Attached files
| file | filename |
|---|---|
| EX-10.19 - EX-10.19 - ALLOS THERAPEUTICS INC | a2202367zex-10_19.htm |
| EX-10.4 - EX-10.4 - ALLOS THERAPEUTICS INC | a2202367zex-10_4.htm |
| EX-23.02 - EX-23.02 - ALLOS THERAPEUTICS INC | a2202367zex-23_02.htm |
| EX-32.01 - EX-32.01 - ALLOS THERAPEUTICS INC | a2202367zex-32_01.htm |
| EX-31.01 - EX-31.01 - ALLOS THERAPEUTICS INC | a2202367zex-31_01.htm |
| EX-31.02 - EX-31.02 - ALLOS THERAPEUTICS INC | a2202367zex-31_02.htm |
| EX-23.01 - EX-23.01 - ALLOS THERAPEUTICS INC | a2202367zex-23_01.htm |
| EX-10.14.2 - EX-10.14.2 - ALLOS THERAPEUTICS INC | a2202367zex-10_142.htm |
| EX-10.20.1 - EX-10.20.1 - ALLOS THERAPEUTICS INC | a2202367zex-10_201.htm |
| EX-10.21.1 - EX-10.21.1 - ALLOS THERAPEUTICS INC | a2202367zex-10_211.htm |
| EX-10.22.1 - EX-10.22.1 - ALLOS THERAPEUTICS INC | a2202367zex-10_221.htm |
| EX-10.16.1 - EX-10.16.1 - ALLOS THERAPEUTICS INC | a2202367zex-10_161.htm |
| EX-10.23.1 - EX-10.23.1 - ALLOS THERAPEUTICS INC | a2202367zex-10_231.htm |
| EX-10.24.2 - EX-10.24.2 - ALLOS THERAPEUTICS INC | a2202367zex-10_242.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
Allos Therapeutics, Inc. Index to Financial Statements
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
|
For the fiscal year ended December 31, 2010. |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
|
For the transition period from to . |
||
Commission File Number 00029815
Allos Therapeutics, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
54-1655029 (I.R.S. Employer Identification No.) |
11080 CirclePoint Road, Suite 200
Westminster, Colorado 80020
(303) 426-6262
(Address, including zip code, and telephone number, including area code, of principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
| (Title of class) | (Name of each exchange on which registered) | |
|---|---|---|
| Common Stock $.001 Par Value | NASDAQ Stock Market LLC | |
| (NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405) of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of common stock held by nonaffiliates of the registrant (based upon the closing sale price of such shares on the NASDAQ Global Market on June 30, 2010) was $482,335,587. Shares of the registrant's common stock held by each current executive officer and director and by each stockholder who is known by the registrant to own 10% or more of the outstanding common stock have been excluded from this computation in that such persons may be deemed to be affiliates of the registrant. Share ownership information of certain persons known by the registrant to own greater than 10% of the outstanding common stock for purposes of the preceding calculation is based solely on information on Schedules 13D and 13G, if any, filed with the Commission. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of February 25, 2011, there were 105,580,200 shares of the registrant's common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant's definitive Proxy Statement for the 2011 Annual Meeting of Stockholders to be filed within 120 days after the end of the Registrant's fiscal year ended December 31, 2010 are incorporated by reference into Part III of this Annual Report on Form 10-K to the extent stated therein.
2
Allos Therapeutics, Inc., the Allos Therapeutics, Inc. logo, FOLOTYN, the FOLOTYN logo and all other Allos names are trademarks of Allos Therapeutics, Inc. in the United States and in other selected countries. All other brand names or trademarks appearing in this report are the property of their respective holders. Unless the context requires otherwise, references in this report to "Allos," the "Company," "we," "us," and "our" refer to Allos Therapeutics, Inc.
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These forward-looking statements include, but are not limited to, statements regarding our commercialization of FOLOTYN for patients with relapsed or refractory peripheral T-cell lymphoma; statements regarding our Marketing Authorisation Application, or MAA, for FOLOTYN in Europe; our projected operating costs and expenses for fiscal year 2011; other statements regarding our future product development and regulatory strategies, including our intent to develop or seek regulatory approval for FOLOTYN for additional indications; the ability of our third-party manufacturers to support our requirements for drug supply; any statements regarding our future financial performance, results of operations or sufficiency of capital resources to fund our operating requirements; and any other statements that are other than statements of historical fact. In some cases, these statements may be identified by terminology such as "may," "will," "should," "expects," "plans," "anticipates," "believes," "estimates," "predicts," "potential" or "continue," or the negative of such terms and other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. These statements involve known and unknown risks and uncertainties that may cause our, or our industry's results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Factors that may cause or contribute to such differences include, among other things, those discussed under the captions "Business," "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations." Forward-looking statements not specifically described above also may be found in these and other sections of this report. All forward-looking statements included in this report are based on information available to us as of the date hereof and we undertake no obligation to revise any forward-looking statements in order to reflect any subsequent events or circumstances. You are advised, however, to consult any further disclosures we make on related subjects in our Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and our website.
We incorporated in the Commonwealth of Virginia on September 1, 1992 as HemoTech Sciences, Inc. and filed amended Articles of Incorporation to change our name to Allos Therapeutics, Inc. on October 19, 1994. We reincorporated in Delaware on October 28, 1996. We completed our initial public offering in 2000 and our shares are listed on the NASDAQ Global Market, where our symbol is ALTH. Our corporate headquarters are located in Westminster, Colorado, a suburb of Denver. Our mailing address is 11080 CirclePoint Road, Suite 200, Westminster, Colorado 80020. Our website address is www.allos.com; however, information found on our website is not incorporated by reference into this report. We operate as a single business segment.
Corporate Overview and Business Strategy
We are a biopharmaceutical company committed to the development and commercialization of innovative anti-cancer therapeutics. Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. We strive to develop proprietary products that have the potential to improve the standard of care in cancer therapy.
3
We are currently focused on the development and commercialization of FOLOTYN® (pralatrexate injection). FOLOTYN is a targeted folate inhibitor designed to accumulate preferentially in cancer cells. FOLOTYN targets the inhibition of dihydrofolate reductase, or DHFR, an enzyme critical in the folate pathway, thereby interfering with DNA and RNA synthesis and triggering cancer cell death. FOLOTYN can be delivered as a single agent, for which we currently have approval for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL, and has the potential to be used in combination therapy regimens. We believe that FOLOTYN's unique mechanism of action offers us the ability to target the drug for development in a variety of hematological malignancies and solid tumor indications. We currently retain exclusive worldwide commercial rights to FOLOTYN for all indications. We may also seek to grow our product portfolio through product acquisition and in-licensing efforts.
On September 24, 2009, the U.S. Food and Drug Administration, or FDA, granted accelerated approval of FOLOTYN for use as a single agent for the treatment of patients with relapsed or refractory PTCL. This approval was based on overall response rate from our pivotal Phase 2 trial known as PROPEL (Pralatrexate in patients with Relapsed Or refractory PEripheral T-cell Lymphoma). Clinical benefit such as improvement in progression-free survival or overall survival has not been demonstrated. FOLOTYN represents our first drug approved for marketing in the United States. FOLOTYN is the first and only drug approved by the FDA for this indication. In connection with the accelerated approval, we are required to conduct post-approval studies that are intended to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma and to determine whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment.
We began making FOLOTYN available for commercial sale in the United States in October 2009 and commenced our commercial launch of FOLOTYN in January 2010. We have established a commercial organization, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize FOLOTYN in the United States. We believe the market for relapsed or refractory PTCL is addressable with a targeted U.S. sales and marketing organization, and we intend to continue promoting FOLOTYN ourselves in the United States.
We are also seeking regulatory approval to market FOLOTYN in Europe for the treatment of patients with relapsed or refractory PTCL. In December 2010, our Marketing Authorisation Application, or MAA, was accepted by the European Medicines Agency, or EMA. Acceptance of the MAA by the EMA indicates that the application is complete and initiates the EMA's regulatory review process. The MAA is based on clinical data from our pivotal PROPEL trial. We may also seek regulatory approval to market FOLOTYN for the treatment of patients with relapsed or refractory PTCL in Japan and other countries. We intend to secure a strategic partner for the potential co-development of FOLOTYN globally and commercialization outside the United States.
We are currently prioritizing our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies. We also intend to complete our ongoing Phase 2 studies in bladder and breast cancer, and investigators are evaluating FOLOTYN in solid tumor indications through our collaboration with the National Comprehensive Cancer Network, or NCCN, Oncology Research Program.
4
The following table summarizes the target indications and clinical development status of the FOLOTYN development program, including our planned post-approval studies:
| Company Sponsored Studies |
Phase |
Status |
||
|---|---|---|---|---|
HEMATOLOGIC MALIGNANCIES |
||||
Peripheral T-cell Lymphoma |
||||
2nd line+: PROPEL Pivotal Study |
2 | FDA accelerated approval on 9/24/09; marketed in U.S. and MAA accepted for review. | ||
1st Line: CHOP Sequential Study* |
3 | Planned initiation in 2011 | ||
Cutaneous T-cell Lymphoma |
||||
2nd Line+: Single Agent Study |
1 | Enrollment completed; results reported Q4 2010 | ||
2nd Line+: Bexarotene Combination* |
1/3 | Enrollment ongoing in Phase 1 study | ||
Lymphoma |
||||
2nd line+: Non-Hodgkin Lymphoma combination Pralatrexate + Gemcitabine |
1/2a | Study ongoing | ||
2nd Line+: B-cell Non-Hodgkin Lymphoma |
2 | Enrollment ongoing | ||
SOLID TUMORS |
||||
Non-Small Cell Lung Cancer |
||||
2nd & 3rd Line: Pralatrexate vs. Erlotinib |
2b | Enrollment completed; results reported Q4 2010 | ||
Bladder Cancer |
||||
2nd Line: Single Agent Study |
2 | Study ongoing; data expected 2H 2011 | ||
Breast Cancer |
||||
2nd Line+: Single Agent Study |
2 | Enrollment ongoing; interim analysis data expected 2H 2011 | ||
NCCN Studies** |
Phase |
Status |
||
Upper GI 1st Line: Pralatrexate + Oxaliplatin |
2 | Ongoing | ||
Upper GI 2nd Line: Pralatrexate + Docetaxel |
2 | Ongoing | ||
Ovarian Recurrent Platinum Sensitive: Carboplatin + Pralatrexate |
2 | Ongoing | ||
Head & Neck: Single Agent Pralatrexate |
2 | Ongoing | ||
Solid Tumors (GI enriched): Sequential Pralatrexate/5-Fluorouracil |
1 | Ongoing | ||
Multiple Myeloma: Bortezomib + Pralatrexate |
1 | Ongoing | ||
- *
- These
studies are required by the FDA as a condition of the accelerated approval of FOLOTYN for the treatment of patients with relapsed or refractory PTCL and
must verify the clinical benefit of FOLOTYN.
- **
- These are investigator-sponsored studies being conducted under a collaboration with the NCCN Oncology Research Program.
5
Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. The key elements of our strategy are to:
- •
- Increase sales of FOLOTYN in the United States. We
commenced the U.S. commercial launch of FOLOTYN in January 2010 and reported net product sales of $35.2 million for the year ended December 31, 2010. We have identified approximately
2,000 accounts and 3,000 physicians as priority targets for our sales and marketing efforts. Our commercial organization is currently focused on increasing account penetration, improving brand and
disease state awareness among physicians, and optimizing the duration of treatment with FOLOTYN. Our goal is to position FOLOTYN as the second-line standard of care for PTCL.
- •
- Obtain regulatory approval to market FOLOTYN in foreign
jurisdictions. We are currently seeking regulatory approval to market FOLOTYN in Europe for the treatment of patients with relapsed or
refractory PTCL. Our MAA was accepted for review by the EMA in December 2010. We may also seek regulatory approval to market FOLOTYN in Japan and other countries.
- •
- Secure a strategic partner for the co-development of FOLOTYN globally and commercialization outside the United
States. We currently retain exclusive worldwide rights to develop and commercialize FOLOTYN for all indications. We intend to secure a
strategic partner for the potential co-development of FOLOTYN globally and commercialization outside the United States.
- •
- Advance our FOLOTYN development program in hematologic malignancies and pursue new
indications. We are currently prioritizing our resources on the development and commercialization of FOLOTYN in hematologic
malignancies. FOLOTYN can be delivered as a single agent, for which we currently have approval for the treatment of patients with relapsed or refractory PTCL, and has the potential to be used in
combination therapy regimens. We plan to complete our ongoing clinical trials and initiate new clinical trials to pursue additional indications for FOLOTYN.
- •
- Explore the activity of FOLOTYN in solid tumor indications through targeted
investments. We intend to complete our ongoing Phase 2 studies in bladder and breast cancer, and investigators are evaluating
FOLOTYN in solid tumor indications through our collaboration with the NCCN Oncology Research Program.
- •
- Expand our product portfolio. We may pursue opportunities from time to time to expand our product portfolio by identifying and evaluating new compounds that have demonstrated potential in preclinical or clinical studies and are strategically aligned with our existing oncology portfolio. Our intent is to build a portfolio of proprietary product candidates that have the potential to improve the standard of care in cancer therapy and provide commercial, regulatory or geographic exclusivity.
FOLOTYN (pralatrexate injection)
FOLOTYN is a targeted folate inhibitor designed to accumulate preferentially in cancer cells. Based on preclinical studies, we believe that FOLOTYN selectively enters cells expressing RFC-1, a protein that is frequently over expressed on cancer cells compared to normal cells. Once inside cancer cells, FOLOTYN is efficiently polyglutamylated, which makes it less susceptible to efflux-based drug resistance and leads to high intracellular drug retention compared to other antifolates. Inside the cell, FOLOTYN targets the inhibition of DHFR, an enzyme critical in the folate pathway, thereby interfering with DNA and RNA synthesis and triggering cancer cell death.
The antimetabolites, including antifolates such as FOLOTYN, are a group of low-molecular weight compounds that exert their effect by virtue of their structural or functional similarity to naturally occurring molecules involved in DNA synthesis. Because the cell mistakes them for a normal
6
metabolite, the antimetabolites either inhibit critical enzymes involved in DNA synthesis or become incorporated into the nucleic acid, producing incorrect codes. Both mechanisms result in inhibition of DNA synthesis and ultimately, cell death. Because of their primary effect on DNA synthesis, the antimetabolites are most effective against actively dividing cells and are largely cell-cycle phase specific. There are three classes of antimetabolites; purine analogs, pyrimidine analogs and folic acid analogs, also termed antifolates. FOLOTYN is a folic acid analog.
The selectivity of antifolates for tumor cells involves their conversion to a polyglutamylated form by the enzyme folypolyglutamyl synthetase. Polyglutamylation is a time- and concentration-dependent process that occurs in tumor cells, and to a lesser extent, normal tissue. The selective activity of the folic acid analogs in malignant cells versus normal cells likely is due to the relative difference in polyglutamylate formation. Polyglutamylated metabolites have prolonged intracellular half-life, increased duration of drug action and are potent inhibitors of several folate-dependent enzymes, including DHFR.
We believe that the resistance of malignant cells to the effects of the folic acid analogs may, in part, be due to impaired polyglutamylation. We believe the improved antitumor effects of FOLOTYN in comparison to methotrexate, as observed in preclinical studies, is likely due to the more effective uptake and transport of FOLOTYN into the cell followed by the greater accumulation of FOLOTYN and its metabolites within the tumor cell through the formation of the polyglutamylated derivatives.
FOLOTYN for the Treatment of Patients with Relapsed or Refractory PTCL
United States
On September 24, 2009, the FDA granted accelerated approval of FOLOTYN for use as a single agent for the treatment of patients with relapsed or refractory PTCL. FOLOTYN is the first and only drug approved by the FDA for this indication. We began making FOLOTYN available for commercial sale in the United States on October 5, 2009 and commenced our commercial launch of FOLOTYN in January 2010.
T-cell lymphomas comprise a biologically diverse group of blood cancers that account for approximately 10% to 15% of all cases of non-Hodgkin lymphoma, or NHL, in the United States. The American Cancer Society estimated that approximately 66,000 new cases of NHL were expected to be diagnosed in the U.S. in 2010. We estimate the current annual incidence of PTCL to be approximately 5,900 patients in the United States. There are currently no pharmaceutical agents approved for use in the treatment of first-line PTCL and, prior to the September 2009 approval of FOLOTYN, there were no pharmaceutical agents approved for use in the treatment of patients with relapsed or refractory PTCL. The outcome of patients with PTCL is poor and the majority of patients ultimately have relapsed or refractory disease to a variety of agents, including multi-agent chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) or CHOP-like regimens. The five-year overall survival rate in these patients is approximately 25% to 40%, depending on sub-type.
We have established a commercial organization, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize FOLOTYN in the United States. We believe the U. S. market for relapsed or refractory PTCL is addressable with a targeted sales and marketing organization, and we intend to continue promoting FOLOTYN ourselves in the United States.
We have also established the Allos Support for Assisting Patients, or ASAP program, to facilitate access to FOLOTYN by providing reimbursement resources to uninsured, underinsured and insured patients, and reimbursement support to healthcare providers. In addition, through the ASAP program, indigent patients who are not eligible for reimbursement may obtain free drug.
In October 2009, the NCCN updated its Clinical Practice Guidelines in Oncology™ for Non-Hodgkin Lymphomas to include FOLOTYN as a suggested treatment regimen for all patients with
7
second-line PTCL. NCCN is recognized by the Centers for Medicare and Medicaid Services and private payers as a mandated reference for oncology coverage policies. Managed care, medical directors, pharmacy benefit directors and other healthcare professionals also reference NCCN compendia when making treatment and reimbursement decisions.
FOLOTYN was approved by the FDA based on the results from PROPEL, an open-label, single-arm, multi-center, international clinical trial that enrolled 115 patients with relapsed or refractory PTCL, 109 of whom were considered evaluable for efficacy according to the trial protocol. Patients were considered evaluable if they received at least one dose of FOLOTYN, their diagnosis of PTCL was confirmed by independent pathology review, and they had relapsed or refractory disease after at least one prior treatment. Patients were treated with FOLOTYN at 30 mg/m2 once weekly by intravenous push over 3-5 minutes for 6 weeks in 7-week cycles until disease progression or unacceptable toxicity. In addition, patients received 1 mg of vitamin B12 intramuscularly every 8-10 weeks and 1.0-1.25 mg of folic acid orally on a daily basis.
The primary efficacy endpoint of the trial was overall response rate (complete response, complete response unconfirmed and partial response) as assessed by International Workshop Criteria, or IWC. The key secondary efficacy endpoint was duration of response. Response assessments were scheduled at the end of cycle 1 and then every other cycle (every 14 weeks). Duration of response was measured from the first day of documented response to disease progression or death. Response and disease progression were evaluated by independent central review using the IWC. The results of the trial demonstrated that 29 of 109 evaluable patients, or 27%, responded to FOLOTYN. The median duration of response was 287 days, or 9.4 months (range 1-503 days). Thirteen of 109 evaluable patients had a duration of response greater-than or equal to 14 weeks (range 98-503 days). The most common grade 3/4 adverse events were thrombocytopenia, which was observed in 33% of patients; mucositis in 21% of patients; neutropenia in 20% of patients; and anemia in 17% of patients.
In December 2009, updated results from the PROPEL trial were presented at the 51st Annual Meeting of the American Society of Hematology, or ASH. The updated results demonstrated that patients treated with FOLOTYN achieved an overall response rate of 29% (32 of 109 evaluable patients) with 63% of patients responding within the first cycle of therapy. The median duration of response was 10.1 months and median overall survival was 14.5 months.
The FDA has awarded orphan drug status to FOLOTYN for the treatment of patients with T-cell lymphoma, which includes patients with relapsed or refractory PTCL. Orphan drug designation is granted by the FDA to drugs intended to treat a rare disease or condition, which for this program is defined as having a prevalence of less than 200,000 individuals in the United States. Under the U.S. Orphan Drug Act, the first company to receive FDA approval for an orphan drug for a designated indication obtains seven years of marketing exclusivity during which the FDA may not approve another company's application for the same orphan drug for the same orphan indication. Because the FDA approved pralatrexate (which we market as FOLOTYN) for the treatment of patients with relapsed or refractory PTCL, a subset of T-cell lymphoma, we expect to receive seven years of marketing exclusivity for that indication. However, if a competitive product that is the same as FOLOTYN, as defined under the applicable regulations, is shown to be clinically superior to our product in the treatment of patients with relapsed or refractory PTCL, or if a competitive product is different from FOLOTYN, as defined under the applicable regulations, the orphan drug exclusivity we have obtained may not block the approval of such competitive product.
Europe and Other Countries
We are currently seeking regulatory approval to market FOLOTYN in Europe for the treatment of patients with relapsed or refractory PTCL. In December 2010, our MAA was accepted for review by the EMA. Acceptance of the MAA indicates that the application is complete and initiates the EMA's regulatory review process. The EMA has granted orphan medicinal product designation to FOLOTYN
8
for the treatment of nodal, other extranodal, and leukaemic/disseminated PTCL. We may also seek regulatory approval to market FOLOTYN for the treatment of patients with relapsed or refractory PTCL in Japan and other countries.
There is a high unmet medical need with no approved agents in either Europe or Japan for the treatment of patients with relapsed or refractory PTCL. We estimate the annual incidence of PTCL in the top five European markets (Germany, France, Italy, Spain and the United Kingdom) in 2010 to be approximately 6,000 to 7,000 patients, with an estimated 4,500 to 6,000 second-line PTCL patients. Likewise, based on our internal research, we estimate the incidence of PTCL in Japan in 2010 to be approximately 3,700 to 4,100 patients, with an estimated 2,800 to 3,500 second-line PTCL patients.
We currently retain exclusive worldwide rights to develop and commercialize FOLOTYN for all indications. One of our key objectives for 2011 is to secure a strategic partner for the potential co-development of FOLOTYN globally and commercialization outside the United States.
We currently provide healthcare professionals outside of the United States with access to FOLOTYN through a named patient program, which is a mechanism through which physicians can prescribe investigational drugs under individual country-specific guidelines for patients prior to marketing approval.
FOLOTYN Post-approval Clinical Studies
FOLOTYN was approved for the treatment of patients with relapsed or refractory PTCL in the United States under the FDA's accelerated approval program, which allows the FDA to approve products for cancer or other life-threatening diseases based on initial positive clinical data. As a condition of approval, we are required to conduct the following post-approval studies that are intended to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphomas and assess whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment:
- •
- A Phase 3, multi-center, randomized clinical study of sequential FOLOTYN versus observation in patients with newly
diagnosed aggressive PTCL who have responded following initial treatment with chemotherapy based on CHOP. Patients responding (either a complete response or a partial response) after
CHOP-based treatment will be randomized 2:1 to FOLOTYN versus observation. In the first quarter of 2011, we reached agreement with the FDA under its Special Protocol Assessment process
(SPA) on the design of this trial. The SPA process provides an agreement that the study design, including trial size, clinical endpoints and/or data analyses are acceptable to the FDA. The SPA
agreement is not a guarantee of approval, and we cannot assure you that the design of, or data collected from, this trial will be adequate to demonstrate the safety and efficacy of FOLOTYN in this
patient population, or otherwise be sufficient to support FDA or any foreign regulatory approval. We plan to initiate this study in 2011. We have agreed to submit the results of this study to the FDA
by June 30, 2017.
- •
- A Phase 3, multi-center, randomized clinical study comparing FOLOTYN in combination with systemic bexarotene versus systemic bexarotene alone in patients with cutaneous T-cell lymphoma, or CTCL, who are refractory to at least one prior systemic therapy. Prior to initiation of the Phase 3 study, we will conduct a Phase 1 study to determine the maximum tolerated dose, or MTD of the combination. We initiated the Phase 1 study in 2010 and patient enrollment is ongoing. We have agreed to complete the Phase 1 study by August 31, 2011. The final design of the Phase 3 study, including number of patients, clinical endpoints and other study details, remains subject to review by the FDA. We have agreed to submit the results of the Phase 3 study to the FDA by September 30, 2015.
9
- •
- A Phase 1 clinical study to evaluate the pharmacokinetics of FOLOTYN in relapsed or refractory advanced solid tumor
or advanced lymphoma patients (B-cell, T-cell and Hodgkin lymphoma) with mild to severe renal impairment. The trial will have four cohorts of six patients for a total of 24
patients. Cohorts will be based on the severity of renal impairment: severely impaired, moderately impaired, mildly impaired and normal. We plan to initiate this study in 2011. We have agreed to
submit the results of this study to the FDA by January 31, 2013.
- •
- Completion of an ongoing Phase 1 mass balance clinical study to evaluate the excretion and metabolic profile of FOLOTYN.
Failure to complete these post-approval studies or adhere to the timelines set by the FDA could result in penalties, including fines or withdrawal of FOLOTYN from the market, unless we are able to demonstrate good cause for not completing the studies or adhering to the timelines. The FDA may also initiate proceedings to withdraw approval if our Phase 3 post-approval studies fail to verify the clinical benefit of FOLOTYN. Further, the FDA may require us to strengthen the warnings and precautions section of the FOLOTYN package insert based on the results of the Phase 1 studies.
FOLOTYN Clinical Development Program
We are developing FOLOTYN both as a single agent and in combination therapy regimens in a variety of hematologic malignancies and solid tumor indications. In addition to the post-approval clinical studies discussed above, the following is a summary of the target indications and clinical development status of the FOLOTYN development program.
Cutaneous T-Cell Lymphoma
Cutaneous T-cell lymphoma, or CTCL, is comprised of a number of indolent non-Hodgkin T-cell lymphomas, including mycosis fungoides and Sézary syndrome. In its early stages, CTCL primarily affects the skin, causing patches, plaques, and tumors, as well as redness and itching; however, as it progresses, CTCL can spread to the blood, lymph nodes and internal organs. For some patients, their disease will progress despite treatment with topical and skin directed therapies and will require systemic treatments, including chemotherapies. Even when CTCL is found in an early stage, it can be difficult to treat and usually returns after initial treatment. According to the Lymphoma Research Foundation, CTCL accounts for approximately 2% to 3% of the estimated 66,000 new cases of NHL diagnosed each year in the United States. According to the Cutaneous Lymphoma Foundation, the prevalence of CTCL in the United States is estimated between 16,000 and 20,000 cases.
In August 2007, we initiated patient enrollment in a Phase 1, open-label, multi-center study of FOLOTYN with vitamin B12 and folic acid supplementation in patients with relapsed or refractory CTCL. In this study, patients with either relapsed or refractory CTCL receive FOLOTYN as part of a weekly schedule for two or three weeks followed by one week of rest. In the Phase 1 dose de-escalation portion, patients received a starting dose of FOLOTYN at 30 mg/m2, with dose reduction in subsequent cohorts to identify an optimal dose for patients with CTCL based on tolerability and efficacy. The dosing regimen of 15 mg/m2 weekly for three weeks out of a four-week cycle was determined to be the optimal starting dose and schedule that provided activity with tolerability.
In December 2010, data from this Phase 1 study were presented at the 52nd Annual ASH Meeting. Of the total 54 patients enrolled in the trial (which includes patients who received lower than the optimal dose), objective responses were observed in 22 patients (41%), including three complete responses and 19 partial responses. In total, 41 of the 54 patients were treated at the optimal dose of FOLOTYN or higher; of these 41 patients, responses were observed in 21 patients (51%). Responses to FOLOTYN were observed in patients whose disease failed to respond to key prior systemic therapies, including 46% of patients whose disease failed to respond to oral bexarotene, 46% whose disease failed to respond to methotrexate, 41% whose disease failed to respond to HDAC inhibitors, and 36% whose
10
disease failed to respond to interferon. Patients in the study were heavily pretreated, having received a median of 6.5 prior therapies (range 1-25) and 4.0 prior systemic therapies (range 1-11). For the 29 patients treated at the optimal starting dose and schedule:
- •
- overall response rate was 45% (13 out of 29 patients);
- •
- responses were durable, with a Kaplan-Meier estimate for duration of response of 73% at six months; and
- •
- median progression-free survival had not been reached at the time of this analysis. Progression-free survival ranged from 1-429 days.
In the 29 patients treated at the optimal dose and schedule, Grade 3-4 adverse events observed were mucositis (17%), thrombocytopenia (3%) and fatigue (3%); and the most common Grade 1-2 adverse events observed were fatigue (34%), mucositis (31%), nausea (31%), epistaxis/nose bleeds (24%), edema (14%) and vomiting (14%). There was no neutropenia reported in patients at the optimal dose or higher.
As part of our post-approval clinical studies discussed above, we plan to initiate a Phase 3, multi-center, randomized clinical study comparing FOLOTYN in combination with systemic bexarotene versus systemic bexarotene alone in patients with CTCL who are refractory to at least one prior systemic therapy. Prior to initiation of the Phase 3 study, we will conduct a Phase 1 study to determine the maximum tolerated dose of the combination. We initiated the Phase 1 study in 2010 and patient enrollment is ongoing. We have agreed to complete the Phase 1 study by August 31, 2011. The final design of the Phase 3 Study, including number of patients, clinical endpoints and other study details remains subject to review by the FDA. We have agreed to submit the results of the Phase 3 study to the FDA by September 30, 2015.
In June 2010, the European Commission, or EC, granted orphan medicinal product designation for pralatrexate for the treatment of CTCL. The EMA orphan medicinal product designation is intended to promote the development of drugs that may provide significant benefit to patients suffering from rare diseases identified as life-threatening or very serious. Under EMA guidelines, orphan medicinal product designation provides ten years of potential market exclusivity once the product is approved for marketing for the designated indication in the European Union.
Non-Hodgkin Lymphoma and Hodgkin Lymphoma
According to the American Cancer Society, an estimated 66,000 new cases of NHL were expected to be diagnosed in the United States in 2010. Approximately 85% of NHL patients represent patients with B-cell lymphoma. Patients with indolent or low-grade NHL may have survival rates as long as 10 years, yet the disease is usually not curable in advanced stages. Aggressive lymphomas generally result in shorter median survival times although patients with these malignancies can be cured in 30% to 60% of cases.
In May 2007, we initiated patient enrollment in a Phase 1/2a, open-label, multi-center study of FOLOTYN and gemcitabine with vitamin B12 and folic acid supplementation in patients with relapsed or refractory NHL or Hodgkin's lymphoma. In the Phase 1 portion of this study, patients with either relapsed or refractory NHL or Hodgkin's lymphoma received FOLOTYN either concurrently on the same day with or followed on sequential days by gemcitabine as part of a weekly schedule for three or four weeks or every two weeks with concurrent vitamin B12 and folic acid supplementation. We enrolled 35 evaluable patients in the Phase 1 portion of the study with the objective of determining the maximum tolerated dose, or MTD, safety, tolerability, and pharmacokinetic profile of escalating doses of sequential or same day administration of FOLOTYN and gemcitabine. The MTD for the sequential dosing regimen was determined to be 10 mg/m2 of FOLOTYN followed on sequential days by 400 mg/m2 of gemcitabine in an every two week schedule. The MTD for the same-day regimen was
11
determined to be 15 mg/m2 of FOLOTYN and 600 mg/m2 of gemcitabine in an every two week schedule. The Phase 2a portion of the trial is closed to enrollment, and we expect to report a final analysis of the data for this trial by the first half of 2012.
In September 2009, we initiated patient enrollment in a Phase 2, open-label, single-arm, multi-center trial of FOLOTYN in patients with relapsed or refractory B-cell NHL. This trial will seek to enroll approximately 27 evaluable patients in up to 10 investigative sites worldwide. The primary endpoint of the study is objective response rate (complete and partial response) as assessed by IWC. Secondary endpoints include duration of response, progression-free survival, overall survival, and the safety and tolerability of FOLOTYN.
In October 2008, the FDA granted orphan drug designation to FOLOTYN for the treatment of patients with follicular lymphoma and for the treatment of patients with diffuse large B-cell lymphoma. In October 2010, the EC granted orphan medicinal product designation for pralatrexate for the treatment of Hodgkin Lymphoma.
