Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a6636525.htm |
Exhibit 99.1

Cowen and Company 31st Annual Health Care Conference Stanley G. Miele President, Sucampo Pharma Americas March 7, 2011 1 Sucampo Pharmaceuticals, Inc.

Forward-looking statements contained in this presentation are based on Sucampo’s assumptions events Sucampo’s and expectations concerning future events. They are subject to significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those reflected in forward-statements Sucampo’s forward-looking the looking statements. Sucampo s statements could be affected by numerous foreseeable and unforeseeable events and developments such as regulatory delays, the failure of clinical trials, the inability to fund drug development initiatives, competitive products and other factors identified in the “Risk Factors” section of Sucampo’s Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. While Sucampo may elect to update these statements at some point in the future Sucampo specifically disclaims any obligation to do so, whether as a result of new information, future events or otherwise. In light of the significant uncertainties inherent in the forward-looking information in this presentation, you are cautioned not to place undue reliance on these forward-looking statements. 2
Sucampo Has a Solid FoundationTwo FDA approved Platform of proprietary Solid financial Global compounds prostone technology position company 3

Unmet Medical Needs Gastroenterology Constipation One of the most common GI complaints in the U.S. Up to 42MM, of U.S. adults experience constipation IBS-C IBS – IBS affects 58 million people in the U.S and ~1/3 or ~19 million IBS-C Approximately 15% of IBS patients seek medical attention Ophthalmology Glaucoma One of the leading causes of blindness in the U.S Prevalence of 4.4MM U.S. Patients 1.3MM diagnosed with POAG 1.8MM diagnosed with Ocular Hypertension 4 NDDC. Constipation. Available at http://www.digestive.niddk.nih.gov/ddiseases/pubs/constipation. Accessed Dec. 3,2009 Higgins PD, et al. Am Journal of Gastroenterology. 2004; 99: 758 Freeman, Cathleenan, Cleveland Clinic, monograph. Aug 1, 2010 Arch of Opthalmology (2004) 122: 532-538
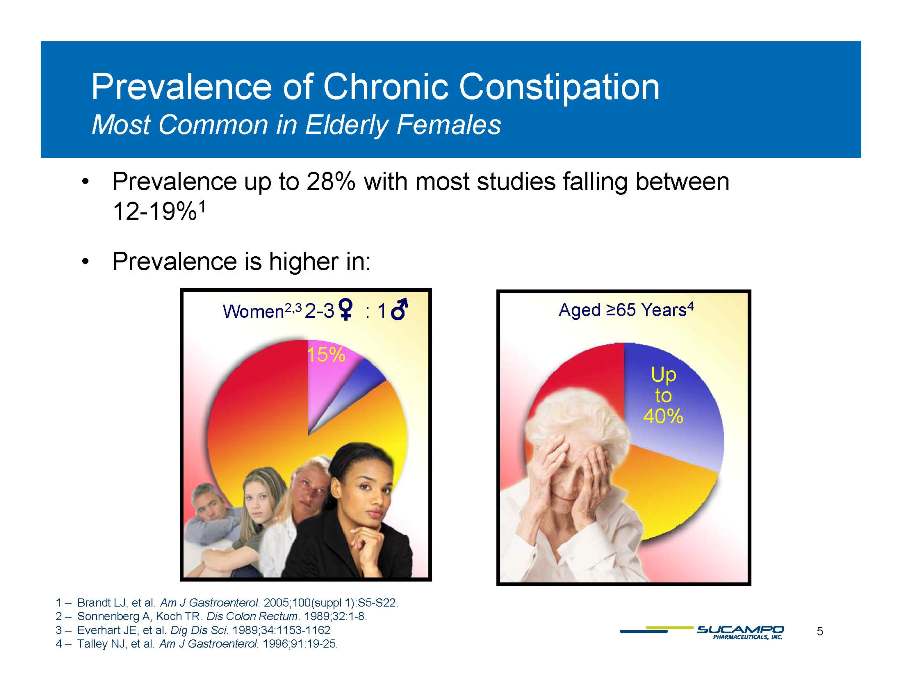
Prevalence of Chronic Constipation Common Females Most in Elderly Prevalence up to 28% with most studies falling between 12-19% 1 Agedd ≥65 Y 4 W 2 3 2 3 1 12 Prevalence is higher in: Aged ≥Years4 Up to 15% Women2,3 2-: 40% 5 1 – Brandt LJ, et al. Am J Gastroenterol. 2005;100(suppl 1):S5-S22. 2 – Sonnenberg A, Koch TR. Dis Colon Rectum. 1989;32:1-8. 3 – Everhart JE, et al. Dig Dis Sci. 1989;34:1153-1162 4 – Talley NJ, et al. Am J Gastroenterol. 1996;91:19-25. 5
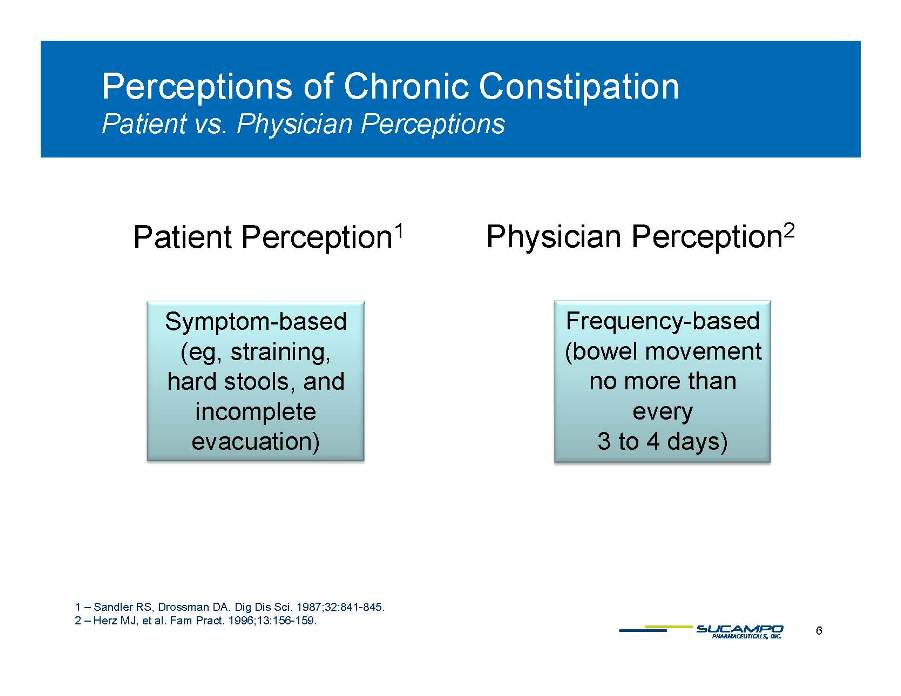
Perceptions of Chronic Constipation Patient vs vs. Physician Perceptions Physician Perception2 Patient Perception1 Frequency-based (bowel movement no more than Symptom-based (eg, straining, hard stools, and every 3 to 4 days), incomplete evacuation) 6 1 – Sandler RS, Drossman DA. Dig Dis Sci. 1987;32:841-845. 2 – Herz MJ, et al. Fam Pract. 1996;13:156-159. Treatment
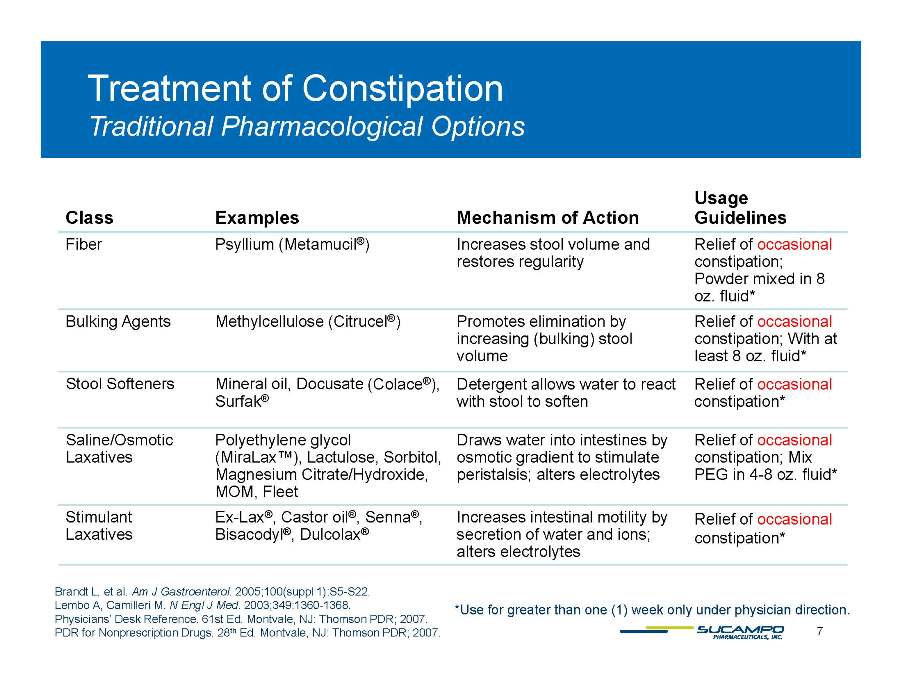
Treatment of Constipation Traditional Class Examples Mechanism of Usage Guidelines Pharmacological Options Action Fiber Psyllium (Metamucil®) Increases stool volume and restores regularity Relief of occasional constipation; Powder mixed in 8 oz. fluid* Bulking Agents Methylcellulose (Citrucel®) Promotes elimination by increasing (bulking) stool volume Relief of occasional constipation; With at least 8 oz. fluid* Stool Softeners Mineral oil, Docusate ( Colace®), Detergent allows water to react Relief of occasional Surfak® g with stool to soften constipation* Saline/Osmotic Laxatives Polyethylene glycol (MiraLax™), Lactulose, Sorbitol, Magnesium Citrate/Hydroxide, Draws water into intestines by osmotic gradient to stimulate peristalsis; alters electrolytes Relief of occasional constipation; Mix PEG in 4-8 oz. fluid* g y , MOM, Fleet p ; y Stimulant Laxatives Ex-Lax®, Castor oil®, Senna®, Bisacodyl®, Dulcolax® Increases intestinal motility by secretion of water and ions; alters electrolytes Relief of occasional constipation* 7 Brandt L, et al. Am J Gastroenterol. 2005;100(suppl 1):S5-S22. Lembo A, Camilleri M. N Engl J Med. 2003;349:1360-1368. Physicians’ Desk Reference. 61st Ed. Montvale, NJ: Thomson PDR; 2007. PDR for Nonprescription Drugs. 28th Ed. Montvale, NJ: Thomson PDR; 2007. *Use for greater than one (1) week only under physician direction.
