Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - AUXILIUM PHARMACEUTICALS INC | dex991.htm |
| 8-K - AUXILIUM PHARMACEUTICALS, INC. -- FORM 8-K - AUXILIUM PHARMACEUTICALS INC | d8k.htm |
 1
January 2011
(NASDAQ: AUXL)
Exhibit 99.2 |
 2
Safe Harbor Statement
We will make various remarks during this presentation that constitute “forward-looking
statements” for purposes of the safe harbor provisions under The Private Securities
Litigation Reform Act of 1995, including statements regarding: the pricing, time to market, size of market, growth potential and therapeutic
benefits of the Company’s products and product candidates, including those for the treatment of
Peyronie’s disease and Frozen Shoulder syndrome; the size and development of the
Dupuytren’s market in the U.S., the EU and the rest of the world; the potential for XIAFLEX to become the standard of care for
Dupuytren’s contracture; the potential for XIAFLEX to be used in multiple indications; the effect
of the identified leading indicators on the success of the XIAFLEX launch and future net
revenues; the ability to obtain reimbursement in the U.S. for XIAFLEX for the treatment of Dupuytren’s; the timing of new
reimbursement codes for XIAFLEX and the effect of the reimbursement process on the success of the
XIAFLEX launch; physicians and sites that are moving from test drive to increasing usage; the
generation of cash through licensing of XIAFLEX in other territories and for new indications; the timing and
likelihood of approval of the Marketing Authorization Application for XIAFLEX for the treatment of
Dupuytren’s contracture in the European Union, and timing and likelihood of success of
launch, if approved; future Testim market share, prescriptions and sales growth and factors that may drive such growth; size
and growth potential of the testosterone replacement therapy market and the gel segment thereof and
factors that may drive such growth; the likelihood of generic competition in the testosterone
replacement therapy gel market; competitive developments affecting the Company’s products and product
candidates, including generic competition in the testosterone replacement therapy market; the scope,
timing, methodology, endpoints, safety, execution and results of the phase III studies for
XIAFLEX for the treatment of Peyronie’s disease; the size of the Peyronie’s market and any increase in treatment for
Peyronie’s; business development efforts and opportunities to build out the Company’s
pipeline; the Company’s development and operational goals and strategic priorities for
2011, including building out its pipeline in specialty therapeutic areas; the ability to fund future operations; the opportunities and
strategies to build shareholder value; the Company’s expected financial performance during 2011
and financial milestones that it may achieve for 2011, including 2011 XIAFLEX U.S. net revenues
and Pfizer royalty and contract revenues; and the likelihood or timing of the Company becoming profitable. All
remarks other than statements of historical facts made during this presentation, including but not
limited to, statements regarding future expectations, plans and prospects for the Company,
statements regarding forward-looking financial information and other statements containing the words “believe,” “may,”
“could,” “will,” “estimate,” “continue,”
“anticipate,” “intend,” “should,” “plan,” “expect,” and similar expressions, as they relate to the Company, constitute
forward-looking statements. Actual results may differ materially from those reflected in
these forward-looking statements due to various factors, including general financial,
economic, regulatory and political conditions affecting the biotechnology and pharmaceutical industries and those discussed in Auxilium's
Annual Report on Form 10-K for the year ended December 31, 2009 and in Auxilium’s Quarterly
Report on Form 10-Q for the period ended September 30, 2010 under the heading “Risk
Factors,” which are on file with the Securities and Exchange Commission (the “SEC”) and may be accessed electronically by
means of the SEC’s home page on the Internet at http://www.sec.gov or by means of the
Company’s home page on the Internet at http://www.auxilium.com under the heading “For
Investors - SEC Filings.” There may be additional risks that the Company does not presently know or that the Company currently
believes are immaterial which could also cause actual results to differ from those contained in the
forward-looking statements. Given these risks and uncertainties, any or all of these
forward-looking statements may prove to be incorrect. Therefore, you should not rely on any such factors or forward-
looking statements.
In addition, forward-looking statements provide the Company’s expectations, plans or
forecasts of future events and views as of the date of this presentation. The Company
anticipates that subsequent events and developments will cause the Company’s assessments to change. However, while the
Company may elect to update these forward-looking statements at some point in the future, the
Company specifically disclaims any obligation to do so. These forward-looking
statements should not be relied upon as representing the Company’s assessments as of any date subsequent to the date of this
presentation. |
 3
Our Strategy Is to Become a Highly Profitable
and Sustainable Biopharmaceutical Company
Maximize Value of
Testim
and XIAFLEX
Deliver on
Current Pipeline
Build Out
Pipeline in
Specialty
Therapeutic
Areas |
 XIAFLEX and Testim
Are Global Product
Opportunities
U.S.
