Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CTI BIOPHARMA CORP | d8k.htm |
| EX-99.1 - PRESS RELEASE - CTI BIOPHARMA CORP | dex991.htm |
| EX-99.3 - CELL THERAPEUTICS, INC. PRESENTATION SLIDES (FOR MEDIA) - CTI BIOPHARMA CORP | dex993.htm |
 Tosedostat
Exclusive Marketing License and Co-Development Agreement
Exhibit 99.2 |
 Forward looking statements
2
This presentation contains forward-looking statements within the meaning of the “safe
harbor” provisions of the Private Securities Litigation Reform Act of 1995. The
forward-looking statements contained in this presentation include statements about future financial and operating results,
and risks and uncertainties that could affect Cell Therapeutic Inc.’s (“CTI”) products
under development. These statements are based on management’s current expectations and
beliefs and are subject to a number of factors and uncertainties that could cause actual results to differ
materially from those described in the forward-looking statements. These statements are not
guarantees of future performance, involve certain risks, uncertainties and assumptions that are
difficult to predict, and are based upon assumptions as to future events that may not prove accurate.
Therefore, actual outcomes and results may differ materially from what is expressed herein. In any
forward-looking statement in which CTI expresses an expectation or belief as to future
results, such expectation or belief is expressed in good faith and believed to have a reasonable
basis, but there can be no assurance that the statement or expectation or belief will result or be
achieved or accomplished. The following factors, among others, could cause actual results to differ materially from those
described in the forward-looking statements: risks associated with preclinical, clinical
and sales and marketing developments in the biopharmaceutical industry in general and in particular including,
without limitation, the potential failure of Tosedostat to prove safe and effective (including
complete and overall response rates) for the treatment of blood related cancers and selected
tumors as determined by the U.S. Food and Drug Administration (the “FDA”) or other regulatory agencies in
the territories where CTI has exclusive rights, that the FDA may not accept the proposed clinical
trial design of Tosedostat and/or may request additional clinical trials, that clinical trials
may not demonstrate the safety and effectiveness of Tosedostat, that CTI cannot predict or guarantee
the pace or geography of enrollment of clinical trials of Tosedostat, including whether or not the
majority of the patients will be enrolled in the U.S., that CTI cannot predict or guarantee the
outcome or results of clinical trials of Tosedostat, that CTI’s cannot predict or guarantee whether the
exclusive marketing license and co-development agreement with Chroma Therapeutics Ltd (the
“Agreement”) will strengthen CTI’s business, financial condition, operating
results and prospects or the trading price of CTI’s securities, that CTI cannot predict or guarantee whether
milestones will be achieved pursuant to the Agreement, CTI’s ability to continue to raise capital
as needed to fund its operations, determinations by regulatory, patent and administrative
governmental authorities, competitive factors, technological developments, costs of developing, producing
and selling CTI’s products under development; and other economic, business, competitive, and/or
regulatory factors affecting CTI’s business generally, including those set forth in
CTI’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K
for its most recent fiscal year and its most recent Quarterly Report on Form 10-Q, especially in
the “Factors Affecting Our Operating Results” and “Management’s Discussion
and Analysis of Financial Condition and Results of Operations” sections, and its Current Reports on Form 8-K. Except
as may be required by law, CTI does not intend to update or alter its forward-looking statements
whether as a result of new information, future events, or otherwise.
|
 Product/Deal Highlights
Tosedostat: Novel tumor-selective targeted therapy
•
Oral, once daily dosing, well tolerated
Encouraging phase I/II data
•
27% mono-therapy response rate elderly r/r AML
•
Ongoing trials provide clinical news flow through 2012
Potentially rapid registration timeline: pivotal study
estimated to start Q4-2011
Leverages CTI’s access to and expertise in blood-related
cancer market
•
Potential development cost and sales synergies with pixantrone
Exclusive rights in Americas on reasonable deal terms
Targets billion dollar market applications
3 |
 Chroma Therapeutics
Public company expertise in a private company
•
Seasoned management and Board of Directors
•
Facilitates co-development interactions and synergies
Investor group
•
Abingworth, Wellcome Trust, Essex Woodlands, Nomura, GSK,
Gilde
Innovators in new targeted therapies harnessing
chromatin biology and novel cell accumulation (ESM*)
technology
Validating partnership with GlaxoSmithKline
4
* Esterase Sensitive Motifs |
 Tosedostat: Mechanism of Action
5
-
Targets members of aminopeptidase family
-
Induces genes characteristic of Amino Acid Deprivation response
(AADR)
-
AADR changes only seen in transformed (tumor) cells and not normal cells
|
 Consequences of amino acid
deprivation in leukemic cells
Cells attempt to solve deficiency (up-regulation of amino
acid transporters –
AADR response)
Cells switch off protein synthesis (e.g. remove mTOR
drive)
Cells switch on stress-related pathways (NFKB,
etc.) Cells produce pro-apoptotic regulators, CHOP, NOXA,
etc., that lead to cell death
Studies indicate that these effects are not seen
in either normal human cells treated
with Tosedostat or in insensitive tumor cells.
