Attached files
| file | filename |
|---|---|
| EX-31.2 - China Polypeptide Group, Inc. | v208015_ex31-2.htm |
| EX-32.2 - China Polypeptide Group, Inc. | v208015_ex32-2.htm |
| EX-31.1 - China Polypeptide Group, Inc. | v208015_ex31-1.htm |
| EX-32.1 - China Polypeptide Group, Inc. | v208015_ex32-1.htm |
| EX-10.13 - China Polypeptide Group, Inc. | v208015_ex10-13.htm |
| EX-10.17 - China Polypeptide Group, Inc. | v208015_ex10-17.htm |
| EX-10.15 - China Polypeptide Group, Inc. | v208015_ex10-15.htm |
| EX-10.16 - China Polypeptide Group, Inc. | v208015_ex10-16.htm |
| EX-10.14 - China Polypeptide Group, Inc. | v208015_ex10-14.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
(Mark
One)
x ANNUAL REPORT PURSUANT TO
SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the
fiscal year ended: September 30,
2010
¨ TRANSITION REPORT PURSUANT
TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the
transition period from ____________ to _____________
Commission
File No. 333-151148
CHINA
POLYPEPTIDE GROUP, INC.
(Exact
name of registrant as specified in its charter)
|
Delaware
|
20-8731646
|
|
(State or other jurisdiction of incorporation or
organization)
|
(I.R.S. Employer Identification No.)
|
No.
11 Jiangda Road
Jianghan
Economic Development Zone
Wuhan
430023
People’s
Republic of China
(Address of principal executive offices)
(86)
27 8351-8396
(Registrant’s telephone number, including area code)
Securities
registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which
registered
|
|
|
None
|
None
|
Securities
registered pursuant to Section 12(g) of the Exchange Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act.
Yes ¨ No x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90
days.
Yes x No ¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Website, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes ¨ No ¨
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K.
x
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting
company. See the definitions of “large accelerated filer,”
“accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.
|
Large
Accelerated Filer ¨
|
Accelerated
Filer ¨
|
|
Non-Accelerated
Filer ¨ (Do not check
if a smaller reporting company)
|
Smaller
reporting company x
|
Indicate
by check mark whether registrant is a shell company (as defined in Rule 12b-2 of
the Act).
Yes ¨ No x
As of
March 31, 2010 (the last business day of the registrant’s most recently
completed second fiscal quarter), the aggregate market value of the shares of
the registrant’s common stock held by non-affiliates (based upon the closing
price of such shares as quoted on the OTC Bulletin Board maintained by the
Financial Industry Regulatory Authority) was approximately $207,863. Shares of
the registrant’s common stock held by each executive officer and director and
each by each person who owns 10% or more of the outstanding common stock have
been excluded from the calculation in that such persons may be deemed to be
affiliates of the registrant. This determination of affiliate status is not
necessarily a conclusive determination for other purposes.
There
were a total of 11,939,967 shares of the registrant’s common stock outstanding
as of January 12, 2011.
None.
CHINA
POLYPEPTIDE GROUP, INC.
Annual
Report on FORM 10-K
For the Fiscal
Year Ended September 30, 2010
TABLE
OF CONTENTS
|
PART
I
|
|||
|
Item
1.
|
Business.
|
2
|
|
|
Item
1A.
|
Risk
Factors.
|
15
|
|
|
Item
1B.
|
Unresolved
Staff Comments.
|
28
|
|
|
Item
2.
|
Properties.
|
29
|
|
|
Item
3.
|
Legal
Proceedings.
|
29
|
|
|
Item
4.
|
(Removed
and Reserved).
|
29
|
|
|
PART
II
|
|||
|
Item
5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters and Issuer
Purchases of Equity Securities.
|
29
|
|
|
Item
6.
|
Selected
Financial Data.
|
30
|
|
|
Item
7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations.
|
30
|
|
|
Item
7A.
|
Quantitative
and Qualitative Disclosures About Market Risk.
|
38
|
|
|
Item
8.
|
Financial
Statements and Supplementary Data.
|
38
|
|
|
Item
9.
|
Changes
in and Disagreements with Accountants on Accounting and Financial
Disclosure.
|
38
|
|
|
Item
9A.
|
Controls
and Procedures.
|
38
|
|
|
Item
9B.
|
Other
Information.
|
40
|
|
|
PART
III
|
|||
|
Item
10.
|
Directors,
Executive Officers and Corporate Governance.
|
40
|
|
|
Item
11.
|
Executive
Compensation.
|
42
|
|
|
Item
12.
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters.
|
44
|
|
|
Item
13.
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
45
|
|
|
Item
14.
|
Principal
Accounting Fees and Services.
|
47
|
|
|
PART
IV
|
|||
|
Item
15.
|
Exhibits,
Financial Statement Schedules.
|
47
|
|
Special
Note Regarding Forward Looking Statements
In addition to historical
information, this report contains forward-looking statements within the meaning
of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the
Securities Exchange Act of 1934, as amended. We use words such as “believe,”
“expect,” “anticipate,” “project,” “target,” “plan,” “optimistic,” “intend,”
“aim,” “will” or similar expressions which are intended to identify
forward-looking statements. Such statements include, among others, those
concerning market and industry segment growth and demand and acceptance of new
and existing products; any projections of sales, earnings, revenue, margins or
other financial items; any statements of the plans, strategies and objectives of
management for future operations; and any statements regarding future economic
conditions or performance, as well as all assumptions, expectations,
predictions, intentions or beliefs about future events. You are cautioned that
any such forward-looking statements are not guarantees of future performance and
involve risks and uncertainties, including those identified in Item 1A, “Risk
Factors” included herein, as well as assumptions, which, if they were to ever
materialize or prove incorrect, could cause the results of the Company to differ
materially from those expressed or implied by such forward-looking
statements.
Although
we believe the expectations reflected in the forward-looking statements are
reasonable, we cannot guarantee future results, level of activity, performance,
or achievements. Moreover, neither we nor any other person assumes
responsibility for the accuracy or completeness of any of these forward-looking
statements. You should not rely upon forward-looking statements as predictions
of future events. We are under no duty to update any of these forward-looking
statements after the date of this report to conform our prior statements to
actual results or revised expectations.
Use
of Terms
Except as
otherwise indicated by the context, references in this report to:
|
|
·
|
“Company,”
“we,” “us” and “our” are to the combined business of China Polypeptide
Group, Inc., a Delaware corporation, and its subsidiaries: Cantix,
Moneyeasy, Tallyho, Wuhan Anti-Aging, Hopsun and
Xingpu;
|
|
|
·
|
“Cantix”
are to our wholly-owned direct subsidiary, Cantix International Limited, a
British Virgin Islands limited
company;
|
|
|
·
|
“Moneyeasy”
are to our wholly-owned indirect subsidiary, Moneyeasy Industries Limited,
a Hong Kong limited company;
|
|
|
·
|
“Tallyho”
are to our wholly-owned indirect subsidiary, Wuhan Tallyho Biological
Product Co., Ltd., a PRC limited
company;
|
|
|
·
|
“Wuhan
Anti-Aging” or “Anti-Aging”
are to our wholly-owned indirect subsidiary, Wuhan
Polypeptide Anti-Aging Research & Development Co., Ltd., a PRC limited
company;
|
|
|
·
|
“Hopsun”
are to our wholly-owned indirect subsidiary, Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd., a PRC limited
company;
|
|
|
·
|
“Xingpu”
are to our variable interest entity (VIE) indirect subsidiary, Guangdong
Xingpu Polypeptide Research Co., Ltd., a PRC limited
company;
|
|
|
·
|
“SEC”
are to the United States Securities and Exchange
Commission;
|
|
|
·
|
“Securities
Act” are to the Securities Act of 1933, as amended, and “Exchange Act” are
to the Securities Exchange Act of 1934, as
amended.
|
|
|
·
|
“China,”
“Chinese” and “PRC” are to People’s Republic of
China;
|
|
|
·
|
“BVI”
are to the British Virgin Islands;
|
|
|
·
|
“Hong
Kong” are to the Hong Kong Special Administrative Region of the People’s
Republic of China;
|
|
|
·
|
“RMB”
are to Renminbi, the legal currency of China;
and
|
|
|
·
|
“U.S.
dollar,” “$” and “US$” are to the legal currency of the United
States.
|
1
PART
I
|
ITEM
1.
|
BUSINESS.
|
Through
our operating subsidiaries in China, we are engaged in the research,
development, production and sale of polypeptide-based nutritional supplements,
health foods, functional foods, nutricosmetics products and other related health
and wellness products. We believe we are one of the leading providers of
polypeptide-based health and wellness products in China.
Polypeptides
are small molecular structures consisting of 10-50 amino acids and have been
found to have high nutritional value and support body functions such as
regulating immunological functions. We focus on enzyme engineering in order to
produce micromolecular protein structures in the form of certain functional
peptides for use as nutritional ingredients, additives and supplements. We have
developed over 70 different types of polypeptide-based nutritional products. Our
key products include Polypeptide Protein Powder, which has been approved by the
PRC State Food and Drug Administration, or SFDA, and Shenguo Polypeptide
Capsules, which has been approved by the relevant health administration
authority of Hubei province and is in the process of gaining SFDA approval.
Other functional polypeptide-based nutritional supplement products include lipid
lowering soy peptide products. These products are manufactured using our
proprietary processing methods.
We
believe that, in China and internationally, we are one of the few companies that
have combined strong research and development capabilities, modernized
manufacturing facilities, a series of high quality diversified nutritional
products, superior customer services and competitive prices for
polypeptide-based products. According to the Polypeptide Industry Competitive
Landscape and Investment Strategy Research Report (2009-2012) by the Chinese
Industry Consulting Network, we are one of the largest companies focusing on
developing, producing and marketing polypeptide-based products in
China.
Our
products are primarily manufactured in our owned and operated
production facilities located on 16,477 square meters of land in the Hannan
Economic Development Zone in Wuhan, China which are planned to be further
expanded. Some of our nutritional supplements and personal care products are
manufactured for us through contractual relationships with other manufacturing
companies, in accordance with our own proprietary formulations. Our products
currently being marketed and sold in China have been tested and approved by the
relevant Chinese governmental hygiene and safety agencies such as the local
bureaus of Ministry of Health and the SFDA, where necessary. Our research and
development efforts are conducted in three different R&D centers from basic
peptide research to anti-aging focused applied research and related product
development in Wuhan, China.
Our
products are sold to customers both in China and internationally, with China
currently being the primary market, through a combined network of sales
personnel in our headquarters and throughout our branch sales offices in 15
provinces in China, wholesalers, distributors and private labeled
partners.
History
and Corporate Structure
China
Polypeptide Group, Inc., formerly known as Hamptons Extreme, Inc., was
incorporated under the laws of the State of Delaware in March 2007. The Company
was formed to engage in the design, manufacturing, distribution and marketing of
surfboards and related equipment. Prior to the reverse acquisition described
below, the Company was considered to be a development stage company with no
material assets or operations and had generated no revenues since its inception.
On July
28, 2008, China Polypeptide Group, Ltd., or CPG, our former parent company,
completed a reverse acquisition transaction through a share exchange with
Ablepeak International Limited, or Ablepeak, a British Virgin Islands company,
and Cantix, whereby CPG issued 55,487,956 shares of its common stock to Ablepeak
in exchange for all of the issued and outstanding capital stock of Cantix.
Cantix thereby became CPG’s wholly-owned subsidiary and Ablepeak became CPG’s
controlling stockholder.
2
On
November 13, 2009, we completed a reverse acquisition of Cantix whereby we
issued to CPG 1,100,000 shares of our common stock, constituting approximately
88% of our then issued and outstanding shares of common stock, in exchange for
all of the issued and outstanding capital stock of Cantix. Cantix thereby became
our wholly-owned subsidiary and its four direct and indirect subsidiaries,
Moneyeasy, Tallyho, Wuhan Anti-Aging and Hopsun, became our indirect
subsidiaries. As a result of the reverse acquisition, our principal business
became the business of Cantix, which, through its PRC-based indirect operating
subsidiaries, Tallyho, Hopsun and Wuhan Anti-Aging, engages in the research and
development, manufacturing, sales and marketing of polypeptide-based nutritional
supplements and health foods.
Cantix
was incorporated in the BVI as a startup stage company on January 29, 2007.
Moneyeasy is a Hong Kong business organization that was incorporated as a
startup stage company on August 25, 2006. Tallyho was organized under the laws
of the PRC on November 28, 1996 under the name of Wuhan Xinrongxin Biological
Product Co., Ltd. The name was changed to its current name on January 6, 1999.
Tallyho’s scope of business includes research and development, production and
sale of biological and nutritional supplements. Tallyho’s business license is
valid through December 24, 2037. Wuhan Anti-Aging was organized under the laws
of the PRC on June 27, 2007. Its primary business is research and development of
protein and polypeptide anti-aging biological products, sales and distribution
of developed products. Wuhan Anti-Aging’s business license is valid through June
13, 2037.
On
November 5, 2007, Moneyeasy entered into an equity purchase agreement to acquire
all the equity interest of Tallyho for RMB 40,230,000 (approximately
$5,854,361). An Approval for Establishment of Enterprise with Investments from
Taiwan, Hong Kong, Macao and Overseas Chinese was issued by the People’s
Government of Wuhan Municipality on November 27, 2007, in connection with
Moneyeasy’s acquisition of Tallyho. The transaction was also approved by the
Wuhan City Bureau of Commerce, subject to the PRC Regulations on Merger with and
Acquisition of Domestic Enterprises by Foreign Investors, which require the
equity interest transfer price to be paid in full by a date certain (July 26,
2008), or in the absence of such express date, within one (1) year commencing
from the issuance of the new business license. On March 25, 2008, a renewed
Business License was issued by the Wuhan Administration for Industry and
Commerce, and Tallyho was converted into a wholly foreign owned
enterprise.
On
December 18, 2007, Moneyeasy entered into another equity purchase agreement to
acquire all the capital of Wuhan Anti-Aging for RMB 8,000,000 (approximately
$1,045,246). Wuhan Anti-Aging received the Certificate of Approval issued by the
People’s Government of Wuhan Municipality on December 29, 2007, and a renewed
Business License from the Wuhan Administration for Industry and Commerce on
January 3, 2008. The acquisition was approved by the Wuhan City Bureau of
Commerce, subject to the PRC Regulations on Merger with and Acquisition of
Domestic Enterprises by Foreign Investors. In this case, the Wuhan City Bureau
of Commerce did not specify an exact date on which the full purchase price was
to be paid for the transferred equity. As such, Moneyeasy was not required to
pay the full purchase price under the agreement for one (1) year, commencing
from the issue of the new business license (i.e. January 2, 2009). By October
30, 2009, Moneyeasy had paid the full purchase price.
On
November 13, 2007, Tallyho established its operating PRC subsidiary, Hopsun and
contributed RMB 14,000,000 (approximately $2,008,227). Hopsun specializes in
service-based sales of health and anti-aging products.
Effective
September 28, 2010, Hopsun entered into a series of contractual arrangements
with Xingpu and its shareholders, Mr. Dongliang Chen, the Company’s Chief
Executive Officer and Board Chairman, and Mr. Shengfan Yan, the Company’s
President and Director, pursuant to which Xingpu became the Company’s variable
interest entity, or VIE. The VIE agreements include an exclusive business
cooperation agreement, through which Hopsun has the right to advise, consult
with, manage and operate Xingpu, and to collect fees based on its profits.
Xingpu's shareholders have also granted their voting rights over Xingpu to
Hopsun. In order to further reinforce Hopsun's rights to control and operate
Xingpu, Xingpu and its shareholders have granted Hopsun the exclusive right and
option to acquire all of their equity interests in Xingpu. Further, Xingpu’s
shareholders have pledged all of their rights, titles and interests in Xingpu to
Hopsun. In accordance with Accounting Standards Codification (“ASC”) Topic 810
and its related subtopics (formerly FASB Interpretation No. 46R, Consolidation
of Variable Interest Entities), Xingpu is now a VIE of the Company and the
Company is the primary beneficiary of Xingpu through Hopsun.
Xingpu is
a development stage company established on March 2, 2010. Its registered capital
is RMB 200,000,000 (approximately $29,255,164), of which RMB 40,000,000
(approximately $5,851,033) was contributed as of September 30, 2010. Xinpu’s
current purpose is to develop the Company’s regional headquarters and its
research and development center. The shareholders of Xingpu each hold a 50%
equity interest in Xingpu.
3
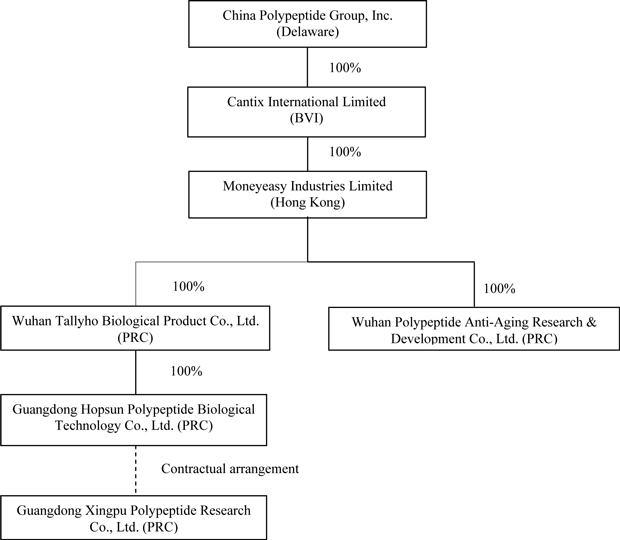
The
following chart reflects our organizational structure as of the date of this
annual report:
Our
Industry
Our
products are targeted for use in the anti-aging and health and wellness markets
and specifically, the nutraceutical (or the nutritional supplements) market, the
cosmetics market and other healthcare markets with a primary geographical focus
in China.
China’s
Economy
Despite
the global economic downturn and fluctuation, China’s economy has outpaced the
economy of many other countries and has seen a GDP growth rate of 8.7% for 2009.
According to the latest statistics, China’s economic growth was 10.6% during the
first 9 months of 2010 and according to a quarterly update report issued by the
World Bank in November 2010, China’s annual economic growth is expected to reach
10% for 2010, fuelled by a transformed growth model featuring less
government-led investment, a recovery in exports and solid domestic consumption.
The Chinese government has put increasing emphasis on domestic consumption to
support further economic growth and recognized it as an import area for economic
transformation. China’s retail sales in 2009 rose 16.9% in real terms, adjusted
for inflation, to approximately US$1.84 trillion and continued to grow 18.3%
year over year during the first 9 months of 2010. As a result of the continued
economic growth, the disposal incomes of Chinese people have kept rising and the
wealthy population has been increasing. We believe that this has led to
increasing awareness of the quality of living and health and driven the demand
for the types of products that we develop and offer.
4
The
Anti-aging Market
We
believe that, in view of the world demographic trends, anti-aging products and
services have become one of the most important and fastest growing areas across
the medical, cosmetic, nutraceutical and other related industries.
According
to a report by the United Nations, the number of seniors over 65 years old has
been increasing at an annual rate of 2.5% in recent years. Statistics and
predictions from the World Health Organization estimate that the elderly
population will reach two billion by 2050. According to the China National
Committee for Aging, an inter-ministry aging matter focused agency under the
State Council of China, the number of seniors over 60 years old reached 167
million by the end of 2009, representing more than 12% in the total population
of China. At an annual growth rate of more than 3% in recent years, China has
become the country with the largest aging population in the world.
A
nationwide nutrition and health status survey conducted by the Ministry of
Health of China which was issued in October 2004 disclosed that the number of
residents in China with chronic non-communicable diseases had been rising
rapidly as a result of unhealthy lifestyle. For example, the prevalence of
hypertension was increasing and the number of hypertension patients amounted to
over 160 million patients, an increase of 31% compared to that of 1991 when the
last same nationwide survey was conducted. The report also showed that chronic
diseases replaced infectious diseases as a major cause of death of residence in
China. The chronic non-communicable diseases are aging effects. The trends of
younger people getting such diseases has also led to increasing anti-aging needs
among middle-aged or even young people in China in recent years.
We
believe that due to lifestyle changes, deterioration of the environment,
increasing life expectancy of Chinese citizens, increasing awareness of quality
of life and health, the demands for anti-aging products such as nutritional
supplements, health foods and cosmetics products, are growing in China. Such
trends have the following characteristics:
|
|
a)
|
expansion
from wealthy population to massive
population;
|
|
|
b)
|
expansion
from aging people to younger
people;
|
|
|
c)
|
extension
from traditional products to new technology
products;
|
|
|
d)
|
migration
of focus from post-disease medical treatments to preventive treatments;
and
|
|
|
e)
|
change
from primary medicine taking to nutritional adjustments both inside and
outside the body.
|
The
Nutraceutical (or Nutritional Supplements) Market
According
to the China Health Care Association, or CHCA, a national association under the
Ministry of Health of China, a nutraceutical market research report released at
the Natural Products Expo West held in Anaheim, California in March 2010,
estimated the size of the nutraceutical market in China to reach US$13.4 billion
in 2009. As a result, China has exceed Japan to be the second largest country
market, following the U.S. Further, pursuant to other releases of CHCA, it is
estimated that the annual growth rate of health supplement consumption by
Chinese people in recent years lies between 15% and 30%, much higher than the
growth in developed countries.
Based on
our review of the experience of other developed countries, when per capita GDP
reaches $1,500 to $3,000, the nutraceutical industry will emerge and closely
follow the country’s economic growth. China’s per capita GDP exceeded $3,000 in
2008 to reach $3,260 and amounted to approximately $3,500 in 2009. The
nutraceutical industry in China has entered into a fast growing stage. Based on
the current growth rate, it is estimated that by 2010, there would be almost 20
cities of an aggregate 100 million in population with per capita GDP exceeding
$10,000. With increasing awareness of living and health quality and the growing
level of disposable income, people in these Chinese cities and other high income
regions present a very appealing market for nutraceutical companies such as
ours. We believe that we will be competitive in this market due to our unique
competitive advantages and our offering of quality products that are favored by
the market.
The
Cosmetic Market
The
cosmetics products industry has been one of the fastest growing industries in
China. According to an investment analysis and forecast report on the Chinese
cosmetics market issued by Chinese Investment Consultants, a leading Chinese
industry research institution, in 2008, the sales of the cosmetics market
increased annually by more than 20% in the past 10 years. It is expected that
the annual sales of cosmetics products would amount to approximately RMB 80
billion with an annual growth of 8.9% in 2010 and reach approximately RMB 110
billion in 2015 with an average annual growth 6.22%.
5
We
believe collagen products will be a major growth segment within the cosmetics
market in China in the next few years. According to a market research report on
collagen in China, it is forecasted that consumption of collagen products in
China will grow as fast as 20% annually. Taiwan pioneers mainland China in using
collagen products with annual consumption of more than NT40 billion ($1.24
billion). We believe that collagen-based nutricosmetics products that contain
small molecular weight collagen, such as Collagen Tripeptide, or CTP, will stand
out from other collagen products in such a trend. We have developed our
proprietary CTP technology and built one of the largest state-of-the-art CTP
production facilities in Asia to capitalize this market
opportunity.
Our
Competitive Strengths
We
believe that our competitive strengths include the following:
|
|
·
|
Advanced
polypeptide technology and research and development capabilities.
We have been researching and developing polypeptides since 1996. Our core
research team’s polypeptide research experience averages over 20 years. We
believe we are one of the leading companies developing polypeptide
technologies in China. For example, our company was the main institution
appointed to set the first peptide-related national standard, i.e., “Soy
Peptides Powder” (GB/T22492-2008) issued by the General Administration of
Quality Supervision, Inspection and Quarantine of China and the National
Standardization Administration of China. In addition, the Collagen
Tripeptide project conducted by our Company won Second Prize at the
Governmental Science and Technology Award given by the Wuhan Municipal
Government in December 2008, and the Polypeptide Beauty-slimming Capsule
product developed by our Company won the Technology Advancement Award in
October 2003 by the Organization Committee of the Third China
International Healthcare Festival. Our Research Center of Peptide
Materials, Anti-aging Research & Development Center and Applied
Research Center of Polypeptide-based Functional Food and Cosmetic have
gathered excellent researchers and scientists in the respective research
areas. We have three patents of invention and have applied for an
additional twelve patents with State Intellectual Property Office of The
PRC.
|
|
|
·
|
Experience
in the production of functional peptides. Unlike our competitors,
which offer soy and animal based peptide products, we provide a wide range
of functional peptide products that are extracted from many different
natural protein ingredients with our proprietary bio-engineering
technologies. Functional polypeptides create functional proteins which
shape themselves in stable, distinctive and discrete structure to perform
specific functions in the body. We believe our technologies and know-how
in the production of functional peptides allow us to produce products that
provide more body regulating and enhancement functions for consumers of
varying age groups and different health needs. We have developed over 70
kinds of functional polypeptide products, which provide a variety of
benefits such as basic nutrition, health-care and anti-aging
benefits.
|
|
|
·
|
Well known
brand names. We believe that two of our trademarks, “Tallyho” and
“Puzhongren” have achieved strong name recognition in local markets. The
provinces of Hubei and Guangdong issued us certificates recognizing these
two brands as “famous brands.” We believe that recognition of “Tallyho”
and “Puzhongren” brand names helps drive the sales of our products and
facilitates acceptance and market penetration of our new products. In
order to protect and enhance our “Tallyho” and “Puzhongren” brand names,
we rely on stringent quality control and our ongoing marketing,
advertising and public relations efforts and continue to stress the
quality, safety and innovativeness of our products. We believe that brand
awareness in our target markets and dedicated distribution channels, will
enable us to leverage goodwill from our brands into future
growth.
|
|
|
·
|
Proven and
stable management team. Our management team has a proven track
record of successful management and has a great deal of experience in the
nutritional supplement and anti-aging industry and business development.
Our management team is led by Dongliang Chen, who became our Chief
Executive Officer in November, 2009, after spending thirteen
years with one of our subsidiaries, Tallyho, where he most recently served
as the Chairman of the Board and Chief Executive Officer. Mr. Chen is the
inventor of two key patents, and the author of a number of science and
biology educational books on polypeptides. Since joining our Company, Mr.
Chen has assembled a team of experienced executives, including: Mr.
Shengfan Yan, our President, who has worked in the nutritional supplements
and related industries in China for over 15 years and has abundant
experience in sales and marketing of nutraceutical products; Mr. Richard
Liu, our Chief Financial Officer, who has over 12 years of experience in
accounting and financial management, including financial executive
experience with publicly-traded companies; Ms. Lirong Hu, our Treasurer,
who brings more than 25 years of accounting and financial management
experience to the Company; Mr. Jun Li, our Company Secretary, who brings
over 15 years of corporate and business development experience in
nutritional supplements and food industries in China. Our core management
and operation team at our operating subsidiaries have been stable and
working together for over 8
years.
|
6
|
|
·
|
Integrated
sales channel. We have a diversified sales network that allows us
to effectively market our products and services to our customers. Our
sales and marketing teams currently consist of approximately 256 full-time
employees and over 300 part-time employees. We sell our end-consumer
products at our community shops or customer service stations and we
usually organize events or seminars at which we provide complimentary
nutrition and anti-aging consultation. Our sales offices, community shops
and customer service stations are strategically located in densely
populated residential areas, within or near local shopping destinations or
in other easy access locations. Our customers place orders for our
products with our own sales staff at our sales locations and we deliver
the products directly to them or purchase them at our community shops. Our
sales network currently covers 15 provinces in China. We also use
third-party distributors to sell our end-consumer products to the selected
local markets. In addition, we leverage on our strong research and product
capabilities to develop and sell polypeptide ingredients and semi-final
products to other nutraceutical, food and cosmetics companies under the
OEM/ODM model, which we referred to as Project
Sales.
|
|
|
·
|
Productive
technology equipment and the advantage of processing technique. Our
products are produced in our own production facilities using modern
production lines, techniques and equipment. Our production facilities are
Good Manufacturing Practice, or GMP, certified for manufacturing health
foods and have the ISO 9001:2000 quality management system certification
and the Hazard Analysis and Critical Control Point, or HACCP, food safety
certification. We believe that our production facilities are satisfactory
for our current production needs but we are preparing to increase capacity
in the future.
|
Our
Growth Strategy
We are
committed to growing our business in the coming years. We will implement the
following strategic plans to take advantage of industry opportunities and our
competitive strengths:
|
|
·
|
Enhance
marking efforts. We plan to enhance our marketing and promotional
campaigns and increase advertisements to strengthen the market awareness
of our brands through both traditional and online media. We also plan to
leverage on more innovative and efficient marketing partners to expand our
market reach, especially for our new products targeted at the middle-aged
to youth markets. We intend to position ourselves as the market thought
leader in the polypeptide product category in China and plan to expand
into new overseas markets for functional polypeptide
products.
|
|
|
·
|
Introduce
new products. We plan to develop new polypeptide technologies and
products according to our market intelligence feedbacks, supported by our
strong research and product development capabilities, with a focus on
products with anti-aging functions that could maintain the health of the
middle and old aged people, ameliorate age related disease and improve
life quality, and the products that delay senility of skin and body,
maintain vigor of the middle aged and the youth. We also plan to develop
new prevalent nutraceutical and nutricosmetics products from our
proprietary functional peptide ingredients such as Collagen Tripeptide,
skin longevity factor peptide, corn peptide, etc. targeted at the
middle-aged to youth markets.
|
|
|
·
|
Further
expand our distribution network to increase the prevalence of our
products. We plan to continue to enhance and expand our
service-based sales network, in both existing and new regional markets,
and the third-party distributor network in selected local markets which
are not adequately covered by our own sales force. We plan to convert and
expand selected community shops or customer service stations into
exclusive shops at highly populated locations to gain additional sales
growth points. We also plan to expand the existing sales network of
functional polypeptide ingredients and OEM/ODM products to supply to more
major nutraceutical, food and cosmetics
partners.
|
In
addition to our organic growth strategies, we also intend to support and
accelerate our growth through acquisitions of potential target companies with
complementary strengths which can create synergies with our existing operations,
product offerings and technologies.
7
Our
Products
We have
developed over 70 different types of polypeptide-based nutritional products. Our
key products include Polypeptide Protein Powder, which has been approved by the
SFDA, and Shenguo Polypeptide Capsules, which has been approved by the relevant
health administration authority of Hubei province and is in the process of
gaining SFDA approval. Other functional polypeptide-based nutritional supplement
products include lipid lowering soy peptide products. These products are
manufactured using our proprietary processing methods.
Polypeptides
are small molecular structures consisting of 10-50 amino acids that have been
found to have nutritional value and support body functions such as regulating
immunological functions. We focus on enzyme engineering in order to produce
micromolecular protein structures in the form of certain functional peptides for
use as nutritional ingredients, additives and supplements.
