Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a11-12286_28k.htm |
Exhibit 99.1
|
Poster No. C81 |
Dose-related efficacy and optimal once-daily dosing interval of the long-acting beta2 |
|
|
|
agonist (LABA), vilanterol trifenatate (VI), in adults with persistent asthma |
|
|
Sterling R,(1) Lim J,(2) Frith L,(2) Snowise NG,(2) |
|
(1)Carolina Research, Respiratory Medicine, Orangeburg, SC, USA; (2)Respiratory Medicines Development Centre, GlaxoSmithKline, Uxbridge, UK |
INTRODUCTION
· Patients with asthma may remain symptomatic despite controller therapy.
· Addition of a LABA to an ICS is recommended for patients symptomatic on ICS alone.(1),(2)
· Combination therapy is currently licensed for twice-daily use; once-daily therapy may increase treatment adherence.
· VI (GW642444M) is a LABA with inherent 24h activity in development as a once-daily treatment in combination with the novel ICS, fluticasone furoate, for asthma and COPD.
OBJECTIVES
1. To evaluate the relative effects in trough FEV1 versus placebo of VI at doses of 6.25mcg once daily, 6.25mcg twice daily, 12.5mcg once daily and 25mcg once daily, each administered for 7 days.
2. To evaluate the relative effects versus placebo on weighted mean for 24h serial FEV1 of selected doses and dose intervals of VI.
3. To assess the safety of VI at selected doses and dose intervals of VI.
METHODS
· Multicenter, randomized, double-blind, placebo-controlled, five-period crossover study in adult patients (>18 years old) with persistent asthma.
· Eligible patients had asthma according to NIH criteria,(1) FEV1 reversibility of >12% and >200mL following albuterol and had been taking ICS at a stable dose for >4 weeks prior to screening.
· Patients were randomized to one of five treatment sequences with each 7 (+3)-day treatment period separated by a 7 (–3/+7)-day wash-out period; all patients received VI 6.25mcg twice daily (morning and evening), VI 6.25mcg once daily, VI 12.5mcg once daily, VI 25mcg once daily and placebo. All VI once-daily doses were given in the evening.
· VI was given via a novel single-step activation dry powder inhaler.
Efficacy endpoints and safety measures
· Primary: trough (pre-bronchodilator and pre-dose) FEV1 at the end of the 7-day treatment period (mean of 23h and 24h assessments post-evening dose).
· Secondary: weighted mean for 24h serial FEV1 on Day 7.
· Exploratory analysis: repeated measures analysis of serial FEV1 on Day 7.
· Analyses of trough and weighted mean serial FEV1 were performed using mixed effects analysis of covariance (ANCOVA) models with fixed effects for treatment period, sex and age. Subject was fitted as a random effect and the period baseline measurement (pre-dose FEV1 on Day 1) was included as part of a bivariate response.
· AE terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA) dictionary.
RESULTS
· Seventy-five patients were randomized (intent-to-treat population [ITT]); 96% of patients completed the study.
· Mean treatment compliance was >98% in each treatment group.
· Table 1 shows demographic and baseline clinical characteristics.
Table 1. Patient baseline demographics and screening lung function (ITT population).
|
|
|
N=75 |
|
Age, years |
|
38.9 (14.37) |
|
Female, n (%) |
|
47 (63) |
|
Race, n (%) |
|
|
|
White |
|
51 (68) |
|
African American/African heritage |
|
23 (31) |
|
Asian |
|
1 (1) |
|
Screening lung function |
|
|
|
Percent predicted FEV1 (%) |
|
66.4 (10.37) |
|
Percent reversibility in FEV1 (%) |
|
27.9 (15.24) |
Values are mean (standard deviation) unless otherwise stated
Efficacy
· VI was associated with statistically significant increases in trough FEV1 versus placebo (p<0.001; Figure 1).
· VI was associated with statistically significant increase in weighted mean serial 24h FEV1 versus placebo (p<0.001; Figure 2).
Figure 1. Least squares mean change from baseline in trough FEV1 (mL) on Day 7 (ITT population).

Figure 2. Weighted mean 24h serial FEV1 (mL) on Day 7 (ITT population).

