Attached files
| file | filename |
|---|---|
| EX-31.1 - HUIHENG MEDICAL, INC. | v217619_ex31-1.htm |
| EX-32.2 - HUIHENG MEDICAL, INC. | v217619_ex32-2.htm |
| EX-32.1 - HUIHENG MEDICAL, INC. | v217619_ex32-1.htm |
| EX-31.2 - HUIHENG MEDICAL, INC. | v217619_ex31-2.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
x
|
ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2010
|
|
|
¨
|
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from to
|
Commission File Number: 333-132056
HUIHENG MEDICAL INC.
(Exact name of registrant as specified in its charter)
|
Nevada
|
20-4078899
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
|
incorporation or organization)
|
Identification No.)
|
|
|
Huiheng Building, Gaoxin 7 Street South
|
||
|
Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057
|
||
|
(Address of principal executive offices)
|
||
86-755-25331366
(Registrant’s Telephone Number, Including Area Code)
Securities registered under Section 12(b) of the Act:
None
Securities registered under Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
x Yes ¨ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
¨Yes x No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Date File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
¨ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.
¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
Non-accelerated filer ¨ (Do not check if a smaller reporting company)
|
Smaller reporting company x
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes x No
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant as of March 15, 2011 was approximately $2,052,573 based upon the closing price of $0.90 per shares as quoted for such date on the Over-the-Counter Bulletin Board maintained by the Financial Industry Regulatory Authority (“FINRA”). Shares of common stock held by each officer and director and by each person who is known to own 10% or more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 29, 2011, there were 14,030,637 shares of the registrant’s $0.001 par value common stock issued and outstanding.
TABLE OF CONTENTS
|
|
Page
|
|||
|
PART I
|
||||
|
ITEM 1.
|
BUSINESS
|
3 | ||
|
ITEM 1A.
|
RISK FACTORS
|
10 | ||
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
23 | ||
|
ITEM 2.
|
PROPERTIES
|
23 | ||
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
23 | ||
|
ITEM 4
|
(REMOVED AND RESERVED)
|
|||
|
PART II
|
||||
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
23 | ||
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
25 | ||
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
25 | ||
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
38 | ||
|
ITEM 8.
|
FINANCIAL STATEMENTS
|
38 | ||
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
38 | ||
|
ITEM 9A.
|
CONTROLS AND PROCEDURES | 38 | ||
|
ITEM 9B.
|
OTHER INFORMATION | 39 | ||
|
PART III
|
||||
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
39 | ||
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
41 | ||
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
43 | ||
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
44 | ||
|
ITEM 14.
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
45 | ||
|
PART IV
|
||||
|
ITEM 15.
|
EXHIBITS
|
45 | ||
|
SIGNATURES
|
47 |
2
PART I
Item 1. Business.
In this Annual Report on Form 10-K, references to “dollars” and “$” are to United States dollars and, unless the context otherwise requires, references to “we,” “us” and “our” refer to Huiheng Medical, Inc. and its consolidated subsidiaries.
This Annual Report contains certain forward-looking statements. When used in this Annual Report, statements which are not historical in nature, including the words “anticipate,” “estimate,” “should,” “expect,” “believe,” “intend” “may,” “project,” “plan” or “continue,” and similar expressions are intended to identify forward-looking statements. They also include statements containing anticipated business developments, a projection of revenues, earnings or losses, capital expenditures, dividends, capital structure or other financial terms.
The forward-looking statements in this Annual Report are based upon our management’s beliefs, assumptions and expectations of our future operations and economic performance, taking into account the information currently available to them. These statements are not statements of historical fact. Forward-looking statements involve risks and uncertainties, some of which are not currently known to us that may cause our actual results, performance or financial condition to be materially different from the expectations of future results, performance or financial condition we express or imply in any forward-looking statements. These forward-looking statements are based on our current plans and expectations and are subject to a number of uncertainties and risks that could significantly affect current plans and expectations and our future financial condition and results.
We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this filing might not occur. We qualify any and all of our forward-looking statements entirely by these cautionary factors. As a consequence, current plans, anticipated actions and future financial conditions and results may differ from those expressed in any forward-looking statements made by or on our behalf. You are cautioned not to unduly rely on such forward-looking statements when evaluating the information presented herein.
Overview of Our Business
We design and sell precision radiotherapy equipment used for the treatment of cancerous tumors in the People’s Republic of China (“PRC” or “China”). Our products include a patented line of gamma treatment systems (“GTS”) that accurately deliver well-defined conforming doses of radiation to the target tissue and a multileaf collimator (“MLC”) used in conjunction with a linear accelerator for radiation therapy applications. We currently have 42 patents issued in the PRC, 3 patents issued in the U.S., one patent issued in the European Union, 1 patent in Sweden, and additional patent applications pending.
We are dependent on a certain number of distributors consisting of hospital equipment financing companies who purchase our equipment and, in turn, sell our products to hospitals and clinics as end users. In addition to providing precision radiotherapy equipment, we offer comprehensive post-sale services for our products as well as products manufactured by others. These services include radioactive cobalt source replacement and disposal, medical expert training, clinical trial analysis, patient tumor treatment analysis, software upgrades and patient care consulting.
Our total revenues decreased from $9.38 million in 2009 to $8.64 million in 2010. Our net income decreased from $3.64 million in 2009 to $2.18 million in 2010. The decrease in revenues and corresponding net income was primarily the result of different products and a decrease in the unit price of products sold during 2010 as compared to the prior year.
Our operating subsidiaries in the PRC were founded in 2001 by Mr. Hui Xiaobing, our Chairman and a pioneer in the GTS industry in the PRC. Mr. Hui served as president and Chairman of Shenzhen OUR Technology, Co., Ltd., the first PRC company to develop a gamma treatment system. Mr. Hui is also the former CEO of Everbright Securities, a major Chinese financial institution.
The majority of our product sales in the PRC are made to Shenzhen Jiancheng Investment Co, Ltd. (“Jiancheng”) who sell our products to hospital and clinics. During the fiscal years ended December 31, 2010 and 2009, Jiancheng accounted for 67% and 71% of our total revenues, respectively.
3
Our Products
We currently have developed five products: the Super Gamma System (“SGS”), the Body Gamma Treatment System (“BGTS”), OPEN Stereotactic Gamma-ray Radiotherapy System, the Head Gamma Treatment System (“HGTS”), and a multileaf collimator device (“MLC”) used in conjunction with a linear accelerator (“LINAC”). On February 8, 2010, we filed an application with the PRC State Food and Drug Administration (“SFDA”) seeking approval of a LINAC product manufactured by us. Although we have been informed that the LINAC unit is in the final stage of SFDA approval, we do not have an anticipated timeframe for receiving such approval. Upon receipt of regulatory approval and available funding for research and development, our intent is to market and sell the LINAC product initially only in the PRC. In addition, the MLC has been approved by SFDA for sale and we launched the sale of the MLC in 2011 targeting primarily the PRC market. As to our advanced magnetic resonance imaging (“MRI”) and industrial LINAC products, we have temporarily suspended the development of these two projects due to the changing of market conditions and our lack of funding available for these projects. We anticipate that our future research and development efforts will focus on developing our advanced MRI device and an industrial LINAC unit used for, among other things, preserving food through irradiation. We will pursue these projects pending availability of funding for future research and development.
Recently, we have taken steps to expand our product offering by acquiring Portola Medical, Inc., a Delaware company whose primary asset consists of its rights to develop, manufacture and sell an adjustable Multi-Catheter Source Applicator under the ClearPath trademark which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer. We plan to market and sell this product in the United States and in Asia. This product is still in its initial phase and there have been no sales of this product.
Our Strategies
Our goal is to gain greater market share for our GTS products by pursuing the following strategies:
|
|
·
|
continuing to offer high quality, competitively priced products and services;
|
|
|
·
|
broadening our product offering and improving our existing technologies;
|
|
|
·
|
expanding our sales and distribution force in the PRC;
|
|
|
·
|
exploring opportunities to develop international markets including the United States, South America, Eastern Europe and Southeast Asia; and
|
|
|
·
|
expanding our product portfolio through relationships with foreign medical equipment technology leaders.
|
Our Strengths
We believe, based solely on management’s knowledge of our industry, that we are a leader in the GTS market in the PRC. We consider our core competitive strengths to be:
|
|
·
|
experienced leadership;
|
|
|
·
|
strong customer relationships and networks;
|
|
|
·
|
proprietary technology with patent protection;
|
|
|
·
|
high quality product line;
|
|
|
·
|
strong gross margins and pricing flexibility;
|
|
|
·
|
consistent, high quality post-sale customer service and support;
|
|
|
·
|
experienced research and development team; and
|
|
|
·
|
strong R&D partnerships with Beijing University, China Science & Technology University and Public Healthcare Institute of Jilin University.
|
4
Our Industry
The market for medical equipment and supplies in the PRC is segmented into geographical regions. Hospitals with greater spending power tend to be located in large towns and cities in the eastern part of the PRC, where rapid economic growth has taken place during the last two decades and where the population tends to have higher income. Medical equipment and supplies distribution is a very specialized and localized business sector in the PRC. Distributors of medical equipment and supplies operate in the PRC within relatively small and geographically dispersed markets, each based in a wealthy eastern city to cover the surrounding areas, with few distributors willing or able to cover the entire country. Most distributors focus on the PRC’s eastern cities, where the bulk of purchasing power is concentrated, while the western part of the PRC has very limited coverage. The fact that different areas of the PRC have their own medical and insurance practices, purchasing policies and regulatory issues further increases the complexity of medical equipment and supplies distribution in the PRC.
Some of the key factors contributing to the growth of the medical equipment market in the country include the PRC’s aging population, the popularization of private hospitals and clinics and the growing demand for high-quality medical equipment and efficient healthcare services. Additionally, initiatives by the government, such as reforms in the national healthcare system, are also fueling growth in the industry.
The PRC Radiotherapy Market
The Ministry of Health identified cancer as the leading cause of death in the PRC for the past years. Further, the presence of both cancer related illnesses and deaths is expected to increase in the future due to certain environmental factors, such as air and water pollution, associated with the PRC’s rapid industrial expansion. As a result, effective treatment of cancer is a high priority for the PRC healthcare system.
Radiotherapy is used in approximately 50% of all cancer treatments worldwide. Based on information most recently available to us, it is estimated that the United States has 13 radiotherapy units per million people. The World Health Organization has previously recommended 6 radiotherapy units per million people for “developed” countries. The PRC remains well below the recommended threshold. In 2007, there was less than one radiotherapy unit per million people in the PRC.
The PRC radiotherapy industry has the following characteristics:
|
|
·
|
consolidation of the market as small suppliers find it difficult to compete;
|
|
|
·
|
high degree of government regulation with respect to unit pricing and approval process;
|
|
|
·
|
large discrepancy between the need for and access to radiation oncology systems; and
|
|
|
·
|
high barriers to entry due to both high technology requirements and established relationships.
|
Trends in the radiotherapy market in the PRC include:
|
|
·
|
increasing ability of the PRC medical community to detect cancer at treatable stages;
|
|
|
·
|
increasing acceptance of the use of Western style medicine and treatment approaches;
|
|
|
·
|
emerging middle class with increased financial ability to pay for medical procedures and improved cancer care; and
|
|
|
·
|
government indications to increase permissible spending on medical equipment procurement.
|
Our Corporate History
We were incorporated as a limited liability company in November 2002 under the name of Pinewood Imports, Ltd. and converted into a corporation under the laws of the State of Nevada on August 29, 2005. The Company was originally engaged in the business of importing molding and door component products, such as framing materials, made from pine wood from Brazil for resale and distribution. On September 6, 2006, we change our name to Mill Basin Technologies, Ltd. and became an inactive company that was in pursuit of merger opportunities or business operations.
5
On May 15, 2007, we entered into a Share Exchange Agreement to acquire all of the outstanding shares of Allied Moral Holdings Limited, a company with limited liability incorporated in the British Virgin Islands on July 26, 2006, with operating subsidiaries in the PRC. Allied Moral Holdings Limited became our wholly owned subsidiary and we changed our name to Huiheng Medical, Inc. At the time we acquired Allied Moral Holdings Limited, it, through its operating subsidiaries in the PRC, designed, developed and manufactured, and provided sales and services in connection with, radiation therapy medical equipment used for the treatment of cancer.
We operate as a Nevada holding company and we conduct all of our business through our operating subsidiaries as described in the chart below. As described above, we own 100% of the equity interest of Allied Moral Holdings, Ltd. (“Allied Moral”), a British Virgin Islands company that, in turn, owns 100% of the equity interest of Tibet Changdu Huiheng Development Company, Ltd., a Tibetan holding company (“Changdu Huiheng”) that, in turn, directly owns 100%, 75% and 50%, respectively, of our operating subsidiaries Wuhan Kangqiao Medical New Technology Company, Ltd., a PRC company (“Wuhan Kangqiao”), Shenzhen Hyper Technology Company, Ltd., a PRC company (“Shenzhen Hyper”), and Beijing Yuankang Kbeta Nuclear Technology Co., Ltd., a PRC company (“Beijing Kbeta”).
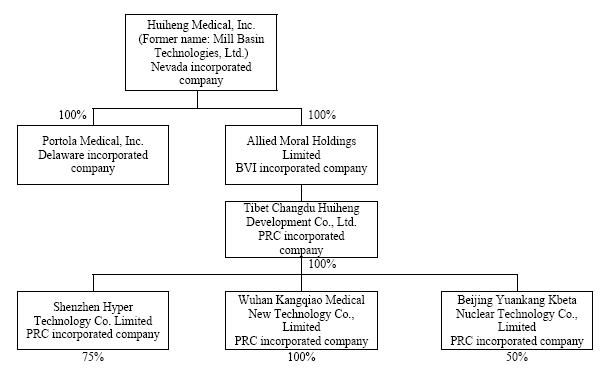
Wuhan Kangqiao focuses on research and development and production management of the Head Gamma Treatment System and Body Gamma Treatment System. Shenzhen Hyper focuses on research and development and production management of the Super Gamma System and our multileaf collimator product that is used in conjunction with a linear accelerator for cancer treatment. Beijing Kbeta focuses on installation and replacement of the Cobalt 60 radioactive sources used in our gamma treatment systems products. The remaining equity interests in Shenzhen Hyper and Beijing Kbeta are owned by unrelated, unaffiliated parties.
6
As described previously, Portola Medical, Inc.’s primary asset consists of its rights to develop, manufacture and sell an adjustable Multi-Catheter Source Applicator which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer. We plan to market and sell this product in the United States and in Asia. Our development and marketing plan is in its initial phases.
Government Regulation
The sale and marketing of our products are subject to regulation in the PRC and in most other countries where we intend to conduct business. For a significant number of our products, we need to obtain and renew licenses and registrations with SFDA and its equivalent in other markets.
In the event we begin to market and sell the Multi-Catheter Source Applicator owned by Portola Medical, Inc. in the United States, we will need to ensure compliance with various federal and state laws.
Regulation
Our products are subject to regulatory controls governing medical devices. Our SGS, BGTS and GTS products are authorized by the SFDA which is the regulatory institution for medical devices in the PRC. As a developer of medical equipment, we are subject to regulation and oversight by different levels of the SFDA. We are also subject to other government laws and regulations which are applicable to developers in general. SFDA requirements include obtaining production permits, compliance with clinical testing standards, development practices, quality standards, applicable industry and adverse reporting, and advertising and packing standards. In addition, the installation of our products require the approval by the Ministry of Health and local provincial government.
On April 1, 2000, SFDA formulated a set of regulations for the medical device market - “Regulations for the Supervision and Administration of Medical Devices” - which included general provisions, administration of medical devices, administration of production, distribution and use of medical devices, supervision of medical devices, penalties, and supplementary provisions. Recent regulatory changes in the PRC include improvements in the supervision and efficiency of medical devices testing, introduction of a new pricing policy for medical devices, and introduction of a new regulation - “Provisions on Daily Supervision and Administration of Medical Devices Manufacturing Enterprises,” by SFDA in 2006. In addition, among other recently issued regulations, “Measure for the Examination of Medical Apparatus Advertisements” came into effect on May 20, 2009. Also on May 20, 2009, the “Standard for the Examination of Medical Apparatus Advertisements” came into effect.
Classification of medical devices
In China, medical devices are classified into three different categories, Class I, Class II and Class III, depending on the degree of risk associated with each medical device and the extent of control needed to ensure safety and effectiveness. Classification of a medical device is important because the class to which a medical device is assigned determines, among other things, whether a developer needs to obtain a production permit and the level of regulatory authority involved in obtaining such permit. Classification of a device also determines the types of registration required and the level of regulatory authority involved in effecting the registration.
Class I devices are those with low risk to the human body and are subject to “general controls.” Class I devices are regulated by the local food and drug administration of the city where the manufacturer is located. Class II devices are those with medium risk to the human body and are subject to “special controls.” Class II devices require product certification, usually through a quality system assessment, and are regulated by the food and drug administration of the province where the manufacturer is located. Class III devices are those with high risk to the human body, such as life-sustaining, life-supporting or implantable devices, and are regulated by the SFDA under the strictest regulatory control.
Our products are classified as Class III devices, and are therefore subject to all regulatory controls governing Class III medical devices.
Production permit for medical devices
A developer must obtain a production permit from the provincial food and drug administration before commencing the development of Class II or Class III medical devices. A production permit, once obtained, is valid for four years and is renewable upon expiration. To renew a production permit, a developer must submit to the provincial food and drug administration an application to renew the permit, along with required information six months before the expiration date of the permit. In September 2007. we applied for a renewal of our production permit, which was promptly renewed in December 2007.
7
Registration requirements of medical devices
In accordance with the “Administration Measures Regarding Medical Device Registration” implemented on August 9, 2004, before a medical device can be developed for commercial distribution, a developer must obtain medical device registration by establishing, to the satisfaction of respective levels of the food and drug administration, the safety and effectiveness of the medical device. In addition, in order to conduct a clinical trial on a Class II or Class III medical device, the SFDA requires developers to apply and to obtain in advance a favorable inspection result for the device from a third party inspection center approved by the SFDA. The application to the inspection center must be supported by required data, such as animal and laboratory testing results, as well as certain pre-clinical and clinical trial data. If approved, the medical device registration is valid for four years.
The SFDA occasionally changes its policies, adopts additional regulations, revises existing regulations or tightens enforcement, each of which could block or delay the approval process for a medical device.
All of the products that we currently sell, including the OPEN Stereotactic Gamma-ray Radiotherapy System, Stereotactic Gamma-ray Whole Body Therapeutic System (SGS-1), Multi-leaf Collimator, TOP Stereotactic Gamma-ray Radiotherapy System, Whole Body Sterotactic Gamma System (SGS-1+), Head Gamma Treatment System (HGTS) and Body Gamma Treatment System (BGTS) have obtained SFDA approval. The Medical Linear Accelerator (LINAC) we currently sell is manufactured by Shinwa. We filed an application with the SFDA seeking approval of a LINAC product manufactured by the Company. In February 2011, the SFDA approved our LINAC product and we intend to begin selling the LINAC product.
Continuing SFDA regulations
We are subject to continuing regulation by the SFDA. In the event of significant modification to an approved medical device, its labeling or its manufacturing process, a new pre-market approval or pre-market approval supplement may be required. Our GTS products are subject to, among others, the following regulations:
|
|
·
|
SFDA’s quality system regulations, which require developers to create, implement and follow certain design, testing, control, documentation and other quality assurance procedures;
|
|
|
·
|
medical device reporting regulations, which require that developers report to the SFDA certain types of adverse reactions and other events involving their products; and
|
|
|
·
|
SFDA’s general prohibition against promoting products for unapproved uses.
|
Class II and III devices may also be subject to special controls applicable to them, such as supply purchase information, performance standards, quality inspection procedures and product testing, which may not be required for Class I devices. We believe we are in compliance with the applicable SFDA guidelines, but we could be required to change our compliance activities or be subject to other special controls if the SFDA changes or modifies its existing regulations or adopts new requirements.
We are also subject to inspection and market surveillance by the SFDA to determine compliance with regulatory requirements. If the SFDA decides to enforce its regulations and rules, the agency can institute a wide variety of enforcement actions, such as:
|
|
·
|
fines, injunctions and civil penalties;
|
|
|
·
|
recall or seizure of our products;
|
|
|
·
|
the imposition of operating restrictions, partial suspension or complete shutdown of production; and
|
|
|
·
|
criminal prosecution.
|
Regulatory requirements for developing international markets
We believe that the regulatory requirements of some international markets we are targeting will be satisfied at least in part by the regulatory approvals granted by the SFDA. We believe that compliance with regulatory requirements in the international markets that we are targeting in the near term will ultimately be the responsibility of the local distributor, especially in markets where regulators will rely on the SFDA approval process. We have not fully evaluated the regulatory requirements of these markets and our ability to comply with the requirements of a jurisdiction will be a factor in our expansion plans. These requirements may include: the need for additional clinical trials; compliance with labeling, advertising and promotion restrictions; manufacturing and quality control obligations; and post-market reporting and record keeping obligations.
8
Customers
China
We sell our products through distributors who primarily consist of third party hospital equipment financing companies and who purchase our equipment and services directly, and resell such equipment and services to hospitals and clinics. Thus, even though these distributors are the purchasers of our equipment, the end users of our products and services are hospitals and clinics that treat various types of tumors. Further, although these distributors are contractually obligated to pay us for the equipment and services, their ability to pay us is dependent upon their receipt of the payments from hospitals and clinics who utilize the equipment and services.
In addition, before our equipment can be installed, the hospital or clinic must obtain approval from both the Ministry of Health and local provincial government, which approval process may range from three to twenty-four months. Because of the approval process, the receipt of payment under a particular purchase agreement may be delayed until the equipment is installed and the hospital or and clinic starts using such equipment for treatment since the third party hospital financing company is unable to make payment to us until it receives payment from the hospital or clinic. We are exploring different ways to resolve this delay in payment as it creates long accounts receivable collection cycle and puts a strain on our cash flow.
Currently, we have a limited number of distributors in the PRC (with two distributors accounting for approximately 90% of our sales for the year ending December 31, 2010) While we are expanding our own sales and marketing capabilities and developing an internal distribution network to facilitate direct sales, we will still need to cooperate with these distributors who provide lease financing to the end users consisting of hospitals and clinics.
Outside China
In an effort to expand our customer base outside of China, we have entered into distribution agreements with companies located in India, Argentina, Ukraine, Chile, Russia, Hungary, South Korea and Peru. These agreements were entered into from September 2005 through October 2007. Based on our experience with our distributor in India (where obtaining regulatory approval took more than two years from the execution of the agreement in January 2006), we expect that it will take at least two years from commencement of a distribution agreement before sales commence in those countries, and substantially longer if the applicable regulatory authorities require clinical trials or additional data beyond what was required to obtain SFDA approval. In addition, we understand that in several countries the local distributors have delayed filing for regulatory approval until they have reasonable assurance that there is a hospital or treatment center in the country that wants to acquire the equipment and there is a feasible source of financing. In general, regulatory approval delays concerning client facility preparation needed to install our products can often delay the sale and installation of our products. In certain instances, such as in Peru, the distributor has obtained regulatory approval, but shipment of our products has been delayed as a result of delays in completion of the treatment center where our products will be installed.
Materials and Components
Our manufacturing activities are outsourced and performed by others. We obtain components for our products and assembly services from a network of third party suppliers located in Shenzhen, Beijing, Wuhan and Chengdu, which are considered some of the industrialized areas of China. We obtain our Cobalt 60 sources from Beijing Shuangyuan Isotope Co. Ltd. and Chengdu Zhonghe Isotope Co. Ltd.; electrical cabinets from Wuhan Shankuo Mechanical & Electrical Equipment Co. Ltd; main engines from Jiangsu Duoling Numerical Control Machine Tool Co. Ltd and Chengdu Aviation Plastic Modeling Co. Ltd; and positioning beds from Shenzhen Tianda High-Tech Material Co. Ltd. Although we work closely with these organizations, component shortages may occur from time to time. We do not have long-term agreements with our suppliers. Instead, we obtain these products and services on a purchase order basis. Because of the knowledge of our suppliers concerning our requirements, we would have some difficulties, in the short run, replacing a supplier.
