Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-12998_48k.htm |
Exhibit 99.1
|
|
STEPHAN RÖSSNER, MD, PhD1; CRAIG A. PETERSON, MS2; BARBARA TROUPIN, MD, MBA2 1PROFESSOR EMERITUS IN HEALTH RELATED BEHAVIORAL SCIENCE, KAROLINSKA INSTITUTET, STOCKHOLM, SE; 2VIVUS INC. MOUNTAIN VIEW, CA, USA Cardiometabolic Risk Reduction is Directly Related to Magnitude of Weight Loss. A Study Using an Investigational Weight Loss Combination Phentermine/Topiramate CR |
|
|
Disclosures Dr. Stephen Rössner has been a Vivus Inc. Advisory board member. |
|
|
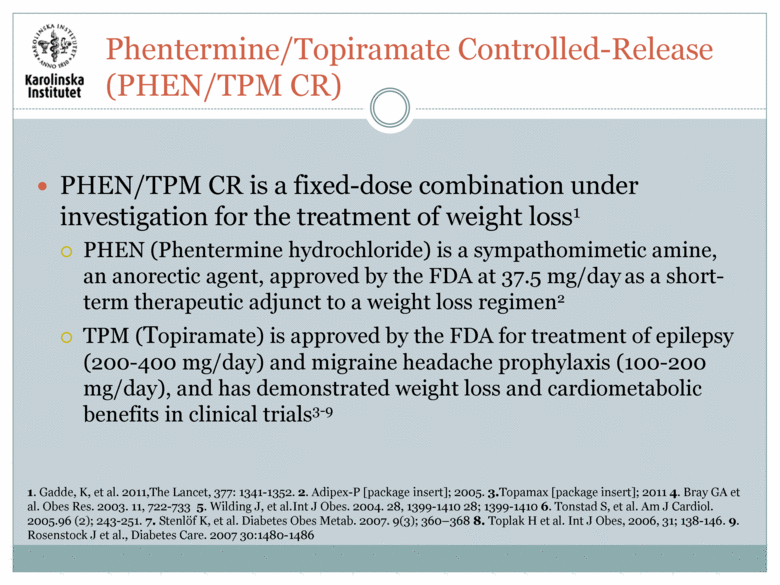
PHEN/TPM CR is a fixed-dose combination under investigation for the treatment of weight loss1 PHEN (Phentermine hydrochloride) is a sympathomimetic amine, an anorectic agent, approved by the FDA at 37.5 mg/day as a short-term therapeutic adjunct to a weight loss regimen2 TPM (Topiramate) is approved by the FDA for treatment of epilepsy (200-400 mg/day) and migraine headache prophylaxis (100-200 mg/day), and has demonstrated weight loss and cardiometabolic benefits in clinical trials3-9 1. Gadde, K, et al. 2011,The Lancet, 377: 1341-1352. 2. Adipex-P [package insert]; 2005. 3.Topamax [package insert]; 2011 4. Bray GA et al. Obes Res. 2003. 11, 722-733 5. Wilding J, et al.Int J Obes. 2004. 28, 1399-1410 28; 1399-1410 6. Tonstad S, et al. Am J Cardiol. 2005.96 (2); 243-251. 7. Stenlöf K, et al. Diabetes Obes Metab. 2007. 9(3); 360–368 8. Toplak H et al. Int J Obes, 2006, 31; 138-146. 9. Rosenstock J et al., Diabetes Care. 2007 30:1480-1486 Phentermine/Topiramate Controlled-Release (PHEN/TPM CR) |
|
|
