Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AUXILIUM PHARMACEUTICALS INC | d8k.htm |
 1
April 2011
(NASDAQ: AUXL)
Exhibit 99.1 |
 2
Safe Harbor Statement
We will make various remarks during this presentation that constitute “forward-looking
statements” for purposes of the safe harbor provisions under The Private Securities
Litigation Reform Act of 1995, including statements regarding: the pricing, time to market, size of market, growth potential and therapeutic benefits of the
Company’s products and product candidates, including those for the treatment of Peyronie’s
disease and Frozen Shoulder syndrome; the size and development of the Dupuytren’s market
in the U.S., the EU and the rest of the world; the potential for XIAFLEX to become the standard of care for Dupuytren’s contracture; the
potential for XIAFLEX to be used in multiple indications and the success of efforts to advance any
such new indications; the effect of the identified leading indicators on the success of the
XIAFLEX launch and future net revenues; the ability to obtain reimbursement in the U.S. for XIAFLEX for the treatment of
Dupuytren’s; the timing and potential effect on sales of new reimbursement codes for XIAFLEX and
the effect of the reimbursement process on the success of the XIAFLEX launch; physicians and
sites that are moving from test drive to increasing usage; the generation of cash through licensing of XIAFLEX in other territories
and for new indications; the timing and likelihood of success of launch of XIAPEX for the treatment of
Dupuytren’s contracture in the European Union and of the development of XIAFLEX for the
treatment of Dupuytren’s contracture and Peyronie’s disease in Japan; additional Phase IV clinical trials for XIAFLEX; peer to
peer dialogue programs for U.S. physicians and our U.S. patient education campaign; future Testim
market share, prescriptions and sales growth and factors that may drive such growth; size and
growth potential of the testosterone replacement therapy market and the gel segment thereof and factors that may drive such
growth; the likelihood of generic competition in the testosterone replacement therapy gel market;
competitive developments affecting the Company’s products and product candidates,
including generic competition in the testosterone replacement therapy market; the scope, timing, methodology, endpoints, safety, execution
and results of the phase III studies for XIAFLEX for the treatment of Peyronie’s disease; the
size of the Peyronie’s market and any increase in treatment for Peyronie’s; business
development efforts and opportunities to build out the Company’s pipeline; the Company’s development and operational goals and strategic
priorities for 2011, including building out its pipeline in specialty therapeutic areas; the ability
to fund future operations; the opportunities and strategies to build shareholder value; the
status, timing, potential counterclaims and appeals, potential outcomes, resulting consequence and costs of, and effects on our business
from, the litigation with BioSpecifics Technologies Corp.; the Company’s expected financial
performance during 2011 and financial milestones that it may achieve for 2011, including 2011
XIAFLEX U.S. net revenues and Pfizer royalty and contract revenues; and the likelihood or timing of the Company becoming profitable
rapidly growing, profitable and sustainable biopharmaceutical company. All remarks other than
statements of historical facts made during this presentation, including but not limited to,
statements regarding future expectations, plans and prospects for the Company, statements regarding forward-looking financial
information and other statements containing the words “believe,” “may,”
“could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “expect,” and
similar expressions, as they relate to the Company, constitute forward-looking statements.
Actual results may differ materially from those reflected in these forward-looking
statements due to various factors, including general financial, economic, regulatory and political conditions affecting the biotechnology and
pharmaceutical industries and those discussed in Auxilium's Annual Report on Form 10-K for the
year ended December 31, 2010 under the heading “Risk Factors, which is on file with the
Securities and Exchange Commission (the “SEC”) and may be accessed electronically by means of the SEC’s home page on the Internet
at http://www.sec.gov or by means of the Company’s home page on the Internet at
http://www.auxilium.com under the heading “For Investors - SEC Filings.”
There may be additional risks that the Company does not presently know or that the Company currently
believes are immaterial which could also cause actual results to differ from those contained in
the forward-looking statements. Given these risks and uncertainties, any or all of these forward-looking statements may
prove to be incorrect. Therefore, you should not rely on any such factors or forward-looking
statements. In addition, forward-looking statements provide the Company’s
expectations, plans or forecasts of future events and views as of the date of this presentation. The Company anticipates that subsequent events and
developments will cause the Company’s assessments to change. However, while the Company may
elect to update these forward-looking statements at some point in the future, the Company
specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the
Company’s assessments as of any date subsequent to the date of this presentation. |
 3
Our Vision Is to Become a Rapidly Growing,
Profitable and Sustainable Biopharmaceutical
Company
Maximize Value of
Testim and XIAFLEX
Deliver on
Current Pipeline
Build Out
Pipeline in
Specialty
Therapeutic
Areas |
 4
XIAFLEX and Testim Are Global Product
Opportunities
U.S.
