Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CTI BIOPHARMA CORP | d8k.htm |
| EX-99.2 - NOTICE, DATED DECEMBER 3, 2010 - CTI BIOPHARMA CORP | dex992.htm |
Exhibit 99.1
REGISTRATION DOCUMENT
ISSUER
CELL THERAPEUTICS INC.
Registration Document filed with CONSOB on December 2, 2010 following authorization to the publication thereof
communicated by means of CONSOB note no. 10098480 of December 1, 2010
The fulfillment of the publication of this Registration Document does not entail any opinion of CONSOB as to the opportunity of the proposed investment and the merit of the data and information related thereto.
1
WARNINGS TO INVESTORS
THE SCOPE OF THESE WARNINGS IS TO ALLOW THE INVESTORS TO IMMEDIATELY CATCH THE ISSUES CONNECTED TO THE CAPACITY OF THE ISSUER TO REGULARLY CARRY ON ITS ACTIVITIES IN THE TWELVE MONTHS FOLLOWING THE DATE OF THIS REGISTRATION DOCUMENT. IN PARTICULAR, BELOW IS A SUMMARY OF THE MAIN RISKS AND UNCERTAINTIES WHICH MIGHT RAISE MATERIAL DOUBTS CONCERNING THE CAPABILITY OF THE ISSUER TO CONTINUE TO CARRY ON ITS ACTIVITIES AS AN ONGOING CONCERN.
| • | THE COMPANY DOES NOT EXPECT THAT ITS EXISTING CASH AND CASH EQUIVALENTS, INCLUDING THE CASH RECEIVED FROM THE ISSUANCE OF ITS SERIES 7 PREFERRED STOCK AND WARRANTS IN OCTOBER 2010, WILL BE SUFFICIENT TO FUND ITS PRESENTLY ANTICIPATED OPERATIONS BEYOND THE FIRST QUARTER OF 2011. THIS RAISES SUBSTANTIAL DOUBT ABOUT THE COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN. PLEASE REFER TO RISK FACTOR 4.1.1 FOR MORE INFORMATION. |
| • | THE COMPANY’S INDEPENDENT REGISTERED PUBLIC ACCOUNTING AUDITOR HAS INCLUDED AN EXPLANATORY PARAGRAPH IN ITS AUDIT REPORTS ON THE COMPANY’S DECEMBER 31, 2007, 2008 AND 2009 CONSOLIDATED FINANCIAL STATEMENTS AND IN ITS LIMITED REVIEW REPORT ON THE COMPANY’S FINANCIAL STATEMENTS CONCERNING THE SIX-MONTH PERIOD ENDED JUNE 30, 2010 WHERE, TAKEN INTO CONSIDERATION WHAT HAS BEEN REPORTED BY THE DIRECTORS IN THE RELEVANT ACCOUNTING DOCUMENTS AND THE COMPANY’S INABILITY TO DEMONSTRATE ITS ABILITY TO SATISFY THE MONETARY LIABILITIES DUE, IT RAISED SUBSTANTIAL DOUBT AS TO THE COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN. THIS MAY HAVE A NEGATIVE IMPACT ON THE TRADING PRICE OF THE COMPANY’S COMMON STOCK AND AS A RESULT, THE COMPANY MAY HAVE A DIFFICULT TIME OBTAINING NECESSARY FINANCING. |
| • | THE COMPANY HAS INCURRED A NET OPERATING LOSS EVERY YEAR SINCE ITS INCORPORATION IN 1991. AS OF SEPTEMBER 30, 2010, THE COMPANY HAD AN ACCUMULATED DEFICIT OF APPROXIMATELY $1.5 BILLION. THE COMPANY HAS NOT DECLARED OR PAID ANY DIVIDENDS ON SHARES OF ITS COMMON STOCK AND DOES NOT CURRENTLY EXPECT TO DO SO IN THE FUTURE. THE COMPANY MAY CONTINUE TO INCUR NET LOSSES, AND IT MAY NEVER ACHIEVE PROFITABILITY. PLEASE REFER TO RISK FACTOR 4.1.1 FOR MORE INFORMATION. |
| • | THE COMPANY DOES NOT HAVE ANY APPROVED DRUGS ON THE MARKET AND DOES NOT GENERATE REVENUES FROM ITS PRODUCTS AND THE COMPANY MAY NOT BE ABLE TO REPAY THE COMPANY’S DEBT OR THE RELEVANT INTEREST, LIQUIDATED DAMAGES OR OTHER PAYMENTS WITH RESPECT TO ITS DEBT. PLEASE REFER TO RISK FACTOR 4.1.1 FOR MORE INFORMATION. |
| • | THE COMPANY HAS A SUBSTANTIAL AMOUNT OF DEBT OUTSTANDING, AND ITS ANNUAL INTEREST EXPENSE WITH RESPECT TO ITS DEBT IS SIGNIFICANT. THE AGGREGATE PRINCIPAL BALANCE OF THE COMPANY’S CONVERTIBLE NOTES AS OF SEPTEMBER 30, 2010 IS APPROXIMATELY $21.1 MILLION, CORRESPONDING TO APPROXIMATELY $10.2 MILLION IN THE 7.5% CONVERTIBLE SENIOR NOTES DUE IN APRIL 2011 (THEREFORE WITHIN THE NEXT 12 |
2
| MONTHS) AND TO APPROXIMATELY $10.9 MILLION IN THE 5.75% CONVERTIBLE SENIOR NOTES DUE IN DECEMBER 2011. PLEASE REFER TO THE FOLLOWING PARAGRAPH 10.3.3 FOR MORE INFORMATION. AS OF SEPTEMBER 30, 2010 THE TOTAL ESTIMATED AND UNAUDITED NET FINANCIAL POSITION OF THE COMPANYWAS APPROXIMATELY NEGATIVE $6.7 MILLION. |
| • | THE COMPANY ESTIMATES ITS AVERAGE CASH BURN-RATE (CORRESPONDING TO ITS MONTHLY FINANCIAL NEED) FOR THE REMAINDER OF 2010 AND THE FIRST QUARTER OF 2011 TO BE ABOUT $5 MILLION PER MONTH. THE COMPANY IS REVIEWING WAYS TO REDUCE ITS BURN RATE. |
| • | WITH RESPECT TO THE PERIODS TO WHICH THE HISTORIC FINANCIAL INFORMATION REPORTED IN THIS REGISTRATION DOCUMENT REFERS, THE COMPANY BORE THE FOLLOWING TOTAL NET LOSS ATTRIBUTABLE TO CTI COMMON SHAREHOLDERS: $148.3 MILLION IN THE YEAR 2007, $202.9 MILLION IN THE YEAR 2008, AND $116.8 MILLION IN THE YEAR 2009. WITH RESPECT TO SUCH AMOUNT OF TOTAL NET LOSS ATTRIBUTABLE TO CTI COMMON SHAREHOLDERS, THE PERCENTAGE OF REMUNERATIONS AND BENEFITS TO DIRECTORS AND THE NAMED EXECUTIVE OFFICERS (AS DEFINED IN THE FOLLOWING GLOSSARY) IS RESPECTIVELY: 3% FOR THE YEAR 2007, 2% FOR THE YEAR 2008, AND 36% FOR THE YEAR 2009. THE TOTAL AMOUNT OF RENUMERATION AND BENEFITS TO DIRECTORS AND THE NAMED EXECUTIVE OFFICERS IS DERIVED FROM INFORMATION PROVIDED IN OUR PROXY STATEMENTS PREPARED IN ACCORDANCE WITH THE APPLICABLE RULES AND REGULATIONS OF THE U.S. SECURITIES AND EXCHANGE COMMISSION AND IS NOT NECESSARILY INDICATIVE OF THE AMOUNT OF COMPENSATION AND BENEFITS RECORDED IN THE FINANCIAL STATEMENTS FOR SUCH PERIODS. PLEASE REFER TO THE FOLLOWING PARAGRAPH 15.1 FOR MORE INFORMATION. |
| • | DURING THE LAST THREE YEAR PERIOD AND THE NINE-MONTH PERIOD ENDING IN SEPTEMBER 30, 2010, THE COMPANY HAS DECREASED ITS RESEARCH AND DEVELOPMENT EXPENSES AS FOLLOWS, SPECIFICALLY FROM $72.019 MILLION IN THE FINANCIAL YEAR 2007 TO $51.614 MILLION IN THE FINANCIAL YEAR 2008, $30.179 IN THE FINANCIAL YEAR 2009 AND, LASTLY, TO $19.375 MILLION IN THE NINE-MONTH PERIOD FROM DECEMBER 31, 2009 TO SEPTEMBER 30, 2010. PLEASE REFER TO THE FOLLOWING PARAGRAPHS 9.2.1, 9.2.3 AND 20.1 FOR MORE INFORMATION. |
| • | OBTAINING REGULATORY APPROVAL TO MARKET DRUGS TO TREAT CANCER IS EXPENSIVE, DIFFICULT AND RISKY. ON APRIL 9, 2010, THE FOOD AND DRUG ADMINISTRATION (FDA) INFORMED THE COMPANY ABOUT THE REJECTION OF THE NEW DRUG APPLICATION (NDA) FOR THE MARKETING OF PIXANTRONE (THAT IS THE PRODUCT IN THE MOST ADVANCED PHASE OF THE MARKETING AUTHORIZATION PROCESS AMONG THE CANDIDATE DRUGS OF CTI) IN THE USA AND RECOMMENDED THAT THE COMPANY CONDUCT AN ADDITIONAL TRIAL TO DEMONSTRATE THE SAFETY AND EFFECTIVENESS OF PIXANTRONE. THE COMPANY DOES NOT KNOW WHAT THIS TRIAL WILL COST OR WHETHER THE FDA WILL ACCEPT THE COMPANY’S DESIGN FOR THIS TRIAL. PLEASE REFER TO THE FOLLOWING PARAGRAPH 6.1 FOR MORE INFORMATION. |
| • | ON NOVEMBER 1, 2010, THE COMPANY SUBMITTED A MARKETING AUTHORIZATION APPLICATION (MAA) TO THE EUROPEAN MEDICINES AGENCY (EMA) FOR PIXUVRI(R) (PIXANTRONE DIMALEATE) AS MONOTHERAPY FOR THE TREATMENT OF ADULT PATIENTS WITH MULTIPLY RELAPSED OR REFRACTORY AGGRESSIVE NON-HODGKIN’S LYMPHOMA |
3
| (NHL). THE APPLICATION SUBMISSION FOLLOWS A POSITIVE OPINION FROM THE EMA’S PEDIATRIC COMMITTEE (PDCO), WHERE PDCO AGREED TO CTI’S PEDIATRIC INVESTIGATION PLAN (PIP) FOR PIXUVRI. MOREOVER ON NOVEMBER 17, 2010 THE EMA APPROVED THE AFORESAID PIP. AS OF THE DATE OF THIS REGISTRATION DOCUMENT THE COMPANY CAN NOT ANTICIPATE THE DURATION OF SUCH MARKETING AUTHORIZATION PROCEEDINGS OR WHETHER THE MAA WILL RECEIVE APPROVAL IN THE NEXT 12 MONTHS. PLEASE REFER TO THE FOLLOWING PARAGRAPH 6.1 FOR MORE INFORMATION. |
| • | THE COMPANY IS NOT YET PURSUING OBTAINING MARKETING APPROVAL FOR ITS DRUG CANDIDATE OPAXIO. THE COMPANY PLANS TO MEET WITH THE FDA TO EXPLORE A POTENTIAL PHASE III REGISTRATION STUDY UTILIZING OPAXIO AS A RADIATION SENSITIZER IN THE TREATMENT OF ESOPHAGEAL CANCER AND HAS ENTERED INTO A CLINICAL TRIALS AGREEMENT WITH THE GYNECOLOGIC ONCOLOGY GROUP, TO PERFORM A PHASE III TRIAL OF OPAXIO AS MAINTENANCE THERAPY IN PATIENTS WITH OVARIAN CANCER. AS OF THE DATE OF THIS REGISTRATION DOCUMENT THE COMPANY CAN NOT ANTICIPATE IF IT WILL BE ABLE TO FILE A NDA IN THE USA OR A MAA IN THE EUROPEAN UNION IN THE NEXT 12 MONTHS. PLEASE REFER TO THE FOLLOWING PARAGRAPH 6.1 FOR MORE INFORMATION. |
4
INDEX
| INDEX |
5 | |||||
| GLOSSARY |
C-1 | |||||
| SECTION I |
REGISTRATION DOCUMENT | C-13 | ||||
| CHAPTER 1 |
PERSONS RESPONSIBLE FOR THIS PROSPECTUS | C-13 | ||||
| CHAPTER 2 |
AUDITORS | C-14 | ||||
| CHAPTER 3 |
SELECTED FINANCIAL INFORMATION | C-15 | ||||
| CHAPTER 4 |
RISK FACTORS | C-20 | ||||
| CHAPTER 5 |
COMPANY INFORMATION | C-53 | ||||
| CHAPTER 6 |
BUSINESS OVERVIEW | C-66 | ||||
| CHAPTER 7 |
ORGANIZATIONAL STRUCTURE | C-98 | ||||
| CHAPTER 8 |
PROPERTY, PLANT AND EQUIPMENT | C-100 | ||||
| CHAPTER 9 |
OPERATING AND FINANCIAL REVIEW | C-102 | ||||
| CHAPTER 10 |
CAPITAL RESOURCES | C-112 | ||||
| CHAPTER 11 |
RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES | C-134 | ||||
| CHAPTER 12 |
TREND INFORMATION | C-136 | ||||
| CHAPTER 13 |
PROFIT FORECASTS OR ESTIMATES | C-140 | ||||
| CHAPTER 14 |
ADMINISTRATIVE, MANAGEMENT, AND SUPERVISORY BODIES AND SENIOR MANAGEMENT | C-141 | ||||
| CHAPTER 15 |
REMUNERATION AND BENEFIT | C-152 | ||||
| CHAPTER 16 |
PRACTICES OF THE BOARD OF DIRECTORS | C-163 | ||||
| CHAPTER 17 |
EMPLOYEES | C-169 | ||||
| CHAPTER 18 |
MAJOR SHAREHOLDERS | C-173 | ||||
| CHAPTER 19 |
RELATED PARTY TRANSACTIONS | C-174 | ||||
| CHAPTER 20 |
FINANCIAL INFORMATION CONCERNING THE ISSUER’S ASSETS AND LIABILITIES, FINANCIAL POSITION AND PROFITS AND LOSSES | C-177 | ||||
| CHAPTER 21 |
ADDITIONAL INFORMATION | C-266 | ||||
| CHAPTER 22 |
MATERIAL CONTRACTS | C-304 | ||||
| CHAPTER 23 |
THIRD PARTY INFORMATION AND STATEMENT BY EXPERTS AND DECLARATIONS OF ANY INTEREST | C-314 | ||||
| CHAPTER 24 |
DOCUMENTS AVAILABLE TO THE PUBLIC | C-315 | ||||
| CHAPTER 25 |
INFORMATION ON HOLDING | C-316 | ||||
5
DEFINITIONS
The important terms and definitions used in this Registration Document are listed below. These definitions and terms shall have the meaning set out below unless otherwise provided.
| Auditors: | Stonefield Josephson, Inc., a company with its registered office in Los Angeles, California. | |
| Authorized Common Shares: | The total number of shares of the Common Stock that the Company is permitted to issue (1,200,000,000 as of the date of the Registration Document, including the issued and outstanding Common Shares) without obtaining stockholder approval (except as may be required by the NASDAQ Marketplace Rules). | |
| Authorized Preferred Shares: | The total number of shares of the Company’s preferred stock that the Company is permitted to issue (10,000,000 as of the date of the Registration Document, including the issued and outstanding Preferred Shares) without obtaining stockholder approval (except as may be required by the NASDAQ Marketplace Rules). The Board of Directors may determine the terms of the preferred shares in its sole discretion. | |
| Borsa Instructions: | The Istruzioni al Regolamento dei Mercati organizzati e gestiti da Borsa Italiana S.p.A. effective as of August 9, 2010. | |
| Borsa Italiana: | Borsa Italiana S.p.A. with registered office in Milan, Piazza Affari 6. | |
| Borsa Regulation: | The Regolamento dei Mercati organizzati e gestiti da Borsa Italiana S.p.A. effective as of June 28, 2010. | |
| Business Day: | Each day between Monday and Friday, inclusive, of any given week in which banks are open and stock exchanges are open for trading in Italy and the United States. | |
| Common Stock: | The shares of common stock of the Company (ISIN Code US1509345039), no par value per share, listed on the NASDAQ Capital Market and on MTA. | |
| CONSOB: | Commissione Nazionale per le Societa e la Borsa (Italian National Commission for Companies and the Stock Exchange). | |
| CONSOB Regulation: | Regulations implementing Italian Legislative Decree No 58 of February 24, 1998, regarding issuers regulation (adopted by CONSOB with resolution No 11971 dated May 14, 1999 as subsequently amended). | |
| CTI Europe: | The Company’s branch in Italy (formerly known as Cell Therapeutics Europe S.r.l., which was merged into the Company in November 2007) with registered office in Via Valentino Mazzola n. 18, Rome (before the registered office was in Bresso). | |
| CTI or the Issuer or the Company: | Cell Therapeutics, Inc. having its registered office located in the State of Washington at 501 Elliott Avenue West, Suite 400, Seattle, Washington 98119 (U.S.). | |
C-1
| Date of the Registration Document: | The date on which the Registration Document has been published. | |
| EBITDA: | Earnings before interest, taxes, depreciation and amortization (EBITDA) is a non-GAAP metric that can be used to evaluate a company’s profitability. | |
| Exchange Act: | The U.S. Securities Exchange Act of 1934, as amended. | |
| Group: | The Company and the Company’s Subsidiaries. | |
| Italian Shareholders | The Company’s shareholders who hold shares in Italy through Monte Titoli. | |
| Issuance: | Any issuance, from time to time, which will be authorized by the Board of Directors prior to the commencement thereof and which will be described in detail in one more securities notes to be disclosed to the public, together with the relevant summaries and the possible updated information, according to the applicable laws and regulations. | |
| Monte Titoli: | Monte Titoli S.p.A., having its registered office in Milan, via Mantegna, 6. | |
| MTA: | MTA, Standard 1, organized and managed by Borsa Italiana. | |
| NASDAQ: | National Association of Securities Dealers Automated Quotations system and the NASDAQ Capital Market (the U.S. exchange on which the Company’s stock is listed). | |
| Named Executive Officers: | Means the Chief Executive Officer (the “CEO”), the Chief Financial Officer (the “CFO”) and the Company’s next three most highly compensated executive officers for fiscal year 2009. | |
| New Common Shares: | The newly issued Common Shares pursuant to the Share Capital Increase. | |
| NASDAQ Marketplace Rules: | The rules of the NASDAQ Capital Market. | |
| Preferred Shares: | Shares of 3% Convertible Preferred Series A, Series B and Series C Stock, shares of 7% Convertible Preferred Series D Stock, shares of Convertible Preferred Series F Stock, shares of Series 1 Preferred Stock, shares of Series 2 Preferred Stock, shares of Series 3 Preferred Stock, shares of Series 4 Preferred Stock, shares of Series 5 Preferred Stock, shares of Convertible Preferred Series 6 Stock and shares of Convertible Preferred Series 7 Stock. As of the date of this Registration Document there were no shares of any series of the Company’s Preferred Stock remaining outstanding. | |
| RIT Oncology: | RIT Oncology, LLC, a joint venture formed by the Company and Spectrum Pharmaceuticals, Inc. in December 2008 for the marketing and commercialization of Zevalin; all Zevalin-specific assets owned by | |
C-2
| the Company (including the supplemental biologics license application submitted on October 2, 2008 for the use of Zevalin as consolidation therapy after remission induction in previously untreated patients with follicular non-Hodgkin’s lymphoma) were transferred to RIT Oncology in connection with this formation. The Company subsequently sold its interest in RIT Oncology to Spectrum Pharmaceuticals, Inc. in March 2009, although it will continue to perform transition services for Zevalin through May 31, 2009. | ||
| Share Capital Increase: | The share capital increase resolved upon for the purposes of the Issuances that may be made under this Registration Document. | |
| SEC: | The U.S. Securities and Exchange Commission. | |
| Subscriber: | A single purchaser, a limited number of purchasers, underwriters or dealers of the Offer. | |
| 2003 Listing Prospectus: | Listing Prospectus filed by the Company with CONSOB in 2003. | |
| Unified Financial Act or TUF: | Italian Legislative Decree No 58 of February 24, 1998, as amended and supplemented. | |
| US GAAP: | U.S. generally accepted accounting principles. | |
GLOSSARY OF THE MEDICAL AND TECHNICAL TERMS USED IN THIS REGISTRATION DOCUMENT
In consideration of the activity that the Company carries out in the biotechnology sector, it has been deemed convenient to prepare the following glossary of the technical terms which will be most frequently used in this Registration Document.
