH. Patients with untreated HoFH have extremely high LDL-C levels, typically between 500 mg/dL and 1,000 mg/dL, and, as a result, are at severely high risk of experiencing premature cardiovascular events, such as a heart attack or stroke. In a report that we commissioned, L.E.K. Consulting LLC, or LEK, an international business consulting firm, estimated that the total number of addressable patients with symptoms consistent with HoFH in each of the United States and, collectively, Germany, the United Kingdom, France, Italy and Spain, which are referred to in this prospectus as the European Union Five, is approximately 3,000 patients, or a combined total of approximately 6,000 patients. LEK’s estimate of the addressable patient population is primarily based on, among other things, physician estimates of patients who have symptoms customarily present in patients definitively diagnosed with HoFH, meaning patients with one of the following: (i) documented functional mutations in both LDL receptor alleles or alleles known to affect LDL receptor functionality, (ii) skin fibroblast LDL receptor activity that is 20 percent less than normal, (iii) untreated total cholesterol greater than 500 mg/dL and triglycerides less than 300 mg/dL with both parents having documented total cholesterol greater than 250 mg/dL or (iv) average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy as decided by the treating physician. Our pivotal Phase III clinical trial specifically included patients
2
Table of Contents
meeting criteria (i), (ii) or (iii). While many patients in our ongoing pivotal Phase III clinical trial also met diagnostic criterion (iv), it was not explicitly one of the inclusion criteria for the trial. The FDA has stated that defining HoFH patients as those with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy, in other words diagnosing patients through method (iv), closely resembles the severe refractory heterozygous familial hypercholesterolemia population. Therefore, these patients may be outside of the HoFH population. The total addressable market opportunity for lomitapide for the treatment of patients with HoFH will ultimately depend upon, among other things, the diagnosis criteria included in the final label for lomitapide, if approved for sale in this indication, acceptance by the medical community, patient access, product pricing and reimbursement.
We also believe that lomitapide has the potential to treat patients with the rare genetic disorder FC, who have extremely high levels of blood triglycerides, or TGs, generally greater than 2,000 mg/dL. These patients are at an increased risk of developing acute pancreatitis, a significant and sometimes life-threatening inflammation of the pancreas. In the report that we commissioned, LEK estimated that, subject to certain factors, there are a total of approximately 1,000 patients in the United States and the European Union Five with FC who could be eligible for treatment with lomitapide.
We believe that lomitapide may also be useful for the treatment of elevated lipid levels in broader patient populations, such as those suffering from heterozygous familial hypercholesterolemia, patients who are statin intolerant and patients with severe hypertriglyceridemia that is brought on by factors other than FC. If we elect to develop lomitapide for broader patient populations, we would plan to do so selectively either on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources.
Limitations of Currently Available Treatment Options
Currently available treatment options for patients with HoFH are extensive but, even when combined together, are often ineffective in significantly reducing LDL-C levels to recommended target levels. The clinical approach for patients with HoFH typically involves an aggressive treatment plan to reduce lipid levels as much as possible through dietary modifications, a combination of available lipid lowering drug therapies and, in many cases, plasma apheresis, which is a mechanical filtration of the blood similar to kidney dialysis. Drug therapies include statins, cholesterol absorption inhibitors and bile acid sequestrants.
Similarly, currently available treatment options for patients with FC are often ineffective in lowering TG levels to recommended target levels. The clinical approach for patients with FC, whose TG levels are generally greater than 2,000 mg/dL, typically involves dietary modifications to lower the intake of dietary fat, the use of omega-3 fatty acids and fibrates. However, these treatments are often inadequate to lower TG levels below 500 mg/dL, the level at which patients are at an increased risk for developing acute pancreatitis. Because of the severely elevated TG levels in this patient population, reducing TG levels to this extent may require reductions in TG levels of 75% or more from baseline.
Our Strategy
Our objective is to develop and commercialize drugs to treat patients with rare lipid related disorders who are at very high risk of experiencing life threatening events at an early age. To achieve this objective, we plan to:
| § | Prepare and submit our NDA for lomitapide for the treatment of patients with HoFH based on our Filing Data. |
| § | Complete our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH and, submit the data collected through week 78 of this trial to the FDA to complete our NDA submission and to the EMA to complete our MAA submission. |
| § | Prepare to commercialize lomitapide for the treatment of patients with HoFH through our planned direct medical education, marketing, and sales force in the Unites States and the European Union. |
3
Table of Contents
| § | Initiate a Phase III pediatric clinical trial in pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH. |
| § | Initiate a Phase III clinical trial of lomitapide for the treatment of adult patients with FC. |
| § | Apply to make lomitapide available for use in France under a cohorte Autorisation Temporaire d’Utilisation or Temporary Authorization to Use, or ATU, to permit the compassionate use of a non-approved drug. |
| § | Selectively seek to expand our geographic distribution capabilities and potentially address broader patient populations for lomitapide. |
Risks Associated with Our Business
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary, including the following:
| § | We currently depend entirely on the success of our lead compound, lomitapide, which we are developing initially for the treatment of patients with HoFH. We are also developing a protocol for a clinical trial of lomitapide for the treatment of adult patients with FC. We may not receive marketing approval for, or successfully commercialize, lomitapide for any indication. |
| § | The numbers of patients suffering from HoFH and FC are small and have not been established with precision. If the actual number of patients with either of these conditions is smaller than we estimate or if any approval that we obtain is based on a narrower definition of these patient populations, our revenue and ability to achieve profitability will be adversely affected, possibly materially. For example, the FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. This means that these patients may ultimately be considered to be outside the HoFH population, reducing the estimated 6,000-patient addressable population. |
| § | In earlier preclinical studies and clinical trials, lomitapide was associated with undesirable side effects, such as adverse gastrointestinal events, elevated liver enzymes and increases in mean hepatic fat levels. Patients in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH have also experienced such undesirable side effects. Lomitapide may continue to cause such side effects or have other properties that could delay or prevent its marketing approval or result in adverse limitations in any approved labeling or on distribution and use of the product. |
| § | We plan to submit our NDA for lomitapide based on our Filing Data, which is prior to completion of our pivotal Phase III clinical trial. If data obtained during the last 22 weeks of the trial are unfavorable, our ability to commercialize lomitapide may be adversely affected. |
| § | Failures or delays in the commencement or completion of preclinical or clinical testing could result in increased costs to us and delay, prevent or limit our ability to generate revenue. |
| § | The preparation and submission of our NDA may take longer than we currently believe it will, which could result in increased costs to us and delay, prevent or limit our ability to generate revenue. |
| § | Our NDA may be subject to review by an FDA advisory committee, which may not render positive feedback to the FDA or could result in increased costs to us and delay, prevent or limit our ability to generate revenue. |
| § | If we receive marketing approval for lomitapide or any other product candidate, our sales will be limited unless the product achieves broad market acceptance for its indications. |
4
Table of Contents
| § | If we fail to obtain or maintain orphan drug exclusivity for lomitapide, we will have to rely on our data and marketing exclusivity, if any, and on our intellectual property rights, which may reduce the length of time we can prevent competitors from selling lomitapide. |
| § | We currently depend on a single third-party manufacturer to produce our preclinical and clinical drug supplies and intend to rely upon third-party manufacturers to produce commercial supplies of lomitapide. This may increase the risk that we will not have sufficient quantities of lomitapide or such quantities at an acceptable cost, which could delay, prevent or impair our clinical development and commercialization of lomitapide. |
| § | We may not obtain an ATU, or obtaining an ATU may adversely affect our ability to set market prices outside of France, either of which could have an adverse effect on our financial results. |
Corporate Information
We were founded in 2005 as a Delaware corporation. Our principal executive offices are located at 101 Main Street, Cambridge, Massachusetts 02142, and our telephone number is (617) 500-7867. Our web site address is www.aegerion.com. The information on, or that can be accessed through, our web site is not part of this prospectus. We have included our web site address as an inactive textual reference only.
Aegerion is a registered trademark of Aegerion Pharmaceuticals, Inc. in the United States and a trademark in other countries. This prospectus also includes other trademarks of Aegerion Pharmaceuticals, Inc. and other persons. Except where the context requires otherwise, in this prospectus “Company,” “Aegerion,” “we,” “us” and “our” refer to Aegerion Pharmaceuticals, Inc.
5
Table of Contents
THE OFFERING
| Common stock offered by us |
3,250,000 shares |
| Common stock offered by the Selling Stockholders |
1,000,000 shares |
| Common stock to be outstanding after this offering |
20,914,641 shares |
| Over-allotment option |
We and the Selling Stockholders have granted the underwriters an option for 30 days from the date of this prospectus to purchase up to 637,500 additional shares of common stock to cover over-allotments. |
| Use of proceeds |
We plan to use the net proceeds of this offering to prepare and submit our NDA and complete the marketing approval process, to complete clinical and non-clinical studies needed for our NDA submission and our pivotal Phase III clinical trial of lomitapide for the treatment of HoFH, to commercially launch lomitapide for the treatment of patients with HoFH, to advance the clinical development of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH and of adult patients with FC and any remainder to fund working capital, capital expenditures and other general corporate purposes, including repayment of the principal loan balance as it comes due under our loan and security agreement, or the Loan and Security Agreement, with Hercules Technology II, L.P. and Hercules Technology III, L.P., collectively the Hercules Funds. We will not receive any proceeds from the shares sold by the Selling Stockholders. For a more complete description of our intended use of the net proceeds from this offering, see “Use of Proceeds.” |
| NASDAQ Global Market symbol |
“AEGR” |
| Risk factors |
You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
The number of shares of common stock to be outstanding after this offering is based on 17,664,641 actual shares of common stock outstanding as of May 31, 2011. The number of shares of common stock to be outstanding after this offering excludes:
| § | 2,174,642 shares of common stock issuable upon the exercise of options outstanding as of May 31, 2011 at a weighted average exercise price of $4.79 per share; |
| § | 29,890 shares of restricted common stock subject to vesting as of May 31, 2011; |
| § | 107,779 shares of common stock subject to outstanding warrants outstanding as of May 31, 2011, with a exercise price of $6.68 per share; and |
| § | 2,814,971 additional shares of common stock available for future issuance under our 2010 Stock Option and Incentive Plan, or 2010 Option Plan, as of May 31, 2011, which is subject to an annual increase of shares of common stock available for issuance under the 2010 Option Plan equal to four percent of the number of shares of common stock issued and outstanding on the immediately preceding December 31. |
Except as otherwise indicated, all information in this prospectus reflects or assumes no exercise of the underwriters’ over-allotment option.
6
Table of Contents
SUMMARY FINANCIAL DATA
You should read the following summary financial data together with the “Capitalization,” “Selected Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our financial statements and the related notes appearing at the end of this prospectus. We have derived the statement of operations data for the years ended December 31, 2008, 2009 and 2010 from our audited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the three months ended March 31, 2010 and 2011 and the balance sheet data as of March 31, 2011 from our unaudited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the period from February 4, 2005 (inception) to March 31, 2011 from our unaudited financial statements appearing at the end of this prospectus. The unaudited financial statements have been prepared on the same basis as our audited financial statements and include, in the opinion of management, all adjustments that management considers necessary for a fair presentation of the financial information set forth in those statements. Our historical results for any prior period are not necessarily indicative of results expected in any future period and our interim results are not necessarily indicative of results for a full year.
| Year Ended December 31, | Three Months Ended March 31, |
Period from February 4, 2005 (inception) to March 31, |
||||||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | 2011 | |||||||||||||||||||
| (in thousands, except share and per share data) | (Unaudited) | |||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||
| Costs and Expenses: |
||||||||||||||||||||||||
| Research and Development |
$ | 17,712 | $ | 7,041 | $ | 7,629 | $ | 1,066 | $ | 3,297 | $ | 53,164 | ||||||||||||
| General and administrative |
5,185 | 3,075 | 5,921 | 972 | 3,490 | 28,036 | ||||||||||||||||||
| Total costs and expenses |
22,897 | 10,116 | 13,550 | 2,038 | 6,787 | 81,200 | ||||||||||||||||||
| Loss from operations |
(22,897 | ) | (10,116 | ) | (13,550 | ) | (2,038 | ) | (6,787 | ) | (81,200 | ) | ||||||||||||
| Interest expense |
(1,127 | ) | (2,083 | ) | (2,404 | ) | (601 | ) | (113 | ) | (7,010 | ) | ||||||||||||
| Interest income |
533 | 177 | 109 | 18 | 68 | 2,784 | ||||||||||||||||||
| Change in fair value of warrant liability |
91 | (174 | ) | (416 | ) | — | — | (263 | ) | |||||||||||||||
| Other than temporary impairment on securities |
(1,665 | ) | — | (30 | ) | — | — | (2,466 | ) | |||||||||||||||
| Other income, net |
31 | — | 244 | — | — | 275 | ||||||||||||||||||
| Loss before income taxes |
(25,035 | ) | (12,196 | ) | (16,047 | ) | (2,622 | ) | (6,832 | ) | (87,879 | ) | ||||||||||||
| Benefit from income taxes |
— | — | 1,793 | 1,793 | — | 1,793 | ||||||||||||||||||
| Net loss |
(25,035 | ) | (12,196 | ) | (14,254 | ) | (829 | ) | (6,832 | ) | (86,086 | ) | ||||||||||||
| Less accretion of preferred stock dividends and other deemed dividends |
(6,242 | ) | (3,287 | ) | (8,751 | ) | (862 | ) | — | (23,663 | ) | |||||||||||||
| Net loss attributable to common stockholders |
$ | (31,277 | ) | $ | (15,483 | ) | $ | (23,005 | ) | $ | (1,691 | ) | $ | (6,832 | ) | $ | (109,750 | ) | ||||||
| Net loss attributable to common stockholders per share — basic and diluted |
$ | (20.92 | ) | $ | (9.35 | ) | $ | (5.07 | ) | $ | (0.99 | ) | $ | (0.39 | ) | |||||||||
| Weighted-average shares outstanding — basic and diluted |
1,495,375 | 1,656,732 | 4,537,407 | 1,701 | 17,642 | |||||||||||||||||||
7
Table of Contents
| As of March 31, 2011 | ||||||||
| (Actual) | (As adjusted)(1) | |||||||
| (Unaudited) | ||||||||
| Balance Sheet Data: |
||||||||
| Cash and cash equivalents |
$ | 46,828 | $ | 100,505 | ||||
| Total assets |
50,014 | 103,691 | ||||||
| Long term debt |
10,000 | 10,000 | ||||||
| Deficit accumulated during the development stage |
(97,821 | ) | (97,821 | ) | ||||
| Total stockholders’ equity |
$ | 35,613 | 89,290 | |||||
| (1) | As adjusted to give effect to the sale by us in this offering of 3,250,000 shares of common stock at an assumed public offering price of $17.72 per share, after deducting underwriting discounts and commissions and the estimated offering expenses payable by us, and the application of the net proceeds as described under “Use of Proceeds.” |
Each $1.00 increase (decrease) in the public offering price per share would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity by approximately $3.1 million, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same and that the underwriters do not exercise their over-allotment option. Depending on market conditions and other considerations at the time we price this offering, we may sell a greater or lesser number of shares than the number set forth on the cover page of this prospectus. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of cash and cash equivalents, total assets and total stockholders’ equity by approximately $16.7 million, assuming the public offering price per share remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the public offering price per share, would increase each of cash and cash equivalents, total assets and total stockholders’ equity by approximately $20.7 million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the public offering price per share, would decrease each of cash and cash equivalents, total assets and total stockholders’ equity by approximately $18.8 million. This information is illustrative only, and following the pricing of this offering, we will update this information based on the actual public offering price and other terms of this offering.
8
Table of Contents
Investing in our common stock involves a high degree of risk. Before you decide to invest in our common stock, you should consider carefully the risks described below, together with the other information contained in this prospectus, including our financial statements and the related notes appearing at the end of this prospectus. We believe the risks described below are the risks that are material to us as of the date of this prospectus. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Financial Position and Capital Requirements
We have incurred significant operating losses since our inception, and anticipate that we will incur continued losses for the foreseeable future.
We are a development stage company with a limited operating history. To date, we have primarily focused on developing our lead compound, lomitapide. We have funded our operations to date primarily through proceeds from the private placement of convertible preferred stock, convertible debt, venture debt and the proceeds from our initial public offering. We have incurred losses in each year since our inception in February 2005. Our net losses were approximately $25.0 million, $12.2 million and $14.3 million for the years ended December 31, 2008, 2009 and 2010, respectively, and $0.8 million and $6.8 million for the quarters ended March 31, 2010 and 2011, respectively. As of March 31, 2011, we had an accumulated deficit of approximately $97.8 million. Substantially all of our operating losses resulted from costs incurred in connection with our development programs and from general and administrative costs associated with our operations.
The losses we have incurred to date, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital. We expect our research and development expenses to increase in connection with our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH and our planned Phase III pediatric clinical trial to evaluate lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH, Phase III clinical trial of lomitapide for the treatment of adult patients with FC and other potential studies or clinical trials of lomitapide. In addition, if we obtain marketing approval for lomitapide, we will likely incur significant sales, marketing, in-licensing and outsourced manufacturing expenses, as well as continued research and development expenses. In addition, we have incurred and expect to continue to incur additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when we will become profitable, if at all.
We have not generated any revenue from lomitapide or any other product candidate and may never be profitable.
Our ability to become profitable depends upon our ability to generate revenue. To date, we have not generated any revenue from our development stage product candidates, including our lead compound, lomitapide, and we do not know when, or if, we will generate any revenue. We do not expect to generate significant revenue unless or until we obtain marketing approval of, and commercialize, lomitapide. Our ability to generate revenue depends on a number of factors, including our ability to:
| § | submit our NDA based on our Filing Data with the FDA, and submit our MAA with the EMA; |
| § | successfully complete our ongoing pivotal Phase III clinical trial for the treatment of patients with HoFH; |
| § | develop and obtain regulatory approval for a protocol for a Phase III pediatric clinical trial to evaluate lomitapide for treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH; |
9
Table of Contents
| § | develop and obtain regulatory approval for a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC; |
| § | contract for the manufacture of commercial quantities of lomitapide at acceptable cost levels if marketing approval is received; and |
| § | establish sales and marketing capabilities to effectively market and sell lomitapide in the United States and the European Union. |
Even if lomitapide is approved for commercial sale in one or both of the initial indications that we are pursuing, the diagnosis criteria in the final label may define the patient populations narrowly thus limiting intended users, or lomitapide may not gain market acceptance or achieve commercial success. In addition, we anticipate incurring significant costs associated with commercializing any approved product. We may not achieve profitability soon after generating product revenue, if ever. If we are unable to generate product revenue, we will not become profitable and may be unable to continue operations without continued funding.
We may need substantial additional capital in the future. If additional capital is not available, we will have to delay, reduce or cease operations.
We may need to raise additional capital to fund our operations and to develop and commercialize lomitapide. Our future capital requirements may be substantial and will depend on many factors including:
| § | the results of our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH; |
| § | the cost, timing and outcomes of seeking marketing approval of lomitapide for the treatment of patients with HoFH in the United States and the European Union; |
| § | our ability to develop and obtain regulatory approval for a protocol for a Phase III pediatric clinical trial to evaluate lomitapide for treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH; |
| § | our ability to develop and obtain regulatory approval for a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC; |
| § | if regulatory approval for the Phase III clinical trial of lomitapide for the treatment of adult patients with FC is obtained, the cost and timing associated with conducting such clinical trial; |
| § | the cost of filing, prosecuting and enforcing patent claims; |
| § | exploration, clinical trials and possible label expansion of lomitapide for use in broader patient populations; |
| § | the costs associated with commercializing lomitapide if we receive marketing approval, including the cost and timing of establishing sales and marketing capabilities to market and sell lomitapide for the treatment of patients with HoFH; and |
| § | subject to receipt of marketing approval, revenue received from sales of approved products, if any, in the future. |
Based on our current operating plan, we anticipate that the net proceeds of this offering, together with our existing cash, cash equivalents and borrowing capacity under our Loan and Security Agreement with the Hercules Funds, will be sufficient to enable us to maintain our currently planned operations, including our continued product candidate development, at least through the next 24 months. However, changing circumstances may cause us to consume capital significantly faster than we currently anticipate. In February 2011, we entered into the Loan and Security Agreement with the Hercules Funds, for a $25.0 million credit facility. At the closing of the Loan and Security Agreement, we received an initial advance of $10.0 million, with interest-only payments for thirteen months. We may request additional term loan advances of up to $15.0 million. As of the date of this prospectus, we have a principal amount of $10.0 million outstanding under the
10
Table of Contents
Loan and Security Agreement. Other than the Loan and Security Agreement with the Hercules Funds, we have no committed external sources of funds. Additional financing may not be available when we need it or may not be available on terms that are favorable to us. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. If adequate funds are not available to us on a timely basis, or at all, we may be required to:
| § | terminate or delay clinical trials or other development activities for lomitapide for one or more indications for which we are developing lomitapide, in particular any clinical trial we initiate for the treatment of adult patients with FC; or |
| § | alter or scale back our continued establishment of sales and marketing capabilities or other activities that may be necessary to commercialize lomitapide. |
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaboration, strategic alliance and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Our limited operating history makes it difficult to evaluate our business and prospects.
We were incorporated in February 2005. Our operations to date have been limited to organizing and staffing our company and conducting product development activities, primarily for lomitapide. Consequently, any predictions about our future performance may not be as accurate as they could be if we had a longer operating history and experience in generating revenue. In addition, as a relatively young business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors.
Risks Associated with Product Development and Commercialization
We currently depend entirely on the success of our lead compound, lomitapide, which we are developing initially for the treatment of patients with HoFH. We are also developing a protocol for a clinical trial of lomitapide for the treatment of adult patients with FC. We may not receive marketing approval for, or successfully commercialize, lomitapide for any indication.
We are initially developing our lead compound, lomitapide, for the treatment of patients with HoFH, and are developing protocols for clinical trials of lomitapide for the treatment of pediatric patients with HoFH and adult patients with FC. Our business currently depends entirely on the successful development and commercialization of lomitapide. We have not yet demonstrated an ability to obtain marketing approval for any product candidate, we have no drug products for sale currently and we may never be able to develop marketable drug products. The research, testing, manufacturing, labeling, approval, sale, marketing and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. We are not permitted to market lomitapide or any other product candidate in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. We have not submitted an NDA to the FDA or comparable applications to other regulatory authorities or received marketing approval for lomitapide or any other product candidate.
11
Table of Contents
Obtaining approval of an NDA is an extensive, lengthy, expensive and uncertain process, and the FDA may delay, limit or deny approval of lomitapide for many reasons, including:
| § | we may not be able to demonstrate to the satisfaction of the FDA that lomitapide is safe and effective for any indication; |
| § | the results of clinical trials may not meet the level of statistical significance or clinical significance required by the FDA for approval; |
| § | the FDA may disagree with the number, design, size, conduct or implementation of our clinical trials; |
| § | the FDA may not find the data from preclinical studies and clinical trials sufficient to demonstrate that lomitapide’s clinical and other benefits outweighs its safety risks; |
| § | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials and require that we conduct one or more additional trials; |
| § | the FDA may not accept data generated at our clinical trial sites; |
| § | the data collected from preclinical studies and clinical trials of any product candidate that we develop may not be sufficient to support the submission of an NDA; |
| § | the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions; |
| § | the FDA may impose limitations on approved labeling, such as narrowing the diagnosis criteria for an indication thus limiting intended users; |
| § | the FDA may require development of a risk evaluation and mitigation strategy, or REMS, as a condition of approval; |
| § | the FDA may identify deficiencies in the manufacturing processes or facilities of third party manufacturers with which we enter into agreements for clinical and commercial supplies; or |
| § | the FDA may change its approval policies or adopt new regulations. |
Before we submit an NDA to the FDA for lomitapide for the treatment of patients with HoFH, we must complete our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH. In addition, we must complete other preclinical and clinical studies, such as a thorough QT study in healthy volunteers to evaluate the effect of lomitapide on the heart’s electrical cycle, known as the QT interval, studies to evaluate the interaction of lomitapide with other drugs, and a study of lomitapide in patients who are renally impaired. If, following submission, our NDA is not accepted for substantive review or approved, the FDA may require that we conduct additional clinical and non-clinical studies before it will reconsider our application.
In particular, it is possible that the FDA may not consider the results of our ongoing single-arm, dose titration, open-label pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, once completed, to be sufficient for approval of lomitapide for this indication. For example, because the FDA normally requires two pivotal clinical trials to approve an NDA, even if we achieve favorable results in our ongoing pivotal Phase III clinical trial, the FDA may require that we conduct a second Phase III clinical trial if the FDA does not find the results to be sufficiently persuasive. Although the FDA has informed us that it is not opposed to our submitting an NDA based on the results of our ongoing Phase III clinical trial, it is possible that, even if we achieve favorable results in our ongoing Phase III clinical trial, the FDA may require us to conduct a second Phase III clinical trial, possibly using a different design, including if the FDA does not find the results from the single trial to be sufficiently persuasive. In addition, it is possible that the FDA could require that we conduct a clinical outcomes study for lomitapide for the treatment of patients with HoFH, demonstrating a reduction in cardiovascular events, either prior to or after the submission of our NDA. If the FDA requires additional studies or trials, we would incur increased costs and delays in the marketing approval process, which may require us to
12
Table of Contents
expend more resources than we have available. In addition, the FDA may not consider as sufficient our clinical trial strategy or any additional required studies or trials that we perform and complete. For example, the FDA may not accept our plan for a single Phase III clinical trial of lomitapide for the treatment of adult patients with FC.
Finally, any final labeling may be approved with limitations. For example, FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. Thus, FDA may narrow the diagnosis criteria for HoFH included in the final label for lomitapide and these patients may be considered to be outside the HoFH population, reducing the estimated 6,000-patient addressable population.
The FDA may require an advisory committee to review and render an opinion on the NDA, which may be negative or may delay approval or limit lomitapide’s marketability.
The FDA has informed us that review of the NDA for lomitapide for the treatment of patients with HoFH at an FDA advisory committee meeting is highly likely. The FDA is not bound by the recommendation of an advisory committee, which is composed of clinicians, statisticians and other experts, but it generally follows such recommendations. The FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions. This would delay and increase the cost of the review process. Any delay in obtaining, or an inability to obtain, marketing approval could prevent us from commercializing lomitapide, generating revenue and achieving profitability.
We plan to submit our NDA for lomitapide based on our Filing Data, which is prior to completion of our pivotal Phase III clinical trial. If data obtained during the last 22 weeks of the trial are unfavorable, our ability to commercialize lomitapide may be adversely affected.
We are currently evaluating lomitapide in a pivotal Phase III clinical trial for the treatment of patients with HoFH. We anticipate that this trial will be completed in the third quarter of 2011. Unfavorable results or outcomes in this or other trials for lomitapide prior to its 78-week completion point would be a major set-back for the lomitapide development programs and for us. An unfavorable outcome in this trial may require us to delay, reduce the scope of, or eliminate this product development program, which could have a material adverse effect on us and the value of our common stock.
The numbers of patients suffering from HoFH and FC are small and have not been established with precision. If the actual number of patients with either of these conditions is smaller than we estimate or if any approval that we obtain is based on a narrower definition of these patient populations, our revenue and ability to achieve profitability will be adversely affected, possibly materially. For example, the FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. This means that these patients may ultimately be considered to be outside the HoFH population.
There is no patient registry or other method of establishing with precision the actual number of patients with HoFH or FC in any geography. In a report that we commissioned, L.E.K. Consulting LLC, or LEK, an international business consulting firm, estimated that the total number of addressable patients with symptoms consistent with HoFH in each of the United States and, collectively, Germany, the United Kingdom, France, Italy and Spain, which are referred to herein as the European Union Five, is approximately 3,000 patients, or a combined total of approximately 6,000 patients. In the report that we commissioned, LEK estimated that there are approximately 1,000 patients in the United States and the European Union Five with FC who could be eligible for treatment with lomitapide. The total addressable market opportunity for lomitapide for the treatment of patients with HoFH or FC will ultimately depend upon, among other things, the diagnosis criteria included in the final label for lomitapide, if approved for sale for these indications, acceptance by the medical community
13
Table of Contents
and patient access, product pricing, and reimbursement. If the actual number of HoFH or FC patients is lower than we believe or if any approval that we obtain is based on a narrower definition of these patient populations, then the potential markets for lomitapide for these indications will be smaller than we anticipate. For example, the FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. This means these patients may ultimately be considered to be outside the HoFH population, reducing the estimated 6,000-patient addressable population. Moreover, the medical literature has historically estimated a prevalence of HoFH, based solely on persons with the HoFH genotype, at approximately one person per million. In any event, if lomitapide is approved for either HoFH or FC, our product revenue may be limited, and it may be more difficult for us to achieve or maintain profitability.
In addition, we currently plan to seek approval of lomitapide initially for the treatment of patients with HoFH who are 18 years of age or older. To support approval for younger patients, we plan to initiate a Phase III pediatric clinical trial of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH, but we do not expect to submit a supplemental NDA to expand our labeling to include this patient population prior to 2014. As a result, any FDA approval would likely, at least initially, be limited to use for treating adult patients with lomitapide. This would limit our initial product revenue and may make it more difficult for us to achieve or maintain profitability. We expect that our approach to seeking approval of lomitapide for FC also will involve initially seeking marketing approval solely for the adult patient population.
We may not obtain an ATU, or obtaining an ATU may adversely affect our ability to set market prices outside of France, either of which could have an adverse effect on our financial results.
We currently intend to apply for a Temporary Authorization for Use for lomitapide in France based on the Phase III clinical trial. An ATU is intended for drugs that treat serious or rare diseases where no suitable therapeutic alternative is available in France, and it allows such drugs to be used in a small number of patients prior to marketing approval. Obtaining an ATU from the French Health Products Safety Agency, or Afssaps, requires satisfying a number of conditions and involves a lengthy and complicated application process. Specifically, we will need to demonstrate, among other things, the following:
| § | lomitapide treats a serious or rare pathology; |
| § | other suitable treatments for such a pathology are not available in France; and |
| § | the benefit of lomitapide outweighs the risk. |
Though we believe lomitapide qualifies under the ATU’s requirements, the Afssaps may not agree that any or all of these conditions are satisfied. Specifically, the Afssaps may determine that apheresis or the drug Zocor are suitable treatments for HoFH, both of which are now available in France. Also, the Afssaps may find the data from the pivotal Phase III trial less compelling than data from a clinical trial conducted in France. Even if Afssaps were to grant an ATU for lomitapide, the Afssaps may suspend, withdraw or modify the terms of the ATU for reasons of public health or if the conditions leading to the granting of the ATU change.
If Afssaps grants an ATU for lomitapide, a price level will be negotiated with the French authorities. This negotiated price may adversely affect the market prices in other countries or jurisdictions where we may sell lomitapide if it is lower than the price that would have otherwise been set in such geographies.
We may not be able to generate enough revenue to cover our operating costs due to pricing or demand issues for lomitapide.
In 2007, the FDA granted lomitapide “orphan drug” status for the treatment of HoFH. “Orphan drug” designation is generally given to drugs that treat a rare disease or condition, defined, in part, as a patient population of fewer than 200,000 in the United States. In a report that we commissioned, LEK estimated that the total number of addressable patients with symptoms consistent with HoFH in each of the United States and, collectively, Germany, the United Kingdom, France, Italy and Spain, which are referred to in this prospectus as the European Union Five, is approximately 3,000 patients, or a combined total of approximately 6,000 patients. However, the
14
Table of Contents
FDA may not agree with the diagnostic criteria LEK employed to estimate the number of patients with HoFH and may define the HoFH population more narrowly. Regardless, with such a small potential patient market, we will need to set and charge a price for lomitapide that is significantly higher than that of most pharmaceuticals in order to generate enough revenue to fund our operating costs.
If our estimate regarding the number of patients in the United States are too high, or if our estimates about the achievable per-dose price for lomitapide are too high, we may not be able to generate revenue and meet our operating costs in the timeframe that we expect, or at all.
In earlier preclinical studies and clinical trials, lomitapide was associated with undesirable side effects, such as adverse gastrointestinal events, elevated liver enzymes and increases in mean hepatic fat levels. Patients in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH have also experienced such undesirable side effects. Lomitapide may continue to cause such side effects or have other properties that could delay or prevent its marketing approval or result in adverse limitations in any approved labeling or on distribution and use of the product.
Undesirable side effects caused by lomitapide or any other product candidate that we develop could cause us, regulatory authorities or institutional review boards to interrupt, delay or halt clinical trials and could result in the delay or denial of marketing approval by the FDA or other regulatory authorities. In earlier studies conducted by us, researchers at the University of Pennsylvania, or UPenn, and Bristol-Myers Squibb Company, or BMS, lomitapide was associated with undesirable side effects. We have also observed some of these side effects to a lesser extent in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH.
For example, in early Phase I and Phase II clinical trials of lomitapide conducted by BMS and UPenn, doses between 25 mg and 100 mg were associated with a very high rate of gastrointestinal adverse events, such as diarrhea, nausea and vomiting, as well as the accumulation of fat in the liver, or hepatic fat, in a significant percentage of patients and elevated liver enzymes in some patients. Although we believe that the high rate of discontinuations in these early Phase I and Phase II clinical trials due to gastrointestinal adverse events resulted in large part from the failure to employ dose titration, which is the gradual increase in dosing over time to allow the body to adapt to the impact of a higher dose, this may not be the case. Patients in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, where dose titration has been employed, have also experienced adverse gastrointestinal events, but to a lesser extent than those experienced in earlier clinical trials. Even if lomitapide gains marketing approval, if marketing experience or future clinical trials demonstrate an increased risk of gastrointestinal side effects, lomitapide may not gain market acceptance over other drugs approved for the treatment of the same indications that do not have such side effects and could be subject to adverse regulatory action by the FDA or foreign regulatory authorities.
A subset of patients in earlier Phase I and Phase II clinical trials of lomitapide experienced increased levels of liver enzymes, an indicator of liver cell damage and, in some cases, liver toxicity. Increases in liver enzymes observed in these earlier clinical trials were typically greater with higher doses of lomitapide, but occurred in some cases at lower doses. For example, in a Phase II clinical trial of lomitapide for the treatment of patients with HoFH, clinically significant elevations in the liver enzyme alanine transaminase, or ALT, were observed in three of six patients. In one patient, the dose of lomitapide was temporarily reduced per protocol, after which ALT returned to lower levels. The patient subsequently was able to resume the earlier, higher dose and continue to be titrated to the maximum dose. In the other two patients, transient elevations in ALT returned to lower levels with continued treatment. In our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, as of May 31, 2011, four of the 23 patients remaining in the trial had experienced ALT elevations greater than five times the upper limit of normal. Of these four patients, three underwent a temporary dose reduction and have maintained study drug at a stable dose. One patient discontinued treatment for a period of seven weeks, after which time treatment was reinstated and the patient was able to maintain a stable dose and complete the trial per protocol. No patients have been removed from the trial due to liver function test elevations.
A subset of patients in earlier Phase I and Phase II clinical trials of lomitapide also experienced increases in mean hepatic fat levels. Although increases in hepatic fat in earlier clinical trials were typically greater with higher
15
Table of Contents
doses of lomitapide, increases also occurred in some cases at lower doses. For example, in a completed 12 week Phase II clinical trial of lomitapide, mean hepatic fat levels increased from week zero to week four, but then plateaued. In our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, as of September 30, 2010, 22 patients who had hepatic fat measurements taken experienced an increase in hepatic fat from a mean of 1.0% to 9.0% at 26 weeks of treatment. Of the 23 patients that completed 56 weeks of treatment as of May 31, 2011 21 patients had hepatic fat measurements available. These 21 patients had a mean hepatic fat level of 7.3% at this measurement time. The threshold for mild steatosis, a condition of hepatic fat accumulation, is in the range of 5 to 6% fat. In addition, the median change in hepatic fat from baseline in these patients was 5.7% at 26 weeks of treatment and 4.4% at 56 weeks of treatment. Some studies suggest that patients who have hepatic steatosis that results from pre-existing conditions, such as obesity and type 2 diabetes, may be at an increased risk for more severe long-term liver consequences, such as hepatic inflammation and fibrosis. However, the consequences of hepatic steatosis that results from other factors, are unclear.
We will also provide to the FDA an analysis of biomarkers for hepatic inflammation and fibrosis using stored samples from a prior Phase II clinical trial as well as samples from our ongoing Phase III trial to better determine lomitapide’s impact on these biomarkers as a proxy for more significant liver complications. If the results of this analysis indicate a risk of hepatic inflammation and fibrosis, or if patients in our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH or future clinical trials have clinically significant hepatic fat accumulation or significantly elevated liver enzymes or other liver related side effects, the FDA or other regulatory authorities could delay or deny marketing approval for lomitapide. Also, even if lomitapide is approved, if marketing experience or future clinical trials demonstrate hepatic fat accumulation, significantly elevated liver enzymes or other liver related side effects, lomitapide may not gain market acceptance over other drugs approved for the treatment of the same indications that do not have such side effects and could be subject to adverse regulatory action by the FDA or foreign regulatory authorities.
In a 104 week dietary carcinogenicity study of lomitapide in mice, significantly increased incidences of tumors in the small intestine and liver were observed. The relationship of these findings in mice is uncertain with regard to human safety because they did not occur in a dose-related manner and liver tumors are common spontaneous findings in the strain of mice used in this study. In a 104 week oral carcinogenicity study of lomitapide in rats, there were no significant incidences of tumors. We submitted the results of both studies to the FDA in March 2011. Although the clinical significance of the findings in mice is unknown, marketing approval could be delayed, limited, or prevented as a result of these carcinogenicity data or carcinogenicity data from the rat study or ongoing clinical trials. Also, even if lomitapide is approved, if marketing experience or future preclinical studies or clinical trials demonstrate potential carcinogenicity, lomitapide may not gain market acceptance over other drugs approved for the treatment of the same indications that do not have such side effects and could be subject to adverse regulatory action by the FDA or foreign regulatory authorities.
Even if lomitapide or any other product candidate that we develop receives marketing approval, we or others may later identify undesirable side effects caused by the product, and in that event a number of potentially significant negative consequences could result, including:
| § | regulatory authorities may suspend or withdraw their approval of the product; |
| § | regulatory authorities may require the addition of labeling statements, such as warnings or contraindications or distribution and use restrictions; |
| § | regulatory authorities may require us to issue specific communications to healthcare professionals, such as “Dear Doctor” letters; |
| § | regulatory authorities may issue negative publicity regarding the affected product, including safety communications; |
| § | we may be required to change the way the product is administered, conduct additional preclinical studies or clinical trials or restrict the distribution or use of the product; |
| § | we could be sued and held liable for harm caused to patients; and |
| § | our reputation may suffer. |
16
Table of Contents
Any of these events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could substantially increase commercialization costs.
Failures or delays in the commencement or completion of preclinical or clinical testing could result in increased costs to us and delay, prevent or limit our ability to generate revenue.
Failures or delays in the commencement or completion of preclinical studies or clinical testing could significantly affect our product development costs and delay, prevent or limit our ability to generate revenue. We do not know whether planned preclinical studies or clinical trials will begin on time or be completed on schedule, if at all. The commencement and completion of preclinical studies or clinical trials can be delayed or prevented for a number of reasons, including:
| § | findings in preclinical studies, such as the existence of pulmonary phospholipidosis, which is the accumulation in lung cells of phospholipids, or lipids with attached phosphate groups, or carcinogenicity; |
| § | difficulties obtaining regulatory approval to commence a clinical trial or complying with conditions imposed by a regulatory authority regarding the scope or term of a clinical trial; |
| § | delays in reaching or failing to reach agreement on acceptable terms with prospective contract research organizations, or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| § | insufficient or inadequate supply or quality of a product candidate or other materials necessary to conduct our clinical trials; |
| § | difficulties obtaining institutional review board, or IRB, approval to conduct a clinical trial at a prospective site; |
| § | challenges recruiting and enrolling patients to participate in clinical trials for a variety of reasons, including size and nature of patient population, proximity of patients to clinical sites, eligibility criteria for the trial, nature of trial protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications; |
| § | severe or unexpected drug-related side effects experienced by patients in a clinical trial; and |
| § | difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the trials, lack of efficacy, side effects or personal issues, or who are lost to further follow-up. |
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a trial, or a data safety monitoring board, or DSMB, overseeing the clinical trial at issue, or other regulatory authorities due to a number of factors, including:
| § | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| § | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities; |
| § | unforeseen safety issues or lack of effectiveness; and |
| § | lack of adequate funding to continue the clinical trial. |
For example, in June 2007, we discontinued a Phase II clinical trial of lomitapide because of suspected microbial contamination, which we believe resulted in patients experiencing gastrointestinal adverse events at a rate, severity and time of onset inconsistent with prior clinical data for lomitapide. After extensive testing and other investigation, we believe that the bacterium B. cereus, which is most often identified with food borne illness, was introduced into the active pharmaceutical ingredient, or API, of the clinical supplies of the drug used in this trial. The existence of microbial contamination by B. cereus is consistent with the nature and intensity of the adverse events experienced in this trial. In previous clinical trials, all of which were conducted using a different lot of API, adverse events were milder, less frequent and typically experienced only after a few days of treatment. We subsequently manufactured a new lot of API and a new lot of clinical supplies utilizing a previously tested lot of
17
Table of Contents
API and instituted new quality control and testing procedures, including tests for microbial contamination, as part of our manufacturing process. A subsequent clinical trial of lomitapide conducted using these newly manufactured clinical supplies did not result in a discontinuation rate consistent with that experienced in the halted trial. However, we may not have identified the actual cause of these adverse events, and it is possible that microbial contamination was not the only cause of these adverse events.
Positive results in preclinical studies and earlier clinical trials of lomitapide may not be replicated in later clinical trials of lomitapide, which could result in development delays or a failure to obtain marketing approval.
Positive results in preclinical studies of lomitapide or any other product candidate that we develop may not be predictive of similar results in humans during clinical trials, and promising results from early clinical trials of lomitapide or any other product candidate may not be replicated in later clinical trials. Interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage development. Accordingly, the results from the completed preclinical studies and clinical trials and ongoing clinical trials for lomitapide may not be predictive of the results we may obtain in later stage trials. Our preclinical studies or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain FDA approval for their products.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, which may result in necessary changes to clinical trial protocols, which could result in increased costs to us, delay our development timeline or reduce the likelihood of successful completion of the clinical trial.
Changes in regulatory requirements and guidance or unanticipated events during our clinical trials may occur, as a result of which we may need to amend clinical trial protocols. Amendments may require us to resubmit our clinical trial protocols to IRBs for review and approval, which may impact the cost, timing or successful completion of a clinical trial. If we experience delays in completion of, or if we terminate, any of our clinical trials, the commercial prospects for lomitapide may be harmed and our ability to generate product revenue will be delayed, possibly materially.
Recently enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize lomitapide or any other product candidate that we develop and affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for lomitapide or any other product candidate that we develop, restrict or regulate post-approval activities and affect our ability to profitably sell lomitapide or any other product candidate for which we obtain marketing approval.
Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of lomitapide, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on
18
Table of Contents
average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
More recently, in March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, beginning in 2011, the new law imposed a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Even if lomitapide or any other product candidate that we develop receives marketing approval, we will continue to face extensive regulatory requirements and the product may still face future development and regulatory difficulties.
Even if marketing approval is obtained, a regulatory authority may still impose significant restrictions on a product’s indications, conditions for use, distribution or marketing or impose ongoing requirements for potentially costly post-market surveillance, post-approval studies or clinical trials. For example, any labeling ultimately approved by the FDA for lomitapide, if it is approved for marketing, may include restrictions on use, such as limitations on how HoFH is defined and diagnosed or limiting lomitapide to second-line or concomitant therapy. The FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. Thus, FDA may narrow the diagnosis criteria for HoFH included in the final label for lomitapide and these patients may be considered to be outside the HoFH population, reducing the estimated 6,000-patient addressable population. In addition, the labeling may include restrictions based upon evidence of hepatic fat accumulation, liver function test elevations, gastrointestinal distress, phospholipidosis or carcinogenicity. Lomitapide will also be subject to ongoing FDA requirements governing the labeling, packaging, storage, advertising, distribution, promotion, recordkeeping and submission of safety and other post-market information, including adverse events, and any changes to the approved product, product labeling, or manufacturing process. The FDA has significant post-market authority, including, for example, the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate serious safety risks related to the use of a drug. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current Good Manufacturing Practice, or cGMP, and other regulations.
If we, our drug products or the manufacturing facilities for our drug products fail to comply with applicable regulatory requirements, a regulatory agency may:
| § | issue warning letters or untitled letters; |
| § | seek an injunction or impose civil or criminal penalties or monetary fines; |
19
Table of Contents
| § | suspend or withdraw marketing approval; |
| § | suspend any ongoing clinical trials; |
| § | refuse to approve pending applications or supplements to applications submitted by us; |
| § | suspend or impose restrictions on operations, including costly new manufacturing requirements; |
| § | seize or detain products, refuse to permit the import or export of products or request that we initiate a product recall; or |
| § | refuse to allow us to enter into supply contracts, including government contracts. |
The FDA has the authority to require a REMS plan as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. Based on our discussions with the FDA, we believe lomitapide, if approved by the FDA for the treatment of patients with HoFH, is likely to be subject to a REMS plan. In particular, we believe that any REMS plan for lomitapide, if it is approved for the treatment of patients with HoFH, will focus on the FDA’s concern that lomitapide not be used in broader patient populations with less severely elevated lipid levels because of the potentially different risk-benefit profile of the drug.
Even if lomitapide or any other product candidate that we develop receives marketing approval in the United States, we may never receive approval or commercialize lomitapide or any other product candidate outside of the United States.
In order to market any product outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy and governing, among other things, clinical trials and commercial sales, pricing and distribution of the product. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The marketing approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. In particular, in many countries outside the United States, it is required that a product receive pricing and reimbursement approval before the product can be commercialized. This can result in substantial delays in such countries.
Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may have a negative effect on the regulatory process in others. Failure to obtain marketing approval in other countries or any delay or setback in obtaining such approval would impair our ability to develop foreign markets for lomitapide. This would reduce our target market and limit the full commercial potential of lomitapide. Many countries are undertaking cost-containment measures that could affect pricing or reimbursement of a product.
If we receive marketing approval for lomitapide or any other product candidate, our sales will be limited unless the product achieves broad market acceptance for these indication.
The commercial success of lomitapide and any other product candidate for which we obtain marketing approval from the FDA or other regulatory authorities will depend upon the acceptance of the product by the medical community, including physicians, patients and healthcare payors. The degree of market acceptance of any approved product will depend on a number of factors, including:
| § | limitations or warnings contained in the product’s approved labeling; |
| § | distribution and use restrictions imposed by the FDA or agreed to by us as part of a mandatory REMS or voluntary risk management plan; |
| § | demonstration of clinical safety and efficacy compared to other products; |
20
Table of Contents
| § | the prevalence and severity of any adverse side effects; |
| § | the relative convenience and ease of administration; |
| § | availability of alternative treatments, including, in the case of lomitapide, a number of competitive products already approved for the treatment of hyperlipidemia or expected to be commercially launched in the near future; |
| § | pricing and cost effectiveness; |
| § | the effectiveness of our or any future collaborators’ sales and marketing strategies; |
| § | our ability to obtain sufficient third-party coverage or reimbursement; and |
| § | the willingness of patients to pay out of pocket in the absence of third-party coverage. |
We plan to seek marketing approval for lomitapide for the treatment of both HoFH and FC based on achieving surrogate endpoints in pivotal clinical testing, as opposed to demonstrating improved clinical outcomes. The absence of outcome data may limit market acceptance of lomitapide, if approved for one or both of these indications, by physicians and patients.
If lomitapide or any other product candidate that we develop is approved but does not achieve an adequate level of acceptance by physicians, healthcare payors and patients, we may not generate sufficient revenue from the product, and we may not become or remain profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of the product may require significant resources and may never be successful.
The FDA and other regulatory agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. If we are found to have improperly promoted off-label uses, we may become subject to significant liability.
The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA or such other regulatory agencies as reflected in the product’s approved labeling. In particular, any labeling approved by the FDA for lomitapide may include restrictions on use, such as limitations on how HoFH is defined and diagnosed or limiting lomitapide to second-line or concomitant therapy. For example, the FDA has stated that our functional HoFH definition of patients with average fasting LDL-C greater than 300 mg/dL on maximally tolerated lipid lowering therapy closely resembles the severe refractory heterozygous familial hypercholesterolemia population. Thus, FDA may narrow the diagnosis criteria for HoFH included in the final label for lomitapide and these patients may be considered to be outside the HoFH population, reducing the estimated 6,000-patient addressable population. The FDA may impose further requirements or restrictions on the distribution or use of lomitapide as part of a REMS plan, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. If we receive marketing approval for lomitapide, physicians may nevertheless prescribe lomitapide to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed.
It will be difficult for us to profitably sell lomitapide or any other product for which we obtain marketing approval if reimbursement for the product is limited.
Market acceptance and sales of lomitapide or any other product candidate that we develop will depend on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and
21
Table of Contents
elsewhere is cost containment. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that reimbursement will be available for lomitapide or any other product that we develop and, if reimbursement is available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. Obtaining reimbursement for lomitapide may be particularly difficult because of the higher prices often associated with drugs directed at orphan populations. In addition, third-party payors are likely to impose strict requirements for reimbursement in order to limit off label use of a higher priced drug. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize lomitapide or any other product candidate that we develop.
If we are unable to establish sales and marketing capabilities to market and sell lomitapide, we may be unable to generate product revenue.
We have begun hiring a sales and marketing team in the United States and the European Union, but we do not currently have a fully developed organization for the sale, marketing or distribution of pharmaceutical products. We have not yet demonstrated an ability to commercialize any product candidate. In order to market any products that may be approved by the FDA or any other regulatory authority, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If lomitapide is approved by the FDA or the EMA, we plan to continue building a commercial infrastructure to launch lomitapide in the United States and the European Union, as the case may be, with a relatively small specialty sales force. The establishment and development of our commercial infrastructure will be expensive and time consuming, and we may not be able to successfully develop this capability. Although our current plan is to hire most of our sales force only if lomitapide is approved by the FDA or the EMA, we will incur expenses prior to product launch in recruiting this sales force and developing a marketing and sales infrastructure. If the commercial launch of lomitapide is delayed as a result of FDA or EMA requirements or other reasons, we would incur these expenses prior to being able to realize any revenue from product sales. Even if we are able to effectively hire a sales force and develop a marketing and sales infrastructure, our sales force may not be successful in commercializing lomitapide or any other product candidate that we develop. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not become profitable.
To the extent we rely on third parties to commercialize lomitapide, if marketing approval is obtained, we may receive less revenue than if we commercialized the product ourselves. In addition, we would have less control over the sales efforts of any third parties involved in our commercialization efforts. In the event we are unable to collaborate with a third-party marketing and sales organization to commercialize lomitapide, particularly for broader patient populations, our ability to generate product revenue may be limited either in the United States or internationally.
If we pursue development of lomitapide for broader patient populations, we likely will be subject to stricter regulatory requirements, product development will be more costly and commercial pricing for any approved indication would likely be lower.
Although we are initially pursuing development of lomitapide for the treatment of patients with HoFH and FC, we believe that lomitapide may be useful for the treatment of elevated lipid levels in broader patient populations. However, our potential development and commercialization of lomitapide in these broader patient populations would be more costly than for HoFH and FC and would be subject to development and commercialization risks that are not applicable to HoFH and FC.
Clinical development of lomitapide in broader patient populations would involve clinical trials with larger numbers of patients possibly taking the drug for longer periods of time. This would be costly and could take many years to complete. In addition, we believe that the FDA and, in some cases, the EMA would require a clinical outcomes study demonstrating a reduction in cardiovascular events either prior to or after the submission of an application for marketing approval for these broader indications. Clinical outcomes studies are particularly expensive and time consuming to conduct because of the larger number of patients required to establish that the
22
Table of Contents
drug being tested has the desired effect. It may also be more difficult for us to demonstrate the desired outcome in these studies than to achieve validated surrogate endpoints, such as the primary efficacy endpoint of our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH of percent change in LDL-C levels. In addition, in considering approval of lomitapide for broader patient populations with less severely elevated lipid levels, the FDA and other regulatory authorities may place greater emphasis on the side effect and risk profile of the drug in comparison to the drug’s efficacy and potential clinical benefit than in smaller, more severely afflicted patient populations. These factors may make it more difficult for us to achieve marketing approvals of lomitapide for these broader patient populations.
If we are able to successfully develop and obtain marketing approval of lomitapide in these broader patient populations, we likely will not be able to obtain the same pricing level that we secure for use of lomitapide for orphan indications. The pricing of some drugs intended for orphan populations is often related to the size of the patient population, with smaller patient populations often justifying higher prices. If the pricing for lomitapide is lower in broader patient populations, we may not be able to maintain higher pricing in the population of more severely afflicted patients. This would lead to a decrease in revenue from sales to more severely afflicted patients and could make it more difficult for us to achieve or maintain profitability.
In addition, if one of our product candidates receives marketing approval for a broader indication than its orphan designation, we may not be able to maintain any orphan drug exclusivity that we obtain or such orphan drug exclusivity may be circumvented by a third-party competitor.
If we fail to obtain or maintain orphan drug exclusivity for lomitapide, we will have to rely on our data and marketing exclusivity, if any, and on our intellectual property rights, which may reduce the length of time that we can prevent competitors from selling lomitapide.
As part of our business strategy, we have obtained in the United States orphan drug designation for lomitapide for the treatment of HoFH. In March 2011, the FDA granted lomitapide orphan drug designation for the treatment of FC. In October 2007, the FDA granted lomitapide orphan drug designation for the treatment of HoFH. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a drug intended to treat a rare disease or condition, defined, in part, as a patient population of fewer than 200,000 in the United States.
In the United States, the company that first obtains FDA approval for a designated orphan drug for the specified rare disease or condition receives orphan drug marketing exclusivity for that drug for a period of seven years. This orphan drug exclusivity prevents the FDA from approving another application, including a full NDA, to market the same drug for the same orphan indication, except in very limited circumstances. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active molecule and is intended for the same use as the drug in question. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
The EMA grants orphan drug designation to promote the development of products that may offer therapeutic benefits for life-threatening or chronically debilitating conditions affecting not more than five in 10,000 people in the European Union. Orphan drug designation from the EMA provides ten years of marketing exclusivity following drug approval, subject to reduction to six years if the designation criteria are no longer met. In October 2010, we withdrew our application to the EMA for orphan drug designation for lomitapide for the treatment of HoFH, based on guidance we received from the EMA that lomitapide is not eligible for orphan drug designation for this indication since lomitapide has the potential to treat hypercholesterolemia in broader patient populations. In October 2010, the EMA granted lomitapide orphan drug designation for the treatment of FC. Our failure to obtain orphan drug designation for lomitapide for the treatment of HoFH in the European Union means that we will not have the benefit of the orphan drug market exclusivity for this indication in the European Union, and, as a result, will need to rely on our intellectual property rights and other exclusivity provisions. Our European
23
Table of Contents
patents directed towards the composition of matter of lomitapide are scheduled to expire in 2016, and may additionally qualify for a supplemental certificate that would provide extended patent protection for up to five years after patent expiration upon marketing approval in the European Union. In addition, lomitapide may qualify as a new chemical entity in the European Union. In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from assessing a generic application for eight years, after which generic marketing authorization can be submitted but not marketed for two years. See also “— If we do not obtain protection under the Hatch-Waxman Amendments and similar foreign legislation by extending the patent terms and obtaining data exclusivity for our product candidates, our business may be materially harmed.”
