Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HeartWare International, Inc. | c10778e8vk.htm |
Exhibit 99.1

| 29th Annual JP Morgan Healthcare ConferenceDoug Godshall, CEO and PresidentJanuary 10, 2011 CAUTION: Investigational device. Limited by United States law to investigational use. |

| Safe Harbor Statement Forward-Looking Statements This presentation contains forward-looking statements that are based on our management's beliefs, assumptions and expectations and on information currently available to our management. Generally, you can identify forward-looking statements by terms such as "may," "will," "should," "could," "would," "expects," "plans," "anticipates," "believes," "estimates," "projects," "predicts," "potential" and similar expressions intended to identify forward-looking statements, which generally are not historical in nature. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation, our expectations regarding (a) regulatory submissions and approvals; (b) our clinical trials, including enrollment in our clinical trials and estimates of regulatory filing dates; (c) our intellectual property position; (d) our perceived ability to develop, receive approval for and commercialize our existing and new products; (e) our expected lead times for achieving milestones and (f) our estimates regarding our preliminary financial results. The forward-looking statements reflect our current view about future events and are subject to risks, uncertainties and assumptions. Accordingly, you should not place undue reliance on our forward-looking statements. Except as required by law, we do not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. We may not actually achieve the plans, projections or expectations disclosed in our forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, including without limitation those described in Part I, "Item 1A. Risk Factors" of our Form 10- K for the year ended December 31, 2009 and subsequent filed reports. For instance, the following important factors could prevent us from achieving our goals and cause the assumptions underlying the forward- looking statements and the actual results to differ materially from those expressed in or implied by those forward looking statements: (a) inability to fund ongoing operations, including raising additional capital to support further development and growth; (b) inability to recruit, retain and motivate appropriately qualified employees; (c) unsatisfactory or uncompetitive clinical outcomes for our products (e.g. high stroke, device failure or adverse event rates); (d) lack of physician acceptance or adoption of our products; and, (e) inability to receive and maintain regulatory approvals and consents (e.g. ISO, FDA etc). Additional information concerning our risk factors and uncertainties is contained in our filings with the SEC and ASX. CAUTION: Investigational device. Limited by United States law to investigational use. |

| HeartWare: Strengthened Base for Future Growth More than 800 patients globally implanted with HVAD(r) > 700 in past 2 yearsMaturing commercial organization in EuropeBolstered manufacturing to accommodate clinical ramp in USAugmented field team to educate and service larger commercial and clinical