| Anthracyclines | Antibiotics which act to inhibit tumor growth by causing damage to DNA. | |
| Biologic Products | Biologic products, like other drugs, are used for the treatment, prevention or cure of disease in humans. In contrast to chemically synthesized small molecular weight drugs, which have a well-defined structure and can be thoroughly characterized, biological products are generally derived from living material – human, animal, or microorganism – are complex in structure, and thus are usually not fully characterized. | |
| Bisplatinates | Bisplatinates are new analogues of the dinuclear-platinum complex that is more potent than cisplatin. Cisplatin is a platinum-based chemotherapy drug used to treat a wide variety of cancers. The Company is currently researching bisplatinates. | |
C-3
| BLA | Biologics License Application, the method by which the FDA reviews and approves biological products for marketing in the U.S. | |
| Chemotherapy | The treatment of a tumor through the use of various chemical compounds, especially cytotoxic agents, which prevent cellular proliferation and reduce tumor growth. | |
| CHOP-R | A chemotherapy regimen used in the treatment of non-Hodgkin’s lymphoma. CHOP-R stands for cyclophosphamide, doxorubicin, vincristine, prednisone, rituximab. | |
| CPOP-R | CPOP-R is the acronym for a chemotherapy regimen. CPOP-R stands for cyclophosphamide, pixantrone, vincristine, prednisone, rituximab and differs from the standard regimen as pixantrone is substituted for doxorubicin. | |
| cGMP/GLP/GCP (current good manufacturing practice/good laboratory practice/ good clinical practice) |
International rules aimed at ensuring the quality of, respectively: (i) the pharmaceutical production processes; (ii) the carrying out of non-clinical trials for regulatory purposes; and (iii) the trials on humans and the definition of trial guidelines. | |
| Clinical data | Patient and other information collected and recorded during and as part of an ongoing clinical trial. | |
| Clinical development (or clinical experimentation) | Series of consequential studies regulated by the regulatory health authorities involving the trials of experimental drugs on humans with the aim of their possible introduction to the market. Clinical trials are typically conducted in three sequential phases, but the phases may overlap or be combined. Phase I usually involves the initial introduction of the investigational drug into people to evaluate its short-term safety, dosage tolerance, metabolism, pharmacokinetics and pharmacologic actions, and, if possible, to gain an early indication of its effectiveness. Phase II usually involves trials in a limited patient population to (i) evaluate dosage tolerance and appropriate dosage, (ii) identify possible adverse effects and safety risks, and (iii) evaluate preliminarily the efficacy of the drug for specific indications. Phase III trials usually further evaluate clinical efficacy and test further for safety by using the drug in its final form in an expanded patient population. | |
C-4
| Confirmed Complete Remission (CR) | The total disappearance of all evidence of disease using physical examination, laboratory studies and radiologic imaging and a criterion for evaluating the efficacy of a particular anti-cancer therapy. | |
| Corporate Integrity Agreement | An agreement with the federal government whereby the Company’s actions with respect to promotion and sales practices are reviewed and reported to the federal government for a period of time. | |
| Cytotoxic agents | Chemicals or compounds that are directly toxic to cells, preventing their reproduction or growth. | |
| Data Safety Monitoring Board, or DSMB | The Data Safety Monitoring Board, or DSMB, is a group consisting of external investigators and company personnel which periodically reviews safety data from an ongoing clinical trial. The DSMB may make recommendations with regard to an ongoing study based on their review of the safety data and their knowledge of treatment of the disease. | |
| Data Monitoring Committee | See Data Safety Monitoring Board | |
| EMA | European Medicines Agency. The inter-governmental agency responsible for approving drug candidates for marketing in the European Union. | |
| Endpoint | In a clinical research trial, a clinical endpoint refers to occurrence of a disease, symptom, sign or laboratory abnormality that constitutes one of the target outcomes of the trial. The results of a clinical trial generally indicate the number of people enrolled who reached the pre-determined clinical endpoint during the study interval, compared with the overall number of people who were enrolled. | |
| EORTC | European Organization for Research and Treatment of Cancer. | |
| Fast Track | Fast track designation is granted to expedite the review process of applications for approval of new drugs intended to treat serious or life-threatening conditions where potential to address an unmet medical need is demonstrated. | |
| FDA | U.S. Food and Drug Administration, the governmental agency in the United States responsible for approving new drug applications. | |
| First-line | Preferred treatment for the diagnosed illness. | |
C-5
| Formulation | Pharmaceutical preparation which enables the administration of a drug’s active principles (i.e., of the components of a drug with pharmacological activity) in the desired manner, due to the use of excipients; the final preparation is ready for clinical use. | |
| GOG | The Gynecologic Oncology Group is a non-profit organization with the purpose of promoting excellence in the quality and integrity of clinical and basic scientific research in the field of gynecologic malignancies. | |
| Hazard ratio | The relative likelihood of experiencing a particular event. In a survival study, this is the relative likelihood of death. A hazard ratio (HR) of 0.5 indicates that one group has half the risk of the other group. | |
| HIF-1/P300 | HIF-1 (hypoxia-inducible factor 1) is a transcription factor that senses the low oxygen concentration and activates a series of genes which helps the tumor survive these conditions | |
| “Intent to treat” analysis | Intent to treat analysis means including all patients randomized to an arm of a clinical trial, regardless of their adherence to the entry criteria, regardless of the treatment they actually received, and regardless of the subsequent withdrawal from treatment or deviation from the protocol. | |
| Investigational New Drug | An application that a drug sponsor must submit to FDA before beginning tests of a new drug on humans. The IND contains the plan for the study and is supposed to give a complete picture of the drug, including its structural formula, animal test results, and manufacturing information. | |
| In vitro | Activities performed outside the living body and in an artificial environment. | |
| In vivo | Experimentation carried out on animals or humans, (meaning, “in the living body”). | |
| Leucopenia | A low white blood cell count, or leucopenia, is a decrease in disease-fighting cells (leukocytes) circulating in your blood. | |
C-6
| Log rank | Log rank describes a standard statistical method for testing the null hypothesis that there is no difference between the populations in the probability of an event at any given time point. A log rank test is most likely to detect a difference between groups when the risk of an event is consistently greater for one group than another. | |
| Marker | Indicator of the presence of a specific kind of tumor, identifiable by means of specific laboratory tests. | |
| Metastatic | Spread of a tumor by cells migrating from a primary site (primary tumor) to a secondary site where a new tumor develops (metastatic secondary tumor). | |
| Mitoxantrone | Intercalating cancer drug with a high level of activity on leukemia and lymphoma, but also used in the combined treatment of breast, prostate and ovarian cancer. | |
| Milestone | A scheduled event that is used to measure progress toward a final set target. | |
| MAA or Marketing authorization application | An application submitted to the EMA for review and approval to begin marketing a drug in Europe. An MAA is similar to the NDA in the U.S. | |
| NDA or New drug application | When the sponsor of a new drug believes that enough evidence on the drug’s safety and effectiveness has been obtained to meet FDA’s requirements for marketing approval, the sponsor submits to FDA a new drug application (NDA). The application must contain data from specific technical viewpoints for review, including chemistry, pharmacology, medical, biopharmaceutics, and statistics. If the NDA is approved, the product may be marketed in the United States. | |
| Neutropenia | An abnormally low count of neutrophils, white blood cells that protect your body from bacteria and fungi. Neutrophils usually constitute about 45 to 75% of all white blood cells in the bloodstream. | |
| Non-Hodgkin’s lymphoma, or NHL | A particular type of malignant tumor that starts in the lymphoid tissue (also called lymph or lymphatic tissue). | |
C-7
| Non-small cell lung cancer, or NSCLC | Non-small cell lung cancer, or NSCLC, is one of two main types of lung cancer. There are three sub-types of NSCLC. The cells in these sub-types differ in size, shape, and chemical make-up.
• squamous cell carcinoma: linked to smoking and tend to be found near the bronchus.
• adenocarcinoma: usually found in the outer part of the lung.
• large-cell undifferentiated carcinoma: tends to grow and spread quickly. | |
| NSCLC | Non-small cell lung cancer. | |
| Off-label | Use of a drug for a disease or condition other than the indication for which it was approved by a regulatory authority, such as the FDA or EMA. | |
| Oncology | Branch of medicine which studies the characteristics of tumors, their classification, diagnosis and therapy. | |
| OPAXIO™ | Paclitaxel poliglumex, previously referred as XYOTAX. In May 2008 the European Medecines Agency (EMA) expressed a positive opinion for the brand name OPAXIO which replaces XYOTAX as the brand name. | |
| Partial Remission (PR) | A decrease in the size of a tumor, or in the extent of cancer in the body, in response to treatment. | |
| Pivotal study or Pivotal trial | Clinical trial on which a drug’s safety and efficacy is based and which provides the data that the FDA uses to decide whether or not to approve a drug. A pivotal study will generally be a phase III, well-controlled, randomized trial. | |
| Pixantrone or Pixuvri™ | A novel anthracycline derivative, BBR 2778, for the treatment of non-Hodgkin’s lymphoma, or NHL, and various other hematologic malignancies, solid tumors and immunological disorders. | |
| Pharmacokinetics | Branch of pharmacology which studies the behavior of compounds in living organisms by means of the evaluation of ADME (absorption, distribution, metabolism and elimination) of the drug in a living organism. If the tests are carried out using toxic doses, the appropriate term is toxicokinetics. | |
C-8
| Pharmacology | Science of the study of drugs, their preparation, their effects and their action mechanisms. | |
| Phase I, Phase II, Phase III | See “Clinical Development”. | |
| PolaRx | PolaRx, a single product company that owned the rights to TRISENOX. | |
| Polyglutamate | A biodegradable polymer of glutamic acid, a naturally occurring amino acid. | |
| Pooled analysis | Pooled analysis means combining analyses from more than one clinical trial. | |
| Preclinical data | Data collected from studies performed in the laboratory or in animals, which is used to predict the potential safety, tolerability, and activity of a potential drug before it has entered clinical trials in humans. (See also preclinical development). | |
| Preclinical development | Laboratory tests (in vitro and in vivo) before testing on humans. This phase involves in vitro and in vivo experimentation to evaluate the activity and toxicity of the compounds and the first stages in the study of the formulation. | |
| Product | Depending on the context, (i) a pharmaceutical or biopharmaceutical substance which is already on the market; or (ii) a pharmaceutical or biopharmaceutical substance which is potentially marketable (potential product or product in clinical development). | |
| Product candidate | A pharmaceutical or biopharmaceutical substance in any phase of research and development between initial research and the completion of the clinical trials and market approval. | |
| Progression-free survival | The time interval during which a patient’s disease is determined to be stable until the time when the disease is determined to be progressing. | |
C-9
| PS2 Patient | A poor performance status patient. Performance status (PS) is a measure of disease symptoms defined by the Eastern Cooperative Oncology Group. It is rated on a scale from PS0 to PS5. Patients who are considered “good performance status” or PS0 or PS1 have minimal or no symptoms of disease and are able to maintain their activities of daily living. Patients with performance status 2 or greater are considered “poor performance status.” PS2 patients are ambulatory and capable of self care and up and about more than 50% of their waking hours, but are unable to carry out work activities. | |
| Radiopharmaceutical | A radioactive compound used in radiotherapy or diagnosis. | |
| Randomized study | A clinical trial, typically conducted at several different centers, in which patients have been randomly assigned to receive either the study drug or control drug. | |
| Refractory | Not responsive to the relevant treatment. | |
| Regulatory health authorities | The authorities which regulate the various stages of experimentation (pre-clinical and clinical) of potential pharmaceutical products and grant/revoke the relative authorization for marketing and sale of the products. In Italy the relevant authority is the Ministry of Health (Ministero della Sanità) and the Superior Institute of Health (Istituto Superiore della Sanità), in Europe, the European Medicines Agency (Agenzia Europea per i Medicinali) (EMA) and, in the U.S., the Food and Drug Administration (FDA). | |
| Resistance (to anti-tumor compounds) | Capability of cancer cells to become less sensitive to the effect of compounds which are intended to weaken them. | |
| sBLA | Supplemental Biologics License Application; the process for approval by the FDA of additional marketed uses for a biologic product which already has an approved BLA from the FDA for another indication. | |
| Second-line | Treatment following first-line treatment; used if the initial treatment is unsuccessful or if the disease re-occurs. | |
C-10
| Shelf registration statement | A registration statement that allows an issuer in the United States to offer securities from time to time, provided that the securities have been described in the registration statement and the registration statement has been declared effective by the SEC. | |
| Special Protocol Assessment, or SPA | The SPA process allows for an agreement between companies and the FDA on the design of a study, including clinical drug supply, pivotal trial design, clinical endpoints, conduct, data analysis, and other clinical trial issues, and is intended to provide assurance that if pre-specified trial results are achieved, they may serve as the primary basis for an efficacy claim in support of an NDA. | |
| STELLAR clinical trials 2, 3, and 4 | Clinical studies for OPAXIO. | |
| Taxol | Anti-cancer agent very effective on numerous forms of solid tumors such as breast, ovarian and lung cancers. | |
| TRISENOX | Arsenic trioxide, a commercial product the Company divested in 2005. | |
| Unconfirmed complete remission (CRu) | The patient has achieved a complete remission, but may not have a completely clean bone marrow test. | |
| United States Attorney’s Office or USAO | The principal litigators of the United States, under the U.S. Attorney General. | |
| United States Public Company Accounting Oversight Board | A private-sector, non-profit corporation, created by the U.S. Sarbanes-Oxley Act of 2002, to oversee the auditors of U.S. public companies in order to protect the interests of investors and further the public interest in the preparation of informative, fair, and independent audit reports. | |
C-11
| Zevalin | Ibritumomab Tiuxetan, a CD20-directed, radiotherapeutic antibody indicated as part of the therapeutic regimen for treatment of relapsed or refractory, low-grade or follicular B-cell non-Hodgkin’s lymphoma. The Company acquired the U.S. sales, marketing and development rights to Zevalin from Biogen Idec in December 2007 and subsequently transferred those rights, along with all assets owned or licensed to the Company specific to Zevalin (including the supplemental biologics license application submitted on October 2, 2008 for the use of Zevalin as consolidation therapy after remission induction in previously untreated patients with follicular non-Hodgkin’s lymphoma), to RIT Oncology in December 2008. As of the date of this Registration Document the Company no longer owns any interest, directly or indirectly, in Zevalin. |
C-12
CHAPTER 1 PERSONS RESPONSIBLE FOR THIS REGISTRATION DOCUMENT
| 1.1 | Persons responsible for this Registration Document |
Cell Therapeutics Inc, a company incorporated in 1991 under the laws of the State of Washington (U.S.), with its registered office located in the State of Washington at 501 Elliott Avenue West, Suite 400, Seattle, Washington 98119 (U.S.) (the “Company”), is responsible, in its quality as the issuer, for the completeness and truthfulness of the information contained in this Registration Document.
| 1.2 | Statement of responsibility for this Registration Document |
The Company declares that this Registration Document is consistent with the copy filed with CONSOB on December 2, 2010, following the communication of the release of the relevant authorization by means of notice no. 10098480 of December 1, 2010.
Having adopted all reasonable diligence for such purposes, the Company declares that the information contained herein is–so far as it is aware–in accordance with the facts and there is no omission that may change the import hereof.
C-13
CHAPTER 2 AUDITORS
| 2.1 | Auditors appointed by the Company to audit the financial statements contained in this Registration Document |
The Company’s financial statements for the years ended December 31, 2007, December 31, 2008 and December, 31 2009 were audited by Stonefield Josephson, Inc., a company with its registered office in Los Angeles, California (U.S.) and a certified independent public accounting firm registered with the United States Public Company Accounting Oversight Board (the “Auditors”). Moreover the Auditor performed a review of the unaudited condensed consolidated financial statements of the Company for the six-month period ended June 30, 2010.
| • | The external auditor opinions for the financial statements for the years ended December 31, 2007 is included in the Company’s Annual Report on Form 10-K, as amended and filed with the SEC on March 26, 2008; |
| • | the external auditor opinions for the financial statements for the years ended December 31, 2008 is included in the Company’s Annual Report on Form 10-K, as amended and filed with the SEC on March 16, 2009; |
| • | the external auditor opinion for the financial statements for the year ended December 31, 2009 is included in the Company’s Annual Report on Form 10-K filed with the SEC on February 26, 2010; |
| • | the external auditor limited review report for the financial information regarding the six-month period ended June 30, 2010 is included in the Company’s Quarterly Report on Form 10-Q as filed with the SEC on August 5, 2010. |
The aforesaid Reports can be found on the Company’s website at http://investors.celltherapeutics.com. The external auditor opinions for the Company’s 2007, 2008 and 2009 financial statements and the external auditor interim review report for the financial information regarding the six-month period ended June 30, 2010 are also attached to this Registration Document as Appendix A.
Stonefield Josephson, Inc.’s address is 2049 Century Park East, Suite 400, Los Angeles, CA 90067.
C-14
CHAPTER 3 SELECTED FINANCIAL INFORMATION
| 3.1 | Introduction |
The selected financial information contained in this Chapter 3 is taken from the Company’s financial statements for the periods ended December 31, 2009, 2008 and 2007 as well as the Company’s reports for the six-months periods ended June 30, 2010 and 2009 and for the nine-month periods ended September 30, 2010 and 2009.
The information in this Chapter must be read alongside the information provided in other parts of this Prospectus and in the documents incorporated by reference.
| 3.2 | Financial information selected from the condensed consolidated balance sheets for the years ended December 31, 2009, 2008 and 2007, for the six-month period ended June 30, 2010 and for the nine-month period ended September 30, 2010 |
| Year ended December 31, 2007 |
Year ended December 31, 2008 |
Year ended December 31, 2009 |
Six-month period ended June 30, 2010 |
Nine- month period ended September 30, 2010 |
||||||||||||||||
| (in thousands of U.S. dollars) |
||||||||||||||||||||
| ASSETS |
||||||||||||||||||||
| Current assets |
22,637 | 28,161 | 42,165 | 67,943 | 20,081 | |||||||||||||||
| Property and equipment, net |
6,025 | 4,324 | 3,430 | 3,507 | 3,114 | |||||||||||||||
| Goodwill |
17,064 | 17,064 | 17,064 | 17,064 | 17,064 | |||||||||||||||
| Other intangibles, net |
15,957 | — | — | — | — | |||||||||||||||
| Investment in joint venture |
— | 5,830 | — | — | — | |||||||||||||||
| Other assets |
11,830 | 8,864 | 6,936 | 6,064 | 6,354 | |||||||||||||||
| Total assets |
$ | 73,513 | $ | 64,243 | $ | 69,595 | $ | 94,578 | $ | 46,613 | ||||||||||
| LIABILITIES AND SHAREHOLDERS’ DEFICIT |
||||||||||||||||||||
| Current liabilities |
53,546 | 42,302 | 63,859 | 64,648 | 25,873 | |||||||||||||||
| Deferred revenue, less current portion |
398 | 319 | 239 | — | — | |||||||||||||||
| Long-term obligations, less current portion |
9,879 | 2,907 | 1,861 | 1,179 | 1,010 | |||||||||||||||
| Convertible notes |
117,579 | 142,373 | 21,779 | 11,880 | 11,985 | |||||||||||||||
| Preferred stock, no par value |
26,236 | 8,403 | — | — | — | |||||||||||||||
| Common stock purchase warrants |
— | — | 626 | 12,255 | 13,461 | |||||||||||||||
| Shareholders’ deficit: |
||||||||||||||||||||
| Series F preferred stock, no par value |
— | — | — | — | — | |||||||||||||||
| Common Stock, no par value |
979,295 | 1,188,071 | 1,418,931 | 1,539,510 | 1,545,324 | |||||||||||||||
| Accumulated other comprehensive income (loss) |
(4,007 | ) | (7,812 | ) | (8,412 | ) | (7,667 | ) | (8,159 | ) | ||||||||||
| Accumulated deficit |
(1,109,413 | ) | (1,312,320 | ) | (1,429,083 | ) | (1,526,919 | ) | (1,542,526 | ) | ||||||||||
| Total CTI shareholders’ deficit |
(134,125 | ) | (132,061 | ) | (18,564 | ) | 4,924 | (5,361 | ) | |||||||||||
| Noncontrolling interest |
— | — | (205 | ) | (308 | ) | (355 | ) | ||||||||||||
| Total shareholders’ deficit |
(134,125 | ) | (132,061 | ) | (18,769 | ) | 4,616 | (5,716 | ) | |||||||||||
| Total liabilities and shareholders’ deficit |
$ | 73,513 | $ | 64,243 | $ | 69,595 | $ | 94,578 | $ | 46,613 | ||||||||||
C-15
| 3.3 | Financial information selected from the condensed consolidated statements of operations for the years ended December 31, 2009, 2008 and 2007, for the six-month periods ended June 30, 2009 and 2010 and for the nine-month periods ended September 30, 2009 and 2010, including the disclosure of the EBITDA for the relevant financial periods |
| Year
ended December 31, 2007 (1) |
Year
ended December 31, 2008 (1) |
Year
ended December 31, 2009 (1) |
Six-month period ended June 30, 2009 (1) |
Six-month period ended June 30, 2010 (1) |
Nine-month period ended September 30, 2009 (1) |
Nine-month period ended September 30, 2010 (1) |
||||||||||||||||||||||
| (in thousands of U.S. dollars) | ||||||||||||||||||||||||||||
| Net Revenue |
$ | 127 | $ | 11,432 | $ | 80 | $ | 40 | $ | 319 | $ | 60 | $ | 319 | ||||||||||||||
| Operating expense (excl. depreciation and amortization) |
128,158 | 83,487 | 79,868 | 27,358 | 44,793 | 53,973 | 57,340 | |||||||||||||||||||||
| Loss from operations (excl. depreciation and amortization) |
(128,031 | ) | (72,055 | ) | (79,788 | ) | (27,318 | ) | (44,474 | ) | (53,913 | ) | (57,021 | ) | ||||||||||||||
| Other income (expenses), net |
9,627 | 42,460 | 2,851 | 10,013 | (3,068 | ) | 3,060 | (2,091 | ) | |||||||||||||||||||
| Preferred Stock: |
||||||||||||||||||||||||||||
| Gain on restructuring of Preferred Stock |
— | — | 2,116 | 2,116 | — | 2,116 | — | |||||||||||||||||||||
| - Beneficial conversion feature |
(9,549 | ) | (1,067 | ) | — | — | — | — | — | |||||||||||||||||||
| - Dividend |
(648 | ) | (662 | ) | (24 | ) | (24 | ) | — | (24 | ) | — | ||||||||||||||||
| - Deemed Dividends on Conversion of Preferred Stock |
— | (21,149 | ) | (23,460 | ) | (9,648 | ) | (47,434 | ) | (23,460 | ) | (50,519 | ) | |||||||||||||||
| EBITDA (2) |
(128,601 | ) | (52,473 | ) | (98,305 | ) | (24,861 | ) | (94,976 | ) | (72,221 | ) | (109,631 | ) | ||||||||||||||
| Depreciation and amortization |
(4,955 | ) | (5,228 | ) | (1,771 | ) | (948 | ) | (966 | ) | (1,424 | ) | (1,413 | ) | ||||||||||||||
| Make-whole interest expense |
(2,310 | ) | (70,243 | ) | (6,345 | ) | (6,345 | ) | — | (6,345 | ) | — | ||||||||||||||||
C-16
| Amortization of debt discount and issuance costs |
(4,280 | ) | (66,530 | ) | (5,788 | ) | (5,348 | ) | (434 | ) | (5,575 | ) | (600 | ) | ||||||||||||||
| Interest expense |
(8,237 | ) | (8,559 | ) | (4,806 | ) | (3,200 | ) | (1,563 | ) | (4,026 | ) | (1,948 | ) | ||||||||||||||
| Non controlling interest |
78 | 126 | 252 | 152 | 103 | 205 | 149 | |||||||||||||||||||||
| Net loss attributable to CTI common shareholders |
$ | (148,305 | ) | $ | (202,907 | ) | $ | (116,763 | ) | $ | (40,550 | ) | $ | (97,836 | ) | $ | (89,386 | ) | $ | (113,443 | ) | |||||||
| Basic and diluted net loss per common share |
$ | (32.75 | ) | $ | (7.00 | ) | $ | (0.25 | ) | $ | (0.11 | ) | $ | (0.15 | ) | $ | (0.21 | ) | $ | (0.17 | ) | |||||||
| Shares used in calculation of basic and diluted net loss per common share |
4,529 | 28,967 | 458,356 | 366,293 | 632,658 | 420,520 | 659,244 |
| (1) | The Company’s financial information was retroactively restated to reflect a one-for-four reverse stock split that occurred on April 15, 2007 and a one-for-ten reverse stock split that occurred on August 31, 2008. Shares outstanding and per share information has been adjusted to reflect these reverse stock splits. |
| (2) | Earnings before interest, taxes, depreciation and amortization (EBITDA) is a non-GAAP metric that can be used to evaluate a company’s profitability. EBITDA differs from the operating cash flow in a cash flow statement primarily by excluding payments for taxes or interest as well as changes in working capital. EBITDA also differs from free cash flow because it excludes cash requirements for replacing capital assets. |
| 3.4 | Net financial standing for the years ended December 31, 2009, 2008 and 2007, for the six-month period ended June 30, 2010 and for the nine-month period ended September 30, 2010 (3) |
| Net Financial Standing |
Year
ended December 31, 2007 |
Year
ended December 31, 2008 |
Year
ended December 31, 2009 |
Six-month period ended June 30, 2010 |
Nine-month period ended September 30, 2010 |
|||||||||||||||
| (in thousands of U.S. dollars) | ||||||||||||||||||||
| Cash and cash equivalents |
(15,798 | ) | (10,072 | ) | (37,811 | ) | (64,534 | ) | (17,268 | ) | ||||||||||
| Restricted cash |
— | (6,640 | ) | — | — | — | ||||||||||||||
| Securities available-for-sale (4) |
(2,548 | ) | (599 | ) | — | — | — | |||||||||||||
| Long term obligations, current portion |
1,020 | 757 | 1,312 | 784 | 812 | |||||||||||||||
| Convertible senior notes, |
10,158 | 10,187 | ||||||||||||||||||
| Convertible senior subordinate notes, current portion |
16,907 | — | 40,363 | 38,515 | ||||||||||||||||
| Convertible subordinate notes, current portion |
2,910 | — | — | — | — | |||||||||||||||
C-17
| Net Financial Standing, current portion |
2,491 | (16,554 | ) | 3,864 | (15,077 | ) | (6,269 | ) | ||||||||||||
| Convertible senior subordinated notes |
55,150 | 55,150 | — | — | — | |||||||||||||||
| Long term obligations, less current portion |
9,879 | 2,907 | 1,861 | 1,179 | 1,010 | |||||||||||||||
| Convertible senior notes, less current portion |
62,429 | 87,223 | 21,779 | 11,880 | 11,985 | |||||||||||||||
| Net Financial Standing, less current portion |
127,458 | 145,280 | 23,640 | 13,059 | 12,995 | |||||||||||||||
| Net Financial Indebtedness |
129,949 | 128,726 | 27,504 | (2,018 | ) | 6,726 | ||||||||||||||
| (3) | Not reviewed by the Company’s auditors. |
| (4) | Securities available-for-sale consist of the following debt securities (in thousands of U.S. dollars): |
| Year ended December 31, 2007 |
Year ended December 31, 2008 |
Year ended December 31, 2009 |
Six period ended June 30, 2010 |
Nine-month period ended September 30, 2010 |
||||||||||||||||
| Type of Security |
||||||||||||||||||||
| Corporate obligations |
1,000 | 599 | — | — | — | |||||||||||||||
| U.S.government obligations |
746 | — | — | — | — | |||||||||||||||
| Municipal obligations |
802 | — | — | — | — | |||||||||||||||
| Total |
2,548 | 599 | — | — | — | |||||||||||||||
As of December 31, 2008 and 2007, all securities available-for-sale had contractual maturities of less than one year. Gross realized gains and losses to date were not material. As of these dates there were no impairment provisions on securities available-for-sale and all securities available-for-sale were held in the United States and were denominated in U.S. dollars.