Non-Small Cell Lung Cancer
Lung cancer is the most common cause of cancer death in the United States. According to the American Cancer Society, an estimated 222,520 new cases of lung cancer were expected to be diagnosed in the United States in 2010. Non-small cell lung cancer, or NSCLC, accounts for the majority of lung cancers, or approximately 85%. The three most common subtypes of NSCLC are squamous cell carcinoma, which accounts for 25% to 30% of all lung cancers; adenocarcinoma, which is the most common type of lung cancer and accounts for about 40% of lung cancers; and large-cell undifferentiated carcinoma, which accounts for 10% to 15% of lung cancers. The majority of people are diagnosed with advanced stage disease and only one to five percent of people with advanced stage (IIIB/IV) NSCLC survive to five years. The most widely used therapies to date remain surgery, chemotherapy and radiation therapy.
In January 2008, we initiated patient enrollment in a Phase 2b, randomized, international, multi-center study comparing FOLOTYN and erlotinib in second or third line patients with Stage IIIB/IV NSCLC who are, or have been, cigarette smokers who have failed treatment with at least one prior platinum-based chemotherapy regimen. We completed enrollment of this study in July 2009 with 201 patients. The objectives of this Phase 2b study were to estimate the efficacy of FOLOTYN relative to that of erlotinib as assessed by overall survival, or OS, the primary endpoint of the trial, and to determine the treatment effect of both drugs in predefined patient cohorts, including light vs. heavy smokers; current vs. former smokers; squamous vs. non-squamous histology; and patients who received prior pemetrexed vs. those who have not. In the fourth quarter of 2010, we announced the presentation of favorable survival data from this trial. The results demonstrated that patients receiving FOLOTYN had a 16% lower risk of death than those treated with erlotinib in the overall (intent-to-treat) population (n=201; hazard ratio (HR)=0.84) and a 13% lower risk of death in the primary efficacy analysis population (n=166; HR=0.87). At six months, 56% of patients treated with FOLOTYN were alive and 51% of patients treated with erlotinib were alive; at one year, 28% of patients treated with FOLOTYN were alive and 18% of patients treated with erlotinib were alive. The median overall survival (OS) time was 6.7 months for patients who received FOLOTYN and 7.0 months for patients who received erlotinib.
Secondary endpoints included progression-free survival (PFS) (HR=0.91; median PFS=3.4 months and 2.8 months for FOLOTYN and erlotinib, respectively) and objective response rate (2% and 7%, respectively). Analyses were also performed according to the statistical analysis plan to assess the activity of FOLOTYN and erlotinib in predefined patient cohorts. These analyses indicated that the majority of cohorts responded favorably to FOLOTYN relative to erlotinib. The most favorable data were observed in patients with non-squamous cell carcinoma (n=107), which demonstrated that
12
patients who received FOLOTYN showed a 35% lower risk of death (OS HR=0.65) and 42% reduction in the risk of disease progression (PFS HR=0.58) relative to erlotinib. In patients with squamous cell carcinoma, a HR for OS of 1.06 was observed, which suggests activity of FOLOTYN given that erlotinib has historically shown a survival benefit in these patients. In the small subset of patients who received prior pemetrexed (n=30), a HR for OS of 1.15 was observed.
The safety profile of FOLOTYN was consistent with that observed and reported in previous FOLOTYN solid tumor studies. The most common Grade 3-4 adverse event observed in patients treated with FOLOTYN was mucositis (23%). Other Grade 3-4 adverse events occurring in more than 5% of patients were fatigue (9%), dyspnea (6%), neutropenia (6%), thrombocytopenia (5%) and anemia (5%) in patients treated with FOLOTYN, and rash (8%), dyspnea (8%), anemia (8%) and fatigue (5%) in patients treated with erlotinib. Of those patients treated with FOLOTYN (n=97) in the trial, 32 patients (33%) discontinued treatment due to adverse events.
In January 2011, we announced that we will not pursue Phase 3 studies for NSCLC at this time based on our assessment of the costs of future development and the potential clinical, regulatory and commercial opportunities in this indication.
Bladder Cancer
According to the American Cancer Society, an estimated 70,530 new cases of bladder cancer were expected to be diagnosed in the United States in 2010. Transitional cell carcinoma, or TCC, is the most common form of bladder cancer, accounting for more than 97% of all bladder cancers. There are currently no agents approved in the United States for the treatment of advanced or metastatic relapsed TCC of the urinary bladder.
In July 2008, we initiated patient enrollment in a Phase 2, open-label, single-arm, multi-center study of FOLOTYN in patients with advanced or metastatic relapsed TCC of the urinary bladder. The primary endpoint of the study is objective response rate (complete and partial response). Secondary endpoints include duration of response, clinical benefit rate, progression-free survival, overall survival and the safety and tolerability of FOLOTYN. Patients received FOLOTYN as an IV push administered on days 1 and 15 of a 4-week/28 day cycle. The initial dose of FOLOTYN is 190 mg/m2, which may be adjusted based on criteria defined in the protocol. Patients received concurrent vitamin therapy of B12 and folic acid. This Phase 2 study is closed to enrollment and we expect to report top line data for this trial in the second half of 2011.
In March 2009, the EC granted orphan medicinal product designation to pralatrexate for the treatment of patients with non-papillary TCC of the urinary bladder. In May 2010, the FDA granted orphan drug designation to FOLOTYN for the treatment of patients with advanced or metastatic TCC of the urinary bladder.
Breast Cancer
Breast cancer is the second most common cancer among American women. According to the American Cancer Society, an estimated 207,090 new cases of invasive breast cancer were expected to be diagnosed in women in the United States in 2010.
In the second quarter of 2010, we initiated patient enrollment in a Phase 2, open-label, single-arm, multi-center international study of FOLOTYN in female patients with advanced or metastatic breast cancer who have failed prior chemotherapy. The primary endpoint is objective response. Secondary endpoints include duration of response, overall survival, safety and pharmacokinetic parameters. Patients will be dosed at 190 mg/m2 every two weeks of a four-week cycle. The study will seek to enroll approximately 30 patients. Patient enrollment is ongoing and we expect to report top line data for this trial in the second half of 2011.
13
NCCN Program
In addition to our ongoing company-sponsored clinical studies, investigators are evaluating FOLOTYN in a variety of solid tumor indications and multiple myeloma through our collaboration with the NCCN Oncology Research Program. We have agreed to provide the NCCN with a research grant of approximately $2.3 million for the study of FOLOTYN as a single agent and in combination therapy regimens, of which approximately $860,000 was paid as of the end of 2010. The current investigator-sponsored studies include a:
- •
- Phase 1/2 study of Carboplatin and FOLOTYN in patients with recurrent platinum sensitive ovarian, fallopian or primary
peritoneal cancer,
- •
- Phase 1 study of Bortezomib in combination with FOLOTYN in relapsed or refractory multiple myeloma,
- •
- Phase 1 study of sequential FOLOTYN followed by a 48-hour infusion of 5-Fluorouracil given
every other week in adult patients with solid tumors,
- •
- Phase 2, multi-center, study of FOLOTYN with vitamin B12 and folic acid supplementation for previously
treated recurrent or metastatic head and neck squamous cell cancer,
- •
- Phase 2 study of FOLOTYN in combination with Oxaliplatin in advanced esophago-gastric cancer, and
- •
- Phase 2 study of FOLOTYN and Docetaxel in patients with advanced esophageal and gastroesophageal carcinoma who have failed prior platinum-based therapy.
There can be no assurances that we will pursue the development of FOLOTYN for one or more of these indications or that such development efforts will be ultimately successful.
Manufacturing
The production of FOLOTYN employs small molecule organic chemistry procedures standard for the pharmaceutical industry. We have arrangements with two third-party manufacturers to produce FOLOTYN bulk drug substance and two third-party manufacturers to produce FOLOTYN formulated drug product. We believe these third-party manufacturers have the capability to meet our projected worldwide clinical trial and commercial requirements for FOLOTYN although we cannot assure you of this. Prior to receiving FDA approval of FOLOTYN, all costs related to purchases of the active pharmaceutical ingredient and the manufacturing of FOLOTYN were recorded as research and development expense. As such, we have established supplies of FOLOTYN bulk drug substance and formulated drug product that are not recorded on our balance sheet as inventory.
We plan to continue to outsource manufacturing responsibilities for FOLOTYN and any additional future product candidates. We believe this manufacturing strategy allows us to direct our financial and managerial resources to the development and commercialization of products rather than to the establishment of a manufacturing infrastructure. We believe it also enables us to minimize fixed costs and capital expenditures, while gaining access to advanced manufacturing process capabilities and expertise. However, if our third party suppliers become unable or unwilling to provide sufficient future drug supply or meet regulatory requirements relating to the manufacture of pharmaceutical agents, we would be forced to incur additional expenses to secure alternative third party manufacturing arrangements and may suffer delays in our ability to conduct clinical trials or commercialize FOLOTYN or future products.
14
Sales and Marketing
We have established a commercial organization, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize FOLOTYN in the United States. We believe the U. S. market for relapsed or refractory PTCL is addressable with a targeted sales and marketing organization, and we intend to continue promoting FOLOTYN ourselves in the United States for this and any additional indications we may obtain in the future. We intend to secure a strategic partner for the co-development of FOLOTYN globally and commercialization outside the United States.
Intellectual Property
We believe that patent protection and trade secret protection are important to our business and that our future success will depend, in part, on our ability to maintain our technology licenses, maintain trade secret protection, obtain and maintain patents and operate without infringing the proprietary rights of others both in the United States and abroad. We believe that obtaining identical patents and protection periods for a given technology throughout all markets of the world will be difficult because of differences in patent laws. In addition, the protection provided by non-U.S. patents, if any, may be weaker than that provided by U.S. patents.
In order to protect the confidentiality of our technology, including trade secrets and know-how and other proprietary technical and business information, we require all of our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the use or disclosure of confidential information. The agreements also oblige our employees, consultants, advisors and collaborators to assign or license to us ideas, developments, discoveries and inventions made by such persons in connection with their work with us. We cannot be sure that these agreements will maintain confidentiality, will prevent disclosure, or will protect our proprietary information or intellectual property, or that others will not independently develop substantially equivalent proprietary information or intellectual property.
The pharmaceutical industry is highly competitive and patents have been applied for by, and issued to, other parties relating to products or new technologies that may be competitive with those being developed by us. Therefore, FOLOTYN may give rise to claims that it infringes the patents or proprietary rights of other parties now or in the future. Furthermore, to the extent that we, our consultants, or manufacturing and research collaborators, use intellectual property owned by others in work performed for us, disputes may also arise as to the rights to such intellectual property or in related or resulting know-how and inventions. An adverse claim could subject us to significant liabilities to such other parties and/or require disputed rights to be licensed from such other parties. A license required under any such patents or proprietary rights may not be available to us, or may not be available on acceptable terms. If we do not obtain such licenses, we may encounter delays in product market introductions, or may find that we are prevented from the development, manufacture or sale of products requiring such licenses. In addition, we could incur substantial costs in defending ourselves in legal proceedings instituted before patent and trademark offices in the United States, the European Union, or other ex-U.S. territories, or in a suit brought against us by a private party based on such patents or proprietary rights, or in a suit by us asserting our patent or proprietary rights against another party, even if the outcome is not adverse to us.
FOLOTYN License Agreement
In December 2002, we entered into a license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, as amended, under which we obtained exclusive worldwide rights to a portfolio of patents and patent applications related to FOLOTYN and its uses. The portfolio currently consists of three issued patents in the United States, two granted
15
patents in Europe, a single granted patent in each of Mexico, New Zealand, Singapore and South Africa, and pending patent applications in the United States, Canada, Europe, Australia, Japan, China, Brazil, Indonesia, India, South Korea, Mexico, Norway, New Zealand and the Philippines. The licensed patents and applications, which expire at various times between July 2017 and May 2025, contain claims covering FOLOTYN substantially free of 10-deazaaminopterin, methods to treat tumors with FOLOTYN substantially free of 10-deazaaminopterin, treatment of breast, lung, and prostate cancer and leukemia with a combination of FOLOTYN and a taxane, treatment of T-cell lymphoma with FOLOTYN, treatment of lymphoma with a combination of FOLOTYN and gemcitabine, methods of assessing sensitivity of a tumor to FOLOTYN, and other methods and compositions.
Under the terms of the agreement, we paid an up-front license fee of $2.0 million upon execution of the agreement and have made aggregate milestone payments of $2.5 million based on the passage of time. Additionally, in May and September 2009, we made milestone payments of $1.5 million based on the FDA accepting our New Drug Application for review and $5.8 million based on the FDA approval to market FOLOTYN, respectively. The up-front license fee and all milestone payments under the agreement prior to FDA approval to market FOLOTYN were recorded to research and development expense as incurred. The $5.8 million milestone payment based on the FDA approval was capitalized as an intangible asset and is being amortized over the expected useful life of the composition of matter patent for FOLOTYN, which we expect to last until July 16, 2022. The only remaining potential milestone payment under the license agreement is for $3.5 million upon regulatory approval to market FOLOTYN in Europe, which, if made would be capitalized and amortized over the expected useful life. In addition, we will pay the licensors royalties based on graduated annual levels of net sales of FOLOTYN to our distributors, net of actual rebates and chargebacks, or distributor sales, which may be different than our net product revenue recognized in accordance with U.S. generally accepted accounting principles, or GAAP, or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur. Royalties are 8% of annual distributor sales up to $150.0 million; 9% of annual distributor sales of $150.0 million through $300.0 million; and 11% of annual distributor sales in excess of $300.0 million. In 2010 and 2009, our royalties were 8% of our net distributor sales.
Customers
We sell FOLOTYN to a limited number of pharmaceutical wholesale distributors, or distributors, who then resell FOLOTYN to patients' respective health care providers. We had $35.2 million and $3.6 million of net product sales for the years ended December 31, 2010 and 2009, respectively. Three distributors affiliated with AmerisourceBergen Corporation accounted for approximately 99% and 100% of our net product sales for the years ended December 31, 2010 and 2009, respectively. We anticipate that affiliates of AmerisourceBergen Corporation will continue to account for substantially all of our net product sales in 2011. We had less than 1% of sales outside of the United States during the year ended December 31, 2010 and none outside the United States during the year ended December 31, 2009.
Competition
There are currently no FDA-approved drugs other than FOLOTYN for the treatment of patients with relapsed or refractory PTCL. However, we are aware of multiple investigational agents that are currently being studied in clinical trials for peripheral T-cell lymphoma, including romidepsin, belinostat and brentuximab vedotin, which, if successful, may compete with FOLOTYN in the United States in 2011. In addition, there are many existing approaches used in the treatment of relapsed or refractory PTCL, including combination chemotherapy and single agent regimens, which represent competition for FOLOTYN.
16
Many companies of all sizes, including a number of large pharmaceutical companies and several biotechnology companies, are developing product candidates that have disease targets similar to those we are pursuing. Some of these competitive product candidates are in clinical trials and others are approved. There are products and technologies currently on the market that will compete directly with FOLOTYN. Universities, governmental agencies and other public and private research organizations also conduct research and may market commercial products on their own or through joint ventures. These companies and institutions also compete with us in recruiting qualified scientific personnel. Many of these entities may have:
- •
- substantially greater financial and other resources;
- •
- larger research and development staffs;
- •
- lower labor costs; and/or
- •
- more extensive sales, marketing and manufacturing organizations.
Many of these companies and organizations have significant experience in preclinical testing, human clinical trials, product manufacturing, marketing, sales and distribution and other regulatory approval and commercial procedures. They may also have a greater number of significant patents and greater legal resources to seek remedies for cases of alleged infringement of their patents by us to block, delay, or compromise our own drug development process.
We expect technology developments in our industry to continue to occur at a rapid pace. Commercial developments by our competitors may render FOLOTYN obsolete or non-competitive, which would have a material adverse effect on our business and financial condition.
Government Regulation
We operate in a highly regulated industry, which is subject to significant federal, state, local and foreign regulation. Our present and future business has been, and will continue to be, subject to a variety of laws and regulations, including the Federal Food, Drug, and Cosmetic Act, or FDC Act, the Medicaid rebate program, the Veterans Health Care Act of 1992, and the Occupational Safety and Health Act, among others.
As a result of these laws and regulations, product development and product approval processes are very expensive and time consuming.
FDA Approval Process
In the U.S., pharmaceutical products are subject to extensive regulation by the FDA. The FDC Act and other federal and state statutes and regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, marketing and promotion, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications or NDAs, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development in the U.S. typically involves preclinical laboratory and animal tests, the submission to the FDA of a notice of claimed investigational exemption or an investigational new drug application or IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease.
17
Preclinical tests include laboratory evaluation, as well as animal trials to assess the characteristics and potential pharmacology and toxicity of the product. The conduct of the preclinical tests must comply with federal regulations and requirements including good laboratory practices. The results of preclinical testing are submitted to the FDA as part of an IND along with other information including information about product chemistry, manufacturing and controls and a proposed clinical trial protocol. Long term preclinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has not objected to the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted in compliance with federal regulations, good clinical practices or GCP, as well as under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary or permanent discontinuation of a clinical trial at any time or impose other sanctions if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an institutional review board, or IRB, for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB's requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses and, if possible, early evidence on effectiveness. In the case of product candidates for severe or life-threatening diseases such as cancer, the initial human testing is often conducted in patients rather than in healthy volunteers. Since these patients already have the target disease, these studies may provide initial evidence of efficacy traditionally obtained in Phase 2 trials and thus these trials are frequently referred to as Phase 1b trials. Additionally, when product candidates can do damage to normal cells, it is not ethical to administer such drugs to healthy patients in a Phase 1 trial. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication or indications, dosage tolerance and optimum dosage, and identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit the FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all preclinical, clinical and other testing and a compilation of data relating to the product's pharmacology, chemistry, manufacture, and controls.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for non-priority drug products are reviewed within ten months. The review process may be
18
extended by the FDA for three additional months to consider new information submitted during the review or clarification regarding information already provided in the submission. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. The FDA will not approve the product unless compliance with current good manufacturing practices or cGMPs, is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After the FDA evaluates the NDA and the manufacturing facilities, it issues an approval letter or a complete response letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require substantial post-approval testing and surveillance to monitor the drug's safety or efficacy and may impose other conditions, including labeling restrictions which can materially affect the potential market and profitability of the drug. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
A complete response letter outlines the deficiencies in an NDA submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If and when those deficiencies have been addressed in a resubmission of the NDA, FDA will re-initiate review. If it is satisfied that the deficiencies have been addressed, the FDA will issue an approval letter. The FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included. It is not unusual for the FDA to issue a complete response letter because it believes that the drug is not safe enough or effective enough or because it does not believe that the data submitted are reliable or conclusive.
Accelerated Approval
Under the FDA's accelerated approval regulations, the FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit. In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions or survives. Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-approval studies, will allow the FDA to withdraw the drug from the market on an expedited basis. In addition, all promotional materials for drug candidates approved under accelerated regulations are subject to prior review by the FDA.
FOLOTYN was approved for the treatment of patients with relapsed or refractory PTCL under the FDA's accelerated approval regulations. The approval was based on overall response rate from our PROPEL trial. In connection with the accelerated approval, we are required to conduct several post-approval studies that are intended to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma and assess whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment.
19
Other Regulatory Requirements
Once an NDA is approved, a product is subject to certain post-approval requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet.
Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs. We cannot be certain that the FDA or any other regulatory agency will grant approval for FOLOTYN for any additional indications or any other product candidate for any indication on a timely basis, if at all.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, risk evaluation and mitigation strategies, and surveillance to monitor the effects of an approved product or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control as well as drug manufacture, packaging, and labeling procedures must continue to conform to current good manufacturing practices, or cGMPs, after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA during which the agency inspects manufacturing facilities to access compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs. Regulatory authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Orphan Drug Designation
Orphan drug designation is granted by the FDA to drugs intended to treat a rare disease or condition, which for this program is defined as having a prevalence of less than 200,000 individuals in the U.S. Orphan drug designation must be requested before submitting a marketing application. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
Orphan drug designation does not shorten the regulatory review and approval process for an orphan drug, nor does it give that drug any advantage in the regulatory review and approval process. However, if an orphan drug later receives the first approval for the indication for which it has orphan drug designation, the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years in the U.S. Orphan drug exclusivity may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug. The FDA has granted orphan drug designation to FOLOTYN for the treatment of patients with T-cell lymphoma, follicular lymphoma, diffuse large B-cell lymphoma and advanced or metastatic TCC of the urinary bladder. Upon FDA approval of FOLOTYN for the treatment of relapsed or refractory PTCL, FOLOTYN received seven years of orphan drug exclusivity for this indication until September 24, 2016.
20
Although obtaining approval to market a product with orphan drug exclusivity may be advantageous, we cannot be certain:
- •
- that we will be the first to obtain approval for any other drugs or indications for which we obtain orphan drug
designation;
- •
- that orphan drug designation will result in any commercial advantage or reduce competition; or
- •
- that the limited exceptions to this exclusivity will not be invoked by the FDA.
The Hatch-Waxman Act
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent with claims that cover the applicant's product or FDA approved method of using this product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application, or ANDA. An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown through bioequivalence testing to be therapeutically equivalent to the listed drug. ANDA applicants are not required to conduct or submit results of pre-clinical or clinical tests to prove the safety or effectiveness of their drug product, other than the requirement for bioequivalence testing. Drugs approved in this way are commonly referred to as "generic equivalents" to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA's Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. A certification that the new product will not infringe the already approved product's listed patents or that such patents are invalid is called a Paragraph IV certification. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification notification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit or a decision in the infringement case that is favorable to the ANDA applicant. The ANDA application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. Federal law provides a period of five years of exclusivity following approval of a drug containing no previously approved active moiety, during which ANDAs for generic versions of those drugs cannot be submitted unless the submission contains a Paragraph IV challenge to a listed patent, in which case the submission may be made four years following the original product approval. Federal law also provides for a period of three years of exclusivity following approval of a listed drug that contains previously approved active ingredients that is approved in a new dosage form, route of administration or combination, or for a new use, the approval of which was required to be supported by new clinical trials conducted by or for the sponsor, during which the FDA cannot grant effective approval of an ANDA based on that listed drug.
21
U.S. Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Federal and State Fraud and Abuse Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical and medical device industries in recent years. These laws include anti-kickback statutes and false claims statutes.
The federal health care program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any health care item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Recently, several pharmaceutical and other health care companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company's marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer's products from reimbursement under government programs, criminal fines, and imprisonment.
Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Such a challenge could have a material adverse effect on our business, financial condition and results of operations.
In addition, as part of the sales and marketing process, pharmaceutical companies frequently provide samples of approved drugs to physicians. This practice is regulated by the FDA and other governmental authorities, including, in particular, requirements concerning record keeping and control procedures. Any failure to comply with the regulations may result in significant criminal and civil penalties as well as damage to our credibility in the marketplace.
22
Foreign Regulation and Product Approval
Outside the United States, our ability to market FOLOTYN is contingent upon receiving marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. At present, foreign marketing authorizations are applied for at a national level, although within the European Union centralized registration procedures are available to companies wishing to market a product in more than one European Union member state. If the regulatory authority is satisfied that adequate evidence of safety, quality and efficacy has been presented, a marketing authorization will be granted. In some countries in the European Union, pricing of prescription drugs is subject to government control and agreements must be reached on a national level before marketing may begin in that country. If we are unable to reach agreement on an acceptable price for our products, we may choose not to pursue marketing of FOLOTYN in that country. The foreign regulatory approval process involves all of the risks associated with FDA approval discussed above.
Similar to the United States, a system for orphan drug designation exists in the European Union. FOLOTYN received orphan medicinal product designation by the European Committee for Orphan Medicinal Products for patients with nodal, other extranodal, and leukaemic/disseminated PTCL, CTCL, Hodgkin lymphoma and non-papillary TCC of the urinary bladder. Orphan designation does not shorten the regulatory review and approval process for an orphan drug, nor does it give that drug any advantage in the regulatory review and approval process. However, if an orphan drug later receives approval for the indication for which it has designation, the relevant regulatory authority may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for 10 years in the European Union.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
Results of Operations
Please see financial statements and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in this report for a discussion of financial information about our business segment and our expenses on research and development.
Employees
As of February 25, 2011, we had a total of 156 full-time employees. No employee is represented by a labor union.
Other Information
Our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, as well as any amendments to those reports, are available free of charge through our website as soon as reasonably practicable after we file them with, or furnish them to, the Securities and Exchange Commission, or SEC. Once at www.allos.com, go to Investors/Financial Reports to locate copies of such reports. You may also read and copy materials that we file with SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding us and other issuers that file electronically with the SEC.
23
Our business faces significant risks. These risks include those described below and may include additional risks of which we are not currently aware or that we currently do not believe are material. If any of the events or circumstances described in the following risk factors actually occurs, they may materially harm our business, financial condition, operating results and cash flow. As a result, the market price of our common stock could decline. Additional risks and uncertainties that are not yet identified or that we think are immaterial may also materially harm our business, operating results and financial condition. The following risks should be read in conjunction with the other information set forth in this report.
We have a history of net losses and an accumulated deficit, and we may never generate sufficient revenue to achieve or maintain profitability in the future.
We have incurred significant net losses and negative cash flows from operations. To date, we have financed our operations primarily through the public and private sale of securities and net product sales. For the years ended December 31, 2010, 2009 and 2008, we had net losses of $77.4 million, $73.6 million and $51.7 million, respectively. As of December 31, 2010, we an accumulated deficit of $450.6 million. We have incurred these losses principally from costs incurred in our research and development programs and from our selling, general and administrative expenses.
On September 24, 2009, we obtained accelerated approval from the FDA for FOLOTYN for use as a single agent for the treatment of patients with relapsed or refractory PTCL. Our ability to achieve profitability is dependent on our ability, alone or with partners, to significantly increase sales of FOLOTYN for the treatment of patients with relapsed or refractory PTCL. We are also developing FOLOTYN for use as a single agent and in combination therapy regimens in a range of hematologic malignancies and solid tumor indications, which may or may not lead to obtaining the necessary regulatory approvals to market FOLOTYN for additional indications. We expect to continue to spend substantial amounts on research and development, including amounts spent on conducting clinical trials and seeking additional regulatory approvals for FOLOTYN, and commercializing FOLOTYN for the treatment of patients with relapsed or refractory PTCL. As a result, we may never generate sufficient revenue from product sales to become profitable or generate positive cash flows.
Our near-term prospects are dependent on FOLOTYN. If we are unable to significantly increase sales of FOLOTYN for the treatment of patients with relapsed or refractory PTCL our ability to achieve profitability will be adversely affected.
FOLOTYN is our only product approved for marketing by the FDA and our ability to generate revenue in the near term is entirely dependent upon sales of FOLOTYN. We may not be able to significantly increase sales of FOLOTYN for a number of reasons, including:
- •
- we may not be able to establish or demonstrate in the medical community the safety and efficacy of FOLOTYN and any
potential advantages over existing therapeutics and products currently in clinical development;
- •
- doctors may be hesitant to prescribe FOLOTYN until results from our post-approval studies are available or
other long term data regarding efficacy and safety exists;
- •
- results from our Phase 3 post-approval studies may fail to verify the clinical benefit of FOLOTYN for
the treatment of T-cell lymphoma;
- •
- we may not be able to establish FOLOTYN as the second-line standard of care for PTCL;
- •
- our limited experience in marketing, selling and distributing FOLOTYN;
- •
- reimbursement and coverage policies of government and private payers such as Medicare, Medicaid, insurance companies, health maintenance organizations and other plan administrators;
24
- •
- the relative price of FOLOTYN as compared to alternative treatment options;
- •
- the relatively low incidence and prevalence rates of relapsed or refractory PTCL, including the reliability of our
estimates;
- •
- we may not have adequate financial or other resources to significantly increase sales of FOLOTYN; and
- •
- we may not be able to continue to manufacture FOLOTYN in commercial quantities or at acceptable costs.
If we are unable to significantly increase sales of FOLOTYN for the treatment of patients with relapsed or refractory PTCL, our ability to achieve profitability will be adversely affected and our stock price would likely decline.
Our operating results are unpredictable and may fluctuate. If our operating results are below the expectations of securities analysts or investors, the trading price of our stock could decline.
Our operating results to date have fluctuated from quarter to quarter and year to year. We believe that our quarterly and annual results of operations may continue to fluctuate and will be difficult to predict due to a variety of factors, including:
- •
- the timing and amount of revenue generated from sales of FOLOTYN;
- •
- the timing and costs associated with our sales and marketing activities for promoting FOLOTYN;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of FOLOTYN;
- •
- the timing and costs associated with conducting preclinical and clinical development of FOLOTYN, including the
post-approval clinical studies required by the FDA;
- •
- the timing and costs associated with our evaluation of, and decisions with respect to, the potential development of
FOLOTYN for additional therapeutic indications;
- •
- the timing, costs and potential revenue associated with a potential strategic partnership for the
co-development of FOLOTYN globally and commercialization outside the United States; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
In addition, we measure compensation cost for stock-based awards made to employees at the grant date of the award, based on the fair value of the award, and recognize the cost as an expense over the employee's requisite service period. As the variables that we use as a basis for valuing these awards change over time, including our underlying stock price, the magnitude of the expense that we must recognize may vary significantly. Any such variance from one period to the next could cause a significant fluctuation in our operating results.
For these reasons, it is difficult for us to accurately forecast future profits or losses. As a result, it is possible that in some quarters our operating results could be below the expectations of securities analysts or investors, which could cause the trading price of our common stock to decline, perhaps substantially.
25
If we are unable to maintain adequate sales, marketing or distribution capabilities or enter into agreements with third parties to perform some of these functions, we will not be able to commercialize FOLOTYN effectively.
The approval of FOLOTYN for the treatment of patients with relapsed or refractory PTCL is our first U.S. approval. Accordingly, we have limited experience in sales, marketing and distribution of pharmaceutical products. We may not be able to adequately maintain the necessary sales, marketing, supply chain management and reimbursement capabilities on our own or enter into arrangements with third parties to perform these functions in a timely manner or on acceptable terms. Additionally, maintaining sales, marketing and distribution capabilities may be more expensive than we anticipate, requiring us to divert capital from other intended purposes or preventing us from building our sales, marketing and distribution capabilities to the desired levels. To be successful we must:
- •
- recruit and retain adequate numbers of effective sales personnel;
- •
- effectively train our sales personnel in the benefits of FOLOTYN;
- •
- establish and maintain successful sales and marketing and education programs that encourage physicians to recommend
FOLOTYN to their patients; and
- •
- manage geographically dispersed sales and marketing operations.
The commercialization of FOLOTYN requires us to manage relationships with an increasing number of collaborative partners, suppliers and third-party contractors. If we are unable to successfully establish and maintain the required infrastructure, either internally or through third parties, and successfully manage an increasing number of relationships, we will have difficulty growing our business. In addition, we intend to enter into co-promotion or out-licensing arrangements with other pharmaceutical or biotechnology partners where necessary to reach foreign market segments and when deemed strategically and economically advisable. To the extent that we enter into co-promotion or other licensing arrangements, our product revenues are likely to be lower than if we directly marketed and sold FOLOTYN, and some or all of the revenues we receive will depend upon the efforts of third parties, which may not be successful. If we are unable to develop and maintain adequate sales, marketing and distribution capabilities, independently or with others, we may not be able to significantly increase sales of FOLOTYN or become profitable.
Even though we have obtained accelerated approval to market FOLOTYN for the treatment of patients with relapsed or refractory PTCL, we are subject to ongoing regulatory obligations and review, including post-approval requirements.
FOLOTYN was approved for the treatment of patients with relapsed or refractory PTCL under the FDA's accelerated approval regulations, which allow the FDA to approve products for cancer or other life threatening diseases based on initial positive data from clinical trials. Under these provisions, we are subject to certain post-approval requirements pursuant to which we are required to conduct two randomized Phase 3 trials to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma. The FDA has also required that we conduct two Phase 1 trials to assess whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment. Failure to complete the studies or adhere to the timelines established by the FDA could result in penalties, including fines or withdrawal of FOLOTYN from the market. The FDA may also initiate proceedings to withdraw approval if our Phase 3 studies fail to verify clinical benefit. Further, the FDA may require us to strengthen the warnings and precautions section of the FOLOTYN package insert or institute a Risk Evaluation and Mitigation Strategy based on the results of these studies or clinical experience. We are also subject to additional, continuing post-approval regulatory obligations, including the possibility of additional clinical studies required by the FDA, safety reporting requirements and regulatory oversight of the promotion and marketing of FOLOTYN.