customer basePositioning organization for FDA audit process and US launch preparationExpanded R&D capabilities to accelerate pipelineUpgrading Clinical team to manage expanding trial activities CAUTION: Investigational device. Limited by United States law to investigational use. |
| 2010 - A Look Back Completed PMA submission for BTT - December 27, 2010Launched DT study - 50+ patients enrolledInitiated CAP - received two patient allotmentsAdvanced next generation MVAD platform toward commencement of GLP animal studies this quarter (Q1 2011)Preliminary Q4 revenues of ~$20 million from $13.8 million in Q3Projecting $54 million in 2010 revenues vs. $24 million in 2009 CAUTION: Investigational device. Limited by United States law to investigational use. |
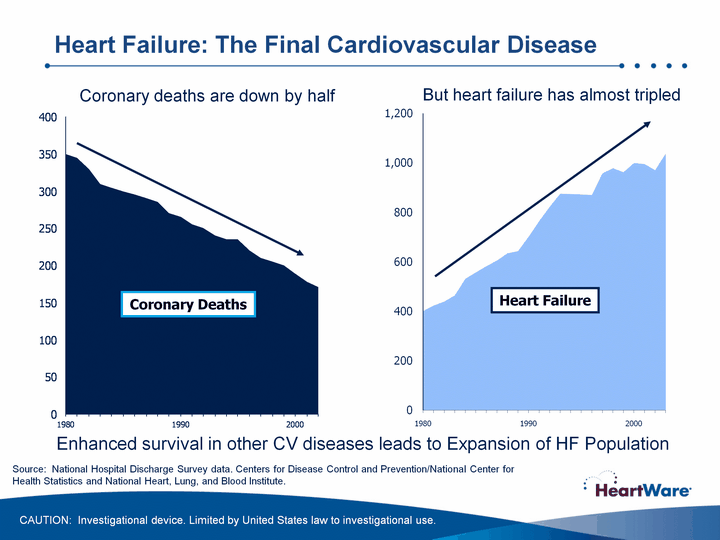
| 1st Qtr 2nd Qtr 1980 401 350 423 345 439 330 464 310 531 305 557 300 582 295 605 290 634 285 643 270 1990 701 265 764 255 822 250 875 240 874 235 872 235 870 220 957 210 978 205 962 200 2000 999 189 995 178 970 171 2003 1037 Coronary Deaths Coronary deaths are down by half But heart failure has almost tripled (CHART) Heart Failure Source: National Hospital Discharge Survey data. Centers for Disease Control and Prevention/National Center for Health Statistics and National Heart, Lung, and Blood Institute. Enhanced survival in other CV diseases leads to Expansion of HF Population Heart Failure: The Final Cardiovascular Disease CAUTION: Investigational device. Limited by United States law to investigational use. |
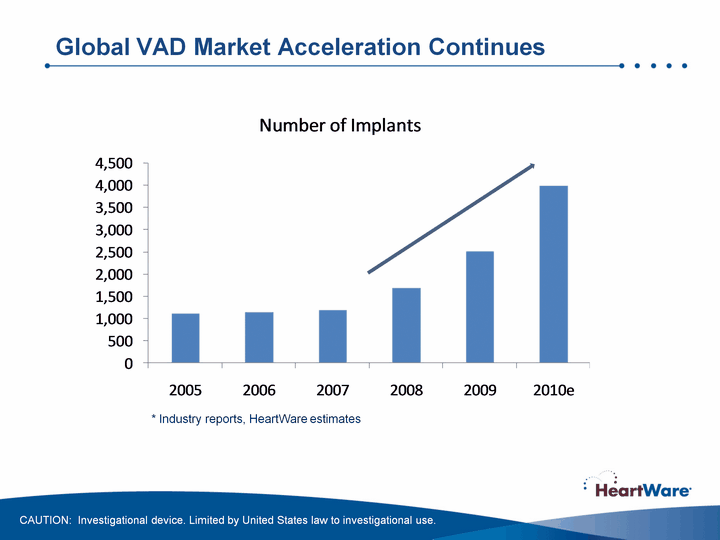