There were no securities outstanding as of December 31, 2009, as of June 30, 2010 and as of September 30, 2010. As of December 31, 2008, the securities outstanding had a fixed interest rate of 7.5%. As of December 31, 2007, the securities outstanding had fixed interest rates ranging from 2.3% to 7.0%.
C-18
| 3.5 | Financial information selected from the consolidated statements of cash flows for the years ended December 31, 2009, 2008 and 2007, for the six-month periods ended June 30, 2010 and 2009 and for the nine-month periods ended September 30, 2010 and 2009 |
| (in thousands of U.S. Dollars) |
Year
ended December 31, 2007 |
Year
ended December 31, 2008 |
Year
ended December 31, 2009 |
Six-month period ended June 30, 2009 |
Six-month period ended June 30, 2010 |
Nine-month period ended September 30, 2009 |
Nine-month period ended September 30, 2010 |
|||||||||||||||||||||
| Net cash used in operating activities |
(103,618 | ) | (80,207 | ) | (88,186 | ) | (50,705 | ) | (39,281 | ) | (70,644 | ) | (50,356 | ) | ||||||||||||||
| Net cash provided by (used in) investing activities |
21,477 | 4,408 | 21,840 | 22,244 | (1,042 | ) | 22,176 | (1,150 | ) | |||||||||||||||||||
| Net cash provided by financing activities |
84,700 | 73,726 | 94,775 | 30,707 | 65,707 | 94,202 | 30,616 | |||||||||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(3,890 | ) | (3,653 | ) | (690 | ) | (338 | ) | 1,339 | (814 | ) | 347 | ||||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
(1,331 | ) | (5,726 | ) | 27,739 | 1,908 | 26,723 | 44,920 | (20,543 | ) | ||||||||||||||||||
C-19
CHAPTER 4 RISK FACTORS
| 4.1 | RISK FACTORS RELATING TO THE ISSUER AND ITS GROUP |
THE REGISTRATION DOCUMENT DISCLOSES RISKS AND ESSENTIAL CHARACTERISTICS CONNECTED TO THE COMPANY. THE INVESTMENT IN FINANCIAL INSTRUMENTS OF THE COMPANY IMPLIES THE TYPICAL RISKS OF AN INVESTMENT IN FINANCIAL INSTRUMENTS. POTENTIAL INVESTORS SHOULD READ THE WHOLE REGISTRATION DOCUMENT AND THE SECURITIES NOTE FROM TIME TO TIME PUBLISHED.
FOR THE PURPOSES OF A CORRECT ASSESSMENT OF THE INVESTMENTS, INVESTORS ARE INVITED TO EVALUATE THE SPECIFIC RISK FACTORS RELATING TO THE COMPANY, TO ITS GROUP, TO THE INDUSTRY IN WHICH IT CARRIES OUT ITS ACTIVITIES AND TO THE ISSUANCE. THE RISK FACTORS BELOW SHOULD BE READ TOGETHER WITH THE OTHER INFORMATION INCLUDED IN THIS REGISTRATION DOCUMENT.
ANY REFERENCE TO CHAPTER AND PARAGRAPH SHALL BE UNDERSTOOD AS REFERENCE TO CHAPTERS AND PARAGRAPHS OF THIS REGISTRATION DOCUMENT.
| 4.1.1 | RISKS RELATED TO THE COMPANY’S ABILITY TO CONTINUE ITS OPERATIONS, TO ITS FINANCIAL SITUATION, TO FUTURE LOSSES AND TO HIGH COSTS FOR RAISING ADDITIONAL FUNDS |
THE COMPANY EXPECTS THAT ITS EXISTING CASH AND CASH EQUIVALENTS, SECURITIES AVAILABLE-FOR-SALE, INTEREST RECEIVABLE AS WELL AS PROCEEDS RECEIVED FROM THE COMPANY’S OFFERINGS TO DATE, INCLUDING THE ISSUANCE OF SHARES OF PREFERRED STOCK OF SERIES 7 AND RELATED WARRANTS, WILL NOT BE SUFFICIENT TO FUND THE COMPANY’S PRESENTLY ANTICIPATED OPERATIONS BEYOND FIRST QUARTER OF 2011.
THE COMPANY’S INDEPENDENT REGISTERED PUBLIC ACCOUNTING AUDITOR HAS INCLUDED AN EXPLANATORY PARAGRAPH IN ITS AUDIT REPORTS ON THE COMPANY’S DECEMBER 31, 2007, 2008 AND 2009 CONSOLIDATED FINANCIAL STATEMENTS AND IN ITS LIMITED REVIEW REPORT ON THE COMPANY’S FINANCIAL STATEMENTS CONCERNING THE SIX-MONTH PERIOD ENDED JUNE 30, 2010 WHERE, TAKEN INTO CONSIDERATION WHAT HAS BEEN REPORTED BY THE DIRECTORS IN THE RELEVANT ACCOUNTING DOCUMENTS AND THE COMPANY’S INABILITY TO DEMONSTRATE ITS ABILITY TO SATISFY THE MONETARY LIABILITIES DUE, IT RAISED SUBSTANTIAL DOUBT AS TO THE COMPANY’S ABILITY TO CONTINUE AS A GOING CONCERN. THIS MAY HAVE A NEGATIVE IMPACT ON THE TRADING PRICE OF THE COMPANY’S COMMON STOCK AND AS A RESULT, THE COMPANY MAY HAVE A DIFFICULT TIME OBTAINING NECESSARY FINANCING.
C-20
LOSSES, REVENUES AND DIVIDENDS
THE COMPANY WAS INCORPORATED IN 1991 AND HAS INCURRED A NET OPERATING LOSS EVERY YEAR. AS OF SEPTEMBER 30, 2010, THE COMPANY HAD AN ACCUMULATED NET DEFICIT OF APPROXIMATELY $1.543 BILLION1.
WITH RESPECT TO THE PERIODS TO WHICH THE HISTORIC FINANCIAL INFORMATION REPORTED IN THIS REGISTRATION DOCUMENT REFERS, THE COMPANY BORE THE FOLLOWING TOTAL NET LOSS ATTRIBUTABLE TO CTI COMMON SHAREHOLDERS: $148.3 MILLION IN THE YEAR 2007, $202.9 MILLION IN THE YEAR 2008, AND $116.8 MILLION IN THE YEAR 2009. WITH RESPECT TO SUCH AMOUNT OF TOTAL NET LOSS ATTRIBUTABLE TO CTI COMMON SHAREHOLDERS, THE PERCENTAGE OF REMUNERATIONS AND BENEFITS TO DIRECTORS AND THE NAMED EXECUTIVE OFFICERS (AS DEFINED IN THE FOLLOWING GLOSSARY) IS RESPECTIVELY: 3% FOR THE YEAR 2007, 2% FOR THE YEAR 2008, AND 36% FOR THE YEAR 2009. THE TOTAL AMOUNT OF REMUNERATION AND BENEFITS TO DIRECTORS AND THE NAMED EXECUTIVE OFFICERS IS DERIVED FROM INFORMATION PROVIDED IN OUR PROXY STATEMENTS PREPARED IN ACCORDANCE WITH THE APPLICABLE RULES AND REGULATIONS OF THE U.S. SECURITIES AND EXCHANGE COMMISSION AND IS NOT NECESSARILY INDICATIVE OF THE AMOUNT OF COMPENSATION AND BENEFITS RECORDED IN THE FINANCIAL STATEMENTS FOR SUCH PERIODS. PLEASE REFER TO THE FOLLOWING PARAGRAPH 15.1 FOR MORE INFORMATION.THE COMPANY IS PURSUING REGULATORY APPROVAL FOR PIXANTRONE, OPAXIO AND BROSTALLICIN. THE COMPANY WILL NEED TO CONDUCT RESEARCH, DEVELOPMENT, TESTING AND REGULATORY COMPLIANCE ACTIVITIES AND UNDERTAKE MANUFACTURING AND DRUG SUPPLY ACTIVITIES, EXPENSES WHICH, TOGETHER WITH PROJECTED GENERAL AND ADMINISTRATIVE EXPENSES, WILL RESULT IN OPERATING LOSSES FOR THE FORESEEABLE FUTURE. THE COMPANY’S ABILITY TO REACH PROFITABILITY IS DEPENDENT UPON ALL OF THESE FACTORS, AND THE COMPANY CANNOT PREDICT WHEN IT MAY REACH PROFITABILITY. FURTHER, THE COMPANY MAY NEVER BECOME PROFITABLE, EVEN IF IT IS ABLE TO COMMERCIALIZE PRODUCTS CURRENTLY IN DEVELOPMENT OR OTHERWISE.
THE COMPANY, FOLLOWING THE TRANSFER TO SPECTRUM PHARMACEUTICAL OF ITS INTEREST IN THE JOINT VENTURE RIT ONCOLOGY RELATED TO THE DRUG PHARMACEUTICAL PRODUCT ZEVALIN, OCCURRED IN MARCH 2009, DOES NOT CURRENTLY HAVE ANY PRODUCTS APPROVED FOR SALE IN THE MARKET. MOREOVER, THE COMPANY DOES NOT GENERATE REVENUES FROM ITS PRODUCTS AND MAY NOT BE ABLE TO REPAY ITS DEBT OR THE RELEVANT INTEREST, LIQUIDATED DAMAGES OR OTHER PAYMENTS WITH RESPECT TO ITS DEBT.
1 It is noted that, as of September 30, 2010, the negative accumulated deficit balance of $1.543 billion is offset by the positive value of the capital stock of the Company since inception of its business amounting to $1.545 billion as it can be observed in the table Consolidated balance sheets as of September 30, 2010 reported under subsequent Paragraph 20.6.2. Moreover, the aforesaid value of the capital stock of the Company (i.e. $1.545 billion) represents historical costs of capital stock transactions as they occurred since inception of its business.
C-21
THE COMPANY HAS NOT DECLARED OR PAID ANY DIVIDENDS ON SHARES OF COMMON STOCK AND DOES NOT CURRENTLY EXPECT TO DO SO IN THE FUTURE. FOR FURTHER INFORMATION ABOUT THE DIVIDEND POLICY PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.7 OF THIS REGISTRATION DOCUMENT.
OVERALL FACE VALUE OF OUTSTANDING NOTES OF THE COMPANY
THE COMPANY HAS A SUBSTANTIAL AMOUNT OF DEBT OUTSTANDING. THE AGGREGATE PRINCIPAL BALANCE OF THE COMPANY’S CONVERTIBLE NOTES AS OF SEPTEMBER 30, 2010 IS APPROXIMATELY $21 MILLION IN CONVERTIBLE NOTES WITH INTEREST RATES RANGING FROM 5.75% TO 7.5%. FOR FURTHER INFORMATION ABOUT THE CONVERTIBLE NOTES OF THE COMPANY PLEASE REFER TO CHAPTER 10, PARAGRAPH 10.3.3 OF THIS REGISTRATION DOCUMENT.
COMPANY BURN RATE
THE COMPANY ESTIMATES ITS AVERAGE CASH BURN-RATE FOR THE REMAINDER OF 2010 TO BE ABOUT $5 MILLION PER MONTH. NEVERTHELESS, THE COMPANY IS REVIEWING ALTERNATIVE OPTIONS TO REDUCE ITS BURN RATE.
COMPANY’S ABILITY TO RAISE ADDITIONAL FUNDS
CTI HAS SUBSTANTIAL OPERATING EXPENSES ASSOCIATED WITH THE DEVELOPMENT OF ITS PRODUCT CANDIDATES AND AS OF SEPTEMBER 30, 2010 CTI HAD APPROXIMATELY $17.3 MILLION IN CASH AND CASH EQUIVALENTS AND ITS TOTAL CURRENT LIABILITIES WERE APPROXIMATELY $25.9 MILLION.
TO CONTINUE ITS OPERATIONS THE COMPANY NEEDS TO RAISE ADDITIONAL FUNDS AND EXPECTS THAT IT WILL NEED TO CONTINUE TO RAISE FUNDS IN THE FUTURE, AND ADDITIONAL FUNDS MAY NOT BE AVAILABLE ON ACCEPTABLE TERMS, OR AT ALL; THE FAILURE TO RAISE SIGNIFICANT ADDITIONAL FUNDS MAY CAUSE THE COMPANY TO CEASE DEVELOPMENT OF OUR PRODUCTS AND OPERATIONS.
SINCE THE COMPANY CURRENTLY DOES NOT HAVE FINANCIAL RESOURCES SUFFICIENT TO FUND ITS OPERATIONS BEYOND FIRST QUARTER OF 2011, IT NEEDS TO RAISE ADDITIONAL CAPITAL. THE COMPANY MAY SEEK TO RAISE SUCH CAPITAL THROUGH PUBLIC OR PRIVATE EQUITY FINANCINGS, PARTNERSHIPS, JOINT VENTURES, DISPOSITIONS OF ASSETS, DEBT FINANCINGS OR RESTRUCTURINGS, BANK BORROWINGS OR OTHER SOURCES. HOWEVER, ADDITIONAL FUNDING MAY NOT BE AVAILABLE ON FAVORABLE TERMS OR AT ALL. IF ADEQUATE FUNDS ARE NOT OTHERWISE AVAILABLE, THE COMPANY WILL FURTHER CURTAIL OPERATIONS SIGNIFICANTLY, INCLUDING THE DELAY, MODIFICATION OR CANCELLATION OF OPERATIONS AND PLANS RELATED TO PIXANTRONE, OPAXIO AND BROSTALLICIN, AND MAY BE FORCED TO CEASE OPERATIONS, LIQUIDATE OUR ASSETS AND POSSIBLY SEEK BANKRUPTCY PROTECTION. A BANKRUPCY MAY RESULT IN THE TERMINATION OF THE AGREEMENTS PURSUANT TO WHICH THE COMPANY LICENSE CERTAIN INTELLECTUAL PROPERTY RIGHTS, INCLUDING THE RIGHTS TO PIXANTRONE, OPAXIO AND BROSTALLICIN. TO OBTAIN ADDITIONAL FUNDING, THE COMPANY MAY NEED TO ENTER INTO ARRANGEMENTS THAT REQUIRE THE COMPANY TO RELINQUISH RIGHTS TO CERTAIN TECHNOLOGIES, DRUG CANDIDATES, PRODUCTS AND/OR POTENTIAL MARKETS, SUCH AS
C-22
THE COMPANY’S TRANSFER OF ZEVALIN ASSETS TO RIT ONCOLOGY AND ITS SUBSEQUENT SALE OF OUR 50% INTEREST IN RIT ONCOLOGY. IN ADDITION, SOME FINANCING ALTERNATIVES MAY REQUIRE THE COMPANY TO MEET ADDITIONAL REGULATORY REQUIREMENTS IN ITALY AND THE UNITED STATES, WHICH MAY INCREASE THE COMPANY’S COSTS AND ADVERSELY AFFECT ITS ABILITY TO OBTAIN FINANCING. TO THE EXTENT THAT ADDITIONAL CAPITAL IS RAISED THROUGH THE SALE OF EQUITY, OR SECURITIES CONVERTIBLE INTO EQUITY, SHAREHOLDERS MAY EXPERIENCE DILUTION OF THEIR PROPORTIONATE OWNERSHIP OF THE COMPANY.
MAIN PRODUCTS, CONNECTED REGULATORY APPROVALS FOR THEIRMANUFACTURE AND MARKETING AND RESEARCH AND DEVELOPMENT EXPENSES
OBTAINING REGULATORY APPROVAL TO MARKET DRUGS TO TREAT CANCER IS EXPENSIVE, DIFFICULT AND RISKY, AS FURTHER DESCRIBED IN RISK FACTOR 4.2.5 BELOW. IF CTI’S PRODUCTS ARE NOT APPROVED QUICKLY ENOUGH TO PROVIDE NET REVENUES TO DEFRAY ITS DEBT AND OPERATING EXPENSES, ITS BUSINESS AND FINANCIAL CONDITION MAY BE ADVERSELY AFFECTED.
C-23
WITH RESPECT TO OPAXIO, THE COMPANY ON THE ONE HAND ON SEPTEMBER 21, 2009, NOTIFIED THE EMA ITS DECISION TO WITHDRAW ITS MARKETING AUTHORIZATION APPLICATION (MAA) FOR A NON-INFERIORITY INDICATION IN NON-SMALL CELL LUNG CANCER (NSCLC), ON THE OTHER HAND, (AS REPORTED IN THE SCHEDULE “CTI’S ONGOING CLINICAL TRIALS” THAT CAN BE CONSULTED UNDER PARAGRAPH 6.1.1 OF THIS REGISTRATION DOCUMENT) IS PRESENTLY CARRYING ON PHASE III TRIALS FOR THE TREATMENT OF OVARIAN CANCER FOR WOMEN WHO HAVE PRE-MENOPAUSAL ESTROGEN LEVELS.
WITH RESPECT TO PIXANTRONE:
(I) ON APRIL 9, 2010, THE FDA GAVE NOTICE TO THE COMPANY OF THE REJECTION OF THE NDA FOR PIXANTRONE AND RECOMMENDED THAT THE COMPANY CONDUCT AN ADDITIONAL CLINICAL TRIAL TO DEMONSTRATE THE SAFETY AND EFFECTIVENESS OF PIXANTRONE. THE COMPANY DOES NOT KNOW WHAT THIS TRIAL WILL COST OR WHETHER THE FDA WILL ACCEPT OUR SPA FOR THIS TRIAL;
(II) ON NOVEMBER 1, 2010 THE COMPANY SUBMITTED A MARKETING AUTHORIZATION APPLICATION (MAA) TO THE EUROPEAN MEDICINES AGENCY (EMA) FOR PIXUVRI (PIXANTRONE DIMALEATE) AS MONOTHERAPY FOR THE TREATMENT OF ADULT PATIENTS WITH MULTIPLE RELAPSED OR REFRACTORY AGGRESSIVE NON-HODGKIN’S LYMPHOMA (NHL). THE APPLICATION SUBMISSION FOLLOWED A POSITIVE OPINION FROM THE EMA’S PEDIATRIC COMMITTEE (PDCO), WHERE PDCO AGREED TO THE COMPANY’S PEDIATRIC INVESTIGATION PLAN (PIP) FOR PIXUVRI. MOREOVER ON NOVEMBER 17, 2010 THE EMA APPROVED THE AFORESAID PIP. AS OF THE DATE OF THIS REGISTRATION DOCUMENT THE COMPANY CAN NOT ANTICIPATE THE DURATION OF SUCH MARKETING AUTHORIZATION PROCEEDINGS OR WHETHER THE MAA WILL RECEIVE APPROVAL IN THE NEXT 12 MONTHS.
FOR FURTHER INFORMATION WITH RESPECT TO THE RESEARCH AND DEVELOPMENT PHASES AND THE AUTHORIZATION PROCEEDINGS OF THE CANDIDATE PRODUCTS OF THE COMPANY, PLEASE REFER TO CHAPTER 6.1 OF THIS REGISTRATION DOCUMENT.
DURING THE LAST THREE YEAR PERIOD AND THE NINE-MONTH PERIOD ENDING IN SEPTEMBER 30, 2010, THE COMPANY HAS DECREASED ITS RESEARCH AND DEVELOPMENT EXPENSES AS FOLLOWS, SPECIFICALLY FROM $72.019 MILLION IN THE FINANCIAL YEAR 2007 TO $51.614 MILLION IN THE FINANCIAL YEAR 2008, $30.179 MILLION IN THE FINANCIAL YEAR 2009 AND LASTLY, TO $19.375 MILLION IN THE NINE-MONTH PERIOD FROM DECEMBER 31, 2009 TO SEPTEMBER 30, 2010.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTERS 6, 9, 10, 20 AND 22 OF THIS REGISTRATION DOCUMENT. PLEASE ALSO REFER TO RISK FACTORS 4.1.5 OF THIS REGISTRATION DOCUMENT.
| 4.1.2 | RISKS RELATED TO A SUBSTANTIAL AMOUNT OF DEBT AND SHAREHOLDER DEFICIT |
THE COMPANY HAS A SUBSTANTIAL AMOUNT OF DEBT OUTSTANDING, AND ITS ANNUAL INTEREST EXPENSE WITH RESPECT TO ITS DEBT IS SIGNIFICANT. THE COMPANY NEEDS TO RAISE SUBSTANTIAL ADDITIONAL CAPITAL TO CONTINUE TO FUND ITS OPERATIONS AS ITS CURRENT CASH RESOURCES, INCLUDING THE PROCEEDINGS RELATED TO THE ISSUANCE OF SHARES OF PREFERRED STOCK OF SERIES 7 AND RELATED WARRANTS, WOULD NOT FUND
C-24
THE COMPANY BEYOND FIRST QUARTER OF 2011. UNLESS THE COMPANY RAISES SUBSTANTIAL ADDITIONAL CAPITAL AND REDUCES ITS OPERATING EXPENSES, IT WILL NOT BE ABLE TO PAY ITS OPERATING EXPENSES OR REPAY ITS DEBT OR THE INTEREST, LIQUIDATED DAMAGES OR OTHER PAYMENTS WITH RESPECT TO ITS DEBT.
THE CONSOLIDATED NET FINANCIAL STANDING OF THE GROUP AS OF SEPTEMBER 30, 2010 IS NEGATIVE $6.726 MILLION AS COMPARED TO THE CONSOLIDATED ASSETS AND LIABILITIES OF THE GROUP OF $46.6 MILLION AND $38.9 MILLION, RESPECTIVELY, AS OF SEPTEMBER 30, 2010.
THE CONSOLIDATED NET FINANCIAL STANDING OF THE GROUP AS OF DECEMBER 31, 2009 AND THE NINE MONTHS ENDED SEPTEMBER 30, 2010 AMOUNTS TO A NEGATIVE VALUE OF RESPECTIVELY 27,504 AND 6,726 THOUSANDS OF US DOLLARS.
