Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
[X] ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE OF 1934
For the fiscal year ended December 31, 2010
[ ] TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE OF 1934
For the transition period from _____ to _____
Commission file number: 001-32581
LOTUS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
Nevada | 20-0507918 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
No. 16 Cheng Zhuang Road, Feng Tai District, Beijing, People's Republic of China | 100071 |
(Address of principal executive offices) | (Zip Code) |
Registrant's telephone number, including area code: | 86 (10) 63899868 |
Securities registered under Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered |
None | Not applicable |
Securities registered under Section 12(g) of the Act:
Common stock, $0.001 Par Value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [√]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes [ ] No [√]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [√] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [ ] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company:
Large accelerated filer | [ ] | Accelerated filer | [ ] |
Non-accelerated filer (Do not check if smaller reporting company) | [ ] | Smaller reporting company | [√] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [√]
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was sold, or the average bid and asked prices of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter: $24,964,911 on June 30, 2010.
As of March 28, 2011, the registrant had 27,747,131 shares of common stock issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
ii
LOTUS PHARMACEUTICALS, INC.
FORM 10-K
TABLE OF CONTENTS
|
| Page No. |
Part I | ||
Item 1. | Business. | 4 |
Item 1A. | Risk Factors. | 16 |
Item 1B. | Unresolved Staff Comments. | 28 |
Item 2. | Properties. | 28 |
Item 3. | Legal Proceedings. | 28 |
Item 4. | [Removed and Reserved.] | 28 |
Part II | ||
Item 5. | Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. | 29 |
Item 6. | Selected Financial Data. | 30 |
Item 7. | Management's Discussion and Analysis of Financial Condition and Results of Operation. | 30 |
Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. | 40 |
Item 8. | Financial Statements and Supplementary Data. | 40 |
Item 9. | Changes In and Disagreements With Accountants on Accounting and Financial Disclosure. | 41 |
Item 9A.(T) | Controls and Procedures. | 41 |
Item 9B. | Other Information. | 43 |
Part III | ||
Item 10. | Directors, Executive Officers and Corporate Governance. | 43 |
Item 11. | Executive Compensation. | 47 |
Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. | 49 |
Item 13. | Certain Relationships and Related Transactions, and Director Independence. | 50 |
Item 14. | Principal Accountant Fees and Services. | 50 |
Part IV | ||
Item 15. | Exhibits, Financial Statement Schedules. | 51 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
This report contains forward-looking statements. These forward-looking statements are subject to known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous assumptions and other factors that could cause our actual results to differ materially from those in the forward-looking statements. These factors include, but are not limited to, economic, political and market conditions and fluctuations, government and industry regulation, interest rate risk, global competition, and other factors as relate to our doing business within the People's Republic of China. Most of these factors are difficult to predict accurately and are generally beyond our control. You should consider the areas of risk described in connection with any forward-looking statements that may be made herein. Readers are cautioned not to place undue reliance on these forward-looking statements and readers should carefully review this report in its entirety, including the risks described in "Item 1A. - Risk Factors" Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this report, and you should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
2
OTHER PERTINENT INFORMATION
We maintain a web site at www.lotuspharma.com. Information on this website is not a part of this annual report.
All share and per share information in this report gives effect to the 2:1 reverse stock split of our common stock which was effective on December 31, 2010.
INDEX OF CERTAIN DEFINED TERMS USED IN THIS REPORT
Unless specifically set forth to the contrary, when used in this report the terms:
• "Lotus," "we," "us," "our," the "Company," and similar terms refer to Lotus Pharmaceuticals, Inc., a Nevada corporation formerly known as S.E. Asia Trading Company, Inc., and its subsidiary,
• "Lotus International" refers to Lotus Pharmaceutical International, Inc., a Nevada corporation and a subsidiary of Lotus,
• "Lotus Century" refers to Lotus Century Pharmaceutical (Beijing) Technology co., Ltd., a wholly foreign-owned enterprise (WFOE) Chinese company which is a subsidiary of Lotus,
• "Liang Fang" refers to Beijing Liang Fang Pharmaceutical Co., Ltd., a Chinese limited liability company formed on June 21, 2000,
• “En Ze Jia Shi” refers to Beijing En Ze Jia Shi Pharmaceutical Co., Ltd., a Chinese limited liability company formed on September 17, 1999 and an affiliate of Liang Fang,
• "Lotus East" collectively refers to Liang Fang and En Ze Jia Shi,
• "Consulting Services Agreements" refers to the Consulting Services Agreements dated September 20, 2006 between Lotus and Lotus East.
• "Operating Agreements" refers to the Operating Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East,
• "Equity Pledge Agreements" refers to the Equity Pledge Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East,
• "Option Agreements" refers to the Option Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East,
• "Proxy Agreements" refers to the Proxy Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East,
• "Contractual Arrangements" collectively refers to the Consulting Services Agreements, Operating Agreements, Equity Pledge Agreements, Option Agreements and the Proxy Agreements,
• "China" or the "PRC" refers to the People's Republic of China,
• “SFDA” refers to The State Food and Drug Administration,
• "RMB" refers to the renminbi which is the currency of mainland PRC of which the yuan is the principal currency,
• "2009" generally refers to the fiscal year ended December 31, 2009;
• "2010" generally refers to the fiscal year ended December 31, 2010; and
• "2011" generally refers to the fiscal year ended December 31, 2011.
3
PART I
ITEM 1. BUSINESS.
Overview
Lotus Pharmaceuticals, Inc. is a holding company incorporated in Nevada with its principal place of business in the People’s Republic of China (the “PRC”). We operate, control and beneficially own the pharmaceutical business of Lotus East. Lotus East develops, manufactures, markets and sells pharmaceutical products in the PRC. Lotus East produces crude medicine and drugs in forms of tablets, capsules, granules, eye-drops, and freeze-dried powder injection. Lotus East has established markets for several drugs that are self-branded or self-patented, including (i) Maixin - valsartan capsules for the treatment of hypertension, (ii) Muxin – eye drops for the treatment of glaucoma and (iii) Yipubishan - octreotide Acetate Injection solution for the treatment of gastric ulcers. Lotus East’s drug development is focused on the treatment of cerebro-cardiovascular disease, asthma, and diabetes. Lotus East has a nationwide sales network to directly and indirectly sell to hospitals, clinics and drugs stores in approximately 30 provinces in China. Additionally, through its 10 retail pharmacy locations in Beijing, China, Lotus East sells western and traditional Chinese medications and medical treatment equipment.
On December 15, 2010, we received notification that our certificate of change was accepted by the Secretary of State of Nevada. Pursuant to the certificate of change, we effected a two-for-one reverse split of our common stock. Pursuant to Section 72.209 of the Nevada Revised Statutes, no shareholder approval or amendment to our articles of incorporation was required for the certificate of change. On December 31, 2010, the reverse stock split was effectuated. Following the reverse stock split, the total number of shares of our common stock outstanding was reduced from 53,399,407 shares to approximately 26,700,000 shares and the maximum number of shares of common stock that the Company is authorized to issue was also reduced from 200,000,000 to 100,000,000. Our financial statements have been retroactively adjusted to reflect the reverse split. Additionally, all share representations are on a post-split basis hereinafter.
Corporate Structure
To be in compliance with China’s regulations on foreign ownership in the pharmaceutical industry, we operate our business in China through the Contractual Arrangements with Lotus East. The contractual relationship among the above companies as follows:
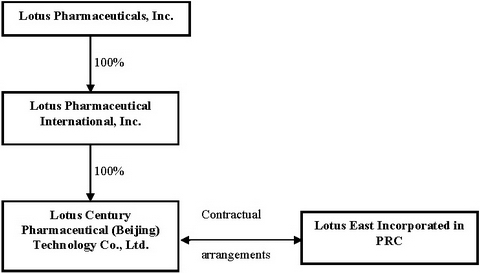
Notwithstanding that Lotus, Liang Fang and En Ze Jia Shi are separate legal entities and the legal obligations of the parties are governed by the Contractual Arrangements, there is commonality of control between Lotus and Lotus East as set forth in the following table:
4
Name | Executive Officer/Director of Lotus | Principal Stockholder of Lotus | Stockholder of Liang Fang | Stockholder of En Ze Jia Shi |
Dr. Zhongyi Liu | √ | √ | √ | √ |
Mrs. Zhenghong Song 1 |
| √ | √ | √ |
Wenli Xian |
|
| √ |
|
(1) Mrs. Zhenghong Song is the spouse of Dr. Zhongyi Liu.
Our principal executive offices are located at No. 16 Cheng Zhuang Road, Feng Tai District, Beijing, PRC 100071 and our telephone number is 86-10-63899868. Our fiscal year end is December 31.
The Contractual Arrangements are comprised of a series of agreements, including Consulting Services Agreements and Operating Agreements, through which we have the right to advise, consult, manage and operate Lotus East, and collect and own all of their respective net profits. Additionally, under Proxy Agreements, the stockholders of Lotus East have vested their voting control over Lotus East to us. In order to further reinforce our rights to control and operate Lotus East, these companies and their stockholders have granted us, under Option Agreements, the exclusive right and option to acquire all of their equity interests in Lotus East, alternatively, all of the assets of Lotus East. Further the Lotus East stockholders have pledged all of their rights, titles and interests in Lotus East to us under Equity Pledge Agreements.
Under PRC laws, each of Lotus Century, Liang Fang and En Ze Jia Shi is an independent legal person and none of them is exposed to liabilities incurred by the other party. Other than pursuant to the Contractual Arrangements, Lotus East does not transfer any other funds generated from their respective operations to us.
On September 6, 2006, we entered into the following Contractual Arrangements:
Consulting Services Agreements. Pursuant to the exclusive Consulting Services Agreements we have the exclusive right to provide to Lotus East general pharmaceutical business operations services as well as consulting services related to the technological research and development of pharmaceutical products as well as general business operation advice and strategic planning (the "Services"). Under these agreements, we own the intellectual property rights developed or discovered through research and development, in the course of providing the Services, or derived from the provision of the Services. Lotus East is to pay us a quarterly consulting service fees in RMB that is equal to Lotus East's net profits, as defined, for such quarter.
Operating Agreements. Pursuant to the Operating Agreements we provide guidance and instructions on Lotus East's daily operations, financial management and employment issues. The stockholders of Lotus East must designate the candidates recommended by us as their representatives on each of Lotus East's Board of Directors. We have the right to appoint senior executives of Lotus East. In addition, we agreed to guarantee Lotus East's performance under any agreements or arrangements relating to Lotus East's business arrangements with any third party. Lotus East, in return, agreed to pledge its accounts receivable and all of its assets to us. Moreover, Lotus East agreed that without our prior consent, Lotus East would not engage in any transaction that could materially affect the assets, liabilities, rights or operations of Lotus East, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party. The term of these agreements is 10 years from September 6, 2006 and may be extended only upon our written confirmation prior to the expiration of the these agreements, with the extended term to be mutually agreed upon by the parties.
Equity Pledge Agreements. Under the Equity Pledge Agreements, the stockholders of Lotus East pledged all of their equity interests in Lotus East to us to guarantee Lotus East's performance of its obligations under the Consulting Services Agreements. If Lotus East or Lotus East's stockholders breach its respective contractual obligations, we, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Lotus East's stockholders also agreed that upon occurrence of any event of default, we will be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of Lotus East's stockholders to carry out the security provisions of the Equity Pledge Agreements and take any action and execute any instrument that we might deem necessary or advisable to accomplish the purposes of The Equity Pledge Agreements. The stockholders of Lotus East agreed not to dispose of the pledged equity interests or take any actions that would prejudice our interest. The Equity Pledge Agreements expire two years after Lotus East's obligations under the Consulting Services Agreements have been fulfilled.
5
Option Agreements. Under the Option Agreements, the stockholders of Lotus East irrevocably granted us or our designated person exclusive options to purchase, to the extent permitted under PRC law, all or part of the equity interests in Lotus East for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. We, or our designated person, have sole discretion to decide when to exercise the option, whether in part or in full. The term of these agreements is 10 years from September 6, 2006 and may be extended prior to their expiration by written agreement of the parties.
Proxy Agreements. Pursuant to the Proxy Agreements, Lotus East's stockholders agreed to irrevocably grant a person to be designated by us with the right to exercise Lotus East's stockholders' voting rights and their other rights, including the attendance at and the voting of Lotus East's stockholders' shares at the stockholders' meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and their Articles of Association, including but not limited to the rights to sell or transfer all or any of his equity interests of Lotus East, and appoint and vote for the directors and Chairman as the authorized representative of the stockholders of Lotus East. The term of these Proxy Agreements is 10 years from September 6, 2006 and may be extended prior to their expiration by written agreement of the parties.
On May 25, 2007, Lotus International’s wholly-owned foreign enterprise (WFOE) Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. (“Lotus Century”) was duly incorporated in Beijing, China. On August 20, 2007, an assignment agreement was signed between Lotus International and Lotus Century. Pursuant to the terms of the assignment agreement, Lotus Century was assigned all of Lotus International’s right, title and interest to control the management and voting and to be entitled to all of the profits and losses of Lotus East.
LOTUS EAST
Based in Beijing, China, Liang Fang is engaged in the production, trade and retailing of pharmaceuticals, focusing on the development of innovative medicines and investing in strategic growth to address various medical needs. Liang Fang owns and operates 10 drug stores throughout Beijing, China, that sell western and traditional Chinese medications and medical treatment equipment and generate revenues from the leasing of retail space to third party vendors at its retail stores. En Ze Jia Shi is the sole manufacturer for Liang Fang and maintains facilities for the production of medicines, patented Chinese medicine, as well as the research and production of other new medicines.
Lotus East's business is composed of two parts:
| · | manufacturing and distribution of pharmaceutical products, |
| · | retailing of western and traditional Chinese medications, and medical treatment equipment through retail locations and direct sales to other Over-the-Counter drug stores in Beijing |
MANUFACTURING AND DISTRIBUTION OF PHARMACEUTICAL PRODUCTS
Lotus East's production enterprise was located in the Chaoyang District of Beijing and covers a floor space of approximately 50,000 square feet until the end of November 2009 when Lotus decided to enhance its operations with a new production facility and office building so as to consolidate its previously separated operation units in Beijing. Lotus East intended to build a new complex building in 2009 and expected to finish the construction of the new building in the fourth quarter of 2011. Until completed, production is outsourced to Shuanghe Pharmaceutical Group which is located near Lotus East’s existing facility. The new facility is designed to be similar to the previous plant, with it containing liquid phase, gas phase, spectrum, and mass spectrum equipment and a variety of types of purification and distilling equipment, all of which can be used for production and research and development of biochemical medicines, Chinese traditional medicines, chemical compound medicines, antibiotics and other new medicines. As one of the first enterprises to be authenticated by the National China Good Manufacturing Practices (GMP), Lotus East believes it has an advanced automatic pharmacy product line which is GMP authenticated under certificate numbers C0849, C0850, D1645, and G3452. In June 2009, Lotus East received a Good Supply Practices (GSP) certificate from the Chinese State Food & Drug Administration (SFDA). Medicines produced by Lotus East include:
| · | crude medicine, |
| · | tablets, |
| · | capsules, |
| · | granules; |
| · | freeze-dried powder for injections and |
| · | eye drops. |
6
Different production control zones are used with separated air conditioning system according to different production and control needs. Production and sale of its self-branded drugs and wholesale distribution of third party manufactured pharmaceuticals is Lotus East's largest and most profitable business. Net revenues from our wholesale distribution account for approximately 70.7% and 79.4% of our total net revenues in fiscal 2010 and fiscal 2009, respectively.
Currently, Lotus East markets and sells its products only in PRC. Lotus East's currently manufactures four branded drugs, and own intellectual property right to one branded drug produced by a third party. All of which are listed in China’s national medical insurance catalog. The five branded drugs include:
Valsartan
In 2000, Lotus East obtained approval from the State Food and Drug Administration (SFDA) of China to sell Valsartan as a raw material and as a capsule (brand name: Maixin). Among its best selling products, Valsartan is a drug that treats hypertension or high blood pressure. This product is globally recognized as the ideal anti-high blood pressure medication by the medical industry due to its most stable and longest lasting therapeutic effects and limited side-effects.
High blood pressure adds to the workload of the heart and arteries. If it continues for a long time, the heart and arteries may not function properly. This can damage the blood vessels of the brain, heart, and kidneys, resulting in a stroke, heart failure, or kidney failure. High blood pressure may also increase the risk of heart attacks. These problems may be less likely to occur if blood pressure is controlled. Valsartan works by blocking a substance in the body that causes blood vessels to tighten. As a result, Valsartan relaxes blood vessels. This lowers blood pressure and increases the supply of blood and oxygen to the heart. Lotus East's goal is to make Valsartan a leading prescribed brand in its class of high blood pressure medications in the PRC.
Lotus East holds current manufacturing rights to produce Valsartan. It estimates that there are currently 10 enterprises producing single regent dosage and fixed-dose combinations of Valsartan in China. The foreign pharmacy Novartis Pharma sells Valsartan under its brand name, Diovan. According to clinical verification, the two products, Maixin and Diovan, have the same clinical effects. Lotus East’s currently holds approximately 20% of the total China market share for Valsartan. Lotus East believes that it will maintain its current market share due to increased brand recognition and continued sales efforts. Even if the market does face fiercer competition in the future, Lotus East believes that its sales revenue from Valsartan can stay relatively stable because of:
| · | Good product quality; |
| · | Stable sales teams; |
| · | The existing customers; |
| · | Stable sales agents; |
| · | Gradual increase of customers at all levels (such as province, municipal and county-level hospitals); |
| · | Gradual increase of sales agents at all levels (such as province, municipal and county-level cities); |
| · | Good corporate image; and |
| · | The relative stability of China national policies. |
Brimonidine Tartrate Eye Drops
Brimonidine Tartrate is a drug used to constrict adrenaline receptors, an important step in treating glaucoma. Lotus East sells Brimonidine Tartrate eye drops under its brand name "Muxin". The drug was first put into market in the U.S. in 1998 and in August 2004, Lotus East received the rights to manufacture and release the drug in the Chinese market. A fast and long lasting therapeutic effect, very few side-effects, a very high exponent of cure and high endurement are prime features of the drug. It will produce no harmful effects of reducing blood pressure, resulting in calmness and so on, much like diazepam. The imported product of its kind is Alphagan and is produced by Allergan (Hangzhou) Pharmaceutical Co., Ltd. According to clinical experiments of People's Hospital of Peking University and Tianjin Eye Hospital, Muxin and imported Alphagan have exactly the same curative effect but the side-effects of Muxin are fewer than Alphagan. As a result, Lotus East believes that its product has a stronger competitive advantage.
Currently, Lotus East holds approximately 50% of the total China market share for Brimonidine Tartrate. Even if the market does face fiercer competition in the future, Lotus East believes that its sales revenue from Brimonidine Tartrate can stay relatively stable because of:
| · | Excellent product quality; |
| · | Stable sales teams; |
| · | The existing customers; |
7
| · | Stable sales agents; |
| · | Gradual increase of customers in the next few years, mainly from tertiary hospitals in Beijing and other provinces; |
| · | Gradual increase or change in provincial and municipal sales agents; |
| · | Good corporate image; and |
| · | The relative stability of China national policies. |
Levofloxacin Lactate for Injection
Levofloxacin is a popular anti-bacterial drug for the treatment of mild, moderate, and severe infections caused by susceptible trains of the designated microorganisms in the conditions such as acute maxillary sinusitis, acute bacterial exacerbation of chronic bronchitis, community-acquired pneumonia, complicated and uncomplicated skin and skin structure infections, complicated and uncomplicated urinary tract infections, and acute pyelonephritis. Lotus East received the rights to manufacture and release the drug in the Chinese market in 2005. Lotus East sells Levofloxacin Lactate for Injection under its brand name "Junxin". The imported product of its kind is manufactured by Japanese pharmaceutical company, Daiichi Pharmaceutical Co.. Our Levofloxacin Lactate for Injection is covered by the National Health Insurance Program, therefore, its sale price is controlled by Chinese government. In 2010, our manufacture facility was removed from service in order to construct our new building in Beijing. If we use third party manufacture service to produce Levofloxacin Lactate for Injection, the gross profit margin which we could get from the sale of the drug would be very low. So, we did not use any third party manufacture service to produce the drug during fiscal 2010. Lotus East management anticipate that Lotus East can continue to produce Levofloxacin at its own new factory in 2012 after construction of the new building is completed. Lotus East expects to sell Levofloxacin after 2011.
Nicergoline for Injection
Nicergoline for Injection is an alpha-receptor blockage nerve system blood-brain medicine with a curative effect. On the cerebral level, it prompts a lowering of vascular resistance, an increase in arterial flow and stimulates the use of oxygen and glucose. Nicergoline also improves blood circulation in the lungs and limbs and has been shown to inhibit blood platelet aggregation. It is used to treat senile dementia, migraines of vascular origin, transient ischemia, platelet hyper-aggregability and macular degeneration. Lotus East received the rights to manufacture this product in 2006 and released the drug in the Chinese market in 2007. Lotus East sells Nicergoline for Injection under its brand name "Ni Mai Jiao Lin". The sale price of our Nicergoline for Injection is controlled by Chinese government since it is covered by the National Health Insurance Program. In 2010, our manufacture facility was removed from service in order to construct our new building in Beijing. If we use third party manufacture service to produce Nicergoline for Injection, the gross profit margin which we could get from the sale of the drug would be very low. Therefore, we did not use any third party manufacture service to produce the drug during fiscal 2010. Lotus East management anticipate that Lotus East can continue to produce Nicergoline at its own new factory in 2012 after construction of the new building is completed. Lotus East expects to sell Nicergoline after 2011.
Yipubishan -Octreotide Acetate Injection Solution
In December 2008, Lotus East entered into an intellectual rights transfer contract with Beijing Yipuan Bio-Medical Technology Co., Ltd. to acquire the drug Yipubishan, a highly effective and stable octreotide acetate injection solution, according to a clinical research report issued by Beijing Union Medical College Hospital Center for Clinical Pharmacology, used to treat the symptoms of gastric ulcers and hemorrhages of the upper digestive tract since 2004. Lotus East paid RMB 54 million (approximately $8 million) for the intellectual property rights of Yipubishan. Yipuan has a manufacturing agreement with Beijing Si Huan pharmaceutical Co., Ltd. to manufacture Yipubishan. Si Huan continues to manufacture Yipubishan for Lotus East after Lotus East purchased the intellectual property rights of Yipubishan. Even if the market does face fiercer competition in the future, Lotus East believes that its sales revenue from Yipubishan can stay relatively stable because of:
| · | Good product quality; |
| · | Stable sales team; |
| · | The existing customers; |
| · | Stable sales agents; |
| · | Gradual increase of customers in third tier cities and small towns or county-level cities; |
| · | Gradual increase of sales agents in third tier cities and small towns or county-level cities; |
| · | Good corporate image; and |
| · | The relative stability of China national policies. |
8
In fiscal 2010 and fiscal 2009, net revenues from wholesale distribution of the above five principal products (Valsartan, Brimonidine Tartrate Eye Drops, Levofloxacin Lactate for Injection, Nicergoline for Injection and Yipubishan – Octreotide Acetate Injection Solution) were approximately 38.0% and 53.8% of our total net revenues, respectively.
Lotus East also acts as a wholesale distributor to distribute various pharmaceutical products manufactured by third party manufacturers. Currently, the third party manufactured products distributed through Lotus East’s wholesale distribution channel are summarized below:
Product Name |
| Indication |
| Type of Distribution Rights |
Recombinant Human Erythropoietin Injection (EPO) |
|
|
| Exclusive Distribution Rights |
Recombinant Human Interleukin-2 Injection |
|
|
| Exclusive Distribution Rights |
Potassium Aspartate and Magnesium Aspartate for Injection |
|
|
| Non-exclusive Distribution Rights |
Omeprazole enteric-coated capsules |
|
|
| Non-exclusive Distribution Rights |
Recombinant Human Granulocyte Stimulating Factor Injection |
| Cancer |
| Exclusive Distribution Rights |
Cervus and Cucumis Polypeptide Injection |
| Rheumatism |
| Non-exclusive Distribution Rights |
Deproteinized Calfblood Extractives Injection |
| Nerves |
| Non-exclusive Distribution Rights |
Cefotaxime Sodium For Injection |
|
|
| Non-exclusive Distribution Rights |
Qingkailing Paotengpian |
| Respiratory |
| Non-exclusive Distribution Rights |
NINGXIN YISHEN Oral Liquid |
| Immune System |
| Non-exclusive Distribution Rights |
Cold Capsules |
|
|
| Non-exclusive Distribution Rights |
Small Adjustable Film |
| Joint Disease |
| Non-exclusive Distribution Rights |
Prostate Capsules |
| Prostate |
| Non-exclusive Distribution Rights |
LGTNG Gel |
| Psoriasis |
| Non-exclusive Distribution Rights |
Shuanghuanglian Oral Liquid |
| Influenza |
| Non-exclusive Distribution Rights |
The fifteen prescription drugs produced by third parties and distributed through our wholesale distribution channel are covered under National Health Insurance Program.
In addition to the commercialized products mentioned above, we have an innovative pipeline of drugs which are under development and focused on the treatment of chronic diseases. The innovative pipeline of drugs is summarized below:
Product candidate | Indication | Expected launch |
R-Bambuterol-Class 1 new drug (1) | Asthma | 2013-2014 |
Gliclazide-Controlled Release Tablets (2) | Diabetes | 2014 |
Isosorbide Mononitrate-Tablets (3) | Cardiovascular-(coronary artery) | 2013-2014 |
Lovastatin-Tablets | Cardiovascular-Hyperlipemia | In development |
Verapamil Hydrochloride-Tablets | Cardiovascular-Irregular Angina | In development |
Valsartan-Controlled Release Tablets | Cardiovascular-Hypertension | In development |
Hawthorn Flavonoids-Tablets (TCM) | Cardiovascular-Hyperlipemia | In development |
(1) It is subject to SFDA approval and currently in Phase I clinical trial. Its patent term is from 2002 to 2022.
(2) It is subject to SFDA approval and currently awaiting for approval to initiate clinical trials. The patent covers the composition and preparation methods for the drug through 2028.
(3) It is subject to SFDA approval and currently awaiting for approval to initiate clinical trials.
RETAILING BUSINESS
Lotus East owns and operates 10 drug stores throughout many different districts of Beijing, including:
| · | Xin Zhong Tai Drugstore |
| · | Nan Gong Drugstore |
| · | Wan Shou Road Drugstore |
| · | He Ping Li Drugstore |
| · | Feng Lin Lv Zhou Drugstore |
| · | Youth Lake Drugstore |
| · | Capital Airport Drugstore |
9
| · | Yong An Zhong Sheng Drugstore |
| · | Cheng Zhuang Road Drugstore |
| · | Feng Tai Drugstore |
These 10 drug stores all offer in excess of 8,000 types of western and traditional Chinese medicines, and medical treatment items. The drug stores attempt to compete based on lower pricing and more efficient distribution and management practices.
Through its retail operations, Lotus East also generates revenue by the leasing of space to licensed physicians, the lease of store front areas to various merchants in the retail pharmacies.
At the end of fiscal 2009, we engaged a general manager for our Over-the-Counter Drug Division that manages our own ten drug stores’ sales, and the newly created direct sales to other Over-the-Counter drug stores in Beijing. The general manager has strong management skills in medical sales and marketing and logistics and is an expert in delivery of services to drug stores in Beijing. In the fiscal 2010, we served more than 1,000 other Over-the-Counter drug stores in Beijing. Due to the growth and success of our OTC Drug Division’s sales force, our retail revenue for fiscal 2010 was substantially increased as compared to our retail revenue for fiscal 2009. We expect our retail revenue from our direct sales to other drug stores in Beijing will continue to increase in the fiscal 2011.
Net revenues from our retail business account for approximately 29.3% and 20.6% of our total net revenues in fiscal 2010 and fiscal 2009, respectively.
RESEARCH AND DEVELOPMENT
Lotus East places great emphasis on product research and development and it has established a research and development center. Lotus East's research and development team is comprised of seven individuals who are focused on developing new drugs and generic drugs, as well as monitoring and improving Lotus East’s drugs sold in the market based on their market research. Lotus East maintains strategic relationships with several research institutions in PRC developing new drugs, such as China Academy of Science and Military Medical Science Academy. Lotus East also cooperates with top research institutions, such as the Third Hospital of Peking University School of Medicine, to perform some testing and clinical trials. We spent $86,545 and $0 on research and development during the years ended December 31, 2010 and 2009, respectively.
THE CHINESE MARKET FOR DRUGS
According to 2009 PRC National Bureau of Statistics and PRC National Population & Family Planning Commission statistics, currently, the Chinese market trends which support Louts East’s future growth include:
| · | Strong demand for pharmaceuticals due to growing affluence, larger middle class, and aging population; |
| · | Elderly population (60+) has grown from 130 million (10.5% of population) in 2000 to 144 million (11% of population) in 2005; |
| · | Cardio-cerebrovascular disease has been on the rise in the PRC due to changing diet habits and moving to a younger age profile; |
| · | Growing government support for Chinese healthcare industry; |
| · | Government’s Universal Health Plan package of $125 billion delivered by next three years; and |
China government’s healthcare reform has begun to take into effect which includes: (i) promoting separation of prescribing and dispensing functions; (ii) encouraging development of pharmacies; and (iii) limiting over-prescription and reducing exorbitant hospital mark-ups and abating corruption. Therefore, many Chinese domestic companies have begun to build chain drug store networks through acquisition. Further, there is a trend to consolidate China’s fragmented pharmaceutical industry in China based on the following factors:
| · | There are approximately 4,000 pharmaceutical manufacturers in China. The average revenue of each pharmaceutical manufacturer is approximately $7 million and the average R&D of each pharmaceutical manufacture is approximately 5% of average revenue of each pharmaceutical manufacturer. Hence, it is hard to form a leadership in China pharmaceutical industry due to the lack of innovation; |
| · | Since GMP certification compliance announced that, the number of drug manufactures was reduced from 6,000 to less than 4,000 in 2009 and we expect that this number will continue to decrease; |
| · | Market-driven competition may eliminate smaller companies. |
10
As a result, the characteristics of future market leaders may be: (i) well-established nationwide sales and distribution network; (ii) strong product development capabilities; and (iii) access to capital. Lotus East believes it is well-positioned to emerge as a leader in the industry.
DISTRIBUTION METHODS OF THE PRODUCTS OR SERVICES AND LOTUS EAST'S CUSTOMERS
Currently, Lotus East’s national sales network includes:
| · | Direct sales of prescription drugs to over 40 hospitals; and |
| · | 10 regional centers use 100+ third party independent distributors to reach hospitals, clinics in 1st, 2nd, 3rd tier cities, and remote villages. A regional sales director for each regional center teamed with 3-10 salespeople. |
Lotus East’s ten regional centers include:
| · | Mongolia Regional Center |
| · | Anhui Regional Center |
| · | Guangdong Regional Center |
| · | Central Regional Center |
| · | Northern Regional Center |
| · | Eastern Regional Center |
| · | South Western Regional Center |
| · | North Eastern Regional Center |
| · | Guangxi Regional Center |
| · | North Western Regional Center |
Lotus East’s sales force will be expanded to increase direct sales of pharmaceuticals to hospitals and third-party OTC drug stores. Lotus East plans to add distributors to national sales network.
