Attached files
| file | filename |
|---|---|
| EX-3.3 - Tanke Biosciences Corp | e608029_ex3-3.htm |
| EX-3.2 - Tanke Biosciences Corp | e608029_ex3-2.htm |
| EX-4.1 - Tanke Biosciences Corp | e608029_ex4-1.htm |
| EX-4.3 - Tanke Biosciences Corp | e608029_ex4-3.htm |
| EX-4.2 - Tanke Biosciences Corp | e608029_ex4-2.htm |
| EX-99.3 - Tanke Biosciences Corp | e608029_ex99-3.htm |
| EX-10.1 - Tanke Biosciences Corp | e608029_ex10-1.htm |
| EX-10.2 - Tanke Biosciences Corp | e608029_ex10-2.htm |
| EX-10.9 - Tanke Biosciences Corp | e608029_ex10-9.htm |
| EX-10.6 - Tanke Biosciences Corp | e608029_ex10-6.htm |
| EX-10.8 - Tanke Biosciences Corp | e608029_ex10-8.htm |
| EX-99.1 - Tanke Biosciences Corp | e608029_ex99-1.htm |
| EX-99.2 - Tanke Biosciences Corp | e608029_ex99-2.htm |
| EX-10.3 - Tanke Biosciences Corp | e608029_ex10-3.htm |
| EX-10.5 - Tanke Biosciences Corp | e608029_ex10-5.htm |
| EX-10.7 - Tanke Biosciences Corp | e608029_ex10-7.htm |
| EX-10.4 - Tanke Biosciences Corp | e608029_ex10-4.htm |
| EX-10.10 - Tanke Biosciences Corp | e608029_ex10-10.htm |
|
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________
|
||
|
FORM 8-K
|
||
|
CURRENT REPORT
|
||
|
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
|
||
| Date of Report (Date of earliest event reported) February 9, 2011 | ||
|
Tanke Biosciences Corporation
|
||
|
(Exact name of registrant as specified in its charter)
|
||
|
Nevada
|
000-53529
|
26-3853855
|
|
(State or other jurisdiction
of incorporation)
|
(Commission
File Number)
|
(I.R.S. Employer
Identification No.)
|
|
Room 2801, East Tower of Hui Hao Building, No. 519 Machang Road, Pearl River New City, Guangzhou, People’s Republic of China
|
510627
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
|
|
Registrant’s telephone number, including area code +86-20-38859025
|
|||
|
19 East 200 South, Suite #1080, Salt Lake City, Utah 84111
|
|||
|
(Former name or former address, if changed since last report.)
|
|||
|
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|||
|
¨
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
|
||
|
¨
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
|
||
|
¨
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
|
||
|
¨
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13a-4(c))
|
||
1
EXPLANATORY NOTE
This Current Report on Form 8-K is being filed in connection with a series of transactions consummated by Tanke Biosciences Corporation (formerly known as Greyhound Commissary, Inc.) (the “Company”), and with certain events and actions taken by the Company.
This Current Report on Form 8-K includes the following items on Form 8-K:
|
Item 1.01
|
Entry into a Material Definitive Agreement
|
|
Item 2.01
|
Completion of Acquisition or Disposition of Assets
|
|
Item 3.02
|
Unregistered Sales of Equity Securities
|
|
Item 5.01
|
Changes in Control of Registrant
|
|
Item 5.02
|
Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers
|
|
Item 5.03
|
Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
|
|
Item 5.06
|
Change in Shell Company Status
|
|
Item 9.01
|
Financial Statements and Exhibits
|
When used in this Current Report on Form 8-K, the terms “we,” “us,” “our” and similar terminology reference to the Company.
Item 1.01 Entry into a Material Definitive Agreement
Securities Purchase Agreement
On February 9, 2011, in connection with the closing of the transactions described in Item 3.02 of this Current Report on Form 8-K (the “Private Placement”), Greyhound Commissary, Inc. entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with the purchasers identified on Schedule A thereto (each, including their respective successors and assigns, an “Investor” and collectively, the “Investors”) and, with respect to certain sections thereof, Euro Pacific Capital, Inc. (the “Lead Placement Agent”) and Newbridge Securities Corporation (the “Co-Placement Agent”), relating to a private placement by the Company of 6,669,627 units (the “Units”), with each Unit consisting of a $1.15 principal amount 8% Senior Convertible Note (each, a “Note”) and a Common Stock Purchase Warrant (each, a “Warrant”) to purchase one share of the Company’s common stock, par value $0.001 per share (the “Common Stock”) with an exercise price of $1.40 per share. The Private Placement was not a “public offering” as defined in Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) and met the requirements to qualify for exemption under Regulation D promulgated under the Securities Act (“Regulation D”).
2
In addition to the other terms described in Item 3.02 of this Current Report on Form 8-K, pursuant to the Securities Purchase Agreement, the Company has agreed, among other things:
(a) that for so long as the Investors continue to hold at least a majority in principal amount of the outstanding Notes, the Lead Placement Agent shall be entitled to nominate one member to the Company’s board of directors, which member shall be an “independent” director as defined under Nasdaq Marketplace Rules;
(b) that during the six months following the closing of the Private Placement, the Company shall not issue any “Future Priced Securities” as such term is described by the rules and regulations of the Financial Industry Regulatory Authority (“FINRA”);
(c) to use its commercially reasonable best efforts to have its Common Stock listed or quoted for trading on any of the NYSE AMEX, the NASDAQ Global Market or the NASDAQ Capital Market as soon as is reasonably practicable following the date that the Company meets the requirements of any such trading markets;
(d) that within six (6) months of the closing of the Private Placement, the Company shall appoint to its board of directors individuals constituting a majority of “independent” directors (as defined under Nasdaq Marketplace rules), with one director designated by the Lead Placement Agent (who may qualify as one such independent director) and with at least two of such directors being fluent in English;
(e) that within six (6) months of the closing of the Private Placement, the Company shall enter into a twenty-four (24) month agreement with a new Chief Financial Officer of the Company who is reasonably satisfactory to the Lead Placement Agent and who is proficient in: (i) generally accepted accounting principles in the United States of America (“GAAP”); (ii) transactions similar to the ones contemplated by the Securities Purchase Agreement; and (iii) U.S. public company listings and the related filing and compliance requirements; and
(f) that within three (3) months of the closing of the Private Placement, the Company shall enter into a 12 month agreement with an investor and public relations firm that is reasonably satisfactory to the Lead Placement Agent.
Notes
On February 9, 2011, the Company offered and sold $7,670,071.50 worth of Notes convertible into up to 6,669,627 shares of our Common Stock, in conjunction with a purchase of the Units in the Private Placement. The Notes are payable 24 months from February 9, 2011 with an interest rate of 8% per annum payable semiannually in arrears. The Company shall place in escrow with Escrow, LLC (the “Escrow Agent”) an amount of the proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments. Until such time as 75% of the Notes are converted into shares of Common Stock, if such escrow is depleted in order to make interest payments, the Company will replenish such escrow amount. At the option of the holder, the Notes may be converted into Common Stock at a price of $1.15 per share, which is subject to customary weighted average and stock based anti-dilution protection. The issuance of the Notes was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
3
The Notes contain customary events of default and affirmative and negative covenants of the Company, including negative covenants which restrict the Company’s ability to do the following (among other things) without the consent of the Lead Placement Agent, as representative of the Investors: (i) incur, or permit to exist, any indebtedness for borrowed money in excess of (A) US$3,000,000 during the twelve (12) month period beginning on February 9, 2011, or (B) US$5,000,000 during the two-year period beginning on February 9, 2011 and ending on February 9, 2013 (the maturity date of the Notes), except in the ordinary course of the Company’s business; (ii) lend or advance money, credit or property to or invest in (by capital contribution, loan, purchase or otherwise) any person or entity in excess of US$1,000,000 except: (A) investments in United States Government obligations, certificates of deposit of any banking institution with combined capital and surplus of at least $200,000,000; (B) accounts receivable arising out of sales in the ordinary course of business; (C) inter-company loans between and among the Company and its subsidiaries; and (D) the loan described under the heading “Certain Relationships and Related Transactions – Loans to Affiliates” under Item 2.01 of this Current Report on Form 8-K, which disclosure is incorporated herein by reference; (iii) pay dividends or make any other distribution on shares of the capital stock of the Company; (iv) create, assume or permit to exist, any lien on any of the Company’s property or assets now owned or hereafter acquired, subject to existing liens and certain exceptions; (v) assume guarantees, subject to certain exceptions; (vi) engage in “sale-leaseback” transactions, subject to certain exceptions; (vii) make capital expenditures in excess of US$5,000,000 in any fiscal year, subject to certain exceptions; and (viii) materially alter the Company’s business.
The above description of Notes is qualified in its entirety by reference to the actual agreement, a copy of which is filed as Exhibit 4.1 hereto and incorporated herein by reference.
Warrants
On February 9, 2011, the Company offered and sold Warrants to purchase 6,669,627 shares of Common Stock in conjunction with a purchase of the Units in the Private Placement. Each Warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable in whole or in part, at an initial exercise price per share of $1.40, which is subject to customary weighted average and stock based anti-dilution protection. The Warrants may be exercised at any time upon the election of the holder, beginning on the date of issuance and ending of the third anniversary of the closing of the Private Placement. The issuance of the Warrants was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
4
Upon the expiration of the Warrant exercise period, the Warrants will expire and become void. In order to exercise the Warrants, the Warrant must be surrendered at the office of the Warrant Agent (as defined in the Warrants) prior to the expiration of the Warrant exercise period, with the form of exercise appearing with the Warrant completed and executed as indicated, accompanied by payment of the full exercise price for the number of Warrants being exercised. Payment shall be by wire transfer or certified check payable to the Company. In the case of partial exercise, the Company will issue a new warrant to the exercising warrant holder, or assigns, evidencing the Warrants which remain unexercised.
In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate in the distribution of our assets.
Holders of Warrants do not have voting, pre-emptive, subscription or other rights of stockholders in respect of the Warrants, nor shall the holders of the Warrants be entitled to receive dividends.
The above description of Warrants is qualified in its entirety by reference to the actual agreement, a copy of which is filed as Exhibit 4.2 hereto and incorporated herein by reference.
Agent Warrants
In connection with the Private Placement, on February 9, 2011, the Company issued to certain affiliates of the Lead Placement Agent three-year warrants (the “Agent Warrants”) to purchase an aggregate of 666,963 shares of Common Stock at an exercise price of $1.15 per share. The Agent Warrants also contain a cashless exercise option. The issuance of the Agent Warrants was not registered under the Securities Act. The issuance of the Agent Warrants was exempt from registration under Section 4(2) of the Securities Act.
The above description of Agent Warrants is qualified in its entirety by reference to the actual agreement, a copy of which is filed as Exhibit 4.3 hereto and incorporated herein by reference.
Registration Rights Agreement
On February 9, 2011, the Company entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the Investors which sets forth the rights of the Investors to have their shares of Common Stock underlying the Notes and the Warrants (and certain other securities as described below) registered with the Securities and Exchange Commission (the “SEC”) for public resale.
Pursuant to the Registration Rights Agreement, the Company has agreed to file a Registration Statement on Form S-1 (the “Registration Statement”) with the SEC within 60 days of the closing of the Private Placement registering the total number of shares of Common Stock underlying the Units sold in the Private Placement (including such shares that are issuable upon exercise of the Warrants). In the event that the SEC provides comments pursuant to Rule 415 of the Securities Act, the Company shall cutback the number of shares it registers pursuant to the applicable SEC guidance. The Company has agreed to use its best efforts to have the Registration Statement declared effective within 160 days after the initial filing with the SEC (or, if the Registration Statement is not reviewed by the SEC, then the Company shall cause the Registration Statement to be declared effective within 5 business days of notification from the SEC that there will not be a review). The Company has also agreed to maintain the effectiveness of the Registration Statement until all of the securities covered by the Registration Statement may be sold by investors under Rule 144 of the Securities Act (“Rule 144”) without any restriction (including volume restrictions).
5
The Registration Rights Agreement provides that in the event the Registration Statement has not been filed or declared effective within the prescribed time period or if the Company has failed to maintain the effectiveness of the Registration Statement as required, the Company shall pay to the Investors liquidated damages equal to 1.0% of the amount invested for each subsequent 30-day period until the Company cures the failure to file, go effective or maintain effectiveness, as applicable, up to a maximum of 6.0%, and prorated for any period of less than 30 days.
Interest Escrow Agreement
On February 9, 2011, in connection with the Private Placement, the Company entered into an Escrow Agreement (the “Interest Escrow Agreement”) with the Lead Placement Agent and the Escrow Agent, as escrow agent. Pursuant to the terms of the Interest Escrow Agreement, the Company deposited into escrow an amount of proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments under the Notes. Until such time as 75% of the Notes are converted into shares of Common Stock, if such escrow is depleted in order to make interest payments, the Company has agreed to promptly replenish such escrow amount.
Securities Escrow Agreement
On February 9, 2011, in connection with the Private Placement, the Company entered into a Securities Escrow Agreement (the “Securities Escrow Agreement”) with the Lead Placement Agent, as agent, Golden Genesis Limited, a British Virgin Islands company (“Golden Genesis”) and the Escrow Agent, as escrow agent. Golden Genesis was the sole shareholder of China Flying Development Limited, a Hong Kong incorporated company (“China Flying”), which became a wholly-owned subsidiary of the Company under the share exchange (the “Share Exchange”) described in Item 2.01 of this Current Report on Form 8-K, pursuant to the Share Exchange Agreement, dated January 3, 2011 (the “Share Exchange Agreement”) and described in the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011.
6
Pursuant to the Securities Escrow Agreement, Golden Genesis will place in escrow 2,000,000 shares of Common Stock (the “Escrow Shares”), to be disbursed to either the Investors on a pro rata basis or to Golden Genesis based on the financial performance of Guangzhou Tanke Industry Co., Ltd. (“Tanke”), a company organized under the laws of the People’s Republic of China (“China” or the “PRC”) and the Company’s principal operating business through the VIE Agreements (as defined and more fully described in Item 2.01 of this Current Report on Form 8-K under the heading “Completion of Acquisition of Assets”). If the Company’s “Adjusted Income” (as defined below) for the year ending December 31, 2011 is (i) at least $4,652,410, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $4,652,410, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. If Tanke’s Adjusted Income for the year ending December 31, 2012 is (i) at least $7,571,111, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $7,571,111, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. For the purposes of the Securities Escrow Agreement, “Adjusted Income” means the sum of: (A) the Company’s net income; plus (B) any expense incurred in connection with the transactions contemplated by the Securities Purchase Agreement in connection with the Private Placement, including, without limitation, expenses related to the filing of a registration statement; plus (C) any depreciation and amortization expenses related to the expenses described in (B) above for the fiscal year ending December 31, 2011 or December 31, 2012 (as applicable), in each case as determined in accordance with GAAP, as reported in the Company’s Annual Report on Form 10-K as filed with the SEC.
7
Item 2.01Completion of Acquisition or Disposition of Assets
Completion of Acquisition of Assets
Pursuant to the Share Exchange Agreement, the Company acquired China Flying and its indirect, controlled subsidiary Tanke, a leading animal nutrition and innovative feed additive provider in China. The closing of the Share Exchange took place on February 9, 2011. On February 9, 2011, pursuant to the terms of the Share Exchange Agreement, the Company acquired all of the outstanding equity securities of China Flying (the “China Flying Shares”) from Golden Genesis, which was the sole shareholder of China Flying immediately prior to the closing of the Share Exchange, and Golden Genesis transferred and contributed all of its China Flying Shares to the Company. In exchange, the Company issued to Golden Genesis 10,758,000 newly issued shares of Common Stock. In addition, pursuant to the terms of the Share Exchange Agreement, the Company effected a 1 for 8.512 reverse stock split to modify the Company’s capital structure to accommodate the transactions contemplated by the Share Exchange and the Private Placement and to put in place an appropriate capital structure for the Company following the closing of the Share Exchange and the Private Placement. Such securities were not registered under the Securities Act. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance of securities by us did not involve a public offering.
China Flying owns 100% of the issued and outstanding capital stock of Guangzhou Kanghui Agricultural Technology Co., Ltd. (“Kanghui Agricultural” or the “WFOE”), a wholly foreign owned enterprise incorporated as a limited liability company under the laws of the PRC. On January 3, 2011, the WFOE entered into a series of variable interest entity contractual agreements (the “VIE Agreements”) with Tanke and the shareholders of Tanke, namely Mr. Guixiong Qiu (“Mr. Qiu”), Mr. Bi Gao (“Mr. Gao”), Ms. Xiuzhen Liang (“Ms. Liang”) and Mr. Bing Teng (“Mr. Teng”) (collectively referred to as the “Tanke Shareholders”) who are all PRC citizens. Pursuant to the VIE Agreements, Kanghui Agricultural effectively assumed management of the business activities of Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Tanke. The VIE Agreements are comprised of a series of agreements, including a Consulting Services Agreement, Operating Agreement, Voting Rights Proxy Agreement, Equity Pledge Agreement and Option Agreement, through which Kanghui Agricultural has the right to advise, consult, manage and operate Tanke for an annual fee in the amount of Tanke’s yearly net profits after tax. Additionally, the Tanke Shareholders have pledged their rights, titles and equity interest in Tanke as security for Kanghui Agricultural to collect consulting and services fees provided to Tanke through an Equity Pledge Agreement. In order to further reinforce Kanghui Agricultural’s rights to control and operate Tanke, the Tanke Shareholders have granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Tanke through an Option Agreement. The terms of the VIE Agreements are more fully described below:
|
|
·
|
Consulting Services Agreement. Tanke and Kanghui Agricultural have entered into a Consulting Services Agreement which provides that Kanghui Agricultural will be the exclusive provider of entrusted management service to Tanke and Tanke will pay all of its net income based on the quarterly financial statements to Kanghui Agricultural for such services. Any such payment to Kanghui Agricultural from the Company would need to comply with applicable Chinese laws affecting payments from Chinese companies to non-Chinese companies. See “Risk Factors – Risks Associated With Doing Business in China.”
|
8
|
|
·
|
Equity Pledge Agreement. Kanghui Agricultural, Tanke and the Tanke Shareholders have entered into an Equity Pledge Agreement, pursuant to which each Tanke Shareholder has pledged all of his shares of Tanke to Kanghui Agricultural in order to guarantee cash-flow payments under the applicable Consulting Services Agreement. The Equity Pledge Agreement further entitles Kanghui Agricultural to collect dividends from Tanke during the term of the pledge.
|
|
|
·
|
Operating Agreement. Pursuant to the operating agreement among Kanghui Agricultural, Tanke and each Tanke Shareholder, Kanghui Agricultural provides guidance and instructions on Tanke’s daily operations and financial affairs. The Tanke Shareholders must designate the candidates recommended by Kanghui Agricultural as their representatives on their respective boards of directors. Kanghui Agricultural has the right to appoint senior executives of Tanke. In addition, Kanghui Agricultural agrees to guarantee Tanke’s performance under any agreements or arrangements relating to Tanke’s business arrangements with any third party. Tanke, in return, agrees to pledge its accounts receivable and all of its assets to Kanghui Agricultural. Moreover, Tanke agrees that without the prior consent of Kanghui Agricultural, Tanke will not engage in any transactions that could materially affect its assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party.
|
|
|
·
|
Option Agreement. Tanke, the Tanke Shareholders and Kanghui Agricultural have entered into an Option Agreement which provides that the Tanke Shareholders granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Tanke.
|
|
|
·
|
Voting Rights Proxy Agreement. Tanke, the Tanke Shareholders and Kanghui Agricultural entered into a Voting Rights Proxy Agreement which provides that the Tanke Shareholders irrevocably appoint the persons designated by Kanghui Agricultural with exclusive right to exercise the Tanke Shareholders’ voting rights.
|
As a result of the VIE Agreements described above, we have consolidated Tanke’s historical financial results in our financial statements as a variable interest entity pursuant to U.S. GAAP following the date of the agreements and combined such results prior to the date of the agreements.
9
We have been advised by PRC legal counsel Martin Hu & Partners LLP, in an opinion dated January 4, 2011, that: (1) our inner-PRC shareholding structure complies with PRC laws and regulations; (2) the contractual arrangements between Kanghui Agricultural, Tanke and the Tanke Shareholders are valid and binding on all parties to these arrangements and do not violate relevant PRC laws or regulations; (3) each of Kanghui Agricultural and Tanke has the requisite corporate power to own, lease and operate its properties, to enter into contracts and to conduct its business and (4) each of Kanghui Agricultural and Tanke is qualified to do business in the respective jurisdiction of its establishment.
