Attached files
Exhibit 10.5
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Execution Copy
CO-DEVELOPMENT AND LICENSE AGREEMENT
This CO-DEVELOPMENT AND LICENSE AGREEMENT (the “Agreement”) is entered into as of March 11, 2011 (the “Effective Date”) by and between CELL THERAPEUTICS, INC., a Washington corporation, with its principal place of business at 501 Elliott Ave. W. #400, Seattle, Washington 98119, U.S.A. (“CTI”), and CHROMA THERAPEUTICS LTD, a company registered under the laws of England and Wales, with its principal place of business at 93 Milton Park, Abingdon, Oxon OX14 4RY, UK (“Chroma”). Chroma and CTI are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, Chroma is developing its proprietary Tosedostat product for cancer;
WHEREAS, CTI possesses substantial resources and expertise in the development, marketing, and commercialization of pharmaceutical products for the treatment of cancer in the Licensed Territory (as defined below); and
WHEREAS, CTI desires to collaborate with Chroma on the further development of the Product (as defined below) in the Field (as defined below) through regulatory approval in the Licensed Territory, and to obtain commercialization rights to the Product in the Field in the Licensed Territory, and Chroma is willing to so collaborate and to grant such rights on the terms and conditions hereof.
NOW THEREFORE, in consideration of the foregoing premises and the mutual promises, covenants and conditions contained in this Agreement, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
1.1 “Accounting Standards” means US GAAP (United States Generally Accepted Accounting Principles) as generally and consistently applied throughout each Party’s organization.
1.2 “Additional Product” means any (a) new formulation, dosage form, improvement, or mode of administration of the Compound, or (b) pharmaceutical composition that contains a derivative or modified form of the Compound including those analogues of the Compound with the structures set forth in Exhibit A-2.
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.3 “Additional Studies” means, other than the Currently Ongoing Studies, Licensed Territory Specific Studies, Extraterritorial Studies, and studies that can be both an Extraterritorial Study and a Licensed Territory Specific Study agreed to be conducted by the Parties as part of the Development Plan under the terms and conditions of this Agreement.
1.4 “Affiliate” means, with respect to a particular Party, a person, corporation, partnership, or other entity that controls, is controlled by or is under common control with such Party. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such entity, whether by the ownership of fifty percent (50%) or more of the voting stock of such entity, or by contract or otherwise.
1.5 “Agreement” has the meaning set forth in the preamble.
1.6 “Applicable Transaction” has the meaning set forth in Section 9.2(a).
1.7 “Approval Date” has the meaning set forth in Section 15.9.
1.8 “Audited Party” has the meaning set forth in Section 4.3(d).
1.9 “Best Knowledge” means, as applied to a Party, that the applicable Party’s senior management with operational responsibility for the Development or Commercialization of the Product is actually aware of a particular fact or other matter following reasonably diligent inquiry of its management employees with primary responsibility for the applicable subject matter.
1.10 “Chroma” has the meaning set forth in the preamble.
1.11 “Chroma Indemnitees” has the meaning set forth in Section 11.2.
1.12 “Chroma Know-How” means all Know-How that is Controlled by Chroma or its Affiliates as of the Effective Date or during the Term and is necessary or reasonably useful for the Development or Commercialization of the Product in the Field in accordance with the terms of this Agreement. For clarity, Chroma Know-How includes Know-How relating to previously conducted Non-Clinical Studies for the Product, Past Studies, and Currently Ongoing Studies, but excludes Information contained within the Chroma Patents. Chroma Know-How shall not include any rights to any independent Know-How of any Third Party entity which obtains “control” (as defined in the definition of Affiliate) of Chroma after the Effective Date, provided that (i) such transaction is not entered into in an effort to circumvent the license granted under this Agreement and (ii) any Know-How constituting “Chroma Know-How” immediately prior to such acquisition of “control”, or would become “Chroma Know-How” but for such acquisition of “control”, shall continue to be licensed under the terms of this Agreement.
2
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.13 “Chroma Patents” means any Patent that (a) is Controlled by Chroma or its Affiliates as of the Effective Date or at any time during the Term (including any Patent arising from Chroma’s Sole Inventions but excluding Joint Patents), and (b) would, but for the license granted by Chroma hereunder, be infringed by the Development, Manufacture, have manufacture, use, sale, offer for sale, having sold, distribution, import, or any other Commercialization of the Product by or on behalf of CTI or its sublicensee(s) in the Field. Chroma Patents shall include without limitation those Patents listed on Exhibits B-1 and the Manufacturing Patent (except that the Manufacturing Patent will be licensed non-exclusively to CTI under the terms of Section 2.1(a)(ii)), and any patent issuing from an application claiming priority thereto or otherwise continuing therefrom. “Chroma Patents” shall not include any rights to any independent Patents of any Third Party entity which acquires “control” (as defined in the definition of Affiliate) of Chroma after the Effective Date, provided that (i) such transaction is not entered into in an effort to circumvent the license granted under this Agreement and (ii) any Patents constituting “Chroma Patents” immediately prior to such acquisition of “control”, or would become “Chroma Patents” but for such acquisition of “control”, shall continue to be licensed under the terms of this Agreement.
1.14 “Chroma Technology” means the Chroma Patents and Chroma Know-How.
1.15 “Claims” has the meaning set forth in Section 11.1.
1.16 “CMC” means chemistry, manufacturing and controls as specified by the FDA.
1.17 “Commercial Information” means sales call activity reports, listing of major accounts including addresses, lists and contact details of distributors and volume of sales activity over the preceding twelve (12) months, list of reimbursement assistance vendors and contact details, list of ongoing investigator sponsored trials including study title and location, managed care and HMO agreements and contact lists, copies of current sales promotional material as well as master production materials, any other similar general information that is solely related to the Commercialization of the Product in the Licensed Territory. Commercial Information expressly excludes any proprietary CTI Know-How. For the avoidance of doubt, no rights under Patents Controlled by CTI are licensed to Chroma in connection with any provision of Commercial Information to Chroma under this Agreement.
1.18 “Commercialization,” with a correlative meaning for “Commercialize” and “Commercializing,” means all activities undertaken before and after obtaining Regulatory Approvals relating specifically to the pre-launch, launch, promotion, Detailing, medical education and medical liaison activities, publication, marketing, pricing, reimbursement, sale, offering for sale, distribution and import of the Product, including: (a) strategic marketing, sales force detailing, advertising, medical education and liaison, and market and Product support; and (b) all customer support, Product distribution, invoicing and sales activities.
1.19 “Commercialization Sublicensee” means any Third Party to which CTI or its Affiliate sublicenses its rights under Sections 2.1(a)(i) and 2.1(a)(ii).
3
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.20 “Committee” has the meaning set forth in Section 3.3(a).
1.21 “Competing Product” means any product, other than the Product, containing tosedostat as an active ingredient, or any other product which has as its primary mode of action the inhibition of aminopeptidases.
1.22 “Compound” means tosedostat (designated by Chroma as CHR-2797), which has the structure set forth on Exhibit A-1, its prodrugs and metabolites, as well as its and their acids, bases, isomers, enantiomers, esters, salts, hydrates, solvates and polymorphs, in any dosage form or form of administration.
1.23 “Confidential Information” means, with respect to a Party, all non-public Information of such Party that is disclosed to the other Party under this Agreement, whether in oral, written, graphic, or electronic form. All non-public Information disclosed by either Party pursuant to the Mutual Confidential Disclosure Agreement between the Parties dated October 1, 2010 (the “CDA”), or the Non-binding Proposal for the Licensing of Tosedostat dated February 2, 2011, shall be deemed to be such Party’s Confidential Information disclosed hereunder.
1.24 “Control” means, with respect to any material, Know-How, or intellectual property right, that a Party (a) owns or (b) has a license to such material, Know-How, or intellectual property right and, in each case, has the ability to grant to the other Party access, a license, or a sublicense (as applicable) to the foregoing on the terms and conditions set forth in this Agreement without violating the terms of any then-existing agreement or other arrangement with any Third Party.
1.25 “Corresponding Share” has the meaning set forth in Section 4.4(b).
1.26 “CTI” has the meaning set forth in the preamble.
1.27 “CTI Indemnitees” has the meaning set forth in Section 11.1.
1.28 “Currently Ongoing Studies” means, collectively, the following clinical studies: (a) “Extension Study With Tosedostat in Relapsed/Refractory Acute Myeloid Leukemia”; NCT01180426 [Recruiting], and (b) “Safety and Anti-Disease Activity of Oral Tosedostat (CHR-2797) in Elderly Subjects With Refractory or Relapsed AML (OPAL)”, NCT00780598 [Active, not recruiting], and (c) “A program of randomized phase II multicenter studies to assess the tolerability and efficacy of the addition of new drugs to standard induction chemotherapy in AML and RAEB > 66 years and very poor risk AML > 18 years”, Hovon 103, 2009-014455-68 [Recruiting].
1.29 “Defaulting Party” has the meaning set forth in Section 13.3.
1.30 “Designated Executive” has the meaning set forth in Section 3.1(b).
4
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.31 “Detail” means a face-to-face or electronic presentation and any associated in-service training regarding the features of the Product by a Party’s sales representative to one or several medical professional(s) having prescribing authority in the Field (including pharmacists), as well as to other individuals or entities that have significant impact or influence on prescribing decisions in the Field.
1.32 “Develop” or “Development” means all research and development activities relating to preparing and conducting and documenting Non-Clinical Studies, human clinical studies, CMC development and regulatory activities (e.g., regulatory applications) with respect to the Product.
1.33 “Development Budget” means the binding annual budget for the Development activities to be undertaken by the Parties under the Development Plan, which Development Budget shall be included in and based on the then-current Development Plan.
1.34 “Development Costs” means reasonable expenses and other costs, including external regulatory expenses, directly incurred by or on behalf of a Party in connection with the Development activities to be undertaken by the Parties in accordance with the approved Development Plan and Development Budget, including, without limitation, the external costs of clinical trials, the preparation, collation and/or validation of data from such clinical trials and the preparation of medical writing and publishing, but excluding any Indirect Costs and any costs of external fees related to the filing of Regulatory Materials. Without limitation of the generality of the foregoing, Development Costs shall include:
(a) all Out-of-Pocket Costs incurred by the Parties or their Affiliates;
(b) the cost of clinical supply, including without limitation (i) costs of clinical supplies of Product, (ii) expenses incurred to purchase and/or package comparator drugs, and (iii) costs and expenses of disposal of clinical samples; and
(c) the costs of consultation and pre-submission meetings with Regulatory Authorities, including any costs related to pharmacovigilance, to the extent such costs are to be considered Development Costs in accordance with the Development Plan.
1.35 “Development Designee” means a Third Party appointed by a Party to conduct Development activities on behalf of such Party as permitted under Section 4.6.
1.36 “Development Documentation” means all Development Information for the Product, including any documentation containing test data for the Product (including pharmacological, biological, chemical, biochemical, clinical study data and data resulting from Non-Clinical Studies), Regulatory Materials, CMC information, drug master files, stability data, and other manufacturing or study data for the Product.
1.37 “Development Plan” has the meaning set forth in Section 4.3(a). For clarity, the Development Plan includes the Initial Development Plan as well as any amendments or updates
5
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
thereto provided under the terms and conditions of this Agreement, including those provided under Sections 4.3(a)(iii), 4.4, and 4.9.
1.38 “Diligent Efforts” means, with respect to a Party’s obligation under this Agreement to Develop or Commercialize the Product, efforts and resources which would normally be used by a pharmaceutical company in the pharmaceutical industry for a product owned by or licensed to it, and activities related to the development and commercialization of such product, which is of similar commercial potential at a similar stage in its development or product lifecycle, taking into account various issues, such as its safety and efficacy, product profile, cost to develop, cost and availability of supply, the time required to complete development, the competitiveness of the marketplace, the company’s patent position with respect to such product (including the company’s ability to obtain or enforce, or have obtained or enforced, such patent rights), the third-party patent landscape relevant to the product, the regulatory structure involved, the likelihood of regulatory approval, the anticipated or actual profitability of the applicable product, and all other relevant factors, all as measured by the facts and circumstances at the time such efforts are due provided that in relation to CTI’s obligations under this Agreement CTI shall not be entitled to factor in sums owed to Chroma under this Agreement or any sums due to any Third Party licensor of Intellectual Property which CTI licenses and is used in relation to the Product.
1.39 “Dollar” means a U.S. dollar, and “$” shall be interpreted accordingly.
1.40 “Effective Date” has the meaning set forth in the preamble.
1.41 “Effective Date of Termination” means the date that this Agreement is terminated under the terms of any of Section 13.2, 13.3, 13.4 or 13.5, as applicable, including the expiration of any cure period or dispute resolution provided thereunder.
1.42 “Executive Steering Committee” or “ESC” means the committee formed by the Parties as described in Section 3.1.
1.43 “Extraterritorial Studies” means all Non-Clinical Studies and clinical studies wheresoever conducted during the Term, whether conducted prior to or following Regulatory Approval of the Product, pertaining to the Regulatory Approval of the Product in the Field solely for the ROW Territory including: Phase 1, 2, 3 or 4 Clinical Studies or pivotal studies (including studies for additional indications or label expansion); investigator-sponsored trials, safety or surveillance studies; pharmacoeconomic studies; pharmacoepidemiology studies; reimbursement studies; and other studies.
1.44 “FDA” means the U.S. Food and Drug Administration or any successor entity.
1.45 “FD&C Act” means the U.S. Federal Food, Drug and Cosmetic Act, as amended.
1.46 “Field” means cancer therapy (including, without limitation, tumor metastasis and growth).
6
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.47 “First Commercial Sale” means the first sale to a Third Party of a Product in a given regulatory jurisdiction after Regulatory Approval has been obtained in such jurisdiction, excluding any pre-regulatory named patient sales, expanded access sales or MTA sales.
1.48 “First Line” means use of the Product as the initial treatment for a given condition or indication.
1.49 “Funding Cap” has the meaning set forth in Section 4.3(b)(iii).
1.50 “Generic Version” has the meaning set forth in Section 8.4(c).
1.51 “Good Clinical Practices” or “GCP” means the then-current standards, practices and procedures for good clinical practice promulgated or endorsed or required by the Regulatory Authority in the Licensed Territory and the ROW Territory as applicable including by the FDA as set forth in the guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” including related regulatory requirements imposed by the FDA and comparable regulatory standards, practices and procedures in jurisdictions outside the U.S. including the European Union, as they may be updated from time to time, including applicable quality guidelines promulgated under the International Conference on Harmonization (“ICH”).
1.52 “Good Laboratory Practices” or “GLP” means the then-current good laboratory practice standards promulgated or endorsed or required by the Regulatory Authority in the Licensed Territory and the ROW Territory as applicable including by the FDA as defined in 21 C.F.R. Part 58, and comparable regulatory standards in jurisdictions outside the U.S. including the European Union, as they may be updated from time to time, including applicable quality guidelines promulgated under the ICH.
1.53 “Good Manufacturing Practices,” “cGMP” or “GMP” means the then-current good manufacturing practices as required by the Regulatory Authority in the Licensed Territory and ROW Territory, as applicable, including by the FDA as defined in the U.S. Current Good Manufacturing Practices, 21 CFR Parts 210 and 211 including related regulatory requirements imposed by the FDA for the manufacture and testing of pharmaceutical materials, and comparable regulatory standards in jurisdictions outside the U.S. including the European Union, as they may be updated from time to time, including applicable quality guidelines promulgated under the ICH and other applicable regulations.
1.54 “Governmental Authority” means any multi-national, federal, state, local, municipal, provincial or other government authority of any nature (including any governmental division, prefecture, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal).
1.55 “HSR Filing Date” has the meaning set forth in Section 15.9.
1.56 “IND” means (a) an Investigational New Drug application as defined in the FD&C Act and applicable regulations promulgated hereunder by the FDA, or (b) the equivalent
7
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
application to the equivalent Governmental Authority in any other regulatory jurisdiction outside the U.S., the filing of which is necessary to commence or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction.
1.57 “Indemnified Party” has the meaning set forth in Section 11.3.
1.58 “Indemnifying Party” has the meaning set forth in Section 11.3.
1.59 “Indirect Costs” means any indirect costs and expenses incurred by either Party, including internal personnel costs (such as compensation and benefits) or other internal overhead costs, rental expenses, lease expenses, utilities, travel and depreciation.
1.60 “Information” means any data, results, technology, business and financial information and information of any type whatsoever, in any tangible or intangible form, including, without limitation, specifications, software, algorithms, marketing reports, test data (including pharmacological, biological, chemical, biochemical, clinical study data and data resulting from Non-Clinical Studies), CMC information, stability data, and other study data.
1.61 “Initial Development Plan” has the meaning set forth in Section 4.3(a)(ii).
1.62 “Initial Notice” has the meaning set forth in Section 9.2(a).
1.63 “Initial Period” has the meaning set forth in Section 9.2(a).
1.64 “Initiation” of a clinical trial shall mean the first dosing of the first patient in such trial.
1.65 “Joint Development Committee” or “JDC” means the committee formed by the Parties as described in Section 3.2.
1.66 “Joint Inventions” has the meaning set forth in Section 9.1.
1.67 “Joint Patent” has the meaning set forth in Section 9.4(b).
1.68 “Know-How” means all technical Information and know-how, including inventions, discoveries, trade secrets, instructions, processes, formulae, materials, expertise and other technology applicable to formulations, compositions, products or their Manufacture, Development, registration, use or Commercialization or methods of assaying or testing them or processes for their manufacture, formulations containing them, compositions incorporating or comprising them and including all biological, chemical, pharmacological, biochemical, toxicological, pharmaceutical, physical and analytical, safety, quality control, manufacturing, preclinical and clinical data, instructions, processes, formulae, expertise and information, Regulatory Materials and copies thereof, relevant to the Development, Manufacture, use or Commercialization of and/or which may be useful in studying, testing, Development, production or formulation of products, or intermediates for the synthesis thereof.
8
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.69 “Laws” means all laws, statutes, rules, regulations, ordinances and other pronouncements having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision, domestic or foreign, including without limitation 21 CFR part 54, HIPAA.
1.70 “Licensed Territory” means North, Central and South Americas, including their respective territories and possessions. This includes, without limitation, the United States, Canada, Mexico, Brazil, Argentina, Venezuela and their respective territories and possessions.
1.71 “Licensed Territory Specific Studies” means all Non-Clinical Studies and clinical studies wheresoever conducted during the Term, whether conducted prior to or following Regulatory Approval of the Product, pertaining to the Regulatory Approval of the Product in the Field solely for the Licensed Territory including; Phase 1, 2, 3, 4 Clinical Studies or pivotal studies (including studies for additional indications or label expansions; investigator sponsored trials, safety or surveillance studies, pharmacoeconomic studies; pharmacoepidemiology studies, reimbursement studies; and other studies.
1.72 “Manufacture” with a correlative meaning for “Manufacturing,” means all activities related to the manufacturing of a pharmaceutical product, or any ingredient thereof, including manufacturing Product in finished form for Development, manufacturing finished Product for Commercialization, packaging, in-process and finished product testing, release of product or any component or ingredient thereof, quality assurance activities related to manufacturing and release of product, all stability studies including those for registration, the development and validation of testing methods used for, but not limited to, release test, stability test and every testing method for commercial use, preparation of the documents of any “Manufacture” related reports in “common technical document” form described in the ICH guidelines, and documents necessary for clinical and market authorization development including but not limited to development history reports for drug substance and drug product, comparability studies and reports, in the Licensed Territory and ROW Territory and regulatory activities related to any of the foregoing.
1.73 “Manufacturing Patent” means US Patent 5,912,360.
1.74 “Marketing Authorization Application” or “MAA” means an application to the appropriate Regulatory Authority for approval to market the Product (but excluding pricing approval) in any particular jurisdiction.
1.75 “Meetings” has the meaning set forth in Section 6.8.
1.76 “NDA” means a New Drug Application in the United States for authorization for marketing of a pharmaceutical product, as defined in the applicable Laws and filed with the FDA.
1.77 “Negotiation Period” has the meaning set forth in Section 9.2(a).
9
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.78 “Net Sales” means, with respect to a particular time period, the total amounts invoiced by CTI, its Affiliates and their respective sublicensees for sales of Products made during such time period to unaffiliated Third Parties, less the following deductions in each case to the extent reasonable and customary and actually allowed or incurred with respect to such sales:
(a) **;
(b) **;
(c) **;
(d) **;
(e) **;
(f) **; and
(g) **.
Notwithstanding the foregoing, amounts invoiced by CTI, its Affiliates, or their sublicensees for the sale of Product among CTI, its Affiliates or their respective sublicensees for resale shall not be included in the computation of Net Sales hereunder and such amounts shall be accounted for only once. For purposes of determining Net Sales, a “sale” shall not include reasonable transfers or dispositions, at no cost, as samples or for charitable purposes, or transfers or dispositions at no cost for Non-Clinical Studies, clinical or regulatory purposes. Net Sales shall be accounted for in accordance with standard CTI practices for operation by CTI, its Affiliates or sublicensees, as practiced in the relevant country in the Licensed Territory, but in any event in accordance with Accounting Standards, consistently applied in such country in the Licensed Territory. Product sales are recognized when persuasive evidence of an arrangement with a Third Party at a fixed or determinable price exists, title and risk of loss has passed to the Third Party (generally upon receipt by the Third Party, and collectability of amounts billed is reasonably assured). Provisions for ** shall be recorded at the time of sale.
1.79 “Non-Clinical Studies” means in vivo animal or in vitro pharmacology, pharmacokinetic, or toxicology testing.
1.80 “Non-Defaulting Party” has the meaning set forth in Section 13.3.
1.81 “Non-Opted In Additional Product Development” has the meaning set forth in Section 4.9(a).
1.82 “Non-Opted In Study” has the meaning set forth in Section 4.4(a).
1.83 “Non-Proposing Party” has the meaning set forth in Section 4.4(a).