Epidemiological data indicates that obesity is an independent risk factor for cardiovascular disease and is associated with increased mortality.1 Modest weight loss of 5%-10% can result in a marked reduction in obesity-related metabolic and cardiovascular risk factors.2 Analysis from a Phase 3 study (CONQUER) assessed effects of magnitude of weight loss (WL) categories on cardiometabolic risk factors in this study population (overweight/obese subjects with >2 weight-related comorbidities) treated with controlled-release phentermine/topiramate (PHEN/TPM CR).3 Logue J, et al. Heart. 2011;97(7):564-568. 2. Goldstein DJ. Int. J Obes. 1992;16:397-415. 3. Gadde K, et al. Lancet. 2011;377:1341-1352. Introduction 1. |
|
|
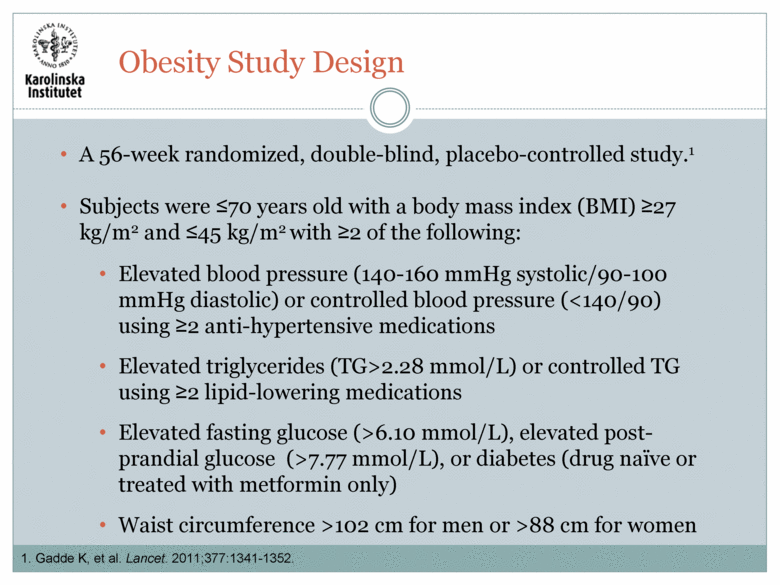
A 56-week randomized, double-blind, placebo-controlled study.1 Subjects were <70 years old with a body mass index (BMI) >27 kg/m2 and <45 kg/m2 with >2 of the following: Elevated blood pressure (140-160 mmHg systolic/90-100 mmHg diastolic) or controlled blood pressure (<140/90) using >2 anti-hypertensive medications Elevated triglycerides (TG>2.28 mmol/L) or controlled TG using >2 lipid-lowering medications Elevated fasting glucose (>6.10 mmol/L), elevated post-prandial glucose (>7.77 mmol/L), or diabetes (drug naïve or treated with metformin only) Waist circumference >102 cm for men or >88 cm for women 1. Gadde K, et al. Lancet. 2011;377:1341-1352. Obesity Study Design |
|
|
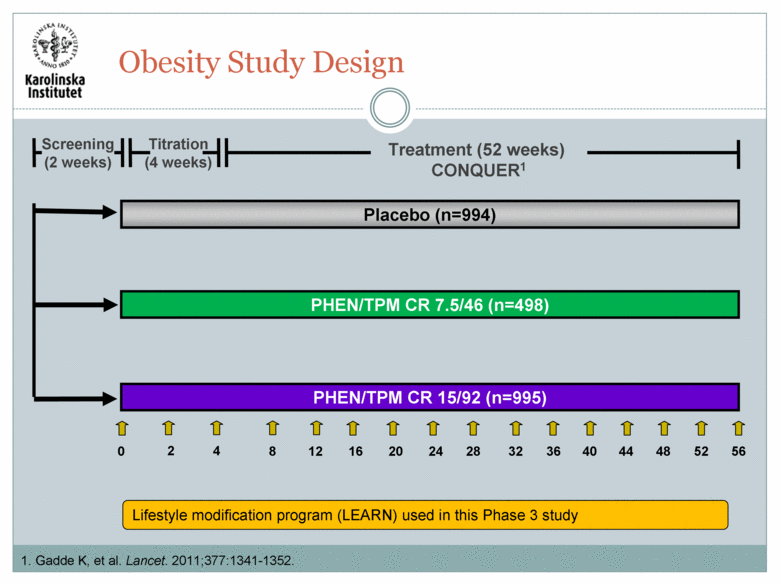