26
In addition, we or our third-party manufacturers are required to adhere to regulations setting forth the FDA's current Good Manufacturing Practices, or cGMP. These regulations cover all aspects of the manufacturing, storage, testing, quality control and record keeping relating to FOLOTYN. Furthermore, we or our third-party manufacturers are subject to periodic inspection by the FDA and foreign regulatory authorities to ensure compliance with cGMP or other applicable government regulations and corresponding foreign standards. We have limited control over a third-party manufacturer's compliance with these regulations and standards. If we or our third-party manufacturers fail to comply with applicable regulatory requirements, we may be subject to fines, suspension, modification or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
The status of coverage and reimbursement from third-party payers for newly approved health care drugs is uncertain and failure to obtain adequate coverage and reimbursement could limit our ability to generate revenue.
Our ability to successfully commercialize FOLOTYN for the treatment of patients with relapsed or refractory PTCL or for other future indications will depend, in part, on the extent to which coverage and reimbursement for FOLOTYN is available from government and health administration authorities, private health insurers, managed care programs and other third-party payers. Significant uncertainty exists as to the coverage and reimbursement of newly approved health care products. In addition, in March 2010, the U.S. Congress enacted legislation to reform the health care system that includes cost containment measures that may adversely affect the amount of reimbursement for pharmaceutical products, including FOLOTYN. These measures include increasing the minimum rebates for products covered by Medicaid programs and extending such rebates to drugs dispensed to Medicaid beneficiaries enrolled in Medicaid managed care organizations as well as expansion of the 340B Public Health Services drug discount program.
Healthcare providers and third-party payers use coding systems to identify diagnoses, procedures, services, drugs, pharmaceutical devices, equipment and other health-related items and services. Proper coding is an integral component to receiving appropriate reimbursement for the administration of FOLOTYN and related services. The majority of payers use nationally recognized code sets to report medical conditions, services and drugs. We obtained transitional pass-through status that enables FOLOTYN to be reimbursed under the hospital outpatient prospective payment system. In addition, in January 2011 we received a permanent reimbursement J-Code for FOLOTYN, although healthcare providers prescribing FOLOTYN were recently required to submit claims for reimbursement using a temporary J-Code, which may result in payment delays or incorrect payment levels. We cannot predict at this time whether our customers will receive adequate reimbursement for FOLOTYN.
Third-party payers, including Medicare, are challenging the prices charged for medical products and services. Government and other third-party payers increasingly are attempting to contain health care costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for disease conditions for which the FDA has not granted labeling approval. Third-party insurance coverage may not be available to patients for FOLOTYN. If government and other third-party payers do not provide adequate coverage and reimbursement levels for FOLOTYN, FOLOTYN's market acceptance may be adversely affected.
We are dependent upon a small number of customers for a significant portion of our revenue, and the loss of, or significant reduction or cancellation in sales to, any one of these customers could adversely affect our results of operations and financial condition.
In the United States, we sell FOLOTYN to a small number of distributors who in turn sell-through to patient health care providers. These distributors also provide multiple logistics services relating to the distribution of FOLOTYN, including transportation, warehousing, cross-docking,
27
inventory management, packaging and freight-forwarding. We do not promote FOLOTYN to these distributors and they do not set or determine demand for FOLOTYN. For the years ended December 31, 2010 and 2009, three companies affiliated with AmerisourceBergen Corporation accounted for substantially all of our FOLOTYN sales. We expect significant customer concentration to continue for the foreseeable future. Our ability to generate sales of FOLOTYN will depend, in part, on the extent to which these distributors are able to provide adequate distribution of FOLOTYN to patient health care providers. Although we believe we can find alternative distributors on a relatively short notice, our revenue during that period of time may suffer and we may incur additional costs to replace a distributor. The loss of any large customer, a significant reduction in sales we make to them, any cancellation of orders they have made with us or any failure to pay for the products we have shipped to them could materially and adversely affect our results of operations and financial condition.
If the distributors that we rely upon to sell FOLOTYN fail to perform, our business may be adversely affected.
Our success depends on the continued customer support efforts of our network of distributors. The use of distributors involves certain risks, including, but not limited to, risks that these distributors will:
- •
- not provide us with accurate or timely information regarding their inventories, the number of patients who are using
FOLOTYN or complaints about FOLOTYN;
- •
- not effectively distribute or support FOLOTYN;
- •
- reduce or discontinue their efforts to sell or support FOLOTYN;
- •
- be unable to satisfy financial obligations to us or others; and
- •
- cease operations.
Any such failure may result in decreased sales of FOLOTYN, which would harm our business.
If we fail to comply with healthcare fraud and abuse laws, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
As a biopharmaceutical company, even though we do not and will not control referrals of health care services or bill directly to Medicare, Medicaid or other third-party payers, certain federal and state healthcare laws and regulations pertaining to fraud and abuse are applicable to our business. These laws and regulations, include, among others:
- •
- the federal Anti-Kickback statute, which prohibits, among other things, persons from soliciting, receiving or
providing remuneration, directly or indirectly, to induce either the referral of an individual for an item or service or the purchasing or ordering of a good or service, for which payment may be made
under federal health care programs such as the Medicare and Medicaid programs;
- •
- federal false claims laws that prohibit, among other things, individuals or entities from knowingly presenting, or causing
to be presented, claims for payment from Medicare, Medicaid, or other third-party payers that are false or fraudulent;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits executing a scheme to
defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain requirements relating to the privacy, security and transmission of
individually identifiable health information;
- •
- federal self-referral laws, such as STARK, which prohibit a physician from making a referral to a provider of certain health services with which the physician or the physician's family member has a financial interest; and
28
- •
- state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payer, including commercial insurers, and state laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA.
Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution under the federal Anti-Kickback statute, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescriptions, purchases or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Further, the recently enacted health care reform law known as the Patient Protection and Affordable Care Act, as modified by the Health Care and Education Affordability Reconciliation Act of 2010, or PPACA, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims laws. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Although physicians are permitted to, based on their medical judgment, prescribe products for indications other than those cleared or approved by the FDA, manufacturers are prohibited from promoting their products for such off-label uses. We market FOLOTYN for the treatment of patients with relapsed or refractory PTCL and provide promotional materials and training programs to physicians regarding the use of FOLOTYN for the treatment of patients with relapsed or refractory PTCL. Although we believe our marketing, promotional materials and training programs for physicians do not constitute off-label promotion of FOLOTYN, the FDA may disagree. If the FDA determines that our promotional materials, training or other activities constitute off-label promotion of FOLOTYN, the FDA could request that we modify our training or promotional materials or other activities or subject us to regulatory enforcement actions, including the issuance of a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they believe that the alleged improper promotion led to the submission and payment of claims for an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. Even if it is later determined we are not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our position and have to divert significant management resources from other matters.
The PPACA imposes new reporting and disclosure requirements for pharmaceutical and device manufacturers with regard to payments or other transfers of value made to physicians and teaching hospitals, effective March 30, 2013. Such information will be made publicly available in a searchable format beginning September 30, 2013. In addition, pharmaceutical and device manufacturers will also be required to report and disclose investment interests held by physicians and their immediate family members during the preceding calendar year. Failure to submit required information may result in civil monetary penalties of up to $150,000 per year (and up to $1 million per year for "knowing failures"), for all payments, transfers of value or ownership or investment interests not reported in an annual submission.
In recent years, several states and localities, including California, the District of Columbia, Maine, Massachusetts, Minnesota, Nevada, Vermont, and West Virginia, have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, and file periodic reports with the state or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Similar legislation is being considered in other states. Many of these requirements are new and uncertain, and the penalties for failure to comply with these requirements are unclear. Nonetheless, if we are found not to be in full compliance with these laws, we could face enforcement action and fines and other penalties, and could receive adverse publicity.
29
If our operations are found to be in violation of any of the healthcare fraud and abuse laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. Moreover, achieving and sustaining compliance with all applicable federal and state fraud and abuse laws may be costly.
If our competitors develop and market products that are more effective than FOLOTYN, our commercial opportunity will be reduced or eliminated.
Even though we have obtained approval to market FOLOTYN for the treatment of patients with relapsed or refractory PTCL, our commercial opportunity will be reduced or eliminated if our competitors develop and market products that are more effective, have fewer side effects or are less expensive than FOLOTYN for this or any other potential indication. Our potential competitors include large, fully-integrated pharmaceutical companies and more established biotechnology companies, each of which have significant resources and expertise in research and development, manufacturing, testing, obtaining regulatory approvals and marketing. Academic institutions, government agencies, and other public and private research organizations conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and marketing. It is possible that competitors will succeed in developing technologies that are more effective than those being developed by us or that would render our technologies obsolete or noncompetitive.
We cannot predict when or if we will obtain regulatory approval to market FOLOTYN in the United States for any additional indications or in any other countries.
We are subject to stringent regulations with respect to product safety and efficacy by various international, federal, state and local authorities. FOLOTYN has not been approved for marketing in the United States for any indication other than the treatment of patients with relapsed or refractory PTCL. In addition, FOLOTYN has not been approved for marketing for this or any other indication in any other country. A pharmaceutical product cannot be marketed in the United States or most other countries until it has completed a rigorous and extensive regulatory review and approval process. Satisfaction of regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. Of particular significance are the requirements covering research and development, preclinical and clinical testing, manufacturing, quality control, labeling and promotion of drugs for human use. We may not obtain the necessary regulatory approvals to market FOLOTYN in the United States for any additional indications or in any other countries. If we fail to obtain or maintain regulatory approvals to market FOLOTYN in the United States for any additional indications or in any other countries, our ability to generate significant revenue or achieve profitability may be adversely affected.
Reports of adverse events or safety concerns involving FOLOTYN or similar small molecule chemotherapeutic agents could delay or prevent us from obtaining or maintaining regulatory approval or negatively impact sales of FOLOTYN.
FOLOTYN may cause serious adverse events. These adverse events could interrupt, delay or halt clinical trials of FOLOTYN, including the FDA-required post-approval studies, and could result in the FDA or other regulatory authorities denying or withdrawing approval of FOLOTYN for any or all indications, including for the treatment of patients with relapsed or refractory PTCL. Adverse events
30
may also negatively impact the sales of FOLOTYN. The FDA, other regulatory authorities or we may suspend or terminate clinical trials at any time. We may also be required to update the warnings and precautions section of the FOLOTYN package insert based on reports of adverse events or safety concerns or implement a Risk Evaluation and Mitigation Strategy, which could adversely affect FOLOTYN's acceptance in the market. We cannot assure you that FOLOTYN will be safe for human use.
At present, there are a number of clinical trials being conducted by other pharmaceutical companies involving small molecule chemotherapeutic agents. If other pharmaceutical companies announce that they observed frequent adverse events or unknown safety issues in their trials involving compounds similar to, or competitive with, FOLOTYN, we could encounter delays in the timing of our clinical trials or difficulties in obtaining or maintaining the necessary regulatory approvals for FOLOTYN. In addition, the public perception of FOLOTYN might be adversely affected, which could harm our business and results of operations and cause the market price of our common stock to decline, even if the concern relates to another company's product or product candidate.
If FOLOTYN fails to meet safety or efficacy endpoints in clinical trials for additional indications, it will not receive regulatory approval and we will be unable to market FOLOTYN for those indications studied.
We have ongoing clinical trials involving FOLOTYN and plan to initiate additional trials to evaluate FOLOTYN's potential clinical utility in other hematologic malignancies. FOLOTYN may not prove to be safe and efficacious in clinical trials for other indications and may not meet all of the applicable regulatory requirements needed to receive regulatory approval for those indications. The clinical development and regulatory approval process is expensive and takes many years. Failure can occur at any stage of development, and the timing of any regulatory approval cannot be accurately predicted. In addition, failure to comply with the FDA and other applicable U.S. and foreign regulatory requirements applicable to clinical trials may subject us to administrative or judicially imposed sanctions.
As part of the regulatory approval process, we must conduct clinical trials for FOLOTYN and any other product candidate to demonstrate safety and efficacy to the satisfaction of the FDA and other regulatory authorities abroad. The number and design of clinical trials that will be required varies depending on the product candidate, the condition being evaluated, the trial results and regulations applicable to any particular product candidate. The designs of our clinical trials for FOLOTYN are based on many assumptions about the expected effect of FOLOTYN, and if those assumptions prove incorrect, the clinical trials may not demonstrate the safety or efficacy of FOLOTYN. Preliminary results may not be confirmed upon full analysis of the detailed results of a trial, and prior clinical trial program designs and results may not be predictive of future clinical trial designs or results. Product candidates in later stage clinical trials may fail to show the desired safety and efficacy despite having progressed through initial clinical trials with acceptable endpoints. For example, we terminated the development of EFAPROXYN, one of our former product candidates, when it failed to demonstrate statistically significant improvement in overall survival in the targeted patients in a Phase 3 clinical trial. If FOLOTYN fails to show clinically significant benefits in any clinical trial or for any particular indication, it may not be approved for marketing for such indication. Additionally, if FOLOTYN is demonstrated to be unsafe in clinical trials for other indications, such demonstration could negatively impact FOLOTYN's existing approval for the treatment of patients with relapsed or refractory PTCL.
Even if we achieve positive interim results in clinical trials, these results do not necessarily predict final results, and acceptable results in early trials may not be repeated in later trials. Data obtained from preclinical and clinical activities are susceptible to varying interpretations that could delay, limit or prevent regulatory clearances, and the FDA can request that we conduct additional clinical trials. A number of companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. In addition, negative or inconclusive results
31
or adverse safety events during a clinical trial could cause a clinical trial to be repeated or terminated. Also, failure to construct clinical trial protocols to screen patients for risk profile factors relevant to the trial for purposes of segregating patients into the patient populations treated with the drug being tested and the control group could result in either group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated. If we have to conduct additional clinical trials for FOLOTYN for any particular indication, it will significantly increase our expenses and may delay marketing of FOLOTYN for such indication.
Even if FOLOTYN meets safety and efficacy endpoints in clinical trials for additional indications, regulatory authorities may not approve FOLOTYN, or we may face post-approval problems that require withdrawal of FOLOTYN from the market.
We will not be able to market FOLOTYN in the United States for any additional indications or in any other countries for any indications until we have obtained the necessary regulatory approvals. Our receipt of approval of FOLOTYN in the United States for the treatment of patients with relapsed or refractory PTCL does not guarantee that we will obtain regulatory approval to market FOLOTYN in the United States for any additional indications or in any other countries. We have limited experience in filing and pursuing applications necessary to gain regulatory approvals, which may place us at risk of delays, overspending and human resources inefficiencies.
FOLOTYN may not be approved for any additional indications even if it achieves its endpoints in clinical trials. Regulatory agencies, including the FDA, or their advisors, may disagree with our interpretations of data from preclinical studies and clinical trials. The FDA has substantial discretion in the approval process, and when or whether regulatory approval will be obtained for any drug we develop. Regulatory agencies also may approve a product candidate for fewer conditions than requested or may grant approval subject to the performance of post-approval studies or Risk Evaluation and Mitigation Strategies for a product candidate. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of FOLOTYN.
Following regulatory approval for any additional indication, FOLOTYN may later produce adverse events that limit or prevent its widespread use or that force us to withdraw FOLOTYN from the market for that indication or other indications. In addition, a marketed product continues to be subject to strict regulation after approval and may be required to undergo post-approval studies. For example, we are required to conduct two randomized Phase 3 trials to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma as well as two Phase 1 trials to assess whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment. Any unforeseen problems with an approved product, any failure to meet the post-approval study requirements or any violation of regulations could result in restrictions on the product, including its withdrawal from the market. Any delay in or failure to obtain or maintain regulatory approvals for FOLOTYN in the United States for any additional indication or in any other countries could harm our business and prevent us from ever generating significant revenues or achieving profitability.
When we seek approval for FOLOTYN in other countries, we are subject to numerous complex regulatory requirements and if approval is denied or limited in another country, or if another country imposes post-marketing requirements, that decision could affect our ability to market FOLOTYN in other countries.
We have filed an MAA with the EMA for FOLOTYN for the treatment of patients with relapsed or refractory PTCL, using the centralized procedure. If major objections are raised during the review procedure, we may not receive marketing approval and would be unable to commercialize FOLOTYN in the European Union. Alternatively, the marketing authorization may be subject to conditions for approval or post authorization obligations. Such conditions or obligations may be costly and time consuming to fulfill and may affect our operations. For example, additional clinical data may be required to confirm the safety or efficacy profile of FOLOTYN in the target patient population. In
32
addition, marketing authorizations are subject to periodic reviews, which, if negative, could affect our ability to commercialize FOLOTYN in the European Union.
Additionally, failure to comply with, or changes to, the regulatory requirements that are applicable to FOLOTYN may result in a variety of consequences, including the following:
- •
- restrictions on FOLOTYN or our manufacturing processes;
- •
- warning letters;
- •
- withdrawal of FOLOTYN from the market;
- •
- voluntary or mandatory recall of FOLOTYN;
- •
- fines against us;
- •
- suspension or withdrawal of regulatory approvals for FOLOTYN;
- •
- suspension or termination of any of our ongoing clinical trials of FOLOTYN;
- •
- refusal to permit import or export of FOLOTYN;
- •
- refusal to approve pending applications or supplements to approved applications that we submit;
- •
- denial of permission to file an application or supplement in a jurisdiction;
- •
- product seizure; and
- •
- injunctions, consent decrees, or the imposition of civil or criminal penalties against us.
We may experience delays in our clinical trials that could adversely affect our financial position and our commercial prospects.
We do not know when our current clinical trials will be completed, if at all. We also cannot accurately predict when other planned clinical trials will begin or be completed. Many factors affect patient enrollment, including the size of the patient population, the proximity of patients to clinical sites, the eligibility criteria for the trial, competing clinical trials and new drugs approved for the conditions we are investigating. Other companies are conducting clinical trials and have announced plans for future trials that are seeking or likely to seek patients with the same diseases as those we are studying. Competition for patients in some cancer trials is particularly intense because of the limited number of leading specialist physicians and the geographic concentration of major clinical centers.
As a result of the numerous factors that can affect the pace of progress of clinical trials, our trials may take longer to enroll patients than we anticipate, if they can be completed at all. Delays in patient enrollment in the trials may increase our costs and slow our product development and approval process. Our product development costs will also increase if we need to perform more or larger clinical trials than planned. If other companies' product candidates show favorable results, we may be required to conduct additional clinical trials to address changes in treatment regimens or for our products to be commercially competitive. Any delays in completing our clinical trials will delay our ability to obtain regulatory approval to market FOLOTYN in the United States for any additional indications or in any other countries, which may adversely affect our ability to generate significant revenues or achieve profitability.
We may be required to suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the trials are not well designed.
Clinical trials must be conducted in accordance with current Good Clinical Practices, or cGCP, or other applicable foreign government guidelines and are subject to oversight by the FDA, foreign governmental agencies and Institutional Review Boards at the medical institutions where the clinical
33
trials are conducted. In addition, clinical trials must be conducted with product candidates produced under cGMP and may require large numbers of test subjects. Clinical trials may be suspended by the FDA, foreign governmental agencies, or us for various reasons, including:
- •
- deficiencies in the conduct of the clinical trials, including failure to conduct the clinical trial in accordance with
regulatory requirements or clinical protocols;
- •
- deficiencies in the clinical trial operations or trial sites;
- •
- the product candidate may have unforeseen adverse side effects;
- •
- the time required to determine whether the product candidate is effective may be longer than expected;
- •
- fatalities or other adverse events arising during a clinical trial due to medical problems that may not be related to
clinical trial treatments;
- •
- the product candidate may appear to be less effective than current therapies;
- •
- the quality or stability of the product candidate may fall below acceptable standards; or
- •
- insufficient quantities of the product candidate to complete the trials.
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to Institutional Review Boards for reexamination, which may impact the costs, timing or successful completion of a clinical trial. Due to these and other factors, FOLOTYN could take a significantly longer time to gain regulatory approval for any additional indications than we expect or we may never gain approval for additional indications, which could reduce our revenue by delaying or terminating the commercialization of FOLOTYN for additional indications.
Due to our reliance on contract research organizations and other third parties to conduct our clinical trials, we are unable to directly control the timing, conduct and expense of our clinical trials.
We rely primarily on third parties to conduct our clinical trials. As a result, we have had and will continue to have less control over the conduct of our clinical trials, the timing and completion of the trials, the required reporting of adverse events and the management of data developed through the trial than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may have staffing difficulties, may undergo changes in priorities or may become financially distressed, any of which may adversely affect their willingness or ability to conduct our trials. We may experience unexpected cost increases that are beyond our control. Problems with the timeliness or quality of the work of a contract research organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials, and contractual restrictions may make such a change difficult or impossible. Additionally, it may be impossible to find a replacement organization that can conduct our trials in an acceptable manner and at an acceptable cost.
We may need to raise additional capital to support our future operations. If we fail to obtain the capital necessary to fund our operations, we will be unable to successfully develop or commercialize FOLOTYN.
Based upon the current status of our product development and commercialization plans, we believe that our cash, cash equivalents, and investments as of December 31, 2010, should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished. We anticipate continuing our current development programs and beginning other long-term development projects involving FOLOTYN, including the post-approval
34
clinical studies required for FOLOTYN. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we expect to incur significant costs relating to the commercialization of FOLOTYN, including costs related to our sales and marketing, medical affairs and manufacturing operations. Therefore, we may need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and amount of revenue generated from sales of FOLOTYN;
- •
- the timing and costs associated with our sales and marketing activities for promoting FOLOTYN;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of FOLOTYN;
- •
- the timing and costs associated with conducting preclinical and clinical development of FOLOTYN, including the
post-approval clinical studies required by the FDA;
- •
- the timing and costs associated with our evaluation of, and decisions with respect to, the potential development of
FOLOTYN for additional therapeutic indications;
- •
- the timing, costs and potential revenue associated with a potential strategic partnership for the
co-development of FOLOTYN globally and commercialization outside the United States; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we might otherwise seek to develop or commercialize ourselves, on terms that are less favorable than might otherwise be available. If we are unable to significantly increase sales of FOLOTYN or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for profitability may be harmed.
Budget constraints may force us to delay our efforts to develop FOLOTYN for additional indications while we complete the post-approval clinical studies required by the FDA, which may prevent us from commercializing FOLOTYN for all desired indications as quickly as possible.
Because we have limited resources, and because research and development is an expensive process, we must regularly assess the most efficient allocation of our research and development budget. In particular, our approval of FOLOTYN in patients with relapsed or refractory PTCL is conditioned upon us undertaking two additional Phase 3 studies and two additional Phase 1 studies which will result in significant additional expense. As a result of our limited resources, we may have to prioritize the development of FOLOTYN for additional indications and may not be able to fully realize the value of FOLOTYN for other indications in a timely manner, if at all.
For example, in January 2011, we announced that we will not pursue Phase 3 studies for NSCLC at this time in order to prioritize our resources on the development and commercialization of FOLOTYN for the treatment of hematologic malignancies, and to manage our operating costs and expenses.
35
We do not have manufacturing facilities or capabilities and are dependent on third parties to fulfill our manufacturing needs, which could result in the delay of clinical trials, regulatory approvals, product introductions and commercial sales.
We are dependent on third parties for the manufacture and storage of FOLOTYN for clinical trials and for commercial sale. If we are unable to contract for a sufficient supply of FOLOTYN on acceptable terms, or if we encounter delays or difficulties in the manufacturing process or our relationships with our manufacturers, we may not have sufficient product to conduct or complete our clinical trials or support commercial requirements for FOLOTYN.
FOLOTYN is cytotoxic, which requires the manufacturers of FOLOTYN to have specialized equipment and safety systems to handle such a substance. In addition, the starting materials for FOLOTYN require custom preparations, which require us to manage an additional set of suppliers to obtain the needed supplies of FOLOTYN.
We have arrangements with two third-party manufacturers to produce FOLOTYN bulk drug substance and two third-party manufacturers to produce FOLOTYN formulated drug product. We believe these third-party manufacturers have the capability to meet our projected worldwide clinical trial and commercial requirements for FOLOTYN although we cannot assure you of this. In particular, our third party manufacturers may not be able to fulfill our potential commercial needs or meet our deadlines, or the components they supply to us may not meet our specifications and quality policies and procedures. If we need to find additional alternative suppliers of FOLOTYN or its components, we may not be able to contract for those components on acceptable terms, if at all. Any such failure to supply or delay caused by such suppliers would have an adverse effect on our ability to continue clinical development of FOLOTYN or commercialize FOLOTYN.
Our current or future manufacturers may be unable to accurately and reliably manufacture commercial quantities of FOLOTYN at reasonable costs, on a timely basis and in compliance with the FDA's cGMP. If our current or future contract manufacturers fail in any of these respects, our ability to timely complete our clinical trials, obtain or maintain required regulatory approvals and successfully commercialize FOLOTYN may be materially and adversely affected. This risk may be heightened with respect to FOLOTYN as there are a limited number of manufacturers with the ability to handle cytotoxic products such as FOLOTYN. Our reliance on contract manufacturers exposes us to additional risks, including:
- •
- our current and future manufacturers are subject to ongoing, periodic, unannounced inspections by the FDA and
corresponding state and international regulatory authorities for compliance with strictly enforced cGMP regulations and similar state and foreign standards, and we do not have control over our
contract manufacturers' compliance with these regulations and standards;
- •
- our manufacturers may not be able to comply with applicable regulatory requirements, which would prohibit them from
manufacturing products for us;
- •
- our manufacturers may have staffing difficulties, may undergo changes in control or may become financially distressed,
adversely affecting their willingness or ability to manufacture products for us;
- •
- our manufacturers might not be able to fulfill our commercial needs, which would require us to seek new manufacturing
arrangements and may result in substantial delays in meeting market demands;
- •
- if we need to change to other commercial manufacturing contractors, the FDA and comparable foreign regulators must approve our use of any new manufacturer, which would require additional testing, regulatory filings and compliance inspections, and the new manufacturers
36
- •
- we may not have intellectual property rights, or may have to share intellectual property rights, to any improvements in the manufacturing processes or new manufacturing processes for our products.
would have to be educated in, or themselves develop substantially equivalent processes necessary for, the production of our products; and
Any of these factors could result in the delay of clinical trials, regulatory submissions, required approvals or commercialization of FOLOTYN. They could also entail higher costs and result in our being unable to effectively commercialize FOLOTYN.
If we are unable to effectively protect our intellectual property, we will be unable to prevent third parties from using our technology, which would impair our competitiveness and ability to commercialize FOLOTYN. In addition, enforcing our proprietary rights may be expensive and result in increased losses.
Our success will depend in part on our ability to obtain and maintain meaningful patent protection for FOLOTYN, both in the United States and in other countries. We rely on patents to protect a large part of our intellectual property and our competitive position. Any patents issued to or licensed by us could be challenged, invalidated, infringed, circumvented or held unenforceable, based on, among other things, obviousness, inequitable conduct, anticipation or enablement. In addition, it is possible that no patents will issue on any of our licensed patent applications. It is possible that the claims in patents that have been issued or licensed to us or that may be issued or licensed to us in the future will not be sufficiently broad to protect our intellectual property or that the patents will not provide protection against competitive products or otherwise be commercially valuable. Failure to obtain and maintain adequate patent protection for our intellectual property would impair our ability to be commercially competitive.
Our commercial success will also depend in part on our ability to commercialize FOLOTYN without infringing patents or other proprietary rights of others or breaching the licenses granted to us. We may not be able to obtain a license to third-party technology that we may require to conduct our business or, if obtainable, we may not be able to license such technology at a reasonable cost. If we fail to obtain a license to any technology that we may require to commercialize FOLOTYN, or fail to obtain a license at a reasonable cost, we will be unable to commercialize FOLOTYN or to commercialize at a price that will allow us to become profitable.
In addition to patent protection, we also rely upon trade secrets, proprietary know-how and technological advances that we seek to protect through confidentiality agreements with our collaborators, employees, advisors and consultants. Our employees and consultants are required to enter into confidentiality agreements with us. We also enter into non-disclosure agreements with our collaborators and vendors, which agreements are intended to protect our confidential information delivered to third parties for research and other purposes. However, these agreements could be breached and we may not have adequate remedies for any breach, or our trade secrets and proprietary know-how could otherwise become known or be independently discovered by others.
37
Furthermore, as with any pharmaceutical company, our patent and other proprietary rights are subject to uncertainty. Our patent rights related to FOLOTYN might conflict with current or future patents and other proprietary rights of others. For the same reasons, the products of others could infringe our patents or other proprietary rights. Litigation or patent interference proceedings, either of which could result in substantial costs to us, may be necessary to enforce any of our patents or other proprietary rights, or to determine the scope and validity or enforceability of other parties' proprietary rights. We may be dependent on third parties, including our licensors, for cooperation and information that may be required in connection with the defense and prosecution of our patents and other proprietary rights. The defense and prosecution of patent and intellectual property infringement claims are both costly and time consuming, even if the outcome is favorable to us. Any adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease selling our future products. We are not currently a party to any patent or other intellectual property infringement claims.
We may explore strategic partnerships that may never materialize or may fail.
We may, in the future, periodically explore a variety of possible strategic partnerships in an effort to gain access to additional product candidates or resources. For example, we are currently seeking a strategic partnership for the potential co-development of FOLOTYN globally and commercialization outside the United States. At the current time, we cannot predict what form such a strategic partnership might take. We are likely to face significant competition in seeking appropriate strategic partners, and these strategic partnerships can be complicated and time consuming to negotiate and document. We may not be able to negotiate strategic partnerships on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic partnerships because of the numerous risks and uncertainties associated with establishing strategic partnerships.
If we enter into one or more strategic partnerships, we may be required to relinquish important rights to and control over the development of FOLOTYN or otherwise be subject to unfavorable terms.
Any future strategic partnerships we enter into could subject us to a number of risks, including:
- •
- we may be required to undertake the expenditure of substantial operational, financial and management resources in
integrating new businesses, technologies and products;
- •
- we may be required to issue equity securities that would dilute our existing stockholders' percentage ownership;
- •
- we may be required to assume substantial actual or contingent liabilities;
- •
- we may not be able to control the amount and timing of resources that our strategic partners devote to the development or
commercialization of FOLOTYN;
- •
- strategic partners may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a
product candidate, repeat or conduct new clinical trials or require a new version of a product candidate for clinical testing;
- •
- strategic partners may not pursue further development and commercialization of products resulting from the strategic
partnering arrangement or may elect to discontinue research and development programs;
- •
- strategic partners may not commit adequate resources to the marketing and distribution of FOLOTYN or any other products,
limiting our potential revenues from these products;
- •
- disputes may arise between us and our strategic partners that result in the delay or termination of the research, development or commercialization of FOLOTYN or any other product
38
- •
- strategic partners may experience financial difficulties;
- •
- strategic partners may not properly maintain or defend our intellectual property rights or may use our proprietary
information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation;
- •
- business combinations or significant changes in a strategic partner's business strategy may also adversely affect a
strategic partner's willingness or ability to complete its obligations under any arrangement;
- •
- strategic partners could independently move forward with a competing product candidate developed either independently or
in collaboration with others, including our competitors; and
- •
- strategic partners could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing FOLOTYN or any other product candidate.
candidate or that result in costly litigation or arbitration that diverts management's attention and consumes resources;
Health care reform measures could adversely affect our business.
The business and financial condition of pharmaceutical and biotechnology companies are affected by the efforts of governmental and third-party payers to contain or reduce the costs of health care. The U.S. Congress recently enacted legislation to reform the health care system. While we anticipate that this legislation may, over time, increase the number of patients who have insurance coverage for pharmaceutical products, it also imposes cost containment measures that may adversely affect the amount of reimbursement for pharmaceutical products, including FOLOTYN. These measures include increasing the minimum rebates for products covered by Medicaid programs and extending such rebates to drugs dispensed to Medicaid beneficiaries enrolled in Medicaid managed care organizations as well as expansion of the 340B Public Health Services drug discount program. In foreign jurisdictions there have been, and we expect that there will continue to be, a number of legislative and regulatory proposals aimed at changing the health care system. For example, in some countries other than the United States, pricing of prescription drugs is subject to government control and we expect to see continued efforts to reduce healthcare costs in international markets.
