Attached files
As filed with the Securities and Exchange Commission on June 10, 2011
Registration Statement No. 333-______
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
SCRIPSAMERICA, INC.
(Exact Name of Small Business Issuer in its Charter)
|
Delaware
|
5122
|
26-2598594
|
|
(State of Incorporation)
|
(Primary Standard Classification Code)
|
(IRS Employer ID No.)
|
77 McCullough Drive, Suite 7
New Castle, Delaware 19720
(800) 957-7622
(Address and Telephone Number of Registrant’s Principal
Executive Offices and Principal Place of Business)
Robert Schneiderman, CEO
ScripsAmerica, Inc.
77 McCullough Drive, Suite 7
New Castle, Delaware 19720
(800) 957-7622
(Name, Address and Telephone Number of Agent for Service)
Copies of communications to:
Fox Law Offices, P.A.
61 Knickerbocker Lane
Peaks Island, ME 04108
(207) 766-0944
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933, please check the following box and list the Securities Act registration Statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
|
|
Non-accelerated filer o (do not check if a smaller reporting company)
|
Smaller reporting company x
|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class Of Securities to be Registered
|
Amount to be
Registered (1)
|
Proposed Maximum
Aggregate
Offering Price
per share (2)
|
Proposed Maximum
Aggregate
Offering Price
|
Amount of
Registration fee
|
||||||||||||
|
Common Stock, par value $.001
|
5,229,000
|
$
|
0.20
|
$
|
1,045,800
|
$
|
121.42
|
|||||||||
(1) In the event of a stock split, stock dividend, or similar transaction involving the common stock, the number of shares registered shall automatically be increased to cover the additional shares of common stock issuable pursuant to Rule 416 under the Securities Act. The amount of shares to be registered represents the Company’s good faith estimate of the number of shares that the registrant may issue pursuant to a Letter Agreement with the selling security holder.
(2) The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457(o). Our common stock is not currently trading on any national exchange. Therefore, in accordance with Rule 457, the offering price of $0.20 was determined by the price shares of common stock that we sold in a Regulation S offering that closed in May 2011. The price of $0.20 is a fixed price at which the selling security holders may sell their shares until our common stock is quoted on the OTC Bulletin Board at which time the shares may be sold at prevailing market prices or privately negotiated prices.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the securities act of 1933 or until the registration statement shall become effective on such date as the commission, acting pursuant to said section 8(a), may determine.
|
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and no offer to buy these securities is being solicited in any state where the offer or sale is not permitted.
PRELIMINARY SUBJECT TO COMPLETION, DATED JUNE 10, 2011
PROSPECTUS
SCRIPSAMERICA, INC.
5,229,000 shares of Common Stock
This prospectus covers the offer and sale of up to 5,229,200 shares of our common stock from time to time by the selling security holders named in this prospectus. The shares of common stock covered by this prospectus are shares that are held, beneficially and of record, by the selling security holders. We are not offering any shares of common stock. The selling security holders will receive all of the net proceeds from sales of the common stock covered by this prospectus.
Our common stock is presently not traded on any national market or securities exchange or in the over-the-counter market. The sales price to the public of the shares of our common stock offered by the selling security holders under this prospectus is fixed at $0.20 per share until such time as our common stock is quoted on the Over-The-Counter (OTC) Bulletin Board and/or the OTCQB or OTC Pink markets. Although we intend to request a registered broker-dealer to apply with the Financial Industry Regulatory Authority to have our common stock eligible for quotation on the OTC Bulletin Board, public trading of our common stock may never materialize or, even if materialized, trading may not be sustained. If our common stock is quoted on the OTC Bulletin Board, then the sale price to the public will vary according to prevailing market prices or privately negotiated prices by the selling security holders. To the best of our knowledge, none of the selling security holders are broker-dealers, underwriters or affiliates thereof.
As of May 30, 2011 we had 51,218,680 shares of common stock issued and outstanding and 2,990,252 shares of Series A Preferred Stock issued and outstanding. The Series A Preferred Stock is convertible into shares of our common stock but issuance and/or resale of such conversion shares are not covered by this prospectus.
INVESTING IN OUR COMMON STOCK IS SPECULATIVE AND INVOLVES A HIGH DEGREE OF RISK AND SHOULD BE CONSIDERED ONLY BY PERSONS WHO CAN AFFORD THE LOSS OF THEIR ENTIRE INVESTMENT. PLEASE REFER TO “RISK FACTORS” BEGINNING ON PAGE 4.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THE PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Our offices are located at 77 McCullough Drive, New Castle< Delaware 19720. Our telephone number is (800) 957-7622. Our website can be found at www.scripsamerica.com.
The Date of This Prospectus Is: _______ __, 2011
|
TABLE OF CONTENTS
|
PAGE
|
|
|
Prospectus Summary
|
1
|
|
Summary Financial Data
|
2
|
|
Risk Factors
|
3
|
|
Use of Proceeds
|
7
|
|
Determination of Offering Price
|
7
|
|
Selling Shareholders
|
7
|
|
Plan of Distribution
|
12
|
|
Description of Securities to be Registered
|
15
|
|
Interest of Named Experts and Counsel
|
15
|
|
Description of Business
|
15
|
|
Description of Property
|
25
|
|
Legal Proceedings
|
25
|
|
Market for Common Equity and Related Stockholder Matters
|
25
|
|
Where You Can Find More Information
|
26
|
|
Financial Statements
|
27
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
28
|
|
Changes In and Disagreements with Accountants on Accounting and Financial Disclosure
|
37
|
|
Directors, Executive Officers, Promoters and Control Persons
|
38
|
|
Executive Compensation
|
41
|
|
Security Ownership of Certain Beneficial Owners and Management
|
41
|
|
Certain Relationships and Related Transactions
|
41
|
|
Disclosure of Commission Position of Indemnification for Securities Act Liabilities
|
42
|
You should rely only on the information contained in this prospectus. We have not, and the selling security holder has not, authorized anyone to provide you with different information. If anyone provides you with different information, you should not rely on it. We are not, and the selling security holder is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information contained in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date. In this prospectus, “ScripsAmerica”, “the Company”, “we”, “us” and “our” refer to ScripsAmerica, Inc., a Delaware corporation, unless the context otherwise requires.
i
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in the common stock. You should carefully read the entire prospectus, including “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements, before making an investment decision .
About ScripsAmerica, Inc.
We are ScripsAmerica, Inc., a Delaware corporation that was formed on May 12, 2008. We are a distributor of prescription and over the counter (OTC) pharmaceuticals.
We implement efficient supply chain management on behalf of our clients, from strategic sourcing to delivering niche generic pharmaceuticals to market. Positioned in the center of the pharmaceutical value chain, we receive purchase orders from pharmaceutical distributors, contract with pharmaceutical packagers and their manufacturers to process orders to the end user’s specifications, and deliver product to a wide range of customers across the health care industry.
Our primary value lies in our growing portfolio of end users to whom we market our services. For end users such as hospitals and home care agencies, custom packaging such as unit of use can save staff time and cost, as well as eliminate dispensing errors at the pharmacist level. Further, we maintain a strategic relationship with the largest pharmaceutical distributor in North America.
In addition to purchase order fulfillment, ScripsAmerica seeks to diversify the Company’s revenue sources by securing FDA approval for and bringing to market so-called DESI drugs, while minimizing clinical risk, and by developing rapid melt formulations of vitamins, OTC drugs and certain generic products. We also plan to acquire pharmaceutical packagers and pharmaceutical manufacturers for a vertical expansion of our business as well.
Principal Executive Offices
Our principal executive offices are located at 77 McCullough Drive, New Castle, Delaware 19720. Our telephone number is (800) 957-7622 and our fax number is (215) 405-2650. Our website address is www.scripsamerica.com. The information on our website is not incorporated by reference into this prospectus and should not be relied upon with respect to this offering.
Recent Developments
On April 1, 2011 we closed on the sale of 2,990,252 shares of our Series A Preferred Stock to a single accredited investor for a purchase price of $1,043,000. The sale of shares was exempt under Section 4(2) of the Securities Act as an offer and sale not involving a public offering. Each share of Series A Preferred Stock is convertible into one share of our common stock. The Series A Preferred Stock is paid a dividend at annual rate of 8% of the purchase price, which dividend is paid at the end of each fiscal quarter. Of the seven members of our board of directors, the holder of the Series A Preferred Stock, as a single class, gets to elect one (1) director to the board and will vote with the common stockholders to four (4) directors (the common stockholders will elect, as a single class, two (2) directors). The Series A Preferred Stockholder will have approval right over certain corporate actions, namely our liquidation or dissolution, any merger, share exchange or asset sale that results in a change of control, the payment of any dividends or the redemption of stock (except for stock dividends, change of control transaction and termination of employment or service). The Series A Preferred Stock is convertible into 5,989,680 shares of our common stock (based on a conversion price of $0.1744, which was adjusted as a result of the forward stock split (as described below). The conversion price of the Series A Preferred Stock will be adjusted for any issuances of stock by us at a price per share less than $0.1744 (subject to certain exemptions such as securities issued under an employee stock option plan or securities issued in business transactions approved by our board). The Series A Preferred Stock has priority to assets over the common stockholders in the event of a liquidation, dissolution or any merger, share exchange or consolidation in which we are not the surviving entity or there is a change in control of us). These rights of the Series A Preferred Stockholder continue until all of the shares of Series A Preferred Stock are converted into our common stock.
On April 15, 2011, we had a forward two-for-one stock split. In addition, as a result of the forward stock split, we adjusted the conversion price of the Series A Preferred Stock to $0.1744 (reduced from $0.3488).
In April 2011, we sold 5,200,000 shares of our common stock to four purchasers for an aggregate purchase price of $176,000. Each of the purchasers was a corporation formed outside of the United States with a business address located outside of the United States. This transaction was exempt from the registration provisions of the Securities Act pursuant to Regulation S as an offshore transaction with non-U.S. persons (as such term is defined in Rule 902 of Regulation S).
In May 2011, we sold 29,000 shares of our common stock to 58 purchasers for an aggregate purchase price of $5,800. Each of the purchasers was a non-U.S. citizen with a residence address located outside of the United States. This transaction was exempt from the registration provisions of the Securities Act pursuant to Regulation S as an offshore transaction with non-U.S. persons (as such term is defined in Rule 902 of Regulation S).
1
SUMMARY FINANCIAL DATA
The following tables summarize the financial data for our business. You should read this summary financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes, all included elsewhere in this prospectus.
We derived the statements of income data for the years ended December 31, 2009 and 2010 and the balance sheet data as of December 31, 2009 and 2010, from our audited financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future.
|
For the Year
Ended
|
For the Year
Ended
|
For the Three Months Ended | ||||||||||||||
|
December 31, 2010
(Audited) |
December 31, 2009
(Audited) |
March 31,
2011
(Unaudited)
|
March 31,
2010
(Unaudited)
|
|||||||||||||
|
Net Sales
|
$ | 3,221,320 | $ | -- | $ | 1,641,233 | $ | 310,227 | ||||||||
|
Gross Profit
|
$ | 677,399 | $ | -- | $ | 465,844 | $ | 51,850 | ||||||||
|
Total Operating Expenses
|
$ | 360,900 | $ | (25,599 | ) | $ | 382,428 | $ | 142 | |||||||
|
Net Income
|
$ | 127,072 | $ | 16,090 | $ | 12,056 | $ | 34,128 | ||||||||
|
Earnings Per Common Share – Basic and Diluted
|
$ | -- | $ | -- | $ | -- | $ | -- | ||||||||
|
BALANCE SHEET DATA:
|
As of
December 31, 2010
(Audited) |
As of
December 31, 2009
(Audited) |
As of
March 31, 2011
(Unaudited)
|
|||||||||
|
Cash
|
$
|
171,898
|
$
|
503
|
$ | 532,540 | ||||||
|
Working Capital
|
$
|
55,895
|
$
|
56,503
|
$ | 100,451 | ||||||
|
Notes payable – related parties
|
$
|
60,000
|
$
|
--
|
$ | 80,000 | ||||||
|
Total Current Liabilities
|
$
|
428,310
|
$
|
--
|
$ | 629,375 | ||||||
|
Stockholders’ equity
|
$
|
264,895
|
$
|
63,803
|
$ | 309,451 | ||||||
The Offering
|
Shares of common stock being registered
|
5,229,000 shares of our common stock offered by selling security holders
|
|
|
Total shares of common stock outstanding as of the date of this prospectus
|
51,218,680
|
|
|
Total proceeds raised by us from the disposition of the common stock by the selling security holders or their transferees
|
We will not receive any proceeds from the sale of shares by the selling security holders
|
2
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy and plans and objectives of management for future operations, the development of the market for our products and the acceptance of our products in these markets, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
This prospectus contains industry data and other statistical information regarding packaging, distribution and sales and marketing services for the pharmaceutical industry that we obtained from independent publications, government publications, press releases, reports by market research firms or other published independent sources. Although we believe these sources are reliable, we have not independently verified their data.
RISK FACTORS
An investment in our common stock involves a high degree of risk. You should carefully consider the risks described below and the other information in this prospectus before investing in our common stock. If any of the following risks occur, our business, operating results and financial condition could be seriously harmed. Please note that throughout this prospectus, the words “we”, “our” or “us” refer to the Company and its subsidiary not to the selling stockholders.
Risk Related to Our Company
Disruptions in our supply chain or among other companies providing services to us could adversely affect our ability to fill purchase orders, which would have a negative impact on our financial performance. The failure of a single source in the supply chain would cause only minor delays in our ability to fill purchase orders. In the event of a supply gap, we would either procure product in the market, if available at a reasonable cost, or work with other sources to formulate the drug in question. Such fixes to the supply gap would cause delay of shipment and increase costs, both of which would have negative impact on our profitability and our results of operations.
We have significant credit and sales concentration as we only have one main customer. Substantial defaults in payments by such customer, a material reduction in purchases or the loss of such customer as a customer could have a material adverse impact on ScripsAmerica’s financial condition, results of operations, and liquidity. For the year ended December 31, 2010, McKesson accounted for 100% of our sales. At present, McKesson is our only customer and they provide us access to the end users of our products and services. As a result, our sales and credit concentration is significant. In the event that McKesson experiences difficulties that would result in its default on payments due to us, a material reduction in purchase orders, or a termination of the relationship, our operations would come to a halt until we established an equivalent relationship with another large distributor. Development of additional distributor relationships for risk diversification purposes will be a focus for us as we expand our operations.
Competition from horizontal and vertical markets involved in pharmaceutical distribution business may erode our profit. Our distribution arm faces competition, both in price and service, from national, regional, and local full-line, short-line, and specialty wholesalers, service merchandisers, self-warehousing chains, manufacturers engaged in direct distribution, and large payor organizations. In addition, competition exists from various other service providers and from pharmaceutical and other healthcare manufacturers (as well as other potential customers) which may from time to time decide to develop, for their own internal needs, supply management capabilities that would otherwise be provided by us. Price, quality of service, and in some cases convenience to the customer are generally the principal competitive elements in this segment.
3
Any acquisitions of technologies, products and businesses that we may acquire to expand or complement our business may be difficult to integrate, could adversely affect our relationships with key customers, and/or could result in significant charges to earnings as well potential dilution to existing stockholders. One element of our business strategy is to identify, pursue and consummate acquisitions that either expand or complement our business. Integration of acquisitions entails a number of risks including the diversion of management’s attention to the assimilation of the operations of acquired businesses; difficulties in the integration of operations and systems; the realization of potential operating synergies; the retention of the personnel of the acquired companies; accounting, regulatory or compliance issues that could arise; challenges in retaining the customers of the combined businesses; and a potential material adverse impact on operating results. If we are not able to successfully integrate our acquisitions, we may not obtain the advantages and synergies that the acquisitions were intended to create, which may have a material adverse effect on our business, results of operations, financial condition and cash flows, our ability to develop and introduce new products and the market price of our stock. In addition, in connection with acquisitions, we could experience disruption in our business, technology and information systems, customer or employee base, including diversion of management’s attention from our continuing operations. There is also a risk that key employees of companies that we acquire or key employees necessary to successfully commercialize technologies and products that we acquire may seek employment elsewhere, including with our competitors. Furthermore, In addition, we will require additional financing in order to fund future acquisitions, which may or may not be attainable. In addition, if we acquire businesses or products, or enter into other significant transactions, we expect to experience significant charges to earnings for merger and related expenses. These costs may include substantial fees for investment bankers, attorneys, accountants and financial printing costs and severance and other closure costs associated with the elimination of duplicate or discontinued products, operations and facilities. Charges that we may incur in connection with acquisitions could adversely affect our results of operations for particular quarterly or annual periods. Finally, we may use shares of our common stock to finance some or all of the purchase price of an acquisition, which may result in a downward trend in our stock price, especially if our results of operations are negatively impacted by such acquisition(s).
We could suffer reputational and financial damage in the event of product recalls. We may be held liable if any product we develop or market causes illness or injury or is found otherwise unsuitable. In addition to any reputational damage we would suffer, we cannot guarantee that our supplier’s product liability insurance would fully cover potential liabilities. However, we are named as an additional insured on the product liability insurance policies of our suppliers. Although our management believes that coverage limits specified by these policies are sufficient. In the event of litigation, any adverse judgments against us would have a material adverse effect on our financial condition, including our cash balances, and results of operations.
Our ability to operate effectively could be impaired if we were to lose the services of our key personnel, or if it were unable to recruit key personnel in the future. Our near-term success will depend to a significant extent on the skills and efforts of Robert Schneiderman and Jeffrey Andrews. While the Company plans to enter into employment agreements with Messrs. Schneiderman, and Andrews in the coming months, such agreements do not assure the services of such personnel as employees may voluntarily terminate their employment with us at any time. The loss of one or more current key employees could have a material adverse effect on our business even if replacements were hired. Our success also depends on its ability to attract and retain additional qualified employees in the future. Competition for such personnel is intense, and we will compete for qualified personnel with numerous other employers, many of whom have greater financial and other resources than the Company does. Our plans to incentivize employees to engage in a long-term relationship with the Company through awarding equity as part of overall compensation.
We may not successfully manage any growth that we may experience through the potential acquisitions we are evaluating, which may result in poor results of operations and may harm our growth. Our future success will depend upon not only product development but also on the expansion of our pharmaceutical supply chain management service business and the effective management of any such growth, which will place a significant strain on our management and on our administrative, operational, and financial resources. To manage any such growth, we will need to integrate into our existing new facilities, employees and operational, financial and management systems. For such integration to be done successfully, our management will need to devote its resources and time to the process. That focus may draw management’s attention from other aspects of the business, such as revenue trends, expense management and/or strategic decisions. If we are unable to manage our growth effectively, our business and results of operations would be harmed as our growth could be adversely affected by such mismanagement.
4
Risks Related to Our Industry
Changes in the U.S. healthcare environment could have a material adverse impact on our results of operations. In recent years, the U.S. healthcare industry has changed significantly in an effort to reduce costs. These changes include increased use of managed care, cuts in Medicare and Medicaid reimbursement levels, consolidation of pharmaceutical and medical-surgical supply distributors, and the development of large, sophisticated purchasing groups. Some of these changes, such as adverse changes in government funding of healthcare services, legislation or regulations governing the delivery or pricing of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to reduce the amount of our products and services they purchase or the price they are willing to pay for our products and services. Changes in the healthcare industry’s or our pharmaceutical suppliers’ pricing, selling, inventory, distribution or supply policies or practices could also significantly reduce our revenues and net income. Healthcare and public policy trends indicate that the number of generic drugs will increase over the next few years as a result of the expiration of certain drug patents. While this is expected to be a positive development for us, changes in pricing of certain generic drugs could have a material adverse impact on our revenues and our results of operations.
Regulation of our distribution business could impose increased costs, delay the introduction of new products, which could negatively impact our business. The healthcare industry is highly regulated. As a result, we and our suppliers and distributor are subject to various local, state and federal laws and regulations, which include the operating and security standards of the Drug Enforcement Administration (DEA), the FDA, various state boards of pharmacy, state health departments, the HHS, CMS, and other comparable agencies. The process and costs of maintaining compliance with such operating and security standards could impose increased costs, delay the introduction of new products and negatively impact our business. For example, there have been increasing efforts by various levels of government agencies, including state boards of pharmacy and comparable government agencies, to regulate the pharmaceutical distribution system in order to prevent the introduction of counterfeit, adulterated and/or mislabeled drugs into the pharmaceutical distribution system. Certain states have adopted or are considering laws and regulations that are intended to protect the integrity of the pharmaceutical distribution system, while other government agencies are currently evaluating their recommendations. In addition, the U.S. Food and Drug Administration (“FDA”) Amendments Act of 2007, which went into effect on October 1, 2007, requires the FDA to establish standards and identify and validate effective technologies for the purpose of securing the pharmaceutical supply chain against counterfeit drugs. These standards may include any track-and-trace or authentication technologies, such as radio frequency identification devices and other similar technologies. These pedigree tracking laws and regulations could increase the overall regulatory burden and costs associated with our pharmaceutical distribution business, and would have a material adverse impact on our operating expenses and our results of operations.
Risks Related to Our Stock
We may need to raise additional capital by sales of our common stock, which may adversely affect the market price of our common stock and your rights in us may be reduced.
We expect to continue to incur product development and selling, general and administrative costs, and as well as funding for potential acquisitions. In order to satisfy our funding requirements we may consider issuing additional debt or equity securities. If we issue equity or convertible debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. If we incur additional debt, it may increase our leverage relative to our earnings or to our equity capitalization, requiring us to pay additional interest expenses and potentially lower our credit ratings. We may not be able to market such issuances on favorable terms, or at all, in which case, we may not be able to develop or enhance our products, execute our business plan, take advantage of future opportunities, or respond to competitive pressures or unanticipated customer requirements.
There is currently no public market for our shares and if such a market materializes, our stockholders may still not be able to resell their shares at or above the price at which they purchased their shares.
There is currently no established public trading market for our securities and an active trading market in our securities may not develop or, if developed, may not be sustained. We intend to apply for admission to quotation of our securities on the OTC Bulletin Board, as well as the OTCQB and OTCPink, after this prospectus is declared effective by the SEC. If for any reason our common stock is not quoted on the OTC Bulletin Board (or the OTCQB and OTC Pink) or a public trading market does not otherwise develop, purchasers of the shares may have difficulty selling their common stock should they desire to do so. No market makers have committed to becoming market makers for our common stock and none may do so.
State securities laws may limit secondary trading, which may restrict the states in which and conditions under which you can sell the shares offered by this prospectus.
Secondary trading in common stock sold in this offering will not be possible in any state until the common stock is qualified for sale under the applicable securities laws of the state or there is confirmation that an exemption, such as listing in certain recognized securities manuals, is available for secondary trading in the state. If we fail to register or qualify, or to obtain or verify an exemption for the secondary trading of, the common stock in any particular state, the common stock could not be offered or sold to, or purchased by, a resident of that state. In the event that a significant number of states refuse to permit secondary trading in our common stock, the liquidity for the common stock could be significantly impacted thus causing you to realize a loss on your investment.
5
Our board of directors has the power to designate a series of preferred stock without shareholder approval that could contain conversion or voting rights that adversely affect the voting power of holders of our common stock and may have an adverse effect on our stock price.
Our Certificate of Incorporation provide for the authorization of 10,000,000 shares of “blank check” preferred stock. Pursuant to our Certificate of Incorporation, our Board of Directors is authorized to issue such “blank check” preferred stock with rights that are superior to the rights of stockholders of our common stock, at a purchase price then approved by our Board of Directors, which purchase price may be substantially lower than the market price of shares of our common stock, without stockholder approval. In March 2011, our Board of Directors authorized 2,990,252 shares of Series A Preferred Stock for a private placement of such shares for an aggregate purchase price of $1,043,000. Though we currently do not have any plans to issue any additional shares of preferred stock, such issuance could give the holders of such preferred stock voting control of the Company which would have a negative effect on the voting power of the holders of our common stock and may cause our stock price to decline.