Our major assembly processes are “dry,” meaning that they do not involve significant quantities of solvents, plating solutions or other types of materials that lead to the generation of large amounts of hazardous wastes, process wastewater discharges or air pollutant emissions. Our GTS devices use radioactive isotopes, and the proper handling of this material, both prior to installation and after removing it from a device for replacement, which must occur periodically, is initially our responsibility. However, under current Chinese law and the arrangements that we have with our waste handler, the responsibility and liability for management of that waste transfers to the waste handler with the waste itself.
9
We periodically review the performance of our suppliers and manufacturers, which includes an evaluation of any quality issues and corrective actions.
Intellectual Property
We have been issued 42 patents in China covering radiotherapy devices, switching devices, and various other aspects of the Gamma Treatment System. We have also been issued three patents in the United States, one patent in the European Union and one patent in Sweden. These foreign patents expire between 2020 and 2027. We intend to patent our new inventions both in China and internationally and have filed a total of approximately 15 patent applications in the PRC, U.S. and EU.
In addition, pursuant to our acquisition of Portola Medical, Inc. in June of 2010, we acquired one additional U.S. patent and eight pending U.S. patent applications (“Portola Patents”). The Portola Patents relate to an adjustable Multi-Catheter Source Applicator which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer.
Our patents cover the intellectual property we use in our products. We do not license patent rights from others for our products. Management is not aware of any current or previous infringement of the existing patents. If any infringement occurs, our management intends to vigorously prosecute actions to halt the infringement and recover damages if the value of the patent is judged at the time to be sufficient to justify that effort.
Employees
As of December 31, 2010, we had 79 employees, of which 79 were full-time employees and 0 were part-time employees. Of such employees, 45 were in research and development, 9 were in sales and customer support, and 25 were in finance and administration. We consider our relations with our employees to be strong.
Corporate Information
Our principal executive office is located at: Huiheng Building, Gaoxin 7 Street South, Keyuannan Road, Nanshan District, Shenzhen Guangdong, P.R. China 518057. Our telephone number at that address is 86-755-25331366. Our website address is www.huihengmedical.com. The information on our website is not a part of this Annual Report.
Item 1A. Risk Factors.
Risks Related to our Business
We extend credit to our customers and often do not collect outstanding receivables on a timely basis. Our inability to collect such receivables has had an adverse effect on our immediate and long-term liquidity.
The contracts covering the sale of our equipment provide for the distributor to make a deposit at the time that the order is placed and to make progress payments at various stages of the manufacturing, shipping, installing and testing process, typically with the final 10% payment due 12 months after the customer accepts the product as meeting the specifications. However, in the PRC we have rarely collected payments under those stated terms and our distributors have historically made most of their payments following the installation of our equipment ranging from 12 to 24 months. The payment by our distributors to us for the purchase of our equipment is dependent upon our distributor’s client’s, consisting of hospitals and clinics, ability to pay. Our two largest distributor, who are third party equipment financing companies, had aggregate outstanding accounts receivable balance of $19.75 million and $14.1 million as of December 31, 2010 and 2009, respectively. Of these amounts approximately 58.8% and 58.9% of the accounts receivable as of December 31, 2010 and 2009 were older than 365 days. Accordingly, we have reclassified that portion of accounts receivable that is older than 365 days as a long-term asset. The delay in receipt of customer payments places pressure on our working capital requirements. Although legal action may be available to collect on our accounts receivable, the duration and outcome of litigation is inherently uncertain, particularly in the PRC, where the civil justice system continues to evolve. If we are unable to collect on these accounts, this will adversely affect our liquidity and profitability.
10
Adverse trends in the medical equipment industry, such as an overall decline in sales or a shift away from the therapies that our products support, may reduce our revenues and profitability.
Our business depends on the continued growth of the radiotherapy equipment industry in the PRC. We operate in an industry characterized by technological change, short product life cycles and margin pressures. It is possible that innovations in the treatment of tumors or improvements in radiotherapy equipment developed by others will make our products uncompetitive, reducing our revenues and profits.
We do not have long-term purchase commitments from our customers and we have to rely on obtaining orders from new customers for our products.
Our medical equipment is generally sold one unit at a time through a limited number of hospital equipment financing companies to a particular hospital or clinic, and they generally do not reorder our products. As a result, the continued growth of our business involves making sales to an increasing number of new hospitals and clinics each year. The failure to find and sell to a significant number of new end users each year would limit our revenues and profits.
We also make significant decisions related to production schedules, component procurement commitments, facility requirements, personnel needs and other resource requirements, based upon our estimates of future sales. Because many of our costs and operating expenses are fixed, a reduction in customer demand would reduce our gross margins and operating results. Additionally, from time to time, we may purchase quantities of supplies and materials greater than the amount that is required to fulfill our customer orders in an attempt to secure more favorable pricing, delivery or credit terms. These purchases could expose us to losses from cancellation costs, inventory carrying costs or inventory obsolescence if the expected demand does not materialize, and hence, adversely affect our business and operating results.
The majority of our products sold in the PRC are made to one entity.
The majority of our product sales in the PRC are made to Jiancheng. During the years ended December 31, 2010 and 2009, sales to Jiancheng accounted for 66.84% and 71.27%, respectively, of our total revenues. Any reduction in sales to Jiancheng will negatively impact our results of operations and could harm our business.
The Installation of our Radiotherapy Equipment in Hospitals by our Customers is Subject to Prior Approval by the Chinese Government.
In China, before our radiotherapy equipment may be installed, each hospital or clinic must obtain approval from the Ministry of Health and the local provincial government. The obligation to obtain approval from the Ministry of Health and the local provincial government is the responsibility of our customer, who is usually a third party hospital equipment financing company and distributes our equipment. As part of the application process, however, we will assist in responding to technical questions raised from time to time . Questions raised during the process can include ensuring that equipment has been approved by the SFDA, that the equipment meets the specifications stated and that the equipment has been properly installed. Because there is no standard application and approval procedure, the processing time for obtaining approval from the local provincial government will vary from province to province. Based on the Company’s prior experience, and depending on the priority of and interest of the local provincial government, obtaining the Ministry of Health and local provincial government approval by our customers has taken from 3 to 24 months. As a result, our ability to sell, install and recognize revenue for sales of our radiotherapy equipment through our customers is largely dependent on the customer’s and hospital’s ability to obtain approval by the Ministry of Health and the local provincial government.
We need additional capital, and we may be unable to obtain such capital in a timely manner or on acceptable terms, or at all.
In order to grow, remain competitive, develop new products and expand distribution, we require additional capital. Our ability to obtain additional capital will be subject to a variety of uncertainties, including:
|
|
·
|
our future financial condition, results of operations and cash flows;
|
|
|
·
|
general market conditions for capital raising activities by medical equipment and related companies;
|
|
|
·
|
investor demand for new offerings by issuers with small market capitalizations; and
|
|
|
·
|
economic, political and other conditions in China and elsewhere.
|
11
As a result of such uncertainties, we may be unable to obtain additional capital in a timely manner, or on acceptable terms or at all. Furthermore, the terms and amount of any additional capital raised through issuances of equity securities may result in significant stockholder dilution.
The price and the sales of our products may be adversely affected by reductions in treatment fees by the Chinese government.
Treatment fees for our radiotherapy systems are subject to prices set by provincial governments in China, and these prices can be adjusted downward or upward from time to time. If the treatment fees for our products are reduced by the government, some customers may be discouraged from buying our products, which would reduce our sales. We may need to decrease the price of our products to provide our customers with acceptable financial returns on their purchases. Our business or results of operations may be adversely affected by a reduction in treatment fees for our products in the future.
Failure to optimize our sourcing activities and cost structure could materially increase our expenses, causing a decline in our margins and profitability.
We strive to utilize our parts suppliers and manufacturing services in an efficient manner. The efficiency of our operations depends in part on our success in accurately forecasting demand for our products and planning component parts and outsourced manufacturing services for new products that we intend to produce. Failure to optimize our sourcing activities and cost structure could materially and adversely affect our business and operating results.
Moreover, our cost structure is subject to fluctuations from inflationary pressures. Recently, China has experienced dramatic growth in its economy. Future instances of similar growth may lead to pressure on wages and salaries that may exceed increases in productivity. In addition, these pressures may be exacerbated by exchange rate movements.
Our business is subject to intense competition, which may reduce demand for our products and materially and adversely affect our business, financial condition, results of operations and prospects.
The medical equipment market is highly competitive, and we expect the level of competition to remain at its current level or intensify. We face direct competition in China and will do so in other markets should we expand internationally. This competition is across all product lines and at all price points. Our competitors also vary significantly according to business segment. For domestic sales, our competitors include publicly-traded and privately-held multinational companies, as well as domestic Chinese companies. To date, our international sales have been very limited (zero international sales in 2009 and zero sales in 2010) and while we are planning to engage in additional international sales in Latin American and Eastern Europe in the near future, there is no guarantee that we will meet with success. Our competitors are primarily publicly-traded and privately-held multinational companies. We also face competition in international sales from companies that have local operations in the markets in which we sell our products.
Some of our larger competitors, especially the multinational companies, have, among them:
|
|
·
|
greater financial and other resources;
|
|
|
·
|
a larger variety of products that have received regulatory approvals;
|
|
|
·
|
more extensive research and development and technical capabilities;
|
|
|
·
|
patent portfolios that may present an obstacle to our conduct of business;
|
|
|
·
|
greater knowledge of local market conditions where we seek to increase our international sales;
|
|
|
·
|
stronger brand recognition; and
|
|
|
·
|
larger sales and distribution networks.
|
As a result, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, and/or market our products as effectively as our competitors or otherwise respond successfully to competitive pressures. In addition, our competitors may be able to offer discounts on competing products as part of a “bundle” of non-competing products, systems and services that they sell to our customers, and we may not be able to match those discounts while remaining profitable. Furthermore, our competitors may develop technologies and products that are more effective than ours or that render our products obsolete or uncompetitive. In addition, the timing of the introduction of competing products into the market could affect the market acceptance and market share of our products. Our failure to compete successfully could materially and adversely affect our business, financial condition, results of operation and prospects.
12
Moreover, some of our internationally-based competitors have established or are in the process of establishing production and research and development facilities in China, while others have entered into cooperative business arrangements with Chinese manufacturers. If we are unable to develop competitive products, obtain regulatory approval or clearance and supply sufficient quantities to the market as quickly and effectively as our competitors, market acceptance of our products may be limited, which could result in decreased sales. In addition, we may not be able to maintain our outsourced manufacturing cost advantage.
In addition, we believe that corrupt practices in the medical equipment industry in China still occur, although it is difficult to quantify. To increase sales, certain manufacturers or distributors of medical equipment may pay kickbacks or provide other benefits to hospital personnel who make procurement decisions. Our company policy prohibits these practices. As a result, as competition intensifies in the medical equipment industry in China, we may lose sales, customers or contracts to competitors who engage in these practices, and there may be no remedy we can pursue to prevent this.
We may fail to effectively develop and commercialize new products, which would materially and adversely affect our business, financial condition, results of operations and prospects.
Our success depends on our ability to anticipate technology development trends and to identify, develop and commercialize in a timely and cost-effective manner new and advanced products that our customers demand. Moreover, it may take an extended period of time for our new products to gain market acceptance, if at all. Furthermore, as the life cycle for a product matures, the average selling price generally decreases. Although we have previously offset the effect of declining average sales prices through increased sales volumes and reductions in outsourced manufacturing costs, we may be unable to continue to do so. Lastly, during a product’s life cycle, problems may arise regarding regulatory, intellectual property, product liability or other issues which may affect its continued commercial viability.
Whether we will be successful in developing and commercializing new products will depend on our ability to:
|
|
·
|
obtain regulatory clearances or approvals in a timely and cost efficient manner;
|
|
|
·
|
optimize our procurement processes to control costs;
|
|
|
·
|
manufacture and deliver quality products in a timely manner;
|
|
|
·
|
increase customer awareness and acceptance of our new products;
|
|
|
·
|
compete effectively with other medical equipment companies;
|
|
|
·
|
price our products competitively; and
|
|
|
·
|
effectively integrate customer feedback into our research and development planning.
|
If we fail to obtain or maintain applicable regulatory clearances or approvals for our products, or if such clearances or approvals are delayed, we will be unable to distribute our products in a timely manner, or at all, which could significantly disrupt our business and materially and adversely affect our sales and profitability.
The sale and marketing of our products are subject to regulation in the PRC and in most other countries where we intend to conduct business. For a significant portion of our products, we need to obtain and renew licenses and registrations with the SFDA and its equivalent in other markets. The processes for obtaining regulatory clearances or approvals can be lengthy and expensive, and the results are unpredictable. All of the products that we currently sell, including the OPEN Stereotactic Gamma-ray Radiotherapy System, Stereotactic Gamma-ray Whole Body Therapeutic System (SGS-1), Multi-leaf Collimator, TOP Stereotactic Gamma-ray Radiotherapy System, and Whole Body Sterotactic Gamma System (SGS-1+) have obtained SFDA approval. The Medical Linear Accelerator (LINAC) we currently sell is manufactured by Shinwa. As of February 8, 2010, we filed an application with SFDA seeking approval of a LINAC product manufactured by the Company which was approved in February 2011. We intend to sell our LINAC soon. In addition, relevant regulatory authorities may introduce additional requirements or procedures that could have the effect of delaying or prolonging the approval for our existing or new products. For example, in 2007 the SFDA introduced a new safety standard to its approval process for new medical equipment which we believe has increased the typical time period required to obtain approval by approximately three months. If we are unable to obtain approvals needed to market existing or new products, or obtain such approvals in a timely fashion, our business would be significantly disrupted, and our sales and profitability could be materially and adversely affected.
In particular, as we enter foreign markets, we lack the experience and familiarity with both the regulators and the regulatory systems, which could make the process more difficult, more costly, more time consuming and less likely to succeed.
13
Failure to manage our growth could strain our management, operational and other resources, which could materially and adversely affect our business and prospects.
Our growth strategy includes building our brand, increasing market penetration of our existing products, developing new products, increasing our targeting of hospitals in the PRC, and expanding internationally. Pursuing these strategies has resulted in, and will continue to result in, substantial demands on management resources.
In addition, the management of our growth will require, among other things:
|
|
·
|
enhancement of our research and development capabilities;
|
|
|
·
|
stringent cost controls;
|
|
|
·
|
adequate working capital and financial resources;
|
|
|
·
|
strengthening of financial and management controls and information technology systems;
|
|
|
·
|
increased marketing, sales and sales support activities; and
|
|
|
·
|
hiring and training of new personnel.
|
If we are not able to manage our growth successfully, our business and prospects would be materially and adversely affected.
If we are unable to effectively and efficiently improve and maintain our controls and procedures, there could be a material adverse effect on our operations or financial results.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the Sarbanes-Oxley Act of 2002 (“SOX”). These requirements may place a strain on our systems and resources, especially as we grow into other markets and develop our own manufacturing capabilities. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and financial condition. SOX requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are currently reviewing and further documenting our internal control procedures. We expect to devote significant resources to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting.
Effective internal controls are necessary for us to provide reliable financial reports. If we cannot provide reliable financial reports, our business and operating results could be harmed. For year ended December 31, 2010, we noted that we needed improvement in our enterprise risk management system to address enterprise risk and internal policies over enterprise level controls. Because of these deficiencies in our controls and procedures, we have undertaken the following remedial measures: (1) we have hired an outside consultant to help us improve internal procedures; and (2) we are working with professional advisors to analyze, re-design and remediate key internal controls over financial reporting in order to standardize internal policies and procedures.
Any failure to implement and maintain the improvements in the controls over our financial reporting, or difficulties encountered in the implementation of these improvements in our controls, could cause us to fail to meet our reporting obligations. Any failure to improve our internal controls to address these identified deficiencies could also cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our stock.
Compliance with rules and regulations concerning corporate governance may be costly, which could harm our business.
We will continue to incur significant legal, accounting and other expenses to comply with regulatory requirements. SOX, together with rules implemented by the Securities and Exchange Commission (“SEC”), has required and will require us to make changes in our corporate governance, public disclosure and compliance practices. In addition, we have incurred significant costs and will continue to incur costs in connection with ensuring that we are in compliance with rules promulgated by the SEC regarding internal controls over financial reporting pursuant to Section 404 of SOX. Compliance with these rules and regulations has increased our legal and financial compliance costs, which have had, and may continue to have, an adverse effect on our profitability.
14
We generate a significant portion of our revenues from a small number of products, and a reduction in demand for any of these products could materially and adversely affect our financial condition and results of operations.
We derive a substantial percentage of our revenues from a small number of products. We currently have just five in our portfolio, and we only recently both launched the fifth product, the SGS-I+, and updated our Medical Linear Accelerator, in 2009. As a result, continued market acceptance and popularity of these products are critical to our success. A reduction in demand due to, among other factors, the introduction of competing products, the entry of new competitors, or customer dissatisfaction with the quality of our products, could materially and adversely affect our financial condition and results of operations.
If we experience a significant number of warranty claims, our costs could substantially increase and our reputation and brand could suffer.
We typically sell our products with warranty terms covering 12 months after purchase. Our product warranty requires us to repair all mechanical malfunctions and, if necessary, replace defective components. If we experience an increase in warranty claims or if our repair and replacement costs associated with warranty claims increase significantly, we may have to accrue a greater liability for potential warranty claims. Moreover, an increase in the frequency of warranty claims could substantially increase our costs and harm our reputation and brand. Our business, financial condition, results of operations and prospects may suffer materially if we experience a significant increase in warranty claims on our products.
Products we develop may contain design or manufacturing defects, which could result in reduced demand for our products and services and increased customer claims against us, causing us to sustain additional costs, loss of business reputation and legal liability.
Our products are highly complex and may at times contain design or manufacturing errors or failures. Any defects in the products we develop, whether caused by a design, manufacturing or component failure or error, may result in claims, delayed shipments to customers or reduced or cancelled customer orders. If these defects occur, we will incur additional costs, and if they occur in large quantity or frequently, we may sustain additional costs, loss of business reputation and legal liability.
Any product recall could have a material adverse effect on our business, results of operations and financial condition.
Complex medical equipment, such as our radiotherapy systems, can experience performance problems that require review and possible corrective action by the manufacturer. From time to time, we receive reports from users of our products relating to performance problems they have encountered. We expect that we will continue to receive customer reports regarding performance problems they encounter through the use of our products. Furthermore, component failures, manufacturing errors or design defects could result in an unsafe condition or injury to the patient. Any serious failures or defects could cause us to withdraw or recall products, which could result in significant costs such as repair and product replacement costs. We cannot assure investors that market withdrawals or product recalls will not occur in the future. The occurrence of such events could have a material adverse effect on our business, financial condition and results of operations. We are currently unable to insure against this type of liability in China.
We could become involved in intellectual property disputes, resulting in substantial costs and diversion of our management resources. Such disputes could materially and adversely affect our business by increasing our expenses and limiting the resources devoted to expansion of our business, even if we ultimately prevail.
We currently possess 42 patents issued in the PRC, three patents in the U.S., one patent in the European Union and one patent in Sweden. We also have a number of patents pending. If one of our patents is infringed upon by a third party, we may need to devote significant time and financial resources to attempt to halt the infringement. We may not be successful in defending the patents involved in such a dispute. Similarly, while we do not knowingly infringe on patents, copyrights or other intellectual property rights owned by other parties, we may be required to spend a significant amount of time and financial resources to resolve any infringement claims against us. We may not be successful in defending our position or negotiating an alternative remedy. Any litigation could result in substantial costs and diversion of our management resources and could reduce our revenues and profits and harm our financial condition.
We also rely on trade secrets, proprietary know-how and other non-patentable technology, which we seek to protect through non-disclosure agreements with employees. We cannot assure investors that these non-disclosure agreements will not be breached, that we will have adequate remedies for any breach, or that our trade secrets, proprietary know-how and other non-patentable technology will not otherwise become known to, or be independently developed by, our competitors.
15
Enforcement of PRC intellectual property-related laws has historically been deficient and ineffective, and is hampered by corruption and local protectionism. Accordingly, intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other countries. Policing unauthorized use of proprietary technology is difficult and expensive, and we might need to resort to litigation to enforce or defend patents issued to us or to determine the enforceability, scope and validity of our proprietary rights or those of others. The experience and capabilities of PRC courts in handling intellectual property litigation varies, and outcomes are unpredictable. Further, such litigation may require significant expenditure of cash and management efforts and could harm our business, financial condition and results of operations. An adverse determination in any such litigation will impair our intellectual property rights and may harm our business, prospects and reputation.
We may develop new products that do not gain market acceptance, and the significant costs in designing and manufacturing such product solutions could hurt our profitability and operations.
We operate in an industry characterized by frequent technological advances, the introduction of new products and new design and manufacturing technologies. We expect to introduce two new products over the next several years. As a result, we are expending funds and committing resources to research and development activities, possibly requiring additional engineering and other technical personnel; purchasing new design, production, and test equipment; expanding our manufacturing capabilities and continually enhancing design processes and techniques. Delays in product approval by regulatory authorities could further increase our costs. We may invest in equipment employing new production techniques for existing products and new equipment in support of new technologies that fail to generate adequate returns on the investment due to insufficient productivity, functionality or market acceptance of the products for which the equipment may be used. We could, therefore, incur significant costs in design and manufacturing services for new products that do not result in sufficient revenue to make those investments profitable, or in any revenue at all.
Our limited operating history makes evaluating our business and prospects difficult.
Shenzhen Hyper commenced operations in September 2001, and we installed our first SGS in that year and installed our first MLC in March 2007. Wuhan Kangqiao also commenced operations in September 2001, and we installed our first BGTS in 2003 and our first HGTS in 2004. As a result, we have a limited operating history which may not provide a meaningful basis for investors to evaluate our business, financial performance and prospects. We may not have sufficient experience to address the risks frequently encountered by early-stage companies, and as a result we may not be able to:
|
|
·
|
maintain profitability;
|
|
|
·
|
preserve what we believe (based solely on management’s knowledge of the industry) is our leading position in the GTS market in China;
|
|
|
·
|
acquire and retain customers;
|
|
|
·
|
attract, train, motivate and retain qualified personnel;
|
|
|
·
|
keep up with evolving industry standards and market developments;
|
|
|
·
|
increase the market awareness of our products;
|
|
|
·
|
respond to competitive market conditions;
|
|
|
·
|
maintain adequate control of our expenses;
|
|
|
·
|
manage our relationships with our suppliers and hospital equipment investors; or
|
|
|
·
|
protect our proprietary technologies.
|
If we are unsuccessful in addressing any of these risks, our business may be materially and adversely affected.
Our component and materials suppliers may fail to meet our needs and quality standards, causing us to experience outsourced manufacturing delays, which may harm our relationships with current or prospective customers and reduce sales.
We acquire the components of our equipment from third parties. This exposes us to supply risk and to price increases that we may not be able to pass on to our customers. There may be shortages of some of the materials and components that we use. If we are unable to obtain sufficient amounts of components or materials on a timely and cost effective basis, we may experience outsourced manufacturing delays, which could harm our relationships with current or prospective customers and reduce sales.
16
We are subject to product liability exposure and have no product liability insurance coverage.
As our main products are medical equipment used for the treatment of patients, we are exposed to potential product liability claims in the event that the use of our products causes or is alleged to have caused personal injuries or other adverse effects. A successful product liability claim against us could require us to pay substantial damages. Product liability claims, whether or not successful, are often costly and time-consuming to defend. Also, in the event that our products prove to be defective, we may be required to recall or redesign such products. As the insurance industry in China is still in an early stage of development, we do not have any product liability insurance. A product liability claim, with or without merit, could result in significant adverse publicity against us, and could have a material adverse effect on the marketability of our products and our reputation, which in turn, could have a material adverse effect on our business, financial condition and results of operations. In addition, we do not have any business interruption insurance coverage for our operations. Any business disruption or natural disaster could result in substantial costs and diversion of resources.
New product development in the medical equipment and supply industry is both costly and labor-intensive and has a very low rate of successful commercialization.
Our success will depend in part on our ability to enhance our existing products and technologies and to develop and acquire new products or technologies. The development process for medical technology is complex and uncertain, as well as time-consuming and costly. Product development requires the accurate assessment of technological and market trends as well as precise technological execution. We cannot assure investors that:
|
|
·
|
our product or technology development will be successfully completed;
|
|
|
·
|
necessary regulatory clearances or approvals will be granted by the SFDA or other regulatory bodies as required on a timely basis, or at all; or
|
|
|
·
|
any product or technology we develop can be commercialized or will achieve market acceptance.
|
Also, we may be unable to develop suitable products or technologies or to acquire such products or technologies on commercially reasonable terms. Failure to develop or acquire, obtain necessary regulatory clearances or approvals for, or successfully commercialize or market potential new products or technologies could have a material adverse effect on our financial condition and results of operations.