6 |
 Tosedostat Clinical Program
Blood-Related Cancers
7 |
 Acute Myeloid Leukemia (AML) Overview
One of the most common types of leukemia in adults
U.S. Incidence >12,000/yr
Median age at diagnosis –
67 years
Poor prognostic factors include
•
Elderly (>60 yo.)
•
Elevated WBC counts (>100,000/mm
3
)
•
Secondary AML resulting from other primary cancers
•
History of MDS or another antecedent hematologic disorder
Response rates and survival in elderly r/r disease is grim
(10%, <3months)
8 |
 Acute Myeloid Leukemia (AML) Overview
Cytarabine
(Ara-C)
and
anthracyclines
mainstay
1
st
line
•
~75% relapse within 1 year
Approximately 2/3 of elderly patients do not tolerate
intensive chemotherapy
•
70% of AML patients are >60yrs old
•
Up to 35% treatment related mortality
Significant unmet medical need in elderly AML for
•
Better tolerated agents
•
Oral, chronic dosing
•
Better efficacy
9 |
 Phase I/II (002) Study
10 |
 Phase I/II Study (002)
Objectives
•
Define MTD and anti-leukemic activity of oral Tosedostat
Study population
•
Elderly (>60yrs), r/r AML, MDS, MM not eligible for chemo
Phase I (3+3) designed for up to 84 days of dosing
•
60mg, 90mg, 130mg, 180mg (oral, 1xdaily)
•
MTD determined during first 28 days
Phase II determines activity in AML, high risk MDS
•
Bone marrow assessment days 28, 56, 84
•
Extended dosing >84 days permissible
11 |
 Phase I/II: Patient characteristics
* Not reported for 1 patient
Phase I
(n = 16)
Phase II
(n = 43)
Median age, years (range)
68.4 (45-84)
68 (35-83)
Male/Female
13/3
28/15
Diagnosis
No. (%)
No. (%)
AML
13 (81.3)
40 (93)
MDS
1 (6.3)
3 (7)
MM
2 (12.5)
0 (0)
ECOG at baseline
No. (%)
No. (%)*
0
8 (50.0)
16 (37.2)
1
6 (37.5)
18 (41.9)
2
2 (12.5)
8 (18.6)
12 |
 Phase I/II: Patient characteristics (cont)
* Excluding the 2 patients that did not receive CHR-2797 in phase II
Phase I
(n = 16)
Phase II
(n = 43)
Median time from diagnosis
3.1 years (0.1-8.8)
7 months (0-39)
Previous treatment*
Untreated
4 (25%)
12 (29.3%)
Cytotoxic Therapy
12 (75%)
29 (70.7%)
Median # priors
2 (range 0-8)
1 (range 1-8)
13 |
 Tosedostat: Treatment duration*
* Including
extended treatment
Dose
No. of patients
Median duration of
therapy, days (range)
Phase I
60 mg
3
140 (119-196)
90 mg
4
40 (13-174)
130 mg
6
69 (42-222)
180 mg
3
21 (9-77)
Phase II
130 mg
41
72 (1-471)
14 |
 Phase I/II: Dose Limiting Toxicities
The recommended dose of 130 mg for the phase II part was determined during
phase I and was based on the following DLT’s:
DLT
Dose
No. of patients
No. of
occurrences
ALT elevation
(Grade 3)
130 mg
1
1
Thrombocytopenia
(Grade 4)
180 mg
2
2
15 |
 Most common adverse events
in phase I-II: time of occurrence
Adverse Event
(Preferred Term)
Incidence
Day 1 -
28
Incidence
Day 28 -
End
Patients (n=57)
%
Patients (n=57)
%
Fatigue
11
19
19
33
Thrombocytopenia
23
40
4
7
Edema –
peripheral
15
26
7
12
Diarrhea
11
19
9
16
Pyrexia
9
16
11
19
Cough
3
5
12
21
Dyspnea
10
18
5
9
Epistaxis
8
14
7
12
Anemia
11
19
3
5
16 |
 Phase I/II: Efficacy (response rates)
Disease
Total #
patients
CR
PR
SD
AML
51
7*
7**
MDS
4
1
1
Myeloma
2
1
Phase I/II
AML
ORR (CR+PR)