The sale of shares of common stock issuable upon the conversion of our outstanding shares of our Series A Preferred Stock could have a negative impact on the market price of our stock if sold. We have 2,990,252 shares of Series A Preferred Stock that are convertible into 5,980,504 shares of common stock (representing approximately 10.5% of the outstanding stock on a fully diluted basis). On October 1, 2011, the shares of common stock may issuable upon the conversion of the Series A Preferred Stock may be sold to the public under Rule 144 (subject only to the volume limitations of Rule 144(d)). If our common stock does not trade with large enough share volume, the sales of the common stock issued upon the conversion of the Series A Preferred Stock may cause the price of our stock to drop significantly. Additionally, the holder of the Series A Preferred Stock has registration rights for the shares of common stock issuable upon the conversion of the Series A Preferred Stock. If the Series A Preferred stockholder exercises such registration rights, such stockholder could sell a large number of shares of our common stock which could cause significant drop in our stock price.
The outstanding shares of our Series A Preferred Stock are entitled to rights and privileges in regard to distribution of assets and protective provisions which may result in actions adverse to the holders of our common stock. So long as there are shares of Series A Preferred Stock outstanding, the holders of such security are entitled to an annual dividend of 8% of the original purchase price, as well as priorities to our distribution of cash and other assets. The holder Series A Preferred Stockholder also has veto power over certain corporate matters, such as redeeming or repurchasing capital stock or any merger, consolidation or share exchange that would result in a change of control. The rights of the Series A Preferred Stockholder will continue until all of the shares are converted into our common stock (either voluntarily or upon an underwritten IPO in which we have gross proceeds of at least $25 million and a price per share of at least $0.872). The holder of such rights of the Series A Preferred Stock may have interests adverse to the common stockholders and the exercise of such rights may have a negative impact on the value of our common stock or the amount of cash or other assets our common stockholders may receive in connection with a distribution or merger, consolidation or share exchange.
Our principal shareholders have significant voting power and may take actions that may not be in the best interest of our other shareholders, who will have no influence over shareholder decisions. Robert Schneiderman, our Chief Executive Officer and a director, and Steve Urbanski, our Executive Vice President and a director, each own 19,980,000, which is approximately 39% of the outstanding shares of our common stock. Messrs. Schneiderman and Urbanski have the ability to exert virtual control over all matters requiring approval of our shareholders, including the election and removal of directors and the approval of mergers or other business combinations (in each case subject to the rights of the Series A Preferred Stockholder as long as there are any such shares outstanding). This concentration of control could be disadvantageous to other shareholders whose interests are different from those of Messrs. Schneiderman and Urbanski. Although there is no voting arrangement between Messrs. Schneiderman and Urbanksi, this concentration of ownership, nonetheless, may have the effect of delaying, deferring, or preventing a change in control, impeding a merger, consolidation, takeover, or other business combination involving us, or discouraging a potential acquirer from making a tender offer, or otherwise attempting to obtain control of us or our business, even if such a transaction would benefit other shareholders.
We do not intend to pay dividends for the foreseeable future except to the Series A Preferred Stockholder.
We have never declared or paid any cash dividends on our common stock and do not intend to pay any cash dividends in the foreseeable future. Except for any dividends owed to the holder of our Series A Preferred Stock, we anticipate that we will retain all of our future earnings for use in the development of our business and for general corporate purposes. Any determination to pay dividends in the future will be at the discretion of our board of directors. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments.
6
Our common stock is expected to be considered “a penny stock” and, as a result, it may be difficult to trade a significant number of shares of our common stock.
The Securities and Exchange Commission (“SEC”) has adopted regulations that generally define “penny stock” to be an equity security that has a market price of less than $5.00 per share, subject to specific exemptions. When our common stock becomes eligible for quotation on the OTC markets (such as the bulletin board or OTCQB), we expect the market price of our common stock to be less than $5.00 per share. As a result of our prior private placements and our forward stock split, we have increased the number of shares outstanding by almost three-fold. Consequently, when our common stock becomes eligible for quotation on the OTC markets it is likely that the market price for our common stock will remain less than $5.00 per share for the foreseeable future and, therefore, may be a “penny stock” according to SEC rules. This designation requires any broker or dealer selling these securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities. These rules may restrict the ability of brokers or dealers to sell our common stock and may affect the ability of investors hereunder to sell their shares. In addition, because we are seeking to have our common stock trade on the OTC markets, investors may find it difficult to obtain accurate quotations of the stock and may experience a lack of buyers to purchase such stock or a lack of market makers to support the stock price.
USE OF PROCEEDS
We will not receive any proceeds from the sale of the shares by the selling stockholders.
DETERMINATION OF OFFERING PRICE
Our common stock is presently not traded on any national market or securities exchange or in the over-the-counter market. As there is no existing public market for our securities, the shares offered for resale hereunder by the selling security holders must initially be offered at a fixed price.
The sales price to the public of the shares of our common stock offered by the selling security holders under this prospectus is fixed at $0.20 per share until such time as our common stock is quoted on the Over-The-Counter (OTC) Bulletin Board, the OTCQX and/or the OTCQB and a public market exists for our common stock. This fixed sales price was determined by using the most recent price paid in cash that we received for our stock, which was the price in our Regulation S offering to the selling security holders as described below in the “Selling Security Holders” section. We expect that the selling security holders will offer their stock in lots of at least 100 shares at the fixed price set forth in the Amendment. It is uncertain, however, how much demand there will be for these shares prior to the commencement of the public trading market.
SELLING SHAREHOLDERS
The following table sets forth the name of the selling shareholders, the number of shares of common stock owned, the number of shares of common stock registered by this prospectus and the number and percent of outstanding shares that the selling shareholder will own after the sale of the registered shares, assuming all of the shares are sold. The information provided in the table and discussions below has been obtained from the selling shareholder. As used in this prospectus, “selling shareholder” includes donees, pledges, transferees or other successors in interest selling shares of our common stock received from the named selling shareholder as a gift, pledge, distribution or other non-sale related transfer. Within the past three years, none of the selling security holders has held any position, office, or other material relationship with us or any of our predecessors or affiliates.
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the Securities and Exchange Commission under the Exchange Act. Unless otherwise noted, each person or group identified possesses sole voting and investment power with respect to the shares, subject to community property laws where applicable. As of May 30, 2011, there were 51,218,680 shares of our common stock issued and outstanding.
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Baypointe Investments Ltd. (4)
|
1,300,000
|
2.5%
|
1,300,000
|
0
|
--
|
|
Burnt Rock Investments Ltd. (5)
|
1,300,000
|
2.5%
|
1,300,000
|
0
|
--
|
|
Blue Shade Inc. (6)
|
1,300,000
|
2.5%
|
1,300,000
|
0
|
--
|
|
Harvest Enterprises Limited (8)
|
1,300,000
|
2.5%
|
1,300,000
|
0
|
--
|
7
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Zahid Abdullatif
Apt. 33
7030 Ch. De La Cote-Saint Luc
Montreal, Quebec H4V 1J3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Marc Aboaf
21 Har Rotem Street
44539 Kear Saba
Israel
|
500
|
*
|
500
|
0
|
--
|
|
Claude N. Allard
2 Willow Cresc
Orangeville, Ontario L9V 1A5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Lori Dawn Allard
2 Willow Crescent
Orangeville, Ontario L9V 1A5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Arnel Asube
30 Halldorson Avenue
Aurora, Ontario L4G 7Z1
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Rachelle Bat-Amy
55 Russell Avenue
Ottawa, Ontario K1N TW9
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Karen Bell
1614 Avonmore Square
Pickering, Ontario
Canada L1V 7H4
|
500
|
*
|
500
|
0
|
--
|
|
Charles Benedek
5659 Av Jellico
Cote Saint-Luc, Quebec
H4W 1Z5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Naomi Berkowitz
5755 Leger
Cote-Saint-Luc, Quebec
H4W 2E8
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Robert Biancolin
77 Castlewood Road
Toronto, Ontario M5N 2L3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Iola Biancolin
77 Castlewood Road
Toronto, Ontario M5N 2L3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Layla Biancolin
Apt. 804
135 George St. South, Toronto, Ontario M5A 4E8 Canada
|
500
|
*
|
500
|
0
|
--
|
8
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Maurice Biancolin
Apt. 804
135 George St. South, Toronto, Ontario M5A 4E8 Canada
|
500
|
*
|
500
|
0
|
--
|
|
Dimitrios Catsiliras
241 Westwood Avenue
Toronto, Ontario M4J 2H3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Morris Chaikelson
4950 Ponsard Avenue
Montreal, Quebec H4W 2A5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Roberto Ciancibello
50 Old Mill Road, GLA-6, Oakville, Ontario, L6J 7W1
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Louise Clement
2293 Avenue D'Oxford
Montreal, Quebec H4A 2X7
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Leonard Cohen
Apt. 29
7030 Ch. De La Cote-Saint-Luc
Montreal, Quebec H4V 1J3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Rosario Cojuangco
30 Halldorson Avenue
Aurora, Ontario L4G 7Z1
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Lucinda Daly
900 - 150 Ferrand Drive
Toronto, Ontario M3C 3E5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Avraham Einhoren
750 Bay Street
Toronto, Ontario M5G 1N6
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Oher Einhoren
6 Joe Amar Street
Kefar-Saba, Israel
|
500
|
*
|
500
|
0
|
--
|
|
Menahem Einhoren
Am Bergfried 1
Langen Germany 63225
|
500
|
*
|
500
|
0
|
--
|
|
Naama Einhoren
750 Bay Street
Toronto, Ontario M5G 1N6
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Robert Gold
900 - 150 Ferrand Drive
Toronto, Ontario M3C 3E5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Alpha Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Linh Tu Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
9
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Mai Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Sinh Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Van Tu Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Xuan Thi Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Yome Huynh
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Gerald Issenman
225 Stanstead Avenue
Montreal, Quebec H3R 1X4
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Elisa Maguolo
Via Chiarin 101b
Campalto, Venezia
Italy
|
500
|
*
|
500
|
0
|
--
|
|
Shari McMaster
601 - 130 Bloor Street West
Toronto, Ontario M5S 1N5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Jodi Parnass
32 Stephenson
Dollard des Ormeaux, Quebec
H9A 2V9 Canada
|
500
|
*
|
500
|
0
|
--
|
|
Paul Rubin
402-3495 Du Musee
Montreal, Quebec H3G 2C8
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Binh Quach
1568 Princelea Place
Mississauga, Ontario L5M 3P2
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Cuc Quach
1568 Princelea Place
Mississauga, Ontario L5M 3P2
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Pat Rubin
402-3495 Du Musee
Montreal, Quebec H3G 2C8
Canada
|
500
|
*
|
500
|
0
|
--
|
10
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Stephen Smith
755 Leger
Cote-Saint-Luc, Quebec
H4W 2E8
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Cheryl Stoyanovski
1673 Pepperwood Gate
Pickering, Ontario L1X 2G1
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Tom Stoyanovski
1673 Pepperwood Gate
Pickering, Ontario L1X 2G1
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Jeff Suissa
32 Stephenson
Dollard des Ormeaux, Quebec H9A 2V9
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Chu Thai
4118 Sunset Valley Court
Mississauga, Ontario L4W 3L5
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Andrew Thomas
2559 Burnford Trail
Mississauga, Ontario L5M 5E3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Paul Thomas
24 Wellesley St. W.
Suite 2110
Toronto, Ontario M4Y 2X6
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Ronald Thomas
350 Doon Valley Drive
Unit 7B
Kitchener, Ontario L5M 3P2
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Shirley Thomas
350 Doon Valley Drive
Unit 7B
Kitchener, Ontario L5M 3P2
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Shizuka Thomas
2559 Burnford Trail
Mississauga, Ontario L5M 5E3
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Sherri Trager
225 Stanstead Avenue
Montreal, Quebec H3R 1X4
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Lorne Wolf
2567 Bedford
Montreal, Quebec H35 1E8
Canada
|
500
|
*
|
500
|
0
|
--
|
11
|
Beneficial Ownership of Common Stock
Prior to this Offering
|
Number of Shares
to be Sold
|
Beneficial Ownership of Common Stock
after this Offering
|
|||
|
Selling Shareholder
|
Number
of Shares
|
Percent
of Class
|
Under this
Prospectus (1)
|
Number of
Shares (2)
|
Percent of
Class (3)
|
|
Gayle Wolf
2567 Bedford
Montreal, Quebec H35 1E8
Canada
|
500
|
*
|
500
|
0
|
--
|
|
Paul Zammit
601 - 130 Bloor Street West
Toronto, Ontario M5S 1N5
Canada
|
500
|
500
|
0
|
--
|
|
|
Peggy Zammit
601 - 130 Bloor Street West
Toronto, Ontario M5S 1N5
Canada
|
500
|
500
|
0
|
--
|
|
|
Rosana Zammit
33 Rosehill Avenue
Apt. 2908
Toronto, Ontario M4T 1G4
|
500
|
500
|
0
|
--
|
|
|
Sondra Zammit
5795 Yonge Street
Apt. 1108
Toronto, Ontario M2m 4J3
Canada
|
500
|
500
|
0
|
--
|
|
|
Robert Zirbi
55 Russell Avenue
Ottawa, Ontario K1N TW9
Canada
|
500
|
500
|
0
|
--
|
*Less than one percent (1%)
|
(1)
|
The number of shares set forth in the table represents an estimate of the number of common shares to be offered by the selling shareholder. We have assumed the sale of all of the common shares offered under this prospectus will be sold. However, as the selling shareholder can offer all, some or none of its common stock, no definitive estimate can be given as to the number of shares that the selling shareholder will offer or sell under this prospectus.
|
|
(2)
|
These numbers assume the selling shareholder sells all of its shares after the completion of the offering.
|
|
(3)
|
Based on 45,989,680 shares of common stock outstanding after the completion of the offering.
|
|
(4)
|
Chang Yong You, the owner of Baypointe Investments Ltd., has the power to vote and dispose of the Company’s securities held by Baypointe Investments Ltd. Baypointe Investments Ltd. has a business address at P.O. Box CR 56766, Suite 1252, #33 Harbour Bay Plaza, East Bay Street, Nassau, Bahamas
|
|
(5)
|
Young Hae Shin, the owner of Burnt Rock Investments Ltd., has the power to vote and dispose of the Company’s securities held by Burnt Rock Investments Ltd. Burnt Rock Investments Ltd. has a business address at PO Box AP 59205, Suite 321, Norfork House, Frederick Street, Nassau Bahamas.
|
|
(6)
|
Wilfred Gatambia Kamau, the owner of Blue Shade Inc., has the power to vote and dispose of the Company’s securities held by Blue Shade Inc. Blue Shade Inc. has a business address at PO Box 14, Clarkes Estate, Cades Bay, Nevis, West Indies.
|
|
(7)
|
Hyo Ki Lim, the owner and Chief Executive Officer of Harvest Enterprises Limited, has the power to vote and dispose of the Company’s securities held by Harvest Enterprises Limited. Harvest Investments Ltd. has a business address at PO Box 14, Clarkes Estate, Cades Bay, Nevis, West Indies.
|
PLAN OF DISTRIBUTION
As of the date of this prospectus, there is no market for our securities. After the date of this prospectus, we expect to have an application filed with the Financial Industry Regulatory Authority for our common stock to be eligible for trading on the OTC Bulletin Board as well as the OTCQB and the OTC Pink. Until our common stock becomes eligible for trading on the OTC Bulletin Board or the OTCQB or the OTC Pink, the selling shareholders will be offering our shares of common stock at a fixed price of $0.20 per share of common stock. After our common stock becomes eligible for trading on the OTC Bulletin Board, the OTCQB or the OTC Pink, the selling shareholders may, from time to time, sell all or a portion of the shares of common stock on OTC Bulletin Board, the OTCQB and/or the OTC Pink or any market upon which the shares of common stock may be listed or quoted currently the OTC Bulletin Board, the OTCQB and/or the OTC Pink in the United States, in privately negotiated transactions or otherwise. After our common stock becomes eligible for trading on the OTC Bulletin Board, the OTCQB and/or the OTC Pink, such sales may be at fixed prices prevailing at the time of sale, at prices related to the market prices or at negotiated prices.
12
After our common stock becomes eligible for trading on the OTC Bulletin Board, the OTCQB or the OTC Pink, the shares of common stock being offered for resale by this prospectus may be sold by the selling shareholders by one or more of the following methods, without limitation:
| ● |
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
| ● |
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
| ● |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
| ● |
an exchange distribution in accordance with the rules of the applicable market or exchange;
|
|
| ● |
privately negotiated transactions;
|
|
| ● |
settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part;
|
|
| ● |
broker-dealers may agree with the selling shareholders to sell a specified number of shares at a stipulated price per share;
|
|
| ● |
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
| ● |
a combination of any of these methods of sale; or
|
|
| ● |
any other method permitted pursuant to applicable law.
|
The selling shareholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the selling shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling shareholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA/NASD Rule 2440 in the FINRA Manual; and in the case of a principal transaction a markup or markdown in compliance with FINRA/NASD IM-2440. Before our common stock becomes eligible for trading on the OTC Bulletin Board, broker-dealers may agree with a selling shareholder to sell a specified number of the shares of common stock at a price per share of $0.00533. After our common stock becomes eligible for trading on the OTC Bulletin Board, broker-dealers may agree with a selling shareholder to sell a specified number of the shares of common stock at a stipulated price per share.
In connection with the sale of shares, the selling shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares in the course of hedging the positions they assume. The selling shareholders may also sell shares short and deliver these shares to close out their short positions, or loan or pledge shares to broker-dealers that in turn may sell these shares. The selling shareholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to that broker-dealer or other financial institution of shares offered by this prospectus, which shares that broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect that transaction).
We will be paying certain fees and expenses incurred by us incident to the registration of the shares.
We will keep this prospectus effective until the earlier of (i) the date on which the shares may be resold by the selling shareholders without registration and without regard to any volume limitations by reason of Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the shares have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
13
Under applicable rules and regulations under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), any person engaged in the distribution of the shares may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the selling shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations there under, including Regulation M, which may limit the timing of purchases and sales of the shares by the selling shareholders or any other person. We will make copies of this prospectus available to the selling shareholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale or provide adequate notice of this prospectus (in each case in compliance with Rule 172 under the Securities Act).
Blue Sky Restrictions on Resale
When a selling shareholder wants to sell shares of common stock under this registration statement, the selling shareholders will also need to comply with state securities laws, also known as "Blue Sky laws," with regard to secondary sales. All states offer a variety of exemption from registration for secondary sales. Many states, for example, have an exemption for secondary trading of securities registered under Section 12(g) of the Securities Exchange Act of 1934 or for securities of issuers that publish continuous disclosure of financial and non-financial information in a recognized securities manual, such as Standard & Poor's. The broker for a selling shareholder will be able to advise a selling shareholder which states our shares of common stock is exempt from registration with that state for secondary sales.
Any person who purchases shares of common stock from a selling shareholder under this registration statement who then wants to sell such shares will also have to comply with Blue Sky laws regarding secondary sales. When the registration statement becomes effective, and a selling shareholder indicates in which state(s) he desires to sell his shares, we will be able to identify whether it will need to register or will rely on an exemption there from.
Penny Stock Regulations
The Securities and Exchange Commission has adopted Rule 15g-9 which establishes the definition of a "penny stock," for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| ● |
That a broker or dealer approve a person’s account for transactions in penny stocks; and
|
|
| ● |
That the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
|
In order to approve a person's account for transactions in penny stocks, the broker or dealer must:
| ● |
Obtain financial information and investment experience objectives of the person; and
|
|
| ● |
Make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
|
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Securities and Exchange Commission relating to the penny stock market, which, in highlight form:
| ● |
Sets forth the basis on which the broker or dealer made the suitability determination; and
|
|
| ● |
Specifies that the broker or dealer received a signed, written agreement.
|
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
14
DESCRIPTION OF SECURITIES TO BE REGISTERED
Under our Certificate of Incorporation, as amended, we are authorized to issue up to 150,000,000 shares of Common Stock, and 10,000,000 shares of Preferred Stock. As of May 30, 2011 there were 51,218,680 shares of common stock issued and outstanding and 2,990,252 shares of Series A Preferred Stock issued and outstanding. The Series A Preferred Stock is convertible into shares of our common stock but issuance and/or resale of such conversion shares are not covered by this prospectus
Common Stock
The holders of Common Stock are entitled to one vote for each share of such stock held of record by them. The holders of record of the shares of common stock, exclusively and as a separate class, shall be entitled to elect two (2) of our directors, who may only be removed by the affirmative vote of the holders of the shares of common stock. Although the holder of the Series A Preferred Stock has the right to elect one (1) of our director, the holders of record of the shares of Common Stock and of any other class or series of voting stock (including the Series A Preferred Stock), exclusively and voting together as a single class, are entitled to elect the balance of the total number of our directors (subject to the rights of any additional series of Preferred Stock that may be established from time to time).
Subject to the preferences of the outstanding shares of Series A Preferred Stock, the holders of Common Stock are entitled to receive dividends when, as and if declared by the Board of Directors out of funds legally available therefore, subject to the prior rights of the holders of outstanding shares of Preferred Stock. Upon our liquidation or dissolution, and Subject to the preferences of the outstanding shares of Series A Preferred Stock, holders of Common Stock are entitled to receive all assets available for distribution to security holders, after payment of creditors and preferential liquidation distributions to preferred security holders, if any exist at the time of such liquidation. The Common Stock has no preemptive or other subscription rights or redemption or sinking fund provisions with respect to such shares. All outstanding shares of Common Stock are fully paid and non-assessable.
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
The financial statements of ScripsAmerica, Inc. as of December 31, 2010 and 2009 and for each of the years then ended has been included herein and in the Registration Statement in reliance upon the report of Raich Ende Malter & Co. LLP, an independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
Certain legal matters in connection with this offering and Registration Statement are being passed upon by Fox Law Offices, P.A., Peaks Island, Maine.
Overview
We are ScripsAmerica, Inc., a Delaware corporation, and we were formed in May 2008. We currently provide distribution of pharmaceutical products. We are in the process of expanding our operations to two new areas: (i) developing of a line of rapidly dissolving drug formulations for vitamins, Over The Counter (OTC) drugs and certain generic products and (ii) securing approval of the United States Drug and Food Administration (the “FDA”) and developing drugs under the Drug Efficacy Study Implementation (DESI) Program.
We are focused on efficient pharmaceutical supply chain management services, from strategic sourcing to delivering niche generic pharmaceuticals to market. Through the largest pharmaceutical distributors in North America, we deliver pharmaceutical products to a wide range of customers across the health care industry, including physicians’ offices, retail pharmacies, long-term care sites, hospitals, and Government and home care agencies. Current therapeutic categories include pain, arthritis, prenatal, urinary, and hormonal replacement drugs.
15
We offer fulfillment of prescription and over the counter (OTC) products. To fulfill purchase orders from customers, we entered into distribution agreements with an FDA-approved suppliers, which process orders to the end user’s desired specifications. Capabilities range from unit of use packaging for in-patient nursing homes and hospitals to bulk packaging for government and internatioanal organizations.
Our value lies in our growing portfolio of end user relationships. We market directly to these customers. For the end user, custom packaging such as unit of use can save staff time and cost, as well as eliminate dispensing errors at the pharmacist level.
In March 2010, we entered into a product development, manufacturing and supply agreement with a pharmaceutical company, which develops and manufactures generic drug products. Under this agreement with this pharmaceutical company, we are developing a pain relief orally disintegrating rapidly dissolving 80 mg and 160 mg tablets. This pain relief formulation is for a widely-used OTC drugs.