In addition, on January 3, 2011, the Tanke Shareholders each entered into a call option agreement (the “Call Option Agreement”) with Golden Genesis, which became effective upon the closing of the Share Exchange. Under the Call Option Agreement, Golden Genesis shall transfer up to 100% of the shares of Common Stock that it receives in the Share Exchange within the next 3 years to the Tanke Shareholders for nominal consideration, resulting in the Tanke Shareholders owning a majority of the outstanding shares of the Common Stock. The Call Option Agreement provides that Golden Genesis shall not dispose of the respective portion of the shares of Common Stock without the Tanke Shareholders’ prior written consent.
Through Kanghui Agricultural and China Flying, the Company will operate and control Tanke as a result of the VIE Agreements. Control of Tanke was acquired through the VIE Agreements, rather than an acquisition of Tanke’s assets or equity, because: (i) the tax and other consequences of a share exchange with a foreign entity that result in the acquisition of a Chinese company are uncertain due to PRC laws that were effective on September 8, 2006 and (ii) if Tanke is not acquired via a share exchange transactions, PRC authorities may require it to be acquired for cash, however the Company was not able to raise a sufficient amount of cash to purchase Tanke.
The foregoing descriptions of the VIE Agreements and the Call Option Agreement, and the transactions contemplated thereby, are subject to the more detailed provisions set forth in the VIE Agreements, which are attached as Exhibits 10.5, 10.6, 10.7, 10.8, 10.9 and 10.10 to this Current Report on Form 8-K and which are incorporated herein by reference.
Pursuant to the Share Exchange Agreement, China Flying became a wholly-owned subsidiary of the Company. The board of directors and majority stockholder of the Company immediately prior to the closing of the Share Exchange approved the Share Exchange Agreement and the Share Exchange. The directors and shareholders of China Flying also approved the Share Exchange Agreement and the Share Exchange.
As a further condition of the closing of the Share Exchange, the officers and directors of the Company resigned and Mr. Qiu was appointed as the a new executive officer of the Company and also its sole director upon effectiveness of an information statement required by Rule 14f-1 (the “Information Statement”) promulgated under the Exchange Act of 1934, as amended (the “Exchange Act”).
10
The foregoing descriptions of the Share Exchange Agreement and the transactions contemplated thereby are subject to the more detailed provisions set forth in the Share Exchange Agreement, which is attached as exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011. All references to the Share Exchange Agreement and to the exhibits to this Current Report on Form 8-K are qualified, in their entirety, by the text of such exhibits.
CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS
Certain information contained in this Current Report on Form 8-K include forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The statements herein which are not historical reflect our current expectations and projections about the Company’s future results, performance, liquidity, financial condition, prospects and opportunities and are based upon information currently available to the Company and their management and their interpretation of what is believed to be significant factors affecting the businesses, including many assumptions regarding future events. Such forward-looking statements include statements regarding, among other things:
|
|
·
|
our ability to produce, market and generate sales of our products;
|
|
|
·
|
our ability to develop, acquire and/or introduce new products;
|
|
|
·
|
our projected future sales, profitability and other financial metrics;
|
|
|
·
|
our future financing plans;
|
|
|
·
|
our plans for expansion of our facilities;
|
|
|
·
|
our anticipated needs for working capital;
|
|
|
·
|
the anticipated trends in our industry;
|
|
|
·
|
our ability to expand our sales and marketing capability;
|
|
|
·
|
acquisitions of other companies or assets that we might undertake in the future;
|
|
|
·
|
our operations in China and the regulatory, economic and political conditions in China;
|
|
|
·
|
our ability as a U.S. company to operate our business in China through an indirect wholly-owned subsidiary; and
|
|
|
·
|
competition existing today or that will likely arise in the future.
|
11
Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” “seek,” or “project” or the negative of these words or other variations on these words or comparable terminology. Actual results, performance, liquidity, financial condition and results of operations, prospects and opportunities could differ materially from those expressed in, or implied by, these forward-looking statements as a result of various risks, uncertainties and other factors, including the ability to raise sufficient capital to continue the Company’s operations. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this Current Report on Form 8-K generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this Current Report on Form 8-K will in fact occur. Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
The specific discussions herein about the Company include financial projections and future estimates and expectations about the Company’s business. The projections, estimates and expectations are presented in this Current Report on Form 8-K only as a guide about future possibilities and do not represent actual amounts or assured events. All the projections and estimates are based exclusively on the Company management’s own assessment of its business, the industry in which it works and the economy at large and other operational factors, including capital resources and liquidity, financial condition, fulfillment of contracts and opportunities. The actual results may differ significantly from the projections.
Potential investors should not make an investment decision based solely on the Company’s projections, estimates or expectations.
Business
Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including the disclosures set forth in this Item 2.01 to this Current Report on Form 8-K, under the headings “Cautionary Note on Forward Looking Statements” and “Risk Factors”, which disclosures are incorporated herein by reference. As a result of the Share Exchange and the VIE Agreements, the Company, through Kanghui Agricultural, its indirect wholly owned subsidiary, assumed management of the business activities of Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Tanke. As used in this section, the terms “we”, “our”, “us” and the “Company” refer to the Company, our direct and indirect subsidiaries and Tanke, our principal operating business.
12
Company Background
The Company was organized on May 24, 1989 under the laws of the State of Idaho and was re-incorporated under the laws of the State of Nevada on November 1, 2007.
The Company was initially created to provide a variety of services related to the operation of a nearby greyhound dog-racing track. Following inception, Greyhound raised funds to assist it in providing food, shelter, healthcare and other services to animals used in the greyhound racing. Subsequently the track was closed and the business was curtailed. Since 1995, Greyhound has engaged in an ongoing search for suitable business opportunities, including a potential merger.
General
Through Tanke, our principal operating business, we are one of the leading animal nutrition and innovative feed additive providers in China. Our products are distinguished from traditional artificial feed additives in that they are environmentally-friendly and are designed to optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish. One of our most popular products is an organic trace mineral additive that we believe is one of the few Chinese-developed organic products in the trace mineral market.
Our target customers are mid-to-large sized feed product factories and large scale producers. The market for our products is significant and growing, as China seeks to meet the demand of its over 1.3 billion citizens for safe and reasonably priced food. With feed additives used in China at less than half the rate of that of the United States and Europe, we are seeking to capitalize on a significant market opportunity as Chinese feed producers modernize and expand the use of modern and more effective additives.
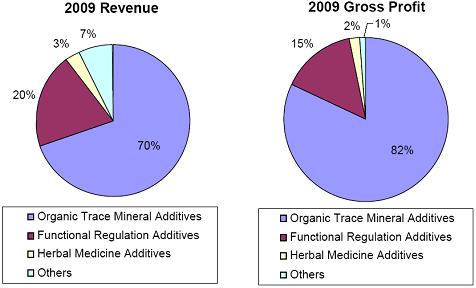
The Company currently produces 21 branded feed additives, with each brand available in seven different mixes that correspond to different stages of an animal’s life cycle. Our major products address most key market categories within China’s animal feed additive industry including: Organic Trace Mineral Additives, which in 2009 accounted for approximately 70% of our revenue, Feed Acidifiers and Flavor Enhancers, which accounted for approximately 20% of our revenue, and Herbal Medicine Additives, which accounted for approximately 3% of our revenue. Our extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. While the majority of our sales are domestic in the PRC, international sales, mainly in Southeast Asia, Latin American and other developing countries, currently account for approximately 5% of our total sales.
Currently, we estimate that we hold about 1% of the feed acidifier market in China and believe we are well positioned to take advantage of the changing marketplace and regulatory environment as the use of feed acidifiers increases.
13
Overview
Through Tanke, our principal operating business, the Company is engaged in the development, production, marketing and distribution of a broad range of innovative feed additive products that foster the growth of healthy and profitable animals. A PRC government certified hi-tech company, the Company’s headquarters are in Guangzhou and it operates in a modern 34,000 square-meter manufacturing facility in the Huadu Economic District, both of which are in Guangdong province. As the Chinese economy continues to evolve and prosper, the opportunity for technology companies like the Company should increase dramatically.
The Company’s feed additive products are distinguished from traditional artificial feed additives in that they are non-hazardous, environmentally friendly and safe for livestock and their human consumers, making them compatible with China's efforts to develop a safer food supply. Such feed additive products are environmentally friendly because animals that consume them produce less waste products than other animals and a decrease in the amount of waste produced is beneficial to the environment.
As a growing player in providing advanced, environmentally-friendly and innovative feed additives, the Company believes sales will rapidly increase as more large scale farms and feed processing and production companies in China seek “Pollution-Free” and “Green Food” certifications from the Chinese government. In addition, feed additives are utilized in China at less than half the rate of the United States and Europe, and the Company has a significant growth opportunity as Chinese farmers and ranchers include a greater amount of increasingly sophisticated additives in their feeds.
The Company’s major products address most key market categories within China’s animal feed additive industry, including:
|
|
·
|
Organic Trace Mineral Additives, which accounted for approximately 70% of the Company’s revenue in 2009;
|
|
|
·
|
Feed Acidifiers, Seasonings and Flavor Enhancers, which accounted for approximately 20% of the Company’s revenue in 2009; and
|
|
|
·
|
Herbal Medicine Additives, which account for approximately 3% of the Company’s revenue in 2009.
|
The Company’s extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. As a result of the Company’s diversified products and extensive distribution network, we believe the Company is ideally positioned to help meet China’s growing demand for safe and reasonably priced food.
14
From Tanke’s incorporation in April 1997, when it manufactured one product utilizing a small rented facility in Gaotang in the Guangdong province, Tanke has grown into one of China’s largest feed additive producers. As of December 31, 2009, the Company marketed 21 different brands of feed additives and had an aggregate production capacity of approximately 400 metric tons per week of feed additive, of which 250 metric tons are organic trace minerals. We estimate that we are currently operating at a blended average across our product lines of approximately 44% of our manufacturing capacity at our current facility. We expect our manufacturing output to increase in 2011 and beyond, and are thus seeking to acquire land for and to build a second facility, which is currently scheduled to be operational in the fourth quarter of 2011.
Currently, the Company plans to introduce two new products into the market in 2011. The first, CA-13, is antibiotic free and designed to enhance the breeding of early-wean piglets by improving their immune system and reducing the days needed for the production of fully-grown pigs. The second, a vitamin additive, enhances the effectiveness of the Company’s trace minerals by promoting greater growth, reducing animal stress and improving the immune system. We expect both products to enhance our future growth.
Overview of the Chinese Feed Additive Market
Over the past decade, as a result of a series of market-based reforms, China’s economy has experienced unprecedented growth, with an average annual GDP growth rate of over 10%. As China has become more prosperous, the rapid growth in per capita income and consumer choices has led to a dramatic improvement in living standards and dietary patterns. Chinese consumers have significantly increased their consumption of high-protein food such as meat and other livestock and in the place of traditional staple grain-based foods. This growing demand for high-protein foods has had a material impact on the growth of the feed additive market.
Beginning in the mid-1970s, in response to China’s increasing and diversifying food consumption, its domestic feed industry began to experience rapid development and transitioned into the world’s second largest feed producer behind the United States. According to IBIS, in 2014, projected animal feed production in China is expected to grow to 160 million tons from 110 million tons in 2009, a compound annual growth rate of 7.7%. Also, according to market research firm IBIS, in revenue terms, the Chinese feed market is expected to grow from annual sales of $40 billion in 2009 to $69 billion in 2014, a compound annual revenue growth rate of 11.4%.
In the past decade, feed producers have become more efficient, with new, high production mills replacing older, smaller mills. As part of their effort to improve the quality of agricultural output and the efficiency of animal production, commercial feed producers have increased their use of feed additives. According to the Chinese Ministry of Agriculture, the Chinese market for all feed additives in 2009 was $4.6 billion.
According to data published on the website of Chinafeed Industrial Information, China’s current annual consumption of feed additives as a percentage of total feed output measured in tonnage is only 2%, compared to 5% in developed countries. Of this 2%, more than 85% represents low-profit products like inorganic mineral materials, with the remaining 15% representing organic trace products, such as amino acids and vitamins, that have higher prices and profit margins. Over 70% of these higher-margin products are imported, creating a significant opportunity for domestic manufacturers, like the Company, to increase their market share. Additionally, while the United States and Western Europe have over 300 feed additive formulas approved for use, to date China has only approved approximately 200 feed additive formulas, of which most is imported. As a result of China’s approval of fewer feed additive formulas, there is an ample market opportunity for new Chinese-manufactured additives.
15
One of the primary components of the Company’s business is Feed Acidifiers and it has gained significant momentum in recent years as a substitute for growth promoters that rely on antibiotics as the primary ingredient. The desirability of Feed Acidifiers is a result of growing concerns about drug-resistant “superbugs” in humans and animals resulting from the indiscriminate use of antibiotics. The global feed industry has been under scrutiny for years for its use of antibiotics as growth promoters in the rearing process of livestock, prompting the European Union to ban the use of Antibiotic Growth Promoters (AGPs) in January 2006. The Chinese government is currently tightening the industry standard and may follow the EU’s lead to restrict or ban the use of AGPs. The Company’s Feed Acidifiers differ from other growth promoters because they contain alkaloids to stimulate acid production in an animal’s stomach, lowering the pH levels to improve overall animal health. Such Feed Acidifiers do not rely on antibiotics as a primary ingredient. Rather, the ingredients in the Company’s Feed Acidifiers comply with the more restrictive requirements of the EU and the increased regulation that the Company anticipates in the future from China’s regulatory authorities.
In response to quality control breakdowns from isolated Chinese manufacturers in 2007, in June, 2009, the Chinese Ministry of Agriculture announced Bulletin No. 1224 (the Safe Use of Feed Additive Specification), which tightened feed additive quality control standards by specifying “norms” of trace elements and other feed additives usage. The maximum normative amounts set by the Chinese government are required to be strictly followed and implemented. As a result of these regulations, the Company expects this new regulation to drive the market to shift from high dosage, low absorption rate inorganic products to low dosage, high absorption rate organic products.
As one of the pioneers in the organic trace minerals additive segment, Tanke, as a member of the Chinese Feed Association, participated in setting the national standards on the usage of organic trace minerals additives. As a result, we are well positioned to further expand our market position.
Competition in the Chinese Market
The Chinese feed additive market is highly fragmented, with approximately 2,500 feed additive companies nationwide and no participant having a greater than 1.2% market share. While many of the Company’s domestic competitors are smaller businesses that operate in relatively specialized niche product areas, the industry is in the process of transforming itself from small, family-based operations into large, enterprise based businesses.
Foreign firms are also attempting to gain a foothold in the Chinese feed additive market, but generally charge higher prices than those of domestic manufacturers.
16
The Company’s largest Chinese competitors include:
|
|
·
|
Changsha Xingjia Bio Tech Co., Ltd., which is engaged in developing, marketing and producing safe, environmental friendly trace mineral feed additives. Changsha offers compound acidifier, amino acid chelated trace elements, copper chloride and other products. Changsha has sales office nationwide and subsidiaries in Thailand and Singapore.
|
|
|
·
|
Debon Bio Tech Co., Ltd., which was established in 2004 and is a Sino-German joint venture engaged in feed additive development and raw material trading. Debon has a long term partnership with its German partner and imports piglet nutrition and feed additives from overseas.
|
The Company also competes with the following large international manufacturers:
|
|
·
|
Zinpro Corporation, a manufacturer of trace minerals. Zinpro offers iron, copper, manganese, zinc and cobalt products used in the dairy, beef, poultry, swine, and equine industries. Headquartered in Eden Prairie, Minnesota, Zinpro has sales offices in the United States, Canada, Mexico, the Netherlands, China, Japan, Thailand, Brazil, Australia and New Zealand.
|
|
|
·
|
Alltech Inc., an animal health and nutrition company. Alltech manufactures nutritional products and solutions for the feed industry. It provides natural feed ingredients and Sel-Plex organic selenium for use in animal species with selenium deficiencies for feed and food manufactures in North America, Latin America, the Asia-Pacific, Europe, the Middle East, and Africa. Alltech is headquartered in Nicholasville, Kentucky and has bioscience centers in the United States, Ireland, and Thailand.
|
Growth Strategy
The Company’s goal is to become the leading provider of feed additives in China. The Company’s primary growth strategy is as follows:
Strengthen our leading position in the organic trace mineral market and substantially increase our Chinese market share within the next three years.
In recent years, Chinese inorganic minerals have been linked to contamination by heavy metals and dioxins. As a result, farmers and feed producers are increasingly switching to organic trace minerals based on research that indicates that quality organic minerals are superior to inorganic minerals in bioavailability, health and performance.
As the largest organic trace minerals producer in China, accounting for approximately 6.6% of total production on an annual basis, the Company is ideally positioned to benefit from the substantial growth it anticipates in the organic trace mineral market. To meet the expected demand, we plan to build a second manufacturing facility that would double our organic trace mineral production capacity.
17
Expand sales of the Company’s products to more regions within China.
As of the end of 2009, the Company had sales representatives in six of the major agriculture centers in China, including China’s northeastern and southern regions. To expand our reach into China’s other regions, we intend to establish sales offices in the northern and central regions of China and hire 25 additional sales personnel in 2011.
Increase the Company’s production capacity
Our current production capacity is 400 metric tons per week and our current production capacity is 250 metric tons per week for organic trace minerals. We estimate we are currently operating at a blended average across our product lines of approximately 44% of our manufacturing capacity at our current facility. We expect our manufacturing output to increase in 2011 and beyond, and are thus seeking to acquire land for and to build a second facility, which is currently scheduled to be operational in the fourth quarter of 2011. To acquire this second facility, and as part of the Company’s expansion strategy, the Company has entered into a letter of intent to purchase a land use right in Qingyuan, Guangdong to build our second manufacturing facility. Upon the completion of the project, we plan to move our manufacturing of organic trace minerals from our existing manufacturing facility in Huadu to the new facility in Qingyuan. This significant evolution of the Company’s manufacturing structure is expected to allow us to devote the Qingyuan facility to our core business of organic trace minerals while providing sufficient production capacity at the Huadu facility for the production of our other products. The Company expects this new facility, when completed, to significantly increase our total production capacity on an annual basis.
Increase the Company’s investment in research and development.
To maintain a competitive advantage in the marketplace, we plan to devote greater resources to our in-house research and development team and to enhance and expand our collaborations with institutions and universities. Our in-house research team typically requires one to two years to develop a new product and bring it to market. We intend to enhance our trial testing program by acquiring a farm operation to streamline our testing.
Additionally, we plan to strengthen our collaboration with institutions and universities as part of our effort to stay on the cutting edge of the feed additive business. The Company believes that as the Chinese market continues to grow and mature, companies will face increasing competitive pressure both in the area of technology and talented personnel. Our partnership with institutions and universities will not only provide the Company with skilled human resources, but also will assist us in staying on the cutting edge of the industry. Currently, the majority of the Company’s research partners are located in Guangzhou, and the Company intends to establish similar cooperative relationships with schools in other regions.
18
Strengthen international sales.
We plan to increase our attendance at industry exhibitions worldwide to market our products to a broader market of potential customers. In our experience, participating in these exhibitions is an effective way to introduce the Company’s products overseas and develop new customers.
Competitive Strengths
We believe that the following competitive strengths have contributed to our current market position and enables us to capitalize on the growth opportunities in the feed additive market in China:
We have a leading market position in the organic trace mineral market.
We are the largest provider and producer of organic trace minerals in China with production capacity of 250 metric tons per week. We entered into a letter of intent to acquire land use rights in Qingyuan, Guangdong province, to build a second manufacturing facility, a project we expect to take one year. Upon the completion of this facility, we expect to double our production capacity of organic trace minerals.
We offer a diversified product portfolio.
Following the introduction of Tanke’s first flavor enhancer into the market in 1997, we have introduced a broad product portfolio to the market, including organic trace mineral, functional regulation additives and herbal medicine additives. Within each of these segments, we produce a diverse array of products.
We have strong research and development capabilities.
We have made significant investments in research and development. We focus our research and development efforts on creating new products with large potential markets and on improving existing technologies, both with a view towards increasing market share and growing the business.
We have built a reputation for exceptional quality.
Our customers depend on our products’ reputation for exceptional quality and uniformity. We have an ISO 9001/2000 International Quality Management System Certificate for our operations management system and GMP for our manufacturer compliance for animal drugs, certifying our commitment to the integrity of our products.
19
Our management team has extensive knowledge of, and experience in, the feed additive industry.
Our management team, led by Guixiong Qiu, Tanke’s founder and the Company’s Chief Executive Officer, match their academic backgrounds in agriculture and chemistry with extensive knowledge of the feed additive industry in China and a proven track record of developing and marketing quality feed additive products.
Products and Services
We currently market 21 different brands of feed additives at various price points to meet the demands of existing and prospective customers. Within each brand there are seven different mixes that correspond to the different growth stages of an animal’s life cycle. Our business focuses on four key business areas: organic trace minerals additives, functional regulation additives, herbal medicine additives and other. The following chart shows each segment’s contributions to fiscal 2009 net sales and gross profit.
Organic Trace Mineral Additives
We are China’s largest domestic provider of organic trace mineral additives, specializing in the development and production of chelated organic trace minerals additives. Our current trace mineral manufacturing facility is the largest chelating facility in China and has the capacity to produce approximately 250 metric tons of organic trace mineral per week.