10
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.84 “Out-of-Pocket Costs” means reasonable, direct and documented expenses paid to Third Parties and specifically identifiable and incurred in connection with the Development of the Product. Such expenses shall have been recorded as income statement items in accordance with the applicable Accounting Standards and, for the avoidance of doubt, shall not include **.
1.85 “Party” and “Parties” has the meaning set forth in the preamble.
1.86 “Past Studies” means, collectively, the following clinical studies that have been completed or terminated prior to the Effective Date: (a) “Clinical Trial to Test the Safety and Effectiveness of an Investigational Drug CHR-2797 With Erlotinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer”, NCT00522938 [Terminated–Poor Recruitment], (b) “Safety Study to Evaluate CHR-2797 in Patients With Advanced Tumours”, NCT00692354 [Completed], (c) “A Study of the Safety and Tolerability of the Addition of CHR-2797 to Paclitaxel in Patients With Advanced or Refractory Tumours”, NCT00737555 [Completed], and (d) “ A Phase I-II Study to Evaluate the Safety, Tolerability and Anti-Disease Activity of the Aminopeptidase Inhibitor, CHR-2797, in Elderly and/or Treatment Refractory Patients with Acute Myeloid Leukaemia or Multiple Myeloma”, NCT00689000 [Completed].
1.87 “Patents” means (a) pending patent applications (and patents issuing therefrom), issued patents, utility models and designs; and (b) reissues, substitutions, confirmations, registrations, validations, re-examinations, additions, continuations, continued prosecution applications, continuations-in-part, or divisions of or to any patents, patent applications, utility models or designs, in each case being enforceable within the applicable territory.
1.88 “Patent Term Extension” means any term extensions, supplementary protection certificates, and equivalents thereof offering patent or patent-like protection beyond the initial term with respect to any issued Patents.
1.89 “Phase 1 Clinical Trial” means a clinical trial of a pharmaceutical product on healthy subjects or patients with the primary purpose of determining safety, metabolism and pharmacokinetic properties and clinical pharmacology of such product.
1.90 “Phase 2 Clinical Trial” means a clinical trial of a pharmaceutical product on patients, including possibly pharmacokinetic studies, the principal purposes of which are to make a preliminary determination that such product is safe for its intended use and to obtain sufficient information about such product’s efficacy to permit the design of a Phase 3 Clinical Trial.
1.91 “Phase 3 Clinical Trial” means a clinical trial on sufficient numbers of patients, which trial(s) are designed to (a) establish that a drug is safe and efficacious for its intended use; (b) define warnings, precautions and adverse reactions that are associated with the drug in the dosage range to be prescribed; and (c) support approval of an application to a Regulatory Authority for the commercial marketing of such drug.
1.92 “Phase 4 Clinical Trial” means a clinical trial of a pharmaceutical product conducted after Regulatory Approval of the product has been obtained from an appropriate
11
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Regulatory Authority, which trial is (a) conducted voluntarily by a Party to enhance marketing or scientific knowledge of such product (e.g., for expansion of product labeling or dose optimization), or (b) conducted as a condition for sale or post-approval commitment to or requirement of a Regulatory Authority. For clarity, a human clinical trial conducted to support a new Regulatory Approval for a new indication of a product shall not be considered a Phase 4 Clinical Trial.
1.93 “Product” means (a) the Compound or (b) any Additional Product, in finished dosage pharmaceutical form, whether administered together as a single pharmaceutical product or co-administered together with one or more other biologic or pharmaceutically active products or agents.
1.94 “Product Complaint” means any written, verbal or electronic expression of dissatisfaction regarding the Product, including without limitation reports of actual or suspected product tampering, contamination, mislabeling or inclusion of improper ingredients.
1.95 “Product Infringement” has the meaning set forth in Section 9.6(b).
1.96 “Product Mark” has the meaning set forth in Section 6.6.
1.97 “Proposing Party” has the meaning set forth in Section 4.4(a).
1.98 “Quality Agreement” has the meaning set forth in Section 7.2.
1.99 “Regulatory Approval” means all approvals necessary, excluding price approval, for the commercial sale of the Product for the Field in a given country or regulatory jurisdiction.
1.100 “Regulatory Authority” means, in a particular country or jurisdiction, any applicable Governmental Authority involved in granting Regulatory Approval in such country or jurisdiction.
1.101 “Regulatory Exclusivity” means any exclusive marketing rights or data exclusivity rights conferred by any Governmental Authority with respect to the Product, in a country under the jurisdiction of such Government Agency in the Licensed Territory, other than a Patent right, including, without limitation, rights conferred in the U.S. under the Hatch-Waxman Act or the FDA Modernization Act of 1997, or rights similar thereto outside the U.S.
1.102 “Regulatory Materials” means regulatory applications, submissions, notifications, communications, correspondence, registrations, Regulatory Approvals and/or other filings made to, received from or otherwise conducted with a Governmental Authority in order to Develop, Manufacture, market, sell or otherwise Commercialize the Product in a particular country, territory or possession. Regulatory Materials include, without limitation, INDs, NDAs and MAAs.
12
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1.103 “Retained Liabilities” has the meaning set forth in Section 11.4.
1.104 “Reviewing Party” has the meaning set forth in Section 4.3(d).
1.105 “ROFR Notice” has the meaning set forth in Section 9.2(b).
1.106 “ROFR Notice Period” has the meaning set forth in Section 9.2(b).
1.107 “ROW Territory” means worldwide except the Licensed Territory.
1.108 “Royalty Term” means, with respect to a particular Product within a particular country in the Licensed Territory, the period of time beginning upon the date of First Commercial Sale of such Product in such particular country and continuing until the later of: (a) the date of expiration of the last Valid Claim in such country that would be infringed by the Development, Manufacture, use or sale of such Product in such country, (b) the expiration of all Regulatory Exclusivity periods with respect to the Product in such country, or (c) ten (10) years after the First Commercial Sale in such country. Thereafter, no further royalties shall be due with respect to such Product in such country.
1.109 “r/r AML” means relapsed/refractory acute myelogenous leukemia.
1.110 “r/r MDS” means relapsed/refractory myelodysplastic syndromes.
1.111 “r/r MM” means relapsed/refractory multiple myeloma.
1.112 “Sales and Marketing Plan” has the meaning set forth in Section 6.2.
1.113 “Sales Event 1” has the meaning set forth in Section 8.3.
1.114 “Sales Event 2” has the meaning set forth in Section 8.3.
1.115 “Sales Event 3” has the meaning set forth in Section 8.3.
1.116 “Sole Inventions” has the meaning set forth in Section 9.1.
1.117 “Study Report IP” means the copyright in study report C09/IIC/001 to manufacture BB-76163 in accordance with the route described in that study report.
1.118 “Subject CTI Rights” has the meaning set forth in Section 9.2.
1.119 “Supply Agreement” has the meaning set forth in Section 7.2.
1.120 “Take the Lead” shall mean, with respect to a particular Party, that such Party is primarily responsible for, and has the authority to make, all day-to-day decisions (in accordance with the approved Development Plan and Sales and Marketing Plan) as they relate to such Party’s responsibilities, rights and/or obligations hereunder; provided, however, the Parties shall
13
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
consult with each other with respect to any matters as requested by either Party or as otherwise required by the terms of this Agreement.
1.121 “Taxes” means taxes (other than income taxes), duties, tariffs or other governmental charges levied on the sale of Products, including, without limitation, consumption taxes.
1.122 “Term” means the term of this Agreement, as determined in accordance with Article 13.
1.123 “Third Party” means any entity other than Chroma or CTI or an Affiliate of either of them.
1.124 “Third Party Royalties” has the meaning set forth in Section 8.4(d)(ii).
1.125 “Threshold” has the meaning set forth in Section 8.4(c).
1.126 “Transition Development Plan” has the meaning set forth in Section 13.8(a).
1.127 “Transition Period” has the meaning set forth in Section 13.7(e).
1.128 “Transition Plan” has the meaning set forth in Section 13.7(e).
1.129 “Upstream Agreements” means the agreements listed on Exhibit B-2.
1.130 “Valid Claim” means a pending or issued claim of a Patent within the Chroma Patents which: (a) has not been held unpatentable, invalid or unenforceable by a court or other government agency of competent jurisdiction in a decision from which no appeal can or has been taken; and (b) which has not been admitted to be invalid or unenforceable through reissue, re-examination, disclaimer or otherwise. Notwithstanding the foregoing, if a claim of a pending patent application within the Patents has not issued as a claim of a patent within five (5) years from first filing of the application, such claim shall not be a Valid Claim for the purposes of this Agreement, unless and until such claim issues as a claim of an issued patent (from and after which time the same shall be deemed a Valid Claim subject to paragraphs (a) and (b) above). With respect to a Valid Claim of a pending patent application, the phrase to “infringe a Valid Claim” means to engage in an activity that would infringe (i.e., by either directly infringing, contributorily infringing, or inducing infringement of) such Valid Claim if it were contained in an issued patent.
1.131 “Vernalis” means Vernalis (R&D) Limited (formerly Vernalis (Oxford) Limited) registered in England with number 1985479.
1.132 “Vernalis Agreement” means the agreement between Vernalis and Chroma dated 24 November 2003 as amended by amendment No.1 dated 30 March 2007.
14
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
ARTICLE 2
LICENSES AND EXCLUSIVITY
2.1 Licenses to CTI under Chroma Technology.
(a) License Grants.
(i) Subject to the terms and conditions of this Agreement, Chroma hereby grants, and shall cause each of its Affiliates to grant CTI, a royalty-bearing, exclusive license under the Chroma Technology, with the right to sublicense as provided below, to Develop, Manufacture, use, make, and have made the Product in the Field in the Licensed Territory, in each case in accordance with the Development Plan and the Supply Agreement and to sell, offer for sale, have sold, distribute, import and otherwise Commercialize the Product in the Field in the Licensed Territory.
(ii) Subject to the terms and conditions of this Agreement, Chroma hereby grants and shall cause each of its Affiliates to grant CTI a royalty-bearing, non-exclusive license under the Manufacturing Patent and the Study Report IP, with the right to sublicense as provided below, to Manufacture and have made the Product in the Field in the Licensed Territory.
(iii) The Chroma Patents identified on Exhibit B-1 are licensed to Chroma by Vernalis under the Vernalis Agreement. CTI acknowledges and agrees that its sublicense rights to such Patents under this Agreement are at all times subject to the applicable terms of the Vernalis Agreement, current copies of which have been provided to CTI as of the Effective Date, provided that Chroma shall at all times be responsible for any payment obligations to Vernalis pursuant to the Vernalis Agreement. Chroma shall provide prior notice to CTI of any proposed amendment of the Vernalis Agreement and shall not, without CTI’s prior written consent, amend or terminate the Vernalis Agreement, which consent shall not be unreasonably withheld, conditioned or delayed. If Chroma becomes aware of any amendment or termination of any other Upstream Agreement, Chroma shall promptly notify CTI of the same. If Chroma receives a notice of default or termination from Vernalis, Chroma shall promptly provide a copy of such notice to CTI. Chroma will use commercially reasonable efforts to cure any such default to avoid termination of the Vernalis Agreement. At CTI’s option, but without any obligation and without limiting any remedies CTI may have against Chroma, CTI may cure any payment default on behalf of Chroma under the Vernalis Agreement. Any such payments made by CTI, whether to cure any default of Chroma or to pay royalties on behalf of Chroma under the Vernalis Agreement, may be deducted from royalties and milestone payments otherwise due to Chroma under Article 8.
(b) Chroma Retained Rights. Notwithstanding the rights granted to CTI in Section 2.1(a) and without limiting the generality of Section 2.4, Chroma retains the right to (i) conduct the Development and Commercialization activities in the ROW Territory as expressly permitted to be conducted by Chroma under this Agreement, including the conduct of any
15
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Extraterritorial Studies designated to be performed by Chroma under Section 4.3(c)(ii) of this Agreement; (ii) to Develop, Manufacture, use, make and have made the Product in the Field in the Licensed Territory for the purposes of exercising its rights in relation to the Product in the ROW Territory or to supply Product to CTI in the Licensed Territory; and (iii) the right to Develop, Manufacture, use make, have made, import and use Product in the Licensed Territory for the purposes of complying with its obligations under this Agreement with respect to any Additional Studies, or with the prior written approval of CTI (such approval not to be unreasonably withheld, conditioned or delayed), to carry out clinical trials in the Licensed Territory for the exclusive purpose of supporting regulatory activities in the ROW Territory.
(c) Sublicense Rights. CTI shall have the right to sublicense any of the rights set forth in Section 2.1(a), subject to Chroma’s prior written approval, which shall not be unreasonably withheld, delayed or conditioned provided that any such sublicense which is granted to an Affiliate of CTI shall not require Chroma’s prior written approval unless and until such Affiliate ceases to be an Affiliate of CTI. Promptly after the execution of any sublicense agreement, CTI shall notify Chroma and, if requested in writing by Chroma, provide Chroma with a copy of such agreement; provided, that any information CTI reasonably deems to be confidential may be redacted. CTI hereby covenants that it will include in all agreements granting sublicenses under the rights granted in Section 2.1(a) provisions consistent with the terms of this Agreement as applicable to a sublicense, including without limitation those contained in Article 4, Article 5, Article 6 and Article 8. CTI shall be liable to Chroma for all acts or omissions of any such sublicensee in connection with such sublicense.
2.2 Negative Covenants.
(a) No Grant of Rights. Chroma agrees not to grant to any Third Party (i) any rights inconsistent with the rights and licenses granted to CTI under this Agreement, including any rights or licenses in or to the Chroma Technology in the Licensed Territory, whether under a material transfer agreement or otherwise; and (ii) subject to Section 2.1(b) above without CTI’s prior written approval (which shall not be unreasonably withheld, delayed or conditioned), any rights or licenses in or to the Chroma Technology to any Third Party in the Field in the ROW Territory. Chroma hereby covenants that it will include in all agreements granting sublicenses under the Chroma Technology in the Field in the ROW Territory provisions consistent with the terms of this Agreement as applicable to a sublicense, including without limitation those contained in Article 4, Article 5 and Article 6.
(b) Non-Competition.
(i) Chroma shall not during the term of this Agreement anywhere in the Licensed Territory, Develop, Manufacture, market, promote, advertise, sell or offer to sell, or otherwise Commercialize (or license or collaborate with a Third Party to do any of the foregoing) a Competing Product. Chroma shall not, during the term of this Agreement, Commercialize the Product to or with a particular entity in the ROW Territory that Chroma knows or reasonably should know will distribute or sell the Product in the Licensed Territory,
16
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
provided that this obligation applies to Chroma only and does not require Chroma to police its sublicensees in this regard. For the purposes of this Section 2.2(b)(i), any act or activity undertaken, or failure to act, by an Affiliate of Chroma, which, if committed by Chroma would constitute a breach of this Section, shall constitute a breach by Chroma.
(ii) CTI shall not during the term of this Agreement anywhere in the ROW Territory, Develop, Manufacture, market, promote, advertise, sell or offer to sell, or otherwise Commercialize (or license or collaborate with a Third Party to do any of the foregoing) a Competing Product. CTI shall not, during the term of this Agreement, Commercialize the Product to or with a particular entity in the Licensed Territory that CTI knows or reasonably should know will distribute or sell the Product in the ROW Territory, provided that this obligation applies to CTI only and does not require CTI to police its sublicensees in this regard. For the purposes of this Section 2.2(b)(ii), any act or activity undertaken, or failure to act, by an Affiliate of CTI, which, if committed by CTI would constitute a breach of this Section, shall constitute a breach by CTI.
(c) No Liens. During the Term, Chroma shall not grant any liens, security interests or other encumbrances on the Chroma Technology, other than security interest to lenders similar to the one described on Exhibit B-1 that is granted in Chroma’s ordinary course of business covering Chroma’s assets generally.
2.3 Registration of License. Notwithstanding anything to the contrary in Article 12, CTI, at its expense, may register the licenses granted under this Agreement in any country of the Licensed Territory. Upon request by CTI, Chroma agrees promptly to, and to cause each of its Affiliates to, execute any “short form” licenses consistent with the terms and conditions of this Agreement submitted to it by CTI, or effect any registrations of its own, which are reasonably necessary to effect the foregoing registration in such country.
2.4 No Implied Licenses. Except as explicitly set forth in this Agreement, neither Party grants any license, express or implied, under its intellectual property rights to the other Party.
ARTICLE 3
OVERVIEW; MANAGEMENT
3.1 Executive Steering Committee.
(a) Formation and Role. The Parties agree to establish and convene a Executive Steering Committee (or “ESC”) for the overall coordination and oversight of the Parties’ activities under this Agreement, promptly after the Effective Date. Each Party shall have an equal number of representatives on the ESC. The ESC shall operate by the procedures set forth in Section 3.3. Except as otherwise provided in Section 14.3(b) and subject to Section 14.2(b), the role of the Executive Steering Committee shall be:
17
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(i) For the Licensed Territory and the ROW Territory, to review and discuss the overall strategy for the Development, Manufacture, Regulatory Approval (including the initial approval and any supplements and expansions thereof) and Commercialization of the Product;
(ii) to review, discuss and approve the Development Plans (including the Development Budget) on or before March 1 of each calendar year;
(iii) to review, discuss and approve any changes or revisions to the Development Plan (including the Development Budget);
(iv) to review and discuss the Sales and Marketing Plan, and any proposed amendments or revisions to such plan;
(v) to resolve any disputes arising within the JDC; and
(vi) to establish such subcommittees, including without limitation the regulatory working group as provided in Section 5.3 and the Publication Team as provided in Section 6.7, and to perform such other functions as appropriate to further the purposes of this Agreement, as mutually agreed by the Parties in writing.
(b) ESC Decisions and Actions. Actions to be taken by the Executive Steering Committee shall be taken only following unanimous vote, with each Party having one (1) vote per representative. Except as otherwise provided in Section 14.3(b), if the Executive Steering Committee fails to reach unanimous agreement on a matter before it for decision for a period in excess of fifteen (15) days from the date first presented to the ESC in writing, the matter shall be submitted immediately to the Chief Executive Officer of each Party (each, the “Designated Executive”) for resolution in accordance with the decision-making procedures described in Section 14.2, including the specific decision-making rights of each Party as described in such section.
3.2 Joint Development Committee.
(a) Formation and Role. The Parties also agree to establish a Joint Development Committee (or “JDC”) which will monitor and coordinate communication and operations regarding the Parties’ efforts with respect to the Development, Manufacture, and Regulatory Approval of the Product in the Field and in the Licensed Territory and the ROW Territory. Each Party shall have an equal number of representatives on the Joint Development Committee. The Joint Development Committee shall operate by the procedures set forth in Section 3.3. The role of the Joint Development Committee shall be:
(i) to facilitate the exchange of Information between the Parties under this Agreement with respect to their Product-related activities (including activities conducted in the Licensed Territory and the ROW Territory), including as and to the extent necessary for each Party to perform its obligations under this Agreement;
18
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(ii) to review and comment on the Initial Development Plan and all updates thereto, and to submit such plan to the ESC for approval (it being understood that the JDC shall submit such plan with sufficient time for the ESC to review and approve such plan on or before March 1 of each calendar year);
(iii) to review and comment on any proposed changes or revisions to the Development Plan (including the Development Budget);
(iv) to review and comment on the Sales and Marketing Plans, and CTI’s Commercialization activities for the Product in the Licensed Territory and Chroma’s Commercialization activities for the Product in the ROW Territory;
(v) to establish a global branding strategy for the Product at least twelve (12) months prior to any Product launch in the Licensed Territory or the ROW Territory, including the determination of ownership rights related to Product Marks and related trademark registrations.
(vi) to establish such working teams or subcommittees and to perform such other functions as appropriate to further the purposes of this Agreement, as determined by the Parties in writing.
(b) JDC Decisions and Actions. Except as expressly provided in this Section 3.2, actions to be taken by the JDC shall only be taken following unanimous vote, with each Party having one (1) vote per representative. Except as otherwise provided in Section 14.3(b), if the Joint Development Committee fails to reach unanimous agreement on a matter before it for decision for a period in excess of ten (10) days from the date first presented to the JDC in writing, the matter shall be referred immediately to the Executive Steering Committee.
3.3 Committee Membership and Procedures.
(a) Membership. Chroma and CTI shall each designate an equal number of representatives to serve on each of the ESC and JDC (each, a “Committee”) by written notices to the other Party. Initially, each Party shall designate three (3) such representatives. Each Committee may elect to vary the number of representatives from time to time during the Term. Either Party may designate substitutes for its Committee representatives if one (1) or more of such Party’s designated representatives is unable to be present at a meeting. From time to time each Party may replace its Committee representatives by written notice to the other Party specifying the prior representative(s) and their replacement(s). Any such substitutes or replacements shall be designated consistent with the following principles: at least one (1) representative shall have appropriate expertise in the clinical development of pharmaceutical products; provided, that each Committee may vary the expertise required for the representatives of each Party as it deems appropriate as the Parties gain experience with the Product. Each Committee will have a chairperson, to be designated as described below. The chairperson shall be responsible for (i) calling meetings, and (ii) preparing and circulating an agenda for the upcoming meeting, but shall have no special authority over the other members of the Committee, and shall have no additional
19
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
voting rights. The alliance managers described in Section 3.4 shall be responsible for preparing and issuing minutes of each ESC and JDC meeting within thirty (30) days thereafter. Such minutes shall not be finalized until each Committee representative reviews and approves such minutes in writing; provided that any minutes shall be deemed approved unless a member of the ESC or the JDC (as applicable) objects to the accuracy of such minutes within fifteen (15) days after the circulation of the minutes by the chairperson. The chairpersons of other subcommittees will be responsible for generating minutes from their respective meetings.
(b) Chairperson.
(i) The chairperson of the ESC shall be appointed for a calendar year, with Chroma appointing the initial chairperson. On January 1 of each year after the Effective Date, the Parties shall rotate designation of the chairperson for the ESC for the commencing year.
