Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
_____________________
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
______________________
| CURAXIS PHARMACEUTICAL CORPORATION |
| (Exact name of registrant as specified in its charter) |
|
Nevada
|
2834
|
26-1919261
|
||
|
(State or other jurisdiction of
|
(Primary Standard Industrial
|
(I.R.S. Employer Identification Number)
|
||
|
incorporation or organization)
|
|
Classification Code Number)
|
|
_______________________
4819 Emperor Blvd., Suite 400
Durham, NC 27703
(888) 919-2873
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices) ________________________
Business Filings Incorporated
6100 Neil Road, Suite 500
Reno, NV 89511
(608) 827-5300
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_________________________
Copies to:
Anslow & Jaclin, LLP
195 Route 9 South, 2nd Floor
Manalapan, NJ 07726
(732) 409-1212
__________________________
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If delivery of the prospectus is expected to be made pursuant to Rule 434, please check the following box. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
¨
|
Accelerated filer
|
¨
|
|
Non-accelerated filer
|
¨
|
Smaller reporting company
|
þ
|
CALCULATION OF REGISTRATION FEE
|
Title of Each Class Of Securities to be Registered
|
Amount to be
Registered (1)
|
Proposed
Maximum
Aggregate
Offering Price
per share
|
Proposed
Maximum
Aggregate
Offering Price
|
Amount of
Registration fee
|
||||||||||||
|
Common Stock, $0.0001 par value per share, issuable pursuant to the Equity Credit Agreement
|
14,500,000
|
$
|
$0.74
|
(2)
|
$
|
10,730,000
|
(3)
|
$
|
$765.05
|
|||||||
|
(1)
|
We are registering 14,500,000 shares of our common stock (the “Put Shares”) that we will put to Southridge Partners II, LP (“Southridge” or “Selling Security Holder”) pursuant to a private equity credit agreement (the “Equity Credit Agreement”) between Selling Security Holder and the registrant entered into on September 16, 2010 and subsequently amended on December 6, 2010. In the event of stock splits, stock dividends, or similar transactions involving the common stock, the number of common shares registered shall, unless otherwise expressly provided, automatically be deemed to cover the additional securities to be offered or issued pursuant to Rule 416 promulgated under the Securities Act of 1933, as amended (the “Securities Act”). In the event that adjustment provisions of the Equity Credit Agreement require the registrant to issue more shares than are being registered in this registration statement, for reasons other than those stated in Rule 416 of the Securities Act, the registrant will file a new registration statement to register those additional shares.
|
|
(2)
|
The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457(c) of the Securities Act on the basis of the closing bid price of common stock of the registrant as reported on the Over-the-Counter BB (the “OTCBB”) on December 7, 2010.
|
|
(3)
|
This amount represents the maximum aggregate value of common stock, which may be put to Southridge by the registrant pursuant to the terms and conditions of the Equity Credit Agreement between Southridge and the registrant.
|
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(a) OF THE SECURITIES ACT OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE COMMISSION, ACTING PURSUANT TO SUCH SECTION 8(a), MAY DETERMINE.
PRELIMINARY PROSPECTUS, SUBJECT TO COMPLETION DATED DECEMBER 8, 2010
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission (“SEC”) is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
PROSPECTUS
CURAXIS PHARMACEUTICAL CORPORATION
14,500,000 Shares of Common Stock
This prospectus relates to the resale of up to 14,500,000 shares of our common stock, par value $0.0001 per share, by Southridge Partners II, LP (“Southridge” or “Selling Security Holder”), which are Put Shares that we will put to Southridge pursuant to the Equity Credit Agreement.
The Equity Credit Agreement with Southridge provides that Southridge is committed to purchase up to $25 million of our common stock. We may draw on the facility from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Equity Credit Agreement.
Southridge is an “underwriter” within the meaning of the Securities Act in connection with the resale of our common stock under the Equity Credit Agreement. No other underwriter or person has been engaged to facilitate the sale of shares of our common stock in this offering. This offering will terminate thirty-six (36) months after the registration statement to which this prospectus is made a part is declared effective by the SEC. Southridge will pay us 95% of the average of the lowest closing bid price of our common stock reported by Bloomberg, LP in any three trading days, consecutive or inconsecutive, of the five consecutive trading day period commencing the date a put notice is delivered.
We will not receive any proceeds from the sale of these shares of common stock offered by Selling Security Holder. However, we will receive proceeds from the sale of our Put Shares under the Equity Credit Agreement. The proceeds will be used for working capital or general corporate purposes. We will bear all costs associated with this registration.
Our common stock is quoted on the OTC Bulletin Board under the symbol “CURX.OB.” The shares of our common stock registered hereunder are being offered for sale by Selling Security Holder at prices established on the OTC Bulletin Board during the term of this offering. On December 7, 2010, the closing bid price of our common stock was $0.74 per share. These prices will fluctuate based on the demand for our common stock.
This investment involves a high degree of risk. You should purchase shares only if you can afford a complete loss. See “Risk Factors” beginning on page 5.
Neither the SEC nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is________, 2010
|
TABLE OF CONTENTS
|
Page
|
|
PROSPECTUS SUMMARY
|
1 |
|
RISK FACTORS
|
5 |
|
FORWARD LOOKING STATEMENTS
|
25 |
|
USE OF PROCEEDS
|
25 |
|
DIVIDEND POLICY
|
25 |
|
MARKET FOR OUR COMMON STOCK
|
25 |
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION
|
27 |
|
BUSINESS
|
41 |
|
MANAGEMENT
|
64 |
|
BENEFICIAL OWNERSHIP OF CURAXIS SHARES
|
71 |
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
73 |
|
DESCRIPTION OF SECURITIES
|
73 |
|
SELLING SECURITY HOLDER
|
74 |
|
PLAN OF DISTRIBUTION
|
76 |
|
LEGAL MATTERS
|
78 |
|
EXPERTS
|
78 |
|
AVAILABLE INFORMATION
|
78 |
|
INTERIM CONDENSED FINANCIAL STATEMENTS
|
F-1 |
| AUDITED FINANCIAL STATEMENTS | F-15 |
You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with information different from that which is contained in this prospectus. This prospectus may be used only where it is legal to sell these securities. The information in this prospectus may only be accurate on the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of securities. This prospectus contains important information about us that you should read and consider carefully before you decide whether to invest in our common stock. If you have any questions regarding the information in this prospectus, please contact Patrick S. Smith, our President and Chief Executive Officer, at: Curaxis Pharmaceutical Corporation, 4819 Emperor Blvd., Suite 400, Durham, NC 27703 or by phone at (888) 919-2873.
All dealers that effect transactions in these securities whether or not participating in this offering may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as underwriters.
i
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all the information that a person should consider before investing in the Company’s securities. A potential investor should carefully read the entire prospectus, including “Risk Factors” and the Consolidated Financial Statements, before making an investment decision.
In this prospectus, “CURX,” “Curaxis,” the “Company,” “our Company,” “we,” “us” and “our” refers to Curaxis Pharmaceutical Corporation.
The Company
We are an emerging specialty pharmaceutical company with a hormone drug product candidate for the treatment of Alzheimer’s disease and multiple cancers. Our therapeutic platform is based on the hypothesis that many diseases of aging may be caused by age-related changes in the function of the hypothalamic-pituitary-gonadal (HPG) axis. The HPG axis is a hormonal endocrine feedback loop that controls development, reproduction and aging in animals. This drug development platform is built on the premise that hormones associated with this feedback loop are beneficial early in life, when they promote growth and development, but are harmful later in life when the mechanism for feedback is compromised, thereby leading to disease processes, including pathologies associated with Alzheimer’s disease and various cancers. We believe our discovery of similar hormonal signaling mechanisms at the cellular level in brain tissue from Alzheimer’s patients and in multiple tumors will enable us to develop significant new treatments for Alzheimer’s disease as well as many cancers.
Corporate History
We were incorporated in Nevada on February 1, 2008 under the name Auto Search Cars, Inc. We engaged in the development of a web-based e-commerce site platform to provide information for the sale of vehicles and vehicle financing and warranties. By providing such platform, we believed that we could be a price leader in the online vehicle sales and service market and bring vehicle sellers and other industry participants (such as vendors of automotive services and advertisers) together with consumers actively engaged in a search for a vehicle or vehicle-related products. Despite offering free listings on our website as an incentive for consumers to use our product, there had been no significant activities related to our website development. Our executive offices were located at 164 Eleven Levels Road, Ridgefield, Connecticut 06877.
On February 8, 2010, we entered into a merger agreement (as amended on July 29, 2010, the “Merger Agreement”) by and among Auto Search Cars, Inc., Auto Search Cars Acquisition Corp., a Delaware Corporation (“Acquisition Corp.”), and Curaxis Pharmaceutical Corporation, a corporation incorporated in Delaware on February 27, 2001 (“Curaxis”) (the “Merger”).
On July 29, 2010 (the “Closing Date”), pursuant to the Merger Agreement, (i) the common stock of Curaxis (the “Curaxis Common Stock”) became no longer outstanding and existing, and was cancelled and retired, and (ii) each holder of Curaxis Common Stock ceased to have any rights with respect to their shares of Curaxis Common Stock, except the right to receive shares of our common stock. Each holder of Curaxis Common Stock received one (1) share of Auto Search common stock for each share of Curaxis Common Stock they own immediately prior to completion of the Merger. Each share of the Auto Search Common Stock issued to the Curaxis stockholders pursuant to the Merger Agreement was restricted from trading or resale for a period of one (1) year commencing at the effective date of the Merger. Auto Search stockholders continued to own their existing shares, which were not affected by the Merger.
On the Closing Date, Curaxis and Acquisition Corp. merged with and into one another, with the surviving corporation being Curaxis. Simultaneously with the filing of a Certificate of Merger with the State of Delaware, Curaxis amended its Certificate of Incorporation in order to change its name to Curaxis Pharma Corp. On the Closing Date, Curaxis Pharma Corp. became our subsidiary.
1
On July 30, 2010, we entered into an agreement and plan of merger with Curaxis Pharmaceutical Corporation, a Nevada corporation formed solely for the purpose of a name change (the “Short-Form Merger”). Pursuant to the Short-Form Merger, we changed our name to Curaxis Pharmaceutical Corporation.
Our principal business offices are located at 4819 Emperor Blvd., Suite 400, Durham, NC 27703, and our telephone number at that address is (888) 919-2873.
The Offering
|
Common stock offered by Selling Security Holder
|
14,500,000 shares of common stock.
|
|
Common stock outstanding before the offering
|
72,216,489 as of November 30, 2010
|
|
Common stock outstanding after the offering
|
86,716,489 shares.
|
|
Terms of the Offering
|
Selling Security Holder will determine when and how it will sell the common stock offered in this prospectus.
|
|
Termination of the Offering
|
Pursuant to the Equity Credit Agreement, this offering will terminate thirty six (36) months after the registration statement to which this prospectus is made a part is declared effective by the SEC.
|
|
Use of proceeds
|
We will not receive any proceeds from the sale of the shares of common stock offered by Selling Security Holder. However, we will receive proceeds from sale of our common stock under the Equity Credit Agreement. The proceeds from the offering will be used for the clinical development of our drug candidate, Memryte, for the treatment of mild to moderate Alzheimer’s disease and general corporate purposes. See “Use of Proceeds.”
|
|
Risk Factors
|
The common stock offered hereby involves a high degree of risk and should not be purchased by investors who cannot afford the loss of their entire investment. See “Risk Factors” beginning on page 5.
|
|
OTC Bulletin Board Symbol
|
CURX.OB
|
This offering relates to the resale of up to 14,500,000 shares of our common stock, par value $0.0001 per share, by Selling Security Holder, which are the Put Shares that we will put to Southridge pursuant to the Equity Credit Agreement. Assuming the resale of all of the shares being registered in this Registration Statement, such shares would constitute approximately 17% of our outstanding common stock.
On September 16, 2010, our Company and Southridge entered into the Equity Credit Agreement pursuant to which we have the opportunity, for a three -year period, commencing on the date on which the SEC first declares effective this registration statement, to which this prospectus is made a part registering the resale of our common shares by Southridge, to resell shares of our common stock purchased under the Equity Credit Agreement. For each share of our common stock purchased under the Equity Credit Agreement, Southridge will pay 95% of the average of the three lowest closing bid price (the “Bid Price”) of any three applicable trading days, consecutive or inconsecutive, during the five (5) trading day period (the “Valuation Period”), commencing on the date a put notice (the “Put Notice”) is delivered to Southridge (the “Put Date”) in the manner prescribed in the Equity Credit Agreement. Subject to the limitations and floor price reductions outlined below, we may, at our sole discretion, issue a Put Notice to Southridge, after which Southridge will be irrevocably bound to acquire such shares. The Company and Southridge subsequently amended certain terms of the Equity Credit Agreement on December 6, 2010.
2
We must specify a floor price in each Put Notice, which shall not be less than sixty percent (60%) of the average of the closing Bid Prices for the three (3) trading days ending immediately prior to the Put Date (the “Floor Price”). In the event that during a Valuation Period for any Put Notice, the closing Bid Price on any trading day falls below the Floor Price specified in such Put Notice, then for each such trading day we shall be under no obligation to sell and Selling Security Holder shall be under no obligation to purchase one fifth (1/5th) of the amount specified in the Put Notice, and the Investment Amount shall accordingly be deemed reduced by such amount. In the event that during a Valuation Period the closing Bid Price falls below the Floor Price for any three (3) trading days - not necessarily consecutive – then the balance of each party’s rights and obligations to purchase and sell the Investment Amount under such Put Notice shall terminate on such third trading day (“Put Termination Day”), and the Investment Amount shall be adjusted to include only one-fifth (1/5) of the initial Investment Amount for each trading day during the Valuation Period prior to the Put Termination Day that the closing Bid Price equals or exceeds the Floor Price.
Furthermore, subject to the terms and conditions of the Equity Credit Agreement, at any time or from time to time after the effectiveness of this registration statement, we can notify Southridge in writing of the existence of a potential material event based upon the good faith determination of our board of directors (the “Blackout Notice”), and Southridge shall not offer or sell any of our securities acquired under the Equity Credit Agreement from the time the Blackout Notice was provided to Southridge until Southridge receives our written notice that such potential material event has either been disclosed to the public or no longer constitutes a potential material event. If we deliver a Blackout Notice within fifteen trading days, commencing the sixth day following a Put Date (the “Closing Date”), and the Bid Price immediately preceding the applicable Blackout Period (the “Old Bid Price”) is greater than the Bid Price on the first trading day immediately following such Blackout Period (the “New Bid Price”), then we are obligated to issue to Southridge a number of additional common shares (the “Blackout Shares”) equal to the difference between (i) the product of (X) the shares that were issued to the Southridge on the most recent Closing Date and held by the Southridge immediately prior to the Blackout Period (the “Remaining Put Shares”), multiplied by (Y) the Old Bid Price, and divided by (Z) the New Bid Price, and (ii) the Remaining Put Shares.
We are relying on an exemption from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), and/or Rule 506 of Regulation D promulgated thereunder. The transaction does involve a private offering, Southridge is an “accredited investor” and/or qualified institutional buyer and Southridge has access to information about our Company and its investment.
At the assumed offering price of $.74 per share, we will be able to receive up to $10,193,500 in gross proceeds, assuming the sale of the entire 14,500,000 shares of our common stock being registered hereunder pursuant to the Equity Credit Agreement. We would be required to register additional shares to obtain the balance of $25,000,000 under the Equity Credit Agreement if the market price of the stock remains stable or falls below the assumed offering price of $.74. Neither the Equity Credit Agreement nor any rights or obligations of the parties under the Equity Credit Agreement may be assigned by either party to any other person.
There are substantial risks to investors as a result of the issuance of shares of our common stock under the Equity Credit Agreement. These risks include dilution of stockholders, significant decline in our stock price and our inability to draw sufficient funds when needed.
Southridge will periodically purchase our common stock under the Equity Credit Agreement and will, in turn, sell such shares to investors in the market at the market price. This may cause our stock price to decline, which will require us to issue increasing numbers of common shares to Southridge to raise the same amount of funds, as our stock price declines.
3
SUMMARY FINANCIAL AND OPERATING INFORMATION
The following selected financial information is derived from our Financial Statements appearing elsewhere in this prospectus and should be read in conjunction with the Company’s Financial Statements, including the notes thereto, appearing elsewhere in this prospectus. (Except as noted, all numbers are reported in thousands)
Summary of Operations
For the Nine Months Ended September 30,
|
2010
|
2009
|
|||||||
|
Total Revenue
|
$
|
0
|
$
|
0
|
||||
|
Loss from operations
|
$
|
(1,555
|
)
|
$
|
(525
|
)
|
||
|
Other expense
|
$
|
(133
|
)
|
$
|
(299
|
)
|
||
|
Gain on debt restructuring
|
$
|
204
|
$
|
6,562
|
||||
|
Interest from derivative conversion feature
|
$
|
(2,586
|
)
|
$
|
0
|
|||
|
Change in fair value of derivatives
|
$
|
429
|
$
|
0
|
||||
|
Net Income (loss)
|
$
|
(3,641
|
)
|
$
|
5,738
|
|||
|
Net income (loss) per common share (basic)
|
$
|
(0.06
|
)
|
$
|
0.10
|
|||
|
Net income (loss) per common share (diluted)
|
$
|
(0.06
|
)
|
0.10
|
||||
|
Weighted average common shares outstanding (basic)
|
65,690
|
56,134
|
||||||
|
Weighted average common shares outstanding (diluted)
|
65,690
|
59,016
|
||||||
For the Years Ended December 31,
|
2009
|
2008
|
|||||||
|
Total Revenue
|
$
|
0
|
$
|
0
|
||||
|
Loss from operations
|
$
|
(1,209
|
)
|
$
|
(1,508
|
)
|
||
|
Other expense
|
$
|
(366
|
)
|
$
|
(478
|
)
|
||
|
Gain on debt restructuring
|
$
|
6,562
|
$
|
0
|
||||
|
Net income (loss)
|
$
|
4,987
|
$
|
(1,986
|
)
|
|||
|
Net income (loss) per common share (basic)
|
$
|
0.09
|
$
|
(0.04
|
)
|
|||
|
Net income (loss) per common share (diluted)
|
$
|
0.08
|
$
|
(0.04
|
)
|
|||
|
Weighted average common shares outstanding (basic)
|
57,699
|
54,070
|
||||||
|
Weighted average common shares outstanding (diluted)
|
60,701
|
54,070
|
||||||
For the Cumulative period from February 27, 2001 (Inception) to:
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Total Revenue
|
$
|
0
|
$
|
0
|
||||
|
Loss from operations
|
$
|
(94,750
|
)
|
$
|
(93,195
|
)
|
||
|
Other expense
|
$
|
(1,050
|
)
|
$
|
(917
|
)
|
||
|
Gain on debt restructuring
|
$
|
6,766
|
$
|
6,562
|
||||
|
Interest from derivative conversion feature
|
$
|
(2,586
|
)
|
$
|
0
|
|||
|
Change in fair value of derivatives
|
$
|
429
|
$
|
0
|
||||
|
Net loss
|
$
|
(91,191
|
)
|
$
|
(87,550
|
)
|
||
Statement of Financial Position
|
September 30,
2010
|
December 31,
2009
|
|||||||
|
Cash and cash equivalents
|
$
|
273
|
$
|
631
|
||||
|
Total assets
|
$
|
1,195
|
$
|
781
|
||||
|
Total Liabilities
|
$
|
13,193
|
$
|
10,371
|
||||
|
Stockholders’ equity ( deficit )
|
$
|
(11,998
|
)
|
$
|
(9,590)
|
|||
4
RISK FACTORS
The shares of our common stock being offered for resale by Selling Security Holder are highly speculative in nature, involve a high degree of risk and should be purchased only by persons who can afford to lose the entire amount invested therein. Before purchasing any of these securities, you should carefully consider the following factors relating to our business and prospects. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, the trading price of our common stock could decline, and you may lose all or part of your investment.
RISKS RELATED TO OUR BUSINESS
We expect to incur losses at least for the foreseeable future and may never achieve or maintain profitability.
We expect to incur operating losses at least for the foreseeable future. If we are unable to raise the funds necessary to pay our expenses, including expenses accumulated in our clinical trial activities to date, we may be unable to continue operations.
To become and remain profitable, we must succeed in developing and commercializing drugs with significant market potential. This will require us to be successful in a range of challenging activities for which we are only in the preliminary stages: developing drugs, obtaining regulatory approval for them, and manufacturing, marketing and selling them. We may never succeed in these activities and may never generate revenues that are significant or large enough to achieve profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable could impair our ability to raise capital, expand our business, diversify our product offerings or continue out operations.
Due to a lack of financial resources, we have been unable to advance the clinical development of our lead product candidate since 2006.
Our lead product candidate is a hormone for the treatment of Alzheimer’s disease. We have previously conducted several clinical trials for this indication, the most recent of which we were forced to terminate in 2006 due to financial constraints. Since terminating those trials, we have been unable to advance the clinical development of our Alzheimer’s disease candidate due to a lack of financial resources. We anticipate that we will be able to raise the funds necessary to restart the clinical development of our Alzheimer’s disease candidate, but there can be no assurance that we will be able to do so. Furthermore, even if we are successful in raising the funds necessary to restart the clinical development of our Alzheimer’s disease candidate, there can be no assurance that we will be able to successfully complete all phases of such clinical development. Drug development is extremely costly and complex and requires multiple clinical trials and we may be unable to obtain suitable financing for all such trials and, even if we are successful in obtaining suitable financing, we may be unable to complete all required clinical trials due to an inability to recruit an adequate number of clinical trial sites or an adequate number of patients to participate in those trials or other reasons.
We will need substantial additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our research and development expenses to increase in connection with our ongoing activities, particularly as we conduct clinical trials for our candidate for the treatment of mild to moderate Alzheimer’s disease. In addition, subject to regulatory approval of any of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, securing commercial quantities of product from our manufacturers and distribution. We will need substantial additional funding to complete clinical trials and fund initial commercialization costs of our therapeutic candidates and to advance our earlier stage preclinical programs, and may be unable to raise capital when needed or on attractive terms, which would force us to delay, reduce or eliminate our research and development programs or commercialization efforts. Our management believes that it has sufficient funds to continue operations through December 31, 2010.
5
Our future capital requirements will depend on many factors, including:
|
·
|
the progress and results of clinical trials of our lead candidate, VP4896, for mild to moderate Alzheimer’s disease;
|
|
·
|
the costs of establishing sales and marketing functions, outsourcing manufacturing needs and obtaining pre-launch inventory of VP4896;
|
|
·
|
the scope, progress, results and cost of pre-clinical development and laboratory testing and clinical trials for our other product candidates;
|
|
·
|
the costs, timing and outcome of regulatory review of VP4896 for mild to moderate Alzheimer’s disease and of our other product candidates;
|
|
·
|
the number and development requirements of other product candidates;
|
|
·
|
the costs of preparing, filing and prosecuting patent applications and maintaining, enforcing and defending intellectual property-related claims;
|
|
·
|
our success in entering into collaboration agreements with leading pharmaceutical and biotechnology companies to assist in furthering the development of product candidates; and
|
|
·
|
the timing, receipt and amount of sales or royalties, if any, from VP4896 and other potential products.
|
Until such time, if ever, when we can generate substantial product revenues, we expect to finance our cash needs through public or private equity offerings, debt financings and possibly corporate collaboration and licensing arrangements. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to our Company or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
We are still in the initial stages of our business plan, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our operations have been limited to organizing and staffing our Company, acquiring, developing and securing our technology and undertaking pre-clinical studies and limited clinical trials of our most advanced product candidate, VP4896. We have not yet demonstrated our ability to successfully complete large-scale, pivotal clinical trials, obtain regulatory approval, manufacture a commercial scale product or arrange for a third party to do so on our behalf or conduct sales and marketing activities necessary for successful product commercialization.
In addition, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from our research focus to supporting commercial activities, and we may not be successful in such transition.
6
We depend heavily on the success of our most advanced product candidate, VP4896 for mild to moderate Alzheimer’s disease, which is still in clinical development. If we are unable to commercialize VP4896 for this indication, or experiences significant delays in doing so, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources in the development of our most advanced product candidate, VP4896, for mild to moderate Alzheimer’s disease. The success of our VP4896 Alzheimer’s disease program will depend on several factors, including the following:
|
·
|
successful patient enrollment in, and completion of, clinical trials;
|
|
·
|
receipt of marketing approvals from the FDA and similar foreign regulatory authorities;
|
|
·
|
obtaining commercial quantities of leuprolide acetate, the active ingredient of VP4896;
|
|
·
|
obtaining commercial quantities of VP4896 from our supplier, Durect Corporation;
|
|
·
|
establishing a sales and marketing infrastructure to market and sell VP4896 in the United States and internationally, whether alone or in collaboration with others; and
|
|
·
|
acceptance of the product by patients, the medical community and third party payors.
|
Our ability to generate product revenues, which we do not expect will occur for at least the next several years, if ever, will depend heavily on the successful development and commercialization of VP4896. If we are unable to commercialize VP4896 for mild to moderate Alzheimer’s disease, or experience significant delays in doing so, our business will be materially harmed.
If we are unable to achieve statistical significance on the primary efficacy endpoints in clinical trials of VP4896 for the treatment of mild to moderate Alzheimer’s disease, we may not be successful in obtaining FDA approval for VP4896, which would materially harm our business.
In ALADDIN I, our Phase II clinical trial for women with mild to moderate Alzheimer’s disease involving an injectable formulation of leuprolide acetate, we identified a trend in favor of the high dose leuprolide acetate group versus placebo, but we did not achieve statistical significance on the primary efficacy endpoints or any of the secondary efficacy endpoints in this clinical trial. The primary efficacy endpoints of the trial were a patient’s score on each of the ADAS-cog, a test of memory and cognition, and the ADCS-CGIC, a global measure of a subject’s change in condition, at 48 weeks compared to baseline. There were various secondary efficacy endpoints, including a patient’s score on the ADCS-ADL, a measurement of a patient’s capacity to perform activities of daily living, at 48 weeks compared to baseline. Analysis of the same primary efficacy endpoints at the completion of the 48-week men’s Phase II study, ALADDIN II, also did not demonstrate statistical significance.
We performed a preplanned subgroup analysis of 78 women out of the 108 women who participated in ALADDIN I, the Phase II clinical trial in women. Of this subgroup of 78 women, which included all of the patients who were taking an acetylcholinesterase inhibitor (AChEI) and excluded the 30 patients who were not taking an AChEI, 28 patients were taking an AChEI plus 11.25 mg of leuprolide acetate, 24 patients were taking an AChEI plus 22.5 mg of leuprolide acetate and 26 patients were taking an AChEI plus placebo. The results for the group of 28 patients that received an AChEI plus the 11.25 mg dose of leuprolide acetate were not statistically significantly different from the results for the group that received an AChEI plus placebo. The results for the group of 24 patients that received an AChEI plus the 22.5 mg of leuprolide acetate compared to the group of 26 patients that received an AChEI plus placebo were statistically significant with respect to the patients’ scores on both primary endpoints, the ADAS-cog and ADCS-CGIC as well as on the ADCS-ADL, a secondary endpoint, prior to applying any statistical adjustment. However, because we performed several comparisons, we were required to adjust the p-values (a mathematical calculation used to determine the likelihood that the measured result was obtained by chance) of the results to account for multiple comparisons. This was done by using what is referred to as the Bonferroni correction, which applies an estimated statistical penalty to account for the number of comparisons made. Specifically, the unadjusted p-values for this subgroup were 0.026 for the ADAS-cog, 0.031 for the ADCS-CGIC and 0.015 for ADCS-ADL. The adjusted p-values for this subgroup were 0.078 for the ADAS-cog, 0.093 for the ADCS-CGIC and 0.044 for ADCS-ADL. Therefore, after applying the Bonferroni correction, we were only able to demonstrate statistical significance with respect to the ADCS-ADL.
7
We likewise performed an analysis of 90 men out of 119 men who participated in ALADDIN II, the Phase II clinical trial in men. Of this subgroup of 90 men, which included all of the patients who were taking an AChEI and excluded the 29 patients who were not taking an AChEI, 27 patients were taking an AChEI plus a 22.5 mg dose of leuprolide acetate, 34 patients were taking an AChEI plus a 33.75 mg dose of leuprolide acetate and 29 patients were taking an AChEI plus placebo. The results for both leuprolide-treated groups were not significantly different from the results for the group that received an AChEI plus placebo. The treated groups of 27 and 34 patients given 22.5 mg and 33.75 mg of leuprolide acetate, respectively, demonstrated a slight positive signal on the ADAS-cog assessment compared to placebo at 48 weeks. Results on the ADCS-CGIC rating demonstrated that 44% of the men who received 22.5 mg of leuprolide acetate plus AChEIs, and 47% of the men who received 33.75 mg of leuprolide acetate plus AChEIs, were either improved or showed no change compared to 31% of the men who received placebo plus AChEIs.
In addition, we used a commercially available formulation of leuprolide acetate administered by injection in its Phase II trials for the treatment of mild to moderate Alzheimer’s disease, while our planned clinical trials will test VP4896, a biodegradable polymeric implant formulation of leuprolide acetate, for this indication. Leuprolide acetate administered through an implant may not have the same effect as leuprolide acetate injections had in our earlier Phase II clinical trials and may cause side effects not seen in those Phase II clinical trials. ALADDIN 105 was a Phase I trial performed by us to test the safety and tolerability of the VP4896 implant and the implantation procedure as well as the pharmacokinetics (absorption, distribution, metabolism, and elimination), of leuprolide delivery from the implant. The results of that study suggest that the VP4896 implant is safe and well tolerated. ALADDIN 105 also demonstrated that the VP4896 implant releases leuprolide acetate in a steady and sustained way over the 8-week dosing period. In addition, our planned clinical trials may be at dosing levels that are higher than the doses of leuprolide acetate used in ALADDIN I and ALADDIN II.
The differences in dosing levels and methods of administration, as well as other factors, may have unexpected effects on the outcome of our clinical trials. If we are unable to achieve statistical significance on the primary efficacy endpoints in pivotal Phase III clinical trials, we may not be successful in obtaining FDA approval for VP4896, which would materially harm our business.
Our program for VP4896 is based on the LH Hypothesis, which is not viewed as the predominant hypothesis regarding the possible causes of Alzheimer’s disease. This adds to the risk of our development effort and may affect physician and patient acceptance and use of VP4896 and the willingness of third parties to provide reimbursement.
Our program for VP4896 is based on the LH hypothesis, which is not the predominant view regarding the possible causes of Alzheimer’s disease. The LH hypothesis proposes that the known neurological and biochemical changes associated with Alzheimer’s disease are caused by elevated levels of the pituitary hormone, leuteinizing hormone (LH). This hypothesis suggests that when LH levels increase as the body ages, the neurological changes seen in Alzheimer’s disease result. The beta amyloid hypothesis, however, proposes that amyloid beta protein, which makes up the plaques present in the brains of Alzheimer’s disease patients, is toxic, and is the causative agent of the disease. Under this hypothesis, inhibiting the production of, and enhancing the clearance of, amyloid beta protein plaques may prevent or treat Alzheimer’s disease. The beta amyloid hypothesis is the predominant view. While preclinical data now support the notion that the LH hypothesis may be interrelated with the amyloid hypothesis and other hypotheses of Alzheimer’s disease pathology, the unproven nature of the LH hypothesis adds to the risk that our development effort may not be successful. In particular, no drugs for the treatment of Alzheimer’s disease have been successfully developed based on the LH hypothesis. Moreover, the predominant status of the beta amyloid hypothesis may impede our development and commercialization efforts. For example, the predominant status of the beta amyloid hypothesis in the medical community may adversely affect our ability to recruit patients for our clinical trials or, if we successfully complete clinical development of this product candidate and obtain regulatory approval, may adversely affect our ability to recruit sales and marketing personnel, may affect physician and patient acceptance and use of the product and the willingness of third parties to provide reimbursement, any of which could materially harm our business.
8
If Curaxis’ preclinical studies do not produce successful results or its clinical trials do not demonstrate safety and efficacy in humans, Curaxis will not be able to commercialize its product candidates.
Before obtaining regulatory approval for the sale of its product candidates, Curaxis must conduct, at its own expense, extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of its product candidates. Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. Specifically, the results of Curaxis’ Phase II trials were collected from a limited number of subjects and may not be indicative of results that it will obtain in later studies which are expected to be of greater size and scope.
A failure of one or more of our clinical trials can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize VP4896 or our other product candidates, including:
|
·
|
regulatory authorities, institutional review boards, or ethics committees may not authorize us to commence a clinical trial or conduct a clinical trial at a prospective trial site;
|
|
·
|
our clinical trials may produce negative or inconclusive results, and we may decide, or regulatory authorities may require us, to conduct additional clinical trials or we may abandon projects that we expect to be promising;
|
|
·
|
enrollment in our clinical trials may be slower than we currently anticipate, resulting in significant delays. Additionally, participants may drop out of our clinical trials at a higher rate than we anticipate;
|
|
·
|
our third party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner;
|
|
·
|
we might have to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks;
|
|
·
|
regulatory authorities, institutional review boards, or ethics committees may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements;
|
|
·
|
the cost of our clinical trials may be greater than we anticipate;
|
|
·
|
the supply or quality of our product candidates or other materials necessary to conduct our clinical trials may be insufficient or inadequate; and
|
|
·
|
the effects of our product candidates may not achieve the desired effects or may include undesirable side effects or the product candidates may have other unexpected characteristics.
|
If we are required to conduct additional clinical trials or other testing of VP4896 or our other product candidates beyond those that we currently contemplate, if we are unable to successfully complete our clinical trials or other testing, or if the results of these trials or tests are not positive or are only modestly positive, we may:
|
·
|
be delayed in obtaining marketing approval for our product candidates;
|
9
|
·
|
not be able to obtain marketing approval; or
|
|
·
|
obtain approval for indications that are not as broad as we intended.
|
Our product development costs will also increase if we experience delays in testing or approvals. We do not know whether any clinical trials will begin as planned, need to be restructured or will be completed on schedule, if at all. Significant clinical trial delays could also shorten the patent protection period during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to commercialize our products or product candidates.
Use of third parties to manufacture our product candidates, including VP4896, may increase the risk that we will not have sufficient quantities of our product candidates or such quantities at an acceptable cost, and clinical development and commercialization of our product candidates could be delayed, prevented or impaired.
We do not own or operate manufacturing facilities for clinical or commercial production of our product candidates. We have limited personnel with experience in drug manufacturing and we lack the resources and the capabilities to manufacture any of our product candidates on a clinical or commercial scale. Our strategy is to outsource all manufacturing of our product candidates and products, including VP4896, to third parties.
Reliance on third party manufacturers entails risks to which we would not be subject if we manufactured product candidates or products ourselves, including:
|
·
|
reliance on the third party for regulatory compliance and quality assurance;
|
|
·
|
the possible breach of the manufacturing agreement by the third party because of factors beyond our control; and
|
|
·
|
the possible termination or non-renewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us.
|
Our manufacturers may not be able to comply with current Good Manufacturing Practice, or cGMP, regulations or other regulatory requirements or similar regulatory requirements outside the United States. Our failure, or the failure of our third party manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure of regulatory authorities to grant marketing approval of our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates.
Our product candidates and any products that we may develop may compete with other product candidates with respect to the availability of manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations that are both capable of manufacturing for us and willing to do so. If the third parties that we engage to manufacture product for our clinical trials should cease to continue to do so for any reason, we likely would experience delays in advancing these trials while we identify and qualify replacement suppliers and we may be unable to obtain replacement supplies on terms that are favorable to us. In addition, if we are not able to obtain adequate supplies of our product candidates or the drug substances used to manufacture them, it will be more difficult for us to develop our product candidates and compete effectively.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that receive regulatory approval on a timely and competitive basis.
10
We rely on Durect Corporation, our sole source provider of VP4896, to produce VP4896 for our clinical trials and will rely on Durect to produce commercial drug supplies of VP4896. Durect has limited experience producing biodegradable implants and may not be able to provide VP4896 to satisfy our requirements.
We rely on Durect Corporation, or Durect, as our sole source provider of VP4896 for use in our clinical trials and, if we receive marketing approval, will rely on Durect as our sole source provider for commercial supply of VP4896. VP4896 requires precise, high quality manufacturing. Durect’s failure to achieve and maintain satisfactory standards could result in patient injury or death, product recalls or withdrawals, delays or failures in product testing or delivery, cost overruns or other problems that could materially harm our business. Durect may encounter manufacturing difficulties involving production yields, quality control and quality assurance. Durect is subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign agencies to ensure strict compliance with cGMP and other government regulations and corresponding foreign standards. We do not control Durect’s compliance with these regulations and standards.
To date, Durect has not produced commercial supply of a biodegradable implant for any drug approved for human use. If we receive marketing approval for and commercially launch VP4896, we anticipate that Durect will need to expand its manufacturing capacity for this drug, possibly materially. Durect may not be able to increase its manufacturing capacity for VP4896 in a timely or economic manner, or at all. Moreover, significant scale up of manufacturing may require additional validation studies, which the FDA must review and approve.
If Durect is unable to successfully increase the manufacturing capacity for VP4896 and we are unable to establish alternative manufacturing capabilities, the commercial launch of VP4896 may be delayed or there may be a shortage in supply. If Durect is unable to successfully increase manufacturing capacity for VP4896 at an acceptable cost, our commercialization of VP4896 could be delayed, prevented or impaired, including through a reduction of our gross margins on product sales.
We could lose supply of VP4896 from Durect if Durect elects to discontinue supply to us pursuant to Durect’s right to do so beginning two years after the first commercial sale or use of VP4896 or if Durect simply fails to or is unable to supply us at the levels required under the agreement. If we lose supply of VP4896 from Durect, we will need to establish an alternative source of supply. The loss of supply and need to establish alternative supply could result in lengthy delays in clinical trials, significant interruption of commercial supplies and substantial additional costs. Switching manufacturers may be difficult because, among other reasons, the number of potential manufacturers is limited, and the FDA must approve any replacement manufacturer. Such approval would require new clinical trials and compliance inspections. If we are able to transfer Durect’s manufacturing technology and process to a new manufacturer, which we have the right to do in circumstances specified in our agreement with Durect, to receive FDA approval would require bioequivalence or similar clinical trials, which are generally simpler and less costly than pivotal clinical trials. If we are not able to obtain such transfer, including as a result of Durect’s refusal to adhere to its contractual obligations, such approval would require new pivotal clinical trials. Accordingly, it may be difficult or impossible for us to find a replacement manufacturer for Durect on acceptable terms, in a manner that avoids supply interruption or possibly at all.
We could also lose our supply of VP4896 from Durect if our agreement with Durect terminates for any reason. In that case, in addition to the risks and consequences associated with having to secure alternative supply arrangements for VP4896 described above, we would be subject to the risks and consequences associated with losing our license to the Durect technology that is included in VP4896, and having to find and test alternative sustained release technology, as described in detail in the risk factor below entitled “If we fail to comply with our obligations in our intellectual property agreements with Durect or other third parties, we could lose license rights that are important to our business.”
11
We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such trials.
We do not have the ability to independently conduct clinical trials for our product candidates and we rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions, and clinical investigators, to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we will not be able to obtain, or may be delayed in obtaining, regulatory approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We also rely on other third parties to store and distribute drug supplies for our clinical trials for VP4896. Any performance failure on the part of our existing or future distributors could delay clinical development or regulatory approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We may not be successful in establishing collaborations, which could adversely affect our ability to discover, develop and, particularly in international markets, commercialize products.
We intend to selectively enter into collaboration agreements with leading pharmaceutical and biotechnology companies to assist us in furthering the development of our product candidates. In particular, we intend to enter into these third-party arrangements for target indications in which our potential collaborator has particular expertise or that involve a large, primary care market that must be served by large sales and marketing organizations. In entering into these collaboration agreements, our goal will be to maintain co-promotion or co-commercialization rights in the United States and, in some cases, other markets. If we are unable to reach agreements with suitable collaborators, we may fail to meet our business objectives for the affected product or program. We face, and will continue to face, significant competition in seeking appropriate collaborators. Moreover, collaboration arrangements are complex and time consuming to negotiate, document and implement. We may not be successful in our efforts to establish and implement collaborations or other alternative arrangements. The terms of any additional collaborations or other arrangements that we establish may not be favorable to us. Moreover, these collaborations or other arrangements may not be successful.
If we are unable to obtain and enforce patent protection for our discoveries, our ability to successfully commercialize our product candidates will be harmed and we may not be able to operate our business profitably.
Our success depends, in part, on our ability to protect proprietary methods and technologies that we develop under the patent and other intellectual property laws of the United States and other countries, so that we can prevent others from using our inventions and proprietary information. However, we may not hold proprietary rights to some patents related to our current and/or future products and technologies. Because patent applications in the United States and many foreign jurisdictions are typically not published until 18 months after filing, or in some cases not at all, and because publications of discoveries in scientific literature lag behind actual discoveries, we cannot be certain that we were the first to make the inventions claimed in our issued patent or pending patent applications, or that we were the first to file for protection of the inventions set forth in our patent applications. As a result, we may be required to obtain licenses under third-party patents to market our proposed products. If licenses are not available to us on acceptable terms, or at all, we will not be able to market the affected products.
Our strategy depends on our ability to rapidly identify and seek patent protection for our discoveries. This process is expensive and time consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. Despite our efforts to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. The issuance of a patent does not guarantee that it is valid or enforceable, so even if we obtain patents, they may not be valid or enforceable against third parties. In addition, the issuance of a patent does not guarantee that we have the right to practice the patented invention. Third parties may have blocking patents that could be used to prevent us from marketing our own patented-product or method and practicing our own patented technology.
12
Our pending patent applications and the pending patent applications of Durect that we have licensed may not result in issued patents. If patents do not issue with respect to such patent applications or if the claims allowed are too narrow, our business may be harmed. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the United States Patent and Trademark Office and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in those foreign countries. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims allowed in any patents issued to us or to others. The allowance of broader claims may increase the incidence and cost of patent interference proceedings and/or opposition proceedings, and the risk of infringement litigation. On the other hand, the allowance of narrower claims may limit the value of our proprietary rights.