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care.
In June 2007, the FDA instituted a partial clinical hold with respect to clinical trials of longer than six months duration for our product candidates. Although the partial clinical hold was lifted with respect to lomitapide, it remains in effect with respect to implitapide. The FDA’s concerns that resulted in this clinical hold may lead to a significant delay in the commencement of clinical trials by us or the failure of our product candidates to obtain marketing approval.
In June 2007, we received notice from the FDA of a partial clinical hold with respect to clinical trials of longer than six months duration for our product candidates. The FDA issued such notices to all sponsors of MTP-Is. At that time, the FDA did not apply this partial clinical hold to our pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH. In connection with the partial clinical hold, the FDA requested that we collect additional preclinical data to assess the risk for pulmonary phospholipidosis, with long-term use of these compounds. We understand that the FDA has undertaken a broader initiative to understand phospholipidosis in those drugs commonly evidencing this phenomenon, typically characterized as cationic (positively charged) and lipophilic (soluble in lipids). MTP-Is are both cationic and lipophilic.
In connection with the partial clinical hold, the FDA requested that we conduct a three month, repeat-dose rat toxicology study that included a recovery group and a sufficient number of active doses to establish the level of exposure at which there is no biologically or statistically significant increase in the frequency or severity of pulmonary phospholipidosis, which is commonly referred to as the no observable adverse effect level. The FDA also requested an electron microscopy lung tissue analysis. We submitted the requested preclinical data for lomitapide in December 2008, and the FDA removed the partial clinical hold with respect to this compound in February 2010. The partial clinical hold remains in effect with respect to implitapide.
If we decide to advance the clinical development of implitapide, the additional preclinical studies requested by the FDA may result in significant increases in estimated time and expense of obtaining FDA approval for this product candidate. In addition, if we are not able to successfully complete the preclinical studies required by the FDA, or otherwise satisfy the FDA’s concerns related to pulmonary phospholipidosis, we could be delayed in, or prevented from, obtaining marketing approval of implitapide. Furthermore, notwithstanding that the FDA has removed the partial clinical hold with respect to lomitapide, if the FDA has further or renewed concerns regarding pulmonary phospholipidosis, it could raise this issue as part of the NDA review process for lomitapide, which could delay or prevent marketing approval of lomitapide or result in adverse limitations in any approved labeling or on distribution and use of the product.
24
Table of Contents
Recent federal legislation and actions by state and local governments may permit re-importation of drugs from foreign countries into the United States, including foreign countries where the drugs are sold at lower prices than in the United States, which could materially adversely affect our operating results and our overall financial condition.
We may face competition in the United States for lomitapide or any other product candidate, if approved, from lower priced products from foreign countries that have placed price controls on pharmaceutical products. This risk may be particularly applicable to drugs such as lomitapide that are formulated for oral delivery and expected to command a premium price. The MMA contains provisions that may change U.S. importation laws and expand pharmacists’ and wholesalers’ ability to import lower priced versions of an approved drug and competing products from Canada, where there are government price controls. These changes to U.S. importation laws will not take effect unless and until the Secretary of Health and Human Services certifies that the changes will pose no additional risk to the public’s health and safety and will result in a significant reduction in the cost of products to consumers. The Secretary of Health and Human Services has not yet announced any plans to make this required certification.
A number of federal legislative proposals have been made to implement the changes to the U.S. importation laws without any certification, and to broaden permissible imports in other ways. Even if the changes do not take effect, and other changes are not enacted, imports from Canada and elsewhere may continue to increase due to market and political forces, and the limited enforcement resources of the FDA, U.S. Customs and Border Protection and other government agencies. For example, Pub. L. No. 111-83, which was signed into law in October 2009 and provides appropriations for the Department of Homeland Security for the 2010 fiscal year, expressly prohibits U.S. Customs and Border Protection from using funds to prevent individuals from importing from Canada less than a 90-day supply of a prescription drug for personal use, when the drug otherwise complies with the Federal Food, Drug, and Cosmetic Act, or FDCA. Further, several states and local governments have implemented importation schemes for their citizens, and, in the absence of federal action to curtail such activities, we expect other states and local governments to launch importation efforts.
The importation of foreign products that compete with lomitapide or any other product candidate for which we obtain marketing approval could negatively impact our revenue and profitability, possibly materially.
Our market is subject to intense competition. If we are unable to compete effectively, lomitapide or any other product candidate that we develop may be rendered noncompetitive or obsolete.
Our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. All of these competitors currently engage in, have engaged in or may engage in the future in the development, manufacturing, marketing and commercialization of new pharmaceuticals, some of which may compete with lomitapide or other product candidates. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Key competitive factors affecting the commercial success of lomitapide and any other product candidates that we develop are likely to be efficacy, safety and tolerability profile, reliability, convenience of dosing, price and reimbursement.
The market for lipid lowering therapeutics is large and competitive with many applicable drug classes. However, our products, if approved, will be focused, at least initially, on niche orphan markets where they will be positioned for use in combination with existing approved therapies, such as statins, to provide incremental efficacy in currently underserved patient populations. We believe that lomitapide will face distinct competition for the treatment of both HoFH and FC. Although there are no MTP-I compounds currently approved by the FDA for the treatment of hyperlipidemia, we are aware of other MTP-I compounds in early stage clinical trials and other pharmaceutical companies that are developing the following potentially competitive product candidates:
| § | HoFH — Isis Pharmaceuticals, Inc., or Isis, is developing an antisense apoB-100 inhibitor, mipomersen, as a weekly subcutaneous injection for lowering high cholesterol, LDL-C and apolipoprotein B and has completed four Phase III clinical trials for this product candidate. The FDA has granted mipomersen |
25
Table of Contents
| orphan drug designation for the treatment of patients with HoFH. Therefore, if mipomersen is approved by the FDA, Isis will be entitled to seven years of marketing exclusivity for such indication as to competitive products that are the same drug as mipomersen. In January 2008, Isis formed a partnership with Genzyme Corporation, now a subsidiary of Sanofi-Aventis, to develop and sell mipomersen. |
| § | FC — In December 2009, Amsterdam Molecular Therapeutics Holding N.V., or AMT, filed an application for marketing approval for Glybera, an injectable gene therapy, with the EMA for the treatment of patients with FC. AMT has stated that it is expecting approval of this MAA in 2011. This product candidate has been tested in a total of 27 patients in three different clinical trials. It would represent the first gene therapy approved in the European Union. |
| § | PCSK9 Defects — Several companies are developing molecules that attempt to mimic the impact observed in patients with defects in their PCSK9 gene. Such patients have lower LDL-C levels and an observed reduction in cardiovascular events, and some believe that medicines that duplicate this behavior may effectively reduce LDL-C levels with a similar benefit. Regeneron Pharmaceuticals, Inc. is among the companies thought to have one of the more advanced PCSK9 programs, with recent Phase I data from an injectable antibody it is developing having been presented at the American College of Cardiology meeting. |
If we obtain marketing approval of lomitapide for the treatment of patients with HoFH in the United States and Isis obtains marketing approval of mipomersen for the treatment of patients with HoFH in the United States, lomitapide would compete in the same market with mipomersen. If Isis obtains marketing approval of mipomersen for the treatment of patients with HoFH in the United States prior to us, Isis could obtain a significant competitive advantage associated with being the first to market and using Genzyme’s marketing and sales force. In connection with obtaining marketing approval for mipomersen, Isis will also obtain orphan drug exclusivity for mipomersen, but we do not believe that Isis’ obtaining orphan drug exclusivity for mipomersen prior to our receiving orphan drug exclusivity for lomitapide would have an adverse effect on our business as mipomersen and lomitapide are different drugs under FDA rules and any exclusivity applicable to either drug will not apply to the other drug. Thus, because mipomersen is a different drug than lomitapide, we could obtain both approval and orphan drug exclusivity for lomitapide even if Isis has already obtained orphan drug exclusivity for mipomersen and Isis could obtain both approval and orphan drug exclusivity for mipomersen even if we have already obtained orphan drug exclusivity for lomitapide.
Similarly, in the European Union, notwithstanding the fact that the EMA has granted lomitapide orphan drug designation for the treatment of FC, if a competitor subsequently obtains approval and market exclusivity for its orphan designated drug for the treatment of FC, our marketing application for FC would not be accepted and we would be excluded from the market only if lomitapide was a “similar medicinal product” to our competitor’s product. In the European Union, a drug is a “similar medicinal product” if it contains a “similar active substance” or substances and is intended for the same therapeutic indication. A “similar active substance” is an identical active substance, or an active substance with the same principal molecular structural features and which acts via the same mechanism. We do not believe orphan drug exclusivity for mipomersen in the European Union would have an adverse effect on our business because we do not believe that lomitapide would be considered a “similar medicinal product” to mipomersen.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drug candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining FDA and other marketing approvals for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render lomitapide or any other product candidate that we develop obsolete or non-competitive before we can recover the expenses of developing and commercializing the product. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render lomitapide or any other product candidate that we develop non-competitive or obsolete.
26
Table of Contents
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
The use of lomitapide or any other product candidate in clinical trials and the sale of lomitapide or any other product candidate for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers or others selling or otherwise coming into contact with our products. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| § | decreased demand for lomitapide or any other product candidate for which we obtain marketing approval; |
| § | impairment of our business reputation and exposure to adverse publicity; |
| § | increased warnings on product labels; |
| § | withdrawal of clinical trial participants; |
| § | costs of related litigation; |
| § | distraction of management’s attention from our primary business; |
| § | substantial monetary awards to patients or other claimants; |
| § | loss of revenue; and |
| § | the inability to successfully commercialize lomitapide or any other product candidate for which we obtain marketing approval. |
We have obtained product liability insurance coverage for our clinical trials with a $5.0 million annual aggregate coverage limit. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for lomitapide or any other product candidate, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain this product liability insurance on commercially reasonable terms. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
A variety of risks associated with our planned international business relationships could materially adversely affect our business.
We may enter into agreements with third parties for the development and commercialization of lomitapide or other product candidates in international markets. If we do so, we would be subject to additional risks related to entering into international business relationships, including:
| § | differing regulatory requirements for drug approvals in foreign countries; |
| § | potentially reduced protection for intellectual property rights; |
| § | the potential for so-called parallel importing, which is what happens when a local seller, faced with high or higher local prices, opts to import goods from a foreign market, with low or lower prices, rather than buying them locally; |
| § | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| § | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
27
Table of Contents
| § | compliance with tax, employment, immigration and labor laws for employees traveling abroad; |
| § | foreign taxes; |
| § | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| § | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| § | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| § | business interruptions resulting from geo-political actions, including war and terrorism, or natural disasters, including earthquakes, volcanoes, typhoons, floods, hurricanes and fires. |
These and other risks of international business relationships may materially adversely affect our ability to attain or sustain profitable operations.
Our relationships with customers and payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with third-party payors and customers will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
| § | The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. |
| § | The federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government. |
| § | The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. |
| § | The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. |
| § | The federal transparency requirements under the Health Care Reform Law requires manufacturers of drugs, devices, biologics, and medical supplies to report to the Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests. |
| § | Analogous state laws and regulations, such as state anti-kickback and false claims laws and transparency laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws |
28
Table of Contents
| require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and drug pricing. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations could be costly. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations, including anticipated activities conducted by our sales team, are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
We lack experience in commercializing products, which may have an adverse effect on our business.
We will need to transition from a company with a development focus to a company capable of supporting commercial activities. We may not be successful in such a transition. We have not yet demonstrated an ability to obtain marketing approval for or commercialize a product candidate. As a result, we may not be as successful as companies that have previously obtained marketing approval for drug candidates and commercially launched drugs.
Risks Related to Our Intellectual Property
If our patent position does not adequately protect our product candidates, others could compete against us more directly, which would harm our business, possibly materially.
As of the date of this prospectus, our lomitapide patent portfolio consists of five issued U.S. patents and related issued patents in Europe, Canada, Israel and Japan and related pending applications in the U.S., Europe, Australia, Japan, Canada, Israel, South Korea and New Zealand, all of which have been licensed to us in a specific field. The issued U.S. patents are scheduled to expire between 2013 and 2027. The U.S. patent covering the composition of matter of lomitapide is scheduled to expire in 2015. The non-U.S. patents directed to lomitapide are scheduled to expire in 2016. As of the date of this prospectus, our implitapide patent portfolio consists of four issued U.S. patents, two pending U.S. non-provisional applications, and related patents and pending applications in Europe, Australia, Asia, Africa, and South America. The issued U.S. patents are scheduled to expire between 2015 and 2017. The U.S. patent and non-U.S. patents covering the composition of matter of implitapide are scheduled to expire in 2015. We also have filed four non-provisional U.S. patent applications and two international applications directed to pharmaceutical combinations of a MTP-I, such as lomitapide or implitapide, and other cholesterol lowering drugs, and to methods of using such combinations in certain dosing regimens to reduce serum cholesterol or TG concentrations.
Our commercial success will depend significantly on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, product candidates and any future products in the United States and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. Our ability to use the patents and patent applications licensed to us and described above to protect our business will depend on our ability to comply with the terms of the applicable licenses and other agreements. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with
29
Table of Contents
certainty. Patents may be challenged, deemed unenforceable, invalidated or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, product candidates and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| § | we will be able to successfully commercialize our products before some or all of our relevant patents expire; |
| § | we or our licensors were the first to make the inventions covered by each of our pending patent applications; |
| § | we or our licensors were the first to file patent applications for these inventions; |
| § | others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
| § | any of our or our licensors’ pending patent applications will result in issued patents; |
| § | any of our or our licensors’ patents will be valid or enforceable; |
| § | any patents issued to us or our licensors and collaborators will provide a basis for commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| § | we will develop additional proprietary technologies or product candidates that are patentable; or |
| § | the patents of others will not have an adverse effect on our business. |
If we do not obtain protection under the Hatch-Waxman Amendments and similar foreign legislation by extending the patent terms and obtaining data exclusivity for our product candidates, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval, if any, of lomitapide or implitapide, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. Our U.S. composition of matter patent for lomitapide is scheduled to expire in 2015, and we plan to seek patent term extension for this patent. In the future, we intend to apply for restorations of patent term for some of our other currently owned or licensed patents to add patent life beyond their current expiration dates, depending on the expected length of clinical trials and other factors involved in the filing of the relevant NDA. However, we may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.
In addition, we believe that lomitapide and implitapide are new chemical entities in the United States and may be eligible for data exclusivity under the Hatch-Waxman Amendments. A drug can be classified as a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety. Under sections 505(c)(3)(E)(ii) and 505(j)(5)(F)(ii) of the FDCA, as amended, a new chemical entity that is granted marketing approval may, even in the absence of patent protections, be eligible for five years of data exclusivity in the United States following marketing approval, which period is reduced to four years if certain patents covering the new chemical entity or its method of use are challenged by a generic applicant. This data exclusivity, if granted, would preclude submission during the exclusivity period of 505(b)(2) applications or abbreviated new drug applications submitted by another company that references the new chemical entity application. In the
30
Table of Contents
European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from assessing a generic application for eight years, after which generic marketing authorization can be submitted but not marketed for two years. However, our compounds may not be considered to be new chemical entities for these purposes or be entitled to the period of data exclusivity. If we are not able to gain or exploit the period of data exclusivity, we may face significant competitive threats to our commercialization of these compounds from other manufacturers, including the manufacturers of generic alternatives. Further, even if our compounds are considered to be new chemical entities and we are able to gain the prescribed period of data exclusivity, another company nevertheless could also market another version of the drug if such company can complete a full NDA with a complete human clinical trial process and obtain marketing approval of its product.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and products could be significantly diminished.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, currently is considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing our products.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. There could be issued patents of which we are not aware that our products infringe. There also could be patents that we believe we do not infringe, but that we may ultimately be found to infringe. Moreover, patent applications are in some cases maintained in secrecy until patents are issued. The publication of discoveries in the scientific or patent literature frequently occurs substantially later than the date on which the underlying discoveries were made and patent applications were filed. Because patents can take many years to issue, there may be currently pending applications of which we are unaware that may later result in issued patents that our products infringe. For example, pending applications may exist that provide support or can be amended to provide support for a claim that results in an issued patent that our product infringes.
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that our products or the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization. Likewise, third parties may challenge or infringe upon our existing or future patents.
Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| § | the patentability of our inventions relating to our product candidates; and |
| § | the enforceability, validity or scope of protection offered by our patents relating to our product candidates. |
Even if we are successful in these proceedings, we may incur substantial costs and divert management’s time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to
31
Table of Contents
avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| § | incur substantial monetary damages; |
| § | encounter significant delays in bringing our product candidates to market; and |
| § | be precluded from manufacturing or selling our product candidates. |
In such event, our business could be adversely affected, possibly materially.
If we fail to comply with our obligations in our license agreements for our product candidates, we could lose license rights that are important to our business.
Our existing license agreements impose, and we expect any future license agreements that we enter into will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. In addition, our license agreement with The Trustees of the University of Pennsylvania, or The Trustees of UPenn, limits the field of use for lomitapide as a monotherapy or in combination with other dyslipidemic therapies for treatment of patients with severe hypercholesterolemia unable to come within 15% of the National Cholesterol Education Program, or NCEP, LDL-C goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe combined hyperlipidemia unable to come within 15% of the NCEP non-high density lipoprotein cholesterol goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe hypertriglyceridemia unable to reduce TG levels to less than 1,000 mg/dL on maximal tolerated therapy. If we fail to comply with the obligations and restrictions under our license agreements, including the limited field of use under our license agreement with The Trustees of UPenn, the applicable licensor may have the right to terminate the license, in which case we might not be able to market any product that is covered by the licensed patents. Any breach or termination of our license agreement with The Trustees of UPenn would have a particularly significant adverse effect on our business because of our reliance on the commercial success of lomitapide. Although we intend to comply with the restrictions on field of use in our license agreement with The Trustees of UPenn by seeking product labels for lomitapide, if approved, that are consistent with the license field, we may still be subject to the risk of breaching the license agreement if we are deemed to be promoting or marketing lomitapide for an indication not covered by any product label that we are able to obtain. In addition, because this restriction on the field of use limits the indications for which we can develop lomitapide, the commercial potential of lomitapide may not be as great as without this restriction.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such clinical trials. We may become involved in commercial disputes with these parties.
We do not have the ability to independently conduct clinical trials, and we rely on third parties such as CROs, medical institutions, academic institutions, such as UPenn, and clinical investigators to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities. However, if we sponsor clinical trials, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial, even if we use a CRO. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, if they need to be
32
Table of Contents
replaced or if the quality or accuracy of the data they provide is compromised due to the failure to adhere to regulatory requirements or our clinical trial protocols, or for other reasons, our development programs may be extended, delayed or terminated, marketing approvals for lomitapide or any other product candidate may be delayed or denied, and we may be delayed or precluded in our efforts to successfully commercialize lomitapide for targeted indications or any other product candidate.
In addition, we may from time to time become involved in commercial disputes with these third parties, for example regarding the quality of the services provided by these third parties or our ultimate liability to pay for services they purported to do on our behalf, or the value of such services. For example, in September 2010, a dispute arose with one of our third-party service providers regarding preliminary work they performed on our behalf related to a planned clinical trial for lomitapide in patients with HeFH. Although we do not expect that the ultimate resolution of this matter will be material to our financial position, cash flow or results of operations, we have not yet resolved this matter or agreed on the amount that we may ultimately pay. Due to our reliance on third-party service providers, we may experience commercial disputes such as these in the future. In some cases, we may be required to pay for work that was not performed to our specifications or not utilized by us, and these obligations may be material.
We do not have drug research or discovery capabilities, and will need to acquire or license existing drug compounds from third parties to expand our product candidate pipeline.
Both of our current product candidates have been licensed to us from third parties: lomitapide has been licensed to us by UPenn and implitapide has been licensed to us by Bayer Healthcare AG. We currently have no drug research or discovery capabilities. Accordingly, if we are to expand our product candidate pipeline beyond our two current drug candidates, we will need to acquire or license existing compounds from third parties. In addition, although we have the right to use implitapide for any indication, our right to use lomitapide is limited to specified patient populations, such as patients with HoFH, severe hypercholesterolemia or severe hypertriglyceridemia. Accordingly, if we wished to expand the development of lomitapide to address other indications, we would need to expand our license agreement with The Trustees of UPenn. We will face significant competition in seeking to acquire or license promising drug compounds. Many of our competitors for such promising compounds may have significantly greater financial resources and more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products, and thus, may be a more attractive option to a potential licensor than us. If we are unable to acquire or license additional promising drug compounds, we will not be able to expand our product candidate pipeline.
We currently depend on a single third-party manufacturer to produce our preclinical and clinical drug supplies and intend to rely upon third-party manufacturers to produce commercial supplies of lomitapide. This may increase the risk that we will not have sufficient quantities of lomitapide or such quantities at an acceptable cost, which could delay, prevent, or impair our clinical development and commercialization of lomitapide.
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We rely on a contract manufacturer to produce lomitapide required for our clinical trials and plan to rely on a contract manufacturer for initial commercial supplies. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of lomitapide if and when approved for marketing by the applicable regulatory authorities.
If we are unable to arrange for third-party manufacturing, or unable to do so on commercially reasonable terms, we may not be able to successfully complete development of lomitapide or market it. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured lomitapide ourselves, including:
| § | reliance on the third party for regulatory compliance and quality assurance; |
| § | the possibility of breach of the manufacturing agreement by the third party because of factors beyond our control; and |
33
Table of Contents
| § | the possibility of termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or damaging to us. |
In addition, the FDA and other regulatory authorities require that product candidates be manufactured according to cGMP. Any failure by our third-party manufacturers to comply with cGMP or failure to scale up our manufacturing processes could lead to a delay in, or failure to obtain, marketing approval of lomitapide or any other product candidate that we develop. In addition, such failure could be the basis for action by the FDA to withdraw approvals for product candidates previously granted to us and for other regulatory action, including seizure, injunction or other civil or criminal penalties.
We currently rely on a single manufacturer for the preclinical and clinical supplies of lomitapide. We purchase these supplies from this manufacturer on a purchase order basis and do not have a long-term supply agreement in place. We also do not have agreements in place for redundant supply or second source for lomitapide. Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval of lomitapide or commercialization of lomitapide. If for some reason our current contract manufacturer cannot perform as agreed, we may be required to replace that manufacturer. Although we believe there are a number of potential replacements that could manufacture the clinical and commercial supply of lomitapide, we may incur added costs and delays in identifying and qualifying any such replacements. Furthermore, although we generally do not begin a clinical trial unless we have a sufficient supply of a product candidate for the trial, any significant delay in the supply of a product candidate for an ongoing trial due to the need to replace a third-party manufacturer could delay completion of the trial.
For example, in June 2007, we voluntarily halted a Phase II clinical trial of lomitapide because of suspected microbial contamination, which we believe resulted in patients experiencing gastrointestinal adverse events at a rate, severity and rapidity of onset inconsistent with prior clinical data for lomitapide. See “Risk Factors — Risks Associated with Product Development and Commercialization — Failures or delays in the commencement or completion of preclinical or clinical testing could result in increased costs to us and delay, prevent or limit our ability to generate revenue” for more information.
If we receive marketing approval of lomitapide, we currently expect to continue to rely upon third-party manufacturers to produce commercial supplies of the product. Accordingly, we will need to identify commercial- scale manufacturers, or our manufacturers of clinical supplies will need to increase their scale of production to meet our projected needs for commercial manufacturing.
Lomitapide and any other product candidate that we develop may compete with other products and product candidates for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that are both capable of manufacturing for us and willing to do so. We may not be able to identify, or reach agreement with, commercial scale manufacturers on commercially reasonably terms, or at all. If we are unable to do so, we will need to develop our own commercial-scale manufacturing capabilities, which likely would: delay commercialization of lomitapide, if approved, possibly materially; require a capital investment by us that could be quite costly; and increase our operating expenses.
If our existing third-party manufacturers, or the third parties that we engage in the future to manufacture a product for commercial sale or for our clinical trials, should cease to continue to do so for any reason, we likely would experience delays in obtaining sufficient quantities for us to meet commercial demand or to advance our clinical trials while we identify and qualify replacement suppliers. If for any reason we are unable to obtain adequate supplies of lomitapide or any other product candidate that we develop or the drug substances used to manufacture it, it will be more difficult for us to develop lomitapide and compete effectively. In addition, if, following approval, if any, we are unable to assure a sufficient quantity of the drug for patients with rare diseases or conditions, we may lose any orphan drug exclusivity to which the product otherwise would be entitled.
If we do not establish successful collaborations, we may have to alter our development plans.
Our drug development programs and potential commercialization of lomitapide will require substantial additional cash to fund expenses. We may selectively seek to establish collaborations to reach patients with HoFH or FC in
34
Table of Contents
geographies that we do not believe we can cost effectively address with our own sales and marketing capabilities. If we elect to develop lomitapide for broader patient populations, we would plan to do so selectively either on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources. If we determine to enter into these collaborations, we may do so in order to:
| § | fund our research and development activities; |
| § | access manufacturing capabilities of third parties; |
| § | help us conduct clinical trials; and |
| § | successfully commercialize lomitapide, if approved. |
We face significant competition in seeking appropriate collaborators and these collaborations are complex and time-consuming to negotiate, document and implement. We may not be able to negotiate collaborations on acceptable terms, or at all. If that were to occur, we may have to curtail the development of a particular product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring lomitapide to market and generate product revenue.
If a collaborative partner terminates or fails to perform its obligations under an agreement with us, the development and commercialization of lomitapide could be delayed or terminated.
We are not currently party to any collaborative arrangements. However, we plan to pursue such arrangements if we decide to develop lomitapide in broader patient populations. If we are successful in entering into collaborative arrangements and any of our collaborative partners does not devote sufficient time and resources to a collaboration arrangement with us, we may not realize the potential commercial benefits of the arrangement, and our results of operations may be adversely affected. In addition, if any future collaboration partner were to breach or terminate its arrangements with us, the development and commercialization of the affected product candidate could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue development and commercialization of the product candidate on our own.
Much of the potential revenue from future collaborations may consist of contingent payments, such as payments for achieving development milestones and royalties payable on sales of drugs developed. The milestone and royalty revenue that we may receive under these collaborations will depend upon our collaborator’s ability to successfully develop, introduce, market and sell new products. In addition, collaborators may decide to enter into arrangements with third parties to commercialize products developed under collaborations using our technologies, which could reduce the milestone and royalty revenue that we may receive, if any. Future collaboration partners may fail to develop or effectively commercialize products using our products or technologies because they:
| § | decide not to devote the necessary resources due to internal constraints, such as limited personnel with the requisite scientific expertise, limited cash resources or specialized equipment limitations, or the belief that other drug development programs may have a higher likelihood of obtaining marketing approval or may potentially generate a greater return on investment; |
| § | decide to pursue other technologies or develop other product candidates, either on their own or in collaboration with others, including our competitors, to treat the same diseases targeted by our own collaborative programs; |
| § | do not have sufficient resources necessary to carry the product candidate through clinical development, marketing approval and commercialization; or cannot obtain the necessary marketing approvals. |
Competition may negatively impact a partner’s focus on and commitment to our product candidate and, as a result, could delay or otherwise negatively affect the commercialization of our product candidate. If future
35
Table of Contents
collaboration partners fail to develop or effectively commercialize lomitapide or any other product candidate for which we obtain marketing approval for any of these reasons, we may not be able to replace the collaboration partner with another partner to develop and commercialize the product candidate under the terms of the collaboration. We may also be unable to obtain, on terms acceptable to us, a license from such collaboration partner to any of its intellectual property that may be necessary or useful for us to continue to develop and commercialize a product candidate.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to hire and retain our key executives and to attract, retain, and motivate qualified personnel.
We are highly dependent on Marc Beer, our Chief Executive Officer, and the other principal members of our executive and development teams. We have entered into employment agreements with certain members of our executive and development teams, but any employee may terminate his or her employment with us at any time. For example, in June 2011, William H. Lewis resigned as our President to pursue other interests. We do not maintain “key man” life insurance for any of our employees. The loss of the services of any of these persons might impede the achievement of our research, development and commercialization objectives.
We expect to continue hiring qualified development personnel. Recruiting and retaining qualified development personnel and sales and marketing personnel will be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of development personnel from universities and research institutions. Failure to succeed in clinical trials may make it more challenging to recruit and retain qualified development personnel.
In addition, as a result of becoming a public company, we need to continue to establish and maintain effective disclosure and financial controls and make changes in our corporate governance practices. We will need to hire additional accounting and financial personnel with appropriate public company experience and technical accounting knowledge, and it may be difficult to recruit and maintain such personnel. Under the Nasdaq Stock Market, Inc. Marketplace Rules Audit Committee requirements, we need to appoint a chairman of our Audit Committee by October 2011.
In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
We will need to grow our organization, and we may encounter difficulties in managing this growth, which could disrupt our operations.
We currently have only twenty employees, and we expect to experience significant growth in the number of our employees and the scope of our operations. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel, which may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of lomitapide. If our management is unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize lomitapide and compete effectively will depend, in part, on our ability to effectively manage any future growth.
36
Table of Contents
Risks Related to the Securities Markets and Investment in Our Common Stock
The market price of our common stock has been, and may continue to be, highly volatile.
Our stock price is volatile, and from October 22, 2010, the first day of trading of our common stock, to May 6, 2011, the trading prices of our stock have ranged from $9.00 to $25.92 per share. This is in part because there has been a public market for our common stock only since our initial public offering in October 2010, and our stock could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including the following:
| § | plans for, progress in and results from clinical trials of our product candidates generally; |
| § | the results of our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH; |
| § | the initiation and results of our planned Phase III clinical trial of lomitapide for the treatment of adult patients with FC; |
| § | the timing of regulatory filings related to these indications; |
| § | announcements of new products, services or technologies, commercial relationships, acquisitions or other events by us or our competitors; |
| § | failure of lomitapide, if approved, to achieve commercial success; |
| § | fluctuations in stock market prices and trading volumes of similar companies; |
| § | general market conditions and overall fluctuations in U.S. equity markets; |
| § | variations in our quarterly operating results; |
| § | changes in our financial guidance or securities analysts’ estimates of our financial performance; |
| § | changes in accounting principles; |
| § | sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; |
| § | additions or departures of key personnel; |
| § | success or failure of products within our therapeutic area of focus; |
| § | discussion of us or our stock price by the financial press and in online investor communities; and |
| § | other risks and uncertainties described in these risk factors. |
In addition, the stock market in general, and the market for small pharmaceutical and biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, following periods of volatility in the market, securities class-action litigation has often been instituted against companies. Such litigation, if instituted against us, could result in substantial costs and diversion of management’s attention and resources, which could materially and adversely affect our business and financial condition.
We may allocate the net proceeds from this offering in ways that you and other stockholders may not approve.
We currently plan to use the net proceeds of this offering to prepare and submit our NDA for lomitapide for the treatment of patients with HoFH based on our Filing Data, to complete our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH and submit the data collected through week 78 of this trial to the FDA to complete our NDA submission and to the EMA to complete our MAA submission, to commercially launch lomitapide for the treatment of patients with HoFH, to advance the clinical development of
37
Table of Contents
lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH and of adult patients with FC and any remainder to fund working capital, capital expenditures and other general corporate purposes, including the repayment of the principal loan balance as it comes due under the Loan and Security Agreement with the Hercules Funds. Because of the number and variability of factors that will determine our use of the proceeds from this offering, their ultimate use may vary substantially from their currently intended use. As such, our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our operating results or enhance the value of our common stock. For a further description of our intended use of the proceeds of the offering, see “Use of Proceeds.”
Our directors and management will exercise significant control over our company, which will limit your ability to influence corporate matters.
After this offering, our executive officers, directors, 5% stockholders and their affiliates will collectively control approximately % of our outstanding common stock. As a result, these stockholders, if they act together, will be able to influence our management and affairs and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control of our company that other stockholders may desire and might negatively affect the market price of our common stock.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and by-laws may delay or prevent an acquisition of us or a change in our management. These provisions include a classified board of directors, a prohibition on actions by written consent of our stockholders and the ability of our board of directors to issue preferred stock without stockholder approval. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Although we believe these provisions collectively provide for an opportunity to obtain greater value for stockholders by requiring potential acquirors to negotiate with our board of directors, they would apply even if an offer rejected by our board were considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
We do not intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our common stock and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
Future sales of our common stock may cause our stock price to decline.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could significant reduce the market price of our common stock and impair our ability to raise adequate capital through the sale of additional equity securities.
If our existing stockholders sell, or if the market believes our existing stockholders will sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline significantly.
38
Table of Contents
As of May 31, 2011, there were:
| § | 2,174,642 shares subject to outstanding options under our 2006 Stock Option and Award Plan and 2010 Stock Option and Incentive Plan; |
| § | 29,890 shares of restricted common stock subject to vesting as of May 31, 2011; |
| § | 107,779 shares of common stock subject to warrants outstanding; and |
| § | an aggregate of 2,814,971 shares reserved for future issuance under our 2010 Stock Option and Incentive Plan, as well as any automatic increases in the number of shares of our common stock reserved for future issuance under these benefit plans. |
If additional shares are sold, or if it is perceived that they will be sold, in the public market, the price of our common stock could decline substantially.
Some of our existing stockholders have demand and piggyback rights to require us to register with the SEC up to approximately 9.1 million shares of our common stock, including shares issuable upon exercise of outstanding options. If we register these shares of common stock, the stockholders would be able to sell those shares freely in the public market.
We also registered approximately 4.4 million shares of our common stock that are subject to outstanding stock options and reserved for issuance under our equity plans. These shares can be freely sold in the public market upon issuance, subject to vesting restrictions.
If you purchase our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
You will suffer immediate and substantial dilution in the net tangible book value of the common stock you purchase in this offering. The public offering price of our common stock is substantially higher than the net tangible book value per share of our outstanding common stock immediately after this offering. Purchasers of common stock in this offering will experience immediate dilution of approximately $13.50 per share in net tangible book value of the common stock. In addition, investors purchasing common stock in this offering will contribute approximately $57.6 million of the total amount invested by stockholders since inception, but will only own approximately 20% of the shares of common stock outstanding. In the past, we issued restricted stock and options to acquire common stock at prices significantly below the public offering price. To the extent these outstanding options are ultimately exercised, investors purchasing common stock in this offering will sustain further dilution. See “Dilution” for a more detailed description of the dilution to new investors in the offering.
39
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) and are made pursuant to the safe harbors of the PSLRA. All statements, other than statements of historical facts, contained in this prospectus, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by terminology such as “may,” “will,” “would,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar words, although not all forward-looking statements contain these identifying words.
The forward-looking statements contained in this prospectus include, among other things, statements about:
| § | our expectations related to the use of proceeds from this offering; |
| § | the progress and timing of our development and commercialization activities; |
| § | the timing and conduct of our clinical trials for our lead compound, lomitapide and the related timing of our NDA and MAA submissions seeking marketing approval in the United States and European Union, respectively; |
| § | our ability to obtain U.S. and foreign marketing approval for lomitapide and the ability of lomitapide to meet existing or future regulatory standards; |
| § | the potential benefits and effectiveness of lomitapide; |
| § | the accuracy of our estimates of the size and characteristics of the markets that may be addressed by lomitapide; |
| § | our ability to manufacture sufficient amounts of lomitapide for clinical trials and commercialization activities; |
| § | our ability to recruit a sales and marketing team for the commercialization of lomitapide once marketing approval has been obtained; |
| § | our potential need for additional capital to fund operations and develop our product candidates; |
| § | risks associated with undesirable side effects experienced by some patients in clinical trials for our product candidates; |
| § | risks associated with our intellectual property rights and the extent to which such intellectual property rights protect our product candidates; |
| § | risks associated with our plan to apply for an ATU for lomitapide in France; |
| § | risks associated with difficulty in setting a market price for lomitapide; |
| § | risks associated with unfavorable results in our ongoing clinical trials of lomitapide; |
| § | risks associated with delayed or failed clinical trials of lomitapide; and |
| § | risks associated with volatility in our stock price as a recently public company. |
We have based these forward-looking statements largely on our current plans, intentions, expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations described in the forward-looking statements that we make. We have included important factors in the cautionary statements included in this prospectus, particularly in the “Risk Factors” section, that we believe could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements that we make. In
40
Table of Contents
light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified timeframe, or at all. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. We do not assume any obligation to update any forward-looking statements.
41
Table of Contents
We estimate that the net proceeds of the sale of the common stock that we are offering will be approximately $53.7 million, or $62.0 million if the underwriters exercise their over-allotment option in full, based on an assumed public offering price of $17.72 per share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
Each $1.00 increase (decrease) in the public offering price per share would increase (decrease) the net proceeds to us from this offering, after deducting underwriting discounts and commissions and our estimated offering expenses, by approximately $3.1 million, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same and that the underwriters do not exercise their over-allotment option. Depending on market conditions and other considerations at the time we price this offering, we may sell a greater or lesser number of shares than the number set forth on the cover page of this prospectus. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting underwriting discounts and commissions and our estimated offering expenses, by approximately $16.7 million, assuming the public offering price per share remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the public offering price per share, would increase the net proceeds to us from this offering, after deducting underwriting discounts and commissions and our estimated offering expenses, by approximately $20.7 million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the public offering price per share, would decrease the net proceeds to us from this offering, after deducting underwriting discounts and commissions and our estimated offering expenses, by approximately $18.8 million. This information is illustrative only, and following the pricing of this offering, we will update this information based on the actual public offering price and other terms of this offering.
We estimate that we will use the proceeds of this offering as follows:
| § | to prepare and submit our NDA for lomitapide for the treatment of patients with HoFH based on our Filing Data; |
| § | to complete our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH and submit the data collected through week 78 of this trial to the FDA to complete our NDA submission and to the EMA to complete our MAA submission; |
| § | to commercially launch lomitapide for the treatment of patients with HoFH; |
| § | to advance the clinical development of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH and of adult patients with FC; and |
| § | any remainder to fund working capital, capital expenditures and other general corporate purposes, including the repayment of the principal loan balance as it comes due under the Loan and Security Agreement with the Hercules Funds. |
Pursuant to the Loan and Security Agreement, the Hercules Funds will make available to us term loans in an aggregate principal amount of up to $25.0 million. The $25.0 million credit facility provides for an initial advance at closing of $10.0 million, with interest-only payments for twelve months, and bears per annum interest at the greater of 10.4% or 10.4% plus prime minus 4.75%. We may request additional term loan advances of up to $15.0 million. As of the date of this prospectus, we have $10.0 million outstanding under the Loan and Security Agreement. We plan to begin repaying the principal loan balance in April of 2012, in accordance with the terms of the Loan and Security Agreement.
As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the proceeds from this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our development and commercialization efforts, the status of and results from our clinical trials, whether or not we enter into strategic collaborations or partnerships, and our operating costs and expenditures. Accordingly, our management will have significant flexibility in applying the net proceeds of this offering.
42
Table of Contents
The costs and timing of drug development and marketing approval, particularly conducting clinical trials, are highly uncertain, are subject to substantial risks and can often change. Accordingly, we may change the allocation of use of these proceeds as a result of contingencies such as the progress and results of our clinical trials and other development activities, the establishment of collaborations, our manufacturing requirements and regulatory or competitive developments.
Based on our current operating plan, we anticipate that the net proceeds of this offering, together with our existing cash, cash equivalents and borrowing capacity under our Loan and Security Agreement with the Hercules Funds, will be sufficient to enable us to maintain our currently planned operations, including our continued product candidate development, at least through the next 24 months. We believe that these available funds will allow us to complete our ongoing pivotal Phase III clinical trial of, seek marketing approval for and commercially launch lomitapide for the treatment of patients with HoFH. In addition, we believe that these available funds will allow us to initiate a Phase III pediatric clinical trial of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH and a Phase III clinical trial of lomitapide for the treatment of adult patients with FC. However, we may require additional funds in order to complete our planned clinical trial of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH and adult patients with FC or to conduct additional clinical trials or seek marketing approval of lomitapide. We may seek these funds through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. Other than the Loan and Security Agreement with the Hercules Funds, we have no committed external sources of funds. Additional financing may not be available when we need it or may not be available on terms that are favorable to us. If adequate funds are not available to us on a timely basis, or at all, we may be required to terminate or delay clinical trials or other development activities for lomitapide for one or more indications for which we are developing lomitapide, or delay our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize lomitapide, if we obtain marketing approval. Because of the risks and uncertainties associated with the development and commercialization of lomitapide, we are unable to estimate the amounts of additional funds we may require in connection with our planned clinical trial of lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH or treatment of adult patients with FC or our future anticipated clinical trials or to achieve FDA approval and commercial introduction of lomitapide. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources — Future Funding Requirements.”
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in short-term, interest-bearing, investment grade securities.
We will not receive any proceeds from the shares sold by the Selling Stockholders.
43
Table of Contents
Our common stock has been listed on The NASDAQ Global Market under the symbol “AEGR” since our initial public offering on October 22, 2010. Prior to that offering, there was no public market for our common stock. The following table sets forth, for the periods indicated, the high and low intraday sales prices of our common stock as reported by The NASDAQ Global Market:
| High | Low | |||||||
| Year ended 2010 |
||||||||
| Fourth Quarter (beginning October 22) |
$ | 15.24 | $ | 9.00 | ||||
| Year ended 2011 |
||||||||
| First Quarter |
$ | 18.00 | $ | 11.54 | ||||
| Second Quarter (through June 15) |
$ | 25.92 | $ | 15.76 | ||||
On June 17, 2011, the closing price of our common stock as reported on The NASDAQ Global Market of our common stock was $17.72. As of the date of this prospectus, we had approximately 450 holders of record of our common stock.
We have never declared or paid dividends on our common stock. Our board of directors will continue to have discretion in determining whether to declare or pay dividends, which will depend upon our financial condition, results of operations, capital requirements and other factors our board of directors deems relevant. We currently anticipate that we will retain any future earnings for the development, operation and expansion of our business and the repayment of indebtedness. In addition, under our Loan and Security Agreement with the Hercules Funds, we are prohibited from declaring or paying any cash dividends. Accordingly, we do not anticipate declaring or paying any cash dividends for the foreseeable future.
44
Table of Contents
The following table sets forth our capitalization as of March 31, 2011:
| § | on an actual basis; and |
| § | on an as adjusted basis to give effect to the sale of 3,250,000 shares of our common stock we are offering at an assumed public offering price of $17.72 per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read the following table together with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of this prospectus and our financial statements and the related notes appearing at the end of this prospectus.
| March 31, 2011 | ||||||||
| Actual | As Adjusted | |||||||
| (Unaudited) (In thousands, except share |
||||||||
| Cash and cash equivalents |
$ | 46,828 | $ | 100,505 | ||||
| Long term debt |
10,000 | 10,000 | ||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value; 5,000,000 shares authorized; no shares issued and outstanding, actual and as adjusted |
— | — | ||||||
| Common stock, $0.001 par value; 125,000,000 shares authorized; 17,745,300 shares issued and 17,641,543 shares outstanding, actual and 20,995,300 shares issued and 20,891,543 shares outstanding, as adjusted |
18 | 21 | ||||||
| Additional paid-in capital |
132,721 | 186,395 | ||||||
| Accumulated other comprehensive loss |
695 | 695 | ||||||
| Deficit accumulated during the development stage |
(97,821 | ) | (97,821 | ) | ||||
| Total stockholders’ equity |
35,613 | 89,290 | ||||||
| Total capitalization |
$ | 45,613 | $ | 99,290 | ||||
Each $1.00 increase (decrease) in the public offering price per share would increase (decrease) each of cash and cash equivalents, additional paid in capital, total stockholders’ equity and total capitalization by approximately $3.1 million, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same and that the underwriters do not exercise their over-allotment option. Depending on market conditions and other considerations at the time we price this offering, we may sell a greater or lesser number of shares than the number set forth on the cover page of this prospectus. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of cash and cash equivalents, additional paid in capital, total stockholders’ equity and total capitalization by approximately $16.7 million, assuming the public offering price per share remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the expected public offering price per share, would increase each of cash and cash equivalents, additional paid in capital, total stockholders’ equity and total capitalization by approximately $20.7 million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the public offering price per share, would decrease each of cash and cash equivalents, additional paid in capital, total stockholders’ equity and total capitalization by approximately $18.8 million. This information is illustrative only, and following the pricing of this offering, we will update this information based on the actual public offering price and other terms of this offering.
45
Table of Contents
The table above does not include:
| § | 2,174,642 shares of common stock issuable upon the exercise of options outstanding as of May 31, 2011 at a weighted average exercise price of $4.79 per share; |
| § | 29,890 shares of restricted common stock subject to vesting as of May 31, 2011; |
| § | 107,779 shares of common stock subject to outstanding warrants outstanding as of May 31, 2011, with a exercise price of $6.68 per share; and |
| § | 2,814,971 additional shares of common stock available for future issuance under our 2010 Option Plan, as of May 31, 2011, which is subject to an annual increase of shares of common stock available for issuance under the 2010 Option Plan equal to four percent of the number of shares of common stock issued and outstanding on the immediately preceding December 31. |
46
Table of Contents
If you invest in our common stock, you will experience dilution to the extent of the difference between the public offering price per share of our common stock you pay in this offering and the net tangible book value per share of our common stock after this offering.
Our net tangible book value as of March 31, 2011 was $34.7 million, or $1.96 per share, based on shares of common stock outstanding as of March 31, 2011. Net tangible book value per share represents the amount of our total tangible assets less total liabilities, all divided by the total number of shares of our common stock outstanding on March 31, 2011.
After giving effect to the sale by us of shares of common stock we are offering at an assumed public offering price of $17.72 per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of March 31, 2011 would have been approximately $88.3 million, or approximately $4.22 per share of common stock. This represents an immediate increase in net tangible book value of approximately $2.26 per share to existing stockholders and an immediate dilution of approximately $13.50 per share to new investors purchasing shares of common stock in this offering. The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
$ | 17.72 | ||||||
| Net tangible book value per share as of March 31, 2011 |
1.96 | |||||||
| Increase per share attributable to this offering |
2.26 | |||||||
| As adjusted net tangible book value per share after this offering |
4.22 | |||||||
| Dilution per share to investors in this offering |
$ | 13.50 | ||||||
If the underwriters fully exercise their over-allotment option, as adjusted net tangible book value after this offering would increase to approximately $4.51 per share, and there would be an immediate dilution of approximately $13.21 per share to new investors.
A $1.00 increase (decrease) in the public offering price per share would increase (decrease) our as adjusted net tangible book value by approximately $3.1 million, or $0.15 per share, and would decrease (increase) dilution to investors in this offering by $0.15 per share, assuming that the number of shares we are offering, as set forth on the cover page of this prospectus, remains the same and that the underwriters do not exercise their over-allotment option. Depending on market conditions and other considerations at the time we price this offering, we may sell a greater or lesser number of shares than the number set forth on the cover page of this prospectus. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) our as adjusted net tangible book value by approximately $16.7 million, or $0.57 per share, and would decrease (increase) dilution to investors in this offering by $0.57 per share, assuming the public offering price per share remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the expected public offering price per share, would increase our as adjusted net tangible book value as of March 31, 2011 by approximately $20.7 million, or $0.76 per share, and would decrease dilution to investors in this offering by approximately $0.76 per share. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the initial public offering price per share, would decrease our as adjusted net tangible book value as of March 31, 2011 by approximately $18.8 million, or approximately $0.72 per share, and would increase dilution to investors in this offering by approximately $0.72 per share. This information is illustrative only, and following the pricing of this offering, we will update this information based on the actual public offering price and other terms of this offering.
To the extent that outstanding options or warrants with an exercise price per share that is less than the as adjusted net tangible book value per share are exercised, you will experience further dilution. If all of our outstanding options and warrants described above were exercised, our as adjusted net tangible book value as of March 31, 2011 after this offering would have been approximately $91.2 million, or approximately $4.04 per share, representing dilution to new investors of approximately $13.68 per share.
47
Table of Contents
The information and tables in this section are based on 17,641,543 shares of common stock issued and outstanding as of March 31, 2011 and exclude:
| § | 2,174,642 shares of common stock issuable upon the exercise of options outstanding as of May 31, 2011 at a weighted average exercise price of $4.79 per share; |
| § | 29,890 shares of restricted common stock subject to vesting as of May 31, 2011; |
| § | 107,779 shares of common stock subject to outstanding warrants outstanding as of May 31, 2011, with a exercise price of $6.68 per share; and |
| § | 2,814,971 additional shares of common stock available for future issuance under our 2010 Option Plan, as of May 31, 2011, which is subject to an annual increase of shares of common stock available for issuance under the 2010 Option Plan equal to four percent of the number of shares of common stock issued and outstanding on the immediately preceding December 31. |
48
Table of Contents
You should read the following selected financial data together with the “Capitalization,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of this prospectus and our financial statements and the related notes appearing at the end of this prospectus. We have derived the statement of operations data for the years ended December 31, 2008, 2009 and 2010 and balance sheet data as of December 31, 2009 and 2010 from our audited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the three months ended March 31, 2010 and 2011 and the balance sheet data as of March 31, 2011 from our unaudited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the period from February 4, 2005 (inception) to March 31, 2011 from our unaudited financial statements appearing at the end of this prospectus. We have derived the statement of operations data for the year ended December 31, 2006 and 2007 and the balance sheet data as of December 2006, 2007 and 2008 from our audited financial statements not included in this prospectus. We have derived the statement of operations data for the year ended December 31, 2006 and 2007 and the balance sheet data as of December 31, 2006, 2007 and 2008 from our audited financial statements not included in this prospectus. The unaudited financial statements have been prepared on the same basis as our audited financial statements and include, in the opinion of management, all adjustments that management considers necessary for a fair presentation of the financial information set forth in those statements. Our historical results for any prior period are not necessarily indicative of results expected in any future period and our interim results are not necessarily indicative of results for a full year.
| Year Ended December 31, | Three Months Ended March 31, |
Period from February 4, (inception) 2005 to March 31, |
||||||||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2010 | 2011 | 2011 | |||||||||||||||||||||||||
| (in thousands, except share and per share data) | (Unaudited) | |||||||||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||||||||||
| Costs and Expenses: |
||||||||||||||||||||||||||||||||
| Research and Development |
$ | 3,801 | $ | 13,542 | $ | 17,712 | $ | 7,041 | $ | 7,629 | $ | 1,066 | $ | 3,297 | $ | 53,164 | ||||||||||||||||
| General and administrative |
3,238 | 6,079 | 5,185 | 3,075 | 5,921 | 972 | 3,490 | 28,036 | ||||||||||||||||||||||||
| Total costs and expenses |
7,039 | 19,621 | 22,897 | 10,116 | 13,550 | 2,038 | 6,787 | 81,200 | ||||||||||||||||||||||||
| Loss from operations |
(7,039 | ) | (19,621 | ) | (22,897 | ) | (10,116 | ) | (13,550 | ) | (2,038 | ) | (6,787 | ) | (81,200 | ) | ||||||||||||||||
| Interest expense |
— | (1,048 | ) | (1,127 | ) | (2,083 | ) | (2,404 | ) | (601 | ) | (113 | ) | (7,010 | ) | |||||||||||||||||
| Interest income |
871 | 1,008 | 533 | 177 | 109 | 18 | 68 | 2,784 | ||||||||||||||||||||||||
| Change in fair value of warrant liability |
— | 237 | 91 | (174 | ) | (416 | ) | — | — | (263 | ) | |||||||||||||||||||||
| Other than temporary impairment or securities |
— | (770 | ) | (1,665 | ) | — | (30 | ) | — | — | (2,466 | ) | ||||||||||||||||||||
| Other income, net |
— | — | 31 | — | 244 | — | — | 275 | ||||||||||||||||||||||||
| Loss before income taxes |
(6,168 | ) | (20,194 | ) | (25,035 | ) | (12,196 | ) | (16,047 | ) | (2,622 | ) | (6,832 | ) | (87,879 | ) | ||||||||||||||||
| Benefit from income taxes |
— | — | — | — | 1,793 | 1,793 | — | 1,793 | ||||||||||||||||||||||||
| Net loss |
(6,168 | ) | (20,194 | ) | (25,035 | ) | (12,196 | ) | (14,254 | ) | (829 | ) | (6,832 | ) | (86,086 | ) | ||||||||||||||||
| Less accretion of preferred stock dividends and other deemed dividends |
(1,652 | ) | (3,658 | ) | (6,242 | ) | (3,287 | ) | (8,751 | ) | (862 | ) | — | (23,663 | ) | |||||||||||||||||
| Net loss attributable to common stockholders |
$ | (7,820 | ) | $ | (23,852 | ) | $ | (31,277 | ) | $ | (15,483 | ) | $ | (23,005 | ) | $ | (1,691 | ) | $ | (6,832 | ) | $ | (109,750 | ) | ||||||||
| Net loss attributable to common stockholders per share — basic and diluted |
$ | (8.30 | ) | $ | (19.15 | ) | $ | (20.92 | ) | $ | (9.35 | ) | $ | (5.07 | ) | $ | (0.99 | ) | $ | (0.39 | ) | |||||||||||
| Weighted-average shares outstanding — basic and diluted |
941,283 | 1,245,246 | 1,495,375 | 1,656,732 | 4,537,407 | 1,701 | 17,642 | |||||||||||||||||||||||||
49
Table of Contents
| As of December 31, | As of March 31, |
|||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
| (in thousands) | (Unaudited) | |||||||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 16,563 | $ | 24,393 | $ | 9,005 | $ | 1,429 | $ | 44,101 | $ | 46,828 | ||||||||||||
| Total assets |
16,771 | 28,479 | 10,252 | 2,650 | 45,747 | 50,014 | ||||||||||||||||||
| Debt financing and convertible notes |
— | 9,180 | 16,098 | 20,096 | — | 10,000 | ||||||||||||||||||
| Deficit accumulated during the development stage |
(8,443 | ) | (30,466 | ) | (58,664 | ) | (70,857 | ) | (90,989 | ) | (97,821 | ) | ||||||||||||
| Total stockholders’ equity (deficiency) |
$ | (8,439 | ) | $ | (28,717 | ) | $ | (55,621 | ) | $ | (70,127 | ) | $ | 41,078 | $ | 35,613 | ||||||||
50
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing at the end of this prospectus. In addition to historical information, some of the information in this discussion and analysis contains forward-looking statements reflecting our current expectations and involves risk and uncertainties. For example, statements regarding our expectations as to our plans and strategy for our business, future financial performance, expense levels and liquidity sources are forward-looking statements. Our actual results and the timing of events could differ materially from those discussed in our forward-looking statements as a result of many factors, including those set forth under the “Risk Factors” section and elsewhere in this prospectus.
