Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d8k.htm |
| EX-99.3 - PRESS RELEASE - INFINITY PHARMACEUTICALS, INC. | dex993.htm |
| EX-99.2 - PRESS RELEASE - INFINITY PHARMACEUTICALS, INC. | dex992.htm |
 Building a Sustainable, Fully Integrated
Biotechnology Company
June 7, 2011
Exhibit 99.1 |
 Forward Looking Statements
2
•
This presentation contains forward-looking statements within the meaning of The Private
Securities Litigation Reform Act of 1995. •
These statements involve risks and uncertainties that could cause actual results to be materially
different from historical results or from any future results expressed or implied by such
forward-looking statements. •
Such forward-looking statements include statements regarding: the therapeutic potential of
Infinity’s Hedgehog pathway, Hsp90, PI3K and FAAH inhibitors; the potential of
IPI-926 in addressing chondrosarcoma , pancreatic cancer and other cancers; clinical trial enrollment
expectations; plans for additional Phase 2 trials of IPI-926; the potential of IPI-504 in
addressing non-small cell lung cancer; the commencement of additional clinical development
of IPI-504; the commencement of Phase 1 development of IPI-145 and Phase 2
development of IPI-940 by Purdue; completing transition activities to enable Phase 2 development
by Purdue; the naming of a new drug development candidate; estimates of 2011 financial
performance (including cash burn and year-end cash and investments balance); and the
expectation that Infinity will have capital to support its current operating plan into 2014.
•
Such forward-looking statements are subject to numerous factors, risks and uncertainties that may
cause actual events or results to differ materially from the company's current expectations.
For example, there can be no guarantee that Infinity’s strategic alliance with
Purdue/Mundipharma will continue for its expected term or that these entities will fund
Infinity’s programs as agreed, or that any product candidate Infinity is developing will
successfully complete necessary preclinical and clinical development phases. Further, there can be no
guarantee that any positive developments in Infinity’s product portfolio will result in stock
price appreciation. Infinity’s expectations could also be affected by risks and
uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of
existing data and new data received from ongoing and future studies; the content and timing of
decisions made by the U.S. Food and Drug Administration and other regulatory authorities,
investigational review boards at clinical trial sites, and publication review bodies;
Infinity's ability to enroll patients in its clinical trials; unplanned cash requirements and
expenditures, including in connection with business development activities; market acceptance
of any products Infinity or its partners may successfully develop; and, Infinity's ability to obtain,
maintain and enforce patent and other intellectual property protection for any product candidate it
is developing. •
These and other risks which may impact management's expectations are described in greater detail
under the caption "Risk Factors" included in Infinity's quarterly report on Form
