Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Capstone Therapeutics Corp. | f8k_042711.htm |
| EX-99 - EXHIBIT 99.1 - Capstone Therapeutics Corp. | exh_991.htm |
1
Capstone Therapeutics
Clinical & Operating Update
April 28, 2011
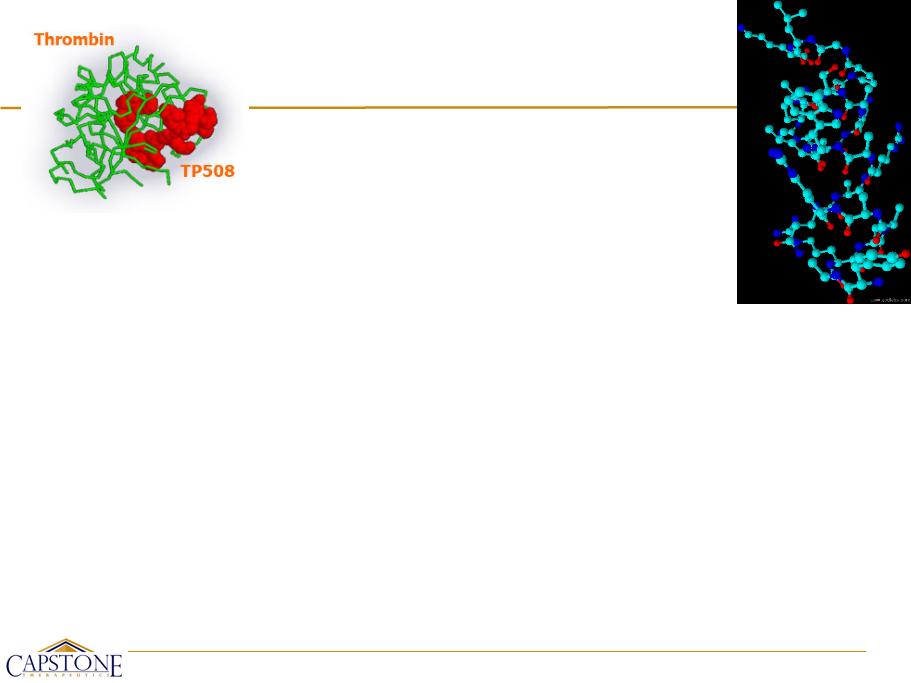
AZX100
AZX100

2
Safe Harbor Statement
u Statements in this presentation or otherwise attributable to Capstone regarding our
business that are not historical facts are made pursuant to the safe harbor provisions of the
Private Securities Litigation Reform Act of 1995. These forward-looking statements, which
include the timing and acceptability of FDA filings and the efficacy and marketability of
potential products, involve risks and uncertainties that could cause actual results to differ
materially from predicted results. These risks include: delays in obtaining or inability to
obtain FDA, institutional review board or other regulatory approvals of pre-clinical or clinical
testing; unfavorable outcomes in our pre-clinical and clinical testing; the development by
others of competing technologies and therapeutics that may have greater efficacy or lower
cost; delays in obtaining or inability to obtain FDA or other necessary regulatory approval of
our products; our inability to successfully and cost effectively develop or outsource
manufacturing and marketing of any products we are able to bring to market; changes in
FDA or other regulations that affect our ability to obtain regulatory approval of our products,
increase our manufacturing costs or limit our ability to market our product; effects of the
Capstone Stockholder Put Rights or ongoing qui tam litigation on our stock price, liquidity or
our ability to continue operations; effects on our stock price and liquidity if we are unable to
meet the requirements for continued listing on the NASDAQ Capital Market; our need for
additional capital in the future to fund the continued development of our product candidates;
and other factors discussed in our Form 10-K for the fiscal year ended December 31, 2010,
and other documents we file with the Securities and Exchange Commission.
business that are not historical facts are made pursuant to the safe harbor provisions of the
Private Securities Litigation Reform Act of 1995. These forward-looking statements, which
include the timing and acceptability of FDA filings and the efficacy and marketability of
potential products, involve risks and uncertainties that could cause actual results to differ
materially from predicted results. These risks include: delays in obtaining or inability to
obtain FDA, institutional review board or other regulatory approvals of pre-clinical or clinical
testing; unfavorable outcomes in our pre-clinical and clinical testing; the development by
others of competing technologies and therapeutics that may have greater efficacy or lower
cost; delays in obtaining or inability to obtain FDA or other necessary regulatory approval of
our products; our inability to successfully and cost effectively develop or outsource
manufacturing and marketing of any products we are able to bring to market; changes in
FDA or other regulations that affect our ability to obtain regulatory approval of our products,
increase our manufacturing costs or limit our ability to market our product; effects of the
Capstone Stockholder Put Rights or ongoing qui tam litigation on our stock price, liquidity or
our ability to continue operations; effects on our stock price and liquidity if we are unable to
meet the requirements for continued listing on the NASDAQ Capital Market; our need for
additional capital in the future to fund the continued development of our product candidates;
and other factors discussed in our Form 10-K for the fiscal year ended December 31, 2010,
and other documents we file with the Securities and Exchange Commission.

