Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - TearLab Corp | ex32_1.htm |
| EX-31.1 - EXHIBIT 31.1 - TearLab Corp | ex31_1.htm |
| EX-31.2 - EXHIBIT 31.2 - TearLab Corp | ex31_2.htm |
| EX-32.2 - EXHIBIT 32.2 - TearLab Corp | ex32_2.htm |
| EX-23.1 - EXHIBIT 23.1 - TearLab Corp | ex23_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2010
Or
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from _________________ to _______________________
Commission file number: 000-51030
TearLab Corp.
(Exact name of registrant as specified in its charter)
| Delaware | 59-3434771 |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
| 7360 Carroll Rd., Suite 200 | |
| San Diego, California | 92121 |
| (Address of principal executive offices) | (Zip Code) |
Registrant's telephone number, including area code: (858) 455-6006
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| Title of each class | Name of each exchange on which registered |
| COMMON STOCK, $0.001 PAR VALUE |
The Nasdaq Stock Market LLC
(The Nasdaq Capital Market)
|
SECURITIES REGISTERED PURSUANT TO SECTION 12(G) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer o | Smaller reporting company x |
| (Do not check if a smaller reporting company) | |||
Indicate by check mark if the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the voting common stock held by non-affiliates of the Registrant (assuming officers, directors and 10% stockholders are affiliates), based on the last sale price for such stock on June 30, 2010: $30,269,878. The Registrant has no non-voting common stock.
As of March 22, 2011, there were 14,775,366 shares of the Registrant's common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant's Proxy Statement for the 2011 Annual Meeting of Stockholders of the Registrant to be held on June 30, 2011 are incorporated by reference into Part III of this Form 10-K.
The Registrant makes available free of charge on or through its website (http://www.occulogix.com) its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. The material is made available through the Registrant's website as soon as reasonably practicable after the material is electronically filed with or furnished to the U.S. Securities and Exchange Commission, or SEC. All of the Registrant's filings may be read or copied at the SEC's Public Reference Room at 100 F Street, N.E., Room 1580, Washington D.C. 20549. Information on the hours of operation of the SEC's Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website (http://www.sec.gov) that contains reports and proxy and information statements of issuers that file electronically.
TEARLAB CORPORATION.
Form 10-K – ANNUAL REPORT
For the Fiscal Year Ended December 31, 2010
Table of Contents
Page
|
PART I
|
|||
|
Item 1.
|
3
|
||
|
Item 1A.
|
12
|
||
|
Item 2.
|
21
|
||
|
Item 3.
|
22
|
||
|
Item 4.
|
22 | ||
|
PART II
|
|||
|
Item 5.
|
23
|
||
|
Item 6.
|
26
|
||
|
Item 7.
|
28
|
||
|
Item 7A.
|
46
|
||
|
Item 8.
|
47
|
||
|
Item 9.
|
77
|
||
|
Item 9A.
|
77
|
||
|
Item 9B.
|
77
|
||
|
PART III
|
|||
|
Item 10.
|
78
|
||
|
Item 11.
|
78
|
||
|
Item 12.
|
78
|
||
|
Item 13.
|
78
|
||
|
Item 14.
|
78
|
||
|
PART IV
|
|||
|
Item 15.
|
79
|
||
PART I
SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements relating to future events and our future performance within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. In some cases, you can identify forward-looking statements by terms such as "may", "will", "should", "could", "would", “hope”, "expects", "plans", "intends", "anticipates", "believes", "estimates", "projects", "predicts", "potential" and similar expressions intended to identify forward-looking statements. These forward-looking statements include, without limitation, statements relating to future events, future results, and future economic conditions in general and statements about:
|
|
·
|
Our future strategy, structure, and business prospects;
|
|
|
·
|
The planned commercialization of our current product;
|
|
|
·
|
The size and growth of the potential markets for our product and technology;
|
|
|
·
|
The adequacy of current, and the development of new distributor, reseller, and supplier relationships, and our efforts to expand relationships with distributors and resellers in additional countries;
|
|
|
·
|
Our anticipated expansion of United States and international sales and operations;
|
|
|
·
|
Our ability to obtain and protect our intellectual property and proprietary rights;
|
|
|
·
|
Our efforts to assist our customers in obtaining their moderate complexity CLIA certification or providing them with support from certified professionals;
|
|
|
·
|
Our efforts to obtain CLIA waiver;
|
|
|
·
|
The anticipated sufficiency of our current office space, and our ability to find additional space as needed; and
|
|
|
·
|
Use of cash, cash needs and ability to raise capital.
|
These statements involve known and unknown risks, uncertainties and other factors, including the risks described in Part I, Item 1A. of this Annual Report on Form 10-K, which may cause our actual results, performance or achievements to be materially different from any future results, performances, time frames or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements. Information regarding market and industry statistics contained in this Annual Report on Form 10-K is included based on information available to us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. We have not reviewed or included data from all sources and cannot assure you of the accuracy of the market and industry data we have included.
See accompanying notes
Corporate Information
TearLab Corp. was incorporated as OccuLogix, Inc. in Delaware in 2002. Unless the context requires otherwise, in this report the terms "the Company," "we," "us" and "our" refer TearLab Corp. and our subsidiaries. References to "$" or "dollars" shall mean U.S. dollars unless otherwise indicated. References to "C$" shall mean Canadian dollars.
ITEM 1. Business.
Overview
We are an in-vitro diagnostic company based in San Diego, California. We are commercializing a proprietary tear testing platform, the TearLab® Osmolarity System that enables eye care practitioners to test for highly sensitive and specific biomarkers using nanoliters of tear film at the point-of-care. Our first product measures tear film osmolarity for the diagnosis of Dry Eye Disease, or DED. Prior to 2008, we had been seeking to commercialize treatments for age-related eye diseases through our Retina and Glaucoma business divisions. Our results by segment are included in our financial statements, which are included under Item 8 to this Annual Report on Form 10-K.
TearLab Research, Inc. (formerly TearLab, Inc.)
TearLab Research, Inc. our wholly-owned subsidiary, develops technologies to enable eye care practitioners to test a wide range of biomarkers (chemistries, metabolites (i.e. glucose), genes and proteins) at the point-of-care. Commercializing that tear testing platform is now the focus of our business.
The Company’s first product, the TearLab Osmolarity System, enables the rapid measurement of tear osmolarity in the doctor's office. Osmolarity is a quantitative and highly specific biomarker that has been shown to assist in the diagnosis and disease management of DED. There are estimated to be between 20 million and 40 million DED patients in the United States, and less than 5% of those patients are currently diagnosed and treated. The innovation of the TearLab Osmolarity System is its ability to precisely and rapidly measure osmolarity in nanoliter volumes of tear samples, using a highly efficient and novel tear collection system. Historically, eye care researchers have relied on expensive instruments to perform tear biomarker analysis. In addition to their cost, these conventional systems are slow, highly variable in their measurement readings, and not categorized as waived by the United States Food and Drug Administration, or FDA, under regulations promulgated under the Clinical Laboratory Improvement Amendments, or CLIA.
The TearLab Osmolarity System consists of the following three components: (1) the TearLab disposable, which is a single-use microfluidic microchip; (2) the TearLab Pen, which is a hand-held device that interfaces with the TearLab disposable; and (3) the TearLab Reader, which is a small desktop unit that allows for the docking of the TearLab Pen and provides a quantitative reading for the operator.
In October 2008, the TearLab Osmolarity System received CE mark approval, clearing the way for sales in the European Union and all countries recognizing the CE mark. In connection with the CE mark clearance, we have entered into multi-year agreements with numerous distributors for distribution of the TearLab Osmolarity System. Currently, we have signed distribution agreements in each of the following countries: Spain, Portugal, Germany, France, Turkey, Ukraine, Bulgaria, Belgium, Netherlands, Switzerland, Finland, Sweden, Korea, Australia, Russia, Hungary, Greece, Canada, Slovakia, Argentina, Czech Republic and the United Kingdom and a sales representation agreement in Japan.
On May 19, 2009, we announced that we received 510(k) clearance from the FDA. The 510(k) clearance allows us to market the TearLab® Osmolarity System to those reference and physician operated laboratories with CLIA certifications allowing them to perform moderate and high complexity tests. Considering that most of our target customers are eye care practitioners without such certifications, we intend to seek a CLIA waiver from the FDA for the TearLab Osmolarity System, although obtaining such a waiver does not preclude us from commercializing the technology. We submitted a CLIA waiver application for the TearLab™ Osmolarity System to the FDA on May 27, 2010. On March 4, 2011, we announced that we had received a communication from the the FDA that the data we submitted was not sufficient to gain approval of our CLIA waiver application. We are reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver. We anticipate receiving the FDA approval of our revised waiver application during either the second half of 2011 or the first half of 2012. A CLIA waiver would reduce the regulatory paperwork for future customers.
On December 8, 2009 we announced that Health Canada issued a Medical Device License for the TearLab Osmolarity System. The Health Canada license allowed us to immediately begin marketing the system in Canada. On August 20, 2009, we entered into an agreement with a distributor, Science with Vision, for exclusive distribution of the TearLab Osmolarity System in Canada. We began selling products through the Canadian distributor in 2010.
Retina Division
Prior to 2008, our Retina division was in the business of developing and commercializing a treatment for dry age-related macular degeneration, or Dry AMD. Our product for Dry AMD, the RHEO™ System, was designed to improve microcirculation in the eye by filtering high molecular weight proteins and other macromolecules from the patient's plasma, which was intended to increase the supply of oxygen and nutrients to the compromised retina and facilitate the removal of cellular waste material from the retina.
We conducted a pivotal clinical trial, called MIRA-1, or Multicenter Investigation of Rheopheresis for AMD, which, if successful, was expected to support our application to the FDA, to obtain approval to market the RHEO™ System in the United States.
After we completed an in-depth analysis of the MIRA-1 study data we met with representatives of the FDA to discuss the impact of the MIRA-1 study results on our Pre-Market Approval Application submission strategy. In light of the MIRA-1's failure to meet its primary efficacy endpoint, the FDA advised us that it would require an additional study of the RHEO™ System to be performed.
On November 1, 2007, we announced the indefinite suspension of our RHEO™ System clinical development program. This decision was made following a comprehensive review of the respective costs and development timelines associated with the products in our portfolio and in light of our financial position. There is no reasonable prospect that the RHEO™ System clinical development program will be relaunched in the foreseeable future.
Current Status and 2008, 2009 & 2010 Financing
Following the suspension of our RHEO™ System clinical development program in 2007 and the consequent winding-down of the RHEO-AMD study in 2008, our only operating business was through our ownership stake in TearLab.
On January 9, 2008, we announced the departure, or pending departure, of seven members of our executive team. By December 31, 2008, a total of 14 employees of the Company had left the Company's employment.
On October 6, 2008, we sold an aggregate of 869,200 shares of common stock at a per share purchase price of $2.50 with total proceeds of $2,173,000.
Also on October 6, 2008, we prepaid our then outstanding $6,703,500 aggregate principal amount bridge loan, or the Bridge Loan, and accrued interest by issuing 3,304,511 shares of common stock to the lenders at a per share price of $2.125. At the time of the prepayment, the Company also paid $481,200 of the commission remaining owed for placement agency services rendered by Marchant through the issuance of 192,480 shares of common stock at a per share price of $2.50.
On July 16, 2009, we announced that we had entered into and closed an agreement with certain investors whereby the investors agreed to provide financing (the “Financing”) to the Company through the purchase of convertible secured notes, in the aggregate amount of $1.55 million. The convertible secured notes (the “Notes”) evidencing the Financing, mature on the second anniversary of their issuance (“the Maturity Date”), bear interest at a rate of 12% per annum and are convertible into shares of the Company’s common stock upon the request of holders of 51% or more of the outstanding principal amount of the Notes at any time after August 31, 2009 and prior to the Maturity Date. The conversion price of the Notes (the “Discount Price”) is $1.3186 per share. Any such conversion is limited to prevent the number of shares issued upon conversion of the Notes from exceeding 19.9% of the outstanding common stock of the Company, measured prior to the date of the Financing. In connection with the Financing, the Company will issue warrants to purchase shares of common stock equal in value to ten percent of the aggregate principal amount of the notes (the “Warrants”). The exercise price of the Warrants is $1.60 per common share.
On January 8, 2010, we obtained commitments for the sale of 3,244,766 shares of our common stock for an aggregate of approximately $3,000,000 in a private placement. 1,886,291 of such shares were issued in January 2010 with the remainder being issued in March 2010, following stockholder approval for the sale of such additional shares. The per share price of the shares, sold in the private placement was $0.92456, which is equal to 80% of the volume weighted average price of our common stock for the 10 trading days ending on the day immediately preceding the January 8, 2010 closing date.
On March 18, 2010, we sold 1,552,795 shares of our common stock and warrants to purchase an additional 621,118 shares of our common stock for gross proceeds of approximately $5,000,000. The purchase price for the shares and warrants was $3.22 per unit (each unit consisting of one share and a warrant to purchase 0.4 shares of common stock). The exercise price of the warrants is $4.00 per share. The warrants are exercisable at any time during the period commencing on September 19, 2010 and ending on September 19, 2011.
Industry
Point-of-care Testing and Dry Eye Disease, or DED
The global market for point-of-care testing is currently $4.5 billion annually or 15% of the $30 billion global market for in-vitro diagnostic products. Approximately 75% of all laboratory tests today are performed at centralized clinical laboratories. However, there is an increasing frequency of diagnostic testing being performed at the point-of-care due to several factors, including a need for rapid testing in acute care situations, the benefits of patient monitoring and disease management, streamlining therapeutic decision making and the overall trend toward personalized medicine. We believe that advances in biodetection technologies that can simplify and accelerate the rate of performing complex diagnostic tests at the point-of-care, and that are reimbursed, will drive utilization and overall point-of-care testing market growth.
TearLab's first product is the TearLab® Osmolarity System. This test can be performed at the point-of-care for the measurement of osmolarity, a quantitative and highly specific biomarker that has shown to assist in the diagnosis and disease management of DED. There are estimated to be between 20 and 40 million people with DED in the United States alone, and this condition is estimated to account for up to one-third of all visits to U.S. eye care professionals.
Each time a person blinks, his or her eyes are resurfaced with a thin layer of a complex fluid known as the tear film. The tear film works to protect eyes from the outside world. Bacteria, viruses, sand, freezing winds and salt water will not damage eyes when the tear film is intact. However, when compromised, a deficient tear film can be an exceedingly painful and disruptive condition. The tear film consists of three components: (i) an innermost mucin layer (produced by the surface cells); (ii) the aqueous layer (the water in tears, produced by the lacrimal gland); and (iii) an oily lipid layer which limits evaporation of the tears (produced by the meibomian glands, located at the margins of the eyelids). The apparatus of the ocular surface forms an integrated unit. When working correctly, the tear film presents a smooth optical surface essential for clear vision and proper immunity. Androgen deficiency and chronic inflammation of the lacrimal or meibomian gland may lead to the condition known as dry eye, which has been likened to arthritis of the eye, and results in a compromised, fragile tear film.
DED is often seen as a result of aging, diabetes, prostate cancer therapy, HIV, autoimmune diseases such as Sjögren's syndrome and rheumatoid arthritis, LASIK surgery, contact lens wear, menopause and as a side effect of hormone replacement therapy. Numerous commonly prescribed and over-the-counter medications also can cause, or contribute to, the manifestation of DED.
There are millions of Americans who suffer from contact lens-induced DED, and 10% to 15% of these patients revert to frame wear annually due to dryness and discomfort. There are between 500,000 and 1.5 million LASIK procedures performed in the U.S each year, and about 50% of patients experience DED post-operatively.
Diagnostic Alternatives for Dry Eye Disease
Existing diagnostic assays are highly subjective, do not correlate well with symptoms, are invasive for patients and may require up to an hour of operator time to perform. All of these factors have constrained the diagnosis and treatment of the DED patient population. As physicians have not had access to objective, quantitative diagnostic assays that correlate well with symptoms and disease pathogenesis, it has been difficult for them to differentiate DED symptoms from other eye diseases that present with very similar symptoms, such as non-infectious ocular allergies or infectious bacterial or viral diseases. To treat DED effectively and to mitigate the emotional and physical effects of this disease, it will be critical to equip physicians with objective, quantitative measurements of disease pathogenesis so they can determine more accurately the most efficacious treatments for their patients.
Osmolarity in DED presents itself as an increase in the salt concentration of the tear film. For over 50 years, studies have shown that tear film osmolarity is the ideal clinical marker for diagnosing DED, providing an objective, quantitative measurement of disease pathogenesis. Measuring osmolarity also serves as an effective disease management tool by providing physicians with an ability to personalize therapeutic intervention and to track patient outcomes quantitatively. Osmolarity testing could also provide physicians with a tool to identify patients at risk for dropping out of contact lens wear early in disease progression, as well as an invaluable test to guide the type and duration of therapy prior to, and following refractive surgery.
The main challenge in measuring osmolarity at the point-of-care is the vanishingly small volume of tear available for testing. Older laboratory osmometers require upwards of ten microliters of fluid to produce a single reading. In addition, these instruments are not particularly suitable for use in a physician's office, since they require continual calibration, cleaning and maintenance. Existing osmometers currently are marketed primarily to reference and hospital laboratories for the measurement of osmolarity in blood, urine and other serum samples.
TearLab's Product
The TearLab® Osmolarity System is an integrated testing system comprised of: (1) the TearLab disposable, which is a single-use microfluidic microchip; (2) the TearLab Pen, which is a hand-held device that interfaces with the TearLab disposable; and (3) the TearLab Reader, which is a small desktop unit that allows for the docking of the TearLab Pen and provides a quantitative reading for the operator. The innovation of the TearLab Osmolarity System is its ability to measure precisely and rapidly, and inexpensively, biomarkers in nanoliter volumes of tear samples or approximately 1,000 times less volume than required forolder laboratory devices.
The operator of the TearLab Osmolarity System, most likely a technician, collects the tear sample from the patient's eye in the TearLab disposable, using the TearLab Pen. After the tear has been collected, the operator places the Pen into the Reader. The TearLab Reader then will display an osmolarity reading to the operator. Following the completion of the test, the TearLab disposable will be discarded and a new TearLab disposable will be readied for the next test. The entire process, from sample to answer, should require approximately two minutes or less to complete.
We are currently engaged in commercial manufacturing of the TearLab Osmolarity System. In October 2008, the TearLab Osmolarity System received CE mark approval, clearing the way for sales in the European Union and all countries recognizing the CE mark. In connection with the CE mark clearance, we have entered into multi-year agreements with numerous distributors for distribution of the TearLab Osmolarity System. In December 2009, Health Canada issued a Medical Device License for the TearLab Osmolarity System allowing us to market our product in Canada.
In May 2009, we received 510(k) approval from the FDA to aid in the diagnosis of patients with signs and symptoms of DED. The 510(k) enables us to sell our product to customers that have moderate or high complexity CLIA certificates in the United States. In addition, we have been awarded ISO 13485 certification for our quality management system. ISO 13485 is an internationally-accepted standard of quality management for medical device manufacturers.
On March 4, 2011, the Company announced that it was in receipt of a communication from the U.S. Food and Drug Administration (“FDA”) indicating that the data submitted by the Company was not sufficient to gain approval of its CLIA Waiver categorization application for the TearLab® Osmolarity System. The Company is reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver. The Company will also continue to pursue it strategy to assist its customers in obtaining moderate complexity CLIA certificates.
We cannot be sure of when, or whether, TearLab will be successful in obtaining a CLIA waiver certificate for the TearLab Osmolarity System.
Currently, we have signed distribution agreements for the TearLab Osmolarity System in each of the following countries: Spain, Portugal, Germany, France, Turkey, Ukraine, Bulgaria, Belgium, Netherlands, Switzerland, Finland, Sweden, South Korea, Australia, Russia, Hungary, Greece, Canada, Slovakia, Argentina, the Czech Republic and the United Kingdom and a sales representation agreement in Japan.
Competition
To date, we have identified one laboratory technology that claims to be able to measure the osmolarity of nanoliter tear samples. This technology is being developed at Advanced Instruments, Inc., Norwood Massachusetts. It is a freezing point osmometer (Model 3100) that requires 500 nanoliter samples and requires upwards of 15 minutes to generate a single reading. The Model 3100 tear osmometer is an FDA Class I exempt device, intended for use in laboratory facilities categorized as moderate or highly complex under the U.S. CLIA regulations. Class I Exempt osmometers are not FDA cleared for use in non-laboratory physician office facilities.
We believe that this device will be limited to institutional research, hospital or clinical laboratory facilities. As it will be impractical to transport tear fluid from the physician office to the laboratory, it will be necessary to send the patient to the laboratory for tear collection and testing. As most laboratories are not trained or staffed in tear collection techniques, it is envisioned that this device will be limited for use in facilities dedicated to tear research.
As there are no commercially available instruments to measure tear film osmolarity at the point-of-care, TearLab views existing DED diagnostic tests, such as the Schirmer Test and ocular surface staining, as its primary source of competition.
Tear film break-up time, or TBUT, is another assay meant as an indication of tear film stability. However, it is subjective, requires a physician to instill a carefully controlled amount of fluorescein dye into the eye and requires a stopwatch to determine the endpoint. TBUT has been shown to be unreliable as a determinant of DED since shortened TBUT does not always correlate well with other signs or symptoms.
Tests like impression cytology and corneal staining, although indicative of relatively late stage phenomena in DED, are subjective, qualitative and generally do not correlate to disease pathogenesis. The Schirmer Test is an imprecise marker of tear function since its diagnostic results vary significantly.
Although, at the present time, there does not appear to be a direct competitor to the TearLab® Osmolarity System, many industry participants have much greater resources than us. This means that those industry participants may be able to make greater investments in research and development, marketing, promotion and sales, than we are capable of right now or will be capable of during the foreseeable future.
Principal Suppliers
We rely on a single supplier, Sparton Medical Systems located in the United States, for the manufacture of the Readers and pens which are key components of the TearLab® Osmolarity System. We also rely on a single supplier, MiniFAB (Aust) Pty Ltd.located in Australia, for the manufacture of the test cards which is also a key component of the TearLab Osmolarity System.
Patents and Proprietary Rights
We own or have exclusive licenses to multiple patents and applications relating to the TearLab Osmolarity System and related technology and processes:
|
|
·
|
eight issued U.S. patents; relating to the TearLab Osmolarity System and related technology and processes and have applied for a number of other patents in the United States and other jurisdictions.
|
|
|
·
|
three pending U.S. patent applications;
|
|
|
·
|
eighteen pending patent applications in countries/regions other than the UnitedStates, including six applications in Europe, four applications in Japan, three applications in Canada, two Australia, one application in Brazil and two applications in Mexico;
|
|
|
·
|
one granted Australian patent, one granted Chinese patent and one granted Mexican patent.
|
We intend to rely on know-how, continuing technological innovation and in-licensing opportunities to further develop our proprietary position. Our ability to obtain intellectual property protection for the TearLab® Osmolarity System and related technology and processes, and our ability to operate without infringing the intellectual property rights of others and to prevent others from infringing our intellectual property rights, will have a substantial impact on our ability to succeed in our business. Although we intend to seek to protect our proprietary position by, among other methods, continuing to file patent applications, the patent position of companies like TearLab is generally uncertain and involves complex legal and factual questions. Our ability to maintain and solidify a proprietary position for our technology will depend on our success in obtaining effective claims and enforcing those claims once granted. We do not know whether any part of our patent applications will result in the issuance of any patents. Our issued patents or those that may issue in the future, or those licensed to us, may be challenged, invalidated or circumvented, which could limit our ability to stop would-be competitors from marketing tests identical to the TearLab Osmolarity System.
In addition to patent protection, we have registered the TearLab trademark in the United States, the European Union, Japan, Korea, Mexico, the Russian Federation and Turkey. Our TearLab trademark applications are pending in Canada and China.
Government Regulation
Government authorities in the United States and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing of our product, which is a medical device. In the United States, the Food and Drug Administration, or FDA, regulates medical devices under the Federal Food, Drug, and Cosmetic Act and implementing regulations. Failure to comply with the applicable FDA requirements, both before and after approval, may subject us to administrative and judicial sanctions, such as a delay in approving or refusal by the FDA to approve pending applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, administrative fines or criminal prosecution.
Unless exempted by regulation, medical devices may not be commercially distributed in the United States unless they have been cleared or approved by the FDA. Medical devices are classified into one of the three classes, Class I, II or III, on the basis of the controls necessary to reasonably assure their safety and effectiveness. Class I devices are subject to general controls, such as labeling, pre-market notification and adherence to good manufacturing practices. The TearLab Osmolarity System is a Class I, non-exempt device. Under the FDA’s Section 510(k) procedure, the manufacturer provides a pre-market notification that it intends to begin marketing the product, and shows that the product is substantially equivalent to another legally marketed product, that it has the same intended use and is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness than does a legally marketed device. In some cases, the submission must include data from human clinical studies. Marketing may commence when the FDA issues a clearance letter finding substantial equivalence. On May 19, 2009 we announced that we received FDA 510(k) clearance of the TearLab Osmolarity System.
After a device receives 510(k) clearance, any modification to the device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, would require a new 510(k) clearance or an approval of a PMA. Although the FDA requires the manufacturer to make the initial determination regarding the effect of a modification to the device that is subject to 510(k) clearance, the FDA can review the manufacturer's determination at any time and require the manufacturer to seek another 510(k) clearance or an approval of a PMA.
The TearLab® Osmolarity System is a Class I, non-exempt device and qualifies for the 510(k) procedure.
CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality control, quality assurance and inspections. The regulations promulgated under CLIA establish three levels of in vitro diagnostic tests: (1) waiver; (2) moderately complex; and (3) highly complex. The standards applicable to a clinical laboratory depend on the level of diagnostic tests it performs. A CLIA waiver is available to clinical laboratory test systems if they meet certain requirements established by the statute. Waived tests are simple laboratory examinations and procedures employing methodologies that are so simple and accurate as to render the likelihood of erroneous results negligible or to pose no reasonable risk of harm to patients if the examinations or procedures are performed incorrectly. These tests are waived from regulatory oversight of the user other than the requirement to follow the manufacturer's labeling and directions for use.