| Global VAD Market Acceleration Continues * Industry reports, HeartWare estimates CAUTION: Investigational device. Limited by United States law to investigational use. |
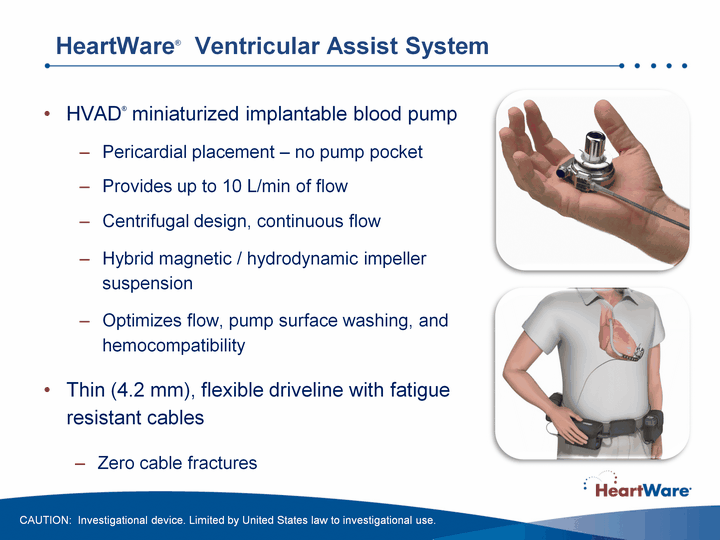
| HeartWare(r) Ventricular Assist System HVAD(r) miniaturized implantable blood pump Pericardial placement - no pump pocketProvides up to 10 L/min of flowCentrifugal design, continuous flowHybrid magnetic / hydrodynamic impeller suspensionOptimizes flow, pump surface washing, and hemocompatibilityThin (4.2 mm), flexible driveline with fatigue resistant cablesZero cable fractures CAUTION: Investigational device. Limited by United States law to investigational use. |
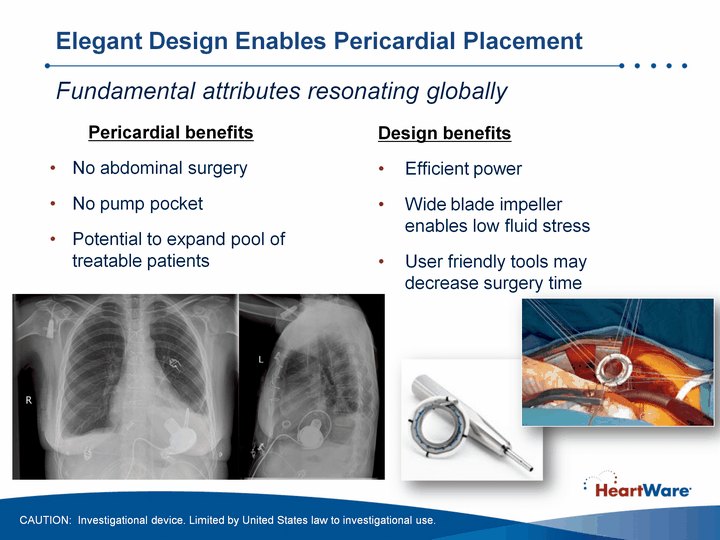
| Elegant Design Enables Pericardial Placement Design benefitsEfficient powerWide blade impeller enables low fluid stressUser friendly tools may decrease surgery time Pericardial benefitsNo abdominal surgeryNo pump pocketPotential to expand pool of treatable patients Fundamental attributes resonating globally CAUTION: Investigational device. Limited by United States law to investigational use. |
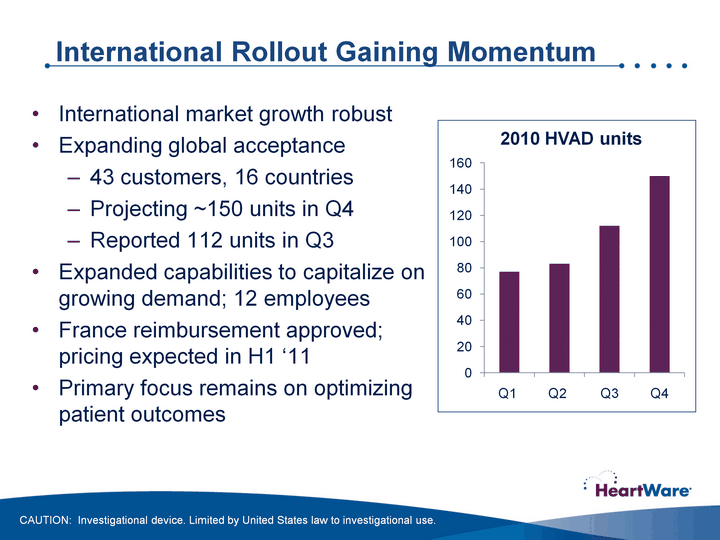
| International Rollout Gaining Momentum International market growth robustExpanding global acceptance43 customers, 16 countriesProjecting ~150 units in Q4Reported 112 units in Q3Expanded capabilities to capitalize on growing demand; 12 employees France reimbursement approved; pricing expected in H1 '11Primary focus remains on optimizing patient outcomes (CHART) CAUTION: Investigational device. Limited by United States law to investigational use. |
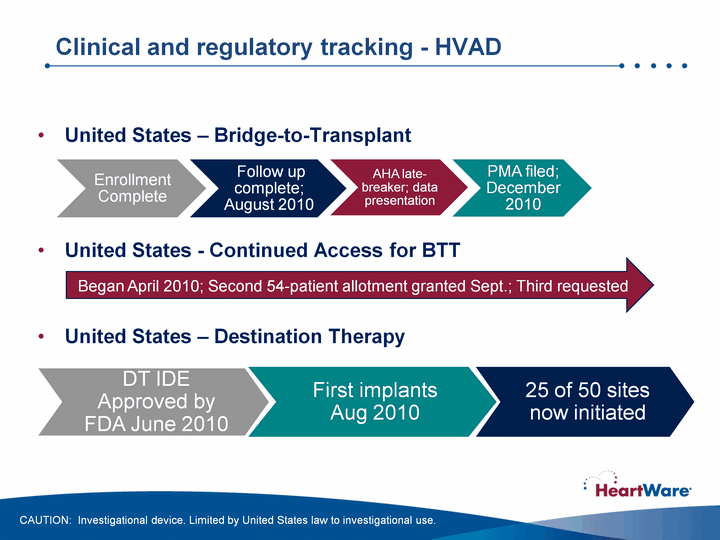
