Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF INDEPENDENT CERTIFIED AUDITOR - China BCT Pharmacy Group, Inc. | fs1a9ex23i_chinabct.htm |
| EX-10.64 - ENTRUSTMENT AGREEMENT - China BCT Pharmacy Group, Inc. | fs1a9ex10lxiv_chinabct.htm |
Registration No. 333-165161
SECURITIES AND EXCHANGE COMMISSION
|
AMENDMENT NO. 9 TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
|
CHINA BCT PHARMACY GROUP, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
5912
|
20-8067060
|
||
|
(State or other Jurisdiction of Incorporation)
|
(Primary Standard Classification Code)
|
(IRS Employer Identification No.)
|
No. 102, Chengzhan Road
Liuzhou City, Guangxi Province, P.R.C. 545007
Tel.: (86) 772-363-8318
(Address and Telephone Number of Registrant’s Principal
Executive Offices and Principal Place of Business)
Corporation Service Company
2711 Centerville Road Suite 400
Wilmington, Delaware 19808
(Name, Address and Telephone Number of Agent for Service)
Copies of communications to:
Thomas Wardell, Esq.
McKenna Long & Aldridge LLP
303 Peachtree Street
Atlanta, Georgia 30308
Tel No.: (404) 527-4990
Fax No.: (404) 527-8890
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective. If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. T
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act of 1933, please check the following box and list the Securities Act registration Statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act of 1933, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. £
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer £
|
Non-accelerated filer £
|
Accelerated filer £
|
Smaller reporting company S
|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class Of Securities to be Registered
|
Amount to be Registered
|
Proposed Maximum Aggregate Offering Price per share
|
Proposed Maximum Aggregate Offering Price
|
Amount of Registration Fee
|
||||||||||||
|
Common stock, $0.001 par value per share
|
3,519,340
|
(1)
|
$
|
2.00
|
(2)
|
$
|
7,038,680
|
$
|
501.86
|
|||||||
|
Common stock, $0.001 par value per share, issuable upon exercise of investor warrants
|
1,759,301
|
$
|
3.81
|
(3)
|
$
|
6,702,937
|
$
|
477.92
|
||||||||
|
Common stock, $0.001 par value per share, issuable upon exercise of co-placement agent warrants
|
351,934
|
(4)
|
$
|
3.05
|
(5)
|
$
|
1,073,399
|
$
|
76.53
|
|||||||
|
Total
|
5,630,575
|
(6)
|
$
|
14,815,016
|
$
|
1,056.31
|
(7)
|
|||||||||
(1) Represents the total number of shares of common stock issued to 135 investors in the registrant’s private placement of 893.91 units. Each unit consists of (i) 3,937 shares of common stock, and (ii) a warrant to purchase 1,968 shares of common stock at an exercise price of $3.81 per share.
(2) Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457. Although there is an established public trading market for our common stock on the Over-the-Counter Bulletin Board (the “OTCBB”), the trading has been limited. Accordingly, the proposed maximum offering price is based on the market price of our shares of common stock on March 2, 2010.
(3) Represents the number of shares of common stock issuable upon exercise of the investor warrants at an exercise price of $3.81 per share.
(4) Calculated pursuant to Rule 457(g).
(5) Represents the number of shares of common stock issuable upon exercise of the co-placement agent warrants at an exercise price of $3.05 per share.
(6) In the event that the total number of shares of common stock registered herein (the “Registrable Securities”) exceeds the limitation set forth pursuant to Rule 415, the number of Registrable Securities to be registered herein will be reduced first by the Registrable Securities owned by the co-placement agents and second, on a pro rata basis, among the investors based on the total number of unregistered shares of common stock underlying the investor warrants on a fully-diluted basis.
(7) A fee of $1,191.81 was previously paid with the initial filing.
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SUCH SECTION 8(a), MAY DETERMINE.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission (“SEC”) is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
Subject to completion, dated January 25, 2011

CHINA BCT PHARMACY GROUP, INC.
5,630,575 Shares of Common Stock
This prospectus relates to the resale by the selling stockholders identified in this prospectus of up to 5,630,575 shares (the “Shares”) of our common stock, par value $0.001 per share, including (i) 3,519,340 shares of our common stock currently issued and outstanding, (ii) 1,759,301 shares of common stock issuable upon exercise of warrants issued to our investors (the “Investor Warrants”), and (iii) 351,934 shares of our common stock issuable upon exercise of the warrants issued to our co-placement agents in the private placement (the “Agent Warrants” and, together with the Investors Warrants, the “Warrants”). The Shares were issued to the selling stockholders in private placement transactions which were exempt from the registration and prospectus delivery requirements of the Securities Act of 1933, as amended.
The selling stockholders may offer all or part of their Shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices. We will not receive any of the proceeds from the Shares by the selling stockholders, but we will receive funds from the exercise of the Warrants if and when those Warrants are exercised on a cash exercise basis. However, there is no assurance that such Warrants will be exercised. In addition, we will not receive any additional proceeds to the extent the Warrants are exercised on a cashless exercise basis. We are paying all of the registration expenses incurred in connection with the registration of the Shares, but we will not pay any of the selling commissions, brokerage fees and related expenses.
Our common stock is quoted on the OTCBB under the symbol “CNBI”. On January 21, 2011 , the last reported sale of our common stock quoted on the OTCBB was $ 2.75 per share.
The selling stockholders, and any broker-dealer executing sell orders on behalf of the selling stockholders, may be deemed to be “underwriters” within the meaning of the Securities Act of 1933, as amended. Commissions received by any broker-dealer may be deemed underwriting commissions under the Securities Act of 1933, as amended.
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page 6 to read about factors you should consider before investing in shares of our common stock.
NEITHER THE SECURITIES AND EXCHANGE COMMITTEE NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The Date of This Prospectus is __________, 201 1
TABLE OF CONTENTS
Page No.
|
1
|
|
|
7
|
|
|
21
|
|
|
21
|
|
|
39
|
|
|
56
|
|
|
58
|
|
|
58
|
|
|
64
|
|
|
64
|
|
|
85
|
|
|
87
|
|
|
90
|
|
|
97
|
|
|
98
|
|
|
104
|
|
|
108
|
|
|
112
|
|
|
112
|
|
|
112
|
|
|
113
|
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that you should consider before investing in the common stock. You should carefully read the entire prospectus, including “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements before making an investment decision.
References in this prospectus to the “PRC” or “China” are to the People’s Republic of China. Except as otherwise specifically stated or unless the context otherwise requires, the terms “Company,” “we,” “us” and “our” refer to collectively (i) China BCT Pharmacy Group, Inc. (f/k/a China Baicaotang Medicine Limited), a corporation incorporated in the State of Delaware; (ii) Ingenious Paragon Global Limited (“Ingenious”), a British Virgin Islands company which is a wholly-owned subsidiary; (iii) Forever Well Asia Pacific Limited (“Forever Well”), a Hong Kong company which is a wholly-owned subsidiary of Ingenious; (iv) Guangxi Liuzhou Baicaotang Medicine Limited (“Liuzhou BCT”), a PRC wholly foreign-owned enterprise (“WFOE”) which is a wholly-owned subsidiary of Forever Well; (v) Hefeng Pharmaceutical Co. Limited (“Hefeng Pharmaceutical”), a PRC company which is a wholly-owned subsidiary of Liuzhou BCT; and (vi) Guangxi Liuzhou Baicaotang Medicine Retail Limited (“BCT Retail”), a PRC company of which 49% of its registered capital was contributed by Liuzhou BCT and 51% of its registered capital was contributed by Baicaotang Property Management Limited (“Property Management”), an affiliated company.
References to BCT Retail’s “registered capital” are to the equity of BCT Retail, which under PRC law is measured not in terms of shares owned but in terms of the amount of capital that has been or will be contributed to a company by a particular shareholder or all shareholders. The portion of a limited liability company’s total capital contributed by a particular shareholder represents that shareholder’s ownership of the company and the total amount of capital contributed by all shareholders is the company’s total equity. Capital contributions are made to a company by deposits into a dedicated account in the company’s name, which the company may access in order to meet its financial needs. When a company’s accountant certifies to PRC authorities that a capital contribution has been made and the company has received the necessary government permission to increase its contributed capital, the capital contribution is registered with regulatory authorities and becomes a part of the company’s “registered capital.”
Business Overview
We are engaged in pharmaceutical distribution, retail pharmacy and manufacturing of pharmaceuticals through our three subsidiaries Liuzhou BCT, Hefeng Pharmaceutical, and BCT Retail, each of which is located in Guangxi Province, China.
We have integrated operations in the following business segments:
|
●
|
Pharmaceutical distribution.
|
Pharmaceutical distribution is our principal business. We conduct our wholesale business through Liuzhou BCT by purchasing from pharmaceutical product suppliers and then distributing the products to our wholesale customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals. Our pharmaceutical distribution business is focused on the market of Guangxi province, which includes major cities such as Nanning, Liuzhou and Guilin, and which has approximately 50 million people. We operate a large regional wholesale pharmaceutical networks in Guangxi province supported by strategically placed warehouse facilities. For the year ended December 31, 2009, revenue generated from our pharmaceutical distribution segment was $97.1 million, or 71.4% of our total revenues for the year. For the nine months ended September 30, 2010 our pharmaceutical distribution segment accounted for approximately 70.6% of our total revenue after elimination of inter-segment sales.
1
We distribute over 8,000 products from nearly 4,000 suppliers through our wholesale distribution in compliance with applicable PRC regulations. Hefeng Pharmaceutical, which is one of our wholly owned subsidiaries, is also one of our suppliers. In 2009 revenue derived from the distribution of third-party products constituted 99% of our pharmaceutical distribution segment revenue.
PRC rules and regulations require most public hospitals and healthcare institutions to purchase medicines from pharmaceutical distributors through a centralized tendering process, which includes the implementation of government-mandated price controls. The manufacturers of provincial catalog medicines that are on the hospitals’ formularies are invited to bid and participate in the centralized tendering process, which they must do directly. The bidding process covers multiple categories of medicines used by the hospitals. A duly organized committee of pharmaceutical and clinical medical experts is responsible for bid evaluations. Selection is based on a number of factors, including bid price, quality, clinical effectiveness, and manufacturer’s reputation and service. The supply of a particular type of medicine is generally made on a non-exclusive basis by multiple manufacturers and distributors. We typically advise and assist pharmaceutical manufacturers in the hospital tendering process and distribute products of pharmaceutical manufacturers upon purchase orders being made by the hospitals after the bidding process.
The Guangxi centralized-online tendering system was started in 2006, and in 2009 the tendering started to be applied also under the New Rural Co-operative Health Insurance Plan. At the first tendering in 2009 we were awarded distribution rights for six counties and townships under the New Rural Co-operative Health Insurance Plan, including Liuzhou, Yizhou, Lipu, Gongchen, Luzhai Laibin and Heshan, and were selected as one of two exclusive distributors for these territories.
|
●
|
Retail pharmacy.
|
Established in 2001, BCT Retail operates a large regional pharmaceutical retail network in Guangxi province, consisting of 137 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” Our retail stores provide convenient, high quality and professional pharmaceutical services and supply a wide variety of medicines, including western medicine, traditional Chinese medicine (“TCM”), dried Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and seasonal medicine. Among the 137 stores, there are 26 stores that are medi-care qualified stores, where customers are able to make their purchases either by cash or by using their medi-care insurance card for payment. For the year ended December 31, 2009, revenue generated from our retail pharmacy segment was $31.2 million, or 22.9% of our total revenues for the year. For the nine months ended September 30, 2010 our retail pharmacy segment accounted for approximately 23.9% of our total revenue after elimination of inter-segment sales.
|
●
|
Manufacturing of pharmaceuticals.
|
Hefeng Pharmaceutical has a manufacturing facility on approximately 40,000 square meters of land and manufactures four types of products:
|
●
|
A Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity (Maximum daily unit production: 2.5 tons per day; maximum days of operation per year: 270 days);
|
|
●
|
A granular formulation unit with an annual production capacity of 0.25 billion packages (Maximum daily unit production: 768,960 packages per day; maximum days of operation per year: 324 days);
|
|
●
|
A pill formulation unit with an annual production capacity of 0.36 billion pills (Maximum daily unit production: 1,252,800 pieces per day; maximum days of operation per annum: 288 days), and
|
|
●
|
A liquid formulation unit with an annual production capacity of 0.1 billion injections (Maximum daily unit production : 347,500 pieces per day; maximum days of operation per annum : 288 days).
|
Hefeng Pharmaceutical produces and sells pharmaceutical products under the registered name “Asio (亚太)” including: traditional anti-inflammatory and antibacterial drugs, cancer treatment drugs, cardio-vascular disease drugs and hepatitis drugs. Hefeng Pharmaceutical’s best-selling products include:
2
|
●
|
Tabellae Sarcandrae, a TCM drug that has similar anti-inflammatory and antibacterial effects as anti-biotics in Western medicine;
|
|
●
|
Corydalis Saxicola Bunting (Yanhuanglian), an important component in various hepatitis prescriptions in TCM;
|
|
●
|
Hydroxycamptotbecine Injection, which is used to treat cancers such as carcinoma ventricui, carcinoma hepatitis and colon cancer;
|
|
●
|
Ethacridine Lactate Injection which is used for second trimester pregnancy termination from week 12-26; and
|
|
●
|
Levodopa, a TCM drug that is used to treat stiffness, tremors, spasms and poor muscle control related to Parkinson’s disease.
|
In addition, Hefeng Pharmaceutical collaborates with several renowned medical research universities in China to continuously improve its raw material abstraction efficiency and production process, and to develop alternative formulas for existing drugs. For the year ended December 31, 2009, revenue generated from our manufacturing segment was $7.7 million, or 5.7% of our total revenues for the year. For the nine months ended September 30, 2010, our manufacturing segment accounted for approximately 5.5% of our total revenue after elimination of inter-segment sales.
The growth profile of Guangxi province is based on the following three factors:
|
●
|
According to data published by the National Bureau of Statistics, Guangxi Province’s GDP was RMB770 billion ($112 billion) in 2009. GDP per capita in Guangxi Province was RMB15,821 ($2,316) in 2009 as compared with GDP per capita of RMB42,141 ($6,169) in the coastal regions (including Fujian Province, Guangdong Province, Hainan Province, Jiangsu Province, Shandong Province, Shanghai, and Zhejiang Province). In 2009 Guangxi Province’s GDP growth rate was 13.9% as compared to an average GDP growth rate of 10.7% in the coastal regions in 2009. In 2009 Guangxi Province had a population of 48.16 million. In 2009 the population growth rate of Guangxi Province was 7.2% as compared with a population growth rate of 5.17% in the coastal regions. (See http://www.stats.gov.cn/tjsj/ndsj/2009/indexch.htm).
|
|
●
|
the inflation rate in China in 2010 is projected to be 3.5% to 4% based upon the World Bank “China Quarterly Update” from March 17, 2010.
(See http://siteresources.worldbank.org/CHINAEXTN/ Resources/318949-1268688634523/CQU_march2010.pdf). , and
|
|
●
|
the general pharmaceutical industry growth rate resulting from the RMB850 billion healthcare reform bill passed by the Chinese government.
|
Corporate Structure and History
We were originally incorporated in the State of Delaware on November 30, 2006 under the name Purden Lake Resource Corp. to engage in the acquisition, exploration and development of natural resource properties. Prior to December 23, 2009 we were a blank check company with nominal assets. We changed our name to China Baicaotang Medicine Limited on December 24, 2009 and to China BCT Pharmacy Group, Inc. on March 25, 2010.
3
Corporate Structure
Our wholly-owned subsidiary, Ingenious is a British Virgin Islands corporation that owns 100% of the issued and outstanding capital stock of Forever Well, a Hong Kong company. Forever Well is the sole shareholder of Liuzhou BCT, a PRC wholly foreign-owned enterprise. Liuzhou BCT contributed 100% of the registered capital of Hefeng Pharmaceutical and 49% of the registered capital of BCT Retail. The remaining 51% of the registered capital of BCT Retail was contributed by Property Management, an affiliate of Liuzhou BCT.
We do not have direct 100% ownership interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores. We have entered into contractual arrangements with Property Management pursuant to which we loaned money equal to 51% of the registered capital of BCT Retail to the shareholders of Property Management and such Property Management shareholders pledged their 51% equity interests in BCT Retail. In addition, pursuant to a Proxy Agreement we entered into with Property Management, we effectively control BCT Retail and therefore, have consolidated BCT Retail with China BCT Pharmacy, Group and its subsidiaries.
The chart below illustrates the current structure of the Company:
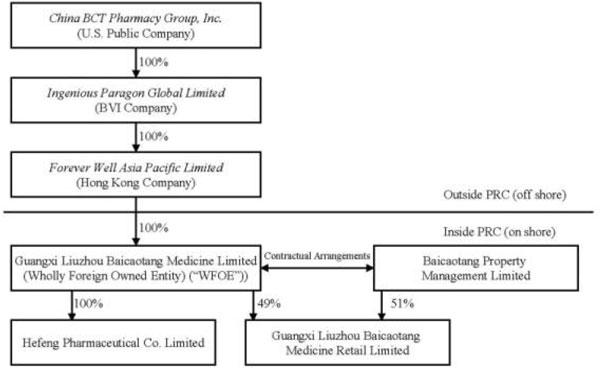
Reorganization
In 2008, the shareholders of Liuzhou BCT (the “Liuzhou BCT Shareholders”) and Xiaoyan Zhang, our CFO, developed a restructuring plan for Liuzhou BCT to obtain the benefits available to a U.S. public company (the “Restructuring”). The first step was for Forever Well to acquire 100% of the equity interests of Liuzhou BCT and its subsidiaries (the “PRC Operating Companies”).
In this step, Forever Well, a Hong Kong company formed by Mr. Ping-Ki Yue, was to acquire the PRC Operating Companies. The second step in the Restructuring was for Ingenious, which was 100% owned by Ms Zhang, to acquire Forever Well and the third step was for Ingenious to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious.
The first step was completed in conjunction with the second step so that as the PRC Companies became subsidiaries of Forever Well, Forever Well was acquired by Ingenious. As part of the second step of the Restructuring, the Liuzhou BCT Shareholders entered into an earn-in agreement (the “Earn-In Agreement”) which provided the Liuzhou BCT Shareholders with a process under which they could purchase for a nominal amount the shares of common stock held by Ms. Zhang.
Under the Earn-In Agreement, Ms. Zhang holds legal title to the shares for the five-year term of the agreement or until the shares have been purchased by the Liuzhou BCT Shareholders. Thereafter, the shares can only be transferred upon the agreement of both Ms. Zhang and the Liuzhou BCT Shareholders. For the term of the Earn-In Agreement or until the shares are purchased by the Liuzhou BCT Shareholders, Ms. Zhang holds all of the governance rights with respect to the shares.
4
After completion of the first and second steps in the Restructuring, the parties concluded that they needed another agreement to complete the Earn-In Agreement and the restructuring process. They therefore entered into the so-called "entrust shareholding" agreements with respect to each of the first and second steps; they made these agreements effective as of the effective date of the first and second steps. These agreements recited that a third-party would maintain the governance rights and economic benefits of the Liuzhou BCT Shareholders during the period of the Earn-In Agreement. The two entrust shareholding agreements were identical except that the third party in the first was Mr. Ping Ki Yue and in the second was Ms. Zhang. The first agreement had been supplanted by the second even at the time it was signed. Under the second agreement, Ms. Zhang, as owner of the shares, had all voting power and, upon notice from her with respect to a matter, the former shareholders might choose to express a preference as to how she would vote the shares; the agreement also confirmed that the former shareholders’ economic interest in the shares was preserved as in the Earn-In Agreement.
These agreements were in fact never utilized and the parties determined that they had been mistaken in their decision to create them and that the Earn-In Agreement expresses entirely the parties’ relationship with respect to the shares and with each other. Representatives of the Liuzhou BCT Shareholders hold the majority of seats on the board of the Company. The Earn-In Agreement reflects the intent and purpose of the parties in undertaking and accomplishing the Restructuring. And following the accomplishment of the third step in the Restructuring, the Earn-In Agreement is the operative agreement for all purposes with respect to the relationship of the Liuzhou BCT Shareholders to the Company. The parties having recognized the mistake, for all of these reasons, the entrust shareholding agreements were rescinded as of their effective dates and the Earn-In Agreement governs the rights of the Liuzhou BCT Shareholders with respect to the shares held by Ms. Zhang.
Under the Earn-in Agreement, Ms. Zhang has legal title to the shares and there are no limits on her voting rights with respect to the shares. Under the Earn-In Agreement, the Liuzhou BCT Shareholders have the right to obtain the economic benefits of the shares by purchasing the shares upon the Company's attaining the low financial thresholds in the agreement which trigger their purchase rights. Ms. Zhang also has no authority to transfer the shares. This can only be done with the agreement of the Liuzhou BCT Shareholders. Therefore, were the Company not to attain the thresholds that have been placed in the Earn-In Agreement, we anticipate that the agreement would be further modified to establish thresholds that were or are attainable.
On December 30, 2009, the goal of the Restructuring was realized when we took the third step and entered into a share exchange agreement with Ingenious, pursuant to which we acquired 100% of the equity of Ingenious in exchange for the issuance of an aggregate of 32,000,000 shares of our common stock to Ms. Zhang and to certain former Liuzhou BCT Shareholders (the “Share Exchange”). As of the date of this prospectus, Ms. Zhang owns 58.9% of our common stock although, as a consequence of the Earn-In Agreement ownership of these shares also resides in the Liuzhou BCT Shareholders. As a result of this transaction, we are a holding company which, through our direct and indirect ownership of Ingenious, Forever Well, Liuzhou BCT, Hefeng Pharmaceutical, and BCT Retail, now has operations based in the PRC.
Private Placement
On October 23, 2009, we entered into a subscription agreement (the “Subscription Agreement”) with certain investors (the “Investors”) for the sale of up to an aggregate of 1,147 units (the “Units”) in a private placement (the “Private Placement”). Simultaneously with the closing of the Share Exchange, we completed the initial closing of the Private Placement of approximately $6.3 million or 632.3 Units (the “Initial Closing”). Upon the Initial Closing, we issued an aggregate of 2,489,370 shares of our common stock and Investor Warrants exercisable for up to 1,244,368 shares of our common stock at an exercise price of $3.81 per share. In addition, in connection with the Initial Closing of the Private Placement, we issued Agent Warrants to the placement agents (the “Co-Placement Agents”) that are exercisable for 248,937 shares of our common stock at an exercise price of $3.05 per share. The funds were held with Signature Bank, acting as escrow agent, and were released to us upon the consummation of the Initial Closing. The closing of the Share Exchange was a condition precedent to the closing of the Private Placement.
On February 1, 2010, we completed the second closing of the Private Placement of approximately $2.6 million (the “Second Closing”) of 261.61 Units, consisting of an aggregate of 1,029,970 shares of our common stock and Investor Warrants exercisable for 514,933 shares of our common stock at an exercise price of $3.81. In connection with the Second Closing, we issued Agent Warrants to the Co-Placement Agents that are exercisable for 102,997 shares of our common stock at an exercise price of $3.05 per share on a cash or cashless basis. The funds were held with Signature Bank, acting as escrow agent, and were released to us upon the consummation of the Second Closing.
We entered into a placement agency agreement (the “Placement Agent Agreement”) with the Co-Placement Agents on October 21, 2009 whereby the Co-Placement Agents received as compensation for acting as placement agent in the Private Placement (i) a total cash fee and a non-accountable marketing allowance in the amount of approximately $0.86 million; and (ii) Agent Warrants to purchase up to 302,521 shares of common stock. Pursuant to participating agent agreements by and among Charles Vista, LLC, May Davis and American Capital, Charles Vista, LLC received as compensation for acting as a sub-agent in the Private Placement (i) a cash fee in the amount of approximately $0.22 million; and (ii) Agent Warrants to purchase up to 49,413 shares of common stock at an exercise price of $3.65 per share. The Co-Placement Agents were responsible for raising the minimum offering amount of $5,820,000 of Units and were compensated as set forth above.
Agreement to Sell Preferred Shares
On January 18, 2011, the Company entered into an agreement (the “Preferred Purchase Agreement”) with Milestone Longcheng Limited (“Milestone”) pursuant to which Milestone will purchase 9,375,000 shares of the Company’s Series A Convertible Preferred Shares, par value $.001 per share (the “Preferred Shares”), for an aggregate purchase price of $30 million. The Preferred Shares carry a dividend of 5% and are convertible initially into an equal number of shares of our Common Stock at an initial conversion price of $3.20 per share. The transaction is subject to a number of closing conditions, including the completion of the amendment of our Certificate of Incorporation to increase our authorized capital to 170 million shares of capital stock consisting of 150 million shares of Common Stock (an increase of 50 million shares) and 20 million shares of blank-check preferred stock which our board of directors will have the authority to issue (the “Amendment”) and the filing of the Amendment and of the Certificate of Designation adopting the terms of the Preferred Shares. All necessary board and stockholder action to approve adoption of the Amendment have been taken and the Amendment will be complete upon filing with the Delaware Secretary of State which will occur 20 days after the mailing to our stockholders of an information statement pursuant to Section 14(c) of the Exchange Act. We filed this information statement with the SEC on January 18, 2011 and will mail it at the earliest date available in accordance with provisions of Section 14(c) and the regulations thereunder. The board has also approved the Certificate of Designation, subject to the filing of the Amendment. The Certificate of Designation will be filed after the filing of the Amendment in connection with the closing of the sale of the Preferred Shares.
5
In connection with this transaction, the Company and Milestone also entered into a registration rights agreement and, with certain of the Company’s principal stockholders, a shareholders agreement. The registration rights agreement provides for registration under the Securities Act of 1933, under various circumstances, of the shares of Common Stock into which the Preferred Shares can be converted. Under the Preferred Purchase Agreement, Milestone (or successor holders of the Preferred Shares) will have the right to name one director and to recommend an additional independent director to the Company’s board of directors, which we expect will be increased to seven directors after closing of the transaction. The Purchase Agreement also provides that Milestone (or successor holders) will have consent rights with respect to certain operating matters. The shareholders agreement provides limitations on the rights of the principal shareholders to engage in competing businesses in the event that they are no longer employed with the Company and also provides a process for their sale of their shares, including first offering them to the holders of the Preferred Shares and co-sale rights of the holders. Under the Certificate of Designation, the holders of Preferred Shares have pre-emptive rights with respect to future offerings and have voting rights with the Common Stock as well as separate voting rights with respect to certain extraordinary transactions. After issuance, the Preferred Shares will represent 18.5% of the outstanding share capital of the Company. These shares, together with the shares of Common Stock owned by Ms. Zhang (representing approximately 44.3% of the outstanding share capital after this transaction) will hold the majority voting interest in the Company. The principal agreements with respect to this transaction have been filed as exhibits to our report on Form 8-K describing the transaction and filed with the SEC on January 18, 2011. We anticipate that the transaction will be closed approximately 30 days after signing or as soon there after as we and Milestone determine.
Where You Can Find Us
Our principal executive office is located at No. 102 Chengzhan Road, Liuzhou City, Guangxi province, China and our telephone number is (86) 772-363-8318. Our corporate website is www.china-bct.com. Information contained on, or accessed through our website is not intended to constitute and shall not be deemed to constitute part of this prospectus.
The Offering
|
Common stock offered by selling stockholders
|
5,630,575 shares of common stock. This includes (i) 3,519,340 shares of our issued and outstanding common stock; (ii) 1,759,301 shares of common stock issuable upon exercise of outstanding Investor Warrants; and (iii) 351,934 shares of common stock issuable upon exercise of outstanding Agent Warrants.
|
|
Common stock outstanding before the offering
|
38,154,340 shares of common stock as of December 29 , 2010.
|
|
Common stock outstanding after the offering
|
40,230,575 shares.
|
|
(assuming full exercise of all of the Warrants)
|
|
|
Terms of the Offering
|
The selling stockholders will determine when and how they will sell the common stock offered in this prospectus.
|
|
Termination of the Offering
|
The offering will conclude upon the earliest of (i) such time as all of the common stock has been sold pursuant to the registration statement or (ii) such time as all of the common stock becomes eligible for resale without volume limitations pursuant to Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), or any other rule of similar effect.
|
|
Use of proceeds
|
We are not selling any shares of the common stock covered by this prospectus, and, as a result, will not receive any proceeds from this offering. We may, however, receive proceeds in the event that some or all of the Warrants held by the selling stockholders are exercised for cash. However, there is no assurance that such Warrants will be exercised. In addition, we will not receive any additional proceeds to the extent the Warrants are exercised on a cashless exercise basis. To the extent that the selling stockholders exercise in cash all of the Warrants, we would receive approximately $7,776,336 in the aggregate from such exercise. The proceeds from the cash exercise of such Warrants, if any, will be used by us for working capital and other general corporate purposes.
|
|
OTCBB Trading Symbol
|
CNBI.OB
|
|
Risk Factors
|
The common stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors” beginning on page 6.
|
6
Risks Relating to Our Business
OUR OPERATING HISTORY MAY NOT SERVE AS AN ADEQUATE BASIS TO JUDGE OUR FUTURE PROSPECTS AND RESULTS OF OPERATIONS.
Our operating history may not provide a meaningful basis on which to evaluate our business. We cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in significant operating losses.
WE MAY BE UNABLE TO COMPETE SUCCESSFULLY AGAINST NEW AND EXISTING COMPETITORS.
The major market for our products and business operations is the cities, villages and towns of Guangxi province. We may face increasing competition in the future as we expand our business in Guangxi province and our adjacent provinces. As we expand our operations in wholesale and retail distribution and manufacture of pharmaceutical products, we will encounter competition from other companies existing in our target markets, such as Sinopharma and Liuzhou Medical and Pharmaceutical Limited in the pharmaceutical distribution area, Shenzhen Accordance Pharmacy Chain Store and Hunan Laobaixing Pharmacy Chain in the retail area and Harbin Pharmaceutical Group, Guangdong Boluo Xianfeng Pharmaceutical Group and Jiangsu Chia Tai Tianqing Pharmaceutical and Sixth Pharma Factory in the manufacturing area, and may face future competition from new foreign and domestic competitors entering the pharmaceutical promotion and distribution market in China. Our current and future competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional and distribution activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. Competition could reduce our market share or force us to lower our prices to unprofitable levels.
OUR CURRENT BUSINESS OPERATIONS RELY HEAVILY UPON MR. HUI TIAN TANG, OUR CHIEF EXECUTIVE OFFICER AND CHAIRMAN, AND ADDING OTHER KEY EMPLOYEES ARE ESSENTIAL TO GROWING OUR BUSINESS.
We have been heavily dependent upon the expertise and management of Mr. Hui Tian Tang, our Chairman and Chief Executive Officer, and his continued services. The loss of Mr. Tang’s services could seriously interrupt our business operations. Although we have entered into an employment contract with Mr. Tang, pursuant to which Mr. Tang agrees to serve as our full time Chief Executive Officer, and Mr. Tang has not indicated any intention of leaving us, the loss of his service for any reason could have a very negative impact on our ability to fulfill our business plan. In addition, we face competition for attracting skilled personnel. If we fail to attract and retain qualified personnel to meet current and future needs, this could slow our ability to grow our business, which could result in a decrease in market share.
THE LIMITED PUBLIC COMPANY EXPERIENCE OF OUR MANAGEMENT TEAM COULD ADVERSELY IMPACT OUR ABILITY TO COMPLY WITH THE REPORTING REQUIREMENTS OF U.S. SECURITIES LAWS.
Our management team has limited public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. Our senior management has never had sole responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately respond to such increased legal, regulatory compliance and reporting requirements, including the establishing and maintaining internal controls over financial reporting. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our ability to comply with the reporting requirements of the Securities Exchange Act of 1934 (the “Exchange Act”), which is necessary to maintain our public company status. If we were to fail to fulfill those obligations, our ability to continue as a U.S. public company would be in jeopardy in which event you could lose your entire investment in our company.
7
IF WE NEED ADDITIONAL CAPITAL TO FUND OUR GROWING OPERATIONS, WE MAY NOT BE ABLE TO OBTAIN SUFFICIENT CAPITAL AND MAY BE FORCED TO LIMIT THE SCOPE OF OUR OPERATIONS.
In connection with our growth strategies, we may experience increased capital needs and accordingly, we may not have sufficient capital to fund our future operations without additional capital investments. Our capital needs will depend on numerous factors, including (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment in research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we cannot obtain additional funding, we may be required to: (i) limit our investments in research and development; (ii) limit our marketing efforts; and (iii) decrease or eliminate capital expenditures. Such reductions could materially adversely affect our business and our ability to compete.
Even if we do find a source of additional capital, we may not be able to negotiate terms and conditions for receiving the additional capital that are acceptable to us. Any future capital investments could dilute or otherwise materially and adversely affect the holdings or rights of our existing shareholders. In addition, new equity or convertible debt securities issued by us to obtain financing could have rights, preferences and privileges senior to our common stock. We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us.
IF WE FAIL TO INCREASE OUR BRAND RECOGNITION, WE MAY FACE DIFFICULTY IN OBTAINING NEW CUSTOMERS AND BUSINESS PARTNERS.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and our ability to provide reliable and useful services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition would be materially adversely affected.
AS A DISTRIBUTOR AND MANUFACTURER OF PHARMACEUTICAL PRODUCTS, WE ARE EXPOSED TO INHERENT RISKS RELATING TO PRODUCT LIABILITY AND PERSONAL INJURY CLAIMS.
Pharmacies are exposed to risks inherent in the manufacturing and distribution of pharmaceutical and other healthcare products, such as with respect to improper filling of prescriptions, labeling of prescriptions, adequacy of warnings, unintentional distribution of counterfeit drugs. In addition, product liability claims may be asserted against us with respect to any of the products we sell and as a retailer, we are required to pay for damages for any successful product liability claim against us, although we may have the right under applicable PRC laws, rules and regulations to recover from the relevant manufacturer for compensation we paid to our customers in connection with a product liability claim. We may also be obligated to recall affected products. If we are found liable for product liability claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation as well as our brand name may also suffer. We, like many other similar companies in China, do not carry product liability insurance. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance due to the limited coverage of any business interruption insurance in China, and as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
8
THE RETAIL PRICES OF SOME OF OUR PRODUCTS ARE SUBJECT TO CONTROL, INCLUDING PERIODIC DOWNWARD ADJUSTMENT, BY PRC GOVERNMENTAL AUTHORITIES.
A number of our pharmaceutical products, primarily those included in the national and provincial medical insurance catalogs, are subject to price controls in the form of fixed retail prices or retail price ceilings. Approximately 60% to 70% of our total retail sales are subject to these price controls. In addition, the retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Since May 1998, the relevant PRC governmental authorities have ordered price reductions of thousands of pharmaceutical products. The latest price reduction occurred in October 2008 and affected 1,357 different pharmaceutical products. Any future price controls or government mandated price reductions may have a material adverse affect on our financial condition and results of operations, including significantly reducing our revenue and profitability.
THE AVERAGE NUMBER OF DAYS DURING WHICH OUR ACCOUNTS RECEIVABLE ARE OUTSTANDING HAS INCREASED WHICH MAY NEGATIVELY AFFECT OUR BALANCE SHEET AND OUR ABILITY TO COLLECT OUR RECEIVABLES AND FUND OUR OPERATIONS AND.
Across all of our pharmaceutical, distributing and manufacturing segments the numbers of days during which our receivables are outstanding has increased significantly in 2009 and in 2010. In the pharmaceutical distribution segment we are obtaining a greater percentage of revenue from hospitals and community health centers who traditionally have a slower payment cycle. In our manufacturing segment, we have also experienced an increase in the days during which our receivables are outstanding, because we have extended more generous credit terms to certain of our customers based upon their history with us and in view of the overall financial crisis. If this slower time to collection should result in an inability to collect a greater percentage of our receivables than previously, the result would be a decrease in the cash available to fund our operations as well as an increase in reserves for receivables which would reduce their value on our balance sheet.
WE MAY BE SUBJECT TO FINES AND PENALTIES IF WE FAIL TO COMPLY WITH THE APPLICABLE PRC LAWS AND REGULATIONS GOVERNING SALES OF MEDICINES UNDER THE PRC NATIONAL MEDICAL INSURANCE PROGRAM.
Eligible participants in the PRC national medical insurance program, mainly consisting of urban residents in China, are entitled to buy medicines using their medical insurance cards in an authorized pharmacy, provided that the medicines they purchase have been included in the national or provincial medical insurance catalogs. The pharmacy, in turn, obtains reimbursement from the relevant government social security bureaus. Moreover, the applicable PRC laws, rules and regulations prohibit pharmacies from selling goods other than pre-approved medicines when purchases are made with medical insurance cards, as well as providing cash to the customer for the medical insurance card. We have established procedures to prohibit our drugstores from selling unauthorized goods to customers who make purchases with medical insurance cards. However, we cannot assure you that those procedures will be strictly followed by all of our employees in all of our stores. If we fail to observe the above laws, rules and regulations with respect to purchases made with medical insurance cards, we may be fined and our qualification for selling medicines included in the national or provincial medical insurance catalogs may be withdrawn by competent authorities.
OUR RETAIL OPERATIONS REQUIRE A NUMBER OF PERMITS AND LICENSES IN ORDER TO CARRY ON THEIR BUSINESS.
Drugstores in China are required to obtain certain permits and licenses from various PRC governmental authorities, including GSP certification. We are also required to obtain food hygiene certificates for the distribution of nutritional supplements and food products other than medicine. We cannot assure you that we can maintain all required licenses, permits and certifications to carry on our business at all times, and from time to time we may have not been in compliance with all such required licenses, permits and certifications. Moreover, these licenses, permits and certifications are subject to periodic renewal and/or reassessment by the relevant PRC governmental authorities and the standards of such renewal or reassessment may change from time to time. We intend to apply for the renewal of these licenses, permits and certifications when required by then applicable laws and regulations. Any failure by us to obtain and maintain all licenses, permits and certifications necessary to carry on our business at any time could have a material adverse effect on our business, financial condition and results of operations. In addition, any inability to renew these licenses, permits and certifications could severely disrupt our business, and prevent us from continuing to carry on our business. Any changes in the standards used by governmental authorities in considering whether to renew or reassess our business licenses, permits and certifications, as well as any enactment of new regulations that may restrict the conduct of our business, may also decrease our revenue and/or increase our costs and materially reduce our profitability and prospects. Furthermore, if the interpretation or implementation of existing laws and regulations changes or if new regulations come into effect requiring us to obtain any additional licenses, permits or certifications that were previously not required to operate our existing businesses, we cannot assure you that we may successfully obtain such licenses, permits or certifications.
9
WE MAY HAVE DIFFICULTY DEFENDING OUR INTELLECTUAL PROPERTY RIGHTS FROM INFRINGEMENT RESULTING IN LAWSUITS REQUIRING US TO DEVOTE FINANCIAL AND MANAGEMENT RESOURCES THAT WOULD HAVE A NEGATIVE IMPACT ON OUR OPERATING RESULTS.
We regard our trademarks and other similar intellectual properties as critical to our success. We rely on trademark and other similar intellectual properties, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights. We have received trademark registration for certain of our products in the PRC. No assurance can be given that our licenses will not be challenged, invalidated, infringed or circumvented, or that our intellectual property rights will provide competitive advantages to us. There can be no assurance that we will be able to obtain a license from a third-party technology that we may need to conduct our business or that such technology can be licensed at a reasonable cost.
Presently, we sell our products mainly in PRC. To date, no trademark or patent filings have been made other than in PRC. To the extent that we market our products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate and we cannot give you any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our own or copy our products.
WE MAY BE ADVERSELY AFFECTED BY COMPLEXITY, UNCERTAINTIES AND CHANGES IN CHINESE REGULATION OF DRUGSTORES AND THE PRACTICE OF MEDICINE.
The Chinese government regulates drugstores, including foreign ownership, and the licensing and permit requirements. These laws and regulations are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be a violation of applicable laws and regulations. Issues, risks and uncertainties relating to Chinese government regulation of the industry include the following:
|
●
|
We only have 49% ownership interest in BCT Retail. We are not able to own 100% interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores and foreign ownership of medical practices. If the Chinese government challenges our control of BCT Retail through contractual relationships, our business could be harmed; and
|
|
●
|
uncertainties relating to the regulation of drugstores, including evolving licensing practices, means that permits, licenses or operations at our company may be subject to challenge. This may disrupt our business, or subject us to sanctions, requirements to increase capital or other conditions or enforcement, or compromise enforceability of related contractual arrangements, which could materially and adversely affect our business, financial condition and results of operations.
|
THERE IS NO ASSURANCE THAT WE WILL PAY DIVIDENDS TO SHAREHOLDERS IN THE FUTURE.
Although Liuzhou BCT, our wholly owned subsidiary, declared and paid to its then existing shareholders cash dividends in the amount of $6,940,000 and $2,044,056 for the years ended December 31, 2008 and 2007, respectively, our board of directors does not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon, among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and, if dividends are paid, there is no assurance with respect to the amount of any such dividend.
10
WE MAY INCUR SIGNIFICANT COSTS TO ENSURE COMPLIANCE WITH UNITED STATES CORPORATE GOVERNANCE AND ACCOUNTING REQUIREMENTS.
We may incur significant costs associated with our public company reporting requirements, costs associated with newly applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these newly applicable rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Risks Related to Our Corporate Structure
WE CONTROL BCT RETAIL THROUGH A SERIES OF CONTRACTUAL ARRANGEMENTS, WHICH MAY NOT BE AS EFFECTIVE IN PROVIDING CONTROL OVER THE ENTITY AS DIRECT OWNERSHIP AND MAY BE DIFFICULT TO ENFORCE.
We operate our retail pharmacy business in the PRC through BCT Retail which holds the licenses, approvals and assets necessary to operate our retail pharmacy business in the PRC. We have a 49% minority equity ownership interest in BCT Retail and rely on contractual arrangements with Property management that allow us to substantially control and operate BCT Retail. These contractual arrangements may not be as effective as direct ownership in providing control over BCT Retail because BCT Retail or its shareholders could breach the arrangements.
Our contractual arrangements with BCT Retail are governed by PRC law and provide for the resolution of disputes through arbitration in the PRC. Accordingly, these contracts would be interpreted in accordance with PRC law and any disputes would be resolved in accordance with PRC legal procedures. If BCT Retail or its shareholders fail to perform their respective obligations under these contractual arrangements, we may have to
|
●
|
incur substantial costs to enforce such arrangements, and
|
|
●
|
rely on legal remedies under PRC law, including seeking specific performance or injunctive relief, and claiming damages.
|
The legal environment in the PRC is not as developed as in the United States and uncertainties in the Chinese legal system could limit our ability to enforce these contractual arrangements. In the event that we are unable to enforce these contractual arrangements, our business, financial condition and results of operations could be materially and adversely affected.
IF THE PRC GOVERNMENT DETERMINES THAT THE CONTRACTUAL ARRANGEMENTS THROUGH WHICH WE CONTROL BCT RETAIL DO NOT COMPLY WITH APPLICABLE REGULATIONS, OUR BUSINESS COULD BE ADVERSELY AFFECTED.
Although we believe our contractual relationships through which we control BCT Retail comply with current licensing, registration and regulatory requirements of the PRC, we cannot assure you that the PRC government would agree, or that new and burdensome regulations will not be adopted in the future. If the PRC government determines that our structure or operating arrangements do not comply with applicable law, it could revoke our business and operating licenses, require us to discontinue or restrict our operations, restrict our right to collect revenues, require us to restructure our operations, impose additional conditions or requirements with which we may not be able to comply, impose restrictions on our business operations or on our customers, or take other regulatory or enforcement actions against us that could be harmful to our business.
11
Risks Relating to the People’s Republic of China
THE SALES PRICES OF SOME MEDICINES ARE CURRENTLY CONTROLLED BY THE CHINESE GOVERNMENT AND THAT MAY ADVERSELY AFFECT OUR BUSINESS.
Prices paid by end consumers for many of our medicines are regulated by PRC’s State Development and Reform Commission. PRC justifies its need to control the drug prices on the basis that, at present, only employees at state or private companies have health insurance. About 900 million rural Chinese people and 35 million urban unemployed Chinese people lack insurance coverage and cannot afford expensive drugs. Our future profitability might suffer if a significant portion of our revenues were to be derived from products whose final selling prices were state-controlled and if those prices were held at levels close to or below our cost of sales.
SALES OF OUR PRODUCTS COULD BE HARMED BY THE WIDESPREAD PRESENCE OF COUNTERFEIT MEDICATION IN PRC NEGATIVELY IMPACTING OUR PROFITABILITY.
Chinese counterfeiting of pharmaceuticals and other products affecting public health has grown in tandem with counterfeiting and piracy of goods such as brand-name clothing, compact discs and computer software. This situation negatively affects China Baicaotang and other major domestic and foreign drug manufacturers in PRC, especially for products marketed through the over the counter rather than hospital channel. With the expansion of our business and increased recognition of our brand name, such risks may increase. Currently, active pharmaceutical ingredients are governed only by chemical regulations. Our ability to increase sales as rapidly as we would like, and our profitability, could be affected if this problem persists or worsens.
SUBSTANTIALLY ALL OF OUR OPERATING ASSETS ARE LOCATED IN CHINA AND SUBSTANTIALLY ALL OF OUR REVENUE WILL BE DERIVED FROM OUR OPERATIONS IN CHINA SO OUR BUSINESS, RESULTS OF OPERATIONS AND PROSPECTS ARE SUBJECT TO THE ECONOMIC, POLITICAL AND LEGAL POLICIES, DEVELOPMENTS AND CONDITIONS IN CHINA.
The PRC’s economic, political and social conditions, as well as government policies, could impair our business. The PRC economy differs from the economies of most developed countries in many respects. China’s GDP has grown consistently since 1978 (National Bureau of Statistics of China). However, we cannot assure you that such growth will be sustained in the future. If, in the future, China’s economy experiences a downturn or grows at a slower rate than expected, there may be less demand for spending in certain industries. A decrease in demand for spending in certain industries could impair our ability to remain profitable. The PRC’s economic growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall PRC economy, but may have a negative effect on us. For example, our financial condition and results of operations may be hindered by PRC government control over capital investments or changes in tax regulations.
The PRC economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the PRC government has implemented measures emphasizing the use of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in China is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. It also exercises significant control over PRC economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
12
IF THE MINISTRY OF COMMERCE, OR MOFCOM, CHINA SECURITIES REGULATORY COMMISSION, OR CSRC, OR ANOTHER PRC REGULATORY AGENCY, DETERMINES THAT MOFCOM AND CSRC APPROVAL OF OUR MERGER WAS REQUIRED OR IF OTHER REGULATORY OBLIGATIONS ARE IMPOSED UPON US, WE MAY INCUR SANCTIONS, PENALTIES OR ADDITIONAL COSTS WHICH WOULD DAMAGE OUR BUSINESS.
On August 8, 2006, six PRC regulatory agencies, including the MOFCOM and the CSRC, promulgated the Rules on Acquisition of Domestic Enterprises by Foreign Investors, or the M&A Rules, a new regulation with respect to the mergers and acquisitions of domestic enterprises by foreign investors that became effective on September 8, 2006. Article 11 of the New M&A Rules requires when a domestic company, enterprise or natural person uses an offshore company legally established or controlled by the domestic company, enterprise or natural person to engage in the merger and acquisition of a related domestic company, the application must be submitted to MOFCOM for approval. Article 40 of the New M&A Rules requires that an offshore special purpose vehicle formed for overseas listing purposes and controlled directly or indirectly by PRC companies or individuals should obtain the approval of the CSRC prior to the listing and trading of such offshore special purpose vehicle’s securities on an overseas stock exchange, especially in the event that the offshore special purpose vehicle acquires shares of or equity interests in the PRC companies in exchange for the shares of offshore companies. Article 39 of the New M&A Rules defines an offshore special purpose vehicle as an offshore company directly or indirectly controlled by a PRC domestic company or natural person for the purpose of the offshore listing of their equity interests in the domestic company. On September 21, 2006, the CSRC published on its official website procedures and filing requirements for offshore special purpose vehicles seeking CSRC approval of their overseas listings.
We believe, based on the opinion of our PRC legal counsel, Broad & Bright Law Firm, that MOFCOM and CSRC approvals were not required for our merger transaction or for the listing and trading of our securities on a trading market because we are not an offshore special purpose vehicle that is directly or indirectly controlled by PRC companies or individuals. Although the merger and acquisition regulations provide specific requirements and procedures, there are still many ambiguities in the meaning of many provisions. Further regulations are anticipated in the future, but until there has been clarification either by pronouncements, regulation or practice, there is some uncertainty in the scope of the regulations and the regulators have wide latitude in the enforcement of the regulations and approval of transactions. If the MOFCOM, CSRC or another PRC regulatory agency subsequently determines that the MOFCOM and CSRC approvals were required, we may face sanctions by the MOFCOM, CSRC or another PRC regulatory agency. If this happens, these regulatory agencies may impose fines and penalties on our operations in China, limit our operating privileges in China, delay or restrict the repatriation of the net proceeds (after the payment of fees and expenses in connection with the Private Placement) received by us from the Private Placement into China, restrict or prohibit payment or remittance of dividends paid by Liuzhou BCT, or take other actions that could damage our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our securities.
THE NEW M&A REGULATIONS ESTABLISH MORE COMPLEX PROCEDURES FOR SOME ACQUISITIONS OF CHINESE COMPANIES BY FOREIGN INVESTORS, WHICH COULD MAKE IT MORE DIFFICULT FOR US TO PURSUE GROWTH THROUGH ACQUISITION IN CHINA.
The New M&A Rules establish additional procedures and requirements that could make some acquisitions of PRC companies by foreign investors, such as ours, more time-consuming and complex, including requirements in some instances that the approval of the Ministry of Commerce shall be required for transactions involving the shares of an offshore listed company being used as the acquisition consideration by foreign investors. In the future, we may grow our business in part by acquiring complementary businesses. Complying with the requirements of the New M&A Rules to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
13
IF THE PRC IMPOSES RESTRICTIONS DESIGNED TO REDUCE INFLATION, FUTURE ECONOMIC GROWTH IN THE PRC COULD BE SEVERELY CURTAILED WHICH COULD HURT OUR BUSINESS AND PROFITABILITY.
While the economy of the PRC has experienced rapid growth, this growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth often can lead to growth in the supply of money and rising inflation. In order to control inflation in the past, the PRC has imposed controls on bank credits, limits on loans for fixed assets and restrictions on state bank lending. Imposition of similar restrictions may lead to a slowing of economic growth, a decrease in demand for our products and generally damage our business and profitability.
FLUCTUATIONS IN EXCHANGE RATES COULD HARM OUR BUSINESS AND THE VALUE OF OUR SECURITIES.
The value of our ordinary shares will be indirectly affected by the foreign exchange rate between U.S. dollars and RMB and between those currencies and other currencies in which our sales may be denominated. Because substantially all of our earnings and cash assets are denominated in RMB and the net proceeds (after the payment of fees and expenses in connection with the Private Placement) received by us from the Private Placement will be denominated and our financial results are reported in U.S. dollars, fluctuations in the exchange rate between the U.S. dollar and the RMB will affect the relative purchasing power of these proceeds, our balance sheet and our earnings per share in U.S. dollars following this offering. In addition, appreciation or depreciation in the value of the RMB relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business or results of operations. Fluctuations in the exchange rate will also affect the relative value of any dividend we issue that will be exchanged into U.S. dollars as well as earnings from, and the value of, any U.S. dollar-denominated investments we make in the future. Since July 2005, the RMB has not been pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the RMB may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future PRC authorities may lift restrictions on fluctuations in the RMB exchange rate and lessen intervention in the foreign exchange market.
Very limited hedging transactions are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions. While we may enter into hedging transactions in the future, the availability and effectiveness of these transactions may be limited, and we may not be able to successfully hedge our exposure at all. In addition, our foreign currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert RMB into foreign currencies.
EXCHANGE CONTROLS THAT EXIST IN THE PRC MAY LIMIT OUR ABILITY TO UTILIZE OUR CASH FLOW EFFECTIVELY.
We are subject to the PRC’s rules and regulations on currency conversion. In the PRC, the State Administration for Foreign Exchange, or SAFE, regulates the conversion between Renminbi and foreign currencies. Currently, foreign investment enterprises, or FIEs, are required to apply to the SAFE for “Foreign Exchange Registration Certificates for FIEs.” As a result of our ownership of Liuzhou BCT, Liuzhou BCT is a FIE. With such registration certificates, which need to be renewed annually, FIEs are allowed to open foreign currency accounts including a “current account” and “capital account.” Currency conversion within the scope of the “current account,” such as remittance of foreign currencies for payment of dividends, can be effected without requiring the approval of the SAFE. However, conversion of currency in the “capital account,” including capital items such as direct foreign investment, loans and securities, still require approval of the SAFE. Further, any capital contributions to Liuzhou BCT by its offshore shareholder must be approved by the Ministry of Commerce in China or its local counterpart. We cannot assure you that the PRC regulatory authorities will not impose further restrictions on the convertibility of the Renminbi. Any future restrictions on currency exchanges may limit our ability to use our cash flow for the distribution of dividends to our shareholders or to fund operations it may have outside of the PRC.
14
In August 2008, SAFE promulgated Circular 142, a notice regulating the conversion by FIEs of foreign currency into Renminbi by restricting how the converted Renminbi may be used. Circular 142 requires that Renminbi converted from the foreign currency-dominated capital of a FIE may only be used for purposes within the business scope approved by the applicable government authority and may not be used for equity investments within the PRC unless specifically provided for otherwise. In addition, SAFE strengthened its oversight over the flow and use of Renminbi funds converted from the foreign currency-dominated capital of a FIE. The use of such Renminbi may not be changed without approval from SAFE, and may not be used to repay Renminbi loans if the proceeds of such loans have not yet been used. Violations of Circular 142 may result in severe penalties, including substantial fines as set forth in the SAFE rules.
PRC REGULATIONS RELATING TO THE ESTABLISHMENT OF OFFSHORE SPECIAL PURPOSE COMPANIES BY PRC RESIDENTS MAY SUBJECT OUR PRC RESIDENT SHAREHOLDERS TO PERSONAL LIABILITY AND LIMIT OUR ABILITY TO INJECT CAPITAL INTO OUR PRC SUBSIDIARIES, LIMIT OUR PRC SUBSIDIARIES’ ABILITY TO DISTRIBUTE PROFITS TO US, OR OTHERWISE ADVERSELY AFFECT US.
SAFE issued a public notice in October 2005, or the SAFE notice, requiring PRC residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of PRC companies, referred to in the notice as an “offshore special purpose company.” PRC residents that are shareholders of offshore special purpose companies established before November 1, 2005 were required to register with the local SAFE branch before March 31, 2006.The failure of our beneficial owners to timely amend their SAFE registrations pursuant to the SAFE notice or the failure of future beneficial owners of our company who are PRC residents to comply with the registration procedures set forth in the SAFE notice may subject such beneficial owners to fines and legal sanctions and may also limit our ability to contribute additional capital into our PRC subsidiaries, limit our PRC subsidiaries’ ability to distribute dividends to our company or otherwise adversely affect our business.
BECAUSE CHINESE LAW GOVERNS MANY OF OUR MATERIAL AGREEMENTS, WE MAY NOT BE ABLE TO ENFORCE OUR RIGHTS WITHIN THE PRC OR ELSEWHERE, WHICH COULD RESULT IN A SIGNIFICANT LOSS OF BUSINESS, BUSINESS OPPORTUNITIES OR CAPITAL.
Chinese law governs many of our material agreements, some of which may be with Chinese governmental agencies. We cannot assure you that we will be able to enforce any of our material agreements or that remedies will be available outside of the PRC. The system of laws and the enforcement of existing laws and contracts in the PRC may not be as certain in implementation and interpretation as in the United States. The Chinese judiciary is relatively inexperienced in enforcing corporate and commercial law, leading to a higher than usual degree of uncertainty as to the outcome of any litigation. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital.
BECAUSE OUR FUNDS ARE HELD IN BANKS IN UNINSURED PRC BANK ACCOUNTS, THE FAILURE OF ANY BANK IN WHICH WE DEPOSIT OUR FUNDS COULD AFFECT OUR ABILITY TO CONTINUE IN BUSINESS.
Funds on deposit at banks and other financial institutions in the PRC are often uninsured. A significant portion of our assets are in the form of cash deposited with banks in the PRC, and in the event of a bank failure, we may not have access to our funds on deposit. Depending upon the amount of money we maintain in a bank that fails, our inability to have access to our cash could impair our operations, and, if we are not able to access funds to pay our suppliers, employees and other creditors, we may be unable to continue in business.
15
OUR BUSINESS COULD BE SEVERELY HARMED IF THE CHINESE GOVERNMENT CHANGES ITS POLICIES, LAWS, REGULATIONS, TAX STRUCTURE OR ITS CURRENT INTERPRETATIONS OF ITS LAWS, RULES AND REGULATIONS RELATING TO OUR OPERATIONS IN CHINA.
Our business is located in Guangxi province, China and virtually all of our assets are located in China. We generate our sales revenue only from customers located in China. Our results of operations, financial state of affairs and future growth are, to a significant degree, subject to China’s economic, political and legal development and related uncertainties. Our operations and results could be materially affected by a number of factors, including, but not limited to
|
●
|
Changes in policies by the Chinese government resulting in changes in laws or regulations or the interpretation of laws or regulations,
|
|
●
|
changes in taxation,
|
|
●
|
changes in employment restrictions,
|
|
●
|
import duties, and
|
|
●
|
currency revaluation.
|
Over the past several years, the Chinese government has pursued economic reform policies including the encouragement of private economic activities and greater economic decentralization. If the Chinese government does not continue to pursue its present policies that encourage foreign investment and operations in China, or if these policies are either not successful or are significantly altered, then our business could be harmed. Following the Chinese government’s policy of privatizing many state-owned enterprises, the Chinese government has attempted to augment its revenues through increased tax collection. It also exercises significant control over China’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Continued efforts to increase tax revenues could result in increased taxation expenses being incurred by us. Economic development may be limited as well by the imposition of austerity measures intended to reduce inflation, the inadequate development of infrastructure and the potential unavailability of adequate power and water supplies, transportation and communications. In addition, the Chinese government continues to play a significant role in regulating industry by imposing industrial policies.
THE CHINESE LAWS AND REGULATIONS WHICH GOVERN OUR CURRENT BUSINESS OPERATIONS ARE SOMETIMES VAGUE AND UNCERTAIN AND MAY BE CHANGED IN A WAY THAT HURTS OUR BUSINESS.
China’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents, unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of Chinese laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are considered an FIE under Chinese laws, and as a result, we must comply with Chinese laws and regulations. We cannot predict what effect the interpretation of existing or new Chinese laws or regulations may have on our business. If the relevant authorities find us to be in violation of Chinese laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation: levying fines; revoking our business and other licenses; requiring that we restructure our ownership or operations; and requiring that we discontinue any portion or all of our business.
16
A SLOWDOWN OR OTHER ADVERSE DEVELOPMENTS IN THE CHINESE ECONOMY MAY MATERIALLY AND ADVERSELY AFFECT OUR CUSTOMERS’ DEMAND FOR OUR SERVICES AND OUR BUSINESS.
All of our operations are conducted in China and all of our revenues are generated from sales to businesses operating in China. Although the Chinese economy has grown significantly in recent years, such growth may not continue. We do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the Chinese economy which may affect demand for our products. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in China may materially reduce the demand for our services and in turn reduce our results of operations.
FAILURE TO COMPLY WITH THE U.S. FOREIGN CORRUPT PRACTICES ACT AND CHINESE ANTI-CORRUPTION LAWS COULD SUBJECT US TO PENALTIES AND OTHER ADVERSE CONSEQUENCES.
Our executive officers, employees and other agents may violate applicable law in connection with the marketing or sale of our products, including China’s anti-corruption laws and the U.S. Foreign Corrupt Practices Act, or the FCPA, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, we are required to maintain records that accurately and fairly represent our transactions and have an adequate system of internal accounting controls. Foreign companies, including some that may compete with us, are not subject to these prohibitions, and therefore may have a competitive advantage over us. The PRC also strictly prohibits bribery of government officials. However, corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in the PRC.
While we intend to implement measures to ensure compliance with the FCPA and Chinese anti-corruption laws by all individuals involved with our company, our employees or other agents may engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations. In addition, our brand and reputation, our sales activities or the price of our ordinary shares could be adversely affected if we become the target of any negative publicity as a result of actions taken by our employees or other agents.
THE IMPLEMENTATION OF THE NEW PRC EMPLOYMENT CONTRACT LAW AND INCREASES IN THE LABOR COSTS IN CHINA MAY HURT OUR BUSINESS AND PROFITABILITY.
A new employment contract law became effective on January 1, 2008 in China. It imposes more stringent requirements on employers in relation to entry into fixed-term employment contracts, recruitment of temporary employees and dismissal of employees. In addition, under the newly promulgated Regulations on Paid Annual Leave for Employees, which also became effective on January 1, 2008, employees who have worked continuously for more than one year are entitled to paid vacation ranging from 5 to 15 days, depending on the length of the employee’s service. Employees who waive such vacation entitlements at the request of the employer will be compensated for three times their normal daily salaries for each vacation day so waived. As a result of the new law and regulations, our labor costs may increase. There is no assurance that disputes, work stoppages or strikes will not arise in the future. Increases in the labor costs or future disputes with our employees could damage our business, financial condition or operating results.
UNDER THE PRC EIT LAW, WE, INGENIOUS AND/OR FOREVER WELL MAY BE CLASSIFIED AS A “RESIDENT ENTERPRISE” OF THE PRC. SUCH CLASSIFICATION COULD RESULT IN TAX CONSEQUENCES TO US, OUR NON-PRC RESIDENT SHAREHOLDERS, INGENIOUS AND FOREVER WELL.
On March 16, 2007, the National People’s Congress approved and promulgated a new tax law, the PRC Enterprise Income Tax Law, or “EIT Law,” which took effect on January 1, 2008. Under the EIT Law, enterprises are classified as resident enterprises and non-resident enterprises. An enterprise established outside of China with “de facto management bodies” within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define “de facto management bodies” as a managing body that in practice exercises “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise; however, it remains unclear whether the PRC tax authorities would deem our managing body as being located within China. Due to the short history of the EIT Law and lack of applicable legal precedents, the PRC tax authorities determine the PRC tax resident treatment of a foreign company on a case-by-case basis.
17
If the PRC tax authorities determine that we, Ingenious and/or Forever Well are a “resident enterprise” for PRC enterprise income tax purposes, a number of PRC tax consequences could follow. First, we, Ingenious and/or Forever Well could be subject to the enterprise income tax at a rate of 25 percent on our, Ingenious’ and/or Forever Well’s worldwide taxable income, as well as PRC enterprise income tax reporting obligations. Second, under the EIT Law and its implementing rules, dividends paid between “qualified resident enterprises” are exempt from enterprise income tax. As a result, if we, Ingenious and Forever Well are treated as PRC “qualified resident enterprises,” all dividends paid from Liuzhou BCT to us (through Forever Well and Ingenious) should be exempt from PRC tax.