On March 4, 2011, the Company announced that it was in receipt of a communication from the U.S. Food and Drug Administration (“FDA”) indicating that the data submitted by the Company was not sufficient to gain approval of its CLIA Waiver categorization application for the TearLab® Osmolarity System. The Company is reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver.
We cannot be sure of when, or whether, TearLab will be successful in obtaining a CLIA waiver certificate for the TearLab Osmolarity System.
Regardless of whether a medical device requires FDA clearance or approval, a number of other FDA requirements apply to the device, its manufacturer and those who distribute it. Device manufacturers must be registered and their products listed with the FDA, and certain adverse events and product malfunctions must be reported to the FDA. The FDA also regulates the product labeling, promotion and, in some cases, advertising, of medical devices. In addition, manufacturers and their suppliers must comply with the FDA's quality system regulation which establishes extensive requirements for quality and manufacturing procedures. Thus, suppliers, manufacturers and distributors must continue to spend time, money and effort to maintain compliance, and failure to comply can lead to enforcement action. The FDA periodically inspects facilities to ascertain compliance with these and other requirements.
Research and Development Expenditure
Our research and development expense was $1.4 million and $1.1 million in the years ended December 31, 2010 and 2009, respectively.
Employees
On December 31, 2010, we had 14 full-time employees.
Available Information
Our corporate Internet address is www.tearlab.com. At the Investor Relations section of this website, we make available free of charge our Annual Report on Form 10-K, our Annual Proxy statement, our quarterly reports on Form 10-Q, any Current Reports on Form 8-K, and any amendments to these reports, as soon as reasonably practicable after we electronically file them with, or furnish them to, the Securities and Exchange Commission, or the SEC. The information found on our website is not part of this Annual Report on Form 10-K. In addition to our website, the Securities and Exchange Commission, or the SEC, maintains an Internet site at www.sec.gov that contains reports, proxy and information statements, and other information regarding us and other issuers that file electronically with the SEC.
ITEM 1A. RISK FACTORS
This Annual Report contains forward-looking statements that involve risks and uncertainties that could cause our actual results to differ materially from those discussed in this Annual Report. These risks and uncertainties include the following:
Risks Relating to our Business
Our near-term success is highly dependent on the success of the TearLab® Osmolarity System, and we cannot be certain that it will receive regulatory approval or be successfully commercialized in the United States.
The TearLab Osmolarity System is currently our only product. Our product is currently sold outside of the United States pursuant to CE mark approval; in Canada pursuant to a Health Canada Medical Device License; and in the United States as a result of having received 510(k) approval from the FDA, to market the TearLab Osmolarity System to those reference and physician operated laboratories with moderate and high complexity, Clinical Laboratory Improvement Act, or CLIA, certifications. We applying for a CLIA waiver from the U.S. Food and Drug Administration, or the FDA, which will allow us to sell to those eye care professionals in the United States who do not have CLIA certifications to perform moderate and high complexity tests. Even if the TearLab Osmolarity System receives all regulatory approvals in the United States, it may never be successfully commercialized. If the TearLab Osmolarity System does not receive all regulatory approvals or is not successfully commercialized, we may not be able to generate revenue, become profitable or continue our operations. Any failure of the TearLab Osmolarity System to receive regulatory approvals or to be successfully commercialized in the United States would have a material adverse effect on our business, operating results, financial condition and cash flows and could result in a substantial decline in the price of our common stock.
On March 4, 2011, the Company announced that it was in receipt of a communication from the U.S. Food and Drug Administration (“FDA”) indicating that the data submitted by the Company was not sufficient to gain approval of its CLIA Waiver categorization application for the TearLab® Osmolarity System. The Company is reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver.
Our near-term success is highly dependent on increasing sales of the TearLab Osmolarity System outside the United States, and we cannot be certain that we will successfully increase such sales.
Our product is currently sold outside of the United States pursuant to CE mark approval and Health Canada Approval in Canada. Our near-term success is highly dependent on increasing our international sales. We may also be required to register our product with health departments in our foreign market countries. A failure to successfully register in such markets would negatively affect our sales in any such markets. In addition, import taxes are levied on our product in certain foreign markets. These foreign markets include Turkey, Spain, Italy and France. Other countries may adopt taxation codes on imported products. Increases in such taxes or other restrictions on our product could negatively affect our ability to import, distribute and price our product.
Our limited working capital and history of losses have resulted in our auditors expressing doubts as to whether we will be able to continue as a going concern.
In the years ended December 31, 2008, 2009 and 2010, we had prepared our consolidated financial statements on the basis that we would continue as a going concern. However, we have sustained substantial losses for each of the years ended December 31, 2005, 2006, 2007, 2008, 2009 and 2010. Our net working capital balance at December 31, 2010 was $0.4 million which represents a $2.6 million increase from our working capital deficit at December 31, 2009. During the year ended December 31, 2010, the Company raised gross proceeds of $8.0 million in private placement and registered direct financings. As a result of our history of losses and current financial condition, there is substantial doubt about our ability to continue as a going concern.
Included in the net working capital at December 31, 2010 is $1,669,000 in convertible notes payable and accrued interest which if not converted to common stock when it comes due for conversion in the third quarter of 2011 would utilize available cash resources. We believe that our cash and cash equivalents will be sufficient to meet our operating activities and other demands only until July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and only until December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011.
Our consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary if we were not able to continue as a going concern.
We have incurred losses since inception and anticipate that we will incur continued losses for the foreseeable future.
We have incurred losses in each year since our inception. As of December 31, 2010, we had an accumulated deficit of $379.0 million. Our losses have resulted primarily from expenses incurred in research and development of our product candidates from the former retina and glaucoma business divisions. We do not know when or if we will receive CLIA waiver approval from the FDA for the TearLab Osmolarity® System or successfully commercialize it in the United States. As a result, and because of the numerous risks and uncertainties facing us, it is difficult to provide the extent of any future losses or the time required to achieve profitability, if at all. Any failure of our product candidate to obtain regulatory approval and any failure to become and remain profitable would adversely affect the price of our common stock and our ability to raise capital and continue operations.
We are leveraged financially, which could adversely affect our ability to adjust our business to respond to competitive pressures and to obtain sufficient funds to satisfy our future research and development needs, and to defend our intellectual property.
As of December 31, 2010, our total indebtedness was approximately $3.7 million. Included in our total indebtedness is $1.7 million of convertible notes payable and accrued interest which comes due in the third quarter of 2011 and if not converted into common shares of the Company could adversely impact our cash reserves needed to operate the business. Our significant debt and debt service requirements could adversely affect our ability to operate our business and may limit our ability to take advantage of potential business opportunities. For example, our high level of debt presents the following risks:
|
·
|
our substantial leverage increases our vulnerability to economic downturns and adverse competitive and industry conditions and could place us at a competitive disadvantage compared to those of our competitors that are less leveraged;
|
|
·
|
our debt service obligations could limit our flexibility in planning for, or reacting to, changes in our business and our industry and could limit our ability to pursue other business opportunities, borrow more money for operations or capital in the future and implement our business strategies; and
|
|
·
|
our level of debt may restrict us from raising additional financing on satisfactory terms to fund working capital, capital expenditures, product development efforts, strategic acquisitions, investments and alliances, and other general corporate requirements.
|
If we are at any time unable to generate sufficient cash flow to service our indebtedness when payment is due, we may be required to attempt to renegotiate the terms of the instruments relating to the indebtedness, seek to refinance all or a portion of the indebtedness or obtain additional financing. There can be no assurance that we will be able to successfully renegotiate such terms, that any such refinancing would be possible or that any additional financing could be obtained on terms that are favorable or acceptable to us.
We may not be able to raise the capital necessary to fund our operations.
Since inception, we have funded our operations through debt and equity financings, including 2008 common stock and debt bridge financings, 2009 convertible secured debt financings and the 2010 private placement and registered direct common stock financings. As of the date of this annual report on Form 10-K, we estimate that our cash and cash equivalents will be sufficient to meet our operating activities and other demands only until July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and only until December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011. however, our prospects for obtaining additional financing are uncertain. Additional capital may not be available on terms favorable to us, or at all. If financing is available, it may not be sufficient for us to continue as a going concern and it may be on terms that adversely affect the interest of our existing stockholders. In addition, future financings could result in significant dilution of existing stockholders and adversely affect the economic interests of existing stockholders.
We will face challenges in bringing the TearLab® Osmolarity System to market in the United States and may not succeed in executing our business plan.
There are numerous risks and uncertainties inherent in the development of new medical technologies. In addition to our requirement for additional capital, our ability to bring the TearLab Osmolarity System to market in the United States and to execute our business plan successfully is subject to the following risks, among others:
|
·
|
Our clinical trials may not succeed. Clinical testing is expensive and can take longer than originally anticipated. The outcomes of clinical trials are uncertain, and failure can occur at any stage of the testing. We could encounter unexpected problems, which could result in a delay in the follow-up submission of our application for the sought-after CLIA waiver from the FDA or prevent its submission altogether.
|
|
·
|
We may not receive the CLIA waiver for the TearLab Osmolarity System from the FDA, in which case our ability to market the TearLab Osmolarity System in the United States will be impacted.
|
|
·
|
The TearLab Osmolarity System is rated under a moderately complex CLIA certification which requires our customers to be certified under the moderately complex CLIA requirements, including certain parallel state requirements. If our customers are unwilling or unable to comply with such requirements, it could have an adverse effect on our ability to market the TearLab Osmolarity System in the United States.
|
|
·
|
Our suppliers and we will be subject to numerous FDA requirements covering the design, testing, manufacturing, quality control, labeling, advertising, promotion and export of the TearLab Osmolarity System and other matters. If our suppliers or we fail to comply with these regulatory requirements, the TearLab Osmolarity System could be subject to restrictions or withdrawals from the market and we could become subject to penalties.
|
|
·
|
Even if we succeed in obtaining the sought-after FDA approvals, we may be unable to commercialize the TearLab® Osmolarity System successfully in the United States. Successful commercialization will depend on a number of factors, including, among other things, achieving widespread acceptance of the TearLab Osmolarity System among physicians, establishing adequate sales and marketing capabilities, addressing competition effectively, the ability to obtain and enforce patents to protect proprietary rights from use by would-be competitors, key personnel retention and ensuring sufficient manufacturing capacity and inventory to support commercialization plans.
|
If we are subject to regulatory enforcement action as a result of our failure to comply with regulatory requirements, our commercial operations would be harmed.
While we received the 510(k) clearance that we were seeking, we will be subject to significant ongoing regulatory requirements, and if we fail to comply with these requirements, we could be subject to enforcement action by the FDA or state agencies, including:
|
·
|
adverse publicity, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
·
|
repair, replacement, refunds, recall or seizure of our product;
|
|
·
|
operating restrictions or partial suspension or total shutdown of production;
|
|
·
|
delay or refusal of our requests for 510(k) clearance or premarket approval of new products or of new intended uses or modifications to our existing product;
|
|
·
|
refusal to grant export approval for our products;
|
|
·
|
withdrawing 510(k) clearances or premarket approvals that have already been granted; and
|
|
·
|
criminal prosecution.
|
If any of these enforcement actions were to be taken by the government, our business could be harmed.
We are required to demonstrate and maintain compliance with the FDA’s Quality System Regulation, or the QSR. The QSR is a complex regulatory scheme that covers the methods and documentation of the design, testing, control, manufacturing, labeling, quality assurance, packaging, storage and shipping of our products. The FDA must determine that the facilities which manufacture and assemble our products that are intended for sale in the United States, as well as the manufacturing controls and specifications for these products, are compliant with applicable regulatory requirements, including the QSR. The FDA enforces the QSR through periodic unannounced inspections. Our facilities have not yet been inspected by the FDA, and we cannot assure you that we will pass any future FDA inspection. Our failure, or the failure of our suppliers, to take satisfactory corrective action in response to an adverse QSR inspection could result in enforcement actions, including a public warning letter, a shutdown of our manufacturing operations, a recall of our product, civil or criminal penalties or other sanctions, which would significantly harm our available inventory and sales and cause our business to suffer.
Our patents may not be valid, and we may not be able to obtain and enforce patents to protect our proprietary rights from use by would-be competitors. Patents of other companies could require us to stop using or pay to use required technology.
Our owned and licensed patents may not be valid, and we may not be able to obtain and enforce patents and to maintain trade secret protection for our technology. The extent to which we are unable to do so could materially harm our business.
We have applied for, and intend to continue to apply for, patents relating to the TearLab® Osmolarity System and related technology and processes. Such applications may not result in the issuance of any patents, and any patents now held or that may be issued may not provide adequate protection from competition. Furthermore, it is possible that patents issued or licensed to us may be challenged successfully. In that event, if we have a preferred competitive position because of any such patents, any preferred position would be lost. If we are unable to secure or to continue to maintain a preferred position, the TearLab Osmolarity System could become subject to competition from the sale of generic products.
Patents issued or licensed to us may be infringed by the products or processes of others. The cost of enforcing patent rights against infringers, if such enforcement is required, could be significant and the time demands could interfere with our normal operations. There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical, biotechnology and medical technology industries. We could become a party to patent litigation and other proceedings. The cost to us of any patent litigation, even if resolved in our favor, could be substantial. Some of our would-be competitors may be able to sustain the costs of such litigation more effectively than we can because of their substantially greater financial resources. Litigation may also absorb significant management time.
Unpatented trade secrets, improvements, confidential know-how and continuing technological innovation are important to our future scientific and commercial success. Although we attempt to, and will continue to attempt to, protect our proprietary information through reliance on trade secret laws and the use of confidentiality agreements with corporate partners, collaborators, employees and consultants and other appropriate means, these measures may not effectively prevent disclosure of our proprietary information, and, in any event, others may develop independently, or obtain access to, the same or similar information.
Certain of our patent rights are licensed to us by third parties. If we fail to comply with the terms of these license agreements, our rights to those patents may be terminated, and we will be unable to conduct our business.
It is possible that a court may find us to be infringing upon validly issued patents of third parties. In that event, in addition to the cost of defending the underlying suit for infringement, we may have to pay license fees and/or damages and may be enjoined from conducting certain activities. Obtaining licenses under third-party patents can be costly, and such licenses may not be available at all.
We may face future product liability claims.
The testing, manufacturing, marketing and sale of therapeutic and diagnostic products entail significant inherent risks of allegations of product liability. Our past use of the RHEO™ System and the components of the SOLX Glaucoma System in clinical trials and the commercial sale of those products may have exposed us to potential liability claims. Our use of the TearLab® Osmolarity System and its commercial sale could also expose us to liability claims. All of such claims might be made directly by patients, health care providers or others selling the products. We carry clinical trials and product liability insurance to cover certain claims that could arise, or that could have arisen, during our clinical trials or during the commercial use of our products. We currently maintain clinical trials and product liability insurance with coverage limits of $2,000,000 in the aggregate annually. Such coverage, and any coverage obtained in the future, may be inadequate to protect us in the event of successful product liability claims, and we may not be able to increase the amount of such insurance coverage or even renew it. A successful product liability claim could materially harm our business. In addition, substantial, complex or extended litigation could result in the incurrence of large expenditures and the diversion of significant resources.
We have entered into a number of related party transactions with suppliers, creditors, stockholders, officers and other parties, each of which may have interests which conflict with those of our public stockholders.
We have entered into several related party transactions with our suppliers, creditors, stockholders, officers and other parties, each of which may have interests which conflict with those of our public stockholders.
If we do not introduce new commercially successful products in a timely manner, our products may become obsolete over time, customers may not buy our products and our revenue and profitability may decline.
Demand for our products may change in ways we may not anticipate because of:
|
·
|
evolving customer needs;
|
|
·
|
the introduction of new products and technologies; and
|
|
·
|
evolving industry standards.
|
Without the timely introduction of new commercially successful products and enhancements, our products may become obsolete over time, in which case our sales and operating results would suffer. The success of our new product offerings will depend on several factors, including our ability to:
|
·
|
properly identify and anticipate customer needs;
|
|
·
|
commercialize new products in a cost-effective and timely manner;
|
|
·
|
manufacture and deliver products in sufficient volumes on time;
|
|
·
|
obtain and maintain regulatory approval for such new products;
|
|
·
|
differentiate our offerings from competitors’ offerings;
|
|
·
|
achieve positive clinical outcomes; and
|
|
·
|
provide adequate medical and/or consumer education relating to new products.
|
Moreover, innovations generally will require a substantial investment in research and development before we can determine the commercial viability of these innovations and we may not have the financial resources necessary to fund these innovations. In addition, even if we are able to successfully develop enhancements or new generations of our products, these enhancements or new generations of products may not produce revenue in excess of the costs of development and they may be quickly rendered obsolete by changing customer preferences or the introduction by our competitors of products embodying new technologies or features.
We rely on a single supplier of each of the key components of the TearLab® Osmolarity System and are vulnerable to fluctuations in the availability and price of our suppliers’ products and services.
We purchase each of the key components of the TearLab Osmolarity System from single third-party suppliers. Our supplier may not provide the components or other products needed by us in the quantities requested, in a timely manner or at a price we are willing to pay. In the event we were unable to renew our agreements with our supplier or they were to become unable or unwilling to continue to provide important components in the required volumes and quality levels or in a timely manner, or if regulations affecting the components were to change, we would be required to identify and obtain acceptable replacement supply sources. We may not be able to obtain alternative suppliers or vendors on a timely basis, or at all, which could disrupt or delay, or halt altogether, our ability to manufacture or deliver the TearLab Osmolarity System. If any of these events should occur, our business, financial condition, cash flows and results of operations could be materially adversely affected.
We face intense competition, and our failure to compete effectively could have a material adverse effect on our results of operations.
We face intense competition in the markets for ophthalmic products and these markets are subject to rapid and significant technological change. Although we have no direct competitors, we have numerous potential competitors in the United States and abroad. We face potential competition from industry participants marketing conventional technologies for the measurement of osmolarity and other in-lab testing technologies, as well as industry participants developing and marketing point-of-care tests, such as the technology being developed by the Aborn Eye Clinic, which is reportedly able to measure the osmolarity of nanoliter tear samples, and commercially available methods, such as the Schirmer Test and ocular surface staining. Many of our potential competitors have substantially more resources and a greater marketing scale than we do. If we are unable to develop and produce or market our products to effectively compete against our competitors, our operating results will materially suffer.
If we lose key personnel, or we are unable to attract and retain highly qualified personnel on a cost-effective basis, it would be more difficult for us to manage our existing business operations and to identify and pursue new growth opportunities.
Our success depends, in large part, upon our ability to attract and retain highly qualified scientific, clinical, manufacturing and management personnel. In addition, any difficulties retaining key personnel or managing this growth could disrupt our operations. Future growth will require us to continue to implement and improve our managerial, operational and financial systems, and to continue to recruit, train and retain, additional qualified personnel, which may impose a strain on our administrative and operational infrastructure. The competition for qualified personnel in the medical technology field is intense. We are highly dependent on our continued ability to attract, motivate and retain highly qualified management, clinical and scientific personnel.
Due to our limited resources, we may not be able to effectively recruit, train and retain additional qualified personnel. If we are unable to retain key personnel or manage our growth effectively, we may not be able to implement our business plan.
Furthermore, we have not entered into non-competition agreements with our key employees. In addition, we do not maintain “key person” life insurance on any of our officers, employees or consultants. The loss of the services of existing personnel, the failure to recruit additional key scientific, technical and managerial personnel in a timely manner, and the loss of our employees to our competitors would harm our research and development programs and our business.
If we fail to establish and maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired, which would adversely affect our consolidated operating results, our ability to operate our business and our stock price.
Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Failure on our part to maintain effective internal financial and accounting controls would cause our financial reporting to be unreliable, could have a material adverse effect on our business, operating results, financial condition and cash flows, and could cause the trading price of our common stock to fall dramatically.
Maintaining proper and effective internal controls will require substantial management time and attention and may result in our incurring substantial incremental expenses, including with respect to increasing the breadth and depth of our finance organization to ensure that we have personnel with the appropriate qualifications and training in certain key accounting roles and adherence to certain control disciplines within the accounting and reporting function. Any failure in internal controls or any additional errors or delays in our financial reporting would have a material adverse effect on our business and results of operations and could have a substantial adverse impact on the trading price of our common stock.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP. Our management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Our management has identified a control deficiency in the past and may identify additional deficiencies in the future.
We cannot be certain that the actions we are taking to improve our internal controls over financial reporting will be sufficient or that we will be able to implement our planned processes and procedures in a timely manner. In future periods, if the process required by Section 404 of the Sarbanes-Oxley Act of 2002 reveals material weaknesses or significant deficiencies, the correction of any such material weaknesses or significant deficiencies could require additional remedial measures which could be costly and time-consuming. In addition, we may be unable to produce accurate financial statements on a timely basis. Any of the foregoing could cause investors to lose confidence in the reliability of our consolidated financial statements, which could cause the market price of our common stock to decline and make it more difficult for us to finance our operations and growth.
The trading price of our common stock may be volatile.
The market prices for, and the trading volumes of, securities of medical device companies, such as ours, have been historically volatile. The market has experienced, from time to time, significant price and volume fluctuations unrelated to the operating performance of particular companies. The market price of our common shares may fluctuate significantly due to a variety of factors, including:
|
·
|
the results of pre-clinical testing and clinical trials by us, our collaborators and/or our competitors;
|
|
·
|
technological innovations or new diagnostic products;
|
|
·
|
governmental regulations;
|
|
·
|
developments in patent or other proprietary rights;
|
|
·
|
litigation;
|
|
·
|
public concern regarding the safety of products developed by us or others;
|
|
·
|
comments by securities analysts;
|
|
·
|
the issuance of additional shares to obtain financing or for acquisitions;
|
|
·
|
general market conditions in our industry or in the economy as a whole; and
|
|
·
|
political instability, natural disasters, war and/or events of terrorism.
|
In addition, the stock market has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of individual companies. Broad market and industry factors may seriously affect the market price of companies’ stock, including ours, regardless of actual operating performance. In the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
Because we do not expect to pay dividends on our common stock, stockholders will benefit from an investment in our common stock only if it appreciates in value.
We have never paid cash dividends on our common stock and have no present intention to pay any dividends in the future. We are not profitable and do not expect to earn any material revenues for at least several years, if at all. As a result, we intend to use all available cash and liquid assets in the development of our business. Any future determination about the payment of dividends will be made at the discretion of our board of directors and will depend upon our earnings, if any, our capital requirements, our operating and financial conditions and on such other factors as our board of directors may deem relevant. As a result, the success of an investment in our common stock will depend upon any future appreciation in its value. There is no guarantee that our common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
Warrant holders will not be entitled to any of the rights of common stockholders, but will be subject to all changes made with respect thereto.
If you hold warrants, you will not be entitled to any rights with respect to our common stock (including, without limitation, voting rights and rights to receive any dividends or other distributions on our common stock), but you will be subject to all changes affecting our common stock. You will have rights with respect to our common stock only if you receive our common stock upon exercise of the warrants and only as of the date when you become a record owner of the shares of our common stock upon such exercise. For example, if an amendment is proposed to our charter or bylaws requiring stockholder approval and the record date for determining the stockholders of record entitled to vote on the amendment occurs prior to the date you are deemed to be the owner of the shares of our common stock due upon exercise of your warrants, you will not be entitled to vote on the amendment, although you will nevertheless be subject to any changes in the powers, preferences or special rights of our common stock.
We can issue shares of preferred stock that may adversely affect the rights of holders of our common stock.
Our certificate of incorporation authorizes us to issue up to 10,000,000 shares of preferred stock with designations, rights, and preferences determined from time to time by our board of directors. Accordingly, our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting or other rights superior to those of holders of our common stock. For example, an issuance of shares of preferred stock could:
|
·
|
adversely affect the voting power of the holders of our common stock;
|
|
·
|
make it more difficult for a third party to gain control of us;
|
|
·
|
discourage bids for our common stock at a premium;
|
|
·
|
limit or eliminate any payments that the holders of our common stock could expect to receive upon our liquidation; or
|
|
·
|
otherwise adversely affect the market price or our common stock.
|
ITEM 2. Properties.
Our world-wide headquarters, occupying approximately 6,500 square feet and used for administrative, sales, marketing, research and development, and finance activities, is located in San Diego, California. The current arrangement ends on June 30, 2015 but the Company has the option to terminate the lease at the end of June 2013. The total future minimum obligation under this lease is $125,000 for 2011.
Our marketing office in Roswell, Georgia consists of approximately 468 square feet and is used for sales and marketing activities. Our current lease obligation ends on April 30, 2011 and the Company has the option to renew for another 12 month term. The future obligation for this lease is $4,000.
We believe that the San Diego, California and Roswell, Georgia facilities are suitable and adequate to support our current operations. We believe that if our existing facilities are not adequate to meet our business requirements long-term, additional space will be available on commercially reasonable terms.
ITEM 3. Legal Proceedings.
We are not aware of any material litigation involving us that is outstanding, threatened or pending.
ITEM 4. (Removed and Reserved).
ITEM 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market for Common Equity
Our common stock trades on the NASDAQ Global Market ("NASDAQ") under the symbol "TEAR" and the Toronto Stock Exchange ("TSX") under the symbol "TLB".