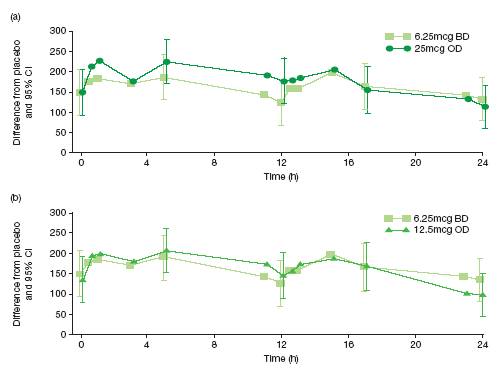
· Repeated measures analysis (difference from placebo in change from period baseline in FEV1 with VI over 0–24h on Day 7)
· there were greater changes in lung function with VI 25mcg once daily than 6.25mcg twice daily at 0-13h; the changes were similar during the 13–23h period (Figure 3a)
· there were greater changes with 12.5mcg once daily than 6.25mcg twice daily during the first 12h but the overall 24h profiles were similar for the two dosing regimens (Figure 3b).
Figure 3. Repeated measures analysis of serial FEV1 (0-24h) on Day 7 (ITT population), treatment differences (mL) from placebo; (a) 6.25mcg twice daily and 25mcg once daily (b) 6.25mcg twice daily and 12.5mcg once daily.
Safety
· VI was well tolerated at all doses; incidence of AEs was low in each VI treatment group and not dose dependent (5–9%; placebo = 18%) (Table 2).
· No drug-related AEs or serious AEs were reported.
Table 2. Summary of most common on-treatment AEs (>3% in any treatment group) (ITT population).
|
|
|
|
|
6.25mcg |
|
6.25mcg |
|
12.5mcg |
|
25mcg |
|
|
|
Placebo |
|
OD |
|
BD |
|
OD |
|
OD |
|
|
|
(n=74) |
|
(n=73) |
|
(n=74) |
|
(n=73) |
|
(n=73) |
|
Any event, n (%) |
|
13 (18) |
|
5 (7) |
|
7 (9) |
|
4 (5) |
|
6 (8) |
|
Number of patients with most frequent event (%) |
|
4 (5) |
|
5 (7) |
|
4 (5) |
|
2 (3) |
|
4 (5) |
|
Nasopharyngitis |
|
0 |
|
1 (1) |
|
1 (1) |
|
2 (3) |
|
1 (1) |
|
Upper respiratory tract infection |
|
1 (1) |
|
0 |
|
3 (4) |
|
0 |
|
0 |
|
Road traffic accident |
|
0 |
|
0 |
|
0 |
|
0 |
|
3 (4) |
|
Back pain |
|
2 (3) |
|
0 |
|
0 |
|
0 |
|
0 |
|
Headache |
|
1 (1) |
|
2 (3) |
|
1 (1) |
|
0 |
|
0 |
|
Rhinitis perennial |
|
0 |
|
2 (3) |
|
0 |
|
0 |
|
0 |
CONCLUSIONS
· All doses of VI were associated with significant improvements in trough FEV1 and weighted mean 24h serial FEV1 versus placebo.
· The greatest numerical improvements in trough FEV1 were seen with VI 6.25mcg twice daily; the greatest numerical improvements in weighted mean 24h serial FEV1 were seen with 25mcg once daily.
· Once-daily dosing with VI is supported by the observation of a sustained benefit over 24h; no substantial difference in weighted mean 24h serial FEV1 was seen between a total daily dose of 12.5mcg given once daily (168mL) or twice daily (166mL) versus placebo.
REFERENCES
(1) NIH. Expert Panel Report 3. Guidelines for the Diagnosis and Management of Asthma. Full report 2007. NIH publication No. 07-4051. http://www.nhlbi.nih.gov.
(2) GINA. Global Strategy for Asthma Management and Prevention - updated 2009. www.ginasthma.org.
ACKNOWLEDGMENTS
· This study was funded by GlaxoSmithKline. ClinicalTrials.gov: NCT00980200; protocol number: HZA113310.
· Richard Sterling (lead author) has no conflicting interests to declare.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Lisa Moore at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
![]()
Presented at the Annual Conference of the American Thoracic Society (ATS), Denver, Colorado, USA, May 13–18, 2011
