Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-30755
CEPHEID
(Exact name of Registrant as Specified in its Charter)
| California | 77-0441625 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification Number) | |
| 904 Caribbean Drive, Sunnyvale, California | 94089-1189 | |
| (Address of Principal Executive Office) | (Zip Code) | |
(408) 541-4191
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, no par value and the associated Stock Purchase Rights
(Title of Class)
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (229.405 of this chapter) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K, or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated Filer | x | Accelerated Filer | ¨ | |||
| Non-accelerated Filer | ¨ (Do not check if a Smaller Reporting Company) | Smaller Reporting Company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 30, 2010, the last business day of the Registrant’s most recently completed second fiscal quarter, the aggregate market value of the common stock held by non-affiliates of the registrant was approximately $931,354,772 based on the closing sale price for the registrant’s common stock on the NASDAQ Global Select Market on that date of $16.02 per share. This number is provided only for the purpose of this report on Form 10-K and does not represent an admission by either the registrant or any such person as to the status of such person.
As of February 7, 2011 there were 61,133,020 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
| Document Description |
10-K Part | |
| Portions of the Proxy Statement for the Annual Meeting of Shareholders (the “Proxy Statement”) to be held on April 26, 2011, and to be filed pursuant to Regulation 14A within 120 days after registrant’s fiscal year ended December 31, 2010 are incorporated by reference into Part III of this Report. | III |
Table of Contents

2010 ANNUAL REPORT ON FORM 10-K
| Page | ||||||
| Item 1. |
Business | 3 | ||||
| Item 1A. |
Risk Factors | 12 | ||||
| Item 1B. |
Unresolved Staff Comments | 21 | ||||
| Item 2. |
Properties | 21 | ||||
| Item 3. |
Legal Proceedings | 22 | ||||
| Item 4. |
(Removed and Reserved) | 22 | ||||
| Item 5. |
23 | |||||
| Item 6. |
25 | |||||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
26 | ||||
| Item 7A. |
39 | |||||
| Item 8. |
40 | |||||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
70 | ||||
| Item 9A. |
70 | |||||
| Item 9B. |
70 | |||||
| Item 10. |
71 | |||||
| Item 11. |
71 | |||||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
71 | ||||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
71 | ||||
| Item 14. |
71 | |||||
| Item 15. |
Exhibits and Financial Statement Schedules | 72 | ||||
| 74 | ||||||
Cepheid®, the Cepheid logo, GeneXpert®, Xpert®, Xpertise, SmartCycler®, SmartCycler II, SmartCap®, I-CORE®, SmartMix®, Smart EBV, Smart VZV, and OmniMix® are trademarks of Cepheid.
2
Table of Contents
FORWARD-LOOKING STATEMENTS
The following discussion of our business, and other parts of this report, contain forward-looking statements that are based upon current expectations. These statements are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “estimate”, “intend”, “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. Forward-looking statements are based upon current expectations that involve risks and uncertainties. Our actual results and the timing of events could differ materially from those anticipated in our forward-looking statements as a result of many factors, including, but not limited to, the following: continued market acceptance of our healthcare associated infection products; changes in the protocols, best practices or level of testing for healthcare associated infections (“HAI”); testing volumes for our other products; development and manufacturing problems; the need for additional intellectual property licenses for new tests and other products and the terms of such licenses; the environment for capital spending by hospitals and other customers for our diagnostic instruments; our ability to successfully sell additional products in the Clinical market; lengthy sales cycles in certain markets; the performance and market acceptance of our new products; our ability to obtain regulatory approvals and introduce new products into the Clinical market; our ability to respond to changing laws and regulations affecting our industry and changing enforcement practices related thereto; the level of testing at existing clinical customer sites; the mix of products sold, which can affect gross margins; our reliance on distributors to market, sell and support our products in certain geographic locations; the occurrence of unforeseen expenditures, asset impairments, acquisitions or other transactions; litigation costs; our ability to integrate the businesses, technologies, operations and personnel of acquired companies; our success in increasing our direct sales and the effectiveness of our sales personnel; the impact of competitive products and pricing; our ability to manage geographically-dispersed operations; our ability to continue to realize manufacturing efficiencies, which is an important factor in improving gross margins; underlying market conditions worldwide; the scope and timing of actual United States Postal Service (“USPS”) funding of the Biohazard Detection System (“BDS”) in its current configuration; the rate of environmental testing using the BDS conducted by the USPS, which will affect the amount of consumable products sold; and the other risks set forth under “Risk Factors” and elsewhere in this report. We neither undertake, nor assume any obligation to update any of the forward-looking statements after the date of this report or to conform these forward-looking statements to actual results.
OVERVIEW
We are a broad-based molecular diagnostics company that develops, manufactures, and markets fully-integrated systems for testing in the Clinical market, as well as for application in our legacy Biothreat, Industrial and Partner markets. Our systems enable rapid, sophisticated molecular testing for organisms and genetic-based diseases by automating otherwise complex manual laboratory procedures. Molecular testing historically has involved a number of complicated and time-intensive steps, including sample preparation, DNA amplification and detection. Our easy-to-use systems integrate these steps and analyze complex biological samples in our proprietary test cartridges. We were first to the United States (“U.S.”) market with a Clinical Laboratory Improvement Amendments (“CLIA”) moderate complexity categorization for an amplified molecular test, and the majority of our products are moderately complex, expanding our market opportunity beyond high complexity laboratories.
Our two principal systems are the GeneXpert and SmartCycler. The GeneXpert system, our primary offering in the Clinical market, integrates sample preparation in addition to DNA amplification and detection. The GeneXpert system is designed for a broad range of user types ranging from reference laboratories and hospital central laboratories to satellite testing locations, such as emergency departments and intensive care units within hospitals and doctors’ offices. The GeneXpert system is also our main system in the Biothreat market. The SmartCycler system integrates DNA amplification and detection to allow rapid analysis of a sample.
In September 2009, we launched the GeneXpert Infinity System (“Infinity”) for high volume testing. The Infinity uses robotic cartridge handling and a full touch screen driven menu, to run up to 1,300 different molecular tests during any 24-hour period, depending upon test selection. The GeneXpert system, including the Infinity, represents a paradigm shift in molecular diagnostics in terms of ease-of-use, time-to-result and flexibility, producing accurate results with minimal risk of contamination. Our Xpert tests for use on the GeneXpert system are unique in that they typically require less than two minutes of hands-on time for unprecedented ease of use, rapid results, in many cases under an hour, and the ability to do testing in any workflow environment: full random access; on-demand; or traditional batch testing.
3
Table of Contents
The paradigm shift represented by the GeneXpert system includes (1) the ability to perform multiple, highly accurate and fast time-to-result molecular diagnostic tests, at any time, even if the system is simultaneously performing other tests, either in a batch or on-demand mode, (2) the system’s ease-of-use, which enables it to be operated without the need for highly-trained laboratory technicians, and (3) the scalability of the instrument, currently from one to 48 modules, enabling it to serve high volume testing requirements as well as lower volume requirements for testing at the point of patient care. Our GeneXpert system can provide rapid results with superior test specificity and sensitivity over comparable systems on the market today that are integrated but have open architectures. Our GeneXpert system operates with an entirely closed cartridge, reducing the likelihood of human error and contamination. In addition, the system is particularly well suited to perform “nested” polymerase chain reaction (“PCR”), a detection method that provides an enhanced level of sensitivity. This method performs an additional amplification of the target specimen after the first PCR amplification. This second amplification is designed to selectively amplify only the desired target sequence. The routine laboratory use of nested PCR has been discouraged because of the high risk of cross-contamination during processing in an open lab environment. We have developed a method for performing the entire test procedure for both of these amplifications in a single closed vessel. This has led to the commercial release of two in vitro diagnostic (“IVD”) products based on nested PCR.
We currently have available a broad and expanding menu of tests and reagents for use on our systems, spanning infectious disease, HAIs, women’s health, genetics and oncology. Our reagents and tests are marketed along with our systems on a worldwide basis.
OUR STRATEGY
Our strategy is to become the leading supplier of integrated systems and tests for molecular diagnostics. Key elements of our strategy to achieve this objective include:
| • | Provide a fully-integrated molecular testing solution to the Clinical market. We believe our GeneXpert system will continue to significantly expand our presence in the Clinical market due to its ease of use, flexibility, and ability to deliver rapid and accurate results. We believe this system is currently the only commercially available closed, self-contained, fully-integrated and automated system for molecular testing. Features of the GeneXpert system and Xpert tests include: |
| • | an approach by which the reagents are typically prepackaged in a single vessel (the test cartridge) into which the specimen is added; |
| • | no further user intervention once the Xpert cartridge is loaded into the GeneXpert system; |
| • | all three phases of PCR, (1) sample preparation, (2) amplification and (3) detection, are performed within the single sealed test cartridge automatically; |
| • | moderate complexity CLIA categorized, amplified molecular tests, which means the GeneXpert system can be operated without the need for highly-trained laboratory technicians; |
| • | commercial availability in a variety of configurations ranging from one to 48 individual test modules, which enables testing in environments ranging from low volume, near-patient testing to high volume, core or central lab testing, and system capacity that can be expanded in support of growing test volumes by adding additional modules; and |
| • | notably, to our knowledge, the only real-time PCR system that operates entirely within a closed system architecture, reducing hands-on time, reducing the likelihood of human error and contamination, and enabling nested PCR capability, a proven process for maximizing real-time PCR sensitivity. |
We also believe that the GeneXpert is the only currently available system that enables molecular testing in any workflow environment: full random access; on-demand; or traditional batch testing. With full random access, different tests for different targets may be run simultaneously in different cartridges in the same GeneXpert instrument. Additional tests may be added by the user at any time. This increases potential utilization and throughput of the instrument and also enables on-demand or “stat” testing, whereby the user can add a new test to the instrument at any time without regard to the stage of processing of any other test on the instrument.
| • | Continue to develop and market new tests. We plan to capitalize on our strengths in nucleic acid chemistry and molecular biology to continue to develop new tests for our systems. In addition, in order to more rapidly expand our test pipeline, we work with strategic partners and major academic institutions and commercial organizations to develop and validate additional tests. |
| • | Obtain additional target rights. We expect to continue to expand our collaborations with academic institutions and commercial organizations to develop and obtain target rights to various infectious disease and cancer targets. In addition, we will be focusing key business development activities on identifying infectious disease and cancer targets held by academic institutions or commercial organizations for potential license or acquisition. |
| • | Extend geographic reach. Internationally, our primary focus is the European Clinical market. Our European sales and marketing operations are headquartered in France. We have direct sales forces in the United Kingdom (“UK”), France and the Benelux region. We will continue to expand our distribution capability in Europe on both a direct and distributor basis. We also have and will continue to develop programs for other international markets, including selling through distributors. |
4
Table of Contents
| • | High Burden Developing Countries sales programs. We are developing and expect to continue to expand our presence in High Burden Developing Countries (“HBDCs”) following the World Health Organization’s endorsement of the Xpert MTB/RIF test in late 2010. In addition, in continued collaboration with the Foundation for Innovative New Diagnostics (“FIND”), we expect to broaden the menu of tests available to HBDC customers at special pricing considerations. |
| • | Continue to maintain applications in the Industrial and Biothreat markets. We currently sell products into our legacy Industrial and Biothreat markets and expect to continue our offerings in these markets. |
PRODUCTS
Our product portfolio consists of tests, reagents and systems for the Clinical, Industrial, Biothreat and Partner markets. Our two main systems are (1) the GeneXpert system, including the Infinity, which incorporates sample preparation, nucleic acid extraction and purification, DNA amplification, and detection into a small self-contained cartridge enabling rapid molecular testing 24 hours a day, 7 days a week, offering medically relevant results when and where they are needed most, and (2) the SmartCycler, which is a system that integrates DNA amplification and detection for rapid batch or random access analysis in “real-time”.
In the Clinical market, we offer tests for both the GeneXpert and the SmartCycler systems in the areas of healthcare associated infections, critical infectious disease, genetics, women’s health, and oncology. These tests include U.S. Food and Drug Administration (“FDA”) cleared products, such as IVD medical devices, CE Marked products (“CE IVD”), Analyte Specific Reagents (“ASRs”), and Research Use Only (“RUO”) tests.
Prior to 2010, the menu of tests for our GeneXpert system in the U.S. consisted of our Xpert MRSA surveillance test in addition to the following diagnostic tests: Xpert GBS for Group B Streptococcus, Xpert EV for detection of Enterovirus, Xpert MRSA/SA-SSTI (Skin and Soft Tissue Infection), Xpert MRSA/SA-BC (Blood Culture), Xpert Clostridium difficile, Xpert HemosIL Factor II and Factor V Leiden. These tests are also available as European CE IVD Marked products under the European Directive on IVD medical devices. Additionally, Xpert Mycobacterium tuberculosis/rifampicin (“MTB/RIF”), Xpert Flu Panel, and Xpert BCR-ABL Monitor are available as European CE IVD Marked products.
In January 2010, we announced that we had received FDA clearance to market our Xpert test for the vanA resistant genes of vancomycin resistant enterocci (“VRE”).
In June 2010, we announced that we had received FDA clearance to market our Xpert SA Nasal Complete test, the first and only molecular test designed for the simultaneous detection and differentiation of both Staphylococcus aureus (SA) and Methicillin-resistant Staphylococcus aureus (MRSA) colonization in a little more than an hour. Staphylococcus aureus is widely recognized as one of the most common causes of HAIs worldwide. Surgical units are high-risk areas for potentially serious consequences of postoperative complications, including surgical site infections. Our on-demand Xpert MRSA/SA nasal test provides rapid determination of pre-operative carrier status by detecting MRSA and SA, enabling clinicians to determine the best course of management for colonized patients.
In December 2010, we introduced Xpert Flu A/B under European CE IVD Marked products.
In the Industrial market, we sell our SmartCycler system along with general use PCR reagents and reaction tubes.
In the Biothreat market, the GeneXpert system is the main platform. GeneXpert modules have been integrated into the BDS purchased by the USPS utilizing our Bacillus anthracis test.
In the Partner market, we sell a variety of products primarily to partners who resell them to end-users for clinical applications.
RESEARCH AND DEVELOPMENT
The principal objective of our research and development program is to develop high-value clinical diagnostic products for the GeneXpert system. We focus our efforts on four main areas: a) assay development efforts to design, optimize, and produce specific tests that leverage the systems and chemistry we have developed, b) target discovery research to identify novel micro RNA targets to be used in the development of future assays, c) chemistry research to develop innovative and proprietary methods to design and synthesize oligonucleotide primers, probes and dyes to optimize the speed, performance and ease-of-use of our assays and d) engineering efforts to extend the multiplexing capabilities of our systems and to develop new low and high throughput systems. Total research and development expense was $42.5 million, $39.3 million and $43.3 million for the years ended December 31, 2010, 2009 and 2008, respectively.
5
Table of Contents
SALES
Sales for products within our specific markets are conducted through both direct sales and indirect distribution channels worldwide. Clinical market sales in the U.S., the UK, France and the Benelux region are handled primarily through our direct sales force, while sales in all other markets are handled primarily through distributors. As international Clinical markets continue to develop, we expect to expand our direct sales efforts. Our marketing programs are managed on a direct basis. No single country outside of the U.S. represented more than 10% of our total revenues and one customer accounted for 11%, 15% and 23% of our total product sales in 2010, 2009 and 2008, respectively.
Distribution and collaboration arrangements
Novartis. In October 2010, we entered into a collaboration with Novartis for the commercialization of a test for monitoring the BCR-ABL gene transcript in peripheral blood specimens from patients diagnosed with Philadelphia chromosome-positive chronic myelogenous leukemia (“Ph+ CML”). To fund development and clinical trial costs, we received an upfront fee of $5.0 million in October 2010 from Novartis with up to $3.0 million in milestone payments over the ensuing 12 months. If the FDA grants us clearance to commercially release the product in the U.S., under the collaboration, Novartis will have exclusive global distribution rights to the Xpert BCR-ABL test under a Cepheid/Novartis label.
Foundation for Innovative New Diagnostics. In May 2006, we entered into an agreement with the FIND to develop a simple, rapid test that can detect mycobactrium tuberculosis and associated rifampin resistance from human sputum samples. Under the agreement, we were responsible for the development of a 6-color GeneXpert instrument to accomplish such test and the development of an enhanced manufacturing line for the manufacture of test cartridges used in the test. FIND reimbursed us at agreed upon amounts. The term of the development portion of the agreement was 30 months, which was subsequently extended an additional five months. In July 2009, the agreement was extended for another year for further specified enhancements. The supply term of the agreement is for 12 years, unless terminated by either party in accordance with relevant provisions of the agreement. In January 2011, the agreement was extended for another year and a new agreement was signed for the development of our Xpert HIV Viral Load test. Under the Xpert HIV agreement, FIND will fund $5.0 million in development costs throughout the two-year contract.
Life Technology Corporation. In October 2002, we entered into a collaboration agreement with Life Technologies Corporation previously known as Applied Biosystems Group (“LIFE”), to develop reagents for use in the USPS BDS program, which was developed by the consortium led by Northrop Grumman Corporation. Under the agreement, reagents are manufactured by LIFE for packaging by us into our GeneXpert test cartridges and sold by us for use in the BDS. This agreement calls for the computed gross margin on sales of anthrax cartridges for the USPS BDS program to be equally shared between the two parties.
USPS Program. In 2003, a Northrop Grumman-led consortium that included Cepheid and other subcontractors developed the BDS for the USPS. This consortium was awarded a production contract, and installations were completed at the end of 2005. In August 2007, we entered into a five-year master purchase order with Northrop Grumman for the purchase of up to $200 million in anthrax test cartridges and associated materials used in BDS. The agreement, and subsequent purchase orders, cover the period through September 30, 2011.
MANUFACTURING
Our facilities and manufacturing processes are designed to comply with the quality standard set by the International Organization for Standardization and the FDA’s Quality System Regulations, enabling us to market our systems in the Clinical, Industrial, Biothreat and Partner testing markets worldwide. In our manufacturing facilities, we assemble our systems and produce reagents and tests for use on our GeneXpert and SmartCycler systems. We assemble our disposable reaction tubes and cartridges on custom, automated assembly lines that are designed with expandable capacity. We depend on suppliers for various components used in the manufacture of the GeneXpert and SmartCycler systems, disposable reaction tubes and cartridges, some of which are our sole source for such components.
6
Table of Contents
BACKLOG
Our aggregate backlog of $42.7 million as of December 31, 2010 is comprised of firm orders which are expected to be converted into sales in 2011. We do not believe that aggregate backlog as of any particular date is necessarily indicative of future results.
COMPETITION
We face intense competition from a number of companies that offer products in our target markets, some of which have substantially greater financial resources and larger, more established marketing, sales and service organizations than we do. These competitors include:
| • | companies developing and marketing sequence detection systems for industrial research products; |
| • | diagnostic and pharmaceutical companies; |
| • | companies developing drug discovery technologies; and |
| • | companies developing or offering biothreat detection technologies. |
Several companies provide systems and reagents for DNA amplification or detection. LIFE and F. Hoffmann-La Roche Ltd. (“Roche”) sell systems integrating DNA amplification and detection (sequence detection systems) to the commercial market. Roche, Abbott Laboratories (“Abbott”), Becton, Dickinson and Company (“BDC”), Qiagen N.V. (“Qiagen”), Gen-Probe, Inc. (“GenProbe”) and Meridian Bioscience, Inc. (“Meridian”) sell sequence detection systems, some with separate robotic batch DNA purification systems and sell reagents to the Clinical market. Other companies, including Siemens AG (“Siemens”), Hologic, Inc. (“Hologic”), and bioMerieux, offer molecular tests.
We also face emerging competition from both established companies such as Beckman Coulter, Inc., and development stage companies that are entering these markets. Several companies are currently making or developing products that may or will compete with our products. Our competitors may succeed in developing, obtaining FDA approval for, or marketing technologies or products that are more effective or commercially attractive than our potential products or that render our technologies and potential products obsolete. As these companies develop their technologies, they may develop proprietary positions that prevent us from successfully commercializing our products.
In order to compete effectively, we will need to demonstrate the advantages of our products over alternative well-established technologies and products. We will also need to demonstrate the potential economic value of our products relative to these technologies and products.
In many instances, particularly in the clinical genetics assessment area, our competitors have substantially greater financial, technical, research and other resources, and larger, more established marketing, sales, distribution and service organizations than we have. Moreover, these competitors may offer broader product lines and tactical discounts and have greater name recognition. If we fail to compete effectively against these and other competitors, we could lose sales, and our business will be harmed.
We believe that the principal competitive factors affecting sales of genetic and DNA analysis systems include the speed, integrated functionality and portability of the equipment, ease of use, the quality of the test results, price, market acceptance of the technology, regulatory approvals, particularly in the Clinical market, possession of the necessary intellectual property licenses for specific markets, collaborations and distributor relationships for specific markets and tests, and the selection of tests available for the system. We believe our products better integrate the various processes associated with DNA and RNA analysis than other currently available equipment, and that the speed, portability, flexibility, reliability and ease of use of our products are competitive.
7
Table of Contents
GOVERNMENT REGULATION
In the Clinical market, our products are regulated as medical device products by the FDA and comparable agencies of other countries. In particular, FDA regulations govern activities such as product development, product testing, product labeling, product storage, premarket clearance or approval, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution. Some of our products, depending on their intended use, will require premarket approval (“PMA”) or 510(k) clearance from the FDA prior to marketing in the U.S. The 510(k) clearance process usually takes from three to four months from submission but can take longer.
To date, we have received FDA clearance on Smart GBS, Xpert GBS, Xpert EV, Xpert MRSA, Xpert MRSA/SA-SSTI, Xpert MRSA/SA-BC, Xpert SA Nasal Complete, Xpert vanA, Xpert C. difficile and Xpert HemosIL Factor II, Factor V, as well as Emergency Use Authorization for the Xpert FluA Panel (during the authorization period in 2010). In addition, we have CE IVD-marked products for sale in Europe for Xpert BCR/ABL, Xpert GBS, Xpert EV, Xpert MRSA, Xpert MRSA/SA-BC, Xpert MRSA/SA-SSTI, Xpert C. difficile, Xpert vanA/vanB, Xpert SA Nasal Complete, Xpert MTB/RIF and Xpert Flu Panel on the GeneXpert instrument. We also have CE IVD-marked products for Smart GBS, Epstein-Barr virus (“EBV”), cytomegalovirus (“CMV”) and varicella zoster virus (“VZV”) on the SmartCycler system. We have CE-IVD marked the GeneXpert system and SmartCycler system for IVD use in EU countries.
For the Industrial and Biothreat markets, some of our products may not need FDA or other regulatory approval; however, all of our products are being produced under ISO 13485 and Quality System Regulations.
INTELLECTUAL PROPERTY
We integrate capabilities in system design, development, production and DNA amplification technologies, along with design, development and manufacture of primers, probes, dyes, quenchers and other individual reagent components. We have and are continuing to develop our own proprietary intellectual property along with licensing specific third-party technologies.
Our competitive success will be affected in part by our continued ability to obtain and maintain patent protection for our inventions, technologies and discoveries, including intellectual property that includes technologies that we license. We have patents covering technologies of our own and have licensed technologies from others. Our pending patent applications may lack priority over applications submitted by third parties or may not result in the issuance of patents. Even if issued, our patents may not be sufficiently broad to provide protection against competitors with similar technologies and may be challenged, invalidated or circumvented.
In addition to patents, we rely on a combination of trade secrets, copyright and trademark laws, nondisclosure agreements, licenses and other contractual provisions and technical measures to maintain and develop our competitive position with respect to intellectual property. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. For example, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries, as many countries do not offer the same level of legal protection for intellectual property as the U.S. Furthermore, for a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside of the U.S. Our trade secrets could become known through other unforeseen means. Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology. Our competitors may also develop similar products without infringing on any of our intellectual property rights or design around our proprietary technologies. Furthermore, any efforts to enforce our proprietary rights could result in disputes and legal proceedings that could be costly and divert attention from our business. We could also be subject to third-party claims that we require additional licenses for our products, and such claims could interfere with our business. From time to time, third parties have contacted us regarding their intellectual property, whether to license intellectual property, or in some instances, alleging potential infringement. If our products infringe the intellectual property rights of others, we could face costly litigation, which could cause us to pay substantial damages and limit our ability to sell some or all of our products. Even if our products were determined not to infringe the intellectual property rights of others, we could incur substantial costs in defending any such claims. Please refer to Item 3 “Legal Proceedings” for a discussion of current litigation surrounding intellectual property rights.
We hold an exclusive license to key technologies from Lawrence Livermore National Laboratory (“LLNL”) related to thermal cycling with integrated optical detection. This license is limited to the fields of nucleic acid analysis and ligand binding tests and contains diligence and U.S. preference provisions. These technologies have resulted in three issued U.S. patents and two pending international patent families. The LLNL technologies are the basis of our I-CORE module and encompass the key I-CORE features.
8
Table of Contents
In April 2004, we entered into a patent license agreement with Applera Corporation (“Applera”) for a non-exclusive worldwide license to make, use, and sell our products incorporating technology covered by Applera patents. In June 2006, the patent license agreement was expanded to include additional products. The Applera patents that we license expire in the U.S in May of 2011, and in Europe the patents expire in April of 2012.
In July 2004, we entered into an agreement with Roche that provides us with rights under a broad range of Roche patents, which include patents relating to the PCR process, reverse transcription-based methods, nucleic acid quantification methods, real-time PCR detection process and composition, and patents relating to methods for detection of viral and cancer targets. A number of the Roche patents that we license expired in the U.S. in August of 2010 and in Europe those patents will expire in August 2011.
In September 2006, we entered into a sublicense agreement with Abbott, pursuant to which Abbott granted us a non-exclusive, world-wide, non-transferable right to Abbott’s exclusive license to certain patents from the Baylor College of Medicine. Under the sublicense agreement, we will be able to make, use, distribute and sell products incorporating the patented technology generally characterized as multiple genomic DNA amplification. In September 2006, we also entered into a license agreement with Abbott, pursuant to which Abbott granted us a non-exclusive, world-wide, non-transferable right to a certain Abbott patent. Under the license agreement, we will be able to make, use, distribute and sell products incorporating the patented technology generally characterized as detection of cervical chlamydia trachomatis infection.
In January 2007, we entered into a sublicense agreement with bioMerieux SA, pursuant to which bioMerieux SA granted us a non-exclusive, worldwide, irrevocable sublicense to certain patents that relate to the diagnosis of MRSA. The patents are owned by Kainos Laboratories Inc. and Professor Keiichi Hiramatsu and have been exclusively licensed to bioMerieux SA with the right for bioMerieux SA to sublicense. Under the sublicense agreement, and subject to certain limitations set forth therein, we will be able to use the licensed rights to develop and sell products for use with our GeneXpert and SmartCycler systems.
We intend to actively pursue acquisitions of additional molecular markers and/or complementary products, technologies or companies in the fields of oncology, infectious diseases and other fields appropriate for molecular diagnostics. Under this program, we made our first significant technology acquisition during 2006 in the emerging field of micro RNA technology. We have identified microRNA sequences that are not in the public miRBase database of microRNA’s. We are using a database of these microRNA sequences, combined with the microRNA sequences in the public miRBase to evaluate diagnostic panels for various cancers and infectious diseases. This research is expected to lead to specific potential test opportunities in the cancer and infectious disease areas. We have recently filed patent applications on lung cancer assays based on microRNA’s.
BUSINESS COMBINATIONS
In 2010, the Company completed acquisitions of certain assets of two companies. Acquisitions are approximately 1% of total assets for each respective period presented.
EMPLOYEES
As of December 31, 2010, we had 576 full-time equivalent employees worldwide. At December 31, 2010, none of our U.S. employees were represented by a labor union. Many of our employees in Sweden are under a collective bargaining agreement. We consider our employee relations to be good.
