Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-12358_18k.htm |
Exhibit 99.1
Below is a graphical representation of the poster entitled “An Open-label, Long-term Evaluation of the Safety and Efficacy of Avanafil in Men With Erectile Dysfunction”:

Below is a reproduction of the contents of the poster entitled “An Open-label, Long-term Evaluation of the Safety and Efficacy of Avanafil in Men With Erectile Dysfunction”:
Authors: Laurence H. Belkoff, DO(a); Andrew McCullough, MD(b); LeRoy A. Jones, MD(c); Irwin Goldstein, MD(d); Charles H. Bowden, MD(e); Karen DiDonato, RN, MSN(e); Brenda Trask(e)
(a)Urologic Consultants of Southeastern Pennsylvania, Bala Cynwyd, PA; (b)Albany College of Medicine, Albany, NY; (c)Urology San Antonio Research, San Antonio, TX; (d)San Diego Sexual Medicine, San Diego, CA; (e)Vivus, Inc., Mountain View, CA
· Introduction
· Erectile dysfunction (ED) affects up to 18 million men in the United States.(1)
· In men with diabetes, the prevalence of ED may be more than 70%.(2)
· First-line treatments for ED are phosphodiesterase type 5 (PDE5) inhibitors that work by increasing blood flow to the penis.
· Most currently available PDE5 inhibitors must be administered 60-120 minutes prior to sexual activity.(3),(4)
· Avanafil is an investigational, rapidly absorbed PDE5 inhibitor that is highly specific for the PDE5 isoenzyme.
· Objectives
· To evaluate the long-term safety, efficacy, and tolerability of avanafil in men with mild-to-severe ED.
· Methods
Study Design
· Avanafil was evaluated for up to 52 weeks in a preplanned, open-label extension study (TA-314) of two 12-week studies.
· TA-301: double-blind, placebo-controlled, Phase 3 trial of men with mild-to-severe ED.
· TA-302: double-blind, placebo-controlled, Phase 3 trial of men with mild-to-severe ED and type 1 or type 2 diabetes.
· Enrollment was terminated once the desired number of subjects enrolled into the extension study.
· Per FDA requirements, 300 subjects were planned through 26 weeks and 100 subjects through 52 weeks.
· All subjects were initially assigned to avanafil 100 mg but were permitted to increase dose to 200 mg (for increased efficacy) or decrease dose to 50 mg (for improved tolerability), if requested.
· Subjects were instructed to take 1 dose of avanafil 30 minutes prior to initiation of sexual activity and were to make >4 attempts at sexual activity per month.
Assessments
· Efficacy endpoints were evaluated at baseline and follow-up examinations using subject diaries with questions from the Sexual Encounter Profile (SEP) and the International Index of Erectile Function (IIEF) questionnaires.
· Baseline represents run-in period from the qualifying study (TA-301 or TA-302).
· Results
Baseline Demographics
· In total, 712 subjects enrolled in this open-label extension study from the 2 qualifying studies (Figure 1).

· Baseline demographics were similar in each treatment group (Table 1).
Table 1. TA-314 Baseline Demographics (Enrolled Subjects)
|
|
|
100 mg Only |
|
100 mg and 200 mg |
|
Total |
|
|
|
|
(n=171) |
|
(n=536)* |
|
(N=712) |
|
|
Mean age, years (SD) |
|
54.2 (10.9) |
|
57.1 (9.9) |
|
56.4 (10.2) |
|
|
Caucasian, n (%) |
|
147 (86.0) |
|
459 (85.6) |
|
610 (85.7) |
|
|
ED severity, n (%) |
|
|
|
|
|
|
|
|
Mild |
|
64 (37.4) |
|
142 (26.5) |
|
207 (29.1) |
|
|
Moderate |
|
59 (34.5) |
|
177 (33.0) |
|
238 (33.4) |
|
|
Severe |
|
48 (28.1) |
|
217 (40.5) |
|
267 (37.5) |
|
|
Mean ED duration, months (SD) |
|
63.7 (58.6) |
|
79.8 (72.3) |
|
75.9 (69.4) |
|
|
History of diabetes, n (%) |
|
63 (36.8) |
|
162 (30.2) |
|
226 (31.7) |
|
|
Type 1 |
|
6 (3.5) |
|
14 (2.6) |
|
20 (2.8) |
|
|
Type 2 |
|
57 (33.3) |
|
148 (27.6) |
|
206 (28.9) |
|
*Subjects who were dosed with both avanafil 100 mg and 200 mg over the course of the study. Some subjects switched immediately (Visit 2) whereas others waited until Visit 3.
Efficacy Endpoints
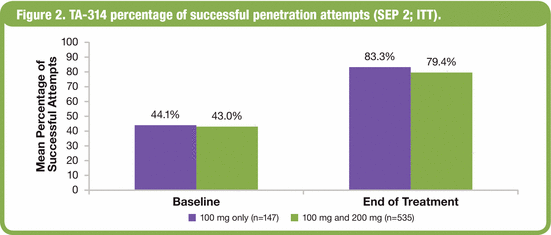
· Treatment with avanafil was associated with improvements in the percentage of successful sexual attempts in which subjects were able to insert the penis into the partner’s vagina (SEP 2; Figure 2).
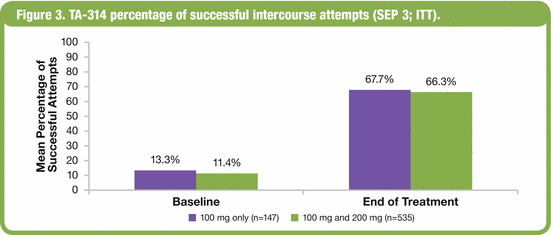
· Similarly, avanafil demonstrated improvements in the percentage of successful sexual attempts in which subjects were able to maintain an erection of sufficient duration to have successful intercourse (SEP 3; Figure 3).
· Improvements in the erectile function (EF) domain score of the IIEF were also observed at the end of treatment (Figure 4).

