Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a59601e8vk.htm |
| EX-99.1 - EX-99.1 - Ardea Biosciences, Inc./DE | a59601exv99w1.htm |
| EX-99.2 - EX-99.2 - Ardea Biosciences, Inc./DE | a59601exv99w2.htm |
Exhibit
99.3

| EFFICACY AND SAFETY OF LESINURAD (RDEA594), A NOVEL URICOSURIC AGENT, GIVEN IN COMBINATION WITH ALLOPURINOL IN ALLOPURINOL-REFRACTORY GOUT PATIENTS: RANDOMIZED, DOUBLE-BLIND, PLACEBO- CONTROLLED, PHASE 2B STUDY F. Perez-Ruiz, J. Sundy, E. Krishnan, V. Hingorani, J. Welp, M. Suster, K. Manhard, Z. Shen, L. Yeh, B. Quart. Annual European Congress of Rheumatology EULAR 201125-28 May, 2011 London |

| Disclosures F. Perez-Ruiz: Grant / Research support from: Asociacion de Reumatologos del Hospital de Cruces y Ministerio de Sanidad, Gobierno de Espana, Consultant for: Ardea Biosciences, Inc., Menarini, Novartis, Speakers Bureau: Menarini, Novartis. J. Sundy: Grant / Research support from: Ardea Biosciences, Inc., Nuon Therapeutics, Regeneron, Celgene, Genentech, Savient, Consultant for: Ardea Biosciences, Inc., Nuon Therapeutics, Pharmos, Novartis, BioCryst, Savient, Roche. E. Krishnan: Shareholder of: Savient, Grant / Research support from: Ardea Biosciences, Inc., Consultant for: Takeda, Savient, URL Pharma, Employees of Ardea Biosciences: V. Hingorani , J. Welp, M. Suster , K. Manhard, Z. Shen , L.-T. Yeh, B. Quart 2 |

| >99%Reabsorption Excretion ~100% of uric acid is initially filtered through glomerular filtration URAT1 Inhibitors Increase the Urinary Excretion of Uric Acid URAT1 Enomoto; Urat1 identification in Nature May2002 Proximal Tubule LesinuradBenzbromarone 3 |

| Objective and Endpoints To assess safety and efficacy of various doses of lesinurad in combination with allopurinol in gout patients not adequately responding to allopurinol alone Endpoints:Primary Percent reduction from baseline in sUA levels at Week 4Key secondary Proportion of subjects whose urate level is < 6.0 mg/dL (~360 µmol/L) following 4 weeks of dosing Absolute and percent reduction from baseline in sUA levels at each weekly visit Incidence of gout flares 4 |

| Study Population 208 gout patients with sUA ^ 6 mg/dL (~360 µmol/L) while receiving a stable dose of allopurinol (200 to 600 mg qd) for at least 6 weeksDiagnosis of gout: ARA criteria (1977) 1Estimated Creatinine Clearance (CrCl) by Cockroft -Gault (C-G) method (actual body weight) ^ 60 mL/min based on screening blood sample was used for enrollmentNormal: ^ 90 mL/minMild: 60-89 mL/min 5 Wallace, et al. Arthritis and Rheum 1977; 20:895-900. |

| Study Design and Dose Titration Schema 2-4 weeks 4 weeks 2 weeks Screening & Run-in Period - 2-4 weeks: initiate colchicineinitiate study supplied allopurinol at same stable doseTreatment Period - 4 weeks: weekly titration up to randomized dose for higher dosesOptional blinded extension period - 44 weeks Colchicine Treatment (0.5 mg - 0.6 mg/day) Randomize within a dose cohort if no gout flare during 2 weeks of colchicine Allopurinol Treatment (200 mg - 600 mg/day) Placebo 200 mg 200 mg Placebo 400 mg 200 mg 400 mg 600 mg Off drug Off drug Off drug Placebo ScreeningPeriod:Patients must have sUA > 6 mg/dL on stable dose of allopurinol 6 |
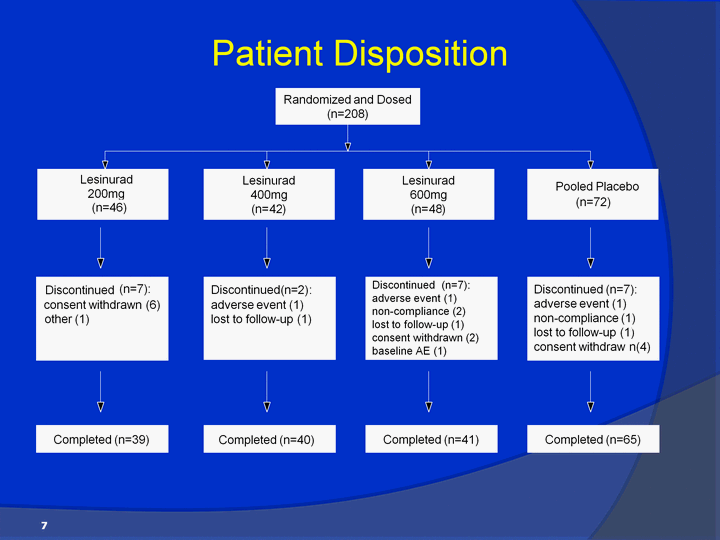
| Patient Disposition 7 Randomized and Dosed ( n =208 ) Lesinurad 600 mg ( n = 48 ) Discontinued (n=7): consent withdrawn (6) other (1) Discontinued (n=7):adverse event (1)non-compliance (2)lost to follow-up (1)consent withdrawn (2)baseline AE (1) Completed (n=39) Completed (n=40) Completed (n=41) Completed (n=65) Pooled Placebo (n=72) Lesinurad200mg (n=46) Discontinued (n=7):adverse event (1)non-compliance (1)lost to follow-up (1)consent withdraw n(4) Lesinurad 400 mg ( n = 42 ) Discontinued ( n = 2 ) : adverse event (1) lost to follow-up (1) |

