Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AGENUS INC | d8k.htm |
 Corporate Presentation
March 2011
Exhibit 99.1 |
 2
Forward-Looking Statement
This presentation contains forward-looking statements including statements
regarding the company’s lead product candidates Oncophage
®
, Prophage Series, formerly known as
Oncophage, (vitespen; formerly HSPPC-96), QS-21 Stimulon
®
and HerpV, formerly AG-707,
(including, but not limited to, the timing of product development, launch and
product revenues), the successful development of the Company’s products
by collaborative partners, the company’s clinical trials (including,
but not limited to, trial initiation, enrollment, completion, analysis and
interpretation of results), the confirmation of Oncophage subgroup analyses, the
regulatory approval process (including, but not limited to, dates and locations of
filings), research programs and other future events and operations. These
forward-looking statements involve uncertainties and risks that are
described under Risk Factors of the Company’s Form 10-Q as filed
with the Securities and Exchange Commission for the period ended September 30,
2010. Actual
results could differ materially from these forward-looking statements.
|
 3
A New Name
•
Our pipeline has moved beyond
autologous
antigen-based technologies
•
Our strategy focuses advancing our platform
technologies through collaboration
•
www.agenusbio.com |
 4
Agenus Investment Highlights
•
QS-21
vaccine
adjuvant:
Royalty
potential
in
2013-2014
•
Oncophage®
•
Prophage Series of therapeutic cancer vaccines advancing in
clinic
•
HerpV in genital herpes |
 5
Product and Clinical Pipeline
Product
Indication
Phase 1
Phase 2
Phase 3
Market
Oncophage for Renal Cell Carcinoma
in Russia
Prophage for Glioma (recurrent)
Prophage for Glioma (newly diagnosed)
QS-21 in Non-Small Cell Lung Cancer QS-21 in
Melanoma QS-21 in Malaria
QS-21 in Shingles
partnered with GSK
partnered with GSK
partnered with GSK
partnered with GSK
QS-21 in Alzheimer’s Disease QS-21 in multiple undisclosed
vaccines partnered with Janssen
HerpV for Genital Herpes |
 6
QS-21: Maturing Pipeline,
Addressing
10B+ Market
•
Clinical indications under
investigation (global incidence)
–
NSCLC (lung: 1.5m)*
–
Alzheimer's disease (prevalence:
26.6m)**
–
Malaria (250m)**
–
HIV (2.6m)**
–
AML (leukemias: 331k)*
–
Tuberculosis (9.3m)**
–
Varicella-zoster (US: 1m)***
–
Prostate cancer (783k)*
–
Breast cancer (1.3m)*
–
Melanoma (132k)****
22
14
4
0
5
10
15
20
25
Total
Vaccines
Clinical
Phase 3
QS-21 Pipeline
*ACS Global Cancer Facts & Figures 2007
**Wikipedia
***NIAID
****WHO |
 7
QS-21: Significant Clinical Progress
•
GlaxoSmithKline
–
Phase 3 NSCLC study launched in 2007 (MAGE-A3)
•
Phase 3 (n=2,270) ongoing; largest trial conducted in NSCLC
•
Phase 2b (n=180): 25% reduction in recurrence vs. placebo