Overview
We are an emerging biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat severe lipid disorders. Lipids are naturally occurring molecules, such as cholesterol and triglycerides, which are transported in the blood. Elevated levels of cholesterol, or hypercholesterolemia, and elevated levels of triglycerides, or hypertriglyceridemia, can dramatically increase the risk of experiencing a potentially life threatening cardiovascular event, such as a heart attack or stroke in the case of hypercholesterolemia or pancreatitis in the case of hypertriglyceridemia. Our lead compound, lomitapide, is a microsomal triglyceride transfer protein inhibitor, or MTP-I, which limits secretion of cholesterol and triglycerides from the intestines and the liver, the main sources of lipids in the body. We are initially developing lomitapide, as an oral, once-a-day treatment for patients with a rare inherited lipid disorder called homozygous familial hypercholesterolemia, or HoFH. These patients are at very high risk of experiencing life threatening cardiovascular events as a result of extremely elevated cholesterol levels in the blood, and as a result, have a substantially reduced life span relative to unaffected individuals. We also plan to develop lomitapide for the treatment of patients with a rare genetic lipid disorder called familial chylomicronemia, or FC. Patients with FC have extremely high levels of triglycerides, or TGs, and, as a result, typically experience recurrent episodes of acute pancreatitis and other serious conditions.
We are currently evaluating lomitapide in a pivotal Phase III clinical trial for the treatment of patients with HoFH. On May 31, 2011, we announced the results of this trial, through 56 weeks of treatment. We believe based on our prior discussions with the U.S. Food and Drug Administration, or FDA, that these results demonstrate sufficient long-term safety and efficacy to support the submission of our New Drug Application, or NDA, for lomitapide. We refer to these week 56 results as our Filing Data, and we will later supplement the Filing Data with data reflecting the full 78-week trial duration. Before we can submit an NDA, we must complete additional clinical and non-clinical studies to assess various other aspects of lomitapide. On June 15, 2011, we met with the FDA, which informed us that it is not opposed to our submitting our NDA based on the Filing Data. We plan to submit our NDA to the FDA and a Marketing Authorization Application, or MAA, to the European Medicines Agency, or EMA, before the end of 2011.
Assuming we obtain approval, in anticipation of our commercial launch of lomitapide initially in the United States and European Union, we have begun to recruit a team comprised of sales representatives and medical education specialists who are experienced in marketing drugs for the treatment of rare, often genetic, disorders. We initially plan to hire a medical education, marketing and sales force of approximately 15 people in the United States and approximately 18 people in the European Union. We also are evaluating other markets to determine other geographies where we will commercialize lomitapide, either alone or in partnership with others.
In addition to HoFH, we are also in the process of developing a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with a severe genetic form of elevated triglycerides, or hypertriglyceridemia, called familial chylomicronemia, or FC. In October 2010, the EMA granted lomitapide orphan drug designation for the treatment of FC. In March 2011, the FDA also granted lomitapide orphan drug designation for this indication. In October 2007, the FDA granted lomitapide orphan drug status for the treatment of HoFH.
51
Table of Contents
We are a development stage company with a limited operating history. To date, we have primarily focused on developing our lead compound, lomitapide. We have funded our operations to date primarily from the private placement of convertible preferred stock, convertible debt, venture debt and the proceeds from our initial public offering, or IPO. From inception through March 31, 2011, we received net proceeds of $40.3 million from the sale of convertible preferred stock, $21.3 million from the sale of convertible debt, and $20.0 million from venture debt.
In the fourth quarter of 2010, we completed our IPO, selling 5,750,000 shares of our common stock at a public offering price of $9.50 per share. Net cash proceeds from the IPO were approximately $48.7 million after deducting underwriting discounts, commissions and offering expenses payable by us. In connection with the closing of the IPO, all of our shares of redeemable convertible preferred stock outstanding at the time of the offering were automatically converted into 7,051,635 shares of common stock and all of our convertible promissory notes were automatically converted to 3,093,472 shares of common stock.
We have incurred losses in each year since our inception in February 2005. Our net losses were approximately $25.0 million, $12.2 million and $14.3 million for the years ended December 31, 2008, 2009 and 2010, respectively and $0.8 million and $6.8 million for the three months ended March 31, 2010 and 2011, respectively. As of March 31, 2011, we had an accumulated deficit of approximately $97.8 million. Substantially all of our operating losses resulted from costs incurred in connection with our development programs and from general and administrative costs associated with our operations.
We expect our research and development expenses to increase in connection with our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, our planned Phase III pediatric clinical trial to evaluate lomitapide for the treatment of HoFH pediatric and adolescent patients (> 7 to < 18 years of age) and our planned Phase III clinical trial of lomitapide for the treatment of adult patients with FC and other potential studies or clinical trials of lomitapide. If we obtain marketing approval for lomitapide, we will likely incur significant sales, marketing, in-licensing and outsourced manufacturing expenses, as well as continued research and development expenses. Furthermore, we are now incurring additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future.
Financial Overview
Revenue
To date, we have not generated any revenue from the sale of any products, and we do not expect to generate significant revenue unless or until we obtain marketing approval of, and commercialize, lomitapide.
Research and Development Expenses
Since our inception, we have focused on our clinical development programs. We recognize research and development expenses as they are incurred. Our research and development expenses consist primarily of:
| § | salaries and related expenses for personnel; |
| § | fees paid to consultants and contract research organizations, or CROs, in conjunction with independently monitoring our clinical trials and acquiring and evaluating data in conjunction with our clinical trials, including all related fees, such as for investigator grants, patient screening, laboratory work and statistical compilation and analysis; |
| § | costs related to production of clinical materials, including fees paid to contract manufacturers; |
| § | costs related to upfront and milestone payments under in-licensing agreements; |
| § | costs related to compliance with regulatory requirements in the United States, the European Union and other foreign jurisdictions; |
| § | consulting fees paid to third parties; and |
| § | costs related to stock options or other stock-based compensation granted to personnel in development functions. |
52
Table of Contents
We expense both internal and external development costs as incurred. We have been developing lomitapide and our other product candidate, implitapide, in parallel, and we typically use our employee and infrastructure resources across several projects. Thus, some of our research and development expenses are not attributable to an individual project but rather are allocated across our clinical stage programs based on management estimates. These allocated expenses include salaries, stock-based compensation charges and related fringe benefit costs for our employees, consulting fees and the fees paid to clinical suppliers.
The following table summarizes our research and development expenses for the years ended December 31, 2008, 2009 and 2010 and the three months ended March 31, 2010 and 2011.
| For the Year Ended December 31, |
For the Three Months Ended March 31, |
|||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (in thousands) | (Unaudited) | |||||||||||||||||||
| Lomitapide |
||||||||||||||||||||
| Clinical development (including regulatory) expenses |
$ | 11,819 | $ | 3,016 | $ | 4,966 | $ | 216 | $ | 2,247 | ||||||||||
| Preclinical development expenses |
2,428 | 1,612 | 935 | 237 | 247 | |||||||||||||||
| Administrative expenses |
1,840 | 1,896 | 1,327 | 337 | 502 | |||||||||||||||
| Total |
$ | 16,087 | $ | 6,524 | $ | 7,228 | $ | 790 | $ | 2,997 | ||||||||||
| Implitapide |
||||||||||||||||||||
| Clinical development (including regulatory) expenses |
$ | 698 | $ | 159 | $ | — | $ | — | $ | — | ||||||||||
| Preclinical development expenses |
24 | — | — | — | — | |||||||||||||||
| Administrative expenses |
903 | 357 | 401 | 276 | 300 | |||||||||||||||
| Total |
$ | 1,625 | $ | 516 | $ | 401 | $ | 276 | $ | 300 | ||||||||||
| Total |
$ | 17,712 | $ | 7,041 | $ | 7,629 | $ | 1,066 | $ | 3,297 | ||||||||||
We expect our research and development expenses will increase as we complete our pivotal Phase III trial of lomitapide for the treatment of patients with HoFH and seek marketing approval for lomitapide in this indication, including our completion of studies and trials required prior to the filing of our NDA for lomitapide, and as we further develop and initiate our planned Phase III pediatric clinical trial to evaluate lomitapide for the treatment of HoFH pediatric and adolescent patients (> 7 to < 18 years of age) and our planned Phase III clinical trial of lomitapide for the treatment of adult patients with FC. In addition, we intend to explore and pursue label expansion in patient populations broader than HoFH and FC. Due to the numerous risks and uncertainties associated with timing and costs to completion of clinical trials, we cannot determine these future expenses with certainty and the actual range may vary significantly from our forecasts.
Our research and development expenditures are subject to numerous uncertainties in timing and cost to completion. Completion of clinical trials may take several years or more, but the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
| § | the number of trials required for approval; |
| § | the number of sites included in the trials; |
| § | the length of time required to enroll suitable patients; |
| § | the number of patients that participate in the trials; |
| § | the number of doses that patients receive; |
| § | the drop-out or discontinuation rates of patients; |
| § | the duration of patient follow-up; |
| § | the number of analyses and tests performed during the trial; |
53
Table of Contents
| § | the phase of development the product candidate is in; and |
| § | the efficacy and safety profile of the product candidate. |
Our expenses related to clinical trials are based on estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation and vary from contract to contract and may result in uneven payment flows. Generally, these agreements set forth the scope of work to be performed at a fixed fee or unit price. Payments under the contracts depend on factors such as the successful enrollment of patients or the completion of clinical trial milestones. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity according to the protocol. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis.
We have not received marketing approval for lomitapide from the FDA, the EMA, or any other foreign regulatory authority. Obtaining marketing approval is an extensive, lengthy, expensive and uncertain process, and the FDA, the EMA or any other foreign regulatory authority, may delay, limit or deny approval of lomitapide for many reasons.
As a result of the uncertainties discussed above, we are unable to determine with certainty the duration and completion costs of our development projects or when and to what extent we will receive revenue from the commercialization and sale of lomitapide.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation for employees in executive and operational functions, including executive, finance, and legal. Other significant costs include facilities costs and professional fees for accounting and legal services, including legal services and expenses associated with obtaining and maintaining patents.
We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates and as we continue to operate as a public company. These increases will likely include increased costs for insurance, costs related to the hiring of additional personnel and payment to outside consultants, lawyers and accountants.
Interest Income and Interest Expense
Interest income consists of interest earned on our cash and cash equivalents. Interest expense consists primarily of cash and non-cash interest costs related to our outstanding debt. In addition, we capitalize costs incurred in connection with the issuance of debt. We amortize these costs over the life of our debt agreements as interest expense in our statement of operations. In November 2010, we repaid in full approximately $3.3 million of outstanding principal and interest and associated expenses under our loan and security agreements with Hercules Technology Growth Capital, or the old Hercules loan agreement. In February 2011, we entered into the Loan and Security Agreement with the Hercules Funds for a $25.0 million credit facility. At the closing of the Loan and Security Agreement, we received an initial advance of $10.0 million, with interest-only payments for thirteen months. The Company may request additional term loan advances of up to $15.0 million. As of the date of this prospectus, we have a principal amount of $10.0 million outstanding under the Loan and Security Agreement.
Net Operating Losses and Tax Carryforwards
As of December 31, 2010, we had approximately $72.4 million of federal net operating loss carryforwards. We also had federal and state research and development tax credit carryforwards of approximately $1.1 million available to offset future taxable income. These federal and state net operating loss and federal and state tax credit carryforwards will begin to expire at various dates beginning in 2028, if not utilized. The Tax Reform Act of 1986 provides for a limitation on the annual use of net operating loss and research and development tax credit carryforwards following certain ownership changes that could limit our ability to utilize these carryforwards. We
54
Table of Contents
have not completed a study to assess whether an ownership change has occurred, or whether there have been multiple ownership changes since our inception, due to the significant costs and complexities associated with such study. Accordingly, our ability to utilize the aforementioned carryforwards may be limited. Additionally, U.S. tax laws limit the time during which these carryforwards may be utilized against future taxes. As a result, we may not be able to take full advantage of these carryforwards for federal and state tax purposes.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses. On an ongoing basis, we evaluate these estimates and judgments, including those described below. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results and experiences may differ materially from these estimates.
While our significant accounting policies are more fully described in Note 2 to our financial statements appearing at the end of this prospectus, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our reported financial results and affect the more significant judgments and estimates that we use in the preparation of our financial statements.
Accrued Expenses
As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with applicable vendor personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued expenses include:
| § | fees paid to CROs in connection with clinical trials; |
| § | fees paid to investigative sites in connection with clinical trials; |
| § | fees paid to contract manufacturers in connection with the production of clinical trial materials; and |
| § | professional service fees. |
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we will adjust the accrual accordingly. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates. We do not anticipate the future settlement of existing accruals to differ materially from our estimates.
Valuation of Financial Instruments
Valuation of Investments
We have an investment in an auction rate security and in non-cumulative redeemable perpetual preferred stock. Valuing these securities requires the use of estimates and assumptions that are subjective. Fair value estimates of
55
Table of Contents
these securities are made at a specific point in time, based on relevant market information. These estimates are subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision.
The estimated fair value of the auction rate security based on discounted cash flows. Our discounted cash flow model considers, among other things, the quality of the underlying collateral, the credit rating of the issuer, an estimate of when these securities are either expected to have a successful auction or otherwise return to par value, the expected interest income to be received over this period and the estimated required rate of return for investors that may be willing to purchase such a security. We also consider third-party valuations, to the extent available, and similar securities priced in the marketplace when arriving at the estimated fair value.
The estimated fair value of our auction rate security that was converted to preferred stock is estimated by analyzing similar securities in the marketplace. We also consider the fair value of the underlying collateral and credit rating of the issuer.
Although our investments have significant discounts as compared to the par value of the securities, changes in inputs or other data that are used to derive the valuation can have a significant effect on the valuation of our investments.
Preferred Stock Warrant Liability
We initially accounted for our preferred stock warrant in accordance with Accounting Standards Codification, or ASC, 480-10, Distinguishing Liabilities from Equity, which required that a financial instrument, other than an outstanding share, that, at inception, is indexed to an obligation to repurchase the issuer’s equity shares, regardless of the timing or likelihood of the redemption shall be classified as a liability. We measured the fair value of the warrant liability using an option-pricing model with changes in fair value recognized in earnings. Any modifications to the warrant liability are recorded in earnings during the period of the modification. The significant assumptions used in estimating the fair value of the warrant liability include the strike price, estimate for volatility, risk free interest rate, estimated fair value of the preferred stock, and the estimated life of the warrant.
In connection with our IPO, the warrant became exercisable for shares of our common stock. In accordance with ASC 480-10 and ASC 815-40, Derivatives and Hedging, we reclassifed the warrant liability to additional paid-in-capital, as the settlement of the warrant is fixed to a specific number of shares of common stock, has no contingency and does not require any cash settlement.
Stock-Based Compensation
We recognize as compensation expense the fair value, for accounting purposes, of stock options, restricted stock awards and other stock-based compensation issued to employees. For service type awards, compensation expense is typically recognized over the requisite service period, which is the vesting period. For performance type awards, the compensation expense is recognized beginning in the period when management has determined it is probable the performance factor will be achieved.
Equity instruments issued to non-employees are recorded at their fair value. The associated compensation expense is recognized over the related service period.
56
Table of Contents
For equity awards that have previously been revalued, any incremental increase in the fair value has been recorded as compensation expense on the date of the modification (for vested awards) or over the remaining service (vesting) period (for unvested awards). The incremental compensation cost is the excess of the fair-value-based measure of the modified award on the date of modification over the fair-value-based measure of the original award immediately before the modification. Stock-based compensation expense includes stock options granted to employees and non-employees and has been reported in our statements of operations as follows:
| For the Year Ended December 31, |
For the Three Months Ended March 31, |
|||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (in thousands) | (Unaudited) | |||||||||||||||||||
| Research and development |
$ | 655 | $ | 497 | $ | 337 | $ | 92 | $ | 146 | ||||||||||
| General and administrative |
592 | 436 | 1,501 | 108 | 1,116 | |||||||||||||||
| Total |
$ | 1,247 | $ | 933 | $ | 1,838 | $ | 200 | $ | 1,262 | ||||||||||
We calculate the fair value of stock-based compensation awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including stock price volatility and the expected life of stock options. As a newly public company, we do not have sufficient history to estimate the volatility of our common stock price or the expected life of our options. We calculate expected volatility based on reported data for selected reasonably similar publicly traded companies, or guideline peer group, for which the historical information is available. We will continue to use the guideline peer group volatility information until the historical volatility of our common stock is relevant to measure expected volatility for future option grants. The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. We determine the average expected life of stock options according to the “simplified method” as described in Staff Accounting Bulletin 110, which is the mid-point between the vesting date and the end of the contractual term. We determine the risk-free interest rate by reference to implied yields available from five-year and seven-year U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. We estimate forfeitures based on our historical analysis of actual stock option forfeitures. The assumptions used in the Black-Scholes option-pricing model for the years ended December 31, 2008, 2009 and 2010 and three months ended March 31, 2010 and 2011 are set forth in our financial statements appearing at the end of this prospectus.
There is a high degree of subjectivity involved when using option-pricing models to estimate stock-based compensation. There is currently no market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of employee stock-based awards is determined using an option-pricing model, that value may not be indicative of the fair value observed in a market transaction between a willing buyer and willing seller. If factors change and we employ different assumptions when valuing our options, the compensation expense that we record in the future may differ significantly from what we have historically reported.
Results of Operations
Comparison of the Three Months Ended March 31, 2011 and the Three Months Ended March 31, 2010
Revenue
We did not recognize any revenue for the three months ended March 31, 2011 or the three months ended March 31, 2010.
Research and Development Expenses
Research and development expenses were $3.3 million for the three months ended March 31, 2011, compared with $1.1 million for the three months ended March 31, 2010. The $2.2 million increase for the three months ended March 31, 2011 was primarily due to the development expenses related to our lomitapide development
57
Table of Contents
program, including a $1.0 million increase in clinical consultant expense, $0.3 million increase in pre-clinical expense, $0.3 million increase in clinical trial supplies, $0.2 million increase in clinical monitoring costs, and $0.4 million increase in administrative expenses.
General and Administrative Expenses
General and administrative expenses were $3.5 million for the three months ended March 31, 2011 as compared to $1.0 million for the three months ended March 31, 2010. The $2.5 million increase was primarily due to increases in compensation costs of $0.3 million, $0.5 million in consulting fees, $0.2 million for rent for our new office facilities, $1.1 million of stock-based compensation, and $0.2 million for legal and accounting services.
Interest Expense
Interest expense was $0.1 million for the three months ended March 31, 2011, compared with $0.6 million for the three months ended March 31, 2010. The $0.5 million decrease was primarily due to reduced borrowings.
Interest Income
Interest income was $0.1 million for the three months ended March 31, 2011, compared with $0 million for the three months ended March 31, 2010. The increase was due to higher cash and cash equivalent balances.
Benefit from income taxes
Our income tax benefit was $0.0 for the three months ended March 31, 2011 compared with $1.8 million for the three months ended March 31, 2010. The $1.8 million represents proceeds received from the sale of New Jersey state net operating losses to a third party during the first quarter of 2010.
Comparison of the Year Ended December 31, 2010 and the Year Ended December 31, 2009
Revenue
We did not recognize any revenue for the year ended December 31, 2010 or the year ended December 31, 2009.
Research and Development Expenses
Research and development expenses were $7.6 million for the year ended December 31, 2010, compared with $7.0 million for the year ended December 31, 2009. The $0.6 million increase is primarily related to our lomitapide development program, including a $2.0 million increase in clinical development expenses, partially offset by a $0.7 million decrease in preclinical development expenses and $0.5 million decrease in administrative expenses and a decrease of $0.2 million in clinical development expenses related to our implitapide development program. The increase in expenses related to our lomitapide development program was higher during 2010 primarily due to costs associated with clinical study work.
General and Administrative Expenses
General and administrative expenses were $5.9 million for the year ended December 31, 2010 and $3.1 million for the year ended December 31, 2009. The $2.8 million increase in 2010 was due to an increase in stock compensation expense of $0.9 million, recruiting and public relations fees of $0.6 million, outside consultant fees of $0.4 million, legal expenses of $0.3 million and other related expenses of $0.6 million.
Interest Expense
Interest expense was $2.4 million for the year ended December 31, 2010, compared with $2.1 million for the year ended December 31, 2009. The $0.3 million increase for the year ended December 31, 2010 was primarily attributable to the write-off of non-cash deferred financing fees associated with the conversion of our preferred stock and convertible notes into shares of common stock at the closing of our IPO, and repayment of debt owed under our old Hercules loan agreement.
58
Table of Contents
Interest Income
Interest income was $0.1 million for the year ended December 31, 2010, compared with $0.2 million for the year ended December 31, 2009. The $0.1 million decrease was due to lower average cash and cash equivalent balances during the year ended December 31, 2010.
Income Tax Benefit
Our income tax benefit was $1.8 million for the year ended December 31, 2010, compared with $0 for the year ended December 31, 2009. The $1.8 million represents proceeds received from the sale of New Jersey state net operating losses to a third party.
Other Income
Other income was $0.2 million for the year ended December 31, 2010, compared with $0 for the year ended December 31, 2009. The $0.2 million increase was due to the receipt of funds from the U.S. Treasury department in 2010 for the Qualified Therapeutics Discovery Projects Grant program.
Comparison of the Year Ended December 31, 2009 and the Year Ended December 31, 2008
Revenue
We did not recognize any revenue for the year ended December 31, 2009 or the year ended December 31, 2008.
Research and Development Expenses
Research and development expenses were $7.0 million for the year ended December 31, 2009, compared with $17.7 million for the year ended December 31, 2008. The $10.7 million decrease for 2009 reflects a decrease of $9.6 million in development expenses related to our lomitapide development program, including a $8.8 million decrease in clinical development expenses and a $0.8 million decrease in preclinical development expenses, and a decrease of $1.1 million in development expenses related to our implitapide development program, including a $0.5 million decrease in clinical development expenses and a $0.6 million decrease in administrative expenses. Development expenses related to our lomitapide development program were higher in 2008 primarily because of our initiation and completion of three Phase II clinical trials for lomitapide in broader patient populations in 2008.
General and Administrative Expenses
General and administrative expenses were $3.1 million for the year ended December 31, 2009, compared with $5.2 million for the year ended December 31, 2008. The $2.1 million decrease was due to approximately $1.0 million of deferred financing fees expensed in 2008 associated with our initial public offering that was not completed, a $0.7 million decrease in salary and related expenses resulting from lower employee headcount associated with our reduction in workforce by five employees in September 2009 and a $0.4 million decrease in professional fees.
Interest Expense
Interest expense was $2.1 million for the year ended December 31, 2009, compared with $1.1 million for the year ended December 31, 2008. The $1.0 million increase for 2009 was primarily attributable to an increase in non-cash accrued interest expense associated with our convertible notes, including the issuance of additional convertible notes in an aggregate principal amount of $5.0 million in July 2009.
Interest Income
Interest income was $0.2 million for the year ended December 31, 2009, compared with $0.5 million for the year ended December 31, 2008. The $0.3 million decrease for 2009 was due to lower cash and cash equivalent balances.
59
Table of Contents
Change in Fair Value of Warrant Liability
We recorded $0.2 million of other expense during the year ended December 31, 2009, compared with $0.1 million of other income during the year ended December 31, 2008. This variance primarily resulted from our amendment of the preferred stock warrant agreement with Hercules Growth Capital to increase the number of shares of preferred stock issuable under the preferred stock warrant and decrease the exercise price per share of the preferred stock warrant from $5.27 to $1.86. This warrant automatically became a warrant to purchase common stock at an exercise price of $6.68 upon the closing of our IPO, at which time the existing liability was reclassified to additional paid-in-capital.
Liquidity and Capital Resources
Since our inception in 2005, we have funded our operations primarily through proceeds from the private placement of convertible preferred stock, convertible debt, venture debt and the proceeds from our IPO. To date, we have not generated any revenue from the sale of products. We have incurred losses and generated negative cash flows from operations since inception. As of March 31, 2011, our cash and cash equivalents totaled $46.8 million.
From inception through March 31, 2011, we received net proceeds of $40.3 million from the sale of convertible preferred stock and $21.3 million from the sale of convertible debt. In March 2007, we entered into the $15.0 million old Hercules loan agreement and borrowed $10.0 million under this agreement. The loan originally bore interest at the prime lending rate, as published by the Wall Street Journal, plus 2.5% per annum, and had a maturity date of August 19, 2010. In connection with entering into the old Hercules loan agreement, we granted Hercules a first priority security interest on all of our assets, except for our intellectual property. We amended the old Hercules loan agreement in September 2008 and July 2009 to extend the maturity date, provide for forbearance with respect to an insolvency covenant and increase the interest rate. In addition, we granted Hercules a first priority security interest in our intellectual property. In October 2010, Hercules waived the existing defaults under the old Hercules loan agreement. In November 2010, we used $3.3 million of the proceeds from the IPO to pay off all our outstanding principal and interest and associated expenses under the old Hercules loan agreement.
On October 27, 2010 we completed our IPO, selling 5,000,000 shares at an offering price of $9.50 per share. On November 2, 2010, the underwriters exercised in full their over-allotment option to purchase an additional 750,000 shares at an offering price of $9.50 per share. Aggregate gross proceeds from the IPO, including the exercise of the over-allotment option, were $54.6 million and net proceeds received after underwriting fees and offering expenses were approximately $48.7 million.
On February 28, 2011, we entered into a Loan and Security Agreement with the Hercules Funds. Pursuant to the Loan and Security Agreement, the Hercules Funds will make available to us term loans in an aggregate principal amount of up to $25.0 million. The $25.0 million credit facility provides for an initial advance at closing of $10.0 million, with interest-only payments for twelve months, and bears per annum interest at the greater of 10.4% or 10.4% plus prime minus 4.75%. We may request additional term loan advances of up to $15.0 million. As of the date of this prospectus, we have $10.0 million outstanding under the Loan and Security Agreement.
We are required to repay the aggregate principal balance of the loan that is outstanding on March 31, 2012 in monthly installments starting on April 1, 2012 and continuing through September 1, 2014. The entire term loan principal balance and all accrued but unpaid interest will be due and payable on September 1, 2014. At our option, we may prepay all or any part of the outstanding advances subject to a prepayment charge.
In connection with entry into the Loan and Security Agreement, we granted the Hercules Funds a security interest in all of our personal property now owned or hereafter acquired, excluding intellectual property.
60
Table of Contents
Cash Flows
The following table sets forth the major sources and uses of cash for the periods set forth below:
| Year Ended December 31, | 3 Months Ended March 31, |
|||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| (in thousands) | (Unaudited) | |||||||||||||||||||
| Net cash provided by (used in): |
||||||||||||||||||||
| Operating activities |
$ | (21,990 | ) | $ | (10,532 | ) | $ | (8,499 | ) | $ | (35 | ) | $ | (7,010 | ) | |||||
| Investing activities |
— | — | (12 | ) | — | (73 | ) | |||||||||||||
| Financing activities |
6,602 | 2,956 | 51,183 | 2,281 | 9,810 | |||||||||||||||
| Net increase\(decrease) in cash and cash equivalents |
$ | (15,388 | ) | $ | (7,576 | ) | $ | 42,672 | $ | 2,246 | $ | 2,729 | ||||||||
Net cash used in operating activities amounted to $22.0 million for 2008, $10.5 million for 2009, $8.5 million for 2010, $0.04 million for the three months ended March 31, 2010 and $7.0 million for the three months ended March 31, 2011. The primary use of cash was to fund our net operating losses, primarily related to the development of our product candidates. The net cash used in operating activities increased in 2008 mainly due to increased development costs, decreased in 2009 mainly due to reduced development costs, and decreased in 2010 as a result of lower development expenses as we concentrated our resources on our Phase III clinical trial activities and, due to cash constraints, extended our payment terms with vendors. The $7.0 million net cash used in operating activities for the three months ended March 31, 2011 was primarily due to the $6.8 million net loss for the period related to the development of our lomitapide lead product candidate.
Net cash used in investing activities was $0 for 2008, $0 for 2009, approximately $12,000 for 2010, consisting of net purchases of property and equipment and $0.1 million for three months ended March 31, 2011, due to net purchases of property and equipment. No cash was used in investing activities for three months ended March 31, 2010
Net cash provided by financing activities amounted to $6.6 million for 2008, $3.0 million for 2009, $51.7 million for 2010, $2.3 million for three months ended March 31, 2010 and $9.8 million for three months ended March 31, 2011. The cash provided by financing activities in 2008 consisted mainly of the issuance of $8.8 million aggregate principal amount of convertible notes, partially offset by $2.2 million of principal payments under the loan and security agreement with Hercules. The cash provided by financing activities in 2009 consisted mainly of the issuance of $5.0 million aggregate principal amount of convertible notes, partially offset by $2.0 million of principal payments under the old Hercules loan agreement. The cash provided by financing activities for 2010 consisted mainly of the issuance of $7.5 million aggregate principal amount of convertible notes and $50.8 million from the IPO, partially offset by $5.5 million of principal payments under the old Hercules loan agreement and $1.1 million of deferred financing fees related to the IPO. The cash provided by financing activities for the three months ended March 31, 2011 consisted of $10.0 million proceeds received from a term loan with Hercules offset by $0.2 million in deferred financing fees. The cash provided by financing activities for the three months ended March 31, 2010 consisted of $3.0 million proceeds received from the issuance of senior subordinated convertible notes offset by $0.7 million of principal payments to Hercules.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations and commitments as of March 31, 2011:
| Payments Due By Period | ||||||||||||||||||||
| Total | Less than 1 year |
1-3 years |
3-5 years |
More than 5 years |
||||||||||||||||
| Operating Leases(1) |
$ | 3,058,655 | $ | 478,624 | $ | 1,113,171 | $ | 1,074,328 | $ | 392,532 | ||||||||||
| License Obligations(2) |
— | — | — | — | — | |||||||||||||||
|
Total(3) |
$ | 3,058,655 | $ | 478,624 | $ | 1,113,171 | $ | 1,074,328 | $ | 392,532 | ||||||||||
61
Table of Contents
| (1) | Operating lease obligations reflect our obligation to make payments in connection with leases that commenced at the end of 2010 and the beginning of 2011. |
| On October 15, 2010, we entered into an agreement with Symphony Suites, as amended, to provide office space in Morristown, New Jersey from the period of October 15, 2010 to March 31, 2011 at an initial rate of $18,361 per month and increasing to $25,706 per month as of February 2011. |
| On November 24, 2010, we entered into an 89 month lease with S/K One Bed Associates, LLC. Under the lease, we lease office space for our New Jersey office in Bedminster, New Jersey. The lease provides for an initial base rent of $11,904 per month plus certain operating expenses and taxes beginning on April 1, 2011, and shall increase on an annual basis beginning in January 2012. |
| On January 1, 2011, we entered into a five year lease with RREEF America REIT II CORP. PPP. Under the lease, we lease office space for our headquarters in Cambridge, Massachusetts. The lease provides for an initial base rent of $30,594 per month, plus certain operating expenses and taxes, and shall increase on an annual basis beginning in January 2012. |
| (2) | License obligations reflect our obligation to make annual payments under our license agreement with Bayer regarding the patent rights and know-how owned or controlled by Bayer applicable to implitapide. Under this agreement, we have been obligated to pay Bayer, in addition to the amounts described below, annual payments through 2011 which become non-refundable when paid totaling $1.0 million in the aggregate. Final payment was made on March 31, 2011. |
| (3) | This table does not include (a) any milestone payments which may become payable to third parties under license agreements as the timing and likelihood of such payments are not known, (b) any royalty payments to third parties as the amounts, timing and likelihood of such payments are not known and (c) contracts that are entered into in the ordinary course of business which are not material in the aggregate in any period presented above. |
Under our license agreement with UPenn, we are required to make development milestone payments to UPenn of up to an aggregate amount of $150,000 when a licensed product’s indication is limited to HoFH or severe refractory hypercholesterolemia, and an aggregate amount of approximately $2.6 million for all other indications within the licensed field. All such development milestone payments for these other indications are payable only once, no matter how many licensed products for these other indications are developed. In addition, we are required to make specified royalty payments on net sales of products covered by the license (subject to a variety of customary reductions), and share with UPenn specified percentages of sublicensing royalties and other consideration that we receive under any sublicenses that we may grant.
Under our license agreement with Bayer, we are required to make certain development milestone payments of up to an aggregate of approximately $4.5 million upon the achievement of certain development milestones. Each development milestone payment is payable only once, no matter how many licensed products are developed. In addition, we are required to make annual payments through 2011 of $1.0 million in the aggregate, with each annual payment creditable in full against any development milestones that are or become payable, a one-time commercialization milestone payment of $5.0 million if we achieve aggregate annual net sales of licensed products of $250.0 million (payable only once, no matter how many licensed products are commercialized), and specified royalty payments on net sales of licensed products (subject to a variety of reductions). Our annual payments to Bayer described in footnote 2 to the contractual obligations table set forth above for 2011 through 2016 will be creditable in full against any development milestones that are or become payable to Bayer.
Future Funding Requirements
We may need to raise additional capital to fund our operations and to develop and commercialize lomitapide. Our future capital requirements may be substantial and will depend on many factors, including:
| § | the results of our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH; |
| § | our ability to develop and obtain regulatory approval for a protocol for a Phase III pediatric clinical trial to evaluate lomitapide for treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH; |
| § | the cost, timing and outcomes of seeking marketing approval of lomitapide for the treatment of patients with HoFH in the United States and the European Union; |
| § | our ability to develop and obtain regulatory approval for a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC; |
| § | if regulatory approval for the Phase III clinical trial of lomitapide for the treatment of adult patients with FC is obtained, the cost and timing associated with conducting such clinical trial; |
62
Table of Contents
| § | the cost of filing, prosecuting and enforcing patent claims; |
| § | exploration and possible label expansion of lomitapide in broader patient populations; |
| § | the costs associated with commercializing lomitapide if we receive marketing approval, including the cost and timing of establishing sales and marketing capabilities to market and sell lomitapide for the treatment of patients with HoFH; and |
| § | subject to receipt of marketing approval, revenue received from sales of approved products, if any, in the future. |
Under the February 2011 Loan and Security Agreement with the Hercules Funds, it will make available term loans of up to $25.0 million. The agreement allows an initial advance of $10.0 million, with interest-only payments for twelve months, and bears per annum interest. We may request additional term loan advances of up to $15.0 million.
To date, we have not generated any revenue from our development stage product candidates. We do not know when, or if, we will generate any revenue. We do not expect to generate significant revenue unless or until we obtain marketing approval of, and commercialize, lomitapide. We expect our continuing operating losses to result in increases in cash used in operations over the next several years. Based on our current operating plan, we anticipate that the net proceeds of this offering, together with our existing cash, cash equivalents and borrowing capacity under our Loan and Security Agreement with the Hercules Funds, will be sufficient to enable us to maintain our currently planned operations, including our continued product candidate development, at least through the next 24 months. We believe that these available funds will allow us to complete our ongoing pivotal Phase III clinical trial of, seek marketing approval for and commercially launch lomitapide for the treatment of patients with HoFH. In addition, we believe that these available funds will allow us to initiate a Phase III pediatric clinical trial to evaluate lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH as well as a Phase III clinical trial of lomitapide for the treatment of patients with FC. However, changing circumstances may cause us to consume capital significantly faster than we currently anticipate. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our planned clinical trial of lomitapide for the treatment of patients with FC or our future anticipated clinical trials.
Other than the Loan and Security Agreement with the Hercules Funds, we have no committed external sources of funds. Additional financing may not be available when we need it or may not be available on terms that are favorable to us. We may seek to raise additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of existing stockholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations and strategic and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates, or grant licenses on terms that are not favorable to us.
Off-Balance Sheet Arrangements
We do not currently have, nor have we ever had, any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. In addition, we do not engage in trading activities involving non-exchange traded contracts. Therefore, we are not materially exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in these relationships.
63
Table of Contents
Quantitative and Qualitative Disclosure About Market Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because the majority of our investments are in long-term investments. We do not have any foreign currency or other derivative financial instruments.
Recent Accounting Pronouncements
See Note 3 to our audited financial statements contained at the end of this prospectus for a summary of recent accounting pronouncements applicable to us.
64
Table of Contents
Overview
We are an emerging biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat severe lipid disorders. Lipids are naturally occurring molecules, such as cholesterol and triglycerides, which are transported in the blood. Elevated levels of cholesterol, or hypercholesterolemia, and elevated levels of triglycerides, or hypertriglyceridemia, can dramatically increase the risk of experiencing a potentially life threatening event, such as a heart attack or stroke in the case of hypercholesterolemia or pancreatitis in the case of hypertriglyceridemia. We are initially developing our lead compound, lomitapide, as an oral, once-a-day treatment for patients with a rare inherited lipid disorder called homozygous familial hypercholesterolemia, or HoFH. These patients are at very high risk of experiencing life threatening cardiovascular events at an early age as a result of extremely elevated cholesterol levels in the blood and, as a result have a substantially reduced life span relative to unaffected individuals.
We are currently evaluating lomitapide in a pivotal Phase III clinical trial for the treatment of patients with HoFH. We completed enrollment for this single-arm, open-label trial in March 2010 with a total of 29 patients. Three of these patients withdrew their consent to participate in the trial and three patients discontinued treatment due to gastrointestinal adverse events. The 23 patients remaining in the trial have completed the 26 week period of therapy after which the primary efficacy endpoint is measured, and the 56 week period of therapy during which the Filing Data were collected. As of the date of this prospectus, 19 of these patients have completed the entire 78 weeks of the trial. We expect to complete this trial in the third quarter of 2011.
On May 31, 2011, we announced the results of this trial, through 56 weeks of treatment. We believe based on our prior discussions with the U.S. Food and Drug Administration, or FDA, that these results demonstrate sufficient long-term safety and efficacy to support the submission of our New Drug Application, or NDA, for lomitapide. We refer to these week 56 results as our Filing Data, and we will later supplement the Filing Data with data reflecting the full 78-week trial duration. Before we can submit an NDA, we must complete additional clinical and non-clinical studies to assess various other aspects of lomitapide. On June 15, 2011, we met with the FDA, which informed us that it is not opposed to our submitting our NDA based on our Filing Data. We plan to submit our NDA to the FDA and a Marketing Authorization Application, or MAA, to the European Medicines Agency, or EMA, before the end of 2011
In October 2007, the FDA granted lomitapide orphan drug designation for the treatment of HoFH. Untreated HoFH patients have extremely high levels of LDL-C, typically between 500 mg/dL and 1,000 mg/dL, and, as a result, are at a severely high risk of experiencing cardiovascular events, such as heart attack or stroke, at a young age. All of the patients enrolled in our current pivotal Phase III clinical trial are using combinations of other lipid-lowering therapies. Nonetheless, these patients had an average LDL-C level at baseline, before treatment with lomitapide, of 354 mg/dL. In the United States, the National Cholesterol Education Program, or NCEP, guidelines currently recommend that patients at high risk of experiencing a heart attack should seek to lower their LDL-C levels below 100 mg/dL with an optional therapeutic target of 70 mg/dL. Aggressive treatment, including dietary modifications plus combination therapy with currently approved lipid lowering drugs at maximum tolerated doses nearly always fails to reduce LDL-C levels to their recommended targets in these patients.
Because drug therapy and dietary modifications are insufficient to lower LDL-C to target levels, many of these patients regularly undergo an expensive, time consuming and invasive procedure called apheresis, a process similar to kidney dialysis whereby LDL-C particles are mechanically filtered from the blood. However, this provides only temporary reductions in LDL-C levels and is not readily available to all patients due to the limited number of treatment centers that perform this procedure. We believe lomitapide has the potential to provide significant reductions in LDL-C levels in this high-risk patient population, thereby reducing or potentially even eliminating the need for apheresis. In a report that we commissioned, L.E.K. Consulting LLC, or LEK, an international business consulting firm, estimated that the total number of patients with HoFH in each of the United States and, collectively, Germany, the United Kingdom, France, Italy and Spain, referred to herein as the European Union Five, is approximately 3,000 patients, or a combined total of approximately 6,000 patients.
65
Table of Contents
We believe that lomitapide also has the potential to treat patients with other life-threatening lipid disorders who are unable to achieve recommended lipid levels on currently available therapies, particularly patients with a severe genetic form of hypertriglyceridemia called familial chylomicronemia, or FC. Untreated FC patients have extremely high levels of triglycerides, or TGs, generally greater than 2,000 mg/dL, and, as a result, experience recurrent episodes of acute pancreatitis and other serious conditions. National Cholesterol Education Program, or NCEP, guidelines suggest that normal TG levels in adults should be less than 150 mg/dL, as higher levels are associated with various health conditions. In particular, patients with TG levels greater than 500 mg/dL are at increased risk of acute pancreatitis, with TG levels of greater than 1,000 mg/dL representing a more definitive risk. However, even with aggressive treatment, many patients with FC are unable to achieve TG levels that meaningfully reduce their risk for pancreatitis and, in some cases, must be treated with acute apheresis. In October 2010, the EMA granted lomitapide orphan drug designation for the treatment of FC. In March 2011, the FDA also granted lomitapide orphan drug designation for this indication. We are in the process of developing a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC. In the report that we commissioned, LEK estimated that, subject to certain factors, there are approximately 1,000 patients in the United States and the European Union Five with FC who could be eligible for treatment with lomitapide.
We believe that lomitapide also may be useful for the treatment of elevated lipids in broader patient populations, such as those suffering from heterozygous familial hypercholesterolemia, or HeFH, patients who are statin intolerant and patients with severe hypertriglyceridemia, which we define herein as patients with TG levels above 2,000 mg/dL, due to defects other than FC. If we elect to develop lomitapide for broader patient populations, we would plan to do so selectively either on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources.
Hyperlipidemic Disorders
Lipids are naturally occurring molecules, such as cholesterol and TGs, which are transported in the blood. The liver and the intestines are the two main sites where lipids are packaged and released within the body. The liver synthesizes cholesterol and TGs and provides the body’s intrinsic supply of lipids. The intestines are the conduit through which dietary lipids enter the body for metabolism. The delivery of cholesterol and TGs to peripheral cells in the body provides necessary sources of cellular energy and cell structure. However, excess levels of lipids in the blood can be the source of significant diseases in humans. The general term for excess lipids is hyperlipidemia. Specific elevations of cholesterol in the blood are termed hypercholesterolemia, and specific elevations of TGs in the blood are termed hypertriglyceridemia.
The direct relationship between lower LDL-C levels and reduced risk for major cardiovascular events has been consistently demonstrated for more than a decade based on over 14 trials involving more than 90,000 patients. These studies have shown about a 1% reduction in risk for every 2 mg/dL drop in LDL-C. As a result, physicians are highly focused on lowering levels of LDL-C in their patients. In the United States, for example, organizations such as the NCEP, the American Heart Association, and the American College of Cardiology have emphasized aggressive management of LDL-C. NCEP guidelines currently recommend that patients at high risk of experiencing a heart attack achieve LDL-C levels of 100 mg/dL or lower through lifestyle changes and drug therapy as appropriate based on their starting levels. Both the Canadian Cardiovascular Society and the Joint British Society have supported LDL-C treatment targets as low as 77 mg/dL for high-risk patients.
NCEP guidelines define normal TG levels in adults as less than 150 mg/dL. TG levels above 150 mg/dL are thought to be associated with obesity and insulin resistance and to confer additional risk for cardiovascular disease. Patients with TG levels greater than 500 mg/dL are at increased risk of acute pancreatitis, with TG levels of greater than 1,000 mg/dL representing a more definitive risk. Based on a 2001 article from the Online Metabolic and Molecular Bases of Inherited Disease, an online database of genetic research, we estimate that up to approximately 20,000 adults in the United States have severe hypertriglyceridemia with TG levels above 2,000 mg/dL.
66
Table of Contents
Homozygous Familial Hypercholesterolemia (HoFH)
HoFH is a rare genetic lipid disorder usually caused by defects in both copies of the low-density lipoprotein, or LDL, receptor genes, resulting in impaired or total loss of function in the LDL receptor. The LDL receptor is a protein on the surface of cells that is responsible for binding and removing LDL from the blood. A loss of LDL receptor function results in accumulation of LDL-C in the blood. Untreated HoFH patients have extremely high LDL-C levels, typically between 500 mg/dL and 1,000 mg/dL. These patients are at severely high risk of experiencing premature cardiovascular events, such as heart attack or stroke, often experiencing their first cardiovascular event in their twenties. If untreated, patients with HoFH generally die before the age of thirty. There is no patient registry or other method of establishing with precision the actual number of patients with HoFH in any geography. We recently commissioned LEK to prepare a commercial report on lomitapide for us. In this report, which is dated September 2, 2010, LEK estimated that the total number of addressable patients with symptoms consistent with HoFH in each of the United States and the European Union Five is approximately 3,000 patients, or a combined total of approximately 6,000 patients, using an approach that is consistent with the characteristics of patients that participated in our pivotal Phase III clinical trial of lomitapide. LEK derived its estimates from interviews of 51 physicians, primarily lipid disorder experts, and 23 third-party payors in the United States and the European Union Five, confirmed by its own secondary research. The total addressable market opportunity for lomitapide for the treatment of patients with HoFH will ultimately depend upon, among other things, the diagnosis criteria included in the final label for lomitapide, if approved for sale for this indication, acceptance by the medical community and patient access, product pricing, and reimbursement.
In our pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, we applied three diagnostic methods to identify patients with HoFH, any one of which would qualify a patient for admission into the trial. These methods are genotyping, fibroblast activity tests and clinical criteria. The genotyping method identifies patients on the basis of gene defects, typically mutations in each of the patient’s two LDL-receptor alleles. However, in clinical practice it is often difficult to discern which patients have HoFH based solely upon a genetic test because there are more than 900 known mutations of these genes. Additionally, rare cases of HoFH have also been attributed to defects in genes other than those of the LDL receptor. Because receptor function is diminished as a result of these mutations, clinicians also may identify patients as having HoFH by testing LDL-receptor activity in skin fibroblast cells. LDL-receptor activity of less than 20% is typically considered to be consistent with LDL-receptor defects leading to HoFH. Alternatively, physicians can utilize phenotypic criteria including a patient’s own cholesterol levels and those of both parents. We refer to patients as having HoFH if they have been diagnosed with HoFH through genotypic, functional (fibroblast activity) or phenotypic (clinical/medical) criteria.
Familial Chylomicronemia (FC)
FC is a rare genetic lipid disorder typically caused by defects in genes that reduce chylomicron clearance, including, most commonly, lipoprotein lipase, or LPL, resulting in extremely low or absent LPL activity. LPL is an enzyme that facilitates the removal of TGs from the blood. Low levels or lack of this enzyme result in the accumulation of TGs in the blood. Patients with FC have extremely high levels of TGs in the blood, generally greater than 2,000 mg/dL, that typically result in recurrent episodes of acute pancreatitis, a significant and sometimes life-threatening inflammation of the pancreas. Acute pancreatitis results in significant abdominal pain and may be associated with clinically meaningful complications, such as organ failure, respiratory complications, significant enlargement of the liver and spleen and eruptive xanthomas, or poolings of triglycerides around the tendons in the body to such a degree that the swelling is easily visible. In the report that we commissioned, LEK estimated that, subject to certain factors, there are approximately 1,000 patients in the United States and the European Union Five with FC who could be eligible for treatment with lomitapide.
67
Table of Contents
Broader Patient Populations
We believe that there is a need for additional lipid-lowering agents to address broader hyperlipidemic patient populations, where patients are unable to achieve their recommended target lipid levels on maximum tolerated doses of currently approved oral therapies. These broader patient populations include:
| § | Heterozygous Familial Hypercholesterolemia (HeFH). In the report that we commissioned, LEK estimated that approximately 600,000 patients in the United States have a single defective copy of the gene that causes familial hypercholesterolemia, and are thus deemed to have the genetic condition called heterozygous familial hypercholesterolemia, or HeFH. These patients also have very high LDL-C levels, typically between 250 mg/dL and 500 mg/dL if untreated. |
| § | Statin Intolerant. Based on a 2009 review published in the Annals of Internal Medicine, a peer-reviewed medical journal, we estimate that up to approximately 10% of hyperlipidemic patients are unable to sustain statin usage due to intolerance, resulting from unacceptable responses such as muscular aches and pains. Physicians are often wary of patients who present with muscular aches and pains, as these can be an early warning sign of a rare but serious side effect seen with statin therapy called rhabdomyolysis, a condition in which a significant amount of muscle tissue rapidly breaks down, which can cause kidney failure and potentially even death. As a result, a significant population of patients in need of LDL-C lowering may not be able to utilize statins, currently the most effective of the LDL-C-lowering therapies. |
| § | Severe hypertriglyceridemia. Based on a 2001 article from the Online Metabolic and Molecular Bases of Inherited Disease, an online database of genetic research, we estimate that up to approximately 20,000 patients in the United States suffer from severe hypertriglyceridemia, which we define herein as TG levels above 2,000 mg/dL. A number of factors can contribute to TG levels exceeding 2,000 mg/dL, including FC, diabetes and alcohol abuse. Patients with this degree of TG elevation are at an increased risk of acute pancreatitis. |
Limitations of Currently Available Treatment Options
High-Risk Hypercholesterolemia
Currently available treatment options for patients at very high risk of experiencing life threatening cardiovascular events as a result of elevated cholesterol levels are extensive but, even when combined together, are often ineffective in significantly reducing LDL-C levels. The clinical approach for patients with HoFH typically involves an aggressive treatment plan to reduce lipid levels as much as possible through dietary modifications and a combination of available lipid lowering drug therapies. These drug therapies include statins, cholesterol absorption inhibitors and bile acid sequestrants. Less frequently, other drugs, such as niacin and fibrates, can be added to provide some incremental reductions in LDL-C levels, although these agents are typically used to modify other lipids. High-risk patients who are unable to reach their recommended target lipid levels on drug therapy often are supplemented with apheresis. Apheresis is expensive, costing up to approximately $150,000 per patient per year in the United States, time consuming and invasive. Because apheresis provides only temporary reductions in LDL-C levels, it must be repeated frequently, typically one or two times per month. In addition, apheresis is not readily available to all patients due to the limited number of treatment centers that perform this procedure and is associated with other complications, such as risk of infection.
High-risk hypercholesterolemia patients are particularly vulnerable for two main reasons. First, their initial LDL-C levels are so high that even with all of the available treatments they still remain very far from their recommended target LDL-C levels. Second, some treatments are not as effective at lowering LDL-C levels for these very severely affected patients due to the specific nature of their condition, which often manifests itself in the form of resistance to the existing treatments. For example, because patients with HoFH generally have mutated forms of the LDL-C receptor genes that regulate hypercholesterolemia, these patients are often resistant, or refractory, to statin therapy. High dose statin therapies that typically produce 46% to 55% reductions in LDL-C levels in the broad hypercholesterolemic patient population on average produce 18% to 24% reductions in patients with HoFH, and sometimes much less.
68
Table of Contents
Severe Hypertriglyceridemia
For patients with severe hypertriglyceridemia, the goal of treatment is to provide significant reductions in blood TG levels. Currently available treatments consist of dietary modifications to lower the intake of dietary fat and the use of omega-3 fatty acids and fibrates. However, these treatments are often inadequate to lower TG levels below 500 mg/dL, a level that predisposes patients to developing acute pancreatitis. Because of the severely elevated TG levels in this patient population, reducing TG levels below 500 mg/dL may require reductions in TG levels of 75% or more. Few individual therapies can reduce TG levels this degree. For example, Lovaza, which is comprised of omega-3 fatty acids, and marketed by GlaxosmithKline, has been shown to reduce TG levels by only approximately 45% to 52% in patients with baseline TG levels between 500 mg/dL and 2,000 mg/mL. Although fibrates have been shown to reduce TG levels by approximately 55% in patients with baseline TG levels between 500 mg/dL and 1,500 mg/dL, the effect of fibrates in patients with baseline TG levels greater than 2,000 mg/dL is not known with certainty. Moreover, patients with TG elevations of intestinal origin, such as FC patients, may be less responsive to fibrates, which act in the liver. Given the need for significant drops in TG levels in these patients, single or even combination therapies are often insufficient for many of these patients.
Our Strategy
Our objective is to develop and commercialize drugs to treat patients with rare lipid related disorders who are at very high risk of experiencing life threatening cardiovascular events, such as heart attack and stroke, at an early age. To achieve this objective, we plan to:
| § | Prepare and submit an NDA to the FDA and an MAA to the EMA before the end of 2011. On May 31, 2011, we announced the results of our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of HoFH, through 56 weeks of treatment, which, we believe based on our discussions with the FDA demonstrate sufficient long-term efficacy and continued safety to support our planned NDA submission for lomitapide. Based on our on-going discussions with the FDA, we believe that the Filing Data provide a sufficient basis on which to submit our NDA. On June 15, 2011, we met with the FDA, which informed us that it is not opposed to our submitting our NDA based on the Filing Data. Before we can submit an NDA, we must complete additional non-clinical studies to assess various other aspects of lomitapide. |
| § | Complete our ongoing pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH, and submit the data collected through week 78 of this trial to the FDA to complete our NDA submission and to the EMA for our MAA submission. We currently plan to complete this trial in the third quarter of 2011. |
| § | Prepare to commercialize lomitapide for the treatment of patients with HoFH. In anticipation of our commercial launch of lomitapide initially in the United States and European Union, we have begun to recruit a highly targeted team, comprised of sales representatives and medical education specialists who are experienced in marketing drugs for the treatment of rare, often genetic, disorders. We initially plan to hire a medical education, marketing and sales force of approximately 15 people in the United States and approximately 18 people in the European Union. We also plan to evaluate other markets to determine other geographies where we will commercialize lomitapide, either alone or in partnership with others. |
| § | Initiate a Phase III pediatric clinical trial in HoFH patients. Subject to obtaining regulatory approval for our Phase III protocol we intend to initiate a Phase III pediatric clinical trial in 2011 to evaluate lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH to enable label expansion. |
| § | Initiate a Phase III clinical trial of lomitapide for the treatment of adult patients with FC. Subject to completing and obtaining regulatory approval for a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC, we plan to initiate such a trial in 2011. Because of the limited number of patients with FC and the fact that they typically are treated by the same specialty physicians who treat patients with HoFH, we expect to access the FC market using the same targeted team that we use to market lomitapide for HoFH. |
69
Table of Contents
| § | Apply to have lomitapide made available for use under a cohorte Autorisation Temporaire d’Utilisation, or Temporary Authorization for Use, or ATU, in France. Under an ATU, the French Health Products Safety Agency, or Afssaps, allows the use of a drug before it has obtained marketing approval in France in order to treat serious or rare diseases for which no other treatment is available in that country. We believe the granting of an ATU and subsequent use of lomitapide by patients in France prior to marketing approval In the United states and European Union may allow us to recognize product sales revenue in 2011. |
| § | Selectively seek to expand our distribution capabilities and potentially address broader patient populations for lomitapide. We may selectively seek to establish collaborations to reach the patients with HoFH or FC in geographies that we do not believe we can cost effectively address with our own sales and marketing capabilities. If we elect to develop lomitapide for broader patient populations, we would plan to do so selectively either on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources. |
Lomitapide
Overview
Our lead product compound, lomitapide, is a small molecule microsomal triglyceride transfer protein, or MTP, inhibitor, or MTP-I, that we are developing as an oral once-a-day treatment for patients with severe lipid disorders. We are conducting a single-arm, dose titration, open-label pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH. We plan to submit an NDA to the FDA and an MAA to the EMA, before the end of 2011. We also plan to commence a Phase III pediatric clinical trial in 2011 to evaluate lomitapide for the treatment of pediatric and adolescent patients (> 7 to < 18 years of age) with HoFH. In addition, we are in the process of developing a protocol for a Phase III clinical trial of lomitapide for the treatment of adult patients with FC.
Lomitapide has been evaluated in thirteen Phase I and six Phase II clinical trials, as well as the on-going Phase III clinical trial, with an extension study as a separate protocol. Approximately 940 patients have been treated with lomitapide as part of these clinical trials.