10-Q filed with the U.S. Securities and Exchange Commission on May 10, 2011.
•
Further, any forward-looking statements contained in this presentation speak only as of the date
hereof, and Infinity expressly disclaims any obligation to update any forward-looking
statements, whether as a result of new information, future events or otherwise.
•
All trademarks used in this presentation are the property of their respective owners. •
Our Internet website is http://www.infi.com. We regularly use our website to post information
regarding our business, product development programs and governance. We encourage
investors to use www.infi.com, particularly the information in the section entitled
“Investors/Media,” as a source of information about Infinity. References to www.infi.com in
this presentation are not intended to, nor shall they be deemed to, incorporate information on
www.infi.com into this presentation by reference. |
    •
Clear registration paths
•
Multiple possible indications
•
Substantial market potential
•
Full U.S. commercial rights
in oncology/inflammation
INFI in 2011: Considerable Near-Term
Momentum
•
Well-financed, with capital into 2014
•
Purdue/Mundipharma alliance enables robust clinical development and approval
strategies; provides access to markets ex-US & those best served by a GP
sales force •
Compelling scientific rationale
•
Intriguing Phase 1b clinical data
•
Underserved markets
Novel
candidates in
development
•
Rigorous, adaptive trials
•
Companion biomarker strategy
Active
phase
2 trials
+ |
 Advancing Pipeline with Broad Commercial
Potential
4 |
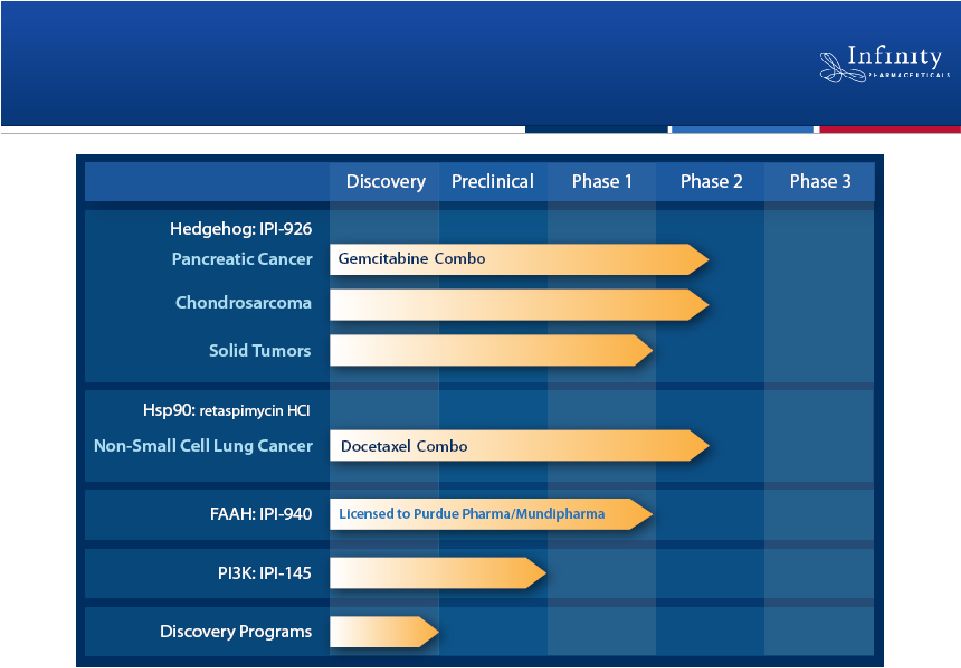
 IPI-926:
Addressing Difficult-to-Treat Cancers by
Targeting the Hedgehog Pathway
5 |
 IPI-926: Overview
6
•
Disrupts malignant activation of the Hedgehog pathway by inhibiting
Smoothened
–
Hedgehog pathway known to drive multiple difficult-to-treat cancers
•
Phase 2 development ongoing
–
Rigorous Phase 2 trial in combination with gemcitabine in pancreatic
cancer
–
Single-agent Phase 2 trial in chondrosarcoma
–
Investigator-sponsored trial program under way
–
Additional development planned for 2011
•
Strong rationale for IPI-926 in broad range of cancers with limited
treatment options
–
Clinical responses seen in two clinical trials; well-tolerated
–
Strong preclinical rationale for each registration path
|
 Large unmet need
•
~35,000 deaths /
year in the U.S. alone
•
Average survival
<6 months
•
5-year survival <5%
Resistant to therapy
•
Gemcitabine
approved Rx with
only a ~6 wk survival
benefit
•
Multiple failures in
Phase 2 and Phase 3
Treating Pancreatic Cancer Requires a
Fundamentally Novel Approach
7
Source: Xiong et. al, 2006. Drugs 66 (8): 1059-72.
Multiple Failures in Pancreatic Cancer
Single Agents
•
Topotecan
•
Irinotecan
•
Cisplatin
•
Oxaliplatin
•
Ifosfamide
•
Epirubicin
•
Docetaxel
•
Paclitaxel
•
Capeticabine
•
Pemetrexed
Combinations with Gemcitabine (Gem) |
 IPI-926 Enhances Delivery of Gemcitabine
to Tumor
8
Vehicle
Gemcitabine alone
IPI-926 +
gemcitabine
Current standard of care
in pancreatic cancer
Tumor cell nuclei
Fluorescent contrast agent
IPI-926 + gemcitabine doubles median survival in a mouse model of pancreatic
cancer (Olive et al. 2009, Science 324: 1457-61.)