(ii) The chairperson of the JDC shall be appointed for a calendar year. The initial chairperson of the JDC will be designated by CTI. On January 1 of each year after the Effective Date, the Parties shall rotate designation of the chairperson for the JDC for the commencing year, provided that promptly following submission of the first NDA or other Regulatory Approval for a Product in the Licensed Territory, CTI will be entitled to replace the chairperson with a CTI representative to serve for at least twelve (12) months after which time Chroma shall again be entitled to designate the chairperson on the following January 1. Thereafter, the Parties shall rotate designation of the chairperson for the JDC for the subsequent years.
(c) Meetings. Meetings of a Committee shall be effective only if at least two (2) representatives of each Party are present or participating. A Committee may meet either (i) in person at either Party’s facilities or at such locations as the Parties may otherwise agree; or (ii) by audio or video teleconference. With the prior consent of the other Party’s representatives (such consent not to be unreasonably withheld or delayed), each Party may invite non-members to participate in the discussions and meetings of a Committee, provided that such participants shall have no voting rights or powers and shall be subject to the confidentiality provisions set forth in Article 12. Additional meetings of a Committee may be held with the consent of each Party, as required under this Agreement, or to resolve any dispute referred to it and neither Party will unreasonably withhold or delay its consent to hold such an additional meeting (and in the case of any dispute referred to the ESC, such meeting shall be held within five (5) business days following referral to the ESC, or as soon as reasonably possible). Each Party shall be responsible for all of its own expenses incurred in connection with participating in the Committees including expenses associated with an initial alliance kick-off meeting.
(i) The ESC shall hold at least three (3) meetings per year, or as otherwise agreed to by the Parties, with at least one (1) of such meetings being held in person.
20
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(ii) The JDC shall each hold a meeting at least every other month, or as otherwise agreed to by the Parties. Unless otherwise agreed to by the Parties, the JDC shall hold at least four (4) meetings in person each year to facilitate alignment and communication.
3.4 Alliance Managers. Promptly following the Effective Date, each Party shall designate in writing an individual to facilitate communication and coordination of the Parties’ activities under this Agreement relating to Products.
3.5 Authority. The ESC and JDC shall each perform its responsibilities under this Agreement based on the principles of prompt and diligent Development, Manufacture and Commercialization of Products in the Licensed Territory and the ROW Territory. The Committees shall each have only the powers assigned expressly to it in this Article 3 and elsewhere in this Agreement, and shall not have any power to amend, modify or waive compliance with this Agreement.
3.6 Collaboration Guidelines. Subject to the terms of this Agreement, the activities and resources of each Party shall be managed by such Party, acting independently and in its individual capacity. The relationship between Chroma and CTI is that of independent contractors and neither Party shall have the power to bind or obligate the other Party in any manner, other than as may be expressly set forth in this Agreement.
3.7 Diligence. CTI shall use Diligent Efforts to Develop, to seek Regulatory Approval for and to Commercialize Products for use in the Field in the Licensed Territory after receipt of applicable Regulatory Approval in accordance with the terms of this Agreement and the Development Plan, consistent with good pharmaceutical practices. Notwithstanding the foregoing sentence, CTI’s obligation to use Diligent Efforts shall not apply to the extent of any delay or failure by or on behalf of Chroma to (i) perform its Development obligations or responsibilities under the Development Plan, (ii) timely supply the Product for Development purposes pursuant to the terms of the Supply Agreement, or (iii) timely transfer manufacturing technology to CTI or its designee. Chroma shall use Diligent Efforts to conduct, in accordance with the terms of this Agreement, the Development to be performed by it under this Agreement in accordance with the Development Plan.
3.8 Sublicensing. In the event that either Party sublicenses a material portion of its rights in the Product to a Third Party in accordance with the terms of this Agreement, the Parties shall in good faith discuss and, as appropriate, amend the structures of the ESC, JDC and any subcommittees to reflect the revised division of responsibilities following execution of such sublicenses.
ARTICLE 4
PRODUCT DEVELOPMENT
4.1 Overview of Product Development. The Parties desire and intend to collaborate with respect to the Development of the Product in the Field, as and to the extent set forth in this
21
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Agreement. The general allocation of responsibilities for conducting Development of the Product shall be as follows: (a) CTI will oversee and shall be responsible for performing the Development activities in the Licensed Territory, including for the sponsorship of Licensed Territory Specific Studies; and (b) Chroma will oversee and shall be responsible for performing the Development activities in the ROW Territory, including for the sponsorship of Extraterritorial Studies; in each case unless otherwise agreed to by the JDC.
4.2 Principles of Product Development. Each Party’s Development of the Product in the Field shall be conducted in a manner consistent with the following principles: (a) seeking Regulatory Approval that includes the appropriate label for such Product in light of the clinical data, and (b) obtaining Regulatory Approval for such Product consistent with the preceding clause and in a timely manner.
4.3 Development Operations and Expenses.
(a) Development Plan.
(i) General. The Parties shall collaboratively conduct the Development of the Product pursuant to a mutually agreed written development plan (the “Development Plan”). The Development Plan will contain the following information, to the extent such information is available:
(1) scope and target timelines for all Development activities in reasonable detail as agreed by the Parties supporting Regulatory Approvals in the Field for the Product in the Licensed Territory and the ROW Territory, including the performance of Currently Ongoing Studies and any Additional Studies as mutually agreed upon by the Parties under the terms of Section 4.4;
(2) a Development Budget; and
(3) plans and timeline for preparing the necessary Development Regulatory Materials in support of obtaining Regulatory Approval in the Licensed Territory and the ROW Territory for the first indication and any label expansion.
(ii) Initial Development Plan. The Parties have agreed upon an initial Development Plan, which is attached hereto as Exhibit C (the “Initial Development Plan”).
(iii) Updates to Development Plan. On at least an annual basis and in the timeframe provided in Section 3.2(a)(ii), commencing in 2012, and at such other times as deemed necessary by the JDC, the JDC shall update and amend, as appropriate (taking into account relevant issues, such as safety and efficacy results, costs of development, the time required to complete development, etc.), the then-current Development Plan. The JDC shall submit all proposed updates and amendments to the Development Plan approved by it to the ESC for review and final approval. Once approved by the ESC, each updated or amended
22
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Development Plan shall become effective and supersede the previous Development Plan as of the date of such approval or at such other time as decided by the ESC.
(b) Development Costs.
(i) Allocation of Costs. As between the Parties, as outlined in the Initial Development Plan and subsequent Development Plans, subject to the Funding Cap, CTI shall be responsible for seventy-five percent (75%) of all Development Costs in the Licensed Territory and the ROW Territory, and Chroma (or its Development Designee) shall be responsible for twenty-five percent (25%) of all Development Costs in the Licensed Territory and the ROW Territory, provided that such cost sharing shall not apply to development costs related to the Currently Ongoing Studies incurred prior to April 1, 2011. Each Party will bear their own Indirect Costs. For clarity, (A) neither Party shall be responsible to the other Party for any costs or expenses incurred by the other Party for any Development activities that are not specifically subject to an agreed-upon Development Plan and Development Budget, and (B) if actual Development Costs for either Party will exceed the applicable Development Budget, the cost overruns must be reviewed and approved by the JDC and the ESC as set forth in Sections 3.1(a)(iii) and 3.2(a)(iii). No Party shall be obliged to fund its applicable proportion of an overrun unless such overrun has been prior approved by the JDC and the ESC.
(ii) Quarterly True-up. Within thirty (30) days of the end of each calendar quarter, each Party will submit to the other Party a report detailing such Party’s Development Costs incurred during such calendar quarter, including copies of invoices and any other supporting evidence necessary to substantiate the actual Development Costs. In the event that a Party’s Development Costs have exceeded its applicable allocation as set forth in Section 4.3(b)(i), as evidenced by the report submitted to the other Party, then, within sixty (60) days of the delivery of the report from such Party, the other Party shall pay to such Party the difference between the actual Development Costs and the applicable portion that is allocated to such Party under Section 4.3(b)(i). Any such difference not paid within such sixty (60) day period will accrue simple interest until the date of payment at the per annum rate of two percent (2%) over the then-current prime rate quoted by Citibank in New York City or the maximum rate allowable by applicable Law, whichever is lower.
(iii) Initial Funding Cap. The total Development Costs under the Initial Development Plan and subsequent Development Plans, from the period commencing with the Effective Date and continuing for three (3) years after such date, shall not exceed $50,000,000 (or such greater amount as the Parties may agree to in writing) (the “Funding Cap”). For clarity, as between the Parties, seventy-five percent (75%) of the Funding Cap ($37,500,000) is the responsibility of CTI, and twenty-five percent (25%) ($12,500,000) is the responsibility of Chroma.
(c) Conduct of Studies.
23
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(i) Currently Ongoing Studies. The Parties intend and agree that any and all Currently Ongoing Studies shall be conducted by Chroma in consultation with the JDC, and in accordance with the Development Plan.
(ii) Extraterritorial Studies. The Parties intend and agree that any and all Extraterritorial Studies shall be conducted by Chroma, in collaboration and consultation with CTI and in consultation with the JDC, and in accordance with the Development Plan.
(iii) Licensed Territory Specific Studies. The Parties intend and agree that any and all Licensed Territory Specific Studies shall be conducted by CTI, in collaboration and consultation with Chroma and in consultation with the JDC, and in accordance with the Development Plan.
(iv) Performance. Each Party agrees to conduct the studies allocated to it as described in this Section 4.3(c) in accordance with the Development Plan. Chroma and CTI each shall provide the JDC with annual reports detailing its Development activities under the Development Plan and the results thereof. Where Chroma licenses any of its rights under the Chroma Technology in the ROW Territory in accordance with Section 2.2(a)(ii) Chroma will use its Diligent Efforts to obtain such detailed reports related to the Development activities from the relevant Third Party on the same basis. However, where Chroma, despite using such Diligent Efforts, is unable to obtain such reports Chroma will provide CTI such information as it does receive from the Third Party, provided that this in no event limits Chroma’s obligation to obtain safety or other pharmacovigilance related data from its sublicensees.
(v) Phase 4 Clinical Studies. If a Party wishes to carry out Phase 4 Clinical Studies on a voluntary basis such studies will be carried out by that Party in consultation with the other Party through the JDC and the Party carrying out such study shall, unless otherwise agreed by the Parties, be responsible for all of the Development Costs associated with such study.
(d) Records; Audits. Each Party (the “Audited Party”) will maintain complete and accurate records in sufficient detail to permit the other Party (the “Reviewing Party”) to confirm the accuracy of the Development Costs under this Agreement. Upon reasonable prior notice, such records shall be available during regular business hours for a period of three (3) years from the end of the calendar year to which they pertain for examination at the expense of the Reviewing Party, and not more often than once each calendar year, by an independent certified public accountant selected by the Reviewing Party and reasonably acceptable to the Audited Party, for the sole purpose of verifying the accuracy of the financial reports furnished by the Audited Party pursuant to this Agreement. Any such auditor shall not disclose the Audited Party’s Confidential Information, except to the extent such disclosure is necessary to verify the accuracy of the financial reports furnished by the Audited Party or the amount of Development Costs allocated under this Agreement.
4.4 Development Opt-In.
24
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(a) Prior to Initiation of Development. In the event that either Party proposes (the “Proposing Party”) to conduct any Extraterritorial Study, any Licensed Territory Specific Study or any study that is both an Extraterritorial Study and a Licensed Territory Specific Study (each of the foregoing for the Product but not an Additional Product) that is not subject to the then-current Development Plan or Development Budget (including with respect to any additional clinical studies regarding indications other than r/r AML or r/r MDS), prior to beginning such Development activities, such Party shall provide notice to the JDC and ESC of its intensions thereof, and copies of any Information of such Party reasonably requested by the JDC and/or ESC. Within sixty (60) days of delivery of such notice, in accordance with the terms of Section 4.3(a)(iii), the JDC and ESC will review and decide whether such additional Extraterritorial Study or Licensed Territory Specific Study will become part of and incorporated into the Development Plan and Development Budget. In the event that the JDC and ESC approve the proposed amendments to the Development Plan and Development Budget, then any such additional Extraterritorial Study or Licensed Territory Specific Study will be performed and paid for subject to the terms and conditions of such amended Development Plan and the terms and conditions of this Agreement, provided that if any such study is requested or required by any Regulatory Authority, the JDC and ESC must include such study in an amended Development Plan and Development Budget. Subject to the foregoing, in the event that the JDC and ESC do not approve the proposed amendments to the Development Plan and Development Budget to include the newly proposed Extraterritorial Study or Licensed Territory Specific Study, but the Proposing Party proceeds on its own accord to conduct such additional Extraterritorial Study or any Licensed Territory Specific Study that it otherwise has the right to conduct under the terms of this Agreement (“Non-Opted In Study”), then the non-proposing Party (“Non-Proposing Party”) will not be obligated to pay for or assume any Development Costs and expenses under Section 4.3(b) related to any Non-Opted In Study. The Non-Proposing Party will also not be entitled to have access to or use for any purpose any data, information or results developed by or on behalf of the Proposing Party as a result of any Non-Opted In Study except for routine adverse event reporting as provided in Section 5.5.
(b) Subsequent to Initiation of Development. For any Non-Opted In Study as provided in paragraph (a), the Non-Proposing Party may subsequently provide written notice to the Proposing Party that it wishes to opt in to the Non-Opted In Study. If such notice is provided at or prior to the mid point of enrolled patients as described in the protocol of such Non-Opted In Study, then the Non-Proposing Party shall pay (i) the Non-Proposing Party’s corresponding share of the Development Cost up to the effective date of opting in, as provided in Section 4.3(b), had the Non-Proposing Party opted to participate in such Non-Opted In Study from day one (“Corresponding Share”) and (ii) an additional payment of ** of its Corresponding Share. If such notice is provided subsequent to the mid point of enrolled patients as described in the protocol of such Non-Opted In Study, then the Non-Proposing Party shall pay (i) its Corresponding Share and (ii) an additional payment of ** of its Corresponding Share.
(c) After Opting In. Following opt in to any previously Non-Opted In Study as provided in paragraph (b) above, including payment of the applicable amounts specified therein, the Non-Proposing Party will have the same rights, as provided under the terms of this
25
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Agreement, to the data, information and results generated from any such previously Non-Opted In Study as though the Non-Proposing Party had participated from day one. Within thirty (30) days after the opt in notice and payment by the Non-Proposing Party as provided in paragraph (b) above, the Parties shall amend the Development Plan and Development Budget to include the previously Non-Opted In Study. The Non-Proposing Party shall, from the date of the opt in notice, also pay for its Corresponding Share of the future Development Costs set out in such amended Development Plan and Development Budget for such previously Non-Opted In Study. Each Party acknowledges and agrees that it shall obligate its sublicensees to agree to comply with the terms and conditions of this Section 4.4 and provide the other Party the benefits under this Section 4.4.
4.5 Cooperation; Compliance with Laws. Each Party shall conduct its Development activities under this Agreement in good scientific manner and in compliance in all material respects with all applicable Laws, including without limitation applicable national and international (e.g., ICH, GCP, GLP, and GMP) guidelines.
4.6 Subcontracting.
(a) Chroma Development Designees. CTI acknowledges and agrees that portions of the Development to be performed by Chroma under this Agreement may be performed on behalf of Chroma by a Development Designee appointed by Chroma, provided that (i) Chroma shall first have obtained CTI’s prior written consent (which consent may not be unreasonably withheld, conditioned or delayed) with respect to the proposed subcontractor, (ii) Chroma shall first have obtained written confidentiality agreements with any such subcontractor and written assignments of, or equivalent rights under, all Patent rights and Know-How which relate to the Product that such subcontractor may develop by reason of work performed under this Agreement, and (iii) Chroma shall be and remain responsible to CTI for the performance of its subcontractors.
(b) CTI Development Designees. Chroma acknowledges and agrees that portions of the Development to be performed by CTI under this Agreement may be performed on behalf of CTI by a Development Designee appointed by CTI, provided that (i) CTI shall first have obtained Chroma’s prior written consent (which consent may not be unreasonably withheld, conditioned or delayed) with respect to the proposed subcontractor, (ii) CTI shall first have obtained written confidentiality agreements with any such subcontractor, and (iii) CTI shall be and remain responsible to Chroma for the performance of its subcontractors.
4.7 Records, Reports and Information. Each Party shall maintain, and cause its Affiliates and sublicensees to maintain, complete, current and accurate records of all Development activities conducted by it hereunder, and all data and other Information resulting from or relating to such activities, including but not limited to the investigator brochure, IND annual report, and any safety plans established during Development. Such records shall fully and properly reflect all work done and results achieved in the performance of the Development activities in good scientific manner appropriate for regulatory purposes. Each Party shall document, and cause its Affiliates and sublicensees to document, all Non-Clinical Studies and
26
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
clinical trials in formal written study reports according to applicable national and international (e.g., ICH, GCP, GLP, and GMP) guidelines. Each Party shall have the right to review such records maintained by the other Party at reasonable times, upon written request, which shall not exceed once a year.
4.8 Data Exchange and Use. Each Party shall promptly provide the other Party with copies of all final reports with respect to the conduct of any Development of the Product in the Field and with access to, upon request of a Party, all data with respect to the conduct of any Development of the Product in the Field, in each case as such data and reports become available to the Party performing the corresponding studies. Each Party will have the right to (a) use such data and reports for the purposes of carrying out its obligations and exercising its rights under this Agreement and (b) to provide all such data and reports generated by the other Party to such Party’s sublicensees or prospective sublicensees subject to the applicable provisions of this Agreement. All data and reports disclosed by one Party to the other under this Agreement shall be deemed Confidential Information of the disclosing Party and may be used by the receiving Party for the purposes of this Agreement, subject to the permitted uses and disclosures described in Section 4.4 and in this Section 4.8 and disclosure to any Regulatory Authority in connection with a Party’s obligations under Article 5.
4.9 Designation of Additional Products. If either Party desires to commence development of an Additional Product in the Field, then such Party shall notify the JDC and ESC in writing and provide a draft development plan for such Additional Product to the JDC and ESC. The JDC and ESC shall consider such development plan in good faith in accordance with the terms of this Agreement. Upon approval by the JDC and ESC, the Parties shall either amend the then-current Development Plans and Sales and Marketing Plans to include such Additional Product or prepare separate plans to include terms relating to such Additional Product, including allocation of costs and such other terms similar to those provided in Section 4.3. The Parties shall determine a reasonable process for the timely delivery of Information regarding any potential Additional Products by each Party to the JDC and/or ESC.
(a) If Not Approved by ESC. In the event that the JDC and ESC do not approve the development of any Additional Product after consideration as provided above, the Proposing Party may pursue any such development on its own (“Non-Opted In Additional Product Development”). If the Proposing Party proceeds on its own accord to pursue any Non-Opted In Additional Product Development, then the Non-Proposing Party will not be obligated to pay for or assume any development costs and expenses related to such Non-Opted In Additional Product Development. The Non-Proposing Party will not be entitled to use for any purpose any data, information or results developed by or on behalf of the Proposing Party as a result of such Non-Opted In Additional Product Development except for routine adverse event reporting as provided in Section 5.5.
(b) Subsequent to Initiation of Development. The Non-Proposing Party may, however, subsequently provide written notice to the Proposing Party that it wishes to opt in to the Non-Opted In Additional Product Development. If such notice is provided at or prior to any
27
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
clinical studies, then the Non-Proposing Party shall pay (i) its Corresponding Share of development costs for such Non-Opted In Additional Product Development under the same terms as those contained in Section 4.3(b) and (ii) an additional payment of ** of such Corresponding Share. If such notice is provided subsequent to the Initiation of clinical studies, then the Non-Proposing Party shall pay (i) its Corresponding Share of development costs for such Non-Opted In Additional Product Development under the same terms as those contained in Section 4.3(b) and (ii) an additional payment of ** of such Corresponding Share.
(c) After Opting In. Following opt in to any previously Non-Opted In Additional Product Development, the Non-Proposing Party will have the same rights, as provided under the terms of this Agreement, to the data, information and results generated from any such previously Non-Opted In Additional Product Development as though the Non-Proposing Party had participated from day one. Within thirty (30) days after the opt in notice and payment by the Non-Proposing Party as provided in paragraph (b) above, the Parties shall either amend the then-current Development Plans and Sales and Marketing Plans to include such Additional Product or prepare separate plans to include terms relating to such Additional Product, including allocation of costs and such other terms similar to those provided in Section 4.3. The Non-Proposing Party shall, from the date of the opt in notice, also pay for its Corresponding Share of the future development costs for such Non-Opted In Additional Product Development set out in such plan. Each Party acknowledges and agrees that it shall obligate its sublicensees to agree to comply with the terms and conditions of this Section 4.9 and provide the other Party the benefits under this Section 4.9.
ARTICLE 5
REGULATORY MATTERS
5.1 Initial Transfer. Within sixty (60) days after the Effective Date, Chroma shall make available to CTI copies of all Development Documentation that are Controlled by Chroma generated as of the Effective Date and relating to the use of the Product in the Field, including any drug master files and Regulatory Materials for the Past Studies. Within ninety (90) days after the Effective Date, Chroma shall submit to the FDA or equivalent foreign Regulatory Authority in the Licensed Territory to transfer to CTI ownership of, and CTI shall own in CTI’s name, any IND, NDA or MAA submissions for the Product in the Licensed Territory including, IND number 75,503 or any foreign equivalents thereof (as applicable) in the Licensed Territory, at no additional charge to CTI. Chroma shall execute and deliver to the applicable Regulatory Authority such documents as are required to notify such Regulatory Authority of the transfer of such IND or any foreign equivalent thereof to CTI.
5.2 Preparation of Regulatory Materials.
(a) Licensed Territory.