Microsomal triglyceride transfer protein exists in both the liver and intestines where it plays a role in the formation of lipoproteins containing cholesterol and TGs. The liver and the intestines are the two main sources of circulating lipids in the body. The liver synthesizes cholesterol and TGs and provides the body’s intrinsic supply of lipids. The intestines are the conduit through which dietary lipids enter the body for metabolism. Given the fact that MTP is involved in the formation of cholesterol-carrying lipoproteins from both liver-related, or hepatic, and intestinal sources, we believe the inhibition of MTP makes an attractive target for lipid lowering therapy. Currently, there are no MTP-Is approved by the FDA for any indication.
Although we believe lomitapide has clinical potential for treatment of broader hyperlipidemic patient populations and may seek to selectively address such broader patient populations in the future, we are currently developing lomitapide as an oral, once-a-day treatment for patients with HoFH and FC. In October 2007, the FDA granted lomitapide orphan drug designation for the treatment of HoFH. In October 2010, we withdrew our application to the EMA for orphan drug designation for lomitapide for the treatment of HoFH, based on guidance we received from the EMA that lomitapide is not eligible for orphan drug designation for this indication since lomitapide has the potential to treat hypercholesterolemia in broader patient populations. At the same time, the EMA granted lomitapide orphan drug designation for the treatment of FC. In March 2011, the FDA also granted orphan drug designation for this indication.
Ongoing Pivotal Phase III Clinical Trial (HoFH)
We are currently studying lomitapide in a pivotal Phase III clinical trial to evaluate its efficacy and long-term safety for the treatment of patients with HoFH at the maximum tolerated dose of up to 60 mg. The FDA is funding approximately $1.0 million of the cost of this trial under its orphan drug product grant program. The trial is being conducted at 11 sites in four countries. We expect to complete this trial in the third quarter of 2011.
70
Table of Contents
This pivotal Phase III trial is a single-arm, dose titration, open-label clinical trial. We completed enrollment in March 2010 with a total of 29 patients. The patients in the trial are adult males and females with a mean age of 31. After a six week run-in period to stabilize lipid lowering therapy (including apheresis if applicable) and diet, patients are given ascending doses of lomitapide beginning at 5 mg/day and then titrated individually up to the maximum tolerated dose, or 60 mg/day over the first 26 weeks of the clinical trial. Patients are then maintained at a maximum tolerated dose for an additional 52 week safety phase. The efficacy and safety phases combined will last 78 weeks. After this time, eligible patients will be given the option to enroll in a separate protocol for a long-term, open-label extension trial to evaluate the long-term efficacy and safety of lomitapide at the maximum tolerated dose beyond 78 weeks. For patients who do not enter the optional open-label extension trial, there will be a six week wash-out period during which lomitapide will be discontinued and patients will remain on concomitant lipid lowering therapy.
The primary efficacy endpoint of this trial is percent change in LDL-C at the maximum tolerated dose compared to baseline after 26 weeks of treatment in combination with other lipid lowering therapies. Background therapies are maintained during the 26 week efficacy phase, but may be modified during the safety phase at the investigator’s discretion. As of May 31, 2011, the background therapy regimens of 14 patients have been reduced during the safety phase. LDL-C levels are measured at Weeks 0, 2, 6, 10, 14, 18, 22, 26, 36, 46, 56, 66 and 78. Because some patients in the trial also receive apheresis, only LDL-C levels prior to apheresis treatment are used in the trial analyses. The secondary endpoints of this trial include the evaluation of other lipid parameters, including percent change in TG levels from baseline, long-term safety, percent change in hepatic fat, as measured by magnetic resonance spectroscopy, or MRS, and pharmacokinetics in combination with other lipid lowering agents. Pharmacokinetics refers to a drug’s absorption, distribution and metabolism in, and excretion from, the body and measures, among other things, bioavailability of a drug, or concentration in the plasma.
Of the 29 patients originally enrolled in the trial, three patients have withdrawn their consent to participate in the trial and three patients have discontinued treatment due to gastrointestinal adverse events. The 23 patients remaining in the trial have completed the 26 week period of therapy after which the primary efficacy endpoint was measured, and the 56 week period of therapy during which the Filing Data were collected. As of the date of this prospectus, 19 of these patients have completed the entire 78 weeks of the trial. We expect to complete this trial in the third quarter of 2011.
Under our protocol and statistical analysis plan for this clinical trial, all primary analyses will be calculated using intention-to-treat principles, which means that each patient that begins treatment is considered part of the trial, whether or not they finish the trial. For purposes of presenting our clinical trial results in this filing, we also present these results on a completer analysis basis, which means that only those results from patients who actually complete the relevant period of treatment are presented. We are also presenting the data in this manner because we believe it can be important to understand the clinical results of those patients who actually completed the full treatment period, especially for a trial with a small number of patients. Although we believe that both presentations are fair representations of the data we have received in this clinical trial through May 31, 2011, the intention-to-treat analysis method is generally the primary statistical method used by the FDA.
As shown in the graph below, using the intention-to-treat analysis method, at the end of the 26 week efficacy phase of the trial, the 29 patients who began treatment in the trial experienced a mean reduction in LDL-C levels of 40% in comparison with baseline, with the baseline measurements reflecting the effect of maximum tolerated background therapy. Mean baseline LDL-C levels of the 29 patients who began treatment in the trial were 336 mg/dL, and mean LDL-C levels of these patients at Week 26 were 190 mg/dL.
Through week 56, the 23 patients currently participating in the trial experienced a mean reduction in LDL-C levels of 44% in comparison with baseline. Mean LDL-C levels of these patients at Week 56 were 199 mg/dL.
As shown in the graph below, the ‘completer’ analysis at the end of the 26 week efficacy phase of the trial, showed for the 23 completer patients a mean reduction in LDL-C levels of 50% in comparison with baseline, with the baseline measurements reflecting the effect of maximum tolerated background therapy. Mean baseline LDL-C levels of the 23 patients who completed the 26 week efficacy phase of the trial were 352 mg/dL, and mean LDL-C levels of these patients at week 26 were 168 mg/dL.
71
Table of Contents
Eight of the 23 patients who completed the 26 week efficacy phase of the trial achieved an LDL-C level below 100 mg/dL at week 26 and 15 of these patients achieved an LDL-C level below 175 mg/dL at week 26. The mean daily dose of lomitapide for the 23 patients who completed the 26 week efficacy phase of the trial was 45 mg at week 26 of the trial. The mean daily dose of lomitapide for these 23 patients at week 56 of the trial was 40 mg. The mean daily dose of lomitapide for the full 29 patients who began treatment in the trial was 38 mg at week 26 of the trial.
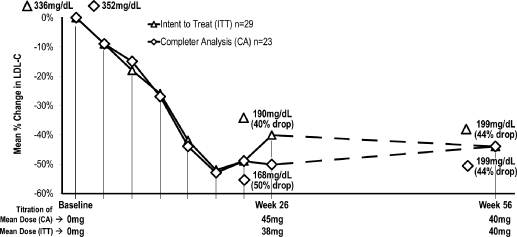
Using the intention-to-treat analysis method, at the end of the 26 week efficacy phase of the trial, the 29 patients who began treatment in the trial experienced median reduction in TG levels of 45% in comparison with baseline. Among the 29 patients who began treatment in the trial, median TG levels were 92 mg/dL at baseline, and 57 mg/dL at week 26.
Using the completer analysis method, at the end of the 26 week efficacy phase of the trial, the 23 patients who completed the 26 week efficacy phase of the trial experienced median reduction in TG levels of 56% in comparison with baseline. Among the 23 patients who completed the 26 week efficacy phase of the trial, median TG levels were 101 mg/dL at baseline, and 57 mg/dL at Week 26.
Through Week 56, the 23 patients still participating in the trial experienced a median reduction in TG levels of 33% in comparison with baseline. Median TG levels for these patients at week 56 were 61 mg/dL.
Mild to moderate gastrointestinal adverse events have been the most commonly reported side effect in this trial. The majority of these gastrointestinal adverse events occurred during the first days following the introduction of a higher dose. We have observed a reduction in gastrointestinal adverse effects after the 26 week efficacy phase in which patients are titrated to the maximum tolerated dose.
Because changes in liver function are an area of interest for this class of drugs, we are also examining the number of instances in which there are significant increases in ALT (alanine transaminase) or AST (aspartate transaminase) observed for any patient at any time during the course of this trial. ALT and AST are liver enzymes that are commonly measured clinically as a part of a diagnostic liver function test to determine liver health. Significantly elevated plasma liver enzymes are indicative of some degree of liver cell damage and in some instances can be indicative of liver toxicity. Although some drugs, such as the cholesterol-lowering class of drugs known as statins, cause an increased incidence of liver enzyme elevations, these are generally not clinically significant. Drug therapies that have high rates of clinically significant liver transaminase elevations may indicate a potential to cause more significant liver toxicity in some patients. The risk of liver damage is increased when clinically significant elevations in liver transaminases are seen with clinically significant elevations of bilirubin, which we have not observed in our trials. As of May 31, 2011, four of the 23 patients remaining in the trial had experienced ALT elevations greater than five times the upper limit of normal. Of these four patients, three underwent a temporary dose reduction and have maintained study drug at a stable dose. One patient discontinued
72
Table of Contents
treatment for a period of seven weeks, after which the treatment was reinstated and the patient was able to maintain a stable dose and complete the trial per protocol. No patients have been removed from the trial due to liver function test elevations.
In accordance with the trial protocol, we are also measuring hepatic fat levels and pulmonary function at Weeks 0, 26, 56 and 78, and after the six week wash-out period for those patients who do not enter the optional open-label extension trial. The 22 patients who had hepatic fat measurements taken experienced an increase in hepatic fat from a mean of 1.0% to 9.0% at 26 weeks of treatment. The threshold for mild steatosis, a condition of hepatic fat accumulation, is in the range of 5 to 6% fat. Of the 23 patients who had completed 56 weeks of treatment, 21 had hepatic fat measurements taken. These 21 patients had a mean hepatic fat level of 7.3% at this measurement time. In addition, the median change in hepatic fat from baseline in these patients was 5.7% at 26 weeks of treatment and 4.4% at 56 weeks of treatment. Some studies suggest that patients who have hepatic steatosis that results from pre-existing conditions, such as obesity and type 2 diabetes, may be at an increased risk for more severe long-term liver consequences, such as hepatic inflammation and fibrosis. However, the consequences of hepatic steatosis that results from other factors is unclear. For example, in an observational study published in the Journal of Lipid Research, a peer-reviewed medical journal, patients who suffer from a genetic MTP deficiency have been shown to have some degree of hepatic steatosis, with average hepatic fat levels of 14.8%, without long-term liver complications. In addition, there are FDA approved drugs for sale in the United States that are known to induce hepatic steatosis, including tamoxifen, which is used for the treatment of breast cancer, and amiodarone, which is used for the treatment of ventricular fibrillation. We expect to complete the population pharmacokinetics analysis at the end of the trial.
Additional Pre-NDA Studies
Before we submit our NDA, we must complete additional preclinical and clinical studies, including a thorough QT study in healthy volunteers to evaluate the effect of lomitapide on the heart’s electrical cycle, known as the QT interval, and a study of lomitapide in patients who are renally impaired. We plan to complete each of these studies prior to the submission of our NDA for HoFH.
Completed Phase II Clinical Trial (HoFH)
In February 2004, UPenn completed a Phase II clinical trial of lomitapide for the treatment of patients with HoFH. In this single-arm, dose titration, open-label clinical trial, six patients were given ascending daily doses based upon body weight of 0.03 mg/kg, 0.1 mg/kg, 0.3 mg/kg and 1.0 mg/kg of lomitapide at four-week intervals for a total of 16 weeks. Given the weight-based dosing, the average dose at 1 mg/kg was 67 mg/day.
73
Table of Contents
In January 2007, the results of this trial were published in The New England Journal of Medicine and are summarized in the chart below. Percentage change represents the average percentage change from baseline in the four lipid parameters for the six patient group. P-value is a measure of the likelihood that reduction in LDL-C levels versus baseline is due to random chance. A p-value of less than 0.05 means the probability that the difference is due to random chance is less than 5%, and is a commonly accepted threshold for denoting a statistically meaningful difference.
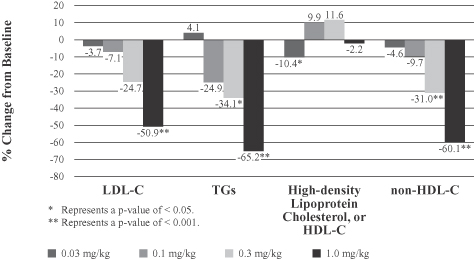
We believe these results demonstrate a dose-dependent effect of lomitapide on lipid levels. In addition, patients treated with lomitapide experienced a mean reduction in body weight of 4.4% (2.8 kg) over the 16 weeks of therapy.
No patient withdrew from the trial and all patients were titrated to the maximum planned dose. Adverse events that were judged to be associated with drug therapy were primarily gastrointestinal, typically transient episodes of increased stool frequency of mild or moderate severity. Clinically significant elevations in the liver enzyme ALT were observed in three of the six patients. In one patient, the dose of lomitapide was temporarily reduced per protocol, after which ALT returned to lower levels. This patient subsequently was able to resume the earlier, higher dose and continue to be titrated to the maximum dose. In the other two patients, the elevations in ALT returned to lower levels with continued lomitapide treatment. Increases in hepatic fat levels were seen in four patients, whereas the other two patients had minimal changes in hepatic fat levels. With the exception of values in one patient, elevated ALT and hepatic fat levels returned to baseline levels upon cessation of therapy. The nonconforming patient, consumed large quantities of alcohol (self-reported to be 6 – 7 oz. of ethanol per day) during the trial and it is suggested that alcohol consumption may have contributed to the elevations during and after the clinical trial. Because a forced-titration scheme of treating the patients at increasing doses with four week intervals was employed, we believe it is reasonable to infer that greater increases in hepatic fat are seen with the higher doses of lomitapide.
An increase in the international normalized ratio, which measures the blood’s ability to form clots, was observed in the two patients receiving warfarin, an anti-coagulation therapy, which may be due to a drug-drug interaction. It is possible that patients who use lomitapide with warfarin may require monitoring of the international normalized ratio, as is commonly experienced by patients taking warfarin with other drugs and adjust dosages as necessary. In addition, pulmonary function tests were conducted at baseline, at the end of each dose and four weeks after study treatment. Pulmonary function tests remained unchanged for the duration of treatment compared to baseline in all patients.
74
Table of Contents
Historical Development of Lomitapide
Although we are currently focused on the development of lomitapide for the treatment of severe forms of elevated cholesterol and TGs, we, BMS and UPenn previously pursued extensive development of lomitapide for potentially broader use or the treatment of high cholesterol for patients at moderately high risk of a cardiovascular event or for patients who were unable to tolerate a statin and therefore required additional LDL-C lowering in pursuit of their recommended target LDL-C levels.
In the mid-to-late 1990s, BMS developed lomitapide as a monotherapy treatment aimed at producing LDL-C lowering efficacy equal to or greater than that seen at maximum dosing with statins. Early clinical trials produced meaningful percent reductions in LDL-C levels, but participants discontinued at a high rate due to gastrointestinal adverse effects. We believe this resulted in large part from the failure to employ dose titration, which is the gradual increase in dosing over time to allow the body to adapt to the impact of a higher dose coupled with a low fat diet. In 2003, BMS donated certain patent rights and other rights related to this product candidate to The Trustees of the University of Pennsylvania, or The Trustees of UPenn. In May 2006, we entered into an exclusive, worldwide patent license with The Trustees of UPenn for the right to develop and commercialize lomitapide to treat specified patient populations.
The historical clinical program for lomitapide consisted of:
| § | eleven Phase I clinical trials involving 251 patients who received single or multiple doses of lomitapide of between 1 mg/day and 200 mg/day; and |
| § | six Phase II clinical trials, including the Phase II clinical trial in patients with HoFH sponsored by UPenn and a Phase II clinical trial in patients with hypercholesterolemia sponsored by BMS, involving 452 patients who received lomitapide. Patients in five of these Phase II trials received lomitapide at daily doses between 2.5 mg/day and 67 mg/day over four and 12 weeks, while the patients in the HoFH Phase II trial sponsored by UPenn received weight-based dosing with mean doses of 2 mg/day to 67 mg/day during the 16 week trial. |
The following table summarizes the four Phase II trials of lomitapide that we completed for the treatment of hypercholesterolemia:
| Trial Description |
Dose Range and Concomitant Drugs |
Duration of Trial |
Number of Patients Dosed with Lomitapide | |||
|
Combination use of lomitapide with ezetimibe |
Lomitapide, 5 mg to 10 mg with or without ezetimibe, 10 mg |
12 weeks |
56 | |||
| Combination use of lomitapide with atorvastatin |
Lomitapide, 5 mg to 10 mg with or without atorvastatin, 20 mg |
8 weeks | 104 | |||
| Combination use of lomitapide and other lipid lowering therapies |
Lomitapide, 2.5 to 10 mg; Lomitapide, 5 mg with atorvastatin, 20 mg, fenofibrate, 145 mg, or ezetimibe, 10 mg |
12 weeks | 227 | |||
| Impact of titration on efficacy, safety and tolerability of lomitapide in combination with atorvastatin |
Lomitapide, 2.5 mg and 5 mg, with or without atorvastatin, 20 mg |
8 weeks | 21 |
75
Table of Contents
In the Phase II clinical trials summarized in the table above, using an intent-to-treat analysis, lomitapide at doses ranging from 2.5 mg/day to 10 mg/day reduced LDL-C levels in a dose dependent manner by 9% to 37% from baseline, respectively, when given as a monotherapy, by 35% to 46% from baseline, respectively, when used with ezetimibe, a drug that inhibits intestinal absorption of cholesterol, and by 47% to 51% from baseline, respectively, when used with atorvastatin. In comparison, ezetimibe monotherapy at a dose of 10 mg/day reduced LDL-C levels by 22% from baseline, and atorvastatin monotherapy at a dose of 20 mg/day reduced LDL-C levels by 42% from baseline after 12 weeks of treatment. Modest reductions in TG levels and body weight were also observed in patients treated with lomitapide in these trials. Most of the reductions in LDL-C levels from baseline were statistically significant. However, many of the changes in TG levels were not statistically significant due to the naturally high degree of variability in TG levels in the blood, the small sample size and the fact that the trials were designed to evaluate effect on LDL-C levels and not TG levels.
In all six Phase II clinical trials and the eleven Phase I clinical trials, the most common adverse events reported were gastrointestinal, including diarrhea, nausea and vomiting. These adverse events were generally mild to moderate in nature. In addition, liver enzyme elevations occurred in a small proportion of patients and led to discontinuations from study drug. In these Phase I clinical trials and the earlier Phase II clinical trials, persistent elevation in liver transaminase levels at three times the upper limit of normal was used as a cutoff for discontinuation of lomitapide treatment. In these trials, out of a total of 351 patients treated with lomitapide at doses of 5 mg/day to 200 mg/day, 14 patients, or 4%, discontinued treatment. In later Phase II clinical trials, based on discussions with the FDA, persistent elevations in liver transaminase levels at five times the upper limit of normal was used as a cutoff for discontinuation of lomitapide treatment. In these trials, out of a total of 352 patients treated with lomitapide at doses of 2.5 mg/day to 10 mg/day alone or in combination with other lipid lowering agents, five patients, or 1.4%, discontinued treatment. In the majority of patients who experienced liver enzyme elevations and remained on study drug, the levels returned to baseline while continuing dosing.
In the six Phase II and eleven Phase I clinical trials, patients with baseline hepatic fat concentrations of less than 6.2% experienced increased mean hepatic fat four weeks after initiation of dosing, which then plateaued at eight weeks and at 12 weeks of dosing to levels of 6.2% to 9.7% for study doses of 2.5 mg/day to 10 mg/day. Higher levels of hepatic fat increases were occasionally observed at higher dose levels.
Familial Chylomicronemia (FC)
We also plan to develop lomitapide for use as an oral, once-a-day treatment for adult patients with FC. In October 2010, the EMA granted lomitapide orphan drug designation for the treatment of FC. In March 2011 the FDA also granted orphan drug designation for this indication. We are currently treating two patients with lomitapide for severe hypertriglyceridemia under the FDA’s compassionate use program, through which a licensed physician may request from a manufacturer an investigational drug for the diagnosis, monitoring or treatment of a serious disease or condition in an individual patient. The FDA will authorize the use if it determines that (1) the patient has no comparable or satisfactory alternative therapy; (2) the potential benefit justifies the potential risks of the treatment; (3) the probable risk to the patient from the investigational drug is not greater than the probable risk from the disease or condition; (4) the patient cannot obtain the drug under another investigational new drug application, or IND, or protocol; and (5) providing the investigational drug will not interfere with the initiation, conduct or completion of clinical investigations to support marketing approval. The physician must obtain written informed consent from the patient and institutional review board, or IRB, approval prior to administering the investigational drug, and a summary of the results of such use, including adverse events, must be provided to the FDA.
Based on the TG reductions seen in these patients and the TG reductions seen in other clinical trials of lomitapide, we believe lomitapide has potential for treating patients suffering from extremely high levels of TGs leading to life threatening pancreatitis.
We are in the process of developing a protocol for a Phase III clinical trial for the treatment of adult patients with FC. Based on our current plans, the protocol would provide for adult patients with FC to enter the trial on their existing treatment regimen and then be randomized to a lomitapide or a placebo treatment arm for 12 weeks.
76
Table of Contents
Patients receiving lomitapide would start at 5 mg and be titrated to 10 mg and 20 mg at four week intervals. The primary efficacy endpoint would be the percent reduction in TG levels at week 12. After week 12, patients would remain on lomitapide as part of a long-term safety phase of the trial.
Potential Future Product Candidate
Implitapide
We believe that we have the opportunity to develop implitapide, our second MTP-I, for the treatment of the same indications as lomitapide. To date, we have focused our efforts with implitapide on optimizing its manufacturing process. Based on clinical data developed by Bayer Healthcare AG, or Bayer, we believe this compound may have a role in addressing LDL-C and TG lowering needs of severe and high-risk patients. Because a lower concentration of implitapide was needed to inhibit the activity of MTP to 50% of its baseline activity in the intestines than in the liver, we believe implitapide may be slightly more active in the intestines than the liver, perhaps positioning it as a preferable treatment of hypertriglyceridemia of intestinal origin.
In June 2007, we received notice from the FDA of a partial clinical hold with respect to clinical trials of longer than six months duration for our product candidates, lomitapide and implitapide. At that time, the FDA did not apply this partial clinical hold to our pivotal Phase III clinical trial of lomitapide for the treatment of patients with HoFH. The FDA removed the partial clinical hold with respect to lomitapide in February 2010, but this partial clinical hold remains in effect with respect to implitapide.
Sales and Marketing
Given our stage of development, we have not yet established a commercial organization or distribution capabilities. In the United States and the European Union, due to the rare nature of the diseases we are seeking to address and the limited options for treatment, patients suffering from these diseases, such as HoFH and FC, together with their physicians, often have a high degree of organization and are well informed, which may make it easier to identify target populations after a drug is approved.
Most patients with diagnosed HoFH or FC, based on the severity of the condition and recommended treatments, will mostly likely be treated at academic centers or otherwise by physicians who specialize in the treatment of highly elevated lipid levels. If approved for the treatment of patients with HoFH or FC, we believe that it will be possible to commercialize lomitapide for these indications with a relatively small specialty sales force that calls on a limited and focused group of physicians. In anticipation of our commercial launch of lomitapide initially in the United States and European Union, we have begun to recruit a team comprised of sales representatives and medical education specialists who are experienced in marketing drugs for the treatment of rare, often genetic, disorders, for the commercialization of lomitapide. We initially plan to hire a medical education, marketing and sales force of approximately 15 people in the United States and approximately 18 people in the European Union.
As a result of the ongoing release of our Phase III clinical data, we have been engaged in dialogue with many of the specialists who serve patients with HoFH and FC. We believe that these activities have provided us with a growing knowledge of the physicians we plan to target for commercial launch of lomitapide for these conditions, subject to marketing approval in the United States and the European Union.
Outside of the United States and the European Union, subject to obtaining necessary marketing approvals, we likely will seek to commercialize lomitapide through distribution or other collaboration arrangements for HoFH and FC. If we elect to develop lomitapide for broader patient populations, we would plan to do so selectively either on our own or by establishing alliances with one or more pharmaceutical company collaborators, depending on, among other things, the applicable indications, the related development costs and our available resources.
We plan on developing a web-linked patient community forum in which patients with HoFH and FC will be able to access educational materials about their disease and communicate with each other. This community may allow us to contact patients with information about their disease and provide an ongoing forum to discuss their condition, as well as alert patients to clinical trials and general educational resources.
77
Table of Contents
Additionally, we are supporting the development of a global disease registry for patients with severe inherited hypercholesterolemia or hypertriglyceridemia, including FC. This may allow the collection of patient specific information, disease and other pertinent medical information to support physician research, ongoing disease management including treatment, and publications. All patient identity information would be confidential to the attending physician and respect the confidentiality laws and regulations of each country.
Through these two web-based products, the patient community and disease registry, we may be able to access patients and potentially add them to our growing list of eligible patients for the time when the drug is approved for use. This will benefit patients with increased information and also us by enabling the rapid identification and enrollment in trials and treatment of willing patients subject to all applicable laws and regulations.
Manufacturing and Supply
Lomitapide is a small molecule drug that is synthesized with readily available raw materials using conventional chemical processes. Hard gelatin capsules are prepared at 5 mg, 10 mg and 20 mg strength by filling the capsule shell with formulated drug product.
We currently have no manufacturing facilities and limited personnel with manufacturing experience. We currently rely on a single contract manufacturer to produce both drug substances and drug products required for our clinical trials and our commercial supply. All lots of drug substance and drug products used in clinical trials are manufactured under current good manufacturing practices, with oversight by our internal managers. We plan to continue to rely upon contract manufacturers and, potentially, collaboration partners to manufacture commercial quantities of our drug substances and drug product candidates if and when approved for marketing by the applicable regulatory authorities.
We currently rely on a single manufacturer for the preclinical and clinical supplies of each of our product candidates and for our registration batches of the drugs. We purchase these supplies from this manufacturer on a purchase order basis and do not have a long-term supply arrangement in place. We also do not have agreements in place for redundant supply or a second source for any of our product candidates although we have identified a possible secondary supplier of drug substance. We believe that there are alternate sources of supply that can satisfy our clinical trial requirements without significant delay or material additional costs.
Competition
Our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. Key competitive factors affecting the commercial success of our product candidates are likely to be efficacy, safety and tolerability profile, reliability and durability of response, convenience of dosing and price and reimbursement.
The market for lipid lowering therapeutics is large and competitive with many applicable drug classes. However, our products, if approved, will be focused, at least initially, on niche orphan markets where they will be positioned for use in combination with existing approved therapies, such as statins, to provide incremental efficacy in currently underserved patient populations. We believe that lomitapide will face distinct competition for the treatment of both HoFH and FC. Although there are no MTP-I compounds currently approved by the FDA for the treatment of hyperlipidemia, we are aware of other MTP-I compounds in early stage clinical trials and other pharmaceutical companies that are developing the following potentially competitive product candidates:
| § | HoFH — Isis Pharmaceuticals, Inc., or Isis, is developing an antisense apoB-100 inhibitor, mipomersen, as a weekly subcutaneous injection for lowering high cholesterol, LDL-C and apolipoprotein B and has completed four Phase III clinical trials for this product candidate. The FDA has granted mipomersen orphan drug designation for the treatment of patients with HoFH. Therefore, if mipomersen is approved by the FDA, Isis will be entitled to seven years of marketing exclusivity for such indication as to competitive products that are the same drug as mipomersen. In January 2008, Isis formed a partnership with Genzyme Corporation, now a subsidiary of Sanofi-Aventis, wherein Genzyme will develop and sell mipomersen. |
78
Table of Contents
| § | FC — In December 2009, Amsterdam Molecular Therapeutics Holding N.V., or AMT, filed an application for marketing approval for Glybera, an injectable gene therapy, with the EMA for the treatment of patients with FC. AMT has stated that it is expecting approval of this MAA in 2011. This product candidate has been tested in a total of 27 patients in three different clinical trials. It would represent the first gene therapy approved in the European Union. |
| § | PCSK9 Defects — Several companies are developing molecules that attempt to mimic the impact observed in patients with defects in their PCSK9 gene. Such patients have lower LDL-C levels and an observed reduction in cardiovascular events, and some believe that medicines that duplicate this behavior may effectively reduce LDL-C levels with a similar benefit. Regeneron Pharmaceuticals, Inc. is among the companies thought to have one of the more advanced PCSK9 programs, with recent Phase I data from an injectable antibody it is developing having been presented at the American College of Cardiology meeting. |
If we obtain marketing approval of lomitapide for the treatment of patients with HoFH in the United States and Isis obtains marketing approval of mipomersen for the treatment of patients with HoFH in the United States, lomitapide would compete in the same market with mipomersen. If Isis obtains marketing approval of mipomersen for the treatment of patients with HoFH in the United States prior to us, Isis could obtain a significant competitive advantage associated with being the first to market and utilizing Genzyme’s marketing and sales force. In connection with obtaining marketing approval for mipomersen, Isis will also obtain orphan drug exclusivity for mipomersen, but we do not believe that Isis’ obtaining orphan drug exclusivity for mipomersen prior to our receiving orphan drug exclusivity for lomitapide would have an adverse effect on our business as mipomersen and lomitapide are different drugs under FDA rules and any exclusivity applicable to either drug will not apply to the other drug. Thus, because mipomersen is a different drug than lomitapide, we could obtain both approval and orphan drug exclusivity for lomitapide even if Isis has already obtained orphan drug exclusivity for mipomersen and Isis could obtain both approval and orphan drug exclusivity for mipomersen even if we have already obtained orphan drug exclusivity for lomitapide.
Similarly, in the European Union, notwithstanding the fact that the EMA has granted lomitapide orphan drug designation for the treatment of FC, if a competitor subsequently obtains approval and market exclusivity for its orphan designated drug for the treatment of FC, our marketing application for FC would not be accepted and we would be excluded from the market only if lomitapide was a “similar medicinal product” to our competitor’s product. In the European Union, a drug is a “similar medicinal product” if it contains a “similar active substance” or substances and is intended for the same therapeutic indication. A “similar active substance” is an identical active substance, or an active substance with the same principal molecular structural features and which acts via the same mechanism. We do not believe orphan drug exclusivity for mipomersen in the European Union would have an adverse effect on our business because we do not believe that lomitapide would be considered a “similar medicinal product” to mipomersen.
If lomitapide receives marketing approval for the treatment of patients with HoFH or FC, we believe that the reductions in LDL-C levels and TG levels that have been observed in clinical trials of this product candidate, its oral form of administration and mechanism of action as a small molecule will be important features that physicians and patients will consider in comparing lomitapide with an injectable and drugs employing complex mechanisms of action, such as antisense compounds and gene therapy.
If we decide to develop and commercialize our product candidates for broader patient populations, we likely will compete more directly with other lipid lowering drugs in these indications. There are a range of drugs in this category, some of which, such as statins, are inexpensive, safe and effective and widely accepted by patients and physicians.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other marketing approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining FDA and other marketing approvals for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effectively marketed and sold
79
Table of Contents
than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render our drugs non-competitive or obsolete.
Intellectual Property
Our policy is to pursue patents, developed internally and licensed from third parties, and other means to protect our technology, inventions and improvements that are commercially important to our business. We also rely on trade secrets that may be important to our business.
Our success will depend significantly on our ability to:
| § | obtain and maintain patent and other proprietary protection for the technology, inventions and improvements we consider important to our business; |
| § | defend our patents; |
| § | preserve the confidentiality of our trade secrets; and |
| § | operate without infringing the patents and proprietary rights of third parties. |
As of the date of this prospectus, our lomitapide patent portfolio consists of five issued U.S. patents and related issued patents in Europe, Canada, Israel and Japan and related pending applications in the U.S., Europe, Australia, Japan, Canada, Israel, South Korea and New Zealand. We hold an exclusive worldwide license from The Trustees of UPenn to these patents and patent applications. This license is described below. The issued U.S. patents described above contain claims directed to the compound, lomitapide, and various methods of use, including methods of treating atherosclerosis, hyperlipidemia or hypercholesterolemia, methods of using certain dosing regimens for treating hyperlipidemia or hypercholesterolemia, and methods of reducing serum lipid levels, cholesterol or TGs, and are scheduled to expire between 2013 and 2027. The U.S. patent covering the composition of matter of lomitapide is scheduled to expire in 2015. The non-U.S. patents directed to lomitapide are scheduled to expire in 2016.
As of the date of this prospectus, our implitapide patent portfolio consists of four issued U.S. patents, two pending U.S. non-provisional applications, and related patents and pending applications in Europe, Australia, Asia, Africa, and South America. We hold an exclusive worldwide license from Bayer to these patents and patent applications. This license is described below. The issued U.S. patents described above contain claims directed to the compound, implitapide, methods for treating obesity and atherosclerosis, and processes for making implitapide, and are scheduled to expire between 2015 and 2017. The U.S. patent and non-U.S. patents covering the composition of matter of implitapide are scheduled to expire in 2015.
In addition to the patents and patent applications described above, we have filed four non-provisional U.S. patent applications and related foreign applications in Australia, Canada, Europe and Japan and two international applications directed to pharmaceutical combinations of a MTP-I, such as lomitapide or implitapide, and other cholesterol lowering drugs, and to methods of using such combinations in certain dosing regimens to reduce serum cholesterol or TG concentrations.
Depending upon the timing, duration and specifics of FDA approval of the use of lomitapide or implitapide, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. See “ — Regulatory Matters —Patent Term Restoration and Marketing Exclusivity.”
Licenses
University of Pennsylvania
In May 2006, we entered into a license agreement with The Trustees of UPenn, pursuant to which we obtained an exclusive, worldwide license from The Trustees of UPenn to certain know-how and a range of patent rights
80
Table of Contents
applicable to lomitapide. In particular, we obtained a license to certain patents and patent applications owned by The Trustees of UPenn relating to the dosing of MTP-Is, including lomitapide, and certain patents and patent applications and know-how covering the composition of matter of lomitapide that were assigned to The Trustees of UPenn by BMS for use in the field of monotherapy or in combination with other dyslipidemic therapies for treatment of patients with severe hypercholesterolemia unable to come within 15% of NCEP LDL-C goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe combined hyperlipidemia unable to come within 15% of NCEP non-HDL-C goal on maximal tolerated oral therapy, as determined by the patient’s prescribing physician, or with severe hypertriglyceridemia unable to reduce TG <1,000 on maximal tolerated therapy. We also have the right to use lomitapide either as a monotherapy or with other dyslipidemic therapies to treat patients with HoFH. We refer to the patents and patent applications assigned by BMS to The Trustees of UPenn and licensed to us by The Trustees of UPenn as the BMS-UPenn assigned patents.
To the extent that rights under the BMS-UPenn assigned patents were not licensed to us under our license agreement with The Trustees of UPenn or were retained by The Trustees of UPenn for non-commercial education and research purposes, those rights, other than with respect to lomitapide, were licensed by The Trustees of UPenn back to BMS on an exclusive basis pursuant to a technology donation agreement between The Trustees of UPenn and BMS. In the technology donation agreement, BMS agreed not to develop or commercialize any compound, including lomitapide, covered by the composition of matter patents included in the BMS-UPenn assigned patents in the field licensed to us exclusively by The Trustees of UPenn. Through our license with The Trustees of UPenn, as provided in the technology donation agreement, we have the exclusive right with respect to the BMS-UPenn assigned patents regarding their enforcement and prosecution in the field licensed exclusively to us by The Trustees of UPenn.
The license from The Trustees of UPenn covers, among other things, the development and commercialization of lomitapide alone or in combination with other active ingredients in the licensed field. The license is subject to customary non-commercial rights retained by The Trustees of UPenn for non-commercial educational and research purposes. We may grant sublicenses under the license, subject to certain limitations.
We are obligated under this license agreement to use commercially reasonable efforts to develop, commercialize, market and sell at least one product covered by the licensed patent rights, such as lomitapide. Pursuant to this license agreement, we paid The Trustees of UPenn a one-time license initiation fee of $56,250. We will be required to make development milestone payments to The Trustees of UPenn of up to an aggregate of $150,000 when a licensed product’s indication is limited to HoFH or severe refractory hypercholesterolemia, and an aggregate of approximately $2.6 million for all other indications within the licensed field. All such development milestone payments for these other indications are payable only once, no matter how many licensed products for these other indications are developed. In addition, we will be required to make royalty payments in a range of levels not greater than 10% on net sales of products covered by the license (subject to a variety of customary reductions), and share with The Trustees of UPenn specified percentages of sublicensing royalties and other consideration that we receive under any sublicenses that we may grant.
This license agreement will remain in effect on a country-by-country basis until the expiration of the last-to-expire licensed patent right in the applicable country. We have the right to terminate this license agreement for convenience upon 60 days prior written notice to The Trustees of UPenn or for The Trustees of UPenn’s uncured material breach of the license agreement. The Trustees of UPenn may terminate this license agreement for our uncured material breach of the license agreement, our uncured failure to make payments to The Trustees of UPenn or if we are the subject of specified bankruptcy or liquidation events.
Bayer Healthcare AG
In May 2006, we entered into a license agreement with Bayer pursuant to which we obtained an exclusive, worldwide license from Bayer under the patent rights and know-how owned or controlled by Bayer applicable to implitapide. This license covers the development and commercialization of implitapide alone or in combination with other active ingredients. We may grant sublicenses under the license, subject to certain limitations. Pursuant
81
Table of Contents
to this license agreement, we granted Bayer a first right of negotiation in the event Aegerion desires to commercialize any licensed products through or with a third party, which expires after 90 days if the parties are unable to come to an agreement. This first right of negotiation applies only to the specific products and in the specific countries potentially subject to third-party commercialization.
We are obligated under this license agreement to use commercially reasonable efforts to develop and commercialize, at our cost, at least one licensed product, such as implitapide. Pursuant to this license agreement, we paid Bayer a one-time initial license fee of $750,000. We will be required to make certain development milestone payments of up to an aggregate of approximately $4.5 million upon the achievement of certain development milestones. Each development milestone payment is payable only once, no matter how many licensed products are developed. In addition, we are required to make annual payments through 2011 of $1.0 million in the aggregate, with each annual payment creditable in full against any development milestones that are or become payable, a one-time commercialization milestone payment of $5.0 million if we achieve aggregate annual net sales of licensed products of $250.0 million (payable only once, no matter how many licensed products are commercialized), and royalty payments in a range of levels not greater than 10% on net sales of licensed products (subject to a variety of reductions).
This license agreement will remain in effect, and royalties will be payable on sales of licensed products, on a country-by-country and product-by-product basis until the later of the expiration of the last-to-expire licensed patent right in the applicable country or ten years after the first commercial sale of a licensed product in that country. This license agreement may be terminated by either party for the other party’s uncured material breach of this license agreement, except that Bayer may only terminate the license agreement for our material breach on a country-by-country or product-by-product basis if our breach is reasonably specific to one or more countries or licensed products, or if the other party becomes the subject of a specified bankruptcy or liquidation event. We have the right to terminate the license agreement for convenience upon 60 days prior written notice to Bayer.
Regulatory Matters
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of products such as those we are developing. Our drugs must be approved by the FDA through the NDA process before they may be legally marketed in the United States and must be approved by foreign regulatory authorities via various procedures before they can be marketed in the applicable country.
U.S. Drug Development Process
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and implementing regulations. The process of obtaining marketing approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include, among other things, the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, civil money penalties, fines, refusals of government contracts, debarment, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| § | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable regulations; |
| § | submission to the FDA of an IND which must become effective before human clinical trials may begin; |
82
Table of Contents
| § | performance of human clinical trials, including adequate and well-controlled trials, according to Good Clinical Practices to establish the safety and efficacy of the proposed drug for its intended use; |
| § | submission to the FDA of an NDA; |
| § | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current good manufacturing practice, or cGMP, to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| § | FDA review and approval of the NDA. |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
Once a pharmaceutical candidate is identified for development it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor will also include a protocol detailing, among other things, the objectives of the first phase of the clinical trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated, if the first phase lends itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during studies due to safety concerns or non-compliance.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with good clinical practice regulations. These regulations include the requirement that all research subjects provide informed consent. Further, an IRB must review and approve the plan for any clinical trial before it commences at any institution. An IRB considers, among other things, whether the risks to individuals participating in the trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative and must monitor the study until completed.
Each new clinical protocol must be submitted to the IND for FDA review, and to the IRBs for approval. Protocols detail, among other things, the objectives of the study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| § | Phase I. The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| § | Phase II. This phase involves trials in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| § | Phase III. This phase involves trials undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population, often at geographically dispersed clinical trial sites. These trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. |
Progress reports detailing developments associated with the clinical testing program must be submitted at least annually to the FDA, and safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events or animal test results that suggest a significant risk to human subjects, such as
83
Table of Contents
carcinogenicity. Phase I, Phase II, and Phase III testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Sponsors of all controlled clinical trials, except for Phase I trials, are required to submit certain clinical trial information for inclusion in the public clinical trial registry and results data bank maintained by the National Institutes of Health.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA generally is subject to the payment of a user fee, although NDAs for designated orphan drugs are exempt from this fee.
In addition, under the Pediatric Research Equity Act of 2003, or PREA, an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant deferrals for submission of data or full or partial waivers. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan designation has been granted.
The FDA could also require a risk evaluation and mitigation strategy, or REMS, plan to mitigate serious risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools.
The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. FDA is required to refer an NDA for a new chemical entity to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions, or explain why such review is not necessary. The FDA has informed us that discussion of the NDA for lomitapide for the treatment of patients with HoFH at an advisory committee meeting is highly likely. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and difficult and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. Before approving an NDA, the FDA will inspect the facility or facilities
84
Table of Contents
where the product is manufactured. In addition, FDA often will conduct a bioresearch monitoring inspection of the clinical trial sites involved in conducting pivotal studies to ensure data integrity and compliance with applicable GCP requirements.
NDAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. In addition, products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval and may be approved on the basis of adequate and well-controlled clinical trials establishing that the drug product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, a sponsor of a drug receiving accelerated approval must perform post-marketing studies to validate the surrogate endpoint or otherwise confirm the effect of the drug on a clinical endpoint, and the drug may be subject to accelerated withdrawal procedures. Priority review and accelerated approval do not change the standards for approval, but may expedite the approval process.
If a product receives marketing approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require us to conduct Phase IV testing which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized. In addition, FDA may impose distribution and use restrictions and other limitations on labeling and communication activities with respect to an approved drug product via a REMS plan.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of lomitapide or implitapide, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the extension must be applied for prior to expiration of the patent and within 60 days of the NDA approval. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the filing of the relevant NDA.
Market and data exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent data exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company that references the previously approved drug. However, an ANDA or 505(b)(2) NDA may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA or 505(b)(2) NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, strengths or dosage forms of an existing drug. This three-year exclusivity covers only the conditions of use associated with the new clinical investigations and, as a general matter, does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for generic versions of the original, unmodified drug product. Five-year
85
Table of Contents
and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
In the European Union, certain patents may qualify for a supplemental protection certificate that would extend patent protection for up to five years after patent expiration upon marketing approval in the European Union.
In the European Union, new chemical entities qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from assessing a generic application for eight years, after which generic marketing authorization can be submitted but not marketed for two years.
Pediatric exclusivity is another type of exclusivity available in the United States. Pediatric exclusivity, if granted, provides an additional six months to an existing exclusivity period, including orphan drug exclusivity, or statutory delay in approval resulting from certain patent certifications. This six-month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial or trials in accordance with an FDA-issued “Written Request” for such a trial or trials. The current pediatric exclusivity provision was reauthorized on September 27, 2007 and, unless reauthorized again, will sunset on October 1, 2012.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug designated as an orphan drug receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan drug exclusivity.
The FDA also administers a clinical research grants program, whereby researchers may compete for funding to conduct clinical trials to support the approval of drugs, biologics, medical devices, and medical foods for rare diseases and conditions. An application for an orphan grant should propose one discrete clinical study to facilitate FDA approval of the product for a rare disease or condition. The study may address an unapproved new product or an unapproved new use for a product already on the market.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval, following notice and an opportunity for a hearing, if, among other things, compliance with certain regulatory standards is not maintained or if new information indicates that the drug is not safe or effective. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. If new safety issues are identified following approval, the FDA can require the NDA sponsor to revise the approved labeling to reflect the new safety information; conduct post-market studies or clinical trials to assess the new safety information; and implement a REMS program to mitigate newly-identified risks. After approval, most changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to prior FDA review and approval. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and
86
Table of Contents
certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. FDA strictly regulates labeling, advertising, promotion and other types of information on products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved labeling. FDA may impose significant civil and monetary penalties for the dissemination of false or misleading direct-to-consumer advertisements. Manufacturers of approved drug products also are subject to annual establishment and product user fees.
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing and marketing of products regulated by the FDA. In addition to new legislation, FDA regulations and guidance are often revised or interpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. For example, in the European Union, a clinical trial application, or CTA, must be submitted to each member state’s national health authority and an independent ethics committee. The CTA must be approved by both the national health authority and the independent ethics committee prior to the commencement of a clinical trial in the member state. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. In addition, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. In all cases, clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain marketing approval of a drug under European Union regulatory systems, we may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The centralized procedure is compulsory for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, and products with a new active substance indicated for the treatment of certain diseases, and optional for those products that are highly innovative or for which a centralized process is in the interest of patients. If we receive orphan designation for our products in the European Union, they will qualify for this centralized procedure, under which each product’s marketing authorization application will be submitted to the EMA. Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Scientific Advice Working Party of the Committee of Medicinal Products for Human Use, or the CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, defined by three cumulative criteria: the seriousness of the disease, such as heavy disabling or life-threatening diseases, to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, the EMA ensures that the opinion of the CHMP is given within 150 days.
87
Table of Contents
The decentralized procedure provides for approval by one or more other, or concerned, member states of an assessment of an application performed by one member state, known as the reference member state. Under this procedure, an applicant submits an application, or dossier, and related materials, including a draft summary of product characteristics, and draft labeling and package leaflet, to the reference member state and concerned member states. The reference member state prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. Within 90 days of receiving the reference member state’s assessment report, each concerned member state must decide whether to approve the assessment report and related materials. If a member state cannot approve the assessment report and related materials on the grounds of potential serious risk to public health, the disputed points may eventually be referred to the European Commission, whose decision is binding on all member states.
For the EMA, a Pediatric Investigation Plan, or a request for waiver or deferral, is required for submission prior to submitting a marketing authorization application for use for drugs in pediatric populations.
In the European Union, the EMA has granted lomitapide orphan drug designation for the treatment of FC. In October 2010, we withdrew our application to the EMA for orphan drug designation for lomitapide for the treatment of HoFH, based on guidance we received from the EMA that lomitapide is not eligible for orphan drug designation for this indication since lomitapide has the potential to treat hypercholesterolemia in broader patient populations. The EMA grants orphan drug designation to promote the development of products that may offer therapeutic benefits for life-threatening or chronically debilitating conditions affecting not more than five in 10,000 people in the European Union. In addition, orphan drug designation can be granted if the drug is intended for a life threatening, seriously debilitating or serious and chronic condition and without incentives it is unlikely that sales of the drug in the European Union would be sufficient to justify developing the drug. Orphan drug designation is only available if there is no other satisfactory method approved in the European Union of diagnosing, preventing or treating the condition, or if such a method exists, the proposed orphan drug will be of significant benefit to patients. Orphan drug designation provides opportunities for free protocol assistance and fee reductions for access to the centralized regulatory procedures before and during the first year after marketing authorization. The fee reductions are not limited to the first year after authorization for small and medium enterprises. Orphan drug designation also provides ten years of market exclusivity following drug approval. The exclusivity period may be reduced to six years if the designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
ATU
We plan to apply to make lomitapide available for use under a cohort Autorisation Temporaire d’Utilisation, or Temporary Authorization for Use, or ATU, in France. Under an ATU, the French Health Products Safety Agency, or Afssaps, allows the use of a drug in France before marketing approval has been obtained in France in order to treat serious or rare diseases for which no other treatment is available in that country. Afssaps will only grant an ATU where the benefit of the product outweighs the risk. An ATU is granted for one year and may be renewed. If an ATU is granted for lomitapide, we will gather and analyze data concerning lomitapide’s use and submit a periodic report to Afssaps. We also will be responsible for submitting pharmacovigilance reports, as necessary. An ATU may be modified, suspended, or withdrawn for reasons of public health or if the conditions under which the ATU was granted are no longer met. We believe the granting of an ATU and subsequent use by patients in France prior to marketing approval would enable us to begin recognizing some product sales revenue for lomitapide prior to its approval in the United States and the remainder of the European Union.
Reimbursement
Sales of pharmaceutical products depend in significant part on the availability of third-party reimbursement. Third-party payors include government health administrative authorities, including at the federal and state level, managed care providers, private health insurers and other organizations. We anticipate third-party payors will provide reimbursement for our products. However, these third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct expensive
88
Table of Contents
pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Our product candidates may not be considered cost-effective. It is time consuming and expensive for us to seek reimbursement from third-party payors. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, the U.S. Congress and state legislatures from time to time propose and adopt initiatives aimed at cost containment, which could impact our ability to sell our products profitably. For example, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act and the associated reconciliation bill, which we refer to collectively as the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states once the provision is effective. Further, beginning in 2011, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may require us to modify our business practices with healthcare practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, imposed new requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries, and included a major expansion of the prescription drug benefit under a new Medicare Part D. Medicare Part D went into effect on January 1, 2006. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which will provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee.
It is not clear what effect the MMA will have on the prices paid for currently approved drugs and the pricing options for new drugs approved after January 1, 2006. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
The cost of pharmaceuticals continues to generate substantial governmental and third-party payor interest. We expect that the pharmaceutical industry will experience pricing pressures due to the trend toward managed healthcare, the increasing influence of managed care organizations, and additional legislative proposals. Indeed, we expect that there will continue to be a number of federal and state proposals to implement governmental pricing controls and limit the growth of healthcare costs, including the cost of prescription drugs. At the present time, Medicare is prohibited from negotiating directly with pharmaceutical companies for drugs. However, Congress is currently considering passing legislation that would lift the ban on federal negotiations. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
89
Table of Contents
Some third-party payors also require pre-approval of coverage for new or innovative drug therapies before they will reimburse healthcare providers that use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, the announcement or adoption of these proposals could have a material adverse effect on our ability to obtain adequate prices for our product candidates and operate profitably.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. In some countries, pricing negotiations with governmental authorities can take six to 12 months or longer after the receipt of marketing approval. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products.
Employees
As of May 31, 2011, we had 20 employees. All of our employees are engaged in administration, finance, clinical, regulatory, quality and business development functions. We believe our relations with our employees are good.
Property and Facilities
Our principal executive office, which is located in Cambridge, Massachusetts, consists of 8,741 square feet. In January 2011, we entered into a five year lease relating to the Massachusetts property. In March 2011, we moved into 6,802 square feet of newly leased office space in Bedminister, New Jersey. From October 15, 2010 to March 31, 2011, we were leasing interim space in Morristown, New Jersey. We believe these new office spaces are adequate for our current needs and that additional or replacement space will be available on commercially reasonable terms as needed.
Legal Proceedings
We are currently not party to any material legal proceedings, although from time to time we may become involved in disputes in connection with the operation of our business.
90
Table of Contents
Executive Officers, Key Employee and Directors
The following table sets forth information regarding our directors, executive officers and key employee, including their ages, as of the date of this prospectus.
| Name | Age | Position | ||||
| Executive Officers |
||||||
| Marc D. Beer |
46 | Chief Executive Officer and Director | ||||
| Mark J. Fitzpatrick |
48 | Chief Financial Officer | ||||
| Christine A. Pellizzari |
43 | Executive Vice President, General Counsel and Secretary | ||||
| Martha J. Carter |
58 | Chief Regulatory Officer | ||||
| John T. Cavan |
52 | Vice President and Chief Accounting Officer | ||||
| Key Employee |
||||||
| Diane L. Tribble, Ph.D., M.M.Sc |
53 | Chief Scientific Officer | ||||
| Directors |
||||||
| David I. Scheer(1)(2) |
58 | Chairman of the Board | ||||
| Sol J. Barer, Ph.D.(3) |
64 | Director | ||||
| Antonio M. Gotto Jr., M.D., D.Phil.(1)(2) |
75 | Director | ||||
| Alison Kiley(1)(2) |
39 | Director | ||||
| Michèle Ollier, M.D.(3) |
52 | Director | ||||
| (1) | Member of the nominating and corporate governance committee. |
| (2) | Member of the audit committee. |
| (3) | Member of the compensation committee. |
Marc D. Beer has served as our Chief Executive Officer since August 2010. Prior to joining Aegerion, from April 2000 to November 2007, he served as the President and Chief Executive Officer of ViaCell, Inc., a cellular therapy company. Prior to that, he held marketing and business development roles at Genzyme Corporation, most recently serving as Vice President of Global Marketing. Prior to joining Genzyme, he served as Vice President, Sales and Marketing at Biostar, Inc. and held a variety of sales and marketing roles in the pharmaceutical and diagnostic devices divisions of Abbott Laboratories. Mr. Beer holds a B.S. from Miami University (Ohio). Our Board of Directors believes that Mr. Beer’s qualifications to sit on our Board of Directors include his extensive experience in the life sciences industry.
Mark J. Fitzpatrick has served as our Chief Financial Officer since May 9, 2011. From 2007 to 2011, he was Vice President, Chief Financial Officer and Assistant Secretary at Proteon Therapeutics, Inc. From 2005 to 2007, prior to his role at Proteon, Mr. Fitzpatrick was Vice President and Chief Financial Officer, Treasurer and Assistant Secretary with RenaMed Biologics, Inc. He also held Chief Financial Officer positions with Dynogen Pharmaceuticals, WorldStreet Corporation and Diacrin. He previously was a senior auditor with Arthur Andersen & Co. Mr. Fitzpatrick graduated cum laude with a B.S. in Accounting from Boston College School of Management and obtained his CPA license in the Commonwealth of Massachusetts in 1987.
Christine A. Pellizzari has served as our Executive Vice President, General Counsel and Secretary since February 2010. Prior to that, she served as our Vice President, General Counsel and Secretary from August 2007 to February 2009 and as our Senior Vice President, General Counsel and Secretary from February 2009 to February 2010. Prior to joining Aegerion, Ms. Pellizzari was employed by Dendrite International, Inc., a provider of sales effectiveness, promotional and compliance solutions to the global pharmaceutical industry, as Associate Counsel and Assistant Secretary from 1998 to 2000, Vice President, General Counsel and Secretary from 2000 to 2004 and Senior Vice President, General Counsel and Secretary from 2004 until the sale of the company to Cegedim S.A. in May 2007, during which time she oversaw the company’s global legal operations and was a member of the company’s Executive Operations Management Committee. Ms. Pellizzari holds a B.A. from the University of Massachusetts at Amherst and a J.D. from the University of Colorado School of Law at Boulder.