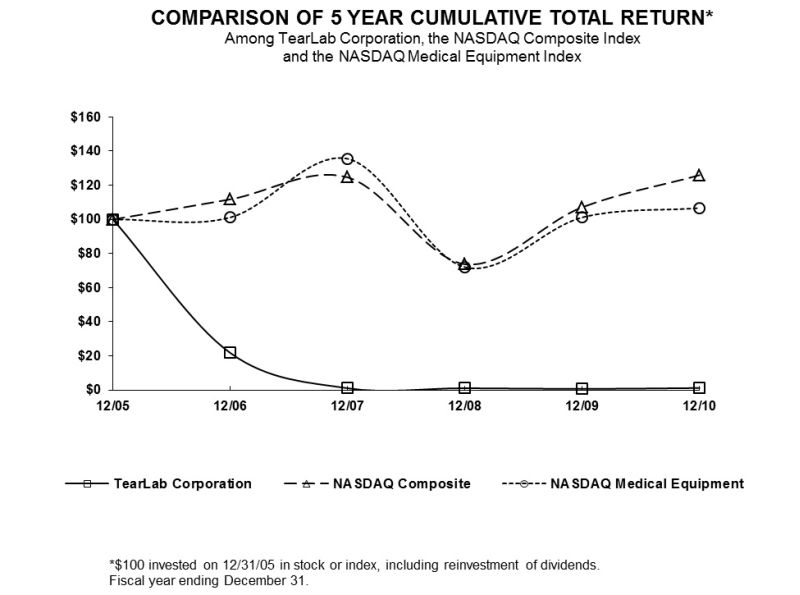
The following table sets forth the range of high and low sales prices per share of our common stock on both the NASDAQ and the TSX for the fiscal periods indicated.
|
Common Stock Prices
|
||||||||||||||||
|
Fiscal 2010
|
Fiscal 2009
|
|||||||||||||||
|
High
|
Low
|
High
|
Low
|
|||||||||||||
|
NASDAQ Capital Market
|
||||||||||||||||
|
First Quarter
|
$ | 5.14 | $ | 0.90 | $ | 3.24 | $ | 0.70 | ||||||||
|
Second Quarter
|
3.10 | 1.88 | 3.60 | 1.58 | ||||||||||||
|
Third Quarter
|
2.92 | 1.76 | 2.45 | 0.98 | ||||||||||||
|
Fourth Quarter
|
3.25 | 2.05 | 1.47 | 1.0075 | ||||||||||||
|
TSX
|
||||||||||||||||
|
First Quarter
|
C$ | 5.16 | C$ | 1.02 | C$ | 3.70 | C$ | 1.50 | ||||||||
|
Second Quarter
|
3.09 | 2.10 | 3.75 | 1.50 | ||||||||||||
|
Third Quarter
|
2.98 | 1.95 | 2.50 | 1.23 | ||||||||||||
|
Fourth Quarter
|
3.33 | 2.07 | 1.69 | 1.15 | ||||||||||||
The closing share price for our common stock on March 17, 2011 as reported by NASDAQ, was $1.60. The closing share price for our common stock on March 17, 2011, as reported by TSX was C$1.58.
As of March 15, 2011, there were approximately 116 stockholders of record of our common stock.
Dividend Policy
We have never declared or paid any cash dividends on shares of our capital stock. We currently intend to retain all available funds to support operations and to finance the growth and development of our business. Any determination related to payments of future dividends will be at the discretion of our board of directors after taking into account various factors that our board of directors deems relevant, including our financial condition, operating results, current and anticipated cash needs, plans for expansion and debt restrictions, if any.
Sales of Unregistered Securities
On January 8, 2010, we obtained commitments for the sale of 3,244,766 shares of our common stock for an aggregate of approximately $3,000,000 in a private placement, of which 1,886,291 shares were issued on January 8, 2010, with the remainder being issued on March 18, 2010, following stockholder approval for the sale of such additional shares. The per share price of the shares sold in the private placement was $0.92456, which is equal to 80% of the volume weighted average price of our common stock for the 10 trading days ending on the day immediately preceding the January 8, 2010 closing date.
Pursuant to the terms of an amended and restated agency agreement (the “Canadian Placement Agency Agreement”), dated as of January 8, 2010, by and between the Company and Marchant Securities Inc. (“Marchant”), Marchant acted as the Canadian placement agent in connection with the private placement. Under the Canadian Placement Agency Agreement, Marchant was issued a number of shares of our common stock as a placement fee equal to the quotient of : (A) 9% of the aggregate purchase price of shares sold at such closing to investors who are resident in Canada, and (B) the closing consolidated bid price per share on the NASDAQ Capital Market immediately prior to such closing. As a result, we issued a total of 101,548 shares of our common stock to Marchant in connection with the private placement as consideration for its services in connection therewith.
The shares were offered in the United States to “accredited investors” pursuant to the exemption from the registration requirements under the Securities Act of 1933, as amended (the “Securities Act”), afforded by Regulation D promulgated thereunder, in Canada to “accredited investors” in reliance on National Instrument 45-106 – Prospectus and Registration Exemptions and in Canada and other jurisdictions outside of the United States in reliance on Regulation S promulgated under the Securities Act.
Repurchases of Equity Securities
None.
Equity Compensation Plans Information
The information required by this item will be contained in an amendment to this Annual Report Form 10-K to be filed with the Securities and Exchange Commission not later than 120 days after the end of our fiscal year ended December 31, 2010.
Stock Performance Graph
The following graph compares the cumulative total stockholder return data for our common stock to the cumulative return of (i) the NASDAQ Composite Index and (ii) the NASDAQ Medical Equipment Index for the period beginning December 9, 2004, and ending on December 31, 2010. The graph assumes that $100 was invested on January 1, 2004, and assumes reinvestment of dividends. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
ITEM 6. Selected Financial Data.
The following selected financial data should be read in conjunction with our consolidated financial statements, the related notes thereto and the information contained in "Item 7 – Management's Discussion and Analysis of Financial Condition and Results of Operations".
|
Year Ended December 31,
|
||||||||||||||||||||
|
2006
|
2007
|
2008
|
2009
|
2010
|
||||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Consolidated Statements of Operations Data:
|
||||||||||||||||||||
|
Revenue
|
$ | 174 | $ | 92 | $ | 458 | $ | 869 | $ | 1,701 | ||||||||||
|
Cost of goods sold
|
3,529 | 2,398 | 163 | 568 | 849 | |||||||||||||||
|
Gross profit (loss)
|
(3,355 | ) | (2,306 | ) | 295 | 301 | 852 | |||||||||||||
|
Operating expenses
|
||||||||||||||||||||
|
Amortization of intangible assets
|
1,824 | 2,577 | 1,207 | 1,215 | 1,215 | |||||||||||||||
|
General and administrative
|
6,651 | 5,528 | 4,233 | 3,245 | 3753 | |||||||||||||||
|
Clinical, regulatory and research and development
|
4,922 | 8,675 | 2,965 | 1,121 | 1,365 | |||||||||||||||
|
Sales and marketing
|
1,625 | 1,413 | 820 | 646 | 1,463 | |||||||||||||||
|
Impairment of goodwill
|
65,946 | — | — | — | — | |||||||||||||||
|
Impairment of intangible asset
|
— | 20,923 | — | — | — | |||||||||||||||
|
Restructuring charges
|
820 | 1,313 | 2,441 | — | — | |||||||||||||||
|
Total operating expenses
|
81,788 | 40,429 | 11,666 | 6,227 | 7,796 | |||||||||||||||
|
Other income (expense)
|
1,547 | 2,769 | 1,665 | (362 | ) | 261 | ||||||||||||||
|
Loss from continuing operations before income taxes
|
(83,596 | ) | (39,966 | ) | (9,706 | ) | (6,288 | ) | (6,683 | ) | ||||||||||
|
Recovery of income taxes
|
2,916 | 5,566 | 338 | 1,903 | — | |||||||||||||||
|
Loss from continuing operations
|
(80,680 | ) | (34,400 | ) | (9,368 | ) | (4,385 | ) | (6,683 | ) | ||||||||||
|
Loss from discontinued operations
|
(1,542 | ) | (35,429 | ) | — | — | — | |||||||||||||
|
Net loss
|
$ | (82,222 | ) | $ | (69,829 | ) | $ | (9,368 | ) | $ | (4,385 | ) | $ | (6,683 | ) | |||||
|
Per Share Data:
|
||||||||||||||||||||
|
Loss from continuing operations per share — basic and diluted
|
$ | (44.84 | ) | $ | (15.19 | ) | $ | (2.29 | ) | $ | (0.44 | ) | $ | (0.47 | ) | |||||
|
Loss from discontinued operations per share — basic and diluted
|
(0.86 | ) | (15.64 | ) | — | — | — | |||||||||||||
|
Net loss per share — basic and diluted
|
$ | (45.70 | ) | $ | (30.83 | ) | $ | (2.29 | ) | $ | (0.44 | ) | $ | (0.47 | ) | |||||
|
Weighted average number of shares used in per share calculations — basic and diluted
|
1,799 | 2,265 | 4,084 | 9,855 | 14,098 | |||||||||||||||
|
As at December 31,
|
||||||||||||||||||||
|
2006
|
2007
|
2008
|
2009
|
2010
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Consolidated Balance Sheet Data:
|
||||||||||||||||||||
|
Cash and cash equivalents of continuing operations
|
$ | 5,741 | $ | 2,236 | $ | 2,565 | $ | 106 | $ | 2,726 | ||||||||||
|
Short-term investments
|
9,785 | — | — | — | — | |||||||||||||||
|
Working capital (deficiency) of continuing operations
|
13,539 | (997 | ) | 1,550 | (2,132 | ) | 420 | |||||||||||||
|
Total assets of continuing operations
|
98,525 | 15,313 | 13,405 | 9,733 | 11,535 | |||||||||||||||
|
Long-term debt (including current portion due to stockholders)
|
152 | 33 | 23 | 1,281 | 1,697 | |||||||||||||||
|
Other long-term obligations (including amount classified as current portion of other liability)
|
6,421 | — | — | ― | ― | |||||||||||||||
|
Total liabilities of continuing operations
|
31,247 | 6,358 | 3,446 | 2,976 | 3,658 | |||||||||||||||
|
Non-controlling interest
|
6,111 | 4,954 | ― | ― | ― | |||||||||||||||
|
Contingently redeemable stock
|
― | ― | 250 | — | ― | |||||||||||||||
|
Common stock
|
2 | 2 | 10 | 10 | 15 | |||||||||||||||
|
Additional paid-in capital
|
354,224 | 362,287 | 377,356 | 378,790 | 386,588 | |||||||||||||||
|
Accumulated deficit
|
(293,059 | ) | (358,289 | ) | (367,657 | ) | (372,043 | ) | (378,726 | ) | ||||||||||
|
Total stockholders' equity
|
61,167 | 4,000 | 9,709 | 6,757 | 7,877 | |||||||||||||||
ITEM 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes, included in Item 8 of this Report. Unless otherwise specified, all dollar amounts are U.S. dollars.
Overview
We are an in vitro diagnostic company that has developed a proprietary tear testing platform, the TearLab® Osmolarity System. The TearLab test measures tear film osmolarity for diagnosis of Dry Eye Disease, or DED. Tear osmolarity is a quantitative and highly specific biomarker that has been shown to correlate with DED. The TearLab test enables the rapid measurement of tear osmolarity in a doctor's office. We refer to the results of our TearLab testing platform as our Point-of-Care business division in the discussion of our operating results below. Commercializing our Point-of-Care tear testing platform is now the focus of our business.
In October 2008, the TearLab Osmolarity System received CE mark approval, clearing the way for sales in the European Union and all countries recognizing the CE mark. In connection with the CE mark clearance, we have entered into multi-year agreements with numerous distributors for distribution of the TearLab Osmolarity System. Currently, we have signed distribution agreements in each of the following countries: Spain, Portugal, Germany, France, Turkey, Ukraine, Bulgaria, Belgium, Netherlands, Switzerland, Finland, Sweden, South Korea, Australia, Russia, Hungary, Greece, Canada, Slovakia, Argentina, the Czech Republic and the United Kingdom and a sales representation agreement in Japan.
On May 19, 2009, we announced that we received 510(k) clearance from the U.S. Food and Drug Administration, (“FDA”). The 510(k) clearance allows us to market the TearLab Osmolarity System to those reference and physician operated laboratories with CLIA certifications allowing them to perform moderate and high complexity tests. Considering that most of our target customers currently are eye care practitioners without such certifications, we intend to seek a CLIA waiver from the FDA for the TearLab Osmolarity System as well as assisting our customers in obtaining their moderate complexity CLIA certification or providing them with support from certified professionals. A CLIA waiver would greatly reduce the regulatory compliance for our future customers. Until we receive the CLIA Waiver Certification, doctors using the TearLab Osmolarity System must be certified under themoderate complexity CLIA certification.
On March 4, 2011, the Company announced that it was in receipt of a communication from the U.S. Food and Drug Administration (“FDA”) indicating that the data submitted by the Company was not sufficient to gain approval of its CLIA Waiver categorization application for the TearLab® Osmolarity System. The Company is reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver. The Company will also continue to pursue it strategy to assist its customers in obtaining moderate complexity CLIA certificates.
On December 8, 2009 we announced that Health Canada issued a Medical Device License for the TearLab Osmolarity System. The Health Canada license allowed us to immediately begin marketing the system in Canada. On August 20, 2009, we entered into an agreement with a distributor, Science with Vision, for exclusive distribution of the TearLab Osmolarity System in Canada. We began selling products through the Canadian distributor in 2010.
On October 19, 2010 we announced that a unique new Current Procedural Terminology (“CPT”) code that will apply to the TearLab Osmolarity test has now been be published by the American Medical Association (“AMA”). The new code will become effective January 1, 2011. The new CPT code for the TearLab Osmolarity test is: 83861; Microfluidic analysis utilizing an integrated collection and analysis device, tear osmolarity (For microfluidic tear osmolarity of both eyes, report 83861 twice). This code falls under the Chemistry sub-section of the Pathology and Laboratory section of the CPT Codebook and will be listed under the 2010 Clinical Laboratory Fee Schedule by the Centers for Medicare and Medicaid Services (CMS). Reimbursement by CMS has been set at $24.01 per eye and will only be available for offices that have a Moderate Complex CLIA certificate until TearLab receives a CLIA Waiver categorization from the FDA. Under the 2011 Clinical Laboratory Fee Schedule listed by CMS the reimbursement rate was reduced to $23.58 and was applicable to a majority of states in the U.S.
Our success is highly dependent on our ability to increase sales of our testing platform in European and other countries recognizing the CE mark, in Canada where we have a Medical Device License and on our receipt of a CLIA waiver which will enable us to begin full commercialization efforts in the United States. Meeting these objectives requires that we have sufficient capital to fund our operations. Our working capital of $420,000 as of December 31, 2010 is insufficient to fund our planned operations and our ability to generate increased revenues is uncertain. If our revenues do not increase sufficiently to meet operational needs we would be required to raise additional capital to fund our operations. We have sufficient cash to fund our operations at current levels through approximately July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and only until December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011. We continue to actively evaluate and pursue various finacing possibilities at this time.
Recent Developments
On January 11, 2011 the Company announced that it and COLA, a private, non-profit clinical laboratory education, consultation and accreditation organization, had partnered to help make the TearLab® Osmolarity System available in the U.S. at the point-of-care. As previously announced, the TearLab Osmolarity test will be reimbursed by the CMS, effective January 1, 2011. Reimbursement will only be available, however, for offices that have a Moderate Complexity CLIA certificate until such time that TearLab receives a CLIA Waiver categorization from the FDA. TearLab’s customers are able to obtain their moderate complexity CLIA certification by working with COLA who will assist them in filing the required documentation, using the COLA on-line CME program and completing the required regulatory process. With the moderate complexity CLIA certification, our customers will be able to be reimbursed for performing the TearLab Osmolarity test on their patients.
On March 4, 2011, the Company announced that it had received communication from the FDA indicating that the data submitted by the Company did not meet the requirements to gain approval of its CLIA Waiver categorization application for the TearLab Osmolarity System. The Company is reviewing the comments made by the FDA and developing the necessary responses or strategies required to continue to pursue the CLIA waiver.
RESULTS OF OPERATIONS
Revenues, Cost of Goods Sold and Gross Margin
For the years ended December 31,
(in thousands)
|
2010
|
Change
|
2009
|
Change
|
2008
|
||||||||||||||||
|
Revenue
|
||||||||||||||||||||
|
TearLab
|
$ | 1,701 | 96 | % | $ | 869 | 190 | % | $ | 300 | ||||||||||
|
Retina
|
— | N/M | * | — | N/M | * | 158 | |||||||||||||
|
Total revenues
|
$ | 1,701 | 96 | % | $ | 869 | 90 | % | $ | 458 | ||||||||||
|
Cost of goods sold
|
||||||||||||||||||||
|
TearLab
|
$ | 849 | 49 | % | $ | 568 | 318 | % | $ | 136 | ||||||||||
|
Retina
|
— | N/M | * | — | N/M | * | 27 | |||||||||||||
|
Total cost of goods sold$ 163
|
$ | 849 | 49 | % | $ | 568 | 248 | % | $ | 163 | ||||||||||
|
Gross margin
|
$ | 852 | 183 | % | $ | 301 | 2 | % | $ | 295 | ||||||||||
|
Percentage of total revenues
|
50 | % | 35 | % | 64 | % | ||||||||||||||
*N/M – Not meaningful
Revenues
TearLab
TearLab revenue consists of sales of the TearLab® Osmolarity System, which is a hand-held tear film test for the measurement of tear osmolarity, a quantitative and highly specific biomarker that has shown to correlate with DED.
The TearLab Osmolarity System consists of the following three components: (1) the TearLab disposable, which is a single-use microfluidic labcard; (2) the TearLab pen, which is a hand-held device that interfaces with the TearLab disposable; and (3) the TearLab reader, which is a small desktop unit that allows for the docking of the TearLab disposable and the TearLab pen and provides a quantitative reading for the operator.
We are working with our established distributors in Europe and Asia to increase sales. The ability for re-imbursement to be obtained in many of those countries where we have distributors will facilitate our ability to increase sales and stimulate the commercialization process. We intend to expand our distribution network to include additional European, Asian and Latin American countries in the future. The Company is supporting these distributors in local clinical trials and in providing them with the required guidance to understand the relationship between DED and osmolarity and how to manage their patients with objective diagnostic data.
Having received 510(k) approval from the FDA in the United States, we have begun selling to customers in the United States who hold CLIA moderate and high complexity certificates and are actively supporting and assisting our customers to obtain their moderate complexity CLIA certificates or providing them with support from certified professionals. We are seeking to obtain a CLIA waiver certificate which will allow us to sell our product to the approximately 50,000 eye care practitioners in the United States that are candidates to operate under a CLIA waiver certification.
Having received a medical device license from Health Canada in December 2009, we have been selling the TearLab® Osmolarity Systems into Canada via a distributor in 2010 and have established a co-operative marketing agreement with AMO Canada to market our product and support the sale of AMO drugs related to DED in Canada..
We believe that it is important that eye care professionals in the United States develop a clinical understanding of the TearLab Osmolarity System. In December 2009, we put in place a program to facilitate this education process in which we provide customers with a free test card(s) for every card purchased to allow them to utilize these free cards to better understand its effectiveness and how it can improve their business practices. As a result, of this program, the average selling price per card decreased to reflect the free cards being provided.
During the years ended December 31, 2010, 2009 and 2008 the Company recognized revenue from the sale of TearLab equipment of $1,701,000, $869,000 and $300,000 respectively.
Retina
Retina revenue, which ended in 2008, consisted of revenue generated from the sale of components of the RHEO System which consists of (1) a Rheofilter filter, a Plasmaflo filter, and tubing which, together, comprise a disposable treatment set, and (2) an Octo Nova pump.
During the year ended December 31, 2008, the Company sold a total of 123 treatment sets on consignment resulting in revenue of $18,500. In addition, the Company sold 115 Octo Nova pumps resulting in revenues of $139,800 in the year ended December 31, 2008. All pumps and disposable treatment sets had previously been fully reserved in the fourth quarter of 2007 when we suspended all RHEO System-related activities.
On November 1, 2007, we announced an indefinite suspension of the RHEO System clinical development program for Dry AMD and are in the process of winding down all remaining RHEO related clinical studies. As a result, we did not generate any retina revenue for the years ended December 31, 2010 and 2009 and do not expect to generate revenue from RHEO related products in the future.
Cost of Goods Sold
TearLab
TearLab cost of goods sold includes costs of goods sold and royalty costs. Our cost of goods sold consists primarily of costs for the manufacture of the TearLab test, including the costs we incur for the purchase of component parts from our suppliers, applicable freight and shipping costs, fees related to warehousing and logistics inventory management. Also included in TearLab costs of goods sold for the year ended December 31, 2009 were inventory reserve costs for excess inventory of $56,000.
Retina
Cost of goods sold includes costs of goods sold and royalty costs. Our cost of goods sold typically consisted primarily of costs for the manufacture of the RHEO System, including the costs we incurred for the purchase of component parts from our suppliers, applicable freight and shipping costs, fees related to warehousing, logistics inventory management and recurring regulatory costs associated with conducting business and ISO certification.
Cost of goods sold for the year ended December 31, 2008 consisted of $1,500 of shipping in handling costs, $25,000 of royalty fees payable and did not contain any inventory charges since the inventory had already been fully written off in 2007.
Gross Margin
During fiscal 2010 as compared with fiscal 2009, our combined gross margin increased from 35% to 50% due to a 96% increase in sales over the prior year, as well as a charge to record a provision for excess TearLab inventory of $56,000 in 2009 that wasn’t repeated in the current year.
During fiscal 2009 as compared with fiscal 2008, our combined gross margin decreased 29%, largely impacted by the provision recorded for excess TearLab inventory of $56,000 in 2009 and the sale of Retina product in 2008 that had been previously written down to nil-value.
Operating Expenses
For the years ended December 31,
(in thousands)
|
2010
|
Change
|
2009
|
Change
|
2008
|
||||||||||||||||
|
Amortization of intangible assets
|
$ | 1,215 | 0 | % | $ | 1,215 | 1 | % | $ | 1,207 | ||||||||||
|
General and administrative
|
3,753 | 16 | % | $ | 3,245 | (23 | )% | $ | 4,233 | |||||||||||
|
Clinical, regulatory and research and development
|
1,365 | 22 | % | 1,121 | (62 | )% | 2,965 | |||||||||||||
|
Sales and marketing
|
1,463 | 126 | % | 646 | (21 | )% | 820 | |||||||||||||
|
Restructuring charges
|
— | N/M | * | — | N/M | * | 2,441 | |||||||||||||
|
Total operating expenses
|
$ | 7,796 | 25 | % | $ | 6,227 | (47 | )% | $ | 11,666 | ||||||||||
*N/M – Not meaningful
Amortization of Intangible Assets
Amortization expense of intangible assets remained relatively unchanged for the years ended December 31, 2010, 2009, and 2008, as there were no adjustments to the Company’s cost basis or estimated useful life of the underlying assets.
General and Administrative Expenses
General and administrative expenses increased by $508,000 or 16% during the year ended December 31, 2010, as compared with the corresponding period in fiscal 2009.
Non-cash stock option and director fees for named executives increased by $692,000 in the current year, offset by a $410,000 reduction in salary expense for executive compensation. Additionally, operations and overhead costs of the manufacturing oversight department increased by $135,000 over the prior year, along with increases in employee bonuses and professional services of $54,000 and $25,000 respectively, due to increased headcount and operational activities related to maintaining the TearLab commercial product.
General and administrative expenses decreased by $988,000 or 23% during the year ended December 31, 2009, as compared with the corresponding period in fiscal 2008.
Finance, tax, and compliance costs were reduced by $605,000 as part of the consolidation of operations and reductions in spending. This was offset by a $382,000 increase in operations and costs which related to commercialization efforts. Additionally legal, executive, and administration costs were reduced by $447,000, $259,000, and $56,000 respective due to reductions in departmental headcount and consolidation of operations.
We are continuing to focus our efforts on controlling costs by reviewing and improving upon our existing business processes and cost structure.
Clinical, Regulatory and Research and Development
Clinical, regulatory and research and development expenses increased by $244,000 or 22% during the year ended December 31, 2010, as compared with 2009. Costs related to product development and patent expense increased by $57,000 over the prior year period, along with a $160,000 increase in employee and consultant related costs primarily related to bonuses. Additionally, regulatory costs in support of the Company’s goal of obtaining CLIA waiver certification increased by $30,000 over the prior year.
Clinical, regulatory and research and development expenses decreased by $1,844,000 or 62% during the year ended December 31, 2009, as compared with the corresponding prior year period. Research and development expenditures specific to the TearLab product for the years ended December 31, 2009 and 2008 were $349,000 and $2,188,000, respectively. The decrease of $1,839,000 was reflective of the maturing stage of TearLab technological development in that the development of the TearLab® Osmolarity System ended at the end of September 2008 and moved to a production or commercial focus. This decrease was offset by a $144,000 increase in labor and development expenses for on-going engineering support. An additional decrease of $108,000 relates to the indefinite suspension of our RHEO™ System clinical development program. This was offset by an $11,000 increase in TearLab clinical trial expenses related to FDA 510K and CLIA certification. These achievements allowed the Company to move forward with clinical trials to attain the CE Mark in Europe and 510(k) approval from the FDA in the United States, in advance of commercialization but resulted in a reduced need for development activities. Lastly, clinical and regulatory overhead cost decreased by approximately $56,000 due to reduced departmental headcount and expenses.
Sales and Marketing Expenses
Sales and marketing expenses increased by $817,000 or 126% during the year ended December 31, 2010, as compared with the prior period in fiscal 2009 driven by the Company’s commercialization efforts in the Unithed States. The increase is due to $579,000 in sales and business development costs that were not present in the prior year. Additionally there was a net $156,000 increase in marketing advertising and travel expense, reflecting additional marketing production and trade show activity over the prior year period and a net increase of $82,000 in employee and consultant related costs over the prior year.
Sales and marketing expenses decreased by $174,000 or 21% during the year ended December 31, 2009, as compared with the prior period in fiscal 2008. The decrease in TearLab marketing costs was reflective of a reduction in advertising and trade show costs. These costs reductions were realized as a result of the Company needing to utilize a reduced level of financial resources for marketing purposes following the launch of the TearLab product in Europe, Asia, and North America.