(i) CTI shall Take the Lead and be the responsible party, in consultation with Chroma, for preparing, filing and holding any and all Regulatory Materials in
28
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
the Licensed Territory associated with any IND, NDA or MAA submissions for the Product or amendments or supplements thereto. Such Regulatory Materials and related Regulatory Approvals shall be owned solely by CTI and held in its name, subject to Chroma’s rights of reference under this Agreement. Except as required by applicable Law or Regulatory Authority, CTI shall not withdraw any such Regulatory Materials or Regulatory Approvals without prior consultation with and approval of Chroma, which shall not be unreasonably withheld, delayed or conditioned. CTI shall be primarily responsible, in consultation with Chroma, for performing all activities required by such Regulatory Authority with respect to (1) maintaining the IND, NDA and MAA submissions, maintaining the Regulatory Approval following its receipt, safety monitoring as further described in Section 5.5 below, promotional activities, compliance, and annual reporting to such Regulatory Authorities, as well as associated document retention, and (2) filing any and all Regulatory Materials for subsequent indications in the Licensed Territory, and holding any such Regulatory Materials, amendments or supplements thereto. CTI shall be responsible for any field alert reporting in the Licensed Territory and Chroma shall reasonably assist CTI with its annual reporting obligations.
(ii) Upon the request of Chroma, CTI shall inform each applicable Regulatory Authority in the Licensed Territory that one (1) or more representatives of Chroma will attend and, to the extent permitted by applicable Law, participate in all major meetings between CTI and such Regulatory Authority, subject to the confidentiality provisions set forth under Article 12. CTI shall timely inform Chroma of any such scheduled meetings, as soon as practicably possible.
(iii) CTI and Chroma shall as soon as reasonably possible following the Effective Date, through the JDC establish a plan and schedule of regulatory activities to be performed by the Parties in connection with obtaining approval for the first NDA or MAA for the Product in the Licensed Territory and for amendments or supplements thereto.
(b) ROW Territory.
(i) Chroma shall retain sponsorship of the clinical trial applications currently active in Europe. Except as required by applicable Law or Regulatory Authority, Chroma shall not withdraw any Regulatory Materials or Regulatory Approvals without prior consultation with and approval of CTI, which shall not be unreasonably withheld, delayed or conditioned.
(ii) Chroma shall Take the Lead and be the responsible party in consultation with CTI, for preparing, filing, holding and maintaining any and all Regulatory Materials for the Product in the ROW Territory associated with any IND or MAA for the Product or amendments or supplements thereto. Such Regulatory Materials and related Regulatory Approvals shall be owned solely by Chroma and held in its name, subject to CTI’s rights of reference under this Agreement. Chroma shall be primarily responsible, in consultation with CTI, for performing all activities required by such Regulatory Authority with respect to (1) maintaining the MAA submissions, maintaining the Regulatory Approval following its receipt,
29
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
safety monitoring as further described in Section 5.5 below, promotional activities, compliance, and annual reporting to such Regulatory Authorities, as well as associated document retention, and (2) filing any and all Regulatory Materials for subsequent indications in the ROW Territory, and holding any such Regulatory Materials, amendments or supplements thereto. Chroma shall be responsible for any field alert reporting in the ROW Territory and CTI shall reasonably assist Chroma with its annual reporting obligations.
(iii) Upon the request of CTI, Chroma shall inform each applicable Regulatory Authority in the ROW Territory that one (1) or more representatives of CTI will attend and, to the extent permitted by applicable Law, participate in all major meetings between Chroma and such Regulatory Authority, subject to the confidentiality provisions set forth under Article 12. Chroma shall timely inform CTI of any such scheduled meetings, as soon as practicably possible.
(iv) The Parties agree and acknowledge that the regulatory strategy for the ROW Territory shall be coordinated with CTI’s activities in the Licensed Territory and consistent with the overall objective of facilitating Regulatory Approval in both the ROW Territory and Licensed Territory.
5.3 Cooperation, Consultation and Review. The Parties shall establish a joint regulatory working group as soon as reasonably possible following the Effective Date and shall cooperate with each other to achieve the regulatory objectives contemplated herein in a timely, accurate and responsive manner. Each Party shall assist the other Party as such other Party may reasonably request in connection with the preparation and filing of all Regulatory Materials contemplated in this Article 5. Each Party shall provide the other Party with copies of any proposed Regulatory Materials to be submitted by such Party (other than routine correspondence) and shall reasonably consider any comments thereto provided by the other Party to the extent practicable.
5.4 Rights of Reference to Regulatory Materials. Subject to the provisions of Sections 4.4 and 4.9, Chroma hereby grants to CTI a right of reference to all Regulatory Materials filed by or for Chroma for the Product solely for the purpose of seeking, obtaining and maintaining Regulatory Approvals for, and the Commercialization of, the Products in the Licensed Territory, consistent with the roles of the Parties set forth in this Agreement. Subject to the provisions of Sections 4.4 and 4.9, CTI hereby grants to Chroma a right of reference to all Regulatory Materials filed by or for CTI for the Product solely for the purpose of seeking, obtaining and maintaining Regulatory Approvals for, and the Commercialization of, the Products in the ROW Territory, consistent with the roles of the Parties set forth in this Agreement.
5.5 Adverse Event Reporting and Safety Data Exchange.
(a) Licensed Territory. The Parties agree that CTI will be primarily responsible for (i) maintaining the global safety database and (ii) monitoring of all clinical experiences in the Licensed Territory and (iii) safety monitoring, pharmacovigilance surveillance, compliance and filing of all required safety reports to Regulatory Authorities in the Licensed
30
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Territory, including without limitation annual safety reports, throughout the Development of the Product in the Licensed Territory as and to the extent required by applicable Law for any study conducted under an IND held by CTI and in respect of the Commercialization of the Product by CTI in the Licensed Territory. The Parties shall cooperate to develop methods and/or procedures for transitioning the activities described in this section to CTI. Chroma will be responsible for all of such matters (except the global safety database) in the ROW Territory.
(b) Safety Information Exchange; Agreement. The Parties shall cooperate to develop methods and/or procedures for sharing information relating to the clinical experiences and in accordance with safety reporting requirements of the respective Regulatory Authorities and as necessary for a Party to comply with applicable Law.
(c) Regulatory Reporting. Each Party shall be permitted, and have the right, to perform pharmacovigilance activities and/or make such safety reports to applicable Regulatory Authorities, to comply with applicable Laws, international best practices for pharmacovigilance activities and/or other activities that the Party, in its reasonable and good faith judgment, believes necessary for the health, safety and protection of patients and/or clinical trial subjects. The Parties shall work together to agree to one opinion with respect to safety issues and to report said opinion to safety boards of any nature, investigators, and to applicable Regulatory Authorities. In the event that, after reasonable medical and scientific consultation, the Parties cannot agree to one opinion with respect to safety issues to be reported to any applicable Regulatory Authority, including but not limited to, individual adverse events or other matters affecting the health, safety or welfare of a patient, then, notwithstanding the provisions of Article 3, the Parties shall follow the dispute resolution procedure provided in Section 14.3(b). For clarity, the obligation to follow such dispute resolution procedure shall not limit either Party’s right, as provided in the first sentence of this Section 5.5(c), to report safety matters to Regulatory Authorities that may be necessary prior to the conclusion of the dispute resolution procedure.
(d) Separate Pharmacovigilance Agreement. Within ninety (90) days of the Effective Date, the Parties shall negotiate in good faith and enter into a separate pharmacovigilance agreement to further detail the provisions of this Section 5.5.
5.6 Recalls. Any decision to initiate a recall of the Product in the Licensed Territory shall be made by CTI and in the ROW Territory shall be made by Chroma, in each case subject to the remaining provisions of this Section 5.6. Before initiating a recall of the Product each Party will consult with the other Party and shall take into account any reasonable comments made by the other Party. The costs of any recall in the Licensed Territory shall be borne by CTI and the costs of any recall in the ROW Territory shall be borne by Chroma, subject to any provisions to the contrary contained in the Supply Agreement.
5.7 Regulatory Authority Communications Received by a Party. Each Party shall keep the other Party informed in a timely manner compliant with the reporting requirements of Regulatory Authorities of notification of any action by, or notification or other information which it receives (directly or indirectly) from any Regulatory Authority which: (a) raises any
31
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
material concerns regarding the safety or efficacy of the Product; (b) indicates or suggests a potential material liability of either Party to Third Parties in connection with the Product; (c) is reasonably likely to lead to a recall or market withdrawal of the Product; or (d) relates to expedited and periodic reports of adverse events with respect to the Product, or Product Complaints, and which may have a material impact on Development of the Product, Regulatory Approval or the continued Commercialization of the Product. The other Party will fully cooperate with and assist such Party in complying with regulatory obligations and communications, including by providing to such Party, in a timely manner after a request, such information and documentation in the other Party’s possession as may be necessary or helpful for the Party to prepare a response to an inquiry from a Regulatory Authority. Each Party will provide the other Party in a timely manner with a copy of all correspondence received from a Regulatory Authority specifically regarding the matters referred to above.
5.8 Audit. If a Regulatory Authority desires to conduct an inspection or audit of a Party’s facility or a facility under contract with such Party with regard to the Product, then the audited Party shall notify the other Party as soon as practicably possible after receipt of such notification of such audit or inspection and provide copies of any materials provided to it by the applicable Regulatory Authority; provided, that the audited Party shall not be required to notify the other Party of audits or inspections that are of a routine nature or that do not relate to the Product, except where such audits result in communications or actions of such Regulatory Authority which have an impact upon the Product. In addition, if a Regulatory Authority conducts an unannounced inspection or audit of a Party’s facility or a facility under contract with such Party with regard to the Product, then the audited Party shall notify the other Party within twenty-four (24) hours of commencement of such audit or inspection. The audited Party shall cooperate, and shall use reasonable efforts to cause the contract facility to cooperate, with such Regulatory Authority and the other Party during such inspection or audit. Following receipt of the inspection or audit observations of such Regulatory Authority (a copy of which the audited Party will immediately provide to the other Party), the audited Party will also provide the other Party with copies of any written communications received from Regulatory Authorities with respect to such facilities in a timely manner after receipt, to the extent such written communications relate to the Product or the Manufacture thereof, and will prepare the response to any such observations. The audited Party will provide the other Party with a copy of any proposed response to such communications and will implement such other Party’s reasonable comments with respect to such proposed response. The audited Party agrees to conform its activities under this Agreement to any commitments made in such a response.
ARTICLE 6
COMMERCIALIZATION
6.1 Overview of Commercialization in the Licensed Territory. Subject to the other terms and conditions of this Article 6, as between the Parties, CTI control all aspects of the Commercialization of the Product in the Field in the Licensed Territory, including, without limitation: (a) developing and executing a commercial launch and pre-launch plan; (b) marketing
32
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
and promotion; (c) booking sales and distribution and performance of related services; (d) handling all aspects of order processing, invoicing and collection, inventory and receivables; (e) publications; (f) providing customer support, including handling medical queries, and performing other related functions; and (g) conforming its practices and procedures in all material respects to the applicable Laws relating to the marketing, detailing and promotion of the Products in the Field in the countries of the Licensed Territory. Except as otherwise provided in this Article 6, CTI shall bear all of the costs and expenses incurred in connection with all such Commercialization activities. For clarity, it is understood that CTI would be not responsible for any Commercialization of the Product in the ROW Territory, and as between the Parties, all costs and expenses thereof shall be borne by Chroma and the responsibility therefore shall be Chroma’s.
6.2 Sales and Marketing Plan. Each Party will Commercialize the Products in its applicable territory pursuant to a detailed plan prepared by such Party and submitted to the JDC for review and comment (the “Sales and Marketing Plans”), but for clarity, not approval or consent. The Sales and Marketing Plans will include information, budgets and timelines regarding the applicable Party’s Commercialization activities, including Product life cycle management, market research, sales training, distribution channels, customer service and sales force matters related to the launch and sale of the Product in the Field and in the applicable territory. The initial Sales and Marketing Plan for each Party shall be delivered to the JDC not later than three (3) months prior to the planned date for NDA submission to the FDA. On at least an annual basis (no later than March 1 of each calendar year thereafter), each Party shall update and amend, as appropriate, its then-current Sales and Marketing Plan. Each Party shall submit all updates and amendments to the Sales and Marketing Plan to the JDC for review and comment. The Parties agree that, as between the Parties (a) CTI will have planning, oversight, and final decision making authority (as provided expressly in Section 14.2) and responsibility for all sales, marketing, and promotional activities related to the Product in the Licensed Territory, and (b) Chroma will have planning, oversight, and final decision making authority (as provided expressly in Section 14.2) and responsibility for all sales, marketing, and promotional activities related to the Product in the ROW Territory, as expressly described in this Agreement. Each Party will have the opportunity, through the JDC, to confer with the other Party on such sales, marketing and promotional matters.
6.3 Pricing; Reimbursement.
(a) As between the Parties, CTI shall have the sole right to determine all pricing of the Product in the Licensed Territory. Notwithstanding anything in this Agreement express or implied to the contrary, Chroma shall not have any right to direct, control, or approve CTI’s pricing of Products for the Licensed Territory.
(b) As between the Parties, CTI shall have the sole right to determine the reimbursement strategy for the Product in the Licensed Territory. Chroma shall confer with CTI on the development of the reimbursement strategy for the Product for the initial indication in the
33
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Licensed Territory in order to share the research and planning information relating to reimbursement processes already developed by Chroma.
(c) Chroma shall have the sole right to determine all pricing and reimbursement of the Product in the ROW Territory.
6.4 Commercial Diligence. CTI shall devote Diligent Efforts to Commercialize the Product and to maximize Net Sales of the Product, subject to compliance with applicable Laws, throughout the Licensed Territory following receipt of Regulatory Approval, including price approval, of such Product in accordance with this Agreement. CTI will launch the Product in the United States within ninety (90) days of receiving Regulatory Approval, including price approval, for the Product in the United States.
6.5 Compliance.
(a) Licensed Territory. CTI, its Affiliates and sublicensees shall comply with all applicable Laws and guidelines in the Licensed Territory applicable to the Commercialization of pharmaceutical products.
(b) ROW Territory. Chroma, its Affiliates and sublicensees shall comply with all applicable Laws and guidelines in the ROW Territory applicable to the Commercialization of pharmaceutical products.
(c) No Obligation. No Party shall be required to take any action, undertake any obligation, or incur any cost or reimbursement obligation, in connection with any activity under this Agreement that such Party believes, in good faith, may violate any applicable Laws.
6.6 Trademark Matters.
(a) Product Mark. It is the intention of the Parties that the Product be sold and marketed under a single worldwide brand, where practicable, legally and commercially viable, in accordance with the global branding strategy to be established by the JDC under Section 3.2(a)(v). Notwithstanding the foregoing, the Parties acknowledge such a single brand approach may not be viable for some countries. Unless determined otherwise by the JDC in the global branding strategy, as between the Parties, CTI will own the trademarks for the names of the Product to be used in the Licensed Territory and Chroma will own the trademarks for the names of the Products to be used in the ROW Territory (each a “Product Mark”), provided that in no event shall either Party be permitted to use the name of the other Party or any brand, logo or trademark of the other Party not solely used for the Product without such Party’s express prior written consent. Each Party will be responsible at its own expense to perform any trademark clearance or registration for any Product Mark in its respective territory.
(b) Marks. Each Party shall provide the other with samples of any materials that incorporate the Product Marks prior to distributing such materials for use. Each Party acknowledges the other’s exclusive ownership of the Product Marks in its respective territory and
34
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
agrees not to take any action inconsistent with such ownership, and any goodwill arising from the use of the Product Marks shall inure to the benefit of the owning Party. Neither Party shall use any Product Mark in a way that would adversely affect its value. Each Party covenants that it shall not use any trademark confusingly similar to or containing any Product Mark in connection with any products (including the Product).
6.7 Publication.
(a) Strategy. The Publication Team, established pursuant to Section 6.7(b), in close cooperation with the JDC will agree in advance on any publication of study results from the Currently Ongoing Studies, any Additional Studies, Licensed Territory Specific Studies or Extraterritorial Studies and prepare a joint publication strategy and publication plan, subject to the provisions of this Section 6.7.
(b) Publication Team. The ESC shall establish a Publication Team composed of an equal number of representatives from each of CTI and Chroma with the reasonable balance of medical and scientific qualifications to agree on the detailed planning of the publication strategy and publication plan detailing the intended timing, venue, media, authors etc. for all publications of matters covered under this Section 6.7 including scientific articles, abstracts and posters intended for presentation at congresses. All health economic publications and communication at any congress shall be an integrated part of the publication strategy and plan. The Publication Team shall be co-chaired by representatives from both Parties and shall convene as deemed necessary to accomplish the above. In case of any dispute concerning publication the matter shall be referred to the ESC for resolution; provided, however, any such resolution shall be consistent with the guidelines set forth in Section 6.7(d).
(c) Publication Review. Except for disclosures permitted pursuant to Section 12.1 and consistent with the mutually agreed publication strategy pursuant to Section 6.7(a), either Party, its employees or consultants wishing to make a publication of matters covered under this Section 6.7 shall deliver to the other Party a copy of the proposed written publication or an outline of an oral disclosure at least sixty (60) days (or earlier if reasonably practicable, or within forty-five (45) days with respect to agreements existing as of the Effective Date that Chroma has with a Third Party performing clinical trials, research or the like in connection with such publication) prior to submission for publication or presentation. The reviewing Party shall have the right (a) to propose modifications to the publication or presentation for patent reasons or trade secret reasons and/or (b) to request a reasonable delay in publication or presentation in order to protect patentable information. If the reviewing Party requests a delay, the Parties shall in good faith discuss and agree on the timing of such publication and if the Parties cannot agree the publishing Party shall delay submission or presentation for a period of sixty (60) days (or forty-five (45) days with respect to agreements existing as of the Effective Date that Chroma has with a Third Party performing clinical trials, research or the like in connection with such publication) to enable patent applications protecting each Party’s rights in such information to be filed in accordance with Article 9 below. Upon expiration of such sixty (60) days (or forty-five (45) days as provided above), the publishing Party shall be free to proceed with the
35
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
publication or presentation. If the reviewing Party requests modifications to the publication or presentation, the publishing Party shall edit such publication to prevent disclosure of trade secret or proprietary business information prior to submission of the publication or presentation.
(d) Standards. All publications of matters covered under this Section 6.7 shall be prepared, presented and/or published in accordance with pharmaceutical industry accepted guidelines including, but not limited to: (i) International Committee of Medical Journal Editors (ICMJE) guidelines, (ii) Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication, (iii) Pharmaceutical Research and Manufacturers of America (PhRMA) guidelines, and (iv) PhRMA Principles on Conduct of Clinical Trials and Communication of Clinical Trial Results.
6.8 International Meetings. The Parties shall consult with each other and coordinate their attendance at meetings, symposia, conferences and the like (“Meetings”) with regard to the Product. CTI will Take the Lead with regard to any Meetings with regard to the Product which are held in the Licensed Territory and Chroma will Take the Lead with regard to any Meetings with regard to the Product which are held in the ROW Territory.
6.9 Key Opinion Leaders. Each Party shall coordinate its activities with the other Party with regard to interactions with key opinion leaders in the Field related to the Product provided that neither Party shall be restricted from approaching or otherwise dealing with any particular key opinion leader.
ARTICLE 7
MANUFACTURE AND SUPPLY
7.1 General Supply Terms. As between the Parties Chroma will be solely responsible for the Manufacture of the Product for Development purposes in both the Licensed Territory and the ROW Territory in accordance with the terms of the Supply Agreement. As between the Parties, CTI will be solely responsible, by itself or through one or more Third Party contract manufacturers, for the Manufacture of the Product for Commercialization in the Licensed Territory. As between the Parties, Chroma will be solely responsible for the Manufacture of the Product for Commercialization in the ROW Territory.
7.2 Supply Agreement. The Parties shall decide, through the JDC, and as reflected in the terms of the Supply Agreement, a mutually acceptable supply chain for the manufacture of the Product for use in clinical trials, including which Party will be responsible for oversight, negotiations and management of Third Party vendors responsible for manufacturing the Product; provided, however, that neither Party will be obligated to use the same supply chain or to enter into joint contractual arrangements with one another or Third Parties relating to the manufacture of the Product. On or before a date to be established by the ESC but in no event later than ninety (90) days after the Effective Date, the Parties shall enter into a supply agreement governing the supply of Product to CTI for Development purposes (the “Supply Agreement”) and (b) a quality agreement governing the quality control, quality assurance and validation of such Product (the
36
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
“Quality Agreement”). The terms of such Supply Agreement and such Quality Agreement shall be negotiated in good faith by the Parties and will contain customary terms and conditions that are consistent with this Agreement. The purchase price for the Product under the Supply Agreements will be Chroma’s cost of manufacture and such purchase price will be considered part of the Development Costs. In the event that CTI wishes to have Chroma supply the Product for Commercialization purposes, the initial purchase price for such supply shall not exceed Chroma’s cost of manufacture plus **. The Supply Agreement will provide appropriate technology transfer provisions (including provision of DMFs and other customary terms in the industry) sufficient to enable CTI to Manufacture the Product for Commercialization in the Licensed Territory in advance of any anticipated Product launch in the Licensed Territory. The Supply Agreement will provide that such technology transfer may be initiated by CTI at any time during the term of the Supply Agreement at CTI’s option by written notice and that Chroma will be obligated to provide such technology transfer. The Supply Agreement will also provide appropriate provisions to address supply related issues post termination under each of the termination scenarios addressed in Sections 13.7 and 13.8.
ARTICLE 8
COMPENSATION
8.1 Upfront Fee. Upon the date this Agreement is signed by both Parties, CTI shall pay to Chroma a one-time non-creditable, non-refundable (subject to the provisions of Section 15.9) upfront fee of $5,000,000.