Finally, the new “resident enterprise” classification could result in a situation in which a 10 percent PRC tax is imposed on dividends we pay to our non-PRC stockholders that are not PRC tax “resident enterprises” and gains derived by them from transferring our common stock, if such income is considered PRC-sourced income by the relevant PRC authorities. In such event, we may be required to withhold a 10 percent PRC tax on any dividends paid to non-PRC resident stockholders. Our non-PRC resident stockholders also may be responsible for paying PRC tax at a rate of 10 percent on any gain realized from the sale or transfer of our common stock in certain circumstances. We would not, however, have an obligation to withhold PRC tax with respect to such gain.
Moreover, the State Administration of Taxation (“SAT”) released Circular Guoshuihan No. 698 (“Circular 698”) on December 15, 2009 that reinforces the taxation of non-listed equity transfers by non-resident enterprises through overseas holding vehicles. Circular 698 addresses indirect share transfers as well as other issues. Circular 698 is retroactively effective from January 1 2008. According to Circular 698, where a foreign (non-PRC resident) investor who indirectly holds shares in a PRC resident enterprise through a non-PRC offshore holding company indirectly transfers equity interests in a PRC resident enterprise by selling the shares of the offshore holding company, and the latter is located in a country or jurisdiction where the effective tax burden is less than 12.5 percent or where the offshore income of his, her, or its residents is not taxable, the foreign investor is required to provide the PRC tax authority in charge of that PRC resident enterprise with certain relevant information within 30 days of the transfer. The tax authorities in charge will evaluate the offshore transaction for tax purposes. In the event that the tax authorities determine that such transfer is abusing forms of business organization and a reasonable commercial purpose for the offshore holding company other than the avoidance of PRC income tax liability is lacking, the PRC tax authorities will have the power to re-assess the nature of the equity transfer under the doctrine of substance over form. A reasonable commercial purpose may be established when the overall international (including U.S.) offshore structure is set up to comply with the requirements of supervising authorities of international (including U.S.) capital markets. If the SAT’s challenge of a transfer is successful, it may deny the existence of the offshore holding company that is used for tax planning purposes and subject the seller to PRC tax on the capital gain from such transfer. Since Circular 698 has a short history, there is uncertainty as to its application. We (or a foreign investor) may become at risk of being taxed under Circular 698 and may be required to expend valuable resources to comply with Circular 698 or to establish that we (or such foreign investor) should not be taxed under Circular 698, which could have a material adverse effect on our financial condition and results of operations (or such foreign investor’s investment in us).
If any such PRC tax applies, a non-PRC resident investor may be entitled to a reduced rate of PRC tax under an applicable income tax treaty and/or a deduction against such investor’s domestic taxable income or a foreign tax credit against such investor’s domestic income tax liability (subject to applicable conditions and limitations). In the case of a U.S. Holder (as defined in the section of this prospectus captioned “Material United States Federal Income Tax Considerations—General”), if a PRC tax applies to dividends paid on our common stock, or to gain from the disposition of our common stock, such tax should be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to applicable conditions and limitations). In addition, the U.S. Holder should be entitled to certain benefits under the Agreement between the Government of the United States of America and the Government of the People’s Republic of China for the Avoidance of Double Taxation and the Prevention of Tax Evasion with Respect to Taxes on Income (the “U.S.-PRC Tax Treaty”), including the treatment of any such income as arising in the PRC for purposes of calculating such foreign tax credit, if such holder is considered a resident of the United States for the purposes of the U.S.-PRC Tax Treaty. Prospective investors should consult with their own tax advisors regarding the applicability of any such taxes, the effects of any applicable income tax treaties, and any available foreign tax credits. For further information, see the discussion in the sections of this prospectus entitled “Material United States Federal Income Tax Considerations” and “Material PRC Income Tax Considerations” below.
18
IT MAY BE DIFFICULT TO AFFECT SERVICE OF PROCESS AND ENFORCEMENT OF LEGAL JUDGMENTS UPON OUR COMPANY AND OUR OFFICERS AND DIRECTORS BECAUSE THEY RESIDE OUTSIDE THE UNITED STATES.
As our operations are presently based in PRC and a majority of our directors and all of our officers reside in PRC, service of process on our company and such directors and officers may be difficult to effect within the United States. Also, our main assets are located in PRC and any judgment obtained in the United States against us may not be enforceable outside the United States.
Risks Associated with this Offering and our Common Stock in General
OUR SHARES OF COMMON STOCK HAVE LITTLE TRADING AND THERE CAN BE NO ASSURANCE THAT THERE WILL BE AN ACTIVE MARKET FOR OUR SHARES OF COMMON STOCK EITHER NOW OR IN THE FUTURE.
Our shares of common stock have little trading, and the price if traded may not reflect our value. There can be no assurance that there will be an active market for our shares of common stock either now or in the future. The market liquidity will be dependent on the perception of our operating business and any steps that our management might take to bring us to the awareness of investors. There can be no assurance given that there will be any awareness generated. Consequently, investors may not be able to liquidate their investment or liquidate it at a price that reflects the value of the business. If a more active market should develop, the price may be highly volatile. Because there may be a low price for our shares of common stock, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in the shares of our common stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of such shares of common stock as collateral for any loans.
WE MAY BE SUBJECT TO THE PENNY STOCK RULES WHICH WILL MAKE THE SHARES OF OUR COMMON STOCK MORE DIFFICULT TO SELL.
We may be subject now and in the future to the SEC’s “penny stock” rules if our shares of common stock sell below $5.00 per share. Penny stocks generally are equity securities with a price of less than $5.00. The penny stock rules require broker-dealers to deliver a standardized risk disclosure document prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer must also provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson, and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information must be given to the customer orally or in writing prior to completing the transaction and must be given to the customer in writing before or with the customer’s confirmation.
In addition, the penny stock rules require that prior to a transaction, the broker dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. The penny stock rules are burdensome and may reduce purchases of any offerings and reduce the trading activity for shares of our common stock. As long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
OUR SHAREHOLDERS WILL EXPERIENCE DILUTION AS A RESULT OF THE CONVERSION OF OUR CLASS A WARRANTS OR ISSUANCE OF SECURITIES IN FUTURE FINANCINGS.
As of the date hereof, we have Investor Warrants and Agent Warrants outstanding which are exercisable for 2,111,235 shares of common stock. To the extent such Warrants are exercised, there will be further dilution. In the event that any future financing should be in the form of securities convertible into, or exchangeable for, equity securities, investors may experience additional dilution upon the conversion or exchange of such securities. In addition, the issuance and sale of the Preferred Shares, when converted, will result in the issuance of an additional 9,375,000 shares of common stock.
19
There are additional authorized but unissued shares of our common stock (and also, after amendment of our certificate of incorporation as described above, additional but unissued shares of our preferred stock) that may be later issued by our management for any purpose without the consent or vote of the stockholders. Our current shareholders may be further diluted in their percentage ownership in the event additional shares are issued by us in the future.
OUR COMMON STOCK IS SUBJECT TO PRICE VOLATILITY UNRELATED TO OUR OPERATIONS.
The market price of our common stock could fluctuate substantially due to a variety of factors, including market perception of our ability to achieve our planned growth, quarterly operating results of other companies in the same industry, trading volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting our competitors or us. In addition, the stock market is subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons unrelated to their operating performance and could have the same effect on our common stock.
WE CANNOT ASSURE YOU THAT OUR COMMON STOCK WILL BECOME LIQUID OR THAT IT WILL BE LSITED ON A SECURITIES EXCHANGE.
Currently, we are quoted on the OTC Bulletin Board, where an investor may find it difficult to obtain accurate quotations as to the market value of our common stock. In addition, if we fail to meet the criteria set forth in SEC regulations, by law, various requirements would be imposed on broker-dealers who sell its securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our common stock, which may further affect its liquidity.
OUR CHIEF FINANCIAL OFFICER OWNS A SUBSTANTIAL PORTION OF OUR OUTSTANDING COMMON STOCK, WHICH WILL ENABLE HER TO INFLUENCE MANY SIGNIFICANT CORPORATE ACTIONS AND IN CERTAIN CIRCUMSTANCES MAY PREVENT A CHANGE IN CONTROL THAT WOULD OTHERWISE BE BENEFICIAL TO OUR SHAREHOLDERS.
As of the date hereof, our Chief Financial Officer, Ms. Zhang, controls approximately 58.9% of our outstanding shares of common stock that are entitled to vote on all corporate actions. After the sale of the Preferred Shares, Ms. Zhang will control approximately 44.3% of the total voting rights of our capital stock and the holders of the Preferred Shares will have approximately 18.5% of the voting rights of our capital stock, so that together these parties will control a majority of the voting rights. This could have a substantial impact on matters requiring the vote of the shareholders, including the election of our directors and most of our corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our shareholders and us. This control could adversely affect the voting and other rights of our other shareholders and could depress the market price of our common stock.
IF WE FAIL TO MAINTAIN AN EFFECTIVE SYSTEM OF INTERNAL CONTROLS, WE MAY NOT BE ABLE TO ACCURATELY REPORT OUR FINANCIAL RESULTS OR PREVENT FRAUD.
Since we operated as a private enterprise without public reporting obligations prior to the Share Exchange, we have committed limited personnel and resources to the development of the external reporting and compliance obligations that would be required of a public company. If our financial reporting systems or procedures fail, we may not be able to provide accurate financial statements on a timely basis or comply with the Sarbanes-Oxley Act of 2002 as it applies to us. Any failure of our ability to provide accurate financial statements could cause the trading price of our common stock to decrease substantially.
20
This prospectus contains forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Business.” These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. These risks and uncertainties include, but are not limited to, the factors described in the section captioned “Risk Factors” above.
In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “would” and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements include, among other things, statements relating to:
|
●
|
our expectations regarding the market for our products and services;
|
|
●
|
our expectations regarding the continued growth of the healthcare industry in PRC;
|
|
●
|
our beliefs regarding the competitiveness of our products;
|
|
●
|
our expectations regarding the expansion of our manufacturing operations;
|
|
●
|
our expectations with respect to increased revenue growth and our ability to achieve profitability resulting from increases in our production volumes;
|
|
●
|
our future business development, results of operations and financial condition; and
|
|
●
|
competition from other companies engaged in manufacturing and distribution of pharmaceutical products.
|
Also, forward-looking statements represent our estimates and assumptions only as of the date of this prospectus. You should read this prospectus and the documents that we reference in this prospectus, or that we filed as exhibits to the registration statement of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
Except as required by law, we assume no obligation to update any forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in any forward-looking statements, even if new information becomes available in the future.
Use of Proceeds
We will not receive any of the proceeds from the sale of our common stock by the selling stockholders. The selling stockholders will receive all of the net proceeds from the sales of common stock offered by them under this prospectus. To the extent that the selling stockholders exercise in cash all of the Warrants covering the 2,111,235 shares of common stock registered for resale under this prospectus, we would receive $7,776,336 in the aggregate from such exercises. However, there is no assurance that such Warrants will be exercised. In addition, we will not receive any additional proceeds to the extent the Warrants are exercised on a cashless exercise basis. We intend to use such proceeds for working capital and other general corporate purposes. We will have complete discretion over how we may use the proceeds, if any, from any cash exercise of the Warrants.
We are registering for resale shares of our common stock that are issued and outstanding held by the selling stockholders identified below. We are registering the shares to permit the selling stockholders and their pledgees, donees, transferees and other successors-in-interest that receive their shares from a selling stockholder as a gift, partnership distribution or other non-sale related transfer after the date of this prospectus to resell the shares when and as they deem appropriate in the manner described in the “Plan of Distribution”. As of the date of this prospectus there are 38,154,340 shares of common stock issued and outstanding.
21
The following table sets forth:
|
●
|
the name of the selling stockholders,
|
|
●
|
the number of shares of our common stock that the selling stockholders beneficially owned prior to the offering for resale of the shares under this prospectus,
|
|
●
|
the maximum number of shares of our common stock that may be offered for resale for the account of the selling stockholders under this prospectus, and
|
|
●
|
the number and percentage of shares of our common stock to be beneficially owned by the selling stockholders after the offering of the shares (assuming all of the offered shares are sold by the selling stockholders).
|
May Davis Partners, LLC (“May Davis”), American Capital Partners, LLC (“American Capital”) and Charles Vista, LLC are broker dealers and received the securities registered herein for their services to us acting as placement agent in our Private Placement.
Except for May Davis, American Capital and Charles Vista, LLC, none of the selling stockholders is a broker dealer or an affiliate of a broker dealer. None of the selling stockholders had any agreement or understanding, directly or indirectly, to distribute any of the shares being registered at the time of purchase.
None of the selling stockholders has been an officer or director of the Company or any of its predecessors or affiliates within the last three years and, except for May Davis, American Capital and Charles Vista, LLC acting in their capacities as our placement agent, no selling stockholder has had a material relationship with the Company.
We entered into a placement agency agreement (the “Placement Agent Agreement”) with May Davis and American Capital “the Co-Placement Agents” on October 21, 2009 whereby May Davis and American Capital received as compensation for acting as placement agent in the Private Placement (i) a total cash fee and a non-accountable marketing allowance in the amount of approximately $0.86 million; and (ii) Agent Warrants to purchase up to 302,521 shares of common stock. Pursuant to participating agent agreements by and among Charles Vista, LLC, May Davis and American Capital, Charles Vista, LLC received as compensation for acting as a sub-agent in the Private Placement (i) a cash fee in the amount of approximately $0.22 million; and (ii) Agent Warrants to purchase up to 49,413 shares of common stock at an exercise price of $3.65 per share. The Co-Placement Agents were responsible for raising the minimum offering amount of $5,820,000 of Units and were compensated as set forth above. The funds in connection with the Private Placement were held with Signature Bank, acting as escrow agent, and were released to us upon the consummation of each closing under the Subscription Agreement.
Each selling stockholder may offer for sale all or part of the shares from time to time. The table below assumes that the selling stockholders will sell all of the shares offered for sale. A selling stockholder is under no obligation, however, to sell any shares pursuant to this prospectus.
22
|
Name
|
Shares of Common Stock Beneficially Owned prior to Offering
(1)
|
Maxim Number of Shares of Common Stock to be Offered
(2)
|
Number of Shares of Shares Common Stock Beneficially Owned after Offering
|
Percent Ownership after Offering
(3)
|
||||||||||||
|
Common Stock and Investor Warrants
|
||||||||||||||||
|
Renald Anelle and Catherine Anelle *(4)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Stratos Antoniadis (5)
|
5,982
|
5,982
|
0
|
0
|
%
|
|||||||||||
|
Steven Benkovsky (6)
|
59,052
|
59,052
|
0
|
0
|
%
|
|||||||||||
|
Warren Boyer (7)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Vincent Caracci and Denise Caracci *(8)
|
14,999
|
14,999
|
0
|
0
|
%
|
|||||||||||
|
Steve Carver (9)
|
14,999
|
14,999
|
0
|
0
|
%
|
|||||||||||
|
Vincent Cicero (10)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Joseph Denora (11)
|
8,859
|
8,859
|
0
|
0
|
%
|
|||||||||||
|
Daniel Faubion (12)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Mark Feingold (13)
|
14,880
|
14,880
|
0
|
0
|
%
|
|||||||||||
|
Vito Gargano and Teresa Gargano *(14)
|
14,999
|
14,999
|
0
|
0
|
%
|
|||||||||||
|
Albert & Heidi Gentile* (15)
|
29,999
|
29,999
|
0
|
0
|
%
|
|||||||||||
|
David Gibbs (16)
|
29,997
|
29,997
|
0
|
0
|
%
|
|||||||||||
|
Viveka Goolcharan (17)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Reshma Goolcharan (18)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Thomas Gormley and Norva Gormley *(19)
|
149,987
|
149,987
|
0
|
0
|
%
|
|||||||||||
|
Gary House (20)
|
56,808
|
56,808
|
0
|
0
|
%
|
|||||||||||
|
Larry Imamshah (21)
|
17,998
|
17,998
|
0
|
0
|
%
|
|||||||||||
|
Larry Juette (22)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Brian Keller and Debbie Keller *(23)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
James Lorenzo and Patricia Lorenzo (24)
|
89,999
|
89,999
|
0
|
0
|
%
|
|||||||||||
|
Frank Mazza (25)
|
449,991
|
449,991
|
0
|
0
|
%
|
|||||||||||
|
Duane Meyer (26)
|
50,432
|
50,432
|
0
|
0
|
%
|
|||||||||||
|
Robert Mezzatesta and Maria Mezzatesta *(27)
|
14,999
|
14,999
|
0
|
0
|
%
|
|||||||||||
|
Jim Moon (28)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Roslyn Parmasad and Vishal Goolcharan *(29)
|
29,997
|
29,997
|
0
|
0
|
%
|
|||||||||||
|
Arlene Phillips (30)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
David Roberts (31)
|
14,763
|
14,763
|
0
|
0
|
%
|
|||||||||||
|
Marc Rotter (32)
|
8,858
|
8,858
|
0
|
0
|
%
|
|||||||||||
|
Bryan Schiff (33)
|
29,527
|
29,527
|
0
|
0
|
%
|
|||||||||||
|
Richard Smee (34)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Aart Snijders (35)
|
17,716
|
17,716
|
0
|
0
|
%
|
|||||||||||
|
Eugene Spiegal and Frances Spiegal *(36)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Alex Stanyek and Carolyn Stanyek *(37)
|
37,497
|
37,497
|
0
|
0
|
%
|
|||||||||||
|
Weijia Su (38)
|
37,497
|
37,497
|
0
|
0
|
%
|
|||||||||||
|
Elliot Tuckel (39)
|
8,858
|
8,858
|
0
|
0
|
%
|
|||||||||||
|
Michael Van den Driessche (40)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Pieter Visser (41)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Steven Wallitt (42)
|
17,717
|
17,717
|
0
|
0
|
%
|
|||||||||||
|
Wade Walter (43)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Kevin Bedassie (44)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Nikita Zdanow (45)
|
17,716
|
17,716
|
0
|
0
|
%
|
|||||||||||
|
David J. Beyer (46)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Lewis, David and Inez (47)
|
88,575
|
88,575
|
0
|
0
|
%
|
|||||||||||
|
Thomas M. DePuy (48)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
Thomas H. Burke (49)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
Steven and Donna Cortese (50)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
23
|
Name
|
Shares of Common Stock Beneficially Owned prior to Offering
(1)
|
Maxim Number of Shares of Common Stock to be Offered
(2)
|
Number of Shares of Shares Common Stock Beneficially Owned after Offering
|
Percent Ownership after Offering
(3)
|
||||||||||||
|
RBC Capital Markets Corp. FBO Bruce R. Shafer IRA (51)
|
11,812
|
11,812
|
0
|
0
|
%
|
|||||||||||
|
Richard W. Lewis (52)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Stephen Bushansky (53)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Dennis Deromedi PSP (54)
|
17,715
|
17,715
|
0
|
0
|
%
|
|||||||||||
|
Neil T. Gutekunst & Teresa A. Gutekunst* (55)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
LJW Limited Partnership (56)
|
159,435
|
159,435
|
0
|
0
|
%
|
|||||||||||
|
Hermes Payne (57)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
|
Wade and Tracy Harris (58)
|
147,625
|
147,625
|
0
|
0
|
%
|
|||||||||||
|
Randy Ackman (59)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Ruthmarie Zimmerman Individual Retirement Account RBC Capital Markets Corp. Cust. (60)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Roy C Neuman (61)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Craig E. Harrison (62)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Andrew & Janet Pace (63)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Donald H. Gregory (64)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
|
Gregory M. Chubon (65)
|
206,675
|
206,675
|
0
|
0
|
%
|
|||||||||||
|
Jeffrey T. Webster (66)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
|
Randall Toig (67)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
RBC Capital Markets Corp FBO Warren Zimmerman IRA (68)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Victor W. and Lynn B. Gumper* (69)
|
14,763
|
14,763
|
0
|
0
|
%
|
|||||||||||
|
Howard Reinsch (70)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Larry V. Coleman (71)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Dean N. Browning (72)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
John M. Gentry (73)
|
17,715
|
17,715
|
0
|
0
|
%
|
|||||||||||
|
George C. Eilers & Polly A. Eilers* (74)
|
17,715
|
17,715
|
0
|
0
|
%
|
|||||||||||
|
Scott Duffney (75)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Tom J. Atkinson (76)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Dale Cripps (77)
|
17,715
|
17,715
|
0
|
0
|
%
|
|||||||||||
|
Harry O. Unger, Jr. (78)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Robert L. Van Horn (79)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Daniel & Deborah Gibson (80)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
|
Stubbs, Steven & Renee (81)
|
88,575
|
88,575
|
0
|
0
|
%
|
|||||||||||
|
John Trone (82)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
Lee Polster & Natasha F. Polster*(83)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Christine D. Whelan (84)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Gregory T. and Anne A. Jones* (85)
|
23,620
|
23,620
|
0
|
0
|
%
|
|||||||||||
|
Linda Mae Alexander (86)
|
17,715
|
17,715
|
0
|
0
|
%
|
|||||||||||
|
Anton Kimball (87)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Kevin Bell (88)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Edward P Aguilar (89)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
John J. Hubbard (90)
|
29,525
|
29,525
|
0
|
0
|
%
|
|||||||||||
|
Lisa Ehlers (91)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
John J. DiLorenzo (92)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Robert L. Oetter (93)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Troy Stubbs (94)
|
159,435
|
159,435
|
0
|
0
|
%
|
|||||||||||
|
Pam & Cliff Halbert (95)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
24
|
Name
|
Shares of Common Stock Beneficially Owned prior to Offering
(1)
|
Maxim Number of Shares of Common Stock to be Offered
(2)
|
Number of Shares of Shares Common Stock Beneficially Owned after Offering
|
Percent Ownership after Offering
(3)
|
||||||||||||
|
Hans Apel (96)
|
82,670
|
82,670
|
0
|
0
|
%
|
|||||||||||
|
Marco A. Aguilar (97)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Daybreak Special Situations Master Fund, Ltd. (98)
|
177,150
|
177,150
|
0
|
0
|
%
|
|||||||||||
|
Paragon Capital, LP (99)
|
118,100
|
118,100
|
0
|
0
|
%
|
|||||||||||
|
Jayhawk Private Equity Fund II, LP (100)
|
885,750
|
885,750
|
0
|
0
|
%
|
|||||||||||
|
Chestnut Ridge Partners, LP (101)
|
295,250
|
295,250
|
0
|
0
|
%
|
|||||||||||
|
Stephen Grant (102)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Arthur Mitchell (103)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Paul Sipple & Joan F. Rae* (104)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Akita Capital LLC (105)
|
29,999
|
29,999
|
0
|
0
|
%
|
|||||||||||
|
Buyers Advantage Inc. (106)
|
7,500
|
7,500
|
0
|
0
|
%
|
|||||||||||
|
Andrew Cimmino & Carla Cimino* (107)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Steve Furer (108)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Mark Goodridge (109)
|
11,810
|
11,810
|
0
|
0
|
%
|
|||||||||||
|
Kevin C. Kopp Trust (110)
|
15,000
|
15,000
|
0
|
0
|
%
|
|||||||||||
|
Terrance Lalchan (111)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Claude Kerry McCan, Jr. (112)
|
44,291
|
44,291
|
0
|
0
|
%
|
|||||||||||
|
Hansraji Nandlal and Siew Nandlal* (113)
|
12,000
|
12,000
|
0
|
0
|
%
|
|||||||||||
|
Darryl Persad (114)
|
5,964
|
5,964
|
0
|
0
|
%
|
|||||||||||
|
Daniel C. Ruda Irr. Tr. (115)
|
88,578
|
88,578
|
0
|
0
|
%
|
|||||||||||
|
Andrew J. & Kim M. Savage * (116)
|
209,050
|
209,050
|
0
|
0
|
%
|
|||||||||||
|
Cary A. Williams & Suzanne Marie Williams* (117)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Paulette Zdanow (118)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Bradley Siegel (119)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Robert Bagshaw (120)
|
8,859
|
8,859
|
0
|
0
|
%
|
|||||||||||
|
Ralph Arch (121)
|
5,905
|
5,905
|
0
|
0
|
%
|
|||||||||||
|
Richmond Capital LP (122)
|
150,000
|
150,000
|
0
|
0
|
%
|
|||||||||||
|
Luppino Landscaping LLC(123)
|
59,050
|
59,050
|
0
|
0
|
%
|
|||||||||||
|
Placement Agent Warrants
|
0
|
%
|
||||||||||||||
|
May Davis Partners, LLC (124)
|
111,940
|
111,940
|
0
|
0
|
%
|
|||||||||||
|
Charles Vista, LLC (125)
|
49,413
|
49,413
|
0
|
0
|
%
|
|||||||||||
|
American Capital Partners, LLC (126)
|
190,581
|
190,581
|
0
|
0
|
%
|
|||||||||||
|
Total
|
5,630,575
|
5,630,575
|
0
|
|||||||||||||
25
* The shares are owned by respective holders as joint tenants with right of survivorship.
|
(1)
|
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, securities that are currently convertible or exercisable into shares of our Common Stock, or convertible or exercisable into shares of our Common Stock within 60 days of the date hereof are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. Except as indicated in the footnotes to the following table, each stockholder named in the table has sole voting and investment power with respect to the shares set forth opposite such stockholder’s name. The percentage of beneficial ownership is based on 38,119,340 shares of Common Stock outstanding as of May 21, 2010.
|
|
(2)
|
Includes the number of shares set forth opposite each Selling Stockholders’ name, and the number of shares that may be issued pursuant to the Warrants.
|
|
(3)
|
Pursuant to the terms of the Warrants, the number of shares of our Common Stock that may be acquired by the warrant holder upon any exercise of the Investor Warrant (or otherwise in respect hereof) will be limited to the extent necessary to insure that, following such exercise (or other issuance), the total number of shares of Common Stock then beneficially owned by such holder and its affiliates and any other persons whose beneficial ownership of Common Stock would be aggregated with the Holder’s for purposes of Section 13(d) of the Securities Exchange Act of 1934, as amended does not exceed 4.9% of the total number of issued and outstanding shares of our Common Stock.
|
|
(4)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(5)
|
Consists of 3,988 shares of our Common Stock and 1,994 shares of our Common Stock underlying the Warrants to purchase up to 1,994 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(6)
|
Consists of 39,370 shares of our Common Stock and 19,682 shares of our Common Stock underlying the Warrants to purchase up to 19,682 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(7)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(8)
|
Consists of 10,000 shares of our Common Stock and 4,999 shares of our Common Stock underlying the Warrants to purchase up to 4,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(9)
|
Consists of 10,000 shares of our Common Stock and 4,999 shares of our Common Stock underlying the Warrants to purchase up to 4,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(10)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(11)
|
Consists of 5,906 shares of our Common Stock and 2,953 shares of our Common Stock underlying the Warrants to purchase up to 2,953 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(12)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(13)
|
Consists of 9,921 shares of our Common Stock and 4,959 shares of our Common Stock underlying the Warrants to purchase up to 4,959 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(14)
|
Consists of 10,000 shares of our Common Stock and 4,999 shares of our Common Stock underlying the Warrants to purchase up to 4,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
26
|
(15)
|
Consists of 20,000 shares of our Common Stock and 9,999 shares of our Common Stock underlying the Warrants to purchase up to 9,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(16)
|
Consists of 20,000 shares of our Common Stock and 9,997 shares of our Common Stock underlying the Warrants to purchase up to 9,997 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(17)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(18)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(19)
|
Consists of 100,000 shares of our Common Stock and 49,987 shares of our Common Stock underlying the Warrants to purchase up to 49,987 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(20)
|
Consists of 37,874 shares of our Common Stock and 18,934 shares of our Common Stock underlying the Warrants to purchase up to 18,934 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(21)
|
Consists of 12,000 shares of our Common Stock and 5,998 shares of our Common Stock underlying the Warrants to purchase up to 5,998 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(22)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(23)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(24)
|
Consists of 60,000 shares of our Common Stock and 29,999 shares of our Common Stock underlying the Warrants to purchase up to 29,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(25)
|
Consists of 299,999 shares of our Common Stock and 149,992 shares of our Common Stock underlying the Warrants to purchase up to 149,992 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(26)
|
Consists of 33,622 shares of our Common Stock and 16,810 shares of our Common Stock underlying the Warrants to purchase up to 16,810 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(27)
|
Consists of 10,000 shares of our Common Stock and 4,999 shares of our Common Stock underlying the Warrants to purchase up to 4,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(28)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(29)
|
Consists of (i) 12,000 shares of our Common Stock and 5,998 shares of our Common Stock underlying the Warrants to purchase up to 5,998 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above, owned by Vishal Goolcharan, and (ii) 8,000 shares of our Common Stock and 3,999 shares of our Common Stock underlying the Warrants to purchase up to 3,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above, owned by Roslyn Parmasad and Vishal Goolcharan as joint tenants with right of survivorship.
|
|
(30)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
27
|
(31)
|
Consists of 9,843 shares of our Common Stock and 4,920 shares of our Common Stock underlying the Warrants to purchase up to 4,920 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(32)
|
Consists of 5,906 shares of our Common Stock and 2,952 shares of our Common Stock underlying the Warrants to purchase up to 2,952 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(33)
|
Consists of 19,685 shares of our Common Stock and 9,842 shares of our Common Stock underlying the Warrants to purchase up to 9,842 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(34)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(35)
|
Consists of 11,811 shares of our Common Stock and 5,905 shares of our Common Stock underlying the Warrants to purchase up to 5,905 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(36)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(37)
|
Consists of 25,000 shares of our Common Stock and 12,497 shares of our Common Stock underlying the Warrants to purchase up to 12,497 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(38)
|
Consists of 25,000 shares of our Common Stock and 12,497 shares of our Common Stock underlying the Warrants to purchase up to 12,497 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(39)
|
Consists of 5,906 shares of our Common Stock and 2,952 shares of our Common Stock underlying the Warrants to purchase up to 2,952 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(40)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(41)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(42)
|
Consists of 11,811 shares of our Common Stock and 5,906 shares of our Common Stock underlying the Warrants to purchase up to 5,906 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(43)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(44)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(45)
|
Consists of 11,811 shares of our Common Stock and 5,905 shares of our Common Stock underlying the Warrants to purchase up to 5,905 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(46)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(47)
|
Consists of 59,055 shares of our Common Stock and 29,520 shares of our Common Stock underlying the Warrants to purchase up to 29,520 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
28
|
(48)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(49)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(50)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(51)
|
Consists of 7,876 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(52)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(53)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(54)
|
Consists of 11,811 shares of our Common Stock and 5,904 shares of our Common Stock underlying the Warrants to purchase up to 5,904 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(55)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(56)
|
Consists of 106,299 shares of our Common Stock and 53,136 shares of our Common Stock underlying the Warrants to purchase up to 53,136 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Robert W. Lucas, President of LJW Partnership LLC and has voting and dispositive power over the shares held by LJW Partnership LLC. Robert W. Lucas may be deemed to beneficially own the shares of Common Stock held by LJW Partnership LLC. Robert W. Lucas disclaims beneficial ownership of such shares. The address for this selling stockholder is 7409 S. Russet Dr., Sioux Fall, SD 57108.
|
|
(57)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(58)
|
Consists of 98,425 shares of our Common Stock and 49,200 shares of our Common Stock underlying the Warrants to purchase up to 49,200 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(59)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(60)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(61)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(62)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(63)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
29
|
(64)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(65)
|
Consists of 137,795 shares of our Common Stock and 68,880 shares of our Common Stock underlying the Warrants to purchase up to 68,880 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(66)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(67)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(68)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(69)
|
Consists of 9,843 shares of our Common Stock and 4,920 shares of our Common Stock underlying the Warrants to purchase up to 4,920 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(70)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(71)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(72)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(73)
|
Consists of 11,811 shares of our Common Stock and 5,904 shares of our Common Stock underlying the Warrants to purchase up to 5,904 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(74)
|
Consists of 11,811 shares of our Common Stock and 5,904 shares of our Common Stock underlying the Warrants to purchase up to 5,904 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(75)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(76)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(77)
|
Consists of 11,811 shares of our Common Stock and 5,904 shares of our Common Stock underlying the Warrants to purchase up to 5,904 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(78)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(79)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(80)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
30
|
(81)
|
Consists of 59,055 shares of our Common Stock and 29,520 shares of our Common Stock underlying the Warrants to purchase up to 29,520 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(82)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(83)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(84)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(85)
|
Consists of 15,748 shares of our Common Stock and 7,872 shares of our Common Stock underlying the Warrants to purchase up to 7,872 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(86)
|
Consists of 11,811 shares of our Common Stock and 5,904 shares of our Common Stock underlying the Warrants to purchase up to 5,904 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(87)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(88)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(89)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(90)
|
Consists of 19,685 shares of our Common Stock and 9,840 shares of our Common Stock underlying the Warrants to purchase up to 9,840 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(91)
|
Consists of 3,837 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(92)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(93)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(94)
|
Consists of 106,299 shares of our Common Stock and 53,136 shares of our Common Stock underlying the Warrants to purchase up to 53,136 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(95)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(96)
|
Consists of 55,118 shares of our Common Stock and 27,552 shares of our Common Stock underlying the Warrants to purchase up to 27,552 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(97)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
31
|
(98)
|
Consists of 118,110 shares of our Common Stock and 59,040 shares of our Common Stock underlying the Warrants to purchase up to 59,040 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Larry Butz and John Prinz, managing partners of this selling stockholder share voting and dispositive power over the shares held by this selling stockholder. Larry Butz and John Prinz may be deemed to beneficially own the shares of Common Stock held by this selling stockholder. Larry Butz and John Prinz disclaim beneficial ownership of such shares. The address for this selling stockholder is 100 E. Cook Ave, Suite 100, Libertyville, IL 60048.
|
|
(99)
|
Consists of 78,740 shares of our Common Stock and 39,370 shares of our Common Stock underlying the Warrants to purchase up to 39,370 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Alan Donenfeld, managing member of this selling stockholder and has voting and dispositive power over the shares held by this selling stockholder. Mr. Donenfeld may be deemed to beneficially own the shares of Common Stock held by this selling stockholder. Mr. Donenfeld disclaims beneficial ownership of such shares. The address for this selling stockholder is 110 East 59th St. 29th Fl., New York, NY 10022.
|
|
(100)
|
Consists of 590,550 shares of our Common Stock and 295,200 shares of our Common Stock underlying the Warrants to purchase up to 295,200 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above Kent C. McCarthy has voting and dispositive power over the shares held by this selling stockholder, Mr. McCarthy may be deemed to beneficially own the shares of Common Stock held by this selling stockholder. Mr. McCarthy disclaims beneficial ownership of such shares. The address for this selling stockholder is 930 Tahoe Blvd 802-281, Incline Village, NV 89451.
|
|
(101)
|
Consists of 196,850 shares of our Common Stock and 98,400 shares of our Common Stock underlying the Warrants to purchase up to 98,400 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Kenneth Holz, CFO of this selling stockholder, has voting and dispositive power over the shares held by Chestnut Ridge Partners, LP. Kenneth Holz may be deemed to beneficially own the shares of Common Stock held by this selling stockholder. Kenneth Holz disclaims beneficial ownership of such shares. The address for this selling stockholder is 10 Forest Avenue, Ste 220, Paramus, NJ 7652.
|
|
(102)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(103)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(104)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(105)
|
Consists of 20,000 shares of our Common Stock and 9,999 shares of our Common Stock underlying the Warrants to purchase up to 9,999 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Cary A. Gotto and Mark D. Samson, Members of this selling stockholder, share voting and dispositive power over the shares held by the selling stockholder. Messrs. Gotto and Samson may be deemed to beneficially own the shares of Common Stock held by this selling stockholder. Messrs. Gotto and Samson disclaim beneficial ownership of such shares. The address for this selling stockholder is 3101 N. Central Ave #1400, Phoenix, AZ 85012.
|
|
(106)
|
Consists of 5,000 shares of our Common Stock and 2,500 shares of our Common Stock underlying the Warrants to purchase up to 2,500 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Claus Demeus, President of the selling stockholder has voting and dispositive power over the shares held by this selling stockholder. Mr. Demeus may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. Demeus disclaims beneficial ownership of such shares. The address for this selling stockholder is 123 Riviera Drive, Brick, NJ 8724.
|
32
|
(107)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(108)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(109)
|
Consists of 7,874 shares of our Common Stock and 3,936 shares of our Common Stock underlying the Warrants to purchase up to 3,936 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(110)
|
Consists of 10,000 shares of our Common Stock and 5,000 shares of our Common Stock underlying the Warrants to purchase up to 5,000 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Kevin C Kopp, Trustee of Kevin C. Kopp Trust and has voting and dispositive power over the shares held by Kevin C. Kopp Trust. Kevin C. Kopp may be deemed to beneficially own the shares of Common Stock held by Kevin C. Kopp Trust. Kevin C. Kopp disclaims beneficial ownership of such shares. The address for this selling stockholder is 480 81st Street, Burr Ridge, IL 60527.
|
|
(111)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(112)
|
Consists of 29,527 shares of our Common Stock and 14,764 shares of our Common Stock underlying the Warrants to purchase up to 14,764 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(113)
|
Consists of 8,000 shares of our Common Stock and 4,000 shares of our Common Stock underlying the Warrants to purchase up to 4,000 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(114)
|
Consists of 3,976 shares of our Common Stock and 1,988 shares of our Common Stock underlying the Warrants to purchase up to 1,988 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(115)
|
Consists of 59,055 shares of our Common Stock and 29,523 shares of our Common Stock underlying the Warrants to purchase up to 29,523 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Daniel C. Ruda, Trustee of Daniel C. Ruda Revocable Trust and has voting and dispositive power over the shares held by Daniel C. Ruda Revocable Trust. Daniel C. Ruda may be deemed to beneficially own the shares of Common Stock held by Daniel C. Ruda Revocable Trust. Daniel C. Ruda disclaims beneficial ownership of such shares. The address for Daniel C. Ruda Revocable Trust is 245 50 Wildwood Drive, Branson, Mo 65616.
|
|
(116)
|
Consists of 139,370 shares of our Common Stock and 69,680 shares of our Common Stock underlying the Warrants to purchase up to 69,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(117)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(118)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(119)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(110)
|
Consists of 5,906 shares of our Common Stock and 2,953 shares of our Common Stock underlying the Warrants to purchase up to 2,953 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
|
(121)
|
Consists of 3,937 shares of our Common Stock and 1,968 shares of our Common Stock underlying the Warrants to purchase up to 1,968 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above.
|
33
|
(122)
|
Consists of 100,000 shares of our Common Stock and 50,000 shares of our Common Stock underlying the Warrants to purchase up to 50,000 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. David B. Kass, managing member of the selling stockholder has voting and dispositive power over the shares held by the selling stockholder. Mr. Kass may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. Kass disclaims beneficial ownership of such shares. The address for the selling stockholder is One Hawthorne Lane, Westport, CT 06880.
|
|
(123)
|
Consists of 39,370 shares of our Common Stock and 19,680 shares of our Common Stock underlying the Warrants to purchase up to 19,680 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Carmelo Luppino has voting and dispositive power over the shares held by the selling stockholder. Mr. Luppino may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. Luppino disclaims beneficial ownership of such shares. The address for the selling stockholder is 77 Sheather Rd, Mt. Kisco, NY 10549.
|
|
(124)
|
Consists of 111,940 shares of our Common Stock underlying the Warrants to purchase up to 111,940 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Owen May has voting and dispositive power over the shares held by the selling stockholder. Mr. May may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. May disclaims beneficial ownership of such shares. The address for the selling stockholder is 825 Third Avenue, 2nd Floor, Suite 231, New York, NY 10022.
|
|
(125)
|
Consists of 49,413 shares of our Common Stock underlying the Warrants to purchase up to 49,413 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Gregg Lorenzo has voting and dispositive power over the shares held by the selling stockholder. Mr. Lorenzo may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. Lorenzo disclaims beneficial ownership of such shares. The address for the selling stockholder is 100 William Street 18th Floor, New York, NY 10038.
|
|
(126)
|
Consists of 190,581 shares of our Common Stock underlying the Warrants to purchase up to 190,581 shares of our Common Stock, subject to a 4.9% limitation on beneficial ownership of our Common Stock as more fully described in note 3 above. Anthony M. Gardini has voting and dispositive power over the shares held by the selling stockholder. Mr. Gardini may be deemed to beneficially own the shares of Common Stock held by the selling stockholder. Mr. Gardini disclaims beneficial ownership of such shares. The address for the selling stockholder is 205 Oser Avenue, Hauppauge, NY 11788.
|
34
PLAN OF DISTRIBUTION
This prospectus relates to the resale of up to 5,630,575 shares (i) issued or (ii) to be issued upon the exercise of certain outstanding Warrants, each held by certain selling stockholders.
May Davis and American Capital are registered broker dealers and FINRA member firms and each is listed as a selling stockholder in this prospectus.
Neither May Davis nor American Capital has an underwriting agreement with us and/or the selling stockholders and no selling stockholder is required to execute transactions through May Davis and American Capital. Further, other than any existing brokerage relationship as customers with May Davis and American Capital, no selling stockholder has any pre-arranged agreement, written or otherwise, with May Davis or ACP to sell their securities through May Davis or American Capital.
The selling stockholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions,
|
●
|
on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale;
|
|
●
|
in the over-the-counter market;
|
|
●
|
in transactions otherwise than on these exchanges or systems or in the over-the-counter market;
|
|
●
|
through the writing of options, whether such options are listed on an options exchange or otherwise;
|
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
●
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
●
|
privately negotiated transactions;
|
|
●
|
short sales;
|
|
●
|
Sales pursuant to Rule 144;
|
|
●
|
broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share;
|
|
●
|
a combination of any such methods of sale; and
|
|
●
|
any other method permitted pursuant to applicable law.
|
If the selling stockholders effect such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares.
35
The selling stockholders may pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock from time to time pursuant to this prospectus or any amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act of 1933, as amended, amending, if necessary, the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer and donate the shares of common stock in other circumstances in which case the transferees, donees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
The selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or reallowed or paid to broker-dealers.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration statement, of which this prospectus forms a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder, including, without limitation, Regulation M of the Exchange Act, which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We will pay all expenses of the registration of the shares of common stock pursuant to the registration rights agreement, estimated to be approximately $135,192 in total, including, without limitation, Securities and Exchange Commission filing fees and expenses of compliance with state securities or “blue sky” laws; provided, however, that a selling stockholder will pay all underwriting discounts and selling commissions, if any. We will indemnify the selling stockholders against liabilities, including some liabilities under the Securities Act, in accordance with the registration rights agreements, or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against civil liabilities, including liabilities under the Securities Act, that may arise from any written information furnished to us by the selling stockholder specifically for use in this prospectus, in accordance with the related registration rights agreement, or we may be entitled to contribution.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
36
DESCRIPTION OF SECURITIES
Common Stock
Our authorized capital stock consists of 100,000,000 shares of common stock, par value $0.001. In connection with the sale of the Preferred Shares, we have approved an amendment to our certificate of incorporation that will increase the number of authorized shares of common stock to 150 million. The holders of shares of our common stock currently (i) have equal ratable rights to dividends from funds legally available therefore, when, as and if declared by the board of directors of the company; (ii) are entitled to share ratably in all of the assets of the company available for distribution to holders of common stock upon liquidation, dissolution or winding up of the affairs of the company; (iii) do not have preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights applicable thereto; and (iv) are entitled to one non-cumulative vote per share on all matters on which stockholders may vote. Please refer to the Articles of Incorporation.
Please refer to our By-Laws which have been filed with the SEC on August 22, 2007 as an exhibit to our Registration Statement on Form SB-2, and the applicable statutes of the State of Delaware for a more complete description of the rights and liabilities of holders of the company’s securities.
The holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of such outstanding shares, voting for the election of directors, can elect all of the directors to be elected, if they so choose, and, in such event, the holders of the remaining shares will not be able to elect any of the company’s directors.
Preferred Stock
Currently, we do not have any shares of preferred stock authorized. However, in connection with the sale of the Preferred Shares, we have approved an amendment to our certificate of incorporation which authorizes 20 million shares of blank-check preferred stock to be issued upon authorization by our board on such terms and in such amounts as the board may from time to time authorize; this amendment will be filed and become effective upon the completion of the process of notification to stockholders provided under Section 14(c) of the Exchange Act and the regulations thereunder.
Holders
As of the date hereof, we have 165 shareholders holding 38,154,340 shares of our issued and outstanding common stock.
Warrants
Investor Warrants
Currently we have outstanding Investor Warrants which are exercisable for 1,759,301 shares of our common stock at an exercise price of $3.81 per share and outstanding Agent Warrants which are exercisable for 351,934 shares of our common stock at an exercise price of $3.05 per share. The Investor Warrants, at the option of the holder, may be exercised by cash payment of the exercise price or, commencing six months following the original issuance date, if a registration statement under the Securities Act of 1933, as amended, covering the shares of common stock underlying the Investor Warrants is not then declared effective by the SEC, in lieu of exercising the Investor Warrants by payment of cash, a holder may exercise the Investor Warrant by a cashless exercise by surrender of the Investor Warrant, in which event we will issue to the holder a number of shares of our common stock computed using the following formula:
|
X =
|
Y - (A)(Y)
|
|
|
B
|
||
|
Where
|
X =
|
The number of shares of common stock to be issued to the Holder.
|
|
Y =
|
The number of shares of common stock issuable upon exercise of this Warrant in accordance with the terms of this Warrant by means of a cash exercise rather than a cashless exercise.
|
|
|
A =
|
The Exercise Price.
|
|
|
B =
|
The Per Share Market Value of one share of common stock on the Business Day immediately preceding the date of such election.
|
37
We will not receive any additional proceeds to the extent that Investor Warrants are exercised by cashless exercise.
The exercise price and number of shares of our common stock issuable upon exercise of the Investor Warrants may be adjusted in certain circumstances, including in the event of a stock dividend, or our recapitalization, reorganization, merger or consolidation and the issuance of rights to purchase additional shares of our common stock or to receive other securities convertible into additional shares of common stock.
In the event we issue any additional stock at a price per share less than $2.54 or without consideration, then the exercise price then in effect upon each such trigger issuance shall be changed to a price equal to 150% of the consideration per share received by us in respect of the shares issued in such trigger issuance. Such adjustment shall be made successively whenever such an issuance is made.
Pursuant to the terms of the Investor Warrants, the number of shares of common stock that may be acquired by the warrant holder upon any exercise of the Investor Warrant (or otherwise in respect hereof) will be limited to the extent necessary to insure that, following such exercise (or other issuance), the total number of shares of common stock then beneficially owned by such holder and its affiliates and any other persons whose beneficial ownership of common stock would be aggregated with the Holder’s for purposes of Section 13(d) of the Securities Exchange Act of 1934, as amended does not exceed 4.9% of the total number of issued and outstanding shares of our common stock.
Placement Agent Warrants
Similarly, the Agent Warrants at the option of the holder, may be exercised by cash payment of the exercise price or, commencing six months following the original issuance date, if a registration statement under the Securities Act of 1933, as amended, covering the shares of common stock underlying the Agent Warrants is not then declared effective by the SEC, in lieu of exercising the Agent Warrants by payment of cash, a holder may exercise the Agent Warrants by a cashless exercise by surrender of the Agent Warrants, in which event we will issue to the holder a number of shares of our common stock computed using the following formula:
|
X =
|
Y - (A)(Y)
|
|
|
B
|
||
|
Where
|
X =
|
the number of shares of common stock to be issued to the Holder.
|
|
Y =
|
the number of shares of common stock issuable upon exercise of this Warrant in accordance with the terms of this Warrant by means of a cash exercise rather than a cashless exercise.
|
|
|
A =
|
the Exercise Price.
|
|
|
B =
|
the Per Share Market Value of one share of common stock on the Business Day immediately preceding the date of such election.
|
We will not receive any additional proceeds to the extent that Agent Warrants are exercised by cashless exercise.
The exercise price and number of shares of our common stock issuable upon exercise of the Agent Warrants may be adjusted in certain circumstances, including in the event of a stock dividend, or our recapitalization, reorganization, merger or consolidation and the issuance of rights to purchase additional shares of our common stock or to receive other securities convertible into additional shares of common stock.
Pursuant to the terms of the Agent Warrants, the number of shares of common stock that may be acquired by the warrant holder upon any exercise of the Agent Warrant (or otherwise in respect hereof) will be limited to the extent necessary to insure that, following such exercise (or other issuance), the total number of shares of common stock then beneficially owned by such holder and its affiliates and any other persons whose beneficial ownership of common stock would be aggregated with the Holder’s for purposes of Section 13(d) of the Securities Exchange Act of 1934, as amended does not exceed 4.9% of the total number of issued and outstanding shares of our common stock.
38
Options
As of the date hereof, we have 1,110,000 options exercisable for shares of our common stock issued and outstanding.
Dividend
During the fiscal years ended December 31, 2009 and 2008, Liuzhou BCT declared and paid to its original shareholders cash dividends in the aggregate amount of nil and $6,940,000, respectively.
The declaration or payment of any future cash dividend will be at the discretion of our board of directors and will depend upon the earnings (if any), capital requirements and financial position of the company, general economic conditions, and other pertinent factors. It is our present intention not to declare or pay any cash dividends in the foreseeable future, but rather to reinvest earnings (if any), in our business operations.
Interests of Named Experts and Counsel
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or is to receive, in connection with the offering, a substantial interest, direct or indirect, in the registrant or any of its parents or subsidiaries. Nor was any such person connected with the registrant or any of its parents or subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
The financial statements included in this prospectus and the registration statement have been audited by PKF, Certified Public Accountants, Hong Kong, China, a member firm of PKF International Limited network of legally independent firms, to the extent and for the periods set forth in their report appearing elsewhere herein and in the registration statement, and are included in reliance upon such report given upon the authority of said firm as experts in auditing and accounting.
We are engaged in pharmaceutical distribution, retail pharmacy and manufacturing of pharmaceuticals through our two wholly-owned subsidiaries Liuzhou BCT and Hefeng Pharmaceutical, and BCT Retail, a retail company that we control through a series of contractual arrangements, each of which is located in Guangxi province, China. Since January 2, 2008, we have three operating segments based on our major lines of businesses: pharmaceutical distribution, retail pharmacy and manufacturing pharmacy. For additional information regarding our segments please refer to our financial statements.
|
●
|
Pharmaceutical distribution
|
Pharmaceutical distribution is our principal business. We conduct our wholesale business through Liuzhou BCT by purchasing pharmaceutical products from suppliers and then distributing them to our wholesale customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals. Our pharmaceutical distribution business is focused on the market of Guangxi province, which includes major cities such as Nanning, Liuzhou and Guilin and which has a population of approximately 50 million people. We operate a large regional wholesale network in Guangxi Province supported by strategically placed warehouse facilities. For the year ended December 31, 2009, revenue generated from our pharmaceutical distribution segment was $97.1 million, or 71.4% of our total revenues for the year.
39
We distribute over 8,000 products from nearly 4,000 suppliers through our wholesale distribution in compliance with PRC regulations. Hefeng Pharmaceutical which is one of our wholly-owned subsidiaries is also one of our suppliers. In 2009 revenue derived from the distribution of third-party products constituted 99% of our pharmaceutical distribution segment revenue. The terms of our distribution agreements vary between supplier and vary in terms of payment period, arrangement of delivery, pricing and quality requirements. The general payment terms vary from advance deposit, to cash on delivery, to payment up to 90 days from delivery, and the payment can be settled by means of bank collection, remittance, bills payable, postal check. The delivery is either to our warehouse, railway station, or prescribed destination within Liuzhou City. The quality of drugs supplied is in accordance with the prescribed national standard requirement. Our top 10 suppliers in our pharmaceutical distribution segment accounted for 27% of our purchases in 2009.
Liuzhou BCT’s GSP Certificate, which is a certification that drugstores in China are required to obtain was issued on September 18, 2009 and will expire on September 17, 2013, subject to renewal for an additional five-year term.
|
●
|
Retail Pharmacy
|
Established in 2001, BCT Retail operates a large regional retail network in Guangxi province, consisting of 170 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” BCT Retail’s GSP Certificate was issued on February 18, 2009 and will expire on February 17, 2014, subject to renewal for an additional five-year term. Our retail stores provide convenient, high quality and professional pharmaceutical services, and supply a wide variety of medicines, including western medicine, TCM, dried Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and seasonal medicine. For the year ended December 31, 2009, revenue generated from our retail pharmacy segment was $31.2 million, or 22.9% of our revenues for the year.
Among the 170 stores, there are 21 stores that are medi-care qualified stores, where customers are able to make their purchase either by cash or by using their medi-care insurance card for payment. With respect to medi-care insurance card payments, we issue an invoice to the national medi-care for reimbursement for those drugs included under the medi-care insurance catalogue. Only medi-care card payments from these 26 stores are entitled to reimbursement. The medi-care insurance catalogue used are provincial-based and developed and based on the national insurance catalogue. The medi-care insurance program is also known as National Medical Insurance Program.
The National Medical Insurance Program is funded primarily by provincial governments and, to a lesser degree, by program participants and their employers. The program has two types of accounts: individual accounts and social pool accounts. Each participant has an individual account that holds all contributions from the participant and part was contributed from his or her employer. National medicine catalog of the National Medical Insurance Program provides guidance to what extent the purchases of these medicines are reimbursable. The implementation of the National Medical Insurance Program is delegated to provincial governments, each of which has established its own medicine catalog. The medicine catalog comprises two lists of drugs which are named as Tier A and Tier B. People can consume and reimburse the drugs on these two lists by means of the insurance card at the medi-care qualified retail stores. When the customers purchase the drugs at the medi-care qualified stores listed under Tier B, they need to pay 20% co-payment by themselves and 80% is deducted from their individual account under the insurance card, whereas 100% is deducted from their individual account for the drugs listed under Tier A. From July 2009, onward, such policy was cancelled and the cost of drugs listed under Tier B can be deducted from the card in full should the balance of the individual account on the card exceed the cost of drugs purchased. If there is not a sufficient balance on the insurance card to cover the cost of the drug, the customer must personally pay the difference at the time of purchase. Our retail stores participate in both of these two tiers of drugs. Thus, subject to an individual having an adequate balance on their card for Tier B drugs, there is no difference between the treatment of Tier A and Tier B drugs from July 2009 onwards.
In order to get reimbursement from the National insurance program, we have to extract the total amount of sales derived from the amount spent by insurance card under our billing system. Our system is linked with the one at the National program. Each month, we only need to reconcile our record with the system and issue the invoice to the National program for reimbursement approval. Then the money is remitted to our designated account with no difference between these two tiers, subject to an individual having an adequate balance on their card for Tier B drugs.
40
The retail price under the medi-care qualified stores is subject to price controls administered by the Price Control Office under the National Development and Reform Commission. Our retail price for Tier A and Tier B drugs under qualified stores is fixed at an agreed ceiling of an agreed percentage over the prescribed one issued by the Guangxi Price Control Office. The prescribed drug prices issued by the Guangxi Price Control Office are in accordance with the price upon the provincial collective tender result which is held annually.
As for the New Rural Cooperative Medicare Plan, the participants in the scheme are people living in rural areas. The program also has individual accounts in which more subsidies are contributed by the provincial government and no employers are involved. It resembles the schemes under the National Medical Insurance Program in which people consume and reimburse the drugs by their individual account. The transaction is not recorded by a card; we instead login their account under the system of the New Rural Cooperative plan to check their balance to ensure that the limit of the account has not been reached.. Unlike National Medical Insurance Program, the eligible person is entitled to consume the drugs under the list of Basic Drugs Catalogue rather than the Tier A and Tier B catalogue,
The retail prices under the New Rural Cooperative Medicare Plan are subject to price controls administered by the Price Control Office under the National Development and Reform Commission. The retail prices under the list of Basic Drugs Catalogue are fixed at an agreed ceiling of percentage over the prescribed one issued by the Guangxi Price Control Office. The prescribed drugs price issued by the Guangxi Price Control Office are in accordance with the price upon the provincial collective tender result which is held annually. In order to get reimbursement from the New Rural Cooperative Medicare Plan, our stores have to extract the sales figures from the billing system and issue the reimbursement notes with the original vouchers and invoices to the relevant Committee for approval. Then the Committee remits the money to the designated account opened by the stores.
|
●
|
Manufacturing of Pharmaceuticals
|
Hefeng Pharmaceutical has a manufacturing facility located on approximately 40,000 square meters of land, and manufactures four types of products:
|
●
|
A Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity (Maximum daily unit production: 2.5 tons per day; maximum days of operation per year: 270 days);
|
|
●
|
A granular formulation unit with an annual production capacity of 0.25 billion packages (Maximum daily unit production: 768,960 packages per day; maximum days of operation per year: 324 days);
|
|
●
|
A pill formulation unit with an annual production capacity of 0.36 billion pills (Maximum daily unit production: 1,252,800 pieces per day; maximum days of operation per year: 288 days); and
|
|
●
|
A liquid formulation unit with an annual production capacity of 0.1 billion injections (Maximum daily unit production: 347,500 pieces per day; maximum days of operation per year: 288 days).
|
The above capacity figures have been derived by our production department. The maximum days of operation is determined after taking into account the days of repairs, sterilization and rinsing process particular to each of the above production lines.
Hefeng Pharmaceutical produces and sells pharmaceutical products under the registered name “Asio (亚太)” including: traditional anti-inflammatory and antibacterial drugs, cancer treatment drugs, cardio-vascular disease drugs and hepatitis drugs.
41
Hefeng Pharmaceutical’s best-selling products include:
|
●
|
Tabellae Sarcandrae, a TCM drug that has similar anti-inflammatory and antibacterial effects as anti-biotics in Western medicine;
|
|
●
|
Corydalis Saxicola Bunting (Yanhuanglian), an important component in various hepatitis prescriptions in TCM;
|
|
●
|
Hydroxycamptotbecine Injection; which is used to treat cancers such as esophagus cancer, carcinoma ventriculi, carcinoma hepatis and colon cancer.
|
|
●
|
Yinge Tongmai Tea, which is made of gingko biloba, kudzu rot and Chinese tea and is used to clear up blood vessels and treat cardio-vascular diseases.; and
|
|
●
|
Levodopa, a TCM drug that is used to treat stiffness, tremors, spasms and poor muscle control related to Parkinson’s disease.
|
In addition, Hefeng Pharmaceutical collaborates with several renowned medical research universities in China to continuously improve its raw material abstraction efficiency and production process, and to develop alternative formulas for existing drugs. For the year ended December 31, 2009, revenue generated from our manufacturing segment was $7.7 million , or 5.7% of our total revenues for the year.
Hefeng Pharmaceutical’s GMP Certificate was issued on July 14, 2009 and will expire on July 13, 2014, and its GMP Certificate for Small Volume Parental Solution was issued on July 10, 2006 and will expire on July 9, 2011; both certificates are subject to renewal of additional five-year term. The renewal process requires us to apply through the State Food and Drug Administration and furnish an application form together with relevant supporting documents, such as our product license, business registration certificate, a summary of our management over drugs manufacturing, a self quality control report, an organization chart, education graphic data, a floor plan, assembly line, production flow and control points, inspection detail over key processes and a list of products produced. The Food and Drugs Administration will consider and issue the notice of acceptance upon the formality check and then carry out inspection on our technical know-how. Thereafter, GMP inspection unit will undertake an on-site inspection and issue an on-site inspection report in compliance with the GMP inspection standards. The State Food and Drug Administration then reviews the on-site inspection report and announces the result of inspection to us. The State Food and Drug Administration will issue the GMP certificate renewal if the reports are satisfactory.
The following table sets forth a breakdown of our external segment revenue after elimination of inter-segment sales, and each segment revenue item as a percentage of our total revenue, as well as our inter-segment sales for the year ended December 31, 2009 and December 31, 2008. For the year ended December 31, 2009, we had approximately $22.5 million of inter-segment revenue, which includes approximately $21.7 million in sales from our pharmaceutical distribution segment to our retail pharmacy segment, and approximately $762,000 in sales from our manufacturing segment to our retail pharmacy segment External segment revenue refers to segment revenue after inter-segment elimination.
|
December 31,
|
||||||||||||||||
|
2009
|
2008
|
|||||||||||||||
|
External Segment revenue
|
‘000
|
%
|
‘000
|
%
|
||||||||||||
|
Pharmaceutical distribution
|
$
|
97,137
|
71.4
|
$
|
72,807
|
66.8
|
||||||||||
|
Retail pharmacy
|
31,223
|
22.9
|
28,593
|
26.2
|
||||||||||||
|
Manufacturing pharmacy
|
7,727
|
5.7
|
7,592
|
7
|
||||||||||||
|
136,087
|
100.0
|
108,992
|
100.0
|
|||||||||||||
|
Inter-segment revenue eliminated
|
22,492
|
N/A
|
21,656
|
N/A
|
||||||||||||
42
Our Products
Products Offered by Hefeng Pharmaceutical
Manufacturing both Chinese medicine and Western medicine, Hefeng Pharmaceutical maintains valid production licenses for 76 drugs. Below is the description of the five (5) best-selling drugs.
|
1.
|
Corydalis Saxicola Bunting (Yanhuanglian)
|
|
Corydalis Saxicola Bunting is an important component in various prescriptions in TCM. Yanhuanglian has been demonstrated to possess many pharmacological activities, including antibacterial, antiviral and anticancer activities. The active ingredients are dehydrocavidine, coptisine, dehydroapocavidine and tetradehydroscoulerine. Systemic clearance of the four active alkaloids in plasma was over 93% of hepatic blood flow, indicating they may be quickly eliminated via hepatic clearance. Less than 10% of the drug was excreted via urine following intravenous and oral administration, suggesting that these four alkaloids may undergo significant metabolism in the body or the drug may be excreted via routes other than urine. Intravenous administration of Yanhuanglian is the most common clinical practice, because it can improve absorption of the four active alkaloids into systemic circulation.
|
 |
We are the sole licensed producer for this drug in China, and the market demand for this drug has been extremely strong due to the effectiveness of the drug to treat hepatic diseases.
|
2.
|
Tabellae Sarcandrae
|
|
Tabellae Sarcandrae, a TCM protected drug, has similar anti-inflammatory and antibacterial effects as anti-biotics in Western medicine. Tabellae Sarcandrae possesses marked inhibition effect to auricular inflammation in mice caused by croton oil, footpad inflammation in rats caused by carragheenin and granuloma in mice by cotton ball. It could also relieve obvious abdominal pain caused by acetic acid and inhibit bacterial growth. The TCM protection is valid from December 19, 2006 to August 1, 2012 and is renwable.
|
 |
|
3.
|
Hydroxycamptotbecine Injection
|
|
Hydroxycamptotbecine Injection is used to treat cancers such as esophagus cancer, carcinoma ventriculi, carcinoma hepatis and colon cancer. We are one of three licensed producers of this drug in China.
|
 |
43
|
4.
|
Ethacridine Lactate Injection
|
|
This family planning drug is very popular in China; it’s used for second trimester pregnancy termination from week 12-26 at hospitals. We are one of three licensed producers in China.
|
 |
|
5.
|
Rotandine
|
|
This product is non-prescription analgesic drug. Used for headaches, menstrual pain, and aiding sleep.
|
 |
Products and services offered by Retail Chain
Our Retail Chain provides our customers with high-quality, professional and convenient pharmaceutical services and supplies of a wide variety of medicines, including Western medicine, TCM, raw materials of dried herbal products, roughly processed herbal medicine, family planning products, as well as convenient seasonal and promotional items. A typical retail drug store of Retail Chain carries approximately 2,800 to 3,200 different products. Management regularly reviews and refines the product selection in order to respond to change in demographics, lifestyles, shopping habits and product preferences of our customers.
