Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOVEST INTERNATIONAL INC | d8k.htm |
 Carlos F. Santos, Ph.D.
VP Regulatory Affairs and
Product Development
www.biovest.com
OTCQB: “BVTI”
Fighting Lymphoma, and Making it Personal.
OneMedForum
Investor Conference
Wednesday, January 12
th
, 2011
Exhibit 99.1 |
 Safe
Harbor Statement Statements
in
this
presentation
that
are
not
strictly
historical
in
nature
constitute
"forward-
looking
statements."
Such
statements
include,
but
are
not
limited
to
statements
about
BiovaxID®,
AutovaxID™,
events
occurring
after
dates
hereof,
and
any
other
statements
relating
to
products,
product
candidates,
product
development
programs,
the
FDA
or
clinical
study
process
including
the
commencement,
process,
or
completion
of
clinical
trials
or
the
regulatory
process.
Such
statements
may
include,
without
limitation,
statements
with
respect
to
the
Company's
plans,
objectives,
expectations
and
intentions,
and
other
statements
identified
by
words
such
as
"may,"
"could,"
"would,"
"should,"
"believes,"
"expects,"
"anticipates,"
"estimates,"
"intends,"
"plans,"
or
similar
expressions.
Such
forward-looking
statements
involve
known
and
unknown
risks,
uncertainties,
and
other
factors
that
may
cause
the
actual
results
of
Biovest
to
be
materially
different
from
historical
results
or
from
any
results
expressed
or
implied
by
such
forward-looking
statements.
These
factors
include,
but
are
not
limited
to,
risks
and
uncertainties
related
to
the
progress,
timing,
cost,
and
results
of
clinical
trials
and
product
development
programs;
difficulties
or
delays
in
obtaining
regulatory
approval
for
product
candidates;
competition
from
other
pharmaceutical
or
biotechnology
companies;
and
the
additional
risks
discussed
in
filings
with
the
Securities
and
Exchange
Commission.
All
forward-looking
statements
are
qualified
in
their
entirety
by
this
cautionary
statement,
and
Biovest
undertakes
no
obligation
to
revise
or
update
this
presentation
to
reflect
events
or
circumstances
after
the
date
hereof.
The
product
names
used
in
this
statement
are
for
identification
purposes
only.
All
trademarks
and
registered
trademarks
are
the
property
of
their
respective
owners. |
 Exchange/Ticker:
OTCQB: BVTI
Price per share:
$0.90
Market Cap:
$123 million
52-week Range:
Low $0.60 / High $2.11
Shares Outstanding:
136.6M shares
Float:
45.6 million shares**
Avg. Daily Volume:
177,000 shares
Biovest Market Data*
* Market data as of
11/30/10
** Accentia Biopharmaceuticals, Inc,. (OTCQB: ABPI) owns 91M shares
|
 Overview
•
Dendreon’s
Provenge
approval in April 2010 represents first
successful therapeutic cancer vaccine (positive results + approval)
•
BiovaxID
Phase 3 trial represents landmark in lymphoma for
vaccines (1
st
vaccine to show DFS benefit in Phase 3)
•
Therapeutic vaccines are extremely safe, focus powerful immune
response against tumor cells to alter the natural progression of
cancer.
•
BiovaxID
proves the hypothesis for cancer vaccines
•
Multiple cancer vaccines in development representing multi-billion
dollar market opportunity with only one successful Phase 3 in NHL
(BiovaxID)
Therapeutic Cancer Vaccines |
 Investment Highlights
•
BiovaxID
personalized lymphoma vaccine holds the potential to be
the next cancer vaccine approved and first vaccine for non-
Hodgkin’s lymphoma
•
Completed Phase 3 clinical trial proves that therapeutic vaccination
actives the immune system and extends disease-free survival in FL
(Schuster, et al. ASCO 2009)
•
Exemplary safety record
•
IgM-Id delivers extraordinary benefit to vaccinated patients and is
fundamental discovery for next-generation vaccines.
Therapeutic Vaccines for Non-Hodgkin’s Lymphoma
“Therapeutic cancer vaccines have gained new momentum in the clinic and gotten
serious attention from previously wary investors…
with several vaccines on a
promising path to commercialization, the market could see a surge of regulatory
activity
and
an
influx
of
market
opportunities.