Our issued patent and any future patents that we may own or license may not contain claims sufficiently broad to protect us against third parties with similar technologies or products, or provide us with any competitive advantage. Moreover, our patents and any patent for which we have licensed rights may be challenged, narrowed, invalidated or circumvented. For example, we are aware of one issued third party U.S. patent, filed after our issued U.S. patent was filed, which may nonetheless be prior art to our patent and which might form the basis of an interference proceeding or invalidity challenge. We believe that our claimed methods of treatment for Alzheimer’s disease are patentably distinct over the third party patent, that the third party patent does not enable our methods of treatment and, therefore, that our patent claims should be found valid and enforceable. However, the patent office or a court might disagree with us. An interference or invalidity challenge to our issued patent could result in the narrowing or loss of our patent claims.
If the patents that we own or license are invalidated or otherwise limited, other companies will be better able to develop products and technologies that compete with ours, which could adversely affect our competitive business position, business prospects and financial condition.
There are limitations on our patent rights relating to our product candidates and technologies, including with respect to the use of VP4896 to treat Alzheimer’s disease, that may affect our ability to exclude third parties from competing against us if we receive approval to market these product candidates or technologies.
Our proprietary rights relating to our product candidates and technologies, including with respect to the use of VP4896 to treat Alzheimer’s disease, are limited in ways that may affect our ability to exclude third parties from competing against us if we receive regulatory approval to market these product candidates and technologies. In particular:
|
·
|
We do not own or license composition of matter patents covering leuprolide acetate, the active pharmaceutical ingredient of VP4896, or methods of making leuprolide acetate. Moreover, the active ingredient that we are testing in our most advanced preclinical programs is also leuprolide acetate. Leuprolide acetate is currently marketed for a number of other indications. As a result, competitors can offer and sell leuprolide acetate products so long as they do not infringe, or contribute to the infringement of, or actively induce infringement of, any patents or other proprietary rights that we or others may have the rights to enforce;
|
|
·
|
The principal patent protection that covers, or that we expect will cover, the use of VP4896 to treat Alzheimer’s disease and leuprolide acetate to treat other indications stems from a method of use patent and patent applications. This type of patent only protects specified methods of using specified products according to the patent claims. This type of patent does not limit a competitor from making and marketing a product that is identical to our product for an indication that is outside of the patented method. Moreover, physicians may prescribe such a competitive identical product for off label indications that are covered by the applicable patents. In particular, leuprolide acetate, the active pharmaceutical ingredient in VP4896 and the drug being tested by us in our most advanced preclinical programs, is currently marketed for a number of other indications. Although off label prescriptions may infringe or contribute to or actively induce infringement of method of use patents, the practice is common and such infringement is difficult to prevent or prosecute. Consequently, VP4896, if approved, could face competition from leuprolide acetate products that are already on the market, or may later be approved, through off label usage of these products to treat Alzheimer’s disease that may be difficult or impossible for us to prevent because our primary patent protection is limited to method of use claims; and
|
13
|
·
|
We have not applied for method of use patent protection outside of the United States and Canada for use of VP4896 to treat Alzheimer’s disease. As a result, there is a significant risk that we will face early generic competition for VP4896 for the treatment of Alzheimer’s disease in international markets. This risk may be increased if competitors are able to develop formulations of leuprolide acetate, such as other polymeric implants, that are as effective as VP4896 may prove to be or if competitors can demonstrate that commercially available leuprolide acetate can be administered by injection in a manner that is as effective as VP4896 may prove to be.
|
These limitations on our patent rights may result in competitors taking product sales away from us, which would reduce our revenues and harm our business.
If we fail to comply with our obligations in our intellectual property agreements with Durect or other third parties, we could lose license rights that are important to our business.
We are a party to a license agreement with Durect pursuant to which Durect grants to us a worldwide, royalty-bearing exclusive license relating to Durect’s polymeric implant technology to develop and commercialize VP4896 for the treatment of Alzheimer’s disease. This technology includes patent applications covering the VP4896 formulation and the method of making VP4896. If we fail to perform our obligations under our agreement with Durect, Durect may terminate the agreement and our license to its patents and its manufacturing know-how. If this happened, we would be required to develop or license an alternative sustained release technology for VP4896. This would likely involve reformulating or otherwise changing VP4896. As a result, the FDA would likely require us to perform new pivotal clinical trials of the reformulated or otherwise changed version of VP4896. This could result in lengthy delays in clinical trials, significant interruption of commercial supplies and substantial additional costs. This would substantially harm our business. Furthermore, acceptable alternative technology may not exist, may not be something we are able to develop and may not be available to us on reasonable terms or possibly at all.
In addition to clinically developing VP4896 for mild to moderate Alzheimer’s disease, we also are working on the pre-clinical development of leuprolide acetate for the treatment of various cancers. We may determine to develop an implantable formulation of leuprolide acetate for these additional indications. However, because our license from Durect is limited to the field of Alzheimer’s disease, we will not be able to develop a leuprolide acetate implant based on Durect’s technology for these additional indications without obtaining an appropriate amendment of our license from Durect. If Durect will not agree to such an amendment, we could seek to develop other polymeric implants of leuprolide acetate that are outside of Durect’s patents and know-how. However, it might be time consuming and expensive for us to do so and might deprive us of the clinical and regulatory benefits that we believe will be available to us from developing a leuprolide acetate implant based on Durect’s technology for additional indications.
We may also enter into additional licenses in the future. Our existing license with Durect imposes, and we expect future licenses with third parties will impose, various diligence, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with these obligations, the licensor may have the right to terminate the license, in which event we might not be able to market any product that is covered by the licensed patents.
14
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely upon unpatented proprietary technology, processes and know-how, including particularly Durect’s manufacturing know-how relating to the production of VP4896. We seek to protect our unpatented proprietary information in part by confidentiality agreements with our employees, consultants and third parties. These agreements may be breached and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently developed by competitors. If we are unable to protect the confidentiality of our proprietary information and know-how, competitors may be able to use this information to develop products that compete with our products, which could adversely impact our business. We could be similarly harmed if Durect’s confidential know-how relating to the production of VP4896 becomes known to our competitors.
If we infringe or are alleged to infringe intellectual property rights of third parties, it will adversely affect our business.
Our research, development and commercialization activities, as well as any product candidates or products resulting from these activities, may infringe or be claimed to infringe patents or patent applications under which we do not hold licenses or other rights. Third parties may own or control these patents and patent applications in the United States and abroad. These third parties could bring claims against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us or our collaborators, we or they could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we or our current or future collaborators may choose or be required to seek a license from the third party and be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly.
For example, we are aware of one issued third party U.S. patent, filed after our issued patent was filed, which includes a claim directed to a method for the treatment of a neurodegenerative disorder which could form the basis of an infringement claim. We believe that if this patent were asserted against us in an infringement action relating to VP4896 for the treatment of mild to moderate Alzheimer’s disease, the claims should be held invalid. An issued U.S. patent, however, is entitled to a presumption of validity as a matter of law. If the claims of this third party patent were found valid and interpreted to cover the use of VP4896 for the treatment for Alzheimer’s disease, we would be liable for past damages for infringement and might be required to obtain a license under the patent to manufacture and market VP4896. If a license were not available to us on acceptable terms, or at all, we might not be able to market VP4896.
Moreover, the field of polymeric implants for sustained drug release is highly competitive, and we are aware of third party patents directed to technologies similar to that of Durect. Although we are not aware of any third party patents that would be literally infringed by the commercial development of the VP4896 implant, we are aware of third party patents which include claims directed to implants containing a copolymer of lactic acid and glycolic acid, that could be asserted against our product in the United States under the doctrine of equivalents. Even if a U.S. patent claim is not literally infringed, the doctrine of equivalents permits a court to find infringement if the differences between the accused product or process and the patent claims are insubstantial. If a court were to find a third party patent infringed under the doctrine of equivalents, the consequences could be the same as those of a finding of literal infringement, including liability for past damages and the possibility of being enjoined from commercializing the affected product or process. In the event of an injunction, we would be required to cease marketing VP4896, but could develop an alternative sustained release formulation which would not infringe any third party patents. Development of an alternative sustained release formulation would require substantial additional time and expense, including additional regulatory review, all of which could harm our business significantly.
15
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical and biotechnology industries. In addition to infringement claims against us, we may become a party to other patent litigation and other proceedings, including interference proceedings declared by the United States Patent and Trademark Office and opposition proceedings in the European Patent Office, regarding intellectual property rights with respect to our products and technology. The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other proceedings may also absorb significant management time.
If we are not able to obtain required regulatory approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates and the activities associated with their development and commercialization, including their testing, manufacture, safety, efficacy, record keeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. Failure to obtain regulatory approval for a product candidate will prevent us from commercializing the product candidate.
We have not received regulatory approval to market any of our product candidates in any jurisdiction. We have only limited experience in filing the applications necessary to gain regulatory approvals and expect to rely on third party contract research organizations to assist us in this process. Securing FDA approval requires the submission of extensive pre-clinical and clinical data, information about product manufacturing processes and inspection of facilities and supporting information to the FDA for each therapeutic indication to establish the product candidate’s safety and efficacy. Our future products may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining regulatory approval or prevent or limit commercial use.
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon, among other things, the type, complexity and novelty of the product candidates involved. Changes in the regulatory approval policy during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional pre-clinical, clinical or other studies. In addition, varying interpretations of the data obtained from pre-clinical and clinical testing could delay, limit or prevent regulatory approval of a product candidate. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable.
Our products could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our products, when and if any of them are approved.
Any product for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and comparable regulatory bodies. These requirements include submissions of safety and other post-marketing information and reports, registration requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents and requirements regarding the distribution of samples to physicians and record keeping. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. Later discovery of previously unknown problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in:
16
|
·
|
restrictions on such products, manufacturers or manufacturing processes;
|
|
·
|
warning letters;
|
|
·
|
withdrawal of the products from the market;
|
|
·
|
refusal to approve pending applications or supplements to approved applications that we submit;
|
|
·
|
recall;
|
|
·
|
fines;
|
|
·
|
suspension or withdrawal of regulatory approvals;
|
|
·
|
refusal to permit the import or export of our products;
|
|
·
|
product seizure; and
|
|
·
|
injunctions or the imposition of civil or criminal penalties.
|
Failure to obtain regulatory approval in international jurisdictions would prevent us from marketing our products abroad.
We intend to have our products marketed outside the United States. In order to market our products in the European Union and many other foreign jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ from that required to obtain FDA approval. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or jurisdictions or by the FDA. We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any market.
The commercial success of any products that we may develop will depend upon the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community.
Any products that we bring to the market may not gain market acceptance by physicians, patients, healthcare payors and others in the medical community. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenues and we may not become profitable. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
|
·
|
the prevalence and severity of any side effects;
|
|
·
|
the efficacy and potential advantages over alternative treatments;
|
|
·
|
the ability to offer our product candidates for sale at competitive prices;
|
|
·
|
relative convenience and ease of administration;
|
17
|
·
|
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
|
|
·
|
the strength of marketing and distribution support; and
|
|
·
|
sufficient third party coverage or reimbursement.
|
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our product candidates, we may be unable to generate product revenues.
We do not currently have a sales or marketing organization and have limited experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. We intend to selectively enter into collaboration agreements with leading pharmaceutical and biotechnology companies to assist us in furthering the development of our product candidates. In particular, we intend to enter into these third-party arrangements for target indications in which our potential collaborator has particular expertise or that involve a large, primary care market that must be served by large sales and marketing organizations. Our goal in these collaborations will be to maintain co-promotion or co-commercialization rights in the United States and, in some cases, other markets. There are risks involved with establishing our own sales and marketing capabilities, as well as in entering into arrangements with third parties to perform these services. For example, developing a sales force is expensive and time consuming and could delay any product launch. We will begin to incur many sales and marketing costs prior to receiving marketing approval for our product candidates. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing capabilities is delayed or prohibited as a result of FDA requirements or other reasons, our investment in these functions would be lost. If we enter into arrangements with third parties to perform sales and marketing services, our product revenues could be lower than if we directly sold and marketed our products. In addition, any revenues received under such arrangements will depend on the skills and efforts of others, and we do not know whether these efforts will be successful.
If third party payors do not provide coverage for products we may develop, or if the amount that a third party payor may reimburse health care providers who use any products that we may develop is not adequate to compensate the health care provider for the use of any such product, our revenues and prospects for profitability will suffer.
Our revenues and profits will depend heavily on purchases of any products we may develop by health care providers who receive reimbursement from third party payors, including governmental and private payors, both in the United States and abroad. Whether health care providers will purchase any products we develop will depend in part upon the coverage policies and availability of adequate reimbursement from such third party payors. Obtaining reimbursement from a third party payor will depend on whether a product is covered under the third party payor’s benefit plan. In general, to be covered, a product must fall within a designated benefit category and be medically reasonable and necessary for treatment. However, even if covered, the amount and method of reimbursement will vary based on factors such as the particular site of service in which the product is used.
Obtaining a determination that a product is covered is a time-consuming and costly process that could require us to provide supporting scientific, clinical and cost-effectiveness data for the use of our products to each payor. We may not be able to provide data sufficient to gain coverage. Even when a payor determines that a product is covered, the payor may impose limitations that preclude payment for some uses that are approved by the FDA or comparable authorities but are determined by the payor to not be medically reasonable and necessary. Moreover, eligibility for coverage does not imply that any product will be covered in all cases or that reimbursement will be available at a rate that permits the health care provider to cover its costs of using the product.
18
Most persons suffering from Alzheimer’s disease are elderly, and therefore we expect that coverage and reimbursement for VP4896 for this indication in the United States will be primarily through the Medicare program. Our business would be materially adversely affected if the Medicare program were to determine that it will not: (1) cover VP4896 for the treatment of Alzheimer’s disease, or any other products we may develop; or (2) adequately reimburse health care providers for the use of VP4896 or any other products we may develop.
Beginning in 2005, pursuant to the Medicare Prescription Drug, Improvement and Modernization Act of 2003, Medicare changed the formula used to calculate the reimbursement for physician-administered drugs. The price established for VP4896 under the Medicare program under the new formula will probably be less than under the previous formula. In addition, alternative methods for calculating reimbursement are used under the Medicare program depending on the benefit category and site of service, which could lower reimbursement still further. In addition, starting in January 2006, physicians now have the option of being reimbursed directly by Medicare or participating in the Competitive Acquisition Program, or CAP, whereby the physician has the option to obtain drugs from a CAP vendor who negotiates prices with drug companies and obtains reimbursement from Medicare for the drug. If substantial numbers of physicians participate in the CAP, it will likely decrease the price we can obtain for VP4896 from the CAP vendor, if it is approved for marketing.
Any products we may develop may also receive reimbursement under Medicaid. If the state-specific Medicaid programs do not provide adequate coverage and reimbursement for any products we may develop, it may have a negative impact on our operations.
The scope of coverage and payment policies varies among third-party private payors, including indemnity insurers, employer group health insurance programs and managed care plans. Such third party carriers may base their coverage and reimbursement on the coverage and reimbursement rate paid by carriers for Medicare beneficiaries. Furthermore, many such payors are investigating or implementing methods for reducing health care costs, such as the establishment of prospective payment systems. Cost containment pressures have led to an increased emphasis on the use of cost-effective products by health care providers. If private payors do not provide adequate coverage or reimbursement for any products we may develop, it could have a negative effect on revenues and results of operations.
Foreign governments tend to impose strict price controls, which may adversely affect our revenues, if any.
In some foreign countries, particularly the countries of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate to other available therapies. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. Because VP4896 is being developed as an implant that will contain eight weeks’ supply of drug, we may face higher product liability risk than if our product was administered in another form, such as a daily pill. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
|
·
|
decreased demand for any product candidates or products that we may develop;
|
|
·
|
injury to our reputation;
|
19
|
·
|
withdrawal of clinical trial participants;
|
|
·
|
costs to defend the related litigation;
|
|
·
|
substantial monetary awards to trial participants or patients;
|
|
·
|
loss of revenue; and
|
|
·
|
the inability to commercialize any products that we may develop.
|
We have product liability insurance that covers our clinical trials up to a $10 million annual aggregate limit and subject to a per claim deductible. The amount of insurance that we currently hold may not be adequate to cover all liabilities that may incur. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for any products. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost and we may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drugs is highly competitive. We face competition with respect to our current product candidates and any products we may seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization. Our competitors may develop products that are more effective, safer, more convenient or less costly than any that we are developing or that would render our product candidates obsolete or non-competitive. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours.
We believe that many competitors are attempting to develop therapeutics for Alzheimer’s disease, including academic institutions, government agencies, public and private research organizations, large pharmaceutical companies and smaller more focused companies.
There are currently five drugs approved for the treatment of Alzheimer’s disease in the United States. In many cases, these products have well-known brand names, are distributed by large pharmaceutical companies with substantial resources and experience and have achieved widespread acceptance among physicians and patients. See “Business—Alzheimer’s disease.” In addition, we are aware of product candidates of third parties that are in development, which, if approved, would compete against our product candidates, if approved.
Durect has made a commitment to us not to develop, commercialize or license to someone else formulations of leuprolide acetate that release a similar dose per unit of time as does VP4896, but has otherwise not committed to us that it will not develop other leuprolide acetate products, including sustained release leuprolide acetate products, using its technologies. As a result, Durect could collaborate with a third party to develop a product having a different dose that competes with VP4896, in some cases using the same Durect sustained release technology as is included in VP4896. Finally, as described under “Risk Factors—There are limitations on our patent rights relating to our product candidates and technologies, including with respect to the use of VP4896 to treat Alzheimer’s disease, that may affect our ability to exclude third parties from competing against us if we receive approval to market these product candidates or technologies,” to the extent that we market products with active ingredients that do not have composition of matter patent protection and are approved for other indications, such as leuprolide acetate, it is possible that we will experience competition from off label sales of products containing these active ingredients.
20
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for our programs or advantageous to our business.
VP4896, our lead product candidate for the treatment of mild to moderate Alzheimer’s disease, is a small biodegradable implant that is administered once every eight weeks by a physician in the physician’s office through a minor surgical procedure. All drugs currently marketed for the treatment of Alzheimer’s disease, and many of the drugs under development for this indication, are orally self-administered pills. If VP4896’s administration method is not accepted by patients or physicians, we may not generate significant product revenues and our business may be materially harmed.
Our business activities involve the use of hazardous materials, which require compliance with environmental and occupational safety laws regulating the use of such materials. If we violate these laws, we could be subject to significant fines, liabilities or other adverse consequences.
Our research and development programs involve the controlled use of hazardous materials. Accordingly, we are subject to federal, state and local laws governing the use, handling and disposal of these materials. Although we believe that our safety procedures for handling and disposing of these materials comply in all material respects with the standards prescribed by state and federal regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In addition, our collaborators may not comply with these laws. In the event of an accident or failure to comply with environmental laws, we could be held liable for damages that result, and any such liability could exceed our assets and resources.
We must comply with federal, state and foreign laws, regulations, and other rules relating to the health care business and, if we are unable to fully comply with such laws, regulations and other rules, we could face substantial penalties.
We are or will be, directly or indirectly through our customers, subject to extensive regulation by the federal government, the states and foreign countries in which we may conduct our business. The laws that directly or indirectly affect our ability to operate our business include the following:
|
·
|
the federal Medicare and Medicaid Anti-Kickback law, which prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual, or furnishing or arranging for a good or service, for which payment may be made under federal healthcare programs such as the Medicare and Medicaid Programs;
|
|
·
|
other Medicare laws, regulations, rules, manual provisions and policies that prescribe the requirements for coverage and payment for services performed by our customers, including the amount of such payment;
|
|
·
|
the federal False Claims Act, which imposes civil and criminal liability on individuals and entities who submit, or cause to be submitted, false or fraudulent claims for payment to the government;
|
|
·
|
the federal False Statements Act, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; and
|
|
·
|
state and foreign law equivalents of the foregoing.
|
21
If our operations are found to be in violation of any of the laws, regulations, rules or policies described above or any other law or governmental regulation to which we or our customers are or will be subject, or if the interpretation of the foregoing changes, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. Similarly, if our customers are found non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on us. Any penalties, damages, fines, curtailment or restructuring of our operations would adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations, and additional legal or regulatory change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
We have substantial indebtedness to trade creditors and several trade creditors have obtained judgments against us.
We have substantial indebtedness to trade creditors relating to goods and services provided to us in connection with our truncated Phase III trials. In general, our relationship with our trade creditors have remained cordial despite this indebtedness and the vast majority of our trade creditors have cooperated with us in our efforts to defer payments to them until we can obtain further financing. However, there can be no assurance that our trade creditors will continue to be cooperative and one or more of them may choose to bring suit to enforce their claims. One trade creditor won a judgment of approximately $1,100,000 against us and we subsequently entered into a negotiated payment arrangement with this creditor, which reduced the ultimate liability to $900,000. In addition, several other trade creditors have obtained default judgments against us, although to our knowledge none of such creditors have initiated any court proceedings to enforce those judgments. To date, the total amount of judgments rendered against us is $1.4 million.
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Patrick S. Smith, our Chairman, President and Chief Executive Officer, David J. Corcoran, our Chief Financial Officer, and the other principal members of our executive and scientific teams. Although we do not have any reason to believe that we may lose the services of any of these persons in the foreseeable future, the loss of the services of any of these persons might impede the achievement of our research, development, and commercialization objectives. At this time, we do not have employment agreements with any of our key personnel, including Mr. Smith and Mr. Corcoran.
Recruiting and retaining qualified scientific personnel and sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
RISKS RELATED TO OUR COMMON STOCK
Any additional funding we arrange through the sale of our common stock will result in dilution to existing security holders.
We have no committed source of financing. We will have to raise additional capital in order for our business plan to succeed. Wherever possible, our board of directors will attempt to use non-cash consideration to satisfy obligations. Therefore, our most likely source of additional capital will be through the sale of additional shares of our common stock. Our board of directors has authority, without action or vote of the shareholders, to issue all or part of the authorized 480,000,000 shares. In addition, if a trading market develops for our common stock, we may attempt to raise capital by selling shares of our common stock, possibly at a discount to market. Such issuances will cause our security holders’ interests in our Company to be diluted, which may negatively affect the value of their shares.
22
Currently, there is no public market for our shares of common stock, and there can be no assurance that any public market will ever develop or that our common stock will be quoted for trading.
Our common stock is listed on the Over-the-Counter Bulletin Board under the symbol “CURX.OB.” However, there is a limited trading market for our shares of common stock. Even if we are successful in developing a public market, there may not be enough liquidity in such market to enable our security holders to sell their stock. If a public market for our common stock does not develop, investors may not be able to re-sell the shares of its common stock, rendering their shares effectively worthless and resulting in a complete loss of their investment. We cannot predict the extent to which investor interest in our Company will lead to the development of an active, liquid trading market. Active trading markets generally result in lower price volatility and more efficient execution of buy and sell orders for investors.
In the event that a public market does develop, the price of our common stock will be subject to significant price fluctuations.
Until an orderly market develops in our common stock, if ever, the price at which our common stock trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these Risk Factors, investor perception of our Company and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our stock.
In addition, our common stock may not be followed by any market analysts, and there may be few institutions acting as market makers for our common stock. Currently, there are seven institutions acting as market makers, but there can be no guarantee that they will continue to do so or that any other institutions in the future will act as a market make for our common stock. Either of these factors could (i) adversely affect the trading price of our common stock and (ii) severely limit the liquidity of our common stock.
Any trading market that may develop may be restricted by virtue of state securities “Blue Sky” laws, making it difficult or impossible for our Security Holders to sell shares of its common stock in those states.
There is a limited public market for our shares of common stock, and there can be no assurance that any public market will develop in the foreseeable future. Transfer of our common stock may also be restricted under the securities regulations and laws promulgated by various states and foreign jurisdictions, commonly referred to as “Blue Sky” laws, which prohibit trading absent compliance with individual state laws. Absent compliance with such individual state laws, our common stock may not be traded in such jurisdictions.
Because we are subject to the “penny stock” rules, the level of trading activity in our common stock may be reduced.
Broker-dealer practices in connection with transactions in “penny stocks” are regulated by penny stock rules adopted by the Securities and Exchange Commission. Penny stocks generally are equity securities with a price of less than $5.00 (other than securities registered on some national securities exchanges). The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction, and, if the broker-dealer is the sole market maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market, and monthly account statements showing the market value of each penny stock held in the customer’s account. In addition, broker-dealers who sell these securities to persons other than established customers and “accredited investors” must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. Consequently, these requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security subject to the penny stock rules, and investors in our common stock may find it difficult to sell their shares.
23
We do not expect to pay dividends to holders of our common stock in the foreseeable future. As a result, holders of our common stock must rely on stock appreciation for any return on their investment.
We have not declared any dividends since our inception, and we do not plan to declare any dividends in the foreseeable future. If we were to become profitable, it would be expected that all of such earnings would be retained to support our business. Accordingly, holders of our common stock will have to rely on capital appreciation, if any, to earn a return on their investment in our common stock.
Risks Related To Market Conditions
We are registering an aggregate of 14,500,000 shares of common stock to be issued under the Equity Credit Agreement. The sale of such shares could depress the market price of our common stock.
We are registering an aggregate of 14,500,000 shares of common stock under the registration statement of which this prospectus forms a part for issuance pursuant to the Equity Credit Agreement. The 14,500,000 shares of our common stock will represent approximately 17% of our shares outstanding immediately after our exercise of the put right. The sale of these shares into the public market by Southridge could depress the market price of our common stock.
Because Southridge will be paying less than the then-prevailing market price for our common stock, your ownership interest may be diluted and the value of our common stock may decline by exercising the put right pursuant to the Equity Credit Agreement.
The Company may not have access to the full amount available under the Equity Credit Agreement.
We have not drawn down funds and have not issued shares of our common stock under the Equity Credit Agreement with Southridge. Our ability to draw down funds and sell shares under the Equity Credit Agreement requires that the registration statement, of which this prospectus is a part, be declared effective by the SEC, and that this registration statement continue to be effective. In addition, the registration statement of which this prospectus is a part registers 14,500,000 total shares of our common stock issuable under the Equity Credit Agreement, and our ability to access the Equity Credit Agreement to sell any remaining shares issuable under the Equity Credit Agreement is subject to our ability to prepare and file one or more additional registration statements registering the resale of these shares, which we may not file until the later of 60 days after Southridge and its affiliates have resold substantially all of the common stock registered for resale under the registration statement of which this prospectus is a part, or six months after the effective date of the registration statement of which this prospectus is a part. These subsequent registration statements may be subject to review and comment by the Staff of the SEC, and will require the consent of our independent registered public accounting firm. Therefore, the timing of effectiveness of these subsequent registration statements cannot be assured. The effectiveness of these subsequent registration statements is a condition precedent to our ability to sell the shares of common stock subject to these subsequent registration statements to Southridge under the Equity Credit Agreement. Even if we are successful in causing one or more registration statements registering the resale of some or all of the shares issuable under the Equity Credit Agreement to be declared effective by the SEC in a timely manner, we will not be able to sell shares under the Equity Credit Agreement unless certain other conditions are met. Accordingly, because our ability to draw down amounts under the Equity Credit Agreement is subject to a number of conditions, there is no guarantee that we will be able to draw down any portion or all of the $25 million available to us under the Equity Credit Agreement.
The common stock to be issued to Southridge pursuant to the Equity Credit Agreement will be purchased at an 5% discount to the average of the lowest closing price of the common stock of any three trading days, consecutive or inconsecutive, during the five consecutive trading days immediately following the date of our notice to Southridge of our election to put shares pursuant to the Equity Credit Agreement. Because the put price is lower than the prevailing market price of our common stock, to the extent that the put right is exercised, your ownership interest may be diluted. Southridge has a financial incentive to sell our common stock immediately upon receiving the shares to realize the profit equal to the difference between the discounted price and the market price. If Southridge sells the shares, the price of our common stock could decrease. If our stock price decreases, Southridge may have a further incentive to sell the shares of our common stock that it holds. These sales may have a further impact on our stock price.
The Equity Credit Agreement’s pricing structure may result in dilution to our stockholders.
Pursuant to the Equity Credit Agreement, Southridge committed to purchase, subject to certain conditions, up to the $25 million of our common stock over a three-year period. If we sell shares to Southridge under the Equity Credit Agreement, or issue shares in lieu of any blackout payment (as described below), it will have a dilutive effect on the holdings of our current stockholders, and may result in downward pressure on the price of our common stock. If we draw down amounts under the Equity Credit Agreement, we will issue shares to Southridge at a discount of 5% from the average price of our common stock. If we draw down amounts under the Equity Credit Agreement when our share price is decreasing, we will need to issue more shares to raise the same amount than if our stock price was higher. Issuances in the face of a declining share price will have an even greater dilutive effect than if our share price were stable or increasing, and may further decrease our share price. In addition, we are entitled in certain circumstances to deliver a “blackout” notice to Southridge to suspend the use of the registration statements that we have filed or may in the future file with the SEC registering for resale the shares of common stock to be issued under the Equity Credit Agreement. If we deliver a blackout notice in the fifteen trading days following a settlement of a draw down, then we must issue Southridge additional shares of our common stock.
Certain provisions of our charter could discourage potential acquisition proposals or change in control.
Our board of directors, without further stockholder approval, may issue preferred stock that would contain provisions that could have the effect of delaying or preventing a change in control or which may prevent or frustrate any attempt by stockholders to replace or remove the current management. The issuance of additional shares of preferred stock could also adversely affect the voting power of the holders of our common stock, including the loss of voting control to others.
24
FORWARD LOOKING STATEMENTS
Information included or incorporated by reference in this prospectus may contain forward-looking statements. This information may involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from the future results, performance or achievements expressed or implied by any forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend” or “project” or the negative of these words or other variations on these words or comparable terminology.
This prospectus contains forward-looking statements, including statements regarding, among other things, (a) our projected sales and profitability, (b) our technology, (c) our manufacturing, (d) the regulations to which we are subject, (e) anticipated trends in our industry and (f) our needs for working capital. These statements may be found under “Management’s Discussion and Analysis or Plan of Operations” and “Business,” as well as in this prospectus generally. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this prospectus will in fact occur.
USE OF PROCEEDS
Selling Security Holder is selling all of the shares of our common stock covered by this prospectus for its own account. Accordingly, we will not receive any proceeds from the resale of our common stock. However, we will receive proceeds from any sale of the common stock to Southridge under the Equity Credit Agreement. We intend to use the net proceeds received for the clinical development of our drug candidate, Memrtye, for the treatment of mild to moderate Alzheimer’s disease, working capital or general corporate needs.
DETERMINATION OF OFFERING PRICE
Our common stock currently trades on the OTC Bulletin Board under the symbol “CURX.OB.” The proposed offering price of the Put Shares is $.74, which is the closing bid price of our common stock on December 7, 2010, as reported by the OTC Bulletin Board. Selling Security Holder may sell shares in any manner at the current market price.
DIVIDEND POLICY
We have never declared dividends or paid cash dividends on our common stock. We intend to retain and use any future earnings for the development and expansion of our business and do not anticipate paying any cash dividends on the common stock in the foreseeable future. The declaration of dividends will be at the discretion of our board of directors and will depend upon our earnings, financial position, general economic conditions and other pertinent factors.
MARKET FOR OUR COMMON STOCK
Our common stock is listed on the Over-the-Counter BB market and trades under the symbol CURX.OB.
The following table sets forth the range of the high and low bid quotations of our common stock for the past two years in the Over-the-Counter BB market, as reported by the OTC Bulletin Board and in the Pink Sheets. The quotations reflect inter-dealer prices without retail mark-up, markdown or commission, and may not represent actual transactions.
25
Calendar Quarter Ended:
|
High
|
Low
|
|||||||
|
2010
|
||||||||
|
September 30
|
$
|
1.90
|
0.90
|
|||||
|
June 30
|
$
|
1.30
|
0.40
|
|||||
|
March 31
|
$
|
1.00
|
0.20
|
|||||
|
2009
|
||||||||
|
December 31
|
$
|
--
|
--
|
|||||
|
September 30
|
$
|
--
|
--
|
|||||
|
June 30
|
$
|
--
|
--
|
|||||
|
March 31
|
$
|
--
|
--
|
|||||
|
2008
|
||||||||
|
December 31
|
$
|
--
|
--
|
|||||
|
September 30
|
$
|
0.002747
|
0.002747
|
|||||
As of November 30, 2010, we had approximately 1,250 stockholders of record.
26
MANAGEMENTS DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion and analysis of our financial condition and results of our operations should be read in conjunction with the financial statements and the notes thereto of Curaxis Pharmaceutical Corporation which appear elsewhere in this prospectus. The results shown herein are not necessarily indicative of the results to be expected for any future periods.
Forward Looking Statements
We are including the following cautionary statement in this registration statement for any forward-looking statements made by, or on behalf of, our Company. Forward-looking statements include statements concerning plans, objectives, goals, strategies, future events or performance and underlying assumptions and other statements which are other than statements of historical facts. Certain statements contained herein are forward-looking statements and accordingly involve risks and uncertainties which could cause actual results or outcomes to differ materially from those expressed in the forward-looking statements. Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” or “project” or the negative of these words or other variations on these words or comparable terminology.
Our expectations, beliefs and projections are expressed in good faith and are believed by us to have a reasonable basis, including without limitation, management’s examination of historical operating trends, data contained in our records and other data available from third parties, but there can be no assurance that management’s expectation, beliefs or projections will result or be achieved or accomplished. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this prospectus generally. In addition to other factors and matters discussed elsewhere herein, the following are important factors that, in our view, could cause actual results to differ materially from those discussed in the forward-looking statements: technological advances by our competitors; anticipated trends in our industry; changes in reimbursement programs; market acceptance of our products; the loss of any key employees; delays in obtaining federal or foreign regulatory clearance; changes in laws, including increased tax rates, regulations or accounting standards, third party relations and approvals, and decisions of courts, regulators and governmental bodies; the effects of local and national economic, credit and capital market conditions on the economy in general, and on the pharmaceutical industry in particular, and the effects of foreign exchange rates and interest rates; and availability of capital on terms satisfactory to us.
Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update these forward-looking statements, or to update the reasons actual results could differ materially from those anticipated from these forward-looking statements, even if new information becomes available in the future.
Overview
We are an emerging specialty pharmaceutical company with a pipeline of product candidates in Alzheimer’s disease and oncology. Curaxis’ most advanced product candidate is Memryte (generic name VP4896), a proprietary, small, biodegradable implant that is comprised of leuprolide acetate and a polymer. Curaxis’ therapeutic platform is based on the hypothesis that many diseases of aging may be caused by age-related changes in the function of the hypothalamic-pituitary-gonadal (HPG) axis. The HPG axis is a hormonal endocrine feedback loop that controls development, reproduction and aging in animals. We believe that Curaxis’ discovery of similar hormonal signaling mechanisms at the cellular level in brain tissue from Alzheimer’s patients and in multiple tumors may enable us to develop significant new treatments for Alzheimer’s disease as well as many tumors.
From inception through early 2006, Curaxis completed two Phase II clinical trials, Aladdin I and Aladdin II, to support the efficacy of its drug candidate in women and men, respectively. In addition, a Phase I trial was completed to support safety and the drug release profile of the proprietary subcutaneous implant. In August 2006, Curaxis completed enrollment in Aladdin 301, the first of its two Phase III clinical trials of the VP4896 implant for the treatment of mild to moderate Alzheimer’ s disease. In October 2006, this trial was discontinued due to financial constraints and converted to a Phase II trial. At the time of termination, approximately 625 patients had been enrolled at 62 sites located throughout the United States and Canada. Curaxis has not conducted material research and development activities for Memryte since terminating its clinical trials and closing its research facility in 2007.
27
Since Curaxis’ inception, it has not received approval to market any product, has had no revenues from product sales and has devoted substantially all of its efforts to the development of its treatment for mild to moderate Alzheimer’s disease. Curaxis funded its operations primarily through the sale of common stock and warrants to private investors, which have provided net cash proceeds of approximately $74.7 million as of September 30, 2010. Curaxis has never been profitable and, as September 30, 2010, it had an accumulated deficit of $91.2 million. Curaxis had net losses of $2.0 million, $6.7 million and $28.2 million for the three years ended December 31, 2008, 2007 and 2006, respectively, and a net loss of $55.6 million for the cumulative period from inception through December 31, 2005. Net income of $5.0 million was recognized for the year ended December 31, 2009 as a result of successful restructuring of trade debt. A net loss of $3.6 million was recognized for the nine-month period ended September 30, 2010. At September 30, 2010 it had $0.3 million in cash and cash equivalents
Subsequent to the Merger, we, through our subsidiary Curaxis, are continuing Curaxis’ business plan of developing for the treatment of mild to moderate Alzheimer’s disease. As we attempt to restart the clinical development program for Memryte after the Merger, this suspension may adversely impact our clinical development and commercialization efforts due to, among other factors, potential difficulties in attracting clinical personnel in the future and in attracting clinical sites to conduct clinical trials of Memryte.
We expect our minimum cash needs to fund our operating expenses and capital expenditures for the twelve-month period ending September 30, 2011 to be approximately $3.1 million. We expect to incur significant and increasing operating losses for at least the foreseeable future as we advance our drug candidates from research through pre-clinical testing and clinical trials and seek regulatory approval and eventual commercialization for any drug candidates that may receive regulatory approval. We will need to generate significant revenues to achieve profitability and we may never do so.
We do not expect to generate product revenue for at least the next several years, if at all. If any of our programs experience delays or do not result in a commercial product, we would not generate revenue from that program in a timely manner or at all. We expect that our operating expenses will continue to increase and may vary substantially from quarter to quarter and year-to-year based on the timing of clinical trial patient enrollment and our other research and development activities. In particular, as we initiate a second Phase II and pivotal Phase III trials of Memryte, our lead drug candidate for the treatment of mild to moderate Alzheimer’s disease, we expect that our research and development expenses will increase significantly. We expect general and administrative costs also to increase as we add personnel. In connection with our preparation for the commercial launch of Memryte, we expect that our capital expenditures may increase as a result of increased manufacturing equipment costs. We plan to establish our own commercialization capability, including a large field sales force in the United States, and expect to incur significant costs related to marketing and sales activities prior to the time we generate any revenue. We believe that period-to-period comparisons of our results of operations are not meaningful and should not be relied on as indicative of our future performance.
The successful development of pharmaceuticals, including our lead product candidate, Memryte, for the treatment of mild to moderate Alzheimer’s disease, is uncertain. We estimate that we will incur expenses of at least $48 million between 2010 and 2014 in order to complete the clinical development of Memryte in accordance with our current development plan and seek FDA approval to market Memryte. If we or the FDA cause a change or delay in our development plan for Memryte, our costs could increase substantially. We cannot reasonably estimate or know the nature, timing and estimated expenses of the efforts necessary to complete the remainder of the development of, or the period, if ever, in which material net cash inflows will commence from Memryte for this indication or other product candidates due to the numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
|
·
|
the scope, rate of progress and expense of our clinical trials and other research and development activities;
|
|
·
|
future clinical trial results;
|
|
·
|
the expense, timing and outcome of seeking regulatory approvals;
|
|
·
|
the expense of establishing clinical and commercial supplies of our product candidates and any products that we may develop;
|
|
·
|
the effect of competing technological and market developments; and
|
|
·
|
the expense of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights.
|
28
Critical Accounting Policies and Estimates
The discussion and analysis of Curaxis’ financial condition and results of operations set forth below are based on its financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America, or “GAAP.” The preparation of these financial statements requires Curaxis to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. Curaxis based its estimates on historical experience and on various other assumptions that it believed to be reasonable under the circumstances. These estimates and assumptions form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe the following accounting policies are the most critical to Curaxis’ financial statements. We believe they are important to the presentation of its financial condition and require the greatest degree of management judgment to make the estimates necessary to ensure their fair presentation.
Accrued Expenses
As part of the process of preparing financial statements, Curaxis is required to estimate accrued expenses. This process involves identifying services which have been performed on its behalf and estimating the level of service performed and the associated cost incurred for such service as of each balance sheet date in its financial statements. Services for which it must estimate accrued expenses include:
|
·
|
costs of services performed by contract research organizations and clinical sites in conjunction with clinical trials;
|
|
·
|
costs of services performed by contract manufacturers in conjunction with the production of clinical trial materials; and
|
|
·
|
professional service fees and expenses.
|
In accruing service fees, Curaxis estimated the time period over which services will be provided and the level of effort in each period. If the actual timing of the provision of services or the level of effort varies from the estimate, it will adjust the accrual accordingly. The majority of its service providers invoice Curaxis monthly in arrears for services performed. In the event that Curaxis does not identify costs that have begun to be incurred or it underestimates or overestimates the level of services performed or the costs of such services, its actual expenses could differ from such estimates. The date on which some services commence, the level of services performed on or before a given date and the cost of such services are often subjective determinations. Curaxis made judgments based upon the facts and circumstances known to it in accordance with GAAP.
Research and Development
Curaxis expenses all research and development costs as incurred. Research and development expense includes, among other things, clinical trial costs. It accounts for its clinical trial costs by estimating the total cost to treat a patient in each clinical trial and recognizing this cost, based on a variety of factors, beginning with the preparation for the clinical trial. This estimated cost includes payments to its contract research organizations for trial site and patient-related costs, including laboratory costs related to the conduct of the trial, and other costs. Its cost per patient varies based on the type of clinical trial, the site of the clinical trial and the length of the treatment period for each patient. As actual costs become known to it, it adjusts its accrual. These changes in estimates may result in a material change in its clinical study accrual, which could materially affect its results of operations. Research and development expenses includes those costs described under “Financial Operations Overview—Research and Development” below.
Stock-Based Compensation
As a normal practice, Curaxis compensates employees and non-employee directors through stock-based compensation. It applies Statement of Financial Accounting Standards No. 123R Share-based Payment, codified in ASC 718 Compensation – Stock Compensation , to stock-based compensation awards. ASC 718 requires the measurement and recognition of non-cash compensation expense for all share-based payment awards made to employees and directors, including employee stock options related to its 2001 Stock Option Plan and 2005 Stock Option Plan, based on fair values. Curaxis adopted this Statement on January 1, 2006 using the modified prospective application.