91
Table of Contents
Martha J. Carter has served as our Chief Regulatory Officer and Senior Vice President since February 2011. From 2006 to February, 2011, Ms. Carter served as Senior Vice President and Chief Regulatory Officer at Proteon Therapeutics, where she was responsible for worldwide regulatory and quality functions. Prior to this role, she was Senior Vice President, Regulatory Affairs for Trine Pharmaceuticals. Prior to that, Ms. Carter was Vice President, Regulatory Affairs for Gel Tex Pharmaceuticals, Inc. where she led the regulatory and quality control functions for both commercial and investigational drug products.
John T. Cavan has served as our Chief Accounting Officer and Vice President since February 2009. Prior to that, he served as our Corporate Controller from May 2006 to February 2009. Prior to joining Aegerion, Mr. Cavan served as Controller of AlgoRx Pharmaceuticals, an emerging biotechnology company. Mr. Cavan served in a variety of financial and operational positions through his prior work with large multinational public companies, including Sony, American Express, International Specialty Products and Nestle. Mr. Cavan holds a B.B.A in Accountancy from Iona College and an M.B.A. in Finance from Seton Hall University.
Diane L. Tribble, Ph.D., M.M.Sc. has served as our Chief Scientific Officer since February 2011. From 2006 to February, 2011, Dr. Tribble was Vice President of Clinical Development at Isis Phamraceuticals, where she led the development of the cholesterol-reducing antisense therapeutic, mipomersen, which is currently in late-stage clinical development. Prior to that, she served as Director of Clinical Research at the Merck Research Laboratories, of Merck & Co. where she worked on the development of several cholesterol-reducing drug candidates. At Merck, Dr. Tribble was also a member of the Cholesterol Task Force of the Merck/Schering-Plough joint venture.
David I. Scheer a co-founder of Aegerion, has served as our Chairman of the Board since February 2005. Since 1981, Mr. Scheer has served as President at Scheer & Company, Inc., a company that provides corporate strategic advisory services in the life sciences industry. Mr. Scheer serves as the Chairman of the Board of Tengion, Inc. and Achillion Pharmaceuticals, Inc. Mr. Scheer also serves on the boards of directors of several private companies. Mr. Scheer is also a member of the Advisory Committee to the Harvard Malaria Initiative and the Leadership Council for the Harvard School of Public Health. Mr. Scheer holds an A.B. cum laude from Harvard College and an M.S. from Yale University. Our Board of Directors believes that Mr. Scheer’s qualifications to sit on our Board of Directors include his years of experience working with life science companies.
Sol J. Barer, Ph.D. became a member of our Board effective May 1, 2011. Dr. Barer has served as the Chairman of Celgene Corporation and the Managing Partner of SJBarer Consulting. He previously served as Chairman and Chief Executive Officer of Celgene from May 2006 until June 2010, when he was appointed Executive Chairman and transitioned to his current position in January 2011. Prior to that, he held several positions within Celgene, including President and Chief Operating Officer. Dr. Barer joined the Celanese Research Company in 1974 and formed the biotechnology group that was subsequently spun out to form Celgene. Dr. Barer also currently serves on the Board of Directors of Amicus Therapeutics, Inc. Dr. Barer received a Ph.D. in Organic Chemistry from Rutgers University and a B.S. from Brooklyn College. Dr. Barer’s significant scientific and executive leadership experience in the pharmaceutical industry contributed to the Board of Directors conclusion that Dr. Barer should serve on our Board.
Antonio M. Gotto, Jr., M.D., D.Phil. has served as a member of our Board of Directors since January 2006. Since January 1997, Dr. Gotto has served as the Stephen and Suzanne Weiss Dean of the Joan and Sanford I. Weill Medical College of Cornell University and Provost for Medical Affairs of Cornell University. Previously, Dr. Gotto served as J.S. Abercrombie Chair of Atherosclerosis and Lipoprotein Research and Chairman and Professor of the Department of Medicine at Baylor College of Medicine and Methodist Hospital. Dr. Gotto currently serves as a member of the Institute of Medicine of the National Academy of Sciences and a Fellow of the American Academy of Arts and Sciences. Dr. Gotto is also a past president of the International Atherosclerosis Society and a past president of the American Heart Association. Dr. Gotto holds a B.A. from Vanderbilt University, a D.Phil. from Oxford University in England, where he was a Rhodes Scholar, and an M.D. from Vanderbilt University School of Medicine. He completed his residency training at Massachusetts General Hospital in Boston, Massachusetts. Our Board of Directors believes that Dr. Gotto’s qualifications to sit on our Board of Directors include his extensive experience and expertise in the lipid area.
92
Table of Contents
Alison Kiley has served as a member of our board of directors since December 2005. Ms. Kiley is a director of Alta Partners, a venture capital firm whose investments are focused on life science companies. She joined Alta Partners in 2001 and her primary investment focus at Alta Partners is on medical technology and biopharmaceuticals. Prior to joining Alta Partners, Ms. Kiley served as a Senior Associate at Robertson Stephens & Company in the Life Sciences Investment Banking Group, where she was responsible for various corporate finance transactions. She serves on the boards of directors of several private companies and she served on the board of directors of Insulet Corporation from 2004 to 2008. Ms. Kiley holds a B.A. from Colgate University and an M.B.A. from Columbia University. Our Board of Directors believes that Ms. Kiley’s qualifications to sit on our Board of Directors include her experience in evaluating and advising life science companies.
Michèle Ollier, M.D. has served as a member of our board of directors since October 2007. Dr. Ollier is a Life Science partner at Index Ventures, a venture capital firm whose investments are focused in information technology and life science companies, which she joined in February 2006. From January 2003 to January 2006, Dr. Ollier was Director of Investment in Life Sciences at Edmond de Rothschild Investment Partners in Paris. Prior to that, Dr. Ollier held various positions relating to strategy, development and commercialization of pharmaceutical products at several biotechnology and pharmaceutical companies, including International CNS Product Manager at Sanofi, Lipid Lowering Agents Group Director at Bristol Myers Squibb France, International Oncology Director at Rhone Poulenc Rorer/RPR Gencell and International Vice President Reproductive Health at Serono. Dr. Ollier holds a Medical Degree from Paris-Ouest University (France). Our Board of Directors believes that Dr. Ollier’s qualifications to sit on our Board of Directors include her experience in evaluating and advising life science companies.
Board of Directors
We currently have six directors and the authorized size of our board of directors is six. The Board of Directors is divided into three classes with members of each class serving for staggered three-year terms, as follows:
| § | Ms. Kiley and Dr. Ollier serve as Class II directors, and their initial terms will expire at our 2012 annual meeting of stockholders; |
| § | Messrs. Beer and Scheer serve as Class III directors, and their initial terms will expire at our 2013 annual meeting of stockholders; and |
| § | Dr. Gotto and Dr. Barer serve as Class I directors, and their initial terms will expire at our 2014 annual meeting of stockholders. |
Our by-laws provide that any vacancies in our Board of Directors and newly created directorships may be filled only by our Board of Directors and that the authorized number of directors may be changed only by our Board of Directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes, so that, as nearly as possible, each class will consist of one-third of the total number of directors. These provisions of our by-laws and the classification of the Board of Directors may have the effect of delaying or preventing changes in the control or management of Aegerion.
There are no family relationships among any of our directors or officers.
Under applicable NASDAQ Marketplace Rules, a director will only qualify as an “independent director,” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our board of directors has considered the relationships of all directors and, where applicable, the transactions involving them described below under “Transactions with Related Persons,” and determined that each of Drs. Gotto, Barer and Ollier, Mr. Scheer and Ms. Kiley does not have any relationship which would interfere with the exercise of independent judgment in carrying out his or her responsibility as a director and that each director qualifies as an independent director under the applicable rules of The NASDAQ Global Market.
93
Table of Contents
Board Leadership Structure and Board’s Role in Risk Oversight
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. Our Board of Directors is actively involved in oversight of risks that could affect us. This oversight is conducted primarily through committees of the Board of Directors, as disclosed in the descriptions of each of the committees below, but the full Board of Directors has retained responsibility for general oversight of risks. Our Board of Directors satisfies this responsibility through full reports by each committee chair regarding the committee’s considerations and actions, as well as through regular reports directly from officers responsible for oversight of particular risks within our company. Our Board of Directors believes that full and open communication between management and the Board of Directors is essential for effective risk management and oversight.
We have separated the positions of chairman of the board and chief executive as we believe that this allows our chief executive officer to focus on our day-to-day business, while allowing the chairman of the board to lead the Board of Directors in its fundamental role of providing advice to and independent oversight of management. We believe that this structure ensures an enhanced role for the independent directors in the oversight of our company and active participation of the independent directors in setting agendas and establishing priorities and procedures for the work of our Board of Directors.
Board Committees
Our Board of Directors has established an Audit Committee, a Compensation Committee and a Nominating Committee. Each of these committees operates pursuant to a separate written charter adopted by our Board of Directors. The charters of the Audit Committee, Compensation Committee and Nominating Committee are available on our website at www.aegerion.com. Information contained on, or accessible through, our website is not a part of this prospectus. We have included our website address as an inactive textual reference only.
The composition and functioning of all of our committees comply with all applicable requirements of the Sarbanes-Oxley Act of 2002, the NASDAQ Global Market and SEC rules and regulations, except that with respect to the independent audit committee requirements our Audit Committee is relying upon the phase in rules of the NASDAQ Global Market and the SEC, as further described below.
Audit Committee
Dr. Gotto, Mr. Scheer and Ms. Kiley serve on the Audit Committee. Our Board of Directors has determined that Mr. Scheer qualifies as an “audit committee financial expert” for purposes of the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Under the applicable rules of the NASDAQ Global Market, as a result of our recent listing in connection with our initial public offering on October 27, 2010, we are permitted to phase in our compliance with the independent audit committee requirements set forth in Marketplace Rule 5615(b)(1) on the same schedule as we are permitted to phase in our compliance with the independent audit committee requirement pursuant to Rule 10A-3(b)(1)(iv)(A) under the Exchange Act, that is, (1) one independent member at the time of listing; (2) a majority of independent members within 90 days of listing; and (3) all independent members within one year of listing.
Our Board of Directors has determined that each of Dr. Gotto and Mr. Scheer is an independent director under the rules of the NASDAQ Global Market and Rule 10A-3 of the Exchange Act. Within one year of our listing on the NASDAQ Global Market, we expect that Ms. Kiley will resign from our Audit Committee and be replaced with a new director, who is independent under Rule 10A-3.
The Audit Committee’s responsibilities include, among other things:
| § | appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm; |
| § | pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
94
Table of Contents
| § | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| § | coordinating the oversight and reviewing the adequacy of our internal control over financial reporting; |
| § | establishing policies and procedures for the receipt and retention of accounting related complaints and concerns; and |
| § | preparing the audit committee report required by SEC rules to be included in our annual proxy statement. |
Compensation Committee
Drs. Barer and Ollier currently serve on the Compensation Committee and Dr. Barer serves as the chairman of the Compensation Committee. Both Drs. Barer and Ollier are independent under the applicable rules of the NASDAQ Global Market. The Compensation Committee’s responsibilities include, among other things:
| § | annually reviewing and approving corporate goals and objectives relevant to compensation of our chief executive officer; |
| § | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and determining the compensation of our chief executive officer; |
| § | reviewing and approving the compensation of all our other officers; |
| § | overseeing and administering our employment agreements, severance arrangements, compensation, welfare, benefit and pension plans and similar plans; and |
| § | reviewing and making recommendations to the Board of Directors with respect to director compensation. |
Nominating and Corporate Governance Committee
Mr. Scheer, Ms. Kiley and Dr. Gotto serve on our Nominating Committee. Dr. Gotto serves as the chairman of the Nominating Committee. Each of Mr. Scheer, Ms. Kiley and Dr. Gotto is independent under the applicable rules of the NASDAQ Global Market. The Nominating Committee’s responsibilities include, among other things:
| § | developing and recommending to the Board of Directors criteria for selecting Board and committee membership; |
| § | establishing procedures for identifying and evaluating director candidates including nominees recommended by stockholders; |
| § | identifying individuals qualified to become Board members; |
| § | recommending to the Board the persons to be nominated for election as directors and to each of the board’s committees; |
| § | overseeing our code of business conduct and ethics and a set of corporate governance guidelines; and |
| § | overseeing the evaluation of the Board, its committees and management. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee, or other committee serving an equivalent function, of any other entity that has one or more of its executive officers serving as a member of our Board of Directors or who will serve on our compensation committee. None of the members of our compensation committee have ever been one of our employees.
95
Table of Contents
Compensation Discussion and Analysis
In this Compensation Discussion and Analysis, we address the compensation programs we have implemented for our current executive officers. Our named executive officers for the year as of December 31, 2010:
| § | Marc D. Beer, Chief Executive Officer |
| § | William H. Lewis, President; |
| § | Christine A. Pellizzari, Executive Vice President, General Counsel and Secretary; |
| § | John T. Cavan, Vice President and Chief Accounting Officer; and |
| § | William J. Sasiela, Ph.D., former Chief Medical Officer and Executive Vice President, Clinical. |
Mr. Beer joined the Company as Chief Executive Officer on August 19, 2010. Dr. Sasiela resigned from his position as Chief Medical Officer and Executive Vice President, Clinical effective September 30, 2010. Mr. Lewis resigned from his position as President effective June 8, 2011.
Background and Overview of Our Executive Compensation Objectives, Policies and Procedures
Our primary objective with respect to executive compensation is to attract and retain individuals who possess knowledge, experience and skills that we believe are important to our business of developing and commercializing novel therapeutics to treat severe lipid disorders.
Specifically our compensation programs are designed to:
| § | attract and retain individuals with superior ability and managerial experience; |
| § | align executive officers’ incentives with our corporate strategies, business objectives and the long-term interests of our stockholders; and |
| § | increase the incentive to achieve key strategic performance measures by linking incentive award opportunities to the achievement of performance objectives and by providing a portion of total compensation for executive officers in the form of ownership in our company. |
To achieve these objectives, we seek to provide a competitive compensation package that ties a substantial portion of the executive’s overall compensation to both our company objectives and the executive’s individual performance. Base salary increases and annual performance bonuses are tied to our company and individual performance in relation to competitive market conditions. Equity awards are primarily used to promote long-term stockholder value and employee retention through the use of multi-year vesting schedules that provide an incentive to the executive to remain in our employ through the end of the vesting period in order to share in any increase in our company value over time.
Our Compensation Committee oversees our compensation and benefit plans and policies, administers our equity incentive plans and reviews and approves annually all compensation decisions relating to all executive officers. The Compensation Committee is not currently using any third-party executive compensation specialists to help it in making compensation-related decisions other than certain industry survey data and does not receive compensation-related recommendations from any executive officers. In reviewing compensation levels of our executive officers for 2010, our Compensation Committee considered our financial status, the contributions that the management team had made to our business, their collective knowledge of compensation trends in the industry in which we compete, and their experiences and business judgment.
As we gain experience as a public company, we expect that the specific direction, emphasis and components of our executive compensation program will evolve. Our Compensation Committee will continue to be primarily responsible for developing and implementing our compensation policies and establishing and approving the compensation for all of our executive officers. In the future, our Compensation Committee may review the
96
Table of Contents
compensation packages offered by other similar companies based on aggregate survey data and may choose to retain the services of third-party executive compensation specialists from time to time, as it sees fit, in connection with the establishment of cash and equity compensation and related policies. While market data and reports from third-party consultants provide useful starting points for compensation decisions, our Compensation Committee may also take into account factors such as level of responsibility, prior experience and individual performance in arriving at final compensation decisions.
Executive Compensation Components
Our executive compensation consists of base salary, cash incentives, equity incentive compensation, broad-based benefits programs and change in control and severance benefits. Each of the elements of our executive compensation is discussed in detail below, including a description of the particular element and how it fits into our overall executive compensation and a discussion of the amounts of compensation paid to our named executive officers in 2010 under each of these elements.
Although we have not adopted any formal guidelines for allocating total compensation between long-term and short-term cash compensation and non-cash compensation, or among different forms of non-cash compensation, we intend to implement and maintain compensation plans that tie a substantial portion of our executives’ overall compensation to the achievement of corporate goals and value-creating milestones, such as advancement of the clinical development of our product candidates and achievement of commercial targets.
Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, places a limit of $1.0 million on the amount of compensation that public companies may deduct in any one year with respect to certain of its named executive officers. Certain performance-based compensation approved by stockholders is not subject to this deduction limit. Our Compensation Committee’s strategy in this regard is to be cost and tax efficient. Therefore, the Compensation Committee intends to preserve corporate tax deductions, while maintaining the flexibility in the future to approve arrangements that it deems to be in our best interests and the best interests of our stockholders, even if such arrangements do not always qualify for full tax deductibility.
Annual Cash Compensation
Base Salary
Base salaries for our named executive officers are intended to be competitive with those received by other individuals in similar positions at the companies with which we compete for talent. Base salaries are originally established at the time the executive is hired based on our understanding of what executives at other similar companies were being paid at such time based on data from Radford collected from the Radford Global Life Sciences Pre-IPO Survey and Dow Jones Venture One Compensation Pro Survey, or the Radford data. The base salaries of our named executive officers are reviewed annually and adjusted to reflect individual roles and contribution to our clinical, regulatory, commercial and operational performance. We may also increase the base salary of an executive officer at other times due to market conditions or if a change in the scope of the officer’s responsibilities justifies such consideration. We believe that a competitive base salary is a necessary element of any compensation program that is designed to attract and retain talented and experienced executives. We also believe that attractive base salaries can motivate and reward executives for their overall performance. Base salaries are established in part based on the individual experience, skills and expected contributions of our executives and our executives’ performance during the prior year.
In early 2010, in connection with setting the 2010 base salaries for the named executive officers, the Compensation Committee considered the prevailing market conditions and our financial position, including our need to raise additional funds, and decided to increase the base salary of each of the named executive officers, who would be serving as such in 2010 by two percent over their 2009 base salaries. In connection with our initial public offering, we increased the base salaries of Messrs. Lewis and Cavan and Ms. Pellizzari in light of their additional responsibilities as officers of a public company.
97
Table of Contents
The base salaries of our named executive officers during the years ended December 31, 2009 and 2010 are summarized in the table below.
| Base Salary 2009 | Base Salary 2010(1)(2) | |||||||
| Marc D. Beer(1). |
$ | — | $ | 450,000 | ||||
| William H. Lewis(3) |
290,700 | 300,000 | ||||||
| Christine A. Pellizzari |
249,900 | 260,000 | ||||||
| John T. Cavan |
162,024 | 175,000 | ||||||
| William J. Sasiela, Ph.D(4). |
290,700 | 296,514 | ||||||
| (1) | In connection with our initial public offering, the 2010 base salaries of Messrs. Lewis and Cavan and Ms. Pellizzari were increased to the amounts reflected above. |
| (2) | Mr. Beer’s employment commenced on August 19, 2010. Pursuant to his employment agreement, Mr. Beer’s annual base salary is $450,000 per year of which he earned a pro rated amount for 2010. |
| (3) | Mr. Lewis resigned from his position as President effective June 8, 2011. |
| (4) | Dr. Sasiela resigned from his position as Chief Medical Officer and Executive Vice President, Clinical effective September 30, 2010. |
Cash Incentive Bonuses
Our Compensation Committee has the authority to award annual performance-based cash bonuses to our executive officers and certain non-executive employees. Each of the named executive officers has a target cash bonus amount that is based on a percentage of his or her base salary. The target bonuses for Messrs. Beer, Lewis and Cavan and Ms. Pellizzari are set forth in such executive officer’s employment agreement. In accordance with such agreements, each executive officer can earn a cash bonus equal to a percentage of his or her base salary upon the achievement of performance goals to be set by the Board of Directors or the Compensation Committee. In addition, if we achieve performance goals in excess of target levels, or if the professional effectiveness of a specific executive officer helped us achieve specific corporate objectives or otherwise contributed to our overall success, the bonuses paid to our executive officers can exceed the target amounts.
The target cash bonus percentages for 2010, as a percentage of base salary, were as follows: Mr. Beer, 50%, Mr. Lewis, 40%; Ms. Pellizzari, 30%; and Mr. Cavan, 25%. However, the Compensation Committee has the discretion to award cash bonuses that are greater than or less than a named executive officer’s target bonus amount. Dr. Sasiela resigned from his position as Chief Medical Officer and Executive Vice President, Clinical effective September 30, 2010, and as a result was not entitled to a bonus for 2010. Cash bonus amounts are based upon our achievement of corporate objectives that are established at the beginning of the fiscal year by our Board of Directors.
The bonus payments for 2010 were based on the achievement of the following corporate objectives, which were equally weighted:
| § | completion of our initial public offering; |
| § | continuation of our pivotal Phase III trial in patients with HoFH; |
| § | advancement of our European regulatory strategy; |
| § | obtaining approval and grant from federal government for therapeutic discovery project for lomitapide; and |
| § | finalization and submission of an end of Phase II package to the FDA for the severe HeFH patient population. |
In determining cash bonuses for the year ended December 31, 2010, the Compensation Committee did not assess the degree to which each executive officer contributed to each of our corporate objectives. Instead, the compensation committee began with the assumption that so long as we met the majority of the corporate objectives set forth above, each executive officer was entitled to at least his or her target bonus, as achievement of the corporate objectives is dependent upon the work of the overall management team. Once this determination was made, the Compensation Committee assessed whether any executive officer deserved a greater bonus as a result of such executive officer’s professional effectiveness in helping us achieve specific corporate objectives or otherwise contributed to our overall success.
98
Table of Contents
For the year ended December 31, 2010, the Compensation Committee determined that we achieved the following corporate objectives:
| § | completion of our initial public offering in October 2010; |
| § | continued our Phase III clinical trial in patients with HoFH; |
| § | advanced our European regulatory strategy; |
| § | obtained approval and grant from federal government for therapeutic discovery project for lomitapide; and |
| § | finalized and submitted an end of Phase II package to the FDA for the severe HeFH patient population. |
In light of the foregoing, the Compensation Committee concluded that each of our named executive officers, with the exception of Dr. Sasiela, was at least entitled to his or her target bonus. For the year ended December 31, 2010 the executive officers were paid the following cash bonus amounts: Mr. Beer, $99,863, Mr. Lewis, $144,000, Ms. Pellizzari, $93,600, and Mr. Cavan, $43,750. Dr. Sasiela did not receive a cash bonus for 2010 due to his resignation on September 30, 2010. Mr. Beer’s bonus amount exceeded his target bonus amount by $16,644. Mr. Lewis’ bonus amount exceeded his target bonus amount by $24,000. Ms. Pellizzari’s bonus amount exceeded her target bonus amount by $15,600. The Compensation Committee determined that Mr. Beer, Mr. Lewis and Ms. Pellizzari deserved bonus amounts greater than their target bonus amounts because they contributed extensively to the success of our initial public offering and clinical and business development programs. In addition, in November 2010 Mr. Cavan was awarded a one-time cash bonus of $25,000 for his role in successfully securing federal government grants in connection with our qualifying therapeutic discovery project program and for his extensive contributions to the success of our initial public offering.
Pursuant to their employment agreements, Messrs. Lewis and Cavan and Ms. Pellizzari are entitled to a special cash bonus of 10% of their base salaries upon the FDA’s acceptance of an NDA for lomitapide. Since no NDA for lomitapide was filed during the fiscal year ended December 31, 2010, Messrs. Lewis and Cavan and Ms. Pellizzari did not receive such bonus for the fiscal year ended December 31, 2010.
Equity Incentive Compensation
Each of our named executive officers has either received restricted stock grants or stock options to purchase shares of common stock in connection with their initial employment with us. We grant equity incentive compensation to our executive officers because we believe doing so will motivate the executive by aligning the executive’s interests more closely with our own. We chose to sell restricted stock to Mr. Lewis and Dr. Sasiela, our initial hires, instead of granting them options because we believed that it was appropriate for our initial key employees to have an immediate equity stake in the company and because we believed owning restricted stock would more closely align their interests with ours. Now that we are a more mature company, we believe it is generally more appropriate to grant options to employees since it is the practice at other companies with which we compete for talent. However, we may continue to grant restricted stock when we deem it appropriate and in our best interest. The restricted stock and stock options held by each executive are subject to vesting in order to encourage the executive to remain with us for several years, and subject to other provisions of their respective restricted stock and stock option agreements, which are described below.
During 2010, equity incentive grants were made to our executives and other employees at the discretion of our Board of Directors. Our Board of Directors grants of stock options have not been formula-based but have been based on the recommendation of the Compensation Committee, which generally takes into account the Compensation Committee’s subjective determination of a mixture of the following qualitative factors that are not weighted: the executive’s level of responsibility, the competitive market for the executive’s position and the executive’s potential contribution to the advancement of our clinical and business development programs. Typically, larger awards have been made to the executive officers who have areas of responsibility and functions that are more likely to build long-term stockholder value as determined by how directly linked their areas of responsibility are to our growth. Certain equity awards are based on achievement of specific milestone events, some which are in the control of the Company and others which are not.
99
Table of Contents
In 2010, based on the recommendations of the Compensation Committee, our Board of Directors granted the named executive officers options to purchase shares of common stock, as follows: Mr. Beer, 885,653 shares; Mr. Lewis, 278,796 shares; Ms. Pellizzari, 124,037 shares; Mr. Cavan, 34,893 shares; and Dr. Sasiela, 24,573 shares. The options were granted to Mr. Beer and Dr. Sasiela, in one allotment on different dates at exercise prices of $1.54 and $2.37 per share, respectively, and to the other executives in two allotments, at exercise prices of $2.37 and $1.54 per share. The option award granted to Mr. Beer was a new hire award and was granted to Mr. Beer in connection with his becoming our Chief Executive Officer. With respect to the equity awards granted to our other named executive officers, the Compensation Committee determined that Mr. Lewis should receive the largest award since he was the most senior member of the management team and was deemed by the Compensation Committee to be most critical to the success of our initial public offering and clinical programs, specifically our ongoing Phase III clinical trial in patients with HoFH, and our business development programs, specifically our discussions with potential strategic collaborators. The Compensation Committee concluded that Ms. Pellizzari should receive the next largest award since she was the next most senior member of the management team, and it was expected that she would have significant responsibilities related to the advancement of our initial public offering and our clinical and business development programs. The Compensation Committee concluded that Mr. Cavan should receive the next largest award, relative to the other members of the management team, since he was the next most senior member of the management team and was expected to contribute to the success of our initial public offering and our clinical and business development programs. Dr. Sasiela resigned in September 2010 and therefore did not receive an option grant in September 2010. As a result, Dr. Sasiela received the smallest aggregate award for 2010. Forty percent (40%) of the shares of common stock underlying the options granted to Ms. Pellizzari in September 2010, or 39,783 shares, are currently unearned as half of this portion shall vest over a period of four years on a monthly basis following the filing of a New Drug Application for lomitapide and the other half of this portion shall vest upon approval of lomitapide by the FDA. In addition, the Compensation Committee subjectively determined, with reference to the Radford data that these awards were appropriately reflective of the competitive market for executives with similar responsibilities at similarly situated companies. We expect that our Compensation Committee will in future periods continue to grant equity awards, at least in part, with reference to market surveys and with the assistance of third-party consultants.
Stock option awards provide our executive officers with the right to purchase shares of our common stock at a fixed exercise price, subject to continued employment with our company. Stock options are earned on the basis of continued service to us and generally vest over four years, beginning with one-fourth vesting one year after the date of grant, then pro-rata vesting quarterly thereafter. See also “— Potential Payments Upon Termination or Change-in-Control” for a discussion of the change-in-control provisions related to stock options.
We have an equity award grant policy that formalizes how we will grant equity-based awards to our officers and employees after this offering. Under our equity award grant policy all grants will need to be approved by our Board of Directors or Compensation Committee. All stock options will be awarded at fair value and calculated based on our closing market price on the grant date. In addition, equity awards will typically be made on a regularly scheduled basis, as follows:
| § | grants made in conjunction with the hiring of a new employee or the promotion of an existing employee will be made on the first trading day of the month following the later of (1) the hire date or the promotion date or (2) the date on which such grant is approved; and |
| § | grants made to existing employees other than in connection with a promotion will be made, if at all, on an annual basis. |
Other Compensation
We currently maintain broad-based benefits and perquisites that are provided to all employees, including health insurance, life and disability insurance, dental insurance and a 401(k) plan.
As discussed below in “Employment Agreements and Severance Agreements with Executive Officers” and in “Potential Payments Upon Termination in Connection with Change-in-Control”, we have agreements with some
100
Table of Contents
of our named executive officers providing certain benefits to them upon termination of their employment and in relation to a change in control of Aegerion, including the acceleration of vesting of restricted stock and options. Our goal in providing severance and change in control benefits is to offer sufficient cash continuity protection such that an officer’s full time and attention will focus on the requirements of the business rather than the potential implications for his or her position. We prefer to have certainty regarding the potential severance amounts payable to the named executive officers under certain circumstances, rather than negotiating severance at the time that a named executive officer’s employment terminates. We have also determined that accelerated vesting provisions in connection with a termination following a change of control are appropriate because it will encourage our restricted stock and option holders, including our named executive officers, to stay focused in such circumstances, rather than the potential implications for them.
Summary Compensation
The following table sets forth information with respect to compensation for the year ended December 31, 2009 and 2010 earned by or awarded to our named executive officers.
Summary Compensation Table – 2010
| Name and Principal Position | Year | Salary ($) |
Bonus ($)(1)(2) |
Option Awards ($)(3) |
Non-Equity Incentive Plan Compensation ($)(1)(4) |
All Other Compensation ($)(5) |
Total Compensation ($) |
|||||||||||||||||||||
| Marc D. Beer(7) |
2010 | $ | 167,885 | $ | 16,644 | (2) | $ | 11,646,337 | $ | 83,219 | $ | 10,087 | (6) | $ | 11,924,172 | |||||||||||||
| Chief Executive Officer |
2009 | — | — | — | — | — | — | |||||||||||||||||||||
| William H. Lewis(8) |
2010 | 297,422 | 24,000 | (2) | 3,396,373 | 120,000 | — | 3,837,795 | ||||||||||||||||||||
| Former President |
2009 | 290,525 | — | 36,715 | 72,675 | — | 399,915 | |||||||||||||||||||||
| Christine A. Pellizzari |
2010 | 256,212 | 15,600 | (2) | 1,361,796 | 78,000 | — | 1,711,608 | ||||||||||||||||||||
| Executive Vice President, General Counsel and Secretary |
2009 | 249,750 | — | 38,207 | 62,475 | — | 350,432 | |||||||||||||||||||||
| John T. Cavan |
2010 | 167,748 | 25,000 | (9) | 301,033 | 43,750 | — | 537,531 | ||||||||||||||||||||
| Vice President and Chief Accounting Officer |
2009 | 161,616 | 10,000 | (10) | 24,084 | 32,404 | — | 228,104 | ||||||||||||||||||||
| William J. Sasiela, Ph.D.(11) |
2010 | 216,012 | — | 54,137 | — | — | 270,149 | |||||||||||||||||||||
| Former Chief Medical Officer and Executive Vice President, Clinical |
2009 | 290,525 | — | 37,886 | 72,675 | — | 401,086 | |||||||||||||||||||||
| (1) | Bonus and non-equity incentive compensation amounts are for performance during the fiscal year ended December 31, 2010, as applicable, whether or not paid in the year the compensation was earned. |
| (2) | Represents cash incentive payments in excess of such named executive officer’s target bonus, paid at the discretion of our Board of Directors in February 2011 for performance in 2010. |
| (3) | Represents the grant date fair value of option awards granted in 2010 in accordance with ASC Topic 718, or ASC 718, formerly Statement of Financial Accounting Standards No. 123R. Our named executive officers will only realize compensation to the extent the fair value of our common stock is greater than the exercise price of such stock options. For information regarding assumptions underlying the valuation of equity awards, see Note 14 of the Financial Statements. |
| (4) | Represents cash incentive payments earned based upon the achievement of corporate objectives established by our Board of Directors and paid in 2011 for performance in 2010. |
| (5) | Excludes medical, dental and certain other benefits received by the named executive officers that are available generally to all of our employees and certain perquisites and other personal benefits received by the named executive officers which do not exceed $10,000 in the aggregate. |
| (6) | Represents the following amounts for Mr. Beer: (i) $5,414 in reimbursable legal expenses incurred by Mr. Beer in connection with the negotiation of his employment arrangement with us and (ii) $4,673 in company contributions to a defined contribution plan. |
| (7) | Mr. Beer joined the Company as Chief Executive Officer on August 19, 2010. |
| (8) | Mr. Lewis resigned from his position as President effective as of June 8, 2011. |
101
Table of Contents
| (9) | Represents a one-time bonus paid to Mr. Cavan in connection with his role in successfully securing federal government grants relating to our qualifying therapeutic discovery project program and for his extensive contributions to the success of our initial public offering. |
| (10) | Represents a one-time bonus paid to Mr. Cavan in connection with obtaining approval from the State of New Jersey for the sale of net operating losses. |
| (11) | Dr. Sasiela resigned from his position as Chief Medical Officer and Executive Vice President, Clinical, effective as of September 30, 2010 and was not eligible to receive an annual cash bonus for 2010. |
The following table sets forth certain information with respect to awards under our non-equity incentive plan made by us to our named executive officers and stock options awarded to our named executive officers for the year ended December 31, 2010.
Grants of Plan-Based Awards – 2010
| Name |
Grant Date | Estimated Future Payouts Under Non-Equity Incentive Plan Awards |
Estimated Future Payouts Under Equity Incentive Plan Awards |
All Other Option Awards: Number of Securities Underlying Options (#) |
Exercise or Base Price of Option Awards ($/Sh) |
Grant Date Fair Value of Option Awards ($)(5) |
||||||||||||||||||||||
| Target ($)(1)(2)(3) | Target (#)(4) | |||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| Marc D. Beer |
$ | 83,219 | ||||||||||||||||||||||||||
| 9/15/2010 | 885,653 | $ | 1.54 | 11,646,337 | ||||||||||||||||||||||||
| William H. Lewis(6) |
$ | 120,000 | ||||||||||||||||||||||||||
| 2/16/2010 | 24,573 | $ | 2.37 | 54,137 | ||||||||||||||||||||||||
| 9/15/2010 | 101,684 | 152,539 | $ | 1.54 | 3,342,236 | |||||||||||||||||||||||
| Christine A. Pellizzari |
$ | 78,000 | ||||||||||||||||||||||||||
| 2/16/2010 | 24,573 | $ | 2.37 | 54,137 | ||||||||||||||||||||||||
| 9/15/2010 | 39,783 | 59,681 | $ | 1.54 | 1,307,659 | |||||||||||||||||||||||
| John T. Cavan |
$ | 43,750 | ||||||||||||||||||||||||||
| 2/16/2010 | 14,416 | $ | 2.37 | 31,760 | ||||||||||||||||||||||||
| 9/15/2010 | 20,477 | $ | 1.54 | 269,273 | ||||||||||||||||||||||||
| William J. Sasiela, Ph.D. |
||||||||||||||||||||||||||||
| 2/16/2010 | 24,573 | $ | 2.37 | 54,137 | ||||||||||||||||||||||||
| (1) | Represents the amount determined by our Compensation Committee as the target annual cash bonus payable to each named executive officer for 2010 as a percentage of 2010 base salary. |
| (2) | Mr. Beer joined the Company as Chief Executive Officer on August 19, 2010. As a result, his target annual cash bonus amount is based on a pro rated 2010 base salary. |
| (3) | Dr. Sasiela resigned from his position as Chief Medical Officer and Executive Vice President, Clinical effective September 30, 2010 and was not eligible to receive an annual cash bonus for 2010. |
| (4) | Represents portion of option grant that is currently unearned as half of this portion shall vest over a period of four years on a monthly basis following the filing of a New Drug Application for lomitapide and the other half of this portion shall vest upon approval of lomitapide by the FDA. |
| (5) | Represents the grant date fair value calculated in accordance with ASC 718. |
| (6) | Mr. Lewis resigned from his position as President effective as of June 8, 2011. |
102
Table of Contents
The following table sets forth certain information with respect to outstanding options held by our named executive officers at December 31, 2010.
Outstanding Equity Awards at Fiscal Year End – 2010
| Option Awards | ||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Number of Securities |
Option Exercise Price |
Option Expiration Date |
|||||||||||||||
| Marc D. Beer |
55,352 | (1) | 830,301 | — | 1.54 | 9/15/2020 | ||||||||||||||
| William H. Lewis(2) |
13,046 | (3) | 870 | — | 2.37 | 3/13/2017 | ||||||||||||||
| 13,822 | (4) | 10,751 | — | 2.37 | 10/1/2018 | |||||||||||||||
| 7,704 | (5) | 9,906 | — | 2.37 | 3/13/2019 | |||||||||||||||
| — | (6) | 24,573 | — | 2.37 | 2/16/2020 | |||||||||||||||
| 9,533 | (7) | 143,006 | 101,684 | 1.54 | 9/15/2020 | |||||||||||||||
| Christine A. Pellizzari |
62,188 | (8) | 14,352 | — | 2.37 | 10/25/2017 | ||||||||||||||
| 8,063 | (9) | 6,271 | — | 2.37 | 10/1/2018 | |||||||||||||||
| 6,719 | (10) | 8,639 | — | 2.37 | 3/13/2019 | |||||||||||||||
| — | (11) | 24,573 | — | 2.37 | 2/16/2020 | |||||||||||||||
| 3,730 | (12) | 55,951 | 39,783 | 1.54 | 9/15/2020 | |||||||||||||||
| John T. Cavan |
37,574 | (13) | — | — | .90 | 5/16/2016 | ||||||||||||||
| 243 | (14) | 35 | — | 2.37 | 4/24/2017 | |||||||||||||||
| 2,303 | (15) | 1,792 | — | 2.37 | 10/1/2018 | |||||||||||||||
| 5,464 | (16) | 7,027 | — | 2.37 | 3/13/2019 | |||||||||||||||
| — | (17) | 14,416 | — | 2.37 | 2/16/2020 | |||||||||||||||
| 1,279 | (18) | 19,198 | — | 1.54 | 9/15/2020 | |||||||||||||||
| William J. Sasiela, Ph.D. |
— | (98) | — | — | — | — | ||||||||||||||
| (1) | Represents option to purchase up to 885,653 shares of our common stock granted to Mr. Beer on September 15, 2010. The shares underlying this option vest in forty eight equal monthly annual installments beginning immediately after the grant date. |
| (2) | Mr. Lewis resigned from his position as President effective as of June 8, 2011. |
| (3) | Represents option to purchase up to 13,916 shares of our common stock granted to Mr. Lewis on February 26, 2007. The shares underlying this option vest as follows: 25% vested on February 26, 2008, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (4) | Represents option to purchase up to 24,573 shares of our common stock granted to Mr. Lewis on October 1, 2008. The shares underlying this option vest as follows: 25% vested on October 1, 2009, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (5) | Represents option to purchase up to 17,610 shares of our common stock granted to Mr. Lewis on March 13, 2009. The shares underlying this option vest as follows: 25% vested on March 13, 2010, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (6) | Represents option to purchase up to 24,573 shares of our common stock granted to Mr. Lewis on February 16, 2010. The shares underlying this option vest as follows: 25% vested on February 16, 2011, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (7) | Represents option to purchase up to 254,223 shares of our common stock granted to Mr. Lewis on September 15, 2010. The shares underlying this option vest as follows: 60% of the shares vesting in equal monthly installments for the four years following the date of grant, 20% of the shares vesting in equal monthly installments for a four year period following the filing of a New Drug Application for lomitapide; and 20% of the shares vesting immediately upon the approval of lomitapide by the Federal Drug Administration. |
| (8) | Represents option to purchase up to 76,540 shares of our common stock granted to Ms. Pellizzari on October 25, 2007. The shares underlying this option vest as follows: 25% vested on August 1, 2008, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (9) | Represents option to purchase up to 14,334 shares of our common stock granted to Ms. Pellizzari on October 1, 2008. The shares underlying this option vest as follows: 25% vested on October 1, 2009, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (10) | Represents option to purchase up to 15,358 shares of our common stock granted to Ms. Pellizzari on March 13, 2009. The shares underlying this option vest as follows: 25% vested on March 13, 2010, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
103
Table of Contents
| (11) | Represents option to purchase up to 24,573 shares of our common stock granted to Ms. Pellizzari on February 16, 2010. The shares underlying this option vest as follows: 25% vested on February 16, 2011, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (12) | Represents option to purchase up to 99,464 shares of our common stock granted to Ms. Pellizzari on September 15, 2010. The shares underlying this option vest as follows: 60% of the shares vesting in equal monthly installments for the four years following the date of grant, 20% of the shares vesting in equal monthly installments for a four year period following the filing of a New Drug Application for Lomitapide; and 20% of the shares vesting immediately upon the approval of Lomitapide by the Federal Drug Administration. |
| (13) | Represents option to purchase up to 37,574 shares of our common stock granted to Mr. Cavan on May 16, 2006. The shares underlying this option vest as follows: 25% vested on May 16, 2007, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (14) | Represents option to purchase up to 278 shares of our common stock granted to Mr. Cavan on April 24, 2007. The shares underlying this option vest as follows: 25% vested on April 24, 2008, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (15) | Represents option to purchase up to 4,095 shares of our common stock granted to Mr. Cavan on October 1, 2008. The shares underlying this option vest as follows: 25% vested on October 1, 2009, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (16) | Represents option to purchase up to 12,491 shares of our common stock granted to Mr. Cavan on March 13, 2009. The shares underlying this option vest as follows: 25% vested on March 13, 2010, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (17) | Represents option to purchase up to 14,416 shares of our common stock granted to Mr. Cavan on February 16, 2010. The shares underlying this option vest as follows: 25% vested on February 16, 2011, with the remainder of the shares vesting in equal quarterly installments for the following three years. |
| (18) | Represents option to purchase up to 20,477 shares of our common stock granted to Mr. Cavan on September 15, 2010. The shares underlying this option vest in equal monthly installments for the four years following the date of grant. |
| (19) | Dr. Sasiela resigned from the Company on September 30, 2010. |
The following table provides information regarding the exercise of options with respect to our named executive officers for the fiscal year ended December 31, 2010. We have intentionally omitted columns regarding the vesting of restricted stock during the year ended December 31, 2010, as no such restricted stock vested during that time period.
Option Exercises and Stock Vested – 2010
| Option Awards | ||||||||
| Name | Number of Shares Acquired on Exercise | Value Realized on Exercise(1)(2) | ||||||
| William J. Sasiela, Ph.D. |
38,307 | $ | 474,624 | |||||
| (1) | Dr. Sasiela’s resigned from the Company on September 30, 2010. |
| (2) | Value realized on exercising is computed by multiplying the aggregate number of shares by $12.39, or the fair value per share of our common stock less the exercise price as of December 28, 2010, the exercise date. |
Pension Benefits and Non-Qualified Deferred Compensation
We have intentionally omitted tables concerning pension benefits and non-qualified deferred compensation because no compensation in these categories was paid in 2010.
Stock and Benefit Plan Information
2006 Stock Option and Grant Plan
Our 2006 Option and Grant Plan, or 2006 Option Plan, was adopted by our Board of Directors in May 2006 and approved by our stockholders in June 2006. We have reserved 1,929,046 shares of our common stock for the issuance of awards under the 2006 Option Plan.
Our 2006 Option Plan is administered by our Board of Directors. Our Board of Directors has the authority to delegate full power and authority to a committee of the Board to select the individuals to whom awards will be granted, to make any combination of awards to participants, to accelerate the exercisability or vesting of any award, to provide substitute awards and to determine the specific terms and conditions of each award, subject to the provisions of the 2006 Option Plan.
104
Table of Contents
The 2006 Option Plan permits us to make grants of incentive stock options, non-qualified stock options, restricted stock awards and unrestricted stock awards to officers, employees, directors, consultants and other key persons. Stock options granted under the 2006 Option Plan have a maximum term of ten years from the date of grant and incentive stock options have an exercise price of no less than the fair value of our Common Stock on the date of grant.
Upon a sale event in which all awards are not assumed or substituted by the successor entity, the 2006 Option Plan and all stock options issued thereunder will terminate upon the effective time of such sale event following an exercise period. Restricted stock shall be treated as provided in the relevant award agreement. Under the 2006 Option Plan, a sale event is defined as the consummation of:
| § | the dissolution or liquidation of Aegerion; |
| § | the sale of all or substantially all of our assets and our subsidiaries on a consolidated basis to an unrelated person or entity; |
| § | a merger, reorganization or consolidation in which the outstanding shares of our Common Stock are converted into or exchanged for securities of the successor entity and the holders of our outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of the successor entity immediately upon completion of such transaction, taking into account only ownership interests resulting from pre-transaction interests in Aegerion; |
| § | the sale, in a single transaction or series of related transactions, of all or a majority of our stock to an unrelated person or entity; or |
| § | any other transaction in which the holders of our outstanding voting power immediately prior to such transaction do not own at least a majority of the outstanding voting power of Aegerion or a successor entity immediately upon completion of the transaction, taking into account only ownership interests resulting from pre-transaction interests in Aegerion. |
2010 Stock Option and Grant Plan
Our 2010 Option Plan was adopted by the Board of Directors in September 2010, and approved by the stockholders in October 2010. The 2010 Option Plan allows the Company to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock awards, restricted stock units, unrestricted stock awards, cash-based awards, performance share awards and dividend equivalent rights.
The 2010 Option Plan may be administered by either the Compensation Committee or a similar committee of at least two non-employee directors who are independent or by the full Board of Directors, or the administrator. The administrator has full power and authority to select the participants to whom awards will be granted, to make any combination of awards to participants, to accelerate the exercisability or vesting of any award and to determine the specific terms and conditions of each award, subject to the provisions of the 2010 Option Plan.
All full-time and part-time officers, employees, non-employee directors and other key persons, including consultants and prospective employees, are eligible to participate in the 2010 Option Plan, subject to the sole discretion of the administrator. There are certain limits on the number of awards that may be granted under the 2010 Option Plan. For example, no more than 1,500,000 shares of common stock may be granted in the form of stock options or stock appreciation rights to any one individual during any one-calendar-year period.
The exercise price of stock options awarded under the 2010 Option Plan may not be less than the fair value of the Company’s common stock on the date of the option grant, and the term of each option may not exceed ten years from the date of grant. The administrator will determine at what time or times each option may be exercised and, subject to the provisions of the 2010 Option Plan, the period of time, if any, after retirement, death, disability or other termination of employment during which options may be exercised.
To qualify as incentive options, stock options must meet additional federal tax requirements, including a $100,000 limit on the value of shares subject to incentive options which first become exercisable in any calendar year, and a shorter term and higher minimum exercise price in the case of certain large stockholders.
105
Table of Contents
In the event of a merger, sale or dissolution, or a similar sale event, unless assumed or substituted, all stock options and stock appreciation rights granted under the 2010 Option Plan that are not exercisable immediately prior to the effective time of a sale event will automatically become fully exercisable, all other awards granted under the 2010 Option Plan will become fully vested and non-forfeitable and awards with conditions and restrictions relating to the attainment of performance goals may become vested and non-forfeitable in connection with a sale event in the administrator’s discretion. In addition, upon the effective time of any such sale event, the 2010 Option Plan and all awards will terminate unless the parties to the transaction, in their discretion, provide for appropriate substitutions or assumptions of outstanding awards.
Our Board of Directors is not granting any further awards under the 2006 Option Plan. We expect to make all future awards under the 2010 Option Plan.
401(k) Savings Plan
Our employee savings plan is intended to qualify under Section 401 of the Code. Our 401(k) plan permits employees to make contributions up to the statutory limit. We have the discretion to match up to 50% of the first 6% of gross wages that an employee contributes, resulting in a maximum match by us that totals up to 3% of an employee’s gross wages. We did match employee contributions in 2010. We may make matching contributions or additional contributions to our 401(k) plan in amounts determined annually.
Employment Agreements and Severance Agreements with Executive Officers
Marc D. Beer. On August 19, 2010, we entered into an employment agreement with Mr. Beer for the position of Chief Executive Officer. Mr. Beer currently receives a base salary of $450,000 per year, which is reviewed annually and is subject to increase but not decrease. Mr. Beer is also eligible for a merit bonus of up to 50% of his base salary, payable at the discretion of the Board of Directors or the Compensation Committee. Mr. Beer’s employment agreement provided for him to receive a stock option award to purchase a fixed number of shares of our Common Stock representing approximately 6.5% of the total number of shares of our outstanding capital stock, on an as-converted basis, anticipated to be outstanding immediately prior to the Company’s initial public offering. For purposes of determining the shares subject to this stock option award, certain estimates and assumptions were mutually agreed upon by us and Mr. Beer. The Board of Directors or the Compensation Committee may also, at its discretion, make additional grants of stock incentives to Mr. Beer. Mr. Beer is eligible to participate in our employee benefit plans to the extent he is eligible for those plans, on the same terms as similarly-situated executive officers of Aegerion.
Mr. Beer’s employment agreement also provides that subject to nomination, election and removal procedures, he will serve on our Board of Directors for as long as he is our Chief Executive Officer.
If Mr. Beer terminates his employment for good reason or if we terminate his employment without cause, he is entitled to receive as severance compensation 12 months’ base salary, which will be reduced by any compensation that Mr. Beer receives from another employer within one year of his separation, continuation of health benefits for the earlier of 12 months or the date he becomes re-employed with substantially comparable benefits and 25% acceleration of any unvested equity incentive awards. These payments and benefits are conditioned upon his execution of a separation agreement that includes a general release within 35 days of his termination.
If within 18 months of a change in control, Mr. Beer is terminated without cause or he terminates his employment for good reason, he will be entitled to a severance payment of (1) 1.5 times the sum of his base salary (which has not been pro-rated) plus his bonus payment for the prior year (which has not been pro-rated), (2) continuation of health benefits until the earlier of 18 months from the termination date or the date he becomes re-employed with substantially comparable benefits and (3) 100% acceleration of any unvested equity incentive awards. Change in control is defined in Mr. Beer’s employment agreement as any person acquiring beneficial ownership of 50% or more of the voting power of Aegerion, the date a majority of the members of our Board of Directors is replaced during any 12-month period by directors whose appointment or election was not endorsed by the majority of the incumbent members of the Board of Directors, the sale of all or substantially all of our assets, or a consolidation or merger of Aegerion where following such event the stockholders who held more than 50% of the voting control of Aegerion prior to such event no longer have such voting control.
106
Table of Contents
The definition of good reason in Mr. Beer’s employment agreement includes a material diminution in his responsibilities, authority or duties, removal or non-election to the Board of Directors, a material diminution in his base salary, the need for him to report to someone other than the board of directors, our material breach of a material provision of his employment agreement, a requirement that he relocate without his consent, or the failure by us to establish our corporate headquarters in Massachusetts within three months of a triggering event (as defined below), or thereafter, a relocation of our corporate headquarters outside of Massachusetts. A triggering event is defined in Mr. Beer’s employment agreement as the completion of the Company’s initial public offering that took place on October 27, 2010, our receipt of $20 million of private and strategic investments or the filing of our first NDA with the FDA.
The definition of cause as set forth in Mr. Beer’s employment agreement includes dishonesty, embezzlement, misappropriation of our assets or property, gross negligence, willful misconduct, neglect of duties, theft, fraud, breach of fiduciary duty, violation of federal or state security laws, conviction or plea of guilty or nolo contendere of a felony, material breach of his employment agreement or material breach of our written policies relating to conduct or ethics.
William H. Lewis. On October 5, 2010, we entered into an employment agreement with Mr. Lewis for the position of President. Prior to his resignation effective June 8, 2011, Mr. Lewis received a base salary of $300,000. Mr. Lewis was also eligible for an annual cash bonus of up to 40% of his base salary, payable at the discretion of the Board of Directors or the Compensation Committee, and a cash bonus equal to 10% of his base salary upon the FDA’s acceptance of an NDA for lomitapide. Mr. Lewis was eligible to participate in our employee benefit plans, to the extent he was eligible for those plans, on the same terms as our other similarly-situated executive officers. Per the terms of his employment agreement, if we terminated Mr. Lewis’ employment without cause or he terminated his employment for good reason, he will be entitled to receive as severance compensation 12 months base salary and health benefits continuance, subject to his signing of a release. If Mr. Lewis’ employment was terminated as a result of death or disability, he or his heirs will be entitled to receive as severance compensation six months base salary and health benefits continuance, subject to his or his heirs signing of a release. In addition, if we terminated Mr. Lewis’ employment without cause or he terminated his employment for good reason within 12 months of the effective date of the agreement, 25% of his outstanding unvested equity awards will vest and become nonforfeitable as of such termination date. Finally, in the event Mr. Lewis’ employment is terminated without cause or he terminates his employment for good reason within 24 months following a sale event, as defined in the applicable equity agreement, 100% of his unvested equity grants shall become fully exercisable as of such termination date.
Christine A. Pellizzari. On October 5, 2010, we entered into an employment agreement with Ms. Pellizzari for the position of Executive Vice President and General Counsel. Ms. Pellizzari’s employment may be terminated by her or by us at will. Ms. Pellizzari currently receives a base salary of $260,000, which is reviewed annually by the Board of Directors or the Compensation Committee. Ms. Pellizzari is also eligible for an annual cash bonus of up to 30% of her base salary, payable at the discretion of the Board of Directors or the Compensation Committee, and a cash bonus equal to 10% of her base salary upon the FDA’s acceptance of an NDA for lomitapide. Ms. Pellizzari is eligible to participate in our employee benefit plans, to the extent she is eligible for those plans, on the same terms as our other similarly-situated executive officers. If we terminate Ms. Pellizzari’s employment without cause or she terminates her employment for good reason, she will be entitled to receive as severance compensation 12 months base salary and health benefits continuance, subject to her signing of a release. In addition, if we terminate Ms. Pellizzari’s employment without cause or she terminates her employment for good reason within 12 months of the effective date of the agreement, 25% of her outstanding unvested equity awards will vest and become nonforfeitable as of such termination date. Finally, in the event Ms. Pellizzari’s employment is terminated without cause or she terminates her employment for good reason within 24 months following a sale event, as defined in the applicable equity agreement, 100% of her unvested equity grants shall become fully exercisable as of such termination date.
John T. Cavan. On October 5, 2010, we entered into an employment agreement with Mr. Cavan for the position of Vice President and Chief Accounting Officer. Mr. Cavan’s employment may be terminated by him or by us at will. Mr. Cavan currently receives a base salary of $175,000, which is reviewed annually by the Board of
107
Table of Contents
Directors or the Compensation Committee. Mr. Cavan is also eligible for an annual cash bonus of up to 25% of his base salary, payable at the discretion of the Board of Directors or the Compensation Committee, and a cash bonus equal to 10% of his base salary upon the FDA’s acceptance of an NDA for lomitapide. Mr. Cavan is eligible to participate in our employee benefit plans, to the extent he is eligible for those plans, on the same terms as our other similarly-situated executive officers. If we terminate Mr. Cavan’s employment without cause or he terminates his employment for good reason, he will be entitled to receive as severance compensation six months base salary and health benefits continuance, subject to his signing of a release. In addition, if we terminate Mr. Cavan’s employment without cause or he terminates his employment for good reason within 12 months of the effective date of the agreement, 25% of his outstanding unvested equity awards will vest and become nonforfeitable as of such termination date. Finally, in the event Mr. Cavan’s employment is terminated without cause or he terminates his employment for good reason within 24 months following a sale event, as defined in the applicable equity agreement, 100% of his unvested equity grants shall become fully exercisable as of such termination date.