Potential strategic alliances may not achieve their objectives, which could lead to wasted effort or involvement in ventures that are not profitable and could harm our company’s reputation.
We are currently exploring strategic alliances intended to enhance or complement our technology, to provide additional know-how, components or supplies, and to introduce and distribute products and services utilizing our technology and know-how. Any strategic alliances entered into may not achieve their strategic objectives, and parties to our strategic alliances may not perform as contemplated. As a result, the alliances themselves may run at a loss, which would reduce our profitability, and if the products or customer service provided by such alliances were of inferior quality, our reputation in the marketplace could be harmed, affecting our existing and future customer relationships.
We may not be able to retain, recruit and train adequate management and production personnel. We rely heavily on those personnel to help develop and execute our business plans and strategies, and if we lose such personnel, it would reduce our ability to operate effectively.
Our success is dependent, to a large extent, on our ability to retain the services of our executive management, who have contributed to our growth and expansion to date. Our Chairman, Mr. Hui Xiaobing, has been, and will continue to be, instrumental to our success. In addition, the executive directors play an important role in our operations and the development of our new products. Accordingly, the loss of their services, without suitable replacements, will have an adverse effect on our business generally, operating results and future prospects.
In addition, our continued operations are dependent upon our ability to identify and recruit adequate management and production personnel in China. We require trained graduates of varying levels and experience and a flexible work force of semi-skilled operators. Many of our current employees come from the more remote regions of China as they are attracted by the wage differential and prospects afforded by Shenzhen, Beijing and our operations. With the economic growth currently being experienced in China, competition for qualified personnel will be substantial, and there can be no guarantee that a favorable employment climate will continue and that wage rates we must offer to attract qualified personnel will enable us to remain competitive. The inability to attract such personnel or the increased cost of doing so could reduce our competitive advantage relative to our competitors, reducing or eliminating our growth in revenues and profits.
17
Risks Related to our Activities in China
The Chinese legal system may have inherent uncertainties that could materially and adversely impact our ability to enforce the agreements governing our operations.
We are subject to oversight at the provincial and local levels of government in China. Our operations and prospects would be materially and adversely affected by the failure of the local government to honor our agreements or an adverse change in the laws governing them. In the event of a dispute, enforcement of these agreements could be difficult in China. China tends to issue legislation, which is followed by implementing regulations, interpretations and guidelines that can render immediate compliance difficult. Similarly, on occasion, conflicts arise between national legislation and implementation by the provinces that take time to reconcile. These factors can present difficulties in our ability to achieve compliance. Unlike the United States, China has a civil law system based on written statutes in which judicial decisions have limited precedential value. The Chinese government has enacted laws and regulations to deal with economic matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, our experience in interpreting and enforcing our rights under these laws and regulations is limited, and our future ability to enforce commercial claims or to resolve commercial disputes in China is therefore unpredictable. These matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces and factors unrelated to the legal merits of a particular matter or dispute may influence their determination.
It will be extremely difficult to acquire jurisdiction and enforce liabilities against our officers, directors and assets based in China.
Substantially all of our assets are located outside of the United States and most of our officers and directors reside outside of the United States. As a result, it may not be possible for United States investors to enforce their legal rights, to effect service of process upon our directors or officers or to enforce judgments of United States courts predicated upon civil liabilities and criminal penalties of our directors and officers under Federal securities laws of the United States. Moreover, we have been advised that the PRC does not have treaties providing for the reciprocal recognition and enforcement of judgments of courts with the United States. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement of criminal penalties of the Federal securities laws of the United States.
Our management may fail to meet the obligations under Chinese law that enable the distribution of profits earned in the PRC to entities outside of the PRC.
A circular promulgated by the State Administration for Foreign Exchange (“SAFE”), which is the PRC governmental body that regulates the conversion of RMB into foreign currencies, has increased the ability of foreign holding companies to receive distributions of profits earned by Chinese operating subsidiaries. We qualify for this treatment, but remaining qualified for it will require the Chinese principals involved in our business to meet annual filing obligations. While they have agreed to meet those annual requirements, it is possible that they will fail to do so, which could limit our ability to gain access to the profits earned by our subsidiary Allied Moral. The result could be the inability to pay dividends to our stockholders or to deploy capital outside of the PRC in a manner that would be beneficial to our business as a whole.
The Chinese government could change its policies toward, or even nationalize, private enterprise, which could leave us unable to use the assets we have accumulated for the purpose of generating profits for the benefit of our stockholders.
Over the past several years, the Chinese government has pursued economic reform policies, including the encouragement of private economic activities and decentralization of economic regulation. The Chinese government may not continue to pursue these policies or may significantly alter them to our detriment without notice. Changes in policies by the Chinese government that result in a change of laws, regulations, their interpretation, or the imposition of confiscatory taxation, restrictions on currency conversion or imports and sources of supply could materially reduce the value of our business by making us uncompetitive or, for example, by reducing our after-tax profits. The nationalization or other expropriation of private enterprises by the Chinese government could result in the total loss of our investment in China, where a significant portion of our profits are generated.
18
National, provincial and local governments have established many regulations governing our business operations.
We are also subject to numerous national, provincial and local governmental regulations, including environmental, labor, waste management, health and safety matters and product specifications and regulatory approvals from healthcare agencies. We are subject to laws and regulations governing our relationship with our employees including: wage requirements, limitations on hours worked, working and safety conditions, citizenship requirements, work permits and travel restrictions. These local labor laws and regulations may require substantial resources for compliance. We are subject to significant government regulation with regard to property ownership and use in connection with our facilities in the PRC, import restrictions, currency restrictions and restrictions on the volume of domestic sales and other areas of regulation. These regulations can limit our ability to react to market pressures in a timely or effective way, thus causing us to lose business or miss opportunities to expand our business.
There may be corrupt practices in the healthcare industry in China, which may place us at a competitive disadvantage if our competitors engage in such practices.
There may be corrupt practices in the healthcare industry in China. For example, in order to secure agreements with hospitals or to increase direct sales of medical equipment, our competitors may engage in corrupt practices in order to influence hospital personnel or other decision-makers in violation of the anti-corruption laws of China and the U.S. Foreign Corrupt Practices Act (“FCPA”). As competition persists and intensifies in our industry, we may lose customers and other opportunities to the extent that our competitors engage in such practices or other illegal activities.
Risks Related to International Operations
We currently conduct most of our business in the PRC. However, we have plans to expand internationally, and the rate and degree of that expansion could be substantial. For that reason, risks related to international operations may be relevant to an investment decision.
The failure of China to continue its policy of economic reforms could, among other things, result in an increase in tariffs and trade restrictions on our products, making our products less attractive in international markets and potentially reducing our future revenues and profits.
China’s government has been reforming its economic system since the late 1970s. The economy of China has historically been a nationalistic, “planned economy,” meaning it has functioned according to governmental plans and pre-set targets or quotas.
However, in recent years, the Chinese government has implemented measures emphasizing the utilization of market forces for economic reform and the reduction of state ownership in business enterprises. Although we believe that the changes adopted by the government of China have had a positive effect on the economic development of China, additional changes still need to be made. For example, a substantial portion of productive assets in China are still owned by the Chinese government. Additionally, the government continues to play a significant role in regulating industrial development. We cannot predict the timing or extent of any future economic reforms that may be proposed, but should they occur, they could reduce our operating flexibility or require us to divert our efforts to products or ventures that are less profitable than those we would elect to pursue on our own.
A recent positive economic change has been China’s entry into the World Trade Organization, the global international organization dealing with the rules of trade between nations. It is believed that China’s entry will ultimately result in a reduction of tariffs for industrial products, a reduction in trade restrictions and an increase in trading with the United States and other western countries. However, China has not fully complied with all of its WTO obligations to date, including fully opening its markets to American goods and easing the current trade imbalance between the two countries. If actions are not taken to rectify these problems, trade relations between the United States and China may be strained, and this may have a negative impact on China’s economy and our business by leading to the imposition of trade barriers on items that incorporate our products, which would reduce the revenues and profits we might otherwise generate from international sales.
In order to market our products internationally, we will be required to obtain relevant regulatory approvals for our products in the countries in which we intend to sell.
Our products will be subject to review by governmental agencies prior to being eligible for sale in new markets. We lack the expertise to navigate such approval processes outside of China and we will be required to rely on our distributors and advisors to obtain such approvals. We will have very little influence over their activities. The failure of our partners to obtain and maintain required approvals will limit our ability to sell our products internationally and harm our prospects for future revenues and profitability.
19
International expansion may be costly, time consuming and difficult. If we do not successfully expand internationally, our profitability and prospects would be materially and adversely affected.
Our success depends to a significant degree upon our ability to expand into international markets. In expanding our business internationally, we intend to enter markets in which we have limited or no experience and in which our brand is not recognized. To promote our brand and generate demand for our products so as to attract distributors in international markets, we expect to spend significantly more on marketing and promotion than we do in our existing markets. We may be unable to attract a sufficient number of distributors, and our selected distributors may not be suitable for selling our products. Furthermore, in new markets we may fail to anticipate competitive conditions that are different from those in China. These competitive conditions may make it difficult or impossible for us to effectively operate in these markets. If our expansion efforts in existing and new markets are unsuccessful, our profitability and prospects would be materially and adversely affected.
As we begin to do business internationally, we will be subject to significant worldwide political, economic, legal and other uncertainties.
Today, substantially all of our assets are located in the PRC. As we begin selling our products to customers worldwide, we will have receivables from, and goods in transit, to those locations.
In addition, international operations will expose us to other risks, including:
|
|
·
|
political instability;
|
|
|
·
|
economic instability and recessions;
|
|
|
·
|
changes in tariffs;
|
|
|
·
|
difficulties of administering foreign operations generally;
|
|
|
·
|
limited protection for intellectual property rights;
|
|
|
·
|
obligations to comply with a wide variety of foreign laws and other regulatory requirements;
|
|
|
·
|
increased risk of exposure to terrorist activities;
|
|
|
·
|
reliance on our international distributors for compliance with foreign regulatory approvals and sales;
|
|
|
·
|
export license requirements;
|
|
|
·
|
unauthorized re-export of our products;
|
|
|
·
|
potentially adverse tax consequences; and
|
|
|
·
|
the inability to effectively enforce contractual or legal rights.
|
The additional risks and costs of our intended international expansion could harm our profitability and financial condition.
Fluctuation of the Renminbi could make our pricing less attractive, causing us to lose sales, or could reduce our profitability when stated in terms of another currency, such as the U.S. dollar.
The value of the Renminbi, or RMB, the main currency used in China, fluctuates and is affected by, among other things, changes in the PRC’s political and economic conditions. The conversion of RMB into foreign currencies such as the U.S. dollar has been generally based on rates set by the People’s Bank of China, which are set daily based on the previous day’s interbank foreign exchange market rates and current exchange rates on the world financial markets. The official exchange rate had remained stable over the past several years. However, the PRC recently adopted a floating rate with respect to the RMB. As a result, the average monthly exchange rate of the RMB to the U.S. dollar was about RMB $6.7604 to one U.S. dollar for the year ended December 31, 2010 as compared to an average of approximately RMB $6.8303 during 2009. This floating exchange rate, and any appreciation of the RMB that may result from such rate, could have various effects on our international business, which include making our products more expensive relative to those of our competitors. It is not possible to predict if the net effects of the appreciation of the RMB, to whatever extent it occurs, would be positive or negative for our business.
20
Changes in foreign exchange regulations in China may affect our ability to pay dividends in foreign currency or conduct other business for which we would need access to foreign currency exchange.
The Renminbi is not currently a freely convertible currency, and the restrictions on currency exchanges may limit our ability to use revenues generated in RMB to fund business activities outside the PRC or to make dividends or other payments in United States dollars. The Chinese government strictly regulates conversion of RMB into foreign currencies. For example, RMB cannot be converted into foreign currencies for the purpose of expatriating the foreign currency, except for purposes such as payment of debts lawfully owed to parties outside of the PRC. Over the years, foreign exchange regulations in the PRC have significantly reduced the government’s control over routine foreign exchange transactions under current accounts.
We cannot provide any assurance that Chinese regulatory authorities will not impose further restrictions on the convertibility of the RMB. Since one of our PRC subsidiaries currently generates virtually all of our revenues and these revenues are denominated mainly in RMB, any future restrictions on currency exchanges may limit our ability to repatriate such revenues for the distribution of dividends to our stockholders or for funding our other business activities outside the PRC.
We are subject to various tax regimes, which may adversely affect our profitability and tax liabilities in the future.
Huiheng is incorporated in the U.S. and has subsidiaries and other operations in the PRC and the British Virgin Islands. We are subject to the tax regimes of these countries. Although virtually all of Huiheng’s profits will be earned outside of the U.S., under U.S. tax laws Huiheng’s earnings generally will be subject to U.S. taxation, because U.S. companies are generally taxed on their world-wide income. This may be true even if Huiheng does not repatriate any of its foreign earnings to the U.S. For certain types of income (generally, income from an active trade or business), U.S. companies are not required to pay tax on that income until they repatriate those earnings to the U.S. (such as for use in paying dividends or repurchasing shares). As a result, repatriation of earnings would trigger more immediate tax obligations. As a result of the imposition of U.S. taxes, Huiheng’s after-tax profits could decrease and could be below the level that would have been obtained if Huiheng were incorporated outside the U.S. The amount of taxes payable in the U.S. generally depends on the profitability of our various operations and the application of available tax credits and tax treaties. We are not currently receiving the benefit of any U.S. tax credit, and we are not currently conducting a material amount of business in a country with an advantageous tax treaty. Since the effect of tax credits and tax treaties depends on the profitability of operations in various jurisdictions, the amount of our tax will vary over time as we change the geographic scope of our activities. However, for the near term we expect that our total tax rate will be significantly influenced by the taxes we pay in China, so that our total tax obligation might decrease as a result of favorable tax treatment in China even though we were subject to additional U.S. taxes. In the future, Huiheng may pay significantly higher taxes than we have paid historically. In addition, any change in tax laws and regulations or the interpretation or application thereof, either internally in one of those jurisdictions or as between those jurisdictions, may adversely affect Huiheng’s profitability and tax liabilities in the future.
Because Chinese law will govern almost all of our material agreements, we may not be able to enforce our legal rights internationally, which could result in a significant loss of business, business opportunities, or capital.
Chinese law will govern almost all of our material agreements. We cannot assure investors that we will be able to enforce any of our material agreements or that remedies will be available outside of the PRC. The system of laws and the enforcement of existing laws in the PRC may not be as certain in implementation and interpretation as in the United States. The Chinese judiciary is relatively inexperienced in enforcing corporate and commercial law, leading to a higher than usual degree of uncertainty as to the outcome of any litigation. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital.
Imposition of trade barriers and taxes may reduce our ability to do business internationally, and the resulting loss of revenue could harm our profitability.
We may experience barriers to conducting business and trade in our targeted emerging markets in the form of delayed customs clearances, customs duties and tariffs. In addition, we may be subject to repatriation taxes levied upon the exchange of income from local currency into foreign currency, substantial taxes of profits, revenues, assets and payroll, as well as value-added tax. Further obstacles still may arise in obtaining the approval for the use of our equipment in the health care systems of various countries. The markets in which we plan to operate may impose onerous and unpredictable duties, tariffs and taxes on our business and products, and there can be no assurance that this will not reduce the level of sales that we achieve in such markets, which would reduce our revenues and profits.
The PRC has agreed that foreign companies will be allowed to import most products into any part of the PRC. In the sensitive area of intellectual property rights, the PRC has agreed to implement the trade-related intellectual property agreement of the Uruguay Round. There can be no assurances that the PRC will implement any or all of the requirements of its membership in the World Trade Organization in a timely manner, if at all. If the PRC does not fulfill its obligations to the World Trade Organization, we may be subject to retaliatory actions by the governments of the countries into which we sell our products, which could render our products less attractive, thus reducing our revenues and profits.
21
Risks Related to our Securities
The market price of our shares is subject to significant price and volume fluctuations.
The price of our common stock may be subject to wide fluctuations due to variations in our operating results, news announcements, our limited trading volume, general market trends both domestically and internationally, currency movements, sales of common shares by our officers, directors and our principal stockholders, and sales of common shares by existing investors. Certain events, such as the issuance of common shares upon the exercise of our outstanding stock options, could also materially and adversely affect the prevailing market price of our common shares. Further, the stock markets in general have recently experienced extreme price and volume fluctuations that have affected the market prices of equity securities of many companies and that have been unrelated or disproportionate to the operating performance of such companies. In addition, a change in sentiment by U.S. investors for China-based companies could have a negative impact on the stock price. These fluctuations may materially and adversely affect the market price of our common shares and the ability to resell shares at or above the price paid, or at any price.
One of our stockholders, which is controlled by our Chief Executive Officer, currently owns approximately 85% of our common stock and has the ability to prevent certain types of corporate actions, to the detriment of other stockholders.
Clear Honest International Limited, a company controlled by Mr. Hui Xiaobing (our Chief Executive Officer) owns 11,750,000 shares of our common stock, which represents approximately 84% of our outstanding shares of common stock. Mr. Hui is able to exercise significant influence over all matters requiring stockholder approval, including the election of a majority of the directors and determination of significant corporate actions. This ownership percentage and level of influence will increase if the earnout shares are issued. If all 928,575 of the earnout shares are issued as additional consideration under the Allied Moral share exchange (which would occur from 2009 through 2011) and assuming that there are no other issuances of shares, Clear Honest would own approximately 86% of our issued and outstanding common stock. This concentration of ownership could also have the effect of delaying or preventing a change in control that could otherwise be beneficial to our stockholders.
Our Articles of Incorporation authorize our board of directors to issue new series of preferred stock that may have the effect of delaying or preventing a change of control, which could adversely affect the value of your shares.
Our articles of incorporation provide that our board of directors will be authorized to issue from time to time, without further stockholder approval, up to 700,000 additional shares of preferred stock in one or more series and to fix or alter the designations, preferences, rights and any qualifications, limitations or restrictions of the shares of each series, including the dividend rights, dividend rates, conversion rights, voting rights, rights of redemption, including sinking fund provisions, redemption price or prices, liquidation preferences and the number of shares constituting any series or designations of any series. Such shares of preferred stock could have preferences over our common stock with respect to dividends and liquidation rights. We may issue additional preferred stock in ways which may delay, defer or prevent a change of control of our company without further action by our stockholders. Such shares of preferred stock may be issued with voting rights that may adversely affect the voting power of the holders of our common stock by increasing the number of outstanding shares having voting rights, and by the creation of class or series voting rights.
Our common stock has been thinly traded and we cannot predict the extent to which a trading market will develop.
Our common stock is quoted on the Over-the-Counter Bulletin Board. Our common stock is thinly traded compared to larger, more widely known companies. Thinly traded common stock can be more volatile than common stock trading in an active public market. We cannot predict the extent to which an active public market for our common stock will develop or be sustained.
We do not expect to pay dividends.
We expect to apply our future earnings, if any, toward the further expansion and development of our business. The likelihood of paying dividends is further reduced by the fact that, in order to pay dividends, we would need to repatriate profits earned outside of the U.S., and in doing so those profits generally would become subject to U.S. taxation. Thus, the liquidity of an investment in our Company is dependent upon the investor’s ability to sell their shares at an acceptable price, rather than receiving an income stream from their investment. The price of our stock may decline and fluctuations in market price coupled with limited trading volume in our shares may limit your ability to realize any value from your investment, including recovering the initial purchase price.
22
Not applicable.
Item 2. Properties.
We currently operate our business out of two properties located in the PRC. Wuhan Kangqiao is headquartered in Wuhan, China where it owns a 287 square meter office building that houses management, research and development personnel, marketing, finance and administrative support staff.
Shenzhen Hyper is headquartered in Shenzhen, China, where it leases office space of 4,000 square meters, with a monthly rental of RMB $160,000 (USD 24,200) per month. The property houses management, research and development personnel, marketing, finance and administrative support staff. The lease will expire at the end of December 2027. We relocated to this space at the beginning of 2008.
Neither facility contains equipment or specialized improvements that would be difficult to move to a new location. If we decided to relocate, we believe that there are many facilities in these locations that would be suitable for our needs.
Item 3. Legal Proceedings.
To the best of management’s knowledge, the Company is not involved in any legal proceedings.
Item 4. (Removed and Reserved).
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market for Registrant’s Common Equity
Our common stock is currently quoted on the Over-the-Counter Bulletin Board maintained by the Financial Industry Regulatory Authority (“FINRA”) under the Symbol: “HHGM.” The table below lists the high and low closing prices per share of our common stock for each quarterly period during the past two fiscal years as quoted on the Over-the-Counter Bulletin Board.
|
Fiscal 2009
|
High
|
Low
|
||||||
|
First Quarter
|
$ | 5.50 | $ | 1.98 | ||||
|
Second Quarter
|
$ | 1.95 | $ | 0.30 | ||||
|
Third Quarter
|
$ | 2.00 | $ | 0.50 | ||||
|
Fourth Quarter
|
$ | 3.00 | $ | 1.00 | ||||
|
Fiscal 2010
|
High
|
Low
|
||||||
|
First Quarter
|
$ | 2.59 | $ | 1.65 | ||||
|
Second Quarter
|
$ | 1.85 | $ | 1.10 | ||||
|
Third Quarter
|
$ | 2.15 | $ | 1.20 | ||||
|
Fourth Quarter
|
$ | 1.60 | $ | 0.80 | ||||
Trading in our common stock has been sporadic and the quotations set forth above are not necessarily indicative of actual market conditions. All prices reflect inter-dealer prices without retail mark-up, mark-down, or commission and may not necessarily reflect actual transactions.
Holders
At March 15, 2011 there were 14,030,637 shares of common stock issued and outstanding that were held by approximately 37 shareholders of record.
23
Dividends
We have never declared any cash dividends and we have no intention of issuing cash dividends in the foreseeable future.
China’s SAFE and Foreign Acquisition Regulation All of our business is conducted through our subsidiaries based in China. Renminbi, or RMB, is not a freely convertible currency currently, and the restrictions on currency exchanges may limit our ability to make dividends or other payments in United States dollars. However, in accordance with the existing foreign exchange regulations in China, Allied Moral is able to pay dividends in foreign currencies, without prior approval from the SAFE, by complying with certain procedural requirements. The current foreign exchange measures may be changed in a way that will make payment of dividends and other distributions outside of China more difficult or unlawful. As a result, if we intend to distribute profits outside of China, we may not be able to obtain sufficient foreign exchange to do so. Additionally, China regulatory authorities may impose further restrictions on the convertibility of the RMB. Since our subsidiaries in China, both direct and indirect, generate virtually all of our revenue, and these revenues are currently denominated in RMB, any future restrictions on currency exchanges may limit our ability to repatriate such revenues for the distribution of dividends to our stockholders.
SAFE regulates the conversion of the RMB into foreign currencies. Currently, Foreign Invested Enterprises (“FIE”) are required to apply for “Foreign Exchange Registration Certificates,” which permit the conversion of RMB into foreign exchange for the purpose of expatriating profits earned in the PRC to a foreign country. Our PRC subsidiary, Changdu Huiheng, is a FIE that has obtained the registration certifications, and with such registration certifications, which need to be renewed annually, Changdu Huiheng is allowed to open foreign currency accounts including a “current account” and “capital account.” Currently, conversion within the scope of the “current account,” such as remittance of foreign currencies for payment of dividends, can be effected without the approval of SAFE. However, conversion of currency in the “capital account,” for capital items such as direct investments, loans, and securities, still requires the approval of SAFE. In accordance with the existing foreign exchange regulations in the PRC, Changdu Huiheng is able to pay dividends in foreign currencies, without prior approval from SAFE, by complying with certain procedural requirements.
SAFE regulations have required extensive documentation and reporting, some of which was burdensome and slowed payments. If there is a return to burdensome payment restrictions and reporting, the ability of a company with its principal operations in China to attract investors will be reduced. Also, current investors may not be able to obtain the profits of the business in which they own for other reasons. Relevant Chinese law and regulation permit payment of dividends only from retained earnings, if any, determined in accordance with Chinese accounting standards and regulations. It is possible that Chinese tax authorities may require changes in our reported income that would limit our ability to pay dividends and other distributions. Chinese law requires companies to set aside a portion of net income to fund certain reserves, which amounts are distributable as dividends. These rules and possible changes could restrict a company in China from repatriating funds to us and our stockholders as dividends.
