Attached files
| file | filename |
|---|---|
| EX-14 - ALTEROLA BIOTECH INC. | v208051_ex14.htm |
| EX-23.1 - ALTEROLA BIOTECH INC. | v208051_ex23-1.htm |
| EX-32.1 - ALTEROLA BIOTECH INC. | v208051_ex32-1.htm |
| EX-31.2 - ALTEROLA BIOTECH INC. | v208051_ex31-2.htm |
| EX-32.2 - ALTEROLA BIOTECH INC. | v208051_ex32-2.htm |
| EX-31.1 - ALTEROLA BIOTECH INC. | v208051_ex31-1.htm |
| EX-21.1 - ALTEROLA BIOTECH INC. | v208051_ex21-1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
DC 20549
Form
10-K
(Mark
one)
|
x
|
Annual Report
Under Section 13 or 15(d) of The Securities Exchange Act of
1934
|
For the
fiscal year ended September 30, 2010
|
¨
|
Transition Report Under Section
13 or 15(d) of The Securities Exchange Act of
1934
|
For the
transition period from ______________ to _____________
Alterola Biotech
Inc.
(Exact
Name of Registrant as Specified in Its Charter)
|
Nevada
|
333-156091
|
N/A
|
||
|
(State or other jurisdiction
of
incorporation)
|
(Commission
File
Number)
|
(IRS
Employer
Identification
No.)
|
|
228
Hamilton Avenue, 3rd Floor
Palo
Alto, California
|
94301
|
|
|
(Address
of principal executive offices)
|
(Zip
Code)
|
Registrant’s
telephone number, including area code: +45-8842 9181
Securities
registered pursuant to Section 12 (b) of the Act - None
Securities
registered pursuant to Section 12(g) of the Act: - None
Indicate
by check mark if the registrant is a well known seasoned issuer, as defined in
Rule 405 of the Securities Act.
Yes
¨ No
x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Exchange Act.
Yes x No
¨
Indicate
by check mark whether the registrant has (1) filed all reports required to be
filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or
for such shorter period the Company was required to file such reports), and (2)
has been subject to such filing requirements for the past 90
days.
Yes x No
¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of the registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K. ¨
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting company. See
the definitions of large accelerated filer”, accelerated filer” and smaller
reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large
accelerated filer ¨
|
Accelerated
filer
|
¨
|
|
|
Non-accelerated
filer ¨
|
Smaller
reporting
company
|
x
|
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act):
Yes
x No
¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes
¨ No
¨
The
aggregate market value of the outstanding common stock, other than shares held
by persons who may be deemed affiliates of the registrant, computed by reference
to the closing sales price for the Registrant’s Common Stock on December 1,
2010, as reported on the OTC Bulletin Board, was not applicable as the Common
Stock was not quoted on the OTC Bulletin Board at that time.
As of
January 10, 2011, there were 92,980,000 shares of Common Stock issued and
outstanding.
Index
to Contents
|
Page Number
|
||||
|
Part
I
|
||||
|
Item
1
|
Business
|
3
|
||
|
Item
1A
|
Risk
Factors
|
7
|
||
|
Item
1B
|
Unresolved
Staff Comments
|
11
|
||
|
Item
2
|
Properties
|
11
|
||
|
Item
3
|
Legal
Proceedings
|
11
|
||
|
Item
4
|
Submission
of Matters to a Vote of Security Holders
|
11
|
||
|
Part
II
|
||||
|
Item
5
|
Market
for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities
|
11
|
||
|
Item
6
|
Selected
Financial Data
|
12
|
||
|
Item
7
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
12
|
||
|
Item
7A
|
Quantitative
and Qualitative Disclosures About Market Risk
|
14
|
||
|
Item
8
|
Financial
Statements and Supplementary Data
|
15
|
||
|
Item
9
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure
|
15
|
||
|
Item 9A
|
Controls
and Procedures
|
15
|
||
|
Item 9B
|
Other
Information
|
16
|
||
|
Part III
|
||||
|
Item
10
|
Directors,
Executive Officers and Corporate Governance
|
16
|
||
|
Item
11
|
Executive
Compensation
|
17
|
||
|
Item
12
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters
|
18
|
||
|
Item
13
|
Certain
Relationships and Related Transactions, and Director
Independence
|
19
|
||
|
Item
14
|
Principal
Accountant Fees and Services
|
20
|
||
|
Part
IV
|
||||
|
Item
15
|
Exhibits
and Financial Statement Schedules
|
20
|
||
|
Signatures
|
|
22
|
||
Caution
Regarding Forward-Looking Information
Certain
statements contained in this annual filing, including, without limitation,
statements containing the words "believes", "anticipates", "expects", “intend”,
“estimate”, “plan” and words of similar import, constitute forward-looking
statements. Such forward-looking statements involve known and unknown risks,
uncertainties and other factors that may cause the actual results, performance
or achievements of the Company, or industry results, to be materially different
from any future results, performance or achievements expressed or implied by
such forward-looking statements.
Forward-looking
statements are based on our current expectations and assumptions regarding our
business, potential target businesses, the economy and other future conditions.
Because forward-looking statements relate to the future, by their nature, they
are subject to inherent uncertainties, risks and changes in circumstances that
are difficult to predict. Our actual results may differ materially from those
contemplated by the forward-looking statements. We caution you therefore that
you should not rely on any of these forward-looking statements as statements of
historical fact or as guarantees or assurances of future performance. Important
factors that could cause actual results to differ materially from those in the
forward-looking statements include changes in local, regional, national or
global political, economic, business, competitive, market (supply and demand)
and regulatory conditions and the following:
2
|
|
•
|
our
status as a development stage
company;
|
|
|
•
|
removal
of our securities from OTC Bulletin Board quotation system, or the ability
to have our securities quoted on OTC Bulletin Board or listed on another
exchange following our business
combination;
|
|
|
•
|
conflicts
of interest of our officers and
directors;
|
|
|
•
|
potential
current or future affiliations of our officers and directors with
competing businesses;
|
|
|
•
|
our
ability to obtain additional financing if
necessary;
|
|
|
•
|
the
control by our existing stockholders of a substantial interest in
us;
|
|
|
•
|
our
being deemed an investment company;
|
|
|
•
|
our
dependence on our key personnel;
|
|
|
•
|
business
and market outlook;
|
|
|
•
|
future
effective tax rates; and
|
|
|
•
|
compliance
with applicable laws.
|
These
risks and others described under “Risk Factors” are not exhaustive.
Given
these uncertainties, readers of this Form 10-K and investors are cautioned not
to place undue reliance on such forward-looking statements. The Company
disclaims any obligation to update any such factors or to publicly announce the
result of any revisions to any of the forward-looking statements contained
herein to reflect future events or developments.
PART
I
Item
1 - Business
General
Alterola
Biotech Inc. (“Alterola”, the “Company”, “we”, “us” or “our”) was incorporated
in the State of Nevada on July 21, 2008 under the name “Jedediah Resources
Corp.” We are a development stage company. The Company’s
fiscal year-end is September 30.
The
Company was formed for the purpose of acquiring exploration and development
stage mineral properties. On October 1, 2008, the Company
incorporated JRE Exploration Ltd (“JRE”), a wholly owned subsidiary in Canada
for the purpose of holding its Canadian mineral claims. On May 3,
2010, the Company changed its focus to the development of intellectual property
and accordingly sold JRE to Ola Juvkam-Wold (“Juvkam-Wold”), the former
chief executive officer of the Company. In keeping with the change of business
focus, on July 9, 2010 the Company changed its name to Alterola Biotech
Inc.
Prior to
May 3, 2010, we were in the business of mineral exploration. On May
3, 2010, the Company entered into a Stock Purchase Agreement (“Stock Purchase
Agreement”) and General Release and Settlement Agreement (“Release and
Settlement”) with Juvkam-Wold who was then the chief executive officer and
a director of the Company. Pursuant to the Stock Purchase Agreement,
Juvkam-Wold agreed to transfer all 55,000,000 shares of common stock of the
Company (“Common Stock”) owned by him to the Company in consideration for all
the issued and outstanding stock of JRE, and the cancellation of all debt owed
by JRE to the Company. Pursuant to the Release and Settlement,
Juvkam-Wold released the Company from any and all claims Juvkam-Wold may have
against the Company or its affiliates. Simultaneously with the
consummation of the Stock Purchase Agreement, Juvkam-Wold resigned from his
positions as Chief Executive Officer of the Company and as a member of the
Board.
On May 3,
2010, we entered into an Intellectual Property Assignment Agreement (“IP
Agreement”) with Mr. Soren Nielsen, our Chief Executive Officer, President and
sole director (“Nielsen”), pursuant to which Nielsen transferred his right,
title and interest in all intellectual property relating to certain chewing gum
compositions having appetite suppressant activity (“the Intellectual Property”
or “IP”) to the Company in consideration for the issuance of 55,000,000 newly
issued shares of Common Stock. Following the acquisition of the IP we
changed our business direction and are now pursuing the development of a chewing
gum with nutraceutical delivery
properties. The term nutraceutical combines the words ‘nutrition’ and
‘pharmaceutical’ and refers to food or food products that provide health and
medical benefits, including the prevention and treatment of
disease.
3
Effective
July 9, 2010, the Company’s Board of Directors authorized a 10 for 1 forward
stock split of the issued and outstanding Common Stock. The
authorized number of common shares was increased from 90,000,000 to 140,000,000
with a par value of $0.001 per share. The number of authorized shares
of preferred stock remained unchanged at 10,000,000 with a par value of $0.001
per share.
Subsequent
to the September 30 fiscal year end, on November 17, 2010, Nielsen entered into
a stock cancellation agreement with the Company whereby 40,000,000 shares of
Common Stock were returned to treasury and cancelled. In
consideration the Company will issue to Nielsen options to acquire Common Stock
pursuant to a stock option plan which the Company intends to adopt in the future
on such terms as determined by the Company’s Board of Directors. In
accordance with SAB Topic 4-C, the Company recorded the cancellation
retroactively as of September 30, 2010, as a reduction to the par value of
common stock with a corresponding increase to additional paid-in
capital.
On
December 21, 2010, the Company issued 250,000 shares at $0.20 for aggregate
proceeds of $50,000.
Overview
and Plan of Operation
Alterola
owns IP in the field of medicament-containing chewing gums for the treatment of
obesity and eating disorders and is in the process of drafting and filing
patents to protect the IP initially in Europe and the United States. The Company
may seek IP protection in other jurisdictions in the
future. Medicament refers to something that treats or prevents or
alleviates the symptoms of disease.
Our
business plan is to establish partnerships with laboratory testing outsourcing
partners (LTO’s) to create and develop nutraceutical patents and concepts based
on expert knowledge of active ingredients, and chewing gum with a taste and
texture on par with traditional confectionery products. The concept areas which
Alterola will focus on in the primary stage are gum products where the
ingredients are already FDA approved, which we expect will hasten FDA approval
and shorten the time to market of Alterola; gum products. Alterola is focusing
on seven new product developments which are in the areas of:
|
|
1.
|
Treatment
of mild depression
|
|
|
2.
|
Immune
defense
|
|
|
3.
|
Pick
me up gum/Energy boost
|
|
|
4.
|
Motion
sickness treatment
|
|
|
5.
|
Vitamins
C delivery
|
|
|
6.
|
Vitamin
B12 delivery
|
|
|
7.
|
Vitamin
D delivery
|
Alterola
is currently in discussion with a number of LTO’s as potential partners for
development in the above - listed product areas. Alterola has identified the
active ingredients for each product area and the LTO’s will measure the amount
of ingredients required and test the timing of release of the active
ingredients. They will also test the taste and shelf life of the products. We
intend to select LTO’s based upon their pricing, availability and
timing.
It is
intended that the gum will be branded under the brands DR GUM and
HERBAGUM. Alterola expects to have the first products available for
consumers in 2011 in North America, Europe and Asia. There may also be an
opportunity to ‘white label’ the gum products and allow the major gum companies
to sell generic versions of the products under their own brands.
To take
the products to market Alterola will need to complete a three phase process
including product development, product production and product
distribution.
Phase
One (Product Development)
In this
phase, we will work with LTO’s to develop new products. We expect it will take
approximately 6-9 per product months to complete lab testing and get to the
production stage.
We have
identified the active ingredients for those products we intend to develop
initially in the areas of stress relief and weight loss and we are in
negotiations with LTO’s. To complete phase one, we will need a finished product
from the LTO which has been fully tested by their labs. We expect
this will take 6-9 months and we are awaiting pricing terms from the various
LTO’s we have met with. We believe the major hurdle to completing product
development is our ability to raise funds. We believe the cost of
product development to bring each product through the LTO development stage is
approximately $25,000 based on discussions with prospective LTOs. We
anticipate raising such capital through the private placement of our equity or
debt securities. However, there is no assurance we will be able to
raise sufficient capital on favorable terms, or at all. If we
consummate private placements of our securities, our current warrant and stock
holders would experience dilution.
4
Phase
Two (Product Production)
To take
the products to production our board will consult with nutritionists and other
industry professionals to determine the best methodology. Alterola is in
discussion with possible nutraceutical manufacturers and will select production
partners able to manufacture our gum products for the market in commercial
volumes. The source of raw materials and location of manufacture will
most likely be China or Malaysia. We have identified several potential partners
who can deliver materials in volumes to meet our demands and we do not
anticipate any problems with obtaining the required supply. At this
time we have not signed any binding agreements so we cannot identify suppliers
until such time as we have signed final agreements. We anticipate that we would
initially produce 500,000 gum packs each containing 10 pieces of gum and we will
need approximately $150,000 to produce 500,000 gum packs. The costs outlined are
based upon initial discussions and quotations from product manufacturers and we
anticipate that the cost for each product will be relatively
constant.