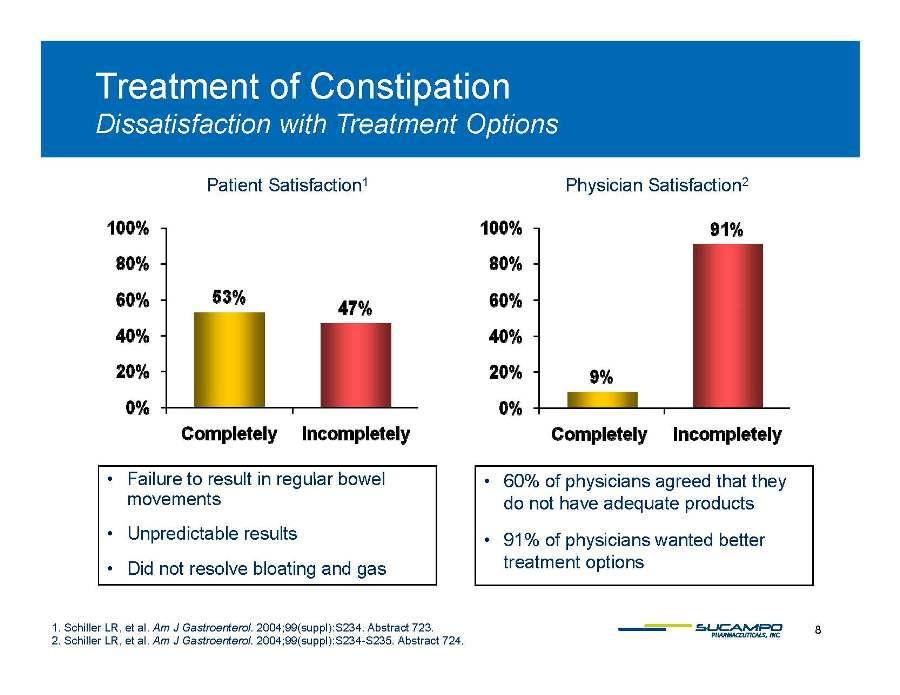
Treatment of Constipation Patient Satisfaction1 Physician Satisfaction2 Dissatisfaction with Treatment Options • • 60% of Failure to result in regular bowel movements • Unpredictable results • physicians agreed that they do not have adequate products • 91% of physicians wanted better 8 1. Schiller LR, et al. Am J Gastroenterol. 2004;99(suppl):S234. Abstract 723. 2. Schiller LR, et al. Am J Gastroenterol. 2004;99(suppl):S234-S235. Abstract 724. Did not resolve bloating and gas treatment options 0% 20% 40% 60% 80% 100% 53% 47% 9% 91% Completely Incompletely
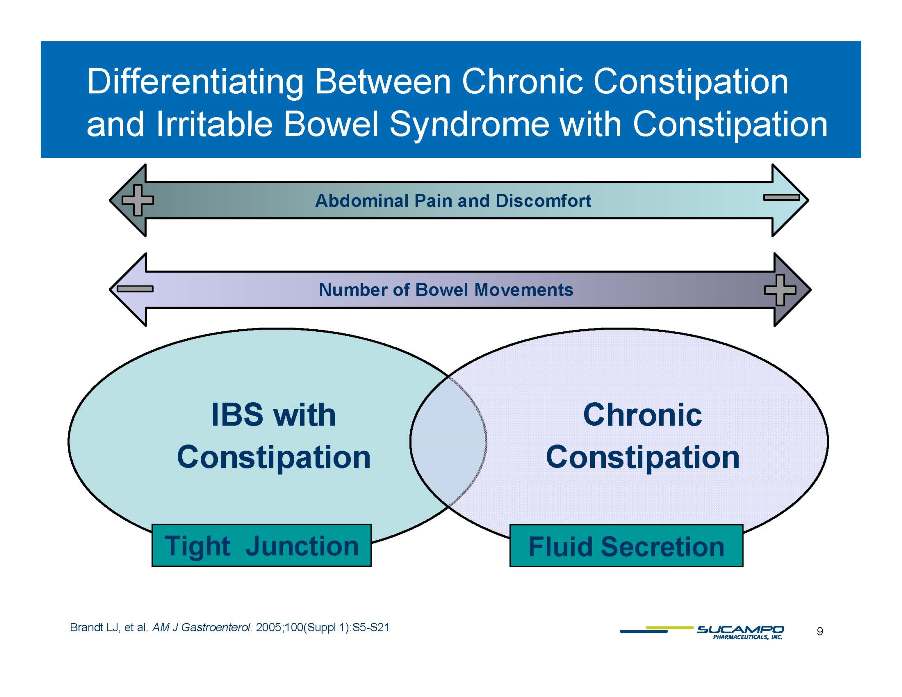
Differentiating Between Chronic Constipation Irritable Bowel Syndrome Constipation with and Abdominal Pain and Discomfort Number of Bowel Movements IBS with Constipation Chronic Constipation Tight Junction Fluid Secretion 9 Brandt LJ, et al. AM J Gastroenterol. 2005;100(Suppl 1):S5-S21
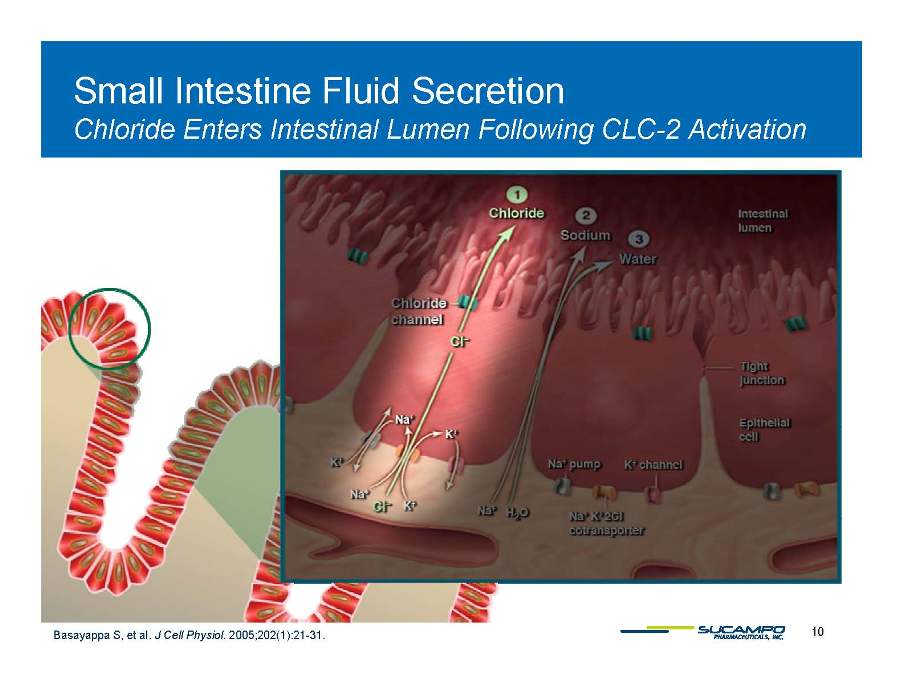
Small Intestine Fluid Secretion Lumen CLC 2 Chloride Enters Intestinal Following CLC-Activation 10 Basayappa S, et al. J Cell Physiol. 2005;202(1):21-31.