|
 Rapid Translation of Preclinical Insights to
Randomized Phase 2 Clinical Trial
9
•
Primary endpoint is overall survival
–
Secondary endpoints include progression free survival, time to progression,
overall response rate
•
Rigorous design to mitigate Phase 3 risk
•
On track to complete enrollment by YE 2011
Phase 1b
Dose
Escalation
Phase 2
MTD
IPI-926 (QD) + gemcitabine
(n = 60)
Placebo + gemcitabine
(n = 60)
1:1
Randomization |
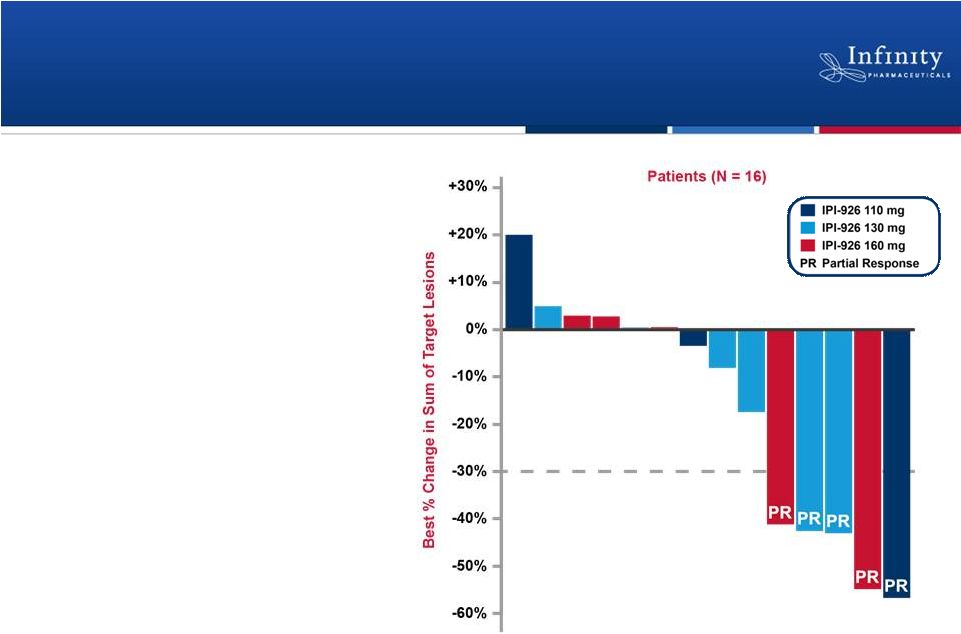 Encouraging Activity Observed in Phase 1b
•
IPI-926 + gemcitabine
led to a 31% response
rate
•
Overall response rate to
gemcitabine is
historically <10%*
10
Stephenson et al., ASCO 2011.
*Moore, et al. J Clin Oncol 25:1960-6.; Seitz et al. Oncology
18:43-7. |
 Liver
metastasis
Pancreas
Evidence of IPI-926 Activity in
Pancreatic Cancer
Pre-treatment
After 4 months of treatment
Pancreas enlarged by cancer and
fibrotic reaction plus liver metastases
Reduced pancreas, fewer liver metastases
11 |
 Phase 1b Trial: Favorable Pharmacokinetic
and Safety Profile
•
No interaction between IPI-926 and gemcitabine
•
Adverse events consistent with the known safety profile of
each agent
•
Most common adverse events were Grades 1 and 2 and
included fatigue, nausea and elevated transaminases
12 |
 IPI-926: First and Only Randomized Trial in
Chondrosarcoma
•
Significant unmet need
–
Chondrosarcoma is highly resistant to chemotherapy
and radiotherapy
–
Therapeutic standard is surgery
–
No effective treatments and no established standard of care for
patients with metastatic or locally advanced, inoperable disease
•
Preclinical data suggests inhibiting the Hedgehog pathway
reduces tumor volume and leads to calcification
•
IPI-926 granted Orphan Drug Designation by FDA and EMA in
this indication
13 |
 Rigorous Phase 2 Trial Under Way
•
Randomized, double-blind, placebo controlled study in patients with
metastatic or locally advanced, inoperable chondrosarcoma
–
Trial design reviewed with FDA and EMA prior to study
•
Primary endpoint is progression free survival
•
Secondary endpoints include time to progression, overall survival,
overall response rate and response duration
~100
Patients
IPI-926 (QD)
Placebo
Progression -
crossover to IPI-926
14
2:1
Randomization |
 IPI-926: Summary
•
Pursuing areas of significant unmet need with strong scientific
and clinical rationale
•
On-target clinical activity demonstrated in multiple solid tumors
•
Phase 2 study in pancreatic cancer advancing
–
On track to complete enrollment by YE 2011
•
Phase 2 trial in chondrosarcoma advancing
•
Additional Phase 2 development planned for 2011
–
Company-
and investigator-sponsored
15 |
 Retaspimycin HCl (IPI-504)
Targeting Non-Small Cell Lung Cancer Through
Hsp90 Inhibition
16 |
 •
Significant unmet need
•
Current standard of care provides limited benefit
–
Docetaxel response rate is ~8%, and PFS is ~3 months*
–
Patients with squamous cell carcinoma or a history of heavy
smoking have poorer prognoses
Retaspimycin HCl: Potential Breakthrough