Our total revenue for organic trace minerals in 2009 was approximately $8.5 million. Such revenue accounted for approximately 70% of our 2009 revenues and net sales and carried a gross profit margin of approximately 52%.
20
Minerals play an important role in the growth and development of fish, livestock, pigs and cattle and are routinely used by breeders to supplement their animals’ diets. Animals require two classes of minerals: major minerals, which include sodium, potassium, chloride, calcium, magnesium, phosphorus, and sulfur, and trace minerals, which include copper, iron, manganese, molybdenum, zinc, chromium, fluorine, selenium and silicon. Major and trace minerals are differentiated primarily by the amount of a particular mineral that an animal requires. Animals require a minimum of 100 milligrams per day of the major minerals to carry out normal bodily functions and less than 100 milligrams per day of trace minerals.
While major minerals are typically present in most feed products provided to animals, Chinese farmers and ranchers are placing an increasing emphasis on the consumption of trace minerals, which help the animal’s body perform its daily routines more efficiently.
Trace minerals are widely available as feed additives in two main forms: organic and inorganic. Although both forms are commonly used, important differences exist in their bioavailability and environmental impact. Organic trace minerals increase bioavailability, reducing feed costs and minimizing nutrient buildup in the soil. Environmentally, new restrictions are likely to be imposed on producers to reduce nutrient excretion, making the Company’s organic feed additives more appealing to breeders. The Company’s products are more appealing because they achieve similar production results to inorganic feed additives while requiring smaller amounts of trace materials.
Our organic trace minerals are marketed primarily in, but also outside of, China to large scale feed producers and farmers under the brand name “Qili”. Our principle organic trace minerals products are:
|
|
·
|
Iron glycine chelate (G/Fe-140);
|
|
|
·
|
Iron glycine chelate (G/Fe-185);
|
|
|
·
|
Zinc glycine chelate (G/Zn-220);
|
|
|
·
|
Manganese glycine chelate (G/Mn-220);
|
|
|
·
|
Copper glycine chelate (G/Cu-210);
|
|
|
·
|
Chromium glycine chelate (G/Cr-001);
|
|
|
·
|
Iron methionine chelate (M/Fe-155);
|
|
|
·
|
Zinc methionline chelate (M/Zn-190);
|
|
|
·
|
Manganese methionine chelate (M/Mn-155);
|
|
|
·
|
Copper methionine chelate (M/Cr-001);
|
|
|
·
|
Zinc lysine chelate (L/Zn-105);
|
|
|
·
|
Zinc lysine chelate (L/Zn-145); and
|
|
|
·
|
Copper lysine chelates (L/Cu-100).
|
Qili products provide the essential minerals (zinc, copper, manganese and chromium) and lysine, which is an amino acid essential to a nutritious livestock feed program. Such products provide nutritional balance for the animals in order for them to have a healthier life. Qili products also help animals absorb these essential minerals and lysine in order to slow the process by which nutrients pass through the animal.
21
We also produce and market multiple trace mineral premix products for livestock and poultry under the trademark “Qilimix,” which has been particularly successful in foreign markets. These products contain highly bio-available minerals and result in the lowest excretion of minerals into the environment, especially for high content copper and zinc. Qilimix products are used to improve the reproductive performance of sows and breeder poultry, the growth and reproductive performance of pigs and the quality and color of animal carcasses.
Functional Regulation Additives
We are the one of the leading developers and providers of functional regulation additives in China. According to the Chinese Ministry of Agriculture, the Chinese market for functional feed additives in 2009, including feed acidifiers and flavor enhancers, was $328 million. Our total revenues and net sales of functional feed additives in 2009 was $2.4 million, accounting for approximately 1% of China’s total production.
Sales from functional regulation additives represent approximately 20% of our 2009 revenues and net sales and carry a gross profit margin of approximately 35%.
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers.
Feed Acidifiers
Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. We produce and market feed acidifiers under the trademark “Qilicid”. Qilicid products consist of alkaloids that stimulate acid production and lower pH levels, inhibiting the development of pathogenic bacteria in the stomach and stimulating endogenous pepsin activities in the stomach and enzyme production in the intestine.
Qilicid products slow the passage of the feed through the animal’s intestine, allowing ample time for digestion, increasing feed intake and nutritional efficiency, reducing undesired gut microorganisms, supporting endogenous digestive enzymes and improving animal growth performance.
Flavor Enhancers
Flavor enhancers are widely used throughout the world as an important agent in the production of blended and high-grade feed to ensure animals obtain the required nutrients and to improve feed efficiency. There are two types of flavor agents: aroma agents, which impart a pleasant scent to feed and come from the roots, stems, leaves, and fruits of natural plants and from artificial compounds, and taste agents, which include sweeteners, which improve the feed’s taste and promotes continuous eating and come from flavor agents, salty agents and other flavoring materials.
22
All flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption and yield from production animals.
As animals grow, their nutritional needs change, requiring corresponding changes to feed. Such feed changes often result in reduced intake by animals accustomed to the flavor of prior feeds. Flavor agents can be mixed with different feeds to result in the same or similar flavor as previous feeds, which helps animals maintain their food intake and successfully switch to a new formula. Additionally, during periods of weaning and transportation, animals and fish normally reduce feed intake. Adding a flavor agent to blended feeds can help alleviate stress and unease, increasing feed consumption and ensuring that an animal obtains the nutrients it requires.
We produce and sell the following (non-sugar) natural sweeteners, feed flavor enhancers, and attractants for use with feed for pigs, piglets, fish and other aquatic animals under the trademark “Tankeball™” Functional Flavoring Series:
|
|
·
|
Tanksweet ST (a mixed sweetener designed to improve the palatability and acceptability of all pig feed);
|
|
|
·
|
Tankarom ST (a feed flavor enhancer and functional physiological regulator that assists animals in overcoming the negative effects of weaning, stress, disease, medications or mal-flavored feedstuffs);
|
|
|
·
|
Tankmix SA (a co-mixed product with sweetener and flavoring that makes feed more attractive);
|
|
|
·
|
Tankebaal sweet (a mixed sweetener to improve the palatability and acceptability of all pig feed);
|
|
|
·
|
Tankarom (a functional physiological regulator);
|
|
|
·
|
Fishy Spicy (an aroma agent added to fishmeal to enhance fishy taste, cover-up mal-flavors in feeds and improve the palatability of feed products);
|
|
|
·
|
Aquatic Lives’ Attractants (consisting of concentrated extracts from natural seafood and high efficient attractants rich in amino acids that improve the feed intake of fish); and
|
|
|
·
|
Kimyso™ (a micro-granulated solid dispersion Kitasamycin premix).
|
Herbal Medicine Additives
We have placed an emphasis on developing and promoting herbal medicine additives blended with feed products in China. Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health.
23
Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives for animal consumption. Herbal Medicine Additives represent approximately 2.2% of our 2009 revenues and net sales and carry a gross profit margin of approximately 23.7%.
We produce and sell the following trademarked products in the Herbal Medicine Additives sector, all of which are derived from Chinese natural plants, herbs and minerals:
|
|
·
|
Extra-Health™ (improves animal immune system and functions);
|
|
|
·
|
Qilimix™(a natural feed additive for livestock and poultry designed to improve the reproductive and growth performance of farm animals); and
|
|
|
·
|
Recoccider™(a highly efficient anticoccidial premix containing Ethopabate and Diclazuril designed to inhibit DHSS and DHRS).
|
Our total revenue for Herbal Medicine Additives in 2009 was approximately $341,000. Such revenue accounted for approximately 3% of our total 2009 revenue, and carried a gross profit margin of approximately 31%.
In January of 2010, we successfully developed and introduced a new antioxidant with the active element extracted from an Asian tree vine, which can be used to maintain the quality of feed products. In the first nine months of 2010, we produced and delivered more than 20 tons of this new antioxidant product from sales to more than 30 customers, generating revenues and net sales of 4 million Renminbi (“RMB”) or approximately $588,235.30.
Other Revenues
We also engage in various businesses including the domestic distribution of raw materials and providing technical support and know-how to our customers. As one of the leading producers and distributors of feed additives in China, the Company is a well-respected brand and able to engage in certain distribution activities to other Chinese companies. In connection with this, the Company purchases raw materials from certain manufacturers and sells such materials domestically to other feed additive manufacturers. As a result of our well known brand and our great reputation, we are able to purchase raw material at a relatively lower price. Subsequently, we sell those raw materials to other customers at a premium.
Our total revenue for Other Revenue in 2009 was approximately $894,000. Such revenue accounted for approximately 7% of our total 2009 revenue, and carried a gross profit margin of approximately 7%. Revenue from raw material trading accounted for about 50% of Other Revenues.
24
In 2011, we intend to import dried distillers’ grains with soluble (DDGS) from North America. DDGS, a bi-product of ethanol production, is a high nutrient feed valued by the livestock industry. When ethanol factories manufacture ethanol, they use only starch from corn and grain sorghum. The remaining nutrients – protein, fiber and oil representing a third of the grain– are used to create DDGS. Because of the increased demand for ethanol, the production of DDGS is expected to double within the next several years, further increasing the quantity of DDGS available for use in livestock feeds.
As feed costs in China for breeders and broilers have reached record highs, there has been a gradual shift among breeders towards the use of alternative feedstuffs such as DDGS. Recognizing this trend, we plan to supply DDGS to mid- and large-size farmers and feed producers throughout China. We believe that importing DDGS and other similar products is a natural extension of our business of selling feed additives to producers. We anticipate significantly growing our raw materials importing business as Chinese demand grows and importation becomes simpler. We expect our raw materials importing business to eventually become a meaningful revenue contributor.
Marketing
The majority of our marketing in China is conducted through sales visits to feed producers, farmers and other potential customers. During these sales meetings, the Company’s sales team distributes marketing materials and shares its extensive knowledge on husbandry and cultivation of farm animals.
As part of our marketing effort, every two years, we co-sponsor the Chinese Academy’s international seminar on Animal Health Products and Feed Additives, inviting speakers and participants who are academic professionals, industry experts or key managers in the agriculture business. The seminar is designed to introduce recent developments and trends in the feed additive business and to provide a platform for increasing awareness of our products. Since beginning the seminar in 2002, it has become one of the important events in the industry and typically attracts more than five hundred professionals.
Outside of China, we market our products mainly through participation in industry exhibitions.
Sales and Distribution
Our target customers are mid-to-large sized feed product factories and large scale producers. These customers have substantial bargaining power and require the feed additive products that they use to meet the highest standards of quality, productivity and efficiency, which we believe gives us a competitive advantage over our smaller competitors. In total, we employ 43 sales or sales-related employees, including 21 in regional sales, 5 in distribution, 4 in the aquatic group, 4 in international sales, 3 in marketing and 6 in technical support.
As of December 31, 2009, we had one customer, Jin Yin Ka (Guangzhou) Biao-Tech Co., Ltd., that accounted for more than ten percent of its consolidated revenues, with such customer accounting for 15.28% of revenue.
25
We sell our products throughout China with a combination of our internal sales teams and a network of agents in 28 regions, including Guangdong, Sichuan, Shandong and Liaoning provinces, which have significant animal breeding industries. Presently, about 80% of our sales are generated through our internal sales force, with the remaining 20% via our agents. The Company currently has sales offices in the cities of Shengyang, Chengdu, Xiameng, Xi’an, Nanning and Zhengjiang.
The maps below serve as a snapshot to demonstrate the diversified market regions of our products within China.
For the 2009 fiscal year, sales to our top 10 customers accounted for approximately 18.11% of our revenue with the top two largest customers (Guangdong Huanong Wens Animal Husbandry Co. and Wens Food Group) accounting for approximately 11% of our revenue.
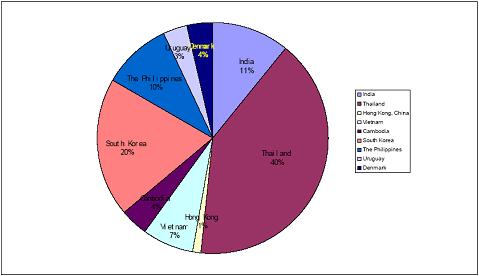
The map below depicts the diversified international market regions where we presently sell our products or intend to expand our distribution in the future. While the vast majority of our sales are domestic, international sales, mainly in Southeast Asia, Latin America and other developing countries, currently account for approximately 5% of sales.
26
The following chart depicts the current distribution of our products outside of China.
Customer Service
Our service and support infrastructure quickly and efficiently provides clients with customized products, technical support and advice. We assign our technicians to prospective customers to conduct a thorough analysis of the customer’s needs, followed by a detailed customized product manual. While the manuals vary according to the specific product, they typically include a product introduction that includes nutrition facts, a user guide, expiration dates and storage and packaging information, among other things. For the large-scale farmer or feed producer, our team also provides training courses to help our customers understand our products and how to use them most effectively.
27
Upon the delivery of our products to new customers, we provide after-sales support, which not only serves to resolve any technical issues, but also helps identify other opportunities for increasing business with current customers. We utilize a multi-tiered product strategy pursuant to which we tailors our products to the needs and preferences of the feed market.
Raw Materials and Suppliers
The raw materials for our products include agricultural commodities and fine powders like amino acid, organic trace minerals and organic acid. Although most of our principal raw materials are widely available in China, the price for certain raw materials can fluctuate. We have adopted measures to reduce our risks in both raw material supply costs and availability, including establishing long-term relationships with suppliers and diversifying supply sources.
To assure the consistency of our raw material supplies, we source most of our materials from mid-to large size companies. Before making any purchase with a new vendor, we evaluate the vendor’s products and attempt to select the most reliable and reputable vendor. We regularly conduct similar evaluations throughout our purchasing process to ensure that we are purchasing high quality raw materials at competitive prices. Because we source our raw materials from several vendors, we are not dependent on any particular vendor or merchant as a sole provider for our raw materials.
Our top ten suppliers constitute 66.7% of our total raw material suppliers in 2009 and 51.37% in 2008. The following table identifies our top suppliers of our products regarding the amount obtained from each supplier equal or more than 10% of the total suppliers during the years ended December 31, 2009 and 2008:
|
2009
|
2008
|
|||||||||||||||
|
Suppliers
|
Amount
|
% of
|
Amount
|
% of
|
||||||||||||
|
|
(ton)
|
Total
|
($ ,000)
|
Total
|
||||||||||||
|
Shandong Baoyuan
|
550 | 10.00 | % | 398 | 10.41 | % | ||||||||||
|
Feicheng A Shi De
|
605 | 11.00 | % | 450 | 11.77 | % | ||||||||||
|
Shandong Kai Sheng
|
825 | 15.00 | % | 85 | 2.22 | % | ||||||||||
Research and Development
We are strongly committed to the development of new products and processes and the enhancement of our existing products and technology. We conduct research and development and acquire new technologies through our in-house research team in collaboration with various universities and research institutions and through technology acquisitions from third parties.
28
Our in-house development team consists of five PhD’s and over 30 researchers responsible for developing new products and responding to customer needs. The in-house team has contributed to the establishment of five national standards for feed additives, has applications pending for five Chinese patents covering synthetic methods for manufacturing additives and holds two Chinese patents covering new products, methodologies and machines used to mix and dry feed additives. We believe that our participation in the development of national standards provides us with an insight into Chinese regulators’ focus and a competitive advantage versus our competitors.
Our most recent in-house development is CA-13 (Weanling King), a new type of transitional feed for early-wean piglets. CA-13 is an antibiotic-free product that improves a piglet’s immune system while reducing the days needed for the production of finished pigs. The initial debut of CA-13 is scheduled in the beginning of 2011, with production expected to be fully operational in the second half of 2011.
To further expand our development platform, we have entered into development agreements with several universities and research institutions, including the Institute of Subtropical Agro-ecology of the Chinese Academy of Sciences, Guangdong University of Technology, Zhongkai Institute of Agriculture Engineering, Northeast Agriculture University, Southeast Agriculture University, and Guelph University in Canada. These cooperative ventures are conducted under agreements that grant us the exclusive right to commercially exploit these new processes and procedures in exchange for licensing fees to the research partner. Our recent developments with universities and research institutions include:
|
|
·
|
A new antioxidant product introduced in 2010 that is used to maintain the quality of feed products, prevent various diseases and promote growth that was developed with the assistance of the Guangdong University of Technology.
|
|
|
·
|
An organic plant pigment designed to improve the skin color of farm animals and fish that was developed in cooperation with Zhongkai Institute of Agriculture Engineering. The Company has finished the laboratory stage of development of this product and is currently field testing the product.
|
|
|
·
|
An organic selenium feed additive developed with the Chinese Academy that is currently in the initial laboratory stage of development.
|
Manufacturing
We operate from a modern 34,000 square-meter manufacturing facility in the Huadu Economic District, in Guangdong province. As of December 31, 2009, we had an aggregate production capacity of approximately 400 metric tons per week of feed additive, of which 250 metric tons are organic trace minerals. We estimate that we are currently operating at a blended average across our product lines of approximately 44% of our manufacturing capacity at our current facility. We expect our manufacturing output to increase in 2011 and beyond, and are thus seeking to acquire land for and to build a second facility, which is currently scheduled to be operational in the fourth quarter of 2011.
29
In 2003, Tanke entered into a Land Use Agreement valid from May 20, 2006 until May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao town granted Tanke a land use right covering the land where Tanke’s manufacturing facility currently stands. This agreement will be superseded by a Land Use Right Certificate and will be automatically terminated once the Company obtains such certificate. We are currently negotiating with the local government to obtain such certificate, which would permit us to operate our business as it is currently conducted. If the Company is not able to obtain a Land Use Right Certificate, the PRC government may declare the Land Lease Agreement invalid, evict the Company’s personnel from the premises and remove the Company’s manufacturing facilities.
Intellectual Property
As a result of our extensive research and development, we own valuable patents, trademarks and licenses and regard our intellectual property as a major component of our competitive strategy. Our granted patents include:
|
|
·
|
A Chinese utility model patent (ZL200620154038.5) the Company owns for a mixed drier of feed additives. The patent was issued in November 2007 for a term of 10 years.
|
|
|
·
|
A Chinese patent (ZL200710030121.0) the Company jointly owns with the Guangdong University of Technology for the methodology of preparation of copper and zinc glycine complexes by ball milling and solid-static doping. The patent was issued in July 2010 for a term of 20 years.
|
We have five pending Chinese patent applications that were developed in collaboration with certain Chinese research institutions or universities.
On June 17, 2008, Guangzhou Tanke Bio-Tech Co., Ltd. (“Tanke Bio-Tech”), a subsidiary of Tanke, entered into an exclusive Licensing Agreement with the Institute of Subtropical Agro-ecology of the Chinese Academy of Sciences (the “Institute”), whereby the Institute granted Tanke Bio-Tech an exclusively license for the use of (a) Chinese Patent ZL200410013212.X which governs the methodology to extract metal sulfur protein from pork liver and (b) Chinese Patent ZL200510120505.2 which is a formula and methodology to enhance the immune system of piglets. Tanke Bio-Tech agreed to pay the Institute an aggregate amount of RMB 6.6 million for the exclusive use of the two patents. The term of the exclusive license for the patents is from January 1, 2008 to December 30, 2012 unless earlier terminated by Tanke Bio-Tech pursuant to the terms of the agreement.
We have also registered seven trademarks with the Trademark Office of the State Administration for Industry and Commerce of China, including “Tanke”, “Qilimix,” “Qili” and “CTanke”.
30
Seasonality
Although our business is not significantly affected by seasonality, demand for our products tends to be lower from January to April because large amount of animals raised by husbandry farms are sold during the Chinese New Year in February. The demand for our products tends to be higher from May to December, because many feed manufactures will complete stocking by December before the Chinese New Year.
Quality Control
Following quality control breakdowns from isolated Chinese manufacturers in the past several years and the resultant negative publicity, we have placed a strong emphasis on maintaining the quality and integrity of our products. To that end, the Company’s internal quality controls are implemented in accordance with the requirements of ISO 9001/2000. The Company has also received the ISO 9001/2000 International Quality Management System and HACCP management system certificates and the GMP certification for an animal drug production lines.
Our quality control center reviews the quality of the factors involved in production of our products, including the examination of raw material, product testing and sampling. Our laboratory maintains samples of each of our delivered products in the event of customer issues or a regulatory review.
Government Regulation
Our domestic business activities are regulated by various governmental agencies in China and Guangdong province and our foreign sales are subject to similar requirements in the countries in which the Company does business. These laws govern current operations and product safety and may require remediation of environmental incidents.
The animal feed additive market in China is managed under a legal system that includes registration, permits, supervision and inspection. The Company believes that it has satisfied all material regulatory requirements and is in material compliance with environmental and agricultural laws and regulations.
Employees
As of September 30, 2010, we employed 151 full-time personnel. We believe that we maintain a satisfactory and safe working environment that it has a history of low turnover. We believe that we complies in all material respects with applicable Chinese labor laws.