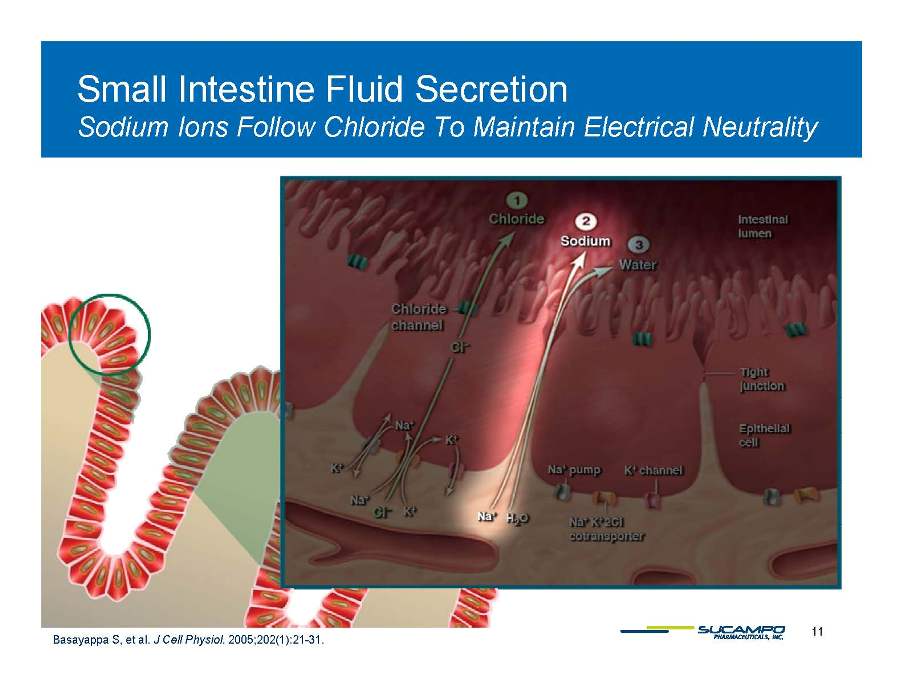
Small Intestine Fluid Secretion Sodium Ions Follow Chloride To Maintain Electrical Neutrality 11 Basayappa S, et al. J Cell Physiol. 2005;202(1):21-31.
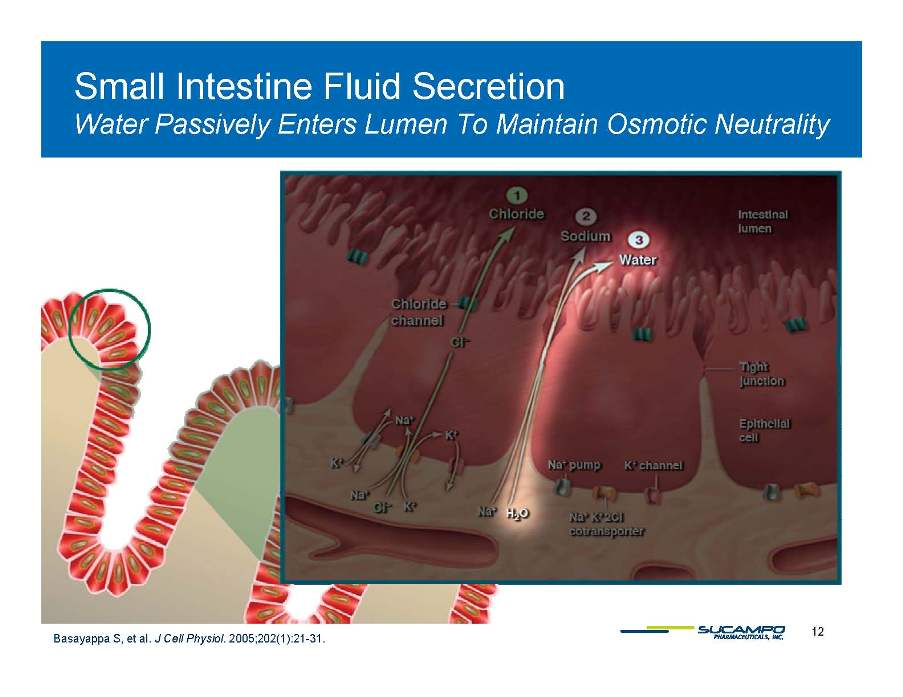
Small Intestine Fluid Secretion Water Lumen To Passively Enters Maintain Osmotic Neutrality 12 Basayappa S, et al. J Cell Physiol. 2005;202(1):21-31.
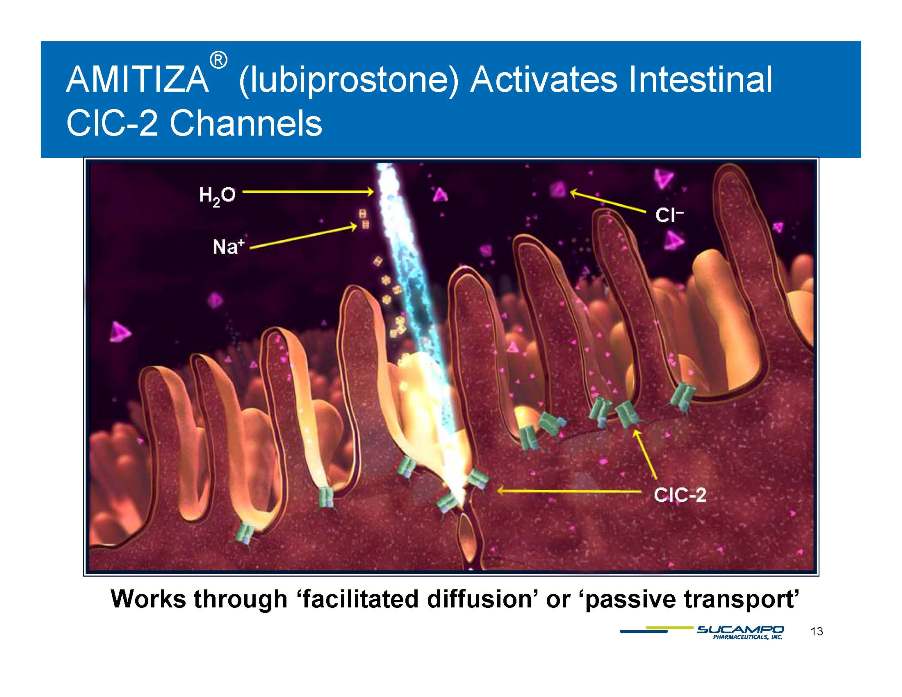
AMITIZA® (lubiprostone) Activates Intestinal ClC-2 Channels ClC 13 Works through ‘facilitated diffusion’ or ‘passive transport’
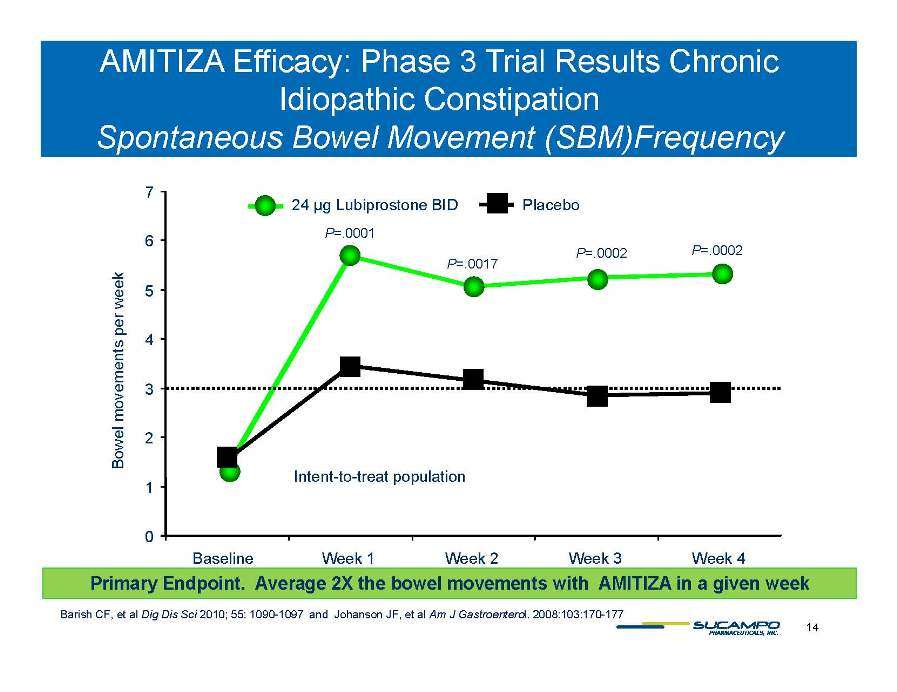
AMITIZA Efficacy: Phase 3 Trial Results Chronic Idiopathic Constipation S t B l M t F 7 24 µg Lubiprostone BID Placebo Spontaneous Bowel Movement (SBM)Frequency P=.0001 P=.0017 P=.0002 P=.0002 5 6 week 3 4 ements per w Intent-to-treat 2 Bowel move population 0 1 Baseline Week 1 Week 2 Week 3 Week 4 14 Barish CF, et al Dig Dis Sci 2010; 55: 1090-1097 and Johanson JF, et al Am J Gastroenterol. 2008:103:170-177 Primary Endpoint. Average 2X the bowel movements with AMITIZA in a given week
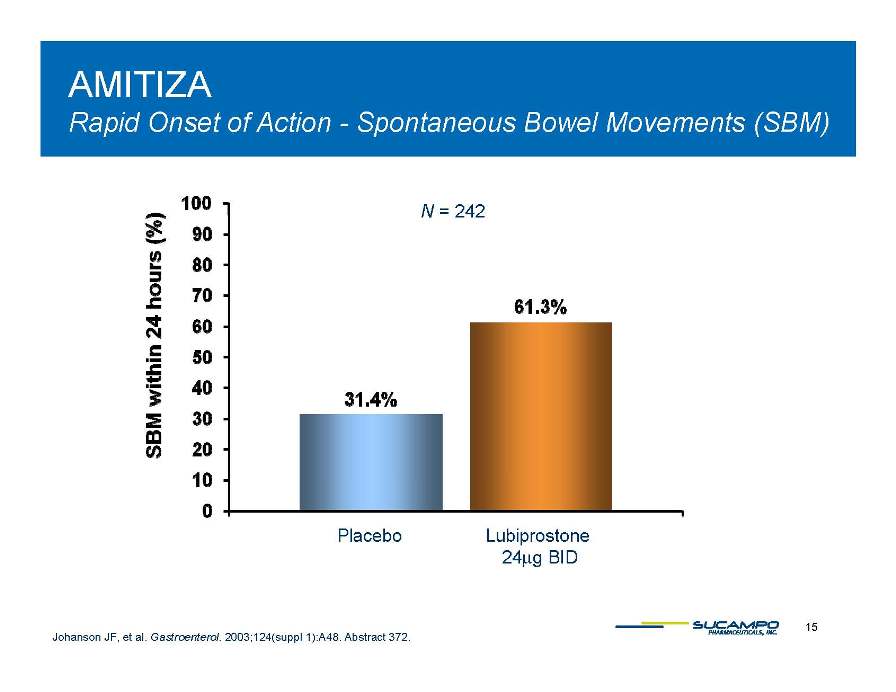
AMITIZA - Rapid Onset of Action Bowel Movements (SBM) Spontaneous N = 242 Placebo Lubiprostone 24μg BID 15 Johanson JF, et al. Gastroenterol. 2003;124(suppl 1):A48. Abstract 372. Μg 0 10 20 30 40 50 60 70 80 90 SBM within 24 hours (%)
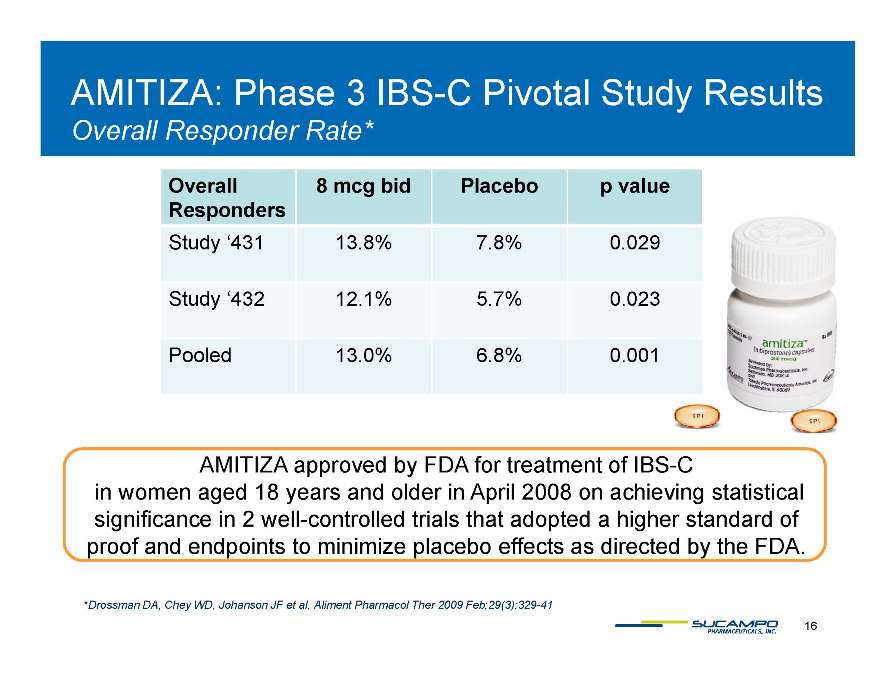