27.5%
* 4 went into remission (duration 4-9 months); 1 cytogenetic response; 1
patient (0101) had a de facto CR (low volume BM tap) ** 1 PR was
short 17 |
 Overall Survival For AML Patients*
NR –
non responders
*Overall survival data for 2 out of the 38 AML patients is not known
**Overall survival data for 1 patient unknown
JCO 28:4333-4338
Disease
Number of
patients
Median Overall Survival
from first treatment with
Tosedostat
to death, days (range)
Phase I
All patients
AML
13
135 (40-420)
•
CR
AML
4
334 (73-420)
•
NR
AML
9
121 (40-402)
Phase II
All patients
AML
38*
126 (8-696)
•
CR
AML
3
272 (73-835)
•
PR
AML
7**
170 (31-505)
•
NR
AML
27**
100 (8-941)
18 |
 Phase I/II Study (002): Conclusions
Well-tolerated mono-therapy
•
Oral once-a-day dosing
•
Transient reduction in platelets
Encouraging phase I/II mono-therapy data
•
27% response rate in elderly r/r AML
•
All responders >60yrs, 79% with r/r disease
•
Overall survival data encouraging
Similar results in MDS warrant separate studies with
hypo-methylating agents
19 |
 Tosedostat Clinical Program
Blood Related Cancers -
AML
Randomized Controlled Trials
20 |
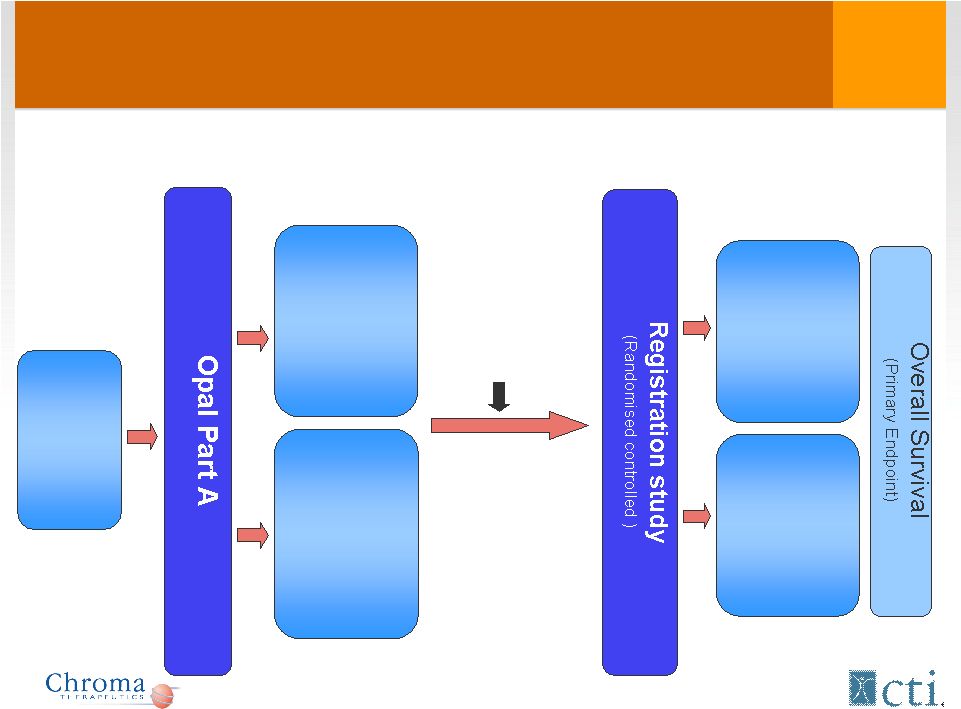 Proposed Pivotal Study Design
Tosedostat
+
Ara-C
6 months
Ara-C
6 months
Relapsed/
refractory
AML
(>60yrs)
35 patients
120 mg
6 months
35 patients
240 mg
(2 months)
120 mg
(4 months)
120 mg
240/120 mg
Complete: Q1 2011
Est. start: Q4 2011
Risk/benefit
review,
Dose
selection |
 1
st
Line AML (HOVON)
Phase I
Assessment of optimal
induction dosing regimen
Dose-finding
Tosedostat
+
dauno/ara-C
Phase II
Optimum induction
regimen and
maintenance
Optimal
Tosedostat
+
dauno/ara-C
regimen
Tosedostat
maintenance
Standard
dauno/ara-C
regimen
consolidation
Arm A
Arm B
•
Patients with AML, RAEB or
RAEB-t, previously untreated,
age >60 yrs, fit for standard
chemo
•
N=~270
•
Study open
22 |
 AML
Competitive Landscape 7 candidates in Phase III mostly cytotoxics
Elderly AML life-threatening
disease with poor survival (~3 months) low tolerability to
chemotherapy •
clofarabine iv NDA and laromustine iv NDA: Rejected
•
Mylotarg®
iv approval in relapsed AML: Withdrawn
•
decitabine iv: Failed primary O.S. endpoint in front-line AML •
lintuzumab iv: Failed primary O.S. endpoint in front-line AML •
sapcitibine : 1
st
line +/-decitabine elderly AML
•
Flt-3 kinase inhibitor
Oral, well-tolerated, chronic treatments improving
survival addresses a major unmet medical need
Tosedostat highly differentiated profile addressing
unmet medical need
23 |
 Tosedostat Clinical Program
Other Blood-Related Cancers
Phase I/II
24 |
 Myelodysplastic Syndrome (MDS)
Disease of elderly :
•
Lower-risk patients-
Lenalidomide;
•
Higher-risk patients -
HSCT (<10%)/ De-methylating agents
•
Age (>60yrs) –
high-intensity therapies not generally offered
Unmet medical need: low-intensity therapies
with improved efficacy and toxicity profiles
Tosedostat:
‘High risk MDS’
is attractive target
Two MDS (RAEB) patients did very well in study 002
Two AML patients were ex-MDS (AHD)
Phase I/II combination with decitabine planned
25 |
 Multiple Myeloma (MM)