| Clinical and regulatory tracking - HVAD United States - Bridge-to-Transplant United States - Continued Access for BTTUnited States - Destination Therapy DT IDE Approved by FDA June 2010 First implants Aug 2010 Began April 2010; Second 54-patient allotment granted Sept.; Third requested CAUTION: Investigational device. Limited by United States law to investigational use. 25 of 50 sites now initiated |
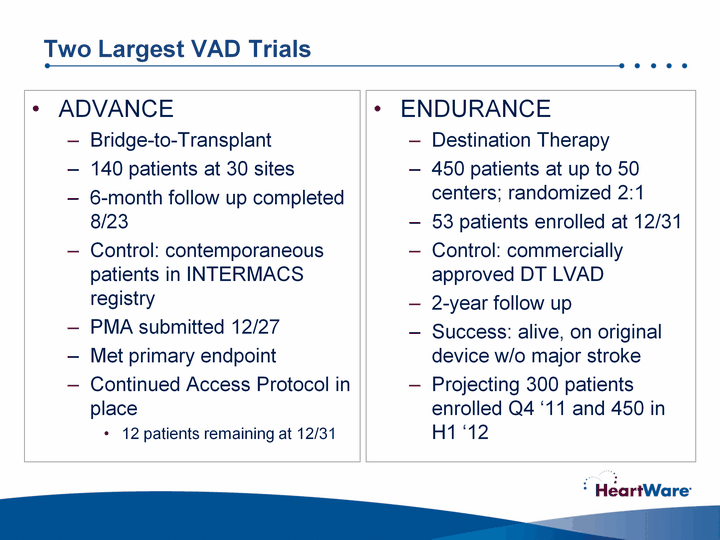
| Two Largest VAD Trials ADVANCEBridge-to-Transplant140 patients at 30 sites6-month follow up completed 8/23Control: contemporaneous patients in INTERMACS registryPMA submitted 12/27Met primary endpointContinued Access Protocol in place12 patients remaining at 12/31 ENDURANCEDestination Therapy450 patients at up to 50 centers; randomized 2:153 patients enrolled at 12/31Control: commercially approved DT LVAD2-year follow upSuccess: alive, on original device w/o major strokeProjecting 300 patients enrolled Q4 '11 and 450 in H1 '12 |
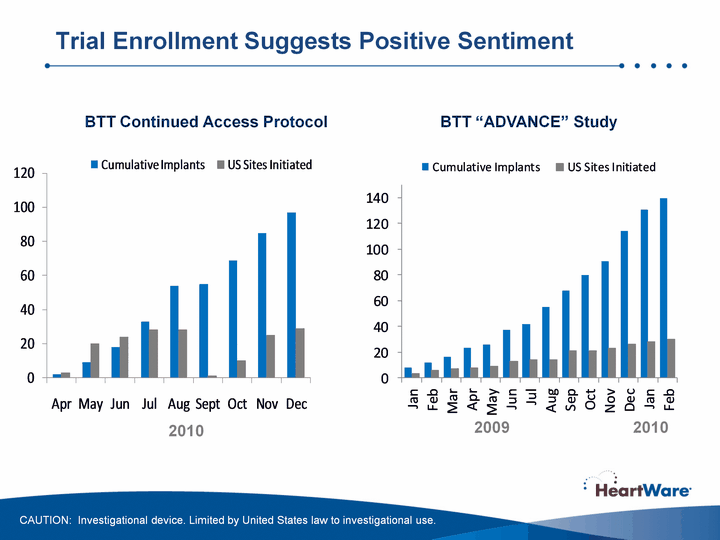
| Trial Enrollment Suggests Positive Sentiment BTT Continued Access Protocol BTT "ADVANCE" Study CAUTION: Investigational device. Limited by United States law to investigational use. 2010 2009 2010 |
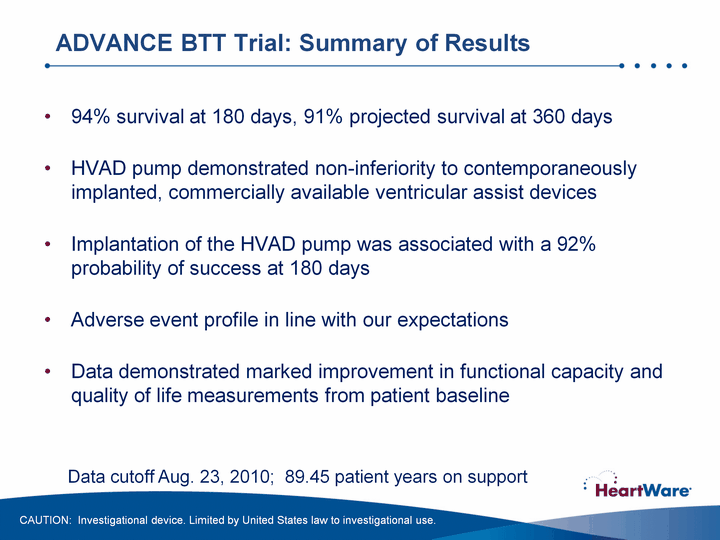
| 94% survival at 180 days, 91% projected survival at 360 daysHVAD pump demonstrated non-inferiority to contemporaneously implanted, commercially available ventricular assist devicesImplantation of the HVAD pump was associated with a 92% probability of success at 180 daysAdverse event profile in line with our expectationsData demonstrated marked improvement in functional capacity and quality of life measurements from patient baseline ADVANCE BTT Trial: Summary of Results CAUTION: Investigational device. Limited by United States law to investigational use. Data cutoff Aug. 23, 2010; 89.45 patient years on support |