8.2 Development Milestone Payments. CTI shall make non-creditable, non refundable milestone payments to Chroma based on achievement of certain milestone events for the Product as set forth in this Section 8.2. CTI shall pay to Chroma the amounts set forth below within thirty (30) days after receipt of Chroma’s invoice following the achievement of the corresponding milestone event. Each milestone payment by CTI to Chroma hereunder shall be payable only once, regardless of the number of times achieved by the Products, and shall not be cumulative.
| Milestone Event |
Milestone Payment | |||
| Indication: Acute Myelogenous Leukemia (AML) Milestone Payments | ||||
| Upon Initiation of the first pivotal study (Phase 2 Clinical Trial and/or Phase 3 Clinical Trial) for AML, as set forth in the Development Plan |
$ | 5,000,000 | ||
37
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Upon first successful completion of a first pivotal study (Phase 2 Clinical Trial and/or Phase 3 Clinical Trial) for AML, as set forth in the Development Plan (measured by FDA acceptance to file NDA for AML) |
$ | * | * | |
| Within 90 days of first Regulatory Approval of an NDA by the FDA in r/r AML |
$ | * | * | |
| Upon first Regulatory Approval of an NDA by the FDA in First Line AML |
$ | * | * | |
| Indication: Myelodysplastic Syndromes (MDS) Milestone Payments | ||||
| Upon first successful randomized Phase 2 Clinical Trial safety/efficacy results in MDS, as set forth in the Development Plan |
$ | * | * | |
| Upon first successful completion of Phase 3 Clinical Trial in MDS, as set forth in the Development Plan (measured by FDA acceptance to file NDA for MDS) |
$ | * | * | |
| Upon first Regulatory Approval by the FDA of an NDA in r/r MDS |
$ | * | * | |
| Upon first Regulatory Approval by the FDA of an NDA in First Line MDS |
$ | * | * | |
| Indication: Multiple Myeloma (MM) Milestone Payments | ||||
| Upon first successful Phase 2 Clinical Trial safety/efficacy results in MM, as set forth in the Development Plan |
$ | * | * | |
| Upon first successful completion of Phase 3 Clinical Trial in MM, as set forth in the Development Plan (measured by FDA acceptance to file NDA for MM) |
$ | * | * | |
| Upon first Regulatory Approval by the FDA of an NDA in r/r MM |
$ | * | * | |
| Upon first Regulatory Approval by the FDA of an NDA in First Line MM |
$ | * | * | |
| Indication: Solid Tumor Milestone Payments |
38
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Upon first successful randomized Phase 2 Clinical Trial safety/efficacy results for solid tumors, as set forth in the Development Plan |
$ | * | * | |
| Upon first successful completion of Phase 3 Clinical Trial for solid tumors, as set forth in the Development Plan (measured by FDA acceptance to file NDA for the relevant solid tumor indication) |
$ | * | * | |
| First Regulatory Approval by the FDA of an NDA in r/r solid tumors affecting <10,000 pts/yr, as per the FDA approved label indication |
$ | * | * | |
| First Regulatory Approval by the FDA of an NDA in First Line solid tumors affecting > 10,000 <50,000 pts/yr, as per the per FDA approved label indication |
$ | * | * | |
| First Regulatory Approval by the FDA of an NDA in First Line solid tumors affecting > 50,000 pts/yr, as per the per FDA approved label indication |
$ | * | * |
For the avoidance of doubt, “acceptance” as used above shall mean acceptance for review of the applicable regulatory filing by the FDA, and “successful safety/efficacy results” means meeting the primary endpoint(s) of the applicable protocol as set forth in the final analysis plan submitted to the applicable Regulatory Authority.
If upon achievement of any development milestone for each indication described above, a previous development milestone for that indication has not been paid, then each such previous development milestone for that indication shall be payable along with the payment for the milestone then achieved for such indication.
CTI will provide written notice to Chroma of the achievement of any milestone above within thirty (30) calendar days of such achievement.
8.3 Sales Milestones. CTI shall make the following one-time, milestone payments to Chroma. Each milestone payment by CTI to Chroma hereunder shall be payable only once, regardless of the number of times achieved by the Products, and shall not be cumulative except for last milestone which is payable on cumulative Net Sales.
39
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Milestone Event |
Milestone Payment | |||
| Upon the first achievement of annual Net Sales in the Licensed Territory of $75,000,000 (“Sales Event 1”). |
$ | * | * | |
| Upon the first achievement of annual Net Sales in the Licensed Territory of $150,000,000 (“Sales Event 2”). |
$ | * | * | |
| Upon the first achievement of cumulative Net Sales in the Licensed Territory of $250,000,000 (“Sales Event 3”). |
$ | * | * | |
If Sales Event 1 and Sales Event 2 are achieved in a single calendar year, milestone payments for each of such events will be payable in that same calendar year. However, if Sales Event 2 and Sales Event 3 are achieved in a single calendar year, only the milestone payment for Sales Event 3 will be due in that same calendar year and the milestone payment for Sales Event 2 in such case will not be due until 1 January in the next calendar year.
CTI shall provide written notice to Chroma of the achievement of any sales milestone above within forty-five (45) calendar days after the end of the calendar quarter in which such sales milestone was achieved. Except as otherwise provided above in this Section 8.3, each sales milestone payment will be due within thirty (30) days of the date of Chroma’s invoice therefor. Each milestone payment shall be non-refundable and non-creditable.
8.4 Royalties.
(a) Royalty Rates for Licensed Territory. Subject to Sections 8.4(b), 8.4(c) and 8.4(d) below, and during the applicable Royalty Term, CTI shall pay to Chroma a running royalty at the following incremental royalty rates, on aggregate, annual Net Sales of the Products in the Licensed Territory.
| Net Sales in the Licensed Territory |
Royalty Rate | |||
| For that portion of annual Net Sales less than or equal to $150,000,000 |
* | *% | ||
| For that portion of annual Net Sales greater than $150,000,000 but less than or equal to $350,000,000 |
* | *% | ||
| For that portion of annual Net Sales greater than $350,000,000 |
* | *% | ||
40
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(b) Single Royalty. Royalties payable under Section 8.4(a) will be payable only once with respect to a particular unit of the Product and will be paid only once regardless of the number of Chroma Patents applicable to such Product.
(c) Reduction of Royalty Following Entry of Generic Product. Following (i) (1) expiration of the last-to-expire Chroma Patent or (2) any lack of protection or enforcement of all non-expired Chroma Patents (as provided in Article 9) covering the Development, Manufacture, use or sale of Products in a country of the Licensed Territory (including any Patent Term Extensions thereof) or (ii) expiration of all Regulatory Exclusivity periods with respect to the Product in such country, the royalty rates set forth in Section 8.4(a) for Net Sales during the remainder of the Royalty Term in such country shall be reduced by ** at the end of the first to occur six (6) month period during which one or more Third Parties sells a number of equivalent units of a Generic Version of the Product in such country of the Licensed Territory comprising, during such time period, ** or more of the aggregate combined number of equivalent units of such Product and such Generic Version(s) of such Product sold in such time period in such country (“the Threshold”), provided that in no event shall the royalty rates set forth in Section 8.4(a) be reduced below a figure which is the sum of ** and the applicable royalty payable by Chroma to Vernalis under the Vernalis Agreement for any such period during the Royalty Term. All such determinations of unit volume shall be based upon a mutually acceptable calculation method and using market share data provided by a reputable and mutually agreed upon provider, such as IMS Health. As used in this Section 8.4(c), “Generic Version” means, with respect to the Product, a product sold by a Third Party that (A) contains a Compound as an active ingredient, and (B) has been approved for sales introduction into interstate commerce by reference to the Product pursuant to (1) Section 505(b)(2) or Section 505(j) of the United States Food, Drug, and Cosmetic Act (21 U.S.C. 355(b)(2) and 21 U.S.C. 355(j), respectively), or (2) any similar approval in any other country of the Licensed Territory, which similar approval is based on reference to the Regulatory Approval for the Product in such country and a demonstration of bio-equivalence or similarity to the Product. At any time following introduction of the initial Generic Version of the Product in a country achieving a market share of at least the Threshold and upon written notice of CTI expressing concern regarding the continued commercial viability of the Product in such country, the Parties agree to negotiate in good faith a mutually acceptable strategy to address CTI’s concerns with a view toward preserving the historic economic balance and profit splits between the Parties.
(d) Third Party Royalties.
(i) During the Term, Chroma shall remain responsible for the payment of royalties and other payment obligations, if any, due to Third Parties under any Chroma Technology which has been licensed to Chroma and is sublicensed to CTI hereunder, including without limitation any payments due under the Upstream Agreements.
(ii) Except as set forth in clause (i) or (iii) of this Section 8.4 (d), or to the extent of any Claim for which Chroma provides indemnification under Section 11.1, or as the Parties may otherwise agree in writing, CTI shall bear any payments associated with any
41
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
royalties owed to any Third Party for intellectual property that is necessary or useful for the Development, Manufacture, storage, handling, use, promotion, sale, offer for sale, importation or other Commercialization of Products for sale in the Licensed Territory (the “Third Party Royalties”).
(iii) During the Term, if CTI determines, in good faith, that it is necessary to seek or exercise any license from any Third Party for intellectual property that is necessary for the Development, Manufacture, storage, handling, use, promotion, sale, offer for sale, importation of other Commercialization of Products for sale in the Licensed Territory, CTI may credit up to ** of the amount of any Third Party Royalties paid by CTI for Third Party intellectual property pursuant to clause (ii) above against royalties payable to Chroma under Section 8.4(a) provided that in no event shall the royalty rates set forth in Section 8.4(a) be reduced below the sum of ** and the applicable royalty rate payable by Chroma to Vernalis under the Vernalis Agreement. CTI may take such credit during any calendar quarter for which royalties are payable hereunder; provided, that in no event will such credit reduce the royalties payable to Chroma for such calendar quarter by more than **. Any share of such Third Party Royalties that remains uncredited due to the application of such floor may be carried forward to subsequent calendar quarters.
8.5 No Projections. Chroma and CTI acknowledge and agree that nothing in this Agreement shall be construed as representing an estimate or projection of anticipated sales of any Product, and that the milestones and Net Sales levels set forth in Sections 8.3 and 8.4 or elsewhere in this Agreement or that have otherwise been discussed by the Parties are merely intended to define the milestone payments and royalty obligations to Chroma in the event such milestones and/or Net Sales levels are achieved.
8.6 Royalty Reports and Payment. Within forty five (45) calendar days following the end of each calendar quarter during the Term, CTI shall provide Chroma with a report containing the following information for the applicable calendar quarter: the amount of gross sales of Product on a country-by-country basis in the Licensed Territory, an itemized calculation of Net Sales in the Licensed Territory showing deductions, to the extent practicable, provided for in the definition of “Net Sales,” a calculation of the royalty payment due on such sales, an accounting of the number of units and prices for Product sold, the exchange rate for each country in which Product was sold, the application of the reductions, if any, made in accordance with the terms of Section 8.4(c) or Section 8.4(d), and any other information reasonably required by Chroma for the purpose of calculating royalties and other amounts due under this Agreement. Any royalty payments due to Chroma will be paid on the date of delivery of such report. In the event that either party determines that the calculation of Net Sales for a calendar quarter deviates from the amounts previously reported to Chroma for any reason (such as, on account of additional amounts collected or Product returns), CTI and Chroma shall reasonably cooperate to reconcile any such deviations to the extent necessary under applicable legal or financial reporting requirements.
42
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
8.7 Foreign Exchange. The rate of exchange to be used in computing the amount of currency equivalent in Dollars owed to a Party under this Agreement shall be the monthly average exchange rate between each currency of origin and U.S. Dollars as reported by Bloomberg or an equivalent resource as agreed by the Parties.
8.8 Payment Method; Late Payments. All payments due to Chroma under Article 8 shall be made in U.S. Dollars by wire transfer of immediately available funds into an account designated by Chroma. If Chroma does not receive payment of any sum due to it under Article 8 on or before the due date, simple interest shall thereafter accrue on the sum due to Chroma until the date of payment at the per annum rate of two percent (2%) over the then-current prime rate quoted by Citibank in New York City or the maximum rate allowable by applicable Law, whichever is lower.
8.9 Records; Audits. CTI will maintain complete and accurate records in sufficient detail to permit Chroma to confirm the accuracy of the calculation of royalty and milestone payments under this Article 8. Upon reasonable prior notice, such records shall be available during regular business hours for a period of four (4) years from the end of the calendar year to which they pertain for examination at the expense of Chroma, and not more often than once each calendar year, by an independent certified public accountant selected by Chroma and reasonably acceptable to CTI, for the sole purpose of verifying the accuracy of the financial reports furnished by CTI pursuant to this Article 8. Any such auditor shall not disclose CTI’s Confidential Information. Any amounts shown to be owed but unpaid shall be paid within thirty (30) days from the accountant’s report, plus interest (as set forth in Section 8.8) from the original due date. Any amounts shown to have been overpaid shall be refunded within thirty (30) days from the accountant’s report. Chroma shall bear the full cost of such audit unless such audit discloses an underpayment by CTI of more than ** of the amount due, in which case CTI shall bear the full cost of such audit.
8.10 Taxes.
(a) Cooperation and Coordination. The Parties acknowledge and agree that it is their mutual objective and intent to appropriately calculate, to the extent feasible and legal, taxes payable with respect to their collaborative efforts under this Agreement and that they shall use all commercially reasonable efforts to cooperate and coordinate with each other to achieve such objective. Without limiting the foregoing, Chroma agrees to provide to CTI reasonable assistance and information regarding any Value Added Tax assessments and requirements.
(b) Payment of Tax. A Party receiving a payment pursuant to this Article 8 shall pay any and all taxes levied on such payment. If applicable Laws require that taxes be deducted and withheld from a payment made pursuant to this Article 8, the remitting Party shall (i) deduct those taxes from the payment; (ii) pay the taxes to the proper taxing authority; and (iii) send evidence of the obligation together with proof of payment to the other Party within sixty (60) days following that payment. The paying Party shall cooperate with and provide reasonable assistance to the receiving Party to prevent the application of any withholding, to obtain the
43
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
benefit of any double taxation treaty or to obtain repayment of any tax which is required to be withheld.
(c) Tax Residence Certificate. A Party (including any entity to which this Agreement may be assigned, as permitted under Section 15.5) receiving a payment pursuant to this Article 8 shall provide the remitting Party appropriate certification from relevant revenue authorities that such Party is a tax resident of that jurisdiction, if such receiving Party wishes to claim the benefits of an income tax treaty to which that jurisdiction is a party. Upon the receipt thereof, any deduction and withholding of taxes shall be made at the appropriate treaty tax rate.
(d) Assessment. Either Party may, at its own expense, protest any assessment, proposed assessment, or other claim by any Governmental Authority for any additional amount of taxes, interest or penalties or seek a refund of such amounts paid if permitted to do so by applicable Law. The Parties shall cooperate with each other in any protest by providing records and such additional information as may reasonably be necessary for a Party to pursue such protest.
ARTICLE 9
INTELLECTUAL PROPERTY MATTERS
9.1 Ownership of Inventions. Each Party shall own any inventions made and funded solely by its or its Affiliates own employees, agents, or independent contractors in the course of conducting its activities under this Agreement, together with all intellectual property rights therein (“Sole Inventions”). The Parties shall jointly own any inventions that are (a) made jointly by employees, agents, or independent contractors of each Party or their Affiliates or (b) jointly funded by the Parties (including as a result of any Development activities jointly funded by the Parties pursuant to Article 4) in the course of performing activities under this Agreement, together with all intellectual property rights therein (“Joint Inventions”). Inventorship shall be determined in accordance with U.S. patent laws. Subject to the terms of this Agreement (including without limitation the licenses granted in Article 2), each Party may use and practice the Joint Inventions for any purpose and may assign, license or otherwise transfer or exploit its rights to the Joint Inventions to an Affiliate or Third Party, without the other Party’s consent and without a duty of accounting to the other Party. For the avoidance of doubt, Chroma’s interest in the Joint Inventions (including its interest in the Joint Patents) will be exclusively licensed to CTI under the same terms the licenses granted in Section 2.1 but will not be subject to royalty payments set forth in Section 8.4. CTI grants to Chroma an exclusive, royalty free, sublicensable (in accordance with the terms of this Agreement) license under CTI’s interest in any Joint Invention to Develop, Manufacture, use, make, have made, sell, offer for sale, have sold, distribute, import and otherwise Commercialize the Product in the Field in the ROW Territory.
9.2 Right of First Negotiation and Refusal. With respect to CTI’s Sole Inventions necessary or useful in the Development, Manufacture or Commercialization of the Product in the Field in the ROW Territory (“Subject CTI Rights”), Chroma has the following right of first negotiation and right of first refusal during the Term, subject to any rights CTI has already
44
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
granted to Third Parties as of the Effective Date with respect to Patents and Know-How Controlled by CTI.
(a) CTI shall not propose, negotiate or enter into a contract or arrangement with any Third Party to license or otherwise dispose of the Subject CTI Rights, unless CTI has first notified Chroma in writing (“Initial Notice”) of its intent to license or otherwise dispose of the Subject CTI Rights (“Applicable Transaction”). The Initial Notice shall provide the material commercially reasonable terms for the Applicable Transaction. Chroma will have ** days from the date of the Initial Notice (“Initial Period”) to notify CTI whether Chroma wishes to engage in negotiations for the Applicable Transaction. If Chroma elects to proceed with such discussions, Chroma shall so notify CTI in writing and the Parties will use good faith efforts to enter into the Applicable Transaction within ** days from the date of Chroma’s notice (“Negotiation Period”). If Chroma notifies CTI in writing that it does not wish to enter into the Applicable Transaction or if CTI does not receive a written notice from Chroma at the end of the Initial Period or if the Parties cannot execute a definitive agreement for the Applicable Transaction by the end of the Negotiation Period, CTI will be free to pursue the Applicable Transaction with any Third Party, subject to Chroma’s right of first refusal below.
(b) Prior to consummating any Applicable Transaction with any Third Party after Chroma’s right of first negotiation provided in Section (a) above has ceased, CTI shall provide Chroma written notice of the proposed material terms in any proposed Applicable Transaction with such Third Party (“ROFR Notice”). If Chroma notifies CTI within ** business days of the date of the ROFR Notice (“ROFR Notice Period”) that Chroma wishes to consummate the Applicable Transaction described in the ROFR Notice, then CTI and Chroma shall enter into a definitive agreement including the material terms described in the ROFR Notice for the proposed Applicable Transaction in a period of no greater than ** days. Subsequent to such ** day time period, or if Chroma does not notify CTI in writing that it wishes to enter into the Applicable Transaction described in the ROFR Notice within the ROFR Notice Period, or if Chroma rejects the Applicable Transaction described the ROFR Notice, then CTI may enter into negotiations and/or an agreement regarding the Applicable Transaction as described in the ROFR Notice with the Third Party without further obligations to Chroma.
9.3 Disclosure of Inventions. Each Party shall promptly disclose to the other any invention disclosures, or other similar documents, submitted to it by its employees, agents or independent contractors describing inventions that are either Sole Inventions or Joint Inventions, and all information relating to such inventions to the extent necessary for the preparation, filing and maintenance of any Patent with respect to such invention.
9.4 Prosecution of Patents.
(a) Chroma Patents. The Chroma Patents in existence as of the Effective Date are listed in Exhibit B-1 hereto. The Parties shall update such Exhibit as appropriate (and at least once per calendar quarter) to add to Exhibit B-1 each Patent filed after the Effective Date by Chroma or its Affiliates (or any applicable licensor) which would constitute an Chroma Patent
45
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
hereunder. Except as otherwise provided in this Section (a), as between the Parties, Chroma shall have the sole right and authority to prepare, file, prosecute and maintain the Chroma Patents on a worldwide basis. Chroma shall bear all costs of preparation, filing, prosecution and maintenance of Chroma Patents. Chroma shall provide CTI reasonable opportunity to review and comment on such efforts regarding such Chroma Patents, including by providing CTI with a copy of material communications from any patent authority regarding such Chroma Patent, and by providing drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses. If Chroma determines in its sole discretion to abandon or not maintain any Chroma Patent(s) that is being prosecuted or maintained by Chroma, then Chroma shall provide CTI with written notice of such determination within a period of time reasonably necessary to allow CTI to determine its interest in such Chroma Patent(s). In the event CTI provides written notice expressing its interest in obtaining such Chroma Patent(s), Chroma shall free of charge assign and transfer to CTI the ownership of, and interest in, such Chroma Patent(s), at CTI’s sole expense, and Chroma shall cooperate with CTI for assignment and transfer of such Chroma Patent(s) at CTI’s sole expense. CTI shall thereafter bear all costs of preparation, filing, prosecution and maintenance of such assigned and transferred Patents. In the event that CTI decides to abandon or not maintain any such Patent(s), CTI shall promptly provide Chroma with written notice of such decision. Chroma shall only be obliged to comply with the provisions of this Section 9.4(a) and 9.4(c) below with respect to any Chroma Patent which is licensed to Chroma under the Vernalis Agreement to the extent that Chroma is not prohibited to do so pursuant to the terms of the Vernalis Agreement, provided that in any event Chroma shall use commercially reasonable efforts to cause Vernalis to comply with these provisions. If, despite Chroma’s commercially reasonable efforts, Chroma is not able to cause Vernalis to so comply, CTI’s commercial diligence obligations under Section 6.4 shall be equitably adjusted in light of such lack of compliance.
(b) Joint Patents. Except as otherwise provided in this Section 9.4(b), CTI shall have the primary right and authority to prepare, file, prosecute and maintain the Patents filed in relation to the Joint Inventions (“Joint Patents”) on a worldwide basis. Chroma and CTI shall share equally all reasonable costs of preparation, filing, prosecution and maintenance of the Joint Patents. CTI shall provide Chroma with the reasonable opportunity (and unless necessary to avoid a material adverse impact on such Joint Patents, at least thirty (30) days) to review and comment on such efforts regarding such Joint Patent, including by providing Chroma with a copy of material communications from any patent authority in such country(ies) regarding such Joint Patent, and by providing drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses, and CTI shall give due consideration to any reasonable comments made by Chroma. If CTI determines in its sole discretion to abandon or not maintain any Joint Patent(s) in any country(ies) of the world, then CTI shall provide Chroma with written notice of such determination within a period of time reasonably necessary to allow Chroma to determine its interest in such Joint Patent(s). In the event Chroma provides written notice expressing its interest in obtaining such Joint Patent(s), CTI shall free of charge assign and transfer to Chroma the ownership of, and interest in, such Joint Patent(s) in such country(ies), at Chroma’s sole expense, and CTI shall cooperate with Chroma with regard to the assignment and transfer of such Joint Patent(s) in such country.