EXECUTIVE OFFICERS OF THE REGISTRANT
The names of our executive officers and their ages, titles and biographies as of February 7, 2011 appear below:
The following table and discussion set forth certain information with regard to our current executive officers.
| Name |
Age | Position | ||||
| John L. Bishop |
66 | Chief Executive Officer and Director | ||||
| Nicolaas (‘Nico’) Arnold | 59 | Executive Vice President, Worldwide Commercial Operations | ||||
| Russel K. Enns, Ph.D. | 62 | Senior Vice President, Chief Regulatory Officer | ||||
| Andrew D. Miller | 50 | Senior Vice President, Chief Financial Officer | ||||
| David H. Persing, M.D., Ph.D. | 55 | Executive Vice President and Chief Medical and Technology Officer and Director | ||||
| Humberto Reyes | 65 | Executive Vice President, Chief Operating Officer | ||||
| Joseph H. Smith | 66 | Senior Vice President, Business Development and General Counsel | ||||
9
Table of Contents
John L. Bishop. Mr. Bishop joined us as Chief Executive Officer and as a director in April 2002. Mr. Bishop served as President and a director of Vysis, Inc., a genomic disease management company that was acquired by Abbott, from 1993 to 2002 and as Chief Executive Officer from 1996 to 2002. From 1991 until 1993, Mr. Bishop was Chairman and Chief Executive Officer of MicroProbe Corporation, a biotechnology company, and, from 1987 until 1991, of Source Scientific Systems, a biomedical instrument manufacturing company. From 1984 to 1986, Mr. Bishop was President and Chief Operating Officer of Gen-Probe From 1968 to 1984, Mr. Bishop held various management positions with American Hospital Supply Company and its affiliates, including a three-year assignment in Japan as an Executive Vice President and Chief Executive Officer of International Reagents Corp., a joint venture between American Hospital Supply Company and Green Cross Corporation. Mr. Bishop currently serves as a director of Conceptus, Inc. In addition, he is a member of the AdvaMed Dx Board.
Nicolaas (‘Nico’) Arnold. Mr. Arnold joined us as our Executive Vice President, Worldwide Commercial Operations in October 2009. Prior to joining us, Mr. Arnold was Regional Vice President for Siemens Healthcare Diagnostics in Northwest Europe, responsible for molecular diagnostics, central lab and point of care sales, from December 2007 to October 2009 and, prior to that, Managing Director of European Sales for Siemens Medical Diagnostics from January 2007 to December 2007. From February 1998 to January 2007, he was the Vice President of Global Marketing and U.S. Sales at Diagnostic Products Corporation, a manufacturer of immuno-diagnostic tests, which was acquired by Siemens Medical Solutions in July 2006.
Russel K. Enns, Ph.D. Dr. Enns was promoted to Senior Vice President, Chief Regulatory Officer in May 2010, and was our Senior Vice President, Regulatory, Clinical & Government Affairs & Quality Systems from April 2004 until May 2010, after having joined us in June 2003 as Vice President, Regulatory, Clinical & Government Affairs & Quality Systems. Prior to joining Cepheid, Dr. Enns was an Abbott Divisional Vice President for Regulatory and Clinical Affairs, Quality Systems, and Medical Reimbursement from 2001 to 2003 at the Vysis Division. From 1995 to 2001, Dr. Enns was Vice President at Vysis, Inc., a genomic disease management company, was acquired by Abbott in 2001. Before joining Vysis, he was Vice President, Technical Affairs of MicroProbe Corporation, a biotechnology company, from 1992 to 1995. Before joining MicroProbe Corporation, he was Director of Product Development, Clinical Programs and Technical Affairs at Gen-Probe, Inc., a biotechnology diagnostic company, from 1984 to 1992. From 1979 to 1984, Dr. Enns was the Director of Cell Biology at Alpha Therapeutics Corporation, and from 1975 to 1979 he was a Senior Biochemist at Monsanto Corporation. He received his Ph.D. in Biochemistry from University of California at Davis in 1976. Dr. Enns is a charter member and past chair of the Clinical and Laboratory Standards Institute (“CLSI”) Area Committee on Molecular Methods, and he is currently a member of the CLSI Board of Directors serving his second term.
Andrew D. Miller. Mr. Miller joined us in April 2008 as Senior Vice President, Chief Financial Officer. Prior to joining Cepheid, Mr. Miller was Vice President of Finance and Chief Accounting Officer for Autodesk, an enterprise software company, from March 2005 to April 2008 and Vice President of Finance and Corporate Controller from May 2003 to March 2005. At Autodesk, he was responsible for global accounting and compliance operations. Before joining Autodesk, from 2000 to 2003, Mr. Miller was Senior Vice President and Chief Financial Officer for MarketFirst Software, Inc., a leading provider of enterprise marketing automation software. Prior to MarketFirst, Mr. Miller served as Vice President of Worldwide Finance for Cadence Design Systems, Vice President of Finance and Corporate Controller for Adaptive Broadband Corporation, and held senior financial roles for Silicon Graphics, Inc. Mr. Miller was a Certified Public Accountant.
David H. Persing, M.D., Ph.D. Dr. Persing first joined us as a director in May 2004, and became our Executive Vice President and Chief Medical and Technology Officer in August 2005. From 1999 to 2005, Dr. Persing was Senior Vice President and Chief Scientific Officer at Corixa Corporation, a Seattle-based biotechnology company, until its acquisition by GlaxoSmithKline. From 1990 to 1999 he was a member of the Clinical and Research Faculty of the Mayo Clinic in Rochester, Minnesota where he conducted research on hepatitis viruses, tick-borne infections and molecular diagnostics. In 1992 he founded and directed the Molecular Microbiology Laboratory at Mayo Clinic. He has authored over 240 peer-reviewed articles and served as Editor in Chief for four textbooks on Molecular Diagnostics, the most recent of which was published by ASM press in October 2010.
Humberto Reyes. Mr. Reyes joined us as Senior Vice President of Operations in November 2004, became our Executive Vice President, Operations in November 2006 and became our Executive Vice President, Chief Operating Officer in May 2008. Prior to joining Cepheid, Mr. Reyes was an Operations Consultant with Brownsboro Group, LLC. from 2003 to 2004. Prior to joining Brownsboro, Mr. Reyes was a Senior Operations Consultant for EXPERTech Associates, consulting in medical devices and biotech industries from 2001 to 2003. Prior to that, he was Head of Operations for OXIS Health Products Inc., which developed, manufactured and marketed products for oxidative research and wellness programs from 1997 to 2001. He is an experienced operations executive with more than 25 years of progressive management experience in the diagnostic and related industries. Mr. Reyes’ work experience also includes Vice President, Operations, Dade Diagnostics at Baxter; Vice President/General Manager, Chromatography Division, Varian and Associates; and Sr. Vice President, Operations, Microgenics Corporation.
10
Table of Contents
Joseph H. Smith. Mr. Smith joined us in June 2003 as Vice President, General Counsel and has served as our Senior Vice President of Business Development and General Counsel since April 2004. He has been Secretary of the Corporation since March 2004. From 1989 to 2002, Mr. Smith was Vice President of Intellectual Property at Applied Biosystems (now Life Technologies Corporation) and its predecessors, a biotechnology research equipment company, and from 2002 to 2003 was its Senior Vice President for Business Development. Prior to Applied Biosystems, Mr. Smith was a partner in the law firm of Wiseman, Jones, and Smith; and prior to that he was a member of the Technical Legal Department of Hewlett-Packard.
AVAILABLE INFORMATION
Our website is located at www.cepheid.com. We make available free of charge on our web site our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file them with or furnish them to the SEC. Information contained on our web site is not part of this Annual Report on Form 10-K or our other filings with the SEC. The public may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov.
The charters of our Audit Committee, our Compensation Committee and our Nominating/Governance Committee, are available on the Investor Relations section of our website under “Corporate Governance”. Also available on that section of our website is our Code of Business Conduct and Ethics, which we expect every employee, officer, director, staffing agency worker and consultant to read, understand and abide by. This information is also available by writing to us at the address on the cover of this Annual Report on Form 10-K.
11
Table of Contents
You should carefully consider the risks and uncertainties described below, together with all of the other information included in this Report, in considering our business and prospects. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations. The occurrence of any of the following risks could harm our business, financial condition or results of operations.
We have a history of operating losses and may not achieve profitability for 2011.
We have incurred operating losses in each fiscal year since our inception. We experienced net losses of approximately $22.4 million in 2008, $22.5 million in 2009 and $5.9 million in 2010. We may achieve profitability for 2011. As of December 31, 2010, we had an accumulated deficit of approximately $208.2 million. Our ability to become profitable for 2011 will likely depend on our ability to continue to increase our revenues, which is subject to a number of factors including our ability to continue to successfully penetrate the Clinical market, our ability to successfully market the GeneXpert system and develop additional effective GeneXpert tests, continued growth in sales of our HAI and other tests, the extent of our participation in the USPS BDS program and the operating parameters of the USPS BDS program, which will affect the rate of our consumable products sold, our ability to compete effectively against current and future competitors, the development of our HBDC sales programs, and global economic and political conditions. Our ability to become profitable for 2011 also depends on our expense levels and product gross margin, which are also influenced by a number of factors, including the resources we devote to developing and supporting our products, the continued progress of our research and development of potential products, the ability to gain FDA clearance for our new products, our ability to improve manufacturing efficiencies, license fees or royalties we may be required to pay and the potential need to acquire licenses to new technology or to use our technology in new markets, which could require us to pay unanticipated license fees and royalties in connection with these licenses. Our expansion efforts may prove more expensive than we currently anticipate, and we may not succeed in increasing our revenues to offset higher expenses. These expenses, among other things, may cause our net income and working capital to decrease. If we fail to grow our revenue, manage our expenses and improve our product gross margin, we may not achieve profitability. If we fail to do so, the market price of our common stock will likely decline.
The current uncertainty in global economic conditions makes it particularly difficult to predict product demand and other related matters, and makes it more likely that our actual results could differ materially from expectations.
Our operations and performance depend on global economic conditions, which have been adversely impacted by the global macroeconomic downturn. These conditions have and may continue to make it difficult for our customers and potential customers to accurately forecast and plan future business activities, and have caused our customers and potential customers to slow or reduce spending, particularly for systems. Furthermore, during economic uncertainty, our customers have experienced and may continue to experience issues gaining timely access to sufficient credit, which could result in their unwillingness to purchase products or an impairment of their ability to make timely payments to us. If that were to continue to occur, we may experience decreased sales, be required to increase our allowance for doubtful accounts and our days sales outstanding would be negatively impacted. Even with economic recovery, it may take time for our customers to establish new budgets and return to normal purchasing patterns. We cannot predict the reoccurrence of any economic slowdown or the strength or sustainability of the economic recovery, worldwide, in the U.S. or in our industry. These and other economic factors could have a material adverse effect on demand for our products and on our financial condition and operating results.
Our operating results may fluctuate significantly and any failure to meet financial expectations may result in a decline in our stock price.
Our quarterly operating results may fluctuate in the future as a result of many factors, such as those described elsewhere in this section, many of which are beyond our control. Because our revenue and operating results are difficult to predict, we believe that period-to-period comparisons of our results of operations are not a good indicator of our future performance. Our operating results may be affected by the inability of some of our customers to consummate anticipated purchases of our products, whether due to adverse economic conditions, changes in internal priorities or, in the case of governmental customers, problems with the appropriations process and variability and timing of orders, changes in procedures or protocols with respect to testing or manufacturing inefficiencies. If revenue declines in a quarter, whether due to a delay in recognizing expected revenue, adverse economic conditions, and unexpected costs or otherwise, our results of operations will be harmed because many of our expenses are relatively fixed. In particular, research and development, sales and marketing and general and administrative expenses are not significantly affected by variations in revenue. If our quarterly operating results fail to meet or exceed the expectations of securities analysts or investors, our stock price could drop suddenly and significantly.
Our sales cycle can be lengthy, which can cause variability and unpredictability in our operating results.
The sales cycles for our systems products can be lengthy, particularly during uncertain economic conditions, which makes it more difficult for us to accurately forecast revenues in a given period, and may cause revenues and operating results to vary significantly from period to period. For example, sales of our products involving our corporate accounts within the Clinical market
12
Table of Contents
and those within the Industrial market often involve purchasing decisions by large public and private institutions, and any purchases can require many levels of pre-approval. In addition, certain Industrial sales may depend on these institutions receiving research grants from various federal agencies, which grants vary considerably from year to year in both amount and timing due to the political process. As a result, we may expend considerable resources on unsuccessful sales efforts or we may not be able to complete transactions on the schedule anticipated.
If we cannot successfully commercialize our products, our business could be harmed.
If our tests for use on our systems do not gain continued market acceptance, we will be unable to generate significant sales, which will prevent us from achieving profitability. While we have received FDA clearance for a number of tests, these products may not continue to experience increased sales. Many factors may affect the market acceptance and commercial success of our products, including:
| • | timely expansion of our menu of tests and reagents; |
| • | the results of clinical trials needed to support any regulatory approvals of our tests; |
| • | our ability to obtain requisite FDA or other regulatory clearances or approvals for our tests under development on a timely basis; |
| • | demand for the tests and reagents we introduce; |
| • | the timing of market entry for various tests for the GeneXpert and the SmartCycler systems; |
| • | our ability to convince our potential customers of the advantages and economic value of our systems and tests over competing technologies and products; |
| • | the breadth of our test menu relative to competitors; |
| • | changes to policies, procedures or what are considered best practices in clinical diagnostics, including practices for detecting and preventing HAIs; |
| • | the extent and success of our marketing and sales efforts; |
| • | the functionality of new products that address market requirements and customer demands; |
| • | level of reimbursement for our products by third-party payers; and |
| • | publicity concerning our systems and tests. |
In particular, we believe that the success of our business will depend in large part on our ability to continue to increase sales of our Xpert tests and our ability to introduce additional tests for the Clinical market. We believe that successfully expanding our business in the Clinical market is critical to our long-term goals and success. We have limited ability to forecast future demand for our products in this market. In addition, we have committed substantial funds to licenses that are required for us to compete in the Clinical market. If we cannot successfully penetrate the Clinical market to fully exploit these licenses, these investments may not yield significant returns, which could harm our business.
Regulations applicable to our products and operations are expensive, time-consuming, and uncertain and may prevent us from obtaining required approvals for the commercialization of some of our products.
In the Clinical market, our products are regulated as medical device products by the FDA and comparable agencies of other countries. In particular, FDA regulations govern activities such as product development, product testing, product labeling, product storage, premarket clearance or approval, manufacturing, advertising, promotion, product sales, reporting of certain product failures and distribution. Some of our products, depending on their intended use, will require premarket approval (“PMA”) or 510(k) clearance from the FDA prior to marketing. The 510(k) clearance process usually takes from three to four months from submission but can take longer. The PMA process is much more costly, lengthy and uncertain and generally takes from six months to one year or longer from submission. Clinical trials are generally required to support both PMA and 510(k) submissions. Certain of our products for use on our GeneXpert and SmartCycler systems, when used for clinical purposes, may require PMA, and all such tests will most likely, at a minimum, require 510(k) clearance. We are planning clinical trials for other proposed products. Clinical trials are expensive and time-consuming. In addition, the commencement or completion of any clinical trials may be delayed or halted for any number of reasons, including product performance, changes in intended use, changes in medical practice and the opinion of evaluator Institutional Review Boards. Additionally, since 2009, the FDA has significantly increased the scrutiny applied to its oversight of companies subject to its regulations, including 510(k) submissions, by hiring new investigators and increasing inspections of manufacturing facilities.
13
Table of Contents
Failure to comply with the applicable requirements can result in, among other things, warning letters, administrative or judicially imposed sanctions such as injunctions, civil penalties, recall or seizure of products, total or partial suspension of production, refusal to grant premarket clearance or PMA for devices, withdrawal of marketing clearances or approvals, or criminal prosecution. With regard to future products for which we seek 510(k) clearance or PMA from the FDA, any failure or material delay to obtain such clearance or approval could harm our business. If the FDA were to disagree with our regulatory assessment and conclude that approval or clearance is necessary to market the products, we could be forced to cease marketing the products and seek approval or clearance. With regard to those future products for which we will seek 510(k) clearance or PMA from the FDA, any failure or material delay to obtain such clearance or approval could harm our business. In addition, it is possible that the current regulatory framework could change or additional regulations could arise at any stage during our product development or marketing, which may adversely affect our ability to obtain or maintain approval of our products and could harm our business.
Comprehensive healthcare reform legislation, signed into law on March 23, 2010, imposes stringent compliance, recordkeeping, and reporting requirements on companies in various sectors of the life sciences industry with which we may need to comply and enhanced penalties for non-compliance with the new healthcare regulations. Complying with new regulations may divert management resources, and inadvertent failure to comply with new regulations may result in penalties being imposed on us. The impact and durability of this legislation, in its current form, remains unclear, and costs of compliance with this legislation, or any future amendments thereto, could result in certain risks and expenses that we may have to assume. Various healthcare reform proposals have also emerged at the state level, and it is unclear which, if any, of these proposals will be enacted and what impact any resulting laws would have on our operations.
Our manufacturing facilities located in Sunnyvale, California, Bothell, Washington, Bromma and Solna, Sweden, where we assemble and produce the GeneXpert and SmartCycler systems, cartridges and other molecular diagnostic kits and reagents, are subject to periodic regulatory inspections by the FDA and other federal and state and foreign regulatory agencies. For example, these facilities are subject to Quality System Regulations (“QSR”) of the FDA and are subject to annual inspection and licensing by the States of California and Washington and European regulatory agencies. If we fail to maintain these facilities in accordance with the QSR requirements, international quality standards or other regulatory requirements, our manufacturing process could be suspended or terminated, which would prevent us from being able to provide products to our customers in a timely fashion and therefore harm our business.
The federal medical device tax by the U.S. government could adversely affect our business, profitability and stock price.
The comprehensive healthcare reform legislation includes an annual excise tax on the sale of medical devices equal to 2.3% of the price of the device, starting on January 1, 2013, which we believe will include all of our Clinical products sold in the U.S. The exact impact of this excise tax is not currently clear. We may seek to pass this tax onto our customers. If we are unsuccessful, our future operating results could be harmed, which in turn could cause the price of our stock to decline. Additionally, because of the uncertainty surrounding these issues, the impact of this tax has not been reflected in our forward guidance.
We rely on licenses of key technology from third parties and may require additional licenses for many of our new product candidates.
We rely on third-party licenses to be able to sell many of our products, and we could lose these third-party licenses for a number of reasons, including, for example, early terminations of such agreements due to breaches or alleged breaches by either party to the agreement. If we are unable to enter into a new agreement for licensed technologies, either on terms that are acceptable to us or at all, we may be unable to sell some of our products or access some geographic or industry markets. We also need to introduce new products and product features in order to market our products to a broader customer base and grow our revenues, and many new products and product features could require us to obtain additional licenses and pay additional license fees and royalties. Furthermore, for some markets, we intend to manufacture reagents and tests for use on our systems. We believe that manufacturing reagents and developing tests for our systems is important to our business and growth prospects but may require additional licenses, which may not be available on commercially reasonable terms or at all. Our ability to develop, manufacture and sell products, and our strategic plans and growth, could be impaired if we are unable to obtain these licenses or if these licenses are terminated or expire and cannot be renewed. We may not be able to obtain or renew licenses for a given product or product feature or for some reagents on commercially reasonable terms, if at all. Furthermore, some of our competitors have rights to technologies and reagents that we do not have which may put us at a competitive disadvantage in certain circumstances and could adversely affect our performance.
Our participation in the USPS BDS program may not result in predictable revenues in the future.
Our participation in the USPS BDS program involves significant uncertainties related to governmental decision-making and timing of deployment and is highly sensitive to changes in national priorities and budgets. Budgetary pressures may result in reduced allocations to projects such as the BDS program, sometimes without advance notice. We cannot be certain that actual funding and operating parameters, or product purchases, will occur at currently expected levels or in the currently expected timeframe.
14
Table of Contents
We may face risks associated with acquisitions of companies, products and technologies, and our business could be harmed if we are unable to address these risks.
If we are presented with appropriate opportunities, we intend to acquire or make other investments in complementary companies, products or technologies. We may not realize the anticipated benefit of any acquisition or investment. We will likely face risks, uncertainties and disruptions associated with the integration process, including difficulties in the integration of the operations and services of an acquired company, integration of acquired technology with our products, diversion of our management’s attention from other business concerns, the potential loss of key employees or customers of the acquired businesses and impairment charges if future acquisitions are not as successful as we originally anticipate. If we fail to successfully integrate other companies, products or technologies that we acquire, our business could be harmed. Furthermore, we may have to incur debt or issue equity securities to pay for any additional future acquisitions or investments, the issuance of which could be dilutive to our existing shareholders. In addition, our operating results may suffer because of acquisition-related costs or amortization expenses or charges relating to acquired intangible assets.
If we are unable to manufacture our products in sufficient quantities and in a timely manner, our operating results will be harmed and our ability to generate revenue could be diminished.
Our revenues and other operating results will depend in large part on our ability to manufacture and assemble our products in sufficient quantities and in a timely manner. Any interruptions we experience in the manufacturing or shipping of our products could delay our ability to recognize revenues in a particular quarter. Manufacturing problems can and do arise, and as demand for our products increases, any such problems could have an increasingly significant impact on our operating results. In the past, we have experienced problems and delays in production that have impacted our product yield and caused delays in our ability to ship finished products, and we may experience such delays in the future. We may not be able to react quickly enough to ship products and recognize anticipated revenues for a given period if we experience significant delays in the manufacturing process. In addition, we must maintain sufficient production capacity in order to minimize such delays, which carries fixed costs that we may not be able to offset if orders slow, which would adversely affect our operating margins. If we are unable to manufacture our products consistently, in sufficient quantities, and on a timely basis, our revenues from product sales, gross margins and our other operating results will be materially and adversely affected.
If certain single source suppliers fail to deliver key product components in a timely manner, our manufacturing ability would be impaired and our product sales could suffer.
We depend on several single source suppliers that supply some of the components used in the manufacture of our systems and our disposable reaction tubes and cartridges. Strategic purchases of components are necessary for our business. If we need alternative sources for key component parts for any reason, these component parts may not be immediately available to us. If alternative suppliers are not immediately available, we will have to identify and qualify alternative suppliers, and production of these components may be delayed. We may not be able to find an adequate alternative supplier in a reasonable time period or on commercially acceptable terms, if at all. Shipments of affected products have been limited or delayed as a result of such problems in the past, and similar problems could occur in the future. In addition, many companies are experiencing financial difficulties as a result of the global economic slowdown. We cannot assure you that our suppliers will not be adversely affected by these conditions or that they will be able to continue to provide us with the components we need. Our inability to obtain our key source supplies for the manufacture of our products may require us to delay shipments of products, harm customer relationships or force us to curtail or cease operations.
If certain of our products fail to obtain an adequate level of reimbursement from third-party payers, our ability to sell products in the Clinical market would be harmed.
Our ability to sell our products in the Clinical market will depend in part on the extent to which reimbursement for tests using our products will be available from government health administration authorities, private health coverage insurers, managed care organizations, and other organizations. There are efforts by governmental and third-party payers to contain or reduce the costs of health care through various means, and the continuous growth of managed care, together with efforts to reform the health care delivery system in the U.S. and Europe, has increased pressure on health care providers and participants in the health care industry to reduce costs. Consolidation among health care providers and other participants in the healthcare industry has resulted in fewer, more powerful health care groups, whose purchasing power gives them cost containment leverage. Additionally, third-party payers are increasingly challenging the price of medical products and services. Furthermore, we are unable to predict what effect the current or any future healthcare reform will have on our business, or the effect these matters will have on our customers. If purchasers or users of our products are not able to obtain adequate reimbursement for the cost of using our products, they may forego or reduce their use. Significant uncertainty exists as to the reimbursement status of newly approved health care products and whether adequate third-party coverage will be available.
15
Table of Contents
The life sciences industry is highly competitive and subject to rapid technological change, if our competitors and potential competitors develop superior products and technologies, our competitive position and results of operations would suffer.
We face intense competition from a number of companies that offer products in our target markets, some of which have substantially greater financial resources and larger, more established marketing, sales and service organizations than we do. These competitors include:
| • | companies developing and marketing sequence detection systems for industrial research products; |
| • | diagnostic and pharmaceutical companies; |
| • | companies developing drug discovery technologies; and |
| • | companies developing or offering biothreat detection technologies. |
Several companies provide systems and reagents for DNA amplification or detection. LIFE and Roche sell systems integrating DNA amplification and detection (sequence detection systems) to the commercial market. Roche, Abbott, BDC, Qiagen, GenProbe and Meridian sell sequence detection systems, some with separate robotic batch DNA purification systems and sell reagents to the Clinical market. Other companies, including Siemens, Hologic, and bioMerieux, offer molecular tests.
The life sciences industry is characterized by rapid and continuous technological innovation. We may need to develop new technologies for our products to remain competitive. One or more of our current or future competitors could render our present or future products obsolete or uneconomical by technological advances. In addition, the introduction or announcement of new products by us or others could result in a delay of or decrease in sales of existing products, as we await regulatory approvals and as customers evaluate these new products. We may also encounter other problems in the process of delivering new products to the marketplace such as problems related to design, development or manufacturing of such products, and as a result we may be unsuccessful in selling such products. Our future success depends on our ability to compete effectively against current technologies, as well as to respond effectively to technological advances by developing and marketing products that are competitive in the continually changing technological landscape.
If our products do not perform as expected or the reliability of the technology on which our products are based is questioned, we could experience lost revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high-quality molecular test systems. We believe that customers in our target markets are likely to be particularly sensitive to product defects and errors. Our reputation and the public image of our products or technologies may be impaired if our products fail to perform as expected or our products are perceived as difficult to use. Despite testing, defects or errors could occur in our products or technologies. Furthermore, with respect to the BDS program, our products are incorporated into larger systems that are built and delivered by others; we cannot control many aspects of the final system.
In the future, if our products experience a material defect or error, this could result in loss or delay of revenues, delayed market acceptance, damaged reputation, diversion of development resources, legal claims, increased insurance costs or increased service and warranty costs, any of which could harm our business. Such defects or errors could also prompt us to amend certain warning labels or narrow the scope of the use of our products, either of which could hinder our success in the market. Furthermore, any failure in the overall BDS, even if it is unrelated to our products, could harm our business. Even after any underlying concerns or problems are resolved, any widespread concerns regarding our technology or any manufacturing defects or performance errors in our products could result in lost revenue, delayed market acceptance, damaged reputation, increased service and warranty costs, and claims against us.
If product liability lawsuits are successfully brought against us, we may face reduced demand for our products and incur significant liabilities.
We face an inherent risk of exposure to product liability claims if our technologies or systems are alleged to have caused harm or do not perform in accordance with specifications, in part because our products are used for sensitive applications. We cannot be certain that we would be able to successfully defend any product liability lawsuit brought against us. Regardless of merit or eventual outcome, product liability claims may result in:
| • | decreased demand for our products; |
| • | injury to our reputation; |
16
Table of Contents
| • | costs of related litigation; and |
| • | substantial monetary awards to plaintiffs. |
Although we carry product liability insurance, if we become the subject of a successful product liability lawsuit, our insurance may not cover all substantial liabilities, which could harm our business.
If our direct selling efforts for our products fail, our business expansion plans could suffer, and our ability to generate revenue will be diminished.
We have a relatively small sales force compared to our competitors. If our direct sales force is not successful, or new additions to our sales team fail to gain traction among our customers, we may not be able to increase market awareness and sales of our products. If we fail to establish our systems in the marketplace, it could have a negative effect on our ability to sell subsequent systems and hinder the planned expansion of our business.
If our distributor relationships are not successful, our ability to market and sell our products would be harmed and our financial performance will be adversely affected.