Research
studies conducted by scientists at Hanyang University in Korea, the University
of Milan and the University of California (Davis), found that soy protein
peptide extracted from certain soybeans could inhibit the growth of metastatic
tumor cells, that the addition of soy protein to the diet could have the effect
of lowering total cholesterol concentrations in the body, and that soybean
peptide may prevent cancer from occurring in cultured cells and animals.
Polypeptide can be extracted from the tissues and organs of animals and plants
and processed in powder, capsule/tablet or injection form for internal use, or
as creams and lotions for topical use. Our polypeptide-based products are
produced in powder, capsule/tablet and liquid forms. Different combination of
polypeptides may be combined with other nutritional factors to form different
products with different benefits.
We have
built our reputation on developing formulas that blend the best of nature with
innovative techniques from nutrition science, to appeal to the growing base of
consumers desiring a healthier lifestyle and seeking differentiated nutritional
supplement and health food products. Our products are manufactured using our
proprietary processing methods.
Our
products can be divided into three (3) categories according to their service
target and functional features: (i) Functional Peptide Ingredients Products;
(ii) Polypeptide-based Healthy Foods and (iii) Polypeptide-based Special
Nutritional Foods.
Functional
Peptide Ingredient Products
Functional
peptide ingredient products are peptide-based ingredients which perform certain
physiological functions including regulating immunological functions, reducing
cholesterol, etc. We produce twelve (12) different functional peptide ingredient
products that we supply to other food, nutritional supplement, medicine and
cosmetics manufacturers. Our functional peptide ingredient products are used in
a variety of products ranging from nutritional supplements to skincare
products.
Polypeptide-based
Health Foods
Polypeptide-based
health foods are edible products with specific health care functions. Included
in this category is one of our key products, Polypeptide Protein Powder. These
products are verified, approved and licensed by SFDA. We produce three
polypeptide-based health food products and mainly sell them under our own brands
primarily through Hopsun’s nationwide sales network.
The
following is a brief description of our domestic polypeptide health food
products:
|
|
·
|
PZR
Protein Powder, which is marketed as a supplement to strengthen the immune
system;
|
|
|
·
|
Tallyho
Ziqing Soft Capsule, which is marketed as a supplement to aid in the
regulation of blood fat; and Anya
Taihe Slimming Capsule, which is marketed as a weight management
supplement.
|
8
Polypeptide-based
Special Nutritional Foods
Polypeptide-based
special nutritional foods are edible products with what we believe to have
special nutritional features which we develop for sale either directly to
consumers or to other nutraceutical distributors and marketers under an OEM/ODM
arrangement. Included in this category are some of our other key products,
Shenguo (Holy Fruit) Polypeptide Capsule, Hebaisui (Crane Longevity) Polypeptide
Tablet, Yanbaimei (All Charming Beauty) Polypeptide Tablet. We produce over
sixty different polypeptide-based special nutritional food products. Certain of
these products are sold under our own brands through Hopsun’s nationwide sales
network. We also sell these off-the-shelf products with our formula and
standards to other healthy food or nutraceutical companies, most of which are
primarily distribution or marketing companies with little development and
manufacturing capacities, under an ODM arrangement. In addition, we also utilize
the traditional OEM model to manufacture specific products according to formula
and the standards provided to us by our customers.
We are
developing orally administrated skin care and anti-aging nutritional products,
or nutricosmetics products, targeted at middle aged and young customers based on
new polypeptide ingredients such as Collagen Tripeptide, or CTP, which is
becoming very popular as a nutricosmetics supplement among young and middle aged
women in China and Asia.
Manufacturing
Our
products are primarily manufactured in our owned and operated production
facilities on a 16,477 square meter site located in the Hannan Economic
Development Zone, Wuhan City, Hubei Province, China. Upon the full commissioning
of our newly built start-of-the-art facilities in July 2010, we have a
designated production capacity of 1,000 tons of protein powder per annum, a
Collagen Tripeptide designated production capacity of 300 tons of polypeptide
ingredient per annum, a designated production capacity of 300 million of
capsule/tablet per annum. We are planning for further expansion of our
manufacturing capabilities for CTP ingredient and oral liquid form products to
meet the increasing market demands in the next few years. Our production facilities are
GMP certified for manufacturing health foods and have the ISO 9001:2000 quality
management system certification and the HACCP food safety
certification.
Some of
our nutritional supplements and personal care products are manufactured for us
through contractual relationships with other manufacturing companies, in
accordance with our own proprietary formulations. We work closely with such
production facilities in an effort to achieve the highest quality standards and
product availability.
Raw
Materials and Suppliers
Raw
materials for our products come from natural animal and vegetable proteins. For
example, collagen peptides and Collagen Tripeptides are extracted from marine
fish such as tilapia, soybean polypeptide products come from soybeans, albumin
polypeptide comes from eggs, and peanut peptides and corn peptides are taken
from peanuts and corn.
Raw
materials used in our products and our product packing materials are developed
in accordance with established criteria, including national standards, industry
standards, and enterprise standards. For products to be exported, international
standards must be followed.
Our
suppliers of raw materials and packing materials must provide us with the
following certificates of qualifications: production licenses, sanitary licenses
and other necessary certificates issued by relevant governmental authorities,
product quality standards, product test reports and certificates of conformance
of products.
Personnel
from our quality control department check all incoming raw materials against
specifications provided by the producers. Both the raw materials and its
packaging are inspected, and after this inspection, raw materials are sampled
according to national standards on sampling and are sent to our center test
room. Visual inspection, physical, chemical and hygiene tests are conducted
according to relevant standards. We submit items we are unable to test to
national test institutions for testing. Raw materials that fail testing are
returned to the suppliers and if we return a supplier’s raw materials twice,
they are no longer used by us.
We
currently maintain a sufficient supply of main raw materials for approximately
seven days of production. Prices for some of our key input materials, such as
soybean, eggs, herbs and packaging materials are increasing. However, we are
confident we can offset these increases with our cost reduction programs and by
raising the prices of our products.
The table
below lists as of September 30, 2010, the suppliers who supply more than 5% of
our products, raw materials and services, showing the cumulative dollar amount
of raw materials purchased from them during the 2010 fiscal year, and the
percentage of raw materials purchased from each supplier as compared to
procurement of all raw materials.
9
|
Rank
|
Supplier
|
Cumulative
Amount Purchased
(USD)
|
Percentage
of Total
Purchases
|
|||||||
|
1
|
Shandong
Linyi Shansong Biological Product Co., Ltd.
|
507,483 | 38 | % | ||||||
|
2
|
Hubei
Shenzhou Scientific, Industrial and Trading Development Co.,
Ltd.
|
145,420 | 9 | % | ||||||
|
3
|
Shenzhen
Jiuzhou Penda Can Manufactring Co., Ltd.
|
119,529 | 8 | % | ||||||
|
4
|
JiangXi
Cosen Biochemical Co., Ltd.
|
109,868 | 7 | % | ||||||
|
5
|
Bozhou
Zhigang Chinese Herb Distribution Center
|
108,925 | 7 | % | ||||||
Our
Customers
Our
products are sold to consumers both in China and internationally, with China
currently being the primary market, through a combined network of sales
personnel in our headquarters and throughout our branch sales offices in 15
provinces in China, and through third-party wholesalers, distributors and
private labeled partners.
The
geographic distribution of our customer base in China as of September 30, 2010
is as depicted in the table below:
|
Area
|
%
of Customers
|
|||
|
Southeast
|
30 | % | ||
|
South
|
19 | % | ||
|
East
|
19 | % | ||
|
North
|
15 | % | ||
|
Northeast
|
7 | % | ||
|
West
|
5 | % | ||
|
Middle
|
4 | % | ||
The
largest customers of ours by sales revenues are usually our local wholesalers,
distributors and private labeled partners. During the fiscal year ended
September 30, 2010, our six largest customers were Shenyang Zhongpu
Biological Product Co., Ltd., Nanjing Puhangtianqu Commercial Trading Co., Ltd.,
Chongqing Xinmi Commercial Trading Co., Ltd., Suzhou Qiqi Trading Co., Ltd.,
Kunming Youkun Commercial Trading Co., Ltd. and
Zhengzhou
Dinggeng Commercial Trading Co.,
Ltd..
In the aggregate, these customers accounted for
approximately $5.5 million, or 15%, of our total sales revenue in
2010.
We intend
to export our Collagen Tripeptide product to Japan. This product has passed a
qualification inspection and has been approved and certified by the Ministry of
Health, Labour and Welfare of Japan. We have appointed a Japanese professional
who has rich experience in the cosmetic and health food markets in Japan to help
us penetrate into the Japanese market and we have developed good relationships
with a number of relevant corporations in Japan.
Our
Sales and Marketing Efforts
We are
committed to building and expanding our sales network, customer base and brand
loyalty by providing a diverse portfolio of high quality polypeptide-based
health and wellness products. The breadth of our product offerings enables our
sales team and our distributors to sell a comprehensive package of nutritional
supplements and health foods that provide a variety of benefits, from
anti-oxidants to weight management and nutrition. Many of our product
formulations have been in existence for years and achieve good market acceptance
and loyalty. However, we continually review, and if necessary, improve our
product formulations based upon developments in nutritional science. We believe
that the longevity and variety in our product portfolio could significantly
enhance our sale effectiveness to expand our business.
We have a
diversified sales network allowing us to effectively market products and
services to our customers. Our sales and marketing department currently consists
of approximately 256 full-time employees and over 300 part-time employees. For
our own brand end consumer products, in addition to sales efforts conducted
directly by our internal sales team and other employees, we also use wholesalers
and distributors. We sell our own brand end consumer products from our
nationwide branch sales network, at community shops or customer service
stations, at exclusive shops, etc. and we usually organize promotional events or
informative seminars at which we provide complimentary nutrition and anti-aging
consultation, under the guidance and control of our consumer sales operation
headquarters in Guangzhou, i.e., Hopsun. These programs target specific consumer
market segments, such as women, men or children, as well as weight-management
customers and individuals seeking to enhance their overall well-being. Our
branch sales offices, service stations and exclusive shops are strategically
located in residential areas of high density, within or near local shopping
destinations and in other easy access locations. Our customers place orders for
our products with our own sales staff at our sales locations and we deliver the
products directly to them or purchase them at our community shops. Our
nationwide sales network covers 15 provinces in China. We also use third-party
distributors to sell our end-consumer products to the selected local markets. We
plan to expand our sales network or partner with more local wholesalers and
distributors to cover more Chinese cities.
10
The sales
team in our Wuhan office oversees sales of our ingredient and OEM/ODM products.
They provide one-stop-shop professional services from product formulation design
to packaging and marketing strategy for our enterprise customers.
Traditional
Sales Methods
We market
and sell some of our products under our own brand names to consumers through
regional dealers and network agents. In addition, we also provide manufacturing
services where we are contracted to manufacture products for third parties
either according to formulas and standards provided to us by such third parties
or according to our formula and standards for sale under our customers’ brand
names.
Innovative
Sales Methods
We have
developed two new sales models: project-based sales model and service-oriented
sales model. Currently, about 83% of our sales are derived through our
service-oriented model. We are also working to develop other sales channels as
well.
The
service-oriented sales model is a sales model through which we work to establish
strong relationships with customers by providing a series of value-added
services such as: health consultation and education services, psychological
counseling services, and physical examination services, among other services. We
believe that providing these services will help us establish brand image,
promote our products, convey product efficacy and benefits, and thus stimulate
demands and increase sales of our products.
The
project-based sales model is a sales model used in connection with the
manufacturing services we provide to customers. In the project-based sales
model, we define each customer’s business as one project, and provide each
customer with project-based one-stop-shop services, including market
consultation, technical demonstration, market positioning, product development,
manufacturing, test and evaluation, market access consultation, package design,
market planning, marketing and after-sale service. We work to assist our
customers in making reasonable use of polypeptide ingredients to develop
products and promote growth of the customer’s market. We believe that by working
to increase our contract manufacturing customer’s sales, sales of our
polypeptide ingredients will increase as well.
Our
Competition
We
believe that the nutraceutical or nutritional supplement business, both within
PRC and globally, is highly fragmented and intensely competitive. In our
industry, we compete based upon product quality, product cost, ability to
produce a diverse range of products and logistical capabilities. Many of our
competitors, both domestic and international, have greater research and
development capabilities and financial, scientific, manufacturing, marketing and
sales resources than we do. Although our marketing and sales efforts of
polypeptide-based products are limited as compared to our competition, we
believe that we have a competitive advantage due to the price and quality of our
products and our ability to produce a diverse range of products and customer
services.
Internationally,
there are companies that apply polypeptide technology in developing new drugs,
but we believe there are few companies that apply polypeptide technology to the
nutritional supplement field. We believe that in China, we are one of the
largest companies focusing on developing, producing and marketing functional
peptide nutritional products. We believe the other companies that sell
polypeptide products are either small size companies or large nutritional
products companies with only small percentage of revenue deriving from
polypeptide products.
11
Our
Research and Development Efforts
We
conduct our research and development efforts independently at our research and
development facilities in Wuhan, China. Our research and development department
is responsible for developing advanced technologies and new polypeptide based
products and for training and testing. As of September 30, 2010, we have a total
of thirty-six research personnel, including retired senior researchers who serve
as consultants for us, two with doctorate degrees and six with master degrees.
The rest are college graduates. Among the research personnel, twelve people are
of senior researcher level, four people are of intermediate researcher level,
and eleven people are of assistant researcher level.
Research
Centers
We have
set up the following three research and development centers.
Research Center of Peptides
Materials. This research center was set up in 1998. The primary purpose
of this research center is to (i) study the methods and conditions of extracting
polypeptides from animals and plants, (ii) test and measure the functional
polypeptides’ physiological regulation functions on humans, and (iii) test the
physical and chemical indicators, from both basic and applied research and
development perspective.
Anti-aging Research &
Development Center. This center was set up in 2007. The center engages in
the research and development of anti-aging products and pilot plant test work.
This center focuses on developing new products with anti-aging functions (such
as Shengguo polypeptide capsule, youth vigor tablet) through screening of
special antioxidants and research of combination of special antioxidants with
the polypeptide materials.
Applied Research Center of
Polypeptide-based Functional Foods & Cosmetics. This center was set
up in 2008 and is primarily engaged in (i) the study and application of
polypeptide-based functional foods & cosmetics, (ii) the development of new
products that are anti-aging and capable of ameliorating fundamental nourishment
supply & health conditions, and (iii) improvement and innovation of
functional food production & processing methods.
Research
Group
We employ
a group of senior in-service professionals in above research centers, most of
who are experts in the polypeptide industry. The leading researchers include
follows:
|
Name
|
Title
|
Specialty
|
||
|
Wang
Ajing
|
Distinguished
Professor
|
Biochemistry
|
||
|
Sun
Xiufa
|
Chief
Scientist
|
Biochemistry
|
||
|
Xia
Wenshui
|
Chief
Scientist
|
Food
Engineering
|
||
|
Mo
Zhaohui
|
Chief
Engineer
|
Processing
Technology
|
||
|
Ji
Jinlin
|
Researcher
|
Electrophorisis,
Chromatography, High Efficiently Liquid Chromatographym,
etc.
|
||
|
Yu
Chenggao
|
Researcher
|
Physiology
and Nutrition
|
During
the fiscal years ended on September 30, 2010 and 2009, we incurred $311,226 and
$ 285,496, respectively, in research and development expenses with respect to
our polypeptide-based products.
Our
Intellectual Property
The
continued success of our business is dependent on our intellectual property
portfolio consisting of registered trademarks, design patents and utility
patents related to our products.
We
currently have three patents which have been granted by the State Intellectual
Property Office of the PRC. The first, for a “processing method of soy
polypeptide dry powder with the function of blood lipid lowering,” expires in
December 2020, the second, for a “processing method of non-bitter soy
polypeptide powder,” expires in May 2022 and the third, for a “processing method
of coleseed peptide with direct enzymolysis of colza meal,” expires in April
2030. We have also applied for an addition twelve patents, which are still in
process.
12
We also
have registered trademarks for “Tallyho”, “AnErYa,” “TuoNiAoMoLiJian” and
“PuZhongRen.”
In
addition, we protect our know-how technologies through confidentiality
agreements we entered into with our key employees that have access to our know
how information among our management members and in our research and production
departments.
Our
Employees
As of
September 30, 2010, we had approximately 455 full-time employees and 300
part-time employees. The following table sets forth the number of our full-time
employees by function:
|
Function
|
Number
|
|
|
Executive
|
5
|
|
|
Administrative
|
19
|
|
|
Sales
and Marketing
|
256
|
|
|
Research
and Development
|
25
|
|
|
Production
|
98
|
|
|
Accounting*
|
52
|
* Including the accounting staff at our sales branches.
Our
ability to achieve our operational and financial objectives depends in part upon
our ability to retain key technical, marketing and operations personnel, and to
attract new employees as required to support growth. Working capital constraints
may impair our ability to retain and attract the staff needed to maintain
current operations and meet the needs of anticipated growth.
In
addition, we rely on consultants to a significant extent to supplement our
regular employee staff in certain key functional areas and to support management
in the execution of our business strategy. These consultants are independent
contractors. There can be no assurance that, if one or more of the consultants
were to terminate their services, we would be able to identify suitable
replacements. Failure to do so could materially and adversely affect our
operating and financial results.
As
required by applicable Chinese law, we have entered into employment contracts
with all of our officers, managers and employees. The remuneration payable to
employees includes basic salaries and allowances. In addition, our employees in
China participate in a state pension scheme organized by Chinese municipal and
provincial governments. We are required to contribute to the scheme at a rate of
28% of the average city monthly salary. In addition, we are required by Chinese
law to cover employees in China with various types of social insurance. We have
purchased social insurance for all of full time our employees.
We
believe that our relationship with our employees is good. We have not
experienced any significant problems or disruption to our operations due to
labor disputes, nor have we experienced any difficulties in recruitment and
retention of experienced staff.
Environmental
Matters
Our
manufacturing facilities are subject to various pollution control regulations
with respect to noise, water and air pollution and the disposal of waste and
hazardous materials. We are also subject to periodic inspections by local
environmental protection authorities. Our operating subsidiaries have received
certifications from the relevant PRC government agencies in charge of
environmental protection indicating that our business operations are in material
compliance with the relevant PRC environmental laws and regulations. We are not
currently subject to any pending actions alleging any violations of applicable
PRC environmental laws.
13
Regulation
General
Regulation of Business
Polypeptide
based products are classified under PRC laws into three categories, based on
potential uses, and each category is subject to separate PRC laws and
regulations. All our polypeptide products must obtain approvals from local
administration authority of health, prior to distribution to customers.
Additionally, for those health food products, we are required to obtain approval
from the SFDA before we can make specific claims regarding the health benefits
of the product. Currently, all of our products have received local
administration authority of health approvals and may be distributed to
customers.
If our
polypeptide products are sold for further food manufacturing, they are
considered to be food additives under the relevant PRC laws and regulations, and
the Company is required to obtain two licenses prior to the distribution of such
products to customers: the Food Hygiene License issued by the relevant local
administration authority of health and the Food Production License issued by the
provincial branch of the General Administration of Quality Supervision,
Inspection and Quarantine, or the Quality Administration. We have obtained both
the Food Hygiene License issued by the Hubei Ministry of Health and the Food
Production License issued by the provincial Quality Administration.
Taxation
On March
16, 2007, the National People’s Congress of China passed a new Enterprise Income
Tax Law, or EIT Law, and on November 28, 2007, the State Council of China passed
its implementing rules, which took effect on January 1, 2008. Before the
implementation of the EIT Law, foreign invested enterprises, or FIEs,
established in the PRC, unless granted preferential tax treatments by the PRC
government, were generally subject to an enterprise income tax, or EIT, rate of
33.0%, which included a 30.0% state income tax and a 3.0% local income tax. The
EIT Law and its implementing rules impose a unified EIT of 25.0% on all
domestic-invested enterprises and FIEs, unless they qualify under certain
limited exceptions. Despite these changes, the EIT Law gives FIEs established
before March 16, 2007, or Old FIEs, a five-year grandfather period during which
they can continue to enjoy their existing preferential tax treatments. During
this five-year grandfather period, the Old FIEs which enjoyed tax rates lower
than 25% under the original EIT law will be subject to gradually increased EIT
rates over a 5-year period until their tax rate reaches 25%. In addition, the
Old FIEs that are eligible for other preferential tax treatments by the PRC
government under the original EIT law are allowed to continue enjoying their
preference until these preferential treatment periods expire.
In
addition to the changes to the current tax structure, under the EIT Law, an
enterprise established outside of China with “de facto management bodies” within
China is considered a resident enterprise and will normally be subject to an EIT
of 25% on its global income. The implementing rules define the term “de facto
management bodies” as “an establishment that exercises, in substance, overall
management and control over the production, business, personnel, accounting,
etc., of a Chinese enterprise.” If the PRC tax authorities subsequently
determine that we should be classified as a resident enterprise, then our
organization's global income will be subject to PRC income tax of 25%. For
detailed discussion of PRC tax issues related to resident enterprise status, see
Item 1A, “Risk Factors – Risks Related to Doing Business in China – Under the
Enterprise Income Tax Law, we may be classified as a ‘resident enterprise' of
China. Such classification will likely result in unfavorable tax consequences to
us and our non-PRC stockholders.
In
addition, the EIT Law and its implementing rules generally provide that a 10%
withholding tax applies to China-sourced income derived by non-resident
enterprises for PRC enterprise income tax purposes unless the jurisdiction of
incorporation of such enterprises’ shareholder has a tax treaty with China that
provides for a different withholding arrangement. Tallyho and Wuhan Anti-Aging
are considered FIEs and are directly held by our subsidiary in Hong Kong.
According to a 2006 tax treaty between the Mainland and Hong Kong, dividends
payable by an FIE in China to the company in Hong Kong who directly holds at
least 25% of the equity interests in the FIE will be subject to a no more than
5% withholding tax. We expect that such 5% withholding tax will apply to
dividends paid to Moneyeasy by Tallyho and Wuhan Anti-Aging, but this treatment
will depend on our status as a non-resident enterprise.
14
Foreign
Currency Exchange
Substantially,
all of our sales revenue and expenses are denominated in RMB. Under the PRC
foreign currency exchange regulations applicable to us, RMB is convertible for
current account items, including the distribution of dividends, interest
payments, trade and service-related foreign exchange transactions. Currently,
our PRC operating subsidiaries may purchase foreign currencies for settlement of
current account transactions, including payments of dividends to us, without the
approval of the PRC State Administration of Foreign Exchange, or SAFE, by
complying with certain procedural requirements. Conversion of RMB for capital
account items, such as direct investment, loan, security investment and
repatriation of investment, however, is still subject to the approval of SAFE.
In particular, if our PRC operating subsidiaries borrow foreign currency through
loans from us or other foreign lenders, these loans must be registered with
SAFE, and if we finance the subsidiaries by means of additional capital
contributions, these capital contributions must be approved by certain
government authorities, including the PRC Ministry of Commerce, or MOFCOM, or
their respective local branches. These limitations could affect our PRC
operating subsidiaries’ ability to obtain foreign exchange through debt or
equity financing.
Dividend
Distributions
Our
revenues are earned by our PRC subsidiaries. However, PRC regulations restrict
the ability of our PRC subsidiaries to make dividends and other payments to
their offshore parent company. PRC legal restrictions permit payments of
dividend by our PRC subsidiaries only out of their accumulated after-tax
profits, if any, determined in accordance with PRC accounting standards and
regulations. Each of our PRC subsidiaries is also required under PRC laws and
regulations to allocate at least 10% of our annual after-tax profits determined
in accordance with PRC GAAP to a statutory general reserve fund until the
amounts in such fund reaches 50% of its registered capital. These reserves are
not distributable as cash dividends. Our PRC subsidiaries have the discretion to
allocate a portion of their after-tax profits to staff welfare and bonus funds,
which may not be distributed to equity owners except in the event of
liquidation.
The
Company intends on reinvesting profits, if any, and does not intend on making
cash distributions of dividends in the near future.
|
RISK
FACTORS.
|
RISKS
RELATED TO OUR BUSINESS
Our
operations, sales, profit and cash flow will be adversely affected if our
polypeptide products fail inspection or are delayed by regulators.
We are
responsible for product testing in our own facility for polypeptide products
sold in China and each batch of our products requires inspection by our Quality
Control center prior to shipment to our customers. The results of product
testing is recorded and approved by the local Quality and Technical Supervision
Department, and such testing must be in accordance with the national quality
standards issued by the National Standardization Administration of China and the
relevant industry quality standards where applicable, which require testing of
such factors including, but not limited to, appearance, packing capacity,
thermal stability, PH value, protein content and percentage of purity of the
product. Government regulators routinely inspect samples from each batch of our
polypeptide products and for export products, e.g., a sample of each shipment is
tested by the China Entry/Exit Inspection and Quality department. In the event that the
regulators delay the approval of our products, change the requirements in such a
way that we are unable to comply with those requirements, or require our other
products to be inspected by regulators before we can ship them to our customers,
our operations, sales, profit and cash flow will be adversely
affected.
15
The nutritional supplement industry in
the PRC is strictly regulated and changes in such regulations may have an
adverse effect on our business.
The
nutritional supplement industry in the PRC is strictly regulated by the state.
The regulatory regime requires administrative approval of supplements, food
additives and production, and comprises a series of regulations and
administrative rules. From time to time, the PRC regulatory authorities may
amend such regulations and administrative rules or promulgate new ones. Our
inability to comply with any such changes in regulations and administrative
rules would have an adverse impact on our business.
We
may not be able to carry on our business if we lose any of the permits and
licenses required by the PRC Government in order to carry on our
business.
All
nutritional supplement manufacturing and distribution enterprises in the PRC are
required to obtain from various PRC governmental authorities certain permits and
licenses, including, in the case of manufacturing enterprises and distribution
enterprises such as ours, a Food Manufacturing Permit, a Food Product
Distribution Permit, and a GMP certificate.
We have
obtained permits and licenses and the GMP certificates, required for the
manufacture of our nutritional supplement products. However, the permits and
licenses held by us are subject to periodic renewal and/or reassessment by the
relevant PRC governmental authorities and the standards of compliance required
in relation thereto may from time to time be subject to change. We intend to
apply for the renewal of such permits and licenses when required by applicable
laws and regulations. Any changes in compliance standards, or any new laws or
regulations that may prohibit or render it more restrictive for us to conduct
our business or increase our compliance costs may adversely affect our
operations or profitability. Any failure by us to obtain such renewals may have
a material adverse effect on the operation of our business. In addition, we may
not be able to carry on business without such permits and business licenses
being renewed.
Our inability to
successfully research and develop new polypeptide based
products could have an adverse effect on our future growth.
We
believe that the successful development of nutritional supplements and health
food products can be affected by many factors. Products that appear to be
promising in the early phases of research and development may fail to be
commercialized for various reasons, including, but not limited to, the failure
to obtain the necessary regulatory approvals. There is no assurance that our
future research and development projects will be successful or that they will be
completed within the anticipated timeframe or budget. Also, there is no
guarantee that we will receive the necessary approvals from relevant authorities
for the production of our newly developed products. Even if such products could
be successfully commercialized, there is no assurance that they will be accepted
by the market as anticipated.
We
may not be able to adequately finance the significant costs associated with the
development of new polypeptide-based nutritional supplement
products.
The
products in the nutritional supplement market can change dramatically with new
technological advancements. We are currently conducting research and development
on new products, which requires a substantial outlay of capital. To remain
competitive, we must continue to incur significant costs in product development,
equipment, facilities and invest in research and development of new products.
These costs may increase, resulting in greater fixed costs and operating
expenses.
In
addition to research and development costs, we could be required to expend
substantial funds for and commit significant resources to the
following:
|
|
·
|
additional
engineering and other technical
personnel;
|
|
|
·
|
advanced
design, production and test
equipment;
|
|
|
·
|
manufacturing
services that meet changing customer
needs;
|
|
|
·
|
technological
changes in manufacturing processes;
and
|
|
|
·
|
manufacturing
capacity.
|
Our
future operating results will depend to a significant extent on our ability to
continue to provide new and competitive products that compare favorably on the
basis of cost and performance with the design and manufacturing capabilities of
competitive third-party technologies. We will need to sufficiently increase our
net sales to offset these increased costs, the failure of which would negatively
affect our operating results.
16
We
may not be able to execute our business expansion plan successfully.
Furthermore, expansion of our business may put added pressure on our management,
financial resources and operational infrastructure, thus impeding our ability to
meet any increased demand for our products and hurting our operating
results.
Our
business plan is intended to significantly grow our operations to meet
anticipated growth in demand for existing products, and to introduce new product
offerings. Our planned growth includes the construction of several new
production lines to be put into operation over the next five years. Growth in
our business may place a significant strain on our personnel, management,
financial systems and other resources. We may be unable to successfully and
rapidly expand sales to potential customers in response to potentially
increasing demand or control costs associated with our growth.
To
accommodate any such growth and compete effectively, we may need to obtain
additional funding to improve information systems, procedures and controls and
expand, train, motivate and manage our employees, and such funding may not be
available in sufficient quantities, if at all. If we are not able to manage
these activities and implement these strategies successfully to expand to meet
any increased demand, our operating results would suffer.
If
we fail to effectively manage our growth, our business and operating results
could be harmed.
We have
experienced, and continue to experience, growth in our operations which has
placed, and will continue to place, significant demands on our management,
operational and financial infrastructure. If we do not effectively manage our
growth, the quality of our products and services could suffer, which could
negatively affect our operating results. To effectively manage this growth, we
will need to continue to improve our operational, financial, and management
controls and our reporting systems and procedures. These systems enhancements
and improvements may require significant capital expenditures and management
resources. Failure to implement these improvements could hurt our ability to
manage our growth and our financial position.
Future
acquisitions may have an adverse effect on our ability to manage our
business.
Selective
acquisitions form part of our strategy to further expand our business. If we are
presented with appropriate opportunities, we may acquire additional companies,
products or technologies. Future acquisitions and the subsequent integration of
new companies into ours would require significant attention from our management.
Our company has little experience with integrating newly acquired businesses.
Potential problems encountered by each organization during mergers and
acquisitions would be unique, posing additional risks to the company. The
diversion of our management’s attention and any difficulties encountered in any
integration process could have an adverse effect on our ability to manage our
business. Future acquisitions would expose us to potential risks, including
risks associated with the assimilation of new operations, technologies and
personnel, unforeseen or hidden liabilities, the diversion of resources from our
existing businesses and technologies, the inability to generate sufficient
revenue to offset the costs and expenses of acquisitions, and potential loss of,
or harm to, relationships with employees, customers and suppliers as a result of
integration of new businesses.