AMITIZA: Phase 3 IBS-C Pivotal Study Results Overall Responder Rate* Amitiza Overall Responders 8 mcg bid Placebo p value Study ‘431 13.8% 7.8% 0.029 Study ‘432 12 1% 5 7% 0 023 12.1% 5.7% 0.023 Pooled 13.0% 6.8% 0.001 AMITIZA approved by FDA for treatment of IBS-C pp y in women aged 18 years and older in April 2008 on achieving statistical significance in 2 well-controlled trials that adopted a higher standard of proof and endpoints to minimize placebo effects as directed by the FDA. 16 *Drossman DA, Chey WD, Johanson JF et al, Aliment Pharmacol Ther 2009 Feb;29(3):329-41 Amitiza
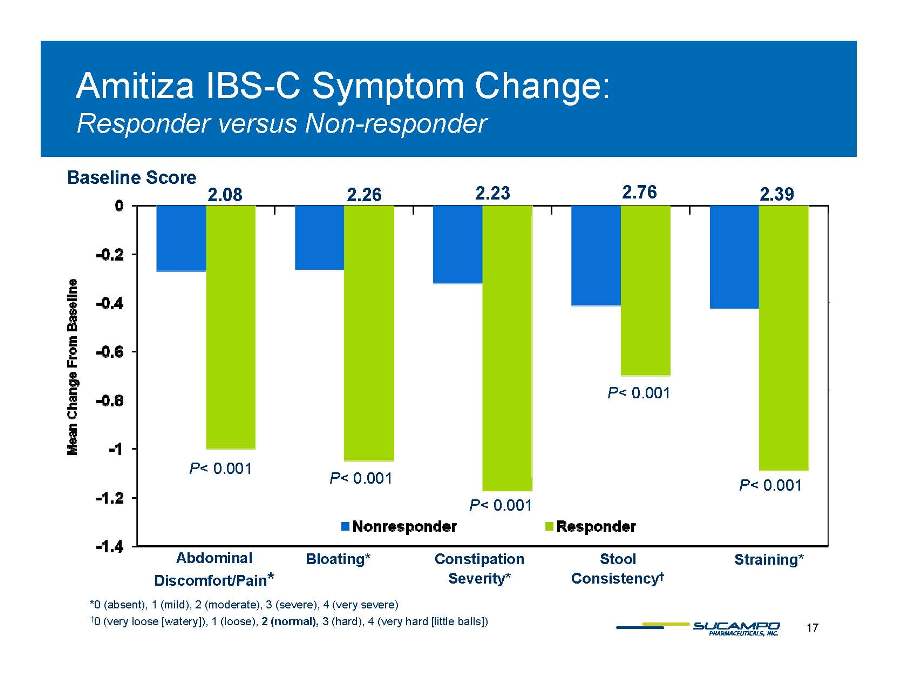
Amitiza IBS-C Symptom Change: Responder Non-responder versus Non 2.08 2.23 2.76 2.39 2.26 Baseline Score P< 0 001 P< 0.001 P< 0 001 0.001 0.001 P< 0.001 P< 0.001 Abdominal Bloating* Constipation Stool Straining* 17 *0 (absent), 1 (mild), 2 (moderate), 3 (severe), 4 (very severe) †0 (very loose [watery]), 1 (loose), 2 (normal), 3 (hard), 4 (very hard [little balls]) Discomfort/Pain* g p Severity* Consistency† g
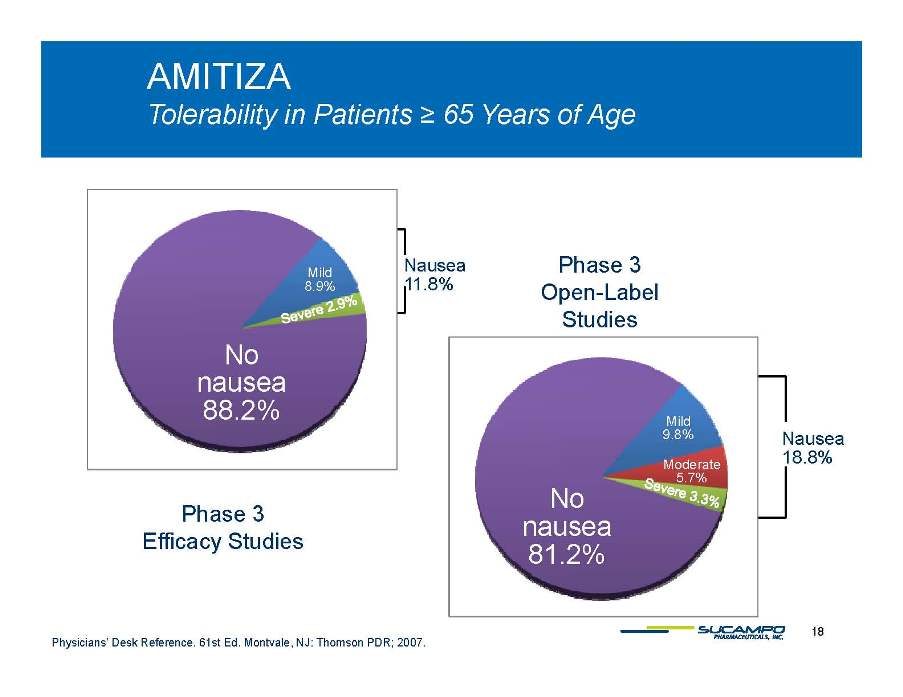
AMITIZA Tolerability in Patients ≥ 65 Years of Age y g Nausea 11.8% Mild 8.9% Phase 3 Open-Label No nausea Studies 88.2% Nausea 18.8% Mild 9.8% Moderate 5 7% No nausea 81.2% 5.7% Phase 3 Efficacy Studies 18 Physicians’ Desk Reference. 61st Ed. Montvale, NJ: Thomson PDR; 2007.
