Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - INFINITY PHARMACEUTICALS, INC. | d8k.htm |
 Building a Sustainable, Fully Integrated
Biotechnology Company
April 2011
Exhibit 99.1 |
 Forward Looking Statements
•
This presentation contains forward-looking statements within the meaning of The
Private Securities Litigation Reform Act of 1995. •
These statements involve risks and uncertainties that could cause actual results to
be materially different from historical results or from any future results
expressed or implied by such forward-looking statements. •
Such
forward-looking
statements
include
statements
regarding:
the
therapeutic
potential
of
Infinity’s
Hedgehog
pathway,
FAAH,
PI3K
and
Hsp90
chaperone inhibitors; the potential of IPI-926 in addressing chondrosarcoma,
pancreatic cancer and other cancers, presentation of rationale for
development of IPI-926 in chondrosarcoma, clinical trial enrollment
expectations, an investigator sponsored trial program with IPI-926,
presentation of data from Infinity’s trials of IPI-926 pancreatic cancer
and chondrosarcoma and of IPI-504 in combination with docetaxel, plans for
additional Phase 2 trials of IPI-926, announcement of data and future plans for
the company’s Hsp90 program, the commencement of Phase 1
development of IPI-145, Phase 2 development of IPI-940 by Purdue;
completing transition activities to enable Phase 2 development by Purdue;
the naming of a new drug development candidate, estimates of 2011 financial
performance (including cash burn and year-end cash and investments
balance), and the expectation that Infinity will have capital to support its current operating plan into 2014.
•
Such forward-looking statements are subject to numerous factors, risks and
uncertainties that may cause actual events or results to differ
materially
from
the
company's
current
expectations.
For
example,
there
can
be
no
guarantee
that
Infinity’s
strategic
alliance with
Purdue/Mundipharma will continue for its expected term or that these entities will
fund Infinity’s programs as agreed, or that any product candidate
Infinity is developing will successfully complete necessary preclinical and
clinical development phases. Further, there can be no guarantee that any
positive developments in Infinity’s product portfolio will result in stock price appreciation. Infinity’s expectations could also be affected by risks
and uncertainties relating to: results of clinical trials and preclinical studies,
including subsequent analysis of existing data and new data received from
ongoing and future studies; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory
authorities, investigational review boards at clinical trial sites, and publication
review bodies; Infinity's ability to enroll patients in its clinical trials;
unplanned
cash
requirements
and
expenditures,
including
in
connection
with
business
development
activities;
market
acceptance
of
any
products
Infinity or its partners may successfully develop; and, Infinity's ability to
obtain, maintain and enforce patent and other intellectual property
protection for any product candidate it is developing.
•
These
and
other
risks
which
may
impact
management's
expectations
are
described
in
greater
detail
under
the
caption
"Risk
Factors"
included
in
Infinity's annual report on Form 10-K filed with the U.S. Securities and
Exchange Commission on March 16, 2011. •
Further, any forward-looking statements contained in this presentation speak
only as of the date hereof, and Infinity expressly disclaims any obligation
to update any forward-looking statements, whether as a result of new information, future events or otherwise.
•
All trademarks used in this presentation are the property of their respective
owners. •
Our Internet website is http://www.infi.com. We regularly use our website to post
information regarding our business, product development programs
and
governance.
We
encourage
investors
to
use
www.infi.com,
particularly