As of the
date of this report, there have been discussions with several manufacturers who
we believe can produce the products at commercial levels at a reasonable cost,
and we are confident there are a number of suitable partners for manufacture of
our intended gum products. Until we have signed
with the LTO and have developed, tested and received such governmental and other
approvals as necessary for our products, there is no value in signing the
production agreements as this will put an undue financial burden on the Company
before we are ready for the manufacturing stage. We believe one of the hurdles
to realizing our business plan is being a small company amongst the major gum
producers, and that manufacturers may consider our products as competitive,
which may limit our ability to work with those manufacturers that have
experience in this area but already have pre-existing relationships with the
major gum producers and manufacturers. However, we believe our niche
positioning and willingness to ‘white label’ products should minimize this
risk. We believe our membership with the ingredient suppliers will
help facilitate in the creation of joint ventures with major gum producers and
could help to expedite our products entry to market; international association
of chewing gum manufacturers and ingredient suppliers. Funding
production will require a significant investment of cash which we do not
currently have. We anticipate raising capital through equity or debt financings
but there are no assurances we will be successful in raising sufficient capital
to finance our business plan on acceptable terms or at all.
Phase
Three (Distribution)
Alterola
is in discussion with distributors for the gum products mentioned above, selling
to both the online and retail markets. Alterola expects to have an agreement in
place with distributors when the products are ready for
production. There is no assurance that Alterola will be able to
capture any portion of the gum market.
We have
identified numerous online distributors worldwide with specific access to sales
channels in the areas of health foods, well being products and alternative
remedies, all of which we believe are the proper channels to market our initial
seven product areas. We have also identified two offline distributors in the US
with access to direct marketing and retail distribution, and one in
Scandinavia. We are continuing to research further distribution
partners to ensure the Company has the best routes to market upon completion of
our products. We expect additional capital will be needed to secure initial
orders, set up an information technology system and consummate and increase
sales and marketing for the initial product launch. We intend to raise
additional capital to complete the distribution of our products and we
anticipate that we will need $200,000 for this phase. We may not be
able to raise sufficient capital on favorable terms or at all.
During
the next 12 months Alterola intends to partner up with between two to three LTO
partners in development of the IP and partner up with international and/or local
manufacturers of nutraceuticals to produce the gum products. Finally, we intend
to find local and global distribution partners for online and local sales. With
the anticipated development of these partnerships, Alterola seeks to be able to
provide an end-to-end supply chain for our gum products.
Employees
To
achieve our business plan over the next 12 months, we will need to appoint
professional management and advisors to the Company’s board of directors and we
anticipate an organizational structure as below will be
required:
5
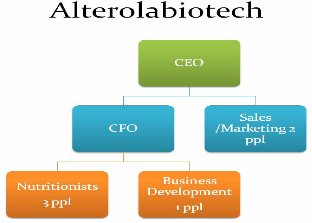
At this
time we have insufficient funds to hire the professionals we require to execute
our business plan, and we will require substantial additional funding in order
to undertake the commercialization of our products. In the next 12 months, we
anticipate spending approximately $500,000 on business expenses, including fees
payable in connection with complying with our Securities and Exchange Commission
(“SEC”) reporting obligations. The risky nature of this enterprise and lack of
tangible assets other than our IP places debt financing beyond the
credit-worthiness required by most banks or typical investors of corporate debt
until such time as an economically viable commercial product can be
demonstrated. We cannot provide investors with any assurances on our ability to
raise funding for the Company, however, we aim to raise sufficient funding
through equity or debt financing and advances from related parties to fund all
of our anticipated expenses. We do not have any arrangements in place for
any financing at this time. Currently there are no full time or part
time employees.
Competition
The
chewing gum and Nutraceutical businesses are highly
competitive. Many of our competitors have substantially greater financial,
marketing, personnel and other resources than we do. The principal methods of
competition include brand recognition, price and price promotion, new product
introductions, packaging changes, distribution methods, and advertising. We will
also compete for distributors, shelf space and customers primarily with other
gum companies. We believe our main competitors are Gumlink and
Wrigley.
Government
Regulation
At this
time, our products are not at the manufacturing stage so we are not subject to
government regulation. However, once production begins we will need
to ensure that only safe and approved ingredients are used in the manufacturing
of the chewing gum. We must ensure quality controls are in place during all
phases of production to ensure that the ingredients and the end products comply
with the requirements and limits set by regulatory authorities. As our products
will be manufactured by third party suppliers who already produce gum products,
we believe their systems and procedures are already substantially compliant with
regulations. In addition to confirming this and monitoring the
manufacturing process, Alterola will ensure that all ingredients are sourced
from reputable suppliers and that controls are in place to ensure high quality
production processes are followed.
The
ingredients we plan to use in our gum products fall under the GRAS (Generally
Regarded As Safe) Regulations of the FDA. Under Sections 201(s) and
409 of the Federal Food, Drug, and Cosmetic Act (the Act), any substance that is
intentionally added to food is a food additive, is subject to premarket review
and approval by the FDA, unless the substance is generally recognized, among
qualified experts, as having been adequately shown to be safe under the
conditions of its intended use, or unless the use of the substance is otherwise
excluded from the definition of a food additive. Upon producing
commercial ready products, we will need to follow the FDA GRAS process to obtain
clearance for the sale of our products. The GRAS clearance process typically
takes 180 days but there is no guarantee that the notice process will not take
longer than anticipated or that the final gum products will be recognized under
the GRAS regulations.
A patent
application for chewing gum having appetite suppressant qualities has been filed
with the relevant US and UK intellectual property offices. At this time, the
patents are pending approval.
Research
and Development
Approximately
2,200 hours in the aggregate have been spent on research and development during
the previous two years.
6
Item
1A - Risk Factors
RISKS
RELATED TO OUR BUSINESS
We
are still in an early stage of development and have a limited history of
operations.
We were
founded in 2008 and changed our business focus in 2010 from mining to the
development of a chewing gum with nutraceutical delivery properties and
have only recently commenced operations under our new business plan. We expect
to incur losses in the future as we implement our business plans and improve our
development production, distribution, sales and marketing capabilities. We
cannot anticipate when, or if, we will generate adequate revenues to achieve
positive cash flow or profitability.
The
success of our business depends on our ability to successfully develop and
commercialize our intellectual property relating to certain chewing gum
compositions having appetite suppressant activity.
The
success of our chewing gum business is dependent upon our ability to develop
final products that receive market acceptance, sell and distribute our products
and overcome all production issues related to high-volume
production. All we have at this time is intellectual property
relating to certain chewing gum compositions having appetite suppressant
activity properties. If we are unable to accomplish these
developmental milestones our business will be adversely affected and we may not
realize any profit from our operations.
If
we are unable to develop and commercialize our products within our anticipated
time frame we will not be able to generate any sales or revenues and may be
forced to cease operations.
Although
we anticipate we will develop products and commercialize our intellectual
property within the time frame set forth herein, we may not be able to meet our
timeline and it may take significantly longer for us to develop and
commercialize our products. If we are unable to timely bring our
products to market, we will not generate any revenue or make any sales and our
future operating prospects may be materially harmed.
We
face substantial capital requirements that we may not be able to satisfy, and
which may cause us to delay, scale back or eliminate some or all of our existing
development or future business initiatives, and which creates substantial doubt
as to our ability to fund future operations and continue as a going
concern.
We are
engaged in developing a nutraceutical chewing
gum. Our operating and capital requirements in connection with
planned development, testing, production, distribution, sales and marketing
activities will be significant. We are not currently generating any revenues
from operations to fund these activities and are dependent on the receipt of
funding from cash raised in equity and debt financings to continue with our
business plan. We currently do not have any commitment for such financing. No
assurance can be given that we will be successful in obtaining additional
financing under terms acceptable to us or in amounts sufficient to fund our
operating activities. The funds that we may raise, if any, may not allow us to
maintain our current and planned operations and such additional financing could
result in the dilution of shareholders’ percentage interests in the Company. If
we are unable to obtain additional capital, we may be required to delay, scale
back or eliminate some or all of our development of existing or future business
initiatives, and therefore there is substantial doubt as to the Company’s
ability to fund future operations and continue as a going concern.
The
chewing gum business is highly competitive.
The
chewing gum and nutraceutical businesses are highly
competitive. Many of our competitors have substantially greater financial,
marketing, personnel and other resources than we do. The principal methods of
competition include brand recognition, price, new product introductions,
packaging changes, distribution methods and advertising. We will also compete
for distributors, shelf space and customers primarily with other gum
companies.
We
compete in an industry that is brand-conscious, so brand name recognition and
acceptance of our products are critical to our success.
Our
business is substantially dependent upon awareness and market acceptance of our
products and brands by our targeted consumers. Our chewing gum products are too
early in the product development cycle to determine whether they will achieve
and maintain satisfactory levels of acceptance by independent distributors and
retail consumers. We believe that the success of our product name brands will
also be substantially dependent upon acceptance of our product name brands.
Accordingly, any failure of our brands to achieve, maintain or increase
acceptance or market penetration would likely have a material adverse affect on
our revenues and financial results.
7
We compete in an industry
characterized by rapid changes in consumer preferences and public perception, so
our ability to develop new products to satisfy consumers’ changing preferences
will determine our long-term success.
Our
future success will depend, in part, upon our ability to develop and introduce
different and innovative chewing gum products. In order to achieve and expand
our market share, we must continue to develop and introduce various products,
although there can be no assurance of our ability to do so. Product lifecycles
for chewing gum brands and/or products and/or packages may be limited to a few
years before consumers’ preferences change. There is no assurance that we will
be able to develop and produce a product that achieves acceptance in the market
place and will become profitable for us. Our inability to respond to
changes in consumer preferences can be expected to harm our results of
operations and future growth.
We
may face risks associated with product liability claims and product
recalls.
We may
experience product liability litigation and product recalls arising from
defectively manufactured products or packaging. We expect to obtain and maintain
product liability insurance insuring our operations from any claims associated
with product liability in an amount that will be sufficient to protect us.
Although we expect to have product liability insurance, we may not have
insurance coverage sufficient in amount and scope against potential liabilities
or the claims may be excluded from coverage under the terms of the policy.
Furthermore, product liability insurance is becoming increasingly expensive. As
a result, we may not be able to obtain sufficient amounts of insurance coverage,
obtain additional insurance when needed, or obtain insurance at a reasonable
cost, which could prevent or inhibit the commercialization of our products. If
we are sued for any injury caused by our products, our liability could equal a
material portion, or even exceed, our total assets. Any claims against us,
regardless of their merit or eventual outcome, could have a detrimental effect
upon our business, operating results and financial condition. We do
not maintain product recall insurance. In the event we were to experience
product liability or product recall claims, our business operations and
financial condition could be materially and adversely affected.
If
we are not able to retain the full time services of Soren Nielsen, it will be
more difficult for us to manage our operations and our operating performance
could suffer.
Our
business is dependent, to a large extent, upon the services of Soren Nielsen,
our President, Chief Executive Officer and Chairman of the Board. We do not have
a written employment agreement with Mr. Nielsen. In addition, we do not maintain
key person life insurance on Mr. Nielsen. Therefore, in the event of the loss or
unavailability of Mr. Nielsen, there can be no assurance that we would be able
to locate in a timely manner or employ qualified personnel to replace him. The
loss of the services of Mr. Nielsen or our failure to attract and retain other
key personnel over time would jeopardize our ability to execute our business
plan and could have a material adverse effect on our business, results of
operations and financial condition.
Future
financings could adversely affect common stock ownership interest and rights in
comparison with those of other security holders.
Our board
of directors has the power to issue additional shares of common or preferred
stock without stockholder approval. If additional funds are raised through the
issuance of equity or convertible debt securities, the percentage ownership of
our existing stockholders will be reduced, and these newly issued securities may
have rights, preferences or privileges senior to those of existing
stockholders. If we issue any additional common stock or securities
convertible into common stock, such issuance will reduce the proportionate
ownership and voting power of each other stockholder. In addition, such stock
issuances might result in a reduction of the book value of our common
stock.
The
manufacturing process we expect to use is not patented.
None of
the manufacturing processes we expect to use in producing our products are
subject to a patent or similar intellectual property protection. Although we are
in the process of obtaining intellectual property protection for our various
chewing gum products and their component parts, invention, currently our only
protection against a third party using our recipes and processes is
confidentiality agreements we expect to enter into with the companies that
produce our products and with our employees who have knowledge of such
processes. If our competitors develop substantially equivalent proprietary
information or otherwise obtain access to our knowledge, we will have greater
difficulty in competing with them for business, and creating our market
share.
Our
intellectual property patent pending does not assure that competitors or others
cannot eventually develop chewing gum similar or superior to the products we
expect to manufacture.
We regard
our intellectual property as critical to our future success. Our patents are
pending with the U.S. Patent and Trademark Office and the UK Intellectual
Property Office. We attempt to protect such property with registered
and common law protections, restrictions on disclosure and other actions to
prevent infringement. We believe product packages and artwork will be
important to our success and we would take action to protect against imitation
of our packaging and trade dress and to protect our trademarks and copyrights,
as necessary. We also expect to rely on a combination of laws and contractual
restrictions, such as confidentiality agreements, to establish and protect our
proprietary rights, trade dress and trade secrets. However, laws and contractual
restrictions may not be sufficient to protect the exclusivity of our
intellectual property rights, trade dress or trade secrets.
8
We cannot
be certain that any of our intellectual property will be granted a patent or if
they are so granted that such patent will provide meaningful protection. The
status of patents involves complex legal and factual questions and the breadth
of claims issued is uncertain. Accordingly, there can be no assurance any
patents that may be issued to us in the future will afford protection against
competitors with similar intellectual property. In addition, no assurances can
be given that patents to be issued in the future to us will not be infringed
upon, reverse engineered or designed around by others or that others will not
obtain patents that we would need to license reverse engineer or design around.
If future patents containing broad claims are upheld by the courts, the holders
of such patents could require other companies, which could potentially include
us, to obtain intellectual property licenses from them or else to reverse
engineer or design around those patents. If we are found to be infringing upon
third-party patents, there can be no assurance that we would be able to design
around or reverse engineer such patents or that any necessary licenses would be
available on reasonable terms, if at all.
There can
be no assurance that other third parties will not infringe or misappropriate our
trademarks and similar proprietary rights. We could incur substantial costs in
defending ourselves and potentially others in litigation or prosecuting
infringement claims against third parties. If the outcome of any such litigation
were unfavorable to us, our business, financial condition and results of
operations could be seriously impacted. If we lose some or all of our
intellectual property rights, our business may be materially and adversely
affected.