Lotus East sells four branded prescription drugs produced and packaged by En Ze Jia Shi (brand name: Maixin, Junxin, Muxin, Ni Mai Jiao Lin) and one branded prescription drug produced and packaged by Si Huan Pharmaceutical Co., Ltd. (brand name: Yipubishan) by its direct sales channel. Lotus East owns the intellectual property rights for the five prescription drugs. Lotus East’s drug products are also sold through its retail drug stores. Lotus East sells over 8,000 kinds of drugs through its 10 drugstores in Beijing.
Lotus East recognizes the importance of branding as well as packaging. All of its products bear a uniform brand but it also brands and packages its products with specialized designs to differentiate the different categories of its products. Lotus East relies on a combination of trademark, copyright and trade secret protection laws in PRC and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect its intellectual property and its brand. Lotus East intends to apply for patent protection of certain of its special technologies to protect its core technologies. There are no assurances, however, that if such applications are made that the patents will be granted. Lotus East also enters into confidentiality, non-compete and invention assignment agreements with its employees and consultants and nondisclosure agreements with third parties. "Maixin", “Muxin”, “En Ze Jia Shi” and "Liang Fang" are its registered trademarks in the PRC.
Lotus East conducts promotional marketing activities to publicize and enhance the company's image as well as to reinforce the recognition of its brand name, including:
| · | publishing advertisements and articles in national as well as specialized and provincial pharmaceutics and Biotech newspapers and magazines, and in other media, including the Internet; |
| · | participation in national meetings, seminars, symposiums, exhibitions for bio-pharmaceutical and other related industries; |
| · | organizing cooperative promotional activities with distributors; |
| · | Educational and seminar training sessions to physicians and pharmacists to introduce and explain its drugs; and |
| · | Establishment of direct sales to resell various retail pharmaceutical products to other third party pharmacies. |
Excluding customers in its retail operations, Lotus East currently has over 200 customers, including over 30 direct customers in Beijing, Shanghai, Chongqing, Guangdong, Inner Mongolia, Ningxia, Henan, Hubei, Liaoning, Heilongjiang, Guangxi, Jiangsu, Hebei, Anhui, Yunnan, Sichuan, Shanxi provinces in the PRC. We did not have any customer that accounted for over 10% of our revenues for fiscal 2010 and 2009, respectively. In fiscal 2010, Lotus East spent $0 on advertising expenses as compared to $46,450 in fiscal 2009.
11
GROWTH STRATEGIES
Lotus East’s growth strategies include, but not limited to, the following areas:
| · | Expansion in sales network: Lotus East plans to add more distributors to its nation-wide sales channels. Lotus East attends substantially every major drug expos in China. Lotus East’s sales representatives are experienced with medical education backgrounds, and are located in ten regional sales centers providing sales and post-sales services to its customers. Regional distributors come into contact with Lotus East through words of mouth and public information. Further, government hospitals are Lotus East’s important direct sales channels. Although Lotus East believes it will encounter high entry barriers to increase new additional hospitals direct sales channels, Lotus East attempts to add new hospital channels in the future; |
|
|
|
| · | Increase in quality drugs offerings: Lotus East’s brand quality stems from its commitment to quality and efficacy of products. Lotus East plans to increase the kinds of prescription drugs and sell them through its national wholesale channel and sales network. In addition, developing drugs to treat cardio-cerebrovascular, asthma and diabetes diseases remain the strategic focus in Lotus East’s pipeline development; |
|
|
|
| · | Building strong brands; |
|
|
|
| · | Leverage economies of scale and cost reduction to gain market share; |
|
|
|
| · | Bringing new drugs into the market, such as: cooperation with top R&D institutions; focus on intellectual property acquisition and sponsoring SFDA application and clinical trial testing; |
AGREEMENT WITH WU LAN CHA BU EMERGENCY HOSPITAL
In October 2006, Lotus East entered into a five-year loan agreement and contract with Wu Lan Cha Bu Emergency Hospital whereby Lotus East agreed to lend to Wu Lan Cha Bu Emergency Hospital approximately $4.5 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan Cha Bu Emergency Hospital agreed that Lotus East would be the exclusive provider for all medicines and disposable medical treatment apparatus to it for a period of 20 years. In October 2006, Dr. Zhongyi Liu, our Chief Executive Officer and the Chairman and principal stockholder of Lotus East, loaned these funds to Wu Lan Cha Bu Emergency Hospital on behalf of Lotus East. Accordingly, in October 2006 Lotus East entered into an assignment agreement whereby it assigned all of its rights, obligations, and receipts under the loan agreement to Dr. Liu, except for the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with Lotus East. As compensation to Dr. Liu for accepting the assignment under the loan agreement including all of the risks and obligations and for Dr. Liu not accepting the rights to revenues from the sale of medical and disposable medical treatment apparatus which will remain with Lotus East, Lotus East agreed to pay Dr. Liu an aggregate of approximately $1.3 million (RMB 9 million) to be paid in five equal annual installments of approximately $272,000 (RMB 1.8 million). The construction project of Wu Lan Cha Bu Emergency Hospital is slowly keeping going down and it is beyond Lotus East’s control. Currently, we estimate the hospital construction project will be completed by 2012 and we anticipate that Lotus East will begin generating revenue from the hospital in 2013.
COMPETITION
Vertically integrated pharmaceutical operations are still at an early stage of development in China due to heavy state involvement in the past and Lotus East believes that the industry is fragmented. Lotus East faces competition from domestic drug research and development companies and drug manufacturing companies which are growing rapidly. Its direct competitors are domestic pharmaceutical companies and new drug research and development institutes that have fairly strong research and development capabilities in new drugs such as Beijing Venture Biopharma Technology Co., Ltd., Fosun Group Co., Ltd., Zhuhai Lizhu and Beijing Nohua. Lotus East also faces competition of foreign companies who have strong proprietary pipeline and strong financial resources. These companies have significantly greater assets than Lotus East has and have a larger current market share than Lotus East has. Lotus East believes the following are its competitive advantage:
| · | Lotus East is one of the first facilities that have been GMP certified by SFDA; |
| · | Lotus East has established products and brand name that are familiar to doctors and pharmacists; |
| · | Good product quality and reputation and generally has not experienced any quality control issue; and |
| · | Extensive distribution network and lower prices. |
Lotus East believes that it is able to compete as a result of its advantages mentioned above.
12
SOURCES AND AVAILABILITY OF RAW MATERIALS, THIRD PARTY MANUFACTURED FINISHED GOODS AND PRINCIPAL SUPPLIERS
Lotus East designs, creates prototypes and manufactures its products at its manufacturing facilities located at Beijing, PRC. Its principal raw materials include Brimonidine Tartrate, Levofloxacin Lactate, Nicergoline and Valsartan. Lotus East requires a supply of quality raw materials to manufacture its products. Historically, Lotus East has not had difficulty in obtaining raw materials from suppliers. Currently, Lotus East relies on numerous suppliers to deliver its required raw materials. The prices for these raw materials are subject to market forces largely beyond Lotus East's control, including energy costs, organic chemical prices, market demand, and freight costs. The prices for these raw materials have varied significantly in the past and may vary significantly in the future.
Third party manufactured finished goods needed to Lotus East’s wholesale distribution channel and retail drugstores are relatively easy to purchase from a number of suppliers. While Lotus East does not have long-term contracts with these suppliers, Lotus East has long-term and good business relationship with them and these companies have generally met Lotus East’s supply requirements. Generally, the factors that affect third party manufactured finished goods price are market demand and freight costs, etc… The price of drugs in China, while unstable and beyond Lotus East’s control, historically did not affect Lotus East’s business significantly, because Lotus East pass along any significant change in drugs price to its customers. However, Lotus East cannot guarantee that the present conditions of the drugs market will continue. Any significant rise in the price of or demand for drugs could have an adverse affect on our results of operations.
INTELLECTUAL PROPERTY
Lotus East relies on a combination of trademark, copyright and trade secret protection laws in PRC and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect its intellectual property and brand. Lotus East intends to apply for patent protection of certain of its special technologies to protect its core technologies. There are no assurances, however, that if such applications are made that the patents will be granted. Lotus East also enters into confidentiality, non-compete and invention assignment agreements with its employees and consultants and nondisclosure agreements with third parties. "Maixin", “Muxin”, “En Ze Jia Shi” and "Liang Fang" are its registered trademarks in the PRC.
GOVERNMENT REGULATION
(A). General regulations related to the pharmaceutical industry in the PRC
The Drug Administration Law of the PRC governs Lotus East and its products. The State Food & Drug Administration of the PRC regulates and implements PRC drug laws. As a developer, producer and distributor of medicinal products, we are subject to regulation and oversight by the SFDA and its provincial and local branches. The Law of the PRC on the Administration of Pharmaceuticals provides the basic legal framework for the administration of the production and sale of pharmaceuticals in China and covers the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products. Its implementing regulations set forth detailed rules with respect to the administration of pharmaceuticals in China. The quality and production control standards must be in compliance with PRC’s GMP requirements. We are also subject to other PRC laws and regulations that are applicable to business operators, manufacturers and distributors in general.
| · | Registration and approval of medicine: A medicine must be registered and approved by the SFDA before it can be manufactured. The registration and approval process requires the manufacturer to submit to the SFDA a registration application containing detailed information concerning the efficacy and quality of the medicine and the manufacturing process and the production facilities the manufacturer expects to use. To obtain the SFDA registration and approval necessary for commencing production, the manufacturer is also required to conduct pre-clinical trials, apply to the SFDA for permission to conduct clinical trials, and, after clinical trials are completed, file clinical data with the SFDA for approval. The SFDA has granted Lotus East six government permits for it to produce the following products: Valsartan capsules; the material of Valsartan; Levofloxacin Lactate for Injection; the material of Levofloxacin Lactate; Brimonidine Tartrate Eyes Drops with a density of 0.1; and Brimonidine Tartrate Eyes Drops with the density of 0.3. |
|
|
|
| · | New medicine: If a medicine is approved by the SFDA as a new medicine, the SFDA will issue a new medicine certificate to the manufacturer and impose a monitoring period which shall be calculated starting from the day of approval for manufacturing of the new medicine and may not exceed five years. The length of the monitoring period is specified in the new medicine certificate. During the monitoring period, the SFDA will monitor the safety of the new medicine, and will neither accept new medicine certificate applications for an identical medicine by another pharmaceutical company, nor approve the production or import of an identical medicine by other pharmaceutical companies. For new medicines approved prior to September 2002, the monitoring period could be longer than five years. As a result of these regulations, the holder of a new medicine certificate effectively has the exclusive right to manufacture the new medicine during the monitoring period. |
13
| · | Provisional national production standard: In connection with the SFDA’s approval of a new medicine, the SFDA will normally direct the manufacturer to produce the medicine according to a provisional national production standard, or a provisional standard. A provisional standard is valid for two years, during which the SFDA closely monitors the production process and quality consistency of the medicine to develop a national final production standard for the medicine, or a final standard. Three months before the expiration of the two-year period, the manufacturer is required to apply to the SFDA to convert the provisional standard to a final standard. Upon approval, the SFDA will publish the final standard for the production of this medicine. In practice, the approval for conversion to a final standard is a time-consuming process. However, during the SFDA’s review period, the manufacturer may continue to produce the medicine according to the provisional standard. |
|
|
|
| · | Transitional period: Prior to the latter of (1) the expiration of a new medicine’s monitoring period or (2) the date when the SFDA grants a final standard for a new medicine after the expiration of the provisional standard, the SFDA will not accept applications for an identical medicine nor will it approve the production of an identical medicine by other pharmaceutical companies. Accordingly, the manufacturer will continue to have an exclusive production right for the new medicine during this transitional period. |
|
|
|
| · | Continuing SFDA regulation: Pharmaceutical manufacturers in China are subject to continuing regulation by the SFDA. If the labeling or manufacturing process of an approved medicine is significantly modified, a new pre-market approval or pre-market approval supplement will be required by the SFDA. A pharmaceutical manufacturer is subject to periodic inspection and safety monitoring by the SFDA to determine compliance with regulatory requirements. The SFDA has a variety of enforcement actions available to enforce its regulations and rules, including fines and injunctions, recall or seizure of products, the imposition of operating restrictions, partial suspension or complete shutdown of production and criminal prosecution. |
(B). Pharmaceutical product manufacturing
| · | Permits and licenses for pharmaceutical manufactures: A pharmaceutical manufacturer must obtain a pharmaceutical manufacturing permit from the SFDA’s relevant branch. This permit is valid for five years and is renewable upon its expiration. Lotus East has the requisite approval and licenses from the Beijing SFDA in order to operate its production facilities. Lotus East does not anticipate any difficulty in renewing its pharmaceutical manufacturing permits upon expiration. |
|
|
|
| · | Good manufacturing practice: A pharmaceutical manufacturer must meet Good Manufacturing Practice standards, or GMP standards, for each of its production facilities in China in respect of each form of pharmaceutical products it produces. GMP standards include staff qualifications, production premises and facilities, equipment, raw materials, environmental hygiene, production management, quality control and customer complaint administration. If a manufacturer meets the GMP standards, the SFDA will issue to the manufacturer a Good Manufacturing Practice certificate, or a GMP certificate, with a five-year validity period. However, for a newly established pharmaceutical manufacturer that meets the GMP standards, the SFDA will issue a GMP certificate with only a one-year validity period. Lotus East is one of the first enterprises to be authenticated by the National China Good Manufacturing Practices (GMP). |
|
|
|
| · | Pharmaceutical distribution: A distributor of pharmaceutical products in China must obtain a pharmaceutical operating permit from the relevant provincial or local SFDA branches. After the operating permit is obtained, the distributor must follow the Good Supply Practice (“GSP”) requirements to establish a complete quality assurance system included product management, personnel, equipments, purchasing, storage and sales processes. Distributors will have to apply for accreditation to the provincial FDA. The provincial FDA will engage experts to conduct on-site inspection in accordance with the standards of pharmaceutical quality management specifications. The GSP certification will be granted to the distributor after it passes the inspections and the GSP certificate is valid for 5 years. Pharmaceutical distributors must obtain Drug dealers must obtain operating permits and GSP certificate of drugs prior to starting the distribution. Otherwise, it is a violation of the Drug Administration Law and the distributor will be prosecuted accordingly. |
|
|
|
| · | Good supply practice standards: The SFDA applies Good Supply Practice standards, or GSP standards, to all pharmaceutical wholesale and retail distributors to ensure the quality of distribution in China. The currently applicable GSP standards require pharmaceutical distributors to implement controls on the distribution of medicine, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. A certificate for GSP standards, or GSP certificate, is valid for five years, except for a newly established pharmaceutical distribution company, for which the GSP certificate is valid for only one year. |
14
| · | Price controls: The retail prices of prescription and over-the-counter medicines sold in China, primarily those included in the national and provincial medical insurance catalogs and those pharmaceutical products whose production or distribution are deemed to constitute monopolies, are subject to price controls in the form of fixed prices or price ceilings administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities. The controls over the retail price of a medicine effectively set the limits for the wholesale price of that medicine. From time to time, the NDRC publishes and updates a national list of medicines that are subject to price control. Fixed prices and price ceilings on medicines are determined based on profit margins that the NDRC deems reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicines and the extent of the manufacturer’s compliance with the applicable GMP standards. The NDRC directly regulates the price of some of the medicines on the list, and delegates the power to provincial price control authorities to regulate the remainder on the list. For those medicines under the authority of provincial price control authorities, each provincial price control authority regulates medicines manufactured by manufacturers registered in that province. Provincial price control authorities have the discretion to authorize price adjustments based on the local conditions and the level of local economic development. Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine and it must apply either to the NDRC, if the price of the medicine is nationally regulated, or to the provincial price control authorities in the province where it is registered, if the price of the medicine is provincially regulated. For a provincially regulated medicine, when provincial price control authorities approve an application, they will file the new approved price with the NDRC for confirmation and thereafter the newly approved price will become binding and enforceable across China. |
|
|
|
| · | Tendering requirement for hospital purchases of medicines: Provincial and municipal government agencies such as provincial or municipal health departments also operate a mandatory tendering process for purchases by state-owned hospitals of a medicine included in provincial medicine catalogs. These government agencies organize a tendering process once every year in their province or city and typically invite manufacturers of provincial catalog medicines that are on the hospitals’ formularies and are in demand by these hospitals to participate in the tendering process. A government-approved committee consisting of physicians, experts and officials is delegated by these government agencies the power to review bids and select one or more medicines for the treatment of a particular medical condition. The selection is based on a number of factors, including bid price, quality and manufacturer’s reputation and service. The bidding price of a winning medicine will become the price required for purchases of that medicine by all state-owned hospitals in that province or city. The tendering requirement was first introduced in 2001 and has since been implemented across China. We understand that the level of present implementation of the tendering requirement varies among different provinces in China. |
|
|
|
| · | Reimbursement under the national medical insurance program: As of March 2011, approximately 402 million people were enrolled into the National Medical Insurance Program. The Ministry of Labor and Social Security, together with other government authorities, determines which medicines are to be included in or removed from the national medicine catalog for the National Medical Insurance Program, and under which tier a medicine should fall, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are based on a number of factors, including price and efficacy. A National Medical Insurance Program participant can be reimbursed for the full cost of a Tier 1 medicine and 80 to 90% of the cost of a Tier 2 medicine. Although it is designated as a national program, the implementation of the National Medical Insurance Program is delegated to various provincial governments, each of which has established its own medicine catalog. A provincial government must include all Tier 1 medicines listed in the national medicine catalog in its provincial medicine catalog, but may use its discretion based on its own selection criteria to add other medicines to, or exclude Tier 2 medicines listed in the national medicine catalog from, its provincial medicine catalog, so long as the combined numbers of the medicines added and excluded do not exceed 15% of the number of the Tier 2 medicines listed in the national catalog. In addition, provincial governments may use their discretion to upgrade a nationally classified Tier 2 medicine to Tier 1 in their provincial medicine catalogs, but may not downgrade a nationally classified Tier 1 medicine to Tier 2. The total amount of reimbursement for the cost of prescription and over-the-counter medicines, in addition to other medical expenses, for an individual program participant in a calendar year is capped at the amount in that participant’s individual account. The amount in a participant’s account varies, depending upon the amount of contributions from the participant and his or her employer. Generally, program participants who are from relatively wealthier eastern parts of China and relatively wealthier metropolitan centers have greater amounts in their individual accounts than those from less developed provinces. |
15
COMPLIANCE WITH ENVIRONMENTAL LAW
Lotus East complies with the Environmental Protection Law of China as well as the applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. The costs for PRC environmental regulation compliance in the past two fiscal years has been immaterial and mainly for the waste discharge processing, industrial exhaust treatment and dust cleaning in connection with our production facilities. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
EMPLOYEES
As of the report filing date, Lotus East has about 533 employees (355 exclusive sales representatives, 111 retail store staff, 35 administrative staff, 25 factory workers and 7 research and development staff), including 233 full time employees and 300 part time employees. Majority of our employees are organized into a union under the labor laws of China and can bargain collectively with Lotus East. Lotus East has not experienced a work stoppage since inception and does not anticipate any work stoppage in the foreseeable future. Management believes that its relations with its employees and union are good.
HISTORY OF OUR COMPANY
Lotus Pharmaceuticals, Inc. (“Lotus” or “the Company”), formerly S.E. Asia Trading Company, Inc. (“S.E.”), was incorporated on January 28, 2004 to sell jewelry and home accessories under the laws of the State of Nevada. S.E. operated as a retailer of jewelry, framed art and home accessories.
Lotus International was incorporated under the laws the State of Nevada on August 28, 2006 to develop and market pharmaceutical products in China. On September 6, 2006, S.E. entered into a definitive Share Exchange Agreement with Lotus International, whereby S.E. would acquire all of the outstanding common stock of Lotus International in exchange for newly-issued shares of S.E.’s stock to Lotus International's stockholders. On September 28, 2006, Lotus International became S.E.’s wholly-owned subsidiary and Lotus International's stockholders own the majority of S.E.’s voting stock. The acquisition of Lotus International by S.E. was accounted for as a reverse merger because on a post-merger basis, the former stockholders of Lotus International held a majority of S.E.’s outstanding common stock on a voting and fully-diluted basis. As a result, Lotus International is deemed to be the acquirer for accounting purposes.
We changed S.E.’s name to Lotus Pharmaceuticals, Inc. on December 6, 2006.
On May 25, 2007, Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. (“Lotus Century”), a wholly foreign-owned enterprise (“WFOE”), was incorporated in Beijing, China. Lotus Century is a Chinese limited liability company and a wholly-owned subsidiary of Lotus International. On August 20, 2007, an assignment agreement was signed between Lotus International and Lotus Century. Lotus Century obtained assignment of Lotus International’s Contractual Agreements to control the management and voting and to take all the profits and bear all the losses of Lotus East.
ITEM 1A. RISK FACTORS
Investing in our securities involves a great deal of risk. Careful consideration should be made of the following factors as well as other information included in this report before deciding to purchase our common stock. You should pay particular attention to the fact that we conduct all of our operations in China and are governed by a legal and regulatory environment that in some respects differs significantly from the environment that may prevail in other countries. Our business, financial condition or results of operations could be affected materially and adversely by any or all of these risks.
Risks relating to Our Business
We may need additional financing to execute our business plan.
The revenues from the production and sale of pharmaceutical products and the projected revenues from these products may not be adequate to support our expansion and product development programs. We may need substantial additional funds to build our new production facilities, pursue further research and development, obtain regulatory approvals, market our products, and file, prosecute, defend and enforce our intellectual property rights. We will seek additional funds through public or private equity or debt financing, strategic transactions and/or from other sources. We could enter into collaborative arrangements for the development of particular products that would lead to our relinquishing some or all rights to the related technology or products.
16
There are no assurances that future funding will be available on favorable terms or at all. If additional funding is not obtained, we will need to reduce, defer or cancel development programs, planned initiatives or overhead expenditures, to the extent necessary. The failure to fund our capital requirements would have a material adverse effect on our business, financial condition and results of operations.
Our success depends on collaborative partners, licensees and other third parties over whom we have limited control.
Due to the complexity of the process of developing pharmaceuticals, our core business depends on arrangements with pharmaceutical institutes, corporate and hospital collaborators, licensors, licensees and others for the research, development, clinical testing, technology rights, manufacturing, marketing and commercialization of our products. We have several research collaborations. Our license agreements could obligate us to diligently bring potential products to market, make milestone payments and royalties that, in some instances, could be substantial, and incur the costs of filing and prosecuting patent applications. There are no assurances that we will be able to establish or maintain collaborations that are important to our business on favorable terms, or at all.
A number of risks arise from our dependence on collaborative agreements with third parties. Product development and commercialization efforts could be adversely affected if any collaborative partner:
| · | terminates or suspends its agreement with us; |
| · | causes delays; |
| · | fails to timely develop or manufacture in adequate quantities a substance needed in order to conduct clinical trials; |
| · | fails to adequately perform clinical trials; |
| · | determines not to develop, manufacture or commercialize a product to which it has rights; or |
| · | otherwise fails to meet its contractual obligations. |
Our collaborative partners could pursue other technologies or develop alternative products that could compete with the products we are developing.
The profitability of our products will depend in part on our ability to protect proprietary rights and operate without infringing the proprietary rights of others.
The profitability of our products will depend in part on our ability to obtain and maintain patents and licenses and preserve trade secrets, and the period our intellectual property remains exclusive. We must also operate without infringing the proprietary rights of third parties and without third parties circumventing its rights. The patent positions of pharmaceutical enterprises, including ours, are uncertain and involve complex legal and factual questions for which important legal principles are largely unresolved. The biotechnology patent situation outside the US is uncertain, is currently undergoing review and revision in many countries, and may not protect our intellectual property rights to the same extent as the laws of the US. Because patent applications are maintained in secrecy in some cases, we cannot be certain that we or our licensors are the first creators of inventions described in our pending patent applications or patents or the first to file patent applications for such inventions.
Other companies may independently develop similar products and design around any patented products we develop. We cannot assure you that:
| · | any of our patent applications will result in the issuance of patents; |
| · | we will develop additional patentable products; |
| · | the patents of others will not impede our ability to do business; or |
| · | third parties will not be able to circumvent our patents. |
A number of pharmaceutical, research, and academic companies and institutions have developed technologies, filed patent applications or received patents on technologies that may relate to our business. If these technologies, applications or patents conflict with ours, the scope of our current or future patents could be limited or our patent applications could be denied. Our business may be adversely affected if competitors independently develop competing technologies, especially if we do not obtain, or obtain only narrow, patent protection. If patents that cover our activities are issued to other companies, we may not be able to obtain licenses at a reasonable cost, or at all; develop our technology; or introduce, manufacture or sell the products we have planned.
17
Patent litigation is becoming widespread in the pharmaceutical industry. Such litigation may affect our efforts to form collaborations, to conduct research or development, to conduct clinical testing or to manufacture or market any products under development. There are no assurances that our patents would be held valid or enforceable by a court or that a competitor's technology or product would be found to infringe our patents in the event of patent litigation. Our business could be materially affected by an adverse outcome to such litigation. Similarly, we may need to participate in interference proceedings declared by the U.S. Patent and Trademark Office or equivalent international authorities to determine priority of invention. We could incur substantial costs and devote significant management resources to defend our patent position or to seek a declaration that another company's patents are invalid.
Much of our know-how and technology may not be patentable, though it may constitute trade secrets. There are no assurances that we will be able to meaningfully protect our trade secrets. We cannot assure you that any of our existing confidentiality agreements with employees, consultants, advisors or collaborators will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Collaborators, advisors or consultants may dispute the ownership of proprietary rights to our technology, for example by asserting that they developed the technology independently.
We may encounter difficulties in manufacturing our products
Before our products can be profitable, they must be produced in commercial quantities in a cost-effective manufacturing process that complies with regulatory requirements, including GMP, production and quality control regulations. If we cannot arrange for or maintain commercial-scale manufacturing on acceptable terms, or if there are delays or difficulties in the manufacturing process, we may not be able to conduct clinical trials, obtain regulatory approval or meet demand for our products. Production of our products could require raw materials which are scarce or which can be obtained only from a limited number of sources. If we are unable to obtain adequate supplies of such raw materials, the development, regulatory approval and marketing of our products could be delayed.
We could need more clinical trials or take more time to complete our clinical trials than we have planned.
Clinical trials vary in design by factors including dosage, end points, length, and controls. We may need to conduct a series of trials to demonstrate the safety and efficacy of our products. The results of these trials may not demonstrate safety or efficacy sufficiently for regulatory authorities to approve our products. Further, the actual schedules for our clinical trials could vary dramatically from the forecasted schedules due to factors including changes in trial design, conflicts with the schedules of participating clinicians and clinical institutions, and changes affecting product supplies for clinical trials.
We rely on collaborators, including academic institutions, governmental agencies and clinical research organizations, to conduct, supervise, monitor and design some or all aspects of clinical trials involving our products. Since these trials depend on governmental participation, we have less control over their timing and design than trials we sponsor. Delays in or failure to commence or complete any planned clinical trials could delay the ultimate timelines for our product releases. Such delays could reduce investors' confidence in our ability to develop products, likely causing our share price to decrease.
We may not be able to obtain the regulatory approvals or clearances that are necessary to commercialize our products.
The PRC and other countries impose significant statutory and regulatory obligations upon the manufacture and sale of pharmaceutical products. Each regulatory authority typically has a lengthy approval process in which it examines pre-clinical and clinical data and the facilities in which the product is manufactured. Regulatory submissions must meet complex criteria to demonstrate the safety and efficacy of the ultimate products. Addressing these criteria requires considerable data collection, verification and analysis. We may spend time and money preparing regulatory submissions or applications without assurances as to whether they will be approved on a timely basis or at all.
Our product candidates, some of which are currently in the early stages of development, will require significant additional development and pre-clinical and clinical testing prior to their commercialization. These steps and the process of obtaining required approvals and clearances can be costly and time-consuming. If our potential products are not successfully developed, cannot be proven to be safe and effective through clinical trials, or do not receive applicable regulatory approvals and clearances, or if there are delays in the process:
| · | the commercialization of our products could be adversely affected; |
| · | any competitive advantages of the products could be diminished; and |
| · | revenues or collaborative milestones from the products could be reduced or delayed. |
18
Governmental and regulatory authorities may approve a product candidate for fewer indications or narrower circumstances than requested or may condition approval on the performance of post-marketing studies for a product candidate. Even if a product receives regulatory approval and clearance, it may later exhibit adverse side effects that limit or prevent its widespread use or that would force us to withdraw the product from the market.
Any marketed product and its manufacturer will continue to be subject to strict regulation after approval. Results of post-marketing programs may limit or expand the further marketing of products. Unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market and possible civil actions.
In manufacturing our products we will be required to comply with applicable good manufacturing practices regulations, which include requirements relating to quality control and quality assurance, as well as the maintenance of records and documentation. If we cannot comply with regulatory requirements, including applicable good manufacturing practice requirements, we may not be allowed to develop or market the product candidates. If we or our manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, we may be subject to sanctions, including fines, product recalls or seizures, injunctions, refusal of regulatory agencies to review pending market approval applications or supplements to approve applications, total or partial suspension of production, civil penalties, withdrawals of previously approved marketing applications and criminal prosecution.
Competitors may develop and market pharmaceutical products that are less expensive, more effective or safer, making our products obsolete or uncompetitive.
Some of our competitors and potential competitors have greater product development capabilities and financial, scientific, marketing and human resources than we do. Technological competition from pharmaceutical companies is intense and is expected to increase. Other companies have developed technologies that could be the basis for competitive products. Some of these products have an entirely different approach or means of accomplishing the desired curative effect than products we are developing. Alternative products may be developed that are more effective, work faster and are less costly than our products. Competitors may succeed in developing products earlier than us, obtaining approvals and clearances for such products more rapidly than us, or developing products that are more effective than ours. In addition, other forms of treatment may be competitive with our products. Over time, our technology or products may become obsolete or uncompetitive.
Our products may not gain market acceptance.
Our products may not gain market acceptance in the pharmaceutical community. The degree of market acceptance of any product depends on a number of factors, including establishment and demonstration of clinical efficacy and safety, cost-effectiveness, clinical advantages over alternative products, and marketing and distribution support for the products. Limited information regarding these factors is available in connection with our products or products that may compete with ours.
To directly market and distribute our pharmaceutical products, we or our collaborators require a marketing and sales force with appropriate technical expertise and supporting distribution capabilities. We may not be able to further establish sales, marketing and distribution capabilities or enter into arrangements with third parties on acceptable terms. If we or our partners cannot successfully market and sell our products, our ability to generate revenue will be limited.
Our operations and the use of our products could subject us to damages relating to injuries or accidental contamination.
Our research and development processes involve the controlled use of hazardous materials. We are subject to PRC national, provincial and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and waste products. The risk of accidental contamination or injury from handling and disposing of such materials cannot be completely eliminated. In the event of an accident involving hazardous materials, we could be held liable for resulting damages. We are not insured with respect to this liability. Such liability could exceed our resources. In the future we could incur significant costs to comply with environmental laws and regulations.