3
Agenda
A. Final Results:
AZX100 OL-ASCAR-03 - Trocar Sites of
Arthroscopic Shoulder Surgery Patients
AZX100 OL-ASCAR-03 - Trocar Sites of
Arthroscopic Shoulder Surgery Patients
B. Status: Stockholder Put Right
C. Status: NASDAQ Listing
D. Operating Results: December 31, 2010
E. Summary

4
A) Twelve-Month Results:
AZX100 OL-ASCAR-03 - Trocar Sites
of Arthroscopic Shoulder Surgery
Patients
AZX100 OL-ASCAR-03 - Trocar Sites
of Arthroscopic Shoulder Surgery
Patients
5
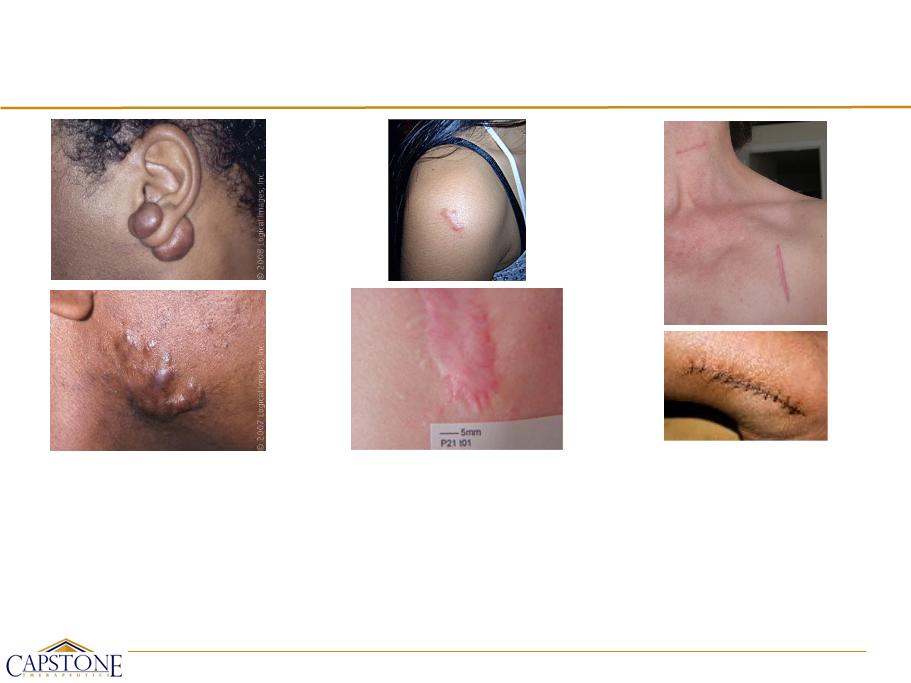
Dermal Scarring: Range of Indications
Keloid Scars
• Overgrowth of dense fibrous
tissue
tissue
• Extend beyond borders of
original wound
original wound
• Do not regress
spontaneously
spontaneously
• Tend to recur after excision
Hypertrophic Scars
• Red, itching, raised fibrous lesions
• Typically do not expand beyond
boundaries of initial injury
boundaries of initial injury
• May undergo partial spontaneous
resolution
resolution
• Common after thermal and other
injuries that involve deep dermis
injuries that involve deep dermis
Normal / Common Scars
• Various types:
• Flat, pale
• Sunken
• Red, dark, raised
• Result from surgery, burns
acne
acne