AS OF DECEMBER 31, 2009 AND SEPTEMBER 30, 2010, CTI HAD A TOTAL SHAREHOLDER DEFICIT OF APPROXIMATELY $18.769 MILLION AND $5.716 MILLION RESPECTIVELY. THERE ARE NO PROVISIONS UNDER US LAW THAT GOVERN THE INTEGRITY OF THE COMPANY’S SHARE CAPITAL AND THEREFORE, THE COMPANY’S SHAREHOLDER DEFICIT MAY CONTINUE AND MAY INCREASE WITHOUT THE NEED FOR SHARE CAPITAL TO BE ADJUSTED.
AS INDICATED IN 4.1.1, CTI WAS INCORPORATED IN 1991 AND HAS INCURRED A NET OPERATING LOSS EVERY YEAR. AS OF SEPTEMBER 30, 2010, CTI HAD AN ACCUMULATED NET DEFICIT OF APPROXIMATELY $1.5 BILLION. REVENUES FOR THE NINE MONTH PERIOD ENDED SEPTEMBER 30, 2010 AND 2009 IN THE AMOUNTS OF 319 AND 60 THOUSANDS OF US DOLLARS, RESPECTIVELY, WHILE INTEREST EXPENSE FOR THE SAME PERIODS WAS 1,948 AND 4,026 THOUSANDS OF US DOLLARS, RESPECTIVELY.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTERS 10 AND 20 OF THIS REGISTRATION DOCUMENT.
| 4.1.3 | RISKS RELATED TO THE NEED TO REDUCE EXPENSES |
THE COMPANY NEEDS TO RAISE SUBSTANTIAL ADDITIONAL CAPITAL TO FUND ITS OPERATIONS. IF THE COMPANY IS UNABLE TO SECURE ADDITIONAL FINANCING ON ACCEPTABLE TERMS IN THE NEAR FUTURE, THE COMPANY EXPECTS TO IMPLEMENT A NUMBER OF COST REDUCTION INITIATIVES, SUCH AS FURTHER REDUCTIONS IN THE COST OF THE COMPANY’S WORKFORCE AND THE DISCONTINUATION OF A NUMBER OF BUSINESS INITIATIVES TO FURTHER REDUCE ITS RATE OF CASH UTILIZATION AND EXTEND THE COMPANY’S EXISTING CASH BALANCES. THE COMPANY BELIEVES THAT THESE ADDITIONAL COST REDUCTION INITIATIVES, IF UNDERTAKEN, WILL PROVIDE IT WITH ADDITIONAL TIME TO CONTINUE ITS PURSUIT OF ADDITIONAL FINANCING SOURCES AND STRATEGIC ALTERNATIVES. IN THE EVENT THE COMPANY IS UNABLE TO OBTAIN FINANCING ON ACCEPTABLE TERMS AND REDUCE ITS EXPENSES, IT MAY BE REQUIRED TO LIMIT OR CEASE OPERATIONS, PURSUE A PLAN TO SELL ITS OPERATING ASSETS OR OTHERWISE MODIFY ITS BUSINESS STRATEGY, WHICH COULD MATERIALLY HARM ITS FUTURE BUSINESS PROSPECTS.
C-25
| 4.1.4 | RISKS RELATING TO EXECUTIVE COMPENSATION |
THE FOLLOWING TABLE SETS FORTH INFORMATION CONCERNING COMPENSATION EARNED FOR SERVICES RENDERED TO THE COMPANY BY THE CHIEF EXECUTIVE OFFICER, THE CHIEF FINANCIAL OFFICER, AND THE COMPANY’S NEXT FOUR MOST HIGHLY COMPENSATED EXECUTIVE OFFICERS FOR FISCAL YEAR 2009, 2008 AND 2007, INCLUDING MR. STROMATT WHO WAS NOT SERVING AS AN EXECUTIVE OFFICER OF THE COMPANY AS OF DECEMBER 31, 2008, NOTWITHSTANDING THE LACK OF ANY POSITIVE ECONOMICAL OUTCOME OBTAINED BY THE COMPANY FROM THE DATE OF ITS INCORPORATION TO THE DATE OF THIS REGISTRATION DOCUMENT.
| Name |
Principal position |
Fiscal Year |
Total compensation($) | |||
| James A. Bianco, M.D. |
Chief Executive Officer | 2009 2008 2007 |
12,592,030 1,506,156 2,197,804 | |||
| Louis A. Bianco |
Chief Financial Officer | 2009 2008 2007 |
5,059,961 539,972 757,816 | |||
| Dan Eramian |
Executive Vice President | 2009 2008 2007 |
3,879,210 485,768 697,793 | |||
| Craig W. Philips |
President of the Company | 2009 2008 |
7,423,518 405,147 | |||
| Jack W. Singer, M.D. |
Executive Vice President | 2009 2008 2007 |
5,011,602 568,248 811,063 | |||
| Scott Stromatt, M.D. |
Executive Vice President | 2008 2007 |
435,711 532,802 | |||
THE TOTAL COMPENSATION AMOUNTS DISCLOSED IN THE ABOVE TABLE FOR EACH FISCAL YEAR INCLUDES CASH COMPENSATION AND EQUITY COMPENSATION FOR EACH EXECUTIVE. FOR THESE PURPOSES, EQUITY COMPENSATION IS INCLUDED USING THE GRANT DATE FAIR VALUE OF EQUITY AWARDS GRANTED TO THE EXECUTIVE IN THE FISCAL YEAR AS DETERMINED IN ACCORDANCE WITH UNITED STATES GENERALLY ACCEPTED ACCOUNTING PRINCIPLES USED TO CALCULATE THE VALUE OF EQUITY AWARDS FOR PURPOSES OF THE COMPANY’S FINANCIAL STATEMENTS.
C-26
WITH SPECIFIC REFERENCE TO FISCAL YEAR 2009, IT IS NOTED THAT THE PERCENTAGE OF TOTAL COMPENSATION AND BENEFITS PAID TO THE COMPANY’S DIRECTORS AND THE NAMED EXECUTIVE OFFICERS (AS DEFINED IN THE GLOSSARY OF THIS REGISTRATION DOCUMENT) WITH RESPECT TO THE TOTAL NET LOSS ATTRIBUTABLE TO CTI COMMON SHAREHOLDERS RECORDED IN SUCH FISCAL YEAR (AMOUNTING TO $116.8 MILLION) IS EQUAL TO 36%. IN THIS REGARD IT IS SPECIFIED THAT THE AFORESAID TOTAL AMOUNT OF COMPENSATION AND BENEFITS PAID TO DIRECTORS AND THE NAMED EXECUTIVE OFFICERS FOR THE FISCAL YEAR 2009 IS DERIVED FROM INFORMATION PROVIDED IN THE COMPANY’S PROXY STATEMENTS PREPARED IN ACCORDANCE WITH THE APPLICABLE RULES AND REGULATIONS OF THE U.S. SECURITIES AND EXCHANGE COMMISSION AND IS NOT NECESSARILY INDICATIVE OF THE AMOUNT OF COMPENSATION AND BENEFITS RECORDED IN THE FINANCIAL STATEMENTS FOR SUCH PERIOD OR, IN THE CASE OF EQUITY COMPENSATION, THE VALUE THAT WILL ACTUALLY BE REALIZED BY THE DIRECTOR OR EXECUTIVE OFFICER (DUE TO THE VESTING REQUIREMENTS OF AWARDS THAT MAY RESULT IN THEIR BEING FORFEITED OR FLUCTUATIONS IN STOCK PRICE THAT MAY AFFECT THE ULTIMATE VALUE OF THE AWARD).
THE COMPANY HAS HISTORICALLY ATTRACTED AND RETAINED, AND CONTINUES TO ATTRACT AND RETAIN, KEY EMPLOYEES THROUGH COMPENSATION ARRANGEMENTS AS APPROVED AS DESCRIBED IN CHAPTER 15 OF THIS REGISTRATION DOCUMENT, WHICH ALSO INCLUDES A DESCRIPTION OF THE COMPANY’S COMPENSATION ARRANGEMENTS WITH SENIOR MANAGEMENT IN THE PAST THREE YEARS. IN PARTICULAR, THE COMPANY INTERNALLY ESTABLISHED A COMPENSATION COMMITTEE COMPOSED BY THREE INDEPENDENT DIRECTORS THAT EVALUATE AND APPROVE THE COMPENSATION ARRANGEMENTS FOR THE EXECUTIVE OFFICERS AFTER (I) RETAINING EXTERNAL CONSULTANTS TO PROVIDE RECOMMENDATIONS ABOUT COMPARABLE MARKET PRACTICES; (II) EVALUATING, ALSO WITH THE ASSISTANCE OF SUCH CONSULTANTS, THE COMPENSATION GRANTED TO THE MANAGERS OF COMPANIES COMPARABLE TO CTI; AND (III) CONSIDERING SPECIFIC PARAMETERS SUCH AS THE PERFORMANCE OF THE COMPANY.
WITH SPECIFIC RESPECT TO THIS LAST PARAMETER, IT HAS TO BE CONSIDERED THAT ANNUAL CASH INCENTIVES FOR OUR EXECUTIVE OFFICERS ARE DESIGNED TO REWARD PERFORMANCE FOR ACHIEVING KEY CORPORATE GOALS, WHICH THE COMPANY BELIEVES IN TURN SHOULD INCREASE SHAREHOLDER VALUE. THE MAIN PERFORMANCE METRICS AGAINST WHICH THE EXECUTIVES ARE EVALUATED BY THE COMPENSATION COMMITTEE ARE (I) THE FINANCIAL PERFORMANCE OF THE COMPANY, (II) THE DRUG DEVELOPMENT AND (III) THE INDIVIDUAL EXECUTIVE’S PERFORMANCE.
MORE IN PARTICULAR AND AT AN ANALYTICAL LEVEL, IT IS NOTED THAT THE MAIN PARAMETERS TAKEN INTO ACCOUNT BY THE COMPENSATION COMMITTEE FOR THE DETERMINATION OF CASH INCENTIVES PAID TO THE COMPANY’S EXECUTIVE OFFICERS IN 2010 (FOR THE ACTIVITIES PERFORMED IN 2009) WERE: (I) THE POSSIBLE EXECUTION OF A LICENSE AGREEMENT FOR PIXANTRONE; (II) THE COMPLETENESS OF THE NEW DRUG APPLICATION (NDA) SUBMISSION FOR THE SAME DRUG; (III) THE RAISING OF OPERATING CAPITAL; AND (IV) THE PERCENTAGE OF THE COMPANY’S THEN-OUTSTANDING NOTES DUE IN 2010-2011 THAT WERE TENDERED IN THE COMPANY’S PUBLICLY-REGISTERED TENDER OFFERS DESCRIBED UNDER SUBSEQUENT PARAGRAPH 21.1.4. MOREOVER IT IS NOTED THAT A
C-27
LOWER SIGNIFICANCE WAS ASSUMED BY THE PARAMETERS LINKED TO THE INDIVIDUAL PERFORMANCE AND THE P&L RESULTS OF THE COMPANY
IN LIGHT OF THE GENERAL CURRENT ECONOMIC CLIMATE, THE COMPANY’S COMPENSATION PHILOSOPHY AND OBJECTIVES FOR FISCAL 2009 CONTINUED TO FOCUS HEAVILY ON RETENTION OF THE COMPANY’S SENIOR MANAGEMENT TEAM THROUGH THIS CHALLENGING TIME WHILE FURTHER LINKING MANAGEMENT’S POTENTIAL REWARDS WITH SHAREHOLDER VALUE. IN 2010, THE COMPANY’S SHAREHOLDERS AND BOARD OF DIRECTORS APPROVED AN AMENDMENT TO THE COMPANY’S 2007 EQUITY INCENTIVE PLAN, AS AMENDED AND RESTATED (THE “2007 EQUITY PLAN”) THAT INCREASED THE NUMBER OF SHARES OF THE COMPANY’S COMMON STOCK THAT MAY BE DELIVERED PURSUANT TO AWARDS GRANTED UNDER THE 2007 EQUITY PLAN BY AN ADDITIONAL 40,000,000 SHARES.
MOREOVER IT IS OBSERVED THAT THE COMPANY’S PROFITABILITY AND FINANCIAL PERFORMANCE COULD DECREASE DUE TO SEVERANCE PAYMENTS THE COMPANY MAY OWE SUCH MANAGEMENT PERSONNEL, NOTWITHSTANDING THE LACK OF ANY POSITIVE ECONOMICAL OUTCOME OBTAINED BY THE COMPANY FROM THE DATE OF ITS INCORPORATION TO THE DATE OF THIS REGISTRATION DOCUMENT, UPON THE EMPLOYEES TERMINATION OR EXIT FROM THE COMPANY PURSUANT TO THE COMPANY’S EMPLOYMENT CONTRACTS.
NOTE THAT THE COMPANY, ITS BOARD OF DIRECTORS, AND THE COMPENSATION COMMITTEE OF ITS BOARD OF DIRECTORS HAVE THE DISCRETION TO, AND MAY, CHANGE THE COMPANY’S COMPENSATION PRACTICES IN THE FUTURE. THESE CHANGES MAY RELATE TO CHANGES IN THE COMPANY’S PERFORMANCE, CHANGES IN LAW, RULES OR REGULATIONS, CHANGES IN COMPENSATION PRACTICES GENERALLY OR AT COMPANIES THAT COMPETE WITH THE COMPANY (GENERALLY OR WITH RESPECT TO HIRING AND RETAINING DIRECTORS, OFFICERS, AND EMPLOYEES), CHANGES IN COMPANY FOCUS OR GOALS, THE TERMINATION OR EXPIRATION OF EXISTING COMPENSATION ARRANGEMENTS, THE NEED TO HIRE NEW OR RETAIN EXISTING DIRECTORS, OFFICERS OR EMPLOYEES, OR ANY OTHER FACTS OR CIRCUMSTANCES THAT THE COMPANY, ITS BOARD OF DIRECTORS OR THE COMPENSATION COMMITTEE OF ITS BOARD OF DIRECTORS MAY DETERMINE TO BE APPROPRIATE.
FOR FURTHER INFORMATION PLEASE REFER TO CHAPTER 15, PARAGRAPH 15.1, OF THIS REGISTRATION DOCUMENT.
| 4.1.5 | RISKS RELATED TO THE LICENSE AND CO-DEVELOPMENT AGREEMENT ENTERED INTO WITH NOVARTIS PHARMACEUTICAL COMPANY LTD. |
ON SEPTEMBER 15, 2006, THE COMPANY ENTERED INTO A LICENSE AND CO-DEVELOPMENT AGREEMENT RELATED TO OPAXIO AND PIXANTRONE WITH NOVARTIS PURSUANT TO WHICH THE COMPANY GRANTED TO NOVARTIS AN EXCLUSIVE WORLDWIDE LICENSE FOR THE DEVELOPMENT AND COMMERCIALIZATION OF OPAXIO AND AN OPTION TO ENTER INTO AN EXCLUSIVE WORLDWIDE LICENCE TO DEVELOP AND COMMERCIALIZE PIXATRONE ON AGREED UPON TERMS.
IF NOVARTIS DOES NOT ELECT TO EXERCISE ITS OPTION WITH REGARD TO PIXANTRONE OR TO PARTICIPATE IN THE DEVELOPMENT AND COMMERCIALIZATION OF OPAXIO (AS
C-28
DESCRIBED IN RISK FACTOR 4.1.6 OF THIS REGISTRATION DOCUMENT), THE COMPANY MAY NOT BE ABLE TO FIND ANOTHER SUITABLE PARTNER FOR THE COMMERCIALIZATION AND DEVELOPMENT OF THOSE PRODUCTS, WHICH MAY HAVE AN ADVERSE EFFECT ON THE COMPANY’S ABILITY TO BRING THOSE DRUGS TO MARKET. IN ADDITION, THE COMPANY WOULD NEED TO OBTAIN A RELEASE FROM NOVARTIS PRIOR TO ENTERING INTO ANY AGREEMENT TO DEVELOP AND COMMERCIALIZE PIXANTRONE OR OPAXIO WITH A THIRD PARTY. THE COMPANY MAY NEVER RECEIVE THE NECESSARY REGULATORY APPROVALS AND THE COMPANY’S PRODUCTS MAY NOT REACH THE NECESSARY SALES LEVELS TO GENERATE ROYALTY OR MILESTONE PAYMENTS EVEN IF NOVARTIS ELECTS TO EXERCISE ITS OPTION WITH REGARD TO PIXANTRONE OR TO PARTICIPATE IN THE DEVELOPMENT AND COMMERCIALIZATION OF OPAXIO.
FOR MORE INFORMATION CONCERNING THE ABOVE MENTIONED AGREEMENT, PLEASE REFER TO CHAPTER 22, PARAGRAPH 22.1.1 OF THIS REGISTRATION DOCUMENT.
| 4.1.6 | RISKS RELATED TO DILUTION RESULTING FROM ISSUANCES OF NEW COMMON SHARES PURSUANT TO THE SECURITIES NOTES WHICH WILL BE DRAFTED, TOGETHER WITH THE RELEVANT SUMMARIES AND THE POSSIBLE UPDATED INFORMATION, ACCORDING TO THIS REGISTRATION DOCUMENT |
TO THE EXTENT THAT THE COMPANY RAISES ADDITIONAL CAPITAL THROUGH THE SALE OF NEW COMMON SHARES PURSUANT TO AN ISSUANCE IN CONNECTION WITH THE SECURITIES NOTES WHICH WILL BE DRAFTED, TOGETHER WITH THE RELEVANT SUMMARIES AND THE POSSIBLE UPDATED INFORMATION, ACCORDING TO THIS REGISTRATION DOCUMENT, THE OWNERSHIP INTEREST OF THE EXISTING COMPANY’S SHAREHOLDERS MAY BE DILUTED, AS THE CURRENT SHAREHOLDERS OF THE COMPANY DO NOT POSSESS ANY RIGHT TO SUBSCRIBE TO NEW SHARES ISSUANCES THAT MAY BE OFFERED TO OTHER INVESTORS, AND THE PRICE OF THE NEW COMMON SHARES SOLD TO SUBSCRIBERS IN SUCH AN ISSUANCE MAY BE AT A PRICE THAT IS DISCOUNTED COMPARED TO THE MARKET VALUE OF THE STOCK.
| 4.1.7 | RISKS RELATED TO MAINTAINING THE LISTING OF THE COMPANY’S COMMON STOCK ON THE NASDAQ CAPITAL MARKET |
EFFECTIVE WITH THE OPENING OF TRADING ON JANUARY 8, 2009, THE U.S. LISTING OF CTI’S COMMON STOCK WAS TRANSFERRED TO THE NASDAQ CAPITAL MARKET. ON MAY 3, 2010 THE COMPANY RECEVEID NOTICE FROM NASDAQ INDICATING THAT FOR THE LAST 30 CONSECUTIVE BUSINESS DAYS THE CLOSING BID PRICE OF COMPANY’S COMMON STOCK WAS BELOW THE MINIMUM $1.00 PER SHARE REQUIREMENT FOR CONTINUED LISTING OF THE COMPANY’S COMMON STOCK ON THE NASDAQ CAPITAL MARKET. THIS NOTIFICATION HAS NO IMMEDIATE EFFECT ON THE LISTING OF OR THE ABILITY TO TRADE THE COMPANY’S COMMON STOCK ON THE NASDAQ CAPITAL MARKET. IN ACCORDANCE WITH NASDAQ MARKETPLACE RULE 5810(C)(3)(A), THE COMPANY WAS PROVIDED A GRACE PERIOD OF 180 CALENDAR DAYS, OR UNTIL NOVEMBER 1, 2010, TO REGAIN COMPLIANCE. ALTHOUGH THE COMPANY DID NOT REGAIN COMPLIANCE WITHIN THE NOVEMBER 1, 2010, ON NOVEMBER 3, 2010 THE NASDAQ GRANTED A FURTHER GRACE PERIOD OF 180 CALENDAR DAYS. THE COMPANY WILL THEREFORE ACHIEVE COMPLIANCE IF THE BID PRICE OF OUR COMMON STOCK CLOSES AT $1.00 PER SHARE OR MORE FOR A MINIMUM OF TEN CONSECUTIVE TRADING DAYS BEFORE MAY 2, 2011. IF THE COMPANY IS UNABLE TO ATTAIN COMPLIANCE
C-29
WITH THE MINIMUM BID PRICE, WHETHER BY EFFECTING A REVERSE STOCK SPLIT OF OUR COMMON STOCK OR OTHERWISE, THE COMPANY MAY BE DELISTED.
IN ADDITION, EVEN THOUGH THE COMPANY CONTINUES TO BE LISTED ON THE NASDAQ CAPITAL MARKET, TRADING IN ITS COMMON STOCK MAY BE HALTED OR SUSPENDED DUE TO MARKET CONDITIONS OR IF NASDAQ, CONSOB OR THE BORSA ITALIANA DETERMINES THAT TRADING IN ITS COMMON STOCK IS INADVISABLE (FOR EXAMPLE, THE TRADING OF THE COMPANY’S STOCK WAS HALTED BOTH BY BORSA ITALIANA AND NASDAQ ON FEBRUARY 10,2009 AND MARCH 23, 2009).
IF CTI’S COMMON STOCK CEASES TO BE LISTED FOR TRADING ON NASDAQ, THE MTA, OR BOTH FOR ANY REASON OR IF TRADING IN CTI’S STOCK IS HALTED OR SUSPENDED ON NASDAQ CAPITAL MARKET, THE MTA, OR BOTH, IT MAY HARM CTI’S STOCK PRICE, INCREASE THE VOLATILITY OF ITS STOCK PRICE AND MAKE IT MORE DIFFICULT FOR INVESTORS TO BUY OR SELL SHARES OF THE COMPANY COMMON STOCK. MOREOVER, IF THE COMPANY’S COMMON STOCK CEASES TO BE LISTED FOR TRADING ON NASDAQ OR IF TRADING IN ITS STOCK IS HALTED OR SUSPENDED ON NASDAQ, THE COMPANY MAY BECOME SUBJECT TO OBLIGATIONS TO REDEEM CERTAIN SHARES OF PREFERRED STOCK AT A PREMIUM AND/OR REPAY ON AN ACCELERATED BASIS CERTAIN CONVERTIBLE NOTES. IN ADDITION, IF THE COMPANY IS NOT LISTED ON NASDAQ AND/OR IF ITS PUBLIC FLOAT FALLS BELOW $75 MILLION (THAT IS THE AMOUNT ESTABLISHED BY SEC RULES FOR A COMPANY TO BE ELIGIBLE TO FILE SHELF REGISTRATION STATEMENTS ON SEC FORM S-3), THE COMPANY WILL BE LIMITED IN ITS ABILITY TO FILE NEW SHELF REGISTRATION STATEMENTS ON SEC FORM S-3 AND/OR TO FULLY USE ONE OR MORE REGISTRATION STATEMENTS ON SEC FORM S-3. THE COMPANY HAS RELIED SIGNIFICANTLY ON SHELF REGISTRATION STATEMENTS ON SEC FORM S-3 FOR MOST OF ITS FINANCINGS IN RECENT YEARS, SO ANY SUCH LIMITATIONS MAY HAVE A MATERIAL ADVERSE EFFECT ON THE COMPANY’S ABILITY TO RAISE THE CAPITAL IT NEEDS.