We depend on relationships with distributors for the marketing and sales of our products in the Industrial and Clinical markets in various geographic regions, and we have a limited ability to influence their efforts. We expect to continue to rely substantially on our distributor relationships for sales into other markets or geographic regions, which is key to our long-term growth potential. Relying on distributors for our sales and marketing could harm our business for various reasons, including:
| • | agreements with distributors may terminate prematurely due to disagreements or may result in litigation between the partners; |
| • | we may not be able to renew existing distributor agreements on acceptable terms; |
| • | our distributors may not devote sufficient resources to the sale of products; |
| • | our distributors may be unsuccessful in marketing our products; |
| • | our existing relationships with distributors may preclude us from entering into additional future arrangements with other distributors; and |
| • | we may not be able to negotiate future distributor agreements on acceptable terms. |
We may be subject to third-party claims that require additional licenses for our products and we could face costly litigation, which could cause us to pay substantial damages and limit our ability to sell some or all of our products.
Our industry is characterized by a large number of patents, claims of which appear to overlap in many cases. As a result, there is a significant amount of uncertainty regarding the extent of patent protection and infringement. Companies may have pending patent applications, which are typically confidential for the first eighteen months following filing, that cover technologies we incorporate in our products. Accordingly, we may be subjected to substantial damages for past infringement or be required to modify our products or stop selling them if it is ultimately determined that our products infringe a third party’s proprietary rights. Moreover, from time to time, we receive correspondence and other communications from companies that ask us to evaluate the need for a license of patents they hold, and indicating or suggesting that we need a license to their patents in order to offer our products and services or to conduct our business operations. For example, we are currently engaged in litigation as further described in Item 3 “Legal Proceedings.” Even if we are successful in defending against claims, we could incur substantial costs in doing so. Any litigation related to this claim and others that may arise in the future of patent infringement could likely consume our resources and could lead to significant damages, royalty payments or an injunction on the sale of certain products. Any additional licenses to patented technology could obligate us to pay substantial additional royalties, which could adversely impact our product costs and harm our business.
If we fail to maintain and protect our intellectual property rights, our competitors could use our technology to develop competing products and our business will suffer.
Our competitive success will be affected in part by our continued ability to obtain and maintain patent protection for our inventions, technologies and discoveries, including our intellectual property that includes technologies that we license. Our ability to do so will depend on, among other things, complex legal and factual questions. We have patents related to some of our technology and have licensed some of our technology under patents of others. Our patents and licenses may not successfully preclude others from using our technology. Our pending patent applications may lack priority over applications submitted by third parties or may not result in the issuance of patents. Even if issued, our patents may not be sufficiently broad to provide protection against competitors with similar technologies and may be challenged, invalidated or circumvented.
17
Table of Contents
In addition to patents, we rely on a combination of trade secrets, copyright and trademark laws, nondisclosure agreements, licenses and other contractual provisions and technical measures to maintain and develop our competitive position with respect to intellectual property. Nevertheless, these measures may not be adequate to safeguard the technology underlying our products. For example, employees, consultants and others who participate in the development of our products may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach. We also may not be able to effectively protect our intellectual property rights in some foreign countries, as many countries do not offer the same level of legal protection for intellectual property as the U.S. Furthermore, for a variety of reasons, we may decide not to file for patent, copyright or trademark protection outside of the U.S. Our trade secrets could become known through other unforeseen means. Notwithstanding our efforts to protect our intellectual property, our competitors may independently develop similar or alternative technologies or products that are equal or superior to our technology. Our competitors may also develop similar products without infringing on any of our intellectual property rights or design around our proprietary technologies. Furthermore, any efforts to enforce our proprietary rights could result in disputes and legal proceedings that could be costly and divert attention from our business.
The U.S. Government has certain rights to use and disclose some of the intellectual property that we license and could exclusively license it to a third party if we fail to achieve practical application of the intellectual property.
Aspects of the technology licensed by us under agreements with third party licensors may be subject to certain government rights. Government rights in inventions conceived or reduced to practice under a government-funded program may include a non-exclusive, royalty-free worldwide license to practice or have practiced such inventions for any governmental purpose. In addition, the U.S. government has the right to require us or our licensors (as applicable) to grant licenses which would be exclusive under any of such inventions to a third party if they determine that: (1) adequate steps have not been taken to commercialize such inventions in a particular field of use; (2) such action is necessary to meet public health or safety needs; or (3) such action is necessary to meet requirements for public use under federal regulations. Further, the government rights include the right to use and disclose, without limitation, technical data relating to licensed technology that was developed in whole or in part at government expense. At least one of our technology license agreements contains a provision recognizing these government rights.
We may need to initiate lawsuits to protect or enforce our patents, which would be expensive and, if we lose, may cause us to lose some, if not all, of our intellectual property rights, and thereby impair our ability to compete.
We rely on patents to protect a large part of our intellectual property. To protect or enforce our patent rights, we may initiate patent litigation against third parties, such as infringement suits or interference proceedings. These lawsuits could be expensive, take significant time and divert management’s attention from other business concerns. They would also put our patents at risk of being invalidated or interpreted narrowly, and our patent applications at risk of not issuing. We may also provoke these third parties to assert claims against us. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in our industry are generally uncertain. We cannot assure you that we would prevail in any of these suits or that the damages or other remedies awarded, if any, would be commercially valuable. During the course of these suits, there may be public announcements of the results of hearings, motions and other interim proceedings or developments in the litigation. Any public announcements related to these suits could cause our stock price to decline.
Our international operations subject us to additional risks and costs.
We conduct operations on a global basis. These operations are subject to a number of difficulties and special costs, including:
| • | compliance with multiple, conflicting and changing governmental laws and regulations; |
| • | laws and business practices favoring local competitors; |
| • | foreign exchange and currency risks; |
| • | difficulty in collecting accounts receivable or longer payment cycles; |
| • | import and export restrictions and tariffs; |
| • | difficulties staffing and managing foreign operations; |
| • | difficulties and expense in enforcing intellectual property rights; |
| • | business risks, including fluctuations in demand for our products and the cost and effort to conduct international operations and travel abroad to promote international distribution and overall global economic conditions; |
18
Table of Contents
| • | multiple conflicting tax laws and regulations; and |
| • | political and economic instability. |
We intend to expand our international sales and marketing activities, including through our subsidiary in France and our direct sales force in the UK, France and the Benelux region, and enter into relationships with additional international distribution partners. We may not be able to attract international distribution partners that will be able to market our products effectively.
Our international operations could also increase our exposure to international laws and regulations. If we cannot comply with foreign laws and regulations, which are often complex and subject to variation and unexpected changes, we could incur unexpected costs and potential litigation. For example, the governments of foreign countries might attempt to regulate our products and services or levy sales or other taxes relating to our activities. In addition, foreign countries may impose tariffs, duties, price controls or other restrictions on foreign currencies or trade barriers, any of which could make it more difficult for us to conduct our business.
The nature of some of our products may also subject us to export control regulation by the U.S. Department of State and the Department of Commerce. Violations of these regulations can result in monetary penalties and denial of export privileges.
Our sales to customers outside the U.S. are subject to government export regulations that require us to obtain licenses to export such products internationally. If the U.S. government were to amend its export control regulations, such amendments could create uncertainty in our industry, result in increased costs of compliance, and further restrict our ability to sell our products abroad. In particular, we are required to obtain a new license for each purchase order of our biothreat products that are exported outside the U.S. Delays or denial of the grant of any required license, or changes to the regulations that make such delays or denials more likely or frequent, could make it difficult to make sales to foreign customers and could adversely affect our revenue. In addition, we could be subject to fines and penalties for violation of these export regulations if we were found in violation. Such violation could result in penalties, including prohibiting us from exporting our products to one or more countries, and could materially and adversely affect our business.
If we fail to retain key members of our staff, our ability to conduct and expand our business would be impaired.
We are highly dependent on the principal members of our management and scientific staff. The loss of services of any of these persons could seriously harm our product development and commercialization efforts. In addition, we require skilled personnel in areas such as microbiology, clinical and sales, marketing and finance. We generally do not enter into employment agreements requiring these employees to continue in our employment for any period of time. Attracting, retaining and training personnel with the requisite skills remains challenging, particularly in the Silicon Valley area of California, where our main office is located. If at any point we are unable to hire, train and retain a sufficient number of qualified employees to match our growth, our ability to conduct and expand our business could be seriously reduced.
If we become subject to claims relating to improper handling, storage or disposal of hazardous materials, we could incur significant cost and time to comply.
Our research and development processes involve the controlled storage, use and disposal of hazardous materials, including biological hazardous materials. We are subject to foreign, federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. We may incur significant costs complying with both existing and future environmental laws and regulations. In particular, we are subject to regulation by the Occupational Safety and Health Administration (“OSHA”) and the Environmental Protection Agency (“EPA”), and to regulation under the Toxic Substances Control Act and the Resource Conservation and Recovery Act in the U.S. OSHA or the EPA may adopt regulations that may affect our research and development programs. We are unable to predict whether any agency will adopt any regulations that would have a material adverse effect on our operations.
The risk of accidental contamination or injury from hazardous materials cannot be eliminated completely. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of our workers’ compensation insurance. We may not be able to maintain insurance on acceptable terms, if at all.
If a catastrophe strikes our manufacturing facilities, we may be unable to manufacture our products for a substantial amount of time and we would experience lost revenue.
Our manufacturing facilities are located in Sunnyvale, California, Bothell, Washington, Bromma and Solna, Sweden. Although we have business interruption insurance, our facilities and some pieces of manufacturing equipment are difficult to replace and could require substantial replacement lead-time. Various types of disasters, including earthquakes, fires, floods and acts of terrorism, may affect our manufacturing facilities. Earthquakes are of particular significance since our primary manufacturing facilities in California are located in an earthquake-prone area. In the event our existing manufacturing facilities or equipment are affected by man-made or natural disasters, we may be unable to manufacture products for sale or meet customer demands or sales projections. If our manufacturing operations were curtailed or ceased, it would seriously harm our business.
19
Table of Contents
Failure to comply with covenants in our loan agreement with Silicon Valley Bank could result in our inability to borrow additional funds and adversely impact our business.
We have entered into a loan and security agreement (the “Loan Agreement”) with Silicon Valley Bank (“SVB”). This loan agreement imposes numerous financial and other restrictive covenants on our operations, including covenants relating to our general profitability and our liquidity. We were in compliance with these covenants as of December 31, 2010. If we violate these or any other covenants, any outstanding amounts under these agreements could become due and payable prior to their stated maturity dates, the bank could proceed against any collateral in our operating accounts and our ability to borrow funds in the future may be restricted or eliminated. These restrictions may also limit our ability to pursue business opportunities or strategies that we would otherwise consider to be in our best interests.
We might require additional capital to respond to business challenges or acquisitions, and such capital might not be available.
We may need to engage in additional equity or debt financing, such as the loan agreement with SVB we entered into in June 2010, to respond to business challenges or acquire complementary businesses and technologies. Equity and debt financing, however, might not be available when needed or, if available, might not be available on terms satisfactory to us. In addition, to the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in dilution to our shareholders. In addition, these securities may be sold at a discount from the market price of our common stock and may include rights, preferences or privileges senior to those of our common stock. If we are unable to obtain adequate financing or financing on terms satisfactory to us, our ability to continue to support our business growth and to respond to business challenges could be significantly limited.
We rely on relationships with collaborative partners and other third parties for development, supply and marketing of certain products and potential products, and such collaborative partners or other third parties could fail to perform sufficiently.
We believe that our success in penetrating our target markets depends in part on our ability to develop and maintain collaborative relationships with other companies. Relying on collaborative relationships is risky to our future success for these products because, among other things:
| • | our collaborative partners may not devote sufficient resources to the success of our collaboration; |
| • | our collaborative partners may not obtain regulatory approvals necessary to continue the collaborations in a timely manner; |
| • | our collaborative partners may be acquired by another company and decide to terminate our collaborative partnership or become insolvent; |
| • | our collaborative partners may develop technologies or components competitive with our products; |
| • | components developed by collaborators could fail to meet specifications, possibly causing us to lose potential projects and subjecting us to liability; |
| • | disagreements with collaborators could result in the termination of the relationship or litigation; |
| • | collaborators may not have sufficient capital resources; |
| • | collaborators may pursue tests or other products that will not generate significant volume for us, but may consume significant research and development and manufacturing resources; and |
| • | we may not be able to negotiate future collaborative arrangements, or renewals of existing collaborative agreements, on acceptable terms. |
Because these and other factors may be beyond our control, the development or commercialization of these products may be delayed or otherwise adversely affected.
If we or any of our collaborative partners terminate a collaborative arrangement, we may be required to devote additional resources to product development and commercialization or we may need to cancel some development programs, which could adversely affect our product pipeline and business.
20
Table of Contents
We enter into collaborations with third parties that may not result in the development of commercially viable products or the generation of significant future revenues.
In the ordinary course of our business, we enter into collaborative arrangements to develop new products or to pursue new markets. These collaborations may not result in the development of products that achieve commercial success, and these collaborations could be terminated prior to developing any products. In addition, our collaboration partners may not necessarily purchase the volume of products that we expect. Accordingly, we cannot be assured that any of our collaborations will result in the successful development of a commercially viable product or result in significant additional future revenues in the future.
Compliance with regulations governing public company corporate governance and reporting is complex and expensive.
Many laws and regulations, notably those adopted in connection with the Sarbanes-Oxley Act of 2002 by the SEC and the NASDAQ Global Market, impose obligations on public companies, such as ours, which have increased the scope, complexity, and cost of corporate governance, reporting, and disclosure practices. Compliance with these reforms and enhanced new disclosures has required and will continue to require substantial management time and oversight and requires us to incur significant additional accounting and legal costs. Furthermore, on July 21, 2010, President Obama signed into law the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010. While this new law is primarily focused on bank reform and consumer protection, it also makes significant changes to corporate governance and executive compensation rules for public companies across all industries. The effects of this new law remain unclear and will likely require substantial management time and oversight and require us to incur significant additional accounting and legal costs. Additionally, changes to existing accounting rules or standards, such as the potential requirement that U.S. registrants prepare financial statements in accordance with International Financial Reporting Standards, may adversely impact our reported financial results and business, and may further require us to incur greater accounting fees.
Our operating results could be materially affected by unanticipated changes in our tax provisions or exposure to additional income tax liabilities.
Our determination of our tax liability (like any company’s determination of its tax liability) is subject to review by applicable tax authorities. Any adverse outcome of such a review could have an adverse effect on our operating results and financial condition. In addition, the determination of our provision for income taxes and other tax liabilities requires significant judgment including our determination of whether a valuation allowance against deferred tax assets is required. Although we believe our estimates and judgments are reasonable, the ultimate tax outcome may differ from the amounts recorded in our financial statements and may materially affect our financial results in the period or periods for which such determination is made.
Our stock price is highly volatile and investing in our stock involves a high degree of risk, which could result in substantial losses for investors.
The market price of our common stock, like the securities of many other medical products companies, fluctuates over a wide range, and will continue to be highly volatile in the future. During 2010, the closing sale price of our common stock on the NASDAQ Global Market ranged from $12.48 to $23.70 per share. Because our stock price has been volatile, investing in our common stock is risky. Furthermore, volatility in the stock price of other companies has often led to securities class action litigation against those companies. Any future securities litigation against us could result in substantial costs and divert management’s attention and resources, which could seriously harm our business, financial condition and results of operations.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
In Sunnyvale, California, the base for our manufacturing, product support and research and development efforts, we lease approximately 76,000 square feet of office and laboratory space pursuant to a lease that expires in March 2015, approximately 27,000 square feet of office space pursuant to a lease that expires in July 2012 and approximately 25,000 square feet of office and manufacturing space pursuant to a sublease that expires in October 2014. We also sublease approximately 44,000 square feet in Sunnyvale to support warehousing and distribution efforts pursuant to a sublease that expires in September 2015. In Bothell, Washington we sublease approximately 16,000 square feet of laboratory space for advanced chemistry research and development pursuant to a sublease that expires in August 2012. In addition, we lease cold storage space in Sunnyvale, California and office space in Illinois and Washington, D.C.
Outside of Toulouse, France we own an approximately 32,000 square-foot building. In Bromma, Sweden we lease approximately 45,000 square feet of office and manufacturing space pursuant to a lease that expires in July 2011 and approximately 2,000 square feet of office space pursuant to a lease that expires in September 2011. In April 2010, we began the planned multi-year, phased occupancy of certain portions of our 129,000 square foot leased office and manufacturing facility in Solna, Sweden that expires in December 2020. In addition, we lease office space in Derby and Buckinghamshire, UK.
21
Table of Contents
During early 2011, we anticipate moving into an additional approximately 29,000 square feet of leased office and laboratory space in Sunnyvale and approximately 1,000 square feet of leased office space in Shanghai, China. We believe we will be able to obtain additional facilities space on commercially reasonable terms, if and when they are required.
The Company believes its existing facilities and equipment are in good operating condition and are suitable for the conduct of its business.
On June 28, 2010, Abaxis, Inc. filed suit in U.S. District Court for the Northern District of California against us, alleging that our Xpert MRSA product infringes U.S. Patent No. 5,413,732, U.S. Patent No. 5,624,597, U.S. Patent No. 5,776,563 and U.S. Patent No. 6,251,684. On July 12, 2010, we filed our response to the suit, denying Abaxis’ allegations of infringement and asked the Court to find Abaxis’ patents invalid and not infringed. On August 5, 2010, Abaxis filed its response to our answer and counterclaim. On November 19, 2010, Abaxis filed an amended complaint in which it added allegations that we breached a licensing contract for the above-referenced patents. On December 17, 2010, we answered Abaxis’ amended complaint, denying that we were in breach of the licensing contract and further amended our counterclaims against Abaxis. On January 14, 2011, Abaxis filed a motion to dismiss certain of our defenses and counterclaims. We believe that the possibility that this legal proceeding will result in a material adverse effect on our business is remote.
On January 10, 2011, Troll Busters LLC filed suit in United States District Court for the Southern District of California against us and twelve other named defendants alleging that Cepheid falsely marked its Omnimix and 3-Agent Biothreat Assay products with expired patents owned by Roche Molecular Systems relating to certain PCR processes. Our initial deadline for answering this complaint is April 11, 2011. We believe that the possibility that this legal proceeding will result in a material adverse effect on our business is remote.
We may be subject to additional various claims, complaints and legal actions that arise from time to time in the normal course of business. Other than as described above, we do not believe we are party to any currently pending legal proceedings that will result in a material adverse effect on our business. There can be no assurance that existing or future legal proceedings arising in the ordinary course of business or otherwise will not have a material adverse effect on our business, consolidated financial position, results of operations or cash flows.
ITEM 4. (REMOVED AND RESERVED)
Not Applicable.
22
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF THE EQUITY SECURITIES |
PRICE RANGE OF COMMON STOCK
Our common stock trades on the NASDAQ Global Select Market under the symbol CPHD. The high and low sale prices for our common stock for each quarter of our two most recent fiscal years, as reported on the NASDAQ Global Select Market, were as follows:
| High | Low | |||||||
| Fiscal year ended December 31, 2010 |
||||||||
| First Quarter |
$ | 19.56 | $ | 12.38 | ||||
| Second Quarter |
21.02 | 15.94 | ||||||
| Third Quarter |
19.81 | 13.81 | ||||||
| Fourth Quarter |
23.99 | 17.45 | ||||||
| Fiscal year ended December 31, 2009 |
||||||||
| First Quarter |
$ | 10.81 | $ | 4.93 | ||||
| Second Quarter |
11.05 | 6.23 | ||||||
| Third Quarter |
15.00 | 8.26 | ||||||
| Fourth Quarter |
15.98 | 11.93 | ||||||
On February 7, 2011, the last reported sale price of our common stock on the NASDAQ Global Select Market was $ 24.70 per share. On February 7, 2011, there were approximately 131 holders of record of our common stock. The actual number of shareholders is greater than the number of record holders, and includes shareholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. The number of holders of record also does not include shareholders whose shares may be held in trust by other entities.
We have never declared or paid any cash dividends on our capital stock. Under our SVB Loan Agreement, if we were to pay dividends, it must be in the form of common stock. We currently intend to retain future earnings, if any, for development of our business and, therefore, do not anticipate that we will declare or pay cash dividends on our capital stock in the foreseeable future.
EQUITY COMPENSATION PLAN INFORMATION
The following table summarizes information about our equity compensation plans as of December 31, 2010. All outstanding awards relate to our common stock.
| Plan Category |
Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuances under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders (1)(2)(3) |
9,314,200 | $ | 12.99 | 3,280,951 | ||||||||
| Equity compensation plans not approved by security holders |
200,000 | $ | 14.93 | — | ||||||||
| Total |
9,514,200 | $ | 13.03 | 3,280,951 | ||||||||
| (1) | The number of securities remaining available for future issuance in column (c) includes 1,081,203 shares of common stock authorized and available for issuance under our Employee Stock Purchase Plan (“ESPP”). The number of shares authorized for issuance under the ESPP is subject to an annual increase equal to the lesser of (i) 200,000 shares, (ii) 0.75% of the outstanding shares on the date of the annual increase or (iii) an amount determined by the Board of Directors. The number of securities to be issued to participants in column (a) does not include shares of common stock to be issued to participants in consideration of aggregate participant contributions under the ESPP as of December 31, 2010. |
23
Table of Contents
| (2) | We issue securities under our 2006 Equity Incentive Plan (“2006 Plan”) in forms other than options, warrants or rights. We may issue stock awards, including but not limited to restricted stock awards, restricted stock units, stock bonus awards, stock appreciation rights and performance share awards. Under the 2006 Plan, non-employee directors are automatically granted options to purchase 18,750 shares of common stock and restricted stock units covering 2,100 shares of common stock upon initial election or appointment to the Board. On the date of the first Board meeting following each annual shareholder meeting each non-employee director then in office for longer than six months will automatically be granted options to purchase 9,375 shares of common stock and restricted stock units covering 1,050 shares of common stock. The Board may also make discretionary grants to purchase common stock to any non-employee director. Under the terms of our 2006 Plan, each award other than a stock option or stock appreciation right will reduce the number of shares remaining available for future issuance in column (c) by 1.75 shares for each share subject to such award. |
| (3) | We have made awards of restricted stock under our 2006 Plan in forms which do not require a payment by the recipient to us at the time of exercise or vesting. Accordingly, the weighted average exercise price in column (b) does not take these awards into consideration. |
STOCK PRICE PERFORMANCE GRAPH
The graph below matches Cepheid’s cumulative 5-year total shareholder return on common stock with the cumulative total returns of the NASDAQ Composite index and the NASDAQ Biotechnology index. The graph tracks the performance of a $100 investment in our common stock and in each of the indexes (with the reinvestment of all dividends) from 12/31/2005 to 12/31/2010.
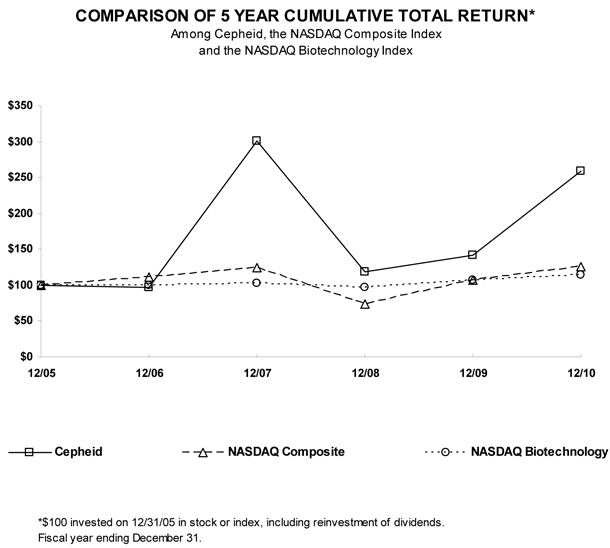
24
Table of Contents
| 12/05 | 12/06 | 12/07 | 12/08 | 12/09 | 12/10 | |||||||||||||||||||
| Cepheid |
100.00 | 96.81 | 300.11 | 118.22 | 142.14 | 259.11 | ||||||||||||||||||
| NASDAQ Composite |
100.00 | 111.74 | 124.67 | 73.77 | 107.12 | 125.93 | ||||||||||||||||||
| NASDAQ Biotechnology |
100.00 | 99.71 | 103.09 | 96.34 | 106.49 | 114.80 | ||||||||||||||||||
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data have been derived from our audited consolidated financial statements. The information below is not necessarily indicative of the results of future operations, and should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of this Form 10-K and the consolidated financial statements and related notes thereto included in Item 8 of this Form 10-K in order to fully understand factors that may affect the comparability of the information presented below.
| Years Ended December 31, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Product sales |
$ | 206,876 | $ | 165,185 | $ | 159,383 | $ | 116,532 | $ | 82,403 | ||||||||||
| Other revenues |
5,592 | 5,442 | 10,244 | 12,941 | 4,949 | |||||||||||||||
| Total revenues |
212,468 | 170,627 | 169,627 | 129,473 | 87,352 | |||||||||||||||
| Cost of product sales |
105,135 | 95,542 | 89,714 | 69,851 | 49,519 | |||||||||||||||
| Gross profit on product sales |
107,333 | 75,085 | 79,913 | 59,622 | 37,833 | |||||||||||||||
| Loss from operations |
(5,344 | ) | (23,366 | ) | (23,650 | ) | (25,164 | ) | (30,986 | ) | ||||||||||
| Net loss |
(5,917 | ) | (22,502 | ) | (22,387 | ) | (22,100 | ) | (26,704 | ) | ||||||||||
| Basic and diluted net loss per common share |
$ | (0.10 | ) | $ | (0.39 | ) | $ | (0.39 | ) | $ | (0.40 | ) | $ | (0.51 | ) | |||||
| Shares used in computing basic and diluted net loss per share |
59,712 | 58,206 | 57,101 | 55,263 | 52,325 | |||||||||||||||
| December 31, | ||||||||||||||||||||
| 2010 | 2009 | 2008 | 2007 | 2006 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents, investments and put option |
$ | 79,538 | $ | 60,717 | $ | 48,017 | $ | 44,026 | $ | 94,936 | ||||||||||
| Working capital |
95,565 | 60,852 | 32,211 | 56,109 | 90,362 | |||||||||||||||
| Total assets |
220,611 | 198,126 | 183,979 | 162,778 | 165,871 | |||||||||||||||
| Short-term obligations: |
||||||||||||||||||||
| Bank borrowing |
— | 14,618 | 14,639 | — | — | |||||||||||||||
| Current portion of note payable |
1,679 | 108 | — | — | — | |||||||||||||||
| Current portion of deferred revenue |
8,207 | 2,923 | 2,834 | 3,728 | 3,913 | |||||||||||||||
| Long-term obligations: |
||||||||||||||||||||
| Note payable, less current portion |
4,991 | 732 | — | 2 | 44 | |||||||||||||||
| Deferred revenue, less current portion |
4,057 | 2,279 | 1,753 | 2,054 | 2,663 | |||||||||||||||
| Accumulated deficit |
(208,182 | ) | (202,265 | ) | (179,763 | ) | (157,376 | ) | (135,276 | ) | ||||||||||
| Total shareholders’ equity |
153,662 | 127,566 | 128,824 | 124,468 | 130,916 | |||||||||||||||
25
Table of Contents
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 that relate to future events or our future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “estimate”, “intend”, “potential,” “project” or “continue” or the negative of these terms or other comparable terminology. Forward-looking statements are based upon current expectations that involve risks and uncertainties. Our actual results and the timing of events could differ materially from those anticipated in our forward-looking statements as a result of many factors, including, but not limited to, the following: changes in the protocols, best practices or level of testing for HAI; testing volume for our other products; development and manufacturing problems; the need for additional intellectual property licenses for new tests and other products and the terms of such licenses; the environment for capital spending by hospitals and other customers for our diagnostic instruments; our ability to successfully sell additional products in the Clinical market; lengthy sales cycles in certain markets; the performance and market acceptance of our new products; our ability to obtain regulatory approvals and introduce new products into the Clinical market; our ability to respond to changing laws and regulations affecting our industry and changing enforcement practices related thereto; the level of testing at existing clinical customer sites; the mix of products sold, which can affect gross margins; our reliance on distributors to market, sell and support our products in certain geographic locations; the occurrence of unforeseen expenditures, asset impairments, acquisitions or other transactions; litigation costs; our ability to integrate the businesses, technologies, operations and personnel of acquired companies; our success in increasing our direct sales and the effectiveness of our sales personnel; the impact of competitive products and pricing; our ability to manage geographically-dispersed operations; our ability to continue to realize manufacturing efficiencies, which are an important factor in improving gross margins; underlying market conditions worldwide; the scope and timing of actual USPS funding of the BDS in its current configuration; the rate of environmental testing using the BDS conducted by the USPS, which will affect the amount of consumable products sold; and the other risks set forth under “Risk Factors” and elsewhere in this report. We assume no obligation to update any of the forward-looking statements after the date of this report or to conform these forward-looking statements to actual results.