Canada
EU
ROW
Open
Open
Territory
XIAFLEX
Testim
4 |
 Auxilium
Has a 6 Year Net Revenue CAGR
of 40.2%
4Q10 XIAFLEX Net
Revenues $8.4M
(including $7.3M in U.S.
revenues)
4Q10 Testim
Net
Revenues $53.4M,
13.3% y/y
growth
5 |
 6 |
 7
•
Excessive collagen in fascia of hand
•
Nodules or pits are an early, active
presentation
•
Rope-like cords develop in the finger
and result in contractures
•
Quality of life and daily activities
affected
•
Surgery has been reserved for
advanced disease due to the nature of
the disease, unpredictable results,
complications, long recovery time and
recurrence/additional surgeries
Dupuytren’s Contracture Is Debilitating for Patients
and Surgery Has Been the Standard of Care
Immediately post-operative
Intra-operative open fasciectomy
Pictures
courtesy
of
Dr.Clayton
Peimer
pre-operative open fasciectomy |
 8
We Believe XIAFLEX Is a Paradigm
Changing Treatment
•
Simple, non-invasive injection of XIAFLEX
•
Established mechanism of action and selective for specific types of collagen
•
XIAFLEX’s
post-approval profile is consistent with clinical trial experience
Pre XIAFLEX injection
30 days following XIAFLEX
injection |
 9
Strategy for Establishing XIAFLEX as the
Standard of Care
•
Disseminate excellent clinical efficacy and safety data
•
Facilitate peer to peer dialogue
•
Educate patients on a non-invasive option
•
Smooth the reimbursement process
•
Activate a critical mass of experienced prescribers |
 10
XIAFLEX Launch Strategy is Tailored to
Customer Needs
•
~ 6,000 target physicians
–
Hand surgeons, plastic surgeons, orthopedic
surgeons, general surgeons, rheumatologists
•
~ 100 field based personnel
–
Sales reps, sales managers, and reimbursement
specialists
•
Medical science liaisons
–
Key opinion leader and regional opinion leader
support |
 11
We
Believe
the
U.S.
Dupuytren’s
Opportunity
Is
Significant
70,000 Surgical
2,3,4
Patients Annually
300,000 Diagnosed
Patients Annually
2,3,4
>1 Million Diagnosed
Patients
2,4
1.
Dupuytren’s
Disease
–
Tubiana,
LeClerq,
Hurst,
Badalamente,
Mackin
2.
SDI Claims Data Based Projections
3.
Medicare
Data
Based
Projections
(BESS
database
used,
Medicare
5%
database
also
used
to
validate
numbers)
4.
Auxilium
Research
(Patient
Segmentation,
Forecast
Research,
WK/AMA
Databases)
Sources:
$1 Billion Annual
Market Opportunity
>$3 Billion Market
Opportunity
$350 Million
Market
Opportunity |
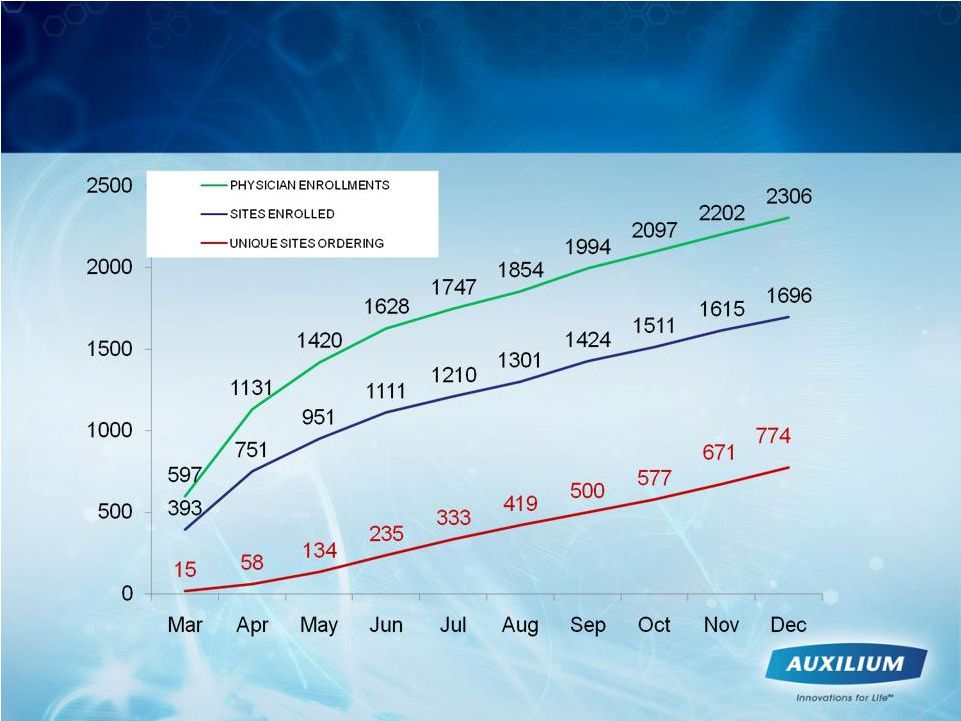 12
Enrollment and Sites that Have Ordered
XIAFLEX Are Trending Positively |
 We