In addition, on October 21, 2005, SAFE promulgated Notice 75, Notice on Issues concerning Foreign Exchange Management in People’s Republic of the PRC Residents’ Financing and Return investments through Offshore Special Purpose Vehicle (“OSPV”). Notice 75 provides that Chinese residents shall apply for foreign investment exchange registration before establishing or controlling an OSPV, which is defined by Notice 75 as a foreign enterprise directly established or indirectly controlled by Chinese residents for foreign equity capital financing with their domestic enterprise assets and interests. Notice 75 further requires that an amendment to the registration of these Chinese residents be made upon the occurrence of any of the following: (i) when any of these Chinese residents contributes equity interests or assets of a Chinese company to the OSPV, (ii) when the OSPV completes an offshore equity financing, or (iii) when there is a material change involving a change in the capital of the OSPV, such as any increase or decrease in its capital, a share swap, a merger or division, or the creation of any security interests over the relevant assets located in China. Pursuant to Notice 75, Chinese residents are prohibited, among other things, from distributing profits or proceeds from a liquidation, paying bonuses, or transferring shares of the OSPV outside of the PRC if Chinese residents have not completed or do not maintain the foreign investment exchange registration.
Mr. Hui Xiaobing, our principal executive officer and director, has filed the requisite application for foreign investment exchange registration under the relevant laws of the PRC and the regulations of Notice 75, and his registration application has been approved by SAFE. His foreign investment exchange registration is valid, legal and effective for the purpose of Notice 75.
Securities authorized for issuance under equity compensation plans
On June 6, 2009, the Board of Directors and the shareholders of the Company approved the Huiheng Medical, Inc. 2009 Share Plan (the “Plan”), under which employees and directors of the Company are eligible to receive direct awards of shares or grants of stock options (either Incentive Stock Options or Nonstatutory Stock Options, as determined by the administrator of the Plan at the time of grant). Under the Plan, 1,566,666 shares have been reserved. The following equity compensation table summarizes the foregoing:
24
|
Number of securities remaining
|
||||||||||||
|
available for future issuance
|
||||||||||||
|
Number of securities to be issued
|
Weighted-average exercise price
|
under equity compensation
|
||||||||||
|
upon exercise of outstanding
|
of outstanding options, warrants
|
plans (excluding securities
|
||||||||||
|
options, warrants and rights
|
and rights
|
reflected in column (a))
|
||||||||||
|
Plan category
|
(a)
|
(b)
|
(c)
|
|||||||||
|
Equity compensation plans approved by security holders
|
30,000 | $ | 5.00 | 1,536,666 | ||||||||
|
Equity compensation plans not approved by security holders
|
- | - | - | |||||||||
|
Total
|
30,000 | $ | 5.00 | 1,536,666 | ||||||||
Issuance of Unregistered Shares
During 2010, we issued a total of 95,347 shares of our common stock in connection with the exercise of conversion rights by certain holders of 9,077 shares of our Series A Convertible Preferred Stock (“Series A Preferred Stock”). The conversion of the Series A Preferred Stock occurred at a conversion rate of 10.5042 shares of common stock for each share of Series A Preferred Stock. The shares of common stock issued in connection with the conversion were exempt from the registration requirements pursuant to Section 3(a)(9) of Securities Act of 1933 since no commission or other remuneration was paid or given directly or indirectly for soliciting such exchange.
Item 6. Selected Financial Data.
Not applicable.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
In addition to historical information, the following discussion contains forward-looking statements that are subject to risks and uncertainties. Actual results may differ substantially from those referred to herein due to a number of factors, including but not limited to risks described in the section entitled Risks Related to Our Business and elsewhere in this Annual Report.
OVERVIEW
We are a China-based medical equipment company that develops, designs and markets radiation therapy systems used for the treatment of cancer. We currently have five products: the Super Gamma System (“SGS”), the Body Gamma Treatment System (“BGTS”), OPEN Stereotactic Gamma-ray Radiotherapy System, the Head Gamma Treatment System (“HGTS”) and a multileaf collimator device (“MLC”) used in conjunction with a linear accelerator.
In 2005, the ownership interests of Shenzhen Hyper, Wuhan Kangqiao and Beijing Kbeta were reorganized under Changdu Huiheng. Upon the completion of the reorganization, Changdu Huiheng owned 75% of the equity interest in Shenzhen Hyper, 100% of the equity interest of Wuhan Kangqiao and 50% of the equity interest of Beijing Kbeta.
In 2006, we established Allied Moral Holdings, Ltd. in the British Virgin Islands as a holding company and Allied Moral acquired 100% of the ownership interests of Changdu Huiheng as part of an ownership restructuring to facilitate investments by foreign investors. As discussed above in “Business - Huiheng Medical’s Background,” the stockholders of Allied Moral engaged in a share exchange transaction with our company in May 2007.
On June 7, 2010, we completed the acquisition of Portola Medical, Inc. whose primary asset consists of its rights to develop, manufacture and sell an adjustable Multi-Catheter Source Applicator which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer. Portola Medical is in its initial stages and we plan to market and sell this product in the United States and in Asia.
25
Our company is led by Mr. Hui Xiaobing, a pioneer in the GTS industry in the PRC. Through his experience and relationships, Mr. Hui maintains access to China’s hospitals and our company’s principal customers. As a result of his leadership, we have successfully developed a strong sales and marketing force, which covers the entire country and maintains relationships with China’s top medical institutions.
Our expansion plans include broadening our product offering. Our research and development team is focused on developing and producing technologically advanced radiotherapy and GTS products. We currently have 42 patents issued in the PRC, three patents in the U.S., one patent in the European Union, one patent in Sweden and additional patents applications pending. Our SGS, BGTS and GTS products are approved for use in China by the State Food and Drug Administration (“SFDA”), an agency of the Ministry of Healthcare of the PRC.
Our future research and development efforts will focus on developing an advanced magnetic resonance imaging (“MRI”) device and an industrial linear accelerator (“LINAC”) unit used for, among other things, preserving food through irradiation. We will pursue these projects pending the availability of funds for future research and development.
We currently sell our products primarily to a small number of hospital equipment investors in China, who install our systems in hospitals or clinics. We also offer comprehensive post-sales services for our medical equipment to our customers. The service contracts are negotiated and signed independently and separately from the sales of medical equipment. Our post-sales services include radioactive cobalt source replacement and disposal, medical expert training, clinical trial analysis, patient tumor treatment analysis, product maintenance, software upgrades, and consulting. Due to the poor economy during the past years, there has been a decrease in the sales of our products and the majority of our total revenues is attributed to service contract revenue. In addition, in China, before our radiotherapy equipment may be installed, each hospital or clinic must obtain approval from the Ministry of Health and the local provincial government. The obligation to obtain approval from the Ministry of Health and the local provincial government is the responsibility of our customer, who is usually a third party hospital equipment financing company and a distributor of our equipment. As part of the application process, however, we will assist in responding to technical questions raised from time to time. Questions raised during the process can include ensuring that equipment has been approved by the SFDA, that the equipment meets the specifications stated and that the equipment has been properly installed. Because there is no standard application and approval procedure, the processing time for obtaining approval from the local provincial government will vary from province to province. Based on our prior experience the time from application to final installation of our radiotherapy equipment is as follows:
|
|
·
|
Once the sale contract has been signed, the hospital or clinic will submit an application for approval with the Ministry of Health and local provincial government for the installation of our radiotherapy equipment. Depending on the priority of and interest of the local provincial government, obtaining the Ministry of Health and local provincial government approval by our customers has taken from as fast as 3 months to as long as 24 months. Unfortunately there is no set time frame in which the local regulatory authorities must respond. As such, the length of the approval process varies dramatically from province to province as each local province has its own approval process and what it deems to be a priority at the local level.
|
|
|
·
|
After both regulatory approvals have been obtained from the Ministry of Health and local provincial government, and assuming the hospital or clinic has properly prepared the facility for installation, it typically takes us 1 to 2 months to install and test our products.
|
As a result, our ability to sell, install and recognize revenue for sales of our radiotherapy equipment through our customers is largely dependent on the customer’s and hospital’s ability to obtain approval by the Ministry of Health and the local provincial government.
Many of the key research and development personnel who developed our products are currently employed by our company.
We will continue to pursue relationships with foreign medical equipment technology leaders to provide high quality, low cost manufacturing services and China-based distribution for their products.
Our Operating Subsidiaries
Shenzhen Hyper Technology Co. Limited. Shenzhen Hyper was established in September of 2001 as a domestic Chinese company based in Shenzhen, China. From inception, it has been engaged in designing, developing and servicing radiotherapy medical equipment used for the treatment of cancerous tumors for customers throughout China. Shenzhen Hyper developed the SGS, our most advanced and versatile technology, in 2001. The SGS is a radiotherapy device that uses gamma radiation to non-invasively treat tumors located in the head and the body and to treat certain functional disorders of the head and neck areas. It utilizes stereotactic, or three-dimensional imaging, principles to enhance the accuracy of the targeting of the radiation beams. Shenzhen Hyper has also developed a MLC device that is used in conjunction with a LINAC to provide conformal shaping of radiotherapy treatment beams, which increases the precision of the beam and reduces the damage caused to surrounding tissues. We are currently developing our own LINAC with which we will integrate our MLC.
Shenzhen Hyper is currently working on the development of additional radiotherapy and diagnostic equipment that will be sold in China and abroad, including the next-generation SGS unit, capable of treating cancerous tumors in the head and body by advanced radiotherapy functions. We have a 75% interest in Shenzhen Hyper.
Wuhan Kangqiao Medical New Technology Co. Wuhan Kangqiao was established in September of 2001 as a domestic Chinese company based in Wuhan, China. From inception, it has been engaged in designing, developing and servicing radiotherapy medical equipment for customers throughout China. Wuhan Kangqiao developed the BGTS in 2003 and the HGTS in 2004 and currently designs, markets and services these products. The BGTS is a stereotactic radiotherapy device that uses gamma sourced radiation to non-invasively treat tumors located in the body. The HGTS is a stereotactic radiotherapy device that uses gamma sourced radiation to non-invasively treat tumors in the head and to treat other functional disorders of the head and neck area. We have a 100% interest in Wuhan Kangqiao.
26
Beijing Yuankang Kbeta Nuclear Technology Co., Limited. Beijing Kbeta was established in December 2004 and supplies the Cobalt-60 radioactive material used as the radioactive source in the SGS, BGTS and HGTS. We have a 50% interest in Beijing Kbeta.
PRICING
Treatment fees for radiotherapy are set by provincial governments in China, a factor we consider when pricing our systems. To gain market penetration, we price our radiotherapy treatment systems at levels that we believe offer attractive economic returns to our customers, taking into account the prices of competing products in the market. We believe that our products offer quality features at a competitive price in China.
If the provincial governments in China reduce the treatment fee rates for radiotherapy, some hospitals and hospital equipment investors may be discouraged from purchasing our products, which would reduce our sales. In that event, we may need to decrease the price of our systems to provide our customers acceptable returns on their purchases. We cannot assure you that our business, financial condition and results of operations will not be adversely affected by any reduction in treatment fees for radiotherapy in the future.
Under existing regulations, we are permitted to install a limited number of units, and to be reimbursed for the equipment and installation costs, prior to the receipt of the regulatory registration for that equipment. Those installations frequently are used to conduct clinical trials or for other research purposes. Our installations of the HGTS units and the MLC units have been made under this exemption. While those limited installations are not considered “sales” or commercial distribution for regulatory purposes, they do result in revenues to us as a result of the cost reimbursements. However, this cost reimbursement is below the price at which we intend to sell our products following regulatory approval.
REVENUES
We primarily derive our revenues from selling our products to third party hospital equipment investors and from selling service contracts to the end users of our products.
Our revenues are net of Value Added Tax (“VAT”), but include VAT refunds on the sales of self-developed software embedded in our medical equipment products. In addition, our revenues include regional VAT and Business tax subsidies.
The sales price of our devices includes basic training and installation services. These services are ancillary to the purchase of medical equipment by our customers and are normally considered by the customers to be an integral part of the acquired equipment. As the delivered items (training and installation services) do not have determinable fair values, revenues for the entire arrangement is recognized upon customer acceptance, which occurs after delivery and installation.
Our revenues, growth and results of operations depend on many factors, including the level of acceptance of our products among doctors, hospitals and patients and our ability to maintain prices for our products at levels that provide favorable margins. The level of acceptance among doctors, hospitals and patients is influenced by the performance and pricing of our products, our ability to educate the medical community about our products, our relationships with hospitals and hospital equipment investors, government reimbursement levels as well as other factors.
Our sales have historically been achieved on a unit-by-unit basis. We expect that in any given period a relatively small, and changing, number of third party investors will continue to account for a significant portion of our revenues. Our customers for products primarily consists of hospital equipment investors. For the year ended December 31, 2009, two customers accounted for 90% of our revenues and for the year ended December 31, 2010, two customers accounted for 90% of our revenues.
We extend credit to our customers and often do not collect outstanding receivables on a timely basis. The contracts covering the sale of our products provide for the customer to make a deposit at the time that the order is placed and to make progress payments at various stages of the manufacturing, shipping, installation and testing process, typically with the final 10% payment due 12 months after the customer accepts the product as meeting the specifications. However, in the PRC we have rarely collected payments under those stated terms and our customers have historically made most of their payments following the installation of our units. Our largest customer had an outstanding accounts receivable balance of $14.7 million and $10.5 million as of December 31, 2010 and 2009. The delay in receipt of customer payments places pressure on our working capital requirements. Legal action is available, but the time it takes and the outcome of any litigation is inherently uncertain, particularly in the PRC, where the civil justice system continues to evolve. Should we be unable to collect on these accounts, it would reduce our liquidity and profitability.
27
COSTS
Cost of revenues
Our cost of revenues primarily consists of material and component costs. It also includes amortization of intangible assets and direct costs incurred in the assembly, installation and service of our products, such as salaries and related personnel expenses and depreciation costs of plant and equipment used for production purposes. Depreciation of property, plant and equipment attributable to manufacturing activities is capitalized and expensed as cost of revenues when product is sold.
As we source a significant portion of our components and raw materials in China, we currently have a relatively low cost base compared to medical technology companies in more developed countries. We expect the costs of components and raw materials in China will increase in the future as a result of further economic development in China. In addition, our focus on new generations and applications of our products may require higher cost components and raw materials. We plan to offset increases in our cost of raw materials and components through more efficient product designs and product assembly enhancements as well as through savings due to economies of scale.
Operating expenses
Our operating expenses primarily consist of research and development expenses, sales and marketing expenses and general and administrative expenses.
Research and development. Research and development expenses primarily consist of costs associated with the design, development, testing and enhancement of both our existing products and our new products under development. These costs consist of expenditures for purchases of supplies, clinical trials, salaries and related personnel expenses, and other relevant costs. Going forward, we expect to increase our research and development expenses, both on an absolute basis and as a percentage of revenues, to develop new products and applications and to improve the designs of our existing products.
Sales and marketing. Sales and marketing expenses consist primarily of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with advertising and other marketing activities. Our sales are currently made primarily to hospital equipment investors, rather than directly to hospitals or clinics. As a result, our sales and marketing expenses as a percentage of revenues are significantly lower than medical equipment companies that operate their own marketing and distribution networks and sell directly to hospitals. Going forward, we expect to increase our expenditures on sales and marketing, both on an absolute basis and as a percentage of revenues, to promote our products in China. Furthermore, we anticipate aggressively pursuing new markets outside the PRC and expect to increase our expenditures on sales and marketing for this purpose as well.
General and administrative. General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management, fees and expenses of our outside advisers, including legal, audit and valuation expenses, expenses associated with our administrative offices and the depreciation of equipment used for administrative purposes. We expect that our general and administrative expenses will increase, both on an absolute basis and as a percentage of revenues, as we hire additional personnel and incur costs related to the anticipated growth of our business and ongoing expenses associated with being a publicly listed company in the U.S.
TAXES AND INCENTIVES
The discussion of taxes that follows denotes financial obligations and thresholds in RMB, since that is the currency in which those amounts are measured. These amounts have been translated into U.S. dollars using the exchange rate in effect on December 31, 2010. The actual future value of taxes expressed in USD will vary depending upon future rates of exchange.
28
Allied Moral Holdings
Under the current laws of the British Virgin Islands, we are not subject to tax on our income or capital gains. In addition, no British Virgin Islands withholding tax would be imposed on payments of dividends.
Changdu Huiheng
Under the current PRC laws, Changdu Huiheng is subject to the Enterprise Income Tax (“EIT”) and the Value Added Tax (“VAT”). Changdu Huiheng is established in the western region of the PRC and, as such, it is currently subject to an EIT rate of 15%, compared to a statutory rate of 25% for most companies in China. However, pursuant to an agreement with the Tibet Finance Bureau, Changdu Huiheng will be refunded any amounts of its annual EIT payment that exceed RMB 900,000 (US$133,000). In addition, the Tibet Finance Bureau will refund Changdu Huiheng’s annual 31% business tax payment and its 38.75% VAT payment under the condition that the total annual business tax and the annual VAT owed exceed RMB 1 million (US$148,000) and RMB 1.5 million (US$222,000), respectively. This tax incentive policy will be valid for 5 years from the commencement of the tax refund, which began in fiscal 2006.
Shenzhen Hyper
Shenzhen Hyper is classified as a high technology company and currently operates in an approved economic-technological development area. As such, it is currently subject to an EIT rate of 15%, compared to a statutory rate of 25% for most companies in China. Furthermore, this classification, according to local tax regulations, entitles Shenzhen Hyper to a tax-free period for two years, commencing on its first profitable year, and a 50% reduction in EIT for the following six years. Although Shenzhen Hyper was profitable in 2007, accumulated losses from prior years eliminated its tax liability for 2007. Shenzhen Hyper began the first year of its tax-free period in 2008.
The VAT is charged based on the selling price of products at a general rate of 17% and our revenues are recorded net of this VAT. Shenzhen Hyper, however, is entitled to a 14% refund of VAT on the sales of self-developed software embedded in device systems. This is a result of a PRC government program to promote the development of the high technology sector of China’s economy. The program phases out for companies after five years of profitable operations.
The VAT refund is recorded as part of revenues under U.S. GAAP. For the fiscal year ended December 31, 2010, the VAT refunds that we applied for in 2009 were booked as other revenue.
Wuhan Kangqiao
Wuhan Kangqiao is classified as a high technology company in Wuhan, and currently operates in an approved economic-technological development area. As such, it was subject to an EIT rate of 15% before 2009, compared to a statutory rate of 25% for most companies in China; however, the local tax regulation is revised in 2009 and the current rate is 25%
The PRC tax system is subject to uncertainties and has been subject to recently enacted changes, the interpretation and enforcement of which are also uncertain. There can be no assurance that changes in PRC tax laws or their interpretation or their application will not subject us to tax increases in the future.
SIGNIFICANT ACCOUNTING POLICIES
Significant Accounting Policies and Estimates
The discussion and analysis of our financial condition presented in this section are based upon our financial statements, which have been prepared in accordance with the generally accepted accounting principles in the United States. During the preparation of our financial statements we are required to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates and judgments, including those related to sales, returns, pricing concessions, bad debts, inventories, investments, fixed assets, intangible assets, income taxes and other contingencies. We base our estimates on historical experience and on various other assumptions that we believe are reasonable under current conditions. Actual results may differ from these estimates under different assumptions or conditions.
29
In response to the SEC’s Release No. 33-8040, “Cautionary Advice Regarding Disclosure About Critical Accounting Policy,” we identified the most critical accounting principles upon which our financial status depends. We determined that those critical accounting principles are related to the use of estimates, inventory valuation, revenue recognition, income tax and impairment of intangibles and other long-lived assets. We present these accounting policies in the relevant sections in this management’s discussion and analysis, including the Significant Accounting Policies discussed below.
(a) Principles of Consolidation The consolidated financial statements include the Company and its subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
(b) Cash Cash consists of cash on hand and in the bank.
(c) Accounts Receivable Accounts receivable are recorded at the invoiced amount, net of allowances for doubtful accounts, sales returns, trade discounts and value added tax. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in the Company’s existing accounts receivable. The Company performs ongoing credit evaluations of its customers’ financial conditions.
Outstanding account balances are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
(d) Inventories The Company values inventories, consisting of work in process and raw materials, at the lower of cost or market. Cost of material is determined on the weighted average cost method. Cost of work in progress includes direct materials, direct production cost and an allocated portion of production overhead.
The final steps of assembly of our products, including installation of radioactive service materials, are completed at customer locations. Accordingly, the Company generally does not carry finished goods (inventory held for sale in the ordinary course of business) inventory.
(e) Property, Plant, and Equipment Property, plant and equipment are recorded at cost less accumulated depreciation. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to general and administrative expenses as incurred. Depreciation of property, plant and equipment is computed by the straight-line method (after taking into account their respective estimated residual values) over the assets estimated useful lives ranging from three to twenty years. Building improvements are amortized on a straight-line basis over the estimated useful life. Depreciation of property, plant and equipment are stated at cost less accumulated depreciation. Upon sale or retirement of property, plant and equipment, the related cost and accumulated depreciation are removed from the accounts and any gain or loss is reflected in operations. Construction in progress represents the costs of property, plant and equipment under construction or installation. The accumulated costs are reclassified as property, plant and equipment when installation or construction is completed. All borrowing costs, which include interest and foreign exchange differences incurred that are attributable to qualifying assets, are capitalized as cost of construction in progress. Capitalization of borrowing costs ceases when the construction is completed and the constructed or installed asset is ready for its intended use.
(f) Intangible Assets Intangible assets are stated at cost, representing the fair value at the time such intangibles were contributed to the Company by the minority owner of a subsidiary in exchange for equity interests. Fair value was supported by cash contributed contemporaneously by another investor. Intangible assets are carried net of accumulated amortization and impairment losses. Amortization expense is recognized on the straight-line basis over the estimated respective useful lives of these intangible assets.
(g) Investment in Affiliated Company The company owns a 50% equity interest of Beijing Kbeta and accounts for the investment using the equity method of accounting. The equity method is utilized as the Company has the ability to exercise significant influence over the investee, but does not have a controlling financial interest.
If circumstances indicate that the carrying value of the Company’s investment in Beijing Kbeta may not be recoverable, the Company would recognize an impairment loss by writing down its investment to its estimated net realizable value if management concludes such impairment is other than temporary.
30
(h) Impairment of Long-Lived Assets Long-lived assets, which include property, plant and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of the asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of, if any, are separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and are no longer depreciated.
(i) Fair Value of Financial Instruments The fair value of a financial instrument is the amount at which the instrument could be exchanged in a current transaction between willing parties. The carrying amounts of financial assets and liabilities, such as cash, accounts receivable, current income tax assets, prepayments and other current assets, accounts payable, income taxes payable, accrued expenses and other current liabilities, approximate their fair values because of the short maturity of these instruments and market rates of interest.
(j) Revenue Recognition The Company generates revenue primarily from sales of medical equipment and provision of maintenance and support services. Revenue is recognized as follows:
Sales of medical equipment
The Company recognizes revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable. The sales price of the medical equipment includes the training services, which generally take about one month. These services are ancillary to the purchase of medical equipment by customers and are normally considered by the customers to be an integral part of the acquired equipment. As training services do not have separately determinable fair values, the Company recognizes revenue for the entire arrangement upon customer acceptance, which occurs after delivery and installation.
In the PRC, value added tax (“VAT”) of 17% on invoice amount is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities.
The medical equipment sold by the Company has embedded self-developed software which can also be sold on a standalone basis.
Provision of maintenance and support services
The Company also provides comprehensive post-sales services to certain distributors for medical equipment used by hospitals. These contracts are negotiated and signed independently and separately from the sales of medical equipment. Under existing service agreements, the Company provides the exchange of cobalt sources, training to users of the medical equipment, maintenance of medical equipment, upgraded software and consulting. Fees for the services are recognized over by the life of the contract on a monthly basis.
(k) Research and Development Costs Research and development costs are charged to expense as incurred. Research and development costs mainly consist of remuneration for the research and development staff and material costs for research and development. The Company incurred $80,071 and $485,290 for the years ended December 31, 2010 and 2009, respectively.
(l) Income Taxes The Company accounts for income taxes under ASC 740 “Income Taxes.” Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the year that includes the enactment date.
