Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a58282e8vk.htm |
| EX-99.1 - EX-99.1 - Ardea Biosciences, Inc./DE | a58282exv99w1.htm |
Exhibit 99.2

| Ardea Biosciences Company Update January 6, 2011 |

| Safe Harbor Statement Statements contained in this presentation regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Ardea's goals, its preclinical and clinical trial plans, timelines and milestones, its expectations about the size of its markets and commercial potential of its compounds, expected results of future clinical trials, expected properties of compounds under development, financial position, cash usage, licensing and partnering opportunities, liquidity and anticipated milestones. Risks that contribute to the uncertain nature of the forward-looking statements include: risks related to the outcomes of preclinical and clinical trials, risks related to regulatory approvals, delays in commencement of preclinical and clinical tests, costs associated with internal development, and the outcome of our business development activities, including collaboration or licensing agreements. These and other risks and uncertainties are described more fully in Ardea's most recently filed SEC documents, including its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, under the headings "Risk Factors." All forward-looking statements contained in this presentation speak only as of the date of this presentation, and Ardea undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof or otherwise. 2 |
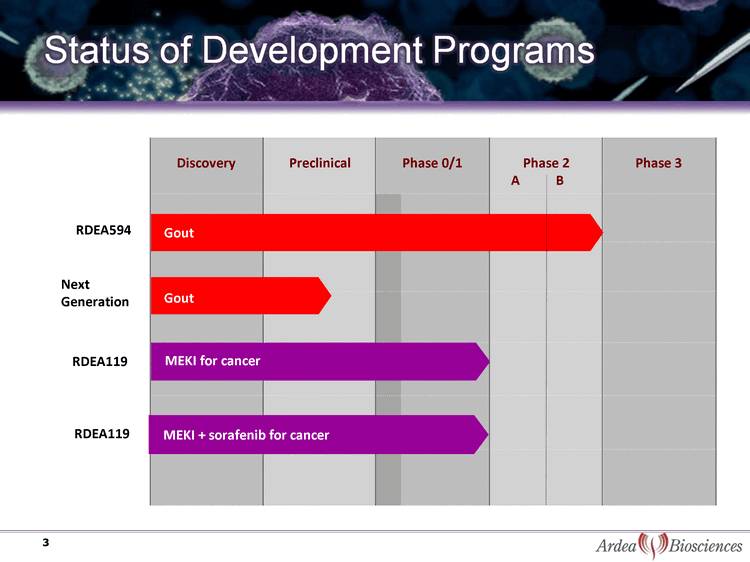
| Status of Development Programs Discovery Preclinical Phase 0/1 Phase 2 A B Phase 3 MEKI for cancer 3 Gout RDEA594 Next Generation MEKI + sorafenib for cancer RDEA119 RDEA119 Gout |
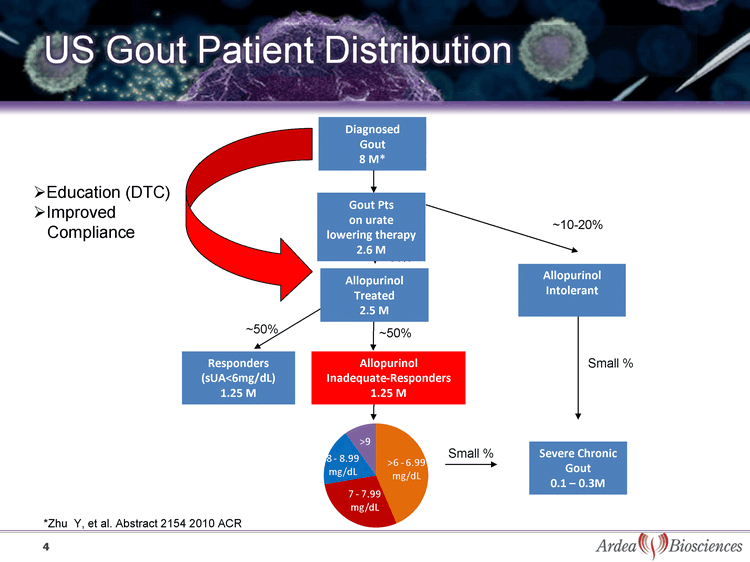
| US Gout Patient Distribution 4 Diagnosed Gout 8 M* ~90% ~50% ~50% Allopurinol Treated 2.5 M Responders (sUA<6mg/dL) 1.25 M Severe Chronic Gout 0.1 - 0.3M Allopurinol Inadequate-Responders 1.25 M ~10-20% Small % Allopurinol Intolerant Gout Pts on urate lowering therapy 2.6 M Small % Education (DTC) Improved Compliance *Zhu Y, et al. Abstract 2154 2010 ACR |