Believe that Quarterly XIAFLEX Unit Sales Growth Increased Steadily in
2010 1Q10
2Q10
3Q10
4Q10
13 |
 14
Sites Are Increasing XIAFLEX Usage
Injections per Site |
 15
We Expect the Global Dupuytren’s Market to
Develop in 2011 and 2012
U.S.
•
XIAFLEX specific J-code J0775 effective Jan. 1
•
U.S. CPT code guidance expected for Jan. 2012
•
Increasing peer to peer dialogue programs for U.S.
physicians
•
Targeted U.S. patient education campaign planned
ROW
•
Pfizer launches XIAFLEX in EU for Dupuytren’s
following expected Q1 2011 approval |
 16
XIAFLEX Revenue Guidance for 2011
U.S. Net Revenues
$50 –
$60 million
Pfizer Royalties &
Contract Revenues
$5 –
$7 million
Total Revenues
$55 –
$67 million |
 Testim
®
1% Testosterone Gel
•
Indicated for testosterone replacement therapy in adult
males for hypogonadism
•
Launched in U.S. in 2003 and EU in 2006
•
>7 yrs of use with established safety and efficacy
>
~100M daily doses since launch
>
16 clinical studies involving approx. 1,800 patients
•
Applied once daily at 5-10mg
>
90% stay on starting dose of 5mg (one tube)
>
Simple application process
17 |
 18
Hypogonadism
Is an Unmet Need and
Under-penetrated Market
•
39% of U.S. males over 45 yrs are
hypogonadal
>Less than 10% of affected population
receives treatment
•
Increasing physician and patient education
and awareness should result in increased
treatment
1
Mulligan T. et al. Int J. Clin Pract
2006
1 |
 19
Gels Continue to Drive Significant Growth in
TRT Marketplace
Source: IMS data
$35
$117
$198
$281
$334
$371
$439
$545
$685
$892
$1,025
$49
$59
$77
$118
$210
$302
$390
$451
$483
$554
$663
$810
$1,041
0
200
400
600
800
1,000
1,200
1,400
1997
1998
1999
2000
2001
2002
2003
2004
2005
2006
2007
2008
2009
2010
Nov
Gel
Patch
Oral
Injectables
($ in millions)
Gel Segment Growth ($)
Nov 2010 L12M:
29.3%
($ in millions)
Gel Segment Growth ($)
Nov 2010 L12M:
29.3%
($ in millions)
Gel Segment Growth ($)
Nov 2010 L12M:
29.3%
($ in millions)
Gel Segment Growth ($)
Nov 2010 L12M:
29.3%
$1,185 |
 20
Testosterone Replacement Therapy Landscape
is Changing
•
New competition in 2011 should help
increase overall market
–
Axiron 2% solution
–
Fortesta 2% gel
–
Androgel 1.6% gel seeking approval
•
Expected further increase in physician and
patient education and awareness |
 21
Delivering on Current Pipeline
PRODUCT
LATE RESEARCH
PRE-CLINICAL PHASE I PHASE II PHASE
III MARKET
TESTIM®
GEL
XIAFLEX®
XIAFLEX®
XIAFLEX®
Hypogonadism
Dupuytren’s contracture
Peyronie’s disease
Frozen Shoulder Syndrome |
 22
1
Smith BH. Am J Clin
Pathol. 1966;45:670-678.