46
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(c) Cooperation in Prosecution. Each Party shall provide the other Party all reasonable assistance and cooperation in the Patent prosecution efforts provided above in this Section 9.4, including providing any necessary powers of attorney and executing any other required documents or instruments for such prosecution, as well as further actions as set forth below.
(i) The Parties shall respectively prepare, file, maintain and prosecute the Chroma Patents and Joint Patents as set forth in this Section 9.4. As used herein, “prosecution” of such Patents shall include, without limitation, all communication and other interaction with any patent office or patent authority having jurisdiction over a patent application in connection with pre-grant proceedings. Post-grant proceedings shall be governed by Section 9.9(b).
(ii) All communications between the Parties relating to the preparation, filing, prosecution or maintenance of the Chroma Patents and Joint Patents, including copies of any draft or final documents or any communications received from or sent to patent offices or patenting authorities with respect to such Patents, shall be considered Confidential Information and subject to the confidentiality provisions of Article 12.
9.5 Patent Term Extensions. The ESC will discuss and recommend for which, if any, of the Patents within the Chroma Patents and Joint Patents the Parties should seek Patent Term Extensions. Chroma, in the case of the Chroma Patents, and CTI in the case of the Joint Patents, shall have the final decision-making authority with respect to applying for any such Patent Term Extensions, and will act with reasonable promptness in light of the development stage of Products to apply for any such Patent Term Extensions, where it so elects; provided, however, that if in a particular country or jurisdiction only one such Patent can obtain a Patent Term Extension, then the Parties will consult in good faith to determine which such Patent should be the subject of efforts to obtain a Patent Term Extension, and in any event CTI’s decision on such matter will control in the case of a disagreement with regard to the Licensed Territory and Chroma’s decision on such matters will control in the case of disagreement with regard to the ROW Territory. The Party that does not apply for an extension hereunder will cooperate fully with the other Party in making such filings or actions, for example and without limitation, making available all required regulatory data and information and executing any required authorizations to apply for such Patent Term Extension. All expenses incurred in connection with activities of each Party with respect to the Patent(s) for which such Party seeks Patent Term Extensions pursuant to this Section 9.5 shall be entirely borne by such Party. Chroma shall only be obliged to comply with the provisions of this Section 9.5 with respect to any Chroma Patent which is licensed to Chroma under the Vernalis Agreement to the extent that Chroma is not prohibited to do so pursuant to the terms of the Vernalis Agreement, provided that in any event Chroma shall use commercially reasonable efforts to cause Vernalis to comply with these provisions. If, despite Chroma’s commercially reasonable efforts, Chroma is not able to cause Vernalis to so comply, CTI’s commercial diligence obligations under Section 6.4 shall be equitably adjusted in light of such lack of compliance. CTI’s rights under this Section 9.5 will
47
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
be correspondingly limited by the rights which Chroma is able to exercise pursuant to the Vernalis Agreement.
9.6 Infringement of Patents by Third Parties.
(a) Notification. Each Party shall promptly notify the other Party in writing of any existing or threatened infringement of the Chroma Patents or Joint Patents of which it becomes aware, and shall provide all evidence in such Party’s possession demonstrating such infringement.
(b) Infringement of Chroma Patents or Joint Patents in the Licensed Territory.
(i) If a Third Party infringes any Chroma Patent or Joint Patent in the Licensed Territory by making, using, importing, offering for sale or selling the Product or a competitive product (a “Product Infringement”), each Party shall share with the other Party all information available to it regarding such alleged infringement. CTI shall have the first right, but not the obligation, to bring an appropriate suit or other action against any person or entity engaged in such Product Infringement in the Licensed Territory, subject to Section 9.6(b)(ii) through 9.6(b)(v), below.
(ii) CTI shall have a period of thirty (30) days after the first notice under 9.6(a) to elect to enforce such Chroma Patent or Joint Patent against such Product Infringement. In the event CTI does not so elect, CTI shall so notify Chroma in writing, and Chroma shall have the right to commence a suit or take action to enforce the applicable Patent against such Third Party perpetrating such Product Infringement in the Licensed Territory at its own cost and expense. If one Party elects to bring suit or take action against the Product Infringement, then the other Party shall have the right, prior to commencement of the trial, suit or action, to join any such suit or action.
(iii) Each Party shall provide to the Party enforcing any such rights under this Section 9.6(b) reasonable assistance in such enforcement, at such enforcing Party’s request and expense, including joining such action as a party plaintiff if required by applicable Law to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, shall reasonably consider the other Party’s comments on any such efforts, and shall seek consent of the other Party in any important aspects of such enforcement including, without limitation, determination of litigation strategy, filing of important papers to the competent court, which shall not be unreasonably withheld or delayed.
(iv) Each Party shall bear all of its own internal costs incurred in connection with its activities under this Section 9.6(b); provided, that in the event that the Parties are joined in suit or action against the Product Infringement and represented by the same outside counsel, then the Parties shall share equally in the external costs and expenses for such action.
48
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(v) The Party not bringing an action with respect to Product Infringement in the Licensed Territory under this Section 9.6(b) shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the Party bringing such action.
(c) Settlement. Neither Party shall settle any claim, suit or action that it brought under this Section 9.6 involving Chroma Patents or Joint Patents without the prior written consent of the other Party, which consent shall not be unreasonably withheld or delayed.
(d) Allocation of Proceeds. If either Party recovers monetary damages from any Third Party in a suit or action brought under Sections 9.6(b) or 9.6(c), whether such damages result from the infringement of Chroma Patents or Joint Patents, such recovery shall be allocated first to the reimbursement of any expenses incurred by the Parties in such litigation and any remaining amounts shall be split as follows: such amounts shall be allocated ** to Chroma and ** to CTI.
(e) Upstream Agreements. Chroma shall only be obliged to comply with the provisions of this Section 9.6 with respect to any Chroma Patent which is licensed to Chroma under the Vernalis Agreement to the extent that Chroma is not prohibited to do so pursuant to the terms of the Vernalis Agreement, provided that in any event Chroma shall use commercially reasonable efforts to cause Vernalis to comply with these provisions. If, despite Chroma’s commercially reasonable efforts, Chroma is not able to cause Vernalis to so comply, CTI’s commercial diligence obligations under Section 6.4 shall be equitably adjusted in light of such lack of compliance. CTI’s rights under Section 9.6 will be correspondingly limited by the rights which Chroma is able to exercise pursuant to the Vernalis Agreement.
9.7 Infringement of Third Party Rights. If any Product Developed, Manufactured, made, have made, stored, handled, used, promoted, sold, offered for sale, imported or otherwise Commercialized by either Party, its Affiliates, licensees or sublicensees becomes the subject of a Third Party’s claim or assertion of infringement of a Patent granted by a jurisdiction within the Licensed Territory or the ROW Territory, the Party first having notice of the claim or assertion shall promptly notify the other Party, the Parties shall agree on and enter into an “identity of interest agreement” wherein such Parties agree to their shared, mutual interest in the outcome of such potential dispute, and thereafter, the Parties shall promptly meet to consider the claim or assertion and the appropriate course of action provided that each Party shall be entitled to defend any such Third Party claim or assertion made against it as it reasonably determines.
9.8 Patent Marking. CTI (or its Affiliate, sublicensee or distributor) shall mark Products marketed and sold by CTI (or its Affiliate, sublicensee or distributor) hereunder with appropriate patent numbers or indicia at Chroma’s reasonable request; provided, however, that CTI shall only be required to so mark such Products to the extent such markings or such notices would impact recoveries of damages or equitable remedies available under applicable Law with respect to infringements of Patents in the Licensed Territory.
9.9 Patent Oppositions and Other Proceedings.
49
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(a) Third-Party Patent Rights. If either Party desires to bring, anywhere in the world, an opposition, action for declaratory judgment, nullity action, interference, declaration for non-infringement, reexamination or other attack upon the validity, title or enforceability of a Patent owned or controlled by a Third Party and having one or more claims that covers the Product, or the use, sale, offer for sale or importation of the Product, such Party shall so notify the other Party and the Parties shall promptly confer to determine whether to bring such action or the manner in which to settle such action. CTI shall have the exclusive right, but not the obligation, to bring at its own expense and in its sole control such action in the Licensed Territory. If CTI does not bring such an action in the Licensed Territory, within ninety (90) days of notification thereof pursuant to this Section 9.9(a) (or earlier, if required by the nature of the proceeding), then Chroma shall have the right, but not the obligation, to bring, at Chroma’s sole expense, such action. The Party not bringing an action under this Section 9.9(a) shall be entitled to separate representation in such proceeding by counsel of its own choice and at its own expense, and shall cooperate fully with the Party bringing such action. Any awards or amounts received in bringing any such action shall be first allocated to reimburse the initiating Party’s expenses in such action, and any remaining amounts shall be retained by such Party.
(b) Parties’ Patent Rights. If any Chroma Patent or Joint Patent becomes the subject of any proceeding commenced by a Third Party within the Licensed Territory or the ROW Territory in connection with an opposition, reexamination request, action for declaratory judgment, nullity action, interference or other attack upon the validity, title or enforceability thereof (except insofar as such action is a counterclaim to or defense of, or accompanies a defense of, an action for infringement against a Third Party under Section 9.6, in which case the provisions of Section 9.6 shall govern), then the Party responsible for filing, preparing, prosecuting and maintaining such Patent as set forth in Section 9.4 hereof, shall control such defense at its own cost and expense. The controlling Party shall permit the non-controlling Party to participate in the proceeding to the extent permissible under applicable Law, and to be represented by its own counsel in such proceeding, at the non-controlling Party’s expense.
9.10 Vernalis Agreement. Within ** days after the execution of this Agreement, Chroma shall use commercially reasonable efforts to either (i) ** or (ii) amend the Vernalis Agreement to address issues related to **, in each case subject to CTI’s prior written approval, not to be unreasonably withheld, delayed or conditioned.
ARTICLE 10
REPRESENTATIONS AND WARRANTIES
10.1 Mutual Representations and Warranties. Each Party hereby represents, warrants, and covenants (as applicable) to the other Party as follows:
(a) Organization, Existence and Power. As of the Effective Date, it is a company or corporation duly organized, validly existing, and in good standing under the laws of the jurisdiction in which it is organized, and has full organizational (whether corporate or
50
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
otherwise) power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as contemplated in this Agreement.
(b) Authority and Binding Agreement. As of the Effective Date, (i) it has the organizational power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary organizational action on its part required to authorize the execution and delivery of the Agreement and the performance of its obligations hereunder; and (iii) the Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms.
(c) No Conflict; Covenant. It is not a party to any agreement that would prevent it from granting the rights granted to the other Party under this Agreement or performing its obligations under the Agreement.
(d) No Debarment. In the course of the development of Products, each Party shall not use, during the Term, any employee or consultant who has been debarred by any Regulatory Authority, or, to the best of such Party’s knowledge, is the subject of debarment proceedings by a Regulatory Authority.
10.2 Additional Representations and Warranties of Chroma. Chroma represents and warrants, on its behalf and on behalf of each of its Affiliates, to CTI as follows:
(a) Chroma Patents. Exhibits B-1 sets forth a complete and accurate list of all Chroma Patents in existence as of the Effective Date. Chroma is the exclusive licensee of those Chroma Patents listed on Exhibit B-1, free and clear of any liens, security interests, encumbrances (other than the security interest described on Exhibit B-1). To Chroma’s Best Knowledge, no party to any Upstream Agreement has granted any liens, security interests or encumbrances to the Chroma Technology. Chroma is listed in the records of the appropriate United States and/or foreign governmental agencies in the Licensed Territory as the sole and exclusive licensee of record for each registration, grant and application included in the Chroma Patents.
(b) Chroma Know-How. Chroma is the sole and exclusive owner of all of the Chroma Know-How, free and clear of any liens, security interests, encumbrances or claims of ownership of license from a Third Party (other than the security interest specifically described on Exhibit B-1). Chroma has the right to use and disclose and to enable CTI to use and disclose (in each case under appropriate conditions of confidentiality) the Chroma Know-How. Chroma has taken all reasonable precautions to preserve the confidentiality of the Chroma Know-How, and has not disclosed to any Third Party any Chroma Know-How that is or was confidential except under terms which preserved its confidentiality.
(c) Rights to Grant Licenses. Chroma has the right to grant to CTI the licenses under the Chroma Technology that it purports to grant hereunder.
51
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(d) No Out-Bound Agreements. Chroma has not granted any Third Party, including any academic organization or agency, any rights to the Product other than the material transfer agreements disclosed to CTI prior to the Effective Date.
(e) Sufficiency of Chroma Technology. As of the Effective Date, to Chroma’s Best Knowledge, the Chroma Technology constitutes all intellectual property necessary for, and no Third Party Know-How is needed to, Develop, Manufacture, use, make, have made, sell, offer for sale, have sold, distribute, import and otherwise Commercialize the Product in the Licensed Territory. Without limiting the foregoing, Chroma represents and warrants that the Chroma Patents listed on Exhibit B-1 have all been licensed to Chroma under the Vernalis Agreement and, to Chroma’s Best Knowledge, none of the intellectual property (including Patents) licensed to **, is necessary or useful to Develop, Manufacture, use, make, have made, sell, offer for sale, have sold, distribute, import and otherwise Commercialize the Product in the Field in the Licensed Territory. Chroma further represents and warrants that none of its Affiliates, as of the Effective Date, have any ownership of or any rights to any intellectual property (including Patents) in the Licensed Territory relating to the Chroma Technology.
(f) Non-Infringement of Chroma Technology by Third Parties. As of the Effective Date, to Chroma’s Best Knowledge, there are no ongoing activities by Third Parties that would constitute infringement or misappropriation of the Chroma Technology within the Licensed Territory.
(g) Non-Infringement. As of the Effective Date, to Chroma’s Best Knowledge, none of the manufacture, use or the sale of the Product in the Licensed Territory infringes any Patent owned by a Third Party or infringes or misappropriates any other Third Party intellectual property. As of the Effective Date, Chroma has not received any verbal or written claim or demand of any person or entity that the manufacture, use, or sale of the Product in the Licensed Territory infringes a Third Party Patent.
(h) Chroma Patents Not Invalid or Unenforceable. As of the Effective Date, the Chroma Patents exist, and Chroma has not received any written notice from a Government Authority or from any Third Party that the Chroma Patents are invalid or unenforceable, in whole or in part. To Chroma’s Best Knowledge, Chroma has filed and prosecuted patent applications within the Chroma Patents in good faith and complied with all duties of disclosure with respect thereto and Chroma is not aware of any prior art or third party rights that would challenge the validity of the Chroma Patents. In addition, to Chroma’s Best Knowledge, Chroma has not committed any act, or omitted to commit any act, that may cause the Chroma Patents to expire prematurely or be declared invalid or unenforceable.
(i) Disclosure. Prior to the Effective Date, Chroma made available to CTI, or provided CTI with, copies of all information with respect to the Product as requested by CTI in writing. In addition, as of the Effective Date Chroma has disclosed to CTI any material information known to Chroma as of such date with respect to (i) the safety of the Product, (ii) the efficacy of the Product, (iii) any then-existing circumstance which would be reasonably likely to
52
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
prohibit or prevent the Development, Manufacturing and/or Commercialization of the Product in the Licensed Territory.
(j) Non-Action or Claim. As of the Effective Date, to Chroma’s Best Knowledge, there are no actual, pending, alleged or threatened adverse actions, suits, claims, interferences or formal governmental investigations involving the Product and/or the Chroma Technology by or against Chroma or any of its Affiliates in or before any court, governmental or Regulatory Authority. In particular, as of the Effective Date, to its Best Knowledge, there is no pending or threatened product liability action involving the Product. As of the Effective Date, there are no claims, judgments or settlements against or owed by Chroma relating to the Chroma Technology or the Product.
(k) Regulatory Materials and Studies. To Chroma’s Best Knowledge, all Regulatory Materials Controlled by Chroma in existence as of the Effective Date and to which CTI has rights of use or reference hereunder, including the Regulatory Materials described in Section 5.1, have been prepared, maintained and retained in accordance with applicable Laws. All preclinical and clinical studies conducted with respect to the Product, including such studies from which the data described in Section 5.1 are derived, have to Chroma’s Best Knowledge been conducted substantially in accordance with applicable Laws by persons with appropriate education, knowledge and experience.
(l) Upstream Agreements. (i) Chroma has provided CTI with a complete, current and accurate copy of each of the Upstream Agreements, including all amendments of each, as such agreements exist on the date hereof; (ii) Chroma has complied at all times with its obligations under the Vernalis Agreement, (iii) Chroma is not in default or breach, and there are no circumstances existing on the date hereof which, with notice or the passage of time or both, could reasonably be expected to result in a default under the Vernalis Agreement, (iv) the Upstream Agreements are in full force and effect and are legal, valid and binding agreements, and enforceable in accordance with their terms, (v) as of the date hereof, Chroma or its Affiliates have not received any rights to any Chroma Technology from any Third Party except under the terms of the Vernalis Agreement provided to CTI and (vi) as of the date hereof, Chroma is not aware of any agreement related to the ownership or rights in the Chroma Technology other than the Upstream Agreements. The warranties given in (i) and (iv) of this paragraph (l) with respect to the other Upstream Agreements that are not the Vernalis Agreement are given to Chroma’s Best Knowledge, provided that no such knowledge qualification applies to the Vernalis Agreement.
10.3 No Other Representations or Warranties. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT, NO REPRESENTATIONS OR WARRANTIES WHATSOEVER, WHETHER EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT, NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS OR ACHIEVEMENT OF ANY PARTICULAR SALES LEVEL, IS MADE OR GIVEN BY OR ON BEHALF OF A PARTY. ALL
53
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
REPRESENTATIONS AND WARRANTIES, WHETHER ARISING BY OPERATION OF LAW OR OTHERWISE, ARE HEREBY EXPRESSLY EXCLUDED.
ARTICLE 11
INDEMNIFICATION
11.1 Indemnification by Chroma. Chroma shall defend, indemnify, and hold CTI and its Affiliates and CTI’s and its Affiliates’ officers, directors, employees, and agents (the “CTI Indemnitees”) harmless from and against any and all Third Party claims, suits, proceedings, damages, expenses (including court costs and reasonable attorneys’ fees and expenses), and recoveries (collectively, “Claims”) to the extent that such Claims arise out of, are based on, or result from (a) the Development, Manufacture, storage, handling, use, promotion, sale, offer for sale, importation or other Commercialization of Products by or on behalf of Chroma or its Affiliates, distributors (other than CTI), sublicensees (other than CTI), or contract manufacturers (unless and to the extent such liability for Manufacturing activities are covered by separate indemnification pursuant to the Supply Agreement, which in such event will control), or (b) the breach of any representation, warranty or covenant of Chroma in this Agreement, or (c) the willful misconduct or negligent acts of Chroma, its Affiliates, or the officers, directors, employees, or agents of Chroma or its Affiliates. The foregoing indemnity obligation shall not apply to the extent that (i) the CTI Indemnitees fail to comply with the indemnification procedures set forth in Section 11.3 and Chroma’s defense of the relevant Claims is prejudiced by such failure, or (ii) any Claim arises from, is based on, or results from any activity for which CTI is obligated to indemnify the Chroma Indemnitees under Section 11.2.
11.2 Indemnification by CTI. CTI shall defend, indemnify, and hold Chroma and its Affiliates and Chroma’s and its Affiliates’ officers, directors, employees, and agents (the “Chroma Indemnitees”) harmless from and against any and all Claims to the extent that such Claims arise out of, are based on, or result from (a) the Development, storage, handling, distribution, use, Manufacture (unless and to the extent liability for Manufacturing activities are covered by separate indemnification pursuant to the Supply Agreement, which in such event will control) promotion, sale, offer for sale, importation or other Commercialization of Products by CTI or its Affiliates, or its or their sublicensees, or distributors or contract manufacturers, or (b) the breach of any representation, warranty or covenant of CTI set forth in this Agreement, or (c) the willful misconduct or negligent acts of CTI or its Affiliates, or the officers, directors, employees, or agents of CTI or its Affiliates. The foregoing indemnity obligation shall not apply to the extent that (i) the Chroma Indemnitees fail to comply with the indemnification procedures set forth in Section 11.3 and CTI’s defense of the relevant Claims is prejudiced by such failure, or (ii) any Claim arises from, is based on, or results from any activity for which Chroma is obligated to indemnify the CTI Indemnitees under Section 11.1.
11.3 Indemnification Procedures. The Party claiming indemnity under this Article 11 (the “Indemnified Party”) shall give written notice to the Party from whom indemnity is being sought (the “Indemnifying Party”) in a reasonably timely manner after learning of such
54
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Claim. The Indemnified Party shall provide the Indemnifying Party with reasonable assistance, at the Indemnifying Party’s expense, in connection with the defense of the claim for which indemnity is being sought. The Indemnified Party may participate in and monitor such defense with counsel of its own choosing at its sole expense; provided, however, the Indemnifying Party shall have the right to assume and conduct the defense of the claim with counsel of its choice. The Indemnifying Party shall not settle any claim without the prior written consent of the Indemnified Party, not to be unreasonably withheld, unless the settlement involves only the payment of money. So long as the Indemnifying Party is actively defending the claim in good faith, the Indemnified Party shall not settle any such claim without the prior written consent of the Indemnifying Party. If the Indemnifying Party does not assume and conduct the defense of the claim as provided above, (a) the Indemnified Party may defend against, and consent to the entry of any judgment or enter into any settlement with respect to the claim in any manner the Indemnified Party may deem reasonably appropriate (and the Indemnified Party need not consult with, or obtain any consent from, the Indemnifying Party in connection therewith), and (b) the Indemnifying Party will remain responsible to indemnify the Indemnified Party as provided in this Article 11.