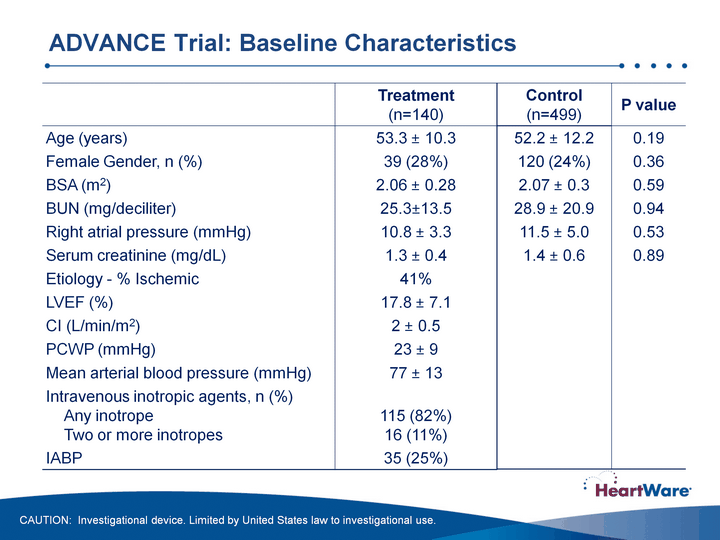
| Control(n=499) P value 52.2 +- 12.2 0.19 120 (24%) 0.36 2.07 +- 0.3 0.59 28.9 +- 20.9 0.94 11.5 +- 5.0 0.53 1.4 +- 0.6 0.89 ADVANCE Trial: Baseline Characteristics ADVANCE Trial: Baseline Characteristics CAUTION: Investigational device. Limited by United States law to investigational use. |
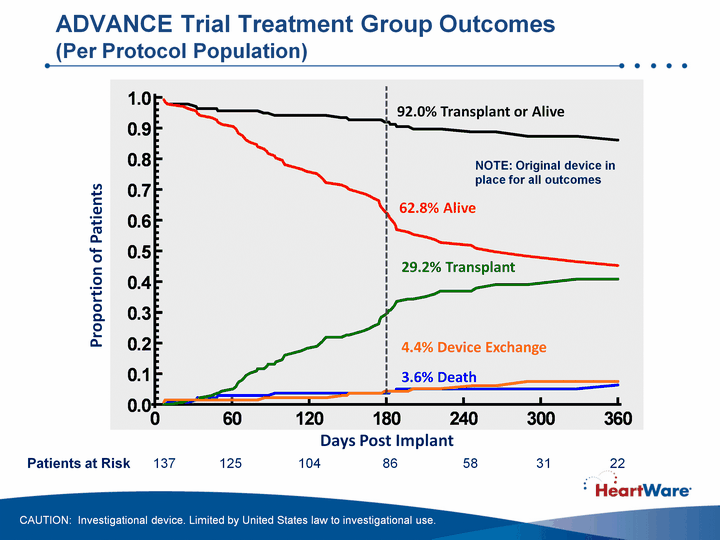
| Days Post Implant Proportion of Patients 62.8% Alive 29.2% Transplant 3.6% Death 4.4% Device Exchange 92.0% Transplant or Alive ADVANCE Trial Treatment Group Outcomes (Per Protocol Population) Patients at Risk 137 125 104 86 58 31 22 NOTE: Original device in place for all outcomes CAUTION: Investigational device. Limited by United States law to investigational use. |
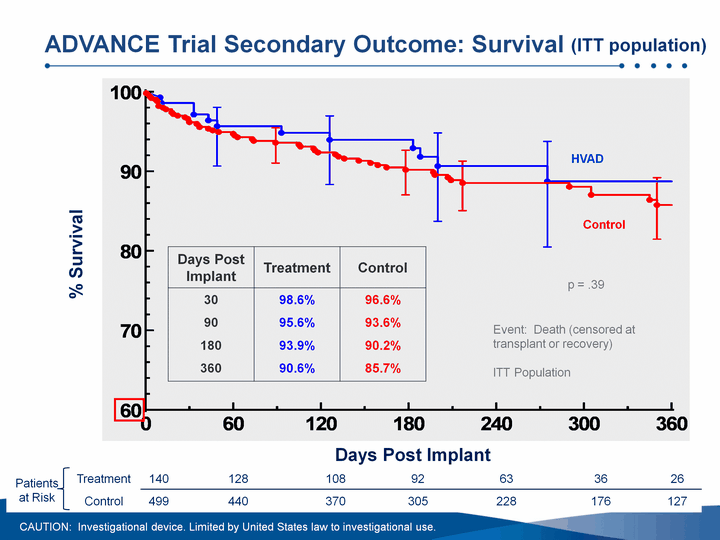
| Days Post Implant % Survival Event: Death (censored at transplant or recovery)ITT Population Patients at Risk Treatment 140 128 108 92 63 36 26 Patients at Risk Control 499 440 370 305 228 176 127 ADVANCE Trial Secondary Outcome: Survival Days Post Implant Treatment Control 30 98.6% 96.6% 90 95.6% 93.6% 180 93.9% 90.2% 360 90.6% 85.7% p = .39 HVAD Control CAUTION: Investigational device. Limited by United States law to investigational use. (ITT population) |
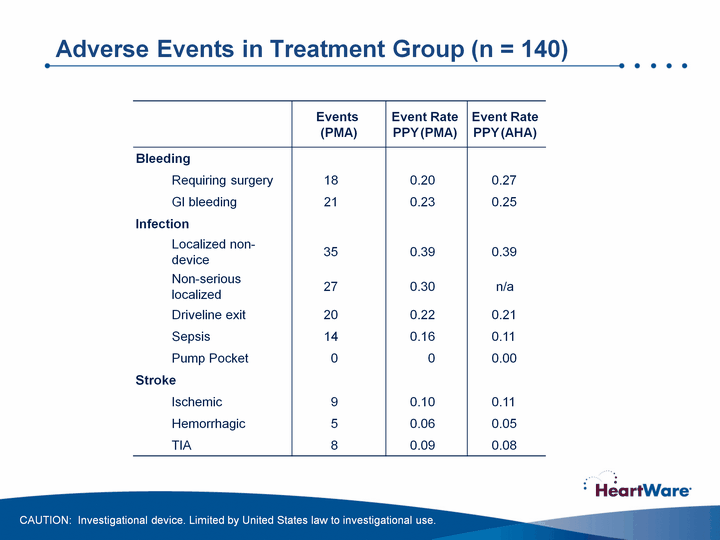
| Adverse Events in Treatment Group (n = 140) Adverse Events in Treatment Group (n = 140) CAUTION: Investigational device. Limited by United States law to investigational use. |