If we were successfully sued for product liability, we could face substantial liabilities that may exceed our resources.
We may be held liable if any product we develop, or any product which is made using our technologies, causes injury or is found unsuitable during product testing, manufacturing, marketing, sale or use. These risks are inherent in the development of agricultural and pharmaceutical products. We currently do not have product liability insurance. We are not insured with respect to this liability. If we choose to obtain product liability insurance but cannot obtain sufficient insurance coverage at an acceptable cost or otherwise protect against potential product liability claims, the commercialization of products that we develop may be prevented or inhibited. If we are sued for any injury caused by our products, our liability could exceed our total assets.
19
We have limited business insurance coverage.
The insurance industry in China is still at an early stage of development. Insurance companies in China offer limited business insurance products. We do not have any business liability or disruption insurance coverage for our operations in China. Any business disruption, litigation or natural disaster may result in our incurring substantial costs and the diversion of our resources.
Our business depends substantially on the continuing efforts of our executive officers and our ability to maintain a skilled labor force, and our business may be severely disrupted if we lose their services.
Our future success depends substantially on the continued services of our executive officers, especially Dr. Liu, our chief executive officer and the chairman of our board. We do not maintain key man life insurance on any of our executive officers. If one or more of our executive officers are unable or unwilling to continue in their present positions, we may not be able to replace them readily, if at all. Therefore, our business may be severely disrupted, and we may incur additional expenses to recruit and retain new officers. In addition, if any of our executives joins a competitor or forms a competing company, we may lose some of our customers.
Our success depends on attracting and retaining qualified personnel.
We depend on a core management and scientific team. The loss of any of these individuals could prevent us from achieving our business objective of commercializing our product candidates. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing and government regulation. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. If our recruitment and retention efforts are unsuccessful, our business operations could suffer.
Risks Related to Our Corporate Structure
PRC laws and regulations governing our businesses and the validity of certain of our contractual arrangements are uncertain. If we are found to be in violation, we could be subject to sanctions. In addition, changes in such PRC laws and regulations may materially and adversely affect our business.
There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including, but not limited to, the laws and regulations governing our business, or the enforcement and performance of our contractual arrangements with our Chinese entity, Lotus East and its shareholders. We are considered a foreign person or foreign invested enterprise under PRC law. As a result, we are subject to PRC law limitations on foreign ownership of Chinese companies. These laws and regulations are relatively new and may be subject to change, and their official interpretation and enforcement may involve substantial uncertainty. The effectiveness of newly enacted laws, regulations or amendments may be delayed, resulting in detrimental reliance by foreign investors. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively.
The PRC government has broad discretion in dealing with violations of laws and regulations, including levying fines, revoking business and other licenses and requiring actions necessary for compliance. In particular, licenses and permits issued or granted to us by relevant governmental bodies may be revoked at a later time by higher regulatory bodies. We cannot predict the effect of the interpretation of existing or new PRC laws or regulations on our businesses. We cannot assure you that our current ownership and operating structure would not be found in violation of any current or future PRC laws or regulations. As a result, we may be subject to sanctions, including fines, and could be required to restructure our operations or cease to provide certain services. Any of these or similar actions could significantly disrupt our business operations or restrict us from conducting a substantial portion of our business operations, which could materially and adversely affect our business, financial condition and results of operations.
The PRC government restricts foreign investment in pharmaceutical businesses in China. Accordingly, we operate our business in China through Lotus East. Lotus East holds the licenses and approvals necessary to operate our pharmaceutical business in China. We have contractual arrangements with Lotus East and its shareholders that allow us to substantially control Lotus East. We cannot assure you, however, that we will be able to enforce these contracts.
Although we believe we comply with current PRC regulations, we cannot assure you that the PRC government would agree that these operating arrangements comply with PRC licensing, registration or other regulatory requirements, with existing policies or with requirements or policies that may be adopted in the future. If the PRC government determines that we do not comply with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or on our customers, or take other regulatory or enforcement actions against us that could be harmful to our business.
20
We may be adversely affected by complexity, uncertainties and changes in PRC regulation of pharmaceutical business and companies, including limitations on our abilities to own key assets.
The PRC government regulates the pharmaceutical industry including foreign ownership of, and the licensing and permit requirements pertaining to, companies in the pharmaceutical industry. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to PRC government regulation of the pharmaceutical industry include the following:
| · | we only have contractual control over Lotus East. We do not own it due to the restriction of foreign investment in Chinese businesses; and |
|
|
|
| · | uncertainties relating to the regulation of the pharmaceutical business in China, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, or have other harmful effects on us. |
The interpretation and application of existing PRC laws, regulations and policies and possible new laws, regulations or policies have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, pharmaceutical businesses in China, including our business.
Our contractual arrangements with Lotus East and its shareholders may not be as effective in providing control over these entities as direct ownership.
Since the law of the PRC limits foreign equity ownership in pharmaceutical companies in China, we operate our business through Lotus East. We have no equity ownership interest in Lotus East and rely on contractual arrangements to control and operate such business. These contractual arrangements may not be effective in providing control over Lotus East as direct ownership. For example, Lotus East could fail to take actions required for our business despite its contractual obligation to do so. If Lotus East fails to perform under its agreements with us, we may have to incur substantial costs and resources to enforce such arrangements and may have to rely on legal remedies under the law of the PRC, which may not be effective. In addition, we cannot assure you that Lotus East’s shareholders would always act in our best interests.
Certain of our executive officers, directors and principal stockholders are also officers, directors and principal stockholders of Lotus East. There are no assurances that the conflicts of interest between obligations to our company and obligations to Lotus East will be resolved in our favor.
While pursuant to the Contractual Arrangements we have the ability to control the daily operations and financial affairs of Lotus East, appoint each of their senior executives and approve all matters requiring approval by their respective members, these actions on our behalf are determined by our Board of Directors. Dr. Liu, our CEO, is a member of our Board of Directors and principal stockholder of our company and he is also officer, director and principal stockholder of Lotus East. Conflicts of interests between his duties to our company and Lotus East may arise. We cannot assure you, however, that when conflicts of interest arise, Dr. Liu will act completely in our interests or that conflicts of interests will be resolved in our favor. In addition, Dr. Liu could violate his legal duties by diverting business opportunities from us to others.
Risks Related to Doing Business in China
If the PRC enacts regulations which forbid or restrict foreign investment, our ability to grow may be severely impaired.
We intend to expand our business in areas relating to our present business. We may also expand by making acquisitions of companies in related industries. Many of the rules and regulations that we would face are not explicitly communicated, and we may be subject to rules that would affect our ability to grow, either internally or through acquisition of other Chinese or foreign companies. There are also substantial uncertainties regarding the proper interpretation of current laws and regulations of the PRC. New laws or regulations that forbid foreign investment could severely impair our businesses and prospects. Additionally, if the relevant authorities find us in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
| · | levying fines; |
| · | revoking our business and other licenses; and |
| · | requiring that we restructure our ownership or operations. |
21
Any deterioration of political relations between the United States and the PRC could impair our operations and your investment in us.
The relationship between the United States and the PRC is subject to sudden fluctuation and periodic tension. Changes in political conditions in the PRC and changes in the state of Sino-U.S. relations are difficult to predict and could adversely affect our operations or cause potential acquisition candidates or their goods and services to become less attractive. Such a change could lead to a decline in our profitability. Any weakening of relations between the United States and the PRC could have a material adverse effect on our operations and your investment in us, particularly in our efforts to raise capital to expand our other business activities.
Adverse changes in economic and political policies of the PRC government could have a material adverse effect on the overall economic growth of China, which could adversely affect our business.
Substantially all of our business operations are conducted in China. Accordingly, our results of operations, financial condition and prospects are subject to a significant degree to economic, political and legal developments in China. China's economy differs from the economies of most developed countries in many respects, including with respect to:
| · | the amount of government involvement; |
| · | level of development; |
| · | growth rate; |
| · | control of foreign exchange; and |
| · | allocation of resources. |
While the PRC economy has experienced significant growth in the past 20 years, growth has been uneven across different regions and among various economic sectors of China. The PRC government has implemented various measures to encourage economic development and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may also have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations that are applicable to us. Since early 2004, the PRC government has implemented certain measures to control the pace of economic growth. Such measures may cause a decrease in the level of economic activity in China, which in turn could adversely affect our results of operations and financial condition.
Price controls may affect both our revenues and net income.
The laws of the PRC provide for the government to fix and adjust prices. Although we are not presently subject to price controls in connection with the sale of our products, it is possible that price controls may be imposed in the future. To the extent that we are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and, unless there is also price control on the products that we purchase from our suppliers, we may face no limitation on our costs. Further, if price controls affect both our revenue and our costs, our ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
Our operations may not develop in the same way or at the same rate as might be expected if the PRC economy were similar to the market-oriented economies of OECD member countries.
The economy of the PRC has historically been a nationalistic, “planned economy,” meaning it functions and produces according to governmental plans and pre-set targets or quotas. In certain aspects, the PRC’s economy has been making a transition to a more market-oriented economy, although the government imposes price controls on certain products and in certain industries. However, we cannot predict the future direction of these economic reforms or the effects these measures may have. The economy of the PRC also differs from the economies of most countries belonging to the Organization for Economic Cooperation and Development (the “OECD”), an international group of member countries sharing a commitment to democratic government and market economy. For instance:
| · | the level of state-owned enterprises in the PRC, as well as the level of governmental control over the allocation of resources is greater than in most of the countries belonging to the OECD; |
| · | the level of capital reinvestment is lower in the PRC than in other countries that are members of the OECD; |
| · | the government of the PRC has a greater involvement in general in the economy and the economic structure of industries within the PRC than other countries belonging to the OECD; |
| · | the government of the PRC imposes price controls on certain products and our products may become subject to additional price controls; and |
| · | the PRC has various impediments in place that make it difficult for foreign firms to obtain local currency, as opposed to other countries belonging to the OECD where exchange of currencies is generally free from restriction. |
22
As a result of these differences, our business may not develop in the same way or at the same rate as might be expected if the economy of the PRC were similar to those of the OECD member countries.
Because some of our officers and directors reside outside of the United States, it may be difficult for you to enforce your rights against them or enforce United States court judgments against them in the PRC.
Most of our executive officers and directors reside in the PRC and a substantial portion of our assets are located in the PRC. It may therefore be difficult for United States investors to enforce their legal rights, to effect service of process upon our directors or officers or to enforce judgments of United States courts predicated upon civil liabilities and criminal penalties of our directors and officers under federal securities laws. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement of criminal penalties of the federal securities laws.
We may have limited legal recourse under Chinese law if disputes arise under contracts with third parties.
Almost all of our agreements with our employees and third parties, including our supplier and customers, are governed by the laws of the PRC. The legal system in the PRC is a civil law system based on written statutes. Unlike common law systems, such as we have in the United States, it is a system in which decided legal cases have little precedential value. The government of the PRC has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the PRC, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance or to seek an injunction under Chinese law are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations.
Because we may not be able to obtain business insurance in the PRC, we may not be protected from risks that are customarily covered by insurance in the United States.
Business insurance is not readily available in the PRC. To the extent that we suffer a loss of a type which would normally be covered by insurance in the United States, such as product liability and general liability insurance, we would incur significant expenses in both defending any action and in paying any claims that result from a settlement or judgment.
Failure to comply with the United States Foreign Corrupt Practices Act could subject us to penalties and other adverse consequences.
We are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some that may compete with us, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the PRC. We can make no assurance, however, that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
A downturn in the economy of the PRC may slow our growth and profitability.
The growth of the Chinese economy has been uneven across geographic regions and economic sectors. There can be no assurance that growth of the Chinese economy will be steady or that any downturn will not have a negative effect on our business especially if it results in either a decreased use of products such as ours or in pressure on us to lower our prices. The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the Chinese government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in China is still owned by the Chinese government. The continued control of these assets and other aspects of the national economy by the Chinese government could materially and adversely affect our business. The Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Efforts by the Chinese government to slow the pace of growth of the Chinese economy could result in reduced demand for our products.
23
Any adverse change in the economic conditions or government policies in China could have a material adverse effect on the overall economic growth and the level of pharmaceutical investments and expenditures in China, which in turn could lead to a reduction in demand for our products and consequently have a material adverse effect on our businesses.
Downturns in the economies of the U.S. and Europe may affect the PRC economy which could reduce the demand for our products.
The rapid growth of the PRC economy in recent years has been partially related to the U.S. and European countries’ demand for goods made in and exported from the PRC. The downturns in the U.S. and European economies may reduce the demand for goods exported by the PRC which could eventually affect the PRC economy as overseas orders decrease. The downturn in the PRC economy may in turn negatively impact the demand for our products.
If certain tax exemptions within the PRC regarding withholding taxes are removed, we may be required to deduct corporate withholding taxes from any dividends we may pay in the future.
Under the PRC’s current tax laws, regulations and rulings, companies are exempt from paying withholding taxes with respect dividends paid to stockholders outside of the PRC. However, if the foregoing exemption is removed, we may be required to deduct certain amounts from any dividends we may pay to our stockholders.
Uncertainties with respect to the PRC legal system could adversely affect us.
We conduct our business primarily through our Chinese entity, Lotus East. Our operations in China are governed by PRC laws and regulations. We are generally subject to laws and regulations applicable to foreign investments in China and, in particular, laws applicable to wholly foreign-owned enterprises. The PRC legal system is based on written statutes. Prior court decisions may be cited for reference but have limited precedential value.
Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, because these laws and regulations are relatively new, and because of the limited volume of published decisions and their nonbinding nature, the interpretation and enforcement of these laws and regulations involve uncertainties. In addition, the PRC legal system is based in part on government policies and internal rules (some of which are not published on a timely basis or at all) that may have a retroactive effect. As a result, we may not be aware of our violation of these policies and rules until some time after the violation. In addition, any litigation in China may be protracted and result in substantial costs and diversion of resources and management attention.
You may experience difficulties in effecting service of legal process, enforcing foreign judgments or bringing original actions in China based on United States or other foreign laws against us, our management or the experts named in the report.
We conduct substantially all of our operations in China and substantially all of our assets are located in China. In addition, most of our senior executive officers reside within China. As a result, it may not be possible to effect service of process within the United States or elsewhere outside China upon our senior executive officers, including with respect to matters arising under U.S. federal securities laws or applicable state securities laws. Moreover, our PRC counsel has advised us that the PRC does not have treaties with the United States or many other countries providing for the reciprocal recognition and enforcement of judgment of courts.
Fluctuation in the value of RMB may have a material adverse effect on your investment.
The value of RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions. All of Lotus East's financial assets and its revenues and costs are denominated in RMB. We rely entirely on fees paid to us by Lotus East. Any significant fluctuation in value of RMB may materially and adversely affect our cash flows, revenues, earnings and financial position, and the value of the consulting fees payable to us by Lotus East.
We face risks related to health epidemics and other outbreaks.
Our business could be adversely affected by the effects of H1N1 virus or another epidemic or outbreak. Since all of our operations are in China, H1N1 virus, Asian Bird Flu or other epidemic in China in the future may disrupt our business operations and have a material adverse effect on our financial condition and results of operations. For instance, health or other government regulations adopted in response may require temporary closure of our production facilities or of our offices. Such closures would severely disrupt our business operations and adversely affect our results of operations. We have not adopted any written preventive measures or contingency plans to combat any future outbreak of health epidemics or any other outbreaks.
24
Risks Related to an Investment in Our Common Stock
We do not anticipate paying any cash dividends.
We presently do not anticipate that we will pay any dividends on any of our capital stock in the foreseeable future. The payment of dividends, if any, would be contingent upon our revenues and earnings, if any, capital requirements, and general financial condition. The payment of any dividends is within the discretion of our Board of Directors. We presently intend to retain all earnings, if any, to implement our business plan; accordingly, we do not anticipate the declaration of any dividends in the foreseeable future.
Our common shares are thinly traded and, you may be unable to sell at or near ask prices or at all if you need to sell your shares to raise money or otherwise desire to liquidate your shares.
Our common shares have historically been sporadically or "thinly-traded”, meaning that the number of persons interested in purchasing our common shares at or near bid prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained.
The market price for our common stock is particularly volatile given our status as a relatively small company with a small and thinly traded “float”. The price at which you purchase our common stock may not be indicative of the price that will prevail in the trading market. You may be unable to sell your common stock at or above your purchase price if at all, which may result in substantial losses to you.
The market for our common shares is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. The volatility in our share price is attributable to a number of factors. First, as noted above, our common shares are sporadically and/or thinly traded. As a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our shareholders may disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously in the event that a large number of our common shares are sold on the market without commensurate demand, as compared to a seasoned issuer which could better absorb those sales without adverse impact on its share price. Secondly, we are a speculative or "risky" investment due to uncertainty of future market acceptance for our current and potential products. As a consequence of this enhanced risk, more risk-adverse investors may, under the fear of losing all or most of their investment in the event of negative news or lack of progress, be more inclined to sell their shares on the market more quickly and at greater discounts than would be the case with the stock of a seasoned issuer. The following factors may add to the volatility in the price of our common shares: actual or anticipated variations in our quarterly or annual operating results; adverse outcomes; and additions or departures of our key personnel, as well as other items discussed under this "Risk Factors" section, as well as elsewhere in this report. Many of these factors are beyond our control and may decrease the market price of our common shares, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common shares will be at any time, including as to whether our common shares will sustain their current market prices, or as to what effect that the sale of shares or the availability of common shares for sale at any time will have on the prevailing market price.
Certain of our officers and directors own a substantial portion of our outstanding common stock, which enables them to influence many significant corporate actions and in certain circumstances may prevent a change in control that would otherwise be beneficial to our shareholders.
As of the report date, our directors and executive officers control approximately 37.5% of our outstanding shares of common stock. These shareholders, acting individually or as a group, could exert substantial influence over matters such as electing directors and approving mergers or other business combination transactions. In addition, because of the percentage of ownership, elections of our board of directors will generally be within the control of these shareholders. While all of our shareholders are entitled to vote on matters submitted to our shareholders for approval, the concentration of shares presently lies with these principal shareholders. As such, it would be difficult for shareholders to propose and have approved proposals not supported by management. There can be no assurances that matters voted upon by our officers and directors in their capacity as shareholders will be viewed favorably by all shareholders of our company.
25
Because the OTC Bulletin Board is a quotation system, not an issuer listing service, market or exchange, it may be difficult for you to sell your common stock or you may not be able to sell your common stock for an optimum trading price.
The OTC Bulletin Board is a regulated quotation service that displays real-time quotes, last sale prices and volume limitations in over-the-counter securities. Because trades and quotations on the OTC Bulletin Board involve a manual process, the market information for such securities cannot be guaranteed. In addition, quote information, or even firm quotes, may not be available. The manual execution process may delay order processing and intervening price fluctuations may result in the failure of a limit order to execute or the execution of a market order at a significantly different price. Execution of trades, execution reporting and the delivery of legal trade confirmations may be delayed significantly. Consequently, one may not be able to sell shares of our common stock at the optimum trading prices.
The dealer’s spread (the difference between the bid and ask prices) may be large and may result in substantial losses to the seller of securities on the OTC Bulletin Board if the common stock or other security must be sold immediately. Further, purchasers of securities may incur an immediate “paper” loss due to the price spread. Moreover, dealers trading on the OTC Bulletin Board may not have a bid price for securities bought and sold through the OTC Bulletin Board. Due to the foregoing, demand for securities that are traded through the OTC Bulletin Board may be decreased or eliminated.
The exercise of outstanding warrants and the conversion of those outstanding preferred stocks will be dilutive to our existing stockholders.
At March 28, 2011, we had 27,747,131 shares of our common stock issued and outstanding and the following securities which are convertible or exercisable into shares of our common stock were outstanding:
| · | 303,554 shares of our common stock issuable upon the conversion of preferred stock; and |
|
|
|
| · | 2,001,000 of our common stock issuable upon the exercise of common stock purchase warrants with exercise prices ranging from $1.74 to $3.82 per share. |
The exercise of the warrants and the conversion of preferred stock may materially adversely affect the market price of our common stock and will have a dilutive effect on our existing stockholders.
Because our stock currently trades below $5.00 per share, and is quoted on the OTC Bulletin Board, our stock is considered a “penny stock” which can adversely affect its liquidity.
As the trading price of our common stock is less than $5.00 per share, our common stock is considered a "penny stock," and trading in our common stock is subject to the requirements of Rule 15g-9 under the Securities Exchange Act of 1934. Under this rule, broker/dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements. The broker/dealer must make an individualized written suitability determination for the purchaser and receive the purchaser's written consent prior to the transaction.
SEC regulations also require additional disclosure in connection with any trades involving a "penny stock," including the delivery, prior to any penny stock transaction, of a disclosure schedule explaining the penny stock market and its associated risks. These requirements severely limit the liquidity of securities in the secondary market because few broker or dealers are likely to undertake these compliance activities. In addition to the applicability of the penny stock rules, other risks associated with trading in penny stocks could also be price fluctuations and the lack of a liquid market.
The market price for our stock may be volatile and the volatility in our common share price may subject us to securities litigation.
The market price for our stock may be volatile and subject to wide fluctuations in response to factors including the following:
| · | actual or anticipated fluctuations in our quarterly operating results; |
| · | changes in financial estimates by securities research analysts; |
| · | conditions in pharmaceutical and agricultural markets; |
| · | changes in the economic performance or market valuations of other pharmaceutical companies; |
26
| · | announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | addition or departure of key personnel; |
| · | fluctuations of exchange rates between RMB and the U.S. dollar; |
| · | intellectual property litigation; and |
| · | general economic or political conditions in China. |
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
The market for our common stock is characterized by significant price volatility when compared to seasoned issuers, and we expect that our share price will continue to be more volatile than a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
Potential new accounting pronouncements may impact our future financial position and results of operations.
There have been regulatory changes, including the Sarbanes-Oxley Act of 2002, and there may potentially be new accounting pronouncements or additional regulatory rulings that will have an impact on our future financial position and results of operations. The Sarbanes-Oxley Act of 2002 and other rule changes are likely to increase general and administrative costs and expenses. In addition, there could be changes in certain accounting rules. These and other potential changes could materially increase the expenses we report under generally accepted accounting principles, and adversely affect our operating results.
We may need additional capital, and the sale of additional shares or other equity securities could result in additional dilution to our shareholders.
We believe that our current cash and cash equivalents and anticipated cash flows from operations will be sufficient to meet our anticipated cash needs for the near future. We may, however, require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in increased debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
If we fail to maintain an effective system of internal controls, we may not be able to accurately report our financial results or prevent fraud.
We are subject to reporting obligations under the U.S. securities laws. The SEC, as required by Section 404 of the Sarbanes-Oxley Act of 2002, adopted rules requiring every public company to include a management report on such company's internal controls over financial reporting in its annual report, which contains management's assessment of the effectiveness of our internal controls over financial reporting. In addition, for issuers that are not “smaller reporting companies”, an independent registered public accounting firm must attest to and report on management's assessment of the effectiveness of our internal controls over financial reporting. As of December 31, 2010, we were a smaller reporting company. However, our status may change in the future. In any case, our management may conclude that our internal controls over our financial reporting are not effective. Moreover, in the event that our status changes from that of a smaller reporting company and we are required to provide an auditor attestation report, even if our management concludes that our internal controls over financial reporting are effective, our independent registered public accounting firm may still decline to attest to our management's assessment or may issue a report that is qualified if it is not satisfied with our controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. Our reporting obligations as a public company will place a significant strain on our management, operational and financial resources and systems for the foreseeable future. Effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent fraud. As a result, our failure to achieve and maintain effective internal controls over financial reporting could result in the loss of investor confidence in the reliability of our financial statements, which in turn could harm our business and negatively impact the trading price of our stock. Furthermore, we anticipate that we will incur considerable costs and use significant management time and other resources in an effort to comply with Section 404 and other requirements of the Sarbanes-Oxley Act.
27
We incur increased costs as a result of being a public company.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and other new rules subsequently implemented by SEC have required changes in corporate governance practices of public companies. We expect these new rules and regulations to increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we incur additional costs associated with our public company reporting requirements. We are currently evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
Not applicable to a smaller reporting company.
ITEM 2. PROPERTIES.
Our principal executive offices are located in Liang Fang's headquarters.
Lotus East leases all of its office and retail locations. Lease terms are generally one to fifteen years, with renewal options. All of its leases provide for a fixed annual rent. Lotus East intends to continue to lease all of its retail locations. In March 2010, Lotus East began substantial construction on a 250,000-square-foot facility in Beijing after receiving permission from the Beijing Chaoyang District Planning Bureau. The new building will house Lotus East’s R&D center, manufacturing operations, storage facilities, sales and administrative offices and employee apartments. The building will become Lotus East’s new corporate headquarters following its completion. Management expects to move into the building by the end of fiscal 2011.
Lotus East has the following properties leased in Beijing, China:
Property Location (District of Beijing, China) | Lease Expiration Period | Purpose |
Fengtai District | December 31, 2011 | Liang Fang warehouse |
Fengtai District | September 18, 2011 | Liang Fang headquarter |
Fengtai District | May 17, 2019 | Retail - Xinzhong Taita Pharmacy |
Fengtai District | December 31, 2011 | Retail - Nangong Pharmacy |
Fengtai District | Month-to-Month (related party) | Retail - Chenzhuang Rd. Pharmacy |
Haidian District | December 31, 2012 | Retail - Wanshou Rd. Pharmacy |
Dongcheng District | May 31, 2012 | Retail - Qingnianhu Pharmacy |
Dongcheng District | December 31, 2011 | Retail - Hepingli Pharmacy |
Chaoyang District | December 31, 2011 | Retail - Capital Airport Pharmacy |
Chaoyang District | Month-to-Month (related party) | Retail - Fenglinlvzhou Pharmacy |
Fangshan District | December 31, 2015 | Retail - Yonganzhongshen Pharmacy |
Liujia Village | September 28, 2012 | Retail – Fengtai Pharmacy |
Chaoyang District | Property Owned by Lotus | En Ze Jia Shi production and manufacturing facility |
ITEM 3. LEGAL PROCEEDINGS.
We are not a party to any pending legal proceedings.
ITEM 4. REMOVED AND RESERVED.
28
PART II
ITEM 5. MARKET FOR COMMON EQUITY; RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES.
MARKET INFORMATION
Our common stock is quoted on the OTCBB under the symbol LTUS. The reported high and low sales prices for the common stock as reported on the OTCBB are shown below for the periods indicated. The quotations reflect inter-dealer prices, without retail mark-up, markdown or commission, and may not represent actual transactions.
| High | Low |
Fiscal 2009 |
|
|
First quarter ended March 31, 2009 | $0.76 | $0.26 |
Second quarter ended June 30, 2009 | $1.30 | $0.52 |
Third quarter ended September 30, 2009 | $1.96 | $0.80 |
Fourth quarter ended December 31, 2009 | $2.70 | $1.50 |
|
|
|
Fiscal 2010 |
|
|
First quarter ended March 31, 2010 | $3.90 | $2.68 |
Second quarter ended June 30, 2010 | $2.52 | $1.78 |
Third quarter ended September 30, 2010 | $2.20 | $1.80 |
Fourth quarter ended December 31, 2010 | $2.98 | $2.04 |
On the date immediately preceding the filing of this annual report, the last sale price for share of our common stock as reported on the OTCBB was $1.52.
STOCKHOLDERS
As of the report filing date, there were approximately 58 record owners of our common stock based upon the shareholders’ listing provided by our transfer agent. Our transfer agent is Signature Stock Transfer, Inc, 2632 Coachlight Court, Plano, Texas 75093, and its telephone number is (972) 612-4120.
DIVIDEND POLICY
We have never paid cash dividends on our common stock since we became public through a reverse merger. We intend to keep future earnings to finance the expansion of our business, and we do not anticipate that any cash dividends will be paid in the foreseeable future.
Our future payment of dividends will depend on our earnings, capital requirements, expansion plans, financial condition and relevant factors that our board of directors may deem relevant. Our retained earnings limit our ability to pay dividends.
UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
The following private placements of the Company’s securities were made in reliance upon the exemption from registration under Section 4(2) of the Securities Act of 1933, as amended, and/or, Rule 506 of Regulation D promulgated under the Securities Act. The Company did not use underwriters in any of the following private placements.
During the period from October 1, 2010 through December 31, 2010, 77,069 shares of preferred stock were converted into 38,535 shares of common stock.
On December 31, 2010, the Company issued 25,000 shares of its common stock to RedChip in connection with investor relations service rendered. The shares were valued at the fair value of $2.59 per share on the grant date. In connection with the issuance of these shares, the Company recorded professional fees of $64,750.
29
Equity Compensation Plan Information
The following table provides information as of December 31, 2010 with respect to the shares of common stock that may be issued under our existing equity compensation plans:
Plan Category |
| Number of securities to be issued upon exercise of outstanding options, warrants and rights |
| Weighted-average exercise price of outstanding options, warrants and rights |
| Number of securities remaining available for future issuance |
Equity compensation plan approved by security holders |
| — |
| — |
| — |
Equity compensation plan not yet approved by security holders (1) |
| — |
| — |
| 1,500,000 |
Total |
| — |
| — |
| 1,500,000 |
(1) Represents the Company's 2010 Stock Incentive Plan.
ITEM 6. SELECTED FINANCIAL DATA.
Not applicable to a smaller reporting company.
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
The following analysis of our consolidated financial condition and results of operations for the years ended December 31, 2010 and 2009, should be read in conjunction with our audited consolidated financial statements, including footnotes, and other information presented elsewhere in this annual report on Form 10-K. This discussion contains forward-looking statements that involve significant risks and uncertainties. As a result of many factors, such as those set forth under “Forward Looking Statements” and “Item 1A. Risk Factors” and elsewhere in this Form 10-K, our actual results may differ materially from those anticipated in these forward-looking statements. When used in this section, "fiscal 2010" means our fiscal year ended December 31, 2010 and "fiscal 2009" means our fiscal year ended December 31, 2009. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements.
OVERVIEW
We develop, manufacture, and sell pharmaceuticals in the PRC. We produce medicine and drugs in the forms of tablets, capsules, granules, eye-drops, and freeze-dried powder injection. We have established markets for several drugs that are self-branded or self-patented, including (i) Maixin - valsartan capsules for the treatment of hypertension, (ii) Muxin – eye drops for the treatment of glaucoma and (iii) Yipubishan - octreotide Acetate Injection solution for the treatment of gastric ulcers. Our drug development is focused on the treatment of cerebro-cardiovascular disease, asthma, and diabetes. We have a nationwide sales network to directly and indirectly sell to hospitals, clinics and drug stores in approximately 30 provinces in China. In fiscal 2010, we added five new prescription drugs to our distributed products delivered through our national wholesale channels. Additionally, through our 10 retail pharmacy locations and direct sales to other drug stores in Beijing, China, we sell western and traditional Chinese medications and medical treatment equipment, and generate ancillary revenues from the leasing of retail space to third party vendors at our retail stores. At the end of fiscal 2009, we engaged a general manager for our Over-the-Counter Drug Division that manages our own ten drug stores’ sales, and the direct sales to other Over-the-Counter drug stores in Beijing. By the end of fiscal 2010, we served more than 1,000 other Over-the-Counter drug stores in Beijing. We expect our retail revenue from our own ten drug stores will remain in its current level with small growth and our retail revenue from our direct sales to other drug stores in Beijing will continue to increase in the future.