Some states are also considering legislation that would control the prices of drugs, and state Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Managed care organizations continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for drugs, including FOLOTYN. It is likely that federal and state legislatures and health agencies will continue to focus on additional health care reform in the future although we are unable to predict what additional legislation or regulation, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such legislation or regulation would have on our business. The pendency or approval of such proposals or reforms could result in a decrease in our stock price or limit our ability to raise capital or to obtain strategic partnerships or licenses.
39
We may not obtain orphan drug exclusivity or we may not receive the full benefit of orphan drug exclusivity even if we obtain such exclusivity.
The FDA has awarded orphan drug status to pralatrexate, which we market under the tradename FOLOTYN, for the treatment of patients with relapsed or refractory PTCL. In addition, the FDA has awarded orphan drug designation to pralatrexate for the treatment of patients with follicular lymphoma and diffuse large B-cell lymphoma and advanced or metastatic TCC of the urinary bladder, for which we do not have approval. Under the Orphan Drug Act, the first company to receive FDA approval for pralatrexate for a designated orphan drug indication will obtain seven years of marketing exclusivity during which the FDA may not approve another company's application for pralatrexate for the same orphan indication. Because the FDA approved FOLOTYN for the treatment of patients with relapsed or refractory PTCL, we have received seven years of marketing exclusivity for that indication. Orphan drug exclusivity does not prevent FDA approval of a different drug for the orphan indication or the same drug for a different indication. In addition, the FDA may void orphan drug exclusivity under certain circumstances.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit commercialization of FOLOTYN.
The testing and marketing of pharmaceutical products entail an inherent risk of product liability. Product liability claims might be brought against us by consumers or health care providers or by pharmaceutical companies or others selling FOLOTYN or any future products. If we cannot successfully defend ourselves against such claims, we may incur substantial liabilities or be required to limit the commercialization of FOLOTYN. We have obtained limited product liability insurance coverage for our human clinical trials and commercial sales of FOLOTYN. However, product liability insurance coverage is becoming increasingly expensive, and we may be unable to maintain such insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
Our success depends on the retention of our President and Chief Executive Officer and other key personnel.
We are highly dependent on our President and Chief Executive Officer, Paul L. Berns, and other members of our management team. We are named as the beneficiary on a term life insurance policy covering Mr. Berns in the amount of $10.0 million. We also depend on key employees and academic collaborators for each of our research and development programs. The loss of any of our key employees or academic collaborators could delay the development and commercialization of FOLOTYN or result in the termination of our FOLOTYN development program in its entirety. Mr. Berns and others on our executive management team have employment agreements with us, but the agreements provide for "at-will" employment with no specified term. Our future success also will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, governmental regulation and commercialization of pharmaceutical products. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations. Additionally, we have recently implemented a strategic reduction in workforce and we may have a more difficult time in attracting and retaining the employees we need as a result of a perceived risk of future workforce and expense reductions. If we are unsuccessful in our recruitment and retention efforts, our business will be harmed.
We also rely on consultants, collaborators and advisors to assist us in formulating and conducting our research and development programs. All of our consultants, collaborators and advisors are employed by other employers or are self-employed and may have commitments to or consulting contracts with other entities that may limit their ability to contribute to our company.
40
We cannot guarantee that we will be in compliance with all potentially applicable regulations.
The development, manufacturing, pricing, marketing, sale and reimbursement of FOLOTYN, together with our general operations, are subject to extensive regulation by federal, state and other authorities within the United States and numerous entities outside of the United States. We have fewer employees than many other companies that have one or more product candidates that are approved for marketing and we rely heavily on third parties to conduct many important functions.
As a publicly-traded company, we are subject to significant regulations including the Sarbanes Oxley Act of 2002 and the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act. The Dodd-Frank Act contains significant corporate governance and executive compensation-related provisions, some of which the Securities and Exchange Commission, or SEC, has recently implemented by adopting additional rules and regulations in areas such as the compensation of executives ("say-on-pay"). We cannot assure you that we are or will be in compliance with all potentially applicable regulations. If we fail to comply with the Sarbanes Oxley Act of 2002, the Dodd-Frank Act and associated SEC rules, or any other regulations, we could be subject to a range of consequences, including restrictions on our ability to sell equity securities or otherwise raise capital funds, the de-listing of our common stock from The NASDAQ Global Market, or NASDAQ, suspension or termination of our clinical trials, failure to obtain approval to market FOLOTYN, restrictions on future products or our manufacturing processes, significant fines, or other sanctions or litigation. Our efforts to comply with these requirements have resulted in, and are likely to continue to result in, an increase in expenses and a diversion of management's time from other business activities.
If our internal controls over financial reporting are not considered effective, our business and stock price could be adversely affected.
Section 404 of the Sarbanes-Oxley Act of 2002 requires us to evaluate the effectiveness of our internal controls over financial reporting as of the end of each fiscal year, and to include a management report assessing the effectiveness of our internal controls over financial reporting in our annual report on Form 10-K for that fiscal year. Section 404 also requires our independent registered public accounting firm to attest to, and report on, management's assessment of our internal controls over financial reporting.
Our management, including our chief executive officer and principal financial officer, does not expect that our internal controls over financial reporting will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system's objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud involving a company have been, or will be, detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and we cannot assure you that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become ineffective because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. We cannot assure you that we or our independent registered public accounting firm will not identify a material weakness in our internal controls in the future. A material weakness in our internal controls over financial reporting would require management and our independent registered public accounting firm to consider our internal controls as ineffective. If our internal controls over financial reporting are not considered effective, we may experience a loss of public confidence, which could have an adverse effect on our business and on the market price of our common stock.
41
Our reserves and estimates depend upon the accuracy and consistency of third party data as well as dependence upon key finance and accounting personnel to maintain and implement the surrounding controls.
We have reserves and estimates that incorporate a significant amount of third party data from our wholesalers. To effectively maintain the reserves and estimates, we depend to a considerable degree upon the timely and accurate reporting to us of such data from these third parties and our key accounting and finance personnel to accurately interpolate such data into the reserves and estimates. If the third party data is not calculated on a consistent basis and reported to us on an accurate or timely basis or we lose any of our key accounting and finance personnel, the accuracy of our consolidated financial statements could be materially affected. This could cause future delays in our earnings announcements, regulatory filings with the SEC and delisting with NASDAQ.
If we do not progress in our programs as anticipated, our stock price could decrease.
For planning purposes, we estimate the timing of a variety of clinical, regulatory and other milestones, such as when a certain product candidate will enter clinical development, when a clinical trial will be initiated or completed, or when an application for regulatory approval will be filed. Some of our estimates are included in this report. Our estimates are based on information available to us as of the date of this report and a variety of assumptions. Many of the underlying assumptions are outside of our control. If milestones are not achieved when we estimated that they would be, investors could be disappointed and our stock price may decrease.
Warburg Pincus Private Equity VIII, L.P. controls a substantial percentage of the voting power of our outstanding common stock.
On March 2, 2005, we entered into a Securities Purchase Agreement with Warburg Pincus Private Equity VIII, L.P., or Warburg, and certain other investors in connection with an equity financing. In connection with this financing, Warburg and certain of its affiliates entered into a standstill agreement pursuant to which they agreed not to pursue, for so long as they continue to own a specified number of shares of our common stock, certain activities the purpose or effect of which may be to change or influence the control of our company.
As of February 25, 2011, we had 105,580,200 shares of common stock outstanding, of which Warburg owned 26,124,430 shares, or approximately 25% of the voting power of our outstanding common stock. Although Warburg has entered into a standstill agreement with us, Warburg is, and will continue to be, able to exercise substantial influence over any actions requiring stockholder approval.
Anti-takeover provisions in our charter documents and under Delaware law could discourage, delay or prevent an acquisition of us, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of our amended and restated certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. In addition, these provisions may make it more difficult for stockholders to replace members of our board of directors. Because our board of directors is responsible for appointing the members of our management team, these provisions could in turn affect any attempt by our stockholders to replace current members of our management team. These provisions include:
- •
- authorizing the issuance of "blank check" preferred stock that could be issued by our board of directors to increase the number of outstanding shares or change the balance of voting control and thwart a takeover attempt;
42
- •
- prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of
stockholders to elect director candidates;
- •
- prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of
our stockholders;
- •
- eliminating the ability of stockholders to call a special meeting of stockholders; and
- •
- establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings.
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with an interested stockholder for a period of three years following the date on which the stockholder became an interested stockholder. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders. Notwithstanding the foregoing, the three-year moratorium imposed on business combinations by Section 203 will not apply to Warburg because, prior to the date on which Warburg became an interested stockholder, our board of directors approved the transactions that resulted in Warburg becoming an interested stockholder. However, in connection with Warburg's participation in an equity financing we completed in March 2005, Warburg and certain of its affiliates entered into a standstill agreement pursuant to which they agreed not to pursue, for so long as they continue to own a specified number of shares of our common stock, certain activities the purpose or effect of which may be to change or influence the control of our company.
We have adopted a stockholder rights plan that may discourage, delay or prevent a merger or acquisition that is beneficial to our stockholders.
In May 2003, our board of directors adopted a stockholder rights plan that may have the effect of discouraging, delaying or preventing a merger or acquisition of us that our stockholders may consider beneficial by diluting the ability of a potential acquirer to acquire us. Pursuant to the terms of the stockholder rights plan, when a person or group, except under certain circumstances, acquires 15% or more of our outstanding common stock or 10 business days after announcement of a tender or exchange offer for 15% or more of our outstanding common stock, the rights (except those rights held by the person or group who has acquired or announced an offer to acquire 15% or more of our outstanding common stock) would generally become exercisable for shares of our common stock at a discount. Because the potential acquirer's rights would not become exercisable for our shares of common stock at a discount, the potential acquirer would suffer substantial dilution and may lose its ability to acquire us. In addition, the existence of the plan itself may deter a potential acquirer from acquiring or making an offer to acquire us. As a result, either by operation of the plan or by its potential deterrent effect, mergers and acquisitions of our company that our stockholders may consider in their best interests may not occur.
Because Warburg owns a substantial percentage of our outstanding common stock, we amended the stockholder rights plan in connection with Warburg's participation in an equity financing we completed in March 2005 to provide that Warburg and its affiliates will be exempt from the stockholder rights plan, unless Warburg and its affiliates become, without the prior consent of our board of directors, the beneficial owner of more than 44% of our common stock.
Unstable market conditions may have serious adverse consequences on our business.
Market instability has made the business climate more volatile and more costly. Our general business strategy may be adversely affected by unpredictable and unstable market conditions. If the current equity and credit markets deteriorate further, or do not improve, it may make any necessary
43
equity or debt financing more difficult, more costly, and more dilutive. While we believe we have adequate capital resources to meet our expected working capital and capital expenditure requirements for at least the next 12 months, a radical economic downturn or increase in our expenses could require additional financing on less than attractive rates or on terms that are excessively dilutive to existing stockholders. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. There is a risk that one or more of our current service providers, manufacturers or other partners may encounter difficulties during challenging economic times, which could have an adverse effect on our business, results of operations and financial condition.
The market price for our common stock has been and may continue to be highly volatile, and an active trading market for our common stock may never exist.
We cannot assure you that an active trading market for our common stock will exist at any time. Holders of our common stock may not be able to sell shares quickly or at the market price if trading in our common stock is not active. The trading price of our common stock has been and is likely to continue to be highly volatile and could be subject to wide fluctuations in price in response to various factors, many of which are beyond our control, including:
- •
- the timing and amount of revenues generated from sales of FOLOTYN;
- •
- actual or anticipated variations in quarterly operating results;
- •
- actual or anticipated regulatory approvals or non-approvals of FOLOTYN or of competing product candidates;
- •
- the loss of regulatory approval for FOLOTYN in patients with relapsed or refractory PTCL;
- •
- actual or anticipated results of our clinical trials involving FOLOTYN;
- •
- changes in laws or regulations applicable to FOLOTYN;
- •
- changes in the expected or actual timing of our development programs;
- •
- announcements of technological innovations by us or our competitors;
- •
- changes in financial estimates or recommendations by securities analysts;
- •
- conditions or trends in the biotechnology and pharmaceutical industries;
- •
- changes in the market valuations of similar companies;
- •
- announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
- •
- additions or departures of key personnel;
- •
- disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to
obtain patent protection for our technologies;
- •
- developments concerning any of our research and development, manufacturing and marketing collaborations;
- •
- sales of large blocks of our common stock;
- •
- sales of our common stock by our executive officers, directors and five percent stockholders; and
- •
- economic and other external factors, including disasters or crises.
44
Public companies in general and companies included on NASDAQ in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. There has been particular volatility in the market prices of securities of biotechnology and other life sciences companies, and the market prices of these companies have often fluctuated because of problems or successes in a given market segment or because investor interest has shifted to other segments. These broad market and industry factors may cause the market price of our common stock to decline, regardless of our operating performance. We have no control over this volatility and can only focus our efforts on our own operations, and even these may be affected due to the state of the capital markets. In the past, following large price declines in the public market price of a company's securities, securities class action litigation has often been initiated against that company, including in 2004 against us. Litigation of this type could result in substantial costs and diversion of management's attention and resources, which would hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
Substantial sales of shares may impact the market price of our common stock.
If our stockholders sell substantial amounts of our common stock, the market price of our common stock may decline. These sales also might make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we consider appropriate. We are unable to predict the effect that sales may have on the then prevailing market price of our common stock. We have entered into a Registration Rights Agreement with Warburg pursuant to which Warburg is entitled to certain registration rights with respect to shares of our common stock. On July 20, 2009, we filed a Registration Statement on Form S-3 with the SEC providing for the registration for resale by Warburg of up to 26,124,430 shares of our common stock, which registration statement was declared effective on August 28, 2009.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not Applicable.
Our corporate headquarters facility consists of approximately 34,536 square feet in Westminster, Colorado. We lease our corporate headquarters facility pursuant to a lease agreement that expires on January 31, 2012. We also lease an office in Princeton, New Jersey which consists of approximately 9,458 square feet. The lease for this office expires on September 30, 2011. We believe that our leased facilities are adequate to meet our needs at this time.
None.
45
ITEM 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information and Holders
Our common stock is traded on the NASDAQ Global Market under the symbol "ALTH." The following table sets forth, for the periods indicated, the high and low sales prices for our common stock as reported on the NASDAQ Global Market:
Year Ended December 31, 2010
|
HIGH | LOW | |||||
|---|---|---|---|---|---|---|---|
First Quarter |
$ | 8.15 | $ | 6.47 | |||
Second Quarter |
$ | 8.79 | $ | 6.10 | |||
Third Quarter |
$ | 6.18 | $ | 3.58 | |||
Fourth Quarter |
$ | 4.81 | $ | 3.71 | |||
Year Ended December 31, 2009
|
HIGH | LOW | |||||
|---|---|---|---|---|---|---|---|
First Quarter |
$ | 9.30 | $ | 5.62 | |||
Second Quarter |
$ | 8.50 | $ | 5.34 | |||
Third Quarter |
$ | 8.79 | $ | 6.60 | |||
Fourth Quarter |
$ | 7.39 | $ | 5.46 | |||
On February 25, 2011, we had approximately 53 holders of record of our common stock.
46
Stock Performance Measurement Comparison(1)
The following graph shows the total stockholder return of an investment of $100 in cash on December 31, 2005 for (i) the Company's common stock, (ii) the NASDAQ Composite Index and (iii) the NASDAQ Biotechnology Index. All values assume reinvestment of the full amount of all dividends and are calculated as of December 31 of each year:
Comparison of 5 year Cumulative Total Return on Investment
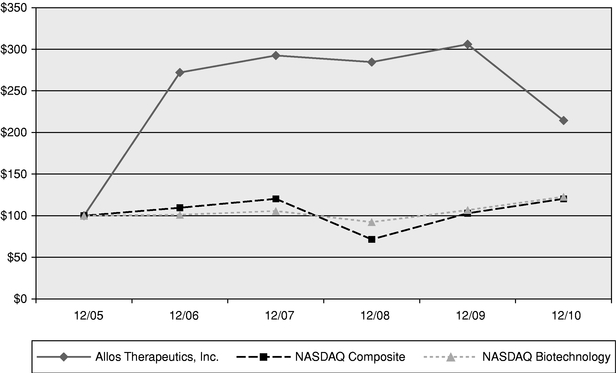
Total Return Analysis
|
12/31/2005 | 12/31/2006 | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Allos Therapeutics, Inc. |
$ | 100.00 | $ | 272.09 | $ | 292.56 | $ | 284.65 | $ | 306.05 | $ | 214.42 | |||||||
NASDAQ Composite |
$ | 100.00 | $ | 109.52 | $ | 120.27 | $ | 71.51 | $ | 102.89 | $ | 120.29 | |||||||
NASDAQ Biotechnology |
$ | 100.00 | $ | 101.02 | $ | 105.65 | $ | 92.31 | $ | 106.74 | $ | 122.76 | |||||||
- (1)
- The information in this section is not "soliciting material," is not deemed "filed" with the SEC and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Dividends
We have never paid dividends to holders of our common stock, and we do not anticipate paying any cash dividends in the foreseeable future as we intend to retain any earnings for use in our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
47
ITEM 6. SELECTED FINANCIAL DATA
The selected financial data set forth below should be read in conjunction with our financial statements and the related notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in this report. The statement of operations data for the years ended December 31, 2010, 2009 and 2008, and the balance sheet data as of December 31, 2010 and 2009, are derived from, and should be read in conjunction with, our audited financial statements and related notes included elsewhere in this report. The statement of operations data for the years ended December 31, 2007 and 2006, and the balance sheet data as of December 31, 2008, 2007 and 2006, are derived from our audited financial statements that do not appear in this report. The historical results are not necessarily indicative of the operating results to be expected in the future.
| |
Years Ended December 31, | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||
| |
(in thousands, except share and per share data) |
|||||||||||||||||
Statement of Operations Data: |
||||||||||||||||||
Net product sales |
$ | 35,227 | $ | 3,585 | $ | — | $ | — | $ | — | ||||||||
Operating costs and expenses: |
||||||||||||||||||
Cost of sales, excluding amortization expense presented below |
3,647 | 408 | — | — | — | |||||||||||||
Research and development |
31,359 | 32,618 | 30,595 | 22,992 | 16,606 | |||||||||||||
Selling, general and administrative |
78,782 | 44,448 | 23,044 | 19,672 | 14,876 | |||||||||||||
Amortization of intangible asset |
454 | 121 | — | — | — | |||||||||||||
Restructuring and separation costs |
— | — | — | — | 646 | |||||||||||||
Total operating costs and expenses |
114,242 | 77,595 | 53,639 | 42,664 | 32,128 | |||||||||||||
Operating loss |
(79,015 | ) | (74,010 | ) | (53,639 | ) | (42,664 | ) | (32,128 | ) | ||||||||
Interest and other income, net |
1,520 | 380 | 1,909 | 3,294 | 1,916 | |||||||||||||
Loss before income taxes |
(77,495 | ) | (73,630 | ) | (51,730 | ) | (39,370 | ) | (30,212 | ) | ||||||||
Income tax benefit |
78 | 77 | — | — | — | |||||||||||||
Net loss |
$ | (77,417 | ) | $ | (73,553 | ) | $ | (51,730 | ) | $ | (39,370 | ) | $ | (30,212 | ) | |||
Net loss per share: basic and diluted |
$ | (0.74 | ) | $ | (0.81 | ) | $ | (0.69 | ) | $ | (0.60 | ) | $ | (0.55 | ) | |||
Weighted average shares: basic and diluted |
105,123,420 | 90,469,720 | 75,399,774 | 65,188,913 | 55,299,614 | |||||||||||||
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||
| |
(in thousands) |
|||||||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents and investments |
$ | 98,565 | $ | 158,544 | $ | 83,965 | $ | 57,756 | $ | 32,796 | ||||||
Working capital |
90,612 | 151,305 | 77,981 | 51,958 | 28,897 | |||||||||||
Total assets |
120,756 | 175,384 | 89,340 | 61,460 | 36,382 | |||||||||||
Common stock and additional paid-in capital |
548,827 | 532,756 | 379,123 | 300,508 | 238,109 | |||||||||||
Accumulated deficit |
(450,629 | ) | (373,212 | ) | (299,659 | ) | (247,929 | ) | (208,559 | ) | ||||||
Total stockholders' equity |
98,198 | 159,544 | 79,464 | 52,579 | 29,550 | |||||||||||
48
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We are a biopharmaceutical company committed to the development and commercialization of innovative anti-cancer therapeutics. Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. We strive to develop proprietary products that have the potential to improve the standard of care in cancer therapy.
We are currently focused on the development and commercialization of FOLOTYN® (pralatrexate injection). FOLOTYN is a targeted folate inhibitor designed to accumulate preferentially in cancer cells. FOLOTYN targets the inhibition of dihydrofolate reductase, or DHFR, an enzyme critical in the folate pathway, thereby interfering with DNA and RNA synthesis and triggering cancer cell death. FOLOTYN can be delivered as a single agent, for which we currently have approval for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL, and has the potential to be used in combination therapy regimens. We believe that FOLOTYN's unique mechanism of action offers us the ability to target the drug for development in a variety of hematological malignancies and solid tumor indications. We currently retain exclusive worldwide commercial rights to FOLOTYN for all indications. We may also seek to grow our product portfolio through product acquisition and in-licensing efforts.
On September 24, 2009, the U.S. Food and Drug Administration, or FDA, granted accelerated approval of FOLOTYN for use as a single agent for the treatment of patients with relapsed or refractory PTCL. This approval was based on overall response rate from our pivotal Phase 2 trial known as PROPEL (Pralatrexate in patients with Relapsed Or refractory PEripheral T-cell Lymphoma). Clinical benefit such as improvement in progression-free survival or overall survival has not been demonstrated. FOLOTYN represents our first drug approved for marketing in the United States. FOLOTYN is the first and only drug approved by the FDA for this indication. In connection with the accelerated approval, we are required to conduct post-approval studies that are intended to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma and to determine whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment.
We began making FOLOTYN available for commercial sale in the United States in October 2009 and commenced our commercial launch of FOLOTYN in January 2010. We have established a commercial organization, including sales, marketing, supply chain management and reimbursement capabilities, to commercialize FOLOTYN in the United States. We believe the market for relapsed or refractory PTCL is addressable with a targeted U.S. sales and marketing organization, and we intend to continue promoting FOLOTYN ourselves in the United States.
We are also seeking regulatory approval to market FOLOTYN in Europe for the treatment of patients with relapsed or refractory PTCL. In December 2010, our Marketing Authorisation Application, or MAA, was accepted by the European Medicines Agency, or EMA. Acceptance of the MAA by the EMA indicates that the application is complete and initiates the EMA's regulatory review process. The MAA is based on clinical data from our pivotal PROPEL trial. We may also seek regulatory approval to market FOLOTYN for the treatment of patients with relapsed or refractory PTCL in Japan and other countries. We intend to secure a strategic partner for the potential co-development of FOLOTYN globally and commercialization outside the United States.
We are currently prioritizing our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies. We are committed to evaluating FOLOTYN for oncology use as a single agent and in combination with other therapies. We also intend to complete our ongoing Phase 2 studies in bladder
49
and breast cancer, and investigators are evaluating FOLOTYN in solid tumor indications through our collaboration with the National Comprehensive Cancer Network, or NCCN, Oncology Research Program.
Results of Operations
We have incurred significant net losses and negative cash flows from operations. We have incurred these losses principally from costs incurred in our research and development programs and from our selling, general and administrative expenses. Our primary business activities have been focused on the development of FOLOTYN for the three year period ended December 31, 2010. For the years ended December 31, 2010, 2009 and 2008, we had net losses of $77.4 million, $73.6 million and $51.7 million, respectively. Research and development expenses for the years ended December 31, 2010, 2009 and 2008 were $31.4 million, $32.6 million and $30.6 million, respectively. As of December 31, 2010, we had accumulated a deficit of $450.6 million.
Our ability to achieve profitability is dependent on our ability, alone or with partners, to significantly increase sales of FOLOTYN for the treatment of patients with relapsed or refractory PTCL in the United States. The amount of our future product sales are subject to significant uncertainty. We may never generate sufficient revenue from product sales to become profitable.
We expect to continue to spend substantial amounts on research and development, including amounts spent on conducting clinical trials and seeking additional regulatory approvals for FOLOTYN. We also expect to continue to spend substantial amounts on selling, general and administrative expenses to promote FOLOTYN for the treatment of patients with relapsed or refractory PTCL in the United States. Therefore, we may need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including those discussed under the "Liquidity and Capital Resources" section below.
If we are unable to significantly increase sales of FOLOTYN or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our future prospects for profitability may be harmed.
Comparison of Years Ended December 31, 2010, 2009 and 2008
Net product sales. Net product sales represent total revenue less distributor fees and estimated allowances for product returns, government rebates and chargebacks to be incurred on the selling price of FOLOTYN related to the respective revenue, as further described in the "Critical Accounting Policies" section below. We began making FOLOTYN available for commercial sale in the United States in October 5, 2009 and commenced our commercial launch of FOLOTYN in January 2010.
We sell FOLOTYN to a limited number of pharmaceutical wholesale distributors, or distributors, the three largest of which are affiliates under common control of an unrelated party. Title to the product passes upon delivery to our distributors, when the risks and rewards of ownership are assumed by the distributor (freight on board destination). These distributors then resell FOLOTYN to the patients' respective health care providers. Prior to the fourth quarter of 2010, product sales to distributors were recorded as deferred revenue until the product was sold through from our distributors to health care providers because we did not have sufficient history to be able to reasonably estimate returns. Beginning in the fourth quarter of 2010, we began recognizing revenue as product is sold to distributors as we established a sufficient history in order to reasonably estimate returns from our distributors. Consequently, for the year ended December 31, 2010, we recognized a one time increase of $604,000 in net product sales of FOLOTYN, or $0.01 per common share, representing product sales previously deferred as of December 31, 2009, net of distributor fees and estimated product returns, government rebates and chargebacks. Through December 31, 2010, product returns have been
50
negligible. Our distributors' contractual return rights are limited to defective product or product that was shipped in error. Returns are not allowed for expired product. Given these limited contractual return rights, the price of FOLOTYN and the limited number of patients in the United States, FOLOTYN distributors and their customers generally carry limited inventory.
Balances and activity in the deferred revenue account and a reconciliation of gross to net product sales for the years ended December 31, 2010, 2009 and 2008 are as follows:
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
| |
(in millions) |
||||||||||
Deferred revenue, beginning of the period |
$ | 0.7 | $ | — | $ | — | |||||
Gross product sales to distributors |
39.5 | 4.9 | — | ||||||||
Gross product sales recognized due to change in revenue recognition methodology |
(0.7 | ) | — | — | |||||||
Gross product sales recognized related to current year |
(39.5 | ) | (4.2 | ) | — | ||||||
Deferred revenue, end of the period |
$ | — | $ | 0.7 | $ | — | |||||
Gross product sales |
$ | 40.2 | $ | 4.2 | $ | — | |||||
Gross to Net Sales Adjustments: |
|||||||||||
Government rebates and chargebacks |
(3.4 | ) | (0.5 | ) | — | ||||||
Distribution fees |
(1.2 | ) | (0.1 | ) | — | ||||||
Product returns allowance |
(0.4 | ) | — | — | |||||||
Net product sales |
$ | 35.2 | $ | 3.6 | $ | — | |||||
The $35.2 million of net product sales in 2010 represents the first full calendar year of sales of FOLOTYN. The $3.6 million of net product sales in 2009 relates to sales of FOLOTYN commencing in the fourth quarter of 2009. There were no corresponding net product sales in 2008.
Balances and activity for the components of our gross to net sales adjustments for the years ended December 31, 2010 and 2009 are as follows:
| |
Product Returns |
Government Rebates and Chargebacks |
Distribution Fees |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in millions) |
||||||||||
Balance at December 31, 2008 |
$ | — | $ | — | $ | — | |||||
Reserve for current period sales |
— | 0.5 | 0.1 | ||||||||
Credits made for sales/payments |
— | (— | ) | (— | ) | ||||||
Balance at December 31, 2009 |
— | 0.5 | 0.1 | ||||||||
Reserve for current period sales |
0.4 | 3.6 | 1.2 | ||||||||
Change in estimate for prior period sales |
0.0 | (0.3 | ) | 0.0 | |||||||
Credits/payments made for prior period sales |
— | (0.1 | ) | (0.1 | ) | ||||||
Credits/payments made for current period sales |
(— | ) | (1.5 | ) | (0.9 | ) | |||||
Balance at December 31, 2010 |
$ | 0.4 | $ | 2.2 | $ | 0.3 | |||||
During the first quarter of 2010, we obtained additional market research and were able to refine our estimated Medicaid utilization, which resulted in a reversal of Medicaid rebate allowances related to 2009 sales totaling $208,000. In March 2010, the Patient Protection and Affordable Care Act, as modified by the Health Care and Education Affordability Reconciliation Act of 2010, or PPACA, was enacted, which increased the Medicaid rebate percentage from 15.1% to 23.1%, retroactive to January 1, 2010.
51
Product returns, government rebates and chargebacks reflect management estimates which are further discussed in the "Critical Accounting Policies" section below.
Cost of sales, excluding amortization expense. Cost of sales, excluding amortization expense, includes royalties, inventory packaging and labeling, warehousing and shipping costs associated with FOLOTYN product revenue.
| |
Years Ended December 31, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
| |
(in millions) |
|||||||||
Cost of sales, excluding amortization expense |
$ | 3.6 | $ | 0.4 | $ | — | ||||
Prior to receiving FDA approval of FOLOTYN on September 24, 2009, all costs related to purchases of the active pharmaceutical ingredient and manufacturing of the product were recorded as research and development expense. Until we sell the inventory for which the costs were previously expensed, our cost of sales will reflect only royalties and other incremental costs incurred subsequent to the FDA approval date. Accordingly, our cost of sales of FOLOTYN will be lower with respect to product that was manufactured prior to FDA approval. This occurred with respect to all sales of FOLOTYN in the fourth quarter of 2009 and a significant amount of sales of FOLOTYN in 2010.
The $3.6 million and $408,000 of cost of sales, excluding amortization expense in 2010 and 2009, respectively, was primarily due to an 8% royalty on our product sales payable to the licensors of FOLOTYN under the terms of our license agreement. There were no corresponding cost of sales in 2008.
Cost of sales for 2011 is expected to approximate 10% of gross product sales to distributors, which includes the current 8% royalty on FOLOTYN sales.
Research and development. Research and development expenses include the costs of certain personnel, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis, third-party manufacturing costs for development of drug materials for use in preclinical studies and clinical trials, and manufacturing costs and licensing fees incurred for FOLOTYN prior to receipt of FDA approval.
| |
Years Ended December 31, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
| |
(in millions) |
|||||||||
Research and development expenses |
$ | 31.4 | $ | 32.6 | $ | 30.6 | ||||
The $1.3 million decrease in research and development expenses in 2010 as compared to 2009 was primarily due to the following:
- •
- a $2.6 million decrease in third-party manufacturing costs for clinical trial material and manufacturing
pre-commercial product in preparation for the approval and commercial availability of FOLOTYN in 2009;
- •
- a $2.5 million decrease in costs related to clinical trials involving FOLOTYN that have closed enrollment,
including decreased costs for our Phase 2b non-small cell lung cancer, or NSCLC, study, which completed patient enrollment in July 2009;
- •
- a $1.5 million decrease in licensing costs resulting from the milestone payment made under the license agreement for FOLOTYN upon FDA acceptance of our New Drug Application, or NDA, for review in May 2009, with no corresponding amount in 2010; and
52
- •
- a $709,000 decrease in consulting, professional fees and grants, primarily resulting from regulatory affairs and preparations related to the filing of our NDA and the FDA's Oncologic Drugs Advisory Committee, or ODAC, meeting for FOLOTYN in September 2009, with no corresponding amounts in 2010.
This decrease was partially offset by:
- •
- a $3.8 million increase in costs related to clinical trials involving FOLOTYN, including start-up costs
for the post-approval studies required by the FDA and other trials with ongoing enrollment;
- •
- a $1.2 million increase in personnel and related travel costs, mainly attributable to additional headcount and
increases in compensation costs year-over-year; and
- •
- a $919,000 increase in non-cash stock-based compensation expense, primarily resulting from a one-time $0.9 million reversal of stock-based compensation expense in connection with the resignation of our former Chief Medical Officer, or CMO, in September 2009, as discussed in more detail in the Stock-based Compensation Expense section below.