Stock compensation arrangements with non-employee service providers are accounted for in accordance with EITF No. 96-18, Accounting for Equity Instruments that are issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, codified in ASC 505-50 Equity-Based Payments to Non-Employees, using a fair value approach. The compensation costs of these arrangements are subject to re-measurement over the vesting terms as earned.
29
The measurement of stock-based compensation requires the use of complex option pricing models and application of judgment in selecting the appropriate valuation assumptions, such as volatility and expected term. Curaxis values its stock-based compensation using the Black-Scholes option pricing model and the single option award approach, in accordance with the requirements of ASC 718.
Curaxis does not have sufficient history of exercise behavior to develop estimates of expected terms since as of June 30, 2010, no options issued since inception of the plans have been exercised. The expected remaining terms based on the estimated time period to elapse prior to commercialization of Curaxis’ initial product. For options to outside consultants, Curaxis uses a term which is equal to the average period required for the options to fully vest and the contractual life of the option. As Curaxis gathers more history regarding its option exercise pattern, and as information regarding the option pattern of comparable companies in its industry becomes publicly available, it will review these estimates and revise them as appropriate. Different estimates of volatility and expected term could materially change the value of an option and the resulting expense.
In addition, Curaxis monitors equity instruments with non-standard provisions, such as performance-based vesting conditions, accelerated vesting based on achievement of performance milestones and features that require instruments to be accounted for as liabilities. Curaxis accounts for transactions in which services are received from non-employees in exchange for equity instruments based on the fair value of such services received or of the equity instruments issued, whichever is more reliably measured, in accordance with ASC 718 and ASC 505-50.
Accounting for equity instruments granted or issued by us under ASC 718 and ASC 505-50 requires fair value estimates of the equity instrument granted or sold. If Curaxis’ estimates of the fair value of these equity instruments are too high or too low, its expenses may be over or understated. The fair value of its common stock was determined by its board of directors. In the absence of a public trading market for its common stock, its board of directors considered objective and subjective factors in determining the fair value of its common stock. In all periods, the board of directors evaluated events that provided indicators of the fair value of its common stock. These events included, depending on the period, the purchase price of its common stock that was issued in private placements during the three years ended December 31, 2009 and the results of its clinical trials. Based on these factors, its board of directors determined that the value of the common stock underlying the stock options granted to employees through September 30, 2010 had a fair value that was equal to the exercise price.
Recently Issued Accounting Pronouncements
Curaxis’ management does not believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying financial statements.
Financial Operations Overview
Revenue
Curaxis had not yet generated any product revenue and does not expect to generate any product revenue for at least the next several years. It plans to seek to generate revenue from product sales if any of its drug candidates receive marketing approval and it begins commercial activities. It expected that any revenue it generates would fluctuate from quarter-to-quarter as a result of the timing, nature and amount of its commercialization activities, whether through direct sales by it or by payments under distribution or other collaboration agreements.
30
Research and Development Expense
Research and development expense consists primarily of:
|
·
|
salaries and related expenses for personnel engaged in research and development activities;
|
|
·
|
fees paid to contract research organizations and clinical sites in conjunction with clinical trials;
|
|
·
|
fees paid to research organizations in conjunction with preclinical studies;
|
|
·
|
costs of materials used in research and development;
|
|
·
|
consulting and sponsored research fees paid to third parties;
|
|
·
|
fees paid to external legal counsel and third parties filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and
|
|
·
|
depreciation of capital assets used to discover and develop our product candidates.
|
Curaxis expensed both internal and external research and development costs as incurred. Research and development expenses have diminished over the last several years due to termination of its clinical trials and closure of its research facility. As the result of the Merger, Curaxis expected research and development expenses to increase substantially in 2010 and thereafter and due to:
|
·
|
initiation of the Phase IIB clinical trials for Memryte for the treatment of mild to moderate Alzheimer’s disease in women;
|
|
·
|
initiation of the Phase III pivotal trials for Memrtye for the treatment of mild to moderate Alzheimer’s disease in women;
|
|
·
|
manufacture of clinical trial materials for these trials and continued development of the production process for Memryte; and
|
|
·
|
increased pre-clinical testing and clinical trials of its other oncology drug candidates.
|
Substantially all research and development expenses incurred by Curaxis to date relate to its efforts to develop Memryte for the treatment of mild to moderate Alzheimer’s disease. Curaxis expected that a substantial percentage of its research and development expense in the future will be incurred in support of its current and future pre-clinical and clinical development programs. These expenditures are subject to numerous uncertainties in timing and cost to completion. Curaxis tested compounds in numerous preclinical studies for safety, toxicology and efficacy. It then will conduct clinical trials for each drug candidate. As it obtains results from trials, it may elect to discontinue or delay clinical trials for some product candidates in order to focus its resources on more promising product candidates. Completion of clinical trials may take several years or more, but the length of time generally varies substantially according to the type, complexity, novelty and intended use of a drug candidate. The cost of clinical trials may vary significantly over the life of a project as a result of a variety of factors, including:
|
·
|
the number of patients who participate in the trials;
|
|
·
|
the number of sites included in the trials;
|
|
·
|
the length of time required to enroll trial participants;
|
|
·
|
the duration of the treatment period in the trials;
|
|
·
|
the effect of regulatory requirements on the trials;
|
31
|
·
|
the duration of patient follow-up; and
|
|
·
|
the efficacy and safety profile of the product candidate.
|
None of the drug candidates has received FDA or foreign regulatory marketing approval. In order to achieve marketing approval, the FDA or foreign regulatory agencies must conclude that the clinical data establishes the safety and efficacy of the drug candidates.
As a result of the uncertainties discussed above, although duration and costs will be substantial, Curaxis was unable to predict accurately the duration and completion costs of its research and development projects or if, when and to what extent it will receive cash inflows from the commercialization and sale of a product.
General and Administrative
General and administrative expense consists primarily of salaries and related expenses for personnel in administrative, finance, operations and human resource functions. Other costs include fees for general legal and other professional services, accounting fees, and directors’ and officers’ liability insurance premiums.
Marketing
Marketing expense consists primarily of salaries and related expenses for personnel responsible for clinical trial recruitment and costs of attendance at various scientific and industry meetings. Other costs include advertising for patient enrollment of clinical trials, promotion materials relating to scientific publications and fees for professional services related to public relations. Curaxis expects these expenses to increase significantly as it begins to develop its own commercialization capabilities.
Net Interest Income (expense)
Interest income consists of interest earned on Curaxis’ cash and cash equivalents. Interest expense consists of interest incurred on notes and mortgage debt payable and equipment and insurance premium debt financing.
Results of Operations
Nine Months Ended September 30, 2010 and 2009
The table below summarizes the results of operations of Curaxis for the nine month periods ended September 30, 2010 and 2009.
|
For the Nine Months ended
September 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
|
(Unaudited – Dollars in thousands)
|
|||||||
|
Operating expenses:
|
||||||||
|
Research and development
|
$
|
62
|
$
|
74
|
||||
|
General and administrative
|
1,493
|
451
|
||||||
|
Marketing
|
-
|
-
|
||||||
|
Total operating expenses
|
1,555
|
525
|
||||||
|
(Loss) income from operations
|
(1,555
|
)
|
(525
|
) | ||||
|
Gain on debt restructuring
|
204
|
6,562
|
||||||
|
Interest income (expense), net
|
(133
|
)
|
(299)
|
|||||
|
Interest from derivative conversion feature
|
(2,586
|
)
|
-
|
|||||
|
Change in fair value of derivatives
|
429
|
-
|
||||||
|
Net income (loss)
|
$
|
(3,641
|
)
|
$
|
5,738
|
|||
32
Curaxis accounts for equity awards to employees and non-employee directors and service providers under ASC 718 Compensation – Stock Compensation. As of September 30, 2010 there was $0.4 million of total unrecognized compensation costs related to unvested stock options. That cost is expected to be amortized on a straight-line basis over a weighted average service period of 1.7 years. Share-based compensation expenses recorded in the nine months ended September 30, 2010 and 2009 were $167 and $295 thousand, respectively.
Nine Months Ended September 30, 2010 Compared to Nine Months Ended September 30, 2009
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
Research and development costs
|
$
|
62
|
$
|
74
|
$
|
(12)
|
(16.2)
|
%
|
||||||||
|
Percent of total operating expenses
|
3.9
|
%
|
14.1
|
%
|
||||||||||||
During 2007, Curaxis terminated all clinical trial activities and closed its’ research facility. Further, the Company did not employ any personnel devoted to research and development activities in 2009 and 2010. As a result, research and development expenses in 2009 and 2010 were limited to legal fees associated with the pursuit and maintenance of various patents and pending patent applications related to our Alzheimer’s disease and oncology drug candidates and depreciation of remaining capital assets used in the manufacturing of our proprietary implant. All capital assets used in the manufacturing of our propriety implant were fully depreciated at December 31, 2009.
The decrease in research and development expenses for the nine months ended September 30, 2010 as compared to the same period for 2009 is due to a decrease in depreciation of the remaining capital assets of $40,000. In addition, during the nine-months ended September 30, 2009, expense of $9,000 was recognized on the ultimate disposal of remaining clinical trial material. These decreases were offset by an increase of $37,000 in legal and professional fees relating to our pending patent applications and maintenance of our existing patents.
General and Administrative Expenses
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
General and administrative expenses
|
$
|
1,493
|
$
|
451
|
$
|
1,042
|
231.0
|
%
|
||||||||
|
Percent of total operating expenses
|
96.1
|
%
|
85.9
|
%
|
||||||||||||
The increase in general and administrative expenses for the nine-months ended September 30, 2010 as compared to the same period for 2009 is primarily related to an increase in payroll and related benefit costs, an increase in legal and professional fees and an increase in occupancy and general administrative expense. Payroll and related benefit costs totaled $0.8 million for the nine months period ended September 30, 2010 compared to $40,000 for the respective period of 2009; resulting in an increase of $0.7 million. No compensation was paid or accrued for any employees including senior officers of Curaxis for the seven month period ended July 31, 2009. In addition $0.4 million in bonus expense was reversed in January of 2009 upon the termination of certain employees who had not fulfilled service requirements related to the 2004 Stock Bonus Plan.
Legal and Professional fees totaled $0.6 million for the nine month period ended September 30, 2010 compared to $0.3 for the comparable period of 2009; an increase of $0.3 million. Legal and professional fees reported for the nine month periods ended September 30, 2010 and 2009 include payments and stock based compensation due under agreements executed between the Curaxis and Southridge Business Solutions and Canterbury Investment Partners totaling $320,000 and $220,000, respectively; investor relations and web site development fees totaling $100,000 and $40,000, respectively; and legal and accounting fees of $230,000 and $40,000, respectively. Under the agreements executed in 2009, Southridge and Canterbury were to assist Curaxis in negotiating settlements with its creditors to eliminate or substantially reduce and defer its trade date and assist Curaxis in merging with a publicly traded corporation to establish a public trading market for its stock. Compensation for the services rendered includes monthly fees plus warrants issued for the purchase of the Company’s stock at agreed upon terms upon closing of the Merger transaction effective July 29, 2010.
Occupancy and general administrative expense totaled $70,000 for the nine months ended September 30, 2010 compared to $31,000 for the nine month period ended September 30, 2009. The increase is attributed to the lease of temporary corporate office space commencing in late 2009 and an increase in the operational activities of the company.
33
Gain on Debt Restructuring
|
Nine Months Ended
|
|||||||||||||
|
September 30,
|
|||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
||||||||||
|
(Unaudited - Dollars in thousands)
|
|||||||||||||
|
Gain on debt restructuring
|
$
|
204
|
$
|
6,562
|
$
|
(6,358
|
)
|
(96.9 )
|
%
|
||||
On January 22, 2010 the Company and a Creditor reached a settlement agreement where by the Company will make payments totaling $900,000 over the next two years to satisfy, in full, its obligation. Further, the agreement restricts the Company from transferring or pledging its intellectual property without prior consent of the Creditor. Immediately preceding the settlement balances recorded on the Company’s financial records with respect to this vendor account included accrued interest and notes payable of $135,000 and $924,000, respectively. The company evaluated the settlement under ASC 470-60, Troubled Debt Restructuring by Debtors. A gain totaling $159,000 was realized on the vendor settlement.
During 2009, one of the Company’s creditors filed a law suit against the Company relating to a claim for $45,000 for services rendered in connection with the Company’s terminated Phase III clinical trial, VP-AD-301. On March 3, 2010, the suit was dismissed with prejudice. A gain of $45,000 was realized on the vendor settlement.
On September 23, 2009, Curaxis reached an agreement with one of its vendors to satisfy related trade debt outstanding. The agreement requires Curaxis to make payments totaling $2 million commencing September 1, 2010 and ending March 31, 2012. Curaxis has the right to prepay any balances due without penalty. In addition, Curaxis agreed to prepay the entire balance of such payments in the event of an acquisition of all or substantially all of Curaxis’ assets or stock; in the event its lead product candidate is licensed to a third party; in the event any patent related to its lead product candidate is sold to any third party; or in the event that it obtains financing in excess of $20 million. Any payment not made in accordance with this agreement shall bear interest at 18% per annum. Immediately preceding the settlement, balances recorded on the financial records with respect to the vendor account included accounts payable, accrued interest and notes payable of $4.7 million, $1.1 million and $2.8 million, respectively. Curaxis evaluated the settlement under ASC 470-60, Troubled Debt Restructurings by Debtors. As a result, a gain totaling $6.6 million was realized on the vendor settlement.
Interest expense, Net
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
Interest expense, net
|
$
|
133
|
$
|
299
|
$
|
(166
|
)
|
(55.5)
|
%
|
|||||||
Interest expense, net for the nine months ended September 30, 2010 and 2009 includes mainly interest accrued on Curaxis’ outstanding promissory notes payable to certain vendors and clinical sites. The majority of the notes outstanding were issued during 2007 and 2008 to satisfy, in part, liabilities generated for services performed in conjunction with the terminated Phase III clinical trial.
The decrease in expense for the nine month period ended September 30, 2010, as compared to the respective period of 2009, can be attributed to a decrease in interest expense related to restructured trade debt of $227,000. This decrease was offset by an increase in interest expense of $59,000 resulting from two judgments awarded on behalf of trade creditors in April and October, 2009 and interest on debt issued in connection with the Merger transaction completed in July 2010 of $2,000.
Interest from derivative conversion feature
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
Interest from derivative conversion feature
|
$
|
2,586
|
$
|
-
|
$
|
2,586
|
100.0
|
%
|
||||||||
34
Interest from derivative conversion feature for the nine months ended September 30, 2010 represents the fair value adjustment recorded upon the initial sale of the preferred securities. We have an obligation to make cash payments to the holders of the preferred stock for any loss that could be realized if the holder converts the preferred stock and we subsequently fail to deliver the certificates representing the shares to be issued upon the conversion by the third trading day of such conversion. Accordingly, the preferred stock has been accounted for as a derivative liability. The fair value of the derivative liability is based on the number of potential common shares issuable upon conversion multiplied by the quoted market price of the Company’s stock as of the date each preferred stock is issued and revalued at each reporting period thereafter.
Change in fair value of derivatives
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
Change in fair value of derivatives
|
$
|
429
|
$
|
-
|
$
|
429
|
100.0
|
%
|
||||||||
During the nine months ended September 30, 2010, the Company issued Series A and Series B Convertible preferred stock. Because the Series A and Series B stock provide for a potential cash settlement in certain circumstances, the preferred stock has been classified as a liability at September 30, 2010. The liability has been recorded at fair value based on potential shares of common stock issuable upon conversion multiplied by the current quoted market price of the company’s common stock and is subject to re measurement. The change in fair value recognized reflects the decrease in the quoted market price of the Company’s stock from the date of the initial issuance of the stock through September 30, 2010.
Net Income (Loss)
|
Nine Months Ended
|
||||||||||||||||
|
September 30,
|
||||||||||||||||
|
2010
|
2009
|
$ Change
|
% Change
|
|||||||||||||
|
(Unaudited - Dollars in thousands)
|
||||||||||||||||
|
Net income (loss)
|
$
|
(3,641
|
)
|
$
|
5,738
|
|
$
|
(9,379
|
)
|
(163.5)
|
%
|
|||||
Net loss for the nine months ended September 30, 2010, was $3.6 million, including a $0.2 million relating gain to the restructuring of trade debt, compared net income $5.7 million, including a $6.6 million gain relating to the restructuring of trade debt, for the same period in 2009. The decrease net income is primarily due to the interest and fair value adjustments recognized on convertible preferred stock issued and the increase in general and administrative expenses offset by the gain recognized on the restructuring of debt.
Years Ended December 31, 2009, 2008 and 2007
The results of operations of Curaxis for each of the years ended December 31, 2009, 2008 and 2007 are as follows:
|
Year Ended December 31,
|
||||||||||||
|
2009
|
2008
|
2007
|
||||||||||
|
(Dollars in thousands)
|
||||||||||||
|
Operating expenses:
|
||||||||||||
|
Research and development
|
$
|
132
|
75
|
$
|
3,057
|
|||||||
|
General and administrative
|
1,077
|
1,433
|
2,472
|
|||||||||
|
Marketing
|
-
|
-
|
357
|
|||||||||
|
Loss on lease termination
|
-
|
-
|
374
|
|||||||||
|
Total operating expenses
|
1,209
|
1,508
|
6,260
|
|||||||||
|
Loss from operations
|
(1,209
|
)
|
(1,508
|
)
|
(6,260
|
)
|
||||||
|
Gain on debt restructuring
|
6,562
|
-
|
-
|
|||||||||
|
Interest income (expense), net
|
(366
|
)
|
(478
|
)
|
(484
|
)
|
||||||
|
Net income (loss)
|
$
|
4,987
|
(1,986
|
)
|
$
|
(6,744
|
)
|
|||||
During 2007, Curaxis terminated all clinical trial activities and closed its research facility. As a result, research and development expenses in 2009 and 2008 were limited to salary and benefit costs associated with remaining personnel devoted to research activities, legal fees associated with the pursuit and maintenance of its patents related to its Alzheimer’s disease and oncology drug candidates and depreciation of remaining capital assets used in the manufacturing of its proprietary implant.
For fiscal year 2007, research and development costs included expenses associated with the closure of clinical trials related to its lead drug candidate Memryte, and development of new products as well as enhancements to existing products.
For fiscal year 2007, marketing expenses primarily consisted of salaries and related benefits and overhead costs for employees associated with marketing activities. Such activities were limited to coordination efforts related to the closure of clinical sites.
Curaxis accounts for equity awards to employees and non-employee directors and service providers under ASC 718 Compensation – Stock Compensation and ASC 505-50 Equity-based Payments to Non-Employees. As of December 31, 2009, there was $0.4 million of total unrecognized compensation costs related to unvested stock options. That cost is expected to be amortized on a straight-line basis over a weighted average service period of 1.03 years. Stock-based compensation expense recorded in the years ended December 31, 2009, 2008 and 2007 was $0.6 million, $0.5 million and $0.9 million, respectively.
35
Year Ended December 31, 2009 Compared to Year Ended December 31, 2008
Research and Development Expense
|
Year ended
December 31, |
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Research and development costs
|
$
|
132
|
$
|
75
|
$
|
57
|
76.0
|
%
|
||||||||
|
Percent of total operating expenses
|
10.9
|
%
|
5.0
|
%
|
||||||||||||
During 2007, Curaxis terminated all clinical trial activities and closed its research facility. As a result, research and development expenses in 2008 and 2009 were limited to salary and benefits costs associated with personnel devoted to research activities, legal fees associated with the pursuit and maintenance of various patents and pending patent applications related to its Alzheimer’s disease and oncology drug candidates and depreciation of remaining capital assets used in the manufacturing of its proprietary implant.
In May of 2008, Curaxis received a refund of $31,000 upon termination of its product liability insurance coverage obtained in anticipation of enrollment of Aladdin 302, the Phase III international clinical trial, and reversed $34,000 in certain costs previously recorded for Aladdin 301, the Phase III domestic clinical trial, upon final reconciliation and settlement with certain clinical sites. In addition, in October 2009, a default judgment was issued on behalf of a creditor seeking payment of amounts due for services rendered with respect to Curaxis' terminated Phase III clinical trial; additional expenses totaling $37,000 were recognized with respect to the terminated trial. The resulting increase in expenses for the year ended December 31, 2009 of $102,000 was offset by a decrease in salaries and related benefit costs of $24,000 due to the ultimate termination of all research personnel in early 2008; a decrease of $19,000 in depreciation of capital assets due to the ultimate sale of certain lab equipment in early 2008; and an aggregate decrease of $2,000 in legal and miscellaneous fees associated with Curaxis’ research and development activities.
General and Administrative Expenses
|
Year Ended
December 31, |
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
General and administrative expenses
|
$
|
1,077
|
$
|
1,433
|
$
|
(356)
|
(24.8)
|
%
|
||||||||
|
Percent of total operating expenses
|
89.1
|
%
|
95.0
|
%
|
||||||||||||
The decrease in general and administrative expenses for the year ended December 31, 2009 as compared to the same period for 2008 was primarily related a reduction in payroll and related benefit costs of $.8 million and occupancy expense of $0.1 million. For the seven-month period ended July 31, 2009, no compensation was paid or accrued for any employees including senior officers of Curaxis. In addition $0.4 million in bonus expense was reversed in January of 2009 upon the termination of certain employees who had not fulfilled service requirements related to the 2004 Stock Bonus Plan.
The decreases noted above were offset by consulting and investment advisory fees recognized, professional fees associated with the proposed merger transaction, investor relations fees to develop Curaxis’ new corporate image and website, retention payments made to current officers and a stock grant to a former employee. During the year ended December 31, 2009, expenses of $0.4 million were recognized under agreements executed between Curaxis and Southridge and Canterbury Investment Partners. Under the agreements executed, Southridge and Canterbury were to assist Curaxis in negotiating settlements with its creditors to eliminate or substantially reduce and defer its trade date and assist Curaxis in merging with a publicly traded corporation to establish a public trading market for its stock. The expense recognized reflects retainers and monthly fees paid and the costs accrued to date associated with warrants to be issued to Southridge upon consummation of the Merger.
36
Fees paid for audit and investor relation services totaled $100,000 for the year ended December 31, 2009; no similar services were provided in 2008. Retention payments to current officers totaled $22,000 for the year ended December 31, 2009. In addition, in December, 2009, the board of directors granted 150,000 shares of common stock to a former executive officer to satisfy, in full, amounts due under an original five-year employment contract; the expense recognized on the grant totaled $30,000.
Gain on Debt Restructuring
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Gain on debt restructuring
|
$
|
6,562
|
$
|
-
|
$
|
6,562
|
100
|
%
|
||||||||
On September 23, 2009, Curaxis reached an agreement with one of its vendors to satisfy related trade debt outstanding. The agreement requires Curaxis to make payments totaling $2 million commencing September 1, 2010 and ending March 31, 2012. Curaxis has the right to prepay any balances due without penalty. In addition, Curaxis agreed to prepay the entire balance of such payments in the event of an acquisition of all or substantially all of Curaxis’ assets or stock; in the event its lead product candidate is licensed to a third party; in the event any patent related to its lead product candidate is sold to any third party; or in the event that it obtains financing in excess of $20 million. Any payment not made in accordance with this agreement shall bear interest at 18% per annum. Immediately preceding the settlement, balances recorded on the financial records with respect to the vendor account included accounts payable, accrued interest and notes payable of $4.7 million, $1.1 million and $2.8 million, respectively. Curaxis evaluated the settlement under ASC 470-60, Troubled Debt Restructurings by Debtors. As a result, a gain totaling $6.6 million was realized on the vendor settlement.
Interest expense, Net
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Interest expense, net
|
$ | 366 | $ | 478 | $ | (112 | ) | (23.4 | )% | |||||||
Interest expense, net for the year ended December 31, 2009 and 2008 includes mainly interest accrued on Curaxis’ outstanding promissory notes payable to certain vendors and clinical sites. The majority of the notes outstanding were issued during 2007 and 2008 to satisfy, in part, liabilities generated for services performed in conjunction with the terminated Phase III clinical trial. The decrease in expense for the year ended December 31, 2009, as compared to the respective period of 2008, can be attributed to judgments issued in late 2008 and early 2009 on two notes outstanding resulting in a decrease in related interest expense of $28,000 and a decrease in interest expense related to the restructured trade debt of $115,000. These decreases were offset by additional interest expense of $28,000 recognized as a result of default judgment issued in late 2009 on behalf of a creditor and an increase interest expense recognized on notes to clinical sites of $3 thousand.
Net Income (Loss)
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2009
|
2008
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Net income (loss)
|
$
|
4,987
|
$
|
(1,986
|
)
|
$
|
6,973
|
351.1
|
%
|
|||||||
Net income for the year ended December 31, 2009, was $5.0 million, including $6.6 million relating to the restructuring of trade debt, compared to $2.0 million net loss for the same period in 2008. The increase in net income is primarily related to the restructuring of debt and a decrease in operating expenses.
37
Year Ended December 31, 2008 Compared to Year Ended December 31, 2007
Research and Development Expense
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Research and development expense
|
$
|
75
|
$
|
3,057
|
$
|
(2,982
|
)
|
(97.6
|
)%
|
|||||||
|
Percent of total operating expenses
|
5.0
|
%
|
48.8
|
%
|
||||||||||||
The decrease in research and development was primarily related to the termination of the clinical trial activities of Curaxis and closure of its research facility in 2007. Research and development expense for fiscal year 2008 includes salary and related benefit costs of $28,000 compared to $660,000 for fiscal year 2007; a decrease of $632,000 reflecting the elimination of nine full time staff equivalents from January 1, 2007 to March 31, 2008.
Costs associated with the clinical trials, specifically VP-AD-103 and VP-AD-301, storage and destruction of the related drug supply, and related insurance costs totaled $1.3 million for fiscal year 2007; no costs related to these activities were recognized in fiscal year 2008. Further, in 2008, Curaxis received a refund of $31,000 upon termination of its product liability insurance coverage obtained in anticipation of enrollment of Aladdin 302, the Phase III international clinical trial, and reversed $34,000 in certain costs previously recorded for Aladdin 301, the Phase III domestic clinical trial, upon final reconciliation and settlement with certain clinical sites.
Expenses associated with Curaxis’ research facility, including rent, utilities, depreciation, external research and allocated overhead costs totaled $706,000 for fiscal year 2007 compared to $60,000 for fiscal year 2008; a decrease of $646,000. The 2008 expense relates solely to the depreciation of capital assets used in the manufacturing process.
In addition, for the year ended December 31, 2008, services and activities provided by outside legal counsel and the Scientific Advisory Board were curtailed significantly, resulting in decreased expenses of $200,000 and $30,000, respectively.
General and Administrative Expense
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
General and administrative expense
|
$
|
1,433
|
$
|
2,472
|
$
|
(1,039
|
) |
(42.0
|
)% | |||||||
|
Percent of total operating expenses
|
95.0
|
% |
39.5
|
% | ||||||||||||
The majority of the decrease in general and administrative expense can be attributed to reductions in general and administrate staff, a reduction in salary for all officers, and downsizing of the corporate office. General and administrative expense for fiscal year 2008 includes salary and related benefit costs of $672,000 compared to $1.2 million for fiscal year 2007; a decrease of $528,000 reflecting the elimination of five full time staff equivalents from January 1, 2007 to March 31, 2008 and a 15% reduction in the salary of all exempt employees effective April 1, 2008. In addition, share-based compensation expenses recognized for fiscal year 2008 totaled $469,000 compared to $783,000 for fiscal year 2007, a decrease of $314,000.
General and administrative expense for fiscal year 2008 includes rent, utilities, depreciation and maintenance expenses totaling $121,000 compared to $605,000 for fiscal year 2007; a decrease of $484,000 reflecting relocation and downsizing of the corporate office location and the transfer related furniture and fixtures to the landlord upon early termination of Curaxis’ sublease agreement.
38
Due to the reduction in personnel and corporate activities decreases in legal and professional fees, corporate insurance, travel expense, board of directors fees and other miscellaneous expenses of $81,000, $83,000, $37,000, $30,000, and $16,000, respectively, were recognized for fiscal year 2008 as compared to fiscal year 2007. Further, in 2008, Curaxis recognized $50,000 income on terminated sponsorships, which reduced the reported general and administrative expense.
The above decreases in general and administrative expense were offset by an increase in allocated corporate overhead of $584,000 for fiscal year 2008 as compared to fiscal year 2007 due to the elimination of research and development and marketing activities of Curaxis in 2008.
Marketing Expense
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Marketing expense
|
$
|
-
|
$
|
357
|
$
|
(357
|
)
|
(100.0
|
)%
|
|||||||
|
Percent of total operating expenses
|
0.0
|
%
|
5.7
|
%
|
||||||||||||
The decrease in marketing expense can be attributed to the decision of management to cease all marketing related activities due to financial restraints in the third quarter of 2007 and to terminate all related employees. The expense recognized for fiscal year 2007 includes salaries and related benefit costs of $204,000, share-based compensation expense of $60,000, travel expense of $19,000 and allocation of corporate expenses including rent, utilities, supplies and insurance totaling $173,000. These expenses were offset by the reversal of $98,000 in bonus expense upon the termination of certain marketing employees who had not fulfilled service requirements related to the 2004 Stock Bonus Plan as of their termination date.
Loss on Lease Termination
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Loss on Lease Termination
|
$
|
-
|
$
|
374
|
$
|
(374
|
)
|
(100.0
|
)%
|
|||||||
|
Percent of total operating expense
|
0.0
|
%
|
6.0
|
%
|
||||||||||||
On June 30, 2007, Curaxis entered into a Termination of Sublease Agreement with its sub landlord for release on its obligation related to its corporate office location. The original lease term expired on March 31, 2011. In consideration for acceptance of the early release, Curaxis conveyed furniture and equipment located on the premises and executed a promissory note in the amount of $300,000. A loss on the early termination of the sublease agreement of $374,000 was recognized by Curaxis.
Interest expense, net
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Interest expense, net
|
$
|
478
|
$
|
484
|
$
|
(6
|
)
|
(1.2
|
)%
|
|||||||
Interest expense, net for fiscal year 2008 reflects interest expense recognized on promissory notes payable outstanding to certain vendors and clinical trial sites. Interest expense, net for fiscal year 2007 reflects interest expenses recognized on promissory notes payable to certain vendors and clinical sites totaling $510,000 net of interest income recognized on cash available for investment totaling $26,000.
39
The majority of the promissory notes outstanding were issued during 2007 and 2008 to satisfy, in part, liabilities generated for services performed in conjunction with Curaxis’ terminated Phase III clinical trial. The decrease in interest expense recognized for the year ended December 31, 2008, compared to the year ended December 31, 2007, of $32,000 can be attributed to a decline in the average balance outstanding approximating $200,000 coupled with a decrease in the weighted average rate of current borrowings from 11.8% to 10.8%.
Net Loss
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2008
|
2007
|
$ Change
|
% Change
|
|||||||||||||
|
(Dollars in thousands)
|
||||||||||||||||
|
Net loss
|
$
|
(1,986
|
)
|
$
|
(6,744
|
)
|
$
|
(4,578
|
)
|
(70.6
|
)%
|
|||||
Net loss for the year ended December 31, 2008, was $2.0 million compared to net loss of $6.7 million for the same period in 2007. The reduction in the net loss of $4.6 million resulted from a decrease in research and development expenses of $3.0 million, a decrease in general and administrative expenses of $1.0 million, a decrease in marketing expense of $0.4 million and the elimination of the loss of $0.4 million recognized on the early termination of a lease agreement in June of 2007.
Liquidity and Capital Resources
Curaxis has been a developmental stage pharmaceutical company, has had no FDA approved products and has generated no commercial revenue. Since inception, Curaxis has incurred losses from operations and has reported negative cash flows.
As of September 30, 2010, Curaxis had an accumulated deficit of $91.2 million and cash and cash equivalents of $0.3 million. The cash position of Curaxis continues to be insufficient to satisfy its obligations with many vendors. Curaxis will continue to incur operating losses and be unencumbered by revenue until it is successful in developing and commercializing its lead product candidate, Memryte. Curaxis estimated that expenses to complete the clinical development of Memryte and to seek FDA approval to market Memryte will total at least $48 million. Such expenses will be incurred over a four-year period. Historically, Curaxis had relied on private placements of its common stock to fund operations.
In 2009, Curaxis entered into a series of agreements with Southridge and Canterbury Investment Partners LLC (“Canterbury”) in order to restructure its balance sheet and establish a trading market for its common stock. Under the terms of those agreements, Southridge and Canterbury have assisted and will continue to assist Curaxis in negotiating settlements with its creditors to eliminate or substantially reduce and defer its trade debt. To date, Curaxis had realized reductions in excess of $6 million in liabilities as the result of the combined efforts. In addition, Southridge assisted Curaxis in securing a $25 million equity facility under which Curaxis can periodically, over a period of three years, sell up to $25 million of its common stock to a third party or affiliate of Southridge. The proceeds of the equity facility will be used to fund the next step in Curaxis' clinical development plan, a Phase IIb study in approximately 200-250 women. Assuming the study commences in early 2011, it is anticipated that the results of the study would be available during 2013. Assuming favorable results from the second Phase II trial are achieved, Curaxis’ management expected to fund the remaining development of its product candidate through public equity offerings, debt financings and possibly corporate collaborations and licensing arrangements. However, the ability to secure such funding on a prospective basis is uncertain. If Curaxis is unable to secure funding as intended, Curaxis will be forced to delay, reduce or eliminate its research and development programs or commercialization efforts.
Southridge has continued to assist Curaxis in meeting its interim cash flow needs. To date, Southridge has advanced the Company $885,000 to assist with its operating cash needs. The funds advanced have been used by the Company to fund certain costs associated with the Merger transaction completed, initiation of public and investor relations initiatives, required payments under trade debt agreements and general operational expenses. In return, the Company issued shares of Series A and Series B 4% Convertible Preferred Stock.
40
Off-Balance Sheet Arrangements
Curaxis had no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on its financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to stockholders.
Controls and Procedures
Management, including our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a – 15(f).
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements should they occur. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the control procedure may deteriorate.
BUSINESS
Corporate History
We were incorporated on February 1, 2008 under the laws of the State of Nevada under the name Auto Search Cars, Inc. On February 8, 2010, we entered into a merger agreement (as amended on July 29, 2010, the “Merger Agreement”) by and among Auto Search Cars, Inc., Auto Search Cars Acquisition Corp., a Delaware Corporation (“Acquisition Corp.”), and Curaxis Pharmaceutical Corporation, a corporation incorporated in Delaware on February 27, 2001 (“Curaxis”) (the “Merger”).
On July 29, 2010 (the “Closing Date”), pursuant to the Merger Agreement, (i) the common stock of Curaxis (the “Curaxis Common Stock”) became no longer outstanding and existing, and was cancelled and retired, and (ii) each holder of Curaxis Common Stock ceased to have any rights with respect to their shares of Curaxis Common Stock, except the right to receive shares of our common stock. Each holder of Curaxis Common Stock received one (1) share of Auto Search common stock for each share of Curaxis Common Stock they own immediately prior to completion of the Merger. Each share of the Auto Search Common Stock issued to the Curaxis stockholders pursuant to the Merger Agreement was restricted from trading or resale for a period of one (1) year commencing at the effective date of the Merger. Such restriction did not apply to Auto Search Common Stock issued in exchange for shares of Curaxis Common Stock issued in Curaxis' Bridge Financing (as defined in the Merger Agreement). Auto Search stockholders continued to own their existing shares, which were not affected by the Merger.
On the Closing Date, Curaxis and Acquisition Corp. merged with and into one another, with the surviving corporation being Curaxis. Simultaneously with the filing of a Certificate of Merger with the State of Delaware, Curaxis amended its Certificate of Incorporation in order to change its name to Curaxis Pharma Corp. On the Closing Date, Curaxis Pharma Corp. became our subsidiary.
On the Closing Date, Curaxis and Acquisition Corp. merged with and into one another, with the surviving corporation being Curaxis. Simultaneously with the filing of a Certificate of Merger with the State of Delaware, Curaxis amended its Certificate of Incorporation in order to change its name to Curaxis Pharma Corp. On the Closing Date, Curaxis Pharma Corp. became our subsidiary.
On July 30, 2010, we entered into an agreement and plan of merger with Curaxis Pharmaceutical Corporation, a Nevada corporation formed solely for the purpose of a name change (the “Short-Form Merger”). Pursuant to the Short-Form Merger, we changed our name to Curaxis Pharmaceutical Corporation.
As the result of the Merger, we acquired Curaxis Pharmaceutical Corporation (“Curaxis”). Curaxis was incorporated on February 27, 2001 under the laws of the state of Delaware.
Curaxis is an emerging specialty pharmaceutical company with a hormone drug product candidate for the treatment of Alzheimer’s disease and multiple cancers. Its therapeutic platform is based on the hypothesis that many diseases of aging may be caused by age-related changes in the function of the hypothalamic-pituitary-gonadal (HPG) axis. The HPG axis is a hormonal endocrine feedback loop that controls development, reproduction and aging in animals. This drug development platform is built on the premise that hormones associated with this feedback loop are beneficial early in life, when they promote growth and development, but are harmful later in life when the mechanism for feedback is compromised, thereby leading to disease processes, including pathologies associated with Alzheimer’s disease and various cancers. Curaxis believed that its discovery of similar hormonal signaling mechanisms at the cellular level in brain tissue from Alzheimer’s patients and in multiple tumors would enable it to develop significant new treatments for Alzheimer’s disease as well as many cancers.
41
Curaxis is a development stage company and has not yet (i) received FDA approval for any of its products; and (ii) generated any commercial revenue through the sale of its products.
After the Merger, we decided that we will not be pursuing the business activities of Auto Search, but instead will be pursuing the business activities of Curaxis. Curaxis’ lead product candidate is a hormone for the treatment of Alzheimer’s disease. It had previously conducted several clinical trials for this indication, the most recent of which it were forced to terminate in 2006 due to financial constraints. Since terminating those trials, it had been unable to advance the clinical development of its Alzheimer’s disease candidate due to a lack of financial resources. We anticipate that we will be able to raise the funds necessary to restart the clinical development of Curaxis’ Alzheimer’s disease candidate, but there can be no assurance that we will be able to do so.
Our Alzheimer’s Disease Program
Leuprolide acetate, a hormone analogue of naturally occurring gonadotropin-releasing hormone (GnRH), has been widely used over the past twenty years for the treatment of a number of hormone-related disorders, most notably prostate cancer and endometriosis and precocious puberty, and has a well-established safety profile. Curaxis had conducted extensive pre-clinical and clinical studies exploring the use of leuprolide acetate for the treatment of Alzheimer’s disease in mild-to-moderate patients. The results to date are encouraging and point especially to a potentially significant new treatment for women with Alzheimer’s disease. Women represent approximately two-thirds of Alzheimer’s patients. However, our ability to conduct further clinical development of Curaxis’ Alzheimer’s disease candidate will be dependent on our ability to raise the funds required to pay for such further clinical development activities. While our management believes that, following the Merger, we will be able to do so, there can be no assurance that we will be successful in raising any such funds subsequent to the Merger.
Men present greater challenges in the use of leuprolide to treat Alzheimer’s disease because leuprolide suppresses their production of testosterone, which could necessitate patient self-administration of supplemental testosterone and which can lead to wide swings in testosterone blood serum levels. Therefore, in the near-term, we plan to concentrate our development efforts on the use of leuprolide acetate to treat women, although we will continue our efforts to better understand mechanisms that might lead to optimum outcomes in men.
Alzheimer’s Disease
Background and Demographics
Alzheimer’s disease is named after Dr. Alois Alzheimer, a German physician, who first described the disease in 1906. Alzheimer’s disease is a progressive, degenerative and ultimately terminal brain disorder that gradually destroys a person’s memory and ability to learn, reason, make judgments, communicate and carry out daily activities. Alzheimer’s disease patients may also experience changes in personality and behavior, such as anxiety, suspiciousness and agitation, as well as delusions or hallucinations as the disease progresses. Alzheimer’s disease is invariably associated with, and defined by, the loss of connections between, and the death of, neurons, as well as deposits of the beta amyloid protein into plaques and the formation of neurofibrillary tangles (consisting of tau protein) in the brain. Existing approved therapies treat the symptoms of some patients with Alzheimer’s disease by temporarily enhancing a patient’s cognitive function and general behavior for a period of time; however, there is no existing treatment that stops or materially slows Alzheimer’s disease progression. Unless the patient first succumbs to some other disease, Alzheimer’s disease eventually leads to the patient’s total incapacitation and ultimately to death.Alzheimer’s disease is an age-related disease. The Alzheimer’s Association estimates that 13% of all individuals over the age of 65 suffer from Alzheimer’s disease and that nearly 50% of all individuals who reach age 85 suffer from Alzheimer’s disease. According to the 2009 World Alzheimer Report by Alzheimer’s Disease International, an international coalition of national Alzheimer’s organizations, 35 million people worldwide suffer from Alzheimer’s disease, including an estimated 5.3 million in the United States, and the number of people with Alzheimer’s is expected to nearly double every 20 years, to 65.7 million in 2030 and 115.4 million in 2050. The Alzheimer’s Association estimates that approximately 450,000 new cases of Alzheimer’s disease are diagnosed annually in the United States. More women suffer from Alzheimer’s disease than do men. Alzheimer’s disease onset has been reported in Down’s syndrome individuals aged as young as 30, with a dramatic increase in prevalence with aging.
42
The Alzheimer’s Association reports that Alzheimer’s disease patients live an average of eight years, with many patients living as much as 20 years, from the initial onset of symptoms. Direct and indirect annual costs of caring for individuals with Alzheimer’s disease in the United States are at least $140 billion, according to estimates used by the Alzheimer’s Association and the National Institute on Aging. The Alzheimer’s Association estimates the average lifetime cost of care for an individual with Alzheimer’s disease in the United States to be approximately $174,000 and have predicted that the costs associated with caring for Alzheimer’s patients will increase dramatically as the population, on average, becomes older.