31
During 2008, the Company adopted ASC740 “Income Taxes,” which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken in the tax return. This interpretation also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures.
(m) Retirement and Other Postretirement Benefits Contributions to retirement schemes are compulsory and are charged to consolidated statements of operations as general and administrative expenses when the related employee service is provided. The Company does not have a separate retirement benefit scheme for any employees.
(n) Warranty The Company provides a product warranty to its customers to repair any product defects that occur generally within twelve months from the date of sale. The Company’s purchase contracts generally allow the customer to withhold up to 10% of the total purchase price for the duration of the warranty period. Based on the limited number of actual warranty claims and the historically low cost of such repairs, the Company has not recognized a liability for warranty claims, but rather recognizes such cost when product repairs are made.
(o) Foreign Currency Translation Assets and liabilities of foreign subsidiaries are translated at the rate of exchange in effect on the balance sheet date; income and expenses are translated at the average rate of exchange prevailing during the year. The related transaction adjustments are reflected in “Accumulated other comprehensive income/(loss)” in the stockholders’ equity section of the Company consolidated balance sheet.
The average monthly exchange rates for year ended December 31, 2010 and the closing rate as of December 31, 2010 were RMB$6.7604and RMB$6.6000 to one USD, respectively. The average monthly exchange rates for year ended December 31, 2009 and the closing rate as of December 31, 2009 were RMB$6.8303 and RMB$6.8270 to one USD, respectively.
(p) Deferred Offering Costs Deferred offering costs are those costs directly attributable to the Company’s proposed public offering. Such costs consist principally of professional fees. Deferred costs accumulated through December 31, 2008 were charged to operations in 2009 as the Company’s proposed offering was deemed unsuccessful.
(q) Comprehensive Income The Company has adopted ASC 220 “Comprehensive Income.” This statement establishes rules for the reporting of comprehensive income and its components. Comprehensive income consists of net income and foreign currency translation adjustments and is presented in the Consolidated Statements of Income and the Consolidated Statement of Stockholders’ Equity.
(r) Financial Instruments with Characteristics of both Liabilities and Equity The Company accounts for its Series A Preferred Stock in accordance with ASC 480 “Distinguishing Liabilities from Equity” and ASC 815 “Derivatives and Hedging.” We have determined that our Series A Preferred Stock is not mandatorily redeemable. Accordingly, the Company accounts for the Preferred stock as permanent equity.
(s) Non-controlling interest in consolidated financial statements In December 2007, the FASB issued authoritative guidance related to noncontrolling interests in consolidated financial statements, which was an amendment of ARB No. 51. This guidance is set forth in Topic 810 in the Accounting Standards Codification (ASC 810). ASC 810 establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. This accounting standard is effective for fiscal years beginning after December 15, 2008.
(t) Impact of New Accounting Standards We describe below recent pronouncements that have had or may have a significant effect on our financial statements. We do not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to our financial condition, results of operations, or disclosures.
In June 2009, the FASB issued Updates No. 2009-01, which establishes the FASB Accounting Standards Codification TM (the Codification) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with generally accepted accounting principles (GAAP). The Codification is effective for interim and annual periods ending after September 15, 2009. We adopted the Codification when referring to GAAP in this report on Form 10-K for the fiscal period ending December 31, 2009. The adoption of the Codification did not have an impact on our consolidated results.
32
The FASB issued authoritative guidance related to subsequent events in May 2009, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before the financial statements are issued or are available to be issued. This guidance is set forth in Topic 855 in the Accounting Standards Codification (ASC 855). ASC 855 provides guidance on the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. We adopted ASC 855 and its application had no impact on our consolidated financial statements.
The FASB issued authoritative guidance for fair value measurements in September 2006, which defines fair value, establishes a framework of measuring fair value, and expands disclosures assets and liabilities measured at fair value in financial statements. This guidance is set forth in Topic 820 in the Accounting Standards Codification (ASC 820). ASC 820 does not require any new fair value measurements but rather eliminates inconsistencies in guidance found in various prior accounting pronouncements. ASC 820 is effective for fiscal years beginning after November 15, 2007. However, on February 6, 2008, the FASB issued authoritative guidance which deferred the effective date of ASC 820 for one year for nonfinancial assets and nonfinancial liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. The adoption of ASC 820 did not have a material impact on our consolidated financial position, results of operations or cash flows.
In December 2007, the FASB issued revised authoritative guidance for business combinations, which establishes principles and requirements for how the acquirer in a business combination: i) recognizes and measures in its financial statements the identifiable assets acquired, the liabilities assumed, an any noncontrolling interest in the acquiree, ii) recognizes and measures the goodwill acquired in the business combination or a gain from a bargain purchase, and iii) determines what information to disclose to enable users of the financial statements to evaluate the nature and financial effects of the business combination. This guidance is set forth in Topic 805 in the Accounting Standards Codification (ASC 805). This accounting standard is effective for fiscal years beginning after December 15, 2008. We adopted ASC 805 and the adoption of ASC 805 did not have a material impact on our consolidated financial position, results of operations or cash flows as of the date of adoption. ASC 805 will be applied to any future business combinations.
In December 2008, ASC 715 “Retirement Benefits” was issued. This statement amends SFAS No. 132(R), Employers’ Disclosures about Pensions and Other Postretirement Benefits, to require additional disclosure in sponsor’s financial statements about pension plan assets, obligations, benefit payments, contributions and net benefit cost and other postretirement benefits. The requirements of this statement are effective for the Company for the fiscal year ending January 31, 2010. Since this statement only pertains to disclosures in the notes to consolidated financial statements, it will not impact the Company’s consolidated financial position and results of operations.
In June 2009, the FASB issued ASC 105 “Generally Accepted Accounting Principles.” ASC 105 establishes the FASB Accounting Standards Codification TM (Codification) as the single source of authoritative U.S. generally accepted accounting principles (U.S. GAAP) recognized by the FASB to be applied by nongovernmental entities. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. ASC 105 and the Codification are effective for financial statements issued for interim and annual periods ending after September 15, 2009, which corresponds to the Company’s second quarter of fiscal 2010. When effective, the Codification will supersede all existing non-SEC accounting and reporting standards. All other non-grandfathered non-SEC accounting literature not included in the Codification will become non-authoritative. Following ASC 105, the FASB will not issue new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, the FASB will not issue new standards in the form of Statements, FASB Staff Positions, or Emerging Issues Task Force Abstracts. Instead, the FASB will issue Accounting Standards Updates, which will serve only to: (a) update the Codification; (b) provide background information about the guidance; and (c) provide the bases for conclusions on the change(s) in the Codification. The adoption of ASC 105 will not have an impact on the Company’s consolidated financial statements.
In October 2009, the FASB issued authoritative guidance that amends existing guidance for identifying separate deliverables in a `-generating transaction where multiple deliverables exist, and provides guidance for allocating and recognizing revenue based on those separate deliverables. The guidance is expected to result in more multiple-deliverable arrangements being separable than under current guidance and is required to be applied prospectively to new or significantly modified revenue arrangements. This guidance, for which the Company is currently assessing the impact on its financial condition and results of operations, will become effective for the Company on January 1, 2011.
33
In January 2010, the FASB issued authoritative guidance intended to improve disclosures about fair value measurements. The guidance requires entities to disclose significant transfers in and out of fair value hierarchy levels and the reasons for the transfers and to present information about purchases, sales, issuances and settlements separately in the reconciliation of fair value measurements using significant unobservable inputs (Level 3). Additionally, the guidance clarifies that a reporting entity should provide fair value measurements for each class of assets and liabilities and disclose the inputs and valuation techniques used for fair value measurements using significant other observable inputs (Level 2) and significant unobservable inputs (Level 3). This guidance is effective for interim and annual periods beginning after December 15, 2009 except for the disclosures about purchases, sales, issuances and settlements in the Level 3 reconciliation, which will be effective for interim and annual periods beginning after December 15, 2010. As this guidance provides only disclosure requirements, the adoption of this guidance will not impact the Company’s financial condition or results of operations.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Incentive Share and After-tax Profit Targets
As an additional payment under the Allied Moral Holdings Securities Exchange Agreement, the previous holders of common stock of Allied Moral Holdings will be issued, on an all or none basis, 309,525 shares of common stock each year over three years (up to an aggregate of 928,575 shares of common stock), if on a consolidated basis, the Company has after-tax profits in the following amounts for the indicated periods:
|
Years Ending December 31,
|
After-tax Profit in USD
|
|||
|
2009
|
18,500,000 | * | ||
|
2010
|
26,200,000 | * | ||
|
2011
|
34,060,000 | |||
* This after-tax profit goal was not achieved for the years ended December 31, 2009 and 2010.
OUR RESULTS OF OPERATIONS
Operating revenues
For the year ended December 31, 2010, total product sales and service revenues were $8.64 million, a decrease of $0.74 million, or 8%, compared to $9.38 million for the prior year. This decrease was due to less revenue from product sales in 2010 compared with 2009.
Total revenues from product sales were $2.47 million for 2010, a decrease of $0.99 million or 29%, compared to $3.46 million for the prior year. In 2010, we sold 3 units of our products, the same number with 2009; however, the products we sold in 2010 were TOP, OPEN and LINAC, whose sales prices were lower than our another product SGS-I, which was sold in 2009.
Total service revenues were $6.17 million for 2010, an increase of $0.25 million or 4.2%, compared to $5.92 million for the prior year. For the year ended December 31, 2010, we managed two more service contract than in the prior year 2009. The increase in revenue from services in 2010 was due to the appreciation of the RMB against the USD and the entering into a new service contract compared to the prior year.
Revenue Backlog
Revenue backlog represents the total amount of unrecognized revenue associated with existing purchase orders for our products. Any deferral of revenue recognition is reflected in an increase in backlog as of the end of that period. The backlog as of December 31, 2010 amounted to $8.94 million, representing a decrease of 2.82 million or 24%, compared to $11.76 million as of December 31, 2009. The backlog has decreased but remained in place in large part due to the newly installation in the last quarter of 2010 and regulatory approval delays concerning clients’ facility preparation as necessary to install our products, which caused some of the units to stay in our backlog longer than we expected. Subject to any further delays in obtaining regulatory approval, we anticipate that the installation of the first SGS-I in Peru will be completed by 2011.
34
The purchase orders are not cancellable and are subject to certain conditions, including but not limited to, customers obtaining regulatory approval from their local government for our products. Notwithstanding, we expect the purchase orders comprising the revenue backlog to be installed during 2011.
Below is a summary of our revenue backlog broken down by product, unit price, quantity and total amount owed as of December 31, 2010, based on an exchange rate of 6.6000 RMD per dollar:
|
Products
|
|
Unit Price
(RMB)
|
Quantity
|
Total Amount
(RMB)
|
Value of Backlog
(USD)
|
|||||||||||
|
SGS-I
|
9,000,000
|
2
|
18,000,000
|
2,727,272.73
|
||||||||||||
|
SGS-I
|
14,000,000
|
1
|
14,000,000
|
2,121,212.12
|
||||||||||||
|
SGS-I
|
14,000,000
|
1
|
14,000,000
|
2,121,212.12
|
||||||||||||
|
LINAC
|
6,500,000
|
2
|
13,000,000
|
1,969,696.97
|
||||||||||||
|
Total
|
6
|
59,000,000
|
8,939,343.94
|
|||||||||||||
Cost of revenues
The total cost of revenues amounted to $2.18 million for the year ended December 31, 2010, a decrease of $0.17 million or 7.2%, compared to $2.35 million for the prior year. The decrease was due to different products we sold and the cost of our products sold in 2010 was lower than those sold in 2009.
Gross margin
As a percentage of total revenues, the overall gross margin decreased to 74.80% for the year ended December 31, 2010 from 74.91% for the prior year. The gross margin remained steadily was due to higher margin service revenues but lower margin sales revenue accounting for a proximal percentage of total revenues in 2010 compared to 2009.
Operating expenses
Sales and marketing expenses Sales and marketing expenses consist primarily of salaries and related expenses for personnel engaged in sales, marketing and customer support functions and costs associated with advertising and other marketing activities.
Sales and marketing expenses were approximately $281,927 for the year ended December 31, 2010, a decrease of 10.9%, or roughly $34,320 compared to approximately $316,247 for the prior year. The decrease was due to all products sold in 2010 were in the PRC domestic market, less marketing expenses related to international sales incurred in 2010 when compared to 2009.
We expect that our selling expenses will remain at or increase above the 2010 levels as we increase our efforts to expand sales, particularly internationally, where our brand is not as well known, and the resulting resources devoted to establish a presence in new markets will be greater.
General and administrative expenses General and administrative expenses consist primarily of salaries and benefits and related costs for our administrative personnel and management, fees and expenses of our outside advisers, including legal, audit and valuation expenses, expenses associated with our administrative offices. General and administrative expenses amounted to approximately $3.38 million for the year ended December 31, 2010, an increase of roughly $0.03 million compared to approximately $3.35 million for the prior year, representing an increase of 0.9%. The general and administrative expenses remaining steadily were due primarily to that the there was no significant change in the personnel number and the overall business kept in a steady level.
Research and development expenses . Research and development expenses include employee compensation, materials consumed and experiment expenses for specific new product research and development, and any expenses incurred for basic research on advanced technologies. Research and development expenses were $80,071 for the year ended December 31, 2010, compared to $485,290 for the prior year. This decrease was mainly due to the decrease in our research and development activities during 2010. As discussed above, our research and development in product development, including the development of our MLC, linear accelerator and SGS-1+ device, was curtailed during 2010 due to the unavailability of financial resources. However, subject to the availability of funds, we intend to focus our future research and development efforts on developing an advanced MRI device and an industrial LINAC unit.
35
Income tax provision
Our effective tax rate was 28.88% for the year ended December 31, 2010, compared to 16.27% for the year ended December 31, 2009. This increase in effective tax rate resulted from a combination of factors. The tax rate payable at each level of our operations is different. In 2010, the tax rate payable at Changdu Huiheng is 15%, compared to 12% in 2009. The effective tax rate is the result of a combination of taxes paid in different items and at different levels. Although the net income before income tax decreased this year because of the provision for doubtful accounts, the accrued tax payable increased to $974,328 in 2010 from $389,632 in 2009 as a result of less tax refund incurred in 2010 compared with 2009.
Net income
For the year ended December 31, 2010, the Company’s net income amounted to $2.18 million, a decrease of $1.46 million or 40% compared to $3.64 million for the prior year. This decrease was attributable primarily to the increase of allowance provisions for doubtful accounts, from $375,679 in 2009 to $594,491 in 2010. The decrease in reversal of provision for bad debt was another reason for the decrease of net income. In 2009, we received reversal of provision for bad debt with amount of $907,720, compared with $31,951 in 2010.
Comprehensive Income
For the year ended December 31, 2010, the Company’s comprehensive income amounted to $1.96 million, a decrease of $1.49 million or 43.19% compared to $3.45 million for the prior year. The decrease was due to a decrease in net income and foreign currency translation adjustments over the period.
LIQUIDITY AND CAPITAL RESOURCES
Our principal sources of liquidity are from cash reserves, current assets such as accounts receivable and from operating activities including the sale of our products and comprehensive post-sales services fees for our products as well as products manufactured by others. Based on the forgoing, we believe that we have sufficient working capital to meet our needs for the year 2010.
As further discussed in detail below, we have historically been unable to collect our accounts receivables on a timely basis. However, due to our positive history with our customers, we keep apprised of such customers’ financial condition and due to our long-standing relationships we are confident that we will collect all the money owed by these customers. So far, most of our clients can pay their outstanding accounts receivable with some delays. In addition to regular meetings with our customers, we have oral agreements with certain customers about payment on their outstanding receivables balance, and thus far we have had success in receiving certain payments on a quarterly basis.
Certain reclassifications have been made to the financial statement presentation as of December 31, 2009 and for the year then ended to correspond to the current year’s format. Total equity and net income are unchanged due to these reclassifications.
Our relatively high margins have historically provided us with sufficient cash to purchase various raw materials, meet our component inventory needs and pay our vendors. In addition, there are very few direct costs associated with our service business which further enhances our margins. We have long-standing, positive relationships with our vendors and have maintained favorable payment terms and believe that we will continue to maintain such favorable payment terms. Also, we believe that we can defer certain tax payments, if we choose to do so, to provide additional cash.
In order for us to implement our current business growth strategy, we will need additional capital to finance a number of expansion initiatives, including new product development, expansion of our Wuhan facility and possible strategic acquisitions. No assurance can be given that we will be able to raise such additional capital, or if raised, that it will be on favorable terms. In the event that we are unable to raise capital, we may not be able to complete some or any of our expansion initiatives.
As of December 31, 2010, we had total assets of $31,415,904. Our cash was $294,542, current portion accounts receivable were $8,130,681, prepaid expenses and current portion other receivables were $2,853,121 and inventories were $2,832,282. Working capital was approximately $9,744,273. The current ratio was approximately 3.23. The quick ratio was approximately 2.58. Our present policy is to apply cash to investment in product development, and further acquisitions or expansion of our business. Consequently, we have not paid and do not expect to pay dividends on Common Stock in the foreseeable future. We do not have any outstanding long term or short term bank loans, and continue to have minimal long-term capital expenditure obligations.
Net cash used in operating activities totaled $624,124 for the year ended December 31, 2010, an increase of $58,168 from $565,956 for the prior year. This increase resulted primarily from the following changes in the operating assets and liabilities:
36
• $4,065,076 increase in accounts receivables;
• $1,472,382 increase in inventories;
• $819,386 decrease in prepaid expense and other receivables;
• $422,235 increase in accounts payable;
• $584,696 increase in tax payable; and
• $63,587 increase in accrued expenses and other current liabilities;
The increase in accounts receivable was due to a combination of factors:
First, the majority of our product sales and installations in 2009 occurred during the last quarter of the year. As a result of the short amount of time between the time of installation and the end of the period, our cash collection this year was lower than our cash collected in the prior year.
Second, due to our strong, long-standing relationships with our customers, we have extended their payment terms. Our sales contracts for our products provide for the customer to make payments based on milestones, which includes a deposit at the time that the order is placed and payments at various stages of the manufacturing, shipping, installation and testing process, typically with the final 10% payment due 12 months after the customer accepts the product as meeting the specifications. However, in the PRC we have rarely collected payments under those stated terms and our customers have historically made most of their payments following the installation of our units, which final installation is often delayed due to the length of time it takes to obtain regulatory approval. Further, although a customer is legally obligated to pay for our products, their ability to pay for the products is dependent on their ability to collect payments from their customers consisting of hospitals and clinics. For such reasons, we often do not collect outstanding receivables on a timely basis.
The delay in receipt of customer payments places pressure on our working capital requirements. In particular, our two major customers (“Customer A” and “Customer B”) collectively accounted for 90% and 90% of our revenues for the years ended December 31, 2010 and 2009. During the year ended December 31, 2010, Customer A paid approximately $2.4 million on its accounts receivable and as of December 31, 2010 had an outstanding balance of approximately $14. 1 million (with provision for bad debts of approximately $0.54 million). Of Customer A’s outstanding accounts receivable balance as of December 31, 2010, 21.27% related to the providing of services and 78.73% related to product sales. During the year ended December 31, 2010, Customer B paid approximately $790,909 on its accounts receivable and as of December 31, 2010 had an outstanding balance of $4,231,211, (with provision for bad debts of approximately $290,758). Of Customer B’s outstanding accounts receivable balance, 100% related to the providing of services. Although we do not yet have an exact payment schedule or a written collection policy, we seek to collect on these outstanding accounts receivables by reviewing our customers’ business operations and financial situation, and having meetings and paying regular visits to our customers and their customers to seek payment on our accounts. Due to these efforts, and our prior positive history with our customers, we are confident that we will collect our accounts receivable owed by these customers. In addition, although legal action is available, the duration and outcome of litigation is inherently uncertain, particularly in the PRC, where the civil justice system continues to evolve.
We are in discussion with certain distributors to settle our accounts receivables by surrendering the equipment and contracts with the hospitals and clinics to us so that we can collect the fees directly. We believe that this will greatly improve our cash flow and reduce the time to collection. However, no agreement has yet to be entered into.
Even though we believe that our accounts receivable are collectible from our customers, of the accounts receivable outstanding as of December 31, 2010 and 2009, 58.8% and 58.9% of such accounts receivable , respectively, were more than 365 days. Accordingly, we reclassified that portion of the accounts receivable more than 365 days as a long-term asset for the year ended December 31, 2009, will account for that portion of accounts receivable more than 365 days as a long-term asset of the year ended December 31, 2010 and years thereafter. See note 2 to our financial statements. This reclassification has no effect to the total amount of assets or to our consolidated statements of income.
The decrease in prepaid expense and other receivables was attributed to a decrease in payments made to our manufacturing suppliers for parts and services needed to manufacture the radiotherapy units that comprise our backlog.
Net cash provided by/(used in) investing activities was $54,481 and ($358,401) for the year ended December 31, 2010 and 2009, respectively. The cash provided by investing activities was from a related party advance. The cash used in investing activities was primarily used for the acquisition of Portola Medical, Inc. this year, compared to land usage rights acquired for our new manufacturing facility in Wuhan last year.
37
There were no cash flows from financing activities for the year ended December 31, 2010 and 2009.
On June 7, 2010, we completed the acquisition of Portola Medical, Inc. (see Note 1 to the Notes to the Consolidated Financial Statements), whose primary asset consists of its rights to develop, manufacture and sell an adjustable Multi-Catheter Source Applicator which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer. We plan to market and sell this product in the United States and in Asia. Under the terms of the purchase agreement, we acquired all of the outstanding shares of Portola Medical for $2,600 per share in cash (without interest) for a total purchase price of $260,000. The purchase price and related costs were funded by Mr. Hui Xiaobing, our chairman and chief executive officer, pursuant to an unsecured promissory note with a maturity date one year from its effective date, unless sooner accelerated upon an event of default.
Cash flows from financing activities both amounted to nil for the year ended December 31, 2010 and 2009.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable.
Item 8. Financial Statements and Supplementary Data.
Our financial statements are annexed to this report beginning on page F-1.
Item 9. Changes In and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
The Company’s management, under the supervision and with the participation of its chief executive officer and chief financial officer, evaluated the effectiveness of the design and operation of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2010. Based on that evaluation, the Company’s chief executive officer and chief financial officer concluded that the disclosure controls and procedures employed at the Company were effective to ensure that the information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined under Exchange Act Rules 13a-15(f). The Company’s internal control system is designed to provide reasonable assurance to its management and board of directors regarding the preparation and fair presentation of published financial statements. Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
The contracts covering the sale of the Company’s equipment (which are primarily sold through the Company’s third party distributors) provide for the distributor to make a deposit at the time that the order is placed and to make progress payments at various stages of the manufacturing, shipping, installing and testing process, typically with the final 10% payment due 12 months after the distributor accepts the product as meeting the customer’s specifications. However, the Company has rarely collected payments under those stated terms and the Company’s distributor has historically made most of its payments following the installation of the Company’s equipment. As a result in delays in collecting its accountants receivable, a substantial portion of the Company’s accounts receivable were more than 365 days old even though the accounts receivable was classified as a current asset.
38
Upon further review and analysis of its accounts receivable, the Company decided to reclassify that portion of the accounts receivable more than 365 days old to a non-current asset to reflect more accurately the time it takes for the Company to collect on its accounts receivable. The reclassification has no effect to the Company’s Consolidated Statements of Income.
Based on our evaluation under that framework, our management concluded that our internal control over financial reporting was not effective as of December 31, 2010.
We believe that our consolidated financial statements for the year ended December 31, 2010 included in this report fairly present our financial position, results of operations and cash flows as of and for the periods presented in all material respects.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. The Company’s internal control over financial reporting was not subject to attestation by the Company’s registered public accounting firm pursuant to temporary rules of the Securities and Exchange Commission that permit the Company to provide only management’s report in this annual report.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. It should be noted that any system of controls, however well designed and operated, can provide only reasonable, and not absolute, assurance that the objectives of the system will be met. In addition, the design of any control system is based in part upon certain assumptions about the likelihood of future events. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Controls Over Financial Reporting
There have been no changes in our internal controls over financial reporting that occurred during the quarter ended December 31, 2010, that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
Item 9B. Other Information.