FOR FURTHER INFORMATION ABOUT THE REQUIREMENTS FOR CONTINUED LISTING OF THE COMPANY’S COMMON STOCK ON THE NASDAQ CAPITAL MARKET PLEASE REFER TO CHAPTER 5, PARAGRAPH 5.1.5, SUBPARAGRAPH D OF THIS REGISTRATION DOCUMENT.
| 4.1.8 | RISKS RELATED TO THE GLOBAL FINANCIAL CRISIS |
THE ONGOING CREDIT CRISIS AND RELATED TURMOIL IN THE GLOBAL FINANCIAL SYSTEM HAS HAD AND MAY CONTINUE TO HAVE AN IMPACT ON THE COMPANY’S BUSINESS AND FINANCIAL CONDITION. THE COMPANY MAY FACE SIGNIFICANT CHALLENGES IF CONDITIONS IN THE FINANCIAL MARKETS DO NOT IMPROVE OR CONTINUE TO WORSEN. IN PARTICULAR, THE COMPANY’S ABILITY TO ACCESS THE CAPITAL MARKETS AND RAISE FUNDS REQUIRED FOR ITS OPERATIONS MAY BE SEVERELY RESTRICTED AT A TIME WHEN THE COMPANY WOULD LIKE, OR NEED, TO DO SO. SUCH RESTRICTION COULD HAVE AN ADVERSE EFFECT ON OUR ABILITY TO MEET OUR CURRENT AND FUTURE FUNDING REQUIREMENTS AND ON OUR FLEXIBILITY TO REACT TO CHANGING ECONOMIC AND BUSINESS CONDITIONS.
C-30
| 4.1.9 | RISKS RELATED TO THE EXERCISE OF THE VOTING RIGHTS AND DELIVERY OF THE MEETING DOCUMENTATION |
DUE TO CERTAIN RESTRICTIONS UNDER ITALIAN LAW WHICH PROHIBIT IDENTIFYING INDIVIDUAL THE COMPANY SHAREHOLDERS WHO HOLD SHARES IN ITALY THROUGH MONTE TITOLI (THE “ITALIAN SHAREHOLDERS”), THE ITALIAN SHAREHOLDERS MAY BE LIMITED IN THEIR ABILITY TO RECEIVE INFORMATION ABOUT THE COMPANY (FOR INSTANCE, ITALIAN SHAREHOLDERS ARE GENERALLY NOT ABLE TO RECEIVE THE SHAREHOLDER MEETING DOCUMENTATION AT THEIR OWN ADDRESS) AND TO AVAIL THEMSELVES OF CERTAIN VOTING MODALITIES SUCH AS INTERNET AND PHONE. THESE LIMITATIONS DO NOT APPLY TO SHAREHOLDERS WHO HOLD THEIR SHARES IN THE COMPANY DIRECTLY OR THROUGH A U.S. INTERMEDIARY.
FOR DETAILED INFORMATION ABOUT THE SHAREHOLDERS’ MEETING DOCUMENTATION AND THE VOTING PROCEDURES, PLEASE REFER TO CHAPTER 21, PARAGRAPH 21.2.5 OF THIS REGISTRATION DOCUMENT.
| 4.1.10 | RISKS RELATED TO THE COMPANY’S NEED TO OBTAIN A QUORUM FOR ITS MEETINGS OF SHAREHOLDERS |
CTI’S ARTICLES REQUIRE THAT A QUORUM, CONSISTING OF ONE-THIRD OF THE OUTSTANDING SHARES OF VOTING STOCK, BE REPRESENTED IN PERSON OR BY PROXY IN ORDER TO TRANSACT BUSINESS AT A MEETING OF ITS SHAREHOLDERS. IN ADDITION, AMENDMENTS TO CTI’S ARTICLES, SUCH AS AN AMENDMENT TO INCREASE THE COMPANY’S AUTHORIZED CAPITAL STOCK, REQUIRE THE APPROVAL OF A MAJORITY OF THE OUTSTANDING SHARES OF THE COMPANY. HOWEVER, UNDER ITALIAN LAWS AND REGULATIONS, IT IS DIFFICULT FOR THE COMPANY TO COMMUNICATE WITH THE BENEFICIAL HOLDERS OF THOSE SHARES TO OBTAIN VOTES. IN 2006 THE COMPANY SCHEDULED TWO ANNUAL MEETINGS OF SHAREHOLDERS BUT WAS UNABLE TO OBTAIN QUORUM AT EITHER MEETING. FOLLOWING THAT FAILURE TO OBTAIN QUORUM, THE COMPANY CONTACTED CERTAIN DEPOSITORY BANKS IN ITALY WHERE SIGNIFICANT NUMBERS OF CTI’S COMMON STOCK WERE HELD AND ASKED THEM TO COOPERATE BY MAKING A BOOK ENTRY TRANSFER OF THEIR SHARE POSITIONS AT MONTE TITOLI TO THEIR U.S. CORRESPONDENT BANK, WHICH WOULD THEN TRANSFER THE SHARES TO AN ACCOUNT OF THE ITALIAN BANK AT A U.S. AFFILIATE BROKER-DEALER. CERTAIN OF THE BANKS CONTACTED AGREED TO PARTICIPATE IN THE CUSTODY TRANSFER PURSUANT TO THESE ARRANGEMENTS AS OF THE RECORD DATE, SUBJECT TO THE RELEVANT BENEFICIAL OWNER TAKING NO ACTION TO DIRECT THE VOTING OF SUCH SHARES. UNDER RULE 452 OF THE NYSE, THE U.S. AFFILIATE BROKER-DEALER MAY VOTE SHARES ABSENT DIRECTION FROM THE BENEFICIAL OWNER ON CERTAIN MATTERS, SUCH AS THE UNCONTESTED ELECTION OF DIRECTORS, AN AMENDMENT TO THE COMPANY’S ARTICLES OF INCORPORATION TO INCREASE AUTHORIZED SHARES THAT ARE TO BE USED FOR GENERAL CORPORATE PURPOSES, AND THE RATIFICATION OF THE COMPANY’S AUDITORS. AS A RESULT OF THE CUSTODY TRANSFER ARRANGEMENT, THE COMPANY WAS ABLE TO HOLD SEVERAL SPECIAL MEETINGS OF THE SHAREHOLDERS AND ANNUAL MEETINGS OF THE SHAREHOLDERS AS OF YEAR 2007. NEVERTHELESS, NOTWITHSTANDING THE CUSTODY TRANSFER ARRANGEMENT,
C-31
THE COMPANY WAS UNABLE TO HOLD THE SPECIAL MEETING OF SHAREHOLDERS IN THE FIRST HALF OF 2010, DUE TO THE FAILURE TO ACHIEVE THE RELEVANT QUORUM.
AS A RESULT OF THE CUSTODY TRANSFER PROCEDURE DESCRIBED ABOVE, THE ANNUAL SHAREHOLDERS’ MEETING OF SEPTEMBER 16, 2010 WAS CALLED (BY MEANS OF A PRELIMINAY NOTICE AND TWO FINAL NOTICE PUBLISHED ON THE ITALIAN NEWSPAPER “IL SOLE 24 ORE” RESPECTIVELY ON JULY 1, JULY 23 AND AUGUST 26, 2010) AND TOOK PLACE WITH THE APPROVAL OF ALL THE RESOLUTIONS ON THE AGENDA.
OBTAINING A QUORUM AT FUTURE MEETINGS WILL DEPEND IN PART UPON THE WILLINGNESS OF ITALIAN DEPOSITORY BANKS TO CONTINUE PARTICIPATING IN THE CUSTODY TRANSFER ARRANGEMENT, AND THERE IS NO ASSURANCE THAT THOSE BANKS THAT HAVE PARTICIPATED IN THE PAST WILL CONTINUE TO PARTICIPATE IN CUSTODY TRANSFER ARRANGEMENTS IN THE FUTURE. THE COMPANY IS EXPLORING OTHER ALTERNATIVES TO OBTAIN QUORUM AT ITS MEETINGS, HOWEVER, THERE IS NO GUARANTEE THAT IT WILL BE ABLE TO FIND AN ALTERNATE METHOD IF THE COMPANY IS UNABLE TO CONTINUE USING THE CUSTODY TRANSFER ARRANGEMENT. AS A RESULT, THE COMPANY MAY BE UNABLE TO GET A QUORUM AT FUTURE ANNUAL OR SPECIAL MEETINGS OF SHAREHOLDERS OR OBTAIN SHAREHOLDER APPROVALS OF PROPOSALS WHEN NEEDED. IF THE COMPANY IS UNABLE TO OBTAIN A QUORUM AT FUTURE SHAREHOLDER MEETINGS AND THUS FAILS TO GET SHAREHOLDER APPROVAL OF CORPORATE ACTIONS, SUCH FAILURE COULD HAVE A MATERIALLY ADVERSE EFFECT ON THE COMPANY. IN ADDITION, BROKERS MAY ONLY VOTE ON THOSE MATTERS FOR WHICH BROKER DISCRETIONARY VOTING IS ALLOWED UNDER RULE 452. THE COMPANY MAY NOT BE ABLE TO OBTAIN THE REQUIRED NUMBER OF VOTES TO APPROVE CERTAIN PROPOSALS THAT REQUIRE A MAJORITY OF ALL OUTSTANDING SHARES TO APPROVE AND, DEPENDING ON THE PROPOSAL IN QUESTION, SUCH FAILURE COULD HAVE A MATERIALLY ADVERSE EFFECT ON THE COMPANY. FOR EXAMPLE, A PROPOSAL TO APPROVE A REVERSE STOCK SPLIT FAILED TO RECEIVE SUFFICIENT VOTES TO PASS AT THE MARCH 2009 SHAREHOLDERS MEETING.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 21, PARAGRAPH 21.2.5 OF THIS REGISTRATION DOCUMENT.
| 4.1.11 | RISKS OF FAILURE IN FINANCING EFFORTS FOR FAILURE TO RECEIVE SHAREHOLDER APPROVAL WHEN NEEDED |
THE COMPANY IS REQUIRED UNDER THE NASDAQ MARKETPLACE RULES TO OBTAIN SHAREHOLDER APPROVAL FOR ANY ISSUANCE OF ADDITIONAL EQUITY SECURITIES THAT WOULD COMPRISE MORE THAN 20% OF ITS TOTAL SHARES OF COMMON STOCK OUTSTANDING BEFORE THE ISSUANCE OF THE SECURITIES AT A DISCOUNT TO THE GREATER OF BOOK OR MARKET VALUE IN AN OFFERING THAT IS NOT DEEMED TO BE A “PUBLIC OFFERING” BY NASDAQ. FUNDING OF ITS OPERATIONS IN THE FUTURE MAY REQUIRE ISSUANCE OF ADDITIONAL EQUITY SECURITIES THAT WOULD COMPRISE MORE THAN 20% OF CTI’S TOTAL SHARES OF COMMON STOCK OUTSTANDING, BUT THE COMPANY MAY NOT OBTAIN THE SHAREHOLDER APPROVAL FOR PURPOSES OF COMPLYING WITH THE NASDAQ MARKETPLACE RULES, PARTICULARLY IN LIGHT OF THE DIFFICULTIES THE COMPANY HAS HAD OBTAINING QUORUM AND HOLDING SHAREHOLDER MEETINGS AS OUTLINED ABOVE. IF THE COMPANY IS NOT ABLE TO OBTAIN FINANCING DUE TO SHAREHOLDER APPROVAL
C-32
DIFFICULTIES, SUCH FAILURE MAY HAVE AN ADVERSE EFFECT ON ITS ABILITY TO CONTINUE OPERATIONS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 21, PARAGRAPH 21.2 OF THIS REGISTRATION DOCUMENT.
| 4.1.12 | RISKS RELATED TO RELIANCE ON THIRD PARTY FOR CONTRACTUAL PAYMENTS |
BECAUSE THE COMPANY DOES NOT CURRENTLY HAVE ANY MARKETED PRODUCTS PRODUCING REVENUE, THE COMPANY’S BUSINESS IS DEPENDENT ON THE PERFORMANCE BY CONTRACTUAL COUNTERPARTIES OF THEIR RESPONSIBILITIES UNDER CONTRACTUAL RELATIONSHIPS. IF THIRD PARTIES DEFAULT ON THEIR PERFORMANCE OF THEIR CONTRACTUAL OBLIGATIONS, THE COMPANY COULD SUFFER SIGNIFICANT FINANCIAL LOSSES AND OPERATIONAL PROBLEMS, WHICH COULD IN TURN ADVERSELY AFFECT OUR FINANCIAL PERFORMANCE, CASH FLOWS OR RESULTS OF OPERATIONS AND MAY JEOPARDIZE OUR ABILITY TO MAINTAIN OUR OPERATIONS.
FOR FURTHER INFORMATION ABOUT THE MAIN AGREEMENTS ENTERED INTO BY THE COMPANY PLEASE REFER TO CHAPTER 22 OF THIS REGISTRATION DOCUMENT.
| 4.1.13 | RISKS RELATED TO THE POSSIBILITY OF DELAY, LIMITATION OR PRECLUSION FROM OBTAINING REGULATORY APPROVAL OF OPAXIO AS A MAINTENANCE THERAPY FOR ADVANCED STAGE OVARIAN CANCER |
FUTURE FINANCIAL SUCCESS OFF THE COMPANY DEPENDS IN PART ON OBTAINING REGULATORY APPROVAL OF OPAXIO. THE PAST CLINICAL TRIALS FOR OPAXIO DID NOT RESULT IN THE EXPECTED OUTCOMES. THE COMPANY IS CURRENTLY FOCUSING ITS DEVELOPMENT OF OPAXIO AS A POTENTIAL MAINTENANCE THERAPY FOR WOMEN WITH ADVANCED STAGE OVARIAN CANCER WHO ACHIEVE A COMPLETE REMISSION FOLLOWING FIRST-LINE THERAPY WITH PACLITAXEL AND CARBOPLATIN ( THE “GOG12 TRIAL”) THERE ARE NO ASSURANCES THAT THE GOG0212 WILL PROVIDE COMPELLING EVIDENCE OR ANY POSITIVE RESULTS, WHICH WOULD PRECLUDE OUR PLANNED SUBMISSION OF AN NDA TO THE FDA. IN ADDITION, THE COMPANY CANNOT PREDICT THE OUTCOME OF THE GOG0212 STUDY AND THAT STUDY MAY NOT DEMONSTRATE OR BE ADEQUATE TO SUPPORT REGULATORY APPROVAL OF OPAXIO BY THE FDA.
FOR FURTHER INFORMATION WITH RESPECT TO PROGRESS IN THE RESEARCH AND DEVELOPMENT RELATED TO OPAXIO AND THE PREVIOUS RELEVANT AUTHORIZATION PROCEDURES, PLEASE REFER TO PARAGRAPH 6.1.1 BELOW.
| 4.1.14 | RISKS RELATED TO THE HARM OF THE BUSINESS OF THE COMPANY IN CASE OF AN ADVERSE OUTCOME IN THE SECURITIES CLASS ACTIONS AND SHAREHOLDER DERIVATIVE LITIGATION THAT HAVE BEEN FILED AGAINST THE COMPANY |
THE COMPANY AND CERTAIN OF ITS OFFICERS AND DIRECTORS ARE NAMED AS DEFENDANTS IN PURPORTED SECURITIES CLASS ACTION AND SHAREHOLDER DERIVATIVE LAWSUITS FILED IN THE U.S. DISTRICT COURT FOR THE WESTERN DISTRICT OF WASHINGTON. THE SECURITIES CLASS ACTION LAWSUITS ARE BROUGHT ON BEHALF OF A PUTATIVE CLASS OF
C-33
PURCHASERS OF OUR SECURITIES FROM MAY 5, 2009 THROUGH MARCH 19, 2010, AND SEEK UNSPECIFIED DAMAGES.
THE SECURITIES COMPLAINTS ALLEGE THAT THE DEFENDANTS VIOLATED THE FEDERAL SECURITIES LAWS BY MAKING CERTAIN ALLEGED FALSE AND MISLEADING STATEMENTS. SPECIFICALLY, PLAINTIFFS CLAIM THAT DEFENDANTS MISLED THE PUBLIC ABOUT THE VALIDITY OF A SPA—A PROCESS BY WHICH THE COMPANY GAINED THE FDA’S AGREEMENT ON CLINICAL TRIAL DESIGN IN ADVANCE OF INITIATING THE TRIAL—FOR A PIXANTRONE PHASE III CLINICAL TRIAL (“EXTEND TRIAL”). PLAINTIFFS CLAIM THAT DEFENDANTS KNEW OR SHOULD HAVE KNOWN THAT THE SPA WAS INVALIDATED WHEN THE TRIAL CLOSED EARLY, AND THEREFORE DEFENDANTS SHOULD HAVE DISCLOSED THAT THE EXTEND TRIAL WAS NO LONGER OPERATING UNDER THE SPA. PLAINTIFFS’ CONSOLIDATED AMENDED COMPLAINT ALLEGES THAT BEGINNING ON MARCH 25, 2008, DEFENDANTS ENGAGED IN A FRAUDULENT SCHEME TO INFLATE THE COMPANY’S STOCK PRICE BY MISREPRESENTING THAT THE SPA WAS STILL VALID AFTER THE COMPANY STOPPED THE EXTEND TRIAL ENROLLMENT EARLY.
THE DERIVATIVE ACTIONS ALLEGE THAT DEFENDANTS BREACHED THEIR FIDUCIARY DUTIES TO THE COMPANY UNDER WASHINGTON LAW BY MAKING OR FAILING TO PREVENT THE DISCLOSURE OF THE SAME ALLEGED FALSE AND MISLEADING STATEMENTS THAT ARE THE BASIS OF THE SECURITIES COMPLAINTS.
THE LAWSUITS ARE AT A PRELIMINARY STAGE IN THE PROCEEDINGS. THE COMPANY BELIEVES THAT THE SECURITIES ACTIONS ARE WITHOUT MERIT AND INTEND TO DEFEND THEM VIGOROUSLY. AS IS TYPICAL IN THIS TYPE OF LITIGATION, ADDITIONAL PURPORTED SECURITIES CLASS ACTION AND SHAREHOLDER DERIVATIVE LAWSUITS CONTAINING SUBSTANTIALLY SIMILAR ALLEGATIONS COULD BE FILED IN THE NEAR FUTURE. THE COMPANY EXPECTS THAT ALL OF THE PURPORTED SECURITIES CLASS ACTIONS WILL BE CONSOLIDATED INTO ONE CONSOLIDATED SECURITIES CLASS ACTION AND ALL OF THE PURPORTED SHAREHOLDER DERIVATIVE LAWSUITS WILL BE CONSOLIDATED INTO ONE CONSOLIDATED DERIVATIVE ACTION. AS WITH ANY LITIGATION PROCEEDING, THE COMPANY CANNOT PREDICT WITH CERTAINTY THE EVENTUAL OUTCOME OF PENDING LITIGATION. NO ESTIMATE OF A LOSS, IF ANY, CAN BE MADE AT THIS TIME IN THE EVENT THAT THE COMPANY DOES NOT PREVAIL. FURTHERMORE, THE COMPANY MAY HAVE TO INCUR SUBSTANTIAL EXPENSES IN CONNECTION WITH THESE LAWSUITS. IN THE EVENT OF AN ADVERSE OUTCOME, THE BUSINESS OF THE COMPANY COULD BE MATERIALLY HARMED.
FOR FURTHER INFORMATION ABOUT THE CONVERTIBLE NOTES OF THE COMPANY PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.8 OF THIS REGISTRATION DOCUMENT.
| 4.1.15 | RISKS RELATED TO THE INABILITY OF THE COMPANY TO OBTAIN THE RAW MATERIALS NECESSARY TO PRODUCE ITS OPAXIO PRODUCT CANDIDATE IN SUFFICIENT QUANTITY TO MEET DEMAND WHEN AND IF SUCH PRODUCT IS APPROVED |
THE COMPANY MAY NOT BE ABLE TO CONTINUE TO PURCHASE THE MATERIALS NECESSARY TO PRODUCE OPAXIO, INCLUDING PACLITAXEL, IN ADEQUATE VOLUME AND QUALITY. PACLITAXEL IS DERIVED FROM CERTAIN VARIETIES OF YEW TREES AND THE SUPPLY OF
C-34
PACLITAXEL IS CONTROLLED BY A LIMITED NUMBER OF COMPANIES. THE COMPANY PURCHASES THE RAW MATERIALS PACLITAXEL AND POLYGLUTAMIC ACID FROM SINGLE SOURCES. SHOULD THE PACLITAXEL OR POLYGLUTAMIC ACID PURCHASED FROM ITS SOURCES PROVE TO BE INSUFFICIENT IN QUANTITY OR QUALITY, SHOULD A SUPPLIER FAIL TO DELIVER IN A TIMELY FASHION OR AT ALL, OR SHOULD THESE RELATIONSHIPS TERMINATE, THE COMPANY MAY NOT BE ABLE TO QUALIFY AND OBTAIN A SUFFICIENT SUPPLY FROM ALTERNATE SOURCES ON ACCEPTABLE TERMS, OR AT ALL.
FOR FURTHER INFORMATION ABOUT THE CONVERTIBLE NOTES OF THE COMPANY PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1 OF THIS REGISTRATION DOCUMENT.
| 4.1.16 | RISKS RELATED TO DEPENDENCE ON THIRD-PARTY MANUFACTURERS |
THE COMPANY DOES NOT CURRENTLY HAVE INTERNAL ANALYTICAL LABORATORY OR MANUFACTURING FACILITIES TO ALLOW THE TESTING OR PRODUCTION OF DRUG PRODUCTS IN COMPLIANCE WITH CGMPS. BECAUSE THE COMPANY DOES NOT DIRECTLY CONTROL ITS SUPPLIERS, THESE VENDORS MAY NOT BE ABLE TO PROVIDE THE COMPANY WITH FINISHED PRODUCT WHEN THE COMPANY NEEDS IT.
THE COMPANY WILL BE DEPENDENT UPON THESE THIRD PARTIES TO SUPPLY IT IN A TIMELY MANNER WITH PRODUCTS MANUFACTURED IN COMPLIANCE WITH CGMPS OR SIMILAR MANUFACTURING STANDARDS IMPOSED BY FOREIGN REGULATORY AUTHORITIES WHERE ITS PRODUCTS WILL BE TESTED AND/OR MARKETED. WHILE THE FDA AND OTHER REGULATORY AUTHORITIES MAINTAIN OVERSIGHT FOR CGMP COMPLIANCE OF DRUG MANUFACTURERS, CONTRACT MANUFACTURERS MAY AT TIMES VIOLATE CGMPS. THE FDA AND OTHER REGULATORY AUTHORITIES MAY TAKE ACTION AGAINST A CONTRACT MANUFACTURER WHO VIOLATES CGMPS. ONE OF CTI’S PRODUCTS UNDER DEVELOPMENT, OPAXIO, HAS A COMPLEX MANUFACTURING PROCESS, WHICH MAY PREVENT THE COMPANY FROM OBTAINING A SUFFICIENT SUPPLY OF DRUG PRODUCT FOR THE CLINICAL TRIALS AND COMMERCIAL ACTIVITIES CURRENTLY PLANNED OR UNDERWAY ON A TIMELY BASIS, IF AT ALL.