the
information
in
the
section
entitled
“Investors/Media,”
as
a source of information about Infinity. References to www.infi.com in this
presentation are not intended to, nor shall they be deemed to, incorporate
information on www.infi.com into this presentation by reference. 2
|
 Building a Sustainable, Fully Integrated
Biotechnology Company
3
EXPERIENCED TEAM
•
Seasoned cross-functional leadership
•
Augmented in 2010 with key hires in clinical, medical
affairs and commercial
FINANCIAL STRENGTH
•
$345M in current and committed capital
•
Strong strategic alliances
•
Infinity retains U.S. rights for oncology/inflammation
INNOVATIVE PRODUCT PORTFOLIO
•
Diverse and growing, with 4 candidates in the clinic
•
Broad commercial potential in oncology/inflammation |
 Advancing Pipeline with Broad
Commercial Potential
4 |
 Hedgehog Program
A Fundamentally New Approach to
Treating a Broad Range of Cancers
5 |
 6
IPI-926: Significant Anti-Cancer Opportunity by Inhibiting
Malignant Activation of the Hedgehog Pathway
Targeting the Tumor Cell
(ligand independent)
Targeting the Tumor Cell
(ligand dependent)
Targeting the Microenvironment
Status:
Phase 2 trial ongoing in
pancreatic cancer
Next milestone:
Presentation of Phase 1b
data at ASCO 2011
Status:
On-target activity in
Phase 1 trial in solid tumors
Next milestone:
Follow-up Phase 1 data in
basal cell carcinoma at
ASCO 2011
Status:
Phase 2 trial ongoing in
chondrosarcoma
Next milestone:
Additional Phase 2 trials
planned for in 2011 |
 Targeting the Tumor Cell:
Phase 1 Objectives Achieved
Well tolerated
–
Majority of related adverse events were Grade 1 or 2
–
Primary related adverse events: Grade 1 and 2 fatigue and nausea
–
No Grade 4 or 5 related adverse events observed
Pharmacokinetic profile supports once daily dosing
Evidence of on target clinical activity observed in BCC patients
–
4 of 17 BCC patients who were naïve to treatment with a Hedgehog inhibitor
showed a clinical partial response
7 |
 Evidence of Clinical Activity in BCC Patients
8
Patient A
Baseline
6 Months
Rudin et al. ESMO 2010 |
 Targeting the Tumor:
Major Unmet Medical Need in Chondrosarcoma
•
Rare, life-threatening bone cancer with limited treatment options
–
Highly resistant to chemotherapy and radiotherapy
–
Therapeutic standard is surgery
–
No effective treatments and no established standard of care for
patients with metastatic or locally advanced, inoperable disease
•
Strong rationale for IPI-926 in chondrosarcoma
•
Granted orphan drug designation by FDA in this indication
9 |
 Preclinical Data
Supports Development of IPI-926 in Chondrosarcoma
In xenografts derived from human primary chondrosarcoma tumors,
administration of IPI-926 led to:
•
Down-regulation of the Hedgehog pathway in tumor cells
•
Tumor growth inhibition
•
Calcification and loss of cellularity
10
IPI-926
Control
Read AACR 2011 |
 2:1
Randomization
Rigorous Phase 2 Trial of IPI-926 in
Chondrosarcoma Underway
•
Randomized, double blind, placebo controlled study in patients with
metastatic or locally advanced, inoperable chondrosarcoma
•
Primary endpoint is progression free survival
•
Secondary endpoints include time to progression, overall survival,
overall response rate and response duration
~100
Patients
IPI-926 (QD)
Placebo
Progression -
crossover to IPI-926 |
 Targeting the Tumor
Microenvironment: Pancreatic Cancer
•
Devastating disease
–
4
th
leading cause of cancer death in the U.S.
–
Estimated 43,000 new cases annually
•
Lowest survival rate of all major cancers
–
Average survival is < 6 months
–
5-year survival < 5%
•
Highly resistant to treatment with many drugs
•
Historically considered an “undruggable”
tumor
–
Gemcitabine approved with median survival of 5.7 months
12 |
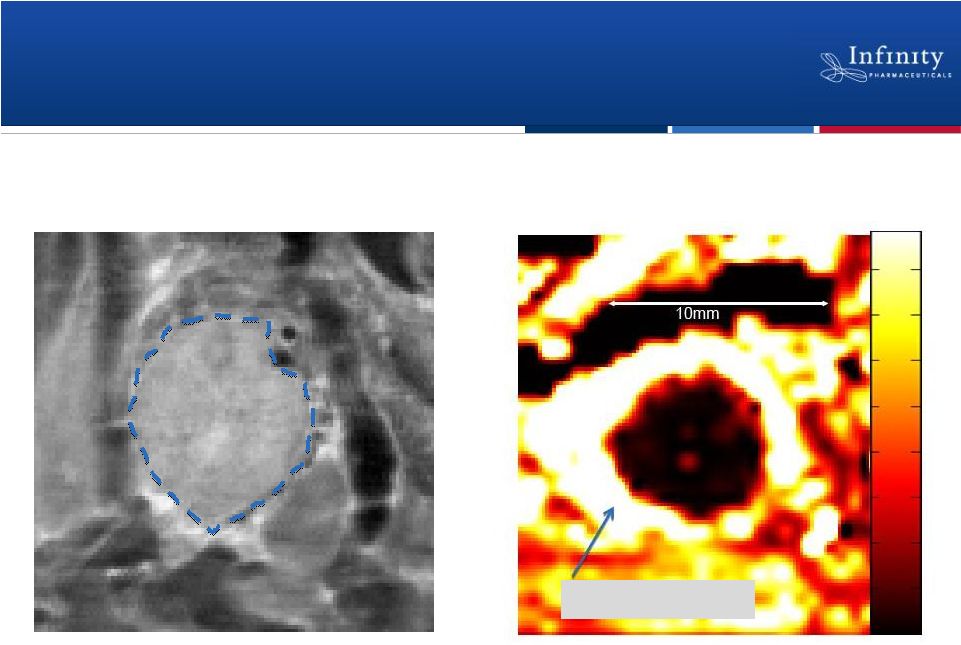   Tumor
with Circulating Contrast Agent
MRI Tumor Image
Dense Pancreatic Tumor Microenvironment
Limits Perfusion
13
Tumor
Contrast Agent
MRI images of transgenic mouse model of pancreatic cancer.