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The following table and text set forth the names and ages as of December 31, 2010 of all of our directors and executive officers. The Board of Directors is comprised of only one class. All of the directors will serve until the next annual meeting of stockholders and until their successors are elected and qualified, or until their earlier death, retirement, resignation or removal. Except for Mr. Huang being the brother-in-law of Mr. Hui, there are no family relationships among directors and executive officers.
Each of our directors, other than Mr. Hui and Mr. Huang, is “independent” under the independence standards adopted by the Nasdaq Stock Market Rules. Also provided herein are brief descriptions of the business experience of each director and executive officer during the past five years and an indication of directorships held by each director in other companies subject to the reporting requirements under United States Federal securities laws.
|
Name
|
Age
|
Position
|
||
|
Hui Xiaobing
|
57
|
Chairman and CEO
|
||
|
Huang Jian
|
54
|
Vice President and Director
|
||
|
Richard Shen
|
45
|
Chief Financial Officer, Current Corporate Secretary
|
||
|
Cui Zhi
|
41
|
Chief Technology Officer
|
||
|
Tang Shucheng
|
48
|
Director of Marketing
|
||
|
Li Daxi
|
63
|
Director
|
39
Hui Xiaobing, Chairman of the Board and Chief Executive Officer
Mr. Hui currently serves as our Chairman of the Board and Chief Executive Officer, positions he has held (including with Allied Moral) since the inception of that company in 2006, and Chairman of the Board and Chief Executive Officer of Changdu Huiheng, positions he has held since 2005. In addition, Mr. Hui has been the Chief Executive Officer of Huiheng Industry since 2004. Mr. Hui also served from 1999 to 2006 as the President and Chairman of the Board of Shenzhen OUR Technology Co., Ltd., the pioneer of the radiotherapy industry in China. He is the former CEO of Everbright Securities, a major Chinese financial institution. Mr. Hui holds a Masters degree in Regional Economics from Tongji University.
Huang Jian, Vice President and Director
Mr. Huang, a director since November 2007, has a background in business and management. He currently serves as the Vice President of the Company, a position he assumed in 2007. Mr. Huang is also the President and Director of Wuhan Kangqiao, positions he has held since 2006. In addition, Mr. Huang has been a director of Shenzhen Hyper since 2006 and has been the President of Shenzhen Hyper since 2001. Mr. Huang has a degree from Beijing Broadcast and Television University.
Richard Shen, Chief Financial Officer, Current Corporate Secretary
Mr. Shen serves as our Chief Financial Officer and, as of June 6, 2009, also serves as the Company’s Corporate Secretary. In addition, he is also a managing partner of Sunlight Investment Limited, an asset management and investment consultant business, where he has served since 2005. From 2002 through 2005, Mr. Shen was a Vice President and Director of New Tech & Telecom Investment Limited. Previously, he served as the General Manager of Touchstone Investment Limited. Mr. Shen received his MBA from York University in Toronto, Canada.
Cui Zhi, Chief Technology Officer
Mr. Cui oversees Changdu Huiheng’s research and development operations as the Chief Technology Officer, a position he has held since 2005. From 2002 to 2005, Mr. Cui was the Chief Engineer for Shenzhen Hyper, where he played a key role in the development of the Super Gamma System. Mr. Cui holds a Ph.D. in Physics from China Science and Technology University.
Tang Shucheng, Director of Marketing
Mr. Tang is responsible for the sales and marketing functions of Tibet Changdu as Director of Marketing, a position he has held since 2005. From 2000 to 2005, Mr. Tang was the Vice General Manager of SZ Jiancheng Investment Co., Ltd., a former affiliate of Huiheng. Mr. Tang studied at the Austria National Science and Technology Academy, where he earned a Ph.D. degree in physics.
Dr. Li Daxi, Director
Dr. Li has been a director since November 2007 and is a member of the Company’s Audit Committee and Compensation Committee. He founded the Chinese Association of Science and Business, an organization devoted to bridging science with business and bridging China with the world, in 1997. Dr. Li has 14 years experience in investment banking and venture capital, including ten years on Wall Street with Salomon Brothers and Lehman Brothers. He is a director of the United Orient Bank where he oversees investments and auditing of the bank. In March 2005, he was invited as an overseas representative to participate in the China National Chinese People’s Political Consultative Conference. He is also a co-founder of the Shenzhen Overseas Chinese Student Venture Park, a joint-venture with the Shenzhen city government, which hosts 250 high-tech startup companies. Dr. Li is a member of the Company’s Audit Committee. Dr. Li received a Ph.D. in high energy physics from the City University of New York.
Involvement in Certain Legal Proceedings
To the best of our knowledge, during the past ten (10) years, none of our directors or executive officers were involved in any of the following: (1) any federal or state bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; (2) any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); (3) being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; and (4) being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
40
Compliance with Section 16(a) of the Exchange Act
Because we have not registered a class of securities under Section 12 of the Securities Exchange Act, our officers and directors are not subject to the reporting requirements of Section 16(a) of the Securities Exchange Act of 1934.
Code of Conduct and Ethics
We have not yet adopted a Code of Conduct and Ethics that applies to all of our employees, officers and directors. However, our board of directors are currently discussing and working to adopt such Code of Conduct and Ethics.
Nominations to the Board of Directors
There were no material changes to the procedures by which security holders may recommend nominees to our Board of Directors.
Audit Committee
Our Board of Directors has established an Audit Committee in accordance with section 3(a)(58)(A) of the Exchange Act, which currently consists of Li Daxi, Hui Xiaobing and Huang Jian. Dr. Li Daxi satisfies the independence requirements established by the Nasdaq Marketplace Rules. The Audit Committee conducts an annual review of the committee’s overall performance. The primary purpose of the Audit Committee is to oversee our accounting and financial reporting processes and the function of the Audit Committee includes retaining our independent auditors, reviewing their independence, reviewing and approving the planned scope of our annual audit, reviewing and approving any fee arrangements with our auditors, overseeing their audit work, reviewing and pre-approving any non-audit services that may be performed by them, reviewing the adequacy of accounting and financial controls, reviewing our critical accounting policies and reviewing and approving any related party transactions. The Audit Committee held one meeting(s) during the fiscal year ended December 31, 2010.
Audit Committee Financial Expert
Our Board of Directors has determined that Li Daxi is an “audit committee financial expert,” as defined in Section 401(h) of Regulation S-K. As disclosed above, Li Daxi is “independent,” as defined by the Nasdaq Stock Market Rules.
Item 11. Executive Compensation.
Summary Compensation Table
The following table sets forth the information, on an accrual basis, with respect to the compensation of our executive officers for the years ended December 31, 2010 and 2009.
|
Name and
Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-
Equity
Incentive
Plan
Compen-
sation
($)
|
Non-
Qualified
Deferred
Compen-
sation
Earnings
|
All Other
Compen-
sation
($)
|
Total
($)
|
||||||||||||
|
Hui Xiaobing,
|
2010
|
$
|
56,866
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
56,866
|
||||||||||
|
Chief Executive Officer
|
2009
|
$
|
56,284
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
56,284
|
||||||||||
|
Huang Jian,
|
2010
|
$
|
35,542
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
35,542
|
||||||||||
|
Vice President
|
2009
|
$
|
35,178
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
35,178
|
||||||||||
|
Richard Shen
|
2010
|
$
|
37,719
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
37,719
|
||||||||||
|
Chief Financial Officer and Corporate Secretary
|
2009
|
$
|
37,333
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
37,333
|
||||||||||
41
Employment Agreements
None of our executive officers have employment agreements.
Equity Awards
During the last fiscal year, we have not granted any stock options or Stock Appreciation Rights (“SARS”) to any executive officers or other individuals listed in the table above. No long term incentive awards were granted during the last fiscal year.
Outstanding Equity Awards at Fiscal Year End
None of our executive officers, directors or employees have exercised options or SARs during the last fiscal year. There are no outstanding SARS for any executive officer or person listed in the table above.
Stock Option Plan
As discussed previously, on June 6, 2009, the Board of Directors and the shareholders of the Company approved the Huiheng Medical, Inc. 2009 Share Plan (the “Plan”), under which employees and directors of the Company are eligible to receive direct awards of shares or grants of stock options (either Incentive Stock Options or Nonstatutory Stock Options, as determined by the administrator of the Plan at the time of grant). The Plan replaces the Company’s 2007 Share Plan which expired under its terms since the 2007 Share Plan was never approved by the Company’s shareholders. Under the Plan, 1,566,666 shares have been reserved, which is the same number of shares that was reserved under the Company’s 2007 Share Plan.
Compensation of Directors
Each director (other than the Chair of the Compensation Committee or Audit Committee) will receive $3,000 per month and a stock option to purchase 30,000 shares vesting quarterly over a period of three years. The Chair of the Compensation Committee will receive $3,500 per month and options for 36,000 shares (with the same vesting schedule as the other directors) and the Chair of the Audit Committee will receive $4,000 per month and options for 36,000 shares (with the same vesting schedule as the other directors). While the Company has agreed to compensate our outside directors for their service through a combination of cash and stock options, as noted above, in addition to the reimbursement of their expenses incurred in performing their duties, we have not made any payment to our directors nor have we awarded any stock options in 2010 except as noted below.
The following table sets forth compensation paid to our non-executive directors for the fiscal year ended December 31, 2010.
|
Name
|
Fees
Earned
or Paid
in Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compen-
sation
($)
|
Total
($)
|
|||||||||||||||||||||
|
Li Daxi
|
$ | 36,000 | $ | - | $ | - | (1) | $ | - | $ | - | $ | - | $ | 36,000 | |||||||||||||
Defined benefit or actuarial plan disclosure
As required by Chinese law, our Chinese subsidiaries contribute 10% of an individual employee’s monthly salary to pension insurance.
42
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
As used in this section, the term beneficial ownership with respect to a security is defined by Rule 13d-3 under the Exchange Act as consisting of sole or shared voting power (including the power to vote or direct the vote) and/or sole or shared investment power (including the power to dispose of or direct the disposition of) with respect to the security through any contract, arrangement, understanding, relationship or otherwise, subject to community property laws where applicable.
As of February 25, 2011, we had a total of 14,030,637 shares of common stock outstanding and 211,390 shares of Series A Preferred Stock issued and outstanding. Each share of Series A Preferred Stock is convertible into 10.5042 shares of common stock, and the 211,390 shares of Series A Preferred Stock outstanding will convert into 2,220,483 shares of common stock in the aggregate.
Common Stock
The following table sets forth, as of March 15, 2011: (a) the names and addresses of each beneficial owner of more than five percent of our common stock known to us, the number of shares of common stock beneficially owned by each such person, and the percent of our common stock so owned; and (b) the names and addresses of each director and executive officer, the number of shares our common stock beneficially owned, and the percentage of our common stock so owned, by each such person, and by all of our directors and executive officers as a group. Unless otherwise indicated, the business address of each of our directors and executive offices is c/o Huiheng Medical, Inc., Huiheng Building, Gaoxin 7 Street South, Keyaunnan Road, Nanshan District, Shenzhen Guangdong, P.R. China 51807. Each person has sole voting and investment power with respect to the shares of our common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
|
Name and Address of Beneficial Owner (Includes Management)
|
Shares Owned
Beneficially
|
Percentage
Ownership
|
||||
|
Hui Xiaobing (1)
|
11,750,000
|
83.75
|
%
|
|||
|
Huang Jian
|
0
|
*
|
||||
|
Richard Shen
|
0
|
*
|
||||
|
Cui Zhi
|
0
|
*
|
||||
|
Tang Shucheng
|
0
|
*
|
||||
|
Li Daxi
|
20,000(2)
|
*
|
||||
|
All Officers & Directors as a Group (7 people)
|
11,765,000
|
83.75
|
%
|
|||
* Individual owns less than 1% of our securities.
(1) Includes shares held by Clear Honest International Limited, a company controlled by Mr. Hui Xiaobing, Chief Executive Officer of Huiheng.
(2) Represents stock options exercisable at March 15, 2011 or within sixty (60) days of March 15, 2011.
Preferred Stock
The following table sets forth, as of March 15, 2011, the names and addresses of each beneficial owner of more than five percent (5%) of our Series A Preferred Stock known to us, the number of shares of Series A Preferred Stock beneficially owned by each such person, the percent of the Series A Preferred Stock so owned, and the number of shares of common stock issuable upon conversion of the Series A Preferred Stock. To our knowledge, none of our directors or executive officers have any direct or beneficial ownership interest in shares of Series A Preferred Stock or shares of common stock issuable upon conversion of the Series A Preferred Stock:
43
|
Name and Address
|
Shares of
Series A
Preferred
Beneficially
Owned
|
Percentage
Ownership
|
Shares of
Common
Stock
Issuable on
Conversion
|
|||||
|
Genesis Capital Advisors LLC
546 Fifth Ave., 5th Floor
New York, NY 10036
|
11,237
|
5.32
|
%
|
118,036
|
||||
|
Platinum Partners Value Arbitrage Fund, L.P.
152 West 57th St.
New York, NY 10019
|
65,317
|
30.9
|
%
|
686,103
|
||||
|
Kenneth Greif (1)
240 Maple Street
Englewood, NJ 07631
|
26,667
|
12.61
|
%
|
280,116
|
||||
|
Atlas Master Fund, Ltd.
135 East 57th St.
New York, NY 1002
|
20,116
|
9.52
|
%
|
211,302
|
||||
|
Sherleigh Associates Inc..
Profit Sharing Plan
80 Columbus Circle PH 76A
New York, NY 10023
|
15,663
|
7.41
|
%
|
164,527
|
||||
|
Hanover Overseas Fund/OHL Ltd.
Level 2, 20 Augustus Terrace
Parnell, Auckland 1142 New Zealand
|
15,663
|
7.41
|
%
|
164,527
|
||||
|
Orion KF Partners
11990 San Vicente Blvd. Ste 200
Los Angeles, CA 90049
|
15,663
|
7.41
|
%
|
164,527
|
||||
(1) Includes 6,667 shares held in the name of Greif China PE, LLC of which Mr. Grief exercise voting control and investment control.
Securities Authorized for Issuance under Equity Compensation Plans
See Item 5 for this information.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
A summary of related party payables at December 31, 2010 and 2009 were as follows:
Amounts due to related parties at December 31, 2010 and December 31, 2009 represents the remaining balance due to a former shareholder of Allied Moral Holdings Limited ("Allied Moral"), a subsidiary of the Company, pursuant to the 2007 share redemption as well as advances related to the acquisition of Changdu Huiheng.
In June 2010, the cash consideration of $260,000 and related expenses amounting to $30,000 for acquiring the new subsidiary, Portola Medical Inc, were paid by the director, Hui Xiaobing directly on behalf of the Company. As of December 31, 2010, such director is owed $359,620.
We believe that Mr. Li. is an independent director as defined under the Nasdaq Stock Market Rules.
44
Item 14. Principal Accounting Fees and Services.
Relationship with Independent Registered Public Accounting Firm
Our Audit Committee has responsibility for the appointment, compensation and oversight of the work of our independent registered public accounting firm, UHY Vocation HK CPA Limited (“UHY”). We retained the firm of UHY as our Independent Registered Public Accounting Firm for the fiscal year ending December 31, 2010. We have appointed UHY as our independent registered public accounting firm for our fiscal year 2011.
Audit Fees
For the fiscal years ended 2010 and 2009, the aggregate fees billed for services rendered for the audits of the annual financial statements and the review of the financial statements included in the quarterly reports on Form 10-Q and the services provided in connection with the statutory and regulatory filings or engagements for those fiscal years and registration statements filed with the SEC were $120,000 and $120,000, respectively.
Audit-Related Fees
For the fiscal years ended December 31, 2010 and 2009, there were no fees billed for the audit or review of the financial statements that are not reported above under Audit Fees.
Tax Fees
For the fiscal years ended December 31, 2010 and 2009, fees billed for tax compliance services were $5,404 and $13,270, respectively.
All Other Fees
For the fiscal years ended December 31, 2010 and 2009, there were no fees billed for services other than services described above.
Audit Committee Approval of Audit and Non-Audit Services of Independent Accountants
The Audit Committee approves all audit and non-audit services provided by the independent auditors. These services may include audit services, audit-related services, tax services and other services. The independent accountants and management are required to periodically report to the Audit Committee regarding the extent of services provided by the independent accountants, and the fees for the services performed to date.
Item 15. Exhibits, Financial Statements and Schedules.
The following documents are filed as part of this Annual Report:
(a) Financial Statements:
|
Page
|
||
|
Report of Independent Registered Accounting Firm
|
F-1
|
|
|
Consolidated Balance Sheets at December 31, 2010 and 2009 and
|
F-2
|
|
|
Consolidated Statements of Income for the Years Ended December 31, 2010 and 2009
|
F-3
|
|
|
Consolidated Statements of Changes in Stockholders’ Equity and Other Comprehensive Income for the Years Ended December 31, 2010 and 2009
|
F-4
|
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2010 and 2009
|
F-5
|
|
|
Notes to Consolidated Financial Statements
|
|
F-6 to F-26
|
(b) Exhibits:
45
|
Exhibit No.
|
Document Description
|
|
|
3.1
|
Articles of Incorporation, as revised (incorporated by reference to the exhibit to the Company’s annual report on Form 10-KSB filed with the SEC on April 10, 2008)
|
|
|
3.2
|
Amended and Restated By-Laws (incorporated by reference to the exhibit to the Company’s annual report on Form 10-KSB filed with the SEC on April 10, 2008)
|
|
|
4.1
|
Amended and Restated Certificate of Designations of Rights and Preferences of the Series A 7% Convertible Preferred Stock (incorporated by reference to the exhibit to the Company’s current report on Form 8-K filed with the SEC on January 16, 2008)
|
|
|
4.2
|
Form of Common Stock Purchase Warrant (incorporated by reference to the exhibit to the Company’s current report on Form S-1 filed August 29, 2008)
|
|
|
10.1
|
Office Lease (incorporated by reference to the exhibit to the Company’s Amendment No. 1 to the Form SB-2 filed with the SEC on December 5, 2007)
|
|
|
10.2
|
Common Stock Purchase Agreement, dated May 7, 2010 by and among the Company, Three Arch Capital, L.P., TAC Associates, L.P., Three Arch Partners IV, L.P., and Three Arch Associates IV, L.P. (incorporated by reference to the exhibit to the Company’s Form 8-K filed with the SEC on May 13, 2010)
|
|
|
10.3
|
Huiheng 2009 Share Plan (incorporated by reference to the exhibit to the Company’s current report on Form 8-K filed with the SEC on June 10, 2009)
|
|
|
10.4
|
Purchase Contract of Cobalt-60 Radiation Sources Used in SGS-1, dated December 1, 2005, by and between Shenzhen Hyper Technology Incorporation and Beijing Atom High-Tech Co., Ltd. (incorporated by reference to the exhibit to the Company’s current report on Form 10-Q filed with the SEC on November 11, 2010)
|
|
|
31.1
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes Oxley Act of 2002
|
|
|
31.2
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes Oxley Act of 2002
|
|
|
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
|
32.2
|
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
46
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
Huiheng Medical, Inc.,
|
||
|
a Nevada corporation
|
||
|
Date: April 15, 2011
|
By:
|
/s/ Hui Xiaobing
|
|
Hui Xiaobing
|
||
|
Chief Executive Officer (Principal Executive Officer)
|
||
|
Date: April 15, 2011
|
By:
|
/s/ Richard Shen
|
|
Richard Shen
|
||
|
Chief Financial Officer (Principal Financial & Accounting
|
||
|
Officer)
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Signature(s)
|
Title(s)
|
Date
|
|||
|
/s/ Hui Xiaobing
|
|||||
|
Hui Xiaobing
|
Chairman
|
April 15, 2011
|
|||
|
/s/ Huang Jian
|
|||||
|
Huang Jian
|
Director
|
April 15, 2011
|
|||
|
/s/ Li Daxi
|
|||||
|
Li Daxi
|
Director
|
April 15, 2011
|
47
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Contents
|
Page(s)
|
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
|
Consolidated Balance Sheets at December 31, 2010 and 2009
|
F-2
|
|
|
Consolidated Statements of Income for the Years Ended December 31, 2010 and 2009
|
F-3
|
|
|
Consolidated Statement of Changes in Stockholders' Equity and Other Comprehensive Income
|
||
|
for the Years Ended December 31, 2010 and 2009
|
F-4
|
|
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2010 and 2009
|
F-5
|
|
|
Notes to the Consolidated Financial Statements
|
F-6 to F-27
|
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
TO THE BOARD OF DIRECTORS AND STOCKHOLDERS
HUIHENG MEDICAL INC. AND SUBSIDIARIES
We have audited the accompanying consolidated balance sheets of Huiheng Medical Inc. and its subsidiaries (collectively the “Company”) as of December 31, 2010 and 2009, and the related consolidated statements of income, changes in stockholders’ equity and comprehensive income, and cash flows for the years then ended. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial positions of the Huiheng Medical Inc. and its subsidiaries. as of December 31, 2010 and 2009, the consolidated results of its operations and its consolidated cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
/s/ UHY VOCATION HK CPA LIMITED
Hong Kong, the People’s Republic of China
April 15, 2011
F-1
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
( IN US DOLLARS)
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash
|
$ | 294,542 | $ | 84,962 | ||||
|
Accounts receivable
|
8,130,681 | 6,777,868 | ||||||
|
Prepaid expenses
|
2,743,602 | 3,339,701 | ||||||
|
Other receivables, net of allowance for doubtful accounts of
|
||||||||
|
$757,576 and $732,386 as of December 31, 2010
|
||||||||
|
and December 31, 2009, respectively
|
109,519 | 92,031 | ||||||
|
Inventories
|
2,832,282 | 1,359,900 | ||||||
|
Total Current Assets
|
14,110,626 | 11,654,462 | ||||||
|
ACCOUNTS RECEIVABLE, net of allowance for doubtful accounts of $1,451,364 and $897,319 as of December 31, 2010 and December 31, 2009, respectively
|
11,617,798 | 9,721,951 | ||||||
|
OTHER RECEIVABLES, net of current portion
|
1,216,841 | 1,457,616 | ||||||
|
INVESTMENT IN AFFILIATE
|
137,032 | 43,152 | ||||||
|
PROPERTY, PLANT AND EQUIPMENT, NET
|
2,419,348 | 2,520,787 | ||||||
|
LAND USE RIGHT, NET
|
952,057 | 939,575 | ||||||
|
INTANGIBLE ASSETS, NET
|
962,202 | 777,086 | ||||||
|
Total Assets
|
$ | 31,415,904 | $ | 27,114,629 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Accounts payable
|
$ | 1,266,774 | $ | 844,539 | ||||
|
Due to related parties
|
385,025 | 24,560 | ||||||
|
Income tax payable
|
974,328 | 389,632 | ||||||
|
Accrued liabilities and other payables
|
1,740,226 | 1,676,639 | ||||||
|
Total Current Liabilities
|
4,366,353 | 2,935,370 | ||||||
|
STOCKHOLDERS' EQUITY:
|
||||||||
|
Preferred stock, $0.001 par value; 1,000,000 shares authorized;
|
||||||||
|
Designated 300,000 shares of Series A convertible preferred stock; 211,390 shares issued and outstanding with liquidation preference of $7,927,125 at December 31, 2010; and 220,467 shares issued and outstanding with liquidation preference of $8,267,513 at December 31, 2009
|
211 | 220 | ||||||
|
Common stock, $0.001 par value; 74,000,000 shares authorized; 23,730,637 shares issued and 14,030,637 shares outstanding at December 31, 2010; and 23,635,290 shares issued and 13,935,290 shares outstanding at December 31, 2009
|
23,731 | 23,635 | ||||||
|
Treasury stock, 9,700,000 common shares, at cost
|
(9,700 | ) | (9,700 | ) | ||||
|
Additional paid-in capital
|
7,498,086 | 7,498,086 | ||||||
|
Statutory surplus reserve
|
1,676,867 | 1,310,516 | ||||||
|
Retained earnings
|
14,298,693 | 12,488,965 | ||||||
|
Accumulated other comprehensive income
|
||||||||
|
Foreign currency translation gain
|
2,633,236 | 1,756,510 | ||||||
|
Total Huiheng's stockholders' equity
|
26,121,124 | 23,068,232 | ||||||
|
Non-controlling interests
|
928,427 | 1,111,027 | ||||||
|
Total Stockholders' Equity
|
27,049,551 | 24,179,259 | ||||||
|
Total Liabilities and Stockholders' Equity
|
$ | 31,415,904 | $ | 27,114,629 | ||||
See accompanying notes to the consolidated financial statements.