We have identified Compound SA 1022, a non-steroidal anti-inflammatory drug, as our initial drug candidate for the DESI program approval. We will need to raise approximately $2,000,000 -$3,000,000 in additional funding to obtain FDA approval of Compound SA-1022 under the DESI program.
Market Opportunity
Pharmaceutical Supply Chain Management Services
The United States constitutes the largest market in the world for generic pharmaceuticals, with and its aging population represents a key driver for the growth of the global pharmaceuticals and domestic consumer products markets. Competitive pressures among U.S. generics providers are continuing to increase as a result of the number of new market entrants growing faster than the generics market as a whole, leading to cost competition on the manufacturing side and squeezed profit margins. On the sales side, generics prices are eroding due to low-cost suppliers from India and China capturing market share, as well as the success of health insurers and health maintenance organizations in negotiating lower reimbursement rates. Finally, large direct purchase customers such as chain drugstores demand product variety and reliability of supply that allows them to lower their inventory levels.
Current trends force generics players to focus on growth of their distribution networks, customer retention, and cost minimization.
We are ideally suited to compete in the current environment by providing a low cost system of broad-based marketing, sales, and distribution capabilities for generics, branded pharmaceuticals, over the counter medicines, vitamins, and nutraceuticals. We have built strong relationships with local customers through a detailed understanding of and demonstrated ability to serve customer needs. These qualities have allowed us to post exceptional growth since commencing operations in February 2010.
We cater to a large and growing customer base while enforcing strict inventory control and pursuing fast turn-around times on every order. The final component of our lean and efficient organizational structure is a workforce minimized in size to essential business functions.
DESI Program
DESI was a program begun by the FDA in the 1960s based on the requirement of the Kefauver-Harris Drug Control Act that all drugs be efficacious as well as safe. The DESI program was intended to classify all pre-1962 drugs that were already on the market as either effective, ineffective, or needing further study. To date, DESI has evaluated over 3,000 separate products and over 16,000 therapeutic claims. By 1984, final action had been completed on 3,443 products; of these, 2,225 (64.6%) were found to be effective, 1,051 (30.5%) were found not effective, and 167 (4.9%) were pending.
Once a DESI drug has been FDA approved and granted market exclusivity, the applicant will have the right to be the single source producer of the drug and control market supply and pricing.
Based on market exclusivity, pricing power and extent of use, DESI drugs represent revenue potential to the manufacturer, on average, of approximately $60 million per drug. Financing is currently the rate-limiting variable to our ability to develop Compound SA 1022 and other DESI drug targets
16
We estimate that there are approximately 200 drugs that have yet to be evaluated under the DESI program. If the prior trends of efficacy for DESI drugs continues (meaning approximately 65% of the DESI drugs will be effective), the potential market size for manufacturing the remaining DESI drugs is approximately $7.8 billion.
Rapidly Dissolving Drug Formulations
During 2011 and 2012, many large pharmaceutical companies will lose patent protection of some of their best-selling products. One of the ways that these large pharmaceutical companies to protect and expand the market for the brand name drugs is through new methods of delivery – a new dosage form. According to IMS Health, a health care information and consulting company, drugs representing approximately $89.5 billion in sales during 2010 will be losing patent protection during the five year period ending December 31, 2014. The pharmaceutical industry’s collective drug pipeline is simultaneously in decline. One of approaches that drug companies may consider for offsetting loss of patent protection and weak drug pipeline is updating old drugs with reformulations, which secures revenues for extended time periods and have a higher likelihood of success as compared to new drug development. Reformulation refers to the process of altering a drug’s characteristics just enough to qualify for a new patent, while keeping the same to use previous clinical testing results for the purpose of FDA approval.
Intellectual property protection for new deliveries includes patents based on the new components added to the drug (“composition of matter patents”), and patents based on the way a medical condition is treated using the new delivery system (“method patents”). If an NDA sponsor of a new delivery drug declares bioequivalence to a previously approved drug, the NDA sponsor, like an ANDA (abbreviated new drug application) filer, may incorporate efficacy and safety data previously submitted to the FDA for the bioequivalent drug. Consequently, reformulation by new delivery, particularly when incorporating a patentable delivery system, is an attractive method of life cycle enhancement.
Drug delivery technology can be an effective defensive strategy when a company adds a relevant therapeutic benefit to a marketed drug, such as improved efficacy or dosing frequency, or new therapeutic indications or target user groups. Additional benefits include boosting a drug’s value, reviving its marketplace position, or rejuvenating products that are in their mature life-cycle stage. Drug delivery technology can also enable or accelerate market entry by overcoming issues such as insolubility, formulation difficulties or high dosing frequency.
Rapid melt technology is an example of a reformulation technique aimed at advanced drug delivery and product differentiation. The FDA’s Center for Drug Evaluation and Research (CDER) Data Standards Manual defines orally disintegrating tablets (ODTs) as “a solid dosage form containing medical substances which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue.” The Agency recommends that, in addition to the original definition, ODTs be considered solid oral preparations that disintegrate rapidly in the oral cavity, with an in-vitro disintegration time of approximately 30 seconds or less, without the need for chewing or liquids. Rapid melt tablets are generally characterized by a hydrophilic matrix, which allows prompt disintegration of tablets as they come into contact with saliva. Disintegration releases the active drug moiety trapped in the matrix, permitting the patient to swallow the product in the form of a liquid, or a suspension in the case of non-soluble components. The rapid disintegration tablet excipient offers extremely fast dissolution from tablets with good hardness made with standard techniques. The current market for oral fast-melt drugs in the U.S. is $1.5 billion.
Clinically, rapid melts improve the pharmacoeconomics of drugs by providing faster onset of action as the dosage form is disintegrated prior to reaching the stomach. This is particularly applicable for acute diseases and to manage breakthrough symptoms. Other potential benefits include superior bioavailability, improved therapy through sustained release and high active dose capabilities, safety, efficacy, convenience, and compliance. With medications for chronic diseases that display time-dependent symptoms, such as ulcers or asthma, drug delivery systems can control the formulation release according to the timing of symptoms. For instance, they could enable a drug to release when asthma attacks occur, generally in the middle of the night. This capability can provide valuable and clinically proven therapeutic benefits, as well as a means for marketers to differentiate their product. ustained release action also reduces dosing frequency, which in turn can serve to decrease the frequency of caregiver interactions. Fewer visits from doctors and nurses save administration costs and time and increase convenience for both patients and caregivers.
Orally disintegrating and fast-dissolving dosage forms have continued to expand as they address a combination of issues traditionally associated with pharmaceuticals administered as oral solid dosages. These include dysphagia (difficulty swallowing), lack of patient compliance and lack of consumer convenience. In addition, the market for rapidly dissolving formulations maintains its strength as an innovative concept for either brand or generic companies with access to dissolving technology.
17
As a result, the combined US, EU and Japanese ODT market has doubled in size over the past four years to surpass $6.4 billion in 2009. Increased generic competition has further expanded the ODT market volume. The number of commercial over-the-counter and prescription ODT products has ballooned to over 450, which is attributable to the rapid genericization of multiple products by a large number of generics companies.
The ODT market is one of the fastest growing sectors of the drug delivery market, with industry experts projecting a 12-15% annual growth rate for the next several years. Based on upward global growth trends of the past decade, the ODT market could produce revenues of $13 billion by 2015. Growth is fueled by patient demand, with recent market studies indicating that more than half of the patients prefer ODTs to other dosage forms and most consumers would ask their doctors for ODTs (70%), purchase ODTs (70%), or prefer ODTs to regular tablets or liquids (80%).
Patient demand is driven by the fact that as many as 40% of Americans experience difficulty swallowing traditional tablets, even though most have no problems swallowing food or liquid. Results from a 2003 nationwide survey of 679 adults on pill-swallowing difficulties, conducted by Harris Interactive, indicated that of those who experienced difficulty swallowing pills, 14% had delayed taking doses of their medication, 8% had skipped a dose, and 4% had discontinued their medication.
Orally dissolving tablets have emerged as a patient-friendly, convenient method of administering medications. In addition to adults, the fast dissolving tablet market will prove particularly applicable to children and the elderly and anyone else who has trouble swallowing regular pills, tablets, or capsules; for example patients whose swallowing is compromised as a clinical symptom of disease. Other groups who benefit from this dosing form include the mentally ill, developmentally disabled, and uncooperative patients. ODTs can also be used in the field, for example in combat zones or for relief efforts following natural disasters, where clean sources of water may be unavailable and rapid onset of action is desirable.
Pharmaceutical Supply Chain Management Services
We currently provide efficient supply chain management on behalf of our clients, from strategic sourcing to delivering niche generic pharmaceuticals to market. Positioned in the center of the pharmaceutical value chain, we receive purchase orders from large pharmaceutical distributors to process orders to the end user’s specifications, and deliver products to a wide range of customers across the health care industry.
Our primary value lies in our growing portfolio of end users to whom ScripsAmerica markets its services. For end users such as hospitals and home care agencies, custom packaging such as unit of use can save staff time and cost, as well as eliminate dispensing errors at the pharmacist level. Further, we maintain a strategic relationship with the largest pharmaceutical distributor in North America, which acts as intermediary between us and our end customers.
Potential Acquisitions
We are currently exploring acquisitions to expand our supply chain management business and improve revenue and profitability. We believe that these potential acquisitions would eliminate fees we currently pay to our suppliers. We expect that the resulting cost savings will flow through to our bottom line as well accelerate our revenue growth. Our management expects each of the two acquisitions to improve our gross profit margin by at least 10%. While we expect that if we go ahead with these acquisitions, such transactions will be done after the date of this prospectus. However, we believe the acquisitions will improve our valuation as a public company and provide us with greater control of the manufacturing, research & development, prescription development and consumer product markets.
We also believe our acquisition will great expand our customers from Compound SA 1022 because it enables in-house manufacturing of Compound SA 1022 and afford us both first priority and exclusive rights on our other manufacturing orders. In addition, we will gain access to such manufacturer’s full customer list as well as the manufacturer’s state-of-the-art R&D facilities.
18
Pharmaceutical Packager. We are currently evaluating under a non-binding letter of intent the purchase of a pharmaceutical packager, which would add to our order processing capacity and profitability. We anticipate that this acquisition would provide us with the processing capacity to reach up to 4,000,000 doses per day, which is a 100% increase over current levels. The targeted pharmaceutical packager has a facility which consists of a temperature-controlled warehouse and a 12,000 square foot area dedicated to pharmaceutical packaging, contract packaging and private labeling, which is also fully temperature-controlled. There are five packaging lines suitable for high and low speed packaging, semi-automatic custom packaging, folding, gluing, assembly, and kitting. This facility has passed inspection by the FDA, DEA and the State Board of Pharmacy. We believe that this planned acquisition will be completed during 2011, pending satisfactory results from an audit of the pharmaceutical packager’s operations and our raising adequate funding for the acquisition.
Pharmaceutical Manufacturer, Laboratory, and R&D Operations. The company is an FDA-registered developer and manufacturer of prescription, over the counter and generic pharmaceutical products in various dosage forms, all of which meet current manufacturing standards. It operates a 30,000 square foot cGMP facility with bulk supplies and contract manufacturing capabilities. It also performs in-house drug product development and accelerated and long-term stability testing. The facilities include state of the art tablet compression machines, blenders, coaters, various sizes of process tanks, as well as a temperature controlled finished goods warehouse.
Strategic Relationship with Our Customer.
Our sole customer is a billion dollar company offering distribution and technology solutions to the health care industry. As the largest pharmaceutical distributor in North America, our customer distributes approximately half of the medicines used every day and supplies more than 40,000 U.S. pharmacy locations, from Wal-Mart to the Department of Veterans Affairs to community pharmacies and hospitals. On the technology side, our customer develops and installs health care information technology systems that eliminate the need for paper prescriptions and paper medical records. Our customer’s software and hardware solutions are used in more than 70% of the nation’s hospitals with more than 200 beds.
In filling purchase orders for our customer (which distributes to hospitals, nursing homes, pharmacy chains, and government agencies, among others), we embody part of the bridge from drug manufacturers and pharmaceutical packaging companies to end users. Despite our customer’s position as intermediary, we market directly to the end users to secure continuing demand for its products.
Our interactions with our suppliers on the one hand, and intermediaries such as our customer and various health care facilities on the other hand, are shown in the charts below.

We plan to create alliances with other major health care organizations for mutual benefit.
19
Purchase Order Processing
Order Flow:
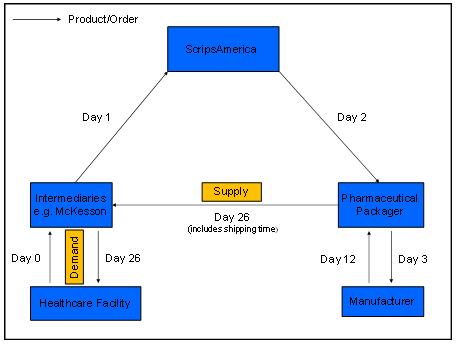
|
Day
|
Event
|
|
|
0
|
Healthcare Facility places order with intermediary (e.g. McKesson, AmeriSource Bergen and Cardinal Health)
|
|
|
1
|
ScripsAmerica receives purchase order from intermediary
|
|
|
2
|
ScripsAmerica commissions packaging distributor to coordinate all activities
|
|
|
3
|
Packaging distributor orders bulk materials from manufacturer
|
|
|
12
|
Packaging distributor receives bulk materials
|
|
|
13-21
|
Packaging distributor packages product to order specifications
|
|
|
22
|
Packaging distributor distributes product to intermediary
|
|
|
26
|
Upon receipt of product, intermediary issues proof of delivery to ScripsAmerica
|
|
|
26
|
Intermediary delivers product to healthcare facility
|
20
Dollar Flow:
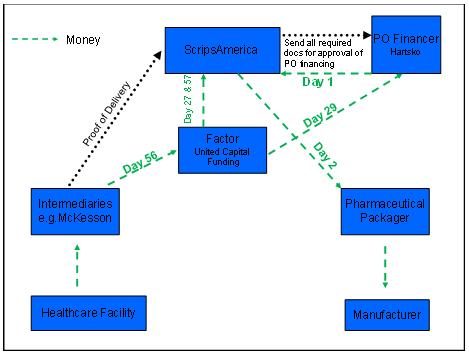
|
Day
|
Event
|
|
|
1
|
ScripsAmerica secures purchase order financing
|
|
|
2
|
ScripsAmerica pays Packaging Distributor negotiated amount to fill purchase order
|
|
|
27
|
With proof of delivery from intermediary, ScripsAmerica secures funding from Accounts Receivable Factor
|
|
|
29
|
Factor settles ScripsAmerica account with Purchase Order Financer
|
|
|
56
|
Intermediary pays Factor for Product received on Day 26
|
|
|
57
|
Factor pays ScripsAmerica any balance due
|
Drug Efficacy Study Implementation (DESI) Program.
In addition to providing its core pharmaceutical packaging, distribution, and sales and marketing services, we seek to secure FDA approval and worldwide market exclusivity for a number of DESI drug candidates.
The FDA encourages manufacturers to submit ANDAs (Abbreviated New Drug Applications) for the DESI drugs that are still being produced and marketed today, and, post approval, provides manufacturers with worldwide market exclusivity ranging from a minimum of three to a maximum of five years.
Preparing for a DESI drug ANDA filing requires an assessment study, which entails a review of the information on the drug of choice, as it exists in the public domain, often from a period of dozens of years. The goal of such a study is to find data that can be used in lieu of sponsor studies. Most DESI drugs have a proven history of safety and efficacy.
Available information is categorized into toxicology, safety, and efficacy data and analyzed with respect to completeness and ANDA requirements. Taking into account known information on similar compounds, the applicant then identifies any gaps in the data and decides what additional studies may be necessary, along with their cost and duration. If additional clinical trials need to be conducted, they will be designed to close existing gaps in the data required for ANDA submission.
The applicant presents his analysis during a pre-IND (“Investigational New Drug”) meeting with the FDA. Upon IND clearance, enrollment of patients into clinical trials can begin. Data from such trials, together with data existing in the public domain will be compiled for the final ANDA submission.
21
We initially seek to gain FDA approval for Compound SA 1022, a non-steroidal anti-inflammatory drug (NSAID). Compound SA 1022 has been in use for decades and is currently being used by thousands of people in the United States. The estimated cost of gaining FDA approval for Compound SA 1022 is $2-3 million, with an anticipated time to approval of two to three years (see chart below). We have retained a reputable firm, which is a provider of medical and regulatory consulting services to pharmaceutical, biotechnology, and device companies, to advise us on our interaction with regulators, clinical program design, and future FDA submissions.
A typical DESI drug development timeline is depicted below:

In addition to existing palliative indications for Compound SA 1022, trials are being conducted by various agencies investigating the anti-inflammatory effects of Compound SA 1022 either as monotherapy or in combination with other drugs for use in cardiovascular disease and Type 2 diabetes.
With hundreds of other DESI drugs still awaiting FDA approval, we have identified additional drug candidates for development and regulatory review. Based on market exclusivity, pricing power and extent of use, DESI drugs represent revenue potential to the manufacturer, on average, of approximately $60 million per drug. Financing is currently the rate-limiting variable to our ability to develop Compound SA 1022 and other DESI drug targets.
Rapidly Dissolving Drug Formulations
Rapidly dissolving drug formulation refers to an oral drug delivery formulation, which entails drugs in tablet form that can be taken without water and will dissolve in the mouth in less than 30 seconds. Rapidly dissolving drug formulations represent a convenient dosing form for patients who have difficulty swallowing – a widespread phenomenon among the elderly - or have sustained injuries to the esophagus. Other groups who benefit from this dosing form include the mentally ill, developmentally disabled, and uncooperative patients. Rapidly dissolving drug formulations can also be used in the field, where a clean source of water may be unavailable.
Rapidly dissolving drug formulations’ primary advantages over other oral dosage forms include patient friendliness, sustained release and high active dose capabilities, low manufacturing cost, manufacture using conventional equipment and blister packaging, and low moisture sensitivity. Rapidly dissolving drug formulations for children can be customized to solve common overdosing or under dosing issues that 2-11-year-olds frequently experience when taking pain relievers or fever reducers in doses intended for adults.
Oral drug delivery remains the preferred dosing method among patients and physicians, with more than 80% of all drugs administered in this manner. Rapidly dissolving drug technology provides pharmaceutical companies with the opportunity for product line extensions for a wide variety of drugs, and we believe there is a significant opportunity as a contract developer of rapidly dissolving drug formulation for specialty prescription pharmaceuticals. The current market for oral fast-melt drugs in the U.S. is $1.5 billion.
22
In March 2010, we entered into a product development, manufacturing, and supply agreement with a pharmaceutical company for a pain relief 80mg orally disintegrating rapid dissolve tablets under which the pharmaceutical company will develop and supply the product, while we will fund all development costs and retain all ownership rights in the pain relief rapidly dissolving drug formulation (including any patent and trademark rights). Current therapeutic categories available as a rapidly dissolving drug formulation through our pharmaceutical partner are allergy, anti-inflammatory, and sleep.
We anticipate project completion and ANDA approval in approximately nine months, with market rollout scheduled for 2012. Revenue projections for our pain relief 80mg orally disintegrating rapid dissolve tablets amount to $1.4 million in 2012 and approximately $6 million in 2013.
After launching pain relief 80mg orally disintegrating rapid dissolve tablets, we plan to develop and launch additional products as quickly as cash flows allow. Vitamins, OTC products, and generics are among the product categories currently under consideration. The rapidly dissolving drug formulation for vitamins can be developed within one to two months, as no regulatory approval is required to bring such products to market, and can be distributed through the existing network. OTC rapidly dissolving drug formulations can be launched with a five- to six-month lead time, including three months of accelerated studies and one month of process validation. Launch of generics as a rapidly dissolving drug formulation requires filing of an ANDA or NDA under Section 505(b)(2) of the Federal Food, Drug and Cosmetics Act, which covers new pharmaceutical products with innovative dosage forms or delivery routes. Generics will require at least two years of time to approval as well as a $1-$2 million investment in safety and efficacy studies.
An overview of potential rapidly dissolving drug formulation products is shown below for each of the three categories:
|
Vitamin
|
Chemical Name
|
Function
|
|
B1
|
Thiamine Mononitrate
|
Breakdown of sugars in the diet
|
|
B2
|
Riboflavin
|
Energy metabolism; metabolism of fats, ketone bodies, carbohydrates, and proteins
|
|
B3
|
Niacinamide
|
Anti-inflammatory
|
|
B6
|
Pyridoxine HCl
|
Balancing of sodium and potassium, promotion of red blood cell production
|
|
B12
|
Cobalamin
|
Key role in the normal functioning of the brain and nervous system, and for the formation of blood
|
|
C
|
Ascorbic Acid
|
Antioxidant used for treatment and prevention of scurvy
|
|
D2
|
Ergocalciferol
|
Promotion of the active absorption of calcium and phosphorus by the small intestine to permit bone mineralization
|
|
D3
|
Cholecalciferol
|
Treatment of Vitamin D deficiency
|
|
OTC Product
|
Function
|
|
Aspirin
|
Analgesic for minor aches and pains, fever reducer, anti-inflammatory medication
|
|
Ibuprofen
|
Non-steroidal anti-inflammatory drug for relief of symptoms of arthritis, menstrual pain, fever, and as an analgesic
|
|
Tylenol
|
Analgesic for relieving pain, reducing fever, and relieving the symptoms of allergies, cold, cough, and flu
|
|
Guaifenesin
|
Expectorant used to relieve chest congestion
|
|
Dextromethophan
|
Cough suppressant
|
|
Chlorpheniramine
|
Control of symptoms of cold or allergies
|
|
Claritin (Loratidine)
|
Antihistamine for treatment of allergies
|
|
Benadryl (Diphenhydramine)
|
Antihistamine for treatment of allergies
|
|
Potassium Chloride
|
Treatment for low potassium blood levels
|
|
Ferrous Sulfate
|
Treatment for iron deficiency anemia
|
|
Glucosamine
|
Treatment for osteoarthritis; non-vitamin, non-mineral dietary supplement; an amino acid sugar and a prominent precursor in the biochemical synthesis of glycosylated proteins and lipids
|
23
|
OTC Product
|
Function
|
|
Chondroitin Sulfate
|
Treatment for osteoarthritis; structural component of cartilage that provides much of its resistance to compression
|
|
Zantac (Ranitdine)
|
Histamine H2-receptor antagonist that inhibits stomach acid production
|
|
Bisacodyl
|
Stimulant laxative for relief of constipation and for the management neurogenic bowel dysfunction; part of bowel preparation before medical examinations such as colonoscopy
|
|
Caffeine
|
Central nervous system stimulant
|
|
Generic Product
|
Function
|
|
Fazaclo (Clozapine)
|
Antipsychotic drug for treatment of the symptoms of schizophrenia
|
|
Remeron SolTab
|
Antidepressant for treatment of major depressive disorders in adults
|
|
Zomig (Zolmitriptan Oral)
|
Serotonin receptor agonist for treatment of the symptoms of migraine headache
|
|
Tempra Firstab Pediatric
|
Non-salicylate analgesic-antipyretic for the temporary relief of pain from headache, muscle strain, bursitis, sprains, and menstrual cramps; fever reducer
|
|
Triaminic Soft Chews
|
Pain reliever, fever reducer, and cough suppressant
|
|
Parcopa
|
Treatment of Parkinson's disease and syndrome
|
|
Zofran (Ondansetron)
|
5-HT3 receptor antagonist used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, and surgery
|
Development Agreement/Intellectual Property
We own the Website www.scripsamerica.com. We do not have any other intellectual property except as described below.