Approach to NSCLC
17
*Hanna, et al. J Clin Oncol
22:1589-97. |
 •
Large market opportunity
–
NSCLC accounts for ~85% of all lung cancers
–
~260K stage IIIB/IV patients diagnosed every year
Non-smokers &
15 pack years
30%
Retaspimycin HCl: Potential Breakthrough
Approach to NSCLC
18
Estimates in G7 countries (US, UK, IT, DE, ES, FR, JP)
Large cell
carcinoma
(18%)
> 15 pack years
70%
% NSCLC Patients by Cell Type (2009)
% NSCLC Patients by Smoking Status (2009) |
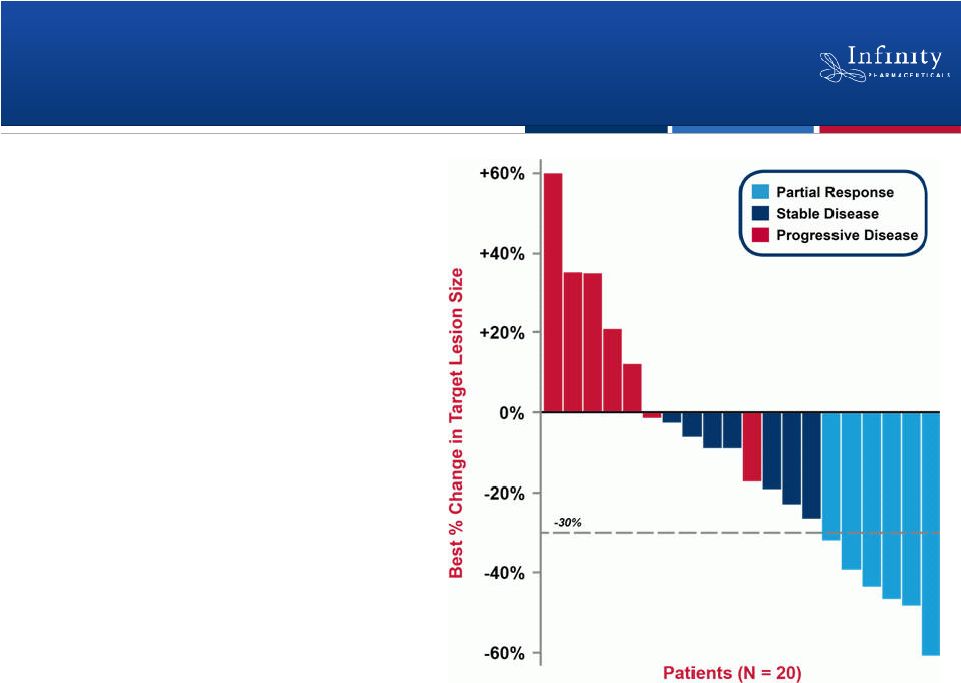 Phase 1b Trial: Retaspimycin HCl Is Clinically
Active in Combination with Docetaxel
19
Compelling Phase 1b data
•
Partial response seen in 6
patients (ORR = 26%)
•
Stable disease seen in 7
patients
Riely et al., ASCO 2011. |
 Responses Observed in Patients with
Historically Poor Prognoses
•
Patients with historically poor prognoses and limited treatment options showed
the best response rates
–
Current or former smokers (ORR = 33%)
–
Patients with squamous cell carcinoma (ORR = 43%)
20
Riely et al., ASCO 2011. |
 Phase 1b Trial: Favorable Safety Profile
•
All toxicities were manageable
•
No unexpected or overlapping toxicities seen
•
GI toxicities were primarily Grade 1 or Grade 2
•
No significant ocular toxicities were observed
•
No dose reductions or discontinuations in response to liver
function tests
21
Riely et al., ASCO 2011. |
 Adaptive Phase 2 Trial Ongoing in NSCLC
22
•
Interim analysis planned to evaluate the relationship between
efficacy and certain patient characteristics, including
–
Histology
–
Tobacco exposure
–
Certain biomarkers
•
Trial may be expanded following interim analysis
~100 smokers with
2
or
3 line
NSCLC
(docetaxel naïve)
Retaspimycin HCl (QW) +
Docetaxel (Q3W)
Placebo (QW) +
Docetaxel (Q3W)
1:1
Randomization
nd
rd |
 Retaspimycin HCl: Summary
•
NSCLC is significantly underserved
•
Combination therapy based on retaspimycin HCl may provide
important therapeutic benefit
–
Promising safety and efficacy profile in combination with docetaxel in
NSCLC patients
–
Higher response rate in heavy smokers / squamous cell carcinoma
•
Adaptive, randomized, placebo-controlled Phase 2 trial of
retaspimycin HCl and docetaxel ongoing
•
Second trial to begin in 2011
•
Phase 1b/2 combination trial in molecularly-defined subset of patients
23 |
 Additional Pipeline Opportunities
24 |
 IPI-145: Novel PI3K Inhibitor Rapidly
Advancing to the Clinic
25
•
IPI-145 is a potent, oral inhibitor of PI3K
and
PI3K
–
PI3K plays a role in hematologic
malignancies and inflammatory conditions
•
Compelling
human
proof-of-concept
of
PI3K
inhibition in heme malignancies
–
IPI-145 is 10-
to 30-fold more potent inhibitor of
PI3K
than PI3K
inhibitor with reported data
•
Data suggest inhibition of PI3K
is important,
particularly in inflammation
–
IPI-145 has shown compelling preclinical activity in
several models of inflammation
•
Phase 1 development expected to begin in 2H
2011 |
 •
IPI-940 inhibits FAAH and is designed to potentiate the effect
of anandamide
–
Constitutively active FAAH degrades anandamide, the body’s