Our product selection is designed to offer choices and convenience to our customers and to achieve high gross margins for us. We offer our customers a broad range of choices in two respects. First, we offer a wide range of complementary products in each therapeutic category so that customers have more choices to suit their needs. For example, a customer looking for a cough remedy will be able to find a wide variety of choices including different OTC drugs, nutritional supplements and herbal products. Second, for products with the same therapeutic purpose, we offer choices in each of the high, medium and low price ranges to suit the needs of customers with different spending power.
|
●
|
Packaged Western and TCM. We offer approximately 2,750 packaged drugs including prescription and OTC drugs. We accept prescriptions only from licensed healthcare providers and do not prescribe medications or otherwise practice medicine. Our in-store pharmacists verify the validity, accuracy and completeness of all prescription drug orders. We ask all prescription drug customers to provide us with information regarding drug allergies, current medical conditions and current medications.
|
|
●
|
Chinese Herbal Medicine. We offer approximately 450 types of various drinkable herbal remedies and packages of assorted herbs for making soup, which are used by consumers as health supplements. Herbal products typically have higher margins than prescription and OTC drugs.
|
|
●
|
Family Planning Products. We offer approximately 40 family planning products, which include family care products such as portable medical devices for family use, birth control and early pregnancy test products and convenience products. Our family planning products also include seasonal and promotional items tailored to local consumer demand for convenience and quality. We believe offering these products increases customer visits by increasing the shopping convenience for our customers.
|
44
Products offered by our wholesaler
Our wholesale business provides Retail Chain with the majority of the pharmaceutical products sold in retail drugstores. Approximately 95% of the packaged Western medicine and TCM, 100% of the Chinese Herbal Medicine and 100% of the family planning products are supplied by our wholesale business.
Besides providing procurement to our retail business, the majority of the sales revenue of our wholesale business arises from supplying pharmaceutical products to hospitals, clinics and healthcare centers at provincial, city, county and district levels. In addition, our wholesale business also exchanges our products with other wholesale networks to obtain products that we do not produce. Further, our wholesale business also distributes our products to other retail networks.
|
●
|
Increasing coverage of social medical insurance in China
|
The National Medical Insurance Program (“National Program”), which was introduced in 1999, is the largest medical insurance program in China. The National Program is funded with varying levels of contributions from the PRC Government, individual program participants and their employers. The National Program provides guidance on which prescription and over-the-counter medicines are included in the program and to what extent the purchases of these medicines are reimbursable.
We believe that only a small percentage of the Chinese population can afford commercial insurance plans. Therefore, the National Program coverage is expected to expand in the future. Provincial and municipal authorities who are responsible for administering social medical insurance funds to cover such reimbursements have been gradually increasing funding in recent years. According to the PRC Ministry of Labor and Social Security, total funding under the national insurance program reached RMB225.7 billion, or $28.9 billion, in 2008, representing an increase of 29.2% from 2007. The availability of funding is expected to increase significantly in the near future, primarily as a result of increased financial and policy support from various levels of the PRC Government.
As a major portion of sales from our pharmaceutical distribution segment are derived from hospitals, the increase in coverage of social medical insurance and the roll-out of the New Rural Cooperative Medicare Plan has increased the demand for drugs, including those we distribute, by hospitals as more patients visit hospitals for treatment, knowing that the costs related to the treatment will be reimbursed under the social medical insurance scheme. In addition, we also believe that our retail business segment will benefit if the social medical insurance scheme covers a larger population base.
|
·
|
Increasing access to healthcare in rural areas
|
At the fifth meeting of the tenth National People’s Congress held in March 2007, the PRC Government announced its goal to accelerate the reform and development of healthcare services in the PRC and focus on building a basic healthcare system that covers both rural and urban areas. The PRC Government’s plans include providing expanded healthcare services for its rural citizens and establishing comprehensive community healthcare service centers that would provide basic medical treatment and pharmaceutical services, as well as upgrading existing class-two hospitals and state owned medical facilities. The public health service centers would be allocated based on demand and population. In addition, the PRC Government has actively promoted the implementation of the New Rural Cooperative Medical Insurance Scheme (“New Rural Insurance Scheme”), which seeks to provide healthcare services to the vast rural areas of China. The program extends to cover approximately 2,729 counties in the PRC, which account for 95.4% of the total number of counties in the PRC. In addition, the program covers approximately 814 million rural residents, which accounts for approximately 91.5% of the total population engaged in the agricultural industry in China as of December 31, 2008.
Because we operate in Guangxi Province, which has a large number of small cities and rural areas, we believe that the New Rural Insurance Scheme will have a positive impact on the demand for our products in all of our segments.
45
|
●
|
PRC Healthcare Reform Plan
|
In September 2008, the State Council published a draft plan to ease the difficulties and minimize the costs for PRC citizens to obtain proper healthcare treatment. On 17 March 2009, the PRC Government issued the Opinion on Deepening the Healthcare System Reform (the “Opinion”). The State Council subsequently released the Notice on Important Implementing Plans for the Healthcare System Reform 2009-2011 (the “Implementing Plan”). The goal of the healthcare reform plan is to establish a basic, universal healthcare framework to provide Chinese citizens with safe, efficient, convenient and affordable healthcare. The Opinion calls for healthcare reform to be carried out in two steps:
|
●
|
Step One, which will be completed by 2011, aims to increase the accessibility while reducing the cost of healthcare. During this phase, the PRC Government will build up a network of basic healthcare facilities, expand coverage of the public medical insurance system to cover 90% or more of the population, and reform the drug supply and public hospital system.
|
|
●
|
Step Two, which will take place between 2011 and 2020, envisions the establishment of a universal healthcare system. The entire population should be covered by public medical insurance; drugs and medical services should be accessible and affordable to citizens in all public healthcare facilities.
|
While the PRC Government has neither provided a concrete timetable nor steps to implement certain tasks, such as the public hospital reform, it has released execution guidance for other tasks. Most notably, the PRC Government has announced it will spend an additional approximately RMB 850 billion, or $125 billion from 2009 to 2011 on the healthcare industry. A significant portion will be expended to establish a basic healthcare medical insurance regime, which aims to cover over 90% of the national population by 2011, mainly through the Urban Worker Program, Urban Resident Program and the New Rural Insurance Scheme. The PRC Government further announced that the annual subsidy for each participant will be increased from approximately RMB 40, or $5.90 to approximately RMB120, or $17.60 for Urban Resident Program participants, and from approximately RMB 80, or $11.76 to approximately RMB120 RMB, or $17.60 for New Rural Insurance Scheme participants, starting from 2010. The reform plan will also raise the cap on claim payments from four times the local average annual income to six times such income. Another significant part of the spending plan focuses on healthcare facilities. The PRC Government plans to build 29,000 rural clinics in 2009. In the next three years, the PRC government plans to build an additional 5,000 rural clinics, 2,000 county-level hospitals and 2,400 urban community clinics in under-developed areas. This substantial increase in healthcare spending is expected to expedite the growth of the healthcare industry in China.
Under the healthcare reform plan, the additional funding for the healthcare industry will primarily target four fundamental healthcare systems in China:
|
●
|
The public health services system. This system focuses on preventing disease and promoting health as a complementary alternative to medical treatment. The public health services system will provide services such as immunizations, regular physical check-ups (for senior citizens over 65 years of age and children under three years of age), pre-natal and post-natal check-ups for women, prevention of infectious or chronic diseases and other preventative and fitness activities.
|
|
●
|
The public medical insurance system. This system covers drugs and medical treatments for the majority of the population. The healthcare reform plan will retain the framework of the current public medical insurance schemes under the National Program, but will expand them to cover more of the population and increase the scope of treatments, raise the cap on claim payments and cover more claims at higher percentages.
|
|
●
|
The public healthcare delivery system. One of the primary goals of the Implementing Plan is to build more healthcare facilities and to improve the training of healthcare professionals. Beyond additional public wellness centers, the reform plan aims to place a medical clinic in every village and a hospital in every county by 2011. In addition, the PRC Government will encourage private investors to establish public non-profit hospitals.
|
46
|
●
|
The drug supply system. This system regulates pricing and how drugs will be procured, prescribed and dispensed in healthcare facilities. The healthcare reform plan will focus on pricing, procurement, prescription and dispensing of essential drugs.
|
Although the healthcare reform plan is expected to benefit our pharmaceutical distribution, retail pharmacy and other business operations and improve our competitive position, the full effect of the healthcare reform plan on our operations is as yet unclear.
Industry Overview
We operate in the large and growing pharmaceutical wholesale and retail industry in China, which we believe offers compelling industry fundamentals and benefits from favorable demographics. With approximately one-fifth of the world’s population and one of the world’s fastest growing economies, China presents significant potential for the retail drugstore industry.
Market Overview
In China, retail pharmaceutical and other healthcare related products may be purchased at either hospital pharmacies or non-hospital drugstores, including independent drugstores and drugstore chains. Historically, sales by hospital pharmacies accounted for a larger percentage of retail sales of pharmaceutical products in China. This is because out-patients typically purchase their prescription drugs at hospital pharmacies in accordance with doctors’ prescriptions. However, if a medical condition can be treated with OTC drugs, many Chinese people typically choose to purchase OTC drugs from non-hospital drugstores instead of consulting a doctor in a hospital for prescription medicines.
Fragmentation of the Pharmaceutical Chain Store Industry and the Trend for Consolidation
The drugstore industry in China is highly fragmented. Retail pharmacies in China include chain drugstores, individual stores, and OTC counters in retail chain stores and supermarkets. While pharmacy chain stores and retail chain stores with OTC counters are expanding quickly, neither format has developed a nationwide presence in China. The NDRC reported that as of December 31, 2004, 7,445 pharmaceutical product wholesalers, 1,410 pharmacy chain stores and 58,065 individual pharmaceutical product retailers have obtained Good Supply Practices certification. According to a White Paper entitled “Status Quo of Drug Supervision in China” dated July 18, 2008 and issued by the PRC Information Office of the State Council, there were more than 340,000 retail pharmaceutical stores in China in 2007. (See http://former.sfda.gov.cn/cmsweb/webportal/W205/A64028182.html). Given the level of fragmentation and increased regulatory requirements, the Company believes retailers with an effective regional presence and a strong reputation are likely to thrive.
Non-Pharmaceutical Sales Opportunity at Retail Pharmacies
We believe drugstore non-pharmaceutical merchandise, combined with prescription and non-prescription drugs, provides customers with a complete wellness solution. Non-pharmaceutical merchandise includes nutrition supplements, beauty, cosmetics and fragrance products, personal care products, as well as consumable, seasonal, promotional and other non-prescription products.
47
Challenges for the Drugstore Industry in China and Increased Competition
While the Chinese economy in general and the drugstore industry in particular have grown significantly in the past decade, such growth may not continue in the future. The drugstore industry in China faces a number of challenges, including:
|
●
|
Competition in the retail drugstore market in China may also intensify;
|
|
●
|
Industry reforms aimed to meet China’s commitments under WTO may foster increased competition from multinational pharmacy chains at the expense of China-based pharmacy chains; and
|
|
●
|
Current PRC laws and regulations limit any foreign investor’s ownership of drugstores to 49.0% if the foreign investor owns interests in more than 30 drugstores in China that sell a variety of branded pharmaceutical products sourced from different suppliers. If this restriction is relaxed or eliminated, there may be increasing competition from large foreign drugstore chains which intend to enter into the drugstore industry in China.
|
The growth profile of Guangxi province is based on the following three factors:
|
●
|
According to data published by the National Bureau of Statistics, Guangxi Province’s GDP was RMB770 billion ($112 billion) in 2009. GDP per capita in Guangxi Province was RMB15,821 ($2,316) in 2009 as compared with GDP per capital of RMB42,141 ($6,169) in the coastal regions (including Fujian Province, Guangdong Province, Hainan Province, Jiangsu Province, Shandong Province, Shanghai, and Zhejiang Province). In 2009 Guangxi Province’s GDP growth rate was 13.9% as compared to an average GDP growth rate of 10.7% in the coastal regions in 2009. In 2009 Guangxi Province had a population of 48.16 million. In 2009 the population growth rate of Guangxi Province was 7.2% as compared with a population growth rate of 5.17% in the coastal regions. (See http://www.stats.gov.cn/tjsj/ndsj/2009/indexch.htm);
|
|
●
|
the inflation rate in China in 2010 is projected to be 3.5% to 4% based upon the World Bank “China Quarterly Update” from March 17, 2010.
(See http://siteresources.worldbank.org/CHINAEXTN/ Resources/318949-1268688634523/CQU_march2010.pdf); and
|
|
●
|
the general pharmaceutical industry growth rate resulting from the RMB850 billion healthcare reform bill passed by the Chinese government.
|
Manufacturing Facility
We have land use rights to the 40,000 square meters of land on which our manufacturing facilities are located that are granted and allocated to us by the government. According to Chinese law, the government owns all the land in China and companies or individuals are authorized to use the land only through land use rights granted or allocated by, or leased from, the PRC government. We currently have adequate manufacturing capacity for our marketed products.
Our production plant maintains Good Manufacturing Practice (“GMP”) certification authorized by the national accreditation bodies of the PRC. A GMP-certified facility operates under the GMP parameters prescribed by the institution granting such certification. GMP parameters are operating standards that are formed to ensure product quality, by regulating the manufacturing space, the storage warehouse for raw materials and finished products, and laboratory areas of the production facility. Hefeng Pharmaceutical operates our production line and holds a general GMP Certificate that was issued on July 14, 2009 and will expire on July 13, 2014, and also holds a GMP Certificate for Small Volume Parental Solution that was issued on July 10, 2006 and will expire on July 9, 2011. Both GMP Certificates will be subject to renewal for an additional five-year term.
Raw Materials
We require a supply of quality raw materials to manufacture our products. Historically, we have not had difficulty obtaining raw materials from suppliers. Currently, we rely on numerous suppliers to deliver our required raw materials. We typically enter into one-year contracts with numerous suppliers in China to secure a steady supply of raw materials throughout the year.
48
Target Market
Our business operations are located in Guangxi province which hosts many second and third-tier cities with less competition in the market of manufacture and distribution of pharmaceutical products. Through our experience in operating in such a business environment, we have accumulated extensive business operating experience in developing a market in second- and third-tier cities and rural areas, and have built a strong reputation and brand name awareness in Guangxi province. Moreover, we have not only gained valuable experience in operational management, but also built up a strong sales network in Guangxi provision. With the brand name and leading position we have established in Guangxi province, we will continue building and expanding our retail and wholesale business in the second- and third-tier cities and the rural areas in Guangxi province through our current retail stores and the new stores that we may acquire in the future. Based on continued forecasted growth in Guangxi province, we may apply the business model we have established in Guangxi province to our business expansion in the second- and third-tier cities and the rural areas of our contiguous provinces, such as Yunnan or Huainan provinces.
Marketing and Sales
We manufacture and market 19 products, consisting of prescription and over-the-counter pharmaceuticals. Our pharmaceutical products are marketed to hospitals, clinics, pharmacies and retail stores. We maintain 22 sales offices throughout Guangxi Province and employ approximately 53 sales and marketing professionals. Where appropriate, we leverage the synergies between complementary products and distribution channels to accelerate the market penetration of our new products.
With respect to our retail stores, our marketing and promotion strategy is to build brand recognition, increase customer traffic to our stores, attract new customers, build strong customer loyalty, maximize repeat customer visits and develop incremental revenue opportunities. We work with vendors to organize promotional campaigns, and vendors typically assign sales persons to our retails stores for these activities. We also hold promotional functions, including price reductions and free gifts, during major Chinese holidays. We also place advertisements in local newspapers regarding our promotions.
Competition
We believe that we are well positioned to compete in the fast-developing Chinese pharmaceutical market with our strong brand, diverse product portfolio, research and development capabilities, established sales and marketing network and favorable cost structure. We believe that competition and leadership in our industry are based on managerial and technological expertise, and the ability to identify and exploit commercially viable products. Other factors affecting our competitive position include time to market, patent position, product efficacy, safety, convenience, reliability, availability and pricing.
Retail Pharmacy
The pharmaceutical industry in China is intensely competitive, rapidly evolving and highly fragmented. In many large cities in China, we need to not only compete with other retail drugstores, but also face increasing competition pressure from discount stores, convenience stores and supermarkets. In order to maintain our competitive position in the market, we have increasingly diversified products and services by offering some non-drug products that are provided in regular convenience stores. In addition, we also increased our competitiveness through careful selection of store location, merchandise, and services.
With the continuous consolidation of the pharmaceutical industry and opening of new drugstore chains in large cities, we will face more competition in the industry. However, in many of our targeted second- and third- tier cities and rural areas, we are facing less competition because major drugstore chains have not entered into the market. We are in a good position to establish our standing and reputation in these targeted markets. In addition, the pharmaceutical industry has entrance barriers for new entrants due to the requirements for such resources as capital, brand name and management expertise. Further, PRC laws and regulations limit a foreign investor’s ownership in retail drugstores to the maximum of 49.0% if such investor holds ownership interest in more than 30 drug stores that sell a variety of branded drugs sourced from different suppliers. This limitation, together with the complexity of the Chinese market, creates a barrier for foreign retail drugstore chain operators to enter into the PRC market. As a result, currently we do not face notable competition from foreign owned drugstore chains.
49
Because our network covers many cities and areas, and many drugstore groups are regional, our competitors vary from region to region. Each region can have its own, among others, distinct demographics, local regulations and shopping style. We do not consider any individual regional drugstores as our major competitor, but we compete with them on an aggregate basis. Our main competitors in Guangxi province are Sinopharma Liuzhou Branch and Sinopharma Nanning Branch, Liuzhou Medical and Pharmaceutical Limited on wholesale side; Shenzhen Accordance Pharm. Chain Store Inc., and Hunan Laobaixing Pharmacy Chain on retail side.
Manufacturing of Pharmaceutical Products
In the pharmaceutical manufacturing business, we compete in general with Harbin Pharmaceutical Group Co. Ltd., Sixth Pharma Factory, Guangdong Boluo Xianfeng Pharmaceutical Group and Jiangsu Chia Tai Tianqing Pharmaceutical.
We also compete with other manufacturers in each specific drug category. For instance, although we are the sole authorized producer of Corydalis Saxicola Bunting (Yanhuanglian), which is the preferred drug treating chronic hepatitis A, B and C, there are drugs that have a similar medical effect for treating hepatitis.
The following table lists the primary competitors for our best selling products produced by Hefeng Pharmaceutical:
|
Our Product
|
Competitor’s Product - Company
|
|
|
Yanhuanglian Injenction
|
Haimingwe Interferon - Qindao Haier Pharmaceutical
Handadang - Lianyungang Shentiantang Pharmaceutical Group
Tianqing Fuxing Marine and Glucose Injection - Jiangsu Chia-tai Tiangqing Pharmaceutical
|
|
|
We are the only manufacturer of our Yanhuanglian product. These competitors produce alternative products.
|
||
|
Tabellae Sarcandrae
|
Shanghai Xingcheng Jiahua Pharmaceutical Co. Ltd
Jiangxi Tianshi Chinese Medicine Co. Lt
Zhejiang Guojing Pharmaceutical Co. Ltd
|
|
|
These companies rely solely on their in-house sales force. We believe that our wholesale network enables us to gain market share more quickly.
|
||
|
Hydroxycamp-totbecine Injection
|
Wuhan Lishizhen Pharmaceutical Co. Ltd
Guizhou Hanfang Pharmaceutical Co. Ltd
Huangshi Feiyun Pharmaceutical Co. Ltd
|
|
|
Ethacridine Lactate Injection
|
Jiangsu Tianmiao Disainuo Pharmaceutical Co. Ltd
Qinghai Pharmaceutical Manufacturing Co. Ltd
|
|
|
We believe we are the leading supplier of this product in Guanxi Province because these competitors have not been able to sustain their production from time to time.
|
||
|
Rotandine Sulfate Injection
|
Guangdong Boluo Xianfeng Pharmaceutical Group Ltd
Guangxi Nanning Baihui Pharmaceutical Group Ltd
|
50
Pharmaceutical Distribution
There are relatively well capitalized and established players in the pharmaceutical distribution business, such as Sinopharm Group Co. Ltd, Liuzhou Medical and Pharmaceutical Limited and Jointtown Pharmaceutical group, which have built an intensive nationwide network while our distribution business is relatively regionally strong. Jointtown Pharmaceutical Group does not have any subsidiaries in Guangxi Province. Sinopharm Group has two subsidiaries in Guangxi Province, in Liuzhou Branch and Nanning. Sinopham specializes in large volume, low margin bulk sales to regional distributors, while we specialize in higher margin end customers, including direct sales to hospitals.
Government Regulation
We are subject to various Chinese laws and regulations pertaining to the pharmaceutical industry. We have attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in China.
In 1998, the PRC State Food and Drug Administration (“SFDA”) introduced the GMP Certificate in order to promote quality and safety of pharmaceutical production. Good Manufacturing Practices were revised in July and October, 2004. We are required to meet GMP standards in order to continue manufacturing pharmaceutical products and health foods. For each new product, we prepare documentation of pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to the provincial food and drug administration. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial food and drug administration, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation and tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years.
The GMP certificate is valid for a term of five years, the pharmaceutical products production permits are subject to renewal every five years, and the health food production permits are valid for three-year terms, and each must be renewed before its expiration, if applicable. If our GMP certificate expires without renewal, we will not be able to continue manufacturing pharmaceutical products, which will cause our production operations to be terminated.
In addition, a distributor of pharmaceutical products in China must obtain a pharmaceutical distribution permit from the relevant provincial or local SFDA branches. The distribution permit is granted if the relevant SFDA provincial branch receives satisfactory inspection results of the distributor’s facilities, warehouse, hygiene environment, quality control systems, personnel and equipment. A pharmaceutical distribution permit is valid for five years.
The SFDA applies Good Supply Practice (“GSP”) standards to all pharmaceutical wholesale distributors as well as to retail to ensure the quality of distribution in China. The currently applicable GSP standards require pharmaceutical distributors to implement controls on the distribution of medicine, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. A certificate for GSP standards, or GSP certificate, is valid for five years, except for a newly established pharmaceutical distribution company, for which the GSP certificate is valid for only one year. If our GSP certificate expires without renewal, we will not be able to continue distributing pharmaceutical products, which will cause our wholesale and retail distribution to be terminated.
Competitive Advantages
As a leading pharmaceutical distributor in the region, we are well-positioned to benefit from the strong growth, consolidation, and regulatory reform in the PRC pharmaceutical and healthcare industry.
51
The PRC healthcare market is one of the fastest-growing healthcare markets in the world, driven by China’s rapidly growing economy, rising living standards, increased health consciousness, large aging population and proactive government policies. Furthermore, the PRC Government recently announced a reform plan to spend RMB850 billion on healthcare in addition to the regular healthcare budget from 2009 to 2011, in order to increase the availability of healthcare, basic medicines and health insurance coverage for people in China. As a comparison, in 2007, the total healthcare expenditure in China was approximately RMB1.1 trillion, of which approximately RMB230 billion was government spending, according to the Ministry of Health. The healthcare reform plan is expected to accelerate growth in the PRC pharmaceutical industry not only by the increased government spending, but also by the expected increases in private healthcare spending stimulated by larger government subsidies to PRC residents, as per capita healthcare spending remains much lower than in developed countries. We are well-positioned to capture business opportunities resulting from this fast growing market.
Building up a modernized logistic, a streamlined supply chain and increased capital entrance barrier for smaller competitors to further strengthen our leading position and differentiate in the region.
In addition, China’s pharmaceutical distribution market is highly fragmented and is characterized by inefficient supply chains. The highly fragmented pharmaceutical distribution industry has recently commenced a process of consolidation, which has led to an increase in market share of the pharmaceutical distributors in the market, we expect the PRC pharmaceutical distribution market to continue to consolidate into one with larger and more efficient distributors. In addition, we expect the healthcare reform plan to promote further consolidation, as it calls for reducing the number of layers between manufacturers and consumers of medicines. We believe that we have the scale, industry standing, brand and financial strength to compete effectively during this process of consolidation. The PRC Government has also adopted measures to raise the operating standards of pharmaceutical companies and promote the quality of distribution of pharmaceutical products in China, in order to ensure a stable supply of safe, effective medicines at reasonable prices. We believe we will benefit from future regulatory reforms, which require pharmaceutical manufacturers and distributors to implement more stringent standards on the manufacturing and distribution of pharmaceutical products. Unlike smaller distributors, we have a large-scale distribution network, high quality equipment and facilities, leading management and qualified personnel, which are required to satisfy the higher standards. These attributes also provide us with a competitive edge over our competitors.
We plan to invest in a modernized logistics center. We estimate the costs of the center to be approximately RMB 150 million. We have not committed to specific timing for our implementation of this advanced logistics center. The timing will depend on the availability of funding, which may come from cash generated from operations, private or public financing or bank loans.
Leading Product and Brand Name in Diversified Area
Over the years, we have developed and introduced a number of pharmaceutical products under the brand name Asio (亚太), which we believe are the leading products in their respective market segments, including Levodopa, which treats the stiffness, tremors, spasms, and poor muscle control of Parkinson’s disease; Hydroxycamptotbecine Injection, which we believe is one of the best choices to fight cancers of various kinds with minimal side effects; Tabellae Sarcandrae, which is called Chinese anti-biotic and has similar anti-inflammatory and antibacterial effects as anti-biotics used in Western medicine; Corydalis Saxicola Bunting (Yanhuanglian), which treats hepatitis, liver cirrhosis, liver ascites and liver cancer; and Yinge Tongmai Tea, which improves cardio-vascular condition and blood circulation, and can be taken as a normal healthy drink on a daily basis. As a result, “Asio (亚太)” has become a widely recognized brand name for our self-developed drugs in China. Among our five best-selling drugs, we are the sole producer of Corydalis Saxicola Bunting (Yanhuanglian) and Hydroxycamptotbecine Injection.
In addition, our sales and marketing teams are specialized in promoting products in different therapeutic categories. The teams have strong relationships with healthcare executives, doctors and pharmacies in their respective target markets and possess extensive sales and marketing experience in promoting prescription and non-prescription pharmaceutical products.
52
Strong Research and Development Capability
Our research and development department relies upon a research institute jointly formed by us and several renowned Chinese medicine universities, including China Medicine University located in Nanjing China, which focuses on utilizing Guangxi’s natural herbal medicine resources to produce drugs that meet the demand of the Chinese domestic market. Currently we pay for such research and development on a project by project basis, which helps us to reduce costs related to an in-house research and development team and to have access to the latest advances in this area. In 2008 and 2009 we spent approximately $0.1 million and $0.7 million, respectively on research and development.
Guangxi is a province with a wealth of natural resources for developing traditional Chinese medicine. In addition, they also used great efforts to discover advanced production process of Levodopa and Corydalis Saxicola Bunting (Yanhuanglian), to develop better understanding of the composition of Hydroxycamptotbecine and its derivatives, to develop key technology of figure print quality control, and to research the production technology of herbal medicine in limestone mountainous areas.
Currently, we are working with the research team for the development of Corydalis Saxicola Bunting Injection Powder by means of vein injection on the foundation of our current Corydalis Saxicola Bunting Injection. Pre-clinical research is now undertaken by the research team in respect of the production tactics, quality standard research, samples inspection, stability testing, package screening process development and the pre-requisite research work required before the application of clinical research. No exact timetable is available for the launch of this new drug in the market.
Extensive Wholesale and Retail Distribution Network and medi-care qualified stores
We operate wholesale and retail distribution networks covering major cities, townships, and counties of Guangxi province. Our wholesale network offers more than 10,000 pharmaceutical products to our clients. Further, BCT Retail offers more than 3,200 pharmaceutical products in 170 retail stores, holding the leading market position in many major cities of Guangxi province. Among the 170 stores, 21 stores are covered by medi-care insurance plan for citizens and employees. We’ve also signed an MOU with local authorities to be authorized to open 30 retail stores under the New Rural Cooperative Health Issuance Plan.
Economic Scope and Integration Ability
We believe we have the potential to expand our scale by acquiring more wholesale and retail networks. By acquisition of a production facility in 2008, we have established a full-integrated operation. Established in the early 1970s, our production facility has accumulated a large reserve of production licenses, established an experienced management team, formed a strong research and development team, and developed solid relationships with local raw material providers. This integration enables us to enjoy economies of scale and also provides us with an ability to increase our operating efficiency in order to sustain strong profit margins and favorable growth rates.
Experienced Management Team with Proven Track Record
Over the past decade, Mr. Hui Tian Tang, Chairman of our board of directors and other members of our senior management team have provided successful leadership in our operations and increased our revenue and profit. Many members of our senior management team have worked with us since our inception or otherwise have broad experience in the retail industry, and have developed extensive expertise in operating a national chain of drugstores, which is important to our future development. The Chief Operating Officer of Liuzhou BCT, Jing Hua Li, has extensive experience in chain store retailing, gained from his four years service with Meidong Bio-technology (AOB), a publicly held multinational pharmaceutical corporation. In addition, the majority of our mid-level managers and managers of our regional operations and stores have been with us for many years. These managers have obtained extensive experience through our internal management training system and real practice in managing retail stores and distribution centers.
53
Well-established Good Supply Practices (GSP) control system
Our logistics system is established in line with GSP. We also have a full-time GSP supervision body comprised of professionals with professional pharmacist qualification or pharmacist-in-charge titles. They are familiar with national laws and regulations on medicine quality, and have extensive knowledge and practical experience in the implementation of GSP in the enterprise.
Professional storage, advanced transportation equipment, comprehensive management system and detailed flow records are integrated with hardware and software of the pharmaceutical business. The medicine warehouse occupies an area of 4,800 square meters, including warehouses of room temperature (0-30°C), cool (<20°C) and cold (2-8°C), stock control and subsidiary operation area, chemical laboratory and has advanced instruments and facilities.
GSP, which can be called a total quality control system for the pharmaceutical industry, is strictly observed from purchase to sale to storage, forming a secure tunnel for the medicine transportation and guaranteeing medicine quality.
Growth Strategy
Expand Sales Networks targeting the Second and Third Tier Cities and Rural Markets
We believe that maintaining a large number of retail stores in desirable geographic markets is essential to our competitiveness and our ability to increase our profitability. We are attempting to significantly expand our market presence in our contiguous provinces in China by effectively leveraging our existing operating infrastructure.
Maintain and Improve Customer Loyalty with Effective Marketing and Promotional Programs
We believe that a strong brand name is critical to obtaining customers’ trust in our business, as well as building customer loyalty and increasing customer visits to our stores. As a result, we intend to continue promoting aggressively and effectively both our brand name and our private label products. Specifically, we will continue to deploy the following marketing and promotional initiatives:
1. adopting advanced category management by focusing on seasonal and cross-merchandising, and offering a wider selection of products;
2. offering services that are carefully tailored to our customers’ healthcare needs, including integrated health programs focused on health supplements, weight management, diabetes, infant care and birth control;
3. enhancing our customer loyalty by organizing community-based activities and targeted promotion programs;
4. using data mining techniques to tailor relevant promotional offers to our target customers, especially our loyalty members;
5. enlarging the number of our promotional partners and developing additional promotional campaigns with these partners; and
6. advertising our brand and private label products in selected newspapers that service our targeted cities.
54
Selectively Pursue Complementary Acquisitions of Chain Stores
We currently plan to selectively acquire drugstore chains or independently operated drugstores that complement our existing store network or help us to establish a presence in new markets. In particular, we plan to grow through first acquiring similar businesses in the cities in Guangxi province and then acquiring business targets outside of the Guangxi province. We target retail chains or individual stores in prime locations and with good brand names, well-developed facilities and customer bases that are complementary to ours, and which are commercially attractive. We believe that our relationship with many industry participants and our knowledge of, and operational expertise in, the drugstore market in China will assist us in making acquisitions. We also believe that we can rapidly and successfully integrate newly acquired stores into our current distribution network and quickly realize operating and financial benefits.
Invest in more advanced logistics and information management systems to improve cost and operating efficiencies
We intend to invest in an advanced logistic center. In Liuzhou city first, we plan to establish regional and provincial logistics facilities equipped with leading technology and information systems. We believe that these improvements will help to provide a direct and high-speed information exchange channel that connects us to our customers and suppliers throughout China, covering each stage of our pharmaceutical distribution operations, including electronic order entry, invoice preparation, purchasing, inventory tracking, GSP-certified warehousing and logistics and delivery arrangements. Once these improvements are completed, we believe that we will be able to shorten delivery lead-times, increase responsiveness to customer demands and reduce distribution and selling expenses.
We have not adopted a specific timetable for implementing the logistic center project, and the expansion plan with respect to our retail stores is our first priority to be carried out in the short term. The timing of the capital investment of the modernized logistic center is subject to two principal factors:
|
●
|
We believe that we must have sufficient scale to justify the investment in modernizing our logistic center. In order to optimize the return on our investment in a modernized logistics center, we will continue to analyze the return on our potential investment and the timing of the modernization.
|
|
●
|
The timetable for the enforcement by the Chinese FDA on requiring modernized logistic centers to be built in order to qualify as a distributor participating in the centralized bid tendering process. We intend to closely monitor the relevant policies, in order to make timely plans for implementing the modernized logistic center. Currently, we do not have an indication of when such a requirement will be put in place as the policy is still in the discussion stage.
|
In addition, the timing will depend on the availability of funding, which may come from cash generated from operations, private or public financing or bank loans.
Continue to build upon our integrated business platform in order to enhance the synergies between our pharmaceutical distribution, retail pharmacy and other businesses
We will continue to build upon our integrated business platform in order to enhance the synergies that arise from our pharmaceutical operations spanning the distribution, retail and manufacturing of pharmaceutical and healthcare products and expand our reach to end-customers. We plan to leverage our existing businesses to capitalize on particular opportunities that may arise and create efficiencies and cost savings in our business operations. We also plan to utilize our extensive distribution network to provide reliable supply channels for our retail drug stores which, in turn, will sell the pharmaceutical and healthcare products supplied by our distribution network. Further, we intend to utilize our pharmaceutical manufacturing operations to produce private-label products for our retail pharmacy operations. We expect to maintain the flexibility to reallocate manufacturing capacity of our products in response to potential changes in supply and demand, as well as to control inventory in a way that enables us to meet expected demand for our products. With operations in multiple segments of the pharmaceutical industry, we are able to maintain control over the quality, supply chain management and marketing of our products. We believe that our integrated business platform maximizes the synergies between our businesses by optimizing our operational efficiency while reducing the costs of our customers, allowing us to further solidify our leadership in the PRC pharmaceutical distribution industry.
55
Intellectual Property
We believe our rights to our trade names and trademarks are the most important factors in implementing our marketing strategy. Our company’s name, Baicaotang (百草堂), means “Place for all kind of Chinese Herbal Medicines” in Chinese. We registered “Baicaotang (百草堂)” as the trademark for our business group, and Asio (亚太) as the trademark for the medicines that our production plant produces. The extension of the Baicaotang (百草堂) trademark was granted on May 5, 2008 and expires on July 13, 2018. The Asio (亚太) trademark was granted on August 15, 2005 and expires on August 15, 2015.
Under the PRC law, we have the exclusive right to use a trademark for products and services for which the trademark has been registered with the State Administration for Industry and Commerce. Trademark registration is valid for 10 years, starting from the day the registration is approved. For renewal of registration, an application shall be made within 6 months before the expiration of such 10 years. If no application is made within that period, an extension of 6 months may be granted. We also rely on trade secrets to protect our know-how and other proprietary information.
One of our products, Tabellae Sarcandrae, has protection as a Traditional Chinese Medicine which was granted on December 19, 2006 and expires on August 1, 2012 and is renewable. During the period of the protection, only the company which has been granted the right of protection can manufacture the specified product during the protection period. When we undertake the renewal process, we apply to the local authority and then to the Ministry of Public Health which assigns the National Committee on the Assessment of the Protected TCM Products to inspect our facilities and announce whether their report satisfies the renewal criteria. In order to apply for this certificate, we need to provide certain information such as the GMP certificate, research data, details of the protected drugs, and a safety assessment.
We currently have no patents or pending patent applications.
Employees
As of the date of this prospectus, we had 1,180 full-time employees and no part-time employees.
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants or allocates landholders a “land use right,” which we sometimes refer to informally as land ownership. There are four ways of acquiring land use rights in the PRC:
|
●
|
Grant of the right to use land;
|
|
●
|
Assignment of the right to use land;
|
|
●
|
Lease of the right to use land; and
|
|
●
|
Allocation of the right to use land.
|
Granted land use rights are provided by the government in exchange for a grant fee, and carry the rights to pledge, mortgage, lease and transfer the land within the term of the grant. Land is granted for a fixed term, generally 70 years for residential use, 50 years for industrial use, and 40 years for commercial and other use. The term is renewable in theory. Unlike in western nations, granted land must be used for the specific purpose for which it was granted.
Allocated land use rights are generally provided by the government for an indefinite period (usually to state-owned entities) and cannot be pledged, mortgaged, leased, or transferred by the user unless otherwise approved by the competent government authorities. Allocated land can be reclaimed by the government at any time. Allocated land use rights may be converted into granted land use rights upon the payment of a grant fee to the government.
56
Our land use rights are set forth below:
Liuzhou BCT
Liuzhou BCT owns a 2,753.5 square meter land use rights, the term of which expires on November 14, 2053, located at No. 102, Chengzhan Road, Liuzhou City, Guangxi Province, PRC, as its corporate headquarter. In addition, Liuzhou Baicaotang also owns other properties listed below:
|
1.
|
Approximately 321.7 square meter land use rights, the term of which expires on March 8, 2047, located at Building 2, 197 No. 3 Middle Road, Liuzhou City, Guangxi province;
|
|
2.
|
Approximately 10.7 square meter land use rights, the term of which expires on December 28, 2044, located at No. 1-3 XingLong Building, Zhongshan Middle Rd., Liuzhou City, Guangxi province;
|
|
3.
|
Approximately 75.2 square meter land use rights, the term of which expires on August 4, 2043, located at Floor 1, 197 No. 3 Middle Road, Liuzhou City, Guangxi province;
|
|
4.
|
Approximately 346.85 square meter land use rights, located at No. 10 Shizi Rd., Luzhai Town, Luzhai County, Guangxi province;
|
|
5.
|
Approximately 5,655.6 square meter land use rights, the term of which expires on November 14, 2053, located at No. 6 Changfeng Rd. Liuzhou City, Guangxi province;
|
|
6.
|
Approximately 886.7 square meter land use rights, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
7.
|
Approximately 1,308.5 square meter land use rights, the term of which expires on August 4, 2053, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
8.
|
Approximately 1,084.5 square meter land use rights, the term of which expires on November 14, 2053, located at No. 4 Changfeng Rd., Liuzhou City, Guangxi province;
|
|
9.
|
Approximately 1,558.05 square meter land use rights, the term of which expires on May 28, 2057, located at Desheng Village, Litang Town Bingyang County, Guangxi province;
|
|
10.
|
Approximately 380.6 square meter land use rights, the term of which expires on August 4, 2043, located at No. 15 May First Rd,, Liuzhou City, Guangxi province.
|
Hefeng Pharmaceutical
Hefeng Pharmaceutical owns a 44,982.18 square meter business facility located at 3 Development District, Donglan County, which is used as its principal executive offices and plant.
BCT Retail
The principal executive offices of BCT Retail are located at No. 102, Chengzhan Road, Liuzhou City, Guangxi Province, PRC. The office space that BCT Retail is using is owned by Liuzhou BCT. BCT Retail has used the office space free of charge and without any lease agreement. BCT Retail currently operates 170 retail chain stores. Most of the chain stores managed by BCT Retail are located in the following towns, counties and municipal cities: Liuzhou City, Ronghui County, Sangjiang County, Liujiang County, Nandan County, Yongfu County, Bama County, Binyang County, Yongan County, Laibin City, Zhaoping Country and Rongan County.
57
BCT Retail's retail chain stores range in size from 85 square meters to 168 square meters. Except for two retail stores which are owned by Liuzhou BCT and used by BCT Retail free of charge and without any lease agreement, all other chain stores are leased. The monthly rents of the chain stores range from approximately $140.00 to $2,500.00, and most of the leases have a three year term. All the lease agreements have similar terms and provisions.
We are currently not involved in any litigation that we believe could have a material adverse effect on our financial condition or results of operations. To our knowledge, there is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of our executive officers or any of our subsidiaries, threatened against or affecting our company, our common stock, any of our subsidiaries or any of our companies or their subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
We were originally incorporated in the State of Delaware on November 30, 2006 under the name Purden Lake Resource Corp. to engage in the acquisition, exploration and development of natural resource properties. We ceased our operations because we had not commenced any exploration activities, and as a result prior to December 30, 2009 we were a “blank check” company with nominal assets. On December 24, 2009, we changed our name to China Baicaotang Medicine Limited and to China BCT Pharmacy Group, Inc. on March 25, 2010.
Our wholly-owned subsidiary, Ingenious, was incorporated under the laws of the British Virgin Islands on May 29, 2008. Ingenious owns 100% of the issued and outstanding capital stock of Forever Well, a Hong Kong company incorporated on January 10, 2008. Forever Well is the sole shareholder of Liuzhou BCT a PRC limited company established on April 3, 1986. Liuzhou BCT contributed 100% of the registered capital of Hefeng Pharmaceutical, a PRC company established on September 18, 2000 and 49% of the registered capital of BCT Retail, a PRC company established on October 30, 2001. The remaining 51% of the registered capital of BCT Retail was contributed by Property Management, an affiliate of Liuzhou BCT.
The chart below illustrates the current structure of the Company:
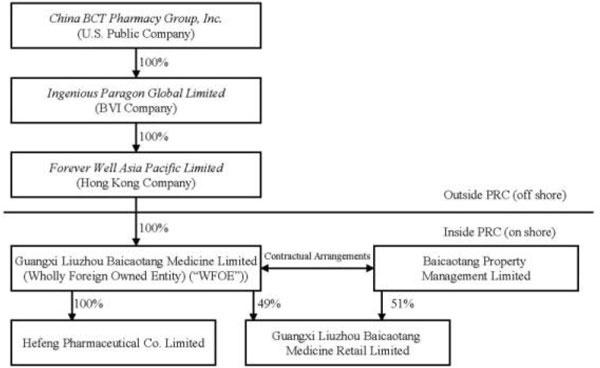
58
The Reorganization
In 2008, the shareholders of Liuzhou BCT (the “Liuzhou BCT Shareholders”) and Xiaoyan Zhang, our CFO, developed a restructuring plan (the “Restructuring”). The goal of the Restructuring was to obtain the benefits of a U.S. public company by entering into a transaction with a public shell company in the United States by which we, the public shell company, would acquire operations based in the PRC, all in compliance with PRC law.
At that time, Ms. Zhang, who is a citizen of Hong Kong, was the sole shareholder of Ingenious, which had no assets or operations and owned 100% of Forever Well as the result of Ms Zhang and the Liuzhou BCT Shareholders having completed in the first two steps of a three-step Restructuring.
The first step was for Forever Well to acquire 100% of the equity interests of Liuzhou BCT and its subsidiaries (the “PRC Operating Companies”). Liuzhou BCT was owned at that time by the Liuzhou BCT Shareholders, certain former and current employees and directors of Liuzhou BCT.
In this step, Forever Well, a Hong Kong company formed by Mr. Ping-Ki Yue, was to acquire the PRC Operating Companies. The second step in the Restructuring was for Ingenious, which was 100% owned by Ms Zhang, to acquire Forever Well and the third step was for Ingenious to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious.
The first step was completed in conjunction with the second step so that as the PRC Companies became subsidiaries of Forever Well, Forever Well was acquired by Ingenious. As part of the second step of the Restructuring, the Liuzhou BCT Shareholders entered into an earn-in agreement (the “Earn-In Agreement”) which provided the Liuzhou BCT Shareholders with a process under which they could purchase for a nominal amount the shares of common stock held by Ms. Zhang. Thereafter Ingenious could undertake the third and final step of the Restructuring to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious.
The Restructuring and the acquisition of Liuzhou BCT was structured to comply with the PRC M&A Laws. Under the PRC M&A Laws, the acquisition of PRC companies by foreign companies that are controlled by PRC citizens who are affiliated with the PRC companies is strictly regulated and requires approval from the Ministry of Commerce, which approval is burdensome to obtain. Such restrictions however, do not apply to foreign entities which are controlled by foreign persons. These restrictions apply only at a “snapshot in time” that occurs at the time PRC companies are acquired by a foreign entity. In our case this was effective August 4, 2008 when Forever Well acquired 100% of the equity interest of Liuzhou BCT from the 15 Liuzhou BCT Shareholders for aggregate consideration of RMB10,000,000 (approximately $1,470,588) which was the registered and fully paid up capital of Liuzhou BCT. At that time Forever Well was owned 100% by Ingenious, and Ingenious was owned 100% by Ms. Zhang, a Hong Kong citizen. Therefore this transaction was a pure cross-border transaction governed by and permitted under the 2006 PRC M&A regulations and the acquisition had been approved by the Ministry of Commerce on June 13, 2008.
Since PRC M&A Laws would have prohibited the Liuzhou BCT Shareholders who were PRC citizens from immediately receiving a controlling interest in Ingenious in a share exchange as consideration for the sale of their interest in Liuzhou BCT, Liuzhou BCT Shareholders holding a majority of the equity interest in Liuzhou BCT and Ms. Zhang instead agreed that they would enter into an Earn-In Agreement to grant those Liuzhou BCT Shareholders a call right to acquire up to all of Ms. Zhang’s interest in Ingenious (or a public parent company of Ingenious, as the case may be) after the acquisition of Liuzhou BCT was consummated in compliance with PRC law. Because all of the Liuzhou BCT shareholders were PRC citizens, a majority of Liuzhou BCT shareholders would not have been permitted to immediately receive shares in Forever Well or in Ingenious in exchange for their interests in Liuzhou BCT. However, there is no prohibition under PRC laws for those Liuzhou BCT shareholders to earn an interest in the Company after the acquisition of Liuzhou BCT was consummated.
59
As noted above at "Prospectus Summary - Corporate Structure and History", as part of the first and second steps of Restructuring, the Liuzhou BCT Shareholders entered into an earn-in agreement. The earn-in agreement was succeeded by the earn-in agreement developed in connection with the Share Exchange (the “Earn-In Agreement”). It, like its successor, provided the Liuzhou BCT Shareholders with a process under which they could purchase for a nominal amount the shares held by Ms. Zhang. The Earn-In Agreement provides for the Liuzhou BCT Shareholders to obtain legal ownership of their proportionate number of our shares issued to Ms. Zhang in the Share Exchange. Ms. Zhang, however, has all of the governance rights to the shares and has elected the present directors of the Company.
On October 22, 2009 Ms. Zhang and certain Liuzhou BCT Shareholders entered into the Earn-In Agreement, which was amended on December 30, 2009 to extend the performance targets from 2009 and 2010 fiscal years to 2010 and 2011 fiscal years because the 2009 performance target was not going to be met. The Earn-in Agreement was further amended on May 19, 2010 to decrease the performance targets for the 2010 and 2011 fiscal years. The amendments to the Earn-in Agreement were made to increase the likelihood that the Liuzhou BCT Shareholders would all be able to earn back their shares in the Company, which was the goal and purpose of the reorganization. These amendments were made without additional consideration being paid
The Earn-in Agreement enables those Liuzhou BCT Shareholders to purchase shares of Ingenious (or its public parent company) from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $15 million and $19 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT Shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met, the Liuzhou BCT Shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT Shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Ms. Zhang may not transfer the shares during the five-year term of the Earn-In Agreement and in the event that all of the shares have not been delivered to the Liuzhou BCT Shareholders, Ms. Zhang can only transfer them as she and the Liuzhou BCT Shareholders agree.
After completion of the first and second steps in the Restructuring, the parties concluded that they needed another agreement to complete the Earn-In Agreement and the restructuring process. They therefore entered into the so-called "entrust shareholding" agreements with respect to each of the first and second steps; they made these agreements effective as of the effective date of the first and second steps. These agreements recited that a third-party would maintain the governance rights and economic benefits of the Liuzhou BCT Shareholders during the period of the Earn-In Agreement. The two entrust shareholding agreements were identical except that the third party in the first was Mr. Ping Ki Yue and in the second was Ms. Zhang. The first agreement had been supplanted by the second even at the time it was signed. Under the second agreement, Ms. Zhang, as owner of the shares, had all voting power and, upon notice from her with respect to a matter, the former shareholders might choose to express a preference as to how she would vote the shares; the agreement also confirmed that the former shareholders’ economic interest in the shares was preserved as in the Earn-In Agreement.
These agreements were in fact never utilized and the parties determined that they had been mistaken in their decision to create them and that the Earn-In Agreement expresses entirely the parties’ relationship with respect to the shares and with each other. Representatives of the Liuzhou BCT Shareholders hold the majority of seats on the board of the Company. The Earn-In Agreement reflects the intent and purpose of the parties in undertaking and accomplishing the Restructuring. And following the accomplishment of the third step in the Restructuring, the Earn-In Agreement is the operative agreement for all purposes with respect to the relationship of the Liuzhou BCT Shareholders to the Company. The parties having recognized the mistake, for all of these reasons, the entrust shareholding agreements were rescinded as of their effective dates and the Earn-In Agreement governs the rights of the Liuzhou BCT Shareholders with respect to the shares held by Ms. Zhang.
Under the Earn-in Agreement, Ms. Zhang has legal title to the shares and there are no limits on her voting rights with respect to the shares. Under the Earn-In Agreement, the Liuzhou BCT Shareholders have the right to obtain the economic benefits of the shares by purchasing the shares upon the Company's attaining the low financial thresholds in the agreement which trigger their purchase rights. Ms. Zhang also has no authority to transfer the shares. This can only be done with the agreement of the Liuzhou BCT Shareholders. Therefore, were the Company not to attain the thresholds that have been placed in the Earn-In Agreement, we anticipate that the agreement would be further modified to establish thresholds that were or are attainable.
On December 30, 2009, the goal of the Restructuring was realized when we entered into and completed a share exchange agreement with Ingenious. At that time we were controlled by our then president and CEO, Lisa Lopomo, who owned 54.5% of our common stock. Pursuant to the share exchange agreement, we acquired 100% of the equity of Ingenious in exchange for the issuance of an aggregate of 32, 000,000 shares of our common stock to Ms. Zhang and to certain Liuzhou BCT Shareholders. As of the date of this prospectus, Ms. Zhang owns 58.9% of our common stock all of which is subject to the provisions of the Earn-In Agreement. As a result of this transaction, we are a holding company which, through our direct and indirect ownership of Ingenious, Forever Well, Liuzhou BCT, Hefeng Pharmaceutical and BCT Retail, now has operations based in the PRC.
Private Placement
Simultaneously with the closing of the Share Exchange, we completed the Initial Closing of the Private Placement of approximately $6.3 million or 632.3 Units. Each Unit consists of (i) 3,937 shares of common stock, and (ii) a Warrant to purchase 1,968 shares of common stock at an exercise price of $3.81 per share. Upon the Initial Closing of the Private Placement, we issued an aggregate of 2,489,370 shares of our common stock and Warrants exercisable for 1,224,368 shares of our common stock at an exercise price of $3.81 per share. In addition, in connection with the Initial Closing of the Private Placement, we issued Agent Warrants to the Co-Placement Agents that are exercisable for 248,937 shares of common stock at an exercise price of $3.05 per share, on a cash or cashless basis. The closing of the Share Exchange was a condition precedent to the closing of the Private Placement.
60
On February 1, 2010, we completed the Second Closing of the Private Placement of approximately $2.6 million or 261.61 Units with the issuance of a total of 1,029,970 shares of our common stock and Investor Warrants exercisable for 514,933 shares of our common stock at an exercise price of $3.81. In connection with the Second Closing of the Private Placement, we issued Agent Warrants to the Co-Placement Agents that are exercisable for 102,997 shares of common stock at an exercise price of $3.05 per share, on a cash or cashless basis.
Agreement to Sell Preferred Shares
On January 18, 2011, the Company entered into the Preferred Purchase Agreement with Milestone pursuant to which Milestone will purchase the Preferred Shares, for an aggregate purchase price of $30 million. The Preferred Shares carry a dividend of 5% and are convertible initially into an equal number of shares of our Common Stock at an initial conversion price of $3.20 per share. The transaction is subject to a number of closing conditions, including the completion of the amendment of our Certificate of Incorporation to increase our authorized capital to 170 million shares of capital stock consisting of 150 million shares of Common Stock (an increase of 50 million shares) and 20 million shares of blank-check preferred stock which our board of directors will have the authority to issue (the “Amendment”) and the filing of the Amendment and of the Certificate of Designation adopting the terms of the Preferred Shares. All necessary board and stockholder action to approve adoption of the Amendment have been taken and the Amendment will be complete upon filing with the Delaware Secretary of State which will occur 20 days after the mailing to our stockholders of an information statement pursuant to Section 14(c) of the Exchange Act. We filed this information statement with the SEC on January 18, 2011 and will mail it at the earliest date available in accordance with provisions of Section 14(c) and the regulations thereunder. The board has also approved the Certificate of Designation, subject to the filing of the Amendment. The Certificate of Designation will be filed after the filing of the Amendment in connection with the closing of the sale of the Preferred Shares.
In connection with this transaction, the Company and Milestone also entered into a registration rights agreement and, with certain of the Company’s principal stockholders, a shareholders agreement. The registration rights agreement provides for registration under the Securities Act of 1933, under various circumstances, of the shares of Common Stock into which the Preferred Shares can be converted. Under the Preferred Purchase Agreement, Milestone (or successor holders of the Preferred Shares) will have the right to name one director and to recommend an additional independent director to the Company’s board of directors, which we expect will be increased to seven directors after closing of the transaction. The Purchase Agreement also provides that Milestone (or successor holders) will have consent rights with respect to certain operating matters. The shareholders agreement provides limitations on the rights of the principal shareholders to engage in competing businesses in the event that they are no longer employed with the Company and also provides a process for their sale of their shares, including first offering them to the holders of the Preferred Shares and co-sale rights of the holders. Under the Certificate of Designation, the holders of Preferred Shares have pre-emptive rights with respect to future offerings and have voting rights with the Common Stock as well as separate voting rights with respect to certain extraordinary transactions. After issuance, the Preferred Shares will represent 18.5% of the outstanding share capital of the Company. These shares, together with the shares of Common Stock owned by Ms. Zhang (representing approximately 44.3% of the outstanding share capital after this transaction) will hold the majority voting interest in the Company. The principal agreements with respect to this transaction have been filed as exhibits to our report on Form 8-K describing the transaction and filed with the SEC on January 18, 2011. We anticipate that the transaction will be closed approximately 30 days after signing or as soon there after as we and Milestone determine.
Contractual Arrangements with Property Management
We do not hold a 100% direct ownership interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores. We have entered into contractual arrangements with Property Management pursuant to which the shareholders of Property Management pledged to us their equity interests in BCT Retail and provide us with the ability to effectively control BCT Retail. The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wan have an aggregate 67.2% interest in the registered share capital of Property Management, and thus controlled BCT Retail prior to the Proxy Agreement being entered into. In addition, the directors of Liuzhou BCT are the same as the directors of BCT Retail. We have been advised by our PRC legal counsel that under PRC corporate law we do not own 100% of BCT Retail. As a result of the related party control, however, as well as the contractual arrangements meeting the provisions regarding consolidation of entities controlled by contract set forth in FASB ASC 810-10-15-18 through 810-10-15-22, BCT Retail is effectively, although not legally, 100% owned by Liuzhou BCT, and therefore, BCT Retail has been included in our consolidated group.
The contractual agreements entered into by us and the Property Management include:
Share Transfer Agreement. Under this agreement dated April 1, 2008 by and between Liuzhou BCT, which held 100% equity interests of BCT Retail, and Property Management, Property Management acquired from Liuzhou BCT 51% equity interests in BCT Retail. The total amount of transfer price was RMB153,000, which was 51% of the registered share capital of BCT Retail. Within twenty (20) business days from the date when Property Management paid the transfer price, all the parties were to amend the articles of association of BCT Retail and register such equity transfer with the competent Authority of Industry and Commerce. Such amendment was made and equity transfer was registered on June 18, 2008.
Shares Pledge Agreement. Under this agreement dated May 3, 2008 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of their equity interests in BCT Retail to Liuzhou BCT to guarantee its obligations to repay a loan in the amount of RMB153,000 made from Liuzhou BCT to Property Management. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement. We intend to extend the agreement prior to its expiration on December 31, 2015.
Share Repurchase Agreement. Under this agreement dated July 31, 2008 by and between Liuzhou BCT and Property Management, Liuzhou BCT was granted a preemption right to repurchase the 51% equity interests in BCT Retail held by Property Management. The term of the preemption right is two years from the date that Property Management deregisters the pledge as a result of paying off loans under the related share pledge agreements. The repurchase price shall be equal to 51% of the registered capital of BCT retail at the time of the repurchase.
61
Shares Pledge Agreement. Under this agreement dated March 31, 2009 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interests in BCT Retail to Liuzhou BCT to guarantee its obligations under a loan in the amount of RMB 1.377 million made from Liuzhou BCT to Property Management. The loan was used by Property Management to increase its registered share capital in BCT Retail and to maintain its 51% interest. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement. We intend to extend the agreement prior to its expiration on December 31, 2015.
Agreement terminating the Shares Pledge Agreements. Under this agreement, dated March 2, 2010 by and between Liuzhou BCT, the shareholders of Property Management and Property Management, Share Pledge Agreements entered into on May 3, 2008 and March 31, 2009 between Liuzhou BCT and the shareholders of Property Management were terminated because they were entered into by error and were duplicative of the Shares Pledge Agreements entered into by Property Management on such dates as described above.
Proxy Agreement. Under this agreement, dated May 19, 2010 by and between Liuzhou BCT and Property Management, Property Management agreed to grant an irrevocable proxy to Liuzhou BCT to appoint the directors, officers and management of BCT Retail and to vote all shares of capital stock of BCT Retail. The Proxy Agreement was entered into in order to make the contractual relationship between the parties consistent with past practices and understandings between the parties as it relates to the transfer of 51% of the equity interest of China BCT to Property Management in April 2008. The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wan have an aggregate 67.2% interest in the registered share capital of Property Management, and thus controlled BCT Retail prior to the Proxy Agreement being entered into.
Additional Provisions Relating to the Private Placement
Registration Rights
Pursuant to the Subscription Agreement, on or prior to March 3, 2010 (the “Registration Statement Filing Date”), we were required to file with the Securities and Exchange Commission (the “SEC”) a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended (the “Securities Act”), (i) registering for resale by the Investors (a) the shares of common stock issued to the Investors in the Private Placement and (b) the shares of common stock underlying the Investor Warrants; and (ii) registering for resale for the Co-Placement Agents of the Private Placement and other agents, the shares of common stock underlying the Agent Warrants (all of the foregoing securities being collectively referred to herein as the “Registrable Securities”). We are required to use our best efforts to have the Registration Statement declared effective prior to the 150th day following February 1, 2010, provided, however, that in the event of a “full review” by the SEC, which we have received, we have an additional 30 days and shall have the Registration Statement declared effective prior to the 180th day following February 1, 2010 (the “Registration Effective Date”).
In the event that (i) the Registration Statement has not been (x) filed on or prior to the Registration Filing Date or (y) declared effective by the SEC on or before the Registration Effective Date; and (ii) the Registrable Securities included in such Registration Statement are not saleable under Rule 144, we shall pay to each Investor as liquidated damages, a cash payment equal to 1% of the aggregated amount invested by such Investor in the Private Placement for the first 30 days and 1% of the aggregated amount vested by such Investors in the Private Placement for every 30-day period thereafter until the Registration Statement has been filed and/or declared effective, or such proportionate percentage for any period less than 30 days. Through September 30, 2010 the liquidated damages pursuant this provision would be approximately $178,782.
62
Make Good Escrow
In addition, pursuant to the Subscription Agreement, at the closing of the Private Placement, Xiaoyan Zhang, our CFO placed 4,000,000 shares of our common stock owned by her (the “Make Good Shares”) in an escrow account administrated by an escrow (the “Make Good Escrow Agent”). In the event we fail to achieve a performance target of $26,000,000 recurring operating net income under the U.S. GAAP before any extra-ordinary gain and excluding any non-cash expenses for our fiscal year ending December 31, 2010 (the “Performance Target”), the Make Good Escrow Agent shall distribute 1,000,000 Make Good Shares to all Investors on a pro rata basis for every $1,000,000 shortfall under the Performance Target. The Make Good shares attach solely to the Investors and not to the securities issued in the Private Placement. Any Make Good Shares released to Investors would not be included in the Registration Statement of which this prospectus forms a part. If the Performance Target is met, the Make Good Shares shall be returned to Ms. Zhang.
Anti-Dilution Protection
As a result of anti-dilution protection included in the Subscription Agreement, we may be obligated to issue additional shares of common stock to the Investors. Pursuant to the Subscription Agreement, the Investors have certain anti-dilution protection from February 1, 2010 until the date that is the earlier of: (i) the effectiveness of the Registration Statement of which this prospectus forms a part, or (ii) the date on which the shares being registered on the Registration Statement may be sold under rule 144 (the “Anti-Dilution Period”). During the Anti-Dilution Period, if we sell additional shares of common stock (subject to certain exceptions) at a price per share of less than $2.54 per share (“Additional Shares”), then we are obligated to issue to each Investor that is still a holder of shares issued in the Private Placement, that number of shares of common stock, equal to the difference between (i) the aggregate purchase price paid by such Investor for each share of common stock underlying Units purchased in the Private Placement divided by the Weighted Average Adjusted Price, less (ii) the number of shares of common stock underlying Units actually purchased in the Private Placement by such Investor.
The “Weighted Average Adjusted Price” equals the quotient obtained by dividing (i) an amount equal to the sum of the aggregate purchase price of all of the shares of common stock underling Units sold in the Private Placement plus the aggregate consideration received by us for such issuance of Additional Shares during the Anti Dilution Period,; by (ii) an amount equal to the sum of the aggregate number of shares of common stock underlying Units sold in the Private Placement plus the aggregate number of Additional Shares sold by us during the Anti-Dilution Period.