The cornerstone of our sales and marketing strategy to date has been to increase awareness of our products among eye care professionals and, in particular, the key opinion leaders in the eye care professions. We assist key opinion leaders in performing clinical trials to generate increased data to provide an increased understanding in the use of the TearLab® Osmolarity System for diagnostic, treatment and monitoring of patients. Presently we are expanding our commercialization efforts in North America, Europe, and Asia. We will continue to develop and execute our conference and podium strategy to ensure visibility and evidence-based positioning of the TearLab Osmolarity System among eye care professionals.
Restructuring Charges
With the suspension of our RHEO System clinical development program, and the ongoing winding-down of the RHEO-AMD study, and our disposition of our glaucoma business operated under SOLX during the year ended December 31, 2007, we reduced our workforce considerably. In accordance with applicable accounting guidance, we recognized a total of $2,441,000 in restructuring charges for the year ended December 31, 2008. The restructuring charges consisted solely of severance and benefit costs related to the termination of a total of 13 employees at our Mississauga office. There were no new restructuring charges during the years ended December 31, 2010 and 2009. All amounts have been paid and all restructuring activities were completed under the restructuring plan before December 31, 2009 .
The table below details the activity affecting our restructuring liability during the two years ended December 31, 2009 and 2008.
|
2009
|
2008
|
|||||||
|
Accrued restructuring liability – beginning of period
|
$ | 13 | $ | 1,313 | ||||
|
Restructuring charges during the period
|
— | 2,441 | ||||||
|
Restructuring charges settled by issuance of stock options
|
— | (2,278 | ) | |||||
|
Foreign exchange adjustment
|
— | (78 | ) | |||||
|
Restructuring charges settled in cash
|
(13 | ) | (1,385 | ) | ||||
|
Accrued restructuring liability – end of period
|
$ | — | $ | 13 | ||||
Other Income (Expense), Net
For the years ended December 31,
(in thousands)
|
2010
|
Change
|
2009
|
Change
|
2008
|
||||||||||||||||
|
Interest income
|
$ | 21 | 600 | % | $ | 3 | (96 | )% | $ | 77 | ||||||||||
|
Changes in fair value of warrant obligation
|
701 | 1,198 | % | 54 | 193 | % | (58 | ) | ||||||||||||
|
Gain on sale of investments
|
— | N/M | * | — | N/M | * | 1,036 | |||||||||||||
|
Interest expense
|
(210 | ) | (126 | %) | (93 | ) | 71 | % | (318 | ) | ||||||||||
|
Amortization of financing costs and beneficial conversion features
|
(228 | ) | 25 | % | (303 | ) | 68 | % | (1,419 | ) | ||||||||||
|
Other income
|
(23 | ) | 0 | % | (23 | ) | (106 | )% | 369 | |||||||||||
|
Non-controlling interest
|
— | N/M | * | — | N/M | * | 1,978 | |||||||||||||
| $ | 261 | 172 | % | $ | (362 | ) | (122 | )% | $ | 1,665 | ||||||||||
*N/M – Not meaningful
Interest Income
Interest income consists of interest income earned on our cash, cash equivalent and investment positions. The fluctuations in interest income during years ended December 31, 2010, 2009 and 2008, are due to fluctuations in the Company’s average cash and investment balances and variations in average interest rates.
Changes in Fair Value of Warrant Obligation
On February 6, 2007, pursuant to securities purchase agreement, or the 2007 Securities Purchase Agreement, between the Company and certain institutional investors, the Company issued 131,497 warrants to these investors. The per share exercise price of these warrants is $46.25.
On March 18, 2010, the Company closed a registered direct financing in which it sold approximately 1,552,796 shares of its common stock and warrants to purchase approximately 621,118 shares of its common stock for gross proceeds of approximately $5,000,000. The exercise price of the warrants is $4.00 per share.
The Company accounts for all of its outstanding warrants in accordance with the provisions of FASB ASC Topic 815 Derivatives and Hedging. The FASB guidance requires every derivative instrument within its scope (including certain derivative instruments embedded in other contracts) to be recorded on the balance sheet as either an asset or liability measured at its fair value, with changes in the derivative's fair value recognized in earnings unless specific hedge accounting criteria are met. The estimated fair value was determined using the Black-Scholes option-pricing model. Based on US GAAP guidance applicable to derivatives, the Company determined that none of the outstanding warrants meet the criteria for classification as equity. Accordingly, the Company classified its outstanding warrants as current liabilities at December 31, 2010, 2009 and 2008.
In addition, ASC Topic 815 requires us to record the outstanding derivatives at fair value at the end of each reporting period resulting in an adjustment to the recorded liability of the derivative, with any gain or loss recorded in earnings of the applicable reporting period. We therefore estimated the aggregate fair value of outstanding warrants for the years ended December 31, 2010, 2009, and 2008 and determined them to be $39,000, $3,000, and $58,000, respectively. The changes in estimated fair values reported in other income (expense) for the years ended December 31, 2010, 2009, and 2008 are $701,000, $54,000, and ($58,000), respectively.
Gain on Sale/(Impairment) of Investments
At December 31, 2007, we had investments in the aggregate principal amount of $1,900,000 which consisted of investments in four separate asset-backed auction rate securities, or ARS, yielding an average return of 5.865% per annum. However, as a result of market conditions, all of these investments had failed to settle on their respective settlement dates. Based on discussions with our advisors and the lack of liquidity for ARS of this type, we concluded that the carrying value of these investments was higher than its fair value as of December 31, 2007. Accordingly, these ARS were recorded at their estimated fair value of $864,000. We considered this to be an other-than-temporary reduction in the value of these ARS. Accordingly, the impairment associated with these ARS of $1,036,000 was included as an impairment of investments in our consolidated statement of operations for the year ended December 31, 2007. In the fourth quarter of 2008, Credit Suisse purchased our outstanding ARS for an aggregate amount of $1,900,000. As a result of the realization of the original principal amount of the ARS, we recorded a gain of $1,036,000 in our 2008 consolidated statement of operations. There was no similar activity in the years ended December 31, 2010 and 2009.
Interest Expense
In the third quarter of 2009, the Company closed an agreement with certain investors whereby the investors agreed to provide financing (the “Financing”) to the Company through the purchase of convertible secured notes, in the aggregate amount of $1.75 million. The convertible secured notes (the “Notes”) evidencing the Financing, mature on the second anniversary of their issuance (“the Maturity Date”), bear interest at a rate of 12% per annum and are convertible into shares of the Company’s common stock upon the request of holders of 51% or more of the outstanding principal amount of the Notes at any time after August 31, 2009 and prior to the Maturity Date. Accrued interest as of December 31, 2010 and 2009 was $303,000 and $93,000, respectively, and is included in short-term liabilities.
Interest expense for the year ended December 31, 2008 consists of accrued interest on Bridge Loans that were converted into our common stock in October 2008.
Amortization of Finance Costs
The Convertible notes payable contains a beneficial conversion feature valued at $728,000 and the accretion of this amount is recorded as an expense over the two year life of the convertible secured notes using the effective interest method. For the years ended December 31, 2010 and 2009, the amortization expense was $177,000 and $237,000, respectively. No similar expense was reported for the year ended December 31, 2008.
Investors in the Financing will receive warrants at an exercise price of $1.60 upon conversion of the convertible secured notes. The Company has valued these warrants using the Black-Scholes value model at a value of $163,000. The accretion of this amount is recorded as an expense over the two year life of the convertible secured notes. For the years ended December 31, 2010 and 2009, the amortization expense was is $41,000 and $53,000, respectively. No similar expense was reported for the year ended December 31, 2008.
Issuance costs of the Financing totaled $87,000 and have been allocated to cost of equity and to deferred finance charges. The amortization of the deferred finance charges as an expense over the two year life of the convertible secured notes for the years ended December 31, 2010 and 2009 is $10,000 and $14,000, respectively. No similar expense was recorded for the year ended December 31, 2008.
Amortization of finance costs for the year ended December 31, 2008 consists of $180,000 paid to Marchant Securities Inc., or Marchant, a related party, for introducing us to the bridge loan lenders who participated in the February 19, 2008 bridge financing. The finance costs had been fully expensed at the time of the conversion of the bridge loans in October 2008.
In 2008, under the terms of certain bridge loans, the conversion price was set at $2.125 per share, a 15% discount to the $2.50 per share paid by PIPE investors. Accordingly, following FASB guidance, we recorded a beneficial conversion on the sale of common stock of $1,239,000 in October 2008, which is equal to the number of shares of common stock issued upon conversion of the Bridge Loans multiplied by the difference between the estimated fair value of the common stock and the bridge loan conversion price per share. The beneficial conversion resulted in a charge to the statement of operations and is included in additional paid-in capital in the same amount.
Other Income (Expense)
Other income for the year ended December 31, 2010 consists primarily of foreign exchange loss of $34,000 due to exchange rate fluctuations on our foreign currency transactions, offset by a gain totaling $7,000 for the disposal of fixed assets.
Other income for the year ended December 31, 2009 consists primarily of a charge of $26,000 for the write-off of retainer fees.
Other income for the year ended December 31, 2008 consists primarily of foreign exchange gain of $325,000 due to exchange rate fluctuations on our foreign currency transactions and a one-time non-recurring income of $47,000 resulting from the termination of RHEO activities.
Non-controlling Interest
On October 6, 2008 we acquired the remaining ownership interest of TearLab Research, Inc. in exchange for the issuance of 3,169,938 shares of common stock. Subsequent to October 6, 2008, no further amounts were reported as non-controlling interest.
Recovery of Income Taxes
For the years ended December 31,
(in thousands)
|
2010
|
Change
|
2009
|
Change
|
2008
|
||||||||||||||||
|
Recovery of income taxes for continuing operations
|
$ | — | 100 | % | $ | 1,903 | 463 | % | $ | 338 | ||||||||||
Pursuant to Internal Revenue Code Section 382 and 383, the annual use of the net operating loss carryforwards could be limited by any greater than 50% ownership change during any three-year testing period. As a result of any such ownership change, portions of the Company’s net operating loss carryforwards are subject to annual limitations. The Company believes an ownership change occurred in 2008 and the annual limitations on net operating loss carryforwards will materially impact the future utilization of such carryforwards and will expire unused. Accordingly, the Company has removed the net operating loss carryforwards generated prior to the ownership change from their deferred tax assets accompanied by a corresponding reduction of the valuation allowance.
The recovery of income taxes decreased by $1,903,000 in the year ended December 31, 2010 to $0 from $1,903,000 in the year ended December 31, 2009.
This decrease in the year ended December 31, 2010 arose because:
|
|
·
|
In the year ended December 31, 2009 tax losses benefitted were $1,322,000, while in the year ended December 31, 2010 tax losses were no longer benefitted as tax losses benefitted fully offset deferred tax liability balances.
|
|
|
·
|
In the year ended December 31, 2009, amortization of deferred tax liabilities of $581,000 was recorded as a recovery of income taxes while in 2010, all amortization of deferred tax liabilities (i.e. $557,000) was completely offset by the reversal of previously benefitted tax losses. Tax losses benefitted in prior years were reversed to ensure that cumulative tax losses benefitted did not exceed cumulative deferred tax liability balances.
|
The recovery of income taxes increased by $1,565,000 in the year ended December 31, 2009 to $1,903,000 from $338,000 in the year ended December 31, 2008.
This increase in the year ended December 31, 2009 arose because:
|
|
·
|
In the year ended December 31, 2008, issuance of shares in association with the PIPE, conversion of Bridge Loans and acquisition of the non-controlling shareholding of TearLab Research, Inc., resulted in a change of control for tax purposes and a Section 382 restriction on the amount of tax losses prior to these transactions that can be recovered. As a result of the Section 382 restrictions, a net reversal of benefits (i.e. a tax expense) of $2,305,000 occurred in the year ended December 31, 2008. There was no reversal of benefits reported in the year ended December 31, 2009.
|
|
|
·
|
In the year ended December 31, 2009 tax losses benefitted decreased by $838,000 to $1,322,000 in the year ended December 31, 2009 from $2,160,000 in the year ended December 31, 2008. Tax losses were no longer benefitted during the year ended December 31, 2009 once tax losses benefitted had fully offset deferred tax liabilities in the year.
|
|
|
·
|
In the year ended December 31, 2009, amortization of deferred tax liabilities increased by $98,000 as compared to the year ended December 31, 2008.
|
To date, we have recognized income tax benefits in the aggregate amount of $3.0 million net of any reversals associated with the recognition of the deferred tax asset from the availability of net operating losses in the United States which may be utilized to reduce taxes in future years. The benefits associated with the balance of the net operating losses are subject to a full valuation allowance since it is not more likely than not that these losses can be utilized in future years.
Recovery of income taxes for the years ended December 31, 2010 and 2009 also includes the amortization of the deferred tax liability of $557,000 and $581,000, respectively, which was recorded based on the a) the difference between the fair value of intangible asset acquired upon the acquisition of a majority interest in TearLab Research, Inc. on November 30, 2006 and its tax base. This difference produces a deferred tax liability totaling $4,834,000 arising from intangible assets being amortized over a period of approximately 10 years, the estimated useful life of the intangible asset, and b) an additional deferred tax liability arose in 2009 totaling $291,000, which is related to the beneficial conversion feature associated with the notes payable and is being amortized over the two year life of the short-term liabilities.
Liquidity and Capital Resources
|
As at December 31,
($ in thousands)
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash and cash equivalents
|
$ | 2,726 | $ | 106 | ||||
|
Percentage of total assets
|
24 | % | 1 | % | ||||
|
Working capital (deficiency)
|
$ | 420 | $ | (2,132 | ) | |||
Financial Condition
Management believes that we have sufficient cash to fund our operations at current levels through approximately July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and only until December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011.
Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties. Actual results could vary as a result of a number of factors. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. Our future funding requirements will depend on many factors, including but not limited to:
|
|
·
|
the cost and results, and the rate of progress, of the clinical trials of the TearLab® Osmolarity System that will be required to support TearLab, Inc.'s application to obtain a CLIA waiver from the FDA;
|
|
|
·
|
TearLab, Inc.'s ability to obtain a CLIA waiver from the FDA for the TearLab Osmolarity System and the timing of such approval, if any;
|
|
|
·
|
whether government and third-party payers agree to reimburse the TearLab Osmolarity System;
|
|
|
·
|
whether eye care professionals engage in the process of obtaining their moderate complex CLIA certification;
|
|
|
·
|
the costs and timing of building the infrastructure to market and sell the TearLab Osmolarity System;
|
|
|
·
|
the cost and results of continuing development of TearLab Corp.'s TearLab Osmolarity System;
|
|
|
·
|
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and
|
|
|
·
|
the effect of competing technological and market developments.
|
At the present time, our only product is the TearLab Osmolarity System, and although we have received 510(k) approval from the FDA it only allows limited commercialization of our product in the United States until we receive a CLIA waiver approval from the FDA. At this time, we do not know when we can expect to begin to generate significant revenues from the TearLab Osmolarity System in the United States.
We will need additional capital in approximately July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and approximately December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011 and our prospects for obtaining that capital are uncertain. Additional capital may not be available on terms favorable to us, or at all. In addition, future financings could result in significant dilution of existing stockholders. However, unless we succeed in raising additional capital, we anticipate that we will be unable to continue our operations beyond approximately July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and approximately December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011.
On October 6, 2008, we raised $2,173,000 in a private placement, or PIPE, transaction in which we issued 869,200 of our common shares at a per share price of $2.50.
During 2008, we entered into a series of bridge loan transactions that resulted in the receipt of an aggregate of $6,704,000. The bridge loans accrued interest at 12% per annum through the date of their conversion. On October 6, 2008, the $6,704,000 of outstanding bridge loan's principal and $318,000 of accrued interest converted into 3,304,511 shares of our common stock. Under the terms of the bridge loans, the conversion price was set at $2.125 per share, a 15% discount to the $2.50 per share paid by the PIPE investors.
In July and August 2009, we raised $1,750,000 in convertible debt funding transactions. The convertible debt accrue interest at 12% per annum through the date of their conversion. On December 31, 2009 $93,248 of accrued interest was earned. Under the terms of the convertible debt , the conversion price has been set at $1.32 per share, a 20% discount to the $1.65 per share market price at July 15, 2009 and upon conversion 1,327,212 shares of our common stock will be issued. Upon conversion of the convertible debt, 109,389 warrants with an exercise price of $1.60 each will be issued to investors in the convertible debt.
On January 8, 2010, we raised $1,744,000 in the first tranche of a private placement transaction in which 1,886,291 shares of common stock were issued at a share price of $0.92456. On March 19, 2010, we raised an additional $1,256,000 in the second tranche of the private placement transaction in which 1,358,475 shares of common stock were issued at a share price of $0.92456 as these funds were in escrow prior to the first closing on January 8, 2010.
Additionally on March 18, 2010, we closed a registered direct financing in which 1,552,795 shares of common stock and warrants to purchase 621,118 shares of common stock for gross proceeds of approximately $5,000,000 were purchased. The investors purchased the shares and warrants for $3.22 per unit (each unit consisting of one share and a warrant to purchase 0.4 shares of common stock). The exercise price of the warrants is $4.00 per share. The warrants are exercisable at any time on or after the sixth-month anniversary of the closing date through and until the 18-month anniversary of the closing of the offering.
Ongoing Sources and Uses of Cash
We anticipate that our cash and cash equivalents and cash generated from increased revenues, will be sufficient to sustain our operations until approximately July 2011 if our convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and approximately December 2011 if our convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011. We continually evaluate various financing possibilities but we typically expect our primary sources of cash will be related to the collection of accounts receivable and, to a lesser degree, interest income on our cash and investment balances. Our accounts receivable collections will be impacted by our ability to grow our point-of-care revenue.
We expect our primary uses of cash will be to fund our operating expenses and pursuing and maintaining our patents and trademarks. In addition, dependent on available funds, we expect to expend cash to improve production capability of the TearLab test, to further improve the performance of the TearLab test, and to pursue additional applications for the lab-on-a-chip technology.
Changes in Cash Flows
Years ended December 31,
(in thousands)
|
2010
|
Change
|
2009
|
Change
|
2008
|
||||||||||||||||
|
Cash used in operating activities
|
$ | (4,540 | ) | $ | (442 | ) | $ | (4,098 | ) | $ | 5,337 | $ | (9,435 | ) | ||||||
|
Cash (used in) provide by investing activities
|
(55 | ) | (31 | ) | (24 | ) | (1,638 | ) | 1,614 | |||||||||||
|
Cash provided by financing activities
|
7,215 | 5,552 | 1,663 | (6,487 | ) | 8,150 | ||||||||||||||
|
Net increase (decrease) in cash and cash equivalents during the year
|
$ | 2,620 | $ | 5,079 | $ | (2,459 | ) | $ | (2,788 | ) | $ | 329 | ||||||||
Cash Used in Operating Activities
Net cash used to fund our operating activities during the year ended December 31, 2010 was $4,540,000 which is lower than our loss from continuing operations of $6,683,000 primarily due to non-cash charges. The non-cash charges which comprise a portion of the net loss during that period consist primarily of the amortization of intangible assets, fixed assets, patents and trademarks, accrued interest on the convertible debt funding, amortization of deferred financing charges, warrants & beneficial conversion values and stock-based compensation expense in the aggregate total of $3,147,000 offset partially by income arising from the revaluation of obligation under warrants and a gain on disposal of fixed assets in the aggregate total of $704,000.
Net cash used to fund our operating activities during the year ended December 31, 2009 was $4,098,000 which approximates our loss from continuing operations of $4,385,000. The non-cash charges which comprise a portion of the net loss during that period consist primarily of the amortization of intangible assets, fixed assets, patents and trademarks, accrued interest on the convertible debt funding, amortization of deferred financing charges, warrants & beneficial conversion values and stock-based compensation expense in the aggregate total of $2,250,000 offset partially by income arising from the revaluation of obligation under warrants, deferred tax benefits and a gain on disposal of fixed assets in the aggregate total of $1,961,000.
The net change in non-cash working capital and non-current asset balances related to operations for the years ended December 31, 2010, 2009 and 2008 consists of the following (in thousands):
|
2010
|
2009
|
2008
|
||||||||||
|
Accounts receivable
|
(163 | ) | 184 | 42 | ||||||||
|
Due from related parties
|
(130 | ) | — | — | ||||||||
|
Inventory
|
(359 | ) | (48 | ) | (148 | ) | ||||||
|
Prepaid expenses
|
16 | (22 | ) | 165 | ||||||||
|
Other current assets
|
17 | (9 | ) | (11 | ) | |||||||
|
Other non-current assets
|
100 | (170 | ) | — | ||||||||
|
Accounts payable
|
(95 | ) | 133 | (878 | ) | |||||||
|
Accrued liabilities
|
347 | 2 | (1,672 | ) | ||||||||
|
Deferred revenue
|
(23 | ) | (87 | ) | 237 | |||||||
|
Due to stockholders
|
(10 | ) | 15 | (10 | ) | |||||||
| $ | (300 | ) | $ | (2 | ) | $ | (2,275 | ) | ||||
Explanations of the more significant net changes in working capital and non-current asset balances are as follows:
|
|
·
|
Accounts receivable increased in 2010 as a result of increased revenues in 2010 related to sales of the TearLab Osmolarity System while improved collections of outstanding receivables in 2009 resulted in a decrease from 2008 to 2009.
|
|
|
·
|
Inventory increased in each of 2010, 2009 and 2008 primarily due to the increase in commercialization of the TearLab product arising from receiving health Canada approval allowing commercialization in Canada and receipt of the 510(k) approval from the FDA in the United States in May 2009 allowing limited commercialization in the United States.
|
|
|
·
|
Non-current assets decreased from 2010 to 2009 reflecting 2009 expenditures incurred related to 2010 fundraising transactions being applied in 2010 and as a result of non-current assets becoming current.
|
|
|
·
|
Accounts payable movement year over year is primarily due to the timing of payment cycles and the increase in commercial activities.
|
|
|
·
|
Accrued liabilities have increased in 2010 primarily as a result of increased accruals for amounts due to employees and increased levels of operations in line with increased commercialization efforts, while 2009 accured liability levels were consistent with 2008.
|
|
|
·
|
Decrease in deferred revenue from 2008 to 2009 and again in 2010 arose from the amortization of licensing fees and from the shipment of product previously paid for.
|
Cash Provided by (used in) Investing Activities
Net cash used in investing activities for the year ended December 31, 2010 and 2009 was $55,000 and $24,000, respectively, used to acquire fixed assets, net of disposals.
Net cash provided by investing activities for the year ended December 31, 2008 is $1,614,000 and consists of the net sale of short-term investments of $1,900,000 offset by $133,000 used to acquire fixed assets and $153,000 used to protect and maintain patents and trademarks.
Cash Provided by Financing Activities
Net cash provided by financing activities for the year ended December 31, 2010 was $7,215,000 and is made up primarily of $3,000,000 raised during 2010 from the issuance of common stock in a PIPE transaction and the $5,000,000 raised from the issuance of common stock in a registered direct fundraising, offset by $807,000 in financing fees and fees for services related to the PIPE and registered direct transactions. In addition, there was $22,000 in funds related to the exercise of options.
Net cash provided by financing activities for the year ended December 31, 2009 was $1,663,000 and is made up of $1,750,000 raised during 2009 from the issuance of convertible debt securities, offset by $87,000 in financing fees to the convertible debt financing.
Net cash provided by financing activities for the year ended December 31, 2008 was $8,150,000 and is made up of $6,704,000 raised during 2008 from the issuance of bridge loans (converted to common stock in October 2008) and $2,173,000 from the sale of common stock in a PIPE transaction, offset by $269,000 in financing fees and fees for services related to the bridge loans and $458,000 in share issuance costs related to the PIPE, bridge loans and acquisition of the non-controlling shareholding of TearLab, Inc. transactions.
Borrowings
As of December 31, 2010, we had a balance of $1,669,000 in convertible notes payable and accrued interest arising from an agreement with investors in July and August of 2009, (as described in Note 8 in the Notes to the financial statements). The convertible notes payable and accrued interest mature in the third quarter of 2011. There are no other outstanding long-term debt or balances available under credit agreements.
Contractual Obligations and Contingencies
The following table summarizes our contractual commitments as of December 31, 2010 and the effect those commitments are expected to have on liquidity and cash flow in future periods.
|
Payments Due by Period
|
||||||||||||||||
|
Total
|
Less than
1 year
|
1 to 3
years
|
More than
3 years
|
|||||||||||||
|
Operating leases
|
$ | 577 | $ | 130 | $ | 383 | $ | 64 | ||||||||
|
Royalty payments
|
1,190 | 70 | 210 | 910 | ||||||||||||
|
Total
|
$ | 1,767 | $ | 200 | $ | 593 | $ | 974 | ||||||||
If the $1,669,000 convertible notes payable and accrued interest at December 31, 2010 is not converted to common stock in the third quarter of 2011, then the liability will have to be satisfied in cash and will significantly impact our liquidity and cash flow in 2011.
Off-Balance-Sheet Arrangements
As of December 31, 2010, we did not have any material off-balance-sheet arrangements as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our audited consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amount of assets, liabilities, sales and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to our intangible assets, uncollectible receivables, inventories, debt and equity instruments and stock-based compensation. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Because this can vary in each situation, actual results may differ from these estimates under different assumptions or conditions.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our audited consolidated financial statements.
Revenue Recognition
We recognize revenue when all four of the following criteria are met: (i) persuasive evidence that an arrangement exists; (ii) delivery of the products has occurred; (iii) the selling price is fixed or determinable; and (iv) collectability is reasonably assured. Amounts received in excess of revenue recognizable are deferred.