| Subject Characteristics Placebo Lesinurad Lesinurad Lesinurad Baseline Parameter Placebo 200 mg 400 mg 600 mg Baseline Parameter (N=72) (N=46) (N=42) (N=48) Mean age (years) 51 53 51 48 Male (%) 99 96 98 100 Caucasian (%) 93 91 93 83 Median sUA (on allopurinol alone) 6.8 6.3 6.9 7.1 Renal Impairment (%)* 54 48 50 40 Allopurinol dose (median, mg) (Range) 300(200-400) 300(200-300) 300(200-300) 300(100-300) Presence of tophus (%) 26 15 17 23 History of gout (years) 7.3 10.2 6.7 8.0 Mean # gout flares, prior year (n, range) 4.2(0-20) 4.1(0-18) 3.5 (0-15) 4.2(1-25) 8 * Mild to moderate renal impairment estimated by Cockroft-Gault using ideal body weight. |

| (CHART) Primary Endpoint - Mean Percent Change in Serum Urate at Week 4 - Intent to Treat p<0.0001 9 p<0.0001 p<0.0001 p-values are for comparison to allopurinol alone (placebo group) using an ANCOVA model N = 72 N = 46 N = 42 N = 48 |

| (CHART) Percent of Patients with sUA < 6 mg/dL at Week 4 - Intent to Treat Analysis* 10 p<0.0001 p<0.0001 p<0.0001 * Patients with missing Week 4 results are analyzed as treatment failures, regardless of the reason for the missing data p-values are for comparison to allopurinol alone (placebo group) using a Fisher's exact test (N = 72) (N = 46) (N = 42) (N = 48) |

| (CHART) Percent of Patients with Serum Urate < 6 mg/dL at Last Visit - LOCF Analysis* 11 p<0.0001 p<0.0001 p<0.0001 * Last observation carried forward analysis (LOCF) uses a prior week's sUA results when Week 4 results are missing p-values are for comparison to allopurinol alone (placebo group) using a Fisher's exact test (N = 72) (N = 46) (N = 42) (N = 48) |

| Lesinurad Works Equally Well With Low Dose Allopurinol LOCF Analysis - Week 4 12 * One patient received allopurinol dose > 300 mg/day: 400 mg/day * n=12 n=60 n=6 n=40 n=4 n=38 n=12 n=36 |
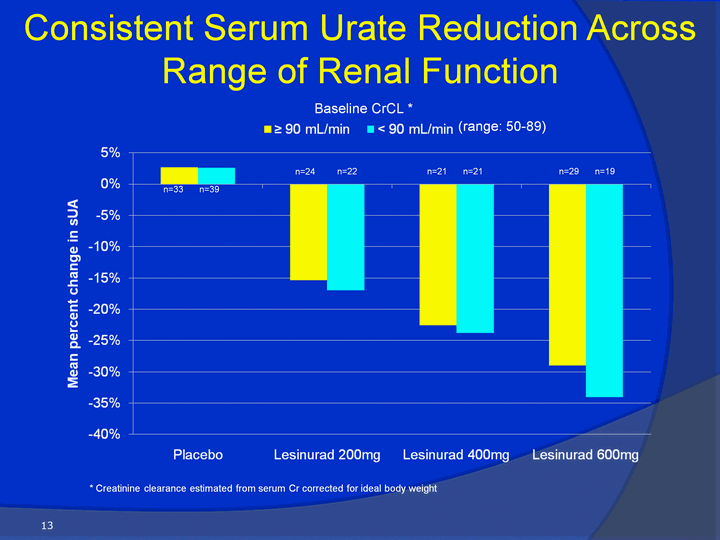
| Consistent Serum Urate Reduction Across Range of Renal Function 13 * Creatinine clearance estimated from serum Cr corrected for ideal body weight Baseline CrCL * n=33 n=39 n=24 n=22 n=21 n=21 n=29 n=19 (range: 50-89) |

| 14 Gout Flares Requiring Treatment Double-blind period (N = 72) (N = 46) (N = 42) (N = 48) |

| Incidence of the Most Frequent Adverse Events* 15 Adverse Events Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Lesinurad Dose Group Allopurinol + Adverse Events 200mg (N=46)n (%) 400mg (N=42) n (%) 600mg (N=48)n (%) Combined Lesinurad(N=136)n (%) Pooled Placebo (N=72)n (%) Any Adverse Event 1 (2.2) 4 (9.5) 5 (10.4) 10 (7.4) 10 (13.9) Diarrhea 0 1 (2.4) 0 1 (0.7) 2 (2.8) Dyspepsia 0 0 1 (2.1) 1 (0.7) 1 (1.4) Lipase Increased 0 2 (4.8) 0 2 (1.5) 1 (1.4) Hematuria 0 1 (2.4) 0 1 (0.7) 2 (2.8) Nausea 0 0 0 0 2 (2.8) *AEs reported by a total of 2 or more subjects that were considered possibly related to drug No Serious Adverse Events (SAEs) in main study5 discontinuations due to adverse events:2 combination patients (urticaria, elevated lipase)1 allopurinol patient (hematuria)2 baseline (allopurinol + colchicine) patients (QT prolongation, elevated CK) One patient died on allopurinol and colchicine prior to receiving lesinurad |