Canada
EU
Japan
Open
ROW
Open
Open
Territory
XIAFLEX
Testim |
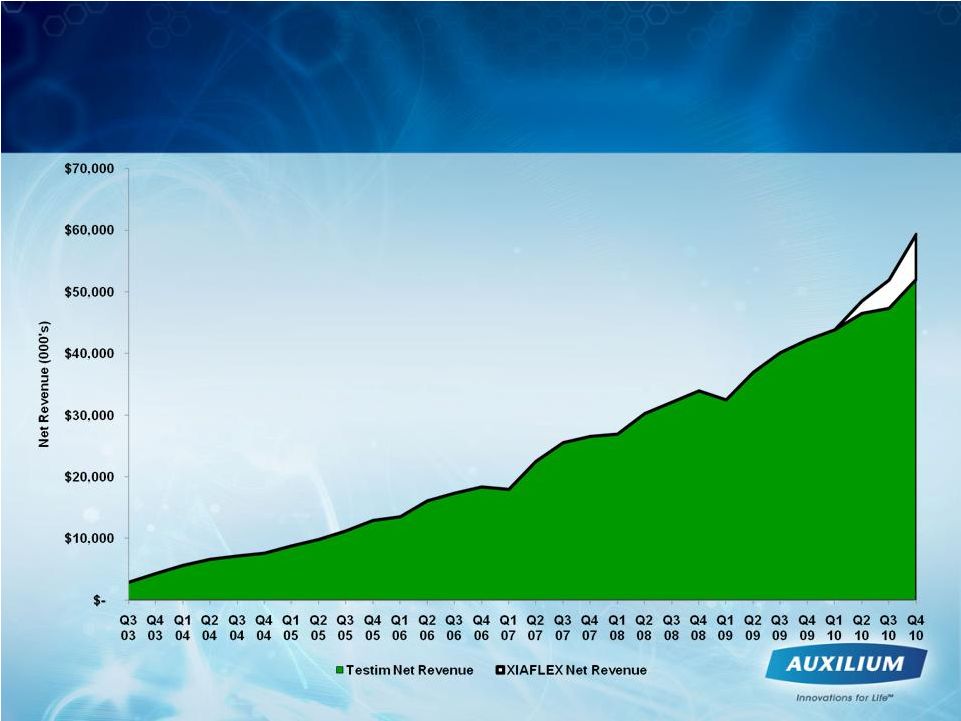 5
Auxilium Has a 6 Year Net Revenue CAGR
of 40.2%
4Q10 XIAFLEX Net
Revenues $8.4M
(including $7.3M in U.S.
revenues)
4Q10 Testim Net
Revenues $53.4M,
13.3% y/y growth |
 6 |
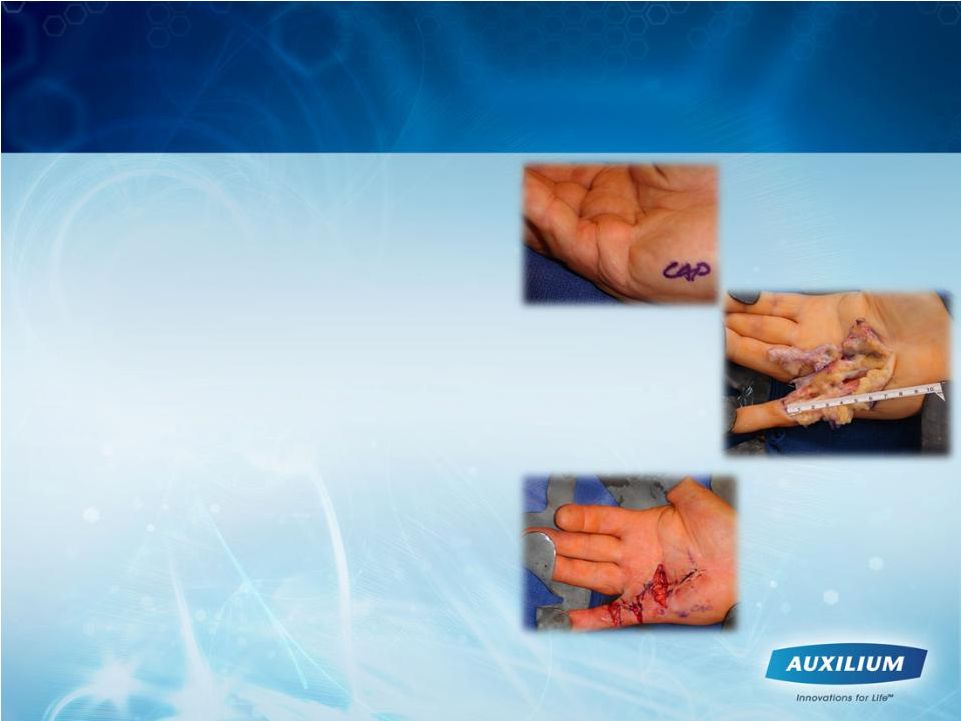 7
•
Excessive collagen in fascia of hand
•
Nodules or pits are an early, active
presentation
•
Rope-like cords develop in the finger
and result in contractures
•
Quality of life and daily activities
affected
•
Surgery has been reserved for
advanced disease due to the nature of
the disease, unpredictable results,
complications, long recovery time and
recurrence/additional surgeries
Dupuytren’s Contracture Is Debilitating for Patients
and Surgery Has Been the Standard of Care
Immediately post-operative
Intra-operative open fasciectomy
Pictures courtesy
of Dr. Clayton Peimer
pre-operative open fasciectomy |
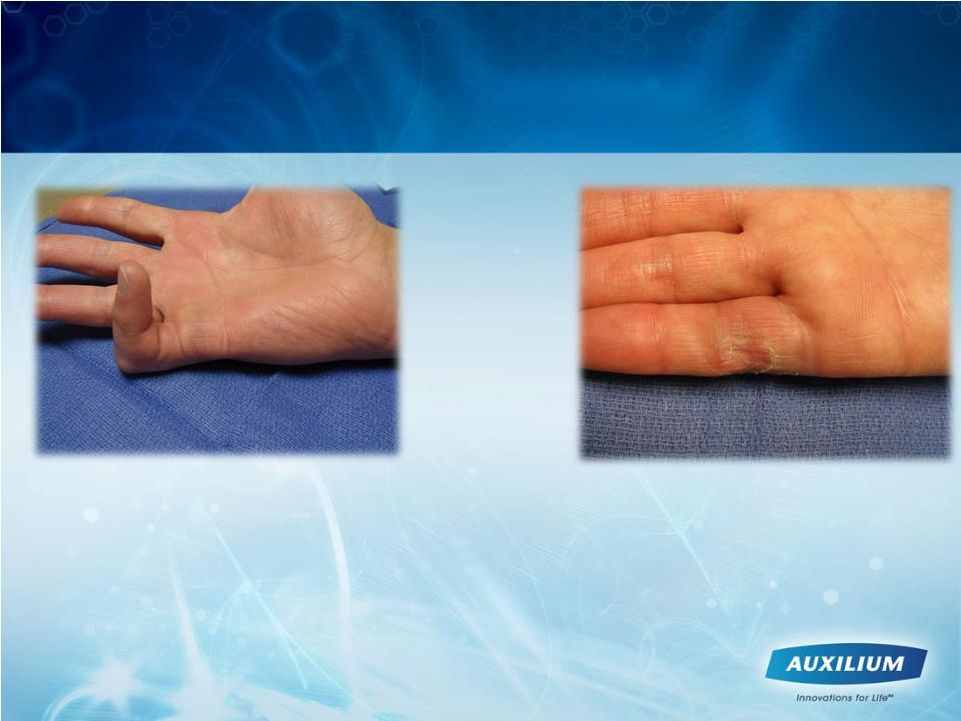 8
We Believe XIAFLEX Is a Paradigm
Changing Treatment
•
Simple, non-invasive injection of XIAFLEX
•
Established mechanism of action and selective for specific types of collagen
•
XIAFLEX’s post-approval profile is consistent with clinical trial
experience Pre XIAFLEX injection
30 days following XIAFLEX
injection |
 9
Strategies for Establishing XIAFLEX as the
Standard of Care
•
Disseminate excellent clinical efficacy and safety data
•
Facilitate peer to peer dialogue
•
Educate patients and health care providers on a non-
invasive option
•
Smooth the reimbursement process
•
Activate a critical mass of experienced prescribers |
 10
XIAFLEX Launch Strategy is Tailored to
Customer Needs
•
~ 6,000 target physicians
–
Hand surgeons, plastic surgeons, orthopedic
surgeons, general surgeons, rheumatologists
•
~ 100 field based personnel
–
Sales reps, sales managers, and reimbursement
specialists
•
Medical science liaisons
–
Key opinion leader and regional opinion leader
support |
 11
We Believe the U.S. Dupuytren’s Opportunity Is
Significant
70,000 Surgical
2,3,4
Patients Annually
300,000 Diagnosed
Patients Annually
2,3,4
>1 Million Diagnosed
Patients
2,4
1.