We
may require additional financing to expand and/or fund our operations and our
failure to obtain necessary financing may impair our operations.
As of
September 30, 2010, we had working capital of approximately $22.4 million. Our
capital requirements in connection with the development of our business are
significant. We may require additional financing to fund and expand our
operations. If we seek to obtain additional financing through sales of our
securities, it may be necessary for us to sell our securities at a price which
is at a significant discount from the market price and on other terms which may
be disadvantageous to us. In connection with any such financing, we may be
required to provide registration rights to the investors and pay damages to the
investor in the event that the registration statement is not filed or declared
effective by specified dates. The price and terms of any financing which would
be available to us could result in both the issuance of a significant number of
shares and significant downward pressure on our stock prices. The inability to
obtain additional financing on favorable terms, if at all, could have a material
adverse effect on our business.
17
We
may need to obtain additional debt or equity financing which may result in
dilution to our shareholders and have a material adverse economic effect on our
business.
We may
need to obtain additional debt or equity financing to fund our capital
expenditures. Additional equity financing may result in dilution to our
shareholders. Additional debt financing may be required, which, if obtained,
may:
|
|
·
|
limit
our ability to pay dividends or require us to seek consents for the
payment of dividends;
|
|
|
·
|
increase
our vulnerability to general adverse economic and industry
conditions;
|
|
|
·
|
limit
our ability to pursue our growth
plan;
|
|
|
·
|
require
us to dedicate a substantial portion of our cash flow from operations as
payment for our debt, thereby reducing availability of our cash flow to
fund capital expenditures, working capital and other general corporate
purposes; and/or
|
|
|
·
|
limit
our flexibility in planning for, or reacting to, changes in our business
and our industry.
|
We cannot
assure you that we will be able to obtain the additional financing on terms that
are acceptable to us, if at all.
We
may encounter increased competition from both local and overseas enterprises as
a result of a relaxation of the PRC regulatory approval process for
polypeptide-based biopharmaceutical products or due to an ease in international
trade restrictions.
Our
continued ability to compete depends on the development of the polypeptide-based
nutritional supplement manufacturing industry in China. The polypeptide-based
nutritional supplement manufacturing industry in China is highly regulated by
both provincial and central governments. Prior to engaging in the production and
distribution of polypeptide-based nutritional supplement products, companies
such as ours are required to obtain production permits and certificates for each
new product formulation from the various provincial food and drug authorities.
We have the advantage of having been approved by the state to produce and
distribute polypeptide-based nutritional supplement products in Hubei Province
and Guangdong Province, and our research and development department has become
familiar with the provincial product approval process. However, while we believe
that the regulatory requirements pose a competitive barrier to entry into the
nutritional supplement industry, there may be new entrants over time. If the
government relaxes these restrictions and allow more competitors to enter into
the market, these competitors may have more capital, better research and
development resources, manufacturing and marketing capability and experience
than us. Our profitability may be adversely affected if (i) competition
intensifies; (ii) competitors drastically reduce prices; or (iii) competitors
develop new products having comparable applications or benefits which are more
effective and/or less costly than those produced by us.
In
addition we expect that competition from imported products will increase as a
result of a trend towards lower import tariffs and China’s admission as a member
of the WTO in December 2001. We believe that lower import tariffs will result in
more affordable pricing for imported polypeptide-based nutritional supplement
products manufactured overseas as compared to domestically manufactured products
such as ours. In addition, China’s membership in the WTO makes it more
accessible to foreign biopharmaceutical manufacturers who may wish to set up
production facilities in the PRC and compete directly with domestic
manufacturers. The expected increased supply of both domestic and foreign
competitively priced biopharmaceutical products in the PRC will result in
increased competition. There is no assurance that our strategies to remain
competitive can be implemented successfully as scheduled, if at all. Our
inability to remain competitive may have an adverse effect on our profitability
and prospects.
We
engage in international sales, which expose us to trade restrictions that could
harm our business and competitive position.
As a
result of our product sales in various geographic regions, we may be subject to
the risks associated with customs duties, export quotas and other trade
restrictions that could have a significant impact on our revenue and
profitability. While we have not encountered significant difficulties in
connection with the sales of our products in international markets, the future
imposition of, or significant increases in, the level of custom duties, export
quotas or other trade restrictions could have an adverse effect on us. Further,
we cannot assure you that the laws of foreign jurisdictions where we sell and
seek to sell our products afford similar or any protection of our intellectual
property rights as may be available under the U.S. laws. We are directly
impacted by the political, economic, military and other conditions in the
countries where we sell or seek to sell our products.
18
We
depend on key personnel, and turnover of key employees and senior management
could harm our business.
Our
future business and results of operations depend in significant part upon the
continued contributions of our key technical and senior management personnel,
including specifically, Dongliang Chen, our Chairman and Chief Executive
Officer, Shengfan Yan, our President, Richard Liu, our Chief Financial Officer,
and Lirong Hu, our Treasurer and Jun Li, our Company Secretary. They also depend
in significant part upon our ability to attract and retain additional qualified
management, technical, marketing and sales and support personnel for our
operations. If we lose a key employee or if a key employee fails to perform in
his or her current position, or if we are unable to attract and retain skilled
employees as needed, our business could suffer. Significant turnover in our
senior management could significantly deplete our institutional knowledge held
by our existing senior management team. We depend on the skills and abilities of
these key employees, as well as the intellectual property owned by Chen and
licensed to us on an exclusive and free of charge basis for the life of the
patent rights granted, in managing the development, manufacturing, technical,
marketing and sales aspects of our business, any part of which could be harmed
by further turnover. In addition, we rely on consultants to a significant extent
to supplement our regular employee staff in certain key functional areas and to
support management in the execution of our business strategy. These consultants
are independent contractors. There can be no assurance that, if one or more of
the consultants were to terminate their services, we would be able to identify
suitable replacements. Failure to do so could materially and adversely affect
our operating and financial results.
We
may incur material product liability claims, which could increase our costs and
harm our financial condition and operating results.
Our
products consist of polypeptides, proteins, herbs, vitamins and minerals and
other ingredients that are classified as foods or dietary supplements and are
not subject to pre-market regulatory approval in China and other countries to
which we have or will export our products such as the United States. Our
products could contain contaminated substances, and some of our products contain
innovative ingredients that do not have long histories of human consumption. We
generally do not conduct or sponsor clinical studies for our products and
previously unknown adverse reactions resulting from human consumption of these
ingredients could occur. As a marketer of dietary and nutritional supplements
and other products that are ingested by consumers or applied to their bodies, we
have been, and may again be, subjected to various product liability claims,
including that the products contain contaminants, the products include
inadequate instructions as to their uses, or the products include inadequate
warnings concerning side effects and interactions with other substances. It is
possible that widespread product liability claims could increase our costs, and
adversely affect our revenues and operating income. Moreover, liability claims
arising from a serious adverse event may increase our costs through higher
insurance premiums and deductibles, and may make it more difficult to secure
adequate insurance coverage in the future. In addition, our product liability
insurance may fail to cover future product liability claims, thereby requiring
us to pay substantial monetary damages and adversely affecting our business.
Finally, given the higher level of self-insured retentions that we have accepted
under our current product liability insurance policies, which are as high as
approximately $10 million, in certain cases we may be subject to the full amount
of liability associated with any injuries, which could be
substantial.
An
increase in the cost of raw materials will affect sales and
revenues.
Raw
materials required for polypeptide production include soybeans, collagen
extract, eggs, herbs, fish and packaging materials. Any increase in the prices
of these raw materials will affect the price at which we can sell our products.
If we are not able to raise our prices to pass on increased costs, we would be
unable to maintain our margins.
A
disruption in the supply of utilities, fire or other calamity at our
manufacturing plant would disrupt production of our products and adversely
affect our sales.
Our
products are manufactured at our production facility located in Hubei Province
in the PRC. While we have not in the past experienced any calamities which
disrupted production, any disruption in the supply of utilities, particularly
electricity or power supply, or any outbreak of fire, flood or other calamity or
natural disaster resulting in significant damage at our facilities would
severely affect our production and have a material adverse effect on our
business, financial condition and results of operations.
We
maintain insurance policies covering losses with respect to damages to our
certain properties such as vehicles. We are currently planning to purchase
insurance coverage for other properties including buildings, machinery and
inventories. There is no assurance that our insurance would be sufficient to
cover all of our potential losses.
19
Our
intellectual property rights are valuable, and any inability to protect them
could reduce the value of our products, services, and brand.
Our
patents, trademarks, trade secrets, copyrights and other intellectual property
rights are important assets for us. Various events outside of our control pose a
threat to our intellectual property rights as well as to our products and
services. For example, effective intellectual property protection may not be
available in China and other countries in which our products are sold. Also, the
efforts we have taken to protect our proprietary rights may not be sufficient or
effective. Any significant impairment of our intellectual property rights could
harm our business or our ability to compete. Also, protecting our intellectual
property rights is costly and time consuming. Any increase in the unauthorized
use of our intellectual property could make it more expensive to do business and
harm our operating results.
If
we are not able to adequately secure and protect, or if we fail to prevent the
loss or misappropriation of, or disputes over, our patent, trademark and other
proprietary rights, we will lose our competitive advantage and our business will
be materially affected.
The
continued success of our business is dependent on our intellectual property
portfolio, which consists of registered trademarks, design patents and utility
patents related to polypeptide-based products. We currently have three patents,
a process technology for producing Soy Polypeptide Powder with lipid lowering
function, a method for producing Soybean Polypeptide Powder without
bitterness and a
preparation method of rapeseed peptide from defatted rapeseed by direct
enzymatic hydrolysis. We also have an additional nine patents in the application
process. The patent applications are subject to approval from the relevant PRC
authorities. We may not be able to successfully obtain the approval of the PRC
authorities for our patent applications. Furthermore, third parties may assert
claims to our proprietary procedures, technologies and systems. These
proprietary procedures, technologies and systems are important to our business
as they allow us to maintain our competitive edge over our
competitors.
While we
are not aware of any infringement on our intellectual property and we have not
been notified by any third party that we are infringing on their intellectual
property, our ability to compete successfully and to achieve future revenue
growth will depend, in significant part, on our ability to protect our
proprietary technology and operate without infringing upon the intellectual
property rights of others. The legal regime in China for the protection of
intellectual property rights is still at its early stage of development.
Intellectual property protection became a national effort in China in 1979 when
China adopted its first statute on the protection of trademarks. Since then,
China has adopted its Patent Law, Trademark Law and Copyright Law and
promulgated related regulations such as the Regulation on Computer Software
Protection, Regulation on the Protection of Layout Designs of Integrated
Circuits and Regulation on Internet Domain Names. China has also acceded to
various international treaties and conventions in this area, such as the Paris
Convention for the Protection of Industrial Property, Patent Cooperation Treaty,
Madrid Agreement and its Protocol Concerning the International Registration of
Marks. In addition, when China became a party to the World Trade Organization in
2001, China amended many of its laws and regulations to comply with the
Agreement on Trade-Related Aspects of Intellectual Property Rights. Despite many
laws and regulations promulgated and other efforts made by China over the years
with a view to tightening up its regulation and protection of intellectual
property rights, private parties may not enjoy intellectual property rights in
China to the same extent as they would in many Western countries, including the
United States, and enforcement of such laws and regulations in China have not
achieved the levels reached in those countries. Both the administrative agencies
and the court system in China are not well-equipped to deal with violations or
handle the nuances and complexities between compliant technological innovation
and non-compliant infringement.
We rely
on confidentiality agreements with our management and employees to protect our
confidential proprietary information. However, the protection of our
intellectual properties may be compromised as a result of:
|
|
·
|
departure
of any of our management members or employees in possession of our
confidential proprietary
information;
|
|
|
·
|
breach
by such departing management member or employee of his or her
confidentiality and non-disclosure undertaking to
us;
|
|
|
·
|
infringement
by others of our proprietary information and intellectual property rights;
or
|
|
|
·
|
refusal
by relevant regulatory authorities to approve our patent or trademark
applications.
|
20
Any of
these events or occurrences will have a material adverse effect on our
operations and the measures that we have put into place to protect our
intellectual property rights may not be sufficient. Litigation to enforce our
intellectual property rights could result in substantial costs to us and may not
be successful. If we are not able to successfully defend our intellectual
property rights, we might lose rights to technology that we need to conduct and
develop our business. This would seriously harm our business, operating results
and financial condition, and enable our competitors to use our intellectual
property to compete against us.
Furthermore,
if third parties claim that our products infringe their patents or other
intellectual property rights, we may be required to devote substantial resources
to defend against such claims. If we are unsuccessful in defending against such
infringement claims, we may be required to pay damages, modify our products or
suspend the production and sale of such products. We cannot guarantee that we
will be able to modify our products on commercially reasonable terms, if at
all.
Our
cash flow could be negatively affected as a result of our extension of
relatively long payment terms to customers that we believe are credit
worthy.
As is
customary in our industry, we extend relatively long payment terms (up to six
months) to customers that we believe are credit worthy. The dollar amount of our
accounts receivable net of allowance for doubtful accounts as of September 30,
2010 was $7,990,490. Although we attempt to establish appropriate reserves for
our receivables, those reserves may not prove to be adequate in view of actual
levels of bad debts. The failure of our customers to pay us timely would
negatively affect our working capital, which could in turn adversely affect our
cash flow.
We
may be exposed to liabilities under the Foreign Corrupt Practices Act, and any
determination that we violated the Foreign Corrupt Practices Act could have a
material adverse effect on our business.
We are
subject to the Foreign Corrupt Practice Act, or FCPA, and other laws that
prohibit improper payments or offers of payments to foreign governments and
their officials and political parties by U.S. persons and issuers as defined by
the statute for the purpose of obtaining or retaining business. We have
operations, agreements with third parties and make sales in China, which may
experience corruption. Our activities in China create the risk of unauthorized
payments or offers of payments by one of the employees, consultants, sales
agents or distributors of our company, because these parties are not always
subject to our control. It is our policy to implement safeguards to discourage
these practices by our employees. Also, our existing safeguards and any future
improvements may prove to be less than effective, and the employees,
consultants, sales agents or distributors of our Company may engage in conduct
for which we might be held responsible. Violations of the FCPA may result in
severe criminal or civil sanctions, and we may be subject to other liabilities,
which could negatively affect our business, operating results and financial
condition. In addition, the government may seek to hold our Company liable for
successor liability FCPA violations committed by companies in which we invest or
that we acquire.
RISKS
RELATED TO DOING BUSINESS IN CHINA
Adverse
changes in political and economic policies of the PRC government could impede
the overall economic growth of China, which could reduce the demand for our
products and damage our business.
We
conduct substantially all of our operations and generate most of our revenue in
China. Accordingly, our business, financial condition, results of operations and
prospects are affected significantly by economic, political and legal
developments in China. The PRC economy differs from the economies of most
developed countries in many respects, including:
|
|
·
|
a
higher level of government
involvement;
|
|
|
·
|
a
early stage of development of the market-oriented sector of the
economy;
|
|
|
·
|
a
rapid growth rate;
|
|
|
·
|
a
higher level of control over foreign exchange;
and
|
|
|
·
|
the
allocation of resources.
|
As the
PRC economy has been transitioning from a planned economy to a more
market-oriented economy, the PRC government has implemented various measures to
encourage economic growth and guide the allocation of resources. While these
measures may benefit the overall PRC economy, they may also have a negative
effect on us.
21
Although
the PRC government has in recent years implemented measures emphasizing the
utilization of market forces for economic reform, the PRC government continues
to exercise significant control over economic growth in China through the
allocation of resources, controlling the payment of foreign currency-denominated
obligations, setting monetary policy and imposing policies that impact
particular industries or companies in different ways.
Any
adverse change in economic conditions or government policies in China could have
a material adverse effect on the overall economic growth in China, which in turn
could lead to a reduction in demand for our products and services and
consequently have a material adverse effect on our business and
prospects.
Uncertainties
with respect to the PRC legal system could limit the legal protections available
to you and us.
We
conduct substantially all of our business through our operating subsidiaries in
the PRC. Our operating subsidiaries are generally subject to laws and
regulations applicable to foreign investments in China and, in particular, laws
applicable to foreign-invested enterprises. The PRC legal system is based on
written statutes, and prior court decisions may be cited for reference but have
limited precedential value. Since 1979, a series of new PRC laws and regulations
have significantly enhanced the protections afforded to various forms of foreign
investments in China. However, since the PRC legal system continues to rapidly
evolve, the interpretations of many laws, regulations and rules are not always
uniform and enforcement of these laws, regulations and rules involve
uncertainties, which may limit legal protections available to you and us. In
addition, any litigation in China may be protracted and result in substantial
costs and diversion of resources and management attention. In addition, all of
our executive officers and all of our directors are residents of China and not
of the United States, and substantially all the assets of these persons are
located outside the United States. As a result, it could be difficult for
investors to affect service of process in the United States or to enforce a
judgment obtained in the United States against our Chinese operations and
subsidiaries.
If
we are found to have failed to comply with applicable laws, we may incur
additional expenditures or be subject to significant fines and
penalties.
Our
operations are subject to PRC laws and regulations applicable to us. However,
many PRC laws and regulations are uncertain in their scope, and the
implementation of such laws and regulations in different localities could have
significant differences. In certain instances, local implementation rules and/or
the actual implementation are not necessarily consistent with the regulations at
the national level. Although we strive to comply with all the applicable PRC
laws and regulations, we cannot assure you that the relevant PRC government
authorities will not later determine that we have not been in compliance with
certain laws or regulations.
In
addition, our facilities and products are subject to many laws and regulations.
Our failure to comply with these and other applicable laws and regulations in
China could subject us to administrative penalties and injunctive relief, as
well as civil remedies, including fines, injunctions and recalls of our
products. It is possible that changes to such laws or more rigorous enforcement
of such laws or with respect to our current or past practices could have a
material adverse effect on our business, operating results and financial
condition. Further, additional environmental, health or safety issues relating
to matters that are not currently known to management may result in
unanticipated liabilities and expenditures.
The
PRC government exerts substantial influence over the manner in which we must
conduct our business activities.
The PRC
government has exercised and continues to exercise substantial control over
virtually every sector of the Chinese economy through regulation and state
ownership. Our ability to operate in China may be harmed by changes in its laws
and regulations, including those relating to taxation, import and export
tariffs, environmental regulations, land use rights, property and other matters.
We believe that our operations in China are in material compliance with all
applicable legal and regulatory requirements. However, the central or local
governments of the jurisdictions in which we operate may impose new, stricter
regulations or interpretations of existing regulations that would require
additional expenditures and efforts on our part to ensure our compliance with
such regulations or interpretations.
Accordingly,
government actions in the future, including any decision not to continue to
support recent economic reforms and to return to a more centrally planned
economy or regional or local variations in the implementation of economic
policies, could have a significant effect on economic conditions in China or
particular regions thereof and could require us to divest ourselves of any
interest we then hold in Chinese properties or joint ventures.
22
Future
inflation in China may inhibit our ability to conduct business in
China.
In recent
years, the Chinese economy has experienced periods of rapid expansion and highly
fluctuating rates of inflation. These factors have led to the adoption by the
Chinese government, from time to time, of various corrective measures designed
to restrict the availability of credit or regulate growth and contain inflation.
High inflation may in the future cause the Chinese government to impose controls
on credit and/or prices, or to take other action, which could inhibit economic
activity in China, and thereby harm the market for our products and our
company.
Restrictions
on currency exchange may limit our ability to receive and use our sales
effectively.
The
majority of our revenues will be settled in RMB, and any future restrictions on
currency exchanges may limit our ability to use revenue generated in RMB to fund
any future business activities outside China or to make dividend or other
payments in U.S. dollars. Although the Chinese government introduced regulations
in 1996 to allow greater convertibility of the RMB for current account
transactions, significant restrictions still remain, including primarily the
restriction that foreign-invested enterprises may only buy, sell or remit
foreign currencies after providing valid commercial documents, at those banks in
China authorized to conduct foreign exchange business. In addition, conversion
of RMB for capital account items, including direct investment and loans, is
subject to governmental approval in China, and companies are required to open
and maintain separate foreign exchange accounts for capital account items. We
cannot be certain that the Chinese regulatory authorities will not impose more
stringent restrictions on the convertibility of the RMB.
Fluctuations in
exchange rates could adversely affect our business and the value of our
securities.
The value
of our common stock will be indirectly affected by the foreign exchange rate
between the U.S. dollar and RMB and between those currencies and other
currencies in which our revenues may be denominated. Appreciation or
depreciation in the value of the RMB relative to the U.S. dollar would affect
our financial results reported in U.S. dollar terms without giving effect to any
underlying change in our business or results of operations. Fluctuations in the
exchange rate will also affect the relative value of any dividend we issue that
will be exchanged into U.S. dollars, as well as earnings from, and the value of,
any U.S. dollar-denominated investments we make in the future.
Since
July 2005, the RMB has no longer been pegged to the U.S. dollar. Although the
People’s Bank of China regularly intervenes in the foreign exchange market to
prevent significant short-term fluctuations in the exchange rate, the RMB may
appreciate or depreciate significantly in value against the U.S. dollar in the
medium to long term. Moreover, it is possible that in the future PRC authorities
may lift restrictions on fluctuations in the RMB exchange rate and lessen
intervention in the foreign exchange market.
Very
limited hedging transactions are available in China to reduce our exposure to
exchange rate fluctuations. To date, we have not entered into any hedging
transactions. While we may enter into hedging transactions in the future, the
availability and effectiveness of these transactions may be limited, and we may
not be able to successfully hedge our exposure at all. In addition, our foreign
currency exchange losses may be magnified by PRC exchange control regulations
that restrict our ability to convert RMB into foreign currencies.
Restrictions
under PRC law on our PRC subsidiaries’ ability to make dividends and other
distributions could materially and adversely affect our ability to grow, make
investments or acquisitions that could benefit our business, pay dividends to
you, and otherwise fund and conduct our business.
Substantially
all of our revenues are earned by our PRC subsidiaries. However, PRC regulations
restrict the ability of our PRC subsidiaries to make dividends and other
payments to its offshore parent company. PRC legal restrictions permit payments
of dividends by our PRC subsidiaries only out of their accumulated after-tax
profits, if any, determined in accordance with PRC accounting standards and
regulations. Our PRC subsidiaries are also required under PRC laws and
regulations to allocate at least 10% of their annual after-tax profits
determined in accordance with PRC generally accepted accounting principles to a
statutory general reserve fund until the amounts in said fund reaches 50% of our
registered capital. Allocations to these statutory reserve funds can only be
used for specific purposes and are not transferable to us in the form of loans,
advances, or cash dividends. Any limitations on the ability of our PRC
subsidiaries to transfer funds to us could materially and adversely limit our
ability to grow, make investments or acquisitions that could be beneficial to
our business, pay dividends and otherwise fund and conduct our
business.
23
You may have
difficulty enforcing judgments against us.
We are a
Delaware holding company and most of our assets are located outside of the
United States. Almost all of our operations are conducted in the PRC. In
addition, most of our directors and officers are nationals and residents of
countries other than the United States. A substantial portion of the assets of
these persons is located outside the United States. As a result, it may be
difficult for you to effect service of process within the United States upon
these persons. It may also be difficult for you to enforce in U.S. courts
judgments on the civil liability provisions of the U.S. federal securities laws
against us and our officers and directors, most of whom are not residents in the
United States and the substantial majority of whose assets are located outside
of the United States. In addition, there is uncertainty as to whether the courts
of the PRC would recognize or enforce judgments of U.S. courts. Although the
recognition and enforcement of foreign judgments are generally provided for
under the PRC Civil Procedures Law, courts in China may recognize and enforce
foreign judgments in accordance with the requirements of the PRC Civil
Procedures Law based on treaties between China and the country where the
judgment is made or on reciprocity between jurisdictions. China does not have
any treaties or other arrangements that provide for the reciprocal recognition
and enforcement of foreign judgments with the United States. In addition,
according to the PRC Civil Procedures Law, courts in the PRC will not enforce a
foreign judgment against us or our directors and officers if they decide that
the judgment violates basic principles of PRC law or national sovereignty,
security or the public interest. So it is uncertain whether a PRC court would
enforce a judgment rendered by a court in the United States.
Failure to comply
with PRC regulations relating to the establishment of offshore special purpose
companies by PRC residents may subject our PRC resident stockholders to personal
liability, limit our ability to acquire PRC companies or to inject capital into
PRC subsidiaries, limit our PRC subsidiary's ability to distribute profits to us
or otherwise materially adversely affect us.
In
October 2005, SAFE issued the Notice on Relevant Issues in the Foreign Exchange
Control over Financing and Return Investment Through Special Purpose Companies
by Residents Inside China, generally referred to as Circular 75, which required
PRC residents to register with the competent local SAFE branch before
establishing or acquiring control over an offshore special purpose company, or
SPV, for the purpose of engaging in an equity financing outside of China on the
strength of domestic PRC assets originally held by those residents. Internal
implementing guidelines issued by SAFE, which became public in June 2007 (known
as Notice 106), expanded the reach of Circular 75 by (1) purporting to cover the
establishment or acquisition of control by PRC residents of offshore entities
which merely acquire “control” over domestic companies or assets, even in the
absence of legal ownership; (2) adding requirements relating to the source of
the PRC resident’s funds used to establish or acquire the offshore entity; (3)
covering the use of existing offshore entities for offshore financings; (4)
purporting to cover situations in which an offshore SPV establishes a new
subsidiary in China or acquires an unrelated company or unrelated assets in
China; and (5) making the domestic affiliate of the SPV responsible for the
accuracy of certain documents which must be filed in connection with any such
registration, notably, the business plan which describes the overseas financing
and the use of proceeds. Amendments to registrations made under Circular 75 are
required in connection with any increase or decrease of capital, transfer of
shares, mergers and acquisitions, equity investment or creation of any security
interest in any assets located in China to guarantee offshore obligations, and
Notice 106 makes the offshore SPV jointly responsible for these filings. In the
case of an SPV which was established, and which acquired a related domestic
company or assets, before the implementation date of Circular 75, a retroactive
SAFE registration was required to have been completed before March 31, 2006.
This date was subsequently extended indefinitely by Notice 106, which also
required that the registrant establish that all foreign exchange transactions
undertaken by the SPV and its affiliates were in compliance with applicable laws
and regulations. Failure to comply with the requirements of Circular 75, as
applied by SAFE in accordance with Notice 106, may result in fines and other
penalties under PRC laws for evasion of applicable foreign exchange
restrictions. Any such failure could also result in the SPV’s affiliates being
impeded or prevented from distributing their profits and the proceeds from any
reduction in capital, share transfer or liquidation to the SPV, or from engaging
in other transfers of funds into or out of China.
We have
asked some of our stockholders, who are PRC residents as defined in Circular 75,
to register with the relevant branch of SAFE as currently required in connection
with their equity interests in us and our acquisitions of equity interests in
our PRC subsidiaries. However, we cannot provide any assurances that they can
obtain the above SAFE registrations required by Circular 75 and Notice 106.
Moreover, because of uncertainty over how Circular 75 will be interpreted and
implemented, and how or whether SAFE will apply it to us, we cannot predict how
it will affect our business operations or future strategies. For example, our
present and prospective PRC subsidiaries' ability to conduct foreign exchange
activities, such as the remittance of dividends and foreign currency-denominated
borrowings, may be subject to compliance with Circular 75 and Notice 106 by our
PRC resident beneficial holders.
24
In
addition, such PRC residents may not always be able to complete the necessary
registration procedures required by Circular 75 and Notice 106. We also have
little control over either our present or prospective direct or indirect
stockholders or the outcome of such registration procedures. A failure by our
PRC resident beneficial holders or future PRC resident stockholders to comply
with Circular 75 and Notice 106, if SAFE requires it, could subject these PRC
resident beneficial holders to fines or legal sanctions, restrict our overseas
or cross-border investment activities, limit our subsidiaries' ability to make
distributions or pay dividends or affect our ownership structure, which could
adversely affect our business and prospects.
Under
the Enterprise Income Tax Law, we may be classified as a “resident enterprise”
of China. Such classification will likely result in unfavorable tax consequences
to us and our non-PRC stockholders.
On March
16, 2007, the National People’s Congress of China passed the EIT Law and on
November 28, 2007, the State Council of China passed its implementing rules,
both of which took effect on January 1, 2008. Under the EIT Law, an enterprise
established outside of China with “de facto management bodies” within China is
considered a “resident enterprise,” meaning that it can be treated in a manner
similar to a Chinese enterprise for enterprise income tax purposes. The
implementing rules of the EIT Law define de facto management as “substantial and
overall management and control over the production and operations, personnel,
accounting, and properties” of the enterprise.
On April
22, 2009, the State Administration of Taxation issued the Notice Concerning
Relevant Issues Regarding Cognizance of Chinese Investment Controlled
Enterprises Incorporated Offshore as Resident Enterprises pursuant to Criteria
of de facto Management Bodies, or the Notice, further interpreting the
application of the EIT Law and its implementation against non-Chinese enterprise
or group controlled offshore entities. Pursuant to the Notice, an enterprise
incorporated in an offshore jurisdiction and controlled by a Chinese enterprise
or group will be classified as a “domestically incorporated resident enterprise”
if (i) its senior management in charge of daily operations reside or perform
their duties mainly in China; (ii) its financial or personnel decisions are made
or approved by bodies or persons in China; (iii) its substantial assets and
properties, accounting books, corporate chops, board and shareholder minutes are
kept in China; and (iv) at least half of its directors with voting rights or
senior management often resident in China. A resident enterprise would be
subject to an enterprise income tax rate of 25% on its worldwide income and its
non-PRC stockholders would be subject to a withholding tax at a rate of 10% when
dividends are paid to such non-PRC stockholders. However, no detailed measures
on enforcement of PRC tax against non-domestically incorporated resident
enterprises are available. Therefore, it is unclear how tax authorities will
determine tax residency based on the facts of each case.
We may be
deemed to be a resident enterprise by Chinese tax authorities. If the PRC tax
authorities determine that we are a “resident enterprise” for PRC enterprise
income tax purposes, a number of unfavorable PRC tax consequences could follow.