6
Evidence of AZX100 Impact on Dermal Scarring
u Cellular (in vitro) (2007)
►AZX100 decreased expression of CTGF & Type 1 collagen
►AZX100 decreased stress fiber formation & altered morphology of
human dermal keloid fibroblasts*
human dermal keloid fibroblasts*
u Pre-Clinical (2007)
►AZX100 significantly improved collagen organization in Siberian
hamster scarring model*
hamster scarring model*
u Clinical (2008-2010)
►Phase 1a & 1b Safety Profile - No drug-related SAEs
►Phase 1b - Strong pharmacologic signal & early signal of activity
►Phase 2a - Two studies (now completed) demonstrated safety
& observed signals of efficacy
& observed signals of efficacy
u Total Clinical Subjects (to date): N = 338
*Lopes, L., et al. “Cell Permeant Peptide Analogues of the Small Heat Shock Protein, HSP20, Reduce TGF-β1-Induced CTGF Expression in
Keloid Fibroblasts.” Journal of Investigative Dermatology 129, 590-598 (11 September 2008) doi:10.1038/jid.2008.264 Original Article
Keloid Fibroblasts.” Journal of Investigative Dermatology 129, 590-598 (11 September 2008) doi:10.1038/jid.2008.264 Original Article

7
u Appearance
► Aesthetic improvement
u Scar Strength
► Reduce risk of dehiscence/scar splitting &
subsequent infection
subsequent infection
u Acceleration of Time to Maximum Healing /
Improvement
Improvement
► Avoid post-surgical risks
Goals of Dermal Scar Therapy

8
Goals of Phase 2a
u Safety & Tolerability
u Initial Investigation of Dose & Administration
u Analyses to Detect Any Signal
or Trend of Efficacy
or Trend of Efficacy
u Phase 2a is not a registration trial

9
AZX100 Phase 2a Pilot Clinical Trial in
Surgical (Trocar Site) Scars
Surgical (Trocar Site) Scars
u One Study:
► OL-ASCAR-03: 3.0 mg, 10.0 mg or placebo
u Objective:
► Scar reduction in trocar sites
u Study Design:
► Comparison of three trocar site scars following arthroscopic
shoulder surgery
shoulder surgery
► Placebo-controlled; n = 150 dosed
► Two active doses per patient (intra-patient control)
3.0 mg, 10.0 mg
3.0 mg, 10.0 mg
► Placebo-only cohort (n=25)
► Two intradermal injections into each trocar site
● 9 ± 2 days following surgery
● 21 ± 2 days following surgery
10
AZX100 Dermal Scarring Phase 2a Clinical Program
Deliverables
OL-ASCAR-03
(Trocar Sites)
Final Data: 2Q2011
OL-ASCAR-04 / -05
(Keloids)
Final Data: 4Q2010
3D Digital Photography
Height, Length,
Volume, Width
Safety
(Subjective)
(Objective)
(“Sub/Objective”)
POSAS*
@ 12 months
2D Digital Photography
VAS**
* POSAS: Patient and Observer Scar Assessment Scale; ** VAS: Visual Analog Scale