31
Organization and Consolidated Subsidiaries
The Company’s organizational structure was carefully developed to abide by Chinese laws and to maintain Tanke’s tax benefits as well as internal organizational efficiencies. The Company’s post Share Exchange organization structure is summarized below:

Recent Events
The disclosure set forth in Item 3.02 of this Current Report on Form 8-K is incorporated herein by reference.
32
Risk Factors
Our business, as well as shares of Common Stock, are highly speculative in nature and involve a high degree of risk. Our securities should be purchased only by persons who can afford to lose their entire investment. Accordingly, prospective investors should carefully consider, along with other matters referred to herein, the following risk factors in evaluating our business before purchasing any Common Stock.
Risks Related to Our Business
We may not possess all the licenses required to operate our business, or may fail to maintain the licenses we currently hold. This could subject us to fines and other penalties, which could have a material adverse effect on our results of operations.
Although we are required to hold a variety of permits, licenses and certificates to conduct our business in China, we may not possess all of the permits, licenses and certificates required for our business. We entered into a Land Lease Agreement valid from May 20, 2006 to May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao Town granted us a land use right covering the land where our manufacturing facility currently stands. This lease will be superseded by a Land Use Right Certificate and will be automatically terminated once we obtain such certificate. Due to changes in the relevant PRC regulations, we have not been granted such certificate for this land. Therefore, pursuant to applicable PRC law, we are not permitted to operate our manufacturing facility without such certificate and as a result, there is a risk that the PRC government may declare our Land Lease Agreement invalid.
We are currently negotiating with the local government to obtain a Land Use Right Certificate, which would permit us to operate our business as it is currently conducted. Without the Land Use Right Certificate, we are unable to apply for a Property Ownership Certificate for our manufacturing facilities. Until we obtain the Land Use Right Certificate, the PRC government may evict our personnel from the premises and remove our manufacturing facilities that we built on the premises. Such action would have a very significant and negative impact on our operations and business.
In addition, there may be other circumstances under which the approvals, permits, licenses or certificates granted by the governmental agencies are subject to change without substantial advance notice, and it is possible that we could fail to obtain the approvals, permits, licenses or certificates that are required to expand our business as we intend. If we fail to obtain or to maintain such permits, licenses or certificates, or renewals are granted with onerous conditions, we could be subject to fines and other penalties and be limited in the number or the quality of the products that we would be able to offer. As a result, our business, result of operations and financial condition could be materially and adversely affected.
33
Concerns with the safety and quality of agricultural feed additive products could cause customers to avoid our products.
We could be adversely affected if our customers and the ultimate consumers of our feed additive products lose confidence in the safety and quality of various feed additive products. Adverse publicity about these types of concerns may discourage our customers from buying our products or cause production and delivery disruptions. Any negative change in customer perceptions about the safety and quality of our feed additive products could adversely affect our business and financial condition.
If our feed additive products become adulterated or misbranded, we would need to recall those items and may experience product liability claims if consumers are injured as a result.
Animal feed products occasionally contain contaminants due to inherent defects in those products or improper storage or handling. Under adverse circumstances, animal feed manufacturers may need to recall some of their products if they become adulterated or misbranded, and may also be liable if the consumption of any of their products causes injury. While we have never been required to recall any of our feed additive products, a widespread product recall could result in changes to one or more of our business processes, product shortages, loss of customer confidence in our products or other adverse effects on our business. If we are required to defend against a product liability claim, whether or not we are found liable under the claim, we could incur substantial costs, our reputation could suffer and our customers might substantially reduce their existing or future orders from us.
We face significant competition in the sales of our agricultural feed additive products.
Competition in the feed additive industry, especially with companies with greater resources, may make us unable to compete successfully, which could adversely affect our business.
In general, the competitive factors in the feed additive industry in China include:
|
·
|
price;
|
|
·
|
product quality;
|
|
·
|
brand identification;
|
|
·
|
breadth of product line; and
|
|
·
|
customer service.
|
To the extent that our products and services do not exhibit these qualities, our ability to compete will be hindered.
34
The markets in which we operate are highly competitive and fragmented and we may not be able to maintain market share.
We operate in highly competitive markets and compete with numerous local Chinese feed additive manufacturers. We expect competition to persist and intensify in the future. Our domestic competitors are mainly leaders in the feed additive markets in China. Our small local competitors may have better access in certain local markets to customers and prospects, an enhanced ability to customize products to a particular region or locality and more established local distribution channels within a small region. We also compete with large Chinese national and multi-national competitors who may have competitive advantages over us in certain areas such as access to capital, technology, product quality, economies of scale and brand recognition and may also be better positioned than us to develop superior product features and technological innovations and to exploit and adapt to market trends. Due to the lack of publicly available information about our competitors and industry, we may not be able to conduct in-depth research and analysis on our current or new markets. Therefore, we may not be able to determine our direct competitors, such competitors' revenues or market share.
In addition, China’s entry into the World Trade Organization may lead to increased foreign competition for us. International producers and traders import products into China that generally are of higher quality than those produced in the local Chinese market. We may face additional competition if these products are considered to be better than the type of feed additives we produce. If we are not successful in our marketing and advertising efforts to increase awareness of our brands, our revenues could decline, which could have a material adverse effect on our business, financial condition, results of operations and share price. We cannot assure you that we will be able to compete successfully against existing or new competition in our markets.
We may not be able to fully implement our current business strategy if we are unable to acquire and develop a second manufacturing facility.
As part of our current business strategy, we intend to continue to increase our production volume in order to gain additional market share. In connection with that strategy, we plan to build a second manufacturing facility that would double our organic trace mineral production capacity. There is a risk that we may be unable to acquire the land use rights for this facility or to commence or finish construction of the new facility, or operate it at a profit. If we are unable to achieve any or all of the foregoing it could have a material adverse effect on our business and results of operations.
We cannot be certain that our feed additive product innovations and marketing achievements to date will continue.
We believe that our past performance has been based on, and our future prospects will depend upon, in large part, our ability to continue to improve our existing feed additive products or develop new feed products. We cannot assure you that we will be successful in introducing, marketing and producing any new feed products or feed additive product innovations, or that we will develop and introduce, in a timely manner, innovations to our existing products which satisfy customer needs or achieve market acceptance. Our failure to develop new feed additive products and introduce them successfully and in a timely manner could harm our ability to grow our business and could have a material adverse effect on our business, results of operations and financial condition.
35
We purchase many commodities that we use for raw materials and packaging; price changes for such commodities may adversely affect our profitability.
When necessary, we attempt to recover our commodity cost increases by increasing prices, promoting a higher-margin product mix and creating additional operating efficiencies. Nevertheless, the raw materials used in our feed additive business are largely commodities that experience price fluctuations caused by external conditions and changes in governmental agricultural programs which we have no control over. Substantial increases in the prices of packaging materials, such as corrugated cardboard, aluminum products, films and plastics, or higher prices of our raw materials could adversely affect our operating performance and financial results. Any substantial fluctuation in the prices of raw materials, if not offset by increases in our sales prices, could adversely affect our profitability.
Outbreaks of livestock disease can adversely affect sales of our products.
Outbreaks of livestock diseases can significantly affect demand for our feed additive products and could result in governmental restrictions on the sale of livestock products to or from customers, or require our customers to destroy their feeds. This could result in the cancellation of orders of feed additive products by our customers and create adverse publicity that may have a material adverse effect on the agricultural products industry and our ability to market our products successfully.
We do not typically have long-term sales contracts with our customers and our customers could at any time reduce purchases of, or entirely cease purchasing, our products, harming our operating results and business.
We typically do not have long-term volume sales contracts with our customers. Accordingly, our customers could reduce their purchases from us or cease purchasing our products altogether when a particular contract expires. A variety of factors, including economic, health, regulatory, political and social instability, could contribute to a slowdown in the demand or a reduction in the market price for our products because poultry demand and pricing is highly correlated with general economic activities. If any of our customers experience serious financial difficulties, it may lead to a decline in sales and write-offs of accounts receivable, which could harm our results of operations.
The cessation of tax exemptions and deductions by the Chinese government may affect our profitability.
On March 16, 2007, the National People’s Congress of China enacted a new tax law, or the New Tax Law, whereby both foreign investment enterprises, or FIEs, and domestic companies will be subject to a uniform income tax rate of 25%. On November 28 2007, the State Council of China promulgated the Implementation Rules of the New Tax Law, the “Implementation Rules”. Both the New Tax Law and the Implementation Rules have become effective on January 1, 2008. Both the New Tax Law and the Implementation Rules provide tax exemption treatment for enterprises engaged in agricultural industries, such as farming, foresting, fishing and animal husbandry. We have been informed that certain of our Chinese subsidiaries are eligible for relevant preferential tax treatment, including tax reduction and exemption, and certain of our products are exempted from value added tax. In the future, if the relevant tax authorities determine that Tanke, our principal operating business, is not eligible for tax exemption treatment it may materially and adversely affect our profits, business and financial performance.
36
Potential environmental liability could have a material adverse effect on our operations and financial condition.
As a manufacturer, we are subject to various Chinese environmental laws and regulations on air emission, waste water discharge, solid wastes and noise. The Environmental Protection Bureau of Huadu District, Guangzhou issued the Opinion on the Environmental Impact Statement regarding the Construction Project of Tanke Group on February 16, 2004. However, we have not obtained any documentation of environment appraisal and acceptance inspection with respect to the completion of projects of the factory buildings and the production lines, because we have not obtained a Land Use Right Certificate for such site nor have we been granted Property Ownership Certificate. We are in the process of applying for a Land Use Right Certificate, which will enable us to obtain an environmental appraisal and acceptance inspection with respect to our facilities. Following our receipt of a Land Use Right Certificate, we anticipate that we will be able to obtain the necessary environmental approvals, but there can be no assurance in this regard. We further can not assure you that we will be able to comply with environmental regulations at all times as the Chinese environmental legal regime is evolving and becoming more stringent. Therefore, if the Chinese government imposes more stringent regulations in future, we may have to incur additional and potentially substantial costs and expenses in order to comply with new regulations, which may negatively affect our results of operations. Furthermore, no assurance can be given that all potential environmental liabilities have been identified or properly quantified or that any prior owner, operator, or tenant has not created an environmental condition unknown to us. If we fail to obtain our Land Use Right Certificate or to comply with any of the present or future environmental regulations in any material aspects, we may suffer from negative publicity and be subject to claims for damages that may require us to pay substantial fines or have our operations suspended or even be forced to cease operations.
We do not presently maintain business disruption insurance and any disruption of the operations in our production facility would damage our business.
All of our feed additive products are currently manufactured in our production facility in the Huadu Economic Development Zone near the capital city of Guangzhou in the province of Guangdong, China. Our operations could be interrupted by fire, flood, earthquake and other events beyond our control. Any disruption of the operations in our production facility would have a significant negative impact on our ability to manufacture and deliver products as we would likely be unable to outsource our production on terms favorable to us, if at all. Failure to replace any lost production capability would cause a potential reduction in sales, the cancellation of orders, loss of valuable employees, damage to our reputation and potential lawsuits.
37
Our products and processes can expose us to product liability claims.
Product liability claims or product recalls can adversely affect our business reputation and expose us to increased scrutiny by local, provincial, and central governmental regulators. The packaging, marketing and distribution of agricultural feed additive products entail an inherent risk of product liability and product recall and the resultant adverse publicity. We may be subject to significant liability if the consumption of any of our products causes injury, illness or death of livestock, other animals or humans. We could be required to recall certain of our feed additive products in the event of contamination or damage to the products. In addition to the risks of product liability or product recall due to deficiencies caused by our production or processing operations, we may encounter the same risks if any third party tampers with our feed additive products. We cannot assure you that we will not be required to perform product recalls, or that product liability claims will not be asserted against us in the future. Any claims that may be made may create adverse publicity that would have a material adverse effect on our ability to market our feed additive products successfully or on our business, reputation, prospects, financial condition and results of operations. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on us and could prevent us from obtaining adequate product liability insurance in the future on commercially reasonable terms.
Our current and future operations substantially depend on our management team and other key personnel, the loss of any of whom could disrupt our business operations.
Our business does and will depend in substantial part on the continued service of our senior management and founder, including but not limited to Guixiong Qiu, Xugang Shu and Bo Jun. The loss of the services of one or more of our key personnel could impede implementation and execution of our business strategy and result in the failure to reach our goals. We do not carry key person life insurance for any of our officers or employees. Our future success will also depend on the continued ability to attract, retain and motivate highly qualified personnel in the diverse areas required for continuing our operations. The rapid growth of the economy in China has caused intense competition for qualified personnel. We cannot assure you that we will be able to retain our key personnel or that we will be able to attract, train or retain qualified personnel in the future.
Volatile energy prices could adversely affect our operating results.
In the last few years, energy prices have risen dramatically and are now volatile, which has resulted in increased and unpredictable increases for our raw materials costs. Continued or volatile increases in energy prices could adversely affect demand for our feed products and increase our operating costs, both of which would reduce our operating income.
If we need additional financing, we may not be available to find such financing on satisfactory terms or at all.
Our capital requirements may be accelerated as a result of many factors, including the growth and timing of our business development activities, budget shortfalls, unanticipated expenses or capital expenditures, future product opportunities, future licensing opportunities and future business combinations. Consequently, we may need to seek additional debt or equity financing, which may not be available on favorable terms, if at all, and which may be dilutive to our stockholders.
38
We may seek to raise additional capital through public or private equity offerings, debt financings or additional corporate collaboration and licensing arrangements. To the extent we raise additional capital by issuing equity securities, our stockholders may experience dilution. To the extent that we raise additional capital by issuing debt securities, we may incur substantial interest obligations, may be required to pledge assets as security for the debt and may be constrained by restrictive financial and/or operational covenants. Any provider of debt financing would also be superior position to our stockholders’ interest in the event of a bankruptcy or liquidation. To the extent we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or product candidates, or grant licenses on unfavorable terms.
We face risks associated with currency exchange rate fluctuations; any adverse fluctuation may adversely affect our operating margins
Almost all of our revenues are denominated in Renminbi. Conducting business in currencies other than US dollars subjects us to fluctuations in currency exchange rates that could have a negative impact on our reported operating results. Fluctuations in the value of the US dollar relative to other currencies impact our revenues, cost of revenues and operating margins and result in foreign currency translation gains and losses. If the exchange rate of the Renminbi is affected by lowering its value as against the US dollar, our reported profitability when stated in US dollars will decrease. Historically, we have not engaged in exchange rate hedging activities and have no current intention of doing so.
We may not be able to adequately protect and maintain our intellectual property, trademark, and brand names.
Our business has and will depend on our ability to continue to develop and market feed additive products. We currently own two patents and have entered into an exclusive licensing agreement with respect to two other patents covering aspects of the manufacturing and production of feed additives. We have filed applications for five additional patents. Our inability to protect our rights to this intellectual property may adversely affect our ability to prevent competitors from using our products and developments.
Intellectual property rights in China are still developing, and there are uncertainties involved in the protection and the enforcement of such rights. We will need to pay special attention to protecting our intellectual property and trade secrets. Failure to do so could lead to the loss of a competitive advantage that could not be compensated by our damages award.
Risks Related to Our Corporate Structure
The Chinese government may determine that the VIE Agreements which we utilize to operate Tanke are not in compliance with applicable Chinese laws, rules and regulations and that they are therefore unenforceable.
39
In China it is widely understood that foreign investment enterprises, or FIEs, are forbidden or restricted from engaging in certain businesses or industries which are sensitive to the economy. As we intend to centralize our management and operation in China without being restricted to conduct certain business activities which are important for our current or future business but are restricted or might be restricted in the future, we believe our VIE Agreements will be essential for our business operation. Pursuant to the terms of the VIE Agreement, almost all of our business activities in China are managed and operated by China Flying though Kanghui Agricultural, and almost all economic benefits and risks arising from the business of Tanke are transferred to China Flying and Kanghui Agricultural.
There are risks involved with the operation of Tanke under the VIE Agreements. We have been advised by Chinese legal counsel that if the Chinese government determines the VIE Agreement used to control the operating company to be unenforceable as they circumvent the Chinese restrictions relating to foreign investment restrictions, the relevant regulatory authorities would have broad discretion in dealing with such breach, including:
|
·
|
imposing economic penalties;
|
|
·
|
discontinuing or restricting the operations of China Flying, Kanghui Agricultural or Tanke;
|
|
·
|
imposing conditions or requirements in respect of the VIE Agreements with which Kanghui Agricultural or Tanke may not be able to comply;
|
|
·
|
requiring us to restructure the relevant ownership structure or operations;
|
|
·
|
taking other regulatory or enforcement actions that could adversely affect our business; and
|
|
·
|
revoking the business license and/or the licenses or certificates of China Flying or Kanghui Agricultural, Tanke, and/or voiding the VIE Agreements.
|
Any of these actions could have a material adverse impact on our business, financial condition and results of operations.
We depend upon the VIE Agreements in conducting our operations in China, which may not be as effective as direct ownership .
We conduct our business through our Chinese operating subsidiaries and generate the revenues through the VIE Agreements. The VIE Agreements may not be as effective in providing us with control over Tanke as direct ownership. The VIE Agreements are governed by Chinese laws and provide for the resolution of disputes through arbitration proceedings pursuant to Chinese laws. Accordingly, the VIE Agreements will be interpreted in accordance with Chinese laws. If Tanke or its shareholders fail to perform the obligations under the VIE Agreements, we may have to rely on legal remedies under Chinese laws, including seeking specific performance or injunctive relief, and claiming damages, and there is a risk that we may be unable to obtain these remedies. The legal environment in China is not as developed as in other jurisdictions. As a result, uncertainties in the Chinese legal system could limit our ability to enforce the VIE Agreements.
40
The pricing arrangement under the VIE Agreements may be challenged by Chinese tax authorities.
We could face adverse tax consequences if Chinese tax authorities determine that the VIE Agreements were not entered into based on arm’s length negotiations. If the Chinese tax authorities determine that the VIE Agreements were not entered into on an arm’s length basis, they may adjust the income and expenses of our company for Chinese tax purposes which could result in higher tax liability.
Any deterioration of the relationship between Kanghui Agriculture and Tanke could materially and adversely affect the overall business operation of our company.
Our relationship with Tanke is governed by the VIE Agreements, which are intended to provide us, through our ownership of China Flying and indirect ownership of Kanghui Agricultural, with effective control over the business operations of Tanke. Tanke could violate the VIE Agreements, go bankrupt, suffer from difficulties in its business, fail to renew necessary permits and certifications or otherwise become unable to perform its obligations under the VIE Agreements and, as a result, our operations, reputation, business and stock price could be severely harmed.
If Kanghui Agricultural exercises the purchase options over Tanke’s equity pursuant to the VIE Agreements, the payment of purchase prices could materially and adversely affect our financial position.
Under the VIE Agreements, China Flying, through its ownership of Kanghui Agricultural, holds an option to purchase all or a portion of the equity of Tanke at a price, pro rata in case of not all, based on the capital paid in by the Tanke Shareholders ($1,147,704 or 9.5 million RMB). If applicable Chinese laws and regulations require an appraisal of the equity interest or provide other restrictions on the purchase price, the purchase price shall be the lowest price permitted under the applicable Chinese laws and regulations. As Tanke is already a contractually controlled affiliate to our company, Kanghui Agricultural’s purchase of Tanke’s equity would not bring immediate benefits to us and the exercise of the option and payment of the purchase prices could adversely affect our financial position.
Risks Associated With Doing Business in China
If Tanke’s land use rights are revoked, we would have no operational capabilities.
Under Chinese law, land is owned by the state or rural collective economic organizations. The rural collective economic organizations issue to tenants the rights to use rural land. Use rights can be revoked and the tenants forced to vacate at any time when redevelopment of the land is in the public interest. The public interest rationale is interpreted quite broadly and the process of land appropriation may be less than transparent. We rely heavily on Tanke’s land use rights for our operations, and the loss of such rights would have a material adverse effect on our business.
41
We entered into a Land Use Right Grant Agreement with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao Town granted us certain land use rights for an area of approximately 60 mu where our manufacturing facility is built. The land use right of such parcel of land is owned collectively by local farmers. However, we currently do not maintain a Land Use Right Certificate for such parcel of land. Having no use right certificate of our land would have a material adverse effect on us as we would be required to relocate our facilities and obtain new land use rights, and there is a risk that we would not be able to accomplish such a relocation with reasonable cost or at all.
In addition, we currently do not maintain a building ownership certificate for our manufacturing facility. Because Tanke does not have land use right certificate on this parcel of land, it neither applied for nor will be granted a building ownership certificate for the manufacturing facility it built on this parcel of land. We can not assure you that we will eventually obtain the building ownership certificate for the foresaid land with reasonable cost.
Economic, political and social conditions in China are subject to significant uncertainty and could affect our business.