· In total, 130 (19.0%) subjects with mild ED, 124 (18.1%) subjects with moderate ED, and 93 (13.6%) subjects with severe ED at baseline had normalized their IIEF-EF domain score (>26) by their last study visit (intent-to-treat [ITT] population; n=686).
Successful Intercourse by Postdose Time Interval
· In subjects attempting sexual intercourse within 15 minutes of dosing, 80% of the sexual attempts were successful (Figure 5).

· In subjects attempting sexual intercourse >6 hours after dosing, 71% of attempts were successful.
· 84% of all sexual attempts were made within 60 minutes of dosing.
Treatment Responders
· In total, 512 subjects started on avanavil 100 mg, increased their dose to avanafil 200 mg, and had at least 1 diary entry for each dose.
– 172 of the 512 subjects who took both 100 mg and 200 mg were considered non-responders on treatment with avanafil 100 mg.
· Responders were defined as having a positive change from baseline in the percentage of sexual attempts in which subjects were able to maintain an erection of sufficient duration to have successful intercourse.
– Of the subjects considered to be avanafil 100 mg non-responders, 65.1% (112/172) responded to subsequent treatment with avanafil 200 mg.
Safety Summary
· Avanafil was generally well tolerated; 493 (69.2%) subjects were exposed to avanafil for >6 months and 153 (21.5%) for >12 months.
· The most common treatment-emergent adverse events (TEAEs) are presented in Table 2.
Table 2. TA-314 Most Common TEAEs (Safety Population)
|
Subjects With Any TEAE, |
|
Total |
|
|
n (%) |
|
(N=711) |
|
|
Headache |
|
40 (5.6) |
|
|
Nasopharyngitis |
|
24 (3.4) |
|
|
Flushing |
|
25 (3.5) |
|
|
Nasal congestion |
|
15 (2.1) |
|
|
Influenza |
|
11 (1.5) |
|
|
Back pain |
|
11 (1.5) |
|
· 2.8% of subjects discontinued treatment due to AEs.
· There were no reports of hearing loss or priapism and one report of cyanopsia.
· The overall incidence of serious AEs was 1.5%, although none was considered drug related.
· There were no deaths during study.
· Conclusions
· These data suggest that avanafil was an effective, long-term treatment for ED, with improvements observed for up to 1 year.
· Avanafil was effective as rapidly as 15 minutes post dose and, in some subjects, beyond 6 hours after dosing.
· Across the study, 84% of all sexual attempts were made within 60 minutes of dosing.
· Avanafil was generally well tolerated over 52 weeks, with a low dropout rate due to AEs (<3%).
· Avanafil may represent a PDE5 inhibitor with a fast onset and good tolerability.
This trial is registered at ClinicalTrials.gov, number NCT00853606.
Acknowledgements: We would like to acknowledge and thank the TA-314 investigators and study coordinators, the Quintiles team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
References: (1) Selvin E, et al. Am J Med. 2007;120(2):151-157. (2) Giuliano FA, et al. Urology. 2004;64(6):1196-1201. (3) Corbin JD. Int J Impot Res. 2004;16(suppl 1):S4-S7. (4) Rosen RC, Kostis JB. Am J Cardiol. 2003;92(9A):9M-18M.