STRATEGY
We are a broad-based molecular diagnostics company that develops, manufactures, and markets fully-integrated systems for testing in the Clinical market, as well as for application in our legacy Biothreat, Industrial and Partner markets. Our systems enable rapid, sophisticated molecular testing for organisms and genetic-based diseases by automating otherwise complex manual laboratory procedures. Our strategy is to become the leading supplier of integrated systems and tests for molecular diagnostics. Key elements of our strategy to achieve this objective include:
| • | Provide a fully-integrated molecular testing solution to the Clinical market. We believe our GeneXpert system will continue to significantly expand our presence in the Clinical market due to its ease of use, flexibility, and ability to deliver rapid and accurate results. We believe this system is currently the only commercially available closed, self-contained, fully-integrated and automated system for molecular testing. Features of the GeneXpert system and Xpert tests include: |
| • | an approach by which the reagents are typically prepackaged in a single vessel (the test cartridge) into which the specimen is added; |
| • | no further user intervention once the Xpert cartridge is loaded into the GeneXpert system; |
| • | all three phases of PCR, (1) sample preparation, (2) amplification and (3) detection, are performed within the single sealed test cartridge automatically; |
| • | moderate complexity CLIA categorized, amplified molecular tests, which means the GeneXpert system can be operated without the need for highly-trained laboratory technicians; |
| • | commercial availability in a variety of configurations ranging from one to 48 individual test modules, which enables testing in environments ranging from low volume, near-patient testing to high volume, core or central lab testing, and system capacity that can be expanded in support of growing test volumes by adding additional modules; and |
| • | notably, to our knowledge, the only real-time PCR system that operates entirely within a closed system architecture, reducing hands-on time, reducing the likelihood of human error and contamination, and enabling nested PCR capability, a proven process for maximizing real-time PCR sensitivity. |
We also believe that the GeneXpert is the only currently available system that enables molecular testing in any workflow environment: full random access; on-demand; or traditional batch testing. With full random access, different tests for different targets may be run simultaneously in different cartridges in the same GeneXpert instrument. Additional tests may be added by the user at any time. This increases potential utilization and throughput of the instrument and also enables on-demand or “stat” testing, whereby the user can add a new test to the instrument at any time without regard to the stage of processing of any other test on the instrument.
26
Table of Contents
| • | Continue to develop and market new tests. We plan to capitalize on our strengths in nucleic acid chemistry and molecular biology to continue to develop new tests for our systems. In addition, in order to more rapidly expand our test pipeline, we work with strategic partners and major academic institutions and commercial organizations to develop and validate additional tests. |
| • | Obtain additional target rights. We expect to continue to expand our collaborations with academic institutions and commercial organizations to develop and obtain target rights to various infectious disease and cancer targets. In addition, we will be focusing key business development activities on identifying infectious disease and cancer targets held by academic institutions or commercial organizations for potential license or acquisition. |
| • | Extend geographic reach. Internationally, our primary focus is the European Clinical market. However, we also have and will continue to develop programs for other international markets. Our European sales and marketing operations are headquartered in France. We have direct sales forces in the UK, France and the Benelux region. We continue to sell through distributors in other international markets. We will continue to expand our distribution capability in Europe on both a direct and distributor basis. We also have and will continue to develop programs for other international markets, including selling through distributors. |
| • | High Burden Developing Countries sale programs. We are developing and expect to continue to expand our presence in HBDCs following the World Health Organization’s endorsement of the Xpert MTB/RIF test in late 2010. In addition, in continued collaboration with FIND, we expect to broaden the menu of tests available to HBDC customers at special pricing considerations. |
| • | Continue to maintain applications in the Industrial and Biothreat markets. We currently sell products into our legacy Industrial and Biothreat markets and expect to continue our offerings in these markets. |
CRITICAL ACCOUNTING POLICIES, ESTIMATES AND ASSUMPTIONS
We consider our accounting policies related to revenue recognition, impairment of intangible assets and goodwill, inventory valuation, warranty accrual, stock-based compensation and income taxes to be critical accounting policies. A number of significant estimates, assumptions, and judgments are inherent in our determination of when to recognize revenue, how to evaluate our intangible assets and goodwill, the calculation of our inventory valuation adjustments, warranty accrual, and stock-based compensation expense. These estimates, assumptions and judgments include deciding whether the elements required to recognize revenue from a particular arrangement are present, estimating the fair value of an intangible asset, which represents the future undiscounted cash flows to be derived from the intangible asset, estimating the amount of inventory obsolescence and warranty costs associated with shipped products and estimating the useful life and volatility of stock awards. We base our estimates and judgments on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results could differ materially from these estimates.
Revenue Recognition
We recognize revenue from the sale of our products and contract arrangements. Our revenue arrangements with multiple elements are divided into separate units of accounting if certain criteria are met, including whether the delivered element has stand-alone value to the customer and whether there is objective and reliable evidence of the fair value of the undelivered items. The consideration we receive is allocated among the separate units based on their respective fair values, and the applicable revenue recognition criteria are applied to each of the separate units. Advance payments received in excess of amounts earned are classified as deferred revenue until earned.
Determining whether the criteria for recognizing revenue have been met, including, for example, determining whether there is sufficient evidence that an arrangement exists, the collectibility of billings are reasonably assured and whether contractual performance obligations and milestones have been satisfied, requires us to make estimates, assumptions and judgments that affect our operating results. For example, our determination of the probability of collection is based upon assessment of the customer’s financial condition through review of their current financial statements or publicly-available credit reports, as well as approvals from government agencies and availability of budgets. For sales to existing customers, prior payment history is also considered in assessing probability of collection. We are required to exercise significant judgment in deciding whether collectibility is reasonably assured, and such judgments may materially affect the timing of our revenues and our results of operations.
27
Table of Contents
Product sales. We recognize revenue from product sales when goods are shipped, there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable and collectibility is reasonably assured. No right of return exists for our products except in the case of damaged goods. We have not experienced any significant returns of our products.
Contract revenues. Contract revenues consist of fees earned under technology license arrangements, services rendered under research and development arrangements, grants and government sponsored research agreements, and milestone payments and royalties received under license and collaboration agreements. Deferred revenue is recorded when funds are received in advance of technologies to be delivered or services to be performed.
License revenue is generally recognized only after both the license period has commenced and the technology has been delivered. However, in multiple-element revenue arrangements, if the delivered technology does not have stand-alone value or if we do not have objective and reliable evidence of the fair value of the undelivered products or services, the amount of revenue allocable to the delivered technology is deferred and amortized over the related involvement period in which the remaining products or services are provided to the customer.
Research and development and government sponsored research contract revenues are recognized as the related services are performed based on the performance requirements of the relevant contract. Under the agreements, we are required to perform specific research and development activities and are compensated either based on the costs or costs plus a mark-up associated with each specific contract over the term of the agreement.
Incentive milestone payments are recognized as revenue upon the achievement of the specified milestone, assuming there are no continuing performance obligations related to that milestone. Incentive milestone payments are substantially at risk at the inception of the arrangement and are normally triggered by events external to Cepheid.
Royalties are typically based on licensees’ net sales of products that utilize our technology and are recognized as earned in accordance with the contract terms when royalties from licensees can be reliably measured and collectibility is reasonably assured, such as upon the receipt of a royalty statement from the customer.
Service revenue is recognized when the services have been provided or for service contracts, ratably over the term of the contract.
Realizability of Long-Lived Assets
Our intangible assets consist primarily of rights to certain patented technologies that we purchased. Intangible assets are recorded at cost, less accumulated amortization. Intangible assets are amortized over their estimated useful lives, ranging from 5 to 20 years, on a straight-line basis except for intangible assets acquired in an acquisition, which are amortized on the basis of economic useful life. Amortization of intangible assets is primarily included in cost of product sales in the consolidated statements of operations.
We review our intangible assets for impairment and conduct an impairment review when events or circumstances indicate the carrying value of a long-lived asset may be impaired, by estimating the future undiscounted cash flows to be derived from an asset to assess whether or not a potential impairment exists. If the carrying value exceeds our estimate of future undiscounted cash flows, we then calculate the impairment as the excess of the carrying value of the asset over our estimate of its fair market value. Events or circumstances which could trigger an impairment review include a significant adverse change in business climate, an adverse action or assessment by a regulator, unanticipated competition, significant changes in the manner of our use of acquired assets, the strategy for our overall business, or significant negative industry or economic trends. There is significant judgment in estimating future cash flows and fair value. In 2010, we recorded an impairment charge of $0.3 million related to a license termination. No impairment charge was recorded in 2009. In 2008, we recorded an impairment charge of $0.4 million related to certain licenses with no future use.
As of December 31, 2010, our carrying value of goodwill was $18.6 million. We annually review our goodwill for impairment. If our fair value exceeds our net book value including goodwill, then goodwill is not considered impaired. The initial step is to compare our fair value as determined by our market capitalization to our net book value. If the market capitalization exceeds the net book value, goodwill is presumed to be unimpaired. Otherwise, we would estimate expected future cash flows of our business, which operates in a number of markets and geographical regions. We would then determine the carrying value of our business and compare the carrying value including goodwill and other intangibles to the discounted future cash flows. If the total of future cash flows is less than the carrying amount of the assets, we would recognize an impairment loss based on the excess of the carrying amount over the fair value of the assets. Estimates of the future cash flows associated with the assets are critical to these assessments. Changes in these estimates based on changed economic conditions or business strategies could result in material impairment charges in future periods. At December 31, 2010, we determined that goodwill was not impaired.
28
Table of Contents
Inventory and Warranty Provisions
We maintain provisions for inventory obsolescence and warranty costs that we believe are reasonable and that are based on our historical experience and current expectations for future performance. The inventory provision is established using management’s estimate of the potential future obsolescence or excess inventory. A substantial decrease in demand for our products or the introduction of new products could lead to excess inventories and could require us to increase our provision for inventory obsolescence. Our current estimates and assumptions are consistent with prior periods. In the past, there have not been significant adjustments of the actual results to our estimates.
We warrant our systems to be free from defects for a period of generally 12 to 15 months from the date of sale and our disposable products to be free from defects, when handled according to product specifications, for the stated life of such products. Accordingly, a provision for the estimated cost of warranty repair or replacement is recorded at the time revenue is recognized. Our warranty provision is established using management’s estimate of future failure rates and of the future costs of repairing any system failures during the warranty period or replacing any disposable products with defects. Significant increases in the failure rates of our products could lead to increased warranty costs and require us to increase our warranty provision. As of December 31, 2010 and 2009, the accrued warranty liability was $1.0 million and $0.7 million, respectively.
Stock-Based Compensation
We account for stock-based compensation in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718-25. Under the fair value recognition provisions of this statement, stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period, which is generally the vesting period. ASC 718-25 requires companies to estimate the fair value of stock-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in our consolidated statements of operations. We recognize the fair value of our stock option awards as compensation expense over the requisite service period of each award, which is generally four years.
In determining fair value of the stock-based compensation payments, we use the Black–Scholes model and a single option award approach, which requires the input of subjective assumptions. These assumptions include: estimating the length of time employees will retain their vested stock options before exercising them (expected term), the estimated volatility of our common stock price over the expected term (volatility), risk-free interest rate and the number of shares subject to options that will ultimately not complete their vesting requirements (forfeitures). Changes in the following assumptions can materially affect the estimate of fair value of stock–based compensation.
| • | Expected term is determined based on historical experience, giving consideration to the contractual terms of the stock-based awards, vesting schedules and expectations of future employee behavior as influenced by changes to the terms of its stock-based awards. |
| • | Expected volatility is based on the historical volatility for the past 5 years, which approximates the expected term of the option grant. |
| • | Risk-free interest rate is based on the implied yield currently available on U.S. Treasury zero-coupon issues with a remaining term equivalent to the expected term of a stock award. |
| • | Estimated forfeitures are based on voluntary termination behavior as well as analysis of actual option forfeitures. |
Income Taxes
We account for income taxes using an asset and liability approach, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our consolidated financial statements, but have not been reflected in our taxable income. A valuation allowance is established to reduce deferred tax assets to their estimated realizable value. Therefore, we provide a valuation allowance to the extent that we do not believe it is more likely than not that it will generate sufficient taxable income in future periods to realize the benefit of its deferred tax assets.
29
Table of Contents
We recognize interest and penalties related to uncertain tax positions in income tax expense. For the year ended December 31, 2010, 2009 and 2008, we did not recognize significant interest or penalties related to uncertain tax positions in the consolidated statements of operations, and at December 31, 2010 and 2009, the we had no significant accrued interest or penalties.
Recent Accounting Pronouncements
For recent accounting pronouncements, see Note 1, “Organization and Summary of Significant Accounting Policies” to the consolidated financial statements appearing in Item 8 to this annual report, which are incorporated by reference into this Item 7.
Results of Operations
Comparison of Years Ended December 31, 2010 and 2009
Revenues
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Revenues: |
||||||||||||
| System sales |
$ | 46,416 | $ | 42,993 | 8 | % | ||||||
| Reagent and disposable sales |
160,460 | 122,192 | 31 | % | ||||||||
| Total product sales |
206,876 | 165,185 | 25 | % | ||||||||
| Other revenues |
5,592 | 5,442 | 3 | % | ||||||||
| Total Revenues |
$ | 212,468 | $ | 170,627 | 25 | % | ||||||
Product Sales
The following table illustrates product sales in the Clinical, Industrial, Biothreat and Partner markets:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Product sales by market: |
||||||||||||
| Clinical Systems |
$ | 31,266 | $ | 26,241 | 19 | % | ||||||
| Clinical Reagents |
131,254 | 89,701 | 46 | % | ||||||||
| Total Clinical |
162,520 | 115,942 | 40 | % | ||||||||
| Industrial |
18,117 | 19,165 | (5 | )% | ||||||||
| Biothreat |
22,206 | 24,762 | (10 | )% | ||||||||
| Partner |
4,033 | 5,316 | (24 | )% | ||||||||
| Total product sales |
$ | 206,876 | $ | 165,185 | 25 | % | ||||||
Total product sales were $206.9 million in 2010, an increase of 25% from $165.2 million in 2009. The increase was due to an increase in our Clinical sales of $46.6 million, or 40%. Clinical Reagents grew $41.6 million, or 46%, driven primarily by an increase in sales of our HAI portfolio of tests as well as our Xpert MTB/RIF test. Clinical Systems grew $5.0 million, or 19%, primarily due to increased GeneXpert System sales. Industrial sales decreased $1.0 million, or 5%, primarily due to both decreased reagent and systems sales. In the Biothreat market, product sales decreased $2.6 million, or 10%, primarily due to reduced anthrax test cartridge sales to Northrop Grumman/USPS. Partner sales decreased $1.3 million, or 24%, due to an overall decline in this legacy business.
We expect our Clinical product sales to continue to increase in 2011 with the continued expansion of the HAI and infectious disease markets, the breadth of our test menu, and the growth of our installed base of customers. We expect both Industrial and Partner sales will decline in 2011 compared to 2010 due to the overall decline in these legacy businesses. For our Biothreat business, we expect a decline in sales as we expect contract revisions to reduce the annual number of tests from about one million under the previous contract, to about six hundred thousand in 2011, partially offset by an increase in test price.
30
Table of Contents
The following table provides a breakdown of our product sales by geographic regions:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Product Sales Geographic Information: |
||||||||||||
| Clinical |
$ | 127,326 | $ | 88,562 | 44 | % | ||||||
| Other |
37,328 | 40,227 | (7 | )% | ||||||||
| Total North America |
$ | 164,654 | $ | 128,789 | 28 | % | ||||||
| Clinical |
$ | 35,194 | $ | 27,380 | 29 | % | ||||||
| Other |
7,028 | 9,016 | (22 | )% | ||||||||
| Total International |
$ | 42,222 | $ | 36,396 | 16 | % | ||||||
| Total product sales |
$ | 206,876 | $ | 165,185 | 25 | % | ||||||
Product sales in North America increased $35.9 million, or 28%, from $128.8 million in 2009 to $164.7 million in 2010. The increase in North American product sales was primarily driven by a $38.8 million, or 44%, increase in Clinical sales, driven by increases in both instrument and reagent sales. The increase in Clinical sales was partially offset by a $2.9 million, or 7%, decrease in Other product sales, due to a decrease in anthrax test cartridge sales to Northrop Grumman/USPS in the Biothreat market.
International product sales, which primarily represent sales in Europe, increased $5.8 million, or 16%, from $36.4 million in 2009 to $42.2 million in 2010. International Clinical sales growth of $7.8 million, or 29% was primarily driven by higher Clinical Reagent sales due to an increase in sales of our HAI tests as well as Xpert MTB/RIF.
No single country outside of the U.S. represented more than 10% of our total revenues in any period presented and one customer accounted for 11% and 15% of our total product sales in 2010 and 2009, respectively.
Other Revenue
Other revenue remained flat in 2010 from 2009. We expect that our other revenue in 2011 will increase as we receive more funding for the research and development of our Xpert BCR-ABL collaboration with Novartis and HIV Viral Load test collaboration with FIND.
Costs and Operating Expenses
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Costs and operating expenses: |
||||||||||||
| Cost of product sales |
$ | 105,135 | $ | 95,542 | 10 | % | ||||||
| Collaboration profit sharing |
6,806 | 8,200 | (17 | )% | ||||||||
| Research and development |
42,503 | 39,313 | 8 | % | ||||||||
| Sales and marketing |
38,840 | 29,156 | 33 | % | ||||||||
| General and administrative |
24,528 | 21,278 | 15 | % | ||||||||
| Restructuring charge |
— | 747 | 100 | % | ||||||||
| Gain from legal settlement |
— | (243 | ) | (100 | )% | |||||||
| Total costs and operating expenses |
$ | 217,812 | $ | 193,993 | 12 | % | ||||||
Cost of Product Sales
Cost of product sales primarily consists of raw materials, direct labor and stock-based compensation expense, manufacturing overhead, facility costs and warranty costs. Cost of product sales also includes royalties on product sales and amortization of intangible assets related to technology licenses and intangibles acquired in the purchases of Sangtec and Stretton. Cost of product
31
Table of Contents
sales increased 10%, or $9.6 million, from $95.5 million in 2009 to $105.1 million in 2010, as a result of increased overall product sales. Our product margin percentage was 49% in 2010 compared to 42% in 2009. The increase in product gross margin in 2010 from 2009 was primarily due to favorable product mix with higher margin Clinical reagent sales comprising a higher percentage of our total product revenue, the phase out in August 2010 of royalty payments to Roche related to U.S. PCR sales, and a decrease in royalties to Abaxis, Inc. due to a manufacturing process change as well as Clinical reagent volume increases and manufacturing efficiencies driving lower Clinical reagent unit costs. We expect the product gross margin percentage to continue to increase in 2011 as we continue to benefit from the phase out of certain royalties, improve our manufacturing efficiencies and anticipate continuous favorable product mix with higher margin Clinical reagent sales comprising a higher percentage of our total product revenue.
Collaboration Profit Sharing
Collaboration profit sharing represents the amount that we pay to LIFE under our collaboration agreement to develop and manufacture reagents for use in the USPS BDS program. Under the agreement, computed gross margin on anthrax cartridge sales are shared equally between the two parties. Collaboration profit sharing expense was $6.8 million and $8.2 million in 2010 and 2009, respectively. The decrease in collaboration profit sharing was the result of decreased anthrax cartridge sales under the USPS BDS program. We expect our collaboration profit sharing expenses will decline in 2011 due to expected lower anthrax cartridge sales to the USPS.
Research and Development Expenses
Research and development expenses primarily consist of salaries and employee-related expenses, which include stock-based compensation, clinical trials, research and development materials, facility costs and depreciation. Research and development expenses increased 8% to $42.5 million in 2010 as compared to $39.3 million in 2009. The increase in research and development expenses of $3.2 million was primarily due to a $1.8 million increase of salaries and employee-related expenses and a $1.3 million increase in research and development supplies for product trials. We expect our research and development expenses will increase in 2011 due to an increase in our trial costs for products currently under development, as well as expected increases on partner-funded development projects.
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of salaries and employee-related expenses, which include commissions and stock-based compensation, travel, facility-related costs and marketing and promotion expenses. Sales and marketing expenses increased 33% to $38.8 million in 2010 from $29.2 million in 2009. The increase in sales and marketing expenses was primarily due to a $4.7 million increase of salaries and employee-related expenses, a $1.4 million increase of travel expenses, a $1.0 million increase of advertising expenses and a $0.7 million increase of consulting expenses. We expect our sales and marketing expenses will increase in 2011 as we continue to expand our efforts in the Clinical market, with particular emphasis on pursuing the market opportunities for our HAI and infectious disease products.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and employee-related expenses, which include stock-based compensation, travel, facility costs, legal, accounting and other professional fees. General and administrative expenses increased 15% to $24.5 million in 2010 from $21.3 million in 2009. The increase was primarily due to a $2.5 million increase in salaries and employee-related expenses and a $0.9 million increase in corporate legal expenses. We expect our general and administrative expenses to remain relatively flat as a percentage of revenues in 2011.
Restructuring Charge
During the first quarter of 2009, the Company eliminated 47 positions that impacted employees, contractors and replacement positions, which resulted in $0.7 million of restructuring expense, primarily related to severance. As of December 31, 2010 and 2009, the activities under this restructuring plan were complete and the Company had no outstanding restructuring expenses to be paid.
Gain from Legal Settlement
There was no gain from legal settlement in 2010. During the third quarter of 2009, the Company entered into a settlement with a vendor regarding certain issues under multiple agreements. Pursuant to the settlement, the vendor paid the Company $0.2 million, which the Company recorded as a gain.
32
Table of Contents
Other Income (Expense), Net
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Other income (expense), net: |
||||||||||||
| Interest income |
$ | 168 | $ | 406 | (59 | )% | ||||||
| Interest expense |
(340 | ) | (332 | ) | 2 | % | ||||||
| Foreign currency gain (loss) and other |
(820 | ) | 350 | 334 | % | |||||||
| Total other income (expense), net |
$ | (992 | ) | $ | 424 | (334 | )% | |||||
Other income (expense), net consists of interest income, interest expense and foreign currency exchange gain (loss) and other. Interest income decreased to $0.2 million in 2010 from $0.4 million in 2009. The decrease is primarily due to lower interest rates in 2010 as compared to 2009. The interest expense remained flat in 2010 from 2009. Foreign currency exchange gain (loss) and other decreased from a $0.4 million gain in 2009 to a $0.8 million loss in 2010 primarily due to a $0.7 million, net of foreign currency forward contracts gain of $0.8 million, increase in foreign currency exchange losses in 2010 due to the strengthening of the U.S. dollar against other currencies, primarily the Euro, a $0.7 million swing related to our auction rate securities, from a $0.6 million gain in 2009 to a $0.1 million loss in 2010, offset by a $0.2 million increase in other income.
Income Taxes
The income tax benefit of $0.4 million in 2010 is primarily comprised of $0.8 million of income tax benefit primarily related to a tax credit for research and development in France, as well as to the amortization of acquired intangibles in Sweden and UK, partially offset by $0.3 million of foreign tax expense and state income tax expense of $0.1 million. The income tax benefit of $0.4 million in 2009 represented U.S. federal income tax benefit of $0.2 million related to a refundable research and development (“R&D”) credit as provided by the Housing and Economic Recovery Act of 2008 (“Act”). The Act, signed into law in July 2008, allowed taxpayers to claim refundable alternative minimum tax or R&D credit carryovers if they had forgone bonus depreciation on certain qualified property and equipment placed in service beginning in April 2008. The income tax benefit in 2009 also represented $0.7 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden partially offset by $0.4 million of foreign tax expense and state income tax expense of $0.1 million. The income tax benefit of $0.9 million in 2008 represented current U.S. federal income tax benefit of $0.2 million related to a refundable R&D credit as provided by the Act. The income tax benefit in 2008 also represented $0.9 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden and a refundable R&D credit in France, partially offset by $0.1 million of state income tax expense.
As of December 31, 2010 and 2009, we had deferred tax assets of approximately $77.1 million and $81.6 million, respectively, which were offset by valuation allowances of $77.0 million and $81.6 million, respectively. We also had a deferred tax liability of $1.7 million as of December 31, 2010 in connection with the acquisition of the assets and licensed intellectual properties of Sangtec and Stretton, which we acquired in February 2007 and November 2008, respectively. As of December 31, 2010, we had net operating loss carryforwards for federal income tax purposes of approximately $154.2 million, which expire in the years 2011 through 2028, federal research and development tax credits of approximately $5.3 million, which expire in the years 2019 through 2030, and foreign tax credits of $0.1 million which expire in 2019. As of December 31, 2010, we had net operating loss carryforwards for state income tax purposes of approximately $69.7 million, which expire in the years 2014 through 2031, and state research and development tax credits of approximately $6.8 million, which have no expiration date.
Utilization of our net operating loss may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such annual limitation may result in the expiration of net operating losses before utilization.
The Company had no undistributed earnings from its foreign subsidiaries for which tax has not been provided as of December 31, 2010.
33
Table of Contents
Comparison of Years Ended December 31, 2009 and 2008
Revenues
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Revenues: |
||||||||||||
| System sales |
$ | 42,993 | $ | 51,766 | (17 | )% | ||||||
| Reagent and disposable sales |
122,192 | 107,617 | 14 | % | ||||||||
| Total product sales |
165,185 | 159,383 | 4 | % | ||||||||
| Other revenues |
5,442 | 10,244 | (47 | )% | ||||||||
| Total Revenues |
$ | 170,627 | $ | 169,627 | 1 | % | ||||||
Product Sales
The following table illustrates product sales in the Clinical, Industrial, Biothreat and Partner markets:
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Product sales by market: |
||||||||||||
| Clinical Systems |
$ | 26,241 | $ | 32,084 | (18 | )% | ||||||
| Clinical Reagents |
89,701 | 58,167 | 54 | % | ||||||||
| Total Clinical |
115,942 | 90,251 | 28 | % | ||||||||
| Industrial |
19,165 | 15,437 | 24 | % | ||||||||
| Biothreat |
24,762 | 35,797 | (31 | )% | ||||||||
| Partner |
5,316 | 17,898 | (70 | )% | ||||||||
| Total product sales |
$ | 165,185 | $ | 159,383 | 4 | % | ||||||
Total product sales were $165.2 million in 2009, an increase of 4% from $159.4 million in 2008. The increase was a result of an increase in our Clinical sales of $25.7 million, or 28%. Clinical Reagents grew $31.5 million, or 54%, driven primarily by an increase in sales of our HAI tests, offset by a $5.8 million, or 18%, decrease in Clinical Systems product sales, as Veterans Affairs (“VA”) hospital system placements substantially declined in 2009 compared to the strong placements in 2008 and as customers were constrained by the effect of the challenging economic environment on capital purchase decisions. Industrial sales increased $3.7 million, or 24%, due to an unanticipated purchase of approximately $2 million of SmartCyclers in Japan, funded by the Japanese government’s stimulus investment. The increase in product sales was offset by a decline in sales in our legacy Biothreat and Partner markets. The decrease of $12.6 million, or 70%, in Partner product sales was due to the expiration of our contract with BDC in the fourth quarter of 2008 and the cancellation of certain contracted purchases by Roche during 2008. In the Biothreat market, product sales decreased $11.0 million, or 31%, primarily due to reduced anthrax test cartridge sales to Northrop Grumman/USPS.