In March 2010, we entered into a product development, manufacturing, and supply agreement with a pharmaceutical company for the development of pain relief 80mg orally disintegrating rapid dissolve tablets under which the pharmaceutical company will develop and supply the pain relief rapidly dissolving drug formulation, while we will fund all development costs and own all rights to the pain relief rapidly dissolving drug formulation, including any patent and trademark rights. Current therapeutic categories available as rapidly dissolving drug formulation through our pharmaceutical partner are allergy, anti-inflammatory, and sleep.
Under our arrangement with our pharmaceutical partner, we will be the exclusive distributor of the pain relief rapidly dissolving drug formulations that are developed under the agreement. Our pharmaceutical partner has agreed not to develop or manufacture any pain relief rapidly dissolving drug formulations with anyone else, and we agreed not to have any pain relief rapidly dissolving drug formulation, or a generic drug product that would compete with such pain relief rapidly dissolving drug formulation, with any other party other than our pharmaceutical partner. During the development of the pain relief rapidly dissolving drug formulation, we may terminate the development agreement on thirty (30) days prior written notice, however, we would be responsible for any development costs incurred by our pharmaceutical partner through the date of notice of termination. After the pain relief rapidly dissolving drug formulation has been developed, either party may terminate the development agreement on 12 months prior written notice.
In the next 12 months, we expect to file a develop and file a trademark application, for a trade mark to use for our rapidly dissolving drug formulation products prior to the sales of the pain relief rapidly dissolving drug formulation we are developing with our pharmaceutical partner.
Competitors
Large, vertically integrated industry players engaged in the development, manufacture, marketing, sale and distribution of generic and/or branded specialty pharmaceuticals in various therapeutic categories include Actavis Group, Apotex, Dr. Reddy’s, Glenmark Pharmaceuticals, Jubilant Life Sciences, Mylan, Par Pharmaceutical Companies, Ranbaxy Laboratories, Sandoz (a division of Novartis), Sun Pharmaceutical Industries, Teva Pharmaceutical Industries, Barr Laboratories (a division of Teva), and Watson Pharmaceuticals.
The group of small and middle market companies within which we compete for market share consists of Adams Laboratories, Blu Pharmaceuticals, Boca Pharmacal, Caraco, Hi-Tech Pharmacal, Impax Laboratories, Lanett Company, Missionpharma, Pharmaceutical Laboratories, Purepac Pharmaceutical, Qualitest Pharmaceuticals, and URL Pharma.
24
What sets ScripsAmerica apart from the latter group is risk diversification and significant return potential through its R&D focus on DESI drugs and rapidly dissolving drug formulation technology.
Employees
As of May 30, 2011, we have three (3) full time employees and five (5) consultants. We plan to hire more persons on as-needed basis.
DESCRIPTION OF PROPERTY
We have rent-free use of 1,500 square feet office space at 77 McCullough Drive, New Castle, Delaware 19720, which is our principal place of business. This space is being made available to us by our contract packager. There is no term for our use of the space, which can be terminated at any time.
LEGAL PROCEEDINGS
There are no legal proceedings pending or threatened against us or our officers and directors.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Admission to Quotation on the OTC Bulletin Board
We intend to have our common stock be quoted on the OTC Bulletin Board, the OTCQB and the OTC Pink. If our securities are not quoted on the OTC Bulletin Board, the OTCQB and/or the OTC Pink a security holder may find it more difficult to dispose of, or to obtain accurate quotations as to the market value of our securities. The OTC Bulletin Board and the OCTQB differ from national and regional stock exchanges in that they:
(1) are not situated in a single location but operates through communication of bids, offers and confirmations between broker-dealers, and
(2) securities admitted to quotation are offered by one or more Broker-dealers rather than the "specialist" common to stock exchanges.
The OTC Bulletin Board is a regulated quotation service that displays real-time quotes, last-sale prices, and volume information in over-the-counter (OTC) equity securities. An OTC Bulletin Board equity security generally is any equity that is not listed or traded on NASDAQ or a national securities exchange.
The OTCQB is the middle tier of the OTC market. OTCQB companies are reporting with the SEC or a U.S. banking regulator, making it easy for investors to identify companies that are current in their reporting obligations. There are no financial or qualitative standards to be in this tier. Issuers with securities within OTCQB Market Tier must be SEC, Bank or Insurance reporting, and they must be current in their disclosure.
OTC Pink is the third tier of the OTC market. OTC Pink is the speculative trading marketplace that has no financial standards or reporting requirements. OTC Pink companies choose the level of information they provide to investors and may have current, limited or no public disclosure. We will provide the same disclosure for the OTC Pink as we do for the OTCQB.
To qualify for quotation on the OTC Bulletin Board, the OTCQB and the OTC Pink an equity security must have one registered broker-dealer, known as the market maker, willing to list bid or sale quotations and to sponsor the company listing. We expect to have an agreement with Westminster Securities, a registered broker-dealer, as the market maker, willing to list bid or sale quotations and to sponsor the Company listing. If it meets the qualifications for trading securities on the OTC Bulletin Board, the OTCQB and the OTCPink, our securities will trade on the OTC Bulletin Board, the OTCQB and the OTCPink until a future time, if at all, that we apply and qualify for admission to quotation on the NASDAQ Global Market. We may not now and it may never qualify for quotation on the OTC Bulletin Board or be accepted for listing of our securities on the NASDAQ Global Market.
Our Transfer Agent
We have appointed Olde Monmouth Stock Transfer Company, with offices at 200 Memorial Parkway, Atlantic Highlands, New Jersey 07716, phone number 732-872-2727, as transfer agent for our shares of common stock. The transfer agent is responsible for all record-keeping and administrative functions in connection with our shares of common stock.
25
Dividend Policy
We have never declared or paid any cash dividends on our shares of common stock nor do we anticipate paying any in the foreseeable future. Except for any dividends owed to the holder of our Series A Preferred Stock, we anticipate that we will retain all of our future earnings to finance our operations and expansion. The payment of cash dividends in the future will be at the discretion of our Board of Directors (subject to the approval of the holder of the Series A Preferred Stock) and will depend upon our earnings levels, capital requirements, any restrictive loan covenants and other factors the Board considers relevant. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any future gains on their investments. Furthermore, we expect to retain any future earnings
Holders of Common Stock
As of May 15, 2011, the shareholders' list of our shares of common stock showed 15 registered shareholders and 51,218,680 shares of our common stock issued and outstanding. We also have 2,990,252 shares of Series A Preferred Stock issued and outstanding, which are convertible into 5,980,504.
Securities authorized for issuance under equity compensation plans
We currently do not have any equity compensation plans.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock to be sold in this offering. This prospectus, which constitutes part of the registration statement, does not include all of the information contained in the registration statement and the exhibits, schedules and amendments to the registration statement.
Some items are omitted in accordance with the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement and to the exhibits and schedules to the registration statement filed as part of the registration statement. Statements contained in this prospectus about the contents of any contract or any other document filed as an exhibit are not necessarily complete and, and in each instance, we refer you to the copy of the contract or other documents filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You may read and copy the registration statement of which this prospectus is a part at the SEC’s public reference room, which is located at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You can request copies of the registration statement by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the SEC’s public reference room. In addition, the SEC maintains an Internet website, located at www.sec.gov, which contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. You may access the registration statement of which this prospectus is a part at the SEC’s Internet website.
Upon the effectiveness of the registration statement of which this prospectus is a part, we will become subject to the full informational and periodic reporting requirements of the Exchange Act. We will fulfill our obligations with respect to such requirements by filing periodic reports and other information with the SEC. We intend to furnish our stockholders with annual reports containing consolidated financial statements certified by an independent registered public accounting firm. We also maintain a website at www.scripsamerica.com. Our website is not a part of this prospectus.
26
| SCRIPSAMERICA, INC. |
Table of Contents
| Page | ||
| Financial Statements - March 31, 2011 and 2010 | ||
| Balance Sheets | F-1 | |
| Statements of Income | F-2 | |
| Statements of Changes in Stockholders’ Equity | F-3 | |
| Statements of Cash Flows | F-4 | |
| Notes to Financial Statements | F-5 - F-9 | |
| Independent Auditors’ Report | F-10 | |
| Financial Statements - December 31, 2010 and 2009 | ||
| Balance Sheets | F-11 | |
| Statements of Income |
F-12
|
|
| Statements of Changes in Stockholders’ Equity | F-13 | |
| Statements of Cash Flows | F-14 | |
| Notes to Financial Statements - December 31, 2010 and 2009 | F-15 - F-24 | |
27
SCRIPSAMERICA, INC.
Balance Sheets
|
March 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
(Unaudited)
|
||||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$ | 532,540 | $ | 171,898 | ||||
|
Receivable due from factor
|
131,244 | 91,341 | ||||||
|
Prepaid expenses and other current assets
|
66,042 | 220,966 | ||||||
|
Deferred income taxes
|
- | - | ||||||
| 729,826 | 484,205 | |||||||
|
Other Assets
|
||||||||
|
Notes receivable - related party - net
|
9,000 | 9,000 | ||||||
|
Deposits
|
200,000 | 200,000 | ||||||
| 209,000 | 209,000 | |||||||
| $ | 938,826 | $ | 693,205 | |||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable and accrued expenses
|
$ | 81,615 | $ | 82,930 | ||||
|
Convertible notes payable - net of discount of $32,240 and $14,620, respectively
|
467,760 | 285,380 | ||||||
|
Notes payable - related parties
|
80,000 | 60,000 | ||||||
| 629,375 | 428,310 | |||||||
|
Stockholders' Equity
|
||||||||
|
Preferred stock - $0.001 par value; 10,000,000 shares authorized; none issued and outstanding
|
||||||||
|
Common stock - $0.001 par value; 150,000,000 shares authorized; 45,989,680 and 45,859,680 shares issued and outstanding as of March 31, 2011 and December 31, 2010, respectively
|
45,990 | 45,860 | ||||||
|
Additional paid-in capital
|
234,140 | 201,770 | ||||||
|
Retained earnings (deficit)
|
29,321 | 17,265 | ||||||
| 309,451 | 264,895 | |||||||
| $ | 938,826 | $ | 693,205 | |||||
F-1
SCRIPSAMERICA, INC.
Statements of Income (Unaudited)
|
For the three months ended, March 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Product Sales - net
|
$ | 1,641,233 | $ | 310,227 | ||||
|
Cost of Goods Sold
|
1,175,389 | 258,377 | ||||||
|
Gross Profit
|
465,844 | 51,850 | ||||||
|
General and Administrative Expenses
|
98,459 | 142 | ||||||
|
Research and Development
|
283,969 | - | ||||||
| 382,428 | 142 | |||||||
|
Income (Loss) Before Other Income (Expenses)
|
83,416 | 51,708 | ||||||
|
Other Income (Expenses)
|
||||||||
|
Interest expense
|
(68,860 | ) | - | |||||
| (68,860 | ) | - | ||||||
|
Income Before Provision for Income taxes
|
14,556 | 51,708 | ||||||
|
Provision for Income Taxes
|
2,500 | 17,580 | ||||||
|
Net Income
|
$ | 12,056 | $ | 34,128 | ||||
|
Earnings Per Common Share
|
||||||||
|
Basic
|
$ | - | $ | - | ||||
|
Diluted
|
$ | - | $ | - | ||||
|
Weighted Average Number of Common Shares
|
||||||||
|
Basic
|
45,940,902 | 80,771,600 | ||||||
|
Diluted
|
48,260,902 | 80,771,600 | ||||||
F-2
SCRIPSAMERICA, INC.
Statements of Changes in Stockholders' Equity
|
For the Three Months ended March 31, 2011 (Unaudited)
|
||||||||||||||||||||
|
Common Stock
|
Additional
|
Retained Earnings
|
Stockholders'
|
|||||||||||||||||
|
Shares
|
Amount
|
Paid-In Capital
|
(Deficit)
|
Equity
|
||||||||||||||||
|
Balance - January 1, 2011
|
45,859,680 | $ | 45,860 | $ | 201,770 | $ | 17,265 | $ | 264,895 | |||||||||||
|
Common stock issued for services
|
30,000 | 30 | 7,470 | - | 7,500 | |||||||||||||||
|
Common stock issued for interest
|
100,000 | 100 | 24,900 | 25,000 | ||||||||||||||||
|
Net income
|
- | - | - | 12,056 | 12,056 | |||||||||||||||
|
Balance - March 31, 2011
|
45,989,680 | $ | 45,990 | $ | 234,140 | $ | 29,321 | $ | 309,451 | |||||||||||
F-3
SCRIPSAMERICA, INC.
Statements of Cash Flows (Unaudited)
|
For the three months ended, March 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Cash Flows from Operating Activities
|
||||||||
|
Net income
|
$ | 12,056 | $ | 34,128 | ||||
|
Adjustments to reconcile net income to net cash (used) by operating activities:
|
||||||||
|
Amortization of discount on convertible note payable
|
7,380 | - | ||||||
|
Common stock issued for services
|
7,500 | - | ||||||
|
Deferred Income tax
|
17,580 | |||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
(Increase) decrease in:
|
||||||||
|
Receivable due from factor
|
(39,903 | ) | (171,979 | ) | ||||
|
Prepaid expenses
|
154,924 | - | ||||||
|
Increase (decrease) in:
|
- | |||||||
|
Accounts Payable and accrued expenses
|
(1,315 | ) | 125,386 | |||||
| 140,642 | 5,115 | |||||||
|
Cash Flows from Investing Activities
|
- | - | ||||||
|
Cash Flows from Financing Activities
|
||||||||
|
Proceeds from convertible notes payable
|
200,000 | - | ||||||
|
Proceeds from notes payable - related parties
|
20,000 | - | ||||||
| 220,000 | - | |||||||
|
Net Increase (Decrease) in Cash and Cash Equivalents
|
360,642 | 5,115 | ||||||
|
Cash and Cash Equivalents - beginning
|
171,898 | 503 | ||||||
|
Cash and Cash Equivalents - end
|
$ | 532,540 | $ | 5,618 | ||||
|
Supplemental Disclosures of Cash Flow Information
|
||||||||
|
Cash Paid:
|
||||||||
|
Interest
|
$ | 61,480 | $ | - | ||||
|
Noncash financing and investing activities:
|
||||||||
|
Common stock issued for services
|
$ | 7,500 | $ | - | ||||
|
Discount on convertible note payable
|
$ | 25,000 | $ | - | ||||
F-4
SCRIPSAMERICA, INC.
| Notes to Financial Statements | For the Three Months Ended March 31, 2011 |
|
1 -
|
Basis of Presentation
|
The accompanying financial statements reflect financial information of ScripsAmerica, Inc., (the “Company” or “ScripsAmerica” or “we”).
The Company was incorporated in the State of Delaware on May 12, 2008, and primarily engages in the sale of generic pharmaceutical drugs to our customer’s various distribution centers located throughout the United States. The Company began shipment of products in February 2010.
The accompanying unaudited interim financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America and the instructions to regulation S-X and should be read in conjunction with the audited financial statements of ScripsAmerica and related notes thereto contained in the Company’s audited financials for the year ended December 31, 2010 filed within this S-1 document. Certain information and note disclosure required in financial statements prepared in accordance with generally accepted accounting principles have been condensed or omitted pursuant to regulation S-X. In the opinion of management, all adjustments, consisting of normal recurring adjustments, necessary for a fair presentation of financial position and the results of operations for the interim periods presented have been reflected herein. The results of operations for interim periods are not necessarily indicative of the results to be expected for the full year.
|
2 -
|
Summary of Significant Accounting Policies
|
A summary of significant accounting policies follows:
|
|
a.
|
Use of Estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
|
|
|
b.
|
Revenue Recognition - Revenue is recognized when persuasive evidence of an arrangement exists, when the selling price is fixed or determinable, when shipment or delivery or performance of services has been rendered, and when collectability is reasonably assured.
|
|
|
c.
|
Research and Development - Expenditures for research and development associated with contract research and development provided by third parties are expensed as operating expenses, as incurred. The Company had charges of $283,969 and $0 for research and development expenses for the three months ended March 31, 2011 and 2010, respectively.
|
|
|
d.
|
Recent Accounting Pronouncements - Management does not believe that any recently issued, but not yet effective accounting pronouncements, if currently adopted, would have a material effect on the accompanying condensed financial statements.
|
F-5
SCRIPSAMERICA, INC.
| Notes to Financial Statements | For the Three Months Ended March 31, 2011 |
|
3 -
|
Receivable Due from Factor
|
In April 2010, the Company entered into a factoring and security agreement. The Company agreed to sell its qualified accounts receivable with recourse in exchange for advances of funds equivalent to 78% of the value of receivables, leaving 22% of the receivables as a reserve by the factor for potential non-payment of the Company’s receivables. The factoring facility is for a term of one year, cancellable by either party upon one month’s written notice, which provided maximum financing of $500,000. The factoring line was increased to $1,000,000 as of February 20, 2011. As collateral for the repayment of advances for receivables sold, the purchaser has a priority security interest in all present and future assets and rights of the Company. The purchaser has required that the Company notify all customers that all payments must be made to a lock-box controlled by the purchaser. The factoring fee is .345% every five days. Factoring fees charged to interest expense for the three months ended March 31, 2011 was $30,480.
As of March 31, 2011, $481,185 of accounts receivable that had been sold to the factor remained uncollected. The factor advanced $349,941 to the Company against the open receivables, leaving $131,244 due from the factor on these receivables. Management has reviewed the open receivables for collectability and has determined that an allowance for doubtful accounts for these receivables is not necessary as of March 31, 2011.
|
4 -
|
Convertible Notes Payable
|
During the three month ended March 31, 2011, the Company issued two one year $100,000 promissory notes payable, aggregating $200,000. The notes are to be used exclusively for the Company’s purchase orders. The notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the note until maturity. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. The notes can be extended by mutual consent of the lender and the Company.
During 2010, the Company issued three one year $100,000 promissory notes payable, aggregating $300,000. These notes are to be used exclusively for the Company’s purchase orders. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. The notes can be extended by mutual consent of the lender and the Company.
Interest expense associated with these notes for the three months ended March 31, 2011 and 2010, was $7,380 and $0, respectively. Additionally, the Company issued to the note holder 168,080 shares of common stock. These shares of common stock are valued at $.25 per share and are being amortized over the life of the notes. Total value assigned to these shares was $42,020, of which $9,780 has been amortized as interest expense, the unamortized discount on these notes as of March 31, 2011 is $32,240.
|
5 -
|
Notes Payable - Related Parties
|
During the three month period ended March 31, 2011, the Company issued two one year promissory notes aggregating $20,000. The notes consist of $10,000 issued to the Company’s president and CEO and $10,000 issued to a company owned by the Company’s president and CEO. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. Interest expense associated with these notes for the three months ended March 31, 2011 was approximately $60.
F-6
SCRIPSAMERICA, INC.
| Notes to Financial Statements | For the Three Months Ended March 31, 2011 |
During 2010, the Company issued six one year promissory notes aggregating $60,000. The notes consist of $40,000 issued to the Company’s president and CEO and $20,000 issued to a company owned by the Company’s president and CEO. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. The notes can be extended by mutual consent of the lender and the Company. Interest expense associated with these notes for the three months ended March 31, 2011 and 2010 was $300 and $0, respectively.
|
6 -
|
Income Taxes
|
Our provision for income taxes at March 31, 2011 and 2010 consisted of the following
| March 31, 2011 | March 31, 2010 | |||||||
| Current | ||||||||
| Federal | $ | 2,500 | $ | 17,580 | ||||
| State | - | - | ||||||
|
Deferred
|
||||||||
| Federal | - | - | ||||||
| Income Tax Expense | $ | 2,500 | $ | 17,580 | ||||
The effective tax rates differ from the statutory rates for 2011 and 2010 primarily due to the following:
|
2011
|
2010
|
|||||||||||||||
|
Effective
|
Effective
|
|||||||||||||||
|
Amount
|
Tax Rate
Percentage
|
Amount
|
Tax Rate
Percentage
|
|||||||||||||
|
Federal income tax liability (benefit)
|
$ | 4,949 | 34.0% | $ | 17,580 | 34.0% | ||||||||||
|
Effect of graduated rates
|
(2,449 | ) | -16.8% | -- | - | |||||||||||
| $ | 2,500 | 17.2% | $ | 17,580 | 34.0% | |||||||||||
|
7 -
|
Stockholders’ Equity
|
Common Stock
In 2011, the board of directors authorized a two-for-one forward stock split and a change in par value. The change in par value and the forward stock split have been retroactively applied as of January 1, 2011 in the balance sheet and the statement of changes in stockholders’ equity.
During the three months ended March 31, 2011, the Company issued 30,000 shares in connection with services provided by a non-employee . The Company charged its operations $7,500.
During the three month period ended March 31, 2011, the Company issued 100,000 shares for interest related to the issuance of note payable. The Company accounted for this transaction as a discount on notes payable totaling $25,000, of which $3,125 was amortized to interest expense for the three month period ended March 31, 2011.
F-7
SCRIPSAMERICA, INC.
| Notes to Financial Statements | For the Three Months Ended March 31, 2011 |
|
8 -
|
Commitments and Contingencies
|
In March 2010, the Company signed a “Product, Manufacturing and Supply Agreement” with a contract packager and labeler for orally disintegrating tablets. The total value of this contract is $935,000. As of March 31, 2011, the Company has paid a total of $732,104, of which $200,000 is considered a stand still fee that has been reflected as a deposit on the balance sheet as of March 31, 2011 and December 31, 2010. An expense of $264,769 was recorded to operations as research and development costs for the three months period ended March 31, 2011. No expense was recorded for the three month period ended March 31, 2010. The balance of $202,896 at March 31, 2011 is expected to be completed and paid in 2011. Upon the commencement of product shipped, a 7% royalty on the gross profit related to the orally disintegrating tablet sales will be due on a quarterly basis. The $200,000 deposit will be applied towards this royalty payment.
The Company utilizes the office space of its product supply vendor at no cost. There is no agreement on the use of this office space and it may not be available to us in the future.
|
9 -
|
Earning Per Common Share
|
The basic earnings per share are computed using the weighted average number of common shares outstanding. The dilutive effect of potential common shares outstanding is included in the diluted earnings per share, The computations of basic earnings and diluted earnings per share for 2011 and 2010 are as follows:
| For the Three Months Ended March 31, 2011 | ||||||||||||
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per Share
Amount
|
||||||||||
|
Net Income
|
$ | 12,055 | ||||||||||
|
Basic earnings per common share
|
$ | 12,055 | 45,940,902 | $ | 0.00 | |||||||
|
Effect of dilutive securities - notes payable
|
- | 2,320,000 | - | |||||||||
|
Diluted earnings per common share
|
$ | 12,055 | 48,260,902 | $ | 0.00 | |||||||
| For the Three Months Ended March 31, 2010 | ||||||||||||
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per Share
Amount
|
||||||||||
|
Net Income
|
$ | 34,128 | ||||||||||
|
Basic earnings per common share
|
$ | 34,128 | 80,771,600 | $ | 0.00 | |||||||
|
Effect of dilutive securities - notes payable
|
- | - | - | |||||||||
|
Diluted earnings per common share
|
$ | 34,128 | 80,771,600 | $ | 0.00 | |||||||
F-8
SCRIPSAMERICA, INC.
| Notes to Financial Statements | For the Three Months Ended March 31, 2011 |
|
10 -
|
Subsequent Events
|
In April 2011, the Company issued notes payable aggregating $86,000 with a 24% annual interest rate.
In April 2011, the Company issued 2,990,252 shares of voting convertible preferred stock for $1,043,000 with an 8% dividend rate, payable quarterly. The preferred stockholder appointed a director to the board of directors.
In April 2011, the Company’s board authorized a two-for-one forward stock split. All share and per share information for all periods presented have been adjusted to reflect the two-for-one stock split.
In April 2011, the Company sold 5,200,000 shares of common stock for an aggregate purchase price of $176,000.