To be in compliance with China’s regulations on foreign ownership in the pharmaceutical industry and to consolidate the financial information of our operating entities, Lotus East, we operate our business in China through the Contractual Arrangements with Lotus East. The contractual relationship among the above companies as follows:
30

The term of Contractual Arrangements was approved and extended by the board of directors in April 2010 from 10 years to 30 years, i.e. 2006-2036.
When used in this section, and except as may be set forth otherwise, the terms "we," "us," "ours," and similar terms includes Lotus Pharmaceuticals Inc. and its subsidiaries, Lotus International and Lotus Century, as well as Lotus East.
BASIS OF PRESENTATION
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). These accounting principles require us to make certain estimates, judgments and assumptions. We believe that the estimates, judgments and assumptions upon which we rely are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities as of the date of the financial statements as well as the reported amounts of revenues and expenses during the periods presented. Our financial statements would be affected to the extent there are material differences between these estimates and actual results. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP and does not require management’s judgment in its application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. Significant estimates in 2010 and 2009 include the allowance for doubtful accounts, the allowance for obsolete inventory, the useful life of property and equipment and intangible assets, fair value of warrants granted and beneficial conversion features related to the issuance of convertible preferred stock. We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of the consolidated financial statements.
VARIABLE INTEREST ENTITIES
Pursuant to ASC 810 “Consolidation” (“ASC 810”), we are required to include in our consolidated financial statements the financial statements of variable interest entity (“VIE”). ASC 810 requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns. VIE is the entity in which we, through contractual arrangements, bear the risk of, and enjoy the rewards normally associated with ownership of the entity, and therefore we are the primary beneficiary of the entity.
31
Lotus East is considered VIE and we are the primary beneficiary. In September 2006, we entered into agreements with Lotus East pursuant to which we shall receive 100% of Lotus East’s net income. In accordance with these agreements, Lotus East shall pay consulting fees equal to 100% of its net income to us and we shall supply business consulting and other general business operation services needed to Lotus East.
The accounts of Lotus East are consolidated in the accompanying consolidated financial statements. As a VIE, Lotus East’s sales are included in our total sales, Lotus East’s income from operations is consolidated with ours, and our net income includes all of Lotus East’s net income, and Lotus East’s assets and liabilities are included in our consolidated balance sheet. The VIE is entirely controlled by us and accordingly, none of the VIE’s net income is subtracted in calculating the net income attributable to us. Because of the contractual arrangements, we have pecuniary interest in Lotus East that requires consolidation of Lotus East financial statements with our consolidated financial statements.
REVENUE RECOGNITION
Product sales are generally recognized when title to the product has transferred to customers in accordance with the terms of the sale. We recognize revenue in accordance with the SEC Staff Accounting Bulletin (SAB) No. 101, “Revenue Recognition in Financial Statements” as amended by SAB No. 104 (together, “SAB 104”), and ASC 605 “Revenue Recognition When Right of Return Exists.” SAB 104 states that revenue should not be recognized until it is realized or realizable and earned. In general, we record revenue when persuasive evidence of an arrangement exists, product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
ASC 605 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
ACCOUNTS RECEIVABLE
Accounts receivable are carried at original invoice amount less an estimate made for doubtful receivables based on a review of all outstanding amounts on a monthly basis. Management judgment and estimates are made in connection with establishing the allowance for doubtful accounts. Specifically, we analyze the aging of accounts receivable balances, historical bad debts, customer concentrations, customer credit-worthiness, current economic trends and changes in our customer payment terms. Significant changes in customer concentration or payment terms, deterioration of customer credit-worthiness or weakening in economic trends could have a significant impact on the collectability of receivables and our operating results. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
INVENTORIES
Inventories are stated at the lower of cost or market with cost determined under the weighted average costing method. Inventory consists of raw material, packaging material, work-in-process, finished capsules, liquids, finished oral suspension powder and other western and traditional Chinese medicines and medical equipments. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or slow-moving or quantities in excess of expected demand, we will record additional reserves for the difference between the cost and the market value. These reserves are recorded based on estimates. At least on a quarterly basis, we review and evaluate our inventory levels relative to product demand, remaining shelf life, future marketing plans and other factors. If the results of the review and evaluation determine that a write-down is necessary, we recognize a loss in the period in which the loss is identified, whether or not the inventory is retained. Our inventory reserves establish a new cost basis for inventory and are not reversed until we sell or dispose of the related inventory.
PROPERTY AND EQUIPMENT
Property and equipment are stated at cost less accumulated depreciation. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the period of disposition.
32
The construction-in-progress which consists of factories and office buildings under construction in China was included in property and equipment. No provision for depreciation is made on construction-in-progress until such time as the relevant assets are completed and ready for their intended use.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets. The useful lives for property and equipment are estimated as follows:
Building and building improvements |
|
| 20 to 40 years |
|
Manufacturing equipment |
|
| 10 to 15 years |
|
Office equipment and furniture |
|
| 5 to 8 years |
|
LAND USE RIGHT HELD FOR DEVELOPMENT
We purchased 1,000 MU (approximately 667,000 square meters) of land in Inner Mongolia in 2008. The area for Inner Mongolia land which was held for development is 900 MU (approximately 600,000 square meters). The area for the rest of Inner Mongolia land on which we expect to build a facility is 100 MU (approximately 67,000 square meters).
Land use right held for development is accounted for at the lower of cost or market. It is considered as long-term asset and free of amortization as it is not used in operations. Management would like to co-develop the land with another entity, but such partner has not been found yet.
LAND USE RIGHT AND OTHER INTANGIBLE ASSETS
Other intangible assets consist of revenue right, intellectual right and software. Revenue right is amortized over 20 years, which is the term we would benefit from it. Intellectual right is being amortized over 10 years as based on the transfer agreement. Software is amortized over 3 years, its estimated useful life. Land use rights are carried at cost and charged to expense on a straight-line basis over the period the rights are granted, 40 - 50 years.
IMPAIRMENT OF LONG-LIVED ASSETS
In accordance with ASC 360, “Accounting for the Impairment or Disposal of Long-Lived Assets”, we examine the possibility of decreases in the value of property and equipment when events or changes in circumstances reflect the fact that their recorded value may not be recoverable. We recognize an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value.
RESEARCH AND DEVELOPMENT
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and depreciation for facilities related to research and development activities and salaries paid for the development of our products and fees paid to third parties.
INCOME TAXES
The Company applies the accounting standard regarding accounting for income taxes and the accounting standard regarding accounting for uncertainty in income taxes for income taxes. This accounting standard requires recognition of deferred income tax liabilities and assets for the expected future tax consequences of temporary differences between the income tax basis and financial reporting basis of assets and liabilities. Provision for income taxes consist of taxes currently due plus deferred taxes. The charge for taxation is based on the results for the year as adjusted for items that are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date. Deferred taxes are accounted for using the balance sheet liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences, and deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which deductible temporary differences can be utilized.
33
Under the accounting standard regarding, accounting for uncertainty in income taxes, a tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the year incurred. GAAP also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure and transition.
STOCK-BASED COMPENSATION
The Company accounts for stock options and other equity based compensation issued to employees in accordance with ASC 718. ASC 718 requires companies to recognize in the statement of income the grant-date fair value of stock options and other equity based compensation issued to employees. The Company accounts for non-employee share-based awards in accordance with ASC 505-50.
FOREIGN CURRENCY TRANSLATION
Our financial statements are expressed in U.S. dollars and the functional currency of our parent company is U.S. dollars, but the functional currency of our operating subsidiaries and affiliates is Chinese Renminbi (“RMB”). For the subsidiaries and affiliates whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred. All of our revenue transactions are transacted in the functional currency. We do not enter any material transaction in foreign currencies and accordingly, transaction gains or losses have not had, and are not expected to have, a material effect on results of operations.
RECENT ACCOUNTING PRONOUNCEMENTS
In December, 2009, FASB issued ASU No. 2009-17, Improvement to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standard Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No.167, Amendments to FASB Interpretation No. 46 (R). The amendments in this Accounting Standards Update replace the quantitative-based risks and rewards calculation for determining which reporting entity, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which reporting entity has the power to direct the activities of a variable interest entity that most significantly impacts the entity's economic performance and (1) the obligation to absorb losses of the entity or (2) the right to receive benefits from the entity. An approach that is expected to be primarily qualitative will be more effective for identifying which reporting entity has a controlling financial interest in a variable interest entity. The amendments in this Update also require additional disclosures about a reporting entity's involvement in variable interest entities, which will enhance the information provided to users of financial statements. The adoption of this ASU did not have a material impact on our consolidated financial statements.
In April 2010, the FASB issued Accounting Standards Update 2010-13, “Compensation—Stock Compensation (Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades,” or ASU 2010-13. This Update provides amendments to Topic 718 to clarify that an employee share-based payment award with an exercise price denominated in currency of a market in which a substantial porting of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. We do not expect the adoption of ASU 2010-17 to have a significant impact on our consolidated financial statements.
34
RESULTS OF OPERATIONS
Comparison of Year Ended December 31, 2010 (“fiscal 2010”) and Year Ended December 31, 2009 (“fiscal 2009”)
Net Revenues
Total net revenues for the year ended December 31, 2010 were $72,696,618 as compared to total net revenues of $56,482,448 for the year ended December 31, 2009, an increase of $16,214,170 or 28.7%. For the years ended December 31, 2010 and 2009, total net revenues consisted of the following:
|
| 2010 |
| 2009 |
| ||
Wholesale |
| $ | 51,412,468 |
| $ | 44,842,525 |
|
Retail |
|
| 21,284,150 |
|
| 11,639,923 |
|
Total net revenues |
| $ | 72,696,618 |
| $ | 56,482,448 |
|
| · | For the year ended December 31, 2010, wholesale revenues increased $6,569,943 or 14.7%, compared to wholesale revenues for the year ended December 31, 2009. The increase was primarily attributable to the increased sales of approximately $8,681,000 from the five new drugs which were added to our wholesale distribution channel in fiscal 2010 offset by the decreased sales of approximately $2,111,000 from our other fifteen drugs. The decrease of wholesale revenue from other fifteen drugs was primarily attributable to being out of stock of Levofloxacin Lactate for injection (brand name “Jun Xin”) and Nicergoline for injection (brand name “Ni Mai Jiao Lin”). The two prescription drugs are covered by the National Health Insurance Program and their sales prices are controlled by the Chinese government. In fiscal 2010, our manufacturing facility was removed from service in order to construct our new building in Beijing. If we were to use third party manufacturers to produce Jun Xin and Ni Mai Jiao Lin, our gross profit margins from the sales of these two drugs would be very low. Therefore, we did not use any third party manufacturers to produce these two drugs. In fiscal 2010, we added five new prescription drugs to our distributed products delivered through our national wholesale channels. The five new prescription drugs covered by the National Health Insurance Program have proven their market acceptance. One of the five new prescription drugs is Omeprazole Enteric-coated Capsule to treat duodenal ulcer. The other four drugs are traditional Chinese medicine in capsules, tablets and ointment for the treatment of chronic prostate infection, psoriasis, influenza and meridian pain, respectively. As a result, our wholesale revenues for the year ended December 31, 2010 increased. We anticipate that our wholesale revenues will decrease in fiscal 2011since: (i) our new Beijing building is in construction and we cannot manufacture our products in our factory before our new Beijing building may be put into service, (ii) we will lose revenue from Muxin (an eye drop) which is one of our self-branded products due to the termination of our out-sourcing manufacture agreement, and (iii) we have limited warehouse space to store our drugs before our new Beijing building may be put into service. We expect these problems can be solved in the fourth quarter of fiscal 2011. Therefore, we expect our wholesale revenues will increase in fiscal 2012. |
|
|
|
| · | For the year ended December 31, 2010, retail revenues increased by $9,644,227 or 82.9%, compared to retail revenues for the year ended December 31, 2009. At the end of fiscal 2009, we engaged a general manager for our Over-the-Counter Drug Division that manages our own ten drug stores’ sales, and the newly created direct sales to other Over-the-Counter drug stores in Beijing. The general manager has strong management skills in medical sales and marketing and logistics and is an expert in delivery of services to drug stores in Beijing. In fiscal 2010, we served more than 1,000 other Over-the-Counter drug stores in Beijing. Due to the growth and success of our OTC Drug Division’s sales force, our retail revenues for the fiscal year of 2010 substantially increased. We expect our retail revenue from our own ten drug stores will remain in its current level with small growth and our retail revenue from our direct sales to other drug stores in Beijing will continue to increase in the future. |
Cost of Revenues
Overall, cost of revenues for the year ended December 31, 2010 increased $7,864,479 or 31.4% as compared to the total cost of revenues for the year ended December 31, 2009. Our total cost of revenues as a percentage of total net revenues for the year ended December 31, 2010 increased to 45.3% from 44.4% for the year ended December 31, 2009. Cost of revenues as a percentage of net revenues from our wholesale operations decreased from 37.2% for the year ended December 31, 2009 to 32.7% for the year ended December 31, 2010. In the 2010 period, 13% of cost of revenues attributable to our wholesale operations was attributable to the cost of the five new drugs which were added to our wholesale distribution channel in fiscal 2010 and 87% of cost of revenues attributable to our wholesale operations was attributable to the cost of our other fifteen drugs. In the 2009 period, 100% of cost of revenues attributable to our wholesale operations was attributable to the cost of our other fifteen drugs. The different revenue mix in the 2010 period had an effect of lowering cost of revenues as a percentage of revenues as compared to the 2009 period. Cost of revenues as a percentage of net revenues from our retail operations for the year ended December 31, 2010 increased to 75.7% from 71.7% for the
35
year ended December 31, 2009. In fiscal 2010, one of our warehouses was removed from service in order to construct the new building in Beijing. Therefore, our remaining warehouse space could not meet our demand in 2010. We addressed this issue by shortening the storage period for our inventory and by reducing our unit sales price. As a result, the cost of revenues as a percentage of net revenues from our retail operations increased. We expect our total cost of revenues as a percentage of total net revenues will remain in its current level in the near future.
Gross Profit
Gross profit for the year ended December 31, 2010 was $39,780,419 or 54.7% of total net revenues, as compared to $31,430,728 or 55.6% of total net revenues for the year ended December 31, 2009. We expect that our gross profit margin will maintain in its current level with minimal growth in the near future.
Operating Expenses
Total operating expenses for the year ended December 31, 2010 were $25,257,935, as compared to total operating expenses of $13,453,914 for the year ended December 31, 2009, an increase of $11,804,021 or 87.7%. This increase included the following:
For the year ended December 31, 2010, selling expenses amounted to $10,392,378 as compared to $8,040,161 for the year ended December 31, 2009, an increase of $2,352,217 or 29.3%. For the year ended December 31, 2010, selling expenses as a percentage of total net revenues was 14.3% while for the year ended December 31, 2009, selling expenses as a percentage of total net revenues was 14.2%. This increase of selling expenses is primarily attributable to the increase of our sales revenues. We expect our selling expenses will increase in the near future since we anticipate that our sales revenues will increase in the fiscal year of 2011.
For the year ended December 31, 2010, research and development expenses amounted to $86,545 as compared to $0 for the year ended December 31, 2009, an increase of $86,545 or 100%. The increase was attributable to a research and development agreement with a third party for the clinical trial of the Laevo-Bambuterol drug entered into in August 2010, which agreement has a term of six months. We expect our research and development expense to continue to increase in the near future since the Laevo-Bambuterol drug is in clinical trials and Gliclazide-Controlled Release Tablets and Isosorbide Mononitrate Tablets are waiting for approval to initiate clinical trials.
For the year ended December 31, 2010, a one-time loss on impairment of property and equipment amounted to $6,762,659 as compared to $1,719,884 for the year ended December 31, 2009, an increase of $5,042,775 or 293.2%. We purchased 1,000 MU (approximately 667,000 square meters) of land in Inner Mongolia in 2008. We had originally intended to build a pharmaceutical manufacturing and storage facility on a portion of the property. The construction began in August 2008 and stopped because priority of capital expenditure was given to the need to build the new building complex in Beijing as the relevant land was upgraded to industrial use in 2009. In March 2010, we began construction on a facility in Beijing after receiving permission from the Beijing Chaoyang District Planning Bureau. The new building will house our Research and Development center, manufacturing operations, storage facilities, sales and administrative offices and employee apartments. The building will become our new corporate headquarters following its completion and management expects to move into the building by the end of fiscal 2011. Our management currently believes it is probable that we will not move forward with the construction of our planned facility in Inner Mongolia in order to focus our efforts and resources on expanding our core business in Beijing. Therefore, we recorded an impairment loss on the entire construction-in-progress which is located in Inner Mongolia of $6,762,659 in fiscal 2010. We recorded an impairment loss on property and equipment of $1,719,884 to recognize the portion of a Beijing En Ze Jia Shi building that was demolished in order to construct our new building in fiscal 2009.
For the year ended December 31, 2010, general and administrative expenses were $8,016,353, as compared to general and administrative expenses of $3,693,869 for the year ended December 31, 2009, an increase of $4,322,484 or 117.0%. These changes are summarized below:
|
| 2010 |
| 2009 |
Salaries and related benefits | $ | 1,585,943 | $ | 724,125 |
Amortization and depreciation expenses |
| 2,247,768 |
| 1,586,630 |
Rent |
| 308,164 |
| 301,994 |
Travel and entertainment |
| 188,274 |
| 261,115 |
Professional fees |
| 3,441,429 |
| 423,804 |
Other |
| 244,775 |
| 396,201 |
Total | $ | 8,016,353 | $ | 3,693,869 |
36
The changes in these expenses from the year ended December 31, 2010 as compared to the year ended December 31, 2009 included the following:
| · | Salaries and related benefits increased $861,818 or 119.0%, which was mainly attributable to an increase in bonus for employees of approximately $885,000 in order to motivate our staffs and to ensure a stable and loyal work team for our long term benefit and success offset by a decrease in salaries paid to our employees of approximately $18,000 and a decrease in benefits for employees of approximately $5,000. We anticipate that our salaries and related benefits will remain at its current level with minimal increase in the near future. |
|
|
|
| · | Amortization of our intangible assets and depreciation on our property and equipment increased by $661,138 or 41.7%, which was primarily attributable to an increase in amortization from land use rights of approximately $207,000 and an increase in depreciation on fixed assets of approximately $454,000. During the year ended December 31, 2010, we did not generate any production activity and we charged all of depreciation from our manufacturing machinery and equipment to general and administrative expenses. While, for the year ended December 31, 2009, we performed some production and we charged all of depreciation from our manufacturing facilities to finished goods and cost of revenues, if it was sold. Therefore, for the fiscal year 2010, general and administrative expenses from depreciation on property and equipment increased by approximately $454,000 even though total depreciation only increased by approximately $54,000 as compared to fiscal year 2009. |
|
|
|
| · | Travel and entertainment decreased $72,841 or 27.9% which was mainly attributable to less travel and entertainment activities in the year ended December 31, 2010 as compared to the travel and entertainment activities in the year ended December 31, 2009. |
|
|
|
| · | Professional fees increased $3,017,625 or 712.0%, which was primarily attributable to an increase in fees related to our consultants’ service of approximately $2,301,000 for corporate affairs and development, an increase in fees paid for our investor relations service of approximately $119,000, an increase in fees paid for our attorney service of approximately $298,000, and an increase in other miscellaneous items of approximately $300,000. |
|
|
|
| · | Other general and administrative expenses, which included office supplies, general management fees, car insurance, postage and other office expenses, decreased by $151,426 or 38.2% reflecting efforts at reducing non-sales related corporate activities as well as stricter controls on corporate spending. |
Income from Operations
As a result of forgoing, we reported income from operations of $14,522,484 for the year ended December 31, 2010 as compared to income from operations of $17,976,814 for the year ended December 31, 2009, a decrease of $3,454,330 or 19.2%.
Other Income (Expense)
For the year ended December 31, 2010, we had a total net other income of $122,751, while we had a total net other expense of $1,175,840 for the year ended December 31, 2009. The change in total net other income (expense) was primarily attributable to:
| · | For the year ended December 31, 2010, our debt issuance costs amounted to $52,226 as compared to $412,184 for the year ended December 31, 2009, a decrease of $359,958 or 87.3%. The decrease was attributable to the decrease in amortization amount on debt issuance costs associated with the issuance of February 2008 Series A Convertible Preferred Stock for which we amortized on a 24-month period and began our amortization on the February 2008 debt issuance costs in March 2008. Debt issuance costs were fully amortized in February 2010. We recorded two-month period amortization amount in fiscal 2010 while we recorded twelve-month period amortization amount in fiscal 2009. |
|
|
|
| · | For the year ended December 31, 2010, other income was $784,461 as compared to $1,342,197 for the year ended December 31, 2009, a decrease of $557,736 or 41.6%. The decrease in other income was mainly attributable to: (i) a decrease in leasing income of approximately $9,000. We sublease certain portions of our retail stores and counter spaces to various other vendors which generate leasing revenues. The slight decrease in leasing income was primarily attributable to the less spaces were rented out. (ii) a decrease in income from third-party manufacturing activities of approximately $514,000. For third-party manufacturing, customers supply the raw materials and we are paid a fee for manufacturing their products. For the fiscal year of 2010, we did not generate any income from third-party manufacturing since we did not occur any third-party manufacturing activities and our prior customers built their own facilities and can manufacture their products at lower costs in their own factories. We do not anticipate that we will generate income from third-party manufacturing in the near future since we do not expect to find any customer with third-party manufacturing contracts. (iii) a decrease in income from research and development and lab testing services of approximately $35,000. We performed research and development and |
37
|
| lab testing projects for various third parties and performed drug testing and analysis for various third parties in fiscal 2009, while we did not perform any research and development and lab testing project for any third parties in fiscal 2010. Our historical customers for these services purchased related machinery and they can do similar research and development and lab testing in their own labs at a lower price and decided not to use our services in fiscal 2010. We do not expect that we will generate any income from research and development and lab testing in the near future since we do not anticipate finding any new customer with research and development and lab testing project. |
|
|
|
| · | For the year ended December 31, 2010, interest expense was $612,626 as compared to $2,154,373 for the year ended December 31, 2009, a decrease of $1,541,747 or 71.6%. The decrease in interest expense was primarily attributable to a decrease in amortization of debt discount associated with the February 2008 Series A Convertible Preferred Stock of approximately $1,044,000, a decrease in accrued dividend for the February 2008 Series A Convertible Preferred Stock of approximately $347,000, a decrease in interest of approximately $338,000 in connection with the transfer of a portion of escrow shares to the purchasers of February 2008 Series A Convertible Preferred Stock offset by an increase in interest of approximately $185,000 from the beneficial conversion feature of the second year interest shares and an increase in interest of approximately $2,000 in connection with the related parties notes payable. |
Income Taxes
For the year ended December 31, 2010, our income tax expense was $220,292, as compared to $368,680 for the year ended December 31, 2009, a decrease of $148,388 or 40.2%. The decrease in income tax expense was mainly attributable to the decrease in taxable income generated by our operating entities.
Net Income
As a result of these factors, we reported net income of $14,424,943 for the year ended December 31, 2010 as compared to net income of $16,432,294 for the year ended December 31, 2009. This translated to basic earnings per common share of $0.55 and $0.74, and diluted earnings per common share of $0.54 and $0.66, for the years ended December 31, 2010 and 2009, respectively.
Other Comprehensive Income
The functional currency of our operating subsidiaries and affiliates is the Chinese Renminbi (“RMB”). The financial statements of our operating subsidiaries and affiliates are translated to U.S. dollars using period end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. As a result of these translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $2,789,989 for the year ended December 31, 2010 as compared to $131,989 for the year ended December 31, 2009. This non-cash gain had the effect of increasing our reported comprehensive income.
Comprehensive Income
As a result of our foreign currency translation gains, we had comprehensive income for the year ended December 31, 2010 of $17,214,932, compared with $16,564,283 for the year ended December 31, 2009.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis.
At December 31, 2010 and 2009, we had a cash balance of $1,339,972 and $3,945,740, respectively. These funds are distributed in financial institutions located in China.
Our working capital increased $1,443,520 to working capital deficit of $3,509,214 at December 31, 2010 from working capital deficit of $4,952,734 at December 31, 2009. This increase in working capital is primarily attributed to an increase in accounts receivable of approximately $0.19 million, a decrease in taxes payable of approximately $1.11 million due to the payments made to tax authority in fiscal 2010, a decrease in unearned revenue of approximately $0.66 million due to the recognition of unearned revenue in fiscal 2010, a decrease in Series A convertible redeemable preferred stock of approximately $4.17 million due to the conversion of convertible redeemable preferred stock into common shares and reclassification of convertible redeemable preferred stock into permanent equity as discussed in elsewhere in this report; offset by a decrease in cash of approximately $2.61 million, a decrease in inventories of
38
approximately $0.41 million due to a storage room being removed from service in fiscal 2010, a decrease in prepaid expenses (current portion) of approximately $0.28 million, a decrease in deferred debt costs of approximately $0.05 million, an increase in accounts payable of approximately $0.04 million, an increase in other payables and accrued liabilities of approximately $0.75 million which was mainly attributable of the increase in refundable deposit of approximately $5,000, the increase in accrued and unpaid sales commission of approximately $196,000, the increase in accrued and unpaid payroll and employees benefit of approximately $776,000 and the increase in other items of approximately $252,000 offset by the decrease in construction payable of approximately $195,000 and the decrease in accrued interest for convertible redeemable preferred stock of approximately $285,000; and an increase in due to related parties (current portion) of approximately $0.55 million due to the increase in current portion of assignment fee payable of approximately $272,000 and the increase in payments made by Dr. Liu, our CEO, on behalf of the Company of approximately $229,000 and the increase from other items of approximately $51,000.
The changes in asset and liabilities discussed above is based on a comparison of amounts on our balance sheets as of December 31, 2010 and 2009 and does not necessarily reflect changes in assets and liabilities reflected on our cash flow statement, for which we use the average foreign exchange rate during the period to calculate these changes.
Our balance sheet as of December 31, 2010 also reflects notes payable to related parties of $5,241,829 due on December 30, 2015 which was a series of working capital loans made to us since December 31, 2005 by the Company’s Chief Executive Officer, his wife, Chief Executive Officer’s father-in-law and two employees of the Company. These loans bear interest based on a floating annual interest rate, which is 80% of China bank interest rate and are unsecured. During the year ended December 31, 2010, we did not repay any portion of the principal of these loan balances.
Net cash provided by operating activities for the year ended December 31, 2010 was $26,775,776 as compared to net cash provided by operating activities of $31,362,466 for the year ended December 31, 2009. For the year ended December 31, 2010, net cash provided by operating activities was primarily attributable to net income of $14,424,943 and the add back of non-cash charges such as depreciation of $480,046, amortization of intangible assets of $1,767,722, a one-time loss on impairment of $6,762,659 on property and equipment as discussed in elsewhere of the report, amortization of deferred debt issuance costs of $52,226, amortization of discount on convertible redeemable preferred stock of $151,553, warrants issued for service of $170,041, interest expense attributable to beneficial conversion feature of preferred shares of $184,660, stock issued for service of $765,749 and stock issued for compensation of $246,000. In addition, the receipt of cash from changes in operating assets and liabilities such as: a decrease in inventories of $429,879, a decrease in prepaid expenses of $1,654,229, an increase in accounts payable of $36,898, in increase in other payables and accrued liabilities of $1,173,055, and an increase in due to related parties of $467,088, offset by the use of cash from changes in operating assets and liabilities such as: an increase in accounts receivable of $124,976, a decrease in taxes payable of $1,184,209, and a decrease in unearned revenue of $681,787.
For the year ended December 31, 2009, net cash provided by operating activities was primarily attributable to net income of $16,432,294, and the add back of depreciation of $425,895, amortization of intangible assets of $1,560,466, a one-time loss form fixed assets impairment of $1,719,884, amortization of deferred debt issuance costs of $412,184, amortization of discount on convertible redeemable preferred stock of $1,196,106, amortization of prepaid expense attributable to warrants of $14,849, stock based compensation of $282,083 and interest expenses caused by escrow shares transfer of $337,500. In addition, the receipt of cash from operations from assets and liabilities such as: a decrease in accounts receivable of $4,361,619, a decrease in other receivable – related party of $2,031,902, a decrease in inventories of $2,755,869, an increase in other payables and accrued liabilities of $1,054,423, an increase in unearned revenue of $596,414 and an increase in due to related parties of $237,452 offset by the use of cash from changes in operating assets and liabilities such as: a decrease in accounts payable of $172,330 and a decrease in taxes payable of $1,895,451.
Net cash used in investing activities for the year ended December 31, 2010 amounted to $29,448,568 which was attributable to the purchase of property and equipment. For the year ended December 31, 2009, net cash used in investing activities was attributable to installments on intangible assets of $17,581,071, and the purchase of property and equipment of $11,118,884.
Net cash provided by financing activities for the years ended December 31, 2010 and 2009 was $0.
We reported a net decrease in cash for the year ended December 31, 2010 of $2,605,768 as compared to a net increase in cash of $2,666,932 for the year ended December 31, 2009.
We estimate that our working capital is not sufficient to fund our current operations for the next 12 months. Lotus East has historically funded its capital expenditures from its working capital. As of December 31, 2010, Lotus East has contractual commitments of approximately $52 million related to a Technology Transfer Agreement and the construction of the new facility in Inner Mongolia and a New Drug Patent Transfer Agreement and a research and development agreement. While it intends to fund the costs with its existing working capital associated with the Technology Transfer Agreement and the New Drug Patent Transfer Agreement and the research
39
and development agreement and a portion of the construction of the new facility in Inner Mongolia, it is dependent upon the continued growth of its operations and prompt payment of outstanding accounts receivables by its customers to ensure that it has sufficient cash for these commitments. Our ability to fully fund the costs associated with the new facility in Inner Mongolia is materially dependent upon our ability to obtain secured bank financing and/or government grants and/or other third party finance.
There is no guarantee that Lotus East can obtain these financings on favorable terms at the right time. Although the Chinese government has announced an economic stimulation plan, there is no guarantee that we will be awarded the government grants successfully. While Lotus East’s management believes the Company will be successful in securing the necessary funding through its increasing revenue, faster collections on receivables, and continuing discussions with various commercial banks, there are no assurances that the funding will be available in the amounts or at the time required to meet Lotus East’s commitments. In the event that Lotus East is not successful in obtaining the funds it needs for the Technology Transfer Agreement and the New Drug Patent Transfer Agreement, it is possible that it could default under the terms of the two agreements and forfeit any funds paid to date. If Lotus East fails to obtain all of the funding necessary to complete the construction of the new facility in Inner Mongolia, which is estimated to be approximately $48.3 million in the next few years, it could get back approximately $34 million for the payments on the land use right, which is refundable if the Chinese local government does not grant it land use right certificate.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, changing interest rates, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows. The total of contractual obligations and commitments does not include any payments made by us.