All of our operations are located in China and our business is subject to political and economic uncertainties in China. The economy of China differs from the economies of most developed countries in many respects, including the level of government involvement, the level of development, control of foreign exchange, and methods for allocating resources. Although under current leadership the Chinese government has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization, a substantial portion of productive assets in China is still owned by the Chinese government. Changes in Chinese policies, laws and regulations, or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on imports, restrictions or prohibitions on dividend payments to stockholders, devaluations of currency or the nationalization or other expropriation of private enterprises may occur from time to time without notice and could have a material adverse effect on our business. Nationalization or expropriation could result in the total loss of our investment in China. We have no control over most of these risks and may be unable to anticipate changes in Chinese economic and political conditions.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
We are dependent on our relationship with the local government in the province in which we operate our business. Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. The Chinese central or local governments may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
42
Future inflation in China may inhibit our ability to conduct business in China. In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may have an adverse effect on profitability. These factors have led to the adoption by Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
If relations between the United States and China worsen, our share price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Any political or trade controversies between the United States and China in the future could adversely affect the market price of our Common Stock and our ability to access U.S. capital markets.
In the fiscal year ended December 31, 2009, we derived approximately 95% of our sales in China. A slowdown or other adverse developments in China’s economy may materially and adversely affect our customers, demand for our products and our business.
During the fiscal year ended December 31, 2009, we generated 95% of our sales in China. We anticipate that sales of our products in China will continue to represent a significant majority of our total sales in the near future. Although China’s economy has grown significantly in recent years, we cannot assure you that such growth will continue. The industry which we are involved in China is relatively new and growing, but we do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the Chinese economy which may affect demand for our products. In addition, the Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Efforts by the Chinese government to slow the pace of growth of the Chinese economy could result in reduced demand for our products. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in China may materially reduce the demand for our products and materially and adversely affect our business.
43
We may have limited legal recourse under Chinese laws if disputes arise under our contracts with third parties.
The Chinese government has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. If our new business ventures are unsuccessful, or other adverse circumstances arise from these transactions, we face the risk that the parties to these ventures may seek ways to terminate the transactions, or, may hinder or prevent us from accessing important information regarding the financial and business operations of these acquired companies. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance, or to seek an injunction under Chinese laws, in either of these cases, are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations. Although legislation in China over the past 30 years has significantly improved the protection afforded to various forms of foreign investment and contractual arrangements in China, these laws, regulations and legal requirements are relatively new and their interpretation and enforcement involve uncertainties, which could limit the legal protection available to us, and foreign investors, including you. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital and could have a material adverse impact on our operations.
Because our principal assets are located outside of the United States and our sole director and officer and all of our key employees reside in China, outside of the United States, it may be difficult for you to enforce your rights based on the United States federal securities laws against us and our officers and directors in the United States or to enforce judgments of United States courts against us or them in China.
Our sole director and officer and all of our key employees reside in China, outside of the United States. In addition, our principal operating business is located in China and all of our assets are located outside of the United States. China does not have a treaty with United States providing for the reciprocal recognition and enforcement of judgments of courts. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the United States federal securities laws against us in the courts of either the United States or China and, even if civil judgments are obtained in courts of the United States, to enforce such judgments in Chinese courts. Further, it is unclear if extradition treaties now in effect between the United States and Chinese would permit effective enforcement against us or our officers and directors of criminal penalties, under the United States federal securities laws or otherwise.
44
We may have difficulty establishing adequate management, legal and financial controls in China, which could impair our planning processes and make it difficult to provide accurate reports of our operating results.
China historically has been deficient in Western style management and financial reporting concepts and practices, as well as in modern banking, and other control systems. We may have difficulty in hiring and retaining a sufficient number of qualified employees familiar with these concepts, practices and systems to work in China. As a result of these factors, and especially given that we expect to be a publicly listed company in the U.S. and subject to regulation as such, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet Western standards. We may have difficulty establishing adequate management, legal and financial controls in China.
Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert Chinese Renminbi into foreign currencies and, if Chinese Renminbi were to decline in value, reducing our revenue in U.S. dollar terms.
Our reporting currency is the U.S. dollar and our operations in China use their local currency as their functional currency. Substantially all of our revenue and expenses are in Chinese Renminbi. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the Renminbi depends to a large extent on Chinese government policies and China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of Renminbi to the U.S. dollar had generally been stable and the Renminbi had appreciated slightly against the U.S. dollar. However, on July 21, 2005, the Chinese government changed its policy of pegging the value of Chinese Renminbi to the U.S. dollar. Under the new policy, Chinese Renminbi may fluctuate within a narrow and managed band against a basket of certain foreign currencies. It is possible that the Chinese government could adopt a more flexible currency policy, which could result in more significant fluctuation of Chinese Renminbi against the U.S. dollar. We can offer no assurance that Chinese Renminbi will be stable against the U.S. dollar or any other foreign currency.
Our financial statements are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign consolidated subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign consolidated subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks, although we may do so in the future. The availability and effectiveness of any hedging transaction may be limited and we may not be able to hedge our exchange rate risks.
45
The application of Chinese regulations relating to the overseas listing of Chinese domestic companies is uncertain, and we may be subject to penalties for failing to request approval of the Chinese authorities prior to listing our shares in the U.S.
On August 8, 2006, six Chinese government agencies, namely, the Ministry of Commerce, or MOFCOM, the State Administration for Industry and Commerce, or SAIC, the China Securities Regulatory Commission, or CSRC, the State Administration of Foreign Exchange, or SAFE, the State Assets Supervision and Administration Commission, or SASAC, and the State Administration for Taxation, or SAT, jointly issued the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, which we refer to as the “New M&A Rules”, which became effective on September 8, 2006. The New M&A Rules purport, among other things, to require offshore “special purpose vehicles,” that are (1) formed for the purpose of overseas listing of the equity interests of Chinese companies via acquisition and (2) are controlled directly or indirectly by Chinese companies and/or Chinese individuals, to obtain the approval of the CSRC prior to the listing and trading of their securities on overseas stock exchanges. Based on our understanding of current Chinese Laws and as advised by our Chinese counsel, (i) Kanghui Agricultural was incorporated by a foreign owned enterprise, and there was no acquisition of the equity or assets of a “Chinese domestic company” as such term is defined under the New M&A Rules and (ii) no provision in the New M&A Rules clearly classifies the contractual arrangements between Kanghui Agricultural and Tanke as a type of transaction falling within the New M&A Rules. Therefore, we were and are not required to obtain the approval of CSRC under the New M&A Rules in connection with the Private Placement.
However, there are substantial uncertainties regarding the interpretation, application and enforcement of the New M&A Rules and CSRC has yet to promulgate any written provisions or formally declare or state whether the overseas listing of a China-related company structured similar to ours is subject to the approval of CSRC. Any violation of these rules could result in fines and other penalties on our operations in China, restrictions or limitations on remitting dividends outside of China, and other forms of sanctions that may cause a material and adverse effect to our business, operations and financial conditions.
The new mergers and acquisitions regulations also established additional procedures and requirements that are expected to make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the Ministry of Commerce be notified in advance of any change-of-control transaction in which a foreign investor takes control of a Chinese domestic enterprise that owns well-known trademarks or China’s traditional brands. We may grow our business in part by acquiring other businesses. Complying with the requirements of the new mergers and acquisitions regulations in completing this type of transactions could be time-consuming, and any required approval processes, including CSRC approval, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
46
Recent Chinese regulations relating to the establishment of offshore special purpose companies by Chinese residents may subject our Chinese resident shareholders or our Chinese subsidiaries to penalties, limit our ability to distribute capital to our Chinese subsidiaries, limit our Chinese subsidiaries’ ability to distribute funds to us, or otherwise adversely affect us.
The SAFE issued a public notice in October 2005, or the SAFE Circular No. 75, requiring Chinese residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of Chinese companies, referred to in the SAFE Circular No. 75 as special purpose vehicles, or SPVs. Chinese residents who are shareholders of SPVs established before November 1, 2005 were required to register with the local SAFE branch before June 30, 2006. Further, Chinese residents are required to file amendments to their registrations with the local SAFE branch if their SPVs undergo a material event involving changes in capital, such as changes in share capital, mergers and acquisitions, share transfers or exchanges, spin-off transactions or long-term equity or debt investments. To date, the Chinese residents who are shareholders of Tanke do not own any equity in the Company and there is no arrangement of trust or shareholding entrustment and thus, currently such Chinese residents do not need to file registrations with SAFE pursuant to SAFE Circular No. 75. When the Chinese residents exercise their options in the future to receive any share of the Company pursuant to the Call Option Agreement, they will need to file registrations with SAFE. However, any failure by shareholders of our Tanke ordinary shares to amend their registration or the failure of future shareholders of our Chinese subsidiaries who are Chinese residents to comply with the registration procedures set forth in the SAFE Circular No. 75 could subject such beneficial owners and our Chinese subsidiaries to fines and legal sanctions. See “Business—Government Regulation—Foreign Exchange Regulation.”
We face uncertainty from the Circular on Strengthening the Administration of Enterprise Income Tax on Non-resident Enterprises' Share Transfer, or Circular 698, released in December 2009 by China's State Administration of Taxation, or the SAT, effective as of January 1, 2010.
Pursuant to the Circular 698, where a foreign investor transfers the equity interests of a Chinese resident enterprise indirectly via disposing of the equity interests of an overseas holding company, which we refer to as an Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report such Indirect Transfer to the competent tax authority of the Chinese resident enterprise. The Chinese tax authority will examine the true nature of the Indirect Transfer, and if the tax authority considers that the foreign investor has adopted an abusive arrangement in order to avoid Chinese tax, they will disregard the existence of the overseas holding company and re-characterize the Indirect Transfer and as a result, gains derived from such Indirect Transfer may be subject to Chinese withholding tax at the rate of up to 10%. Circular 698 also provides that, where a non-Chinese resident enterprise transfers its equity interests in a Chinese resident enterprise to its related parties at a price lower than the fair market value, the competent tax authority has the power to make a reasonable adjustment to the taxable income of the transaction.
47
Since Circular 698 became effective on January 1, 2010, we cannot assure you that our reorganization will not be subject to examination by Chinese tax authorities or that any direct or indirect transfer of our equity interests in our Chinese subsidiaries via our overseas holding companies will not be subject to a withholding tax of 10%.
Due to various restrictions under Chinese laws on the distribution of dividends by our Chinese operating companies, we may not be able to pay dividends to our stockholders.
The Wholly-Foreign Owned Enterprise Law (1986), as amended, the Wholly-Foreign Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law of China (2006) contain the principal regulations governing dividend distributions by wholly foreign-owned enterprises. Under these regulations, wholly foreign-owned enterprises may pay dividends only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. Additionally, we are required to set aside a certain amount of its accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends except in the event of liquidation and cannot be used for working capital purposes. The Chinese government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of China. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the profits of Tanke. Furthermore, if our subsidiaries in China incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we unable to receive all of the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our Common Stock.
The State Administration of Foreign Exchange restrictions or changes in foreign exchange regulations in China may affect our ability to pay dividends in foreign currency or conduct other foreign exchange business.
We receive substantially all of our revenues in RMB, which is currently not a freely convertible currency. The restrictions on currency exchanges may limit our ability to use revenues generated in RMB to make dividends or other payments in United States dollars. The Chinese government strictly regulates conversion of RMB into foreign currencies. Over the years, foreign exchange regulations in China have significantly reduced the government’s control over routine foreign exchange transactions under current accounts. In China, SAFE regulates the conversion of the RMB into foreign currencies. Pursuant to applicable Chinese laws and regulations, foreign invested enterprises incorporated in China are required to apply for “Foreign Exchange Registration Certificates.” Currently, conversion within the scope of the “current account” (e.g. remittance of foreign currencies for payment of dividends, trade and service-related foreign exchange transactions, etc.) can be effected without requiring the approval of SAFE, instead, need to be registered with the SAFE. However, conversion of currency in the “capital account” (e.g. for capital items such as direct investments, loans, securities, etc.) still requires the approval of SAFE. In addition, failure to obtain approval from SAFE for currency conversion on the capital account may adversely impact our capital expenditure plans and our ability to expand in accordance with our desired objectives.
48
All of our income is derived from the consulting fees we receive from Tanke through the VIE Agreements. SAFE restrictions may delay the payment of dividends, since we have to comply with certain procedural requirement and we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the profits of Kanghui Agricultural, and it thus may delay our payment of dividend to the equity holders.
Foreign exchange transactions by Chinese operating subsidiaries under the capital account continue to be subject to significant foreign exchange controls and require the approval of and need to register with Chinese government authorities, including SAFE. In particular, if Tanke, our Chinese operating subsidiary, borrows foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance Tanke by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the Ministry of Commerce, or their respective local counterparts. These limitations could affect Tanke’s ability to obtain foreign exchange through debt or equity financing.
The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining foreign currency, we may be unable to pay dividends or meet obligations that may be incurred in the future that require payment in foreign currency.
We are subject to Chinese environmental laws that adversely affect our results of operations.
We are subject to multiple Chinese environmental laws, including the Law on Environmental Protection of China and the Law of Prevention of Effluent Pollution of China and other environmental regulation governing the classification and disposal of waste. We expect that national, provincial and local governmental agencies will adopt stricter pollution controls in the future. Our production process may produce waste which may be harmful to the environment. While we believe that we are in compliance with current environmental regulation, there can be no assurance that future changes in environmental laws and regulations will not impose costly compliance requirements on us or otherwise subject us to future liabilities. Our profitability may be adversely affected if additional or modified environmental control regulations are imposed upon us.
49
Our failure to fully comply with Chinese labor laws, including laws relating to social insurance, may expose us to potential liability and increased costs.
Companies operating in China must comply with a variety of labor laws, including the Labor Contract Law of China enacted in June 2007, or the New Labor Contract Law, and laws requiring us to make social insurance (including unemployment insurance, medical insurance, and pension) and other staff welfare-oriented payments (such as housing funds). Our failure to comply with these laws could have a material adverse effect on our business. The New Labor Contract Law, which became effective on January 1, 2008, imposes stricter obligations on employers including a requirement that employers execute written labor contracts with all of their employees. Among our 151 employees, we have paid social insurance for 137 of our employees and 14 employees participated in commercial insurance due to the fact that they are transferred to the Company from other companies who have paid their social insurance. Although we believe we are currently in compliance with Chinese labor laws, including social insurance requirements, our failure to remain in compliance in the future could adversely impact our results of operations.
Furthermore, the New Labor Contract Law governs standard terms and conditions for employment, including termination and lay-off rights, contract requirements, compensation levels and consultation with labor unions, among other topics. In addition, the law limits non-competition agreements with senior management and other employees who have access to confidential information to two years and imposes restrictions or geographical limits. This new labor contract law will increase our labor costs, which could adversely impact our results of operations.
We must comply with the Foreign Corrupt Practices Act.
We are required to comply with the United States Foreign Corrupt Practices Act, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some of our competitors, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in mainland China. If our competitors engage in these practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business or from government officials who might give them priority in obtaining new licenses, which would put us at a disadvantage. Although we inform our personnel that such practices are illegal, we have no formal policies in place to prevent our employees or other agents from engaging in such conduct. If such conduct is undertaken, we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties, which could adversely impact our business and results of operations.
Risks Related to Our Securities
The offering price of the Units, the conversion price of the Notes and the exercise price of the Warrants were determined by Tanke and may not be indicative of the actual value of the securities or the value of the underlying Common Stock.
The offering price of the Units, the Conversion Price (as defined in the Notes) of the Notes and the Exercise Price (as defined in the Warrants) of the Warrants were determined by Tanke and may not be indicative of our actual value or the value of the Common Stock underlying the Notes and Warrants following the closing of the Share Exchange. The Conversion Price bears no relationship to the assets, book value, net worth or any other recognized criteria of our value. Prospective investors should undertake their own analysis of those matters.
50
There are restrictions on the transferability of the securities.
The Private Placement was made pursuant to Section 4(2) of the Act and/or the provisions of Rule 506 of Regulation D and, accordingly, the securities offered thereby were not registered under the Securities Act and may not be re-offered, sold, or otherwise transferred unless they are registered under the Securities Act or an exemption is available therefrom. Further, the securities were offered only to persons who are “accredited investors”. If no registration statement under the Securities Act for the resale of such securities is filed and becomes effective, the resale of such securities may be subject to Rule 144. Because we engaged in the Share Exchange with a “shell company”, no sales may be made under Rule 144 for the first twelve months after the closing of the Share Exchange. Investors must therefore be prepared to bear the economic risk of an investment in our securities for an indefinite period of time.
The registration statement may not be declared effective or remain effective.
Under the terms of the Registration Rights Agreement, we are obligated to include the shares of Common Stock underlying the Notes and the shares of Common Stock issuable when the Warrants are exercised in a registration statement to be filed by us with the SEC and to have such registration statement declared effective within certain time frames. We are subject to penalties in the event we fail to comply with such obligations. Nevertheless, no assurances can be given that the registration statement will ever be declared effective or that the shares of Common Stock underlying the Notes and the shares of Common Stock issuable when the Warrants are exercised will ever be registered under the Securities Act and thereby be available for public resale. Additionally, once the registration statement is filed and declared effective by the SEC, it will be necessary for us to file post-effective amendments to the registration statement when subsequent events so require. We intend to use our best efforts to keep the registration statement current, but may not be able to do so. If the registration statement is not declared effective or is not current in the future, your ability to sell the shares of Common Stock underlying the Notes and the shares of Common Stock issuable when the Warrants are exercised will be limited.
An active and visible trading market for our Common Stock may not develop.
If the Notes are converted into, or the Warrants are exercised for, shares of Common Stock, we cannot predict whether an active market for shares of our Common Stock will develop in the future. In the absence of an active trading market:
|
|
·
|
Investors may have difficulty buying and selling or obtaining market quotations;
|
|
|
·
|
Market visibility for shares of our Common Stock may be limited; and
|
51
|
|
·
|
A lack of visibility for shares of our Common Stock may have a depressive effect on the market price for shares of our Common Stock.
|
The OTCBB is an unorganized, inter-dealer, over-the-counter market that provides significantly less liquidity than NASDAQ or the NYSE AMEX. The trading price of the Common Stock is expected to be subject to significant fluctuations in response to variations in quarterly operating results, changes in analysts’ earnings estimates, announcements of innovations by us or our competitors, general conditions in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions, may have a material or adverse effect on the market price of shares of our Common Stock.
The market price for shares of our Common Stock may be volatile.
The market price for shares of our Common Stock may be volatile and subject to wide fluctuations in response to factors including the following:
|
|
·
|
actual or anticipated fluctuations in our quarterly operating results;
|
|
|
·
|
changes in financial estimates by securities research analysts;
|
|
|
·
|
conditions in commodities markets;
|
|
|
·
|
changes in the economic performance or market valuations of other feed additive technology companies;
|
|
|
·
|
announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
|
·
|
addition or departure of key personnel;
|
|
|
·
|
fluctuations of exchange rates between RMB and the U.S. dollar;
|
|
|
·
|
intellectual property litigation; and
|
|
|
·
|
general economic or political conditions in China.
|
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of shares of our Common Stock.
We do not anticipate paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of Common Stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their shares of Common Stock at or above the price they paid for them
52
We have considerable discretion in the use of proceeds from the Private Placement and we may use these proceeds in ways with which you may not agree.
Our Board of Directors and our management will have considerable discretion in the application of the net proceeds received by us in the Private Placement. Investors will not have the opportunity, as part of their investment decision, to assess whether proceeds are being used appropriately. You must rely on the judgment of our Board of Directors and our management regarding the application of the net proceeds of the Private Placement. The net proceeds may be used for corporate purposes that do not improve our efforts to maintain profitability or increase the price of our securities. The net proceeds from the Private Placement may be placed in investments that do not produce income or that lose value. See “Use of Proceeds” in this Item 2.01 of this Current Report on Form 8-K.
Shares eligible for future sale may adversely affect the market price of our Common Stock, as the future sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our Common Stock.
Pursuant to the terms of the Registration Rights Agreement and the Securities Purchase Agreement, we agreed to file a registration statement with the SEC to register the securities issued in the Private Placement within 60 days of the closing of the Private Placement. All of the shares included in an effective registration statement as described above may be freely sold and transferred except if subject to a lock up agreement. Additionally, concurrently with the closing of the Private Placement, the Company engaged in a Share Exchange. Following the closing of the Share Exchange, the Tanke Shareholders are eligible to sell all or some of their shares of our Common Stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144, subject to certain limitations. In general, pursuant to Rule 144, a stockholder (or stockholders whose shares are aggregated) who has satisfied an one-year holding period may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of Common Stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale. As of the closing of the Private Placement, 1% of our issued and outstanding shares of Common Stock equal approximately 133,241 shares (without giving effect to any conversion of Notes or exercise of Warrants or Agent Warrants). Rule 144 also permits, under certain circumstances, the sale of securities, without any limitations, by a non-affiliate that has satisfied a one-year holding period. Any substantial sale of Common Stock pursuant to any resale prospectus or Rule 144 may have an adverse effect on the market price of our Common Stock by creating an excessive supply.