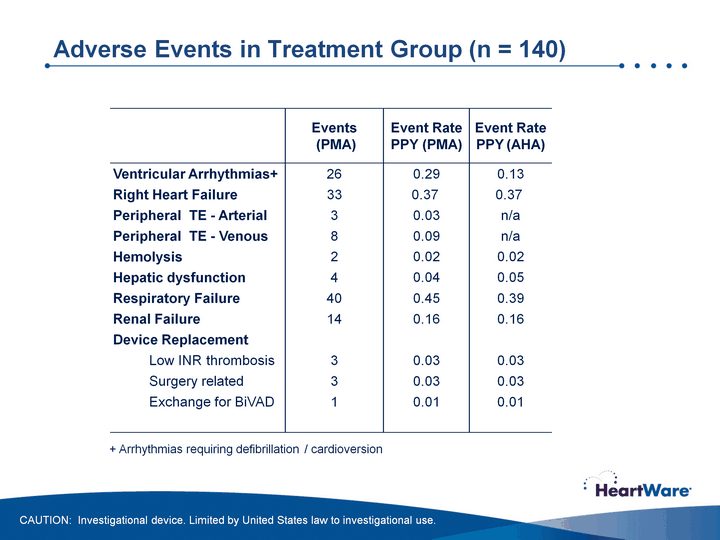
| Adverse Events in Treatment Group (n = 140) + Arrhythmias requiring defibrillation / cardioversion CAUTION: Investigational device. Limited by United States law to investigational use. |
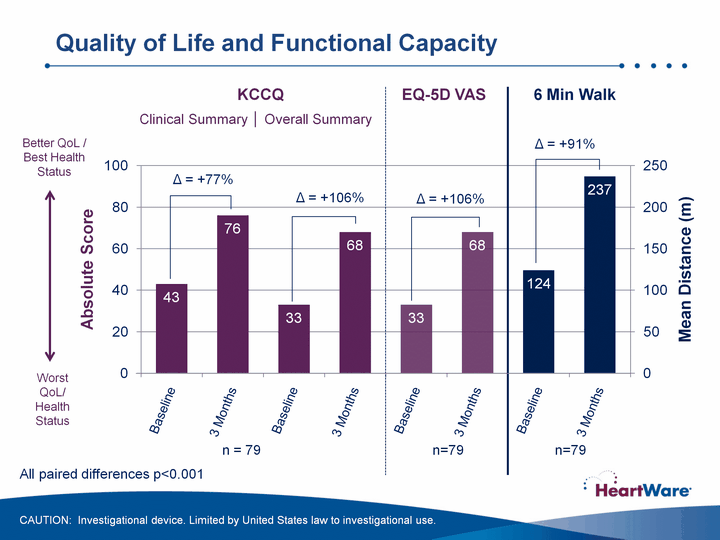
| KCCQ KCCQ n = 79 Better QoL / Best Health Status Worst QoL/ Health Status 6 Min Walk Clinical Summary ^ Overall Summary n=79 n=79 All paired differences p<0.001 EQ-5D VAS ^ = +77% ^ = +106% ^ = +91% ^ = +106% Quality of Life and Functional Capacity CAUTION: Investigational device. Limited by United States law to investigational use. |

| Development Priorities for 2011 Evergreen HVADEnhancements to pump and algorithmsIntroduce new tools to improve implantUpgrade patient accessoriesDrive MVAD to First-in-ManMultiple versions under wayDesign for AE reduction to expand treatable populationAccelerate NG ElectronicsNext Generation ControllerRamping TETS investmentExpanding development on multiple programs Focus areas for accelerated investment |
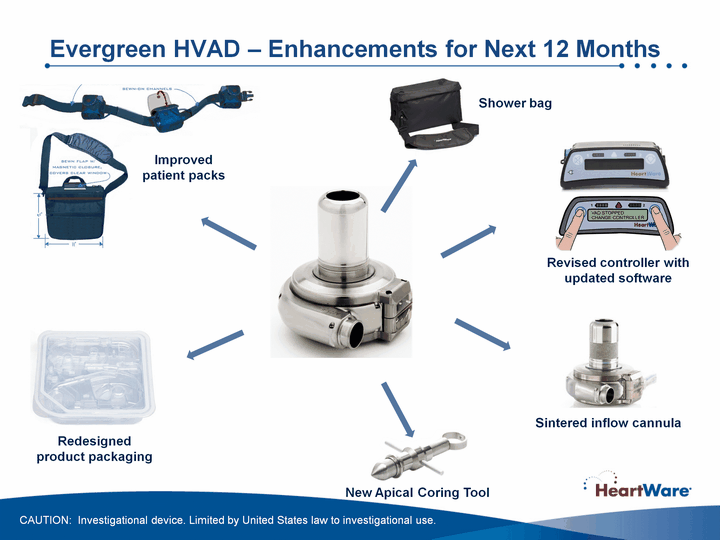
| Evergreen HVAD - Enhancements for Next 12 Months Improved patient packs Redesigned product packaging New Apical Coring Tool Sintered inflow cannula Revised controller with updated software Shower bag CAUTION: Investigational device. Limited by United States law to investigational use. |
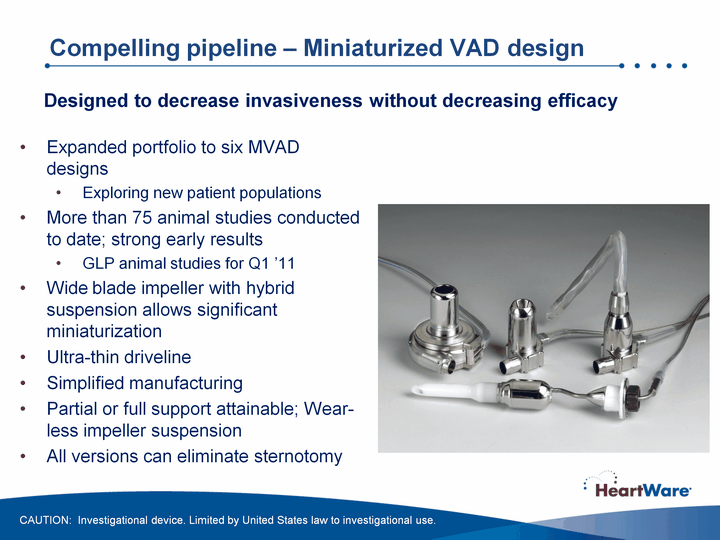