In May 2011, the Company sold 29,000 shares of common stock for an aggregate purchase price of $5,800.
F-9
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
ScripsAmerica, Inc.
New Castle, Delaware
We have audited the accompanying balance sheets of ScripsAmerica, Inc. (the “Company”) as of December 31, 2010, and 2009, and the related statements of income, changes in stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2010. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of ScripsAmerica, Inc. as of December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2010, in conformity with accounting principles generally accepted in the United States of America.
|
/s/ Raich Ende Malter & Co. LLP
|
|
Raich Ende Malter & Co. LLP
|
|
New York, New York
|
|
June 1, 2011
|
F-10
SCRIPSAMERICA, INC.
Balance Sheets
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
171,898
|
$
|
503
|
||||
|
Receivable due from factor
|
91,341
|
-
|
||||||
|
Prepaid expenses and other current assets
|
220,966
|
-
|
||||||
|
Deferred income taxes
|
-
|
56,000
|
||||||
|
484,205
|
56,503
|
|||||||
|
Other Assets
|
||||||||
|
Notes receivable - related party - net
|
9,000
|
7,300
|
||||||
|
Deposits
|
200,000
|
-
|
||||||
|
209,000
|
7,300
|
|||||||
|
$
|
693,205
|
$
|
63,803
|
|||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable and accrued expenses
|
$
|
82,930
|
$
|
-
|
||||
|
Convertible notes payable - net of discount of $14,620
|
285,380
|
-
|
||||||
|
Notes payable - related parties
|
60,000
|
-
|
||||||
|
428,310
|
-
|
|||||||
| Commitments and Contingencies | - | - | ||||||
|
Stockholders' Equity
|
||||||||
|
Preferred stock - $0.001 par value; 10,000,000 shares authorized; none issued and outstanding
|
-
|
-
|
||||||
|
Common stock - $0.001 par value; 150,000,000 shares authorized; 45,859,680 and 80,771,600 shares issued and outstanding as of December 31, 2010 and 2009, respectively; 80,000,000 shares subscribed as of December 31, 2009
|
45,860
|
80,722
|
||||||
|
Subscriptions receivable
|
-
|
(4,000
|
)
|
|||||
|
Additional paid-in capital
|
201,770
|
96,838
|
||||||
|
Retained earnings (deficit)
|
17,265
|
(109,807
|
)
|
|||||
|
264,895
|
63,803
|
|||||||
|
$
|
693,205
|
$
|
63,803
|
|||||
See accompanying notes to financial statements.
F-11
SCRIPSAMERICA, INC.
Statements of Income
|
For the Year Ended December 31, 2010
|
For the Year Ended December 31, 2009
|
|||||||
|
Product Sales - net
|
$
|
3,221,320
|
$
|
-
|
||||
|
Cost of Goods Sold
|
2,543,921
|
-
|
||||||
|
Gross Profit
|
677,399
|
-
|
||||||
|
General and Administrative Expenses
|
93,565
|
25,599
|
||||||
|
Research and Development
|
267,335
|
-
|
||||||
| 360,900 |
25,599
|
|||||||
|
Income (Loss) Before Other Income (Expenses)
|
316,499
|
(25,599
|
)
|
|||||
|
Other Income (Expenses)
|
||||||||
|
Forfeited deposits
|
-
|
(14,500
|
)
|
|||||
|
Interest expense
|
(130,905
|
)
|
-
|
|||||
|
Interest income
|
1,693
|
189
|
||||||
|
(129,212
|
)
|
(14,311
|
)
|
|||||
|
Income (Loss) Before Provision for (Benefit from) Income Taxes
|
187,287
|
(39,910
|
)
|
|||||
|
Provision for (Benefit from) Income Taxes
|
60,215
|
(56,000
|
)
|
|||||
|
Net Income
|
$
|
127,072
|
$
|
16,090
|
||||
|
Earnings Per Common Share
|
||||||||
|
Basic
|
$
|
-
|
$
|
-
|
||||
|
Diluted
|
$
|
-
|
$
|
-
|
||||
|
Weighted Average Number of Common Shares
|
||||||||
|
Basic
|
79,195,292
|
80,771,600
|
||||||
|
Diluted
|
80,635,292
|
80,771,600 | ||||||
See accompanying notes to financial statements.
F-12
SCRIPSAMERICA, INC.
Statements of Changes in Stockholders' Equity
For the Years Ended December 31, 2010 and 2009
|
*Common Stock
|
||||||||||||||||||||||||
|
Shares
|
Amount
|
*Subscritpions
Receivable
|
*Additional
Paid-In Capital
|
Retained
Earnings
(Deficit)
|
Stockholders’ Equity
|
|||||||||||||||||||
|
Balance - January 1, 2009 - as restated *
|
80,771,600 | $ | 80,772 | $ | (4,000 | ) | $ | 96,838 | $ | (125,897 | ) | $ | 47,713 | |||||||||||
|
Net income
|
- | - | - | - | 16,090 | 16,090 | ||||||||||||||||||
|
Balance - December 31, 2009
|
80,771,600 | 80,772 | (4,000 | ) | $ | 96,838 | (109,807 | ) | 63,803 | |||||||||||||||
|
Cancellation of subscriptions receivable
|
(40,000,000 | ) | (40,000 | ) | 2,000 | 38,000 | - | - | ||||||||||||||||
|
Common stock issued for services
|
5,000,000 | 5,000 | - | 45,000 | - | 50,000 | ||||||||||||||||||
|
Common stock issued for services
|
20,000 | 20 | - | 4,980 | - | 5,000 | ||||||||||||||||||
|
Common stock issued for interest
|
68,080 | 68 | - | 16,952 | - | 17,020 | ||||||||||||||||||
|
Collection of subscriptions receivable
|
- | - | 2,000 | - | - | 2,000 | ||||||||||||||||||
|
Net income
|
- | - | - | - | 127,072 | 127,072 | ||||||||||||||||||
|
Balance - December 31, 2010
|
45,859,680 | $ | 45,860 | $ | - | $ | 201,770 | $ | 17,265 | $ | 264,895 | |||||||||||||
*All amounts give retroactive effect to the stock split and change in par value; in addtion, shares of common stock and subscriptions receivable give retroactive effect to a prior-period adjustment discussed in Note 11.
See accompanying notes to financial statements.
F-13
SCRIPSAMERICA, INC.
Statements of Cash Flows
|
For the
Year Ended |
For the
Year Ended |
|||||||
|
Cash Flows from Operating Activities
|
||||||||
|
Net income
|
$ | 127,072 | $ | 16,090 | ||||
|
Adjustments to reconcile net income to net cash (used) by operating activities:
|
||||||||
|
Provision for bad debts
|
2,500 | 18,687 | ||||||
|
Amortization of discount on convertible note payable
|
2,400 | - | ||||||
|
Common stock issued for services
|
30,000 | - | ||||||
|
Deferred income taxes
|
56,000 | (56,000 | ) | |||||
|
Changes in operating assets and liabilities:
|
||||||||
|
(Increase) decrease in:
|
||||||||
|
Receivable due from factor
|
(91,341 | ) | - | |||||
|
Prepaid expenses
|
(195,966 | ) | - | |||||
|
Deposits
|
(200,000 | ) | - | |||||
|
Increase (Decrease) in:
|
||||||||
|
Accounts payable and accrued expenses
|
82,930 | (2,750 | ) | |||||
| (186,405 | ) | (23,973 | ) | |||||
|
Cash Flows from Investing Activities
|
||||||||
|
Loans to related parties
|
(4,200 | ) | (19,487 | ) | ||||
|
Cash Flows from Financing Activities
|
||||||||
|
Proceeds from convertible notes payable
|
300,000 | - | ||||||
|
Proceeds from notes payable - related parties
|
60,000 | - | ||||||
|
Collection of subscriptions receivable
|
2,000 | - | ||||||
| 362,000 | - | |||||||
|
Net Increase (Decrease) in Cash and Cash Equivalents
|
171,395 | (43,460 | ) | |||||
|
Cash and Cash Equivalents - beginning
|
503 | 43,963 | ||||||
|
Cash and Cash Equivalents - end
|
$ | 171,898 | $ | 503 | ||||
|
Supplemental Disclosures of Cash Flow Information
|
||||||||
|
Cash Paid:
|
||||||||
|
Interest
|
$ | 124,061 | $ | - | ||||
| Income taxes | - | - | ||||||
|
Noncash financing and investing activities:
|
||||||||
|
Common stock issued for services
|
$ | 55,000 | $ | - | ||||
|
Discount on convertible note payable
|
$ | 17,020 | $ | - | ||||
|
Cancellation of subscriptions receivable
|
$ | 2,000 | $ | - | ||||
See accompanying notes to financial statements.
F-14
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
| 1 - | Organization and Business |
|
ScripsAmerica, Inc., (the “Company” or “ScripsAmerica” or “we”) was incorporated in the State of Delaware on May 12, 2008, and primarily engages in the sale of generic pharmaceutical drugs to our customer’s various distribution centers located throughout the United States. The Company began shipment of products in February 2010.
The Company receives purchase orders from a national pharmaceutical distributor and fills the orders through a contracted packaging and manufacturing services provider. ScripsAmerica offers fulfillment of prescription and over the counter (OTC) orders, as well as labeling, packaging, and shipping services. The Company maintains its corporate office in New Castle, Delaware.
|
|
| 2 - | Summary of Significant Accounting Policies |
| A summary of significant accounting policies follows: |
|
|
a.
|
Use of Estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the balance sheet and reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
|
|
|
b.
|
Revenue Recognition - Revenue is recognized when persuasive evidence of an arrangement exists, when the selling price is fixed or determinable, when shipment or delivery or performance of services has been rendered, and when collectability is reasonably assured.
|
|
|
c.
|
Customer, Product, and Supplier Concentration - We sell our products directly to a wholesale drug distributor who, in turn, supplies products to pharmacies, hospitals, governmental agencies, and physicians. All of our sales were to Mckesson Corporation.
|
|
|
On January 1, 2010, the Company entered into a Service Agreement, with a term of ten years, cancellable by either party upon twelve months written notice, which provides for the packaging and distribution of goods, received from the Company’s suppliers, to the Company’s customers. The Company used this contract packager exclusively for all of its warehouse, customer service, distribution, and labeling services for 2010.
|
|
|
In March 2010, the Company entered into a Product, Manufacturing, and Supply Agreement with the same company that supplies its packaging and distribution services. See Note 13.
|
|
|
d.
|
Research and Development - Expenditures for research and development associated with contract research and development provided by third parties are expensed as operating expenses, as incurred. The Company had charges of $267,335 and $0 for research and development expenses for the years ended December 31, 2010 and 2009, respectively.
|
|
|
e.
|
Cash and Cash Equivalents - The Company considers all highly liquid financial instruments purchased with an original maturity of three months or less to be cash equivalents.
|
F-15
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
|
|
f.
|
Accounts Receivable - Accounts receivable are stated at estimated net realizable value and net of accounts receivable sold subject to charge-back. Management provides for uncollectible amounts through a charge to earnings and a credit to an allowance for doubtful accounts based on its assessment of the current status of individual accounts and historical collection information. Balances that are deemed uncollectible after management has used reasonable collection efforts are written off through a charge to the allowance and a credit to accounts receivable. The Company has entered into an accounts receivable factoring facility agreement. See Note 4.
|
|
|
g.
|
Income Taxes - The Company provides for income taxes using an asset and liability based approach for reporting for income taxes. Deferred income tax assets and liabilities are computed annually for differences between the financial statement and the tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. See Note 10.
|
|
|
The Company also complies with the provisions of Accounting for Uncertainty in Income Taxes. The accounting regulation prescribes a recognition threshold and measurements process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return.
|
|
|
h.
|
Fair Value Measurements - The Company adopted the Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC) No. 820, Fair Value Measurements and Disclosures (“ASC 820”). ASC 820 clarifies that fair value is an estimate of the exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants (i.e., the exit price at the measurement date). Under ASC 820, fair value measurements are not adjusted for transaction cost. ASC 820 provides for use of a fair value hierarchy that prioritizes inputs to valuation techniques used to measure fair value into three levels:
|
Level 1: Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2: Input other than quoted market prices that are observable, either directly or indirectly, and reasonably available. Observable inputs reflect the assumptions market participants would use in pricing the asset or liability and are developed based on market data obtained from sources independent of the Company.
Level 3: Unobservable inputs. Unobservable inputs reflect the assumptions that the Company develops based on available information about what market participants would use in valuing the asset or liability.
An asset or liability’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Availability of observable inputs can vary and is affected by a variety of factors.
|
|
The Company uses judgment in determining the fair value of assets and liabilities, and level 3 assets and liabilities involve greater judgment than level 1 and level 2 assets and liabilities.
|
|
|
i.
|
Financial Instruments - The carrying values of cash equivalents, receivable due from factor, accounts payable and accrued expenses, and notes payable approximate their fair values due to their short-term maturities.
|
|
|
j.
|
Advertising Expenses - The Company expenses advertising costs as incurred. The Company did not incur any advertising expenses for the years ended December 31, 2010 and 2009.
|
F-16
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
|
|
k.
|
Cost of Goods Sold - The Company purchases all of its product from suppliers at various contracted prices and does not own or maintain any inventory. Upon shipment of product, the Company is charged the contracted price. The Company financed the purchase of inventory based on confirmed purchase orders via a revolving finance agreement and convertible notes payable. See Notes 7 and 9. Shipping and handling costs are included in cost of goods sold.
|
|
|
l.
|
Stock Based Compensation - The Company may exchange its common stock for services rendered by employees, directors, consultants, and others. The Company accounts for stock and stock options issued to employees and non-employees for services and other compensation under the provisions of ASC No. 718, Compensation - Stock Compensation and ASC No. 505-50, Equity-Based Payments to Non-Employees, which establish standards for the accounting for transactions in which an entity exchanges its equity instruments for goods and services at fair value. These standards also address transactions in which an entity incurs liabilities in exchange for goods and services that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of those equity instruments. For employees, stock awarded is valued based on the closing bid price on the date of grant. For non-employees, stock issued for services is valued at either the invoiced or contracted value of services provided, or to be provided, or the fair value of the stock at the due date per the service agreement, or stock issuance date, whichever is more readily determinable. The Company has not issued any warrants or options since inception.
|
|
|
m.
|
Earnings Per Share - Basic net income per common share is computed using the weighted average number of common shares outstanding during the periods. Diluted net income per share is computed using the weighted average number of common and dilutive common equivalent shares outstanding during the period. See Note 15.
|
|
|
n.
|
Recently Issued Accounting Pronouncements -
|
In October 2009, the FASB issued Accounting Standards Update (“ASU”) 2009-13, Multiple-Deliverable Revenue Arrangements, (amendments to FASB ASC Topic 605, Revenue Recognition) (“ASU 2009-13”). ASU 2009-13 requires entities to allocate revenue in an arrangement using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The amendments eliminate the residual method of revenue allocation and require revenue to be allocated using the relative selling price method. ASU 2009-13 should be applied on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, with early adoption permitted. The Company is currently assessing the impact of the standard on its results of operations or financial condition.
In January 2010, the FASB issued ASU 2010-06, Improving Disclosures about Fair Value Measurements. ASU 2010-06 amends ASC 820 to require additional disclosures regarding fair value measurements. The amended guidance requires entities to disclose the amounts of significant transfers between Level 1 and Level 2 of the fair value hierarchy and the reasons for these transfers, the reasons for any transfers in or out of Level 3, and information in the reconciliation of recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis. The ASU also clarifies the requirements for entities to disclose information about both the valuation techniques and inputs used in estimating Level 2 and Level 3 fair value measurements. The amended guidance is effective for interim and annual financial periods beginning after December 15, 2009. The full adoption of ASU 2010-06 is not expected to impact the Company’s operating results, financial position or cash flows, but did impact the Company’s disclosures on fair value measurements.
F-17
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
In April 2010, the FASB issued ASU 2010-17, Revenue Recognition - Milestone Method (“ASU 2010-17”). ASU 2010-17 provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. The followingcriteria must be met for a milestone to be considered substantive. The consideration earned by achieving the milestone should (i) be commensurate with either the level of effort required to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from the vendor’s performance to achieve the milestone; (ii) be related solely to past performance; and (iii) be reasonable relative to all deliverables and payment terms in the arrangement. No bifurcation of an individual milestone is allowed, and there can be more than one milestone in an arrangement. Accordingly, an arrangement may contain both substantive and non-substantive milestones. ASU 2010-17 is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. Management evaluated the potential impact of ASU 2010-17 and does not expect its adoption to have a material effect on the Company’s results of operations or financial condition.
In December 2010, the FASB issued ASU 2010-29, Business Combinations (ASC Topic 805): Disclosure of Supplementary Pro Forma Information for Business Combinations. The amendments in this ASU affect any public entity as defined by ASC Topic 805 that enters into business combinations that are material on an individual or aggregate basis. The amendments in this ASU specify that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. The amendments also expand the supplemental pro forma disclosures to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. The amendments are effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The Company does not expect adoption of ASU 2010-29 to have a material impact on the Company’s results of operations or financial condition.
In December 2010, the FASB issued ASU 2010-28, Intangibles - Goodwill and Other (ASC Topic 350): When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts. The amendments in this ASU modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. The qualitative factors are consistent with the existing guidance and examples, which require that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. For public entities, the amendments in this ASU are effective for fiscal years, and interim periods within those years, beginning after December 15, 2010. Early adoption is not permitted. The Company does not expect the adoption of ASU 2010-28 to have a material impact on the Company’s results of operations or financial condition.
|
|
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying consolidated financial statements.
|
| 3 - |
Prepaid Expenses and Other Current Assets
|
|
The balance as of December 31, 2010 and 2009 was $220,966 and $-0-, respectively. The balance as of December 31, 2010 consisted of an advance of $195,966 to the Company’s packaging and distribution provider, and $25,000 for stock compensation. See Note 12.
|
F-18
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
|
4 -
|
Receivable Due from Factor
|
In April 2010, the Company entered into a factoring and security agreement. The Company agreed to sell its qualified accounts receivable with recourse in exchange for advances of funds equivalent to 78% of the value of receivables, leaving 22% of the receivables as a reserve by the factor for potential non-payment of the Company’s receivables. The factoring facility is for a term of one year, cancellable by either party upon one month’s written notice, which provided maximum financing of $500,000. The factoring line was increased to $750,000 as of December 31, 2010. As collateral for the repayment of advances for receivables sold, the purchaser has a priority security interest in all present and future assets and rights of the Company. The purchaser has required that the Company notify all customers that all payments must be made to a lock-box controlled by the purchaser. The factoring fee is .345% every five days. Factoring fees charged to interest expense for the year ended December 31, 2010 was $65,917.
As of December 31, 2010, $438,088 of accounts receivable had been sold to the factor, of which $346,747 had been advanced to the Company, leaving $91,341 due from the factor on these receivables. Management has reviewed the open receivables for collectibility and has determined that an allowance for doubtful accounts for these receivables is not necessary at December 31, 2010.
|
5 -
|
Notes Receivable and Other Related Party Transactions
|
On December 22, 2008, the Company entered into a loan agreement with a stockholder, who is also an officer of the Company, in the amount of $6,500. The loan bears no interest and is payable on demand. On March 15, 2009, the Company amended the loan agreement to increase the amount to $7,300. On August 31, 2010, the Company amended the loan agreement to increase the amount to $9,000. As of December 31, 2010 and 2009, the outstanding balance is $9,000 and $7,300, respectively, and is classified as notes receivable - related party- net.
The Company entered into revolving loan agreements with JBS Capital, Inc. (“JBS”) and its wholly-owned subsidiary, Verbena Pharmaceuticals, Inc. (“Verbena”) during 2008. The agreement with JBS provided available credit of $30,000, bearing interest at 5.00% per annum on the outstanding principal. The agreement with Verbena provided available credit of $100,000. On June 30, 2009, the agreement with Verbena was amended to provide available credit of $225,000, bearing interest at 5.00% per annum on the outstanding principal. Outstanding principal and accrued interest is to be repaid in full upon the earlier of the completion of a merger or December 31, 2010. At December 31, 2010 and 2009 , the balance outstanding under these agreements is classified as notes receivable - related party - net and consists of advances to and payments on behalf of JBS and Verbena aggregating $21,200 and $118,477, respectively, less an allowance for uncollectible accounts of $21,200 and $118,477, respectively. The outstanding balance was offset by an allowance for uncollectible accounts due to the inability of JBS and Verbena to repay the loans as of December 31, 2010 and 2009. Future collections, if any, will be recognized as other income. The Company’s CEO/director and two of its other directors, all of whom are stockholders of the Company, are the sole stockholders of JBS. The loans to JBS and Verbena were made when the Company was exploring the possibility of a merger with Verbena, a nontrading public “shell company.”
In 2010, the Company paid $8,500 in consultant fees to a consulting firm owned by the Company’s CEO and an additional $8,500 in consulting fees to a consulting firm owned by the Company's Chief Financial Officer.
|
6 -
|
Deposits
|
|
|
Deposits as of December 31, 2010 consist of an advance royalty payment of $200,000 made under the Company’s Product Development, Manufacturing and Supply agreement. See Note 13.
|
F-19
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
|
7 -
|
Convertible Notes Payable
|
|
|
During 2010, the Company issued three one year $100,000 promissory notes payable, aggregating $300,000. These notes are to be used exclusively for the Company’s purchase orders. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity on October 8, 2011, November 22, 2011 and December 31, 2011. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. The notes can be extended by mutual consent of the lender and the Company. Interest expense charged relating to these notes for the year ended December 31, 2010 was $7,494. Additionally, the Company issued to the note holder 68,080 shares of common stock. These shares of common stock are valued at $.25 per share and are being amortized over the life of the notes. Total value assigned to these shares was $17,020, of which $2,400 has been amortized as interest expense for the year ended December 31, 2010. The unamortized discount on these notes as of December 31, 2010 is $14,620.
|
|
8 -
|
Notes Payable - Related Parties
|
During 2010, the Company issued six one year promissory notes aggregating $60,000. The notes consist of $40,000 issued to the Company’s CEO and $20,000 issued to a company owned by the Company’s CEO. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity. The amount of the notes, to the Company’s CEO, and the related maturities are $15,000, May 16, 2011, $5,000, June 3, 2011, $10,000, October 24, 2011, and $10,000, December 22, 2011, respectively. The amount of the notes, to the company owned by the Company’s CEO, and the related maturities are $10,000, May 16, 2011, and $10,000, June 3, 2011, respectively. The principal portion of the notes can be converted into common stock at any time during the one year term at the rate of $.25 per share at the option of the lender. The notes can be extended by mutual consent of the lender and the Company. Interest expense charged relating to these notes for the year ended December 31, 2010 was $6,397.
|
9 -
|
Purchase Order Financing
|
On May 19, 2010, the Company entered into a revolving facility with a financial services company to fund the purchase of inventory products upon the receipt of confirmed purchase orders from customers. The facility provides for maximum financing of $300,000, has no expiration date, and is personally guaranteed by the CEO. During 2010, the Company borrowed and repaid $1,309,626. There is no outstanding balance on this credit facility as of December 31, 2010. The interest rate on the borrowings is 4% every thirty days or portion thereof. Interest expense charged relating to these notes for the year ended December 31, 2010 was $48,697.
|
10 -
|
Income Taxes
|
The Company accounts for income taxes under the asset and liability method as stipulated by ASC 740, Accounting for Income Taxes, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of the events that have been included in the financial statements or tax returns. Deferred income taxes are recognized for all significant temporary differences between tax and financial statements bases of assets and liabilities. Valuation allowances are established against net deferred tax assets when it is more likely than not that some portion or all of the deferred tax asset will not be realized.