| Study 203 - Optional Blinded Extension Period After a minimum of two week washout, patients were eligible for restarting lesinurad / matched placebo in blinded extension periodPatients restarted at a dose of lesinurad 200 mg / matched placebo at Week 0The dose may be escalated for responseAllopurinol continued throughout washout and extension phase 16 Lesinurad 200mg / matched placebo + Allopurinol Lesinurad 400mg / matched placebo + Allopurinol Lesinurad 600mg / matched placebo + Allopurinol Optional - Lesinurad 600mg/ matched placebo + increased Allopurinol (in 100 mg increments) Weeks 0 - 4 Weeks 9 - 12 Weeks 5 - 8 Weeks 15 - 44 |
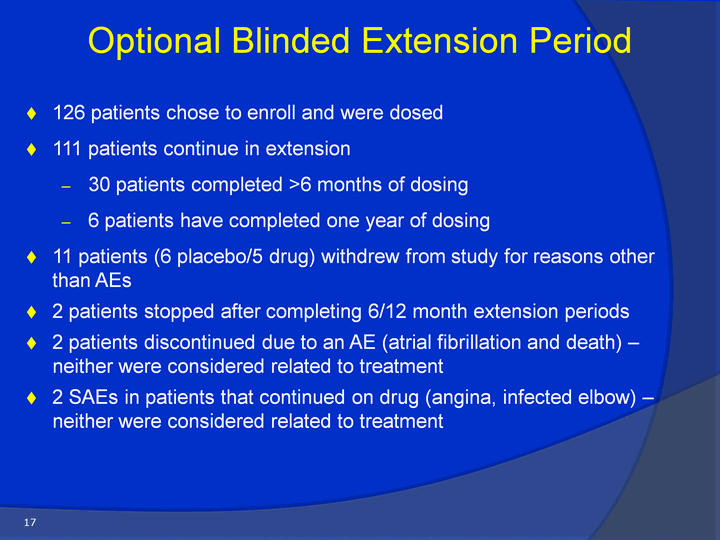
| 126 patients chose to enroll and were dosed111 patients continue in extension30 patients completed >6 months of dosing6 patients have completed one year of dosing 11 patients (6 placebo/5 drug) withdrew from study for reasons other than AEs 2 patients stopped after completing 6/12 month extension periods2 patients discontinued due to an AE (atrial fibrillation and death) - neither were considered related to treatment2 SAEs in patients that continued on drug (angina, infected elbow) - neither were considered related to treatment Optional Blinded Extension Period 17 |

| (CHART) Patients Continue to Respond in Long-Term Extension 18 Main 4-week study presented with last observation carried forward analysis (LOCF) uses a prior week's sUA results when Week 4 results are missingThe extension results are based on observed data at week 28 as of April 8, 2011. (N = 72) (N = 46) (N = 42) (N = 48) N = 8 15 6 1 Percent of Patients with sUA < 6 mg/dL Week 28 Extension |

| Patients on Lesinurad 200mg Continue to Decline for at Least 8 Weeks After 4 weeks, non-responding patients can be escalated to 400 mg and then later to 600 mg; patients that moved to higher doses are excluded from this analysis to see the effect of just the 200 mg dose 19 (CHART) The extension results are based on observed data at week 28 as of April 8, 2011. n 76 73 47 41 28 23 19 |

| Conclusions Lesinurad produced consistent, sustained reductions in serum urate levels when added on to allopurinol in patients who were not adequately responding to allopurinol aloneStatistically significant increases in response rates were observed at all lesinurad combination doses in:all treated patients patients with normal or mildly impaired renal functionpatients on 200 mg or 300 mg allopurinolNo safety concerns and no dose-related side effects were observed with the combinationLesinurad is a promising drug for the treatment of hyperuricemia in gout patients who have an inadequate response to allopurinol 20 |