Subsequent to its launch in the fourth quarter of 2008, the Company’s revenues have been derived from sales of the TearLab Osmolarity System for DED which consists of hardware and related disposables. The Company’s sales are currently to countries in North America, Europe, and Asia, and are generally transacted through distributors. The Company records revenue when all of its obligations are completed which is generally upon shipment of the Company’s products
Accounts receivable and allowance for doubtful accounts
The Company’s accounts receivable consist primarily of trade receivables from customers and are generally unsecured and due within 30 days. The Company evaluates the collectability of its accounts receivable based on a combination of factors. Expected credit losses related to trade receivables are recorded as an allowance for doubtful accounts in the Consolidated Balance Sheets as of December 31, 2010 and 2009. The allowance for doubtful accounts is charged to sales and marketing expense and accounts receivable are written off as uncollectible and deducted from the allowance after appropriate collection efforts have been exhausted. The carrying value of accounts receivable approximates their fair value due to their short term nature. To date, charges for bad debt expense have not been material.
Inventory Valuation
Inventory is recorded at the lower of cost (based on first in, first out basis) or market and consists of finished goods. Inventory is periodically reviewed for evidence of slow-moving or obsolete items, and the estimated reserve is based on management's reviews of inventories on hand, compared to estimated future usage and sales, reviewing product shelf-life, and assumptions about the likelihood of obsolescence.
As of December 31, 2010, 2009 and 2008, we had inventory reserves of $116,000, $124,000 and $68,000, respectively. During the years ended December 31, 2010, 2009 and 2008, we recognized a provision for excess and obsolete inventory of $0, $56,000 and $68,000, respectively, and wrote-off $8,000, $0 and $7,296,000, respectively.
Valuation of Intangible and other Long-lived Assets.
We periodically assess the carrying value of intangible and other long-lived assets, which requires us to make assumptions and judgments regarding the future cash flows of these assets. The assets are considered to be impaired if we determine that the carrying value may not be recoverable based upon our assessment of the following events or changes in circumstances:
|
|
·
|
the asset's ability to continue to generate income from operations and positive cash flow in future periods;
|
|
|
·
|
loss of legal ownership or title to the asset;
|
|
|
·
|
significant changes in our strategic business objectives and utilization of the asset(s); and
|
|
|
·
|
the impact of significant negative industry or economic trends.
|
If the assets are considered to be impaired, the impairment we recognize is the amount by which the carrying value of the assets exceeds the fair value of the assets. Fair value is determined by a combination of third party sources and discontinued cash flows. In addition, we base the useful lives and related amortization or depreciation expense on our estimate of the period that the assets will generate revenues or otherwise be used by us. We also periodically review the lives assigned to our intangible assets to ensure that our initial estimates do not exceed any revised estimated periods from which we expect to realize cash flows from the technologies. If a change were to occur in any of the above-mentioned factors or estimates, the likelihood of a material change in our reported results would increase.
At December 31, 2010, the net book value of identifiable intangible assets that are subject to amortization totaled $7,139,000 and the net book value of fixed assets totaled $126,000.
We determined that, as of December 31, 2010 and 2009, there have been no significant events which may have affected the carrying value of TearLab technology. However, our prior history of losses and losses incurred during the current fiscal year reflects a potential indication of impairment, thus requiring management to assess whether TearLab Research, Inc.'s technology was impaired as of December 31, 2010 and 2009. Based on management's estimates of forecasted undiscounted cash flows as of December 31, 2010 and 2009, we concluded that there was no indication of an impairment of the TearLab technology in either fiscal period.
Stock-based Compensation
We use the fair value method to account for share-based payments in accordance with the authoritative guidance for stock compensation. The fair value of each option award is estimated on the date of grant using a Black-Scholes-Merton option pricing model, or Black-Scholes model, that uses assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, our expected stock price volatility, actual and projected employee stock option exercise behaviors, risk-free interest rate and expected dividends. Expected volatilities are based on the historical volatility of our common stock. The expected term of options granted is based on analyses of historical employee termination rates and option exercises. The risk-free interest rates are based on the U.S. Treasury yield in effect at the time of the grant. Since we do not expect to pay dividends on our common stock in the foreseeable future, we estimated the dividend yield to be 0%. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We estimate pre-vesting forfeitures based on our historical experience.
If factors change and we employ different assumptions for determination of fair value in future periods, the share-based compensation expense that we record may differ significantly from what we have recorded in the current period. There is a high degree of subjectivity involved when using option pricing models to estimate share-based compensation. Certain share-based payments, such as employee stock options, may expire worthless or otherwise result in zero intrinsic value as compared to the fair values originally estimated on the grant date and reported in our consolidated financial statements. Alternatively, values may be realized from these instruments that are significantly in excess of the fair values originally estimated on the grant date and reported in our consolidated financial statements. There is currently no market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of employee share-based awards is determined in accordance with authoritative guidance on stock compensation using an option-pricing model, that value may not be indicative of the fair value observed in a willing buyer/willing seller market transaction.
As of December 31, 2010, $1.6 million of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of 1.74 years.
Income Taxes
We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the consolidated financial statements from such a position should be measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The completion of the reorganization transactions on October 6, 2008 makes it more likely than not that we incurred a change of control for purposes of Section 382 for US Income Taxes. (See Note 7 Income Taxes in the notes to the financial statements.) Rules under Section 382 of the U.S. Internal Revenue Code substantially reduce our ability to utilize prior tax losses.
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the expected future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. We measure deferred tax assets and liabilities using the enacted tax rates expected to apply to taxable income in the years in which we expect to recover or settle those temporary differences. We recognize the effect of a change in tax rates on deferred tax assets and liabilities in income in the period that includes the enactment date. A valuation allowance is established when it is "more likely than not" the future realization of all or some of the deferred tax assets will not be achieved. For further explanation of our provision for income taxes, see Note 7, “Income Taxes”.
For information on the recent accounting pronouncements impacting our business, see Note 2 of the Notes to Consolidated Financial Statements included in Item 8.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk.
Currency Fluctuations and Exchange Risk
All of our sales are in U.S. dollars, while a minor portion of our expenses are in Canadian dollars and Australian dollars. We cannot predict any future trends in the exchange rate of the Canadian dollar or Australian dollar against the U.S. dollar. Any strengthening of the Canadian dollar or Australian dollar in relation to the U.S. dollar would increase the U.S. dollar cost of our operations, and affect our U.S. dollar measured results of operations. We maintain bank accounts in both Canadian dollars and Australian dollars to meet short term operational operating requirements. Based on the balances in the Canadian dollar and Australian dollar denominated bank accounts at December 31, 2010, hypothetical increases of $0.01 in the value of the Canadian dollar and the Australian dollar in relation to the U.S. dollar would have minimal impact on the results of our operations. We do not engage in any hedging or other transactions intended to manage these risks. In the future, we may undertake hedging or other similar transactions or invest in market risk sensitive instruments if we determine that is advisable to offset these risks.
Interest Rate Risk
The primary objective of our investment activity is to preserve principal while maximizing interest income we receive from our investments, without increasing risk. We believe this will minimize our market risk. We do not use interest rate derivative transactions to manage our interest rate risk. We reduce our exposure to interest rate risk by investing in investment grade securities or money market accounts. Declines in interest rates over an extended period of time will reduce our interest income while an increase over an extended period of time will increase our interest income. A reduction of interest rate by 100 basis points over the year ended 2010 would reduce interest income by under $1,000.
ITEM 8. Financial Statements and Supplementary Data.
Consolidated Financial Statements
REPORT OF INDEPENDENT REGISTERED
PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of TearLab Corp.
We have audited the accompanying consolidated balance sheets of TearLab Corporation and its subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of operations, stockholders' equity, and cash flows for each of the three years in the period ended December 31, 2010. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of TearLab Corporation and its subsidiaries at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects the information set forth therein.
The accompanying financial statements have been prepared assuming that TearLab Corporation will continue as a going concern. As more fully described in Note 1, the Company's recurring losses from operations and level of working capital available to fund operations raise substantial doubt about its ability to continue as a going concern. Management’s plans as to these matters also are described in Note 1. The December 31, 2010 consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| /s/ Ernst & Young LLP |
San Diego, California
March 29, 2011
CONSOLIDATED BALANCE SHEETS
(expressed in thousands except number of shares)
|
As of December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$ | 2,726 | $ | 106 | ||||
|
Accounts receivable
|
312 | 149 | ||||||
|
Due from related parties
|
130 | — | ||||||
|
Inventory.
|
555 | 196 | ||||||
|
Prepaid expenses
|
322 | 338 | ||||||
|
Other current assets
|
33 | 55 | ||||||
|
Total current assets
|
4,078 | 844 | ||||||
|
Fixed assets, net
|
126 | 140 | ||||||
|
Patents and trademarks, net
|
192 | 220 | ||||||
|
Other non-current assets
|
— | 176 | ||||||
|
Intangible assets, net
|
7,139 | 8,353 | ||||||
|
Total assets
|
$ | 11,535 | $ | 9,733 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$ | 353 | $ | 448 | ||||
|
Accrued liabilities
|
1,441 | 1,093 | ||||||
|
Due to stockholders
|
28 | 38 | ||||||
|
Deferred revenue
|
128 | 151 | ||||||
|
Obligations under warrants
|
39 | 3 | ||||||
|
Convertible notes payable and accrued interest
|
1,669 | 1,243 | ||||||
|
Total current liabilities
|
3,658 | 2,976 | ||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders' equity
|
||||||||
|
Preferred Stock, $0.001 par value, 10,000,000 authorized, none outstanding
|
— | — | ||||||
|
Common Stock, $0.001 par value, 40,000,000 authorized, 14,775,366 and 9,866,685 issued and outstanding at December 31, 2010 and 2009, respectively
|
15 | 10 | ||||||
|
Additional paid-in capital
|
386,588 | 378,790 | ||||||
|
Accumulated deficit
|
(378,726 | ) | (372,043 | ) | ||||
|
Total stockholders' equity
|
7,877 | 6,757 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 11,535 | $ | 9,733 | ||||
TearLab Corp.
CONSOLIDATED STATEMENTS OF OPERATIONS
(expressed in thousands except number of shares)
|
Years ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Revenue
|
||||||||||||
|
TearLab
|
$ | 1,701 | $ | 869 | $ | 300 | ||||||
|
Retina
|
— | — | 158 | |||||||||
|
Total revenue
|
1,701 | 869 | 458 | |||||||||
|
Cost of goods sold
|
||||||||||||
|
TearLab - product cost
|
849 | 568 | 137 | |||||||||
|
Retina - product cost
|
— | — | 26 | |||||||||
|
Total cost of goods sold (excluding amortization of intangibles)
|
849 | 568 | 163 | |||||||||
|
Gross profit
|
852 | 301 | 295 | |||||||||
|
Operating expenses
|
||||||||||||
|
Amortization of intangibles assets
|
1,215 | 1,215 | 1,207 | |||||||||
|
General and administrative
|
3,753 | 3,245 | 4,233 | |||||||||
|
Clinical, regulatory and research & development
|
1,365 | 1,121 | 2,965 | |||||||||
|
Sales and marketing
|
1,463 | 646 | 820 | |||||||||
|
Restructuring charges
|
— | — | 2,441 | |||||||||
|
Total operating expenses
|
7,796 | 6,227 | 11,666 | |||||||||
|
Loss from operations
|
(6,944 | ) | (5,926 | ) | (11,371 | ) | ||||||
|
Other income (expenses)
|
||||||||||||
|
Interest income
|
21 | 3 | 77 | |||||||||
|
Changes in fair value of warrant obligations
|
701 | 54 | (58 | ) | ||||||||
|
Gain on investments
|
— | — | 1,036 | |||||||||
|
Interest expense
|
(210 | ) | (93 | ) | (318 | ) | ||||||
|
Amortization of deferred financing charges, warrants & beneficial conversion values
|
(228 | ) | (303 | ) | (1,419 | ) | ||||||
|
Other, net
|
(23 | ) | (23 | ) | 369 | |||||||
|
Total other income (expense)
|
261 | (362 | ) | (313 | ) | |||||||
|
Loss before income taxes
|
(6,683 | ) | (6,288 | ) | (11,684 | ) | ||||||
|
Recovery of income taxes
|
— | 1,903 | 338 | |||||||||
|
Net loss
|
(6,683 | ) | (4,385 | ) | (11,346 | ) | ||||||
|
Net loss attributable to non-controlling interest, net of tax
|
— | — | 1,978 | |||||||||
|
Net loss attributable to TearLab Corp.
|
(6,683 | ) | (4,385 | ) | (9,368 | ) | ||||||
|
Excess of purchase price over the carrying value of the non-controlling interest in OcuSense, Inc.
|
— | — | (4,813 | ) | ||||||||
|
Net loss available to common stockholders
|
$ | (6,683 | ) | $ | (4,385 | ) | $ | (14,181 | ) | |||
|
Weighted average number of shares outstanding – basic and diluted
|
14,097,973 | 9,855,045 | 4,083,655 | |||||||||
|
Net loss per common share – basic and diluted
|
$ | (0.47 | ) | $ | (0.44 | ) | $ | (2.29 | ) | |||
|
Net loss available to common stockholders per common share – basic and diluted
|
$ | (0.47 | ) | $ | (0.44 | ) | $ | (3.47 | ) | |||
TearLab Corp.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(expressed in $000’s U.S. dollars)
|
Common stock
|
Additional paid-in capital
|
Accumulated
deficit
|
Non –controlling Interest
|
Stockholders'
equity
|
||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||||||
|
Balance, December 31, 2007
|
2,292,280 | $ | 2 | $ | 362,287 | $ | (358,289 | ) | $ | 4,954 | $ | 8,954 | ||||||||||||
|
Stock-based compensation
|
— | — | 190 | — | 136 | 326 | ||||||||||||||||||
|
Stock-based compensation, related to severances
|
— | — | 2,137 | — | — | 2,137 | ||||||||||||||||||
|
Shares issued on private placement of common stock
|
869,200 | 1 | 2,303 | — | — | 2,304 | ||||||||||||||||||
|
Shares issued on conversion of bridge loans principle and accrued interest
|
3,304,511 | 4 | 8,608 | — | — | 8,612 | ||||||||||||||||||
|
Shares issued to Marchant Securities for services provided in the PIPE and bridge loan transactions
|
192,480 | — | (481 | ) | — | — | (481 | ) | ||||||||||||||||
|
Costs paid to Marchant Securities for services provided in the PIPE and bridge loan transactions
|
— | — | (89 | ) | — | — | (89 | ) | ||||||||||||||||
|
Shares issued on acquisition of non-controlling shareholdings of Ocusense
|
3,050,309 | 3 | 7,672 | — | — | 7,675 | ||||||||||||||||||
|
Excess of fair value of common shares over fair value of non-controlling interest acquired
|
— | — | (4,813 | ) | — | — | (4,813 | ) | ||||||||||||||||
|
Investment by TearLab Corp. to acquire non controlling interest
|
— | — | — | — | (3,112 | ) | (3,112 | ) | ||||||||||||||||
|
Net loss, net of taxes attributable to non controlling interest.
|
— | — | — | — | (1,978 | ) | (1,978 | ) | ||||||||||||||||
|
Share issue costs
|
— | — | (458 | ) | — | — | (458 | ) | ||||||||||||||||
|
Net loss and comprehensive loss attributable to TearLab Corp..
|
— | — | — | (9,369 | ) | — | (9,369 | ) | ||||||||||||||||
|
Balance, December 31, 2008
|
9,708,780 | 10 | 377,356 | (367,658 | ) | — | 9,708 | |||||||||||||||||
|
Stock-based compensation
|
— | — | 519 | — | — | 519 | ||||||||||||||||||
|
Shares issued in negotiated settlement
|
38,276 | — | 110 | — | — | 110 | ||||||||||||||||||
|
Warrant value associated with convertible debt financing
|
— | — | 163 | — | — | 163 | ||||||||||||||||||
|
Beneficial conversion feature value associated with convertible debt financing
|
— | — | 436 | — | — | 436 | ||||||||||||||||||
|
Issue costs of the warrants and beneficial conversion feature
|
— | — | (44 | ) | — | — | (44 | ) | ||||||||||||||||
|
Reclassification of previously contingently redeemable common stock
|
119,629 | — | 250 | — | — | 250 | ||||||||||||||||||
|
Net loss and comprehensive loss
|
— | — | — | (4,385 | ) | — | (4,385 | ) | ||||||||||||||||
|
Balance, December 31, 2009
|
9,866,685 | 10 | 378,790 | (372,043 | ) | — | 6,757 | |||||||||||||||||
|
Stock-based compensation
|
— | — | 1,395 | — | — | 1,395 | ||||||||||||||||||
|
Shares issued in registered direct financing
|
1,552,795 | 2 | 3,616 | — | — | 3,618 | ||||||||||||||||||
|
Shares issued in PIPE financing
|
3,244,766 | 3 | 2,765 | — | — | 2,768 | ||||||||||||||||||
|
Shares issued to Marchant Securities for services provided in the PIPE financing
|
101,548 | — | — | — | — | — | ||||||||||||||||||
|
Options exercised
|
9,572 | — | 22 | — | — | 22 | ||||||||||||||||||
|
Net loss and comprehensive loss
|
— | — | — | (6,683 | ) | — | (6,683 | ) | ||||||||||||||||
|
Balance, December 31, 2010
|
14,775,366 | $ | 15 | $ | 386,588 | $ | (378,726 | ) | $ | — | $ | 7,877 | ||||||||||||
TearLab Corp.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(expressed in thousands except number of shares)
|
Years ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
OPERATING ACTIVITIES
|
||||||||||||
|
Net loss for the year
|
$ | (6,683 | ) | $ | (4,385 | ) | $ | (9,368 | ) | |||
|
Adjustments to reconcile net loss to cash used in operating activities:
|
||||||||||||
|
Stock-based compensation
|
1,395 | 519 | 2,463 | |||||||||
|
Depreciation of fixed assets
|
71 | 71 | 63 | |||||||||
|
Amortization of patents and trademarks
|
28 | 49 | 23 | |||||||||
|
Amortization of intangible assets
|
1,215 | 1,215 | 1,207 | |||||||||
|
Amortization of deferred financing charges, warrants & beneficial conversion values
|
228 | 303 | 180 | |||||||||
|
Beneficial conversion on bridge loan
|
— | — | 1,239 | |||||||||
|
Non-cash interest accrued on convertible debt funding
|
210 | 93 | — | |||||||||
|
Non-cash interest on bridge loans converted to equity
|
— | — | 318 | |||||||||
|
(Gain) Loss on disposal of fixed assets
|
(3 | ) | (4 | ) | 9 | |||||||
|
Change in fair value of warrant obligation
|
(701 | ) | (54 | ) | 58 | |||||||
|
Gain on short term investments
|
— | — | (1,036 | ) | ||||||||
|
Deferred income taxes
|
— | (1,903 | ) | (338 | ) | |||||||
|
Non-controlling interest
|
— | — | (1,978 | ) | ||||||||
|
Net change in working capital and non-current asset balances related to operations
|
(300 | ) | (2 | ) | (2,275 | ) | ||||||
|
Cash used in operating activities
|
(4,540 | ) | (4,098 | ) | (9,435 | ) | ||||||
|
INVESTING ACTIVITIES
|
||||||||||||
|
Proceeds from the sale of investments
|
— | — | 1,900 | |||||||||
|
Additions to fixed assets, net of proceeds
|
(55 | ) | (24 | ) | (133 | ) | ||||||
|
Additions to patents and trademarks
|
— | — | (153 | ) | ||||||||
|
Cash (used in) provided by investing activities
|
(55 | ) | (24 | ) | 1,614 | |||||||
|
FINANCING ACTIVITIES
|
||||||||||||
|
Proceeds from exercise of common stock options
|
22 | — | — | |||||||||
|
Proceeds from issuance of shares in a private placement financing
|
3,000 | — | — | |||||||||
|
Proceeds from issuance of share and warrants in a registered direct financing
|
5,000 | — | — | |||||||||
|
Proceeds from issuance of convertible debt funding
|
— | 1,750 | 6,704 | |||||||||
|
Cash paid for financing fees
|
— | (87 | ) | (269 | ) | |||||||
|
Share issuance costs
|
(807 | ) | — | (458 | ) | |||||||
|
Proceeds from issuance of common stock
|
— | — | 2,173 | |||||||||
|
Cash provided by financing activities
|
7,215 | 1,663 | 8,150 | |||||||||
|
Net increase (decrease) in cash and cash equivalents during the year
|
2,620 | (2,459 | ) | 329 | ||||||||
|
Cash and cash equivalents, beginning of year
|
106 | 2,565 | 2,236 | |||||||||
|
Cash and cash equivalents, end of year
|
$ | 2,726 | $ | 106 | $ | 2,565 | ||||||
Notes to Consolidated Financial Statements
(expressed in U.S. dollars except as otherwise noted)
1. Basis of Presentation
Nature of Operations
TearLab Corp. formerly OccuLogix, Inc. ("TearLab" or the "Company"), a Delaware corporation, is an ophthalmic device company that is commercializing a proprietary in vitro diagnostic tear testing platform, the TearLab® test for dry eye disease, or DED, which enables eye care practitioners to test for highly sensitive and specific biomarkers using nanoliters of tear film at the point-of-care.
On November 30, 2006, the Company acquired 50.1% of the capital stock, on a fully diluted basis, 57.62% on an issued and outstanding basis, of TearLab Research, Inc. (formerly TearLab, Inc.), a San Diego-based company that is in the process of developing technologies that will enable eye care practitioners to test, at the point-of-care, for highly sensitive and specific biomarkers using nanoliters of tear film. On October 6, 2008, the Company acquired the remaining non-controlling interest in TearLab Research, Inc.
The accompanying consolidated financial statements include the accounts of the Company and all of its subsidiaries. Intercompany accounts and transactions have been eliminated on consolidation.
Going concern uncertainty
The consolidated financial statements have been prepared on the basis that the Company will continue as a going concern. However, the Company has sustained substantial losses of $6.7 million, $4.4 million and $14.2 million for the years ended December 31, 2010, 2009 and 2008, respectively. The Company's working capital at December 31, 2010 is $0.4 million. As a result of the Company's history of losses and financial condition, there is substantial doubt about the ability of the Company to continue as a going concern. Management believes the Company’s existing cash will be sufficient to cover its operating and other cash demands until approximately July 2011 if the Company’s convertible note holders do not convert their convertible notes and accrued interest to common shares of the Company in July 2011 and approximately December 2011 if the Company’s convertible note holders do convert their convertible notes and accrued interest to common shares of the Company in July 2011.
A successful transition to attaining profitable operations is dependent upon obtaining sufficient financing to fund the Company’s planned expenses and achieving a level of revenues adequate to support the Company’s cost structure. The Company has been seeking additional debt or equity financing to support its operations until it becomes cash flow positive. There can be no assurances that there will be adequate financing available to the Company on acceptable terms or at all. If the Company is unable to obtain additional financing, the Company would need to significantly curtail or reorient its operations during 2011, which could have a material adverse effect on the Company’s ability to achieve its business objectives and as a result may require the Company to file for bankruptcy or cease operations. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts classified as liabilities that might be necessary should the Company be forced to take any such actions.
2. SIGNIFICANT ACCOUNTING POLICIES
The consolidated financial statements have been prepared by management in conformity with U.S. generally accepted accounting principles, (“U.S. GAAP).
Use of estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenue and expenses during the reporting periods. Some of the Company's more significant estimates include those related to uncollectible receivables, inventory reserves, stock-based compensation, equity instruments, investments and its intangible assets. Actual results could differ from those estimates.
Concentrations and risk
Credit risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and cash equivalents and accounts receivable. The Company limits its exposure to credit loss by placing its cash with high credit quality financial institutions.
During fiscal 2010, the Company derived 100% of its revenue from the sale of the TearLab Osmolarity product. Of this revenue, 11% was from a single distributor. Currently, there is only one supplier for each of the reader and pen components of the TearLab® Osmolarity System and the test cards.
Fair value of financial instruments
The Company’s financial instruments consist principally of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and convertible Notes. The carrying amounts of financial instruments such as cash equivalents, accounts receivable, accounts payable and accrued expenses approximate the related fair values due to the short-term maturities of these instruments.
Cash and cash equivalents
The Company considers all highly liquid investments that are readily convertible into cash and have an original maturity of three months or less at the time of purchase to be cash equivalents.
Accounts receivable and allowance for doubtful accounts
The Company’s accounts receivable consist primarily of trade receivables from customers and are generally unsecured and due within 30 days. The carrying value of accounts receivable approximates their fair value due to their short term nature. The Company evaluates the collectability of its accounts receivable based on a combination of factors. Expected credit losses related to trade receivables are recorded as an allowance for doubtful accounts. The allowance for doubtful accounts is charged to sales and marketing expense and accounts receivable are written off as uncollectible and deducted from the allowance after appropriate collection efforts have been exhausted. To date, charges for bad debt expense have not been material.
Inventory
Inventory is recorded at the lower of cost (based on first in, first out basis) or market and consists of purchased finished goods. Inventory is periodically reviewed for evidence of slow-moving or obsolete items, and the estimated reserve is based on management's reviews of inventories on hand, compared to estimated future usage and sales, reviewing product shelf-life, and assumptions about the likelihood of obsolescence.
Fixed assets
Property and equipment are carried at cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, ranging from three to seven years. Maintenance and repairs are expensed as incurred. The Company reviews property, plant and equipment for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. An impairment loss would be recognized when estimated future undiscounted cash flows relating to the asset are less than its carrying amount. An impairment loss is measured as the amount by which the carrying amount of an asset exceeds its fair value.
Impairment of long-lived assets
The Company periodically assesses the carrying value of intangible and other long-lived assets, and whenever events or changes in circumstances indicate that the carrying amount of an asset might not be recoverable. The assets are considered to be impaired if the Company determines that the carrying value may not be recoverable based upon its assessment, which includes consideration of the following events or changes in circumstances:
|
|
·
|
the asset's ability to continue to generate income from operations and positive cash flow in future periods;
|
|
|
·
|
loss of legal ownership or title to the asset;
|
|
|
·
|
significant changes in the Company’s strategic business objectives and utilization of the asset(s); and
|
|
|
·
|
the impact of significant negative industry or economic trends.
|
If the assets are considered to be impaired, the impairment recognized is the amount by which the carrying value of the assets exceeds the fair value of the assets. Fair value is determined by a combination of data from third party sources and the application of discounted cash flow models to project cash flows from the asset. In addition, the Company bases the useful lives and related amortization or depreciation expense on an estimate of the period that the assets will generate revenues or otherwise be used. The Company also periodically reviews the lives assigned to long-lived assets to ensure that the initial estimates do not exceed any revised estimated periods from which the Company expects to realize cash flows from its assets.