| Compelling pipeline - Miniaturized VAD design Expanded portfolio to six MVAD designsExploring new patient populationsMore than 75 animal studies conducted to date; strong early resultsGLP animal studies for Q1 '11Wide blade impeller with hybrid suspension allows significant miniaturizationUltra-thin driveline Simplified manufacturingPartial or full support attainable; Wear- less impeller suspensionAll versions can eliminate sternotomy Designed to decrease invasiveness without decreasing efficacy CAUTION: Investigational device. Limited by United States law to investigational use. |
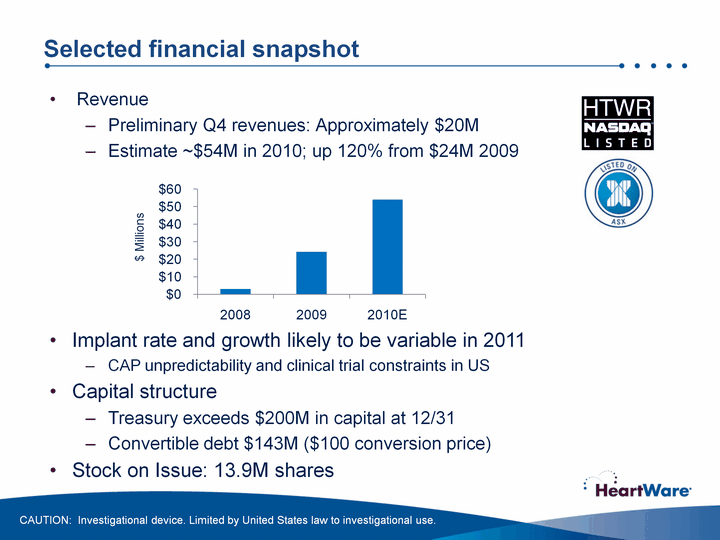
| Selected financial snapshot RevenuePreliminary Q4 revenues: Approximately $20MEstimate ~$54M in 2010; up 120% from $24M 2009 CAUTION: Investigational device. Limited by United States law to investigational use. (CHART) $ Millions Implant rate and growth likely to be variable in 2011CAP unpredictability and clinical trial constraints in USCapital structureTreasury exceeds $200M in capital at 12/31Convertible debt $143M ($100 conversion price)Stock on Issue: 13.9M shares |

| Another Busy Year Ahead Initiation of MVAD GLP animal studies in Q1 2011Secure reimbursement and launch HVAD in FrancePreparation for PMA audit; potential advisory committee panelISHLT presentation of ADVANCE trial and CAP dataDrive enrollment in DT and CAPPublication of ADVANCE dataEnroll 300th patient in ENDURANCE DT study by year endCrystallize BiVad clinical / regulatory pathRoll out multiple HVAD product enhancementsAccelerate electronics portfolioPrepare for US launch CAUTION: Investigational device. Limited by United States law to investigational use. |

| Leading a Small Revolution CAUTION: Investigational device. Limited by United States law to investigational use. |