11
u Final Analysis Reveals Signals of Efficacy
► Monthly statistical tests performed: Months 3-12
► 8 tests yielded statistically significant or near significant results;
7 of these 8 favored AZX100
7 of these 8 favored AZX100
► Significant or nearly significant findings occurred early;
slightly more often at 3.0 mg than at 10.0 mg; primarily at Month 3
slightly more often at 3.0 mg than at 10.0 mg; primarily at Month 3
● Example: Total Scar Volume at Month 3
AZX100 - 3.0 mg vs. placebo; p = 0.0094*
AZX100 - 10.0 mg vs. placebo; p = 0.033*
AZX100 - 3.0 mg vs. placebo; p = 0.0094*
AZX100 - 10.0 mg vs. placebo; p = 0.033*
► Scar histology at Month 12
Collagen Density, Maturity and Organization favored
AZX100 3.0 mg, with p-values of 0.05 to 0.10 for all three variables
Collagen Density, Maturity and Organization favored
AZX100 3.0 mg, with p-values of 0.05 to 0.10 for all three variables
u Favorable Safety Profile
AZX100 Phase 2a Pilot Clinical Trial
in Surgical (Trocar Site) Scars - Final Results
in Surgical (Trocar Site) Scars - Final Results
* p-values ≤ 0.05 are considered statistically significant

12
Is AZX100 a Viable Candidate for
Clinical & Commercial Development?
Clinical & Commercial Development?
u Novel Mechanism: Anti-fibrotic
u Strong Patent Position
u Phase 2a - Early Human Efficacy Trials
►Demonstrated Safety
►Observed Signal of Efficacy Across Multiple Metrics & Time Points
u Cost-Effective Manufacturing Process
u Market Potential…
►Multiple Indications
● Dermal Scarring (Keloid; Hypertrophic; Surgical)
● Other Fibrotic Disorders (e.g., IPF)
►Large Market
● 22.5 Million Surgical Procedures Performed Annually in U.S. Alone
● Incidence of Keloid Susceptibility within Certain Populations: 5% - 15%
● Estimated Addressable Market >$1.0 Billion
►Potential Prophylactic Standard of Care in General Surgery
►Potential Reimbursement for Problem Scars (Keloid)

13
Is AZX100 a Viable Candidate for
Clinical & Commercial Development?
Clinical & Commercial Development?
u Answer: YES
► Capstone believes the AZX100
development program in
dermal scarring should be continued
development program in
dermal scarring should be continued
u To this end…
► Capstone is pursuing a pharma or biotech
collaboration to fund development
collaboration to fund development
► Internal planning & development programs continue
► Clinical Pathway Indicated
● Initiate Phase 2 dose / administration optimization studies

14
B) Status: Stockholder Put Right

15
Novel Corporate Governance
u Stockholder Put Right - Approved May-2010
u Mechanics:
►Gives all CAPS Stockholders as of record date (30-
Jun-2011) the right to “put” their shares of common
stock to the Company on or about 31-Jul-2011 for
cash redemption at formula-based price*
Jun-2011) the right to “put” their shares of common
stock to the Company on or about 31-Jul-2011 for
cash redemption at formula-based price*
● *Net Liquid Assets less Commitments and Contingencies
►Put Right will expire if:
● Material Transaction
● Plan of Dissolution or Liquidation
● Change in Control