53
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002, as well as new rules subsequently implemented by the SEC, has required changes in corporate governance practices of public companies. We expect these new rules and regulations to increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements. We are currently evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley, could have a material adverse effect on our business and stock price.
As a public company, we will be required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of Sarbanes-Oxley, which will require annual management assessments of the effectiveness of our internal control over financial reporting. During the course of our testing, we may identify deficiencies, which we may not be able to remediate in time to meet our deadline for compliance with Section 404. Testing and maintaining internal control can divert our management’s attention from other matters that are important to the operation of our business. We also expect the new regulations to increase our legal and financial compliance costs, make it more difficult to attract and retain qualified officers and members of our Board of Directors, particularly to serve on our Audit Committee, and make some activities more difficult, time consuming and costly. We may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 and, although we are currently exempt from the requirement that our independent auditor provide a report addressing the effectiveness of internal control over financial reporting, our independent registered public accounting firm may not be able or willing to issue an unqualified report on the effectiveness of our internal control over financial reporting if we become subject to such requirement in the future. If we conclude that our internal control over financial reporting is not effective, we cannot be certain as to the timing of completion of our evaluation, testing and remediation actions or their effect on our operations because there is presently no precedent available by which to measure compliance adequacy. If either we are unable to conclude that we have effective internal control over financial reporting, or to the extent we are subject to the requirement, our independent auditors are unable to provide us with an unqualified report as required by Section 404, then investors could lose confidence in our reported financial information, which could have a negative effect on the trading price of our stock.
54
Our Common Stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our Common Stock, which is currently and will be quoted for trading on OTCBB, may be considered to be a “penny stock” if it does not qualify for one of the exemptions from the definition of “penny stock” under Section 3a51-1 of the Exchange Act, as amended. Our Common Stock may be a “penny stock” if it meets one or more of the following conditions: (i) the stock trades at a price less than $5.00 per share; (ii) it is not traded on a “recognized” national exchange; (iii) it is not quoted on the Nasdaq Capital Market, or even if so, has a price less than $5.00 per share; or (iv) is issued by a company that has been in business less than three years with net tangible assets less than $5 million. The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our Common Stock will be subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to: (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our Common Stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
Our majority stockholder and its affiliates control the outcome of matters requiring stockholder approval.
Following the Private Placement, Golden Genesis is our majority stockholder and beneficially owns 10,011,469 shares of Common Stock, or 75.14% of the issued and outstanding shares of Common Stock (without giving effect to any conversion of Notes or exercise of Warrants or Agent Warrants). Golden Genesis is controlled (and will be majority owned, upon consummation of the transactions contemplated in the Call Option Agreements described herein) by our Chairman and Chief Executive Officer, Mr. Qiu. Consequently, Golden Genesis and Mr. Qiu will have the ability, when acting alone or with others, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as changes to our certificate of incorporation and bylaws and a merger or a sale of our company or a sale of all or substantially all of our assets. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those of our officers, directors and affiliates. Golden Genesis and Mr. Qiu also have significant control over our business, policies and affairs. Additionally, this significant concentration of share ownership may adversely affect the trading price for our Common Stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders.
55
Provisions in our Bylaws could discourage, delay or prevent a change of control of our company and may result in an entrenchment of management and diminish the value of our Common Stock.
Our Bylaws provide that, unless otherwise prescribed by statute, special meetings of the stockholders can only be called by the Chairman of our Board of Directors, our President, or by a majority of the Board of Directors. These provisions may discourage, delay or prevent a merger, acquisition or other change of control that our stockholders may consider favorable. Such provisions could impede the ability of our common stockholders to benefit from a change of control and, as a result, could materially adversely affect the market price of our Common Stock and your ability to realize any potential change-in-control premium. See “Description of Registrant’s Securities to Be Registered” in this Item 2.01 of this Current Report on Form 8-K.
We do not presently have a Chief Financial Officer with U.S. public company experience.
We do not presently have a Chief Financial Officer that is familiar with the accounting and reporting requirements of a U.S. publicly-listed company. Although we have agreed to retain the services of such an executive within 6 months of the closing of the Private Placement, no assurances can be given that we will be able to identify or afford the financial requirements of qualified candidates. The position of Chief Financial Officer of a U.S. publicly-listed company is critical to the operations of such a company, and our failure to fill this position in a timely and effective manner will negatively impact our business.
56
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of the results of operations and financial condition of Tanke, our principal operating business, for the nine months ended September 30, 2010 and 2009 and for the fiscal years ended December 31, 2009 and 2008 should be read in conjunction with the financial statements, and the notes to those financial statements, that are included in Item 9.01 of this Current Report on Form 8-K. Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including the disclosures set forth in this Item 2.01 to this Current Report on Form 8-K under the headings “Cautionary Note on Forward Looking Statements” and “Risk Factors”, which are incorporated herein by reference. As a result of the Share Exchange and the VIE Agreements, the Company, through Kanghui Agricultural, its indirect wholly owned subsidiary, assumed management of the business activities of Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Tanke. As used in this section, the terms “we”, “our”, “us” and the “Company” refer to the Company, our direct and indirect subsidiaries and Tanke, our principal operating business.
Overview
The Company is one of the leading animal nutrition and feed additive providers in China. A PRC government certified high-tech company, the Company’s products optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish, and seek to capitalize on China’s growing demand for safe and reasonably priced food. With feed additives used in China at less than half the rate of that of the United States and Europe, the Company is poised to seize a significant market opportunity as Chinese feed producers modernize and expand the use of modern and more effective additives.
The Company has more than 150 employees, with 43 engaged in sale or sales-related activities. The Company’s headquarters are in Guangzhou and it operates in a modern 34,000 square-meter manufacturing facility in the Huadu Economic District, both of which are in Guangdong province. The Company currently produces 21 branded feed additives, with each brand available in seven different mixes that correspond to different stage of an animal’s life cycle.
Our major products address most key market categories within China’s animal feed additive industry including: Organic Trace Mineral Additives, which in 2009 accounted for approximately 70% of our revenue, Feed Acidifiers and Flavor Enhancers, which accounted for approximately 20% of our revenue, and Herbal Medicine Additives, which accounted for approximately 3% of our revenue. Our extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. While the majority of our sales are domestic in the PRC, international sales, mainly in Southeast Asia, Latin American and other developing countries, currently account for approximately 5% of our total sales.
57
Critical Accounting Policies
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements, included in Item 9.01 of this Current Report on Form 8-K, we believe that the following accounting policies are the most critical to aid investors in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Basis of Presentation
The Company’s consolidated financial statements have been stated in US dollars and prepared in accordance with generally accepted accounting principles in the United States of America.
Use of Estimates
In preparing consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of due from related parties, the net realizable value of inventories, the estimation of useful lives of property and equipment, and intangible assets. Actual results could differ from those estimates.
Revenue Recognition
Revenue is recognized when all of the following criteria are met:
|
|
·
|
Persuasive evidence of an arrangement exists;
|
|
|
·
|
Delivery has occurred or services have been rendered;
|
|
|
·
|
The seller’s price to the buyer is fixed or determinable; and
|
|
|
·
|
Collectability is reasonably assured.
|
The Company continually performs analyses of returns and allowances and records a provision at the time of sale if necessary. The Company revisits this estimate regularly and adjusts it if conditions change.
Research and Development Costs
Research and development costs are charged as expense when incurred and included in operating expenses.
58
Foreign Currency Translation
The functional currency of Tanke, our principal operating business, is the Renminbi. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
For financial reporting purposes, the financial statements of the Company which are prepared using the functional currency have been translated into United States dollars (“US$” or “$”). Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates for the period. Stockholders’ equity is translated at historical exchange rates. Any translation adjustments are included as a foreign exchange adjustment in other comprehensive income, a component of stockholders’ equity.
Exchange rates applied for the foreign currency translation during the 2009 and 2008 fiscal years are as follows:
|
US$1 to RMB
|
2009
|
2008
|
|
Closing rate
|
6.8282
|
6.8346
|
|
Average rate
|
6.8314
|
7.0696
|
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US$ at the rates used in translation.
Results of Operations
Results of Operations for the Year Ended December 31, 2009 versus 2008
Revenue, Costs of Sales and Gross Profit
The Company operates in four segments: (1) Organic Trace Mineral Additives, (2) Functional Regulation Additives, (3) Herbal Medicine Additives and (4) Other Revenues. Management tracks each of these operations separately.
Following is comparison of our revenue, costs of sales and gross profit by segment for the years ended December 31, 2009 and 2008.
59
|
Years Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 8,498,716 | $ | 4,840,103 | $ | 3,658,613 | 76 | % | ||||||||
|
Functional Regulation Additives
|
2,436,244 | 2,176,493 | 259,751 | 12 | % | |||||||||||
|
Herbal Medicine Additives
|
340,635 | 474,109 | (133,474 | ) | (28 | )% | ||||||||||
|
Other
|
893,944 | 1,105,164 | (211,220 | ) | (19 | )% | ||||||||||
| $ | 12,169,539 | $ | 8,595,869 | 3,573,670 | 42 | % | ||||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 4,038,472 | $ | 3,270,311 | $ | 768,161 | 23 | % | ||||||||
|
Functional Regulation Additives
|
1,588,669 | 1,485,618 | 103,051 | 7 | % | |||||||||||
|
Herbal Medicine Additives
|
235,641 | 222,315 | 13,326 | 6 | % | |||||||||||
|
Other
|
832,139 | 293,534 | 538,605 | 183 | % | |||||||||||
| $ | 6,694,921 | $ | 5,271,778 | 1,423,143 | 27 | % | ||||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 4,460,244 | $ | 1,569,792 | $ | 2,890,452 | 184 | % | ||||||||
|
Functional Regulation Additives
|
847,575 | 690,875 | 156,700 | 23 | % | |||||||||||
|
Herbal Medicine Additives
|
104,994 | 251,794 | (146,800 | ) | (58 | )% | ||||||||||
|
Other
|
61,805 | 811,630 | (749,825 | ) | (92 | )% | ||||||||||
| $ | 5,474,618 | $ | 3,324,091 | 2,150,527 | 65 | % | ||||||||||
Organic Trace Mineral Additives
Organic trace mineral additives constitute the largest and fastest growing area of our business. We are China’s largest domestic provider of organic trace mineral additives, specializing in the development and production of chelated organic trace mineral additives. The Company’s current trace mineral manufacturing facility is the largest chelating facility in China and has the capacity to produce approximately 250 metric tons of organic trace minerals per week.
Revenue from organic trace mineral additives accounted for approximately 70% of the Company’s total 2009 revenues, up from 56% in 2008, and carried a gross profit margin of approximately 52%, compared to 32% in 2008.
In 2009, revenue from organic trace mineral additives increased by $3,658,613 or 76% from the level in 2008, primarily due to the introduction of new products in 2008 that obtained market acceptance toward the end of 2008 and contributed to the substantial increase in revenue in 2009. Additionally gross profits from this segment increased by $2,890,452, or 184% because of improved productivity in the manufacturing process.
60
Functional Regulation Additives
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system.
We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers. Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. Flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption in order to improve the yield from production animals.
Revenue from functional regulation additives accounted for approximately 20% of the Company’s total 2009 revenues, down from 25% in 2008, and carried a gross profit margin of approximately 35%, compared to 32% in 2008.
In 2009, revenue from functional regulation additives increased by $259,751, or 12% from the level in 2008 due primarily to increases in sales to existing customers. Additionally gross profits from this segment increased by $156,700, or 23% because of improved manufacturing processes creating better efficiencies as revenue increased.
Herbal Medicine Additives
Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health. Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives in feed animal production.
Revenue from herbal medicine additives accounted for approximately 3% of the Company’s total 2009 revenues, down from 6% in 2008, and carried a gross profit margin of approximately 31%, compared to 53% in 2008.
In 2009, revenue from herbal medicine additives decreased by $133,474, or 28% from the level in 2008 due to what management believes is a temporary decrease in demand for the product. Additionally, the decrease in gross profits from this segment of $146,800, or 58% is mainly due to the decrease in revenue in 2009, exacerbated by inefficiencies resulting from smaller production levels.
Other Revenues
As one of the leading sellers of feed additives in China, the Company is a well-respected brand with a great reputation. As a result, we are able to purchase raw materials from certain manufacturers at a relatively low price and sell those raw materials to other feed additive manufacturers at a premium.
61
In addition, we provide technical support and know-how to our customers, and we charge for this service.
In 2009, other revenue decreased by $211,220, or 19% from the level in 2008. The decrease primarily resulted from the slower growth of our technical service business as we focused on the development of our core business in 2009. Additionally gross profits from this segment decreased by $749,825, or 92% primarily because of the decrease in technical services which had historically resulted in higher profit margin.
Reconciliation of Gross Profit to Income Before Taxes
We evaluate the performance of our operating segments based on segment revenue and gross profit, and management uses aggregate segment gross profit as a measure of the overall performance of the business. We believe that information about aggregate segment gross profit assists investors by allowing them to evaluate changes in the gross profit of our various offerings separate from factors other than product offerings that affect net income. The following table reconciles aggregate segment gross profit to income before income taxes.
|
Years Ended
|
||||||||||||||||
|
December 31,
|
Better (Worse)
|
|||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
Aggregate segment gross profit
|
$ | 5,474,618 | $ | 3,324,091 | $ | 2,150,527 | 64.7 | % | ||||||||
|
Selling expenses
|
1,596,439 | 1,081,099 | (515,340 | ) | (47.7 | )% | ||||||||||
|
Administrative expenses
|
783,069 | 653,600 | (129,469 | ) | (19.8 | )% | ||||||||||
|
Depreciation and amortization
|
39,508 | 44,286 | 4,778 | 10.8 | % | |||||||||||
|
Other operating expenses
|
17,601 | 44,176 | 26,575 | 60.2 | % | |||||||||||
|
Income from operations
|
3,038,001 | 1,500,930 | 1,537,071 | 102.4 | % | |||||||||||
|
Foreign exchange gains, net
|
43,159 | 9,304 | 33,855 | 363.9 | % | |||||||||||
|
Interest income
|
4,890 | 1,390 | 3,500 | 251.8 | % | |||||||||||
|
Interest expense
|
(58,626 | ) | (16,305 | ) | (42,321 | ) | (259.6 | )% | ||||||||
|
Income before income taxes
|
3,027,424 | 1,495,319 | 1,532,105 | 102.5 | % | |||||||||||
|
Income tax expense
|
443,423 | 96,991 | (346,432 | ) | (357.2 | )% | ||||||||||
|
Net income
|
$ | 2,584,001 | $ | 1,398,328 | 1,185,673 | 84.8 | % | |||||||||
Cost of Goods Sold
Our cost of sales primarily consist of costs associated with (i) purchase of raw materials from suppliers, which accounts for approximately 90% of the total cost of goods sold, and (ii) production costs such as depreciation, low value consumables, salaries for product departments staff, and water and electricity expenses. Salaries for production department staff cover approximately 4% of the cost of goods sold, while consumption of low value consumables covers 2%. Accordingly, a supplier’s failure to supply raw materials or components in a timely manner, or to supply raw materials or components that meet our quality and quantity requirements or the inability to obtain alternative sources of these products or components on a timely basis or on terms acceptable to us, would create delays in providing of our products. Our cost of goods sold may fluctuate due to a number of factors, including, but not limited to a change in key suppliers for raw materials and the innovation of new substitutable products.
62
Cost of goods sold for the year ended December 31, 2009 amounted to approximately $6.7 million compared with $5.3 million in 2008, an increase of approximately $1.4 million or 27.0%. The increase in 2009 was due to the corresponding increase of sales of 41.6%. Sales increased at a higher rate than the corresponding increase in cost of goods sold as the price of raw materials was lower in 2009 due to the downturn in the economy. In addition, our growth in sales was also due to the technical improvement of our production formula by our research and development department through the development of different combinations of raw material additives without any significant increases in cost. Accordingly, our gross profit margin increased in 2009 to 45.0% from 38.7% in 2008.
Selling and Administrative Expenses
Selling expenses for the year ended December 31, 2009 amounted to approximately $1.6 million compared with $1.1 million in 2008, an increase of approximately $500,000 and 47.7%. The increase in selling expenses was in line with the percentage increase of sales, which was mainly attributed to an increase in travel and meeting expenses for sales department staff to develop new customers and expand the market. Selling expenses primarily include travel expense, salaries, and transportation expense, which covers approximately 50%, 25%, and 12% of the total selling expenses, respectively.
Administrative expenses for the year ended December 31, 2009 amounted to $783,069 compared with $653,600 for 2008, an increase of $129,469 and 19.8%. The increase in administrative expenses is primarily due to bad debt expense related to uncollectible receivables in our subsidiary Guangzhou Tanke Animal Health Co. Ltd. General and administrative expenses primarily represent the salaries for management, travel expenses, and general office expenses, which cover approximately 49%, 15%, and 15% of general and administrative expenses, respectively.
Our selling, general and administrative expenses may fluctuate in the future due to a variety of factors, including, but not limited to:
|
·
|
Additional expenses as a result of becoming a reporting company including, but not limited to, director and officer insurance, compensation for the director, SEC reporting and compliance, transfer agent fees, additional staffing, professional fees and similar expenses; and
|
|
·
|
Expansion by developing new products or expansion of new markets, including travel and entertainment expenses, advertising expenses.
|
Foreign Exchange Gains, Interest Income and Expense
Foreign exchange gains represent primarily the net foreign exchange gains or losses during the year. The amounts for the year ended December 31, 2009 increased by $33,855 and 363.9% as compared to 2008 primarily due to the reimbursement of loan interest by the Guangdong Government, which is one of the policies to encourage development of small and medium size enterprises within Guangdong.
63
Interest income remained consistent in 2009 as compared to 2008 as our average cash savings remained approximately the same in each year. Interest expense increased during the year ended December 31, 2009 by $42,321 and 259.6% as compared to 2008 due to the new long-term borrowing in 2009.
Income Tax Expense
All of our income was generated from mainland China and was generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriate tax adjustments, except for one subsidiary of Tanke, Guangzhou Tanke Bio-Tech Co. Ltd. (“Tanke Bio-Tech”), which obtained tax exemption on April 2007 from the State Tax Bureau of Huadu District, Guangzhou. Tanke Bio-Tech was a joint venture enterprise and was eligible for tax reduction and exemption, which enjoys the “two year exempt, three years half” benefits. The effective tax rate for Tanke Bio-Tech in 2009 and 2008 year end was 12.5% and 0%, respectively.
The income tax expense amounted to $443,423 for the year ended December 31, 2009, an increase of $346,432 and 357.2% compared to 2008 was attributed to the increased taxable income and tax rate for the year ended December 31, 2009.
Results of Operations for the Nine Months Ended September 30, 2010 versus 2009
Revenue, Costs of Sales and Gross Profit
The Company operates in four segments: Organic Trace Mineral Additives, Functional Regulation Additives, Herbal Medicine Additives and Other Revenues. Management oversees each of these operations separately.
Following is comparison of our revenue, costs of sales and gross profit by segment for the nine months ended September 30, 2010 and 2009.
64
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
Better (Worse)
|
|||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 10,596,610 | $ | 6,667,219 | $ | 3,929,391 | 59 | % | ||||||||
|
Functional Regulation Additives
|
1,983,528 | 1,907,579 | 75,949 | 4 | % | |||||||||||
|
Herbal Medicine Additives
|
304,306 | 267,832 | 36,474 | 14 | % | |||||||||||
|
Other
|
658,257 | 620,535 | 37,722 | 6 | % | |||||||||||
| $ | 13,542,701 | $ | 9,463,165 | 4,079,536 | 43 | % | ||||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 6,553,365 | $ | 3,287,412 | (3,265,953 | ) | (99 | )% | ||||||||
|
Functional Regulation Additives
|
1,282,146 | 1,270,935 | (11,211 | ) | (1 | )% | ||||||||||
|
Herbal Medicine Additives
|
197,799 | 188,513 | (9,286 | ) | (5 | )% | ||||||||||
|
Other
|
599,927 | 535,162 | (64,765 | ) | (12 | )% | ||||||||||
| $ | 8,633,237 | $ | 5,282,022 | (3,351,215 | ) | (63 | )% | |||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 4,043,245 | $ | 3,379,807 | 663,438 | 20 | % | |||||||||
|
Functional Regulation Additives
|
701,382 | 636,644 | 64,738 | 10 | % | |||||||||||
|
Herbal Medicine Additives
|
106,507 | 79,319 | 27,188 | 34 | % | |||||||||||
|
Other
|
58,330 | 85,373 | (27,043 | ) | (32 | )% | ||||||||||
| $ | 4,909,464 | $ | 4,181,143 | 728,321 | 17 | % | ||||||||||
Organic Trace Mineral Additives
Organic trace mineral additives constitute the largest and fastest growing area of our business. We are China’s largest domestic provider of organic trace mineral additives, specializing in the development and production of chelated organic trace mineral additives. The Company’s trace mineral manufacturing facility is the largest chelating facility in China and has the capacity to produce approximately 250 metric tons of organic trace minerals per week.