The following table provides a breakdown of our product sales by geographic regions:
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Product Sales Geographic Information: |
||||||||||||
| Clinical |
$ | 88,562 | $ | 70,248 | 26 | % | ||||||
| Other |
40,227 | 55,078 | (27 | )% | ||||||||
| Total North America |
$ | 128,789 | $ | 125,326 | 3 | % | ||||||
| Clinical |
$ | 27,380 | $ | 20,004 | 37 | % | ||||||
| Other |
9,016 | 14,053 | (36 | )% | ||||||||
| Total International |
$ | 36,396 | $ | 34,057 | 7 | % | ||||||
| Total product sales |
$ | 165,185 | $ | 159,383 | 4 | % | ||||||
34
Table of Contents
Product sales in North America increased $3.5 million, or 3%, from $125.3 million in 2008 to $128.8 million in 2009. The increase in North American product sales was primarily driven by an $18.3 million increase in Clinical sales, driven primarily by an increase of reagent sales partially offset by a decrease of systems sales. The increase was also largely offset by an $11.0 million decline in anthrax test cartridge sales to Northrop Grumman/USPS in the Biothreat market and a $5.1 million decrease in Partner sales due to the expiration of our contract with BDC in the fourth quarter of 2008.
International product sales, which primarily represent sales in Europe, increased $2.3 million, or 7%, from $34.1 million in 2008 to $36.4 million in 2009. International Clinical sales growth was primarily driven by higher Clinical Reagent sales due to an increase in sales of our HAI tests, offset by decreased Clinical System sales. The increase in international product sales was offset by a $7.5 million decrease in Partner sales due to the expiration of our contract with BDC in the fourth quarter of 2008 and the cancellation of certain contracted purchases by Roche during 2008.
No single country outside of the U.S. represented more than 10% of our total revenues in any period presented and one customer accounted for 15% and 23% of our total product sales in 2009 and 2008, respectively.
Other Revenue
Other revenue decreased 47% from $10.2 million in 2008 to $5.4 million in 2009. The decrease was primarily due to the completion of a program associated with the development of a 6-color GeneXpert system as well as the conclusion in the first quarter 2009 of the recognition of revenue related to the license fee received from bioMerieux.
Costs and Operating Expenses
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Costs and operating expenses: |
||||||||||||
| Cost of product sales |
$ | 95,542 | $ | 89,714 | 6 | % | ||||||
| Collaboration profit sharing |
8,200 | 11,089 | (26 | )% | ||||||||
| Research and development |
39,313 | 43,310 | (9 | )% | ||||||||
| Sales and marketing |
29,156 | 29,757 | (2 | )% | ||||||||
| General and administrative |
21,278 | 20,861 | 2 | % | ||||||||
| Restructuring charge |
747 | — | 100 | % | ||||||||
| Gain from legal settlement |
(243 | ) | (1,454 | ) | (83 | )% | ||||||
| Total costs and operating expenses |
$ | 193,993 | $ | 193,277 | 0 | % | ||||||
Cost of Product Sales
Cost of product sales consists of raw materials, direct labor and stock-based compensation expense, manufacturing overhead, facility costs and warranty costs. Cost of product sales also includes royalties on product sales and amortization of intangible assets related to technology licenses and intangibles acquired in the purchases of Sangtec and Stretton. Cost of product sales increased 6%, or $5.8 million, from $89.7 million in 2008 to $95.5 million in 2009, as a result of the increased overall product sales. Our product margin percentage was 42% in 2009 compared to 44% in 2008. The decrease in product gross margin in 2009 from 2008 was primarily due to an increase in license fee amortization expense from additional licenses, excess capacity in our European manufacturing facility that manufactures product for Roche, which cancelled certain contract purchases in 2008, and under-absorption of U.S. overhead due to decreased Clinical System sales volume.
Collaboration Profit Sharing
Collaboration profit sharing represents the amount that we pay to LIFE under our collaboration agreement to develop and manufacture reagents for use in the USPS BDS program. Under the agreement, computed gross margin on anthrax cartridge sales are shared equally between the two parties. Collaboration profit sharing expense was $8.2 million and $11.1 million in 2009 and 2008, respectively. The decrease in collaboration profit sharing was the result of decreased anthrax cartridge sales under the USPS BDS program.
35
Table of Contents
Research and Development Expenses
Research and development expenses consist of salaries and employee-related expenses, which include stock-based compensation, clinical trials, research and development materials, facility costs and depreciation. Research and development expenses decreased 9% to $39.3 million in 2009 as compared to $43.3 million in 2008. The decrease in research and development expenses of $4.0 million was primarily due to a $1.1 million decrease in clinical trial costs, a $0.8 million decrease in research and development supplies, a $0.5 million decrease in external services, a $0.3 million decrease in license fee write-offs and a $0.2 million decrease in travel expense.
Sales and Marketing Expenses
Sales and marketing expenses consist primarily of salaries and employee-related expenses, which include commissions and stock-based compensation, travel, facility-related costs and marketing and promotion expenses. Sales and marketing expenses decreased 2% to $29.2 million in 2009 from $29.8 million in 2008. The decrease in sales and marketing expenses was primarily due to a $0.6 million decrease in travel expense.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and employee-related expenses, which include stock-based compensation, travel, facility costs, legal, accounting and other professional fees. General and administrative expenses increased 2% to $21.3 million in 2009 from $20.9 million in 2008. The increase was primarily due to a $1.7 million increase in salaries and employee-related expenses, offset by a $1.1 million decrease of contractor costs as we brought certain tasks in-house.
Restructuring Charge
During the first quarter of 2009, the Company eliminated 47 positions that impacted employees, contractors and replacement positions, which resulted in $0.7 million of restructuring expense, primarily related to severance. As of December 31, 2010 and 2009, the activities under this restructuring plan were complete and the Company had no outstanding restructuring expenses to be paid.
Gain from Legal Settlement
During the third quarter of 2009, the Company entered into a settlement with a vendor regarding certain issues under multiple agreements. Pursuant to the settlement, the vendor paid the Company $0.2 million, which the Company recorded as a gain.
On December 25, 2008, the Company entered into a settlement agreement (the “Settlement Agreement”) with Roche Molecular Systems, Inc. (“RMS”) regarding the cancellation by RMS of outstanding purchase orders as well as future purchasing requirements under a previously negotiated supply agreement with the Company. Pursuant to the Settlement Agreement, RMS agreed to pay the Company $2.1 million as full and complete consideration for all cancelled orders and failure to meet purchasing requirements under the supply agreement. Approximately $0.7 million of the consideration was applied against certain inventory purchases that the Company had made in anticipation of building product to ship to Roche. The remaining $1.4 million was recorded as a gain in 2008, even though payment was not received until in January 2009.
Other Income (Expense), Net
| Years Ended December 31, | ||||||||||||
| 2009 | 2008 | % Change | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Other income (expense), net: |
||||||||||||
| Interest income |
$ | 406 | $ | 1,225 | (67 | )% | ||||||
| Interest expense |
(332 | ) | (13 | ) | 2,454 | % | ||||||
| Foreign currency gain (loss) and other |
350 | (860 | ) | 141 | % | |||||||
| Total other income (expense), net |
$ | 424 | $ | 352 | 20 | % | ||||||
Other income (expense), net consists of interest income, interest expense and foreign currency exchange gain (loss) and other. Interest income decreased to $0.4 million in 2009 from $1.2 million in 2008. The decrease was primarily due to lower interest rates in 2009 as compared to 2008. The interest expense increase of $0.3 million in 2009 compared to 2008 was primarily due to interest expense related to our UBS loan entered into during the fourth quarter of 2008. Foreign currency exchange gain (loss) and other went from a $0.9 million loss in 2008 to a $0.3 million gain in 2009 due to foreign currency exchange losses in 2008 prior to the implementation of our foreign currency hedge program, under which the Company utilizes forward contracts in order to reduce foreign currency exchange market risks.
36
Table of Contents
Income Taxes
The income tax benefit of $0.4 million in 2009 represented a U.S. federal income tax benefit of $0.2 million related to a refundable R&D credit as provided by the Act. The Company recognized the credit based on property and equipment placed into service throughout 2009. The income tax benefit in 2009 also represented $0.7 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden partially offset by $0.4 million of foreign tax expense and state income tax expense of $0.1 million. The income tax benefit of $0.9 million in 2008 represented current U.S. federal income tax benefit of $0.2 million related to a refundable R&D credit as provided by the Act. The income tax benefit in 2008 also represented $0.9 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden and a refundable R&D credit in France, partially offset by $0.1 million of state income tax expense.
LIQUIDITY AND CAPITAL RESOURCES
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Net cash provided by (used in) operating activities |
$ | 27,343 | $ | 13,676 | $ | (4,607 | ) | |||||
| Net cash provided by (used in) investing activities |
9,716 | (8,503 | ) | (15,219 | ) | |||||||
| Net cash provided by financing activities |
6,546 | 6,881 | 26,818 | |||||||||
As of December 31, 2010, we had $79.5 million in cash and cash equivalents. The $43.8 million increase in total cash and cash equivalents consisted primarily of $27.3 million provided by operating activities, $9.7 million provided by investing activities and $6.5 million provided by financing activities. We maintain our portfolio of cash equivalents in money market funds in order to minimize market risk and preserve principal.
Net cash provided by (used in) operating activities was $27.3 million, $13.7 million and $(4.6) million for the years ended December 31, 2010, 2009 and 2008, respectively. The net cash provided by (used in) operating activities was primarily comprised of net loss plus the net effect of non-cash expenses, working capital sources and capital uses of cash. For the year ended December 31, 2010, the primary working capital uses of cash were decreases in accounts payable and other liabilities, an increase in accounts receivable driven by higher revenue than in the prior year, and increases in prepaid expenses and other current assets. The primary working capital sources of cash were increases in deferred revenue and accrued compensation and a decrease in inventory. For the year ended December 31, 2009, the primary working capital sources of cash were increases in accounts payable, other current liabilities and deferred revenue, and decreases in prepaid expenses, other current assets and other non-current assets. The primary working capital uses of cash were increases in accounts receivable and inventory.
Net cash provided by (used in) investing activities was $9.7 million, $(8.5) million and $(15.2) million for the years ended December 31, 2010, 2009 and 2008, respectively. For the year ended December 31, 2010, net cash provided by investing activities consisted of proceeds from the sales and maturities of short-term investments related to the settlement of $24.8 million of our auction rate securities, partially offset by net capital expenditures, the cost of asset acquisitions and payments for technology licenses. For the year ended December 31, 2009, net cash used in investing activities consisted of net capital expenditures and payments for technology licenses partially offset by proceeds from sales and maturities of marketable securities and a transfer of restricted cash to unrestricted cash. The change in net cash provided by investing activities for 2010 from 2009 is primarily due to an increase in proceeds from the maturities of short-term investments offset by an increase in capital expenditures. In 2011, we expect our capital expenditures to be approximately 7% of revenue due to investment in the capacity and continued automation of our manufacturing operations to support the growth in our GeneXpert test revenue and expansion of our HBDC programs.
Net cash provided by financing activities was $6.5 million, $6.9 million and $26.8 million for the years ended December 31, 2010, 2009 and 2008, respectively. The increase in net proceeds from the issuance of common shares under our ESPP and exercises of stock options was partially offset by the increase of principal payment of bank borrowing, as we have paid down the principal balances on our UBS loan due to the settlement of our auction rate securities. The change from the year ended December 31, 2009 to the year ended December 31, 2010 was primarily due to the increase in principal payment of bank borrowing offset by the increase in net proceeds from the issuance of common shares under our ESPP and exercises of stock options and awards.
37
Table of Contents
On June 7, 2010, we entered into a loan and security agreement (the “Loan Agreement”) with SVB, which provided for a total revolving credit line of up to $15.0 million with a maturity date of June 7, 2012 and bearing interest no less than 4.0% with a commitment fee of 0.75% per annum on the unused portion. We had no outstanding balance under the revolving credit line at December 31, 2010.
On November 10, 2008, we accepted a comprehensive settlement arrangement offered by UBS, the fund manager with which we held our auction rate securities. Under the settlement, we had the option (“the put option”) to sell the auction rate securities held in our accounts with UBS to UBS at par value during the period beginning June 30, 2010 and ending July 2, 2012 (“put option exercise period”). Since the settlement agreement was a legally enforceable firm commitment, the put option was recognized as a financial asset at fair value in our financial statements, and accounted for separately from the associated securities. The fair value of the put option was based on the difference in value between the par value and the fair value of the associated auction rate securities. We measured the put option at its fair value, and subsequent changes in fair value also were recognized in respective period financial results. At December 31, 2009, our put option was recorded at fair value, at par for $3.3 million and we had classified these securities as trading. On June 30, 2010, we notified UBS of our intent to exercise our put option to sell our portfolio of auction rate securities, which were classified as trading securities and recorded at fair value, at par to UBS for $11.8 million. In addition, the rights permitted us to establish a demand revolving credit line, payable on demand, in an amount up to 75% of fair value of the securities at a net no cost, meaning that the interest that we paid on the credit line would not exceed the interest that we received on the auction rate securities that we had pledged as security for the credit line. We borrowed $14.7 million on the line of credit on November 10, 2008. On July 1, 2010, we paid down the outstanding loan balance of $1.3 million, such that there was no outstanding balance at December 31, 2010. We had an outstanding loan balance of $14.6 million, which approximated the carrying value, at December 31, 2009.
Contractual Obligations
As of December 31, 2010, our contractual obligations were as follows (in thousands):
| Payments Due by Period | ||||||||||||||||||||
| Total | Less Than 1 Year |
1-3 Years |
3-5 Years |
More Than 5 Years |
||||||||||||||||
| Operating leases |
$ | 42,875 | $ | 5,809 | $ | 11,539 | $ | 10,750 | $ | 14,777 | ||||||||||
| Notes payable |
6,670 | 1,679 | 3,380 | 1,411 | 200 | |||||||||||||||
| Purchase obligations |
16,464 | 11,564 | 4,900 | — | — | |||||||||||||||
| Minimum royalties |
4,387 | 561 | 1,194 | 1,202 | 1,430 | |||||||||||||||
| $ | 70,396 | $ | 19,613 | $ | 21,013 | $ | 13,363 | $ | 16,407 | |||||||||||
Purchase obligations include purchase orders or contracts for the purchase of raw materials and other goods and services. We do not have significant agreements for the purchase of raw materials or other goods specifying minimum quantities or set prices that exceed our expected requirements. Minimum royalty payments represent licensed royalties we are obligated to pay under our license agreements.
Rent expense for all operating leases for 2010, 2009 and 2008 was $4.1 million, $3.7 million and $3.4 million, respectively.
The expected timing of payment of the obligations discussed above is estimated based on current information. Timing of payments and actual amounts paid could vary in some circumstances depending on the timing of receipt of goods or services or changes to agreed-upon amounts for some obligations.
Off-Balance-Sheet Arrangements
As of December 31, 2010, we did not have any off-balance-sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K promulgated under the Securities Act of 1933.
Financial Condition Outlook
We plan to continue to make expenditures to expand our manufacturing capacity, to support our activities in sales and marketing and research and development and to support our working capital needs.
In the future, we may seek additional funds to support our strategic business needs and may seek to raise such additional funds through private or public sales of equity, debt or convertible securities, strategic relationships, bank debt, lease financing arrangements, or other available means. If additional funds are raised through the issuance of equity or equity-related securities, shareholders may experience additional dilution, or such equity securities may have rights, preferences, or privileges senior to those of the holders of our common stock. If adequate funds are not available or are not available on acceptable terms to meet our business needs, our business may be harmed.
38
Table of Contents
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS
Interest rate risk
The primary objective of our investment activities is to preserve principal while at the same time maximizing the income we receive from our investments without significantly increasing risk. Our investments in interest-bearing assets are subject to interest rate risk. This means that a change in prevailing interest rates may cause the fair value of the investment to fluctuate. For example, if we hold a security that was issued with a fixed interest rate at the then-prevailing rate and the prevailing interest rate later rises, the fair value of our investment will probably decline. To minimize this risk, we maintain our interest-bearing portfolio, which consists of cash and cash equivalents, in money market funds. Due to the short-term nature of the investments, we believe we currently have no material exposure to interest rate risk arising from our investments. Therefore we have not included quantitative tabular disclosure in this Form 10-K.
On June 7, 2010, we entered into the Loan Agreement with SVB. We had an outstanding balance of $5.5 million on the term line and no outstanding balance under the revolving credit line at December 31, 2010. If interest rates were to increase (decrease) by 100 basis points, the interest expense would have a corresponding increase (or decrease) of approximately less than $0.1 million.
Foreign currency risk
We operate primarily in the U.S. and a majority of our revenue, cost, expense and capital purchasing activities for 2010 were transacted in U.S. Dollars. As a corporation with international as well as domestic operations, we are exposed to changes in foreign exchange rates. We have operations in Sweden, France, Belgium, and the UK and we pay payroll and other expenses in the local currencies. In 2010, international sales were approximately 20% of our product sales. Our international sales are predominantly made in European countries. Our exposures to foreign currency risks may change over time and could have a material adverse impact on our financial results. We have implemented a foreign currency hedging program to mitigate our exposure to foreign currency gains and losses. As of December 31, 2010, we had two outstanding foreign exchange forward contracts, which were recorded as a derivative asset within prepaid expenses and other current assets with a fair value of approximately $4,000 and derivative liability within accrued and other liabilities with a fair value of approximately $92,000. As of December 31, 2009, we had two outstanding foreign exchange forward contracts, which were recorded as derivative liabilities within accrued and other liabilities, with a fair value of approximately $28,000 representing the estimated loss on those outstanding foreign currency exchange forward contracts. During 2010, the effect of our hedging transactions, consisting entirely of foreign currency forward contracts, on our consolidated statement of operations was a pre-tax gain of $0.8 million to mitigate our foreign currency fluctuations. We will continue to use hedging programs in the future and may use currency forward contracts, currency options, and/or other derivative financial instruments commonly utilized to reduce financial market risks if it is determined that such hedging activities are appropriate to reduce risk. A 10% change in the exchange rates upward or downward in our portfolio of foreign currency contracts would have decreased or increased, respectively our unrealized gain by approximately $1.1 million at December 31, 2010 and unrealized loss by approximately $0.8 million at December 31, 2009. We do not hold or purchase any currency contracts for trading purposes.
39
Table of Contents
ITEM 8. CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The following consolidated financial statements and the related notes thereto, of Cepheid and the Reports of Independent Registered Public Accounting Firm, Ernst and Young LLP, are filed as a part of this Form 10-K.
| Page | ||||
| Management’s Report on Internal Control Over Financial Reporting |
41 | |||
| 42 | ||||
| 44 | ||||
| 45 | ||||
| 46 | ||||
| 47 | ||||
| 48 | ||||
| 68 | ||||
40
Table of Contents
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of management, including the principal executive officer and principal financial officer, management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on management’s evaluation under the framework in Internal Control – Integrated Framework, management has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2010. Ernst & Young LLP, an independent registered public accounting firm, has audited the consolidated financial statements included in this Annual Report on Form 10-K and, as part of its audit, has issued an attestation report, included herein, on the effectiveness of the Company’s internal control over financial reporting.
| February 24, 2011 | ||||||||
| /s/ JOHN L. BISHOP |
/s/ ANDREW D. MILLER |
|||||||
| John L. Bishop | Andrew D. Miller | |||||||
| Chief Executive Officer | Senior Vice President and Chief Financial Officer | |||||||
41
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Cepheid
We have audited Cepheid’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Cepheid’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cepheid maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Cepheid as of December 31, 2010 and 2009, and the related consolidated statements of operations, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010 of Cepheid and our report dated February 24, 2011 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
San Jose, California
February 24, 2011
42
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Shareholders
Cepheid
We have audited the accompanying consolidated balance sheets of Cepheid as of December 31, 2010 and 2009, and the related consolidated statements of operations, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. Our audits also included the financial statement schedule listed in the Index at Part IV Item 15(b). These financial statements and schedule are the responsibility of Cepheid’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cepheid at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cepheid’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 24, 2011 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
San Jose, California
February 24, 2011
43
Table of Contents
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands, except share data) |
||||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 79,538 | $ | 35,786 | ||||
| Short-term investments |
— | 21,636 | ||||||
| Put option |
— | 3,295 | ||||||
| Accounts receivable, less allowance for doubtful accounts of $61 thousand and $7 thousand as of December 31, 2010 and 2009, respectively |
28,010 | 23,014 | ||||||
| Inventory |
37,598 | 38,015 | ||||||
| Prepaid expenses and other current assets |
4,138 | 2,421 | ||||||
| Total current assets |
149,284 | 124,167 | ||||||
| Property and equipment, net |
27,438 | 24,021 | ||||||
| Other non-current assets |
607 | 495 | ||||||
| Intangible assets, net |
24,688 | 30,817 | ||||||
| Goodwill |
18,594 | 18,626 | ||||||
| Total assets |
$ | 220,611 | $ | 198,126 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 21,957 | $ | 22,068 | ||||
| Accrued compensation |
12,594 | 8,869 | ||||||
| Accrued royalties |
7,994 | 12,929 | ||||||
| Accrued other liabilities |
1,288 | 1,800 | ||||||
| Current portion of deferred revenue |
8,207 | 2,923 | ||||||
| Current portion of notes payable |
1,679 | 108 | ||||||
| Bank borrowing |
— | 14,618 | ||||||
| Total current liabilities |
53,719 | 63,315 | ||||||
| Long-term portion of deferred revenue |
4,057 | 2,279 | ||||||
| Note payable, less current portion |
4,991 | 732 | ||||||
| Other liabilities |
4,182 | 4,234 | ||||||
| Total liabilities |
66,949 | 70,560 | ||||||
| Commitments and contingencies (Note 9) |
||||||||
| Shareholders’ equity: |
||||||||
| Preferred stock, no par value; 5,000,000 shares authorized, none issued or outstanding |
— | — | ||||||
| Common stock, no par value; 100,000,000 shares authorized, 60,568,626 and 58,644,990 shares issued and outstanding at December 31, 2010 and 2009, respectively |
288,387 | 273,052 | ||||||
| Additional paid-in capital |
72,731 | 56,408 | ||||||
| Accumulated other comprehensive income |
726 | 371 | ||||||
| Accumulated deficit |
(208,182 | ) | (202,265 | ) | ||||
| Total shareholders’ equity |
153,662 | 127,566 | ||||||
| Total liabilities and shareholders’ equity |
$ | 220,611 | $ | 198,126 | ||||
The accompany notes are an integral part of these consolidated financial statements.
44
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands, except per share data) | ||||||||||||
| Revenues: |
||||||||||||
| Total product sales |
206,876 | 165,185 | 159,383 | |||||||||
| Other revenues |
5,592 | 5,442 | 10,244 | |||||||||
| Total revenues |
212,468 | 170,627 | 169,627 | |||||||||
| Costs and operating expenses: |
||||||||||||
| Cost of product sales |
105,135 | 95,542 | 89,714 | |||||||||
| Collaboration profit sharing |
6,806 | 8,200 | 11,089 | |||||||||
| Research and development |
42,503 | 39,313 | 43,310 | |||||||||
| Sales and marketing |
38,840 | 29,156 | 29,757 | |||||||||
| General and administrative |
24,528 | 21,278 | 20,861 | |||||||||
| Gain from legal settlement |
— | (243 | ) | (1,454 | ) | |||||||
| Restructuring charge |
— | 747 | — | |||||||||
| Total costs and operating expenses |
217,812 | 193,993 | 193,277 | |||||||||
| Loss from operations |
(5,344 | ) | (23,366 | ) | (23,650 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest and other income, net |
168 | 406 | 1,225 | |||||||||
| Interest expense |
(340 | ) | (332 | ) | (13 | ) | ||||||
| Foreign currency exchange gain (loss) and other |
(820 | ) | 350 | (860 | ) | |||||||
| Other income, net |
(992 | ) | 424 | 352 | ||||||||
| Loss before benefit for income taxes |
(6,336 | ) | (22,942 | ) | (23,298 | ) | ||||||
| Benefit for income taxes |
419 | 440 | 911 | |||||||||
| Net loss |
$ | (5,917 | ) | $ | (22,502 | ) | $ | (22,387 | ) | |||
| Basic net loss per share |
$ | (0.10 | ) | $ | (0.39 | ) | $ | (0.39 | ) | |||
| Diluted net loss per share |
$ | (0.10 | ) | $ | (0.39 | ) | $ | (0.39 | ) | |||
| Shares used in computing basic net loss per share |
59,712 | 58,206 | 57,101 | |||||||||
| Shares used in computing diluted net loss per share |
59,712 | 58,206 | 57,101 | |||||||||
The accompany notes are an integral part of these consolidated financial statements.
45
Table of Contents
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
| Common Stock | Additional Paid-In Capital |
Accumulated Other Compre- hensive Income (Loss) |
Accumulated Deficit |
Total Share- holders’ Equity |
||||||||||||||||||||
| (In thousands) | Shares | Amount | ||||||||||||||||||||||
| Balance at December 31, 2007 |
55,611 | $ | 254,807 | $ | 26,697 | $ | 340 | $ | (157,376 | ) | $ | 124,468 | ||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
— | — | — | — | (22,387 | ) | (22,387 | ) | ||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | (363 | ) | — | (363 | ) | ||||||||||||||||
| Total comprehensive loss |
(22,750 | ) | ||||||||||||||||||||||
| Issuance of shares of common stock under employee and director option plans |
1,772 | 9,849 | — | — | — | 9,849 | ||||||||||||||||||
| Stock-based compensation related to stock options and awards and employee stock purchase plan |
— | — | 14,922 | — | — | 14,922 | ||||||||||||||||||
| Issuance of shares of common stock under employee stock purchase plan |
281 | 2,335 | — | — | — | 2,335 | ||||||||||||||||||
| Balance at December 31, 2008 |
57,664 | 266,991 | 41,619 | (23 | ) | (179,763 | ) | 128,824 | ||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
— | — | — | — | (22,502 | ) | (22,502 | ) | ||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | 394 | — | 394 | ||||||||||||||||||
| Total comprehensive loss |
(22,108 | ) | ||||||||||||||||||||||
| Issuance of shares of common stock under employee and director option plans |
562 | 3,436 | — | — | — | 3,436 | ||||||||||||||||||
| Stock-based compensation related to stock options and awards and employee stock purchase plan |
— | — | 14,789 | — | — | 14,789 | ||||||||||||||||||
| Issuance of shares of common stock under employee stock purchase plan |
419 | 2,625 | — | — | — | 2,625 | ||||||||||||||||||
| Balance at December 31, 2009 |
58,645 | 273,052 | 56,408 | 371 | (202,265 | ) | 127,566 | |||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
— | — | — | — | (5,917 | ) | (5,917 | ) | ||||||||||||||||
| Foreign currency translation adjustment |
— | — | — | 355 | — | 355 | ||||||||||||||||||
| Total comprehensive loss |
(5,562 | ) | ||||||||||||||||||||||
| Issuance of shares of common stock under employee and director option plans |
1,450 | 12,280 | — | — | — | 12,280 | ||||||||||||||||||
| Stock-based compensation related to stock options and awards and employee stock purchase plan |
— | — | 16,323 | — | — | 16,323 | ||||||||||||||||||
| Issuance of shares of common stock under employee stock purchase plan |
474 | 3,055 | — | — | — | 3,055 | ||||||||||||||||||
| Balance at December 31, 2010 |
60,569 | $ | 288,387 | $ | 72,731 | $ | 726 | $ | (208,182 | ) | $ | 153,662 | ||||||||||||
The accompany notes are an integral part of these consolidated financial statements.