First, we may be subject to the enterprise income tax at a rate of 25% on our
worldwide taxable income as well as PRC enterprise income tax reporting
obligations. In our case, this would mean that income such as interest on
financing proceeds and non-China source income would be subject to PRC
enterprise income tax at a rate of 25%. Second, although under the EIT Law and
its implementing rules dividends paid to us from our PRC subsidiary would
qualify as “tax-exempt income,” we cannot guarantee that such dividends will not
be subject to a 10% withholding tax, as the PRC foreign exchange control
authorities, which enforce the withholding tax, have not yet issued guidance
with respect to the processing of outbound remittances to entities that are
treated as resident enterprises for PRC enterprise income tax purposes. Finally,
it is possible that future guidance issued with respect to the new “resident
enterprise” classification could result in a situation in which a 10%
withholding tax is imposed on dividends we pay to our non-PRC stockholders and
with respect to gains derived by our non-PRC stockholders from transferring our
shares. We are actively monitoring the possibility of “resident enterprise”
treatment for the 2010 tax year and are evaluating appropriate organizational
changes to avoid this treatment, to the extent possible.
If we
were treated as a “resident enterprise” by PRC tax authorities, we would be
subject to taxation in both the U.S. and China, and our PRC tax may not be
creditable against our U.S. tax.
25
We
face uncertainty from China’s Circular on Strengthening the Administration of
Enterprise Income Tax on Non-Resident Enterprises' Share Transfer, or Circular
698, that was released in December 2009 with retroactive effect from January 1,
2008.
The
Chinese State Administration of Taxation released a circular on December 15,
2009 that addresses the transfer of shares by nonresident companies, generally
referred to as Circular 698. Circular 698, which is effective retroactively to
January 1, 2008, may have a significant impact on many companies that use
offshore holding companies to invest in China. Circular 698, which provides
parties with a short period of time to comply with its requirements, indirectly
taxes foreign companies on gains derived from the indirect sale of a Chinese
company. Where a foreign investor indirectly transfers equity interests in a
Chinese resident enterprise by selling the shares in an offshore holding
company, and the latter is located in a country or jurisdiction where the
effective tax burden is less than 12.5% or where the offshore income of his,
her, or its residents is not taxable, the foreign investor is required to
provide the tax authority in charge of that Chinese resident enterprise with the
relevant information within 30 days of the transfers. Moreover, where a foreign
investor indirectly transfers equity interests in a Chinese resident enterprise
through an abuse of form of organization and there are no reasonable commercial
purposes such that the corporate income tax liability is avoided, the PRC tax
authority will have the power to re-assess the nature of the equity transfer in
accordance with PRC’s “substance-over-form” principle and deny the existence of
the offshore holding company that is used for tax planning purposes. There is
uncertainty as to the application of Circular 698. For example, while the term
"indirectly transfer" is not defined, it is understood that the relevant PRC tax
authorities have jurisdiction regarding requests for information over a wide
range of foreign entities having no direct contact with China. It is also
unclear, in the event that an offshore holding company is treated as a
domestically incorporated resident enterprise, whether Circular 698 would still
be applicable to transfer of shares in such offshore holding company. Moreover,
the relevant authority has not yet promulgated any formal provisions or formally
declared or stated how to calculate the effective tax in the country or
jurisdiction and to what extent and the process of the disclosure to the tax
authority in charge of that Chinese resident enterprise. In addition, there are
not any formal declarations with regard to how to decide “abuse of form of
organization” and “reasonable commercial purpose,” which can be utilized by us
to balance if our Company complies with the Circular 698. If Circular 698 is
determined to be applicable to us based on the facts and circumstances around
such share transfers, we may become at risk of being taxed under Circular 698
and we may be required to expend valuable resources to comply with Circular 698
or to establish that we should not be taxed under Circular 698, which could have
a material adverse effect on our financial condition and results of
operations.
We
have limited insurance coverage for our operations in China.
The
insurance industry in China is still at an early stage of development. Insurance
companies in China offer limited insurance products. We have determined that the
risks of disruption or liability from our business, the loss or damage to our
property, including our facilities, equipment and office furniture, the cost of
insuring for these risks, and the difficulties associated with acquiring such
insurance on commercially reasonable terms make it impractical for us to have
such insurance. As a result, we do not have any business liability, disruption,
litigation or property insurance coverage for our operations in China except for
insurance on some company owned vehicles. Any uninsured occurrence of loss or
damage to property, or litigation or business disruption may result in the
incurrence of substantial costs and the diversion of resources, which could have
an adverse effect on our operating results.
Because
our funds in the PRC are held in banks which do not provide deposit insurance,
the failure of any bank in which we deposit our funds could affect our ability
to continue in business.
Banks and
other financial institutions in the PRC do not provide deposit insurance for
funds held on deposit. As a result, in the event of a bank failure, we may not
have access to funds on deposit in the PRC. Depending upon the amount of money
we maintain in a bank that fails, our inability to have access to our cash could
impair our operations, and, if we are not able to access funds to pay our
suppliers, employees and other creditors, we may be unable to continue in
business.
26
RISKS
RELATED TO THE MARKET FOR OUR STOCK
Our
common stock is quoted on the OTC Bulletin Board which may have an unfavorable
impact on our stock price and liquidity.
Our
common stock is quoted on the OTC Bulletin Board. The OTC Bulletin Board is a
significantly more limited market than the New York Stock Exchange or Nasdaq
system. Though we plan to file an application to list our Common Stock on such
senior exchanges as Nasdaq or New York Stock Exchange as soon as practicable,
however, we cannot assure you that we will be able to meet the initial listing
standards of any stock exchange, or that we will be able to maintain any such
listing. The quotation of our shares on the OTC Bulletin Board may result in a
less liquid market available for existing and potential stockholders to trade
shares of our common stock, could depress the trading price of our common stock
and could have a long-term adverse impact on our ability to raise capital in the
future.
The
market price of our common stock is volatile, leading to the possibility of its
value being depressed at a time when you may want to sell your
holdings.
The
market price of our common stock is volatile, and this volatility may continue.
Numerous factors, many of which are beyond our control, may cause the market
price of our common stock to fluctuate significantly. In addition to market and
industry factors, the price and trading volume for our common stock may be
highly volatile for specific business reasons. Factors such as variations in our
revenues, earnings and cash flow, announcements of new investments, cooperation
arrangements or acquisitions, and fluctuations in market prices for our products
could cause the market price for our shares to change
substantially.
Securities
class action litigation is often instituted against companies following periods
of volatility in their stock price. This type of litigation could result in
substantial costs to us and divert our management’s attention and
resources.
Moreover,
the trading market for our common stock will be influenced by research or
reports that industry or securities analysts publish about us or our business.
If one or more analysts who cover us downgrade our common stock, the market
price for our common stock would likely decline. If one or more of these
analysts cease coverage of us or fail to regularly publish reports on us, we
could lose visibility in the financial markets, which, in turn, could cause the
market price for our common stock or trading volume to decline.
Furthermore,
securities markets may from time to time experience significant price and volume
fluctuations for reasons unrelated to operating performance of particular
companies. These market fluctuations may adversely affect the price of our
common stock and other interests in our company at a time when you want to sell
your interest in us.
We
may be subject to penny stock regulations and restrictions and you may have
difficulty selling shares of our common stock.
The SEC
has adopted regulations which generally define so-called “penny stocks” to be an
equity security that has a market price less than $5.00 per share or an exercise
price of less than $5.00 per share, subject to certain exemptions. If our common
stock becomes a “penny stock”, we may become subject to Rule 15g-9 under the
Exchange Act, or the Penny Stock Rule. This rule imposes additional sales
practice requirements on broker-dealers that sell such securities to persons
other than established customers and “accredited investors” (generally,
individuals with a net worth in excess of $1,000,000 or annual incomes exceeding
$200,000, or $300,000 together with their spouses). For transactions covered by
Rule 15g-9, a broker-dealer must make a special suitability determination for
the purchaser and have received the purchaser's written consent to the
transaction prior to sale. As a result, this rule may affect the ability of
broker-dealers to sell our securities and may affect the ability of purchasers
to sell any of our securities in the secondary market.
For any
transaction involving a penny stock, unless exempt, the rules require delivery,
prior to any transaction in penny stock, of a disclosure schedule prepared by
the SEC relating to the penny stock market. Disclosure is also required to be
made about sales commissions payable to both the broker-dealer and the
registered representative and current quotations for the securities. Finally,
monthly statements are required to be sent disclosing recent price information
for the penny stock held in the account and information on the limited market in
penny stock.
There can
be no assurance that our common stock will qualify for exemption from the Penny
Stock Rule. In any event, even if our common stock were exempt from the Penny
Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act,
which gives the SEC the authority to restrict any person from participating in a
distribution of penny stock, if the SEC finds that such a restriction would be
in the public interest.
27
Consequently,
as long as our Common Stock is regarded as a “penny stock”, such regulations may
deter broker-dealers from recommending or selling our Common Stock, which may
further affect its liquidity. This would also make it more difficult
for us to raise additional capital.
Certain
provisions of our Certificate of Incorporation may make it more difficult for a
third party to effect a change-of-control.
Our
Certificate of Incorporation authorizes our board of directors to issue up to
1,000,000 shares of preferred stock. The preferred stock may be
issued in one or more series, the terms of which may be determined at the time
of issuance by the board of directors without further action by the
stockholders. These terms may include preferences as to dividends and
liquidation, conversion rights, redemption rights and sinking fund
provisions. The issuance of any preferred stock could diminish the
rights of holders of our common stock, and therefore could reduce the value of
such common stock. In addition, specific rights granted to future
holders of preferred stock could be used to restrict our ability to merge with,
or sell assets to, a third party. The ability of our board of directors to issue
preferred stock could make it more difficult, delay, discourage, prevent or make
it more costly to acquire or effect a change-in-control, which in turn could
prevent our stockholders from recognizing a gain in the event that a favorable
offer is extended and could materially and negatively affect the market price of
our common stock.
Our
holding company structure may limit the payment of dividends.
We have
no direct business operations, other than our ownership of our
subsidiaries. While we have no current intention of paying dividends,
should we decide in the future to do so, as a holding company, our ability to
pay dividends and meet other obligations depends upon the receipt of dividends
or other payments from our operating subsidiaries and other holdings and
investments. In addition, our operating subsidiaries, from time to
time, may be subject to restrictions on their ability to make distributions to
us, including as a result of restrictive covenants in loan agreements,
restrictions on the conversion of local currency into U.S. dollars or other hard
currency and other regulatory restrictions. We currently intend to
retain any future earnings for funding growth and, therefore, do not expect to
pay any dividends in the foreseeable future. If we determine that we
will pay dividends to the holders of our Common Stock, we cannot assure that
such dividends will be paid on a timely basis. As a result, you will
not receive any return on your investment prior to selling your shares in our
company and, for the other reasons discussed in this “Risk Factors” section, you
may not receive any return on your investment even when you sell your shares in
our company and your shares may become worthless. If future dividends
are paid in RMB, fluctuations in the exchange rate for the conversion of RMB
into U.S. dollars may reduce the amount received by U.S. stockholders upon
conversion of the dividend payment into U.S. dollars.
We
do not intend to pay dividends for the foreseeable future.
For the
foreseeable future, we intend to retain any earnings to finance the development
and expansion of our business, and we do not anticipate paying any cash
dividends on our common stock. Accordingly, investors must be prepared to rely
on sales of their common stock after price appreciation to earn an investment
return, which may never occur. Investors seeking cash dividends should not
purchase our common stock. Any determination to pay dividends in the future will
be made at the discretion of our board of directors and will depend on our
results of operations, financial condition, contractual restrictions,
restrictions imposed by applicable law and other factors our board deems
relevant.
The
issuance of shares through our stock compensation plans may dilute the value of
existing stockholders and may affect the market price of our stock.
Although
we do not have an option or other equity-based incentive plan at present, in the
future we may use stock options, stock grants and other equity-based incentives,
to provide motivation and compensation to our officers, employees and key
independent consultants. The award of any such incentives will result in an
immediate and potentially substantial dilution to our existing stockholders and
could result in a decline in the value of our stock price. The exercise of these
options and the sale of the underlying shares of Common Stock and the sale of
stock issued pursuant to stock grants may have an adverse effect upon the price
of our stock.
|
ITEM
1B.
|
UNRESOLVED
STAFF COMMENTS.
|
Not
Applicable.
28
|
ITEM
2.
|
PROPERTIES.
|
All land
in China is owned by the State or collectives. Individuals and
companies are permitted to acquire rights to use land or land use rights for
specific purposes. In the case of land used for industrial purposes,
the land use rights are granted for a period of 50 years. This period
may be renewed at the expiration of the initial and any subsequent terms
according to the relevant Chinese laws. Granted land use rights are
transferable and may be used as security for borrowings and other
obligations.
Our
executive offices are located at No. 11 Jiangda Road, Jianghan Economic
Development Zone, Wuhan City, Hubei Province, China. Our executive
offices consist of approximately 2,780 square meters consisting entirely of
administrative office space. The administrative office is owned by
the Company and therefore we do not pay rent for the use of the office
space. The sale offices and customer service stations are leased from
individual or corporate owners on a month-to-month basis, at an aggregate
average monthly cost of $11,090.
Our main
production facilities are located in the Hannan Economic Development Zone, in
Wuhan City, Hubei Province, China. The current total site area
is approximately 16,477 square meters. Tallyho purchased the rights to
use the lands and built the production facilities first in 1998 and later in
2009 for facility expansion.
We
believe that all our properties have been adequately maintained, are generally
in good condition, and are suitable and adequate for our business. We
do not have property insurance on any of our properties.
|
ITEM
3.
|
LEGAL
PROCEEDINGS.
|
From time
to time, we may become involved in various lawsuits and legal proceedings which
arise in the ordinary course of business. However, litigation is
subject to inherent uncertainties, and an adverse result in these or other
matters may arise from time to time that may harm our business. We
are currently not aware of any such legal proceedings or claims that we believe
will have a material adverse affect on our business, financial condition or
operating results.
|
ITEM
4.
|
(REMOVED
AND RESERVED).
|
PART
II
|
ITEM
5.
|
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER
PURCHASES OF EQUITY SECURITIES.
|
Market
Information
Our
common stock is quoted on the OTC Bulletin Board trades under the symbol
“CHPN.” There is not now, and historically there has never been, an
active trading market for our common stock. Since our common stock has only
traded on an extremely limited and sporadic basis, information is not available
for the prices of our common stock.
Approximate
Number of Holders of Our Common Stock
As of
January 12, 2011, there were approximately 57 holders of record of our common
stock. This number excludes the shares of our common stock owned by
stockholders holding stock under nominee security position
listings.
Dividend
Policy
We have
never declared dividends or paid cash dividends. Our board of directors will
make any future decisions regarding dividends. We currently intend to retain and
use any future earnings for the development and expansion of our business and do
not anticipate paying any cash dividends in the near future.
29
Securities
Authorized for Issuance Under Equity Compensation Plans
See Item
12, “Security Ownership of Certain Beneficial Owners and Management and Related
Stockholder Matters — Securities Authorized for Issuance Under Equity
Compensation Plans.”
Recent
Sales of Unregistered Securities
We have
not sold any equity securities during the fiscal year ended September 30, 2010
that were not previously disclosed in a quarterly report on Form 10-Q or a
current report on Form 8-K that was filed during the 2010 fiscal
year.
Purchases
of Equity Securities
No
repurchases of our common stock were made during the fourth quarter of
2010.
|
ITEM
6.
|
SELECTED
FINANCIAL DATA.
|
Not
Applicable.
|
ITEM
7.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS.
|
Cautionary
Notice Regarding Forward-Looking Statements
We make
certain forward-looking statements in this report. Statements concerning our
future operations, prospects, strategies, financial condition, future economic
performance (including growth and earnings), demand for our products, and other
statements of our plans, beliefs, or expectations, including the statements
contained under the captions “Management’s Discussion and Analysis of Financial
Condition and Results of Operations,” “Business,” as well as captions elsewhere
in this document, are forward-looking statements. In some cases these
statements are identifiable through the use of words such as “anticipate,”
“believe,” “estimate,” “expect,” “intend,” “plan,” “project,” “target,” “can”,
“could,” “may,” “should,” “will,” “would,” and similar expressions. We
intend such forward-looking statements to be covered by the safe harbor
provisions contained in Section 27A of the Securities Act of 1933, as amended,
or the Securities Act, and in Section 21E of the Securities Exchange Act of
1934, as amended, or the Exchange Act. The forward-looking statements
we make are not guarantees of future performance and are subject to various
assumptions, risks, and other factors that could cause actual results to differ
materially from those suggested by these forward-looking statements.
Because such statements are subject to risks and uncertainties, actual
results may differ materially from those expressed or implied by the
forward-looking statements. Indeed, it is likely that some of our assumptions
will prove to be incorrect. Our actual results and financial position will
vary from those projected or implied in the forward-looking statements and the
variances may be material. You are cautioned not to place undue reliance
on such forward-looking statements. These risks and uncertainties,
together with the other risks described from time to time in reports and
documents that we file with the SEC should be considered in evaluating
forward-looking statements.
The
nature of our business makes predicting the future trends of our revenues,
expenses, and net income difficult. Thus, our ability to predict results
or the actual effect of our future plans or strategies is inherently uncertain.
The risks and uncertainties involved in our business could affect the
matters referred to in any forward-looking statements and it is possible that
our actual results may differ materially from the anticipated results indicated
in these forward-looking statements. Important factors that could cause
actual results to differ from those in the forward-looking statements include,
without limitation, the following:
|
-
|
The
effect of political, economic, and market conditions and geopolitical
events;
|
|
-
|
Legislative
and regulatory changes that affect our
business;
|
|
-
|
The
availability of funds and working
capital;
|
|
-
|
The
actions and initiatives of current and potential
competitors;
|
|
-
|
Investor
sentiment; and
|
|
-
|
Our
reputation.
|
30
We do not
undertake any responsibility to publicly release any revisions to these
forward-looking statements to take into account events or circumstances that
occur after the date of this report. Additionally, we do not undertake any
responsibility to update you on the occurrence of any unanticipated events which
may cause actual results to differ from those expressed or implied by any
forward-looking statements.
The
following discussion and analysis should be read in conjunction with our
consolidated financial statements and the related notes thereto as filed with
the SEC and other financial information contained elsewhere in this
report.
Overview
CPGI was
incorporated in Delaware in March 2007 to engage in the design, manufacturing,
distribution and marketing of surfboards and related equipment. Prior
to the acquisition of Cantix, discussed below, CPGI was a development stage
company and had no material assets and no revenues. On November 13,
2009, CPGI acquired all of the issued and outstanding capital stock of
Cantix. As a result of the acquisition (i) Cantix became a wholly
owned subsidiary of CPGI, (ii) CPGI’s principal business changed to
be the business of Cantix, (iii) CPGI ceased to be a shell Company, (iv)
CPGI changed its fiscal year end from December 31 to September 30, and (v) the
shareholders of Cantix became the controlling shareholders of CPGI. The share exchange
transaction with Cantix was treated as a reverse acquisition, with Cantix as the
acquirer and CPGI as the acquired party. Because Cantix and its
affiliates’ operations are the only significant operations of the Company,
unless the context suggests otherwise, when we refer in this report to business
and financial information for periods prior to the consummation of the reverse
acquisition, we are referring to the business and financial information of
Cantix and its consolidated subsidiaries.
Through
our direct and indirect operating subsidiaries, Tallyho, Hopsun, Anti-Aging and
Xinpu, we are principally engaged in the research, development, manufacturing,
marketing and sales of polypeptide-based anti-aging nutritional supplements,
health foods, functional foods and related material products. We have
developed over 70 different types of polypeptide-based products. We market and
sell our products through a combined network of sales personnel, wholesalers and
private labeled partners in China and internationally, however China is
currently our primary market.
Our
products are primarily manufactured in our 16,477 square
meters production facilities located in the Hannan Economic Development Zone in
Wuhan, China. Our products currently being marketed and sold in China
have been tested and approved by the applicable Chinese governmental hygiene and
safety agencies, including by the local bureaus of the Ministry of Health and
the State Food and Drug Administration. Our research and development
efforts are conducted at our facilities in Wuhan, China.
We
believe that we are one of the few companies in our industry with competitive
prices and high quality of diversified nutritional products combined with
excellent customer service. We believe that we are one of the largest
companies in China focusing on the development and production of functional
peptide nutritional products.
Our
common stock is eligible for quotation on Over-the-Counter Bulletin Board under
the trading symbol of “CHPN.”
Results
of Operations
The
following tables set forth key components of our results of operations for the
periods indicated, both in dollars and as a percentage of sales
revenues.
31
Comparison
of the Years Ended September 30, 2010 and 2009
|
Years Ended September 30,
|
||||||||||||||||
|
2010
|
2009
|
|||||||||||||||
|
Amount
|
% of
Revenues
|
Amount
|
% of
Revenues
|
|||||||||||||
|
(in dollars, except percentages)
|
||||||||||||||||
|
REVENUES
|
38,061,827 | 100.0 | % | 37,724,869 | 100.0 | % | ||||||||||
|
COST
OF SALES
|
2,986,612 | 7.8 | % | 2,247,048 | 6.0 | % | ||||||||||
|
GROSS
PROFIT
|
35,075,215 | 92.2 | % | 35,477,821 | 94.0 | % | ||||||||||
|
SELLING
AND ADMINISTRATIVES EXPENSES
|
27,311,609 | 71.8 | % | 22,842,559 | 60.6 | % | ||||||||||
|
OPERATING
INCOME
|
7,763,606 | 20.4 | % | 12,635,262 | 33.5 | % | ||||||||||
|
OTHER
INCOME (EXPENSE)
|
||||||||||||||||
|
Interest
income (expense), net
|
(66,682 | ) | -0.2 | % | 177,437 | 0.5 | % | |||||||||
|
Equity
loss in affiliates
|
(30,866 | ) | -0.1 | % | (30,611 | ) | -0.1 | % | ||||||||
|
Gain
on disposal of associates
|
- | 0.0 | % | 42,433 | 0.1 | % | ||||||||||
|
Loss
on impairment of goodwill
|
(113,713 | ) | -0.3 | % | - | 0.0 | % | |||||||||
|
Other
income (expense)
|
(673,241 | ) | -1.8 | % | 412,820 | 1.1 | % | |||||||||
|
|
||||||||||||||||
|
INCOME
BEFORE INCOME TAX EXPENSE
|
6,879,104 | 18.1 | % | 13,237,341 | 35.1 | % | ||||||||||
|
|
||||||||||||||||
|
INCOME
TAX EXPENSE
|
1,387,712 | 3.6 | % | 2,715,274 | 7.2 | % | ||||||||||
|
|
||||||||||||||||
|
NET
INCOME
|
5,491,392 | 14.4 | % | 10,522,067 | 27.9 | % | ||||||||||
|
|
||||||||||||||||
|
OTHER
COMPREHENSIVE INCOME
|
||||||||||||||||
|
Foreign
currency translation gain
|
788,653 | 2.1 | % | 52,102 | 0.1 | % | ||||||||||
|
COMPREHENSIVE
INCOME
|
6,280,045 | 16.5 | % | 10,574,169 | 28.0 | % | ||||||||||
Revenues. Revenues for the
fiscal year ended September 30, 2010 increased $336,958, or 0.9%, to
$38,061,827, from $37,724,869 for the fiscal year ended September 30, 2009. The
increase is mainly attributable to the increase in our business-to-business
(“B2B”) sales of polypeptide-based ingredients and private-labeled products to
other nutraceutical manufacturers and marketers by $1,515,003, or 67.9%, as
compared to that in the fiscal year ended September 30, 2009.
Our
business-to-consumer (“B2C”) sales of own branded polypeptide-based
nutraceutical products decreased by $1,178,044, or 3.3%, as compared to the
fiscal year of 2009. Consumer sales of own branded products through our own
sales network increased by $3,805,604 or 13.6% while such sales through
third-party channels decreased by $4,983,648 or 66.1% as compared to those in
the fiscal year ended September 30, 2009. Management has been
redirecting our sales efforts to focusing on B2C sales through own network and
restructuring the third-party channels so as to enhance future B2C
sales. We believe that the increasing domestic income levels and
corresponding awareness of health and quality of life, among other factors, will
help us achieve sales growth going forward.
Cost of Sales. Cost of sales
for the fiscal year ended September 30, 2010 totaled $2,986,612, or
approximately 7.8% of revenues, compared to $2,247,048, or approximately 6.0% of
revenues, for the fiscal year ended September 30, 2009, an increase of $739,564
or 32.9%. The increase is mainly attributable to (a) the increases in
prices of raw materials and overhead costs and (b) the increase of input VAT
that cannot be used to offset against output VAT and has to be absorbed in costs
as the result of the small scale taxpayer status of some sales branches during
the fiscal year of 2010. Given the overall market inflation in China, especially
for those agricultural products which are the main raw materials for our
products, management expects that our raw material prices will continue to
increase in future periods. However, given the Chinese government’s measures in
recent months to control consumption prices, including consumption of
agricultural products, it is anticipated that such prices will gradually become
stable, if not decrease. We expect that such inflationary impact, if any, on our
results of operations would be minimal or controllable given our historically
high gross margin.
Gross Profit. Gross profit
for the fiscal year ended September 30, 2010 decreased $402,606, or 1.1%, to
$35,075,215, from $35,477,821 for the fiscal year ended September 30,
2009. The respective gross margins are 92.2% and 94.0% for the fiscal
years ended September 30, 2010 and 2009. The decrease in gross profit
in fiscal year 2010 is mainly due to the increase in our costs of
sales.
32
Selling and Administrative Expenses.
Selling and administrative expenses for the fiscal year ended September
30, 2010 totaled $27,311,609, or approximately 71.8% of revenues, compared to
$22,842,559, or approximately 60.6% of revenues, for the fiscal year of 2009, an
increase of $4,469,050, or 19.6%. The increase is mainly attributable to the
increase in bad debt allowance expenses and the increase in professional
expenses related to operations as a public company. The Company assessed and
provided bad debt allowances of $3,859,826 during the fiscal year ended
September 30, 2010 for doubtful accounts receivables and other receivables, as
compared to $579,096 during the fiscal year of 2009, an increase of $3,280,730,
or 566.5%. The Company incurred $787,354 in professional expenses
related to our public company operations in the fiscal year ended September 30,
2010, as compared to $47,306 during the fiscal year of 2009, an increase of
$740,048, or 1,564%, as a result of the reverse merger closed in November
2009.
Operating Income. Operating
income amounted to $7,763,606, or approximately 20.4% of revenues, for the
fiscal year ended September 30, 2010, as compared to $12,635,262, or
approximately 33.5% of revenues, for the fiscal year ended September 30, 2009, a
decrease of $4,871,656, or 38.6%. The decrease in operating income is mainly
attributable to the increases in costs of sales and selling and administrative
expenses in fiscal year 2010.
Interest Income (Expense).
Net interest expense for the year ended September 30, 2010 amounted to
$66,682, as compared to net interest income of $177,437 for the fiscal year
ended September 30, 2009, an absolute change of $244,119. The change is mainly
because Shenzhen Era Bio-technology (Shenzhen) Co. Ltd. (“Era Biotech”) which
owes $4,078,769 in loans to the Company, made interest payments of $234,480 in
fiscal year 2009 but failed to do so in fiscal year 2010.
Equity loss in Affiliates.
Equity loss in affiliates for the fiscal year ended September 30, 2010
amounted to $30,866, an increase of $255, or 0.8%, as compared to equity in loss
in affiliates of $30,611 for the fiscal year ended September 30, 2009. The
increase is due to the increase in the net loss of an affiliate company in which
the Company has a 40% equity interest and uses the equity method to account for
such an investment.
Loss on Impairment of Goodwill.
A loss on impairment of goodwill of $113,713 incurred during the fiscal
year ended September 30, 2010, as compared to $nil during the fiscal year ended
September 30, 2009. Goodwill of $113,713 related to the acquisition of
Anti-Aging was assessed to be impaired as of September 30, 2010. There was no
impairment of goodwill as of September 30, 2009.
Other Income (Expense). Other
expense was $673,241 for the fiscal year ended September 30, 2010, as compared
to other income of $412,820 for the fiscal year ended September 30, 2009, an
absolute change of $1,086,061. The change is mainly attributable to the late
payment of interest and penalty of $728,336 settled for payments in connection
with land use right for our land in Gangzhou Science Park during the fiscal year
ended September 30, 2010, and income of $468,960 earned from transferring
technical know-how of certain products specifically developed for a third party
health food distributor during the fiscal year ended September 30,
2009.
Income before Income Tax
Expense. Income before income tax expense was $6,879,104, or
approximately 18.1% of revenues, for the fiscal year ended September 30, 2010,
as compared to $13,237,341, or approximately 35.1% of revenues, for the fiscal
year ended September 30, 2009, a decrease of $6,358,637, or 48%. The decrease in
income before income tax expense is mainly attributable to the $5,208,614
increase in costs of sales and selling and administrative expenses and the
$1,330,180 decrease in interest income and other income (expense) during the
fiscal year of 2010.
Income Tax Expense. Income
tax expense was $1,387,712, or approximately 3.6% of revenues, for the fiscal
year ended September 30, 2010, as compared to $2,715,274, or approximately 7.2%
of revenues, for the fiscal year ended September 30, 2009, a decrease of
$1,327,562 or 48.9%. The decrease in income tax expense is mainly attributable
to a) a $6,358,237 decrease of our income before income tax expense, and b) the
certification of our main operating subsidiaries, Hopsun and Tallyho, as High
and New Technological Enterprises in December 2009 and 2010, respectively, and
their being subject to a preferential income tax rate of 15% during fiscal year
2010, as compared to the previous rate of 25%.
33
Net Income. Net income
amounted to $5,491,392, or approximately 14.4% of revenues, for the fiscal year
ended September 30, 2010, as compared to $10,522,067, or approximately 27.9% of
revenues, for the fiscal year ended September 30, 2009, a decrease of
$5,030,675, or 47.8%. The decrease in net income is mainly
attributable to: the increase of $4,469,050 in selling and administrative
expenses, especially the $3,859,826 increase in bad debt allowances for doubtful
receivables; the $739,564 increase in costs of sales, the $1,086,061 increase in
other expenses; and the $244,119 decrease in interest income during the fiscal
year of 2010. Although no assurance
can be given, management believes that our revenues and net income will resume
growth in future periods resulting from, among other factors, growing market
demands for anti-aging nutritional supplements, health foods and functional food
products, our increased sales and marketing efforts after the restructuring of
our sales offices, our newly added manufacturing capacity to meet such
increasing demands, our expansion into other high margin peptide-based product
categories, as well as improved economic conditions and the PRC government’s
stimulus measures for domestic consumption.