Revenue from organic trace mineral additives accounted for approximately 78% of the Company’s total 2010 revenues, up from 70% for the corresponding nine month period in 2009, and carried a gross profit margin of approximately 38%, compared to 51% in 2009.
In the nine months ended September 30, 2010, revenue from organic trace mineral additives increased by $3,929,391, or 59% from the level in 2009 due to the success of an extensive promotion program intended to boost sales. We also increased sales personnel during the year, leading to an increase in customer acceptance of our products. Gross profits from this segment increased by only $663,438, or 20% because we focused on increasing market share instead of profitability. We believe the increased market share creates the potential for a higher profit margin in the future.
65
Functional Regulation Additives
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system.
We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers. Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. Flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption in order to improve the yield from production animals.
Revenue from functional regulation additives accounted for approximately 15% of the Company’s total 2010 revenues, down from 20% in the corresponding nine month period ended September 30, 2009, and carried a gross profit margin of approximately 35%, compared to 33% for the nine months ended September 30, 2009.
In 2010, revenue from functional regulation additives increased by $75,949, or 4% from the level in 2009 due to growth in sales to our existing customers, which we believe is sustainable. Additionally gross profits from this segment increased by $64,738, or 10% because of increased manufacturing efficiencies as production levels increased.
The Company’s feed acidifiers differ from other growth promoters because they contain alkaloids to stimulate acid production in an animal’s stomach, lowering the pH levels to improve overall animal health. Such feed acidifiers do not rely on antibiotics as a primary ingredient. Rather, the ingredients in the Company’s feed acidifiers comply with the more restrictive requirements of the EU and the increased regulation that the Company anticipates in the future from China’s regulatory authorities. As a result, the Company expects growth in this particular area.
Herbal Medicine Additives
Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health.
Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives in food animal production.
Revenue from herbal medicine additives accounted for approximately 2% of the Company’s total 2010 revenues, down from 3% in 2009, and carried a gross profit margin of approximately 35%, compared to 30% in the corresponding nine months ended September 30, 2009.
66
In 2010, revenue from herbal medicine additives increased by $36,474, or 14% from the level in 2009 mainly due to a new antioxidant product, which was introduced in January of 2010. We delivered more than 20 metric tons of this new product in the first nine months of 2010. Additionally gross profits from this segment increased by $27,188, or 34% because of the higher profit margin of our new products as well as more effective cost control of the production of our products.
Other Revenues
As one of the leading sellers of feed additives in China, the Company is a well-respected brand with a great reputation. Consequently, we are able to purchase raw materials from certain manufacturers at a relatively low price and sell those raw materials to other feed additive manufacturers at a premium.
In addition, we provide technical support and know-how to our customers, and we charge for this service.
Other revenues accounted for approximately 5% of the Company’s total 2010 revenues, down from 7% in the nine months ended September 30, 2010, and carried a gross profit margin of approximately 9%, compared to 14% the same period in 2009.
In 2010, other revenue increased by $37,722, or 6% from the level in 2009 due to a 23% increase in revenue from our byproducts. However gross profits from this segment decreased by $27,043, or 32% primarily because of the continued decrease in revenue attributable to technical services.
Reconciliation of Gross Profit to Income Before Taxes
We evaluate the performance of our operating segments based on segment revenue and gross profit, and management uses aggregate segment gross profit as a measure of the overall performance of the business. We believe that information about aggregate segment gross profit assists investors by allowing them to evaluate changes in the gross profit of our various offerings separate from factors other than product offerings that affect net income. The following table reconciles aggregate segment gross profit to income before income taxes.
67
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
Better (Worse)
|
|||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(unaudited)
|
(unaudited)
|
|||||||||||||||
|
Aggregate segment gross profit
|
$ | 4,909,464 | $ | 4,181,143 | $ | 728,321 | 17.4 | % | ||||||||
|
Selling expenses
|
1,298,552 | 1,136,969 | 161,583 | 14.2 | % | |||||||||||
|
Administrative expenses
|
591,238 | 566,769 | 24,469 | 4.3 | % | |||||||||||
|
Depreciation and amortization
|
36,516 | 36,600 | (84 | ) | -0.2 | % | ||||||||||
|
Other operating expenses
|
1,344 | 2,323 | (979 | ) | -42.1 | % | ||||||||||
|
Income from operations
|
2,981,814 | 2,438,482 | 543,332 | 22.3 | % | |||||||||||
|
Foreign exchange gains, net
|
186 | - | 186 | 100.0 | % | |||||||||||
|
Interest income
|
33,382 | 3,525 | 29,857 | 847.0 | % | |||||||||||
|
Interest expense
|
(79,921 | ) | (53,168 | ) | (26,753 | ) | 50.3 | % | ||||||||
|
Income before income taxes
|
2,935,461 | 2,388,839 | 546,622 | 22.9 | % | |||||||||||
|
Income tax expense
|
395,930 | 345,534 | 50,396 | 14.6 | % | |||||||||||
|
Net income
|
2,539,531 | 2,043,305 | 496,226 | 24.3 | % | |||||||||||
Cost of Goods Sold
Cost of goods sold for the nine months ended September 30, 2010 amounted to approximately $8.6 million as compared to $5.3 million in 2009, an increase of approximately $3.3 million or 63.4%. The increase in cost of goods sold was partially due to the increase in sales. The cost of goods sold increased at a higher rate than the corresponding increase in sales of 43.1%. The major reason for this difference is due to the increased price of raw materials during 2010, as our costs were lower in 2009 due to the effect of the worsened economic conditions. As a result, the unit price of some major raw materials such as “lemon acid” and “organic herbal material” increased by approximately 25% in 2010. Accordingly, our gross profit margin decreased in 2010 to 36.3% from 44.2% in 2009. Our management will consider amending our selling prices based on the fluctuations of raw material prices in the future.
Selling and Administrative Expenses
Selling expenses for the nine months ended September 30, 2010 amounted to approximately $1.3 million as compared to $1.1 million in 2009, an increase of $161,583 and 14.2%. The increase in selling expenses was primarily due to the growth in sales as well as an increase in the number of sales staff.
Administrative expenses for the nine months ended September 30, 2010 were consistent with the expenses for 2009, as these expenses are not as variable based on sales.
Foreign Exchange Gains, Interest Income and Expense
Interest income increased by $29,857 and 847.0% during the nine months ended September 30, 2010 as compared to 2009. This was due to the increase in average cash savings during 2010. Interest expense increased by $26,753 and 50.3% during the nine months ended September 2010 as compared to 2009. The increase was due primarily to our long-term borrowings in May 2009 which bear interest at 5.4% per annum. Interest expense was higher during the nine months ended September 30, 2010 as compared to 2009, as the loan amounts were outstanding for 9 months during 2010, as compared to only approximately 4 months in 2009.
68
Income Tax Expense
For the nine months ended September 30, 2010, the increase tax expenses were in line with the sales growth.
Liquidity and Capital Resources
As of September 30, 2010, December 31, 2009, and 2008 we had cash and balances of $3,067,051, $1,817,875, and $1,263,517, respectively, primarily consisting of cash on hand and demand deposits. The following table provides detailed information about our net cash flow for all financial statement periods presented in this report. To date, we have financed our operations by cash from operations and capital contributions by our Chinese shareholders.
The following table sets forth a summary of our cash flows for the periods indicated.
|
Year Ended
|
Nine Months Ended
|
|||||||||||||||
|
December 31,
|
December 31,
|
September 30,
|
September 30,
|
|||||||||||||
|
2009
|
2008
|
2010
|
2009
|
|||||||||||||
|
Net cash provided by (used in) operating activities
|
$ | 1,739,570 | $ | 1,263,766 | $ | 2,475,703 | $ | (30,402 | ) | |||||||
|
Net cash provided by (used in) investing activities
|
(363,698 | ) | 286,426 | (73,430 | ) | (229,201 | ) | |||||||||
|
Net cash provided by (used in) financing activities
|
(822,959 | ) | (660,915 | ) | (1,198,991 | ) | 197,524 | |||||||||
|
Effects of exchange rate change on cash
|
1,445 | 52,689 | 45,895 | 5,661 | ||||||||||||
|
Net increase (decrease) in cash
|
$ | 554,358 | $ | 941,966 | $ | 1,249,177 | $ | (56,418 | ) | |||||||
Operating Activities
Net cash provided by operating activities was $1,739,570 for the year ended December 31, 2009 compared to cash provided of $1,263,766 in 2008, an increase of $475,804. The increase was due to the increase in net income of $1.2 million, driven primarily by the market acceptance of our new organic trace mineral products. This was offset by the need to increase inventory to support the increased business, as well as the need to pay down some of our trade accounts payables.
During the nine months ended September 30, 2010, net cash provided by operating activities was $2,475,703 compared to cash used during the same period in 2009 of $30,402, an increase of $2,506,105. The increase in cash flows from operating activities was partially due to the $496,226 increase in net income, but was mostly driven by payments of approximately $1.2 million in 2009 to settle payables accounts, as well as an increase in other receivables of approximately $600,000. The payments in 2009 were settling accounts established in 2008, and therefore, as the accounts were settled, this type of payment was not necessary in 2010, nor will it be necessary in the future.
69
Investing Activities
Net cash used in investing activities was $363,698 for the year ended December 31, 2009 compared to cash provided in 2008 of $286,426, a change of $650,124. The increase in cash used in investing activities in 2009 was partially due to our investment of $146,000 in a new unconsolidated entity during 2009. In addition, as a result of this investment, more cash needed to be set aside as restricted. Together, these two actions absorbed $293,000 of cash in 2009. Also, in 2008, $348,000 of cash we had previously restricted in 2007 was returned to the Company, providing additional cash from investing activities.
During the nine months ended September 30, 2010, net cash used in investing activities amounted to $73,430 as compared to cash used in 2009 of $229,201, a decrease of $155,771. Cash used during 2010 was primarily due to the purchase of new equipment as well as spending on construction in progress. These expenditures totaled $221,000. This was partially offset by the return in 2010 of the $146,000 of cash that was restricted in 2009. Cash used in 2009 was primarily due to investments in unconsolidated entities, as well as cash starting to become restricted related to that investment.
Net cash used in financing activities was $822,959 for the year ended December 31, 2009 compared to cash used in 2008 of $660,915, an increase of $162,044. The cash used during 2009 was due to advances to three shareholders of Tanke in the amount of $3,107,000, partially offset by payments on the Company’s bank loans of $2,196,000. During 2008, the Company borrowed $800,000 from the bank, and made advances to the shareholders of $138,000.
During the nine months ended September 30, 2010, net cash used in financing activities was $1,198,991 compared to cash provided in 2009 of $197,524, an increase of $1,396,515. Cash used in 2010 was due to the Company continuing to repay a bank debt in the amount of $665,000, and also shareholder advances in the amount of $534,000. In 2009, the Company borrowed $2,196,000 from the bank and advanced $1,998,000 to three shareholders. No additional borrowings occurred in 2010.
The Company does not intend to borrow additional money from banks and intends to pay off the existing facility with cash flow from operations. Additionally, the shareholders executed a promissory note and agreed to repay $3,265,559 to the Company by June 30, 2012. As a result, we expect positive cash from financing activities during 2011.
Historically, we have funded our operations primarily through cash generated from operations. Over the next twelve months, we intend to pursue organic and acquisitive growth and increase our market share in mainland China. We believe that our cash on hand and cash flow from operations will meet our present operating cash needs for the next 12 months. However, we will require additional cash resources to meet the cash requirements of our planned growth.
70
We may also require additional cash resources due to unanticipated changes in business conditions, the implementation of our marketing strategy and in order to increase brand awareness, or acquisitions we may decide to pursue. If our financial resources are insufficient to satisfy our capital requirements, we may seek to sell additional equity, securities, convertible notes or warrants in the future.
Effect of Changes in the Foreign Exchange Rate
Upon translation of the Company’s financial statements into US Dollars for the purpose of financial reporting in the United States, the exchange rate between the Chinese Renminbi and the US Dollar can have an impact on the amount of reported cash on hand.
All of our revenue is generated in China, and currently over 90% of our cost is within China. As a result, from an operational standpoint, a change in the exchange rate has relatively little impact on us. Such change is not expected to affect our liquidity in any significant way.
Economy and Inflation
Inflation and changing prices have not had a material effect on our business and we do not expect that inflation or changing prices will materially affect our business in the foreseeable future.
We have not experienced any significant cancellation in orders due to the downturn in the economy. Furthermore, we have also had only a small number of customer-requested delays in delivery or production.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements that have, or are reasonably likely to have a current or future effect on our financial statements.
Seasonality
Our operating results and operating cash flows historically have not been subject to significant seasonal variations. However, sales around Chinese New Year will be comparatively lower than other months. This pattern may change as a result of new market opportunities or new product introduction.
Accounting Pronouncements
See Note 2 of the consolidated financial statements of the Company attached as Exhibit 99.2 to this Current Report on Form 8-K for a summary of our significant accounting policies.
We do not anticipate that the adoption of these recent accounting pronouncements will have a material impact on our financial statements.
71
Properties
The Company’s corporate office is located at East Tower of Hui Hao Building, No. 519 Machang Road, Pearl River New City, Guangzhou, China. The Company’s manufacturing facility is in a modern 34,000 square-meter facility in the Huadu Economic District, in Guangdong province.
We entered into a Land Lease Agreement valid from May 20, 2006 to May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao Town granted us a land use right covering the land where our manufacturing facility currently stands. This lease will be superseded by a Land Use Right Certificate and will be automatically terminated once Tanke obtains such certificate. Due to changes in the relevant PRC regulations, we have not been granted such certificate for this land. Therefore, pursuant to applicable PRC law, we are not permitted to operate our manufacturing facility without such certificate and as a result, there is a risk that the PRC government may declare our Land Lease Agreement invalid.
We are currently negotiating with the local government to obtain a Land Use Right Certificate, which would permit us to operate our business as it is currently conducted. Without the Land Use Right Certificate, we are unable to apply for a Property Ownership Certificate for our manufacturing facilities. Until we obtain the Land Use Right Certificate, the PRC government may evict our personnel from the premises and remove our manufacturing facilities that we built on the premises. Such action would have a very significant and negative impact on our operations and business.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the beneficial ownership of our Common Stock as of February 9, 2011, by (i) each person known by us to be the beneficial owner of 5% or more of the outstanding Common Stock, (ii) each executive officer and director of the Company, and (iii) all of our executive officers and directors as a group.
Unless otherwise indicated, the Company believes that all persons named in the table have sole voting and investment power with respect to all shares of Common Stock beneficially owned by them.
|
Name and Address of Beneficial Owner
|
Shares of Common Stock Owned (1)
|
Percent of Class (2)
|
|
Guixiong Qiu *
Room 401, 5 building cha shan qui
South China Agricultural University dayuan No 483 tianhe qui wu shan road
Guangzhou city Guangdong
P.R.China
|
4,505,161 (3)
|
33.81%
|
|
Xugang Shu *
Room 401, 5 building cha shan qui
South China Agricultural University dayuan No 483 tianhe qui wu shan road
Guangzhou city Guangdong
P.R.China
|
0
|
0.0%
|
|
Bo Jun *
Room 401, 5 building cha shan qui
South China Agricultural University dayuan No 483 tianhe qui wu shan road
Guangzhou city Guangdong
P.R.China
|
0
|
0.0%
|
|
Bi Gao
Room 704 , No 10 tianhe guanghe road
Guangzhou city Guangdong
P.R.China
|
3,203,670 (4)
|
24.04%
|
|
Xiuzhen Liang
Room 404, No 10 quan fu li fang cun qui
Guangzhou city Guangdong
P.R.China
|
2,002,294 (5)
|
15.03%
|
|
All directors and officers as a group
|
4,505,161
|
33.81%
|
72
* Director and/or executive officer.
|
(1)
|
Under Rule 13d-3 of the Exchange Act, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights.
|
|
(2)
|
Based on 13,324,093 shares of Common Stock issued and outstanding after the closing of the Share Exchange and Private Placement.
|
|
(3)
|
Includes (i) 3,605,161 shares of Common Stock held by Golden Genesis that shall be transferred within the next 3 years to Mr. Qiu pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Qiu without the prior written consent of Mr. Qiu, before all of such shares are transferred to Mr. Qiu; and (ii) 900,000 shares of Common Stock over which Mr. Qiu has voting power that are held by Golden Genesis and subject to the terms of the Securities Escrow Agreement, which provides that 450,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Qiu), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012.
|
73
|
(4)
|
Includes (i) 2,563,670 shares of Common Stock held by Golden Genesis that shall be transferred within the next 3 years to Mr. Gao pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Gao without the prior written consent of Mr. Gao, before all of such shares are transferred to Mr. Gao; and (ii) 640,000 shares of Common Stock over which Mr. Gao has voting power that are held by Golden Genesis and subject to the terms of the Securities Escrow Agreement, which provides that 320,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Gao), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012.
|
|
(5)
|
Includes (i) 1,602,294 shares of Common Stock held by Golden Genesis that shall be transferred within the next 3 years to Ms. Liang pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Ms. Liang without the prior written consent of Ms. Liang, before all of such shares are transferred to Ms. Liang; and (ii) 400,000 shares of Common Stock over which Ms. Liang has voting power that are held by Golden Genesis and subject to the terms of the Securities Escrow Agreement, which provides that 200,000 shares will be disbursed in each of 2012 and 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Ms. Liang), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2011 and 2012.
|
Change in Control
The disclosure set forth in Item 5.01 of this Current Report on Form 8-K is incorporated herein by reference.
Directors and Executive Officers
Effective February 9, 2011, in connection with the closing of the Share Exchange, Geoff Williams, our former President and Chief Executive Officer, and Nancy Ah Chong, our former Secretary and Treasurer resigned from their respective offices and Mr. Qiu was appointed as our Chairman of the Board of Directors. On or about February 13, 2011, effective as of the tenth (10th) day following the later of the date of the filing of the Information Statement with the SEC or the date of mailing of the Information Statement to our stockholders, Geoff Williams and Nancy Ah Chong will resign as our directors and Mr. Qiu will be appointed as the sole director of the Company. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
74
Effective February 9, 2011, in connection with the closing of the Share Exchange and the resignations of Mr. Williams and Ms. Chong as our officers, we also appointed new officers of the Company. The following table sets forth the name, age, and position of such officers. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. There are no family relationships between any of our executive officers or directors.
|
Name
|
Age
|
Position
|
|
Guixiong Qiu
|
45
|
Founder and Chief Executive Officer
|
|
Xugang Shu
|
34
|
Vice President of Research and Development
|
|
Bo Jun
|
32
|
Marketing Director
|
Guixiong Qiu. Mr. Qiu has more than 13 years experience in the feed additives industry. Mr. Qiu earned an associate degree from South China Agricultural University with a major in Animal Inspection in 1997 and completed an Advanced Program in Agriculture Industrial and Business Management from Tsinghua University in 2005. After his graduation from college, Mr. Qiu worked for Zhengda Kangdi, a large feed producer, for three years. In 1991, he formed a logistic company trading animal drugs. In 1993, Mr. Qiu assisted an associate in managing a flavor enhancer feed additive company until founding Tanke.
Mr. Qiu served as a Vice President of the China Feed Industry Association and is a trustee of the Guangdong Province & Guangzhou City Feed Industry Association, Vice President of China Animal Health Association and trustee of China Green Industries Union Association. Mr. Qiu is also the President and a Director of Guangzhou Hai Hong Chuang Ltd.
Having worked for over 13 years in the feed additive industry, Mr. Qiu brings specialized knowledge of Tanke’s business to our board of directors.
Xugang Shu. Dr. Shu joined Tanke in 2002 after receiving his Masters Degree in Chemistry from Guangdong University of Technology. From December 2002 until December 2004, he was in charge of Tanke’s product quality meeting ISO 9001 and 2000 standards and compliance with GMP. In 2005, Dr. Shu became Tanke’s Vice President for Research & Development and organized the technology research center that has been responsible for 5 patent applications. He has received numerous awards and received a PhD in Chemistry from Guangdong University of Technology.
Bo Jun. Prior to joining Tanke in 2003, Mr. Jun was a sales manager responsible for the sale of feed products at Hunan Haihong Group. From 2002 to 2003, Mr. Jun was a business development officer of technology for Shunde Zhongtian Feed Industrial Co. He has a BA in veterinary from Chongqing Southwest University.
Executive Compensation
The following table sets forth all cash compensation paid by Tanke, for the fiscal years of 2009 and 2010. The table below sets forth the positions and compensations for each officer and director of Tanke. All the officers were paid in RMB and the amounts reported in this table have been converted from Renminbi to U.S. dollars based on the December 30, 2010 conversion rate of RMB 6.6000 to $1.00, as certified for customs purposes by the Federal Reserve Bank of New York.