As of December 31, 2009, the Company had net operating loss carryforwards of approximately $166,000, available to reduce future federal and state taxable income, expiring through 2029, that were fully utilized in 2010. The Company had $56,000 in deferred income tax assets at December 31, 2009, resulting from net operating loss carryforwards. At December 31, 2009, management believed it was more likely than not that the full benefit would be realized.
F-20
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
The Company files federal and Delaware state income taxes. Currently, there are no tax examinations in progress, nor has the Company had any federal or state examinations since its inception in 2008. All of the Company’s tax years are subject to federal and state tax examination. As of December 31, 2010, the Company did not have any unrecognized tax benefits and does not expect this to change significantly over the next 12 months. The Company recognizes interest and penalties, if any, related to uncertain tax positions in income tax expense. As of December 31, 2010, the Company has no accrued interest or penalties related to uncertain tax positions.
| 2010 | 2009 | |||||||
| Current | ||||||||
| Federal | $ | 2,740 | $ | - | ||||
| State | 1,475 | - | ||||||
| Deferred | ||||||||
| Federal | 56,000 | 56,000 | ||||||
| $ | 60,215 | $ | (56,000 | ) | ||||
The effective tax rates differ from the statutory rates for 2010 and 2009 primarily due to the following:
|
2010
|
2009
|
|||||||||||||||
|
Amount
|
Effective
Tax RatePercentage
|
Amount
|
Effective
Tax RatePercentage
|
|||||||||||||
|
Federal income tax liability (benefit)
|
$ | 63,678 | 34 | % | $ | (13,600 | ) | (34 | )% | |||||||
|
State income tax liability (benefit)
|
1,475 | 1 | % | -- | - | |||||||||||
|
Effect of graduated rates
|
(4,938 | ) | (3 | )% | - | 28 | % | |||||||||
|
Change in valuation allowance
|
- | - | (42,400 | ) | (106 | )% | ||||||||||
| $ | 60,215 | 32 | % | $ | (56,000 | ) | (140 | )% | ||||||||
Significant components of deferred tax assets and liabilities are as follows:
| 2010 | 2009 | |||||||
| Net operating loss carryforwards | $ | - | $ | 56,000 | ||||
|
11 -
|
Prior-Period Adjustment
|
During 2009, the Company’s management discovered that the subscription for 80,000,000 shares of common stock at par to certain officers of the Company on May 15, 2008 was omitted from the previously issued financial statements. Accordingly, an adjustment, as of May 15, 2008, was made to common stock and subscription receivable. There was no effect on total equity.
|
12 -
|
Stockholders’ Equity
|
General
The preferred shares have a par value of $.001 per share, and the Company is authorized to issue 10,000,000 shares. The preferred stock of the Company shall be issued by the board of directors of the
F-21
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
Company in one or more classes or one or more series within any class, and such classes or series shall have such voting powers, full or limited, or no voting powers, and such designations, preferences, limitations or restrictions as the board of directors of the Company may determine, from time to time. As of December 31, 2010, there are no outstanding shares of preferred stock issued. See Note 16.
As of April 2011, common stock shares have a par value of $.001 per share (see Note 16) and the Company is authorized to issue 150,000,000 shares, each share shall be entitled to cast one vote for each share held at all stockholders’ meeting for all purposes, including the election of directors. The common stock does not have cumulative voting rights.
Common Stock
In 2011, the board of directors authorized a two-for-one forward stock split and a change in par value. See Note 16. The change in par value and the forward stock split have been retroactively applied as of January 1, 2009 in the balance sheet and the statement of changes in stockholders’ equity.
During 2010, the Company issued 20,000 shares in connection with services related to the issuance of notes payable. The Company charged its operations $5,000.
During 2010, the Company issued 68,080 shares for interest related to the issuance of notes payable. The Company accounted for this transaction as a discount on notes payable totaling $17,020, of which $2,400 was amortized to interest expense for the year ended December 31, 2010. See Note 7.
During 2010, the Company issued 5,000,000 shares of stock to its current chief financial officer for past and future services. These shares were valued at $50,000 based upon the fair value of the consulting services to be performed. A $25,000 charge was recognized as stock based compensation and the balance of $25,000 is reflected as prepaid as of December 31, 2010, and will be amortized in 2011. The stock issued contains a “claw back” provision under certain circumstances whereby 2,500,000 shares of stock will be cancelled.
In December 2010, half of the stock subscription receivable from the Company’s two founders was received, and the balance of the subscriptions receivable for 40,000,000 shares was cancelled.
There was no common stock issued during the fiscal year ended December 31, 2009.
|
13 -
|
Commitments and Contingencies
|
|
|
In March 2010, the Company signed a “Product, Manufacturing and Supply Agreement” with a contract packager and labeler for orally disintegrating tablets. The total value of this contract is $935,000. As of December 31, 2010, the Company has paid a total of $467,335, of which $200,000 is considered a stand still fee that has been reflected as a deposit on the balance sheet as of December 31, 2010. An expense of $267,335 was recorded to operations as research and development costs for the fiscal year 2010. The balance of $467,665 is expected to be completed and paid in 2011. Upon the commencement of product shipped, a 7% royalty on the gross profit related to the orally disintegrating tablet sales will be due on a quarterly basis. The $200,000 deposit will be applied towards this royalty payment.
The Company utilizes the office space of its vendor at no cost. There is no agreement for the use of this space and it may not be available to us in the future.
|
|
14 -
|
Forfeited Deposits
|
On April 2, 2009, the Company entered into a confidential term sheet agreement to purchase a U.S. based publicly traded company (the “issuer”), currently trading on the OTC exchange. The agreement required that after completion of the purchase, one percent of the outstanding shares would remain with the issuer. In accordance with the agreement, the Company submitted a nonrefundable deposit of $12,000. The agreement expired sixty days from the date of the agreement, and was subsequently extended upon the submission of an additional deposit of $2,500 in May 2009. As of December 31, 2009, the agreement expired and the deposits of $14,500 were recorded in the statement of operations as forfeited deposits.
F-22
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
|
15 -
|
Earning Per Common Share
|
|
|
The basic earnings per share are computed using the weighted average number of common shares outstanding. The dilutive effect of potential common shares outstanding is included in the diluted earnings per share, The computations of basic earnings and diluted earnings per share for 2010 and 2009 are as follows:
|
|
For the Year Ended December 31, 2010
|
||||||||||||
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per Share
Amount
|
||||||||||
|
Net Income
|
$ | 127,072 | ||||||||||
|
Basic earnings per common share
|
$ | 127,072 | $ | 79,195,292 | $ | 0.00 | ||||||
|
Effect of dilutive securities - notes payable
|
- | 1,440,000 | - | |||||||||
|
Diluted earnings per common share
|
$ | 127,072 | $ | 80,635,292 | $ | 0.00 | ||||||
| For the Year Ended December 31, 2009 | ||||||||||||
|
Income
(Numerator)
|
Shares
(Denominator)
|
Per Share
Amount
|
||||||||||
|
Net Income
|
$ | 16,090 | ||||||||||
|
Basic earnings per common share
|
$ | 16,090 | $ | 80,771,600 | $ | 0.00 | ||||||
|
Effect of dilutive securities - notes payable
|
- | - | - | |||||||||
|
Diluted earnings per common share
|
$ | 16,090 | $ | 80,771,600 | $ | 0.00 | ||||||
|
16 -
|
Subsequent Events
|
During 2011, the Company issued notes payable aggregating $320,000 with a 24% annual interest rate. In connection with these notes payable, the Company issued 50,000 shares of common stock.
On February 20, 2011, the Company’s accounts receivable factoring line of credit was increased to $1,000,000.
On February 28, 2011, the Company issued two one year promissory notes aggregating $20,000. The notes consist of $10,000 issued to the Company's CEO and $10,000 issued to a company owned by the Company's CEO. These notes provide for monthly interest only payments of 2%, payable in cash, or common stock of the Company at $0.25 per share, at the option of the lender. There is no required principal payment on the notes until maturity. These notes mature on February 27, 2012.
In March 2011, the Company changed its par value from $.0001 per share to $.001 per share for both preferred and common stock shares. All share and per common share information for all periods presented have been adjusted to reflect this change.
The Company increased its board of directors from five to seven members during 2011.
In April 2011, the Company issued notes payable aggregating $86,000 with a 24% interest rate.
F-23
SCRIPSAMERICA, INC.
| Notes to Financial Statements | December 31, 2010 and 2009 |
In April 2011, the Company issued 2,990,252 shares of voting Series A convertible preferred stock for $1,043,000 with an 8% dividend rate, payable quarterly. The preferred stockholder appointed a director to the board of directors. Each share of Series A Preferred Stock is convertible into one share of the Company’s common stock. Of the seven members of the Company’s board of directors (the “Board”), the holder of the Series A Preferred Stock, as a single class, gets to elect one (1) director to the Board and will vote with the common stockholders to elect four (4) directors (the common stockholders will elect, as a single class, two (2) directors). The Series A Preferred Stockholder will have approval right over certain corporate actions, namely the liquidation or dissolution of the Company, any merger, share exchange or asset sale that results in a change of control, the payment of any dividends or the redemption of stock (except for stock dividends, change of control transaction and termination of employment or service). The Series A Preferred Stock is convertible into 5,989,680 shares of the Company’s common stock (based on a conversion price of $0.1744, which was adjusted as a result of the forward stock split (as described below)). The conversion price of the Series A Preferred Stock will be adjusted for any issuances of stock by the Company at a price per share less than $0.1744 (subject to certain exemptions such as securities issued under an employee stock option plan or securities issued in business transactions approved by the Board). The Series A Preferred Stock has priority to assets over the common stockholders in the event of a liquidation, dissolution or any merger, share exchange or consolidation in which the Company is not the surviving entity or there is a change in control of the Company. These rights of the Series A Preferred Stockholder continue until all of the shares of Series A Preferred Stock are converted into the Company’s common stock.
In April 2011, the Company’s Board authorized a two-for-one forward stock split. All share and per share information for all periods presented have been adjusted to reflect the two-for-one stock split.
In April 2011, the Company sold 5,200,000 shares of common stock for an aggregate purchase price of $176,000.
In May 2011, the Company sold 29,000 shares of common stock for an aggregate purchase price of $5,800.
F-24
AND RESULTS OF OPERATIONS
Caution Regarding Forward-Looking Information
Certain statements contained herein, including, without limitation, statements containing the words “believes”, “anticipates”, “expects” and words of similar import, constitute forward-looking statements. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the Company, or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements.
Such factors include, among others, the following: international, national and local general economic and market conditions: demographic changes; the ability of the Company to sustain, manage or forecast its growth; the ability of the Company to successfully make and integrate acquisitions; raw material costs and availability; new product development and introduction; existing government regulations and changes in, or the failure to comply with, government regulations; adverse publicity; competition; the loss of significant customers or suppliers; fluctuations and difficulty in forecasting operating results; changes in business strategy or development plans; business disruptions; the ability to attract and retain qualified personnel; the ability to protect technology; and other factors referenced in this and previous filings.
Given these uncertainties, readers of this prospectus and investors are cautioned not to place undue reliance on such forward-looking statements.
Overview
ScripsAmerica, Inc., (“ScripsAmerica” or “the Company” or “We”) was incorporated in the State of Delaware on May 12, 2008 and primarily engages in sale of generic pharmaceutical drugs to our customer’s various distribution centers located throughout the United States. The Company began shipment of products in February 2010.
ScripsAmerica, Inc. is a healthcare services company focused on efficient supply chain management, from strategic sourcing to delivering niche generic pharmaceuticals to market. Through the largest pharmaceutical distributor in North America, ScripsAmerica delivers product to a wide range of customers across the health care industry, including physicians’ offices, retail pharmacies, long-term care sites, hospitals, and Government and home care agencies. Current therapeutic categories include pain, arthritis, prenatal, urinary, and hormonal replacement drugs.
The United States constitutes the largest market in the world for generic pharmaceuticals, and its aging population represents a key driver for the growth of the global pharmaceuticals and domestic consumer products markets. Competitive pressures among U.S. generics providers are continuing to increase as a result of the number of new market entrants growing faster than the generics market as a whole, leading to cost competition on the manufacturing side and squeezed profit margins. On the sales side, generics prices are eroding due to low-cost suppliers from India and China capturing market share, as well as the success of health insurers and health maintenance organizations in negotiating lower reimbursement rates. Finally, large direct purchase customers such as chain drugstores demand product variety and reliability of supply that allows them to lower their inventory levels.
The Company is ideally suited to compete in the current environment by providing a low cost system of broad-based marketing, sales, and distribution capabilities for generics, branded pharmaceuticals, over the counter medicines, vitamins, and nutraceuticals. The Company has built strong relationships with local customers through a detailed understanding of and demonstrated ability to serve customer needs. These qualities have allowed ScripsAmerica to post exceptional growth since commencing operations in February 2010.
We are in the process of expanding our operations to two new areas: (i) developing of a line of rapidly dissolving drug formulations for vitamins, Over The Counter (OTC) drugs and certain generic products and (ii) securing approval of the United States Drug and Food Administration (the “FDA”) and exclusive manufacturing rights for a number of drug candidates under the Drug Efficacy Study Implementation (“DESI”) Program.
Oral drug delivery remains the preferred dosing method among patients and physicians, with more than 80% of all drugs administered in this manner. Rapid melt technology provides pharmaceutical companies with the opportunity for product line extensions for a wide variety of drugs, and we believe there is a significant opportunity as a contract developer of rapidly dissolving drug formulations for specialty prescription pharmaceuticals. The current market for oral fast-melt drugs in the U.S. is $1.5 billion.
28
In March 2010, we entered into a product development, manufacturing and supply agreement with a pharmaceutical company, which develops and manufactures generic drug products. Under this agreement with our pharmaceutical partner, we are developing a pain relief orally disintegrating rapidly dissolving 80 mg and 160 mg tablets. This pain relief formulation is for widely-used OTC drugs.
DESI was a program begun by the FDA in the 1960s based on the requirement of the Kefauver-Harris Drug Control Act that all drugs be efficacious as well as safe. The DESI program was intended to classify all pre-1962 drugs that were already on the market as either effective, ineffective, or needing further study. To date, DESI has evaluated over 3,000 separate products and over 16,000 therapeutic claims. By 1984, final action had been completed on 3,443 products; of these, 2,225 (64.6%) were found to be effective, 1,051 (30.5%) were found not effective, and 167 (4.9%) were pending. Once a DESI drug has been FDA approved and granted market exclusivity, the applicant will have the right to be the single source producer of the drug and control market supply and pricing.
We have identified Compound SA 1022, a non-steroidal anti-inflammatory drug, as our initial drug candidate for the DESI program approval. We will need to raise approximately $2,000,000 -$3,000,000 in additional funding to obtain FDA approval of Compound SA-1022 under the DESI program.
Description of Revenues
ScripsAmerica offers fulfillment of prescription and over the counter (OTC) orders. To fulfill purchase orders from customers, ScripsAmerica processes orders to the end user’s desired specifications. Capabilities range from unit of use packaging for in-patient nursing homes and hospitals to bulk packaging for government and internatioanal organizations.
The Company’s revenue is generated from the purchases of product from various suppliers and ships completed product per the end user’s specifications to distribution centers. The Company recognizes revenues in accordance with the guidance in the Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin No. 104. Accordingly, revenue is recognized when persuasive evidence of an arrangement exists, when the selling price is fixed or determinable, when shipment or delivery or performance of services has been rendered and collectability is reasonably assured. Once the Company receives proof of delivery from our customer’s distribution centers revenue is recognized.
Description of Expenses
Our expenses include the following: (a) Costs of Goods sold (the Company purchases all its product form suppliers at various contracted prices and does not own or maintain any inventory. Upon shipment of product the Company is charged the contracted price. The Company finances the purchases of product sold based on confirmed purchase orders via a revolving finance agreement); (b) Costs of services, which consists primarily of salaries and contracted professional fees for various consultants used in management for the Company; (c) Marketing costs associated with contracts and selling product; (d) General administrative costs, which consists of overhead expenses, such as travel, telecommunications and office expenses; (e) Interest expenses mainly related to our us of factoring and facility agreement; and (f) Research and development cost associated with the development of new product delivery forms.
29
Results of Operations
Results for the three months ended March 31, 2011 versus the three months ended March 31, 2010.
|
2011
|
2010
|
|||||||||||||
|
Total Revenue
|
1,641,000 | 100 | % | 310,000 | 100 | % | ||||||||
|
Cost of Goods Sold
|
1,175,000 | 72 | % | 258,000 | 83 | % | ||||||||
|
Gross Profit
|
466,000 | 28 | % | 52,000 | 17 | % | ||||||||
|
Operating Costs and Expenses:
|
||||||||||||||
|
General and Administrative
|
98,000 | 6 | % | 0 | 0 | % | ||||||||
|
Research and Development
|
284,000 | 17 | % | 0 | 0 | % | ||||||||
|
Total Operating expenses
|
382,000 | 23 | % | 0 | 0 | % | ||||||||
|
Total Other Income / (expenses)
|
(69,000 | ) | 4 | % | 0 | |||||||||
|
Income before taxes
|
15,000 | 1 | % | 52,000 | 17 | % | ||||||||
|
Tax Expense
|
3,000 | 18,000 | 6 | % | ||||||||||
|
Net Income
|
12,000 | 1 | % | 34,000 | 11 | % | ||||||||
Operating Revenues
The Company began shipment of products in February 2010 and the majority of the Company’s sales revenue is generated from the sales of generic pharmaceutical prescription orders. For the three months ended March 31, 2011 the Company generated sales revenue of approximately $1,641,000 as compared to sales of approximately $310,000 for the three months ended March 31, 2010.
All the shipments were to one customer, Mckesson Corporation, the largest pharmaceutical distributor in North America, which acts as an intermediary between ScripsAmerica and the end users of its products.
The following table sets forth selected statement of operations data as a percentage of total sales.
|
Products sold
|
2011
|
% to total
|
2010
|
% to total
|
||||||||||||
|
Prescription drug products
|
$ | 1,444,000 | 86% | $ | 278,000 | 87% | ||||||||||
|
OTC & non prescription products
|
235,000 | 14% | 49,000 | 13% | ||||||||||||
|
Gross Sales
|
1,679,000 | 100% | 378,000 | 100% | ||||||||||||
|
Discounts / Charge backs
|
(38,000 | ) | 2% | (68,000 | ) | 18% | ||||||||||
|
Net Sales
|
$ | 1,641,000 | 100% | $ | 310,000 | 100% | ||||||||||
Cost of Product Revenue
Cost of product revenue consist primarily of a contracted price from our supplier and manufacturer to produce all of our products that were shipped in both periods.
30
Gross Profit
Gross profit for the three months ended March 31, 2011 was approximately $466,000 or 28% primarily driven by the sales of prescription generic drugs, as compared to a gross profit of approximately $52,000 for the same three month period in 2010.
Operating Expenses
General and Administrative: For the three months ended March 31, 2011, general and administrative expenses (“G&A”) were approximately $98,000 as compared to approximately $0 for the three months ended March 31, 2010. G&A expenses for the first quarter of 2011 consist of salaries and wages of approximately $57,000, professional fees, which consist mainly of legal and accounting fees, of approximately $38,000 and general office supplies made up the balance of approximately $3,000. For the three months ended March 31, 2010, the Company had virtually no G&A.
Research and Development: The Company’s expenditures for research and development cost were approximately $284,000, for the three months ended March 31, 2011 versus none for the same three months prior year. Our research and development costs can mainly be attributed to on-going research related to new product development with a third party provider.
Total Other Income and Expenses: Other income/expense of approximately $69,000 primarily consists of interest expense associated with our factoring fess related to factoring our accounts receivables for the three months ended March 31, 2011. There was no interest expense for the three months ended March 31, 2010.
Net Income Applicable to Common Shares: The Company recorded a net income of approximately $12,000 for the three months ended March 31, 2011, as compared to a net income of approximately $34,000 for the three months ended March 31, 2010, a decrease of approximately $21,000 over the prior year three month period. This decrease in net income is a result of operating expenses increasing significantly over the prior year expenses. Even though the gross profit increased approximately $414,000 for the three months ended March 31, 2011 over 2010, our research and development costs increased approximately $284,000 and general and administrative expense increased approximately $98,000, for a total increase in operation expenses over prior year of approximately $382,000. We also incurred increased interest expense of approximately $69,000 and a decrease in income tax expense of approximately $15,000. Earnings per common share were $0.00 for basic and diluted for the three months ended March 31, 2011, and $0.00 for basic and diluted for the three months ended March 31, 2010.
Liquidity and Capital Resources
At March 31, 2011, the Company had total current assets of approximately $730,000 and total current liabilities of approximately $629,000, resulting in working capital of approximately $101,000. At March 31, 2011, the Company's current assets consisted of approximately $532,000 in cash, approximately $66,000 in prepaid expenses and other current assets and $131,000 receivable due from factor. Current liabilities at March 31, 2011 consist primarily of approximately $468,000 in convertible notes payable, $80,000 in related party notes payable, and approximately $82,000 in accounts payable and accrued expenses.
During 2010, we met our liquidity needs primarily from operational sales and issuances of the Company’s securities. Current operations are again expected to be funded from sales of the Company’s products. Management plans to pursue sales and profit growth through (a) expansion of its distribution network and in-house research and development of new products (i.e. rapidly dissolving drug formulation pain relief products) and (b) grow through an acquisition of a pharmaceutical packager and distribution. The balance of funds required for our research and development costs for our rapidly dissolving drug formulation program is approximately $0.5 million and will be funded via current operations. Management expects to launch these new rapidly dissolving drug formulation products in the fourth quarter of 2011, which will require additional capital of approximately $1.2 to $1.5 million for marketing launch programs. Management plans to sell the Company’s preferred and/or common stock securities to raise the capital needed to fund these product launch programs. Management’s plan for an acquisition of a pharmaceutical packager and distributor will require approximately $5.0 to $7.0 million of investment capital and the Company expects raise funds for this initiative from the sale of the Company’s preferred and/or common stock securities.
31
In addition to the initiatives identified above, the Company has identified Compound SA 1022, a non-steroidal anti-inflammatory drug, as our initial drug candidate in Drug Efficacy Study Implementation (“DESI) program. In order to obtain FDA approval for Compound SA 1022 under the DESI program Management estimates that it would need to raise approximately $2.0 to $3.0 million in capital funding. This program is also expected to be funded from the sale of the Company’s preferred and/or common stock securities.
These funding efforts will proceed unabated but the Company can provide no guarantee that the funding will be realized.
Operating Activities
Net cash provided by operating activities was approximately $141,000 for the three months ended March 31, 2011, as compared to cash provided by operating activities of approximately $5,000 for the three months ended March 31, 2010, and increase in cash provided of approximately $136,000.
Investing Activities
For the three months ended March 31, 2011 and 200 there was no cash used in investing activities.
Financing Activities
Net cash provided by financing activities was approximately $220,000 for the thee months ended March 31, 2011 compared to none for three months ended March 31, 2010. The increase in cash provided by financing activities is mainly from proceeds from the promissory notes in the sum of $220,000, of which $20,000 was purchased by a related party.
Commitments and Contingencies
In 2010 we signed a two year exclusive contract with our primary supplier/manufacturer in the amount of $935,000. In fiscal year 2010 we made payments of $467,335 to this supplier, with $200,000 to be applied to future purchases and $267,335 was expensed as research and development costs. In the 2011 we are committed to pay the balance of $467,665 which will be spent on additional research and development and the purchase of the new product developed. For the three months period ended March 31, 2011 the Company paid $284,000 which was charged to research and development.
The Company utilizes the office space of its product supply vendor at no cost. There is no agreement on the use of this office space and it may not be available to us in the future.