Placement Agency Agreement
We entered into a placement agency agreement (the “Placement Agent Agreement”) with the Co-Placement Agents on October 21, 2009 whereby the Co-Placement Agents received as compensation for acting as placement agent in the Private Placement (i) a total cash fee and a non-accountable marketing allowance in the amount of approximately $0.86 million; and (ii) Agent Warrants to purchase up to 302,521 shares of common stock. Pursuant to participating agent agreements by and among Charles Vista, LLC, May Davis and American Capital, Charles Vista, LLC received as compensation for acting as a sub-agent in the Private Placement (i) a cash fee in the amount of approximately $0.22 million; and (ii) Agent Warrants to purchase up to 49,413 shares of common stock at an exercise price of $3.65 per share. The Co-Placement Agents were responsible for raising the minimum offering amount of $5,820,000 of Units and were compensated as set forth above. The funds in connection with the Private Placement were held with Signature Bank, acting as escrow agent, and were released to us upon the consummation of each closing under the Subscription Agreement.
63
Our common stock is currently traded on the OTCBB under the trading symbol “CNBI.OB”. The last reported price for our common stock on the OTCBB on January 21, 2011 was $ 2.75 per share.
The following table sets forth the high and low bid information for our common stock for the period from January 1, 2008 through December 31 , 2010. The OTC Bulletin Board quotations reflect inter-dealer prices, are without retail markup, markdowns or commissions, and may not represent actual transactions.
|
Common Stock
|
||||||||
|
High
|
Low
|
|||||||
|
First quarter 2008*
|
||||||||
|
Second quarter 2008*
|
$
|
-
|
$
|
-
|
||||
|
Third quarter 2008*
|
$
|
-
|
$
|
-
|
||||
|
Fourth quarter 2008*
|
$
|
-
|
$
|
-
|
||||
|
First quarter 2009*
|
$
|
-
|
$
|
-
|
||||
|
Second quarter 2009*
|
$
|
-
|
$
|
-
|
||||
|
Third quarter 2009*
|
$
|
-
|
$
|
-
|
||||
|
Fourth quarter 2009*
|
$
|
-
|
$
|
-
|
||||
|
First quarter 2010 (commencing on March 3, 2010)
|
$
|
3.00
|
$
|
2.60
|
||||
|
Second quarter 2010
|
$
|
4.10
|
$
|
2.00
|
||||
|
Third quarter 2010
|
$
|
4.00
|
$
|
2.55
|
||||
| Fourth quarter 2010 | $ | 3.55 | $ | 2.30 | ||||
__________
*No bid information is available for such period. Trading commenced on March 3, 2010.
As of December 29 , 2010, there were 159 holders of record of our common stock.
During the fiscal years ended December 31, 2008 and 2007, Liuzhou BCT declared and paid to its original shareholders cash dividends in the aggregate amount of $6,940,000 and $2,044,056 respectively. No dividend was paid in 2009.
The declaration or payment of any future cash dividend will be at the discretion of our board of directors and will depend upon the earnings (if any), capital requirements and financial position of the company, general economic conditions, and other pertinent factors. It is our present intention not to declare or pay any cash dividends in the foreseeable future, but rather to reinvest earnings (if any), in our business operations.
The following discussion should be read along with our financial statements and notes thereto. This section includes a number of forward-looking statements that reflect our current views with respect to future events and financial performance. Forward-looking statements are often identified by words like believe, expect, estimate, anticipate, intend, project and similar expressions, or words which, by their nature, refer to future events. You should not place undue certainty on these forward-looking statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from our predictions.
64
Business Review
We are engaged in pharmaceutical distribution, retail pharmacy and manufacture of pharmaceuticals through our three subsidiaries Liuzhou BCT, Hefeng Pharmaceutical, and BCT Retail, each of which is located in Guangxi Province, China.
We have integrated operations in the following three business segments.
Pharmaceutical distribution segment:
We provide a comprehensive offering of pharmaceutical and healthcare products, including branded and generic prescription medicines, over-the counter medicines, Western and Chinese medicines, as well as personal care products and medical supplies, Chinese herbs, and medical instrument from manufacturers and suppliers through distribution to our customers, including hospitals, retail drug stores, other pharmaceutical wholesalers, clinics, medical centers, and individuals located mainly in Guangxi Province except for the other pharmaceutical wholesalers. Over 8,000 products are distributed in compliance with China’s regulations over the pharmaceutical industry. For the nine months ended September 30, 2010, our pharmaceutical distribution segment accounted for approximately 70.6% of our total revenue after elimination of inter-segment sales.
Revenue derived from Chinese herbal medicine, family planning products, medical instruments, injection drugs and other packaged medicine drugs constituted 0.96%, 0.1%, 0.49%, 19.06% and 79.38% of our pharmaceutical distribution segment’s total revenue in 2009, respectively. The terms of our distribution agreements vary between supplies and vary in terms of payment period, arrangement of delivery, pricing and quality requirements. The general payment period terms vary from advance deposit, to cash on delivery, and to payment up to 90 days from the date of delivery, and the payment can be settled by means of bank collection, remittance, bills payable, postal check. Our top 10 suppliers in our pharmaceutical distribution segment accounted for 19% of our purchases in for the nine months ended September 30, 2010.
Retail pharmacy segment:
BCT Retail operates a large regional retail pharmacy network of stores in Guangxi province, consisting of 170 directly owned retail stores in Guangxi province under the registered name “Baicaotang 百草堂.” Our retail stores provide high-quality convenient and professional pharmaceutical services, and supply a wide variety of medicines for selling prescription medicines, over-the-counter medicines, Chinese herbal medicine, roughly processed Chinese herbal medicine, family planning products, and other pharmaceutical products and healthcare products. Revenue from the retail pharmacy segment is generated by cash sales or medi-card reimbursement from the national insurance scheme. There is no difference in the sales price of our products in our medi-care qualified stores between cash and medi-card payment. Prior to July 2009 co-payment was collected with respect to certain payments by medi-card. Since July 2009, due to a change in the national insurance scheme, no co-payments are required under the national insurance scheme. For the nine months ended September 30, 2010, our retail pharmacy segment accounted for approximately 23.9% of our total revenue after elimination of inter-segment sales.
The following table sets forth the accounts receivable from the National Program for our retail pharmacy segment as of September 30, 2010, December 31, 2008 and 2009:
|
(in thousands)
|
As of September 30,
|
As of December 31,
|
||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Payor
|
||||||||||||
|
National Program
|
$
|
238
|
$
|
295
|
$
|
194
|
||||||
Our billing system does not currently have the capacity to generate an ageing schedule for all of our receivables. Nevertheless, we are able to create ageing schedules by reference to data from our accounting system. The payment from the National Program is made on a lump sum basis after they are approved. At the end of each month, we reconcile our records with those of the National Program and send invoices to it for reimbursement. Once amounts are confirmed by the National Program, such amounts due for the current month are available for reimbursement. No allowance for bad debts has been made as med-card reimbursement under the national insurance scheme is assured.
65
Manufacturing segment:
On December 31, 2007, in order to diversify its business activities, Liuzhou BCT entered into an agreement with Li Jing Hua to acquire 100% of Hefeng Pharmaceutical at a consideration of RMB36,340,064 (equivalent to $4,982,223) which was satisfied by issuance of 13.44% of the registered capital of Liuzhou BCT. The acquisition was completed on January 2, 2008. Located in Donglan District, Guangxi province and built on approximately 40,000 square meters of land which we own, Hefeng Pharmaceutical has four product processing units: (1) Chinese herbal medicine abstraction unit for raw material and medicine paste with 670 tons of annual abstraction capacity; (2) granular formulation unit with an annual production capacity of 0.25 billion packages; (3) pill formulation unit with annual production capacity of 0.36 billion pills, and (4) liquid formulation unit with an annual production capacity of 0.1 billion injections. We manufacture and sell both the generic and clinic drugs all over the China. For the nine months ended September 30, 2010, our manufacturing segment accounted for approximately 5.5% of our total revenue after elimination of the inter-segment sales.
Growth Strategy
Distribution segment
We pursue a stable and organic growth strategy for our distribution business segment. One component of this strategy is to participate in the centralized tendering process conducted under the New Rural Cooperative Health Insurance Plan. This Program provides for centralized tenders for supplying drugs to hospitals and medical centers in counties, but outside cities which are tendered separately. In this tender, we won distribution rights in six counties and townships, which entitled us to distribute drugs to all the rural hospitals and medical centers in each county and township. We began supplying these institutions in August, 2009. The distribution rights granted in each tender extend until the next tender. The upcoming 2010 tender is the second tender in this program. We will continue to bid aggressively in these tenders for rural hospitals and medical centers.
During September of 2010 we entered into separate distribution agreements with three government-owned hospitals, each for a term of three years, pursuant to which the hospitals agreed to our supplying them with from 30% to 95% of their respective purchase plans for certain drugs. We agree to use our best efforts to fulfill the orders by distributing the drugs under the statutory requirement of the mandated collective tender plan. We were required to make good-faith deposits totaling RMB 23,000,000 ($3,374,100) for the security of these distribution agreements. Under these agreements there are no provisions for forfeiture or offset with respect to these deposits. The hospitals are required to repay the deposit amounts in full when the agreements expire or when terminated, which may be done only under certain circumstances. We entered into such agreements to pursue relationships with these large hospitals in order to achieve a higher share of these customers' purchases. Based on the terms of the agreements, we anticipate the revenues derived from these distribution agreements will contribute additional revenue of approximately $7,000,000 annually, commencing in the fourth quarter of 2010, over the three-year terms of the agreements.
Retail Segment
We currently plan to selectively acquire drugstore chains or independently operated drugstores that complement our existing store network or help us to establish a presence in new markets. In particular, we plan to grow through first acquiring similar businesses in the cities in Guangxi province and then acquiring business targets outside of the Guangxi province. We target retail chains or individual stores in prime locations and with good brand names, well-developed facilities and customer bases that are complementary to ours, and which are commercially attractive. We believe that our relationship with many industry participants and our knowledge of, and operational expertise in, the drugstore market in China will assist us in making acquisitions. We also believe that we can rapidly and successfully integrate newly acquired stores into our current distribution network and quickly realize operating and financial benefits.
In March of this year we closed an asset transfer agreement to acquire 30 existing retail stores under the New Rural Co-operative Health Issuance Plan for approximately $2.4 million. In April of this year we closed an asset transfer agreement to acquire 18 retail pharmacy stores in Hezhou City, Guangxi Province for approximately $1.3 million in cash and in May of this year we entered into and closed an asset acquisition agreement to acquire 11 retail pharmacy stores in Laibin City, Guangxi Province for approximately $0.6 million in cash. In July of this year we further expanded our retail network across Guangxi by opening 12 new retail pharmaceutical stores in the cities of Liuzhou, Guilin, Nanning and Laibin. In September, 2010 we entered into two assets transfer agreements to acquired 33 retail pharmacy stores. The transactions are expected to close in the fourth quarter of 2010.
66
We intend to expend a total of RMB 174 million, or approximately $25.5 million for the opening of 360 additional chain stores (including the estimated 48 new stores already acquired this year) in 2010 and 2011. In order to satisfy and fully implement our growth strategy, we will need to raise money, through equity or debt financing, either in a private placement or a public offering. However, there are no assurances that such financing will be available or available on terms acceptable to us. To the extent that we require additional financing in the future and are unable to obtain such additional financing, we may not be able to fully implement our growth strategy.
Manufacturing Segment
We pursue an organic growth strategy for our manufacturing business segment. We have employed more working capital on developing new client accounts to expand further our sales network. Benefiting from China’s healthcare reform, hospitals’ drug usage has increased dramatically. We will also take this opportunity to further reinforce our sales and marketing.
We invested around $200,000 in a new drug line of Compound Diphenoxylate tablets in May of 2010. Compound Diphenoxylate tablets are used for diarrhea. The Chinese government places quotas on raw material producers for Compound Diphenoxylate Tablets, but does not limit producers who have both production licenses for raw material and finished drugs. There are only two licensed raw material producers of this kind, and Hefeng Pharmaceutical is one of the two. Moreover, Hefeng Pharmaceutical also has a production license for finished Compound Diphenoxylate Tablets. Therefore, Hefeng Pharmaceutical is the only producer in China who owns a production license for both raw material of and finished Compound Diphenoxylate Tablets. We expect that this drug alone can contribute $1.8 million of revenue to Hefeng Pharmaceutical’s revenue annually. We currently have received revenue from this drug in the amount of $0.14 million. We anticipate these sales will contribute about $0.45 million in the fourth quarter of 2010 and the $1.8 million level by the third quarter of 2011.
Principal Factor Affecting Our Operating Results
A number of our pharmaceutical products, primarily those included in the national and provincial medical insurance catalogs, are subject to price controls in the form of fixed retail prices or retail price ceiling controls administered by the Price Control Office under the National Development and Reform Commission, or the NDRC, and provincial price control authorities. Approximately 60% to 70% of our total retail sales are subject to these price controls. The retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Any future price controls or government mandated price reductions may cause potential variability of our earnings and cash flows.
67
RESULTS OF OPERATIONS
Nine months ended September 30, 2010 compared to nine months ended September 30, 2009
The following table sets forth the key components of our results of operations for the periods indicated.
|
Nine months ended September 30,
|
|||||||||||||||
|
2010
|
% of total sales revenue
|
2009
|
% of total sales revenue
|
||||||||||||
|
(000)
|
(000)
|
||||||||||||||
|
Sales revenue
|
$
|
134,049
|
100.0
|
$
|
99,473
|
100.0
|
|||||||||
|
Cost of sales
|
99,341
|
(74.1
|
)
|
72,944
|
(73.3
|
)
|
|||||||||
|
Gross profit
|
34,708
|
25.9
|
26,529
|
26.7
|
|||||||||||
|
Operating expenses
|
|||||||||||||||
|
Administrative expenses
|
5,308
|
(4.0
|
)
|
2,808
|
(2.8
|
)
|
|||||||||
|
Research and development expenses
|
-
|
-
|
53
|
(0.1
|
)
|
||||||||||
|
Selling expenses
|
3,471
|
(2.6
|
)
|
2,577
|
(2.6
|
)
|
|||||||||
|
Total operating expenses
|
8,779
|
(6.6
|
)
|
5,438
|
5.5
|
||||||||||
|
Income from operations
|
25,929
|
19.3
|
21,091
|
21.2
|
|||||||||||
|
Non-operating income (expense)
|
|||||||||||||||
|
Interest income
|
7
|
-
|
66
|
-
|
|||||||||||
|
Other income
|
144
|
-
|
71
|
-
|
|||||||||||
|
Change in fair value of warrants liabilities
|
(458
|
)
|
(0.3
|
)
|
-
|
-
|
|||||||||
|
Other expenses
|
(203
|
)
|
(0.1
|
)
|
(12
|
)
|
-
|
||||||||
|
Finance costs
|
(680
|
)
|
(0.5
|
)
|
(1,091
|
)
|
(1.1
|
)
|
|||||||
|
Total non-operating income (expense)
|
(1,190
|
)
|
(0.9
|
)
|
(966
|
)
|
(0.9
|
)
|
|||||||
|
Income before income taxes
|
24,739
|
18.4
|
20,125
|
20.3
|
|||||||||||
|
Income taxes
|
(6,455
|
)
|
(4.8
|
)
|
(4,749
|
)
|
(4.8
|
)
|
|||||||
|
Net income
|
18,284
|
13.6
|
15,376
|
15.5
|
|||||||||||
|
Other comprehensive income
|
|||||||||||||||
|
Foreign currency translation adjustments
|
(1
|
)
|
-
|
25
|
-
|
||||||||||
|
Total comprehensive income
|
$
|
18,283
|
13.6
|
$
|
15,401
|
15.5
|
|||||||||
The table below sets forth a breakdown of our external segment revenue after elimination of inter-segment sales, and each segment revenue item as a percentage of our total sales revenue, as well as our inter-segment sales for the nine months ended September 30, 2010 and September 30, 2009. For the nine months ended September 30, 2010, we had approximately $25.2 million of inter-segment revenue, which includes approximately $24.6 million in sales from our pharmaceutical distribution segment to our retail pharmacy segment, and approximately $0.6 million in sales from our manufacturing segment to our distribution pharmacy segment. For the nine months ended September 30, 2009, we had approximately $17.9 million of inter-segment revenue from our pharmaceutical distribution segment. External segment revenue refers to segment revenue after inter-segment elimination.
68
|
Nine months ended September 30,
|
|||||||||||||
|
2010
|
% of total sales revenue
|
2009
|
% or total sales revenue
|
||||||||||
|
External Segment revenue
|
(000)
|
(000)
|
|||||||||||
|
Pharmaceutical distribution
|
$
|
94,687
|
70.6
|
$
|
70,773
|
71.1
|
|||||||
|
Retail pharmacy
|
32,031
|
23.9
|
22,352
|
22.5
|
|||||||||
|
Manufacturing
|
7,331
|
5.5
|
6,348
|
6.4
|
|||||||||
|
$
|
134,049
|
100.0
|
$
|
99,473
|
100.0
|
||||||||
|
Inter-segment revenue
|
$
|
25,155
|
$
|
17,948
|
|||||||||
Sales Revenue
During the nine months ended September 30, 2010, we had sales revenue of $134.0 million, as compared to sales revenue of $99.5 million during the nine months ended September 30, 2010, an increase of $34.6 million or approximately 34.8%. This increase was mainly attributable to the respective increase in sales revenue of $23.9 million and $9.7 million from our distribution segment and our retail segment during the period.
Pharmaceutical distribution segment
We derive the majority of our revenue from our pharmaceutical distribution segment from sales of pharmaceutical products to our customers. We distribute a comprehensive offering of pharmaceutical and health care products, including branded and generic prescription medicines, over-the-counter medicines, western and traditional Chinese medicines, as well as personal care products and medical supplies.
The following table sets forth revenue from our pharmaceutical distribution operations by category of customers:
|
Nine months ended September 30,
|
|||||||||||||
|
2010
|
%
of Sales
|
2009
|
%
of Sales
|
||||||||||
|
(000)
|
(000)
|
||||||||||||
|
Hospitals
|
$
|
65,267
|
68.9
|
$
|
48,657
|
68.8
|
|||||||
|
Other drug stores
|
5,215
|
5.5
|
175
|
0.2
|
|||||||||
|
Clinics and health care centers
|
13,904
|
14.7
|
4,247
|
6.0
|
|||||||||
|
Distributors and others
|
10,301
|
10.9
|
17,694
|
25.0
|
|||||||||
|
Total
|
$
|
94,687
|
100.0
|
$
|
70,773
|
100.0
|
|||||||
Revenue from our pharmaceutical distribution segment increased by 33.8% from $70.8 million for the nine months ended September 30, 2009 to $94.7 million for the nine months ended September 30, 2010. The increase in revenue of $23.9 million is the result of the increase in volume by $33.3 million and offset by the reduction in price level by $9.4 million. The average reduction in price is attributed to the change in prices paid by hospitals based upon the PRC Government-mandated collective tender process. The increase in sales revenue from our pharmaceutical distribution segment was attributed primarily to the increase of $16.6 million in sales to hospitals and $9.7 million in sales to clinics and health care centers after offsetting the decrease in sales to distributors by $7.4 million as a result of more of our resources being allocated for the development of hospital customers. The increase in sales to hospitals was the result of an increase in the quantity and range of products sold to our existing hospital clients, which was attributable to the increase in coverage by the national insurance plan and the implementation of the New Rural Cooperative-Medicare plan. The increase in sales to clinics and health care centers was attributed to the penetration of the market at the community region after we won the six city and townships’ distribution business bid from Basic Drugs Catalogue Plan in the middle of 2009. The increase in sales to other drug stores was the result of the increase in the quantity and range of products to the existing and new stores upon our marketing effort.
69
Retail pharmacy segment
Revenue from our retail pharmacy segment increased by 43.3% from $22.4 million for the nine months ended September 30, 2009 to $32.0 million for the nine months ended September 30, 2010. The increase in revenue resulted from the increase in sales volume by $8.4 million and the general increase in our prices by approximately $1.2 million. The slight increase in our retail prices was the result of the adjustment of our selling prices in accordance with the increased cost of merchandise. The increase in sales volume was primarily attributed to the sales which resulted from the opening of 71 stores during the period. In addition, revenues derived from five stores which opened in the second half of 2009 also contributed to the growth of the sales. In addition to acquisition, the growth was derived partly by the increase of sales through medi-care insurance cards as a result of an increase in the portion of pharmacy products entitled to be reimbursed by the PRC government, as well as an increase in the number of people covered by insurance.
The following table sets forth revenue from our retail pharmacy segment by existing stores and new stores opened during each period.
|
Nine months ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
(000
|
)
|
(000
|
)
|
|||||
|
Existing stores (1)
|
$
|
25,881
|
$
|
22,352
|
||||
|
New stores (2)
|
6,150
|
-
|
||||||
|
Total (includes closed stores)
|
32,031
|
22,352
|
||||||
|
No. of stores
|
137
|
66
|
||||||
(1) One store was closed during the nine months ended September 30, 2010. The sales derived from that store for the nine months ended September 30, 2010 was $113,749 while that store represented sales of $328,087 for the nine months ended September 30, 2009.
(2) Represents the 71 stores opened during the nine months period ended September 30, 2010, including the 59 stores acquired during the second quarter ended June 30, 2010.
Manufacturing segment
Revenue from our manufacturing segment increased by 15.5% from $6.3 million for the nine months ended September 30, 2009 to $7.3 million for the nine months ended September 30, 2010. The increase was attributed to the volume of sales solely as there were no changes in prices between the periods. The increase in sales was attributed to the increase in sales through the penetration of sales to new distributors as a result of efforts made by our sales representatives.
Cost of Sales
Cost of sales was $72.9 million for the nine months ended September 30, 2009 as compared to $99.3 million for the nine months ended September 30, 2010, representing an increase of $26.4 million. Our cost of sales consist of the cost of merchandise and raw materials and other costs. Other costs include direct labor and depreciation and miscellaneous costs. The increase was primarily due to higher costs of purchasing merchandise following the increase in our revenue.
70
Gross Profits
Gross profit was $34.7 million for the six months ended September 30, 2010 as compared to $26.5 million for the nine months ended September 30, 2009, representing an increase of $8.2 million or approximately 30.1%. Our gross profit margin was 25.9% and 26.7% for the nine months ended September 30, 2010 and September 30, 2009, respectively. The gross profit margin was relatively stable; we maintain the margin between our cost of purchasing pharmaceutical products from our suppliers and our prices of pharmaceutical products sold to our hospital, pharmaceutical distributor and other customers. For hospital customers, we establish a pricing range aligned with our suppliers through the PRC Government-mandated collective tender process. For other pharmaceutical product distributors, we arrange three party negotiations with distributors and suppliers. The slight decrease in profit margin was mainly attributed to the reduction of the portion of gross margin from our distribution segment.
Pharmaceutical distribution segment
The respective gross margin for our pharmaceutical distribution segment was approximately 20.9% and 21.7% for the nine months ended September 30, 2010 and 2009, respectively. The cost of sales includes only the cost of merchandise. In order to penetrate and capture the growth of the medical insurance plan, we attempted to satisfy the demand of hospital customers for both high-profit margin and low-profit margin products. This resulted in a slight decrease in the gross profit margin for the nine months ended September 30, 2010 compared to the same period in 2009.
Retail pharmacy segment
The respective gross margin for our retail pharmacy segment was approximately 32.6% and 32.8% for the nine months ended September 30, 2010 and 2009, respectively. The cost of sales includes only the cost of merchandise, and we adjust the retail price in accordance with the cost of merchandise. There was no significant change in gross profit between these two periods.
Manufacturing segment
The respective gross margin for our manufacturing segment was approximately 60.6% and 60.3% for the nine months ended September 30, 2010 and 2009, respectively. The cost of sales consists of cost of merchandise and raw materials and other costs. Other costs include direct labor, depreciation and miscellaneous costs. There was no material price change for the cost of sales and our prices, and the gross profit was stable for each of our products manufactured.
Selling, Research and Development and Administrative Expenses - Combined
Selling, research and administrative expenses totaled $8.8 million for the nine months ended September 30, 2010, as compared to $5.4 million for the nine months ended September 30, 2009, representing an increase of $3.4 million or approximately 61.4%. The increase was attributed to the increase of administrative expenses and selling expenses by $2.5 million and $0.9 million, respectively.
Selling Expenses
Selling expenses increased by 34.7% from $2.6 million for the nine months ended September 30, 2009 to $3.5 million for the nine months ended September 30, 2010. The increase was primarily due to the increase in our marketing staff’s wages and salaries, and payment for staff welfare in connection with our increased sales and marketing activities. The percentage of our distribution and selling expenses to our total revenue was 2.6% for both the nine months ended September 30, 2010 and September 30, 2009.
Pharmaceutical distribution segment
The selling expenses of our pharmaceutical distribution segment increased by 45.4% from $1.2 million for the nine months ended September 30, 2009 to $1.8 million for the nine months ended September 30, 2010. The increase was primarily due to the increase in our marketing staff’s wages and salaries, and payment for staff welfare in connection with our marketing activities by $0.4 million
71
Retail pharmacy segment
The selling expenses of our retail pharmaceutical distribution segment increased by 42.4% from $1 million for the nine months ended September 30, 2009 to $1.4 million for the nine months ended September 30, 2010. The increase was primarily due to the increase in our salaries of retail staff by $0.4 million in connection with our increase in the number of stores opened for the period ended September 30, 2010.
Manufacturing segment
The selling expenses of our manufacturing segment decreased by 21.7% from $0.4 million for the six months ended September 30, 2009 to $0.3 million for the nine months ended September 30, 2010. The decrease was primarily due to the reduction in advertising expenses.
Administrative expenses
Administrative expenses increased by 89.0% from $2.8 million for the nine months ended September 30, 2009 to $5.3 million for the nine months ended September 30, 2010. The increase in administrative expenses for the nine months ended September 30, 2010 was primarily due to an increase in the incurrence of fees and salaries in connection with the compliance of being a public company by $0.9 million as well as an increase in share based compensation of $0.7 million. Further there was an increase in staff cost inclusive of wages and salaries and staff benefits of $0.59 million, and the rental expenditures resulting from the opening of new stores, which was approximately $0.35 million. The percentage of our administrative expenses to our total revenue increased from 2.8% in 2009 to 4.0% in 2010 and was mainly attributed to the increase in salary level.
Pharmaceutical distribution segment
The administrative expenses of our pharmaceutical distribution segment increased by $0.44 million or 22.8% from $1.97 million for the nine months ended September 30, 2009 to $2.41 million for the nine months ended September 30, 2010. The increase was primarily due to the increase in staff cost inclusive of wages and salaries and staff benefits by $0.41 million upon the increase of individual salary levels.
Retail pharmacy segment
The administrative expenses of our retail pharmaceutical distribution segment increased by $0.56 million or 157.3% from $0.36 million for the nine months ended September 30, 2009 to $0.92 million for the nine months ended September 30, 2010. The increase was the result of increases in our staff welfare by $0.16 million. In addition, there were increases in rental charges of $0.35 million upon the opening of new stores.
Manufacturing segment
The administrative expenses of our manufacturing segment increased by $0.03 million or 7.9% from $0.41 million for the nine months ended September 30,, 2009 to $0.44 million for the nine months ended September 30, 2010. The increase was the result of increases in our salaries by $0.02 million.
Research and development
Research and development expenses were $0.05 million for the nine months ended September 30, 2009 while no expenses were incurred for the nine months ended September 30, 2010. We only incur the expenditures on research and development for our manufacturing segment. In prior years, most expenditure was spent in connection with the initial phase of research and the feasibility of development, however, we did not have any expenditures for research and development during the nine months ended September 30, 2010.
72
Change in fair value of warrants
For the nine months ended September 30, 2010, we incurred a non-cash charge of $0.4 million unrelated to our operations which resulted from the change in fair value of warrants issued to investors in conjunction with the Company’s issuance of warrants in December 2009 and February 2010 pursuant to provisions of FASB ASC Topic 815, “Derivative and Hedging”. The accounting treatment of the warrants resulted from a provision providing anti-dilution protection to the warrant holders.
Income before income tax
As a result of the foregoing, our income before income tax increased by 22.9% to $24.7 million for the nine months ended September 30, 2010 compared to $20.1 million for the same period in 2009. The percentage of our income before income tax to total revenue decreased slightly from 20.2% in 2009 to 18.5% in 2010 and was mainly attributed to the increase in our staff costs related to expanding our business as well as the incurrence of costs in compliance of being a public company. Further there is the occurrence of charges related to the change in fair value of warrants and share based compensation.
Pharmaceutical distribution segment
Our income before income tax from distribution operations increased by 30.8% from $12.2 million for the nine months ended September 30, 2009 to $16.0 million for the nine months ended September 30, 2010, due to increases in sales. The profit margin decreased to 16.9% from 17.3%.
Retail pharmacy segment
Our income before income tax from retail pharmacy segment operations increased by 36.5% from $5.2 million for the nine months ended September 30, 2009, to $7.1 million for the nine months ended September 30, 2010 due to the substantial increase in sales. The profit margin decreased to 22.0% from 23.1%.
Manufacturing segment
Our income before income tax from manufacturing segment operations increased by 39.9% from $2.8 million for the nine months ended September 30, 2009, to $3.9 million for the nine months ended September 30, 2010. The profit margin increased to 52.6% from 43.4%.
Net Income
As a result of the above factors, we had net income of $18.3 million for the nine months ended September 30, 2010 as compared to $15.4 million for the nine months ended September 30, 2009, representing an increase of $2.9 million or approximately 18.9%. For the nine months ended September 30, 2010, our net income was impacted by a non-cash charge of $0.4 million as a result of warrant liabilities and share-based compensation unrelated to our operations of $0.7 million. Excluding this $1.1 million non-cash charge, our net income for the nine months ended September 30, 2010 would have been $19.4 million, representing an increase of 26.2% from the same period in 2009.
Earnings per share
For the nine months ended September 30, 2010, our basic and diluted earnings per share was $0.48 compared to $0.48 for the same period in 2009.
73
Year Ended December 31, 2009 Compared to Year Ended December 31, 2008
The following table sets forth the key components of our results of operations for the periods indicated.
|
Year ended December 31
|
||||||||||||||||
|
2009
|
2008
|
|||||||||||||||
|
‘000
|
% of total sales
|
‘000
|
% of total sales
|
|||||||||||||
|
Sales revenue
|
$
|
136,087
|
100.0
|
$
|
108,991
|
100.0
|
||||||||||
|
Cost of sales
|
100,579
|
73.9
|
79,362
|
72.8
|
||||||||||||
|
Gross profit
|
35,508
|
26.1
|
29,629
|
27.2
|
||||||||||||
|
Operating expenses
|
||||||||||||||||
|
Administrative expenses
|
4,599
|
3.4
|
3,341
|
3.1
|
||||||||||||
|
Research and development expenses
|
100
|
0.1
|
764
|
0.7
|
||||||||||||
|
Selling expenses
|
3,866
|
2.8
|
2,122
|
1.9
|
||||||||||||
|
8,565
|
6.3
|
6,227
|
5.7
|
|||||||||||||
|
Income from operations
|
26,943
|
19.8
|
23,402
|
21.5
|
||||||||||||
|
Interest income
|
16
|
-
|
29
|
0.1
|
||||||||||||
|
Other income
|
125
|
0.1
|
143
|
0.1
|
||||||||||||
|
Finance costs
|
(1,414
|
)
|
(1.0
|
)
|
(1,260
|
)
|
(1.2
|
)
|
||||||||
|
Income before income taxes
|
25,670
|
18.9
|
22,314
|
20.5
|
||||||||||||
|
Income taxes
|
(6,262
|
)
|
(4.6
|
)
|
(5,657
|
)
|
(5.2
|
)
|
||||||||
|
Net income
|
$
|
19,408
|
14.3
|
$
|
16,657
|
15.3
|
||||||||||
|
Other comprehensive income
|
||||||||||||||||
|
Foreign currency translation adjustments
|
57
|
-
|
1,142
|
1.0
|
||||||||||||
|
Total comprehensive income
|
$
|
19,465
|
14.3
|
$
|
17,799
|
16.3
|
||||||||||
The table below sets forth a breakdown of our external segment revenue after elimination of inter-segment sales, and each segment revenue item as a percentage of our total revenue, as well as our inter-segment sales for the years ended December 31, 2009 and December 31, 2008. For the year ended December 31, 2009, we had approximately $22.5 million of inter-segment revenue, which includes approximately $21.7 million in sales from our pharmaceutical distribution segment to our retail pharmacy segment, and approximately $762,000 in sales from our manufacturing segment to our distribution pharmacy segment. External segment revenue refers to segment revenue after inter-segment elimination.
|
Year ended December 31,
|
||||||||||||||||
|
2009
|
2008
|
|||||||||||||||
|
‘000
|
% of total sales revenue
|
‘000
|
% of total sales revenue
|
|||||||||||||
|
External Segment revenue
|
||||||||||||||||
|
Pharmaceutical distribution
|
$
|
97,137
|
71.4
|
$
|
72,806
|
66.8
|
||||||||||
|
Retail pharmacy
|
31,223
|
22.9
|
28,593
|
26.2
|
||||||||||||
|
Manufacturing pharmacy
|
7,727
|
5.7
|
7,592
|
7.0
|
||||||||||||
|
$
|
136,087
|
100.0
|
$
|
108,991
|
100.0
|
|||||||||||
|
Inter-segment revenue
|
$
|
22,492
|
$
|
21,656
|
||||||||||||
74
Sales Revenue
During the year ended December 31, 2009, we had sales revenue of $136.1 million, as compared to sales revenue of $109 million during the year ended December 31, 2008, an increase of $27.1 million or approximately 24.9%. This increase was mainly attributable to an increase in sales revenue of $24.3 million from our pharmaceutical distribution operations. Sales revenue derived from our pharmaceutical distribution segment amounted to $97.1million, which accounted for 71.4% of our total sales revenue. In addition, the respective increase of $2.6 million and $0.1 million of our retail and manufacturing segments also contributed to the increase in the sales revenue during the period
Pharmaceutical distribution segment
We derive the majority of our revenue from our pharmaceutical distribution segment from sales of pharmaceutical products to our customers. We distribute a comprehensive offering of pharmaceutical and health care products, including branded and generic prescription medicines, over-the-counter medicines, Western and TCM medicines, as well as personal care products and medical supplies.
The following table sets forth revenue from our pharmaceutical distribution operations by category of customers:
|
Year ended December 31
|
||||||||||||||||
|
2009
|
2008
|
|||||||||||||||
|
‘000
|
%
of sales
|
‘000
|
%
of sales
|
|||||||||||||
|
Hospitals
|
$
|
71,541
|
73.6
|
$
|
38,636
|
53.1
|
||||||||||
|
Other drug stores
|
219
|
0.2
|
252
|
0.3
|
||||||||||||
|
Clinics and health care centre
|
4,910
|
5.1
|
2,438
|
3.3
|
||||||||||||
|
Distributors and others
|
20,467
|
21.1
|
31,480
|
43.3
|
||||||||||||
|
Total
|
$
|
97,137
|
100.0
|
$
|
72,806
|
100.0
|
||||||||||
Revenue from our pharmaceutical distribution segment increased by 33.4% from $72.8 million for the year ended December 31, 2008 to $97.1 million for the year ended December 31, 2009. The increase was the result of the increase of price level by approximately $5 million and increase of volume of sales by approximately $19 million. The price change was mainly attributed to the change of price levels paid by hospitals as a result of PRC Government-mandated collective tender process. The increase in sales revenue volume from our pharmaceutical distribution segment was the result of an increase in the quantity and range of products sold to our existing hospital clients, which was attributable to:
|
●
|
China’s expansion of the healthcare security scheme coverage to city residents, including unemployed population, student and self-employed person,
|
|
●
|
the establishment of basic drugs catalogue by hospitals for full reimbursement basis upon medical social insurance scheme,
|
|
●
|
the increase of the reimbursement ratio covered by medical social insurance scheme, and
|
|
●
|
the implementation of New Rural Cooperative Medicare scheme for rural population as well.
|
An additional factor contributing to the increase in our sales revenue from our pharmaceutical distribution segment was additional sales resulting from a six county and township distribution business bid we won by middle of 2009. Further, the penetration of the group’s distribution network into community clinics and health care institutions also contributed to the increase in sales revenue.
75
Retail pharmacy segment
Revenue from our retail pharmacy segment increased by 9.2% from $28.6 million for the year ended December 31, 2008 to $31.2 million for the year ended December 31, 2009. The increase was the result of the increase in average price level by $1 million and in sales volume by approximately $2 million. The price change was the result of the increase of cost of merchandise and in the price level of the national insurance catalogue which our retail prices are sold by reference to. The increase in sales volume was partly contributed by sales derived through medi-care insurance cards as a result of an increase in the portion of pharmacy products entitled to be reimbursed by the PRC government, as well as an increase in the number of people covered by insurance. Further, the prevalence of and concerns related to swine flu in 2009 also boosted the demand for pharmacy products at our drug stores.
The following table sets forth revenue from our retail pharmacy segment by existing stores and new stores opened during each year:
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
| ‘000 | ‘000 | |||||||
|
Existing stores (1)
|
$
|
30,272
|
$
|
28,593
|
||||
|
New stores (2)
|
951
|
-
|
||||||
|
Total (include closed stores)
|
$
|
31,223
|
$
|
28,593
|
||||
|
No. of stores
|
66
|
64
|
||||||
(1) Includes sales derived from the stores which were closed during the fiscal period ended 2009. Four stores were closed during the fiscal period ended 2009 and the sales derived from this said period were $108,000, while their corresponding sales for the period ended 2008 were $1,782,000 approximately.
(2) Represents the stores opened during the fiscal period ended December 31, 2009.
Manufacturing pharmacy segment
Revenue from our manufacturing segment increased by 1.8% from $7.6 million for the year ended December 31, 2008 to $7.7 million for the year ended December 31, 2009. The increase was attributed to the change in volume of sales and not affected by price factors.
Cost of Sales.
Cost of Sales was $100.6 million for the year ended December 31, 2009 as compared to $79.4 million for the year ended December 31, 2009. Our cost of sales consist of the cost of merchandise and raw materials and other costs. Other costs include direct labor, depreciation and other costs. The increase was primarily due to an increase in the costs of purchasing merchandise following the increase in our revenue.
Gross Profit
Gross profit was $35.5 million for the year ended December 31, 2009 as compared to $29.6 million for the year ended December 31, 2008, representing an increase of $5.9 million or approximately 19.8%. Our gross profit margin was 26.1% and 27.2% for the year ended December 31, 2009 and December 31, 2008 respectively. The gross profit margin was relatively stable in which we maintain the margin between our cost of purchasing pharmaceutical products from our suppliers and our prices of pharmaceutical products sold to our hospital, pharmaceutical distributor and other customers. For hospital customers, we establish a pricing range aligned with our suppliers through the PRC Government-mandated collective tender process. For other pharmaceutical product distributors, we arrange three party negotiations with distributors and suppliers. The slight decrease in profit margin was mainly attributed to a larger percentage of wholesale accounts that we sold products to compared to 2008.
76
Pharmaceutical distribution segment
The respective gross profit margin for our pharmaceutical distribution segment was approximately 20.8% and 23% for the year ended December 31, 2009 and 2008, respectively. The cost of sales includes only the cost of merchandise. In order to penetrate and capture the growth of the market upon medical insurance scheme, we attempted to satisfy the demand of hospital customers by procuring from other eligible supplies within the medicine catalogue but at lower margins because of pre-determined/set pricing under the medicine catalogue. This results in slight decrease in the gross profit margin between year 2009 and year 2008.
Retail pharmacy segment
The respective gross margin for our retail pharmacy segment was approximately 32.7% and 28.4% for the year ended December 31, 2009 and 2008, respectively. The cost of sales includes only the cost of merchandise, and we adjust the retail price in accordance with the cost of merchandise. The increase in gross profit margin was primarily attributed to our strategy of promoting higher profit margin goods and selling more private label products which have higher margins.
Manufacturing segment
The respective gross profit margin for our manufacturing segment was approximately 65.9% and 63.1% for the year ended December 31, 2009 and 2008, respectively. The change was not related to the change in the cost of overhead. We believe this increase was due to management focus on optimizing our product portfolio to higher margin products
Selling, Research and Development and Administrative Expenses. Selling, research and development and administrative expenses totaled $8.6 million for the year ended December 31, 2009, as compared to $6.2 million for the year ended December 31, 2008, representing an increase of $2.4 million or approximately 38.7%.
Selling Expenses
Selling expenses increased by 82.2.% from $2.1 million for the year ended December 31, 2008 to $3.9 million for the year ended December 31, 2009. The increase was primarily due to the increase in our marketing staff’s wages and salaries, payment for staff welfare, commission and transportation costs in connection with our increased sales and marketing activities. The percentage of our distribution and selling expenses to our total revenue gradually increased from 1.9% to 2.8% and was primarily due to the increase of our efforts to penetrate and capture market share.
Pharmaceutical distribution segment
The selling expenses of our pharmaceutical distribution segment increased by 157.1% from $0.7 million for the year ended December 31, 2008 to $1.8 million for the year ended December 31, 2009. The increase was primarily due to the increase in our marketing staff’s salaries and welfare by $0.7 million and entertainment expenses by $0.2 million in connection with our marketing activities. Further the increase was contributed to by the increase of transportation charges by $0.1 million and other related selling activities as a result of the increase in sales.
Retail pharmacy segment
The selling expenses of our retail pharmaceutical distribution segment increased by 75.0 % from $0.8 million for the year ended December 31, 2008 to $1.4 million for the year ended December 31, 2009. The increase was primarily due to the increase in our salaries and staff welfare to retail staff by $0.4 million in connection with our increase in sales and the increase of the number of stores opened. Further, the increase was contributed to by the increase in utility charges by $0.1 million due to the opening of the new stores.
77
Manufacturing pharmacy segment
The selling expenses of our manufacturing segment increased by 5.1 % from $0.59 million for the year ended December 31, 2008 to $0.62 million for the year ended December 31, 2009. The increase was primarily due to the increase in commission paid to regional sales representatives by $0.06 million upon the increase in sales. The increase was offset partially by a reduction in advertising.
Administrative expenses
Administrative expenses increased by 37.7% from $3.3 million for the year ended December 31, 2008 to $4.6 million for the year ended December 31, 2009. The increase was primarily due to an increase in wages and salaries, staff benefits, post-employment benefits, and the rental expenditures resulting from the renewal of leases and the opening of new stores. The percentage of our administrative expenses to our total revenue increased slightly from 3.1% in 2008 to 3.4% in 2009.
Pharmaceutical distribution segment
The administrative expenses of our pharmaceutical distribution segment increased by 50% from $2.2 million for the year ended December 31, 2008 to $3.3 million for the year ended December 31, 2009. The increase was primarily due to the increase in our salaries, post-employment benefits to our staff by $0.4 million upon the expansion of our business. In addition, the increase was contributed to by the increase in write offs of long outstanding receivables by $0.3 million. Further, we also wrote off $0.1 million in trade receivables in 2009.
Retail pharmacy segment
The administrative expenses of our retail pharmaceutical distribution segment were increased by 25.0 % from $0.4 million for the year ended December 31, 2008 to $0.5 million for the year ended December 31, 2009. The increase was primarily due to the increase in rental charges by $0.1million upon the increase in the number of new stores and higher rental charges upon renewal of certain leases.
Manufacturing pharmacy segment
The administrative expenses of our manufacturing segment were relative stable and remained $0.6 million for both the year ended December 31, 2008 and 2009.
Research and development
Research and development decreased by 86.9% from $0.8 million for the year ended December 31, 2008 to $0.1 million for the year ended December 31, 2009 We only incur the expenditures on research and development for our manufacturing pharmacy segment. In prior years, most expenditure was spent in connection with the initial phase of research and the feasibility of development of certain attributes was uncertain. During the year, we focus mainly on a few types of drugs which have greater prospect for further refinement upon the results of the initial research being done. Thus, fewer expenditures were spent during 2009.
Income before income tax
As a result of the foregoing, our income before income tax was $ 25.7million for the year ended December 31, 2009, representing an increase of 15.0% from $22.3 million for the year ended December 31, 2008.
Pharmaceutical distribution segment
Our income before income tax from distribution operations increased by 8.5% from $14.0 million for the year ended December 31, 2008, to $15.2 million for the year ended December 31, 2009. The profit margin decreased to 15.6% from 19.2%
78
Retail pharmacy segment
Our income before income tax from retail pharmacy segment operations increased by 21.2% from $5.9 million for the year ended December 31, 2008, to $7.2 million for the year ended December 31, 2009. The profit margin increased to 23.1% from 20.8%.
Manufacturing segment
Our income before income tax from manufacturing segment operations increased by 43.1% from $2.4 million for the year ended December 31, 2008 to $3.5 million for the year ended December 31, 2009. The profit margin increased to 44.9% from 32%
Net Income
As a result of the above factors, we had net income of $19.4 million for the year ended December 31, 2009 as compared to $16.7 million for the year ended December 31, 2008, representing an increase of $2.8 million or approximately 16.5%.
Earnings per share
For the fiscal year ended December 31, 2009, our earnings per share was $0.61, representing an increase of 17.3%, compared to the same period in 2008.
Liquidity and Capital Resources
Our principal source of funds are cash generated from operations and various short-term and long-term bank loan borrowings and certain credit facilities inclusive of bills payable, as well as cash contributions from certain directors and related companies. Our primary liquidity requirements are to finance working capital, to fund the payment of interest and principal due on indebtedness and to finance acquisitions or organic growth to expand our facilities and operations. As of September 30, 2010, $8.8 million of our indebtedness was due within one year and $2.4 million was due beyond one year. During the nine months ended September 30, 2010 we did not experience any difficulties in renewing our bank loans with our lenders.
Restricted cash and cash equivalents
Our restricted cash consists of collateral we provide for bills payable. As of September 30, 2010 and December 31, 2009, our restricted cash was approximately $1.0 million and $1.2 million, respectively. As of September 30, 2010 and December 31, 2009 we had cash of $13.6 million and $13.3 million, respectively exclusive of restricted cash.
We believe that our existing sources of liquidity, along with cash expected to be generated from services will be sufficient to fund our operations, anticipated capital expenditures, working capital and other financing requirements for at least the next twelve months. We will continue to monitor our expenditures and cash flow position.
|
For the nine months ended
September 30,
|
For the year ended
December 31,
|
|||||||||||||||
|
2010
|
2009
|
2009
|
2008
|
|||||||||||||
|
'000
|
'000
|
'000
|
'000
|
|||||||||||||
|
Net cash provided by operating activities
|
$
|
9,919
|
$
|
14,349
|
$
|
5,900
|
$
|
11,734
|
||||||||
|
Net cash used in investing activities
|
(8,462
|
)
|
(336
|
)
|
(346
|
)
|
(831
|
)
|
||||||||
|
Net cash provided by/ (used in) financing activities
|
(1,144
|
)
|
(2,712
|
)
|
6,417
|
(9,982
|
)
|
|||||||||
|
Foreign currency translation
|
(1
|
)
|
7
|
68
|
(176
|
)
|
||||||||||
|
Net increase in cash and equivalents
|
312
|
11,308
|
12,039
|
745
|
||||||||||||
|
Cash and cash equivalents, beginning of period
|
13,304
|
1,265
|
1,265
|
520
|
||||||||||||
|
Cash and cash equivalents, end of period
|
$
|
13,616
|
$
|
12,573
|
$
|
13,304
|
$
|
1,265
|
||||||||
79
We believe that our existing sources of liquidity, along with cash expected to be generated from services will be sufficient to fund our operations, anticipated capital expenditures, working capital and other financing requirements for at least the next twelve months. We will continue to monitor our expenditures and cash flow position.
Operating Activities
We primarily derive our cash flow from operating activities from the sale of our products and services in our three segments. Our cash used in operating activities is primarily from the purchase of raw materials and products, distribution and selling expenses and general administrative expenses and taxes. Cash flows from our operations can be significantly affected by factors such as the timing of payment of accounts receivables and the payment of our accounts payables to suppliers.
Cash from operating activities is primarily affected by our pharmaceutical distribution segment, which has a high demand on working capital while the retail pharmacy segment generates primarily cash and a minority of accounts receivable pending reimbursements for medi-card payments. Because our pharmaceutical manufacturing segment accounted for only 5.5% of our revenues in 2010, it does not have a significant impact on cash from operating activities.
Cash provided by operating activities was $9.9 million for the nine months ended September 30, 2010 compared to $14.3 million provides operating activities for the nine months ended September 30, 2009, representing a decrease of $4.4 million. Operating cash flows for 2010 reflect primarily net cash receipts derived from business operations. Despite the increase in net income for the nine months ended September 30, 2010 as compared to the same period of 2009, the reduction in net cash from operating activities was primarily attributable to the slowdown of accounts receivable payment, which increased to 91 days in 2010 as compared to 65 days in 2009. The increase in turnover days was attributable to an increase in the portion of our sales to hospitals and community health care centers under our pharmaceutical distribution operations segment to 83.6% of total revenue during the nine months ended September 30, 2010 from 74.8% for the nine months ended September 30, 2009.
For our pharmacy distribution segment, accounts receivable turnover days for the nine months ended September 30, 2010 were 118 as compared to 81 for the same period in 2009. Hospitals and the community heath care centers have comparatively longer payment cycles and the portion of sales to both collectively increased to 83.6% for the nine months ended September 30, 2010 from 74.8% for the nine months ended September 30, 2009. Many hospitals have undertaken construction and expansion work. It resulted in the slow down of their payments. However, in the PRC, all hospitals and the community health care centers are owned or controlled by the PRC government. We believe that it is highly unlikely that a liquidation of one of our hospital and community health care center customers will occur, and that the recovery of accounts receivable from them are highly secure. Therefore, it is our customary practice to grant a longer credit period. There have been no instances of write-offs of any receivables owed by a hospital or the community health care centers.
For our retail segment, accounts receivable turnover days for the nine months ended September 30, 2010 were 2 days as compared to 4 days in 2009. We generally receive cash from the customer at our retail store except for our medi-care qualified stores. The accounts receivable comprised only the medi-card reimbursement under the national program. The increase in sales was mainly derived by the increase in the number of stores opened during the first nine months of 2010; nevertheless, not all the newly opened stores were medi-care qualified stores. Thus, the increase in the number of medi-care qualified stores is not in line with the increase in the number of stores. This resulted in the reduction in accounts receivable turnover days between the periods.
For our manufacturing segment, the accounts receivable turnover days for the nine months ended September 30, 2010 were 123 as compared to 105 in 2009. The increase was due to the extension of credit to our customers based on the payment history of the customers.
Cash provided by operating activities was $5.9 million for the year ended December 31, 2009 compared to $11.7 million for fiscal 2008, representing a decrease of $5.8 million or approximately 49.6%. Operating cash flows for 2009 reflects primarily net cash receipts derived from business operations. Despite the increase in net income in 2009 as compared to the same period of 2008, the reduction in net cash from operating activities was primarily attributable to the slowdown of accounts receivable payment, which increased to 95 days in 2009 as compared to 70 days in 2008. Such decrease was, to a certain degree, offset by our delay in cash payments to vendors and the increase in our use of bills and restricted cash for creditor settlement.
80
For our pharmacy distribution segment, accounts receivable turnover days for the year ended December 31, 2009 were 121 as compared to 94 in 2008. The increase in turnover days was attributable to an increase in the portion of our sales to hospitals from 53% in 2008 to 74% in 2009. Hospitals, which are owned by the PRC government, have comparatively longer payment cycles, especially at the period of time when more investment was spent by hospitals to meet the medical reform requirement promulgated by the PRC government.
For our retail segment, accounts receivable turnover days for the year ended December 31, 2009 were 3 days as compared to 2 days in 2008. We generally receive cash from the customer at the retail store except for our medi-care qualified stores. The accounts receivable comprised only the medi-card reimbursement under the national program. The increase in accounts receivable was mainly attributable to the growth of the sales derived from the national insurance scheme upon the increase of the coverage of its scheme and resulted in the increase of the accounts receivable turnover days for these consecutive years.
For our manufacturing segment, the accounts receivable turnover days were 135 in 2009 compared to 108 in 2008. The increase was due to the extension of credit to our customers in light of the global financial crisis from year 2008 onwards.
Investing Activities
Our cash flow from investing activities primarily consists of purchases of property, plant and equipment and disposal of leasehold land. Cash used in investing activities was $8.5 million for the nine months ended September 30, 2010, compared to $0.3 million of cash used by investing activities for the same period in 2009, representing an increase of $8.2 million. The increase in cash used was primarily due to both the deposit and the payment for the acquisition of the chain stores of $5.8 million. The payment of a long term deposit by $3.2 million in connection with the acquisition of retail stores was partly offset by the proceeds from the disposal of land use rights.
Cash used in investing activities was $0.3 million for fiscal 2009, compared to $0.8 million for fiscal year 2008. The decrease in cash used was primarily due to the proceeds obtained from the disposal of land in fiscal year 2009 and the reduction in the expenditures for the acquisition of property, plant and equipment.
Financing Activities
Our cash flow from financing activities is derived primarily from the proceeds and from repayments of bank borrowings. Cash used in financing activities was $1.1 million for the nine months ended September 30, 2010, compared to $2.7 million used in financing activities for the same period in 2009 representing a decrease of cash from financing activities by $1.6 million. The decrease of cash from financing activities was primarily due to the proceeds received from private placement of $2.3 million and the reduction of restricted cash held by banks by $1.8 million. The increase was offset by the increase of repayment of other loans by $1.5 million as well as the net increase of advances to related companies by $1.1 million.
Cash provided by financing activities was $6.4 million for the twelve months of fiscal year 2009, compared to $9.9 million used in financing activities for fiscal year 2008. The substantial increase was primarily due to the proceeds of the Private Placement of shares amounting to $5.3 million in December 2009. In addition, we did not declare or pay any dividends in 2009. In 2008, a dividend of $6,940,000 was paid to former shareholders of Liuzhou BCT. Further, the increase was also attributable to the reduction in loan repayments to directors and other parties.
Working capital
Our working capital as of September 30, 2010 and December 31, 2009 was $44.8 million and $29.6 million respectively. Our working capital is critical to our financial performance. We must maintain sufficient liquidity and financial flexibility to continue our daily operations. Our sales practices with hospitals in our pharmaceutical distribution segment, which have a longer term of payment when compared with other customers and the increase in the proportion of our sales to hospitals have resulted in a significant demand for working capital.
81
The following table sets forth accounts receivable from our pharmaceutical distribution operations by category of customers:
|
As of September 30,
|
As of December 31,
|
|||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
'000
|
‘000 | ‘000 | ||||||||||
|
Hospitals
|
$
|
28,855
|
$
|
27,445
|
$
|
11,171
|
||||||
|
Other drug stores
|
5,635
|
15
|
29
|
|||||||||
|
Clinics and health care centres
|
8,914
|
189
|
148
|
|||||||||
|
Distributors and others
|
7,057
|
4,474
|
7,287
|
|||||||||
|
Total
|
$
|
50,461
|
$
|
32,123
|
$
|
18,635
|
||||||
We intend to continuously spend approximately $21.5 million for the opening of additional chain stores for the rest of year 2010 and in 2011. In order to satisfy and fully implement our growth strategies, we will need to raise capital, through equity or debt financing, either in a private placement or a public offering. However, there are no assurances that such financing will be available or available on terms acceptable to us. To the extent that we require additional financing in the future and are unable to obtain such additional financing, we may not be able to fully implement our growth strategies.
Borrowings and Credit Facilities
The short-term bank borrowings outstanding as of September 30, 2010 and December 31, 2009 were $8.8 million and $7.1 million, respectively while long term bank borrowings outstanding as of September 30, 2010 and December 31, 2009 were $2.4 million and $3.7 million respectively. The loans are from various financial institutions and represent the maximum amount of each facility. The short term loans bore an average interest rate of 5.99% and 7.14% per annum for these two periods, respectively, and the rate was adjusted currently or quarterly in accordance with the loan rate of the People’s Bank of China. These loans do not contain any financial covenants or restrictions. The loans are secured by the land use rights, property, plant and equipment of the Company and the properties from related-parties.
The short-term borrowings have one year terms and expire at various times throughout the year. These facilities contain no specific renewal terms.
The long-term borrowings have three to seven years terms. Part of the borrowings mature at various times over the next two years and part of the borrowings are repayable by monthly installments within the five years before maturity. The loan bears an interest rate from 6.48% to 7.74% and was adjusted currently to annual basis in accordance with the loan rate of the People’s Bank of China
These loans do not contain any financial covenants or restrictions. The loans are secured by the land use rights, property, plant and equipment of the Company and the properties from related-parties.
In addition to bank borrowings mentioned above, we have trade credit facilities in the amount of $1.5 million as at September 30, 2010 and $1.0 million was utilized as at September 30, 2010. The trade facilities are secured by land use rights, property, plant and equipment of the Company.
Critical Accounting Estimates
This section should be read together with the Summary of Significant Accounting Policies included as Notes to the consolidated financial statements included in our financial statements. Our consolidated financial information has been prepared in accordance with generally accepted accounting principles in the United States, which requires us to make judgments, estimates and assumptions that affect (1) the reported amounts of our assets and liabilities, (2) the disclosure of our contingent assets and liabilities at the end of each fiscal period and (3) the reported amounts of revenues and expenses during each fiscal period. We continually evaluate these estimates based on our own historical experience, knowledge and assessment of current business and other conditions, our expectations regarding the future based on available information and reasonable assumptions, which together form our basis for making judgments about matters that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, our actual results could differ from those estimates. Some of our accounting policies require a higher degree of judgment than others in their application.
82
When reviewing our financial statements, you should consider (1) our selection of critical accounting policies, (2) the judgment and other uncertainties affecting the application of those policies, and (3) the sensitivity of reported results to changes in conditions and assumptions. We believe the following accounting policies involve the most significant judgment and estimates used in the preparation of our financial statements.
Goodwill
We account for goodwill in accordance with the provisions of FASB ASC 805, “Goodwill and Intangible Assets” (“ASC 805”). We conduct impairment tests on an annual basis and, in addition, if we notice any indication of impairment, we conduct such test immediately. The application of the impairment test requires judgment, including the identification of reporting units, assignment of assets and liabilities to reporting units and the determination of the fair value of each reporting unit. Fair value is primarily determined by computing the future discounted cash flows expected to be generated by the reporting unit that are believed to be reasonable under current and forecasted circumstances, the results of which form the basis for making judgments about carrying values of the reported net assets of our reporting units. We conduct an impairment test as of December 31, 2009 and no impairment loss was identified.
Long-lived assets
Long-lived assets are tested for impairment in accordance with FASB ASC 360-10-45 “Impairment or Disposal of Long-Lived Assets” (previously SFAS No. 144). We periodically evaluate potential impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The application of the impairment test requires judgment inclusive of the future cash flow attributable from the use of the asset. If the asset is determined not to be recoverable, it is considered to be impaired and the impairment to be recognized.
Depreciation
Depreciation is provided on straight-line basis over estimated useful lives. We determine the estimated useful lives, residual values and related depreciation charges for our property, plant and equipment. This estimate is based on the historical experience of the actual useful lives and residual values of property, plant and equipment of similar nature and functions. We will revise the depreciation charge where useful lives and residual values are different from those previously estimated, or we will write-off or write-down technically obsolete or non-strategic assets that have been abandoned or sold.
Allowances for doubtful accounts
We establishes the general provisioning policy to make allowance equivalent to 40% of gross amount of trade receivables due between half and one year and 100% of gross amount of accounts receivable due over 1 year. Additional specific provision is made against trade receivables whenever they are considered to be doubtful. We make judgments about the customer’s ability to pay its outstanding invoices on a timely basis and whether its financial position might deteriorate significantly in the future affecting its capability for payment.
Allowances for inventories
In assessing the ultimate realization of inventories, we make judgments as to future demand requirements compared to current or committed inventory levels. We estimate the demand requirements based on market conditions, forecasts over demand by our customers, sales contracts and orders in hand. As at December 31, 2009, 7% of our inventory will expire within 7 to 9 months time, 8% will expire within 10 to 12 months time, 85% of inventory will expire in over 1 year. We estimate the extent of provision to be made should the inventory fall within 6 months before the expiration upon assessing the capability of the sales campaign and the possible return of drugs to the vendors. As for the drugs which will expire in over half a year’s time, we judge that the drugs are still marketable and estimate the provision upon the future demand and the current inventory level.
83
Recognition of revenue
Revenue is recognized when the following criteria under Financial Accounting Standards Board(“FASB”) Accounting Standards Codification (“ASC”) 605 “Revenue Recognition” should be met :
|
1)
|
Persuasive evidence of an arrangement exists;
|
|
2)
|
Delivery has occurred or services have been rendered;
|
|
3)
|
The seller’s price to the buyer is fixed or determinable; and
|
|
4)
|
Collectability is reasonably assured.
|
We base our estimates on historical results, taking into consideration the type of customer, the type of transaction and the specifics of each arrangement.
Sales of goods – Pharmaceutical distribution segment
We sell a range of pharmaceutical products to various customers and the majority of revenue results from the contracts signed with various distributors and hospitals which are government-owned. Sales of goods are recognized upon customers' acceptance when the goods have been delivered to the premises of customers and the customers have full discretion over the goods and the significant risks and rewards of ownership have been transferred to the customer. Currently we do not have any sales on consignment and we do not use consignment sales as our sales method. The price to the buyer is fixed in which no cancellation clause exists and no right of return is granted except in rare cases where those drugs are damaged during the delivery process. In the absence of the right of return clause, we estimate that the product return was insignificant other than the clause for damages arising from delivery. Except for damages incurred during delivery, we have no obligation to accept returns if the inventory kept by the customer is excessive or reaches its expiration date without being sold. We grant credit to customers with proven sales records. The collectability is assured by background checks for the new customer.
Sales of goods – Retail Pharmacy segment
We operate a chain of retail stores for selling the pharmacy products. Sales of goods are recognized upon customer acceptance when we sell and deliver the products to the individual customers at our stores. We estimate that no significant post-delivery obligations exist. Retail sales are in cash or medi-insurance card, for which the reimbursement is assured as it is run by the national government agency. No return is allowed after sales.
Sales of goods – Manufacturing segment
The revenue recognition criteria used with our manufacturing segment are the same as the operation under our pharmaceutical distribution segment except the customers comprise only distributors.
During the nine months ended September 30, 2010, $463,784 worth of goods were returned as a result of damage. We have not sold goods to customers as a result of incentives. We do not grant sales discount or any allowances to customers if they don’t re-sell their goods before the date of expiration. No return of goods is allowed except when the goods are damaged during the delivery process. As the return of goods are not allowed, we only assess and estimate the return arising from damage during the delivery process and the estimate of return is negligible. Thus, we do not assess any return derived from the levels of inventory in the distribution channel, estimated shelf life, and the introduction of new products as the factors are not relevant to us for the estimate of return.
Off Balance Sheet Arrangements
We have no off balance sheet arrangements.