The definition of cause as set forth in the employment agreements with Mr. Cavan and Ms. Pellizzari includes dishonesty, embezzlement or misappropriation of our assets; gross negligence, willful misconduct, theft, fraud or breach of a fiduciary duty owed to us; violation of federal or state securities laws; conviction of a felony or any crime involving moral turpitude, including a plea of nolo contendere; any material breach of the executive’s employment agreement; or material breach of any of our written policies relating to conduct or ethics. The definition of good reason as set forth in the employment agreements with Mr. Cavan and Ms. Pellizzari includes a material diminution in the executive’s responsibilities, duties or authority; a material diminution in the executive’s base salary; a material change without the executive’s consent of the location of the principal location of the executive’s office; or a material breach by us of the executive’s employment agreement.
Potential Payments Upon Termination in Connection with Change-in-Control
The table below reflects, as applicable, cash severance, option acceleration and continuation of health benefits payable to our named executive officers (1) in connection with the termination of his or her employment relationship without cause or resignation for good reason, (2) upon a sale event or change in control, as applicable and (3) in connection with termination without cause or resignation for good reason following a sale event or a change in control, as applicable, in each case, and assuming that the triggering event took place on December 31, 2010. See also “— Employment Agreements and Severance Agreements with Executive Officers.”
| Name | Benefit | Termination without Cause |
Resignation for Good Reason |
Termination without Cause in Connection with Sale Event or Change in Control |
Resignation for Good Reason in Connection with Sale Event or Change in Control |
|||||||||||||
| Marc D. Beer |
Cash Severance | $ | 450,000 | (1) | $ | 450,000 | (1) | $ | 945,000 | (2) | $ | 945,000 | (2) | |||||
| Option acceleration | 2,621,675 | (4) | 2,621,675 | (4) | 10,486,702 | (5) | 10,486,702 | (5) | ||||||||||
| Health Benefits | 18,720 | (6) | 18,720 | (6) | 28,080 | (3) | 28,080 | (3) | ||||||||||
| William H. Lewis |
Cash Severance | 300,000 | (7) | 300,000 | (7) | 300,000 | (7) | 300,000 | (7) | |||||||||
| Option acceleration | 908,604 | (4) | 908,604 | (4) | 3,634,415 | (5) | 3,634,415 | (5) | ||||||||||
| Health Benefits | 18,720 | (6) | 18,720 | (6) | 18,720 | (6) | 18,720 | (6) | ||||||||||
| William J. Sasiela |
Cash Severance | — | — | — | — | |||||||||||||
| Option acceleration | — | — | — | — | ||||||||||||||
| Health Benefits | — | — | — | — | ||||||||||||||
| Christine A. Pellizzari |
Cash Severance | 260,000 | (8) | 260,000 | (8) | 260,000 | (8) | 260,000 | (8) | |||||||||
| Option acceleration | 461,093 | (4) | 461,093 | (4) | 1,844,373 | (8) | 1,844,373 | (8) | ||||||||||
| Health Benefits | 18,720 | (6) | 18,720 | (6) | 18,720 | (6) | 18,720 | (6) | ||||||||||
| John T. Cavan |
Cash Severance | 87,500 | (9) | 87,500 | (9) | 87,500 | (9) | 87,500 | (9) | |||||||||
| Option acceleration | 129,264 | (4) | 129,264 | (4) | 517,057 | (5) | 517,057 | (5) | ||||||||||
| Health Benefits | 9,360 | (10) | 9,360 | (10) | 9,360 | (10) | 9,360 | (10) | ||||||||||
108
Table of Contents
| (1) | Represents twelve months continuation of base salary, which was $450,000 per year at December 31, 2010. |
| (2) | Represents 1.5 times the sum of his base salary (which has not been pro-rated) which was $450,000 per year at December 31, 2010, plus his bonus payment for the prior year (which has not been pro-rated). |
| (3) | Represent eighteen months continuation of standard employee benefits, including health insurance and benefits. |
| (4) | Represents the in-the-money value of 25% of the unvested portion of such person’s equity awards as of December 31, 2010. The value is calculated by multiplying the amount (if any) by which $14.17, the closing price of the Company’s common stock on December 31, 2010, exceeds the exercise price of the option, by the number of shares subject to the accelerated portion of the options. |
| (5) | Represents the in-the-money value of 100% of the unvested portion of such person’s equity awards as of December 31, 2010. The value is calculated by multiplying the amount (if any) by which $14.17, the closing price of the Company’s common stock on December 31, 2010, exceeds the exercise price of the option, by the number of shares subject to the accelerated portion of the options. |
| (6) | Represents twelve months continuation of standard employee benefits, including health insurance and benefits. |
| (7) | Represents twelve months continuation of base salary, which was $300,000 per year at December 31, 2010. |
| (8) | Represents twelve months continuation of base salary, which was $260,000 per year at December 31, 2010. |
| (9) | Represents six months continuation of base salary, which was $175,000 per year at December 31, 2010. |
| (10) | Represents twelve months continuation of standard employee benefits, including health insurance and benefits. |
| (11) | Represents six months continuation of standard employee benefits, including health insurance and benefits. |
Proprietary Information and Inventions Agreements
Each of our named executive officers has also entered into a standard form agreement with respect to proprietary information and inventions. Among other things, this agreement obligates each named executive officer to refrain from disclosing any of our proprietary information received during the course of employment and, with some exceptions, to assign to us any inventions conceived or developed during the course of employment.
Director Compensation
Non-Employee Director Compensation Policy
Prior to October 27, 2010, non-employee directors were only compensated if they had an agreement in place with us, and only Mr. Scheer and Dr. Gotto had agreements in place with us pursuant to which they were entitled to receive compensation in connection with attending meetings of the Board of Directors and for other services.
Prior to October 27, 2010, Mr. Scheer was entitled to compensation for his services as a consultant and as a member of our Board of Directors in accordance with an amended and restated consulting agreement with Scheer & Company, Inc., which we entered into in August 2006. Pursuant to this agreement, Mr. Scheer was entitled to receive $1,000 for each Board meeting he attended in person and $500 for each Board meeting he attended telephonically. In addition, Mr. Scheer was entitled to $1,250 per month for providing certain operational and strategic services to us from time to time. Our agreement with Dr. Scheer terminated on October 27, 2010.
Prior to October 27, 2010, Dr. Gotto was entitled to compensation for his services as a consultant and as a member of our Board of Directors in accordance with a consulting agreement with Dr. Gotto that we entered into in April 2006. Pursuant to this agreement, Dr. Gotto was entitled to receive $20,000 annually, $1,000 for each Board meeting he attended in person and $500 for each Board meeting he attended telephonically. In addition, he was entitled to receive $3,000 for each scientific advisory board meeting he attended in person and $1,000 for each scientific advisory board meeting he attended telephonically. Our agreement with Dr. Gotto terminated on October 27, 2010.
Effective October 27, 2010, our Board of Directors adopted a non-employee director compensation policy. This policy is designed to provide a total compensation package that enables us to attract and retain, on a long-term basis, high caliber non-employee directors and to align the directors’ interests with the long-term interests of our stockholders. Non-employee members of the Board of Directors began to receive payments for their service as Board members in accordance with this policy in 2011. Employee directors do not receive additional compensation for their services as directors.
109
Table of Contents
Under the policy, all non-employee directors are entitled to be paid cash compensation as set forth below.
| Annual Retainer |
In-Person Attendance Per Meeting |
Telephonic Attendance Per Meeting |
||||||||||
| Board |
||||||||||||
| Chairman of the Board |
$ | 40,000 | $ | 2,000 | $ | 1,000 | ||||||
| Other Non-Employee Directors |
25,000 | 2,000 | 1,000 | |||||||||
| Audit Committee |
||||||||||||
| Committee Chairman |
35,000 | 1,000 | 1,000 | |||||||||
| Committee Members |
— | 1,000 | 1,000 | |||||||||
| Compensation Committee |
||||||||||||
| Committee Chairman |
35,000 | 1,000 | 1,000 | |||||||||
| Committee Members |
— | 1,000 | 1,000 | |||||||||
| Nominating and Corporate Governance Committee |
||||||||||||
| Committee Chairman |
30,000 | 1,000 | 1,000 | |||||||||
| Committee Members |
— | 1,000 | 1,000 | |||||||||
The annual retainers are paid quarterly, in arrears, or upon the earlier resignation or removal of the non-employee director. The annual retainers are pro-rated based on the number of calendar days served by such director. The annual retainers and meeting fees are additive, meaning that directors earn retainers and fees for both Board and committee participation.
Under the policy, each person who is initially appointed or elected to the Board of Directors is eligible for an option grant to purchase shares of Common Stock, representing 0.1% of Aegerion on a fully-diluted basis, under our stock option plan on the date he or she first becomes a non-employee director. In addition, each director is entitled to an annual option grant to purchase shares of Common Stock representing 0.05% of the company on a fully-diluted basis. So long as the director remains on the Board of Directors, the director option grants will vest one-third on each one-year anniversary of the date of grant. Director option grants become immediately exercisable upon the death, disability or retirement of a director or upon a change in control of Aegerion. In addition, directors have up to one year following cessation of service as a director to exercise the options (to the extent vested at the date of such cessation), provided that the director has not been removed for cause. All of the foregoing options are granted at fair market value on the date of grant.
In April 2011, the Board, based in part on the recommendation of the Nominating Committee, approved an amendment to the Non-Employee Director Compensation Policy providing for the issuance of an additional equity award to the Chairmen of the Audit Committee and the Compensation Committee. Pursuant to this amendment, the Chairmen of the Audit Committee and the Compensation Committee will be entitled to receive a one-time grant of restricted stock equal to 0.15% of the Company on a fully-diluted basis when they are first elected to the position as a Chairman. These restricted stock grants will vest in the same manner as the director option grants described above.
110
Table of Contents
The following table sets forth a summary of the compensation earned by our non-employee directors in 2010. We have intentionally omitted columns from this table pertaining to types of compensation not paid to our non-employee directors in 2010.
Director Compensation Table – 2010
| Name | Fees Earned or Paid in Cash ($) |
Option Awards ($) | All Other Compensation ($) | Total ($) | ||||||||||||
| David I. Scheer |
$ | 3,500 | $ | — | $ | 14,406 | (1) | $ | 17,906 | |||||||
| Antonio M. Gotto, Jr., M.D., D. Phil. |
1,500 | 91,760 | (3) | 20,000 | (2) | 113,260 | ||||||||||
| Jason Fisherman, M.D.(4) |
— | — | — | — | ||||||||||||
| Alison Kiley |
— | — | — | — | ||||||||||||
| Michèle Ollier, M.D. |
— | — | — | — | ||||||||||||
| (1) | Represents consulting fees and reimbursement of expenses paid to Mr. Scheer under a consulting agreement, dated as of August 1, 2006, as amended. This consulting agreement terminated pursuant to its terms upon the closing of our initial public offering on October 27, 2010. |
| (2) | Represents consulting fees paid to Dr. Gotto under a consulting agreement, dated as of April 4, 2006, as amended. This consulting agreement terminated pursuant to its terms upon the closing of our initial public offering on October 27, 2010. |
| (3) | Represents the grant date fair value of option award granted to Dr. Gotto on October 27, 2010 for purchase of up to 10,238 shares at an exercise price of $9.50 per share, calculated in accordance with ASC Topic 718, formerly Statement of Financial Accounting Standards No. 123R. For information regarding assumptions underlying the valuation of equity awards, see Note 13 to the Financial Statements in our Annual Report on Form 10-K for the fiscal year ended December 31, 2010. As of December 31, 2010, this option award was the only one outstanding for Dr. Gotto. |
| (4) | Dr. Fisherman resigned from the Board of Directors in April 2011. |
Limitation of Liability and Indemnification Agreements
As permitted by the Delaware General Corporation Law, provisions in our certificate of incorporation and by-laws limit or eliminate the personal liability of our directors. Consequently, a director will not be personally liable to us or our stockholders for monetary damages or breach of fiduciary duty as a director, except for liability for:
| § | any breach of the director’s duty of loyalty to us or our stockholders; |
| § | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| § | any unlawful payments related to dividends or unlawful stock purchases, redemptions or other distributions; or |
| § | any transaction from which the director derived an improper personal benefit. |
These limitations of liability do not alter director liability under the federal securities laws and do not affect the availability of equitable remedies such as an injunction or rescission.
In addition, our by-laws provide that:
| § | we will indemnify our directors, officers and, in the discretion of our board of directors, certain employees to the fullest extent permitted by the Delaware General Corporation Law; and |
| § | we will advance expenses, including attorneys’ fees, to our directors and, in the discretion of our board of directors, to our officers and certain employees, in connection with legal proceedings, subject to limited exceptions. |
We have entered into indemnification agreements with each of our directors and our executive officers. These agreements provide that we will indemnify each of our directors and executive officers to the fullest extent permitted by law and advance expenses, including attorneys’ fees, to each indemnified director or executive officer in connection with any proceeding in which indemnification is available.
111
Table of Contents
We also maintain general liability insurance which covers certain liabilities of our directors and officers arising out of claims based on acts or omissions in their capacities as directors or officers, including liabilities under the Securities Act.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or persons controlling us, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers under these indemnification provisions. We believe that these provisions, the indemnification agreements and the insurance are necessary to attract and retain talented and experienced directors and officers.
At present, there is no pending litigation or proceeding involving any of our directors or officers where indemnification will be required or permitted. We are not aware of any threatened litigation or proceeding that might result in a claim for such indemnification.
112
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Since January 1, 2008, we have engaged in the following transactions with our directors, executive officers and holders of more than 5% of our voting securities, affiliates or any member of the immediate family of the foregoing persons.
Convertible Note Financings
September 2008
In September 2008, we received gross proceeds of $3,814,760 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes are automatically convertible into common stock upon the closing of this offering at a conversion price equal to 80% of the price to the public in this offering. No payments of principal or interest have been made under these notes. The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 1,005,847 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
944,342 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
1,088,678 | |||
| MVM International Life Sciences Fund No. 1 L.P. and affiliated entities(4) |
324,601 | |||
| William H. Lewis(5) |
42,758 | |||
| (1) | Consists of $403,143 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $591,640 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $11,064 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a former director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $864,938 convertible note purchased by Alta BioPharma Partners III, L.P., $58,088 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $21,316 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | Consists of $7,335 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $716,081 convertible note purchased by Index Ventures III (Delaware) L.P., $352,507 convertible note purchased by Index Ventures III (Jersey) L.P. and $12,755 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $321,287 convertible note purchased by MVM International Life Sciences Fund No. 1 L.P. and $3,314 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
December 2008
In December 2008, we received gross proceeds of $5,000,001 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes are automatically convertible into common stock upon the closing of this offering at a conversion price equal to 80% of the price to the public in this offering. No payments of principal or interest have been made under these convertible notes.
113
Table of Contents
The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 1,313,822 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
1,233,485 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
1,422,013 | |||
| MVM International Life Sciences Fund No. 1 L.P. and affiliated entities(4) |
432,802 | |||
| William H. Lewis(5) |
57,003 | |||
| (1) | Consists of $526,579 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $772,791 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $14,452 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a former director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $1,129,769 convertible note purchased by Alta BioPharma Partners III, L.P., $75,874 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $27,842 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | Consists of $9,579 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $935,333 convertible note purchased by Index Ventures III (Delaware) L.P., $460,440 convertible note purchased by Index Ventures III (Jersey) L.P. and $16,661 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $428,473 convertible note purchased by MVM International Life Sciences Fund No. 1 L.P. and $4,329 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
July 2009
In July 2009, we received gross proceeds of $5,000,000 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes are automatically convertible into common stock upon the closing of this offering at a conversion price equal to 80% of the price to the public in this offering. No payments of principal or interest have been made under these convertile notes.
The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 1,315,787 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
1,235,330 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
1,424,140 | |||
| MVM International Life Sciences Fund No. 1 L.P. and affiliated entities(4) |
429,622 | |||
| William H. Lewis(5) |
56,587 | |||
| (1) | Consists of $527,367 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $773,947 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $14,473 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds including the above listed funds. Dr. Fisherman, a former director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $1,131,459 convertible note purchased by Alta BioPharma Partners III, L.P., $75,987 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $27,884 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
114
Table of Contents
| (3) | Consists of $9,593 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $936,732 convertible note purchased by Index Ventures III (Delaware) L.P., $461,129 convertible note purchased by Index Ventures III (Jersey) L.P. and $16,686 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $425,287 convertible note purchased by MVM International Life Sciences Fund No. 1 L.P. and $4,335 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
January 2010
In January 2010, we received gross proceeds of $3,000,000 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrued interest at a rate equal to 8% per year, compounding daily, and had a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes were automatically converted into shares of our Common Stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public in the offering. No payments of principal or interest were made under the convertible notes. The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 789,472 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
741,198 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
854,484 | |||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
257,773 | |||
| William H. Lewis(5) |
33,952 | |||
| (1) | Consists of $316,420 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $464,368 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $8,684 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a former director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $678,875 convertible note purchased by Alta BioPharma Partners III, L.P., $45,592 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $16,730 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | Consists of $5,756 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $562,039 convertible note purchased by Index Ventures III (Delaware) L.P., $276,677 convertible note purchased by Index Ventures III (Jersey) L.P. and $10,012 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $255,172 convertible note purchased by MVM International Life Sciences Fund No. 1 Limited Partnership and $2,601 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
June 2010
In June 2010, we received gross proceeds of $1,500,000 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes were automatically converted into shares of our Common Stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public in the offering. No payments of principal or interest have been made under these convertible notes.
115
Table of Contents
The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 394,736 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
370,599 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
427,242 | |||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
128,887 | |||
| William H. Lewis(5) |
16,976 | |||
| (1) | Consists of $158,210 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $232,184 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $4,342 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $339,438 convertible note purchased by Alta BioPharma Partners III, L.P., $22,796 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $8,365 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a Director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | Consists of $3,418 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $280,662 convertible note purchased by Index Ventures III (Delaware) L.P., $138,162 convertible note purchased by Index Ventures III (Jersey) L.P. and $5,000 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $127,586 convertible note purchased by MVM International Life Sciences Fund No. 1 Limited Partnership and $1,301 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
August 2010
In August 2010, we received gross proceeds of $1,500,000 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes were automatically converted into shares of our Common Stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public in the offering. No payments of principal or interest have been made under these convertible notes.
The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 394,736 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
370,599 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
427,242 | |||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
128,887 | |||
| William H. Lewis(5) |
16,976 | |||
| (1) | Consists of $158,210 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $232,184 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $4,342 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $339,438 convertible note purchased by Alta BioPharma Partners III, L.P., $22,796 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $8,365 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a Director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
116
Table of Contents
| (3) | Consists of $3,418 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $280,662 convertible note purchased by Index Ventures III (Delaware) L.P., $138,162 convertible note purchased by Index Ventures III (Jersey) L.P. and $5,000 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $127,586 convertible note purchased by MVM International Life Sciences Fund No. 1 Limited Partnership and $1,301 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
October 2010
In October 2010, we received gross proceeds of $1,500,000 from the sale of convertible notes in a private placement to certain of our existing investors. The convertible notes accrue interest at a rate equal to 8% per year, compounding daily, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible notes were automatically converted into shares of our Common Stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public in the offering. No payments of principal or interest have been made under these convertible notes.
The following table sets forth the participation in this financing by our executive officers, entities affiliated with our directors and our 5% stockholders and their affiliates.
| Name | Principal Amount of Note |
|||
| Funds managed by Advent International Corporation(1) |
$ | 394,736 | ||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
370,599 | |||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
427,242 | |||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
128,887 | |||
| William H. Lewis(5) |
16,976 | |||
| (1) | Consists of $158,210 convertible note purchased by Advent Healthcare and Life Sciences III Limited Partnership, $232,184 convertible note purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and $4,342 convertible note purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of $339,438 convertible note purchased by Alta BioPharma Partners III, L.P., $22,796 convertible note purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and $8,365 convertible note purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a Director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | Consists of $3,418 convertible note purchased by Yucca Partners L.P. (Jersey Branch) as Administrator of the Index Co-Investment Scheme, $280,662 convertible note purchased by Index Ventures III (Delaware) L.P., $138,162 convertible note purchased by Index Ventures III (Jersey) L.P. and $5,000 convertible note purchased by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of $127,586 convertible note purchased by MVM International Life Sciences Fund No. 1 Limited Partnership and $1,301 convertible note purchased by MVM Executive Limited. |
| (5) | Mr. Lewis resigned as President on June 8, 2011. |
In October 2010, we amended the terms of all previously issued convertible notes such that the conversion price of the convertible notes was changed from 85% to 80% of the price to the public in the initial public offering.
117
Table of Contents
Purchase of Common Stock in Our Initial Public Offering
October 2010
On October 27, 2010, our chief executive officer and certain of our 5% stockholders and their affiliates purchased an aggregate of 1,131,580 shares of our Common Stock at the closing of our initial public offering at the initial price to the public, as set forth in the table below.
| Name | Number of Shares of Common Stock Purchased |
Aggregate Purchase Price for Shares of Common Stock(3) |
||||||
| Funds managed by Advent International Corporation(1) |
263,159 | 2,500,000 | ||||||
| Alta BioPharma HLS III Limited Partnership and affiliated entities(2) |
315,790 | 3,000,000 | ||||||
| Index Ventures III (Jersey), L.P. and affiliated entities(3) |
315,790 | 3,000,000 | ||||||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
131,579 | 1,252,000 | ||||||
| Marc D. Beer |
105,263 | 999,999 | ||||||
| (1) | Consists of 105,474 shares of Common Stock purchased by Advent Healthcare and Life Sciences III Limited Partnership, 154,790 shares of Common Stock purchased by Advent Healthcare and Life Sciences III-A Limited Partnership and 2,895 shares of Common Stock purchased by Advent Partners HLS III Limited Partnership. Advent International has engaged White Cube Management LLC to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Fisherman, a director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (2) | Consists of 289,236 shares of Common Stock purchased by Alta BioPharma Partners III, L.P., 19,426 shares of Common Stock purchased by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG and 7,128 shares of Common Stock purchased by Alta Embarcadero BioPharma Partners III, LLC. Alison Kiley, a director of Aegerion, is a Director of Alta BioPharma Partners. Ms. Kiley disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (3) | 315,790 shares of Common Stock purchased by Index Ventures III (Delaware) L.P. Michèle Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | Consists of 130,263 shares of Common Stock purchased by MVM International Life Sciences Fund No. 1 Limited Partnership and 1,316 shares of Common Stock purchased by MVM Executive Limited. |
Employment and Consulting Agreements
We have also entered into employment agreements with Messrs. Beer, Cavan and Lewis and Ms. Pellizzari that provide for certain salary, bonus and severance compensation. For more information regarding these agreements, see “Executive Compensation — Employment Agreements and Severance Agreements with Executive Officers.”
Stock Option Awards
For more information regarding stock options awarded to our named executive officers, see “Executive Compensation — Outstanding Equity Awards at Fiscal Year End.”
Indemnification Agreements
We have agreed to indemnify our directors and officers in certain circumstances. See “Executive Compensation — Limitation of Liability and Indemnification Agreements.”
Registration Rights
Certain of our directors, executive officers and holders of more than 5% of our common stock are party to agreements providing for rights to register under the Securities Act certain shares of our capital stock. For more information regarding these agreements, see “Description of Capital Stock — Registration Rights.”
118
Table of Contents
Policies for Approval of Related Person Transactions
Our Board of Directors reviews and approves transactions with directors, officers, and holders of five percent or more of our voting securities and their affiliates, each, a related person. We have adopted a related person transaction approval policy that governs the review of related person transactions. Pursuant to this policy, if we want to enter into a transaction with a related person or an affiliate of a related person, our general counsel will review the proposed transaction to determine, based on applicable NASDAQ and SEC rules, if such transaction requires pre-approval by the Audit Committee and/or Board of Directors. If pre-approval is required, such matters will be reviewed at the next regular or special Audit Committee and/or Board of Directors meeting. We may not enter into a related person transaction unless our general counsel has either specifically confirmed in writing that no further reviews are necessary or that all requisite corporate reviews have been obtained.
119
Table of Contents
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth information with respect to the beneficial ownership of our Common Stock, as of May 31, 2011 with respect to:
| § | the Selling Stockholders |
| § | each beneficial owner of 5% or more of our outstanding Common Stock; |
| § | each of our named executive officers and directors; and |
| § | all of our executive officers and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities and include shares of Common Stock issuable upon the exercise of stock options that are immediately exercisable or exercisable within 60 days after May 31, 2011, although these shares are not deemed outstanding for the purpose of computing the percentage ownership of any other person. Except as otherwise indicated, all of the shares reflected in the table are shares of Common Stock and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. The information is not necessarily indicative of beneficial ownership for any other purpose. Percentage of ownership is based on 17,664,641 shares of Common Stock outstanding on May 31, 2011.
Except as otherwise indicated in the table below, addresses of named beneficial owners are in care of Aegerion Pharmaceuticals, Inc., 101 Main Street, Cambridge, Massachusetts 02142.
| Shares Beneficially Owned Prior to Offering |
Number of Shares Offered |
Shares Beneficially Owned After Offering |
Shares to be Sold if Underwriters’ Option is Exercised in Full |
Shares Beneficially Owned After the Offering if Underwriters’ Option is Exercised in Full |
||||||||||||||||||||||||||||
| Name of Beneficial Owner | Number | Percentage | Number | Percentage | Number | Percentage | ||||||||||||||||||||||||||
| 5% Stockholders |
||||||||||||||||||||||||||||||||
| Funds managed by Advent International Corporation(1) |
2,786,110 | 15.77 | % | 122,277 | 2,663,833 | 12.74 | % | 17,033 | 2,646,800 | 12.36 | % | |||||||||||||||||||||
| Alta BioPharma Partners III, L.P. and affiliated entities(2) |
2,680,551 | 15.17 | % | — | 2,680,551 | 12.82 | % | — | 2,680,551 | 12.52 | % | |||||||||||||||||||||
| Index Venture Associates III Limited and affiliated entities(3) |
3,046,158 | 17.24 | % | 877,723 | 2,168,435 | 10.37 | % | 122,267 | 2,046,168 | 9.56 | % | |||||||||||||||||||||
| MVM International Life Sciences Fund No. 1 Limited Partnership and affiliated entities(4) |
958,937 | 5.43 | % | — | 958,937 | 4.59 | % | — | 958,937 | 4.48 | % | |||||||||||||||||||||
| Wellington Management Company, LLP(5) |
1,360,100 | 7.70 | % | — | 1,360,100 | 6.5 | % | — | 1,360,100 | 6.35 | % | |||||||||||||||||||||
| Named Executive Officers and Directors |
||||||||||||||||||||||||||||||||
| Marc D. Beer(6) |
303,656 | 1.72 | % | — | 303,656 | 1.45 | % | — | 303,656 | 1.42 | % | |||||||||||||||||||||
| William H. Lewis(7) |
385,637 | 2.18 | % | — | 385,637 | 1.84 | % | — | 385,637 | 1.80 | % | |||||||||||||||||||||
| Mark J. Fitzpatrick(8) |
2,750 | * | — | 2,750 | * | — | 2,750 | * | ||||||||||||||||||||||||
| Christine Pellizzari(9) |
110,360 | * | — | 110,360 | * | — | 110,360 | * | ||||||||||||||||||||||||
| John T. Cavan(10) |
56,464 | * | — | 56,464 | * | — | 56,464 | * | ||||||||||||||||||||||||
| David I. Scheer(11) |
650,154 | 3.68 | % | — | 650,154 | 3.11 | % | — | 650,154 | 3.04 | % | |||||||||||||||||||||
| Sol J. Barer, M.D.(12) |
— | * | — | — | * | — | — | * | ||||||||||||||||||||||||
| Alison Kiley(2) |
2,680,551 | 15.17 | % | — | 2,680,551 | 12.82 | % | — | 2,680,551 | 12.52 | % | |||||||||||||||||||||
| Antonio M. Gotto, Jr., M.D., D.Phil(13) |
13,916 | * | — | 13,916 | 0.07 | % | — | 13,916 | 0.07 | % | ||||||||||||||||||||||
| Michèle Ollier, M.D.(3) |
3,046,158 | 17.24 | % | 877,723 | 2,168,435 | 10.37 | % | 122,267 | 2,046,168 | 9.56 | % | |||||||||||||||||||||
| All executive officers and directors as a group (10 persons)(14) |
6,871,342 | 38.90 | % | 877,733 | 5,993,619 | 28.66 | % | 122,267 | 5,871,352 | 27.42 | % | |||||||||||||||||||||
| Other Selling Stockholders |
||||||||||||||||||||||||||||||||
120
Table of Contents
| (1) | The amount shown and the following information are derived from the Schedule 13D, filed on November 10, 2010, by Advent International Corporation, or AIC, reporting beneficial ownership as of October 27, 2010. The address for funds managed by AIC is 75 State Street, Boston, MA 02109. The amount reported consists of 1,116,697 shares held by Advent Healthcare and Life Sciences III Limited Partnership, 1,638,777 shares held by Advent Healthcare and Life Sciences III A Limited Partnership and 30,736 shares held by Advent Partners HLS III Limited Partnership. AIC is the manager of Advent International LLC, or AI LLC, which in turn is the general partner of AHLS III GP Limited Partnership, or AHLS III GP. As such, AIC has the sole power to vote of and dispose of the securities owned by AI LLC and AHLS III GP. The beneficial ownership of AIC, AI LLC and AHLS III GP derive from such power. AHLS III GP is the general partner of Advent Healthcare and Life Sciences III Limited Partnership and Advent Healthcare and Life Sciences III A Limited Partnership. As such AIC has the sole power to vote and dispose of securities of Advent Healthcare and Life Sciences III Limited Partnership and Advent Healthcare and Life Sciences III A Limited Partnership, and the beneficial ownership of AIC, AI LLC and AHLS III GP with respect to such entities derive from such power. AIC is the general partner of Advent Partners HLS III Limited Partnership and as such has the power to vote and dispose of the securities of Advent Partners HLS III Limited Partnership. The beneficial ownership of AIC with respect to such entity derives from such power. AIC has engaged White Cube Management LLC, or White Cube, to advise it with respect to the operation of certain private equity funds, including the above listed funds. Dr. Jason Fisherman, who served as a director of Aegerion until he resigned on April 12, 2011, is a managing director of White Cube. Dr. Fisherman disclaims beneficial ownership except to the extent of his pecuniary interest therein. |
| (2) | The amount shown and the following information are derived from the Schedule 13D, filed on November 8, 2010, by Alta BioPharma Partners III, L.P., or ABPIII, and its affiliated entities reporting beneficial ownership as of October 27, 2010. The address for ABPIII and its affiliated entities is One Embarcadero Center, Suite 3700, San Francisco, CA 94111. The amount owned consisted of 2,455,167 shares held by ABPIII, 164,882 shares held by Alta BioPharma Partners III GmbH & Co. Beteiligungs KG, or ABPIIIK, and 69,502 shares held by Alta Embarcadero BioPharma Partners III, LLC, or AEBPIII. Alta BioPharma Management III, LLC, or ABPMIII, as the general partner of ABPIII and managing limited partner of ABPIIIKG, shares the power to direct the voting and disposition of the shares directly held by ABPIII and ABPIIIKG and may be deemed to beneficially own the shares held by such entities. By virtue of their positions as directors of ABMIII and managers of AEBPIII, each of Farah Champsi, Edward Penhoet and Edward Hurwitz, or collectively, the Directors and each a Director, shares the power to direct the disposition and vote of the shares held by ABPIII, ABPIIIKG and AEBPIII and may be deemed to beneficially own the shares held by such entities. By virtue of her position as a member of ABMIII, Ms. Kiley, a director of Aegerion, may be deemed to share the power to direct disposition and vote the shares held by ABPIII, ABPIIIKG and AEBPIII and may be deemed to beneficially own the shares held by such entities. Each of the Directors and Ms. Kiley disclaims beneficial ownership of the shares held by such entities, except to the extent of his or her pecuniary interest therein. |
| (3) | The amount shown and the following information are derived from the Schedule 13D, filed on November 8, 2010, by Index Venture Associates III Limited, or Index Venture, and affiliated entities reporting beneficial ownership as of October 27, 2010. The address for Index Venture and its affiliated entities is P.O. Box 641, No. 1 Seaton Place, St. Helier, Jersey JE4 8YJ, Channel Islands. The amount owned consisted of 2,111,715 shares held by Index Ventures III (Delaware) L.P., or IVIII Delaware, 884,086 shares held by Index Ventures III (Jersey) L.P., or IVIII Jersey, 31,985 shares held by Index Ventures III Parallel Entrepreneur Fund (Jersey) L.P., or IVIII PEF, and 18,372 shares held by Yucca Partners L.P. Jersey Branch, or Yucca. Index Venture, as the general partner of IVIII Delaware, IVIII Jersey and IVIII PEF and an affiliate of Yucca, may be deemed to share the right to direct the voting and dispositive control over shares held by such entities. Dr. Ollier, a director of Aegerion, is a partner of Index Ventures. Dr. Ollier disclaims beneficial ownership except to the extent of her proportionate pecuniary interest therein. |
| (4) | The amount shown and the following information are derived from the Schedule 13G, filed on March 18, 2011, by MVM International Life Sciences No. 1 Limited Partnership, or MVM International, and affiliated entities reporting beneficial ownership as of December 31, 2010. The address for MVM International is 6 Henrietta Street, London WC2E 8PU. The amount owned consists of 949,326 shares held by MVM International and 9,611 shares held by MVM Executive Limited, or MVM Limited. MVM (GP) (No. 2) Limited, or MVM GP, which is the general partner of MVM International, is a wholly-owned subsidiary of MVM Life Sciences Partners LLP, or MVM Partners. In addition, MVM Limited is a wholly-owned subsidiary of MVM Partners. The shares owned by MVM Limited are held in trust for the benefit of certain individuals, but the shares are controlled by MVM Partners. Therefore, MVM GP may be deemed to beneficially own the shares held by MVM International and MVM Partners may be deemed to beneficially own the shares held by MVM International and MVM Limited. Each of MVM Partners and MVM GP disclaims beneficial ownership of the shares except to the extent of its pecuniary interest therein. |
| (5) | The amount shown and the following information are derived from the Schedule 13G, filed on February 14, 2011, by Wellington Management Company, LLP, or Wellington, reporting beneficial ownership as of December 31, 2010. The address of Wellington is 280 Congress Street, Boston, Massachusetts. Wellington, in its capacity as an investment advisor, may be deemed to beneficially own the 1,360,100 shares held by its clients, as it has shared voting power with respect to 1,307,700 shares and shared dispositive power with respect to all 1,360,100 shares. |
| (6) | Includes (a) 116,752 shares of common stock and (b) 186,904 shares of common stock subject to options exercisable within 60 days of May 31, 2011. |
| (7) | Includes (a) 305,465 shares of common stock and (b) 80,172 shares of common stock subject to options exercisable within 60 days of May 31, 2011. Mr. Lewis resigned as President on June 8, 2011 |
| (8) | Mr. Fitzpatrick joined the Company as Chief Financial Officer on May 9, 2011. |
| (9) | Includes 110,360 shares of common stock subject to options exercisable within 60 days of May 31, 2011. |
| (10) | Includes 56,464 shares of common stock subject to options exercisable within 60 days of May 31, 2011. |
121
Table of Contents
| (11) | The address for Scheer Investment Holdings VII LLC is 555 Long Wharf Drive, 11th Floor, New Haven, CT 06511. Mr. Scheer, our Chairman, is the managing member of Scheer Investment Holdings VII LLC. Mr. Scheer has sole power to vote all shares. Mr. Scheer disclaims beneficial ownership except to the extent of his proportionate pecuniary interest therein. |
| (12) | Dr. Barer joined the Board of Directors of the Company on May 1, 2011. |
| (13) | Includes 13,916 shares of common stock. |
| (14) | Does not include shares held by William Lewis, who resigned as President on June 8, 2011. |
122
Table of Contents
General
The following description of our capital stock is intended as a summary only and is qualified in its entirety by reference to our amended and restated certificate of incorporation and amended and restated by-laws, which are filed as exhibits to the registration statement, of which this prospectus forms a part, and to the applicable provisions of the Delaware General Corporation Law. We refer in this section to our amended and restated certificate of incorporation as our certificate of incorporation, and we refer to our amended and restated by-laws as our by-laws.
Our authorized capital stock consists of 125,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share, all of which shares of preferred stock will be undesignated.
As of April 11, 2011, we had issued and outstanding:
| § | 17,641,543 shares of our common stock outstanding held by 448 stockholders of record. A substantial number of holders of our common stock are “street name” or beneficial holders, whose shares are held by banks, brokers and other financial institutions. |
The number of shares issued and outstanding excludes:
| § | 2,177,813 shares of common stock issuable upon the exercise of options outstanding as of April 11, 2011, at a weighted average exercise price of $4.63 per share; |
| § | 107,779 shares of common stock subject to outstanding warrants outstanding as of April 11, 2011 with a weighted average exercise price of $6.68 per share; and |
| § | 2,864,788 additional shares of common stock available for future issuance under our 2010 Option Plan, as of April 11, 2011, which is subject to an annual increase of shares of common stock available for issuance under the 2010 Option Plan equal to four percent of the number of shares of common stock issued and outstanding on the immediately preceding December 31. |
Common Stock
The holders of our common stock are entitled to one vote for each share held on all matters properly submitted to a vote of the stockholders. The holders of our common stock do not have any cumulative voting rights. Holders of our common stock are entitled to receive proportionally any dividends declared by our board of directors, subject to any preferential dividend rights of any outstanding preferred stock.
In the event of our liquidation, dissolution, or winding up, holders of our common stock are entitled to share ratably in all assets remaining after payment of all debts and other liabilities, subject to the prior rights of any outstanding preferred stock. Holders of our common stock have no preemptive, subscription, redemption or conversion rights and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designated in the future. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and nonassessable.
Preferred Stock
Our board of directors is authorized, without action by the stockholders, to designate and issue up to 5,000,000 shares of preferred stock in one or more series. Our board of directors can fix the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of common stock. The issuance of preferred stock, while
123
Table of Contents
providing flexibility in connection with possible future financings and acquisitions and other corporate purposes could, under certain circumstances, have the effect of delaying, deferring or preventing a change in control and could harm the market price of our common stock.
Our board of directors has made the determination to issue such shares based on its judgment as to our best interests and the best interests of our stockholders. We have no current plans to issue any shares of preferred stock.
Warrants
In September 2008, we entered into a warrant agreement, or the Warrant Agreement, with Hercules Technology Growth Capital. Under the Warrant Agreement, which was amended in July 2009, Hercules Technology Growth Capital owned the right to purchase 387,238 shares of series A redeemable preferred stock, which became, in accordance with its terms, a warrant to purchase 107,779 shares of common stock, at an exercise price of $6.68 per share, upon the closing of our initial public offering.
The Warrant Agreement is exercisable for a period ending on the earlier of (i) a merger or sale of Aegerion in which the consideration consists entirely of cash and/or freely tradeable securities or (ii) the later to occur of (a) September 29, 2018 or (b) five (5) years after an initial public offering. The warrant agreement includes a customary net issuance feature, which allows Hercules to in lieu of payment of the exercise price in cash, to surrender the warrant and receive a net amount of shares based on the fair value of the shares at the time of exercise of the warrant after deduction of the aggregate exercise price.
Registration Rights
Holders of approximately 9.1 million shares of our common stock as of May 31, 2011, after giving effect to the conversion of the outstanding redeemable convertible preferred stock and accumulated dividends thereon and conversion of the aggregate principal and accrued interest outstanding under all of our outstanding convertible notes into common stock upon the closing of this offering, have rights, under the terms of an investor rights agreement or a restricted stock agreement between us and these holders, to require us to use our best efforts file registration statements under the Securities Act or request that their shares be covered by a registration statement that we are otherwise filing. We refer to these shares as registrable securities. The investor rights agreement and the restricted stock agreements do not provide for any liquidated damages, penalties or other rights in the event we do not file a registration statement.
Demand Registration Rights. Subject to certain exceptions, the holders of a majority of the then outstanding registrable securities described above have the right to demand that we file a registration statement covering the offering and sale of their registrable securities. We are not obligated to file a registration statement on more than one occasion upon the request of the holders of a majority of the then outstanding registrable securities.
Form S-3 Registration Rights. If we are eligible to file a registration statement on Form S-3, the holders of the registrable securities described above have the right, on one or more occasions, to request registration on Form S-3 of the sale of the registrable securities held by such holder provided such securities are anticipated to have an aggregate sale price, net of underwriting discounts and commissions, if any, in excess of $2.5 million. We have the ability to delay the filing of such registration statement under specified conditions, if our board of directors reasonably determines, upon the advice of counsel, that the registration would interfere with any material, non-public transaction involving us. The postponement can not exceed 90 days, and we may exercise this right only once in any 12 month period. We are not obligated to effect more than one registration of registrable securities on Form S-3 in any 12 month period.
Piggyback Registration Rights. The holders of the registrable securities described above have piggyback registration rights. Under these provisions, if we register any securities for public sale, including pursuant to any stockholder initiated demand registration, these holders will have the right to include their shares in the registration statement, subject to customary exceptions. The underwriters of any underwritten offering will have the right to limit the number of registrable securities to be included in the registration statement, and piggyback registration rights are also subject to the priority rights of stockholders having demand registration rights in any demand registration.
124
Table of Contents
Expenses of Registration. We will pay all registration expenses, other than underwriting discounts and commissions and selling expenses, related to any demand, Form S-3 or piggyback registration, including reasonable attorney’s fees and disbursements of one counsel for the holders of registrable securities in an amount not to exceed an aggregate of $35,000.
Indemnification. The investor rights agreement contains customary cross-indemnification provisions, under which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions attributable to them.
Certain Anti-Takeover Provisions of our Certificate of Incorporation and By-Laws
Our certificate of incorporation and by-laws includes a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board Composition and Filling Vacancies. In accordance with our certificate of incorporation, our board is divided into three classes serving staggered three-year terms, with one class being elected each year. Our certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of 75% or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board of directors, however occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum.
No Written Consent of Stockholders. Our certificate of incorporation provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of our by-laws or removal of directors by our stockholders without holding a meeting of stockholders.
Meetings of Stockholders. Our certificate of incorporation and by-laws provide that only a majority of the members of our board of directors then in office may call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders. Our by-laws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the meeting.
Advance Notice Requirements. Our by-laws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures provide that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the annual meeting for the preceding year. The notice must contain certain information specified in the by-laws.
Amendment to Certificate of Incorporation and By-Laws. As required by the Delaware General Corporation Law, any amendment of our certificate of incorporation must first be approved by a majority of our board of directors, and if required by law or our certificate of incorporation, must thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions relating to stockholder action, board composition, limitation of liability and the amendment of our certificate of incorporation must be approved by not less than 75% of the outstanding shares entitled to vote on the amendment, and not less than 75% of the outstanding shares of each class entitled to vote thereon as a class. Our by-laws may be amended by the affirmative vote of a majority of the directors then in office, subject to any limitations set forth in the by-laws; and may also be amended by the affirmative vote of at least 75% of the outstanding shares entitled to vote on the
125
Table of Contents
amendment, or, if our board of directors recommends that the stockholders approve the amendment, by the affirmative vote of the majority of the outstanding shares entitled to vote on the amendment, in each case voting together as a single class.
Undesignated Preferred Stock. Our certificate of incorporation provides for 5,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not in the best interests of our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Section 203 of the Delaware General Corporate Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s voting stock. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| § | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| § | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| § | at or after the time the stockholder became interested, the business combination was approved by our board of directors of the corporation and authorized at an annual or special meeting of the stockholders, but not by written consent, by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| § | any merger or consolidation involving the corporation and the interested stockholder; |
| § | any sale, transfer, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| § | subject to exceptions, any transaction that results in the issuance of transfer by the corporation of any stock of the corporation to the interested stockholder; |
| § | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interest stockholder; and |
126
Table of Contents
| § | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
NASDAQ Global Market Listing
Our common stock is listed on The NASDAQ Global Market under the symbol “AEGR.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Registrar and Transfer Company.
127
Table of Contents
Under the terms and subject to the conditions contained in an underwriting agreement dated June , 2011, the underwriters named below have agreed to purchase, and we and the Selling Stockholders have agreed to sell to them, shares of our common stock. Jefferies & Company, Inc. and Deutsche Bank Securities Inc. are acting as joint book-running managers in this offering. The underwriters are offering the common stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the shares offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares offered hereby (other than the shares subject to the underwriters’ over-allotment option) if any such shares are taken. Each underwriter has severally agreed to purchase the number of shares indicated in the following table.
| Underwriters | Number of Shares | |||
| Jefferies & Company, Inc. |
||||
| Deutsche Bank Securities Inc. |
||||
| Leerink Swann LLC |
||||
| Needham & Company, LLC |
||||
| Collins Stewart LLC |
||||
| Total |
4,250,000 | |||
Commissions and Expenses
The underwriters have advised us that they propose to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the offering, the public offering price and concession to dealers may be reduced by the underwriters. No such reduction shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The shares are offered by the underwriters as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part.
The underwriting discount is equal to the public offering price per share of common stock less the amount paid by the underwriters to us and the Selling Stockholders per share of common stock. The following table shows the per share and total public offering price, the underwriting discounts and the proceeds, before expenses, to us and the Selling Stockholders assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares, as discussed below.
| Total | ||||||||||||
| Per Share | Without Over-Allotment Exercise |
With Over-Allotment Exercise |
||||||||||
| Public offering price |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions paid by us |
$ | $ | $ | |||||||||
| Underwriting discounts and commissions paid by Selling Stockholders |
$ | $ | $ | |||||||||
| Proceeds to us (before expenses) |
$ | $ | $ | |||||||||
| Proceeds to Selling Stockholders (before expenses) |
$ | $ | $ | |||||||||
We estimate expenses payable by us in connection with the offering of common stock, other than the underwriting discounts and commissions referred to above, will be approximately $601,095. The Selling Stockholders will bear the cost of all underwriting discounts and selling commissions applicable to the sale of common stock by them.
The underwriters have agreed to reimburse the Company for its expenses incurred in the offering up to a maximum of .25% of the aggregate purchase price, or $ .
128
Table of Contents
Option to Purchase Additional Shares
We and the Selling Stockholders have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to an aggregate of 637,500 additional shares at the same price they are paying for the shares shown in the table above. The underwriters may exercise this option at any time and from time to time, in whole or in part, within 30 days after the date of this prospectus. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , or $ per share, and the total proceeds to us, before expenses, will be $ . The total underwriting discounts and commissions payable by the Selling Stockholders will be $ , or $ per share, and the total proceeds to the Selling Stockholders, before expenses, will be $ .
Indemnification
We and the Selling Stockholders have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933, as amended, and liabilities arising from certain breaches by us of the underwriter agreement. We and the Selling Stockholders have also agreed to contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
We, our officers and directors and holders of substantially all of our outstanding stock, options and warrants, including the Selling Stockholders, have entered into lock-up agreements with the underwriters. Under these agreements, we and these other individuals have agreed, subject to specified exceptions, not to sell or transfer any common stock or securities convertible into, or exchangeable or exercisable for, common stock, during a period ending 90 days after the date of this prospectus, without first obtaining the written consent of Jefferies & Company, Inc. and Deutsche Bank Securities Inc. Specifically, we, the Selling Stockholders and these other individuals have agreed not to:
| § | offer, pledge, sell or contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for common stock; or |
| § | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock, whether any such transaction described above is to be settled by delivery of common stock or other securities, in cash or otherwise. |
The restrictions described above do not apply to:
| § | the sale of shares of common stock to the underwriters pursuant to the underwriting agreement; |
| § | the issuance by us of shares of common stock upon the exercise of an option or warrant outstanding on the date of this prospectus of which the underwriters have been advised in writing or that is described in this prospectus; |
| § | the grant by us of stock options or other stock-based awards, or the issuance of shares of common stock upon exercise thereof, to eligible participants pursuant to employee benefit or equity incentive plans described in this prospectus, provided that, prior to the grant of any such stock options or other stock-based awards that vest within the restricted period, each recipient of such grant shall sign and deliver a lock-up agreement agreeing to be subject to the restrictions on transfer described above; |
| § | transfers of shares of common stock by security holders in connection with a merger, reorganization or consolidation of us with or into another entity, including through the purchase of our outstanding capital stock, pursuant to which our stockholders immediately prior to such transaction will own less than 50% of the surviving entity’s voting power after such transaction; |
| § | transactions by security holders relating to any shares of common stock acquired from the underwriters in connection with this offering; |
129
Table of Contents
| § | transactions by security holders relating to any shares of common stock or other securities acquired in open market transactions after the closing of this offering; |
| § | transfers by security holders of shares of common stock or other securities as a bona fide gift or by will or intestacy; |
| § | transfers by security holders of shares of common stock or other securities to any trust for the direct or indirect benefit of the security holder or the immediate family of the security holder; or |
| § | transfers by distribution by security holders of shares of common stock or other securities to partners, members, or shareholders of the security holder, |
provided that in the case of each of the preceding three types of transactions, the transfer does not involve a disposition for value and each transferee or distributee signs and delivers a lock-up agreement agreeing to be subject to the restrictions on transfer described above; and provided further, that in the case of each of the preceding five types of transactions, no filing or public disclosure reporting under the securities laws will be required or voluntarily made.
The 90-day restricted period is subject to extension if (1) during the last 17 days of the restricted period we issue an earnings release or material news or a material event relating to us occurs or (2) prior to the expiration of the restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the restricted period, in which case the restrictions imposed in the lock-up agreements will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
Electronic Distribution
This prospectus in electronic format may be made available on websites or through other online services maintained by the underwriters of the offering, or by their affiliates. Other than the prospectus in electronic format, the information on the underwriters’ websites and any information contained in any other website maintained by the underwriters or any of their affiliates is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us or the underwriters in their capacity as underwriters and should not be relied upon by investors.
Price Stabilization, Short Positions and Penalty Bids
Until the distribution of the shares of common stock is completed, rules may limit the underwriters from bidding for and purchasing shares of our common stock.
In connection with this offering, the underwriters may engage in transactions that stabilize or maintain the price of our common stock and may make short sales of our common stock or purchase our common stock on the open market to cover positions created by short sales. Short sales involve the sale by an underwriter of a greater number of shares than it is required to purchase in this offering. An underwriter may close out any short position by purchasing shares in the open market or by exercising its over-allotment option.
An underwriter also may impose a penalty bid, whereby the underwriter may reclaim selling concessions allowed to other broker-dealers in respect of the common stock sold in the offering for their account if the underwriter repurchases the shares in stabilizing or covering transactions. These activities may stabilize, maintain or otherwise affect the market price of the common stock, which may be higher than the price that might otherwise prevail in the open market. The imposition of a penalty bid may also affect the price of the shares of our common stock in that it discourages resales of those shares of our common stock.
A short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in this offering. A “stabilizing bid” is a bid for or the purchase of common stock on behalf of the underwriters in the open market prior to the completion of this offering for the purpose of fixing or maintaining
130
Table of Contents
the price of the shares of common stock. A “syndicate covering transaction” is the bid for or purchase of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering.
Similar to other purchase transactions, the underwriters’ purchases to cover syndicate short sales may have the effect of raising or maintaining the market price of our shares or preventing or retarding a decline in the market price of our shares. As a result, the price of our shares may be higher than the price that might otherwise exist in the open market.
In connection with this offering, the underwriters may also engage in passive market making transactions in our common stock on The NASDAQ Global Market in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transaction, if commenced, will not be discontinued without notice.
Affiliations
The underwriters and their affiliates may provide various investment banking, commercial banking, financial advisory and other services to us and our affiliates for which services they have received, and may in the future receive, customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans.
131
Table of Contents
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (as defined below) (each, a Relevant Member State), with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, or the Relevant Implementation Date, an offer of our common stock to the public may not be made in that Relevant Member State prior to the publication of a prospectus in relation to our common stock which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that an offer to the public in that Relevant Member State of any shares of our common stock may be made at any time under the following exemptions under the Prospectus Directive if they have been implemented in the Relevant Member State:
(a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c) to fewer than 100 natural or legal persons per Relevant Member State (other than qualified investors as defined in the Prospectus Directive); or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive; provided that no such offer of our common stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of our common stock to the public” in relation to any shares of our common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and our common stock to be offered so as to enable an investor to decide to purchase or subscribe our common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
France
This prospectus has not been, and will not be, submitted to the clearance procedures of the Autorité des marchés financiers (the “AMF”) in France and may not be directly or indirectly released, issued, or distributed to the public in France, or used in connection with any offer for subscription or sale of our common stock to the public in France, in each case within the meaning of Article L. 411-1 of the French Code monétaire et financier (the “French Financial and Monetary Code”).
The securities have not been, and will not be, offered or sold to the public in France, directly or indirectly, and will only be offered or sold in France (i) to qualified investors (investisseurs qualifiés) investing for their own account, in accordance with all applicable rules and regulations, and in particular in accordance with Articles L. 411-2 and D. 411-2 of the French Financial and Monetary Code; (ii) to investment services providers authorized to engage in portfolio investment on behalf of third parties, in accordance with Article L.411-2 of the French Financial and Monetary Code; or (iii) in a transaction that, in accordance with all applicable rules and regulations, does not otherwise constitute an offer to the public (“appel public à l’épargne”) in France within the meaning of Article L.411-1 of the French Financial and Monetary Code.
This prospectus is not to be further distributed or reproduced (in whole or in part) in France by any recipient, and this prospectus has been distributed to the recipient on the understanding that such recipient is a qualified
132
Table of Contents
investor or otherwise meets the requirements set forth above, and will only participate in the issue or sale of the securities for their own account, and undertakes not to transfer, directly or indirectly, the securities to the public in France, other than in compliance with all applicable laws and regulations and in particular with Articles L.411-1, L.411-2, D.411-1 and D.411-2 of the French Financial and Monetary Code.
United Kingdom
Shares of our common stock may not be offered or sold and will not be offered or sold to any persons in the United Kingdom other than to persons whose ordinary activities involve them in acquiring, holding, managing or disposing of investments (as principal or as agent) for the purposes of their businesses or otherwise in circumstances which have not resulted or will not result in an offer to the public in the United Kingdom within the meaning of the Financial Services and Markets Act 2000, or the FSMA.
In addition, any invitation or inducement to engage in investment activity (within the meaning of section 21 of the FSMA) in connection with the issue or sale of shares of our common stock may only be communicated or caused to be communicated in circumstances in which Section 21(1) of the FSMA does not apply to us. Without limitation to the other restrictions referred to herein, this prospectus is directed only at (1) persons outside the United Kingdom or (2) persons who:
(a) are qualified investors as defined in section 86(7) of FSMA, being persons falling within the meaning of article 2.1(e)(i), (ii) or (iii) of the Prospectus Directive; and
(b) are either persons who fall within article 19(1) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or Order, or are persons who fall within article 49(2)(a) to (d) (“high net worth companies, unincorporated associations, etc.”) of the Order; or
(c) to whom it may otherwise lawfully be communicated in circumstances in which Section 21(1) of the FSMA does not apply.
Without limitation to the other restrictions referred to herein, any investment or investment activity to which this offering circular relates is available only to, and will be engaged in only with, such persons, and persons within the United Kingdom who receive this communication (other than persons who fall within (2) above) should not rely or act upon this communication.