In
addition to patent protection, we will rely on the law of unfair competition and
trade secrets to protect our proprietary rights. We consider several elements of
our product design and production process to be trade secrets. We attempt to
protect our trade secrets and other proprietary information through agreements
with employees, consultants, subcontractors, customers and suppliers,
enforcement of state and federal statutory and common law, and other security
measures. However, third parties may independently develop substantially
equivalent proprietary information and techniques, or otherwise gain access to
our trade secrets or disclose such technology, which could have a detrimental
effect on our business, results of operations and financial condition. We cannot
be certain that our efforts to vigorously protect our rights will always be
successful.
We
may not be able to establish and manage our operations effectively.
We
commenced operations under our new business model in 2010 and anticipate rapid
growth in the future. We are in the process of establishing our business
capabilities in order to finalize the development and production of our products
and introduce our products and capture market opportunities. As we continue to
grow, we must continue to improve our operational and financial systems,
procedures and controls. In order to fund our on-going operations and our future
growth, we need to have sufficient internal sources of liquidity or access to
additional financing from external sources. Furthermore, our management will be
required to maintain and strengthen our relationships with our future LTO’s,
distributors, customers, suppliers and other third parties. As a result, our
anticipated expansion will place significant strains on our management
personnel, systems and resources. We also will need to further strengthen our
internal control and compliance functions to ensure that we will be able to
comply with our legal and contractual obligations and minimize our operational
and compliance risks. Our current and planned operations, personnel, systems,
internal procedures and controls may not be adequate to support our future
growth. If we are unable to manage our growth effectively, we may not be able to
take advantage of market opportunities, execute our business strategies or
respond to competitive pressures.
We
expect to depend on third parties to supply key raw materials to us. Failure to
obtain a sufficient supply of these raw materials in a timely fashion and at
reasonable costs could significantly delay or cancel our production and
shipments, which would have a material adverse impact on our ability to generate
revenue.
We expect
to purchase certain key raw materials from domestic and foreign suppliers on the
basis of purchase orders. We may not be able to obtain sufficient supply of
these raw materials from our suppliers or establish alternate suppliers in a
timely fashion or at a reasonable cost, or these raw materials may not comply
with our specifications. Our failure to secure a sufficient supply of
key raw materials would result in a significant delay or cancellation in our
production and shipments. Failure to obtain a sufficient supply of these raw
materials at a reasonable cost could harm our revenue, gross profit margins and
future growth prospects.
Fluctuations
in prices and availability of raw materials could increase our costs or cause
delays in shipments, which would adversely impact our business and results of
operations.
Our
operating results could be adversely affected by increases in the cost of raw
materials. If we are not been able to fully offset the effects of higher costs
of raw materials through price increases to customers or by way of productivity
improvements, our net profit will be harmed.
We
will be dependent on third-party manufacturers to create our products. If these
factories fail to properly follow the manufacturing process, resulting in
defective gum products, our reputation could be severely damaged and our sales
could be materially and adversely affected.
We expect
third party manufacturers to manufacture our chewing gum products. If
these third-party manufacturers fail to manufacture in accordance with our
specifications, our products will not gain widespread acceptance and our
reputation could be severely damaged. In addition, if these manufacturers are
unable to produce a sufficient number of products and we cannot timely find
qualified alternative manufacturers, our sales and results of operations could
be materially and adversely affected.
9
We
expect to rely on third parties whose operations are outside our
control.
We expect
to rely on arrangements with third-party manufacturers, suppliers, distributors,
shippers and carriers for production, shipment, storage and sales of our
products. As a result, we may be subject to disruptions and increased costs due
to factors that are beyond our control, including operational issues of those
third parties, labor strikes, inclement weather, natural disasters and rapidly
increasing fuel costs. If the services of any of these third parties become
unsatisfactory, we may experience delays in meeting our customers’ product
demands and we may not be able to find a suitable replacement on a timely basis
or on commercially reasonable terms or at all. Any failure of operations at any
such third parties’ business may harm our revenue and our future operating
prospects, damage our reputation and could cause us to lose
customers.
The
success of our business depends on our ability to attract, train and retain
highly skilled employees and key personnel.
Because
of the specialized nature of our business, we must attract, train and retain a
workforce comprised of highly skilled employees and other key personnel. As we
are commencing operations and expect our business to grow rapidly, our ability
to train and integrate new employees into our operations may not meet the
requirements of our growing business. Our failure to attract, train or retain
highly skilled employees and other key personnel in numbers that are sufficient
to satisfy our needs would materially and adversely affect our
business.
Risks
Concerning our Securities
Our
common stock has a limited trading market.
While our
common stock is currently quoted on the Pink Sheets under the symbol
PINK:ALTA, an active trading market has not developed and we do not have a
significant public float. In the absence of an active trading market,
you may have difficulty buying and selling or obtaining market quotations for
our stock, the market visibility for our stock may be limited, and the lack of
visibility for our common stock may have a depressive effect on the market price
for our common stock.
Preferred
Stock may limit financing and business combination opportunities.
Our
ability to issue 10,000,000 shares of preferred stock (none of which are
currently outstanding) may limit our ability to obtain debt or equity financing
as well as impede our potential takeover, which takeover may be in the best
interest of stockholders. Our ability to issue these authorized but unissued
securities may also negatively impact our ability to raise additional capital
through the sale of our debt or equity securities.
Failure
to achieve and maintain effective internal controls in accordance with Section
404 of the Sarbanes-Oxley Act could have a material adverse effect on our
business and operating results. In addition, current and potential
stockholders could lose confidence in our financial reporting, which could have
a material adverse effect on our stock price.
Effective
internal controls are necessary for us to provide reliable financial reports and
effectively prevent financial fraud. We are required to document and test our
internal control procedures in order to satisfy the requirements of Section 404
of the Sarbanes-Oxley Act, which requires annual management assessments of the
effectiveness of our internal controls over financial reporting for the year
ending September 30, 2010. During the course of our testing, we may
identify deficiencies which we may not be able to remediate in time to meet the
deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements
of Section 404. In addition, if we fail to maintain the adequacy of our internal
controls, as such standards are modified, supplemented or amended from time to
time, we may not be able to ensure that we can conclude on an ongoing basis that
we have effective internal controls over financial reporting in accordance with
Section 404 of the Sarbanes-Oxley Act. Failure to achieve and maintain an
effective internal control environment could also cause investors to lose
confidence in our reported financial information, which could have a material
adverse effect on our stock price.
We
are likely to remain subject to “penny stock” regulation and as a consequence
there are additional sales practice requirements and additional warning issued
by the SEC.
As long
as the trading price of our common stock is below $5.00 per share, the
open-market trading of our common stock will be subject to the “penny stock”
rules of the SEC. The “penny stock” rules impose additional sales practice
requirements on broker-dealers who sell securities to persons other than
established customers and accredited investors (generally those with assets in
excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together
with their spouse). For transactions covered by these rules, the broker-dealer
must make a special suitability determination for the purchase of securities and
have received the purchaser’s written consent to the transaction before the
purchase. Additionally, for any transaction involving a penny stock, unless
exempt, the broker-dealer must deliver, before the transaction, a disclosure
schedule prescribed by the SEC relating to the penny stock market. The
broker-dealer also must disclose the commissions payable to both the
broker-dealer and the registered representative and current quotations for the
securities. Finally, monthly statements must be sent disclosing recent price
information on the limited market in penny stocks. These additional burdens
imposed on broker-dealers may restrict the ability of broker-dealers to sell the
common stock and may affect a stockholder’s ability to resell the common
stock.
10
There can
be no assurance that our common stock will qualify for exemption from the “penny
stock” rules. In any event, even if our common stock is exempt from such rules,
we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the
SEC the authority to restrict any person from participating in a distribution of
a “penny stock” if the SEC finds that such a restriction would be in the public
interest.
Stockholders
should be aware that, according to SEC Release No. 34-29093, the market for
penny stocks has suffered in recent years from patterns of fraud and abuse. Such
patterns include (i) control of the market for the security by one or a few
broker-dealers that are often related to the promoter or issuer; (ii)
manipulation of prices through prearranged matching of purchases and sales and
false and misleading press releases; (iii) boiler room practices involving
high-pressure sales tactics and unrealistic price projections by inexperienced
sales persons; (iv) excessive and undisclosed bid-ask differential and markups
by selling broker-dealers; and (v) the wholesale dumping of the same securities
by promoters and broker-dealers after prices have been manipulated to a desired
level, along with the resulting inevitable collapse of those prices and with
consequent investor losses. Our management is aware of the abuses that have
occurred historically in the penny stock market.
Item
1B—Unresolved Staff Comments
None
Item
2 - Properties
The
Company currently maintains a mailing address and rents virtual office
facilities at 228 Hamilton Avenue - 3rd Floor Palo Alto, CA 94301. The Company’s
telephone number there is (650) 209-8084. We also have a rented
office in Denmark at Birkedommervej 30, 2400 Kobenhagen, Denmark.
Item
3 - Legal Proceedings
The
Company and its properties are not a party or subject to any pending legal
proceedings, and no such proceedings are known to be contemplated.
Item
4 - Submission of Matters to a Vote of Security Holders
None.
PART
II
Item
5 - Market for the Registrant’s Common Equity, Related Stockholder Matters and
Issuer Purchases of Equity Securities
Market
for Trading and Eligibility for Future Sale
The
Company’s common stock is currently being quoted on the Pink Sheets under the
trading symbol is PINK:ALTA. Until September 21, 2010, our common stock was
listed for quotation on the Over-the-Counter Bulletin Board under the symbol
OTC:JEDE. As of September 21, 2010, our common stock failed to comply
with Rule 15c2-11 and was delisted to the Pink Sheets. On November 1,
2010, our trading symbol changed to “ALTA.” The following tables set
forth, for the calendar quarter indicated, the range of high and low bid
information for our common stock as reported on the OTC Bulletin
Board. Trading in the common stock on the OTCBB and Pink Sheets has
been limited and sporadic and the quotations set forth below are not necessarily
indicative of actual market conditions. Further, the quotations
merely reflect the prices at which transactions were proposed, and do not
necessarily represent actual transactions.
|
High
|
Low
|
|||||||
|
2010
by Quarter
|
||||||||
|
July
1, 2010 - September 30, 2010
|
$ | 0.25 | $ | 0.10 | ||||
|
April
1, 2010 – June 30, 2010
|
$ | N/A | $ | N/A | ||||
|
January,
2010 - March 31, 2010
|
$ | N/A | $ | N/A | ||||
|
October
1, 2009 - December 31, 2009
|
$ | N/A | N/A | |||||
|
2009
by Quarter
|
||||||||
|
July
1, 2009 - September 30, 2009
|
$ | N/A | N/A | |||||
|
April
1, 2009 – June 30, 2009
|
$ | N/A | $ | N/A | ||||
|
January,
2009 - March 31, 2009
|
$ | N/A | $ | N/A | ||||
|
October
1, 2008 - December 31, 2008
|
$ | N/A | N/A | |||||
11
Holders
As of
January 10, 2011, there were 92,980,000 shares of our common stock outstanding
held by approximately 45 stockholders of record. The number of our
stockholders of record excludes any estimate by us of the number of beneficial
owners of shares held in street name, the accuracy of which cannot be
guaranteed.
Dividend
Policy
We have
not paid any cash dividends on our common stock to date, and we have no
intention of paying cash dividends in the foreseeable future. Whether we will
declare and pay dividends in the future will be determined by our board of
directors at their discretion, subject to certain limitations imposed under the
Nevada Corporations Law. The timing, amount and form of dividends, if
any, will depend on, among other things, our results of operations, financial
condition, cash requirements and other factors deemed relevant by our board of
directors.
Recent
Sales of Unregistered Securities
On
December 21, 2010, we consummated the first closing of 250,000 shares of common
stock of the Company for a purchase price of $0.20 per share for an aggregate of
$50,000 in a private placement of a minimum of 200,000 and a maximum of
2,000,000 shares of our common stock. The issuance was exempt
pursuant to Section 4(2) of the Securities Act of 1933, as amended, and/or Rule
506 promulgated thereunder or Regulation S as it applies to non-U.S.
Persons.
On July
23, 2010, we entered into a $50,000 unsecured convertible promissory note (the
“Promissory Note”) with Paramount Trading Company Inc. (the
“Lender”). Under the terms of the Promissory Note, the Lender will
receive the principal amount of $50,000, plus interest at the rate of 12% per
annum, on July 24, 2011; provided, however, the Lender shall have the right to
convert all, or any portion, of the outstanding principal plus all accrued
interest into a number of fully paid and non-assessable whole shares of the
Company's common stock as derived from the fair market value of the Company’s
common stock at the time of exercise. The Promissory Note may be prepaid by the
Company at any time without penalty or premium. The issuance was exempt pursuant
to Section 4(2) of the Securities Act of 1933, as amended, and/or Rule 506
promulgated thereunder.
On May 3,
2010, we issued to Mr. Nielsen 55,000,000 shares of our common stock in exchange
for Mr. Nielsen having transferred his right, title and interest in all
intellectual property relating to certain chewing gum compositions having
appetite suppressant activity. The issuance was exempt pursuant to Section 4(2)
of the Securities Act of 1933, as amended, and/or Rule 506 promulgated
thereunder.
On
January 5, 2010, we issued 33,330,000 shares of our common stock to Murrayfield
Limited in a private transaction for $50,000. The issuance was exempt
pursuant to Section 4(2) of the Securities Act of 1933, as amended, and/or Rule
506 promulgated thereunder.
Repurchases
of Equity Security
None
Item
6 - Selected Financial Data
Not
applicable.
Item
7 - Management’s Discussion and Analysis of Financial Condition and Results of
Operations
(1) Caution
Regarding Forward-Looking Information
Certain
statements contained in this annual filing, including, without limitation,
statements containing the words "believes", "anticipates", "expects", “intend”,
“estimate”, “plan” and words of similar import, constitute forward-looking
statements. Such forward-looking statements involve known and unknown risks,
uncertainties and other factors that may cause the actual results, performance
or achievements of the Company, or industry results, to be materially different
from any future results, performance or achievements expressed or implied by
such forward-looking statements.