Patents and trademarks
Patents and trademarks are recorded at historical cost and are amortized using the straight-line method over their estimated useful lives, not to exceed 15 years.
Product Warranties
The Company generally provides a 12 month warranty on its TearLab® Osmolarity System and related disposables. The Company accrues the estimated cost of this warranty at the time revenue is recorded and charge warranty expense to cost of goods sold. Warranty reserves are established based on historical experience with failure rates and the number of systems covered by warranty. Warranty reserves are depleted as systems and disposables are replaced. The Company reviews warranty reserves quarterly and, if necessary, make adjustments. The activities in the warranty reserve are as follows (in thousands):
|
Years ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Balance at beginning of year
|
$ | 9 | $ | — | $ | — | ||||||
|
Charges to cost of goods sold
|
55 | 15 | — | |||||||||
|
Costs applied to liability
|
(13 | ) | (6 | ) | — | |||||||
|
balance at end of year
|
$ | 51 | $ | 9 | $ | — | ||||||
Income Taxes
A deferred tax asset or liability is determined based on the difference between the financial statement and tax basis of assets and liabilities as measured by the enacted tax rates which will be in effect when these differences reverse. The Company provides a valuation allowance against net deferred tax assets unless, based upon the available evidence, it is more likely than not that the deferred tax assets will be realized.
Revenue recognition
Revenue is recognized when all four of the following criteria are met: (i) persuasive evidence that an arrangement exists; (ii) delivery of the products has occurred; (iii) the selling price is fixed or determinable; and (iv) collectability is reasonably assured. The Company’s timing of revenue recognition is impacted by factors such as passage of title, payments and customer acceptance. Amounts received in excess of revenue recognizable under SAB No. 104 are deferred.
Subsequent to its launch in the fourth quarter of 2008, the Company’s revenues have been derived from sales of the TearLab® Osmolarity System for DED which consists of hardware and related disposables. The Company’s sales are currently to countries in North America, Europe, and Asia, and are generally transacted through distributors. The Company records revenue when all of its obligations are completed which is generally upon shipment of the Company’s products.
The Company typically has a no return policy for its products, however during 2010, the Company established a return reserve for product sales that contain an implicit right of return. This reserve of $73,000 reduces revenue and is included in accrued liabilities.
Cost of goods sold
Cost of goods sold includes the costs the Company incurs for the purchase of the TearLab Systems sold and related freight and shipping costs, fees related to warehousing, logistics inventory management and recurring regulatory costs associated with conducting business. The Company recorded $142,000, $49,000 and $8,000 in shipping and handling fees for the years ended December 31, 2010, 2009 and 2008, respectively.
Clinical, regulatory and research & development costs
Clinical and regulatory costs attributable to the performance of contract services are recognized as an expense as the services are performed. Non-refundable, up-front fees paid in connection with these contracted services are deferred and recognized as an expense over the estimated term of the related contract.
Stock-based compensation
The Company accounts for stock-based compensation expense for its employees in accordance with US GAAP guidance related to stock-based compensation. Under this guidance, stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as an expense ratably over the requisite service period of the award. The Company has selected the Black-Scholes option-pricing model as its method of determining the fair value for all its awards and will recognize compensation cost on a straight-line basis over the awards' vesting periods.
Foreign currency translation
The Company's functional and reporting currency is the U.S. dollar. The assets and liabilities of the Company's Canadian operations are maintained in U.S. dollars. Monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars at exchange rates in effect at the consolidated balance sheet dates, and non-monetary assets and liabilities are translated at exchange rates in effect on the date of the transaction. Revenue and expenses are translated into U.S. dollars at average exchange rates prevailing during the year. Resulting exchange gains (losses) of: ($34,000), ($2,000), $325,000 are included in other income/(expense) in the years ended December 31, 2010, 2009 and 2008, respectively.
Comprehensive income
Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Comprehensive income (loss) includes unrealized gains or losses on the Company’s marketable securities and foreign currency translation adjustments. In all the periods presented, the Company’s comprehensive loss equaled net loss for the period.
Net loss per share
Basic earnings per share (“EPS”) excludes dilutive securities and is computed by dividing net loss available to common stockholders by the weighted average number of shares of common stock outstanding for the year. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted and the resulting additional shares are dilutive because their inclusion decreases the amount of EPS.
The following are potentially dilutive securities which have not been used in the calculation of diluted loss per share as they are anti-dilutive (in thousands):
|
December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Stock options
|
3,577 | 3,357 | 2,278 | |||||||||
|
Shares reserved for issuance on conversion of debt obligations
|
1,327 | 1,327 | - | |||||||||
|
Warrants to be issued on conversion of debt obligations
|
109 | - | - | |||||||||
|
Warrants
|
753 | 220 | 111 | |||||||||
|
Total
|
5,766 | 4,904 | 2,389 | |||||||||
Recent accounting pronouncements
In October 2009, the Financial Accounting Standards Board (“FASB”) issued an amendment to the accounting standards related to the accounting for revenue in arrangements with multiple deliverables including how the arrangement consideration is allocated among delivered and undelivered items of the arrangement. Among the amendments, this standard eliminates the use of the residual method for allocating arrangement consideration and requires an entity to allocate the overall consideration to each deliverable based on an estimated selling price of each individual deliverable in the arrangement in the absence of having vendor-specific objective evidence or other third party evidence of fair value of the undelivered items. This standard also provides further guidance on how to determine a separate unit of accounting in a multiple-deliverable revenue arrangement and expands the disclosure requirements about the judgments made in applying the estimated selling price method and how those judgments affect the timing or amount of revenue recognition. This standard, for which the Company is currently assessing the impact, became effective for the Company on January 1, 2011.
In January 2010, the FASB issued (ASU) No. 2010-06, Fair Value Measurements and Disclosures (Topic 820), “Improving Disclosures about Fair Value Measurements” (“ASU No. 2010-06”). ASU No. 2010-06 requires new disclosures about significant transfers in and out of Level 1 and Level 2 fair value measurements and the reasons for such transfers and in the reconciliation for Level 3 fair value measurements, the requirement to disclose separately information about purchases, sales, issuances and settlements. The Company adopted the provisions of ASU No. 2010-06 on January 1, 2010, except for disclosures about purchases, sales, issuances and settlements in the reconciliation for Level 3 fair value measurements. Those disclosures will be effective for financial statements issued for fiscal years beginning after December 15, 2010. The Company does not expect the impact of its adoption to be material to its consolidated financial statements.
In July 2010, the FASB issued (ASU) No. 2010-20, “Disclosures about the Credit Quality of Financing Receivables and the Allowance for Credit Losses.” ASU No. 2010-20 requires companies that hold financing receivables, which include loans, lease receivables, and the other long-term receivables to provide more information in their disclosures about the credit quality of their financing receivables and the credit reserves held against them. On December 31, 2010, we adopted all amendments that require disclosures as of the end of a reporting period, and on January 1, 2011, the Company adopted all amendments that require disclosures about activity that occurs during a reporting period (the remainder of this ASU). The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
3. ACQUISITION AND NON-CONTROLLING INTEREST
On November 30, 2006, the Company acquired 50.1% of the capital stock, on a fully diluted basis, 57.62% on an issued and outstanding basis; of TearLab Research, Inc. (formerly TearLab, Inc.). TearLab Research, Inc. was determined to be a Variable Interest Entity, or VIE, and the Company was determined to be the primary beneficiary. As required by ASC Topic 810 Consolidation, the assets, liabilities and non-controlling interest of TearLab Research, Inc. were measured at fair value.
After initial measurement, the non-controlling interests’ stake reflected the initial fair value of the non-controlling's interest less the non-controlling's proportionate interest in losses incurred.
On October 6, 2008, the Company acquired the remaining 42.38% interest in TearLab Research, Inc. that it did not previously own by issuance of shares with a fair value of $7,925,000, which resulted in the elimination of the non-controlling interest in the amount of $3,112,000. Since the Company had consolidated TearLab Research, Inc. as a VIE, the excess of the purchase price over the value of the non-controlling interest acquired was treated as a capital item and was reported as a reduction to Additional Paid In Capital and included in the net loss available to stockholders in the consolidated statement of operations for 2008. There were no acquisitions made during fiscal 2009 or 2010.
Non-controlling stockholders' share of net losses from operations for the year ended December 31, 2008 was $1,978,000. As a result of the Company’s acquisition of the remaining ownership in TearLab Research, Inc., the non-controlling interest was reduced to zero at December 31, 2008 and no non-controlling stockholders’ share of net losses from operations was reported in the years ended December 31, 2010 and 2009.
4. BALANCE SHEET DETAILS
Accounts receivable
|
(in thousands)
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Trade receivables
|
$ | 311 | $ | 153 | ||||
|
Allowance for doubtful accounts
|
(13 | ) | (7 | ) | ||||
|
Other receivables
|
14 | 3 | ||||||
| $ | 312 | $ | 149 | |||||
Inventory
Inventory is recorded at the lower of cost or market and consists of finished goods. Inventory is accounted for on a first-in, first-out basis. Deferred cost of sales (included in finished goods) consists of products shipped but not recognized as revenue because they did not meet the revenue recognition criteria.
|
(in thousands)
|
December 31,
2010
|
December 31,
2009
|
||||||
|
Finished goods
|
$ | 671 | $ | 320 | ||||
|
Inventory reserves
|
(116 | ) | (124 | ) | ||||
| $ | 555 | $ | 196 | |||||
The Company evaluates inventory for estimated excess quantities and obsolescence, based on expected future sales levels and projections of future demand, with the excess inventory provided for. In addition, the Company assesses the impact of changing technology and market conditions.
Prepaid expenses
|
(in thousands)
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Prepaid insurance
|
$ | 122 | $ | 147 | ||||
|
Prepaid financing costs
|
83 | 82 | ||||||
|
Prepaid regulatory fees
|
— | 3 | ||||||
|
Deferred royalty costs
|
36 | 41 | ||||||
|
Other fees and services
|
81 | 65 | ||||||
| $ | 322 | $ | 338 | |||||
Fixed Assets
|
(in thousands)
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Furniture and office equipment
|
$ | 48 | $ | 25 | ||||
|
Computer equipment and software
|
151 | 145 | ||||||
|
Demo equipment
|
33 | — | ||||||
|
Medical equipment
|
317 | 330 | ||||||
| 549 | 500 | |||||||
|
Accumulated depreciation
|
(423 | ) | (360 | ) | ||||
| $ | 126 | $ | 140 | |||||
Depreciation expense was $71,000, $71,000, and $63,000, during the years ended December 31, 2010, 2009, and 2008, respectively.
Patents and trademarks
|
(in thousands)
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Patents
|
$ | 236 | $ | 194 | ||||
|
Trademarks
|
32 | 79 | ||||||
| 268 | 273 | |||||||
|
Accumulated amortization
|
(76 | ) | (53 | ) | ||||
| $ | 192 | $ | 220 | |||||
Amortization expense was $28,000, $49,000, and $23,000 during the years ended December 31, 2010, 2009, and 2008, respectively.
. These patents and trademarks are amortized, using the straight-line method, over an estimated useful life of 10 years from the date of approval of the patents and trademarks.
Estimated aggregate amortization expense for patents and trademarks at December 31, 2010 is as follows (in thousands):
|
2011
|
$ | 28 | ||
|
2012
|
28 | |||
|
2013
|
28 | |||
|
2014
|
28 | |||
|
2015
|
28 | |||
|
Thereafter
|
52 | |||
|
Total
|
$ | 192 |
Accrued liabilities
|
(in thousands)
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Due to professionals
|
$ | 296 | $ | 302 | ||||
|
Clinical trial accruals
|
174 | 195 | ||||||
|
Due to employees and directors
|
332 | 134 | ||||||
|
Accrued capital transaction advisory services
|
200 | 100 | ||||||
|
Accrued royalty payments
|
84 | 18 | ||||||
|
Corporate compliance
|
63 | 81 | ||||||
|
Product return reserve
|
73 | — | ||||||
|
Product warranties
|
51 | 9 | ||||||
|
Obligation to repay advances received
|
50 | 67 | ||||||
|
Miscellaneous
|
118 | 187 | ||||||
| $ | 1,441 | $ | 1,093 | |||||
5. INTANGIBLE ASSETS
The Company's intangible assets consist of the value of TearLab Technology acquired in the acquisition of TearLab Research, Inc. The TearLab Technology consists of a disposable lab card and card reader, supported by an array of patents and patent applications that are either held or in-licensed by the Company. The TearLab Technology is being amortized using the straight-line method over an estimated useful life of 10 years. Amortization expense for the years ended December 31, 2010, 2009 and 2008 was $1,215,000, $1,215,000 and $1,207,000, respectively.
Intangible assets subject to amortization consist of the following (in thousands):
|
December 31, 2010
|
December 31, 2009
|
|||||||||||||||
|
Cost
|
Accumulated Amortization
|
Cost
|
Accumulated Amortization
|
|||||||||||||
|
TearLab™ technology
|
$ | 12,172 | $ | 5,033 | $ | 12,172 | $ | 3,819 | ||||||||
Estimated future amortization expense related to intangible assets with finite lives at December 31, 2010 is as follows (in thousands):
|
Amortization of intangible assets
|
||||
|
2011
|
$ | 1,215 | ||
|
2012
|
1,215 | |||
|
2013
|
1,215 | |||
|
2014
|
1,215 | |||
|
2015
|
1,215 | |||
|
Thereafter
|
1,064 | |||
|
Total
|
$ | 7,139 | ||
The Company determined that, as of December 31, 2010, there have been no significant events which may affect the carrying value of its TearLab technology. However, the Company's prior history of losses and losses incurred during the current fiscal year reflect a potential indication of impairment, thus requiring management to assess whether the Company's TearLab technology was impaired as of December 31, 2010. Based on management's estimates of forecasted undiscounted cash flows as of December 31, 2010, the Company concluded that there is no indication of an impairment of the Company's TearLab technology. Therefore, no impairment charge was recorded during the year ended December 31, 2010.
6. RELATED PARTY TRANSACTIONS
The following are the Company's related party transactions:
TLC Vision
TLC Vision Corporation (“TLC”) held a 2.9% and 7.6% ownership interest in the Company, on an issued and outstanding basis, as of December 31, 2010 and 2009, respectively.
TLC provided computer and administrative support to the Company in the years ended December 31, 2010, 2009, and 2008 for which the Company recorded expense of $6,000, 21,000, and $80,000, respectively. The balances due to TLC Vision Corporation were $28,000 and $38,000 at December 31, 2010 and 2009, respectively.
Other
On August 20, 2009, the Company entered into a distribution agreement with Science with Vision, pursuant to which Science with Vision obtained exclusive Canadian distribution rights with respect to the Company’s products. The Company began selling products through the Canadian distributor in 2010. Sales to this distributor for the years ended December 31, 2010 and 2009 were $96,000 and $4,500, respectively, and the outstanding accounts receivable balances due at December 31, 2010 and 2009 were $33,000 and $4,500, respectively. The Company’s chairman of the board of directors and chief executive officer has a material financial interest in Science with Vision.
Effective November 20, 2009, the Company entered into an agency agreement with Marchant Securities Inc. (“Marchant”). Pursuant to the terms of the agreement, Marchant acted as the Canadian placement agent in connection with the Company’s private placement of up to $3,000,000 of the Company’s common stock. As a result of the closing of the private placement financings in January 2010 and March 2010, under the Agency agreement, the Company issued an aggregate of 101,548 shares to Marchant. The Company’s chairman of the board of directors and chief executive officer has a material financial interest in Marchant.
The Company has also agreed to indemnify Marchant, its affiliates and their respective directors, officers, employees, shareholders and agents against all expenses, losses, claims, actions, damages or liabilities, and the reasonable fees and expenses of their counsel, arising out of the provision of the services pursuant to the agreement.
On November 2, 2009, the Company, entered into a capital advisory agreement for a minimum of two years with Greybrook Capital Inc., or Greybrook, which was amended on January 8, 2010. Pursuant to the terms of the agreement, as amended, Greybrook is entitled to elect to receive in consideration of its provision of capital advisory services to the Company, within 90 days of the agreement and again on November 2, 2010, compensation consisting of (i) $100,000 in cash or (ii) shares of the Company’s common stock equal to the quotient of (A) $100,000 and (B) $1.22, the closing consolidated bid price on the date of the original execution of the agreement. The calculation of the number of shares to be issued under ii) of the amending agreement was 81,967 shares of the Company’s common stock for each of the two years of service for a cumulative total of 163,934 common shares. No shares were issued and no payments were made under this agreement at December 31, 2010 and the Company has accrued $200,000 and $100,000 of a liability to Greybrook as at December 31, 2010 and 2009, respectively. The Company’s chairman of the board of directors and chief executive officer, is a principal with, and holds a material financial interest in Greybrook.
The Company has accounts receivable due from a related party for the years ended December 31, 2010 and 2009 in the amount of $33,000 and $4,500, respectively, for product shipped.
The Company currently has an employee who formerly served as a United States distributor of the Company’s products. This employee is no longer in control of the distributor; however the employee continues to hold a material financial interest in the distributing company. Sales to this distributor for the years ended December 31, 2010 and 2009 were $192,000 and $0, respectively, and the outstanding accounts receivable balances at December 31, 2010 and 2009 were $98,000 and $0, respectively.
7. INCOME TAXES
Significant components of the Company's deferred tax assets and liabilities are as follows (in thousands):
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred tax assets
|
|
|
||||||
|
Intangible assets
|
$ | 263 | $ | 222 | ||||
|
Fixed assets
|
11 | 24 | ||||||
|
Stock options
|
6,178 | 5,749 | ||||||
|
Accruals and other
|
379 | 290 | ||||||
|
Net operating loss carry forwards
|
5,357 | 3,772 | ||||||
| 12,188 | 10,057 | |||||||
|
Valuation allowance
|
(9,228 | ) | (6,519 | ) | ||||
|
Deferred tax asset
|
2,960 | 3,538 | ||||||
|
Deferred tax liability
|
||||||||
|
Beneficial conversion feature
|
(125 | ) | (196 | ) | ||||
|
Intangible assets
|
(2,835 | ) | (3,342 | ) | ||||
|
Deferred tax liability
|
(2,960 | ) | (3,538 | ) | ||||
|
Deferred taxes, net
|
$ | — | $ | — | ||||
The following is a reconciliation of the recovery of income taxes between those that are expected, based on enacted tax rates and laws, to those currently reported (in thousands):
|
December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Loss for the year before income taxes
|
$ | (6,683 | ) | $ | (6,288 | ) | $ | (9,706 | ) | |||
|
Expected recovery of income taxes
|
(2,654 | ) | (2,515 | ) | (3,646 | ) | ||||||
|
Non-controlling interest
|
— | — | (791 | ) | ||||||||
|
Stock-based compensation
|
97 | 144 | — | |||||||||
|
Warrants
|
(278 | ) | — | — | ||||||||
|
Non-deductible interest
|
83 | 37 | — | |||||||||
|
Section 382 limitation of deferred assets
|
— | — | 42,377 | |||||||||
|
Deferred state tax rate adjustment
|
47 | (667 | ) | — | ||||||||
|
Return to provision
|
(48 | ) | 673 | 1,446 | ||||||||
|
Non-deductible expenses & other
|
44 | 29 | 11 | |||||||||
|
Change in valuation allowance
|
2,709 | 396 | (39,735 | ) | ||||||||
|
Total recovery of income taxes
|
$ | — | $ | (1,903 | ) | $ | (338 | ) | ||||
Income taxes are recorded in accordance with authoritative guidance for accounting for income taxes, which requires the recognition of deferred tax assets and liabilities to reflect the future tax consequences of events that have been recognized in the Company’s consolidated financial statements or tax returns. Measurement of the deferred items is based on enacted tax laws. In the event of differences between financial reporting bases and tax bases of the Company’s assets the probability of being able to realize the future benefits indicated by such assets is required. The Company records a valuation allowance to reduce the deferred tax assets to the amount that is more likely than not to be realized. Management has considered future taxable income and ongoing tax planning strategies in assessing the need for the valuation allowance. In the event the Company were to determine that it would be able to realize the deferred tax assets in the future in excess of their net recorded amounts, an adjustment to the deferred tax assets would increase the income in the period such determination was made. Likewise, should the Company determine that it would not be able to realize all or part of the net deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to income in the period such determination was made.
In July 2006, the “FASB issued additional guidance which requires the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, the provisions under this guidance also provides guidance on derecognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. The Company adopted the provisions under this guidance on January 1, 2007. The adoption of these provisions had no impact on the Company’s consolidated financial position or results of operations. At December 31, 2009 and 2008, the Company’s unrecognized income tax benefits and uncertain tax provisions were not material and would not, if recognized, affect the effective tax rate.
The October 6, 2008 closing of the private investment by certain investors, the acquisition of the remaining ownership interest in TearLab, Inc. and the conversion of the bridge loans combined to result in a change of ownership for tax purposes causing Section 382 restrictions on the use of tax losses to apply. Utilization of the net operating loss and capital loss carryforwards will be subject to a substantial annual limitation under Section 382 of the Internal Revenue Code of 1986, and similar state provisions due to ownership change limitations that have occurred. These ownership changes will limit the amount of net operating loss and capital loss carryforwards that can be utilized to offset future taxable income. In general, an ownership change, as defined by Section 382, results from transactions increasing ownership of certain stockholders or public groups in the stock of the corporation by more than 50 percentage points over a three-year period.
At December 31, 2010, the Company had federal net operating loss carryforwards of approximately $87.7 million, of which $65.1 million will expire unused due to the 382 limitation, and California net operating loss carryforwards of approximately $22.8 million, of which $15.2 million will expire unused due to 382 limitation. The federal net operating loss carryforwards begin to expire in 2012, and the California net operating loss carryforwards begin to expire in 2015.
In accordance with authoritative guidance, the impact of an uncertain income tax position on the
income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained
upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if
it has less than a 50% likelihood of being sustained. As of December 31, 2010 and 2009 the Company had no unrecognized tax benefits.
The Company’s policy is to recognize interest and penalties related to income tax matters in other expense. Because the Company has generated net operating losses since inception for both state and federal purposes, no additional tax liability, penalties or interest has been recognized for balance sheet or income statement purposes as of and for the period ended December, 31, 2010.
When applicable, the Company recognizes accrued interest and penalties related to unrecognized tax benefits as other expense in its consolidated statements of operations, which is consistent with the recognition of these items in prior reporting periods. As of December 31, 2010, the Company did not have any liability for the payment of interest and penalties.
The Company does not expect a significant change in the amount of its unrecognized tax benefits within the next 12 months. Therefore, it is not expected that the change in the Company’s unrecognized tax benefits will have a significant impact on the Company’s results of operations or financial position.
All of the federal income tax returns for the Company and its subsidiaries remain open since their respective dates of incorporation due to the existence of net operating losses. The Company and its subsidiaries have not been, nor are they currently, under examination by the Internal Revenue Service or the Canada Revenue Agency.
State and provincial income tax returns are generally subject to examination for a period of between three and five years after their filing. However, due to the existence of net operating losses, all state income tax returns of the Company and its subsidiaries since their respective dates of incorporation are subject to re-assessment. The state impact of any federal changes remains subject to examination by various states for a period of up to one year after formal notification to the states. The Company and its subsidiaries have not been, nor are they currently, under examination by any state tax authority.
8. CONVERTIBLE NOTES PAYABLE AND ACCRUED INTEREST
In July 2009, the Company entered into an agreement with certain investors whereby the investors agreed to provide financing (the “Financing”) to the Company through the purchase of convertible secured notes (“the Notes”), in the aggregate amount of $1.55 million. On August 31, 2009, an additional $200,000 of financing was received by the Company to bring the aggregate total funding received to $1.75 million. The Notes evidencing the Financing, mature on the second anniversary of their issuance (“the Maturity Date”), bear interest at a rate of 12% per annum and are convertible into shares of the Company’s common stock upon the request of holders of 51% or more of the outstanding principal amount of the Notes at any time after August 31, 2009 and prior to the Maturity Date. The conversion price of the Notes (the “Discount Price”) is $1.3186 determined on August 31, 2009 and represents 80% of the volume weighted average price on the NASDAQ stock market for the ten trading days prior to August 31, 2009. The Notes are secured by substantially all of the assets of the Company.
In connection with the Financing, the Company will issue warrants with a life of five years to purchase 109,389 shares of common stock (the “Warrants”). The exercise price of the Warrants is $1.60 per common share representing the price per share equal to the closing bid price per share of the Company’s common stock on the NASDAQ stock market on July 15, 2009. The warrants will not be issued until the Notes are converted to common shares of the Company. The Company has recorded $163,000 representing the fair value of the warrants in additional paid-in capital which has been calculated using the Black-Scholes value model. The value of the warrant is being accreted over the two year term of the Notes and an amortization charge of $41,000 and $53,000 in the years ended December 31, 2010 and 2009, respectively, is included in other income (expense).
As the conversion price of the Notes reflected a price discounted from the fair market value of the Company’s common stock, there is a deemed beneficial conversion feature associated with the Financing. The Company has recorded $728,000 representing the value of the beneficial conversion feature at the date of the Financing in additional paid-in capital. The value of the beneficial conversion is being amortized over the two year term of the Notes with a charge of $177,000 and $237,000 for the years ended December 31, 2010 and 2009, respectively, included in other income (expense).
The Company incurred $87,000 in legal and consulting expenses related to the Financing which was allocated to deferred finance charges for $43,000 and cost of equity for $44,000 in proportion to the allocation of the Financing amount between equity and liabilities. The value of the deferred financing is being amortized over the two year term of the Notes and an expense of $10,000 and $14,000 for the years ended December 31, 2010 and 2009, respectively, is included in other income (expense).