–
Phase 3 melanoma study launched Dec. 2008 (MAGE-A3)
•
Phase 3: n=1,300
–
Phase 3 malaria study launched March 2009
•
Phase 3: n=16,000, last patient expected to be dosed Feb. 2011
•
Phase 2: 53% reduction in clinical episodes of malaria
–
Phase 3 shingles study launched in 2010
•
Two Phase 3 studies, n=15,000 per study
–
GSK/Abbott MAGE-A3 diagnostic agreement
•
JANSSEN(J&J)/Pfizer
–
Phase 2 Alzheimer’s disease study launched in 2007
–
Janssen/GE collaboration to identify early markers of Alzheimer’s
|
 8
The PROPHAGE Series of Cancer Vaccines |
 9
PROPHAGE Series: 1
st
Approved
Cancer Vaccine
•
Patient-specific HSP-based therapeutic cancer vaccine
•
Theoretically applicable to almost any cancer
•
>850 patients worldwide safely treated in 8 cancers
•
Simple out-patient injection
•
Manufactured in less than 10 hours
•
Phase 3 adjuvant RCC data has strengthened over time |
 10
PROPHAGE Series Clinical Overview
•
Signals of clinical activity observed in 8 cancers (US incidence)*
–
Kidney (58,240)
–
Melanoma (68,130)
–
Brain (22,020)
–
Colorectal (142,570)
–
Lung (222,520)**
–
Pancreatic (43,140)
–
Gastric (21,000)
–
Non-Hodgkin’s lymphoma (65,540)
•
Efficacy signals include tumor-specific and innate immune
responses, tumor responses, RFS, PFS, OS across tumor types
•
Efficacy appears most pronounced in earlier-stage patients
•
Combination trials allow for more advanced disease
•
Well tolerated; low toxicity profile
*ACS Cancer Facts Figures 2010
**Manufacturing feasibility only |
 Oncophage®
Demonstrates
Statistical
Significance in ECOG Intermediate Risk RCC Patients |
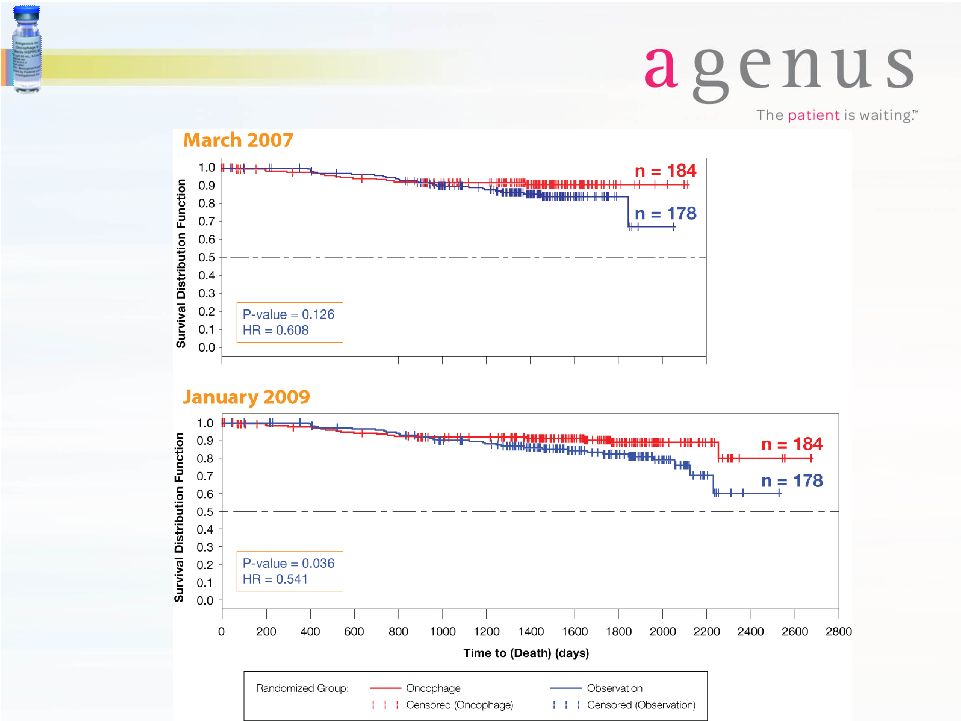 12
Overall Survival, Intermediate-Risk |
 13
PROPHAGE Opportunity: Glioma
•
Over 20,000 cases of brain cancer diagnosed each year in the US
•
Phase 2 trial underway in newly diagnosed glioma
–
Actively enrolling patients (n=50); recently expanded to 10 sites
–
Prophage + standard of care [radiation therapy plus Temodar (temozolomide)]
–
No significant toxicities observed to date
•
Phase 1/2 trial in recurrent glioma
–
Phase 2 portion actively enrolling patients (n=30)
–
Phase 2 survival data
•
Median survival 44 weeks, compared to historical median of 26 weeks
•
70% of patients survived beyond 36 weeks
•
40% survived up to or longer than 1 year
•
All patients tested mounted innate immune response, 92% mounted adaptive
CD4/CD8 t-cell response
•
Potential for accelerated registration pathway in the US
•
Investigator-sponsored (UCSF); funded by NCI, patient advocacy
groups |
 NCCN Practice Guidelines (2011)
Treatment
NCCN Practice Guidelines (2011)
Treatment
Overview: Recurrent GBM
Overview: Recurrent GBM
Agents listed in the practice
guidelines
Median OS Months
Platinum Based Regimens¹
7.4
PCV²
7.6
Cyclophosphamide³
4.0
Carmustine wafer
4
7.5
Temozolomide
5
Not Reported/median PFS 5 mos.