32
Results for the year ended December 31, 2010 versus the year ended December 31, 2009.
|
Statement of Operations Data:
|
2010
|
|||||||
|
Total Revenue
|
$
|
3,221,000
|
100.0
|
%
|
||||
|
Cost of Goods Sold
|
2,544,000
|
79.0
|
%
|
|||||
|
Gross Profit
|
677,000 |
21.0
|
%
|
|||||
|
Operating Costs and Expenses:
|
||||||||
|
General and Administrative
|
94,000
|
2.9
|
%
|
|||||
|
Research and Development
|
267,000
|
8.3
|
%
|
|||||
|
Total Operating expenses
|
361,000
|
11.2
|
%
|
|||||
|
Total Other Income / (expenses)
|
(129,000
|
)
|
4.0
|
%
|
||||
|
Net Income before taxes
|
187,000
|
5.8
|
%
|
|||||
|
Tax Expense
|
60,000
|
1.9
|
%
|
|||||
|
Net Income
|
$
|
127,000
|
3.9
|
%
|
||||
Operating Revenues
The Company began shipment of products in February 2010 and the majority of the Company’s sales revenue is generated from the sales of generic pharmaceutical prescription orders. There were no sales in fiscal year 2009 and the Company incurred approximately $26,000 in general and administrative expenses and approximately another $14,000 in other expenses for a total loss before income taxes of approximately $40,000. This pre-tax loss combined with a reversal of the prior years valuation allowance generated a tax benefit of approximately $56,000 generating net income of approximately $16,000 for the year ended December 31, 2009.
All the shipments were to one customer, Mckesson Corporation, the largest pharmaceutical distributor in North America, which acts as an intermediary between ScripsAmerica and the end users of its products.
The following table sets forth selected statement of operations data as a percentage of total revenues.
|
Products sold
|
2010
|
% to total
|
||||||
|
Prescription drugs products
|
3,174,000 | 85 | % | |||||
|
OTC & nonprescription products
|
539,000 | 15 | % | |||||
| Gross Sales | 3,713,000 | 100 | % | |||||
|
Service fees
|
(492,000 | ) | -15.3 | % | ||||
|
Net Sales Revenues
|
3,221,000 | 100 | % | |||||
Cost of Product Revenue
Cost of product revenue consist primarly of a contracted price from our supplier and manufacturer to produce all of our products that were shipped.
Gross Profit
Gross profit for the year ended December 31, 2010 was approximately $677,000, or 21%, primarily driven by the sales of prescription generic drugs.
33
Operating Expenses
General and Administrative: For the year ended December 31, 2010, general and administrative expenses (“G&A”) were approximately $94,000 as compared to approximately $26,000 for the year ended December 31, 2009. The majority of G&A costs for fiscal year 2010 consist of salaries and wages of approximately $51,000, professional fees, which consist mainly of legal and accounting fees, were approximately $36,000 and all other general office supplies made up the balance of approximately $7,000. For the year ended December 31, 2009, G&A consisted mainly of professional fees and provision for bad debts.
Research and Development: The Company expenditures for research and development cost were approximately $267,000, for the year ended December 31, 2010 versus none from the prior year. Our research and development costs can mainly be attributed to on-going research in the new products development with a third party provider.
Other Income and Expenses: For the year end December 31, 2010, other income/expense was an expense of approximately $129,000 and primarily consist of interest expense associated with our factoring fess related to factoring our purchases and accounts receivables. These factor fees were approximately $115,000 for fiscal year 2010. The other approximately $14,000 was interest expense associated with our outstanding notes payable.
Income taxes provision/benefit. Total income taxes expense was approximately $60,000 for the year ended December 31, 2010, an increase of approximately $116,000, versus the prior year. This increase in taxes is a result of an increase income before taxes of approximately $227,000 compare to the prior year. For the year ended December 31, 2010 income before taxes was approximately $187,000 versus a loss in fiscal year 2009 of approximately $40,000.
Net Income Applicable to Common Shares: The Company recorded a net income of approximately $127,000 for the year ended December 31, 2010, as compared to a net income of approximately $16,000 for the fiscal year 2009, an increase of approximately $111,000 over the prior year. This increase in net income is a result of fiscal year 2010 being our first year of generating sales revenue. The profits were driven by approximately $3,200,000 in sales revenue, which generated a gross profit of approximately $677,000. Our total expenses described above were approximately $3,073,000 resulting in net income of approximately $127,000. Our fiscal year 2009 net income was mainly due to a tax benefit from loss of approximately $40,000 for 2009 and the reversal of prior years' valuation allowance. Earnings per common share were less than $.01 for basic and diluted for the year ended December 31, 2010, and less than $.01 for basic and diluted for the year ended December 31, 2009.
Liquidity and Capital Resources
At December 31, 2010, the Company had total current assets of approximately $484,000 and total current liabilities of approximately $428,000, resulting in working capital of approximately $56,000. At December 31, 2010, the Company's current assets consisted of approximately $172,000 in cash, approximately $221,000 in prepaid expenses asset and $91,000 receivables due from factor. Current liabilities at December 31, 2010 consist primarily of approximately $285,000 in convertible notes payable, $60,000 in notes payable - related parties, and approximately $83,000 in accounts payable and accrued expenses.
During 2010, the Company has met it liquidity needs primarily from operational sales and issuances of the Company’s securities. Current operations are again expected to be funded from sales of the Company’s products. The Company plans to pursue sales and profit growth through (a) expansion of its distribution network and in-house research and development of new products (i.e. rapidly dissolving drug formulation pain relief products) and (b) grow through an acquisition of a pharmaceutical packager and distribution. The balance of funds required for our research and development costs for our rapidly dissolving drug formulation program is approximately $0.5 million and will be funded via current operations. The Company expects to launch these new rapidly dissolving drug formulation products in the fourth quarter of 2011, which will require additional capital of approximately $1.2 to $1.5 million for marketing launch programs. The Company plans to sell Company stock securities to raise the capital needed to fund this rapidly dissolving drug formulation product launch programs. The Company’s plan for an acquisition of a pharmaceutical packager and distributor will require approximately $5 to $7 million of investment capital and the Company expects raise funds for this initiative from the sale of the Company’s stock securities.
In additions to the initiatives identified above, the Company has identified Compound SA 1022, a non-steroidal anti-inflammatory drug, as our initial drug candidate in Drug Efficacy Study Implementation (“DESI) program. In order to obtain FDA approval for Compound 1022 under the DESI program the Company estimates that it would need to raise approximately $2.0 to $3.0 million in capital funding, this program is also expected to be funded from the sale of the Company’s stock securities.
These funding efforts will proceed unabated but the Company can provide no guarantee that the funding will be realized.
34
Operating Activities
Net cash used in operating activities was approximately $186,000 for the year ended December 31, 2010. Cash used by operating activities can be attributable to net income of approximately $239,000 an increase in cash used for advance payments on the purchase of inventory of approximately $200,000 and prepaid expenses of approximately $196,000, an increase in our accounts receivable due from factor of approximately $91,000 and an increase in accounts payable and accrued expenses of approximately $83,000.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2010 was minimal, approximately $4,000 as compared to approximately $19,000 for the fiscal year ended December 31, 2009. This reduction of approximately $15,000 is result of lower loans to related parties.
Financing Activities
Net cash provided by financing activities was approximately $362,000 for the year ended December 31, 2010 which was mainly from proceeds received in 2010 from promissory notes payable of $360,000, of which $60,000 was received for promissory notes purchased by a related party.
Commitments and Contingencies
In 2010 we signed a two year exclusive contract with our primary supplier / manufacturer in the amount of $935,000. In fiscal year 2010 we made payments of $467,335 to this supplier, with $200,000 to be applied to future purchases and $267,335 was expensed as research and development costs. In the 2011 we are committed to pay the balance of $467,665 which will be spent on additional research and development and the purchase of the new product developed.
Off-Balance Sheet Arrangements
We currently have no off-balance sheet arrangements.
Critical Accounting Policies
The preparation of our financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make estimates and judgments that affect our reported assets, liabilities, revenues, and expenses, and the disclosure of contingent assets and liabilities. We base our estimates and judgments on historical experience and on various other assumptions we believe to be reasonable under the circumstances. Future events, however, may differ markedly from our current expectations and assumptions. While there are a number of significant accounting policies affecting our financial statements, we believe the following critical accounting policies involve the most complex difficult and subjective estimates and judgments:
Revenue Recognition - Revenue is recognized when persuasive evidence of an arrangement exists, when the selling price is fixed or determinable, when shipment or delivery or performance of services has been rendered and collectability is reasonably assured.
Customer, Product and Supplier Concentration -We primarily sell our products directly to a wholesale drug distributor who, in turn, supplies products to pharmacies, hospitals, governmental agencies and physicians. On January 1, 2010, the Company entered into a Service Agreement, with a term of ten years, cancellable by either party upon twelve months written notice, which provides for the packaging and distribution of goods, received from the Company’s suppliers, to the Company’s customers. The company uses this contract packager exclusively for all it warehouse, customer service, distribution and labeling services for 2010. In March 2010, the Company entered into a Product, Manufacturing and Supply Agreement with the same company which supplies its packaging and distribution services.
Research and development - Expenditures for research and development associated with contract research and development provided by third parties, are expensed as operating expenses as incurred. The company had charges of $267,335 and $0 for research and development expenses for years ended December 31 2010 and 2009 respectively.
Accounts receivable -Accounts receivable are stated at estimated net realizable value and net of accounts receivable sold subject to charge-back. Management provides for uncollectible amounts through a charge to earnings and a credit to an allowance for doubtful accounts based on its assessment of the current status of individual accounts and historical collection information. Balances that are deemed uncollectible after management has used reasonable collection efforts are written off through a charge to the allowance and a credit to accounts receivable. The Company has entered into an accounts receivable factoring facility agreement.
35
Income Taxes - The Company provides for income taxes using an asset and liability based approach for reporting for income taxes. Deferred income tax assets and liabilities are computed annually for differences between the financial statement and the tax basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company also complies with the provisions of accounting for uncertainty in income taxes. The accounting regulations prescribes a recognition threshold and measurements process for recording in the financial statements uncertain tax positions taken or expected to be taken in a tax return.
Cost of Goods Sold - The Company purchases all its product from suppliers at various contracted prices and does not own or maintain any inventory. Upon shipment of product the Company is charged the contracted price. The Company finances the purchase of inventory based on confirmed purchase orders via a revolving finance agreement.
Two-for-One Stock Split - In April 2011, the Company’s board of directors authorized a 2:1 forward stock split. Also in March 2011 the board of directors amended the certificate of incorporation to increase the authorized number of shares to 150,000,000 before the split with a new par value of $.001 per share. All share and per common share information for all periods presented have been retroactively applied as of January 1, 2009.
Stock based compensation - The Company may exchange its common stock for services render by employees, directors, consultants and others. The Company accounts for stock and stock options issued to employees and non-employees for services and other compensation under the provisions of ASC No. 718, Compensation – Stock Compensation and ASC No. 505-50, Equity-Based Payments to Non-Employees, which establish standards for the accounting for transactions in which an entity exchanges its equity instruments for goods and services at fair value. These standards also address transactions in which an entity incurs liabilities in exchange for goods and services that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of those equity instruments. For employees, stock awarded is valued based on the closing bid price on the date of grant. For non-employees, stock issued for services is valued at either the invoiced or contracted value of services provided, or to be provided, or the fair value of the stock at the due date per the service agreement, or stock issuance date, whichever is more readily determinable. As of December 31, 2010, the Company has no warrants or options.
Recently Issued Accounting Pronouncements - In October 2009, the FASB issued ASU 2009-13, Multiple-Deliverable Revenue Arrangements, (amendments to FASB ASC Topic 605, Revenue Recognition) (“ASU 2009-13”). ASU 2009-13 requires entities to allocate revenue in an arrangement using estimated selling prices of the delivered goods and services based on a selling price hierarchy. The amendments eliminate the residual method of revenue allocation and require revenue to be allocated using the relative selling price method. ASU 2009-13 should be applied on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010, with early adoption permitted. The Company is currently assessing the impact of the standard on its results of operations or financial condition.
January 2010, the FASB issued ASU 2010-06, Improving Disclosures about Fair Value Measurements. ASU 2010-06 amends ASC 820 to require a number of additional disclosures regarding fair value measurements. The amended guidance requires entities to disclose the amounts of significant transfers between Level 1 and Level 2 of the fair value hierarchy and the reasons for these transfers, the reasons for any transfers in or out of Level 3, and information in the reconciliation of recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis. The ASU also clarifies the requirements for entities to disclose information about both the valuation techniques and inputs used in estimating Level 2 and Level 3 fair value measurements. The amended guidance is effective for interim and annual financial periods beginning after December 15, 2009. The full adoption of ASU 2010-06 is not expected to impact the Company’s operating results, financial position or cash flows, but did impact the Company’s disclosures on fair value measurements. See note 4 “Fair Value Measurement of Financial Instruments”.
In February 2010, FASB issued ASU No. 2010-09, Amendments to Certain Recognition and Disclosure Requirements (ASU 2010-09). This update amends Subtopic 855-10 and gives a definition to SEC filer, and requires SEC filers to assess for subsequent events through the issuance date of the financial statements. This amendment states that an SEC filer is not required to disclose the date through which subsequent events have been evaluated for a reporting period. ASU 2010-09 becomes effective upon issuance of the final update. The Company adopted the provisions of ASU 2010-09 for the period ended March 31, 2010.
36
In April 2010, the FASB issued ASU 2010-17, Revenue Recognition — Milestone Method (“ASU 2010-17”). ASU 2010-17 provides guidance on the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. A vendor can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period in which the milestone is achieved only if the milestone meets all criteria to be considered substantive. The following criteria must be met for a milestone to be considered substantive. The consideration earned by achieving the milestone should (i) be commensurate with either the level of effort required to achieve the milestone or the enhancement of the value of the item delivered as a result of a specific outcome resulting from the vendor’s performance to achieve the milestone; (ii) be related solely to past performance; and (iii) be reasonable relative to all deliverables and payment terms in the arrangement. No bifurcation of an individual milestone is allowed and there can be more than one milestone in an arrangement. Accordingly, an arrangement may contain both substantive and non-substantive milestones. ASU 2010-17 is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010. Management evaluated the potential impact of ASU 2010-17 and does not expect its adoption to have a material effect on the Company’s results of operations or financial condition.
In December 2010, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2010-29, “Business Combinations (ASC Topic 805): Disclosure of Supplementary Pro Forma Information for Business Combinations.” The amendments in this ASU affect any public entity as defined by ASC Topic 805 that enters into business combinations that are material on an individual or aggregate basis. The amendments in this ASU specify that if a public entity presents comparative financial statements, the entity should disclose revenue and earnings of the combined entity as though the business combination(s) that occurred during the current year had occurred as of the beginning of the comparable prior annual reporting period only. The amendments also expand the supplemental pro forma disclosures to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. The amendments are effective prospectively for business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2010. Early adoption is permitted. The Company does not expect adoption of ASU 2010-29 to have a material impact on the Company’s results of operations or financial condition.
In December 2010, the FASB issued ASU 2010-28, “Intangibles - Goodwill and Other (ASC Topic 350): When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts.” The amendments in this ASU modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. The qualitative factors are consistent with the existing guidance and examples, which require that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. For public entities, the amendments in this ASU are effective for fiscal years, and interim periods within those years, beginning after December 15, 2010. Early adoption is not permitted. The Company does not expect the adoption of ASU 2010-28 to have a material impact on the Company’s results of operations or financial condition.
Management does not believe that any other recently issued, but not currently effective, accounting standards if currently adopted would have a material effect on the accompanying consolidated financial statements.
ON ACCOUNTING AND FINANCIAL DISCLOSURE
Our accountant is Raich Ende Malter & Co. LLP, an Independent Registered Public Accounting Firm. We do not presently intend to change accountants. At no time have there been any disagreements with such accountants regarding any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure.
37
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Our executive officers and directors as of May 30, 2011 are as follows:
|
NAME
|
AGE
|
POSITION
|
DATE APPOINTED
|
|
Robert Schneiderman (1)
|
68
|
Chief Executive Officer, Director
|
May 12, 2008
|
|
Jeffrey Andrews
|
59
|
Chief Financial Officer
|
October 10, 2010
|
|
Brian Ettinger (1)
|
58
|
Director; Chairman of the Board
|
April 1, 2011 (Chairman since May 17, 2011)
|
|
Brian Anderson (2)
|
59 |
Director
|
May 24, 2011
|
|
Dr. Joseph Camardo (2), (3)
|
58
|
Director
|
April 1, 2011
|
|
Dr. Michael Imperiale (2), (3)
|
51
|
Director
|
April 25, 2011
|
|
Andrius Pranskevicius (1)
|
31
|
Director
|
April 1, 2011
|
|
Steve Urbanski (3)
|
40
|
Executive Vice President and Director
|
May 12, 2008
|
(1) Member of Compensation Committee.
(2) Member of Audit Committee.
(3) Member of Nominating and Corporate Governance Committee.
The following summarizes the occupation and business experience during the past five years for our sole officer and director.
Robert Schneiderman has served as our Chief Executive Officer and has been a director since May 12, 2008 (the date of our inception). Mr. Schneiderman is one of our founding shareholders. From February 2002 until May 2008, Mr. Schneiderman owned and ran a financial consulting firm. From August 1966 to January 2002, Mr. Schneiderman, worked at a prominent Philadelphia recruiting firm, during which he served as CEO from August 1966 to January 2002. Mr. Schneiderman received a B.S. from Temple University in 1964.
Jeffrey J. Andrews has served as our Chief Financial Officer since October 2010. Prior to that time, Mr. Andrews was a financial consultant from February 2010 until October 2010, and he served as the Chief Financial Officer, Treasurer and Secretary of Global Resources Corp., a public company, from September 2006 until February, 2010 and as a director from September 2006 until his resignation on May 21, 2008. Mr. Andrews graduated from Villanova University in May, 1974 with a B.S. in Accounting. He has been a C.P.A. in Pennsylvania since 1978. He commenced his accounting career as an Audit Manager for a regional firm, and over his career has served as the Controller, Treasurer and/or CFO of various companies, and has had experience in corporate restructurings and reorganizations as well as IPO's and SEC periodic reporting. From April, 1999 to June, 2002, Mr. Andrews served as CFO of Collectible Concepts Group, Inc., a public company. From June 2002 to October 2004 Mr. Andrews was the Controller of Encapsulation Systems Inc.
Brian Ettinger has served as a director and our Chairman of its Board of Directors since April 1, 2011. Since 2002, Mr. Ettinger has served as the CEO and General Counsel for Worldwide Strategic Partners, Inc. (“WSP”), a privately-held energy consulting firm involved in domestic and international energy projects involving oil and gas production, exploration, alternative fuels, waste to energy, biofuels, power and pipelines. From June 2007 to August 2009, he also served as an independent consultant to Nuclear Solutions, Inc., a company that developed technology to convert waste coal into diesel fuel. Mr. Ettinger has also had a general practice law office since January 1994. Mr. Ettinger currently serves on the board of directors of WSP and on the board of directors of Texas Pacific Corporation, a privately-held developer of a natural gas and products pipeline from Texas to Arizona and Nevada. Mr. Ettinger received a B.A. degree in Political Science and Economics from LaSalle College in 1974 and a Juris Doctor degree from South Texas College of Law in 1983.
Brian Anderson has served as our director since May 24, 2011. Since February 1, 2011, Mr. Anderson has been the Chief Operating Officer of The Broadsmoore Group, a privately-held diversified merchant bank providing fully integrated business and investment services for private equity, public market and real estate transactions. From November 2007 to December 2010, he was a Director in the fixed income group at Oppenheimer & Co. Inc. From November 2007-December 2009 Mr. Anderson was the Chief Operating Officer at Vanquish Capital Group, LLC, which operated a hedge fund, an investment advisor and a broker dealer. Mr. Anderson was a Director in the Capital Markets Group at Washington Mutual from 2005 to 2007 where he specialized in structured credit transactions. From 1983 to 2005, Mr. Anderson worked in the institutional mortgage business at Lehman Brothers, Morgan Stanley, Drexel Burnham Lambert, Kidder Peabody, Paine Webber and Countrywide Securities. Mr. Anderson received a Bachelor of Science degree from the United States Military Academy at West Point in 1974, and an MBA from the University of Pennsylvania in 1983. Mr. Anderson served in the United States Army from June 1974 to August 1981.
Joseph Camardo, M.D. has served as our director since April 1, 2011. Dr. Camardo worked at Wyeth-Ayerst laboratories in Philadelphia from 1989, when he joined as an Associate Director, Clinical Research, until 2010, when he retired as Senior Vice President of Global Medical Affairs. Dr. Camardo received a Bachelor of Arts degree in Biology from the University of Pennsylvania in Philadelphia in 1974, and an MD from the University of Pennsylvania School of Medicine in 1979. He completed his internship at the Hospital of the University of Pennsylvania in 1980, and his residency at Presbyterian Hospital in Philadelphia in 1988. Between his internship and residency Dr. Camardo worked as a postdoctoral fellow at the Division of Neurobiology and the Department of Pharmacology at the College of Physicians and Surgeons of Columbia University in New York.
38
Michael Imperiale M.D. has served as our director since April 1, 2011. Since 2009 Dr. Imperiale has served as Senior Director, Global Medical Affairs for BioMarin in Novato California. Prior to joining BioMarin from 2007 to 2009, he was Vice President of Clinical research Operations at Hana Biosciences in South San Francisco. Dr. Imperiale has served in a number of positions in the Pharmaceutical and Biotechnology industries including as Senior Director of Medical Services at Nuvelo and Director of Clinical Trials Development at Exelixis from 2004 to 2006. Dr. Imperiale received a M.D. from The Hahnemann University School of Medicine in 1987 and a B.A. from Villanova University in 1982.
Andrius Pranskevicius has served as our director since April 1, 2011. Since October 2010, Mr. Pranskevicius has served as an investment manager at Mart Management LLC, a private equity advisor. From January 2007 to March 2010, he was the Chief Financial Officer at MMM Projecktai UAB, a real estate development and acquisition firm. From January 2004 to January 2007, Mr. Pranskevicius was the Chief Financial Officer at UAB “SP Investicija”, which develops and manages food chains. He received a B.A. in Management and Business Administration in 2001 from Vilnius University in Lithuania.
Steve Urbanski has served as our Executive Vice President since October 2010 and a Director since May 2008. Mr. Urbanski is one of our founding shareholders. From May 2008 to October 2010, Mr. Urbanski served as our Chief Financial Officer. He served as Controller of NWS Corp. from November 2008 to December 2009. From January 2004 to October 2008, Mr. Urbanski was an accountant at Downey, Sweeney Fitzgerald & Co., P.C., an audit, tax and accounting firm in western Massachusetts. He received a B.A. in Accounting and Business Management at Westfield State College.
Board of Directors Committees and Other Information
During our fiscal year ended December 31, 2010, our Board consisted of a two directors, Robert Schneiderman, our Chief Executive Officer, and Mr. Steve Urbanski, our Executive Vice President. On April 1, 2011, our board increased the number of directors to five (5) persons and appointed Mr. Andrius Pranskevicius, Mr. Brian Ettinger and Dr. Joseph Camardo as directors to fill the newly created vacancies. On April 25, 2011, the Board increased the size of the board to seven (7) members and appointed Dr. Michael Imperiale as a director to fill one of the newly created vacancies. On May 24, 2011, the Board appointed Mr. Brian Anderson as a director to fill the remaining vacancy. In accordance with Delaware corporate law, our business and affairs are managed under the direction of the Board. Mr. Brian Ettinger was appointed as Chairman of the Board on May 17, 2011.
The Company's Board consisted of two directors during the fiscal year ended December 31, 2010 and, 11 Board meetings were held and all other resolutions were adopted by unanimous written consent.
Policy Regarding Director Attendance At Annual Meetings
The Company does not have a formal policy regarding the Board attendance at annual meetings. During fiscal 2011, we expect to adopt such a policy.