The $2.0 million increase in research and development expenses in 2009 as compared to 2008 was primarily due to the following:
- •
- a $1.6 million increase in personnel and related travel costs, mainly attributable to additional headcount and
increases in compensation costs year-over-year;
- •
- a $1.6 million increase in consulting and professional fees, primarily related to regulatory affairs, including
preparations related to the filing of our NDA for FOLOTYN and the FDA's ODAC meeting in September 2009;
- •
- a $1.4 million increase in third-party manufacturing costs for clinical trial material and
pre-commercial scale-up activities for FOLOTYN; and
- •
- a $1.0 million increase in licensing costs for FOLOTYN, as $1.5 million of milestone payments under the license agreement for FOLOTYN became due upon FDA acceptance of our NDA for review in May 2009, with a corresponding amount of $500,000 in the same period in 2008.
These increases were partially offset by:
- •
- a $2.1 million decrease in clinical trial costs involving FOLOTYN, including decreased costs for PROPEL, which
completed patient enrollment in April 2008;
- •
- a $724,000 decrease in stock-based compensation, primarily related to the resignation of our former CMO, as discussed in
more detail in the Stock-based Compensation Expense section below; and
- •
- a $617,000 decrease in preclinical study costs involving FOLOTYN.
We expect research and development expenses for 2011, excluding non-cash stock-based compensation expense, to be fairly consistent with the 2010 amount of $28.0 million. Our guidance for 2011 research and development expenses includes our ongoing and planned studies, including the planned initiation in 2011 of our post-marketing Phase 3 study in PTCL.
53
We expect the non-cash stock-based compensation portion of research and development expense to increase in 2011 as compared to 2010, as discussed in more detail in the Stock-based Compensation Expense section below.
In January 2011, we implemented a strategic reduction of our workforce by approximately 13%, or 25 employees. Personnel reductions were primarily focused in research and development and general and administrative functions. The restructuring was a result of our decision to prioritize our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies, and to manage our operating costs and expenses accordingly. We expect to incur total restructuring charges of approximately $0.7 million in connection with the restructuring, all in the form of one-time termination benefits, including approximately $0.3 million of research and development expenses. We expect to record substantially all of these charges in the first quarter of 2011.
We charge direct internal and external research and development expenses to the respective development programs. In December 2002, we entered into a license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, as amended, under which we obtained exclusive worldwide rights to a portfolio of patents and patent applications related to FOLOTYN and its uses. From December 2002 through December 31, 2010, we have incurred direct costs of approximately $71.3 million associated with the development of FOLOTYN, including $65.5 million related to research and development expenses for FOLOTYN and an additional $5.8 million related to a milestone payment as a result of the FDA's approval to market FOLOTYN on September 24, 2009, which was capitalized as an intangible asset on our balance sheet. In addition, we incur indirect costs related to FOLOTYN, which consist primarily of salaries and benefits for all clinical, regulatory affairs, biostatistical analysis and manufacturing personnel, consulting and professional fees, including costs associated with filing our NDA and MAA for FOLOTYN, facilities costs and other internal-shared resources related to the development and maintenance of infrastructure, systems and processes. The research and development expenses of $31.4 million, $32.6 million and $30.6 million for the years ended December 31, 2010, 2009 and 2008 primarily relate to FOLOTYN development including all direct and indirect costs incurred in each of those years.
The timing and costs to complete the successful clinical development of FOLOTYN for additional indications is highly uncertain, and therefore difficult to estimate. The lengthy process of seeking regulatory approvals for additional indications, and the subsequent compliance with applicable regulations, require the expenditure of substantial resources. Clinical development timelines, likelihood of success and total costs vary widely and are impacted by a variety of factors, including those discussed in the "Risk Factors" section of Part I, Item 1A above. Because of these risks and uncertainties, we cannot predict whether or when we will successfully complete the development of FOLOTYN for any additional indications or the ultimate costs of such efforts.
Selling, general and administrative. Selling, general and administrative expenses include costs for sales and marketing activities, corporate development, medical affairs, executive administration, corporate offices and related infrastructure.
| |
Years Ended December 31, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
| |
(in millions) |
|||||||||
Selling, general and administrative expenses |
$ | 78.8 | $ | 44.4 | $ | 23.0 | ||||
54
The $34.3 million increase in selling, general and administrative expenses in 2010 as compared to 2009 was primarily due to the following:
- •
- a $17.8 million increase in personnel and related travel and infrastructure costs, mainly attributable to
additional headcount to support the commercialization of FOLOTYN, including our sales and marketing organization, and increases in compensation costs year-over-year;
- •
- an $11.5 million increase in sales and marketing costs associated with the commercialization of FOLOTYN, including
promotional expenses, advisory boards, market research and costs related to trade shows;
- •
- a $2.4 million increase in grants and sponsored medical education programs;
- •
- a $1.8 million increase in stock-based compensation, net of a one-time $0.8 million reversal
resulting from the departure of our former Chief Commercial Officer in August 2010, as discussed in more detail in the Stock-based Compensation Expense
section below; and
- •
- a $762,000 increase in professional fees primarily related to increased portfolio and intellectual property development activities and administrative compliance associated with commercialization and additional headcount.
The $21.4 million increase in selling, general and administrative expenses in 2009 as compared to 2008 was primarily due to the following:
- •
- an $11.3 million increase in personnel and related travel and infrastructure costs, mainly attributable to
additional headcount for the planned commercialization of FOLOTYN, including our sales and marketing organization, and increases in compensation costs year-over-year;
- •
- a $4.2 million increase in market research and consulting expenses related to the planned commercialization of
FOLOTYN;
- •
- a $3.3 million increase in advertising costs, including promotional expenses and costs related to trade shows;
- •
- a $1.4 million increase in non-cash stock-based compensation expense, as discussed in more detail
below;
- •
- a $1.3 million increase in grants and sponsored medical education programs; and
- •
- a $600,000 increase related to public relations activities.
These increases were partially offset by a $815,000 decrease in portfolio and intellectual property development activities.
We expect selling, general and administrative expenses for 2011, excluding non-cash stock-based compensation expense, to slightly decrease compared to the 2010 amount of $70.7 million.
We expect the non-cash stock-based compensation portion of selling, general and administrative expense to increase in 2011 as compared to 2010, as discussed in more detail in the Stock-based Compensation Expense section below.
In January 2011, we implemented a strategic reduction of our workforce by approximately 13%, or 25 employees. Personnel reductions were primarily focused in research and development and general and administrative functions. We plan to maintain our current level of personnel in sales and marketing. The restructuring was a result of our decision to prioritize our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies, and to manage our operating costs and expenses accordingly. We expect to incur total restructuring charges of approximately $0.7 million in connection with the restructuring, all in the form of one-time termination benefits, including approximately
55
$0.4 million of selling, general and administrative expenses. We expect to record substantially all of these charges in the first quarter of 2011.
Stock-based Compensation Expense. Stock-based compensation expense for the years ended December 31, 2010, 2009 and 2008 has been recognized in our Statements of Operations as follows:
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
| |
(in millions) |
||||||||||
Research and development |
$ | 3.3 | $ | 2.4 | $ | 3.1 | |||||
Selling, general and administrative |
8.1 | 6.3 | 4.9 | ||||||||
Total stock-based compensation expense |
$ | 11.4 | $ | 8.7 | $ | 8.0 | |||||
Effective August 24, 2010, James V. Caruso, our former Executive Vice President, Chief Commercial Officer (CCO), departed the Company. As a result of Mr. Caruso's departure, we adjusted the forfeiture rate applied to his equity compensation, which resulted in a one-time $787,000 reversal of selling, general and administrative stock-based compensation expense during the year ended December 30, 2010, of which $605,000 related to stock option awards and $182,000 related to restricted stock unit awards, or RSUs.
Effective September 30, 2009, Pablo J. Cagnoni, M.D., our former Senior Vice President, Chief Medical Officer (CMO), resigned. As a result of his resignation, we adjusted the forfeiture rate applied to his equity compensation, which resulted in a one-time $906,000 reversal of research and development stock-based compensation expense during the year ended December 31, 2009, of which $699,000 related to stock option awards, $166,000 related to restricted stock awards and $41,000 related to RSUs.
Of the $11.4 million of stock-based compensation recognized in the year ended December 31, 2010, $9.0 million was related to our stock option plans, $2.2 million related to restricted stock and RSUs and $203,000 related to our employee stock purchase plan. Of the $8.7 million of stock-based compensation recognized in the year ended December 31, 2009, $8.1 million was related to our stock option plans, $416,000 related to restricted stock and RSUs and $116,000 related to our employee stock purchase plan. Of the $8.0 million of stock-based compensation recognized in the year ended December 31, 2008, $7.5 million was related to our stock option plans, $423,000 related to restricted stock and $59,000 related to our employee stock purchase plan. The $2.7 million increase in stock-based compensation expense in 2010 as compared to 2009 was primarily due to options granted to new employees during 2010, an increase in the number of options and RSUs granted to existing employees pursuant to our annual grants that occurred in February 2010, and the grant of additional RSUs to existing employees that occurred in October 2010, offset by the reversal related to the departure of our former CCO discussed above. The $660,000 increase in stock-based compensation expense in 2009 as compared to 2008 was primarily due to increases in the number of options granted to new employees during 2009 and to existing employees pursuant to our annual grants that occurred in February 2009, offset by the reversal related to the resignation of our former CMO discussed above.
As of December 31, 2010, the unrecorded stock-based compensation balance related to stock option awards was $7.3 million and will be recognized over an estimated weighted-average amortization period of 1.6 years. As of December 31, 2010, the unrecorded stock-based compensation balance related to RSUs was approximately $8.0 million and will be recognized over an estimated weighted-average amortization period of 1.9 years. As of December 31, 2010, the unrecorded stock-based compensation balance related to restricted stock awards was approximately $10,000 and will be recognized over an estimated weighted-average amortization period of 1.4 years.
56
Stock-based compensation expense in fiscal year 2011 is expected to be approximately $13 to $15 million. The projected increase in stock-based compensation expense in 2011 as compared to 2010 is primarily due to a full year of expense relating to the restricted stock units that were granted to existing employees in October 2010 and the restricted stock units that were granted to existing employees in February 2011 pursuant to our annual grants, which vest over 3 years instead of 4 years and result in a higher fair value and related stock-based compensation expense over the vesting period of the award.
Amortization of intangible asset. Amortization of intangible asset includes amortization of capitalized license costs over the expected patent life of the related product.
Amortization of intangible asset expense for the years ended December 31, 2010 and 2009 was $454,000 and $121,000, respectively. There was no expense for the year ended December 31, 2008. The expense in the years ended December 31, 2010 and 2009 was due to the amortization of the $5.8 million intangible asset resulting from a milestone payment under our license agreement for FOLOTYN in September 2009. Amortization expense is being recorded on a straight line basis over the estimated remaining life of the composition of matter patent for FOLOTYN, which we expect to last until July 16, 2022. This includes the anticipated Hatch-Waxman extension that provides patent protection for drug compounds for a period of up to five years to compensate for time spent in development. This term is our best estimate of the life of the patent. If, however, the Hatch-Waxman extension is not granted, the intangible asset will be amortized over a shorter period.
Interest and Other Income, Net. Interest income, net of interest expense, for the years ended December 31, 2010, 2009 and 2008 was $1.5 million, $380,000, and $1.9 million, respectively. The $1.1 million increase in 2010 as compared to 2009 was primarily due to the Therapeutic Discovery Tax Credit, which we elected to receive in the form of a cash payment, or grant totaling approximately $1,467,000 and a realized loss of approximately $157,000 on the sale of certain of our investments during the year ended December 31, 2009, with no corresponding amount for the same period in 2010, offset by lower yields on our cash, cash equivalents and investments. The $1.5 million decrease in 2009 as compared to 2008 was primarily due to lower yields on our cash, cash equivalents and investments in marketable securities and a $149,000 loss on the disposal of certain software that was no longer in use during the three months ended June 30, 2009, offset by a $395,000 decrease in realized losses on the sale of certain of our investments in marketable securities. We have the ability and intent to hold our remaining investments in marketable securities to recover the entire amortized cost basis of the investments as of December 31, 2010, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs.
Income Taxes. Income tax benefit for the years ended December 31, 2010, 2009 and 2008 was $78,000, $77,000 and $0, respectively. The income tax benefit recorded for both years ended December 31, 2010 and 2009 were related to a refundable research and experimentation income tax credit received during the respective period. The $77,000 increase in income tax benefit in the year ended December 31, 2009 as compared to the same period in 2008 was related to a refundable research and experimentation income tax credit received during 2009, with no corresponding amount in 2008. As of December 31, 2010, we had available approximately $304.7 million of net operating loss, or NOL, carryforwards, after taking into consideration NOLs expected to expire unused due to the limitations under Section 382 of the Internal Revenue Code, and which includes approximately $9.2 million of deductions related to stock-based compensation that are not realized as deferred tax assets until current taxes payable can be reduced. Of these NOL carryforwards, $3.9 million will expire in 2011 and $6.2 million in 2012 and the remaining NOL carryforwards expire in 2018 through 2030. In addition, as of December 31, 2010, we had research and development credit and orphan drug credit carryforwards, after taking into consideration the Section 382 limitation, of $4.8 million and $6.6 million, respectively, to offset future regular tax expense. Since our formation, we have raised
57
capital through the issuance of capital stock on several occasions which, combined with shareholders' subsequent disposition of those shares, has resulted in four changes of control in 1994, 1998, 2001 and 2005, as defined by Section 382. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% within a three-year period. As a result of the most recent ownership change in 2005, utilization of approximately $59.9 million of NOL carryforwards generated prior to the latest change are subject to an annual limitation of approximately $2.2 million under Section 382, determined by multiplying the value of our stock at the time of the ownership change by the applicable long-term tax-exempt rate. Additionally, we have a recognized built-in gain that increased the annual limitation by $3.3 million for each of the five years after the 2005 ownership change. Any unused annual limitation may be carried over to subsequent years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Subsequent ownership changes, as defined in Section 382, could further limit the amount of our NOL carryforwards and research and development credits that can be utilized annually to offset future taxable income.
Liquidity and Capital Resources
As of December 31, 2010, we had $98.6 million in cash, cash equivalents, and investments. Of this amount, $48.2 million was held in money market funds and cash accounts and $50.4 million was held in U.S. Treasury bills and notes and high-grade corporate notes with a weighted average duration of the remaining time to maturity of approximately four months. Until required for use in our business, we invest our cash reserves in bank deposits, money market funds, certificates of deposit, high-grade corporate notes and U.S. government instruments in accordance with our investment policy.
During the three years ended December 31, 2010, we have financed our operations primarily through public sales of our equity securities, which have resulted in net proceeds to us of $205.3 million. In addition, we began generating sales of FOLOTYN in the fourth quarter of 2009. Net product sales were $35.2 million and $3.6 million for the years ended December 31, 2010 and 2009, respectively, which partially offset our operating costs and expenses for the respective periods. Our key objective for 2011 is to increase sales of FOLOTYN in the United States.
Net cash used to fund our operating activities for the years ended December 31, 2010, 2009 and 2008 was $63.7 million, $62.2 million and $42.9 million, respectively.
For fiscal year 2011, total operating costs and expenses are expected to approximate $95 to $98 million, excluding cost of sales and non-cash stock-based compensation expense. Stock-based compensation expense is expected to approximate $13 to $15 million. In January 2011, we implemented a strategic reduction of our workforce by approximately 13%, or 25 employees. Personnel reductions were primarily focused in research and development and general and administrative functions. We plan to maintain our current level of personnel in sales and marketing. The restructuring was a result of our decision to prioritize our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies, and to manage our operating costs and expenses. We expect to incur total restructuring charges of approximately $0.7 million in connection with the restructuring, all in the form of one-time termination benefits. We expect to record substantially all of these charges in the first quarter of 2011. Actual financial results for 2011 will vary based upon many factors, including the growth of FOLOTYN sales and rate of patient enrollment in clinical trials that are ongoing and planned for initiation in 2011.
Net cash used in investing activities for the year ended December 31, 2010 was $34.0 million and consisted primarily of purchases of investments in marketable securities, partially offset by the proceeds
58
from maturities of investments in marketable securities. Net cash provided by investing activities for the year ended December 31, 2009 was $28.0 million and consisted of the proceeds from maturities and sales, net of purchases of investments in marketable securities, partially offset by $5.8 million of cash paid related to the milestone payment made under our license agreement for FOLOTYN upon FDA approval in September 2009 and $1.4 million for the acquisition of property and equipment. Net cash used in investing activities for the year ended December 31, 2008 was $13.2 million and consisted primarily of purchases of investments in marketable securities, partially offset by the proceeds from maturities of investments in marketable securities.
Net cash provided by financing activities for the year ended December 31, 2010 was $4.6 million and consisted primarily of proceeds from the issuance of common stock associated with stock options exercised by our employees and sales of stock under our employee stock purchase plan. Net cash provided by financing activities for the year ended December 31, 2009 was $145.0 million and consisted of $47.0 million of net proceeds from the sale of 7,750,000 shares of common stock in April 2009, $93.1 million of net proceeds from the sale of 14,000,000 shares of common stock in October 2009, and $4.9 million of proceeds from the issuance of common stock associated with stock options exercised by our employees and sales of stock under our employee stock purchase plan. Net cash provided by financing activities for the year ended December 31, 2008 was $70.6 million and consisted primarily of the net proceeds from the sale of 12,420,000 shares of common stock in May 2008 and $5.4 million of proceeds from the issuance of common stock associated with stock options exercised by our employees and sales of stock under our employee stock purchase plan.
Based upon the current status of our product development and commercialization plans, we believe that our cash, cash equivalents, and investments as of December 31, 2010 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and actual results could vary materially.
We anticipate continuing our current development programs and beginning other long-term development projects involving FOLOTYN, including the post-approval clinical studies required for FOLOTYN. These projects may require many years and substantial expenditures to complete and may ultimately be unsuccessful. In addition, we expect to incur significant costs relating to the commercialization of FOLOTYN, including costs related to our sales and marketing, medical affairs and manufacturing operations. Therefore, we may need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and amount of revenue generated from sales of FOLOTYN;
- •
- the timing and costs associated with our sales and marketing activities for promoting FOLOTYN;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of FOLOTYN;
- •
- the timing and costs associated with conducting preclinical and clinical development of FOLOTYN, including the
post-approval clinical studies required by the FDA;
- •
- the timing and costs associated with our evaluation of, and decisions with respect to, the potential development of
FOLOTYN for additional therapeutic indications;
- •
- the timing, costs and potential revenue associated with a potential strategic partnership for the
co-development of FOLOTYN globally and commercialization outside the United States; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
59
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we might otherwise seek to develop or commercialize ourselves, on terms that are less favorable than might otherwise be available. If we are unable to generate meaningful amounts of revenue from future product sales or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our business and future prospects for revenue and profitability may be harmed.
Obligations and Commitments
Below is a schedule of the timing of contractual commitments, by fiscal year, related to our leases, service contracts and license agreements. We currently have no off-balance sheet arrangements.
| |
2011 | 2012 to 2013 | 2014 to 2015 | After 2015 |
Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Operating lease obligations |
$ | 1,271,000 | $ | 521,000 | $ | — | $ | — | $ | 1,792,000 | ||||||
Total obligations |
$ | 1,271,000 | $ | 521,000 | $ | — | $ | — | $ | 1,792,000 | ||||||
Operating lease obligations represent our future minimum rental commitments for non-cancelable operating leases for our facilities and automobiles. We lease our corporate headquarters facility pursuant to a lease agreement that expires on January 31, 2012. Our lease for an office in Princeton, New Jersey expires on September 30, 2011.
We are currently seeking regulatory approval to market FOLOTYN in Europe for the treatment of patients with relapsed or refractory PTCL. In December 2010, our MAA was accepted for review by the EMA. In the event we obtain regulatory approval to market FOLOTYN in Europe, we are required to make an additional milestone payment of $3.5 million under the terms of our license agreement for FOLOTYN.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses. We base our estimates on historical experience, available information and assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions. We have reviewed our critical accounting policies and estimates with the Audit Committee of our Board of Directors. We believe the following policies to be the most critical to an understanding of our financial condition and results of operations because they require us to make estimates, assumptions and informed management judgments about matters that are inherently uncertain:
- •
- revenue recognition;
- •
- accounting for research and development expenses;
- •
- accounting for inventory; and
60
- •
- accounting for stock-based compensation expense.
Revenue Recognition
We generate revenue from product sales. We recognize product revenue when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) our price to the buyer is fixed and determinable; and (4) collectability is reasonably assured. Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if (1) our price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid us, or the buyer is obligated to pay us and the obligation is not contingent on resale of the product, (3) the buyer's obligation to us would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from that provided by us, (5) we do not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated.
We sell FOLOTYN to a limited number of pharmaceutical wholesale distributors, or distributors, the three largest of which are affiliates under common control of an unrelated party. Title to the product passes upon delivery to our distributors, when the risks and rewards of ownership are assumed by the distributor (freight on board destination). These distributors then resell FOLOTYN to the patients' respective health care providers. Prior to the fourth quarter of 2010, product sales to distributors were recorded as deferred revenue until the product was sold through from our distributors to health care providers because we did not have sufficient history to be able to reasonably estimate returns. Beginning in the fourth quarter of 2010, we began recognizing revenue as product is sold to distributors as we established a sufficient history in order to reasonably estimate returns from our distributors. Consequently, for the year ended December 31, 2010, we recognized a one time increase of $604,000 in net product sales of FOLOTYN, or $0.01 per common share, representing product sales previously deferred as of December 31, 2009, net of distributor fees and estimated product returns, government rebates and chargebacks. We monitor inventory levels within our distribution channel and sales to end users, or health care providers, to determine whether deferral of sales is required. No such deferrals were recorded at December 31, 2010.
We estimate gross to net sales adjustments based upon analysis of third-party information, including information obtained from our primary distributors with respect to their inventory levels and sell-through to the distributors' customers.
Net Product Sales
Our net product sales represent total product sales less distributor fees and estimated allowances for product returns, government rebates and chargebacks to be incurred on the selling price of FOLOTYN related to the respective product sales. In addition, we incur distributor fees related to the management of our product by distributors. These distributor fees are recorded within net product sales and are based on definitive contractual agreements. Due to estimates and assumptions inherent in determining the amount of returns, rebates and chargebacks, the actual amount of returns and claims for rebates and chargebacks may be different from our estimates, at which time we would adjust our reserves accordingly. Product sales allowances and accruals are based on definitive contractual agreements or legal requirements (such as Medicaid laws and regulations) related to the purchase and/or utilization of the product by these entities. Allowances and accruals are recorded in the same period that the related revenue is recognized.
61
Product Returns
Our distributors' contractual return rights are limited to defective product or product that was shipped in error. Returns are not allowed for expired product. Given these limited contractual return rights, the price of FOLOTYN and the limited number of patients in the United States, FOLOTYN distributors and their customers generally carry limited inventory. We estimated product returns for FOLOTYN based upon actual returns history within our distribution channel, which were consistent with historical trends of product returns for similar companies in the pharmaceutical industry. The actual returns history within our distribution channel is derived from third-party information obtained from certain distributors with respect to their inventory levels and sell-through to the distributors' customers. We will continue to monitor the historical trend of returns, including the impacts on this trend of product expiry dates and may be required to make future adjustments to our estimates. During the year ended December 31, 2010, we recorded an estimated sales return allowance of approximately 1% of cumulative gross product sales to date, or $444,000.
Medicaid Rebates
Our product is subject to state government-managed Medicaid programs whereby discounts and rebates are provided to participating state governments. We record estimated rebates payable under governmental programs, including Medicaid, as a reduction of revenue at the time revenues are recorded. Our calculations related to these rebate accruals require estimates, including estimates of customer mix primarily based on a combination of market and clinical research, to determine which sales will be subject to rebates and the amount of such rebates. During the first quarter of 2010, we obtained additional market research and were able to refine our estimated Medicaid utilization, which resulted in a reversal of Medicaid rebate allowances related to 2009 sales totaling $208,000. Our estimate of utilization is based on market research and information about our expected patient population. Through December 31, 2010, we have not had sufficient claims from states for rebates with which to update our estimate. However, when we have sufficient claims history, we will consider such history in our estimate which could result in a change in our estimate. We also consider any legal interpretations of the applicable laws related to Medicaid and qualifying federal and state government programs and any new information regarding changes in the Medicaid programs' regulations and guidelines that would impact the amount of the rebates. In March 2010, the PPACA was enacted, which increased the Medicaid rebate percentage from 15.1% to 23.1%, retroactive to January 1, 2010. In addition, the states' ability to early adopt portions of PPACA, and any implementing regulations, could impact future estimates related to our Medicaid rebate allowances. We update our estimates and assumptions each period and record any necessary adjustments to our reserves. Although allowances and accruals are recorded at the time of product sale, certain rebates are typically paid out, on average, up to six months or longer after the sale. For reference purposes, a 10% to 20% increase in the Medicaid utilization percentage within our patient population as of December 31, 2010, would result in an approximate $659,000 to $1,318,000 reduction in cumulative net product sales.
Government Chargebacks
Our products are subject to certain programs with federal government qualified entities whereby pricing on products is discounted below distributor list price to participating entities. These entities purchase products through distributors at the discounted price, and the distributors charge the difference between their acquisition cost and the discounted price back to us. We account for chargebacks by establishing an accrual in an amount equal to our estimate of chargeback claims at the time of product sale. We do not expect the impact of the 340B program expansion included in the PPACA to significantly change our estimated government chargeback accruals because drugs approved under an Orphan Drug designation were specifically excluded from the provisions of the PPACA. The FDA has awarded orphan drug status to FOLOTYN for the treatment of patients with T-cell
62
lymphoma, which includes patients with relapsed or refractory PTCL. Through December 31, 2010 our chargeback experience has not been sufficient to update our estimated chargebacks. However, when we have sufficient history, we will consider such history in our estimate which could result in a change in our estimate. Due to estimates and assumptions inherent in determining the amount of government chargebacks, the actual amount of claims for chargebacks may be different from our estimates, at which time we would adjust our reserves accordingly.
Research and Development
Research and development expenditures are charged to expense as incurred. Research and development expenses include the costs of certain personnel, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis, third party manufacturing costs for development of drug materials for use in clinical trials and preclinical studies and licensing fees for our product candidates prior to FDA approval. All finished drug inventory costs associated with production activities in our third party manufacturing facilities prior to receiving FDA approval for such facilities and prior to receiving regulatory approval to market our product are expensed to research and development expenses. We accrue research and development expenses for activity as incurred during the fiscal year and prior to receiving invoices from clinical sites and third party clinical and preclinical research organizations. We accrue external costs for clinical and preclinical studies based on an evaluation of the following: the progress of the studies, including patient enrollment, dosing levels of patients enrolled, estimated costs to dose patients, invoices received, and contracted costs with clinical sites and third party clinical and preclinical research organizations. Significant judgments and estimates must be made and used in determining the accrued balance in any accounting period. Actual results could differ from those estimates. During the years ended December 31, 2010, 2009 and 2008, we did not have any changes in estimates that resulted in material adjustments to research and development expenses accrued in the prior period.
In accordance with certain research and development agreements, we are obligated to make certain upfront payments upon execution of the agreement. We record these upfront payments as prepaid research and development expenses. Such payments are recorded to research and development expense as services are performed. We evaluate on a quarterly basis whether events and circumstances have occurred that may indicate impairment of remaining prepaid research and development expenses.
Inventory
Costs associated with the production of FOLOTYN bulk drug substance and formulated drug product by our third party manufacturers are recorded as either research and development expense or inventory.
Costs associated with the production of FOLOTYN by our third party manufacturers are expensed to research and development expense at the time of production when the formulated drug product is packaged for clinical trial use.
We capitalize the costs for our marketed products at the lower of cost (first-in, first-out method) or market (current replacement cost) with cost determined on the first-in, first-out basis and then expense the sold inventory as a component of cost of goods sold.
Prior to receiving FDA approval of FOLOTYN, all costs related to purchases of the active pharmaceutical ingredient and the manufacturing of the product were recorded as research and development expense. We have remaining supplies of FOLOTYN drug substance and drug product that are not recorded as inventory on our balance sheet as of December 31, 2010 because they were purchased prior to FDA approval. Accordingly, our cost of sales will be lower with respect to product manufactured prior to FDA approval. Until we sell these supplies for which the costs were previously
63
expensed, our cost of sales will reflect only incremental costs incurred subsequent to the FDA approval date.
Stock-based Compensation Expense
We have several stock-based compensation plans under which incentive and non-qualified stock options, RSUs and restricted shares may be granted, and an employee stock purchase plan. We measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. That cost is recognized over the period during which an employee is required to provide services in exchange for the award, the requisite service period (usually the vesting period). We provide an estimate of forfeitures at initial grant date.
During the years ended December 31, 2010, 2009 and 2008, we recorded stock-based compensation expense of approximately $11.4 million, $8.7 million and $8.0 million, respectively, related to stock-based awards, including stock options, RSUs, restricted stock and our employee stock purchase plan. As of December 31, 2010, the unrecorded deferred stock-based compensation balance related to these stock-based awards was approximately $15.3 million and will be recognized over the remaining vesting periods of the awards. Judgments and estimates must be made and used in determining the factors used in calculating the fair value of stock-based awards, including the expected forfeiture rate of our stock-based awards, the expected life of our stock-based awards, and the expected volatility of our stock price. For more information on stock-based compensation expense during the year ended December 31, 2010, refer to Note 5 "Stock-Based Compensation Plans" of the Notes to our Financial Statements included in Part IV, Item 15 of this report.
Recent Accounting Pronouncements
We reviewed recently issued accounting pronouncements and plan to adopt those that are applicable to us. We do not expect the adoption of these pronouncements to have a material impact on our financial position, results of operations or cash flows.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our financial instruments as of December 31, 2010 consisted of cash, cash equivalents, investments, accounts receivable, prepaid expenses, accounts payable and accrued liabilities. All highly liquid investments with original maturities of three months or less are considered to be cash equivalents. We invest in marketable securities in accordance with our investment policy. The primary objectives of our investment policy are to preserve principal and maintain proper liquidity to meet operating needs. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2010 was approximately four months. As of December 31, 2010, our investments of $50.4 million were all classified as held-to-maturity and were held in a variety of interest-bearing instruments, consisting mainly of U.S. Treasury bills and notes. We did not hold any derivative instruments, foreign exchange contracts, asset backed securities, mortgage backed securities, auction rate securities, or securities of issuers in bankruptcy in our investment portfolio as of December 31, 2010. The value of our investments may be adversely affected by rating downgrades or bankruptcies affecting the issuers of such securities, whether caused by instability in the global financial markets, lack of liquidity in the credit and capital markets, or other factors. We have the ability and intent to hold our remaining investments in marketable securities to recover the entire amortized cost basis of the investments as of December 31, 2010, although we monitor our investment portfolio with the primary objectives of preserving principal and maintaining proper liquidity to meet our operating needs.
64
Investments in fixed-rate interest-bearing instruments carry varying degrees of interest rate risk. The fair market value of our fixed-rate securities may be adversely impacted due to a rise in interest rates. In general, securities with longer maturities are subject to greater interest-rate risk than those with shorter maturities. Due in part to this factor, our interest income may fall short of expectations or we may suffer losses in principal if securities are sold that have declined in market value due to changes in interest rates. Due to the short duration of our investment portfolio, we believe an immediate 10% change in interest rates would not be material to our financial condition or results of operations.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements required by this Item are included in Item 15 of this report and are presented beginning on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of the end of the period covered by this report, an evaluation was carried out under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of our disclosure controls and procedures, as defined in Rule 13(a)-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Based on that evaluation, our management, including our principal executive officer and principal financial officer, concluded that our disclosure controls and procedures were effective as of December 31, 2010 to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining effective internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or Rule 15d-(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company's principal executive officer and principal financial officer and effected by the company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that:
- •
- pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and
dispositions of the assets of the company;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
65
- •
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2010. In making its assessment, management used the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on its assessment, management determined that, as of December 31, 2010, we maintained effective internal control over financial reporting based on those criteria.