11.4 No Assumed Obligations and Liabilities. Notwithstanding the foregoing and anything else to the contrary in this Agreement, Chroma expressly acknowledges and agrees that CTI does not, under the terms of this Agreement or otherwise, assume any liability or obligation related to the Product to the extent relating to periods before the Effective Date, including any liability or obligation with respect to any Products that were used or distributed by or for Chroma, including any liabilities or obligations with respect to the Past Studies (collectively, the “Retained Liabilities”). Chroma shall retain and be solely responsible and liable for all Retained Liabilities.
11.5 Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE, OR INDIRECT DAMAGES ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 11.5 IS INTENDED TO OR SHALL LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF ANY PARTY UNDER SECTION 11.1 OR 11.2, OR DAMAGES AVAILABLE FOR A PARTY’S BREACH OF CONFIDENTIALITY OBLIGATIONS IN ARTICLE 12, A PARTY’S LIABILITY FOR FRAUD, DEATH OR PERSONAL INJURY CAUSED BY THEIR NEGLIGENCE OR ANY OTHER LIABILITY WHICH MAY NOT BE EXCLUDED BY LAW.
11.6 Insurance. Each Party shall procure and maintain insurance, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated at all times during which any Product is being clinically tested in human subjects or commercially distributed or sold by such Party. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 11. Each Party
55
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
shall provide the other Party with written evidence of such insurance upon request. Each Party shall provide the other Party with written notice at least thirty (30) days prior to the cancellation, non-renewal or material change in such insurance or self-insurance which materially adversely affects the rights of the other Party hereunder.
ARTICLE 12
CONFIDENTIALITY
12.1 Confidentiality. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties during a period that is the longer of (i) the Term, or (ii) fifteen (15) years from the Effective Date, each Party agrees that it shall keep confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as provided for in this Agreement any Confidential Information furnished to it by the other Party pursuant to this Agreement except for that portion of such information or materials that the receiving Party can demonstrate by competent written proof:
(a) was already rightfully known to the receiving Party or its Affiliate, other than under an obligation of confidentiality, at the time of disclosure by the other Party;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement;
(d) was disclosed to the receiving Party or its Affiliate by a Third Party who has a legal right to make such disclosure; or
(e) was independently discovered or developed by the receiving Party or its Affiliate without the aid, application, or use of the disclosing Party’s Confidential Information, as evidenced by a contemporaneous writing.
12.2 Authorized Disclosure. Each Party may disclose Confidential Information belonging to the other Party to the extent such disclosure is reasonably necessary in the following situations:
(a) regulatory filings and other filings with Governmental Authorities, including filings with the Securities and Exchange Commission, the Commissione Nazionale per le Società e la Borsa or other securities regulatory authority, The Nasdaq Stock Market LLC, the Mercato Telematico Azionario or other relevant exchange on which such Party is listed;
(b) prosecuting or defending litigation;
56
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(c) complying with applicable Laws;
(d) disclosure to its employees, agents, consultants, and any bona fide Third Party potential (sub)-licensees (including potential Third Party contract manufacturers and other licensees or collaborators) only on a need-to-know basis and solely as necessary in connection with the performance of or as otherwise contemplated by this Agreement, provided that in each case the recipient of such Confidential Information must agree to be bound by similar obligations of confidentiality and non-use at least as equivalent in scope as those set forth in this Article 12 prior to any such disclosure; and
(e) disclosure of the material terms of this Agreement to any bona fide potential investor, investment banker, acquiror, merger partner, licensees, sublicensees or other potential or actual financial or commercial partner; provided that in connection with such disclosure, the disclosing Party shall use all reasonable efforts to inform each recipient of the confidential nature of such Confidential Information and cause each recipient of such Confidential Information to treat such Confidential Information as confidential.
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to clause (a) through (c) of this Section 12.2, it will, except where impracticable, give reasonable advance notice to the other Party of such disclosure and, if reasonably requested by the other Party, use diligent efforts to secure confidential treatment of such information. In any event, the Parties agree to take all reasonable action to avoid disclosure of Confidential Information hereunder except as otherwise provided in this Agreement. Each Party will be responsible for any acts or omissions of any Third Party to which such Party discloses Confidential Information in accordance with this Section 12.2.
12.3 Publicity.
(a) The Parties shall make a joint public announcement of the execution of this Agreement in the form attached as Exhibit D, which shall be issued at a time to be mutually agreed by the Parties. The Parties, will as soon as reasonably possible following the Effective Date but prior to any required filing timelines, agree on a redacted version of this Agreement to be filed by CTI on any public register pursuant to any securities law or regulation, provided that there is no assurance that such redactions will be acceptable to the applicable Governmental Authorities.
(b) Neither Party shall issue any additional press release or other publicity materials, or make any public presentation with respect to the terms or conditions of, this Agreement (but excluding the timelines for the programs being conducted pursuant to this Agreement or efforts and progress against such timelines, which may be the subject of additional press releases, other publicity materials and public presentations without the need to obtain the prior written consent of the other Party), in each case without the prior written consent of the other Party. The restriction in this paragraph (b) shall not apply to any future disclosures required by Law or regulation, including as may be required in connection with any filings made with, or by the requirements of the securities exchange on which such Party’s securities are traded; provided,
57
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
that the disclosing Party uses all reasonable efforts to inform the other Party at least three (3) business days prior to making any such disclosures and, if reasonably requested by the other Party, cooperates with the other Party in seeking a protective order or other appropriate remedy (including redaction). In addition, where required by Law of the applicable securities exchange upon which a Party may be listed, such Party shall have the right to make a press release announcing the achievement of each milestone under this Agreement as it is achieved, and the achievements of Regulatory Approvals in the Licensed Territory as they occur, subject only to the review procedure set forth in the preceding sentence. In relation to the other Party’s review of such an announcement, such other Party may make specific, reasonable comments on such proposed press release within the prescribed time for commentary, but shall not withhold its consent to disclosure of the information that the relevant milestone has been achieved and triggered a payment hereunder. Neither Party shall be required to seek the permission of the other Party to repeat any information regarding the terms of this Agreement that has already been publicly disclosed by such Party, or by the other Party, in accordance with this Section 12.3.
ARTICLE 13
TERM AND TERMINATION
13.1 Term. This Agreement shall (subject to Section 15.9) become effective on the Effective Date and, unless earlier terminated pursuant to this Article 13, shall remain in effect, on a Product-by-Product basis and on a country-by-country basis, until the expiration of the Royalty Term of such Product in such country.
13.2 Early Termination. CTI shall have the right to terminate this Agreement for convenience, upon one hundred and twenty (120) days’ written notice to Chroma.
13.3 Termination for Breach. If either Party is in material breach of the obligations, covenants and representations contained in this Agreement the other Party (the “Non-Defaulting Party”) shall be entitled to give to the Party in default (the “Defaulting Party”) written notice specifying the nature of the default and requiring it to cure such default. If such default is not cured (a) in the case of a failure to make any undisputed payment (other than with respect to any refund contemplated by Section 15.9, which shall be made immediately upon the occurrence of any such termination) or credit due pursuant to this Agreement, within thirty (30) days after receipt of such notice, or (b) in the case of any other default, within ninety (90) days after the receipt of such notice (or, if such breach is not capable of being cured within such ninety (90) day period, within such amount of time as may be reasonably necessary to cure such breach (but no longer than one-hundred and eighty (180) days), so long as the Defaulting Party is making diligent efforts to do so), the Non-Defaulting Party shall be entitled, without prejudice to any other rights conferred on it by this Agreement, and in addition to any other remedies available to it by law or in equity, immediately to terminate this Agreement by giving written notice to the Defaulting Party. The right of a Party to terminate this Agreement, as herein provided, shall not be affected in any way by its waiver or failure to take action with respect to any previous default.
58
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
If either Party disputes the existence of a material breach, then such dispute shall be resolved under the terms of Article 14 before this Agreement may be terminated for such material breach.
13.4 Insolvency. Either Party may terminate this Agreement upon written notice if, at any time, the other Party shall file in any court or agency pursuant to any statute or regulation of any state or country, a petition in bankruptcy or insolvency or for an arrangement or for the appointment of a receiver or trustee of the other Party or of substantially all of its assets, or if the other Party enters into a written agreement of composition, or if the other Party shall be served with an involuntary petition against it, filed in any insolvency proceeding, and such petition shall not be dismissed within ninety (90) calendar days after the filing thereof, or if the other Party is a party to any dissolution or liquidation, or if the other Party shall make an assignment of substantially all of its assets for the benefit of creditors or if the other Party shall be subject to a procedure which is similar to or the same as any of the foregoing in any country.
13.5 Termination for Patent Challenge by CTI. If CTI or any of its Affiliates: (a) commences or otherwise voluntarily determines to participate in (other than as a response to any action or proceeding initiated by Chroma or its Affiliates (other than relating to a matter covered under Section 13.3), or as may be necessary or reasonably required to assert a cross-claim or a counter-claim, or to respond to a court request or order or administrative law request or order) any action or proceeding (including any patent opposition or re-examination proceeding), challenging or denying the validity of any Chroma Patent or any claim thereof; or (b) actively assists any other person (other than as a response to any action or proceeding initiated by Chroma or its Affiliates (other than relating to a matter covered under Section 13.3), or as may be necessary or reasonably required to assert a cross-claim or counter-claim, or to respond to a court request or order or administrative law request or order) in bringing or prosecuting any action or proceeding (including any patent opposition or re-examination proceeding) challenging or denying the validity of any of such Chroma Patent or any claim thereof, Chroma shall have the right to terminate this Agreement on ninety (90) days written notice to CTI unless CTI or its Affiliates promptly terminates any such challenge within thirty (30) days after its receipt of such notice from Chroma. CTI shall include this obligation in its agreements with its sublicensees, provided that if any such sublicense initiates a patent challenge against Chroma as described above, subject to the exceptions noted above, CTI’s termination of such sublicense agreement shall be deemed a cure under this provision and Chroma will not be entitled to terminate this Agreement under this provision in such instance.
13.6 Continuing Rights of Commercialization Sublicensees. Upon any termination of any license rights granted to CTI under this Agreement, each sublicense previously granted by CTI or any of its Affiliates under such license rights to any Commercialization Sublicensee shall remain in effect and shall become a direct license or sublicense, as the case may be, of such rights by Chroma to such Commercialization Sublicensee, provided that such Commercialization Sublicensee is not in breach of its agreement with CTI, and subject to the Commercialization Sublicensee agreeing in writing to assume CTI’s terms, conditions and obligations to Chroma under this Agreement as they pertain to the sublicensed rights.
59
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
13.7 Effect of Termination for CTI. The following provisions shall apply if (a) CTI terminates this Agreement in its entirety pursuant to Section 13.2, or (b) Chroma terminates this Agreement due to CTI’s material uncured breach under Section 13.3 (c) due to CTI’s insolvency under Section 13.4 or, (d) due to CTI’s patent challenge under Section 13.5.
(a) Termination of Licenses. The licenses granted to CTI under this Agreement shall terminate, including restriction on Chroma’s right to exercise rights under the Joint Inventions set forth under Section 9.1. After the Effective Date of Termination and except as provided below, either Party will be free to use and practice the Joint Inventions for any purpose on a worldwide basis and may assign, license or otherwise transfer or exploit its rights to the Joint Inventions to an Affiliate or Third Party, without the other Party’s consent and without a duty of accounting to the other Party.
(b) Outstanding Payment Obligations. CTI shall be responsible for any outstanding payment obligations of CTI under Article 8 that existed or accrued prior to the Effective Date of Termination.
(c) Transfer of Materials.
(i) Development Documentation. CTI will transfer and assign to Chroma all Development Documentation related to the Product developed under the Development Plan.
(ii) Commercial Information. CTI will provide a written and electronic copy of the Commercial Information, to the extent such information has not already been provided to Chroma through its participation in the JDC. All Commercial Information shall be treated as CTI’s Confidential Information under Sections 12.1 and 12.2 and may only be used by or for Chroma in the Commercialization of the Product and for no other purpose.
(iii) Inventory. Chroma shall have the option to purchase existing Product inventories from CTI at the price CTI paid for such inventories.
(iv) Product Marks. For the Product Marks solely used for the Products by or for CTI in the Licensed Territory at the time of termination, CTI will transfer such Product Marks to Chroma without additional consideration, including registrations therefor and any goodwill associated therewith, provided that in no event will such transfer include any rights in CTI’s name or any brand, logo or trademark of CTI not solely used for the Product.
(v) Costs. CTI will be responsible for all costs associated with any transfer or transition pursuant to this paragraph (c) (other than the purchase price for the inventory as provided above).
(d) Option for Rights Not Transferred.
60
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(i) Non-Opted In Study or Non-Opted In Additional Product Development. For any Non-Opted In Study or any Non-Opted In Additional Product Development for which Chroma did not opt in under the terms of Sections 4.4 or 4.9, respectively, Chroma may opt in, during the Transition Period only, to any such Non-Opted In Study or any Non-Opted In Additional Product Development under the respective terms in Sections 4.4 and 4.9. If Chroma opts in under such terms, Chroma’s access to any Regulatory Materials, data, or other information generated from such studies will be the same under the terms of this Agreement as though Chroma had opted in to any such Non-Opted In Study or Non-Opted In Additional Product Development prior to the termination of this Agreement. If Chroma does not opt in to any Non-Opted In Study or any Non-Opted In Additional Product Development during the Transition Period, CTI will have no further obligations to Chroma, and Chroma will not have any right, with respect to such study or product.
(ii) Subject CTI Rights. If Chroma desires to license rights to Subject CTI Rights after the termination of this Agreement, Chroma may request such rights with a written notice to CTI and the Parties will enter into good faith negotiation during the Transition Period to provide such rights to Chroma on customary industry terms, including milestone and royalty payments. If the Parties cannot agree on the terms of any such license during the Transition Period or if Chroma does not request such rights in writing during the Transition Period, CTI will have no further obligations to Chroma, and Chroma will not have any right, with respect to Subject CTI Rights.
(e) Transition Plan. Within ninety (90) days after the Effective Date of Termination, the Parties shall negotiate in good faith and establish a transition plan to effectuate the transfer and transition provided in this Section 13.7 (“Transition Plan”) within one hundred and twenty (120) days after the date of the Transition Plan (“Transition Period”). In addition to the items described above in this Section 13.7, the Transition Plan will also address the transition of the following items, including the allocation of cost and responsibility related to the transition of such items. Other than obligations expressly set forth in the Transition Plan (some of which may continue after the Transition Period), CTI will not have any further obligation related to the Development Manufacture or Commercialization of the Product.
(i) whether and how the existing contracts exclusively related to the supply of the Product or clinical trials of the Product should be transitioned or terminated; and
(ii) what Development activities, including any on going clinical trials, set forth in the then current Development Plan should be continued on the same terms (including allocation of cost provided that where any clinical trials cannot be terminated under applicable Law, CTI will continue to be responsible for 75% of the Development Costs set forth in the then current Development Plan associated with any such clinical trial contained in an agreed Development Plan until the completion of such trial, provided that CTI will not be responsible for the costs associated with the enrollment of additional patients for such trial unless such additional enrollment is required by applicable Law).
61
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
(f) Further Assurances. CTI shall, at Chroma’s request and CTI’s expense, take such other reasonable and customary actions and execute such other reasonable and customary instruments, assignments and documents as may be necessary to effect the transfer of rights and materials expressly provided in this Section 13.7 above. After the Effective Date of Termination and before such items are transferred to Chroma or its designated Affiliate, CTI shall hold them on trust for Chroma, and shall maintain them in force and shall not encumber, amend, cancel or surrender or take any step in relation thereto unless requested to do so by Chroma or any applicable government or Regulatory Authority.
13.8 Effect of Termination for Chroma.
(a) Continuation of Rights. If CTI has the right to terminate this Agreement pursuant to Sections 13.3 or 13.4 and does not exercise such right, CTI will have the right to retain the licenses and rights granted under this Agreement to the Chroma Technology existing at the date of CTI’s notice to Chroma that it wishes to retain such rights and licenses, subject to payment of milestones and royalties under the terms of Article 8. CTI will also have the right to continue to use Chroma’s Regulatory Materials, Development Documentation and Confidential Information existing at the date of CTI’s notice to Chroma that it wishes to retain its rights and licenses under this Agreement, subject to the terms and conditions of this Agreement related to such items. Within ninety (90) days after CTI’s notice that CTI wishes to retain such licenses and rights, the Parties shall negotiate in good faith and establish a transition plan to address any ongoing or remaining Development activities under the then current Development Plan (“Transition Development Plan”). Such activities shall be conducted under same cost allocation terms as provided under this Agreement for the Development Plan. After the completion of Development activities provided under the Transition Development Plan, neither Party will have any further Development obligations under this Agreement.
(b) Termination of Agreement. If CTI has the right to terminate this Agreement pursuant to Sections 13.3 or 13.4 and does exercise such right, the provisions of Sections 13.7(c), 13.7(d), 13.7(e), and 13.7(f) shall apply except that Chroma will be solely responsible for all costs associated with any transfer or transition of such items, including all Development Costs for any Development activities provided to continue in the Transition Plan.
13.9 Rights in Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by Chroma or CTI are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the licensee of such rights under this Agreement shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code or any other applicable Law. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against a Party under the U.S. Bankruptcy Code or any other applicable Law, the other Party shall be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in such other Party’s possession, shall be promptly delivered to it (a) upon any such commencement of a bankruptcy proceeding
62
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
upon the other Party’s written request therefor, unless the Party subject to the proceeding’s elects to continue to perform all of its obligations under this Agreement or (b) if not delivered under clause (a), following the rejection of this Agreement by the Party subject to the proceeding’s upon written request therefor by the other Party. Notwithstanding anything in this Agreement to the contrary, it is the intention of the Parties that upon any commencement of a bankruptcy proceedings, the Party subject to the proceeding shall have the right to assume and assign this Agreement and its rights and obligations hereunder pursuant to Section 365 of the U.S. Bankruptcy Code or any other applicable Law.
13.10 Survival. The following provisions shall survive any expiration or termination of this Agreement for the period of time specified in the applicable section or, if no time is specified, indefinitely: Article 10, Article 11, Article 12, Article 14, and Article 15. To the extent Development or Commercialization obligations or rights survive under the terms of Sections 13.7 or 13.8, Article 4, Article 5 and Article 6 will survive for the duration of such Development or Commercialization obligations or rights. Termination or expiration of this Agreement for any reason shall not release a Party from any liability or obligation that already has accrued prior to such expiration or termination, nor affect the survival of any provision hereto to the extent it is expressly stated to survive such termination.
ARTICLE 14
DISPUTE RESOLUTION
14.1 Disputes. The Parties recognize that disputes as to certain matters may from time to time arise during the Term which relate to either Party’s rights and/or obligations hereunder. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation and without resort to litigation. To accomplish this objective, the Parties agree to follow the procedures set forth in this Article 14 to resolve any controversy or claim arising out of, relating to or in connection with any provision of this Agreement, if and when a dispute arises under this Agreement.
14.2 Referred from Committee.
(a) General. Except for disputes subject to Section 14.3(b), either Party may, by written notice to the other Party, have any dispute arising from a Committee pursuant to the terms of Article 3 referred to each Party’s Designated Executive for attempted resolution by good faith negotiations within thirty (30) days after such notice is received, or in less time if necessary to ensure patient safety or compliance with applicable Laws. If the Designated Executives are not able to resolve such dispute within such thirty (30) day period, either Party may at any time thereafter pursue binding arbitration under the terms of Section 14.5.
(b) Specific Decision-Making Rights. Except for disputes subject to Section 14.3(b), if the Designated Executives of the Parties are not able to resolve a dispute within the thirty (30) day period described above, and the dispute is related to one of the areas listed in
63
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
the immediate subparagraphs below, then each of Chroma and CTI shall have the unilateral right to settle such matter as provided below.
(i) CTI Decisions. The Designated Executive of CTI shall have the right to make the final decision with respect to matters involving the Development, Manufacture and Commercialization of the Product solely in the Licensed Territory; provided that such decision shall be made in good faith, cannot be inconsistent with the terms of this Agreement, and such decision cannot materially and adversely affect Chroma’s right and ability to Develop, Manufacture and Commercialize the Product in the ROW Territory.
(ii) Chroma Decisions. The Designated Executive of Chroma shall have the right to make the final decision with respect to matters involving the Development, Manufacture and Commercialization of the Product solely in the ROW Territory; provided that such decision shall be made in good faith, cannot be inconsistent with the terms of this Agreement, and such decision cannot materially and adversely affect CTI’s right and ability to Develop, Manufacture and Commercialize the Product in the Licensed Territory.
(iii) Development Costs. Notwithstanding the above provisions neither Party’s share of Development Costs can be increased unless both Parties agree to any such increase as part of the Development Plan or any amendments thereto, subject to the Development opt in mechanism described in Sections 4.4.
14.3 Arising Between the Parties.
(a) General Matters. Except for disputes subject to Section 14.3(b) below or referred from Committee and subject to Section 14.2 above, the Parties shall refer all other disputes arising under or in connection with this Agreement, including, without limitation, any claim or controversy relating to the validity, enforceability, interpretation, performance, breach or termination hereof to the Designated Executive for each Party for attempted resolution by good faith negotiations within thirty (30) days after such dispute is first identified by either Party in writing to the other. With respect to any dispute that is not resolved by such Designated Executives within such period, either Party may at any time thereafter pursue binding arbitration under the terms of Section 14.5.
(b) Safety Matters. In the event that the Parties cannot agree to one opinion with respect to an individual adverse event or other matter affecting the health, safety or welfare of a patient, then, the Parties shall convene the relevant Committee to discuss and seek resolution of such matter as expeditiously as possible to ensure patient safety and compliance with applicable Laws. In connection with such discussions, the Parties may convene any joint working groups or outside consultants and/or experts in the subject matter of the disagreement to assist the Parties to reach one opinion. If such discussions do not result in one opinion between the Parties in a reasonably timely fashion, then the most conservative opinion shall prevail.