The following tables summarize our contractual obligations as of December 31, 2010, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
|
| Payments due by period | |||||||||||||
|
| Total |
| Less than |
| 1-3 |
| 3-5 |
| 5+ | |||||
Related parties indebtedness |
| $ | 7,055,535 |
| $ | 1,813,706 |
| $ | — |
| $ | 5,241,829 |
| $ | — |
Interest payment on notes payable – related parties |
| $ | 869,067 |
| $ | — |
| $ | — |
| $ | 869,067 |
| $ | — |
Technology purchase obligations |
| $ | 1,663,692 |
| $ | 831,846 |
| $ | 831,846 |
| $ | — |
| $ | — |
New drug patent purchase obligations |
| $ | 453,734 |
| $ | 453,734 |
| $ | — |
| $ | — |
| $ | — |
Construction obligations in Inner Mongolia |
| $ | 48,398,318 |
| $ | — |
| $ | 18,149,369 |
| $ | 30,248,949 |
| $ | — |
Obligations from research and development agreement |
| $ | 1,480,989 |
| $ | 1,480,989 |
| $ | — |
| $ | — |
| $ | — |
Total contractual obligations |
| $ | 59,921,335 |
| $ | 4,580,275 |
| $ | 18,981,215 |
| $ | 36,359,845 |
| $ | — |
Off-balance Sheet Arrangements
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not applicable to smaller reporting companies.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
Please see our financial statements beginning on page F-1 of this annual report.
40
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A(T). CONTROLS AND PROCEDURES.
Our internal control over financial reporting is a process designed by or under the supervision of our principal executive and principal financial officers to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and dispositions of assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and the directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on our consolidated financial statements.
Our management does not expect that our disclosure controls or our internal controls over financial reporting will prevent all errors and frauds. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Such limitations include the fact that human judgment in decision-making can be faulty and that breakdowns in internal control can occur because of human failures, such as simple errors or mistakes or intentional circumvention of the established process.
Disclosure Controls and Procedures
Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating and implementing possible controls and procedures.
As required by Rule 13a-15 under the Exchange Act, our management has carried out an evaluation, with the participation and under the supervision of our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of December 31, 2010. As discussed in more detail below, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of December 31, 2010, due to the material weaknesses that we identified in internal control over financial reporting.
Report of Management on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. Our management is also required to assess and report on the effectiveness of our internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 ("Section 404"). Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. In making this assessment, we used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control - Integrated Framework. During our assessment of the effectiveness of internal control over financial reporting as of December 31, 2010, management identified material weaknesses and determined that our internal controls over financial reporting were not effective as of that date. The material weaknesses related to the following:
1. Accounting and Finance Personnel Weaknesses - The current staff in our accounting department does not have extensive experience in U.S. GAAP, and needs substantial training so as to meet with the higher demands of being a U.S. public company. The accounting skills and understanding necessary to fulfill the requirements of U.S. GAAP-based reporting, including the skills of subsidiary financial statements consolidation, are inadequate and were inadequately supervised. The lack of sufficient and adequately trained accounting and finance personnel, and segregation of duties resulted in ineffective financial reporting functions.
41
2. Lack of internal audit function– The Company lacks qualified personnel to perform the internal audit functions. We may not be able to detect errors in a timely manner in certain key areas like revenue recognition, inter-company transactions, cash receipt and cash disbursement authorizations, inventory safeguard and proper costing of our products. In addition, the scope of the internal audit function is not yet developed.
During the year ended December 31, 2010, our internal accounting staff was primarily engaged in ensuring compliance with PRC accounting and reporting requirements for our operating subsidiaries and was not required to meet or apply U.S. GAAP requirements. Our current accounting department responsible for financial reporting for the Company, on a consolidated basis, is relatively new to U.S. GAAP and the related internal control procedures required for U.S. public companies. Although our accounting staff is professional and experienced in accounting requirements and procedures generally accepted in the PRC, management has determined that they will require additional training and assistance in U.S. GAAP matters. Management has determined that our internal audit function is also significantly deficient due to insufficient qualified resources to perform internal audit functions.
3. Insufficient segregation of duties in the financial reporting process– We lack accounting staff with adequate US GAAP expertise, the Company was unable to meet the segregation of duties requirements in the US GAAP reporting process to ensure proper review and approval functions.
Remediation Measures of Material Weakness
We have implemented, or plan to implement, the measures described below under the supervision and guidance of our management to remediate the above material weaknesses and to strengthen our internal controls over financial reporting. Key elements of the remediation effort include, but are not limited to, the following activities, which have been implemented, or are in the process of implementation, as of the date of filing of this Annual Report:
| · | We plan to engage a consultant or consulting firm in 2011, to review, evaluate and identify inadequacies of our existing internal control procedures, and to make recommendation and implement changes as necessary for the improvement of our internal controls. |
|
|
|
| · | We will continue to bring in additional qualified financial personnel for the accounting department to further strengthen our financial reporting function. We have started training our internal accounting staff in US GAAP and financial reporting requirements. |
|
|
|
| · | In December 2010, our Board of Directors formed an Audit Committee, which includes three independent directors, two of whom qualify as a “financial expert” by the standard of the SEC. The Audit Committee will assist the Board in fulfilling its oversight responsibility relating to the quality and integrity of our financial reporting. |
|
|
|
| · | Due to our size and nature, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, we will implement procedures to assure that the initiation of transactions, the custody of assets and the recording of transactions will be performed by separate individuals. |
We believe that the foregoing steps will remediate the material weakness identified above, and we will continue to monitor the effectiveness of these steps and make any changes that our management deems appropriate.
A material weakness (within the meaning of PCAOB Auditing Standard No. 5) is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of the company's financial reporting.
Auditor Attestation
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by our registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permanently exempt smaller reporting companies.
42
Changes in Internal Control over Financial Reporting
Except as described above, there were no changes in our internal controls over financial reporting during the three months ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION.
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
CURRENT DIRECTORS AND EXECUTIVE OFFICERS
The following individuals serve as our current executive officers and members of our Board of Directors:
Name |
| Age |
| Positions held: |
Dr. Zhongyi Liu |
| 50 |
| Chief Executive Officer, President, and Chairman of the Board of Directors |
Ms. Yan Zeng |
| 42 |
| Chief Financial Officer |
Mr. Xing Shen |
| 40 |
| Vice President of Corporate Development |
Mr. Michael Toups (1)(2)(3) |
| 45 |
| Director |
Mr. Lijun Fan (1)(2)(3) |
| 42 |
| Director |
Mr. Zhaohui Li (1)(2)(3) |
| 42 |
| Director |
Mr. Jun Lu |
| 31 |
| Director |
(1) Member of Audit Committee.
(2) Member of Nominating Committee.
(3) Member of Compensation Committee.
BIOGRAPHICAL INFORMATION
Dr. Zhongyi Liu. Dr. Liu has served as Chairman of the Board and Chief Executive Officer of Lotus since September 2006 and he has also served in those positions at Lotus International since founding that company in August 2006. Dr. Liu is also the Chairman, Deputy Chief Physician and founder of Liang Fang and founder and General Manager of En Ze Jia Shi. As a researcher and medical student, Dr. Liu excelled in the development of many new drugs which are widely used in China today. While worked for the Chinese Government in 1992, he established the Research Center of Space Flight Biological Engineering Technology, and continued his renowned research related to incretion diseases. Four years later, entering the private sector and invigorated by burgeoning capitalism in China, Dr. Liu started his first pharmaceutical company in Beijing. He earned his Master's Degree in Endocrinology from Beijing Xiehe Medical School, after finished his undergraduate studies in Inner Mongolia Medicine College located in Inner Mongolia, China. He is a majority stockholder in each of Liang Fang and En Ze Jia Shi. The Board believes that Dr. Liu has the experience, qualifications, attributes and skills necessary to serve on the Board because of his extensive experience in the medical research industry, his having provided leadership and strategic direction to the Company and his unparalleled knowledge of the Company and its business. Dr. Liu is not a member of the board of directors for any public company or any investment company, neither has he been a member of the board of directors for such companies for the past five years. Dr. Liu’s experience as Chief Executive Officer led to the conclusion he should serve on the board of directors given the Company’s business and structure.
Ms. Yan Zeng. Ms. Zeng has served as Chief Financial Officer for Lotus Pharmaceuticals, Inc. since September 22, 2010 (except for a period in September 2010). Ms. Zeng has over ten years experience as a financial manager and auditor for Chinese based companies. From September 3, 2010 to September 21, 2010, Ms. Zeng served as an accountant for Beijing Liang Fang Pharmaceutical Ltd.. From May 1, 2009 to September 2, 2010, Ms. Zeng served as Chief Financial Officer of Lotus Pharmaceuticals, Inc.. From 2008 to April 30, 2009, Ms. Zeng has been an accountant for Beijing Liang Fang Pharmaceutical Ltd., a subsidiary of Lotus Pharmaceuticals, Inc.. From 2005 to 2008, Ms. Zeng was a registered accountant in Beijing Topson Certified Public Accountants, where she conducted auditing and provided financial counsel for public companies. From 2004 to 2005, Ms. Zeng was a financial manager for Beijing Unite Youbang Science and Technology Ltd. Ms. Zeng is a CPA in China with bachelor degree in Business from Beijing Information Science & Technology University.
43
Mr. Xing Shen. Mr. Shen has served as Vice President of Corporate Development since September 2010. Mr. Shen has been with the Company since July 2009. From July 2006 through May 2009, Mr. Shen was an associate equity analyst for RBC Capital Markets, where he co-covered approximately 30 companies in the biotech industry. Mr. Shen received a Masters of Business Administration in 2006 and a Doctorate in Molecular Cancer Biology in 2000 from Duke University. Mr. Shen received a Master of Science in plant molecular biology from Peking University in 1995 and a Bachelor of Science in Virology from Wuhan University in 1992. Mr. Shen has received Series 7, 63, 86 and 87 licenses from Finra and is a Chartered Financial Analyst (CFA) level one candidate.
Mr. Michael Toups. Mr. Toups has been a member of our Board of Directors since December 2010. Mr. Toups is an accounting and corporate finance executive with 20 years of experience in senior management with specialties in business strategy, M&A and international trade. He has middle-market corporate finance experience across a variety of industries as both principal and advisor and has served in roles as the CFO, COO, and director of private and publicly traded companies. Mr. Toups’ expertise includes PCAOB audits, SEC reporting, Sarbanes-Oxley compliance and investor relations. Mr. Toups is also well-versed in Chinese business practices and has directed strategic planning for Asia-based companies for over 12 years. Most recently, since June 2010, Mr. Toups has served as the CFO for Longwei Petroleum Investment Holding Limited, a China-based petroleum distributor. Since September 2010, Mr. Toups has also served as the CFO of China Bilingual Technology & Education Group, a China-based education company operating K-12 private boarding schools. Mr. Toups has previously served as Director of Asia Investment Banking, Midtown Partners & Co. from December 2007 to July 2010 and as the CFO and Director of Nork Lighting, a China-based manufacturer and the largest retailer of high-end residential lighting products in China from December 2007 to July 2010. From January 2001 to December 2007, he served as president of Peak Crown, a consulting company for the import of products from Asia and financial services. Mr. Toups holds an MBA in Finance from the University of Notre Dame and a BBA in Finance from Texas Christian University. Mr.Toups’s financial and public company director experience led to the conclusion he should serve on the Company’s board of directors given the Company’s business and structure.
Mr. Lijun Fan. Mr. Fan has been a member of our Board of Directors since December 2010. Since 2009, Mr. Fan has served as President and CEO of Jolyvia International, Inc., which manufactures independent brand cosmetics and operates Jolyvia Beauty SPA national chain stores in China. From 2003 to 2009, he was the general manager and director of Jourdeness International Inc., where he was responsible for the expansion of direct-to-consumer cosmetics and beauty chain SPA in China. Previously, from 1997 to 2003, he was the general manager and founder of Zhuhai Yi Sheng Development Co., a sales distributor for cosmetics and personal care products, and sales manager for Zhuhai Dong Da Biological Pharmaceutical Co.. Mr. Fan holds a Bachelor of Science degree in biology from Jinan University. Mr. Fan’s scientific, marketing and manufacturing experience and knowledge led to the conclusion he should serve on the Company’s board of directors given the Company’s business and structure.
Mr. Zhaohui Li. Mr. Li has been a member of our Board of Directors since December 2010. Since 1998, Mr. Li has served as a professor and the director of the Department of Assisted Reproduction at China Three Gorges University School of Medicine. Previously, from 1995 to 1998, Mr. Li has served as Assistant to the General Manager and Director of Marketing at Guangdong Zhuhai Dong Da Biological Pharmaceutical Co. and General Manager at Guangdong Zhuhai Dong Wu Pharmaceutical Co.. He holds a Master of Science degree from the Institute of Biochemistry at the Chinese Academy of Sciences and a Bachelor of Science degree in biology from Peking University. Mr. Li’s scientific and medical knowledge and experience led to the conclusion he should serve on the Company’s board of directors given the Company’s business and structure.
Mr. Jun Lu. Mr. Lu has been a member of our Board of Directors since December 2010. Mr. Lu has been the head of research and development for Lotus Pharmaceuticals since 2006 and previously, from 2001 to 2003, worked at Beijing Double-Crane Pharmaceutical Co., Ltd. Mr. Lu holds both a Master's and a Bachelor's degree in pharmacy from ShenYang Pharmaceutical University. His research focuses on various new pharmaceutical dosage forms, especially in osmotic pump-controlled release. Mr. Lu has published six papers in well-known peer-reviewed journals and filed four patents, two of which have been granted. Mr. Lu’s experience as head of research and development at the Company led to the conclusion he should serve on the Company’s board of directors given the Company’s business and structure.
INVOLVEMENT IN CERTAIN LEGAL PROCEEDINGS
None of the current officers or directors of the Company have been the subject of litigation over the past ten years nor was any the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
| · | Any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of bankruptcy or within two years prior to that time; or |
44
| · | Any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); or |
|
|
|
| · | Being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or |
|
|
|
| · | Being found by a court of competent jurisdiction (in a civil violation), the SEC or the Commodity Future Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; or |
|
|
|
| · | Being the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: any Federal or State securities or commodities law or regulation; or any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity. This violation does not apply to any settlement of a civil proceeding among private litigants; or |
|
|
|
| · | Being the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
DIRECTOR QUALIFICATIONS
We seek directors with established strong professional reputations and experience in areas relevant to the strategy and operations of our businesses. We seek directors who possess the qualities of integrity and candor, who have strong analytical skills and who are willing to engage management and each other in a constructive and collaborative fashion. We also seek directors who have the ability and commitment to devote significant time and energy to service on the Board and its committees. We believe that all of our directors meet the foregoing qualifications.
FAMILY RELATIONSHIPS
There are no family relationships between or among any of the current directors, executive officers or persons nominated or charged by the Company to become directors or executive officers.
SECTION 16(A) BENEFICIAL OWNERSHIP REPORTING COMPLIANCE
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our executive officers and directors, and persons who beneficially own more than 10% of a registered class of our equity securities to file with the Securities and Exchange Commission initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common shares and other equity securities, on Forms 3, 4 and 5 respectively. Executive officers, directors and greater than 10% stockholders are required by the Securities and Exchange Commission regulations to furnish us with copies of all Section 16(a) reports they file. Based on our review of the copies of such forms furnished to us and to the best of our knowledge, we believe that, during fiscal year 2010, our current and prior directors who are Zhongyi Liu, Ian Ashley, Ping Li, Jin Liu, Xuemei Xian, Zhenghong Song, Michael Toups, Lijun Fan, Zhaohui Li and Jun Lu have not timely filed the required Forms.
CODE OF ETHICS
We have adopted a Code of Ethics which applies to our principal executive officer, principal financial officer, and principal accounting officer. We will provide a copy, without charge, to any person desiring a copy of the Code of Ethics, by written request to 16 Cheng Zhuang Road, Feng Tai District, Beijing, 100071, People’s Republic of China. Attention: Corporate Secretary. In addition, we have filed a copy of the Financial Code of Ethics with the Securities and Exchange Commission as an exhibit to this report, and posted the Code of Ethics on our website at www.lotuspharma.com.
45
COMMITTEES OF THE BOARD OF DIRECTORS
On December 22, 2010 our Board of Directors established an Audit Committee, a Nominating Committee and a Compensation Committee. Prior to the establishment of these committees, our Board did not have any committee and the functions of those committees were being undertaken by the entire Board as a whole. Information concerning the current membership and function of each committee is as follows:
Committee |
| Chair |
| Member |
| Member |
Audit |
| Lijun Fan |
| Zhaohui Li |
| Michael Toups |
Compensation |
| Zhaohui Li |
| Lijun Fan |
| Michael Toups |
Nominating |
| Michael Toups |
| Zhaohui Li |
| Lijun Fan |
Audit Committee
The Audit Committee assists the Board in fulfilling its oversight responsibility relating to the quality and integrity of our financial reporting practices. The primary purposes of the Audit Committee are to oversee on behalf of the Board:
| · | our accounting and financial reporting processes and the integrity of our financial statements; |
| · | the audits of our financial statements and the appointment, compensation, qualifications, independence and performance of our independent auditors; |
| · | our compliance with legal and regulatory requirements; |
| · | the qualitative aspects of financial reports to our shareholders; and |
| · | the performance of our internal audit function and internal control over financial reporting. |
The Audit Committee is composed of three directors, all of whom have been determined by the Board of Directors to be “independent,” as defined by the NYSE Amex Company Guide. The Audit Committee Charter requires that all members of the Audit Committee must be financially literate and at least one member must be an “audit committee financial expert” as defined in the SEC’s rules. The Board has determined that Mr. Fan and Mr. Toups qualifies as an “audit committee financial expert” as defined by the SEC.
Compensation Committee
The Compensation Committee is responsible for overseeing our compensation programs and practices, including our executive compensation plans and incentive compensation plans. The Chief Executive Officer provides input to the Compensation Committee with respect to the individual performance and compensation recommendations for the other executive officers. The Compensation Committee is composed of three directors, all of whom have been determined by the Board of Directors to be “independent,” as defined by the NYSE Amex Company Guide.
Nominating Committee
The Nominating Committee assists the Board in:
| · | identifying individuals qualified to become board members and recommending to the Board the nominees for election as directors at the next annual meeting of shareholders, |
| · | overseeing, reviewing and making periodic recommendations concerning our corporate governance policies, and |
| · | advising on matters of organization, management succession plans, major changes in organizational structure and conduct of Board activities. |
The Nominating Committee is comprised of three members, all of whom have been determined by the Board of Directors to be “independent,” as defined by the NYSE Amex Company Guide.
Board Leadership Structure and Role in Risk Oversight
Although we have not adopted a formal policy on whether the Chairman and Chief Executive Officer positions should be separate or combined, we have traditionally determined that it is in the best interests of the Company and its shareholders to combine these roles. Dr. Liu has served as our Chairman since September 2006. Due to the small size and early stage of the Company, we believe it is currently most effective to have the Chairman and Chief Executive Officer positions combined.
46
Our Audit Committee is primarily responsible for overseeing our risk management processes on behalf of our board of directors. The Audit Committee receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. In addition, the Audit Committee reports regularly to the full Board of Directors, which also considers our risk profile. The Audit Committee and the full Board of Directors focus on the most significant risks facing our company and our company’s general risk management strategy, and also ensure that risks undertaken by our Company are consistent with the Board’s appetite for risk. While the Board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our Board leadership structure supports this approach.
Changes in Nominating Procedures
During the year ended December 31, 2010, the board of directors established the Nominating Committee, as discussed above.
ITEM 11. EXECUTIVE COMPENSATION.
The following table is a summary of the compensation we paid for each of the last two fiscal years ended December 31, 2010 and 2009, respectively (unless otherwise provided) to the persons who acted as our principal executive officers during the two years. No other executive officers received compensation in excess of $100,000 in these two years. The value attributable to any option awards, if any, is computed in accordance with FASB ASC Topic 718. In fiscal 2010 and 2009, we did not grant any option to executive officers or directors.
Summary Compensation Table | |||||||||
Name and | Fiscal | Salary | Bonus | Stock | Option | Non-Equity | Nonqualified | All | Total |
Zhongyi Liu (1) | 2010 | 180,000 | 0 | 0 | 0 | 0 | 0 | 0 | 180,000 |
| 2009 | 180,000 | 0 | 0 | 0 | 0 | 0 | 0 | 180,000 |
(1) | Dr. Liu has served as our Chief Executive Officer and President since September 28, 2006. Dr. Liu’s did not have any other compensation for 2010 and 2009. Compensation amounts reflected for Dr. Liu for each of 2010 and 2009 exclude any payments to him for his services as a director or pursuant to the terms of the assignment agreement entered into between Dr. Liu and Lotus East in October 2006 in conjunction with the loan agreement and contract with Wu Lan Cha Bu Emergency Hospital. See Director Compensation below and Item 13. Certain Relationships and Related Transactions, and Director Independence. |
HOW COMPENSATION IS DETERMINED
We currently have no employment agreements with any of our executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer's responsibilities following a change-in-control. Dr. Liu’s and other employee’s compensation is determined from time to time by the Board of Directors of Lotus Internationals, Inc, of which Dr. Liu is a member. The amount of compensation of any employee is not tied to any performance goals or other traditional measurements and may be increased from time to time at the sole discretion of such Board.
RETIREMENT BENEFITS
We currently have no plans that provide for payments or other benefits at, following, or in connection with retirement of our named executive officers.
PERQUISITES
We have not provided our named executive officers with any material perquisites and other personal benefits and, therefore, we do not view perquisites as a significant or necessary element of our executive’s compensation.
47
NONQUALIFIED DEFINED CONTRIBUTION AND OTHER NONQUALIFIED DEFERRED COMPENSATION PLANS
We currently have no defined contribution or other plans that provide for the deferral of compensation to our named executive officers on a basis that is not tax-qualified.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
There are no unexercised options; stock that has not vested; or equity incentive plan awards held by any named executive officer outstanding as of December 31, 2010.
DIRECTORS COMPENSATION
The following table provides information concerning the compensation of members of our Board of Directors for each of their services as a director during our fiscal year ended December 31, 2010.
Director Compensation | |||||||
Name | Fees | Stock | Option | Non-equity | Nonqualified | All other | Total |
Zhongyi Liu | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Ian Ashley (2) | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Ping Li (3) | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Jin Liu (4) | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Xuemei Xian (5) | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Zhenghong Song (6) | 0 | 11,000 | 0 | 0 | 0 | 0 | 11,000 |
Michael Toups (7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Lijun Fan (8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Zhaohui Li (9) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Jun Lu (10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
(1) | The amounts included in the “Stock awards” column represent the fair market value of 5,000 shares of our common stock issued to each of directors as compensation for their services on our Board of Directors. |
(2) | Ian Ashley resigned as a member of our Board of Directors in October 2010. |
(3) | Ping Li resigned as a member of our Board of Directors in December 2010. |
(4) | Jin Liu resigned as a member of our Board of Directors in December 2010. |
(5) | Xuemei Xian resigned as a member of our Board of Directors in December 2010. |
(6) | Zhenghong Song resigned as a member of our Board of Directors in December 2010. |
(7) | Michael Toups appointed as a member of our Board of Directors in December 2010. |
(8) | Lijun Fan appointed as a member of our Board of Directors in December 2010. |
(9) | Zhaohui Li appointed as a member of our Board of Directors in December 2010. |
(10) | Jun Lu appointed as a member of our Board of Directors in December 2010. |
2010 STOCK INCENTIVE PLAN
The 2010 Stock Incentive Plan provides for the issuance of up to 1,500,000 shares of common stock to employees, directors, consultants and advisors of the Company and its subsidiaries. Awards may be in the form of stock options (including incentive stock options), stock appreciation rights, stock awards, and cash awards. The Plan is administered by the Company’s Board of Directors or a committee of the Board of Directors consisting of at least two directors who shall not be employees of the Company. Subject to the terms of the Plan, the Board of Directors as administrator has the sole discretion to select the directors, officers, employees, consultants and advisors who will receive awards, determine the terms and conditions of the awards, and interpret the provisions of the Plan and outstanding awards. The Board of Directors generally may amend or terminate the Plan at any time and for any reason, except that share and other award limitations cannot be increased and minimum stock option and stock appreciation right exercise prices cannot be changed unless such a plan amendment is approved by the Company’s stockholders. If any award under the Plan is cancelled prior to its exercise or vesting in full, or if the number of shares subject to an award is reduced for any reason, the shares of the Company’s common stock that are no longer subject to such award will be returned to the available pool of shares reserved for issuance under the Plan. As of the filing date of this report, the Company had issued a total of 5,000 shares of common stock.
48
RISK MANAGEMENT
The Company does not believe risks arising from its compensation policies and practices for its employees are reasonably likely to have a material adverse effect on the Company.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
At March 28, 2011 we had 27,747,131 shares of common stock issued and outstanding and 607,107 shares of Series A Preferred Stock issued and outstanding. Each holder of common stock is entitled to one vote for each share of common stock held, and each holder of Series A Preferred Stock is entitled to the number of votes equal to the number of shares of common stock into which such shares of Series A Preferred Stock could be converted. Presently, the Series A Preferred Stock is convertible on a one Series A Preferred Stock for one-second common stock basis. The holders of the common stock and the Series A Preferred Stock vote together on all matters submitted to a vote of our stockholders, except that holders of Series A Preferred Stock are not entitled to vote in the election of our directors. The following table sets forth information known to us as of March 28, 2011 relating to the beneficial ownership of shares of our voting securities by:
| · | each person who is known by us to be the beneficial owner of five percent or more of our outstanding voting stock; |
| · | each current director; |
| · | each current executive officer; and |
| · | all of our current executive officers and directors as a group. |
Unless otherwise indicated, the business address of each person listed is in care of No. 16 Cheng Zhuang Road, Feng Tai District, Beijing, People's Republic of China, 100071. The percentages in the table have been calculated on the basis of treating as outstanding for a particular person, all shares of our common stock outstanding on that date and all shares of our common stock issuable to that holder in the event of exercise of outstanding options, warrants, rights or conversion privileges owned by that person at that date which are exercisable within 60 days of that date. Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of stock owned by them, except to the extent that power may be shared with a spouse.
| Amount and Nature of Beneficial Ownership (1) |
| |||
| Common Stock | Series A Preferred Stock |
| ||
Name | # of Shares | % of Class | # of Shares | % of Class | % of Vote |
Zhongyi Liu (2) | 10,333,738 | 37.2% | 0 | n/a | 37.2% |
Yan Zeng | 0 | * | 0 | n/a | * |
Michael Toups | 20,000 | * | 0 | n/a | * |
Lijun Fan | 20,000 | * | 0 | n/a | * |
Zhaohui Li | 20,000 | * | 0 | n/a | * |
Jun Lu | 20,000 | * | 0 | n/a | * |
Zhenghong Song (3) | 3,464,000 | 12.5% | 0 | n/a | 12.5% |
All current officers and directors as a group (six persons) | 10,413,738 | 37.5% | 0 | n/a | 37.5% |
* represents less than 1%
(1) | Based on 27,747,131 outstanding shares of Common Stock and 607,107 outstanding shares of Series A Preferred Stock as of March 28, 2011. |
(2) | Dr. Liu’s holdings exclude the holdings of his wife, Mrs. Zhenghong Song, over which he disclaims beneficial ownership. |
(3) | Mrs. Zhenghong Song is Dr. Liu’s wife. Mrs. Zhenghong Song’s holdings exclude the holdings of Dr. Liu over which she disclaims beneficial ownership. |
49
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
PRC law currently limits foreign equity ownership of Chinese companies. To comply with these foreign ownership restrictions, we operate our business in China through the Contractual Arrangements with Lotus East and shareholders of Lotus East that were executed on September 6, 2006. Certain of our principal shareholders, executive officers and directors are also principal owners, executive officers and directors of Lotus East, including Dr. Zhongyi Liu.
Dr. Liu leases Lotus East two retail spaces for no charge on a month-to-month basis.
Our Chief Executive Officer and his spouse and several employees, from time to time, provided advances to us for working capital purpose. These advances are non-interest bearing, unsecured and payable on demand. At December 31, 2010, we had a payable to the chief executive officer and his spouse and these employees at an amount of $744,879 which was included in due to related parties on our balance sheet dated December 31, 2010.
On October 9, 2006, the Company entered into a five-year loan agreement and a contract with Wu Lan, whereby the Company agreed to lend Wu Lan approximately $4.5 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan agreed to grant the Company an exclusive right to supply all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. In October 2006, the Company’s Chief Executive Officer, Dr. Liu, lent this loan to Wu Lan on behalf of the Company. On October 21, 2006, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Dr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Dr. Liu accepted the assignment with all the risks and obligations but had no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Dr. Liu compensation for an aggregate of approximately $1.3 million (RMB 9 million) in 5 equal annual installments of approximately $272,000 (RMB 1.8 million) started from October 21, 2006. During fiscal 2010 and 2009, we did not pay anything to Dr. Liu for the liability incurred by the assignment in the agreement mentioned above. Dr. Liu agreed to allow the Company for deferring the installments in fiscal year 2010 and 2009 in order to ease the burden on the Company’s working capital needs. At December 31, 2010, amount due under this assignment agreement was $1,068,827, and was included in current portion of due to related parties on our balance sheet dated December 31, 2010.
Our Chief Executive Officer, Mrs. Zhenghong Song, Mr. Guoan Song who is Mrs. Zhenghong Song’s father and our Chief Executive Officer’s father-in-law, and two employees made a loan to us for working capital purpose on December 31, 2005. The loan is due on December 30, 2015 with variable annual interest at 80% of current bank rate and unsecured. According to the terms set forth in the loan agreement, the interest for the loan will be paid off on due date of the loan. The principal of the loan was included in long-term loan payable – related parties at an amount of $5,241,829 and the accrued and unpaid interest for the loan was included in long-term portion of due to related parties at an amount of $869,067 on our balance sheet dated December 31, 2010.
During the fiscal 2010, our Chief Executive Officer, Dr. Zhongyi Liu, from time to time, made payments to various unrelated third parties on behalf of us. At December 31, 2010, we had a payable to our Chief Executive Officer at an amount of $228,670 which was included in current portion of due to related parties on our balance sheet dated December 31, 2010.