Liquidity
and Capital Resources
Cash
Flows
The
following table sets forth a summary of our cash flows for the years indicated
below:
|
Years Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
(in
dollars)
|
||||||||
|
Net
cash provided by (used in) operating activities
|
$ | 10,135,790 | $ | (125,880 | ) | |||
|
Net
cash used in investing activities
|
(9,133,723 | ) | (1,945,429 | ) | ||||
|
Net
cash provided by financing activities
|
8,721,720 | 559,340 | ||||||
|
Effect
of exchange rate fluctuation on cash and cash equivalents
|
257,493 | 33,287 | ||||||
|
Net
increase (decrease) in cash and cash equivalents
|
9,981,280 | (1,478,682 | ) | |||||
|
Cash
and cash equivalents, beginning of year
|
4,439,732 | 5,918,414 | ||||||
|
Cash
and cash equivalents, end of year
|
$ | 14,421,012 | $ | 4,439,732 | ||||
Operating
Activities
Net cash
provided by operating activities increased to $10,135,790 during the fiscal year
ended September 30, 2010, an increase of $10,261,670, or 8,151.9%, from net cash
used in operating activities of $125,880 during the fiscal year ended September
30, 2009. The increase is mainly attributable to a significant decrease in
accounts receivable of $7,982,851, from $14,666,836 in accounts receivable as of
September 30, 2009, and collections of $8,146,464 accounts receivables related
to the third-party distributors of our own branded products in fiscal year
2010.
Investing
Activities
Net cash
used in investing activities increased by $7,188,294, or 369.5%, to $9,133,723
during the fiscal year ended September 30, 2010, as compared to $1,945,429
during the fiscal year ended September 30, 2009. The significant
increase is mainly due to (a) $4,065,603 investment in our new production
facility in Wuhan, China, and (b) payments of $5,012,076 to acquire the land use
rights for the land lot on which we plan to build our regional headquarters and
research and development center in Guangzhou, China and for the land lot in
Wuhan, China that we have reserved for further manufacturing capacity expansion
in fiscal year 2010.
Financing
Activities
Net cash
provided by financing activities amounted to $8,721,720 during the fiscal year
ended September 30, 2010, as compared to $559,340 during the fiscal year ended
September 30, 2009, an increase of $8,162,380 or 1,459.3%, resulting mainly from
three equity investments during the fiscal year ended September 30, 2010. See
our disclosure under “Capital Resources” below for more details regarding these
equity investments.
34
Capital
Resources
In
January 2010, we sold 666,667 shares of our common stock and a 5-year warrant to
purchase an additional 333,333 shares of our common stock at an exercise price
of $6.75 per share to a non-U.S. institutional investor, for an aggregate
purchase price for $3.6 million. The Company received $2.24
million of the $3.6 million purchase price in December 2009 and the remaining
$1.36 million in January 2010. The net proceeds is approximately $3.3 million
after deducting commissions and other closing expenses.
On April
21, 2010, we consummated a share purchase agreement, dated April 16, 2010,
between the Company and a non-U.S. institutional investor, pursuant to which the
investor purchased (i) 609,557 shares of our common stock, for an aggregate
purchase price of $3 million, and (ii) a five year warrant to purchase up to an
additional 80,956 shares of our common stock at an exercise price of $6.75 per
share. We agreed to give the investor piggy-back resale registration
rights in connection with the purchase, as well as a right of first refusal with
respect to any new shares of common stock issued by us in connection with any
future private offering. We also agreed to grant the investor the right to
equally participate in any future sale by the principal shareholders of shares
in the Company to a third party. The net proceeds were approximately $2.8
million, after deducting commissions and other closing expenses.
On
September 2, 2010, we consummated a share purchase agreement, dated August 25,
2010, between the Company and a non-U.S. institutional investor, pursuant to
which the investor purchased (i) 585,743 shares of our common stock, for an
aggregate purchase price of $3 million, and (ii) a five year warrant to purchase
up to an additional 87,861 shares of our common stock at an exercise price of
$6.75 per share. We agreed to file a registration statement
registering the above common shares and the common shares underlying the warrant
for resale on or before March 1, 2010, the six-month anniversary of the closing.
The investor also received a right of first refusal with respect to any new
Common Stock issued by the Company and the right to participate as a seller in
any sale of shares of the Company by the original principal shareholders to a
third party. The net proceeds were approximately $2.9 million after deducting
commissions and other closing expenses.
Loan
Commitments
As of
September 30, 2010, the Company had the following outstanding bank
loans:
The
Company had an outstanding bank loan of RMB1,980,000 (approximately $295,606)
from the Agriculture Bank of China, Wuhan Branch (“Agriculture Bank”). This bank
loan has been continuously extended from September 2003, the original maturity
date. The Company has been repaying the principal amount of this loan
with payments of RMB 20,000 (approximately $2,932) each month. Interest accrues
on a monthly basis at the rate of 5.84% per annum.
The
Company had an outstanding bank loan of RMB1,500,000 (approximately $223,944)
from the Wuhan Finance Bureau (“WFB”). This loan has been continuously extended
from November 2001, the original maturity date, and such loan will be repaid
when WFB requests repayment. Interest accrues on a monthly basis at the
rate of 5.94% per annum.
We believe
that we will require additional capital to finance any future
manufacturing expansion, market channel expansion, changes in our business plan
or other future capital intensive developments, including any investments or
acquisitions we may decide to pursue. To the extent it becomes necessary to
raise additional capital, we may seek to raise it through the sale of debt
and/or equity securities, funding from joint-venture or strategic partners,
institutional debt financing or loans, or a combination of the foregoing. Other
than as described above, we currently do not have any binding commitments for,
or readily available sources of, additional financing. If we decide to pursue
any of the above projects, we cannot provide any assurances that we will be able
to secure the additional cash or working capital we may require to implement
such project now or in the future, or if we do, the terms thereof.
We
believe that our currently available funds, funds from operations and available
financing is sufficient for us to continue our operations as presently conducted
for at least the next twelve (12) months.
Obligations
under Material Contracts
None.
35
Off
Balance Sheet Arrangements
As of
September 30, 2010, we have not entered into any other financial guarantees or
other commitments to guarantee the payment obligations of any third parties. We
have not entered into any derivative contracts that are indexed to our
shares and classified as stockholder’s equity or that are not reflected in our
consolidated financial statements. Furthermore, we do not have any retained
or contingent interest in assets transferred to an unconsolidated entity that
serves as credit, liquidity or market risk support to such entity. We do
not have any variable interest in any unconsolidated entity that provides
financing, liquidity, market risk or credit support to us or engages in
leasing, hedging or research and development services with us.
Seasonality
In recent
years, the Company’s revenues for the second and forth quarters in each fiscal
year have been higher than revenues for the first and third fiscal
quarters. We believe that this is partially due to a number of major
domestic festivals and holiday celebrations that occur in China around January
and September, such as the Chinese New Year.
Critical
Accounting Policies and Estimates
Our
management’s discussion and analysis of our financial condition and results of
operations above is based on our consolidated financial statements
which have been prepared in accordance with United States generally
accepted accounting principles (“U.S. GAAP”). The preparation of
these financial statements requires us to make estimates and assumptions that
affect the reported amounts of assets and liabilities and the disclosure of
contingent assets and liabilities at the date of the financial statements and
the reported amounts of revenues and expenses during the reporting periods.
We evaluate our estimates and assumptions on an ongoing basis. We base our
estimates on historical experience and on various other factors that we believe
are reasonable under the circumstances, the results of which form the basis for
making judgments about the carrying value of assets and liabilities that are not
readily apparent from other sources. However, actual results could differ
materially from these estimates under different assumptions or
conditions.
While our
significant accounting policies are more fully described in Note 2 of the Notes
to our consolidated financial statements in this Annual Report on Form
10-K, we believe that the following accounting policies are the most critical
accounting policies to assist in fully understanding and evaluating
our management’s discussion and analysis:
Principles
of Consolidation
The
consolidated financial statements, prepared in accordance with U.S. GAAP,
include the assets, liabilities, revenues, expenses and cash flows of the
Company and all its subsidiaries. This basis of accounting differs in certain
material respects from that used for the preparation of the books and records of
the Company's principal subsidiaries, which are prepared in accordance with the
accounting principles and the relevant financial regulations applicable to
enterprises with limited liabilities established in the PRC, the accounting
standards used in the place of such entities’ domicile. The
accompanying consolidated financial statements reflect necessary adjustments not
recorded in the books and records of the Company's subsidiaries to present them
in conformity with US GAAP. All significant inter-company accounts,
transactions and cash flows are eliminated on
consolidation. Investment in affiliates in which the Company
exercises significant influence but do not control and are not the primary
beneficiary are accounted for using the equity method.
In
accordance with Accounting Standards Codification (“ASC”) Topic 810 and its
related subtopics (formerly as FASB Interpretation No. 46R, Consolidation of
Variable Interest Entities) ("ASC Topic 810"), variable interest entities (VIEs)
are generally entities that lack sufficient equity to finance their activities
without additional financial support from other parties or whose equity holders
lack adequate decision making ability. All VIEs with which the Company is
involved must be evaluated to determine the primary beneficiary of the risks and
rewards of the VIE. The primary beneficiary is required to consolidate the VIE
for financial reporting purposes. The Company has concluded that Xinpu is a VIE
and that the Company is the primary beneficiary. Under the requirements of ASC
Topic 810 the Company consolidated the financial statements of Guangdong
Xinpu.
36
Use
of Estimates
The
preparation of financial statements in conformity with U.S. GAAP requires
management to make estimates and assumptions relating to the reported amounts of
assets, liabilities, revenues and expenses, and related disclosure of contingent
assets and liabilities at the date of the financial statements and the reported
amounts of revenues and expenses during the reporting periods. Significant items
subject to such estimates and assumptions include revenues; the allowance for
doubtful receivables; deposit for land use rights; recoverability of the
carrying amount of property and equipment, goodwill and intangible assets; fair
values of financial instruments; and the assessment of contingent
obligations. These estimates are often based on complex judgments and
assumptions that management believes to be reasonable but are inherently
uncertain and unpredictable. Actual results could differ from those
estimates.
Revenue
Recognition
The
Company recognizes revenue in accordance with ASC Topic 605 (formerly Staff
Accounting Bulletin ("SAB") No. 104). All of the following criteria must exist
in order for the Group to recognize revenue: (1) persuasive evidence of an
arrangement exists; (2) delivery has occurred or services have been
rendered; (3) the seller's price to the buyer is fixed or determinable; and
(4) collectability is reasonably assured.
Accounts
Receivable
Accounts
receivable are recorded at net realizable value consisting of the carrying
amount less an allowance for uncollectible accounts, as needed. The allowance
for doubtful accounts is the Company’s best estimate of the amount of probable
credit losses in the Company’s existing accounts receivable. The
Company determines the allowance based on aging data, historical collection
experience, customer specific facts and economic conditions. Account balances
are charged off against the allowance after all means of collection have been
exhausted and the potential for recovery is considered remote. The
Company does not have any off-balance-sheet credit exposure related to its
customers.
Inventories
Inventories
are stated at the lower of cost or market value. Cost is determined using the
weighted average cost method. Cost of finished goods comprises direct material,
direct production cost and an allocated portion of production overheads based on
normal operating capacity. The Company evaluates the need for reserves
associated with obsolete, slow-moving and non-salable inventory by reviewing the
net realizable value on a periodic basis. If inventory is written
down to net realizable value, the write-down is charged to expense.
Foreign
Currency Translation
The
accompanying financial statements are presented in United States dollars. The
functional currency of the Company’s subsidiaries and VIE in the PRC is Renminbi
(“RMB”) and in Hong Kong is the Hong Kong dollar (“HK$”). The financial
statements are translated into United States dollars from RMB at year-end
exchange rates as to assets and liabilities and average exchange rates as to
revenues and expenses. Capital accounts are translated at their historical
exchange rates when the capital transactions occurred.
Recent
Accounting Pronouncements
In
February 2010, FASB issued ASU No. 2010-09 “Subsequent Events” (“ASC Topic 855”)
which removes the requirement for an SEC filer to disclose a date in both issued
and revised financial statements. This amendment shall be applied prospectively
for interim or annual financial periods ending after June 15, 2010. The Group
does not believe the adoption will have a material effect on the Group's
consolidated financial statements.
37
The FASB
has issued ASU 2010-06, Fair Value Measurements and Disclosures (ACS Topic 820):
Improving Disclosures about Fair Value Measurements. This ASU requires some new
disclosures and clarifies some existing disclosure requirements about fair value
measurement as set forth in Codification Subtopic 820-10. The FASB’s objective
is to improve these disclosures and, thus, increase the transparency in
financial reporting. Specifically, ASU 2010-06 amends Codification Subtopic
820-10 to now require:
|
|
-
|
A
reporting entity to disclose separately the amounts of significant
transfers in and out of Level 1 and Level 2 fair value measurements and
describe the reasons for the transfers;
and
|
|
|
-
|
In
the reconciliation for fair value measurements using significant
unobservable inputs, a reporting entity should present separately
information about purchases, sales, issuances, and
settlements.
|
In
addition, ASU 2010-06 clarifies the requirements of the following existing
disclosures:
|
|
-
|
For
purposes of reporting fair value measurement for each class of assets and
liabilities, a reporting entity needs to use judgment in determining the
appropriate classes of assets and liabilities;
and
|
|
|
-
|
A
reporting entity should provide disclosures about the valuation techniques
and inputs used to measure fair value for both recurring and nonrecurring
fair value measurements.
|
ASU
2010-06 is effective for interim and annual reporting periods beginning after
December 15, 2009, except for the disclosures about purchases, sales, issuances,
and settlements in the roll forward of activity in Level 3 fair value
measurements. Those disclosures are effective for fiscal years beginning after
December 15, 2010, and for interim periods within those fiscal years. Early
adoption is permitted.
In July
2010, the FASB issued Accounting Standard Update (“ASU”) 2010-20, “Receivables
(Topic 310): Disclosures about the Credit Quality of Financing Receivables and
the Allowance for Credit Losses”. This ASU amends Topic 310 to
improve the disclosures that an entity provides about the credit quality of its
financing receivables and the related allowance for credit losses. As a result
of these amendments, an entity is required to disaggregate by portfolio segment
or class certain existing disclosures and provide certain new disclosures about
its financing receivables and related allowance for credit losses. For public
entities, the disclosures as of the end of a reporting period are effective for
interim and annual reporting periods ending on or after December 15, 2010. The
disclosures about activity that occurs during a reporting period are effective
for interim and annual reporting periods beginning on or after December 15,
2010, except for the expanded disclosure requirements. The Group is currently
evaluating the potential impact, if any, of this ASU on its consolidated
financial statements.
|
ITEM
7A.
|
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISK.
|
Not
Applicable.
|
ITEM
8.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA.
|
The full
text of our audited consolidated financial statements as of September 30, 2010
and 2009 begins on page F-1 of this report.
|
ITEM
9.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE.
|
None.
|
ITEM
9A.
|
CONTROLS
AND PROCEDURES.
|
Evaluation
of Disclosure Controls and Procedures
We
maintain disclosure controls and procedures (as defined in Rule 13a-15(e) under
the Exchange Act) that are designed to ensure that information that would be
required to be disclosed in Exchange Act reports is recorded, processed,
summarized and reported within the time period specified in the SEC’s rules and
forms, and that such information is accumulated and communicated to our
management, including to our Chief Executive Officer and Chief Financial
Officer, as appropriate, to allow timely decisions regarding required
disclosure.
38
As
required by Rule 13a-15 under the Exchange Act, our management, including Mr.
Dongliang Chen, our Chief Executive Officer and Mr. Richard Liu, our Chief
Financial Officer, evaluated the effectiveness of the design and operation of
our disclosure controls and procedures as of September 30,
2010. Based on that evaluation, Mr. Chen and Mr. Liu concluded that,
because of the material weaknesses described below, as of September 30, 2010,
our disclosure controls and procedures were not effective.
Management’s
annual report on internal control over financial reporting
Management
is responsible for establishing and maintaining adequate internal control over
financial reporting for the Company. Internal control over financial reporting
refers to the process designed by, or under the supervision of, our chief
executive officer and chief financial officer, and effected by our Board of
Directors, management and other personnel, to provide reasonable assurance
regarding the reliability of our financial reporting and the preparation of
financial statements for external purposes in accordance with U.S. GAAP, and
includes those policies and procedures that:
|
|
·
|
pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of our
assets;
|
|
|
·
|
provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with U.S. GAAP, and that
our receipts and expenditures are being made only in accordance with the
authorization of our management and directors;
and
|
|
|
·
|
provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of our assets that could have
a material effect on the financial
statements.
|
Management
conducted an evaluation of the effectiveness of our internal control over
financial reporting as of September 30, 2010 based on the framework set forth in
the report entitled Internal Control - Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission. Based on the
evaluation, management concluded that our internal control over financial
reporting as of September 30, 2010 was not effective. Management identified the
following material weaknesses as of September 30, 2010:
|
|
·
|
Lack
of Internal Audit Function - We lack qualified resources to perform our
internal audit functions properly. In addition, we have not yet fully
developed the scope and effectiveness of our internal audit
function.
|
|
|
·
|
Lack
of Audit Committee - We lack an audit committee within our board to
oversee the financial reporting pursuant to U.S. GAAP and the SEC’s rules
and regulations.
|
|
|
·
|
Insufficient
U.S. GAAP Accounting Skills and Experience - We found that our accounting
staff lacked sufficient accounting skills and experience necessary to
fulfill our public reporting obligations according to U.S. GAAP and the
SEC’s rules and
regulations.
|
Our management is in the process of implementing remediation procedures to improve internal controls over financial reporting. The Company has already taken measures to remediate these material weaknesses by seeking additional financial reporting and accounting staff members with relevant accounting experience, skills and knowledge in the preparation of financial statements in accordance with of U.S. GAAP and financial reporting disclosure requirements under SEC rules. The Company’s first step in this regard is the appointment of Mr. Richard Liu as Chief Financial Officer by our Board of Directors. Mr. Liu is an experienced professional in the field of financial reporting and other related areas from a public accounting professional to financial executive of U.S. listed companies. The Company believes that Mr. Liu brings the sufficient accounting skills and experience necessary to enable us to implement and reinforce a rigorous process for collecting and reviewing information required for the preparation of the financial statement, and help us fulfill our public accounting obligations according to U.S. GAAP and the SEC’s rules and regulations with the support from the board and additional personnel experienced in U.S. GAAP and the SEC’s rules to be hired.
39
The
Company has also engaged a professional consulting firm experienced in handling
compliance with the requirements of the Sarbanes-Oxley Act of 2002 with respect
to internal control over financial reporting in order to assist the Company with
improving its internal controls and meet the requirements of Sarbanes-Oxley Act
of 2002. In the meantime, the Company has been searching and negotiating with
several qualified candidates to serve as the audit committee members so as to
establish an audit committee within the Board of Directors of the
Company. The Company is also in the process of establishing an
internal audit function and will continue to hire more experienced personnel
with expertise in U.S. public company financial reporting.
Because
of its inherent limitations, internal control over financial reporting may not
prevent or detect misstatements. Projections of any evaluation of effectiveness
to future periods are subject to the risk that controls may become inadequate
because of changes in conditions, or that the degree of compliance with the
policies and procedures may deteriorate.
Management
remains committed to improving its internal control over financial reporting and
will continue to work to put effective controls in place.
Because
the Company is a smaller reporting company, this annual report does not include
an attestation report of our registered public accounting firm regarding
internal control over financial reporting. Management’s report was not subject
to attestation by our registered public accounting firm.
Changes
in internal control over financial reporting
There
were no changes in our internal controls over financial reporting during the
fourth quarter of our fiscal year ended September 30, 2010 that have materially
affected, or are reasonably likely to materially affect, our internal control
over financial reporting.
|
ITEM
9B.
|
OTHER
INFORMATION.
|
We have
no information to disclose that was required to be disclosed in a report on Form
8-K during fourth quarter of fiscal year 2010, but was not
reported.
PART
III
|
ITEM
10.
|
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE.
|
Directors
and Executive Officers
The
following sets forth the name and position of each of our current executive
officers and directors.
|
NAME
|
AGE
|
POSITION
|
||
|
Dongliang
Chen
|
48
|
Chairman
of the Board and Chief Executive Officer
|
||
|
Shengfan
Yan
|
43
|
President
and Director
|
||
|
Lirong
Hu
|
52
|
Treasurer
and Director
|
||
|
Richard
Liu
|
36
|
Chief
Financial Officer
|
Dongliang
Chen. Dongliang Chen was
appointed our Chairman and Chief Executive Officer effective November 13, 2009.
Since June 2008, Mr. Chen has served, and he continues to serve, as the Chief
Executive Officer and a director of CPG. Mr. Chen is also the Chairman and Chief
Executive Officer of Tallyho, a position he has held since December 1996. Prior
thereto, Mr. Chen was the CEO of Wuhan Sanrong Group Ltd. From 1990 to 1992, he
was the Chief Technology Officer at Union Technology Limited in Hong Kong from
1990 to 1992. Mr. Chen is a committee member of China Health
Management Association of the Chinese Medical Association and also is a visiting
professor at Wuhan Bioengineering Institute of China. Mr. Chen’s management
experience, having served as our Chairman and Chief Executive Officer since
2009, CPG’s Chairman and Chief Executive Officer since 2008, and as Tallyho’s
Chairman and Chief Executive Officer since 1996, along with his industry
experience, led us to the conclusion that he should serve as a director of our
Company, in light of our business and structure.
40
Shengfan
Yan. Shengfan Yan was appointed our President and a director
effective November 13, 2009. Since June 2008, Shengfan Yan has
served, and he continues to serve, as the President and a director of CPG. Mr.
Yan is also the President and a director of Tallyho, positions he has held since
November 2007. Prior thereto, Mr. Yan was the President of Guangdong Puzhongren
Group, a provider of polypeptide protein and polypeptide capsules, from March
2001 through October 2007. Prior to his employment at Guangdong
Puzhongren, he was the General Sales Manager at Guangdong Zhengguo Group, a
provider of Anti-cancer drugs. Mr. Yan holds an Executive MBA from
San Yat Sen University and a Bachelor degree from Hunan Normal University with a
major in History. Mr. Yan’s management experience, having served as
our President since 2009, CPG’s President since 2008, and as Tallyho’s President
since 2007, along with his industry experience and educational background, led
us to the conclusion that he should serve as a director of our Company, in light
of our business and structure.
Lirong
Hu. Lirong Hu was appointed our Treasurer and a director effective
November 13, 2009. From November 13, 2009 until March 1, 2010, she also served
as our Chief Financial Officer. Since June 2008, Ms. Hu has served, and she
continues to serve, as the Chief Financial Officer and a director of CPG. Ms. Hu
is also the Chief Financial Officer and a director of Tallyho. Ms. Hu
was appointed the Chief Financial Officer of Tallyho in 1999 and became a
director of Tallyho in November 2007. Prior thereto, Ms. Hu was an
accountant at Wuhan Sandi Electronic Apparatus Company from 1991to
1998. Ms. Hu received a Bachelor’s degree in Industrial Accounting
from Hubei University in 1987. Ms. Hu’s management experience, having
served as our Chief Financial Officer and Treasurer, CPG’s Chief Financial
Officer and since 2008, and as Tallyho’s Chief Financial Officer since 1999,
along with her educational background, led us to the conclusion that she should
serve as a director of our Company, in light of our business and
structure.
Richard
Liu. Richard Liu was appointed as our Chief Financial Officer on March 1,
2010. He has served as a senior executive for a number of private and public
U.S. and Chinese companies. From April 2008 to December 2009, Mr. Liu
was the Chief Financial Offer of China Energy Recovery, Inc., a clean technology
and alternative energy company listed on the OTCBB. From October 2006 to
March 2008, Mr. Liu was the Chief Executive Officer and a Director of China
National Credit Information Services, Inc., a credit advisory
company. From August 2004 to September 2006, he co-founded and served
as the Vice President of Finance and Operations and a Director of PanPacifics
Technology Holding Ltd., an on-demand global trade management software
developer. Mr. Liu served as the Director of Finance for Kiwa
Bio-Tech Products Group Corp., an agricultural bio-technological product
producer listed on the OTCBB, from September 2003 to August
2004. Before that, he was an assistant Chief Financial Officer for
YesMobile Holdings Co. Ltd., a Chinese wireless technology company, from July
2000 to July 2001. Mr. Liu began his career at Arthur Andersen, LLP,
a public accounting firm, where he worked from August 1996 to July
2000. Mr. Liu holds an MBA degree from UCLA and obtained his Bachelor
of Engineering from Shanghai Jiao Tong University in China. Mr. Liu
is also a member of the Chinese Institute of Certified Public Accountants
(CICPA).
There are
no agreements or understandings for any of our executive officers or director to
resign at the request of another person and no officer or director is acting on
behalf of nor will any of them act at the direction of any other
person.
Directors
are elected until their successors are duly elected and qualified.
Family
Relationships
There are
no family relationships among our directors or officers.
Involvement
in Certain Legal Proceedings
To the
best of our knowledge, none of our directors or executive officers has, during
the past ten years:
|
|
·
|
been
convicted in a criminal proceeding or been subject to a pending criminal
proceeding (excluding traffic violations and other minor
offences);
|
|
|
·
|
had
any bankruptcy petition filed by or against the business or property of
the person, or of any partnership, corporation or business association of
which he was a general partner or executive officer, either at the time of
the bankruptcy filing or within two years prior to that
time;
|
|
|
·
|
been
subject to any order, judgment, or decree, not subsequently reversed,
suspended or vacated, of any court of competent jurisdiction or federal or
state authority, permanently or temporarily enjoining, barring, suspending
or otherwise limiting, his involvement in any type of business,
securities, futures, commodities, investment, banking, savings and loan,
or insurance activities, or to be associated with persons engaged in any
such activity;
|
41
|
|
·
|
been
found by a court of competent jurisdiction in a civil action or by the
Securities and Exchange Commission or the Commodity Futures Trading
Commission to have violated a federal or state securities or commodities
law, and the judgment has not been reversed, suspended, or
vacated;
|
|
|
·
|
been
the subject of, or a party to, any federal or state judicial or
administrative order, judgment, decree, or finding, not subsequently
reversed, suspended or vacated (not including any settlement of a civil
proceeding among private litigants), relating to an alleged violation of
any federal or state securities or commodities law or regulation, any law
or regulation respecting financial institutions or insurance companies
including, but not limited to, a temporary or permanent injunction, order
of disgorgement or restitution, civil money penalty or temporary or
permanent cease-and-desist order, or removal or prohibition order, or any
law or regulation prohibiting mail or wire fraud or fraud in connection
with any business entity; or
|
|
|
·
|
been
the subject of, or a party to, any sanction or order, not subsequently
reversed, suspended or vacated, of any self-regulatory organization (as
defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))),
any registered entity (as defined in Section 1(a)(29) of the Commodity
Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange,
association, entity or organization that has disciplinary authority over
its members or persons associated with a
member.
|
Except as
set forth in our discussion below in Item 13, “Certain Relationships and Related
Transactions, and Director Independence – Transactions with Related Persons,”
none of our directors, director nominees or executive officers has been involved
in any transactions with us or any of our directors, executive officers,
affiliates or associates which are required to be disclosed pursuant to the
rules and regulations of the SEC.
Section
16(A) Beneficial Ownership Reporting Compliance
We are
not subject to Section 16(A) of the Exchange Act.
Code
of Ethics
We have
not adopted a code of ethics. However, we intend to adopt a code of ethics in
the future. We envision that the code of ethics will apply to all of our
employees, officers and directors.
Material
Changes to Director Nomination Procedures
There
have been no material changes to the procedures by which stockholders may
recommend nominees to our board of directors since such procedures were last
disclosed.
Audit
Committee and Audit Committee Financial Expert
We do not
have an audit committee or an audit committee financial expert serving on the
audit committee. Our entire board of directors currently is responsible for the
functions that would otherwise be handled by an audit committee. However, we
intend to establish an audit committee of the board of directors in the near
future. We envision that the audit committee will be primarily responsible for
reviewing the services performed by our independent auditors, evaluating our
accounting policies and our system of internal controls. Upon the establishment
of an audit committee, the board will determine whether any of the directors
qualify as an audit committee financial expert.
|
ITEM
11.
|
EXECUTIVE
COMPENSATION.
|
Summary Compensation Table – Fiscal
Years Ended September 30, 2010 and 2009
The
following table sets forth information concerning all cash and non-cash
compensation awarded to, earned by or paid to the named persons for services
rendered in all capacities during the noted periods. No other executive officer
received total annual salary and bonus compensation in excess of
$100,000.
42
|
Name
and Principal Position
|
Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards(1)
($)
|
All
Other
Compensation
($)
|
Total
($)
|
||||||||||||||||
|
Dongliang
Chen,
|
2010
|
39,758 | - | - | 1,600 | 41,358 | ||||||||||||||||
|
Chief
Executive Officer(2)
|
2009
|
14,069 | - | - | 1,759 | 15,828 | ||||||||||||||||
|
Richard
Liu,
|
2010
|
51,310 | - | 78,990 | 130,300 | |||||||||||||||||
|
Chief
Financial Officer
|
2009
|
- | - | - | - | - | ||||||||||||||||
|
John
Delaney,
|
2010
|
- | - | - | - | - | ||||||||||||||||
|
Former
Chief Executive Officer(3)
|
2009
|
- | - | - | - | - | ||||||||||||||||
|
(1)
|
Amount
shown does not reflect compensation actually received by the named
executive officer. The amount represents the grant date value for stock
granted to the named executive officer in fiscal years 2010 and 2009
calculated in accordance with Financial Accounting Standards Board (FASB)
Accounting Standards Codification (ASC) Topic 718. The assumptions used to
calculate the value of stock awards are set forth under Note 22 of the
Notes to Consolidated Financial Statements included in this report. The
trading of the Company’s stock on the OTC Bulletin Board is very limited.
The closing stock price as of December 31, 2010 was $0.75 as quoted on the
OTC Bulletin Board maintained by the Financial Industry Regulatory
Authority. Based on this latest closing price, Mr. Liu’s stock award was
$19,125 as of September 30, 2010.
|
|
(2)
|
On
November 13, 2009, we acquired Cantix in a reverse acquisition transaction
that was structured as a share exchange and in connection with that
transaction, Mr. Dongliang Chen became our Chairman and Chief Executive
Officer. Prior to the effective date of the reverse
acquisition, Mr. Chen served at Canitx’s subsidiary Tallyho as its
Chairman and Chief Executive Officer. The annual, long term and other
compensation shown in this table include the amount Mr. Chen received from
Tallyho prior to the consummation of the reverse
acquisition.
|
|
(3)
|
John
Delaney resigned from all offices he held with us upon the closing of the
reverse acquisition of Cantix on November 13,
2009.
|
Employment
Agreements
On
September 1, 2008, we entered into an employment agreement through our PRC
operating subsidiary, Tallyho, with our Chief Executive Officer, Mr. Dongliang
Chen, pursuant to which Mr. Chen is entitled to a base salary of approximately
$3,313 (RMB22,600) per month in fiscal year 2010, subject to increase at our
discretion. Mr. Chen may also receive performance-based bonuses, at
our sole discretion, and is eligible to participate in any stock option plan or
other share incentive program adopted by us. The employment agreement
was renewed on September 1, 2010 and has a 3-year term expiring August 31, 2013,
and is renewable for the same period upon mutual agreement by the expiration
date.