12
Forward-looking
statements are based on our current expectations and assumptions regarding our
business, potential target businesses, the economy and other future conditions.
Because forward-looking statements relate to the future, by their nature, they
are subject to inherent uncertainties, risks and changes in circumstances that
are difficult to predict. Our actual results may differ materially from those
contemplated by the forward-looking statements. We caution you therefore that
you should not rely on any of these forward-looking statements as statements of
historical fact or as guarantees or assurances of future performance. Important
factors that could cause actual results to differ materially from those in the
forward-looking statements include changes in local, regional, national or
global political, economic, business, competitive, market (supply and demand)
and regulatory conditions and the following:
|
|
•
|
our
status as a development stage
company;
|
|
|
•
|
conflicts
of interest of our officers and
directors;
|
|
|
•
|
our
ability to obtain additional financing if
necessary;
|
|
|
•
|
our
dependence on our key personnel;
|
|
|
•
|
business
and market outlook;
|
|
|
•
|
future
effective tax rates; and
|
|
|
•
|
compliance
with applicable laws.
|
These
risks and others described under “Risk Factors” are not exhaustive.
Given
these uncertainties, readers of this Form 10-K and investors are cautioned not
to place undue reliance on such forward-looking statements. The Company
disclaims any obligation to update any such factors or to publicly announce the
result of any revisions to any of the forward-looking statements contained
herein to reflect future events or developments.
Results
of Operations
We have
generated no revenue to date since inception and we do not anticipate earning
revenues until such time that we enter into contract for the purchase of our
chewing gum products. We have recently begun the development stage of
our business and we can provide no assurance that we will be able to develop,
produce or sell commercial gum compositions. The ingredients
Alterola expects to be developing will have documented effect on the treatments
in focus. If the products have proven active composition, we will seek to
enter into commercial production.
We
incurred operating expenses from operations in the amount of $66,169 for the
three months ended September 30, 2010, compared with $12,745 for the comparative
period of 2009. Our expenses for the three months ended September 30, 2010
increased from the same period largely as a result of the change in business
direction which significantly increased legal fees and the concentration on the
commercialization of our Nutraceutical gum IP. We anticipate that
future operating expenses will continue to increase as a result of
commercializing the Nutraceutical gum IP. There will be significant investment
required to develop, produce and distribute the gum products. We
incurred operating expenses from operations in the amount of $126,781 for the
year ended September 30, 2010, compared with $97,328 for the year ended
September 30, 2009.
The main
reasons for the change in expenses incurred from operations for the year ended
September 30, 2010 compared to the prior year were: an increase in accounting
and audit fees to $41,156 (2009- $31,895), and an increase in legal fees to
$38,759 (2009 - $27,064) due to the Company entering into a monthly legal
services agreement rather than paying per diem. Transfer and filing fees
increased to $3,456 (2009 - $3,405). Consulting fees increased to $9,930
(2009 - $4,000) and management fees increased to $19,900 (2009 - $12,000) as a
result of a change in management and the board of directors in May
2010. Travel expenses increased to $6,700 (2009 - $270) due to
our CEO Soren Nielsen making business trips to Singapore, Denmark, Norway, Basle
and Malaysia in order to meet with potential partners, suppliers, manufacturers
and other complimentary businesses to further Alterola’s endeavors; Rent
increased to $2,888 (2009 - $nil) as a result of opening corporate offices in
Copenhagen, Denmark. Communication costs increased to $1,212 (2009 -
$nil) also as a result of opening the office in Copenhagen. Mineral
property option and exploration costs decreased to $1,866 (2009 - $18,007) as a
result of the change in business focus of the Company. Other expenses
incurred were relatively unchanged period to period. We would expect that legal,
accounting, consulting and management fees will increase in the future as a
result of working toward the commercialization of our gum products. We
anticipate adding new staff to our organization and working with consultants to
execute our business plan.
We
incurred operating expenses of $256,550 in the period from July 21, 2008 (Date
of Inception) through September 30, 2010. The operating expenses
consisted primarily of accounting and audit expenses of $73,801, legal fees of
$69,335; management fees of $34,134; consulting fees of $13,930; mineral
property option and exploration costs of $19,873; transfer and filing fees of
$6,861; stock based compensation of $26,000; travel of $6,970; rent of $2,888;
communication costs of $1,212; interest on notes payable of $2,090 and bank
charges of $1,217.
13
We
recorded a net loss of $66,169 for the three months ended September 30, 2010,
compared with $12,745 for the comparative period in 2009. We recorded
a net loss of $128,871 for the year ended September 30, 2010, compared with loss
of $97,238 for the year ended September 30, 2009. We also have
incurred accumulated losses of $258,640 for the period from July 21, 2008 (Date
of Inception) until September 30, 2010. The changes in the net loss between the
periods can be attributed to the change of business direction and focus on the
commercialization of our IP. The focus on creating a commercial gum product will
require significant future investment and will likely result in further net
losses during the development, production and distribution phases.
Liquidity
and Capital Resources
As of
September 30, 2010, we had total current assets of $528. We had
$85,750 in current liabilities as of September 30, 2010. Thus, we had a negative
working capital balance of $85,222 as of September 30, 2010.
At
September 30, 2009, our previous year end, we had current assets of $7,224,
current liabilities of $2,375 and thus we had a working capital balance of
$4,849 at September 30, 2009.
During
the year ended September 30, 2010, the Company issued 33,330,000 shares of
common stock for $0.0015 for gross proceeds of $50,000.
The
Company also raised an additional $30,000 by way of a promissory note, and a
further $50,000 by issuing convertible promissory notes during the year ended
September 30, 2010.
Our
assets changed during the period due to the disposal of our mining operations
and acquisition of the Nutraceutical gum IP. We anticipate that continuing
the commercialization of the gum products and receiving patent protection for
our IP will increase the value of our assets. Our current cash reserve is not
sufficient to continue operations for more than 6 months and we will need to
raise additional capital to execute on our business plan. We expect to raise
additional capital to fund our operations until the point at which we can
commercialize our products and reach operating profitability. In the short term,
we will also rely on related party advances to cover the cost of operations
until we are able to consummate a private placement of our equity securities,
although no related parties are required to or have presently agreed to advance
such funds. There are no assurances that we will be successful in closing
a private placement or related party advances, and this poses a significant risk
to our continuing operations.
We have
not attained profitable operations and are dependent upon obtaining financing to
pursue significant IP development activities beyond those planned for the
current fiscal year.
Off
Balance Sheet Arrangements
As of
September 30, 2010, there were no off balance sheet arrangements.
Going
Concern
These
financial statements have been prepared in accordance with generally accepted
accounting principles applicable to a going concern, which assumes that the
Company will be able to meet its obligations and continue its operations for its
next twelve months. Realization values may be substantially different
from carrying values as shown and these financial statements do not give effect
to adjustments that would be necessary to the carrying values and classification
of assets and liabilities should the Company be unable to continue as a going
concern. At September 30, 2010, the Company has negative working
capital of $85,222, has yet to achieve profitable operations, has accumulated
losses of $258,640 since its inception (July 21, 2008) and expects to incur
further losses in the development of its business, all of which casts
substantial doubt about the Company’s ability to continue as a going
concern.
The
Company’s ability to continue as a going concern is dependent upon its ability
to generate future profitable operations and/or to obtain the necessary
financing from shareholders or other sources to meet its obligations and repay
its liabilities arising from normal business operations when they become
due. Management has no formal plan in place to address this concern
but considers that the Company will be able to obtain additional funds by equity
or debt financing and/or related party advances, however there is no assurance
of additional funding being available or on acceptable terms, if at
all.
Item 7A - Quantitative and
Qualitative Disclosures about Market Risk
We are a
“smaller reporting company” as defined by Regulation S-K and as such, are not
required to provide the information contained in this item pursuant to
Regulation S-K.
14
Item
8 - Financial Statements and Supplementary Data
The
required financial statements begin on page F-1 of this document.
Item
9 - Changes in and Disagreements with Accountants on Accounting and Financial
Disclosure
During
the Company’s fiscal years ended September 30, 2009 and 2008, and through July
22, 2010, there were no disagreements with BDO Canada on any matter of
accounting principles or practices, financial statement disclosure, or auditing
scope and procedure, which disagreements, if not resolved to the satisfaction of
BDO Canada, would have caused BDO Canada to make reference thereto in its
reports on the financial statements. The Company engaged De Joya
Griffith & Company, LLC (“De Joya”) as its auditors as of July 23,
2010. As of July 23, 2010 and through the date of this Annual Report,
there have been no disagreements with De Joya on any matter of accounting
principles or practices, financial statement disclosure, or auditing scope and
procedure, which disagreements, if not resolved to the satisfaction of De Joya,
would have caused De Joya to make reference thereto in its reports on the
financial statements.
Item
9A - Controls and Procedures
Disclosure
Controls and Procedures. Our management, under the supervision and with
the participation of our certifying officer, who is our sole officer and acts as
our chief executive officer and chief financial officer, (the “Certifying
Officer”) has evaluated the effectiveness of our disclosure controls and
procedures as defined in Rules 13a-15 promulgated under the Exchange Act as of
the end of the period covered by this Annual Report. Based on such evaluation,
our Certifying Officer has concluded that, as of the end of the period covered
by this Annual Report, our disclosure controls and procedures were not
effective. Disclosure controls and procedures are controls and procedures
designed to ensure that information required to be disclosed in our reports
filed or submitted under the Exchange Act is recorded, processed, summarized and
reported within the time periods specified in the SEC’s rules and forms and
include controls and procedures designed to ensure that information we are
required to disclose in such reports is accumulated and communicated to
management, including our Certifying Officer, as appropriate to allow timely
decisions regarding required disclosure.
Management’s
Annual Report on Internal Control over Financial Reporting. Management is
responsible for establishing and maintaining adequate internal control over
financial reporting, as such term is defined in Rule 13a-15(f) of the Exchange
Act.
Internal
control over financial reporting is defined under the Exchange Act as a process
designed by, or under the supervision of, our Certifying Officer and effected by
our board of directors, management and other personnel, to provide reasonable
assurance regarding the reliability of financial reporting and the preparation
of financial statements for external purposes in accordance with generally
accepted accounting principles and includes those policies and procedures
that:
|
|
Pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of our
assets;
|
|
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted
accounting principles, and that our receipts and expenditures are being
made only in accordance with authorizations of our management and
directors; and
|
|
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of our assets that could have
a material effect on the financial
statements.
|
Because
of its inherent limitation, internal control over financial reporting may not
prevent or detect misstatements. Also, projections of any evaluations of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies and procedures may deteriorate. Accordingly, even an effective
system of internal control over financial reporting will provide only reasonable
assurance with respect to financial statement preparation.
Our
management, with the participation of our Certifying Officer, evaluated the
effectiveness of the Company’s internal control over financial reporting as of
September 30, 2010. In making this assessment, our management used the criteria
set forth by the Committee of Sponsoring Organizations of the Treadway
Commission in Internal Control - Integrated Framework. Based on this evaluation
and those criteria, our management, with the participation of our Certifying
Officer, concluded that, as of September 30, 2010, our internal control over
financial reporting were not effective based on management’s identification of
the material weaknesses as follows:
Lack of Segregation of
Duties. There is a lack of segregation of duties at the
Company because only one employee, the Certifying Officer of the Company,
is charged with the responsibilities of general administrative and financial
matters. Management will continue to evaluate the material weaknesses and
expects to remedy these weaknesses identified above and have already engaged
Sarbanes Oxley compliance consultants to identify the areas of weakness and to
suggest remedial measures.
15
(b) Changes in Internal
Control over Financial Reporting.
There
were no significant changes in the Company's internal control over financial
reporting that occurred during fiscal 2010 that have materially affected, or are
reasonably likely to materially affect, such control.
This
Annual Report does not include an attestation report of our registered public
accounting firm regarding our internal control over financial reporting.
Management’s report was not subject to attestation by our registered public
accounting firm pursuant to temporary rules of the SEC that permit us to provide
only management’s report in this Annual Report.
There
have not been any changes in our internal control over financial reporting (as
defined in Exchange Act Rules 13a-15(f)) that occurred during the quarter ended
September 30, 2010 that have materially affected, or are reasonably likely to
materially affect, our internal control over financial reporting.
Item.
9B -Other Information.
On
December 21, 2010, we consummated the first closing of 250,000 shares of common
stock of the Company for a purchase price of $0.20 per share for an aggregate of
$50,000 in a private placement of a minimum of 200,000 and a maximum of
2,000,000 shares of our common stock. The issuance was exempt
pursuant to Section 4(2) of the Securities Act of 1933, as amended, and/or Rule
506 promulgated thereunder or Regulation S as it applies to non-U.S.
Persons.
PART
III
Item
10 - Directors, Executive Officers and Corporate Governance
The
directors and executive officers serving the Company are as
follows:
|
Name
|
Age
|
Position Held
|
Tenure
|
||||
|
Soren
Nielsen
|
40
|
President,
Secretary, Treasurer, Chief Executive Officer, and Chief Financial Officer
and Director
|
Mr.
Nielsen has served as our director since October 30, 2009 and was
appointed as President, Secretary, Treasurer, Chief Executive Officer, and
Chief Financial Officer on May 3,
2010.
|
Biographical
Information
Soren Nielsen, age 40, was
appointed and became the President, Secretary, Treasurer, Chief Executive
Officer, and Chief Financial Officer of the Company on May 3,
2010. Nielsen has served as a member of the Board since October 30,
2009. As of November 2010, Mr. Nielsen has served as the CEO and a
director of Firstrank1, a UK entity. From 2008 to 2009, Mr. Nielsen
was a Service Executive for Microsoft where he structured, marketed and sold
strategic information technology projects and complex service
engagements. Mr. Nielsen served as a sales manager of Steria A/S,
Skovlunde in Denmark between 2000 and 2007, where he developed new business
opportunities, prepared sales budgets and negotiated large
transactions. Nielsen received his Bachelors degree in marketing from
the University of Huddersfield in the United Kingdom.