Italy
This prospectus has not been and will not be filed with or cleared by the Italian securities exchange commission (Commissione Nazionale per le societa e la Borsa — the “CONSOB”) pursuant to Legislative Decree No. 58 of 24 February 1998 (as amended, the “Finance Law”) and to CONSOB Regulation No. 11971 of 14 May 1999 (as amended, the “Issuers Regulation”). Accordingly, copies of this prospectus or any other document relating to our common stock may not be distributed, made available or advertised in Italy, nor may our common stock be offered, purchased, sold, promoted, advertised or delivered, directly or indirectly, to the public other than (i) to “Professional Investors (such being the persons and entities as defined pursuant to article 31(2) of CONSOB Regulation No. 11522 of 1 July 1998, as amended, the “Intermediaries Regulation”) pursuant to article100 of the Finance Law; (ii) to prospective investors where the offer of our common stock relies on the exemption from the investment solicitation rules pursuant to, and in compliance with the conditions set out by article 100 of the Finance Law and article 33 of the Issuers Regulation, or by any applicable exemption; provided that any such offer, sale, promotion, advertising or delivery of our common stock or distribution of the prospectus, or any part thereof, or of any other document or material relating to our common stock in Italy is made: (a) by investment firms, banks or financial intermediaries authorized to carry out such activities in the Republic of Italy in accordance with the Finance Law, the Issuers Regulation, Legislative Decree No. 385 of 1 September 1993 (as amended, the “Banking Law”), the Intermediaries Regulation, and any other applicable laws and regulations; and (b) in compliance with any applicable notification requirement or duty which may, from time to time, be imposed by CONSOB, Bank of Italy or by any other competent authority.
133
Table of Contents
Germany
Any offer or solicitation of securities within Germany must be in full compliance with the German Securities Prospectus Act (Wertpapierprospektgesetz — WpPG). The offer and solicitation of securities to the public in Germany requires the publication of a prospectus that has to be filed with and approved by the German Federal Financial Services Supervisory Authority (Bundesanstalt für Finanzdienstleistungsaufsicht — BaFin). This prospectus has not been and will not be submitted for filing and approval to the BaFin and, consequently, will not be published. Therefore, this prospectus does not constitute a public offer under the German Securities Prospectus Act (Wertpapierprospektgesetz). This prospectus and any other document relating to our common stock, as well as any information contained therein, must therefore not be supplied to the public in Germany or used in connection with any offer for subscription of our common stock to the public in Germany, any public marketing of our common stock or any public solicitation for offers to subscribe for or otherwise acquire our common stock. This prospectus and other offering materials relating to the offer of our common stock are strictly confidential and may not be distributed to any person or entity other than the designated recipients hereof.
Sweden
This is not a prospectus under, and has not been prepared in accordance with the prospectus requirements provided for in, the Swedish Financial Instruments Trading Act [lagen (1991:980) om handel med finasiella instrument] nor any other Swedish enactment. Neither the Swedish Financial Supervisory Authority nor any other Swedish public body has examined, approved, or registered this document.
134
Table of Contents
The validity of the shares of common stock offered in this prospectus is being passed upon for us by Goodwin Procter LLP, Boston, Massachusetts. Dewey & LeBoeuf LLP, New York, New York, is counsel for the underwriters in connection with this offering.
Ernst & Young LLP, independent registered public accounting firm, has audited our financial statements at December 31, 2010 and 2009, and for each of the three years in the period ended December 31, 2010, as set forth in their report. We have included our financial statements in the prospectus in reliance on Ernst & Young LLP’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 (File No. 333-174944) under the Securities Act with respect to the shares of common stock we are offering by this prospectus. This prospectus, which forms a part of the registration statement, does not contain all of the information included in the registration statement. For further information pertaining to us and our common stock, you should refer to the registration statement and to its exhibits. For further information with respect to us and the common stock offered by this prospectus, you should refer to the registration statement and the exhibits to the registration statement. Statements contained in this prospectus as to the contents of any contract, agreement or any other document are not necessarily complete, and in each instance, we refer you to the copy of the contract, agreement or any other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We also file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. Additionally, we make our SEC filings available free of charge through our website, www.aegerion.com. Information contained on, or accessible through, our website is not part of this Prospectus. We have included our website address as an inactive textual reference only.
You may read and copy any disclosure we file with the SEC at the SEC’s Public Reference Room, which is located at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website, which is located at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access a copy of the registration statement for this offering at the SEC’s Internet site.
135
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-7 | ||||
| F-8 | ||||
| Unaudited Balance Sheets as of December 31, 2010 and March 31, 2011 |
F-29 | |||
| F-30 | ||||
| F-31 | ||||
| F-32 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Aegerion Pharmaceuticals, Inc.
We have audited the accompanying balance sheets of Aegerion Pharmaceuticals, Inc. (a development stage company) as of December 31, 2010 and 2009, and the related statements of operations, stockholders’ equity (deficiency) and cash flows for each of the three years in the period ended December 31, 2010 and the period from February 4, 2005 (Inception) to December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Aegerion Pharmaceuticals, Inc. at December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010 and the period from February 4, 2005 (Inception) to December 31, 2010, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young, LLP
MetroPark, New Jersey
March 31, 2011
F-2
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Balance Sheets
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 1,429,126 | $ | 44,100,897 | ||||
| Prepaid expenses and other current assets |
535,921 | 504,744 | ||||||
| Total current assets |
1,965,047 | 44,605,641 | ||||||
| Property and equipment, net |
14,731 | 16,471 | ||||||
| Investments in securities |
645,000 | 1,125,000 | ||||||
| Other assets |
25,200 | — | ||||||
| Total assets |
$ | 2,649,978 | $ | 45,747,112 | ||||
| Liabilities and stockholders’ equity (deficiency) |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 403,605 | $ | 3,145,887 | ||||
| Accrued compensation |
343,443 | 492,938 | ||||||
| Other accrued liabilities |
1,426,696 | 1,029,873 | ||||||
| Debt financing |
5,252,423 | — | ||||||
| Convertible notes |
14,843,300 | — | ||||||
| Warrant liability |
567,074 | — | ||||||
| Total current liabilities |
22,836,541 | 4,668,698 | ||||||
| Commitments and contingencies |
||||||||
| Series A redeemable convertible preferred stock, $0.001 par value; 13,000,000 shares authorized, 12,211,604 and 0 shares issued and outstanding at December 31, 2009 and 2010 (aggregate liquidation preference of $29,868,336 and $0 as of December 31, 2009 and 2010, respectively) |
29,633,656 | — | ||||||
| Series B redeemable convertible preferred stock, $0.001 par value; 6,650,000 shares authorized, 3,810,773 and 0 shares issued and outstanding at December 31, 2009 and 2010 (aggregate liquidation preference of $20,361,729 and $0 as of December 31, 2009 and 2010, respectively) |
20,306,416 | — | ||||||
| Stockholders’ equity (deficiency): |
||||||||
| Common stock, $0.001 par value, 30,000,000 and 125,000,000 shares authorized at December 31, 2009 and 2010, respectively; 1,811,886 and 17,745,300 shares issued at December 31, 2009 and 2010, respectively; 1,708,129 and 17,641,543 shares outstanding at December 31, 2009 and 2010, respectively |
1,812 | 17,744 | ||||||
| Treasury Stock, at cost; 103,757 shares at December 31, 2009 and 2010 |
(373 | ) | (373 | ) | ||||
| Subscription Receivable |
— | (90,735 | ) | |||||
| Additional paid-in-capital |
648,514 | 131,549,805 | ||||||
| Deficit accumulated during the development stage |
(70,857,347 | ) | (90,988,786 | ) | ||||
| Accumulated other comprehensive income |
80,759 | 590,759 | ||||||
| Total stockholders’ equity (deficiency) |
(70,126,635 | ) | 41,078,414 | |||||
| Total liabilities and stockholders’ equity (deficiency) |
$ | 2,649,978 | $ | 45,747,112 | ||||
See accompanying notes.
F-3
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Operations
| Years Ended December 31, | Period From February 4, 2005 (Inception) to December 31, |
|||||||||||||||
| 2008 | 2009 | 2010 | 2010 | |||||||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development |
$ | 17,712,353 | $ | 7,040,682 | $ | 7,628,725 | $ | 49,867,264 | ||||||||
| General and administrative |
5,184,970 | 3,074,848 | 5,921,639 | 24,545,035 | ||||||||||||
| Total costs and expenses |
22,897,323 | 10,115,530 | 13,550,364 | 74,412,299 | ||||||||||||
| Loss from operations |
(22,897,323 | ) | (10,115,530 | ) | (13,550,364 | ) | (74,412,299 | ) | ||||||||
| Interest expense |
(1,126,974 | ) | (2,083,208 | ) | (2,403,875 | ) | (6,897,832 | ) | ||||||||
| Interest income |
533,089 | 177,161 | 109,057 | 2,716,436 | ||||||||||||
| Change in fair value of warrant liability |
90,730 | (174,425 | ) | (416,267 | ) | (262,741 | ) | |||||||||
| Other than temporary impairment on securities |
(1,665,307 | ) | — | (30,000 | ) | (2,465,759 | ) | |||||||||
| Other income, net |
30,750 | — | 244,479 | 275,229 | ||||||||||||
| Loss before income taxes |
(25,035,035 | ) | (12,196,002 | ) | (16,046,970 | ) | (81,046,966 | ) | ||||||||
| Benefit from income taxes |
— | — | 1,793,129 | 1,793,129 | ||||||||||||
| Net loss |
(25,035,035 | ) | (12,196,002 | ) | (14,253,841 | ) | (79,253,837 | ) | ||||||||
| Less: accretion of preferred stock dividends and other deemed dividends |
(6,241,923 | ) | (3,286,654 | ) | (8,750,897 | ) | (23,663,413 | ) | ||||||||
| Net loss attributable to common stockholders |
$ | (31,276,958 | ) | $ | (15,482,656 | ) | $ | (23,004,738 | ) | $ | (102,917,250 | ) | ||||
| Net loss attributable to common stockholders per common share—basic and diluted |
$ | (20.92 | ) | $ | (9.35 | ) | $ | (5.07 | ) | |||||||
| Weighted-average shares outstanding—basic and diluted |
1,495,375 | 1,656,732 | 4,537,407 | |||||||||||||
See accompanying notes.
F-4
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity (Deficiency)
Period From February 4, 2005 (Inception) to December 31, 2010
| Common Stock | Subscription Receivable |
Additional Paid-in Capital |
Treasury Stock | Deficit Accumulated During the Development Stage |
Accumulated Other Comprehensive Income |
Total Stockholders’ Equity (Deficiency) |
||||||||||||||||||||||||||||||
| Shares | Capital | Shares | Income | |||||||||||||||||||||||||||||||||
| Balance at February 4, 2005 (inception) |
— | $ | — | $ | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||||
| Issuance of common stock to founders |
1,475,137 | 1,475 | — | 3,825 | — | — | — | — | 5,300 | |||||||||||||||||||||||||||
| Issuance of common stock for services |
62,622 | 63 | — | 139,633 | — | — | — | — | 139,696 | |||||||||||||||||||||||||||
| Subscription receivable |
— | — | (200 | ) | — | — | — | — | — | (200 | ) | |||||||||||||||||||||||||
| Stock-based compensation resulting from restricted stock granted to employees |
139,164 | 139 | — | 6,818 | — | — | — | — | 6,957 | |||||||||||||||||||||||||||
| Shares issued with convertible debt |
12,500 | 12 | — | 27,846 | — | — | — | — | 27,858 | |||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (74,162 | ) | — | — | — | — | (74,162 | ) | |||||||||||||||||||||||||
| Accretion of preferred issuance cost |
— | — | — | (1,097 | ) | — | — | — | — | (1,097 | ) | |||||||||||||||||||||||||
| Beneficial conversion—Series A |
— | — | — | 187,250 | — | — | — | — | 187,250 | |||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (1,406,620 | ) | — | (1,406,620 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2005 |
1,689,423 | 1,689 | (200 | ) | 290,113 | — | — | (1,406,620 | ) | — | (1,115,018 | ) | ||||||||||||||||||||||||
| Stock-based compensation resulting from stock options granted to employees and board of director |
13,916 | 14 | — | 265,158 | — | — | — | — | 265,172 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock granted to nonemployees |
108,547 | 109 | — | 257,508 | — | — | — | — | 257,617 | |||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (786,454 | ) | — | — | (865,571 | ) | — | (1,652,025 | ) | ||||||||||||||||||||||||
| Accretion of preferred issuance costs |
— | — | — | (26,325 | ) | — | — | — | — | (26,325 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (6,168,096 | ) | — | (6,168,096 | ) | ||||||||||||||||||||||||||
| Balance at December 31, 2006 |
1,811,886 | 1,812 | (200 | ) | — | — | — | (8,440,287 | ) | — | (8,438,675 | ) | ||||||||||||||||||||||||
| Stock-based compensation resulting from stock options granted to employees and board of director |
— | — | — | 1,114,977 | — | — | — | — | 1,114,977 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock granted to nonemployees |
— | — | — | 657,099 | — | — | — | — | 657,099 | |||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (1,828,581 | ) | — | — | — | — | (1,828,581 | ) | |||||||||||||||||||||||||
| Accretion of preferred issuance costs |
— | — | — | (28,062 | ) | — | — | — | — | (28,062 | ) | |||||||||||||||||||||||||
| Beneficial conversion—Series B |
— | — | — | 1,829,171 | — | — | (1,829,171 | ) | — | — | ||||||||||||||||||||||||||
| Subscription receivable |
— | — | 200 | — | — | — | — | — | 200 | |||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (20,194,242 | ) | — | (20,194,242 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2007 |
1,811,886 | 1,812 | — | 1,744,604 | — | — | (30,463,700 | ) | — | (28,717,284 | ) | |||||||||||||||||||||||||
F-5
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Stockholders’ Equity (Deficiency)—(Continued)
Period From February 4, 2005 (Inception) to December 31, 2010
| Common Stock | Subscription Receivable |
Additional Paid-in Capital |
Treasury Stock | Deficit Accumulated During the Development Stage |
Accumulated Other Comprehensive Income |
Total Stockholders’ Equity (Deficiency) |
||||||||||||||||||||||||||||||
| Shares | Capital | Shares | Income | |||||||||||||||||||||||||||||||||
| Balance at December 31, 2007 |
1,811,886 | $ | 1,812 | $ | — | $ | 1,744,604 | — | $ | — | $ | (30,463,700 | ) | $ | — | $ | (28,717,284 | ) | ||||||||||||||||||
| Stock-based compensation resulting from stock options granted to employees and board of director |
— | — | — | 1,055,068 | — | — | — | — | 1,055,068 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock granted to nonemployees |
— | — | — | 192,624 | — | — | — | — | 192,624 | |||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (3,079,313 | ) | — | — | — | — | (3,079,313 | ) | |||||||||||||||||||||||||
| Accretion of preferred issuance costs |
— | — | — | (36,748 | ) | — | — | — | — | (36,748 | ) | |||||||||||||||||||||||||
| Beneficial conversion—Series B |
— | — | — | 3,162,610 | — | — | (3,162,610 | ) | — | — | ||||||||||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | 103,757 | (373 | ) | — | — | (373 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (25,035,035 | ) | — | (25,035,035 | ) | |||||||||||||||||||||||||
| Balance at December 31, 2008 |
1,811,886 | 1,812 | — | 3,038,845 | 103,757 | (373 | ) | (58,661,345 | ) | — | (55,621,061 | ) | ||||||||||||||||||||||||
| Stock-based compensation resulting from stock options granted to employees and board of director |
— | — | — | 876,540 | — | — | — | — | 876,540 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock granted to nonemployees |
— | — | — | 56,529 | — | — | — | — | 56,529 | |||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (3,286,654 | ) | — | — | — | — | (3,286,654 | ) | |||||||||||||||||||||||||
| Accretion of preferred issuance costs |
— | — | — | (36,746 | ) | — | — | — | — | (36,746 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | (12,196,002 | ) | — | (12,196,002 | ) | |||||||||||||||||||||||||
| Change in unrealized gain on available-for-sale securities |
— | — | — | — | — | — | — | 80,759 | 80,759 | |||||||||||||||||||||||||||
| Comprehensive loss |
— | — | — | — | — | — | — | — | (12,115,243 | ) | ||||||||||||||||||||||||||
| Balance at December 31, 2009 |
1,811,886 | 1,812 | — | 648,514 | 103,757 | (373 | ) | (70,857,347 | ) | 80,759 | (70,126,635 | ) | ||||||||||||||||||||||||
| Issuance of common stock, IPO |
5,750,000 | 5,750 | — | 48,671,178 | — | — | — | — | 48,676,928 | |||||||||||||||||||||||||||
| Issuance of common stock, options exercised |
38,307 | 38 | (90,735 | ) | 90,697 | — | — | — | — | — | ||||||||||||||||||||||||||
| Issuance of common stock- conversion of convertible notes |
3,093,472 | 3,093 | — | 23,507,808 | — | — | — | — | 23,510,901 | |||||||||||||||||||||||||||
| Issuance of common stock—conversion of preferred stock |
7,051,635 | 7,051 | — | 52,911,777 | — | — | — | — | 52,918,828 | |||||||||||||||||||||||||||
| Reclass of warrant liability |
— | — | — | 983,342 | — | — | — | — | 983,342 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock options granted to employees and board of director |
— | — | — | 1,824,684 | — | — | — | — | 1,824,684 | |||||||||||||||||||||||||||
| Stock-based compensation resulting from stock granted to nonemployees |
— | — | — | 12,966 | — | — | — | — | 12,966 | |||||||||||||||||||||||||||
| Beneficial conversion—convertible notes |
— | — | — | 5,877,598 | — | — | (5,877,598 | ) | — | — | ||||||||||||||||||||||||||
| Accretion of preferred stock dividend |
— | — | — | (2,873,300 | ) | — | — | — | — | (2,873,300 | ) | |||||||||||||||||||||||||
| Accretion of preferred stock issuance costs |
— | — | — | (105,459 | ) | — | — | — | — | (105,459 | ) | |||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | (14,253,841 | ) | — | (14,253,841 | ) | ||||||||||||||||||||||||||
| Change in unrealized gain on available for sale securities |
— | — | — | — | — | — | — | 510,000 | 510,000 | |||||||||||||||||||||||||||
| Comprehensive loss |
— | — | — | — | — | — | — | — | (13,743,841 | ) | ||||||||||||||||||||||||||
| Balance December 31, 2010 |
17,745,300 | $ | 17,744 | $ | (90,735 | ) | $ | 131,549,805 | 103,757 | $ | (373 | ) | $ | (90,988,786 | ) | $ | 590,759 | $ | 41,078,414 | |||||||||||||||||
See accompanying notes.
F-6
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Cash Flows
| Year Ended December 31, | Period From February 4, 2005 (Inception) to December 31, |
|||||||||||||||
| 2008 | 2009 | 2010 | 2010 | |||||||||||||
| Operating activities |
||||||||||||||||
| Net loss |
$ | (25,035,035 | ) | $ | (12,196,002 | ) | $ | (14,253,841 | ) | $ | (79,253,837 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Depreciation and amortization |
24,185 | 28,389 | 9,984 | 87,435 | ||||||||||||
| Noncash stock-based compensation |
1,247,692 | 933,069 | 1,837,650 | 6,460,889 | ||||||||||||
| Noncash interest expense |
477,302 | 1,340,945 | 1,919,100 | 4,189,616 | ||||||||||||
| Mark to market of warrant liability |
(90,730 | ) | 174,425 | 416,267 | 262,741 | |||||||||||
| Impairment loss on investments in securities |
1,665,307 | — | 30,000 | 2,465,759 | ||||||||||||
| Changes in operating assets and liabilities: |
||||||||||||||||
| Prepaid expenses and other assets |
988,022 | (221,232 | ) | (466,085 | ) | (1,539,055 | ) | |||||||||
| Accounts payable |
(744,293 | ) | (425,001 | ) | 1,729,715 | 2,133,321 | ||||||||||
| Accrued compensation |
221,202 | (387,798 | ) | 149,495 | 492,938 | |||||||||||
| Other accrued liabilities |
(744,008 | ) | 220,946 | 128,241 | 1,554,938 | |||||||||||
| Net cash used in operating activities |
(21,990,356 | ) | (10,532,259 | ) | (8,499,474 | ) | (63,145,255 | ) | ||||||||
| Investing activities |
||||||||||||||||
| Purchases of property and equipment |
— | — | (11,724 | ) | (103,906 | ) | ||||||||||
| Purchases of marketable securities |
— | — | — | (24,713,000 | ) | |||||||||||
| Sales of marketable securities |
— | — | — | 21,713,000 | ||||||||||||
| Net cash used in investing activities |
— | — | (11,724 | ) | (3,103,906 | ) | ||||||||||
| Financing activities |
||||||||||||||||
| Debt extinguishment fee |
— | — | (525,000 | ) | (525,000 | ) | ||||||||||
| Proceeds from issuances of convertible notes |
8,814,761 | 4,999,946 | 7,499,935 | 22,063,642 | ||||||||||||
| Proceeds from issuances of notes payable |
7,525,000 | — | — | 17,525,000 | ||||||||||||
| Payment of notes payable |
(9,737,362 | ) | (2,043,539 | ) | (5,481,460 | ) | (17,527,534 | ) | ||||||||
| Payment of subscription receivable |
— | — | — | 200 | ||||||||||||
| Proceeds from issuances of common stock |
— | — | 50,801,250 | 50,805,390 | ||||||||||||
| Deferred financing fees |
— | — | (1,111,756 | ) | (1,111,756 | ) | ||||||||||
| Proceeds from issuances of preferred stock, net |
— | — | — | 39,120,489 | ||||||||||||
| Repurchase of common stock |
(373 | ) | — | — | (373 | ) | ||||||||||
| Net cash provided by financing activities |
6,602,026 | 2,956,407 | 51,182,969 | 110,350,058 | ||||||||||||
| Net increase (decrease) in cash and cash equivalents |
(15,388,330 | ) | (7,575,852 | ) | 42,671,771 | 44,100,897 | ||||||||||
| Cash and cash equivalents, beginning of period |
24,393,308 | 9,004,978 | 1,429,126 | — | ||||||||||||
| Cash and cash equivalents, end of period |
$ | 9,004,978 | $ | 1,429,126 | $ | 44,100,897 | $ | 44,100,897 | ||||||||
| Supplemental disclosures of cash flow information |
||||||||||||||||
| Accretion of preferred stock dividends and issuance costs |
$ | 3,116,061 | $ | 3,323,400 | $ | 2,978,759 | $ | 13,028,473 | ||||||||
| Warrant issued with notes payable |
$ | — | $ | — | $ | — | $ | 720,600 | ||||||||
| Series B redeemable preferred stock beneficial conversion |
$ | 3,162,610 | $ | — | $ | — | $ | 4,991,781 | ||||||||
| Cash paid interest expense |
$ | 649,671 | $ | 742,263 | $ | 484,774 | $ | 2,708,213 | ||||||||
| Warrant liability reclass to additional paid in capital |
$ | — | $ | — | $ | 983,341 | $ | 983,341 | ||||||||
| Deemed dividend—convertible notes beneficial conversion option |
$ | — | $ | — | $ | 5,877,598 | $ | 5,877,598 | ||||||||
F-7
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements
December 31, 2010
| 1. | Organization |
Aegerion Pharmaceuticals, Inc. (the “Company” or “Aegerion”) is an emerging biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat severe lipid disorders. The Company is in its development stage as defined by the FASB Accounting Standards Codification (“ASC”) 915, Development Stage Entities. The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital, establishing facilities and performing research and development activities. Since inception, the Company has incurred significant losses from operations and expects losses to continue for the foreseeable future during its development phase. The Company’s success depends primarily on the successful development and regulatory approval of its product candidates. From February 4, 2005 (inception) through December 31, 2010, the Company had a deficit accumulated during the development stage of $91.0 million. Also, at December 31, 2010, the Company’s current assets totaled approximately $44.6 million compared to current liabilities of $4.7 million resulting in a working capital of $39.9 million. This increase in working capital is a result of the successful initial public offering (“IPO”) in October 2010. The Company believes that its existing cash and cash equivalents will be sufficient to cover its cash flow requirements through the first half of 2012.
| 2. | Summary of Significant Accounting Principles |
Basis of Presentation
The accompanying financial statements have been prepared in accordance with U.S. generally accepted accounting principles (“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position for the periods presented.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist of highly liquid instruments purchased with an original maturity of three months or less.
Investments in Securities
The Company’s investments primarily consist of auction rate securities which are variable-rate debt securities and an investment in preferred stock (see Note 6). These investments are classified as available-for-sale since it is the Company’s intent to sell the securities when a market opportunity is available. They are reported at fair value on the Company’s balance sheet. Unrealized gains and losses on these investments are reported within accumulated other comprehensive income/(loss) as a separate component of stockholders’ equity (deficiency). If a decline in the fair value of a marketable security below the Company’s cost basis is determined to be other than temporary, such marketable security is written down to its estimated fair value as a new cost basis and the amount of the write-down is included in earnings as an impairment charge.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is provided over the estimated useful lives of the respective assets, which range from three to seven years, using the straight-line method.
F-8
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
Deferred Financing Costs
Deferred financing costs include costs directly attributable to the Company’s offering of its equity securities and its debt financing. These costs are deferred and are capitalized as part of other assets. Costs attributable to equity offerings are charged against the proceeds of the offering once completed. Costs attributable to debt financing are deferred and amortized over the term of the financing.
Long-Lived Assets
Long-lived assets to be held and used are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. Long-lived assets to be disposed are reported at the lower of the carrying amount or fair value less cost to sell.
Research and Development Costs
Research and development costs are comprised primarily of costs for personnel, including salaries and benefits; clinical studies performed by third parties; materials and supplies to support the Company’s clinical programs; contracted research; manufacturing; related consulting arrangements; costs related to upfront and milestone payments under license agreements; and other expenses incurred to sustain the Company’s overall research and development programs. Internal research and development costs are expensed as incurred. Third-party research and development costs are expensed at the earlier of when the contracted work has been performed or as upfront and milestone payments are made. Clinical trial expenses require certain estimates. The Company estimates these costs based upon a cost per patient that varies depending on the site of the clinical trial.
Concentration of Credit Risk
The Company’s financial instruments that are exposed to credit risks consist primarily of cash and cash equivalents and available for sale investment securities. The Company maintains its cash and cash equivalents in bank accounts, which, at times, exceed federally insured limits.
Cumulative Redeemable Convertible Preferred Stock
The carrying value of the redeemable convertible preferred stock was increased by periodic accretions and annual dividends so that the carrying amount equals the redemption amount at the earliest redemption date. Historically, these increases were charged to additional paid-in-capital until that balance was reduced to zero, and any remaining accretions were charged to deficit accumulated during the development stage. All of the convertible preferred stock were converted to common stock at the time of the IPO.
Income Taxes
The Company uses the liability method to account for income taxes, including the recognition of deferred tax assets and deferred tax liabilities for the anticipated future tax consequences attributable to differences between financial statements amounts and their respective tax bases. The Company reviews its deferred tax assets for recovery. A valuation allowance is established when the Company believes that it is more likely than not that its deferred tax assets will not be realized. Changes in valuation allowances from period to period are included in the Company’s tax provision in the period of change.
F-9
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
On January 1, 2009, the Company adopted the authoritative guidance under ASC 740-10, Accounting for Uncertainty in Income Taxes. This authoritative interpretation clarified and standardized the manner by which companies are required to account for uncertain income tax positions. Under this guidance, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not to be sustained upon examination based on the technical merits of the position. The amount of the accrual for which an exposure exists is measured as the largest amount of benefit determined on a cumulative probability basis that the Company believes is more likely than not to be realized upon ultimate settlement of the position. In the event the Company recognizes any interest or penalties, related to uncertain tax positions, the accounting policy of the Company is to record the interest or penalty in the financial statements as interest expense. The Company did not incur any interest or penalties related to income tax during the years ended December 31, 2010 and 2009. Tax returns for all years 2005 and thereafter are subject to future examination by tax authorities.
Beneficial Conversion Charges
When the Company issues debt or equity securities that are convertible into capital stock at a discount from the fair value of the capital stock at the date the debt or equity financing is committed, a beneficial conversion charge is measured as the difference between the fair value and the conversion price at the commitment date. The beneficial conversion charge is presented as a discount or reduction to the related debt security or as an immediate charge to earnings, with an offsetting credit to increase additional paid-in-capital. A beneficial conversion charge was recognized at the time of the IPO and represents a discount on the fair value of the common stock of the Company purchased by the convertible noteholders. See Note 11 for further information about the beneficial conversion option.
Stock-Based Compensation
The Company accounts for its stock-based compensation in accordance with ASC 718, Compensation-Stock Compensation. Compensation cost is recognized for all stock-based payments granted and is based on the grant-date fair value estimated using the Black-Scholes option pricing model. The equity instrument is not considered to be issued until the instrument vests. As a result, compensation cost is recognized over the requisite service period with an offsetting credit to additional paid-in-capital. See Note 14 for further information about the Company’s stock option plans.
The Company has from time to time modified the price of its stock options to employees and directors. The Company accounts for the incremental value of the modified option based on the excess of the fair value of the modified award over the fair value of the original option measured immediately before its terms are modified based on current circumstances. That is, the value of the original (pre-modification) option is estimated based on current assumptions, without regard to the assumptions made on the grant date. The incremental value calculated for vested options is charged to expense immediately, while the incremental value associated with unvested options is added to unrecognized compensation costs and recorded in earnings over the remaining vesting period of the award.
The modifications made to the Company’s equity awards did not result in significant incremental compensation costs, either individually or in the aggregate.
Comprehensive Loss
Comprehensive loss for the Company includes net loss and the change in net unrealized gains and losses on available-for-sale securities.
F-10
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
Segment Information
The Company currently operates in one business segment focusing on the development and commercialization of its lead compound. The Company is not organized by market and is managed and operated as one business. A single management team reports to the chief operating decision maker who comprehensively manages the entire business. The Company does not operate any separate lines of business or separate business entities with respect to its products. Accordingly, the Company does not accumulate discrete financial information with respect to separate service lines and does not have separately reportable segments.
| 3. | Recent Accounting Pronouncements |
In December 2010, the FASB issued FASB Accounting Standards Update 2010-27, Other Expenses (Topic 720): Fees Paid to the Federal Government by Pharmaceutical Manufacturers. This update addresses questions concerning how pharmaceutical manufacturers should recognize and classify in the income statement fees mandated by the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act (the “Acts”). The Acts impose an annual fee on the pharmaceutical manufacturing industry for each calendar year beginning on or after January 1, 2011. An entity’s portion of the annual fee is payable no later than September 30 of the applicable calendar year and is not tax deductible. The annual fee is payable to the U.S. Treasury once a pharmaceutical manufacturing entity has a gross receipt from a branded prescription drug sale to any specified government program or in accordance with coverage under any government program for each calendar year beginning on or after January 1, 2011. Management does not believe that this update will have any effect on the accompanying financial statements as the Company is still in the development stage and does not have any sales. Management will continue to evaluate any subsequent effects on the financial statements.
In January 2010, the FASB issued Accounting Standards Update 2010-06, Fair Value Measurements and Disclosures (Subtopic 820). This update requires new disclosures as follows:
| § | Transfers in and out of Levels 1 and 2. Companies should disclose separately the amounts of significant transfers in and out of Levels 1 and 2 fair value measurements and describe the reasons for the transfers. |
| § | Activity in Level 3 fair value measurements. In the reconciliation for fair value measurements using significant unobservable inputs, Level 3, a reporting entity should present separately information about purchases, sales, issuances, and settlements (gross basis vs. one net number). |
This update also clarifies the following:
| § | Level of disaggregation. Companies should provide fair value measurement disclosures for each class of assets and liabilities. A class is often a subset of assets or liabilities within a line item in the statement of financial position. Judgment needs to be used to determine the appropriate classes of assets and liabilities. |
| § | Disclosures about inputs and valuation techniques. Companies should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements for Level 2 or Level 3 inputs. |
The Company’s financial statements include the disclosures required by this update.
F-11
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
| 4. | Prepaid Expenses and Other Current Assets |
The following summarizes assets classified as prepaid expenses and other current assets at December 31, 2009 and 2010:
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| Prepaid Insurance |
$ | 9,948 | $ | 371,370 | ||||
| Debt extinguishment fee on notes payable |
344,308 | — | ||||||
| Deferred financing costs—convertible notes |
178,154 | — | ||||||
| Other prepaid expenses |
3,511 | 133,374 | ||||||
| Total |
$ | 535,921 | $ | 504,744 | ||||
| 5. | Property and Equipment |
Property and equipment consist of the following:
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| Computer and office equipment |
$ | 62,789 | $ | 73,317 | ||||
| Office furniture and equipment |
19,083 | 20,279 | ||||||
| 81,872 | 93,596 | |||||||
| Less accumulated depreciation and amortization |
(67,141 | ) | (77,125 | ) | ||||
| Property and equipment, net |
$ | 14,731 | $ | 16,471 | ||||
Depreciation expense was $24,185, $28,389 and $9,984 for the years ended December 31, 2008, 2009 and 2010, respectively.
| 6. | Investments in Securities |
The following is a summary of investments held by the Company as of December 31, 2009 and 2010:
| Adjusted Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
December 31, 2009 |
|||||||||||||
| Auction rate securities |
$ | 414,241 | $ | 80,759 | $ | — | $ | 495,000 | ||||||||
| Auction rate securities converted to preferred stock |
150,000 | — | — | 150,000 | ||||||||||||
| Total |
$ | 564,241 | $ | 80,759 | $ | — | $ | 645,000 | ||||||||
| Adjusted Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value at December 31, 2010 |
|||||||||||||
| Auction rate securities |
$ | 414,241 | $ | 530,759 | $ | — | $ | 945,000 | ||||||||
| Auction rate securities converted to preferred stock |
120,000 | 60,000 | — | 180,000 | ||||||||||||
| Total |
$ | 534,241 | $ | 590,759 | $ | — | $ | 1,125,000 | ||||||||
In 2007, the Company invested a portion of its cash in investments consisting primarily of investment-grade, asset-backed, variable-rate debt obligations and auction rate securities (“ARS”) as outlined in the Company’s investment policy, which were classified as available-for-sale securities and were reported in the balance sheet at fair value. ARS are variable-rate debt securities that do not mature in the near term, with the interest rate being reset through Dutch auctions that are typically held every 7, 28 or 35 days. The securities trade at par and are
F-12
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
callable at par on any interest payment date at the option of the issuer. Interest is paid at the end of each auction period. During the fourth quarter of 2007, the Company liquidated approximately $8 million of ARS at par value and held the remaining $3 million of ARS. Shortly thereafter the Company’s ARS experienced a failed auction, meaning that there were no buyers willing to purchase the securities at par. Pursuant to the terms of the ARS agreement, in the event of a failed auction in which the holders cannot sell the securities, the interest or dividend rate on the security resets to a “penalty” rate. As a result of the failed auctions, the Company does not believe the ARS are currently liquid. The securities for which the auctions have failed will continue to accrue interest at the contractual penalty rate and will continue to be auctioned every 28 days until the auction succeeds, the issuer calls the securities or the securities mature.
In December 2008, one of the Company’s ARS was subject to a put option, which converted the security into non-cumulative redeemable perpetual preferred stock. The Company’s investment in the underlying debt obligation matures in 2021. In March 2010, the Company was notified that the issuer of the non-cumulative redeemable perpetual preferred stock would not pay dividends as a result of having reported an earned surplus deficit. The Company determined the decline in fair value of its non-cumulative redeemable perpetual preferred stock for the year ended December 31, 2010 to be other than temporary and recorded an impairment charge of $30,000, which is included in net loss.
Subsequent declines in fair value will also be evaluated as to whether they are other than temporary. Given the continued lack of liquidity associated with these investments, the investments are not reasonably available for current operations, as such, the Company classifies these investments as long-term investments in the financial statements.
| 7. | Fair Value of Financial Instruments |
The Company’s financial instruments consist of cash and cash equivalents, securities, accounts payable, accrued liabilities, notes payable, convertible notes and warrant liability. Fair value estimates of these instruments are made at a specific point in time, based on relevant market information. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. The carrying amount of cash and cash equivalents, accounts payable, accrued liabilities, notes payable and convertible notes are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. The fair value of the Company’s investments in securities is based upon observable and unobservable inputs.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. Assets and liabilities that are measured at fair value are reported using a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| § | Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. |
| § | Level 2—Inputs other than quoted prices in active markets that are observable for the asset or liability, either directly or indirectly. |
| § | Level 3—Inputs that are unobservable for the asset or liability. |
As of December 31, 2010, the Company held par value $3.0 million of investments in ARS. The Company’s investments in securities are classified within Level 2 and 3 of the fair value hierarchy using inputs that are
F-13
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
observable or unobservable for the asset or liability. The fair value measurements of the Company’s investments in securities and warrant liability at December 31, 2009 and 2010 are summarized in the tables below:
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other |
Significant Unobservable Inputs (Level 3) |
Balance at December 31, 2009 |
|||||||||||||
| Assets: |
||||||||||||||||
| Auction rate securities |
$ | — | $ | — | $ | 495,000 | $ | 495,000 | ||||||||
| Auction rate securities converted to preferred stock |
— | 150,000 | — | 150,000 | ||||||||||||
| Total assets at fair value |
$ | — | $ | 150,000 | $ | 495,000 | $ | 645,000 | ||||||||
| Liabilities: |
||||||||||||||||
| Warrant |
$ | — | $ | — | $ | 567,074 | $ | 567,074 | ||||||||
| Total liabilities at fair value |
$ | — | $ | — | $ | 567,074 | $ | 567,074 | ||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at December 31, 2010 |
|||||||||||||
| Assets: |
||||||||||||||||
| Auction rate securities |
$ | — | $ | — | $ | 945,000 | $ | 945,000 | ||||||||
| Auction rate securities converted to preferred stock |
— | 180,000 | — | 180,000 | ||||||||||||
| Total assets at fair value |
$ | — | $ | 180,000 | $ | 945,000 | $ | 1,125,000 | ||||||||
The changes in fair value of the Company’s Level 3 financial instruments during the years ended December 31, 2008, 2009 and 2010 were as follows:
| Level 3 | ||||||||
| Auction rate securities |
Warrant liability |
|||||||
| Balance at December 31, 2007 |
$ | 1,434,000 | $ | 483,379 | ||||
| Other-than-temporary impairment included in net loss |
(1,019,759 | ) | — | |||||
| Fair value adjustment included in net loss |
— | (90,730 | ) | |||||
| Balance at December 31, 2008 |
$ | 414,241 | $ | 392,649 | ||||
| Unrealized gain recorded in other comprehensive loss |
80,759 | — | ||||||
| Fair value adjustment included in net loss |
— | 174,425 | ||||||
| Balance at December 31, 2009 |
$ | 495,000 | $ | 567,074 | ||||
| Unrealized gain recorded in other comprehensive loss |
450,000 | — | ||||||
| Fair value adjustment included in net loss |
— | 416,267 | ||||||
| Reclassification of warrant to additional paid-in-capital |
— | (983,341 | ) | |||||
| Balance at December 31, 2010 |
$ | 945,000 | $ | — | ||||
The estimated fair value of the ARS is derived through discounted cash flows, a Level 3 input. The Company’s discounted cash flow analysis considered, among other things, the quality of the underlying collateral, the credit rating of the issuer, an estimate of when these securities are either expected to have a successful auction or otherwise return to par value, the expected interest income to be received over this period, and the estimated required rate of return for investors that may be willing to purchase such a security. The Company also
F-14
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
considered third-party valuations, to the extent available, and similar securities priced in the marketplace when arriving at the estimated fair value. At December 31, 2010, the Company’s auction rate securities were reflected at 63% of par value.
The estimated fair value of the Company’s ARS that was converted to preferred stock is estimated by analyzing similar securities in the marketplace, a Level 2 input. The Company also considers the fair value of the underlying collateral and credit rating of the issuer. Based on observed settlements of similar securities, the preferred stock was recorded at 12% of par value at December 31, 2010.
Warrant Liability/Equity Instrument
The warrant liability outstanding as of December 31, 2009 related to the warrant instrument held by Hercules Technology Growth Capital, Inc., or Hercules, to purchase an aggregate of 387,238 shares of series A redeemable convertible preferred stock at an exercise price of $1.86 per share. In accordance with its terms, the instrument became an equity warrant to purchase 107,779 shares of common stock at an exercise price of $6.68 per share upon the closing of the IPO. The warrant is exercisable over a period of 10 years from the date of issuance and had a remaining life of 6.4 years at October 27, 2010. The estimated fair value of the Company’s warrant was determined using a Black-Scholes option pricing model, a Level 3 input. The significant assumptions used in estimating the fair value of the Company’s warrant liability as of October 27, 2010 included the strike price $6.68, estimate for volatility 150%, risk free interest rate 2.3%, fair value of the common stock $9.50 and the estimated life of the warrant 6.4 years. The Company accounts for its warrant in accordance with ASC 480-10, Distinguishing Liabilities from Equity, which requires that a financial instrument, other than an outstanding share, that, at inception, is indexed to an obligation to repurchase the issuer’s equity shares, regardless of the timing or likelihood of the redemption shall be classified as a liability. Any modification to the warrant liability was recorded in earnings during the period of the modification. On October 27, 2010, the date of the IPO, all of the shares of preferred stock were converted to shares of common stock. The Company revalued the warrant based upon the fair value of the common stock upon the closing of the IPO, and subsequently reclassified the warrant from liability to equity. The warrant has a remaining life of 6.25 years at December 31, 2010.
| 8. | Other Accrued Liabilities |
Other accrued liabilities consist of the following:
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| Accrued research and development costs |
$ | 732,838 | $ | 933,339 | ||||
| Accrued legal |
17,669 | 20,717 | ||||||
| Other accrued liabilities |
151,189 | 75,817 | ||||||
| Accrued financing fees |
525,000 | — | ||||||
| Total |
$ | 1,426,696 | $ | 1,029,873 | ||||
F-15
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
| 9. | Commitments |
Leases
The Company leased certain office facilities and office equipment under operating leases during the year ended December 31, 2010. The future minimum payments for all noncancelable operating leases as of December 31, 2010 are as follows:
| Year Ending December 31: |
||||
| 2011 |
$ | 544,027 | ||
| 2012 |
530,847 | |||
| 2013 |
569,212 | |||
| 2014 |
590,095 | |||
| Thereafter |
1,027,690 | |||
| Total |
$ | 3,261,871 | ||
Rent expense under operating leases was approximately $144,000 for the years ended December 31, 2008 and 2009, and $175,000 for the year ended December 31, 2010.
On October 15, 2010 the Company entered into an agreement with Symphony Suites, as amended, to provide offices in Morristown New Jersey for the period of October 15, 2010 to March 31, 2011 at an initial rate of $18,361 per month increasing to $25,706 per month as of February 2011.
On November 24, 2010, the Company entered into a lease with S/K One Bed Associates, LLC to lease office space for the Company’s office in Bedminster, New Jersey. The lease provides for an initial base rent of $11,904 per month plus certain operating expenses and taxes beginning on April 1, 2011, and shall increase on an annual basis beginning in January 2012.
On January 1, 2011, the Company entered into a lease with RREEF America REIT II CORP. PPP to lease office space for the Company’s corporate headquarters in Cambridge, Massachusetts. The lease provides for an initial base rent of $30,594 per month, plus certain operating expenses and taxes, and shall increase on an annual basis beginning in January 2012.
Other Commitments
Under the Company’s license agreements, the Company could be required to pay up to a total of approximately $15.0 million upon achieving certain milestones, such as the initiation of clinical trials or the granting of patents. From inception through December 31, 2010, the Company has paid $2,446,250 in milestone payments resulting from the execution of certain agreements, patent approvals and the initiation of U.S. clinical trials. Milestone payments will also be due upon the first administration to a subject using licensed technology in a Phase III clinical trial and FDA approvals in addition to sales milestones and royalties payable on commercial sales if any occur.
University of Pennsylvania Licensing Agreements
In May 2006, the Company entered into a license agreement with The Trustees of the University of Pennsylvania (“UPenn”) pursuant to which it obtained an exclusive, worldwide license from UPenn to certain know-how and a range of patent rights applicable to the Company’s lead compound, lomitapide. In particular, the Company obtained a license to certain patent and patent applications owned by UPenn relating to the dosing of micrososmal triglyceride transfer protein inhibitors, including lomitapide and certain patents and patent
F-16
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
applications and know-how covering the composition of matter of lomitapide that were assigned to UPenn by Bristol-Myers Squibb Company (“BMS”) in the field of monotherapy or in combination with other dyslipidemic therapies, which are therapies for the treatment of patients, with abnormally high or low levels of plasma cholesterol or triglycerides.
To the extent that rights under the BMS-UPenn assigned patents were not licensed to the Company under its license agreement with UPenn or were retained by UPenn for non-commercial educational and research purposes, those rights, other than with respect to lomitapide, were licensed by UPenn back to BMS on an exclusive basis pursuant to a technology donation agreement between UPenn and BMS. In the technology donation agreement, BMS agreed not to develop or commercialize any compound, including lomitapide, covered by the composition of matter patents included in the BMS-UPenn assigned patents in the field licensed to the Company exclusively by UPenn. Through the Company’s license with UPenn, as provided in the technology donation agreement, it has the exclusive right with respect to the BMS-UPenn assigned patents regarding their enforcement and prosecution in the field licensed exclusively to the Company by UPenn.
The license from UPenn covers, among other things, the development and commercialization of lomitapide alone or in combination with other active ingredients in the licensed field. The license is subject to customary noncommercial rights retained by UPenn for non-commercial educational and research purposes. The Company may grant sublicenses under the license, subject to certain limitations.
The Company is obligated under this license agreement to use commercially reasonable efforts to develop, commercialize, market and sell at least one product covered by the licensed patent rights, such as lomitapide. Pursuant to this license agreement, the Company paid UPenn a one-time license initiation fee of $56,250, which was included in research and development expense in 2005. The Company will be required to make development milestone payments to UPenn of up to an aggregate of $150,000 when a licensed product’s indication is limited to homozygous familial hypercholesterolemia or severe refractory hypercholesterolemia, and an aggregate of $2,550,000 for all other indications within the licensed field. All such development milestone payments for these other indications are payable only once, no matter how many licensed products for these other indications are developed. In addition, the Company will be required to make specified royalty payments on net sales of products covered by the license (subject to a variety of customary reductions) and share with UPenn specified percentages of sublicensing royalties and other consideration that the Company receives under any sublicenses that it may grant.
This license agreement will remain in effect on a country-by-country basis until the expiration of the last-to-expire licensed patent right in the applicable country. The Company has the right to terminate this license agreement for UPenn’s uncured material breach of the license agreement or for convenience upon 60 days’ prior written notice to UPenn, subject to certain specific conditions and consequences. UPenn may terminate this license agreement for the Company’s uncured material breach of the license agreement, its uncured failure to make payments to UPenn or if the Company is the subject of specified bankruptcy or liquidation events.
Bayer Licensing Agreements
In May 2006, the Company entered into a license agreement with Bayer Healthcare AG (“Bayer”), pursuant to which it obtained an exclusive, worldwide license from Bayer under the patent rights and know-how owned or controlled by Bayer applicable to implitapide. This license covers the development and commercialization of implitapide alone or in combination with other active ingredients. The Company may grant sublicenses under the license, subject to certain limitations. Pursuant to this license agreement, the Company granted Bayer a first right of negotiation in the event the Company desires to commercialize any licensed products through or with a third
F-17
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
party, which expires after 90 days if the parties are unable to come to an agreement. This first right of negotiation applies only to the specific products and in the specific countries potentially subject to third-party commercialization.
The Company is obligated under this license agreement to use commercially reasonable efforts to develop and commercialize, at its cost, at least one licensed product, such as implitapide. Pursuant to this license agreement, the Company paid Bayer a one-time initial license fee of $750,000, which was included in research and development expense in 2006. The Company is required to make certain development milestone payments of up to an aggregate of $4,375,000 upon the achievement of certain development milestones. Each development milestone payment is payable only once, no matter how many licensed products are developed. In addition, the Company is required to make annual payments for 2007 through 2011, which are nonrefundable when paid, totaling $1,000,000 in the aggregate, with each annual payment creditable in full against any development milestones that are or become payable, a one-time commercialization milestone payment of $5,000,000 if the Company achieves aggregate net sales of licensed products of $250,000,000 (payable only once, no matter how many licensed products are commercialized), and specified royalty payments on net sales of licensed products (subject to a variety of deductions).
This license agreement will remain in effect, and royalties will be payable on sales of licensed products, on a country-by-country and product-by-product basis until the later of the expiration of the last-to-expire licensed patent right in the applicable country or ten years after the first commercial sale of a licensed product in that country. This license agreement may be terminated by either party for the other party’s uncured material breach of this license agreement, except that Bayer may only terminate the license agreement for the Company’s material breach on a country-by-country or product-by-product basis if the Company’s breach is reasonably specific to one or more countries or licensed products, or if the other party becomes the subject of a specified bankruptcy or liquidation event. The Company has the right to terminate the license agreement for convenience upon 60 days’ prior written notice to Bayer.
The Company has no material commitments for capital expenditures.
| 10. | Debt Financing |
In March 2007, the Company entered into a $15.0 million loan and security agreement with Hercules and borrowed $10.0 million under this agreement. In 2009, the Company classified the debt with Hercules as a current liability since the Company was in default of its insolvency covenant during 2009 and therefore the debt was callable by the lender as of December 31, 2009. On November 4, 2010, the Company repaid in full approximately $3.3 million of outstanding principal and interest under this loan and security agreement with Hercules.
| 11. | Convertible Notes and Beneficial Conversion Option |
During 2008, 2009, and 2010, the Company entered into several loan agreements with various financial institutions and individuals (“Purchasers”), whereby the Purchasers agreed to purchase, an aggregate principal amount of $21.3 million of senior subordinated convertible notes (“Notes”), subordinate only to certain loans made to the Company by Hercules as specified in the subordination agreement among the Purchasers, the Company and Hercules.
The Notes may not be prepaid in whole or in part without the written consent of the Purchasers. The interest on these Notes was 8.0% per annum and the maturity date was December 31, 2011.
F-18
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
In connection with the Notes, the Company provided to the purchasers a beneficial conversion option, which provided that immediately upon the closing of sales of the Company’s capital stock, in one transaction or series of related transactions, which sale or sales, including without limitation any initial public offering, that results in gross proceeds of at least $10 million, the Notes will automatically convert into such shares of newly issued capital stock. The purchasers were entitled to receive a number of shares determined by dividing the loan balance as of the conversion date by an amount equal to 85% of the price per share of the newly issued capital stock. In October 2010, the Company modified the conversion percentage to 80% of the share price. Upon the closing of the IPO, the outstanding principal and interest under these Notes were converted into 3,093,472 shares of common stock at a conversion price of $7.60 or 80% of the offering price. As a result, a beneficial conversion charge of approximately $5.9 million was recognized in additional paid-in- capital.
| 12. | Net Loss Attributable to Common Stockholders Per Share |
The Company previously determined that its series A and B redeemable convertible preferred stock represented participating securities since both securities participated equally with common stock in dividends and unallocated income. The series A and B redeemable convertible preferred stock were converted to common stock on October 27, 2010 upon the closing of the IPO.
Basic net loss per common share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period, less weighted-average unvested common shares subject to repurchase. Diluted net loss per common share is computed by dividing the net loss applicable to common stockholders by the weighted-average number of unrestricted shares of common stock and dilutive common stock equivalents outstanding for the period, determined using the treasury-stock method and the as if-converted method.
Net loss attributable to common stockholders for each period must be allocated to common stock and participating securities to the extent that the securities are required to share in the losses. The Company’s series A and B redeemable convertible preferred stock do not have a contractual obligation to share in losses of the Company. As a result, basic net loss per common share is calculated by dividing net loss by the weighted-average shares of common stock outstanding during the period.
The following table presents the computation of basic and diluted net loss per share of common stock:
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| Numerator: |
||||||||||||
| Net loss |
$ | (25,035,035 | ) | $ | (12,196,002 | ) | $ | (14,253,841 | ) | |||
| Accretion of preferred stock and other deemed dividends |
(6,241,923 | ) | (3,286,654 | ) | (8,750,897 | ) | ||||||
| Net loss attributable to common stockholders |
$ | (31,276,958 | ) | $ | (15,482,656 | ) | $ | (23,004,738 | ) | |||
| Denominator: |
||||||||||||
| Weighted-average common shares outstanding—basic and diluted |
1,495,375 | 1,656,732 | 4,537,407 | |||||||||
| Basic and diluted net loss per common share |
$ | (20.92 | ) | $ | (9.35 | ) | $ | (5.07 | ) | |||
F-19
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
The following table shows historical dilutive common share equivalents outstanding, which are not included in the above historical calculation, as the effect of their inclusion is anti-dilutive during each period.
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| Convertible preferred stock |
6,233,647 | 6,670,002 | — | |||||||||
| Unvested restricted stock |
93,322 | 6,784 | — | |||||||||
| Options |
451,641 | 355,690 | 1,715,813 | |||||||||
| Warrants |
33,042 | 107,779 | 107,779 | |||||||||
| Total |
6,811,652 | 7,140,255 | 1,823,592 | |||||||||
| 13. | Capital Structure |
Common Stock
At December 31, 2010, the Company was authorized to issue 125,000,000 shares of common stock.
Dividends on common stock will be paid when, and if, declared by the Board of Directors. Each holder of common stock is entitled to vote on all matters and is entitled to one vote for each share held. The Company will, at all times, reserve and keep available, out of its authorized but unissued shares of common stock, sufficient shares to affect the conversion of the shares for stock options. Common stock issued to the founders of the Company vests over a four-year period. Unvested shares are subject to repurchase by the Company at the original issuance price. As of December 31, 2009 and 2010, there were 6,784 and -0- shares, respectively, subject to repurchase at an aggregate price of approximately $24 and $0, respectively.
Initial Public Offering
In October 2010, the Company completed its initial public offering, and in November 2010, the underwriters exercised in full their overallotment option to purchase additional shares of common stock. Including the overallotment, a total of 5,750,000 shares were sold in the offering, resulting in net proceeds to the Company of approximately $48.7 million, net of underwriting discounts and commissions and estimated offering expenses.
Cumulative Convertible Redeemable Preferred Stock
The Company was authorized to issue 19,650,000 shares of preferred stock, of which 13,000,000 shares were designated Series A redeemable convertible preferred stock (“Series A”) and 6,650,000 shares were designated Series B redeemable convertible preferred stock (“Series B” and together with the Series A, the “Designated Preferred”). All of the convertible redeemable preferred stock were converted to common stock on October 27, 2010 upon the closing of the IPO.
Subsequent to the IPO, the Company was authorized to issue 5,000,000 shares of undesignated preferred stock, par value $0.001 per share.
Dividends
The prior holders of Designated Preferred were entitled to receive annual dividends at a rate of 7% of the original purchase price in advance of any distributions to common stockholders. Dividends were payable when, if and as, declared by the Board of Directors and were cumulative. No dividends were declared through October 27, 2010, the date the shares were converted to common stock. The Company, in 2009 and up to October 27, 2010 recorded, in accordance with its redemption value, accrued dividends of $3,079,313, $3,286,654 and $2,873,300 during 2008, 2009 and 2010, respectively.