DIRECTOR INDEPENDENCE
Our Board of Directors has determined that Michael Toups, Lijun Fan and Zhaohui Li are “independent” as the term is used in NYSE Amex Company Guide.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Aggregate fees billed by our current principal accountants, Friedman LLP for audit services related to the most two recent fiscal years, and for other professional services billed in the most recent fiscal year, were as follows:
|
| Fiscal Years Ended December 31, |
| ||||
Category |
| 2010 |
| 2009 |
| ||
Audit Fees1 |
| $ | 70,000 |
| $ | 45,000 |
|
Audit Related Fees2 |
|
| 22,500 |
|
| 21,000 |
|
Tax Fees3 |
|
| — |
|
| — |
|
All Other Fees4 |
|
| — |
|
| — |
|
Total |
| $ | 92,500 |
| $ | 66,000 |
|
50
1 | Consists of fees billed for the audit of our annual financial statements, review of our Form 10-K and services that are normally provided by the independent auditors in connection with year-end statutory and regulatory filings or engagements. | |
|
| |
2 | Consists of fees billed for the review of our quarterly financial statements, review of our forms 10-Q, 8-K and services that are normally provided by the independent auditors in connection with non year-end statutory and regulatory filings or engagements. | |
|
| |
3 | Consists of professional services rendered by our independent auditors for tax compliance, tax advice and tax planning. The services for the fees disclosed under this category include tax return preparation and technical tax advice. | |
|
| |
4 | Consisted of fees for other miscellaneous items. | |
Our Board of Directors has adopted a procedure for pre-approval of all fees charged by our independent auditors. Under the procedure, the Board approves the engagement letter with respect to audit, tax and review services. Other fees are subject to pre-approval by the Board, or, in the period between meetings, by a designated member of Board. Any such approval by the designated member is disclosed to the entire Board at the next meeting. The audit, review and tax fees paid to the auditors with respect to fiscal year 2010 were pre-approved by the entire Board of Directors.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
Exhibits
Exhibit No. | Description |
2.3 | Share Exchange Agreement between S.E. Asia Trading Company, Inc., SEAA Stockholders and Lotus Pharmaceutical International, Inc. and the Lotus Pharmaceutical International, Inc. Stockholders dated September 6, 2006 (1) |
3.1 | Certificate of Amendment effective December 14, 2006 (5) |
3.2 | Charter of S.E. Asia Trading Company, Inc. as filed with the State of Nevada (2) |
3.3 | Bylaws (2) |
3.4 | Certificate of Designation of the Relative Rights and Preferences of the Series A Convertible Redeemable Preferred Stock (8) |
3.5 | Amendment to Bylaws, dated February 18, 2011 (18) |
4.1 | Lotus Pharmaceuticals, Inc. 2010 Stock Incentive Plan (16) |
4.2 | Form of Series A Convertible Redeemable Preferred Stock Certificate (8) |
5.1 | Legal Opinion Concerning Lotus Pharmaceuticals, Inc. and its Subsidiaries/Affiliates dated February 5, 2008. (19) |
10.1 | Termination Agreement, dated as of July 20, 2010, by and between Lotus Pharmaceuticals, Inc. and YA Global Master SPV Ltd (15) |
10.2 | Operating Agreement between Lotus Pharmaceutical International, Inc., and Liang Fang, Liang Fang’s Majority Stockholders dated September 6, 2006 (3) |
10.3 | Proxy Agreement between Lotus Pharmaceutical International, Inc., Liang Fang, and Liang Fang s Majority Stockholders dated September 6, 2006 (3) |
10.4 | Option Agreement between Lotus Pharmaceutical International, Inc. and Liang Fang, Liang Fang Majority Stockholders dated September 6, 2006 (3) |
10.5 | Equity Pledge Agreement between Lotus Pharmaceutical International, Inc. and En Zhe Jia Shi Pharmaceutical Co., Ltd. and En Ze Jia Shi’s Majority Stockholders dated September 6, 2006 (3) |
10.6 | Operating Agreement between Lotus Pharmaceutical International, Inc., and En Zhe Jia Shi, En Zhe Jia Shi’s Majority Stockholders dated September 6, 2006 (3) |
10.7 | Proxy Agreement between Lotus Pharmaceutical International, Inc., En Zhe Jia Shi, and En Zhe Jia Shi’s Majority Stockholders dated September 6, 2006 (3) |
10.8 | Option Agreement between Lotus Pharmaceutical International, Inc. and En Zhe Jia Shi, En Zhe Jia Shi’s Majority Stockholders dated September 6, 2006 (3) |
10.9 | Consulting Services Agreement between Lotus Pharmaceutical International, Inc. and Liang Fang Pharmaceutical Co., Ltd. dated September 6, 2006 (3) |
10.10 | Consulting Services Agreement between Lotus Pharmaceutical International, Inc., En Zhe Jia Shi Pharmaceutical Co., Ltd. dated September 6, 2006 (3) |
10.11 | Letter of Resignation by Mr. Thomas Miller to the Board of Directors of S.E. Asia Trading Company (3) |
51
10.12 | General Partnership Agreement between Genesis Equity Partners, LLC and Liang Fang Pharmaceutical, Ltd. dated March 15, 2006. (3) |
10.13 | Lease Agreement between Beijing Aoshikai Peace Lane Shopping Center and Liang Fang Pharmaceutical Co. Ltd. dated June 1, 2002. (3) |
10.14 | Lease Agreement between Beijing Aoshikai Peace Lane Shopping Center and Liang Fang Pharmaceutical Co. Ltd. dated June 1, 2005. (3) |
10.15 | Lease Agreement between Beijing Fengtai District Retired Officer Management Agency of General Logistics of P.L.A. and Liang Fang Pharmaceutical Co. Ltd. dated September 15, 2003. (3) |
10.16 | Lease Agreement between Beijing Fengtai District 2nd Sanatorium of General Logistics of P.L.A. and Liang Fang Pharmaceutical Co. Ltd. dated April 1, 2003. (3) |
10.17 | Lease Agreement between Beijing Qiji Investment and Management Center and Liang Fang Pharmaceutical Co. Ltd. dated October 10, 2005. (3) |
10.18 | Lease Agreement between Beijing South Palace Marketing Center and Liang Fang Pharmaceutical Co. Ltd. dated January 1, 2006. (3) |
10.19 | Lease Agreement between Beijing Xingfa Food Shop and Liang Fang Pharmaceutical Co. Ltd. dated January 1, 2006. (3) |
10.20 | Lease Agreement between Construction and Repair Agency of Haiying Group and Liang Fang Pharmaceutical Co. Ltd. dated December 31, 2000. (3) |
10.21 | Lease Agreement between Liu Zhongyi and Liang Fang Pharmaceutical Co. Ltd. Dated December 15, 2001. (3) |
10.22 | Lease Agreement between Real Estate Management Agency of General Logistics of P.L.A. and Drugstore (Wanshou Round Branch) of Liang Fang Pharmaceutical Co. Ltd. dated September 1, 2004. (3) |
10.23 | Loan Agreement between Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. and Liu Zhongyi dated December 31, 2005. (3) |
10.24 | Loan Agreement between Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. and Ma Zhaozhao dated December 31, 2005. (3) |
10.25 | Loan Agreement between Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. and Song Guoan dated December 31, 2005. (3) |
10.26 | Loan Agreement between Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. and Song Zhenghong dated December 31, 2005. (3) |
10.27 | Loan Agreement between Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. and Zheng Guixin dated December 31, 2005. (3) |
10.28 | Letter Agreement among S.E. Asia Trading Company, Inc., Lynn Management, LLC and Dynacap Holdings Limited LLC dated September 13, 2006. (3) |
10.29 | Bill of Sale between S.E. Asia Trading Company, Inc. and Charles Smith dated September 25, 2006. (3) |
10.30 | Loan Agreement among Beijing Liang Fang Pharmaceutical Co., Ltd. and Wu Lan Cha Bu Emergency Hospital (English version) (7) |
10.31 | Loan Agreement among Beijing Liang Fang Pharmaceutical Co., Ltd. and Wu Lan Cha Bu Emergency Hospital (Chinese version) (7) |
10.32 | Contract among Wu Lan Cha Bu Emergency Hospital Center and Beijing Liang Fang Pharmaceutical Co., Ltd. dated October 10, 2006 (English version) (7) |
10.33 | Contract among Wu Lan Cha Bu Emergency Hospital Center and Beijing Liang Fang Pharmaceutical Co., Ltd. dated October 10, 2006 (Chinese version) (7) |
10.34 | Assignment agreement among Beijing Liang Fang Pharmaceuticals Co., Ltd. and Dr. Liu Zhong Yi dated October 10, 2006 (English version) (7) |
10.35 | Assignment agreement among Beijing Liang Fang Pharmaceuticals Co., Ltd. and Dr. Liu Zhong Yi dated October 10, 2006 (Chinese version) (7) |
10.36 | Convertible Redeemable Preferred Share and Warrant Purchase Agreement dated February 25, 2008 by and among Lotus Pharmaceuticals, Inc., its founders Dr. Liu Zhong Yi and Mrs. Song Zhenghong, and certain accredited investors (6) |
10.37 | Form of Escrow Agreement (6) |
10.38 | Form of Closing Escrow Agreement (6) |
10.39 | English translation of Technology Transfer Agreement dated April 25, 2008 by and between Bei Jing En Ze Jia Shi Pharmaceuticals LTD and Dong Guan Kai Fa Biologicals Medicine LTD (9) |
10.40 | English translation of Agreement dated June 3, 2008 by and between Beijing Liang Fang Pharmaceutical Co., Ltd., a Chinese limited liability company, and Cha You Qian Qi Economy Commission. (10) |
10.41 | Yipubishan New Drug Certificate and Intellectual Property Right Transfer Contract (12) |
52
10.42 | Standby Equity Distribution Agreement between Lotus Pharmaceuticals Inc. and YA Global Master SPV Ltd (13) |
10.43 | Assignment of Agreement between Lotus Pharmaceutical International, Inc. and Lotus Century Pharmaceutical (Beijing) Technology dated August 20, 2007 (19). |
Lotus Pharmaceuticals, Inc. Code of Business Conduct and Ethics* | |
16.1 | Letter dated January 13, 2010 from Bagell, Josephs, Levine & Company, LLP. (14) |
21.1 | Subsidiaries of the company (7) |
Consent of Friedman LLP * | |
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Executive Officer * | |
Rule 13a-14(a)/ 15d-14(a) Certification of Chief Financial Officer * | |
Section 1350 Certification of Chief Executive Officer * | |
Section 1350 Certification of Chief Financial Officer * | |
99.1 | Certificate of Change (17) |
* | Filed herewith |
(1) | Incorporated by reference from the Current Report on Form 8-K filed on September 7, 2006. |
(2) | Incorporated by reference from the registration statement on Form SB-1, SEC File No. 333-118898, filed on September 10, 2004, as amended. |
(3) | Incorporated by reference from the Current Report on Form 8-K filed on October 5, 2006. |
(4) | Incorporated by reference from the Current Report on Form 8-K filed on November 13, 2006. |
(5) | Incorporated by reference from the Current Report on Form 8-K filed on December 19, 2006. |
(6) | Incorporated by reference from the Current Report on Form 8-K filed on February 26, 2008. |
(7) | Incorporated by reference from the Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006. |
(8) | Incorporated by reference from the Current Report on Form 8-K/A as filed on February 29, 2008. |
(9) | Incorporated by reference from the Current Report on Form 8-K as filed on May 30, 2008. |
(10) | Incorporated by reference from the Current Report on Form 8-K as filed on July 17, 2008. |
(11) | Incorporated by reference from the Current Report on Form S-1 as filed on May 13, 2008. |
(12) | Incorporated by reference from the Current Report on Form 8-K as filed on February 18, 2009. |
(13) | Incorporated by reference from the Current Report on Form 8-K as filed on March 5, 2010 |
(14) | Incorporated by reference from the Current Report on Form 8-K as filed on January 15, 2010 |
(15) | Incorporated by reference from the Current Report on Form 8-K as filed on July 23, 2010 |
(16) | Incorporated by reference from the Registration Statement on Form S-8 as filed on September 3, 2010 |
(17) | Incorporated by reference from the Current Report on Form 8-K as filed on December 22, 2010 |
(18) | Incorporated by reference from the Current Report on Form 8-K as filed on February 23, 2011 |
(19) | Incorporated by reference from the Annual Report on Form 10-K as filed on March 31, 2010 |
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed below on its behalf by the undersigned thereunto duly authorized.
LOTUS PHARMACEUTICALS, INC. | ||
|
|
|
March 28, 2011 | By: | /s/ Zhongyi Liu |
| Zhongyi Liu, Chief Executive Officer and President, | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
Signature |
| Title |
| Date |
|
|
|
|
|
/s/ Dr. Zhongyi Liu |
| Chief Executive Officer and Director, |
| March 28, 2011 |
Dr. Zhongyi Liu |
| principal executive officer |
|
|
|
|
|
|
|
/s/ Ms. Yan Zeng |
| Chief Financial Officer, |
| March 28, 2011 |
Ms. Yan Zeng |
| principal financial and accounting officer |
|
|
|
|
|
|
|
/s/ Mr. Michael Toups |
| Director |
| March 28, 2011 |
Mr. Michael Toups |
|
|
|
|
|
|
|
|
|
/s/ Mr. Lijun Fan |
| Director |
| March 28, 2011 |
Mr. Lijun Fan |
|
|
|
|
|
|
|
|
|
/s/ Mr. Zhaohui Li |
| Director |
| March 28, 2011 |
Mr. Zhaohui Li |
|
|
|
|
|
|
|
|
|
/s/ Mr. Jun Lu |
| Director |
| March 28, 2011 |
Mr. Jun Lu |
|
|
|
|
54
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010 and 2009
CONTENTS
Report of Independent Registered Public Accounting Firm | F-2 |
|
|
Consolidated Financial Statements: |
|
|
|
Consolidated Balance Sheets |
|
As of December 31, 2010 and 2009 | F-3 |
|
|
Consolidated Statements of Income and Comprehensive Income |
|
For the Years Ended December 31, 2010 and 2009 | F-4 |
|
|
Consolidated Statements of Stockholders’ Equity |
|
For the Years Ended December 31, 2010 and 2009 | F-5 |
|
|
Consolidated Statements of Cash Flows |
|
For the Years Ended December 31, 2010 and 2009 | F-6 |
|
|
Notes to Consolidated Financial Statements | F-7 to F-30 |
F-1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Lotus Pharmaceuticals Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of Lotus Pharmaceuticals Inc. and Subsidiaries as of December 31, 2010 and 2009, and the related consolidated statements of income and comprehensive income, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2010. Lotus Pharmaceuticals Inc. and Subsidiaries’ management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Lotus Pharmaceuticals Inc. and Subsidiaries as of December 31, 2010 and 2009, and the results of their operations and their cash flows for each of the years in the two-year period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
/s/ Friedman LLP
New York, New York
March 28, 2011
F-2
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
| As of December 31, |
| ||||
|
| 2010 |
| 2009 |
| ||
ASSETS |
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
|
Cash |
| $ | 1,339,972 |
| $ | 3,945,740 |
|
Accounts receivable |
|
| 1,973,150 |
|
| 1,784,194 |
|
Security deposit |
|
| 16,682 |
|
| 16,132 |
|
Inventories |
|
| 634,583 |
|
| 1,039,867 |
|
Prepaid expenses |
|
| 577,077 |
|
| 856,691 |
|
Deferred debt costs |
|
| — |
|
| 52,226 |
|
|
|
|
|
|
|
|
|
Total Current Assets |
|
| 4,541,464 |
|
| 7,694,850 |
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT, net |
|
| 39,337,935 |
|
| 16,223,775 |
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
Prepaid expenses |
|
| — |
|
| 1,359,583 |
|
Land use right held for development |
|
| 29,236,891 |
|
| — |
|
Deposits and Installments on intangible assets |
|
| 9,528,419 |
|
| 9,214,299 |
|
Land use right, net |
|
| 12,932,421 |
|
| 41,673,492 |
|
Other intangible assets, net |
|
| 7,607,485 |
|
| 8,214,936 |
|
|
|
|
|
|
|
|
|
Total Assets |
| $ | 103,184,615 |
| $ | 84,380,935 |
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
|
Accounts payable |
| $ | 37,829 |
| $ | — |
|
Other payables and accrued liabilities |
|
| 3,441,466 |
|
| 2,690,684 |
|
Taxes payable |
|
| 2,024,565 |
|
| 3,131,908 |
|
Unearned revenue |
|
| 504,442 |
|
| 1,163,771 |
|
Due to related parties |
|
| 2,042,376 |
|
| 1,490,649 |
|
Series A convertible redeemable preferred stock, $.001 par value; 10,000,000 shares authorized; 607,107 and 4,967,959 shares issued and outstanding at December 31, 2010 and 2009, respectively, net of discount |
|
| — |
|
| 4,170,572 |
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
| 8,050,678 |
|
| 12,647,584 |
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
|
Due to related parties |
|
| 869,067 |
|
| 866,102 |
|
Notes payable - related parties |
|
| 5,241,829 |
|
| 5,069,023 |
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
| 14,161,574 |
|
| 18,582,709 |
|
|
|
|
|
|
|
|
|
COMMITMENTS AND CONTIGENCIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY: |
|
|
|
|
|
|
|
Preferred stock ($.001 par value; 10,000,000 shares authorized; 607,107 and 4,967,959 shares issued and outstanding at December 31, 2010 and 2009, respectively) |
|
| 607 |
|
| — |
|
Common stock ($.001 par value; 100,000,000 and 200,000,000 shares authorized; 26,763,485 and 23,653,166 shares issued and outstanding at December 31, 2010 and 2009, respectively) |
|
| 26,763 |
|
| 23,653 |
|
Additional paid-in capital |
|
| 21,679,147 |
|
| 15,672,981 |
|
Statutory reserves |
|
| 6,240,202 |
|
| 5,674,324 |
|
Retained earnings |
|
| 53,925,101 |
|
| 40,066,036 |
|
Accumulated other comprehensive income |
|
| 7,151,221 |
|
| 4,361,232 |
|
|
|
|
|
|
|
|
|
Total stockholders' Equity |
|
| 89,023,041 |
|
| 65,798,226 |
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders' Equity |
| $ | 103,184,615 |
| $ | 84,380,935 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-3
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
|
|
| For the Years Ended |
| |||
|
| December 31, |
| ||||
|
| 2010 |
| 2009 |
| ||
|
|
|
|
|
|
|
|
NET REVENUES: |
|
|
|
|
|
|
|
Wholesale |
| $ | 51,412,468 |
| $ | 44,842,525 |
|
Retail |
|
| 21,284,150 |
|
| 11,639,923 |
|
|
|
|
|
|
|
|
|
Total Net Revenues |
|
| 72,696,618 |
|
| 56,482,448 |
|
|
|
|
|
|
|
|
|
COST OF REVENUES: |
|
|
|
|
|
|
|
Wholesale |
|
| 16,801,703 |
|
| 16,700,366 |
|
Retail |
|
| 16,114,496 |
|
| 8,351,354 |
|
|
|
|
|
|
|
|
|
Total Cost of Revenues |
|
| 32,916,199 |
|
| 25,051,720 |
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 39,780,419 |
|
| 31,430,728 |
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
Selling expenses |
|
| 10,392,378 |
|
| 8,040,161 |
|
Research and development expenses |
|
| 86,545 |
|
| — |
|
Impairment loss |
|
| 6,762,659 |
|
| 1,719,884 |
|
General and administrative expenses |
|
| 8,016,353 |
|
| 3,693,869 |
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
| 25,257,935 |
|
| 13,453,914 |
|
|
|
|
|
|
|
|
|
INCOME FROM OPERATIONS |
|
| 14,522,484 |
|
| 17,976,814 |
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
Debt issuance costs |
|
| (52,226 | ) |
| (412,184 | ) |
Other income |
|
| 784,461 |
|
| 1,342,197 |
|
Interest income |
|
| 3,142 |
|
| 48,520 |
|
Interest expense |
|
| (612,626 | ) |
| (2,154,373 | ) |
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
| 122,751 |
|
| (1,175,840 | ) |
|
|
|
|
|
|
|
|
INCOME BEFORE INCOME TAXES |
|
| 14,645,235 |
|
| 16,800,974 |
|
|
|
|
|
|
|
|
|
INCOME TAXES |
|
| 220,292 |
|
| 368,680 |
|
|
|
|
|
|
|
|
|
NET INCOME |
| $ | 14,424,943 |
| $ | 16,432,294 |
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
Foreign currency translation gain |
|
| 2,789,989 |
|
| 131,989 |
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
| $ | 17,214,932 |
| $ | 16,564,283 |
|
|
|
|
|
|
|
|
|
NET INCOME PER COMMON SHARE: |
|
|
|
|
|
|
|
Basic |
| $ | 0.55 |
| $ | 0.74 |
|
Diluted |
| $ | 0.54 |
| $ | 0.66 |
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: |
|
|
|
|
|
|
|
Basic |
|
| 26,177,900 |
|
| 22,104,928 |
|
Diluted |
|
| 26,996,397 |
|
| 25,023,191 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-4
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
For the Years Ended December 31, 2010 and 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Accumulated |
|
|
|
| |
|
| Series A Preferred Stock |
| Common Stock |
| Additional |
|
|
|
|
|
|
| other |
| Total |
| ||||||||||
|
| Number of |
|
|
|
| Number of |
|
|
|
| Paid-in |
| Retained |
| Statutory |
| Comprehensive |
| Shareholders' |
| ||||||
|
| Shares |
| Amount |
| Shares |
| Amount |
| Capital |
| Earnings |
| Reserves |
| Income |
| Equity |
| ||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2008 |
| — |
| $ | — |
|
| 21,210,120 |
| $ | 21,210 |
| $ | 11,575,591 |
| $ | 25,557,537 |
| $ | 3,750,529 |
| $ | 4,229,243 |
| $ | 45,134,110 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for services |
| — |
|
| — |
|
| 1,805,119 |
|
| 1,805 |
|
| 2,650,528 |
|
| — |
|
| — |
|
| — |
|
| 2,652,333 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible preferred stock |
| — |
|
| — |
|
| 637,928 |
|
| 638 |
|
| 1,109,362 |
|
| — |
|
| — |
|
| — |
|
| 1,110,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Escrow Shares transferred to various investors per 2008 Purchase Agreement |
| — |
|
| — |
|
| — |
|
| — |
|
| 337,500 |
|
| — |
|
| — |
|
| — |
|
| 337,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appropriation to statutory reserve |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| (1,923,795 | ) |
| 1,923,795 |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 16,432,294 |
|
| — |
|
| — |
|
| 16,432,294 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 131,989 |
|
| 131,989 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2009 |
| — |
|
| — |
|
| 23,653,166 |
|
| 23,653 |
|
| 15,672,981 |
|
| 40,066,036 |
|
| 5,674,324 |
|
| 4,361,232 |
|
| 65,798,226 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for services |
| — |
|
| — |
|
| 290,170 |
|
| 290 |
|
| 765,459 |
|
| — |
|
| — |
|
| — |
|
| 765,749 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of convertible preferred stock |
| — |
|
| — |
|
| 2,326,551 |
|
| 2,327 |
|
| 4,045,873 |
|
| — |
|
| — |
|
| — |
|
| 4,048,200 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for warrants exercise |
| — |
|
| — |
|
| 329,557 |
|
| 330 |
|
| (330 | ) |
| — |
|
| — |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants issued for legal service |
| — |
|
| — |
|
| — |
|
| — |
|
| 170,041 |
|
| — |
|
| — |
|
| — |
|
| 170,041 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for compensation |
| — |
|
| — |
|
| 125,261 |
|
| 125 |
|
| 245,875 |
|
| — |
|
| — |
|
| — |
|
| 246,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Beneficial conversion feature of preferred stock issued for debt interest |
| — |
|
| — |
|
| — |
|
| — |
|
| 184,660 |
|
| — |
|
| — |
|
| — |
|
| 184,660 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reclassification of convertible preferred stock |
| 684,176 |
|
| 684 |
|
| — |
|
| — |
|
| 594,549 |
|
| — |
|
| — |
|
| — |
|
| 595,233 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for conversion of preferred stock |
| (77,069 | ) |
| (77 | ) |
| 38,535 |
|
| 38 |
|
| 39 |
|
| — |
|
| — |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for adjustments for 1:2 reverse split |
| — |
|
| — |
|
| 245 |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Appropriation to statutory reserve |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| (565,878 | ) |
| 565,878 |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 14,424,943 |
|
| — |
|
| — |
|
| 14,424,943 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustment |
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| 2,789,989 |
|
| 2,789,989 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2010 |
| 607,107 |
| $ | 607 |
|
| 26,763,485 |
| $ | 26,763 |
| $ | 21,679,147 |
| $ | 53,925,101 |
| $ | 6,240,202 |
| $ | 7,151,221 |
| $ | 89,023,041 |
|
The accompanying notes are an integral part of these consolidated financial statements
F-5
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
| For the Years Ended |
| |||
|
|
| December 31, |
| |||
|
|
| 2010 |
|
| 2009 |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
Net income |
| $ | 14,424,943 |
| $ | 16,432,294 |
|
Adjustments to reconcile net income from operations to net cash provided by operating activities: |
|
|
|
|
|
|
|
Depreciation |
|
| 480,046 |
|
| 425,895 |
|
Amortization of intangible assets |
|
| 1,767,722 |
|
| 1,560,466 |
|
Loss on impairment |
|
| 6,762,659 |
|
| 1,719,884 |
|
Amortization of deferred debt issuance costs |
|
| 52,226 |
|
| 412,184 |
|
Amortization of discount on convertible redeemable preferred stock |
|
| 151,553 |
|
| 1,196,106 |
|
Amortization of prepaid expense attributable to warrants |
|
| — |
|
| 14,849 |
|
Warrants issued for service |
|
| 170,041 |
|
| — |
|
Interest expense attributable to beneficial conversion feature of preferred shares |
|
| 184,660 |
|
| — |
|
Stock issued for service |
|
| 765,749 |
|
| — |
|
Stock issued for compensation |
|
| 246,000 |
|
| 282,083 |
|
Interest expenses caused by escrow shares transfer |
|
| — |
|
| 337,500 |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
Accounts receivable |
|
| (124,976 | ) |
| 4,361,619 |
|
Other receivable |
|
| — |
|
| (336 | ) |
Other receivable - related party |
|
| — |
|
| 2,031,902 |
|
Inventories |
|
| 429,879 |
|
| 2,755,869 |
|
Prepaid expenses |
|
| 1,654,229 |
|
| 11,643 |
|
Accounts payable |
|
| 36,898 |
|
| (172,330 | ) |
Other payables and accrued liabilities |
|
| 1,173,055 |
|
| 1,054,423 |
|
Taxes payable |
|
| (1,184,209 | ) |
| (1,895,451 | ) |
Unearned revenue |
|
| (681,787 | ) |
| 596,414 |
|
Due to related parties |
|
| 467,088 |
|
| 237,452 |
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES |
|
| 26,775,776 |
|
| 31,362,466 |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
Payments on intangible assets |
|
| — |
|
| (17,581,071 | ) |
Purchase of property and equipment |
|
| (29,448,568 | ) |
| (11,118,884 | ) |
|
|
|
|
|
|
|
|
NET CASH USED IN INVESTING ACTIVITIES |
|
| (29,448,568 | ) |
| (28,699,955 | ) |
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE ON CASH |
|
| 67,024 |
|
| 4,421 |
|
|
|
|
|
|
|
|
|
NET (DECREASE) INCREASE IN CASH |
|
| (2,605,768 | ) |
| 2,666,932 |
|
|
|
|
|
|
|
|
|
CASH - beginning of year |
|
| 3,945,740 |
|
| 1,278,808 |
|
|
|
|
|
|
|
|
|
CASH - end of year |
| $ | 1,339,972 |
| $ | 3,945,740 |
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
Cash paid for: |
|
|
|
|
|
|
|
Interest |
| $ | — |
| $ | — |
|
Income taxes |
| $ | 647,189 |
| $ | — |
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
Common stock issued for conversion of convertible redeemable preferred stock |
| $ | 4,048,200 |
| $ | 1,110,000 |
|
Convertible redeemable preferred stock reclassified to permanent equity |
| $ | 595,233 |
| $ | — |
|
The accompanying notes are an integral part of these consolidated financial statements
F-6
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
Lotus Pharmaceuticals, Inc. (“Lotus” or “the Company”), formerly S.E. Asia Trading Company, Inc. (“SEAA”), was incorporated on January 28, 2004 under the laws of the State of Nevada. SEAA originally operated as a retailer of jewelry, framed art and home accessories. In December 2006, SEAA changed its name to Lotus Pharmaceuticals, Inc.
On September 28, 2006, pursuant to a Share Exchange Agreement with Lotus Pharmaceutical International, Inc. (“Lotus International”), the Company acquired all of the outstanding common stock of Lotus International from the Lotus International shareholders in exchange for newly-issued stock of the Company (“Stock Exchange”). Lotus International became a wholly-owned subsidiary of the Company and Lotus International’s shareholders became the owners of the majority of the Company’s voting stock. The acquisition of Lotus International by the Company was accounted for as a reverse merger on a post-merger basis; the former shareholders of Lotus International hold a majority of the outstanding common stock of the Company on a voting and fully-diluted basis. As a result, Lotus International is deemed to be the acquirer for accounting purposes.
Lotus International was incorporated under the laws the State of Nevada on August 28, 2006 to develop and market pharmaceutical products in the People’s Republic of China (“PRC” or “China”). PRC law currently has limits on foreign ownership of certain companies. To comply with these foreign ownership restrictions, Lotus International operates its pharmaceutical business in China through Beijing Liang Fang Pharmaceutical Co., Ltd. (“Liang Fang”) and an affiliate of Liang Fang, Beijing En Ze Jia Shi Pharmaceutical Co., Ltd. (“En Ze Jia Shi”), both of which are pharmaceutical companies headquartered in the PRC and organized under the laws of the PRC (hereinafter, referred to together as “Lotus East”). Lotus International controlled Lotus East through various contracts with Lotus East and its shareholders in September 2006, pursuant to which Lotus International should provide technology consulting and other general business operation services to Lotus East. Lotus International also has the ability to substantially influence Lotus East’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval. As a result, Lotus International is considered the primary beneficiary of Lotus East.
In September 2006, Lotus International entered into the following contractual arrangements:
Operating Agreement. Pursuant to the operating agreement among Lotus, Lotus East and the shareholders of Lotus East, (collectively “Lotus East’s Shareholders”), Lotus provides guidance and instructions on Lotus East’s daily operations, financial management and employment issues. The shareholders of Lotus East must designate the candidates recommended by Lotus as their representatives on Lotus East’s Board of Directors. Lotus has the right to appoint senior executives of Lotus East. In addition, Lotus agreed to guarantee Lotus East’s performance under any agreements or arrangements relating to Lotus East’s business arrangements with any third party. Lotus East, in return, agreed to pledge its accounts receivable and all of its assets to Lotus. Moreover, Lotus East agreed that without the prior consent of Lotus, Lotus East would not engage in any transaction that could materially affect the assets, liabilities, rights or operations of Lotus East, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party. The term of this agreement is ten (10) years from September 6, 2006 and may be extended only upon Lotus’s written confirmation prior to the expiration of the this agreement, with the extended term to be mutually agreed upon by the parties.
Consulting Services Agreement. Pursuant to the exclusive consulting services agreements between Lotus and Lotus East, Lotus has the exclusive right to provide to Lotus East general pharmaceutical business operations services as well as consulting services related to the technological research and development of pharmaceutical products as well as general business operation advice and strategic planning (the “Services”). Under this agreement, Lotus owns the intellectual property rights developed or discovered through research and development, in the course of providing the Services, or derived from the provision of the Services. Lotus East is required to pay a quarterly consulting service fees in Renminbi (“RMB”), the functional currency of the PRC, to Lotus that is equal to Lotus East’s profits, as defined, for such quarter.