Director
Qualification
Mr. Nielsen is well-qualified to serve
as our director because he has over 10 years experience in developing business
opportunities and sales. Mr. Nielsen has experience with early
development stage companies through their growth into multimillion dollar
operating businesses. We believe Mr. Nielsen’s experience in
structuring, marketing and sales of complex business opportunities will further
our purpose of growth and development of the Company.
Compliance
With Section 16(a) of the Exchange Act
Section
16(a) of the Exchange Act requires our executive officers and directors and
person who own more than 10% of our common stock to file reports regarding
ownership of and transactions in our securities with the Commission and to
provide us with copies of those filings. Based solely on our review of the
copies received by or a written representation from certain reporting persons we
believe that during fiscal year ended September 30, 2010, we believe that all
eligible persons are in compliance with the requirements of Section
16(a).
16
Code
Of Ethics
The
Company has adopted a code of ethics. The Company expects to post the text
of such code of ethics to its Internet website as soon as such website is
launched. The Company has filed a copy of its code of ethics as an
exhibit to this Annual Report and will make available without charge, upon
request, a copy of such code of ethics to any person until such time as the code
of ethics is available on the Company’s website.
Corporate
Governance
There are
no material changes to the procedures by which security holders may recommend
nominees to the Company’s board of directors since the Company last reported on
such procedures.
Director
Independence and Board Committees
Based on
the NASDAQ definition of director independence, our sole director, Mr. Nielsen
is not an independent director. Our board of directors presently does
not have a standing audit, nominating or compensation committees and the entire
board is performing the functions normally associated with an audit, nominating
and compensation committee. We expect that we will seek to form audit
and other board committees in a manner consistent with exchange-listed companies
if we apply for a listing on an exchange. It is the Company’s goal to
add at least one director who is a financial expert.
Item
11 - Executive Compensation
The table
below summarizes all compensation awarded to, earned by, or paid to both our
officers and directors for all services rendered in all capacities to us for our
fiscal years ended September 30, 2010 and 2009.
|
SUMMARY
COMPENSATION
TABLE
|
||||||||||||||||||||||||||||||||||||
|
Name
and
principal
position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive
Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||||||||
|
Soren
Nielsen, CEO, CFO, President, Secretary-Treasurer
|
2010
|
0 | 0 | 0 | 0 | 0 | 0 | $ | 26,800 | * | 0 | |||||||||||||||||||||||||
|
Ola
Juvkam-Wold, former
CEO,
CFO, President, Secretary-Treasurer
|
2009
2010**
|
0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
* All
other compensation consisted of: (i) management fees of $15,900; (ii) travel
expenses of $6,700; (iii) rent expenses of $2,888; and (iv) communication costs
of $1,212. FirsRank1, an entity wholly owned and controlled by Mr. Nielsen,
invoices the Company for fees and expenses incurred by Mr. Nielsen in his
capacity as the sole officer and director of the Company. There is no
formal agreement between Firstrank1 and the Company. Mr. Nielsen did
not receive any other compensation from the Company during the two most
recently completed fiscal years. In future periods, the Company anticipates that
it will pay compensation to its officer(s) and/or director(s).
**Prior
to his departure from the Company, Mr. Juvkam-Wold received $1,000 per month
(totaling $4,000 for the year ended September 30, 2010) through his company Oro
Enterprises Ltd. with respect to a Corporate Management Service Agreement with
our company.
17
Outstanding Equity Awards at Fiscal
Year-End
The table
below summarizes all unexercised options, stock that has not vested, and equity
incentive plan awards for each named executive officer for the fiscal year ended
September 30, 2010.
|
OUTSTANDING
EQUITY AWARDS AT FISCAL
YEAR-END
|
||||||||||||||||||||||||||||||||||||
|
OPTION
AWARDS
|
STOCK
AWARDS
|
|||||||||||||||||||||||||||||||||||
|
Name
|
Number
of
Securities
Underlying
Unexercised
Options
(#)
Exercisable
|
Number
of
Securities
Underlying
Unexercised
Options
(#)
Unexercisable
|
Equity
Incentive
Plan
Awards:
Number
of
Securities
Underlying
Unexercised
Unearned
Options
(#)
|
Option
Exercise
Price
($)
|
Option
Expiration
Date
|
Number
of
Shares
or
Units
of
Stock
That
Have
Not
Vested
(#)
|
Market
Value
of
Shares
or
Units
of
Stock
That
Have
Not
Vested
($)
|
Equity
Incentive
Plan
Awards:
Number
of
Unearned
Shares,
Units
or
Other
Rights
That
Have
Not
Vested
(#)
|
Equity
Incentive
Plan
Awards:
Market
or
Payout
Value
of
Unearned
Shares,
Units
or
Other
Rights
That
Have
Not
Vested
(#)
|
|||||||||||||||||||||||||||
|
Soren
Nielsen
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Ola
Juvkam-Wold
|
- | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
Compensation
of Directors
The table
below summarizes all compensation of our directors for the fiscal years
completed September 30, 2010 and 2009.
|
DIRECTOR
COMPENSATION
|
||||||||||||||||||||||||||||
|
Name
|
Fees
Earned
or
Paid
in
Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive
Plan
Compensation
($)
|
Non-
Qualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||
|
Soren
Nielsen
|
- | - | - | - | - | - | - | |||||||||||||||||||||
|
Ola
Juvkam-Wold
|
- | - | - | - | - | - | - | |||||||||||||||||||||
Compensation
Committee Interlocks and Insider Participation
The
Company does not have a compensation committee and our entire board of
directors, consisting of Soren Nielsen, participates in deliberations of the
compensation of the Company’s directors and officers.
Compensation
Committee Report
Soren
Nielsen has reviewed the Compensation Discussion and Analysis required by Item
402(b), and based on such review determined that the Compensation Discussion and
Analysis should not be included in the Company’s Annual Report on Form
10-K.
Item
12. Security Ownership of Certain Beneficial Owners and Management and
Related Stockholder Matters.
The table
sets forth below certain information regarding the beneficial ownership of our
common stock as of January 10, 2011, based on 92,980,000 aggregate shares of
common stock outstanding as of such date, by: (i) each person who is known by us
to own beneficially more than 5% of our outstanding common stock with the
address of each such person, (ii) each of our present directors and officers,
and (iii) all officers and directors as a group.
18
Beneficial
ownership is determined in accordance with SEC rules and generally includes
voting or investment power with respect to securities. Unless otherwise
indicated, the stockholders listed in the table have sole voting and investment
power with respect to the shares indicated. Unless otherwise noted, the
principal address of each of the stockholders, directors and officers listed
below is Alterola Biotech Inc., 228 Hamilton Avenue, 3rd Floor,
Palo Alto, CA 94301.
All share
ownership figures include shares of our Common Stock issuable upon securities
convertible or exchangeable into shares of our Common Stock within sixty (60)
days of January 3, 2011, which are deemed outstanding and beneficially owned by
such person for purposes of computing his or her percentage ownership, but not
for purposes of computing the percentage ownership of any other
person.
|
Name of Beneficial Owner
|
Shares of Common
Stock Owned
|
Percent of Class
Beneficially Owned
|
||||||
|
Soren
Nielsen
|
15,000,000 | 16 | % | |||||
|
Murrayfield
Limited (1)
|
33,330,000 | 36 | % | |||||
|
All
Executive Officers and Directors as a group (1 person)
|
15,000,000 | 16 | % | |||||
|
|
(1)
|
Steven
Drayton is the beneficial owner of Murryfield Limited having a business
address of c/o Eurohelvetia Trust Co. S.A., World Trade Center 1, P.O. Box
69, 1215, Geneva.
|
Equity
Compensation Plans
The
Company does not currently have any equity compensations plans and may adopt
such plans in the future.
Changes
in Control
There are
currently no arrangements which may result in a change in control of the
Company.
Item
13 - Certain Relationships and Related Transactions, and Director
Independence
Transactions
with Related Persons, Promoters and Certain Control Persons
On May 3,
2010, the Company entered into each of the Stock Purchase Agreement and Release
and Settlement with Juvkam-Wold, who was then Chief Executive Officer and a
director of the Company. Pursuant to the Stock Purchase Agreement,
Juvkam-Wold agreed to transfer all 55,000,000 shares of Common Stock owned by
him to the Company in consideration for all the issued and outstanding stock of
the Company’s wholly owned subsidiary, JRE, and the cancellation of all debt
owed by JRE to the Company. Pursuant to the Release and Settlement,
Juvkam-Wold released the Company from any and all claims Juvkam-Wold may have
against the Company or its affiliates. The operations of JRE include
the Property Option Agreement. The sale of all issued and outstanding
stock of JRE was approved by the Board. Simultaneously with the
consummation of the Stock Purchase Agreement Juvkam-Wold resigned from his
positions as Chief Executive Officer of the Company and as a member of the
Board.
On May 3,
2010, the Company entered into an IP Agreement with Mr. Nielsen, our Chief
Executive Officer and director, pursuant to which Mr. Nielsen transferred his
right, title and interest in all IP relating to certain chewing gum compositions
having appetite suppressant activity to the Company in consideration for the
issuance of 55,000,000 newly issued shares of Common Stock.
On
November 17, 2010, Mr. Nielsen entered into a stock cancellation agreement with
the Company whereby 40,000,000 shares of common stock were returned to treasury
and cancelled. In consideration the Company will issue to Mr. Nielsen
options to acquire common stock pursuant to a stock option plan which will be
adopted by the Company at some time in the future. In accordance with
SAB Topic 4-C, the Company recorded the cancellation retroactively as of
September 30, 2010, as a reduction to the par value of common stock with a
corresponding increase to additional paid-in capital.
FirsRank1,
an entity wholly owned and controlled by Mr. Nielsen invoices, the Company for
fees and expenses incurred by Mr. Nielsen in his capacity as the sole officer
and director of the Company. There is no formal agreement between
Firstrank1 and the Company.
Director
Independence
The
Company currently has one director, and is not required to have a majority of
independent directors for such time as the Company’s securities continue to be
quoted on the Pink Sheets.
19
Item
14 - Principal Accountant Fees and Services
The
following table sets forth fees paid or payable to our independent registered
public accounting firm during the fiscal years ended September 30, 2010, 2009
and 2008. On July 22, 2010, the Company notified BDO Canada LLP that
it was dismissed as the Company’s independent registered public accounting
firm. On July 23, 1010, the Board of Directors of the Company
approved the engagement of De Joya Griffith & Company as the Company’s
independent registered public accounting firm.
|
Year
ended
|
Year
ended
|
Year
ended
|
||||||||||
|
September
30,
|
September
30,
|
September
30,
|
||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
1.
Audit fees
|
$ | 41,156 | $ | 31,895 | $ | 13,869 | ||||||
|
2.
Audit-related fees
|
- | - | - | |||||||||
|
3.
Tax fees
|
2,200 | - | 1,575 | |||||||||
|
4.
All other fees
|
- | - | - | |||||||||
|
Totals
|
$ | 43,356 | $ | 31,895 | $ | 15,444 | ||||||
The
category of “Audit Fees” includes fees for our annual audit and quarterly
reviews.
The
category of “Tax Fees” includes tax consultation and planning fees and tax
compliance services.
We have
considered whether the provision of any non-audit services, currently or in the
future, is compatible with De Joya Griffith & Company, LLC maintaining its
independence and have determined that these services do not compromise their
independence.
Financial
Information System Design and Implementation: De Joya Griffith & Company,
LLC did not charge the Company any fees for financial information system design
and implementation fees.
The
Company has no formal audit committee. However, the Board of Directors is the
Company's de facto audit committee. In discharging its oversight responsibility
as to the audit process, the Board obtained from the independent auditors a
formal written statement describing all relationships between the auditors and
the Company that might bear on the auditors' independence as required by the
appropriate Professional Standards issued by the Public Company Accounting
Oversight Board, the U. S. Securities and Exchange Commission and/or the
American Institute of Certified Public Accountants. The Board discussed with the
auditors any relationships that may impact their objectivity and independence,
including fees for non-audit services, and satisfied itself as to the auditors'
independence. The Board also discussed with management, the internal auditors
and the independent auditors the quality and adequacy of the Company's internal
controls.
The
Company’s principal accountant, De Joya Griffith & Company, LLC, did not
engage any other persons or firms other than the principal accountant’s
full-time, permanent employees.
Item
15 - Exhibits, Financial Statement Schedules
|
Exhibit
Number
|
Description
|
|
3.1
|
Articles
of Incorporation, as amended (1)
|
|
3.2
|
Bylaws,
as amended (1)
|
|
10.1
|
Intellectual Property Assignment Agreement entered
into on May 3, 2010 between the Company and Soren
Nielsen(2)
|
|
10.2
|
Stock Purchase Agreement, entered into on May 3,
2010, between the Company and Ola Juvkam-Wold(2)
|
|
10.3
|
Promissory
Note between the Company and Murrayfield Limited, dated June 25,
2010.(3)
|
|
10.4
|
Promissory
Note between the Company and Paramount Trading Company Inc., dated July
23, 2010.(4)
|
|
14
|
Code
of Conduct and Ethics
|
|
21.1
|
Subsidiaries
|
|
31.1
|
Certification of Chief Executive Officer pursuant
to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant
to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2
|
Certification of Chief Financial Officer pursuant
to Securities Exchange Act Rule 13a-14(a)/15d-14(a), as adopted pursuant
to Section 302 of the Sarbanes-Oxley Act of
2002
|
20
|
32.1
|
Certification of Chief Executive Officer pursuant
to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002
|
|
32.2
|
Certification of Chief Executive Officer pursuant
to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002
|
|
1
|
Incorporated
by reference to the Registration Statement on Form S-1 filed on December
12, 2008.
|
|
2
|
Incorporated
by reference to the Current Report on Form 8-K filed on May 7,
2010.
|
|
3
|
Incorporated
by reference to the Current Report on Form 8-K filed on June 29,
2010.
|
|
4
|
Incorporated
by reference to the Current Report on Form 8-K filed on July 28,
2010.
|
21
SIGNATURES
Pursuant
to the requirements of Section 13 or 15(d) of the Securities Exchange Act of
1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned, thereunto duly authorized.
|
ALTEROLA
BIOTECH INC
|
|||||
|
Dated:
January 13, 2011
|
/s/ Soren Nielsen
|
||||
|
Soren
Nielsen
|
|||||
|
President,
Chief Executive Officer
(Principal
Executive Officer)
|
|||||
|
Chief
Financial Officer and Director
(Principal
Financial and Accounting Officer)
|
22
ALTEROLA
BIOTECH INC.