Current Scientific Theories
Curaxis’ LH Hypothesis
Based upon Curaxis’ pre-clinical and clinical studies, we believe that inappropriately elevated levels of pituitary hormones produced in response to normal aging may be important in promoting the development of Alzheimer’s disease. At the time of menopause in females, when estrogen production by the ovaries falls precipitously, gonadotropins (luteinizing hormone (LH) and follicle-stimulating hormone (FSH)) become elevated. In aging males, testosterone production by the testes drops about one percent per year, leading to a more gradual increase in gonadotropins over time. Our preclinical research suggests that LH, which is released by the pituitary gland, is associated with many of the pathologies currently being researched in Alzheimer’s disease, including amyloid beta deposition which leads to formation of plaques in the brain tissue, tau phosphorylation (addition of phosphate groups to tau protein) which leads to formation of neurofibrillary tangles inside neurons, abnormal cell division, oxidative stress, and inflammation. We believe that elevated levels of LH that are known to occur with aging contribute to many of these pathological changes, ultimately resulting in cognitive decline and Alzheimer’s disease. We refer to the hypothesis that links LH to the development of Alzheimer’s disease as the LH Hypothesis.
Our lead product candidate, VP4896, is designed to dramatically reduce levels of LH in the bloodstream and in brain tissue through administration of a long-term, controlled release dose of leuprolide acetate, which suppresses LH production. We hold an issued United States patent with claims directed to the treatment of Alzheimer’s disease by administering any agent, including leuprolide acetate that reduces or eliminates serum levels of gonadotropins. In Curaxis’ Phase I trial, trial 105, its proprietary dosage form of leuprolide acetate, VP4896, has been demonstrated to dramatically suppress or eliminate LH levels in the serum of healthy male and female subjects.
The following summarizes the prominent hypotheses regarding the cause of Alzheimer’s disease and provides an overview of evidence we have that links the LH Hypothesis to each of these hypotheses.
Beta Amyloid Hypothesis
There are several hypotheses regarding the cause of Alzheimer’s disease, the predominant one being the beta amyloid hypothesis. The assumption behind this hypothesis is that amyloid beta protein, which makes up the plaques present in the brains of Alzheimer’s disease patients, is toxic and is the causative agent of the disease. The generally accepted view is that inhibiting the production of, and enhancing the clearance of, amyloid beta protein would reduce the formation of and possibly eliminate amyloid plaques that might be toxic to neurons, therefore preventing or treating Alzheimer’s disease. Based on this hypothesis, many companies have designed therapies to suppress or eliminate amyloid beta protein in order to affect the rate of progression of Alzheimer’s disease. Research based on the beta amyloid hypothesis has been ongoing for two decades without yielding any approved therapies to date.
While we do not specifically target amyloid beta elimination, we have evidence that links leuprolide acetate treatment in animal models to significant reductions in amyloid beta concentrations, and more importantly to a decreased rate of cognitive decline.
43
In a paper published in the Journal of Biological Chemistry in May 2004, Curaxis showed that leuprolide treatment of normal mice significantly reduced the concentrations of brain amyloid beta (1-42) by 71% after four weeks, and amyloid beta (1-40) by 40% after eight weeks. Amyloid beta (1-42) and (1-40) are different segments of amyloid present in the brain, with amyloid beta (1-42) representing a more toxic form of the protein (Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition . J Biol Chem 279(19):20539-20545).
In a paper published in Biochemica Biphysica Acta in April 2006, Curaxis showed that leuprolide, when given to mice with Alzheimer’s-like disease, significantly reduced concentrations of brain amyloid beta, and resulted in stabilized, and in some cases, improved cognition when leuprolide treated mice were compared to those which received placebo. (Luteinizing hormone modulates cognition and amyloid-ß deposition in Alzheimer APP transgenic mice. Bioch Biophys Acta 1762(4)447-452).
Tau Hypothesis
Another prominent theory is the Tau Hypothesis. Tau proteins contribute to the internal structural architecture of neurons. In Alzheimer’s disease, tau proteins gain unusually high levels of phosphate groups (become phosphorylated) and a portion of the protein is apparently sliced off to create protein fragments. These altered tau protein fragments lose their ability to function normally and stick together in structures called neurofibrillary tangles which form within the neuron and are thought to be neurotoxic. These tangles, together with plaques formed by amyloid beta, are the primary hallmarks of Alzheimer’s disease found in the brains of Alzheimer’s disease patients when autopsied.
While we do not target the reduction of neurofibrillary tangles, we have evidence that LH, which is suppressed by VP4896, actively and rapidly induces the phosphorylation of tau proteins and may therefore be one of the causative agents for tangle formation. We have evidence from laboratory experiments that LH treatment of neuroblastoma cell lines results in increased phosphorylation of tau protein, whereas leuprolide acetate reduces the levels and slows the kinetics of tau phosphorylation.
Abnormal Cell Division Hypothesis
Another hypothesis, called the cell cycle hypothesis, proposes that neurological and biochemical changes associated with Alzheimer’s disease are caused by the abnormal re-entry of brain cells into the cell division cycle, a process by which one cell replicates itself and divides into two cells. In general, it is thought that adult brain cells have lost the ability to successfully divide. Thus, this hypothesis suggests that when adult brain cells are stimulated to divide, the neurological changes seen in Alzheimer’s disease result. The proponents of this hypothesis believe that instead of successfully completing cell division, these brain cells die, and this brain cell death is believed to produce the clinical deficits observed in Alzheimer’s disease patients. We have evidence from laboratory experiments that LH treatment of glioblastoma and neuroblastoma cells in culture causes increased cell division that can subsequently be prevented by concomitant treatment with leuprolide acetate.
Oxidative Stress
Oxidative stress results from the production of toxic chemical by-products that arise from normal cellular metabolism and has also been implicated and studied as a potential cause of Alzheimer’s disease. These toxic by-products may cause direct damage to cells or may induce genetic mutations or DNA damage. With respect to the LH hypothesis, treatment of a neuronal cell line with LH in vitro has produced preliminary data that suggests that LH may inhibit enzymes that are essential to the body’s management of oxidative stress. In other words, LH may enhance oxidative stress and the elimination of LH might reduce the impact of oxidative stress on cells.
44
Inflammation
Inflammation, which is a normal physiological reaction to infection, can sometimes produce damage. Autoimmune diseases such as rheumatoid arthritis and multiple sclerosis are examples of undesirable and uncontrolled inflammatory events. Inflammation has been implicated as a possible cause of Alzheimer’s disease and it has been suggested that reducing chronic inflammation might provide benefit. Several anti-inflammatory therapies, including drugs as common as Ibuprofen, have been clinically tested for their ability to benefit Alzheimer’s patients. To date, anti-inflammatory therapies have not proven successful. With respect to the LH hypothesis, laboratory data suggests that LH promotes the production of growth factors (cytokines) that enhance or magnify the inflammatory response and LH may, therefore, contribute to Alzheimer’s disease by exacerbating inflammatory processes in the brain.
LH Hypothesis – The Human Reproductive Hormone Feedback Loop
Most biochemical processes in the body are tightly regulated and are subject to both positive and negative feedback. Positive feedback promotes a certain reaction, such as the synthesis of a hormone, while negative feedback inhibits a reaction or event. The concentration of certain hormones secreted by a region of the brain called the hypothalamus, the pituitary gland and the gonads is regulated by a feedback loop that has both positive and negative feedback components. The loop is initiated when the hypothalamus releases gonadotropin-releasing hormone, or GnRH. GnRH then stimulates the pituitary to secrete the two gonadotropins—LH and FSH. The gonadotropins bind to receptors on the gonads, the ovaries in females and the testicles in males, and stimulate the gonads to produce the sex steroid hormones, estrogen and testosterone.
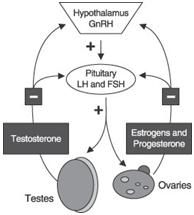
Once the hypothalamus senses that the sex steroid hormones are at an acceptable level, it reduces the release of GnRH. The reduced level of GnRH provides feedback to the pituitary gland to reduce the secretion of gonadotropins, resulting in reduced gonadotropin levels. Reduced gonadotropin levels then provide feedback to the gonads to reduce the production of the sex steroid hormones. Once the hypothalamus senses the sex steroid hormones dropping below a particular level, the hypothalamus increases the release of GnRH, which re-initiates the hormonal feedback loop and the production of the two gonadotropins.
Our Scientific Approach
Our scientific approach is based on the observation that many diseases of aging may be caused by the age-related changes in levels of reproductive hormones that are secreted by the hypothalamus, the pituitary gland and the gonads. This approach is built on the premise that these hormones are beneficial early in life, because they regulate and promote development and growth through cell division and differentiation in order to achieve reproduction, but are harmful later in life because they become unregulated and cause abnormal processes including pathologies associated with AD. We believe that this change in hormone levels is a primary cause of many age-related diseases, including Alzheimer’s disease and various cancers.
45
We believe that the gonadotropin LH is an important and pivotal cause of Alzheimer’s disease. Our research suggests that LH serves as the catalyst that potentially leads to increased production of amyloid beta protein, drives changes in tau protein that may lead to formation of neurofibrillary tangles, and possibly impacts other factors such as oxidative stress and inflammation which, in total, lead to the cognitive decline associated with Alzheimer’s disease. We base these beliefs on both experimental evidence and scientific observations, principally resulting from Curaxis’ work and the work of our consultants.
Preclinical Support for the Combined Treatment of Leuprolide Acetate and Acetylcholinesterase Inhibitors in Alzheimer’s Disease
Curaxis’ Phase II proof of concept study in female patients with mild-to-moderate Alzheimer’s disease demonstrated efficacy of 22.5 mg Lupron Depot™ when administered together with acetylcholinesterase inhibitors (AChEIs), as assessed by the ADAS-cog, ADCS-CGIC and ADCS-ADL outcome measures, when compared to patients receiving AChEIs alone. Administration of AChEIs is the current standard of care for Alzheimer’s patients. AChEIs inhibit the enzymatic activity of acetylcholinesterase, thereby increasing levels of the neurotransmitter acetylcholine. As the positive effect of leuprolide acetate plus AChEIs was an unexpected clinical result, subsequent pre-clinical work has focused on understanding the potential interaction between the LH and acetylcholine biological signaling pathways in neurons. In an attempt to better understand the mechanism of action or potential functional overlap between GnRH analogues and AChEIs, cell culture studies were performed to explore the role of AChEIs in modulation of the hypothalamic-pituitary-gonadal (HPG) axis of hormones and receptors, using brain cancer cells. Curaxis’ findings in these studies demonstrate that an AChEI can modulate HPG axis genes and may support the clinical findings that the two drugs together provide more benefit than either drug alone. A patent application detailing the use of GnRH analogues in combination with AChEIs or NMDA receptor antagonists to treat Alzheimer’s disease and mild cognitive impairment is pending in the United States, Europe and other countries.
Limitations of Current Alzheimer’s Disease Therapies
There are currently five drugs approved for the treatment of Alzheimer’s disease in the United States:
|
·
|
Aricept, marketed by Pfizer, Inc. and Eisai Company, Ltd.;
|
|
·
|
Exelon, marketed by Novartis AG;
|
|
·
|
Reminyl, also known as Razadyne, marketed by Shire Pharmaceuticals Group plc and Janssen Pharmaceutical Products, LP;
|
|
·
|
Cognex, marketed by Sciele Pharmaceuticals Corp.; and
|
|
·
|
Namenda, marketed by Forest Pharmaceuticals, Inc.
|
The first four of these drugs are acetylcholinesterase inhibitors or AChEIs; the fifth is an N-methyl-D-asparate or NMDA receptor antagonist. These drugs target the symptoms of Alzheimer’s disease by enhancing some patients’ cognitive function and general behavior, but generally are not thought to slow progression of the disease.
Our Solution: Memryte
Memryte (generic name: VP4896) is a proprietary, small, biodegradable implant comprised of leuprolide acetate and a polymer (synthetic compound). Each implant is approximately 1.5 millimeters in diameter and 3.0 centimeters in length. Memryte is designed to provide controlled, long-term, sustained release of leuprolide acetate. The polymer in Memryte is similar to the material in resorbable surgical stitches and degrades in the body. As a result, and in contrast to some other types of implantable drug delivery devices, surgical removal is not required. Memryte implants are inserted subcutaneously through a needle in the fleshy region on the side of the patient’s abdomen. The procedure is conducted in a physician’s office using a local anesthetic and takes approximately ten minutes. Administration of leuprolide acetate using the Memryte implant has been demonstrated in our Phase I ALADDIN 105 trial to minimize an initial dosage peak and provide a steady leuprolide acetate level throughout the two-month dosing period.
46
Curaxis administered an injectable formulation of leuprolide acetate in its two Phase II dose-ranging clinical trials for Alzheimer’s disease while it was developing Memryte. Leuprolide acetate has been marketed for almost two decades, primarily as a treatment for advanced prostate cancer. It is also approved for use in other hormone-related conditions, such as endometriosis and anemia caused by uterine fibroids in women, and precocious puberty in children. It is administered for these indications by intramuscular injection. The safety profile of leuprolide acetate has been well established over many years. The most common side effects are similar to those seen with menopause and surgical castration, such as hot flashes and osteoporosis. Since all of the women who enter our trials are post-menopausal, these side effects should be nominal. As a raw material, leuprolide acetate is available from a number of manufacturers.
Clinical Trials
Curaxis previously conducted several clinical studies to test leuprolide acetate and VP4896 for the treatment of mild to moderate Alzheimer’s disease. It branded its clinical trial program as “Antigonadotropin-Leuprolide in Alzheimer’s Disease Drug Investigation,” for which it used the acronym ALADDIN. Curaxis’ clinical program includes: a completed Phase I safety and pharmacokinetic study of VP4896, ALADDIN 105; and three Phase II clinical trials, two of which used an injectable formulation of leuprolide acetate and one of which used the proprietary implant, VP4896, which Curaxis was forced to terminate in 2006 due to financial constraints. Since terminating those trials, Curaxis had been unable to advance the clinical development of its Alzheimer’s disease candidate due to a lack of financial resources. As the result of the Merger, we anticipate that we will be able to raise the funds necessary to restart the clinical development of the Alzheimer’s disease candidate, but there can be no assurance that we will be able to do so.
These clinical trials are summarized in the following table:
|
Trial Name
/ Clinical Phase
|
Enrollment
Criteria
|
Number of
Clinical Sites
|
Number of
Participants
|
Trial
Duration
|
Status
|
|||||
|
ALADDIN 301 / Phase II
|
Men and women aged 60 years or older with mild to moderate Alzheimer’s disease; patients were required to receive AChEIs during and for at least 120 days prior to trial
|
62 in the United States and Canada
|
612 recruited, 370 included in data set due to discontinuation
|
56 weeks
|
Discontinued on October 18, 2006. Approximately 369 patients at 24 weeks, 193 patients at 32/34 weeks and 89 patients at 40 weeks
|
|||||
|
ALADDIN I / 103 Phase II
|
Women aged 65 years or older with mild to moderate Alzheimer’s disease; patients were allowed, but not required, to receive AChEIs during and prior to trial
|
5 in the United States
|
108
|
48 weeks
|
Completed
|
|||||
|
ALADDIN II / 104 Phase II
|
Men aged 65 years or older with mild to moderate Alzheimer’s disease; patients were allowed, but not required, to receive AChEIs during and prior to trial
|
17 in the United States
|
119
|
48 weeks
|
Completed
|
|||||
|
ALADDIN 105 / Phase I
|
Healthy women aged 45 to 70 years and men aged 50 to 70 years
|
1 in the United States
|
50
|
24 weeks
|
Completed
|
47
We employ a variety of clinically validated and FDA accepted efficacy measurements in our Alzheimer’s disease clinical trials. These include:
|
·
|
The Alzheimer’s Disease Assessment Scale-Cognitive Subscale, or ADAS-cog. ADAS-cog is a test of memory and cognition that is designed to measure changes in Alzheimer’s disease subjects that might occur in response to a pharmacological intervention, or the action of a given drug. An increasing score on this measure (over time, compared to the baseline score) indicates cognitive decline, while a decreasing score (over time, compared to the baseline score) indicates cognitive improvement;
|
|
·
|
The Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change, or ADCS-CGIC . ADCS-CGIC is a global measure of a subject’s change in condition from baseline in Alzheimer’s disease. The score is based on information gained from interviews with the subject and the subject’s caregiver; and
|
Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory, or ADCS-ADL. ADCS-ADL is a comprehensive rating scale designed to measure a subject’s capacity to perform activities of daily living, including ability to eat, dress, bathe, telephone, travel, shop and perform other household chores. The score is based on interviews of the subject’s caregiver. An increasing score on this measure (over time, compared to the baseline score) indicates additional capacity to perform activities of daily living, while a decreasing score indicates less capacity.
The ADAS-cog, the ADCS-CGIC and the ADCS-ADL are recognized by the FDA and regulatory agencies outside the United States as the most commonly used assessments for endpoints in Alzheimer’s disease clinical trials. To minimize the side effects associated with the loss of testosterone due to leuprolide acetate therapy, male subjects who received leuprolide acetate in these clinical trials also received testosterone on a daily basis in the form of a gel applied to the skin. To prevent the patients or the study personnel from learning of the treatment assignment, male subjects randomly assigned to placebo received a placebo gel on a daily basis. Since women with Alzheimer’s disease are post-menopausal and are no longer producing much estrogen, side effects associated with the loss of estrogen are minimal and do not require hormonal supplementation.
The intent-to-treat population in these trials consists of participants who received at least one dose of randomized drug and who had at least one assessment beyond baseline of at least one primary efficacy variable. For data analysis purposes, an intent-to-treat analysis is based on the initial treatment intent, not on the treatment eventually administered, and is intended to reduce data artifacts. For patients who do not complete the trial, the last assessment taken is carried forward to each of the subsequent assessment time points and is used in the final analysis data set. These data points are referred to as last observations carried forward (LOCF).
48
In general, the results of Curaxis’ Phase II dose-ranging studies in women were encouraging and pointed to a potentially significant new treatment for women with Alzheimer’s disease. The results in men have been less encouraging, in part because a number of men on placebo unexpectedly performed at or above baseline during the trials. Therefore, for the short-and medium-term, we plan to concentrate our development efforts on the use of leuprolide acetate to treat women, although we will continue our efforts to better understand mechanisms that might lead to improved outcomes in men.
Phase II / ALADDIN I
Curaxis has completed a randomized, double-blind, placebo-controlled, dose-ranging, 48-week, Phase II clinical trial to assess the efficacy and safety of an injectable formulation of leuprolide acetate on cognitive and global function in women with mild to moderate Alzheimer’s disease. We call this clinical trial ALADDIN I or trial 103. The trial was conducted at five investigative study sites in the United States. Women aged 65 or older with mild to moderate Alzheimer’s disease were eligible to participate in the trial. Patients were allowed to receive AChEIs during the trial if they began taking this medication at least 90 days prior to the trial and continued a stable dose throughout the trial.
A total of 109 women were enrolled in this study, 108 of which were included in the intent-to-treat population and assigned to one of three groups comprised of 36 participants each:
|
·
|
a low dose leuprolide acetate group; 11.25 mg of leuprolide every 12 weeks;
|
|
·
|
a high dose leuprolide acetate group; 22.5 mg of leuprolide every 12 weeks; and
|
|
·
|
a placebo group.
|
Each participant was administered an injection of leuprolide acetate or placebo (saline) once every 12 weeks during the trial. The primary efficacy endpoints of the trial were patient scores on the ADAS-cog and the ADCS-CGIC at 48 weeks compared to baseline. There were various secondary efficacy endpoints, including patient scores on the ADCS-ADL at 48 weeks compared to baseline. There was a trend at week 48 in favor of the high dose leuprolide acetate group in this Phase II trial, indicating a relative stabilization of the disease compared to the placebo group. However, Curaxis did not achieve the primary efficacy endpoints or any of the secondary efficacy endpoints in this trial with statistical significance.
In accordance with the written statistical analysis plan Curaxis had previously adopted for ALADDIN I, it also performed an analysis of 78 patients in the intent-to-treat population who were taking AChEIs, comparing results for the group of 24 patients treated with AChEIs plus the high dose of leuprolide acetate used in the study and the group of 28 patients treated with AChEIs plus the 11.25 mg dose of leuprolide acetate against the results for a group of 26 patients who were treated with AChEIs and received placebo in the study. The results for the group that received an AChEI plus the 11.25 mg dose of leuprolide acetate were not statistically significantly different from the results for the group that received an AChEI plus placebo.
As described below, the group that received the 22.5 mg dose of leuprolide acetate plus an AChEI demonstrated a benefit in comparison to the group that received an AChEI plus placebo. In addition, on each of the seven occasions during the 48-week study at which Curaxis assessed these two groups, the mean score of the 22.5 mg dose of leuprolide acetate plus AChEI group was more favorable than the mean score of the placebo plus AChEI group on each of the ADAS-cog, ADCS-CGIC and ADCS-ADL measures.
49
Statistical significance is measured by a p-value, which is a mathematical calculation used to determine the likelihood that the measured result was obtained by chance. A p-value of 0.05 means that the probability that the result occurred by chance is one in twenty. A low p-value indicates a greater likelihood that the observed result did not occur by chance, and therefore implies greater statistical significance.
For purposes of this subgroup analysis of the results of our ALADDIN I trial, Curaxis calculated p-values in two different ways. First it calculated unadjusted p-values, which indicate statistical significance as if this subgroup analysis had been a primary efficacy endpoint. However, because it performed several comparisons, it was required to adjust the p-values of the results to account for multiple statistical comparisons. This was done by using what is referred to as the Bonferroni correction, which applies an estimated statistical penalty to account for the number of comparisons made.
In this subgroup analysis, the mean ADAS-cog score in the group receiving the 22.5 mg dose of leuprolide acetate and an AChEI worsened by 0.18 points at week 48 from baseline compared to a mean worsening of 3.30 points in the group receiving placebo and an AChEI. The p-value for this difference was 0.026 on an unadjusted basis and 0.078 on an adjusted basis.
In the ADCS-CGIC analysis, 58% of the subgroup receiving the 22.5 mg dose of leuprolide acetate and an AChEI scored no change or better at week 48 in comparison with baseline versus 38% of the subgroup receiving placebo and an AChEI. The p-value for this difference was 0.031 on an unadjusted basis and 0.093 on an adjusted basis.
The mean ADCS-ADL score in the subgroup receiving the 22.5 mg dose of leuprolide acetate and an AChEI declined 0.54 points at week 48 from baseline compared to a mean decline of 6.85 points in the subgroup receiving placebo and an AChEI. The p-value for this difference was 0.015 on an unadjusted basis and 0.044 on an adjusted basis.
The following graphs summarize the results of this subgroup analysis.
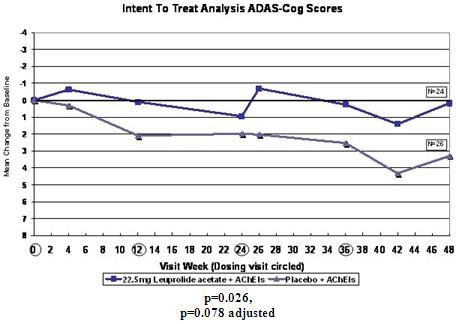
50
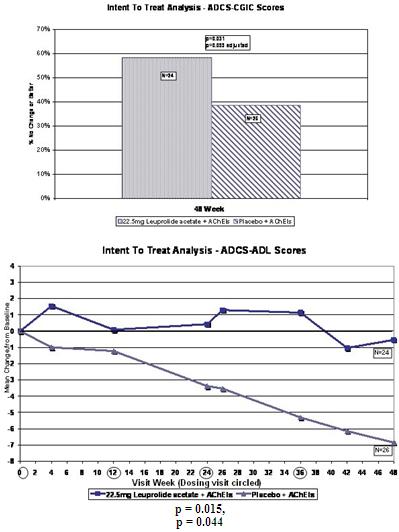
ALADDIN 301
In August 2006, Curaxis completed enrollment in ALADDIN 301, the first of its two randomized, double-blind, placebo-controlled, 56-week, Phase III clinical trials of the VP4896 implant for the treatment of mild to moderate Alzheimer’s disease as adjunctive (combination) therapy to AChEIs. Men and women aged 60 or older who had been diagnosed with mild to moderate Alzheimer’s disease were eligible to participate in the trials. One of the trial inclusion criteria was that all subjects shall have been on an AChEI for at least 120 days prior to baseline and continue to receive that AChEI throughout the trial.
The protocol provided for study participants to be randomly assigned to VP4896 or placebo in a ratio of three to two (3:2). VP4896 or placebo was administered every eight weeks in the form of a subcutaneous implant. Each participant received two implants on each occasion. The dosage level from the two implants was approximately equal to the high dose of leuprolide acetate being administered in ALADDIN II and 150% of the high dose in ALADDIN I. Curaxis method of administration in these trials was different from its ALADDIN I and ALADDIN II clinical trials, in which leuprolide acetate was administered by injection at 12-week intervals over a 48-week period.
51
The primary evaluation of the efficacy of VP4896 in the treatment of mild to moderate Alzheimer’s disease was measured by the difference from placebo in the scores on ADAS-cog and ADCS-CGIC compared to baseline. Secondary efficacy endpoints in each trial were to assess the efficacy of VP4896 compared to placebo as measured by change from baseline in a variety of other commonly used Alzheimer’s disease measurements, including ADCS-ADL, at 50 weeks.
In October 2006, Curaxis discontinued this trial due to financial constraints and converted it to a Phase II trial. At the time this trial was terminated, approximately 625 patients had been enrolled at 62 sites in the United States and Canada. After this discontinuation, Curaxis had approximately 369 patients who had received their 24 week assessment, approximately 193 patients who had received their 32/34 week assessment, and approximately 89 patients who had received their 40 week assessment.
In May 2007, in accordance with the written statistical analysis plan Curaxis had previously adopted for the trial, it completed an analysis of the results of ALADDIN 301 for (1) men and women as a single group and (2) for women separately, for participants who reached their 24, 32/34 or 40 week assessments. The analysis of the combined group of men and women showed no significant differences in favor of the group that received VP4896, in part because a number of men on placebo unexpectedly performed at or above baseline during the trials. However, the results in women who received VP4896 supported the efficacy signal in women that was demonstrated in ALADDIN 103. The following graphs reflect the results of the analysis for women in ALADDIN 301.
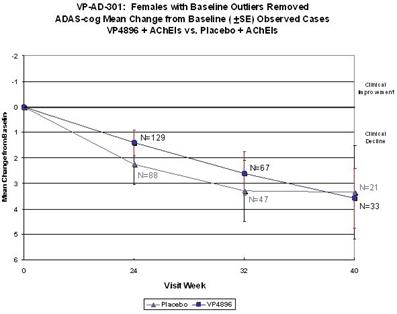
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups; placebo and VP4896). Average (mean) ADAS-cog scores (changes from baseline scores) were plotted and the numbers of patients included at each time point are given. The graph above includes the observed cases (patients that actually had an assessment at the specified visits) at 24, 32/34 and 40 weeks. VP4896-treated females demonstrated better ADAS-cog scores at 24 and 32/34 weeks, compared to placebo females.
52
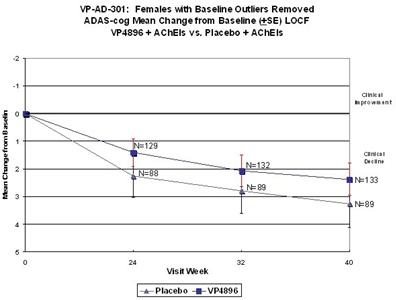
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups; placebo and VP4896). Average (mean) ADAS-cog scores (changes from baseline scores) were plotted and the numbers of patients included at each time point are given. The graph above includes the last-observation-carried-forward (LOCF) cases at 24, 32/34 and 40 weeks, i.e., all of the females completed through week 24 and if they discontinued after weeks 24 or 32/34, their scores are included in the 40 week data point. VP4896-treated females demonstrated better ADAS-cog scores at 24, 32/34 and 40 weeks, compared to placebo females.
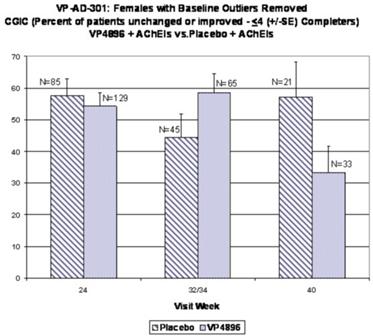
53
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups; placebo and VP4896).
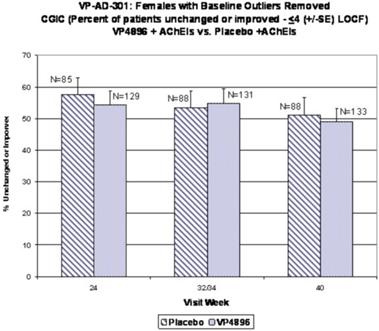
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups; placebo and VP4896). Average (mean) clinical global impression of change (CGIC) scores were plotted and the numbers of patients included at each time point are given. The bars represent the percent of females that were scored as unchanged or better at each time point. The graph above includes the last-observation-carried-forward (LOCF) cases at 24, 32/34 and 40 weeks, i.e., all of the females completed through week 24 and if they discontinued after weeks 24 or 32/34, their scores are included in the 40 week data point. VP4896-treated females demonstrated better CGIC scores at 32/34 weeks, compared to placebo females.
54
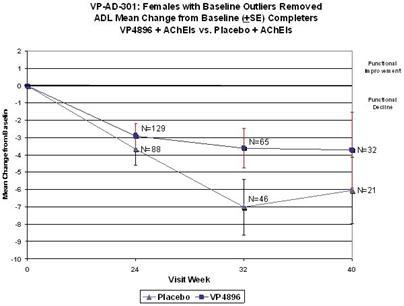
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups; placebo and VP4896). Average (mean) ADCS-ADL scores (changes from baseline scores) were plotted and the numbers of patients included at each time point are given. The graph above includes the observed cases (patients that actually had assessments at the specified visits) at 24, 32/34 and 40 weeks. VP4896-treated females demonstrated better ADL scores at 24, 32/34 and 40 weeks, compared to placebo females.
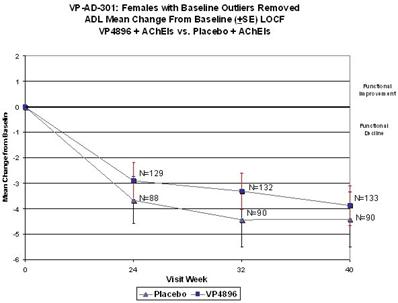
Female patients with ADAS-cog baseline scores that were considered to be extreme observations (too low or too high) were removed from the analysis (10 patients were removed from both groups, placebo and VP4896). Average (mean) ADCS-ADL change from baseline scores were plotted and the numbers of patients included at each time point are given. The graph above includes the last-observation-carried-forward (LOCF) cases at 24, 32/34 and 40 weeks, i.e., all of the females completed through week 24 and if they discontinued after weeks 24 or 32/34, their scores are included in the 40 week data point. VP4896-treated females demonstrated better ADL scores at 24, 32/34 and 40 weeks, compared to placebo females.
55
ALADDIN II
Curaxis had completed a randomized, double-blind, placebo controlled, dose-ranging, 48-week, Phase II clinical trial, ALADDIN II, to assess the efficacy and safety of an injectable formulation of leuprolide acetate on cognitive and global function in men with mild to moderate Alzheimer’s disease. It called this clinical trial ALADDIN II or trial 104. The trial was conducted at 17 investigative study sites in the United States. Men aged 65 or older with mild to moderate Alzheimer’s disease were eligible to participate in the trial. Patients were allowed to receive AChEIs during the trial if they began taking this medication at least 60 days prior to the trial and continued a stable dose throughout the trial.
A total of 119 men were enrolled in this study and were included in the intent-to-treat population and assigned to one of three groups comprised of:
|
·
|
a low dose leuprolide acetate group; 22.5 mg of leuprolide per 12 weeks; 39 men
|
|
·
|
a high dose leuprolide acetate group; 33.75 mg of leuprolide per 12 weeks; 42 men and
|
|
·
|
a placebo group; 38 men.
|
Each participant was administered an injection of leuprolide acetate or placebo (saline) once every 12 weeks during the trial. The primary efficacy endpoints of the trial were a patient’s score on the ADAS-cog and the ADCS-CGIC at 48 weeks compared to baseline. There were various secondary efficacy endpoints, including a patient’s score on the ADCS-ADL at 48 weeks compared to baseline. The low and high doses of leuprolide acetate produced very similar results. Curaxis did not achieve the primary efficacy endpoints or any of the secondary efficacy endpoints in this trial with statistical significance. The p values for the endpoints in this trial were 0.729 for ADAS-cog, 0.530 for ADCS-CGIC and 0.232 for ADCS-ADL.
Curaxis analyzed data from patients receiving either 22.5 mg or 33.75 mg doses of leuprolide acetate in conjunction with AChEIs and compared those to placebo (saline) patients also receiving AChEIs. There was a small but not statistically significant signal on the ADAS-cog in favor of both leuprolide treatment groups at week 48. Curaxis did not achieve its primary or secondary statistical endpoints for either group.Results on the ADCS-CGIC rating demonstrated that 44% of the men who received 22.5 mg of leuprolide acetate plus AChEIs, and 47% of the men who received 33.75 mg of leuprolide acetate plus AChEIs, were either improved or showed no change compared to 31% of the men who received placebo plus AChEIs. The p-value for this difference was 0.18 on an adjusted basis.
In the ALADDIN II study, leuprolide acetate administered as an injection was well tolerated at both dose levels without any evidence of a dose-related increase in adverse events. The safety profile in ALADDIN II was similar to that of ALADDIN I.
Phase I / ALADDIN 105
Curaxis had completed a Phase I single center, randomized, double-blind, placebo-controlled, multiple-dose and formulation comparison study of VP4896. It enrolled 50 healthy volunteers in this study consisting of post-menopausal women aged 45 to 70 years and men between the ages of 50 and 70 years. The primary objective of this trial was to determine the safety and tolerability of VP4896 implants at various dose combinations monitored over a period of 24 weeks. Secondary objectives were to study the pharmacokinetics (the processes by which a drug is absorbed, distributed, metabolized and eliminated by the body) of VP4896 implants over a period of up to 48 weeks and to compare the pharmacokinetic profile of a single administration of a VP4896 implant with the pharmacokinetic profile of a single administration of one-month leuprolide acetate by injection over a period of eight weeks.
56
The implants provided a relatively constant systemic release of leuprolide acetate over an eight-week dosing interval. Following the first 24 hours of dosing, steady concentrations of the drug were established, rising slowly to a peak that occurred at approximately four weeks and, thereafter, declining slowly to the end of the eight-week dosing period to a level similar to the level at the end of 24 hours.
There were no serious adverse events reported in this trial. All safety events in this trial believed to be related to leuprolide acetate were consistent with those listed in the package insert for leuprolide acetate’s approved indications. Some patients experienced minor bruising or irritation as a result of the implant procedure that resolved within two weeks.
Planned Phase IIb Study
The next step in the clinical development plan is a Phase IIb study in approximately 200-250 women. The purpose of this study is to confirm the efficacy results Curaxis attained in women in our ALADDIN I and ALADDIN 301 clinical trials and provide a foundation for determining the size and duration of Phase III studies of VP4896. We anticipate that the study will be a 12-month, double-blind, placebo-controlled study consisting of 1 active treatment arm and 1 placebo arm, with a 60/40 split between the active arm and the placebo arm. Assuming the study commences in the first quarter of 2011, and that enrollment is completed by the end of 2011, we anticipate that the results of the study would be available during the first quarter of 2013. However, in order to complete this study, we will need to raise approximately $20 million during the next several years to cover the costs associated with the study and related administrative and overhead costs. Curaxis had been unable to advance the clinical development of Memryte since 2006 due to a lack of financial resources and there can be no assurance that we will be successful in raising the funds needed to commence and complete the planned Phase IIb study. Furthermore, even if we are successful in raising the funds necessary to restart the clinical development of our Alzheimer’s disease candidate, there can be no assurance that we will be able to successfully complete all phases of such clinical development. Drug development is extremely costly and complex and requires multiple clinical trials and we may be unable to obtain suitable financing for all such trials and, even if we are successful in obtaining suitable financing, we may be unable to complete all required clinical trials, due to an inability to recruit an adequate number of clinical trial sites or an adequate number of patients to participate in those trials or other reasons.
Our Oncology Program
Curaxis had conducted an extensive preclinical research program in the use of leuprolide acetate to treat a number of cancers, including hormone refractory prostate cancer, brain cancers, kidney cancer, pancreatic cancer and non-small-cell lung cancer.
Its work in oncology is based on new insights into the growth of cancer cells that have been discovered by its scientists. In particular, its scientific findings relating to autocrine (cell produces factors that regulate its own functions)-paracrine (cell produces factors that regulate the functions of adjacent cells) signaling and the replication of the hypothalamic-pituitary-gonadal hormonal feedback loop (the HPG axis) inside of cancer cells point to a previously unknown mechanism that drives the growth of cancer cells and an entirely new method of attacking those cancer cells; i.e., using high doses of leuprolide acetate to eliminate the gonadotropins that may be driving the growth of those cells.
We plan to use the same compound for several cancer Phase II clinical trials as Curaxis is using for clinical trials of VP4896. Since Curaxis had completed Phase I safety trials of VP4896, we believe that we will be able to commence our clinical programs for various cancers with Phase II clinical trials instead of Phase I safety trials. The FDA will have to agree to the commencement of these clinical programs with Phase II clinical trials when we submit our Investigational New Drug Applications, or INDs, to the FDA. However, we do not intend to initiate any such Phase II trials until our planned Phase IIb trial of VP4896 for the treatment of mild to moderate Alzheimer’s in women is completed and, in any event, our ability to initiate such trials will be subject to the availability of adequate financial resources to support such trials.
57
Manufacturing
We do not own or intend to own manufacturing facilities for the production of clinical or commercial quantities of leuprolide acetate or any of the compounds that Curaxis had been testing in its pre-clinical programs. We currently rely, and our strategy is to continue to rely, on third parties for the manufacture of our product candidates and any products for which we may obtain regulatory approval to commercially manufacture and market.
In August 2008, Curaxis entered into an agreement with Covidien Ltd. under which Covidien had agreed to supply, and Curaxis had agreed to purchase, 75% of its annual quantities of leuprolide. In the event Covidien is unable to supply leuprolide in the quantities we require on a timely basis, we have the right to purchase leuprolide from other sources.
The implants for VP4896 are manufactured by Durect Corporation using a melt extrusion process in which leuprolide acetate is mixed with a polymer. The mixture is then formed and cut into small rod-shaped implants that are approximately 1.5 millimeters in diameter and 3.0 centimeters in length.
Durect Agreement
In July 2002, Curaxis entered into a Feasibility, Development and Commercialization Agreement with Southern Biosystems, Inc., a company which subsequently merged into its parent corporation, Durect. Under this agreement, Durect produces VP4896 for us using Durect’s biodegradable polymeric implant technology to provide a sustained release formulation of leuprolide acetate to treat Alzheimer’s disease. Durect has the right to subcontract its responsibilities under the agreement, provided that Durect remains responsible for its obligations under the agreement. Under the agreement, Durect has granted Curaxis a worldwide, exclusive license to manufacture, market and sell VP4896.
We must pay all of Durect’s costs associated with development and regulatory approval activities and make payments to Durect upon the achievement of specified development and regulatory milestones for VP4896. Curaxis has made milestone payments to Durect totaling $500,000 and we are obligated to make additional payments, up to an aggregate maximum amount of $2,500,000, if specified additional milestones are met. We have agreed to purchase quantities of VP4896 from Durect at transfer prices equal to specified percentages of Durect’s fully allocated costs to produce it. We are also obligated to pay royalties to Durect on commercial sales of VP4896 based on the following schedule: (i) 10% on annual revenues through $250 million, (ii) 12% on annual revenues in excess of $250 million up through $500 million, and (iii) 14% on annual revenues in excess of $500 million. These royalties are payable to Durect for the maximum time permitted by law in each country, which may be longer than the life of any patents that Durect licensed to us. The term of the agreement is open-ended and the rights and responsibilities of the parties to the agreement continue until the agreement is terminated pursuant to its terms. Either party to the agreement may terminate the agreement in the event of a default by the other party in any of its material obligations under the agreement if the default is not cured within thirty days of receipt of written notice from the other party of the agreement. In addition, either party to the agreement may immediately terminate the agreement if the other party to the agreement files a petition in bankruptcy or insolvency and such petition is not dismissed within sixty days.
Under the agreement, Durect granted Curaxis an exclusive license under its intellectual property rights to use its polymeric implant technology to develop and commercialize VP4896 on a worldwide basis for the treatment of Alzheimer’s disease. The Durect intellectual property licensed by Curaxis includes patent applications covering the VP4896 formulation and the method of making VP4896. Curaxis’ license from Durect of its technology does not extend to the use of VP4896 for the treatment of any other indications. It is required under the agreement to diligently, and in accordance with the timelines for clinical development set by Curaxis, conduct all required clinical trials, obtain all necessary regulatory approvals and commercialize VP4896 in the United States for Alzheimer’s disease. Curaxis and Durect have established a joint development team, consisting of two representatives from each company, to manage the development of VP4896. Ultimately, Curaxis has final decision making authority with respect to VP4896 development matters.
58
Covidien/Mallinckrodt Agreement
On January 1, 2009, Curaxis entered into a supply agreement with Mallinckrodt, Inc. (“Mallinkrodt”), a subsidiary of Covidien, Ltd. (“Covidien”), under which Curaxis had agreed to purchase 75% of its annual requirements of leuprolide acetate from Mallinckrodt for a period of five years beginning January 1, 2009, and ending December 31, 2013, at an initial price of $550 per gram (the “Covidien Agreement”). The initial price is subject to annual adjustment upward and downward based on changes in Mallinckrodt’s costs and production methods. The Covidien Agreement is automatically renewable for a second five-year term beginning January 1, 2014, unless Mallinckrodt elects not to renew the Covidien Agreement for the second five-year term. In connection with the execution of the Covidien Agreement, Mallinckrodt paid Curaxis the sum of $500,000.