Titration (4 weeks) Treatment (52 weeks) CONQUER1 PHEN/TPM CR 15/92 (n=995) Placebo (n=994) 0 2 4 12 8 16 20 24 32 28 36 40 44 48 56 52 Screening (2 weeks) PHEN/TPM CR 7.5/46 (n=498) Lifestyle modification program (LEARN) used in this Phase 3 study Obesity Study Design 1. Gadde K, et al. Lancet. 2011;377:1341-1352. |
|
|
Least squares (LS) mean percent WL in the ITT-LOCF population Percentage of subjects achieving >5% to <10% WL, >10% to <15% WL, and >15% WL by treatment Changes in cardiometabolic parameters categorized by magnitude of weight loss (>5% to <10% WL, >10% to <15% WL, and >15% WL) regardless of treatment Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) Triglycerides (mmol/L) High-density lipoprotein cholesterol (HDL-C; mmol/L) HbA1C (%) Fasting glucose (mmol/L) Assessments at Week 56 |
|
|
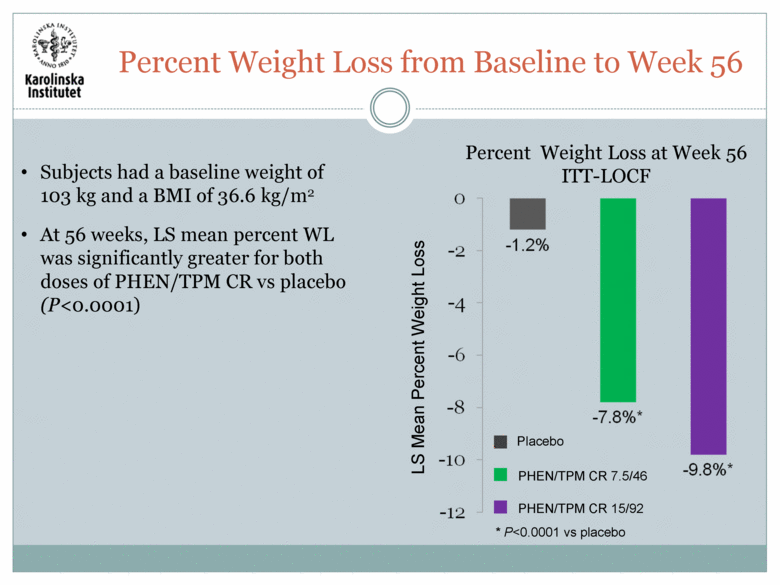
Subjects had a baseline weight of 103 kg and a BMI of 36.6 kg/m2 At 56 weeks, LS mean percent WL was significantly greater for both doses of PHEN/TPM CR vs placebo (P<0.0001) LS Mean Percent Weight Loss Placebo PHEN/TPM CR 7.5/46 PHEN/TPM CR 15/92 * P<0.0001 vs placebo Percent Weight Loss at Week 56 ITT-LOCF Percent Weight Loss from Baseline to Week 56 |
|
|
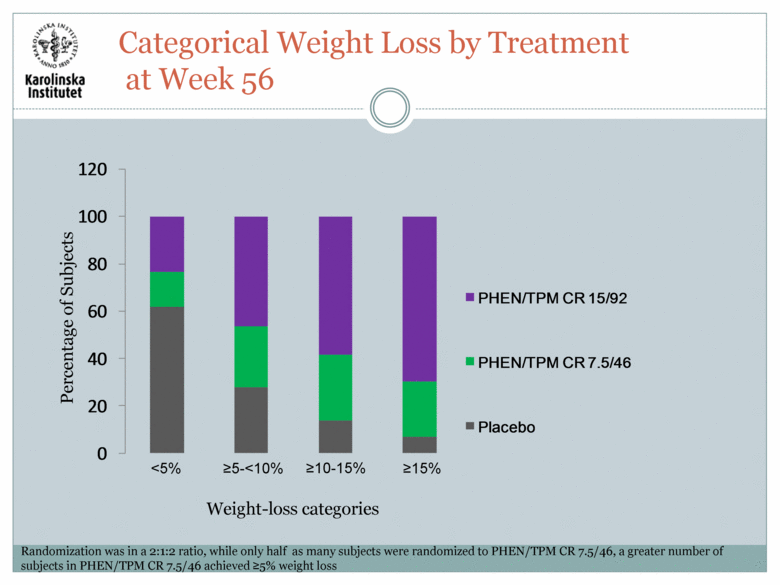
Percentage of Subjects Weight-loss categories Categorical Weight Loss by Treatment at Week 56 <5% >5-<10% >10-15% >15% Randomization was in a 2:1:2 ratio, while only half as many subjects were randomized to PHEN/TPM CR 7.5/46, a greater number of subjects in PHEN/TPM CR 7.5/46 achieved >5% weight loss 0 20 40 60 80 100 120 PHEN/TPM CR 15/92 PHEN/TPM CR 7.5/46 Placebo |
|
|
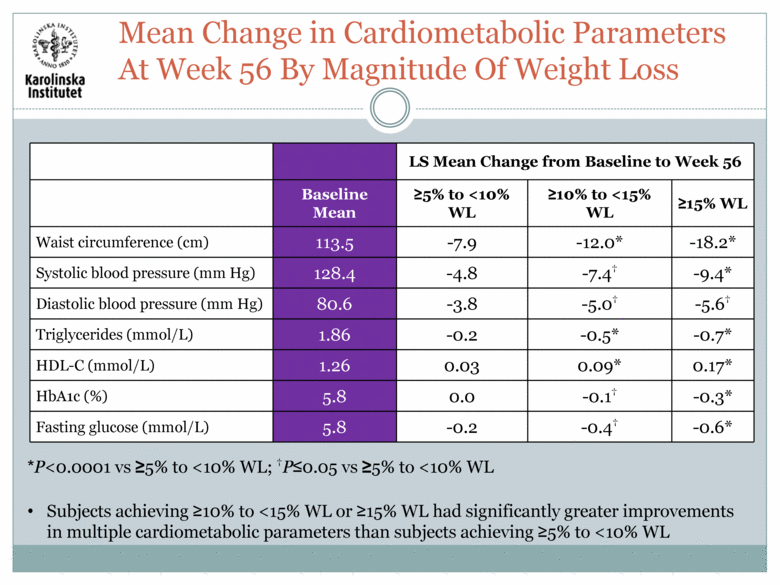