-GEN,
October
2010 |
                       Company
Product
Indication
Phase I
Phase 2
Phase 3
Marketed
Dendreon
Provenge
Prostate
Biovest
BiovaxID
fNHL
Menarini
Abagovomab
Ovarian
Oncothyreon/
Merck KGaA
Stimuvax
NCSLC
GSK
MAGE-A3
NSCLC
Vical
Allovectin-7
Melanoma
Bavarian Nordic
Prostvac
Prostate
New Link
HyperAcute
Pancreatic
Celldex
Rindopepimut
Glioblastoma
Geron
GRNVAC1
AML
TVAX
TVI
Astrocytoma
Prima
Cvac
Ovarian
Oxford Biomedica
TroVax
Prostate
Argos
AGS-003
Renal Cell
Cancer Vaccines in Phase 2/3 Clinical Trials |
 7
2011E
2012E
2013E
# B-cell NHL –
Relapsed/Refractory
285,582
294,150
302,974
# of Relapse/Refractory B-cell NHL
with Active Disease
57,116
58,830
60,595
U.S. Market Opportunity: fNHL
& MCL*
Newly Diagnosed Cases
Relapsed/Refractory
* Source: Cowen & Company Report, “NHL: A Large Market Getting Larger”,
December 2008 All NHL –
Newly Diagnosed
81,277
85,341
89,608
% Indolent Follicular NHL (fNHL)
# Newly Diagnosed fNHL
25%
20,319
25%
21,335
25%
22,402
% Mantle Cell Lymphoma (MCL)
# Newly Diagnosed MCL
6%
4,876
6%
5,120
6%
5,376 |
 BiovaxID Vaccine Manufacture |
 Hybridoma Autologous Production
Process Preserves Isotype
Hollow-Fiber Bioreactor
Cultures Tumor Derived Cells
(Heterohybridoma)
for Id Protein Production
Isotype-Matched Id Protein
Secreted and
Purified |
 |
 Phase 3 Id Vaccine Studies by Isotype |
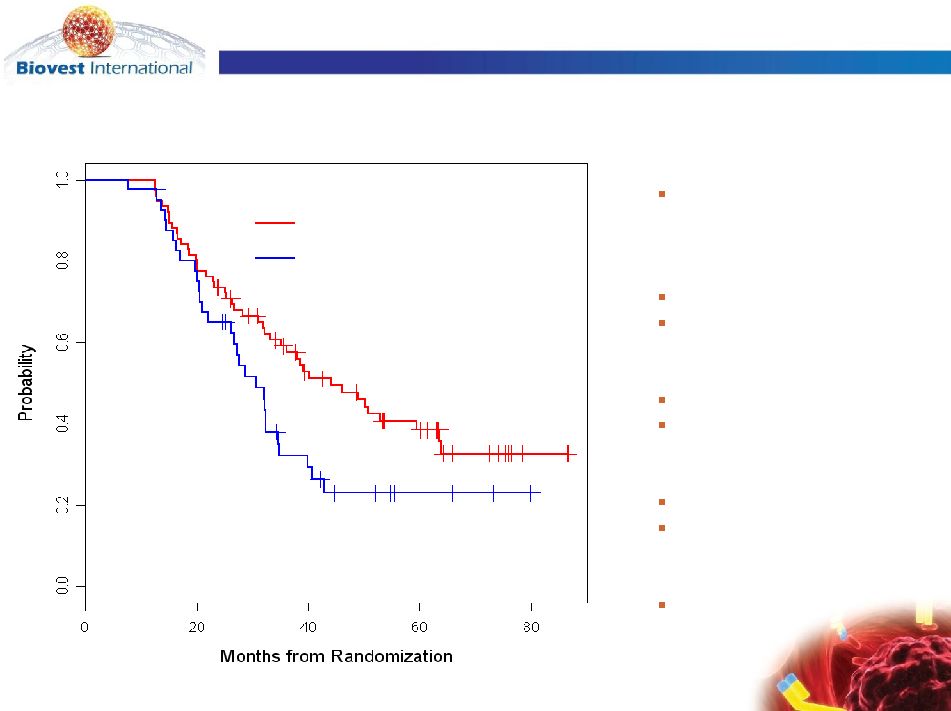 Median Follow-up
56.6 mo (range 12.6 –
89.3)
Median DFS
Id-vaccine = 44.2 mo
Control = 30.6 mo
N = 117
Id-vaccine N = 76
Control N = 41
Events
Id-vaccine = 44
Control = 29
Cox PH Model
HR = 0.62; [95% CI:
0.39,0.99] (p=0.047)
log-rank
p=0.045
Control arm
Id-vaccine arm
Modified ITT Disease Free Survival from Randomization:
Id-vaccine vs. Control (n=117) |
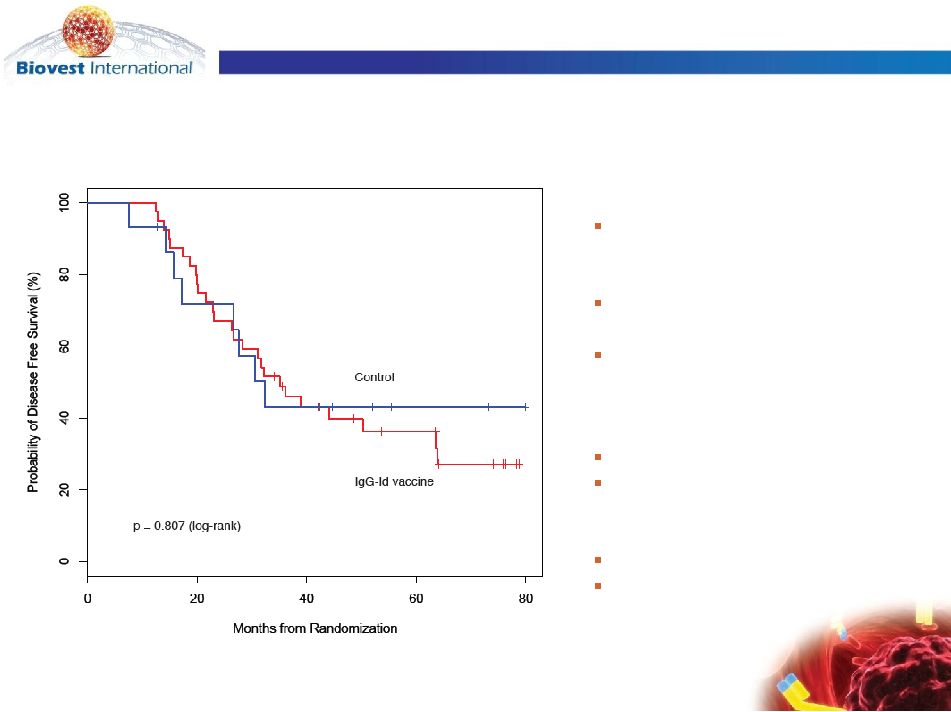 Disease Free Survival for Patients with IgM-isotype
lymphomas:
IgG-Id Vaccine vs. Control Vaccine
Median Follow-up
56.6 mo (range 12.6 –
89.3)
Median DFS
IgG-Id vaccine
35.1 mo [95% CI: 26.7,64]
Control
32.4 mo [95% CI:26.7,NA]
N = 55
IgG-Id vaccine N = 40
Control
N = 15
Events
IgG-Id vaccine = 26
Control = 8 |
 Disease Free Survival for Patients with IgM-isotype lymphomas:
IgM-Id Vaccine vs. Control Vaccine
Median Follow-up
56.6 mo (range 12.6 –
89.3)
Median DFS
IgM-Id vaccine =
52.9 mo [95% CI:40.2,NA
Control =
28.7 mo [95% CI:21.0,39.8]
N = 60
IgM-Id vaccine N = 35
Control
N = 25
Events
IgM-Id vaccine = 17
Control = 20 |
 Disease Free Survival for Patients receiving IgM-Id or IgG-Id
Vaccine vs. All Patients Receiving Control Vaccine
IgM-Id Vaccine