Southridge Agreement
On May 28, 2009, Curaxis entered into a transaction management agreement with Southridge Business Solutions Group, LLC, of Ridgefield, Connecticut (“Southridge” or “Selling Security Holder”), to assist Curaxis in restructuring its balance sheet, principally through negotiations with several large trade creditors to reduce their claims, and to assist Curaxis in effectuating a merger with a suitable public corporation (the “Transaction Management Agreement”). Pursuant to the Transaction Management Agreement, Curaxis had worked with Southridge to reduce its trade debt by approximately $6,000,000 and entered into the Merger Agreement with Auto Search Cars, Inc. Under the terms of the Transaction Management Agreement, Curaxis is obligated to pay Southridge a management fee of $10,000 per month, from June 1, 2009 through the first anniversary of the closing of the Merger with Auto Search, or July 2011. Curaxis may terminate the Transaction Management Agreement at any time by giving 90 days’ advance notice to Southridge. In addition, Curaxis is obligated to issue to Southridge warrants equal to 3% of Curaxis’ outstanding stock as of the date of the Merger with Auto Search at an exercise price of $0.001 per share. A total of 2,149,148 warrants have been issued to Southridge under the agreement.
Canterbury Agreement
On June 12, 2009, Curaxis entered into a letter agreement with Canterbury Investment Partners, LLC of Hingham, Massachusetts (“Canterbury”), to assist Curaxis in a range of issues in connection with its contemplated merger with a public shell corporation, including its negotiations with Southridge, in developing a strategy to negotiate reductions, deferrals and/or equity exchanges with its trade creditors and in raising funds from current or prospective investors and in negotiating the terms of the acquisition of a public corporation (the “Letter Agreement”). Under the terms of the Letter Agreement, Curaxis paid Canterbury a retainer of $50,000 and is also obligated to pay Canterbury a monthly fee of $5,833 for a period of twelve (12) months from September 2009 through August 2010. In addition, under the Letter Agreement, Curaxis has issued a warrant to Canterbury to purchase 854,358 shares of Curaxis Common Stock at a price of $0.22 per share. Following the closing of the Merger with Auto Search, Curaxis is obligated to pay Canterbury the sum of $8,000 per month for a period of six (6) months for Canterbury’s assistance in developing and implementing a strategy to support Curaxis in its dealings with the financial community. Mark Pompeo, who is the manager of Canterbury, is the brother of Ronald Pompeo, who was elected a director of Curaxis on July 3, 2009.
GroupMark Agreement
On September 14, 2009, Curaxis entered into an Investor Development and Corporate Imaging Agreement with GroupMark Financial Services Ltd. of Flemington, New Jersey (“GroupMark”), to assist Curaxis in developing a new website (www.curaxispharma.com) and related blog and to develop an awareness of Curaxis through various avenues of public exposure (the “GroupMark Agreement”). Pursuant to the GroupMark Agreement, Curaxis had launched a completely revised website and a blog devoted to items of interest to the Alzheimer’s community (http://blog.curaxis pharma.com). Under the terms of the GroupMark Agreement, Curaxis has paid GroupMark $50,000 for its website and blog development services. In addition, Curaxis issued GroupMark a warrant for 100,000 shares of Curaxis common stock at a purchase price of $0.30 per share.
59
Sales and Marketing
We intend to maximize the commercial potential of our product candidates by selectively entering into collaboration agreements with leading pharmaceutical and biotechnology companies to assist us in furthering the development of our product candidates. In particular, we intend to enter into these third-party arrangements for target indications in which our potential collaborator has particular expertise or that involve a large, primary care market that must be served by large sales and marketing organizations. In entering into these collaboration agreements, our goal will be to maintain co-promotion or co-commercialization rights in the United States and, in some cases, other markets.
We expect to contract with third parties to warehouse and distribute our products and to provide administrative functions, such as accounts receivable management and other similar activities.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition and a strong emphasis on proprietary products. We face competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies and private and public research institutions.
Many of our competitors have significantly greater financial resources and experience in research and development, manufacturing, pre-clinical testing, clinical trials, regulatory approvals and marketing approved products than we do. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects, are easier to administer or are less expensive than any products that we may develop. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business.
If approved, our Alzheimer’s product will compete against the five drugs currently approved for the treatment of Alzheimer’s disease as monotherapies. Four of the drugs are AChEIs, including Aricept (marketed by Pfizer, Inc.) and Eisai Company, Ltd.; Exelon (marketed by Novartis AG); Reminyl (marketed by Shire Pharmaceuticals Group plc and Janssen Pharmaceutical Products, LP); and Cognex (marketed by Sciele Pharmaceuticals Corporation). The fifth drug, Namenda (marketed by Forest Pharmaceuticals, Inc.) is an NMDA receptor antagonist. These products have well-known brand names, are distributed by large pharmaceutical companies and have achieved widespread acceptance among physicians and patients. In addition, our product could face competition from other leuprolide acetate products that are already on the market or may later be approved for other indications, if they are used or prescribed off label for Alzheimer’s disease.
In addition, there are many companies and academic and research institutions researching and developing potential treatments for Alzheimer’s disease. Some of these companies are large pharmaceutical companies, while others are smaller companies that may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and biotechnology companies. Companies with compounds to treat Alzheimer’s disease in human clinical trials include Pfizer, Eli Lilly, Glaxo SmithKline, Wyeth, Elan and Medivation.. To our knowledge, none of these companies or any other company is working on a potential Alzheimer’s disease treatment that has a mechanism of action that is the same as that of our Alzheimer’s product.
60
Patents and Proprietary Rights
Our success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, technology and know how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
Our patent portfolio includes one issued U.S. patent and numerous patent applications in the United States, foreign countries and regions under the Patent Cooperation Treaty. Use of VP4896 in therapeutically effective amounts to treat Alzheimer’s disease is covered by our U.S. patent, which claims, among other things, the use of leuprolide acetate in therapeutically effective amounts to treat Alzheimer’s disease through a reduction or elimination of blood serum levels of FSH and LH. This U.S. patent expires in 2018. Depending on the timing, duration and specifics of FDA approval of the use of VP4896 for the treatment of Alzheimer’s disease, this patent may be eligible for patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. A foreign counterpart application to this patent is pending only in Canada. We are aware of one issued third party U.S. patent, filed after our issued U.S. patent was filed, which may nonetheless be prior art to our patent and which might form the basis of an interference proceeding or invalidity challenge. See “Risk Factors—If we infringe or are alleged to infringe intellectual property rights of third parties, it will adversely affect our business.”
Our portfolio also includes patent applications directed to the treatment of Alzheimer’s disease with a combination of leuprolide acetate and AChEIs and/or NMDA receptor antagonists, to the treatment of various diseases and disorders associated with senescence, including Parkinson’s disease and prostate cancer, to the treatment of brain cancers, and to the treatment of cancers that express the genes of the HPG axis. Counterpart filings to these patent applications have been made in a number of other jurisdictions, including Europe and Japan. As described above, Curaxis also holds an exclusive license from Durect for the sustained release technology for VP4896 for the treatment of Alzheimer’s disease. Leuprolide acetate, the active pharmaceutical ingredient in VP4896, is off-patent; therefore we expect to rely on method of use and formulation patents to protect our market position.
We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, scientific advisors and other contractors, as well as physical security of our premises and our information technology systems. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Regulatory Matters
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, marketing and export and import of pharmaceutical products, such as those we are developing. The process of obtaining regulatory approvals and the subsequent substantial compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources.
U.S. Government Regulation
In the United States, the information that must be submitted to the FDA in order to obtain approval to market a new drug varies depending on whether the drug is a new product whose safety and effectiveness has not previously been demonstrated in humans or a drug whose active ingredient(s) and certain other properties are the same as those of a previously approved drug. A new drug will follow the New Drug Application, or NDA, route.
61
NDA Approval Processes
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, and implements regulations. Failure to comply with the applicable regulatory requirements at any time may result in administrative or judicial sanctions. These sanctions could include the FDA’s imposition of a clinical hold on trials, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The testing and approval process requires substantial time, effort, and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products. The FDA may limit the indications for use or place other conditions on any approvals that could restrict the commercial application of the products. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval.
Post-Approval Requirements
After regulatory approval of a product is obtained, we are required to comply with a number of post-approval requirements. For example, as a condition of approval of an NDA, the FDA may require post marketing testing and surveillance to monitor the product’s safety or efficacy. In addition, holders of an approved NDA are required to report certain adverse reactions and production problems to the FDA to provide updated safety and efficacy information and to comply with requirements concerning advertising and promotional labeling for their products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes certain procedural, substantive, and record keeping requirements. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursement vary greatly from country to country.
Under European Union regulatory systems, we may submit marketing authorizations either under a decentralized procedure or, if our product is eligible, under a centralized procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize approval.
62
Reimbursement
In both domestic and foreign markets, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third party payors to those who use our products. Third party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. These third party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the coverage status and reimbursement amount of newly approved healthcare product candidates. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Depending on the results of such studies, our product candidates may not be considered cost-effective. Adequate third party reimbursement may not be available to health care providers to cover the costs of using any products we may develop, which may not allow us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Number of Total Employees and Number of Full Time Employees
We believe that our success will depend greatly on our ability to identify, attract, and retain capable employees. As of November 30, 2010, we had three full time employees. Our employees are not represented by any collective bargaining unit, and we believe our relations with our employees are good.
Employment Agreements
We do not have any employment agreements in place and do not anticipate entering into any employment agreements in the foreseeable future.
DESCRIPTION OF PROPERTY
Our executive offices occupy approximately 2,000 square feet in an executive office suite in Durham, North Carolina (the “Office Space”). On November 3, 2009, Curaxis entered into a lease agreement with Regus Management Group LLC (“Regus”) to lease the Office Space from February 1, 2010 to January 31, 2011. The agreement was subsequently amended and extends to February 28, 2011. Curaxis is required to pay monthly rental payments to Regus in the amount of $4,200. We believe our existing facilities are adequate for our current needs and that suitable additional or alternative space will be available in the future on commercially reasonable terms as needed.
LEGAL PROCEEDINGS
Prior to the Merger, we were not involved in any litigation that we believed could have a material adverse effect on our financial condition or results of operations. At that time, there was no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of our Company or any of our subsidiaries, threatened against or affecting our Company, our common stock, any of our subsidiaries or of our Company’s or our subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect.
Curaxis is a party to the legal proceedings described below, which relate primarily to claims by various trade creditors arising in connection with Curaxis’ truncated ALADDIN 301 trial. We assumed these liabilities as the result of the consummation of the Merger.
In 2008, Aptuit, Inc. obtained a judgment for approximately $1.1M in the Circuit Court of Jackson County, Missouri at Kansas City, relating to work it performed for Curaxis in connection with Curaxis’ truncated ALADDIN 301 trial. Aptuit’s claims were based on invoices previously rendered to Curaxis in connection with that work and on a promissory note that Curaxis had delivered to Aptuit in connection with those invoices. Curaxis subsequently entered into a settlement agreement with Aptuit that requires payment of $900,000 over the next two years to satisfy this judgment.
63
In 2009, Genzyme Corporation obtained a judgment for $424,000 in the United States District Court for the District of Massachusetts, relating to leuprolide supplied to Curaxis in 2006 for its truncated ALADDIN 301 trial. Curaxis did not oppose the entry of this judgment, as it had previously acknowledged the validity of this debt to Genzyme. Genzyme has informally agreed through their counsel to defer any collection activities until the fourth quarter of 2010 and we expect to have discussions with Genzyme to establish a payment schedule for this judgment at that time.
In 2009, Margolin Brain Institute obtained a default judgment for $128,469, including interest and attorneys fees, in the Superior Court of the State of California, Fresno County - Civil Division, relating to work it performed in Curaxis. truncated ALADDIN 301 trial. The judgment has since been domesticated in North Carolina, pursuant to the Uniform Enforcement of Foreign Judgments Act. We expect to engage in discussions concerning settlement and payment of this judgment in the fourth quarter of 2010.
In 2009, Premier Research Atlanta, Inc. filed a suit that relates to a claim for approximately $45,000 for statistical services in connection with clinical trials. On March 3, 2010, this suit was dismissed with prejudice in the General Court of Justice, Superior Court Division, County of Wake, State of North Carolina.
MANAGEMENT
| Name | Age | Title | ||
| Patrick S. Smith | 58 | President and Chief Executive Officer and Chairman of the Board | ||
| David J. Corcoran | 62 | Executive Vice President, Chief Financial Officer and a Director | ||
| Judith S. T. Geaslen | 48 | Vice President of Finance | ||
| Sheldon L. Goldberg | 63 | Director | ||
| William E. McConville | 64 | Director | ||
| Ronald V. Pompeo | 57 | Director | ||
| Bert A. Spilker | 69 | Director |
Patrick S. Smith has served as our President and Chief Executive Officer and our Chairman of the Board since the effective date of the Merger. Mr. Smith is a co-founder of Curaxis and has served as Curaxis’ President, Chief Executive Officer and a director since its inception in February 2001. From December 1992 to February 2001, Mr. Smith was involved with various charitable and philanthropic causes, which principally involved developing not-for-profit private schools. In 1981 Mr. Smith founded Critical Care America, an alternative-site healthcare company, and served as Chairman and Chief Executive Officer until November 1992. Mr. Smith has served as a member of the boards of directors of various privately-held corporations including Integrated Care Systems, Cardio-Care America, Women’s Care America, Pediatricare America, Infusion Centers America and Naples Community Hospital System, Inc. Mr. Smith is a graduate of John Carroll University and holds an Honorary Doctorate from New York Medical College.
David J. Corcoran has served as our Vice President of Finance of the Corporation and as one of our directors since the effective date of the Merger. Mr. Corcoran is a co-founder of Curaxis and has served as Curaxis’ Chief Financial Officer and a director since its inception in February 2001. From September 1999 to February 2001, Mr. Corcoran was a self-employed consultant working with Mr. Smith in certain of his charitable activities, which principally involved developing not-for-profit private schools. In June 1996, Mr. Corcoran co-founded MedNet Affiliates, a provider of office-based orthopedic services and served as Vice President and a director until August 1999. He also previously served as general counsel to Critical Care America from its founding in 1981 through 1992. Mr. Corcoran is a graduate of Bowdoin College and of Northwestern University School of Law.
64
Judith S. T. Geaslen has served as our Vice President of Finance since the effective date of the Merger. Ms. Geaslen had been Curaxis’ Vice President of Finance and Chief Accounting Officer since January 2010 after serving as Corporate Controller and Accountant since July 2004. From March 1999 to April, 2000, Ms. Geaslen served as Vice President and Corporate Controller at Wilmington Trust Corporation, a financial holding company engaged in providing a range of banking and other financial services through its banking and other subsidiaries, and from1994 to 1999, served as Vice President and Manager of the Asset Review Division. From September 1984 to September 1994, Ms. Geaslen was employed by Ernst & Young, a registered public accounting firm, mostly recently serving as Audit Senior Manager. Ms. Geaslen is a graduate of Saint Mary’s College, Notre Dame, Indiana.
Sheldon L. Goldberg has served as one of our directors since the effective date of the Merger. Mr. Goldberg had been a director of Curaxis since February 2006 and served as its Senior Vice President of Corporate Development from April 2005 through December 2008. From October 2002 to March 2005, Mr. Goldberg served as President and Chief Executive Officer of the Alzheimer’s Association, a patient advocacy group for Alzheimer’s disease. From September 1999 to May 2005, Mr. Goldberg served as Chairman of the Board of AARP Services Inc., a subsidiary of AARP, which markets products and services to AARP members. From October 1995 to October 2002, Mr. Goldberg served as President and Chief Executive Officer of the Jewish Home and Hospital Lifecare Systems in New York, a long-term care health provider. Mr. Goldberg is a graduate of the University of the Wisconsin.
Father William E. McConville has been one of our directors since the effective date of the Merger. Father McConville had been a director of Curaxis since February 2006. Father McConville has been associate pastor for St. Francis of Assisi since 2001. From July 1989 to June 2001, he was a professor of religious studies at Sienna College. Father McConville has served as a member of the boards of directors and board of trustees of various educational and charitable institutions. Father McConville is a graduate of Catholic University of America and received his M.A. in Theology from Washington Theological Union and his Ph.D. in Systematic Theology from Vanderbilt University. Father McConville is a member of the Order of Friars Minor.
Ronald V. Pompeo has been one of our directors since the effective date of the Merger. Mr. Pompeo had served as a director of Curaxis since July 2009. Mr. Pompeo had been a long-time shareholder of Curaxis. For the past fifteen years, Mr. Pompeo had been the President and Chief Executive Officer of Rudy V. Pompeo, Inc., a general construction contractor based in Weymouth, Massachusetts. In addition, Mr. Pompeo is active in a number of volunteer and not for profit organizations. He is a graduate of Boston College.
Bert A. Spilker was appointed to the Board of Directors effective August 12, 2010. Dr. Spilker is founder and President of Bert A. Spilker & Associates, LLC, a leader in drug development, clinical trials and FDA advisory services. Prior to his current position, Dr. Spilker served as the Senior Vice President of Scientific and Regulatory Affairs for PhRMA (Pharmaceutical Research and Manufacturers of America) based in Washington, D.C. Formerly Dr. Spilker was President and co-founder of Orphan Medical, Inc., a public pharmaceutical company acquired by Jazz Pharmaceuticals in 2005. He is well known as the author of 16 books on clinical trial methods and the processes of drug discovery and development. These books are considered by many as the standard references on clinical trials and drug development.
There are no family relationships among our officers, directors and significant employees.
65
Board of Directors
Members of our board of directors are elected for one-year terms serving until the next annual stockholders’ meeting or until their death, resignation, retirement, removal, disqualification, or until a successor has been elected and qualified. All officers are appointed annually by our board of directors and serve at the pleasure of our board of directors. Currently, our directors receive no cash compensation for their service on our board of directors.
Director Independence
On an annual basis, each director and executive officer will be obligated to disclose any transactions with our Company and any of its subsidiaries in which a director or executive officer, or any member of his or her immediate family, have a direct or indirect material interest. Following completion of these disclosures, our board of directors will make an annual determination as to the independence of each director using the current standards for “independence” that satisfy both the criteria for the Nasdaq and the NYSE Amex Equities.
As of October 31, 2010, the Curaxis board of directors determined that the following directors are independent under these standards: (i) Mr. William E. McConville, (ii) Mr. Ronald V. Pompeo and (iii) Bert Spilker.
Committees of the Board of Directors
Concurrent with having sufficient members and resources, the board of directors intends to establish an audit committee and a compensation committee. The audit committee will review the results and scope of the audit and other services provided by the independent auditors and review and evaluate the system of internal controls. The compensation committee will review and recommend compensation arrangements for the officers and employees. No final determination has yet been made as to the memberships of these committees or when we will have sufficient members to establish committees. We believe that we will need a minimum of three (3) independent directors to have effective committee systems.
EXECUTIVE COMPENSATION
2009 SUMMARY COMPENSATION TABLE
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named executive officers and directors by us during the period ended December 31, 2009, 2008, and 2007.
|
Non-Equity
|
|||||||||||||||||||
|
Incentive
|
|||||||||||||||||||
|
Stock
|
Option
|
Plan
|
All Other
|
||||||||||||||||
|
Name and Principal Position
|
Year
|
Salary ($)
|
Bonus ($)(1)
|
Awards ($)
|
Awards ($)
|
Compensation ($)
|
Compensation ($)
|
Total($)
|
|||||||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
|||||||||||
|
Patrick S. Smith,
|
2009
|
$87,500
|
None
|
None
|
$48,290
|
(2)
|
None
|
$13,000
|
(5)
|
$148,790
|
|||||||||
|
President, Chief Executive Officer
|
2008
|
$126,783
|
None
|
None
|
$53,348
|
(3)
|
None
|
$13,361
|
(6)
|
$193,492
|
|||||||||
|
and Chairman of the Board
|
2007
|
$192,500
|
None
|
None
|
$75,442
|
(4)
|
None
|
$13,361
|
(6)
|
$281,303
|
|||||||||
|
David J. Corcoran,
|
2009
|
$74,375
|
None
|
None
|
$36,219
|
(2)
|
None
|
$10,250
|
(7)
|
$120,844
|
|||||||||
|
Executive Vice President,Chief
|
2008
|
$97,167
|
None
|
None
|
$40,011
|
(3)
|
None
|
$10,361
|
(8)
|
$147,539
|
|||||||||
|
Financial Officer and Director
|
2007
|
$147,500
|
None
|
None
|
$56,583
|
(4)
|
None
|
$10,361
|
(8)
|
$214,444
|
|||||||||
|
Judith S. T. Geaslen
|
2009
|
$50,000
|
None
|
None
|
$52,274
|
(2)
|
None
|
5,460
|
(9)
|
$107,734
|
|||||||||
|
Vice President, Finance
|
2008
|
$80,514
|
None
|
None
|
$35,487
|
(3)
|
None
|
None
|
$116,001
|
||||||||||
|
2007
|
$85,200
|
None
|
None
|
$35,389
|
(4)
|
None
|
None
|
$120,589
|
|||||||||||
|
Sheldon L. Goldberg,
|
2009
|
None
|
None
|
$30,000
|
(11)
|
None
|
(2)
|
None
|
None
|
$30,000
|
|||||||||
|
Director and Former Senior Vice
|
2008
|
$31,950
|
None
|
None
|
$112,253
|
(3)
|
None
|
$1,361
|
(10)
|
$145,564
|
|||||||||
|
President, Corporate Development(1)
|
2007
|
$124,500
|
None
|
None
|
$111,973
|
(4)
|
None
|
$7,361
|
(10)
|
$243,834
|
66
|
(1)
|
Mr. Goldberg resigned as Senior Vice President, Corporate Development effective December 31, 2008. Mr. Goldberg continues as a member of the Board of Directors. The information in this table for Mr. Goldberg relates to compensation he received in his capacity as Senior Vice President, Corporate Development.
|
|
(2)
|
Represents the aggregate expense under ASC 718 recognized by Curaxis for 2009 as a result of option awards held by the applicable executive officer, disregarding estimated forfeitures. Assumptions made in the valuation were consistent with those applied in years 2008 and 2007. For a description of these assumptions, see Notes 1 and 6 to the financial statements for the year ended December 31, 2008.
|
|
(3)
|
Represents the aggregate expense under ASC 718 recognized by Curaxis for 2008 as a result of option awards held by the applicable executive officer, disregarding estimated forfeitures. For a description of the assumptions made in the valuation, see Notes 1 and 6 to the financial statements for the year ended December 31, 2008.
|
|
(4)
|
These options vest as to 20% on May 18, 2007 and 20% vested on each May 18th thereafter based on continued employment through May 18, 2011.
|
|
(5)
|
Represents car allowance of $3,000 plus retention payment of $10,000.
|
|
(6)
|
Represents annual car allowance of $12,000 plus the value of our contributions on behalf of Mr. Smith under our long-term disability insurance plan.
|
|
(7)
|
Represents car allowance of $2,250 plus retention payment of $8,000.
|
|
(8)
|
Represents car allowance of $9,000 plus the value of our contributions on behalf of Mr. Corcoran under our long-term disability insurance plan.
|
|
(9)
|
Represents car allowance of $1,800 plus retention payment of $3,660.
|
|
(10)
|
Represents annual car allowance of $6,000 for 2007 and 2008 plus the value of our contributions on behalf of Mr. Goldberg under our long-term disability insurance plan.
|
|
(11)
|
Represents the fair value of 150,000 shares of Curaxis common stock granted to Mr. Goldberg to satisfy, in full, amounts due under an original five-year employment contract.
|
67
Explanatory Information Relating to 2009 Summary Compensation Table
Please note the following points in connection with the information in the 2009 Summary Compensation Table:
|
●
|
The compensation of the executive officers of Curaxis is reviewed on an annual basis by the Board of Directors. Each year, Curaxis considers whether to adjust the base salaries of senior management, including the executive officers, in order to reward individual performance, keep pace with cost of living increases and respond to competitive considerations. However, due to lack of funding, no increases have been rewarded to senior management and executive officers during the three years ended December 31, 2009. In many cases, base salaries have been reduced significantly. No accruals have been made to compensate officers for the reduced wages in future periods.
|
DIRECTOR COMPENSATION
2009 SUMMARY COMPENSATION TABLE
The following summary compensation table sets forth all compensation awarded to, earned by, or paid to the named directors by us during the period ended December 31, 2009, 2008, and 2007.
|
Non-Equity
|
|||||||||||||||||
|
Incentive
|
|||||||||||||||||
|
Stock
|
Option
|
Plan
|
All Other
|
||||||||||||||
|
Name and Principal Position
|
Year
|
Salary
($) |
Bonus
($)(1) |
Awards
($) |
Awards
($) |
Compensation
($) |
Compensation
($) |
Total
($) |
|||||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
|||||||||
|
Sheldon L. Goldberg
|
2009
|
None
|
None
|
None
|
137,443
|
(3)
|
None
|
None
|
137,443
|
||||||||
|
Director and Former Senior Vice President,
|
2008
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|||||||||
|
Corporate Development (1)
|
2007
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|||||||||
|
William E. McConville
|
2009
|
None
|
None
|
None
|
$64,729
|
(3)
|
None
|
None
|
$64,729
|
||||||||
|
Director
|
2008
|
None
|
None
|
None
|
$89,976
|
(4)
|
None
|
None
|
$89,976
|
||||||||
|
2007
|
None
|
None
|
None
|
$84,982
|
(5)
|
None
|
None
|
$84,982
|
|||||||||
|
Ronald V. Pompeo
|
2009
|
None
|
None
|
None
|
$526
|
(3)
|
None
|
None
|
$526
|
||||||||
|
Director (2)
|
2008
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|||||||||
|
2007
|
None
|
None
|
None
|
None
|
None
|
None
|
None
|
|
|
||
|
(1)
|
Mr. Goldberg resigned as Senior Vice President, Corporate Development effective December 31, 2008. Mr. Goldberg continues as a member of the Board of Directors. The information in this table for Mr. Goldberg relates to compensation he received in his capacity as a member of the Board of Directors.
|
|
|
(2)
|
Mr. Pompeo was elected to the Board of Directors effective July 2009.
|
|
(3)
|
Represents the aggregate expense under ASC 718 recognized by Curaxis for 2009 as a result of option awards held by the applicable director, disregarding estimated forfeitures. Assumptions made in the valuation were consistent with those applied in years 2008 and 2007. For a description of these assumptions, see Notes 1 and 6 to the financial statements for the year ended December 31, 2009.
|
|
(4)
|
Represents the aggregate expense under ASC 718 recognized by Curaxis for 2008 as a result of option awards held by the applicable director, disregarding estimated forfeitures. For a description of the assumptions made in the valuation, see Notes 1 and 6 to the financial statements for the year ended December 31, 2009.
|
|
(5)
|
Represents the aggregate expense under ASC 718 recognized by Curaxis for 2007 as a result of option awards held by the applicable director, disregarding estimated forfeitures. For a description of the assumptions made in the valuation, see Notes 1 and 6 to the financial statements for the year ended December 31, 2009.
|
68
OUTSTANDING EQUITY AWARDS AT 2009 FISCAL YEAR-END
|
Option Awards
|
Stock Awards
|
||||||||||||||||||||||||||||
|
Equity
|
|||||||||||||||||||||||||||||
|
Incentive
|
|||||||||||||||||||||||||||||
|
Equity
|
Plan
|
||||||||||||||||||||||||||||
|
Incentive
|
Awards:
|
||||||||||||||||||||||||||||
|
Plan
|
Market
|
||||||||||||||||||||||||||||
|
Awards:
|
or Payout
|
||||||||||||||||||||||||||||
|
Number
|
Value of
|
||||||||||||||||||||||||||||
|
Market
|
of Unearned
|
Unearned
|
|||||||||||||||||||||||||||
|
Number of
|
Number of
|
Number of
|
Value of
|
Shares,
|
Shares,
|
||||||||||||||||||||||||
|
Securities
|
Securities
|
Shares or
|
Shares or
|
Units or
|
Units or
|
||||||||||||||||||||||||
|
Underlying
|
Underlying
|
Units of
|
Units of
|
Other
|
Other
|
||||||||||||||||||||||||
|
Unexercised
|
Unexercised
|
Option
|
Stock That
|
Stock That
|
Rights
|
Rights That
|
|||||||||||||||||||||||
|
Options
|
Options
|
Exercise
|
Option
|
Have Not
|
Have Not
|
That Have
|
Have
|
||||||||||||||||||||||
| (#) | (#) |
Price
|
Expiration
|
Vested
|
Vested
|
Not Vested
|
Not Vested
|
||||||||||||||||||||||
|
Name
|
Exercisable
|
Unexercisable
|
($)
|
Date
|
(#) |
($)
|
(#) |
($)
|
|||||||||||||||||||||
|
(a)
|
(b)
|
(c)
|
(d)
|
(e)
|
(f)
|
(g)
|
(h)
|
(i)
|
|||||||||||||||||||||
|
Patrick S. Smith(1)
|
240,000 | — | $ | 0.83 |
06/20/12
|
— | — | — | — | ||||||||||||||||||||
|
Patrick S. Smith(2)
|
80,000 | — | $ | 2.00 |
09/15/13
|
— | — | — | — | ||||||||||||||||||||
|
Patrick S. Smith(3)
|
32,000 | 8,000 | $ | 8.00 |
01/17/15
|
— | — | — | — | ||||||||||||||||||||
|
Patrick S. Smith(4)
|
72,000 | 48,000 | $ | 1.51 |
02/05/17
|
— | — | — | — | ||||||||||||||||||||
|
David J. Corcoran(1)
|
180,000 | — | $ | 0.83 |
06/20/12
|
— | — | — | — | ||||||||||||||||||||
|
David J. Corcoran(2)
|
60,000 | — | $ | 2.00 |
09/15/13
|
— | — | — | — | ||||||||||||||||||||
|
David J. Corcoran(3)
|
24,000 | 16,000 | $ | 8.00 |
01/17/15
|
— | — | — | — | ||||||||||||||||||||
|
David J. Corcoran(4)
|
54,000 | 36,000 | $ | 1.51 |
02/05/17
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(7)
|
3,500 | — | $ | 4.00 |
12/01/14
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(3)
|
4,000 | 1,000 | $ | 8.00 |
01/17/15
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(8)
|
800 | 200 | $ | 10.00 |
05/04/15
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(5)
|
20,000 | 5,000 | $ | 6.00 |
05/18/16
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(4)
|
18,000 | 12,000 | $ | 1.51 |
02/05/17
|
— | — | — | — | ||||||||||||||||||||
|
Judith S. T. Geaslen(13)
|
100,000 | — | $ | 0.20 |
02/19/19
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(9)
|
18,000 | 4,500 | $ | 8.00 |
04/18/15
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(10)
|
42,000 | 8,000 | $ | 8.00 |
04/18/15
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(8)
|
8,000 | 2,000 | $ | 10.00 |
05/04/15
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(5)
|
48,000 | 12,000 | $ | 6.00 |
05/18/16
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(4)
|
54,000 | 36,000 | $ | 1.51 |
02/05/17
|
— | — | — | — | ||||||||||||||||||||
|
Sheldon Goldberg(14)
|
150,000 | — | $ | 0.20 |
09/08/19
|
— | — | — | — | ||||||||||||||||||||
|
William E. McConville(11)
|
35,000 | — | $ | 6.00 |
02/20/16
|
— | — | — | — | ||||||||||||||||||||
|
William E. McConville(12)
|
60,000 | 40,000 | $ | 1.51 |
02/05/17
|
— | — | — | — | ||||||||||||||||||||
|
William E. McConville(6)
|
20,000 | 30,000 | $ | 1.00 |
06/11/17
|
— | — | — | — | ||||||||||||||||||||
|
William E. McConville(14)
|
150,000 | — | $ | 0.20 |
09/08/19
|
— | — | — | — | ||||||||||||||||||||
|
Ronald V. Pompeo(15)
|
50,000 | — | $ | 0.20 |
09/08/19
|
— | — | — | — | ||||||||||||||||||||
69
|
(1)
|
These stock options vested as to 20% of the shares on June 20, 2003 and 20% vested on each June 20th thereafter based on continued employment through June 20, 2007.
|
|
(2)
|
These stock options vested as to 20% of the shares on September 15, 2004 and 20% vested on each September 15th thereafter based on continued employment through September 15, 2008.
|
|
(3)
|
These stock options vested as to 20% of the shares on January 17, 2006 and 20% vested on each January 17th thereafter based on continued employment through January 17, 2010.
|
|
(4)
|
These stock options vested as to 20% of the shares on January 1, 2008 and 20% vested on each January 1st thereafter based on continued employment through January 1, 2012.
|
|
(5)
|
These stock options vest as to 20% on May 18, 2007 and 20% vested on each May 18th thereafter based on continued employment through May 18, 2011.
|
|
(6)
|
These stock options granted to Senior officers and non-employee Board members vested as to 20% of the shares on June 11, 2008 and 20% vested on each June 11th thereafter based on continued employment or service as a director of Curaxis through June 11, 2012 provided that such vesting shall be accelerated and each such option shall become fully-vested upon the first to occur of any of the following: (1) a sale by the Corporation of all or substantially all of its assets or similar acquisition or merger transaction approved by the Board; (2) closing of an out licensing transaction or similar collaboration agreement by the Corporation that is approved by the Board and provides Curaxis with at least $5 million in upfront cash payments at closing; or (3) closing of an equity investment in the Corporation by one or more institutional investors totaling at least $15 million at closing.
|
|
(7)
|
These stock options granted to Ms. Geaslen upon employment vested as to 20% of the shares on December 1, 2005 and 20% vested on each December 1st thereafter based on continued employment through December 1, 2009.
|
|
(8)
|
These stock options vested as to 20% of the shares on May 4, 2006 and 20% vested on each May 4th thereafter based on continued employment through May 4, 2010.
|
|
(9)
|
These stock options vested as to 20% of the shares on April 18, 2006 and 20% vested on each April 18th thereafter based on continued employment through April 18th 2010.
|
|
(10)
|
These stock options granted to Mr. Goldberg upon employment vested as to 20% of the shares at April 18, 2005 and 16% vested on each April 18th thereafter based on continued employment through April 18th 2010.
|
|
(11)
|
These stock options vested as to 1/3 of the shares on February 20, 2007 and 1/3 vested on each February 20th thereafter until fully vested based on continued service as a director of Curaxis.
|
|
(12)
|
These stock options vested as to 20% of the shares on January 1, 2008 and 20% vested on each January 1st thereafter through January 1, 2012 based on continued service as a director of Curaxis.
|
|
(13)
|
These stock options vested as to 100% of the shares on the grant date of February 19, 2009.
|
|
(14)
|
These stock options vested as to 100% of the shares on the grant date of September 9, 2009.
|
|
(15)
|
These stock options vested as to 20% of the shares on September 8, 2009 and 20% vested on each September 8th thereafter through September 8, 2014 based on continued service as a director of Curaxis.
|
70
BENEFICIAL OWNERSHIP OF CURAXIS SHARES
|
•
|
each stockholder known to Curaxis to own beneficially more than 5% of Curaxis’ common stock;
|
|
|
•
|
each of Curaxis’ directors;
|
|
|
•
|
each of Curaxis’ executive officers, including each of the Named Executive Officers listed in the “2009 Summary Compensation Table” included in this proxy statement; and
|
|
|
•
|
all of Curaxis’ current directors and executive officers as a group.
|
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or dispositive power with respect to securities. Ordinary shares relating to options or warrants currently exercisable, or exercisable within 60 days of November 30, 2010, are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Except as indicated by footnote, and subject to the community property laws where applicable, the persons or entities named in the tables have sole voting and dispositive power with respect to all shares shown as beneficially owned by them. Except as otherwise noted in the tables below, the address of each person or entity listed in the table is c/o Curaxis Pharmaceutical Corporation, 4819 Emperor Blvd., Suite 400, Durham, NC 27703.
|
Amount and Nature
|
|||||
|
Name and Address
|
of Beneficial Ownership
|
Percentage
|
|||
|
of Ordinary Shares (1)
|
of Total
|
||||
|
DIRECTORS AND EXECUTIVE OFFICERS
|
|||||
|
Patrick S. Smith,
President, Chief Executive Officer and Director
|
13,306,453
|
(2)
|
16.1%
|
||
|
David J. Corcoran,
Executive Vice President, Chief Financial Officer and Director
|
3,657,961
|
(3)
|
4.4%
|
||
|
Judith S. T. Geaslen,
Vice President, Finance
|
188,300
|
(4)
|
—
|
||
|
Sheldon Goldberg,
Former Senior Vice President, Corporate Development and Director
|
659,600
|
(5)
|
—
|
||
|
William E. McConville,
Director
|
295,000
|
(6)
|
—
|
||
|
Ronald V. Pompeo,
Director
|
70,800
|
(7)
|
—
|
||
|
Bert A. Spilker,
Director
|
3,000
|
—
|
|||
|
All directors and executive officers as a group on a pro forma basis to give effect to the Merger (7 persons)
|
18,181,114
|
(8)
|
21.9%
|
||
|
SHAREHOLDERS OWNING MORE THAN 5%
|
|||||
|
Richard L. Bowen
|
10,162,560
|
12.3%
|
|||
|
Jonathan J. Martin
|
4,304,460
|
(9)
|
5.2%
|
||
71
|
—
|
Less than 1%
|
|
|
(1)
|
Based on 82,870,925 outstanding shares assuming the exercise of 10,654,436 warrants issued and outstanding, but assuming no exercise of outstanding options. In addition, shares outstanding do not assume conversion of Series A and B preferred stock.
|
|
|
(2)
|
Includes 432,000 shares of Common Stock issuable upon exercise of stock options exercisable within 60 days of August 31, 2010. Also includes 4,345,000 shares owned by the Patrick S. Smith Charitable Remainder Trust, of which Mr. Smith is the trustee, and 300,000 shares held by RBC Dain Rauscher Inc. as custodian for Patrick S. Smith IRA.
|
|
|
(3)
|
Includes 324,000 shares of Common Stock issuable upon exercise of stock options exercisable within 60 days of August 31, 2010 and 741,382 shares and 36,150 warrants owned by the Patrick S. Smith Irrevocable Trust u/d/t dated June 25, 1991, for which David J. Corcoran serves as trustee. Mr. Corcoran has full investment and voting power over the shares held in the Trust, but disclaims beneficial ownership thereof. Also includes 87,000 shares held by the David J. Corcoran Rollover IRA.
|
|
|
(4)
|
Includes 168,500 shares of Common Stock issuable upon exercise of stock options exercisable within 60 days of August 31, 2010. Also includes 4,000 shares held by Wachovia Securities as custodian fbo Judith S. T. Geaslen.
|
|
|
(5)
|
Includes 364,500 shares of Common Stock issuable upon exercise of stock options exercisable within 60 days of August 31, 2010. Also includes 50,000 shares owned by Shelberg LLC of which Sheldon Goldberg is the general partner and 185,000 shares and 60,000 warrants held by the Sheldon Goldberg Trust for which Sheldon L, Goldberg is the trustee.
|
|
|
(6)
|
Includes 295,000 shares of Common stock issuable upon the exercise of stock options exercisable within 60 days of August 31, 2010.
|
|
|
(7)
|
Includes 10,000 shares of Common stock issuable upon the exercise of stock options exercisable within 60 days of August 31, 2010 and 5,000 shares and 30,000 warrants held by Charles Schwab &Co., Inc. as custodian for the Ronald V. Pompeo IRA.
|
|
|
(8)
|
See notes 1-7 above.
|
|
|
(9)
|
Includes 3,589,460 warrants issued to Mr. Martin in connection with the Merger.
|
72
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Any future transactions or loans between us and our officers, directors, principal stockholders or affiliates will be on terms no less favorable to us than could be obtained from an unaffiliated third party, and will be approved by a majority of disinterested directors.
On June 12, 2009, Curaxis entered into a letter agreement with Canterbury Investment Partners, LLC of Hingham, Massachusetts (“Canterbury”) to assist Curaxis in a range of issues in connection with its contemplated merger with a public shell corporation, including its negotiations with Southridge, in developing a strategy to negotiate reductions, deferrals and/or equity exchanges with its trade creditors, and in raising funds from current or prospective investors and in negotiating the terms of the acquisition of a public corporation (the “Letter Agreement”). Under the terms of the Letter Agreement, Curaxis paid Canterbury a retainer of $50,000 and is also obligated to pay Canterbury a monthly fee of $5,833 for a period of twelve (12) months from September 2009 through August 2010. To date, $64,163 has been paid under the monthly agreement. In addition, under the Letter Agreement, Curaxis has issued a warrant to Canterbury to purchase 854,358 shares of Curaxis Common Stock at a price of $0.22 per share. Following the closing of the Merger, Curaxis is obligated to pay Canterbury the sum of $8,000 per month for a period of six (6) months for Canterbury’s assistance in developing and implementing a strategy to support Curaxis in its dealings with the financial community. Mark Pompeo, who is the manager of Canterbury, is the brother of Ronald Pompeo, who was elected a director of Curaxis on July 3, 2009.
MARKET FOR COMMON STOCK
Our common stock is quoted on the OTCBB under the trading symbol “CURX.OB.” To date, our common stock has traded with limited volume.
DESCRIPTION OF SECURITIES
On November 19, 2009, Mr. Jonathan Martin, our then sole director and majority shareholder, authorized an amendment to our Certificate of Incorporation to increase the authorized common and preferred stock of our Company. On December 9, 2009, Mr. Martin authorized us to undertake a 91-for-1 forward stock split of our issued and outstanding common stock. As a result, our authorized capital stock as stated in our Amended and Restated Certificate of Incorporation consists of 480,000,000 shares of common stock, $0.0001 par value per share, and 20,000,000 shares of preferred stock, $0.0001 par value per share.