On March
1, 2010, we entered into an employment agreement with our Chief Financial
Officer, Mr. Richard Liu, pursuant to which Mr. Liu is entitled to an annual
base salary of approximately $87,850 (RMB 600,000) per year, subject to increase
at our discretion. Under the agreement, we agreed to grant to Mr. Liu
(i) 8,000 shares of our common stock upon execution of the employment agreement
and (ii) an additional 90,000 shares of our common stock, to vest in 6 equal
installments of 15,000 shares at the beginning of each six-month period during
the term of the agreement, with the first 15,000 shares vesting on March 1,
2010. Pursuant to the employment agreement, Mr. Liu may also receive
performance-based bonuses, at our sole discretion, and is eligible to
participate in any stock option plan or other share incentive program adopted by
us. The employment agreement has an initial three-year term expiring
March 1, 2013, and is automatically renewable for the same period unless a
written notice of nonrenewal is given by either Mr. Liu or us at least 90 days
prior to expiration.
We have
not provided retirement benefits (other than a state pension scheme in which all
of our employees in China participate) or severance or change of control
benefits to our named executive officers.
Outstanding
Equity Awards at Fiscal Year End
The
following table sets forth the equity awards outstanding at September 30, 2010
for each of our named executive officers. Market values are based on
our December 31, 2010 closing stock price of $0.75 as quoted on the OTC Bulletin
Board maintained by the Financial Industry Regulatory
Authority.
43
|
OPTION AWARDS
|
STOCK AWARDS
|
|||||||||||||||||||||||||||||||||||
|
Name
|
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable
|
Equity
Incentive
Plan Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options
(#)
|
Option
Exercise
Price
($)
|
Option
Expiration
Date
|
Number of
Shares or
Units of
Stock That
Have Not
Vested
(#)
|
Market Value
of Shares or
Units of Stock
That Have
Not Vested
($)
|
Equity
Incentive Plan
Awards:
Number of
Unearned
Shares, Units
or Other
Rights That
Have Not
Vested
(#)
|
Equity
Incentive
Plan Awards:
Market or
Payout Value
of Unearned
Shares, Units or
Other Rights
That Have Not
Vested
($)
|
|||||||||||||||||||||||||||
|
Richard
Liu
|
- | - | - | - | - | 60,000 | 45,000 | - | - | |||||||||||||||||||||||||||
|
(1)
|
On
March 1, 2010, Mr. Liu received a right to be granted 90,000 shares of our
common stock, to vest in 6 equal installments of 15,000 shares at the
beginning of each six-month period during the term of his employment
agreement, with the first 15,000 shares vesting on March 1,
2010.
|
Compensation
of Directors
No member
of our board of directors received any compensation for his services as a
director during the year ended September 30, 2010.
|
ITEM
12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS.
|
Security
Ownership of Certain Beneficial Owners and Management
The
following table sets forth information regarding beneficial ownership of our
common stock as of December 31, 2010 (i) by each person who is known by us to
beneficially own more than 5% of our common stock; (ii) by each of our officers
and directors; and (iii) by all of our officers and directors as a group. Unless
otherwise specified, the address of each of the persons set forth below is in
care of the Company, No. 11 Jiangda Road, Jianghan Economic Development Zone,
Wuhan 430023, People’s Republic of China.
|
Name & Address of
Beneficial Owner
|
Office, If Any
|
Title of Class
|
Amount &
Nature of
Beneficial
Ownership(1)
|
Percent
of
Class(2)
|
||||||||
|
Officers
and Directors
|
||||||||||||
|
Dongliang
Chen
|
Chairman
and Chief Executive Officer
|
Common
Stock, $0.0001 par value
|
8,800,000 | (3) | 73.7 | % | ||||||
|
Shengfan
Yan
|
President
and Director
|
Common
Stock, $0.0001 par value
|
- | - | ||||||||
|
Lirong
Hu
|
Treasurer
and Director
|
Common
Stock, $0.0001 par value
|
- | - | ||||||||
|
Jun
Li
|
Vice
President and Company Secretary
|
Common
Stock, $0.0001 par value
|
- | - | ||||||||
|
Richard
Liu
|
Chief
Financial Officer
|
Common
Stock, $0.0001 par value
|
38,000 | (4) | * | |||||||
|
All
Officers and Directors as a group (5 persons named above)
|
Common
Stock, $0.0001 par value
|
8,838,000 | (3) | 74.0 | % | |||||||
|
5%
Security Holders
|
||||||||||||
|
China
Polypeptide Group Ltd.
|
Common
Stock, $0.0001 par value
|
8,800,000 | (3) | 73.7 | % | |||||||
|
Dongliang
Chen
|
Common
Stock, $0.0001 par value
|
8,800,000 | (3) | 73.7 | % | |||||||
44
* Less
than 1%
|
(1)
|
Beneficial
ownership is determined in accordance with the rules of the SEC and
generally includes voting or investment power with respect to
securities.
|
|
(2)
|
A
total of 11,939,967 shares of our common stock are considered to be
outstanding pursuant to SEC Rule 13d-3(d)(1) as of December 31, 2010. For
each beneficial owner above, any options exercisable within 60 days have
been included in the denominator.
|
|
(3)
|
Dongliang
Chen is the Chairman and Chief Executive Officer of China Polypeptide
Group Ltd. and he has voting and investment power over securities held by
it. Mr. Chen disclaims beneficial ownership of such shares
except to the extent of his pecuniary interest
therein.
|
|
(4)
|
On
March 1, 2010, Mr. Liu received 8,000 shares of our common stock that
vested immediately, as well as a right to receive 90,000 shares of our
common stock that will vest in 6 equal installments of 15,000 shares at
the beginning of each six-month period during the term of his employment
agreement, with the first 15,000 shares vesting on March 1, 2010. As of
December 31, 2010, 30,000 of these shares have vested and another 15,000
shares will vest on March 1, 2011 during his
employment.
|
Changes in Control
There are
no arrangements known to us, including any pledge by any person of our
securities, the operation of which may at a subsequent date result in a change
in control of the Company.
Securities
Authorized for Issuance Under Equity Compensation Plans
We do not
have in effect any compensation plans under which our equity securities are
authorized for issuance and we do not have any outstanding stock
options.
|
ITEM
13.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE.
|
Transactions
with Related Persons
The
following includes a summary of transactions since the beginning of our 2009
fiscal year, or any currently proposed transaction, in which we were or are to
be a participant and the amount involved exceeded or exceeds the lesser of
$120,000 or one percent of the average of our total assets at year-end for the
last two completed fiscal years, and in which any related person had or will
have a direct or indirect material interest (other than compensation described
under Item 11, “Executive Compensation”). We believe the terms obtained or
consideration that we paid or received, as applicable, in connection with the
transactions described below were comparable to terms available or the amounts
that would be paid or received, as applicable, in arm’s-length
transactions.
|
|
·
|
Mr.
Chen, our Chairman and Chief Executive Officer, is also the Chairman and
Chief Executive Officer of CPG. CPG is the holder of 73.7% of
our outstanding common stock.
|
|
|
·
|
Pursuant
to a Patent License Agreement, dated October 2, 2009, between Mr.
Chen and Tallyho, as amended on May 25, 2010, Mr. Chen has licensed to
Tallyho the rights to use certain processes and technologies for which Mr.
Chen has filed patent applications.
|
|
|
·
|
Wuhan
Hopsun Biological Products Inspection Company Limited (“Wuhan
Inspection”), an affiliate company in which Tallyho, one of the Company’s
subsidiaries, holds 40% shareholding, advanced to Tallyho payments for
relevant expenses for obtaining the necessary special licenses for Wuhan
Inspection’s inspection business through and with the assistance of
Tallyho. The balances recorded as due to related parties – Wuhan
Inspection were $442,689 and $450,642 as of September 30, 2010 and
2009.
|
45
|
|
·
|
Effective
September 28, 2010, Hopsun entered into a series of contractual
arrangements with Xingpu and its shareholders, Mr. Dongliang Chen, the
Company’s Chief Executive Officer and Board Chairman, and Mr.
Shengfan Yan, the Company’s President and Director, pursuant to which
Xingpu became the Company’s variable interest entity, or
VIE. The VIE agreements
include:
|
|
|
a)
|
Exclusive Business
Cooperation Agreement. Xingpu has entered into an Exclusive
Business Cooperation Agreement with Hopsun, which agreement provides that
Hopsun will be the exclusive provider of business support, technical and
consulting services to Xingpu and that Xingpu will pay the service fees
determined based on a percentage of Xingpu’s profit before tax, which is
determined and adjustable at the sole discretion of Hopsun. The time of
payment is also determined at the sole discretion of
Hopsun.
|
|
|
b)
|
Proxy
Agreement. Each of the shareholders of Xingpu has executed a Proxy
Agreement irrevocably granting and entrusting Hopsun, for the maximum
period permitted by law, with all of his voting rights as a shareholder of
Xingpu and to vote on his behalf for all matters requiring shareholder
approval, including but not limited to, the sale, transfer, pledge, or
disposition of his shareholding in Xingpu. Hopsun shall exercise such
rights in accordance with and within the limitations of the laws of the
PRC and the Articles of Association of
Xingpu.
|
|
|
c)
|
Equity Pledge
Agreement. Hopsun and the shareholders of Xingpu have entered into
an Equity Pledge Agreement, pursuant to which each such shareholder
pledges all of his equity interest of Xingpu to Hopsun as a security for
the obligations of Xingpu and each shareholders under the Exclusive
Business Cooperation Agreement and the payment of services fees under such
an agreement. The Pledge shall refer to the rights owned by Hopsun, who
shall be entitled to a priority in receiving payment by the proceeds from
the auction or sale of the equity interest pledged by each shareholders of
Xingpu to Hopsun. Such Xingpu shareholders have agreed not to
dispose of the pledged equity interests or take any actions that would
prejudice Hopsun’s interest.
|
|
|
d)
|
Exclusive Option
Agreement. Each of the shareholders of Xingpu has entered into an
Exclusive Option Agreement, which provides that Hopsun will be entitled to
acquire such shares from the current shareholders upon certain terms and
conditions, to be extent permitted under the PRC law. The Exclusive Option
Agreement also prohibits the current shareholders of Xingpu from disposing
of their equity interests without written consent of
Hopsun. Hopsun has not yet taken any corporate action to
exercise this right of purchase, and there is no guarantee that it will do
so or will be permitted to do so by applicable law at such time as it may
wish to do so.
|
In
accordance with Accounting Standards Codification (“ASC”) Topic 810 and its
related subtopics (formerly FASB Interpretation No. 46R, Consolidation of
Variable Interest Entities), Xingpu is a VIE of the Company and the Company is
the primary beneficiary of Xingpu through Hopsun.
Except as
set forth in our discussion above, none of our directors, director nominees or
executive officers has been involved in any transactions with us or any of our
directors, executive officers, affiliates or associates which are required to be
disclosed pursuant to the rules and regulations of the SEC.
Promoters
and Certain Control Persons
We did
not have any promoters at any time during the past five fiscal
years.
Parents
of the Company
CPG
currently owns 73.7% of our common stock.
Director
Independence
We
currently do not have any independent directors, as the term “independent” is
defined by the rules of the Nasdaq Stock Market.
46
|
ITEM
14.
|
PRINCIPAL
ACCOUNTING FEES AND SERVICES.
|
Independent
Registered Public Accounting Firm’s Fees
The
following is a summary of the fees billed to the Company by its principal
accountants for professional services rendered for the fiscal years ended
September 30, 2010 and 2009:
|
Fiscal Year Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Audit
Fees
|
$ | 195,000 | $ | 116,000 | ||||
|
Audit
Related Fees
|
- | - | ||||||
|
Tax
Fees
|
- | - | ||||||
|
All
Other Fees
|
- | - | ||||||
|
Total
|
195,000 | 116,000 | ||||||
“Audit
Fees” consisted of the aggregate fees billed for professional services rendered
for the audit of our annual financial statements and the reviews of the
financial statements included in our Forms 10-Q and for any other services that
were normally provided in connection with our statutory and regulatory filings
or engagements.
“Audit
Related Fees” consisted of the aggregate fees billed for professional services
rendered for assurance and related services that were reasonably related to the
performance of the audit or review of our financial statements and were not
otherwise included in Audit Fees.
“Tax
Fees” consisted of the aggregate fees billed for professional services rendered
for tax compliance, tax advice and tax planning. Included in such Tax Fees were
fees for preparation of our tax returns and consultancy and advice on other tax
planning matters.
“All
Other Fees” consisted of the aggregate fees billed for products and services
provided and not otherwise included in Audit Fees, Audit Related Fees or Tax
Fees.
Pre-Approval
Policies and Procedures
Under the
Sarbanes-Oxley Act of 2002, all audit and non-audit services performed by our
auditors must be approved in advance by our Board to assure that such services
do not impair the auditors’ independence from us. In accordance with its
policies and procedures, our Board pre-approved the audit service performed by
Bernstein & Pinchuk LLP for our consolidated financial statements as of and
for the year ended September 30, 2010.
PART
IV
|
ITEM
15.
|
EXHIBITS,
FINANCIAL STATEMENT SCHEDULES.
|
Financial
Statements and Schedules
The
financial statements are set forth under Item 8 of this annual report on Form
10-K. Financial statement schedules have been omitted since they are either not
required, not applicable, or the information is otherwise included.
Exhibit
List
The
following exhibits are filed as part of this report or incorporated by
reference:
|
Exhibit No.
|
Description
|
|
|
2.1
|
Stock
Exchange Agreement, dated as of November 13, 2009, by and among the
registrant, China Polypeptide Group Ltd. and Cantix International Limited
[Incorporated by reference to Exhibit 2.1 to the registrant’s Current
Report on Form 8-K filed on November 17, 2009]
|
|
|
3.1*
|
Certificate
of Incorporation of the registrant, as amended to
date
|
47
|
Exhibit No.
|
Description
|
|
|
3.2
|
Bylaws
of the registrant [Incorporated by reference to Exhibit 3.2 to the
registrant’s Registration Statement on Form S-1 filed on May 23,
2008]
|
|
|
4.1
|
Warrant
to Purchase Common Stock issued December 16, 2009 [Incorporated by
reference to Exhibit 99.2 to the registrant’s Current Report on Form 8-K
filed on January 13, 2010]
|
|
|
10.1
|
Share
Purchase Agreement, dated as of August 25, 2010, by and between the
registrant and Wealth Chance Investments Ltd. (English Translation)
[Incorporated by reference to Exhibit 10.1 to the registrant’s Current
Report on Form 8-K filed on September 2, 2010]
|
|
|
10.2
|
Share
Purchase Agreement, dated as of April 16, 2010, by and between the
registrant and Step Best Investments Ltd. (English Translation)
[Incorporated by reference to Exhibit 10.1 to the registrant’s Current
Report on Form 8-K filed on April 22, 2010]
|
|
|
10.3
|
Form
of Securities Purchase Agreement, dated December 16, 2009 [Incorporated by
reference to Exhibit 99.1 to the registrant’s Current Report on Form 8-K
filed on January 13, 2010]
|
|
|
10.4
|
Agreement
for Return on Purchase Price, dated as of July 14, 2008, by and among
Wuhan Tallyho Biological Product Co., Ltd., Wuhan Polypeptide Anti-Aging
Research and Development Co., Ltd., Moneyeasy Industries Limited and the
shareholders of Wuhan Tallyho Biological Product Co., Ltd. and Wuhan
Polypeptide Anti-Aging Research and Development Co., Ltd. (English
Translation) [Incorporated by reference to Exhibit 10.1 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.5
|
Stock
Purchase Agreement, dated November 5, 2007, among Moneyeasy Industries
Limited, Wuhan Tallyho Biological Product Co., Ltd. and its shareholders
(English Translation) [Incorporated by reference to Exhibit 10.2 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.6
|
Stock
Purchase Agreement, December 18, 2007, between Moneyeasy Industries
Limited, Wuhan Polypeptide Anti-Aging Research & Development Co., Ltd.
and its shareholders (English Translation) [Incorporated by reference to
Exhibit 10.3 to the registrant’s Current Report on Form 8-K filed on
November 17, 2009]
|
|
|
10.7
|
Agency
Contract, dated as of June 30, 2009, between Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd. and Jinjiang Shukun Food Trade Co., Ltd
(English Translation) [Incorporated by reference to Exhibit 10.7 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.8
|
Supplementary
Agreement, dated as of October 26, 2009, between Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd. and Jinjiang Shukun Food Trade
Co., Ltd (English Translation) [Incorporated by reference to Exhibit 10.8
to the registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.9
|
Loan
Agreement, dated as of December 5, 2008, between Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd. and Era Biotechnology
(Shenzhen) Co., Ltd. (English Translation) [Incorporated by reference to
Exhibit 10.5 to the registrant’s Current Report on Form 8-K filed on
November 17, 2009]
|
|
|
10.10
|
Supplementary
Agreement, dated as of July 28, 2009, between Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd. and Era Biotechnology (Shenzhen) Co., Ltd.
(English Translation) [Incorporated by reference to Exhibit 10.6 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.11
|
Ceiling
Amount Security and Loan Contract between Wuhan Xianfeng Rural Credit
Cooperative, Wuhan Fanya Peptide Material Research Ltd. and Wuhan Tallyho
Biological Product Co., Ltd. (English Translation) [Incorporated by
reference to Exhibit 10.4 to the registrant’s Current Report on Form 8-K
filed on November 17, 2009]
|
|
|
10.12
|
Employment
Agreement, dated as of March 1, 2010, between the registrant and Mr.
Richard Liu [Incorporated by reference to Exhibit 10.1 to the registrant’s
Current Report on Form 8-K filed on March 5, 2010]
|
|
|
10.13*
|
Patent
License Agreement, dated October 2, 2009, between Dongliang Chen and Wuhan
Tallyho Biological Product Co., Ltd, and the supplementary agreement,
dated May 25, 2010, in relation thereto (English
Translation).
|
48
|
Exhibit No.
|
Description
|
|
|
10.14*
|
Exclusive
Business Cooperation Agreement, dated September 28, 2010, between
Guangdong Hopsun Polypeptide Biological Technology Co., Ltd. and Guangdong
Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
10.15*
|
Proxy
Agreement, dated September 28, 2010, among Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd., Dongliang Chen and Shengfan Yan (English
translation)
|
|
|
10.16*
|
Equity
Pledge Agreement, dated September 28, 2010, among Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd., Dongliang Chen, Shengfan Yan
and Guangdong Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
10.17*
|
Exclusive
Option Agreement, dated September 28, 2010, among Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd., Dongliang Chen, Shengfan Yan
and Guangdong Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
21
|
List
of subsidiaries of the registrant [Incorporated by reference to Exhibit
21.1 to the Registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
31.1*
|
Certifications
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002.
|
|
|
31.2*
|
Certifications
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002.
|
|
|
32.1*
|
Certification
of Chief Executive Officer Pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002.
|
|
|
32.2*
|
Certification
of Chief Financial Officer Pursuant to Section 906 of the
Sarbanes-Oxley Act of
2002.
|
*Filed
herewith.
49
SIGNATURES
In
accordance with section 13 or 15(d) of the Securities Exchange Act of 1934, the
Registrant caused this Report on Form 10-K to be signed on its behalf by the
undersigned, thereto duly authorized individual.
Date:
January 13, 2011
|
CHINA
POLYPEPTIDE GROUP, INC.
|
||
|
By:
|
/s/ Dongliang
Chen
|
|
|
Dongliang
Chen
|
||
|
Chief
Executive Officer
|
||
|
By:
|
/s/
Richard Liu
|
|
|
Richard
Liu
|
||
|
Chief
Financial Officer
|
||
In
accordance with the Securities Exchange Act of 1934, this report has been signed
below by the following persons on behalf of the Registrant and in the capacities
and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Dongliang Chen
|
Chairman
and Chief Executive Officer
|
January
13, 2011
|
||
|
Dongliang
Chen
|
(Principal
Executive Officer)
|
|||
|
/s/ Richard Liu
|
Chief
Financial Officer
|
January
13, 2011
|
||
|
Richard
Liu
|
(Principal
Financial and Accounting Officer)
|
|||
|
/s/ Shengfan Yan
|
President
and Director
|
January
13, 2011
|
||
|
Shengfan
Yan
|
||||
|
/s/ Lirong Hu
|
Treasurer
and Director
|
January
13, 2011
|
||
|
Lirong
Hu
|
|
|
50
CHINA
POLYPEPTIDE GROUP, INC.
Index
to Consolidated Financial Statements of China Polypeptide Group,
Inc.
|
Page
No.
|
|||
|
Report
of Independent Registered Public Accounting Firm
|
F-2
|
||
|
Consolidated
Balance Sheets
|
F-3
|
||
|
Consolidated
Statements of Income and Comprehensive Income
|
F-4
|
||
|
Consolidated
Statements of Shareholders' Equity
|
F-5
|
||
|
Consolidated
Statements of Cash Flows
|
F-6
|
||
|
Notes
to Consolidated Financial Statements
|
F-7
|
F-1
Report
of Independent Registered Public Accounting Firm
To the
Board of Directors and Shareholders of
China
Polypeptide Group, Inc.
We have
audited the accompanying consolidated balance sheets of China Polypeptide Group,
Inc., its subsidiaries and variable interest entity (together the “Group”) as of
September 30, 2010 and 2009 and the related consolidated statements of income
and comprehensive income, changes in shareholders’ equity, and cash flows
(together the “consolidated financial statements”) for each of the years in the
two-year period ended September 30, 2010. The Group’s management is
responsible for these financial statements. Our responsibility is to express an
opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audit to obtain reasonable assurance about whether the
consolidated financial statements are free of material misstatement. The Group
is not required to have, nor were we engaged to perform, an audit of its
internal control over financial reporting. Our audits included consideration of
internal control over financial reporting as a basis for designing audit
procedures that are appropriate in the circumstances, but not for the purpose of
expressing an opinion on the effectiveness of the Group’s internal control over
financial reporting. Accordingly, we express no such opinion. An audit also
includes examining, on a test basis, evidence supporting the amounts and
disclosures in the consolidated financial statements, assessing the accounting
principles used and significant estimates made by management, as well as
evaluating the overall consolidated financial statement presentation. We believe
that our audits provide a reasonable basis for our opinion.
In our
opinion, the consolidated financial statements referred to above present fairly,
in all material respects, the financial position of the Group as of September
30, 2010 and 2009, and the results of their operations and their cash flows for
each of the years in the two-year period ended September 30, 2010 in
conformity with accounting principles generally accepted in the United States of
America.
/s/
Bernstein & Pinchuk LLP
New York,
NY
January
13, 2011
F-2
|
China
Polypeptide Group, Inc.
|
|||||
|
Consolidated
Balance Sheets
|
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Assets
|
||||||||
|
Current
assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 14,421,012 | $ | 4,439,732 | ||||
|
Accounts
receivable, net
|
5,956,039 | 14,666,836 | ||||||
|
Inventories,
net
|
525,319 | 970,685 | ||||||
|
Prepayments
and other receivables, net
|
2,659,871 | 5,404,943 | ||||||
|
Amounts
due from staff
|
7,993,826 | 5,460,867 | ||||||
|
Deferred
tax assets
|
782,355 | 277,335 | ||||||
|
Other
assets
|
419,111 | 411,824 | ||||||
|
Total
Current Assets
|
32,757,533 | 31,632,222 | ||||||
|
Property,
plant and equipment, net
|
11,867,665 | 6,943,440 | ||||||
|
Deposit
for land use rights
|
5,552,160 | 440,100 | ||||||
|
Land
use rights, net
|
279,450 | 280,953 | ||||||
|
Investment
in equity affiliates
|
227,351 | 254,286 | ||||||
|
Intangible
assets, net
|
42,829 | - | ||||||
|
Goodwill
|
- | 113,792 | ||||||
|
Total
Assets
|
$ | 50,726,988 | $ | 39,664,793 | ||||
|
Liabilities
and Shareholders’ Equity
|
||||||||
|
Current
liabilities
|
||||||||
|
Accounts
payable
|
$ | 2,199,212 | $ | 573,401 | ||||
|
Accrued
expenses and other liabilities
|
1,525,934 | 2,449,129 | ||||||
|
Taxes
payable
|
5,597,799 | 6,404,040 | ||||||
|
Short-term
loans
|
519,550 | 566,262 | ||||||
|
Deferred
revenue
|
- | 3,928,804 | ||||||
|
Amounts
due to related parties
|
442,689 | 7,097,865 | ||||||
|
Total
Current Liabilities
|
10,285,184 | 21,019,501 | ||||||
|
Commitments
and Contingencies
|
- | - | ||||||
|
Shareholders’
equity
|
||||||||
|
Common
stock: 120,000,000 shares authorized, $0.0001 par value, 11,939,967 shares
and 8,800,000 shares issued and outstanding as of September 30, 2010 and
2009, respectively
|
1,194 | 880 | ||||||
|
Additional
paid-in capital
|
16,231,912 | 638,404 | ||||||
|
Deferred
compensation
|
(77,355 | ) | - | |||||
|
Unappropriated
retained earnings
|
22,194,795 | 16,842,439 | ||||||
|
Appropriated
retained earnings
|
1,283,484 | 1,144,448 | ||||||
|
Accumulated
other comprehensive income
|
807,774 | 19,121 | ||||||
|
Total
Shareholders’ Equity
|
40,441,804 | 18,645,292 | ||||||
|
Total
Liabilities and Shareholders’ Equity
|
$ | 50,726,988 | $ | 39,664,793 | ||||
See notes
to consolidated financial statements.
F-3
|
China
Polypeptide Group, Inc.
|
||||||
|
Consolidated
Statements of Income and Comprehensive
Income
|
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenues
|
$ | 38,061,827 | $ | 37,724,869 | ||||
|
Cost
of sales
|
2,986,612 | 2,247,048 | ||||||
|
Gross
profit
|
35,075,215 | 35,477,821 | ||||||
|
Operating
expenses
|
||||||||
|
Selling
and administrative expenses
|
27,311,609 | 22,842,559 | ||||||
|
Operating
income
|
7,763,606 | 12,635,262 | ||||||
|
Other
income (expense)
|
||||||||
|
Interest
income (expense), net
|
(66,682 | ) | 177,437 | |||||
|
Equity
loss in affiliates
|
(30,866 | ) | (30,611 | ) | ||||
|
Gain
on disposal of associates
|
- | 42,433 | ||||||
|
Loss
on impairment of goodwill
|
(113,713 | ) | - | |||||
|
Other
income (expense)
|
(673,241 | ) | 412,820 | |||||
| (884,502 | ) | 602,079 | ||||||
|
Income
before income tax expense
|
6,879,104 | 13,237,341 | ||||||
|
Income
tax expense
|
1,387,712 | 2,715,274 | ||||||
|
Net
income
|
5,491,392 | 10,522,067 | ||||||
|
Other
comprehensive income
|
||||||||
|
Foreign
currency translation gain
|
788,653 | 52,102 | ||||||
|
Comprehensive
income
|
$ | 6,280,045 | $ | 10,574,169 | ||||
|
Earnings
per share
|
||||||||
|
Basic
and diluted
|
$ | 0.51 | $ | 1.20 | ||||
|
Weighted
average number of common shares outstanding:
|
||||||||
|
Basic
and diluted
|
10,681,123 | 8,800,000 | ||||||
See notes
to consolidated financial statements.
F-4
China
Polypeptide Group, Inc.
Consolidated
Statements of Shareholders’
Equity
|
Other
|
||||||||||||||||||||||||||||||||
|
Additional
|
Unappropriated
|
Appropriated
|
comprehensive
|
Total
|
||||||||||||||||||||||||||||
|
Shareholders’
|
paid-in
|
Deferred
|
retained
|
retained
|
income
|
Shareholders’
|
||||||||||||||||||||||||||
|
Share
|
capital
|
capital
|
compensation
|
earnings
|
earnings
|
(loss)
|
Equity
|
|||||||||||||||||||||||||
|
Balance
as of September 30, 2008
|
8,800,000 | 880 | 168,964 | - | 6,449,420 | 1,015,400 | (32,981 | ) | 7,601,683 | |||||||||||||||||||||||
|
Net
income for the year
|
- | - | - | 10,522,067 | - | - | 10,522,067 | |||||||||||||||||||||||||
|
Additional
paid-in capital contributed
|
- | 469,440 | - | - | - | - | 469,440 | |||||||||||||||||||||||||
|
Appropriated
retained earnings
|
- | - | - | (129,048 | ) | 129,048 | - | - | ||||||||||||||||||||||||
|
Foreign
currency translation
|
- | - | - | - | - | 52,102 | 52,102 | |||||||||||||||||||||||||
|
Balance
as of September 30, 2009
|
8,800,000 | $ | 880 | $ | 638,404 | $ | - | $ | 16,842,439 | $ | 1,144,448 | $ | 19,121 | $ | 18,645,292 | |||||||||||||||||
|
Reverse
acquisition between the Company and Cantix (Note 1)
|
1,200,000 | 120 | - | - | - | - | - | 120 | ||||||||||||||||||||||||
|
Common
stock issued for financing (Note 22)
|
1,861,967 | 186 | 7,551,624 | - | - | - | - | 7,551,810 | ||||||||||||||||||||||||
|
Warrants
issued related to financing (Note 22)
|
1,257,593 | - | - | - | - | 1,257,593 | ||||||||||||||||||||||||||
|
Common
stock issued for services
|
78,000 | 8 | 187,196 | (77,355 | ) | - | - | - | 109,849 | |||||||||||||||||||||||
|
Conversion
of debt as additional paid-in capital (Note 18 (b))
|
6,597,095 | - | - | - | - | 6,597,095 | ||||||||||||||||||||||||||
|
Net
income for the year
|
- | 5,491,392 | - | - | 5,491,392 | |||||||||||||||||||||||||||
|
Appropriated
retained earnings
|
- | (139,036 | ) | 139,036 | - | - | ||||||||||||||||||||||||||
|
Foreign
currency translation
|
- | - | - | - | - | 788,653 | 788,653 | |||||||||||||||||||||||||
|
Balance
as of September 30, 2010
|
11,939,967 | $ | 1,194 | $ | 16,231,912 | $ | (77,355 | ) | $ | 22,194,795 | $ | 1,283,484 | $ | 807,774 | $ | 40,441,804 | ||||||||||||||||
See notes
to consolidated financial statements.