| Almost Half of Patients Under Treatment are Still Having Flares - Lower sUA Reduces Flares Physicians believe that approximately half of the 2.6M patients receiving treatment are still having flares* This is consistent with less than half of patients reaching target sUA on allopurinol sUA of inadequate-responders on allopurinol:* 5 5 Proportion of Subjects (%) Abstract 758: MA Becker, et al. ACR Boston November 2007 *Ardea Market Research |

| RDEA594 - Clinical Summary 6 Study 201 Phase 2a combination with allopurinol Combination with RDEA594 400 mg produced 100% response for sUA < 6 mg/dL Lower rate of abnormal laboratory values on RDEA594 than control Study 110 Phase 1b PK/PD combination with allopurinol Combination with RDEA594 600 mg produced 100% response for sUA < 6 mg/dL & < 5 mg/dL Study 105 Phase 1 POC combination with febuxostat RDEA594 400 mg plus febuxostat 40 mg achieved >70% reduction of sUA in healthy volunteers Study 111 Phase 1b combination with febuxostat All combinations with RDEA594 and febuxostat produced 100% response for sUA < 6 mg/dL with greater sUA reductions with increasing doses of either agent Study 202 Phase 2b monotherapy study Excellent reduction in uric acid with safety profile similar to placebo Study 203 Phase 2b RDEA594 and allopurinol combination study |

| Placebo RDEA594 Phase 2b Combination Study with Allopurinol in Allopurinol-Refractory Patients 2-4 weeks 4 weeks 2 weeks Population: 208 gout patients with serum urate (sUA) ^ 6 mg/dL while receiving a stable dose of allopurinol for at least 6 weeks Duration: 4-week double-blind treatment period with doses escalated weekly Endpoints: Primary: Mean reduction in sUA at Week 4 Key Secondary: proportion of subjects with sUA < 6.0 mg/dL at Week 4 Safety and tolerability of the combination versus allopurinol alone Colchicine Treatment (0.5 mg - 0.6 mg/day) Randomize if no gout flare during 2 weeks of colchicine 7 200 mg 200 mg Placebo 400 mg 200 mg 400 mg 600 mg Off drug Off drug Off drug Allopurinol Treatment (200 mg - 600 mg/day) Placebo Screening Period: Patients must have sUA > 6 mg/dL on stable dose of allopurinol |

| (CHART) Primary Endpoint - Mean Percent Change in Serum Urate at Week 4 p<0.0001 8 ITT Population p<0.0001 p<0.0001 Baseline sUA = 6.7 6.4 6.9 7.3 (mg/dL) P-values are for comparison to placebo group using an ANCOVA model |

| (CHART) Percent of Patients with Serum Urate < 6 mg/dL at Week 4 9 n = 72 46 42 48 P<0.0001 P<0.0001 P<0.0001 ITT Analysis* *Intent-to-treat analysis considers patients' without Week 4 results as failures, regardless of the reason for the missing data P-values are for comparison to allopurinol alone (placebo group) using a Fisher's exact test |

| (CHART) Percent of Patients with Serum Urate < 6 mg/dL at Last Visit 10 LOCF analysis used in febuxostat US package insert* n = 70 45 42 42 P<0.0001 P<0.0001 P<0.0001 *Last observation carried forward analysis uses a prior week's sUA results when Week 4 results are missing P-values are for comparison to allopurinol alone (placebo group) using a Fisher's exact test |