84
On December 30, 2009, the board of directors of China BCT Pharmacy Group, Inc. terminated Bernstein & Pinchuk LLP, an Independent Member of BDO SEIDMAN Alliance (“Bernstein”) as the independent registered accounting firm, and engaged PKF, Certified Public Accountants, Hong Kong, China, a member firm of PKF International Limited network of legally independent firms (“PKF HK”), to serve as the Company’s independent auditors. Pursuant to Item 304(a) of Regulation S-K under the Securities Act of 1933, as amended, and under the Securities Exchange Act of 1934, as amended:
|
(a)
|
(i)
|
Bernstein was terminated as our independent registered public accounting firm effective on December 30, 2009.
|
|
|
(ii)
|
For the two most recent fiscal years ended March 31, 2009 and 2008, Bernstein’s reports on the financial statements did not contain any adverse opinions or disclaimers of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles, other than for a going concern.
|
||
|
(iii)
|
The termination of Bernstein and engagement of PKF HK was approved by our board of directors.
|
||
|
(iv)
|
We and Bernstein did not have any disagreements with regard to any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure for the audited financials for the fiscal years ended March 31, 2009 and 2008, and subsequent interim periods ended June 30 and September 30, 2009 and through the date of dismissal, which disagreements, if not resolved to the satisfaction of Bernstein, would have caused it to make reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(v)
|
During our fiscal years ended March 31, 2009 and 2008, and subsequent interim periods ended June 30 and September 30, 2009 and through the date of dismissal, we did not experience any reportable events.
|
||
|
(b)
|
(i)
|
On December 30, 2009, we engaged PKF HK to serve as our independent registered public accounting firm.
|
|
|
(ii)
|
Prior to engaging PKF HK, we had not consulted PKF HK regarding the application of accounting principles to a specified transaction, completed or proposed, the type of audit opinion that might be rendered on our financial statements or a reportable event, nor did we consult with PKF HK regarding any disagreements with our prior auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the prior auditor, would have caused it to make a reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(iii)
|
We did not have any disagreements with PKF HK and therefore did not discuss any past disagreements with PKF HK.
|
||
|
(c)
|
Bernstein furnished us with a letter addressed to the SEC stating that it agrees with the statements made by us regarding Bernstein.
|
On July 15, 2010, we engaged PKF, Certified Public Accountants, A Professional Corporation, Glendale, California, a member firm of PKF International Limited network of legally independent firms (“PKF California”), as our principal accountant, and dismissed PKF, Certified Public Accountants, Hong Kong, China, a member firm of PKF International Limited network of legally independent firms (“PKF HK”), from that role.
85
Pursuant to Item 304(a) of Regulation S-K under the Securities Act of 1933, as amended, and under the Securities Exchange Act of 1934, as amended:
|
(a)
|
(i)
|
PKF HK was terminated as our independent registered public accounting firm effective on July 15, 2010.
|
|
|
(ii)
|
For the two most recent fiscal years ended December 31, 2009 and 2008, PKF HK’s reports on the financial statements did not contain any adverse opinions or disclaimers of opinion, and were not qualified or modified as to uncertainty, audit scope, or accounting principles, other than for a going concern.
|
||
|
(iii)
|
The termination of PKF HK and engagement of PKF California was approved by our audit committee.
|
||
|
(iv)
|
We and PKF HK did not have any disagreements with regard to any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure for the audited financials for the fiscal years ended December 31, 2009 and 2008, and subsequent interim period through the date of dismissal, which disagreements, if not resolved to the satisfaction of PKF HK, would have caused it to make reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(v)
|
During our fiscal years ended December 31, 2009 and 2008, and subsequent interim period through the date of dismissal, we did not experience any reportable events.
|
||
|
(vi)
|
The reports of PKF HK on our financial statements for the fiscal years ended December 31, 2009 and 2008 did not contain an adverse opinion or a disclaimer of an opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles.
|
||
|
(b)
|
(i)
|
On July 15, 2010, we engaged PKF California to serve as our independent registered public accounting firm.
|
|
|
(ii)
|
Prior to engaging PKF California, we had not consulted PKF California regarding the application of accounting principles to a specified transaction, completed or proposed, the type of audit opinion that might be rendered on our financial statements or a reportable event, nor did we consult with PKF California regarding any disagreements with our prior auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of the prior auditor, would have caused it to make a reference to the subject matter of the disagreements in connection with its reports.
|
||
|
(iii)
|
We did not have any disagreements with PKF California and therefore did not discuss any past disagreements with PKF California.
|
||
|
(c)
|
PKF HK furnished us with a letter addressed to the SEC stating that it agrees with the statements made by us regarding PKF HK.
|
86
The following table sets forth the name and age of officers and directors as of August 30, 2010. Our executive officers are elected annually by our board of directors. Our executive officers hold their offices until they resign, are removed by the board, or his or her successor is elected and qualified.
Directors and Executive Officers
|
Name
|
Age
|
Position
|
|||
|
Hui Tian Tang
|
49
|
Chairman & Chief Executive Officer
|
|||
|
Xiao Yan Zhang
|
37
|
Director & Chief Financial Officer
|
|||
|
Eng Leong Lee
|
43
|
Independent Director
|
|||
|
Simon Choi
|
50
|
Independent Director
|
|||
|
Man Wai Chiu
|
49
|
Independent Director
|
|||
Mr. Hui Tian Tang
Mr. Tang was appointed as our CEO on December 30, 2009 and as our Chairman on January 14, 2010. Mr. Tang is a registered pharmacist and has been the president of Liuzhou BCT since 2001. In 1993, he was hired as the general manager by Liuzhou BCT (f/k/a Guangxi Liuzhou Wholesaler) and was promoted to president of the company in 2001. Mr. Tang has over 25 years experience in the traditional Chinese medicine industry. As the president of Liuzhou BCT, Mr. Tang has been responsible for the formulation of strategies, decision-making on investment projects and development directions on the operations and overall business management, and led us successfully through the privatization process in 2001. Prior to his employment with Liuzhou BCT in 1993, Mr. Tang was employed by Guangxi Jinchengjiang Medicine Wholesaler Group from 1983 to 1993 where he was deputy general manager. In July 1983, Mr. Tang received a Bachelor of Chinese Pharmacy from Guangxi Chinese Medicine University. As our CEO, we believe that Mr. Tang’s extensive experience in the traditional Chinese medicine industry and his essential knowledge of the Company’s operations provide him with significant insights into our business which make him qualified to be the Chairman of our board of directors.
Ms. Xiao Yan Zhang
Ms. Zhang joined Liuzhou BCT in May 2008 as our Corporate Strategy VP and was appointed as our CFO on December 30, 2009 and as a director on January 14, 2010. Prior to joining Liuzhou BCT, from 2006 to 2008 she was a corporate finance advisor to First Asia Finance Group, in Hong Kong. Ms. Zhang is an Associate Member of CPA Australia. She received a Masters degree in accounting from Curtin University of Technology, Australia in 2007, an MBA in International Business from CMSD Switzerland in 2001 and a BA (Honors) in Marketing from Portsmouth University, UK in 2004. We believe that Ms. Zhang brings extensive finance experience and vital operational experience to the board of directors.
87
Mr. Eng Leong Lee
Mr. Lee was appointed as our director on June 7, 2010. Mr. Lee has been the Group Chief Financial Officer for Alliance Bank Malaysia Berhad since January 2010, responsible for the overall strategy and management of group finance. From 2000 to 2010, he held various positions with Microsoft Corporation, most recently as the CFO of Microsoft Greater China. Mr. Lee also has over 20 years of experience in public accounting, financial reporting and auditing, including tenures with KPMG and Price Waterhouse. Mr. Lee is a Certified Public Accountant and Chartered Accountant of Malaysia and is a member of Malaysian Institute of Accountants and the Malaysian Institute of Internal Auditors. Mr. Lee received a degree in accounting in 1992 from the Malaysian Institute of Certified Public Accountants (MICPA) and qualified as a Certified Public Accountant with MICPA in 1993. We believe that Mr. Lee’s extensive experience in public accounting, financial reporting and auditing provides him with a valuable perspective from which he helps advise the board of directors.
Mr. Simon Choi
Mr. Choi was appointed as our director on June 7, 2010. Mr. Simon Choi has been International Legal Counsel for TCL, a global TV manufacturer, since February 2005. From 1998 to 2005, Mr. Choi was the Legal Counsel and Company Secretary of Simatelex, a Hong Kong OEM small electrical appliances manufacturer, where he advised on a variety of corporate legal matters. Earlier in his career, Mr. Choi was an Assistant Solicitor of Hastings & Co, Solicitors, where he specialized in banking, corporate finance, general commercial and PRC practice. Mr. Choi is also an active PRC legal advisor to the Hong Kong Electrical Appliances Manufacturers Association, a major trade association in Hong Kong. Mr. Choi received an LLB from Peking University in 1991, an LLM from London University in 1992 and a CPEC in Law from Hong Kong University in 1994. Mr. Choi was admitted as a Solicitor of the Supreme Court of England and Wales in 1998 and as a member of the Institute of Linguists in 1996. We believe that Mr. Choi’s diverse legal background and familiarity with PRC law provides him with a unique and valuable legal perspective from which he helps advise the board of directors.
Mr. Man Wai Chiu
Mr. Chiu was appointed as our director on June 7, 2010. Mr. Man Wai Chiu has served as the Chief Operating Officer of Deer Consumer Products, Inc. since May 2007, where he manages sales, sourcing, shipping and accounting. From April 1997 to May 2007, he held various positions at Hamilton Beach Proctor-Silex, Inc., most recently as the Sourcing Director. Mr. Chui received his Bachelor of Laws from the University of London and MBA from Charles Sturt University in Australia. We believe that Mr. Chiu brings vital management and corporate strategy experience to the board of directors.
Board Composition
Our board of directors is currently composed of five members. All actions of the board of directors require the approval of a majority of the directors in attendance at a meeting at which a quorum is present. A quorum is a majority of the board of directors.
Family Relationships
There are no family relationships between any of our directors or executive officers and any other directors or executive officers.
Corporate Governance
Code of Ethics
On June 9, 2010, we adopted a new Code of Business Conduct and Ethics, which we believe is more comprehensive than our prior Code of Ethics. The new Code of Ethics, in accordance with Section 406 of the Sarbanes-Oxley Act of 2002 and Item 406 of Regulation S-K, constitutes our Code of Ethics for our principal executive officer, our principal financial and accounting officer and our other senior financial officers. The Code of Ethics is intended to promote honest and ethical conduct, full and accurate reporting, and compliance with laws as well as other matters. A printed copy of the Code of Ethics may be obtained free of charge by writing to China BCT Pharmacy Group, Inc., No. 102 Chengzhan Road, Liuzhou City, Guangxi province, China.
88
Director Independence
We use the definition for independence set forth in Rule 5605(a)(2) of the NASDAQ Marketplace Rules to determine that we have a majority of the board of directors comprised of “independent” directors, and to determine that the committees of our board of directors are comprised of “independent” directors. Based on those standards set forth in Rule 5605(a)(2), the board of directors has determined that Eng Leong Lee, Simon Choi and Man Wai Chiu are independent directors, who make up a majority of the directors on our board.
Committees of the Board of Directors
Audit Committee
Our Audit Committee consists of Eng Leong Lee, Simon Choi and Man Wai Chiu, each of whom is independent. The Audit Committee assists the board of directors oversight of (i) the integrity of our financial statements, (ii) our compliance with legal and regulatory requirements, (iii) the independent auditor’s qualifications and independence, and (iv) the performance of our internal audit function and independent auditor, and prepares the report that the Securities and Exchange Commission requires to be included in our annual proxy statement. The Audit Committee operates under a written charter. Mr. Lee is the Chairman of our Audit Committee.
The board of directors determined that Mr. Lee possesses accounting or related financial management experience that qualifies him as financially sophisticated within the meaning of Rule 4350(d)(2)(A) of the Nasdaq Marketplace Rules and that he is an “audit committee financial expert” as defined by the rules and regulations of the SEC.
Nominating and Corporate Governance Committee
The purpose of the Nominating and Corporate Governance Committee is to assist the board of directors in identifying qualified individuals to become members of our board of directors, in determining the composition of the board of directors and in monitoring the process to assess board effectiveness. Each of Eng Leong Lee, Simon Choi and Man Wai Chiu are members of the Nominating and Corporate Governance Committee. The Nominating and Corporate Governance Committee operates under a written charter. Mr. Choi is the Chairman of the Nominating Committee.
Compensation Committee
The Compensation Committee is responsible for overseeing and, as appropriate, making recommendations to the board of directors regarding the annual salaries and other compensation of our executive officers and general employees and other policies, and for providing assistance and recommendations with respect to our compensation policies and practices. Each of Eng Leong Lee, Simon Choi and Man Wai Chiu are members of the Compensation Committee. The Compensation Committee operates under a written charter. Mr. Choi is the Chairman of the Compensation Committee.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of our Company during the past ten years.
Conflicts of Interest
Ms. Zhang, one of our directors and our chief financial officer, controls approximately 58.9% of our outstanding shares of common stock that are entitled to vote on all corporate actions. Ms. Zhang’s actions could have a substantial impact on matters requiring the vote of the shareholders, including the election of our directors and most of our corporate actions. This control could delay, defer or prevent others from initiating a potential merger, takeover or other change in our control, even if these actions would benefit our shareholders and us. This control could adversely affect the voting and other rights of our other shareholders and could depress the market price of our common stock.
89
Certain potential conflicts of interest are inherent in the relationships between our officers and directors, and us.
From time to time, one or more of our affiliates may form or hold an ownership interest in and/or manage other businesses both related and unrelated to the type of business that we own and operate. These persons expect to continue to form, hold an ownership interest in and/or manage other businesses which may compete with ours with respect to operations, including financing and marketing, management time and services and potential customers. These activities may give rise to conflicts between or among the interests of us and other businesses with which our affiliates are associated. Our affiliates are in no way prohibited from undertaking such activities, and neither we nor our shareholders will have any right to require participation in such other activities.
Further, because we intend to transact business with some of our officers, directors and affiliates, as well as with firms in which some of our officers, directors or affiliates have a material interest, potential conflicts may arise between the respective interests of us and these related persons or entities. We believe that such transactions will be effected on terms at least as favorable to us as those available from unrelated third parties.
With respect to transactions involving real or apparent conflicts of interest, we have adopted policies and procedures which require that: (i) the fact of the relationship or interest giving rise to the potential conflict be disclosed or known to the directors who authorize or approve the transaction prior to such authorization or approval, (ii) the transaction be approved by a majority of our disinterested outside directors, and (iii) the transaction be fair and reasonable to us at the time it is authorized or approved by our directors.
Compensation Discussion and Analysis
We strive to provide our named executive officers with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in the same or similar locality.
It is not uncommon for companies with operations primarily in China to have base salaries and bonuses as the sole and only form of compensation. Prior to the establishment of our Compensation Committee in June 2010, the base salary level for all of our executive officers, including our Chairman and Chief Executive Officer, Mr. Tang, was established and reviewed. Mr. Tang, based on the level of responsibilities, the experience and tenure of the individual and the current and potential contributions of the individual. However, since the establishment of our Compensation Committee in June 2010, our Compensation Committee has been delegated the authority to make these decisions. The base salary is compared to similar positions within comparable peer companies and with consideration of the executive’s relative experience in his or her position. Based on an evaluation of available information with respect to the base salaries of executives of our competitors, we believe that the base salary and bonus paid to our named executive officers is in line with our competitors. Base salaries are reviewed periodically and at the time of promotion or other changes in responsibilities.
As indicated under the heading “2010 Omnibus Securities and Incentive Plan” below, the board of directors has recently implemented a more comprehensive compensation program appropriate for executives of a public company, which takes into account other elements of compensation, including without limitation, short and long term compensation, cash and non-cash, and other equity-based compensation such as stock options. We believe that such compensation programs are comparable to our peers in the industry and will retain and attract talented individuals.
90
Summary Compensation Table— Fiscal Years Ended December 31, 20 10 and 200 9
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named person for services rendered in all capacities during the noted periods.
|
Name and Principal Position (1)
|
Year Ended December 31
|
Salary
($)
|
Bonus
($) (5)
|
Stock Awards ($)
|
Option Awards ($) (6) (7)
|
Non-Equity Incentive Plan Compensation Earnings ($)
|
Non- Qualified Deferred Compensation Earnings ($)
|
All Other Compensation ($)
|
Total ($)
|
|||||||||||||||||||||||||
|
Lisa Lopomo
|
20 10
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|||||||||||||||||||||||||
|
former CEO and
|
2009
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|||||||||||||||||||||||||
|
Director (1)
|
||||||||||||||||||||||||||||||||||
|
Hui Tian Tang
|
20 10
|
139,200
|
0
|
0
|
969,370
|
0
|
0
|
0
|
1,108,570
|
|||||||||||||||||||||||||
|
Chairman and CEO (2) (3)
|
200 9
|
27,027
|
0
|
0
|
0
|
0
|
0
|
0
|
27,027
|
|||||||||||||||||||||||||
|
Xiaoyan Zhang
|
20 10
|
109,092
|
0
|
0
|
805,070
|
0
|
0
|
0
|
914,162
|
|||||||||||||||||||||||||
|
CFO and Director (2) (4)
|
200 9
|
15,500
|
0
|
0
|
0
|
0
|
0
|
0
|
15,500
|
|||||||||||||||||||||||||
| (1) |
On December 30, 2009, Ms. Lisa Lopomo tendered her letter of resignation to resign as CEO, effective December 30, 2009 and to resign from our board of directors, effective on January 14, 2010.
|
| (2) |
On December 30, 2009, Hui Tian Tang and Xiaoyan Zhang were elected to the board of directors of the Company, effective January 14, 2010 which is 10 days following the filing of an information statement required by Rule 14f-1 promulgated under the Exchange Act. In addition, effective on December 30, 2009, Hui Tian Tang and Xiaoyan Zhang were also elected to serve as the CEO and CFO of the Company, respectively.
|
| (3) |
Represents amounts paid to Mr. Tang by Liuzhou BCT.
|
| (4) |
Represents amounts paid to Ms. Zhang as Corporate Strategy V.P. of Liuzhon BCT.
|
| (5) |
The amount of bonuses due to Mr. Tang and Ms. Zhang for the year ended December 31, 2010, are to be determined at the discretion of the Company’s board of directors and are not calculable as of the date of this prospectus. The Company expects to be able to determine such amounts after the issuance of the audit report, which the Company anticipates will be not later than March 31, 2011.
|
| (6) |
The options granted to each of Mr. Tang and Ms. Zhang will vest and become fully exercisable if the Company achieves after-tax net income of at least $26 million for the 2010 fiscal year and such options will terminate if the company does not meet that target. The date for determination is the date of the audit report, which the Company anticipates will be not later than March 31, 2011.
|
| (7) | Represents the aggregate grant date fair value of option awards made during 2010 computed in accordance with FASB ASC Topic 718. Fair values were estimated using the binomial model. For a description of the assumptions used to determine these amounts, see Note 18 in the Notes to the Consolidated Financial Statements filed herewith for the period ended September 30, 2010. |
Outstanding Equity Awards— Fiscal Year Ended December 31, 2010
The following table sets forth information concerning unexercised options; stock that has not vested equity incentive plan awards for each named executive officer outstanding as of the end of the fiscal year ended December 31, 2010.
|
Name and Principal Position
|
Number of
Securities Underlying Unexercised
options
|
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options
|
Equity Incentive Plan Awards: Number of
Securities Underlying
Unexercised Unearned Options (1)
|
Option Exercise
Price ($)
|
Option Expiration
Date (2)
|
Number of Shares or Units of Stock that have not Vested
|
Market
Value of Shares of Units of Stock that Have not Vested
($)
|
Equity Incentive
Plan Awards:
Number of Unearned Shares, Units or
Other Rights that have not Vested
|
Equity Incentive
Plan Awards:
Market or
Payout Value of Unearned
Shares, Units or other Rights that have not Vested ($)
|
||||||||||||||||||||||||
|
Hui Tian Tang
|
0
|
0
|
590,000
|
4.00
|
June 27, 2020
|
0
|
0
|
0
|
0
|
||||||||||||||||||||||||
|
Chairman and CEO
|
|||||||||||||||||||||||||||||||||
|
Xiaoyan Zhang
|
0
|
0
|
490,000
|
4.00
|
June 27, 2020
|
0
|
0
|
0
|
0
|
||||||||||||||||||||||||
|
CFO and Director
|
|||||||||||||||||||||||||||||||||
|
(1)
|
The options granted to each of Mr. Tang and Ms. Zhang will vest and become fully exercisable if the Company achieves after-tax net income of at least $26 million for the 2010 fiscal year and such options will terminate if the company does not meet that target. The date for determination is the date of the audit report, which the Company anticipates will be not later than March 31, 2011. The exercise price of the option may be paid for in cash or by a cashless exercise.
|
|
(2)
|
If the options do not become exercisable on account of the failure to meet the 2010 after-tax net income target described in footnote 1 above, the options shall terminate on the date on which the audit report is dated. The options otherwise terminate on the earlier of (i) June 27, 2020 or (ii) the date as of which the option has been fully exercised.
|
91
2010 Omnibus Securities and Incentive Plan
On June 27, 2010, our board of directors adopted the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) for the benefit of our employees, nonemployee directors and consultants and the employees, nonemployee directors and consultants of its affiliates, for purposes of assisting us to attract, retain and provide incentives to key management employees and nonemployee directors of, and non-employee consultants to, us and our affiliates, and to align the interests of such individuals with those of our stockholders. Accordingly, the Plan provides for the granting of distribution equivalent rights, incentive stock options, non-qualified stock options, performance share awards, performance unit awards, restricted stock awards, restricted stock unit awards, stock appreciation rights, tandem stock appreciation rights, unrestricted stock awards or any combination of the foregoing, as may be best suited to the circumstances of the particular employee, director or consultant as provided in the Plan.
The following is a summary of the material provisions of the Plan and is qualified in its entirety by reference to the complete text of the Plan, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Administration. The Plan is administered by the compensation committee of the board of directors, which consists of three members of the board of directors, each of whom is a “non-employee director” within the meaning of Rule 16b-3 promulgated under the Exchange Act and an “outside director” within the meaning of Code Section 162(m). Among other things, the compensation committee has complete discretion, subject to the express limits of the Plan, to determine the directors, employees and nonemployee consultants to be granted an award, the type of award to be granted, the number of shares of common stock subject to each award, the exercise price of each option and base price of each stock appreciation right, the term of each award, the vesting schedule for an award, whether to accelerate vesting, the value of the shares of common stock underlying the award, and the required withholding, if any. The compensation committee may amend, modify or terminate any outstanding award, provided that the participant’s consent to such action is required if the action would materially and adversely impair the participant’s rights or entitlements with respect to that award. The compensation committee is also authorized to construe the award agreements, and may prescribe rules relating to the Plan. Notwithstanding the foregoing, the compensation committee does not have any authority to grant or modify an award under the Plan with terms or conditions that would cause the grant, vesting or exercise to be considered nonqualified “deferred compensation” subject to Code Section 409A.
Grant of Awards; Shares Available for Awards. The Plan provides for the grant of options, stock appreciation rights, performance share awards, performance unit awards, distribution equivalent right awards, restricted stock awards, restricted stock unit awards and unrestricted stock awards to directors, officers, employees and nonemployee consultants of us or our affiliates. The aggregate number of shares of common stock that may be issued under the Plan shall not exceed 12.5% of the total shares of common stock which are issued and outstanding and shares of common stock issuable upon the conversion of any outstanding shares of preferred stock of the Company. If any award expires, is cancelled, or terminates unexercised or is forfeited, any shares subject thereto is again available for grant under the Plan.
Currently, there are 7 employees and directors who would be entitled to receive stock options and/or restricted shares under the Plan. Future new hires and additional consultants would be eligible to participate in the Plan as well. The number of options and/or restricted shares to be granted to executives and directors cannot be determined at this time as the grant of stock options and/or restricted shares is dependent upon various factors such as hiring requirements and job performance.
Stock Options. Options granted under the Plan may be either “incentive stock options” (“ISOs”), which are intended to meet the requirements for special federal income tax treatment under the Code, or “nonqualified stock options” (“NQSOs”). Options may be granted on such terms and conditions as the compensation committee may determine; provided, however, that the per share exercise price under an option may not be less than the fair market value of a share of the underlying shares of common stock on the date of grant and the term of the option may not exceed 10 years (110% of such value and 5 years in the case of an ISO granted to an employee who owns (or is deemed to own) more than 10% of the total combined voting power of all classes of our capital stock or our parent or subsidiary). ISOs may only be granted to employees. In addition, the aggregate fair market value of shares of common stock covered by ISOs (determined at the time of grant) which are exercisable for the first time by an employee during any calendar year may not exceed $100,000. Any excess is treated as a NQSO.
Stock Appreciation Rights. A stock appreciation right (“SAR”) entitles the participant, upon exercise, to receive an amount, in cash or stock or a combination thereof, equal to the increase in the fair market value of the underlying shares of common stock between the date of grant and the date of exercise. SARs may be granted in tandem with, or independently of, options granted under the Plan. A SAR granted in tandem with an option (i) is exercisable only at such times, and to the extent, that the related option is exercisable in accordance with the procedure for exercise of the related option; (ii) terminates upon termination or exercise of the related option (likewise, the option granted in tandem with a SAR terminates upon exercise of the SAR); (iii) is transferable only with the related option; and (iv) if the related option is an ISO, may be exercised only when the value of the stock subject to the option exceeds the exercise price of the option. A SAR that is not granted in tandem with an option is exercisable at such times as the compensation committee may specify.
92
Performance Shares or Performance Unit Awards. Performance share or performance unit awards entitle the participant to receive cash or shares of common stock upon attaining specified performance goals. In the case of performance units, the right to acquire the units is denominated in cash values.
Distribution Equivalent Right Awards. A distribution equivalent right award entitles the participant to receive bookkeeping credits, cash payments and/or common stock distributions equal in amount to the distributions that would have been made to the participant had the participant held a specified number of our shares of common stock during the period the participant held the distribution equivalent right. A distribution equivalent right may be awarded as a component of another award, where, if so awarded, such distribution equivalent right will expire or be forfeited by the participant under the same conditions as under such other award.
Restricted Stock Awards or Restricted Stock Unit Award. A restricted stock award is a grant or sale of shares of common stock to the participant, subject to our right to repurchase all or part of the shares at their purchase price (or to require forfeiture of such shares if purchased at no cost) in the event that conditions specified by the compensation committee in the award are not satisfied prior to the end of the time period during which the shares subject to the award may be repurchased by or forfeited to us. A restricted stock unit entitles the participant to receive a cash payment equal to the fair market value of a share of common stock for each restricted stock unit subject to such restricted stock unit award, if the holder satisfies the applicable vesting requirement.
Unrestricted Stock Awards. An unrestricted stock award is a grant or sale of shares of common stock to the participant that is not subject to transfer, forfeiture or other restrictions, in consideration for past services rendered to us or an affiliate or for other valid consideration.
Change-in-Control Provisions. In connection with the grant of an award, the compensation committee may provide that, in the event of a change in control, such award will become fully vested and immediately exercisable.
Amendment and Termination. The compensation committee may adopt, amend and rescind rules relating to the administration of the Plan, and amend, suspend or terminate the Plan, but no such amendment or termination will be made that materially and adversely impairs the rights of the participant with respect to any award without the participant’s consent, other than amendments that are necessary to permit the granting of awards in compliance with Code Sections 162(m) and/or 409A. We have attempted to structure the Plan so that remuneration attributable to options and other awards will not be subject to the deduction limitation contained in Code Section 162(m).
Employment Agreements
We have recently entered into employment agreements with Hui Tian Tang, our CEO, and Xiaoyan Zhang, our CFO. The employment agreements were negotiated on our behalf by Mr. Tang and were approved by our board of directors on May 18, 2010. At that time our board of directors consisted only of Mr. Tang and Ms. Zhang. The following is a summary of the material provisions of the employment agreements for each of Mr. Tang and Ms. Zhang and is qualified in its entirety by reference to the complete text of such employment agreements, copies of which are filed as exhibits to the registration statement of which this prospectus is a part.
Hui Tian Tang
On January 1, 2008, Liuzhou BCT entered into an employment agreement with Mr. Hui Tian Tang pursuant to which Mr. Tang was hired as president of Liuzhou BCT and received a salary of $27,027 per year in 2008 and 2009. That agreement expired on December 31, 2009. From January 1, 2010 to May 18, 2010 we had an oral agreement with Mr. Tang pursuant to which we agreed to pay him 90,000 Hong Kong Dollars ($11,688) per month and a discretionary bonus based upon our 2010 financial performance On May 18, 2010, we and Liuzhou BCT entered into a new employment agreement with Mr. Tang to employ him as our CEO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay him RMB 79,600 ($11,600) per month (or RMB 955,200 ($139,200) per year) and a bonus based upon our 2010 financial performance and for 2011, upon our 2011 financial performance. We believe Mr. Tang deserved this increase in salary because of the Company’s growth over the past several years under Mr. Tang’s leadership and to provide Mr. Tang with a more competitive base salary that is in line with his role and responsibilities at the Company. The agreement provides that if we achieve an after-tax net income of at least $26 million for the 2010 fiscal year, Mr. Tang’s options which may be exercised for 590,000 shares of our common stock will vest and become exercisable, as discussed more specifically below under the heading “Stock Option Agreements.” The Company may, in its discretion, pay a bonus to Mr. Tang, the details of which will be set forth by the Company.
93
The agreement may be terminated upon mutual agreement between the Company and Mr. Tang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Mr. Tang is criminally prosecuted under the law.
The Company may terminate the agreement by serving 30 days' prior written notice to Mr. Tang or giving Mr. Tang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Mr. Tang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out his job responsibilities; (ii) where Mr. Tang is unable to fulfill the duties of his position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Mr. Tang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Mr. Tang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Mr. Tang in accordance with the law; (ii) the Company forces Mr. Tang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
On May 18, 2010, we also entered into a non-disclosure and non-competition agreement with Mr. Tang. Pursuant to this agreement, Mr. Tang agreed to maintain the confidentiality of all confidential information as defined in the agreement and not disclose any of such confidential information without our prior written consent. Mr. Tang also agreed that during the course of his employment and for a period of 24 months immediately following the termination of his relationship with us for any reason, Mr. Tang will not (i) serve as a partner, employee, consultant, officer, director, manager, agent, associate, investor, or otherwise for, (ii) directly or indirectly, own, purchase, organize or take preparatory steps for the organization of, (iii) build, design, finance, acquire, lease, operate, manage, invest in, work or consult for or otherwise affiliate with, any business, in competition with or otherwise similar to our business. The foregoing covenant covers Mr. Tang’s activities in (a) the People's Republic of China, (b) Taiwan, (c) Hong Kong, (d) Macao, (e) the United States of America, and (f) all other countries and/or regions of the world. In addition, Mr. Tang agreed that for a period of 24 months immediately following the termination of his relationship with us for any reason, Mr. Tang shall not solicit any of our employees to leave their employment, either for himself or for any other person or entity. The description of the non-disclosure and non-competition agreement with Mr. Tang is qualified in its entirety by reference to the complete text of such agreement, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Xiaoyan Zhang
Beginning on January 1, 2010, we had an oral agreement with Xiaoyan Zhang to employ her as our CFO, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and a discretionary bonus based upon our 2010 financial performance. On May 18, 2010, we and Forever Well entered into a new employment agreement with Ms. Zhang to employ her as our CFO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and a bonus based upon our 2010 financial performance and for 2011, upon our 2011 financial performance. The agreement provides that if we achieve an after-tax net income of at least $26 million for the 2010 fiscal year, Ms. Zhang shall receive vested options which may be exercised for 490,000 shares of our common stock, as discussed more specifically below under the heading “Stock Option Agreements.” The Company may, in its discretion, pay a bonus to Ms. Zhang, the details of which will be set forth by the Company.
The agreement may be terminated upon mutual agreement between the Company and Ms. Zhang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Ms. Zhang is criminally prosecuted under the law.
94
The Company may terminate the agreement by serving 30 days' prior written notice to Ms. Zhang or giving Ms. Zhang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Ms. Zhang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out her job responsibilities; (ii) where Ms. Zhang is unable to fulfill the duties of her position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Ms. Zhang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Ms. Zhang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Ms. Zhang in accordance with the law; (ii) the Company forces Ms. Zhang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
On May 18, 2010, we also entered into a non-disclosure and non-competition agreement with Ms. Zhang. Pursuant to this agreement, Ms. Zhang agreed to maintain the confidentiality of all confidential information as defined in the agreement and not disclose any of such confidential information without our prior written consent. Ms. Zhang also agreed that during the course of her employment and for a period of 24 months immediately following the termination of her relationship with us for any reason, Ms. Zhang will not (i) serve as a partner, employee, consultant, officer, director, manager, agent, associate, investor, or otherwise for, (ii) directly or indirectly, own, purchase, organize or take preparatory steps for the organization of, (iii) build, design, finance, acquire, lease, operate, manage, invest in, work or consult for or otherwise affiliate with, any business, in competition with or otherwise similar to our business. The foregoing covenant covers Ms. Zhang’s activities in (a) the People's Republic of China, (b) Taiwan, (c) Hong Kong, (d) Macao, (e) the United States of America, and (f) all other countries and/or regions of the world. In addition, Ms. Zhang agreed that for a period of 24 months immediately following the termination of her relationship with us for any reason, Ms. Zhang shall not solicit any of our employees to leave their employment, either for herself or for any other person or entity. The description of the non-disclosure and non-competition agreement with Ms. Zhang is qualified in its entirety by reference to the complete text of such agreement, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Stock Option Agreements
On June 27, 2010, we entered into a stock option agreement with Mr. Tang which provides for the grant to Mr. Tang of an option to purchase 590,000 shares of our common stock at an exercise price of $4.00 per share. The option vests and becomes exercisable with respect to all of the shares solely if our after-tax net income for fiscal 2010 equals at least U.S. $26,000,000 (excluding any non-cash expenses) (the “2010 Income Target”), determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditor in its report on said financial statements (the “Audit Report”). If the 2010 Income Target is met, the option shall vest and become exercisable on the date on which the Audit Report is dated, and if the option does not become exercisable on account of the failure to meet the 2010 Income Target, the option shall terminate on the date on which the Audit Report is dated. The exercise price of the option may be paid for in cash or by a cashless exercise. The option terminates on the earlier of (i) June 27, 2020 or (ii) the date as of which the option has been fully exercised.
On June 27, 2010, we also entered into a stock option agreement with Ms. Zhang which provides for the grant to Ms. Zhang of an option to purchase 490,000 shares of our common stock at an exercise price of $4.00 per share. The option vests and becomes exercisable with respect to all of the shares solely if our after-tax net income for fiscal 2010 equals at least the 2010 Income Target, determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditor in the Audit Report. If the 2010 Income Target is met, the option shall vest and become exercisable on the date on which the Audit Report is dated, and if the option does not become exercisable on account of the failure to meet the 2010 Income Target, the option shall terminate on the date on which the Audit Report is dated. The exercise price of the option may be paid for in cash or by a cashless exercise. The option terminates on the earlier of (i) June 27, 2020 or (ii) the date as of which the option has been fully exercised.
95
On July 16, 2010, we entered into separate stock option agreements with each of our three independent directors, Messrs. Lee, Choi and Chiu, which agreements provide for the grant to each such individual of an option to purchase 10,000 shares of our common stock at an exercise price of $4.00 per share. Each option vests and becomes exercisable with respect to all of the shares on June 6, 2011. The exercise price of each option may be paid for in cash or by cancellation of existing shares of our common stock held by the independent directors. Each option terminates on the earlier of (i) July 16, 2015 or (ii) the date as of which the option has been fully exercised.
Outstanding Equity Awards at Fiscal Year End
None of our executive officers received any equity awards, including, options, restricted stock or other equity incentives during the fiscal year ended December 31, 2009.
Independent Director Agreements
In connection with the appointments of Messrs. Lee, Choi and Chiu to our board of directors, we entered into one year agreements with each of them. Pursuant to these agreements, each of Messrs. Lee, Choi and Chiu will receive a fee of One Hundred Twenty Thousand Hong Kong Dollars (HK$120,000) (US$ 17,647) for the term of the agreement, payable in cash in equal monthly installments. We will also reimburse each independent director for expenses related to his attending meetings of the board, meetings of committees of the board, executive sessions and stockholder meetings.
Director Compensation
Except as set forth above, our directors are reimbursed for reasonable expenses incurred by them in connection with attending meetings of the board, meetings of committees of the board, executive sessions and stockholder meetings, but they do not receive any other compensation for serving on the board of directors. None of the directors has received compensation for their respective services rendered to us for the 2008 and 2009 fiscal years.
Compensation Committee Interlocks and Insider Participation
During the last fiscal year we did not have a standing Compensation Committee. Our board of directors was responsible for the functions that would otherwise be handled by the compensation committee. Other than Mr. Tang, our chairman and chief executive officer, Ms. Zhang, our director and chief financial officer, and Ms. Lopomo, our former director and chief executive officer, no other officers or employees participated in deliberations of our board of directors concerning executive officer compensation.
Indemnification of Directors and Executive Officers and Limitation of Liability
Delaware General Corporation Law does not limit the extent to which a company’s articles of association may provide for indemnification of officers and directors, except to the extent any such provision may be held by the courts of the State of Delaware to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Our articles of association provide for indemnification of our officers and directors for any liability incurred in their capacities as such, except through their own willful negligence or default.
In so far as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is theretofore unenforceable.
96
The following table sets forth certain information regarding beneficial ownership of our common stock effective September 30, 2010 by (i) each person (or group of affiliated persons) who is known by us to own more than five percent of the outstanding shares of our common stock, (ii) each director, and named executive officer, and (iii) all of our directors and executive officers as a group. As of September 30, 2010, we had 38,154,340 shares of common stock issued and outstanding.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Unless otherwise noted, the principal address of each of the stockholders, directors and officers listed below is c/o Guangxi Liuzhou Baicaotang Medicine Limited, No. 102 Chengzhan Road, Liuzhou City, Guangxi Province, PRC.
All share ownership figures include shares of our common stock and securities convertible or exchangeable into shares of our common stock within sixty (60) days of September 30, 2010, which are deemed outstanding and beneficially owned by such person for purposes of computing his or her percentage ownership, but not for purposes of computing the percentage ownership of any other person.
|
Name and Address of Beneficial Owners
|
Amount and Nature of Beneficial Ownership (1)
|
Percent of Class (2)
|
||||||
|
Xiao Yan Zhang (3)
|
22,480,000
|
58.9
|
%
|
|||||
|
Hui Tian Tang (3)
|
2,241,193
|
5.9
|
%
|
|||||
|
Eng Leong Lee
|
–
|
–
|
||||||
|
Man Wai Chiu
|
–
|
–
|
||||||
|
Simon Choi
|
–
|
–
|
||||||
|
Yu Jing Tan (3)
|
2,816,889
|
7.4
|
%
|
|||||
|
Jing Hua Li (3)
|
3,474,237
|
9.1
|
%
|
|||||
|
All directors and executive officers as a group (5 persons)
|
24,721,193
|
64.8
|
%
|
|||||
(1) Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares of common stock subject to securities anticipated to be exercisable or convertible at or within 60 days of the date hereof, are deemed outstanding for computing the percentage of the person holding such option or warrant but are not deemed outstanding for computing the percentage of any other person. The indication herein that shares are anticipated to be beneficially owned is not an admission on the part of the listed stockholder that he, she or it is or will be a direct or indirect beneficial owner of those shares.
(2) Based upon 38,154,340 shares of common stock issued and outstanding.
97
(3) Xiao Yan Zhang is a citizen of Hong Kong and our Chief Financial Officer, Secretary and director and holds the 22,480,000 shares as the legal owner. Pursuant to an Earn-in Agreement dated December 30, 2009, as amended on May 19, 2010, by and among Ms. Zhang and the Liuzhou BCT Shareholders, the Liuzhou BCT Shareholders have a call right to purchase up to 22,480,000 shares of our common stock from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $15 million and $19 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT Shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met the Liuzhou BCT Shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT Shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Our Chairman and CEO, Hui Tian Tang, is one of the Liuzhou BCT Shareholders and has the right to acquire up to 2,241,193 shares of our common stock under the Earn-In Agreement if both performance targets are met. Pursuant to the Earn-in-Agreement, there are no restrictions on Ms. Zhang’s ability to vote these shares. The shares may not be transferred by Ms. Zhang except pursuant to the Earn-in-Agreement and after its expiration only in the manner agreed with the Liuzhou BCT Shareholders. The Liuzhou BCT Shareholders can only obtain legal ownership of the shares under the Earn-In Agreement. Messrs. Tan and Li are Liuzhou BCT Shareholders with rights under the Earn-In Agreement to acquire the number of shares set opposite their names and have the same rights with respect to the shares as Mr. Tan’s rights described above in this footnote and elsewhere in this prospectus.
China BCT Pharmacy Group, Inc.
For a description of the related party agreements we entered into as part of our December 2009 reorganization, please see the section entitled “Our Corporate History”. Effective December 23, 2009, the Company entered into lock-up agreements with the original shareholders of Ingenious, consisting of Xiao Yan Zhang, Lei Ying, Chunqi Cao, Bixun Su, Hui Tian Tang, Yik Kwok Wah, and Yik Li Yee, pursuant to which these shareholders will be refrained from selling any of our securities from the date of the Subscription Agreement for twelve (12) months after the earlier of: (i) the effective date of the Registration Statement of which this prospectus is a part; or (ii) the date that the shares of common stock may be sold under Rule 144, without limitation.
Forever Well
On March 28, 2008, Forever Well entered into an agreement with the stockholders of Liuzhou BCT to acquire their entire equity interest in Liuzhou BCT for cash consideration of approximately $1,470,588 (RMB10,000,000) which is the registered and fully paid up capital of Liuzhou BCT.
Liuzhou BCT
On February 8, 2007, Mr. Hui Tian Tang entered into a loan agreement with Industrial and Commercial Bank of China Guangxi Branch in the amount of approximately $234,720 (RMB1,600,000), pursuant to which Liuzhou BCT, pledged part of its assets to the bank as security interest for the loan. Mr. Hui Tang then lent the full amount of the above loan to Liuzhou BCT for working capital. On December 31, 2008, a mutual agreement was signed among Mr. Tang, Industrial and Commercial Bank of China Guangxi Branch and Liuzhou BCT, pursuant to which Liuzhou BCT assumed the obligation to repay the principal amount and accrued interest from January 1, 2009 onwards.
98
On February 12, 2007, Jiang You Ru, a director of Liuzhoug BCT, entered into a loan agreement with Industrial and Commercial Bank of China Guangxi Branch in the amount of approximately $264,060 (RMB1,800,000), pursuant to which Liuzhou BCT pledged part of its assets to the bank as security interest for the loan. Mr. Ru then lent the full amount of the above loan to the Liuzhou BCT for working capital. On December 31, 2008, a mutual agreement was signed among Mr. Ru, Industrial and Commercial Bank of China Guangxi Branch and Liuzhou BCT, pursuant to which Liuzhou BCT assumed the obligation to repay the principal amount and accrued interest from January 1, 2009 onwards.
On January 15, 2009, Liuzhou BCT entered into a loan agreement with Agricultural Bank of China Liuzhou Branch in the amount of approximately $660,150 (RMB 4,500,000), pursuant to which both the Baicaotang Property Development Limited and Wuxuan Baicaotang Medicine Limited pledged part of their land and property to the bank as security interest for the loan. The loan has been repaid on January 15, 2010
On December 19, 2008, Liuzhou BCT entered into a loan agreement with Liuzhou City Commercial Bank in the amount of approximately $733,500 (RMB 5,000,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
On December 29, 2008, Liuzhou BCT entered into a loan agreement with Rurol Credence Cooperation of Guangxi in the amount of approximately $514,706 (RMB 3,500,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
On January 25, 2010, Liuzhou BCT entered into a loan agreement with Agricultural Bank of China Liuzhou Branch in the amount of approximately $1,290,000 (RMB 8,800,000), pursuant to which Baicaotang Property Development Limited and Liuzhou BCT pledged part of their premises to the bank as security interest for the loan.
On April 30, 2010, Liuzhou BCT entered into a loan agreement with Bank of Communication in the amount of approximately $2,640,600 (RMB 18,000,000), pursuant to which Baicaotang Property Development Limited pledged part of its premises to the bank as security interest for the loan.
In addition, we also entered into the following sales transactions at market prices with related parties, pursuant to a standard form of purchase agreement filed as an exhibit to the registration statement of which this prospectus is a part and we have continued to enter into such transactions.
|
Nine
|
||||||||||||
|
months ended
|
Year ended
|
Year ended
|
||||||||||
|
September 30,
|
December 31,
|
December 31,
|
||||||||||
|
Sales of goods to related parties
|
2010
|
2009
|
2008
|
|||||||||
|
Liucheng Medicine Limited (1)
|
$
|
215,090
|
$
|
311,759
|
$
|
438,371
|
||||||
|
Guangxi Tianhu Medicine Limited (2)
|
$
|
5,763
|
$
|
271,890
|
$
|
821,449
|
||||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch (3)
|
$
|
1,477,978
|
$
|
1,273,381
|
$
|
1,267,429
|
||||||
|
Wuxuan Baicaotang Medicine Limited (4)
|
$
|
29,763
|
$
|
208,475
|
$
|
487,781
|
||||||
|
(1)
|
The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jiang, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an aggregate 34.3% interest in the registered share capital of Liucheng Medicine Limited.
|
|
(2)
|
The directors of Liuzhou BCT, Huii Tian Tang, Jing Hua Li, You Ru Jiang, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an aggregate 46.3% interest in the registered share capital of Guangxi Tianhu Medicine Limited.
|
99
|
(3)
|
The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an aggregate 67.2% interest in the registered share capital of Guangxi Baicaotang Medicine Limited Guigang Branch.
|
|
(4)
|
The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an aggregate 35.3% interest in the registered share capital of Wuxuan Baicaotang Medicine Limited.
|
The amount due from related companies as of September 30, 2010 and December, 31 2009 and 2008 was as follows. Amounts due from related parties represent loans from us to related parties together with amounts representing our accounts receivable from related parties. Such loan amounts are non-interest bearing and have no due date as is often the custom in China.
|
Nine months ended
September 30,
|
Year ended
December 31,
|
Year ended December 31,
|
||||||||||||||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
||||||||||||||||||||||||||||||||||||||
|
Names of related parties
|
Trade receivable
|
Loan receivable
|
Total
|
Trade receivable
|
Loan receivable
|
Total
|
Trade receivable
|
Loan receivable
|
Total
|
Remark
|
||||||||||||||||||||||||||||||
|
Guangxi Tianhu Medicine Limited
|
$
|
125,429
|
$
|
1,397,407
|
$
|
1,522,836
|
$
|
406,519
|
-
|
$
|
406,519
|
$
|
504,937
|
$
|
177,661
|
$
|
682,598
|
(1
|
)
|
|||||||||||||||||||||
|
Liucheng Medicine Limited
|
$
|
174,748
|
-
|
174,748
|
-
|
-
|
-
|
$
|
28,423
|
-
|
$
|
28,423
|
(1
|
)
|
||||||||||||||||||||||||||
|
Wuxuan Baicaotang Medicine Limited
|
$
|
8,851
|
$
|
428,657
|
$
|
437,508
|
$
|
3,978
|
-
|
$
|
3,978
|
-
|
-
|
-
|
(1
|
)
|
||||||||||||||||||||||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch
|
$
|
1,846,741
|
$
|
756,698
|
$
|
2,603,439
|
$
|
117,506
|
$
|
2,279,657
|
$
|
2,397,163
|
$
|
185,472
|
-
|
$
|
185,472
|
(1
|
)
|
|||||||||||||||||||||
|
Property Management
|
-
|
$
|
251,960
|
$
|
251,960
|
-
|
$
|
258,092
|
$
|
258,092
|
-
|
$
|
128,440
|
$
|
128,440
|
(2
|
)
|
|||||||||||||||||||||||
|
Baicaotang Property Development Limited
|
-
|
$
|
253,917
|
253,917
|
-
|
$
|
1,209,834
|
$
|
1,209,834
|
-
|
$
|
3,633,128
|
$
|
3,633,128
|
(3
|
)
|
||||||||||||||||||||||||
|
Total
|
$
|
2,155,769
|
$
|
3,088,639
|
$
|
5,244,408
|
$
|
528,003
|
$
|
3,747,583
|
$
|
4,275,586
|
$
|
718,832
|
$
|
3,939,229
|
$
|
4,658,061
|
||||||||||||||||||||||
|
(1)
|
See Notes 1, 2, 3 and 4 in the table immediately above.
|
|
(2)
|
The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an aggregate 67.2% interest in the registered share capital of Baicaotang Property management.
|
|
(3)
|
The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wang have an interest over the related company by holding 67.2% of its paid up capital collectively
|
100
The amounts due to related companies represent loans from related companies at September 30, 2010, December 31, 2009 and December 31, 2008 was as follows. The amounts are interest free and no interest was paid during the relevant reporting period.
|
Nine months ended
September 30,
|
Year ended
December 31,
|
Year ended December 31,
|
||||||||||||||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
||||||||||||||||||||||||||||||||||||||
|
Names of related parties
|
Repayment of principal
|
Maximum balance
|
Outstanding
|
Repayment of principal
|
Maximum balance
|
Outstanding
|
Repayment of principal
|
Maximum balance
|
Outstanding
|
Remark
|
||||||||||||||||||||||||||||||
|
Wuxuan Baicaotang Medicine Limited
|
-
|
-
|
-
|
$
|
98,861
|
$
|
98,861
|
-
|
-
|
$
|
98,861
|
$
|
98,861
|
(1
|
)
|
|||||||||||||||||||||||||
|
Guangxi Tianhu Medicine Limited
|
-
|
-
|
-
|
$
|
5,134
|
$
|
5,134
|
-
|
-
|
$
|
5,134
|
$
|
5,134
|
(1
|
)
|
|||||||||||||||||||||||||
|
Liucheng Medicine Limited
|
$
|
128,579
|
$
|
128,579
|
-
|
$
|
129,768
|
$
|
229,925
|
$
|
128,579
|
-
|
-
|
-
|
(1
|
)
|
||||||||||||||||||||||||
|
Property Management
|
-
|
-
|
-
|
$
|
22,445
|
$
|
22,445
|
-
|
$
|
30,346
|
$
|
52,791
|
$
|
22,445
|
(1
|
)
|
||||||||||||||||||||||||
|
Baicaotang Property Development Limited
|
$
|
748,018
|
$
|
748,018
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(1
|
)
|
|||||||||||||||||||||||||||
|
(1)
|
See Notes 1, 2 and 3 in the table immediately above.
|
Transactions with Property Management
We do not have a 100% direct ownership interest in BCT Retail due to the restriction of foreign investment in pharmacy chains with 30 or more drugstores. We have entered into contractual arrangements with Property Management pursuant to which the shareholders of Property Management pledged to us their equity interests in BCT Retail and provide us with the ability to effectively control BCT Retail. The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wan have an aggregate 67.2% interest in the registered share capital of Property Management.
The contractual agreements entered into by us and Property Management include:
Share Transfer Agreement. Under this agreement dated April 1, 2008 by and between Liuzhou BCT, which held 100% of the equity interests of BCT Retail, and Property Management, Property Management acquired from Liuzhou BCT 51% equity interests in BCT Retail. The total amount of transfer price was RMB153,000, which was 51% of the registered share capital of BCT Retail. Within twenty (20) business days from the date when Property Management paid the transfer price, all the parties were to amend the articles of association of BCT Retail and register such equity transfer with the competent Authority of Industry and Commerce. Such amendment was made and equity transfer was registered on June 18, 2008.
101
Shares Pledge Agreement. Under this agreement dated May 3, 2008 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interest in BCT Retail to Liuzhou BCT to guarantee its obligations to repay a loan in the amount of RMB153,000 made from Liuzhou BCT to Property Management. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement.
Share Repurchase Agreement. Under this agreement dated July 31, 2008 by and between Liuzhou BCT and Property Management, Liuzhou BCT was granted a preemption right to repurchase the 51% equity interests in BCT Retail held by Property Management. The term of the preemption right is two years from the date that Property Management deregisters the pledge as a result of paying off loans under the related share pledge agreements. The repurchase price shall be equal to 51% of the registered capital of BCT retail at the time of the repurchase.
Shares Pledge Agreement. Under this agreement dated March 31, 2009 and amended on May 19, 2010 among Liuzhou BCT and Property Management, Property Management pledged all of its equity interest in BCT Retail to Liuzhou BCT to guarantee its obligations under a loan in the amount of RMB 1.377 million made from Liuzhou BCT to Property Management. The loan was used by Property Management to increase its registered share capital in BCT Retail and to maintain its 51% interest. The loan must be repaid by December 31, 2015 and may only be paid by transfer by Property Management of its equity interest in BCT retail to Liuzhou BCT. During the term of the Agreement, Liuzhou BCT has the right to receive any dividends that would have been paid by BCT Retail to Property Management. The May 19, 2010 amendment was made to: (i) correct the name of Party B from the Shareholders of Property Management to Property Management, (ii) to clarify that the loan could only be paid off by the transfer of Property Management’s interest in BCT Retail to Liuzhou BCT and (iii) to clarify that Liuzhou BCT had the right to any dividends or distributions that BCT Retail would have otherwise made to Property Management during the term of the Share Pledge Agreement.
Agreement terminating the Shares Pledge Agreements. Under this agreement, dated March 2, 2010 by and between Liuzhou BCT, the shareholders of Property Management and Property Management, Shares Pledge Agreements entered into on May 3, 2008 and March 31, 2009 between Liuzhou BCT and the shareholders of Property Management were terminated because they were entered into by error and were duplicative of the Shares Pledge Agreements entered into by Property Management on such dates as described above.
Proxy Agreement. Under this agreement, dated May 19, 2010 by and between Liuzhou BCT and Property Management, Property Management agreed to grant an irrevocable proxy to Liuzhou BCT to appoint the directors, officers and management of BCT Retail and to vote all shares of capital stock of BCT Retail. The Proxy Agreement was entered into in order to make the contractual relationship between the parties consistent with past practices and understandings between the parties as it relates to the transfer of 51% of the equity interest of China BCT to Property Management in April 2008. The directors of Liuzhou BCT, Hui Tian Tang, Jing Hua Li, You Ru Jing, Chun Lin Liu, Wen De Wei and Bang Fu Wan have an aggregate 67.2% interest in the registered share capital of Property Management, and thus controlled BCT Retail prior to the Proxy Agreement being entered into.
Hefeng Pharmaceutical
From 2006 to 2009, Mr. Li Jing Hua, the General Manager of Liuzhou BCT, entered into a series of loan agreements with Hefeng Pharmaceutical, pursuant to which Hefeng Pharmaceutical borrowed an aggregate of approximately$1,215,285 at monthly interest rates ranging from 5.8% to 6.8%. All of the loan agreements have similar terms and provisions.
102
Liuzhou Retail
On June 18, 2009, Liuzhou Retail entered into a loan agreement with Rurol Credence Cooperation of Guangxi in the amount of approximately $1,613,700 (RMB 11,000,000), pursuant to which Baicaotang Property Development Limited pledge part of its premise to the bank as security interest for the loan.
Transactions with Promoters and Certain Control Persons
Lisa Lopomo, our former director, loaned the Company $1,000 with zero interest. On November 30, 2006, a total of 1,000,000 shares of Common Stock were issued to Lisa Lopomo at $0.005 per share or $5,000. On January 2, 2007 Lisa Lopomo, paid $5,000 on behalf of the Company for the cost of a mining claim. She was issued 1,000,000 shares of common stock at $.005 per share for a total of $5,000 in exchange for the cash paid out. On July 21, 2007, Lisa Lopomo was issued 1,000,000 shares of common stock in exchange for $5,000, or $0.005 per share.
On December 30, 2009 (the “Closing Date”), we entered into a Share Exchange Agreement (the “Exchange Agreement”) with Ingenious Paragon Global Limited, a British Virgin Islands company ("Ingenious”), and the shareholders of Ingenious named in the Exchange Agreement (the “Ingenious Shareholders”), which own shares constituting 100% of the issued and outstanding common shares of Ingenious (the “Ingenious Shares”). Pursuant to the terms of the Exchange Agreement, the Ingenious Shareholders transferred all Ingenious Shares to the Company in exchange for the issuance of 32,000,000 shares of our common stock, $.001 par value to the Ingenious Shareholders. As a result of the Share Exchange, Ingenious became our wholly-owned subsidiary. In connection with the closing of the Share Exchange, Lisa Lopomo cancelled 2,900,000 shares of the Common Stock that she owned in the Company, and resigned from our board of directors and all office positions that she held in the Company.
On October 22, 2009, an Earn-In Agreement was entered into by Xiaoyan. Zhang, our current CFO and certain former Liuzhou BCT Shareholders, namely Hui Tian Tang, our current CEO, Jiang You Ru, Liu Chun Lin, Wei Wen De, Wang Bang Fu, Zhao Ming An, Zhang Qing Qiu, Yang Xiao Jian, Meng Yuan Gang, Jiang Qi Feng, He Wen Heng, Liu Gong Chun, Jia Jun Wen, Tan Yu Jing, Li Jing Hua, Ye Yuan Jian
The Earn-in Agreement was amended on December 30, 2009 to extend the performance targets from 2009 and 2010 fiscal years to 2010 and 2011 fiscal years because the 2009 performance target was not going to be met. The Earn-in Agreement was further amended on May 19, 2010 to decrease the performance targets for the 2010 and 2011 fiscal years. The amendments to the Earn-in Agreement were made to increase the likelihood that the Liuzhou BCT Shareholders would all be able to earn back their shares in the Company, which was the goal and purpose of the reorganization. These amendments were made without additional consideration being paid.
The Earn-in Agreement enables those Liuzhou BCT Shareholders to purchase shares of Ingenious (or its public parent company) from Ms. Zhang for a nominal amount per share provided that the Company meets certain performance targets for fiscal 2010 and 2011. For the 2010 and 2011 fiscal years the performance targets for the Company are $15 million and $19 million after tax audited net income, respectively. If the 2010 performance target is met, the Liuzhou BCT Shareholders have the right to acquire 50% of shares held by Ms. Zhang over which they have a call right. If the 2011 performance target is met, the Liuzhou BCT Shareholders have the right to acquire the other 50% of the shares held by Ms. Zhang over which they have a call right. The number of shares which can be acquired by the Liuzhou BCT Shareholders under the Earn-In Agreement is in proportion to their former relative ownership interest in Liuzhou BCT. Ms. Zhang may not transfer the shares during the five-year term of the Earn-In Agreement and in the event that all of the shares have not been delivered to the Liuzhou BCT Shareholders, Ms. Zhang can only transfer them as she and the Liuzhou BCT Shareholders agree.
103
General
The following is a summary of the material U.S. federal income tax consequences to an investor of the acquisition, ownership and disposition of our common stock purchased by the investor pursuant to this offering. As used in this discussion, references to “we”, “our” and “us” refer only to China BCT Pharmacy Group, Inc.
The discussion below of the U.S. federal income tax consequences to “U.S. Holders” will apply to a beneficial owner of our common stock that is for U.S. federal income tax purposes:
|
●
|
an individual citizen or resident of the United States;
|
|
●
|
a corporation (or other entity treated as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
●
|
an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or
|
|
●
|
a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust, or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
|
If a beneficial owner of our common stock is not described as a U.S. Holder and is not an entity treated as a partnership or other pass-through entity for U.S. federal income tax purposes, such owner will be considered a “Non-U.S. Holder.” The material U.S. federal income tax consequences applicable to Non-U.S. Holders is described below under the heading “Non-U.S. Holders.”
This summary is based on the Internal Revenue Code of 1986, as amended, or the “Code,” its legislative history, Treasury regulations promulgated thereunder, published rulings and court decisions, all as currently in effect. These authorities are subject to change or differing interpretations, possibly on a retroactive basis.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to any particular holder based on such holder’s individual circumstances. In particular, this discussion considers only holders that own our common stock as capital assets within the meaning of Section 1221 of the Code, and does not address the potential application of the Medicare contribution tax on certain unearned income or the alternative minimum tax. In addition, this discussion does not address the U.S. federal income tax consequences to holders that are subject to special rules, including:
|
●
|
financial institutions or financial services entities;
|
|
●
|
broker-dealers;
|
|
●
|
taxpayers who are subject to the mark-to-market accounting rules under Section 475 of the Code;
|
|
●
|
tax-exempt entities;
|
|
●
|
governments or agencies or instrumentalities thereof;
|
|
●
|
insurance companies;
|
|
●
|
regulated investment companies;
|
104
|
●
|
real estate investment trusts;
|
|
●
|
certain expatriates or former long-term residents of the United States;
|
|
●
|
persons that actually or constructively own 5 percent or more of our voting stock;
|
|
●
|
persons that acquired our common stock pursuant to an exercise of employee stock options, in connection with employee stock incentive plans or otherwise as compensation;
|
|
●
|
persons that hold our common stock as part of a straddle, constructive sale, hedging, conversion or other integrated transaction; or
|
|
●
|
persons whose functional currency is not the U.S. dollar.
|
This discussion does not address any aspect of U.S. federal non-income tax laws, such as gift or estate tax laws, state, local or non-U.S. tax laws or, except as discussed herein, any tax reporting obligations of a holder of our common stock. This discussion assumes that any distributions made (or deemed made) by us on our common stock and any consideration received (or deemed received) by a holder in consideration for the sale or other disposition of our common stock will be in U.S. dollars. Additionally, the discussion does not consider the tax treatment of partnerships or other pass-through entities or persons who hold our common stock through such entities. If a partnership (or other entity classified as a partnership for U.S. federal income tax purposes) is the beneficial owner of our common stock, the U.S. federal income tax treatment of a partner in the partnership will generally depend on the status of the partner and the activities of the partnership.
We have not sought, and will not seek, a ruling from the Internal Revenue Service (“IRS”) or an opinion of counsel as to any U.S. federal income tax consequence described herein. The IRS may disagree with the description herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK. IT IS NOT TAX ADVICE. EACH PROSPECTIVE INVESTOR IN OUR COMMON STOCK IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK, INCLUDING THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL, AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS, AND ANY APPLICABLE TAX TREATIES.
U.S. Holders
Taxation of Distributions
A U.S. Holder generally will be required to include in gross income as ordinary income the amount of any cash dividend paid on the shares of our common stock. A cash distribution on such shares generally will be treated as a dividend for U.S. federal income tax purposes to the extent the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Such cash distributions in excess of such earnings and profits generally will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. Holder’s adjusted tax basis in our common stock. Any remaining excess generally will be treated as gain from the sale or other disposition of the common stock and will be treated as described under “—Taxation on the Disposition of Common Stock” below.
105
Any dividends we pay to a U.S. Holder that is treated as a taxable corporation for U.S. federal income tax purposes generally will qualify for the dividends received deduction if the applicable holding period and other requirements are satisfied. With certain exceptions, if the applicable holding period and other requirements are satisfied, dividends we pay to a non-corporate U.S. Holder generally will constitute “qualified dividends” that will be subject to tax at the maximum regular tax rate accorded to long-term capital gains for tax years beginning on or before December 31, 2010, after which the regular U.S. federal income tax rate applicable to dividends is scheduled to return to the regular tax rate generally applicable to ordinary income.