Dupuytren’s
Disease
–
Tubiana,
LeClerq,
Hurst,
Badalamente,
Mackin
2.
SDI Claims Data Based Projections
3.
Medicare
Data
Based
Projections
(BESS
database
used,
Medicare
5%
database
also
used
to
validate
numbers)
4.
Auxilium
Research (Patient
Segmentation,
Forecast
Research,
WK/AMA
Databases)
Sources:
$350 Million
Market
Opportunity
$1 Billion
Annual Market
Opportunity
>$3 Billion Market
Opportunity |
 12
Launch Update Through
March 2011 |
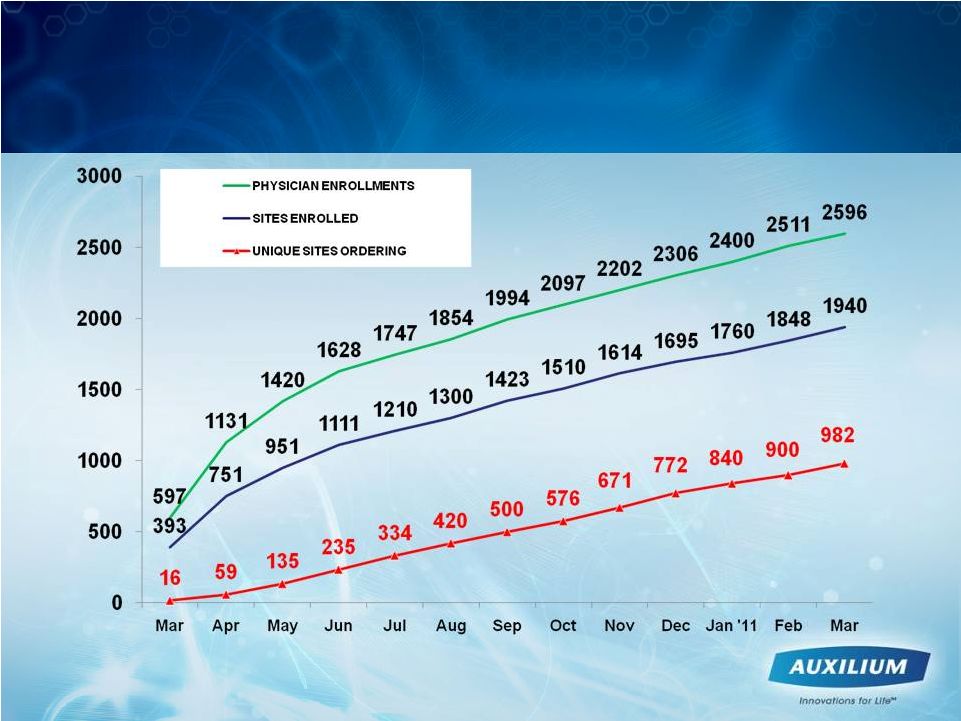 13