Bevacizumab
6
9.2
Prophage Series G-200
7
>10 months
1
Yung
WK,
et
al.
J
Clin
Oncol
1991
(Population
included
AA
and
GBM)
,2
Kappelle
AC,
et
al.
Neurology
2001
(Procarbazine,
Lomustine,
Vincristine
combination),
3
Chamberlain
MC,
Tsao-Wei,
DD.
Cancer
2004
4
Brem
et
al.
Lancet
1995,
5
Perry
et
al.
Cancer
2008,
6
Friedman
et
al.
J
Clin
Oncol
2009,
7
Parsa
et
al.
18th
International
Conference
for
Brain
Tumor
Research
and
Therapy |
 NCCN Practice Guidelines (2011)
NCCN Practice Guidelines (2011)
Treatment Overview: Newly Diagnosed GBM
Treatment Overview: Newly Diagnosed GBM
Agents listed in the practice guidelines
Median PFS/OS
Months
Carmustine
wafer¹
PFS 5.9
OS 13.9
Radiation/Temozolomide²
PFS 6.9
OS 14.6
XRT/TMZ +
Prophage Series G-100 (n=15)
N/A
1
Westphal et al. Neuro-Oncology 2003
2
Stupp et al. NEJM 2005 |
 16
"Significant Advances" (p< 0.05) in Epithelial Malignancies in
Patients Not Selected on the Basis of Molecular Characteristics
Drug
Tumor
Survival Gain (months)
•
Gemcitabine
Pancreas
1.5
•
Bevacizumab
Colon
2.2
•
Erlotinib
Pancreas
0.4
•
Bevacizumab
NSCLC
2
•
Sorafenib
Renal
2
•
Temozolamide
GBM
2.5
•
Docetaxel
Prostate
2.4
•
Topotecan
Cervix
2.3
•
Cetuximab
Colon
1.5
•
Erlotinib
NSCLC
2
•
Cetuximab
NSCLC
1.2
•
Bevacizumab
Breast
1.5
Data
from
Stewart
DJ,
Kurzrock
R.
Cancer:
the
road
to
Amiens.
J
Clin
Oncol
2009;27:328–33. |
 17
A New Era for Cancer Vaccines
•
Provenge FDA approval
•
Ipilimumab imminent FDA PDUFA date
•
Rationale for combinations with vaccines
•
Newly-created opportunity to use combinations |
 18
Genital Herpes and HerpV Overview
•
Genital herpes is the most prevalent viral STD
–
Approximately 55 million Americans/Europeans affected
–
80% of patients suffer symptomatic recurrences
•
Virus establishes life-long latency
•
HerpV is an off-the-shelf HSP-based therapeutic vaccine
candidate for treatment of genital herpes
•
Contains 32 immunogenic peptides of the herpes genome
•
Promising Phase 1 results
–
100% of evaluable patients receiving HerpV+QS-21 had CD4+T-cell
response
–
63% had CD8+ T-cell response; seeing both is unprecedented
–
Safety profile established
•
Potential to partner for Phase 2 and beyond
•
Potential platform technology |
 19
2011 Goals and Objectives
•
Continued clinical advancement of QS-21 vaccine adjuvant
–
Possible
Phase 3 data announcements
•
Partnerships for Oncophage®
in RCC in Europe/Russia
•
Advancement of Prophage in glioma (brain cancer)
–
Recurrent glioma data presentation mid-year
–
Exploration of accelerated US regulatory pathway
–
Initiation of pediatric trial mid-year
–
Potential for partnership
•
Initiation of Prophage Series combination trials
•
Development of HerpV in genital herpes
–
Data publication mid-year
–
Potential for partnership opportunity
•
In-licensing agreements and new corporate/academic
collaborations |
 20
Balance Sheet
December 31, 2010 ($ millions)
Cash and short-term investments
$19.8
Other current assets
1.1
Net plant and equipment
6.2
Other long-term assets
3.8
Total assets
$30.9
Current liabilities
$40.1
Long-term liabilities
5.5
Stockholders’
equity
(14.7) Total liabilities and stockholders’
equity
$30.9 |