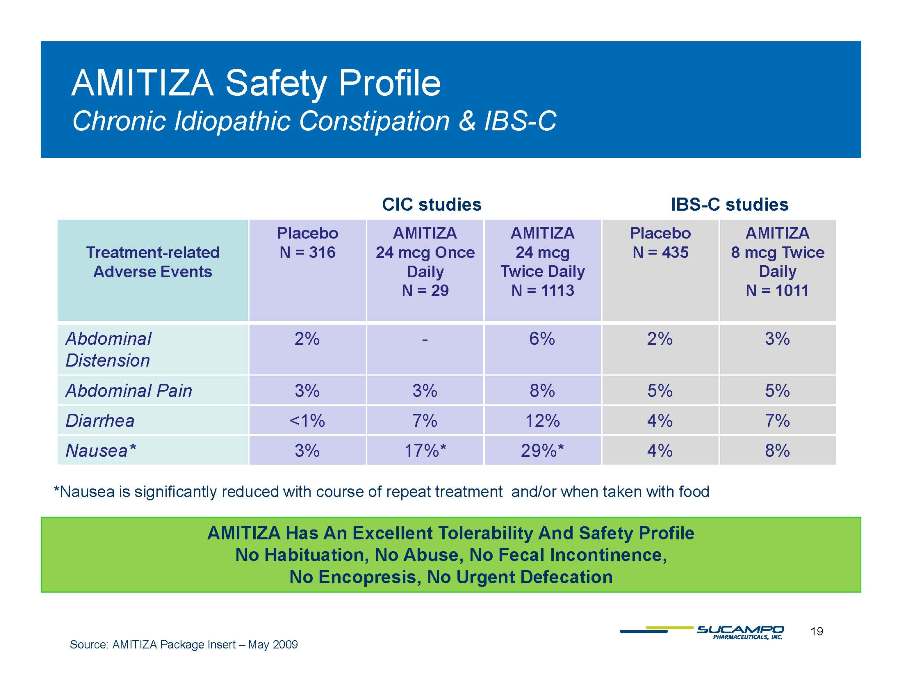
AMITIZA Safety Profile Chronic Idiopathic IBS-C Constipation & IBS CIC studies IBS-C studies Treatment- related Adverse Events Placebo N = 316 AMITIZA 24 mcg Once Daily N = 29 AMITIZA 24 mcg Twice Daily N = 1113 Placebo N = 435 AMITIZA 8 mcg Twice Daily N = 1011 Abdominal Distension 2% - 6% 2% 3% Abdominal 3% 3% 8% 5% 5% Pain Diarrhea <1% 7% 12% 4% 7% Nausea* 3% 17%* 29%* 4% 8% *Nausea is significantly reduced with course of repeat treatment and/or when taken with food AMITIZA Has An Excellent Tolerability And Safety Profile No Habituation, No Abuse, No Fecal Incontinence, 19 Source: AMITIZA Package Insert – May 2009 No Encopresis, No Urgent Defecation
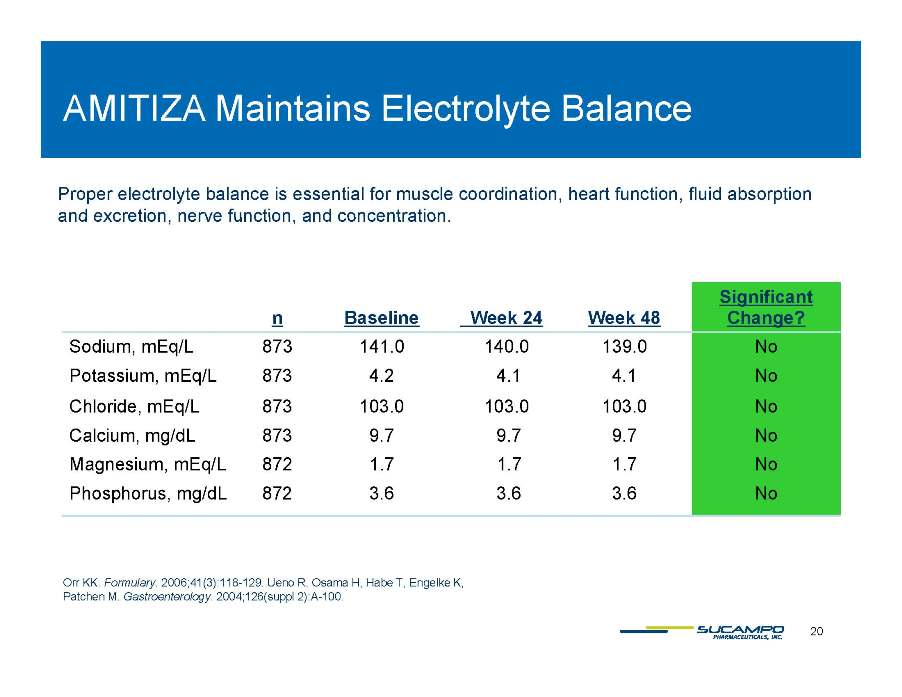
AMITIZA Maintains Electrolyte Balance Proper electrolyte balance is essential for muscle coordination, heart function, fluid absorption and excretion function concentration Significant excretion, nerve function, and concentration. n Baseline Week 24 Week 48 g Change? Sodium, mEq/L 873 141.0 140.0 139.0 No Potassium, mEq/L 873 4.2 4.1 4.1 No Chloride, mEq/L 873 103.0 103.0 103.0 No Calcium, mg/dL 873 9.7 9.7 9.7 No Magnesium, mEq/L 872 1.7 1.7 1.7 No Phosphorus, mg/dL 872 3.6 3.6 3.6 No 20 Orr KK. Formulary. 2006;41(3):118-129. Ueno R, Osama H, Habe T, Engelke K, Patchen M. Gastroenterology. 2004;126(suppl 2):A-100.