Leading Indicators Trending Positively
-
Enrollment & sites that have ordered XIAFLEX |
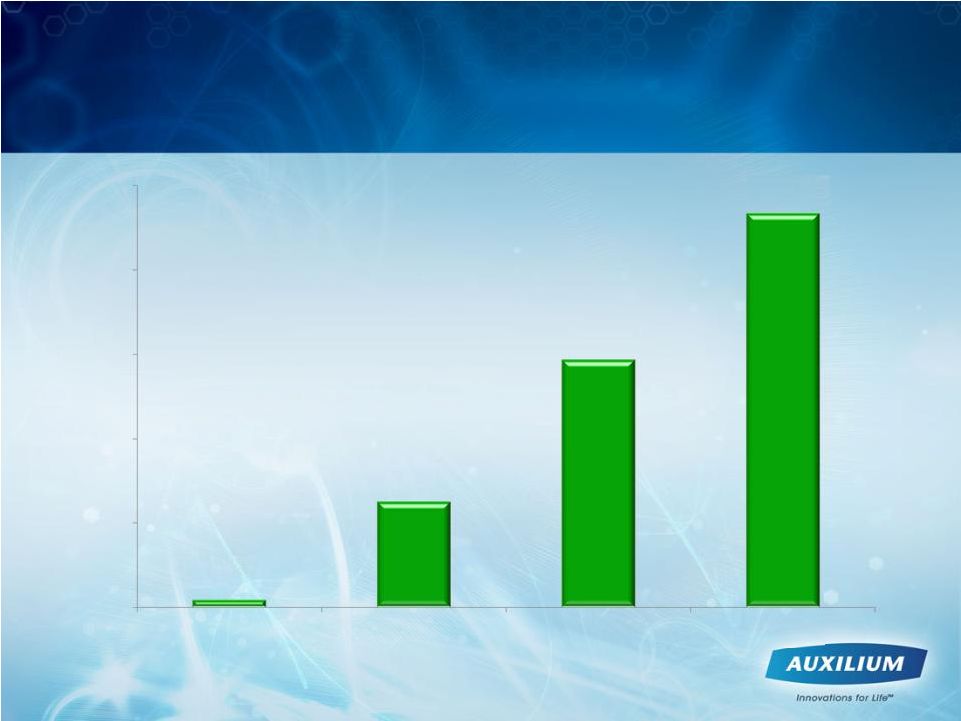 14
Quarterly XIAFLEX Unit Sales Growth
Increased Steadily in 2010
1Q10
2Q10
3Q10
4Q10
53
632
1464
2356
0
500
1000
1500
2000
2500 |
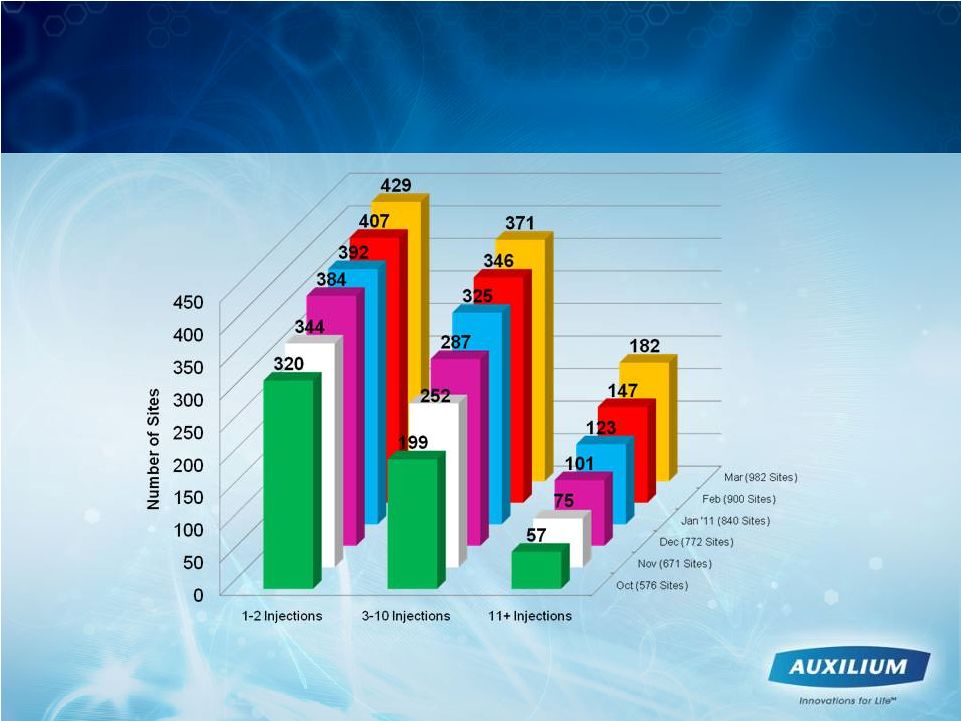 15
Some Sites Moving from Test Drive to
Increasing Usage
Injections per Site |
 16
We Expect the Global Dupuytren’s Market to
Develop in 2011 and 2012
U.S.