F-5
|
China
Polypeptide Group, Inc.
|
||||||||
|
Consolidated
Statements of Cash Flows
|
||||||||
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash
flow from operating activities
|
||||||||
|
Net
income
|
$ | 5,491,392 | $ | 10,522,067 | ||||
|
Adjustments
to reconcile net income to net cash provided by (used in) operating
activities
|
||||||||
|
Depreciation
and amortization
|
379,362 | 295,688 | ||||||
|
Disposal
of property, plant and equipment
|
36,742 | - | ||||||
|
Bad
debts provision
|
3,859,826 | 579,096 | ||||||
|
Amortization
of stock-based compensation
|
60,135 | - | ||||||
|
Gain
on disposal of associates
|
- | (42,433 | ) | |||||
|
Equity
loss in affiliates
|
30,866 | 30,611 | ||||||
|
Loss
on impairment of goodwill
|
113,713 | - | ||||||
|
Changes
in operating assets and liabilities
|
||||||||
|
Accounts
receivable
|
7,982,851 | (13,618,806 | ) | |||||
|
Amounts
due to related parties
|
- | 345,271 | ||||||
|
Inventories
|
454,186 | (415,930 | ) | |||||
|
Prepayments
and other receivables
|
(245,014 | ) | (3,050,809 | ) | ||||
|
Amounts
due from staff
|
(2,392,301 | ) | (2,851,408 | ) | ||||
|
Other
assets
|
(491,076 | ) | (117,259 | ) | ||||
|
Accounts
payable
|
541,747 | 765,195 | ||||||
|
Accrued
expenses and other liabilities
|
(857,597 | ) | (458,725 | ) | ||||
|
Deferred
revenue
|
(3,926,087 | ) | 3,924,787 | |||||
|
Taxes
payable
|
(902,955 | ) | 3,966,775 | |||||
|
Net
cash provided by (used in) operating activities
|
10,135,790 | (125,880 | ) | |||||
|
Cash
flow from investing activities
|
||||||||
|
Purchases
of property, plant and equipment
|
(4,065,603 | ) | (70,818 | ) | ||||
|
Purchases
of intangible assets
|
(56,074 | ) | (1,465,500 | ) | ||||
|
Proceeds
from disposal of associates
|
- | 33,199 | ||||||
|
Cash
paid to acquire land use rights
|
(5,012,046 | ) | (442,310 | ) | ||||
|
Net
cash used in investing activities
|
(9,133,723 | ) | (1,945,429 | ) | ||||
|
Cash
flow from financing activities
|
||||||||
|
Proceeds
from private placements, net
|
8,809,403 | - | ||||||
|
Proceeds
from (Repayment of) related parties loans
|
(31,976 | ) | 594,512 | |||||
|
Repayment
of a bank loan
|
(55,707 | ) | (35,172 | ) | ||||
|
Net
cash provided by financing activities
|
8,721,720 | 559,340 | ||||||
|
Effect
of exchange rate fluctuation on cash and cash equivalents
|
257,493 | 33,287 | ||||||
|
Net
increase (decrease) in cash and cash equivalents
|
9,981,280 | (1,478,682 | ) | |||||
|
Cash
and cash equivalents, beginning of year
|
4,439,732 | 5,918,414 | ||||||
|
Cash
and cash equivalents, end of year
|
$ | 14,421,012 | $ | 4,439,732 | ||||
|
Supplemental
disclosure of cash flow information:
|
||||||||
|
Income
taxes paid
|
$ | 3,199,333 | $ | 1,088,688 | ||||
|
Interest
paid
|
$ | 41,776 | $ | 96,668 | ||||
|
Supplemental
disclosure of non-cash
transactions:
|
||||||||
|
Conversion
of debt as additional paid-in capital (Note 18 (b))
|
$ | 6,597,095 | $ | - | ||||
See notes
to consolidated financial statements.
F-6
China
Polypeptide Group, Inc.
Notes
to Consolidated Financial Statements
Note
1 - Principal Activities and Organization
The
consolidated financial statements include the financial statements of China
Polypeptide Group, Inc. (“CPGI” or the “Company”), its subsidiaries and variable
interest entity (“VIE”), Cantix International Limited (“Cantix”), Moneyeasy
Industries Limited (“Moneyeasy”), Wuhan Tallyho Biological Product Co., Limited
(“Tallyho”), Guangdong Hopsun Polypeptide Biological Technology Co., Limited
(“Hopsun”), Wuhan Polypeptide Anti-Aging Research & Development Co., Limited
(“Anti-Aging”), and Guangdong Xingpu Polypeptide Research Co., Limited
(“Xingpu”). The Company, its subsidiaries and VIE are collectively referred to
as the “Group”.
Principal
Activities
The
Company, formerly known as Hamptons Extreme, Inc., was incorporated under the
laws of the State of Delaware in March 2007. The Company was formed to engage in
the design, manufacturing, distribution and marketing of surfboards and related
equipment. After the Exchange (as defined below), the Company,
through its operating subsidiaries in the People’s Republic of China (the
“PRC”), is principally engaged in research, development,
manufacturing, marketing and sales of polypeptide-based nutritional supplements,
health foods, functional foods and related ingredient products.
Cantix, a
wholly owned subsidiary of the Company, was incorporated in British Virgin
Islands (the “BVI”) on January 29, 2007. Cantix is a holding company with
minimum activities.
Moneyeasy,
a wholly owned subsidiary of Cantix, was incorporated in Hong Kong on
August 25, 2006, is a holding company with minimum activities.
Tallyho
was founded on December 2, 1996 in the PRC with a paid-in capital of $5,854,361
(RMB40,230,000) and has been engaged in research, production and sales of
polypeptide-based nutritional supplements, health food products and related
ingredient products. Tallyho is a wholly owned subsidiary of
Moneyeasy.
Hopsun
was founded on November 13, 2007 in the PRC. It is a wholly owned subsidiary of
Tallyho with $1,424,055 (RMB10,000,000) initial paid-in capital which was later
increased to $2,008,227 (RMB14,000,000) and specializes in service-based sales
of health and anti-aging products.
Anti-Aging
was founded on June 13, 2007 in the PRC, with $1,045,246 (RMB8,000,000) paid-in
capital and specialized in research and development of polypeptide-based health
products and provision of related technological services. Anti-Aging is a wholly
owned subsidiary of Moneyeasy.
Xingpu is
a development stage company established in March 2, 2010. Its registered capital
is approximately $29,255,164 (RMB200,000,000) of which $5,851,033
(RMB40,000,000) was paid up as of September 30, 2010. The equity holders of
Xingpu are Mr. Dongliang Chen, the Company’s Chief Executive Officer and the
Chairman of the Board, and Mr. Shengfan Yan, the President and a director
of the Company, with each holding a 50% equity interest of Xingpu. Xingpu’s
business scope includes polypeptide product development, real estate
investment, technology transfer, technical consultation, import and export, etc.
with the current purpose to develop the Group’s regional headquarter and its
research and development center.
Effective September
28, 2010, Hopsun entered into a series of contractual arrangements with Xingpu
and its equity holders consisting of a series of agreements, including an
Exclusive Business Cooperation Agreement, through which Hopsun has the right to
advise, consult with, manage and operate Xingpu, and to collect fees based on
its profits. Xingpu's equity holders have also granted their voting rights over
Xingpu to Hopsun. In order to further reinforce Hopsun's rights to control and
operate Xingpu, Xingpu and its equity holders have granted Hopsun the exclusive
right and option to acquire all of their equity interests in Xinpu. Further,
Xingpu’s equity holders have pledged all of their rights, titles and interests
in Xingpu to Hopsun. As both companies are under common control, this has been
accounted for as a reorganization of entities and the financial statements have
been prepared as if the reorganization had occurred from the beginning of the
periods presented. CPGI consolidates Xingpu’s results, assets and
liabilities in its financial statements.
F-7
On
November 13, 2009, the Company, Cantix and the shareholders of Cantix entered
into a stock exchange agreement (the “Stock Exchange Agreement”), pursuant to
which the Company acquired all of the issued and outstanding shares of common
stock of Cantix from the Cantix shareholders in exchange for 8,800,000 shares of
common stock of the Company, representing approximately 88% of the issued and
outstanding shares of common stock of the Company (the “Exchange”). After the
Exchange, Cantix became a wholly-owned subsidiary of the Company.
Under
accounting principles generally accepted in the United States of America (“U.S.
GAAP”), the Exchange is treated as a reverse acquisition, and the consolidated
financial statements of the Company have been retroactively adjusted to reflect
the Exchange from the beginning of the periods presented. The Exchange has been
accounted for as a reverse acquisition and recapitalization (the
“Reorganization”) of the Company, whereby Cantix is deemed to be the accounting
acquirer (legal acquiree) and the Company to be the accounting acquiree (legal
acquirer). The historical consolidated financial statements for the periods
prior to November 13, 2009 are solely of Cantix except that the equity section
and earnings per share have been retroactively restated to reflect the
Exchange.
On
December 1, 2009, all shares and per share numbers set forth in this Note 1
reflect an 8-for-1 forward stock split effectuated by the Company, pursuant to
which each one (1) share of the Company’s common stock issued and outstanding on
December 1, 2009 automatically converted into eight(8) shares of the Company’s
common stock.
Note
2 - Summary of Significant Accounting Policies and Practices
(a) Basis
of Presentation
The
consolidated financial statements have been prepared in accordance with U.S.
GAAP.
In
accordance with Accounting Standards Codification (“ASC”) Topic 810 and its
related subtopics “Consolidation of Variable Interest Entities” ("ASC Topic
810"), VIEs are generally entities that lack sufficient equity to finance their
activities without additional financial support from other parties or whose
equity holders lack adequate decision making ability. All VIEs with which the
Company is involved must be evaluated to determine the primary beneficiary of
the risks and rewards of the VIEs. The primary beneficiary is required to
consolidate the VIE for financial reporting purposes. The Company has concluded
that Xingpu is a VIE and that the Company is the primary beneficiary and
loss absorber as of September 30, 2010. Under the requirements of ASC Topic 810
the Company consolidated the financial statements of Xingpu.
The
consolidated financial statements include the financial statements of the
Company, its subsidiaries and VIE. All significant inter-company transactions
and balances have been eliminated in consolidation.
(b)
Use of Estimates
The
preparation of consolidated financial statements in conformity with U.S. GAAP
requires management to make estimates and assumptions relating to the reported
amounts of assets, liabilities, revenues and expenses, and related disclosure of
contingent assets and liabilities at the date of the financial statements and
the reported amounts of revenues and expenses during the reporting periods.
Significant items subject to such estimates and assumptions include revenues;
the allowance for doubtful receivables; deposit for land use rights;
recoverability of the carrying amount of property and equipment, goodwill and
intangible assets; fair values of financial instruments; and the assessment of
contingent obligations. These estimates are often based on complex
judgments and assumptions that management believes to be reasonable but are
inherently uncertain and unpredictable. Actual results could differ
from those estimates.
F-8
(c)
Cash and Cash Equivalents
Cash and
cash equivalents represent cash on hand and current deposits with banks. The
Group considers all highly liquid investments with original maturities of three
months or less at the time of purchase to be cash equivalents.
(d)
Accounts Receivable
Accounts
receivable are recorded at net realizable value consisting of the carrying
amount less an allowance for uncollectible accounts, as needed. The allowance
for doubtful accounts is the Group’s best estimate of the amount of probable
credit losses in the Group’s existing accounts receivable. The Group
determines the allowance based on aging data, historical collection experience,
customer specific facts and economic conditions. Account balances are charged
off against the allowance after all means of collection have been exhausted and
the potential for recovery is considered remote. The Group does not
have any off-balance-sheet credit exposure related to its customers. As of
September 30, 2010 and 2009, $1,570,754 and $717,451 allowances for doubtful
accounts were provided respectively.
(e)
Inventories
Inventories
are stated at the lower of cost or market value. Cost is determined using the
weighted average cost method. Inventories embraces raw materials, low-value
consumables, packaging materials, work in process and finished goods. Cost
of finished goods comprises direct material, direct production cost and an
allocated portion of production overheads based on normal operating
capacity.
The Group
evaluates the need for reserves associated with obsolete, slow-moving and
non-salable inventories by reviewing the net realizable value on a periodic
basis. Where there is evidence that the utility of inventories, in their
disposal in the ordinary course of business, will be less than cost, whether due
to physical deterioration, obsolescence, changes in price levels, or other
causes, a provision is accrued for the difference with charges to cost of
sales.
(f)
Prepayments and Other Receivables
As needed
for normal business purposes, the Group advances predetermined amounts based
upon internal policy to certain employees and unrelated parties to ensure
certain transactions to be performed in a timely manner. The Group has full
oversight and control over the advanced accounts except for the loans to ERA
Bio-Technology (Shenzhen) Co., Ltd. (“ERA Biotech”). As of September 30, 2010
and 2009, $3,145,468 and $54,263 allowances for doubtful accounts were provided
respectively (Note 7).
(g)
Property, Plant and Equipment
Property,
plant and equipment are stated at historical cost less accumulated depreciation
and impairment. The historical cost of acquiring an item of property, plant and
equipment includes the costs necessarily incurred to bring it to the condition
and location necessary for its intended use. If an item of property, plant and
equipment requires a period of time in which to carry out the activities
necessary to bring it to that condition and location, the interest cost incurred
during that period as a result of expenditures for the item is a part of the
historical cost. This item is categorized as construction in progress and is not
depreciated until substantially all the activities necessary to bring it to the
condition and location necessary for its intended use are
completed.
F-9
Depreciation
of property, plant and equipment is calculated using the straight-line method
(after taking into account their respective estimated residual value) over the
estimated useful lives of the assets as follows.
|
Asset
|
Useful
lives
|
|
|
Buildings
and improvements
|
5~40
years
|
|
|
Machinery
and equipment
|
5~10
years
|
|
|
Furniture
and office equipment
|
2~5
years
|
|
|
Motor
vehicles
|
5
years
|
Depreciation
of property, plant and equipment attributable to manufacturing activities is
capitalized as part of inventories, and expensed to cost of goods sold when
inventories are sold.
Expenditure
for maintenance and repairs is expensed as incurred.
The gain
or loss on the disposal of property, plant and equipment is the difference
between the net sales proceeds and the carrying amount of the relevant assets
and is recognized in the consolidated statements of income and comprehensive
income.
Construction
in progress represented capital expenditure in respect of direct costs of
construction or acquisition and design fees incurred. Capitalization
of these costs ceases and the construction in progress is transferred to the
appropriate category of property, plant and equipment when substantially all the
activities necessary to prepare the assets for their intended use are
completed. Construction in progress is not depreciated.
(h) Land
Use Rights
Land use
rights represent payments for the rights to use certain parcels of land for a
certain period of time in the PRC. Land use rights are carried at cost and
charged to expense on a straight-line basis over the period the rights are
granted, i.e., 50 years.
(i)
Investment in Equity Affiliates
Investment
in affiliates consists of ownership in associated companies, which the Group
exercises significant influence, usually a percentage ownership between 20% and
50%, and is accounted for under the equity method of accounting. Under the
equity method of accounting, an investee’s accounts are not reflected within the
Group’s consolidated balance sheets and statements of income and comprehensive
income; however, the Company’s share of the earnings or losses of the investee
are reflected in the caption “Equity in loss in affiliates” in the consolidated
statements of income and comprehensive income. The Group’s carrying value in an
equity method investee is reflected in the caption “Long-term Investment” in the
Group’s consolidated balance sheets.
When the
Group’s carrying value in an equity method investee is reduced to zero, no
further losses are recorded in the Group’s consolidated financial statements
unless the Group guaranteed obligations of the investee or has committed
additional funding. When the investee subsequently reports income, the Group
will not record its share of such income until it equals the amount of its share
of losses not previously recognized.
(j)
Goodwill
Goodwill
represents the excess of acquisition costs over the fair value of tangible net
assets and identifiable intangible assets of businesses acquired. Goodwill and
certain other intangible assets deemed to have indefinite lives are not
amortized. Intangible assets determined to have definite lives are amortized
over their useful lives. Goodwill and indefinite lived intangible assets are
subject to impairment testing annually as of the fiscal year-end or whenever
events or changes in circumstances indicate that the carrying amount may not be
fully recoverable, using the guidance and criteria described in ASC Topic 350,
“Goodwill and Other Intangible Assets”. This testing compares carrying values to
fair values and, when appropriate, the carrying value of these assets is reduced
to fair value.
F-10
The
goodwill related to the acquisition of Anti-Aging of $113,713 was assessed to be
impaired as of September 30, 2010. There was no impairment of
goodwill as of September 30, 2009.
(k) Impairment of
Long-Lived Assets
Long-lived
assets held and used by the Group are reviewed for impairment whenever events or
changes in circumstances indicate that the carrying amount of assets may not be
recoverable. It is reasonably possible that these assets could become impaired
as a result of technology or other industry changes. Determination of
recoverability of assets to be held and used is determined by comparing the
carrying amount of an asset to future undiscounted cash flows to be generated by
the assets. If such assets are considered to be impaired, the impairment to be
recognized is measured by the amount by which the carrying amount of the assets
exceeds the fair value of the assets. Assets to be disposed of are reported at
the lower of the carrying amount or fair value less costs to
sell. There is no impairment of other long-lived assets except for
goodwill (Note 2 (j)) as of September 30, 2010 and 2009.
(l) Appropriated
Retained Earnings
The
income of the Group’s PRC subsidiaries is distributable to its equity holders
after transfer to reserves as required by relevant PRC laws and regulations and
the subsidiaries’ articles of association. Appropriations to the reserves are
approved by the respective boards of directors.
Reserves
include statutory reserves and other reserves. Statutory reserves can be used to
make good previous years’ losses, if any, and may be converted into capital in
proportion to the existing equity interests of shareholders, provided that the
balance after such conversion is not less than 25% of the registered capital.
The appropriation of statutory reserve may cease to apply if the balance of the
fund is equal to 50% of the entity’s registered capital. Pursuant to relevant
PRC laws and articles of association of Tallyho, Hopsun, Anti-Aging and Xingpu,
the appropriation to the statutory reserves and other reserves is 10% of net
profit after taxation of respective entity, as determined in accordance with PRC
accounting standards and regulations. The results of operations reflected in the
consolidated financial statements prepared in accordance with U.S. GAAP
might differ from those reflected in the statutory financial statements of the
Group’s subsidiaries.
As of
September 30, 2010 and 2009, the statutory reserve recorded by the Group’s
PRC subsidiaries amounted to $1,283,484 and $1,144,448,
respectively.
(m)
Revenue Recognition
The Group
recognizes revenue in accordance with ASC Topic 605. All of the following
criteria must exist in order for the Group to recognize revenue:
(1) persuasive evidence of an arrangement exists; (2) delivery has
occurred or services have been rendered; (3) the seller's price to the
buyer is fixed or determinable; and (4) collectability is reasonably
assured.
Revenue
is recognized when products are delivered and the customer takes ownership and
assumes risk of loss, collection of the relevant receivable is probable,
persuasive evidence of an arrangement exists and the sales price is fixed or
determinable.
F-11
(n)
Research and Development Costs
Research
and development costs are expensed as incurred. These expenses
consist of the costs of the Group’s internal research and development
activities and the costs of developing new products and enhancing existing
products. Research and development costs amounted to $311,226 and $285,496 for
the years ended September 30, 2010 and 2009, respectively.
(o)
Advertising
The Group
expenses all advertising costs as incurred. The advertising cost for the years
ended September 30, 2010 and 2009 was $49,947 and $29,421,
respectively.
(p)
Retirement
and Other Postretirement Benefits
Full-time
employees of the Group in the PRC participate in a government mandated defined
contribution plan, pursuant to which certain pension benefits, medical care,
employee housing fund and other welfare benefits are provided to employees.
Chinese labor regulations require that the PRC subsidiaries of the Group make
contributions to the government for these benefits based on certain percentages
of the employees’ salaries. The Group has no legal obligation for the benefits
beyond the contributions made. The total amounts for such employee benefits,
which were expensed as incurred, were approximately $398,053 and $187,853 for
the years ended September 30, 2010 and 2009, respectively.
(q) Foreign
Currency Translation and Transactions
The
Company’s functional currency is the United States dollar (“$” or “US$”). The
functional currency of the Company’s subsidiaries and VIE in the PRC is Renminbi
(“RMB”) and in Hong Kong is the Hong Kong dollar (“HK$”).
At the
date a foreign currency transaction is recognized,
each asset, liability, revenue, expense, gain, or loss arising from the
transaction is measured initially in the functional currency of the recording entity by use of the
exchange rate in effect at that
date. The increase or decrease in
expected functional currency cash flows upon settlement of a transaction
resulting from a change in exchange rates between the functional currency and the currency in which the
transaction is denominated is recognized as foreign currency transaction gain or loss that is included in determining
net income for the period in which the exchange
rate changes. At each balance sheet date, recorded balances that are
denominated in a foreign currency are adjusted to reflect the current exchange
rate.
The
Company’s reporting currency is US$. Assets and liabilities of the PRC
subsidiaries and VIE are translated at the current exchange rate at the balance
sheet dates, and revenues and expenses are translated at the average exchange
rates during the reporting periods. Translation adjustments are reported in
other comprehensive income.
(r)
Income Taxes
The Group
adopted ASC Topic 740 “Accounting for Income Taxes” that requires
recognition of deferred income tax liabilities and assets for the expected
future tax consequences of temporary differences between income tax basis and
financial reporting basis of assets and liabilities. Provision for income taxes
consist of taxes currently due plus deferred taxes.
The
charge for taxation is based on the results for the year as adjusted for items,
which are non-assessable or disallowed. It is calculated using tax rates that
have been enacted or substantively enacted by the balance sheet date. Deferred
tax is accounted for using the balance sheet liability method in respect of
temporary differences arising from differences between the carrying amount of
assets and liabilities in the financial statements and the corresponding tax
basis used in the computation of assessable tax profit. In principle, deferred
tax liabilities are recognized for all taxable temporary differences, and
deferred tax assets are recognized to the extent that it is probably that
taxable profit will be available against which deductible temporary differences
can be utilized.
F-12
Deferred
tax is calculated at the tax rates that are expected to apply to the period when
the asset is realized or the liability is settled. Deferred tax is charged or
credited in the income statement, except when it related to items credited or
charged directly to equity, in which case the deferred tax is also dealt with in
equity. Deferred tax assets and liabilities are offset when they relate to
income taxes levied by the same taxation authority and the Group intends to
settle its current tax assets and liabilities on a net basis.
(s)
Uncertain
Tax Positions
The Group
follows ASC Topic 740 “Accounting for Uncertainty in Income Taxes”.
ASC Topic 740 prescribes a more-likely-than-not threshold for financial
statement recognition and measurement of a tax position taken or expected to be
taken in a tax return. ASC Topic 740 also provides guidance on recognition of
income tax assets and liabilities, classification of current and deferred income
tax assets and liabilities, accounting for interest and penalties associated
with tax positions, accounting for income taxes in interim periods, and income
tax disclosures. The Group did not have any interest and penalties associated
with tax positions as of September 30, 2010 and 2009.
(t)
Earnings
per Share
Earnings
per share are calculated in accordance with ASC Topic 260 “Earnings
Per Share”. Basic earnings per share are computed by dividing income
attributable to holders of common shares by the weighted average number of
common shares outstanding during the period. Diluted earnings per common share
reflects the potential dilution that could occur if securities or other
contracts to issue common shares were exercised or converted into common
shares.
(u)
Comprehensive
Income
The Group
follows ASC Topic 220 “Reporting Comprehensive Income”, which
establishes standards for reporting and display of comprehensive income, its
components and accumulated balances. Comprehensive income is defined to include
all changes in equity except those resulting from investments by owners and
distributions to owners. Among other disclosures, ASC Topic 220 requires that
all items that are required to be recognized under current accounting standards
as components of comprehensive income be reported in a financial statement that
is displayed with the same prominence as other financial statements. During the
periods presented, the Group’s comprehensive income represents its net income
and foreign currency translation adjustments.
(v)
Commitments and Contingencies
In the
normal course of business, the Group is subject to loss contingencies, such as
legal proceedings and claims arising out of its business, that cover a wide
range of matters, including, among others, government investigations, product
and environmental liability, and tax matters. In accordance with ASC Topic 450
(SFAS No. 5, “Accounting for Contingencies”), the Group records accruals
for such loss contingencies when it is probable that a liability has been
incurred and the amount of loss can be reasonably estimated. Historically, the
Group has not experienced any material service liability claims.
(w)
Fair Value of Financial Instruments
Financial
instruments include cash and cash equivalents, accounts and notes receivable,
prepayments and other receivables, short-term loans, accounts and notes payable,
other payables and amounts due to related party. The carrying amounts of these
financial instruments approximate their fair value due to the short term
maturities of these instruments.
The Group
adopted ASC Topic 820-10 “Fair Value Measurements” on January 1,
2008 for all financial assets and liabilities and nonfinancial assets and
liabilities that are recognized or disclosed at fair value in the consolidated
financial statements on a recurring basis (at least annually). ASC Topic 820-10
defines fair value, establishes a framework for measuring fair value, and
expands disclosures about fair value measurements. The Group has not adopted ASC
Topic 820-10 for non-financial assets and non-financial liabilities, as these
items are not recognized at fair value on a recurring basis.
F-13
ASC Topic
820-10 defines fair value as the price that would be received from selling an
asset or paid to transfer a liability in an orderly transaction between market
participants at the measurement date. When determining the fair value
measurements for assets and liabilities required or permitted to be recorded at
fair value, the Group considers the principal or most advantageous market in
which it would transact and it considers assumptions that market participants
would use when pricing the asset or liability.
ASC Topic
820-10 establishes a fair value hierarchy that requires an entity to maximize
the use of observable inputs and minimize the use of unobservable inputs when
measuring fair value. A financial instrument’s categorization within the fair
value hierarchy is based upon the lowest level of input that is significant to
the fair value measurement. ASC Topic 820-10 establishes three levels of inputs
that may be used to measure fair value:
|
-
|
Level
1 applies to assets or liabilities for which there are quoted prices in
active markets for identical assets or
liabilities.
|
|
-
|
Level
2 applies to assets or liabilities for which there are inputs other than
quoted prices included within Level 1 that are observable for the asset or
liability such as quoted prices for similar assets or liabilities in
active markets; quoted prices for identical assets or liabilities in
markets with insufficient volume or infrequent transactions (less active
markets); or model-derived valuations in which significant inputs are
observable or can be derived principally from, or corroborated by,
observable market data.
|
|
-
|
Level
3 applies to assets or liabilities for which there are unobservable inputs
to the valuation methodology that are significant to the measurement of
the fair value of the assets or
liabilities.
|
(x)
Recently Issued Accounting Pronouncements
In
February 2010, FASB issued ASU No. 2010-09 “Subsequent Events” (“ASC Topic 855”)
which removes the requirement for an SEC filer to disclose a date in both issued
and revised financial statements. This amendment shall be applied prospectively
for interim or annual financial periods ending after June 15, 2010. The Group
does not believe the adoption will have a material effect on the Group's
consolidated financial statements.
The FASB
has issued ASU 2010-06, Fair Value Measurements and Disclosures (ACS Topic 820):
Improving Disclosures about Fair Value Measurements. This ASU requires some new
disclosures and clarifies some existing disclosure requirements about fair value
measurement as set forth in Codification Subtopic 820-10. The FASB’s objective
is to improve these disclosures and, thus, increase the transparency in
financial reporting. Specifically, ASU 2010-06 amends Codification Subtopic
820-10 to now require:
|
-
|
A
reporting entity to disclose separately the amounts of significant
transfers in and out of Level 1 and Level 2 fair value measurements and
describe the reasons for the transfers;
and
|
|
-
|
In
the reconciliation for fair value measurements using significant
unobservable inputs, a reporting entity should present separately
information about purchases, sales, issuances, and
settlements.
|
In
addition, ASU 2010-06 clarifies the requirements of the following existing
disclosures:
|
-
|
For
purposes of reporting fair value measurement for each class of assets and
liabilities, a reporting entity needs to use judgment in determining the
appropriate classes of assets and liabilities;
and
|
|
-
|
A
reporting entity should provide disclosures about the valuation techniques
and inputs used to measure fair value for both recurring and nonrecurring
fair value measurements.
|
ASU
2010-06 is effective for interim and annual reporting periods beginning after
December 15, 2009, except for the disclosures about purchases, sales, issuances,
and settlements in the roll forward of activity in Level 3 fair value
measurements. Those disclosures are effective for fiscal years beginning after
December 15, 2010, and for interim periods within those fiscal years. Early
adoption is permitted.
F-14
In July
2010, the FASB issued Accounting Standard Update (“ASU”) 2010-20, “Receivables
(Topic 310): Disclosures about the Credit Quality of Financing Receivables and
the Allowance for Credit Losses”. This ASU amends Topic 310 to
improve the disclosures that an entity provides about the credit quality of its
financing receivables and the related allowance for credit losses. As a result
of these amendments, an entity is required to disaggregate by portfolio segment
or class certain existing disclosures and provide certain new disclosures about
its financing receivables and related allowance for credit losses. For public
entities, the disclosures as of the end of a reporting period are effective for
interim and annual reporting periods ending on or after December 15, 2010. The
disclosures about activity that occurs during a reporting period are effective
for interim and annual reporting periods beginning on or after December 15,
2010, except for the expanded disclosure requirements. The Group is currently
evaluating the potential impact, if any, of this ASU on its consolidated
financial statements.
Note
3 - Significant Risks
(a) Foreign
Currency Risk
The Group
transacts all of its business in RMB, which is not freely convertible into
foreign currencies. On January 1, 1994, the PRC government abolished the
dual rate system and introduced a single rate of exchange as quoted daily by the
People’s Bank of China (the “PBOC”). However, the unification of the exchange
rates does not imply that RMB may be readily convertible into US$ or other
foreign currencies. All foreign exchange transactions continue to take place
either through the PBOC or other banks authorized to buy and sell foreign
currencies at the exchange rates quoted by the PBOC. Approval of foreign
currency payments by the PBOC or other institutions requires submitting a
payment application form together with suppliers’ invoices, shipping documents
and signed contracts.
Additionally,
the value of RMB is subject to changes in central government policies and
international economic and political developments affecting supply and demand in
the PRC foreign exchange trading system market.
(b) Concentration
of Credit Risk
Assets
that potentially subject the Group to significant concentration of credit risk
primarily consist of cash and cash equivalents, accounts and notes receivable.