F-7
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Organization (continued)
Equity Pledge Agreement. Under the equity pledge agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East pledged all of their equity interests in Lotus East to Lotus to guarantee Lotus East’s performance of its obligations under the technology consulting agreement. If Lotus East or Lotus East’s Shareholders breaches its respective contractual obligations, Lotus, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Lotus East’s Shareholders also agreed that upon occurrence of any event of default, Lotus shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of Lotus East’s Shareholders to carry out the security provisions of the equity pledge agreement and take any action and execute any instrument that Lotus may deem necessary or advisable to accomplish the purposes of the equity pledge agreement. The shareholders of Lotus East agreed not to dispose of the pledged equity interests or take any actions that would prejudice Lotus’ interest. The equity pledge agreement will expire two years after Lotus East’s obligations under the exclusive consulting services agreements have been fulfilled.
Option Agreement. Under the option agreement between the shareholders of Lotus East and Lotus, the shareholders of Lotus East irrevocably granted Lotus or its designated person an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Lotus East for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. Lotus or its designated person has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is 10 years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Proxy Agreement. Pursuant to the proxy agreement among Lotus and Lotus East’s Shareholders, Lotus East’s Shareholders agreed to irrevocably grant a person to be designated by Lotus with the right to exercise Lotus East’s Shareholders’ voting rights and their other rights, including the attendance at and the voting of Lotus East’s Shareholders’ shares at the shareholders’ meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and its Article of Association, including but not limited to the rights to sell or transfer all or any of his equity interests of Lotus East, and appoint and vote for the directors and Chairman as the authorized representative of the shareholders of Lotus East. The term of this Proxy Agreement is ten (10) years from September 6, 2006 and may be extended prior to its expiration by written agreement of the parties.
Liang Fang is a Chinese limited liability company and was formed under laws of the People’s Republic of China on June 21, 2000. Liang Fang is engaged in the production, trade and retailing of pharmaceuticals. Further, Liang Fang is focused on developing innovative medicines and investing strategic growth to address various medical needs for patients worldwide. Liang Fang’s operations are based in Beijing, China.
As of December 31, 2010, Liang Fang owns and operates 10 drug stores throughout Beijing, China. These drugstores sell western and traditional Chinese medicines, and medical treatment accessories.
Liang Fang’s affiliate, En Ze Jia Shi is a Chinese limited liability company and was formed under laws of the People’s Republic of China on September 17, 1999. En Ze Jia Shi is the sole manufacturer for Liang Fang and maintains facilities for the production of medicines, patented Chinese medicine, as well as the research and production of other new medicines.
As a result of the management agreements between Lotus International and Lotus East, which enable Lotus International to absorb a majority of the risk of loss from Lotus East’s activities and enable Lotus International to receive a majority of its expected residual returns, Lotus International accounts for Lotus east as a variable interest entity (“VIE”) under FASB ASC 810 “Consolidation of Variable Interest Entities”.
On May 29, 2007, the Company formed a new entity, Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. (‘‘Lotus Century’’), a wholly foreign-owned enterprise (“WFOE”) organized under the laws of the Peoples’ Republic of China. Lotus Century is a Chinese limited liability company and a wholly-owned subsidiary of Lotus Pharmaceutical International, Inc. Lotus Century intends to be engaged in development of innovative medicines, medical technology consulting and outsourcing services, and related training services.
F-8
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Organization (continued)
On August 20, 2007, an assignment agreement was signed between Lotus International and Lotus Century. Pursuant to the terms of the assignment agreement, Lotus Century was assigned all of Lotus International’s right, title and interest to control the management and voting and to be entitled to all of the profits and losses of Lotus East.
Basis of presentation
The consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”). The consolidated statements include the accounts of Lotus Pharmaceuticals, Inc. and its wholly-owned subsidiaries, Lotus international and Lotus Century and variable interest entities under its control (Liang Fang and En Ze Jia Shi). All significant inter-company balances and transactions have been eliminated in consolidation.
The Company follows the provision of ASC 810 "Consolidation of Variable Interest Entities" ("ASC 810"), that requires a Variable Interest Entity (VIE) to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE's residual returns. VIEs are those entities in which the Company, through contractual arrangements, bears the risks of, and enjoys the rewards normally associated with ownership of the entities, and therefore the Company is the primary beneficiary of these entities. As a VIE, Lotus East’s revenues are included in the Company’s total revenues, its income from operations is consolidated with the Company’s, and the Company’s net income includes all of Lotus East’s net income.
Reverse stock split
On December 15, 2010, the Company received notification that its certificate of change was accepted by the Secretary of State of Nevada. Pursuant to the certificate of change, the Company effected a two-for-one reverse split of its common stock on December 31, 2010. Pursuant to Nevada Revised Statutes, no shareholder approval or amendment to the Company’s articles of incorporation was required for the certificate of change. Accordingly, all references to number of shares and to per share information in the consolidated financial statements have been adjusted to reflect the reverse stock split on a retroactive basis.
Use of estimates
The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect certain reported amounts of assets, liabilities, revenues, expenses, and the related disclosures at the date of the financial statements and during the reporting period. Accordingly, actual results could differ from those estimates. Significant estimates include the allowance for doubtful accounts, the allowance for obsolete inventory, the useful life of property and equipment and intangible assets, assumptions used in assessing impairment of long-term assets and valuation of deferred tax assets, fair value of warrants granted and beneficial conversion features related to the issuance of convertible preferred stock.
Fair value of financial instruments
The Company follows the provision of ASC 820, Fair Value Measurements and Disclosures. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
F-9
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair value of financial instruments (continued)
The carrying amounts reported in the balance sheets for cash, accounts receivable, accounts payable and accrued expenses, customer advances, and amounts due to related parties approximate their fair market value based on the short-term maturity of these instruments. The carrying value of the long-term debt approximates fair value based on market rates and terms currently available to the Company. Certain assets are measured at fair value on a non-recurring basis but are subject to fair value adjustments only under certain circumstances. These assets include long-lived assets that are written down to fair value when they are held for sale or determined to be impaired using the Level 3 inputs. During the year ended December 31, 2010, the Company recognized an impairment loss of $6,762,659 associated with the entire construction-in-progress related to the manufacturing plant in Cha Ha Er Industrial Park in Inner Mongolia due to the fact that the Company is planning to co-develop the land with other party (see note 5). For the year ended December 31, 2009, the Company recognized an impairment loss of $1,719,884 associated with the manufacturing building that was demolished in order to construct our new building.
Cash and cash equivalents
The Company considers all highly liquid instruments purchased with a maturity of three months or less and money market accounts to be cash equivalents. The Company maintains cash and cash equivalents with various financial institutions mainly in the PRC. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. Non-performance by these institutions could expose the Company to losses for amounts in excess of insured balances.
Accounts receivable
The Company presents accounts receivable, net of an allowance for doubtful accounts, if necessary. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the age of the balance, customer’s historical payment history, its current credit-worthiness and current economic trends. The amount of the provision, if any, is recognized in the consolidated statement of operations within “General and Administrative Expenses”. Accounts are written off after exhaustive efforts at collection. Because of the Company’s good relationship with its customers and the efforts of the Company’s collection representative to collect outstanding receivables, the majority age of the balance of the Company’s accounts receivable are less than three months. Management believes that the accounts receivable are fully collectable. Therefore, no allowance for doubtful accounts is deemed to be required as of December 31, 2010 and 2009.
Inventories
Inventories, consisting of raw materials, packaging materials, work-in-process and finished goods, are stated at the lower of cost or market utilizing the weighted average costing method. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of sales. The Company did not record any inventory reserve at December 31, 2010 and 2009.
Property and equipment
Property and equipment is carried at cost and depreciated on a straight-line basis over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the period of disposition. The Company reviews property and equipments for impairment when events or changes in circumstances indicate the recorded value may not be recoverable.
The construction-in-progress which consists of factories and office buildings under construction in China is included in property and equipment. No provision for depreciation is made on construction-in-progress until such time as the relevant assets are completed and ready for their intended use.
F-10
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Impairment of long-lived assets
In accordance with ASC 360, “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company periodically reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its carrying value. The Company recorded an impairment charge for the abandonment of construction-in-progress of $6,762,659 for the year ended December 31, 2010 and recorded an impairment loss on property and equipment of $1,719,884 for the year ended December 31, 2009.
Income tax
Deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory tax rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. The effect on deferred income taxes of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is recognized if it is more likely than not that some portion, or all of, a deferred tax asset will not be realized.
US GAAP includes the accounting and disclosure for uncertain tax positions and prescribes a recognition threshold and measurement attribute for recognition and measurement of a tax position taken or expected to be taken in a tax return.
The evaluation of a tax position is a two-step process. The first step is to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the year incurred. The Company did not have any uncertain tax position as of December 31, 2010 and 2009.
Value added tax
The Company is subject to value added tax (“VAT”) for manufacturing products. The applicable VAT rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent for the government.
Revenue recognition
Product sales are recognized when title to the product has transferred to customers in accordance with the terms of the sale. The Company recognizes revenue in accordance with ASC 360. ASC 360 states that revenue should not be recognized until it is realized or realizable and earned. The Company records revenue when persuasive evidence of an arrangement exists, product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated.
F-11
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Unearned revenue
Unearned revenue consists of prepayments from customers for merchandise that had not yet been shipped. The Company will recognize the deposits as revenue when customers take delivery of the goods, in accordance with its revenue recognition policy. At December 31, 2010 and 2009, the Company had unearned revenue of $504,442 and $1,163,771, respectively.
Concentrations of credit risk
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially all of the Company’s cash is maintained with state-owned banks within the PRC, and no deposits are covered by insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts. A significant portion of the Company’s sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables is limited due to generally short payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Stock-based compensation
The Company accounts for stock options and other equity based compensation issued to employees in accordance with ASC 718 “Share-Based Payment”. ASC 718 requires companies to recognize an expense in the statement of income at the grant-date of stock options and other equity based compensation issued to employees. There were no options outstanding as of December 31, 2010 and 2009. The Company accounts for non-employee share-based awards in accordance with ASC 505-50 “Equity-based payments to non-employees”
Shipping
Shipping costs are expensed as incurred and included in selling expenses. Shipping costs amount to $0 and $292 for the years ended December 31, 2010 and 2009, respectively. During the year ended December 31, 2010, the shipping costs were paid by the Company’s customers.
Advertising
Advertising is expensed as incurred and is included in selling expenses. Advertising expenses amounted to $0 and $46,450 for the years ended December 31, 2010 and 2009, respectively.
Research and development
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and salaries paid for the development of the Company’s products and depreciation related to facilities used and fees paid to third parties. Research and development expenses amounted to $86,545 and $0 for the years ended December 31, 2010 and 2009, respectively.
F-12
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Foreign currency translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the parent company is the U.S. dollar, and the functional currency of the Company’s operating subsidiaries and affiliates is Chinese Renminbi (“RMB”). For the subsidiaries and affiliates whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. Translation adjustments resulting from the process of translating the local currency financial statements into U.S. dollars are included in determining comprehensive income. The cumulative translation adjustment and effect of exchange rate changes on cash for the years ended December 31, 2010 and 2009 was $67,024 and $4,421, respectively. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
All of the Company’s revenue transactions are transacted in the functional currency. The Company does not enter any material transaction in foreign currencies and, accordingly, transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
Asset and liability accounts at December 31, 2010 and 2009 were translated at 6.6118 RMB to $1.00 and at 6.8372 RMB to $1.00, respectively, which were the exchange rates on the balance sheet dates. Equity accounts were stated at their historical rate. The average translation rates applied to the statements of income for the years ended December 31, 2010 and 2009 were 6.77875 RMB and 6.84088 RMB to $1.00, respectively. Cash flows from the Company’s operations are calculated based upon the local currencies using the average translation rate. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Earnings per common share
Basic earnings per share is computed by dividing net income available to common shareholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. Potentially dilutive common shares consist of common shares issuable upon the conversion of series A preferred stock (using the if-converted method) and common stock warrants (using the treasury stock method).
All share and per share amounts used in the Company’s consolidated financial statements and notes thereto have been retroactively restated to reflect the 2-to-1 reverse split, which occurred on December 31, 2010.
The following is a reconciliation of the basic and diluted earnings per share computations for the years ended December 31, 2010 and 2009:
| 2010 |
| 2009 | ||
Net income for basic earnings per share | $ | 14,424,943 |
| $ | 16,432,294 |
Weighted average shares used in basic computation |
| 26,177,900 |
|
| 22,104,928 |
Earnings per share: |
|
|
|
|
|
Basic | $ | 0.55 |
| $ | 0.74 |
F-13
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Earnings per common share (continued)
Diluted earnings per share: |
|
|
|
|
|
| 2010 |
| 2009 | ||
Net income for basic earnings per share | $ | 14,424,943 |
| $ | 16,432,294 |
Add: interest expense |
| 24,277 |
|
| — |
Net income for diluted earnings per share |
| 14,449,220 |
|
| 16,432,294 |
Weighted average shares used in basic computation |
| 26,177,900 |
|
| 22,104,928 |
Diluted effect of warrants |
| 113,686 |
|
| 34,100 |
Diluted effect of convertible preferred stock |
| 704,811 |
|
| 2,884,163 |
Weighted average shares used in diluted computation |
| 26,996,397 |
|
| 25,023,191 |
Earnings per share: |
|
|
|
|
|
Diluted | $ | 0.54 |
| $ | 0.66 |
For the years ended December 31, 2010 and 2009, a total of 200,000 and 1,863,500 warrants, respectively, have not been included in the calculation of diluted earnings per share due to their anti-dilutive effect.
Accumulated other comprehensive income
The Company follows ASC 220 “Reporting Comprehensive Income” to recognize the elements of comprehensive income. Comprehensive income is comprised of net income and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. For the Company, accumulated other comprehensive income consisted of unrealized gains on foreign currency translation adjustments from the translation of financial statements from Chinese RMB to US dollars.
Segment reporting
ASC 280 requires use of the “management approach” model for segment reporting. The management approach model is based on how a company’s management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company. During the years ended December 31, 2010 and 2009, the Company operated in two business segments - (1) Wholesales segment and (2) Retail segment.
Recent accounting pronouncements
In December, 2009, FASB issued ASU No. 2009-17, Improvement to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standard Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No.167, Amendments to FASB Interpretation No. 46 (R). The amendments in this Accounting Standards Update replace the quantitative-based risks and rewards calculation for determining which reporting entity, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which reporting entity has the power to direct the activities of a variable interest entity that most significantly impacts the entity's economic performance and (1) the obligation to absorb losses of the entity or (2) the right to receive benefits from the entity. An approach that is expected to be primarily qualitative will be more effective for identifying which reporting entity has a controlling financial interest in a variable interest entity. The amendments in this Update also require additional disclosures about a reporting entity's involvement in variable interest entities, which will enhance the information provided to users of financial statements. The adoption of this ASU did not have a material impact on the Company’s consolidated financial statements.
In April 2010, the FASB issued Accounting Standards Update 2010-13, “Compensation—Stock Compensation (Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades,” or ASU 2010-13. This Update provides amendments to Topic 718 to clarify that an employee share-based payment award with an exercise price denominated in currency of a market in which a substantial porting of the entity’s equity securities trades
F-14
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Recent accounting pronouncements (continued)
should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. The Company does not expect the adoption of ASU 2010-17 to have a significant impact on its consolidated financial statements.
Reclassifications
Certain prior year amounts (such as: rent expense for retail stores was reclassified from cost of revenues to general and administrative expenses; other revenue was reclassified into other income; payments for Inner Mongolia land use right was reclassified from deposits and installments on intangible assets to land use right.) have been reclassified to conform to the current year presentation. These reclassifications have no material impact on the previously reported financial position, results of operations and cash flows.
NOTE 2 – INVENTORIES
At December 31, 2010 and 2009, inventories consisted of the following:
|
| 2010 |
| 2009 |
Raw materials | $ | 157,165 | $ | 773,211 |
Packaging materials |
| 4,830 |
| 16,023 |
Finished goods |
| 472,588 |
| 250,633 |
| $ | 634,583 | $ | 1,039,867 |
NOTE 3 – PREPAID EXPENSES
At December 31, 2010 and 2009, prepaid expenses consist of the following:
|
| 2010 |
| 2009 |
Prepaid service fee | $ | — | $ | 2,121,250 |
Prepaid research and development expenses |
| 453,734 |
| — |
Prepaid rent expense |
| 123,343 |
| 95,024 |
Total |
| 577,077 |
| 2,216,274 |
Less: current portion |
| (577,077) |
| (856,691) |
Long term assets | $ | — | $ | 1,359,583 |
NOTE 4 – PROPERTY AND EQUIPMENT
At December 31, 2010 and 2009, property and equipment consists of the following:
| Useful Life |
| 2010 |
| 2009 |
Office equipment and furniture | 3 - 8Years | $ | 254,564 | $ | 244,490 |
Manufacturing equipment | 10 - 15Years |
| 5,780,040 |
| 5,589,490 |
Building and building improvements | 20 - 40Years |
| — |
| 2,339,612 |
Construction-in-progress |
|
| 43,361,865 |
| 12,620,225 |
Total |
|
| 49,396,469 |
| 20,793,817 |
Less: accumulated depreciation |
|
| (2,798,635) |
| (2,849,233) |
impairment on construction-in-progress |
|
| (7,259,899) |
| (1,720,809) |
|
| $ | 39,337,935 | $ | 16,223,775 |
F-15
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – PROPERTY AND EQUIPMENT (Continued)
At December 31, 2010, construction in progress amounted to $43,361,865, representing (i) costs incurred for construction of a new manufacturing plant of approximately $7 million located in Cha Ha Er Industrial Park in Inner Mongolia, China, and (ii) costs incurred for construction of a new building of approximately $36 million in Beijing, China.
Upon completion of the construction in progress and ready for their intended uses, the assets will be classified to their respective property and equipment categories.
For the year ended December 31, 2010, depreciation expense amounted to $480,046, of which $0 was included in cost of sales since the Company did not perform any production during the year ended December 31, 2010. For the year ended December 31, 2009, depreciation expense amounted to $425,895, of which $399,731 was included in cost of sales.
The Company purchased 1,000 MU (approximately 667,000 square meters) of land in Inner Mongolia in 2008. The Company had originally intended to build a pharmaceutical manufacturing and storage facility on a portion of the property. The construction began in August 2008 and stopped because priority of capital expenditure was given to the need to build the new building complex in Beijing as the relevant land was upgraded to industrial use in 2009. In March 2010, the Company began construction on a facility in Beijing after receiving permission from the Beijing Chaoyang District Planning Bureau. The new building will house the Company’s research and development center, manufacturing operations, storage facilities, sales and administrative offices and employee apartments. The building will become the Company’s new corporate headquarters following its completion and management expects to move into the building by the end of 2011. The Company’s management currently believes it is probable that the Company will not move forward with the construction of the Company’s planned facility in Inner Mongolia in order to focus the Company’s efforts and resources on expanding its core business in Beijing. Therefore, during the year ended December 31, 2010, the Company recorded an impairment loss on the entire construction-in-progress related to the manufacturing plant in Cha Ha Er Industrial Park in Inner Mongolia of $6,762,659. During the year ended December 31, 2009, the Company recorded an impairment loss on property and equipment in the amount of $1,719,884 associated with a portion of a Beijing En Ze Jia Shi building being demolished to make room for the construction of the new building.
NOTE 5 – LAND USE RIGHT HELD FOR DEVELOPMENT
The Company purchased 1,000 MU (approximately 667,000 square meters) of land in Inner Mongolia in 2008 (See note 4). The area for Inner Mongolia land which was held for development is 900 MU (approximately 600,000 square meters). The area for the rest of Inner Mongolia land on which the Company expects to build a facility is 100 MU (approximately 67,000 square meters).
Land use right held for development is accounted for at the lower of cost or market. It is considered as long-term asset and free of amortization as it is not used in operations. Management would like to co-develop the land with another entity, but such partner has not been found yet. The Company evaluated the fair value of the land use right as of December 31, 2010 and concluded that the fair value of the right exceeded the carrying value and no impairment loss is recorded.
As of December 31, 2010 and 2009, land use right held for development consists of the following:
|
| Lower of cost or market | ||
|
| 2010 |
| 2009 |
Inner Mongolia land use right – 900 MU | $ | 29,236,891 | $ | — |
Total | $ | 29,236,891 | $ | — |
NOTE 6 – DEPOSITS AND INSTALLMENTS ON INTANGIBLE ASSETS
Deposit and Installments on a Chinese Class I drug patent-Laevo-Bambuterol
Pursuant to the technology transfer agreement the Company entered into in April 2008 (See note 14 for Technology Transfer Agreement), the Company made a deposit to acquire a Chinese Class I drug patent. Accordingly, the Company recorded $3,024,895 (RMB 20 million) as a deposit on a patent in 2008. Such deposit will be returned to the Company after all milestone payment in connection with the technology transfer agreement has been made.
F-16
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – DEPOSITS AND INSTALLMENTS ON INTANGIBLE ASSETS (Continued)
Also, the Company has arranged an installment payment plan on the Chinese Class I drug patent to obtain the patent based on clinic milestones, as stipulated in the signed contract. Through December 31 2009 and 2010, the Company made $5,596,056 (RMB 37 million) as installment payments on the intangible assets as of December 31, 2010. The Company will need to make additional installment payments of approximately $1.66 million (RMB 11 million) to obtain the patent. The various milestone payments have been recorded as an asset and not expensed because all such payments are fully refundable if the studies are not ratified by the SFDA.
In addition, the Company expects to incur approximately $7.56 million (RMB 50 million) in research and development expense related to the Laevo-Bambuterol drug that has entered in Clinical Trial I in the next two years.
Installments on Gliclazide-Controlled Release Tablets
Pursuant to the new drug patent transfer agreement the Company entered into in February 2009 (See note 13 for New Drug Patent Transfer Agreement), the Company has made the first installment to the transferor to obtain the patent. Hence, the Company recorded $907,468 (RMB 6 million) as installment payment on intangible assets as of December 31, 2010. In order to acquire the patent, the Company needs to make additional installments of approximately $454,000 (RMB 3 million). These payments have been recorded as an asset as they are fully refundable if the studies are not ratified by the SFDA.
In addition, the Company expects to incur approximately $1.21 million (RMB 8 million) in research and development expense related to the Gliclazide-Controlled Release Tablets that was accepted by the China SFDA for medicine registration application in the next two years.
NOTE 7 – LAND USE RIGHT AND OTHER INTANGIBLE ASSETS
On October 9, 2006, the Company entered into a five-year loan agreement (the “Loan Agreement”) and a contract with Wu Lan Cha Bu Emergency Hospital (“Wu Lan”), whereby the Company agreed to lend Wu Lan approximately $4.5 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan agreed to grant the Company an exclusive right to supply all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty years. In October 2006, the Company’s chief executive officer, Dr. Zhongyi Liu (hereafter, “Dr. Liu”), made this loan to Wu Lan on behalf of the Company. On October 21, 2006, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Dr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Dr. Liu accepted the assignment with all the risks and obligations but no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Dr. Liu compensation for approximately $1.3 million (RMB 9 million) in five (5) equal annual installments of approximately $272,000 (RMB 1.8 million) commencing October 21, 2006. Accordingly, the Company recorded an intangible asset of approximately $1.3 million (RMB 9 million) related to the exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. The Company amortizes this exclusive right over a term of 20 years.
The Company entered into an intellectual rights transfer contract with Beijing Yipuan Bio-Medical Technology Co., Ltd. in December 2008 to acquire the drug property right of Yipubishan. The intellectual property right is valued at a fixed amount of RMB 54 million (approximately $8 million). The Company paid the transfer fee in full for the intellectual property right to Yipuan. The intellectual property right has a term of 10 years and will not expire until December 31, 2018. The Company amortizes the intellectual property right over the term of the intellectual property right.
All land in the PRC is owned by the PRC government and cannot be sold to any individual or company. The Company has recorded the amounts paid to acquire long-term interests to utilize land underlying the Company’s facilities as land use rights. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on the straight-line method over the terms of the land use rights, which range from 40 to 50 years. The Company acquired one parcel of land use right in Beijing and the other parcel of land use right in Inner Mongolia, China. The Company has received land use right certificate on the parcel of land in Beijing. However, as of the filing date of this report, the Company has not received land use right certificate on the parcel of land in Inner Mongolia. The delay is common in China. As described in elsewhere in this reporting, the area for the Inner Mongolia land on which the Company expects to build a facility is 100 MU (approximately 67,000 square meters).The Company acquired the parcel of Beijing land use right in the amount of approximately $10.1 million (RMB 66,769,504) and the 100 MU (approximately 67,000 square meters) Inner Mongolia land use right in the amount of approximately $3.4 million (RMB 22,370,000), which are included in intangible assets.
F-17
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – LAND USE RIGHT AND OTHER INTANGIBLE ASSETS (Continued)
At December 31, 2010 and 2009, intangible assets consist of the following:
|
| 2010 |
| 2009 |
Revenue rights | $ | 1,361,203 | $ | 1,316,328 |
Intellectual rights |
| 8,167,216 |
| 7,897,970 |
Land use rights |
| 13,481,277 |
| 42,477,842 |
Software |
| 11,192 |
| 10,823 |
|
| 23,020,888 |
| 51,702,963 |
Less: accumulated amortization |
| (2,480,982) |
| (1,814,535) |
|
|
|
|
|
| $ | 20,539,906 | $ | 49,888,428 |
Amortization expense amounted to approximately $1,767,722 and $1,560,466 for the years ended December 31, 2010 and 2009, respectively.
The projected amortization expense attributed to future years is as follows:
Years ending December 31: |
| Expense |
2011 | $ | 1,202,506 |
2012 |
| 1,200,741 |
2013 |
| 1,200,741 |
2014 |
| 1,200,741 |
2015 and thereafter |
| 15,735,177 |
|
|
|
| $ | 20,539,906 |
NOTE 8 – RELATED PARTY TRANSACTIONS
Notes payable – related parties
Notes payable - related parties consisted of the following at December 31, 2010 and 2009:
|
| 2010 |
| 2009 |
Note to Guoan Song, father of Zhenghong Song who is the spouse of the company’s CEO, Zhongyi Liu, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% and 4.75% at December 31, 2010 and 2009, respectively), and unsecured | $ | 789,826 | $ | 763,788 |
|
|
|
|
|
Note to Guixin Zheng, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% and 4.75% at December 31, 2010 and 2009, respectively), and unsecured |
| 1,709,822 |
| 1,653,455 |
|
|
|
|
|
Note to Zhaozhao Ma, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% and 4.75% at December 31, 2010 and 2009, respectively), and unsecured |
| 684,779 |
| 662,204 |
|
|
|
|
|
Note to Zhongyi Liu, CEO and director, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% and 4.75% at December 31, 2010 and 2009, respectively), and unsecured |
| 1,457,643 |
| 1,409,590 |
|
|
|
|
|
Note to Zhenghong Song, spouse of the Company’s Chief Executive Officer, Zhongyi Liu, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% and 4.75% at December 31, 2010 and 2009, respectively), and unsecured |
| 599,759 |
| 579,986 |
|
|
|
|
|
Total notes payable – related parties, long term | $ | 5,241,829 | $ | 5,069,023 |
F-18
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – RELATED PARTY TRANSACTIONS (Continued)
Notes payable – related parties (continued)
For the years ended December 31, 2010 and 2009, the Company recorded a total interest expense of $239,629 and $237,452 related to those loans, respectively.
Due to related parties
The Chief Executive Officer of the Company Dr. Zhongyi Liu and his spouse and several key employees of the Company, from time to time, provided advances to the Company for working capital purposes. During the years ended December 31, 2010 and 2009, the Company did not repay any of these advances. At December 31, 2010 and 2009, the Company had a payable to its Chief Executive Officer and his spouse and other employees at an amount of $744,879 and $720,323, respectively. These advances are short-term in nature and non-interest bearing and unsecured.
As discussed in Note 6, the Company entered into a five-year loan agreement and a contract with Wu Lan, whereby the Company agreed to lend Wu Lan approximately $4.5 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. The Company’s CEO, Dr. Liu, made this loan to Wu Lan on behalf of the Company. In return, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Dr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Dr. Liu accepted the assignment with all the risks and obligations but had no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Dr. Liu compensation for an aggregate of approximately $1.3 million (RMB 9 million) in 5 equal annual installments of approximately $272,000 (RMB 1.8 million) started from October 21, 2006.
For the years ended December 31, 2010 and 2009, the Company did not pay anything to Dr. Liu for the liability incurred by the assignment in the agreement mentioned above. At December 31, 2010 and 2009, amounts due under this assignment agreement were $1,068,827 and $1,033,592, respectively, of which $0 and $263,266 were included in long-term liabilities, and have been included in due to related parties on the accompanying consolidated balance sheets, respectively.
At December 31, 2010 and 2009, the Company has recorded accrued interest relating to notes payable - related parties of $869,067 and $602,836, respectively, which have been included in due to related parties – long-term on the accompanying consolidated balance sheets.
The accrued and unpaid interest relating to notes payable will be paid in full on the due date of the notes in accordance with the loan agreement. Therefore, the accrued interest is long-term in nature.
During the fiscal year of 2010, the Chief Executive Officer of the Company, Dr. Zhongyi Liu, from time to time, made payments to various unrelated third parties on behalf of the Company. At December 31, 2010, the Company owed its Chief Executive Officer $228,670. These advances are short-term in nature, non-interest bearing and unsecured.
For the years ended December 31, 2010 and 2009, a summary of activities in due to related parties is as follows:
|
| Assignment fee payable |
| Working capital advances |
| Accrued interest on notes payable |
| Payments made on behalf of the Company |
| Total |
Balance – December 31, 2008 | $ | 1,031,028 | $ | 718,536 | $ | 364,350 | $ | — | $ | 2,113,914 |
Additions |
| — |
| — |
| 237,580 |
| — |
| 237,580 |
Foreign currency fluctuations |
| 2,564 |
| 1,787 |
| 906 |
| — |
| 5,257 |
Balance- December 31, 2009 |
| 1,033,592 |
| 720,323 |
| 602,836 |
| — |
| 2,356,751 |
Additions |
| — |
| — |
| 245,680 |
| 228,670 |
| 474,350 |
Foreign currency fluctuations |
| 35,235 |
| 24,556 |
| 20,551 |
| — |
| 80,342 |
Balance- December 31, 2010 | $ | 1,068,827 | $ | 744,879 | $ | 869,067 | $ | 228,670 | $ | 2,911,443 |
F-19
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – OTHER PAYABLES AND ACCRUED EXPENSES
At December 31, 2010 and 2009, other payables and accrued expenses consist of the following:
|
| 2010 |
| 2009 |
Construction payable | $ | 120,996 | $ | 315,718 |
Refundable deposit |
| 153,513 |
| 148,452 |
Accrued sales commission |
| 1,512,447 |
| 1,316,328 |
Accrued payroll and employees benefit |
| 1,280,394 |
| 504,031 |
Accrued interest for convertible redeemable preferred stock |
| — |
| 284,524 |
Other |
| 374,116 |
| 121,631 |
| $ | 3,441,466 | $ | 2,690,684 |
NOTE 10 – CONVERTIBLE REDEEMABLE PREFERRED STOCKS
On February 25, 2008 (“Closing Date”), the Company sold, pursuant to a Convertible Redeemable Preferred Share and Warrant Purchase Agreement (the “Purchase Agreement”) by and among the Company, Dr. Zhongyi Liu and Mrs. Zhenghong Song (the “Founders”), and accredited investors (each a “Purchaser” and collectively, the “Purchasers”), 5,747,118 shares of the Company’s Series A Convertible Redeemable Preferred Stock (the “Preferred Stock”) and warrants (the “Warrants”) to purchase 1,436,777 shares of the Company’s common stock, in a private placement (the “February 2008 Private Placement”) pursuant to Regulation D under the Securities Act of 1933, for an aggregate purchase price of $5 million (the “Transaction”). Net proceeds, exclusive of expenses of the transaction were $4.6 million in cash, after the Company paid fees of approximately $469,000 in cash, of which $400,000 was paid to Maxim Group, LLC, the placement agent for the Transaction. The Company recorded $796,133 in fees, of which $327,565 was related to the value of the warrants granted to the placement agent, as a deferred debt cost and amortized approximately $52,226 and $412,184 of the deferred cost during the years ended December 31, 2010 and 2009, respectively. The convertible redeemable preferred stock was deemed debt due to the mandatory redeemable feature of the Preferred Stock according to ASC 480 "Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity".