F-20
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
Conversion
Designated Preferred stockholders were entitled to convert their shares plus all dividends accrued or declared but unpaid, up to and including the date of conversion into fully paid and nonassessable shares of common stock. The Series A were convertible into a number of shares of the Company’s common stock determined by dividing (1) the Series A per-share purchase price of $2.74 plus an accumulated dividend of 7% per year (which is calculated on a daily basis and compounded annually) by (2) a conversion price equal to $2.74 per share. Each share of Series B was convertible into a number of shares of its common stock determined by dividing (1) the Series B per-share purchase price of $4.62 plus an accumulated dividend of 7% per year (which is calculated on a daily basis and compounded annually) by (2) a conversion price equal to $4.62 per share. On October 27, 2010, the Company completed the IPO and the Designated Preferred converted into 7,051,635 shares of the Company’s common stock.
The following table summarizes preferred stock activity for the Company:
| Series A | Series B | |||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||
| Balance at February 4, 2005 (inception) |
— | $ | — | — | $ | — | ||||||||||
| Issuance of Series A redeemable convertible preferred |
12,211,604 | 22,519,880 | — | — | ||||||||||||
| Less financing fees |
— | (131,627 | ) | — | — | |||||||||||
| Accrued preferred dividends |
— | 74,162 | — | — | ||||||||||||
| Accretion of financing fees |
— | 1,097 | — | — | ||||||||||||
| Balance at December 31, 2005 |
12,211,604 | 22,463,512 | — | — | ||||||||||||
| Accrued preferred dividends |
— | 1,652,025 | — | — | ||||||||||||
| Accretion of financing fees |
— | 26,325 | — | — | ||||||||||||
| Balance at December 31, 2006 |
12,211,604 | 24,141,862 | — | — | ||||||||||||
| Issuance of Series B redeemable convertible preferred |
— | — | 3,810,773 | 17,605,773 | ||||||||||||
| Less financing fees |
— | — | — | (103,668 | ) | |||||||||||
| Accrued preferred dividends |
— | 1,649,629 | — | 178,952 | ||||||||||||
| Accretion of financing fees |
— | 24,606 | — | 3,456 | ||||||||||||
| Balance at December 31, 2007 |
12,211,604 | 25,816,097 | 3,810,773 | 17,684,513 | ||||||||||||
| Accrued preferred dividends |
— | 1,831,175 | — | 1,248,138 | ||||||||||||
| Accretion of financing fees |
— | 16,015 | — | 20,733 | ||||||||||||
| Balance at December 31, 2008 |
12,211,604 | 27,663,287 | 3,810,773 | 18,953,384 | ||||||||||||
| Accrued preferred dividends |
— | 1,954,354 | — | 1,332,300 | ||||||||||||
| Accretion of financing fees |
— | 16,015 | — | 20,732 | ||||||||||||
| Balance at December 31, 2009 |
12,211,604 | 29,633,656 | 3,810,773 | 20,306,416 | ||||||||||||
| Accrued preferred dividends |
— | 1,713,175 | — | 1,160,125 | ||||||||||||
| Accretion of financing fees |
— | 46,710 | — | 58,747 | ||||||||||||
| Balance at October 27, 2010 |
12,211,604 | 31,393,541 | 3,810,773 | 21,525,288 | ||||||||||||
| Conversion to common stock |
(12,211,604 | ) | (31,393,541 | ) | (3,810,773 | ) | (21,525,288 | ) | ||||||||
| Balance at December 31, 2010 |
— | $ | — | — | $ | — | ||||||||||
| 14. | Stock Option Plans |
The Company’s 2006 Stock Option and Grant Plan (the “2006 Plan”) was adopted by the Board of Directors in May 2006 and approved by the stockholders in June 2006. The 2006 Plan provides for the granting of options to purchase common stock in the Company to employees and consultants at a price not less than the estimated fair
F-21
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
value at the date of grant, or 110% of the estimated fair value at the date of grant if the optionee is a 10% owner of the Company. Under the provisions of the 2006 Plan, no option will have a term in excess of ten years.
The 2006 Plan was intended to encourage ownership of stock by employees and consultants of the Company and to provide additional incentives for them to promote the success of the Company’s business. The Board of Directors is responsible for determining the individuals that receive option grants, the number of options each individual will receive, the option price per share and the exercise period of each option. Options granted pursuant to the 2006 Plan generally vest over four years and have been granted at the estimated fair value of the Company’s common stock, as determined by the Board of Directors, as of each grant date. The 2006 Plan allows the option holders to exercise their options early, which are then subject to repurchase by the Company at the original exercise price of such options. No early exercise of options occurred through December 31, 2009. With the adoption of the 2010 plan, the Company will no longer be granting options from this plan.
The Company’s 2010 Stock Option and Incentive Plan (the “2010 Option”) Plan was adopted by the Board of Directors in September 2010, and approved by the stockholders in October 2010. The 2010 Option Plan allows the Company to make grants of incentive stock options, non-qualified stock options, stock appreciation rights, restricted stock awards, restricted stock units, unrestricted stock awards, cash-based awards, performance share awards and dividend equivalent rights.
The 2010 Option Plan may be administered by either the Compensation Committee or a similar committee of at least two non-employee directors who are independent or by the full Board of Directors, or the administrator. The administrator has full power and authority to select the participants to whom awards will be granted, to make any combination of awards to participants, to accelerate the exercisability or vesting of any award and to determine the specific terms and conditions of each award, subject to the provisions of the 2010 Option Plan.
All full-time and part-time officers, employees, non-employee directors and other key persons, including consultants and prospective employees, are eligible to participate in the 2010 Option Plan, subject to the sole discretion of the administrator. There are certain limits on the number of awards that may be granted under the 2010 Option Plan. For example, no more than 1,500,000 shares of common stock may be granted in the form of stock options or stock appreciation rights to any one individual during any one-calendar-year period.
The exercise price of stock options awarded under the 2010 Option Plan may not be less than the fair value of the Company’s common stock on the date of the option grant, and the term of each option may not exceed ten years from the date of grant. The administrator will determine at what time or times each option may be exercised and, subject to the provisions of the 2010 Option Plan, the period of time, if any, after retirement, death, disability or other termination of employment during which options may be exercised.
To qualify as incentive options, stock options must meet additional federal tax requirements, including a $100,000 limit on the value of shares subject to incentive options which first become exercisable in any calendar year, and a shorter term and higher minimum exercise price in the case of certain large stockholders.
In the event of a merger, sale or dissolution, or a similar sale event, unless assumed or substituted, all stock options and stock appreciation rights granted under the 2010 Option Plan that are not exercisable immediately prior to the effective time of a sale event will automatically become fully exercisable, all other awards granted under the 2010 Option Plan will become fully vested and non-forfeitable and awards with conditions and restrictions relating to the attainment of performance goals may become vested and non-forfeitable in connection with a sale event in the administrator’s discretion. In addition, upon the effective time of any such sale event, the 2010 Option Plan and all awards will terminate unless the parties to the transaction, in their discretion, provide for appropriate substitutions or assumptions of outstanding awards.
F-22
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
In connection with the Company’s 2007 initial public offering attempt, the Company retrospectively reassessed the estimated fair value of the Company’s stock for all periods prior to 2006 and subsequently performed contemporaneous valuations of its common stock. The company followed the guidance outlined in the American Institute of Certified Public Accountants (“AICPA”), “Practice Aid for Valuation of Privately-Held Company Equity Securities Issued as Compensation” (the “Practice Aid”). In conducting the reassessment, the Company took into consideration the market and income approaches to valuation as set forth in the Practice Aid. In determining the reassessed fair value of its common stock, the Company established as the fair market value, for accounting purposes, of its common stock at $12.87 per share for all equity awards made during the second half of fiscal year ended 2006.
For all equity compensation arrangements awarded during the six months ended June 30, 2007, the Company established $25.37 per share as the fair market value, for accounting purposes, of the Company’s common stock. Equity awards made during this period were made in the period leading up to the Company’s proposed initial public offering in June 2007 at an anticipated price range between $29.30 and $34.18 per share. On June 15, 2007, the Company withdrew the registration statement for this proposed initial public offering due to market conditions.
During October and November 2007, the Company issued equity awards with an exercise price of $12.45 per share, which the Company determined to be the fair value of its common stock on the date of grant. At that time, the Company also re-priced the equity awards made during the quarter ended June 30, 2007 to an exercise price of $12.45 per share. The significant decrease in enterprise valuation between June 2007 and November 2007 is reflective of the uncertainty surrounding the Company’s clinical development programs during that period.
In the first quarter of 2008, the Company received guidance from its underwriting syndicate that there was a lack of investor demand to complete the proposed initial public offering. As a result, management decided not to continue efforts to complete the proposed initial public offering and withdrew the registration filing in the fourth quarter of 2008. In addition, there was continued uncertainty regarding the regulatory approval to move the Company’s lead compound into phase III trials, including the need to initiate additional clinical trials. These events were coupled with growing unfavorable market conditions with continued limited access to capital as a result of the general market liquidity crisis. These issues contributed to a significant decrease in the enterprise’s valuation of the Company in the second half of 2008. In October 2008, the Company issued equity awards with an exercise price of $5.08, which the Company determined to be the fair value of its common stock on the date of grant.
As of December 31, 2008, the Company’s enterprise valuation continued to decrease due to the continuing general market liquidity issues and the uncertainty regarding the regulatory approval of the Company’s lead compound. As such, the Company valued its common stock at $2.37 per share as of December 31, 2008 and for the first quarter of 2009.
In the first quarter of 2010, the Company issued equity awards with an exercise price of $2.37 per share, which the Company’s board of directors determined to be equal to the fair value of the Company’s common stock on the date of grant. During this period, there was continued uncertainty regarding the regulatory pathway for the Company’s product candidates, including whether the Company could submit an NDA for lomitapide for the treatment of patients with HoFH without the need to conduct additional clinical trials. Also during this period, the Company’s financial resources were increasingly strained.
During the quarter ended September 30, 2010, the Company issued equity awards with an exercise price of $1.54 per share. In connection with its initial public offering, the Company completed a retrospective valuation of the fair value of its common stock. The Company believed that the preparation of the retrospective valuation was
F-23
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
necessary due to the fact that the probability of an initial public offering had accelerated significantly after certain key milestones were achieved. The Company calculated a retrospective value of $13.38 per share of common stock which was used to measure stock-based compensation expense for the stock options granted during the third quarter of 2010.
In October 2010, after the achievement of several regulatory pathway milestones and the successful completion of the IPO, the Company issued equity awards with an exercise price of $9.50 per share, the offering price of shares issued in the IPO.
During the years ended December 31, 2008, 2009 and 2010, the Company recorded stock-based compensation expense of approximately $1.2 million, $0.9 million and $1.8 million, respectively. No related tax benefits of the stock-based compensation costs have been recognized since the Company’s inception.
The Company uses the Black-Scholes option-pricing model to determine the estimated fair value for stock-based awards. The fair value of stock option awards is amortized on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. Expected volatility was calculated based on reported data for selected reasonably similar publicly traded companies, or guideline peer group, for which historical information was available. The Company will continue to use the guideline peer group volatility information until the historical volatility of the Company’s common stock is relevant to measure expected volatility for future option grants. The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The average expected life was determined according to the “simplified method” as described in Staff Accounting Bulletin (“SAB”) 110, which is the mid-point between the vesting date and the end of the contractual term. The risk-free interest rate is determined by reference to implied yields available from the 5-year and 7-year U.S. Treasury securities with a remaining term equal to the expected life assumed at the date of grant. Forfeitures are estimated based on the Company’s historical analysis of actual stock option forfeitures. The weighted-average grant-date fair values of stock options granted during the years ended December 31, 2008, 2009 and 2010 were $5.08, $2.37 and $1.71 per share, respectively. The weighted-average assumptions used in the Black-Scholes option-pricing model are as follows:
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| Risk-free interest rate |
2.29 | % | 2.18 | % | 1.14 | % | ||||||
| Dividend yield |
— | — | — | |||||||||
| Weighted-average expected life of options (years) |
6.25 | 6.25 | 6.26 | |||||||||
| Volatility |
100.00 | % | 150.00 | % | 150.00 | % | ||||||
The Company had 210,408 and 1,420,200 shares of unvested stock options granted to employees at a weighted exercise price of $2.34 and $1.88 per share at December 31, 2009 and 2010, respectively. The Company had 6,784 and 0 shares of unvested restricted common stock granted to employees at December 31, 2009 and 2010, respectively. Total unrecognized stock-based compensation cost related to nonvested stock options and restricted common stock granted to employees as of December 31, 2010 was $15.3 million. This unrecognized cost is expected to be recognized over a weighted-average period of approximately 3.4 years. As of October 27, 2010, no future stock options will be granted from the 2006 Plan; any future stock options and other equity awards will be made from the 2010 Plan.
F-24
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
The following table summarizes stock option activity for the Company:
| Number of Outstanding Stock Options |
Exercise Price Per Share |
Options Outstanding Weighted- Average Exercise Price |
||||||||||
| Balance at December 31, 2007 |
490,478 | $ | 0.90 – $12.87 | $ | 9.40 | |||||||
| Options granted |
115,902 | $ | 5.08 | |||||||||
| Exercised |
— | — | ||||||||||
| Forfeited/cancelled |
(154,759 | ) | $ | 5.08 | ||||||||
| Balance at December 31, 2008 |
451,631 | $ | 0.90 – $12.45 | $ | 6.49 | |||||||
| Options granted |
146,209 | $ | 2.37 | |||||||||
| Exercised |
— | — | ||||||||||
| Forfeited/cancelled |
(242,150 | ) | $ | 2.37 – $12.45 | ||||||||
| Balance at December 31, 2009 |
355,690 | $ | 0.90 – $12.87 | $ | 2.20 | |||||||
| Options granted |
1,455,946 | $ | 2.37 – $9.50 | |||||||||
| Exercised |
(38,307 | ) | $ | 2.37 | ||||||||
| Forfeited/Cancelled |
(57,516 | ) | 2.37 | |||||||||
| Balance at December 31, 2010 |
1,715,813 | $ | 0.90 – $9.50 | $ | 1.88 | |||||||
The aggregate intrinsic value as of December 31, 2010 for options exercisable was $3.6 million and $17.5 million for options expected to vest. The total intrinsic value of all options for the years ended December 31, 2008, 2009 and 2010 was $0, $0 and $21.1 million, respectively. The intrinsic value of the options exercised in 2008, 2009 and 2010 was $0, $0, and $0.5 million, respectively.
The following table summarizes information about stock options outstanding at December 31, 2010:
| Outstanding | Weighted Average Remaining Contractual Life (years) |
Exercisable | ||||||||||||||||||
| Number of Options |
Weighted Average Exercise Price |
Number of Options |
Weighted Average Exercise Price |
|||||||||||||||||
| Exercise Price |
||||||||||||||||||||
| $0.90 |
38,965 | $ | 0.90 | 5.4 | 38,965 | $ | 0.90 | |||||||||||||
| $2.37 |
346,388 | 2.37 | 7.8 | 184,903 | 2.37 | |||||||||||||||
| $1.54 |
1,289,507 | 1.54 | 9.3 | 71,745 | 1.54 | |||||||||||||||
| $9.50 |
40,953 | 9.50 | 9.8 | — | 9.50 | |||||||||||||||
| 1,715,813 | 295,613 | |||||||||||||||||||
The Company periodically remeasures fair value of stock-based awards issued to non-employees and records income or expense over the vesting period of such awards. The Company recorded compensation expense related to non-employees of approximately $193,000, $57,000 and $13,000 for the years ended December 31, 2008, 2009 and 2010, respectively.
F-25
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
Stock-based compensation expense includes stock options granted to employees and non-employees and has been reported in the Company’s statements of operations as follows:
| Years Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| (in thousands) | ||||||||||||
| Research and development |
$ | 655 | $ | 497 | $ | 337 | ||||||
| General and administrative |
592 | 436 | 1,501 | |||||||||
| Total |
$ | 1,247 | $ | 933 | $ | 1,838 | ||||||
| 15. | Employee Benefit Plan |
The Company maintains a defined contribution 401(k) plan (the “Plan”) available to employees. Employee contributions are voluntary and are determined on an individual basis, limited by the maximum amounts allowable under federal tax regulations. As of December 31, 2010, the Company had elected to match up to 3% of the employees’ contributions to the Plan. The Company recorded employer contribution expense of approximately $38,799, $81,891 and $46,317 during the years ended December 31, 2008, 2009 and 2010, respectively.
| 16. | Income Taxes |
As of December 31, 2009 and 2010, the Company had gross deferred tax assets of approximately $25.8 million and $29.9 million, respectively. Realization of the deferred tax assets is dependent upon the Company generating future taxable income, if any, the amount and timing of which are uncertain.
Accordingly, the deferred tax assets have been fully offset by a valuation allowance at December 31, 2009 and 2010. The net valuation allowance increased by approximately $4.5 million and $4.1 million for the years ended December 31, 2009 and 2010, respectively.
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company’s deferred tax assets relate primarily to net operating loss carryforwards. Significant components of the Company’s deferred tax assets are as follows:
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 22,929,000 | $ | 27,757,000 | ||||
| Research credits |
938,000 | 1,136,000 | ||||||
| Other temporary differences |
708,000 | 44,000 | ||||||
| Impairment loss on available for sale securities |
974,000 | 974,000 | ||||||
| Fair value adjustment on warrant liability |
227,000 | — | ||||||
| Total gross deferred tax assets |
25,776,000 | 29,911,000 | ||||||
| Valuation allowance |
(25,776,000 | ) | (29,911,000 | ) | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
F-26
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
A reconciliation of the statutory tax rates and the effective tax rates for the years ended December 31, 2008, 2009 and 2010 is as follows:
| December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| Statutory rate |
(34 | )% | (34 | )% | (34 | )% | ||||||
| State and local income taxes (net of federal tax benefit) |
(6 | ) | (6 | ) | (5 | ) | ||||||
| Other |
(3 | ) | 3 | 2 | ||||||||
| Valuation allowance |
43 | 37 | 26 | |||||||||
| Effective tax rates |
— | % | — | % | (11 | )% | ||||||
As of December 31, 2010, the Company had federal net operating loss carryforwards of $72.4 million. The Company also had federal and state research and development tax credit carryforwards of $1.1 million. The federal net operating loss and tax credit carryforwards will expire at various dates beginning in 2028, if not utilized. The difference between the statutory tax rate and the effective tax rate is primarily attributable to the valuation allowance offsetting deferred tax assets.
During the year ended December 31, 2010, the Company sold its New Jersey net operating loss carryforwards in the amount of approximately $21.9 million for a net tax benefit of approximately $1.8 million under the New Jersey Emerging Technology and Biotechnology Financial Assistance program.
Utilization of the net operating loss carryforwards and credits may be subject to a substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. The Company has not performed a detailed analysis to determine whether an ownership change under Section 382 of the Internal Revenue Code has occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of net operating loss carryforwards attributable to periods before the change.
| 17. | Related Party Transactions |
In July 2005, the Company entered into a consulting agreement with Scheer & Company, Inc., a company affiliated with David Scheer, chairman of the Board of Directors and a stockholder of the Company, to provide general corporate advice and consulting. The Company expensed related consulting fees of approximately $41,000, $42,000, and $19,156 for the years ended December 31, 2008, 2009 and 2010, respectively. This consulting agreement was terminated in the connection with the closing of the IPO.
In April 2006, the Company entered into a consulting agreement with Antonio M. Gotto, Jr., M.D., a member of the Board of Directors, for nonspecific consulting activities on behalf of the Company. The Company expensed related consulting fees of approximately $28,000, $31,000, and $21,500 for the years ended December 31, 2008, 2009 and 2010, respectively. This consulting agreement was terminated in the connection with the closing of the IPO.
| 18. | Contingencies |
During 2010, a dispute arose with a third-party service provider regarding the Company’s obligation to pay for services purported to have been performed on behalf of the Company in connection with a planned clinical trial for lomitapide in patients with HeFH. The Company is working with the service provider to negotiate a resolution to the matter and the Company does not expect that the ultimate resolution will be material to the Company’s financial position, cash flow or results of operations.
F-27
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Financial Statements—(Continued)
December 31, 2010
| 19. | Subsequent Events |
On February 28, 2011, the Company entered into a Loan and Security Agreement (the “Loan and Security Agreement”) with Hercules Technology II, L.P. and Hercules Technology III, L.P. (collectively, the “Hercules Funds”). Pursuant to the Agreement, the Hercules Funds will make available to the Company term loans in an aggregate principal amount of up to $25.0 million. The $25.0 million credit facility provides for an initial advance at closing of $10.0 million, with interest-only payments for twelve months, and bears per annum interest at the greater of 10.4% or 10.4% plus prime minus 4.75%. The Company may request additional term loan advances of up to $15.0 million.
The Company shall repay the aggregate principal balance of the loan that is outstanding in monthly installments starting on April 1, 2012 and continuing through September 1, 2014. The entire term loan principal balance and all accrued but unpaid interest will be due and payable on September 1, 2014. At its option, the Company may prepay all or any part of the outstanding advances subject to a prepayment charge (defined in the Loan and Security Agreement).
In connection with the Loan and Security Agreement, the Company granted the Hercules Funds a security interest in all of the Company’s personal property now owned or hereafter acquired, excluding intellectual property. The Loan and Security Agreement also provides for standard indemnification of the Hercules Funds and contains representations, warranties and certain covenants of the Company. The assertions embodied in those representations and warranties were made for purposes of the Loan and Security Agreement and are subject to qualifications and limitations agreed by the parties in connection with the negotiation of the terms of the Loan and Security Agreement. In addition, certain representations and warranties may be made as of a specific date, may be subject to a contractual standard of materiality different from that which an investor might view as material, or may have been used for purposes of allocating risk between the respective parties, rather than establishing matters as facts.
| 20. | Selected Quarterly Financial Data (Unaudited) |
| Quarters Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| 2009 |
||||||||||||||||
| Net loss attributable to common stockholders |
$ | (3,890,692 | ) | $ | (3,995,771 | ) | $ | (3,281,252 | ) | $ | (4,314,941 | ) | ||||
| Basic and diluted net loss per common share |
$ | (2.40 | ) | $ | (2.43 | ) | $ | (1.96 | ) | $ | (2.56 | ) | ||||
| 2010 |
||||||||||||||||
| Net loss attributable to common stockholders |
$ | (1,690,846 | ) | $ | (3,037,339 | ) | $ | (6,172,314 | ) | $ | (12,104,239 | ) | ||||
| Basic and diluted net loss per common share |
$ | (0.99 | ) | $ | (1.78 | ) | $ | (3.61 | ) | $ | (0.92 | ) | ||||
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
F-28
Table of Contents
PART I. FINANCIAL INFORMATION
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Condensed Balance Sheets
| March 31, 2011 |
December 31, 2010 |
|||||||
| (unaudited) | ||||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 46,827,763 | $ | 44,100,897 | ||||
| Prepaid expenses and other current assets |
795,569 | 504,744 | ||||||
| Total current assets |
47,623,332 | 44,605,641 | ||||||
| Property and equipment, net |
88,479 | 16,471 | ||||||
| Long term investments |
1,230,000 | 1,125,000 | ||||||
| Other assets |
1,072,330 | — | ||||||
| Total assets |
$ | 50,014,141 | $ | 45,747,112 | ||||
| Liabilities and stockholders’ equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 2,309,633 | $ | 3,145,887 | ||||
| Accrued compensation |
298,438 | 492,938 | ||||||
| Other accrued liabilities |
978,722 | 1,029,873 | ||||||
| Total current liabilities |
3,586,793 | 4,668,698 | ||||||
| Long term debt |
10,000,000 | — | ||||||
| Other liabilities |
814,553 | — | ||||||
| Total liabilities |
$ | 14,401,346 | $ | 4,668,698 | ||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value, 5,000,000 authorized at March 31, 2011 and December 31, 2010; 0 shares issued and outstanding at March 31, 2011 and December 31, 2010 |
— | — | ||||||
| Common stock, $0.001 par value, 125,000,000 shares authorized at March 31, 2011 and December 31, 2010; 17,745,300 and 17,745,300 shares issued at March 31, 2011 and December 31, 2010, respectively; 17,641,543 and 17,641,543 shares outstanding at March 31, 2011 and December 31, 2010, respectively |
17,744 | 17,744 | ||||||
| Treasury stock, at cost; 103,757 shares at March 31, 2011 and December 31, 2010 |
(373 | ) | (373 | ) | ||||
| Subscription Receivable |
— | (90,735 | ) | |||||
| Additional paid-in-capital |
132,720,909 | 131,549,805 | ||||||
| Deficit accumulated during the development stage |
(97,821,244 | ) | (90,988,786 | ) | ||||
| Accumulated other comprehensive income |
695,759 | 590,759 | ||||||
| Total stockholders’ equity |
35,612,795 | 41,078,414 | ||||||
| Total liabilities and stockholders’ equity |
$ | 50,014,141 | $ | 45,747,112 | ||||
See accompanying notes.
F-29
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Operations
(Unaudited)
| Three Months Ended March 31, |
Period from February 4, 2005 (inception) to March 31, |
|||||||||||
| 2011 | 2010 | 2011 | ||||||||||
| Operating Expenses: |
||||||||||||
| Research and development |
$ | 3,296,703 | $ | 1,066,176 | $ | 53,163,967 | ||||||
| General and administrative |
3,490,752 | 972,026 | 28,035,787 | |||||||||
| Total operating expenses |
6,787,455 | 2,038,202 | 81,199,754 | |||||||||
| Loss from operations |
(6,787,455 | ) | (2,038,202 | ) | (81,199,754 | ) | ||||||
| Interest expense |
(112,527 | ) | (601,460 | ) | (7,010,359 | ) | ||||||
| Interest income |
67,524 | 17,677 | 2,783,960 | |||||||||
| Other expense, net |
— | — | (2,453,271 | ) | ||||||||
| Loss before income taxes |
(6,832,458 | ) | (2,621,985 | ) | (87,879,424 | ) | ||||||
| Benefit from income taxes |
— | 1,793,129 | 1,793,129 | |||||||||
| Net loss |
(6,832,458 | ) | (828,856 | ) | (86,086,295 | ) | ||||||
| Less: Accretion of preferred stock dividends and other deemed dividends |
— | (861,990 | ) | (23,663,413 | ) | |||||||
| Net loss attributable to common stockholders |
$ | (6,832,458 | ) | $ | (1,690,846 | ) | $ | (109,749,708 | ) | |||
| Net loss attributable to common stockholders per common share - basic and diluted |
$ | (0.39 | ) | $ | (0.99 | ) | ||||||
| Weighted-average shares outstanding - basic and diluted |
17,641,543 | 1,701,355 | ||||||||||
See accompanying notes.
F-30
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Statements of Cash Flows
(Unaudited)
| Three Months Ended March 31, |
Period From February 4, 2005 (Inception) to March 31, |
|||||||||||
| 2011 | 2010 | 2011 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (6,832,458 | ) | $ | (828,856 | ) | $ | (86,086,295 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
1,410 | 3,158 | 88,845 | |||||||||
| Noncash stock-based compensation |
1,261,839 | 199,701 | 7,722,728 | |||||||||
| Noncash interest expense |
22,971 | 444,148 | 4,212,587 | |||||||||
| Impairment loss on securities and other non-cash charges |
— | — | 2,728,500 | |||||||||
| Changes in assets and liabilities: |
||||||||||||
| Prepaid expenses and other current assets |
(290,825 | ) | (31,008 | ) | (1,829,880 | ) | ||||||
| Accounts payable |
(836,254 | ) | 903,110 | 1,297,067 | ||||||||
| Accrued compensation |
(194,500 | ) | (266,397 | ) | 298,438 | |||||||
| Other accrued liabilities |
(51,151 | ) | (459,303 | ) | 1,503,787 | |||||||
| Other long term liabilities |
39,553 | — | 39,553 | |||||||||
| Other long term assets |
(130,501 | ) | — | (130,501 | ) | |||||||
| Net cash used in operating activities |
(7,009,916 | ) | (35,447 | ) | (70,155,171 | ) | ||||||
| Investing activities |
||||||||||||
| Purchases of property and equipment |
(73,418 | ) | — | (177,324 | ) | |||||||
| Purchases of marketable securities |
— | — | (24,713,000 | ) | ||||||||
| Sales of marketable securities |
— | — | 21,713,000 | |||||||||
| Net cash used in investing activities |
(73,418 | ) | — | (3,177,324 | ) | |||||||
| Financing activities |
||||||||||||
| Deferred financing fees |
(189,800 | ) | — | (1,301,556 | ) | |||||||
| Proceeds from issuances of convertible debt |
— | 3,000,000 | 22,063,642 | |||||||||
| Debt extinguishment fee |
— | — | (525,000 | ) | ||||||||
| Proceeds from issuances of debt financing |
10,000,000 | — | 27,525,000 | |||||||||
| Payment of debt financing |
— | (718,930 | ) | (17,527,534 | ) | |||||||
| Payment of subscription receivable |
— | — | 200 | |||||||||
| Proceeds from issuances of common stock |
— | — | 50,805,390 | |||||||||
| Proceeds from issuances of preferred stock, net |
— | — | 39,120,489 | |||||||||
| Repurchase of common stock |
— | — | (373 | ) | ||||||||
| Net cash provided by financing activities |
9,810,200 | 2,281,070 | 120,160,258 | |||||||||
| Net increase in cash and cash equivalents |
2,726,866 | 2,245,623 | 46,827,763 | |||||||||
| Cash and cash equivalents, beginning of period |
44,100,897 | 1,429,126 | — | |||||||||
| Cash and cash equivalents, end of period |
$ | 46,827,763 | $ | 3,674,749 | $ | 46,827,763 | ||||||
| Supplemental disclosures of cash flow information |
||||||||||||
| Accretion of preferred stock dividends and issuance costs |
$ | — | $ | 861,990 | $ | 13,028,473 | ||||||
| Warrant liability reclass to additional paid-in-capital |
$ | — | $ | — | $ | 983,341 | ||||||
| Series B redeemable preferred stock beneficial conversion |
$ | — | $ | — | $ | 4,991,781 | ||||||
| Warrant issued with notes payable |
$ | — | $ | — | $ | 720,600 | ||||||
| Deemed dividend—convertible notes beneficial conversion option |
$ | — | $ | — | $ | 5,887,598 | ||||||
| Deferred charge due on maturity of Hercules term loan |
$ | 775,000 | $ | — | $ | 775,000 | ||||||
| Cash paid interest expense |
$ | 89,556 | $ | 157,312 | $ | 2,797,769 | ||||||
See accompanying notes.
F-31
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements
March 31, 2011
| 1. | Description of Business and Significant Accounting Policies |
Organization and Basis of Presentation
Aegerion Pharmaceuticals, Inc. (the “Company” or “Aegerion”) is an emerging biopharmaceutical company focused on the development and commercialization of novel therapeutics to treat severe lipid disorders. The Company is in its development stage as defined by the FASB Accounting Standards Codification (“ASC”) 915, Development Stage Entities. The Company’s activities since inception have consisted principally of acquiring product and technology rights, raising capital, establishing facilities and performing research and development activities. Since inception, the Company has incurred significant losses from operations and expects losses to continue for the foreseeable future during its development phase. The Company’s success depends primarily on the successful development and regulatory approval of its product candidates. From February 4, 2005 (inception) through March 31, 2011, the Company had a deficit accumulated during the development stage of $97.8 million. The Company believes that its existing cash and cash equivalents will be sufficient to cover its cash flow requirements through the first half of 2012.
Basis of Presentation
The accompanying unaudited financial statements have been prepared in accordance with United States generally accepted accounting principles (“GAAP”) and with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all information and footnotes required by GAAP for complete financial statements. In the opinion of management, the accompanying financial statements include all adjustments (including normal recurring accruals) considered necessary for fair presentation of the Company’s financial position, results of operations and cash flows for the periods presented. Operating results for the current interim period are not necessarily indicative of the results that may be expected for the fiscal year ended December 31, 2011. This Form 10-Q should be read in conjunction with the audited financial statements and accompanying notes in the Company’s Annual Report on Form 10-K for the year ended December 31, 2010 (“Form 10-K”).
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, securities, accounts payable, accrued liabilities, and notes payable. Fair value estimates of these instruments are made at a specific point in time, based on relevant market information. These estimates may be subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. The carrying amount of cash and cash equivalents, securities, accounts payable, accrued liabilities, and notes payable are generally considered to be representative of their respective fair values because of the short-term nature of those instruments. The fair value of the Company’s investments in securities is based upon observable and unobservable inputs.
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. Assets and liabilities that are measured at fair value are reported
F-32
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements—(Continued)
March 31, 2011
using a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| § | Level 1—Quoted prices in active markets for identical assets or liabilities that the Company has the ability to access at the measurement date. |
| § | Level 2—Inputs other than quoted prices in active markets that are observable for the asset or liability, either directly or indirectly. |
| § | Level 3—Inputs that are unobservable for the asset or liability. |
As of March 31, 2011 and December 31, 2010, the Company held par value $3.0 million of investments in auction rate securities. The Company’s investments in securities are classified within Level 2 and 3 of the fair value hierarchy using inputs that are observable or unobservable for the asset or liability. The fair value measurements of the Company’s investments in securities at March 31, 2011 and December 31, 2010 are summarized in the tables below:
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at March 31, 2011 |
|||||||||||||
| Assets: |
||||||||||||||||
| Auction rate securities |
$ | — | $ | — | $ | 1,050,000 | $ | 1,050,000 | ||||||||
| Auction rate securities converted to preferred stock |
— | 180,000 | — | 180,000 | ||||||||||||
| Total assets at fair value |
$ | — | $ | 180,000 | $ | 1,050,000 | $ | 1,230,000 | ||||||||
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Balance at December 31, 2010 |
|||||||||||||
| Assets: |
||||||||||||||||
| Auction rate securities |
$ | — | $ | — | $ | 945,000 | $ | 945,000 | ||||||||
| Auction rate securities converted to preferred stock |
— | 180,000 | — | 180,000 | ||||||||||||
| Total assets at fair value |
$ | — | $ | 180,000 | $ | 945,000 | $ | 1,125,000 | ||||||||
The change in fair value of the Company’s Level 3 financial instruments during the quarter ended March 31, 2011 was as follows:
| Level 3 | ||||
| Auction rate securities |
||||
| Balance at December 31, 2010 |
$ | 945,000 | ||
| Unrealized gain recorded in other comprehensive loss |
105,000 | |||
| Fair value adjustment included in net loss |
— | |||
| Balance at March 31, 2011 |
$ | 1,050,000 | ||
F-33
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements—(Continued)
March 31, 2011
The estimated fair value of the auction rate securities is derived through discounted cash flows, a Level 3 input. The Company’s discounted cash flow analysis considered, among other things, the quality of the underlying collateral, the credit rating of the issuer, an estimate of when these securities are either expected to have a successful auction or otherwise return to par value, the expected interest income to be received over this period, and the estimated required rate of return for investors that may be willing to purchase such a security. The Company also considered third-party valuations, to the extent available, and similar securities priced in the marketplace when arriving at the estimated fair value. At March 31, 2011, the Company’s auction rate securities were reflected at 70% of par value.
The estimated fair value of the Company’s auction rate security that was converted to preferred stock is estimated by analyzing similar securities in the marketplace, a Level 2 input. The Company also considers the fair value of the underlying collateral and credit rating of the issuer. Based on observed settlements of similar securities, the preferred stock was recorded at 12% of par value at March 31, 2011.
| 2. | Long term investments |
The following is a summary of investments held by the Company as of March 31, 2011 and December 31, 2010:
| Adjusted Cost |
Gross Unrealized |
Gross Unrealized Losses |
Fair Value at March 31, 2011 |
|||||||||||||
| Auction rate securities |
$ | 414,241 | $ | 635,759 | $ | — | $ | 1,050,000 | ||||||||
| Auction rate securities converted to preferred stock |
120,000 | 60,000 | — | 180,000 | ||||||||||||
| Total |
$ | 534,241 | $ | 695,759 | $ | — | $ | 1,230,000 | ||||||||
| Adjusted Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value at December 31, 2010 |
|||||||||||||
| Auction rate securities |
$ | 414,241 | $ | 530,759 | $ | — | $ | 945,000 | ||||||||
| Auction rate securities converted to preferred stock |
120,000 | 60,000 | — | 180,000 | ||||||||||||
| Total |
$ | 534,241 | $ | 590,759 | $ | — | $ | 1,125,000 | ||||||||
| 3. | Debt Financing |
On February 28, 2011, the Company entered into a Loan and Security Agreement (the “Loan and Security Agreement”) with Hercules Technology II, L.P. and Hercules Technology III, L.P. (collectively, the “Hercules Funds”). Pursuant to the Agreement, the Hercules Funds will make available to the Company term loans in an aggregate principal amount of up to $25.0 million. The $25.0 million credit facility provides for an initial advance at closing of $10.0 million, with interest-only payments for thirteen months, and bears per annum interest at the greater of 10.4% or 10.4% plus prime minus 4.75%. The Company may request additional term loan advances of up to $15.0 million. The Company will repay the aggregate principal balance of the loan that is outstanding in monthly installments starting on April 1, 2012 and continuing through September 1, 2014. The entire term loan principal balance and all accrued but unpaid interest will be due and payable on September 1, 2014. At its option, the Company may prepay all or any part of the outstanding advances subject to a prepayment charge (defined in the Loan and Security Agreement). In connection with the Loan and Security Agreement, the Company granted the Hercules Funds a security interest in all of the Company’s personal property now owned or hereafter acquired, excluding intellectual property. The Loan and Security Agreement also provides for standard indemnification of the Hercules Funds and contains representations, warranties and certain covenants of the Company.
F-34
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements—(Continued)
March 31, 2011
| 4. | Capital Structure |
At March 31, 2011, the Company was authorized to issue 5,000,000 shares of $0.001 par value preferred stock. There were no shares issued and outstanding. Dividends on the stock will be paid when, and if, declared by the Board of Directors.
At March 31, 2011, the Company was authorized to issue 125,000,000 shares of $0.001 par value common stock. There were 17,745,300 shares issued and 17,641,543 shares outstanding. Dividends on the stock will be paid when, and if, declared by the Board of Directors. Each holder of common stock is entitled to vote on all matters and is entitled to one vote for each share held. The Company will, at all times, reserve and keep available, out of its authorized but unissued shares of common stock, sufficient shares to affect the conversion of the shares for stock options.
| 5. | Comprehensive Loss |
Comprehensive loss for the Company includes net loss and the changes in net unrealized gains and losses on available for sale securities. Comprehensive loss for the three months ended March 31, 2011 and 2010 is detailed below:
| Three Months Ended March 31, | ||||||||
| 2011 | 2010 | |||||||
| Net loss |
$ | (6,832,458 | ) | $ | (828,856 | ) | ||
| Change in unrealized gain on available for sale securities |
105,000 | — | ||||||
| Comprehensive loss |
$ | (6,727,458 | ) | $ | (828,856 | ) | ||
| 6. | Stock Option Plan |
Compensation expense related to stock-based payments awards totaled $1.3 million and $0.2 million during the three months ended March 31, 2011 and 2010, respectively.
On March 1, 2011, the Company awarded certain employees 462,000 stock options with an exercise price of $14.84 per share, the closing price of the Company’s common stock on March 1, 2011, the date of the award. The Company determined the fair value of the stock-based awards using the Black-Scholes option pricing model. The fair value of the stock option will be expensed over the requisite service period. Stock-based compensation expense includes stock options granted to employees and non-employees and has been reported in the Company’s statements of operations as follows:
| Three Months Ended March 31, | ||||||||
| 2011 | 2010 | |||||||
| Research and development |
$ | 146,250 | $ | 92,146 | ||||
| General and administrative |
1,115,589 | 107,555 | ||||||
| Total |
$ | 1,261,839 | $ | 199,701 | ||||
The fair value of the options granted is estimated on the date of grant using a Black-Scholes option pricing model with the following weighted-average assumptions:
| Three Months Ended March 31, | ||||||||
| 2011 | 2010 | |||||||
| Expected stock price volatility |
85.0 | % | 150 | % | ||||
| Risk free interest rate |
2.46 | % | 1.79 | % | ||||
| Expected life of options (years) |
6.46 | 6.25 | ||||||
| Expected annual dividend per share |
$ | 0.00 | $ | 0.00 | ||||
F-35
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements—(Continued)
March 31, 2011
A summary of option activities related to the Company’s stock options for the three months ended March 31, 2011 is as follows:
| Options Outstanding | ||||||||||||
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life |
||||||||||
| Balance at December 31, 2010 |
1,715,813 | $ | 1.88 | 8.1 | ||||||||
| Options granted |
462,000 | 14.84 | 9.9 | |||||||||
| Options exercised |
— | — | — | |||||||||
| Options forfeited/cancelled |
— | — | — | |||||||||
| Balance at March 31, 2011 |
2,177,813 | $ | 4.63 | 9.0 | ||||||||
As of March 31.2011, the Company had approximately $18.3 million of unrecognized compensation expense related to unvested stock options. The Company expects this compensation expense to be recognized over a weighted average period of 3.5 years.
| 7. | Basic and Diluted Net Loss Attributable to Common Stockholders per Common Share |
Basic net loss per common share is calculated by dividing the net loss applicable to common stockholders by the weighted-average number of common shares outstanding for the period. Diluted net loss per common share is computed by dividing the net loss applicable to common stockholders by the weighted-average number of unrestricted common shares and dilutive common share equivalents outstanding for the period, determined using the treasury-stock method and the as if-converted method.
| Three Months Ended March 31, |
||||||||
| 2011 | 2010 | |||||||
| Net loss attributable to common stockholders |
$ | (6,832,458 | ) | $ | (1,690,846 | ) | ||
| Weighted average common shares outstanding—basic and diluted |
17,641,543 | 1,701,355 | ||||||
| Net loss attributable to common stockholders per common share—basic and diluted |
$ | (0.39 | ) | $ | (0.99 | ) | ||
The following table shows historical dilutive common share equivalents outstanding, which are not included in the above historical calculation, as the effect of their inclusion is anti-dilutive during each period.
| Three Months Ended March 31, | ||||||||
| 2011 | 2010 | |||||||
| Convertible preferred stock |
— | 6,784,483 | ||||||
| Options |
2,177,813 | 481,174 | ||||||
| Warrant |
107,779 | 107,779 | ||||||
| Total |
2,285,592 | 7,373,436 | ||||||
F-36
Table of Contents
Aegerion Pharmaceuticals, Inc.
(A Development Stage Company)
Notes to Unaudited Financial Statements—(Continued)
March 31, 2011
| 8. | Contingencies |
During September 2010, a dispute arose with a third-party service provider regarding the Company’s obligation to pay for services purported to have been performed on behalf of the Company in connection with a planned clinical trial for lomitapide in patients with heterozygous familial hypercholesterolemia. The Company is working with the service provider to negotiate a resolution to the matter and the Company does not expect that the ultimate resolution will be material to the Company’s financial position, cash flows or results of operations for the full fiscal year.
| 9. | Subsequent events |
The Company has evaluated all events or transactions that occurred after March 31, 2011 up through the date the Company issued these financial statements.
F-37
Table of Contents
4,250,000 Shares

Common Stock
Joint Book-Running Managers
Jefferies
Deutsche Bank Securities
Co- Managers
Leerink Swann
Needham & Company, LLC
Collins Stewart
, 2011
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of the common stock being registered. All the amounts shown are estimates except the Securities and Exchange Commission registration fee, and the Financial Industry Regulatory Authority, or FINRA, filing fee.
| Total | ||||
| Securities and Exchange Commission registration fee |
$ | 10,055 | ||
| FINRA filing fee |
8,661 | |||
| Blue sky qualification fees and expense |
15,000 | |||
| Printing and engraving expenses |
100,000 | |||
| Legal fees and expenses |
250,000 | |||
| Accounting fees and expenses |
120,000 | |||
| Transfer agent and registrar fees |
1,000 | |||
| Miscellaneous expenses |
96,379 | |||
| Total expenses |
$ | 601,095 | ||
Item 14. Indemnification of Directors and Officers
Section 102(b)(7) of the Delaware General Corporation Law provides that a corporation, in its certificate of incorporation, may limit the personal liability of a director of the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| § | Transaction from which the director derived improper personal benefit; |
| § | Act or omission not in good faith or that involved intentional misconduct or a knowing violation of law; |
| § | Unlawful payment of dividends or redemption of shares; or |
| § | Breach of a director’s duty of loyalty to the corporation or its stockholders. |
Section 145(a) of the Delaware General Corporation Law provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation), because he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding, if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
Section 145(b) of the Delaware General Corporation Law provides, in general, that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor because the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification shall be made with respect to any claim, issue or matter as to which he or she shall have been adjudged to be
II-1
Table of Contents
liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, he or she is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or other adjudicating court shall deem proper.
Section 145(g) of the Delaware General Corporation Law provides, in general, that a corporation may purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would have the power to indemnify the person against such liability under Section 145 of the Delaware General Corporation Law.
Article VII of our Amended and Restated Certificate of Incorporation, or the Charter, provides that no director of our company shall be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to us or our stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) in respect of unlawful dividend payments or stock redemptions or repurchases, or (4) for any transaction from which the director derived an improper personal benefit. In addition, our Charter provides that if the Delaware General Corporation Law is amended to authorize the further elimination or limitation of the liability of directors, then the liability of a director of our company shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
Article VII of the Charter further provides that any amendment, repeal or modification of such article by our stockholders or an amendment to the Delaware General Corporation Law will not adversely affect any right or protection existing at the time of such amendment, repeal or modification with respect to any acts or omissions occurring before such amendment, repeal or modification of a director serving at the time of such amendment, repeal or modification.
Article V of our Amended and Restated By-Laws, or the By-Laws, provides that we will indemnify each of our directors and officers and, in the discretion of our board of directors, certain employees, to the fullest extent permitted by the Delaware General Corporation Law as the same may be amended (except that in the case of an amendment, only to the extent that such amendment permits us to provide broader indemnification rights than the Delaware General Corporation Law permitted us to provide prior to such amendment) against any and all expenses, judgments, penalties, fines and amounts reasonably paid in settlement that are incurred by the director, officer or such employee or on the director’s, officer’s or employee’s behalf in connection with any threatened, pending or completed proceeding or any claim, issue or matter therein, to which he or she is or is threatened to be made a party because he or she is or was serving as a director, officer or employee of our company, or at our request as a director, partner, trustee, officer, employee or agent of another corporation, partnership, limited liability company, joint venture, trust, employee benefit plan, foundation, association, organization or other legal entity, if he or she acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of our company and, with respect to any criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful. Article V of the By-Laws further provides for the advancement of expenses to each of our directors and, in the discretion of our board of directors, to certain officers and employees.
In addition, Article V of the By-Laws provides that the right of each of our directors and officers to indemnification and advancement of expenses will be a contract right and will not be exclusive of any other right now possessed or hereafter acquired under any statute, provision of the Charter or By-Laws, agreement, vote of stockholders or disinterested directors or otherwise. Furthermore, Article V of the By-Laws authorizes us to provide insurance for our directors, officers and employees against any liability, whether or not we would have the power to indemnify such person against such liability under the Delaware General Corporation Law or the provisions of Article V of the By-Laws.
II-2
Table of Contents
We have entered into indemnification agreements with each of our directors and our executive officers. These agreements provide that we will indemnify each of our directors and executive officers to the fullest extent permitted by law and advance expenses, including attorneys’ fees, to each indemnified director or executive officer in connection with any proceeding in which indemnification is available.
We also maintain a general liability insurance policy which covers certain liabilities of directors and officers of our company arising out of claims based on acts or omissions in their capacities as directors or officers.
In the underwriting agreement we will enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act, against certain liabilities.
Item 15. Recent Sales of Unregistered Securities and Use of Proceeds
Recent Sales of Unregistered Securities
The following list sets forth information regarding all securities sold by us in the three years preceding the date of this prospectus:
Convertible Note Financings and Warrant Issuances
In September 2008, we received gross proceeds of $3,814,760 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year, and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price of our initial public offering, or $7.60.
In December 2008, we received gross proceeds of $5,000,001 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
In July 2009, we received gross proceeds of $5,000,000 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
In January 2010, we received gross proceeds of $3,000,000 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
In June 2010, we received gross proceeds of $1,500,000 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
In August 2010, we received gross proceeds of $1,500,000 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
II-3
Table of Contents
In October 2010, we received gross proceeds of $1,500,000 from the sale of convertible promissory notes in a private placement to certain of our existing investors. The convertible promissory notes accrue interest at a rate equal to 8% per year and have a maturity date of December 31, 2011, unless converted prior thereto. The convertible promissory notes are automatically convertible into common stock upon the closing of our initial public offering at a conversion price equal to 80% of the price to the public of our initial public offering.
Stock Option and Restricted Stock Grants
From May 31, 2006 through September 30, 2010, we granted stock options under our 2006 Option Plan to purchase an aggregate of 1,713,167 shares of common stock, net of forfeitures, with a weighted average exercise price of $1.71 per share, to certain of our employees, consultants and directors. During the year ended December 31, 2010, we granted options under our 2010 Option Plan to purchase 40,953 shares of common stock to our employees, consultants and directors with a weighted average exercise price of $9.50. During the five months ended May 31, 2011, we granted options under our 2010 Option Plan to purchase 481,927 shares of common stock to our employees with a weighted average exercise price of $15.02. In May 2011, we awarded 29,890 shares of restricted stock under our 2010 Option Plan.
We deemed the grants of stock options, except to the extent exempt pursuant to Section 4(2) of the Securities Act, to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
Securities Act Exemptions
We deemed the offers, sales and issuances of the securities described in paragraphs (a) through (i) and to the extent applicable a portion of the stock options described in paragraph (j) granted to executive officers to be exempt from registration under the Securities Act in reliance on Section 4(2) of the Securities Act, including Regulation D and Rule 506 promulgated thereunder, relative to transactions by an issuer not involving a public offering. All purchasers of securities in transactions exempt from registration pursuant to Regulation D represented to us that they were accredited investors and were acquiring the shares for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from such registration.
We deemed the grants of stock options described in paragraph (j), except to the extent described above as exempt pursuant to Section 4(2) of the Securities Act, to be exempt from registration under the Securities Act in reliance on Rule 701 of the Securities Act as offers and sales of securities under compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits.
The exhibits to the registration statement are listed in the Exhibit Index to this registration statement and are incorporated herein by reference.
II-4
Table of Contents
Item 17. Undertakings
(a) Insofar as indemnification for liabilities arising under the Securities Act, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(b) The undersigned Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Amendment No. 1 to the registration statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the Township of Bedminster, New Jersey, on June 18, 2011.
| AEGERION PHARMACEUTICALS, INC. | ||
| By: |
/s/ MARC D. BEER | |
| Marc D. Beer President (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities indicated below on the 18th day of June 2011.
| Signature | Capacity | |||
| /s/ MARC D. BEER |
Chief Executive Officer (Principal Executive Officer) and Director | |||
| Marc D. Beer | ||||
| * |
Chief Financial Officer (Principal Financial Officer) | |||
| Mark J. Fitzpatrick | ||||
| * |
Vice President and Chief Accounting Officer (Principal Accounting Officer) | |||
| John T. Cavan | ||||
| * |
Chairman of the Board | |||
| David L. Scheer | ||||
| * |
Director | |||
| Alison Kiley | ||||
| * |
Director | |||
| Sol J. Barer, Ph.D. | ||||
| * |
Director | |||
| Antonio M. Gotto, Jr. | ||||
| * |
Director | |||
| Michèle Ollier | ||||
| *By: | /s/ CHRISTINE A. PELLIZZARI |
|||
| Christine A. Pellizzari | ||||
| Attorney-in-Fact | ||||
II-6
Table of Contents
EXHIBIT INDEX
| Exhibit Number | Description of Document | |||
| 1.1† | Form of Underwriting Agreement. | |||
| 3.1 | Amended and Restated Certificate of Incorporation, attached as Exhibit 3.2 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| 3.2 | Amended and Restated By-laws, attached as Exhibit 3.4 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| 4.1 | Form of specimen certificate evidencing shares of common stock, attached as Exhibit 4.1 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| 5.1** | Opinion of Goodwin Procter LLP | |||
| +10.1 | 2006 Stock Option and Grant Plan, as amended, and forms of agreement thereunder, attached as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.2 | 2010 Stock Option and Incentive Plan and forms of agreement thereunder, attached as Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| 10.3 | Amended and Restated Investor Rights Agreement, dated November 9, 2007, as amended, attached as Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| #10.4 | Patent License Agreement with University of Pennsylvania, dated May 19, 2006, as amended September 27, 2006, attached as Exhibit 10.6 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| #10.5 | License Agreement with Bayer Healthcare AG, dated May 31, 2006, as amended February 15, 2007, attached as Exhibit 10.7 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| 10.6 | Form of Indemnification Agreement, attached as Exhibit 10.12 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.7 | Form of Non-Employee Director Compensation Policy, attached as Exhibit 10.13 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.8 | Employment Agreement with Christine Pellizzari, dated October 5, 2010, attached Exhibit 10.4 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.9 | Employment Agreement with John Cavan, dated October 5, 2010, attached Exhibit 10.5 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.10 | Employment Agreement with Marc D. Beer, dated August 19, 2010, attached Exhibit 10.16 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
Table of Contents
| Exhibit Number | Description of Document | |||
| 10.11 | Lease by and between the Registrant and RREEF America REIT II CORP. PPP, dated January 1, 2011, attached as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on January 6, 2011, and incorporated herein by reference | |||
| 10.12 | Loan and Security Agreement by and between the Registrant, Hercules Technology II, L.P. and Hercules Technology III, L.P., dated February 28, 2011, attached as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 4, 2011, and incorporated herein by reference | |||
| 10.13 | Amended and Restated Warrant Agreement with Hercules Technology Growth Capital, Inc., dated September 29, 2008, as amended July 2, 2009, attached as Exhibit 10.11 to the Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on August 10, 2010, and incorporated herein by reference | |||
| +10.14 | Employment Agreement with Martha Carter, dated February 14, 2011, attached as Exhibit 10.1 to the Registrant’s Annual Report on Form 10-Q, filed with the SEC on May 16, 2011, and incorporated herein by reference | |||
| +10.15 | Employment Agreement with Diane Tribble, dated February 17, 2011, attached as Exhibit 10.2 to the Registrant’s Annual Report on Form 10-Q, filed with the SEC on May 16, 2011, and incorporated herein by reference | |||
| +10.16 | Employment Agreement with Mark Fitzpatrick, dated May 9, 2011, attached as Exhibit 10.3 to the Registrant’s Annual Report on Form 10-Q, filed with the SEC on May 16, 2011, and incorporated herein by reference | |||
| 21.1 | Subsidiaries of Registrant, attached as Exhibit 21.1 to the Registrant’s Annual Report on Form 10-K, filed with the SEC on March 31, 2011, and incorporated herein by reference | |||
| 23.1** | Consent of L.E.K. Consulting, LLC | |||
| 23.2† | Consent of Ernst & Young LLP | |||
| 23.3** | Consent of Goodwin Procter LLP (included in Exhibit 5.1) | |||
| 24.1** | Power of Attorney | |||
| * | To be filed by amendment. |
| ** | Previously filed |
| † | Filed herewith |
| # | Confidential treatment has been received for certain provisions of this Exhibit. Confidential materials have been omitted and filed separately with the SEC. |
| + | Management contract or compensatory plan or arrangement. |