WITH RESPECT TO PIXANTRONE, WE ARE CURRENTLY DISPUTING OUR RIGHT TO CANCEL THE EXCLUSIVE MANUFACTURING CONTRACT BETWEEN US AND THE FORMER MANUFACTURER OF PIXANTRONE, SICOR SOCIETÀ ITALIANA CORTICOSTEROIDI S.R.L. WE ASSERT MULTIPLE GROUNDS FOR TERMINATING THIS EXCLUSIVE MANUFACTURING AGREEMENT, WHICH THE FORMER MANUFACTURER DISPUTES. THE FORMER MANUFACTURER HAS ASSERTED THAT WE DO NOT HAVE THE RIGHT TO TERMINATE THE MANUFACTURING CONTRACTS AND HAS FILED A LAWSUIT IN THE COURT OF MILAN TO COMPEL US TO SOURCE PIXANTRONE FROM THAT MANUFACTURER. A HEARING WAS HELD ON JANUARY 21, 2010 TO DISCUSS PRELIMINARY MATTERS AND SET A SCHEDULE FOR FUTURE FILINGS AND HEARINGS. ON NOVEMBER 11, 2010 A HEARING WAS HELD AIMED AT EXAMINING AND DISCUSSING THE REQUESTS FOR EVIDENCE SUBMITTED BY THE PARTIES IN THE BRIEFS FILED PURSUANT TO ARTICLE 183, PARAGRAPH 6 OF THE ITALIAN CODE OF CIVIL PROCEDURE. AT THE HEARING OF NOVEMBER 11, THE JUDGE DECLARED THAT THE CASE DOES NOT REQUIRE ANY DISCOVERY OR EVIDENTIARY PHASE, AS IT MAY BE DECIDED ON THE BASIS OF THE DOCUMENTS AND PLEADINGS FILED BY THE PARTIES.
C-35
THE JUDGE FIXED ACCORDINGLY THE LAST HEARING FOR OCTOBER 11, 2012, FOR THE PARTIES TO DEFINITIVELY SUBMIT TO THE JUDGE THEIR REQUESTS.
THE CONSEQUENCE FOR CTI IN THE EVENT OF A FINAL NEGATIVE OUTCOME OF THIS LITIGATION WOULD BE THAT THE MANUFACTURING AGREEMENT WITH SICOR WOULD BE STILL BINDING AND EFFECTIVE BETWEEN THE PARTIES. SICOR DID NOT MAKE ANY REQUEST FOR COMPENSATION OF DAMAGES.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1 OF THIS REGISTRATION DOCUMENT.
| 4.1.17 | RISKS RELATED TO THE SUCCESSFUL DEVELOPMENT OF THE COMPANY’S PRODUCT CANDIDATES |
OPAXIO, PIXANTRONE AND BROSTALLICIN ARE CURRENTLY IN CLINICAL TRIALS; THESE CLINICAL TRIALS MAY NOT BE SUCCESSFUL AND EVEN IF THEY ARE, THE COMPANY MAY NOT BE SUCCESSFUL IN DEVELOPING ANY OF THEM INTO A COMMERCIAL PRODUCT. SUCH PRODUCT CANDIDATES WILL BE SUCCESSFUL ONLY IF:
| • | ITS PRODUCT CANDIDATES ARE DEVELOPED TO A STAGE THAT WILL ENABLE IT TO COMMERCIALIZE THEM OR SELL RELATED MARKETING RIGHTS TO PHARMACEUTICAL COMPANIES; |
| • | IT IS ABLE TO COMMERCIALIZE PRODUCT CANDIDATES IN CLINICAL DEVELOPMENT OR SELL THE MARKETING RIGHTS TO THIRD PARTIES; AND |
| • | ITS PRODUCT CANDIDATES, IF DEVELOPED, ARE APPROVED BY THE REGULATORY AUTHORITIES. |
THE COMPANY IS DEPENDENT ON THE SUCCESSFUL COMPLETION OF THESE GOALS IN ORDER TO GENERATE REVENUES. THE FAILURE TO GENERATE SUCH REVENUES MAY PRECLUDE IT FROM CONTINUING ITS RESEARCH AND DEVELOPMENT OF THESE AND OTHER PRODUCT CANDIDATES.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPHS 6.1.1 AND 6.1.2 OF THIS REGISTRATION DOCUMENT.
| 4.1.18 | RISKS RELATED TO THE NEED TO IN-LICENSE DRUG COMPOUNDS |
ONE COMPONENT OF CTI’S BUSINESS STRATEGY IS IN-LICENSING DRUG COMPOUNDS DEVELOPED BY OTHER PHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES OR ACADEMIC RESEARCH LABORATORIES. SUBSTANTIALLY ALL OF CTI’S COMPOUNDS IN CLINICAL DEVELOPMENT ARE IN-LICENSED FROM A THIRD-PARTY, INCLUDING PIXANTRONE, OPAXIO AND BROSTALLICIN.
COMPETITION FOR NEW PROMISING COMPOUNDS AND COMMERCIAL PRODUCTS CAN BE INTENSE. IF THE COMPANY IS NOT ABLE TO IDENTIFY FUTURE IN-LICENSING OPPORTUNITIES AND ENTER INTO FUTURE LICENSING ARRANGEMENTS ON ACCEPTABLE TERMS, ITS FUTURE PRODUCT PORTFOLIO AND POTENTIAL PROFITABILITY COULD BE HARMED.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.4.2 OF THIS REGISTRATION DOCUMENT.
C-36
| 4.1.19 | RISKS RELATED TO THIRD-PARTY COLLABORATIONS |
THE COMPANY HAS ENTERED INTO COLLABORATIVE ARRANGEMENTS WITH THIRD-PARTIES TO DEVELOP AND/OR COMMERCIALIZE PRODUCT CANDIDATES AND IS CURRENTLY SEEKING ADDITIONAL COLLABORATIONS. FOR EXAMPLE, THE COMPANY ENTERED INTO AN AGREEMENT WITH THE GYNECOLOGIC ONCOLOGY GROUP TO PERFORM A PHASE III TRIAL OF OPAXIO IN PATIENTS WITH OVARIAN CANCER. ADDITIONAL COLLABORATIONS MIGHT BE NECESSARY IN ORDER FOR THE COMPANY TO FUND ITS RESEARCH AND DEVELOPMENT ACTIVITIES AND THIRD-PARTY MANUFACTURING ARRANGEMENTS, SEEK AND OBTAIN REGULATORY APPROVALS AND SUCCESSFULLY COMMERCIALIZE ITS EXISTING AND FUTURE PRODUCT CANDIDATES. IF THE COMPANY FAILS TO ENTER INTO ADDITIONAL COLLABORATIVE ARRANGEMENTS OR FAILS TO MAINTAIN ITS EXISTING COLLABORATIVE ARRANGEMENTS, THE NUMBER OF PRODUCT CANDIDATES FROM WHICH IT COULD RECEIVE FUTURE REVENUES WOULD DECLINE.
FOR A DESCRIPTION OF THE TERMS AND CONDITIONS OF THE MAIN COLLABORATION AGREEMENTS, PLEASE REFER TO CHAPTER 22, PARAGRAPH 22.1.1 (COLLABORATION AGREEMENTS).
CTI’S DEPENDENCE ON COLLABORATIVE ARRANGEMENTS WITH THIRD-PARTIES WILL SUBJECT IT TO A NUMBER OF RISKS THAT COULD HARM ITS ABILITY TO DEVELOP AND COMMERCIALIZE PRODUCTS, INCLUDING THAT:
| • | COLLABORATIVE ARRANGEMENTS MAY NOT BE ON TERMS FAVORABLE TO CTI; |
| • | DISAGREEMENTS WITH PARTNERS MAY RESULT IN DELAYS IN THE DEVELOPMENT AND MARKETING OF PRODUCTS, TERMINATION OF CTI’S COLLABORATION AGREEMENTS OR TIME CONSUMING AND EXPENSIVE LEGAL ACTION; |
| • | THE COMPANY CANNOT CONTROL THE AMOUNT AND TIMING OF RESOURCES PARTNERS DEVOTE TO PRODUCT CANDIDATES OR THEIR PRIORITIZATION OF PRODUCT CANDIDATES AND PARTNERS MAY NOT ALLOCATE SUFFICIENT FUNDS OR RESOURCES TO THE DEVELOPMENT, PROMOTION OR MARKETING OF OUR PRODUCTS, OR MAY NOT PERFORM THEIR OBLIGATIONS AS EXPECTED (IN PARTICULAR, WITH RESPECT TO THE TRISENOX SALE AGREEMENT WITH CEPHALON, A DESCRIPTION OF WHICH CAN BE FOUND IN PARAGRAPH 22.1.1.11 BELOW, CEPHALON MAY DECIDE NOT TO SUBMIT ANY ADDITIONAL INFORMATION TO THE FDA TO APPLY FOR LABEL EXPANSION OF TRISENOX, IN WHICH CASE THE COMPANY WOULD NOT RECEIVE A MILESTONE PAYMENT UNDER THE AGREEMENT); |
| • | PARTNERS MAY CHOOSE TO DEVELOP, INDEPENDENTLY OR WITH OTHER COMPANIES, ALTERNATIVE PRODUCTS OR TREATMENTS, INCLUDING PRODUCTS OR TREATMENTS WHICH COMPETE WITH CTI’S; |
| • | CTI’S BUSINESS IS DEPENDENT ON THE PERFORMANCE OF PARTNERS OF THEIR RESPONSIBILITIES UNDER CONTRACTUAL RELATIONSHIPS AND IF THIRD PARTIES DEFAULT ON THEIR PERFORMANCE OF THEIR CONTRACTUAL OBLIGATIONS CTI COULD SUFFER SIGNIFICANT FINANCIAL LOSSES AND OPERATIONAL PROBLEMS; |
C-37
| • | AGREEMENTS WITH PARTNERS MAY EXPIRE OR BE TERMINATED WITHOUT RENEWAL (IN PARTICULAR, PURSUANT TO THE JOINT DEVELOPMENT AGREEMENT ENTERED INTO WITH NOVARTIS, FOR A DESCRIPTION OF WHICH PLEASE REFER TO PARAGRAPH 22.1.1.1, NOVARTIS HAS THE RIGHT TO TERMINATE SUCH AGREEMENT IN ANY MOMENT UPON WRITTEN NOTICE), OR PARTNERS MAY BREACH COLLABORATION AGREEMENTS WITH CTI; |
| • | BUSINESS COMBINATIONS OR SIGNIFICANT CHANGES IN A PARTNER’S BUSINESS STRATEGY MIGHT ADVERSELY AFFECT THAT PARTNER’S WILLINGNESS OR ABILITY TO COMPLETE ITS OBLIGATIONS TO CTI; AND |
| • | THE TERMS AND CONDITIONS OF THE RELEVANT AGREEMENTS MAY NO LONGER BE SUITABLE. |
THE OCCURRENCE OF ANY OF THESE EVENTS COULD ADVERSELY AFFECT THE DEVELOPMENT OR COMMERCIALIZATION OF CTI’S PRODUCTS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.4 OF THIS REGISTRATION DOCUMENT.
| 4.1.20 | RISKS RELATED TO FLUCTUATIONS IN THE EXCHANGE RATE OF THE U.S. DOLLAR RELATIVE TO THE EURO |
THE COMPANY IS EXPOSED TO RISKS ASSOCIATED WITH THE TRANSLATION OF EURO-DENOMINATED FINANCIAL RESULTS AND ACCOUNTS INTO U.S. DOLLARS. AS LONG AS THE COMPANY CONTINUES TO HAVE ASSETS AND LIABILITIES HELD IN ITS ITALIAN BRANCHES THE CARRYING VALUE OF THESE ASSETS AND LIABILITIES WILL BE AFFECTED BY FLUCTUATIONS IN THE VALUE OF THE U.S. DOLLAR AS COMPARED TO THE EURO. CHANGES IN THE VALUE OF THE U.S. DOLLAR AS COMPARED TO THE EURO MIGHT HAVE AN ADVERSE EFFECT ON OUR REPORTED RESULTS OF OPERATIONS AND FINANCIAL CONDITION.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 9, PARAGRAPH 9.2.1 AND CHAPTER 20 OF THIS REGISTRATION DOCUMENT.
| 4.1.21 | RISKS RELATED TO THE POSSIBILITY THAT THE COMPANY MAY OWE ADDITIONAL AMOUNTS FOR VALUE ADDED TAXES RELATED TO ITS OPERATIONS IN EUROPE |
EUROPEAN OPERATIONS OF THE COMPANY ARE SUBJECT TO A VALUE ADDED TAX, OR VAT, WHICH IS USUALLY APPLIED TO ALL GOODS AND SERVICES PURCHASED AND SOLD THROUGHOUT EUROPE. ON APRIL 14, 2009 AND DECEMBER 21, 2009, THE ITALIAN TAX AUTHORITY, OR ITA, ISSUED NOTICES OF ASSESSMENT TO CTI (EUROPE) BASED ON THE ITA’S AUDIT OF CTI (EUROPE)’S VAT RETURNS FOR THE YEARS 2003 AND 2005, RESPECTIVELY. ON JUNE 25, 2010, THE ITA ISSUED NOTICES OF ASSESSMENT TO CTI (EUROPE) FOR THE YEARS 2006 AND 2007 BASED ON SIMILAR FINDINGS OF THE 2003 AND 2005 ASSESSMENTS. THE ITA AUDITS CONCLUDED THAT CTI (EUROPE) DID NOT COLLECT AND REMIT VAT ON CERTAIN INVOICES ISSUED TO NON-ITALIAN CLIENTS FOR SERVICES PERFORMED BY CTI (EUROPE). THE ASSESSMENTS, INCLUDING INTEREST AND PENALTIES, FOR THE YEARS 2003, 2005, 2006 AND 2007 ARE €0.5 MILLION, €5.5 MILLION, €2.5 MILLION AND €0.8 MILLION. THE COMPANY BELIEVES THAT THE SERVICES INVOICED WERE NON-VAT TAXABLE CONSULTANCY SERVICES AND THAT THE VAT RETURNS ARE CORRECT AS ORIGINALLY FILED. THE COMPANY INTENDS
C-38
TO VIGOROUSLY DEFEND ITSELF AGAINST SUCH ASSESSMENT; HOWEVER, IF THE COMPANY IS UNABLE TO DEFEND ITSELF AGAINST THE ASSESSMENTS AND IF THE COMPANY RECEIVES AN ASSESSMENT FOR SUBSEQUENT YEARS, IT MAY HARM COMPANY’S RESULTS OF OPERATIONS AND FINANCIAL CONDITION.
FOR FURTHER INFORMATION ABOUT THE ABOVE MENTIONED TAX ASSESSMENT PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.8 OF THIS REGISTRATION DOCUMENT.
| 4.1.22 | RISKS RELATED TO ANTI-TAKEOVER PROVISIONS |
PROVISIONS OF CTI’S ARTICLES OF INCORPORATION AND BYLAWS MAY HAVE THE EFFECT OF DETERRING OR DELAYING ATTEMPTS BY ITS SHAREHOLDERS TO REMOVE OR REPLACE MANAGEMENT, TO COMMENCE PROXY CONTESTS, OR TO EFFECT CHANGES IN CONTROL. THESE PROVISIONS INCLUDE:
| • | A CLASSIFIED BOARD SO THAT ONLY APPROXIMATELY ONE THIRD OF THE BOARD OF DIRECTORS IS ELECTED EACH YEAR; |
| • | ELIMINATION OF CUMULATIVE VOTING IN THE ELECTION OF DIRECTORS; |
| • | PROCEDURES FOR ADVANCE NOTIFICATION OF SHAREHOLDER NOMINATIONS AND PROPOSALS; |
| • | THE ABILITY OF CTI’S BOARD OF DIRECTORS TO AMEND ITS BYLAWS WITHOUT SHAREHOLDER APPROVAL; AND |
| • | THE ABILITY OF CTI’S BOARD OF DIRECTORS TO ISSUE SHARES OF PREFERRED STOCK WITHOUT SHAREHOLDER APPROVAL UPON THE TERMS AND CONDITIONS AND WITH THE RIGHTS, PRIVILEGES AND PREFERENCES AS THE BOARD OF DIRECTORS MAY DETERMINE. |
THE COMPANY HAS IMPLEMENTED A SHAREHOLDER RIGHTS PLAN, DATED DECEMBER 28, 2009, PURSUANT TO WHICH AN ACQUISITION OF 20% OR MORE OF ITS COMMON STOCK COULD RESULT IN THE EXERCISABILITY OF THE PREFERRED STOCK PURCHASE RIGHT ACCOMPANYING EACH SHARE OF COMMON STOCK OF THE COMPANY (EXCEPT THOSE HELD BY A 20% SHAREHOLDER, WHICH BECOME NULL AND VOID), THEREBY ENTITLING THE HOLDER TO RECEIVE UPON EXERCISE, IN LIEU OF A NUMBER OF UNITS OF PREFERRED STOCK, THAT NUMBER OF SHARES OF COMMON STOCK OF THE COMPANY HAVING A MARKET VALUE OF TWO TIMES THE EXERCISE PRICE OF THE RIGHT. THE EXISTENCE OF OUR RIGHTS PLAN COULD HAVE THE EFFECT OF DELAYING, DEFERRING OR PREVENTING A THIRD PARTY FROM MAKING AN ACQUISITION PROPOSAL FOR THE COMPANY AND MAY INHIBIT A CHANGE IN CONTROL THAT SOME, OR A MAJORITY, OF THE STOCKHOLDERS OF THE COMPANY MIGHT BELIEVE TO BE IN THEIR BEST INTEREST OR THAT COULD GIVE OUR STOCKHOLDERS THE OPPORTUNITY TO REALIZE A PREMIUM OVER THE THEN-PREVAILING MARKET PRICES FOR THEIR SHARES.
IN ADDITION, AS A WASHINGTON CORPORATION, THE COMPANY IS SUBJECT TO WASHINGTON LAW WHICH IMPOSES RESTRICTIONS ON SOME TRANSACTIONS BETWEEN A CORPORATION AND CERTAIN SIGNIFICANT SHAREHOLDERS.
C-39
THESE PROVISIONS, ALONE OR TOGETHER, COULD HAVE THE EFFECT OF DETERRING OR DELAYING CHANGES IN INCUMBENT MANAGEMENT, PROXY CONTESTS OR CHANGES IN CONTROL.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 21, PARAGRAPH 21.2 OF THIS REGISTRATION DOCUMENT.
| 4.1.23 | RISKS RELATING TO THE DEPENDENCE ON MANAGEMENT PERSONNEL |
THE COMPANY DEPENDS HEAVILY ON KEY PERSONNEL, AND THE LOSS OF KEY EMPLOYEES AND SENIOR MANAGEMENT COULD HARM THE COMPANY’S BUSINESS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
THE COMPANY’S FUTURE SUCCESS DEPENDS IN SIGNIFICANT PART UPON THE CONTINUED CONTRIBUTIONS OF ITS SENIOR MANAGEMENT PERSONNEL, INCLUDING JAMES A. BIANCO, M.D., THE COMPANY’S CHIEF EXECUTIVE OFFICER, AND MR. CRAIG PHILIPS, THE COMPANY’S PRESIDENT. IT ALSO DEPENDS IN SIGNIFICANT PART UPON THE COMPANY’S ABILITY TO ATTRACT AND RETAIN ADDITIONAL QUALIFIED MANAGEMENT, TECHNICAL, SALES AND MARKETING AND SUPPORT PERSONNEL FOR OUR OPERATIONS.
COMPETITION FOR SUCH PERSONNEL IS INTENSE AND THE COMPANY MAY FAIL TO RETAIN OUR KEY PERSONNEL OR FAIL TO ATTRACT, ASSIMILATE OR RETAIN OTHER HIGH-QUALIFIED PERSONNEL IN THE FUTURE. IF THE COMPANY LOSES A KEY EMPLOYEE, IF A KEY EMPLOYEE FAILS TO PERFORM IN HIS OR HER CURRENT POSITION OR IF THE COMPANY ARE NOT ABLE TO ATTRACT AND RETAIN SKILLED EMPLOYEES AS NEEDED, THE COMPANY’S BUSINESS COULD SUFFER.
TURNOVER IN THE COMPANY’S SENIOR MANAGEMENT COULD SIGNIFICANTLY DEPLETE INSTITUTIONAL KNOWLEDGE HELD BY THE COMPANY’S EXISTING SENIOR MANAGEMENT TEAM AND IMPAIR THE COMPANY’S OPERATIONS, WHICH COULD HARM ITS BUSINESS.
FOR FURTHER INFORMATION ABOUT THE CONVERTIBLE NOTES OF THE COMPANY PLEASE REFER TO CHAPTER 14 OF THIS REGISTRATION DOCUMENT.
| 4.1.24 | RISKS RELATING TO FORWARD-LOOKING STATEMENTS |
THIS REGISTRATION DOCUMENT CONTAINS FORWARD-LOOKING STATEMENTS WHICH ARE BASED ON CURRENT EXPECTATIONS REGARDING THE COMPANY, THE GROWTH OF ITS BUSINESS, ITS FINANCIAL PERFORMANCE AND THE DEVELOPMENT OF THE INDUSTRY IN WHICH THE COMPANY OPERATES. SINCE THESE FORWARD-LOOKING STATEMENTS REFLECT CTI’S CURRENT ESTIMATES REGARDING FUTURE EVENTS, THEY INVOLVE RISKS AND UNCERTAINTIES.
THEREFORE, NO UNDUE RELIANCE SHOULD BE PLACED ON ANY FORWARD-LOOKING STATEMENTS CONTAINED IN THIS REGISTRATION DOCUMENT AS THEY SPEAK ONLY AS OF THE DATE ON WHICH SUCH STATEMENTS WERE MADE. IN EVALUATING THESE FORWARD-LOOKING STATEMENTS, INVESTORS SHOULD ALSO CONSIDER THE OTHER RISK FACTORS CONTAINED IN THIS REGISTRATION DOCUMENT.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6 OF THIS REGISTRATION DOCUMENT.
C-40
| 4.1.25 | RISKS RELATED TO THE MATERIAL ADVERSE IMPACT ON FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF THE COMPANY IN CASE OF UNFAVORABLE OUTCOME OF LITIGATION AND OTHER CLAIMS AGAINST THE COMPANY |
THE COMPANY IS SUBJECT TO A VARIETY OF CLAIMS AND LAWSUITS FROM TIME TO TIME, SOME OF WHICH ARISE IN THE ORDINARY COURSE OF OUR BUSINESS. ADVERSE OUTCOMES IN SOME OR ALL OF SUCH PENDING CASES MAY RESULT IN SIGNIFICANT MONETARY DAMAGES OR INJUNCTIVE RELIEF AGAINST THE COMPANY. WHILE THE COMPANY CURRENTLY BELIEVES THAT RESOLUTION OF THESE MATTERS, INDIVIDUALLY OR IN THE AGGREGATE, WILL NOT HAVE A MATERIAL ADVERSE IMPACT ON THE FINANCIAL POSITION OF THE COMPANY, RESULTS OF OPERATIONS OR TRADING PRICE OF OUR SECURITIES, THE ULTIMATE OUTCOME OF LITIGATION AND OTHER CLAIMS IS SUBJECT TO INHERENT UNCERTAINTIES, AND THE COMPANY’S VIEW OF THESE MATTERS MAY CHANGE IN THE FUTURE. IT IS POSSIBLE THAT ITS FINANCIAL CONDITION AND RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED IN ANY PERIOD IN WHICH THE EFFECT OF AN UNFAVORABLE FINAL OUTCOME BECOMES PROBABLE AND REASONABLY ESTIMABLE.
IN ACCORDANCE WITH ASC 450, THE COMPANY HAS ACCRUED €110,000 AS OF SEPTEMBER 30, 2010 FOR THOSE MATTERS THAT MANAGEMENT HAS ASSESSED THE LIKELIHOOD OF AN UNFAVORABLE OUTCOME AS PROBABLE.