AMITIZA: Further Opportunities -- A Third Indication Management of Opioid-induced Bowel Dysfunction (OBD) in non-cancer non pain patients • Reported results of successful AMITIZA phase 3 trial (OBD0631) at DDW 2010* L • Lubiprostone achieved a statistically significant p=0.02) greater increase in the mean number of SBMs per week in 8 of the 12 weeks of the trial as compared to placebo patients • The percentage of patients who achieved a SBM within 24 hours and 48 hours was significantly higher with lubiprostone as compared to placebo (p=0.0126 at 24 hours, and p=0.0360 at 48 hours) • Statistical significance was achieved for the overall change from baseline in constipation-associated symptom secondary endpoints 21 * DDW 2010, Abstract #780958

AMITIZA: Further Opportunities – A Third Indication • Sucampo initiated a third phase 3 randomized, placebo-controlled, multi-center trial of AMITIZA for OBD in December 2010* • Trial design: – Almost the same protocol as used in the successful phase 3 trial ( OBD0631) p p ) reported at DDW 2010, except exclusion of patients on methadone – One 24-mcg gel capsule of lubiprostone or placebo twice each day – 12 week treatment period – Permitted concomitant pain medications include: fentanyl, morphine and oxycontin p y, p y but exclude methadone – Goal is to enroll 420 patients in the U.S. and Europe – Primary endpoint: change from baseline in SBM frequency at Week 8 without reduction in dose of study pain medication y p • Sucampo and Takeda to share trial costs 50-50 22 *Sucampo press release, Jan. 7, 2011

Key Terms of Agreements with Takeda For U.S. and Canadian Marketing and Commercialization • Takeda shall exert best efforts to promote, market and sell and to maximize net sales revenue – Currently covers two indications: CIC in adults and IBS-C in women aged 18 years and older – Takeda holds right of first refusal to additional GI indications in US and Canada – Takeda records all U.S. sales, Sucampo receives a royalty – Sucampo retains all other rights – Takeda also has rights to AMITIZA in Canada, but not yet launched • Sucampo’s tiered royalty rate: 18% to 26% of annual net sales • Sucampo reimbursed for majority of GI clinical development costs • Sucampo has total 23 received a of $150 million in upfront and development milestone payments as of Dec. 31, 2010
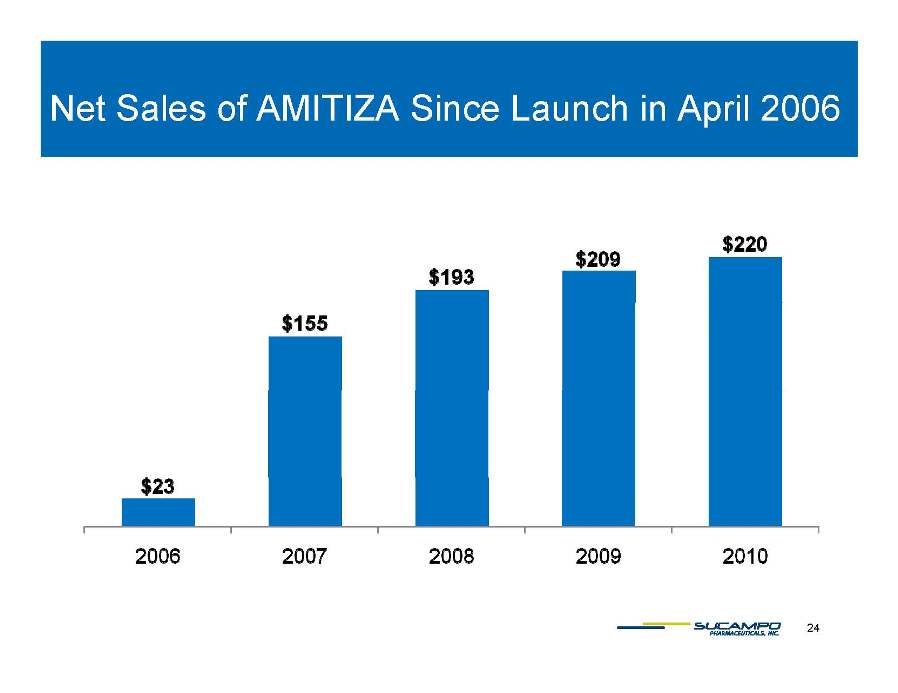
Net Sales of AMITIZA Since Launch in April 2006 24 $23 $155 $193 $209 $220 2006 2007 2008 2009 2010

AMITIZA – Future Global Opportunities Key Terms of Agreement with Abbott Japan • Key element in Sucampo’s growth strategy: increase the number of international market approvals for AMITIZA • Abbott received exclusive rights to commercialize lubiprostone in Japan for CIC, and right of first refusal for additional indications in Japan • If successfully developed, Sucampo will supply finished product to Abbott • Sucampo retains right to co-promote AMITIZA in Japan and to develop AMITIZA for additional indications • Sucampo has received a total of $22.5 million in upfront and milestone payments from Abbott, as of December 31, 2010 25 • Sucampo designed and managed the recently reported successful phase 3 efficacy trial and long-term safety trial in Japanese CIC patients AMITIZA:

AMITIZA: Future Global Opportunities – Japan Japanese Phase 3 efficacy trial * • efficacy p=0 001)* Primary endpoint reached statistical significance (p 0.001) • Double-blind, placebo-controlled multi-center trial, evaluated 124 patients • Dose: Placebo or lubiprostone 24-mcg soft gel capsule, twice daily, for 28 days • Results filed with Japanese authorities in marketing application (Sept. 2010) Japanese Phase 3 long-term safety trial** • An open-label, multi-center trial with 209 patients • lubiprostone 24-mcg gel Dose: one capsule twice a day for 48 weeks • Results demonstrate lubiprostone is safe and well tolerated • There results were added to marketing application (Dec. 2010) Data from phase 3 trials to be presented at DDW in May 2011 26 Sucampo Press Releases: * August 5, 2010 ** Nov. 30, 2010
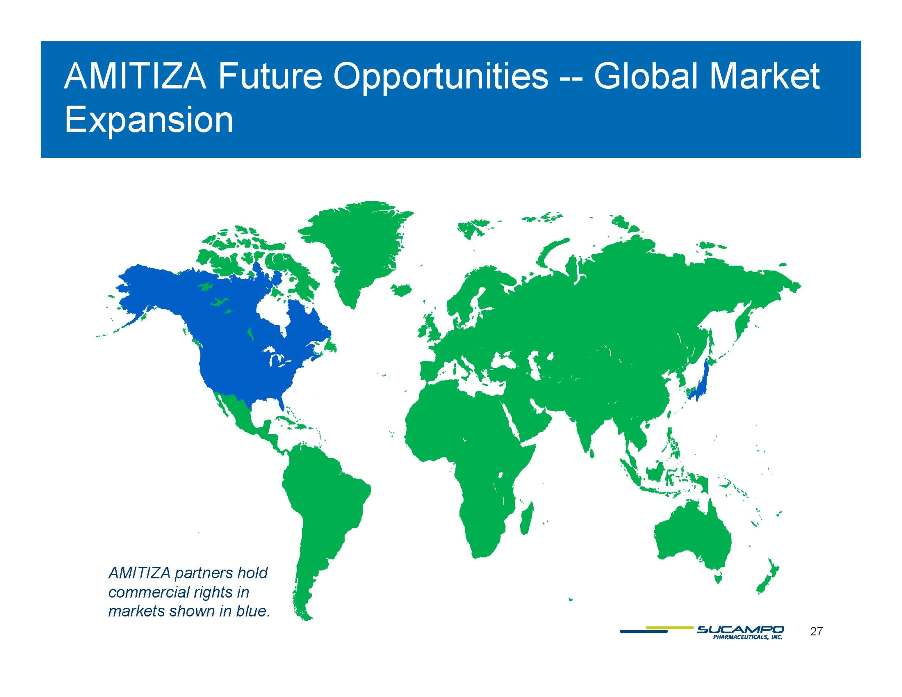
AMITIZA Future Opportunities -- Global Market Expansion AMITIZA 27 partners hold commercial rights in markets shown in blue.