2
Somers KD, Dawson DM. J Urol. 1997;157:311-315.
•
Scarring phenomenon affecting the
tunica
albuginea
•
Plaques
show
excessive
collagen
deposition
•
Potential Symptoms
>
Pain with erection, penile curvature/
deviation, penile shortening, indentations,
and/or erectile dysfunction
>
May experience difficulty with sexual
intercourse, loss of self-esteem,
and depression
Peyronie’s
disease Is a Devastating Disorder
with No Approved Therapies
1
2 |
 23
1
Bella A. Peyronie’s Disease J Sex Med 2007;4:1527–1538
2
Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex
Med 2004;1:6–23. 3
Mulhall JP, et al. Subjective and objective analysis of the prevalence of
Peyronie’s disease in a population of men presenting for prostate
cancer screening. J Urol 2004;171:2350–3. 4
Smith BH. Am J Clin Pathol. 1966;45:670-678.
5
Lindsay MB, J Urol.
1991;146:1007-1009.
6
Nyberg L, J Urol.128: 48, 1982
•
Prevalence of Peyronie’s disease is estimated to be approximately
5% in adult men
1,2,3
>
Actual prevalence may be higher, based on autopsies
4
•
Prevalence rate increases with age
>
The average age of disease onset is 53 years
5
•
High association with other diseases such as:
>
Diabetes, erectile dysfunction (ED), Dupuytren’s contracture,
plantar fascial contracture, tympanosclerosis, gout, and Paget’s
disease
6
We believe Peyronie’s Disease Is Under-
diagnosed and Under-treated |
 24
Peyronie’s Disease Phase III Development
Program
Study
Type
~ #
Subjects
Sites
XIAFLEX:
Placebo
Duration
AUX-CC-803
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-804
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-802
Open Label
250
12 US
6 NZ
18 EU
N/A
36 Wks
AUX-CC-805
Pharmacokinetics
16
1 US
N/A
4 Wks
XIAFLEX 0.58 mg
Two injections per treatment cycle
24 to 72 hours between injections
Penile plaque modeling following each cycle
Up to 4 cycles at 6 week intervals
Co-primary endpoints of disease bother and penile curvature
|
 25
We Believe Changes to the Phase IIb Trial Design
Should Increase the Chance of Success in Phase III
•
4 cycles of therapy in the phase III trial
•
All patients receive modeling in the phase III trial
•
All patients must have greater than 12 months of
disease since diagnosis to enter the phase III trial
Mean net improvements in phase IIb penile
curvature and bother domain for modeling
arm would have met statistical significance
using phase III co-primary endpoint
requirements |
 26
Business Development Efforts Focused on
Building Out Pipeline
•
Leverage current infrastructure of Testim and
XIAFLEX field forces with marketed products
•
Seeking Phase II and later assets in Urology,
Endocrinology, Rheumatology, and Orthopedics
•
Niche products with specialty physician call
points also represent development opportunities |
 27
Q4 ’10 Growth
2010
Growth
($ Millions)
vs. 2009
Full Year vs. 2009
Testim
U.S.
$52.1
23.3%
$190.0 25.0% Testim
Ex U.S. & Contract
$1.3 (73.7%)
$3.0
(64.2%)
Testim
Total
$53.4
13.3%
$193.0 20.3% XIAFLEX
U.S. $7.3
-
$14.1
-
XIAFLEX Contract
$1.1
22.1%
$4.3
22.1%
XIAFLEX
Total
$8.4
845.0%
$18.4
416.7%
Total
Revenues
$61.8
28.7%
$211.4
28.9%
2010
Preliminary
Net
Revenue
Cash as of 9/30/2010 = $142.4 million |
 28
Strategic Priorities in 2011
•
Maximize XIAFLEX net revenues for Dupuytren’s contracture in
the U.S.
•
Support Pfizer in the launch of Dupuytren’s contracture in the
EU
•
Enroll Peyronie’s disease phase III clinical program
•
Projected double-blind study enrollment to be completed in 1Q11
•
Phase III studies top-line results anticipated in 1H12
•
Advance XIAFLEX new indication(s)
•
Maximize global Testim revenues |
 29
Our Strategy Is to Become a Highly Profitable
and Sustainable Biopharmaceutical Company
Maximize Value of
Testim and XIAFLEX
Deliver on
Current Pipeline
Build Out
Pipeline in
Specialty
Therapeutic
Areas |