•
XIAFLEX specific J-code J0775 effective Jan. 1
•
U.S. CPT code guidance expected for Jan. 2012
•
Additional phase IV clinical trials
•
Increasing peer to peer dialogue programs for U.S.
physicians
•
Targeted U.S. patient education campaign planned
ROW
•
Pfizer
launch
of
XIAPEX
®
in
EU
begins
in
2011
•
Asahi Kasei development of XIAFLEX in Japan |
 Testim
®
1% Testosterone Gel
17
•
Indicated for testosterone replacement therapy in adult
males for hypogonadism
•
Launched in U.S. in 2003 and EU in 2006
•
>7 yrs of use with established safety and efficacy
•
Applied once daily at 5-10mg
>
~100M daily doses since launch
>
16 clinical studies involving approx. 1,800 patients
>
90% stay on starting dose of 5mg (one tube)
>
Simple application process |
 18
Hypogonadism Is an Unmet Need and
Under-penetrated Market
•
39% of U.S. males over 45 yrs are
hypogonadal
1
>Less than 10% of affected population
receives treatment
•
Increasing physician and patient education
and awareness should result in increased
treatment
1
Mulligan
T.
et
al.
Int
J.
Clin
Pract
2006 |
 19
Gels Continue to Drive Significant Growth in
TRT Marketplace
Source: IMS data
$35
$117
$198
$281
$334
$371
$439
$545
$685
$892
$1,149
$49
$59
$77
$118
$210
$302
$390
$451
$483
$554
$663
$810
$1,041
0
200
400
600
800
1,000
1,200
1,400
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
($ in millions)
Gel Segment Growth ($)
Feb
2011 L12M:
28.8%
$1,329
Gel
Patch
Oral
Injectables |
 20
Testosterone Replacement Therapy Landscape
is Changing
•
New competition in 2011 should help
increase overall market
–
Axiron 2% solution
–
Fortesta 2% gel
–
Androgel 1.6% gel seeking approval
•
Expected further increase in physician and
patient education and awareness |
 21
Delivering on Current Pipeline
PRODUCT
LATE RESEARCH
PRE-CLINICAL PHASE I PHASE II PHASE
III MARKET
TESTIM®
GEL
XIAFLEX®
XIAFLEX®
XIAFLEX®
Hypogonadism
Dupuytren’s contracture
Peyronie’s disease
Frozen Shoulder Syndrome |
 22
1
Smith BH. Am J Clin Pathol. 1966;45:670-678.
2
Somers KD, Dawson DM. J Urol. 1997;157:311-315.
•
Scarring phenomenon affecting the
tunica albuginea
1
•
Plaques show excessive collagen deposition
2
•
Potential Symptoms
>
Pain with erection, penile curvature/
deviation, penile shortening, indentations,
and/or erectile dysfunction
>
May experience difficulty with sexual
intercourse, loss of self-esteem,
and depression
Peyronie’s disease Is a Devastating Disorder
with No Approved Therapies |
 23
1
Bella A. Peyronie’s Disease J Sex Med 2007;4:1527–1538
2
Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med
2004;1:6–23. 3
Mulhall JP, et al. Subjective and objective analysis of the prevalence of Peyronie’s disease in a
population of men presenting for prostate cancer screening. J Urol 2004;171:2350–3.
4
Smith BH. Am J Clin Pathol. 1966;45:670-678.
5
Lindsay MB, J Urol.
1991;146:1007-1009.