In addition, the effectiveness of our internal control over financial reporting as of December 31, 2010 has been audited by Ernst & Young, LLP, an independent registered public accounting firm, as stated in their report on page 67 of this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
In the fourth quarter of 2010, we changed our revenue recognition policy to begin recording sales as product is received by our distributors, whereas, previously we recorded revenue under the sell-through method. The new revenue recognition methodology required new processes and accounting estimates. We are not aware of any material adverse impacts on our internal controls over financial reporting as a result of the implementation of these new controls. There were no other changes in our internal control over financial reporting during the quarter ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
66
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Allos Therapeutics, Inc.
We have audited Allos Therapeutics, Inc.'s (the "Company") internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Allos Therapeutics, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Allos Therapeutics, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheet of Allos Therapeutics, Inc. as of December 31, 2010, and the related statements of operations, stockholders' equity, and cash flows for the year then ended of Allos Therapeutics, Inc. and our report dated March 3, 2011 expressed an unqualified opinion thereon.
|
/s/ Ernst & Young LLP |
Denver,
Colorado
March 3, 2011
67
None.
68
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item concerning our directors is incorporated by reference to the information to be set forth in the section entitled "Proposal 1—Election of Directors" in our definitive Proxy Statement for the 2011 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the end of our fiscal year ended December 31, 2010, or the Proxy Statement. The information required by this Item concerning compliance with Section 16(a) of the Exchange Act is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Section 16(a) Beneficial Ownership Reporting Compliance." The information required by this Item concerning the procedures by which our stockholders may recommend nominees to our Board of Directors is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Information Regarding the Director Nomination Process." The information required by this Item concerning our Audit Committee is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Audit Committee." The balance of the information required by this Item, except as otherwise set forth below, is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Executive Officers."
Our Board of Directors has adopted a Code of Business Conduct and Ethics for all of our directors, officers and employees. Stockholders may locate a copy of our Code of Business Conduct and Ethics on our website at http://www.allos.com or request a free copy from:
Allos
Therapeutics, Inc.
Attention: Investor Relations
11080 CirclePoint Road, Suite 200
Westminster, CO 80020
Telephone: 303-426-6262
To date, there have been no waivers under our Code of Business Conduct and Ethics. We will post any waivers, if and when granted, of our Code of Business Conduct and Ethics on our website.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item regarding executive compensation is incorporated by reference to the information to be set forth in the sections of the Proxy Statement entitled "Executive Compensation," "Director Compensation," "Compensation Committee Interlocks and Insider Participation," and "Compensation Committee Report."
69
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Securities Authorized for Issuance Under Equity Compensation Plans
The following table provides certain information with respect to our equity compensation plans in effect as of December 31, 2010:
Plan Category
|
Number of securities to be issued upon exercise of outstanding options and rights (a) |
Weighted-average exercise price of outstanding options and rights (b) |
Number of securities remaining available for issuance under equity compensation plans (excluding securities reflected in column (a)) (c) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Equity compensation plans approved by security holders |
11,208,907 | $ | 6.36 | (1) | 13,293,520 | (2)(3) | ||||
Equity compensation plans not approved by security holders |
— | — | — | |||||||
Total |
11,208,907 | $ | 6.36 | (1) | 13,293,520 | (2)(3) | ||||
- (1)
- Weighted-average
exercise price of outstanding options and rights excludes restricted stock unit awards, as they have no exercise price.
- (2)
- Includes
11,191,603 shares of common stock available for future issuance under our 2008 Equity Incentive Plan. All stock awards granted under our 2008
Equity Incentive Plan after the June 24, 2008 effective date thereof, other than stock options and stock appreciation rights granted with an exercise price of at least 100% of such stock
award's fair market value on the date of grant, reduce the number of shares available for issuance under our 2008 Equity Incentive Plan by 1.35 shares per share granted pursuant to the stock award.
Shares of common stock that revert to and again become available for issuance under our 2008 Equity Incentive Plan and that prior to such reversion were granted pursuant to a stock award that reduced
the number of shares available under our 2008 Equity Incentive Plan by 1.35 shares per share granted pursuant to such stock award, will cause the number of shares of our common stock available for
issuance under our 2008 Equity Incentive Plan to increase by 1.35 shares upon such reversion.
- (3)
- Includes 2,101,917 shares of common stock available for future issuance under our 2001 Employee Stock Purchase Plan.
Other Information
Other information required by this Item regarding security ownership of certain beneficial owners and management is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Security Ownership of Certain Beneficial Owners and Management."
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item regarding certain relationships and related transactions and director independence is incorporated by reference to the information to be set forth in the sections of the Proxy Statement entitled "Transactions with Related Persons" and "Information Regarding the Board of Directors and Corporate Governance."
70
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item regarding principal accounting fees and services is incorporated by reference to the information to be set forth in the section of the Proxy Statement entitled "Proposal 2—Ratification of Selection of Independent Registered Public Accounting Firm."
71
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are being filed as part of this report:
- (1)
- Financial Statements.
- (2)
- Financial Statement Schedules.
Reference is made to the Index to Financial Statements of Allos Therapeutics, Inc. appearing on page F-1 of this report.
- (3)
- Exhibits.
All financial statement schedules have been omitted because they are not applicable or not required or because the information is included elsewhere in the Financial Statements or the Notes thereto.
The following is a list of exhibits filed as part of this report on Form 10-K. Where so indicated exhibits that were previously filed are incorporated by reference.
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 3.01 | Amended and Restated Certificate of Incorporation. | 8-K | 7/20/2009 | 3.1 | ||||||||
3.02 |
Certificate of Designation of Series A Junior Participating Preferred Stock. |
8-K |
7/20/2009 |
3.2 |
||||||||
3.03 |
Certificate of Amendment to Restated Certificate of Incorporation. |
8-K |
7/20/2009 |
3.3 |
||||||||
3.04 |
Certificate of Amendment to the Certificate of Designations of Series A Junior Participating Preferred Stock. |
8-K |
7/20/2009 |
3.4 |
||||||||
3.05 |
Certificate of Amendment to Restated Certificate of Incorporation. |
8-K |
6/25/2010 |
3.1 |
||||||||
3.06 |
Amended and Restated Bylaws of Allos Therapeutics, Inc. |
8-K |
6/25/2007 |
3.04 |
||||||||
4.01 |
Form of Common Stock Certificate. |
S-1/A |
3/17/2000 |
4.01 |
||||||||
4.02 |
Reference is made to Exhibits 3.01, 3.02, 3.03, 3.04 and 3.05. |
|||||||||||
4.03 |
Rights Agreement dated May 6, 2003 between Allos and Mellon Investor Services LLC. |
8-K |
5/9/2003 |
99.2 |
||||||||
4.04 |
Form of Rights Certificate. |
8-K |
5/9/2003 |
99.3 |
||||||||
4.05 |
Amendment to Rights Agreement dated March 4, 2005 between Allos and Mellon Investor Services LLC. |
8-K |
3/4/2005 |
4.06 |
||||||||
4.06 |
Amendment to Rights Agreement dated January 29, 2007 between Allos and Mellon Investor Services LLC. |
8-K |
1/30/2007 |
4.1 |
||||||||
72
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 4.07 | Amendment to Rights Agreement, dated as of July 17, 2009, between Allos and Mellon Investor Services LLC. | 8-K | 7/20/2009 | 4.1 | ||||||||
10.1 |
† |
Form of Amended and Restated Indemnity Agreement between Allos and each of its directors and officers. |
8-K |
6/25/2007 |
10.01 |
|||||||
10.2 |
† |
1995 Stock Option Plan, as amended. |
S-1 |
1/26/2000 |
10.11 |
|||||||
10.3 |
† |
2000 Stock Incentive Compensation Plan, as amended. |
8-K |
12/22/2005 |
10.1 |
|||||||
10.3.1 |
† |
Form of Incentive Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
8-K |
2/11/2005 |
99.1 |
|||||||
10.3.2 |
† |
Form of Nonqualified Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
8-K |
2/11/2005 |
99.2 |
|||||||
10.3.3 |
† |
Form of Nonqualified Stock Option Letter Agreement for Non-Employee Directors under 2000 Stock Incentive Compensation Plan. |
8-K |
2/24/2006 |
10.1 |
|||||||
10.4 |
† |
2001 Employee Stock Purchase Plan, as amended and restated effective December 13, 2010. |
X |
|||||||||
10.4.1 |
† |
2001 Employee Stock Purchase Plan Offering (Series Beginning July 1, 2007). |
8-K |
6/25/2007 |
10.12.1 |
|||||||
10.5 |
* |
Office Lease dated April 4, 2001 between Allos and Catellus Development Corporation. |
10-Q |
8/14/2001 |
10.27 |
|||||||
10.5.1 |
* |
Amended and Restated Second Amendment to Lease dated December 9, 2002 between Allos and Catellus Development Corporation. |
10-K |
3/28/2003 |
10.27.1 |
|||||||
10.5.2 |
* |
Third Amendment to Lease dated November 28, 2003 between Allos and Catellus Development Corporation. |
10-K |
3/5/2004 |
10.27.2 |
|||||||
10.5.3 |
* |
Fifth Amendment to Office Lease Agreement dated June 16, 2008 between Allos and Circle Point Properties, LLC. |
10-Q |
8/5/2008 |
10.5.3 |
|||||||
10.6 |
Securities Purchase Agreement dated March 2, 2005 between Allos and the Investors listed on the signature pages thereto. |
8-K/A |
3/10/2005 |
10.41 |
||||||||
10.7 |
Registration Rights Agreement dated March 4, 2005 between Allos and the Investors listed on Schedule I thereto. |
8-K/A |
3/10/2005 |
10.42 |
||||||||
10.8 |
Letter Agreement dated March 4, 2005 among Allos, Warburg Pincus Private Equity VIII, L.P., Warburg Pincus & Co. and Warburg Pincus LLC. |
8-K |
3/4/2005 |
10.43 |
||||||||
73
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.9 | † | Summary of Compensation Arrangements for Non-Employee Directors. | 10-Q | 8/4/2009 | 10.1 | |||||||
10.10 |
† |
Restricted Stock Award Agreement dated March 9, 2006 between Allos and Paul L. Berns. |
8-K |
3/14/2006 |
10.2 |
|||||||
10.11 |
† |
2006 Inducement Award Plan, including forms of Stock Option Grant Notice with Stock Option Agreement and Restricted Stock Grant Notice with Restricted Stock Grant Agreement. |
8-K |
6/6/2006 |
10.1 |
|||||||
10.12 |
* |
License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated December 23, 2002 and amended May 9, 2006 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
10-K |
3/1/2010 |
10.13 |
|||||||
10.12.1 |
* |
Second Amendment to License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated November 6, 2007 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
10-K |
3/1/2010 |
10.13.1 |
|||||||
10.13 |
† |
Corporate Bonus Plan, as amended and restated effective September 15, 2008. |
10-Q |
11/5/2008 |
10.1 |
|||||||
10.14 |
† |
Second Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Paul L. Berns. |
10-K |
2/27/2008 |
10.20 |
|||||||
10.14.1 |
† |
First Amendment to Second Amended and Restated Employment Agreement, effective as of May 20, 2009, between Allos and Paul L. Berns. |
8-K |
5/22/2009 |
10.1 |
|||||||
10.14.2 |
† |
Second Amendment to Second Amended and Restated Employment Agreement, effective as of March 2, 2011, between Allos and Paul L. Berns. |
X |
|||||||||
10.15 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and James V. Caruso. |
10-K |
2/27/2008 |
10.22 |
|||||||
10.15.1 |
† |
First Amendment to Amended and Restated Employment Agreement, dated as of May 20, 2009, between Allos and James V. Caruso. |
8-K |
5/22/2009 |
10.3 |
|||||||
10.15.2 |
† |
Release Agreement, dated as of October 22, 2010, between Allos and James V. Caruso. |
8-K |
10/22/2010 |
10.3 |
|||||||
10.16 |
† |
Second Amended and Restated Employment Agreement, effective June 2, 2010, between Allos and Marc H. Graboyes. |
8-K |
6/3/2010 |
10.1 |
|||||||
74
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.16.1 | † | First Amendment to Second Amended and Restated Employment Agreement, effective as of March 2, 2011, between Allos and Marc H. Graboyes. | X | |||||||||
10.17 |
† |
Executive Compensation and Equity Awards. |
8-K |
2/26/2010 |
10.1 |
|||||||
10.18 |
† |
Allos Therapeutics, Inc. 2008 Equity Incentive Plan, as amended. |
8-K |
6/25/2010 |
10.1 |
|||||||
10.18.1 |
† |
Form of Option Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
S-8 |
6/24/2008 |
99.2 |
|||||||
10.18.2 |
† |
Form of Restricted Stock Award Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
S-8 |
6/24/2008 |
99.3 |
|||||||
10.18.3 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
8-K |
2/27/2009 |
10.2 |
|||||||
10.18.4 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
8-K |
10/22/2010 |
10.2 |
|||||||
10.19 |
† |
Allos Therapeutics, Inc. Severance Benefit Plan, as amended and restated effective December 13, 2010. |
X |
|||||||||
10.19.1 |
† |
Allos Therapeutics, Inc. Change in Control Severance Benefit Schedule, as amended and restated effective February 23, 2008. |
8-K |
2/27/2009 |
10.4 |
|||||||
10.19.2 |
† |
Allos Therapeutics, Inc. Amendment No. 1 to Change in Control Severance Benefit Schedule, adopted May 19, 2009. |
8-K |
5/22/2009 |
10.5 |
|||||||
10.20 |
† |
Employment Agreement, effective June 25, 2009, between the Company and David C. Clark. |
8-K |
6/26/2009 |
10.1 |
|||||||
10.20.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and David C. Clark. |
X |
|||||||||
10.21 |
Employment Agreement, effective April 26, 2010, between the Company and Charles Q. Morris. |
8-K |
4/27/2010 |
10.1 |
||||||||
10.21.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Charles Q. Morris. |
X |
|||||||||
10.22 |
† |
Employment Agreement, effective September 16, 2010, between Allos and Bruce K. Bennett, Jr. |
8-K |
9/17/2010 |
10.1 |
|||||||
10.22.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Bruce K. Bennett, Jr. |
X |
|||||||||
10.23 |
† |
Employment Agreement, effective September 16, 2010, between Allos and Michael E. Schick. |
8-K/A |
9/17/2010 |
10.1 |
|||||||
75
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.23.1 | † | First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Michael E. Schick. | X | |||||||||
10.24 |
† |
Employment Agreement, effective April 29, 2009, between Allos and Bruce A. Goldsmith. |
8-K/A |
9/17/2010 |
10.2 |
|||||||
10.24.1 |
† |
First Amendment to Employment Agreement, effective May 22, 2009, between Allos and Bruce A. Goldsmith. |
8-K/A |
9/17/2010 |
10.3 |
|||||||
10.24.2 |
† |
Second Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Bruce A. Goldsmith. |
X |
|||||||||
10.25 |
† |
Executive Equity Awards. |
8-K |
10/22/2010 |
10.1 |
|||||||
23.01 |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm. |
X |
||||||||||
23.02 |
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
X |
||||||||||
24.01 |
Power of Attorney (included on signature page hereto). |
X |
||||||||||
31.01 |
Rule 13a-14(a)/15d-14(a) Certification. |
X |
||||||||||
31.02 |
Rule 13a-14(a)/15d-14(a) Certification. |
X |
||||||||||
32.01 |
# |
Section 1350 Certification. |
X |
|||||||||
- †
- Indicates
management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of
Form 10-K.
- *
- Indicates
confidential treatment has been granted with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and
Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
- #
- The certifications attached as Exhibit 32.01 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Allos Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.
76
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ALLOS THERAPEUTICS, INC. | ||||
Date: March 3, 2011 |
By: |
/s/ PAUL L. BERNS Paul L. Berns President and Chief Executive Officer |
||
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints Paul L. Berns and David C. Clark, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and all other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons on behalf of the registrant on March 3, 2011, and in the capacities indicated:
Name
|
Title
|
|
|---|---|---|
| /s/ STEPHEN J. HOFFMAN Stephen J. Hoffman |
Chairman of Board of Directors and Director | |
/s/ PAUL L. BERNS Paul L. Berns |
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
/s/ DAVID C. CLARK David C. Clark |
Vice President, Finance and Treasurer (Principal Financial and Accounting Officer) |
|
/s/ NISHAN DE SILVA Nishan de Silva |
Director |
|
/s/ JEFFREY R. LATTS Jeffrey R. Latts |
Director |
|
/s/ JONATHAN S. LEFF Jonathan S. Leff |
Director |
|
/s/ TIMOTHY P. LYNCH Timothy P. Lynch |
Director |
|
/s/ DAVID M. STOUT David M. Stout |
Director |
77
Allos Therapeutics, Inc.
Index to Financial Statements
F-1
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Allos Therapeutics, Inc.
We have audited the accompanying balance sheet of Allos Therapeutics, Inc. (the "Company") as of December 31, 2010, and the related statements of operations, stockholders' equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the 2010 financial statements referred to above present fairly, in all material respects, the financial position of Allos Therapeutics, Inc. at December 31, 2010, and the results of its operations and its cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Allos Therapeutics, Inc.'s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 3, 2011 expressed an unqualified opinion thereon.
|
/s/ Ernst & Young LLP |
Denver,
Colorado
March 3, 2011
F-2
Report of Independent Registered Public Accounting Firm
To
the Board of Directors and Stockholders of
Allos Therapeutics, Inc.:
In our opinion, the accompanying balance sheet as of December 31, 2009 and the related statements of operations, changes in stockholders' equity, and cash flows for each of two years in the period ended December 31, 2009 present fairly, in all material respects, the financial position of Allos Therapeutics, Inc. at December 31, 2009, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2009, in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
/s/
PricewaterhouseCoopers LLP
Denver, Colorado
March 1, 2010
F-3
ALLOS THERAPEUTICS, INC.
BALANCE SHEETS
(Dollars in thousands, except share and per share amounts)
| |
December 31, | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||||
ASSETS |
|||||||||
Current assets: |
|||||||||
Cash and cash equivalents |
$ | 48,164 | $ | 141,185 | |||||
Short-term investments |
50,334 | 17,016 | |||||||
Restricted cash |
238 | 238 | |||||||
Accounts receivable |
12,076 | 4,862 | |||||||
Inventory |
178 | 36 | |||||||
Prepaid expenses and other assets |
2,180 | 3,808 | |||||||
Total current assets |
113,170 | 167,145 | |||||||
Property and equipment, net |
2,245 | 2,169 | |||||||
Long-term investments |
67 | 343 | |||||||
Intangible asset, net |
5,225 | 5,679 | |||||||
Other assets |
49 | 48 | |||||||
Total assets |
$ | 120,756 | $ | 175,384 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||||||
Current liabilities: |
|||||||||
Trade accounts payable |
$ | 4,931 | $ | 2,035 | |||||
Accrued liabilities |
17,627 | 13,136 | |||||||
Deferred revenue |
— | 669 | |||||||
Total current liabilities |
22,558 | 15,840 | |||||||
Commitments and contingencies (Note 9) |
|||||||||
Stockholders' equity: |
|||||||||
Preferred stock, $0.001 par value; 10,000,000 shares authorized; no shares issued or outstanding |
— | — | |||||||
Series A Junior Participating Preferred Stock, $0.001 par value; 1,500,000 shares designated from authorized preferred stock; no shares issued or outstanding |
— | — | |||||||
Common stock, $0.001 par value; 200,000,000 and 150,000,000 shares authorized at December 31, 2010 and December 31, 2009, respectively; 105,493,546 and 104,234,409 shares issued and outstanding at December 31, 2010 and December 31, 2009, respectively |
105 | 104 | |||||||
Additional paid-in capital |
548,722 | 532,652 | |||||||
Accumulated deficit |
(450,629 | ) | (373,212 | ) | |||||
Total stockholders' equity |
98,198 | 159,544 | |||||||
Total liabilities and stockholders' equity |
$ | 120,756 | $ | 175,384 | |||||
The accompanying notes are an integral part of these financial statements.
F-4
ALLOS THERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(Dollars in thousands, except share and per share amounts)
| |
Years Ended December 31, | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||
Net product sales |
$ | 35,227 | $ | 3,585 | $ | — | ||||||
Operating costs and expenses: |
||||||||||||
Cost of sales, excluding amortization expense presented below |
3,647 | 408 | — | |||||||||
Research and development |
31,359 | 32,618 | 30,595 | |||||||||
Selling, general and administrative |
78,782 | 44,448 | 23,044 | |||||||||
Amortization of intangible asset |
454 | 121 | — | |||||||||
Total operating costs and expenses |
114,242 | 77,595 | 53,639 | |||||||||
Operating loss |
(79,015 | ) | (74,010 | ) | (53,639 | ) | ||||||
Interest and other income, net |
1,520 | 380 | 1,909 | |||||||||
Loss before income taxes |
(77,495 | ) | (73,630 | ) | (51,730 | ) | ||||||
Income tax benefit |
78 | 77 | — | |||||||||
Net loss |
$ | (77,417 | ) | $ | (73,553 | ) | $ | (51,730 | ) | |||
Net loss per share: basic and diluted |
$ | (0.74 | ) | $ | (0.81 | ) | $ | (0.69 | ) | |||
Weighted average shares: basic and diluted |
105,123,420 | 90,469,720 | 75,399,774 | |||||||||
The accompanying notes are an integral part of these financial statements.
F-5
ALLOS THERAPEUTICS, INC.
STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
(Dollars in thousands, except share and per share amounts)
| |
Common Stock | |
|
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders' Equity |
||||||||||||||
| |
Shares | Amount | |||||||||||||||
Balance at January 1, 2008 |
67,641,943 | $ | 68 | $ | 300,440 | $ | (247,929 | ) | $ | 52,579 | |||||||
Issuance of common stock upon exercise of stock options for cash |
1,144,041 | 1 | 5,290 | — | 5,291 | ||||||||||||
Issuance of common stock upon exercise of purchase rights |
22,828 | — | 119 | — | 119 | ||||||||||||
Issuance of common stock net of offering costs of $4,864 |
12,420,000 | 12 | 65,173 | — | 65,185 | ||||||||||||
Issuance of restricted stock |
10,000 | — | — | — | — | ||||||||||||
Stock compensation expense |
— | — | 8,020 | — | 8,020 | ||||||||||||
Net loss |
— | — | — | (51,730 | ) | (51,730 | ) | ||||||||||
Balance at December 31, 2008 |
81,238,812 | 81 | 379,042 | (299,659 | ) | 79,464 | |||||||||||
Issuance of common stock upon exercise of stock options for cash |
1,241,034 | 1 | 4,682 | — | 4,683 | ||||||||||||
Issuance of common stock upon exercise of purchase rights |
42,063 | — | 229 | — | 229 | ||||||||||||
Issuance of common stock in April 2009, net of offering costs of $1,868 |
7,750,000 | 8 | 46,949 | — | 46,957 | ||||||||||||
Issuance of common stock in October 2009, net of offering costs of $6,316 |
14,000,000 | 14 | 93,070 | — | 93,084 | ||||||||||||
Forfeiture of restricted stock |
(37,500 | ) | — | — | — | — | |||||||||||
Stock compensation expense |
— | — | 8,680 | — | 8,680 | ||||||||||||
Net loss |
— | — | — | (73,553 | ) | (73,553 | ) | ||||||||||
Balance at December 31, 2009 |
104,234,409 | 104 | 532,652 | (373,212 | ) | 159,544 | |||||||||||
Issuance of common stock upon exercise of stock options for cash |
1,104,474 | 1 | 4,084 | — | 4,085 | ||||||||||||
Issuance of common stock upon exercise of purchase rights |
115,797 | — | 528 | — | 528 | ||||||||||||
Issuance of common stock upon vesting of restricted stock units |
38,866 | — | — | — | — | ||||||||||||
Other |
— | — | 32 | — | 32 | ||||||||||||
Stock compensation expense |
— | — | 11,426 | — | 11,426 | ||||||||||||
Net loss |
— | — | — | (77,417 | ) | (77,417 | ) | ||||||||||
Balance at December 31, 2010 |
105,493,546 | $ | 105 | $ | 548,722 | $ | (450,629 | ) | $ | 98,198 | |||||||
The accompanying notes are an integral part of these financial statements.
F-6
ALLOS THERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(Dollars in thousands)
| |
Years Ended December 31, | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||||||
Cash Flows From Operating Activities: |
||||||||||||||
Net loss |
$ | (77,417 | ) | $ | (73,553 | ) | $ | (51,730 | ) | |||||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||
Depreciation and amortization |
861 | 462 | 393 | |||||||||||
Stock-based compensation expense |
11,426 | 8,680 | 8,020 | |||||||||||
Amortization of intangible asset |
454 | 121 | — | |||||||||||
Realized loss on sale of marketable securities |
— | 157 | 552 | |||||||||||
Loss on disposal of property and equipment |
207 | 152 | 19 | |||||||||||
Inventory reserve |
138 | — | — | |||||||||||
Changes in operating assets and liabilities: |
||||||||||||||
Accounts receivable |
(7,883 | ) | (4,193 | ) | — | |||||||||
Prepaid expenses and other assets |
1,628 | 25 | (930 | ) | ||||||||||
Interest receivable on investments |
(49 | ) | 806 | (168 | ) | |||||||||
Inventory |
(102 | ) | — | — | ||||||||||
Trade accounts payable |
2,769 | 1,692 | (911 | ) | ||||||||||
Accrued liabilities |
4,312 | 3,452 | 1,905 | |||||||||||
Net cash used in operating activities |
(63,656 | ) | (62,199 | ) | (42,850 | ) | ||||||||
Cash Flows From Investing Activities: |
||||||||||||||
Acquisition of property and equipment |
(1,016 | ) | (1,414 | ) | (1,097 | ) | ||||||||
Pledge of restricted cash |
— | — | (54 | ) | ||||||||||
Cash paid for license |
— | (5,800 | ) | — | ||||||||||
Purchases of marketable securities |
(74,974 | ) | (18,208 | ) | (93,938 | ) | ||||||||
Proceeds from maturities of marketable securities |
41,980 | 49,500 | 75,136 | |||||||||||
Proceeds from sales of marketable securities |
— | 3,894 | 6,747 | |||||||||||
Net cash provided by (used in) investing activities |
(34,010 | ) | 27,972 | (13,206 | ) | |||||||||
Cash Flows From Financing Activities: |
||||||||||||||
Proceeds from issuance of common stock associated with stock options and employee stock purchase plan |
4,613 | 4,912 | 5,410 | |||||||||||
Proceeds from issuance of common stock, net of issuance costs |
32 | 140,041 | 65,185 | |||||||||||
Net cash provided by financing activities |
4,645 | 144,953 | 70,595 | |||||||||||
Net (decrease) increase in cash and cash equivalents |
(93,021 | ) | 110,726 | 14,539 | ||||||||||
Cash and cash equivalents, beginning of period |
141,185 | 30,459 | 15,920 | |||||||||||
Cash and cash equivalents, end of period |
$ | 48,164 | $ | 141,185 | $ | 30,459 | ||||||||
Supplemental Schedule of Cash and Non-cash Operating and Financing Activities: |
||||||||||||||
Tax refunds received |
$ | 78 | $ | 77 | $ | — | ||||||||
Deferred revenue in accounts receivable |
$ | (669 | ) | $ | 669 | $ | — | |||||||
Assets recorded for which payment has not yet occurred |
$ | 306 | $ | 152 | $ | — | ||||||||
The accompanying notes are an integral part of these financial statements.
F-7
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
(Dollars shown in tables are in thousands, except per share amounts)
Unless the context otherwise requires, references in this report to "Allos," the "Company," "we," "us" and "our" refer to Allos Therapeutics, Inc.
1. Formation and Business of the Company
We are a biopharmaceutical company committed to the development and commercialization of innovative anti-cancer therapeutics. Our goal is to build a profitable company by generating income from products we develop and commercialize, either alone or with one or more potential strategic partners. We strive to develop proprietary products that have the potential to improve the standard of care in cancer therapy.
We are currently focused on the development and commercialization of FOLOTYN® (pralatrexate injection). FOLOTYN is a targeted folate inhibitor designed to accumulate preferentially in cancer cells. FOLOTYN targets the inhibition of dihydrofolate reductase, or DHFR, an enzyme critical in the folate pathway, thereby interfering with DNA and RNA synthesis and triggering cancer cell death. FOLOTYN can be delivered as a single agent, for which we currently have approval for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma, or PTCL, and has the potential to be used in combination therapy regimens. We believe that FOLOTYN's unique mechanism of action offers us the ability to target the drug for development in a variety of hematological malignancies and solid tumor indications. We currently retain exclusive worldwide commercial rights to FOLOTYN for all indications. We may also seek to grow our product portfolio through product acquisition and in-licensing efforts.
On September 24, 2009, the U.S. Food and Drug Administration, or FDA, granted accelerated approval of FOLOTYN for use as a single agent for the treatment of patients with relapsed or refractory PTCL. This approval was based on overall response rate from our pivotal PROPEL trial. Clinical benefit such as improvement in progression-free survival or overall survival has not been demonstrated. FOLOTYN represents our first drug approved for marketing in the United States. FOLOTYN is the first and only drug approved by the FDA for this indication. In connection with the accelerated approval, we are required to conduct post-approval studies that are intended to verify and describe FOLOTYN's clinical benefit in patients with T-cell lymphoma and to determine whether FOLOTYN poses a serious risk of altered drug levels resulting from organ impairment.
In addition to relapsed or refractory PTCL, we are currently evaluating FOLOTYN in a number of oncology indications, including cutaneous T-cell lymphoma and other hematologic malignancies, bladder and breast cancer.
As of December 31, 2010, we had $98.6 million in cash, cash equivalents, and investments. Based upon the current status of our product development and commercialization plans, we believe that our cash, cash equivalents, and investments in marketable securities as of December 31, 2010 should be adequate to support our operations through at least the next 12 months, although there can be no assurance that this can, in fact, be accomplished.
Our ability to achieve profitability is dependent on our ability, alone or with partners, to significantly increase sales of FOLOTYN for the treatment of patients with relapsed or refractory PTCL in the United States. The amount of our future product sales are subject to significant uncertainty. We may never generate sufficient revenue from product sales to become profitable.
F-8
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
1. Formation and Business of the Company (Continued)
We expect to continue to spend substantial amounts on research and development, including amounts spent on conducting clinical trials and seeking additional regulatory approvals for FOLOTYN. We also expect to continue to spend substantial amounts on selling, general and administrative expenses to promote FOLOTYN for the treatment of patients with relapsed or refractory PTCL in the United States. Therefore, we may need to raise additional capital to support our future operations. Our actual capital requirements will depend on many factors, including:
- •
- the timing and amount of revenue generated from sales of FOLOTYN;
- •
- the timing and costs associated with our sales and marketing activities for promoting FOLOTYN;
- •
- the timing and costs associated with manufacturing clinical and commercial supplies of FOLOTYN;
- •
- the timing and costs associated with conducting preclinical and clinical development of FOLOTYN, including the
post-approval clinical studies required by the FDA;
- •
- the timing and costs associated with our evaluation of, and decisions with respect to, the potential development of
FOLOTYN for additional therapeutic indications;
- •
- the timing, costs and potential revenue associated with a potential strategic partnership for the
co-development of FOLOTYN globally and commercialization outside the United States; and
- •
- our evaluation of, and decisions with respect to, potential in-licensing or product acquisition opportunities or other strategic alternatives.
We may seek to obtain this additional capital through equity or debt financings, arrangements with corporate partners, or from other sources. Such financings or arrangements, if successfully consummated, may be dilutive to our existing stockholders. However, there is no assurance that additional financing will be available when needed, or that, if available, we will obtain such financing on terms that are favorable to our stockholders or us. In the event that additional funds are obtained through arrangements with collaborative partners or other sources, such arrangements may require us to relinquish rights to some of our technologies, product candidates or products under development, which we might otherwise seek to develop or commercialize ourselves, on terms that are less favorable than might otherwise be available. If we are unable to significantly increase sales of FOLOTYN or cannot otherwise raise sufficient additional funds to support our operations, we may be required to delay, reduce the scope of or eliminate one or more of our development programs and our future prospects for profitability may be harmed.
We incorporated in the Commonwealth of Virginia on September 1, 1992 as HemoTech Sciences, Inc. and filed amended Articles of Incorporation to change our name to Allos Therapeutics, Inc. on October 19, 1994. We reincorporated in Delaware on October 28, 1996. We operate as a single business segment.