(formerly Jedediah Resources
Corp.)
(A
Development Stage Company)
CONSOLIDATED
FINANCIAL STATEMENTS
September
30, 2010 and 2009
(Stated in US
Dollars)
(Audited)
CONTENTS
|
Report
of Independent Registered Public Accounting Firm
|
F-1
|
|
|
Consolidated
Balance Sheets
|
F-3
|
|
|
Consolidated
Statements of Operations
|
F-4
|
|
|
Consolidated
Statements of Stockholders’ (Deficit) Equity
|
F-5
|
|
|
Statements
of Cash Flows
|
F-6
|
|
|
Notes
to Financial Statements
|
F-7
|
23

Report of Independent
Registered Public Accounting Firm
To The
Board of Directors and Stockholders
Alterola
Biotech, Inc.
Palo
Alto, CA
We have
audited the accompanying consolidated balance sheet of Alterola Biotech, Inc.
(FKA: Jedediah Resources Corp.) (A Development Stage Company) (the “Company”) as
of September 30, 2010, and the related consolidated statements of operations,
stockholders’ deficit, and cash flows for the year then ended and from inception
(July 21, 2008) to September 30, 2010. These financial statements are the
responsibility of the Company's management. Our responsibility is to express an
opinion on the financial statements based on our audit. We did not
audit the financial statements of Alterola Biotech, Inc. (FKA: Jedediah
Resources Corp.) (A Development Stage Company) for the year ended September 30,
2009 and from inception (July 21, 2008) to September 30, 2009. Those
statements were audited by other auditors whose report has been furnished to us
and our opinion, in so far as it relates to the amounts included in the year
ended September 30, 2009 and from inception (July 21, 2008) to September 30,
2009, is based solely on the report of other auditors.
We
conducted our audit in accordance with standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether
the financial statements are free of material misstatement. An audit
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements. An audit also includes
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audit provides a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Alterola Biotech, Inc. (FKA:
Jedediah Resources Corp.) (A Development Stage Company) as of September 30,
2010, and the results of their operations and cash flows for the year then ended
and from inception (July 21, 2008) to September 30, 2010 in conformity with
accounting principles generally accepted in the United States.
We have
also audited the adjustments to the financial statements as of September 30,
2009 to retrospectively apply the effect of the forward stock split described in
Note 1. In our opinion, such adjustments are appropriate and have
been properly applied. We were not engaged to audit, review, or apply
any procedures to the financial statements as of September 30, 2009 of the
Company other than with respect to the adjustments and, accordingly, we do not
express an opinion or any other form of assurance on the financial statements as
of September 30, 2009 taken as a whole.
The
accompanying consolidated financial statements have been prepared assuming the
Company will continue as a going concern. As discussed in Note 1 to the
financial statements, the Company has suffered recurring losses from operations,
which raise substantial doubt about its ability to continue as a going concern.
Management’s plans in regard to these matters are also described in Note 1. The
financial statements do not include any adjustments that might result from the
outcome of this uncertainty.
De Joya
Griffith & Company, LLC
/s/ De
Joya Griffith & Company, LLC
Henderson,
Nevada
January
7, 2011
2580
Anthem Village Drive, Henderson, NV 89052
Telephone
(702) 563-1600 ● Facsimile (702) 920-8049
F-1
 |
Tel:
604 688 5421
|
BDO
Canada LLP
|
|
Fax:
604 688 5132
|
600
Cathedral Place
|
|
|
www.bdo.ca
|
925
West Georgia Street
|
|
|
Vancouver
BC V6C
3L2 Canada
|
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Stockholders,
Alterola
Biotech Inc.
(formerly
Jedediah Resources Corp.)
(An
Exploration Stage Company)
We have
audited, before the effects of the adjustments to retrospectively apply the
change in accounting described in Note 1 relating to the forward stock split,
the accompanying consolidated balance sheet of Alterola Biotech Inc. (formerly
Jedediah Resources Corp.) (the “Company”) (An Exploration Stage Company) and its
subsidiary as of September 30, 2009 and the related consolidated statements of
operations and comprehensive loss, cash flows and stockholders' equity for the
year ended September 30, 2009 (the 2009 financial statements before the effects
of the adjustments discussed in Note 1 are not presented therein) and for the
period from July 21, 2008 (Date of Inception) to September 30, 2009 (the 2009
financial statements before the effects of the adjustments discussed in Note 1
are not presented therein). These financial statements are the
responsibility of the Company's management. Our responsibility is to
express an opinion on these financial statements based on our
audit.
We
conducted our audit in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require
that we plan and perform the audit to obtain reasonable assurance whether the
financial statements are free of material misstatement. The Company
is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting. Our audit included
consideration of internal control over financial reporting as a basis for
designing audit procedures that are appropriate in the circumstances, but not
for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no
such opinion. An audit also includes examining, on a test basis,
evidence supporting the amounts and disclosures in the financial statements,
assessing the accounting principles used and significant estimates made by
management, as well as evaluating the overall financial statement
presentation. We believe that our audit provides a reasonable basis
for our opinion.
In our
opinion, the financial statements referred to above, before the effects of the
adjustments to retrospectively apply the effect of the forward stock split
described in Note1, present fairly, in all material respects, the financial
position of the Company and its subsidiary as of September 30, 2009 and the
results of their operations and their cash flows for the year ended September
30, 2009 and for the period from July 18, 2008 (Date of Inception) to September
30, 2009, in conformity with accounting principles generally accepted in the
United States of America.
BDO
Canada LLP, a Canadian limited liability partnership, is a member of BDO
International Limited, a UK
company limited by guarantee, and forms part of the international BDO network
of independent
member firms.
The
accompanying consolidated financial statements referred to above have been
prepared assuming that the Company will continue as a going
concern. As discussed in Note 1 to the financial statements, the
Company is in the exploration stage, has working capital which will not be
sufficient to sustain operations over the next twelve months, has yet to achieve
profitable operations, has accumulated losses since its inception and expects to
incur further losses in the development of its business These
factors, along with other matters as set forth in Note 1, raise substantial
doubt that the Company will be able to continue as a going
concern. The financial statements do not include any adjustments that
might result from the outcome of these uncertainties.
We were
not engaged to audit, review or apply any procedures to the adjustments to
retrospectively apply the effect of the forward stock split in Note 1 and,
accordingly, we do not express an opinion or any other form of assurance about
whether such adjustments are appropriate and have been properly
applied. These adjustments were audited by DeJoya Griffith &
Company, LLC.
(signed)
“BDO Canada LLP”
|
Chartered
Accountants
|
|
|
Vancouver,
Canada
|
|
|
December
16, 2009
|
F-2
ALTEROLA
BIOTECH INC.
(formerly Jedediah Resources
Corp.)
(A
Development Stage Company)
CONSOLIDATED
BALANCE SHEETS
(Stated in US
Dollars)
(Audited)
|
September 30,
|
September 30,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
Current
asset
|
||||||||
|
Cash
|
$ | 28 | $ | 7,224 | ||||
|
Prepaid
expenses
|
500 | - | ||||||
|
Total
current assets
|
528 | 7,224 | ||||||
|
Intellectual
property – Note 5
|
21,500 | - | ||||||
|
Website
|
6,200 | - | ||||||
|
Total
assets
|
$ | 28,228 | $ | 7,224 | ||||
|
LIABILITIES
AND STOCKHOLDERS’ (DEFICIT) EQUITY
|
||||||||
|
Current
liabilities
|
||||||||
|
Accounts
payable and accrued liabilities
|
$ | 5,750 | $ | 2,375 | ||||
|
Notes
payable – Note 6
|
80,000 | - | ||||||
|
Total
current liabilities
|
85,750 | 2,375 | ||||||
|
Total
liabilities
|
85,750 | 2,375 | ||||||
|
STOCKHOLDERS’
(DEFICIT) EQUITY
|
||||||||
|
Preferred
stock, $0.001 par value 10,000,000 shares authorized, none
outstanding
|
- | - | ||||||
|
Common
stock, $0.001 par value – Note 8 140,000,000 shares authorized 92,730,000
issued (September 30, 2009: 99,400,000 issued)
|
92,730 | 99,400 | ||||||
|
Additional
paid-in capital
|
56,142 | 35,218 | ||||||
|
Deficit
accumulated during the development stage
|
(206,394 | ) | (129,769 | ) | ||||
|
Total
stockholders’ (deficit) equity
|
(57,522 | ) | 4,849 | |||||
|
Total
liabilities and stockholders’ (deficit) equity
|
$ | 28,228 | $ | 7,224 | ||||
SEE
ACCOMPANYING NOTES
F-3
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
CONSOLIDATED
STATEMENTS OF OPERATIONS
(Stated in US
Dollars)
(Audited)
|
(Cumulative)
|
||||||||||||
|
July 21,
|
||||||||||||
|
2008
|
||||||||||||
|
(Date of
|
||||||||||||
|
Year Ended
|
Year Ended
|
Inception) to
|
||||||||||
|
September 30,
|
September 30,
|
September 30,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
Expenses
|
||||||||||||
|
Administrative
and general
|
$ | 124,915 | $ | 79,231 | $ | 210,677 | ||||||
|
Mineral
property option and exploration costs
|
1,866 | 18,007 | 19,873 | |||||||||
|
Stock
based compensation
|
- | - | 26,000 | |||||||||
|
Operating
expenses
|
(126,781 | ) | (97,238 | ) | (256,550 | ) | ||||||
|
Other
expenses
|
||||||||||||
|
Interest
expense
|
(2,090 | ) | - | (2,090 | ) | |||||||
|
Total
other expenses
|
(2,090 | ) | - | (2,090 | ) | |||||||
|
Net
loss
|
(128,871 | ) | (97,238 | ) | (258,640 | ) | ||||||
|
Basic
loss per share
|
$ | (0.00 | ) | $ | (0.00 | ) | ||||||
|
Weighted
average number of common shares basic
|
107,434,082 | 99,209,315 | ||||||||||
SEE
ACCOMPANYING NOTES
F-4
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
CONSOLIDATED
STATEMENT OF STOCKHOLDERS’ (DEFICIT) EQUITY
(Stated in US
Dollars)
(Audited)
|
Deficit
|
||||||||||||||||||||||||||||
|
Accumulated
|
||||||||||||||||||||||||||||
|
Additional
|
During the
|
|||||||||||||||||||||||||||
|
Preferred Shares
|
Common Shares
|
Paid-In
|
Exploration
|
|||||||||||||||||||||||||
|
Number
|
Amount
|
Number
|
Amount
|
Capital
|
Stage
|
Total
|
||||||||||||||||||||||
|
Balance
July 21, 2008 (Inception)
|
- | $ | - | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
|
Capital
stock issued for cash – at $0.001
|
- | - | 55,000,000 | 55,000 | - | - | 55,000 | |||||||||||||||||||||
|
Capital
stock returned to treasury for cancellation on rescission of subscription
(Note 8)
|
- | - | (55,000,000 | ) | (55,000 | ) | - | - | (55,000 | ) | ||||||||||||||||||
|
Capital
stock issued for cash – at $0.00095
|
- | - | 55,000,000 | 55,000 | (2,754 | ) | - | 52,246 | ||||||||||||||||||||
|
Stock-based
compensation for shares issued on discount
|
- | - | - | - | 26,000 | - | 26,000 | |||||||||||||||||||||
|
Capital
stock issued for cash – at $0.00149
|
- | - | 42,000,000 | 42,000 | 17,207 | - | 59,207 | |||||||||||||||||||||
|
Less:
commission
|
- | - | - | - | (5,700 | ) | - | (5,700 | ) | |||||||||||||||||||
|
Net
loss
|
- | - | - | - | - | (32,531 | ) | (32,531 | ) | |||||||||||||||||||
|
Balance
September 30, 2008
|
- | - | 97,000,000 | 97,000 | 34,753 | (32,531 | ) | 99,222 | ||||||||||||||||||||
|
Capital
stock issued for cash – at $0.00119
|
- | 2,400,000 | 2,400 | 465 | - | 2,865 | ||||||||||||||||||||||
|
Net
loss
|
- | - | - | - | - | (97,238 | ) | (97,238 | ) | |||||||||||||||||||
|
Balance
September 30, 2009
|
- | - | 99,400,000 | 99,400 | 35,218 | (129,769 | ) | 4,849 | ||||||||||||||||||||
|
Capital
stock issued for cash – at $0.0015
|
- | - | 33,330,000 | 33,330 | 16,670 | - | 50,000 | |||||||||||||||||||||
|
Capital
stock returned to treasury for cancellation on sale of Subsidiary JRE
Exploration Ltd - (Note 3)
|
- | - | (55,000,000 | ) | (55,000 | ) | 2,754 | 52,246 | - | |||||||||||||||||||
|
Capital
stock issued for intellectual property - (Note 5) – at
$0.0003
|
- | - | 55,000,000 | 55,000 | (38,500 | ) | - | 16,500 | ||||||||||||||||||||
|
Capital
stock returned to treasury for cancellation - (Note 1)
|
- | - | (40,000,000 | ) | (40,000 | ) | 40,000 | - | - | |||||||||||||||||||
|
Net
loss
|
- | - | - | - | - | (128,871 | ) | (128,871 | ) | |||||||||||||||||||
|
Balance
September 30, 2010
|
- | $ | - | 92,730,000 | $ | 92,730 | $ | 56,142 | $ | (206,394 | ) | $ | (57,522 | ) | ||||||||||||||
SEE
ACCOMPANYING NOTES
F-5
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
STATEMENTS
OF CASH FLOWS
(Stated in US
Dollars)
(Audited)
|
Date of
|
||||||||||||
|
Inception
|
||||||||||||
|
Year Ended
|
Year Ended
|
(July 21,2008)
|
||||||||||
|
September 30,
|
September 30,
|
to September 30,
|
||||||||||
|
2010
|
2009
|
2010
|
||||||||||
|
Cash
flows from operating activities
|
||||||||||||
|
Net
loss
|
$ | (128,871 | ) | $ | (97,238 | ) | $ | (258,640 | ) | |||
|
Adjustment
to reconcile net loss to net cash used in operating
activities:
|
||||||||||||
|
Stock
based compensation
|
- | - | 26,000 | |||||||||
|
Change
in non-cash working capital items:
|
||||||||||||
|
Prepaid
expenses
|
(500 | ) | - | (500 | ) | |||||||
|
Accounts
payable and accrued liabilities
|
3,376 | (1,987 | ) | 5,751 | ||||||||
|
Net
cash used in operating activities
|
(125,995 | ) | (99,225 | ) | (227,389 | ) | ||||||
|
Cash
flows from investing activities
|
||||||||||||
|
Acquisition
of intellectual property
|
(5,000 | ) | - | (5,000 | ) | |||||||
|
Website
development
|
(6,200 | ) | - | (6,200 | ) | |||||||
|
Net
cash used in investing activities
|
(11,200 | ) | - | (11,200 | ) | |||||||
|
Cash
flows from financing activities
|
||||||||||||
|
Capital
stock issued for cash
|
50,000 | 2,865 | 213,618 | |||||||||
|
Capital
stock returned to treasury
|
- | - | (55,000 | ) | ||||||||
|
Proceeds
from issuance of notes payable
|
80,000 | - | 80,000 | |||||||||
|
Net
cash provided by financing activities
|
130,000 | 2,865 | 238,618 | |||||||||
|
Increase
(decrease) in cash during the period
|
(7,196 | ) | (96,360 | ) | 28 | |||||||
|
Cash,
beginning of the period
|
7,224 | 103,584 | - | |||||||||
|
Cash,
end of the period
|
$ | 28 | $ | 7,224 | $ | 28 | ||||||
|
Supplemental
disclosure of cash flow information:
|
||||||||||||
|
Cash
paid for:
|
||||||||||||
|
Interest
|
$ | - | $ | - | $ | - | ||||||
|
Income
taxes
|
$ | - | $ | - | $ | - | ||||||
|
Non-
cash investing and financing activities:
|
||||||||||||
|
Common
stock issued for intellectual property
|
$ | 16,500 | $ | - | $ | 16,500 | ||||||
|
Common
stock cancelled on sale of subsidiary
|
$ | 52,246 | $ | - | $ | 52,246 | ||||||
SEE
ACCOMPANYING NOTES
F-6
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS
September
30, 2010 and 2009
(Stated in US
Dollars)
|
Note
1
|
Nature of Operations
and Ability to Continue as a Going
Concern
|
The
Company was incorporated in the state of Nevada, United States of America on
July 21, 2008. The Company was formed for the purpose of acquiring
exploration and development stage mineral properties. The Company’s
year-end is September 30.