If a PRC tax applies to any dividends paid to a U.S. Holder on our common stock, such tax should be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to applicable conditions and limitations). In addition, such U.S. Holder should be entitled to certain benefits under the U.S.-PRC Tax Treaty, if such holder is considered a resident of the United States for purposes of the U.S.-PRC Tax Treaty. U.S. Holders should consult their own tax advisors regarding the deduction or credit for any such PRC tax and their eligibility for the benefits of the U.S.-PRC Tax Treaty.
Taxation on the Disposition of Common Stock
Upon a sale or other taxable disposition of our common stock, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the common stock.
The regular U.S. federal income tax rate on capital gains recognized by U.S. Holders generally is the same as the regular U.S. federal income tax rate on ordinary income, except that long-term capital gains recognized by non-corporate U.S. Holders are generally subject to U.S. federal income tax at a maximum regular rate of 15 percent for taxable years beginning before January 1, 2011 (and 20 percent thereafter). Capital gain or loss will constitute long-term capital gain or loss if the U.S. Holder’s holding period for the common stock exceeds one year. The deductibility of capital losses is subject to various limitations.
If a PRC tax applies to any gain from the disposition of our common stock by a U.S. Holder, such tax should be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to applicable conditions and limitations). In addition, such U.S. Holder should be entitled to certain benefits under the U.S.-PRC Tax Treaty, if such holder is considered a resident of the United States for purposes of the U.S.-PRC Tax Treaty. U.S. Holders should consult their own tax advisors regarding the deduction or credit for any such PRC tax and their eligibility for the benefits of the U.S.-PRC Tax Treaty.
Non-U.S. Holders
Taxation of Distributions
Any cash distribution we make to a Non-U.S. Holder of shares of our common stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), generally will constitute a dividend for U.S. federal income tax purposes. Unless we are treated as an “80/20 company” for U.S. federal income tax purposes, as described below, any such dividend paid to a Non-U.S. Holder with respect to shares of our common stock that is not effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States, as described below, generally will be subject to U.S. federal withholding tax at a rate of 30 percent of the gross amount of the dividend, unless such Non-U.S. Holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN). In satisfying the foregoing withholding obligation with respect to a cash distribution, we may withhold up to 30 percent of either (i) the gross amount of the entire distribution, even if the amount of the distribution is greater than the amount constituting a dividend, as described above, or (ii) the amount of the distribution we project will be a dividend, based upon a reasonable estimate of both our current and our accumulated earnings and profits for the taxable year in which the distribution is made. If U.S. federal income tax is withheld on the amount of a distribution in excess of the amount constituting a dividend, the Non-U.S. Holder may obtain a refund of all or a portion of the excess amount withheld by timely filing a claim for refund with the IRS. Any such distribution not constituting a dividend generally will be treated, for U.S. federal income tax purposes, first as reducing the Non-U.S. Holder’s adjusted tax basis in its shares of our common stock (but not below zero) and, to the extent such distribution exceeds the Non-U.S. Holder’s adjusted tax basis, as gain from the sale or other disposition of the common stock, which will be treated as described under “—Taxation on the Disposition of Common Stock” below.
106
There is a possibility that we may qualify as an “80/20 company” for U.S. federal income tax purposes. In general, a U.S. corporation is an 80/20 company if at least 80 percent of its gross income earned directly or from subsidiaries during an applicable testing period is “active foreign business income.” The 80 percent test is applied on a periodic basis. If we qualify as an 80/20 company, a percentage of any dividend paid by us generally will not be subject to U.S. federal withholding tax. A Non-U.S. Holder should consult with its own tax advisors regarding the amount of any such dividend subject to withholding tax in this circumstance.
Cash dividends we pay to a Non-U.S. Holder that are effectively connected with such Non-U.S. Holder’s conduct of a trade or business within the United States (and, if certain income tax treaties apply, are attributable to a U.S. permanent establishment or fixed base maintained by the Non-U.S. Holder) generally will not be subject to U.S. withholding tax, provided such Non-U.S. Holder complies with certain certification and disclosure requirements (usually by providing an IRS Form W-8ECI). Instead, such dividends generally will be subject to U.S. federal income tax, net of certain deductions, at the same graduated individual or corporate tax rates applicable to U.S. persons. If the Non-U.S. Holder is a corporation, such dividends that are effectively connected income may also be subject to a “branch profits tax” at a rate of 30 percent (or such lower rate as may be specified by an applicable income tax treaty).
Taxation on the Disposition of Common Stock
A Non-U.S. Holder generally will not be subject to U.S. federal income tax in respect of gain recognized on a sale, exchange or other disposition of our common stock, unless:
|
●
|
the gain is effectively connected with the conduct of a trade or business by the Non-U.S. Holder within the United States (and, under certain income tax treaties, is attributable to a U.S. permanent establishment or fixed base maintained by the Non-U.S. Holder);
|
|
●
|
the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of disposition and certain other conditions are met; or
|
|
●
|
we are or have been a “United States real property holding corporation” (“USRPHC”) for U.S. federal income tax purposes at any time during the shorter of the five year period ending on the date of disposition or the Non-U.S. Holder’s holding period for the common stock disposed of, and, generally, in the case where our common stock is regularly traded on an established securities market, the Non-U.S. Holder has owned, directly or indirectly, more than 5 percent of our common stock at any time during the shorter of the five year period ending on the date of disposition or the Non-U.S. Holder’s holding period for the common stock disposed of. There can be no assurance that our common stock will be treated as regularly traded on an established securities market for this purpose.
|
Unless an applicable tax treaty provides otherwise, gain described in the first and third bullet points above generally will be subject to U.S. federal income tax, net of certain deductions, at the same tax rates applicable to U.S. persons. Any gains described in the first bullet point above of a Non-U.S. Holder that is a foreign corporation may also be subject to an additional “branch profits tax” at a 30 percent rate (or a lower applicable tax treaty rate). Any U.S. source capital gain of a Non-U.S. Holder described in the second bullet point above (which may be offset by U.S. source capital losses during the taxable year of the disposition) generally will be subject to a flat 30 percent U.S. federal income tax rate (or a lower applicable tax treaty rate).
In connection with the third bullet point above, we generally will be classified as a USRPHC if (looking through certain subsidiaries) the fair market value of our “United States real property interests” equals or exceeds 50 percent of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes. We have not made a determination of whether we currently are, or expect to be for the foreseeable future, a USRPHC. Non-U.S. Holders, particularly those Non-U.S Holders that could be treated as actually or constructively holding more than 5 percent of our common stock, should consult their own tax advisors regarding the U.S. federal income tax consequences of owning and disposing of our common stock.
107
Information Reporting and Backup Withholding
We generally must report annually to the IRS and to each holder the amount of dividends and certain other distributions we pay to such holder on our common stock and the amount of tax, if any, withheld with respect to those distributions. In the case of a Non-U.S. Holder, copies of the information returns reporting those distributions and withholding may also be made available to the tax authorities in the country in which the Non-U.S. Holder is a resident under the provisions of an applicable income tax treaty or agreement. Information reporting is also generally required with respect to proceeds from the sales and other dispositions of our common stock to or through the U.S. office (and in certain cases, the foreign office) of a broker.
In addition, backup withholding of U.S. federal income tax, currently at a rate of 28 percent, generally will apply to distributions made on our common stock to, and the proceeds from sales and other dispositions of our common stock by, a non-corporate U.S. Holder who:
|
●
|
fails to provide an accurate taxpayer identification number;
|
|
●
|
is notified by the IRS that backup withholding is required; or
|
|
●
|
in certain circumstances, fails to comply with applicable certification requirements.
|
A Non-U.S. Holder generally may eliminate the requirement for information reporting (other than with respect to distributions, as described above) and backup withholding by providing certification of its foreign status, under penalties of perjury, on a duly executed applicable IRS Form W-8 or by otherwise establishing an exemption.
Backup withholding is not an additional tax. Rather, the amount of any backup withholding will be allowed as a credit against a U.S. Holder’s or a Non-U.S. Holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that certain required information is timely furnished to the IRS. Holders are urged to consult their own tax advisors regarding the application of backup withholding and the availability of and procedure for obtaining an exemption from backup withholding in their particular circumstances.
The following discussion summarizes the material PRC income tax considerations relating to the acquisition, ownership and disposition of our common stock purchased by an investor pursuant to this offering. As used in this discussion, references to “we”, “our” and “us” refer only to China BCT Pharmacy Group, Inc.
Resident Enterprise Treatment
On March 16, 2007, the Fifth Session of the Tenth National People’s Congress passed the Enterprise Income Tax Law of the PRC (“EIT Law”), which became effective on January 1, 2008. Under the EIT Law, enterprises are classified as “resident enterprises” and “non-resident enterprises.” Pursuant to the EIT Law and its implementing rules, enterprises established outside China whose “de facto management bodies” are located in China are considered “resident enterprises” and subject to the uniform 25 percent enterprise income tax rate on worldwide income. According to the implementing rules of the EIT Law, “de facto management body” refers to a managing body that in practice exercises overall management control over the production and business, personnel, accounting and assets of an enterprise.
On April 22, 2009, the State Administration of Taxation issued the Notice on the Issues Regarding Recognition of Enterprises that are Domestically Controlled as PRC Resident Enterprises Based on the De Facto Management Body Criteria, which was retroactively effective as of January 1, 2008. This notice provides that an overseas incorporated enterprise that is controlled domestically will be recognized as a “tax-resident enterprise” if it satisfies all of the following conditions: (i) the senior management responsible for daily production/business operations are primarily located in the PRC, and the location(s) where such senior management execute their responsibilities are primarily in the PRC; (ii) strategic financial and personnel decisions are made or approved by organizations or personnel located in the PRC; (iii) major properties, accounting ledgers, company seals and minutes of board meetings and stockholder meetings, etc., are maintained in the PRC; and (iv) 50 percent or more of the board members with voting rights or senior management habitually reside in the PRC.
108
Given the short history of the EIT Law and lack of applicable legal precedent, it remains unclear how the PRC tax authorities will determine the PRC tax resident status of a company organized under the laws of a foreign (non-PRC) jurisdiction, such as us, Ingenious and/or Forever Well. If the PRC tax authorities determine that we, Ingenious and/or Forever Well are a “resident enterprise” for PRC enterprise income tax purposes, a number of tax consequences could follow. First, we, Ingenious and/or Forever Well could be subject to the enterprise income tax at a rate of 25 percent on our, Ingenious’ and/or Forever Well’s worldwide taxable income, as well as PRC enterprise income tax reporting obligations. Second, the EIT Law provides that dividend income between “qualified resident enterprises” is exempt from income tax. As a result, if we, Ingenious and Forever Well are treated as “qualified resident enterprises,” all dividends paid from Liuzhou BCT to us (through Forever Well and Ingenious) should be exempt from PRC tax.
As of the date of this prospectus, there has not been a definitive determination as to the “resident enterprise” or “non-resident enterprise” status of us, Ingenious or Forever Well. However, since it is not anticipated that we, Ingenious and/or Forever Well would receive dividends or generate other income in the near future, we, Ingenious and Forever Well are not expected to have any income that would be subject to the 25 percent enterprise income tax on global income in the near future. We, Ingenious and Forever Well will make any necessary tax payment if we, Ingenious or Forever Well (based on future clarifying guidance issued by the PRC), or the PRC tax authorities, determine that we, Ingenious or Forever Well are a resident enterprise under the EIT Law, and if we, Ingenious or Forever Well were to have income in the future.
Dividends From Liuzhou BCT
If Forever Well is not treated as a resident enterprise under the EIT Law, then dividends that Forever Well receives from Liuzhou BCT may be subject to PRC withholding tax. The EIT Law and the implementing rules of the EIT Law provide that (A) an income tax rate of 25 percent will normally be applicable to investors that are “non-resident enterprises” (“non-resident investors”) which (i) have an establishment or place of business inside the PRC, and (ii)
have income in connection with their establishment or place of business that is sourced from the PRC or is earned outside the PRC but has an actual connection with their establishment or place of business inside the PRC, and (B) a PRC withholding tax at a rate of 10 percent will normally be applicable to dividends payable to non-resident investors which (i) do not have an establishment or place of business in the PRC or (ii) have an establishment or place of business in the PRC, but the relevant income is not effectively connected with such establishment or place of business, to the extent such dividends are derived from sources within the PRC.
As described above, the PRC tax authorities may determine the resident enterprise status of entities organized under the laws of foreign jurisdictions on a case-by-case basis. We, Ingenious and Forever Well are holding companies and substantially all of our income and that of Ingenious and Forever Well may be derived from dividends. Thus, if we, Ingenious and/or Forever Well are considered a “non-resident enterprise” under the EIT Law and the dividends paid to us, Ingenious and/or Forever Well are considered income sourced within the PRC, such dividends received may be subject to PRC withholding tax as described in the foregoing paragraph.
The State Council of the PRC or a tax treaty between China and the jurisdiction in which the non-PRC investor resides may reduce such income or withholding tax, with respect to a non-resident investor. Pursuant to the Arrangement between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income (the “PRC-Hong Kong Tax Treaty”), if the Hong Kong resident enterprise that is not deemed to be a conduit by the PRC tax authorities owns more than 25 percent of the equity interest in a company in China, the 10 percent PRC withholding tax on the dividends the Hong Kong resident enterprise receives from such company in China is reduced to 5 percent.
However, such preferential tax rate under the PRC-Hong Kong Tax Treaty does not automatically apply. According to the Circular Guoshuifa No. 2009 124, which was released by the State Administration of Taxation (“SAT”) and became effective on October 1, 2009, approvals from competent local tax authorities are required on a case-by-case basis before a Hong Kong resident company can enjoy the above-mentioned tax treatment relating to dividends under the PRC–Hong Kong Tax Treaty.
109
In addition, the principle that the substance prevails over the form shall be observed by a competent tax authority in evaluating whether and recognizing that the eligible dividend recipient will enjoy the preferential tax rate, in accordance with the Circular GuoShuihan 2009 No.81, released by the SAT on February 20, 2009, and the Circular Guoshuihan 2009 No. 601, released by the SAT on October 27, 2009, respectively. Several circumstances relating to the dividend receiving company in Hong Kong will be harmful to such recognition, in particular: (i) it is obligated to distribute all or most (approximately 60 percent or more) of the dividends received to a resident of a third country or region within a prescribed period (say within 12 months following the receipt of the dividends); (ii) it hardly conducts other business, save in possession of properties or rights yielded from the dividends; (iii) its assets, scale, and staff are disproportional to the dividends; (iv) it can hardly control or dispose of, or hardly assumes liability pertaining to, the properties or rights yielded from the dividends; (v) the dividends received are not taxable in Hong Kong, or the effective tax rate there is low; and (vi) it fails to hold any equity interest of the China company for a period of 12 consecutive months. Accordingly, Forever Well may be determined as falling in the above circumstances and subject to the normal PRC withholding tax rate instead of the preferential withholding tax rate under the PRC-Hong Kong Tax Treaty.
We are a U.S. holding company, and we have a subsidiary in the British Virgin Islands (Ingenious), which in turn owns a 100 percent equity interest in a subsidiary in Hong Kong (Forever Well), which in turns owns a 100 percent equity interest in Liuzhou BCT. As a result, if Forever Well were treated as a PRC “non-resident enterprise” under the EIT Law, then dividends that Forever Well receives from Liuzhou BCT (assuming such dividends were considered sourced within the PRC) (i) may be subject to a 5 percent PRC withholding tax, if the PRC-Hong Kong Tax Treaty were applicable, or (ii) if such treaty does not apply (i.e., because the PRC tax authorities may deem Forever Well to be a conduit under the above rules that is not entitled to treaty benefits), may be subject to a 10 percent PRC withholding tax. Similarly, if Ingenious were treated as a PRC “non-resident enterprise” under the EIT Law, and Forever Well were treated as a PRC “resident enterprise” under the EIT Law, then dividends that Ingenious receives from Forever Well (assuming such dividends were considered sourced within the PRC) may be subject to a 10 percent PRC withholding tax. A similar situation would arise if we were treated as a PRC “non-resident enterprise” under the EIT Law, and Ingenious were treated as a PRC “resident enterprise” under the EIT Law. Any such taxes on dividends could materially reduce the amount of dividends, if any, we could pay to our shareholders.
As of the date of this prospectus, there has not been a definitive determination as to the “resident enterprise” or “non-resident enterprise” status of us, Ingenious or Forever Well. As described above, however, Liuzhou BCT is not expected to pay any dividends in the near future. We, Ingenious and Forever Well will make any necessary tax withholding if, in the future, Liuzhou BCT were to pay any dividends and we, Ingenious or Forever Well (based on future clarifying guidance issued by the PRC), or the PRC tax authorities, determine that we, Ingenious or Forever Well are a non-resident enterprise under the EIT Law.
Dividends that Non-PRC Resident Investors Receive From Us; Gain on the Sale or Transfer of Our Common Stock
If dividends payable to (or gains recognized by) our non-resident investors are treated as income derived from sources within the PRC, then the dividends that non-resident investors receive from us and any such gain on the sale or transfer of our common stock may be subject to taxes under the PRC tax laws.
Under the EIT Law and the implementing rules of the EIT Law, PRC withholding tax at the rate of 10 percent is applicable to dividends payable to non-resident investors which (i) do not have an establishment or place of business in the PRC or (ii) have an establishment or place of business in the PRC but the relevant income is not effectively connected with the establishment or place of business, to the extent that such dividends have their sources within the PRC. Similarly, any gain realized on the transfer of our common stock by such investors is also subject to 10 percent PRC income tax if such gain is regarded as income derived from sources within the PRC.
The dividends paid by us to non-resident investors with respect to our common stock, or gain non-resident investors may realize from the sale or transfer of our common stock, may be treated as PRC-sourced income and, as a result, may be subject to PRC tax at a rate of 10 percent. In such event, we may be required to withhold a 10 percent PRC tax on any dividends paid to non-resident investors. In addition, non-resident investors in our common stock may be responsible for paying PRC tax at a rate of 10 percent on any gain realized from the sale or transfer of our common stock if such non-resident investors and the gain satisfy the requirements under the EIT Law and its implementing rules. However, under the EIT Law and its implementing rules, we would not have an obligation to withhold PRC income tax in respect of the gains that non-resident investors (including U.S. investors) may realize from the sale or transfer of our common stock.
110
If we were to pay any dividends in the future, and if we (based on future clarifying guidance issued by the PRC), or the PRC tax authorities, determine that we must withhold PRC tax on any dividends payable by us under the EIT Law, we will make any necessary tax withholding on dividends payable to our non-resident investors. If non-resident investors as described under the EIT Law (including U.S. investors) realize any gain from the sale or transfer of our common stock and if such gain were considered as PRC-sourced income, such non-resident investors would be responsible for paying 10 percent PRC income tax on the gain from the sale or transfer of our common stock. As indicated above, under the EIT Law and its implementing rules, we would not have an obligation to withhold PRC income tax in respect of the gains that non-resident investors (including U.S. investors) may realize from the sale or transfer of our common stock.
On December 15, 2009, the SAT released Circular Guoshuihan No. 698 (“Circular 698”) that reinforces the taxation of non-listed equity transfers by non-resident enterprises through overseas holding vehicles. Circular 698 addresses indirect share transfers as well as other issues. Circular 698 is retroactively effective from January 1, 2008. According to Circular 698, where a foreign (non-PRC resident) investor who indirectly holds shares in a PRC resident enterprise through a non-PRC offshore holding company indirectly transfers equity interests in a PRC resident enterprise by selling the shares of the offshore holding company, and the latter is located in a country or jurisdiction where the effective tax burden is less than 12.5 percent or where the offshore income of his, her, or its residents is not taxable, the foreign investor is required to provide the PRC tax authority in charge of that PRC resident enterprise with certain relevant information within 30 days of the transfer. The tax authorities in charge will evaluate the offshore transaction for tax purposes. In the event that the tax authorities determine that such transfer is abusing forms of business organization and a reasonable commercial purpose for the offshore holding company other than the avoidance of PRC income tax liability is lacking, the PRC tax authorities will have the power to re-assess the nature of the equity transfer under the doctrine of substance over form. A reasonable commercial purpose may be established when the overall international (including U.S.) offshore structure is set up to comply with the requirements of supervising authorities of international (including U.S.) capital markets. If the SAT’s challenge of a transfer is successful, it may deny the existence of the offshore holding company that is used for tax planning purposes and subject the seller to PRC tax on the capital gain from such transfer. Since Circular 698 has a short history, there is uncertainty as to its application. We (or a foreign investor) may become at risk of being taxed under Circular 698 and may be required to expend valuable resources to comply with Circular 698 or to establish that we (or such foreign investor) should not be taxed under Circular 698, which could have a material adverse effect on our financial condition and results of operations (or such foreign investor’s investment in us).
Penalties for Failure to Pay Applicable PRC Income Tax
Non-resident investors in us may be responsible for paying PRC tax at a rate of 10 percent on any gain realized from the sale or transfer of our common stock if such non-resident investors and the gain satisfy the requirements under the EIT Law and its implementing rules, as described above.
According to the EIT Law and its implementing rules, the PRC Tax Administration Law (the “Tax Administration Law”) and its implementing rules, the Provisional Measures for the Administration of Withholding of Enterprise Income Tax for Non-resident Enterprises (the “Administration Measures”) and other applicable PRC laws or regulations (collectively the “Tax Related Laws”), where any gain derived by a non-resident investor from the sale or transfer of our common stock is subject to any income tax in the PRC, and such non-resident investor fails to file any tax return or pay tax in this regard pursuant to the Tax Related Laws, such investor may be subject to certain fines, penalties or punishments, including without limitation: (1) if a non-resident investor fails to file a tax return and present the relevant information in connection with tax payments, the competent tax authorities shall order it to do so within the prescribed time limit and may impose a fine up to RMB 2,000, and in egregious cases, may impose a fine ranging from RMB 2,000 to RMB 10,000; (2) if a non-resident investor fails to file a tax return or fails to pay all or part of the amount of tax payable, the non-resident investor shall be required to pay the unpaid tax amount payable, a surcharge on overdue tax payments (the daily surcharge is 0.05 percent of the overdue amount, beginning from the day the deferral begins) and a fine ranging from 50 percent to 500 percent of the unpaid amount of the tax payable; (3) if a non-resident investor fails to file a tax return or pay the tax within the prescribed time limit according to the order by the PRC tax authorities, the PRC tax authorities may collect and check information about the income items of the non-resident investor in the PRC and other payers (the “Other Payers”) who will pay amounts to such non-resident investor, and send a “Notice of Tax Issues” to the Other Payers to collect and recover the tax payable and impose overdue fines on such non-resident investor from the amounts otherwise payable to such non-resident investor by the Other Payers; (4) if a non-resident investor fails to pay the tax payable within the prescribed time limit as ordered by the PRC tax authorities, a fine may be imposed on the non-resident investor ranging from 50 percent to 500 percent of the unpaid tax payable, and the PRC tax authorities may, upon approval by the director of the tax bureau (or sub-bureau) of, or higher than, the county level, take the following compulsory measures: (i) notify in writing the non-resident investor’s bank or other financial institution to withhold from the account thereof for payment of the amount of tax payable, and (ii) detain, seal off, or sell by auction or on the market the non-resident investor’s commodities, goods or other property in a value equivalent to the amount of tax payable; or (5) if the non-resident investor fails to pay all or part of the amount of tax payable or surcharge for overdue tax payment, and can not provide a guarantee to the tax authorities, the tax authorities may notify the frontier authorities to prevent the non-resident investor or its legal representative from leaving the PRC.
111
The validity of the shares of our common stock offered hereby has been passed upon for us by McKenna Long Aldridge LLP, Atlanta, Georgia.
Our audited financial statements as of and for the years ended December 31, 2009 and 2008 have been included in this prospectus in reliance upon the report of PKF, Certified Public Accountants, Hong Kong, China, a member firm of PKF International Limited network of legally independent firms and their authority as experts in accounting and auditing.
We have filed with the United States Securities and Exchange Commission, 100 F. Street, N.E., Washington, D.C. 20549, a registration statement on Form S-1 under the Securities Act for the common stock offered by this prospectus. We have not included in this prospectus all the information contained in the registration statement and you should refer to the registration statement and its exhibits for further information.
The registration statement and other information may be read and copied at the SEC’s Public Reference Room at 100 F. Street N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a web site (http://www.sec.gov) that contains the registration statements, reports, proxy and information statements and other information regarding registrants that file electronically with the SEC such as us.
You may also read and copy any reports, statements or other information that we have filed with the SEC at the addresses indicated above and you may also access them electronically at the web site set forth above. These SEC filings are also available to the public from commercial document retrieval services.
Disclosure of Commission Position on Indemnification of Securities Act Liabilities
Our Certificate of Incorporation, provides to the fullest extent permitted by Section 145 of the General Corporation Law of the State of Delaware, that our directors or officers shall not be personally liable to us or our stockholders for damages for breach of such director’s or officer’s fiduciary duty. The effect of this provision of our Certificate of Incorporation, is to eliminate our rights and our stockholders (through stockholders’ derivative suits on behalf of our company) to recover damages against a director or officer for breach of the fiduciary duty of care as a director or officer (including breaches resulting from negligent or grossly negligent behavior), except under certain situations defined by statute. We believe that the indemnification provisions in our Certificate of Incorporation, are necessary to attract and retain qualified persons as directors and officers.
Our By Laws also provide that our board of directors may also authorize the Company to indemnify our employees or agents, and to advance the reasonable expenses of such persons, to the same extent, following the same determinations and upon the same conditions as are required for the indemnification of and advancement of expenses to our directors and officers. As of the date of this report, our board of directors has not extended indemnification rights to persons other than directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
112
|
Pages
|
|
|
Condensed Consolidated Statements of Income and Comprehensive Income for the three and nine months ended September 30, 2010 and 2009
|
Q-1
|
|
Condensed Consolidated Balance Sheets as of September 30, 2010 and December 31, 2009
|
Q-2 – Q-3
|
|
Condensed Consolidated Statements of Cash Flows for the six months ended September 30, 2010 and 2009
|
Q-4 – Q-5
|
|
Condensed Consolidated Statements of Stockholders’ Equity as of September 30, 2010
|
Q-6
|
|
Notes to Condensed Consolidated Financial Statements
|
Q-7 – Q-26
|
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
Consolidated Statements of Income and Comprehensive Income for the year ended December 31, 2009 and 2008
|
F-2
|
|
Consolidated Balance Sheets as of December 31, 2009 and 2008
|
F-3 – F-4
|
|
Consolidated Statements of Cash Flows for the year ended December 31, 2009 and 2008
|
F-5 – F-6
|
|
Consolidated Statements of Stockholders’ Equity for the year ended December 31, 2009 and 2008
|
F-7
|
|
Notes to Consolidated Financial Statements
|
F-8 – F-31
|
113
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Consolidated Statements of Income and Comprehensive Income
(Unaudited)
(Stated in US Dollars)
|
Three months ended
|
Nine months ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
(unaudited)
|
(unaudited)
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Sales revenue
|
$
|
52,528,246
|
$
|
33,867,191
|
$
|
134,049,842
|
$
|
99,473,445
|
||||||||
|
Cost of sales
|
39,294,987
|
24,705,066
|
99,341,298
|
72,944,350
|
||||||||||||
|
Gross profit
|
13,233,259
|
9,162,125
|
34,708,544
|
26,529,095
|
||||||||||||
|
Operating expenses
|
||||||||||||||||
|
Administrative expenses
|
2,371,018
|
1,010,753
|
5,307,917
|
2,807,799
|
||||||||||||
|
Research and development expenses
|
-
|
-
|
-
|
52,969
|
||||||||||||
|
Selling expenses
|
1,384,765
|
913,163
|
3,471,200
|
2,577,153
|
||||||||||||
|
Total operating expenses
|
3,755,783
|
1,923,916
|
8,779,117
|
5,437,921
|
||||||||||||
|
Income from operations
|
9,477,476
|
7,238,209
|
25,929,427
|
21,091,174
|
||||||||||||
|
Non-operating income (expense)
|
||||||||||||||||
|
Interest income
|
2,781
|
56,811
|
6,923
|
65,902
|
||||||||||||
|
Other income
|
16,936
|
70,754
|
143,643
|
70,754
|
||||||||||||
|
Change in fair value of warrant liabilities
|
249,092
|
-
|
(457,718
|
)
|
-
|
|||||||||||
|
Other expenses
|
(162,352
|
)
|
(2,520
|
)
|
(203,051
|
)
|
(11,990
|
)
|
||||||||
|
Finance costs - note 4
|
(202,259
|
)
|
(532,590
|
)
|
(679,868
|
)
|
(1,090,930
|
)
|
||||||||
|
Total non-operating income (expense)
|
(95,802
|
)
|
(407,545
|
)
|
(1,190,071
|
)
|
(966,264
|
)
|
||||||||
|
Income before income taxes
|
9,381,674
|
6,830,664
|
24,739,356
|
20,124,910
|
||||||||||||
|
Income taxes
|
(2,474,101
|
)
|
(1,732,491
|
)
|
(6,455,209
|
)
|
(4,748,741
|
)
|
||||||||
|
Net income
|
6,907,573
|
5,098,173
|
18,284,147
|
15,376,169
|
||||||||||||
|
Other comprehensive income
|
||||||||||||||||
|
Foreign currency translation
|
||||||||||||||||
|
adjustments
|
-
|
47,707
|
(1,334
|
)
|
25,143
|
|||||||||||
|
Total comprehensive income
|
$
|
6,907,573
|
$
|
5,145,880
|
$
|
18,282,813
|
$
|
15,401,312
|
||||||||
|
Earnings per share - basic
|
$
|
0.18
|
$
|
0.16
|
$
|
0.48
|
$
|
0.48
|
||||||||
|
Earnings per share - diluted
|
$
|
0.18
|
$
|
0.16
|
$
|
0.48
|
$
|
0.48
|
||||||||
|
Weighted average number of shares
|
||||||||||||||||
|
outstanding - basic
|
38,154,340
|
32,000,000
|
38,032,897
|
32,000,000
|
||||||||||||
|
Weighted average number of shares
|
||||||||||||||||
|
outstanding - diluted
|
38,506,274
|
32,000,000
|
38,384,831
|
32,000,000
|
||||||||||||
See the accompanying notes to consolidated financial statements
Table of Contents
Q-1
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Consolidated Balance Sheets
(Stated in US Dollars)
|
September 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
(*)
|
|||||||
|
Assets
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$
|
13,615,931
|
$
|
13,304,158
|
||||
|
Restricted cash
|
976,157
|
1,155,779
|
||||||
|
Accounts receivable, net
|
53,963,308
|
35,410,039
|
||||||
|
Amounts due from related companies - note 12
|
5,244,408
|
4,275,586
|
||||||
|
Other receivables, prepayments and deposits
|
3,561,591
|
2,526,398
|
||||||
|
Inventories - note 7
|
16,607,838
|
8,745,525
|
||||||
|
Deferred income taxes
|
28,932
|
60,164
|
||||||
|
Total current assets
|
93,998,165
|
65,477,649
|
||||||
|
Property, plant and equipment, net - note 9
|
13,924,862
|
12,171,689
|
||||||
|
Deposit for acquisition of assets of retail chain stores
|
1,290,960
|
-
|
||||||
|
Goodwill - note 8
|
107,968
|
107,968
|
||||||
|
Other intangible assets, net - note 8
|
604,135
|
660,034
|
||||||
|
Land use rights, net - note 10
|
13,093,363
|
13,979,753
|
||||||
|
Long term deposits - note 16
|
3,227,400
|
-
|
||||||
|
Deferred income taxes
|
696,149
|
663,699
|
||||||
|
Total assets
|
$
|
126,943,002
|
$
|
93,060,792
|
||||
(*) Derived from audited financial statements
See the accompanying notes to consolidated financial statements
Q-2
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Consolidated Balance Sheets (Cont’d)
(Stated in US Dollars)
|
September 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
(*)
|
|||||||
|
Liabilities and Stockholders’ Equity
|
||||||||
|
Current liabilities
|
||||||||
|
Accounts payable
|
$
|
33,883,753
|
$
|
21,398,816
|
||||
|
Other payables and accrued expenses
|
3,628,929
|
3,194,612
|
||||||
|
Amounts due to directors - note 11
|
187,426
|
1,008,111
|
||||||
|
Amounts due to related companies - note 12
|
-
|
128,579
|
||||||
|
Income tax payable
|
2,473,001
|
562,603
|
||||||
|
Secured bank loans - note 13
|
8,841,011
|
7,136,069
|
||||||
|
Other loans - note 14
|
154,886
|
2,361,258
|
||||||
|
Retirement benefit costs
|
50,714
|
59,158
|
||||||
|
Total current liabilities
|
49,219,720
|
35,849,206
|
||||||
|
Secured long-term bank loans - note 13
|
2,412,404
|
3,631,957
|
||||||
|
Warrant liabilities - note 15
|
2,313,137
|
-
|
||||||
|
Retirement benefit costs
|
218,693
|
201,320
|
||||||
|
Total liabilities
|
54,163,954
|
39,682,483
|
||||||
|
Commitments and contingencies - note 16
|
||||||||
|
Stockholders’ Equity - notes 15, 17 and 18
|
||||||||
|
Common stock: par value $0.001 per share; 100,000,000 shares authorized; 38,154,340 and 37,089,370 shares issued and outstanding as of September 30, 2010 and December 31, 2009, respectively
|
38,154
|
37,089
|
||||||
|
Additional paid-in capital
|
16,037,760
|
14,920,899
|
||||||
|
Statutory and other reserves
|
2,605,901
|
2,605,901
|
||||||
|
Accumulated other comprehensive income
|
2,108,936
|
2,110,270
|
||||||
|
Retained earnings
|
51,988,297
|
33,704,150
|
||||||
|
Total Stockholders’ Equity
|
72,779,048
|
53,378,309
|
||||||
|
Total Liabilities and Stockholders’ Equity
|
$
|
126,943,002
|
$
|
93,060,792
|
||||
(*) Derived from audited financial statements
See the accompanying notes to consolidated financial statements
Table of Contents
Q-3
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Consolidated Statements of Cash Flows
(Unaudited)
(Stated in US Dollars)
|
Nine months ended September 30,
|
||||||||
|
(Unaudited)
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash flows from operating activities :
|
||||||||
|
Net income
|
$
|
18,284,147
|
$
|
15,376,169
|
||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
provided by operating activities :
|
||||||||
|
Depreciation and amortization
|
908,659
|
864,912
|
||||||
|
Deferred income taxes
|
(1,218
|
)
|
(11,601
|
)
|
||||
|
Gain on sale of land use right
|
(44,919
|
)
|
-
|
|||||
|
Loss on sale of property, plant and equipment
|
-
|
306
|
||||||
|
Change in fair value of warrant liabilities
|
457,718
|
-
|
||||||
|
Share-based compensation expense
|
658,207
|
-
|
||||||
|
Changes in operating assets and liabilities, net of effects of acquisitions of retail stores :
|
||||||||
|
Accounts receivable
|
(18,553,269
|
)
|
(5,239,365
|
)
|
||||
|
Other receivables, prepayments and deposits
|
(1,035,193
|
)
|
(1,878,603
|
)
|
||||
|
Inventories
|
(5,733,211
|
)
|
(3,441,351
|
)
|
||||
|
Amounts due from related companies
|
-
|
50,715
|
||||||
|
Accounts payable
|
12,484,937
|
6,975,375
|
||||||
|
Other payables and accrued expenses
|
574,481
|
1,947,673
|
||||||
|
Retirement benefit costs
|
8,929
|
2,650
|
||||||
|
Income tax payable
|
1,910,398
|
(297,343
|
)
|
|||||
|
Total adjustments
|
(8,364,481
|
)
|
(1,026,632
|
)
|
||||
|
Net cash flows provided by operating activities
|
$
|
9,919,666
|
$
|
14,349,537
|
||||
See the accompanying notes to consolidated financial statements
Table of Contents
Q-4
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Consolidated Statements of Cash Flows (Cont’d)
(Unaudited)
(Stated in US Dollars)
|
Nine months ended September 30,
|
||||||||
|
(Unaudited)
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash flows from investing activities :
|
||||||||
|
Acquisition of property, plant and equipment
|
$
|
(119,641
|
)
|
$
|
(337,228
|
)
|
||
|
Payments to acquire retail stores
|
(4,521,744
|
)
|
-
|
|||||
|
Deposit for acquisition of retail stores assets
|
(1,290,960
|
)
|
-
|
|||||
|
Long term deposits
|
(3,227,400
|
)
|
-
|
|||||
|
Proceeds from sale of property, plant and equipment
|
-
|
407
|
||||||
|
Proceeds from sale of land use right
|
697,495
|
-
|
||||||
|
Net cash flows used in investing activities
|
(8,462,250
|
)
|
(336,821
|
)
|
||||
|
Cash flows from financing activities :
|
||||||||
|
Advance/repayment activities with related companies, net
|
(1,097,401
|
)
|
173,617
|
|||||
|
Proceeds received from private placement
|
2,315,138
|
-
|
||||||
|
Restricted cash
|
179,622
|
(1,997,748
|
)
|
|||||
|
Repayments to directors
|
(820,685
|
)
|
(558,006
|
)
|
||||
|
Proceeds from bank loans
|
8,039,160
|
9,232,962
|
||||||
|
Repayments of bank loans
|
(7,553,771
|
)
|
(8,771,050
|
)
|
||||
|
Proceeds from other loans
|
35,208
|
-
|
||||||
|
Repayments of other loans
|
(2,241,580
|
)
|
(791,968
|
)
|
||||
|
Net cash flows used in financing activities
|
(1,144,309
|
)
|
(2,712,193
|
)
|
||||
|
Effect of foreign currency translation on cash and cash equivalents
|
(1,334
|
)
|
7,205
|
|||||
|
Net increase in cash and cash equivalents
|
311,773
|
11,307,728
|
||||||
|
Cash and cash equivalents - beginning of period
|
13,304,158
|
1,265,184
|
||||||
|
Cash and cash equivalents - end of period
|
$
|
13,615,931
|
$
|
12,572,912
|
||||
|
Supplemental disclosures for cash flow information :
|
||||||||
|
Cash paid for :
|
||||||||
|
Interest
|
$
|
762,622
|
$
|
1,053,134
|
||||
|
Income taxes
|
$
|
4,546,029
|
$
|
5,050,082
|
||||
See the accompanying notes to consolidated financial statements
Table of Contents
Q-5
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Condensed Consolidated Statements of Stockholders’ Equity
Unaudited
(Stated in US Dollars)
|
Statutory
|
Accumulated
|
|||||||||||||||||||||||||||
|
Common stock
|
Additional
|
and
|
other
|
|||||||||||||||||||||||||
|
No. of
|
paid-in
|
other
|
comprehensive
|
Retained
|
||||||||||||||||||||||||
|
shares
|
Amount
|
capital
|
reserves
|
income
|
earnings
|
Total
|
||||||||||||||||||||||
|
Balance, December 31, 2009
|
37,089,370
|
$
|
37,089
|
$
|
14,920,899
|
$
|
2,605,901
|
$
|
2,110,270
|
$
|
33,704,150
|
$
|
53,378,309
|
|||||||||||||||
|
Net income
|
-
|
-
|
-
|
-
|
-
|
18,284,147
|
18,284,147
|
|||||||||||||||||||||
|
Foreign currency translation
|
||||||||||||||||||||||||||||
|
adjustments
|
-
|
-
|
-
|
-
|
(1,334
|
)
|
-
|
(1,334
|
)
|
|||||||||||||||||||
|
Share-based compensation
|
-
|
-
|
658,207
|
-
|
-
|
-
|
658,207
|
|||||||||||||||||||||
|
Shares issued for payment of placement service
|
35,000
|
35
|
(35
|
)
|
-
|
-
|
-
|
-
|
||||||||||||||||||||
|
Private placement
|
1,029,970
|
1,030
|
1,752,831
|
-
|
-
|
-
|
1,753,861
|
|||||||||||||||||||||
|
Reclassification
|
-
|
-
|
(1,294,142
|
)
|
-
|
-
|
-
|
(1,294,142
|
)
|
|||||||||||||||||||
|
Balance, September 30, 2010
|
38,154,340
|
$
|
38,154
|
$
|
16,037,760
|
$
|
2,605,901
|
$
|
2,108,936
|
$
|
51,988,297
|
$
|
72,779,048
|
|||||||||||||||
See the accompanying notes to consolidated financial statements
Table of Contents
Q-6
China BCT Pharmacy Group, Inc.
(Formerly named China Baicaotang Medicine Limited)
Notes to Consolidated Financial Statements
(Unaudited)
(Stated in US Dollars)
1. Corporate information
China BCT Pharmacy Group Inc. (the “Company”), formerly known as China Baicaotang Medicine Limited, and previous to that, Purden Lake Resource Corp., was incorporated in the State of Delaware on November 30, 2006 as a limited liability company.
The Company is principally engaged in the distribution, production, and retail sale of pharmaceutical products in the People’s Republic of China (the “PRC”).
Currently the Company has five subsidiaries:
|
The Company’s
|
||||||||
|
Place/date of
|
effective
|
|||||||
|
incorporation or
|
ownership
|
Common stock/
|
Principal
|
|||||
|
Company name
|
establishment
|
interest
|
registered capital
|
activities
|
||||
|
Ingenious Paragon Global Limited (“Ingenious”)
|
British Virgin Islands (“BVI”) / May 29, 2008
|
100%
|
Authorized, issued and fully paid 50,000 common shares of $1 par value each
|
Investment holding
|
||||
|
Forever Well Asia Pacific Limited (“Forever Well”)
|
Hong Kong / January 10, 2008
|
100%
|
Authorized, issued and fully paid 10,000 common shares of HK$1 each
|
Investment holding
|
||||
|
Guangxi Liuzhou Baicaotang Medicine Limited (“Liuzhou BCT”)
|
PRC / April 3, 1986
|
100%
|
Registered and fully paid up capital RMB10 million
|
Investment holding and distribution of drugs
|
||||
|
Guangxi Liuzhou Baicaotang Medicine (Retail Chain) Limited (“BCT Retail”)
|
PRC / October 30, 2001
|
100%
|
Registered and fully paid up capital RMB300,000
|
Retail sales of drugs
|
||||
|
Guangxi Hefeng Pharmaceutical Company Limited (“Hefeng Pharmaceutical”)
|
PRC / September 18, 2000
|
100%
|
Registered and fully paid up capital RMB5,000,000
|
Production and sales of drugs
|
Q-7
2. Basis of presentation
The accompanying unaudited consolidated financial statements of the Company and its subsidiaries have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (the “SEC”) including the instructions to Form 10-Q and Regulation S-X. Certain information and note disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been condensed or omitted from these statements pursuant to such rules and regulations and, accordingly, they do not include all the information and notes necessary for comprehensive consolidated financial statements and should be read in conjunction with our audited consolidated financial statements, included in our Annual Report on Form 10-K for the years ended December 31, 2009 and 2008.
In the opinion of the management of the Company, all adjustments, which are of a normal recurring nature, necessary for a fair statement of the results for the periods presented herein have been made. Results for the interim periods presented are not necessarily indicative of the results that might be expected for the entire fiscal year.
3. Summary of significant accounting policies
Basis of consolidation
The consolidated financial statements include the accounts of China BCT Pharmacy Group, Inc., and its subsidiaries; Ingenious, Forever Well, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical. All significant inter-company accounts and transactions have been eliminated in consolidation.
Use of estimates
In preparing financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of accounts receivable and inventories, and the estimation on useful lives of property, plant and equipment and intangible assets. Actual results could differ from those estimates.
Cash and cash equivalents
Cash and cash equivalents include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less. As of September 30, 2010 and December 31, 2009, the cash and cash equivalents were mainly denominated in Renminbi (“RMB”) and United States dollars were placed with banks in the PRC and Hong Kong. For those denominated in RMB, they are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government. The remaining insignificant balance of cash and cash equivalents were denominated in Hong Kong dollars.
Restricted Cash
Deposits in banks pledged as securities for bills payable (note 10) that are restricted in use are classified as restricted cash under current assets.
Allowance for doubtful accounts
The Company establishes an allowance for doubtful accounts based on management’s assessment of the collectability of trade receivables. A considerable amount of judgment is required in assessing the amount of the allowance. The Company considers the historical level of credit losses of all segments (Retail, Wholesales and Manufacturing) and applies percentages to aged receivable categories. The Company makes judgments about the creditworthiness of each customer based on ongoing credit evaluations, and monitors current economic trends that might impact the level of credit losses in the future for all segments. If the financial condition of the customers were to deteriorate, resulting in their inability to make payments, a higher allowance may be required.
Q-8
Based on the above assessment, during the reporting periods, management establishes the general provisioning policy for all segments to make allowance equivalent to 40% of the gross amount of trade receivables due between 6 months and 12 months and 100% of the gross amount of accounts receivable due over 12 months. Additional specific provisions are made against trade receivables whenever they are considered to be doubtful.
Bad debts are written off when identified. The Company extends unsecured credit to customers ranging from three to six months in the normal course of business. The Company does not accrue interest on trade accounts receivable.
Inventories
Inventories are stated at the lower of cost or market value. Cost is determined on weighted average basis and includes all expenditures incurred in bringing the goods in a saleable condition to the point of sale. The Company’s inventory reserve requirements generally fluctuate based on projected demands, market conditions and product life cycles. In determining the adequate level of inventories to have on hand, management makes judgments as to the projected inventory demands as compared to the current or committed inventory levels. Inventory quantities and expiration dates are reviewed regularly and provisions for excess or obsolete inventory are recorded based on the expiration dates and the Company’s forecast of future demand and market conditions.
No provisions for excess or obsolete inventory were made as of September 30, 2010 or December 31, 2009.
Property, plant and equipment
Property, plant and equipment is stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
Depreciation is provided on a straight-line basis over their estimated useful lives. The depreciation rates are as follows:
|
Annual rate
|
Residual value
|
|||||||
|
Buildings
|
2.54% - 9.84
|
%
|
Nil - 2
|
%
|
||||
|
Plant and machinery
|
7.00% -18.40
|
%
|
Nil - 10
|
%
|
||||
|
Motor vehicles
|
6.00% -18.40
|
%
|
10
|
%
|
||||
|
Furniture, fixtures and equipment
|
6.00% -18.40
|
%
|
10
|
%
|
||||
Construction in progress mainly represents expenditures related to the construction of a new production line and the improvements of the manufacturing process. All direct costs relating to the new production line and the improvements of the manufacturing process are capitalized as construction in progress. No depreciation is provided in respect of construction in progress.
Maintenance and repairs are charged to cost and expense as incurred. Cost and related accumulated depreciation of property sold or otherwise disposed of are removed from the accounts and any resulting gain or loss is included in operations.
Goodwill and intangible assets
The Company applies the provisions of FASB ASC 805, “Goodwill and Intangible Assets” (“FASB ASC 805”). Under FASB ASC 805, goodwill and intangible assets with indefinite useful lives are not amortized, but instead are tested for impairment at least annually.
Goodwill, with an infinite useful life, is stated at cost less accumulated impairment.
Q-9
Pharmaceutical licenses, customer contracts, trademarks, technology know-how and patents are stated at cost less accumulated amortization. Amortization is provided on a straight-line over their useful lives as follows:
|
Pharmaceutical licenses
|
10 years
|
|
Customer contracts, trademarks, know-how and patents
|
1–3 years
|
Land use rights
Land use rights are stated at cost less accumulated amortization. Amortization is provided using the straight-line method over the terms of the leases ranging from 40 to 70 years. The lease term is obtained from the relevant PRC land authority.
Impairment of long-lived assets
Long-lived assets are tested for impairment in accordance with FASB ASC 360-10-45 “Impairment or Disposal of Long-Lived Assets”. The Company periodically evaluates potential impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book value of such assets exceeds the future undiscounted cash flows attributable to such assets. During the reporting period, the Company has not identified any indicators that would require testing for impairment.
Government grants
Receipts of government grants to encourage research and development activities, which are non-refundable, are credited to deferred income upon receipt. Government grants are used for purchases of property, plant and equipment, to subsidize the research and development expenses incurred, for compensation expenses already incurred or for good performance of the Company.
Grants applicable to purchase of property, plant and equipment are amortized over the life of the depreciable assets. For research and development expenses, the Company matches and offsets the government grants with the expenses of the research and development activities as specified in the grant approval document in the corresponding period when such expenses are incurred. Government grants received as compensation for expenses already incurred in the prior period or for good performance of the Company are recognized as income in the period they become recognizable.
Revenue recognition
Revenue from the sales of the Company’s products in both the wholesales and manufacturing segments is recognized upon customer acceptance. This occurs at the time of delivery to the customer, provided persuasive evidence of an arrangement exists, such as a signed sales contract. The significant risks and rewards of ownership are transferred to the customers at the time when the products are delivered and there is no significant post-delivery obligation to the Company. In addition, the sales price is fixed or determinable and collection is reasonably assured. The Company does not provide customers with contractual rights of return for products. When there are significant post-delivery performance obligations, revenue is recognized only after such obligations are fulfilled. The Company evaluates the terms of the sales agreement with its customer in order to determine whether any significant post-delivery performance obligations exist. Currently the sales under both the wholesale and manufacturing segments do not include any terms which may impose any significant post-delivery performance obligations on the Company.
Revenue from the sales of the Company’s products in its retail segment is recognized upon customer acceptance. This occurs at the time when the product is purchased by the retail customers at the Company’s retail stores, and there is no significant post-delivery obligation, and collection is reasonably assured. The Company does not have a return policy allowing customers to return the products sold. When there are any significant post-delivery performance obligations, revenue is recognized only after such obligations are fulfilled. The Company evaluates the rules and regulations relating to the retail sales of drugs in the PRC in order to determine whether any significant post-delivery performance obligations exist. Currently, the rules and regulations relating to the retail sales of drugs in the PRC does not include any provisions which may impose significant post-delivery performance obligations on the Company.
Q-10
Revenue from the sales of the Company’s products represents the invoiced value of goods, net of the value-added tax (VAT). All of the Company’s products that are sold in the PRC are subject to a Chinese value-added tax at a rate of 17 percent of the gross sales price. This VAT may be offset by the VAT paid by the Company on raw and other materials that are included in the cost of producing the Company’s finished products.
Advertising, research and development expenses
Advertising and research and development expenses are expensed as incurred.
Retirement benefit costs
Liuzhou BCT adopted a retirement plan to provide for eligible employees who were hired prior to April 23, 2002. These eligible employees are entitled to receive certain amounts upon termination or retirement from the Company. The amounts are based on the employee’s years of service in Liuzhou BCT up to April 23, 2002. The obligation for retirement benefit costs is recorded at the present value of the cost that is expected to settle the obligation and is recognized when the retirement plan is approved. The employees hired after April 23, 2002 are not entitled to this retirement plan.
Shipping and handling costs
Shipping and handling costs are expensed as incurred and are included in selling expenses.
Vendor allowances
The Company receives allowances from certain vendors whose products it purchases for resale. These allowances are received for a variety of buying activities such as the volume purchase allowance associated with the vendor programs. Consideration received from a vendor is a reduction in the cost of the inventory and is recognized as a reduction in the cost of goods sold. The Company also receives promotional allowance funds for specific vendor-sponsored programs per the applicable agreements. These promotional allowance funds are recognized as a reduction in the cost of goods sold as the program occurs.
Store opening costs
Costs incurred with store start-up costs, such as travel for recruitments, and training and setup for new store openings, are expensed as incurred.
Dividends
Dividends are recorded in the Company’s financial statements in the period in which they are declared.
Off-balance sheet arrangements
The Company does not have any off-balance sheet arrangements.
Income taxes
The Company uses the asset and liability method of accounting for income taxes pursuant to FASB ASC 740 "Income Taxes”. Under the asset and liability method of FASB ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Q-11
Comprehensive income
The Company has adopted FASB ASC 220, “Comprehensive Income”, which establishes standards for reporting and display of comprehensive income (loss), its components and accumulated balances. Components of comprehensive income (loss) include net income (loss) and foreign currency translation adjustments.
Concentrations of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist of cash and cash equivalents, restricted cash, trade receivables and amounts due from related companies. As of September 30, 2010 and December 31, 2009, substantially all of the Company’s cash and cash equivalents and restricted cash were held by major financial institutions located in the PRC, which management believes are of high credit standing. With respect to trade receivables, the Company extends credit based on an evaluation of the customer’s financial condition. The Company generally does not require collateral for trade receivables and maintains an allowance for doubtful accounts of trade receivables.
During the nine months ended September 30, 2010 and 2009, no single customer accounted for 10% or more of the Company’s consolidated sales and no single customer constituted 10% or more of the Company’s trade receivables.
Foreign currency translation
The functional currency of the Company is RMB and RMB is not freely convertible into foreign currencies. The Company maintains its financial statements in the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet date. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
Assets and liabilities of the Company’s operations are translated into the reporting currency, United States dollars, at the exchange rate in effect at the balance sheet dates. Revenue and expenses are translated at average rates in effect during the reporting periods. The resulting translation adjustment is reflected as accumulated other comprehensive income (loss), a separate component of stockholder’s equity in the statement of stockholder’s equity.
Basic and diluted earnings per share
The Company reports basic earnings per share in accordance with FASB ASC 260, “Earnings Per Share”. Basic earnings per share is computed using the weighted average number of shares outstanding during the reporting periods. The weighted average number of shares of the Company represents the common stock outstanding during the reporting periods. Diluted earnings per share is computed using the weighted average number of common shares outstanding plus the effect of dilutive securities outstanding during the reporting periods.
Fair value of financial instruments
The Company adopted FASB ASC 820 on January 1, 2008. The adoption of FASB ASC 820 did not materially impact the Company's financial position, results of operations or cash flows.
FASB ASC 820 requires the disclosure of the estimated fair value of financial instruments including those financial instruments for which the fair value option was not elected. The carrying amounts of both the financial assets and liabilities approximate to their fair values due to short maturities or the applicable interest rates approximate the current market rates.
Q-12
Recently issued accounting pronouncements
In April 2010, the Financial Accounting Standards Board (the “FASB”) issued guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. The guidance is effective on a prospective basis for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, with early adoption permitted. The Company does not expect a material impact on its consolidated condensed financial statements upon the adoption of the new accounting standard.
Accounting for Transfers of Financial Assets (Included in amended FASB ASC 860 “Transfers and Servicing”, previously SFAS No. 166, “Accounting for Transfers of Financial Assets - an Amendment of Financial Accounting Standard Board (Statement No. 140.”). The amended topic addresses information a reporting entity provides in its financial statements about the transfer of financial assets; the effects of a transfer on its financial position, financial performance, and cash flows; and a transferor’s continuing involvement in transferred financial assets. Also, the amended topic removes the concept of a qualifying special purpose entity, limits the circumstances in which a transferor derecognizes a portion or component of a financial asset, defines participating interest and enhances the information provided to financial statement users to provide greater transparency. The amended topic is effective for the first annual reporting period beginning after November 15, 2009 and was effective for us as of January 1, 2010. The adoption of this amended topic has no material impact on the Company’s financial statements.
Consolidation of Variable Interest Entities - Amended (Included in amended FASB ASC 810 “Consolidation”, previously SFAS 167 “Amendments to FASB Interpretation No. 46(R)”). The amended topic requires an enterprise to perform an analysis to determine the primary beneficiary of a variable interest entity; to require ongoing reassessments of whether an enterprise is the primary beneficiary of a variable interest entity and to eliminate the quantitative approach previously required for determining the primary beneficiary of a variable interest entity. The amended topic also requires enhanced disclosures that will provide users of financial statements with more transparent information about an enterprise’s involvement in a variable interest entity. The amended topic is effective for the first annual reporting period beginning after November 15, 2009 and will be effective for us as of January 1, 2010. The adoption of this amended topic has no material impact on the Company’s financial statements.
The FASB issued Accounting Standards Update (ASU) No. 2009-13, Revenue Recognition (Topic 605): Multiple Deliverable Revenue Arrangements - A Consensus of the FASB Emerging Issues Task Force.” This update provides application guidance on whether multiple deliverables exist, how the deliverables should be separated and how the consideration should be allocated to one or more units of accounting. This update establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence, if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific or third-party evidence is available. The Company will be required to apply this guidance prospectively for revenue arrangements entered into or materially modified after January 1, 2011; however, earlier application is permitted. The management is in the process of evaluating the impact of adopting this ASU update on the Company’s financial statements.
The FASB issued ASU 2010-06, Improving Disclosures about Fair Value Measurements. ASU 2010-06 amends FASB ASC 820 to require the following additional disclosures regarding fair value measurements: (i) the amounts of transfers between Level 1 and Level 2 of the fair value hierarchy; (ii) reasons for any transfers in or out of Level 3 of the fair value hierarchy and (iii) the inclusion of information about purchases, sales, issuances and settlements in the reconciliation of recurring Level 3 measurements. ASU 2010-06 also amends FASB ASC 820 to clarify existing disclosure requirements, requiring fair value disclosures by class of assets and liabilities rather than by major category and the disclosure of valuation techniques and inputs used to determine the fair value of Level 2 and Level 3 assets and liabilities. With the exception of disclosures relating to purchases, sales, issuances and settlements of recurring Level 3 measurements, ASU 2010-06 was effective for interim and annual reporting periods beginning after December 15, 2009. The disclosure requirements related to purchases, sales, issuances and settlements of recurring Level 3 measurements will be effective for financial statements for annual reporting periods beginning after December 15, 2010. The management is in the process of evaluating the effect of ASU 2010-06 on its financial statements and results of operation and is currently not yet in a position to determine such effects.
Q-13
The FASB issued ASU No. 2010-02, “Consolidation (FASB ASC 810) Accounting and Reporting for Decreases in Ownership of a Subsidiary - a Scope Clarification”. This amendment affects entities that have previously adopted FASB 810-10 (formally SFAS 160). It clarifies the decrease in ownership provisions of FASB ASC 810-10 and removes the potential conflict between guidance in that section and asset derecognition and gain or loss recognition guidance that may exist in other US GAAP. An entity will be required to follow the amended guidance beginning in the period that it first adopts FAS 160 (now included in FASB ASC 810-10). The adoption of this ASU update has no material impact on the Company’s financial statements.
In February 2010, the FASB issued ASU 2010-09, Subsequent Events: Amendments to Certain Recognition and Disclosure Requirements, which amends FASB ASC 855, Subsequent Events. The update provides that SEC filers, as defined in ASU 2010-09, are no longer required to disclose the date through which subsequent events have been evaluated in originally issued and revised financial statements. The update also requires SEC filers to evaluate subsequent events through the date the financial statements are issued rather than the date the financial statements are available to be issued. The Company adopted ASU 2010-09 upon issuance. This update had no material impact on the financial position, results of operations or cash flows of the Company.
4. Finance costs
|
Three months ended
|
Nine months ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Bank and other loans interest
|
$
|
187,609
|
$
|
510,522
|
$
|
626,774
|
$
|
1,053,134
|
||||||||
|
Bank charges
|
14,399
|
1,718
|
35,657
|
16,039
|
||||||||||||
|
Finance charges from retirement
|
||||||||||||||||
|
benefits costs
|
251
|
20,350
|
17,437
|
20,350
|
||||||||||||
|
Others
|
-
|
-
|
-
|
1,407
|
||||||||||||
|
$
|
202,259
|
$
|
532,590
|
$
|
679,868
|
$
|
1,090,930
|
|||||||||
5. Income taxes
United States
The Company is subject to the United States of America tax law at tax rates up to 34%. No provision for the US federal income taxes has been made as the Company had no taxable income in this jurisdiction for the reporting periods.
BVI
Ingenious was incorporated in the BVI and, under the current laws of the BVI, is not subject to income taxes.
Hong Kong
Forever Well is subject to the Hong Kong Profits tax at a tax rate of 16.5%. No provision for the Hong Kong Profits tax has been made as the Company had no taxable income in this jurisdiction since its incorporation.
Q-14
PRC
Corporate income tax (“CIT”) to Baicaotang Medicine and Baicaotang Retail, was charged at 33%, of which 30% is for national tax and 3% is for local tax, of the assessable profits before 2008. The PRC’s legislative body, the National People’s Congress, adopted the unified CIT Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rate is set at 25% for both domestic enterprises and foreign-invested enterprises. However, there is a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatments granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25% may continue to enjoy the lower rate and will transition into the new tax rate over a five-year period beginning on the effective date of the CIT Law. Enterprises that are currently entitled to exemptions for a fixed term will continue to enjoy such treatment until the exemption term expires. Preferential tax treatment will continue to be granted to industries and projects that qualify for such preferential treatments under the new tax law. Accordingly, Liuzhou BCT and BCT Retail were subject to a tax rate of 25% starting from fiscal year 2008.
In accordance with the Circular of the State Council on Policies and Measures Pertaining to the Development of the Western Region (“DOWR”), the companies are entitled to a preferential rate of 15% if they are engaged in the projects listed in the Guiding Catalogue, and the revenue derived from accounts for over 70% of total revenue. Hefeng Pharmaceutical met this DOWR requirement; as a result, the tax authority approved and granted a preferential tax rate of 15% for fiscal years from 2003 to 2010. Beginning with the fiscal year 2011, Hefeng Pharmaceutical will be subject to CIT at a rate of 25% under the new tax law.
6. Earnings per share
Basic earnings per share has been computed using the weighted average number of common shares outstanding. Diluted earnings per share has been computed using the weighted average number of common shares and common share equivalents outstanding (which consists of warrants and options to the extent they are dilutive). For the three months and nine months ended September 30, 2010, dilutive warrants to purchase 351,934 shares were included in the diluted earnings per share calculations for those periods, and potentially dilutive options and warrants of 1,080,000 and 1,759,301, respectively, were excluded from the diluted loss per share calculation as the average market price of the Company’s common stock did not exceed the weighted average exercise price of such options and warrants, and to have included them would have been anti-dilutive.
7. Inventories
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Raw materials
|
$
|
887,208
|
$
|
722,109
|
||||
|
Work-in-progress
|
136,536
|
148,954
|
||||||
|
Finished goods
|
15,584,094
|
7,874,462
|
||||||
|
$
|
16,607,838
|
$
|
8,745,525
|
|||||
Q-15
8. Goodwill and Other intangible assets
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Goodwill
|
||||||||
|
Acquisition of Hefeng
|
$
|
107,968
|
$
|
107,968
|
||||
|
Other intangible assets
|
||||||||
|
Pharmaceutical licenses
|
$
|
799,197
|
$
|
799,197
|
||||
|
Customer contracts
|
90,729
|
90,729
|
||||||
|
Trademarks, know-how and patents
|
99,892
|
99,892
|
||||||
|
989,818
|
989,818
|
|||||||
|
Accumulated amortization
|
(385,683
|
)
|
(329,784
|
)
|
||||
|
Net
|
$
|
604,135
|
$
|
660,034
|
||||
During the nine months ended September 30, 2010 and 2009, amortization amounted to $55,899 and $58,871, respectively.