The following summary of our common stock and preferred stock is not complete and may not contain all the information you should consider before investing in our common stock. This description is subject to and qualified in its entirety by the provisions of our Amended and Restated Certificate of Incorporation, amended and restated bylaws and applicable Nevada law. After the Merger, there were 71,667,902 common shares issued, 13,720,597 common shares reserved for issuance, and 394,611,501 common shares authorized and unissued but not reserved. Upon the exercise of all options and warrants, we may be required to issue up to 13,541,501 restricted shares of common stock.
Common Stock
As of November 30, 2010, there were 72,216,489 shares of our common stock issued and outstanding. The holders of shares of common stock have no subscription, redemption, subscription, sinking fund or conversion rights. In addition, the holders of shares of common stock have no preemptive rights to maintain their percentage of ownership in future offerings or sales of our stock. The holders of shares of common stock have one vote per share in all elections of directors and on all other matters submitted to a vote of our stockholders. The holders of common stock are entitled to ratably receive dividends, if any, as and when declared from time to time by our board of directors out of funds legally available therefor. Upon liquidation, dissolution or winding up of our affairs, the holders of common stock will be entitled to participate equally and ratably, in proportion to the number of shares held, in our net assets available for distribution to holders of common stock. The shares of common stock currently outstanding are fully paid and nonassessable.
73
Preferred Stock
Our board of directors, without further stockholder authorization, may issue from time to time up to 20,000,000 shares of preferred stock in one or more series, establish the number of shares to be included in any of these series and fix the designations, powers, preferences and rights of the shares of each of these series and any qualifications, limitations or restrictions thereof, including dividend rights and preferences over dividends on the common stock, conversion rights, voting rights, redemption rights, the terms of any sinking fund therefor and rights upon liquidation.
SELLING SECURITY HOLDER
We agreed to register for resale 14,500,000 shares of our common stock, par value $0.0001 per share, (the “Put Shares”) that we will put to Southridge Partners II, LP (“Southridge” or “Selling Security Holder”) pursuant to a private equity credit agreement (the “Equity Credit Agreement”) between Southridge and the registrant, dated September 16, 2010 and subsequently amended on December 6, 2010. The Equity Credit Agreement with Southridge provides that Southridge is committed to purchase up to $25,000,000 of our common stock. We may draw on the facility from time to time, as and when we determine appropriate in accordance with the terms and conditions of the Equity Credit Agreement. We will not receive any proceeds from the sale of these shares of common stock offered by Selling Security Holder. However, we will receive proceeds from the sale of our Put Shares under the Equity Credit Agreement. The proceeds will be used for working capital or general corporate purposes.
Security Holder Pursuant to the Equity Credit Agreement
Southridge is the potential purchaser of our common stock under the Equity Credit Agreement. The 14,500,000 Put Shares offered in this prospectus are based on the Equity Credit Agreement between Southridge and us. Southridge may from time to time offer and sell any or all of the Put Shares that are registered under this prospectus. The put option price is 95% of the average of the three lowest Bid Prices in the five trading days period immediately following the Put Date.
We are unable to determine the exact number of shares that will actually be sold by Southridge according to this prospectus due to:
|
●
|
the ability of Southridge to determine when and whether it will sell any of the Put Shares under this prospectus; and
|
|
●
|
the uncertainty as to the number of Put Shares that will be issued upon exercise of our put options under the Equity Credit Agreement.
|
The following information contains a description of how Southridge acquired (or shall acquire) the shares to be sold in this offering. Southridge has not held a position or office, or had any other material relationship with us, except as follows.
Southridge is a limited partnership organized and existing under the laws of the state of Delaware. All investment decisions of, and control of, Southridge is held by its general partner Southridge Advisors, LLC. Stephen M. Hicks is the manager of Southridge Advisors, LLC, and he has voting and investment power over the shares beneficially owned by Southridge Partners II, LP. Southridge acquired, or will acquire, all shares being registered in this offering in the financing transactions with us.
74
Southridge intends to sell up to 14,500,000 shares of our common stock pursuant to the Equity Credit Agreement under this prospectus. On September 16, 2010, the Company and Southridge entered into the Equity Credit Agreement pursuant to which we have the opportunity, for a three-year period beginning on the date on which the SEC first declares effective this registration statement registering the resale of our shares by Southridge, to sell shares of our common stock for a total purchase price of $25,000,000. For each share of our common stock purchased under the Equity Credit Agreement, Southridge will pay 95% of the average lowest Bid Price of any three trading days, consecutive or inconsecutive during the Valuation Period. On December 6, 2010, the Company and Southridge subsequently amended the terms of the Equity Credit Agreement.
In addition, pursuant to the Equity Credit Agreement, in each Put Notice, we are required to specify a Floor Price. In the event the Bid Price decreases below the Floor Price during the Valuation Period, Southridge shall not be allowed to fund one-fifth (1/5) of the put amount on the Put Notice for each such trading day, and the put amount on the Put Notice shall be adjusted accordingly.
Furthermore, subject to the terms and conditions of the Equity Credit Agreement, at any time or from time to time after the effectiveness of this registration statement, we can notify Southridge in a Blackout Notice the existence of a potential material event based upon the good faith determination of our board of directors, and Southridge shall not offer or sell any of our shares acquired under the Equity Credit Agreement, or engage in any transaction involving or relating to such shares from the time the Blackout Notice was provided to them until Southridge receives our written notice that such potential material event has either been disclosed to the public or no longer constitutes a potential material event. If we deliver a Blackout Notice within fifteen trading days, commencing on a Closing Date, and the Old Bid Price on the day immediately preceding the applicable Blackout Period is greater than the New Bid price on the first trading day immediately following such Blackout Period, then we are obligated to issue to Southridge a number of Blackout Shares, equal to the difference between (i) the product of (X) the Remaining Put Shares that were issued to Southridge on the most recent Closing Date and held by Southridge immediately prior to the Blackout Period, multiplied by (Y) the Old Bid Price, and divided by (Z) the New Bid Price, and (ii) the Remaining Put Shares.
We are relying on an exemption from the registration requirements of the Securities Act for the private placement of our securities under the Equity Credit Agreement pursuant to Section 4(2) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder. The transaction does not involve a public offering, Southridge is an “accredited investor” and/or qualified institutional buyer and Southridge has access to information about us and its investment.
There are substantial risks to investors as a result of the issuance of shares of our common stock under the Equity Credit Agreement. These risks include dilution of stockholders and significant decline in our stock price.
Southridge will periodically purchase shares of our common stock under the Equity Credit Agreement and will in turn, sell such shares to investors in the market at the prevailing market price. This may cause our stock price to decline, which will require us to issue increasing numbers of shares to Southridge to raise the same amount of funds, as our stock price declines.
Southridge and any participating broker-dealers are “underwriters” within the meaning of the Securities Act. All expenses incurred with respect to the registration of the common stock will be borne by us, but we will not be obligated to pay any underwriting fees, discounts, commission or other expenses incurred by Selling Security Holder in connection with the sale of such shares.
Except as indicated below, neither Selling Security Holder nor any of its associates or affiliates has held any position, office, or other material relationship with us in the past three years.
75
The following table sets forth the name of Selling Security Holder, the number of shares of common stock beneficially owned by Selling Security Holder as of the date hereof and the number of share of common stock being offered by Selling Security Holder. The shares being offered hereby are being registered to permit public secondary trading, and Selling Security Holder may offer all or part of the shares for resale from time to time. However, Selling Security Holder is under no obligation to sell all or any portion of such shares nor is Selling Security Holder obligated to sell any shares immediately upon effectiveness of this prospectus. All information with respect to share ownership has been furnished by Selling Security Holder. The column entitled “Number of Shares Beneficially Owned After the Offering” assumes the sale of all shares offered.
|
(1)
|
The number assumes Selling Security Holder sells all of its shares being offering pursuant to this prospectus.
|
|
(2)
|
Southridge Partners II, LP is a limited partnership organized and exiting under the laws of the state of Delaware. Southridge Advisors, LLC is the general partner of Southridge and has voting and investment power over the shares beneficially owned by Southridge Partners II, LP. Stephen M. Hicks is the manager of Southridge Advisors, LLC, and he has voting and investment power over the shares beneficially owned by Southridge Partners II, LP.
|
The number assumes that Southridge purchases the maximum amount of registrable Put Shares in this registration statement.
This prospectus relates to the resale of up to 14,500,000 shares issued pursuant to the Equity Credit Agreement held by a certain Selling Security Holder.
Selling Security Holder and any of its pledges, donees, assignees and other successors-in-interest may, from time to time, sell any or all of their shares of our common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. Selling Security Holder may use any one or more of the following methods when selling shares:
|
·
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
·
|
block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
·
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
·
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
·
|
privately negotiated transactions;
|
|
·
|
broker-dealers may agree with Selling Security Holder to sell a specified number of such shares at a stipulated price per share;
|
|
·
|
through the writing of options on the shares;
|
|
·
|
a combination of any such methods of sale; and
|
|
·
|
any other method permitted pursuant to applicable law.
|
76
To the extent permitted by law, Selling Security Holder may also engage in short sales against the box after this registration statement becomes effective, puts and calls and other transactions in our securities or derivatives of our securities and may sell or deliver shares in connection with these trades.
Selling Security Holder, or its pledges, donees, transferees or other successors in interest, may also sell the shares directly to market makers acting as principals and/or broker-dealers acting as agents for itself or its customers. Such broker-dealers may receive compensation in the form of discounts, concessions or commissions from Selling Security Holder and/or the purchasers of shares for whom such broker-dealers may act as agents or to whom they sell as principal or both, which compensation as to a particular broker-dealer might be in excess of customary commissions. Market makers and block purchasers purchasing the shares will do so for their own account and at their own risk. It is possible that Selling Security Holder will attempt to sell shares of Common Stock in block transactions to market makers or other purchasers at a price per share which may be below the then market price. Selling Security Holder cannot assure that all or any of the shares offered in this prospectus will be issued to, or sold by, Selling Security Holder. In addition, any brokers, dealers or agents, upon effecting the sale of any of the shares offered in this prospectus are “underwriters” as that term is defined under the Securities Act or the Exchange Act, or the rules and regulations under such acts. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act.
Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by Selling Security Holder. Selling Security Holder may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that person under the Securities Act.
Selling Security Holder may from time to time pledge or grant a security interest in some or all of the shares of our common stock owned by it and, if it defaults in the performance of its secured obligations, the pledgee or secured parties may offer and sell such the shares of common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or any other applicable provision of the Securities Act amending the list of selling security holders to include the pledgee, transferee or other successors in interest as selling security holders under this prospectus.
Selling Security Holder also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares of common stock from time to time under this prospectus after we have filed an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling security holders to include the pledgee, transferee or other successors in interest as selling security holders under this prospectus.
We are required to pay all fees and expenses incident to the registration of the shares of common stock. Otherwise, all discounts, commissions or fees incurred in connection with the sale of our common stock offered hereby will be paid by Selling Security Holder.
Selling Security Holder acquired the securities offered hereby in the ordinary course of business and has advised us that it has not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding the sale of its shares of common stock, nor is there an underwriter or coordinating broker acting in connection with a proposed sale of shares of common stock by Selling Security Holder. We will file a supplement to this prospectus if Selling Security Holder enters into a material arrangement with a broker-dealer for sale of common stock being registered. If Selling Security Holder uses this prospectus for any sale of the shares of common stock, it will be subject to the prospectus delivery requirements of the Securities Act.
77
Pursuant to a requirement by the Financial Industry Regulatory Authority, or FINRA, the maximum commission or discount to be received by any FINRA member or independent broker/dealer may not be greater than eight percent (8%) of the gross proceeds received by us for the sale of any securities being registered pursuant to SEC Rule 415 under the Securities Act.
The anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of our common stock and activities of Selling Security Holder. Selling Security Holder will act independently of us in making decisions with respect to the timing, manner and size of each sale.
Southridge is an “underwriter” within the meaning of the Securities Act in connection with the sale of our common stock under the Equity Credit Agreement. In connection with the Equity Credit Agreement, no fees were paid Southridge nor shares of our restricted common stock issued as additional consideration.
We will pay all expenses incident to the registration, offering and sale of the shares of our common stock to the public hereunder other than commissions, fees and discounts of underwriters, brokers, dealers and agents. If any of these other expenses exists, we expect Southridge to pay these expenses. We have agreed to indemnify Southridge and its controlling persons against certain liabilities, including liabilities under the Securities Act. We estimate that the expenses of the offering to be borne by us will be approximately $35,000. We will not receive any proceeds from the resale of any of the shares of our common stock by Southridge. We may, however, receive proceeds from the sale of our common stock under the Equity Credit Agreement.
Anslow & Jaclin, LLP, 195 Route 9 South, 2nd Floor, Manalapan, NJ, 07726, will pass upon the validity of the shares of our common sock being offered hereby.
EXPERTS
Our financial statements for the years ended December 31, 2009, 2008 and 2007 which are included in this prospectus and the registration statement, were audited by Rosenberg Rich Baker Berman and Company, an independent registered public accounting firm, as stated in their report appearing with the financial statements herein and incorporated in this registration statement, and are included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
We are subject to the informational requirements of the Exchange Act, which requires us to file reports, proxy statements and other information with the Securities and Exchange Commission (the “Commission”). Such reports, proxy statements and other information, along with the registration statement, including the exhibits and schedules thereto, may be inspected at public reference facilities of the Commission at 100 F Street N.E, Washington D.C. 20549. Copies of such material can be obtained from the Public Reference Section of the Commission at prescribed rates. You may call the Commission at 1-800-SEC-0330 for further information on the operation of the public reference room. Because we file documents electronically with the Commission, you may also obtain this information by visiting the Commission’s Internet website at http://www.sec.gov.
78
CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
CURAXIS PHARMACEUTICAL CORPORTATION
(A Development Stage Corporation)
INDEX TO CONDENSED FINANCIAL STATEMENTS
|
PAGE
|
||
|
CONDENSED CONSOLIDATED BALANCE SHEETS AS OF SEPTEMBER 30, 2010 (UNAUDITED) AND DECEMBER 31, 2009
|
F-2
|
|
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS FOR THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2010 AND 2009 AND THE CUMULATIVE PERIOD FROM INCEPTION (FEBRAURY 27, 2001) TO SEPTEMBER 30, 2010
|
F-3
|
|
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT) FOR THE CUMULATIVE PERIOD FROM INCEPTION (FEBRAURY 27, 2001) TO SEPTEMBER 30, 2010
|
F-4
|
|
|
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2010 AND 2009 AND THE CUMULATIVE PERIOD FROM INCEPTION (FEBRAURY 27, 2001) TO SEPTEMBER 30, 2010
|
F-6
|
|
|
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
|
F-8
|
F-1
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands)
|
December 31,
|
September 30,
|
|||||||
|
2009
|
2010
|
|||||||
|
|
(Unaudited)
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 631 | $ | 273 | ||||
|
Accounts receivable
|
11 | 3 | ||||||
|
Prepaid financing costs
|
- | 294 | ||||||
|
Prepaid assets
|
135 | 32 | ||||||
|
Security deposit
|
4 | 6 | ||||||
|
Total current assets
|
781 | 608 | ||||||
|
Property and equipment, net
|
- | - | ||||||
|
Prepaid financing costs, net of current portion
|
- | 587 | ||||||
|
Total assets
|
781 | 1,195 | ||||||
|
Liabilities and Stockholders’ Equity (Deficit)
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
2,619 | 2,572 | ||||||
|
Accrued expenses
|
1,345 | 1,195 | ||||||
|
Current portion of capital lease obligation
|
4 | 4 | ||||||
|
Notes payable
|
2,426 | 2,688 | ||||||
|
Total current liabilities
|
6,394 | 6,459 | ||||||
|
Derivative Instrument
|
- | 3,157 | ||||||
|
Deferred purchase credit
|
500 | 500 | ||||||
|
Deferred revenue
|
1,727 | 1,727 | ||||||
|
Long-term notes payable
|
1,750 | 1,350 | ||||||
|
Total liabilities
|
10,371 | 13,193 | ||||||
|
Commitments and contingencies
|
― | ― | ||||||
|
Stockholders’ equity (deficit):
|
||||||||
|
Preferred Stock, $.0001 par value; 1 authorized; -0- and 1 shares issued and outstanding at December 31, 2009 and September 30, 2010
|
― | ― | ||||||
|
Common stock, $0.001 and $0.0001 par value at December 31, 2009 and September 30, 2010, respectively; 480,000 authorized; 62,811 and 72,031 shares issued and outstanding at December 31, 2009 and September 30, 2010, respectively
|
62 | 7 | ||||||
|
Additional paid-in capital
|
77,898 | 79,376 | ||||||
|
Subscription receivable
|
― | (190 | ) | |||||
|
Deficit accumulated during the development stage
|
(87,550 | ) | (91,191 | ) | ||||
|
Total stockholders’ equity (deficit)
|
(9,590 | ) | (11,998 | ) | ||||
|
Total liabilities and stockholders’ equity (deficit)
|
$ | 781 | $ | 1,195 | ||||
See notes to condensed consolidated financial statements
F-2
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited - in thousands, except per share amounts)
|
Three months ended
September 30,
|
Nine months ended
September 30,
|
Cumulative from
Inception
(February 27, 2001)
to September 30,
|
||||||||||||||||||
|
2009
|
2010
|
2009
|
2010
|
2010
|
||||||||||||||||
|
Operating expenses
|
||||||||||||||||||||
|
Research and development
|
$ | 16 | $ | 19 | $ | 74 | $ | 62 | $ | 61,417 | ||||||||||
|
General and administrative
|
506 | 569 | 451 | 1,493 | 26,311 | |||||||||||||||
|
Marketing
|
- | - | - | - | 5,557 | |||||||||||||||
|
Loss on investment in real estate
|
- | - | - | - | 1,091 | |||||||||||||||
|
Loss on lease termination
|
- | - | - | - | 374 | |||||||||||||||
|
Total operating (income) expenses
|
522 | 588 | 525 | 1,555 | 94,750 | |||||||||||||||
|
Operating (loss) income
|
(522 | ) | (588 | ) | (525 | ) | (1,555 | ) | (94,750 | ) | ||||||||||
|
Gain on debt restructuring
|
6,562 | - | 6,562 | 204 | 6,766 | |||||||||||||||
|
Interest income (expense), net
|
(96 | ) | (41 | ) | (299 | ) | (133 | ) | (1,050 | ) | ||||||||||
|
Interest from derivative conversion feature
|
- | (2,586 | ) | - | (2,586 | ) | (2,586 | ) | ||||||||||||
|
Change in fair value of derivatives
|
-
|
429 | - | 429 | 429 | |||||||||||||||
|
Net income (loss)
|
$ | 5,944 | $ | (2,786 | ) | $ | 5,738 | $ | ( 3,641 | ) | $ | (91,191 | ) | |||||||
|
Basic net income (loss) per share
|
$ | 0.10 | $ | (0.04 | ) | $ | 0.10 | $ | (0.06 | ) | ||||||||||
|
Diluted net income (loss) per share
|
$ | 0.10 | $ | ( 0.04 | ) | $ | 0.10 | $ | (0.06 | ) | ||||||||||
|
Weighted average common shares outstanding – basic
|
57,716 | 69,408 | 56,134 | 65,690 | ||||||||||||||||
|
Weighted average common shares outstanding – diluted
|
61,359 | 69,408 | 59,016 | 65,690 | ||||||||||||||||
See notes to condensed consolidated financial statement
F-3
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOILIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
(Unaudited - in thousands)
|
Preferred stock
|
Common stock
|
Additional
Paid-in-
|
Subscription
|
Deficit Accumulated during the Development
|
||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
receivable
|
Stage
|
Total
|
|||||||||||||||||||||||||
|
Balances at Inception (February 27, 2001)
|
―
|
$
|
―
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
||||||||||||||||||
|
Sale of common stock
|
25,657
|
26
|
1,073
|
―
|
1,099
|
|||||||||||||||||||||||||||
|
Stock-based compensation
|
―
|
―
|
―
|
―
|
100
|
―
|
―
|
100
|
||||||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(1,219
|
)
|
(1,219
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2001
|
―
|
―
|
25,657
|
26
|
1,173
|
―
|
(1,219
|
)
|
(20
|
)
|
||||||||||||||||||||||
|
Sale of common stock
|
―
|
―
|
4,054
|
4
|
4,105
|
―
|
―
|
4,109
|
||||||||||||||||||||||||
|
Issuance of common stock in exchange for consulting services
|
―
|
―
|
12
|
―
|
10
|
―
|
―
|
10
|
||||||||||||||||||||||||
|
Stock-based compensation
|
―
|
―
|
―
|
―
|
272
|
―
|
―
|
272
|
||||||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(3,190
|
)
|
(3,190
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2002
|
―
|
―
|
29,723
|
30
|
5,560
|
―
|
(4,409
|
)
|
1,181
|
|||||||||||||||||||||||
|
Sale of common stock
|
―
|
―
|
4,794
|
5
|
8,239
|
―
|
―
|
8,244
|
||||||||||||||||||||||||
|
Issuance of common stock in exchange for consulting service
|
―
|
―
|
36
|
―
|
55
|
―
|
―
|
55
|
||||||||||||||||||||||||
|
Repurchase of common stock
|
―
|
―
|
(330
|
)
|
―
|
(379
|
)
|
―
|
―
|
(379
|
)
|
|||||||||||||||||||||
|
Stock-based compensation
|
―
|
―
|
―
|
―
|
78
|
―
|
―
|
78
|
||||||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(6,633
|
)
|
(6,633
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2003
|
―
|
―
|
34,223
|
35
|
13,553
|
―
|
(11,042
|
)
|
2,546
|
|||||||||||||||||||||||
|
Sale of common stock
|
―
|
―
|
4,388
|
4
|
12,491
|
―
|
―
|
12,495
|
||||||||||||||||||||||||
|
Issuance of common stock in exchange for consulting services
|
―
|
―
|
20
|
―
|
66
|
―
|
―
|
66
|
||||||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(11,626
|
)
|
(11,626
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2004
|
―
|
―
|
38,631
|
39
|
26,110
|
―
|
(22,668
|
)
|
3,481
|
|||||||||||||||||||||||
|
Sale of common stock
|
―
|
―
|
3,305
|
3
|
28,839
|
―
|
―
|
28,842
|
||||||||||||||||||||||||
|
Issuance of common stock in exchange for consulting services
|
―
|
―
|
4
|
―
|
33
|
―
|
―
|
33
|
||||||||||||||||||||||||
|
Stock-based compensation
|
―
|
―
|
―
|
―
|
10
|
―
|
―
|
10
|
||||||||||||||||||||||||
|
Repurchase of common stock
|
―
|
―
|
(1
|
)
|
―
|
(9
|
)
|
―
|
―
|
(9
|
)
|
|||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(32,904
|
)
|
(32,904
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2005
|
―
|
―
|
41,939
|
42
|
54,983
|
―
|
(55,572
|
)
|
(547
|
)
|
||||||||||||||||||||||
|
Sale of common stock
|
―
|
―
|
1,513
|
1
|
15,127
|
―
|
―
|
15,128
|
||||||||||||||||||||||||
|
Sale of common stock under warrants
|
―
|
―
|
2,886
|
3
|
286
|
―
|
―
|
289
|
||||||||||||||||||||||||
|
Stock-based compensation
|
―
|
―
|
―
|
―
|
1,155
|
―
|
―
|
1,155
|
||||||||||||||||||||||||
|
Repurchase of common stock
|
―
|
―
|
(150
|
)
|
―
|
―
|
―
|
―
|
―
|
|||||||||||||||||||||||
|
Net loss
|
―
|
―
|
―
|
―
|
―
|
―
|
(28,235
|
)
|
(28,235
|
)
|
||||||||||||||||||||||
|
Balances at December 31, 2006
|
―
|
―
|
46,188
|
$
|
46
|
$
|
1,551
|
$
|
―
|
$
|
(83,807
|
)
|
$
|
(12,210
|
)
|
|||||||||||||||||
See notes to condensed consolidated financial statements
F-4
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOILIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’
EQUITY (DEFICIT) - CONTINUED
(Unaudited - in thousands)
|
Preferred stock
|
Common stock
|
Additional
Paid-in-
|
Subscription | Deficit Accumulated during the Development | ||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
receivable
|
Stage
|
Total
|
|||||||||||||||||||||||||
|
Sale of common stock
|
― | $ | ― | 1,822 | $ | 2 | $ | 2,336 | $ | ― | $ | ― | $ | 2,338 | ||||||||||||||||||
|
Sale of common stock under warrants
|
1,971 | 2 | 171 | ― | 173 | |||||||||||||||||||||||||||
|
Stock-based compensation
|
― | ― | ― | ― | 889 | ― | ― | 889 | ||||||||||||||||||||||||
|
Net loss
|
― | ― | ― | ― | ― | ― | (6,744 | ) | (6,744 | ) | ||||||||||||||||||||||
|
Balances at December 31, 2007
|
― | ― | 49,981 | 50 | 74,947 | ― | (90,551 | ) | (15,554 | ) | ||||||||||||||||||||||
|
Sale of common stock
|
― | ― | 5,120 | 5 | 518 | ― | ― | 523 | ||||||||||||||||||||||||
|
Sale of common stock under warrants
|
― | ― | 41 | ― | 4 | ― | ― | 4 | ||||||||||||||||||||||||
|
Stock-based compensation
|
― | ― | ― | ― | 469 | ― | ― | 469 | ||||||||||||||||||||||||
|
Net loss
|
― | ― | ― | ― | ― | ― | (1,986 | ) | (1,986 | ) | ||||||||||||||||||||||
|
Balances at December 31, 2008
|
― | ― | 55,142 | 55 | 75,938 | ― | (92,537 | ) | (16,544 | ) | ||||||||||||||||||||||
|
Sale of common stock, net
|
― | ― | 6,071 | 6 | 1,096 | ― | ― | 1,102 | ||||||||||||||||||||||||
|
Sale of common stock under warrants
|
― | ― | 1,448 | 1 | 143 | ― | ― | 144 | ||||||||||||||||||||||||
|
Issuance of warrants
|
― | ― | ― | ― | 330 | ― | ― | 330 | ||||||||||||||||||||||||
|
Stock grant
|
― | ― | 150 | ― | 30 | ― | ― | 30 | ||||||||||||||||||||||||
|
Stock-based compensation
|
― | ― | ― | ― | 361 | ― | ― | 361 | ||||||||||||||||||||||||
|
Net income
|
― | ― | ― | ― | ― | ― | 4,987 | 4,987 | ||||||||||||||||||||||||
|
Balances at December 31, 2009
|
― | ― | 62,811 | 62 | 77,898 | ― | (87,550 | ) | (9,590 | ) | ||||||||||||||||||||||
|
Sale of common stock, net
|
― | ― | 1,182 | 1 | 219 | ― | ― | 220 | ||||||||||||||||||||||||
|
Sale of common stock under warrants
|
― | ― | 343 | 1 | 33 | ― | ― | 34 | ||||||||||||||||||||||||
|
Issuance of warrants
|
― | ― | ― | ― | 1,111 | ― | ― | 1,111 | ||||||||||||||||||||||||
|
Recapitalization of Company upon Merger effective July 29, 2010
|
― | ― | 7,695 | (57 | ) | (51 | ) | ― | ― | (108 | ) | |||||||||||||||||||||
|
Stock-based compensation
|
― | ― | ― | ― | 166 | ― | ― | 166 | ||||||||||||||||||||||||
|
Sale of Preferred stock
|
1 | ― | ― | ― | ― | (190 | ) | ― | (190 | ) | ||||||||||||||||||||||
|
Net loss
|
― | ― | ― | ― | ― | ― | (3,641 | ) | (3,641 | ) | ||||||||||||||||||||||
|
Balances at September 30, 2010
|
1 | $ | ― | 72,031 | $ | 7 | $ | 79,376 | $ | (190 | ) | $ | (91,191 | ) | $ | (11,998 | ) | |||||||||||||||
See notes to condensed consolidated financial statements
F-5
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited - in thousands)
|
Nine Months
Ended
September 30,
|
Cumulative from Inception (February 27, 2001) to
September 30,
|
|||||||||||
|
2009
|
2010
|
2010
|
||||||||||
|
Cash flows from operating activities
|
||||||||||||
|
Net income (loss)
|
$ | 5,738 | $ | (3,641 | ) | $ | (91,191 | ) | ||||
|
Adjustments to reconcile net income (loss) to net cash used in operating activities:
|
||||||||||||
|
Depreciation
|
40 | ― | 994 | |||||||||
|
Stock-based compensation expense
|
416 | 396 | 4,006 | |||||||||
|
Common stock issued in exchange for consulting service
|
― | ― | 164 | |||||||||
|
Interest from derivative conversion feature
|
― | 2,586 | 2,586 | |||||||||
|
Change in fair value of derivatives
|
(429 | ) | (429 | ) | ||||||||
|
Loss (gain) on disposal of property and equipment
|
(9 | ) | ― | 169 | ||||||||
|
Loss on investment in real estate
|
― | ― | 1,091 | |||||||||
|
Gain on restructuring of trade debt
|
(6,562 | ) | (204 | ) | (6,766 | ) | ||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Prepaid expenses
|
47 | 103 | 51 | |||||||||
|
Accounts receivable
|
(8 | ) | 8 | (3 | ) | |||||||
|
Security deposits
|
― | (2 | ) | (6 | ) | |||||||
|
Other assets
|
3 | ― | ― | |||||||||
|
Accounts payable
|
18 | (10 | ) | 7,516 | ||||||||
|
Accrued expenses
|
298 | (15 | ) | 2,427 | ||||||||
|
Deferred purchase credit
|
― | ― | 500 | |||||||||
|
Deferred revenue
|
― | ― | 1,727 | |||||||||
|
Accrued compensation
|
(351 | ) |
―
|
― | ||||||||
|
Net cash used in operating activities
|
(370 | ) | (1,208 | ) | (77,164 | ) | ||||||
|
Cash flows from investing activities
|
||||||||||||
|
Purchase of property and equipment
|
― | ― | (1,193 | ) | ||||||||
|
Purchase of real estate investment
|
― | ― | (3,155 | ) | ||||||||
|
Proceeds from the sale of property and equipment
|
10 | ― | 50 | |||||||||
|
Net cash provided by (used in) investing activities
|
10 | ― | (2,234 | ) | ||||||||
|
Cash flows from financing activities
|
||||||||||||
|
Issuance of common stock
|
1,239 | 254 | 74,744 | |||||||||
|
Issuance of Preferred Stock
|
810 | 810 | ||||||||||
|
Issuance of notes payable
|
― | ― | 5,011 | |||||||||
|
Issuance of long-term debt
|
― | ― | 592 | |||||||||
|
Payments on notes payable
|
(11 | ) | (214 | ) | (1,079 | ) | ||||||
|
Payments on capital lease obligation
|
(4 | ) | ― | (16 | ) | |||||||
|
Repurchase of common stock
|
― | ― | (388 | ) | ||||||||
|
Repayments of notes payable to related parties
|
― | ― | (202 | ) | ||||||||
|
Payments on long-term debt
|
― | ― | (3 | ) | ||||||||
|
Proceeds from notes payable to related parties
|
― | ― | 202 | |||||||||
|
Repayment of mortgage note payable
|
― | ― | (3,100 | ) | ||||||||
|
Proceeds from the issuance of mortgage note
|
― | ― | 3,100 | |||||||||
|
Net cash provided by financing activities
|
$ | 1,224 | $ | 850 | $ | 79,671 | ||||||
See notes to condensed consolidated financial statements
F-6
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS - CONTINUED
(Unaudited - in thousands)
|
Nine Months Ended
September 30,
|
Cumulative from Inception
(February 27, 2001) to
September 30,
2010
|
|||||||||||
|
2009
|
2010
|
|||||||||||
|
Net increase (decrease) in cash and cash equivalents
|
$ | 864 | $ | (358 | ) | $ | 273 | |||||
|
Cash and cash equivalents, beginning of period
|
5 | 631 | ― | |||||||||
|
Cash and cash equivalents, end of period
|
$ | 869 | $ | 273 | $ | 273 | ||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||
| Cash paid for interest | $ | 2 | $ | ― | $ | 242 | ||||||
|
Cash paid for income taxes
|
$ | ― | $ | ― | $ | ― | ||||||
|
Non-cash financing activities:
|
||||||||||||
|
Conversion of trade payables to notes
|
$ | ― | $ | ― | $ | 5,603 | ||||||
|
Restructuring of trade debt
|
$ | 6,562 | $ | 204 | $ | 6,766 | ||||||
|
Issuance of warrant for accrued liability
|
$ | ― | $ | ― | $ | 129 | ||||||
|
Issuance of warrants for prepaid asset
|
$ | ― | $ | (881 | ) | $ | (881 | ) | ||||
|
Net liabilities acquired in Merger Transaction
|
$ | ― | $ | (92 | ) | $ | (92 | ) | ||||
See notes to condensed consolidated financial statements
F-7
CURAXIS PHARMACEUTICAL CORPORATION
(A Development Stage Corporation)
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Description of business and basis of presentation
The accompanying unaudited condensed consolidated financial statements of Curaxis Pharmaceutical Corporation and its wholly owned subsidiary (the “Company”), have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 10 of Regulation S-X of the Securities and Exchange Commission (the “SEC”). Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. These statements should be read in conjunction with the financial statements and notes thereto included in the Company’s latest audited financial statements. The audited financial statements for the year ended December 31, 2009 are included in the Company’s Registration statement on Form S-4 filed with the Securities and Exchange Commission on June 22, 2010 and within this Registration Statement.
In the opinion of management, all adjustments, consisting only of normal, recurring adjustments, considered necessary for a fair presentation of the results of these interim periods have been included. The results of operations for the nine months ended September 30, 2010 may not be indicative of the results that may be expected for the full year.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
Buisness – The Company is an emerging specialty pharmaceutical company with a hormone drug product candidate for the treatment of Alzheimer’s disease and multiple cancers. Our therapeutic platform is based on the hypothesis that many diseases of aging may be caused by age-related changes in the function of the hypothalamic-pituitary-gonadal (HPG) axis. The HPG axis is a hormonal endocrine feedback loop that controls development, reproduction and aging in animals. This drug development platform is built on the premise that hormones associated with this feedback loop are beneficial early in life, when they promote growth and development, but are harmful later in life when the mechanism for feedback is compromised, thereby leading to disease processes, including pathologies associated with Alzheimer’s disease and various cancers. We believe our discovery of similar hormonal signaling mechanisms at the cellular level in brain tissue from Alzheimer’s patients and in multiple tumors will enable us to develop significant new treatments for Alzheimer’s disease as well as many cancers.
Corporate History – The Company incorporated in Nevada on February 1, 2008 under the name Auto Search Cars, Inc. The Company initially was engaged in the development of a web-based e-commerce site platform to provide information for the sale of vehicles and vehicle financing and warranties. However, no significant activities related to the website development have occurred. On February 8, 2010, the Company entered into an Agreement and Plan of Merger and Plan of Reorganization (the “Merger Agreement”) with Auto Search Cars Acquisition Corp., (“Acquisition Sub”) a Delaware corporation wholly owned by Auto Search Cars, Inc., (“Auto Search”) pursuant to which Acquisition Sub would be merged with and into the Company with the Company continuing as the surviving wholly-owned subsidiary of Auto Search. On July 29, 2010, the parties executed an amendment to the Merger Agreement in order to amend the consideration paid for the cancellation of certain shares of the Corporation’s common stock.
F-8
On July 29, 2010, the merger transaction was closed. Pursuant to the Amended Merger Agreement, Auto Search issued 64.2 million shares of its common stock to the holders of common stock of the Company. Under the terms of the Merger, all but 8.7 million of these shares are restricted from trading for a period of one year from the closing of the Merger. In addition, each issued Curaxis Warrant (as defined in the Merger Agreement) was converted into warrants to purchase an equal number of shares of Auto Search’s common stock at the Exercise Price defined in the Company Warrants. Further the sole officer of Auto Search agreed to cancel 181,285,000 shares of Auto Search common stock in exchange for payment of $100 thousand dollars in the form of a promissory note and the issuance of 3,589,460 warrants to purchase common stock of the surviving corporation.
On the Closing Date, Curaxis and Acquisition Corp. merged with and into one another, with the surviving corporation being Curaxis. Simultaneously to the filing of the certificate of merger with the State of Delaware, Curaxis amended its certificate of incorporation in order to change its name to Curaxis Pharma Corp. On the Closing Date, Curaxis Pharma Corp. became a subsidiary of Auto Search.
On July 30, 2010, Auto Search entered into an agreement and plan of merger with Curaxis Pharmaceutical Corporation, a Nevada corporation formed solely for the purpose of a name change. Pursuant to the Short-Form Merger, Auto Search changed its name to Curaxis Pharmaceutical Corporation.
Basis of Presentation - The Company is a development stage company and has focused its efforts to date on raising capital and research and development. The accompanying financial statements have been prepared on a basis which assumes that the Company will continue as a going concern and which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has a limited operating history and has incurred losses from operations since its inception. The cash position of the Company has deteriorated significantly over the last three years and the Company has been unable to satisfy its outstanding liabilities with many vendors. In late 2006, the company terminated its’ Phase III clinical trial VP-AD-301 and in 2007 closed its’ research facility.
In 2009, Curaxis entered into a series of agreements with Southridge Business Solutions LLC (“Southridge”) and Canterbury Investment Partners LLC (“Canterbury”) in order to restructure its balance sheet and establish a trading market for its common stock. Under the terms of those agreements, Southridge and Canterbury have assisted and will continue to assist Curaxis in negotiating settlements with its creditors to eliminate or substantially reduce and defer its trade debt. To date, Curaxis has realized reductions in excess of $6 million in liabilities as the result of the combined efforts. In addition, Southridge assisted Curaxis in securing a $25 million equity facility under which Curaxis can periodically, over a period of three years, sell up to $25 million of its common stock to an affiliate of Southridge. The proceeds of the equity facility will be used to fund the next step in Curaxis' clinical development plan, a Phase IIb study in approximately 200-250 women. Assuming the study commences in early 2011, it is anticipated that the results of the study would be available during 2013. Assuming favorable results from the second Phase II trial are achieved, Curaxis’ management expected to fund the remaining development of its product candidate through public equity offerings, debt financings and possibly corporate collaborations and licensing arrangements. However, the ability to secure such funding on a prospective basis is uncertain. If Curaxis is unable to secure funding as intended, Curaxis will be forced to delay, reduce or eliminate its research and development programs or commercialization efforts.
Fair Value of Financial Instruments - The carrying amounts for cash, cash equivalents, accounts receivables, prepaid assets, accounts payable, and accrued expenses approximate fair value because of their short-term nature. We have determined that it is not practical to estimate the fair value of our notes payable because of their unique nature and the costs that would be incurred to obtain an independent valuation. We do not have comparable outstanding debt on which to base an estimated current borrowing rate or other discount rate for purposes of estimating the fair value of the notes payable and we have not yet obtained or developed a valuation model. Additionally, we are engaged in research and development activities and have not yet developed products for sale. Accordingly, at this stage of our development, a credit risk assessment is highly judgmental. These factors all contribute to the impracticability of estimating the fair value of the notes payable. At September 30, 2010, the carrying value of the notes payable and accrued interest was $4.0 million and $420 thousand, respectively.
F-9
Fair Value Measurements - The authoritative guidance for fair value measurements defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or the most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Market participants are buyers and sellers in the principal market that are (i) independent, (ii) knowledgeable, (iii) able to transact, and (iv) willing to transact. The guidance describes a fair value hierarchy based on the levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
|
·
|
Level 1 — Quoted prices in active markets for identical assets or liabilities
|
|
·
|
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or corroborated by observable market data or substantially the full term of the assets or liabilities
|
|
·
|
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the value of the assets or liabilities
|
Derivative Instrument - The Derivative instrument consists of the Series A and B convertible preferred stock, which has certain cash settlement provisions. This financial instrument is recorded in the balance sheet at fair value as a liability. Changes in fair value are recognized in earnings in the period of change.
Recently Issued Accounting Standards - Management does not believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying financial statements.
2. Notes payable
Notes payable consist of the following (in thousands):
| December 31, | September 30, | |||||||
|
2009
|
2010
|
|||||||
|
Notes payable to clinical research sites
|
$ | 407 | $ | 393 | ||||
|
Promissory Note dated September 15, 2006
|
2,000 | 1,950 | ||||||
|
Promissory Installment Note dated September 26, 2006
|
924 | 750 | ||||||
|
Promissory Note dated July 1, 2007
|
300 | 300 | ||||||
|
Promissory Note dated August 21, 2007
|
420 | 420 | ||||||
|
Promissory Note and Security Agreement dated September 22, 2008
|
26 | 26 | ||||||
|
Default judgment dated October 29, 2009
|
99 | 99 | ||||||
|
Promissory note dated July 29, 2010
|
- | 100 | ||||||
| 4,176 | 4,038 | |||||||
|
Less: Current portion of notes payable
|
2,426 | 2,688 | ||||||
| $ | 1,750 | $ | 1,350 | |||||
Promissory note dated July 29, 2010 in the amount of $100 thousand was executed in conjunction with the merger transaction consummated on July 29, 2010. The note bears interest at an annual rate of 8% and is payable on or before July 26, 2011.