On
October 1, 2008, the Company incorporated JRE Exploration Ltd, (“JRE”) a wholly
owned subsidiary in Canada for the purpose of holding its Canadian mineral
claims.
On May 3,
2010, the Company changed its focus to the development of intellectual property
and accordingly sold JRE to the former president. (Note 3). In
keeping with the change of business focus, on July 9, 2010 the Company changed
its name to Alterola Biotech Inc.
Effective
July 9, 2010, the Board of Directors authorized a 10 for 1 forward stock split
on the issued common shares. The authorized number of common shares
was increased from 90,000,000 to 140,000,000 common shares with a par value of
$0.001. The number of authorized Preferred shares remained unchanged
at 10,000,000 with a par value of $0.001. All references in the
accompanying financial statements to the number of common shares have been
restated to reflect the forward stock split.
Subsequent
to the year end, on November 17, 2010, the President entered into a stock
cancellation agreement with the Company whereby 40,000,000 common shares were
returned to treasury and cancelled. In consideration the Company will
issue to the President options to acquire common stock pursuant to the stock
option which will be adopted by the Company in the future. Due to the
fact that the shares under this agreement have been cancelled without the
exchange of consideration to reduce number of shares outstanding, the Company
considered the change in capital structure from the cancellation agreement a
reverse stock split. In accordance with SAB Topic 4-C, the Company
recorded the cancellation retroactively as of September 30, 2010 as a
reduction to the par value of common stock with a corresponding increase to
additional paid-in capital.
These
financial statements have been prepared in accordance with generally accepted
accounting principles applicable to a going concern, which assumes that the
Company will be able to meet its obligations and continue its operations for its
next twelve months. Realization values may be substantially different
from carrying values as shown and these financial statements do not give effect
to adjustments that would be necessary to the carrying values and classification
of assets and liabilities should the Company be unable to continue as a going
concern. At September 30, 2010, the Company has a negative working
capital of $85,222, has yet to achieve profitable operations, has accumulated
losses of $258,640 since its inception and expects to incur further losses in
the development of its business, all of which casts substantial doubt about the
Company’s ability to continue as a going concern.
The
Company’s ability to continue as a going concern is dependent upon its ability
to generate future profitable operations and/or to obtain the necessary
financing from shareholders or other sources to meet its obligations and repay
its liabilities arising from normal business operations when they come
due.
F-7
ALTEROLA BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 2
|
Note
1
|
Nature of Operations
and Ability to Continue as a Going Concern –
(cont’d)
|
Management
has no formal plan in place to address this concern but considers that the
Company will be able to obtain additional funds by equity financing and/or
related party advances, however there is no assurance of additional funding
being available or on acceptable terms, if at all. These financial
statements do not include any adjustments related to the recoverability and
classification of assets or the amounts and classification of liabilities that
might be necessary should the Company be unable to continue as a going
concern.
|
Note 2
|
Summary of Significant
Accounting Policies
|
The
following is a summary of significant accounting policies used in the
preparation of these financial statements.
|
|
The
financial statements of the Company have been prepared in accordance with
accounting principles generally accepted in the United States of America
and are stated in US dollars.
|
|
|
Use
of Estimates
|
|
|
The
preparation of financial statements in conformity with accounting
principles generally accepted in the United States of America requires
management to make estimates and assumptions that affect the amounts of
assets and liabilities and disclosures of contingent assets and
liabilities at the date of the financial statements and the reported
amounts of revenues and expenditures during the reporting
period. Actual results could differ from these
estimates.
|
Principles of
Consolidation
These
consolidated financial statements include the results of the Company and JRE
Exploration Ltd., (“JRE”) a wholly owned subsidiary from incorporation on
October 1, 2008 until disposal on May 3, 2010. All significant
inter-company transactions and balances have been eliminated.
Exploration Stage
Company
The
Company is an exploration stage company. All losses accumulated since
inception have been considered as part of the Company’s exploration stage
activities.
Cash
|
|
Cash
consists of all highly liquid investments that are readily convertible to
cash within 90 days when purchased.
|
F-8
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 3
|
Note
2
|
Summary of Significant
Accounting Policies –
(cont’d)
|
Mineral
Property
Mineral
property exploration costs are charged to operations as
incurred. When it has been determined that a mineral property can be
economically developed as a result of establishing proven and probable reserves,
the costs incurred to develop such property, are capitalized. Such
costs will be depleted using the units-of-production method over the estimated
life of the probable reserve.
Research and
Development
|
|
Costs
of research and development are expensed as
incurred.
|
Intangible
assets
Intellectual
Property
The
Company does not amortize intangible assets with indefinite useful lives, rather
such assets are required to be tested for impairment at least annually or sooner
whenever events or changes in circumstances indicate that the assets may be
impaired. The Company amortizes its intangible assets with definite
lives over their estimated useful lives and reviews these assets for impairment.
The Company will amortize its acquired intangible assets with definite
lives over the estimated economic life of the completed product.
Website
Development Costs
Costs
incurred in developing and maintaining a website are charged to expense when
incurred for the planning, content population, and administration or maintenance
of the website. All development costs for the application, infrastructure,
and graphics development are capitalized and subsequently reported at the lower
of unamortized cost or net realizable value. Capitalized costs will be
amortized using straight-line basis over two years, the estimated economic life
of the completed website. As of September 30, 2010, the Company’s
website was not in service; accordingly no amortization has been recorded during
the year ended September 30, 2010.
Stock-based
Compensation
|
|
The
Company is required to record compensation expense, based on the fair
value of the awards, for all awards granted after the date of the
adoption.
|
Foreign Currency
Translation
The
Company’s functional currency is the US dollar as substantially all of the
Company’s operations are in the United States of America. The Company
uses the United States dollar as its reporting currency for consistency with
registrants of the Securities and Exchange Commission (“SEC”).
F-9
ALTEROLA BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 4
|
Note
2
|
Summary of Significant
Accounting Policies –
(cont’d)
|
Assets
and liabilities denominated in a foreign currency are translated at the exchange
rate in effect at the balance sheet date and capital accounts are translated at
historical rates. Income statement accounts are translated at the
average rates of exchange prevailing during the period. Translation
adjustments from the use of different exchange rates from period to period are
included in the Accumulated Other Comprehensive Income account in Stockholder’s
Equity, if applicable. Transactions undertaken in currencies other
than the functional currency of the entity are translated using the exchange
rate in effect as of the transaction date. Any exchange gains and
losses are included in the Statement of Operations and Comprehensive
Loss.
Income
Taxes
The
Company uses the asset and liability method of accounting for income
taxes. Deferred tax assets and liabilities are recognized for the
future tax consequences attributable to temporary differences between the
financial statements carrying amounts of existing assets and liabilities and
loss carry-forwards and their respective tax bases. Deferred tax
assets and liabilities are measured using enacted tax rates expected to apply to
taxable income in the years in which those temporary differences are expected to
be recovered or settled.
The
effect of a change in tax rules on deferred tax assets and liabilities is
recognized in operations in the year of change. A valuation allowance is
recorded when it is “more likely-than-not” that a deferred tax asset will not be
realized.
Basic and Diluted Loss Per
Share
Basic
loss per share is computed using the weighted average number of shares
outstanding during the period. Fully diluted earnings (loss) per
share are computed similar to basic income (loss) per share except that the
denominator is increased to include the number of common stock equivalents
(primarily outstanding options and warrants). Common stock
equivalents represent the dilutive effect of the assumed exercise of the
outstanding stock options and warrants, using the treasury stock method, at
either the beginning of the respective period presented or the date of issuance,
whichever is later, and only if the common stock equivalents are considered
dilutive based upon the Company’s net income (loss) position at the calculation
date. As there are no common stock equivalents outstanding, diluted
and basic loss per share are the same.
Long-Lived
Assets
|
|
Management
reviews useful lives and obsolescence and assesses commercial viability of
these assets periodically, at least annually. Based on these
reviews, management estimates that the undiscounted future cash flows
expected to result from the use of these assets exceeds the current
carrying value of these assets. Any adverse change in the
estimate of these undiscounted future cash flows could necessitate an
impairment charge that would adversely affect operating
results.
|
F-10
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 5
|
Note
2
|
Summary of Significant
Accounting Policies –
(cont’d)
|
|
|
Management
also estimates useful lives for estimates of assets’ commercial lives, and
the likelihood of technological obsolescence. Should the actual
useful life of a class of assets differ from the estimated useful life,
the Company would record an impairment
charge.
|
Comprehensive
Income
The
Company is required to report comprehensive income, which includes net loss as
well as changes in equity from non-owner sources.
Recently Issued Accounting
Pronouncements
In April
2010, the FASB issued ASU No. 2010-17, "Revenue Recognition - Milestone Method
(Topic 605): Milestone Method of Revenue Recognition" (codified within ASC 605 -
Revenue Recognition). ASU 2010-17 provides guidance on defining a milestone and
determining when it may be appropriate to apply the milestone method of revenue
recognition for research or development transactions. ASU 2010-17 is effective
for interim and annual periods beginning after June 15, 2010. The adoption of
ASU 2010-17 is not expected to have any material impact on our financial
position, results of operations or cash flows.
In March
2010, the FASB issued ASU No. 2010-11, "Derivatives and Hedging (Topic 815):
Scope Exception Related to Embedded Credit Derivatives" (codified within ASC 815
- Derivatives and Hedging). ASU 2010-11 improves disclosures originally required
under SFAS No. 161. ASU 2010-11 is effective for interim and annual periods
beginning after June 15, 2010. The adoption of ASU 2010-11 is not expected to
have any material impact on our financial position, results of operations or
cash flows.