6
Nyberg L, J Urol.128: 48, 1982
•
Prevalence of Peyronie’s disease is estimated to be approximately
5% in adult men
1,2,3
>
Actual prevalence may be higher, based on autopsies
4
•
Prevalence rate increases with age
>
The
average
age
of
disease
onset
is
53
years
5
•
High association with other diseases such as:
>
Diabetes, erectile dysfunction (ED), Dupuytren’s contracture,
plantar fascial contracture, tympanosclerosis, gout, and Paget’s
disease
6
We believe Peyronie’s Disease Is Under-
diagnosed and Under-treated |
 24
Peyronie’s Disease Phase III Development
Program
Study
Type
~ #
Subjects
Sites
XIAFLEX:
Placebo
Duration
AUX-CC-803
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-804
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-802
Open Label
250
12 US
6 NZ
18 EU
N/A
36 Wks
AUX-CC-805
Pharmacokinetics
16
1 US
N/A
4 Wks
XIAFLEX 0.58 mg
Two injections per treatment cycle
24 to 72 hours between injections
Penile plaque modeling following each cycle
Up to 4 cycles at 6 week intervals
Co-primary endpoints of disease bother and penile curvature
|
 25
We Believe Changes to the Phase IIb Trial Design
Should Increase the Chance of Success in Phase III
•
4 cycles of therapy in the phase III trial
•
All patients receive modeling in the phase III trial
•
All patients must have greater than 12 months of
disease since diagnosis to enter the phase III trial
Mean net improvements in phase IIb penile
curvature and bother domain for modeling
arm would have met statistical significance
using phase III co-primary endpoint
requirements |
 26
Business Development Efforts Expected to
Focus on Building Out Pipeline
•
Leverage current infrastructure of Testim and
XIAFLEX field forces with marketed products
•
Seeking Phase II and later assets in Urology,
Endocrinology, Rheumatology, and Orthopedics
•
Niche products with specialty physician call
points also represent development opportunities |
 27
BTC Litigation Update
•
On Feb. 15, 2011, AUXL filed a complaint against BioSpecifics Technologies
Corp.
(BTC)
alleging
that
BTC
has
breached
the
parties’
Amended
and
Restated
Development and License Agreement dated as of December 11, 2008 by its
commencement of clinical trials for the use of injectable collagenase to treat
canine
lipomas
without
the
prior
knowledge
and
approval
of
the
parties’
Joint
Development Committee (JDC).
•
AUXL is seeking preliminary and permanent injunctions ordering BTC to, among
other things:
•
suspend its canine lipoma clinical trial known as Chien-803 and
•
refrain from initiating any new clinical trials related to collagenase, both until
such time as
any
such
trial
has
been
reviewed
and
approved
by
the
parties’
JDC
pursuant
to
the
terms of the Agreement.
•
AUXL also seeking a declaratory judgment as to the rights and responsibilities of
the JDC under the Agreement
•
Suit filed in the Court of Common Pleas, Chester County, Pennsylvania
•
Court ordered that papers filed with the court were to be deemed
under seal and
not available to the public until further order |
 28
2010 Financial Results ($ Millions)
Incr. (Decr.)
2010
2009
$
%
Total Revenues
$211.4
$164.0
$47.4
29%
Cost of Goods Sold *
$49.7
$37.1
$12.6
34%
Gross Profit
$161.7
$127.0
$34.7
27%
% of Revenues
76%
77%
R & D Expense *
$48.0
$51.4
($3.4)
-7%
S G & A Expense *
$164.7
$129.2
$35.5
27%
Net Loss
($51.2)
($53.5)
$2.2
4%
Earnings per share
($1.08)
($1.22)
$0.14
12%
* Stock Based Comp Expense
$17.81
$17.90
($0.1)
-1%
Cash & Cash
Equivalents
$128.20 |
 29
2011 Guidance
Global Testim Revenues
$200 -
210 million
Xiaflex:
US Revenues
50 -
60 million
Ex US / Def Rev
5 -
7 million
Total Xiaflex
$55 -
67 million
Total Revenues
$255 -277 million
R & D Expense
$60 -
70 million
S G & A Expense
$170 -
180 million
Net Income (Loss)
$(35) -
(45) million
Stock Base Comp Expense
$15 -
20 million |
 30
We Believe That Auxilium is Well-Positioned
for Long-Term Growth
•
Maximize XIAFLEX net revenues for Dupuytren’s
contracture in the U.S.;
•
Maximize global Testim revenues.
•
Support Pfizer in the launch of Xiapex for Dupuytren’s
contracture in the EU;
•
Complete Peyronie’s disease phase III double-blind
studies, with top-line results anticipated in 1H12; and,
•
Advance XIAFLEX new indication(s). |
 31
Our Vision Is to Become a Rapidly Growing,
Profitable and Sustainable Biopharmaceutical
Company
Maximize Value of
Testim and XIAFLEX
Deliver on
Current Pipeline
Build Out
Pipeline in
Specialty
Therapeutic
Areas |