| Acknowledgements Patients for their participation in the studiesInvestigators and their staff members: 21 Ukraine A. GnylorybovV. KovalenkoI. Shepet'ko M. StanislavchukA. Ter-Vartanian V. Tseluyko Canada W. Arkinstall R. Hart B. Lasko D. Shu G. Tellier I. Wilderman United KingdomJ. Calvert Georgia L. Kilsonia L. Shalamberidze Poland J. Brzezicki S. Jeka W. PorawskaA. Sokalski C. Stepniak M. Stopinska-Polaszewsk SpainF. Perez-Ruiz United StatesA. Adaoag D. MillerR. Anspach J. Pappas H. Baraf M. Prupas M. Baron B. Rankin D. DeSantis H. Rojas R. Fleischmann J. SundyG. Gottschlich R. SurowitzM. Guice M. SzczesnyW. Harper O. TernenyJ. Kirstein M. Turner E. Krishnan B. Walsh S. Lee J. WilsonR. Lidman |
Lesinurad (RDEA594), A Novel Oral Uricosuric Agent, in Combination with Febuxostat Shows Significant Additive
Urate Lowering Effects in Gout Subjects with 100% Response Achieved for All Combination Dose Regimens
R. Fleischmann1, Z. Shen2, L. Yeh2, B. Kerr2, E. Polvent2, M. Suster2, V. Hingorani2, J.N. Miner2, K. Manhard2, B. Quart2
1Metroplex Clinical Research Center, Dallas, 2Ardea Biosciences Inc, San Diego, CA
Urate Lowering Effects in Gout Subjects with 100% Response Achieved for All Combination Dose Regimens
R. Fleischmann1, Z. Shen2, L. Yeh2, B. Kerr2, E. Polvent2, M. Suster2, V. Hingorani2, J.N. Miner2, K. Manhard2, B. Quart2
1Metroplex Clinical Research Center, Dallas, 2Ardea Biosciences Inc, San Diego, CA
Introduction
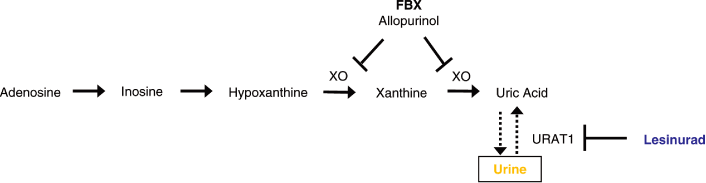
Lesinurad (RDEA594) is a uricosuric agent in development for the treatment of gout, which acts
through inhibition of uric acid transporters (URAT1 and OAT4 — EULAR2011—THU0025) in the proximal
tubule of the kidney. Lesinurad has been well tolerated, with dose-dependent reductions in serum
urate (sUA) in over 500 subjects who have received lesinurad in Phase 1 & 2 clinical trials.
Febuxostat (Adenuric®, Uloric®, FBX) is a xanthine oxidase (XO)
inhibitor recently approved in the EU (80 mg -120 mg once daily (QD) and the US (40 mg — 80 mg QD).
In a published study, an increased speed of tophi reduction was observed when XO and URAT1
inhibitors were given in combination1. The studies described below were designed to
evaluate the benefit of combining two mechanisms of sUA lowering as illustrated in Figure 1. This
dual mechanism approach with lesinurad is expected to produce target sUA reductions (response is
defined as sUA < 6 mg/dL) in gout patients and may ideally be suited to patients with high sUAs
and tophaceous gout. Simultaneously targeting these two different mechanisms for sUA lowering has
produced significantly increased reductions in sUA over either agent alone, providing the potential
for faster tophi resolution and a decrease in the rate of gout flares with chronic
treatment.1,2
| Figure 1. Dual Mechanism of sUA Reduction: Decreasing Urate Production by XO Inhibition and Increasing Urate Excretion by URAT1 Inhibition Leads to Greater sUA Lowering |
In a prior drug-drug interaction study in healthy volunteers, lesinurad 200 mg and 400 mg in
combination with FBX 40 mg showed no clinically relevant pharmacokinetic (PK) drug-drug interaction
between lesinurad and FBX. Lesinurad with a low dose of FBX produced substantial additive effects
on sUA in these subjects, with reductions of up to ~70%. The combination of lesinurad with FBX was
well tolerated.
Methods
RDEA594-111 was a multicenter, open-label, multiple-dose study in subjects with gout designed to
evaluate the sUA lowering effects, PK, drug-drug interaction potential, safety and tolerability of
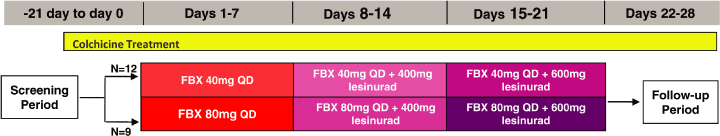
lesinurad in combination with FBX (Figure 2). The study included 21 subjects in 2 panels. All
subjects initially received FBX alone for 7 days, followed by combination treatment with lesinurad
400 mg QD for 7 days, and then combination treatment with lesinurad 600 mg QD for 7 days.
Figure 2. Study design for RDEA594-111
Key Inclusion Criteria:
| Ø | Healthy adult males or post-menopausal/surgically sterile females ³ 18 and £ 80 years of age | |
| Ø | Met one or more criteria for the diagnosis of gout as per the ARA Criteria for the Classification of Acute Arthritis of Primary Gout | |
| Ø | sUA level ³ 8 mg/dL | |
| Ø | Body mass index of £ 40 kg/m2 | |
| Ø | All laboratory parameters within normal limits or not clinically significant |
Assessments:
| Ø | Adverse events (AEs), clinical laboratory test results, vital signs, 12—lead electrocardiograms (ECGs), and physical examination for safety | |
| Ø | PK of lesinurad and of FBX in plasma | |
| Ø | Pharmacodynamics including sUA lowering and urine urate excretion |
Objectives:
| Ø | Primary |