F-10
3. Stock Purchase Warrants
At December 31, 2009 and September 30, 2010 outstanding warrants to purchase the company’s common stock are as follows:
|
December 31,
2009
|
September 30,
2010
|
Exercise Price
|
Expiration Date
|
|||||||||||
|
Warrants issued January 2006 through May 2007 in conjunction with private placement of common stock.
|
3,615,700 | 3,280,900 | $ | 0.10 |
December 31, 2011
|
|||||||||
|
Warrants issued to select vendors to satisfy, in part, liabilities generated from services performed in conjunction with our terminated Phase III clinical trial, VP-AD-301.
|
54,800 | 54,800 | $ | 0.50 |
December 31, 2011
|
|||||||||
|
Warrants issued under Transaction Management Agreement executed with Southridge.
|
1,656,500 | 2,149,100 | $ | 0.001 |
August 31, 2016
|
|||||||||
|
Warrants issued to outside consultants for services rendered in connection with the financing round completed on February 5, 2010.
|
- | 844,400 | $ | 0.22 |
August 31, 2016
|
|||||||||
|
Warrants issued as consideration for the cancellation of 181,285,000 shares of common stock upon consummation of the Merger transaction effective July 29, 2010.
|
- | 3,589,500 | $ | 0.001 |
July 29, 2017
|
|||||||||
|
Warrants issued to Southridge under the Transaction Management Agreement upon execution of Equity Credit Agreement dated September 14, 2010.
|
- | 820,800 | $ | 1.52 |
September 15, 2015
|
|||||||||
|
Warrants issued August 31, 2010 to outside vendor for development of the Company’s website.
|
- | 100,000 | $ | 0.30 |
August 31, 2015
|
|||||||||
| 10,839,500 | ||||||||||||||
During the nine months ended September 30, 2010, 334,800 warrants were exercised at $0.10 per share and 10,000 warrants were exercised at $0.20 per share.
4. Convertible Preferred Stock
On September 23, 2010 and September 29, 2010, the Company amended its certificate of incorporation by filing certificates of designation with Secretary of State of Nevada that designated two series of convertible preferred stock (the “Series A Convertible Preferred Stock” and the “Series B Convertible Preferred Stock”), respectively. Each share of Series A and Series B preferred stock has a par value of $.0001 and an initial stated value equal to $1,000. The Company is to pay cumulative dividends at the rate of 4% per annum payable quarterly in arrears beginning December 15, 2010. Dividends may be paid in cash or at the Company’s irrevocable option, in shares at the lesser of (A) the pre-determined conversion price or (B) 80% of the ten previous days VWAP. Each share of preferred stock shall be convertible at any time at the option of the Holder into common stock. The number of shares of common stock to be issued upon conversion of each share of preferred stock will be determined by dividing the initial stated value of the preferred stock by a pre-determined conversion rate. The predetermined conversion rates for the Series A and Series B preferred stock is $0.35 and $0.50, respectively.
On September 30, 2010, the Company entered into a Series A Convertible Preferred Stock Purchase Agreement (the “Series A Agreement”), by and between the Company and C P Acquisition Partners LP, an affiliate of Southridge, (the “Purchaser”) Pursuant to the terms of the Series A Agreement, the Company sold 500 shares of the Company’s Series A Convertible Preferred Stock for a total purchase price of $500,000.
F-11
On September 30, 2010, the Company entered into a Series B Convertible Preferred Stock Purchase Agreement (the “Series B Agreement”), by and between the Company and the Purchaser. Pursuant to the terms of the Series B Agreement, the Company sold 500 shares of the Company’s Series B Convertible Preferred Stock for a total purchase price of $500,000. Cash proceeds of $310 thousand have been received by the Company as of September 30, 2010. A subscription receivable in the amount of $190 thousand is recognized as of September 30, 2010.
We have an obligation to make a cash payment to the holders of the Series A and Series B preferred stock for any loss that could be realized if the holders convert the preferred stock and we subsequently fail to deliver a certificate representing the shares to be issued upon such conversion by the third trading day after such conversion and the holders purchase, in an arm’s-length open market transaction or otherwise, shares of Common Stock (the “Covering Shares”) in order to make delivery in satisfaction of a sale of Common Stock by the converting Holder (the “Sold Shares”), which delivery such converting Holder anticipated to make using the shares to be issued upon such conversion (a “Buy-In”). “Buy-In Adjustment Amount” means the amount equal to the excess, if any, of (i) the converting Holder’s total purchase price (including brokerage commissions, if any) for the Covering Shares associated with a Buy-In, over (ii) the net proceeds (after brokerage commissions, if any) received by the converting Holder from the sale of the Sold Shares. Accordingly, the Series A and Series B preferred shares have been accounted for as a liability. Their fair value is estimated, at the end of each quarterly reporting period, based on the potential shares of common stock issuable upon conversion multiplied by the current quoted market price of the Company’s common stock. The Company estimated the fair value of the Series A and Series B preferred shares on the grant date to be $3.6 million. The difference between the estimated fair value and the stated value of the preferred stock of $2.6 million was recorded as interest expense. The fair value of the Series A and Series B preferred shares decreased by $429 thousand for the three and nine months ended September 30, 2010, and the fluctuation has been recorded in the statements of operations.
Dividends payable in arrears at September 30, 2010 totaled $400.
5. Accounting for stock-based compensation
Total stock-based compensation expense recognized in the accompanying condensed consolidated statement of operations for the three and nine months ended September 30, 2009 and 2010 was as follows (in thousands):
|
Three-months ended
September 30,
|
Nine months ended
September 30, 2010
|
|||||||||||||||
|
2009
|
2010
|
2009
|
2010
|
|||||||||||||
|
Employee-related stock-based compensation
|
$ | 117 | $ | 53 | $ | 295 | $ | 167 | ||||||||
|
Non-employee related stock based compensation
|
109 | - | 121 | 229 | ||||||||||||
| $ | 226 | $ | 53 | $ | 416 | $ | 396 | |||||||||
As of September 30, 2010 there was $454 thousand of total unrecognized compensation cost related to non-vested share based compensation arrangements granted under the Plans. That cost is expected to be recognized over a weighted–average period of 1.7 years.
6. Earnings per share
In accordance with SFAS No. 128, Earnings per Share (“SFAS No. 128”), codified in ASC 260, basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding. Diluted net loss per common share is computed similarly to basic net loss per share, except that the denominator is increased to include all potential dilutive common shares, including outstanding options and warrants. Potentially dilutive common shares have been excluded from the diluted loss per common share computation for the three and nine month periods ended September 30, 2010 because such securities have an anti-dilutive effect on loss per share due to the Company’s net loss. Potential dilutive securities, which consist of stock options, warrants and shares that could be issued upon conversion of the preferred stock, that are not included in the diluted net loss per share calculation because of their anti dilutive effect total 16,796,200 shares as of September 30, 2010.
F-12
Dilutive securities included in the calculation of diluted net income per share for the three and nine months ended September 30, 2009, which consist of stock options and warrants, represented an aggregate of 3,643,000 and 2,882,000 shares, respectively. Securities excluded from the calculations of diluted net income per share because their effect was anti-dilutive, represented an aggregate of 6,032,000 shares as of September 30, 2009..
7. Equity Credit Agreement
On September 16, 2010, the Company entered into a Private Equity Credit Agreement (the “Equity Credit Agreement”) with Southridge Partners II, LP (the “Investor”), a limited partnership organized and existing under the laws of the State of Delaware and an affiliate of Southridge. Pursuant to this Equity Credit Agreement, the Investor shall commit to purchase upon to $25 million of the Company’s common stock over the course of thirty six months commencing the effective date of the Registration Statement covering the Registrable Securities pursuant to the Equity Credit Agreement. The put option price is 95% of the average of three lowest closing bid price (the “Bid Price”) of any two applicable trading days, consecutive or inconsecutive, during the five trading day period (the “Valuation Period”) commencing the date a put notice (the “Put Notice”) is delivered to the Investor (the “Put Date”) in a manner provided by the Equity Credit Agreement.
In addition, pursuant to the Equity Credit Agreement, in each Put Notice, the Company is required to specify a minimum stock price which in no event shall be less than 60% of the average of the Bid Price during the three trading day period commencing the Put Date (the “Floor Price”). In the event the Bid Price decreases below the Floor Price during the Valuation Period, the Company shall have the right to cancel such Put Notice.
The “Registrable Securities” include the Put Shares, any Blackout Shares (as defined in the Equity Credit Agreement) and any securities issued or issuable with respect to any of the foregoing by way of exchange, stock dividend or stock split or in connection with a combination of shares, recapitalization, merger, consolidation or other reorganization or otherwise.
Upon execution of the Equity Credit Agreement, the Company issued 820,856 warrants to the Investor in accordance with the Transaction Management Agreement with Southridge. The exercise price is equal to 120% of the average closing price of the Company's stock for the previous 20 days or $1.52. The fair value of the warrants totaling $881 thousand has been recognized as prepaid financing costs as of the agreement date and will be amortized over the life of the agreement or 36 months.
8. Fair Value
In accordance with FASB ASC 820, “Fair Value Measurements and Disclosures”, the following table represents the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as September 30, 2010 and December 31, 2009:
|
Level 2
|
Level 2
|
|||||||
|
September 30, 2010
|
December 31, 2009
|
|||||||
|
($ thousands)
|
($ thousands)
|
|||||||
|
Derivative instruments (long term)
|
$
|
3,157
|
$
|
0
|
||||
|
Total
|
$
|
3,157
|
$
|
0
|
||||
F-13
9. Commitments
Management Advisory and Consulting Agreements
Southridge Agreement
On May 28, 2009, Curaxis entered into a transaction management agreement with Southridge Business Solutions Group, LLC, of Ridgefield, Connecticut (“Southridge” or “Selling Security Holder”), to assist Curaxis in restructuring its balance sheet, principally through negotiations with several large trade creditors to reduce their claims, and to assist Curaxis in effectuating a merger with a suitable public corporation (the “Transaction Management Agreement”). Pursuant to the Transaction Management Agreement, Curaxis had worked with Southridge to reduce its trade debt by approximately $6,000,000 and entered into the Merger Agreement with Auto Search Cars, Inc. Under the terms of the Transaction Management Agreement, Curaxis is obligated to pay Southridge a management fee of $10,000 per month, from June 1, 2009 through the first anniversary of the closing of the Merger with Auto Search, or July 2011. Curaxis may terminate the Transaction Management Agreement at any time by giving 90 days’ advance notice to Southridge. In addition, Curaxis is obligated to issue to Southridge warrants equal to 3% of Curaxis’ outstanding stock as of the date of the Merger with Auto Search at an exercise price of $0.001 per share. A total of 2,149,148 warrants have been issued to Southridge under the agreement.
Canterbury Agreement
On June 12, 2009, Curaxis entered into a letter agreement with Canterbury Investment Partners, LLC of Hingham, Massachusetts (“Canterbury”), to assist Curaxis in a range of issues in connection with its contemplated merger with a public shell corporation, including its negotiations with Southridge, in developing a strategy to negotiate reductions, deferrals and/or equity exchanges with its trade creditors and in raising funds from current or prospective investors and in negotiating the terms of the acquisition of a public corporation (the “Letter Agreement”). Under the terms of the Letter Agreement, Curaxis paid Canterbury a retainer of $50,000 and is also obligated to pay Canterbury a monthly fee of $5,833 for a period of twelve (12) months from September 2009 through August 2010. In addition, under the Letter Agreement, Curaxis has issued a warrant to Canterbury to purchase 854,358 shares of Curaxis Common Stock at a price of $0.22 per share. Following the closing of the Merger with Auto Search, Curaxis is obligated to pay Canterbury the sum of $8,000 per month for a period of six (6) months for Canterbury’s assistance in developing and implementing a strategy to support Curaxis in its dealings with the financial community.
10. Related Party Transactions
During the two years ended December 2008, the Company funded travel expenses of an executive officer not directly related to the Company’s business. Travel expenses paid by the Company on behalf of the officer totaled $14 thousand. In addition, during the year ended December 31, 2009, the Company advanced funds to the executive officer for travel and office expenses totaling $16,000. Payments made to the Company by the employee and actual expenses incurred on the Company’s behalf totaled $10 thousand and $9 thousand for the years ended December 31, 2008 and 2009, respectively. During the three months ended September 30, 2010, the executive officer forfeited compensation payments to satisfy the liability to the Company in full.
11. Subsequent Events
Subsequent to September 30, 2010, proceeds of $75 thousand were received reducing the Series B subscription receivable to $115 thousand.
F-14
Curaxis Pharmaceutical Corporation
(A Development Stage Company)
Financial Statements
December 31, 2008 and 2009
F-15
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Index to Financial Statements
|
Report of Independent Registered Public Accounting Firm
|
F-17 | |||
|
Balance Sheets
|
F-18 | |||
|
Statements of Operations
|
F-19 | |||
|
Statements of Changes in Stockholders’ Equity (Deficit)
|
F-20 – F-21 | |||
|
Statements of Cash Flows
|
F-22 – F-23 | |||
|
Notes to Financial Statements
|
F-24 – F-40 |
F-16
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Curaxis Pharmaceutical Corporation
(A Development Stage Company):
We have audited the accompanying balance sheets of Curaxis Pharmaceutical Corporation (a Development Stage Company) as of December 31, 2008 and 2009, and the related statements of operations and cash flows for each of the three years in the period ended December 31, 2009 and the changes in stockholders' equity (deficit), for the period from January 1, 2006 to December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We did not audit the financial statements of Curaxis Pharmaceutical Corporation for the period from inception to December 31, 2005.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, based on our audits, the financial statements referred to above present fairly, in all material respects, the financial position of Curaxis Pharmaceutical Corporation as of December 31, 2008 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, and the changes in stockholders' equity (deficit), for the period from January 1, 2006 to December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company is in the development stage, has incurred losses from operations since its inception and has a net stockholders’ deficit. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Rosenberg Rich Baker Berman & Company
Somerset, New Jersey
March 31, 2010
F-17
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Balance Sheets
(in thousands)
|
December 31,
|
||||||||
|
2008
|
2009
|
|||||||
|
Assets
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 5 | $ | 631 | ||||
|
Accounts receivable from related parties
|
5 | 11 | ||||||
|
Prepaid assets
|
88 | 135 | ||||||
|
Security deposit
|
- | 4 | ||||||
|
Total current assets
|
98 | 781 | ||||||
|
Property and equipment, net
|
46 | - | ||||||
|
Other assets
|
3 | - | ||||||
|
Total assets
|
147 | 781 | ||||||
|
Liabilities and Stockholders’ Equity (Deficit)
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
7,436 | 2,619 | ||||||
|
Accrued expenses
|
1,930 | 1,345 | ||||||
|
Current portion of capital lease obligation
|
4 | 4 | ||||||
|
Notes payable
|
4,728 | 2,426 | ||||||
|
Total current liabilities
|
14,098 | 6,394 | ||||||
|
Accrued compensation
|
351 | - | ||||||
|
Deferred purchase credit
|
500 | 500 | ||||||
|
Deferred revenue
|
1,727 | 1,727 | ||||||
|
Long-term notes payable
|
15 | 1,750 | ||||||
|
Total liabilities
|
16,691 | 10,371 | ||||||
|
Commitments and contingencies
|
― | ― | ||||||
|
Stockholders’ equity (deficit):
|
||||||||
|
Common stock, $0.001 par value; 100,000 authorized; 55,142 and 62,811 issued and outstanding at December 31, 2008 and 2009, respectively
|
55 | 62 | ||||||
|
Additional paid-in capital
|
75,938 | 77,898 | ||||||
|
Deficit accumulated during the development stage
|
(92,537 | ) | (87,550 | ) | ||||
|
Total stockholders’ equity (deficit)
|
(16,544 | ) | (9,590 | ) | ||||
|
Total liabilities and stockholders’ equity (deficit)
|
$ | 147 | $ | 781 | ||||
The accompanying notes are an integral part of these financial statements.
F-18
Curaxis Pharmaceutical Corporation
(formerly known as Voyager Pharmaceutical Corporation)
(A Development Stage Corporation)
Statements of Operations
(in thousands, except per share amounts)
|
For the Years Ended
December 31,
|
Cumulative
from
Inception
(February 27, 2001)
to
December 31,
|
|||||||||||||||
|
2007
|
2008
|
2009
|
2009
|
|||||||||||||
|
(unaudited)
|
||||||||||||||||
|
Operating expenses
|
||||||||||||||||
|
Research and development
|
$ | 3,057 | $ | 75 | $ | 132 | $ | 61,355 | ||||||||
|
General and administrative
|
2,472 | 1,433 | 1,077 | 24,818 | ||||||||||||
|
Marketing
|
357 | - | - | 5,557 | ||||||||||||
|
Loss on investment in real estate
|
- | - | - | 1,091 | ||||||||||||
|
Loss on lease termination
|
374 | - | - | 374 | ||||||||||||
|
Total operating (income) expenses
|
6,260 | 1,508 | 1,209 | 93,195 | ||||||||||||
|
Operating (loss) income
|
(6,260 | ) | (1,508 | ) | (1,209 | ) | (93,195 | ) | ||||||||
|
Gain on debt restructuring
|
- | - | 6,562 | 6,562 | ||||||||||||
|
Interest income (expense), net
|
(484 | ) | (478 | ) | (366 | ) | (917 | ) | ||||||||
|
Net income (loss)
|
$ | (6,744 | ) | $ | (1,986 | ) | $ | 4,987 | $ | (87,550 | ) | |||||
|
Basic net income (loss) per share
|
$ | (0.14 | ) | $ | (0.04 | ) | $ | 0.09 | ||||||||
|
Diluted net income (loss) per share
|
$ | (0.14 | ) | $ | (0.04 | ) | $ | 0.08 | ||||||||
|
Weighted average common shares outstanding – basic
|
47,934 | 54,070 | 57,699 | |||||||||||||
|
Weighted average common shares outstanding – diluted
|
47,934 | 54,070 | 60,701 | |||||||||||||
The accompanying notes are an integral part of these financial statements.
F-19
Curaxis Pharmaceutical Corporation
(formerly known as Voyager Pharmaceutical Corporation)
(A Development Stage Corporation)
Statements of Changes in Stockholders’ Equity (Deficit)
(in thousands)
|
Common Stock
|
Additional Paid-in-Capital
|
Deficit
Accumulated
during the
Development
Stage
|
Total
|
|||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||
|
Balances at Inception (February 27, 2001)
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
|||||||||||
|
Sale of common stock (unaudited)
|
25,657
|
26
|
1,073
|
―
|
1,099
|
|||||||||||||||
|
Stock-based compensation (unaudited)
|
―
|
―
|
100
|
―
|
100
|
|||||||||||||||
|
Net loss (unaudited)
|
―
|
―
|
―
|
(1,219
|
)
|
(1,219
|
)
|
|||||||||||||
|
Balances at December 31, 2001 (unaudited)
|
25,657
|
26
|
1,173
|
(1,219
|
)
|
(20
|
)
|
|||||||||||||
|
Sale of common stock (unaudited)
|
4,054
|
4
|
4,105
|
―
|
4,109
|
|||||||||||||||
|
Issuance of common stock in exchange for consulting services (unaudited)
|
12
|
―
|
10
|
―
|
10
|
|||||||||||||||
|
Stock-based compensation (unaudited)
|
―
|
―
|
272
|
―
|
272
|
|||||||||||||||
|
Net loss (unaudited)
|
―
|
―
|
―
|
(3,190
|
)
|
(3,190
|
)
|
|||||||||||||
|
Balances at December 31, 2002 (unaudited)
|
29,723
|
30
|
5,560
|
(4,409
|
)
|
1,181
|
||||||||||||||
|
Sale of common stock (unaudited)
|
4,794
|
5
|
8,239
|
―
|
8,244
|
|||||||||||||||
|
Issuance of common stock in exchange for consulting services (unaudited)
|
36
|
―
|
55
|
―
|
55
|
|||||||||||||||
|
Repurchase of common stock (unaudited)
|
(330
|
)
|
―
|
(379
|
)
|
―
|
(379
|
)
|
||||||||||||
|
Stock-based compensation (unaudited)
|
―
|
―
|
78
|
―
|
78
|
|||||||||||||||
|
Net loss (unaudited)
|
―
|
―
|
―
|
(6,633
|
)
|
(6,633
|
)
|
|||||||||||||
|
Balances at December 31, 2003 (unaudited)
|
34,223
|
35
|
13,553
|
(11,042
|
)
|
2,546
|
||||||||||||||
|
Sale of common stock (unaudited)
|
4,388
|
4
|
12,491
|
―
|
12,495
|
|||||||||||||||
|
Issuance of common stock in exchange for consulting services (unaudited)
|
20
|
―
|
66
|
―
|
66
|
|||||||||||||||
|
Net loss (unaudited)
|
―
|
―
|
―
|
(11,626
|
)
|
(11,626
|
)
|
|||||||||||||
|
Balances at December 31, 2004 (unaudited)
|
38,631
|
39
|
26,110
|
(22,668
|
)
|
3,481
|
||||||||||||||
|
Sale of common stock (unaudited)
|
3,305
|
3
|
28,839
|
―
|
28,842
|
|||||||||||||||
|
Issuance of common stock in exchange for consulting services (unaudited)
|
4
|
―
|
33
|
―
|
33
|
|||||||||||||||
|
Stock-based compensation (unaudited)
|
―
|
―
|
10
|
―
|
10
|
|||||||||||||||
|
Repurchase of common stock (unaudited)
|
(1
|
)
|
―
|
(9
|
)
|
―
|
(9
|
)
|
||||||||||||
|
Net loss (unaudited)
|
―
|
―
|
―
|
(32,904
|
)
|
(32,904
|
)
|
|||||||||||||
|
Balances at December 31, 2005 (unaudited)
|
41,939
|
42
|
54,983
|
(55,572
|
)
|
(547
|
)
|
|||||||||||||
|
Sale of common stock
|
1,513
|
1
|
15,127
|
―
|
15,128
|
|||||||||||||||
|
Sale of common stock under warrants
|
2,886
|
3
|
286
|
―
|
289
|
|||||||||||||||
|
Stock-based compensation
|
―
|
―
|
1,155
|
―
|
1,155
|
|||||||||||||||
|
Repurchase of common stock
|
(150
|
)
|
―
|
―
|
―
|
―
|
||||||||||||||
|
Net loss
|
―
|
―
|
―
|
(28,235
|
)
|
(28,235
|
)
|
|||||||||||||
|
Balances at December 31, 2006
|
46,188
|
$
|
46
|
$
|
71,551
|
$
|
(83,807
|
)
|
$
|
(12,210
|
)
|
|||||||||
The accompanying notes are an integral part of these financial statements.
F-20
Curaxis Pharmaceutical Corporation
(formerly known as Voyager Pharmaceutical Corporation)
(A Development Stage Corporation)
Statements of Changes in Stockholders’ Equity (Deficit), continued
(in thousands)
|
Additional
Paid-in-Capital
|
Deficit
Accumulated
during the
Development
Stage
|
Total
|
||||||||||||||||||
| Common Stock | ||||||||||||||||||||
|
Shares
|
Amount
|
|||||||||||||||||||
|
Sale of common stock
|
1,822 | $ | 2 | $ | 2,336 | $ | ― | $ | 2,338 | |||||||||||
|
Sale of common stock under warrants
|
1,971 | 2 | 171 | ― | 173 | |||||||||||||||
|
Stock-based compensation
|
― | ― | 889 | ― | 889 | |||||||||||||||
|
Net loss
|
― | ― | ― | (6,744 | ) | (6,744 | ) | |||||||||||||
|
Balances at December 31, 2007
|
49,981 | 50 | 74,947 | (90,551 | ) | (15,554 | ) | |||||||||||||
|
Sale of common stock
|
5,120 | 5 | 518 | ― | 523 | |||||||||||||||
|
Sale of common stock under warrants
|
41 | ― | 4 | ― | 4 | |||||||||||||||
|
Stock-based compensation
|
― | ― | 469 | ― | 469 | |||||||||||||||
|
Net loss
|
― | ― | ― | (1,986 | ) | (1,986 | ) | |||||||||||||
|
Balances at December 31, 2008
|
55,142 | 55 | 75,938 | (92,537 | ) | (16,544 | ) | |||||||||||||
|
Sale of common stock, net
|
6,071 | 6 | 1,096 | ― | 1,102 | |||||||||||||||
|
Sale of common stock under warrants
|
1,448 | 1 | 143 | ― | 144 | |||||||||||||||
|
Issuance of warrants
|
― | ― | 330 | ― | 330 | |||||||||||||||
|
Stock grant
|
150 | ― | 30 | ― | 30 | |||||||||||||||
|
Stock-based compensation
|
― | ― | 361 | ― | 361 | |||||||||||||||
|
Net income
|
― | ― | ― | 4,987 | 4,987 | |||||||||||||||
|
Balances at December 31, 2009
|
62,811 | $ | 62 | $ | 77,898 | $ | (87,550 | ) | $ | (9,950 | ) | |||||||||
The accompanying notes are an integral part of these financial statements.
F-21
Curaxis Pharmaceutical Corporation
(formerly known as Voyager Pharmaceutical Corporation)
(A Development Stage Corporation)
Statements of Cash Flows
(in thousands)
|
For the Years Ended December 31,
|
Cumulative
from Inception
(February 27, 2001)
to
December 31,
|
|||||||||||||||
|
2007
|
2008
|
2009
|
2009
|
|||||||||||||
|
(unaudited)
|
||||||||||||||||
|
Cash flows from operating activities
|
||||||||||||||||
|
Net income (loss)
|
$ | (6,744 | ) | $ | (1,986 | ) | $ | 4,987 | $ | (87,550 | ) | |||||
|
Adjustments to reconcile net income (loss) to net cash used in operating activities:
|
||||||||||||||||
|
Depreciation
|
228 | 81 | 45 | 994 | ||||||||||||
|
Stock-based compensation expense
|
889 | 469 | 637 | 3,610 | ||||||||||||
|
Common stock issued in exchange for consulting services
|
― | ― | ― | 164 | ||||||||||||
|
Loss (gain) on disposal of property and equipment
|
175 | ― | (9 | ) | 169 | |||||||||||
|
Loss on investment in real estate
|
― | ― | ― | 1,091 | ||||||||||||
|
Gain on restructuring of trade debt
|
― | ― | (6,562 | ) | (6,562 | ) | ||||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||||||
|
Prepaid expenses
|
155 | 30 | 36 | (52 | ) | |||||||||||
|
Accounts receivable
|
25 | (5 | ) | (6 | ) | (11 | ) | |||||||||
|
Security deposits
|
― | ― | (4 | ) | (4 | ) | ||||||||||
|
Other assets
|
2 | 16 | 3 | ― | ||||||||||||
|
Accounts payable
|
(144 | ) | (206 | ) | 90 | 7,526 | ||||||||||
|
Accrued expenses
|
89 | 269 | 512 | 2,442 | ||||||||||||
|
Deferred purchase credit
|
― | 500 | ― | 500 | ||||||||||||
|
Deferred revenue
|
1,727 | ― | ― | 1,727 | ||||||||||||
|
Accrued compensation
|
(109 | ) | 73 | (351 | ) |
―
|
||||||||||
|
Net cash used in operating activities
|
(3,707 | ) | (759 | ) | (622 | ) | (75,956 | ) | ||||||||
|
Cash flows from investing activities
|
||||||||||||||||
|
Purchase of property and equipment
|
― | ― | ― | (1,193 | ) | |||||||||||
|
Purchase of real estate investment
|
― | ― | ― | (3,155 | ) | |||||||||||
|
Proceeds from the sale of property and equipment
|
40 | ― | 10 | 50 | ||||||||||||
|
Proceeds from the sale of real estate
|
― | ― | ― | 2,064 | ||||||||||||
|
Decrease in restricted cash
|
― | ― | ― | ― | ||||||||||||
|
Net cash provided by (used in) investing activities
|
$ | 40 | $ | ― | $ | 10 | $ | (2,234 | ) | |||||||
The accompanying notes are an integral part of these financial statements.
F-22
Curaxis Pharmaceutical Corporation
(formerly known as Voyager Pharmaceutical Corporation)
(A Development Stage Corporation)
Statements of Cash Flows, continued
(in thousands)
|
For the Years Ended December 31,
|
Cumulative
from
Inception
(February 27, 2001)
to
December 31,
|
|||||||||||||||
|
2007
|
2008
|
2009
|
2009
|
|||||||||||||
|
(unaudited)
|
||||||||||||||||
|
Cash flows from financing activities
|
||||||||||||||||
|
Issuance of common stock
|
$ | 2,511 | $ | 527 | $ | 1,246 | $ | 74,490 | ||||||||
|
Issuance of notes payable
|
189 | 243 | ― | 5,011 | ||||||||||||
|
Issuance of long-term debt
|
574 | 18 | ― | 592 | ||||||||||||
|
Payments on notes payable
|
(826 | ) | (31 | ) | (8 | ) | (865 | ) | ||||||||
|
Payments on capital lease obligation
|
(4 | ) | (5 | ) | ― | (16 | ) | |||||||||
|
Repurchase of common stock
|
― | ― | ― | (388 | ) | |||||||||||
|
Repayments of notes payable to related parties
|
― | (37 | ) | ― | (202 | ) | ||||||||||
|
Payments on long-term debt
|
― | (3 | ) | (3 | ) | |||||||||||
|
Proceeds from notes payable to related parties
|
― | 37 | ― | 202 | ||||||||||||
|
Repayment of mortgage note payable
|
― | ― | ― | (3,100 | ) | |||||||||||
|
Proceeds from the issuance of mortgage note
|
― | ― | ― | 3,100 | ||||||||||||
|
Net cash provided by financing activities
|
2,444 | 749 | 1,238 | 78,821 | ||||||||||||
|
Net increase (decrease) in cash and cash equivalents
|
(1,223 | ) | (10 | ) | 626 | 631 | ||||||||||
|
Cash and cash equivalents, beginning of period
|
1,238 | 15 | 5 | ― | ||||||||||||
|
Cash and cash equivalents, end of period
|
$ | 15 | $ | 5 | $ | 631 | $ | 631 | ||||||||
|
Supplemental disclosure of cash flow information:
|
||||||||||||||||
|
Cash paid for interest
|
$ | 2 | $ | 2 | $ | ― | $ | 242 | ||||||||
|
Non-cash financing activities:
|
||||||||||||||||
|
Conversion of trade payables to notes
|
$ | 763 | $ | 261 | $ | 238 | $ | 5,841 | ||||||||
|
Restructuring of trade debt
|
$ | ― | $ | ― | $ | 6,562 | $ | 6,562 | ||||||||
|
Issuance of warrant for accrued liability
|
$ | ― | $ | ― | $ | ― | $ | ― | ||||||||
The accompanying notes are an integral part of these financial statements.
F-23
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(1) Organization and Summary of Significant Accounting Policies
Curaxis Pharmaceutical Corporation was incorporated in the State of Delaware on February 27, 2001 under the name Angel Care America, Inc. to develop clinical applications of its Alzheimer’s treatment. On March 20, 2001, a separate corporation called Voyager Pharmaceutical Corporation (“Old Voyager”) was incorporated in Delaware with the intent of focusing exclusively on research, clinical trials, and FDA approval. On November 28, 2001, Old Voyager was merged into Angel Care America, Inc., and the company’s name was changed to Voyager Pharmaceutical Corporation. On December 2, 2009, the name of Voyager Pharmaceutical Corporation was changed to Curaxis Pharmaceutical Corporation (the “Company”). Prior to the merger, the Company and Old Voyager were entities under common control and, accordingly, the accompanying financial statements include the accounts of Old Voyager from March 20, 2001 (the date of Old Voyager’s inception) through November 28, 2001 (the date of its merger with Angel Care America, Inc.). The Company is dedicated to finding cures for major age-related diseases, including Alzheimer’s disease, by bringing treatment to market through FDA approval.
The Company is a development stage company and has focused its efforts to date on raising capital and research and development. The accompanying financial statements have been prepared on a basis which assumes that the Company will continue as a going concern and which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has a limited operating history and has incurred losses from operations since its inception. The cash position of the Company has deteriorated significantly over the last three years and the Company has been unable to satisfy its outstanding liabilities with many vendors. In late 2006, the company terminated its’ Phase III clinical trial VP-AD-301 and in 2007 closed its’ research facility.
In 2009, the Company entered into a series of agreements with Southridge Business Solutions LLC (“Southridge”) and Canterbury Investment Partners LLC (“Canterbury”) in order to restructure its’ balance sheet and establish a trading market for its common stock. Under the terms of those agreements, Southridge and Canterbury will assist the Company in negotiating settlements with its creditors to eliminate or substantially reduce and defer its trade debt and assist the Company in merging with a publicly traded corporation to establish a public trading market for its stock. Further, upon consummation of a merger transaction, Southridge will assist the Company in securing an equity facility under which the Company can periodically, over a period of two years, sell up to $20 million of its common stock to Southridge. The proceeds of the equity facility will be used to fund further development of the Company’s lead drug candidate through to commercialization.
Although management continues to work with Southridge and Canterbury to pursue these plans, there is no assurance that the Company will be successful in completing the proposed merger transaction and obtaining the necessary funding or financing on terms acceptable to the Company. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
In May 2003, the Company affected a 3-for-1 stock split of its common stock in the form of a stock dividend.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
The accompanying notes are an integral part of these financial statements.
F-24
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(1) Organization and Summary of Significant Accounting Policies (continued)
Fair Value of Financial Instruments
The Company’s financial instruments consist mainly of cash, cash equivalents, accounts payable, accrued expenses, capital lease obligations, notes payable and deferred credits. The carrying values of the short-term financial instruments approximate their fair value due to the short-term nature of these instruments. The fair values of the long-term lease and notes payable obligations reflect that which would have been paid if the obligations were settled at year-end.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents are stated at cost and consist of bank deposits and a money market fund that invests in short-term debt securities. The carrying amount of cash and cash equivalents approximates fair value.
Property and Equipment
Property and equipment is stated at cost. Equipment acquired under capital leases is initially recorded at the present value of the minimum lease payments at inception of the lease.
Depreciation is computed using the straight-line method over the estimated useful lives of the assets which range from three to five years. Leasehold improvements and equipment held under capital leases are amortized over the related lease terms, which is shorter than the estimated useful lives of the assets.
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment when events or changes in circumstances indicate the book value of the assets may not be recoverable. In accordance with Statement of Financial Accounting Standards No. 144, (“SFAS No. 144”) codified in ASC 360-10-35-15 Impairment or Disposal of Long-Lived Assets, recoverability is measured by comparing the book value of the asset to the future net undiscounted cash flows expected to be generated by the asset.
No events or changes in circumstances have been identified which would impact the recoverability of the Company’s long-lived assets reported at December 31, 2007, 2008, or 2009.
Stock-Based Compensation
The Company applies Statement of Financial Accounting Standards No. 123R Share-based Payment, codified in ASC 718 Compensation – Stock Compensation, to stock-based compensation awards. SFAS No. 123R requires the measurement and recognition of non-cash compensation expense for all share-based payment awards made to employees and directors, including employee stock options related to our 2001 Stock Option Plan and 2005 Stock Option Plan, based on fair values. The Company adopted this Statement on January 1, 2006 using the modified prospective application.
The accompanying notes are an integral part of these financial statements.
F-25
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(1) Organization and Summary of Significant Accounting Policies (continued)
Stock compensation arrangements with non-employee service providers are accounted for in accordance with EITF No. 96-18, Accounting for Equity Instruments that are issued to Other than Employees for Acquiring, or in Conjunction with Selling, Goods or Services, codified in ASC 505-50 Equity-Based Payments to Non-Employees, using a fair value approach. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
Stock Purchase Warrants
The Company has issued warrants to purchase shares of its common stock. Warrants have been accounted for as equity in accordance with the provisions of EITF Issue No. 00-19: Accounting for Derivative Financial Instruments Indexed to, and potentially Settled in, a Company’s Own Stock, codified in ASC 480, Distinguishing Liabilities from Equity.
Research and Development
Research and development costs include all direct costs related to the development of the Company’s products, including clinical trial costs, salaries and related benefits of personnel, costs of producing drug material for clinical trials and fees paid to consultants. Research and development costs are expensed as incurred.
Segment Reporting
Statement of Financial Accounting Standards No. 131, Disclosure about Segments of an Enterprise and Related Information, codified in ASC 280, Segment Reporting, establishes standards for reporting information about the Company’s operating segments. The Company operates in one business segment, the business of discovery, development and commercialization of pharmaceutical treatments for major age-related diseases.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities, and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company has established a valuation allowance against its deferred tax assets due to the uncertainty surrounding the realization of such assets.
On January 1, 2007, the Company adopted FASB Interpretation No. 48 (FIN 48), “Accounting for Uncertainty Income Taxes, codified in ASC 740-10-25, Income Taxes- Overall-Recognition. The Company did not have any unrecognized tax benefits and there was no effect on our financial condition or results of operations as a result of adopting FIN 48. The Company’s practice is to recognize interest and /or penalties related to income tax matters in income tax expense as incurred.
The accompanying notes are an integral part of these financial statements.
F-26
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(1) Organization and Summary of Significant Accounting Policies (continued)
Comprehensive Loss
The Company has adopted the provisions of Statement of Financial Accounting Standards No. 130, Reporting Comprehensive Income (“SFAS No. 130”), codified in ASC 220, Comprehensive Income which establishes rules for the reporting and display of comprehensive loss and its components. For all periods presented, there were no differences between net loss and comprehensive loss.
Certain Risks and Uncertainties
The Company’s product candidates under development require approval from the United States Food and Drug Administration (“FDA”) or other international regulatory agencies prior to commercial sales. There can be no assurance our products will receive the necessary clearance. If the Company is denied clearance or clearance is delayed, it may have a material adverse impact on the Company.
The Company’s product is concentrated in rapidly changing, highly competitive markets, which are characterized by rapid technological advances, changes in customer requirements and evolving regulatory requirements and industry standards. Any failure by the Company to anticipate or to respond adequately to technological developments in our industry, changes in customer requirements or changes in regulatory requirements or industry standards, or any significant delays in the development or introduction of products or services, could have a material adverse effect on the Company’s business, operating results and future cash flows. The Company relies on one corporation as a sole source provider of Memryte for use in its clinical trials.
Recently Issued Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying financial statements
(2) Property and Equipment, Net
Property and equipment consists of the following at (in thousands):
|
December 31,
|
||||||||
|
2008
|
2009
|
|||||||
|
Furniture and office equipment
|
$ | 189 | $ | 106 | ||||
|
Laboratory and manufacturing equipment
|
243 | 241 | ||||||
|
Vehicles
|
23 | - | ||||||
| 455 | 347 | |||||||
|
Accumulated depreciation and amortization
|
(409 | ) | (347 | ) | ||||
| $ | 46 | $ | - | |||||
Depreciation expense totaled $228 thousand, $81 thousand, and $45 thousand for the years ended December 31, 2007, 2008 and 2009, respectively.
The accompanying notes are an integral part of these financial statements.
F-27
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(3) Accrued Expenses
Accrued expenses consist of the following (in thousands):
|
December 31,
|
||||||||
|
2008
|
2009
|
|||||||
|
Development and clinical trial expenses
|
$ | 806 | $ | 773 | ||||
|
Interest
|
1,124 | 424 | ||||||
|
Professional fees
|
- | 35 | ||||||
|
Costs associated with equity raise
|
- | 113 | ||||||
| $ | 1,930 | $ | 1,345 | |||||
(4) Notes payable
Notes payable consist of the following (in thousands):
|
December 31,
|
||||||||
|
2008
|
2009
|
|||||||
|
Notes payable to clinical research sites
|
$ | 415 | $ | 407 | ||||
|
Promissory Note dated September 15, 2006
|
2,804 | 2,000 | ||||||
|
Promissory Installment Note dated September 26, 2006
|
924 | 924 | ||||||
|
Promissory Noted dated July 1, 2007
|
300 | 300 | ||||||
|
Promissory Note dated August 21, 2007
|
274 | 420 | ||||||
|
Promissory Note and Security Agreement dated September 22, 2008
|
26 | 26 | ||||||
|
Default judgment dated October 29, 2009
|
- | 99 | ||||||
| 4,743 | 4,176 | |||||||
|
Less: Current portion of notes payable
|
4,728 | 2,426 | ||||||
| $ | 15 | $ | 1,750 | |||||
Weighted average interest rates on current notes payable at December 31, 2008 and 2009 were 10.8% and 9.9%, respectively.
Notes payable to clinical sites represent unsecured promissory notes executed during 2007 and 2008 to repay clinical sites for services performed in conjunction with the Company’s terminated Phase III clinical trial, VP-AD-301. The notes were issued for six-month to one year terms and bear interest at an annual rate of 12% to 18%. At December 31, 2009, all notes payable to clinical sites had matured and were in default for lack of payment.
Promissory note dated September 16, 2006 in the amount of $4 million was executed in conjunction with a Repayment agreement between the Company and a significant vendor. The note was issued to satisfy, in part, liabilities generated for service performed in conjunction with our terminated Phase II clinical trial. The unsecured note bears interest at 12.0% and required installment payments commencing October 1, 2006. At the request of the Company, the Repayment Agreement has been amended to cure all events of default for non-payment. However, payment of principal in full and accrued interest to date was due on May 31, 2008. On September 23, 2009, the Company reached an agreement with this vendor to satisfy amounts owed related to notes payable and trade debts outstanding. The agreement requires the Company to make payments totaling $2 million commencing September 1, 2010 and ending March 31, 2012. The Company has the right to prepay any balances due without penalty. In addition, the company agreed to prepay the entire balance of such payments in the event of an acquisition of all or substantially all of the Company’s assets or stock; in the event the company’s lead product candidate is licensed to a third party; in the event any patent related to the Company’s lead product candidate is sold to any third party; or in the event the Company obtains financing in excess of $20 million. Any payment not made in accordance with the agreement shall bear interest at 18% per annum. Immediately preceding the settlement balances recorded on the Company’s financial records with respect to this vendor account included accounts payable, accrued interest and notes payable of $4.7 million, $1.1 million and $2.8 million, respectively. The company evaluated the settlement under Financial Accounting Standards No. 15, Accounting by Debtors and Creditors for Trouble Debt Restructuring, codified in ASC 470-60, Troubled Debt Restructuring by Debtors. As a result, a gain totaling $6.5 million was realized on the settlement. The basic and diluted EPS on the aggregate gain for the year ended December 31, 2009 were $0.11 and $0.11, respectively.
The accompanying notes are an integral part of these financial statements.
F-28
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(4) Notes payable (continued)
Promissory Installment Note dated September 26, 2006 in the amount of $1.1 million was executed in conjunction with Letter of Agreement between the Company and a significant vendor to satisfy liabilities generated for the receiving, distribution and return processing of clinical trial material. The note is secured against inventory held by the vendor and required monthly payments commencing September 26, 2006 of principal plus a 1% fee on the outstanding balance. The Company was in default for non payment as of December 31, 2006. On April 3, 2008, a Final and Amended Judgment was awarded on behalf of the creditor against the Company in the amount $924 thousand principal and $135 thousand in accrued interest and late fees, for a total of $1.059 million. On January 22, 2010 the Company and Creditor reached a settlement agreement where by the Company will make payments totaling $900,000 over the next two years to satisfy, in full, its obligation. Further, the agreement restricts the Company from transferring or pledging its intellectual property without prior consent of the Creditor. As a result of the settlement, the Company recognized a gain of $159 thousand.