F-9
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States of America requires management to make estimates and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amount of net product sales and expenses during the reporting period. Actual results could differ from these estimates.
Cash Equivalents
All highly liquid investments with original maturities of three months or less are considered to be cash equivalents.
Restricted Cash
On August 22, 2008, $238,000 of cash was pledged as collateral on a letter of credit related to a lease for administrative office space and is classified as restricted cash on the Balance Sheet.
Prepaid Expenses and Other Assets
Prepaid expenses and other assets are comprised of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Prepaid sales, marketing and medical affairs expenses |
$ | 1,308 | $ | 1,839 | |||
Prepaid expenses and other assets |
650 | 1,251 | |||||
Prepaid research and development expenses |
222 | 718 | |||||
|
$ | 2,180 | $ | 3,808 | |||
Research and development expenditures are charged to expense as incurred. In accordance with certain research and development agreements, we are obligated to make certain upfront payments upon execution of the agreement. We record these upfront payments as prepaid research and development expenses. Such payments are recorded to research and development expense as services are performed. We evaluate on a quarterly basis whether events and circumstances have occurred that may indicate impairment of remaining prepaid research and development expenses.
Inventory
Costs associated with the production of FOLOTYN bulk drug substance and formulated drug product by our third party manufacturers are recorded as either research and development expense or inventory.
Costs associated with the production of FOLOTYN by our third party manufacturers are expensed to research and development expense at the time of production when the formulated drug product is packaged for clinical trial use.
F-10
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
We capitalize the costs for our marketed products at the lower of cost (first-in, first-out method) or market (current replacement cost) with cost determined on the first-in, first-out basis and then expense the sold inventory as a component of cost of goods sold.
Prior to receiving FDA approval of FOLOTYN, all costs related to purchases of the active pharmaceutical ingredient and the manufacturing of the product were recorded as research and development expense. We have remaining supplies of FOLOTYN drug substance and drug product that are not recorded as inventory on our Balance Sheet as of December 31, 2010 because they were purchased prior to FDA approval. Accordingly, our cost of sales will be lower with respect to product manufactured prior to FDA approval. Until we sell these supplies for which the costs were previously expensed, our cost of sales will reflect only incremental costs incurred subsequent to the FDA approval date.
Inventory consisted of:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Work in process |
$ | 254 | $ | 36 | |||
Finished goods |
38 | — | |||||
|
292 | 36 | |||||
Less reserve |
(114 | ) | — | ||||
Total Inventory |
$ | 178 | $ | 36 | |||
| |
Inventory Reserve |
|||
|---|---|---|---|---|
Balance at December 31, 2009 |
$ | — | ||
Reserve expensed to cost of sales |
138 | |||
Inventory deductions |
(24 | ) | ||
Balance at December 31, 2010 |
$ | 114 | ||
Property and Equipment
Property and equipment is recorded at cost and is depreciated using the straight-line method over estimated useful lives.
F-11
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
The components of property and equipment are as follows:
| |
December 31, | |
||||||
|---|---|---|---|---|---|---|---|---|
| |
Estimated Lives |
|||||||
| |
2010 | 2009 | ||||||
Computer hardware and software |
$ | 2,614 | $ | 2,027 | 3 years | |||
Office furniture and equipment |
1,681 | 1,628 | 3 - 7 years | |||||
Leasehold improvements |
508 | 498 | Lease term | |||||
Software projects in process |
721 | 601 | ||||||
|
5,524 | 4,754 | ||||||
Less accumulated depreciation and amortization |
(3,279 | ) | (2,585 | ) | ||||
Property and equipment, net |
$ | 2,245 | $ | 2,169 | ||||
We realized a loss primarily related to the disposal of certain software that was no longer in use totaling $207,000 and $152,000 for the years ended December 31, 2010 and 2009, respectively, which is recorded in Interest and other income, net on the Statement of Operations.
Long-lived Assets
We review long-lived assets, including acquired product rights and property and equipment when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the assets' book value to future net undiscounted cash flows the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceed their fair value, which is measured based on the projected discounted future net cash flows arising from the assets. Fair value of our long-lived assets is determined using the expected cash flows discounted at a rate commensurate with the risk involved. Assumptions and estimates used in the evaluation of impairment may affect the carrying value of long-lived assets, which could result in impairment charges in future periods. We have not recorded any impairment losses through December 31, 2010.
Accrued liabilities
Accrued liabilities are comprised of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Accrued personnel costs |
$ | 6,103 | $ | 5,133 | |||
Accrued royalties, government rebates and chargebacks, returns and distribution fees |
3,849 | 963 | |||||
Accrued sales and marketing expenses |
3,536 | 2,407 | |||||
Accrued research and development expenses |
2,762 | 3,363 | |||||
Accrued expenses—other |
1,377 | 1,270 | |||||
|
$ | 17,627 | $ | 13,136 | |||
F-12
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
Operating Leases
We recognize lease expense on a straight-line basis over the initial lease term. For leases that contain rent holidays, escalation clauses or tenant improvement allowances, we recognize rent expense on a straight-line basis and record the difference between the rent expense and rental amount payable as deferred rent. As of December 31, 2010 and 2009, we had $135,000 and $243,000, respectively, of deferred rent in accrued liabilities.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The following fair value hierarchy prioritizes the inputs into valuation techniques used to measure fair value. Accordingly, we use valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs when determining fair value. The three levels of the hierarchy are as follows:
| Level 1: | Inputs that reflect unadjusted quoted prices in active markets that are accessible to us for identical assets or liabilities; | |
Level 2: |
Inputs include quoted prices for similar assets and liabilities in active and inactive markets or that are observable for the asset or liability either directly or indirectly; and |
|
Level 3: |
Unobservable inputs that are supported by little or no market activity. |
We have no assets or liabilities that were measured using quoted prices for similar assets and liabilities or significant unobservable inputs (Level 2 and Level 3 assets and liabilities, respectively) as of December 31, 2010. The carrying value of our cash held in money market funds totaling $44.5 million as of December 31, 2010 is included in cash and cash equivalents on our Balance Sheet and approximates market values based on quoted market prices, or Level 1 inputs. Our financial instruments include cash and cash equivalents, investments, accounts receivable, prepaid expenses, accounts payable and accrued liabilities. The carrying amounts of financial instruments approximate their fair value due to their short maturities.
See Note 3—"Investments" for additional details regarding our investments.
Product Sales
We generate revenue from product sales. We recognize product revenue when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) our price to the buyer is fixed and determinable; and (4) collectability is reasonably assured. Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if (1) our price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid us, or the buyer is obligated to pay us and the obligation is not contingent on resale of the product, (3) the buyer's obligation to us would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring
F-13
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
the product for resale has economic substance apart from that provided by us, (5) we do not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated.
We sell FOLOTYN to a limited number of pharmaceutical wholesale distributors, or distributors, the three largest of which are affiliates under common control of an unrelated party. Title to the product passes upon delivery to our distributors, when the risks and rewards of ownership are assumed by the distributor (freight on board destination). These distributors then resell FOLOTYN to the patients' respective health care providers. Prior to the fourth quarter of 2010, product sales to distributors were recorded as deferred revenue until the product was sold through from our distributors to health care providers because we did not have sufficient history to be able to reasonably estimate returns. Beginning in the fourth quarter of 2010, we began recognizing revenue as product is sold to distributors as we established a sufficient history in order to reasonably estimate returns from our distributors. Consequently, for the year ended December 31, 2010, we recognized a one time increase of $604,000 in net product sales of FOLOTYN, or $0.01 per common share, representing product sales previously deferred as of December 31, 2009, net of distributor fees and estimated product returns, government rebates and chargebacks. We monitor inventory levels within our distribution channel and sales to end users, or health care providers, to determine whether deferral of sales is required. No such deferrals were recorded at December 31, 2010.
We estimate gross to net sales adjustments based upon analysis of third-party information, including information obtained from our primary distributors with respect to their inventory levels and sell-through to the distributors' customers.
Net Product Sales
Our net product sales represent total product sales less distributor fees and estimated allowances for product returns, government rebates and chargebacks to be incurred on the selling price of FOLOTYN related to the respective product sales. In addition, we incur distributor fees related to the management of our product by distributors. These distributor fees are recorded within net product sales and are based on definitive contractual agreements. Due to estimates and assumptions inherent in determining the amount of returns, rebates and chargebacks, the actual amount of returns and claims for rebates and chargebacks may be different from our estimates, at which time we would adjust our reserves accordingly. Product sales allowances and accruals are based on definitive contractual agreements or legal requirements (such as Medicaid laws and regulations) related to the purchase and/or utilization of the product by these entities. Allowances and accruals are recorded in the same period that the related revenue is recognized.
Product Returns
Our distributors' contractual return rights are limited to defective product or product that was shipped in error. Returns are not allowed for expired product. Given these limited contractual return rights, the price of FOLOTYN and the limited number of patients in the United States, FOLOTYN distributors and their customers generally carry limited inventory. We estimated product returns for FOLOTYN based upon actual returns history within our distribution channel, which were consistent with historical trends of product returns for similar companies in the pharmaceutical industry. The
F-14
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
actual returns history within our distribution channel is derived from third-party information obtained from certain distributors with respect to their inventory levels and sell-through to the distributors' customers. We will continue to monitor the historical trend of returns, including the impacts on this trend of product expiry dates and may be required to make future adjustments to our estimates. During the year ended December 31, 2010, we recorded an estimated sales return allowance of approximately 1% of cumulative gross product sales to date, or $444,000.
Medicaid Rebates
Our product is subject to state government-managed Medicaid programs whereby discounts and rebates are provided to participating state governments. We record estimated rebates payable under governmental programs, including Medicaid, as a reduction of revenue at the time revenues are recorded. Our calculations related to these rebate accruals require estimates, including estimates of customer mix primarily based on a combination of market and clinical research, to determine which sales will be subject to rebates and the amount of such rebates. During the first quarter of 2010, we obtained additional market research and were able to refine our estimated Medicaid utilization, which resulted in a reversal of Medicaid rebate allowances related to 2009 sales totaling $208,000. Our estimate of utilization is based on market research and information about our expected patient population. Through December 31, 2010, we have not had sufficient claims from states for rebates with which to update our estimate. However, when we have sufficient claims history, we will consider such history in our estimate which could result in a change in our estimate. We also consider any legal interpretations of the applicable laws related to Medicaid and qualifying federal and state government programs and any new information regarding changes in the Medicaid programs' regulations and guidelines that would impact the amount of the rebates. In March 2010, the Patient Protection and Affordable Care Act, as modified by the Health Care and Education Affordability Reconciliation Act of 2010, or PPACA, was enacted, which increased the Medicaid rebate percentage from 15.1% to 23.1%, retroactive to January 1, 2010. In addition, the states' ability to early adopt portions of PPACA, and any implementing regulations, could impact future estimates related to our Medicaid rebate allowances. We update our estimates and assumptions each period and record any necessary adjustments to our reserves. Although allowances and accruals are recorded at the time of product sale, certain rebates are typically paid out, on average, up to six months or longer after the sale. For reference purposes, a 10% to 20% increase in the Medicaid utilization percentage within our patient population as of December 31, 2010, would result in an approximate $659,000 to $1,318,000 reduction in cumulative net product sales.
Government Chargebacks
Our products are subject to certain programs with federal government qualified entities whereby pricing on products is discounted below distributor list price to participating entities. These entities purchase products through distributors at the discounted price, and the distributors charge the difference between their acquisition cost and the discounted price back to us. We account for chargebacks by establishing an accrual in an amount equal to our estimate of chargeback claims at the time of product sale. We do not expect the impact of the 340B program expansion included in the PPACA to significantly change our estimated government chargeback accruals because drugs approved under an Orphan Drug designation were specifically excluded from the provisions of the PPACA. The
F-15
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
FDA has awarded orphan drug status to FOLOTYN for the treatment of patients with T-cell lymphoma, which includes patients with relapsed or refractory PTCL. Through December 31, 2010 our chargeback experience has not been sufficient to update our estimated chargebacks. However, when we have sufficient history, we will consider such history in our estimate which could result in a change in our estimate. Due to estimates and assumptions inherent in determining the amount of government chargebacks, the actual amount of claims for chargebacks may be different from our estimates, at which time we would adjust our reserves accordingly.
Balances and activity in the deferred revenue account and a reconciliation of gross to net product sales for the years ended December 31, 2010, 2009 and 2008 are as follows:
| |
Years Ended December 31, |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Deferred revenue, beginning of the period |
$ | 669 | $ | — | $ | — | |||||
Gross product sales to distributors |
39,533 | 4,875 | — | ||||||||
Gross product sales recognized due to change in revenue recognition methodology |
(669 | ) | — | — | |||||||
Gross product sales recognized related to current year |
(39,533 | ) | (4,206 | ) | — | ||||||
Deferred revenue, end of the period |
$ | — | $ | 669 | $ | — | |||||
Gross product sales |
$ |
40,202 |
$ |
4,206 |
$ |
— |
|||||
Gross to Net Sales Adjustments: |
|||||||||||
Government rebates and chargebacks |
(3,374 | ) | (501 | ) | — | ||||||
Distribution fees |
(1,157 | ) | (120 | ) | — | ||||||
Product returns allowance |
(444 | ) | — | — | |||||||
Net product sales |
$ | 35,227 | $ | 3,585 | $ | — | |||||
Balances and activity for the components of our gross to net sales adjustments for the years ended December 31, 2010 and 2009 are as follows:
| |
Product Returns |
Government Rebates and Chargebacks |
Distribution Fees |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2008 |
$ | — | $ | — | $ | — | |||||
Reserve for current period sales |
— | 501 | 120 | ||||||||
Credits made for sales/payments |
— | (14 | ) | (34 | ) | ||||||
Balance at December 31, 2009 |
— | 487 | 86 | ||||||||
Reserve for current period sales |
437 | 3,650 | 1,141 | ||||||||
Change in estimate for prior period sales |
7 | (276 | ) | 16 | |||||||
Credits/payments made for prior period sales |
— | (126 | ) | (84 | ) | ||||||
Credits/payments made for current period sales |
(16 | ) | (1,511 | ) | (896 | ) | |||||
Balance at December 31, 2010 |
$ | 428 | $ | 2,224 | $ | 263 | |||||
F-16
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
Major Customers and Concentration of Credit Risk
We sell FOLOTYN to a limited number of pharmaceutical wholesale distributors, or distributors, the three largest of which are affiliates under common control of an unrelated party and are detailed below, without requiring collateral. We periodically assess the financial strength of these customers and establish allowances for anticipated losses, if necessary. Substantially all of our sales were made in the United States.
| |
% of total trade accounts receivable at December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Customer A |
53.8 | % | 47.6 | % | |||
Customer B |
23.1 | % | 30.6 | % | |||
Customer C |
22.3 | % | 21.8 | % | |||
| |
% of total gross product sales for the year ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | |||||
Customer A |
51.2 | % | 51.0 | % | |||
Customer B |
23.8 | % | 30.0 | % | |||
Customer C |
24.3 | % | 19.0 | % | |||
Cost of sales
Cost of sales, excluding amortization expense, includes cost of product sold, royalties, inventory packaging and labeling, warehousing and shipping costs associated with FOLOTYN product sales. See discussion in Note 9 regarding the 8% current royalty rates under our license agreement for FOLOTYN. Prior to receiving FDA approval of FOLOTYN, all costs related to purchases of the active pharmaceutical ingredient and the manufacturing of the product were recorded as research and development expense. Until we sell these supplies for which the costs were previously expensed, our cost of sales will reflect only incremental costs incurred subsequent to the FDA approval date and accordingly will be lower than when we sell through the product manufactured prior to FDA approval. We sold a portion of our finished goods that were manufactured subsequent to the FDA approval date totaling $120,000 during the year ended December 31, 2010, which were recorded in cost of sales, excluding amortization expense in the Statement of Operations.
Advertising Costs
Advertising costs are expensed as incurred and are included in selling, general and administrative expenses in our statement of operations. Advertising costs, including promotional expenses and costs related to trade shows were $5.9 million, $4.7 million and $1.4 million for the years ended December 31, 2010, 2009 and 2008, respectively.
F-17
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
Stock-Based Compensation
We have several stock-based compensation plans under which incentive and non-qualified stock options, restricted stock units and restricted shares may be granted, and an employee stock purchase plan. We measure the cost of employee services received in exchange for an award of equity instruments based on the grant date fair value of the award. That cost is recognized over the period during which an employee is required to provide services in exchange for each vesting tranche of the award, the requisite service period (usually the vesting period). We provide an estimate of forfeitures at initial grant date.
See Note 5—"Stock-Based Compensation Plans" for additional details regarding the impact of our stock based compensation plans on our financial statements.
Research and Development
Research and development expenditures are charged to expense as incurred. Research and development expenses include the costs of certain personnel, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis, third party manufacturing costs for development of drug materials for use in clinical trials and preclinical studies and licensing fees for our product candidates prior to FDA approval. All finished drug inventory costs associated with production activities in our third party manufacturing facilities prior to receiving FDA approval for such facilities and prior to receiving regulatory approval to market our product are expensed to research and development expenses. We accrue research and development expenses for activity as incurred during the fiscal year and prior to receiving invoices from clinical sites and third party clinical and preclinical research organizations. We accrue external costs for clinical and preclinical studies based on an evaluation of the following: the progress of the studies, including patient enrollment, dosing levels of patients enrolled, estimated costs to dose patients, invoices received, and contracted costs with clinical sites and third party clinical and preclinical research organizations. Significant judgments and estimates must be made and used in determining the accrued balance in any accounting period. Actual results could differ from those estimates. During the years ended December 31, 2010, 2009 and 2008, we did not have any changes in estimates that resulted in material adjustments to research and development expenses accrued in the prior period.
Income Taxes
Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities at each year end and their respective tax bases using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances have been established to reduce our net deferred tax assets to zero, as we believe that it is more likely than not that such assets will not be realized.
Net Loss Per Share
Basic net loss per share is computed by dividing the net loss attributable to common stockholders for the period by the weighted average number of common shares outstanding during the period.
F-18
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
2. Summary of Significant Accounting Policies (Continued)
Diluted earnings per share is computed by giving effect to all dilutive potential common stock outstanding during the period, including stock options, restricted stock, restricted stock unit awards and shares to be issued under our employee stock purchase plan.
Diluted net loss per share is the same as basic net loss per share for all periods presented because any potential dilutive common shares were anti-dilutive due to our net loss (as including such shares would decrease our basic net loss per share). Such potentially dilutive shares are excluded when the effect would be to reduce net loss per share. Because we reported a net loss for the years ended December 31, 2010, 2009 and 2008, all potentially dilutive common shares have been excluded from the computation of the dilutive net loss per share for all periods presented. Such potentially dilutive common shares consist of the following:
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | |||||||
Common stock options |
8,716,829 | 8,292,496 | 7,236,512 | |||||||
Unvested restricted stock |
12,500 | 125,000 | 293,750 | |||||||
Unvested restricted stock units |
2,492,078 | 155,479 | — | |||||||
|
11,221,407 | 8,572,975 | 7,530,262 | |||||||
Recent Accounting Pronouncements
We reviewed recently issued accounting pronouncements and plan to adopt those that are applicable to us. We do not expect the adoption of these pronouncements to have a material impact on our financial position, results of operations or cash flows.
3. Investments
We do not intend to sell and we do not believe that it is more likely than not that we will be required to sell our investments before recovering the cost of securities, nor do we expect not to recover the entire amortized cost basis of our investments in marketable securities. As such, our investments in marketable securities as of December 31, 2010 and 2009 are classified as held-to-maturity and are carried at cost plus accrued interest. The changes in the value of these securities, other than impairment charges, are not reported on our financial statements. The weighted average duration of the remaining time to maturity for our portfolio of investments in marketable securities as of December 31, 2010 was approximately four months. All of the investments classified as short-term on the balance sheet have an original maturity of longer than 90 days, but less than one year. The investments classified as long-term on the balance sheet have remaining contractual maturities of greater than one year and less than three years as of the balance sheet date.
F-19
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
3. Investments (Continued)
The carrying value of investments in marketable securities by contractual maturity, consisted of the following as of December 31, 2010:
| |
Amortized cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Short-term held-to-maturity securities: |
|||||||||||||
U.S. Treasury bills and notes |
$ | 50,063 | $ | 11 | $ | 1 | $ | 50,073 | |||||
U. S. Government agency securities |
271 | 6 | — | 277 | |||||||||
Total due in one year or less |
$ | 50,334 | $ | 17 | $ | 1 | $ | 50,350 | |||||
Long-term held-to-maturity securities: |
|||||||||||||
Corporate notes |
$ | 305 | $ | 11 | $ | — | $ | 316 | |||||
Less: Amounts classified as restricted cash |
(238 | ) | — | — | (238 | ) | |||||||
Total due in one to two years |
$ | 67 | $ | 11 | $ | — | $ | 78 | |||||
The carrying value of investments in marketable securities by contractual maturity, consisted of the following as of December 31, 2009:
| |
Amortized cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Short-term held-to-maturity securities: |
||||||||||||||
U.S. Treasury bills |
$ | 8,006 | $ | 11 | $ | — | $ | 8,017 | ||||||
Certificates of deposit |
9,010 | 5 | — | 9,015 | ||||||||||
Total due in one year or less |
$ | 17,016 | $ | 16 | $ | — | $ | 17,032 | ||||||
Long-term held-to-maturity securities: |
||||||||||||||
U. S. Government agency securities |
$ | 273 | $ | 11 | $ | — | $ | 284 | ||||||
Corporate notes |
308 | 13 | — | 321 | ||||||||||
Sub-total |
$ | 581 | $ | 24 | $ | — | $ | 605 | ||||||
Less: Amounts classified as restricted cash |
(238 | ) | — | — | (238 | ) | ||||||||
Total due in one to three years |
$ | 343 | $ | 24 | $ | — | $ | 367 | ||||||
As of December 31, 2010 and 2009 there were no material investments in a loss position. Market values were determined for each individual security in the investment portfolio. If a decline in fair value below the amortized cost basis of an investment is judged to be other-than-temporary, the cost basis of the investment is written down to fair value. Additionally, management assesses whether it intends to sell or would more-likely-than-not not be required to sell the investment before the expected recovery of the amortized cost basis. During the year ended December 31, 2009, we realized losses of approximately $157,000 on the sale of certain of our investments in marketable securities, which were sold in order to preserve our principal as the issuers of these securities experienced significant deteriorations in their creditworthiness as evidenced by investment rating downgrades. As of December 31, 2010, we have an unrealized loss of $1,000 on one of our U.S. Treasury bill investments
F-20
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
3. Investments (Continued)
with an aggregate fair value of $10.0 million. As of December 31, 2010, no other than temporary impairment has been recorded on any of our investments since these unrealized losses are on U.S. government issued securities maturing within one year. The decline in value of the investments as of December 31, 2010 was caused by primarily by changes in interest rates. We have the ability and intent to hold our remaining investments in marketable securities to recover the entire amortized cost basis of the investments as of December 31, 2010. There were no investments in an unrealized loss position as of December 31, 2009.
4. Stockholders' Equity
Common Stock
2008 Common Stock Financing
On May 29, 2008, we sold 12,420,000 shares of our common stock in an underwritten public offering at a price of $5.64 per share. The number of shares issued included 1,620,000 shares purchased by the underwriters pursuant to their exercise in full of their overallotment option. We received net proceeds from the offering of $65.2 million, after deducting $4.2 million of underwriting discounts and commissions and $661,000 of offering expenses.
2009 Common Stock Financings
On April 3, 2009, we sold 7,750,000 shares of our common stock in an underwritten public offering at a price of $6.30 per share. We received net proceeds from the offering of $47.0 million, after deducting $1.4 million of underwriting commissions and $473,000 of offering expenses.
On October 13, 2009, we sold 14,000,000 shares of our common stock in an underwritten public offering at a price of $7.10 per share. We received net proceeds from the offering of $93.1 million, after deducting $5.7 million of underwriting commissions and $568,000 of offering expenses.
Common Stock Reserved for Future Issuance
At December 31, 2010, we have reserved shares of common stock for future issuance as follows:
| |
Outstanding at December 31, 2010 |
Available for grant at December 31, 2010 |
Shares of Common Stock Reserved at December 31, 2010 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
2001 Employee Stock Purchase Plan |
— | 2,101,917 | 2,101,917 | ||||||||
2008 Equity Incentive Plan |
11,208,907 | 11,191,603 | 22,400,510 | ||||||||
Total for Equity Incentive Plans |
11,208,907 | 13,293,520 | 24,502,427 | ||||||||
On June 22, 2010, we filed with the Delaware Secretary of State a Certificate of Amendment to our Restated Certificate of Incorporation to increase the number of common shares authorized to 200,000,000 shares.
F-21
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
4. Stockholders' Equity (Continued)
Stockholder Rights Plan
In May 2003, we designated 1,000,000 shares of our authorized Preferred Stock as Series A Junior Participating Preferred Stock, par value $0.001 per share, pursuant to a Stockholder Rights Plan approved by our Board of Directors under which all stockholders of record as of May 28, 2003 received a dividend distribution of one preferred share purchase right, or a Right, for each outstanding share of our common stock. The Rights trade with the common stock and no separate Right certificates will be distributed until such time as the Rights become exercisable in accordance with the Stockholder Rights Plan. In general, the Rights become exercisable when (i) a person or group acquires 15% or more of our common stock or (ii) a tender offer or exchange offer by a person or group for 15% or more of our common stock is commenced or publicly announced. The Stockholder Rights Plan is intended as a means to guard against abusive takeover tactics and to provide for fair and equal treatment for all stockholders in the event that an unsolicited attempt is made to acquire us.
On July 17, 2009, we filed with the Delaware Secretary of State a Certificate of Amendment to our Certificate of Designation of Series A Junior Participating Preferred Stock to increase the number of shares designated as Series A Junior Participating Preferred Stock thereunder from 1,000,000 shares to 1,500,000 shares. In accordance with the terms of our Amended and Restated Certificate of Incorporation, as amended, our Board of Directors has the authority to increase the number of shares of any series of preferred stock. The Certificate of Amendment was approved by our board of directors on July 16, 2009.
In connection with an equity financing we completed in March 2005, we amended the Stockholder Rights Plan to provide that Warburg Pincus Private Equity VIII, L.P., or Warburg, and certain of its affiliates will be exempt from the Stockholder Rights Plan, unless Warburg and its affiliates become, without the prior consent of our Board of Directors, the beneficial owner of more than 44% of our common stock.
In connection with the acquisition of shares of our common stock by Baker Brothers Life Sciences, L.P. and certain other affiliated funds, which are collectively referred to herein as "Baker," in the February 2007 Financing, we amended the Stockholder Rights Plan to provide that Baker will be exempt from the Stockholder Rights Plan, unless Baker becomes, without the Company's prior consent, the beneficial owner of more than 20% of our common stock.
Until the Rights become exercisable, the Rights will have no dilutive impact on our earnings per share data. The Rights are protected by customary anti-dilution provisions. As of December 31, 2010, no shares of Series A Junior Participating Preferred Stock were issued or outstanding.
5. Stock-Based Compensation Plans
At our Annual Meeting of Stockholders held on June 24, 2008, our stockholders approved the Allos Therapeutics, Inc. 2008 Equity Incentive Plan, or the 2008 Plan. The 2008 Plan authorizes the issuance of incentive stock options, nonstatutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance stock awards and forms of equity compensation, which may be granted to employees, directors and consultants. Only employees may receive incentive stock options. The 2008 Plan succeeds and continues prior equity incentive plans. As of June 24, 2008, no additional stock awards will be granted under the prior plans and all outstanding stock awards
F-22
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
5. Stock-Based Compensation Plans (Continued)
granted under the prior plans are deemed to be stock awards granted under the 2008 Plan (but remain subject to the terms of the prior plans with respect to which they were originally granted).
12,550,843 shares of our common stock were eligible to be issued pursuant to stock awards granted under the 2008 Plan, provided that all stock awards granted after the June 24, 2008 effective date of the 2008 Plan, other than stock options and stock appreciation rights granted with an exercise price of at least 100% of such stock award's fair market value on the date of grant, will reduce the number of shares available for issuance under the 2008 Plan by 1.35 shares per share granted pursuant to the stock award. If a stock award under the 2008 Plan expires or otherwise terminates without being exercised in full, the shares of common stock of the Company not acquired pursuant to the stock award will again become available for issuance under the 2008 Plan. In addition, shares issued pursuant to a stock award that are forfeited to or repurchased by us prior to becoming fully vested and shares that are cancelled pursuant to an exchange or repricing program will become available for the grant of new stock awards under the 2008 Plan. Shares of common stock that revert to and again become available for issuance under the 2008 Plan and that prior to such reversion were granted pursuant to a stock award that reduced the number of shares available under the 2008 Plan by 1.35 shares per share granted pursuant to such stock award, shall cause the number of shares of common stock of the Company available for issuance under the 2008 Plan to increase by 1.35 shares upon such reversion.
At our Annual Meetings of Stockholders held on June 22, 2010 and June 23, 2009, our stockholders approved an amendment to the Allos Therapeutics, Inc. 2008 Equity Incentive Plan, or the Plan, to increase the aggregate number of shares of common stock authorized for issuance under the Plan by 7,500,000 and 5,750,000 shares, respectively. Our Board of Directors had previously approved the amendment and recommended its approval to our stockholders.
The 2008 Plan, and one of our prior plans (together, the "Plans"), provide for appropriate adjustments in the number of shares reserved and outstanding options in the event of certain changes to our outstanding common stock by reason of merger, recapitalization, stock split or other similar events. Options granted under the 2008 Plan may be exercised for a period of not more than 10 years from the date of grant or any shorter period as determined by our Board of Directors. Options vest as determined by the Board of Directors, generally over a period of two to four years, subject to acceleration under certain events. The exercise price of any incentive stock option granted under the Plans must equal or exceed the fair market value of our common stock on the date of grant, or 110% of the fair market value per share in the case of a 10% or greater stockholder.
Stock-based compensation expense for the years ended December 31, 2010, 2009 and 2008 has been recognized in the accompanying Statements of Operations as follows:
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Research and development |
$ | 3,314 | $ | 2,395 | $ | 3,118 | |||||
Selling, general and administrative |
8,112 | 6,285 | 4,902 | ||||||||
Total stock-based compensation expense |
$ | 11,426 | $ | 8,680 | $ | 8,020 | |||||
Effective August 24, 2010, James V. Caruso, our former Executive Vice President, Chief Commercial Officer (CCO), departed the Company. As a result of Mr. Caruso's departure, we adjusted
F-23
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
5. Stock-Based Compensation Plans (Continued)
the forfeiture rate applied to his equity compensation, which resulted in a $787,000 reversal of selling, general and administrative stock-based compensation expense during the three months ended September 30, 2010, of which $605,000 related to stock option awards and $182,000 related to restricted stock unit awards.
Effective September 30, 2009, Pablo J. Cagnoni, M.D., our former Senior Vice President, Chief Medical Officer (CMO), resigned. As a result of his resignation, we adjusted the forfeiture rate applied to his equity compensation, which resulted in a $906,000 reversal of research and development stock-based compensation expense during the three months ended September 30, 2009, of which $699,000 related to stock option awards, $166,000 related to restricted stock awards and $41,000 related to restricted stock unit awards.
Stock Options
The following table summarizes our stock option activity and related information:
| |
Options Outstanding | Options Exercisable | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Shares |
Weighted Average Exercise Price |
Number of Shares |
Weighted Average Exercise Price |
||||||||||
Outstanding at December 31, 2009 |
8,292,496 | $ | 5.83 | 3,823,683 | $ | 5.02 | ||||||||
Granted |
2,723,251 | 7.21 | ||||||||||||
Exercised |
(1,104,474 | ) | 3.70 | |||||||||||
Forfeited/Canceled |
(1,194,444 | ) | 7.08 | |||||||||||
Outstanding at December 31, 2010 |
8,716,829 | $ | 6.36 | 4,539,454 | $ | 5.89 | ||||||||
The options outstanding at December 31, 2010 have a weighted average remaining contractual term of 7.4 years.