In May
2010, the FASB (Financial Accounting Standards Board) issued Accounting
Standards Update 2010-19 (ASU 2010-19), Foreign Currency (Topic 830): Foreign
Currency Issues: Multiple Foreign Currency Exchange Rates. The
amendments in this Update are effective as of the announcement date of March 18,
2010. The Company does not expect the provisions of ASU 2010-19 to have a
material effect on the Company's financial position, results of operations or
cash flows of the Company.
|
Note
3
|
Sale of
Subsidiary
|
|
|
Pursuant
to an agreement dated May 3, 2010, the Company sold its wholly-owned
subsidiary, JRE Exploration Ltd., to the Company’s former
president. In consideration for the sale the purchaser returned
55,000,000 shares of Jedediah to the Company for cancellation, and the
Company forgave all amounts owed by JRE to the
Company.
|
F-11
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 6
|
Note 3
|
Sale of Subsidiary
– (cont’d)
|
|
|
The
following table summarizes the identifiable assets and liabilities of JRE
that were disposed of and the consideration
received.
|
|
May 3, 2010
|
||||
|
Identifiable
Assets and Liabilities
|
||||
|
Mineral
Property
|
$ | - | ||
|
Amount
owed to Jedediah Resources Corp
|
(21,843 | ) | ||
|
Net
liabilities of JRE
|
(21,843 | ) | ||
|
Consideration
Received
|
||||
|
Elimination
of consolidated losses of JRE
|
21,843 | |||
|
Gain/(Loss)
on Disposal
|
$ | - | ||
|
Receipt
and cancellation of 55,000,000 shares returned to treasury, recorded in
statement of equity.
|
$ | (52,246 | ) | |
|
Note
4
|
Financial
Instruments
|
|
|
Fair
value is defined as the price that would be received upon sale of an asset
or paid upon transfer of a liability in an orderly transaction between
market participants at the measurement date and in the principal or most
advantageous market for that asset or liability. The fair value
should be calculated based on assumptions that market participants would
use in pricing the asset or liability, not on assumptions specific to the
entity. In addition, the fair value of liabilities should
include consideration of non-
|
|
|
performace
risk including our own credit risk.
|
In
addition to defining fair value, the disclosure requirements around fair value
establishes a fair value hierarchy for valuation inputs which is
expanded. The hierarchy prioritizes the inputs into three levels
based on the extent to which inputs used in measuring fair value are observable
in the market. Each fair value measurement is reported in one of the
three levels which is determined by the lowest level input that is significant
to the fair value measurement in its entirety. These levels
are:
|
Level 1 –
|
inputs
are based upon unadjusted quoted prices for identical instruments traded
in active markets.
|
|
Level
2 –
|
inputs
are based upon significant observable inputs other than quoted prices
included in Level 1, such as quoted prices for identical or similar
instruments in markets that are not active, and model-based valuation
techniques for which all significant assumptions are observable in the
market or can be corroborated by observable market data for substantially
the full term of the assets or
liabilities.
|
F-12
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 7
|
Note
4
|
Financial Instruments
– (cont’d)
|
|
Level 3 –
|
inputs
are generally unobservable and typically reflect management’s estimates of
assumptions that market participants would use in pricing the asset or
liability. The fair values are therefore determined using model-based
techniques that include option pricing models, discounted cash flow
models, and similar
techniques.
|
The
carrying value of the Company’s financial assets and liabilities which consist
of cash, accounts payable and accrued liabilities, and notes payable are valued
using level 1 inputs. The Company believes that the recorded values
approximate their fair value due to the short maturity of such
instruments. Unless otherwise noted, it is management’s opinion that
the Company is not exposed to significant interest, exchange or credit risks
arising from these financial instruments.
|
Note
5
|
Intellectual
Property
|
Pursuant
to an Assignment Agreement dated May 3, 2010 (the “Agreement”), the Company
acquired from the president of the Company a 100% undivided right in and to all
intellectual property relating to certain chewing gum compositions having
appetite suppressant activity. Consideration given for the
acquisition was 55,000,000 Common shares of the Company. Since the
intellectual property was acquired from a related party, the Company recorded
the value of the intellectual property received at $16,500, based on historical
basis. During the year ended September 30, 2010, the Company incurred
a further $5,000 in patent application fees.
|
Note
6
|
Notes
Payable
|
|
2010
|
2009
|
|||||||
|
Note
payable, unsecured, bearing interest at 12% per annum, due on June 26,
2011.
|
$ | 30,000 | $ | - | ||||
|
Convertible
note payable, unsecured, bearing interest at 12% per annum, due on July
24, 2011
|
50,000 | - | ||||||
| $ | 80,000 | $ | - | |||||
The
Convertible note is convertible at the option of the holder. The
number of shares of common stock into which the convertible note will be
converted is determined by the Fair Market Price (“FMV”) of the common stock at
the date of conversion. In the event there is no determinable market
price the FMV shall be:
|
|
a)
|
The
share price at the last private offering of the common stock,
or,
|
|
|
b)
|
The
30 day moving average of the Common Stock in the event a public listing of
the common stock has taken
place.
|
F-13
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 8
|
Note
7
|
Related Party
Transactions and Balances – Notes 3, 5 and
8
|
In
addition to those related party transactions disclosed elsewhere in the
financial statements the Company paid or accrued;
|
|
a)
|
management
fees of $4,000 (2009 - $12,000)(cumulative 2010 - $18,234) charged by a
company controlled by the former president of the
Company.
|
|
|
b)
|
management
fees of $15,900 (2009 - $nil)(cumulative 2010 - $15,900); travel expenses
of $6,700 (2009 – nil)(cumulative 2010 - $6,700), rent of $2,888 (2009 -
$nil)(cumulative 2010 - $2,888), and communication costs of $1,212 (2009 -
$nil)(cumulative - $1,212) charged by a company controlled by the
president of the Company. Additionally, through a company
controlled by the president of the Company, transfer agent and filings
fees of $1,000 (2009 - $nil)(cumulative -
$1,000).
|
|
Note
8
|
Capital Stock –
Notes 3 and 5
|
|
a)
|
Authorized:
|
10,000,000
preferred shares with a par value of $0.001.
140,000,000
common shares with a par value of $0.001.
|
|
b)
|
Issued:
|
|
|
On
August 6, 2008, the Company issued 55,000,000 common shares to the
Company’s president at $0.001 per share for total proceeds of
$55,000.
|
On
September 22, 2008, the incumbent president resigned as both an officer and
director and a new president and director was appointed. At the
request of the departing president, the Company’s board of directors rescinded
his share subscription for 55,000,000 common shares and repaid the subscription
proceeds of $55,000.
On
September 22, 2008, the Company issued 55,000,000 common shares to the Company’s
new president at $0.00095 (CDN$0.001) per share for total proceeds of $52,246
(CDN$55,000).
On
September 22, 2008, the Company issued 39,600,000 common shares at approximately
$0.00149 (CDN$0.0015) per share for total proceeds of $55,740 (CDN$59,400)
pursuant to a private placement. On September 30, 2008, the Company
issued 2,400,000 common shares at approximately $0.00149 (CDN$0.0015) per share
for total proceeds of $3,467 (CDN$3,600) pursuant to a private
placement. The Company paid a commission of $5,700 for net proceeds
of $53,507 for these private placements.
F-14
ALTEROLA
BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 9
|
Note 8
|
Capital
Stock – Note 3 and 5 –
(cont’d)
|
On
October 29, 2008, the Company issued 2,400,000 common shares at approximately
$0.00119 (CDN$0.015) per share for total proceeds of $2,865 (CDN$3,600) pursuant
to a private placement.
On
January 5, 2010, pursuant to a share subscription agreement, the Company issued
33,330,000 Common Shares at $0.0015 for aggregate proceeds of $50,000.
On May 3,
2010, pursuant to the sale of JRE Exploration Ltd. (Note 3) the Company received
55,000,000 of its Common stock from the former Company president with a fair
value of $52,246 for cancellation, as consideration for the sale of JRE, our
wholly owned subsidiary.
On May 3,
2010, pursuant to an assignment agreement for the acquisition of certain
intellectual property, (Note 5) the Company issued 55,000,000 Common shares with
a fair value of $16,500 to the president of the Company.
Subsequent
to the year end, on November 17, 2010, the President entered into a stock
cancellation agreement with the Company whereby 40,000,000 common shares were
returned to treasury and cancelled. In consideration the Company will
issue to the President options to acquire common stock pursuant to the stock
option which will be adopted by the Company in the future. Due to the
fact that the shares under this agreement have been cancelled without the
exchange of consideration to reduce number of shares outstanding, the Company
considered the change in capital structure from the cancellation agreement a
reverse stock split. In accordance with SAB Topic 4-C, the Company
recorded the cancellation retroactively as of September 30, 2010 as a reduction
to the par value of common stock with a corresponding increase to additional
paid-in capital.
|
Note 9
|
Income
Taxes
|
|
|
A
reconciliation of the income tax provision computed at statutory rates to
the reported tax provision is as
follows:
|
|
2010
|
2009
|
|||||||
|
Basic
statutory and provincial income tax rate
|
35.0 | % | 35.0 | % | ||||
|
Approximate
loss before income taxes
|
$ | 129,000 | $ | 97,000 | ||||
|
Expected
approximate tax recovery on net loss, before income tax
|
$ | 45,000 | $ | 34,000 | ||||
|
Valuation
allowance
|
(45,000 | ) | (34,000 | ) | ||||
|
Future
income tax recovery
|
$ | - | $ | - | ||||
F-15
ALTEROLA BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 10
Significant components of the Company’s
future tax assets and liabilities are as follows:
|
|
2010
|
2009
|
||||||
|
Future
income tax assets
|
||||||||
|
Non-capital
losses carried forward
|
$ | 84,000 | $ | 39,000 | ||||
|
Mining
properties
|
6,000 | 6,300 | ||||||
|
Less:
valuation allowance
|
(90,000 | ) | (45,300 | ) | ||||
|
Future
income tax assets
|
$ | - | $ | - | ||||
|
Note 9
|
Income Taxes –
(cont’d)
|
At
September 30, 2009, the Company has incurred accumulated non-capital losses
totalling approximately $241,000 which are available to reduce taxable income in
future taxation years.
These
losses expire as follows:
|
Year of Expiry
|
Amount
|
|||
|
2028
|
$ | 33,000 | ||
|
2029
|
79,000 | |||
|
2030
|
129,000 | |||
| $ | 241,000 | |||
The
amount taken into income as deferred tax assets must reflect that portion of the
income tax loss carryforwards that is more-likely-than-not to be realized from
future operations. The Company has chosen to provide an allowance of
100% against all available income tax loss carryforwards, regardless of their
time of expiry
Uncertain
Tax Positions
The
Company has adopted FASB guidance on "Accounting for Uncertainty in Income
Taxes". The guidance prescribes a recognition threshold and
measurement attribute for the recognition and measurement of tax positions taken
or expected to be taken in income tax returns. The guidance also
provides guidance on de-recognition of income tax assets and liabilities,
classification of current and deferred income tax assets and liabilities, and
accounting for interest and penalties associated with tax
positions.
The
Company filed income tax returns in the U.S. federal jurisdiction, various state
and foreign jurisdictions. The Company’s tax returns are subject to
tax examinations by U.S. federal and state tax authorities, or examinations by
foreign tax authorities until respective statue of limitation. It is
subject to tax examinations by tax authorities for all taxation years commencing
on or after 2008.
Management’s
analysis of the guidance supports the conclusion that the Company does not have
any accruals for uncertain tax positions as of September 30, 2010. As
a result, tabular reconciliation of beginning and ending balances would not be
meaningful. If interest and penalties were to be assessed, we would
charge interest to interest expense, and penalties to other operating expense in
the period of the assessment. It is not anticipated that unrecognized
tax benefits would significantly increase or decrease within 12 months of the
reporting date.
F-16
ALTEROLA BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 11
The
Company estimated the expected amount of loss carry forwards available. The
Company expects to have significant net operating loss carry forwards for income
tax purposes available to offset future taxable income.
|
Note 10
|
Subsequent
Events
|
Commitments
|
|
a)
|
On
October 5, 2010, the Company entered into a exclusive contract with
Glenbarry Holdings Inc. (“Glenbarry”) for Glenbarry to act as the
Company’s Capital Acquisitions Consultant. Glenbarry is to
facilitate introductions of one or more persons whether individuals or
otherwise to the Company for the purpose of investing in the
Company. The agreement is for an initial period of 120 days and
shall renew automatically for successive terms unless either party
delivers 10 days written notice of
cancellation.
|
|
|
As
compensation for successful introductions Glenbarry will receive a 5% cash
commission upon the Company receiving investment
funds. Commission will continue to be payable for a period of 2
years after the expiration of the agreement if investment funds are
received from any investor introduced by
Glenbarry.
|
|
|
b)
|
On
October 6, 2008, the Company’s wholly owned subsidiary, JRE Exploration
Ltd (“JRE”) entered into a property option agreement whereby JRE was
granted an option to earn up to an 85% interest in a mineral claim (the
“Bragg” claim) consisting of 594.1 hectares located in the Omineca Mining
Division of British Columbia. It is located 78 miles north by
north-west of the central British Columbia city of Prince George,
approximately 25 miles south of the town of McKenzie and approximately 5
miles west of the hamlet of McLeod Lake. Access to the property is by way
of logging roads, extending north and west from McLeod Lake. The option
agreement is denominated in Canadian dollars. Consideration for
the option is cash payments totalling $8,623 (CDN$9,000) and aggregate
exploration expenditures of $179,864 (CDN$186,000) as
follows:
|
|
i)
|
Cash
payments as follows:
|
|
§
|
$1,850
(CDN$2,000) upon execution of the Option agreement
(paid);
|
|
§
|
$1,866
(CDN$2,000) on or before October 31, 2009 (paid by way of promissory note
which was paid in full on January 5,
2010);
|
|
§
|
$4,908
(CDN$5,000) on or before October 31,
2010.
|
|
|
ii)
|
Exploration
expenditures of $12,804 (CDN$15,000) on or before October 31, 2009,
$24,787 (CDN$28,000) in aggregate on or before October 31, 2010; $179,864
(CDN$186,000) in aggregate on or before October 31,
2011.
|
F-17
ALTEROLA BIOTECH INC.
(formerly
Jedediah Resources Corp.)
(A
Development Stage Company)
Notes to
the Consolidated Financial Statements
September
30, 2010 and 2009
(Stated
in US Dollars) – Page 12
Upon
earning its 85% interest in the option, the Company shall enter into a joint
venture agreement to develop and operate the property.
As at
March 31, 2010, the Company had incurred, via the operator, exploration
expenditures aggregating $16,157 (CDN$19,207).
|
Note 10
|
Subsequent
Events – (cont’d)
|
The
property option agreement was stated in Canadian dollars. On May 3,
2010, the Company sold JRE to the Company’s former president and all commitments
associated with the mineral property were assumed by the acquirer.
Other Subsequent
Events
|
|
a)
|
On
November 17, 2010, the President entered into a stock cancellation
agreement with the Company whereby 40,000,000 common shares were returned
to treasury and cancelled. In consideration the Company will
issue to the President options to acquire common stock pursuant to the
stock option plan which will be adopted by the Company at some time in the
future. Due to the fact that the shares under this agreement
have been cancelled without the exchange of consideration to reduce number
of shares outstanding, the Company considered the change in capital
structure from the cancellation agreement a reverse stock
split. In accordance with SAB Topic 4-C, the Company recorded
the cancellation retroactively as of September 30, 2010 as a reduction to
the par value of common stock with a corresponding increase to additional
paid-in capital.
|
|
|
b)
|
On
December 21, 2010, the Company issued 250,000 shares at $0.20 for
aggregate proceeds of $50,000.
|
F-18