9. Property, plant and equipment, net
Property, plant and equipment are stated at cost, as follows:
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Buildings
|
$
|
12,331,575
|
$
|
12,331,575
|
||||
|
Plant and machinery
|
1,304,443
|
1,291,734
|
||||||
|
Furniture, fixtures and equipment
|
2,693,533
|
374,615
|
||||||
|
Motor vehicles
|
407,624
|
367,132
|
||||||
|
16,737,175
|
14,365,056
|
|||||||
|
Accumulated depreciation
|
(3,163,366
|
)
|
(2,544,420
|
)
|
||||
|
13,573,809
|
11,820,636
|
|||||||
|
Construction in progress
|
351,053
|
351,053
|
||||||
|
$
|
13,924,862
|
$
|
12,171,689
|
|||||
|
(a)
|
An analysis of buildings, plant and machinery pledged to banks for banking loans (note 13(d)(i)) is as follows :
|
|
September 30, 2010
|
December 31, 2009
|
|||||||
|
Buildings
|
$
|
4,769,920
|
$
|
7,409,774
|
||||
|
Accumulated depreciation
|
(961,279
|
)
|
(1,290,533
|
)
|
||||
|
$
|
3,808,641
|
$
|
6,119,241
|
|||||
Q-16
|
10.
|
Land use rights
|
|
September 30, 2010
|
December 31, 2009
|
|||||||
|
Land use rights
|
$
|
15,362,549
|
$
|
16,039,294
|
||||
|
Accumulated amortization
|
(2,269,186
|
)
|
(2,059,541
|
)
|
||||
|
$
|
13,093,363
|
$
|
13,979,753
|
|||||
The Company has obtained land use rights from the relevant PRC land authority. The lease terms of the land use rights range from 40 to 70 years, and permit the use of the land on which the office premises, production facilities and warehouse of the Company are situated. As of September 30, 2010 and December 31, 2009, land use rights with carrying amounts of $933,609 and $6,205,140, respectively, were pledged to a bank for the bank loans granted to the Company (note 13(d)(ii)).
During the nine months ended September 30, 2010 and 2009, amortization amounted to $233,814 and $255,933, respectively.
During the nine months ended September 30, 2010, land use rights with carrying amounts of $652,576 were sold for consideration of $697,495, net of direct costs, resulting in a gain of $44,919.
11. Amounts due to directors
The amounts due to directors are unsecured and repayable on demand. The balances are interest-free, except for the amounts of $122,771 as of September 30, 2010, and $836,084 as of December 31, 2009, which were interest bearing at fixed rates ranging from 6.96% to 8.16% per annum.
12. Amounts due from/to related companies
The related companies are controlled by several of the Company’s directors including of Mr. Hui Tian Tang, the Company’s Chief Executive Officer and Chairman. These amounts due from or to related companies are interest-free, unsecured and payable on demand.
13. Secured bank loans
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Short-term loans - note 13(a)
|
$
|
7,599,060
|
$
|
7,070,940
|
||||
|
Current maturities of long-term bank loan
|
1,241,951
|
65,129
|
||||||
|
$
|
8,841,011
|
$
|
7,136,069
|
|||||
|
Long-term bank loans - note 13(b)
|
3,654,355
|
3,697,086
|
||||||
|
Less: current maturities
|
(1,241,951
|
)
|
(65,129
|
)
|
||||
|
$
|
2,412,404
|
$
|
3,631,957
|
|||||
Q-17
|
(a)
|
The weighted average interest rates for short-term loans as of September 30, 2010 and December 31, 2009 were 6.34% and 7.14% per annum, respectively.
|
|
(b)
|
The long term loans as of September 30, 2010 were interest bearing at variable rates ranging from HIBOR plus 6.48% to HIBOR plus 7.74% per annum, respectively.
|
|
(c)
|
As of September 30, 2010, the Company’s banking facilities were composed of the following:
|
|
Facilities granted
|
Granted
|
Amount
Utilized
|
Unused
|
|||||||||
|
Secured bank loans
|
$
|
11,253,415
|
$
|
11,253,415
|
$
|
-
|
||||||
|
(d)
|
As of September 30, 2010, the above bank loans were secured by the following:
|
|
(i)
|
Buildings, plant and machinery with a carrying value of $3,808,641 (note 9).
|
|
(ii)
|
Land use rights with a carrying value of $933,609 (note 10).
|
|
(iii)
|
Buildings and land use rights owned by the related companies which are controlled by several of the Company’s directors.
|
|
(e)
|
Long-term borrowings are repayable as follows:
|
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Within one year
|
$
|
1,241,951
|
$
|
66,386
|
||||
|
After one year but within two years
|
2,200,970
|
1,757,354
|
||||||
|
After two years but within three years
|
79,726
|
1,689,590
|
||||||
|
After three years but within four years
|
86,105
|
81,920
|
||||||
|
After four years but within five years
|
45,603
|
88,429
|
||||||
|
After five years
|
-
|
14,664
|
||||||
|
$
|
3,654,355
|
$
|
3,698,343
|
|||||
During the nine months ended September 30, 2010, there was no covenant requirement under the banking loans granted to the Company.
14. Other loans
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Interest bearing
|
||||||||
|
Employee - note 14(a)
|
$
|
-
|
$
|
377,067
|
||||
|
Third parties - note 14(a)
|
-
|
1,544,091
|
||||||
|
-
|
1,921,158
|
|||||||
|
Non interest bearing
|
||||||||
|
Third parties
|
154,886
|
440,100
|
||||||
|
$
|
154,886
|
$
|
2,361,258
|
|||||
|
(a)
|
Interest bearing loans at a fixed rate of 4% to 7.2% per annum.
|
|
(b)
|
All the other loans are unsecured and repayable on demand.
|
Q-18
15. Warrant liabilities
As of December 30, 2009, the Company completed a private placement of 2,489,370 shares of common stock and five-year warrants to purchase up to 1,244,368 shares of common stock (“First Batch Warrants”). The stock was purchased at an exercise price of $3.81 per share for gross proceeds of $6,322,952, which includes issuance expenses of $1,016,290. In accordance with FASB ASC 815, these warrants are not considered indexed to the Company’s own stock and should be classified as financial derivative liabilities at fair value for each reporting period. For the year ended December 31, 2009, the fair value of First Batch Warrants was $1,294,142 and the Company considered the amount to be immaterial to the financial statements. Thus, the net proceeds were allocated to First Batch Warrants resulting in the entire amount recorded as equity.
Upon the completion of a private placement as of February 1, 2010, as stated in Note 17(a), of which five-year warrants to purchase up to 514,933 shares of common stock (“Second Batch Warrants”) were issued to investors and should be classified as financial derivative liabilities at fair value for each reporting period in accordance with FASB ASC 815, the Company determined that the aggregate fair value of warrants issued as of February 1, 2010 was material to the financial statements for the three months ended March 31, 2010. Accordingly, a reclassification was made for the fair value of First Batch Warrants as of December 31, 2009 amounting to $1,294,142 from the Company’s equity to warrant liabilities as of February 1, 2010. For the Second Batch Warrants, part of the net proceeds amounting to $561,277, representing the fair value as of February 1, 2010, was allocated to warrant liabilities at initial recognition.
The fair value of the warrants was calculated using the binomial model. The assumptions that were used to calculate fair value of First Batch Warrants and Second Batch Warrants as of September 30, 2010 are as follows:
|
·
|
Expected volatility of 45.5%
|
|
·
|
Expected dividend yield of 0%
|
|
·
|
Risk-free interest rate of 3.427%
|
|
·
|
Expected lives of 4.25 years and 4.34 years for First Batch Warrants and Second Batch Warrants respectively
|
|
·
|
Exercise price of $3.81 per share
|
As of September 30, 2010, the fair value of warrant liabilities was $2,313,137 and the corresponding loss on the change in fair value of warrant liabilities of $457,718 was recognized in the Company’s statement of operations for the nine months ended September 30, 2010.
Warrants issued and outstanding, all of which are exercisable at September 30, 2010, are summarized as follows:
|
Number of shares
|
||||
|
First Batch Warrants
|
1,244,368
|
|||
|
Second Batch Warrants
|
514,933
|
|||
|
1,759,301
|
||||
Q-19
16. Commitments and contingencies
|
a.
|
Capital commitment
|
As of September 30, 2010, the Company had capital commitments in respect of the acquisition of property, plant and equipment amounting to $274,695, which were contracted for but not provided for in the consolidated financial statements.
|
b.
|
Operating lease commitments
|
As of September 30, 2010, the Company had non-cancelable operating leases for its retail shops and future minimum lease payments to be paid are as follows:
|
Year
|
||||
|
2010
|
$
|
234,320
|
||
|
2011
|
925,494
|
|||
|
2012
|
842,769
|
|||
|
2013
|
288,098
|
|||
|
2014
|
104,143
|
|||
|
$
|
2,394,824
|
|||
The rental expense relating to the operating leases was $558,783 and $227,280 for the nine months ended September 30, 2010 and 2009, respectively.
|
c.
|
Operating lease arrangement
|
As of September 30, 2010, the Company leases part of its building in PRC under an operating lease arrangement until 2010. Future minimum lease payments to be received under non-cancelable operating leases are as follows:
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Within one year
|
$
|
84,700
|
$
|
11,903
|
||||
|
After one year but within two years
|
15,118
|
-
|
||||||
|
After two years but within three years
|
9,547
|
-
|
||||||
|
$
|
109,365
|
$
|
11,903
|
|||||
|
d.
|
Employment agreements
|
During the 2nd quarter, the Company entered into employment agreements with Hui Tian Tang, the Company’s CEO, and Xiaoyan Zhang, the Company’s CFO. The employment agreements were approved by the board of directors and the compensation committee. The following is a summary of the material provisions of the employment agreements for Mr. Tang and Ms. Zhang:
Q-20
On May 18, 2010, the Company entered into a new employment agreement with Mr. Tang to employ him as CEO for a term from January 1, 2010 to January 1, 2012, pursuant to which he will be paid RMB 79,600 ($11,600) per month (or RMB 955,200 ($139,200) per year) and additional share-based compensation based upon our 2010 financial performance (see note 18). The agreement may be terminated upon mutual agreement between the Company and Mr. Tang in writing. In addition, the Company shall have the right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Mr. Tang is criminally prosecuted under the law.
The Company may also terminate the agreement by serving 30 days' prior written notice to Mr. Tang or giving Mr. Tang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Mr. Tang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out his job responsibilities; (ii) where Mr. Tang is unable to fulfill the duties of his position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Mr. Tang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Mr. Tang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Mr. Tang in accordance with the law; (ii) the Company forces Mr. Tang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
On May 18, 2010, the Company entered into a new employment agreement with Ms. Zhang to employ her as CFO for a term from January 1, 2010 to January 1, 2012, pursuant to which we have agreed to pay her HKD70,000 ($9,091) per month (or HKD840,000 ($109,092) per year) and additional share-based compensation based upon our 2010 financial performance (see note 18).
The agreement may be terminated upon mutual agreement between the Company and Ms. Zhang in writing. In addition, the Company shall have right to unilaterally terminate the agreement under certain circumstances, including, among other things, (i) serious violations of the labor laws or the rules or regulations of the Company; (ii) causing serious damage to the interests of the Company; or (iii) Ms. Zhang is criminally prosecuted under the law.
The Company may terminate the agreement by serving 30 days' prior written notice to Ms. Zhang or giving Ms. Zhang one month’s salary in lieu of notice in any one of the following circumstances: (i) where Ms. Zhang, after undergoing a legally prescribed period of medical treatment and recuperation for an illness or a non-work-related injury, remains unable to carry out her job responsibilities; (ii) where Ms. Zhang is unable to fulfill the duties of her position to the standards required under the terms of the agreement; or (iii) where the agreement cannot be performed due to any major changes of any objective circumstances, which includes, but is not limited to, a merger of the Company into another business entity, or sale or transfer by the Company of a substantial portion of the assets it owns to third parties, a material adjustment in operative policy, or a declaration of bankruptcy, dissolution or liquation by the Company.
Ms. Zhang may terminate the agreement during the term upon 30 days prior written notice to the Company. In addition, Ms. Zhang may terminate the agreement in certain circumstances, including if (i) the Company fails to pay the social insurance premiums for Ms. Zhang in accordance with the law; (ii) the Company forces Ms. Zhang to work by means of violence or intimidation; or (iii) the Company fails to pay labor remuneration in full and on time or fails to provide the labor protection or working conditions as agreed under the agreement.
e) Registration payment arrangements
On December 30, 2009, the Company completed a private placement of 2,489,370 shares of common stock and warrants in order to purchase up to 1,244,368 shares of common stock at an exercise price of $3.81 per share. In connection with the private placement, warrants to purchase up to 248,937 shares of common stock at an exercise price of $3.05 per share were issued to the Co-Placement Agents.
Q-21
Pursuant to the subscription agreement, the Company was required to file a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended, (i) registering for resale by the investors 2,489,370 shares of common stock and warrants in order to purchase up to 1,244,368 shares of common stock issued to the investors; and (ii) registering for resale for the Co-Placement Agents for the warrants to purchase up to 248,937 shares of common stock (all of the foregoing securities being collectively referred herein as the “Registrable Securities”). The Company agreed to use its best efforts to file the Registration Statement within 30 days from the Second Closing, dated February 1, 2010 (“Registration Filing Date”) and to have the Registration Statement declared effective prior to the 150th day following the Second Closing, provided, however, that in the event of a “full review” by the SEC, the Company shall be afforded an additional 30 days and shall have the Registration Statement declared effective prior to the 180th day following the Second Closing (the “Registration Effective Date”).
In the event that (i) the Registration Statement has not been filed on or prior to the Registration Filing Date or declared effective by the SEC on or before the Registration Effective Date; and (ii) the Registrable Securities included in such Registration Statement are not saleable under Rule 144, the Company shall pay to each investor as liquidated damages, a cash payment equal to 1% of the aggregated amount invested by such investors in the private placement for the first 30 days and 1% of the aggregated amount vested by such investors in the private placement for every 30-day period thereafter until the registration statement has been filed and/or declared effective, or such proportionate percentage for any period less than 30 days. In accordance with FASB ASC 825-20, if the Company determines a registration payment arrangement is probable and can be reasonably estimated, a liability should be recorded. Provision for registration payment through September 30, 2010 has been provided in the amount of $178,783. As of September 30, 2010 and up to the date of approval of these financial statements, it is not considered probable that the Company will be required to make any further material payments under the registration rights arrangement and therefore no provision for such liability has been made.
f) Distribution agreements
During September of 2010 we entered into separate distribution agreements with these three government-owned hospitals, each for a term of three years, pursuant to which the hospitals agreed to our supplying them with from 30% to 95% of their respective purchase plans for certain drugs. We agree to use our best efforts to fulfill the orders by distributing the drugs under the statutory requirement of the mandated collective tender plan. We were required to make good-faith deposits totaling RMB 23,000,000 ($3,374,100) for the security of these distribution agreements. Under these agreements there are no provisions for forfeiture or offset with respect to these deposits. The hospitals are required to repay the deposit amounts in full when the agreements expire or when terminated, which may be done only under certain circumstances. We entered into such agreements to pursue relationships with these large hospitals in order to achieve a higher share of these customers' purchases. Based on the terms of the agreements, we anticipate the revenues derived from these distribution agreements will contribute additional revenue of approximately $7,000,000 annually, commencing in the fourth quarter of 2010, over the three-year terms of the agreements.
17. Common stock and additional paid-in capital
|
Number of
shares
|
Amount
|
Additional
paid-in
capital
|
||||||||||
|
Balance, January 1, 2010
|
37,089,370
|
$
|
37,089
|
$
|
14,920,899
|
|||||||
|
Share-based compensation expense - note 18
|
-
|
-
|
658,207
|
|||||||||
|
Reclassification - note 15
|
-
|
-
|
(1,294,142
|
)
|
||||||||
|
Private placement - note 17(a)
|
1,029,970
|
1,030
|
1,752,831
|
|||||||||
|
Shares issued for the payment of placement service
|
35,000
|
35
|
(35
|
)
|
||||||||
|
Balance, September 30, 2010
|
38,154,340
|
$
|
38,154
|
$
|
16,037,760
|
|||||||
|
(a)
|
As of February 1, 2010, the Company completed a private placement of 1,029,970 shares of common stock and warrants in order to purchase up to 514,933 shares of common stock at an exercise price of $3.81 per share for gross proceeds of $2,616,108 which includes issuance expenses of $300,970. In accordance with FASB ASC 815, these warrants are not considered indexed to the Company’s own stock and should be classified as financial derivative liabilities at fair value for each reporting period. A part of the net proceeds amounting to $561,277, was allocated to warrant liabilities with the remaining balance of $1,753,861 recorded in the Company’s equity at initial recognition. See also note 15.
|
|
(b)
|
On February 5, 2010, the company issued 35,000 shares to several lawyers for the placement services in connection thereto.
|
Q-22
18. Stock option arrangements
On June 27, 2010, we adopted the China BCT Pharmacy Group, Inc. 2010 Omnibus Securities and Incentive Plan (the “Plan”) for the benefit of our employees, nonemployee directors and consultants and the employees, nonemployee directors and consultants of its affiliates, for purposes of assisting us to attract, retain and provide incentives to key management employees and nonemployee directors of, and non-employee consultants to, us and our affiliates, and to align the interests of such individuals with those of our stockholders. Accordingly, the Plan provides for the granting of distribution equivalent rights, incentive stock options, non-qualified stock options, performance share awards, performance unit awards, restricted stock awards, restricted stock unit awards, stock appreciation rights, tandem stock appreciation rights, unrestricted stock awards or any combination of the foregoing, as may be best suited to the circumstances of the particular employee, director or consultant as provided in the Plan.
On June 27, 2010, we granted options under the Plan to two executive employees. The options vest and become exercisable, for a 10 year period from the grant date, with respect to all of the shares only if our after-tax net income for fiscal 2010 equals at least U.S. $26,000,000 (excluding any non-cash expenses) (the “2010 Income Target”), determined on the basis of our audited financial statements for our 2010 fiscal year, as confirmed by our independent auditors in their report on said financial statements (the “Audit Report”). If the 2010 Income Target is met, the options shall vest and become exercisable on the date on which the Audit Report is dated, and if the options do not become exercisable due to the failure to meet the 2010 Income Target, the option shall terminate on the date on which the Audit Report is dated.
On July 16, 2010, we entered into separate stock option agreements with each of our three independent directors, Messrs. Lee, Choi and Chiu, which agreements provide for the grant to each such individual of an option to purchase 10,000 shares of our common stock at an exercise price of $4.00 per share. Each option vests and becomes exercisable with respect to all of the shares on June 6, 2011. The exercise price of each option may be paid for in cash or by cancellation of existing shares of our common stock held by the independent directors. Each option terminates on the earlier of (i) July 16, 2015 or (ii) the date as of which the option has been fully exercised
The fair value of options granted to the two executive employees at the date of grant was $1,774,440. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of June 27, 2010 were: 0% dividend yield, expected volatility of 30%, risk-free interest rate of 4.78%, an exercise multiple of 2, and a post-vesting forfeiture rate of 0%. The Company expensed $617,197 in share-based compensation for the nine months ended September 30, 2010 based upon the conclusion of management that it is probable the 2010 Income Target will be achieved. The remaining compensation cost of $1,157,243 related to non-vested awards will be recognized over the implied remaining requisite service period of October 1, 2010 through March 29, 2011 for both options.
The fair value of options granted to the three independent directors at the date of grant was $41,010 and was expensed in share-based compensation for the nine months ended September 30, 2010. Fair values were estimated using the binominal model. The assumptions that were used to calculate fair value of stock options as of July 16, 2010 were: 0% dividend yield, expected volatility of 41.8%, risk-free interest rate of 3.427%, an exercise multiple of 2, and a post-vesting forfeiture rate of 0%.
The company expensed share-based compensation included in administrative expense in the amount of $658,207 for the nine months ended September 30, 2010.
There were no options granted with exercise prices below the market value of the stock at the grant date. All outstanding options at September 30, 2010 had no intrinsic value because the Company’s stock price was lower than all option exercise prices. A summary of the Company’s stock options issued and outstanding as of and for the nine months ended September 30, is presented below:
Q-23
|
Number of options
|
Exercise Price
|
|||||||
|
Stock options granted and outstanding
|
1,110,000
|
$
|
4.00
|
|||||
19. Defined contribution plan
Pursuant to the relevant PRC regulations, the Company is required to make contributions at a rate of 30.5% of employees’ salaries and wages to a defined contribution retirement plan organized by a state-sponsored social insurance plan in respect of the retirement benefits for the Company’s employees in the PRC. The only obligation of the Company with respect to the retirement plan is to make the required contributions under the plan. No forfeited contributions are available to reduce the contribution payable in the future years. The contributions of the defined contribution plan were charged to the consolidated statements of income. The Company contributed $351,559 and $331,113 for the nine months ended September 30, 2010 and 2009, respectively.
20. Acquisitions of retail stores
During the second quarter of 2010, the Company finalized the purchase of 59 retail stores from three separate store owners. The total consideration paid for the stores was cash of $4,521,744. Based upon the reports of licensed business appraisers retained by the Company, the purchase price was allocated to the net assets acquired, as follows:
|
Inventory
|
$
|
2,129,102
|
||
|
VAT tax recoverable
|
140,164
|
|||
|
Store improvements and equipment
|
2,252,478
|
|||
|
Total
|
$
|
4,521,744
|
During the third quarter of 2010, the Company entered into two asset transfer agreements to acquire 33 retail pharmacy stores. As of September 30, 2010, total deposits of $1,290,960 were made to the sellers. The transactions are expected to close in the fourth quarter of 2010.
21. Segment information
The Company uses the “management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Management, including the chief operating decision maker, reviews the operating results solely by monthly revenue of pharmaceutical distribution, retail pharmacy and manufacturing segments and the operating results of the Company. As such, the Company has determined that the Company has three operating segments as defined by FASB ASC 280, “Segment Reporting”: Pharmaceutical distribution, retail pharmacy and manufacturing.
|
Three Months Ended,
|
Nine Months Ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Net Sales
|
||||||||||||||||
|
Wholesale Distribution
|
$
|
38,120,898
|
$
|
23,033,227
|
$
|
94,687,187
|
$
|
70,772,780
|
||||||||
|
Retail
|
11,517,739
|
8,583,721
|
32,031,604
|
22,352,150
|
||||||||||||
|
Manufacturing
|
2,889,609
|
2,250,243
|
7,331,051
|
6,348,515
|
||||||||||||
|
$
|
52,528,246
|
$
|
33,867,191
|
$
|
134,049,842
|
$
|
99,473,445
|
|||||||||
Q-24
Total intersegment revenue for the three months ended September 30, 2010 and 2009 was $9,380,775 and $7,530,351, respectively. Total intersegment revenue for the nine months ended September 30, 2010 and 2009 was $25,155 and $17,949,744, respectively.
|
Three Months Ended,
|
Nine Months Ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Operating Income
|
||||||||||||||||
|
Wholesale Distribution
|
$
|
6,628,044
|
$
|
3,910,518
|
$
|
15,994,222
|
$
|
12,225,187
|
||||||||
|
Retail
|
2,224,779
|
1,972,702
|
7,061,789
|
5,171,623
|
||||||||||||
|
Manufacturing
|
1,466,808
|
960,322
|
3,854,902
|
2,755,086
|
||||||||||||
|
$
|
10,319,631
|
$
|
6,843,542
|
$
|
26,910,913
|
$
|
20,151,896
|
|||||||||
|
Three Months Ended,
|
Nine Months Ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Depreciation and Amortization Expenses
|
||||||||||||||||
|
Wholesale Distribution
|
$
|
163,515
|
$
|
163,669
|
$
|
455,865
|
$
|
429,564
|
||||||||
|
Retail
|
26,219
|
982
|
37,627
|
2,209
|
||||||||||||
|
Manufacturing
|
143,944
|
138,531
|
415,167
|
433,139
|
||||||||||||
|
$
|
333,678
|
$
|
303,182
|
$
|
908,659
|
$
|
864,912
|
|||||||||
|
September 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Assets
|
||||||||
|
Wholesale
|
$
|
98,524,678
|
$
|
68,252,951
|
||||
|
Retail
|
13,060,972
|
9,184,032
|
||||||
|
Manufacturing
|
15,028,854
|
10,319,034
|
||||||
|
$
|
126,614,504
|
$
|
87,756,017
|
|||||
Q-25
A reconciliation is provided for unallocated amounts relating to corporate operations which is not included in the segment information.
|
Three months ended
|
Nine months ended
|
|||||||||||||||
|
September 30,
|
September 30,
|
|||||||||||||||
|
(Unaudited)
|
(Unaudited)
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Total consolidated revenue
|
$
|
52,528,246
|
$
|
33,867,191
|
$
|
134,049,842
|
$
|
99,473,445
|
||||||||
|
Total profit for reportable
|
||||||||||||||||
|
segments
|
$
|
10,319,631
|
$
|
6,843,542
|
$
|
26,910,913
|
$
|
20,151,896
|
||||||||
|
Unallocated amounts relating to
|
||||||||||||||||
|
operations:
|
||||||||||||||||
|
Change in fair value of warrant liabilities
|
249,092
|
-
|
(457,718
|
)
|
-
|
|||||||||||
|
Share-based compensation
|
(632,490
|
)
|
-
|
(658,207
|
)
|
-
|
||||||||||
|
Provision for registration payment
|
(178,783
|
)
|
-
|
(178,783
|
)
|
-
|
||||||||||
|
Other general expenses
|
(375,529
|
)
|
(12,692
|
)
|
(873,990
|
)
|
(24,744
|
)
|
||||||||
|
Finance costs
|
(247
|
)
|
(186
|
)
|
(2,859
|
)
|
(2,242
|
)
|
||||||||
|
Income before income taxes
|
$
|
9,381,674
|
$
|
6,830,664
|
$
|
24,739,356
|
$
|
20,124,910
|
||||||||
|
September 30,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
(Unaudited)
|
(Audited)
|
|||||||
|
Assets
|
||||||||
|
Total assets for reportable segments
|
$
|
126,614,504
|
$
|
87,756,017
|
||||
|
Cash and cash equivalents
|
328,498
|
5,078,973
|
||||||
|
Other receivables
|
-
|
225,802
|
||||||
|
$
|
126,943,002
|
$
|
93,060,792
|
|||||
All of the Company’s long-lived assets and revenues are classified based on the customers’ location.
22. Subsequent events
On January 18, 2011, the Company entered into an agreement (the “Preferred Purchase Agreement”) with Milestone Longcheng Limited (“Milestone”) pursuant to which Milestone will purchase 9,375,000 shares of the Company’s Series A Convertible Preferred Shares, par value $.001 per share (the “Preferred Shares”), for an aggregate purchase price of $30 million. The Preferred Shares carry a dividend of 5% and are convertible initially into an equal number of shares of our Common Stock at an initial conversion price of $3.20 per share. The transaction is subject to a number of closing conditions, including the completion of the amendment of the Company’s Certificate of Incorporation to increase its authorized capital to 170 million shares of capital stock consisting of 150 million shares of Common Stock (an increase of 50 million shares) and 20 million shares of blank-check preferred stock which the Company’s board of directors will have the authority to issue (the “Amendment”) and the filing of the Amendment and of the Certificate of Designation adopting the terms of the Preferred Shares. All necessary board and stockholder action to approve adoption of the Amendment have been taken and the Amendment will be complete upon filing with the Delaware Secretary of State which will occur 20 days after the mailing to the Company’s stockholders of an information statement pursuant to Section 14(c) of the Exchange Act. The Company filed this information statement with the SEC on January 18, 2011 and will mail it at the earliest date available in accordance with provisions of Section 14(c) and the regulations thereunder. The board has also approved the Certificate of Designation, subject to the filing of the Amendment. The Certificate of Designation will be filed after the filing of the Amendment in connection with the closing of the sale of the Preferred Shares.
In connection with this transaction, the Company and Milestone also entered into a registration rights agreement and, with certain of the Company’s principal stockholders, a shareholders agreement. The registration rights agreement provides for registration under the Securities Act of 1933, under various circumstances, of the shares of Common Stock into which the Preferred Shares can be converted. Under the Preferred Purchase Agreement, Milestone (or successor holders of the Preferred Shares) will have the right to name one director and to recommend an additional independent director to the Company’s board of directors, which the Company expects will be increased to seven directors after closing of the transaction. The Purchase Agreement also provides that Milestone (or successor holders) will have consent rights with respect to certain operating matters. The shareholders agreement provides limitations on the rights of the principal shareholders to engage in competing businesses in the event that they are no longer employed with the Company and also provides a process for their sale of their shares, including first offering them to the holders of the Preferred Shares and co-sale rights of the holders. Under the Certificate of Designation, the holders of Preferred Shares have pre-emptive rights with respect to future offerings and have voting rights with the Common Stock as well as separate voting rights with respect to certain extraordinary transactions. After issuance, the Preferred Shares will represent 18.5% of the outstanding share capital of the Company. These shares, together with the shares of Common Stock owned by Ms. Zhang (representing approximately 44.3% of the outstanding share capital after this transaction) will hold the majority voting interest in the Company. The Company anticipates that the transaction will be closed approximately 30 days after signing or as soon there after as the Company and Milestone determine. The Company is currently considering the appropriate accounting treatment for this transaction and will disclose such treatment, as appropriate, once finalized.
The Company has evaluated subsequent events since September 30, 2010 and has determined that there were no subsequent events other than as described above to recognize or disclose in these financial statements.
Q-26
Report of Independent Registered Public Accounting Firm
To the Directors and Stockholders of
China BCT Pharmacy Group, Inc.
We have audited the accompanying consolidated balance sheets of China BCT Pharmacy Group, Inc. (the “Company”) and its subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of Income and comprehensive Income, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and its subsidiaries as of December 31, 2009 and 2008, and the consolidated results of their operations and their cash flows for each of the two years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
PKF
Certified Public Accountants
Hong Kong, China
March 31, 2010
F-1
China BCT Pharmacy Group, Inc.
Consolidated Statements of Income and Comprehensive Income
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Revenue
|
||||||||
|
Sales
|
$
|
136,086,708
|
$
|
108,991,329
|
||||
|
Cost of sales
|
100,578,769
|
79,361,987
|
||||||
|
Gross profit
|
35,507,939
|
29,629,342
|
||||||
|
Operating expenses
|
||||||||
|
Administrative expenses
|
4,598,800
|
3,341,605
|
||||||
|
Research and development expenses
|
99,688
|
763,995
|
||||||
|
Selling expenses
|
3,865,980
|
2,122,153
|
||||||
|
8,564,468
|
6,227,753
|
|||||||
|
Income from operations
|
26,943,471
|
23,401,589
|
||||||
|
Interest income
|
16,100
|
29,315
|
||||||
|
Government grants - Note 5
|
29,320
|
-
|
||||||
|
Other income - Note 6
|
95,345
|
143,426
|
||||||
|
Finance costs - Note 7
|
(1,413,873
|
)
|
(1,260,290
|
)
|
||||
|
Income before income taxes
|
25,670,363
|
22,314,040
|
||||||
|
Income taxes - Note 8
|
(6,261,798
|
)
|
(5,656,878
|
)
|
||||
|
Net income attributable to
|
||||||||
|
China BCT Pharmacy Group, Inc. common stockholders
|
19,408,565
|
16,657,162
|
||||||
|
Other comprehensive income
|
||||||||
|
Foreign currency translation adjustments
|
57,322
|
1,142,614
|
||||||
|
Total comprehensive income
|
$
|
19,465,887
|
$
|
17,799,776
|
||||
|
Earnings per share: basic and diluted - Note 9
|
$
|
0.61
|
$
|
0.52
|
||||
|
Weighted average number of shares outstanding: basic and diluted
|
32,013,943
|
31,963,669
|
||||||
See the accompanying notes to consolidated financial statements
F-2
China BCT Pharmacy Group, Inc.
Consolidated Balance Sheets
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
ASSETS
|
||||||||
|
Current assets
|
||||||||
|
Cash and cash equivalents
|
$
|
13,304,158
|
$
|
1,265,184
|
||||
|
Restricted cash - Note 10
|
1,155,779
|
1,228,011
|
||||||
|
Trade receivables, net - Note 11
|
35,410,039
|
20,976,203
|
||||||
|
Amounts due from related companies - Note 20
|
4,275,586
|
4,658,061
|
||||||
|
Other receivables, prepayments and deposits - Note 12
|
2,526,398
|
1,252,527
|
||||||
|
Inventories - Note 13
|
8,745,525
|
6,425,765
|
||||||
|
Deferred taxes - Note 8
|
60,164
|
60,164
|
||||||
|
Total current assets
|
65,477,649
|
35,865,915
|
||||||
|
Goodwill - Note 14
|
107,968
|
107,968
|
||||||
|
Other intangible assets - Note 14
|
660,034
|
786,049
|
||||||
|
Property, plant and equipment, net - Note 15
|
12,171,689
|
12,413,274
|
||||||
|
Land use rights - Note 16
|
13,979,753
|
15,667,168
|
||||||
|
Deposits for acquisition of property, plant and equipment
|
–
|
299,325
|
||||||
|
Deferred taxes - Note 8
|
663,699
|
674,649
|
||||||
|
TOTAL ASSETS
|
$
|
93,060,792
|
$
|
65,814,348
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
LIABILITIES
|
||||||||
|
Current liabilities
|
||||||||
|
Trade payables
|
$
|
19,159,212
|
$
|
15,007,291
|
||||
|
Bills payable - Note 10
|
2,239,604
|
1,448,293
|
||||||
|
Other payables and accrued expenses - Note 17
|
3,194,612
|
3,230,577
|
||||||
|
Amounts due to directors - Note 19
|
1,008,111
|
1,270,929
|
||||||
|
Amounts due to related companies - Note 20
|
128,579
|
126,440
|
||||||
|
Income tax payable
|
562,603
|
954,666
|
||||||
|
Secured bank loans - Note 21
|
7,136,069
|
10,291,005
|
||||||
|
Other loans - Note 22
|
2,361,258
|
2,432,139
|
||||||
|
Retirement benefit costs - Note 5
|
59,158
|
89,016
|
||||||
|
Total current liabilities
|
35,849,206
|
34,850,356
|
||||||
|
Secured long-term bank loans - Note 21
|
3,631,957
|
2,200,500
|
||||||
|
Retirement benefit costs - Note 5
|
201,320
|
180,426
|
||||||
|
TOTAL LIABILITIES
|
39,682,483
|
37,231,282
|
||||||
|
COMMITMENTS AND CONTINGENCIES - Note 23
|
||||||||
See the accompanying notes to consolidated financial statements
F-3
China BCT Pharmacy Group, Inc.
Consolidated Balance Sheets (continued)
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
STOCKHOLDERS’ EQUITY
|
||||||||
|
Common stock: par value $0.001 per share ;
|
||||||||
|
Authorized 100,000,000 shares in 2009 and 2008
|
||||||||
|
37,089,370 shares issued and outstanding as of December 31, 2009
|
||||||||
|
and 32,000,000 shares issued and outstanding as of December 31,
|
||||||||
|
2008 - Note 24
|
37,089
|
32,000
|
||||||
|
Additional paid-in capital - Note 24
|
14,920,899
|
9,596,632
|
||||||
|
Statutory and surplus reserves - Note 25
|
2,605,901
|
1,431,174
|
||||||
|
Accumulated other comprehensive income
|
2,110,270
|
2,052,948
|
||||||
|
Retained earnings
|
33,704,150
|
15,470,312
|
||||||
|
TOTAL STOCKHOLDERS’ EQUITY
|
53,378,309
|
28,583,066
|
||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
93,060,792
|
$
|
65,814,348
|
||||
See the accompanying notes to consolidated financial statements
F-4
China BCT Pharmacy Group, Inc.
Consolidated Statements of Cash Flows
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash flows from operating activities
|
||||||||
|
Net income attributable to China BCT Pharmacy Group, Inc.
|
||||||||
|
common stockholders
|
$
|
19,408,565
|
$
|
16,657,162
|
||||
|
Adjustments to reconcile net income to net cash provided by operating activities
|
||||||||
|
Depreciation
|
598,276
|
632,900
|
||||||
|
Amortization of other intangible assets
|
118,565
|
200,227
|
||||||
|
Amortization of land use rights
|
313,533
|
334,206
|
||||||
|
Deferred taxes
|
10,943
|
(52,835
|
)
|
|||||
|
Gain on disposal of land use rights
|
(17,814
|
)
|
-
|
|||||
|
Recovery of doubtful debts
|
-
|
(84,091
|
)
|
|||||
|
Written off of other receivables
|
174,524
|
-
|
||||||
|
Changes in operating assets and liabilities :-
|
||||||||
|
Trade receivables
|
(14,423,654
|
)
|
(9,394,900
|
)
|
||||
|
Other receivables, prepayments and deposits
|
(1,447,184
|
)
|
199,870
|
|||||
|
Amounts due to (from) related companies
|
(1,020,253
|
)
|
324,261
|
|||||
|
Inventories
|
(2,318,179
|
)
|
3,003,812
|
|||||
|
Trade payables
|
4,149,091
|
192,095
|
||||||
|
Bills payable
|
790,772
|
(180,766
|
)
|
|||||
|
Other payables and accrued expenses
|
(35,940
|
)
|
(442,348
|
)
|
||||
|
Retirement benefit costs
|
(8,959
|
)
|
680
|
|||||
|
Income tax payable
|
(391,796
|
)
|
344,490
|
|||||
|
Net cash flows provided by operating activities
|
5,900,490
|
11,734,763
|
||||||
|
Cash flows from investing activities
|
||||||||
|
Payments to acquire and for deposits for acquisition of property, plant and
|
||||||||
|
equipment and land use rights
|
(71,328
|
)
|
(721,910
|
)
|
||||
|
Net cash received from the RTO
|
22,694
|
-
|
||||||
|
Cash received from disposal of land use rights
|
1,391,588
|
-
|
||||||
|
Cash received from acquisition of Hefeng Pharmaceutical - Note 4
|
-
|
631,818
|
||||||
|
Advanced to related companies
|
(1,689,557
|
)
|
(741,043
|
)
|
||||
|
Net cash flows used in investing activities
|
$
|
(346,603
|
)
|
$
|
(831,135
|
)
|
||
See the accompanying notes to consolidated financial statements
F-5
China BCT Pharmacy Group, Inc.
Consolidated Statements of Cash Flows (continued)
(Stated in US Dollars)
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cash flows from financing activities
|
||||||||
|
Advance from related companies
|
$
|
3,094,189
|
$
|
72,368
|
||||
|
Restricted cash
|
72,183
|
(250,089
|
)
|
|||||
|
Repayments to directors
|
(262,639
|
)
|
(1,016,329
|
)
|
||||
|
Dividend paid to former stockholders of Liuzhou BCT
|
-
|
(6,940,000
|
)
|
|||||
|
Proceeds from bank loans
|
9,587,885
|
10,688,723
|
||||||
|
Repayment of bank loans
|
(11,310,190
|
)
|
(11,229,285
|
)
|
||||
|
Cash received from private placement
|
5,306,662
|
-
|
||||||
|
Proceeds from other loans
|
590,945
|
1,317,891
|
||||||
|
Repayment of other loans
|
(661,777
|
)
|
(2,642,631
|
)
|
||||
|
Proceeds from issue of common stock by Liuzhou BCT
|
-
|
17,569
|
||||||
|
Net cash flows provided by (used in) financing activities
|
6,417,258
|
(9,981,783
|
)
|
|||||
|
Effect of foreign currency translation on cash and cash equivalents
|
67,829
|
(176,397
|
)
|
|||||
|
Net increase in cash and cash equivalents
|
12,038,974
|
745,448
|
||||||
|
Cash and cash equivalents - beginning of year
|
1,265,184
|
519,736
|
||||||
|
Cash and cash equivalents - end of year
|
$
|
13,304,158
|
$
|
1,265,184
|
||||
|
Supplemental disclosures for cash flow information :-
|
||||||||
|
Cash paid for
|
||||||||
|
- Interest
|
$
|
1,291,082
|
$
|
1,227,364
|
||||
|
- Income taxes
|
$
|
6,642,573
|
$
|
5,365,222
|
||||
|
Non-cash investing activity
|
||||||||
|
Acquisition of Hefeng Pharmaceutical in form of non-cash
|
||||||||
|
contribution – Note 4
|
$
|
-
|
$
|
4,982,223
|
||||
See the accompanying notes to consolidated financial statements
F-6
China BCT Pharmacy Group, Inc.
Consolidated Statements of Stockholders’ Equity
(Stated in US Dollars)
|
Common stock
|
Additional
|
Statutory and surplus
|
Accumulated
other
|
|||||||||||||||||||||||||
|
No. of
shares
|
Amount
|
paid-in
capital
|
reserves
(Note 25)
|
comprehensive
income
|
Retained
earnings
|
Total
|
||||||||||||||||||||||
|
Balance, January 1, 2008
|
26,419,200
|
$
|
26,419
|
$
|
4,602,421
|
$
|
329,562
|
$
|
910,334
|
$
|
6,854,762
|
$
|
12,723,498
|
|||||||||||||||
|
Net income
|
-
|
-
|
-
|
-
|
-
|
16,657,162
|
16,657,162
|
|||||||||||||||||||||
|
Foreign currency translation adjustments
|
-
|
-
|
-
|
-
|
1,142,614
|
-
|
1,142,614
|
|||||||||||||||||||||
|
Acquisition of Hefeng Pharmaceutical - Note 3 and 4
|
4,300,800
|
4,301
|
4,977,922
|
-
|
-
|
-
|
4,982,223
|
|||||||||||||||||||||
|
Increase in paid up capital of Liuzhou BCT in form of cash
|
||||||||||||||||||||||||||||
|
consideration before the
Reorganization as stated in
|
||||||||||||||||||||||||||||
|
Note 2
|
1,280,000
|
1,280
|
16,289
|
-
|
-
|
-
|
17,569
|
|||||||||||||||||||||
|
Appropriation to reserves
|
-
|
-
|
-
|
1,101,612
|
-
|
(1,101,612
|
)
|
-
|
||||||||||||||||||||
|
Dividend – Note 18
|
-
|
-
|
-
|
-
|
-
|
(6,940,000
|
)
|
(6,940,000
|
)
|
|||||||||||||||||||
|
Balance, December 31, 2008
|
32,000,000
|
32,000
|
9,596,632
|
1,431,174
|
2,052,948
|
15,470,312
|
28,583,066
|
|||||||||||||||||||||
|
Net income
|
-
|
-
|
-
|
-
|
-
|
19,408,565
|
19,408,565
|
|||||||||||||||||||||
|
Foreign currency translation
adjustments
|
-
|
-
|
-
|
-
|
57,322
|
-
|
57,322
|
|||||||||||||||||||||
|
Appropriation to reserves
|
-
|
-
|
-
|
1,174,727
|
-
|
(1,174,727
|
)
|
-
|
||||||||||||||||||||
|
Recapitalization
|
2,600,000
|
2,600
|
20,094
|
-
|
-
|
-
|
22,694
|
|||||||||||||||||||||
|
Private placement – Note 24(b)
|
2,489,370
|
2,489
|
5,304,173
|
-
|
-
|
-
|
5,306,662
|
|||||||||||||||||||||
|
Balance, December 31, 2009
|
37,089,370
|
$
|
37,089
|
$
|
14,920,899
|
$
|
2,605,901
|
$
|
2,110,270
|
$
|
33,704,150
|
$
|
53,378,309
|
|||||||||||||||
See the accompanying notes to consolidated financial statements
F-7
1. Corporate information
|
(i)
|
China BCT Pharmacy Group, Inc. (the “Company”), formerly known as Purden Lake Resource Corp. which changed its name to China Baicaotang Medicine Limited on December 24, 2009 and to China BCT Pharmacy Group, Inc. on March 25, 2010, was incorporated in the State of Delaware on November 30, 2006 as a limited liability company with authorized capital stock consisting of 100,000,000 shares of Common Stock, par value $0.001. Prior to the completion of reverse takeover transaction (“RTO”) on December 30, 2009 as mentioned in Note 2 (iv), the Company was a development stage company for acquisition, exploration and development of natural resource properties. Following the completion of RTO on December 30, 2009, the Company commenced to be engaged in distribution, retail and production of drugs in the People’s Republic of China (the “PRC”).
|
|
(ii)
|
Forever Well Asia Pacific Ltd. (“Forever Well”) was incorporated in Hong Kong on January 10, 2008 as a limited liability company with authorized, issued and paid up capital of HK$10,000, divided into 10,000 common shares of HK$1 par value each. The principal activity of Forever Well is investment holding.
|
|
(iii)
|
Ingenious Paragon Global Limited (“Ingenious”) was incorporated in the British Virgin Islands (the “BVI”) on May 29, 2008 as a limited liability company with authorized, issued and paid up capital of $50,000, divided into 50,000 common shares of $1 par value each. Prior to the completion of RTO on December 30, 2009, the 50,000 common shares were held by Xiao Yan Zhang, the Chief Financial Officer of the Company.
|
|
(iv)
|
Liuzhou BCT was established on April 3, 1986 in the PRC as a State-Owned Enterprise. On June 20, 2001, Liuzhou BCT was transformed into a joint stock enterprise through management buyout by certain of its management and employees. On December 29, 2007, Liuzhou BCT was transformed into a limited company. Liuzhou BCT is engaged in the distribution of drugs in the PRC. Before the acquisition by Forever Well as stated in Note 2(iii), the registered and paid up capital was RMB10,000,000 which were held as to 23.83% by Hui Tian Tang, 13.44% by Jing Hua Li, 10.81% by Wen De Wei, 6.81% by You Ru Jiang, 6.81% by Chun Lin Liu and 6.81% by Bang Fu Wang, who are also the directors of Ingenious and Liuzhou BCT. The remaining 31.49% were held by nine stockholders, who are the employees of Liuzhou BCT.
|
|
(v)
|
Guangxi Liuzhou Baicaotang Medicine (Retail Chain) Ltd. (“BCT Retail”) is a wholly owned subsidiary of Liuzhou BCT and was established on October 30, 2001 with registered and paid up capital of RMB300,000. BCT Retail is engaged in the retail of drugs in the PRC.
|
|
(vi)
|
Guangxi Hefeng Pharmaceutical Co Ltd. (“Hefeng Pharmaceutical”) was established on September 18, 2000 with registered and paid up capital of RMB5,000,000. Hefeng Pharmaceutical is engaged in the production and sale of drugs for healing of hepatitis, cough, parkinson’s disease in the PRC. On December 31, 2007, Liuzhou BCT entered into an agreement with Jing Hua Li to acquire his entire interest in Hefeng Pharmaceutical at a consideration of RMB36,340,064 (equivalent to $4,982,223) which was satisfied by issuance of 13.44% shares of Liuzhou BCT. The acquisition was completed on January 2, 2008, which is the date Liuzhou BCT obtained the control over Hefeng Pharmaceutical by appointing directors into the board of directors of Hefeng Pharmaceutical.
|
F-8
2. Reorganization
To rationalize the group structure, the Company, Ingenious, Forever Well, Liuzhou BCT and BCT Retail reorganized their group structure (the “Reorganization”) as follows:
|
(i)
|
Due to certain regulatory restrictions on a wholly owned foreign enterprise or its wholly owned subsidiary to hold over 50% equity interest in any PRC company which operates more than 30 drug stores in the PRC (the “Drug Stores Restrictions”), Liuzhou BCT sold its 51% equity interest in BCT Retail to Liuzhou Baicaotang Property Management Company Ltd. (“Baicaotang Property”), of which the directors of Ingenious are the controlling stockholders of Baicaotang Property, at a consideration of RMB153,000 on April 1, 2008. Afterwards, Baicaotang Property pledged its 51% equity interest in BCT Retail to Liuzhou BCT to secure a loan, amounting to RMB153,000, granted by Liuzhou BCT to Baicaotang Property up to December 31, 2015 for the acquisition of 51% equity interest in BCT Retail. According to a Repurchase Agreement dated July 31, 2008, Liuzhou BCT was entitled to a preemptive right to repurchase the 51% equity interest in BCT Retail from Baicaotang Property up to the earlier of December 31, 2017 or the removal of the Drug Stores Restrictions. Before the execution of the preemptive right by Liuzhou BCT, the rights and obligations as a stockholder of the 51% equity interest in BCT Retail are still vested in Liuzhou BCT and the appointment of the board of directors and management is controlled by Liuzhou BCT.
|
|
(ii)
|
On March 28, 2008, Forever Well entered into an agreement with the stockholders of Liuzhou BCT to acquire their entire equity interest in Liuzhou BCT at a cash consideration of RMB10,000,000, which is the registered and fully paid up capital of Liuzhou BCT.
|
|
(iii)
|
On June 30, 2008, Ingenious acquired the entire equity interest in Forever Well at a cash consideration of HK$10,000, which is the issued and fully paid up capital of Forever Well.
|
|
(iv)
|
On December 23, 2009, the Company entered into a Share Exchange Agreement with the shareholders of Ingenious to acquire their 100% of the issued and outstanding common shares in Ingenious by issuance of 32,000,000 shares of the Company’s common stock, par value $0.001 per share (the “Exchange Transaction”).
Following the completion of the Exchange Transaction on December 30, 2009, 2,900,000 shares of the Company’s common stock of $0.001 each, which are held by a Company’s shareholder, Lisa Lopomo, were cancelled on December 30, 2009.
The Exchange Transaction, which was completed on December 30, 2009, constituted a RTO and thereafter Ingenious became a wholly owned subsidiary of the Company.
|
|
(v)
|
On December 23, 2009, Zhang Xiao Yan entered into an Earn-in Agreement with the former stockholders of Liuzhou BCT of which the former stockholders of Liuzhou BCT are given the rights to acquire 22,480,000 common shares (Earn-in Shares) of the Company at $300,000 based on their former respective equity interest in Liuzhou BCT immediate before the acquisition by Forever Well as stated in Note 2 (iii) provided that the Company meets the profit targets, representing audited net income after tax of $26 million for the year of 2010 and $28 million for the year of 2011. The former stockholders are allowed to acquire 50% and 50% of Earn-in Shares upon the profit targets have been met for the years of 2010 and 2011 respectively.
|
Upon the completion of Reorganization on December 23, 2009, the Company, Forever Well, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical are under common control of Tang Hui Tian, Li Jing Hua, Wei Wen De, Jiang You Ru, Liu Chun Lin and Wang Bang Fu, who are the directors of the Company and Liuzhou BCT. The acquisition of Ingenious, Forever Well and Liuzhou BCT have been accounted for using combination of entities under common control as stated in Note 3.
F-9
3. Basis of presentation
Except for Hefeng Pharmaceutical, accounting for recapitalization is adopted for the preparation of consolidated financial statements to present the combined results of operations and financial position of the Company, Ingenious, Forever Well, Liuzhou BCT and BCT Retail as if the current group structure, which means that Ingenious, Forever Well, Liuzhou BCT and BCT Retail are wholly owned subsidiaries of the Company, had been in existence at the beginning of the reporting period. The 13.44% equity interest of Liuzhou BCT transferred for the acquisition of Hefeng Pharmaceutical as stated in Note 1 (vi) was accounted for as 13.44% of issued 32,000,000 common stock of the Company, amounted to $4,301 with $4,977,922 recorded in additional paid in capital during the year ended December 31, 2008. Purchase accounting was adopted to reflect the post-acquisition results of Hefeng Pharmaceutical, which was acquired by Liuzhou BCT on January 2, 2008, in these consolidated financial statements of the Company.
4. Acquisition
On December 31, 2007, in order to diversify its business activities, Liuzhou BCT entered into an agreement with Li Jing Hua to acquire his entire interest in Hefeng Pharmaceutical at a consideration of RMB36,340,064 (equivalent to $4,982,223) which was satisfied by issuance of 13.44% shares of Liuzhou BCT. The acquisition was completed on January 2, 2008.
The following table summarizes the allocation of the purchase price reflecting the amounts assigned to Hefeng Pharmaceutical’s each major class of assets acquired and liabilities assumed at the date of acquisition:
|
January 2, 2008
|
||||
|
Current assets
|
$
|
3,601,575
|
||
|
Property, plant and equipment, net
|
4,696,303
|
|||
|
Land use right
|
1,695,927
|
|||
|
Goodwill
|
107,968
|
|||
|
Intangible assets
|
925,044
|
|||
|
Deferred tax assets
|
136,735
|
|||
|
Current liabilities
|
(3,439,329
|
)
|
||
|
Long-term bank loan
|
(2,742,000
|
)
|
||
|
Net assets acquired
|
$
|
4,982,223
|
||
|
Satisfied by
|
||||
|
The fair value of 13.44% equity interest in Liuzhou BCT
|
$
|
4,982,223
|
||
|
Net cash received from the acquisition of Hefeng Pharmaceutical
|
$
|
631,818
|
||
As of December 31, 2008, the consolidated balance sheet includes a goodwill identified upon the acquisition of 100% equity interest in Hefeng Pharmaceutical amounting to $107,968 which represents the excess of the purchase price representing the fair value of 13.44% equity of the Liuzhou BCT over the attributable share of fair value of acquired identifiable net assets of Hefeng Pharmaceutical of $4.87 million at the time of acquisition on January 2, 2008.
The goodwill arising from the acquisition of Hefeng Pharmaceutical is expected to be non-deductible for income tax purpose.
F-10
The Company with advice from an independent appraiser, has identified all assets acquired (including intangible assets which meets either the separability criterion or the contractual-legal criterion in accordance with ASC 805 as of the date of acquisition with a conclusion that certain significant identifiable intangible assets inclusive of trademark, product licences and customers contracts was identified and recognized.
The following unaudited pro forma financial information presents the combined results of operations of the Company with the operations of Hefeng Pharmaceutical as if the acquisition had occurred as of the beginning of fiscal year 2007:
|
(Pro Forma) Year ended December 31,
|
||||||||
|
2008
|
2007
|
|||||||
|
Revenue
|
$
|
108,991,329
|
$
|
70,522,398
|
||||
|
Net income
|
$
|
16,657,162
|
$
|
8,275,088
|
||||
|
Earnings per share : basic and diluted
|
$
|
0.52
|
$
|
0.27
|
||||
This unaudited pro forma financial information is presented for informational purposes only. The unaudited pro forma financial information may not necessarily reflect the future results of operations or the results of operations had the Company owned and operated this business as of the beginning of the period presented.
5. Summary of significant accounting policies
Basis of consolidation
The consolidated financial statements include the accounts of the Company, Ingenious, Forever Well, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical. All significant inter-company accounts and transactions have been eliminated in consolidation.
Use of estimates
In preparing financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of accounts receivable, inventories and the estimation on useful lives of property, plant and equipment and intangible assets. Actual results could differ from those estimates.
Concentrations of credit risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents, restricted cash, trade receivables and amounts due from related companies. As of December 31, 2009 and 2008, substantially all of the Company’s cash and cash equivalents and restricted cash were held by major financial institutions located in the PRC, which management believes are of high credit quality. With respect to trade receivables, the Company extends credit based on an evaluation of the customer’s financial condition. The Company generally does not require collateral for trade receivables and maintains an allowance for doubtful accounts of trade receivables.
During the years ended December 31, 2009 and 2008, no customers were identified with who accounted for 10% or more of the Company’s consolidated sales and no customers asset for or constituted 10% or more of the Company’s trade receivables.
F-11
Cash and cash equivalents
Cash and cash equivalents include all cash, deposits in banks and other highly liquid investments with initial maturities of three months or less. As of December 31, 2009, the cash and cash equivalents were mainly denominated in Renminbi (“RMB”) and United States Dollars were placed with banks in the PRC and Hong Kong. For those denominated in RMB, they are not freely convertible into foreign currencies and the remittance of these funds out of the PRC is subject to exchange control restrictions imposed by the PRC government. The remaining insignificant balance of cash and cash equivalents were denominated in HK dollars.
Restricted Cash
Deposits in banks pledged as securities for bills payable (Note 10) that are restricted in use are classified as restricted cash under current assets.
Allowance for doubtful accounts
The Company establishes an allowance for doubtful accounts based on management’s assessment of the collectibility of trade receivables. A considerable amount of judgment is required in assessing the amount of the allowance, the Company considers the historical level of credit losses of all segments (Retail, Wholesales and Manufacturing) and applies percentages to aged receivable categories. The Company makes judgments about the creditworthiness of each customer based on ongoing credit evaluations, and monitors current economic trends that might impact the level of credit losses in the future for all segments. If the financial condition of the customers were to deteriorate, resulting in their inability to make payments, a larger allowance may be required.
The Company's general credit sales policy is to provide its customers with credit ranging from one month to six months, meaning that within this period it does not consider the receivables overdue and it does not record a bad debt provision. Once a sale has closed it automatically grants its customers credit for between one month to three months; it does not may give additional credit of between three to six months which is called a credit extension period, and which needs a sales managers' approval to grant. Therefore, for any receivables within the six month period it does not make a provision for bad debt. Any receivable that is over six months, meaning ranging from month six up to one year, it consider overdue, and will make a provision of 40% bad debt for that receivable.
As at December 31, 2009, 77% of the Company's trade receivables were derived from hospitals. In the PRC, all hospitals are owned or controlled by the PRC government. The Company believes that it is highly unlikely that a liquidation of one of its hospital customers will occur, and that the recovery of debts from hospitals is highly secured. Therefore, it is the Company's customary practice to grant a longer credit period without the consideration of making a provision on its accounts receivable.
Based on the above assessment, during the reporting years, the management establishes the general provisioning policy to make allowance equivalent to 40% of gross amount of trade receivables due between half and one year and 100% of gross amount of accounts receivable due over 1 year for all segments. Additional specific provision is made against trade receivables whenever they are considered to be doubtful.
Bad debts are written off when identified. The Company extends unsecured credit to customers ranging from three to six months in the normal course of business. The Company does not accrue interest on trade accounts receivable.
Historically, losses from uncollectible accounts have not significantly deviated from the general allowance estimated by the management and no significant additional bad debts have been written off directly to the profit and loss. This general provisioning policy has not changed in the past since establishment and the management considers that the aforementioned general provisioning policy is adequate and not too excessive and does not expect to change this established policy in the near future.
F-12
Inventories
Inventories are stated at the lower of cost or market value. Cost is determined on weighted average basis and includes all expenditures incurred in bringing the goods to the point of sale and putting them in a saleable condition. In assessing the ultimate realization of inventories, the management makes judgments as to future demand requirements compared to current or committed inventory levels. The Company’s reserve requirements generally increase with its projected demand requirements; decrease due to market conditions, product life cycle changes. The Company estimates the demand requirements based on market conditions, forecasts prepared by its customers, sales contracts and orders in hand. Inventory quantities and expiry dates are regularly reviewed and provisions for excess or obsolete inventory are recorded primarily based on the expiry dates and the Company’s forecast of future demand and market conditions.
No provision for excess or obsolete inventory was made for the years ended December 31, 2009 and 2008.
Property, plant and equipment
Property, plant and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
Depreciation is provided on straight-line basis over their estimated useful lives. The principal depreciation rates are as follows :-
|
Annual rate
|
Residual value
|
|||||||
|
Buildings
|
2.54% - 9.84
|
%
|
Nil - 2
|
%
|
||||
|
Plant and machinery
|
7% - 18.4
|
%
|
Nil - 10
|
%
|
||||
|
Motor vehicles
|
6% -18.4
|
%
|
10
|
%
|
||||
|
Furniture, fixtures and equipment
|
6% -18.4
|
%
|
10
|
%
|
||||
Construction in progress mainly represents expenditures in respect of the construction of a new production line and improving the manufacturing process. All direct costs relating to the new production line and improving the manufacturing process are capitalized as construction in progress. No depreciation is provided in respect of construction in progress.
Maintenance or repairs are charged to expense as incurred. Upon sale or disposition, the applicable amounts of asset cost and accumulated depreciation are removed from the accounts and the net amount less proceeds from disposal is charged or credited to income.
Goodwill and intangible assets
The Company applies the provisions of ASC 805, “Goodwill and Intangible Assets” (“ASC 805”). Under ASC 805, goodwill and intangible assets with indefinite useful lives are not amortized, but instead are tested for impairment at least annually.
Goodwill with infinite useful life are stated at cost less accumulated impairment. The Company completed the annual impairment tests as of December 31, 2009.
Pharmaceutical licenses, customer contracts, trademarks, know-how and patents are stated at cost less accumulated amortization. Amortization is provided on a straight-line over their useful lives as follows:
|
Pharmaceutical licences
|
10 years
|
|
Customer contracts, trademarks, know-how and patents
|
1–3 years
|
F-13
Land use right
Land use rights are stated at cost less accumulated amortization. Amortization is provided using the straight-line method over the terms of the lease of 40 to 70 years obtained from the relevant PRC land authority.
Impairment of long-lived assets
Long-lived assets are tested for impairment in accordance with ASC 360-10-45 “Impairment or Disposal of Long-Lived Assets” (previously SFAS No. 144). The Company periodically evaluates potential impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cashflows attributable to such assets. During the reporting periods, the Company has not identified any indicators that would require testing for impairment.
Government grants
Receipts of government grants to encourage research and development activities, which are non-refundable, are credited to deferred income upon receipt. Government grants are used for purchases of property, plant and equipment, to subsidize the research and development expenses incurred, for compensation expenses already incurred or for good performance of the Company.
Grants applicable to purchase of property, plant and equipment are amortized over the life of the depreciable assets. For research and development expenses, the Company matches and offsets the government grants with the expenses of the research and development activities as specified in the grant approval document in the corresponding period when such expenses are incurred. Government grants received as compensation for expenses already incurred in the prior period or for good performance of the Company are recognized as income in the period they become recognizable.
During the year ended December 31, 2009, the Company received government grants of $29,320 for good performance. The Company recognized this amount as income for the year. No government grant was received and recognized as income for the year ended December 31, 2008.
Revenue recognition
Revenue from sales of the Company’s products in wholesales and manufacturing segments is recognized upon customer acceptance, which occur at the time of delivery to customer, provided persuasive evidence of an arrangement exists, such as signed sales contract, the significant risks and rewards of ownership have been transferred to the buyer at the time when the products are delivered to its customers with no significant post-delivery obligation on our part, the sales price is fixed or determinable and collection is reasonably assured. We do not provide our customers with contractual rights of return for any of our products. When there is any significant post-delivery performance obligations exits, revenue is recognized only after such obligations are fulfilled. The Company evaluates the terms of sales agreement with its customer in order to determine whether any significant post-delivery performance obligations exist. Currently, the sales under wholesales and manufacturing segments do not include any terms which may impose any significant post-delivery performance obligations to the Company.
Revenue from sales of the Company’s products in retail segment is recognized upon customer acceptance, which occur at the time of the product is purchased by the retail customers at our retail stores with no significant post-delivery obligation on our part, and collection is reasonable assured. The Company does not have a return policy allowing customers to return the products sold. When there is any significant post-delivery performance obligations exits, revenue is recognized only after such obligations are fulfilled. The Company evaluates the rules and regulations relating to retail of drugs in the PRC in order to determine whether any significant post-delivery performance obligations exist. Currently, the rules and regulations relating to retail of drugs in the PRC does not include any provisions which may impose any significant post-delivery performance obligations to the Company.
F-14
Revenue from sales of the Company’s product represents the invoiced value of goods, net of the value-added tax (VAT). All of the Company’s products that are sold in the PRC are subject to a Chinese value-added tax at a rate of 17 percent of the gross sales price. This VAT may be offset by VAT paid by the Company on raw materials and other materials included in the cost of producing the Company’s finished product products.