F-2
|
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
|
|
CONSOLIDATED STATEMENTS OF INCOME
|
|
(IN US DOLLARS)
|
|
For the year ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
REVENUES
|
$ | 8,636,573 | $ | 9,378,579 | ||||
|
COST OF REVENUES
|
2,176,184 | 2,353,088 | ||||||
|
GROSS PROFIT
|
6,460,389 | 7,025,491 | ||||||
|
OPERATING EXPENSES:
|
||||||||
|
Sales and marketing expenses
|
281,927 | 316,247 | ||||||
|
General and administrative expenses
|
3,382,617 | 3,351,201 | ||||||
|
Research and development costs
|
80,071 | 485,290 | ||||||
|
Total Operating Expenses
|
3,744,615 | 4,152,738 | ||||||
|
OPERATING INCOME
|
2,715,774 | 2,872,753 | ||||||
|
OTHER INCOME / (EXPENSES):
|
||||||||
|
Other income
|
182,902 | 1,253,747 | ||||||
|
Interest income
|
293 | 375 | ||||||
|
Interest expenses
|
(253,876 | ) | - | |||||
|
Gain on business acquisition
|
21,508 | - | ||||||
|
Equity in income / (loss) of affiliate
|
90,204 | (2,012 | ) | |||||
|
Total Other Income
|
41,031 | 1,252,110 | ||||||
|
NET INCOME BEFORE INCOME TAXES
|
2,756,805 | 4,124,863 | ||||||
|
INCOME TAXES
|
796,213 | 671,078 | ||||||
|
NET INCOME BEFORE ATTRIBUTION OF
|
||||||||
|
NON-CONTROLLING INTERESTS
|
1,960,592 | 3,453,785 | ||||||
|
NET LOSS ATTRIBUTABLE TO NON-CONTROLLING INTERESTS
|
215,574 | 186,631 | ||||||
|
NET INCOME ATTRIBUTABLE TO HUIHENG’S SHAREHOLDERS
|
2,176,166 | 3,640,416 | ||||||
|
EARNINGS PER SHARE
|
||||||||
|
- Basic
|
$ | 0.16 | $ | 0.26 | ||||
|
- Diluted
|
$ | 0.13 | $ | 0.22 | ||||
|
Weighted Common Shares Outstanding
|
||||||||
|
- Basic
|
13,960,476 | 13,918,771 | ||||||
|
- Diluted
|
16,251,113 | 16,251,113 | ||||||
See accompanying notes to the consolidated financial statements.
F-3
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' EQUITY AND OTHER COMPREHENSIVE INCOME
(IN US DOLLARS)
|
Series A Preferred Stock
|
Common Stock
|
Treasury Stock
|
||||||||||||||||||||||||||||||||||||||||||
|
Number of
|
Number of
|
Number of
|
Additional
Paid-in
|
Statutory
|
Retained
|
Accumulated
Other
Comprehensive
|
Total
Huiheng's
stockholders'
|
Non-controlling
|
Total
Stockholders
|
Comprehensive
|
||||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
surplus reserve
|
Earnings
|
Income
|
equity
|
interest
|
'Equity
|
Income
|
|||||||||||||||||||||||||||||||
|
Balance, December 31, 2008
|
229,133 | $ | 229 | 23,544,254 | $ | 23,544 | (9,700,000 | ) | $ | (9,700 | ) | $ | 7,498,086 | $ | 1,310,516 | $ | 8,848,631 | $ | 1,767,573 | $ | 19,438,879 | $ | 1,298,604 | $ | 20,737,483 | $ | - | |||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||||||||||||||
|
Net income/(loss)
|
- | - | - | - | - | - | - | - | 3,640,416 | - | 3,640,416 | (186,631 | ) | 3,453,785 | 3,453,785 | |||||||||||||||||||||||||||||
|
Foreign currency translation loss
|
- | - | - | - | - | - | - | - | - | (11,063 | ) | (11,063 | ) | (946 | ) | (12,009 | ) | (12,009 | ) | |||||||||||||||||||||||||
|
Issuance of common stock in connection with conversion of preferred stock
|
(8,666 | ) | (9 | ) | 91,036 | 91 | - | - | - | - | (82 | ) | - | - | - | - | - | |||||||||||||||||||||||||||
|
Balance, December 31, 2009
|
220,467 | 220 | 23,635,290 | 23,635 | (9,700,000 | ) | (9,700 | ) | 7,498,086 | 1,310,516 | 12,488,965 | 1,756,510 | 23,068,232 | 1,111,027 | 24,179,259 | $ | 3,441,776 | |||||||||||||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||||||||||||||
|
Net income/(loss)
|
- | - | - | - | - | - | - | - | 2,176,166 | - | 2,176,166 | (215,574 | ) | $ | 1,960,592 | 1,960,592 | ||||||||||||||||||||||||||||
|
Foreign currency translation gain
|
- | - | - | - | - | - | - | - | - | 876,726 | 876,726 | 32,974 | 909,700 | 909,700 | ||||||||||||||||||||||||||||||
|
Issuance of common stock in connection with conversion of preferred stock
|
(9,077 | ) | (9 | ) | 95,347 | 96 | - | - | - | - | (87 | ) | - | - | - | - | - | |||||||||||||||||||||||||||
|
Appropriation to statutory reserves
|
- | - | - | - | - | - | - | 366,351 | (366,351 | ) | - | - | - | - | - | |||||||||||||||||||||||||||||
|
Balance, December 31, 2010
|
211,390 | $ | 211 | 23,730,637 | $ | 23,731 | (9,700,000 | ) | $ | (9,700 | ) | $ | 7,498,086 | $ | 1,676,867 | $ | 14,298,693 | $ | 2,633,236 | $ | 26,121,124 | $ | 928,427 | $ | 27,049,551 | $ | 2,870,292 | |||||||||||||||||
See accompanying notes to the consolidated financial statements.
F-4
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(IN US DOLLARS)
|
For the year ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash flows from operating activities:
|
||||||||
|
Net income before attribution of non-controlling interests
|
$ | 1,960,592 | $ | 3,453,785 | ||||
|
Net loss attributable to non-controlling interests
|
215,574 | 186,631 | ||||||
|
Total Huiheng's net income
|
2,176,166 | 3,640,416 | ||||||
|
Adjustments to reconcile net income to net cash used in operating activities:
|
||||||||
|
Depreciation of property, plant and equipment
|
249,185 | 253,991 | ||||||
|
Recovery of bad debts
|
(31,951 | ) | (907,720 | ) | ||||
|
Amortization of land use rights
|
19,364 | 17,569 | ||||||
|
Amortization of intangible assets
|
89,585 | 82,483 | ||||||
|
Bad debt expense
|
594,491 | 375,679 | ||||||
|
Write off of deferred offering costs
|
- | 460,209 | ||||||
|
Interest expenses
|
253,876 | - | ||||||
|
Equity in (income) / loss of affiliate
|
(90,204 | ) | 2,012 | |||||
|
Non-controlling interest
|
(215,574 | ) | (186,631 | ) | ||||
|
Gain on acquisition of subsidiary
|
(21,508 | ) | - | |||||
|
Changes in assets and liabilities:
|
||||||||
|
Accounts receivable
|
(4,065,076 | ) | (5,721,391 | ) | ||||
|
Prepaid expenses and other receivables
|
819,386 | 786,146 | ||||||
|
Inventories
|
(1,472,382 | ) | (6,548 | ) | ||||
|
Accounts payable
|
422,235 | 11,633 | ||||||
|
Income tax payable
|
584,696 | 368,201 | ||||||
|
Accrued liabilities and other payables
|
63,587 | 257,995 | ||||||
|
Net cash used in operating activities
|
(624,124 | ) | (565,956 | ) | ||||
|
Cash flows from investing activities:
|
||||||||
|
Capital expenditures on addition of property, plant and equipment
|
(35,519 | ) | (134,207 | ) | ||||
|
Repayment of advance to third parties
|
- | 541,704 | ||||||
|
Repayment of advances to related party
|
- | 732,032 | ||||||
|
Advance to third party
|
- | (541,704 | ) | |||||
|
Advance from related party
|
90,000 | - | ||||||
|
Payment for land use right
|
- | (956,226 | ) | |||||
|
Net cash provided by / (used in) investing activities
|
54,481 | (358,401 | ) | |||||
|
Net decrease in cash
|
(569,643 | ) | (924,357 | ) | ||||
|
Effect on change of exchange rates
|
779,223 | (9,857 | ) | |||||
|
Cash as of January 1
|
84,962 | 1,019,176 | ||||||
|
Cash as of December 31
|
$ | 294,542 | $ | 84,962 | ||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid during the year for:
|
||||||||
|
Income tax paid
|
$ | 238,472 | $ | 303,041 | ||||
See accompanying notes to the consolidated financial statements.
F-5
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 1 - ORGANIZATION AND OPERATIONS
Huiheng Medical, Inc. (“the Company” or “Huiheng”), formerly known as Mill Basin Technologies, Limited (“Mill Basin”), is a China-based medical device company that, through its subsidiaries, designs, develops and markets radiation therapy systems used for the treatment of cancer. The Company is a Nevada holding company and conducts all of its business through operating subsidiaries in China and a subsidiary in the US (Portola Medical, Inc), which holds the rights to certain patents.
The Company's common stock is listed on the Over-the-counter Bulletin Board ("OTCBB") market and traded under the symbol "HHGM".
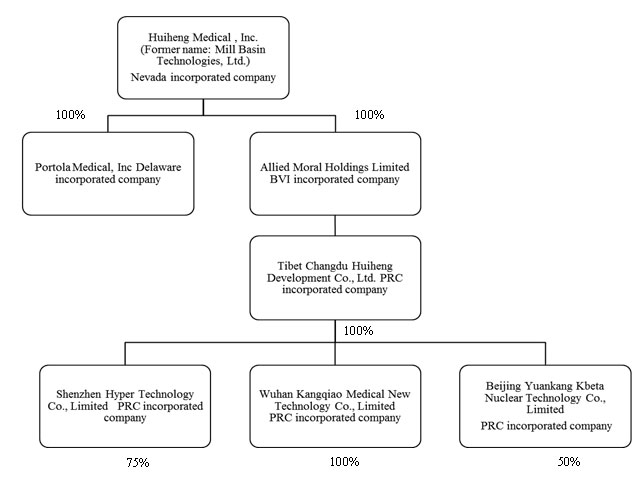
Acquisition of New Subsidiary
On June 7, 2010, the Company acquired a 100% interest in Portola Medical Inc which is incorporated in Delaware and its primary asset consists of its rights to develop, manufacture and sell an adjustable Multi-Catheter Source Applicator under the ClearPath trademark which is intended to provide brachytherapy when a physician chooses to deliver intracavitary radiation to the surgical margins following lumpectomy of breast cancer. The Company plans to market and sell this product in the United States and in Asia. This product is still in its initial phase and there have been no sales of this product.
F-6
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The consolidated financial statements include all accounts of the Company and its wholly-owned and majority-owned subsidiaries. All material inter-company balances and transactions have been eliminated in consolidation.
Reclassifications
Certain reclassifications have been made to the financial statement presentations as of December 31, 2009 and for the year then ended to correspond to the current year’s format. Total equity and net income are unchanged due to these reclassifications.
Summary of significant accounting policies
Estimates
The preparation of the financial statements in accordance with United States Generally Accepted Accounting Principles (“US GAAP”) requires management of the Company to make a number of estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the years. Significant items subject to such estimates and assumptions include the recoverability of the carrying amount and the estimated useful lives of long-lived assets; valuation allowances for receivables and realizable values for inventories. Actual results could differ from those estimates in consolidation.
Accounts receivable
Accounts receivable are recorded at the invoiced amount, net of allowances for doubtful accounts, sales returns, trade discounts and value added tax. The allowance for doubtful accounts is the Company’s best estimate of the amount of probable credit losses in the Company’s existing accounts receivable. The Company performs ongoing credit evaluations of its customers' financial conditions. The Company provided an allowance of $1,451,364 and $897,319 for doubtful accounts respectively as of December 31, 2010 and December 31, 2009, respectively.
Outstanding account balances are reviewed individually for collectability. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote.
The ultimate collection of the Company’s accounts receivable may take more than one year, and any portion of accounts receivable expected to be collected in more than one year is reflected as noncurrent, net of allowance for doubtful accounts relating to that portion of the receivables. The bifurcation between current and noncurrent portions of accounts receivable is based on management’s estimate and predicated on historical collection experience.
Accounts receivables balances at December 31, 2009 amounting to $16,499,819 (net of deducting allowance of doubtful accounts of $897,319) which were previously shown as current in the annual report 10K have been reclassified between current and non-current in order to correspond to the current year’s classification.
The Company had signed agreements with SZ Jiancheng Investment Co., Ltd., “Jiancheng” as on March 14, 2011 to acquire the operating rights of four medical centers valued at approximately $8 million (RMB 54,000,000) for a period from 5 to 8 years. Both parties agreed to use the long outstanding of accounts receivable owed by Jiancheng to the Company as consideration of these operating rights.
F-7
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Inventories
The Company values inventories, consisting of work in process and raw materials, at the lower of cost or market. Cost of material is determined on the weighted average cost method. Cost of work in progress includes direct materials, direct production cost and an allocated portion of production overhead.
The final steps of assembly of our products, including installation of radioactive service materials, are completed at customer locations. Accordingly, the Company generally does not carry finished goods inventory.
Property, plant and equipment
Property, plant and equipment are recorded at cost less accumulated depreciation. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to general and administrative expenses as incurred. Depreciation of property, plant and equipment is computed by the straight-line method (after taking into account their respective estimated residual values) over the assets estimated useful lives ranging from three to twenty years. Building improvements are amortized on a straight-line basis over the estimated useful life. Upon sale or retirement of property, plant and equipment, the related cost and accumulated depreciation are removed from the accounts and any gain or loss is reflected in operations. The estimated useful lives of the assets are as follows:
|
Estimated Life
|
||
|
Buildings and building improvements
|
3 to 20
|
|
|
Production equipment
|
3 to 5
|
|
|
Furniture fixtures and office equipment
|
3 to 5
|
|
|
Motor vehicles
|
5 to 10
|
Land use right
Land use right is recorded at cost less accumulated amortization. Under ASC 350 "Intangibles, Goodwill and Other", land use right is classified as a definite lived intangible asset and is amortized over its useful life. According to the laws of the PRC, the government owns all of the land in the PRC. Companies or individuals are authorized to possess and use the land only through land use rights granted by the PRC government. The Company's land use right is amortized using the straight-line method over the lease term of 50 years.
F-8
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Intangible assets
The Company’s intangible assets include patent and pending patents applications. The Company accounts for its intangible assets pursuant to FASB ASC Subtopic 350-30, “General Intangibles Other Than Goodwill”. Under ASC 350-30-35, intangibles with definite lives are amortized on a straight-line basis over the lesser of their estimated useful lives or contractual terms. Accordingly, the Company amortizes the patent over their legal term of 20 years, on a straight-line basis.
Investment in affiliate
The Company owns a 50% equity interest in Beijing Yuankang Kbeta Nuclear Technology Company, Ltd ("Beijing Kbeta") and accounts for the investment using the equity method of accounting. The equity method is utilized as the Company has the ability to exercise significant influence over the investee, but does not have a controlling financial interest. If circumstances indicate that the carrying value of the Company's investment in Beijing Kbeta may not be recoverable, the Company would recognize an impairment loss by writing down its investment to its estimated net realizable value if management concludes such impairment is other than temporary.
We have invested in Beijing Kbeta since it provides the Company the ability to install and replace the Cobalt 60 radioactive sources used in our gamma treatment systems products.
Impairment of long-lived assets
Long-lived assets, which include tangible assets and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of the asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated undiscounted future cash flows, an impairment charge is recognized by the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of, if any, are separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and are no longer depreciated. At December 31, 2010 and 2009, the Company determined that there was no impairment of value.
F-9
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Fair value of financial instruments
The fair value of a financial instrument is the amount at which the instrument could be exchanged in a current transaction between willing parties. The carrying amounts of financial assets and liabilities, such as cash, accounts receivable, prepaid expenses and other current assets, accounts payable, income taxes payable, accrued expenses and other current liabilities, approximate their fair values because of the short maturity of these instruments and market rates of interest.
Revenue recognition
The Company generates revenue primarily from sales of medical equipment and the sale of maintenance and support services. Revenue is recognized as follows:
|
(i)
|
Sales of medical equipment
|
The Company recognizes revenue when products are delivered and the customer takes ownership and assumes risk of loss, collection of the relevant receivable is probable, persuasive evidence of an arrangement exists and the sales price is fixed or determinable. The sales price of the medical equipment includes the training services, which generally take about 1 month. These services are ancillary to the purchase of medical equipment by customers and are normally considered by the customers to be an integral part of the acquired equipment. As training services do not have separately determinable fair values, the Company recognizes revenue for the entire arrangement upon customer acceptance, which occurs after delivery and installation.
In the PRC, value added tax (“VAT”) of 17% on invoiced amounts is collected in respect of the sales of goods on behalf of tax authorities. The VAT collected is not revenue of the Company; instead, the amount is recorded as a liability on the balance sheet until such VAT is paid to the authorities.
Pursuant to the laws and regulations of the PRC, Shenzhen Hyper Technology Co., Limited ("Shenzhen Hyper") (a subsidiary of the Company) is entitled to a refund of VAT on the sales of self-developed software embedded in medical equipment. The VAT refund represents the amount of VAT collected from customers and paid to the authorities in excess of 3% of relevant sales. The amount of VAT refund is calculated on a monthly basis. As the refund relates directly to the sale of self-developed software that is embedded in the Company’s products, the Company recognizes the VAT refund at the time the product is sold. The amount is included in the line item "Revenues" in the consolidated statements of income and is recorded on an accrual basis.
The medical equipment sold by the Company has embedded self-developed software which can also be sold on a standalone basis.
|
(ii)
|
Sales of maintenance and support services
|
The Company also provides comprehensive post-sales services to certain distributors for medical equipment used by hospitals. These contracts are negotiated and signed independently and separately from the sales of medical equipment. In accordance with the agreements, the Company provides comprehensive services including replace of Cobalt 60 radioactive sources, additional training to users of the medical equipment, maintenance of medical equipment, software upgrades and consulting. Fees for these services are recognized over the life of the contract on a monthly basis.
F-10
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Government tax refund
Pursuant to a tax incentive policy provided by the Tibet Finance Bureau, Tibet Changdu Huiheng Development Co., Ltd (“Changdu Huiheng”), a subsidiary of the Company, is eligible for the following tax refunds for five (5) consecutive years commencing from September 2006:
|
(i)
|
Annual income tax payment in excess of $133,000 (RMB 900,000) will be refundable.
|
|
(ii)
|
31% of business tax payments will be refunded if annual business tax payment in excess of $148,000 (RMB 1.0 million).
|
|
(iii)
|
38.75% of VAT payments will be refunded if annual VAT payment in excess of $222,000 (RMB 1.5 million).
|
The Company records these tax refunds as other income when the refunds are confirmed and paid by Tibet Finance Bureau.
Warranty
The Company provides a product warranty to its customers to repair any product defects that occur generally within twelve months from the date of sale. The Company's sales contracts generally allow the customer to withhold up to 10% of the total selling price for the duration of the warranty period which is included in Accounts receivable. Based on the limited number of actual warranty claims and the historically low cost of such repairs, the Company has not recognized a liability for warranty claims, but rather recognizes such cost when product repairs are made.
Research and development costs
Research and development costs are charged to expense as incurred. Research and development costs mainly consist of salary for the research and development staff and material costs for research and development. The Company incurred $80,071 and $485,290 for the year ended December 31, 2010 and 2009 respectively.
Income taxes
The Company accounts for income taxes under ASC 740 "Income Taxes". Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of operations in the period that includes the enactment date.
During 2008, the Company adopted ASC740 "Income Taxes", which prescribes a more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken in the tax return. This interpretation also provides guidance on de-recognition of income tax assets and liabilities, classification of current and deferred income tax assets and liabilities, accounting for interest and penalties associated with tax positions, accounting for income taxes in interim periods and income tax disclosures.
F-11
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Foreign currency translation
Assets and liabilities of foreign subsidiaries are translated at the rate of exchange in effect on the balance sheet date; income and expenses are translated at the average rate of exchange prevailing during the period. The related transaction adjustments are reflected in "Accumulated other comprehensive income / (loss)" in the equity section of the consolidated balance sheet.
|
2010
|
2009
|
||||
|
Balance sheet items, except for equity accounts
|
RMB6.6000 = $1
|
RMB6.8270 = $1
|
|||
|
Items in statement of income
|
RMB6.7604 = $1
|
RMB6.8303 = $1
|
Cash
Cash consist of cash on hand and at banks. Substantially all of the Company's cash is held with financial institutions located in the PRC. Management believes that these major financial institutions are of high credit quality.
Share-based compensation
The Company awards stock options and other equity-based instruments to its employees, directors and consultants and provides employees the right to purchase common stock (collectively "share-based payments"), pursuant to stockholder approved plans. Compensation cost related to such awards is measured based on the fair value of the instrument on the grant date and is recognized on a straight-line basis over the requisite service period, which generally equals the vesting period. All of the Company's stock-based compensation is based on grants of equity instruments and no liability awards have been granted.
During 2009, the Company adopted a stock option plan (the "Plan") for selected employees, directors, consultants to promote the success of the Company's business by offering these individuals an opportunity to acquire an ownership interest in the Company. The Plan provides both for direct awards of shares and for the granting of options to purchase shares as determined by the Administrator at the time of the grant. Under the Plan, 1,566,666 shares have been reserved for awards.
The Company has elected to use the calculated value method to account for the options issued in 2009. The Company used its historical closing price of 2010 to estimate volatility. Using the Black-Scholes-Merton option pricing model, management has determined that the options issued in 2009 have a calculated value of $0.4683 per share. Total compensation cost associated with these options was $14,049 over the three-year service period that began on the grant date. Compensation cost in 2010 and 2009 was $4,683 and $3,512 respectively. Such amount had not been recorded as it was considered immaterial.
F-12
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
The assumptions used and the weighted average calculated value of options are as follows for the year ended December 31, 2010:
|
Risk-free interest rate
|
2.01 | % | ||
|
Expected dividend yield
|
- | |||
|
Expected volatility
|
152.33 | % | ||
|
Expected life in years
|
3.4 | |||
|
Service period in years
|
3 | |||
|
Weighted average calculated value of options granted
|
$ | 0.4683 |
The following is an analysis of options to purchase shares of the Company’s stock issued and outstanding as of December 31, 2010:
|
Weighted Avergae
|
||||||||
|
Options
|
Price
|
|||||||
|
Options granted on June 6, 2009
|
30,000 | $ | 5 | |||||
|
Exercised
|
- | |||||||
|
Expired/cancelled
|
- | |||||||
|
Options outstanding, as at December 31, 2009
|
30,000 | |||||||
|
Granted
|
- | |||||||
|
Exercised
|
- | |||||||
|
Expired/cancelled
|
- | |||||||
|
Options outstanding, as at December 31, 2010
|
30,000 | |||||||
As of December 31, 2010, options for 17,500 shares at a weighted average exercise price of $5.00 were vested and exercisable. These options have a weighted average remaining contractual term of 1.2 years. Compensation cost of approximately $5,854 had not yet been recognized on nonvested awards. The weighted average period over which it is expected to be recognized is 1.25 years.
Other comprehensive income
The Company has adopted ASC 220 "Comprehensive Income". This statement establishes rules for the reporting of other comprehensive income and its components. Other comprehensive income consists of net income and foreign currency translation adjustments and is presented in the Consolidated Statements of Income and the Consolidated Statement of Changes in Stockholders' Equity.
F-13
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
Earnings per share
The value of basic earnings per share is computed on the basis of the weighted-average number of shares of our common stock outstanding during the year. Diluted earnings per share is computed on the basis of the weighted-average number of shares of our common stock plus the effect of dilutive potential common shares outstanding during the year using the if-converted method. Dilutive potential common shares include Series A Convertible Preferred Stock.