Tumor |
 IPI-926 Enhances Delivery of Gemcitabine
to Tumor
14
Vehicle
Gemcitabine alone
IPI-926 +
gemcitabine
Current standard of care
in pancreatic cancer
Tumor cell nuclei
Fluorescent contrast agent |
 Strong Preclinical Rationale for IPI-926
in Pancreatic Cancer
15
Controls
IPI-926 + Gemzar®
Days
IPI-926 + gemcitabine doubles median survival
in pancreatic cancer model
0
10
20
30
40
50
60
70
80
90
0
20
40
60
80
100 |
 Phase 2 Trial of IPI-926 in Pancreatic
Cancer Ongoing
16
•
Phase 1b: Determine safety profile and MTD
•
Phase 2: Evaluate safety and efficacy
–
Primary endpoint is overall survival; secondary endpoints include
progression free survival, time to progression, overall response
rate
–
Rigorous design to mitigate Phase 3 risk
Dose
Escalation
MTD
1:1
Randomization
IPI-926 (QD) + gemcitabine
(n = 60)
Placebo + gemcitabine
(n = 60)
Phase 1b
Phase 2 |
 IPI-926: Summary
•
Strong preclinical rationale for all three settings of malignant
pathway activation
•
On-target activity demonstrated in basal cell carcinoma
–
Follow-on data to be presented at ASCO 2011
•
Phase 2 trial in chondrosarcoma advancing
–
Preclinical rationale presented at AACR
•
Phase 2 trial in pancreatic cancer advancing
–
Phase 1b data to be presented at ASCO 2011
•
Additional Phase 2 studies planned for 2011
17 |
 Additional Pipeline Opportunities
18 |
 Hsp90 Program
Overview •
IPI-504 well-tolerated in multiple
studies
at
biologically
active
doses
–
Phase 2 mBC with Herceptin
®
–
Phase 2 study NSCLC
–
Phase 1b solid tumor with
docetaxel
•
Two NSCLC studies ongoing
–
Phase 1b study with docetaxel
(data at ASCO 2011)
–
Validation in NSCLC patients with
ALK rearrangements (IST)
•
IPI-493 in two Phase 1 dose
escalation studies
19
Sequist et al., Journal of Clinical Oncology, 2010. |
 IPI-145:
Selectively Inhibiting PI3K Delta and Gamma
20
•
Oral, dual-specific inhibitor of PI3K
delta and gamma
–
PI3K plays a key role in cell proliferation
and survival, differentiation, trafficking
and immunity
–
Delta and gamma isoforms are strongly
implicated in broad range of inflammatory
conditions and hematologic cancers
•
IPI-145 has demonstrated activity in
several preclinical models of
inflammation
•
Phase 1 development expected
to begin in H2’11 |
 •
Novel, oral agent designed to potentiate
the body’s natural pain relief
•
Encouraging preliminary data from
Phase 1 trial
–
Marked FAAH inhibition and increased
anandamide levels
–
No observed dose-limiting toxicities
•
Purdue and Mundipharma exercised rights for
worldwide development and commercialization
–
Expected to begin Phase 2 development in pain in 2011
IPI-940: Leveraging Scientific Insights
Beyond Oncology
21 |
 Strategic Alliances Provide Funding and
Access to Global Markets
22
Hedgehog, PI3K and early discovery
•
R&D funding from Mundipharma
•
INFI to develop and register product candidates globally
•
INFI to commercialize products in the U.S.
•
Mundipharma to commercialize products ex-U.S.
FAAH
•
Transferred to Purdue and Mundipharma following
successful Phase 1 in 2010
•
Purdue and Mundipharma responsible for
development and global commercialization |
 Financial Strength to Drive Value Creation
23
Current and Committed Capital
1
Line of credit may be drawn for any corporate purpose.
$195 M
Committed R&D Funding
for 2011-12
$50 M
Line of Credit
(Balloon note at prime, matures 2019)
Cash and Investments
(as of 12/31/10)
$345 Million
1 |
 2011 Financial Guidance
Cash Runway into 2014
•
Projected 2011 cash burn of $30M -
$40M
•
Anticipate year-end cash and investments balance of
$60M -
$70M
–
Based on current operating plan; excludes $50M line of credit from Purdue
•
Approximately 26.5 million shares outstanding
24 |
 Pipeline Advancements in 2011
Hedgehog (IPI-926)
Begin Phase 2 portion of pancreatic cancer study
Present Phase 1b data in pancreatic cancer
Begin additional Phase 2 studies
Initiate investigator sponsored trial program
Hsp90 (IPI-504 and IPI-493)
Present Phase 1 data of IPI-504 in combination with docetaxel
Announce future plans for Hsp90 program
PI3K (IPI-145)
Begin Phase 1 study in H2
FAAH (IPI-940)
Complete transition activities to Purdue to enable Phase 2 studies in pain
Discovery
Name a new development candidate
25 |
 Achieving Our Mission:
Sustainable, Fully Integrated Biotech
EXPERIENCED TEAM
FINANCIAL STRENGTH
INNOVATIVE PRODUCT PORTFOLIO |
 Building a Sustainable, Fully Integrated
Biotechnology Company
April 2011 |