Advertising, research and development expenses
Advertising and research and development expenses are charged to expense as incurred.
Advertising expenses amounting to $123,195 and $158,997 for the year ended December 31, 2009 and 2008 are included in selling expenses.
Research and development expenses consist primarily of remuneration for research and development staff and material costs for research and development.
Research and development expenses amounting to $99,688 and $763,995 for the year ended December 31, 2009 and 2008 are included in operating expenses.
Retirement benefit costs
Liuzhou BCT adopted a retirement scheme to provide for eligible staff employed prior to April 23, 2002. The eligible staff are entitled to receive certain amounts based on their years of service in Liuzhou BCT up to April 23, 2002, upon their termination of employment relationship with the Company or retirement. The obligation of retirement benefit costs is recorded at the present value of the cost expected to settle the obligation and is recognised when the retirement scheme has been approved. The staff employed after April 23, 2002 is not entitled to this retirement scheme.
As of December 31, 2009, the discount rate of 7.14% (2008: 6.3%), which represented the Company’s weighted average borrowing rate of Renminbi loans in the PRC, was adopted to calculate the present value of retirement benefits cost. An analysis of the provision for retirement benefit costs for the years ended December 31, 2009 and 2008 is as follows:
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Balance at beginning of year
|
$
|
269,442
|
$
|
251,164
|
||||
|
Imputed interest
|
20,349
|
22,594
|
||||||
|
Payments during the year
|
(29,307
|
)
|
(21,914
|
)
|
||||
|
Translation adjustments
|
(6
|
)
|
17,598
|
|||||
|
Balance at end of year
|
$
|
260,478
|
$
|
269,442
|
||||
Shipping and handling costs
Shipping and handling costs of $469,900 and $251,839 for the two years ended December 31, 2009 and 2008 respectively are charged to expenses as incurred and are included in selling expenses.
Vendor allowances
The Company receives allowances from certain of its vendors whose products it purchases for resale. These allowances are received for a variety of buying activities, including vendor program such as volume purchase allowance. Consideration received from a vendor is a reduction in the cost of the vendor’s products and is recognized a reduction in the cost of sales and the related inventory. The Company also receives promotional allowance funds for specific vendor-sponsored programs are recognized as a reduction of cost of sales as the program occurs and the funds are earned per the agreement.
F-15
Store opening costs
Costs incurred in connection with store start-up costs, such as travel for recruitment, training and setup of new store openings, are expensed as incurred.
Income taxes
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC 740 "Income Taxes” (previously SFAS No. 109). Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
Dividends
Dividends are recorded in Company’s financial statements in the period in which they are declared.
Off-balance sheet arrangements
The Company does not have any off-balance sheet arrangements.
Registration payment arrangement
Contingent obligations to make future payments or otherwise transfer consideration under a registration payment arrangement, whether issued as a separate agreement or included as a provision of a financial instrument or other agreement, are separately recognized and measured when they are probable and reasonably estimable.
Comprehensive income
The Company has adopted ASC 220, “Comprehensive Income”, which establishes standards for reporting and display of comprehensive income (loss), its components and accumulated balances. Components of comprehensive income (loss) include net income (loss) and foreign currency translation adjustments.
Foreign currency translation
The functional currency of the Company is RMB and RMB is not freely convertible into foreign currencies. The Company maintains its financial statements in the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet date. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
For financial reporting purposes, the financial statements of the Company which are prepared using the functional currency have been translated into United States dollars. Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustment to other comprehensive income, a component of stockholders’ equity. The exchange rates in effect at December 31, 2009, 2008 and 2007 were RMB1 for $0.1467, $0.1467 and $0.1371 respectively. There is no significant fluctuation in exchange rate for the conversion of RMB to US dollars after the balance sheet date.
F-16
Basic and diluted earnings per share
The Company reports basic earnings per share in accordance with ASC 260, “Earnings Per Share” (previously SFAS No. 128). Basic earnings (loss) per share is computed using the weighted average number of shares outstanding during the periods presented. The weighted average number of shares of the Company represents the common stock outstanding during the reporting periods. Diluted earnings per share is computed using the weighted average number of common shares outstanding during the periods plus the effect of dilutive securities outstanding during the periods. At December 31, 2009, the Company had outstanding warrants exercisable into shares of common stock.
Fair value of financial instruments
The Company adopted ASC 820 (previously Statement of Financial Accounting Standards ("SFAS") No. 157) on January 1, 2008. The adoption of ASC 820 did not materially impact the Company's financial position, results of operations or cash flows.
ASC 820 requires the disclosure of the estimated fair value of financial instruments including those financial instruments for which fair value option was not elected. The carrying amounts of the financial assets and liabilities approximate to their fair values due to short maturities or the applicable interest rates approximate the current market rates.
Recently issued accounting pronouncements
FASB Accounting Standards Codification (Accounting Standards Update “ASU” 2009-1). In June 2009, the Financial Accounting Standard Board (“FASB”) approved its Accounting Standards Codification (“Codification”) as the single source of authoritative United States accounting and reporting standards applicable for all non-governmental entities, with the exception of the SEC and its staff. The Codification is effective for interim or annual financial periods ending after September 15, 2009 and impacts our financial statements as all future references to authoritative accounting literature will be referenced in accordance with the Codification. There have been no changes to the content of our financial statements or disclosures as a result of implementing the Codification.
As a result of our implementation of the Codification during the quarter ended September 30, 2009, previous references to new accounting standards and literature are no longer applicable. In the current financial statements, we will provide reference to both new and old guidance to assist in understanding the impacts of recently adopted accounting literature, particularly for guidance adopted since the beginning of the current fiscal year but prior to the Codification.
Noncontrolling Interests (Included in amended Topic ASC 810 “Consolidation”, previously SFAS No. 160 “Noncontrolling Interests in Consolidated Financial Statements”, an amendment of ARB No. 51). The amended topic establishes accounting and reporting standards for the noncontrolling interest in a subsidiary and for the deconsolidation of a subsidiary. It clarifies that a noncontrolling interest in a subsidiary is an ownership interest in the consolidated entity that should be reported as equity in the consolidated financial statements. The adoption of this amended topic has no material impact on the Company’s financial statements.
Business Combinations (Included in amended Topic ASC 805 “Business Combinations”, previously SFAS No. 141(R)). This ASC guidance addresses the accounting and disclosure for identifiable assets acquired, liabilities assumed, and noncontrolling interests in a business combination. The adoption of this amended topic has no material impact on the Company’s financial statements.
Intangibles-Goodwill and Other (Included in amended Topic ASC 350”, previously FASB staff position (“FSP”) FAS 142-3, Determination of the Useful Life of Intangible Assets). The amended topic amends the factors an entity should consider in developing renewal or extension assumptions used in determining the useful life of recognized intangible assets under FASB Statement No. 142, “Goodwill and Other Intangible Assets”. This new guidance applies prospectively to intangible assets that are acquired individually or with a group of other assets in business combinations and asset acquisitions. The amended topic is effective for financial statements issued for fiscal years and interim periods beginning after December 15, 2008. Early adoption is prohibited. The adoption of this amended topic has no material effect on the Company's financial statements.
F-17
Business Combinations (Included in amended Topic ASC 805, previously FSP No. 141R-1 “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”). Amended topic ASC 805 amends the requirements for the provisions in FASB Statement 141R for the initial recognition and measurement, subsequent measurement and accounting, and disclosures for assets and liabilities arising from contingencies in business combinations. The amended topic eliminates the distinction between contractual and non-contractual contingencies, including the initial recognition and measurement criteria and instead carries forward most of the provisions for acquired contingencies. The amended topic is effective for contingent assets and contingent liabilities acquired in evaluating the impact. The adoption of this amended topic has no material impact on the Company’s financial statements.
Fair Value Measurements and Disclosures (Included in amended Topic ASC 820, previously FSP No. 157-4, “Determining Whether a Market is Not Active and a Transaction Is Not Distressed”.) The amended topic clarifies when markets are illiquid or that market pricing may not actually reflect the “real” value of an asset. If a market is determined to be inactive and market price is reflective of a distressed price then an alternative method of pricing can be used, such as a present value technique to estimate fair value. The amended topic identifies factors to be considered when determining whether or not a market is inactive. The amended topic would be effective for interim and annual periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009 and shall be applied prospectively. The adoption of this amended topic has no material effect on the Company's financial statements.
Investments - Debt and Equity Securities - Overall - Transition and Open Effective Date Information (Included in amended Topic ASC 320, previously FASB Staff Position No. 115-2 and Statement of Financial Accounting Standards No. 124-2, “Recognition and Presentation of Other-Than-Temporary Impairments”). The amended topic amends the other-than-temporary impairment guidance in U.S. GAAP for debt securities through increased consistency in the timing of impairment recognition and enhanced disclosures related to the credit and noncredit components of impaired debt securities that are not expected to be sold. In addition, increased disclosures are required for both debt and equity securities regarding expected cash flows, credit losses, and securities with unrealized losses. The adoption of this amended topic has no material impact on the Company’s financial statements.
Interim Disclosures about Fair Value of Financial Instruments (Included in amended Topic ASC 825 “Financial Instruments”, previously FSP SFAS No. 107-1). This guidance requires that the fair value disclosures required for all financial instruments be included in interim financial statements. This guidance also requires entities to disclose the method and significant assumptions used to estimate the fair value of financial instruments on an interim and annual basis and to highlight any changes from prior periods. The amended topic was effective for interim periods ending after September 15, 2009. The adoption of this amended topic has no material impact on the Company’s financial statements.
Subsequent Events (Included in amended Topic ASC 855 “Subsequent Events”, previously SFAS No. 165). The amended topic establishes accounting and disclosure requirements for subsequent events. The amended topic details the period after the balance sheet date during which the Company should evaluate events or transactions that occur for potential recognition or disclosure in the financial statements, the circumstances under which the Company should recognize events or transactions occurring after the balance sheet date in its financial statements and the required disclosures for such events. The Company adopted this amended topic effective June 1, 2009.
Accounting for Transfers of Financial Assets (Included in amended Topic ASC 860 “Transfers and Servicing”, previously SFAS No. 166, “Accounting for Transfers of Financial Assets - an Amendment of FASB Statement No. 140.”). The amended topic addresses information a reporting entity provides in its financial statements about the transfer of financial assets; the effects of a transfer on its financial position, financial performance, and cash flows; and a transferor’s continuing involvement in transferred financial assets. Also, the amended topic removes the concept of a qualifying special purpose entity, limits the circumstances in which a transferor derecognizes a portion or component of a financial asset, defines participating interest and enhances the information provided to financial statement users to provide greater transparency. The amended topic is effective for the first annual reporting period beginning after November 15, 2009 and will be effective for us as of January 1, 2010. The management is in the process of evaluating the impact of adopting this amended topic on the Company’s financial statements.
F-18
Consolidation of Variable Interest Entities – Amended (Included in amended Topic ASC 810 “Consolidation”, previously SFAS 167 “Amendments to FASB Interpretation No. 46(R)”). The amended topic requires an enterprise to perform an analysis to determine the primary beneficiary of a variable interest entity; to require ongoing reassessments of whether an enterprise is the primary beneficiary of a variable interest entity and to eliminate the quantitative approach previously required for determining the primary beneficiary of a variable interest entity. The amended topic also requires enhanced disclosures that will provide users of financial statements with more transparent information about an enterprise’s involvement in a variable interest entity. The amended topic is effective for the first annual reporting period beginning after November 15, 2009 and will be effective for us as of January 1, 2010. The management is in the process of evaluating the impact of adopting this amended topic on the Company’s financial statements.
In August 2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-05 (“ASU 2009-05”), an update to ASC 820, Fair Value Measurements and Disclosures. This update provides amendments to reduce potential ambiguity in financial reporting when measuring the fair value of liabilities. Among other provisions, this update provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the valuation techniques described in ASU 2009-05. ASU 2009-05 became effective for the Company’s annual financial statements for the year ended December 31, 2009. The adoption of this ASU has no material impact on the Company’s financial statements.
The FASB issued Accounting Standards Update (ASU) No. 2009-13, Revenue Recognition (Topic 605): Multiple Deliverable Revenue Arrangements - A Consensus of the FASB Emerging Issues Task Force.” This update provides application guidance on whether multiple deliverables exist, how the deliverables should be separated and how the consideration should be allocated to one or more units of accounting. This update establishes a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence, if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific or third-party evidence is available. The Company will be required to apply this guidance prospectively for revenue arrangements entered into or materially modified after January 1, 2011; however, earlier application is permitted. The management is in the process of evaluating the impact of adopting this ASU on the Company’s financial statements.
The FASB issued ASU-2010-09 (Topic 855) to amend guidance on subsequent events to remove the requirement for SEC filers (as defined in ASU 2010-09) to disclose the date through which an entity has evaluated subsequent events. This change alleviates potential conflicts with current SEC guidance. An SEC filer is still required to evaluate subsequent events through the date financial statements are issued, but disclosure of that date is no longer required. The amendments in ASU 2010-09 became effective upon issuance of the guidance.
6. Other income
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Rental income
|
$
|
69,086
|
$
|
86,008
|
||||
|
Sales of raw material
|
209
|
36,270
|
||||||
|
Other operating income
|
26,050
|
21,148
|
||||||
|
$
|
95,345
|
$
|
143,426
|
|||||
F-19
7. Finance costs
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Bank loans interest
|
$
|
1,007,423
|
$
|
1,171,373
|
||||
|
Other loans interest
|
257,111
|
58,296
|
||||||
|
Imputed interest on retirement benefit costs
|
20,349
|
22,594
|
||||||
|
Bank charges
|
92,969
|
4,482
|
||||||
|
Discounting charges
|
35,994
|
-
|
||||||
|
Others
|
27
|
3,545
|
||||||
|
$
|
1,413,873
|
$
|
1,260,290
|
|||||
8. Income taxes
United States
China BCT Pharmacy Group, Inc. is subject to the United States of America Tax law at tax rate of 34%. No provision for the US federal income taxes has been made as the Company had no taxable income in this jurisdiction for the reporting period.
BVI
Ingenious was incorporated in the BVI and, under the current laws of the BVI, is not subject to income taxes.
Hong Kong
Forever Well is subject to the Hong Kong Profits tax at tax rate of 16.5% (2008: 16.5%). No provision for the Hong Kong Profits tax has been made as the Company had no taxable income in this jurisdiction since its incorporation.
PRC
Corporate income tax (“CIT”) to Liuzhou BCT and BCT Retail, was charged at 33%, of which 30% is for national tax and 3% is for local tax, of the assessable profits before 2008. The PRC’s legislative body, the National People’s Congress, adopted the unified CIT Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rates is set at 25% for both domestic enterprises and foreign-invested enterprises. However, there is a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatments granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25% may continue to enjoy the lower rate and will transit into the new tax rate over a five-year period beginning on the effective date of the CIT Law. Enterprises that are currently entitled to exemptions for a fixed term will continue to enjoy such treatment until the exemption term expires. Preferential tax treatment will continue to be granted to industries and projects that qualify for such preferential treatments under the new tax law. Accordingly, Liuzhou BCT and BCT Retail was subject to tax rate of 25% starting from fiscal year 2008.
In accordance with the Circular of the State Council on Policies and Measures Pertaining to the Development of the Western Region (“DOWR”), the companies are entitled to preferential rate of 15% if it is engaged in the projects listed in Guiding Catalogue and the revenue derived from it account for the amount over 70% of total revenue. As Hefeng Pharmaceutical met this DOWR requirement, it was approved by the tax authority and was granted a preferential tax rate of 15% for fiscal years 2003 to 2010. From fiscal year 2011, Hefeng Pharmaceutical will be subject to CIT at rate of 25% under the new tax law.
F-20
The components of the provision (benefit) for income taxes are :
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Current taxes - PRC
|
$
|
6,250,855
|
$
|
5,709,713
|
||||
|
Deferred taxes - PRC
|
10,943
|
(52,835
|
)
|
|||||
|
$
|
6,261,798
|
$
|
5,656,878
|
|||||
The effective income tax expenses differs from the PRC statutory income tax rate of 25% for the year ended December 31, 2009 and 2008 in the PRC as follows :
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Provision for income taxes at PRC statutory income tax rate
|
$
|
6,417,591
|
$
|
5,578,510
|
||||
|
Non-deductible items for tax
|
229,848
|
216,461
|
||||||
|
Decrease in deferred tax assets resulting from a reduction of PRC
|
||||||||
|
statutory income tax rate from 33% to 25%
|
-
|
127,379
|
||||||
|
Under provision in prior year
|
122
|
-
|
||||||
|
Tax holiday
|
(338,135
|
)
|
(260,751
|
)
|
||||
|
Others
|
(47,628
|
)
|
(4,721
|
)
|
||||
|
$
|
6,261,798
|
$
|
5,656,878
|
|||||
During the year ended December 31, 2009, the amount of benefit from tax holiday was $338,135 (2008: $260,751) and the effect on earnings per share was $0.01 (2008: $0.01).
In July 2006, the FASB issued ASC 740-10-25 (previously Interpretation No. 48, “Accounting for Uncertainty in Income Taxes”). This interpretation requires recognition and measurement of uncertain income tax positions using a “more-likely-than-not” approach. The Company adopted this ASC 740-10-25 on January 1, 2007. Under the new CIT Law effective on January 1, 2008, the Company may be deemed to be a resident enterprise by the PRC tax authorities. If the Company was deemed to be resident enterprise, the Company may be subject to the CIT at 25% on the worldwide taxable income and dividends paid from PRC subsidiaries to their overseas holding companies may be exempted from 10% PRC withholding tax. Except for certain immaterial interest income from bank deposits placed with financial institutions outside the PRC, all of the Company’s income is generated from the PRC operation. Given the immaterial amount of income generated from outside the PRC and the PRC subsidiaries do not intend to pay dividends in the foreseeable future, the management considers that the impact arising from resident enterprise on the Company’s financial position is not significant. The management evaluated the Company’s overall tax positions and considered that no additional provision for uncertainty in income taxes is necessary as of December 31, 2009.
F-21
Deferred tax assets/(liabilities) as of December 31, 2009 and 2008 are composed of the followings:
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
The PRC
|
||||||||
|
Current deferred tax assets :
|
||||||||
|
Allowance for doubtful debts
|
$
|
60,164
|
$
|
60,164
|
||||
|
Non current deferred tax assets (liabilities) :
|
||||||||
|
Depreciation of property, plant and equipment
|
$
|
1,860,796
|
$
|
1,952,809
|
||||
|
Amortization of land use right
|
(1,380,844
|
)
|
(1,446,503
|
)
|
||||
|
Amortization of intangible assets
|
183,747
|
168,343
|
||||||
|
$
|
663,699
|
$
|
674,649
|
|||||
9. Earnings per share
During the reporting periods, potential dilutive shares are excluded from the computation of earnings per share if their effect is anti-dilutive. 1,493,305 anti-dilutive warrants were excluded from the calculation of earnings per share for year of 2009. Accordingly, the basic and diluted earnings per share are the same.
The per share data reflects the reorganization of stockholders’ equity as if the Reorganization occurred as of the beginning of the first period presented.
10. Restricted cash and bills payable
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Bank deposits held as collateral for bills payable
|
$
|
1,155,779
|
$
|
1,228,011
|
||||
The Company is requested by certain of its suppliers to settle by issuance of bills for which the banks add their undertakings to guarantee their settlement at maturity. These bills are interest free with maturity of three to six months from date of issuance. As security for the banks undertakings, the Company is required to deposit with such banks equal to 50% to 100% of the bills amount at the time of issuance and pay bank charges. These deposits will be used to settle the bills at maturity.
11. Trade receivables, net
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Trade receivables
|
$
|
35,554,436
|
$
|
21,120,600
|
||||
|
Less : allowance for doubtful accounts
|
(144,397
|
)
|
(144,397
|
)
|
||||
|
Net
|
$
|
35,410,039
|
$
|
20,976,203
|
||||
F-22
A summary of the segment trade receivables as at December 31, 2009 and 2008 is as follows :
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Wholesale segment
|
$
|
31,979,147
|
$
|
18,491,596
|
||||
|
Retail segment
|
295,463
|
193,858
|
||||||
|
Manufacturing segment
|
3,135,429
|
2,290,749
|
||||||
|
$
|
35,410,039
|
$
|
20,976,203
|
|||||
An analysis of the allowance for doubtful accounts for the years ended December 31, 2009 and 2008 is as follows :
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Balance at beginning of year
|
$
|
144,397
|
$
|
215,225
|
||||
|
Recovery of doubtful debts
|
-
|
(84,091
|
)
|
|||||
|
Translation adjustments
|
-
|
13,263
|
||||||
|
Balance at end of year
|
$
|
144,397
|
$
|
144,397
|
||||
No trade receivables (2008: US$1,606,516) were pledged as collateral under certain loan agreements as of December 31, 2009.
12. Other receivables, prepayments and deposits
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Advances to staff
|
$
|
432,700
|
$
|
210,322
|
||||
|
Prepayments
|
1,365,600
|
959,289
|
||||||
|
Other receivables
|
728,098
|
82,916
|
||||||
|
$
|
2,526,398
|
$
|
1,252,527
|
|||||
13. Inventories
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Raw materials
|
$
|
722,109
|
$
|
509,394
|
||||
|
Work-in-progress
|
148,954
|
119,680
|
||||||
|
Finished goods
|
7,874,462
|
5,796,691
|
||||||
|
$
|
8,745,525
|
$
|
6,425,765
|
|||||
F-23
14. Goodwill and other Intangible assets
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Goodwill
|
||||||||
|
Acquisition of Hefeng Pharmaceutical - Note 14(a)
|
$
|
107,968
|
$
|
107,968
|
||||
|
Other intangible assets
|
||||||||
|
Pharmaceutical licences
|
$
|
799,197
|
$
|
799,197
|
||||
|
Customer contracts
|
90,729
|
90,729
|
||||||
|
Trademarks, know-how and patents
|
99,892
|
99,892
|
||||||
|
989,818
|
989,818
|
|||||||
|
Accumulated amortization
|
(329,784
|
)
|
(203,769
|
)
|
||||
|
Net
|
$
|
660,034
|
$
|
786,049
|
||||
Notes :
|
(a)
|
The amount represents goodwill identified upon acquisition of Hefeng Pharmaceutical as stated in Note 4.
|
During the years ended December 31, 2009 and 2008 amortization charge was $118,565 and $200,227 respectively.
The estimated aggregate amortization expenses for other intangible assets for the five succeeding years is as follows :
|
Year
|
||||
|
2010
|
$
|
113,217
|
||
|
2011
|
79,920
|
|||
|
2012
|
79,920
|
|||
|
2013
|
79,920
|
|||
|
2014
|
79,920
|
|||
|
$
|
432,897
|
|||
15. Property, plant and equipment, net
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Costs :
|
||||||||
|
Buildings
|
$
|
12,331,575
|
$
|
12,091,574
|
||||
|
Plant and machinery
|
1,291,734
|
1,278,098
|
||||||
|
Furniture, fixtures and equipment
|
374,615
|
348,437
|
||||||
|
Motor vehicles
|
367,132
|
334,466
|
||||||
|
14,365,056
|
14,052,575
|
|||||||
|
Accumulated depreciation
|
(2,544,420
|
)
|
(1,932,154
|
)
|
||||
|
11,820,636
|
12,120,421
|
|||||||
|
Construction in progress
|
351,053
|
292,853
|
||||||
|
Net
|
$
|
12,171,689
|
$
|
12,413,274
|
||||
F-24
|
(a)
|
An analysis of buildings, plant and machinery pledged to banks for banking loans (Note 21(d)(i)) is as follows :
|
|||||||
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Costs :
|
||||||||
|
Buildings
|
$
|
7,409,774
|
$
|
10,782,432
|
||||
|
Furniture, fixtures and equipment
|
-
|
929,092
|
||||||
|
7,409,774
|
11,711,524
|
|||||||
|
Accumulated depreciation
|
(1,290,533
|
)
|
(1,289,333
|
)
|
||||
|
Net
|
$
|
6,119,241
|
$
|
10,422,191
|
||||
| (b) |
During the reporting periods, depreciation is included in :
|
|||||||
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Cost of sales and overheads of inventories
|
$
|
157,256
|
$
|
152,816
|
||||
|
Selling expenses
|
1,198
|
4,701
|
||||||
|
Administrative expenses
|
439,822
|
475,383
|
||||||
|
$
|
598,276
|
$
|
632,900
|
|||||
(c) Construction in progress
Construction in progress mainly represents expenditures in respect of the construction of a new production line and improving the manufacturing process.
16. Land use rights
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Land use rights
|
$
|
16,039,294
|
$
|
17,445,819
|
||||
|
Accumulated amortization
|
(2,059,541
|
)
|
(1,778,651
|
)
|
||||
|
Net
|
$
|
13,979,753
|
$
|
15,667,168
|
||||
The Company has obtained land use rights from the relevant PRC land authority for a period of 40 to 70 years to use the land on which the office premises, production facilities and warehouse of the Company are situated. As of December 31, 2009, and 2008, land use rights with carrying amount of $6,205,140 and $7,788,781 respectively were pledged to a bank for the bank loans granted to the Company (Note 21(d)(ii)).
During the years ended December 31, 2009 and 2008, amortization amounted to $313,533 and $334,206 respectively.
F-25
During the year ended December 31, 2009, land use right with carrying amounts of $1,373,773 were disposed of at considerations, net direct costs, of $1,391,587 resulting in gain of $17,814.
The estimated aggregate amortization expenses for land use right for the five succeeding years is as follows:
|
Year
|
||||
|
2010
|
$
|
312,705
|
||
|
2011
|
312,705
|
|||
|
2012
|
312,705
|
|||
|
2013
|
312,705
|
|||
|
2014
|
312,705
|
|||
|
$
|
1,563,525
|
|||
17. Other payables and accrued expenses
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Accrued audit fee
|
$
|
102,000
|
$
|
160,000
|
||||
|
Other accrued expenses
|
217,256
|
572,684
|
||||||
|
Deposits received
|
995,387
|
819,908
|
||||||
|
Accrued interest expenses
|
16,339
|
36,264
|
||||||
|
Accrued staff costs – Note 17(a)
|
1,046,820
|
772,067
|
||||||
|
VAT and other tax payables
|
471,690
|
407,034
|
||||||
|
Temporary receipt
|
-
|
146,700
|
||||||
|
Other payables
|
345,120
|
315,920
|
||||||
|
$
|
3,194,612
|
$
|
3,230,577
|
|||||
Notes :
|
a)
|
The amount included accrued salaries and wages, staff welfare and accrued social insurance to the PRC municipal and provincial governments which cover pensions, unemployment and medical insurances and staff housing fund.
|
18. Dividends
Dividends of $Nil and $6,940,000 declared during the years ended December 31, 2009 and 2008 respectively were made by Liuzhou BCT to its former stockholders in proportion to the percentage of their holdings of paid up capital.
19. Amounts due to directors
The amounts are unsecured and repayable on demand. Except for the amounts of $836,084 (2008: $744,817) as of December 31, 2009 which were interest bearing at fixed rates ranging from 3.2% to 8.16% (2008: 3.2%) per annum, the remaining balances are interest-free.
20. Amounts due from/to related companies
The related companies are controlled by certain Company’s directors collectively inclusive of Hui Tian Tang. These amounts are interest-free, unsecured and repayable on demand.
F-26
21. Secured bank loans
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Short-term loans - Note 21(a)
|
$
|
7,070,940
|
$
|
8,935,497
|
||||
|
Current maturities of long-term bank loan
|
65,129
|
1,355,508
|
||||||
|
$
|
7,136,069
|
$
|
10,291,005
|
|||||
|
Long-term bank loans - Note 21(b)
|
3,697,086
|
3,556,008
|
||||||
|
Less: current maturities
|
(65,129
|
)
|
(1,355,508
|
)
|
||||
|
$
|
3,631,957
|
$
|
2,200,500
|
|||||
|
(a)
|
The weighted average interest rates for short-term loans as of December 31, 2009 and 2008 were 7.14% and 10.96% per annum respectively.
|
|
(b)
|
Except for loans of $3,726,180 as of December 31, 2009 which were interest bearing at fixed rates ranging from 6.138% to 9.855% per annum, the remaining balances as of December 31, 2009 were interest bearing at variable rates ranging from HIBOR plus 5.841% to HIBOR plus 7.02% per annum respectively.
|
|
(c)
|
As of December 31, 2009, the Company’s banking facilities were composed of the following :
|
|
Amount
|
||||||||||||
|
Facilities granted
|
Granted
|
Utilized
|
Unused
|
|||||||||
|
Secured bank loans
|
$
|
10,768,026
|
$
|
10,768,026
|
$
|
-
|
||||||
|
(d)
|
As of December 31,2009, The above bank loans were secured by the following :
|
|
(i)
|
Property, plant and equipment with carrying value of $6,119,241 (Note 15);
|
|
(ii)
|
Land use rights with carrying value of $6,205,140 (Note 16); and
|
|
(iii)
|
Buildings and land use right owned by a related company which is controlled by certain of the Company's directors.
|
During the reporting periods, there was no covenant requirement under the banking facilities granted to the Company.
The aggregate maturities of long-term bank loans as of December 31, 2009 are as follows:
Fiscal years ending on December 31,
|
Year
|
||||
|
2010
|
$
|
65,129
|
||
|
2011
|
1,757,354
|
|||
|
2012
|
1,689,590
|
|||
|
2013 and afterward
|
185,013
|
|||
|
$
|
3,697,086
|
|||
F-27
22. Other loans
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Interest bearing
|
||||||||
|
- staff - Note 22(a)
|
$
|
377,067
|
$
|
638,145
|
||||
|
- third parties - Note 22(a)
|
1,544,091
|
1,793,994
|
||||||
|
1,921,158
|
2,432,139
|
|||||||
|
Non interest bearing
|
||||||||
|
- third parties
|
440,100
|
-
|
||||||
|
$
|
2,361,258
|
$
|
2,432,139
|
|||||
|
(a)
|
Interest bearing at a fixed rate of 0.2667% to 0.6% per month.
|
|
(b)
|
All the other loans are unsecured and repayable on demand.
|
23. Commitments and contingencies
|
a.
|
Capital commitment
|
As of December 31, 2009, the Company had no capital commitments in respect of the acquisition of property, plant and equipment which were contracted for but not provided in the consolidated financial statements.
|
b.
|
Operating lease commitments
|
As of December 31, 2009, the Company had non-cancelable operating leases for its retail shops and future minimum lease payment to be paid are as follows:
|
Year
|
||||
|
2010
|
$
|
253,495
|
||
|
2011
|
223,273
|
|||
|
2012
|
163,491
|
|||
|
2013 and afterward
|
66,644
|
|||
|
$
|
706,903
|
|||
The rental expense relating to the operating leases was $309,833 and $229,013 for the years ended December 31, 2009 and 2008 respectively.
|
c.
|
Operating lease arrangement
|
As of December 31, 2009, the Company leases its retail shops in PRC under an operating lease arrangement until 2010. Future minimum lease payments to be received under non-cancelable operating lease are as follows:
|
As of December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Within one year
|
$
|
11,903
|
$
|
8,950
|
||||
|
d.
|
Registration payment arrangement
|
F-28
On December 30, 2009, the Company completed a private placement of 2,489,370 shares of common stock and warrants to purchase up to 1,244,368 shares of common stock at an exercise price of $3.81 per share. In connection with the private placement, warrants to purchase up to 248,937 shares of common stock at an exercise price of $3.05 per share were issued to the Co-Placement Agents.
Pursuant to the subscription agreement, the Company was required to file a registration statement (the “Registration Statement”) under the Securities Act of 1933, as amended, (i) registering for resale by the investors for 2,489,370 shares of common stock and the warrants to purchase up to 1,244,368 issued to the investors; and (ii) registering for resale for the Co-Placement Agents for the warrants to purchase up to 248,937 shares of common stock (all of the foregoing securities being collectively referred herein as the “Registrable Securities”). The Company agreed to use its best efforts to file the Registration Statement within 30 days from the Second Closing, dated February 1, 2010 (“Registration Filing Date”) and to have the Registration Statement declared effective prior to the 150th day following the Second Closing, provided, however, that in the event of a “full review” by the SEC, the Company shall be afforded an additional 30 days and shall have the Registration Statement declared effective prior to the 180th day following the Second Closing (the “Registration Effective Date”).
In the event that (i) the Registration Statement has not been filed on or prior to the Registration Filing Date or declared effective by the SEC on or before the Registration Effective Date; and (ii) the Registrable Securities included in such Registration Statement are not saleable under Rule 144, the Company shall pay to each investor as liquidated damages, a cash payment equal to 1% of the aggregated amount invested by such investors in the private placement for the first 30 days and 1% of the aggregated amount vested by such investors in the private placement for every 30-day period thereafter until the registration statement has been filed and/or declared effective, or such proportionate percentage for any period less than 30 days. In accordance with ASC 825-20, if the Company determines a registration payment arrangement is probable and can be reasonably estimated, a liability will recorded. As of December 31, 2009 and up to the date of approval of these financial statements, it is not considered probable that the Company will be required to make any payments under the registration rights arrangement and therefore no provision for such contingent liability has been made.
24. Common stock and additional paid-in capital
|
Additional
|
||||||||||||
|
Number of
|
paid-in
|
|||||||||||
|
shares
|
Amount
|
capital
|
||||||||||
|
Balance, December 31, 2008
|
32,000,000
|
$
|
32,000
|
$
|
9,596,632
|
|||||||
|
Recapitalization - Note 24(d)
|
2,600,000
|
2,600
|
20,094
|
|||||||||
|
Private placement - Note 24(b)
|
2,489,370
|
2,489
|
5,304,173
|
|||||||||
|
Balance, December 31, 2009
|
37,089,370
|
$
|
37,089
|
$
|
14,920,899
|
|||||||
|
(a)
|
On December 30, 2009, the Company issued 32,000,000 shares of common stock at par value $0.001 each to the shareholders of Ingenious in exchange for their 100% issued and outstanding common stock in Ingenious.
|
|
(b)
|
On December 30, 2009, the Company completed a private placement of 2,489,370 shares of common stock and warrants to purchase up to 1,244,368 shares of common stock at an exercise price of $3.81 per share for a gross proceed of $6,322,952 with related issuance expenses of $1,016,290. In connection with the private placement, warrants to purchase up to 248,937 shares of common stock at an exercise price of $3.05 per share were issued to Co-Placement Agent.
|
|
(c)
|
On December 30, 2009, 2,900,000 shares of the Company’s common stock, which were held by a Company’s shareholder, Lisa Lopomo, were cancelled.
|
|
(d)
|
The Company’s issued and outstanding 2,600,000 shares of common stock prior to the RTO were accounted for as $22,694, the net book value at the time of RTO.
|
F-29
25. Statutory surplus reserves
Under PRC regulations, Liuzhou BCT, BCT Retail and Hefeng Pharmaceutical may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC GAAP. In addition, these companies are required to set aside at least 10% of their after-tax net profits each year, if any, to fund the statutory reserves until the balance of the reserves reaches 50% of their registered capital. The statutory reserves are not distributable in the form of cash dividends to the Company and can be used to make up cumulative prior year losses.
26. Defined contribution plan
Pursuant to the relevant PRC regulations, the Company is required to make contributions at a rate of 30.5% to employees’ salaries and wages to a defined contribution retirement scheme organized by a state-sponsored social insurance plan in respect of the retirement benefits for the Company’s employees in the PRC. The only obligation of the Company with respect to retirement scheme is to make the required contributions under the plan. No forfeited contribution is available to reduce the contribution payable in the future years. The defined contribution plan contributions were charged to the consolidated statements of income. The Company contributed $734,379 and $498,598 for the year ended December 31, 2009 and 2008, respectively.
27. Segment information
The Company uses the “management approach” in determining reportable operating segments. The management approach considers the internal organization and reporting used by the Company’s chief operating decision maker for making operating decisions and assessing performance as the source for determining the Company’s reportable segments. Management, including the chief operating decision maker, reviews operating results solely by monthly revenue of wholesales, retail and manufacturing sectors and operating results of the Company and, as such, the Company has determined that the Company has three operating segments as defined by ASC 280, “Segments Reporting” (previously SFAS 131)”: wholesales, retail and manufacturing.
|
Wholesale
|
Retail
|
Manufacturing
|
Eliminations
|
Total
|
||||||||||||||||||||||||||||||||||||
|
Year ended
December 31,
|
Year ended
December 31,
|
Year ended
December 31,
|
Year ended
December 31,
|
Year ended
December 31,
|
||||||||||||||||||||||||||||||||||||
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
|||||||||||||||||||||||||||||||
|
Revenue from external customers
|
$
|
97,137,585
|
$
|
72,806,899
|
$
|
31,222,577
|
$
|
28,592,852
|
$
|
7,726,546
|
$
|
7,591,578
|
$
|
-
|
$
|
-
|
$
|
136,086,708
|
$
|
108,991,329
|
||||||||||||||||||||
|
Revenue from intersegment
|
21,729,943
|
21,470,183
|
-
|
-
|
762,280
|
185,833
|
(22,492,223
|
)
|
(21,656,016
|
)
|
-
|
-
|
||||||||||||||||||||||||||||
|
Interest income
|
15,116
|
25,386
|
984
|
3,332
|
-
|
597
|
-
|
-
|
16,100
|
29,315
|
||||||||||||||||||||||||||||||
|
Interest expenses
|
902,642
|
945,838
|
37,826
|
14,767
|
344,415
|
291,658
|
-
|
-
|
1,284,883
|
1,252,263
|
||||||||||||||||||||||||||||||
|
Amortization
|
274,765
|
298,131
|
-
|
-
|
157,333
|
236,302
|
-
|
-
|
432,098
|
534,433
|
||||||||||||||||||||||||||||||
|
Depreciation
|
180,492
|
225,139
|
3,498
|
3,394
|
414,286
|
404,367
|
-
|
-
|
598,276
|
632,900
|
||||||||||||||||||||||||||||||
|
Segment profit
|
15,187,570
|
13,992,806
|
7,198,703
|
5,938,155
|
3,472,102
|
2,426,031
|
-
|
-
|
25,858,375
|
22,356,992
|
||||||||||||||||||||||||||||||
|
Segment assets
|
68,252,951
|
51,226,243
|
9,184,032
|
1,984,571
|
10,319,034
|
12,586,520
|
-
|
-
|
87,756,017
|
65,797,334
|
||||||||||||||||||||||||||||||
|
Expenditure for segment assets
|
$
|
50,539
|
$
|
149,081
|
$
|
7,502
|
$
|
5,674
|
$
|
275,623
|
$
|
567,155
|
$
|
-
|
$
|
-
|
$
|
333,664
|
$
|
721,910
|
||||||||||||||||||||
F-30
A reconciliation is provided for unallocated amounts relating to corporate operations which is not included in the segment information.
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Total consolidated revenue
|
$
|
136,086,708
|
$
|
108,991,329
|
||||
|
Total profit for reportable segments
|
$
|
25,858,375
|
$
|
22,356,992
|
||||
|
Unallocated amounts relating to operations
|
||||||||
|
Finance costs
|
(2,791
|
)
|
(2,952
|
)
|
||||
|
Other general expenses
|
(185,221
|
)
|
(40,000
|
)
|
||||
|
Income before income taxes
|
$
|
25,670,363
|
$
|
22,314,040
|
||||
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Assets
|
||||||||
|
Total assets for reportable segments
|
$
|
87,756,017
|
$
|
65,797,334
|
||||
|
Cash and cash equivalents
|
5,078,973
|
16,671
|
||||||
|
Other receivables
|
225,802
|
343
|
||||||
|
$
|
93,060,792
|
$
|
65,814,348
|
|||||
All of the Company’s long-lived assets and revenues classified based on the customers are located in the PRC.
28. Related party transactions
Apart from the transactions as disclosed elsewhere in these consolidated financial statements, the Company has entered into following transactions with its related parties:
|
(i)
|
Sales to related companies of which certain Company’s directors have controlling interests.
|
|
Year ended December 31,
|
||||||||
|
Name of related companies
|
2009
|
2008
|
||||||
|
Liucheng Medicine Company
|
$
|
311,759
|
$
|
438,371
|
||||
|
Guangxi Tianhu Medicine Limited
|
$
|
271,890
|
$
|
821,449
|
||||
|
Wuxuan Baicaotang Medicine Limited
|
$
|
208,475
|
$
|
487,781
|
||||
|
Guangxi Liuzhou Baicaotang Medicine Limited, Guigang Branch
|
$
|
1,273,381
|
$
|
1,262,429
|
||||
|
(ii)
|
During the year of 2008, Liuzhou BCT declared dividends of $6,940,000 to the former stockholders of Liuzhou BCT.
|
29. Subsequent event
The Company has evaluated its activities subsequent to December 31, 2009 and has concluded that, except for the transaction described below, no subsequent events have occurred that would require recognition or disclosure in the consolidated financial statements.
On February 1, 2010, the Company completed a private placement of 1,029,970 shares of common stock and warrants to purchase up to 514,933 shares of common stock at an exercise price of $3.81 per share for gross proceeds of $2,616,108. In connection with the private placement, warrants to purchase up to 102,997 shares of common stock at an exercise price of $3.05 were issued to Co-Placement Agent on a cash or cashless basis.
F-31
5,630,575 Shares
Common Stock
CHINA BCT PHARMACY GROUP, INC.
PROSPECTUS
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus or any prospectus supplement. This prospectus is not an offer of these securities in any jurisdiction where an offer and sale is not permitted.
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
|
Securities and Exchange Commission Registration Fee
|
$
|
1,192
|
||
|
Printing Fees
|
$
|
5,000
|
*
|
|
|
Accounting fees
|
$
|
30,000
|
*
|
|
|
Legal fees and expenses
|
$
|
100,000
|
*
|
|
|
Total
|
$
|
136,192
|
*
|
|
|
* estimates
|
||||
All amounts are estimates other than the Commission’s registration fee. We are paying all expenses of the offering listed above. No portion of these expenses will be borne by the selling stockholders. The selling stockholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage commissions or costs of sale.
Item 14. Indemnification of Directors and Officers
Our Certificate of Incorporation, provides to the fullest extent permitted by Section 145 of the General Corporation Law of the State of Delaware, that our directors or officers shall not be personally liable to us or our stockholders for damages for breach of such director’s or officer’s fiduciary duty. The effect of this provision of our Certificate of Incorporation, is to eliminate our rights and our stockholders (through stockholders’ derivative suits on behalf of our company) to recover damages against a director or officer for breach of the fiduciary duty of care as a director or officer (including breaches resulting from negligent or grossly negligent behavior), except under certain situations defined by statute. We believe that the indemnification provisions in our Certificate of Incorporation, are necessary to attract and retain qualified persons as directors and officers.
Our By Laws also provide that our board of directors may also authorize us to indemnify our employees or agents, and to advance the reasonable expenses of such persons, to the same extent, following the same determinations and upon the same conditions as are required for the indemnification of and advancement of expenses to our directors and officers. As of the date of this report, our board of directors has not extended indemnification rights to persons other than directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities.
On July 21, 2007, we issued 1,000,000 shares of our common stock to our former officer and director, Lisa Lopomo, in exchange for $5,000, or $0.005 per share. The issuance of these securities was exempt from registration pursuant to Section 4(2) and Rule 506 of Regulation D promulgated under the Securities Act. No advertising or general solicitation was employed in offering the securities. The offering was made to an accredited investor who was also our executive officer and director at the time of such issuance, and the transfer was restricted in accordance with the requirements of the Securities Act. In addition, we have made an independent determination that such individual was an accredited and sophisticated investor, and that such individual was capable of analyzing the merits and risks of the investment and understood the speculative nature of the investment.
II-1
In August 2008, the shareholders of Liuzhou BCT (the “Liuzhou BCT Shareholders”) and Xiaoyan Zhang, our CFO, developed a restructuring plan (the “Restructuring”). The goal of the Restructuring was to enter into a transaction with a public shell company in the United States so that the public shell company, would acquire operations based in the PRC, all in compliance with PRC law. At that time, Ms. Zhang, who is a citizen of Hong Kong, was the sole shareholder of Ingenious, which had no assets or operations and owned 100% of Forever Well. The first step was for Forever Well to acquire 100% of the equity interests of Liuzhou BCT and its subsidiaries (the “PRC Operating Companies”). Liuzhou BCT was owned at that time by a total of 16 individuals, who were certain former and current employees and directors of Liuzhou BCT. After the acquisition of the PRC Operating Companies by Forever Well was consummated, the second step was for Ingenious to enter into and complete a transaction with a U.S. public reporting company whereby that company would acquire Ingenious. In connection with the Restructuring, Forever Well acquired 100% of the equity interest of Liuzhou BCT from the 16 Liuzhou BCT Shareholders for aggregate consideration of RMB10,000,000 (approximately $1,470,588) which was the registered and fully paid up capital of Liuzhou BCT. On December 30, 2009, the goal of the Restructuring was realized when the Company entered into a share exchange agreement with Ingenious and its shareholders, pursuant to which the Company acquired 100% of the equity of Ingenious in exchange for the issuance of an aggregate of 32,000,000 shares of our common stock to Ms. Zhang and six other Liuzhou BCT Shareholders. The issuance of the securities in the share exchange transaction was exempt from registration pursuant to either (i) Section 4(2) of the Securities Act or (ii) Regulation S promulgated under the Securities Act. Such securities qualified for exemption under Section 4(2) of the Securities Act of 1933 since the issuance of the securities us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which we sold a high number of securities to a high number of investors. In addition, each of the shareholders of Ingenious made representations to us that such Ingenious shareholder carefully reviewed such information as he or she has deemed necessary to evaluate an investment in the Company and its securities, and that such Ingenious shareholder has been furnished all materials that he or she has requested relating to the Company and the issuance of its shares and each Ingenious shareholder was afforded the opportunity to ask questions of the Company’s representatives to obtain any information necessary to verify the accuracy of any representations or information made or given to the Ingenious shareholders. We believe that the Ingenious shareholderswere sophisticated investors. Such issuance qualified for exemption under Regulation S promulgated under the Securities Act with respect to certain investors because such investors were not “U.S. persons” as defined under Regulation S, were located outside the United States at the time of the offer and acceptance thereof and had no plan or intention to sell the securities in the United States or to a “U.S. person.”
On December 30, 2009, simultaneously with the closing of the Share Exchange, we consummated a Private Placement for the issuance and sale of Units, consisting of an aggregate of 2,489,370 shares of common stock and Investor Warrants to purchase up to 1,244,368 shares of common stock at a price per share of $3.81 for gross proceeds in the amount of approximately $6.3 million, to approximately 102 accredited investors. The issuance of these securities was exempt from registration pursuant to Section 4(2) and Rule 506 of Regulation D promulgated under the Securities Act. We made this determination based on the representations of the Investors which included, in pertinent part, that such Investors were “accredited investors” within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, and that such Investors were acquiring our common stock for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Investors understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom. In addition, each Investor represented to us that it has had a reasonable opportunity to receive, and fully and carefully review, all information related to the Company, the securities, the share exchange transaction and Ingenious requested by it and to ask questions of and receive answers from the Company regarding the Company, the share exchange transaction, Ingenious and the terms and conditions of the offering of the securities. We believe that such investors were sophisticated.
II-2
On February 1, 2010, we completed the Second Closing of the Private Placement for the issuance and sale of Units, consisting of an aggregate of 1,029,970 shares of common stock and Investor Warrants to purchase up to 514,933 shares of common stock at a price per share of $3.81 for gross proceeds in the amount of approximately $2.6 million, to approximately 32 accredited investors. The issuance of these securities was exempt from registration pursuant to Section 4(2) and Rule 506 of Regulation D promulgated under the Securities Act. We made this determination based on the representations of the Investors which included, in pertinent part, that such Investors were “accredited investors” within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, and that such Investors were acquiring our common stock for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Investors understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom. In addition, each Investor represented to us that it has had a reasonable opportunity to receive, and fully and carefully review, all information related to the Company, the securities, the share exchange transaction and Ingenious requested by it and to ask questions of and receive answers from the Company regarding the Company, the share exchange transaction, Ingenious and the terms and conditions of the offering of the securities. We believe that such investors were sophisticated.
In connection with the closing of the Private Placement, the Co-Placement Agents received a cash fee equal to 10% of the gross proceeds of the Private Placement plus non-accountable allowance equal to 3% of the gross proceeds, and Agent Warrants to purchase 351,934 shares of our common stock at a price per share of $3.05. The Agent Warrants were issued to a total of 31 entities and individuals. The issuance of the Agent Warrants was exempt from registration pursuant to Section 4(2) of the Securities Act. Such securities qualified for exemption under Section 4(2) of the Securities Act of 1933 since the issuance of the securities us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which it sold a high number of securities to a high number of investors. In addition, Co-Placement Agent had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such securities are restricted pursuant to Rule 144 of the 1933 Securities Act. This restriction ensures that these securities would not be immediately redistributed into the market and therefore not be part of a “public offering.” In addition, we believe that the recipients of the Agent Warrants were sophisticated investors.
On February 5, 2010, we issued an aggregate of 15,000 shares of our common stock to four individuals in exchange for certain legal services provided to the Company by Anslow & Jaclin, LLP, a law firm of which each of the four individuals were a member. These individuals are Richard I. Anslow, Gregg E. Jaclin, Eric M. Stein and Zhuayao Hui. The issuance of the securities was exempt from registration pursuant to Section 4(2) of the Securities Act. Such securities qualified for exemption under Section 4(2) of the Securities Act of 1933 since the issuance of the securities us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which we sold a high number of securities to a high number of investors. We believe that such individuals were sophisticated investors.
On February 5, 2010, we issued 20,000 shares of our common stock to Cuixia Wang in exchange for consulting services provided to us, which included assisting us in obtaining relevant governmental approvals in China during the Restructuring process. The issuance of the securities was exempt from registration pursuant to either (i) Section 4(2) of the Securities Act or (ii) Regulation S promulgated under the Securities Act. Such securities qualified for exemption under Section 4(2) of the Securities Act of 1933 since the issuance of the securities us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of securities offered. We did not undertake an offering in which we sold a high number of securities to a high number of investors. We believe that such individual was a sophisticated investor. Such issuance qualified for exemption under Regulation S promulgated under the Securities Act with respect to certain investors because such investors were not “U.S. persons” as defined under Regulation S, were located outside the United States at the time of the offer and acceptance thereof and had no plan or intention to sell the securities in the United States or to a “U.S. person.”
II-3
Item 16. Exhibits and Financial Statement Schedules
|
2.1
|
Share Exchange Agreement dated December 23, 2009 *
|
|
3.1
|
Certificate of Incorporation (2)
|
|
3.2
|
Certificate of Amendment of Certificate of Incorporation (1)
|
|
3.3
|
By-laws (2)
|
|
4.1
|
Form of Warrant (1)
|
|
4.2
|
Form of Co-placement Agent Warrant *
|
|
5.1
|
Legal Opinion of McKenna Long & Aldridge LLP *
|
|
10.1
|
Subscription Agreement Dated October 23, 2009 (1)
|
|
10.2
|
Co-Placement Agent Agreement Dated October 21, 2009 *
|
|
10.3
|
Escrow Deposit Agreement Dated October 21, 2009 *
|
|
10.4
|
Earn-In Agreement dated October 22, 2009 (1)
|
|
10.5
|
Share Transfer Agreement dated as of April 1, 2008 *
|
|
10.6
|
Shares Pledge Agreement dated May 3, 2008 *
|
|
10.7
|
Shares Pledge Agreement dated March 31, 2009 *
|
|
10.8
|
Shares Repurchase Agreement dated July 31, 2008 *
|
|
10.9
|
Loan Agreement and Pledge Agreement dated December 19, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Liuzhou City Commercial Bank *
|
|
10.10
|
Loan Agreement and Pledge Agreement dated December 29, 2008 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Rurol Credence Cooperation of Guangxi *
|
|
10.11
|
Loan Agreement and Pledge Agreement dated January 8, 2009 by and among Liuzhou Baicaotang, Baicaotang Property Development Limited and Agricultural Bank of China *
|
|
10.12
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated February 13, 2006 *
|
|
10.13
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated March 27, 2006 *
|
|
10.14
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated April 6, 2006 *
|
II-4
|
10.15
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated May 18, 2006 *
|
|
10.16
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 6, 2006 *
|
|
10.17
|
Loan Agreement by and between Li Jin Hua and Guangxi Hefeng Pharmaceutical Co., Limited dated December 29, 2008 *
|
|
10.18
|
Loan Agreement dated February 8, 2007 by and between Hui Tian Tang and Industrial and Commercial Bank of China Guangxi Branch *
|
|
10.19
|
Confirmation Agreement dated December 31, 2008 by and among Hui Tian Tang, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch *
|
|
10.20
|
Loan Agreement dated February 12, 2007 by and between Jiang You Ru and Industrial and Commercial Bank of China Guangxi Branch *
|
|
10.21
|
Confirmation Agreement dated December 31, 2008 by and among Jiang You Ru, Liuzhou Baicaotang and Liuzhou Baicaotang Guigang Branch *
|
|
10.22
|
Lock-Up Agreement *
|
|
10.23
|
Employment Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Pharmaceutical Co., Ltd., China BCT Pharmacy Group, Inc. and Hui Tian Tang (3)
|
|
10.24
|
Employment Agreement dated May 18, 2010 by and between Forever Well Asia Pacific Limited, China BCT Pharmacy Group, Inc. and Xiaoyan Zhang (3)
|
|
10.25
|
Earn-in Agreement dated December 30, 2009 *
|
|
10.26
|
Shares Pledge Agreement dated May 3, 2008 *
|
|
10.27
|
Shares Pledge Agreement dated March 31, 2009 *
|
|
10.28
|
Termination Agreement dated March 2, 2010 *
|
|
10.29
|
Share Transfer Agreement by and between He Wenheng and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
10.30
|
Share Transfer Agreement by and between Jia Junwen and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
10.31
|
Share Transfer Agreement by and between Jiang Qifeng and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
10.32
|
Share Transfer Agreement by and between Jiang Youru and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
II-5
|
10.33
|
Share Transfer Agreement by and between Li Jinghua and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.34
|
Share Transfer Agreement by and between Liu Chunlin and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.35
|
Share Transfer Agreement by and between Liu Gongchun and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.36
|
Share Transfer Agreement by and between Meng Yuangang and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.37
|
Share Transfer Agreement by and between Tan Yujing and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.38
|
Share Transfer Agreement by and between Ye Yuanjian and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.39
|
Share Transfer Agreement by and between Wang Bangfu and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.40
|
Share Transfer Agreement by and between Wei Wende and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.41
|
Share Transfer Agreement by and between Yang Xiaojian and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.42
|
Share Transfer Agreement by and between Zhang Qingqiu and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.43
|
Share Transfer Agreement by and between Zhao Ming’an and Forever Well Asia Pacific Limited dated March 18, 2008 *
|
|
|
10.44
|
Amendment No. 1 to Earn-in Agreement, by and between Xiaoyan Zhang and the Buyers named thererin, dated May 19, 2010 *
|
|
|
10.45
|
Amendment to Share Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 *
|
|
|
10.46
|
Amendment to Share Pledge Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd, dated May 19, 2010 *
|
|
|
10.47
|
Proxy Agreement by and between Guangxi Liuzhou Baicaotang Medicine Ltd. and Liuzhou Baicaotang Property Management Ltd., dated May 19, 2010 *
|
|
|
10.48
|
Form of sales agreement with related parties *
|
|
|
10.49
|
Independent Director Agreement of Eng Leong Lee (4)
|
|
II-6
|
10.50
|
Independent Director Agreement of Simon Choi (4)
|
|
10.51
|
Independent Director Agreement of Man Wai Chiu (4)
|
|
10.52
|
2010 Omnibus Securities and Incentive Plan (5)
|
|
10.53
|
Stock Option Agreement between the Registrant and Hui Tian Tang made as of June 27, 2010 (5)
|
|
10.54
|
Stock Option Agreement between the Registrant and Xiaoyan Zhang made as of June 27, 2010 (5)
|
|
10.55
|
Stock Option Agreement between the Registrant and Eng Leong Lee made as of July 16, 2010 *
|
|
10.56
|
Stock Option Agreement between the Registrant and Simon Choi made as of July 16, 2010 *
|
|
10.57
|
Stock Option Agreement between the Registrant and Man Wai Chiu made as of July 16, 2010 *
|
|
10.58
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Pharmaceutical Co., Ltd., China BCT Pharmacy Group, Inc. and Hui Tian Tang *
|
|
10.59
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Forever Well Asia Pacific Limited, China BCT Pharmacy Group, Inc. and Xiaoyan Zhang *
|
|
10.60
|
Asset Transfer Agreement dated February 28, 2010 by and between Liuzhou Wubaitang Medicine Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.61
|
Asset Transfer Agreement dated March 29, 2010 by and between Zhaoping County Laobaixin Pharmacy Chain store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.62
|
Asset Transfer Agreement dated April 25, 2010 by and between Lai Bing Peng Fei Pharmacy Chain store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.63
|
Form of Retail Chain Leasing Contract*
|
|
10.64
|
Entrustment Agreement Between Liuzhou BCT Shareholders and Ms. Xiaoyan Zhang on Ingenious Dated July 21, 2008 **
|
|
14
|
Code of Ethics and Business Conduct (4)
|
|
21
|
List of Subsidiaries *
|
|
23.1
|
Consent of Independent Certified Auditor **
|
|
23.2
|
Consent of McKenna Long & Aldridge LLP (included in the opinion filed as Exhibit 5.1) *
|
|
23.3
|
Consent of Broad & Bright *
|
|
99.1
|
Retail Chain Store Address Chart (1)
|
|
10.50
|
Independent Director Agreement of Simon Choi (4)
|
|
10.51
|
Independent Director Agreement of Man Wai Chiu (4)
|
|
10.52
|
2010 Omnibus Securities and Incentive Plan (5)
|
|
10.53
|
Stock Option Agreement between the Registrant and Hui Tian Tang made as of June 27, 2010 (5)
|
|
10.54
|
Stock Option Agreement between the Registrant and Xiaoyan Zhang made as of June 27, 2010 (5)
|
|
10.55
|
Stock Option Agreement between the Registrant and Eng Leong Lee made as of July 16, 2010 *
|
|
10.56
|
Stock Option Agreement between the Registrant and Simon Choi made as of July 16, 2010 *
|
|
10.57
|
Stock Option Agreement between the Registrant and Man Wai Chiu made as of July 16, 2010 *
|
|
10.58
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Guangxi Liuzhou Baicaotang Pharmaceutical Co., Ltd., China BCT Pharmacy Group, Inc. and Hui Tian Tang *
|
|
10.59
|
Non-Disclosure and Non-Competition Agreement dated May 18, 2010 by and between Forever Well Asia Pacific Limited, China BCT Pharmacy Group, Inc. and Xiaoyan Zhang *
|
|
10.60
|
Asset Transfer Agreement dated February 28, 2010 by and between Liuzhou Wubaitang Medicine Chain Store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.61
|
Asset Transfer Agreement dated March 29, 2010 by and between Zhaoping County Laobaixin Pharmacy Chain store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.62
|
Asset Transfer Agreement dated April 25, 2010 by and between Lai Bing Peng Fei Pharmacy Chain store Co., Ltd. and Liuzhou Baicaotang Medicine Retail Limited*
|
|
10.63
|
Form of Retail Chain Leasing Contract*
|
|
10.64
|
Entrustment Agreement Between Liuzhou BCT Shareholders and Ms. Xiaoyan Zhang on Ingenious Dated July 21, 2008 **
|
|
10.65
|
Series A Convertible Preferred Shares Purchase Agreement, by and between the Company and Milestone Longcheng Limited (6)
|
|
10.66
|
Registration Rights Agreement, by and between the Company and Milestone Longcheng Limited (6)
|
|
10.67
|
Shareholders Agreement, by and among the Company, Milestone Longcheng Limited, certain shareholders of the Company and Mr. Tian Hui Tang as representative for the shareholders (6)
|
|
14
|
Code of Ethics and Business Conduct (4)
|
|
21
|
List of Subsidiaries *
|
|
23.1
|
Consent of Independent Certified Auditor **
|
|
23.2
|
Consent of McKenna Long & Aldridge LLP (included in the opinion filed as Exhibit 5.1) *
|
|
23.3
|
Consent of Broad & Bright *
|
|
99.1
|
Retail Chain Store Address Chart (1)
|
II-7
|
(1)
|
Incorporated by reference to the Company’s Form 8-K filed on December 31, 2009.
|
|
(2)
|
Incorporated by reference to the Company’s Form SB-2 filed on August 22, 2007.
|
|
(3)
|
Incorporated by reference to the Company’s Form 8-K filed on May 21, 2010.
|
|
(4)
|
Incorporated by reference to the Company’s Form 8-K filed on June 9, 2010.
|
|
(5)
|
Incorporated by reference to the Company’s Form 8-K filed on June 29, 2010.
|
|
(6)
|
Incorporated by reference to the Company’s Form 8-K filed on January 18, 2011.
|
* Previously filed
** Filed herewith.
Item 17. Undertakings.
Undertaking Required by Item 512 of Regulation S-K.
(a) The undersigned registrant will:
(1) File, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to:
(i) include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information in the registration statement; and notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) include any additional or changed material information on the plan of distribution.
(2) For determining liability under the Securities Act, treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
(3) File a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
(b) For determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the registrant undertakes that in a primary offering of securities of the registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the registrant or used or referred to by the registrant;
II-8
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the registrant or its securities provided by or on behalf of the registrant; and
(iv) Any other communication that is an offer in the offering made by the registrant to the purchaser.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(d) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i) Each prospectus filed by the registrant pursuant to 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
II-9
SIGNATURES
In accordance with the requirements of the Securities Act, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and authorized this amendment to the registration statement to be signed on its behalf by the undersigned, in Liuzhou City, Guangxi province on January 25 , 2011.
CHINA BCT PHARMACY GROUP, INC
|
/s/ Hui Tian Tang
|
|
Name: Hui Tian Tang
Position: Chief Executive Officer (Principal Executive Officer)
|
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons and in the capacities and on the dates indicated.
|
Dated: January 25 , 2011
|
By:
|
/s/ Hui Tian Tang
|
|
Name:
|
Hui Tian Tang
|
|
|
Title:
|
Chief Executive Officer (Principal Executive Officer) and Director
|
|
|
Dated: January 25 , 2011
|
By:
|
/s/ Xiaoyan Zhang
|
|
Name:
|
Xiaoyan Zhang
|
|
|
Title:
|
Chief Financial Officer (Principal Financial and Accounting Officer) and Director
|
|
|
Dated: January 25 , 2011
|
By:
|
/s/ Man Wai Chiu*
|
|
Name:
|
Man Wai Chiu
|
|
|
Title:
|
Director
|
|
|
Dated: January 25 , 2011
|
By:
|
/s/ Eng Leong Lee*
|
|
Name:
|
Eng Leong Lee
|
|
|
Title:
|
Director
|
|
|
Dated: January 25 , 2011
|
By:
|
/s/ Simon Choi*
|
|
Name:
|
Simon Choi
|
|
|
Title:
|
Director
|
|
* Signed by Hui Tian Tang as pursuant to his power-of-attorney
II-10