46
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (5,917 | ) | $ | (22,502 | ) | $ | (22,387 | ) | |||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
9,326 | 8,808 | 7,632 | |||||||||
| Amortization of intangible assets |
6,966 | 6,823 | 5,343 | |||||||||
| Amortization of prepaid compensation |
— | 147 | 252 | |||||||||
| Stock-based compensation related to employees and consulting services rendered |
16,615 | 15,215 | 14,064 | |||||||||
| Write-offs of intangible assets |
271 | — | 406 | |||||||||
| Unrealized (gain) loss on auction rate securities |
(1,714 | ) | (6,734 | ) | 9,899 | |||||||
| Unrealized (gain) loss on put option |
1,844 | 6,143 | (9,438 | ) | ||||||||
| Deferred rent |
85 | (26 | ) | 231 | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(4,394 | ) | (4,062 | ) | 2,495 | |||||||
| Inventory |
124 | (4,937 | ) | (8,580 | ) | |||||||
| Prepaid expenses and other current assets |
(1,426 | ) | 2,059 | (2,520 | ) | |||||||
| Other non-current assets |
(113 | ) | 425 | (676 | ) | |||||||
| Accounts payable and other current liabilities |
(5,112 | ) | 10,753 | 1,106 | ||||||||
| Accrued compensation |
3,726 | 949 | (663 | ) | ||||||||
| Deferred revenue |
7,062 | 615 | (1,771 | ) | ||||||||
| Net cash provided by (used in) operating activities |
27,343 | 13,676 | (4,607 | ) | ||||||||
| Cash flows from investing activities: |
||||||||||||
| Capital expenditures |
(13,047 | ) | (8,575 | ) | (14,936 | ) | ||||||
| Acquisition of leasehold improvements |
125 | — | 327 | |||||||||
| Payments for technology licenses |
(1,000 | ) | (1,500 | ) | (418 | ) | ||||||
| Cost of acquisitions, net of cash acquired |
(1,300 | ) | (148 | ) | (1,884 | ) | ||||||
| Proceeds from the sale of fixed assets |
138 | 20 | 125 | |||||||||
| Proceeds from maturities of marketable securities |
24,800 | 200 | 2,550 | |||||||||
| Transfer from (to) restricted cash |
— | 1,500 | (983 | ) | ||||||||
| Net cash provided by (used in) investing activities |
9,716 | (8,503 | ) | (15,219 | ) | |||||||
| Cash flows from financing activities: |
||||||||||||
| Net proceeds from the sale of common shares and exercise of stock options and awards |
15,334 | 6,061 | 12,183 | |||||||||
| Proceeds from bank borrowing |
— | 20 | 14,700 | |||||||||
| Proceeds from notes payable |
6,448 | 849 | — | |||||||||
| Principal payments of bank borrowing |
(14,618 | ) | (40 | ) | (65 | ) | ||||||
| Principal payments of notes payable |
(618 | ) | (9 | ) | — | |||||||
| Net cash provided by financing activities |
6,546 | 6,881 | 26,818 | |||||||||
| Effect of exchange rate change on cash |
147 | 254 | 10 | |||||||||
| Net increase in cash and cash equivalents |
43,752 | 12,308 | 7,002 | |||||||||
| Cash and cash equivalents at beginning of year |
35,786 | 23,478 | 16,476 | |||||||||
| Cash and cash equivalents at end of year |
$ | 79,538 | $ | 35,786 | $ | 23,478 | ||||||
| Supplemental Cash Flow Information: |
||||||||||||
| Cash paid for interest |
$ | 225 | $ | 273 | $ | 11 | ||||||
The accompany notes are an integral part of these consolidated financial statements.
47
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
1. Organization and Summary of Significant Accounting Policies
Organization and Business
Cepheid (the “Company” or “we”) was incorporated in the State of California on March 4, 1996. The Company is a broad-based molecular diagnostics company that develops, manufactures, and markets fully-integrated systems for testing in the Clinical market, as well as for application in the Company’s legacy Industrial, Biothreat and Partner markets. The Company’s systems enable rapid, sophisticated molecular testing for organisms and genetic-based diseases by automating otherwise complex manual laboratory procedures.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries after elimination of intercompany transactions and balances. All gains and losses realized from foreign currency transactions denominated in currencies other than the foreign subsidiary’s functional currency are included in foreign currency exchange gain (loss) and other in the Consolidated Statements of Operations. Adjustments resulting from translating the financial statements of all foreign subsidiaries into U.S. dollars are reported as a separate component of accumulated other comprehensive income (loss) in shareholders’ equity. The assets and liabilities of the Company’s foreign subsidiaries are translated from their respective functional currencies into U.S. dollars at the rates in effect at the balance sheet date, and revenue and expense amounts are translated at rates approximating the weighted average rates during the period.
Within the current liabilities section of the consolidated balance sheets and the cash flow from operating activities section of the consolidated statements of cash flows, certain amounts were reclassified to conform to the current period presentation.
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from these estimates.
Fair Value of Financial Instruments
The Company defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Our valuation techniques used to measure fair value maximized the use of observable inputs and minimized the use of unobservable inputs. The fair value hierarchy is based on the following three levels of inputs:
• Level 1 - Quoted prices in active markets for identical assets or liabilities.
• Level 2 - Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
• Level 3 - Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
See Note 2, “Fair Value,” for information and related disclosures regarding our fair value measurements.
Cash, Cash Equivalents and Short-Term Investments
Cash and cash equivalents consist of cash on deposit with banks and money market instruments. Interest income includes interest, dividends, amortization of purchase premiums and discounts and realized gains and losses on sales of securities.
48
Table of Contents
The Company designates marketable securities and short-term investments as either trading or available-for-sale and records them at fair value. Realized and unrealized gains and losses on investments are determined on the specific identification method. If designated as a trading security, unrealized gains and losses are recorded to current period operating results. If designated as an available-for-sale security, unrealized holding gains or losses are reported as a component of accumulated other comprehensive income. Marketable securities and short-term investments with original maturities greater than 90 days and remaining maturities less than one year are classified as short-term; otherwise they are classified as long-term. When an investment is sold, we recognize the difference between the sales proceeds and its carrying value (determined based on specific identification) as a realized gain or loss. An impairment charge is recognized when the decline in the fair value of a security below the amortized cost basis as a result of credit losses or otherwise is determined to be other-than-temporary. The Company considers various factors in determining whether to recognize an impairment charge, including the duration of time and the severity to which the fair value has been less than our amortized cost basis, any adverse changes in the investees’ financial condition and whether it is more-likely-than-not that the Company will hold the investment for a period of time sufficient to allow for any anticipated recovery in market value.
Auction Rate Securities
At December 31, 2010 we did not hold any auction rate securities.
Prior to July 1,2010, we had a portfolio of auction rate securities that failed to settle at auction since March 2008. We valued our auction rate securities using a discounted cash flow methodology with significant inputs that included credit quality of the issuer, the percentage and the types of guarantees, contractual maturity, the timing and probability of the auction succeeding or security being called and discount factors. We collected interest on the investments that failed to settle at auction at the maximum contractual rate.
On November 10, 2008, we accepted a comprehensive settlement arrangement offered by UBS, the fund manager with which we held our auction rate securities. Under the settlement, we had the put option to sell the auction rate securities held in our accounts with UBS to UBS at par value during the period beginning June 30, 2010 and ending July 2, 2012. Since the settlement agreement was a legally enforceable firm commitment, the put option was recognized as a financial asset at fair value in our financial statements, and accounted for separately from the associated securities. The fair value of the put option was based on the difference in value between the par value and the fair value of the associated auction rate securities. We measured the put option at its fair value, and subsequent changes in fair value also were recognized in respective period financial results. At December 31, 2009, our put option was recorded at fair value, at par for $3.3 million and we had classified these securities as trading. On June 30, 2010, we notified UBS of our intent to exercise our put option to sell our portfolio of auction rate securities, which were classified as trading securities and recorded at fair value, at par to UBS for $11.8 million. In addition, the rights permitted us to establish a demand revolving credit line, payable on demand, in an amount up to 75% of fair value of the securities at a net no cost, meaning that the interest that we paid on the credit line would not exceed the interest that we received on the auction rate securities that we had pledged as security for the credit line. We borrowed $14.7 million on the line of credit on November 10, 2008. On July 1, 2010, we paid down the outstanding loan balance of $1.3 million, such that there was no outstanding balance at December 31, 2010. We had an outstanding loan balance of $14.6 million, which approximated the carrying value, at December 31, 2009.
In 2010, we recorded a gain to other income (expense), net of $1.7 million to increase the value of our auction rate securities investments classified as trading securities, offset by a loss of $1.8 million to reduce the estimated fair value of the put option. In 2009, we recorded a gain to other income, net of $6.7 million to increase the value of our auction rate securities investments classified as trading securities, partially offset by a loss of $6.1 million to reduce the estimated fair value of the put option.
Inventory
Inventory is stated at the lower of standard cost (which approximates actual cost) or market, with cost determined on the first-in-first-out method. Accordingly, allocation of fixed production overheads to conversion costs is based on normal capacity of production. Abnormal amounts of idle facility expense, freight, handling costs and spoilage are expensed as incurred and not included in overhead. In addition, unrecognized stock-based compensation cost of approximately $0.9 million and $1.2 million was included in inventory as of December 31, 2010 and 2009, respectively.
49
Table of Contents
The components of inventories were as follows (in thousands):
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Raw materials |
$ | 14,101 | $ | 16,615 | ||||
| Work in process |
12,601 | 10,933 | ||||||
| Finished goods |
10,896 | 10,467 | ||||||
| $ | 37,598 | $ | 38,015 | |||||
Property and Equipment
Property and equipment are stated at cost. Depreciation is calculated using the straight-line method, and the cost is amortized over the estimated useful lives of the assets, which range from 3 to 10 years. Leasehold improvements are amortized using the straight-line method over the estimated useful lives of the assets or the term of the lease, whichever is shorter.
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Land |
$ | 21 | $ | 21 | ||||
| Building |
3,331 | 3,119 | ||||||
| Scientific equipment |
22,845 | 17,577 | ||||||
| Manufacturing equipment |
19,972 | 20,554 | ||||||
| Office furniture, computers and equipment |
11,764 | 9,473 | ||||||
| Leasehold improvements |
14,862 | 10,706 | ||||||
| 72,795 | 61,450 | |||||||
| Less accumulated depreciation and amortization |
(45,357 | ) | (37,429 | ) | ||||
| $ | 27,438 | $ | 24,021 | |||||
Intangible Assets and Goodwill
As of December 31, 2010, intangible assets consisted primarily of rights to certain patented technologies licensed from Roche and Applera, (see Note 5, “Patent License Agreements” and Note 7, “Collaborative Agreements and Contracts”) and intangible assets acquired in acquisitions (see Note 8, “Acquisitions”).
Intangible assets related to licenses are recorded at cost, less accumulated amortization. Intangible assets related to technology and other intangible assets acquired in acquisitions are recorded at fair value at the date of acquisition, less accumulated amortization. Intangible assets are amortized over their estimated useful lives, ranging from 3 to 20 years, on a straight-line basis, except for intangible assets acquired in acquisitions, which are amortized on the basis of economic useful life. Amortization of intangible assets is included in the accompanying consolidated statements of operations.
The Company reviews its intangible assets for impairment and conducts the impairment review when events or circumstances indicate the carrying value of a long-lived asset may be impaired by estimating the future undiscounted cash flows to be derived from an asset to assess whether or not a potential impairment exists. If the carrying value exceeds the Company’s estimate of future undiscounted cash flows, an impairment value is calculated as the excess of the carrying value of the asset over the Company’s estimate of its fair market value. Events or circumstances which could trigger an impairment review include a significant adverse change in the business climate, an adverse action or assessment by a regulator, unanticipated competition, significant changes in the Company’s use of acquired assets, the Company’s overall business strategy, or significant negative industry or economic trends. In 2010, we recorded an impairment charge of $0.3 million related to a license termination. No impairment charge was recorded in 2009. In 2008, we recorded an impairment charge of $0.4 million related to certain licenses with no future use.
The Company annually reviews its goodwill for impairment. If the fair value of the Company exceeds its net book value including goodwill, then goodwill is not considered impaired. The initial step is to compare the Company’s fair value as determined by its market capitalization to its net book value. If the market capitalization exceeds the net book value, goodwill is presumed to be unimpaired. Otherwise, the Company would estimate expected future cash flows of its business, which operates in a number of markets and geographical regions. The Company would then determine the carrying value of its business and compare its carrying value including goodwill and other intangibles to the discounted future cash flows. If the total of future cash flows is less than the carrying amount of the assets, the Company would recognize an impairment loss based on the excess of the carrying amount over the fair value of the assets. At December 31, 2010, the Company compared its market capitalization to its net book value and determined that goodwill was not impaired.
50
Table of Contents
Warranty Reserve
The Company warrants its systems to be free from defects for a period of generally 12 to 15 months from the date of sale and its disposable products to be free from defects, when handled according to product specifications, for the stated life of such products. Accordingly, a provision for the estimated cost of warranty repair or replacement is recorded at the time revenue is recognized. The Company’s warranty provision is established using management’s estimate of future failure rates and future costs of repairing any failures during the warranty period or replacing any disposable products with defects. The activities in the warranty provision consisted of the following (in thousands):
| 2010 | 2009 | 2008 | ||||||||||
| Balance at beginning of year |
$ | 652 | $ | 655 | $ | 549 | ||||||
| Costs incurred and charged against reserve |
(594 | ) | (573 | ) | (210 | ) | ||||||
| Accrual related to current year product sales |
911 | 570 | 316 | |||||||||
| Balance at end of year |
$ | 969 | $ | 652 | $ | 655 | ||||||
Revenue Recognition
The Company recognizes revenue from product sales when there is persuasive evidence that an arrangement exists, delivery has occurred, the price is fixed or determinable and collectibility is reasonably assured. No right of return exists for the Company’s products except in the case of damaged goods. The Company has not experienced any significant returns of its products. Contract revenues include fees for technology licenses and research and development services, royalties under license and collaboration agreements. Contract revenue related to technology licenses is generally fully recognized only after the license period has commenced, the technology has been delivered and no further involvement of the Company is required. When the Company has continuing involvement related to a technology license, revenue is recognized over the license term. Royalties are typically based on licensees’ net sales of products that utilize the Company’s technology, and royalty revenues are recognized as earned in accordance with the contract terms when the royalties can be reliably measured and their collectibility is reasonably assured, such as upon the receipt of a royalty statement from the customer. Service revenue is recognized when the services have been provided. Shipping and handling costs are expensed as incurred and included in cost of product sales. In those cases where the Company bills shipping and handling costs to customers, the amounts billed are classified as revenue.
The Company recognizes revenue from both one-time product sales and longer-term contract arrangements. The Company enters into certain revenue arrangements with multiple deliverables. Multiple element revenue agreements are evaluated to determine whether the delivered item has value to the customer on a stand-alone basis and whether objective and reliable evidence of the fair value of the undelivered item exists. Deliverables in an arrangement that do not meet the separation criteria must be treated as one unit of accounting for purposes of revenue recognition. Advance payments received in excess of amounts earned, such as funds received in advance of products to be delivered or services to be performed, are classified as deferred revenue until earned.
Grants and government sponsored research revenue and contract revenue related to research and development services are recognized as the related services are performed based on the performance requirements of the relevant contract. Under such agreements, the Company is required to perform specific research and development activities and is compensated either based on the costs or costs plus a mark-up associated with each specific contract over the term of the agreement or when certain milestones are achieved and recoverability is reasonably assured.
Research and Development
Research and development expenses consist of costs incurred for company-sponsored and collaborative research and development activities. These costs include direct and research-related overhead expenses. The Company expenses research and development costs, including the expenses for research under collaborative agreements, as such costs are incurred.
Stock-Based Compensation
Stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period, which is generally the vesting period. We estimate the fair value of stock-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods, generally four years, in our consolidated statements of operations.
51
Table of Contents
In determining fair value of the stock-based compensation payments, we use the Black–Scholes model and a single option award approach, which requires the input of subjective assumptions. These assumptions include: estimating the length of time employees will retain their vested stock options before exercising them (expected term), the estimated volatility of our common stock price over the expected term (volatility), the risk-free interest rate and the number of shares subject to options that will ultimately not complete their vesting requirements (forfeitures). Changes in the following assumptions can materially affect the estimate of fair value of stock–based compensation.
| • | Expected term is determined based on historical experience, giving consideration to the contractual terms of the stock-based awards, vesting schedules and expectations of future employee behavior as influenced by changes to the terms of its stock-based awards. |
| • | Expected volatility is based on the historical volatility for the past 5 years, which approximates the expected term of the option grant. |
| • | Risk-free interest rate is based on the implied yield currently available on U.S. Treasury zero-coupon issues with a remaining term equivalent to the expected term of a stock award. |
| • | Estimated forfeitures are based on voluntary termination behavior as well as analysis of actual option forfeitures. |
Foreign Currency Hedging
The Company recognizes derivative instruments, including foreign exchange contracts, in the balance sheet in other assets or liabilities at their fair value. The Company utilizes foreign exchange forward contracts in order to reduce the impact of fluctuations in the value of non-functional currency monetary assets and liabilities upon its financial statements and cash flows. These instruments are used to hedge foreign currency exposures of underlying non-functional currency monetary assets and liabilities primarily arising from intercompany transactions such as intercompany inventory purchases between Cepheid and its foreign subsidiaries. These foreign exchange contracts, carried at fair value, generally have a maturity of three months or less. The Company’s accounting policies for these instruments are based on whether they are designated as hedging transactions. Our foreign exchange contracts were not designated as hedging transactions, therefore, the changes in fair value of the derivatives are recorded in earnings. The Company enters into approximately two to six derivatives per quarter ranging from $0.1 million to $12.5 million in notional value each with a total notional value of approximately $11.2 million and $8.2 million in 2010 and 2009, respectively. As of December 31, 2010, we had two outstanding foreign exchange forward contracts, which were recorded as a derivative asset within prepaid expenses and other current assets with a fair value of approximately $4,000 and derivative liability within accrued and other liabilities with a fair value of approximately $92,000. As of December 31, 2009, we had two outstanding foreign exchange forward contracts, which were recorded as liabilities within accrued and other liabilities with a fair value of approximately $28,000. The effect of our hedging transactions, consisting entirely of foreign currency forward contracts, on our consolidated statement of operations in 2010 and 2009 was a pre-tax gain of approximately $0.8 million and a pre-tax loss of approximately $0.7 million, respectively.
Comprehensive Income (Loss)
Comprehensive loss includes net loss as well as other comprehensive income. As of December 31, 2010 and 2009, the Company’s accumulated other comprehensive income (loss) consists solely of cumulative foreign currency translation adjustments.
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Net loss |
$ | (5,917 | ) | $ | (22,502 | ) | ||
| Other comprehensive income: |
||||||||
| Foreign currency translation adjustments |
355 | 394 | ||||||
| Comprehensive loss |
$ | (5,562 | ) | $ | (22,108 | ) | ||
Net Loss Per Share
Basic net loss per share has been calculated based on the weighted-average number of common shares outstanding during the period. Shares used in diluted net loss per share calculations exclude anti-dilutive common stock equivalent shares, consisting of stock options. These anti-dilutive common stock equivalent shares totaled 5,651,000, 7,162,000 and 5,287,000 for 2010, 2009 and 2008, respectively.
52
Table of Contents
Income Taxes
We account for income taxes using an asset and liability approach, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in our consolidated financial statements, but have not been reflected in our taxable income. A valuation allowance is established to reduce deferred tax assets to their estimated realizable value. Therefore, we provide a valuation allowance to the extent that we do not believe it is more likely than not that it will generate sufficient taxable income in future periods to realize the benefit of its deferred tax assets.
We recognize interest and penalties related to uncertain tax positions in income tax expense. For the year ended December 31, 2010, 2009 and 2008, we did not recognize significant interest or penalties related to uncertain tax positions in the consolidated statements of operations, and at December 31, 2010 and 2009, we had no significant accrued interest or penalties.
Recent Accounting Pronouncements
In September 2009, the FASB issued ASU No. 2009-13, “Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force” (“ASU 2009-13”). It updates the existing multiple-element revenue arrangements guidance currently included under ASC 605-25, which originated primarily from the guidance in EITF Issue No. 00-21, “Revenue Arrangements with Multiple Deliverables.” The revised guidance primarily provides two significant changes: 1) eliminates the need for objective and reliable evidence of the fair value for the undelivered element in order for a delivered item to be treated as a separate unit of accounting, and 2) eliminates the residual method to allocate the arrangement consideration. In addition, the guidance also expands the disclosure requirements for revenue recognition. We will adopt ASU 2009-13 on January 1, 2011. We do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
In October 2009, the FASB issued ASU No. 2009-14, “Certain Revenue Arrangements That Include Software Elements — a consensus of the FASB Emerging Issues Task Force” (“ASU 2009-14”). It modifies the scope of ASC subtopic 985-605 Software-Revenue Recognition to exclude from its requirements: 1) non-software components of tangible products and 2) software components of tangible products that are sold, licensed, or leased with tangible products when the software components and non-software components of the tangible product function together to deliver the tangible product’s essential functionality. We will adopt ASU 2009-14 on January 1, 2011. We do not expect the adoption of this guidance to have a material impact on our consolidated financial statements.
53
Table of Contents
2. Fair Value
The following table represents the fair value hierarchy for our financial assets (cash equivalents and short-term investments) and financial liabilities (foreign currency derivatives) measured at fair value on a recurring basis as of December 31, 2010 and 2009 (in thousands):
| Balance as of December 31, 2010: |
||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash |
$ | 63,667 | $ | — | $ | — | $ | 63,667 | ||||||||
| Cash equivalent - money market funds |
15,871 | — | — | 15,871 | ||||||||||||
| Foreign currency derivatives |
— | 4 | — | 4 | ||||||||||||
| Total |
$ | 79,538 | $ | 4 | $ | — | $ | 79,542 | ||||||||
| Liabilities: |
||||||||||||||||
| Foreign currency derivatives |
$ | — | $ | 92 | $ | — | $ | 92 | ||||||||
| Total |
$ | — | $ | 92 | $ | — | $ | 92 | ||||||||
| Balance as of December 31, 2009: |
||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets: |
||||||||||||||||
| Cash |
$ | 30,208 | $ | — | $ | — | $ | 30,208 | ||||||||
| Cash equivalent - money market funds |
5,578 | — | — | 5,578 | ||||||||||||
| Short-term investments - taxable auction rate securities |
— | — | 21,636 | 21,636 | ||||||||||||
| Put option |
— | — | 3,295 | 3,295 | ||||||||||||
| Total |
$ | 35,786 | $ | — | $ | 24,931 | $ | 60,717 | ||||||||
| Liabilities: |
||||||||||||||||
| Foreign currency derivatives |
$ | — | $ | 28 | $ | — | $ | 28 | ||||||||
| Total |
$ | — | $ | 28 | $ | — | $ | 28 | ||||||||
The Company recorded derivative assets and liabilities at fair value. The Company’s derivatives consist of foreign exchange forward contracts. The Company has elected to use the income approach to value the derivatives, using observable Level 2 market expectations at the measurement date and standard valuation techniques to convert future amounts to a single present amount assuming that participants are motivated, but not compelled to transact.
Level 2 inputs for the valuations are limited to quoted prices for similar assets or liabilities in active markets (specifically foreign currency spot rate and forward points) and inputs other than quoted prices that are observable for the asset or liability (specifically LIBOR rates, credit default spot rates, and company specific LIBOR spread). Mid-market pricing is used as a practical expedient for fair value measurements. The fair value measurement of an asset or liability must reflect the nonperformance risk of the entity and the counterparty. Therefore, the impact of the counterparty’s creditworthiness when in an asset position and the Company’s creditworthiness when in a liability position has also been factored into the fair value measurement of the derivative instruments and did not have a material impact on the fair value of these derivative instruments. Both the counterparty and the Company are expected to continue to perform under the contractual terms of the instruments.
Level 3 assets consist of auction rate securities whose underlying assets are student loans, most of which are guaranteed by the federal government. In February 2008, auctions began to fail for these securities, and since then each auction in our portfolio failed. Prior to the settlement of exercising our put option to sell the auction rate securities held in our accounts with UBS to UBS at par value on July 1, 2010, we had classified the auction rate securities as short-term assets on our consolidated balance sheet as of December 31, 2009. These investments were valued at fair value. The following table provides a summary of changes in fair value of our auction rate securities and put option for the 2010 and 2009 (in thousands):
54
Table of Contents
| Level 3 | ||||
| Balance at January 1, 2009 |
$ | 24,539 | ||
| Settlements |
(199 | ) | ||
| Unrealized gain of auction rate securities included in current period earnings |
6,734 | |||
| Unrealized loss of put option included in current period earnings |
(6,143 | ) | ||
| Balance at December 31, 2009 |
$ | 24,931 | ||
| Level 3 | ||||
| Balance at January 1, 2010 |
$ | 24,931 | ||
| Settlements |
(24,800 | ) | ||
| Unrealized gain of auction rate securities included in current period earnings |
1,714 | |||
| Unrealized loss of put option included in current period earnings |
(1,845 | ) | ||
| Balance at December 31, 2010 |
$ | — | ||
Our investment portfolio of auction rate securities was structured with short-term interest rate reset dates of generally less than 30 days, but with contractual maturities well in excess of ten years. Our auction rate securities consisted of investments that were backed by pools of student loans, which were principally guaranteed by the Federal Family Educational Loan Program, or insured. We believed that the credit quality of these securities was high based on these guarantees. We determined the fair market values of our financial instruments based on the fair value hierarchy requirements for an entity to maximize the use of observable inputs (Level 1 and Level 2 inputs) and minimize the use of unobservable inputs (Level 3 inputs) when measuring fair value. Until the first quarter of 2008, the fair values of our auction rate securities were determinable by reference to frequent successful Dutch auctions of such securities, which settled at par. Therefore, at the adoption date, we had categorized our investments in auction rate securities as Level 1. Given the failures in the auction markets to provide quoted market prices of the securities, as well as the lack of any correlation of these instruments to other observable market data, we valued these securities using a discounted cash flow methodology with the most significant input categorized as Level 3. Significant inputs that went into the model were the credit quality of the issuer, the percentage and the types of guarantees, contractual maturity, the timing and probability of the auction succeeding or the security being called and discount factors.
On November 10, 2008, we accepted a comprehensive settlement arrangement offered by UBS, the fund manager with which we held our auction rate securities. Under the settlement, we had the option to sell the auction rate securities held in our accounts with UBS to UBS at par value during the put option exercise period. Since the settlement agreement was a legally enforceable firm commitment, the put option was recognized as a financial asset at fair value in our financial statements, and accounted for separately from the associated securities. The fair value of the put option was based on the difference in value between the par value and the fair value of the associated auction rate securities. We measured the put option at its fair value, and subsequent changes in fair value also were recognized in respective period financial results. At December 31, 2009, our put option was recorded at fair value, at par for $3.3 million and we had classified these securities as trading. On June 30, 2010, we notified UBS of our intent to exercise our put option to sell our portfolio of auction rate securities, which were classified as trading securities and recorded at fair value, at par to UBS for $11.8 million. In addition, the rights permitted us to establish a revolving credit line, payable on demand, in an amount up to 75% of fair value of the securities at a net no cost, meaning that the interest that we paid on the credit line would not exceed the interest that we received on the auction rate securities that we had pledged as security for the credit line. We borrowed $14.7 million on the line of credit on November 10, 2008. On July 1, 2010, we paid down the outstanding loan balance of $1.3 million, such that there was no outstanding balance at December 31, 2010. We had an outstanding loan balance of $14.6 million, which approximated the carrying value, at December 31, 2009.