Richard Lindstrom, M.D. and Tom Davidson, both of whom are directors of the Company, invested $100,000 each in the Financing. Greybrook Corporation, an entity controlled by Elias Vamvakas, Chairman and Interim CEO of the Company, or members of his family, invested $310,000 in the Financing.
Accrued interest of $303,000 and $93,000 at December 31, 2010 and 2009, respectively, is included in the convertible notes payable and accrued interest balance on our balance sheet.
9. RESTRUCTURING CHARGES
During 2008, the Company completed its restructuring plan including obtaining the agreement of certain former executives and the shareholder approval for portions of the severance or retention liabilities to be replaced with fully vested options exercisable into common shares of the Company. In accordance with ASC Topic 420 Exit or Disposal Cost Obligations the Company recognized a total of $2,441,000 in restructuring charges during the year ended December 31, 2008 related to severance and benefit costs to terminated employees at the Company's Mississauga, Ontario office. The Company granted to 11 former executives and one current executive options with an equivalent fair value to $2,213,000 in lieu of cash severance payments. In addition, the Company modified the existing options held by the employees to allow for continued vesting under the original terms of the options and to remove the provision requiring the options to expire 90 days after they were terminated. In accordance with applicable accounting guidance, the Company treated the modification as the grant of a new award. As these individuals are no longer employees or rending further service to the Company after termination, the fair value of the reissued options on the modification date was then expensed immediately as no further service is required from the terminated employees. All severance and benefit costs have been paid as of December 31, 2010.
10. COMMITMENTS AND CONTINGENCIES
Commitments
The Company has commitments relating to operating leases recognized on a straight line basis over the term of the lease for rental of office space and equipment from unrelated parties, expiring at various times from January 1, 2011 through June 30, 2015. The total future minimum obligation under these various leases for 2011, 2012, 2013, 2014 and 2015 is $130,000, $126,000, 128,000, 129,000 and $64,000, respectively. Rent expensed under these leases was $145,000, $174,000, and $131,000 for the years ended December 31, 2010, 2009 and 2008, respectively.
On March 12, 2003, TearLab entered into a patent license and royalty agreement with the University of California San Diego to obtain a second exclusive license to make, use, sell, offer for sale, and import TearLab technology in development. Starting in 2009, the Company was required to make minimum royalty payments of $35,000 or 5.5% of gross sales per year, whichever is higher. However, if this new technology is combined with existing technology, the maximum royalty payable on the sale of the combined products would be 5.5% of gross sales per year. As the new technology is currently in development, there is no revenue and the minimum royalty payment of $35,000 is applicable. Future minimum royalty payments under this agreement as of December 31, 2010 are approximately as follows (in thousands):
|
2011
|
$ | 35 | ||
|
2012
|
35 | |||
|
2013
|
35 | |||
|
2014
|
35 | |||
|
2015
|
35 | |||
|
Thereafter
|
420 | |||
|
Total
|
$ | 595 |
Effective October 1, 2006, TearLab entered into a patent license and royalty agreement with the University of California San Diego to obtain an exclusive license to make, use, sell, offer for sale, and import existing TearLab technology. The Company is required to make royalty payments of $35,000 or 5.5% of gross sales per year, whichever is higher. During the year ended December 31, 2010, the Company incurred fees of $99,000 under this agreement. Additionally, the Company is required to pay a royalty of 30% of any sublicense fees it receives prior to receiving FDA approval and 25% of any sub-license fees it receives after FDA approval. Future minimum royalty payments under this agreement as of December 31, 2010 are approximately as follows (in thousands):
|
2011
|
$ | 35 | ||
|
2012
|
35 | |||
|
2013
|
35 | |||
|
2014
|
35 | |||
|
2015
|
35 | |||
|
Thereafter
|
420 | |||
|
Total
|
$ | 595 |
On February 23, 2009, the Company entered into an agreement for the manufacturing of its TearLab® reader and pens with a third party manufacturing company. The agreement is in effect for three years after the start of production and can be renewed for one-year. The Company has no minimum commitment. Upon termination or cancellation of the agreement, the Company is liable for inventory and materials remaining at the manufacturer’s facilities at 110% of the manufacturer’s cost. At December 31, 2010, the manufacturer maintained inventory and materials with a value of approximately $181,000, all of which had been purchased to meet manufacturing requirements of purchase orders issued by the Company.
Contingencies
During the ordinary course of business activities, the Company may be contingently liable for litigation and a party to claims. Currently the Company is not party to any litigation. Management believes that adequate provisions have been made in the accounts where required. Although it is not possible to estimate the extent of potential costs and losses, if any, management believes that the ultimate resolution of any such contingencies will not have a material adverse effect on the financial position and results of operations of the Company.
11. FAIR VALUE MEASUREMENTS
The Company measures certain assets and liabilities in accordance with authoritative guidance which requires fair value measurements be classified and disclosed in one of the following three categories:
|
|
·
|
Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities.
|
|
|
·
|
Level 2: Observable prices that are based on inputs not quoted on active markets, but corroborated by market data.
|
|
|
·
|
Level 3: Unobservable inputs are used when little or no market data is available.
|
Assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurements. The Company reviews the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy. The Company did not have any assets or liabilities in Level 1 and Level 2 and no transfers to or from Level 3 of the fair value measurement hierarchy during the year ended December 31, 2010.
At December 31, 2010, the Company has a liability for warrants to purchase 131,497 shares of common stock at an exercise price of $46.25 per share that are valued at $1,000, as well as a liability for warrants to purchase 621,118 shares of common stock at an exercise price of $4.00 per share that are valued at $38,000 (Note 12). Both liabilities are classified as level 3 fair value measurements.
The following table provides a reconciliation for all liabilities measured at fair value using significant unobservable inputs (level 3) for the year ended December 31, 2010 (in thousands):
|
Fair Value Measurements Using Significant Unobservable Inputs (Level 3)
|
||||
|
Balance of warrant liability at January 1, 2010
|
$ | 3 | ||
|
Issuances
|
737 | |||
|
Change in fair value of warrant liability included in other (income) / expense
|
(701 | ) | ||
|
Balance of warrant liability at December 31, 2010
|
$ | 39 | ||
12. CAPITAL STOCK
(a) Authorized share capital
The total number of authorized shares of common stock of the Company is 40,000,000. Each share of common stock has a par value of $0.001 per share. The total number of authorized shares of preferred stock of the Company is 10,000,000. Each share of preferred stock has a par value of $0.001 per share.
(b) Common stock
On February 11, 2009, the Company filed with the Securities and Exchange Commission (“SEC”), a prospectus as part of a registration statement on Form S-3 using a "shelf" registration process. Under the shelf process, the Company may from time to time offer or sell any combination of common stock, preferred stock, debt securities, depository shares or warrants in one or more offering up to a total dollar value of $30,000,000. The registered direct financing which closed on March 18, 2010 was effected under this “shelf” registration.
On April 21, 2009, the Company issued 38,276 shares of its common stock valued at $110,000 under the terms and conditions of a settlement agreement.
On January 11, 2010, the Company sold 1,886,291 shares of its common stock for an aggregate of approximately $1,744,000. The Company also received commitments to purchase an additional 1,358,475 shares of its common stock for an aggregate of approximately $1,256,000 subject to obtaining stockholder approval which was received on March 3, 2010. The additional shares were issued on March 19, 2010. Related to this equity issuance transaction, the Company issued 101,548 shares to Marchant, a related party, for its placement services (Note 6)and incurred cash issuance costs of $260,000.
On March 18, 2010, the Company closed a registered direct financing in which it sold 1,552,795 shares of its common stock and warrants to purchase 621,118 shares of its common stock for gross proceeds of approximately $5,000,000 with associated cash issuance costs of $617,000. The investors agreed to purchase the shares and warrants for $3.22 per unit (each unit consisting of one share and a warrant to purchase 0.4 shares of common stock). The exercise price of the warrants is $4.00 per share. The warrants are exercisable at any time on or after the sixth-month anniversary of the closing date through and until the 18-month anniversary of the closing of the offering.
(c) Stock Option Plan
The Company has a stock option plan, the 2002 Stock Option Plan (the "Stock Option Plan"), which was most recently amended on June 24, 2010 in order to increase the shares reserved under the Stock Option Plan by 800,000. Under the Stock Option Plan, up to 3,200,000 options are available for grant to employees, directors and consultants. Options granted under the Stock Option Plan may be either incentive stock options or non-statutory stock options. Under the terms of the Stock Option Plan, the exercise price per share for an incentive stock option shall not be less than the fair market value of a share of stock on the effective date of grant and the exercise price per share for non-statutory stock options shall not be less than 85% of the fair market value of a share of stock on the date of grant. No option granted to a holder of more than 10% of the Company's common stock shall have an exercise price per share less than 110% of the fair market value of a share of stock on the effective date of grant.
Options granted are typically service-based options. Generally, options expire 10 years after the date of grant. No incentive stock options granted to a 10% owner optionee shall be exercisable after the expiration of five years after the effective date of grant of such option, no option granted to a prospective employee, prospective consultant or prospective director may become exercisable prior to the date on which such person commences service, and with the exception of an option granted to an officer, director or consultant, no option shall become exercisable at a rate less than 20% per annum over a period of five years from the effective date of grant of such option unless otherwise approved by the Board.
The Company accounts for stock-based compensation under the authoritative guidance which requires that share-based payment transactions with employees be recognized in the financial statements based on their fair value and recognized as compensation expense over the vesting period. The amount of expense recognized during the period is affected by many complex and subjective assumptions, including: estimates of the Company’s future volatility, the expected term for its stock options, option exercise behavior, the number of options expected to ultimately vest, and the timing of vesting for the Company’s share-based awards.
The following table sets forth the total stock-based compensation expense resulting from stock options included in the Company's consolidated statements of operations (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
General and administrative
|
$ | 1,051 | $ | 299 | $ | 152 | ||||||
|
Clinical and regulatory
|
202 | 160 | 109 | |||||||||
|
Sales and marketing
|
142 | 60 | (76 | ) | ||||||||
|
Stock-based compensation expense before income taxes
|
$ | 1,395 | $ | 519 | $ | 185 | ||||||
Net cash proceeds from the exercise of common stock options were $22,000, $0 and $0 for the years ended December 31, 2010, 2009 and 2008, respectively. No income tax benefit was realized from stock option exercises during the years ended December 31, 2010, 2009 and 2008. The Company presents excess tax benefits from the exercise of stock options, if any, as financing cash flows rather than operating cash flows.
The weighted-average fair value of stock options granted during the years ended December 31, 2010, 2009 and 2008 was $1.69, $1.04, and $2.17, respectively.
The estimated fair value of stock options for the periods presented was determined using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Volatility
|
112 | % | 102 | % | 115 | % | ||||||
|
Expected life of options
|
5.55 years
|
5.54 years
|
4.22 years
|
|||||||||
|
Risk-free interest rate
|
2.12 | % | 2.06 | % | 1.82 | % | ||||||
|
Dividend yield
|
0 | % | 0 | % | 0 | % | ||||||
The Company's computation of expected volatility is based on the Company's historical stock prices after its initial public offering in December 2004 and for prior periods a comparable company's historical stock prices were used as the Company did not have sufficient historical data. Due to the lack of sufficient historical data, the Company's computation of expected life was estimated using the "short-cut approach" as provided in SAB No. 110 as options granted by the Company meet the criteria of "plain vanilla" options as defined in SAB No. 110. Under this approach, estimated life is calculated to be the mid-point between the vesting date and the end of the contractual period. The risk-free interest rate for an award is based on the U.S. Treasury yield curve with a term equal to the expected life of the award on the date of grant.
A summary of the options issued during the year ended December 31, 2010 and the total number of options outstanding as of that date and changes since December 31, 2007 are set forth below:
|
Number of Options Outstanding
|
Weighted Average Exercise Price
|
Weighted Average Remaining Contractual Life (years)
|
Aggregate Intrinsic Value
|
|||||||||||||
|
Outstanding, December 31, 2007
|
191,500 | $ | 41.04 | 7.41 | $ | — | ||||||||||
|
Assumption of OcuSense stock options outstanding
|
673,034 | 1.35 | ||||||||||||||
|
Granted
|
1,452,166 | 2.79 | ||||||||||||||
|
Exercised
|
— | — | ||||||||||||||
|
Forfeited/cancelled
|
(38,217 | ) | 40.30 | |||||||||||||
|
Outstanding, December 31, 2008
|
2,278,483 | 4.95 | 8.41 | $ | 522,547 | |||||||||||
|
Granted
|
1,200,864 | 1.39 | ||||||||||||||
|
Exercised
|
— | — | ||||||||||||||
|
Forfeited/cancelled/expired
|
(122,840 | ) | 7.21 | |||||||||||||
|
Outstanding, December 31, 2009
|
3,356,507 | $ | 3.59 | 8.01 | $ | 304,471 | ||||||||||
|
Granted
|
284,383 | 2.14 | ||||||||||||||
|
Exercised
|
(9,572 | ) | 2.25 | |||||||||||||
|
Forfeited/cancelled/expired
|
(54,775 | ) | 5.51 | |||||||||||||
|
Outstanding, December 31, 2010
|
3,576,543 | $ | 3.46 | 7.18 | $ | 899,010 | ||||||||||
|
Vested or expected to vest, December 31, 2010
|
2,979,582 | $ | 3.72 | 7.24 | $ | 899,010 | ||||||||||
|
Exercisable, December 31, 2010
|
2,887,807 | $ | 3.40 | 6.85 | $ | 899,010 | ||||||||||
The aggregate intrinsic value at December 31, 2010 represents the total pre-tax intrinsic value, calculated as the difference between the Company's closing stock price on the last trading day of the respective fiscal year and the exercise price, multiplied by the number of shares that would have been received by the option holders if the options that could be exercised had been exercised on such date.
The total fair value of stock options vested during the years ended December 31, 2010, 2009, and 2008 was $1,218,000, $394,000, and $441,000, respectively.
As of December 31, 2010, $1,639,000 of total unrecognized compensation cost related to stock options is expected to be recognized over a weighted-average period of 1.74 years.
As of December 31, 2010, the Company had 251,000 options remaining in the Stock Option Plan available for grant.
(d) Warrants
On February 6, 2007, pursuant to the 2007 Securities Purchase Agreement between the Company and certain institutional investors, the Company issued warrants to these investors (“2007 Warrants”). The 2007 Warrants are five-year warrants exercisable immediately into an aggregate of 127,050 shares of the Company's common stock at $46.25 per common share. On February 6, 2007, the Company also issued a warrant, (“the Cowen Warrant”) to Cowen and Company, LLC in partial payment of the placement fee for the services it had rendered as the placement agent in connection with the private placement of the shares and the 2007 Warrants pursuant to the 2007 Securities Purchase Agreement. The Cowen Warrant is a five-year warrant exercisable into an aggregate of 4,447 shares of the Company's common stock. The per share exercise price of the Cowen Warrant is $46.25, and the Cowen Warrant became exercisable on August 6, 2007.
The Company accounts for the 2007 Warrants and the Cowen Warrant in accordance with accounting guidance which requires every derivative instrument within its scope to be recorded on the balance sheet as either an asset or liability measured at its fair value, with changes in fair value recognized in earnings unless specific hedge accounting criteria are met. Based on guidance applicable to derivatives, the Company determined that the 2007 Warrants and the Cowen Warrant do not meet the criteria for classification as equity. Accordingly, the Company classified the 2007 Warrants and the Cowen Warrant as current liabilities at December 31, 2010 and 2009, respectively.
The estimated fair value of the 2007 Warrants and the Cowen Warrant at December 31, 2010 was determined using the Black-Scholes option-pricing model with the following weighted average assumptions:
|
Volatility
|
108 | % | ||
|
Expected life of Warrants
|
1.08 years
|
|||
|
Risk-free interest rate
|
0.32 | % | ||
|
Dividend yield
|
0 | % | ||
The Company is required to record the outstanding warrants at fair value at the end of each reporting period, resulting in an adjustment to the recorded liability of the derivative, with any gain or loss recorded in earnings of the applicable reporting period. The Company, therefore, estimated the fair value of the 2007 Warrants and the Cowen Warrant as of December 31, 2010 and determined the aggregate fair value to be $1,000, as compared to $3,000 as of December 31, 2009. This decrease of $2,000 was recorded as a gain in other income (expense) in the consolidated statement of operations during the year ended December 31, 2010.
On March 18, 2010, the Company closed a registered direct financing in which it sold approximately 1,552,796 shares of its common stock and warrants ( “2010 Warrants”) to purchase 621,118 shares of its common stock for gross proceeds of approximately $5,000,000. The investors agreed to purchase the shares and warrants for $3.22 per unit (each unit consisting of one share and a warrant to purchase 0.4 shares of common stock). The exercise price of the 2010 Warrants is $4.00 per share. The 2010 Warrants are exercisable at any time on or after the sixth-month anniversary of the closing date through and until the 18-month anniversary of the closing of the offering.
The Company accounts for the 2010 Warrants in accordance with applicable guidance to derivatives, the Company determined that the 2010 Warrants do not meet the criteria for classification as equity. Accordingly, the Company classified the 2010 Warrants as current liabilities at December 31, 2010.
The estimated fair value of the Warrants at March 18, 2010 was determined to be $737,000, using the Black-Scholes option-pricing model with the following weighted average assumptions:
|
Volatility
|
113 | % | ||
|
Expected life of Warrants
|
1.50 years
|
|||
|
Risk-free interest rate
|
0.70 | % | ||
|
Dividend yield
|
0 | % | ||
The estimated fair value of the Warrants at December 31, 2010 was determined to be $38,000, using the Black-Scholes option-pricing model with the following weighted average assumptions:
|
Volatility
|
53 | % | ||
|
Expected life of Warrants
|
0.75 years
|
|||
|
Risk-free interest rate
|
0.24 | % | ||
|
Dividend yield
|
0 | % | ||
The Company is required to record the outstanding warrants at fair value at the end of each reporting period, resulting in an adjustment to the obligations under warranty, with any gain or loss recorded in earnings of the applicable reporting period. The Company, therefore, estimated the fair value of the 2010 Warrants as of December 31, 2010 and determined the aggregate fair value to be $38,000, with a decrease of approximately $699,000 over the measurement of the aggregate fair value of the 2010 Warrants on March 18, 2010. This decrease was recorded as a gain in other income (expense) in the consolidated statement of operations during the year ended December 31, 2010.
The following table provides activity for the Warrants issued and outstanding during the three years ended December 31, 2010 (in thousands, except weighted average exercise prices):
|
Number of warrants outstanding
|
Weighted average exercise price
|
|||||||
|
Outstanding, December 31, 2008
|
132 | $ | 46.25 | |||||
|
Granted
|
— | — | ||||||
|
Forfeited
|
— | — | ||||||
|
Outstanding, December 31, 2009
|
132 | 46.25 | ||||||
|
Granted
|
621 | 4.00 | ||||||
|
Forfeited
|
— | — | ||||||
|
Outstanding, December 31, 2010
|
753 | $ | 11.38 | |||||
In connection with the Financing which the Company was engaged in July and August 2009, the Company will issue 109,389 warrants (“Financing Warrants”) with a life of five years to purchase 109,389 shares of common stock if the aggregate principal amount of the notes issued in the Financing are converted to common shares of the Company. The exercise price of the Financing Warrants is $1.60 per common share representing the price per share equal to the closing bid price per share of the Company’s common stock on the NASDAQ stock market on July 15, 2009. The warrants will not be issued until the convertible secured notes are converted to common shares of the Company and will be exerciseable upon issuance. The Company has recorded $163,000 representing the fair value of the Financing Warrants at the issuance date which has been calculated using the Black-Scholes value model. The value of the Financing Warrants is being amortized over the two year term of the convertible secured debt and a charge of $41,000 is included in other income (expense) at December 31, 2010.
13. CONSOLIDATED STATEMENTS OF CASH FLOWS
The net change in working capital and non-current asset balances related to operations consists of the following (in thousands):
|
Years ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Accounts receivable
|
(163 | ) | 184 | 42 | ||||||||
|
Due from related parties
|
(130 | ) | — | — | ||||||||
|
Inventory
|
(359 | ) | (48 | ) | (148 | ) | ||||||
|
Prepaid expenses
|
16 | (22 | ) | 165 | ||||||||
|
Other current assets
|
17 | (9 | ) | (11 | ) | |||||||
|
Other non-current assets
|
100 | (170 | ) | — | ||||||||
|
Accounts payable
|
(95 | ) | 133 | (878 | ) | |||||||
|
Accrued liabilities
|
347 | 2 | (1,672 | ) | ||||||||
|
Deferred revenue
|
(23 | ) | (87 | ) | 237 | |||||||
|
Due to stockholders
|
(10 | ) | 15 | (10 | ) | |||||||
| $ | (300 | ) | $ | (2 | ) | $ | (2,275 | ) | ||||
The following table lists those items that have been excluded from the consolidated statements of cash flows as they relate to non-cash transactions and additional cash flow information (in thousands):
|
Years ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Warrant issued in registered direct financing
|
737 | — | — | |||||||||
|
Warrant value arising from issuance of convertible debt financing
|
— | 163 | — | |||||||||
|
Beneficial conversion feature value arising from issuance of convertible debt funding
|
— | 728 | — | |||||||||
|
Common stock issued in a settlement agreement
|
— | 110 | — | |||||||||
|
Common stock issued on merger
|
— | — | 7,925 | |||||||||
|
Non-controlling Interest Acquired
|
— | — | 3,112 | |||||||||
|
Conversion of bridge loans and accrued interest to common stock
|
— | — | 8,261 | |||||||||
|
Common stock issued to Marchant Securities for services provided in the PIPE and bridge loan transactions.
|
155 | — | 481 | |||||||||
There were no interest or income taxes paid for the years ended December 31, 2010, 2009 & 2008.
14. SEGMENT and GEOGRAPHIC INFORMATION
Up to December 31, 2008, the Company had two operating segments: retina and point-of-care. The retina segment was in the business of commercializing the RHEO™ System which was used to perform the Rheopheresis™ procedure, a procedure that selectively removes molecules from plasma, which is designed to treat Dry AMD. On November 1, 2007, the Company announced an indefinite suspension of the RHEO™ System clinical development program for Dry AMD. That decision was made following a comprehensive review of the respective costs and development timelines associated with the products in the Company’s portfolio.The only costs incurred during 2009, 2010 and 2008 in relation to the retina segment are the clean-up costs for clinical sites. As a result of the progressive wind down of RHEO-AMD activities, the Company’s only business segment is the point-of-care business.
The Company’s reportable units in the year ended December 31, 2008 were strategic business units that offered different products and services. They were managed separately, because each business unit required different technology and marketing strategies. The business units’ managements were acquired or developed individually.
The Company’s business units are as follows (in thousands):
|
Year ended December 31, 2008
|
||||||||||||
|
Retina
|
Point-of-care
|
Total
|
||||||||||
|
Revenue
|
$ | 158 | $ | 300 | $ | 458 | ||||||
|
Expenses:
|
||||||||||||
|
Cost of goods sold
|
26 | 137 | 163 | |||||||||
|
Operating
|
3,216 | 4,715 | 7,931 | |||||||||
|
Depreciation and amortization
|
26 | 1,267 | 1,293 | |||||||||
|
Restructuring charges
|
2,441 | — | 2,441 | |||||||||
|
Loss from continuing operations
|
(5,551 | ) | (5,819 | ) | (11,370 | ) | ||||||
|
Interest income
|
71 | 6 | 77 | |||||||||
|
Interest expense and finance fees
|
(498 | ) | — | (498 | ) | |||||||
|
Changes in fair value of warrant obligation
|
(58 | ) | — | (58 | ) | |||||||
|
Gain on short-term investment
|
1,036 | — | 1,036 | |||||||||
|
Beneficial conversion on bridge loan shares issued
|
— | (1,239 | ) | (1,239 | ) | |||||||
|
Other income (expense), net
|
288 | 80 | 368 | |||||||||
|
Non-controlling interest
|
— | 1,978 | 1,978 | |||||||||
|
Recovery of income taxes
|
— | 338 | 338 | |||||||||
|
Loss from continuing operations
|
$ | (4,712 | ) | $ | (4,656 | ) | $ | (9,368 | ) | |||
|
Total assets
|
$ | 2,391 | $ | 11,014 | $ | 13,405 | ||||||
The following table provides geographic information related to the Company's fixed assets and revenues (in thousands):
|
United States
|
Canada
|
Rest of World
|
Total
|
|||||||||||||
|
December 31, 2010
|
||||||||||||||||
|
Fixed assets
|
$ | 126 | $ | - | $ | - | $ | 126 | ||||||||
|
Revenues
|
$ | 1,109 | $ | 97 | $ | 495 | $ | 1,701 | ||||||||
|
December 31, 2009
|
||||||||||||||||
|
Fixed assets
|
$ | 139 | $ | 1 | $ | - | $ | 140 | ||||||||
|
Revenues
|
$ | 184 | $ | 90 | $ | 594 | $ | 868 | ||||||||
|
December 31, 2008
|
||||||||||||||||
|
Fixed assets
|
$ | 179 | $ | 4 | $ | - | $ | 183 | ||||||||
|
Revenue
|
$ | - | $ | - | $ | 458 | $ | 458 | ||||||||
15. QUARTERLY FINANCIAL DATA (UNAUDITED)
The following tables contain selected unaudited consolidated statement of operations data for each quarter of fiscal 2010 and 2009 (in thousands, except per share data):
|
Fiscal 2010 Quarter Ended ($ 000’s)
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenue
|
$ | 275 | $ | 418 | $ | 210 | $ | 798 | ||||||||
|
Gross profit
|
86 | 220 | 105 | 441 | ||||||||||||
|
Loss from operations
|
(1,926 | ) | (1,752 | ) | (1,726 | ) | (1,540 | ) | ||||||||
|
Net loss
|
$ | (2,080 | ) | $ | (1,540 | ) | $ | (2,156 | ) | $ | (907 | ) | ||||
|
Weighted average number of shares outstanding basic and diluted
|
12,053 | 14,766 | 14,766 | 14,770 | ||||||||||||
|
Net loss per common share basic and diluted (i)
|
$ | (0.17 | ) | $ | (0.10 | ) | $ | (0.15 | ) | $ | (0.06 | ) | ||||
|
Fiscal 2009 Quarter Ended ($ 000’s)
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenue
|
$ | 243 | $ | 97 | $ | 263 | $ | 265 | ||||||||
|
Gross profit
|
124 | (34 | ) | 124 | 86 | |||||||||||
|
Loss from continuing operations
|
(1,709 | ) | (1,723 | ) | (1,092 | ) | (1,402 | ) | ||||||||
|
Net loss
|
$ | (1,003 | ) | $ | (1,020 | ) | $ | (655 | ) | $ | (1,707 | ) | ||||
|
Weighted average number of shares outstanding basic and diluted
|
9,828 | 9,858 | 9,867 | 9,867 | ||||||||||||
|
Net loss per common share basic and diluted (i)
|
$ | (0.10 | ) | $ | (0.10 | ) | $ | (0.07 | ) | $ | (0.17 | ) | ||||
|
(i)
|
Net loss per share basic and diluted are computed independently for the quarters presented. Therefore, the sum of the quarterly per share information may not be equal to the annual per share information.
|
ITEM 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not applicable.