| • | To evaluate the pharmacokinetics of FBX alone and in combination with lesinurad | ||
| • | To evaluate the pharmacokinetics and urinary excretion of lesinurad in combination with FBX | ||
| • | To evaluate the pharmacokinetics of colchicine alone and in combination with FBX or both febuxostat and lesinurad. |
Ø Secondary
| • | To measure the effect of FBX alone and in combination with lesinurad on serum urate concentrations and urinary urate excretion | ||
| • | To evaluate the safety and tolerability of multiple doses of lesinurad in combination with FBX |
Results
Disposition and Demographics
Twenty-one subjects enrolled and 20 completed the study. One subject in Panel 1 was discontinued
on Day 14 due to a protocol violation (positive drug screen).
Table 1. Subject Demographics
| Panel 1 (FBX 40 mg) | Panel 2 (FBX 80 mg) | |||||||
| Baseline Variable | (N=12) | (N=9) | ||||||
Age, years [Mean (SD)] |
52 (14.3 | ) | 43 (11.1 | ) | ||||
Body weight, kg [Mean (SD)] |
101.6 (24.44 | ) | 105.5 (19.03 | ) | ||||
BMI, kg/m2 [Mean (SD)] |
32.8 (5.82 | ) | 32.1 (5.06 | ) | ||||
sUA, mg/dL (Median) |
9.2 | 10.4 | ||||||
Race [n (%)] |
||||||||
Asian |
1 (8 | %) | 0 | |||||
Black |
4 (33 | %) | 3 (33 | %) | ||||
White |
7 (58 | %) | 6 (67 | %) | ||||
Ethnicity [n (%)] |
||||||||
Not Hispanic or Latino |
12 (100 | %) | 9 (100 | %) | ||||
Results (cont’d)
Pharmacodynamics
| Table 2. | Combination of Lesinurad + FBX Resulted in Significant sUA Reduction by both Median Percent and Absolute Change at Trough and Peak |
| Trough | 12-hrs post-dose | |||||||||||||||||||||||||||||||
| FBX | FBX | FBX | ||||||||||||||||||||||||||||||
| dose | + lesinurad | + lesinurad | + lesinurad | + lesinurad | ||||||||||||||||||||||||||||
| (mg) | sUA Variable | Baseline | Alone | 400 mg | 600 mg | Alone | 400 mg | 600 mg | ||||||||||||||||||||||||
| 40 | sUA levels (mg/dL) |
9.2 | 5.7 | 3.9 | 3.8 | 5.2 | 2.7 | 2.4 | ||||||||||||||||||||||||
| (n=12 | ) | Absolute reduction (mg/dL) |
— | -3.2 | -5.4 | -5.8 | -3.9 | -6.5 | -6.8 | |||||||||||||||||||||||
Percent change (%) |
— | -35 | % | -56 | % | -61 | % | -44 | % | -68 | % | -71 | % | |||||||||||||||||||
| 80 | sUA levels (mg/dL) |
10.4 | 5.8 | 3.3 | 2.8 | 4.8 | 2.3 | 2.0 | ||||||||||||||||||||||||
| (n=9 | ) | Absolute reduction (mg/dL) |
— | -4.2 | -6.7 | -7.3 | -5.1 | -8.0 | -8.4 | |||||||||||||||||||||||
Percent change (%) |
— | -47 | % | -65 | % | -73 | % | -52 | % | -78 | % | -81 | % | |||||||||||||||||||
Figure 3. Combination of Lesinurad + FBX Significantly Reduced sUA Compared to FBX Alone
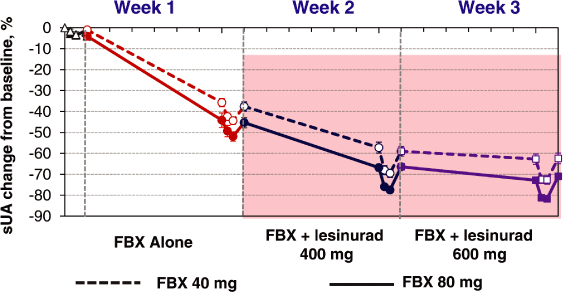
| Ø | Clinically important reductions in sUA of up to 80% were observed in gout subjects with the combination of lesinurad and FBX | |
| Ø | With lesinurad 400 mg dose in panels 1 and 2, subjects achieved median sUA levels of 3.9 mg/dL and 3.3 mg/dL at trough, respectively; and 2.7 mg/dL and 2.3 mg/ dL, respectively, at 12-hrs post-dose | |
| Ø | With lesinurad 600 mg dose in panels 1 and 2, subjects achieved median sUA levels of 3.8 mg/dL and 2.8 mg/dL at trough, respectively; and 2.4 mg/dL and 2.0 mg/ dL, respectively, at 12-hrs post-dose |
| Figure 4. All Subjects Achieved a Clinical Response (sUA < 6 mg/dL) in all Combination Regimens of Lesinurad + FBX at Trough |
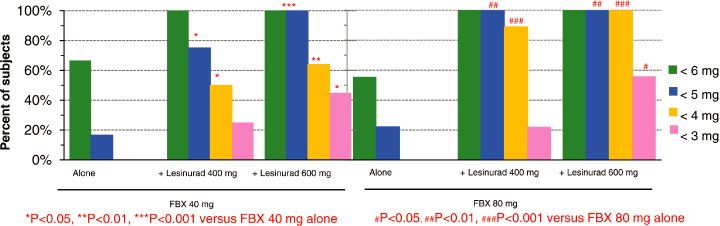
| Ø | At trough; |
| • | 100% of subjects receiving the combination of lesinurad and FBX achieved sUA levels below the clinically important target of 6 mg/dL, compared to 67% and 56% for 40 mg and 80 mg, respectively, with FBX alone | ||
| • | 75 to 100% of subjects receiving the combination of lesinurad and FBX achieved sUA levels below of 5 mg/dL, compared to 17% and 22% for 40 mg and 80 mg, respectively, with FBX alone. Reduction of sUA below 5 mg/dL may be clinically important for subjects with tophi, as the velocity of tophi resolution has been shown to be enhanced with lower sUA 1 |
| Figure 5. Over 90% of Subjects Achieved sUA < 4 mg/dL in all Combination Regimens of Lesinurad + FBX at 12-hrs Post-Dose |
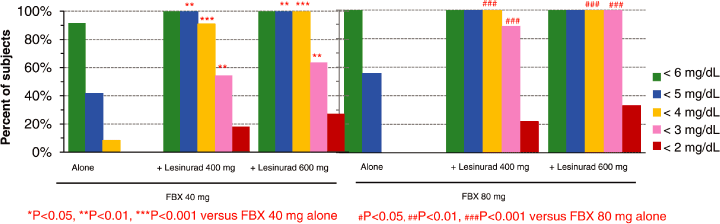
| Ø | At 12-hrs post-dose; |
| • | Even greater responses were seen with combination therapy compared to FBX alone. | ||
| • | 100% of subjects achieved sUA levels below 4 mg/dL on lesinurad 400 mg or 600 mg combined with 80 mg FBX, and 100% of subjects achieved levels below 3 mg/dL on lesinurad 600 mg combined with 80 mg FBX. No subject achieved these reduced sUA levels on either dose of FBX alone. As noted above, lower sUA is associated with faster resolution of tophi in a study by Perez-Ruiz.1 Increased resolution of tophi was also associated with lower sUA in Phase 3 trials of pegloticase, where the regimen producing a mean plasma urate of approximately 3 mg/dL produced the greatest tophi response3 | ||