Stockholder Communications With the Board
The Board currently does not have a formal process for stockholders to send communications to the Board. Nevertheless, the Board desires that the views of shareholders are heard by the Board and that appropriate responses are provided to shareholders on a timely basis. The Board does not recommend that formal communication procedures be adopted at this time because it believes that informal communications are sufficient to communicate questions, comments and observations that could be useful to the Board. However, shareholders wishing to normally communicate with the Board may send communications directly to: c/o ScripsAmerica, Inc., 77 McCullough Drive, New Castle, Delaware 19720, Attention: Corporate Secretary.
Code of Ethics
On May 17, 2011, we adopted a code of ethics that applies to all of our directors, officers (including our chief executive officer and chief financial officer, and any person performing similar functions) and employees. We have made our Code of Ethics available by filing it as Exhibit 14 to the registration statement on Form S-1 of which this prospectus is a part.
Section 16(a) Beneficial Reporting Compliance
Upon the effectiveness of the registration statement in which this prospectus is contained, our executive officers, directors and shareholders beneficially owning more than 10% of our common stock will be required under the Exchange Act to file reports of beneficial ownership of our common stock with the Securities and Exchange Commission. Copies of those reports must also be furnished to us. During the preceding twelve months, none of our executive officers, directors and shareholders beneficially owning more than 10% of our common stock were required to file such reports of beneficial ownership under the Exchange Act.
39
Committees
On May 24, 2011, the Board of Directors appointed Brian Anderson as Chairman and a member of the Audit Committee, and Drs. Joseph Camardo and Michael Imperiale as members of the Audit Committee. Our Chief Financial Officer, Jeffrey Andrews, will be an ex officio member of the Audit Committee. The Audit Committee reviews the scope and results of the Company's financial statements conducted by the Company's independent auditors. The Committee also reviews the scope of other services provided by the Company's independent auditors, proposed changes in the Company's financial and accounting standards and principles, and the Company's policies and procedures with respect to its internal accounting, and auditing and financial controls. The Committee makes recommendations to the Board of Directors on the engagement of the independent auditors, as well as other matters that may come before it or as directed by the Board of Directors. Our Board of Directors has determined that Mr. Anderson is an “audit committee financial expert” within the applicable definition of the Securities and Exchange Commission. Each of Mr. Brenan, Dr. Camardo and Dr. Imperiale qualifies as an independent director under Rule 10A-3 of the Securities Exchange Act of 1934 and as defined in NASD Marketplace Rule 4200(15).
On May 24, 2011, the Board of Directors appointed Brian Ettinger, Andrius Pranskevicius and Robert Schneiderman, our Chief Executive Officer, as members of the Compensation Committee. In addition, Mr. Ettinger was also appointed as Chairman of the Compensation Committee. The Compensation Committee makes decisions concerning matters of executive compensation; administers the Company's executive incentive plans; reviews compensation plans, programs and policies; and monitors the performance and compensation of executive officers. The goal of our Board of Directors executive compensation policy is to ensure that an appropriate relationship exists between executive compensation and the creation of stockholder value, while at the same time attracting, motivating and retaining senior management. Each of Messrs. Ettinger and Pranskevicius qualify as independent directors under Rule 10A-3 of the Securities Exchange Act of 1934 and as defined in NASD Marketplace Rule 4200(15).
On May 24, 2011, the Board of Directors appointed Richard C. Fox, our outside general counsel, Steve Urbanski, Dr. Joseph Camardo and Dr. Michael Imperiale to the Nominating Committee. The Nominating Committee participates in identifying qualified individuals to become directors and determining the composition of the Board and its committees. In addition, the Committee reviews and recommends to the Board of Directors proposed changes to the Company’s Certificate of Incorporation and By-Laws. In consultation with the Chairman of the Board, the committee periodically reviews, revises, interprets and confirms compliance with the Company’s corporate governance policies and corporate governance guidelines. The committee recommends to the Board of Directors ways to enhance services to and improve communications and relations with the Company’s shareholders; and conducts, in consultation with the Chairman of the Board, an annual review of the Corporation’s Code of Ethics and Business Conduct. Each of Dr. Camardo, Dr. Imperiale and Mr. Fox qualifies as an independent director under Rule 10A-3 of the Securities Exchange Act of 1934 and as defined in NASD Marketplace Rule 4200(15).
Family Relationships
None of the directors, executive officers and key employees shares any familial relationship.
Independence of Directors
Messrs. Ettinger, Anderson and Pranskevicius and Drs. Camardo and Imperiale qualify as independent directors under Rule 10A-3 of the Securities Exchange Act of 1934 and as defined in NASD Marketplace Rule 4200(15).
Term of Office
Our directors are appointed for a one-year term to hold office until the next annual general meeting of our shareholders or until removed from office in accordance with our bylaws and our amended and restated certificate of incorporation. Our officers are appointed by our board of directors and hold office until removed by the board.
Our officers and directors have not filed any bankruptcy petition, been convicted of or been the subject of any criminal proceedings or the subject of any order, judgment or decree involving the violation of any state or federal securities laws within the past five (5) years.
40
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officer during the years ended December 31, 2010, and 2009 in all capacities for the accounts of our executive, including the Chief Executive Officer (CEO) and Chief Financial Officer (CFO):
SUMMARY COMPENSATION TABLE
|
Name and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Option
Awards
($)
|
All Other
Compensation
($)
|
Total ($)
|
||||||||||||||||
|
Robert Schneiderman
|
2010
|
--
|
--
|
--
|
$ |
8,500 (1)
|
$ |
8,500
|
||||||||||||||
|
Chief Executive Officer
|
2009
|
--
|
--
|
--
|
--
|
--
|
||||||||||||||||
|
Jeffrey Andrews (2)
|
2010
|
--
|
--
|
$ |
58,500 (2)
|
$ |
58,500
|
|||||||||||||||
|
Chief Financial Officer
|
2009
|
--
|
--
|
--
|
--
|
--
|
||||||||||||||||
|
(1)
|
Consists of consulting fees paid to Harry James Production DBA R S and Associates, a consulting firm owned by Mr. Schneiderman. The consulting fees were for consulting services provided to the Company by R S and Associates.
|
|
(2)
|
Consists of (i) $8,500 in consulting fees paid to Powell Strategic Advisors, Inc., a financial consulting firm owned by Mr. Andrews, and (ii) 2,500,000 shares of common stock valued at $50,000 (of which 1,250,000 shares of common stock are subject to a claw back in the event that we do not acquire a pharmaceutical manufacturer by December 31, 2011).
|
Employment Agreements
We do not have any employment agreements with any of our executive officers.
Outstanding Equity Awards at Year End
We currently do not have any equity compensation plans. Except for 2,500,000 shares of our common stock we granted to our Chief Financial Officer, Jeffrey Andrews, in connection with his hiring, we have not made any equity awards to any of our officers or directors. We do not have any outstanding options or other forms of equity compensation.
Director Compensation
We currently do not pay any compensation to directors for their service on the Board. We may in the future determine to pay our directors' fees, grant them equity compensation and/or reimburse our directors for expenses related to their activities.
Equity Compensation Plan Information
We currently do not have any equity compensation plans. Except for 2,500,000 shares of our common stock we granted to our Chief Financial Officer, Jeffrey Andrews, in connection with his hiring, we have not made any equity awards to any of our officers or directors. We do not have any outstanding options or other forms of equity compensation.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of May 30, 2011, certain information concerning the beneficial ownership of our common stock, by (i) each person known by us to own beneficially five per cent (5%) or more of the outstanding shares of each class, (ii) each of our directors and executive officers, and (iii) all of our executive officers and directors as a group.
41
The number of shares beneficially owned by each 5% stockholder, director or executive officer is determined under the rules of the Securities and Exchange Commission, or SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under those rules, beneficial ownership includes any shares as to which the individual or entity has sole or shared voting power or investment power and also any shares that the individual or entity has the right to acquire within 60 days after May 30, 2011 through the exercise of any stock option, warrant or other right, or the conversion of any security. Unless otherwise indicated, each person or entity has sole voting and investment power (or shares such power with his or her spouse) with respect to the shares set forth in the following table. The inclusion in the table below of any shares deemed beneficially owned does not constitute an admission of beneficial ownership of those shares.
|
Name and Address(1)
|
Shares of
Common
Stock
Beneficially
Owned
|
Percent of
Common
Stock (2)
|
||||||
|
Robert Schneiderman
|
19,980,000
|
39.0
|
%
|
|||||
|
Steve Urbanski
|
19,980,000
|
39.0
|
% | |||||
|
Jeffrey Andrews
|
5,080,000
|
(3)
|
9.9
|
%
|
||||
|
Brian Ettinger
|
--
|
*
|
||||||
|
Brian Anderson
|
--
|
*
|
||||||
|
Dr. Michael Imperiale
|
--
|
*
|
||||||
|
Andrius Pranskivicius
|
5,980,504
|
(4)
|
10.5
|
%
|
||||
|
Dr. Joseph Camardo
|
--
|
*
|
||||||
|
All executive officers and directors as a group (8 persons)
|
50,908,504
|
89.1
|
%
|
|||||
|
*
|
Less than 1%.
|
|
(1)
|
The address for each named person is c/o ScripsAmerica, Inc., 77 McCullough Drive, New Castle, Delaware 19720.
|
|
(2)
|
For each named person and group included in this table, percentage ownership of our common stock is calculated by dividing the number of shares of our common stock beneficially owned by such person or group by the sum of (i) 51,218,680 shares of our common stock outstanding as of May 30, 2011 and (ii) the number of shares of our common stock that such person has the right to acquire within 60 days after May 30, 2011.
|
|
(3)
|
1,250,000 shares of common stock owned by Mr. Andrews are subject to a claw back in the event that we do not acquire a pharmaceutical manufacturer by December 31, 2011.
|
|
(4)
|
Represents shares of common stock issuable upon the conversion of 2,990,252 shares of Series A Preferred Stock held by Development 72, LLC, a limited liability company owned by Mr. Pranskevicus.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
In 2010, we paid $8,500 in consultant fees to Harry James Production DBA R S and Associates, a consulting firm owned by Mr. Schneiderman, our Chief Executive Officer and a director.
In 2010, we paid $8,500 in consulting fees paid to Powell Strategic Advisors, Inc., a financial consulting firm owned by Mr. Andrews, our Chief Financial Officer.
On December 22, 2008, we entered into a loan agreement with a stockholder, who is also one of our officers, in the amount of $6,500. The loan bears no interest and is payable on demand. On March 15, 2009, we amended the loan agreement to increase the amount to $7,300. On August 31, 2010, we amended the loan agreement to increase the amount to $9,000. As of March 31, 2011 and December 31, 2010 and 2009, the outstanding balance was $9,000, $9,000 and $7,300, respectively.
During the year ended 2010, we issued one year promissory notes aggregating $60,000, which notes consist of $40,000 issued to our Chief Executive Officer and $20,000 issued to a company owned by our Chief Executive Officer. These notes provide for monthly interest only payments of 2% of principal payable, at our option, in cash or in shares of our common stock (based on a valuation of $0.50 per share). Upon maturity, outstanding principal is payable and may be converted to common stock of the Company at $0.50 per share at the option of the lenders. The notes can be extended by mutual consent of the note holder and us. These notes mature at various times from May 2011 to December 2011. Interest charged to operations on these promissory notes was $360 and $300 for the three months ended March 31, 2011 and 2010 and $6,397 for the year ended December 31, 2010.
42
On February 28, 2011, we issued one year promissory notes aggregating $20,000, which notes consist of $10,000 issued to our Chief Executive Officer and $10,000 issued to a company owned by our Chief Executive Officer. These notes provide for monthly interest only payments of 2% of principal payable, at our option, in cash or in shares of our common stock (based on a valuation of $0.50 per share). Upon maturity, outstanding principal is payable and may be converted to common stock of the Company at $0.50 per share at the option of the lenders. The notes can be extended by mutual consent of the note holder and us. These notes mature on February 27, 2012.
DISCLOSURE OF COMMISSION POSITION OF INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our Amended and Restated Certificate of Incorporation incorporates certain provisions permitted under the General Corporation Law of Delaware relating to the liability of directors. The provisions eliminate a director's liability for monetary damages for a breach of fiduciary duty, including gross negligence, except in circumstances involving certain wrongful acts, such as the breach of a Director's duty of loyalty or acts or omissions which involve intentional misconduct or a knowing violation of law. These provisions do not eliminate a Director's duty of care. Moreover, the provisions do not apply to claims against a Director for violations of certain laws, including federal securities laws.
Our Amended and Restated Certificate of Incorporation also contains provisions to indemnify the Directors, officers, employees or other agents to the fullest extent permitted by the General Corporation Law of Delaware. These provisions may have the practical effect in certain cases of eliminating the ability of shareholders to collect monetary damages from Directors. We believe that these provisions will assist us in attracting or retaining qualified individuals to serve as directors.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
43
SCRIPSAMERICA, INC.
5,229,000 SHARES OF COMMON STOCK
PROSPECTUS
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS DOCUMENT OR THAT WE HAVE REFERRED YOU TO. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH INFORMATION THAT IS DIFFERENT. THIS PROSPECTUS IS NOT AN OFFER TO SELL COMMON STOCK AND IS NOT SOLICITING AN OFFER TO BUY COMMON STOCK IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
The Date of This Prospectus Is: _______ __, 2011
44
PART II – INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item. 13 Other Expenses Of Issuance And Distribution.
|
Securities and Exchange Commission registration fee
|
$
|
121.42
|
||
|
Transfer Agent Fees
|
$
|
0
|
||
|
Accounting fees and expenses
|
$
|
16,000
|
||
|
Legal fees and expenses
|
$
|
40,000
|
||
|
Blue Sky fees and expenses
|
$
|
0
|
||
|
Miscellaneous
|
$
|
1,500
|
||
|
Total
|
$
|
57,621.42
|
All amounts are estimates other than the Commission’s registration fee. We are paying all expenses of the offering listed above. No portion of these expenses will be borne by the selling shareholders. The selling shareholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage commissions or costs of sale.
Item. 14 Indemnification of Directors and Officers.
ScripsAmerica, Inc.’s Amended and Restated Certificate of Incorporation contains provisions to indemnify the directors, officers, employees or other agents to the fullest extent permitted by the General Corporation Law of Delaware. These provisions may have the practical effect in certain cases of eliminating the ability of shareholders to collect monetary damages from directors. ScripsAmerica believes that these provisions will assist ScripsAmerica in attracting or retaining qualified individuals to serve as directors.
The Amended and Restated and the Amended Bylaws of the Registrant provide that the Registrant shall indemnify its officers, directors and certain others to the fullest extent permitted by the General Corporation Law of Delaware (“DGCL”). Section 145 of the DGCL provides that the Registrant, as a Delaware corporation, is empowered, subject to certain procedures and limitations, to indemnify any person against expenses (including attorney’s fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending or completed action, suit or proceeding (including a derivative action) in which such person is made a party by reason of his being or having been a director, officer, employee or agent of the Registrant (each, an “Indemnitee”); provided that the right of an Indemnitee to receive indemnification is subject to the following limitations: (i) an Indemnitee is not entitled to indemnification unless he acted in good faith and in a manner that he reasonably believed to be in or not opposed to the best interests of the Company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such conduct was unlawful and (ii) in the case of a derivative action, an Indemnitee is not entitled to indemnification in the event that he is judged to be liable to the Company (unless and only to the extent that the court determines that the Indemnitee is fairly and reasonably entitled to indemnification for such expenses as the court deems proper). The statute provides that indemnification pursuant to its provisions is not exclusive of other rights of indemnification to which a person may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors, or otherwise.
Pursuant to Section 145 of the DGCL, the Registrant has purchased insurance on behalf of its present and former directors and officers against any liability asserted against or incurred by them in such capacity or arising out of their status as such.
In accordance with Section 102(b)(7) of the DGCL, the Certificate of Incorporation of the Registrant eliminates personal liability of the Registrant’s directors to the Registrant or its stockholders for monetary damages for breach of their fiduciary duties as a director, with certain limited exceptions set forth in Section 102(b) (7) of the DGCL.
The Registrant has entered into an indemnification agreement with the director elected by the holder of the Series A Preferred Stock. The terms of the agreement require that the Registrant maintain a minimum level of insurance coverage for claims against officers and directors and that the Registrant indemnify the Series A director against claims against them that arise in their service on behalf of the Registrant.
II-1
Item. 15 Recent Sales of Unregistered Securities.
We were incorporated in the State of Delaware on May 12, 2008. On August 14, 2008 we issued to our two founders and two initial investors an aggregate of 20,022,000 shares of our common stock for a total purchase price of $33,000. These shares were issued in reliance on the exemption under Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”). These shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. The recipients of the shares were accredited investors and acknowledged the restricted nature of the shares they acquired. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In September and October 2008, we issued an aggregate of 323,800 shares of our common stock to three (3) investors for a total purchase price $161,900. These shares were issued in reliance on the exemption under Section 4(2) of the Securities Act. These shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. The recipients of the shares were accredited investors and acknowledged the restricted nature of the shares they acquired. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In April 2011, the Company sold 5,200,000 shares of its common stock to four purchasers for an aggregate purchase price of $176,000. Each of the purchasers was a corporation formed outside of the United States with a business address located outside of the United States. This transaction was exempt from the registration provisions of the Securities Act pursuant to Regulation S as an offshore transaction with non-U.S. persons (as such term is defined in Rule 902 of Regulation S).
In May 2011, the Company sold 29,000 shares of its common stock to 58 purchasers for an aggregate purchase price of $5,800. Each of the purchasers was a non-U.S. citizen with a residence address located outside of the United States. This transaction was exempt from the registration provisions of the Securities Act pursuant to Regulation S as an offshore transaction with non-U.S. persons (as such term is defined in Rule 902 of Regulation S).
From May 2010 through April 2011, we issued promissory notes in the aggregate principal amount of $680,000 to four (4) investors. These notes provide for monthly interest only payments of 2% of principal payable, at our option, in cash or in shares of our common stock (based on a valuation of $0.50 per share). Upon maturity, outstanding principal is payable and may be converted to common stock of the Company at $0.50 per share at the option of the lenders. The notes have a one (1) year term and mature at various dates from May 2011 through April 2012. The convertible notes were issued to such investors in reliance on the exemption under Section 4(2) of the Securities Act. The convertible notes qualified for exemption under Section 4(2) of the Securities Act since the issuances by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. The recipients of the convertible notes were accredited investors and acknowledged the restricted nature of the notes they acquired. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In November 2010, we issued 23,040 shares of our common stock to six investors in connection with the purchase by such investors of convertible notes from us (as described above). The shares of common stock were issued to such investors in reliance on the exemption under Section 4(2) of the Securities Act. The shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuances by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. The recipients of the shares were accredited investors and acknowledged the restricted nature of the shares they acquired. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In February 2011, we issued 60,000 shares to two investors in connection with the purchase by such investors of convertible notes from us (as described above). These investors had also purchased convertible notes from us in February 2011. The shares of common stock were issued to such investors in reliance on the exemption under Section 4(2) of the Securities Act. The shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuances by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. The recipients of the shares were accredited investors and acknowledged the restricted nature of the shares they acquired. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In September 2009, November 2010 and February 2011, we issued an aggregate of 30,000 shares of our common stock as fees for assistance provided to us in capital raising efforts. These shares were issued in reliance on the exemption under Section 4(2) of the Securities Act. These shares of our Class A common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
In December 2010, we issued to our Chief Financial Officer, Jeffrey Andrews, 2,500,000 shares of our common stock as a signing bonus in connection with the commencement of his employment as our Chief Financial Officer, and we sold 40,000 shares of common stock to Mr. Andrews for $10,000. All of these shares were issued in reliance on the exemption under Section 4(2) of the Securities Act. These shares of our Class A common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
II-2
Item. 16 Exhibits and Financial Statement Schedules.
|
EXHIBIT NUMBER
|
DESCRIPTION
|
|
3.1
|
Amended and Restated Certificate of Incorporation.
|
|
3.2
|
By-Laws.
|
|
5.1
|
Opinion of Fox Law Offices, P.A.
|
|
10.1
|
Series A Preferred Stock Purchase Agreement, dated as of April 1, 2011, by and between ScripsAmerica, Inc. and Development 72, LLC.
|
|
10.2
|
Investors’ Rights Agreement, dated as of April 1, 2011, by and between ScripsAmerica, Inc. and Development 72, LLC.
|
|
10.3
|
Right of First Refusal and Co-Sale Agreement, dated as of April 1, 2011, by and among ScripsAmerica, Inc., Development 72, LLC, Robert Schneiderman, Jeffrey Andrews and Steve Urbanski.
|
|
10.4
|
Form of Indemnification Agreement by and between ScripsAmerica and each member of its board of directors.
|
|
10.5
|
Form of Restrictive Covenant Agreement by and between ScripsAmerica and each of its officers and directors.
|
|
10.6
|
Product Development, Manufacturing and Supply Agreement, entered into as of March 1, 2010, between ScripsAmerica, Inc. and Marlex Pharmaceuticals, Inc.
|
|
10.7
|
Form of Private Placement Subscription Agreement.
|
|
10.8
|
Form of Promissory Note.
|
|
10.9
|
Consulting Agreement, dated April 1, 2011, by and between Artemis and ScripsAmerica.
|
|
10.10
|
Services Agreement, dated November 23, 2010, by and between Clementi & Associates Ltd. and ScripsAmerica.
|
|
10.11
|
Factoring and Security Agreement with United Capital Factoring.
|
| 10.12 | Form of Regulation S Subscription Agreement (April 2011). |
| 10.13 | Form of Regulation S Subscription Agreement (May 2011). |
|
14.1
|
ScripsAmerica Code of Conduct.
|
|
23.1
|
Consent of Raich Ende Malter & Co. LLP
|
|
23.2
|
Consent of Counsel, contained in Exhibit 5.1.
|
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
II-3
(4) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
i. Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-4
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and authorized this registration statement to be signed on its behalf by the undersigned on June 10, 2011.
|
SCRIPSAMERICA, INC.
|
||
|
By:
|
/s/ Robert Schneiderman
|
|
|
Robert Schneiderman
|
||
|
Chief Executive Officer
|
||
POWER OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Robert Schneiderman and Jeffrey Andrews as his true and lawful attorney-in-fact and agent, with full power of substitution and revocation, to sign on his or her behalf, individually and in each capacity stated below, all amendments and post-effective amendments to this Registration Statement and to file the same, with all exhibits thereto and any other documents in connection therewith, with the Securities and Exchange Commission under the Securities Act of 1933, granting unto each such attorney-in-fact and agent full power and authority to do an perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming each act that said attorney-in-fact and agent may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates stated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Robert Schneiderman
|
Chief Executive Officer and Director
|
June 10, 2011
|
||
|
Robert Schneiderman
|
(Principal Executive Officer)
|
|||
|
/s/ Jeffrey Andrews
|
Chief Financial Officer
|
June 10, 2011
|
||
|
Jeffrey Andrews
|
(Principal Financial and Accounting Officer)
|
|||
|
/s/ Brian Ettinger
|
Chairman of the Board and Director
|
June 10, 2011
|
||
|
Brian Ettinger
|
||||
|
/s/ Brian Anderson
|
Director
|
June 10, 2011
|
||
|
Brian Anderson
|
||||
|
/s/ Joseph Camardo
|
Director
|
June 10, 2011
|
||
|
Joseph Camardo
|
||||
|
/s/ Michael Impreiale
|
Director
|
June 10, 2011
|
||
|
Michael Imperiale
|
||||
|
/s/ Andrius Pranskevicius
|
Director
|
June 10, 2011
|
||
|
Andrius Pranskevicius
|
||||
|
/s/ Steve Urbanski
|
Director
|
June 10, 2011
|
||
|
Steve Urbanski
|
II-5