LS Mean Change from Baseline to Week 56 Baseline Mean >5% to <10% WL >10% to <15% WL >15% WL Waist circumference (cm) 113.5 -7.9 -12.0* -18.2* Systolic blood pressure (mm Hg) 128.4 -4.8 -7.4† -9.4* Diastolic blood pressure (mm Hg) 80.6 -3.8 -5.0† -5.6† Triglycerides (mmol/L) 1.86 -0.2 -0.5* -0.7* HDL-C (mmol/L) 1.26 0.03 0.09* 0.17* HbA1c (%) 5.8 0.0 -0.1† -0.3* Fasting glucose (mmol/L) 5.8 -0.2 -0.4† -0.6* Subjects achieving >10% to <15% WL or >15% WL had significantly greater improvements in multiple cardiometabolic parameters than subjects achieving >5% to <10% WL Mean Change in Cardiometabolic Parameters At Week 56 By Magnitude Of Weight Loss *P<0.0001 vs >5% to <10% WL; †P<0.05 vs >5% to <10% WL |
|
|
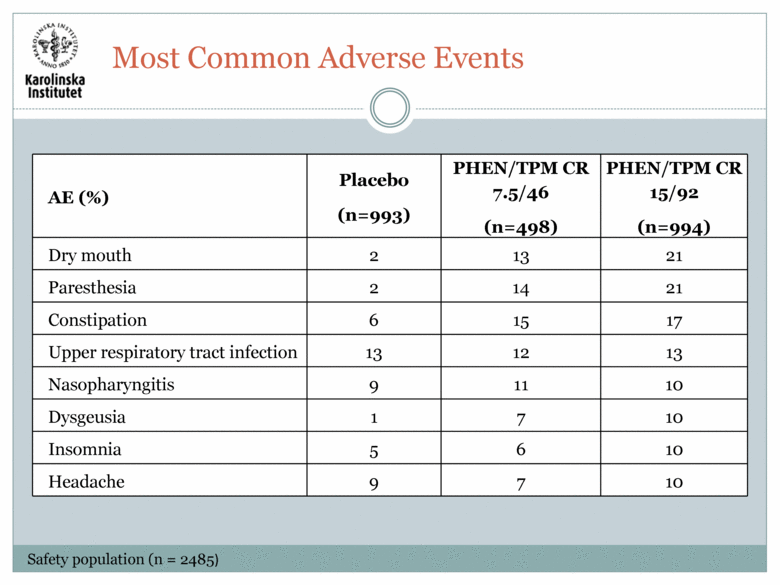
Most Common Adverse Events AE (%) Placebo (n=993) PHEN/TPM CR 7.5/46 (n=498) PHEN/TPM CR 15/92 (n=994) Dry mouth 2 13 21 Paresthesia 2 14 21 Constipation 6 15 17 Upper respiratory tract infection 13 12 13 Nasopharyngitis 9 11 10 Dysgeusia 1 7 10 Insomnia 5 6 10 Headache 9 7 10 Safety population (n = 2485) |
|
|
More subjects treated with PHEN/TPM CR compared to placebo achieved >5% to <10%, or >15% categorical WL Both doses of PHEN/TPM CR resulted in greater WL than placebo Greater improvements in multiple obesity-related cardiometabolic risk factors occurred with increasing WL, which was more common in patients treated with PHEN/TPM CR Conclusions |
|
|
Patients, investigators, and study staff Medpace, study CRO VIVUS European Advisory Board Members, for their input and interpretations Slide development assistance P. Hughes, B. Troupin (Vivus Inc.) Acknowledgments |