| • | The combination of FBX 40 mg plus lesinurad 400 mg was significantly superior to the 80 mg dose of FBX alone (80 mg/day is the highest approved dose in the US) |
| Table 3. | The Combination of Lesinurad + FBX should not Increase Risk for Kidney Stones based on 24 hr Undissociated Urine Urate Levels3 and Urine pH |
Mean (SE) Undissociated 24h Urine Urate Concentration in mg/dL
| FBX | ||||||||||||||||
| Panel | Baseline | Alone | + Lesinurad 400 mg | + Lesinurad 600 mg | ||||||||||||
FBX 40 mg |
2.7 (0.6 | ) | 1.6 (0.4 | ) | 1.9 (0.5 | ) | 2.3 (0.7 | ) | ||||||||
FBX 80 mg |
11 (1.8 | ) | 3.9 (1.0 | ) | 4.8 (1.6 | ) | 2.3 (0.6 | ) | ||||||||
| Mean (SE) 24h Urine pH | ||||||||||||||||
FBX 40 mg |
5.9 (0.1 | ) | 5.9 (0.1 | ) | 6.0 (0.1 | ) | 6.0 (0.1 | ) | ||||||||
FBX 80 mg |
5.5 (0.1 | ) | 5.6 (0.1 | ) | 5.7 (0.2 | ) | 5.8 (0.1 | ) | ||||||||
| Ø | Mean (SE) undissociated urine urate levels (mg/dL) remained well below 10 mg/dL, which decreases the risk of uric acid stones 4 | |
| Ø | Urine pH increased or remained unchanged in gout subjects |
Pharmacokinetics
Table 4. Steady State Plasma FBX Exposures were Mildly Increased by Lesinurad
| Geometric Mean Ratio% (CI90%) | ||||||||||||||||
| Dose (mg) | (Combo/Alone) | |||||||||||||||
| FBX | Lesinurad | N | Cmax | AUC | ||||||||||||
40 |
400 | 11 | 109 (83-143 | ) | 108 (99-117 | ) | ||||||||||
| 600 | 11 | 129 (109-154 | ) | 120 (109-132 | ) | |||||||||||
80 |
400 | 9 | 113 (104-123 | ) | 119 (112-126 | ) | ||||||||||
| 600 | 9 | 118 (93-148 | ) | 121 (107-137 | ) | |||||||||||
| Ø | In gout subjects, a mild increase (< 30%) in FBX exposure was seen with co-administration with lesinurad | |
| Ø | Lesinurad exposure was not altered by FBX |
Safety
| Ø | Overall, the combination of lesinurad and FBX was well tolerated, with no serious adverse events, no discontinuations due to adverse events, and no increase in adverse events with the combination | |
| Ø | No difference in rate of adverse events across dosing periods, with lowest rate observed in the lesinurad 600 mg + FBX arms (Table 5) | |
| Ø | Renal safety: 1 subject had a grade 2 increase in serum creatinine (SCr) on colchicine alone; no subjects with grade 2 or greater SCr increases on lesinurad or the combination | |
| Ø | Liver safety: 1 subject had a grade 3 increase in ALT on FBX 40 mg alone, which normalized during combination treatment | |
| Ø | 12 subjects had at least one CK elevation above the normal range; however, 8 of those subjects had elevated CKs at screening and/or baseline. Of the remaining 4 subjects, 2 subjects had 1 elevation each, 1 subject had 2 elevations, and 1 subject had 4 elevations. None of the elevations were considered by the investigator to be clinically significant |
Table 5. Treatment-emergent Adverse Events Reported in 2 or More Subjects
| 40 mg FBX | 80 mg FBX | |||||||||||||||||||||||
| + Lesinurad | + Lesinurad | + Lesinurad | + Lesinurad | |||||||||||||||||||||
| MedDRA Preferred | _ | 400 mg | 600 mg | _ | 400 mg | 600 mg | ||||||||||||||||||
| Term | (N=12) | (N=12) | (N=11) | (N=9) | (N=9) | (N=9) | ||||||||||||||||||
Dyspepsia |
0 | 1 (8 | %) | 0 | 0 | 2 (22 | %) | 0 | ||||||||||||||||
Gout Flares |
2 (17 | %) | 0 | 0 | 0 | 1 (11 | %) | 3 (33 | %) | |||||||||||||||
Headache |
1 (8 | %) | 1 (8 | %) | 0 | 0 | 3 (33 | %) | 0 | |||||||||||||||
Conclusions
| Ø | In gout subjects, the combination of lesinurad and FBX produced substantial additive sUA lowering with 100% response rate (< 6 mg/dL) achieved at all combination dose levels, 100% of subjects achieved < 4 mg/dL at highest doses at trough, and 100% of subjects achieved sUA < 3 mg/dL intraday. | |
| Ø | Combining drugs with complementary mechanisms produced significantly greater reductions in sUA than increasing the dose of a single agent. | |
| Ø | No clinically relevant pharmacokinetic drug-drug interactions between lesinurad and FBX were noted in gout subjects at the doses tested. | |
| Ø | Given that mono-sodium urate crystals are soluble below 6.8 mg/dL, the ability of the combination of lesinurad and FBX to bring sUA in 100% of subjects to < 3 mg/dL may be particularly useful in subjects who have accumulated large deposits of uric acid, or tophi. | |
| Ø | The combination was generally well tolerated with no deaths or other serious adverse events and no subjects discontinued from the study due to adverse events. | |
| Ø | Multiple QD oral doses of 400 and 600 mg lesinurad were generally safe and well tolerated by gout subjects with hyperuricemia when administered in combination with 40 and 80 mg FBX QD. |