IgG-Id Vaccine
All Controls
Median Follow-up
56.6 mo (range 12.6 –
89.3)
N = 115
IgG-Id vaccine N = 40
IgM-Id vaccine N = 35
Control N = 40
IgM-Id Vaccine vs All
Controls:
p=0.01
IgG-Id Vaccine vs All
Controls:
p=0.30 |
 Expected Key Milestones: BiovaxID
•
Report additional BiovaxID Phase III data to support regulatory filings,
including immune response data (a secondary endpoint) with analysis
ongoing at MD Anderson Cancer Center
•
Accelerate the advancement of BiovaxID through strategic
partnership(s)
•
Meet with FDA and other international regulatory agencies to
determine regulatory pathway in seeking
approval(s)
•
Obtain Orphan Drug designation from EMA for BiovaxID for mantle cell
lymphoma indication
(10-years of market exclusivity in EU and other
regulatory benefits)
•
Announce private/public partnership with non-dilutive funding to
expand Minneapolis Cell Culture Center to accommodate production
of BiovaxID
•
Publish Phase III BiovaxID follicular lymphoma
results in leading
cancer peer-review journal |
 Expected Key Milestones: Cell Culture
•
Report next series of results from Naval Health Research
Center viral growth studies (H1N1) in hollow-fiber bioreactor
and publish results in peer-review journal
•
Announce strategic collaborations including new bioreactor
CRADA contract with U.S. Government, Department of
Defense (development and revenue-generating event)
•
Report multiple new revenue-generating manufacturing
contracts for biologics production (MN Cell Culture Center)
•
Secure new U.S. and foreign distribution agreement(s) with
bio-instruments leader(s) to market Biovest’s line of hollow-
fiber bioreactors |
 Expected Key Milestones: General Corporate
•
Secure equity research analyst coverage from respected
investment banking firm(s)
•
Elevate stock listing to OTCQX Market, the highest market
tier of the Over-the-Counter Market
•
Announce formation of Scientific Advisory Board consisting
of key opinions leaders in cancer vaccine field
•
Initiate proactive investor relations and public relations
campaign to raise awareness and highlight achievement of
key milestones, including attending/presenting at key
investment and scientific conferences
•
Continue to file new patent applications (biologics &
instruments)
•
Actively support lymphoma patient advocacy efforts |
 Contact Biovest
For more
information,
please contact:
Douglas W. Calder
Director, Investor Relations
& Public
Relations 324 S. Hyde Park Avenue; Suite 350
Tampa, Florida, 33606, USA
Phone: 813-864-2558
Email: dwcalder@biovest.com |