natural source of pain relief
•
Encouraging data from Phase 1 trial in healthy volunteers
–
Marked FAAH inhibition and increased anandamide levels
–
No observed dose-limiting toxicities
•
Purdue and Mundipharma exercised rights for
worldwide development and commercialization
–
Purdue expected to begin Phase 2 development in pain in 2011
IPI-940: Phase 2-Ready FAAH Inhibitor
26 |
 Strong Financial Foundation to
Reach Key Inflection Points
27 |
 Strategic Alliances Provide Funding and
Access to Global Markets
28
Hedgehog, PI3K and early discovery
•
R&D funding from Mundipharma
•
INFI to develop and register product candidates globally
•
INFI to commercialize products in the U.S.
•
Access to ex-US markets: Mundipharma to commercialize
products ex-U.S.
•
INFI entitled to royalty of 10% to 20% on ex-U.S. sales
FAAH
•
Purdue and Mundipharma responsible for global development
and commercialization
•
Access to GP sales force: Purdue and Mundipharma
responsible for global commercialization
•
INFI entitled to royalty of 10% to 20% on global sales
|
 Financial Strength to Drive Value Creation
29
$195 M
Committed 2011-12 R&D Funding
$50 M
Line of Credit¹
(Balloon note at prime, matures 2019)
Cash and Investments
(as of 12/31/10)
Current and Committed Capital
$345 Million
1
Line of credit may be drawn for any corporate purpose.
~$100 M |
 2011 Financial Guidance:
Cash Runway into 2014
•
Projected 2011 revenue of $90M -
$95M
•
Projected 2011 cash burn of $30M -
$40M
•
Anticipate year-end cash and investments balance of
$60M -
$70M
–
Based on current operating plan; excludes $50M line of credit from Purdue
and $110M R&D funding commitment from Mundipharma for 2012
•
Approximately 26.6 million shares outstanding
30 |
 Pipeline Advancements in 2011
Hedgehog (IPI-926)
Begin Phase 2 portion of pancreatic cancer study
Present Phase 1b data in pancreatic cancer (June 4 ,
ASCO) Initiate investigator sponsored trial program
Begin additional Phase 2 studies
Hsp90 (Retaspimycin HCl)
Present Phase 1 data of retaspimycin HCl in combination with docetaxel (June 6 , ASCO) Announce future plans for Hsp90
program PI3K (IPI-145)
Begin Phase 1 study in H2
FAAH (IPI-940)
Complete transition activities to Purdue to enable Phase 2 studies in pain
Discovery
Name a new development candidate
31
th
th |
    •
Clear registration paths
•
Multiple possible indications
•
Substantial market potential
•
Full U.S. commercial rights
in oncology/inflammation
INFI in 2011: Considerable Near-Term
Momentum
•
Compelling scientific rationale
•
Intriguing Phase 1b clinical data
•
Underserved markets
Novel
candidates in
development
•
Rigorous, adaptive trials
•
Companion biomarker strategy
Active
phase
2 trials
•
Well-financed, with capital into 2014
•
Purdue/Mundipharma alliance enables robust clinical development and approval
strategies; provides access to markets ex-US & those best served by a GP
sales force + |
 Building a Sustainable, Fully Integrated
Biotechnology Company
June 7, 2011 |