ITEM 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to the our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefit of controls must be considered relative to their costs. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of the end of the period covered by the report, we carried out an evaluation, under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)). Based on that evaluation, our chief executive officer and chief financial officer concluded that, as at December 31, 2010 our disclosure controls and procedures were effective at the reasonable assurance level.
MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Because of its inherent limitations, internal control over financial reporting may not prevent or detect all misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
We conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2010.
This annual report does not include an attestation report of the Company's independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s independent registered public accounting firm pursuant to the rules of the SEC that permit the Company to provide only a management’s report in this report.
There has been no change in our internal control over financial reporting that occurred during the most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. Other Information.
None.
PART III
ITEM 10. Directors, Executive Officers and Corporate Governance.
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
ITEM 11. Executive Compensation.
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
ITEM 14. Principal Accountant Fees and Services.
The information required by this item will be set forth in the Proxy Statement and is incorporated in this report by reference.
PART IV
ITEM 15. Exhibits and Financial Statement Schedules.
|
(1)
|
Financial Statements included in PART II of this report:
|
|
Included in PART II of this report:
|
Page
|
|
|
Reports of Independent Registered Public Accounting firms
|
48
|
|
|
Consolidated Balance Sheets as of December 31, 2010 and December 31, 2009
|
49
|
|
|
Consolidated Statements of Operations for the three years ended December 31, 2010
|
50
|
|
|
Consolidated Statements of Stockholders’ Equity for the three years ended December 31, 2010
|
51
|
|
|
Consolidated Statements of Cash Flows for the three years ended December 31, 2010
|
52
|
|
|
Notes to Consolidated Financial Statements
|
53
|
|
(2)
|
Financial Statement Schedules:
|
SCHEDULE II -- VALUATION AND QUALIFYING ACCOUNTS
|
Balance at beginning of period
|
Provision
|
Write-off and recoveries
|
Balance at end of period
|
|||||||||||||
|
(in thousands)
|
||||||||||||||||
|
Fiscal 2008
|
||||||||||||||||
|
Bad debt reserves
|
$ | 173 | $ | — | $ | (173 | ) | $ | — | |||||||
|
Inventory reserves
|
7,296 | 68 | (7,296 | ) | 68 | |||||||||||
|
Fiscal 2009
|
||||||||||||||||
|
Bad debt reserves
|
— | 9 | (2 | ) | 7 | |||||||||||
|
Inventory reserves
|
68 | 56 | — | 124 | ||||||||||||
|
Product warranties
|
— | 15 | 6 | 9 | ||||||||||||
|
Fiscal 2010
|
||||||||||||||||
|
Bad debt reserves
|
7 | 39 | (33 | ) | 13 | |||||||||||
|
Inventory reserves
|
124 | — | (8 | ) | 116 | |||||||||||
|
Product warranties
|
$ | 9 | $ | 55 | $ | (13 | ) | $ | 51 | |||||||
|
(3)
|
List of exhibits required by Item 601 of Regulation S-K. See part (b) below.
|
(b) Exhibits: The following exhibits are filed as a part of this report:
All other financial statement schedules have been omitted because they are not applicable, not required or the information required is shown in the financial statements or the notes thereto.
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
2.1
|
Form of Plan of Reorganization.
|
Exhibit 2.1 to the Registrant's Registration Statement on Form S-1/A No. 4, filed with the Commission on December 6, 2004 (file no. 333-118024)
|
||
|
3.1
|
Restated Certificate of Incorporation of OccuLogix, Inc., filed with the Secretary of State of the State of Delaware on October 7, 2008.
|
Exhibit 3.3 to the Registrant's Current Report on Form 8-K filed with the Commission on October 9, 2008 (file no. 000-51030)
|
||
|
3.2
|
Amended and Restated By-Laws of the Registrant as currently in effect.
|
Exhibit 10.4 to the Registrant's Registration Statement on Form S-1/A No. 3, filed with the Commission on November 16, 2004 (file no. 333-118024)
|
||
|
3.3
|
Certificate of Amendment of OccuLogix, Inc., filed with the Secretary of State of the State of Delaware on October 7, 2008.
|
Exhibit 3.2 to the Registrant's Current Report on Form 8-K filed with the Commission on October 9, 2008 (file no. 000-51030)
|
||
|
3.4
|
Restated Certificate of Incorporation of OccuLogix, Inc., filed with the Secretary of State of the State of Delaware on October 7, 2008.
|
Restated Certificate of Incorporation of OccuLogix, Inc., filed with the Secretary of State of the State of Delaware on October 7, 2008.
|
||
|
3.5
|
Certificate of Amendment of OccuLogix, Inc., filed with the Secretary of State of the State of Delaware on May 18, 2010.
|
Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed with the Commission on May 19, 2010 (file no. 000-51030)
|
||
|
4.1
|
Form of Common Stock Purchase Warrant Agreement
|
Exhibit A to the Registrant's free writing prospectus filed with the Commission on March 17, 2010 (file no. 333-157269)
|
||
|
4.2
|
Form of Common Stock Purchase Warrant Agreement
|
Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed with the Commission on July 16, 2009 (file no. 000-51030)
|
||
|
10.1
|
Series A Stock Purchase Agreement by and among TearLab, Inc. and the Registrant dated as of November 30, 2006.
|
Exhibit 10.45 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030) (Exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K and will be provided to the Securities and Exchange Commission upon request.)
|
||
|
10.2
|
Securities Purchase Agreement, dated as of February 1, 2007, by and among the Registrant and the investors listed on the Schedule of Investors attached thereto as Exhibit A.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K, filed with the Commission on February 6, 2007 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.3
|
#
|
Employment Agreement between the Registrant and Suh Kim dated as of March 12, 2007.
|
Exhibit 10.47 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030)
|
|
|
10.4
|
License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003.
|
Exhibit 10.48 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030) (Portions of this exhibit have been omitted pursuant to a request for confidential treatment.)
|
||
|
10.5
|
Amendment No. 1, dated June 9, 2003, to the License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003.
|
Exhibit 10.49 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030)
|
||
|
10.6
|
Amendment No. 2, dated September 5, 2005, to the License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003.
|
Exhibit 10.50 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030) (Portions of this exhibit have been omitted pursuant to a request for confidential treatment.)
|
||
|
10.7
|
Amendment No. 3, dated July 7, 2006, to the License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003.
|
Exhibit 10.51 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030)
|
||
|
10.8
|
Amendment No. 4, dated October 9, 2006, to the License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003.
|
Exhibit 10.52 to the Registrant's Annual Report on Form 10-K/A, filed with the Commission on March 29, 2007 (file no. 000-51030)
|
||
|
10.9
|
Terms of Business, dated February 5, 2007, between Invetech Pty Ltd. and TearLab, Inc.
|
Exhibit 10.30 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.10
|
Amendment No. 5, dated June 29, 2007, to the License Agreement between TearLab, Inc. and The Regents of the University of California dated March 12, 2003. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment.)
|
Exhibit 10.31 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.11
|
Lease, dated October 17, 2005, between Penyork Properties III Inc. and the Registrant.
|
Exhibit 10.32 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.12
|
Lease Amending Agreement, dated as of March 9, 2007, between the Registrant and 2600 Skymark Investments Inc., amending the Lease between Penyork Properties III Inc. and the Registrant dated October 17, 2005.
|
Exhibit 10.33 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.13
|
2002 Stock Option Plan, as amended and restated on June 24, 2010.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K, filed with the Commission on July 23, 2010 (file no. 000-51030)
|
||
|
10.14
|
Manufacturing and Development Agreement, dated October 25, 2007, between MiniFAB (Aust) Pty Ltd and TearLab, Inc. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment.)
|
Exhibit 10.35 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.15
|
First Amendment to Series A Preferred Stock Purchase Agreement, dated October 29, 2007, between TearLab, Inc. and the Registrant
|
Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on November 9, 2007 (file no. 000-51030)
|
||
|
10.16
|
Research Agreement, dated as of December 13, 2007, between * and TearLab, Inc. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment.)
|
Exhibit 10.37 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.17
|
Stock Purchase Agreement, dated as of December 19, 2007, between the Registrant and Solx Acquisition, Inc. (Exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K and will be provided to the Commission upon request.)
|
Exhibit 10.38 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.18
|
Amending Agreement, dated as of December 19, 2007, by and among the Registrant, Solx, Inc. and Peter M. Adams, acting for and on behalf of the Stockholder Representative Committee, amending the Agreement and Plan of Merger, dated as of August 1, 2006, by and among the Registrant, OccuLogix Mergeco, Inc., Solx, Inc. and Doug P. Adams, John Sullivan and Peter M. Adams, acting in each case, in his capacity as a member of the Stockholder Representative Committee referred to therein.
|
Exhibit 10.39 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.19
|
#
|
Termination Agreement, dated as of December 19, 2007, between Doug P. Adams and the Registrant, terminating the Employment Agreement between the Registrant and Doug P. Adams dated as of September 1, 2006.
|
Exhibit 10.40 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.20
|
Limited Guaranty, dated as of December 19, 2007, by Doug P. Adams for the benefit of the Registrant.
|
Exhibit 10.41 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.21
|
Security Agreement, dated as of December 19, 2007, by Solx, Inc. in favor of the Registrant.
|
Exhibit 10.42 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.22
|
Letter Agreement, dated December 20, 2007, between the Registrant and Solx Acquisition, Inc.
|
Exhibit 10.43 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.23
|
#
|
Termination Agreement, dated as of January 4, 2008, between John Cornish and the Registrant, terminating the Employment Agreement between the Registrant and John Cornish dated as of April 1, 2005, as amended.
|
Exhibit 10.44 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.24
|
#
|
Termination Agreement, dated as of January 4, 2008, between Julie Fotheringham and the Registrant, terminating the Employment Agreement between the Registrant and Julie Fotheringham dated September 1, 2004.
|
Exhibit 10.45 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.25
|
#
|
Termination Agreement, dated as of January 4, 2008, between Stephen Parks and the Registrant, terminating the Employment Agreement between Stephen Parks and the Registrant dated as of October 4, 2005.
|
Exhibit 10.46 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.26
|
#
|
Termination Agreement, dated as of January 8, 2008, between David C. Eldridge and the Registrant, terminating the Employment Agreement between the Registrant and Dr. David Eldridge dated November 9, 2004.
|
Exhibit 10.47 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.27
|
#
|
Termination Agreement, dated as of January 31, 2008, between Nozhat Choudry and the Registrant, terminating the Employment Agreement between Nozhat Choudry and the Registrant, as amended.
|
Exhibit 10.48 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.28
|
#
|
Termination Agreement, dated as of January 31, 2008, between Stephen Kilmer and the Registrant, terminating the Employment Agreement between the Registrant and Stephen Kilmer dated July 30, 2004.
|
Exhibit 10.49 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.29
|
Loan Agreement, dated as of February 19, 2008, by and among the Registrant, the Lenders named therein and Marchant Securities Inc.
|
Exhibit 10.50 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.30
|
Share Pledge Agreement, dated as of February 19, 2008, by the Registrant in favor of Marchant Securities Inc., as collateral agent.
|
Exhibit 10.51 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
||
|
10.31
|
#
|
Employment Agreement, dated as of February 25, 2008, between the Registrant and William G. Dumencu.
|
Exhibit 10.52 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.32
|
#
|
Termination Agreement, dated as of February 25, 2008, between Asahi Kasei Kuraray Medical Co., Ltd. and the registrant.
|
Exhibit 10.53 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.33
|
#
|
Amending Agreement, dated as of March 3, 2008, between Nozhat Choudry and the Registrant, amending the Termination Agreement between Nozhat Choudry and the Registrant dated as of January 31, 2008.
|
Exhibit 10.54 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.34
|
#
|
Amending Agreement, dated as of March 3, 2008, between John Cornish and the Registrant, amending the Termination Agreement between John Cornish and the Registrant dated as of January 4, 2008.
|
Exhibit 10.55 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.35
|
#
|
Amending Agreement, dated as of March 3, 2008, between David C. Eldridge and the Registrant, amending the Termination Agreement between David C. Eldridge and the Registrant dated as of January 8, 2008.
|
Exhibit 10.56 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.36
|
#
|
Amending Agreement, dated as of March 3, 2008, between Julie Fotheringham and the Registrant, amending the Termination Agreement between Julie Fotheringham and the Registrant dated as of January 4, 2008.
|
Exhibit 10.57 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
|
10.37
|
#
|
Amending Agreement, dated as of March 3, 2008, between Stephen Parks and the Registrant, amending the Termination Agreement between Stephen Parks and the Registrant dated as of January 4, 2008.
|
Exhibit 10.58 to the Registrant's Annual Report on Form 10-K, filed with the Commission on March 17, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.38
|
Agreement and Plan of Merger and Reorganization, dated April 22, 2008, by and among the Registrant, OcuSense Acquireco, Inc. and TearLab, Inc. (formerly known as OcuSense, Inc.) (Exhibits have been omitted pursuant to Item 601(b)(2) of Regulation S-K and will be provided to the Securities and Exchange Commission upon request.)
|
Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on May 12, 2008 (file no. 000-51030)
|
||
|
10.39
|
Amending Agreement, dated as of May 5, 2008, by and among the Registrant, the lenders listed on the Schedule of New Lenders attached there to as Exhibit A, the lenders listed the Schedule of Required Lenders attached thereto as Exhibit B and Marchant Securities Inc., amending the Loan Agreement, dated as of February 19, 2008, by and among the Registrant, the Lenders named therein and Marchant Securities Inc. and the Share Pledge Agreement, dated as of February 19, 2008, by the Registrant in favor of Marchant Securities Inc., as collateral agent.
|
Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on May 12, 2008 (file no. 000-51030)
|
||
|
10.40
|
Securities Purchase Agreement, dated as of May 19, 2008, by and among OccuLogix, Inc., Marchant Securities Inc. and the investors listed on the Schedule of Investors attached thereto as Exhibit A.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K, filed with the Commission on May 21, 2008 (file no. 000-51030)
|
||
|
10.41
|
Amending Agreement by and among OccuLogix, Inc., Marchant Securities Inc. and the investor party thereto.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on July 28, 2008 (file no. 000-51030)
|
||
|
10.42
|
Amending Agreement, dated as of July 28, 2008, by and among OccuLogix, Inc., OcuSense Acquireco, Inc. and TearLab, Inc. (formerly known as OcuSense, Inc.)
|
Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed with the Commission on July 28, 2008 (file no. 000-51030)
|
||
|
10.43
|
#
|
Second Amending Agreement, dated as of June 16, 2008, between John Cornish and the Registrant, amending the Termination Agreement between the Registrant and John Cornish dated as of January 4, 2008, as amended.
|
Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.44
|
#
|
Second Amending Agreement, dated as of June 16, 2008, between Julie Fotheringham and the Registrant, amending the Termination Agreement between the Registrant and Julie Fotheringham dated as of January 4, 2008, as amended.
|
Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.45
|
#
|
Amending Agreement, dated as of June 16, 2008, between Stephen Kilmer and the Registrant, amending the Termination Agreement between the Registrant and Stephen Kilmer dated as of January 31, 2008.
|
Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.46
|
#
|
Second Amending Agreement, dated as of June 16, 2008, between David C. Eldridge and the Registrant, amending the Termination Agreement between the Registrant and David C. Eldridge dated as of January 8, 2008, as amended.
|
Exhibit 10.4 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.47
|
#
|
Second Amending Agreement, dated as of June 16, 2008, between Stephen Parks and the Registrant, amending the Termination Agreement between the Registrant and Stephen Parks dated as of January 4, 2008, as amended.
|
Exhibit 10.5 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.48
|
#
|
Second Amending Agreement, dated as of June 19, 2008, between Nozhat Choudry and the Registrant, amending the Termination Agreement between the Registrant and Nozhat Choudry dated as of January 31, 2008, as amended.
|
Exhibit 10.6 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.49
|
#
|
Termination Agreement, dated as of June 30, 2008, between Thomas P. Reeves and the Registrant, terminating the Employment Agreement between the Registrant and Thomas P. Reeves dated as of August 1, 2004, as amended.
|
Exhibit 10.7 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.50
|
#
|
Third Amending Agreement, dated as of July 25, 2008, between John Cornish and the Registrant, amending the Termination Agreement between the Registrant and John Cornish dated as of January 4, 2008, as amended.
|
Exhibit 10.8 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.51
|
#
|
Third Amending Agreement, dated as of July 25, 2008, between Julie Fotheringham and the Registrant, amending the Termination Agreement between the Registrant and Julie Fotheringham dated as of January 4, 2008, as amended.
|
Exhibit 10.9 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.52
|
#
|
Second Amending Agreement, dated as of July 25, 2008, between Stephen Kilmer and the Registrant, amending the Termination Agreement between the Registrant and Stephen Kilmer dated as of January 31, 2008, as amended.
|
Exhibit 10.10 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.53
|
#
|
Third Amending Agreement, dated as of July 25, 2008, between David C. Eldridge and the Registrant, amending the Termination Agreement between the Registrant and David C. Eldridge dated as of January 8, 2008, as amended.
|
Exhibit 10.11 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.54
|
#
|
Third Amending Agreement, dated as of July 25, 2008, between Stephen Parks and the Registrant, amending the Termination Agreement between the Registrant and Stephen Parks dated as of January 4, 2008, as amended.
|
Exhibit 10.12 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.55
|
#
|
Third Amending Agreement, dated as of July 25, 2008, between Nozhat Choudry and the Registrant, amending the Termination Agreement between the Registrant and Nozhat Choudry dated as of January 31, 2008, as amended.
|
Exhibit 10.13 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.56
|
#
|
Amending Agreement, dated as of July 25, 2008, between Thomas P. Reeves and the Registrant, amending the Termination Agreement between the Registrant and Thomas P. Reeves dated as of June 30, 2008.
|
Exhibit 10.14 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
|
|
10.57
|
Second Amending Agreement, dated as of July 28, 2008, by and among the Registrant, the lenders listed on the Schedule of Second New Lenders attached thereto as Exhibit A, the lenders listed on the Schedule of Required Lenders attached thereto as Exhibit B and Marchant Securities Inc., amending the Loan Agreement, dated as of February 19, 2008, by and among the Registrant, the lenders listed on the Schedule of Lenders attached thereto as Exhibit A and Marchant Securities Inc., as amended, and amending the Share Pledge Agreement, dated as of February 19, 2008, by the Registrant in favor of Marchant Securities Inc., as collateral agent, as amended.
|
Exhibit 10.15 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 11, 2008 (file no. 000-51030)
|
||
|
10.58
|
Loan Agreement, dated as of August 13, 2008, by and among OccuLogix, Inc. and TearLab, Inc. (formerly known as OcuSense, Inc.)
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on August 15, 2008 (file no. 000-51030)
|
||
|
10.59
|
Second Amending Agreement, dated as of October 6, 2008, by and among OccuLogix, Inc., OcuSense Acquireco, Inc. and TearLab, Inc. (formerly known as OcuSense, Inc.)
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on October 9, 2008 (file no. 000-51030)
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
10.60
|
Second Amending Agreement, dated as of October 1, 2008, by and among OccuLogix, Inc., Marchant Securities Inc. and the investors listed on the Schedule of Investors attached thereto as Exhibit A.
|
Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed with the Commission on October 9, 2008 (file no. 000-51030)
|
||
|
10.61
|
Letter Agreement, dated January 8, 2010, amending the Capital Advisory Agreement with Greybrook Capital Inc.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on January 11, 2010 (file no. 000-51030)
|
||
|
10.62
|
Placement Agency Agreement, dated as of March 14, 2010, by and between the Company and Rodman & Renshaw, LLC.
|
Exhibit 99.1 to the Registrant's Current Report on Form 8-K filed with the Commission on March 15, 2010 (file no. 000-51030)
|
||
|
10.63
|
Securities Purchase Agreement, dated as of March 14, 2010, by and between the Company and certain investors.
|
Exhibit A to the Registrant's free writing prospectus filed with the Commission on March 17, 2010 (file no. 333-157269)
|
||
|
10.64
|
Distribution Agreement, dated as of August 20, 2009, by and between the Company and Science with Vision, a Canadian corporation.
|
Exhibit 99.5 to the Registrant's Current Report on Form 8-K filed with the Commission on March 15, 2010 (file no. 000-51030)
|
||
|
10.65
|
Capital Advisory Agreement with Greybrook Capital Inc., dated November 3, 2009
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on November 3, 2009 (file no. 000-51030)
|
||
|
10.66
|
Securities Purchase Agreement, dated as of July 15, 2009, by and between the Company and certain investors.
|
Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed with the Commission on July 16, 2009 (file no. 000-51030)
|
||
|
10.67
|
Form of 12% Convertible Secured Note.
|
Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed with the Commission on July 16, 2009 (file no. 000-51030)
|
||
|
10.68
|
Security Agreement, dated July 15, 2009, by and between the Company and certain investors.
|
Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed with the Commission on July 16, 2009 (file no. 000-51030)
|
||
|
10.69
|
Form of Director and Affiliate Letter Agreement.
|
Exhibit 10.5 to the Registrant's Current Report on Form 8-K filed with the Commission on July 16, 2009 (file no. 000-51030)
|
||
|
14.1
|
Code of Conduct of the Registrant
|
|
Exhibit Number
|
— |
Exhibit Description
|
Incorporated by Reference
|
|
|
14.2
|
Complaint and Reporting Procedures of the Registrant.
|
Exhibit 14.2 to the Registrant's Quarterly Report on Form 10-Q, filed with the Commission on August 8, 2005 (file no. 000-51030)
|
||
|
21.1
|
Subsidiaries of Registrant.
|
|||
|
Consent of Independent Registered Public Accounting Firm.
|
||||
|
24.1
|
Power of Attorney (included on signature page).
|
|||
|
CEO's Certification required by Rule 13A-14(a) of the Securities Exchange Act of 1934.
|
||||
|
CFO's Certification required by Rule 13A-14(a) of the Securities Exchange Act of 1934.
|
||||
|
CEO's Certification of periodic financial reports pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, U.S.C. Section 1350.
|
||||
|
CFO's Certification of periodic financial reports pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, U.S.C. Section 1350.
|
* * *
#Management compensatory plan, contract or arrangement
Copies of the exhibits filed with this Annual Report on Form 10-K or incorporated by reference herein do not accompany copies hereof for distribution to stockholders of the Registrant. The Registrant will furnish a copy of any of such exhibits to any stockholder requesting the same for a nominal charge to cover duplicating costs.
POWER OF ATTORNEY
The registrant and each person whose signature appears below hereby appoint Elias Vamvakas and William G. Dumencu as attorney-in-fact with full power of substitution, severally, to execute in the name and on behalf of the registrant and each such person, individually and in each capacity stated below, one or more amendments to this Annual Report on Form 10-K, which amendments may make such changes in this Annual Report as the attorney-in-fact acting in the premises deems appropriate and to file any such amendments to this Annual Report on Form 10-K with the Securities and Exchange Commission.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned thereunto duly authorized.
| Dated: March 29, 2011 | TearLab Corp. | |
|
By:
|
/s/ Elias Vamvakas | |
|
Elias Vamvakas
|
||
|
Chief Executive Officer
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Dated: March 29, 2011
|
By:
|
/s/ Elias Vamvakas
|
|
Elias Vamvakas
|
||
|
|
Chief Executive Officer and
|
|
|
|
Chairman of Board of Directors
|
|
|
|
||
|
Dated: March 29, 2011
|
By:
|
/s/ William G. Dumencu
|
|
William G. Dumencu
|
||
|
Chief Financial Officer and Treasurer
|
||
|
Dated: March 29, 2011
|
By:
|
/s/ Anthony Altig
|
|
|
Anthony Altig
|
|
|
|
Director
|
|
|
Dated: March 29, 2011
|
By:
|
/s/ Thomas N. Davidson, Jr.
|
|
Thomas N. Davidson, Jr.
|
||
|
Director
|
||
|
Dated: March 29, 2011
|
By:
|
/s/ Adrienne L. Graves
|
|
Adrienne L. Graves
|
||
|
Director
|
||
|
Dated: March 29, 2011
|
By:
|
/s/ Richard L. Lindstrom, M.D.
|
|
Richard L. Lindstrom, M.D.
|
||
|
Director
|
||
|
Dated: March 29, 2011
|
By:
|
/s/ Donald Rindell
|
|
|
Donald Rindell
|
|
|
|
Director
|
|
|
Dated: March 29, 2011
|
By:
|
/s/ Paul Karpecki
|
|
|
Paul Karpecki
|
|
|
|
Director
|
|
|
Dated: March 29, 2011
|
By:
|
/s/ Brock Wright
|
|
Brock Wright
|
||
|
Director
|
-90-