FOR FURTHER INFORMATION ABOUT THE CONVERTIBLE NOTES OF THE COMPANY, PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.8 OF THIS REGISTRATION DOCUMENT.
| 4.1.26 | RISKS RELATED TO PUBLIC HEALTH ISSUES, WARS AND OTHER MILITARY ACTION, AS WELL AS TERRORIST ATTACKS AND THREATS AND GOVERNMENT RESPONSES THERETO, ESPECIALLY IF ANY SUCH ACTIONS WERE DIRECTED AT THE COMPANY OR COMPANY’S FACILITIES OR CUSTOMERS, WHICH MAY ADVERSELY AFFECT FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
PUBLIC HEALTH ISSUES, TERRORIST ATTACKS IN THE UNITED STATES AND ELSEWHERE, GOVERNMENT RESPONSES THERETO, AND MILITARY ACTIONS IN IRAQ, AFGHANISTAN AND ELSEWHERE, MAY DISRUPT OPERATIONS OF THE COMPANY OR THOSE OF COMPANY’S CUSTOMERS AND SUPPLIERS AND MAY AFFECT THE AVAILABILITY OF MATERIALS NEEDED TO MANUFACTURE THE PRODUCTS OF THE COMPANY OR THE MEANS TO TRANSPORT THOSE MATERIALS TO MANUFACTURING FACILITIES AND FINISHED PRODUCTS TO CUSTOMERS. IN JUNE 2009, THE WORLD HEALTH ORGANIZATION DECLARED AN H1N1 INFLUENZA, OR SWINE FLU, PANDEMIC, AND SUCH PANDEMIC COULD CAUSE DAMAGE OR DISRUPTION TO INTERNATIONAL COMMERCE BY CREATING ECONOMIC AND POLITICAL UNCERTAINTIES THAT MAY HAVE A STRONG NEGATIVE IMPACT ON THE GLOBAL ECONOMY, US, AND COMPANY’S CUSTOMERS OR SUPPLIERS. SHOULD THE SEVERITY OF THE H1N1 INFLUENZA PANDEMIC INCREASE OR OTHER PUBLIC HEALTH ISSUES ARISE, THE COMPANY COULD BE NEGATIVELY IMPACTED BY THE NEED FOR MORE STRINGENT EMPLOYEE TRAVEL RESTRICTIONS, ADDITIONAL LIMITATIONS IN THE AVAILABILITY OF FREIGHT SERVICES, GOVERNMENTAL ACTIONS LIMITING THE MOVEMENT OF PRODUCTS BETWEEN VARIOUS REGIONS AND DISRUPTIONS IN THE OPERATIONS OF OUR CUSTOMERS OR SUPPLIERS. THE LONG-TERM EFFECTS OF THE H1N1 PANDEMIC, THE TERRORIST ATTACKS, AND THE ONGOING WAR ON TERRORISM ON OUR BUSINESS AND ON THE GLOBAL ECONOMY REMAIN UNKNOWN.
C-41
IN ADDITION, ANY OF THESE EVENTS COULD INCREASE VOLATILITY IN THE UNITED STATES AND WORLD FINANCIAL MARKETS WHICH MAY DEPRESS THE PRICE OF CTI’S COMMON STOCK AND MAY LIMIT THE CAPITAL RESOURCES AVAILABLE TO THE COMPANY OR COMPANY’S CUSTOMERS OR SUPPLIERS, WHICH COULD RESULT IN DECREASED ORDERS FROM CUSTOMERS, LESS FAVORABLE FINANCING TERMS FROM SUPPLIERS, AND SCARCITY OR INCREASED COSTS OF MATERIALS AND COMPONENTS OF THE PRODUCTS OF THE COMPANY. ADDITIONALLY, TERRORIST ATTACKS DIRECTLY UPON THE COMPANY MAY SIGNIFICANTLY DISRUPT COMPANY’S ABILITY TO CONDUCT OUR BUSINESS. ANY OF THESE OCCURRENCES COULD HAVE A SIGNIFICANT IMPACT ON OPERATING RESULTS, REVENUES AND COSTS OF THE COMPANY AND MAY RESULT IN INCREASED VOLATILITY OF THE TRADING PRICE OF CTI SECURITIES.
| 4.1.27 | RISKS RELATED TO HIGHER HEALTH CARE COSTS |
THE COMPANY WILL BE IMPACTED BY THE RECENT PASSAGE OF THE PATIENT PROTECTION AND AFFORDABLE CARE ACT (PPACA). UNDER THE PPACA, THE COMPANY MAY BE REQUIRED TO AMEND ITS HEALTH CARE PLANS TO, AMONG OTHER THINGS, PROVIDE AFFORDABLE COVERAGE, AS DEFINED IN THE PPACA, TO ALL EMPLOYEES, OR OTHERWISE BE SUBJECT TO A PAYMENT PER EMPLOYEE BASED ON THE AFFORDABILITY CRITERIA IN THE ACT: COVER ADULT CHILDREN OF OUR EMPLOYEES TO AGE 26; DELETE LIFETIME LIMITS; AND DELETE PRE-EXISTING CONDITION LIMITATIONS. MANY OF THESE REQUIREMENTS WILL BE PHASED IN OVER A PERIOD OF TIME. ADDITIONALLY, SOME STATES AND LOCALITIES HAVE PASSED STATE AND LOCAL LAWS MANDATING THE PROVISION OF CERTAIN LEVELS OF HEALTH BENEFITS BY SOME EMPLOYERS. INCREASED HEALTH CARE COSTS COULD HAVE A MATERIAL ADVERSE EFFECT ON COMPANY’S BUSINESS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
| 4.2 | RISK FACTORS RELATING TO THE INDUSTRY IN WHICH THE ISSUER OPERATES |
| 4.2.1 | RISKS RELATED TO EXTENSIVE GOVERNMENT REGULATION |
A) ASPECTS RELATING TO THE BUSINESS OF THE ISSUER
THE COMPANY IS SUBJECT TO RIGOROUS AND EXTENSIVE REGULATION IN THE UNITED STATES OR IN THE COUNTRIES WHERE THE COMPANY OPERATES OR DISTRIBUTES ITS PRODUCTS FOR TRIAL PURPOSES. IN PARTICULAR:
| • | CTI’S PRODUCTS MAY NOT BE MARKETED IN THE UNITED STATES UNTIL THEY HAVE BEEN APPROVED BY THE FDA AND MAY NOT BE MARKETED IN OTHER COUNTRIES UNTIL THEY HAVE RECEIVED APPROVAL FROM THE APPROPRIATE AGENCIES; |
| • | IN THE EVENT THE COMPANY RECEIVES MARKETING APPROVAL FOR ANY OF ITS PRODUCT CANDIDATES THE COMPANY WILL BE SUBJECT TO NUMEROUS REGULATIONS AND STATUTES REGULATING THE MANNER OF SELLING AND OBTAINING REIMBURSEMENT FOR THOSE PRODUCTS; |
| • | THE MARKETING AND PROMOTION OF PHARMACEUTICALS IS ALSO HEAVILY REGULATED, PARTICULARLY WITH REGARD TO PROHIBITION ON THE PROMOTION PRODUCTS FOR OFF-LABEL USES. |
C-42
FAILURE TO COMPLY WITH THE ABOVE REGULATORY REQUIREMENTS BY CTI OR ITS EMPLOYEES COULD RESULT IN VARIOUS ADVERSE CONSEQUENCES, INCLUDING POSSIBLE DELAY IN APPROVAL OR REFUSAL TO APPROVE A PRODUCT, WITHDRAWAL OF APPROVED PRODUCTS FROM THE MARKET, PRODUCT SEIZURES, INJUNCTIONS, MONETARY PENALTIES, OR CRIMINAL PROSECUTION.
THE EXPENSE TO RETAIN AND PAY LEGAL COUNSEL AND CONSULTANTS TO DEFEND AGAINST ANY SUCH POSSIBLE PROCEEDINGS WOULD BE SUBSTANTIAL, AND TOGETHER WITH THE DIVERSION OF MANAGEMENT’S TIME AND ATTENTION TO ASSIST IN ANY SUCH DEFENSE, MAY NEGATIVELY AFFECT THE COMPANY’S FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
IN ADDITION, BOTH BEFORE AND AFTER APPROVAL, CTI’S CONTRACT MANUFACTURERS AND ITS PRODUCTS ARE SUBJECT TO NUMEROUS REGULATORY REQUIREMENTS COVERING, AMONG OTHER THINGS, TESTING, MANUFACTURING, QUALITY CONTROL, LABELING, ADVERTISING, PROMOTION, DISTRIBUTION AND EXPORT. FAILURE TO COMPLY WITH SUCH REQUIREMENTS MAY CAUSE US TO CURTAIL OR STOP THE MANUFACTURE OF SUCH PRODUCTS UNTIL WE OBTAIN REGULATORY COMPLIANCE.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.8 OF THIS REGISTRATION DOCUMENT.
B) FINANCIAL ASPECTS
CTI’S STOCK IS TRADED ON THE MTA MARKET AND IT IS REQUIRED TO ALSO COMPLY WITH THE RULES AND REGULATIONS OF CONSOB AND BORSA ITALIANA, WHICH COLLECTIVELY REGULATE COMPANIES LISTED ON ITALY’S PUBLIC MARKETS. COMPLIANCE WITH ITALIAN LISTING REQUIREMENTS SUCH AS THE PUBLICATION OF A LISTING PROSPECTUS THAT HAS BEEN APPROVED BY CONSOB PRIOR TO ISSUING COMMON STOCK IN ANY TWELVE-MONTH PERIOD THAT EXCEEDS 10% OF THE NUMBER OF SHARES OF COMMON STOCK OUTSTANDING AT THE BEGINNING OF THAT PERIOD (SAVE FOR THE APPLICABLE EXCEPTIONS) MAY DELAY ADDITIONAL ISSUANCES OF CTI’S COMMON STOCK AND ENTAIL THE SEEK FOR ALTERNATIVE FUNDING SOLUTIONS THE RELEVANT EXPENSES OF WHICH MIGHT HAVE A NEGATIVE IMPACT OF THE FINANCIAL SITUATION OF THE COMPANY.
FOR MORE INFORMATION CONCERNING THE APPLICATION OF THE ITALIAN LAW TO THE COMPANY, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.6 AND CHAPTER 16, PARAGRAPH 16.4 OF THIS REGISTRATION DOCUMENT.
| 4.2.2 | RISKS RELATED TO COMPETITION AND RAPID TECHNOLOGICAL CHANGES |
COMPETITION IN THE ONCOLOGY MARKET IS INTENSE AND IS ACCENTUATED BY THE RAPID PACE OF TECHNOLOGICAL DEVELOPMENT. THE COMPANY ANTICIPATES THAT IT WILL FACE INCREASED COMPETITION IN THE FUTURE AS NEW COMPANIES ENTER THE MARKET. CTI’S COMPETITORS IN THE UNITED STATES AND ELSEWHERE ARE NUMEROUS AND INCLUDE, AMONG OTHERS, MAJOR MULTINATIONAL PHARMACEUTICAL COMPANIES, SPECIALIZED BIOTECHNOLOGY COMPANIES AND UNIVERSITIES AND OTHER RESEARCH INSTITUTIONS.
MANY OF CTI’S COMPETITORS, EITHER ALONE OR TOGETHER WITH THEIR COLLABORATORS AND IN PARTICULAR, THE MULTINATIONAL PHARMACEUTICAL COMPANIES, HAVE SUBSTANTIALLY GREATER FINANCIAL RESOURCES AND DEVELOPMENT AND MARKETING
C-43
TEAMS THAN CTI. IN ADDITION, MANY OF CTI’S COMPETITORS, EITHER ALONE OR TOGETHER WITH THEIR COLLABORATORS, HAVE SIGNIFICANTLY GREATER EXPERIENCE THAN THE COMPANY DOES IN DEVELOPING, MANUFACTURING AND MARKETING PRODUCTS. AS A RESULT, THESE COMPANIES’ PRODUCTS MIGHT COME TO MARKET SOONER OR MIGHT PROVE TO BE MORE EFFECTIVE, LESS EXPENSIVE, HAVE FEWER SIDE EFFECTS OR BE EASIER TO ADMINISTER THAN CTI’S. IN ANY SUCH CASE, SALES OF CTI’S PRODUCTS OR EVENTUAL PRODUCTS WOULD LIKELY SUFFER AND IT MIGHT NEVER RECOUP THE SIGNIFICANT INVESTMENTS IT IS MAKING TO DEVELOP THESE PRODUCT CANDIDATES.
FOR MORE INFORMATION, ALSO WITH RESPECT TO DIRECT COMPETING PRODUCTS OF PIXANTRONE, OPAXIO, BROSTALLICIN IN CASE THESE LAST WOULD RECEIVE THE MARKETING AUTHORIZATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.4 (COMPETITION) AND 6.2 OF THIS REGISTRATION DOCUMENT.
| 4.2.3 | RISKS RELATED TO REIMBURSEMENT AND HEALTHCARE COST CONTAINMENT INITIATIVES |
THE ONGOING EFFORTS OF GOVERNMENTAL AND THIRD-PARTY PAYORS TO CONTAIN OR REDUCE THE COST OF HEALTHCARE MAY AFFECT CTI’S ABILITY TO COMMERCIALIZE ITS PRODUCTS SUCCESSFULLY. GOVERNMENTAL AND OTHER THIRD-PARTY PAYORS CONTINUE TO ATTEMPT TO CONTAIN HEALTHCARE COSTS BY:
| • | CHALLENGING THE PRICES CHARGED FOR HEALTH CARE PRODUCTS AND SERVICES, |
| • | LIMITING BOTH COVERAGE AND THE AMOUNT OF REIMBURSEMENT FOR NEW THERAPEUTIC PRODUCTS, |
| • | DENYING OR LIMITING COVERAGE FOR PRODUCTS THAT ARE APPROVED BY THE FDA BUT ARE CONSIDERED EXPERIMENTAL OR INVESTIGATIONAL BY THIRD-PARTY PAYORS, |
| • | REFUSING IN SOME CASES TO PROVIDE COVERAGE WHEN AN APPROVED PRODUCT IS USED FOR DISEASE INDICATIONS IN A WAY THAT HAS NOT RECEIVED FDA MARKETING APPROVAL, AND |
| • | DENYING COVERAGE ALTOGETHER. |
THE CONTINUING EFFORTS OF GOVERNMENT AND INSURANCE COMPANIES, HEALTH MAINTENANCE ORGANIZATIONS AND OTHER PAYERS OF HEALTHCARE COSTS TO CONTAIN OR REDUCE COSTS OF HEALTH CARE MAY AFFECT THE FUTURE REVENUES OF THE COMPANY AND PROFITABILITY, AND THE FUTURE REVENUES AND PROFITABILITY OF ITS POTENTIAL CUSTOMERS, SUPPLIERS AND COLLABORATIVE PARTNERS AND THE AVAILABILITY OF CAPITAL. IN THE UNITED STATES, GIVEN THE COMPREHENSIVE HEALTH CARE REFORM LEGISLATION THAT THE PRESIDENT SIGNED INTO LAW ON MARCH 23, 2010, UNDER THE PATIENT PROTECTION AND AFFORDABLE CARE ACT (HR 3590), OR THE PPACA, THE U.S. CONGRESS AND STATE LEGISLATURES WILL LIKELY CONTINUE TO FOCUS ON HEALTH CARE REFORM, THE COST OF HEALTHCARE SERVICES AND PRODUCTS AND ON THE REFORM OF THE MEDICARE AND MEDICAID SYSTEMS. WHILE THE COMPANY CANNOT PREDICT WHETHER ANY SUCH LEGISLATIVE OR REGULATORY PROPOSALS WILL BE ADOPTED, THE ANNOUNCEMENT OR ADOPTION OF THESE PROPOSALS COULD SIGNIFICANTLY INFLUENCE THE PURCHASE OF HEALTHCARE SERVICES AND PRODUCTS, RESULTING IN LOWER PRICES
C-44
AND REDUCING DEMAND FOR THE COMPANY’S PRODUCTS. IN ADDITION, IN ALMOST ALL EUROPEAN MARKETS, PRICING AND CHOICE OF PRESCRIPTION PHARMACEUTICALS ARE SUBJECT TO GOVERNMENTAL CONTROL. THEREFORE, THE PRICE OF COMPANY’S PRODUCTS AND THEIR REIMBURSEMENT IN EUROPE WILL BE DETERMINED BY NATIONAL REGULATORY AUTHORITIES.
EVEN IF THE COMPANY SUCCEEDS IN BRINGING ANY OF ITS PROPOSED PRODUCTS TO THE MARKET, THEY MAY NOT BE CONSIDERED COST-EFFECTIVE AND THIRD-PARTY REIMBURSEMENT MIGHT NOT BE AVAILABLE OR SUFFICIENT. IF ADEQUATE THIRD-PARTY COVERAGE IS NOT AVAILABLE, THE COMPANY MAY NOT BE ABLE TO MAINTAIN PRICE LEVELS SUFFICIENT TO REALIZE AN APPROPRIATE RETURN ON ITS INVESTMENT IN RESEARCH AND PRODUCT DEVELOPMENT. IN ADDITION, LEGISLATION AND REGULATIONS AFFECTING THE PRICING OF PHARMACEUTICALS MAY CHANGE IN WAYS ADVERSE TO THE COMPANY BEFORE OR AFTER ANY OF ITS PROPOSED PRODUCTS ARE APPROVED FOR MARKETING.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.2 OF THIS REGISTRATION DOCUMENT.
| 4.2.4 | RISKS RELATED TO THE LIMITATION OF MARKET ACCEPTANCE OF THE PRODUCTS OF THE COMPANY AND THE POSSIBILITY THAT THE COMPANY MAY NOT ACHIEVE ANTICIPATED REVENUES IF USERS OF CTI’S PRODUCTS ARE UNABLE TO OBTAIN ADEQUATE REIMBURSEMENT FROM THIRD-PARTY PAYERS, OR IF NEW RESTRICTIVE LEGISLATION IS ADOPTED |
THE COMPANY’S ABILITY TO COMMERCIALIZE ITS PRODUCTS WILL DEPEND IN PART ON THE EXTENT TO WHICH APPROPRIATE REIMBURSEMENT LEVELS FOR THE COST OF THE PRODUCTS OF THE COMPANY AND RELATED TREATMENT ARE OBTAINED BY GOVERNMENTAL AUTHORITIES, PRIVATE HEALTH INSURERS AND OTHER ORGANIZATIONS, SUCH AS HEALTH MAINTENANCE ORGANIZATIONS (“HMOS”). THIRD-PARTY PAYERS ARE INCREASINGLY CHALLENGING THE PRICES CHARGED FOR MEDICAL CARE. ALSO, THE TREND TOWARD MANAGED HEALTH CARE IN THE UNITED STATES AND THE CONCURRENT GROWTH OF ORGANIZATIONS SUCH AS HMOS, WHICH COULD CONTROL OR SIGNIFICANTLY INFLUENCE THE PURCHASE OF HEALTH CARE SERVICES AND PRODUCTS, AS WELL AS LEGISLATIVE PROPOSALS TO FURTHER REFORM HEALTH CARE OR REDUCE GOVERNMENT INSURANCE PROGRAMS, MAY ALL RESULT IN LOWER PRICES FOR THE PRODUCTSOF THE COMPANY . THE COST CONTAINMENT MEASURES THAT HEALTH CARE PAYERS AND PROVIDERS ARE INSTITUTING AND THE EFFECT OF ANY HEALTH CARE REFORM COULD MATERIALLY HARM COMPANY’S ABILITY TO OPERATE PROFITABLY.
| 4.2.5 | RISKS RELATED TO PRODUCTS THAT APPEAR PROMISING IN RESEARCH AND DEVELOPMENT WHICH MAY BE DELAYED OR FAIL TO REACH LATER STAGES OF DEVELOPMENT OR THE MARKET |
THE SUCCESSFUL DEVELOPMENT OF PHARMACEUTICAL PRODUCTS IS HIGHLY UNCERTAIN AND OBTAINING REGULATORY APPROVAL TO MARKET DRUGS TO TREAT CANCER IS EXPENSIVE, DIFFICULT AND RISKY. PRODUCTS THAT APPEAR PROMISING IN RESEARCH AND DEVELOPMENT MAY BE DELAYED OR FAIL TO REACH LATER STAGES OF DEVELOPMENT OR THE MARKET FOR SEVERAL REASONS, INCLUDING:
C-45
| • | CLINICAL TRIAL RESULTS MAY SHOW THE PRODUCT TO BE LESS EFFECTIVE THAN DESIRED OR TO HAVE HARMFUL OR PROBLEMATIC SIDE EFFECTS; |
| • | PRECLINICAL TESTS MAY SHOW THE PRODUCT TO BE TOXIC OR LACK EFFICACY IN ANIMAL MODELS; |
| • | FAILURE TO RECEIVE THE NECESSARY U.S. AND INTERNATIONAL REGULATORY APPROVALS OR A DELAY IN RECEIVING SUCH APPROVALS; |
| • | DIFFICULTIES IN FORMULATING THE PRODUCT, SCALING THE MANUFACTURING PROCESS OR GETTING APPROVAL FOR MANUFACTURING; |
| • | MANUFACTURING COSTS, PRICING, REIMBURSEMENT ISSUES OR OTHER FACTORS MAY MAKE THE PRODUCT UNECONOMICAL TO COMMERCIALIZE; |
| • | OTHER COMPANIES OR PEOPLE HAVE OR MAY HAVE PROPRIETARY RIGHTS TO A PRODUCT CANDIDATE, SUCH AS PATENT RIGHTS, AND WILL NOT LET THE PRODUCT CANDIDATE BE SOLD ON REASONABLE TERMS, OR AT ALL; OR |
| • | THE PRODUCT CANDIDATE IS NOT COST EFFECTIVE IN LIGHT OF EXISTING THERAPEUTICS. |
PRECLINICAL AND CLINICAL DATA CAN BE INTERPRETED IN DIFFERENT WAYS, WHICH COULD DELAY, LIMIT OR PREVENT REGULATORY APPROVAL. NEGATIVE OR INCONCLUSIVE RESULTS OR ADVERSE MEDICAL EVENTS DURING A CLINICAL TRIAL COULD DELAY, LIMIT OR PREVENT REGULATORY APPROVAL. IN ADDITION, ANY SIGNIFICANT PROBLEM IN THE PRODUCTION OF THE PRODUCTS OF THE COMPANY, SUCH AS THE INABILITY OF A SUPPLIER TO PROVIDE RAW MATERIALS OR SUPPLIES USED TO MANUFACTURE OUR PRODUCTS, EQUIPMENT OBSOLESCENCE, MALFUNCTIONS OR FAILURES, PRODUCT QUALITY OR CONTAMINATION PROBLEMS, OR CHANGES IN REGULATORY REQUIREMENTS OR STANDARDS THAT REQUIRE MODIFICATIONS TO MANUFACTURING PROCESS OF THE COMPANY COULD DELAY, LIMIT OR PREVENT REGULATORY APPROVAL WHICH COULD HAVE A MATERIAL ADVERSE EFFECT ON ITS BUSINESS, FINANCIAL CONDITION AND RESULTS OR THE TRADING PRICE OF ITS SECURITIES. THERE CAN BE NO ASSURANCE AS TO WHETHER OR WHEN THE COMPANY WILL RECEIVE REGULATORY APPROVALS FOR ITS PRODUCTS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 11, PARAGRAPH 11.2 OF THIS REGISTRATION DOCUMENT.
| 4.2.6 | RISKS RELATED TO INTELLECTUAL PROPERTY |
A) PROTECTION OF INTELLECTUAL PROPERTY OF CTI
DEVELOPMENT AND PROTECTION OF CTI’S INTELLECTUAL PROPERTY ARE CRITICAL TO ITS BUSINESS. IF THE COMPANY DOES NOT ADEQUATELY PROTECT ITS INTELLECTUAL PROPERTY, COMPETITORS MAY BE ABLE TO PRACTICE ITS TECHNOLOGIES. CTI’S SUCCESS DEPENDS IN PART ON ITS ABILITY TO:
| • | OBTAIN PATENT PROTECTION FOR ITS PRODUCTS OR PROCESSES BOTH IN THE UNITED STATES AND OTHER COUNTRIES, |
| • | PROTECT TRADE SECRETS, AND |
C-46
| • | PREVENT OTHERS FROM INFRINGING ON ITS PROPRIETARY RIGHTS. |
WHEN POLYMERS ARE LINKED, OR CONJUGATED, TO DRUGS, THE RESULTS ARE REFERRED TO AS POLYMER-DRUG CONJUGATES. THE COMPANY IS DEVELOPING DRUG DELIVERY TECHNOLOGY THAT LINKS CHEMOTHERAPY TO BIODEGRADABLE POLYMERS. FOR EXAMPLE, OPAXIO IS PACLITAXEL, THE ACTIVE INGREDIENT IN TAXOL®, ONE OF THE WORLD’S BEST SELLING CANCER DRUGS, LINKED TO POLYGLUTAMATE. THE COMPANY MAY NOT RECEIVE A PATENT FOR ALL OF ITS POLYMER-DRUG CONJUGATES AND IT MAY BE CHALLENGED BY THE HOLDER OF A PATENT COVERING THE UNDERLYING DRUG AND/OR METHODS FOR ITS USE OR MANUFACTURE.