RESCULA®: A Differentiated Ophthalmic Drug A unique mechanism of action • RESCULA activates Maxi K (BK) channels in neurons and contractile cells* • Lowers IOP by increased outflow of aqueous humor through trabecular pathway** meshwork and uveoscleral pathway • Increases both retinal and choroidal components of ocular blood flow to optic nerve*** • Maintains visual field in glaucoma patients; inhibits apoptosis of retinal neurons and ischemia-induced degeneration of optic nerve fibers in nonclinical studies**** * et al. Invest Ophthalmol Vis Sci. 1994;35:4087-4099. Kern TS. Exp Diabetes Res. 2007;2007:95013. Hardy P et al. Prostaglandins Leukot Essent Fatty Acids. 2005;72(5):301-325. • ** Alm A et al. Exp Eye Res. 2009;88:760-768. Toris CB et al. Arch Ophthalmol. 2004;122:1782-1787. Llobet A et al. News Physiol Sci. 2003;18:205-209 28 205 • *** Kojima S et al. Nippon Ganka Bakkai Zasshi. 1997:101;605-610. Makimoto Y et al. Jpn J Ophthalmol. 2002;46:31-35. Kimura I et al. Jpn Ophthalmol. 2005;49:287-293 • **** Sugiyama T et al. Arch Ophthalmol. 2009;127:454-459 Yu DY
RESCULA: Current Status and Potential Opportunities • RESCULA eye-drops are a prostone-based drug, not a prostaglandin • FDA-approved for lowering of intra-ocular pressure (IOP) in primary open angle glaucoma (POAG) and ocular hypertension patients who are intolerant of or are insufficiently responsive to other lowering medications; not currently available in U.S. • data RESCULA’s approval in 2000 Sucampo submitted developed after RESCULA s FDA in an NDA (August 2009) and updated it in December 2010 • Awaiting a commercially viable label from FDA in order to complete U.S. g y p launch plans • Potential indications include Dry Age-related Macular Edema (dry AMD) and 29 Retinitis Pigmentosa (RP) RESCULA:
RESCULA: Phase 2 Clinical Trial Design -- Retinitis Pigmentosa Design of Phase 2 Trial Conducted by Partner • A multi-center, randomized, double-blind, three parallel group, placebo controlled trial conducted by R-Tech Ueno (RTU) at 6 Japanese sites • Enrolled 112 mid- to late-stage Retinitis Pigmentosa (RP) patients with visual acuity 0 5 of 0.5 or more in a narrow visual field • Patients received either one or two drops of active drug or placebo twice a day for 24 week • Primary endpoint: change from baseline in the mean retinal sensitivity of the central 2-degrees of the ocular fundus as measured with an MP-1 microperimeter• Secondary endpoints included y p – Change in retinal sensitivity measured by Humphrey perimeter (10-2) – Visual acuity – Contrast sensitivity Health related Quality of Life (measured VFQ 25) 30 – Health-by VFQ-* R-Tech Ueno press releases of June 3, 2010 and July 15, 2010
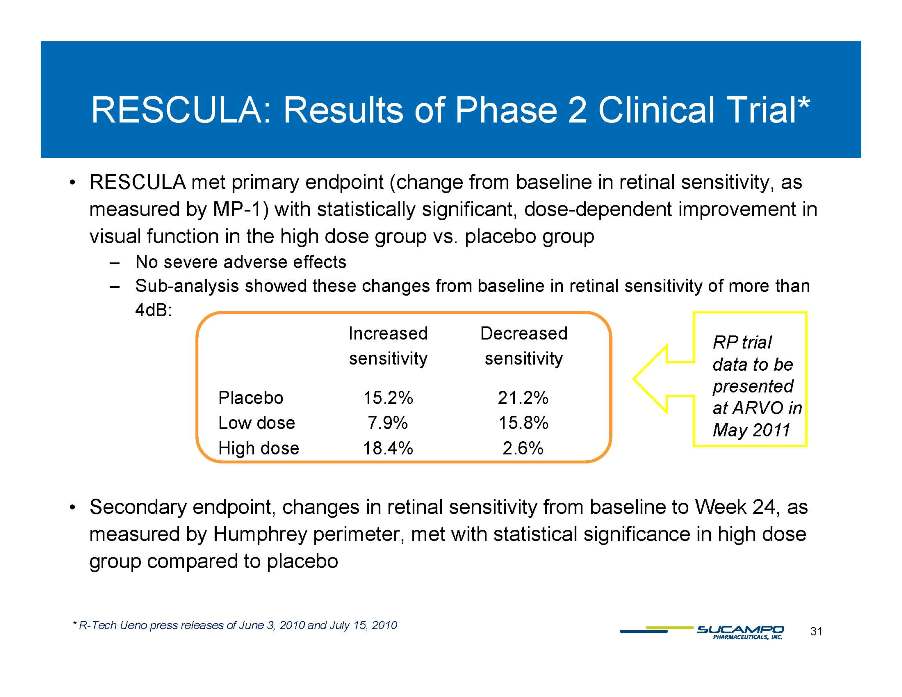
RESCULA: Results of Phase 2 Clinical Trial* • RESCULA met primary endpoint (change from baseline in retinal sensitivity, as measured by MP-1) with statistically significant, dose-dependent improvement in y ) visual function in the high dose group vs. placebo group – No severe adverse effects – Sub-analysis showed these changes from baseline in retinal sensitivity of more than 4dB 4dB: Increased sensitivity Decreased sensitivity Pl b 15 2% 21 2% RP trial data to be presented Placebo 15.2% 21.2% Low dose 7.9% 15.8% High dose 18.4% 2.6% p at ARVO in May 2011 • Secondary endpoint, changes in retinal sensitivity from baseline to Week 24, as measured by Humphrey perimeter, met with statistical significance in high dose group compared to placebo 31 * R-Tech Ueno press releases of June 3, 2010 and July 15, 2010
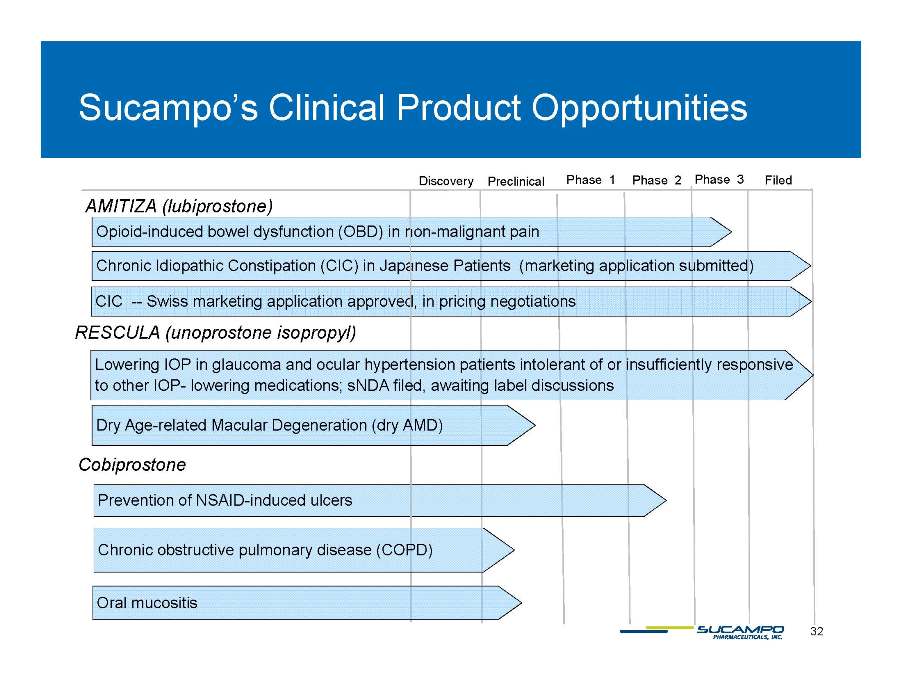
Sucampo’s Clinical Product Opportunities Phase 3 Preclinical Phase 2 Phase 1 Filed AMITIZA (lubiprostone) Discovery Opioid-induced bowel dysfunction (OBD) in non-malignant pain Chronic Idiopathic Constipation (CIC) in Japanese Patients (marketing application submitted) CIC -- Swiss approved marketing application approved, in pricing negotiations RESCULA (unoprostone isopropyl) Lowering IOP in glaucoma and ocular hypertension patients intolerant of or insufficiently responsive to other IOP- lowering medications; sNDA filed, awaiting label discussions Cobiprostone Dry Age-related Macular Degeneration (dry AMD) Prevention of NSAID-induced ulcers Chronic obstructive pulmonary disease (COPD) 32 Oral mucositis
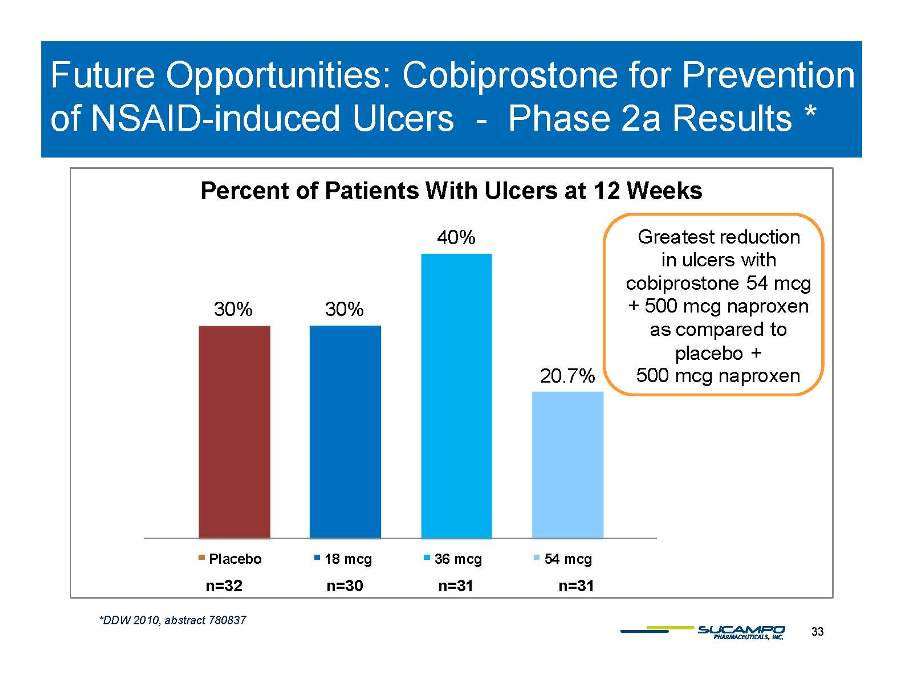