Reference:
| 1. | Perez-Ruiz F, Arthritis Rheum 2002;47:356-360. | |
| 2. | Becker MA, et al. 2007 ACR Annual Meeting, Abstract No. 758. | |
| 3. | FDA Advisory Committee Briefing Book for Pegloticase | |
| Asplin J, Nephrology, 1996; 16: 412-424 |
Annual European Congress of Rheumatology EULAR, London, May 25 — 28, 2011
THU0025
Lesinurad (RDEA594), A Investigational Uricosuric Agent for Hyperuricemia and Gout,
Blocks OAT4 Transport, Mechanism of Hydrochlorothiazide-Dependent Hyperuricemia
P.K. Tan, D. Hyndman, S. Liu, B. Quart and J.N. Miner,
ARDEA BIOSCIENCES, San Diego, CA, United States
Blocks OAT4 Transport, Mechanism of Hydrochlorothiazide-Dependent Hyperuricemia
P.K. Tan, D. Hyndman, S. Liu, B. Quart and J.N. Miner,
ARDEA BIOSCIENCES, San Diego, CA, United States
Introduction
Lesinurad (RDEA594) is a urate lowering therapy in clinical development for the treatment of gout
that blocks reabsorption of urate (UA) within the kidney proximal tubule by inhibiting the URAT1
transporter. Lesinurad shows significant urate lowering activity in both monotherapy and in
combination with xanthine oxidase inhibitors. In addition to URAT1, organic anion transporter 4
(OAT4) is also considered an important regulator of urate excretion. As such, lesinurad was tested
to determine its activity against OAT4 and was found to be active against OAT4 at concentrations
that would be observed at the site of action. Activity against OAT4 may provide important
additional pharmacologic properties, as it has been postulated that OAT4 may be responsible for the
hyperuricemia associated with some diuretics. Hypertension is prevalent in gout patients and they
are often treated with anti-hypertensive agents, such as thiazide diuretics, which have been known
since the 1950’s to elevate serum UA
levels1. Urate lowering therapies are generally believed to
work less efficiently in patients taking diuretics
concomitantly2. This effect is thought to be
mediated by enhanced UA reabsorption due to activation of OAT4 by diuretics via two different
mechanisms3,4.
Figure 1. Two mechanisms for HCTZ-induced hyperuricemia
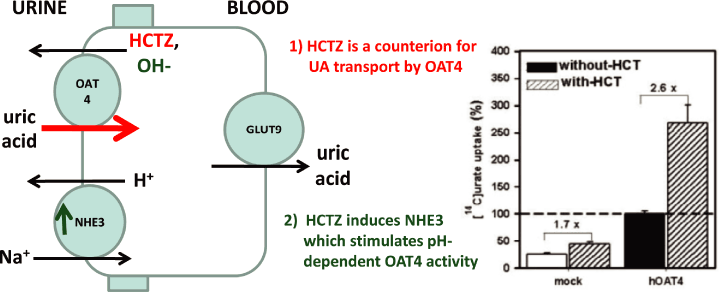
| • | Direct -HCTZ enhancement of Uric acid uptake by OAT42 | |
| • | Indirect -HCTZ enhances an OAT4 stimulatory protein (NHE3)3 |
Data presented with permission Johannes Hagos
Methods
The UA transporter OAT4 was stably expressed in cultured cells and oocytes. For cultured cells,
HEK293 cells stably expressing the transporters were produced through transfection of DNA
constructs carrying the transporters, antibiotic selection, and clonal selection of clones with
high transporter activity. Alternatively, cells transiently expressing the transporters were
produced by reverse transfection of HEK293T cells. Transfectants were plated at high density onto
poly-L-lysine coated multiwell plates and assayed 1-2 days later. Results were similar for stable
and transient expressing cells. Oocytes were injected with cRNA for OAT4 expression and then
assayed 3-4 days later.
Activity assays were performed by incubating the cells with transporter substrates in assay buffer
containing 125 mM sodium gluconate, 4.8 mM potassium gluconate, 1.2 mM monobasic sodium phosphate,
1.2 mM magnesium sulfate, 1.3 mM calcium gluconate, 5.6 mM glucose, and 25 mM HEPES pH 7.1. Drugs
such as lesinurad were added to the cells prior to addition of substrate for the indicated times.
Substrates used were 6-carboxyfluorescein (CF) at 5 µM, 3H-estrone sulfate (ES) at 50 nM, and
14C-uric acid (UA) at 100 µM. For transfected cells, substrates were incubated for 2 minutes and
then removed by aspiration and the cells washed three times in a wash buffer containing 125 mM
sodium gluconate and 25 mM HEPES pH 7.1. Cells were then lysed in 1 M sodium hydroxide prior to
fluorescence measurement for CF transport and liquid scintillation counting for ES and UA. Oocyte
assays were performed similarly, except that drugs such as lesinurad were injected and then
transport was measured after 30 minutes (ES).
Results
Figure 2. Lesinurad inhibits OAT4 transport activity
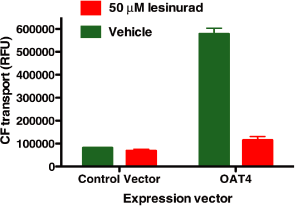
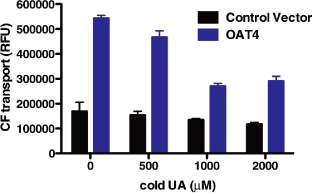
Figure 3. Urate acts as a competitive substrate for OAT4 of CF
Human Kidney cells (HEK293) exhibit OAT4-dependent transport of CF that is blocked by lesinurad.
293T cells transiently transfected with OAT4 or control plasmid lacking OAT4 (pCMV) were incubated
with CF, with vehicle (green) or 50 uM Lesinurad (red).
EC50 ~900 uM
293T cells transiently transfected with OAT4 blue or control plasmid lacking OAT4 (pCMV, black)