3. Intangible Assets
Intangible assets related to licenses are recorded at cost, less accumulated amortization. Intangible assets related to technology and other intangible assets acquired in acquisitions are recorded at fair value at the date of acquisition, less accumulated amortization. Intangible assets are amortized over their estimated useful lives, ranging from 3 to 20 years, on a straight-line basis, except for intangible assets acquired in acquisitions, which are amortized on the basis of economic useful life. Amortization of intangible assets is primarily included in cost of product sales in the accompanying consolidated statements of operations.
55
Table of Contents
The recorded value and accumulated amortization of major classes of intangible assets were as follows (in thousands):
| Gross
Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||
| Balance, December 31, 2010 |
||||||||||||
| Licenses |
$ | 44,507 | $ | 27,173 | $ | 17,334 | ||||||
| Technology acquired in acquisitions |
8,621 | 3,246 | 5,375 | |||||||||
| Other intangible assets acquired in acquisitions |
4,283 | 2,304 | 1,979 | |||||||||
| $ | 57,411 | $ | 32,723 | $ | 24,688 | |||||||
| Balance, December 31, 2009 |
||||||||||||
| Licenses |
$ | 44,507 | $ | 21,792 | $ | 22,715 | ||||||
| Technology acquired in acquisitions |
8,613 | 2,027 | 6,586 | |||||||||
| Other intangible assets acquired in acquisitions |
3,183 | 1,667 | 1,516 | |||||||||
| $ | 56,303 | $ | 25,486 | $ | 30,817 | |||||||
In 2010, the Company completed acquisitions of certain assets of two companies. Acquisitions are approximately 1% of total assets for each respective period presented. Technology acquired in acquisitions and other intangible assets increased by a total of $1.1 million at December 31, 2010 from December 31, 2009.
Included in licenses was $19.9 million in connection with a patent license agreement with Roche, effective July 1, 2004. The net book value of this license was $7.0 million and $9.0 million at December 31, 2010 and 2009, respectively.
We capitalize patent licenses and amortize them over their estimated useful lives on a straight-line basis. Amortization expense of intangible assets was $7.0 million, $6.8 million and $5.3 million for the years ended December 31, 2010, 2009 and 2008, respectively. The expected future annual amortization expense of intangible assets recorded on the Company’s consolidated balance sheet as of December 31, 2009 is as follows, assuming no impairment charges (in thousands):
| For the Years Ending December 31, |
Amortization Expense |
|||
| 2011 |
$ | 6,845 | ||
| 2012 |
5,468 | |||
| 2013 |
4,756 | |||
| 2014 |
3,418 | |||
| 2015 |
1,497 | |||
| Thereafter |
2,704 | |||
| Total expected future annual amortization |
$ | 24,688 | ||
4. Segment and Significant Concentrations
We and our wholly owned subsidiaries operate in one business segment.
The following table illustrates product sales in the Clinical, Industrial, Biothreat and Partner markets:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (Amounts in thousands) | ||||||||||||
| Product sales by market: |
||||||||||||
| Clinical Systems |
$ | 31,266 | $ | 26,241 | $ | 32,084 | ||||||
| Clinical Reagents |
131,254 | 89,701 | 58,167 | |||||||||
| Total Clinical |
162,520 | 115,942 | 90,251 | |||||||||
| Industrial |
18,117 | 19,165 | 15,437 | |||||||||
| Biothreat |
22,206 | 24,762 | 35,797 | |||||||||
| Partner |
4,033 | 5,316 | 17,898 | |||||||||
| Total product sales |
$ | 206,876 | $ | 165,185 | $ | 159,383 | ||||||
56
Table of Contents
We currently sell our products through our direct sales force and through third-party distributors. For the years ended December 31, 2010, 2009 and 2008, there was one direct customer that accounted for 11%, 15% and 23% of total product sales, respectively. We have distribution agreements with several companies to distribute products in the U.S. and have several regional distribution arrangements throughout Europe, Japan, China, the Americas and other parts of the world. The following table provides a summary of product sales by geographic region for the three years ended December 31, 2010, 2009 and 2008 (in thousands):
The following table provides a breakdown of product sales by geographic region (in thousands):
| Years
Ended December 31, |
||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Product Sales Geographic information: |
||||||||||||
| North America |
||||||||||||
| Clinical |
$ | 127,326 | $ | 88,562 | $ | 70,248 | ||||||
| Other |
37,328 | 40,227 | 55,078 | |||||||||
| Total North America |
164,654 | 128,789 | 125,326 | |||||||||
| International |
||||||||||||
| Clinical |
35,194 | 27,380 | 20,004 | |||||||||
| Other |
7,028 | 9,016 | 14,053 | |||||||||
| Total International |
42,222 | 36,396 | 34,057 | |||||||||
| Total product sales |
$ | 206,876 | $ | 165,185 | $ | 159,383 | ||||||
No single country outside of the U.S. represented more than 10% of our total revenues or total assets in any period presented and no customer outside the U.S. accounted for more than 10% of our total product sales.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of bank deposits and accounts receivable. The Company maintains a portfolio of cash equivalents and money market funds.
The Company’s accounts receivable are derived primarily from sales to customers. The Company performs ongoing credit evaluations of its customers and limits the amount of credit extended when deemed necessary, but generally requires no collateral. In addition, the Company maintains an allowance for potential doubtful accounts. There was one customer whose accounts receivable balance represented 3% of total receivables as of December 31, 2010 and 12% of total receivables as of December 31, 2009.
The Company relies on several companies as its sole source for various materials used in its manufacturing process. Any extended interruption in the supply of these materials could result in the failure to meet customer demand.
5. Patent License Agreements
In April 2004, the Company entered into a patent license agreement with Applera, through its LIFE and its Celera Diagnostics joint venture, for a non-exclusive worldwide license to make, use, and sell the Company’s products incorporating technology covered by Applera patents. The Company also entered into a patent license agreement with Roche, effective July 1, 2004, for a non-exclusive worldwide license to make, use, and sell the Company’s products incorporating technology covered by Roche patents. Under the license agreements, the Company agreed to pay aggregate license fees of $32.2 million, of which $23.5 million was paid in 2005 and $8.7 million was paid in 2006. In connection with the license agreements, the Company recorded intangible assets of $31.1 million, representing the present value of license fee obligations which is net of imputed interest of $1.1 million. The effective interest rate used to calculate the present value of the discounted payments was 4.0% for both the Roche and Applera licenses. In June 2006, the Applera patent license agreement was expanded to include additional Company products, for which the Company paid an additional $0.5 million. The intangible assets related to the Applera and Roche licenses are amortized on a straight-line basis over their useful lives of approximately 10 years, with the amortization recorded as part of the cost of product sales. The Company also paid approximately $1.2 million in back royalties related to the Applera license, which was expensed during the quarter ended March 31, 2004.
57
Table of Contents
The Company also agreed to pay Applera and Roche ongoing royalties on sales of any products incorporating the licensed patents. Resulting product royalties are recorded as part of the cost of product sales when the related product sales are recognized.
In September 2006, Cepheid entered into a sublicense agreement with Abbott, pursuant to which Abbott granted Cepheid a non-exclusive, world-wide, non-transferable right to Abbott’s exclusive license to certain patents from the Baylor College of Medicine. Under this sublicense agreement, the Company will be able to make, use, distribute and sell products incorporating the patented technology generally characterized as multiple genomic DNA amplification. In September 2006, Cepheid also entered into a license agreement with Abbott, pursuant to which Abbott granted Cepheid a non-exclusive, world-wide, non-transferable right to a certain Abbott patent. Under this license agreement, the Company will be able to make, use, distribute and sell products incorporating the patented technology generally characterized as detection of cervical chlamydia trachomatis infection. License payments for these agreements totaled $2.0 million. The intangible assets related to these sublicenses are amortized on a straight-line basis over their useful lives of approximately 7 and 9 years, respectively, with the amortization recorded as part of the cost of product sales.
In January 2007, Cepheid entered into a sublicense agreement with bioMerieux SA, pursuant to which bioMerieux SA granted Cepheid a non-exclusive, worldwide, irrevocable sublicense to certain patents that relate to the diagnosis of methicillin resistant staphylococcus aureus. The patents are owned by Kainos Laboratories Inc. and Professor Keiichi Hiramatsu and have been exclusively licensed to bioMerieux SA with the right for bioMerieux SA to sub-license. Under the sublicense agreement, and subject to certain limitations set forth therein, Cepheid is able to use the licensed rights to develop and sell products for use in connection with its GeneXpert and SmartCycler platforms. In exchange for such rights, Cepheid agreed to pay an initial license fee of approximately $4.0 million and quarterly royalties based on net product sales during the term of the sublicense agreement, which expires when the last of the patents licensed under the agreement expires. The license fee was paid in the first quarter of 2007 and is being amortized on a straight-line basis over the useful life of approximately 10 years, with the amortization recorded as part of the cost of product sales.
6. Collaboration Profit Sharing
Collaboration profit sharing represents the amount that the Company pays to LIFE under our collaboration agreement to develop reagents for use in the BDS developed for the USPS. Under the agreement, computed gross margin on anthrax cartridge sales are shared equally between the two parties. Collaboration profit sharing expense was $6.8 million, $8.2 million and $11.1 million for the years ended December 31, 2010, 2009, 2008 respectively. The total revenues and cost of sales related to these cartridge sales are included in the respective balances in the consolidated statement of operations.
7. Collaborative Agreements and Contracts
Novartis
In October 2010, the Company entered into a collaboration with Novartis for the commercialization of a test for monitoring the BCR-ABL gene transcript in peripheral blood specimens from patients diagnosed with Ph+ CML. To fund development and clinical trial costs, Cepheid received an upfront fee of $5.0 million in October 2010 from Novartis with up to $3.0 million in milestone payments over the ensuing 12 months. If the FDA grants us clearance to commercially release the product in the U.S., under the collaboration, Novartis will have exclusive global distribution rights to the Xpert BCR-ABL test under a Cepheid/Novartis label.
Foundation for Innovative New Diagnostics
In May 2006, the Company entered into an agreement with the FIND to develop a simple, rapid test that can detect mycobactrium tuberculosis and associated rifampin resistance from human sputum samples. Under the agreement, Cepheid was responsible for the development of a 6-color GeneXpert instrument to accomplish such test and the development of an enhanced manufacturing line for the manufacture of test cartridges used in the test. FIND reimbursed Cepheid at agreed upon amounts. The term of the development portion of the agreement was 30 months, which was subsequently extended an additional five months. In July 2009, the agreement was extended for another year for further specified enhancements. The supply term of the agreement is for 12 years, unless terminated by either party in accordance with relevant provisions of the agreement. In January 2011, the agreement was extended for another year and a new agreement was signed for the development of our Xpert HIV Viral Load test. Under the Xpert HIV agreement, FIND will fund $5.0 million in development costs throughout the two-year contract.
LIFE and Northrop Grumman Corporation
In October 2002, the Company entered into a collaboration agreement with LIFE to develop reagents for use in the USPS BDS program, which was developed by the consortium led by Northrop Grumman Corporation. Under the agreement, reagents will be manufactured by LIFE for packaging by the Company into its GeneXpert test cartridges and sold by the Company for use in the BDS. This agreement calls for the computed gross margin on sales of anthrax cartridges for the USPS BDS program to be equally shared between the two parties.
58
Table of Contents
In August 2007, the Company entered into a five-year master purchase order with Northrop Grumman for the purchase of up to $200 million in anthrax test cartridges and associated materials used in BDS. The agreement, and subsequent purchase orders, cover the period through September 30, 2011.
8. Acquisitions
In 2010, the Company completed acquisitions of certain assets of two companies. In 2008, the Company completed one acquisition of certain assets of a company. Acquisitions are approximately 1% of total assets for each respective period presented.
9. Commitments, Contingencies and Legal Matters
Facility Leases
The Company leases office space under arrangements expiring through 2020. Certain of these lease arrangements contain escalation clauses whereby monthly rent increases over time. Rent expense is recognized on a straight-line basis over the lease period. Net rent expense for all operating leases, partially offset by subleasing a portion of our office space to a third party, for the years ended December 31, 2010, 2009 and 2008 was $4.1 million, $3.7 million and $3.4 million, respectively.
Minimum annual rental commitments under facility operating leases at December 31, 2010 are as follows (in thousands):
| Years Ending December 31, |
||||
| 2011 |
$ | 5,809 | ||
| 2012 |
5,732 | |||
| 2013 |
5,807 | |||
| 2014 |
5,924 | |||
| 2015 |
4,826 | |||
| Thereafter |
14,777 | |||
| Total minimum payments |
$ | 42,875 | ||
Commitments
As of December 31, 2010, purchase commitments consisted of $11.6 million, $2.5 million and $2.5 million for 2011, 2012 and 2013.
We have certain royalty commitments associated with the shipment and licensing of certain products. Royalty expense is generally based on a dollar amount per unit shipped or a percentage of the underlying revenue.
Contingencies
The Company responds to claims arising in the ordinary course of business. In certain cases, management has accrued estimates of the amounts it expects to pay upon resolution of such matters, and such amounts are included in other accrued liabilities. Should the Company not be able to secure the terms it expects, these estimates may change and will be recognized in the period in which they are identified. Although the ultimate outcome of such claims is not presently determinable, management believes that the resolution of these matters will not have a material adverse effect on the Company’s financial position, results of operations and cash flows.
59
Table of Contents
In the normal course of business, we provide indemnifications of varying scope to customers against claims of intellectual property infringement made by third parties arising from the use of our products. Historically, costs related to indemnification provisions have not been significant and we are unable to estimate the maximum potential impact of these indemnification provisions on our future results of operations.
To the extent permitted under California law, we have agreements whereby we indemnify our directors and officers for certain events or occurrences while the director or officer is, or was serving, at our request in such capacity. The indemnification period covers all pertinent events and occurrences during the director’s or officer’s service. The maximum potential amount of future payments we could be required to make under these indemnification agreements is not specified in the agreements; however, we have director and officer insurance coverage that reduces our exposure and enables us to recover a portion of any future amounts paid. We believe the estimated fair value of these indemnification agreements in excess of applicable insurance coverage is minimal.
Legal Matters
On June 28, 2010, Abaxis, Inc. filed suit in U.S. District Court for the Northern District of California against us, alleging that our Xpert MRSA product infringes U.S. Patent No. 5,413,732, U.S. Patent No. 5,624,597, U.S. Patent No. 5,776,563 and U.S. Patent No. 6,251,684. On July 12, 2010, we filed our response to the suit, denying Abaxis’ allegations of infringement and asked the Court to find Abaxis’ patents invalid and not infringed. On August 5, 2010, Abaxis filed its response to our answer and counterclaim. On November 19, 2010, Abaxis filed an amended complaint in which it added allegations that we breached a licensing contract for the above-referenced patents. On December 17, 2010, we answered Abaxis’ amended complaint denying that we were in breach of the licensing contract and further amended our counterclaims against Abaxis. On January 14, 2011, Abaxis filed a motion to dismiss, countering certain of our defenses and counterclaims. We believe that the possibility that this legal proceeding will result in a material adverse effect on our business is remote.
On January 10, 2011, Troll Busters LLC filed suit in United States District Court for the Southern District of California against us and twelve other named defendants alleging that Cepheid falsely marked its Omnimix and 3-Agent Biothreat Assay products with expired patents owned by Roche Molecular Systems relating to certain PCR processes. Our initial deadline for answering this complaint is April 11, 2011. We believe that the possibility that this legal proceeding will result in a material adverse effect on our business is remote.
During the third quarter of 2009, the Company entered into a settlement with a vendor regarding certain issues under multiple agreements. Pursuant to the settlement, the vendor paid the Company $0.2 million, which the Company recorded as a gain.
10. Shareholders’ Equity
Stock Option Plans
On April 27, 2006, the Company’s shareholders approved the 2006 Plan, which was approved by the Board in February 2006. On April 27, 2006, the Board also terminated the Company’s 1997 Stock Option Plan (“1997 Plan”). No new grants will be made under the 1997 Plan, and options granted or shares issued under the 1997 Plan that were outstanding on the date the 1997 Plan was terminated will remain subject to the terms of the 1997 Plan. Shares of common stock reserved for issuance under the 2006 Plan include (i) an initial authorization of 3,800,000 shares of common stock, (ii) shares reserved but unissued under the 1997 Plan as of the date the 1997 Plan was terminated and (iii) shares subject to awards granted under the 1997 Plan that are cancelled, forfeited or repurchased by the Company or expire after the 1997 Plan termination. On April 24, 2008, shareholders approved an increase to the number of shares of common stock reserved for issuance under the 2006 Plan by 1,800,000. On April 29, 2010, shareholders approved an increase to the number of shares of common stock reserved for issuance under the 2006 Plan by 3,800,000.
Under the 2006 Plan, the Company may grant incentive stock options (“ISOs”) and non-qualified stock options (“NQSOs”), restricted stock awards (“RSAs”), stock bonus awards (“SBAs”), stock appreciation rights (“SARs”), restricted stock units (“RSUs”) and performance share awards (“PSAs”). ISOs may be granted only to employees and directors of the Board, and all other awards may be granted to Company employees and directors and to consultants, independent contractors and advisors of the Company for services rendered. Any award, other than a stock option or a SAR, shall reduce the number of shares available for issuance by 1.75 shares for each share subject to such award (for a stock option or a SAR this ratio shall remain 1:1). The 2006 Plan is administered by the Compensation Committee of the Board (“Committee”). RSAs, SBAs, RSUs and PSAs (collectively, “Full Value Equity Awards”) with vesting or settlement restrictions, as applicable, based upon completion of performance goals, shall have a minimum one-year vesting or settlement restriction period (the “One-Year Restriction Period”) and all other vesting or settlement restrictions, as applicable, for Full Value Equity Awards shall have a minimum three-year vesting or settlement restriction period (the “Three-Year Restriction Period” and together with the One-Year Restriction Period, the “Minimum
60
Table of Contents
Restriction Periods”). The Company may grant Full Value Equity Awards without taking into account the Minimum Restriction Periods, provided, that, the Company does not grant more than 10% of the aggregate shares of common stock reserved and available for grant and issuance under the 2006 Plan without the Minimum Restriction Periods. The following provides a general description of each type of award under the 2006 Plan. As of December 31, 2010, we had 2,199,748 shares of our common stock reserved for future issuance under the 2006 Plan.
Stock options may be granted at no less than the fair market value per share of common stock on the date of the grant (at 110% of fair market value for ISOs granted to 10% shareholders), expire not later than seven years from the date of grant (five years from the date of grant for ISOs granted to 10% shareholders) and generally vest 25% one year after the date of grant and then on a pro rata basis over the following 36 months.
RSAs may be granted at a purchase price that is less than fair market value on the date of grant, and the restrictions are determined by the Committee and may be based on years of service with the Company or completion of performance goals during a period. The Committee will determine the extent that the RSA is earned prior to the payment for the shares awarded.
SBAs may be granted for past or future services and may contain restrictions based on years of service with the Company or completion of performance goals during a period. No payment will be required for shares awarded under an SBA. Payments to recipients of an SBA may be in the form of cash, shares of common stock, or a combination thereof, based on the fair market value of shares earned under the SBA. The Committee will determine the number of shares to be awarded under the SBA and the extent that the SBA is earned prior to the payment for the shares awarded.
SARs are awards for past or future services that may be settled in cash or shares of common stock, including restricted stock, having a value equal to the number of shares subject to the SAR multiplied by the difference between the fair market value on the date of grant and the exercise price. The Committee determines the terms of each SAR, including the number of shares of common stock subject to the SAR, the exercise price and the times during which the SAR may be settled, consideration to be made on settlement, and effect of the participant’s termination. If SARs are awarded based on performance goals, the Committee will determine the extent that the SAR is earned. SARs may be granted at an exercise price that may be less than fair market value per share of common stock on the date of grant, may be exercisable at one time or from time to time, and have a term not to exceed seven years.
RSUs are awards for past or future services that may be settled in cash or shares of common stock, including restricted stock. The Committee determines the terms of each RSU, including the number of shares of common stock subject to the RSU, the times during which the RSU may be settled, consideration to be made on settlement, and effect of the participant’s termination. If RSUs are awarded based on performance goals, the Committee will determine the extent that the RSU is earned. The number of shares subject to the RSU may be fixed or may vary depending on performance goals determined by the Committee. While the RSU shall be paid currently, under certain circumstances the Committee may permit the participant to defer settlement of the RSU.
PSAs are awards denominated in shares of common stock that may be settled in cash or issuance of such shares (which may consist of restricted stock). The Committee will determine the terms of each PSA, including the number of shares of common stock subject to the PSA, the performance factors and period that shall determine the time and extent to which each PSA shall be settled, consideration to be made on settlement, and effect of the participant’s termination. The Committee will determine the extent that the PSA is earned. The number of shares subject to the PSA may be fixed or may vary in accordance with performance goals as determined by the Committee.
61
Table of Contents
We have no outstanding SBAs, SARs, or PSAs as of December 31, 2010 and 2009.
A summary of option activity under all plans is as follows (in thousands, except weighted average exercise price and weighted average remaining contractual term):
| Shares | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (in years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding, December 31, 2007 |
8,905 | $ | 8.48 | |||||||||||||
| Granted |
2,109 | $ | 20.94 | |||||||||||||
| Exercised |
(1,750 | ) | $ | 5.63 | ||||||||||||
| Forfeited |
(258 | ) | $ | 16.47 | ||||||||||||
| Outstanding, December 31, 2008 |
9,006 | $ | 11.72 | |||||||||||||
| Granted |
1,694 | $ | 9.45 | |||||||||||||
| Exercised |
(505 | ) | $ | 6.80 | ||||||||||||
| Forfeited |
(443 | ) | $ | 13.05 | ||||||||||||
| Outstanding, December 31, 2009 |
9,752 | $ | 11.52 | |||||||||||||
| Granted |
1,448 | $ | 19.79 | |||||||||||||
| Exercised |
(1,388 | ) | $ | 8.83 | ||||||||||||
| Forfeited |
(298 | ) | $ | 16.09 | ||||||||||||
| Outstanding, December 31, 2010 |
9,514 | $ | 13.03 | 4.20 | $ | 94,022 | ||||||||||
| Exercisable, December 31, 2010 |
6,538 | $ | 11.30 | 3.59 | $ | 75,915 | ||||||||||
| Vested and expected to vest, December 31, 2010 |
8,954 | $ | 12.81 | 4.13 | $ | 90,425 | ||||||||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (i.e., the difference between our closing stock price of $22.75 on the last trading day of 2010 and the exercise price, times the number of shares for options where the exercise price is below the closing stock price) that would have been received by the option holders had all option holders exercised their options on that date. This amount changes based on the fair market value of our stock. The total intrinsic value of options actually exercised was $14.4 million, $2.5 million, and $37.0 million for the years ended December 31, 2010, 2009, and 2008, respectively.
62
Table of Contents
A summary of all award activity, which consists of RSAs and RSUs, is as follows (in thousands, except weighted average grant date fair value):
| Shares | Weighted Average Grant Date Fair Value |
|||||||
| Outstanding, December 31, 2007 |
91 | $ | 13.55 | |||||
| Granted |
27 | $ | 24.76 | |||||
| Vested |
(42 | ) | $ | 14.80 | ||||
| Cancelled |
(10 | ) | $ | 21.73 | ||||
| Outstanding, December 31, 2008 |
66 | $ | 16.07 | |||||
| Granted |
65 | $ | 12.51 | |||||
| Vested |
(58 | ) | $ | 13.19 | ||||
| Cancelled |
(8 | ) | $ | 19.85 | ||||
| Outstanding, December 31, 2009 |
65 | $ | 14.60 | |||||
| Granted |
415 | $ | 20.05 | |||||
| Vested |
(67 | ) | $ | 15.08 | ||||
| Cancelled |
(10 | ) | $ | 8.43 | ||||
| Outstanding, December 31, 2010 |
403 | $ | 17.92 | |||||
In accordance with the 2006 Plan, RSAs and RSUs granted in 2010 and 2009 reduced the number of shares available for future grant by a factor of 1.75 in 2010, 2009 and 2008 for each share subject to such award, or 725,667, 113,750 and 47,250 shares in 2010, 2009 and 2008.
Employee Stock Purchase Plan
The ESPP was adopted in April 2000, amended in June 2003 and April 2009. The ESPP permits eligible employees of the Company and its participating subsidiaries to purchase common stock at a discount up to a maximum of 15% of compensation through payroll deductions during defined two-year offering periods consisting of four, six-month purchase periods. The price at which stock is purchased under the ESPP is equal to 85% of the fair market value of the common stock on the first day of the two-year offering period or the last day of the six-month purchase period, whichever is lower. The number of shares available for future issuance increase annually equal to the lesser of (a) 200,000 shares, (b) 0.75% of the outstanding shares on the date of the annual increase or (c) an amount determined by the Board.
Reserved Shares
As of December 31, 2010, the Company has reserved shares of common stock for future issuance as follows (in thousands):
| 2006 Plan: |
||||
| Options, RSUs and awards outstanding for all plans |
9,514 | |||
| Reserved for future grants |
2,200 | |||
| ESPP |
1,081 | |||
| 12,795 | ||||
63
Table of Contents
Stock-Based Compensation
Fair Value—The fair value of the Company’s stock options granted to employees and shares purchased by employees under the ESPP for the years ended December 31, 2010, 2009 and 2008 was estimated using the following assumptions:
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| OPTION SHARES: |
||||||||||||
| Expected Term (in years) |
4.61 | 4.52 | 4.46 | |||||||||
| Volatility |
0.55 | 0.74 | 0.60 | |||||||||
| Expected Dividends |
0.00 | % | 0.00 | % | 0.00 | % | ||||||
| Risk Free Interest Rates |
2.13 | % | 1.86 | % | 2.38 | % | ||||||
| Estimated Forfeitures |
7.74 | % | 8.04 | % | 7.74 | % | ||||||
| ESPP SHARES: |
||||||||||||
| Expected Term (in years) |
1.25 | 1.25 | 1.25 | |||||||||
| Volatility |
0.65 | 0.85 | 0.70 | |||||||||
| Expected Dividends |
0.00 | % | 0.00 | % | 0.00 | % | ||||||
| Risk Free Interest Rates |
0.46 | % | 0.65 | % | 2.26 | % | ||||||
| Estimated Forfeitures |
7.74 | % | 8.04 | % | 7.74 | % | ||||||
The weighted average fair value of our stock options granted to employees was $9.38, $5.53 and $6.44 for 2010, 2009 and 2008, respectively. Also, the weighted average fair value for our shares purchased by employees under the ESPP was $6.90, $4.31 and $7.60 for 2010, 2009 and 2008, respectively.
Stock-Based Compensation Cost—The following table is a summary of the major categories of stock compensation expense recognized in accordance with ASC 718, “Compensation—Stock Compensation” (“ASC 718”) for the years ended December 31, 2010, 2009 and 2008 (in thousands).
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Cost of product sales |
$ | 2,523 | $ | 2,411 | $ | 1,016 | ||||||
| Research and development |
5,274 | 5,499 | 5,407 | |||||||||
| Sales and marketing |
3,613 | 2,823 | 3,456 | |||||||||
| General and administrative |
5,206 | 4,482 | 4,185 | |||||||||
| Total stock-based compensation cost |
$ | 16,616 | $ | 15,215 | $ | 14,064 | ||||||
The above stock-based compensation cost includes $1.8 million, $1.0 million, and $1.7 million related to ESPP for 2010, 2009, and 2008, respectively. In addition, stock-based compensation cost of approximately $0.9 million, $1.2 million and $1.6 million was included in inventory as of December 31, 2010, 2009 and 2008, respectively.