The following table summarizes information about outstanding stock options that are fully vested and currently exercisable, and outstanding stock options that are expected to vest in the future:
| |
Number Outstanding |
Weighted Average Remaining Contractual Term |
Weighted Average Exercise Price |
Aggregate Intrinsic Value |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
As of December 31, 2010: |
||||||||||||||
Options fully vested and exercisable |
4,539,454 | 6.1 | $ | 5.89 | $ | 1,509,000 | ||||||||
Options expected to vest, including effects of expected forfeitures |
3,426,857 | 8.7 | $ | 6.89 | 44,000 | |||||||||
Options fully vested and expected to vest |
7,966,311 | 7.2 | $ | 6.32 | $ | 1,553,000 | ||||||||
The aggregate intrinsic value in the tables above represents the total pretax intrinsic value, based on our closing stock price of $4.61 as of December 31, 2010, which would have been received by the option holders had all option holders with in-the-money options exercised their options as of that date. The total number of in-the-money options exercisable as of December 31, 2010 was 979,925. The total intrinsic value of outstanding stock options as of December 31, 2010 was $1,565,000.
F-24
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
5. Stock-Based Compensation Plans (Continued)
The total intrinsic value of options exercised during the years ended December 31, 2010, 2009 and 2008 was $4,085,000, $4,583,000 and $3,168,000, respectively. We settle employee stock option exercises with newly issued common shares.
Stock-based compensation expense related to our stock option plans was $8,981,000, $8,147,000 and $7,539,000 for the years ended December 31, 2010, 2009 and 2008, respectively. The stock-based compensation expense amounts for the years ended December 31, 2010 and 2009 include a $605,000 and $699,000, respectively, reversal related to the departure of our former CCO and CMO discussed above. As of December 31, 2010, the unrecorded stock-based compensation balance related to stock option awards was $7,283,000 and will be recognized over an estimated weighted-average amortization period of 1.6 years.
Valuation assumptions for stock options granted during the years ended December 31, 2010, 2009 and 2008
For stock options granted during the years ended December 31, 2010, 2009 and 2008, the majority vest 25% one year after the date of grant, and the remaining 75% in equal monthly installments thereafter over the next three years, until all such shares are vested and exercisable. Stock-based compensation is calculated according to the FASB issued accounting guidance and is expensed over the vesting period of the individual options using the graded vesting attribution method. During the years ended December 31, 2010, 2009 and 2008, we granted stock options with a weighted-average grant-date fair value of $4.07, $4.12 and $3.88 per share, respectively. The fair value of stock options granted to our employees during the years ended December 31, 2010, 2009 and 2008 was estimated on the date of each grant using the Black-Scholes option pricing model using the following weighted-average assumptions:
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Stock option plans: |
|||||||||||
Expected dividend yield |
0 | % | 0 | % | 0 | % | |||||
Expected stock price volatility |
62 | % | 72 | % | 74 | % | |||||
Risk free interest rate |
2.5 | % | 2.1 | % | 2.9 | % | |||||
Expected life (years) |
5.5 | 5.2 | 5.0 | ||||||||
We used an expected dividend yield of 0%, as we do not expect to pay dividends during the expected life of these awards. The expected stock price volatility is determined using our historical stock volatility over the period equal to the expected life of each award. The risk-free interest rate is based on U.S. Treasury zero-coupon issues with a remaining term equal to the expected life of each award. The expected life of the stock options was estimated using peer data of companies in the life science industry with similar equity plans. Stock-based compensation expense is recognized net of estimated pre-vesting forfeitures, which results in recognition of expense on options that are ultimately expected to vest over the expected option term. Forfeitures were estimated using actual historical forfeiture experience.
F-25
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
5. Stock-Based Compensation Plans (Continued)
Restricted Stock
The following table summarizes activity and related information for our restricted stock, or RS, awards:
| |
Number of Shares |
Weighted Average Grant-Date Fair Value |
||||||
|---|---|---|---|---|---|---|---|---|
Nonvested RS at December 31, 2009 |
125,000 | $ | 3.72 | |||||
Granted |
— | — | ||||||
Vested |
(112,500 | ) | 3.41 | |||||
Nonvested RS at December 31, 2010 |
12,500 | $ | 6.51 | |||||
The shares of restricted stock vest in four equal annual installments from the date of grant. The grant-date fair value of shares granted during the year ended December 31, 2008 was $75,000 based on the closing market price of the Company's common stock on the grant dates of the awards and was $7.49 for the year ended December 31, 2008. The total fair value of shares vested during the year ended December 31, 2010, 2009 and 2008 was $806,000, $869,000 and $751,000, respectively. During the years ended December 31, 2010, 2009 and 2008, we recorded stock-based compensation related to restricted stock awards of $42,000, $48,000 and $423,000, respectively. The stock-based compensation expense amounts for the year ended December 31, 2009 includes the $166,000 reversal related to the resignation of our former CMO discussed above. As of December 31, 2010, the unrecorded stock-based compensation balance related to restricted stock awards was $10,000 and will be recognized over an estimated weighted-average amortization period of 1.4 years.
The following table summarizes activity and related information for restricted stock unit, or RSU, awards:
| |
Number of Shares |
Weighted Average Grant-Date Fair Value |
||||||
|---|---|---|---|---|---|---|---|---|
Nonvested RSU at December 31, 2009 |
155,479 | $ | 6.48 | |||||
Granted |
2,470,718 | 4.98 | ||||||
Vested |
(38,866 | ) | 6.48 | |||||
Forfeited |
(95,253 | ) | 7.03 | |||||
Nonvested RSU at December 31, 2010 |
2,492,078 | $ | 4.97 | |||||
The shares of RSU awards vest in three or four equal annual installments from the date of grant. In October 2010, we issued an aggregate of 2,016,315 RSUs to our employees under the Company's 2008 Equity Incentive Plan, as amended. The October 2010 RSU awards vest in three equal annual installments from the date of grant, subject to the employees' continued service with the Company through such vesting dates. Upon vesting of the restricted stock unit awards, we issue unrestricted shares of our common stock. The total fair value of shares vested during the year ended December 31, 2010 was $278,000. During the year ended December 31, 2010, 2009 and 2008, we recorded stock-based
F-26
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
5. Stock-Based Compensation Plans (Continued)
compensation related to restricted stock unit awards of $2,199,000, $368,000 and $0, respectively. The stock-based compensation expense amounts for the years ended December 31, 2010 and 2009 include a $182,000 and $41,000, respectively, reversal related to the departures of our former CCO and CMO discussed above. As of December 31, 2010, the unrecorded stock-based compensation balance related to restricted stock unit awards was $7,959,000 and will be recognized over an estimated weighted-average amortization period of 1.9 years.
Employee Stock Purchase Plan
On February 28, 2001, our Board of Directors approved the Allos Therapeutics, Inc. 2001 Employee Stock Purchase Plan, or Purchase Plan, which was also approved by our stockholders on April 17, 2001. Under the Purchase Plan, we are authorized to issue up to 2,500,000 shares of common stock to qualified employees. Qualified employees can choose to have up to 10% of their annual base earnings withheld to purchase shares of our common stock during each offering period. The purchase price of the common stock is 85% of the lower of the fair market value of a share of common stock on the first day of the offering or the fair market value of a share of common stock on the last day of the purchase period. We sold 115,797, 42,063 and 22,828 shares to employees in 2010, 2009 and 2008, respectively. There were 2,101,917 shares available for sale under the Purchase Plan as of December 31, 2010. Stock-based compensation expense related to our Purchase Plan was $203,000, $117,000 and $59,000 for the years ended December 31, 2010, 2009 and 2008, respectively. The weighted-average estimated grant date fair value of purchase awards under the Purchase Plan during the years ended December 31, 2010, 2009 and 2008 was $1.75, $2.78 and $2.59 per share, respectively.
The fair value of purchase awards granted to our employees during the years ended December 31, 2010, 2009 and 2008 was estimated using the Black-Scholes option pricing model using the following weighted-average assumptions:
| |
2010 | 2009 | 2008 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
Stock purchase plan: |
|||||||||||
Expected dividend yield |
0 | % | 0 | % | 0 | % | |||||
Expected stock price volatility |
49 | % | 77 | % | 65 | % | |||||
Risk free interest rate |
0.2 | % | 0.4 | % | 2.6 | % | |||||
Expected life (years) |
0.5 | 0.5 | 0.5 | ||||||||
6. Intangible asset, net
Costs incurred for products or product candidates not yet approved by the FDA and for which no alternative future use exists are recorded as expense. In the event a product or product candidate has been approved by the FDA or an alternative future use exists for a product or product candidate, patent and license costs are capitalized and amortized over the shorter of the expected patent life and the expected life cycle of the related product or product candidate.
As a result of the FDA's approval to market FOLOTYN on September 24, 2009, we met a milestone under our license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, discussed in Note 9, which required us to make a milestone payment of $5.8 million. We capitalized the $5.8 million payment as an intangible asset and
F-27
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
6. Intangible asset, net (Continued)
began amortizing the asset following the FDA approval to market FOLOTYN. Amortization expense is being recorded on a straight line basis over the remaining expected life of the patent for FOLOTYN, which we expect to last until July 16, 2022. This includes the anticipated Hatch-Waxman extension that provides patent protection for drug compounds for a period of up to five years to compensate for time spent in development. This term is our best estimate of the life of the patent. If, however, the Hatch-Waxman extension is not granted, the intangible asset will be amortized over a shorter period. The estimated annual amortization expense for the intangible asset is approximately $454,000 per year during 2011 through 2021 and $234,000 in 2022.
7. Income Taxes
We have incurred net losses since inception.
The components of our current tax benefit, for the years ended December 31, 2010 and 2009 was related to a federal and state research and experimentation income tax credit received.
The income tax benefit computed using our net loss and the federal statutory income tax rate differs from our actual income tax benefit, primarily due to the following:
| |
Years ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2010 | 2009 | 2008 | ||||||||
Federal income tax benefit at 35% |
$ | (27,124 | ) | $ | (25,766 | ) | $ | (18,105 | ) | ||
State income tax (benefit), net of federal benefit |
(1,921 | ) | (1,506 | ) | (1,449 | ) | |||||
Stock-based compensation |
2,577 | 1,660 | 555 | ||||||||
Research and development and orphan drug credits |
(979 | ) | (503 | ) | (1,244 | ) | |||||
Change in valuation allowance |
26,884 | 25,689 | 20,208 | ||||||||
Other |
485 | 349 | 35 | ||||||||
Benefit for income taxes |
$ | (78 | ) | $ | (77 | ) | $ | — | |||
The components of our deferred tax assets are as follows, as of December 31:
| |
2010 | 2009 | |||||||
|---|---|---|---|---|---|---|---|---|---|
Deferred tax assets: |
|||||||||
Net operating loss carryforwards |
$ | 111,794 | $ | 87,765 | |||||
Amortization of intangibles |
1,193 | 1,865 | |||||||
Research and development and orphan drug credit carryforwards |
11,421 | 9,915 | |||||||
Stock-based compensation |
6,235 | 5,183 | |||||||
Other |
1,942 | 973 | |||||||
Total deferred tax assets |
132,585 | 105,701 | |||||||
Valuation allowance |
(132,585 | ) | (105,701 | ) | |||||
Net deferred tax assets |
$ | — | $ | — | |||||
F-28
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
7. Income Taxes (Continued)
Our deferred tax assets represent an unrecognized future tax benefit. A valuation allowance has been established for the entire tax benefit as we believe that it is more likely than not that such assets will not be realized.
As of December 31, 2010, we had available approximately $304.7 million of net operating loss, or NOL, carryforwards, after taking into consideration NOLs expected to expire unused due to the limitations under Section 382 of the Internal Revenue Code, and which includes approximately $9.2 million of deductions related to stock-based compensation, not reflected in deferred tax assets, that are not realized as deferred tax assets until current taxes payable can be reduced. Of these NOL carryforwards, $3.9 million will expire beginning in 2011, $6.2 million in 2012 and the remaining NOL carryforwards expire in 2018 through 2030. In addition, as of December 31, 2010, we had research and development credit and orphan drug credit carryforwards, after taking into consideration the Section 382 limitation, of $4.8 million and $6.6 million, respectively, to offset future regular tax expense. Since the Company's formation, it has raised capital through the issuance of capital stock on several occasions which, combined with shareholders' subsequent disposition of those shares, has resulted in four changes of control in 1994, 1998, 2001 and 2005, as defined by Section 382. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50% within a three-year period. As a result of the most recent ownership change in 2005, utilization of approximately $59.9 million of NOL carryforwards generated prior to the latest change are subject to an annual limitation of approximately $2.2 million under Section 382, determined by multiplying the value of our stock at the time of the ownership change by the applicable long-term tax-exempt rate. Additionally, we have a recognized built-in gain that increased the annual limitation by $3.3 million for each of the five years after the 2005 ownership change. Any unused annual limitation may be carried over to subsequent years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Our NOL carryforwards reflected above are adjusted to remove the portion that will expire due to the limitation. Subsequent ownership changes, as defined in Section 382, could further limit the amount of our NOL carryforwards and research and development credits that can be utilized annually to offset future taxable income.
Tax positions must initially be recognized in the financial statements when it is more likely than not that the position will be sustained upon examination by the tax authorities. Such tax positions must initially and subsequently be measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority assuming full knowledge of the position and relevant facts. Based on our evaluation, we have concluded that there are no significant uncertain tax positions requiring recognition in our financial statements. Our evaluation was performed for the periods from December 31, 1993 through December 31, 2010, the tax periods which remain subject to examination by major tax jurisdictions as of December 31, 2010.
We may from time to time be assessed interest or penalties by major tax jurisdictions, although there have been no such assessments historically with material impact to our financial results. In the event we receive an assessment for interest and/or penalties, it would be classified in the financial statements as income tax expense.
F-29
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
8. Employee Benefit Plan
We maintain a defined contribution plan covering substantially all employees under Section 401(k) of the Internal Revenue Code. We provided a 50% match of employees' contributions up to $5,000 per employee per year. We made total contributions of $544,000, $356,000 and $246,000 during the years ended December 31, 2010, 2009 and 2008, respectively. Company contributions are fully vested after four years of employment.
9. Commitments and Contingencies
Lease Commitments
We lease offices and automobiles under agreements that expire at various dates through 2013. These office leases contain clauses for renewal at our option for one additional three year term. Total office rent expense for the years ended December 31, 2010, 2009 and 2008 was $743,000, $786,000 and $715,000, respectively.
The aggregate future minimum rental commitments as of December 31, 2010, for non-cancelable operating leases with initial or remaining terms in excess of one year are as follows:
Year Ending December 31:
|
|
|||
|---|---|---|---|---|
2011 |
$ | 1,271 | ||
2012 |
453 | |||
2013 |
68 | |||
Total |
$ | 1,792 | ||
Royalty and License Fee Commitments
In December 2002, we entered into a license agreement with Memorial Sloan-Kettering Cancer Center, SRI International and Southern Research Institute, as amended, under which we obtained exclusive worldwide rights to a portfolio of patents and patent applications related to FOLOTYN and its uses. Under the terms of the agreement, we paid an up-front license fee of $2.0 million upon execution of the agreement and have made aggregate milestone payments of $2.5 million based on the passage of time. Additionally, in May and September 2009, we made milestone payments of $1.5 million based on the FDA accepting our New Drug Application for review and $5.8 million based on the FDA approval to market FOLOTYN, respectively. The up-front license fee and all milestone payments under the agreement prior to FDA approval to market FOLOTYN were recorded to research and development expense as incurred. As discussed in Note 6, the $5.8 million milestone payment based on the FDA approval was capitalized as an intangible asset and is being amortized over the expected useful life of the composition of matter patent for FOLOTYN, which we expect to last until July 16, 2022. The only remaining potential milestone payment under the license agreement is for $3.5 million upon regulatory approval to market FOLOTYN in Europe, which, if made would be capitalized and amortized over the expected useful life of the licensed patents. Under the terms of the agreement, we are required to fund all development programs and will have sole responsibility for all commercialization activities. In addition, we will pay the licensors royalties based on graduated annual levels of net sales of FOLOTYN to our distributors, net of actual rebates and chargebacks, or distributor sales, which may be different than our net product revenue recognized in accordance with
F-30
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
9. Commitments and Contingencies (Continued)
U.S. generally accepted accounting principles, or GAAP, or sublicense revenues arising from sublicensing the product, if and when such sales or sublicenses occur. Royalties are 8% of annual distributor sales up to $150.0 million; 9% of annual distributor sales of $150.0 million through $300.0 million; and 11% of annual distributor sales in excess of $300.0 million. In 2010 and 2009, our royalties were 8% of our net distributor sales.
Contingencies
We enter into indemnification provisions under our agreements with other companies in our ordinary course of business, typically with business partners, contractors, clinical sites and suppliers. Under these provisions we generally indemnify and hold harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of our activities or the use of our product candidates. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments we could be required to make under these indemnification provisions is unlimited. We have not incurred material costs to defend lawsuits or settle claims related to these indemnification agreements. The estimated fair value of the indemnification provisions of these agreements is minimal as of December 31, 2010, and accordingly, we have no corresponding liabilities recorded as of December 31, 2010.
F-31
ALLOS THERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
(Dollars shown in tables are in thousands, except per share amounts)
10. Quarterly Information (Unaudited)
The results of operations on a quarterly basis for the years ended December 31, 2010 and 2009 were as follows:
| |
March 31, 2010 |
June 30, 2010 |
Sept. 30, 2010 |
Dec. 31, 2010(1) |
March 31, 2009 |
June 30, 2009 |
Sept. 30, 2009 |
Dec. 31, 2009 |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Net product sales |
$ | 7,407 | $ | 7,885 | $ | 8,230 | $ | 11,705 | $ | — | $ | — | $ | — | $ | 3,585 | |||||||||||
Operating costs and expenses: |
|||||||||||||||||||||||||||
Cost of sales, excluding amortization expense presented below |
689 | 752 | 889 | 1,317 | — | — | — | 408 | |||||||||||||||||||
Research and development |
9,285 | 6,522 | 7,249 | 8,303 | 8,360 | 8,776 | 7,538 | 7,944 | |||||||||||||||||||
Selling, general and administrative |
17,932 | 20,517 | 18,702 | 21,631 | 6,963 | 8,037 | 11,327 | 18,121 | |||||||||||||||||||
Amortization of intangible asset |
113 | 114 | 113 | 114 | — | — | 7 | 114 | |||||||||||||||||||
Total operating costs and expenses |
28,019 | 27,905 | 26,953 | 31,365 | 15,323 | 16,813 | 18,872 | 26,587 | |||||||||||||||||||
Operating loss |
(20,612 | ) | (20,020 | ) | (18,723 | ) | (19,660 | ) | (15,323 | ) | (16,813 | ) | (18,872 | ) | (23,002 | ) | |||||||||||
Interest and other income, net |
65 | 66 | (129 | ) | 1,518 | 173 | 6 | 125 | 76 | ||||||||||||||||||
Loss before income taxes |
(20,547 | ) | (19,954 | ) | (18,852 | ) | (18,142 | ) | (15,150 | ) | (16,807 | ) | (18,747 | ) | (22,926 | ) | |||||||||||
Income tax benefit |
— | — | 78 | — | — | — | 77 | — | |||||||||||||||||||
Net loss |
$ | (20,547 | ) | $ | (19,954 | ) | $ | (18,774 | ) | $ | (18,142 | ) | $ | (15,150 | ) | $ | (16,807 | ) | $ | (18,670 | ) | $ | (22,926 | ) | |||
Net loss per share: basic and diluted |
$ | (0.20 | ) | $ | (0.19 | ) | $ | (0.18 | ) | $ | (0.17 | ) | $ | (0.19 | ) | $ | (0.19 | ) | $ | (0.21 | ) | $ | (0.22 | ) | |||
Weighted average shares: basic and diluted |
104,602,134 | 105,187,206 | 105,320,554 | 105,373,147 | 81,096,293 | 89,011,044 | 89,543,949 | 102,007,968 | |||||||||||||||||||
- (1)
- Net
product sales for the fourth quarter includes a one-time increase of approximately $1.1 million related to a change in revenue
recognition methodology. Prior to the fourth quarter, we recognized revenue on the basis of demand sales. During the fourth quarter, we began recognizing revenue on the basis of factory sales as we
established a sufficient history in order to reasonably estimate returns in accordance with GAAP. The $1.1 million one-time increase in net product sales represents the cumulative
difference between factory sales and demand sales at the end of the third quarter, or deferred revenue, less applicable gross to net sales adjustments.
In October 2010, we received the Therapeutic Discovery Tax Credit, which we elected to receive in the form of a cash payment, or grant totaling approximately $1,467,000 and which was recorded in Interest and other income, net in the fourth quarter.
11. Subsequent Events
We have evaluated all subsequent events through the date which these financial statements were issued.
In January 2011, we implemented a strategic reduction of our workforce by approximately 13%, or 25 employees. Personnel reductions were primarily focused in research and development and general and administrative functions. We plan to maintain our current level of personnel in sales and marketing. The restructuring was a result of our decision to prioritize our resources on the development and commercialization of FOLOTYN for the treatment of PTCL, cutaneous T-cell lymphoma and other hematologic malignancies, and to manage our operating costs and expenses accordingly. We expect to incur total restructuring charges of approximately $0.7 million in connection with the restructuring, all in the form of one-time termination benefits. We expect to record substantially all of these charges in the first quarter of 2011.
F-32
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 3.01 | Amended and Restated Certificate of Incorporation. | 8-K | 7/20/2009 | 3.1 | ||||||||
3.02 |
Certificate of Designation of Series A Junior Participating Preferred Stock. |
8-K |
7/20/2009 |
3.2 |
||||||||
3.03 |
Certificate of Amendment to Restated Certificate of Incorporation. |
8-K |
7/20/2009 |
3.3 |
||||||||
3.04 |
Certificate of Amendment to the Certificate of Designations of Series A Junior Participating Preferred Stock. |
8-K |
7/20/2009 |
3.4 |
||||||||
3.05 |
Certificate of Amendment to Restated Certificate of Incorporation. |
8-K |
6/25/2010 |
3.1 |
||||||||
3.06 |
Amended and Restated Bylaws of Allos Therapeutics, Inc. |
8-K |
6/25/2007 |
3.04 |
||||||||
4.01 |
Form of Common Stock Certificate. |
S-1/A |
3/17/2000 |
4.01 |
||||||||
4.02 |
Reference is made to Exhibits 3.01, 3.02, 3.03, 3.04 and 3.05. |
|||||||||||
4.03 |
Rights Agreement dated May 6, 2003 between Allos and Mellon Investor Services LLC. |
8-K |
5/9/2003 |
99.2 |
||||||||
4.04 |
Form of Rights Certificate. |
8-K |
5/9/2003 |
99.3 |
||||||||
4.05 |
Amendment to Rights Agreement dated March 4, 2005 between Allos and Mellon Investor Services LLC. |
8-K |
3/4/2005 |
4.06 |
||||||||
4.06 |
Amendment to Rights Agreement dated January 29, 2007 between Allos and Mellon Investor Services LLC. |
8-K |
1/30/2007 |
4.1 |
||||||||
4.07 |
Amendment to Rights Agreement, dated as of July 17, 2009, between Allos and Mellon Investor Services LLC. |
8-K |
7/20/2009 |
4.1 |
||||||||
10.1 |
† |
Form of Amended and Restated Indemnity Agreement between Allos and each of its directors and officers. |
8-K |
6/25/2007 |
10.01 |
|||||||
10.2 |
† |
1995 Stock Option Plan, as amended. |
S-1 |
1/26/2000 |
10.11 |
|||||||
10.3 |
† |
2000 Stock Incentive Compensation Plan, as amended. |
8-K |
12/22/2005 |
10.1 |
|||||||
10.3.1 |
† |
Form of Incentive Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
8-K |
2/11/2005 |
99.1 |
|||||||
10.3.2 |
† |
Form of Nonqualified Stock Option Letter Agreement under 2000 Stock Incentive Compensation Plan. |
8-K |
2/11/2005 |
99.2 |
|||||||
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.3.3 | † | Form of Nonqualified Stock Option Letter Agreement for Non-Employee Directors under 2000 Stock Incentive Compensation Plan. | 8-K | 2/24/2006 | 10.1 | |||||||
10.4 |
† |
2001 Employee Stock Purchase Plan, as amended and restated effective December 13, 2010. |
X |
|||||||||
10.4.1 |
† |
2001 Employee Stock Purchase Plan Offering (Series Beginning July 1, 2007). |
8-K |
6/25/2007 |
10.12.1 |
|||||||
10.5 |
* |
Office Lease dated April 4, 2001 between Allos and Catellus Development Corporation. |
10-Q |
8/14/2001 |
10.27 |
|||||||
10.5.1 |
* |
Amended and Restated Second Amendment to Lease dated December 9, 2002 between Allos and Catellus Development Corporation. |
10-K |
3/28/2003 |
10.27.1 |
|||||||
10.5.2 |
* |
Third Amendment to Lease dated November 28, 2003 between Allos and Catellus Development Corporation. |
10-K |
3/5/2004 |
10.27.2 |
|||||||
10.5.3 |
* |
Fifth Amendment to Office Lease Agreement dated June 16, 2008 between Allos and Circle Point Properties, LLC. |
10-Q |
8/5/2008 |
10.5.3 |
|||||||
10.6 |
Securities Purchase Agreement dated March 2, 2005 between Allos and the Investors listed on the signature pages thereto. |
8-K/A |
3/10/2005 |
10.41 |
||||||||
10.7 |
Registration Rights Agreement dated March 4, 2005 between Allos and the Investors listed on Schedule I thereto. |
8-K/A |
3/10/2005 |
10.42 |
||||||||
10.8 |
Letter Agreement dated March 4, 2005 among Allos, Warburg Pincus Private Equity VIII, L.P., Warburg Pincus & Co. and Warburg Pincus LLC. |
8-K |
3/4/2005 |
10.43 |
||||||||
10.9 |
† |
Summary of Compensation Arrangements for Non-Employee Directors. |
10-Q |
8/4/2009 |
10.1 |
|||||||
10.10 |
† |
Restricted Stock Award Agreement dated March 9, 2006 between Allos and Paul L. Berns. |
8-K |
3/14/2006 |
10.2 |
|||||||
10.11 |
† |
2006 Inducement Award Plan, including forms of Stock Option Grant Notice with Stock Option Agreement and Restricted Stock Grant Notice with Restricted Stock Grant Agreement. |
8-K |
6/6/2006 |
10.1 |
|||||||
10.12 |
* |
License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated December 23, 2002 and amended May 9, 2006 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. |
10-K |
3/1/2010 |
10.13 |
|||||||
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.12.1 | * | Second Amendment to License Agreement for 10-Propargyl-10-Deazaaminopterin "PDX" dated November 6, 2007 between Allos and SRI International, Sloan-Kettering Institute for Cancer Research and Southern Research Institute. | 10-K | 3/1/2010 | 10.13.1 | |||||||
10.13 |
† |
Corporate Bonus Plan, as amended and restated effective September 15, 2008. |
10-Q |
11/5/2008 |
10.1 |
|||||||
10.14 |
† |
Second Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and Paul L. Berns. |
10-K |
2/27/2008 |
10.20 |
|||||||
10.14.1 |
† |
First Amendment to Second Amended and Restated Employment Agreement, effective as of May 20, 2009, between Allos and Paul L. Berns. |
8-K |
5/22/2009 |
10.1 |
|||||||
10.14.2 |
† |
Second Amendment to Second Amended and Restated Employment Agreement, effective as of March 2, 2011, between Allos and Paul L. Berns. |
X |
|||||||||
10.15 |
† |
Amended and Restated Employment Agreement, effective December 13, 2007, between Allos and James V. Caruso. |
10-K |
2/27/2008 |
10.22 |
|||||||
10.15.1 |
† |
First Amendment to Amended and Restated Employment Agreement, dated as of May 20, 2009, between Allos and James V. Caruso. |
8-K |
5/22/2009 |
10.3 |
|||||||
10.15.2 |
† |
Release Agreement, dated as of October 22, 2010, between Allos and James V. Caruso. |
8-K |
10/22/2010 |
10.3 |
|||||||
10.16 |
† |
Second Amended and Restated Employment Agreement, effective June 2, 2010, between Allos and Marc H. Graboyes. |
8-K |
6/3/2010 |
10.1 |
|||||||
10.16.1 |
† |
First Amendment to Second Amended and Restated Employment Agreement, effective as of March 2, 2011, between Allos and Marc H. Graboyes. |
X |
|||||||||
10.17 |
† |
Executive Compensation and Equity Awards. |
8-K |
2/26/2010 |
10.1 |
|||||||
10.18 |
† |
Allos Therapeutics, Inc. 2008 Equity Incentive Plan, as amended. |
8-K |
6/25/2010 |
10.1 |
|||||||
10.18.1 |
† |
Form of Option Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
S-8 |
6/24/2008 |
99.2 |
|||||||
10.18.2 |
† |
Form of Restricted Stock Award Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
S-8 |
6/24/2008 |
99.3 |
|||||||
10.18.3 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
8-K |
2/27/2009 |
10.2 |
|||||||
10.18.4 |
† |
Form of Restricted Stock Unit Grant Notice and Agreement under the 2008 Equity Incentive Plan. |
8-K |
10/22/2010 |
10.2 |
|||||||
10.19 |
† |
Allos Therapeutics, Inc. Severance Benefit Plan, as amended and restated effective December 13, 2010. |
X |
|||||||||
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 10.19.1 | † | Allos Therapeutics, Inc. Change in Control Severance Benefit Schedule, as amended and restated effective February 23, 2008. | 8-K | 2/27/2009 | 10.4 | |||||||
10.19.2 |
† |
Allos Therapeutics, Inc. Amendment No. 1 to Change in Control Severance Benefit Schedule, adopted May 19, 2009. |
8-K |
5/22/2009 |
10.5 |
|||||||
10.20 |
† |
Employment Agreement, effective June 25, 2009, between the Company and David C. Clark. |
8-K |
6/26/2009 |
10.1 |
|||||||
10.20.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and David C. Clark. |
X |
|||||||||
10.21 |
Employment Agreement, effective April 26, 2010, between the Company and Charles Q. Morris. |
8-K |
4/27/2010 |
10.1 |
||||||||
10.21.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Charles Q. Morris. |
X |
|||||||||
10.22 |
† |
Employment Agreement, effective September 16, 2010, between Allos and Bruce K. Bennett, Jr. |
8-K |
9/17/2010 |
10.1 |
|||||||
10.22.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Bruce K. Bennett, Jr. |
X |
|||||||||
10.23 |
† |
Employment Agreement, effective September 16, 2010, between Allos and Michael E. Schick. |
8-K/A |
9/17/2010 |
10.1 |
|||||||
10.23.1 |
† |
First Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Michael E. Schick. |
X |
|||||||||
10.24 |
† |
Employment Agreement, effective April 29, 2009, between Allos and Bruce A. Goldsmith. |
8-K/A |
9/17/2010 |
10.2 |
|||||||
10.24.1 |
† |
First Amendment to Employment Agreement, effective May 22, 2009, between Allos and Bruce A. Goldsmith. |
8-K/A |
9/17/2010 |
10.3 |
|||||||
10.24.2 |
† |
Second Amendment to Employment Agreement, effective as of March 2, 2011, between Allos and Bruce A. Goldsmith. |
X |
|||||||||
10.25 |
† |
Executive Equity Awards. |
8-K |
10/22/2010 |
10.1 |
|||||||
23.01 |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm. |
X |
||||||||||
23.02 |
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. |
X |
||||||||||
24.01 |
Power of Attorney (included on signature page hereto). |
X |
||||||||||
31.01 |
Rule 13a-14(a)/15d-14(a) Certification. |
X |
||||||||||
| |
|
Incorporated by Reference | |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exhibit No. |
Description | Form | Filing Date |
Number | Filed Herewith |
|||||||
| 31.02 | Rule 13a-14(a)/15d-14(a) Certification. | X | ||||||||||
32.01 |
# |
Section 1350 Certification. |
X |
|||||||||
- †
- Indicates
management contract or compensatory plan or arrangement required to be filed as an exhibit pursuant to Item 15(b) of
Form 10-K.
- *
- Indicates
confidential treatment has been granted with respect to specific portions of this exhibit. Omitted portions have been filed with the Securities and
Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
- #
- The certifications attached as Exhibit 32.01 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Allos Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.