The Company used $2,576,556 of the net proceeds of the Transaction to repay in full all of its outstanding principal obligations including accrued interest under the 14% Secured Convertible Notes due February 2008, and the Company used the remainder of the net proceeds for working capital and general corporate purposes.
Pursuant to the Purchase Agreement, the Company issued to the Purchasers an aggregate of 5,747,118 shares of the Company’s Preferred Stock, par value $0.001 per share, at a price equal to $0.87 per share (the “Preferred Shares”). Each of these Preferred Shares was convertible into 0.5 share of the Company’s common stock (as adjusted for stock splits, stock dividends, reclassification and the like), pays an 8% dividend annually, payable in additional Convertible Preferred Shares and also pays any dividend to be paid on the common shares on an as-converted basis. Until May 25, 2010, the Preferred Shares could be redeemed at the option of the Purchasers at the redemption price of $0.87 per share (as adjusted for stock splits, stock dividends, reclassification and the like), and no other capital stock of the Company may be redeemable prior to the Preferred Shares. Holders of Preferred Shares may not convert Preferred Shares to common shares if the conversion would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of Preferred Shares on not less than 61 days written notice to the Company. In addition, the Company issued to the Purchasers warrants to purchase up to 1,436,777 shares of the Company’s common stock in the aggregate.
The Warrants have an exercise price of $2.41. The warrants are exercisable for a period of five years from the closing date. Holders of the warrants may not exercise the warrants if the exercise would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of the warrants on not less than 61 days written notice to the Company.
The warrants issued to the investors to purchase 1,436,777 shares of the Company’s common stock were treated as a discount on the convertible redeemable preferred stocks and were valued at $1,188,926 and had been amortized over the term of the Purchase Agreement. Additionally, the convertible redeemable preferred stocks were considered to have an embedded beneficial conversion feature (BCF) because the effective conversion price was less than the half of the fair value of the Company’s common stock on February 25, 2008. The value of the beneficial conversion feature was $1,121,337 and was recorded as a discount on the convertible
F-20
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – CONVERTIBLE REDEEMABLE PREFERRED STOCKS (Continued)
redeemable preferred stocks and had been amortized over the term of the Purchase Agreement which was two years. Hence, in connection with the issuance of the Preferred Shares and Warrants, the Company recorded a total debt discount of $2,310,263 that had been amortized over the term of the convertible preferred stocks. For the years ended December 31, 2010 and 2009, amortization of debt discount amounted to $151,553 and $1,196,106, respectively, had been included in interest expense.
The fair value of the warrants granted to the placement agent and issued to the investors with the private placement was computed using the Black-Scholes option-pricing model. Variables used in the option-pricing model include (1) risk-free interest rate at the date of grant (4.64%), (2) expected warrant life of 5 years, (3) expected volatility of 91.85%, and (4) expected dividend yield of 0.
On February 25, 2009, the Company issued 459,772 additional shares of Preferred Stock to the holders of Series A Preferred Stock for the first mandatory 8% annual dividends.
During the period from May 1, 2009 to December 31, 2009, the Company issued 36,925 shares of Preferred Stock to certain of its Series A preferred stockholders for their mandatory dividends since they fully converted their convertible redeemable preferred stock into common stock.
During the fiscal year 2009, 1,275,856 shares of Preferred Stock were converted into 637,928 shares of the Company’s common stock.
At December 31, 2009, 4,967,959 shares of preferred stock were still outstanding.
On February 25, 2010, the Company issued 369,319 additional shares of Preferred Stock to the holders of Series A convertible redeemable preferred stock for the second mandatory 8% annual dividends of $321,308. The price of the Preferred Stock was $0.87 per share which was less than the half of the market price of the Company’s common stock on February 25, 2010 and each of these Preferred Shares was convertible into 0.5 share of the Company’s common stock (as adjusted for stock splits, stock dividends, reclassification and the like). The Company recognized the beneficial conversion feature as an interest expense of $184,660, representing the difference between the fair market value of the 369,319 shares of the Preferred Stock, determined on an “as if converted” basis, and the price of the 369,319 shares of the Preferred Stock ($321,308) with a corresponding credit to additional paid-in capital of $184,660.
During the period from January 1, 2010 to May 25, 2010, 4,653,102 shares of Preferred Stock were converted into 2,326,551 shares of the Company’s common stock.
Under the designations of related agreements, rights and preferences of the Preferred Stock, the Company could be required to redeem the Preferred Stock at the option of the holder for a period of 90 days beginning on February 25, 2010. The redemption price which is equal to $0.87 per share plus any accrued but unpaid dividends must be paid in cash, in one lump sum within one month from the end of the 90 days period. Following the end of said 90 days period, the Series A Preferred Stock is not redeemable. None of preferred stockholders required redeeming the Preferred Stock by the end of the specific redemption period. Since the redemption feature of the Preferred Stock lapsed, the Company reclassified the existing carrying amount of the convertible Preferred Stock from debt to equity on May 26, 2010 in accordance with the provisions of ASC 480. Accordingly, the 684,176 shares of outstanding convertible Preferred Stock were recorded as equity on May 26, 2010. In connection with the reclassification into permanent equity, the Company reduced debt of $595,233 with a corresponding credit to Series A Preferred Stock (par value) of $684 and Additional paid-in capital of $594,549.
NOTE 11 – TAXES
Income Tax
The Company is subject to income taxes on an entity basis on income arising in or derived from the tax jurisdiction in which each entity is domiciled.
Lotus Pharmaceuticals Inc. was incorporated in the United States and has incurred an aggregate U.S. net operating loss of approximately $5,987,000 as of December 31, 2010, subject to the Internal Revenue Code Section 382, which places a limitation on the amount of taxable income that can be offset by net operating losses after a change in ownership. The net operating loss carries forward for United States income taxes, which may be available for offsetting against future taxable U.S. income. These carryforwards will expire, if not utilized, through 2030.
F-21
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – TAXES
Income Tax (continued)
Management believes that the realization of the benefits from these loss carryforwards appears uncertain due to the Company’s limited operating history and continuing losses for United States income tax purposes. Accordingly, the Company has provided a 100% valuation allowance on the deferred tax asset to reduce the asset to zero. Management will review this valuation allowance periodically and make adjustments as needed. The valuation allowance at December 31, 2010 and 2009 was approximately $2,036,000 and $1,252,000, respectively. The net change in the valuation allowance was an increase of approximately $784,000 and $484,000 during the years ended December 31, 2010 and 2009, respectively. The consolidated income is earned overseas and will continue to be indefinitely reinvested in overseas operations. Accordingly, no provision has been made for U.S. deferred taxes related to future repatriation of these earnings, nor is it practicable to estimate the amount of income taxes that would have to be provided if we concluded that such earnings will be remitted in the future.
Deferred tax assets and liabilities are provided for significant income and expense items recognized in different years for income tax and financial reporting purposes. Temporary differences, which give rise to a net deferred tax asset for the Company as of December 31, 2010 and 2009 is as follows:
|
| 2010 |
| 2009 |
Tax benefit of net operating loss carryforward | $ | 2,036,000 | $ | 1,252,000 |
Valuation allowance |
| (2,036,000) |
| (1,252,000) |
Net deferred tax asset | $ | — | $ | — |
Lotus Pharmaceutical International, Inc. was incorporated in the United States and it was an affiliated group of Lotus Pharmaceuticals Inc. for United States income tax purpose and it did not have any business activity. Therefore, no income tax provision was made for Lotus Pharmaceutical International, Inc. U.S. tax returns prior to 2008 are no longer subject to examination by tax authorities.
Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. and Lotus East were incorporated in the PRC and are subject to PRC income tax which is computed according to the relevant laws and regulations in the PRC.
Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. did not have any business activity for PRC income tax purpose. So, no income tax provision was made for Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd according to the related PRC income tax law.
Beijing Liang Fang was subject to 25% income tax rate since January 1, 2009. Located in Inner Mongolia, Liang Fang’s branch received income tax exemption for its fiscal 2010 and 2009 taxable income from the Cha You Qian Qi government situated in Inner Mongolia, P.R.C. on June 3, 2008.
The table below summarizes the differences between the U.S. statutory federal rate and the Company’s effective tax rate for years ended December 31, 2010 and 2009:
| 2010 |
| 2009 |
US statutory rates | 34.00% |
| 34.00% |
Foreign income not recognized in the US | (34.00%) |
| (34.00%) |
China statutory rates | 25.00% |
| 25.00% |
China income tax exemption | (23.63%) |
| (22.81%) |
Effective income tax rates | 1.37% |
| 2.19% |
The estimated tax savings as a result of the income tax exemption from Liang Fang’s branch for the years ended December 31, 2010 and 2009 amounted to $6,216,094 and $4,720,944, respectively. The net effect on earnings per share had the income tax been applied would decrease basic earnings per share for the year ended December 31, 2010 and 2009 from $0.55 to $0.31 and from $0.74 to $0.53, respectively.
F-22
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – TAXES (Continued)
Value Added Tax
Enterprises or individuals who sell commodities, engage in repair and maintenance or import and export goods in the PRC are subject to a value added tax, or VAT, in accordance with Chinese laws. The applicable VAT tax rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT).
VAT on sales and VAT on purchases amounted to $12,670,479 and $1,243,484 for the year ended December 31, 2010 and $9,607,184 and $979,731 for the year ended December 31, 2009, respectively. Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent. As of December 31, 2010 and 2009, the VAT payable amounted to $1,874,078 and $2,411,459, respectively.
Taxes Payable
At December 31, 2010 and 2009, taxes payable (prepaid) are as follows:
|
| 2010 |
| 2009 |
Value added tax | $ | 1,874,078 | $ | 2,411,459 |
Corporation income tax |
| (56,223) |
| 368,878 |
Other taxes |
| 206,710 |
| 351,571 |
Total | $ | 2,024,565 | $ | 3,131,908 |
NOTE 12 – STOCKHOLDERS’ EQUITY
Preferred Stock
During the period from October 1, 2010 through December 31, 2010, 77,069 shares of preferred stock were converted into 38,535 shares of common stock.
Common Stock and Additional Paid-in Capital
In January 2010, the Company issued an aggregate of 329,557 shares of common stock to Longview Fund LP for its cashless exercise of warrants.
In January 2010, the Company issued McLaughlin & Stern, LLP, warrants to purchase 75,000 shares of common stock at an exercise price of $3.82 per share in connection with legal services rendered. The fair value of the warrants granted to McLaughlin & Stern, LLP was computed using the Black-Scholes option-pricing model. Variables used in the option-pricing model include (1) risk-free interest rate at the date of grant (2.58%), (2) expected warrant life of 5 years, (3) expected volatility of 96.77%, and (4) expected dividend yield of 0.
In March 2010, the Company issued YA Global Master SPV Ltd. (“YA”) an aggregate of 104,059 shares of its common Stock as a payment for commitment fee of $300,000 in connection with a Standby Equity Distribution Agreement entered into with YA. The shares were valued at the fair value of $2.88 per share on the grant date.
In March 2010, the Company issued Gragnola Limited an aggregate of 100,000 shares of its common stock in connection with corporate affairs and development services to be rendered. The shares were valued at the fair value of $2.76 per share on the grant date. In connection with the issuance of these shares, the Company recorded consulting expenses of $276,000.
On May 24, 2010, the Company issued 50,000 shares of its common stock to a consultant in connection with the consulting service rendered and to be rendered. The shares were valued at the fair value of $2.10 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expenses of $47,775 and recorded prepaid expenses of $57,225 and amortized it over the rest of service period. For the year ended December 31, 2010, the amortization expenses related to this issuance was $57,225.
F-23
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – STOCKHOLDERS’ EQUITY (Continued)
Common Stock and Additional Paid-in Capital (continued)
In May 2010, the Company issued 95,261 shares of its common stock to its Chief Executive Officer in connection with the service rendered. The shares were valued at the fair value of $1.89 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expenses of $150,000 and recorded compensation expenses of $30,000.
On May 27, 2010, the Company issued 30,000 shares of its common stock to its six directors in connection with the service rendered and to be rendered. The shares were valued at the fair value of $2.20 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expenses of $18,750 and recorded prepaid expenses of $47,250 and amortized it over the rest of service period. For the year ended December 31, 2010, the amortization expenses related to this issuance was $47,250.
In July 2010, the Company issued 11,111 shares of its common stock to Mclaughlin & Stern, LLP in connection with legal service rendered. The shares were valued at the fair value of $1.80 per share on the grant date. In connection with the issuance of these shares, the Company recorded professional fees of $20,000.
During the period from January 1, 2010 through May 25, 2010, the Company issued 2,326,551 shares of common stock upon the conversion of 4,653,102 shares of series A preferred stock.
On December 31, 2010, the Company issued 25,000 shares of its common stock to RedChip in connection with investor relations service rendered. The shares were valued at the fair value of $2.59 per share on the grant date. In connection with the issuance of these shares, the Company recorded professional fees of $64,750.
2010 Stock Incentive Plan
The 2010 Stock Incentive Plan provides for the issuance of up to 1,500,000 shares of common stock to employees, directors, consultants and advisors of the Company and its subsidiaries. Awards may be in the form of stock options (including incentive stock options), stock appreciation rights, stock awards, and cash awards. The Plan is administered by the Company’s Board of Directors or a committee of the Board of Directors consisting of at least two directors who shall not be employees of the Company. Subject to the terms of the Plan, the Board of Directors as administrator has the sole discretion to select the directors, officers, employees, consultants and advisors who will
receive awards, determine the terms and conditions of the awards, and interpret the provisions of the Plan and outstanding awards. The Board of Directors generally may amend or terminate the Plan at any time and for any reason, except that share and other award limitations cannot be increased and minimum stock option and stock appreciation right exercise prices cannot be changed unless such a plan amendment is approved by the Company’s stockholders. If any award under the Plan is cancelled prior to its exercise or vesting in full, or if the number of shares subject to an award is reduced for any reason, the shares of the Company’s common stock that are no longer subject to such award will be returned to the available pool of shares reserved for issuance under the Plan. As of December 31, 2010, the Company has not made any grants under the 2010 Stock Incentive Plan.
Statutory Surplus Reserves
The Company is required to make appropriations to statutory surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory surplus reserve is required to be at least 10% of the after tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entities’ registered capital.
The statutory surplus reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of shares currently held by them, provided that the remaining statutory surplus reserve balance after such issue is not less than 25% of the registered capital.
F-24
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – STOCKHOLDERS’ EQUITY (Continued)
Statutory Surplus Reserves (continued)
Pursuant to the Company’s articles of incorporation, the Company is to appropriate 10% of its net profits as statutory surplus reserve up to 50% of the Company’s registered capital. Prior to June 30, 2010, the Company appropriated the required 50% of its registered capital to statutory reserves for Lotus East.
For the years ended December 31, 2010 and 2009, statutory surplus reserve activity was as follows:
|
| Statutory Surplus Reserve |
Balance – December 31, 2008 | $ | 3,750,529 |
Addition to statutory surplus reserves |
| 1,923,795 |
Balance – December 31, 2009 |
| 5,674,324 |
Addition to statutory surplus reserves |
| 565,878 |
Balance – December 31, 2010 | $ | 6,240,202 |
Stock Warrants
Stock warrants issued, terminated/forfeited, exercised and outstanding during the years ended December 31, 2010 and 2009 are as follows:
|
| Shares |
| Average Exercise price per share | ||
Warrants outstanding, December 31, 2008 |
|
| 2,583,500 |
| $ | 2.25 |
Warrants granted |
|
| — |
|
| — |
Warrants expired/forfeited |
|
| — |
|
| — |
Warrants exercised |
|
| — |
|
| — |
Warrants outstanding, December 31, 2009 |
|
| 2,583,500 |
|
| 2.25 |
Warrants granted |
|
| 75,000 |
|
| 3.82 |
Warrants expired/forfeited |
|
| — |
|
| — |
Warrants exercised |
|
| (657,500) |
|
| 1.74 |
Warrants outstanding, December 31, 2010 |
|
| 2,001,000 |
| $ | 2.47 |
The following table summarizes the shares of the Company’s common stock issuable upon exercise of warrants outstanding and exercisable at December 31, 2010:
Warrants Outstanding and Exercisable | ||||||||
| Exercise |
| Number |
| Weighted |
| Weighted | |
$ | 1.74 |
| 62,500 |
| 1.12 | $ | 1.74 | |
| 2.40 |
| 1,436,777 |
| 2.16 |
| 2.40 | |
| 2.41 |
| 301,723 |
| 2.16 |
| 2.41 | |
| 3.00 |
| 125,000 |
| 1.07 |
| 3.00 | |
$ | 3.82 |
| 75,000 |
| 4.03 |
| 3.82 | |
|
|
| 2,001,000 |
| 2.13 | $ | 2.47 | |
F-25
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – SEGMENT INFORMATION
The following information is presented in accordance with ASC 280, Disclosure about Segments of an Enterprise and Related Information. In the years ended December 31, 2010 and 2009, the Company operated in two reportable business segments: (1) Wholesale segment: the manufacture and distribution of pharmaceutical products, and other ancillary business activities performed in wholesale location and (2) Retail segment: the retailing of western and traditional Chinese medications and medical treatment equipment through its own ten drug stores and direct sales to other drug stores located in Beijing China, and other ancillary revenues generated from retail locations. The Company’s reportable segments are strategic business units that offer different products. They are managed separately based on the fundamental differences in their operations.
Information with respect to these reportable business segments for the years ended December 31, 2010 and 2009 was as follows:
For the year ended December 31, 2010 |
| Wholesale operations |
| Retail operations |
| Unallocated |
| Total |
Net revenues | $ | 51,412,468 | $ | 21,284,150 | $ | — | $ | 72,696,618 |
Cost of revenues |
| (16,801,703) |
| (16,114,496) |
| — |
| (32,916,199) |
Operating expenses |
| — |
| — |
| (25,257,935) |
| (25,257,935) |
Other income (expense) |
| — |
| — |
| 122,751 |
| 122,751 |
Income tax |
| — |
| — |
| (220,292) |
| (220,292) |
Net income | $ | 34,610,765 | $ | 5,169,654 | $ | (25,355,476) | $ | 14,424,943 |
For the year ended December 31, 2009 |
| Wholesale operations |
| Retail operations |
| Unallocated |
| Total |
Net revenues | $ | 44,842,525 | $ | 11,639,923 | $ | — | $ | 56,482,448 |
Cost of revenues |
| (16,700,366) |
| (8,351,354) |
| — |
| (25,051,720) |
Operating expenses |
| — |
| — |
| (13,453,914) |
| (13,453,914) |
Other income (expense) |
| — |
| — |
| (1,175,840) |
| (1,175,840) |
Income tax |
| — |
| — |
| (368,680) |
| (368,680) |
Net income | $ | 28,142,159 | $ | 3,288,569 | $ | (14,998,434) | $ | 16,432,294 |
The Company does not allocate operating expenses, other expense and income tax expense to its reportable segments, because these activities are managed at a corporate level. In addition, the specified amounts for depreciation and amortization, interest expense and income tax expense are not included in the measure of segment profit or loss reviewed by the chief operating decision maker and these specified amounts are not regularly provided to the chief operating decision maker. Therefore, the Company has not disclosed depreciation and amortization, interest expense and income tax expense for each reportable segment.
Asset information by reportable segment is not reported to or reviewed by the chief operating decision maker and, therefore, the Company has not disclosed asset information for each reportable segment. Substantially all of the Company’s assets are located in China.
NOTE 14 – COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company leases office and manufacturing space in Beijing, China and those leases expire through May 2019.
Future minimum rental payments required under these operating leases are as follows:
Years ending December 31, |
|
|
2011 | $ | 238,236 |
2012 |
| 184,846 |
2013 |
| 113,434 |
2014 |
| 113,434 |
2015 |
| 113,434 |
thereafter |
| 382,839 |
|
|
|
Total minimum lease payments | $ | 1,146,223 |
F-26
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – COMMITMENTS AND CONTINGENCIES (Continued)
Operating Leases (continued)
For the years ended December 31, 2010 and 2009, rent expense amounted to $308,164 and $301,994, respectively.
Technology Transfer Agreement
In April 2008, one of the Company’s affiliates, En Ze Jia Shi, entered into a Technology Transfer Agreement with Dong Guan Kai Fa Biological Medicine LTD (“Dong Guan”) pursuant to which Dong Guan agreed to transfer the technology material, new medicine research and rights to the Chinese patent of the anti-asthma new medicine R-BM to En Ze Jia Shi on an exclusive basis in exchange for a transfer technology fee of approximately $7.26 million (RMB 48 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
| · | complete the filing with the SFDA of the medicine’s clinical research ratification document, |
|
|
|
| · | complete the clinical research, |
|
|
|
| · | complete the medicine’s trial production, and |
|
|
|
| · | provide raw materials and formulation related documentation and apply for the new medicine certification and production approval. |
In addition to the payment of the technology transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at $7.56 million (RMB 50 million). Lotus East intends to use its working capital to fund the project’s costs.
Dong Guan is responsible for preparing and transferring the clinical research and application documents as well as assisting En Ze Jia Shi in the completing the clinical research and applying for the new medicine certification and production approval documents.
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
| · | Approximately $1.51 million (RMB 10 million) is due by the 15th business day following the receipt of the processing notice of the receipt of the clinical application and all related material from SFDA is received, |
|
|
|
| · | Approximately $1.21 million (RMB 8 million) is due by the 10th business day after the receipt of the medicine’s clinical ratification document, |
|
|
|
| · | Approximately $1.51 million (RMB 10 million) is due by the 15th business day after the medicine’s Phase I clinical study is completed and ratification from the SFDA is obtained, and |
|
|
|
| · | Approximately $3.03 million (RMB 20 million) is due by the 10th business day after the medicine’s Phase II clinical study is completed and ratification from the SFDA is obtained. |
En Ze Jia Shi paid Dong Guan a deposit of approximately $3.03 million (RMB 20 million) in April 2008 which is to be returned to En Ze Jia Shi within 10 days after the transfer technology fee is fully paid. In the event Dong Guan should be unable to timely return the deposit, it will pay En Ze Jia Shi a late fee and En Ze Jia Shi is entitled to damages for Dong Guan’s failure to timely return the deposit.
The intellectual property arising from the agreement will be jointly shared by the parties. In addition, En Ze Jia Shi has guaranteed that both parties must jointly apply for related government grants prior to when the new medicine is marketed. Upon receipt of the government grants En Ze Jia Shi guaranteed that the grant monies will be shared equally by both parties. As of December 31, 2010, the Company has not received any government grant. The agreement can be terminated by Dong Guan if En Ze Jia Shi should fail to make any of the aforedescribed payments in which event the patent rights would revert to Dong Guan and it is entitled to transfer the project rights to a third party.
F-27
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – COMMITMENTS AND CONTINGENCIES (Continued)
Technology Transfer Agreement (continued)
Once the drug is marketed, the Company is obligated to pay 3% of revenue derived from the drug to Dongguan.
Inner Mongolia New Facility
In June 2008, one of the Company’s affiliates, Liang Fang, entered into an agreement with Cha You Qian Qi Economy Commission, a governmental agency (“Cha You”) related to the construction of a pharmaceutical plant in Cha You’s Cha Ha Er Industrial Garden District in Inner Mongolia. The new facility, which will be comprised of approximately 40,000 square meters situated on 600 MU of land (approximately 400,200 square meters), will be used to expand Liang Fang’s business. The Company was subsequently granted the right to expand the land use right area to 1,000 MU (approximately 667,000 square meters). The project will require a total investment of RMB 320 million, or $48,398,318 besides the payment for the land use right. The construction of the project began in August 2008 and the Company anticipates that it will take a few years to complete the construction of the project.
Liang Fang intends to use its present working capital together with bank loans and/or government grants and/or third party finance to fund the project. The funds are required to be invested over the estimated construction period of the project. As of December 31, 2010, Liang Fang has paid approximately $3.4 million (approximately RMB 22.37 million) of the total investment. Liang Fang, however, has not secured either the bank loans or government grants and does not have sufficient working capital to complete this project without securing substantial funds from third party sources.
Under the terms of the agreement, Cha You agreed to abate fees associated with water resources, waste and other relative supplies for a period of 30 years and agreed to ensure that the land use tax to be paid by Liang Fang after it begins normal production will be at the lowest tax rate imposed for five years. Once the project is completed, for a period of eight years the local reserved portion of the imposed corporation income tax will be returned to Liang Fang.
New Drug Patent Transfer Agreement
In February 2009, one of the Company’s affiliates, En Ze Jia Shi, entered into a New Drug Patent Transfer Agreement with Beijing Huicheng Ruixiang Pharmaceutical Technology Co. LTD (“Huicheng”) pursuant to which Huicheng agreed to transfer the patent technology and related research materials about the Chinese drug of Gliclazide-Controlled Released Tablets to En Ze Jia Shi on an exclusive basis in exchange for a transfer patent fee of approximately $1.3 million (RMB 9 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
| · | Finishing other needed related technical materials of this new medicine and providing the legal invoice for raw materials and purchase agreement, |
|
|
|
| · | Providing enough raw materials, to enable Huicheng to prepare new medicine of 100,000 dosage units, and standard samples for experiments and research, |
|
|
|
| · | Providing the choice basis and quality standards of the package materials, |
|
|
|
| · | Paying for organization, seal, signature, field-exam, registration and related fees including registration and examination fees, registration evaluation fees etc. for the new medicine registration materials, |
|
|
|
| · | Completing clinical research and paying related fees if the new medicine is required for clinical research, and |
|
|
|
| · | Making payment to Huicheng according to specific schedule mentioned in the agreement. |
In addition to the payment of the patent transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at approximately $1.21 million (RMB 8 million). Lotus East intends to use its working capital to fund the project costs.
F-28
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – COMMITMENTS AND CONTINGENCIES (Continued)
New Drug Patent Transfer Agreement (continued)
Huicheng Ruixiang Pharmaceutical Technology Co. Ltd. is obligated to
| · | Provide the patent of the new medicine, |
|
|
|
| · | Provide the related technical materials and the original records of the experiments which satisfy the requirement of the fifth classified chemical drug registration by Drug Registration Administration Method (2005) issued by Chinese SFDA. The Huicheng has to provide above mentioned materials to En Ze Jia Shi in 30 days after it received first installment payment and qualified documents from En Ze Jia Shi, |
|
|
|
| · | Provide the new medicine registration samples with 100,000 dosage units, and |
|
|
|
| · | Supplement and improve the technical materials according to the requirement of the Evaluation Center of Chinese State Drug Administration. |
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
| · | Approximately $0.9 million (RMB 6 million) is due by the 90th business day following the receipt of the Notice of China Accepted Patent and Notice of Medicine Registration Application, |
|
|
|
| · | Approximately $0.2 million (RMB 1.5 million) is due by the 30th business day after the receipt of the medicine’s clinical ratification document, and |
|
|
|
| · | Approximately $0.2 million (RMB1.5 million) is due by the 30th business day after the production ratification from the SFDA is obtained. |
En Ze Jia Shi made the first installment of approximately $0.9 million (RMB 6 million) to Huicheng in May 2009 which is to be returned to En Ze Jia Shi if it cannot obtain the production ratification from the SFDA due to any fault caused by Huicheng.
Other Contingency
The Labor Contract Law of the People’s Republic of China, effective as of January 1, 2008, requires employers to assure the liability of the severance payments if employment relations are terminated by employers and employees have been working for the employers for at least two years. The Company has estimated its possible severance payments of approximately $15,000 as of December 31, 2010, which have not been reflected in its condensed consolidated financial statements.
NOTE 15 – CONCENTRATIONS
Customer
No customer accounted for more than 10% of the Company’s total sales for the year ended December 31, 2010 and 2009, respectively.
Suppliers
One major supplier provided approximately 14% of the Company’s purchases of raw materials and third party manufactured finished goods for the year ended December 31, 2010 and the Company did not have any amount of advances to the supplier as of December 31, 2010.
One major supplier provided approximately 25% of the Company’s purchases of raw materials and third party manufactured finished goods for the year ended December 31, 2009 and the Company did not have advances to this supplier as of December 31, 2009.
F-29
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 16 – SUBSEQUENT EVENTS
In January 2011, the Company issued 5,000 shares of its common stock to a lawyer in connection with legal service rendered. The shares were valued at the fair value of $2.27 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expense of $11,350.
In January 2011, the Company issued 80,000 shares of its common stock to its four newly appointed directors in connection with service rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded director fees of $41,000 and prepaid expense of $123,000 and will amortize it over the service period.
In January 2011, the Company issued 300,000 shares of its common stock to three persons in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded compensation expense of $153,750 and prepaid expense of $461,250 and will amortize it over service period.
In January 2011, the Company issued 40,000 shares of its common stock to a consultant in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded consulting expense of $20,500 and prepaid expense of $61,500 and will amortize it over the service period.
In January 2011, the Company issued 30,000 shares of its common stock to its vice president of corporate development in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded compensation expense of $15,375 and prepaid expense of $46,125 and will amortize it over the service period.
In January 2011, the Company issued 47,383 shares of its common stock to its chief executive officer in connection with services rendered. The shares were valued at the fair value of $2.216 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expense of $105,000.
In January 2011, the Company issued 451,263 shares of its common stock to its chief executive office in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.216 per share on the grant date. In connection with the issuance of these shares, the Company recorded compensation expense of $250,000 and prepaid expense of $750,000 and will amortize it over the service period.
In February 2011, the Company issued 30,000 shares of its common stock to a key person in connection with services rendered and to be rendered. The shares were valued at the fair value of $1.92 per share on the grant date. In connection with the issuance of these shares, the Company recorded compensation expense of $14,400 and prepaid expense of $43,200 and will amortize it over the service period.
F-30