As of December 31, 2010, the total compensation cost related to unvested stock-based grants awarded under the Company’s 1997 Plan and 2006 Plan but not yet recognized was approximately $20.1 million, which is net of estimated forfeitures of $5.3 million. This cost will be amortized on a straight line basis over a weighted average period of approximately 2.52 years and will be adjusted for subsequent changes in estimated forfeitures.
As of December 31, 2010, the total compensation cost related to RSAs and RSUs under the 2006 Plan not yet recognized was approximately $5.7 million, which is net of estimated forfeitures of $1.4 million. This cost will be amortized on a straight line basis over a weighted average period of approximately 1.92 years and 3.80 years for RSAs and RSUs, respectively and will be adjusted for subsequent changes in estimated forfeitures.
At December 31, 2010, the total compensation cost related to options to purchase the Company’s common shares under the ESPP but not yet recognized was approximately $0.3 million. The cost will be amortized on a straight-line basis over the two year offering period, as such term is defined in the ESPP.
64
Table of Contents
11. Employee Benefit Plan
The Company adopted a 401(k) plan that allows eligible employees to contribute a percentage of their qualified compensation subject to IRS limits. The Company has the discretion to make matching contributions each year. Contributions made by the Company for the years ended December 31, 2010, 2009 and 2008 were $0.6 million, $0.5 million, and $0.5, respectively.
12. Income Taxes
The income tax benefit of $0.4 million in 2010 is primarily comprised of $0.8 million of income tax benefit primarily related to a tax credit for research and development in France, as well as to the amortization of acquired intangibles in Sweden and UK, partially offset by $0.3 million of foreign tax expense and state income tax expense of $0.1 million. The income tax benefit of $0.4 million in 2009 represented U.S. federal income tax benefit of $0.2 million related to a refundable R&D credit as provided by the Act. The income tax benefit in 2009 also represented $0.7 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden partially offset by $0.4 million of foreign tax expense and state income tax expense of $0.1 million. The income tax benefit of $0.9 million in 2008 represented current U.S. federal income tax benefit of $0.2 million related to a refundable R&D credit as provided by the Act. The income tax benefit in 2008 also represented $0.9 million of income tax benefit mainly related to the amortization of acquired intangibles in Sweden and a refundable R&D credit in France, partially offset by $0.1 million of state income tax expense.
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets (liabilities) are as follows (in thousands):
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Net operating loss carryforwards |
$ | 38,300 | $ | 49,393 | ||||
| Capitalized research and development costs |
190 | 1,633 | ||||||
| Research and other credit carryforwards |
9,709 | 8,925 | ||||||
| Stock option compensation |
13,952 | 12,512 | ||||||
| Other |
14,945 | 9,120 | ||||||
| Total deferred tax assets |
77,096 | 81,583 | ||||||
| Valuation allowance for deferred tax assets |
(76,982 | ) | (81,583 | ) | ||||
| Total deferred tax liability |
(1,656 | ) | (1,919 | ) | ||||
| Net deferred tax liability |
$ | (1,542 | ) | $ | (1,919 | ) | ||
Realizability of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance with the exception of those in the UK and Sweden relating to the amortization of acquired assets and licensed intellectual property of Sangtec and Stretton. The valuation allowance decreased by approximately $4.6 million, increased by $9.1 million and decreased by $11.4 million during the years ended December 31, 2010, 2009 and 2008, respectively.
As of December 31, 2010, the Company had net operating loss carryforwards for federal income tax purposes of approximately $154.2 million, which expire in the years 2011 through 2028, federal research and development tax credits of approximately $5.3 million, which expire in the years 2019 through 2030, and foreign tax credits of $0.1 million which expire in 2019. As of December 31, 2009, the Company had net operating loss carryforwards for state income tax purposes of approximately $69.7 million, which expire in the years 2014 through 2031, and state research and development tax credits of approximately $6.8 million, which have no expiration date.
Utilization of the Company’s net operating loss may be subject to a substantial annual limitation due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such annual limitation may result in the expiration of net operating loss before utilization.
The Company had no undistributed earnings from its foreign subsidiaries for which tax has not been provided as of December 31, 2010.
The Company maintains liabilities for uncertain tax positions. These liabilities involve considerable judgment and estimation and are continuously monitored by management based on the best information available, including changes in tax regulations, the outcome of relevant court cases, and other information. For federal income tax purposes, the Company has open tax years from
65
Table of Contents
1996 through 2010 due to net operating loss carryforwards relating to these years. Substantially all material state, local and foreign income tax matters have been concluded for years through December 31, 2001. For California state income tax purposes, the open years are from 2000 through 2010 due to either research credit carryovers or net operating loss carryforwards.
The following table summarizes the activity related to our unrecognized tax benefits (in thousands):
| 2010 | 2009 | 2008 | ||||||||||
| Balance at beginning of year |
$ | 4,741 | $ | 4,330 | $ | 3,675 | ||||||
| Increase related to current year tax positions |
518 | 486 | 689 | |||||||||
| Increase (decrease) for tax positions of prior years |
(73 | ) | (75 | ) | (34 | ) | ||||||
| Balance at end of year |
$ | 5,186 | $ | 4,741 | $ | 4,330 | ||||||
All of the unrecognized tax benefits would affect our effective tax rate if recognized, before consideration of certain valuation allowances. The Company anticipates that the total unrecognized tax benefits will not significantly change due to the settlement of audits and the expiration of statutes of limitations prior to December 31, 2011.
The Company’s policy is to recognize interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense. We recognize interest and penalties related to uncertain tax positions in income tax expense. In 2010, 2009 and 2008, we did not recognize any significant interest or penalties related to uncertain tax positions. As of December 31, 2010 and 2009, we had accrued no significant interest or penalties.
13. Derivative Instruments and Hedging Activities
The Company is exposed to global market risks, including the effect of changes in foreign currency exchange rates and uses derivatives to manage financial exposures that occur in the normal course of business. The Company does not hold or issue derivatives for trading or speculative purposes.
The Company enters into foreign exchange forward contracts to mitigate the change in fair value of our net recognized foreign currency assets and liabilities and to reduce the risk that our earnings and cash flows will be adversely affected by changes in exchange rates. These derivative instruments are not designated as hedging instruments. Accordingly, changes in the fair value of these derivative instruments are recognized immediately in other income (expense), net on the statement of operations together with the transaction gain or loss from the hedged balance sheet position. These derivative instruments do not subject us to material balance sheet risk due to exchange rate movements because gains and losses on these derivatives are intended to offset gains and losses on the assets and liabilities being hedged.
The Company’s derivative financial instruments present certain market and counterparty risks; however, concentration of counterparty risk is mitigated as the Company deals with major banks with Standard & Poor’s and Moody’s long-term debt ratings of A or higher. In addition, only conventional derivative financial instruments are utilized. At this time, the Company does not require collateral or any other form of securitization to be furnished by the counterparties to its derivative financial instruments.
14. Gain from Legal Settlement
During the third quarter of 2009, we entered into a settlement with a vendor regarding certain issues under multiple agreements. Pursuant to the settlement, the vendor paid us $0.2 million, which we have recorded as a gain.
15. Restructuring
During the first quarter of 2009, the Company eliminated 47 positions that impacted employees, contractors and replacement positions, which resulted in $0.7 million of restructuring expense, primarily related to severance. As of December 31, 2010 and 2009, the activities under this restructuring plan were complete and the Company had no outstanding restructuring expenses to be paid.
66
Table of Contents
16. Notes payable
Notes payable
On June 7, 2010, the Company entered into a Loan Agreement with SVB, which provided for (i) a total revolving credit line of up to $15.0 million with a maturity date of June 7, 2012 and bearing interest no less than 4.0% with a commitment fee of 0.75% per annum on the unused portion, and (ii) a total term line of $6.0 million, $2.0 million of which was funded on the effective date of the Loan Agreement, $2.0 million of which was funded in the third quarter of 2010 and $2.0 million of which was funded in the fourth quarter of 2010, with a maturity date for each term loan of 48 months following funding and bearing interest no less than 5.5%. The borrowings under the Loan Agreement are collateralized by a first priority security interest in certain assets of the Company. The Company had an outstanding balance of $5.5 million on the term line and no outstanding balance under the revolving credit line at December 31, 2010. The loan agreement requires the Company to maintain no less than 75% of its consolidated cash and cash equivalents in the operating bank accounts in the U.S. as collateral. Also, the loan agreement requires a covenant for the Company to satisfy a minimum consolidated EBITDA – Earnings Before Interest, Taxes, Depreciation and Amortization on a quarterly basis and a minimum liquidity covenant on a monthly basis. If the Company violates any of these covenants or otherwise breaches the loan agreement, the Company may be required to repay the loans prior to their stated maturity dates, and the bank may be able to foreclose on any collateral in the Company’s operating accounts. The Company was in compliance with these covenants as of December 31, 2010. As of December 31, 2010, the minimum SVB loan principal payments are as follows (in thousands):
| Payments Due by Period | ||||||||||||||||||||
| Total | Less Than 1 Year |
1-3 Years |
3-5 Years |
More Than 5 Years |
||||||||||||||||
| Note payable |
5,500 | 1,500 | 3,000 | 1,000 | — | |||||||||||||||
The Company also had an outstanding note payable of $1.2 million and $0.8 million at December 31, 2010 and 2009, respectively, with its real property in France pledged as collateral for the loan.
Bank Borrowing
The UBS comprehensive settlement agreement permitted the Company to establish a revolving credit line, payable on demand, in an amount up to 75% of the fair market value of the auction rate securities at a net no cost, meaning that the interest the Company paid on the credit line would not exceed the interest that the Company received on the auction rate securities that the Company had pledged as security for the credit line. The Company borrowed $14.7 million on the line of credit on November 10, 2008 and had no outstanding balance at December 31, 2010 and an outstanding balance of $14.6 million at December 31, 2009.
67
Table of Contents
CEPHEID
QUARTERLY FINANCIAL INFORMATION
| Quarters Ended | ||||||||||||||||
| Mar 31 | June 30 | Sep 30 | Dec 31 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| 2010 |
||||||||||||||||
| Revenues: |
||||||||||||||||
| Product sales |
$ | 47,182 | $ | 48,648 | $ | 54,877 | $ | 56,169 | ||||||||
| Other revenues |
863 | 994 | 1,178 | 2,557 | ||||||||||||
| Total revenues |
48,045 | 49,642 | 56,055 | 58,726 | ||||||||||||
| Cost and operating expenses: |
||||||||||||||||
| Cost of product sales |
26,071 | 25,215 | 27,279 | 26,570 | ||||||||||||
| Collaboration profit sharing |
1,655 | 1,654 | 2,404 | 1,093 | ||||||||||||
| Research and development |
9,701 | 10,150 | 10,986 | 11,666 | ||||||||||||
| Sales and marketing |
8,985 | 9,260 | 9,969 | 10,626 | ||||||||||||
| General and administrative |
5,715 | 5,848 | 6,033 | 6,932 | ||||||||||||
| Total cost and operating expenses |
52,127 | 52,127 | 56,671 | 56,887 | ||||||||||||
| Income (loss) from operations |
(4,082 | ) | (2,485 | ) | (616 | ) | 1,839 | |||||||||
| Other income (expense), net |
(276 | ) | (261 | ) | (109 | ) | (346 | ) | ||||||||
| Income (loss) before income tax expense |
(4,358 | ) | (2,746 | ) | (725 | ) | 1,493 | |||||||||
| Benefit (provision) for income taxes |
19 | 945 | (397 | ) | (148 | ) | ||||||||||
| Net income (loss) |
$ | (4,339 | ) | $ | (1,801 | ) | $ | (1,122 | ) | $ | 1,345 | |||||
| Basic net income (loss) per share |
$ | (0.07 | ) | $ | (0.03 | ) | $ | (0.02 | ) | $ | 0.02 | |||||
| Diluted net income (loss) per share |
$ | (0.07 | ) | $ | (0.03 | ) | $ | (0.02 | ) | $ | 0.02 | |||||
| Weighted average shares used in computing basic net income (loss) per share |
58,936 | 59,493 | 59,987 | 60,413 | ||||||||||||
| Weighted average shares used in computing diluted net income (loss) per share |
58,936 | 59,493 | 59,987 | 63,372 | ||||||||||||
| Gross profit on product sales: |
||||||||||||||||
| Product sales |
$ | 47,182 | $ | 48,648 | $ | 54,877 | $ | 56,169 | ||||||||
| Cost of product sales |
(26,071 | ) | (25,215 | ) | (27,279 | ) | (26,570 | ) | ||||||||
| $ | 21,111 | $ | 23,433 | $ | 27,598 | $ | 29,599 | |||||||||
Diluted net income per common share is computed by dividing income available to common shareholders by the weighted-average number of shares of common stock outstanding during the period increased to include the number of additional shares of common stock that would have been outstanding if the potentially dilutive securities had been issued.
68
Table of Contents
| Quarters Ended | ||||||||||||||||
| Mar 31 | June 30 | Sep 30 | Dec 31 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||
| 2009 |
||||||||||||||||
| Revenues: |
||||||||||||||||
| Product sales |
$ | 36,588 | $ | 39,560 | $ | 40,798 | $ | 48,239 | ||||||||
| Other revenues |
2,179 | 1,461 | 837 | 965 | ||||||||||||
| Total revenues |
38,767 | 41,021 | 41,635 | 49,204 | ||||||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of product sales |
20,690 | 23,250 | 23,765 | 27,837 | ||||||||||||
| Collaboration profit sharing |
2,629 | 2,612 | 1,306 | 1,653 | ||||||||||||
| Research and development |
10,338 | 10,315 | 8,744 | 9,916 | ||||||||||||
| Sales and marketing |
6,812 | 6,917 | 7,040 | 8,387 | ||||||||||||
| General and administrative |
5,269 | 5,340 | 5,223 | 5,446 | ||||||||||||
| Restructuring charge |
747 | — | — | — | ||||||||||||
| Gain from legal settlement |
— | — | (243 | ) | — | |||||||||||
| Total costs and operating expenses |
46,485 | 48,434 | 45,835 | 53,239 | ||||||||||||
| Loss from operations |
(7,718 | ) | (7,413 | ) | (4,200 | ) | (4,035 | ) | ||||||||
| Other income (expense), net |
72 | 459 | 116 | (223 | ) | |||||||||||
| Loss before income tax expense |
(7,646 | ) | (6,954 | ) | (4,084 | ) | (4,258 | ) | ||||||||
| Benefit (provision) for income taxes |
44 | 175 | 245 | (24 | ) | |||||||||||
| Net loss |
$ | (7,602 | ) | $ | (6,779 | ) | $ | (3,839 | ) | $ | (4,282 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.13 | ) | $ | (0.12 | ) | $ | (0.07 | ) | $ | (0.07 | ) | ||||
| Weighted average shares used in computing basic and diluted net loss per share |
57,832 | 58,053 | 58,335 | 58,596 | ||||||||||||
| Gross profit on product sales: |
||||||||||||||||
| Product sales |
$ | 36,588 | $ | 39,560 | $ | 40,798 | $ | 48,239 | ||||||||
| Cost of product sales |
(20,690 | ) | (23,250 | ) | (23,765 | ) | (27,837 | ) | ||||||||
| $ | 15,898 | $ | 16,310 | $ | 17,033 | $ | 20,402 | |||||||||
69
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of December 31, 2010, we carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer and Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(a) and 15d-15(e) under the Securities and Exchange Act of 1934, as amended). Based on the evaluation, we concluded that the design and operation of our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in the reports filed and submitted under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to our management, including our Principal Executive Officer and Principal Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
The report of management required under this Item 9A is contained in Item 8 of Part II of this Annual Report on Form 10-K under the heading “Management’s Report on Internal Control Over Financial Reporting.”
Attestation Report of Independent Registered Public Accounting Firm
The attestation report required under this Item 9A is contained in Item 8 of Part II of this Annual Report on Form 10-K under the heading “Report of Independent Registered Public Accounting Firm.”
Changes in Internal Control over Financial Reporting
There were no significant changes in our internal control over financial reporting during the fourth quarter of 2010.
None.
70
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information in response to this item is incorporated herein by reference to our definitive proxy statement for our 2011 annual meeting of shareholders to be held on April 26, 2011. Information related to our executive officers also appears under the caption “Executive Officers of the Registrant” in Item 1 to this report.
ITEM 11. EXECUTIVE COMPENSATION
Information in response to this item is incorporated herein by reference to our definitive proxy statement for our 2011 annual meeting of shareholders to be held on April 26, 2011.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
Information in response to this item is incorporated herein by reference to our definitive proxy statement for our 2011 annual meeting of shareholders to be held on April 26, 2011. For the information required by this item with respect to Item 201(d) of Regulation S-K regarding securities authorized for issuance under equity compensation plans, see “Item 5: Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities—Equity Compensation Plan Information.”
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information in response to this item is incorporated herein by reference to our definitive proxy statement for our 2011 annual meeting of shareholders to be held on April 26, 2011.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Information in response to this item is incorporated herein by reference to our definitive proxy statement for our 2011 annual meeting of shareholders to be held on April 26, 2011.
71
Table of Contents
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
The following documents are being filed as part of this report on Form 10-K:
(a) Financial Statements
The following financial statements are filed as part of this report on Form 10-K under “Item 8: Consolidated Financial Statements and Supplementary Data.”
Management’s Report on Internal Control Over Financial Reporting
Reports of Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Shareholders’ Equity
Consolidated Statements of Cash Flows
Notes to Consolidated Financial Statements
Supplementary Data: Quarterly Financial Information
| (b) | Schedule II — Valuation and Qualifying Accounts for the years ended December 31, 2010, 2009 and 2008. |
All other schedules are omitted as the required information is inapplicable or the information is presented in “Item 8: Consolidated Financial Statements and Supplementary Data.”
(c) Exhibits
The exhibit list in the Index to Exhibits is incorporated herein by reference as the list of exhibits required as part of this report on Form 10-K.
72
Table of Contents
CEPHEID
SCHEDULE II — VALUATION AND QUALIFYING ACCOUNTS
| Description |
Balance at Beginning of Year |
Costs and Expenses |
Deductions | Balance at End of Year |
||||||||||||
| (In thousands) | ||||||||||||||||
| Allowance for doubtful accounts: |
||||||||||||||||
| Year ended December 31, 2008 |
$ | 37 | $ | 69 | $ | (86 | ) | $ | 20 | |||||||
| Year ended December 31, 2009 |
20 | (12 | ) | (1 | ) | 7 | ||||||||||
| Year ended December 31, 2010 |
7 | 80 | (26 | ) | 61 | |||||||||||
73
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CEPHEID | ||
| By: |
/s/ ANDREW D. MILLER | |
| Andrew D. Miller | ||
| Senior Vice President and Chief Financial Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints John L. Bishop and Andrew D. Miller or either of them, his or her true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution, for him and in his or her name, place and stead, in any and all capacities to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto the attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that the attorneys-in-fact and agents, or either of them, or their, his or her substitutes or substitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ JOHN L. BISHOP John L. Bishop |
Chief Executive Officer and Director (Principal Executive Officer) |
February 24, 2011 | ||
| /s/ ANDREW D. MILLER Andrew D. Miller |
Senior Vice President and Chief Financial Officer (Principal Financial Officer) |
February 24, 2011 | ||
| /s/ THOMAS L. GUTSHALL Thomas L. Gutshall |
Director and Chairman of the Board | February 24, 2011 | ||
| /s/ THOMAS D. BROWN |
Director | February 24, 2011 | ||
| Thomas D. Brown | ||||
| /s/ CRISTINA H. KEPNER |
Director | February 24, 2011 | ||
| Cristina H. Kepner | ||||
| /s/ ROBERT EASTON |
Director | February 24, 2011 | ||
| Robert Easton | ||||
| /s/ DEAN O. MORTON |
Director | February 24, 2011 | ||
| Dean O. Morton | ||||
| /s/ MITCHELL D. MROZ |
Director | February 24, 2011 | ||
| Mitchell D. Mroz | ||||
| /s/ DAVID H. PERSING, M.D., PH.D. David H. Persing, M.D., Ph.D. |
Executive Vice President and Chief Medical and Technology Officer and Director | February 24, 2011 | ||
| /s/ HOLLINGS C. RENTON |
Director | February 24, 2011 | ||
| Hollings C. Renton | ||||
74
Table of Contents
| Incorporated by Reference | ||||||||||||
| Exhibit Number |
Description of Exhibit |
Form |
File No. |
Exhibit |
Filing Date |
Filed Herewith | ||||||
| 3.1 | Amended and Restated Articles of Incorporation | S-1 | 333-34340 | 3.1 | 4/7/2000 | |||||||
| 3.2 | Amended and Restated Bylaws | 8-K | 3.01 | 10/24/2008 | ||||||||
| 3.3 | Certificate of Determination specifying the terms of the Series A Junior Participating Preferred Stock of registrant, as filed with the Secretary of State to the State of California on October 2, 2002 | 8-A | 3.02 | 10/4/2002 | ||||||||
| 4.1 | Reference is made to Exhibits 3.1 and 3.2 | |||||||||||
| 4.2 | Specimen Common Stock Certificate | 10-Q | 4.01 | 7/31/2002 | ||||||||
| 4.3 | Rights Agreement dated September 26, 2002 between Cepheid and Computershare Trust Company as Rights Agent, which includes as Exhibit A the form of Certificate of Determination of Series A Junior Participating Preferred Stock, as Exhibit B the Summary of Stock Purchase Rights and as Exhibit C the Form of Rights Certificate | 8-A | 3.02 | 10/4/2002 | ||||||||
| 10.1* | 1997 Stock Option Plan, as amended | S-8 | 333-106181 | 4.2 | 6/17/2003 | |||||||
| 10.2* | 2000 Employee Stock Purchase Plan, as amended | 8-K | 99.1 | 5/5/2009 | ||||||||
| 10.3* | 2006 Equity Incentive Plan, as amended, and restated | 8-K | 99.01 | 11/1/2010 | ||||||||
| 10.4* | Form of Indemnification Agreement between Cepheid and its officers and directors | S-1 | 333-34340 | 10.6 | 4/7/2000 | |||||||
| 10.5† | License Agreement, dated January 16, 1996, between Cepheid and The Regents of the University of California, Lawrence Livermore National Laboratory | S-1 | 333-34340 | 10.9 | 6/7/2000 | |||||||
| 10.6† | Thermal Cycler Supplier Agreement, dated April 15, 2000, between Cepheid and PE Biosystems, a division of PE Corporation | S-1 | 333-34340 | 10.16 | 5/18/2000 | |||||||
| 10.7 | Lease Agreement dated October 18, 2001, between Cepheid and Aetna Life Insurance Company | 10-K | 10.17 | 3/22/2002 | ||||||||
| 10.8† | Collaboration Agreement between Applied Biosystems and Cepheid dated October 11, 2002 | 10-K | 10.28 | 3/25/2003 | ||||||||
| 10.9† | IVD Products Patent License Agreement between Cepheid and F. Hoffmann-La Roche Ltd, effective July 1, 2004 | 10-Q | 10.28 | 8/9/2004 | ||||||||
| 10.10† | Real-Time Instrument Patent License Agreement between Applera Corporation and Cepheid, dated April 5, 2004 | 10-Q | 10.29 | 8/9/2004 | ||||||||
| 10.11* | Offer letter dated November 4, 2004 from Cepheid to Mr. Humberto Reyes from Cepheid | 10-K | 10.35 | 2/28/2005 | ||||||||
| 10.12 | Facility lease agreement between Cepheid and Teachers Insurance & Annuity Association of America, Inc. dated May 13, 2005 | 8-K | 99.01 | 5/18/2005 | ||||||||
| 10.13* | Employment offer letter between Cepheid and David H. Persing dated July 21, 2005 | 8-K | 99.01 | 7/26/2005 | ||||||||
| 10.14* | Form of Stock Option Grant Agreement with certain executive officers of Cepheid approved by Cepheid’s Compensation Committee of the Board of Directors on April 27, 2005 | 10-Q | 10.3 | 8/4/2005 | ||||||||
75
Table of Contents
| Incorporated by Reference | ||||||||||||
| Exhibit Number |
Description of Exhibit |
Form |
File No. |
Exhibit |
Filing Date |
Filed Herewith | ||||||
| 10.15* | Employment Agreement dated January 24, 2007, by and between Cepheid and John L. Bishop | 8-K | 10.1 | 1/29/2007 | ||||||||
| 10.16* | Share Purchase Agreement dated February 14, 2007, by and between Cepheid, Altana Technology Projects GmbH, and Altana Pharma AG | 8-K | 2.1 | 2/20/2007 | ||||||||
| 10.17 | Settlement and Cross-License Agreement between Cepheid and Idaho Technology, Inc. dated January 2, 2007 | 10-Q | 10.1 | 5/10/2007 | ||||||||
| 10.18† | Master Purchase Order between Northrop Grumman Security Systems and Cepheid dated August 15, 2007 | 10-Q | 10.1 | 11/5/2007 | ||||||||
| 10.19* | Employment Agreement dated February 6, 2008, by and between Cepheid and Andrew D. Miller | 8-K | 10.01 | 2/11/2008 | ||||||||
| 10.20* | Amended and Restated Form of Change of Control Retention and Severance Agreement between Cepheid and each of its executive officers | 8-K | 99.02 | 5/3/2010 | ||||||||
| 10.21 | Office Lease dated February 28, 2008, between BRCP Caribbean Portfolio, LLC, and Cepheid | 10-Q | 10.1 | 5/7/2008 | ||||||||
| 10.22 | Series C-2 Auction Rate Securities Rights (Incorporated by reference to Exhibit 4.6 to the Registration Statement on Form F-3 (File No. 333-153882) filed by UBS AG with the Securities and Exchange Commission on October 7, 2008) | F-3 | 333-153882 | 4.6 | 10/7/2008 | |||||||
| 10.23 | Credit Line Agreement dated December 5, 2008, by and between Cepheid and UBS | 10-K | 10.38 | 2/26/2009 | ||||||||
| 10.24* | Offer Letter dated June 5, 2003 from Cepheid to Joseph H. Smith | X | ||||||||||
| 10.25* | Amendment to Employment Agreement dated February 21, 2008 from Cepheid to Joseph H. Smith |
X | ||||||||||
| 10.26 | Loan and Security Agreement, dated as of June 7, 2010, by and between Cepheid and Silicon Valley Bank | 8-K | 10.01 | 6/9/2010 | ||||||||
| 21.1 | List of Subsidiaries | X | ||||||||||
| 23.1 | Consent of Independent Registered Public Accounting Firm | X | ||||||||||
| 31.1 | Certification of Chief Executive Officer pursuant to Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 31.2 | Certification of Principal Financial Officer pursuant to Rule 13a-14(a), as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
| 32.2 | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||
76
Table of Contents
| Incorporated by Reference | ||||||||||||
| Exhibit Number |
Description of Exhibit |
Form |
File No. |
Exhibit |
Filing Date |
Filed Herewith | ||||||
| 101.INS§ | XBRL Instance Document | |||||||||||
| 101.SCH§ | XBRL Taxonomy Extension Schema Document | |||||||||||
| 101.CAL§ | XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||||
| 101.DEF§ | XBRL Taxonomy Extension Definition Linkbase Document | |||||||||||
| 101.LAB§ | XBRL Taxonomy Extension Label Linkbase Document | |||||||||||
| 101.PRE§ | XBRL Taxonomy Extension Presentation Linkbase Document | |||||||||||
| * | Management contract or compensatory plan or arrangement. |
| † | Confidential treatment has been granted with respect to portions of the exhibit. A complete copy of the agreement, including the redacted terms, has been separately filed with the Securities and Exchange Commission. |
| § | Pursuant to applicable securities laws and regulations, the Company is deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and is not subject to liability under any anti-fraud provisions of the federal securities laws as long as the Company has made a good faith attempt to comply with the submission requirements and promptly amends the interactive data files after becoming aware that the interactive data files fails to comply with the submission requirements. Users of this data are advised that, pursuant to Rule 406T, these interactive data files are deemed not filed and otherwise are not subject to liability. |
77