Strong biologic and preclinical rationale
•
Tosedostat inhibits myeloma cell proliferation and induces apoptosis
in
cell
lines
and
primary
patient
cells,
with
minimal
effects
on
bone
marrow stroma (Faith Davies)
•
Tosedostat is synergistic with other anti-myeloma therapies
(dexamethasone, bortezomib) in vitro.
HDAC inhibitors being explored in MM
•
Potential synergy between Tosedostat and HDAC inhibitors
2 Planned phase I/II studies
•
Combination trial between Tosedostat and CHR-3996 (HDAC inhibitor)
planned in MM (Royal Marsden)
•
Combination with bortezomib
26 |
 Tosedostat: Conclusion
Tosedostat
is a novel aminopeptidase inhibitor with tumor
selective anti-proliferative activity
Well-tolerated oral agent (once daily)
Demonstrated synergy in combination with currently
approved targeted therapies
•
Bortezomib, decitabine, azacytidine, HDAC inhibitors
Potentially applicable across a variety of blood related
cancers and select solid tumors
Pivotal study (AML) estimated to begin in Q4-2011
27 |
 Summary Deal Terms & Structure
CTI gets exclusive development and marketing rights
in the Americas; Chroma retains ROW
Cost-sharing
•
CTI bears 75% of agreed-upon direct development expenses
•
Trials aimed at US/EU regulatory approval
Joint Governance (Development/Marketing Committees)
•
CTI lead party in America’s/Chroma lead in ROW
Development budget capped at $50mm over 3 years
•
75% CTI -
25% Chroma
Initial Payments
•
$5mm upon execution
•
$5mm upon initiation of r/r AML phase III pivotal trial
28 |
 Summary Deal Terms & Structure
Success-based Milestones
•
Scaled by number of potential patients in a given indication taking
into account line of approval (1
st
line Vs r/r)
•
Successful clinical trial results and NDA approvals
•
Blood-related cancers -
AML, MDS, or Myeloma
•
Solid Tumor indication <10,000 pts/yr >50,000 pts/yr
•
Sales Thresholds
Standard royalty rate scaled to increasing net sales
29 |
 Potential Market Opportunity
Blood-related Cancers is large US market opportunity
•
AML
~12,000 pts/yr
•
MDS
~12,000 pts/yr
•
Myeloma
~26,000 pts/yr
Estimated 2010 WW annual sales for drugs
approved/used in AML/MDS or Myeloma
•
AML/MDS (clofarabine, decitabine, azacytidine)
>$ 900 million
•
Myeloma (lenalidomide/bortezomib)
>$2.4 billion
30 |
 Indication
WW 2010
sales*
Patent
expiration
MDS
$200MM
2013
MDS
$534MM
2011
Ped. ALL
$103MM
2018
Multiple
Myeloma/Mantle Cell
$1.5B
2017
Multiple
Myeloma/Deletion 5q MDS
$2.5B
2019
Market Opportunity: Current agents
VELCADE
*Information from SEC filings.
31 |
 Summary
Late-stage opportunity potentially addressing unmet
medical needs in large markets
•
Potential 1
st
oral non-cytotoxic treatment for AML
•
Synergistic activity with “blockbuster”
products (Velcade®,
Dacogen®, Vidaza®, Revlimid®) in MDS, myeloma
•
Potential rapid registration timeline-
pivotal study to start Q4-2011
Good data visibility and news flow over next
18-24 months while phase III trial completes
Development and sales synergies with pixantrone
makes deal accretive at product launch
Partnership enhanced by Chroma’s seasoned
management and expertise
32 |