Future Opportunities: Cobiprostone for Prevention of NSAID-induced - * Percent of Patients With Ulcers at 12 Weeks NSAID Ulcers Phase 2a Results 30% 30% 40% Greatest reduction in ulcers with cobiprostone 54 mcg + 500 naproxen 20.7% mcg as compared to placebo + 500 mcg naproxen Placebo 18 mcg 36 mcg 54 mcg 33 *DDW 2010, abstract 780837 n=32 n=30 n=31 n=31
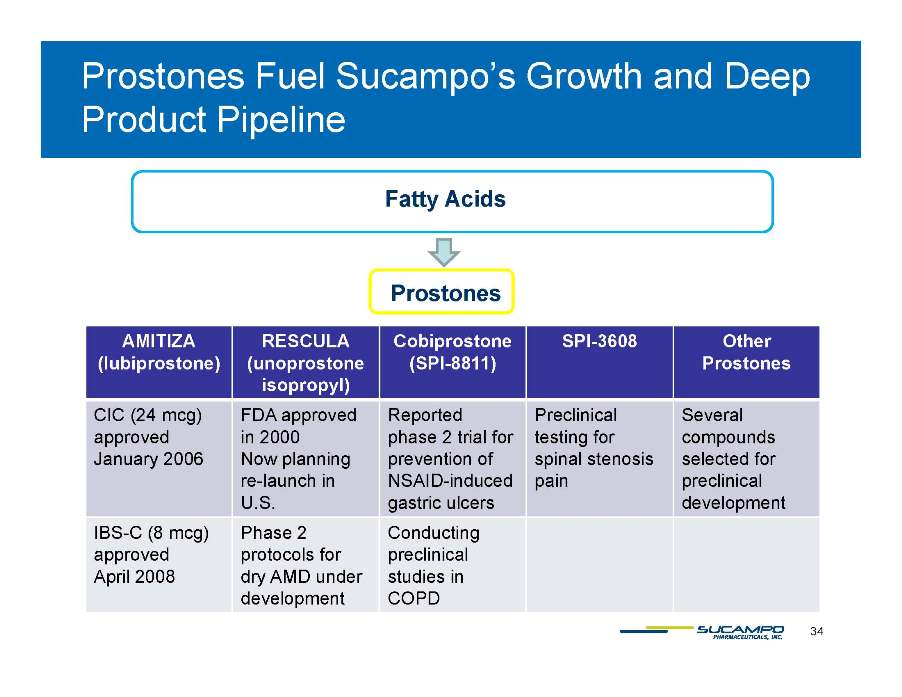
Prostones Fuel Sucampo’s Growth and Deep Pipeline Product Fatty Acids Prostones AMITIZA (lubiprostone) RESCULA (unoprostone isopropyl) Cobiprostone (SPI-8811) SPI-3608 Other Prostones p py) CIC (24 mcg) Approved January 2006 FDA approved in 2000 Now planning re launch Reported phase 2 trial for prevention of NSAID induced Preclinical testing for spinal stenosis pain Several compounds selected for preclinical re-in U.S. NSAID-gastric ulcers development IBS-C (8 mcg) approved Phase 2 protocols for Conducting preclinical 34 April 2008 dry AMD under development studies in COPD
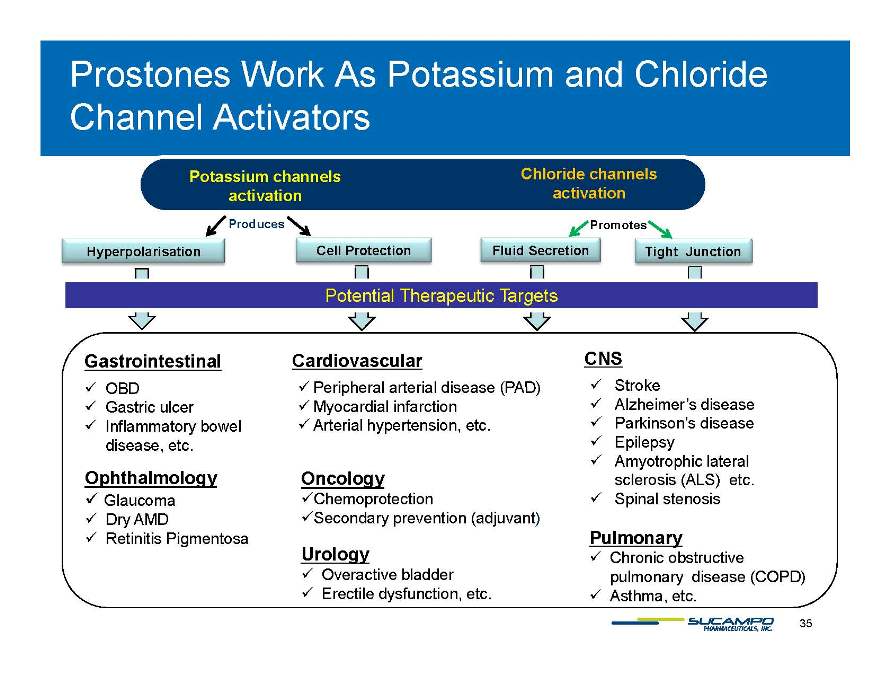
Prostones Work As Potassium and Chloride Channel Activators Chloride channels activation Potassium channels Activation Potential Therapeutic Targets Hyperpolarisation Cell Protection Fluid Secretion Tight Junction Produces Promotes Gastrointestinal OBD Cardiovascular Peripheral arterial CNS Stroke Gastric ulcer Inflammatory bowel disease, etc. disease (PAD) Myocardial infarction Arterial hypertension, etc. Alzheimer’s disease Parkinson’s disease Epilepsy Amyotrophic lateral sclerosis (ALS) etc. Spinal stenosis Pulmonary Chronic Urology Ophthalmology Glaucoma Dry AMD Retinitis Pigmentosa Oncology Chemoprotection Secondary prevention (adjuvant) 35 obstructive pulmonary disease (COPD) Asthma, etc. Overactive bladder Erectile dysfunction, etc.

Sucampo’s Financial Results and Position ( In millions, except per share data) 2009* 2010* , p p ) 2009 2010 Product Royalty Revenue $38.3 $40.3 R&D Revenue* $24.0 $16.5 Total Revenue $67.3 $61.9 Net Income/(Loss) $4 8 ($2 7) 4.8 2.7) Earnings Per Share (diluted) $0.11 ($0.07) Cash, Restricted Cash and $153 0 $123 9** Investments 153.0 123.9 *Results for 2009 and 2010 are consolidated to reflect the acquisition of Sucampo AG in Dec 2010 ** At Dec. 31, 2010, Sucampo had $44.4 million in long-term debt. 36

Sucampo’s 2011 Milestones • Completion of enrollment into third phase 3 clinical trial of lubiprostone for OBD during third quarter • Gain approval of a commercially viable label (sNDA) for RESCULA to support a re-U S re launch in the U.S. for the approved indication of lowering of intraocular pressure (IOP) in open-angle glaucoma and ocular hypertension in patients who are intolerant of or insufficiently responsive to other IOP-lowering medications • Submit a Marketing Approval Application (MAA) for lubiprostone for CIC in the United Kingdom • Sucampo AG Integrate into corporate structure to achieve operational efficiencies afforded by our December 2010 acquisition of it • Make substantial progress towards successfully resolving our dispute 37
Sucampo: A Biopharmaceutical Company AMITIZA® • Only FDA approved drug for chronic idiopathic constipation (CIC) in adults • IBS Only FDA approved drug for irritable bowel syndrome with constipation (IBS-C) in women aged 18 years and older • Marketing authorization approved in Switzerland for CIC, now in pricing negotiations there • Filed NDA for CIC in Japan in Sept 2010 • opioid-induced bowel U S Third Phase 3 trial in opioid dysfunction (OBD) initiated late 2010 in U.S. • U.S + Canadian commercial rights held by Takeda; Abbott holds Japanese commercial rights RESCULA® • FDA-approved for lowering intra-ocular pressure (IOP) in glaucoma and ocular hypertension patients who are intolerant of or insufficiently IOP responsive to other IOP-lowering medications • In-licensed clinical development and commercial rights in April 2009 • Await FDA approval of commercially viable label supplemental NDA (sNDA) to re-launch in U.S. • Designing trials for additional indications, based on partner’s breakthrough phase 2 results A deep pipeline leveraging prostone technology technology, expertise • Cobiprostone for prevention of NSAID-induced gastric ulcers in Phase 2 • SPI-3608 in preclinical development for pain associated with spinal stenosis • Additional prostones in preclinical development 38 Strong financial position • $123.9 million in cash, restricted cash and investments as well as $44.4MM in long term debt as of Dec. 31, 2010

Cowen and Company 31st Annual Health Care Conference Stanley G. Miele President, Sucampo Pharma Americas March 7, 2011 39