were incubated with CF without or with different amounts of cold UA.
Figure 4. Lesinurad inhibits urate transport activity by OAT4
Lesinurad and benzbromarone are inhibitors of OAT4, with IC50’s of 5 and 10 µM, respectively.
Concentrations of compound likely found in proximal tubule are shown shaded (red —lesinurad,
grey — benzbromarone).
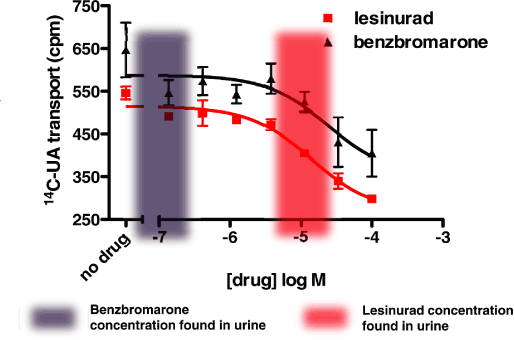
Figure 5. Lesinurad inhibits URAT1 and OAT4 with similar potency
UA transport was measured in 293T cells transiently expressing URAT1 or 293 cells stably expressing
OAT4. Inhibition was measured after a 5 minute incubation in the indicated concentrations of
lesinurad.
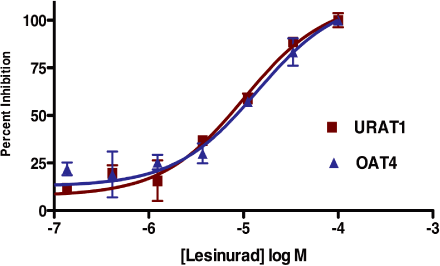
Figure 6. HCTZ has no effect on lesinurad-mediated inhibition of OAT4 transporter activity
293 cells stably expressing OAT4 were incubated with 3H-Estrone sulphate for 5 min in the presence
or absence of 1 mM HCTZ in the media. A dose response was conducted with lesinurad and percent
transported ES was measured and graphed.
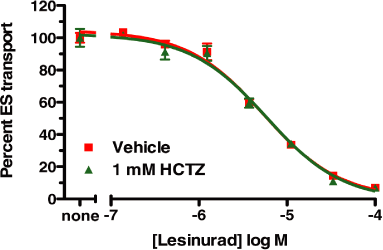
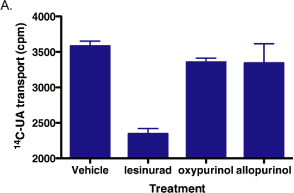
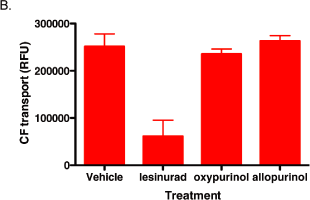
Figure 7. Oxypurinol and allopurinol do not inhibit OAT4 transport activity
Results
(cont’d)
100 µM of lesinurad, oxypurinol, or allopurinol was incubated for 5 minutes with OAT4-expressing
293 cells prior to addition of OAT4 substrates 14C-UA (A) or CF (B).
Figure 8A. Lesinurad likely inhibits OAT4 from the extracellular (apical) side
Oat4-expressing oocytes were injected with 50 nl of various concentrations of lesinurad or vehicle
intracellularly (inside) and uptake of 3H Estrone Sulfate was measured. For extracellular
inhibition (outside) lesinurad or vehicle was added directly to media at the indicated
concentrations.
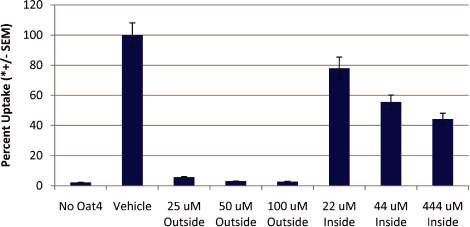
Figure 8B. Control experiment demonstrating that lesinurad remains inside injected oocytes for the
duration of the experiment
Oat4-expressing
oocytes were injected with 50 nl of
14C-labeled lesinurad in water. 14C was
measured in 8 Oocytes immediately (T=0). Remaining oocytes were incubated for 30 minutes, then 14C
levels were measured in the buffer (outside). In addition, the amount of 14C remaining in the
oocytes was measured (inside).
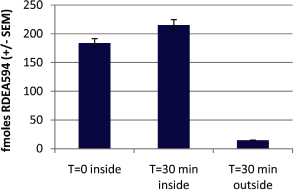
Figure 9. Phase 2 Study 203 — Design
Study 203, a 4-week, double-blind, placebo-controlled trial of lesinurad in 208 gout patients who
were not adequately responding to allopurinol with serum urate (sUA)
³ 6 mg/dL while receiving a
stable dose of allopurinol for at least 6 weeks. One of three doses of lesinurad or matching
placebo was added to the patients allopurinol regimen. Primary endpoint was mean reduction in sUA
at Week 4, with the key secondary endpoint the proportion of subjects with sUA < 6.0 mg/dL at
Week 4. A small number of patients in this trial received a thiazide diuretic during the treatment
period; their response rates were compared to those patients not receiving concomitant diuretics.
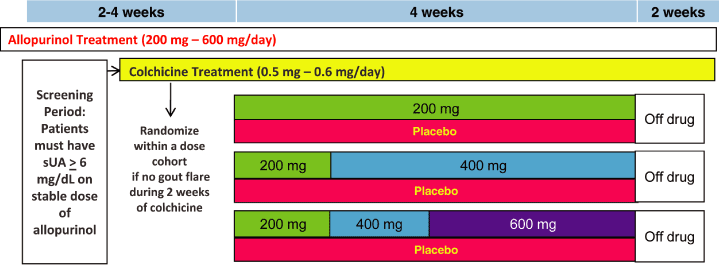
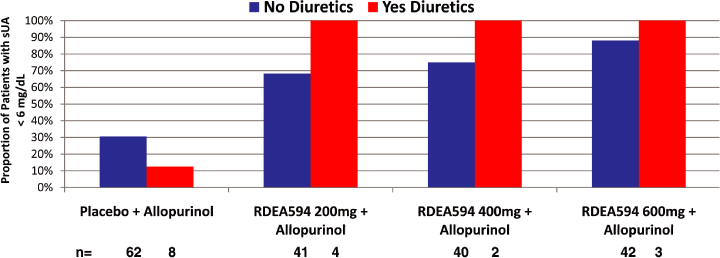
Figure 10. Proportion of patients responding in Study 203. Patients receiving diuretics had an
excellent response to lesinurad
Results (cont’d)
Conclusions
| Ø | The ability of OAT4 to transport urate from the urine side to the blood side is enhanced by thiazide diuretics, which are known to produce hyperuricemia | |
| Ø | Lesinurad inhibits OAT4 from the apical (urine) side in the proximal tubule | |
| Ø | Lesinurad inhibits OAT4 with similar potency as URAT1 at concentrations achieved in treated patients suggesting that lesinurad could lower sUA in patients receiving thiazide diuretics better than both xanthine oxidase inhibitors and other URAT1 inhibitors | |
| Ø | Since thiazide diuretics are one of the most common treatments for hypertension, this additional activity could be beneficial for many patients | |
| Ø | Despite small numbers of patients taking thiazide diuretics in the 203 study, the high response to lesinurad in diuretic-treated patients is consistent with OAT4 inhibition by lesinurad | |
| Ø | The activity of lesinurad in patients receiving thiazide diuretics will be studied further during Phase 3 |
References
| 1. | Healey et al, NEJM 1959; 261:1358-1362 | |
| 2. | Reyes AJ. Cardiovasc Drugs Ther. 2003 Sept-Nov; 17(5-6): 397-414. | |
| 3. | Hagos et al, J Am Soc Nephrol 18: 430, 2007 | |
| 4. | Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. J Clin Invest 2005, 115: 1651-1658. |
 Annual
European Congress of Rheumatology EULAR, London, May 25 —
28, 2011
Annual
European Congress of Rheumatology EULAR, London, May 25 —
28, 2011 