Promissory Note dated July 1, 2007 in the amount of $300 thousand was executed in conjunction with a Termination of Sublease Agreement between the Company and its’ landlord as consideration for early termination of the sublease agreement. The original sublease agreement for corporate office space was executed on April 20, 2005 and was to expire on March 31, 2011. The promissory note bears interest at 10%. Payment of principal and all accrued interest was due June 30, 2009. The Promissory note was in default for non-payment as of July 1, 2009.
Promissory note dated August 21, 2007 in the amount of $274 thousand was executed in conjunction with a Structured Settlement Agreement between the Company and a significant vendor to satisfy, in part, payments due to the vendor for the purchase of clinical trial material. The unsecured note bears interest at an annual rate of 8% and required payment in full not later than July 15, 2012. In addition, the Company was required to make graduated monthly payments commencing June 25, 2007 to satisfy amounts outstanding at the date of the agreement not reflected in the Promissory Note. At December 31, 2008, the Company was in default of the Structured Settlement Agreement for non-payment of the monthly amounts set forth by the agreement. On April 6, 2009, an Order of Default Judgment was awarded on behalf of the creditor against the Company in the amount $420 thousand principal and $5 thousand in accrued interest and fees, for a total of $425 thousand. The principal balance of $420 reflects the balance outstanding on the original Promissory note of $274 thousand and amounts recorded as trade payables immediately preceding the issuance of the Default Judgment of $146 thousand. The principal balance due on the Default Judgment has been reclassified as a current note payable at December 31, 2009.
The accompanying notes are an integral part of these financial statements.
F-29
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(4) Notes payable (continued)
Promissory Note and Security Agreement dated September 22, 2008 in the amount of $27 thousand was executed pursuant to the terms of the Fifth Amendment to Lease between the Company and it’s landlord as consideration for the Company to relocate and reduce the size of its’ leased office space. The Promissory note bears interest at an annual rate of 9% and requires equal monthly installment payments to be made commencing November 1, 2008 though April 11, 2011. The Promissory note balance of $26 thousand at December 31, 2009 is in default for non-payment. As a result, the note has been reclassified as a current liability at December 31, 2009.
A default judgment was issued on October 29, 2009 on behalf of a creditor seeking payment of amounts due for services rendered with respect to the Company’s terminated Phase III clinical trial, VP-AD-301. The judgment requires payment of the principal amount of $99 thousand plus interest accrued at an annual rate of 10%.
(5) Common Stock and Stock Purchase Warrants
At December 31, 2008 and 2009, the Company was authorized to issue 100 million shares, respectively, of $0.001 par value common stock. The holders of common stock are entitled to receive dividends whenever funds are legally available and when declared by the Board of Directors. Each share of common stock is entitled to one vote.
In May 2003, the Company declared a 3 for 1 stock split of its common stock in the form of a stock dividend. All common share and per share information in the financial statements and related notes have been retroactively restated to reflect the stock split.
Upon inception, the Company issued 5,728 million shares of common stock to its founders. Issuances of common stock through private placements are as follows (in thousands except for per share amounts):
|
Shares
|
Proceeds
|
Price per share
|
||||||||||
|
(unaudited)
|
(unaudited)
|
(unaudited)
|
||||||||||
|
March through August 2001
|
1,268 | $ | 846 | $ | 0.66 | |||||||
|
November 2001 (unaudited
|
4 | 3 | 0.83 | |||||||||
|
January through July 2002
|
2,591 | 2,158 | 0.83 | |||||||||
|
August through December 2002
|
1,463 | 1,951 | 1.33 | |||||||||
|
January through May 2003
|
1,719 | 2,292 | 1.33 | |||||||||
|
June through October 2003
|
2,581 | 5,162 | 2.00 | |||||||||
|
December 2003
|
164 | 410 | 2.50 | |||||||||
|
January through April 2004
|
3,371 | 8,429 | 2.50 | |||||||||
|
February through July 2004
|
1,017 | 4,066 | 4.00 | |||||||||
|
January through March 2005
|
2,102 | 16,814 | 8.00 | |||||||||
|
June through July 2005
|
1,203 | 12,028 | 10.00 | |||||||||
In November 2001, the Company issued 18,657,000 shares of common stock in exchange for 3,007,000 shares of Old Voyager common stock which were originally issued in March 2001 for $250 thousand.
In May 2003, the Company repurchased and reissued 120,000 shares of common stock for $100 thousand, or $0.83 per share. During the same period, the Company repurchased and reissued 210 thousand shares of common stock for $280 thousand, or $1.33 per share.
From January 2006 though May 2007, the Company raised $16.6 million through the sale of 1,661,000 units at $10 per unit. A unit consisted of one share of common stock and warrants to purchase two additional shares of common stock at $12 per share. The Company amended this offering on August 21, 2006. Under the amendment each unit consists of one share of common stock and warrants to purchase six additional shares of common stock at $0.10 per share. The warrants to purchase common stock expire in 2011.
The accompanying notes are an integral part of these financial statements.
F-30
(5) Common Stock and Stock Purchase Warrants (continued)
From June 2007 to December 2008, the Company raised $1.4 million through the sale of 6,792,000 shares of common stock at $1.00 per share. This offering was amended on August 22, 2007 and March 6, 2008 to reduce the pricing to $.50 and $.20 per share, respectively and 3,669,000 additional shares were issued on a retroactive basis. The accounting for the issuance of these additional shares followed the character of the initial offering and no expense was recorded.
During 2008, the Company issued stock purchase warrants to select vendors to satisfy, in part, liabilities generated from services performed in conjunction with our terminated Phase III clinical trial, VP-AD-301. The warrants provide for the purchase 54,800 shares of common stock at $.50 per share and expire on December 31, 2011.
From January through December 2009, the Company raised $1.2 million through the sale of 6.0 million shares of common stock at $0.20 per share and the issuance of 1.4 million shares of common stock upon exercise of outstanding warrants at $0.10 per share. These proceeds are net of expenses associated with warrants to be issued to outside consultants for services rendered in connection with the financing round totaling $113 thousand.
On May 28, 2009, the company entered into a Transaction Management Agreement with Southridge Business Solutions Group, LLC (“Southridge”). Under the terms of the agreement, the company retained Southridge, on a non-exclusive basis, to provide services to enable the Company to restructure its balance sheet and execute a potential public offering or other public transaction. As part of the consideration for the services to be provided, the Company issued a total of 1,656,549 warrants to Southridge to purchase the Company’s stock at $.001 per shares. The warrants provide for a cashless exercise and expire seven years after the date of issuance. Per the agreement, additional warrants with be issued upon closing of a public transaction in so that the total warrants issued to Southridge equal 3% of the outstanding stock of the surviving corporation. For the year ended December 31, 2009 expense recognized by the Company on the issuance of the beneficial warrants totaled $246 thousand.
On December 2, 2009, the Board of Directors granted 150,000 shares of common stock, valued at $30 thousand, to a former executive officer to satisfy, in full, amounts due under an original five year employment contract.
At December 31, 2009 outstanding warrants to purchase the company’s common stock are as follows:
|
Number of
shares
|
Exercise
Price
|
Expiration
Date
|
|||||
| 3,615,700 | $ | 0.10 |
December 31, 2011
|
||||
| 54,800 | $ | 0.50 |
December 31, 2011
|
||||
| 1,656,500 | $ | 0.001 |
August 31, 2016
|
||||
| - | $ | 0.22 |
August 31, 2016
|
||||
| 5,327,000 | |||||||
The accompanying notes are an integral part of these financial statements.
F-31
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(6) Stock Option Plan
During 2001, the Company adopted the 2001 Stock Option Plan (the “2001 Plan”) under which 1,500,000 shares of the Company’s common stock was reserved for issuance to employees, directors and consultants. Options granted under the Plan may be incentive stock options or non-qualified stock options. On January 31, 2005, the Company adopted the 2005 Stock Option Plan (the “2005 Plan”) with terms and conditions similar to the 2001 Plan under which 3,500,000 shares of the Company’s common stock are reserved for issuance to employees, directors and consultants. The Board of Directors determines the exercise price, term and the period over which options become exercisable at each grant date. The Board of Directors determines what it deems to be the fair market value of the stock based on the price per share of the most recent private placement of common stock and other relevant information. Since inception, all options were granted to employees with an exercise price equal to the deemed fair value of the common stock on the date of grant. Under the 2001 Plan and the 2005 Plan, options vest over various periods, generally annually over five years, and expire not more than ten years after the date of grant.
The Company has granted 684,000 stock options outside of the approved 2001 Plan and 2005 Plan to several consultants that vested upon completion of certain services. In addition, the Company has granted 53,000 options under the 2005 Plan to other non-employees. Upon adoption of ASC 718, the Company continues to account for the resulting non-employee stock-based compensation for all options granted to non-employees based on the estimated fair value of the options, determined using the Black-Scholes option valuation model, in accordance with ASC 505-50, and amortizes such expense on a straight –line basis. The measurement date for the calculation of compensation expense is considered to be the date when all services have been rendered or the date that options are fully vested.
During the years ended December 31, 2007, 2008 and 2009, the Company recognized stock-based compensation expense/(benefit) related to its stock option plans in the accompanying statements of operations as follows (in thousands):
|
Year ended December 31,
|
||||||||||||
|
2007
|
2008
|
2009
|
||||||||||
|
Employee-related stock-based compensation
|
$ | 931 | $ | 469 | $ | 391 | ||||||
|
Non-employee related stock based compensation
|
(42 | ) | - | 246 | ||||||||
| $ | 889 | $ | 469 | $ | 637 | |||||||
Total stock-based compensation expense/ (benefit) recognized in the accompanying statement of operations is included in the following categories:
|
Year ended December 31,
|
||||||||||||
|
2007
|
2008
|
2009
|
||||||||||
|
Research and development
|
$ | 46 | $ | - | $ | - | ||||||
|
General and administrative
|
783 | 469 | 637 | |||||||||
|
Marketing
|
60 | - | - | |||||||||
| $ | 889 | $ | 469 | $ | 637 | |||||||
The accompanying notes are an integral part of these financial statements.
F-32
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(6) Stock Option Plan (continued)
The Company estimates the fair value of stock options at the date of grant using the Black-Scholes option valuation model. The Company calculated the fair value of employee stock options for the years ended December 31, 2007 and 2009, using the following assumptions:
|
Year ended December 31,
|
||||||||
|
2007
|
2009
|
|||||||
|
Risk-free interest rate
|
3.49 to 5.10
|
% |
2.28 to 3.06
|
% | ||||
|
Expected term (in years)
|
7.5 | 7.5 | ||||||
|
Expected volatility
|
100 | % | 100 | % | ||||
|
Expected dividends
|
0 | % | 0 | % | ||||
No stock options were granted during the year ended December 31, 2008.
The risk-free rate is estimated to equal U.S. Treasury security rates for applicable terms. Estimated volatility is based on the historical stock price volatility of comparable companies’ common stock, as our stock is not publicly traded. The Company does not have sufficient history of exercise behavior to develop estimates of expected term since as of December 31, 2009, no options issued since inception of the plans have been exercised. The expected terms is based on the estimated time period to elapse prior to commercialization of the Company’s initial product. For options to outside consultants, the Company uses a term which is equal to the average the period required for the options to fully vest and the contractual life of the option. As the Company gathers more history regarding its option exercise pattern, and as information regarding the option pattern of comparable companies in its industry becomes publically available, it will review these estimates and revise them as appropriate. Different estimates of volatility and expected term could materially change the value of an option and the resulting expense.
The following table summarizes the Company’s stock option activity under the 2001 Plan, the 2005 Plan, as well as 674,000 options granted to consultants outside of the 2001 and 2005 Plans, including number of shares (“Number”) and weighted average exercise price (“WAEP”) for the years ended December 31, 2007, 2008 and 2009:
|
Number
of
Shares
|
Weighted Average
Exercise
Price
|
Weighted-Average Contractual Term
|
Weighted Average
Grant Date
Fair Value
per Share
|
|||||||||||||
|
Outstanding at December 31, 2006
|
2,730,000 | $ | 3.37 | 6.90 | $ | 3.41 | ||||||||||
|
Options granted
|
2,496,000 | $ | 1.30 | |||||||||||||
|
Options cancelled
|
(1,015,000 | ) | $ | 3.70 | ||||||||||||
|
Outstanding at December 31, 2007
|
4,211,000 | $ | 1.87 | 6.12 | $ | 1.19 | ||||||||||
|
Options granted
|
- | $ | - | |||||||||||||
|
Options cancelled
|
(1,314,000 | ) | $ | 1.78 | ||||||||||||
|
Outstanding at December 31, 2008
|
2,897,000 | $ | 2.21 | 5.69 | $ | 1.26 | ||||||||||
|
Options granted
|
550,000 | $ | 0.20 | |||||||||||||
|
Options cancelled
|
(170,000 | ) | $ | 2.19 | ||||||||||||
|
Outstanding at December 31, 2009
|
3,277,000 | $ | 1.87 | 5.15 | $ | 1.07 | ||||||||||
|
Exercisable at December 31, 2009
|
2,947,000 | $ | 1.87 | 4.91 | $ | 1.05 | ||||||||||
The accompanying notes are an integral part of these financial statements.
F-33
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(6) Stock Option Plan (continued)
The weighted-average grant-date fair value of options granted during the years ended December 31, 2007, 2008 and 2009 were $0.82, $0, $0.17, respectively. No options were exercised during the three years ended December 31, 2009.
A summary of the status of the Company’s non-vested shares as of December 31, 2009:
|
Weighted-
|
||||||||
|
Average
|
||||||||
|
Shares
|
Grant Date
Fair Value
|
|||||||
|
Non-vested at January 1, 2009
|
625,000 | $ | 1.61 | |||||
|
Options Granted
|
550,000 | 0.17 | ||||||
|
Options vested
|
(675,000 | ) | 0.66 | |||||
|
Options cancelled
|
(170,000 | ) | 1.35 | |||||
|
Non-vested at December 31, 2009
|
330,000 | $ | 1.29 | |||||
As of December 31, 2009, there was $347 thousand of total unrecognized compensation cost related to non-vested share based compensation arrangements granted under the Plans. That cost is expected to be recognized over a weighted–average period of 1.03 years. The total fair value of shares vested during the years ended December 31, 2007, 2008, and 2009 was $1.1 million, $550 thousand, and $447 thousand, respectively.
During the years end December 31, 2007, 2008 and 2009, the Company extended the contractual life of 629 thousand, 10 thousand, and 397 thousand vested options, respectively, held by several former employees. No additional expense was recognized as a result of the modifications.
(7) Earnings Per Share
In accordance with SFAS No. 128, Earnings per Share (“SFAS No. 128”), codified in ASC 260, basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding. Diluted net loss per common share is computed similarly to basic net loss per share, except that the denominator is increased to include all potential dilutive common shares, including outstanding options and warrants. Potentially dilutive common shares have been excluded from the diluted loss per common share computation for each of the two years ended December 31, 2007 and 2008 because such securities have an anti-dilutive effect on loss per share due to the Company’s net loss.
The following table sets forth a reconciliation of the number of shares used in the basic and diluted EPS computation for the years ended December 31, 2007, 2008 and 2009:
|
|
at December 31,
|
|||||||||||
|
2007
|
2008
|
2009
|
||||||||||
|
Weighted average common shares outstanding
|
47,934 | 54,070 | 57,699 | |||||||||
|
Dilutive securities - Options and warrants
|
- | - | 3,002 | |||||||||
|
Diluted average common stock equivalents outstanding
|
47,934 | 54,070 | 60,701 | |||||||||
The accompanying notes are an integral part of these financial statements.
F-34
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(7) Earnings Per Share (continued)
The following table sets forth the number of potential shares of common stock that have been excluded from diluted earnings per share because their effect was anti-dilutive (in thousands):
|
|
at December 31,
|
|||||||||||
|
2007
|
2008
|
2009
|
||||||||||
|
Outstanding stock options
|
4,210 | 2,897 | 2,907 | |||||||||
|
Outstanding warrants
|
5,105 | 5,118 | 2,695 | |||||||||
| 9,315 | 8,015 | 5,602 | ||||||||||
(8) Income Taxes
No provision for Federal or state income taxes has been recorded as the Company has incurred net operating losses since inception.
Significant components of the Company’s deferred tax assets at December 31, 2008 and 2009 consist of the following (in thousands):
|
December 31,
|
||||||||
|
2008
|
2009
|
|||||||
|
Domestic and operating loss carryforwards
|
$ | 33,728 | $ | 31,785 | ||||
|
Research and development credits
|
2,756 | 2,756 | ||||||
|
Contributions
|
55 | 53 | ||||||
|
Fixed assets
|
19 | 23 | ||||||
|
Stock based compensation
|
971 | 1,110 | ||||||
|
Accrued compensation
|
135 | - | ||||||
|
Total deferred tax asset
|
37,664 | 35,727 | ||||||
|
Valuation allowance for deferred assets
|
(37,664 | ) | (35,727 | ) | ||||
|
Net deferred tax asset
|
$ | ― | $ | ― | ||||
At December 31, 2008 and 2009, the Company provided a full valuation allowance against its net deferred tax assets since realization of these benefits is not considered to be more likely than not. The increase in the valuation allowance of $2.8 million and $750 thousand for 2007 and 2008, respectively, resulted primarily from the additional net operating loss carryforward generated. The decrease in the valuation allowance of $1.9 million for 2009 resulted from the reduction in the net operating loss carryforward of $6.5 million. The reduction represents the gain recognized on the settlement of trade debt which was excluded from taxable income due to the insolvent status of the Company immediately prior the discharge of debt.
At December 31, 2009, the Company had Federal net operating loss carryforwards of $82.4 million that expire in varying amounts between 2021 and 2029, and state net operating loss carryforwards of $82.4 million that expire in varying amounts between 2017 and 2024. Additionally, the Company has research and development credit carryforwards of $2.8 million as of December 31, 2009 for Federal tax purposes that expire in varying amounts between 2022 and 2027.
The accompanying notes are an integral part of these financial statements.
F-35
(A Development Stage Corporation)
Notes to Financial Statements
(8) Income Taxes (continued)
The Tax Reform Act of 1986 contains provisions which limit the ability to utilize net operating loss carryforwards and tax credit carryforwards in the case of certain events including significant changes in ownership interests. If the Company’s NOLs and/or tax credits are limited, and the Company has taxable income which exceeds the permissible yearly NOL, the Company would incur a Federal income and/or state tax liability even though NOLs would be available in future years.
Taxes computed at the statutory Federal income tax rate of 34 percent are reconciled to the provision for income taxes for the years ended December 31 as follows (in thousands):
|
2007
|
% of
pretax
loss
|
2008
|
% of
pretax
loss
|
2009
|
% of
pretax
loss
|
|||||||||||||||||||
|
United States Federal tax (benefit) at statutory rate
|
$ | (2,293 | ) | 34.0 | $ | (675 | ) | 34.0 | $ | 1,706 | 34.0 | |||||||||||||
|
State taxes (benefit) (net of federal benefit)
|
(104 | ) | 1.6 | (31 | ) | 1.6 | 77 | 1.6 | ||||||||||||||||
|
Change in valuation reserve
|
2,780 | (41.2 | ) | 750 | (37.8 | ) | (1,937 | ) | (38.6 | ) | ||||||||||||||
|
Research and development credits
|
(1,429 | ) | 21.2 | (1,429 | ) | 72.0 | (1,429 | ) | (28.5 | ) | ||||||||||||||
|
Other non-deductible expenses
|
1,046 | (15.6 | ) | 1,385 | (69.8 | ) | 1,583 | 31.5 | ||||||||||||||||
|
Provision for income taxes
|
$ | ― | ― | $ | ― | ― | $ | ― | ― | |||||||||||||||
(9) Commitments
Leases
The Company is obligated under a capital lease that expired in 2009 for office equipment. Included in property and equipment are assets recorded under this capital lease obligation with a cost of $20,200 at December 31, 2009. Depreciation expense related to these assets of $6,700 is reported for the year ended December 31, 2007. The assets were fully depreciated at December 31, 2007. No payments were made under the agreement in 2009.
The Company had several non-cancelable operating leases, primarily for facilities and office equipment. Rental expense, including maintenance charges, for operating leases during 2007, 2008 and 2009 was $513 thousand, $63 thousand and $39 thousand, respectively. The rental expense for the years ended December 31, 2007 and 2008 is reduced by sub lease payments received of $123 thousand and $53 thousand, respectively.
On June 30, 2007, the Company entered into a Termination of Sublease Agreement with its Sub landlord for release on its obligation related to the corporate office location. The original lease term expired on March 31, 2011. In consideration for acceptance of the early release, the Company conveyed furniture and equipment located
on the premises and executed a Promissory note in the amount of $300 thousand. A loss on the early termination of the sublease agreement of $374 thousand was recognized by the Company.
Effective September 22, 2008 the Company agreed to a Fifth Amendment to Lease agreement with its landlord. Pursuant to the terms of the amendment, the Company reduced the size of its’ leased space and relocated its’ office within the building. The term of the amended lease expires on April 30, 2011. In consideration for the reduced lease obligation, the Company agreed to pay the landlord a relocation fee of $27 thousand pursuant to the terms of a Promissory note. The relocation fee has been included as rental expense for the year ended December 31, 2008. No payments were made under the amended agreement in 2009. Annual payments required under the amended lease for the years ended December 31, 2009, 2010 and 2011 total $35 thousand, $36 thousand and $3 thousand, respectively.
The accompanying notes are an integral part of these financial statements.
F-36
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(9) Commitments (continued)
On November 3, 2009, the Company entered into a lease agreement for corporate office space. The one-year lease term commenced February 1, 2010. The annual payments required under the new lease total $44 thousand and $ 4 thousand for the years ended December 31, 2010 and 2011, respectively.
Management Advisory and Consulting Agreements
On May 28, 2009 the Company entered into a Transaction Management Agreement with Southridge Business Solutions Group LLC (“Southridge”). Under the terms of the Agreement, the Company retained Southridge, on a non-exclusive basis, to provide services to enable the Company to restructure its balance sheet and execute a potential public offering or other public transaction. In conjunction with the Transaction Management Agreement, Southridge has agreed to assist the Company in securing an Equity Purchase Agreement with one of its affilialites to be executed in conjunction with a public offering or other public transaction. Under the terms of the proposed Equity Purchase Agreement, a third party to be identified by Southridge would commit to purchase $20 million of the Company’s common stock during a two-year period commencing at a date to be determined subsequent to the closing of the proposed public transaction. The purchase price of the common stock purchased under the Equity Purchase Agreement shall equal 92% of the average of the three low closing bid prices during the ten days commencing on the first trade date that notice is provided by the Company of its intent to draw down on the equity facility.
In consideration for the services to be provided, the Company is required to pay Southridge a monthly management fee of $10 thousand commencing June 1, 2009. Upon closing of a public transaction, the management fee will continue at $10 thousand per month and will be non-cancelable for a period of twelve months subsequent to the transaction date. For the year ended December 31, 2009, management fees of $70 thousand had been paid by the Company. In addition, the Company issued a total of 1.7 million warrants to Southbridge to purchase the company‘s stock at $.001 per share. The warrants provide for a cashless exercise and expire seven years after the date of issuance. Per the agreement, additional warrants will be issued at the transaction date in so that the total warrants issued to Southridge equal 3% of the outstanding shares of commons stock of the surviving corporation on a diluted basis.
On June 12, 2009 the Company engaged Canterbury Investment Partners LLC (“Canterbury’) to assist the Company in negotiating reductions, deferrals or equity exchanges with its trade creditors and in developing a strategy to raise equity from current and prospective investors. In exchange for the services to be provided, the Company paid Canterbury a retainer of $50 thousand. $20 thousand payable on August 7, 2009 when the Company raised a total of $150 thousand in new equity investment and $30 thousand payable on August 21, 2009 when the Company raised a total of $500 thousand in new equity investment. The Company is required to pay a monthly fee of $5,833 for a period of twelve months commencing September 1, 2009 when the Company raised $600 thousand in new equity investment. As of December 31, 2009, monthly fees of $23 thousand had been paid by the Company. Further, the Company is to issue warrants to Canterbury equal to 10% of the number of shares issued by the Company in new equity raised for cash consideration from the date of the agreement to the date a public transaction is completed. The warrants are to be priced at 110% of the per share price for the new equity or $0.22. The warrants are to have registration rights and a cashless exercise feature.
The accompanying notes are an integral part of these financial statements.
F-37
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(9) Commitments (continued)
On September 15, 2009, Curaxis entered into an agreement with GroupMark Financial Services Ltd. (“GroupMark”) to perform investor relation services and develop Curaxis’ new website. The Company is to pay GroupMark an Investor Development fee of $35,000 of which $25,000 had been paid as of December 31, 2009. In addition, the Company paid an affiliate of GroupMark, $15,000 during the year ended December 31, 2009 for the development of its new website. Further, as part for the consideration for the services rendered, upon completion of the website, Curaxis is to issue 100,000 warrants to GroupMark to purchase shares of the Company’s common stock at $.030 per share. The warrants will provide for a cashless exercise and expire five years from the date of issuance.
On November 25, 2009, the Company executed an engagement letter with Anslow & Jaclin for legal services to be provided in conjunction with a proposed Merger transaction. Fees for the work to be performed include the commitment of the Company to issue shares of common stock of the surviving corporation in an amount equivalent to .25% of the anticipated outstanding common shares post Merger.
Supplier Arrangement
The Company has entered into a non-exclusive worldwide agreement with Durect Corporation to develop and manufacture clinical and commercial supplies of the Company’s unique formulation for Alzheimer’s’ treatment. The Company has an exclusive worldwide license to Durect’s sustained release technology used in the production of this formulation. During the years ended December 31, 2004 and 2005, the Company made payments of $250 thousand and $250 thousand, respectively, upon initiation of the first Phase I trial for the product and initiation of the first Phase III trial for the product. These milestone payments were recorded as research and development expense. The Company will make additional payments of up to $2.5 million to Durect upon the achievement of certain development and regulatory milestones, as well as payments for research and development expenditures, and royalties based on product sales. The agreement is non-cancelable by Durect, except for material default by the Company. Research and development expenses recognized under the contract totaled $1.7 million for the year ended December 31, 2006. As of December 31, 2006, the Company was in default of the agreement due to non –payment.
On January 23, 2007 the agreement with Durect Corporation was amended to provide financial assistance to Company. Pursuant to the Amendment, Durect permanently waived its right to receive payments for development costs incurred totaling $727 thousand and its’ right to terminate the agreement as allowed for non-payment for outstanding development costs. Further, Durect remitted $1 million to the Company to assist in funding work required to compile and analyze data relating to the terminated Phase III clinical trial. In consideration for the above, the Company agreed to increase future royalty payments due Durect on product sales from a range of 5% to 7% to a range of 10% to 14% dependent upon volumes and to make a minimum royalty payment of $250 thousand annually commencing the year in which the first commercial sale is made. The liabilities forgiven by Durect and the financial payment made totaling $1.8 million have been recorded as deferred credits by the Company. The liability will be amortized over the projected royalty stream upon commercialization of the respective product.
The accompanying notes are an integral part of these financial statements.
F-38
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(9) Commitments (continued)
On July 28, 2008, the Company entered into a Supply Agreement with Mallinckrodt Inc. to provide the Company with Lueprolide Acetate for a period of five years commencing January 1, 2009. Under the terms of the Agreement, the company is required to purchase 75% of its annual requirements for the product from Mallinckrodt at an agreed upon price of $550 per gram. The price will be adjusted annually to reflect cost increases of the supplier on a dollar-for dollar basis. The price to be paid by the Company will be reduced by $50 per gram from the price in effect once the first volume of 75% of its annual requirements is accepted under the “FMOC” manufacturing process as defined by the agreement. The agreement will be automatically renewed for an additional 5 year term unless Mallinkrodt provides written notice at least six months prior to the end of the initial term. The Agreement may be terminated by written agreement of the parties. Upon execution of the Agreement, Mallinkckrodt paid $500 thousand to the Company with the understanding that the Company would be in a position to accept product as of January 1, 2009. The Company has recorded the payment as a deferred purchase credit at December 31, 2008. The Company was in default of the Agreement at January 1, 2009, as it was unable to accept product.
Clinical Trials
In April 2005, the Company entered into a Master Service Agreement with Quintiles, Inc. (“Quintiles Agreement”) under which Quintiles provides services to the company related to the conduct of the Phase III clinical trials for the Company’s unique formulation for Alzheimer’s treatment. Pursuant to this agreement, Quintiles performs clinical trials and services, and other research and development services, as requested by the Company. The Company is obligated to pay fees, expenses and pass through costs of Quintiles in accordance with work orders issued under the agreement. The term of the agreement is for five years unless terminated sooner by the Company without cause upon 60 days written notice or by either party upon 30 days written notice of any breach of the agreement which is not cured within the 30 day notice.
Due to lack of funding, the Company was forced to terminate the Phase III clinical in September 2006. A final work order was executed on January 25, 2007 to close-out the study and terminate the contract. Research and development expenses recognized with respect to work orders issued under the Master Service Agreement totaled $1.5 million for the year ended December 31, 2007.
(10) Related Party Transactions
During March 2008, an executive officer loaned the Company $37 thousand. The interest free loan was paid in full as of September 30, 2008.
During the two years ended December 2008, the Company funded travel expenses of an executive officer not directly related to the Company’s business. Travel expenses paid by the Company on behalf of the officer totaled $14 thousand. In addition, during the year ended December 31, 2009, the Company advanced funds to the executive officer for travel and office expenses totaling $16,000. Payments made to the Company by the employee and actual expenses incurred on the Company’s behalf totaled $10 thousand and $9 thousand for the years ended December 31, 2008 and 2009, respectively. Receivables in the amount of $5 thousand and $11 thousand have been recorded at December 31, 2008 and 2009, respectively.
The accompanying notes are an integral part of these financial statements.
F-39
Curaxis Pharmaceutical Corporation
(A Development Stage Corporation)
Notes to Financial Statements
(11) Subsequent Events
From January through February 3, 2010, the Company raised $197 thousand through the sale of 1.0 million shares of common stock at $0.20 per share and the issuance of 129 thousand shares of common stock upon exercise of outstanding warrants at $0.10 per share. These proceeds are net of expenses associated with warrants to be issued to outside consultants for services rendered in connection with the financing round totaling $17 thousand.
On February 8, 2010, the Company entered into an Agreement and Plan of Merger and Plan of Reorganization (the “Merger Agreement”) with Auto Search Cars Acquisition Corp., (“Acquisition Sub”) a Delaware corporation wholly owned by Auto Search Cars, Inc., (“Auto Search”) pursuant to which Acquisition Sub will be merged with and into the Company with the Company continuing as the surviving wholly-owned subsidiary of Auto Search. Upon completion of the transaction, Auto Search will change its name to Curaxis Pharmaceutical Corporation.
Auto Search will issue approximately 63,943,574 shares of its common stock to Curaxis shareholders in a stock-for-stock exchange for all outstanding shares of Curaxis common stock. In addition, each issued Curaxis Warrant (as defined in the Merger Agreement) shall be converted into warrants to purchase an equal number of shares of Auto Search’s common stock at the Exercise Price defined in the Company Warrants. Upon completion of the Merger, Curaxis shareholders will own approximately 91%, and the Auto Search shareholders will own approximately 9%, of the aggregate number of shares of common stock of the surviving Corporation.
In addition, the sole officer of Auto Search has agreed to cancel 181,285,000 shares of Auto Search common stock in exchange for payment of $100 thousand dollars and the issuance of 3,589,460 warrants to purchase common stock of the surviving corporation.
The Merger is intended to be a tax-free reorganization under Section 368(a) of the Internal Revenue Code of 1986, as amended.
On January 22, 2010 the Company and a Creditor reached a settlement agreement where by the Company will make payments totaling $900,000 over the next two years to satisfy, in full, its obligation. Further, the agreement restricts the Company from transferring or pledging its intellectual property without prior consent of the Creditor. Immediately preceding the settlement balances recorded on the Company’s financial records with respect to this vendor account included accrued interest and notes payable of $135 thousand and $924 thousand, respectively. A gain totaling $159 thousand was realized on the vendor settlement.
During 2009, one of the Company’s creditors filed a law suit against the Company relating to a claim for $45 thousand for services rendered in connection with the Company’s terminated Phase III clinical trial, VP-AD-301. On March 3, 2010, the suit was dismissed with prejudice.
The accompanying notes are an integral part of these financial statements.
F-40
No dealer, salesperson or other person has been authorized to give any information or to make any representations other than those contained in this prospectus in connection with the offering made by this prospectus, and, if given or made, such information or representations must not be relied upon as having been authorized by our Company or the Selling Security Holder. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than those specifically offered hereby or an offer to sell or a solicitation of an offer to buy any of these securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation. Except where otherwise indicated, this prospectus speaks as of the effective date of the registration statement. Neither the delivery of this prospectus nor any sale hereunder shall under any circumstances create any implication that there has been no change in the affairs of our Company since the date hereof.
14,500,000
Shares of
Common Stock
CURAXIS PHARMACEUTICAL CORPORATION
PROSPECTUS
, 2010
INFORMATION NOT REQUIRED IN PROSPECTUS
The estimated expenses of this offering in connection with the issuance and distribution of the securities being registered, all of which are to be paid by the registrant, are as follows:
|
$
|
5,000
|
|||
|
Legal Fees and Expenses
|
$
|
*
|
20,000
|
|
|
Accounting Fees and Expenses
|
$
|
5,000
|
||
|
Miscellaneous Expenses
|
$
|
*
|
5,000
|
|
|
Total
|
$
|
* |
35,000
|
Indemnification of Directors and Officers
Our directors and officers are indemnified as provided by the Nevada corporate law and our Bylaws. We have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”). Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Recent Sales of Unregistered Securities
In February 2008, the Company issued to Jonathon J. Martin, the Company’s founder and former sole officer, 2,000,000 shares of our common stock, $0.0001 par value (with a value of $200), in exchange for the founding officer’s business plan, business concept and website.
These shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance of shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, the investor had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such shares are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
From February to March 2008, we issued 76,700 shares of common stock to 40 individuals and entities at a price of $0.10 per share, in one offering for total proceeds of $7,670. These security holders had an opportunity to ask questions of and receive answers from our executive officers and were provided with access to our documents and records in order to verify the information provided.
II-1
These shares of our common stock qualified for exemption under Section 4(2) of the Securities Act since the issuance of shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, the investor had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such shares are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
On July 29, 2010, the Company issued a five-year warrant for the purchase of 3,589,460 shares of the Company’s common stock to John Martin, the Company’s former chief executive officer, in consideration for the cancellation of certain shares pursuant to the Merger. The exercise price of the warrant is $0.001 per share.
The warrant qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, the investor had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such shares are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
II-2
On August 31, 2010, the Company issued a six-year warrant for the purchase of 2,149,148 shares of the Company’s common stock to Southridge, issued in consideration for advisory services in connection with the Merger. The exercise price of the warrant is $0.001 per share.
The warrant qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, the investor had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such shares are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
On September 30, 2010, the Company entered into a Series A Convertible Preferred Stock Purchase Agreement (the “Series A Agreement”), by and between the Company and C P Acquisition Partners LP (the “Purchaser”). Pursuant to the terms of the Series A Agreement, the Company sold 500 shares of the Company’s Series A Convertible Preferred Stock for a total purchase price of $500,000.
On September 30, 2010, the Company entered into a Series B Convertible Preferred Stock Purchase Agreement (the “Series B Agreement”), by and between the Company and the Purchaser. Pursuant to the terms of the Series B Agreement, the Company sold 500 shares of the Company’s Series B Convertible Preferred Stock for a total purchase price of $500,000.
The Company relied on an exemption from the registration requirements of the Securities Act for the sale of our securities under the Series A Agreement and Series B Agreement pursuant to Section 4(2) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder. The transaction did not involve a public offering, the Purchaser is an “accredited investor” and/or qualified institutional buyer and the Purchaser had access to information about us and its investment.
On September 15, 2010, the Company issued a five-year warrant for the purchase of 820,856 shares of the Company’s common stock to Southridge Partners II, LP, issued in consideration for execution of the Equity Credit Agreement. The exercise price of the warrant is $1.52 per share.
The warrant qualified for exemption under Section 4(2) of the Securities Act since the issuance shares by us did not involve a public offering. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, the investor had the necessary investment intent as required by Section 4(2) since they agreed to and received share certificates bearing a legend stating that such shares are restricted pursuant to Rule 144 of the Securities Act. This restriction ensures that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act for this transaction.
II-3
EXHIBITS
All references to registrant’s Forms 8-K, 10-K and 10-Q include reference to File No. 000-03125.
|
Exhibit Number
|
Description of Document
|
|
| 2.1 |
Agreement and Plan of Merger and Plan of Reorganization by and Among Auto Search Cars, Inc., Auto Search Cars Acquisition Corp., and Curaxis Pharmaceutical Corporation.
|
|
| 3.1 |
Articles of Incorporation (incorporated by reference to the Company’s Registration Statement on Form S-1 (as filed with the SEC on May 15, 2008 (Registration No. 333-150937)).
|
|
| 3.2 |
By-laws of the Company (incorporated by reference to the Company’s Registration Statement on Form S-1, filed with the SEC on May 15, 2008 (Registration No. 333-150937)).
|
|
| 5.1 |
Legal Opinion of Anslow & Jaclin LLP*
|
|
| 10.1 |
Feasibility, Development and Commercialization Agreement, dated July 22, 2002, by and between Voyager Pharmaceutical Corporation, a Delaware corporation, and Southern Biosystems, Inc., an Alabama corporation (Durect) (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.2 |
Amendment No.1 to Feasibility, Development and Commercialization Agreement, effective January 23, 2007, by and between Durect Corporation and Voyager Pharmaceutical Corporation (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.3 |
Office Service Agreement, dated November 3, 2009, by and between Voyager Pharmaceutical Corporation and Regus Management Group LLC (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.4 |
Supply Agreement, dated January 1, 2009, by and between Voyager, Inc. and Mallinckrodt Inc. (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.5 |
Consulting Agreement, dated June 12, 2009, by and between Voyager Pharmaceutical Corporation and Canterbury Investment Partners LLC (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.6 |
Transaction Management Agreement, dated May 28, 2009, by and between Voyager Pharmaceutical Corporation and Southridge Business Solutions Group LLC.
|
|
| 10.7 |
Investor Development and Corporate Imaging Agreement, dated September 14, 2009, by and between Voyager Pharmaceutical Corporation and GroupMark Financial Services Ltd. (as filed on Form S-4/A with the SEC on June 21, 2010).
|
|
| 10.8 |
Amended and Restated Equity Credit Agreement by and between Curaxis Pharmaceutical Corp. and Southridge Partners II, LP, dated December 6, 2010*
|
|
| 10.9 |
Amended and Restated Registration Rights Agreement by and between Curaxis Pharmaceutical Corp. and Southridge Partners II, LP, dated December 6, 2010*
|
|
| 23.1 |
Consent of counsel (included in Exhibit 5.1)*
|
|
| 23.2 |
Consent of Rosenberg Rich Berman Baker and Company, Independent Registered Public Accounting Firm*
|
II-4
Undertakings
The Registrant hereby undertakes the following:
(a)(1) To file, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to:
(i) include any Prospectus required by Section 10(a)(3) of the Securities Act;
reflect (ii) in the Prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement, but notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) include any additional or changed material information of the plan of distribution.
For (2) determining liability under the Securities Act, treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
File a (3) post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the provisions described under Item 24 above, or otherwise, the Registrant has been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification relative to alleged Securities Act violations (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person, the Registrant will submit to a court of appropriate jurisdiction the question of whether such indemnification is against public policy and will be governed by the final adjudication of such issue.
(c) That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-5
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, as amended, the Registrant certifies that it has reasonable grounds to believe that it meets all of the requirements of filing on Form S-1 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, in the City of Durham, State of North Carolina on December 8, 2010.
|
CURAXIS PHARMACEUTICAL CORPORATION
|
|||
|
By:
|
/s/ Patrick S. Smith
|
||
|
Patrick S. Smith,
|
|||
|
Chief Executive Officer, Principal Accounting Officer
|
|||
|
By:
|
/s/ David J. Corcoran
|
||
|
David J. Corcoran
|
|||
|
Chief Financial Officer, Principal Accounting Officer
|
|||
PURSUANT TO THE REQUIREMENTS OF THE SECURITIES ACT OF 1933, AS AMENDED, THIS REGISTRATION STATEMENT HAS BEEN SIGNED BY OR ON BEHALF OF THE FOLLOWING PERSONS IN THE CAPACITIES AND ON THE DATES INDICATED:
|
Signature
|
Title
|
Date
|
||
|
/s/ Patrick S. Smith
|
President, Chief Executive Officer and Director
|
December 8, 2010
|
||
|
Patrick S. Smith
|
||||
|
/s/ David J. Corcoran
|
Executive Vice President, Chief Financial Officer and Director
|
December 8, 2010
|
||
|
David J. Corcoran
|
||||
|
/s/ Judith S.T. Geaslen
|
Vice President, Finance
|
December 8, 2010
|
||
|
Judith S.T. Geaslen
|
||||
|
/s/ Sheldon L. Goldberg
|
Director
|
December 8, 2010
|
||
|
Sheldon L. Goldberg
|
||||
|
/s/ William E. McConville
|
Director
|
December 8, 2010
|
||
|
William E. McConville
|
||||
|
/s/ Ronald V. Pompeo
|
Director
|
December 8, 2010
|
||
|
Ronald V. Pompeo
|
||||
|
/s/ Bert Spilker
|
Director
|
December 8, 2010
|
||
|
Bert Spilker
|
II-6