16
Stockholder Put Right Status
u 4Q2009 - CAPS publicly disclosed that OrthoLogic (OLGC), its predecessor
legal entity, was named as a defendant in a “qui tam” action against the
orthopedic device industry
legal entity, was named as a defendant in a “qui tam” action against the
orthopedic device industry
► CAPS joined group defense and sought summary dismissal
u Dec-2010 - Judge allowed case to continue to discovery on a technicality
u Feb-2011 - CAPS advised by counsel that Delaware law prohibits stockholder
distribution in face of an undefined contingent liability
distribution in face of an undefined contingent liability
u Apr-2011 - CAPS Board of Directors Determination
► Pending qui tam litigation seeks potentially significant damages that, if awarded,
could exceed financial resources of the Company
could exceed financial resources of the Company
► Pendency of this claim at the time of share repurchases could cause violation Section
160 of Delaware General Corporation Law & Uniform Fraudulent Transfer Act
160 of Delaware General Corporation Law & Uniform Fraudulent Transfer Act
► Although the probability of an unsuccessful outcome is low, the magnitude of potential
damages is such that the value ascribed to this contingent liability would cause per-
share purchase price to be zero upon exercise of the put rights
damages is such that the value ascribed to this contingent liability would cause per-
share purchase price to be zero upon exercise of the put rights
► Absent a settlement, dismissal or other developments by June 30, 2011, the
Company will be unable to purchase shares upon exercise of the put rights as the
per-share purchase price determined by the Board will be zero and the put rights will
expire
Company will be unable to purchase shares upon exercise of the put rights as the
per-share purchase price determined by the Board will be zero and the put rights will
expire

17
CAPS Legal Position re: Qui Tam
u Aggressive defense
u Legacy Issue
► CAPS/OLGC exited bone stimulation device business Nov-2003
with sale to DJ Orthopedics
with sale to DJ Orthopedics
► Notice of lawsuit received six years after selling the business
u OLGC under regulatory compliance mandate with U.S. Dept. of
Health during period in question
Health during period in question
► Filed annual compliance reports
u OLGC not named with other defendants in “follow-on”
lawsuit regarding kickbacks
lawsuit regarding kickbacks
u U.S. Gov’t has not joined plaintiff in lawsuit
► 94% dismissal rate in qui tam cases that lack federal
participation; remaining 6% have moved to judgment / settlement
participation; remaining 6% have moved to judgment / settlement

18
C) Status: NASDAQ Listing

19
NASDAQ Listing Compliance
u Timing
►Compliance period ends 02-May-2011
● NASDAQ discretion regarding length of grace period
►Appeal process of two to three weeks
►Reverse stock split is sole NASDAQ-accepted plan
►CAPS may move to CAPS.OB listing
u Board opposes reverse stock split
►Less float
►Market capitalization often declines
u CAPS polled stockholders via letter seeking input
regarding reverse split as a mechanism to maintain
NASDAQ listing
regarding reverse split as a mechanism to maintain
NASDAQ listing
►Feedback has indicated stockholder preference against the
proposal of a reverse stock split
proposal of a reverse stock split

20
D) Key Financial Facts

21
Capstone Financial Summary
Capstone Key Financial Facts
u $24.4MM cash (31-Dec-2010); within guidance
u No long-term debt
u 52-wk Range: $0.38 - $1.23
u Market Cap as of 26-Apr-2011 : ~$16.7MM
|
|
Guidance
|
Actual
|
|
2006
|
Orig: $35.0MM
Rev: $15.0-$17.5MM
|
$13.4MM
|
|
2007
|
$18.0 - $19.0MM
|
$9.6MM
|
|
2008
|
$13.0 - $15.0MM
|
$12.6MM
(incl. $1.041MM stock repurchase)
|
|
2009
|
$14.0 - $16.0MM
|
$12.8MM
|
|
2010
|
~$13.0MM
|
$10.8MM
|

22
E) Summary

23
Summary
u AZX100 shows safety and signal of efficacy
in dermal scarring
in dermal scarring
u CAPS believes AZX100 is a viable
commercial candidate
commercial candidate
►Industry will cast deciding vote through
partnering
partnering
● If AZX100 is partnered, clinical trials proceed
● If AZX100 is not partnered, CAPS optimizes
clinical program value and preserves cash assets
for benefit of stockholders
clinical program value and preserves cash assets
for benefit of stockholders

24
NASDAQCM: CAPS
www.capstonethx.com
Capstone Therapeutics
1275 West Washington Street - Suite 101
Tempe, AZ 85281
(602) 286-5520