75
|
SUMMARY COMPENSATION TABLE
|
|||||||||
|
Name and Principal
Position
|
Fiscal Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Change in
Pension Value
and
Nonqualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
|
Guixiong Qiu, Founder, CEO and Chairman of the Board of Directors (1)
|
2010
|
31,384.61
|
38,461.53
|
69,846.14
|
|||||
|
2009
|
28,787.88
|
1,188.96
|
29,976.84
|
||||||
|
Geoff Williams, President, CEO and Director (2)
|
2010
|
||||||||
|
2009
|
|||||||||
|
Xugang Shu,
Vice President of Research and Development (1)
|
2010
|
20,307.69
|
15,384.61
|
35,692.30
|
|||||
|
2009
|
10,060.44
|
3,634.55
|
1,188.96
|
14,927.51
|
|||||
|
Bo Jun, Marketing Director (1)
|
2010
|
30,769.23
|
30,769.23
|
||||||
|
2009
|
22,071.67
|
1,188.96
|
23,260,63
|
||||||
(1) The compensation data for Guiziong Qiu, Xugang Shu and Bo Jun prior to the Share Exchange reflects compensation paid by Tanke.
(2) Prior to the Share Exchange, Geoff Williams served as a principal executive officer and director of Greyhound. Since its inception, Greyhound had not paid its officers any salary or consulting fees.
Employment Agreements
Since our inception and prior to the closing of the Share Exchange, we did not pay any salary or consulting fees to our officers. We have signed standard employment agreements in compliance with PRC labor laws and regulations with our current directors and senior executives.
Option/SAR Grants in Last Fiscal Year
We did not grant any stock options to our executive officers or directors from inception through the date of this Current Report on Form 8-K.
76
Director Compensation
We do not pay our directors any fees or other compensation for acting as directors. We have not paid any fees or other compensation to any of our directors for acting as directors to date.
Certain Relationships and Related Transactions
Loans to Affiliates
On December 24, 2010, in satisfaction of the amounts due from related parties, Mr. Guixiong Qiu, Mr. Bi Gao, and Ms. Xiuzhen Liang executed a Promissory Note with Tanke in amount of $3,443,198.67. The loan is unsecured, bears interest at a rate of 4% per annum and will mature on June 30, 2012, at which point the outstanding amount, plus accrued but unpaid interest, is due.
Reorganization-Related Transactions
The disclosures set forth in Item 1.01 of this Current Report on Form 8-K, under the heading “Securities Escrow Agreement”, and in Item 2.01 of this Current Report on Form 8-K, under the heading “Completion of Acquisition of Assets”, are incorporated herein by reference.
Other
Other than employment and the foregoing arrangements, none of the following persons has any direct or indirect material interest in any transaction to which we are a party since our incorporation or in any proposed transaction to which we are proposed to be a party: (i) any of the Company’s directors or officers; (ii) any person who beneficially owns, directly or indirectly, shares carrying more than 10% of the voting rights attached to our Common Stock; or (iii) any relative or spouse of any of the foregoing persons, or any relative of such spouse, who has the same house as such person or who is a director or officer of any parent or subsidiary of the Company.
Director Independence
As part of our obligations under the Securities Purchase Agreement in connection with the Private Placement, we are required to appoint a Board of Directors consisting of a majority of “independent” directors (as defined under the Nasdaq Marketplace Rules) and one director designated by the Lead Placement Agent, with at least two of such directors being fluent in English within six months of the closing of the Private Placement. Upon such appointment, we will seek to form audit and other board committees in a manner consistent with Nasdaq listed companies.
77
Legal Proceedings
We have no material proceedings pending nor are we aware of any pending investigation or threatened litigation by any third party.
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
We are a reporting company under the Exchange Act, and our public filings can be accessed at www.sec.gov. Effective February 10, 2011, our Common Stock is listed for quotation on the Over-The-Counter Bulletin Board under the trading symbol “TNBI” (formerly “GHND”). There has been limited trading in our shares since they became eligible for trading on the OTCBB during the third quarter of 2009.
The following table sets forth the reported high and low bid prices on TNBI (formerly GHND) for the periods indicated as reported by the OTCBB during and after the third quarter of 2009 (N/A indicates no trading during such period):
|
TNBI
(formerly GHND)
|
||||||||
|
High
|
Low
|
|||||||
|
Fiscal Year 2009
|
||||||||
|
Third Quarter
|
N/A | N/A | ||||||
|
Fourth Quarter
|
N/A | N/A | ||||||
|
Fiscal Year 2010
|
||||||||
|
First Quarter
|
N/A | N/A | ||||||
|
Second Quarter
|
N/A | N/A | ||||||
|
Third Quarter
|
$ | 0.10 | $ | 0.10 | ||||
|
Fourth Quarter
|
N/A | N/A | ||||||
|
Fiscal Year 2011
|
||||||||
|
First Quarter (through February 9, 2011)
|
N/A | N/A | ||||||
As of February 9, 2011, we had 57 holders of record of our Common Stock.
Since inception we have not paid any dividends on our Common Stock. We currently do not anticipate paying any cash dividends in the foreseeable future on our Common Stock. Although we intend to retain our earnings, if any, to finance the exploration and growth of our business, our Board of Directors will have the discretion to declare and pay dividends in the future. Payment of dividends in the future will depend upon our earnings, capital requirements, and other factors, which our Board of Directors may deem relevant.
Recent Sales of Unregistered Securities
The disclosure set forth in Item 3.02 of this Current Report on Form 8-K is incorporated herein by reference.
78
Description of Registrant’s Securities to Be Registered
The Common Stock was registered under the Exchange Act pursuant to a Form 10 (File Number 000-53529) that was filed on December 16, 2008.
As of the date of this Current Report on Form 8-K, the Company is authorized to issue 50,000,000 shares of Common Stock. Prior to the closing of the Share Exchange, 3,397,787 shares of Common Stock were issued and outstanding. In connection with the closing of the Share Exchange, the Company completed a 1 for 8.512 reverse stock split.
Common Stock. Our Common Stock is entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required by law, the holders of our Common Stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our Common Stock that are present in person or represented by proxy. Holders representing 50 percent (50%) of our Common Stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation. Our Articles of Incorporation do not provide for cumulative voting in the election of directors. Holders of our Common Stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our Common Stock.
The disclosure set forth in Item 3.02 of this Current Report on Form 8-K is incorporated herein by reference.
Indemnification of Directors and Officers
The current bylaws of the Company provides that the Board of Directors shall cause the Company to indemnify a current or former director, officer and Secretary of the Company, or a current or former director, officer and Company of a corporation of which the Company is or was a stockholder and the heirs and personal representative of any such person against all costs, charges and expenses to settle an action, judgment or proceeding to which they are made a party by reason of their position of director or officer of the Company.
The Company is permitted by the Bylaws to purchase and maintain insurance for any director, officer, employee or agent of the Company or as a director, officer, employee or agent of the Company of which the Company is or was a stockholder and his or her heirs or personal representatives against a liability incurred by him as a Director, officer, employee or agent.
79
Our company is incorporated under the laws of the State of Nevada. Section 78.7502 of the Nevada Revised Statutes (the “NRS”) provides that a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendere or its equivalent, does not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and that, with respect to any criminal action or proceeding, the person had reasonable cause to believe that his conduct was unlawful.
Section 78.7502 of the NRS further provides a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys' fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
Section 78.751 of the NRS provides that any discretionary indemnification under Section 78.7502 of the NRS, unless ordered by a court or advanced pursuant to subsection 2 of Section 78.751 of the NRS, ay be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made by:
|
|
·
|
By the stockholders;
|
|
|
·
|
By the board of directors by majority vote of a quorum consisting of directors - who were not parties to the action, suit or proceeding;
|
|
|
·
|
If a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion; or
|
|
|
·
|
If a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion.
|
80
The Company’s Articles of Incorporation and Bylaws or an agreement made by the Company may provide that the expenses of officers and directors incurred in defending a civil or criminal action, suit or proceeding must be paid by the corporation as they are incurred and in advance of the final disposition of the action, suit or proceeding, upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that the person is not entitled to be indemnified by the corporation. The provisions of this subsection do not affect any rights to advancement of expenses to which corporate personnel other than directors or officers may be entitled under any contract or otherwise by law.
The indemnification and advancement of expenses authorized in or ordered by a court pursuant to Section 78.751 of the NRS:
|
|
·
|
does not exclude any other rights to which a person seeking indemnification or advancement of expenses may be entitled under the articles of incorporation or any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, for either an action in his official capacity or an action in another capacity while holding his office, except that indemnification, unless ordered by a court pursuant to section 78.7502 of the NRS or for the advancement of expenses made pursuant to subsection 2 of section 78.751 of the NRS, may not be made to or on behalf of any director or officer if a final adjudication establishes that his acts or omissions involved intentional misconduct, fraud or a knowing violation of the law and was material to the cause of action; and
|
|
|
·
|
continues for a person who has ceased to be a director, officer, employee or agent and inures to the benefit of the heirs, executors and administrators of such a person.
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of our Company under Nevada law or otherwise, we have been advised the opinion of the SEC is that such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event a claim for indemnification against such liabilities (other than payment by us for expenses incurred or paid by a director, officer or controlling person of our company in successful defense of any action, suit, or proceeding) is asserted by a director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction, the question of whether such indemnification by it is against public policy in the Securities Act and will be governed by the final adjudication of such issue.
Financial Statements and Supplementary Data
See Item 9.01 of this Current Report on Form 8-K for the financial statements required hereunder.
81
Changes in and Disagreements With Accountants on Accounting and Financial Disclosures
None.
Financial Statements and Exhibits
The exhibits are listed and described in Item 9.01 of this Current Report on Form 8-K
82
Item 3.02 Unregistered Sales of Equity Securities
The disclosures set forth in Item 1.01 of this Current Report on Form 8-K, under the headings “Notes”, “Warrants” and “Agent Warrants”, and in Item 2.01 of this Current Report on Form 8-K, under the heading “Completion of Acquisition of Assets”, are incorporated herein by reference.
For their services as placement agents in the Private Placement, the Company paid cash fees of $ 767,007.15 in the aggregate (not including expenses) to the Lead Placement Agent. The Company also agreed to indemnify the Lead Placement Agent and the Co-Placement Agent against certain liabilities, including liabilities under the Securities Act. The Agent Warrants have registration rights identical to the registration rights afforded to the Investors of the Notes and Warrants, as well as weighted average anti-dilution and other customary provisions for warrants of such type.
For a period of eighteen (18) months from the date of the closing of the Private Placement, if the Company consummates any public or private equity or equity-linked financing transactions with, or enters into a definitive agreement to effect, any public or private equity or equity-linked financing transactions with any Investor or Investors introduced to the Company by the Lead Placement Agent in connection with the Private Placement, then, concurrently with the closing of such financing, the Lead Placement Agent shall receive (i) a cash fee equal to 10.0% of the gross proceeds received from such Investor or Investors and (ii) the Company shall issue the Lead Placement Agent warrants to purchase a number of shares of Common Stock derived by dividing (a) an amount equal to 10.0% of the gross proceeds received by the Company from such Investor or Investors by (b) the price per share or unit of the securities sold to such Investor or Investors, unless such payment is precluded by law, rule, regulation or regulatory authority.
Additionally, for a period of eighteen (18) months from the date of the closing of the Private Placement, if the Company proposes to effect any public or private placement of equity-linked securities (a “Subsequent Offering”), the Company has agreed to offer to engage the Lead Placement Agent as the Company’s exclusive lead underwriter or placement agent in connection with such Subsequent Offering; provided that the Lead Placement Agent may decline in writing such engagement in its sole and absolute discretion at such time. If the Lead Placement Agent agrees to serve as lead underwriter or placement agent in connection with a Subsequent Offering, the Lead Placement Agent shall receive (i) a cash fee equal to 10.0% of the gross proceeds received by the Company in such Subsequent Offering and (ii) the Company shall issue the Lead Placement Agent warrants to purchase a number of shares of Common Stock derived by dividing (a) an amount equal to 10.0% of the gross proceeds received by the Company in the Subsequent Offering by (b) the price per share or unit of the securities sold in the Subsequent Offering, unless such payment is precluded by law, rule, regulation or regulatory authority. If the Lead Placement Agent declines to serve as lead underwriter or placement agent in connection with a Subsequent Offering, the Lead Placement Agent shall nevertheless receive (i) a cash fee equal to 5.0% of the gross proceeds received by the Company in such Subsequent Offering and (ii) the Company shall issue the Lead Placement Agent warrants to purchase a number of shares of Common Stock derived by dividing (a) an amount equal to 5.0% of the gross proceeds received by the Company in the Subsequent Offering by (b) the price per share or unit of the securities sold in the Subsequent Offering, unless such payment is precluded by law, rule, regulation or regulatory authority.
83
Item 5.01 Changes in Control of Registrant
Prior to the closing of the Share Exchange, Geoff Williams was the Company’s President, CEO and Director, Nancy Ah Chong was the Company’s Secretary, Treasurer and Director, and H. Deworth Williams was the Company’s majority stockholder with 88.3% of the issued and outstanding Common Stock immediately prior to the closing of the Share Exchange.
As explained more fully in Item 2.01 of this Current Report on Form 8-K, upon the closing of the Share Exchange, pursuant to the Share Exchange Agreement, the Company issued 10,758,000 shares of Common Stock, or 80.74% of the issued and outstanding shares of Common Stock following the Share Exchange (without giving effect to any conversion of Notes or exercise of Warrants or Agent Warrants), to Golden Genesis, in exchange for 100% of the stock of China Flying held by Golden Genesis. Wong Kwai Ho (“Ms. Wong”), a Hong Kong resident, owns 100% of the issued and outstanding shares of Golden Genesis. Under the Call Option Agreement, the Tanke Shareholders hold irrevocable and unconditional options to acquire up to 100% of the Common Stock owned by Golden Genesis for consideration of $0.01 per share.
Following the closing of the Private Placement, Golden Genesis owns 10,011,469 shares, or 75.14%, of the issued and outstanding Common Stock, without taking into account any conversion of Notes or exercise of Warrants or Agent Warrants. As a result of the reverse stock split made in connection with the closing of the Share Exchange, the Company’s former majority stockholder, H. Deworth Williams now owns 2.56% of the Common Stock.
Pursuant to the Share Exchange Agreement, effective February 9, 2011, Geoff Williams resigned as our President and Chief Executive Officer and Nancy Ah Chong resigned as our Secretary and Treasurer, concurrently with the closing of the Share Exchange. In connection with the resignations of Geoff Williams and Nancy Ah Chong from such positions, Guixiong Qiu was appointed as the Company’s Chief Executive Officer. On or about February 13, 2011, effective as of the tenth (10th) day following the later of the date of the filing of the Information Statement and the date of mailing of the Information Statement to our stockholders, Geoff Williams and Nancy Ah Chong will resign as our directors and Mr. Qiu will be appointed as the sole director of the Company. In addition, Xugang Shu was appointed as the Company’s Vice President of Research and Development and Bo Jun was appointed as the Company’s Marketing Director.
The disclosure set forth in Item 2.01 of this Current Report on Form 8-K is incorporated herein by reference.
84
Item 5.02 Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers
The disclosures set forth in Item 2.01 of this Current Report on Form 8-K, under the headings “Directors and Executive Officers” and “Certain Relationships and Related Transactions”, are incorporated herein by reference.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
On February 8, 2011, in connection with the closing of the Share Exchange, the Company filed a Certificate of Amendment to its Articles of Incorporation to reflect the one share for 8.512 shares reverse split of the Common Stock, effective as of February 10, 2011. The Company also filed a Certificate of Amendment to its Articles of Incorporation to change its corporate name from “Greyhound Commissary, Inc.” to “Tanke Biosciences Corporation”, effective as of February 10, 2011.
Item 5.06 Change in Shell Company Status
As explained more fully in Item 2.01 of this Current Report on Form 8-K, the Company was a “shell company” (as such term defined in Rule 12b-2 under the Exchange Act) immediately before the closing of the Share Exchange. As a result of the Share Exchange, China Flying became wholly owned subsidiary and Tanke became the main operational business of the Company.
Item 9.01 Financial Statements and Exhibits
(a) Financial Statements of Business Acquired
The audited consolidated financial statements of Tanke as of and for the years ended December 31, 2009 and 2008 and the unaudited financial statements as of September 30, 2010 and for the nine months ended September 30, 2010 and 2009 and related footnotes are attached hereto as Exhibit 99.2 and are incorporated herein by reference.
The following is an index to such financial statements:
Exhibit 99.2 - Audited financial statements of Tanke as of and for the fiscal years ended December 31, 2009 and 2008 and the unaudited financial statements as of September 30, 2010 and for the nine months ended September 30, 2010 and 2009
|
Report of Independent Registered Public Accounting Firm
|
2
|
|
Consolidated Balance Sheets
|
3
|
|
Consolidated Statements of Income and Comprehensive Income
|
4
|
|
Consolidated Statements of Shareholders’ Equity
|
5
|
|
Consolidated Statements of Cash Flows
|
6
|
|
Notes to the Consolidated Financial Statements
|
7
|
85
(c) Shell Company Transactions – Pro Forma Financial Information
The financial statements and the pro forma financial information required by Item 9.01(c) to Form 8-K are filed with this Current Report on Form 8-K as Exhibit 99.3.
(d) Exhibits
|
Exhibit No.
|
Description
|
|
|
2.1
|
Share Exchange Agreement, dated January 3, 2011, by and among Greyhound Commissary, Inc. (now known as Tanke Biosciences Corporation) (the “Company”), Golden Genesis Limited (“Golden Genesis”) and China Flying Development Limited (“China Flying”) (1)
|
|
|
3.1
|
Articles of Incorporation of the Company (2)
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation for reverse stock split *
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation for change of corporate name *
|
|
|
3.4
|
Bylaws of the Company (3)
|
|
|
4.1
|
Form of Note issued to the investors (the “Investors”) in the private placement of 6,669,627 units (the “Private Placement”), dated February 9, 2011 *
|
|
|
4.2
|
Form of Warrant issued to the Investors in the Private Placement, dated February 9, 2011 *
|
|
|
4.3
|
Form of Agent Warrant issued to certain affiliates of Euro Pacific Capital, Inc., dated February 9, 2011 *
|
|
|
10.1
|
Securities Purchase Agreement, dated February 9, 2011, by and among the Company, the Investors and, with respect to certain sections thereof, Euro Pacific Capital, Inc. and Newbridge Securities Corporation *
|
|
|
10.2
|
Registration Rights Agreement, dated February 9, 2011, by and among the Company and the Investors *
|
|
|
10.3
|
Securities Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, Golden Genesis and Escrow, LLC, as escrow agent *
|
|
|
10.4
|
Interest Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, and Escrow, LLC, as escrow agent *
|
|
|
10.5
|
Consulting Services Agreement, dated January 3, 2011, between Guangzhou Tanke Industry Co., Ltd. (“Tanke”) and Guangzhou Kanghui Agricultural Technology Co., Ltd. (the “WFOE”) *
|
|
|
10.6
|
Operating Agreement, dated January 3, 2011, by and among Guixiong Qiu, Bi Gao, Xiuzhen Liang and Bing Teng (the “Tanke Shareholders”), Tanke and the WFOE *
|
|
|
10.7
|
Voting Rights Proxy Agreement, dated January 3, 2011, by and among Tanke, the Tanke Shareholders and the WFOE *
|
|
|
10.8
|
Equity Pledge Agreement, dated January 3, 2011, by and among Tanke, the Tanke Shareholders and the WFOE *
|
|
|
10.9
|
Option Agreement, dated January 3, 2011, by and among Tanke, the Tanke Shareholders and the WFOE *
|
|
|
10.10
|
Call Option Agreement, dated January 3, 2011, between Golden Genesis, Wong Kwai Ho and the Tanke Shareholders *
|
86
|
99.1
|
Press Release, dated February 10, 2011, announcing the closing of the Share Exchange and the Private Placement *
|
|
|
99.2
|
Audited financial statements of Tanke as of and for the fiscal years ended December 31, 2009 and 2008 and the unaudited financial statements as of September 30, 2010 and the nine months ended September 30, 2010 and 2009 *
|
|
|
99.3
|
Pro forma financial information *
|
|
*
|
Filed herewith
|
|
(1)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011.
|
|
(2)
|
Incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form 10 filed with the SEC on December 16, 2008.
|
|
(3)
|
Incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement Form 10 filed with the SEC on December 16, 2008.
|
87
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
TANKE BIOSCIENCES CORPORATION.
|
||||
|
Date: February 10, 2011
|
By:
|
/s/ Guixiong Qiu | ||
|
Name:
|
Guixiong Qiu
|
|||
|
Title:
|
Chief Executive Officer
|
|||