| Incidence of the Most Frequent Adverse Events* 11 Adverse Events Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + RDEA594 Dose Group Allopurinol + Adverse Events 200mg (N=46)n (%) 400mg (N=42) n (%) 600mg (N=48)n (%) Combined RDEA594(N=136)n (%) Pooled Placebo (N=72)n (%) Any Adverse Event 1 (2.2) 4 (9.5) 5 (10.4) 10 (7.4) 10 (13.9) Diarrhea 0 1 (2.4) 0 1 (0.7) 2 (2.8) Dyspepsia 0 0 1 (2.1) 1 (0.7) 1 (1.4) Dizziness 1 (2.2) 0 0 1 (0.7) 2 (2.8) Lipase Increased 0 1 (2.4) 0 1 (0.7) 1 (1.4) Hematuria 0 1 (2.4) 0 1 (0.7) 2 (2.8) *Adverse events reported by at least 2 subjects that were considered at least possibly related to drug Confidential |
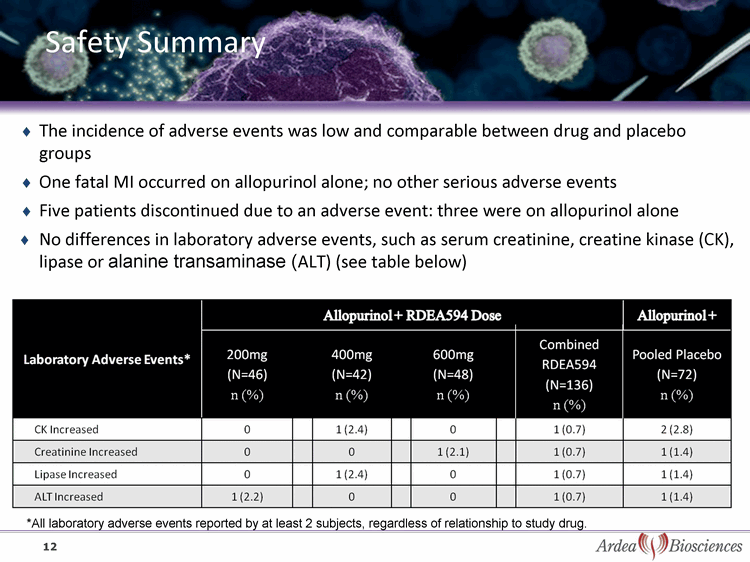
| Safety Summary 12 Laboratory Adverse Events* Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + RDEA594 Dose Allopurinol + Laboratory Adverse Events* 200mg (N=46)n (%) 400mg (N=42) n (%) 600mg (N=48)n (%) Combined RDEA594(N=136)n (%) Pooled Placebo (N=72)n (%) CK Increased 0 1 (2.4) 0 1 (0.7) 2 (2.8) Creatinine Increased 0 0 1 (2.1) 1 (0.7) 1 (1.4) Lipase Increased 0 1 (2.4) 0 1 (0.7) 1 (1.4) ALT Increased 1 (2.2) 0 0 1 (0.7) 1 (1.4) *All laboratory adverse events reported by at least 2 subjects, regardless of relationship to study drug. The incidence of adverse events was low and comparable between drug and placebo groups One fatal MI occurred on allopurinol alone; no other serious adverse events Five patients discontinued due to an adverse event: three were on allopurinol alone No differences in laboratory adverse events, such as serum creatinine, creatine kinase (CK), lipase or alanine transaminase (ALT) (see table below) |
| Study 203 Summary In patients not adequately responding to allopurinol, addition of RDEA594 produced statistically and clinically relevant reductions in sUA All three doses of RDEA594 produced reductions in sUA that were significantly superior to allopurinol alone (p<0.0001) All three doses of RDEA594 significantly increased the proportion of patients with sUA < 6 mg/dL (p<0.0001) With the LOCF analysis, as used in the package insert for febuxostat, 95% of patients responded to the combination of RDEA594 600 mg + allopurinol The combination of RDEA594 + allopurinol was well tolerated, with infrequent adverse events, no SAEs on the combination, and fewer discontinuations due to AEs on the combination than allopurinol alone 13 |