The following table sets forth the computation of basic and diluted net income per common share:
|
For the Year ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Net income per common share
|
$ | 2,176,166 | $ | 3,640,416 | ||||
|
Weighted average outstanding shares of common stock
|
13,960,476 | 13,918,771 | ||||||
|
Dilutive effect of Convertible Preferred Stock
|
2,290,637 | 2,332,342 | ||||||
|
Diluted weighted average outstanding shares
|
16,251,113 | 16,251,113 | ||||||
|
Earnings per common share:
|
||||||||
|
Basic
|
$ | 0.16 | $ | 0.26 | ||||
|
Diluted
|
$ | 0.13 | $ | 0.22 | ||||
For the year ended December 31, 2010, the stock option plan granted in June 2009 to purchase 30,000 common shares were not included in diluted earnings per share because the effect would be anti-dilutive.
Commitments and contingencies
The Company is subject to lawsuits, investigations and other claims related to operations, product, taxing authorities, environmental and other matters out of the normal course of business, and is required to assess the likelihood of any adverse judgments or outcomes to these matters, as well as potential ranges of probable losses and fess. A determination of the amount of reserves and disclosures required, if any, for these contingencies are made after considerable analysis of each individual issue. The Company accrues for contingent liabilities when an assessment of the risk of loss is probable and can be reasonably estimated. The Company discloses contingent liabilities when the risk of loss is reasonably or probable.
All of the Company’s operations are carried out and all of its assets are located in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency fluctuation and remittances and methods of taxation, among other things.
Financial instruments with characteristics of both liabilities and equity
The Company accounts for its Series A Preferred Stock in accordance with ASC 480 "Distinguishing Liabilities from Equity" and ASC 815 "Derivatives and Hedging". We have determined that our Series A Preferred Stock is not mandatorily redeemable. Accordingly, the Company accounts for the Preferred stock as permanent equity.
F-14
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Non-controlling interest in consolidated financial statements
In December 2007, the FASB issued authoritative guidance related to non-controlling interests in consolidated financial statements, which was an amendment of ARB No. 51. This guidance is set forth in Topic 810 in the Accounting Standards Codification (ASC 810). ASC 810 establishes accounting and reporting standards for the non-controlling interest in a subsidiary and for the deconsolidation of a subsidiary. This accounting standard is effective for fiscal years beginning after December 15, 2008. The Company adopted the presentation and disclosure requirements of ASC 810 retrospectively to the December 31, 2008 financial statements.
Fair value measurements
ASC Topic 820, Fair Value Measurement and Disclosures, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. This topic also establishes a fair value hierarchy which requires classification based on observable and unobservable inputs when measuring fair value. There are three levels of inputs that may be used to measure fair value:
|
Level 1 -
|
Quoted prices in active markets for identical assets or liabilities.
|
|
Level 2 -
|
Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
|
|
Level 3 -
|
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Company evaluates its hierarchy disclosures each quarter.
The carrying values of cash and cash equivalents, account receivable, and account payable, approximate fair values due to their short maturities.
There was no asset or liability measured at fair value on a non-recurring basis as of December 31, 2010 and 2009.
F-15
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Impact of new accounting standards
We describe below recent pronouncements that have had or may have a significant effect on our financial statements. We do not discuss recent pronouncements that are not anticipated to have an impact on or are unrelated to our financial condition, results of operations, or disclosures.
The FASB issued authoritative guidance related to subsequent events in May 2009, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before the financial statements are issued or are available to be issued. This guidance is set forth in Topic 855 in the Accounting Standards Codification (ASC 855). ASC 855 provides guidance on the period after the balance sheet date during which management of a reporting entity should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. We adopted ASC 855 and its application had no impact on our consolidated financial statements.
In October 2009, FASB issued authoritative guidance that amends existing guidance for identifying separate deliverables in a revenue-generating transaction where multiple deliverables exist, and provides guidance for allocating and recognizing revenue based on those separate deliverables. The guidance is expected to result in more multiple-deliverable arrangements being separable than under current guidance and is required to be applied prospectively to new or significantly modified revenue arrangements. This guidance, for which the Company is currently assessing the impact on its financial condition and results of operations, will become effective for the Company on January 1, 2011.
In January 2010, the FASB issued ASU 2010-6, Improving Disclosures About Fair Value Measurements, which requires reporting entities to make new disclosures about recurring or nonrecurring fair-value measurements including significant transfers into and out of Level 1 and Level 2 fair-value measurements and information on purchases, sales, issuances, and settlements on a gross basis in the reconciliation of Level 3 fair-value measurements. ASU 2010-6 is effective for annual reporting periods beginning after December 15, 2009, except for Level 3 reconciliation disclosures which are effective for annual periods beginning after December 15, 2010. The adoption of ASU 2010-6 did not have a material impact on our consolidated financial statement disclosures.
F-16
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (…/Cont’d)
Summary of significant accounting policies (…/Cont'd)
Impact of new accounting standards (…/Cont'd)
In December, 2010, the FASB amended its view on performing step two of a goodwill impairment analysis. The amendment does not prescribe a specific method of calculating the carrying value of a reporting unit in the performance of step one of the goodwill impairment test and requires entities with a zero or negative carrying value to assess, considering qualitative factors such as those listed in Accounting Standards Codification (ASC) 350-20-35-30 Intangibles - Goodwill and Other, whether it is more likely than not that a goodwill impairment exists. If an entity concludes that it is more likely than not that a goodwill impairment exists, the entity must perform step two of the goodwill impairment test. For public entities, these amendments are effective for impairment tests performed during entities' fiscal years that begin after December 15, 2010. The Company does not believe the adoption of this amendment will have a material effect on its financial statements.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
NOTE 3 – INVENTORIES
At December 31, 2010 and 2009, inventories consisted of the following:
|
2010
|
2009
|
|||||||
|
Raw materials
|
$ | 578,071 | $ | 281,112 | ||||
|
Work-in-progress
|
2,254,211 | 1,078,788 | ||||||
| $ | 2,832,282 | $ | 1,359,900 | |||||
Final assembly of our products, including installation of radioactive source materials, is conducted on site at our customers' locations. Our products are not considered to be finished good (available for sale in the normal course of business) until such time as the source material is installed in the units.
NOTE 4 – PREPAID EXPENSES
At December 31, 2010 and 2009, prepaid expenses consisted of the following:
|
2010
|
2009
|
|||||||
|
Advances made to suppliers
|
$ | 2,452,693 | $ | 3,058,465 | ||||
|
Construction in progress paid on behalf of landlord (a)
|
||||||||
|
- current portion
|
290,909 | 281,236 | ||||||
| $ | 2,743,602 | $ | 3,339,701 | |||||
|
Non-current portion (a)
|
$ | 1,216,841 | $ | 1,457,616 | ||||
|
(a)
|
Under an agreement signed with the landlord, Shenzhen Hyper will make payment for the construction in progress of the building in advance on behalf the landlord. Meanwhile, Shenzhen Hyper signed a rental agreement with the landlord to rent the building for 20 years at about US$24,200 (RMB160,000) per month. The balance of the amount due from the landlord will be used and amortized as rental expenses incurred by Shenzhen Hyper.
|
F-17
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 4 – PREPAID EXPENSES (…/Cont'd)
The expected amortization of construction in progress for the next five years and thereafter is as follows:
|
Total
|
||||
|
For the year ended
|
||||
|
December 31,
|
||||
|
2011
|
$ | 290,904 | ||
|
2012
|
290,904 | |||
|
2013
|
290,904 | |||
|
2014
|
290,904 | |||
|
2015
|
290,904 | |||
|
Thereafter
|
53,230 | |||
|
Total
|
$ | 1,507,750 | ||
NOTE 5 – OTHER RECEIVABLES
At December 31, 2010 and 2009, other receivables, net, consisted of the following:
|
2010
|
2009
|
|||||||
|
Other receivables
|
||||||||
|
- loan or advance to staff for business travelling
|
$ | 38,758 | $ | 43,072 | ||||
|
- utilities and rental deposits
|
1,515 | 1,948 | ||||||
|
- advances made by director
|
3,030 | 2,930 | ||||||
| - others (net of allowance for doubtful accounts of $757,576 and $732,386) | 66,216 | 44,081 | ||||||
| $ | 109,519 | $ | 92,031 | |||||
NOTE 6 – PROPERTY, PLANT AND EQUIPMENT
At December 31, 2010 and 2009, property, plant and equipment consisted of the following:
|
2010
|
2009
|
|||||||
|
Buildings and building improvements
|
$ | 2,565,072 | $ | 2,479,782 | ||||
|
Production equipment
|
702,900 | 656,423 | ||||||
|
Furniture, fixture and office equipment
|
422,616 | 366,868 | ||||||
|
Motor vehicles
|
193,750 | 187,308 | ||||||
| 3,884,338 | 3,690,381 | |||||||
|
Less: Accumulated depreciation
|
(1,464,990 | ) | (1,169,594 | ) | ||||
| $ | 2,419,348 | $ | 2,520,787 | |||||
For the year ended December 31, 2010 and 2009, depreciation expense amounted to $249,185 and $253,991, respectively.
F-18
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 7 - LAND USE RIGHT
Land use right represents prepaid lease payments to the Local Government for land use right held for a period of 50 years from January 20, 2009 to December 26, 2058 in Wuhan, People's Republic of China. Land use right is amortized using the straight-line method over the lease term of 50 years. The amortization expense for the year ended December 31, 2010 and 2009 was $19,364 and $17,569, respectively.
NOTE 8 – INTANGIBLE ASSETS
At December 31, 2010 and 2009, intangible assets, net consisted of the following:
|
2010
|
2009
|
|||||||
|
Patent technology in Shenzhen Hyper (a)
|
$ | 1,515,152 | $ | 1,464,772 | ||||
|
Patent technology in Portola Medical, Inc.(b)
|
250,000 | - | ||||||
|
Less: Accumulated amortization
|
(802,950 | ) | (687,686 | ) | ||||
| $ | 962,202 | $ | 777,086 | |||||
|
(a)
|
Patent represents a patent technology for the production of a component of the radiation treatment system. Pursuant to the patent certificate, the patent is valid for 20 years from the application date, May 1999.
|
|
(b)
|
Patent acquired in Portola Medical, Inc. The amortization expenses for the years ended December 31, 2010 was $6,250. Patent technology is utilized in the production of medical equipment and is amortized over its estimated useful life of 20 years.
|
For the year ended December 31, 2010 and 2009, amortization expense amounted to $89,585 and $82,483, respectively. The expected amortization for the next five years and thereafter is as follows:
|
Patent technology in
Portola Medical, Inc.
|
Patent technology in
Shenzhen Hyper
|
Total
|
||||||||||
|
For the year ended
|
||||||||||||
|
December 31,
|
||||||||||||
|
2011
|
$ | 12,500 | $ | 85,361 | $ | 97,861 | ||||||
|
2012
|
12,500 | 85,361 | 97,861 | |||||||||
|
2013
|
12,500 | 85,361 | 97,861 | |||||||||
|
2014
|
12,500 | 85,361 | 97,861 | |||||||||
|
2015
|
12,500 | 85,361 | 97,861 | |||||||||
|
Thereafter
|
181,250 | 291,647 | 472,897 | |||||||||
|
Total
|
$ | 243,750 | $ | 718,452 | $ | 962,202 | ||||||
F-19
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 9 – ACCRUED LIABILITIES AND OTHER PAYABLES
At December 31, 2010 and 2009, accrued liabilities and other payables consisted of the following:
|
2010
|
2009
|
|||||||
|
Accrued expenses - operating
|
$ | 311,342 | $ | 365,923 | ||||
|
Accrued payroll and welfare
|
181,255 | 188,125 | ||||||
|
Value added tax, other taxes payable and surcharges
|
1,170,646 | 1,121,406 | ||||||
|
Customer deposits
|
76,983 | 1,185 | ||||||
| $ | 1,740,226 | $ | 1,676,639 | |||||
NOTE 10 – NON-CONTROLLING INTEREST
Non-controlling interest included in the Company’s balance sheets as of December 31, 2010 represents 25% of equity interest in Shenzhen Hyper.
|
2010
|
2009
|
|||||||
|
Net loss attributable to non-controlling interest
|
|
|
||||||
|
Balance at beginning of period
|
$ | 1,111,027 | $ | 1,298,604 | ||||
|
Effect for the current year
|
||||||||
|
- Net loss
|
(215,574 | ) | (186,631 | ) | ||||
|
- Foreign currency translation (gain)/loss
|
32,974 | (946 | ) | |||||
|
Balance at end of period
|
$ | 928,427 | $ | 1,111,027 | ||||
NOTE 11 – DUE TO RELATED PARTIES
A summary of related party payables at December 31, 2010 and 2009 are as follows:
Amounts due to related parties at December 31, 2010 and December 31, 2009 represents the remaining balance due to a former shareholder of Allied Moral Holdings Limited ("Allied Moral"), a subsidiary of the Company, pursuant to the 2007 share redemption as well as advances related to the acquisition of Changdu Huiheng.
In June 2010, the cash consideration of $260,000 and related expenses amounting to $30,000 for acquiring the new subsidiary, Portola Medical Inc, were paid directly by the director, Hui Xiaobing on behalf of the Company. As of December 31, 2010, such director is owed $359,620.
F-20
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 12– STOCKHOLDERS' EQUITY
The Company has authorized 74,000,000 shares of Common stock. Prior to the share exchange in 2007, Mill Basin had 10,150,000 shares of its common stock outstanding. In contemplation of the share exchange, Mill Basin shareholders contributed 9,700,000 common shares to treasury, resulting in 450,000 shares of Mill Basin's common stock outstanding immediately prior to the share exchange. As of December 31, 2010, 23,730,637 shares were issued of which 9,700,000 are deemed treasury shares contributed from Mill Basin's shareholders, and 14,030,637 shares outstanding. As of December 31, 2009, 23,635,290 shares were issued, of which 9,700,000 are deemed treasury shares contributed from Mill Basin's shareholders, and 13,935,290 shares outstanding.
The Company has authorized 1,000,000 shares of Preferred stock, with 300,000 shares designated as Series A convertible preferred stock. As of December 31, 2010, 211,390 shares were issued and outstanding with a liquidation preference of $7,927,125. As of December 31, 2009, 220,467 shares were issued and outstanding with a liquidation preference of $8,267,513.
Series A Conversion
During 2010, certain holders of the Series A Preferred stock converted a total of 9,077 shares of preferred stock into common stock at a rate of 1 share of Series A Preferred Stock to 10.5042 shares of common stock resulting in an issuance of 95,347 shares of common stock.
Statutory surplus reserve
In accordance with PRC Company Law, Changdu Huiheng is required to appropriate at least 10% of the profit to the statutory surplus reserve. Appropriation to the statutory surplus reserve by Changdu Huiheng is based on profits arrived at under PRC accounting standards for business enterprises for each year, after offsetting any prior year's losses.
Appropriation to the statutory surplus reserve must be made before distribution of dividends to owners. The appropriation is required until the statutory surplus reserve reaches 50% of the equity. This statutory surplus reserve is not distributable in the form of cash dividends.
As of December 31, 2010, the Company established and segregated its retained earnings and the amount for the Statutory Surplus Reserve of $1,676,867.
F-21
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 13 - PORTOLA MEDICAL, INC. ACQUISITION
The Company entered into an Agreement to purchase all the common stock of Portola Medical, Inc, dated May 7, 2010, from Three Arch Capital, L.P., TAC Associates, L.P., Three Arch Partners IV, L.P., and Three Arch Associates IV, L.P.
The authorized capital stock of Portola Medical, Inc consists of Common Stock, par value $0.01 per share, of which 100 shares were issued and outstanding. Under the terms of the Agreement, the Company acquired 100% of the common stock in Portola Medical, Inc at $2,600 per share and the total consideration is of $260,000.
The following table presents the allocation of the purchase price to the assets acquired and liabilities assumed, based on their fair values:
|
Property, plant, and equipment
|
$ | 31,508 | ||
|
Intangible assets
|
250,000 | |||
|
Total asset acquired
|
281,508 | |||
|
Total liabilities assumed
|
- | |||
|
Net assets acquired
|
$ | 281,508 |
The net assets acquired exceeded the purchase price by $21,508 which was recorded as a gain on business acquisition. Included in the net assets acquired, $250,000 represents the cost of acquired intangible assets, which is made up of 8 patents with 20-year useful life (Note 8).
NOTE 14 - SEGMENT REPORTING
The Group has two reportable segments: products and services.
The following table presents information about the Company's operating segments for the year ended December 31, 2010 and 2009:
|
2010
|
2009
|
|||||||
|
Revenues:
|
||||||||
|
Products
|
$ | 2,465,337 | $ | 3,456,193 | ||||
|
Services
|
6,171,236 | 5,922,386 | ||||||
| $ | 8,636,573 | $ | 9,378,579 | |||||
|
2010
|
2009
|
|||||||
|
Operating income:
|
||||||||
|
Products
|
$ | 801,603 | $ | 1,652,118 | ||||
|
Services
|
5,541,447 | 5,269,992 | ||||||
| 6,343,050 | 6,922,110 | |||||||
|
Corporate expenses
|
(3,627,276 | ) | (4,049,357 | ) | ||||
|
Operating income
|
$ | 2,715,774 | $ | 2,872,753 | ||||
F-22
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 15 – INCOME TAXES
Huiheng Medical, Inc. is a non-operating holding company. All of the Company’s income before income taxes and related tax expenses are from PRC sources. The Company's PRC subsidiaries file income tax returns under the Income Tax Law of the People's Republic of China concerning Foreign Investment Enterprises and Foreign Enterprises and local income tax laws.
Income tax expense for the years ended December 31, 2010 and 2009 amounted to $796,213 and $671,078, respectively.
As Changdu Huiheng, Huiheng’s subsidiary, is located in the western area in the PRC and is within the industry specified by relevant laws and regulations of the PRC, the tax rate applicable to Changdu Huiheng is 15% (2009: 12%).
Wuhan Kangqiao is a high-tech enterprise with operations in an economic-technological development area in the PRC. Therefore, the applicable tax rate is 25%.
Shenzhen Hyper is a high-tech manufacturing company located in the Shenzhen special economic region. Therefore, the applicable tax rate is also 15% (2009:18%). According to local tax regulation, Shenzhen Hyper is entitled to a tax-free period for the first two years, commencing from the first profit-making year and a 50% reduction in state income tax rate for the next six years.
A reconciliation of the expected income tax expense to the actual income tax expense for the year ended December 31, 2010 and 2009 are as follows:
|
2010
|
2009
|
|||||||
|
Income before income taxes
|
$ | 2,756,805 | $ | 4,124,863 | ||||
|
Expected PRC income tax expense at statutory tax rate of 25%
|
689,201 | 1,031,216 | ||||||
|
Non-deductible expenses
|
480,285 | 347,710 | ||||||
|
Income tax exempted
|
(373,273 | ) | (707,848 | ) | ||||
|
Income tax expense
|
$ | 796,213 | $ | 671,078 | ||||
The PRC tax system is subject to substantial uncertainties and has been subject to recently enacted changes. The interpretation and enforcement of which are also uncertain. The Company remains open to examination by the major jurisdictions to which the Company is subject to, in this case, the PRC tax authorities.
No deferred tax liability has been provided as the amount is deemed immaterial.
F-23
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 16 – CONCENTRATION OF CREDIT RISK
Customers' concentrations
Customers accounting for 10% or more of the Group's net revenue for the year ended December 31, 2010 and 2009 are as follows:
|
2010
|
2009
|
|||||||
|
Customer A
|
67 | % | 71 | % | ||||
|
Customer B
|
23 | % | 19 | % | ||||
Two customers accounted for 90% and two customers accounted for 90% of Revenue for the years ended December 31, 2010 and 2009, respectively. These customers also accounted for 95% and 83% of accounts receivable as of December 31, 2010 and 2009, respectively. A termination or a reduction in orders from any of these customers could have a material impact on the Company’s results of operations and financial condition.
Except as disclosed above, no other single customer accounted for 10% or more of the Group's net revenue for the years ended December 31, 2010 and 2009.
Other credit risks
As of December 31, 2010, all of the Company’s cash and cash equivalents were held by major financial institutions located in the PRC, none of which were insured or collateralized. However, management believes those financial institutions are of high credit quality and has assessed the loss arising from the non-insured cash and cash equivalents from those financial institutions to be immaterial to the consolidated financial statements. Therefore, no loss in respect of the cash and cash equivalent were recognized as of December 31, 2010.
F-24
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 17 - OPERATING LEASE COMMITMENTS
As of December 31, 2010, the total future minimum lease payments under non-cancellable operating leases in respect of premises, which were based on the closing rate as of December 31, 2010 are as follows:
|
For the year ended
|
||||
|
December 31
|
||||
|
2011
|
$ | 290,909 | ||
|
2012
|
290,909 | |||
|
2013
|
290,909 | |||
|
2014
|
290,909 | |||
|
2015
|
290,909 | |||
|
Thereafter
|
3,442,425 | |||
|
TOTAL
|
$ | 4,896,970 | ||
F-25
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 18 – CONDENSED PARENT COMPANY FINANCIAL INFORMATION
The Company records its investment in subsidiaries under the equity method of accounting as prescribed in ASC Topic 323, “Investments – Equity Method and Joint Ventures”. Such investment and long-term loans to subsidiaries are presented on the balance sheet as “Investments in subsidiaries” and the income of the subsidiaries is presented as “Equity in income of subsidiaries” on the statement of income.
These supplemental condensed parent company financial statements should be read in conjunction with the notes to the Company’s Consolidated Financial Statements. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted.
As of December 31, 2009 and 2010, there were no material contingencies, significant provisions for long-term obligations, or guarantees of the Company, except as separately disclosed in the Company’s Consolidated Financial Statements, if any.
|
CONDENSED BALANCE SHEETS
|
As of December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Investments in subsidiaries
|
$ | 27,145,551 | $ | 24,275,259 | ||||
|
Total Assets
|
$ | 27,145,551 | $ | 24,275,259 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Other payable
|
$ | 96,000 | $ | 96,000 | ||||
|
Total Current Liabilities
|
96,000 | 96,000 | ||||||
|
STOCKHOLDERS' EQUITY:
|
||||||||
|
Preferred stock, $0.001 par value; 1,000,000 shares authorized;
|
||||||||
|
Designated 300,000 shares of Series A convertible preferred
stock; 211,390 shares issued and outstanding with liquidation
|
||||||||
|
preference of $7,927,125 at December 31, 2010; and 220,467
|
||||||||
|
shares issued and outstanding with liquidation preference
|
||||||||
|
of $8,267,513 at December 31, 2009
|
211 | 220 | ||||||
|
Common stock, $0.001 par value; 74,000,000 shares authorized;
|
||||||||
|
23,730,637 shares issued and 14,030,637 shares outstanding
|
||||||||
|
at December 31, 2010; and 23,635,290 shares issued and
|
||||||||
|
13,935,290 shares outstanding at December 31, 2009
|
23,731 | 23,635 | ||||||
|
Treasury stock, 9,700,000 common shares, at cost
|
(9,700 | ) | (9,700 | ) | ||||
|
Additional paid-in capital
|
7,498,086 | 7,498,086 | ||||||
|
Statutory surplus reserve
|
1,676,867 | 1,310,516 | ||||||
|
Retained earnings
|
14,298,693 | 12,488,965 | ||||||
|
Accumulated other comprehensive income
|
||||||||
|
Foreign currency translation gain
|
2,633,236 | 1,756,510 | ||||||
|
Total Huiheng's stockholders' equity
|
26,121,124 | 23,068,232 | ||||||
|
Non-controlling interests
|
928,427 | 1,111,027 | ||||||
|
Total Stockholders' Equity
|
27,049,551 | 24,179,259 | ||||||
|
Total Liabilities and Stockholders' Equity
|
$ | 27,145,551 | $ | 24,275,259 | ||||
F-26
HUIHENG MEDICAL, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(IN US DOLLARS)
NOTE 18 – CONDENSED PARENT COMPANY FINANCIAL INFORMATION (…/Con’d)
|
CONDENSED STATEMENT OF INCOME
|
Year ended December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
General and administrative expenses
|
$ | (530,488 | ) | $ | (291,039 | ) | ||
|
Equity in income of subsidiaries
|
2,706,654 | 3,931,455 | ||||||
|
Net income
|
$ | 2,176,166 | $ | 3,640,416 | ||||
|
CONDENSED STATEMENT OF CASH FLOWS
|
Year ended December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Net cash used in operating activities
|
$ | - | $ | - | ||||
|
Net cash used in investing activities
|
- | - | ||||||
|
Net cash provided by financing activities
|
- | - | ||||||
|
Cash, beginning of year
|
- | - | ||||||
|
Cash, end of year
|
$ | - | $ | - | ||||
F-27