As of September 30, 2010 and 2009, substantially all of the Group’s cash and
cash equivalents were deposited in financial institutions located in the PRC,
which management believes are of high credit quality. Accounts receivable are
typically unsecured and are derived from revenue earned from customers in the
PRC. The risk with respect to accounts receivable is mitigated by credit
evaluations the Group performs on its customers and its ongoing monitoring
process of outstanding balances.
(c) Concentration
of Customers
The top
six third-party customers accounted for approximately 15% of the consolidated
revenues for the year ended September 30, 2010 and the top seven third-party
customers accounted for approximately 23% of the consolidated revenues for the
year ended September 30, 2009.
The top
six third-party customers accounted for 67% of the accounts receivable as of
September 30, 2010 and the top seven third-party customers accounted for 91% of
the accounts receivable as of September 30, 2009.
(d)
Concentration of Suppliers
The top
five third-party suppliers accounted for approximately 62% of the total purchase
for the year ended September 30, 2010 while approximately 59% for the year ended
September 30, 2009.
F-15
The top
five third-party suppliers accounted for 56% of the accounts payable as of
September 30, 2010 and the top five third-party suppliers accounted for 46% of
the accounts payable as of September 30, 2009.
Note
4 – Earnings per Share
The Group
reports earnings per share in accordance with ASC Topic 260 “Earnings
per Share”. ASC Topic 260 requires presentation of basic and diluted
earnings per share in conjunction with the disclosure of the methodology used in
computing such earnings per share. Basic earnings per share excludes
dilution and is computed by dividing income available to common shareholders by
the weighted average common shares outstanding during the period. Diluted
earnings per share takes into account the potential dilution that could occur if
securities or other contracts to issue common stock were exercised and converted
into common stock. The following is a reconciliation of the basic and diluted
earnings per share computations for the years ended September 30, 2010 and
2009:
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
For the years ended September 30, 2010 and 2009
|
||||||||
|
Net
income for basic and diluted earnings per share
|
$
|
5,491,392
|
$
|
10,522,067
|
||||
|
Weighted
average number of common shares outstanding – basic and diluted
*
|
10,681,123
|
8,800,000
|
||||||
|
Earnings
per share – basic and diluted
|
$
|
0.51
|
$
|
1.20
|
||||
* All the
outstanding warrant shares do not have dilutive effect on earnings per
share.
Note
5 - Accounts Receivable, net
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Accounts
receivable
|
$
|
7,526,793
|
$
|
15,384,287
|
||||
|
Allowance
for doubtful accounts
|
(1,570,754
|
)
|
(717,451
|
)
|
||||
|
$
|
5,956,039
|
$
|
14,666,836
|
|||||
The
movement of allowance for doubtful accounts for the years ended September 30,
2010 and 2009 is as follows:
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Allowance
for doubtful accounts, beginning of the year
|
$
|
717,451
|
$
|
117,365
|
||||
|
Addition
|
849,221
|
688,158
|
||||||
|
Reduction
|
(23,803
|
)
|
(89,347
|
)
|
||||
|
Translation
adjustment
|
27,885
|
1,275
|
||||||
|
Allowance
for doubtful accounts, end of the year
|
$
|
1,570,754
|
$
|
717,451
|
||||
F-16
Note
6 – Inventories, net
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Raw
materials
|
$
|
179,993
|
$
|
154,874
|
||||
|
Work
in progress
|
226,844
|
99,870
|
||||||
|
Finished
goods
|
29,541
|
599,948
|
||||||
|
Low-value
consumables and packaging materials
|
88,941
|
115,993
|
||||||
|
$
|
525,319
|
$
|
970,685
|
|||||
Note
7 - Prepayments and Other Receivables
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
ERA
Bio-Technology (Shenzhen) Co., Ltd. (a)
|
$
|
4,078,769
|
$
|
4,007,843
|
||||
|
Other
receivables and prepaid expenses
|
1,360,730
|
995,284
|
||||||
|
Advance
to suppliers
|
285,892
|
456,079
|
||||||
|
5,725,391
|
5,459,206
|
|||||||
|
Allowance
for doubtful accounts
|
(3,145,468
|
)
|
(54,263
|
)
|
||||
|
$
|
2,579,923
|
$
|
5,404,943
|
|||||
|
(a)
|
As
of September 30, 2010, the Group lent $4,078,769, in aggregate to ERA
Biotech. $2,314,089 of such loans was due on August 11, 2009
and was extended to July 28, 2010 on July 28, 2009. The remaining
$1,764,680 balance of such loans matured on June 9, 2010. The Group has
entered into a non-binding letter of intent with ERA Biotech to convert
such loans into shares of ERA Biotech. However, no definitive
agreement has been reached. Based on the new development, the
Company evaluated the collectability of such loan balance and determined
to provide a bad debt allowance of $2,585,809 as of September 30,
2010.
|
Note
8 – Amounts Due from Staff
Amounts
due from staff represent various advances to certain employees for business
purposes and receivables from sales managers due to certain amounts of funds
collected from customers by sales managers of Hopsun and kept at respective bank
accounts for working capital purposes. Hopsun has entered into a cash advance
custody agreement with each sales manager which stipulates the ownership and all
the other rights related to such funds belong to Hopsun and each sales manager
bears various legal responsibilities including guarding the safety of such
funds. In addition to other control measures, these bank accounts are operated
by Hopsun instead of sales managers. Amounts due from staff amounted to
$7,993,826 and $5,460,867 as of September 30, 2010 and 2009,
respectively.
F-17
Note
9 – Property, Plant and Equipment
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Buildings
and improvement
|
$
|
9,885,136
|
$
|
2,263,059
|
||||
|
Machinery
and equipment
|
2,315,357
|
629,806
|
||||||
|
Furniture
and office equipment
|
517,850
|
294,680
|
||||||
|
Motor
vehicles
|
786,501
|
629,661
|
||||||
|
13,504,844
|
3,817,206
|
|||||||
|
Accumulated
depreciation
|
(1,637,179
|
)
|
(1,274,766
|
)
|
||||
|
11,867,665
|
2,542,440
|
|||||||
|
Construction
in progress
|
-
|
4,401,000
|
||||||
|
$
|
11,867,665
|
$
|
6,943,440
|
|||||
A
building owned by the Group with net book value of $1,038,167 was pledged, as
renewed as of September 30, 2010, by the Group to Wuhan Rural Commercial Bank
for a loan of $507,606, borrowed by Wuhan Pan-Asia Peptide Material Research Co.
Limited (“Wuhan Pan-Asia”), an unrelated third party. Wuhan Pan-Asia entered
into an agreement with the Group for a loan facility of $746,480 to the Group in
exchange for the pledge. As of September 30, 2010, the balance due to Wuhan
Pan-Asia in this relation was repaid in August 2010 (Note 16).
Depreciation
expense for the years ended September 30, 2010 and 2009 was $358,987 and
$291,720, respectively.
As of
September 30, 2010, the construction of a new factory with estimated total
original value of $8,757,598 located in Wuhan, Hubei Province, the PRC is
completed and transferred from construction in progress to fixed assets but is
still subject to final assessment.
Note
10 – Deposit for Land Use Rights
Deposit
for land use rights represents the payments that are paid to the government to
secure the land use rights of the land lots in Guangzhou Science Park for future
regional headquarters and research and development center purpose and in Wuhan
for future manufacturing facility expansion. The Group has paid deposit for land
use rights, in aggregate, $5,552,160 and $440,100 as of September 30, 2010 and
2009, respectively. The relevant certificates of land use right are to be
issued, and if not, such amount will be refunded.
Note
11 - Land Use Rights
As of
September 30, 2010, land use rights of the Group included certain parcels of
land located in Wuhan City, Hubei Province, the PRC, with a net carrying value
of $279,450. The land use rights for land with area of approximately 11,208
square meters and 7,947 square meters which will expire in November 2048 and
January 2059, respectively. Amortization expense for the years ended September
30, 2010 and 2009 was $6,357 and $3,968, respectively.
Note
12 – Investment in Equity Affiliates
As of
September 30, 2010, the Group’s investment in affiliates accounted for based on
the equity method of accounting represented 40% interest in Wuhan Hopsun
Biological Product Inspection Co., Limited (“Wuhan Inspection”), which engages
in biotechnological health products testing including Tallyho’s
products.
F-18
The
investments in affiliates amounted to $227,351 and $254,286 as of September 30,
2010 and 2009, respectively, which includes the Group’s share of accumulated
losses in the affiliates of $30,866 and $30,611 for the years ended September
30, 2010 and 2009.
Note
13 - Other Assets
Other
assets represented equipment held for sale. The Group acted as a guarantor for
an original loan amount of $419,111 borrowed by Wuhan Sanrong Group Limited
(“Wuhan Sanrong”) from Agriculture Bank of China in 2001. The loan was secured
with elevators that belonged to Wuhan Sanrong at that time. Wuhan Sanrong
encountered some cash flow difficulties and the bank requested that the Group
take over the loan. The loan including the collateral was taken over by the
Group in September 2002. These assets are held for disposal and are carried at
lower of carrying value or fair value less cost to sell. Wuhan Sanrong has
ceased to be a shareholder of Tallyho since March 28, 2001.
Note
14 - Short-term Loans
|
Interest
|
September
30,
|
||||||||||||||||||||
|
rate per
|
2010
|
2009
|
|||||||||||||||||||
|
Lender
|
Annum
|
RMB
|
USD
|
RMB
|
USD
|
||||||||||||||||
|
Agriculture Bank of
China
|
|||||||||||||||||||||
|
The
term of the loan has expired in September 2003 (Note 13)
|
5.84
|
%
|
1,980,000
|
295,606
|
2,360,000
|
346,211
|
|||||||||||||||
|
Wuhan Finance
Bureau
|
|||||||||||||||||||||
|
The
term of the loan has expired in November 2001
|
5.94
|
%
|
1,500,000
|
223,944
|
1,500,000
|
220,051
|
|||||||||||||||
|
Total
bank loans
|
3,480,000
|
519,550
|
3,860,000
|
566,262
|
|||||||||||||||||
Short-term
loans represent those short-term loans from financial institutions. The term of
the loan from Agriculture Bank of China and Wuhan Finance Bureau expired in 2003
and 2001. The loan from Agriculture Bank of China is being repaid by
$2,932 per month, but Wuhan Finance Bureau has not demanded repayment. Interest
accrued on a monthly basis based on the contractual rate.
The
interest expenses for the years ended September 30, 2010 and 2009 were
$76,473 and $96,390, respectively. The interest expenses also
include those related to the loan from Wuhan Pan-Asia, an unrelated party (Note
16).
Note
15 – Deferred Revenue
Deferred
revenue represents the sales related to the products delivered to the
third-party wholesalers or distributors for which collectability could not be
reasonably assured. As of September 30, 2010, management believes that the
collectability of the accounts receivables related to the remaining balance of
deferred revenue could not be reasonably assured. Thus, the remaining
balance of deferred revenue of $1,371,794 (RMB9,188,412) was offset against the
corresponding accounts receivables as of September 30, 2010. As of September 30,
2009, deferred revenue amounted to $3,928,804.
F-19
Note
16 - Accrued Expenses and Other Liabilities
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Advance
from customers
|
$
|
15,355
|
$
|
3,738
|
||||
|
Accrued
payroll
|
243,373
|
87,891
|
||||||
|
Accrued
expense
|
208,852
|
134,146
|
||||||
|
Other
payables
|
||||||||
|
–
Wuhan Pan-Asia (a)
|
-
|
679,223
|
||||||
|
–
Guangzhou Shunfa Garment Factory
|
-
|
564,428
|
||||||
|
–
Wuhan Xinwang Investment Management Group
|
392,909
|
386,077
|
||||||
|
–
Malaysia Phoenix Group
|
119,123
|
117,052
|
||||||
|
Others
|
546,322
|
476,574
|
||||||
|
$
|
1,525,934
|
$
|
2,449,129
|
|||||
|
(a)
|
Wuhan
Pan-Asia borrowed the funds to lend to the Group from Wuhan Rural
Commercial Bank for which the Group pledged an office building as
collateral (Note 9). Interest on the loan is paid by the Group monthly at
the rate of 0.531% during the year ended September 30, 2010. This loan
matured in August 2010 and was repaid, thus as of September 30, 2010, the
Group had a zero balance due to Wuhan Pan-Asia. Such a loan of
Wuhan Pan-Asia was renewed by September 30, 2010 with the same major terms
and the Group later borrowed approximately $0.3 million from Wuhan
Pan-Asia by January 12, 2011.
|
Note
17 - Taxation
The
Company, its subsidiaries and VIE each file tax returns separately.
1)
VAT
Pursuant
to the Provisional Regulation of the PRC on the VAT and its implementing rules,
all entities and individuals (“taxpayers”) that are engaged in the sale of
products in the PRC are generally required to pay the VAT at a rate of 17% of
the gross sales proceeds received, less any deductible the VAT already paid or
borne by the taxpayers. Further, when exporting goods, the exporter is entitled
to a portion of or all the refund of VAT that it has already paid or incurred.
VAT Taxpayers can be divided into two categories, i.e., General VAT Taxpayer and
Small Scale VAT Taxpayer. The Small Scale VAT Taxpayer is subject to a
simplified VAT rate on the gross sales proceeds while all VAT previously paid,
or input VAT, cannot be deductible for tax payment purpose. This simplified VAT
tax rate was reduced to 3% effective January 1, 2009. Some of Hopsun’s branch
offices are Small Scale VAT Taxpayers and subject to the simplified VAT rate of
3%,
2) Income
tax
United
States
CPGI is
incorporated in Delaware and is subject to U.S. federal income tax with a system
of graduated tax rates ranging from 15% to 35%. As CHPN does not conduct any
business in Delaware, it is not subject to Delaware state corporate income
tax.
F-20
BVI
Cantix,
incorporated in BVI, is governed by the income tax law of BVI. According to
current BVI income tax law, the applicable income tax rate for Cantix is
nil.
Hong
Kong
Moneyeasy,
incorporated in Hong Kong, is subject to a corporate income tax rate of
16.5%.
PRC
In
accordance with the relevant tax laws and regulations of the PRC, a company
registered in the PRC is subject to income taxes within the PRC at the
applicable tax rate on the taxable income. The statutory tax rate is
25%.
Tallyho,
registered in the City of Wuhan in the PRC, has obtained the approval and would
be qualified as a New and
High-Tech Enterprise ("NHTE") by relevant governmental authorities in December
2010. According to the PRC income tax law, Tallyho would be eligible to enjoy a
preferential tax rate of 15% for the calendar year of 2010.
Hopsun,
registered in the City of Guangzhou in the PRC, was qualified as a New and
High-Tech Enterprise ("NHTE") by relevant governmental authorities in December
2009. According to the PRC income tax law and its NHTE certificate issued,
Hopsun is subject to income tax at a preferential tax rate of 15% starting from
the calendar year of 2009 for 3 years unless the NHTE status would become
invalid.
Anti-Aging,
registered in the City of Wuhan in the PRC, is subject to the income tax at tax
rate of 25%.
Xingpu,
registered in the City of Guangzhou in the PRC, is subject to the income tax at
tax rate of 25%.
|
a)
|
Tax
payable
|
Taxes
payable consist of the following:
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
VAT
payable
|
$
|
3,113,658
|
$
|
2,629,954
|
||||
|
Income
tax payable
|
2,250,810
|
3,569,913
|
||||||
|
Other
taxes payable
|
233,331
|
204,173
|
||||||
|
$
|
5,597,799
|
$
|
6,404,040
|
|||||
|
b)
|
Taxation
provision
|
The
following table reconciles the Group’s effective tax for the periods
presented:
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Expected
enterprise income tax at statutory tax rate
|
$ | 2,081,631 | $ | 3,337,893 | ||||
|
Effect
of preferential tax rate
|
(832,652 | ) | (659,256 | ) | ||||
|
Non-deductable
expenses
|
117,095 | 51,656 | ||||||
|
Others
|
21,638 | (15,019 | ) | |||||
|
Effective
enterprise income tax
|
$ | 1,387,712 | $ | 2,715,274 | ||||
F-21
The
provision for income taxes consists of taxes on income from operations plus
changes in deferred taxes for the periods presented:
|
Years
Ended September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Current
tax expenses
|
$ | 1,878,788 | $ | 2,832,533 | ||||
|
Deferred
tax benefits
|
(491,076 | ) | (117,259 | ) | ||||
|
Income
tax expenses
|
$ | 1,387,712 | $ | 2,715,274 | ||||
(c)
Deferred tax assets
Deferred
tax assets and deferred tax liabilities reflect the tax effects of temporary
differences between the carrying amounts of assets and liabilities for financial
reporting purpose and the tax bases used for income tax purpose. The following
represents the tax effect of each major type of temporary
difference.
Deferred
tax assets consist of the following:
|
September
30,
|
||||||||
|
|
2010
|
2009
|
||||||
|
|
||||||||
|
Allowance
for doubtful receivables
|
$ | 707,433 | $ | 154,928 | ||||
|
Allowance
for inventory obsolescence
|
24,868 | 39,158 | ||||||
|
Difference
in depreciation
|
50,054 | 83,249 | ||||||
|
Tax
loss carried-forward
|
85,639 | 113,911 | ||||||
| 867,994 | 391,246 | |||||||
|
Valuation
allowance
|
(85,639 | ) | (113,911 | ) | ||||
|
Deferred
tax assets, net
|
$ | 782,355 | $ | 277,335 | ||||
The tax
loss carried-forward incurred by Anti-Aging amounted to $85,639 and $113,911 for
the years ended September 30, 2010 and 2009, respectively, may not be recovered
for the respective future 5 years’ taxable income. Management believes that the
realization of the benefits arising from the loss appear to be remote due to the
research and development nature of Anti-Aging’s operation and its continuing
losses for PRC income tax purpose, and therefore, full provision has been
provided for the tax loss carried-forward amounts.
F-22
Note
18 - Related Party Transactions
The
transactions with the following entities and individuals were made in the
ordinary course of business and were negotiated on an arm’s length basis.
A summary of balances and transactions with related parties is as
follows:
|
September
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Due
to related parties
|
||||||||
|
Wuhan
Inspection (a)
|
$
|
442,689
|
$
|
450,642
|
||||
|
Former
equity holders of Tallyho (b)
|
-
|
5,748,948
|
||||||
|
Xiu
Choi Fa
|
-
|
898,275
|
||||||
|
$
|
442,689
|
$
|
7,097,865
|
|||||
|
(a)
|
Wuhan
Inspection advanced to Tallyho payments for relevant expenses for
obtaining the necessary special licenses for Wuhan Inspection’s inspection
business through and with the assistance of Tallyho.
|
|
(b)
|
Pursuant
to an acquisition agreement between Moneyeasy and the former equity
holders of Tallyho on November 5, 2007, Moneyeasy acquired 100% of
Tallyho’s shares with a total consideration of $5,748,948. The balance was
settled as of December 31, 2009. In November 2009,
pursuant to a letter issued by Xiu Chio Fa to Cantix, as the then
shareholder of Cantix, Xiu Chio Fa waived the receivable from Cantix which
amounted to $6,597,095 (RMB45,030,000), part of which includes the above
amount.
|
All
amounts due to related companies were interest free, unsecured and had no fixed
term of repayment.
Note
20 - Restricted Net Assets
The
Company’s ability to pay dividends is primarily dependent on the Company
receiving distributions of funds from its subsidiaries. Relevant PRC statutory
laws and regulations permit payments of dividends by the Company’s PRC
subsidiary only out of their retained earnings, if any, as determined in
accordance with PRC accounting standards and regulations. The results of
operations reflected in the consolidated financial statements prepared in
accordance with U.S. GAAP differ from those reflected in the statutory financial
statements of the Company’s subsidiaries.
Amounts
restricted include registered paid-in capital, additional paid-in capital and
statutory reserve funds of the Company’s PRC subsidiaries as determined pursuant
to PRC generally accepted accounting principles, totaling approximately
$7,532,767 and $7,262,649, as of September 30, 2010 and 2009,
respectively.
Note
21 - Commitment and Contingency
(a)
Operating lease commitment
The Group
has entered into several tenancy agreements for the operating lease of office
block, workshop and warehouses. The Company’s commitment for minimum lease
payments under these operating leases as of September 30, 2010 for the next five
years is as follows:
|
Year
ended September 30,
|
||||
|
2011
|
$ | 50,640 | ||
|
2012
|
11,576 | |||
|
2013
|
2,977 | |||
|
2014
|
2,977 | |||
|
2015
thereafter
|
1,985 | |||
| $ | 70,155 | |||
(b)
Capital commitment
There is
no capital commitment as of September 30, 2010.
F-23
(c)
Contingency
The Group
is not currently a party to any legal proceeding, investigation or claim which,
in the opinion of the management, is likely to have a material adverse effect on
the business, financial condition or results of operations, The Group did not
record any contingencies as of September 30, 2010 and 2009.
Note
22 – Shareholders’ Equity
Common
stock
On
November 13, 2009, as the result of closing of the Exchange disclosed in Note 1,
the Company acquired all of the issued and outstanding capital stock of Cantix
in exchange for the issuance of 8,800,000 (on a post 8-for-1 forward stock split
basis) shares of the Company’s common stock.
On
January 8, 2010, as the result of closing of an investment of $3,600,000
pursuant to a Securities Purchase Agreement dated December 16, 2009, the Company
issued a) 666,667 shares of the Company’s common stock, and b) a 5 year warrant
to purchase up to an additional 333,333 shares of Common Stock at an exercise
price of $6.75 per share to the investor. The net proceeds was $3,300,000 after
deducting commissions and other closing expenses of $300,000.
On March
1 and September 1, 2010, the Company issued, in aggregate, 38,000 shares of the
Company’s common stock as payments for services which were valued at $137,490
and included in deferred compensation of which $60,135 was expensed during the
year ended September 30, 2010.
On April
21, 2010, as the result of closing of an investment with gross proceeds of
$3,000,000 pursuant to a Share Purchase Agreement dated as of April 16, 2010,
the Company issued (i) 609,557 shares of common stock, par value $.0001 per
share, and (ii) a 5 year warrant to purchase up to an additional 80,956 shares
of Common Stock at an exercise price of $6.75 per share to the investor. The net
proceeds was $2,761,070 after deducting commissions and other closing expenses
of $238,930.
On
September 2, 2010, as the result of closing of an investment with gross proceeds
of $3,000,000 pursuant to a Share Purchase Agreement dated as of August 25,
2010, the Company issued (i) 585,743 shares of common stock,
par value $.0001 per share, and (ii) a 5 year warrant to purchase up to an
additional 87,861 shares of Common Stock at an exercise price of $6.75 per share
to the investor. The net proceeds was $2,935,534 after deducting transaction
related cash commission and expenses of $64,466. The Company also issued 40,000
shares of the Company’s common stock for commissions which were valued at
$187,200 and treated as transaction costs associated with the above
financing.
On
January 8, 2010, the Company issued a 5 year warrant to purchase up to 333,333
shares of Common Stock at an exercise price of $6.75 per share. On
April 21, 2010, the Company issued a 5 year warrant to purchase up to 80,956
shares of Common Stock at an exercise price of $6.75 per share. On
September 2, 2010, the Company issued a 5 year warrant to purchase up to 87,861
shares of Common Stock at an exercise price of $6.75 per share. The fair values
of these warrants were estimated using the Black-Scholes option–pricing
model.
The
following table summarizes the assumptions used in the Black-Scholes
option–pricing model when calculating the fair values of the
warrants:
|
Number of
Shares
Underlying the
Warrant Valued
|
Expected Life
(Years)
|
Exercise Price
|
Expected
Volatility
|
Dividend Yield
|
Risk Free
Interest Rate
|
Grant Date Fair
Value
|
||||||||||||||||||
|
333,333
|
2
|
$
|
6.75
|
125
|
%
|
-
|
0.96
|
%
|
$
|
1,051,434
|
||||||||||||||
|
80,956
|
2
|
$
|
6.75
|
125
|
%
|
-
|
1.03
|
%
|
$
|
225,505
|
||||||||||||||
|
87,861
|
2
|
$
|
6.75
|
125
|
%
|
-
|
0.50
|
%
|
$
|
257,394
|
||||||||||||||
F-24
Due to
the limited trading history of the Company’s common stock, the Company used a
similar public company's (similar industry, similar size and similar length of
operations) market prices to calculate the volatility which was estimated to be
125%.
Following
is a summary of the warrant activity:
|
Outstanding
as of October 1, 2009
|
-
|
|||
|
Granted
|
502,150
|
|||
|
Forfeited
|
-
|
|||
|
Exercised
|
-
|
|||
|
Outstanding
as of September 30, 2010
|
502,150
|
Note
23 - Subsequent Events
Management
has considered all events occurring through the date that the consolidated
financial statements have been issued and has determined that there are no such
events that are material to the consolidated financial statements.
F-25
EXHIBIT
INDEX
|
Exhibit No.
|
Description
|
|
|
2.1
|
Stock
Exchange Agreement, dated as of November 13, 2009, by and among the
registrant, China Polypeptide Group Ltd. and Cantix International Limited
[Incorporated by reference to Exhibit 2.1 to the registrant’s Current
Report on Form 8-K filed on November 17, 2009]
|
|
|
3.1*
|
Certificate
of Incorporation of the registrant, as amended to date
|
|
|
3.2
|
Bylaws
of the registrant [Incorporated by reference to Exhibit 3.2 to the
registrant’s Registration Statement on Form S-1 filed on May 23,
2008]
|
|
|
4.1
|
Warrant
to Purchase Common Stock issued December 16, 2009 [Incorporated by
reference to Exhibit 99.2 to the registrant’s Current Report on Form 8-K
filed on January 13, 2010]
|
|
|
10.1
|
Share
Purchase Agreement, dated as of August 25, 2010, by and between the
registrant and Wealth Chance Investments Ltd. (English Translation)
[Incorporated by reference to Exhibit 10.1 to the registrant’s Current
Report on Form 8-K filed on September 2, 2010]
|
|
|
10.2
|
Share
Purchase Agreement, dated as of April 16, 2010, by and between the
registrant and Step Best Investments Ltd. (English Translation)
[Incorporated by reference to Exhibit 10.1 to the registrant’s Current
Report on Form 8-K filed on April 22, 2010]
|
|
|
10.3
|
Form
of Securities Purchase Agreement, dated December 16, 2009 [Incorporated by
reference to Exhibit 99.1 to the registrant’s Current Report on Form 8-K
filed on January 13, 2010]
|
|
|
10.4
|
Agreement
for Return on Purchase Price, dated as of July 14, 2008, by and among
Wuhan Tallyho Biological Product Co., Ltd., Wuhan Polypeptide Anti-Aging
Research and Development Co., Ltd., Moneyeasy Industries Limited and the
shareholders of Wuhan Tallyho Biological Product Co., Ltd. and Wuhan
Polypeptide Anti-Aging Research and Development Co., Ltd. (English
Translation) [Incorporated by reference to Exhibit 10.1 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.5
|
Stock
Purchase Agreement, dated November 5, 2007, among Moneyeasy Industries
Limited, Wuhan Tallyho Biological Product Co., Ltd. and its shareholders
(English Translation) [Incorporated by reference to Exhibit 10.2 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.6
|
Stock
Purchase Agreement, December 18, 2007, between Moneyeasy Industries
Limited, Wuhan Polypeptide Anti-Aging Research & Development Co., Ltd.
and its shareholders (English Translation) [Incorporated by reference to
Exhibit 10.3 to the registrant’s Current Report on Form 8-K filed on
November 17, 2009]
|
|
|
10.7
|
Agency
Contract, dated as of June 30, 2009, between Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd. and Jinjiang Shukun Food Trade Co., Ltd
(English Translation) [Incorporated by reference to Exhibit 10.7 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.8
|
Supplementary
Agreement, dated as of October 26, 2009, between Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd. and Jinjiang Shukun Food Trade
Co., Ltd (English Translation) [Incorporated by reference to Exhibit 10.8
to the registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.9
|
Loan
Agreement, dated as of December 5, 2008, between Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd. and Era Biotechnology
(Shenzhen) Co., Ltd. (English Translation) [Incorporated by reference to
Exhibit 10.5 to the registrant’s Current Report on Form 8-K filed on
November 17, 2009]
|
|
|
10.10
|
Supplementary
Agreement, dated as of July 28, 2009, between Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd. and Era Biotechnology (Shenzhen) Co., Ltd.
(English Translation) [Incorporated by reference to Exhibit 10.6 to the
registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
10.11
|
Ceiling
Amount Security and Loan Contract between Wuhan Xianfeng Rural Credit
Cooperative, Wuhan Fanya Peptide Material Research Ltd. and Wuhan Tallyho
Biological Product Co., Ltd. (English Translation) [Incorporated by
reference to Exhibit 10.4 to the registrant’s Current Report on Form 8-K
filed on November 17,
2009]
|
|
Exhibit No.
|
Description
|
|
|
10.12
|
Employment
Agreement, dated as of March 1, 2010, between the registrant and Mr.
Richard Liu [Incorporated by reference to Exhibit 10.1 to the registrant’s
Current Report on Form 8-K filed on March 5, 2010]
|
|
|
10.13*
|
Patent
License Agreement, dated October 2, 2009, between Dongliang Chen and Wuhan
Tallyho Biological Product Co., Ltd, and the supplementary agreement,
dated May 25, 2010, in relation thereto (English
Translation).
|
|
|
10.14*
|
Exclusive
Business Cooperation Agreement, dated September 28, 2010, between
Guangdong Hopsun Polypeptide Biological Technology Co., Ltd. and Guangdong
Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
10.15*
|
Proxy
Agreement, dated September 28, 2010, among Guangdong Hopsun Polypeptide
Biological Technology Co., Ltd., Dongliang Chen and Shengfan Yan (English
translation)
|
|
|
10.16*
|
Equity
Pledge Agreement, dated September 28, 2010, among Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd., Dongliang Chen, Shengfan Yan
and Guangdong Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
10.17*
|
Exclusive
Option Agreement, dated September 28, 2010, among Guangdong Hopsun
Polypeptide Biological Technology Co., Ltd., Dongliang Chen, Shengfan Yan
and Guangdong Xinpu Polypeptide Research Co., Ltd. (English
translation)
|
|
|
21
|
List
of subsidiaries of the registrant [Incorporated by reference to Exhibit
21.1 to the Registrant’s Current Report on Form 8-K filed on November 17,
2009]
|
|
|
31.1*
|
Certifications
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002.
|
|
|
31.2*
|
Certifications
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002.
|
|
|
32.1*
|
Certification
of Chief Executive Officer Pursuant to Section 906 of the
Sarbanes-Oxley Act of 2002.
|
|
|
32.2*
|
Certification
of Chief Financial Officer Pursuant to Section 906 of the
Sarbanes-Oxley Act of
2002.
|