14.4 Injunctive Relief. Nothing herein may prevent either Party from seeking a preliminary injunction or temporary restraining order in any court of competent jurisdiction in
64
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
order to prevent any Confidential Information from being disclosed without appropriate authorization under this Agreement or to prevent any termination of this Agreement under Section 13.3 if the existence of a material breach is in dispute.
14.5 Binding Arbitration. Any dispute arising under or in connection with this Agreement, that is not resolved by the Designated Executives pursuant to Section 14.3(a) within the time period set forth therein shall be submitted to binding arbitration under the Rules of Arbitration of the London Court of International Arbitration before an arbitral tribunal of three arbitrators. Each Party shall nominate one arbitrator, and the two co-arbitrators, in consultation with the Party nominating them, shall together nominate the third arbitrator, who shall serve as the chairperson of the arbitral tribunal. The place of arbitration shall be New York, New York, and all hearings in the arbitration shall be conducted there. The arbitral tribunal’s award shall be (i) in writing, stating the reasons for such decision; (ii) based solely on the terms and conditions of this Agreement, as interpreted, if applicable, in accordance with the laws of England and Wales; (iii) final and binding upon the Parties hereto; and (iv) enforceable in any court of competent jurisdiction.
ARTICLE 15
MISCELLANEOUS
15.1 Entire Agreement; Amendment. This Agreement, including the Exhibits hereto, sets forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto with respect to the subject matter hereof and supersedes, as of the Effective Date, all prior and contemporaneous agreements and understandings between the Parties with respect to the subject matter hereof, including the CDA and term sheet. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties with respect to the subject matter hereof other than as are set forth herein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
15.2 Force Majeure. Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented by force majeure and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. For purposes of this Agreement, force majeure shall include conditions beyond the control of the Parties, including without limitation, an act of God, war, civil commotion, terrorist act, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe, and failure of plant or machinery (provided that such failure could not have been prevented by the exercise of skill, diligence, and prudence that would be reasonably and ordinarily expected from a skilled and experienced person engaged in the same type of undertaking under the same or similar
65
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
circumstances). If a force majeure persists for more than ninety (90) days, then the Parties will discuss in good faith the modification of the Parties’ obligations under this Agreement in order to mitigate the delays caused by such force majeure.
15.3 Notices. Any notice required or permitted to be given under this Agreement shall be in writing, shall specifically refer to this Agreement, and shall be addressed to the appropriate Party at the address specified below or such other address as may be specified by such Party in writing in accordance with this Section 15.3, and shall be deemed to have been given for all purposes (a) when received, if hand-delivered or sent by confirmed facsimile or a reputable courier service, or (b) five (5) business days after mailing, if mailed by first class certified or registered airmail, postage prepaid, return receipt requested.
| If to Chroma: | CHROMA THERAPEUTICS LTD 93 Milton Park | |
| Abingdon, Oxon OX14 4RY, UK | ||
| Attn: Chief Executive Officer | ||
| Fax: ** | ||
| with a copy to: | CHROMA THERAPEUTICS LTD 93 Milton Park | |
| Abingdon, Oxon OX14 4RY, UK | ||
| Attn: Chief Financial Officer | ||
| Fax: ** | ||
| If to CTI: | CELL THERAPEUTICS, INC. 501 Elliott Ave. W. #400 | |
| Seattle, Washington 98119, U.S.A. | ||
| Attn: Chief Executive Officer | ||
| Fax: ** | ||
| with a copy to: | CELL THERAPEUTICS, INC. 501 Elliott Ave. W. #400 | |
| Seattle, Washington 98119, U.S.A. | ||
| Attn: General Counsel | ||
| Fax: ** | ||
15.4 No Strict Construction; Headings. This Agreement has been prepared jointly and shall not be strictly construed against either Party. Ambiguities, if any, in this Agreement shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision. The headings of each Article and Section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular Article or Section. Except where the context otherwise requires, the use of any gender shall be applicable to all genders, and the word
66
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
“or” is used in the inclusive sense (and/or). The term “including” as used herein means including, without limiting the generality of any description proceeding such term.
15.5 Assignment. Neither Party may assign or transfer this Agreement or any rights or obligations hereunder without the prior written consent of the other (which shall not be unreasonably withheld, delayed or conditioned), except that a Party may make such an assignment without the other Party’s prior written consent to a successor-in-interest to all or substantially all of the assets of the relevant Party to which this Agreement relates, whether in a merger, sale of stock, sale of assets or other transaction, provided that such transaction is not entered into in an effort to circumvent the requirement to obtain the consent of the other Party. Any successor or assignee of rights and/or obligations permitted hereunder shall, in writing to the other Party, expressly assume performance of such rights and/or obligations, and the assigning party shall remain liable for all obligations of the assignee after the assignment. Any permitted assignment shall be binding on the successors of the assigning Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 15.5 shall be null, void and of no legal effect.
15.6 Non-Solicitation. While the Parties are performing Development and Commercialization activities under this Agreement and for a period of two (2) years thereafter, neither Party shall, without the express written consent of the other Party, recruit, solicit or induce any employee of the other Party to terminate his or her employment with such other Party. The foregoing provision shall not, however, restrict either Party or its Affiliates from advertising employment opportunities in any manner that does not directly target the other Party or its Affiliates or from hiring any persons who respond to such generalized public advertisements.
15.7 Performance by Affiliates. Each Party may discharge any obligations and exercise any right hereunder through any of its Affiliates. Each Party shall cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Agreement shall be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate.
15.8 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.9 HSR Filing. Each of CTI and Chroma agrees to prepare and make appropriate filings under the HSR Act relating to this Agreement and the transactions contemplated hereby as soon as reasonably practicable after the Effective Date (the “HSR Filing Date”). The Parties agree to cooperate in the antitrust clearance process and to furnish promptly to the Federal Trade Commission (FTC), the Antitrust Division of the Department of Justice and any other agency or authority, any information reasonably requested by them in connection with such filings. Other than the provisions of this Section 15.9, Section 8.1 and Articles 11 and 12, the rights and
67
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
obligations of the Parties under this Agreement shall not become effective until the waiting period provided by the HSR Act shall have terminated or expired without any action by any government agency or challenge to the transaction (the date of such termination or expiration shall be the “Approval Date” of this Agreement), provided that neither Party shall grant any rights to engage in any act that would conflict with the terms and conditions of this Agreement until such Approval Date. Upon the occurrence of the Approval Date, all provisions of this Agreement shall become effective as of the Effective Date automatically without the need for further action by the Parties. In the event that antitrust clearance from the FTC and Antitrust Division of the Department of Justice is not obtained within ninety (90) days after the HSR Filing Date, or such other date as the Parties may mutually agree, this Agreement may be terminated by either Party. If this Agreement is terminated for such reason, (a) all rights granted in this Agreement shall revert back to the grantor , (b) Chroma shall repay to CTI immediately the upfront fee of $5,000,000 and (c) the Parties will not have any further rights or obligations to each other under this Agreement except as provided in Section 13.10. In the event a provision of this Agreement needs to be deleted or substantially revised in order to obtain regulatory clearance of this transaction, the Parties will negotiate in good faith in accordance with Section 15.10.
15.10 Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by any court of competent jurisdiction from which no appeal can be or is taken, the provision shall be considered severed from this Agreement and shall not serve to invalidate any remaining provisions hereof. The Parties shall make a good faith effort to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering this Agreement may be realized.
15.11 No Waiver. Any delay in enforcing a Party’s rights under this Agreement or any waiver as to a particular default or other matter shall not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written and signed waiver relating to a particular matter for a particular period of time.
15.12 Independent Contractors. Subject to the terms of this Agreement, the activities and resources of each Party shall be managed by such Party, acting independently and in its individual capacity. The relationship between Chroma and CTI is that of independent contractors and neither Party shall have the power to bind or obligate the other Party in any manner, other than as may be expressly set forth in this Agreement. Nothing herein shall be construed to create the relationship of partners, principal and agent, or joint-venture partners between the Parties.
15.13 English Language; Governing Law. This Agreement, including any dispute, claim or controversy arising out of or related to validity, enforceability, interpretation, performance, breach or termination hereof, shall be governed by and construed under the laws of England and Wales, without giving effect to any choice of law principles that would require the application of the laws of a different state, provided that Laws of any governing Regulatory
68
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Authority, as well as the intellectual property laws of the jurisdiction in which any intellectual property rights at issue were granted, shall also govern to the extent applicable.
15.14 Counterparts. This Agreement may be executed in one (1) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. This Agreement shall be binding upon the delivery by each Party of an executed signature page to the other Party, which may include by facsimile transmission.
[Signature Page Follows]
69
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
IN WITNESS WHEREOF, the Parties have executed this Agreement in duplicate originals by their duly authorized officers as of the Effective Date.
| CELL THERAPEUTICS, INC. | CHROMA THERAPEUTICS LTD | |||||||
| By: | /s/ James A. Bianco | By: | /s/ Ian Nicholson | |||||
| Name: | James A. Bianco, M.D. | Name: | Ian Nicholson | |||||
| Title: | Chief Executive Officer | Title: | Chief Executive Officer | |||||
Signature Page to the Co-Development and License Agreement entered into as of March 11, 2011
S-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT A-1
COMPOUND DESCRIPTION
| Name | Tosedostat | |
| Synonyms | 2S-[2R-(S-Hydroxy-hydroxycarbamoyl-methyl)-4-methylpentanoylamino]-2-phenylethanoic acid cyclopentyl ester | |
| Molecular Structure |
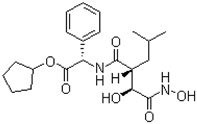
| |
| Molecular Formula | C21H30N2O6 | |
| Molecular Weight | 406.47 | |
| CAS Registry Number | 238750-77-1 | |
A-1-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT A-2
ANALOGUES OF TOSEDOSTAT
****
A-2-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT B-1
CHROMA PATENTS
| BB-76163 (CHR-2797) Patent Portfolio | ||||||||||
| Patents and Patent Applications Derived from International Patent | ||||||||||
| Application WO 98/11063 | ||||||||||
| Country |
Status | Application No | Patent No | |||||||
| Australia | Granted | 41277/97 | 718890 | |||||||
| Belgium | Granted | EP97939052.3 | EP 0925278 | |||||||
| Canada | Granted | 2265666 | 2,265,666 | |||||||
| Switzerland | Granted | EP97939052.3 | EP 0925278 | |||||||
| Czech Republic | Granted | PV 821-99 | 298048 | |||||||
| Germany | Granted | EP97939052.3 | EP 0925278 | |||||||
| Spain | Granted | EP97939052.3 | EP 0925278 | |||||||
| France | Granted | EP97939052.3 | EP 0925278 | |||||||
| UK | Granted | EP97939052.3 | EP 0925278 | |||||||
| Italy | Granted | EP97939052.3 | EP 0925278 | |||||||
| Japan | Granted | 1988-513347 | 4238334 | |||||||
| Norway | Granted | 19991139 | 314227 | |||||||
| New Zealand | Granted | 333923 | 333923 | |||||||
| Poland | Granted | P-333369 | P-333369 | |||||||
| USA | Granted | 08/925584 | 6169075 | |||||||
| USA (divisional) | Granted | 09/514083 | 6790834 | |||||||
| Patents and Patent Applications Derived from International Patent | ||||||||||
| Application WO 99/46241 | ||||||||||
| Country |
Status | Application No | Patent No | |||||||
| Australia | Granted | 64106/98 | 747977 | |||||||
| Canada | Granted | 2323414 | 2,323,414 | |||||||
| China | Granted | 98813847.6 | ZL 98813847.6 | |||||||
| Czech Republic | Granted | PV 2000-3317 | 299610 | |||||||
| Germany | Granted | EP98 909 620.1 | EP 1062202 | |||||||
| France | Granted | EP98 909 620.1 | EP 1062202 | |||||||
| UK | Granted | EP98 909 620.1 | EP 1062202 | |||||||
| Ireland | Granted | EP98 909 620.1 | EP 1062202 | |||||||
| Israel | Granted | 137774 | 137774 | |||||||
| Japan | Granted | 2000-535622 | 4324324 | |||||||
B-1-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Mexico | Granted | 8762 | 224080 | |||
| New Zealand | Granted | 506293 | 506293 | |||
| Poland | Granted | P-342811 | 190637 | |||
| USA | Granted | 09/100539 | 6462023 |
SECURITY INTEREST:
**
B-1-2
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT B-2
UPSTREAM AGREEMENTS
| Other Party(ies) |
Title of Agreement |
Effective Date | ||
| Vernalis (Oxford) Ltd. | Exclusive License Agreement Covering BB-76163 for Use in Cancer | November 24, 2003 | ||
| Vernalis (R&D) Limited (formerly known as Vernalis (Oxford) Limited | Amendment No. 1 to Exclusive License Agreement Covering BB-76163 for Use in Cancer | March 30, 2007 | ||
| 1. **
2. ** |
** | ** | ||
| 1. **
2. ** |
** | ** | ||
B-2-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT C
DEVELOPMENT PLAN
****
C-1-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT D
PRESS RELEASE
Cell Therapeutics Acquires Exclusive Marketing and Co-Development Rights
in the Americas to Chroma Therapeutics’ Tosedostat, a First in Class Tumor
Selective Oral Therapy for Treatment of Blood Related and Other Cancers
Phase III Pivotal Trial in Acute Myeloid Leukemia Expected to Start in Q4 2011
Provides Potential Portfolio and Commercial Synergies with Pixantrone
Conference Call and Webcast to be Held on Monday, March 14, 2011 at 8:30 AM Eastern
time/1:30 PM Central European time/5:30 AM Pacific time
March 14, 2011 Seattle and Oxford, UK—Cell Therapeutics, Inc. (“CTI”) (NASDAQ and MTA: CTIC) and Chroma Therapeutics Ltd. (“Chroma”) announced today that the companies have entered into a co-development and license agreement providing CTI with exclusive marketing and co-development rights to Chroma’s drug candidate tosedostat in North, Central and South America. Tosedostat is an oral, aminopeptidase inhibitor that has demonstrated significant anti-tumor responses in blood related cancers and solid tumors in phase I-II clinical trials. CTI, in collaboration with Chroma, expects to commence a phase III clinical study in the United States and Europe in elderly patients with relapsed or refractory acute myeloid leukemia (“AML”) for potential approval by the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”). The FDA and the EMA have granted tosedostat orphan drug status for AML.
Pursuant to the terms of the agreement, CTI will make an upfront payment of $5 million and a milestone payment of $5 million when the AML pivotal trial is initiated, which is expected to occur in the fourth quarter of 2011. The agreement also includes customary development-based milestone payments related to AML, myelodysplastic syndrome (“MDS”) and certain other indications, as well as royalties on net sales in CTI’s territories. CTI will oversee development operations and commercialization activities in its territories and Chroma will oversee development operations and commercialization activities in the rest of the world. Subject to a funding cap of $50 million for the first three years, CTI will be responsible for 75% of development costs and Chroma will be responsible for 25% of development costs.
“Tosedostat, similar to drugs like bortezomib and lenalidomide, represents a departure from conventional cytotoxic chemotherapy toward more tumor selective targeted therapy that interferes with cellular pathways necessary for tumor survival,” commented James A. Bianco, M.D., CEO of CTI. “In initial clinical studies, tosedostat was well-tolerated, given orally once a day and produced encouraging response
D-1-1
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
rates in difficult to treat patients with acute leukemia and a variety of blood related cancers. We are excited to add tosedostat to our late-stage product pipeline alongside pixantrone as we continue with our strategy of building a pipeline of novel drugs for treating blood-related cancers.”
Chroma is an Oxford, UK based private company led by a management team with extensive public biotechnology and large pharmaceutical company experience, including former executives of Celltech, British Biotech, AstraZeneca and Roche, and backed by leading specialist investors. Chroma is focused on harnessing chromatin biology and its novel cell accumulation (ESM) technology to develop new targeted therapies for cancer and inflammatory disorders.
“We believe that this is a collaboration that should enable the rapid progression of tosedostat toward seeking regulatory approval, given CTI’s development and commercialization capabilities and experience in the blood-related cancer space,” said Ian Nicholson, CEO of Chroma. “In working with clinicians in developing tosedostat we have clearly identified significant unmet medical needs where tosedostat could provide an important therapeutic advance for patients if approved.”
AML is a hematologic cancer that is an aggressive, fast-growing cancer that starts inside the bone marrow with the production of abnormal blood cells. The American Cancer Society estimates that 12,330 new cases of AML will be diagnosed and approximately 8,950 deaths from AML will occur in the U.S. in 2010. AML is generally a disease affecting older people with the average patient age at onset of approximately 67 years. There remain a substantial proportion of elderly patients who do not receive intensive chemotherapy due to their inability to tolerate such regimens, and other risk factors. Therefore, there is a significant unmet medical need in developing a well-tolerated and effective treatment for these patients.
Tosedostat is an orally dosed aminopeptidase inhibitor which blocks the M1/17 family of aminopeptidases. Disrupting aminopeptidases deprives sensitive tumor cells of amino acids by blocking protein recycling resulting in tumor cell death. Tosedostat has been studied in Chroma’s phase I-II clinical trials both as a single agent and in combination with other chemotherapeutic agents. Such studies have demonstrated significant anti-tumor responses without the typical side effects of conventional, non-targeted cytotoxic therapies. Initial target indications include AML, MDS and multiple myeloma.
Conference Call Information
On Monday, March 14, 2011, at 8:30 a.m. Eastern time/1:30 p.m. Central European time/5:30 a.m. Pacific time, members of CTI’s and Chroma’s management teams will host a conference call to discuss the co-development and licensing agreement.
Conference Call Numbers
Monday, March 14, 2011 8:30 a.m. Eastern/1:30 p.m. Central European/5:30 a.m. Pacific Time
D-1-2
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
1-877-941-0843 (US Participants – Toll-Free)
1-480-629-9643 (US Participants)
800-149-038 (Italy Participants – Toll-Free)
39-06-45-210-8364 (Italy Participants)
0800 358 0857 (UK Participants – Toll-Free)
44-20-8515-2302 (UK Participants)
Call-back numbers for post-listening available at 11:30 a.m. Eastern Time:
1-800-406-7325 (US Participants)
1-303-590-3030 (International)
Passcode: 4423141#
Live audio webcast at www.celltherapeutics.com will be archived for post-call listening approximately two hours after call ends.
About Cell Therapeutics, Inc.
Headquartered in Seattle, CTI is a biopharmaceutical company committed to developing an integrated portfolio of oncology products aimed at making cancer more treatable. For additional information, please visit www.CellTherapeutics.com.
Sign up for email alerts and get RSS feeds at our Web site,
http://www.CellTherapeutics.com/investors_alert
About Chroma Therapeutics
Chroma Therapeutics, based in Oxford (UK), is a drug discovery and development company focused in the fields of oncology and inflammatory disorders. Chroma is building a broad pipeline of first- or best-in-class treatments utilizing its expertise in chromatin biology and its novel intracellular accumulation technologies, which include the ability to selectively target drugs’ tomacrophages. Chroma is backed by a number of leading specialist investors, including Abingworth, Essex Woodlands, Gilde, Phase4 and The Wellcome Trust. More information about Chroma can be found at www.chromatherapeutics.com.
This press release includes forward-looking statements that involve a number of risks and uncertainties the outcome of which could materially and/or adversely affect actual future results and the market price of the Company’s securities. Specifically, the risks and uncertainties that could affect the development and commercialization of tosestodat include risks associated with preclinical, clinical and sales and marketing developments in the biopharmaceutical industry in general and in particular, including, without limitation, the potential failure of tosedostat to prove safe and effective (including complete and overall response rates) for the treatment of blood related and other cancers as determined by the FDA and/or the EMA, that the FDA may not accept the proposed clinical trial design of tosedostat and/or may request additional clinical trials, that clinical trials may not demonstrate the safety and effectiveness of tosedostat, that the Company cannot predict or guarantee the pace or geography of enrollment of clinical trials of tosedostat, including whether or not the majority of the patients will be enrolled in the U.S., that the phase III pivotal trial for tosedostat for AML may not start during the fourth quarter of
D-1-3
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
2011, that the Company cannot predict or guarantee the outcome or results of clinical trials of tosedostat, that the Company cannot predict or guarantee whether the co-development and license agreement will strengthen the Company’s business, financial condition, operating results and prospects or the trading price of the Company’s securities, that the Company cannot predict or guarantee whether milestones will be achieved pursuant to the Agreement, the Company’s ability to continue to raise capital as needed to fund its operations and make milestone payments, as applicable, determinations by regulatory, patent and administrative governmental authorities, competitive factors, technological developments, costs of developing, producing and selling the Company’s products under development and co-development, and other economic, business, competitive, and/or regulatory factors affecting the Company’s business generally, including those set forth in the Company’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for its most recent fiscal year and its most recent Quarterly Report on Form 10-Q, especially in the “Factors Affecting Our Operating Results” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections, and its Current Reports on Form 8-K. Except as may be required by law, the Company does not intend to update or alter its forward-looking statements whether as a result of new information, future events, or otherwise.
###
Media Contact:
Dan Eramian
T: 206.272.4343
C: 206.854.1200
E: deramian@ctiseattle.com
www.CellTherapeutics.com/press_room
Investors Contact:
Ed Bell
T: 206.282.7100
Lindsey Jesch Logan
T: 206.272.4347
F: 206.272.4434
E: invest@ctiseattle.com
www.CellTherapeutics.com/investors
Medical Information Contact:
T: 800.715.0944
E: info@askarm.com
Chroma Therapeutics Ltd.:
Ian Nicholson, CEO
Richard Bungay, CFO
T: +44 (0) 1235 829120
Brunswick PR
D-1-4
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Jon Coles
Justine McIlroy
T: +44 (0)20 7404 5959
D-1-5