THE PATENT POSITION OF BIOPHARMACEUTICAL FIRMS GENERALLY IS HIGHLY UNCERTAIN AND INVOLVES COMPLEX LEGAL AND FACTUAL QUESTIONS. THE U.S. PATENT AND TRADEMARK OFFICE HAS NOT ESTABLISHED A CONSISTENT POLICY REGARDING THE BREADTH OF CLAIMS THAT IT WILL ALLOW IN BIOTECHNOLOGY PATENTS. IF IT ALLOWS BROAD CLAIMS, THE NUMBER AND COST OF PATENT INTERFERENCE PROCEEDINGS IN THE UNITED STATES AND THE RISK OF INFRINGEMENT LITIGATION MAY INCREASE. IF IT ALLOWS NARROW CLAIMS, THE RISK OF INFRINGEMENT MAY DECREASE, BUT THE VALUE OF OUR RIGHTS UNDER CTI’S PATENTS, LICENSES AND PATENT APPLICATIONS MAY ALSO DECREASE. PATENT APPLICATIONS IN WHICH THE COMPANY HAS RIGHTS MAY NEVER ISSUE AS PATENTS AND THE CLAIMS OF ANY ISSUED PATENTS MAY NOT AFFORD MEANINGFUL PROTECTION FOR ITS TECHNOLOGIES OR PRODUCTS. IN ADDITION, PATENTS ISSUED TO THE COMPANY OR ITS LICENSORS MAY BE CHALLENGED AND SUBSEQUENTLY NARROWED, INVALIDATED OR CIRCUMVENTED. LITIGATION, INTERFERENCE PROCEEDINGS OR OTHER GOVERNMENTAL PROCEEDINGS THAT THE COMPANY MAY BECOME INVOLVED IN WITH RESPECT TO ITS PROPRIETARY TECHNOLOGIES OR THE PROPRIETARY TECHNOLOGY OF OTHERS COULD RESULT IN SUBSTANTIAL COST TO IT. PATENT LITIGATION IS WIDESPREAD IN THE BIOTECHNOLOGY INDUSTRY, AND ANY PATENT LITIGATION COULD HARM CTI’S BUSINESS. COSTLY LITIGATION MIGHT BE NECESSARY TO PROTECT A PATENT POSITION OR TO DETERMINE THE SCOPE AND VALIDITY OF THIRD-PARTY PROPRIETARY RIGHTS, AND THE COMPANY MAY NOT HAVE THE REQUIRED RESOURCES TO PURSUE ANY SUCH LITIGATION OR TO PROTECT ITS PATENT RIGHTS. ANY ADVERSE OUTCOME IN LITIGATION WITH RESPECT TO THE INFRINGEMENT OR VALIDITY OF ANY PATENTS OWNED BY THIRD-PARTIES COULD SUBJECT THE COMPANY TO SIGNIFICANT LIABILITIES TO THIRD-PARTIES, REQUIRE DISPUTED RIGHTS TO BE LICENSED FROM THIRD-PARTIES OR REQUIRE IT TO CEASE USING A PRODUCT OR TECHNOLOGY.
THE COMPANY ALSO RELIES UPON TRADE SECRETS, PROPRIETARY KNOW-HOW AND CONTINUING TECHNOLOGICAL INNOVATION TO REMAIN COMPETITIVE. THIRD-PARTIES MAY INDEPENDENTLY DEVELOP SUCH KNOW-HOW OR OTHERWISE OBTAIN ACCESS TO ITS TECHNOLOGY. WHILE THE COMPANY REQUIRES ITS EMPLOYEES, CONSULTANTS AND CORPORATE PARTNERS WITH ACCESS TO PROPRIETARY INFORMATION TO ENTER INTO CONFIDENTIALITY AGREEMENTS, THESE AGREEMENTS MAY NOT BE HONORED
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.4.1 AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
C-47
B) RELIANCE ON THIRD PARTIES’ INTELLECTUAL PROPERTY
THE COMPANY HAS LICENSED INTELLECTUAL PROPERTY, INCLUDING PATENT APPLICATIONS RELATING TO INTELLECTUAL PROPERTY FOR PIXANTRONE AND BROSTALLICIN. THE COMPANY HAS ALSO IN-LICENSED THE INTELLECTUAL PROPERTY RELATING TO ITS DRUG DELIVERY TECHNOLOGY RELATING TO OPAXIO THAT USES POLYMERS THAT ARE LINKED TO DRUGS, KNOWN AS POLYMER-DRUG CONJUGATES. SOME OF CTI’S PRODUCT DEVELOPMENT PROGRAMS DEPEND ON ITS ABILITY TO MAINTAIN RIGHTS UNDER THESE LICENSES. EACH LICENSOR HAS THE POWER TO TERMINATE ITS AGREEMENT WITH THE COMPANY IF IT FAILS TO MEET ITS OBLIGATIONS UNDER THESE LICENSES. THE COMPANY MAY NOT BE ABLE TO MEET ITS OBLIGATIONS UNDER THESE LICENSES. IF THE COMPANY DEFAULTS UNDER ANY LICENSE AGREEMENTS, IT MAY LOSE ITS RIGHT TO MARKET AND SELL ANY PRODUCTS BASED ON THE LICENSED TECHNOLOGY.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.1 AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
C) INFRINGEMENT OF THIRD PARTIES’ INTELLECTUAL PROPERTY
THE COMPANY ATTEMPTS TO MONITOR PATENT FILINGS FOR PATENTS THAT MAY BE RELEVANT TO ITS PRODUCTS AND PRODUCT CANDIDATES IN AN EFFORT TO GUIDE THE DESIGN AND DEVELOPMENT OF ITS PRODUCTS TO AVOID INFRINGEMENT BUT HAS NOT CONDUCTED AN EXHAUSTIVE SEARCH. THE COMPANY MAY NOT BE ABLE TO SUCCESSFULLY CHALLENGE THE VALIDITY OF THESE PATENTS AND COULD HAVE TO PAY SUBSTANTIAL DAMAGES, POSSIBLY INCLUDING TREBLE DAMAGES, FOR PAST INFRINGEMENT AND ATTORNEYS’ FEES IF IT IS ULTIMATELY DETERMINED THAT ITS PRODUCTS INFRINGE A THIRD-PARTY’S PATENTS. FURTHER, THE COMPANY MAY BE PROHIBITED FROM SELLING ITS PRODUCTS BEFORE IT OBTAINS A LICENSE, WHICH, IF AVAILABLE AT ALL, MAY REQUIRE IT TO PAY SUBSTANTIAL ROYALTIES. MOREOVER, THIRD PARTIES MAY CHALLENGE THE PATENTS THAT HAVE BEEN ISSUED OR LICENSED TO CTI. EVEN IF INFRINGEMENT CLAIMS AGAINST THE COMPANY ARE WITHOUT MERIT, OR IF THE COMPANY CHALLENGES THE VALIDITY OF ISSUED PATENTS, LAWSUITS TAKE SIGNIFICANT TIME, MAY BE EXPENSIVE AND MAY DIVERT MANAGEMENT ATTENTION FROM OTHER BUSINESS CONCERNS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.4. AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
| 4.2.7 | RISKS RELATED TO CLINICAL TRIALS |
BEFORE REGULATORY APPROVAL FOR ANY POTENTIAL PRODUCT CAN BE OBTAINED, THE COMPANY MUST UNDERTAKE EXTENSIVE PRE-CLINICAL AND CLINICAL TESTING ON HUMANS TO DEMONSTRATE THE SAFETY AND EFFICACY OF THE PRODUCT. SUCH TESTING MAY REQUIRE SIGNIFICANT TIME, RESOURCES OR EXPERTISE TO THOSE ORIGINALLY EXPECTED TO BE NECESSARY, OR BE SUSPENDED AT ANY TIME BY THE REGULATORY AUTHORITIES ON THE BASIS THAT THE PARTICIPANTS ARE BEING EXPOSED TO UNACCEPTABLE HEALTH RISKS OR FOR OTHER REASONS, UNDER THE DISCRETION OF SAME REGULATORY AUTHORITIES.
THE COMPANY HAS LIMITED EXPERIENCE IN CONDUCTING CLINICAL TRIALS. IT EXPECTS TO CONTINUE TO RELY ON THIRD-PARTIES, SUCH AS CONTRACT RESEARCH ORGANIZATIONS, ACADEMIC INSTITUTIONS AND/OR COOPERATIVE GROUPS, TO CONDUCT, OVERSEE AND MONITOR CLINICAL TRIALS AS WELL AS TO PROCESS THE CLINICAL RESULTS AND MANAGE
C-48
TEST REQUESTS, WHICH MAY RESULT IN DELAYS OR FAILURE TO COMPLETE TRIALS, IF THE THIRD-PARTIES FAIL TO PERFORM OR TO MEET THE APPLICABLE STANDARDS.
IF THE COMPANY FAILS TO COMMENCE OR COMPLETE, NEEDS TO PERFORM MORE OR LARGER CLINICAL TRIALS THAN PLANNED OR EXPERIENCES DELAYS IN ANY OF ITS PRESENT OR PLANNED CLINICAL TRIALS, ITS DEVELOPMENT COSTS MAY INCREASE AND/OR ITS ABILITY TO COMMERCIALIZE ITS PRODUCT CANDIDATES MAY BE ADVERSELY AFFECTED. IF DELAYS OR COSTS ARE SIGNIFICANT, CTI’S FINANCIAL RESULTS AND ITS ABILITY TO COMMERCIALIZE ITS PRODUCT CANDIDATES MAY BE ADVERSELY AFFECTED.
FOR MORE INFORMATION, ALSO WITH RESPECT TO THE ELEMENTS NECESSARY IN ORDER TO FULFILLMENT THE CLINICAL STUDIES, PLEASE REFER TO CHAPTER 6, PARAGRAPHS 6.1.1 AND 6.12 AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
| 4.2.8 | RISKS RELATED TO RELIANCE ON NOVEL TECHNOLOGIES |
THE COMPANY BASES SEVERAL OF ITS PRODUCT CANDIDATES UPON NOVEL TECHNOLOGIES THAT IT IS USING TO DEVELOP DRUGS FOR THE TREATMENT OF CANCER. THESE TECHNOLOGIES HAVE NOT BEEN PROVEN. FURTHERMORE, PRECLINICAL RESULTS IN ANIMAL STUDIES MAY NOT PREDICT OUTCOMES IN HUMAN CLINICAL TRIALS. CTI’S PRODUCT CANDIDATES MAY NOT BE PROVEN SAFE OR EFFECTIVE. IF THESE TECHNOLOGIES DO NOT WORK, CTI’S DRUG CANDIDATES MAY NOT DEVELOP INTO COMMERCIAL PRODUCTS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPHS 6.1.1 AND 6.1.2 OF THIS REGISTRATION DOCUMENT.
| 4.2.9 | RISKS RELATED TO PRODUCTS LIABILITY AND INSURANCE |
CTI’S BUSINESS EXPOSES IT TO POTENTIAL PRODUCT LIABILITY RISKS INHERENT IN THE TESTING, MANUFACTURING AND MARKETING OF HUMAN PHARMACEUTICAL PRODUCTS, AND IT MAY NOT BE ABLE TO AVOID SIGNIFICANT PRODUCT LIABILITY EXPOSURE. WHILE IT HAS INSURANCE COVERING USE OF ITS PRODUCT CANDIDATES IN ITS CLINICAL TRIALS, IT IS POSSIBLE THAT THE COMPANY WILL NOT BE ABLE TO MAINTAIN SUCH INSURANCE ON ACCEPTABLE TERMS OR THAT ANY INSURANCE OBTAINED WILL PROVIDE ADEQUATE COVERAGE AGAINST POTENTIAL LIABILITIES. CTI’S INABILITY TO OBTAIN SUFFICIENT INSURANCE COVERAGE AT AN ACCEPTABLE COST OR OTHERWISE TO PROTECT AGAINST POTENTIAL PRODUCT LIABILITY CLAIMS COULD PREVENT OR LIMIT THE COMMERCIALIZATION OF ANY PRODUCTS IT DEVELOPS. IF A PRODUCT LIABILITY CLAIM WERE SUCCESSFUL AGAINST CTI AND THE JUDGMENT AGAINST CTI WAS IN EXCESS OF CTI’S INSURANCE COVERAGE AGAINST THE CLAIM, SUCH RESULT MAY HARM CTI’S FINANCIAL STATEMENTS AND RESULTS OF OPERATIONS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.1 OF THIS REGISTRATION DOCUMENT.
| 4.2.10 | RISKS RELATED TO THE USE OF HAZARDOUS MATERIALS |
CTI’S RESEARCH AND DEVELOPMENT ACTIVITIES INVOLVE THE CONTROLLED USE OF HAZARDOUS MATERIALS, CHEMICALS AND VARIOUS RADIOACTIVE COMPOUNDS. THE COMPANY IS SUBJECT TO INTERNATIONAL, FEDERAL, STATE, AND LOCAL LAWS AND REGULATIONS GOVERNING THE USE, MANUFACTURE, STORAGE, HANDLING AND DISPOSAL
C-49
OF SUCH MATERIALS AND CERTAIN WASTE PRODUCTS. ALTHOUGH THE COMPANY BELIEVES THAT ITS SAFETY PROCEDURES FOR HANDLING AND DISPOSING OF SUCH MATERIALS COMPLY WITH THE STANDARDS PRESCRIBED BY STATE AND FEDERAL REGULATIONS, THE RISK OF ACCIDENTAL CONTAMINATION OR INJURY FROM THESE MATERIALS CANNOT BE ELIMINATED COMPLETELY. IN THE EVENT OF SUCH AN ACCIDENT, IT COULD BE HELD LIABLE FOR ANY DAMAGES THAT RESULT AND ANY SUCH LIABILITY NOT COVERED BY INSURANCE COULD EXCEED ITS RESOURCES. COMPLIANCE WITH ENVIRONMENTAL LAWS AND REGULATIONS MAY BE EXPENSIVE, AND CURRENT OR FUTURE ENVIRONMENTAL REGULATIONS MAY IMPAIR CTI’S RESEARCH, DEVELOPMENT OR PRODUCTION EFFORTS.
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.1 AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
| 4.2.11 | RISKS RELATED TO ANIMAL TESTING |
CERTAIN OF CTI’S RESEARCH AND DEVELOPMENT ACTIVITIES INVOLVE ANIMAL TESTING. SUCH ACTIVITIES HAVE BEEN THE SUBJECT OF CONTROVERSY AND ADVERSE PUBLICITY. ANIMAL RIGHTS GROUPS AND OTHER ORGANIZATIONS AND INDIVIDUALS HAVE ATTEMPTED TO STOP ANIMAL TESTING ACTIVITIES BY PRESSING FOR LEGISLATION AND REGULATION IN THESE AREAS AND BY DISRUPTING ACTIVITIES THROUGH PROTESTS AND OTHER MEANS. TO THE EXTENT THE ACTIVITIES OF THESE GROUPS ARE SUCCESSFUL, CTI’S BUSINESS COULD BE MATERIALLY HARMED BY DELAYING OR INTERRUPTING ITS RESEARCH AND DEVELOPMENT ACTIVITIES
FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 6, PARAGRAPH 6.1.1 AND CHAPTER 11, PARAGRAPH 11.1 OF THIS REGISTRATION DOCUMENT.
| 4.3 | RISK FACTORS RELATING TO THE FINANCIAL INSTRUMENTS ISSUED |
| 4.3.1 | RISKS RELATED TO THE VOLATILITY OF CTI’S STOCK PRICE |
THE MARKET PRICE FOR SECURITIES OF BIOPHARMACEUTICAL AND BIOTECHNOLOGY COMPANIES, INCLUDING CTI’S, HISTORICALLY HAS BEEN HIGHLY VOLATILE, AND THE MARKET FROM TIME TO TIME HAS EXPERIENCED SIGNIFICANT PRICE AND VOLUME FLUCTUATIONS THAT ARE UNRELATED TO THE OPERATING PERFORMANCE OF SUCH COMPANIES. FOR EXAMPLE, DURING THE TWELVE MONTH PERIOD ENDED JULY 30, 2010, CTI’S STOCK PRICE RANGED FROM A LOW OF $0.12 TO A HIGH OF $1.83. FLUCTUATIONS IN THE TRADING PRICE OR LIQUIDITY OF CTI’S COMMON STOCK MAY ADVERSELY AFFECT THE VALUE OF YOUR INVESTMENT IN ITS COMMON STOCK.
FACTORS THAT MAY HAVE A SIGNIFICANT IMPACT ON THE MARKET PRICE AND MARKETABILITY OF CTI’S SECURITIES INCLUDE:
| • | ANNOUNCEMENTS BY THE COMPANY OR OTHERS OF RESULTS OF PRECLINICAL TESTING AND CLINICAL TRIALS AND REGULATORY ACTIONS; |
| • | ANNOUNCEMENTS OF TECHNOLOGICAL INNOVATIONS OR NEW COMMERCIAL THERAPEUTIC PRODUCTS BY CTI, ITS COLLABORATIVE PARTNERS OR ITS PRESENT OR POTENTIAL COMPETITORS; |
C-50
| • | THE ISSUANCE OF ADDITIONAL DEBT, EQUITY OR OTHER SECURITIES WHICH NEED TO BE PURSUED IN 2010 TO GENERATE ADDITIONAL FUNDS TO COVER OUR CURRENT DEBT AND OPERATING EXPENSES; |
| • | CTI’S QUARTERLY OPERATING RESULTS; |
| • | DEVELOPMENTS OR DISPUTES CONCERNING PATENT OR OTHER PROPRIETARY RIGHTS; |
| • | DEVELOPMENTS IN CTI’S RELATIONSHIPS WITH COLLABORATIVE PARTNERS; |
| • | ACQUISITIONS OR DIVESTITURES; |
| • | LITIGATION AND GOVERNMENT PROCEEDINGS; |
| • | ADVERSE LEGISLATION, INCLUDING CHANGES IN GOVERNMENTAL REGULATION; |
| • | THIRD-PARTY REIMBURSEMENT POLICIES; |
| • | CHANGES IN SECURITIES ANALYSTS’ RECOMMENDATIONS; |
| • | SHORT SELLING; |
| • | CHANGES IN HEALTH CARE POLICIES AND PRACTICES; |
| • | ECONOMIC AND OTHER EXTERNAL FACTORS; AND |
| • | GENERAL MARKET CONDITIONS. |
IN THE PAST, FOLLOWING PERIODS OF VOLATILITY IN THE MARKET PRICE OF A COMPANY’S SECURITIES, SECURITIES CLASS ACTION LITIGATION HAS OFTEN BEEN INSTITUTED. FOR EXAMPLE, IN THE CASE OF CTI, CTI AND CERTAIN CTI’S OFFICERS AND DIRECTORS ARE NAMED AS DEFENDANTS IN PURPORTED SECURITIES FROM MAY 5, 2009 THROUGH MARCH 19, 2010. THESE LAWSUITS SEEK UNSPECIEFIED DAMAGES AND, AS WITH ANY LITIGATION PROCEEDING, CTI CAN NOT PREDICT WITH CERTAINTY THE EVENTUAL OUTCOME OF PENDING LITIGATION. FURTHERMORE, WE MAY HAVE TO INCUR SUBSTANTIAL EXPENSES IN CONNECTION WITH THESE LAWSUITS AND THE CTI’S MANAGEMENT’S ATTENTION AND RESOURCES COULD BE DIVERTED FROM CTI’S BUSINESS AS CTI RESPOND TO LITIGATION.
THE COMPANY MAINTAINS SIGNIFICANT INSURANCE TO COVER THESE RISKS FOR THE COMPANY AND ITS DIRECTORS AND OFFICERS, BUT ITS INSURANCE IS SUBJECT TO HIGH DEDUCTIBLES TO REDUCE PREMIUM EXPENSE, AND THERE IS NO GUARANTEE THAT THE INSURANCE WILL COVER ANY SPECIFIC CLAIM THAT THE COMPANY MAY FACE IN THE FUTURE, OR THAT IT WILL BE ADEQUATE TO COVER ALL POTENTIAL LIABILITIES AND DAMAGES.
FOR MORE INFORMATION ON THE SHARE VALUE, PLEASE REFER TO CHAPTER 20, PARAGRAPHS 20.1.3 AND 20.8 OF THIS REGISTRATION DOCUMENT.
| 4.3.2 | RISKS RELATED TO THE MARKET PRICE OF THE COMMON STOCK OF THE COMPANY WHICH MAY BE ADVERSELY AFFECTED BY MARKET CONDITIONS AFFECTING THE STOCK MARKETS IN GENERAL, INCLUDING PRICE AND TRADING FLUCTUATIONS ON THE NASDAQ CAPITAL MARKET |
THE MARKET PRICE OF THE COMMON STOCK OF THE COMPANY MAY BE ADVERSELY AFFECTED BY MARKET CONDITIONS AFFECTING THE STOCK MARKETS IN GENERAL,
C-51
INCLUDING PRICE AND TRADING FLUCTUATIONS ON THE NASDAQ CAPITAL MARKET. THESE CONDITIONS MAY RESULT IN (I) VOLATILITY IN THE LEVEL OF, AND FLUCTUATIONS IN, THE MARKET PRICES OF STOCKS GENERALLY AND, IN TURN, OUR SHARES OF COMMON STOCK, AND (II) SALES OF SUBSTANTIAL AMOUNTS OF THE COMMON STOCK OF THE COMPANY IN THE MARKET, IN EACH CASE THAT COULD BE UNRELATED OR DISPROPORTIONATE TO CHANGES IN OUR OPERATING PERFORMANCE.
| 4.3.3 | RISKS RELATED TO POSSIBILE FUTURE SALES OR OTHER DILUTION OF EQUITY OF THE COMPANY, WHICH MAY ADVERSELY AFFECT THE MARKET PRICE OF SHARES OF ITS COMMON STOCK |
THE COMPANY IS NOT RESTRICTED FROM ISSUING ADDITIONAL SHARES OF COMMON STOCK OR PREFERRED STOCK, INCLUDING ANY SECURITIES THAT ARE CONVERTIBLE INTO OR EXCHANGEABLE FOR, OR THAT REPRESENT THE RIGHT TO RECEIVE, SHARES OF COMMON STOCK OR PREFERRED STOCK OR ANY SUBSTANTIALLY SIMILAR SECURITIES. THE MARKET PRICE OF OUR SHARES OF COMMON STOCK OR PREFERRED STOCK COULD DECLINE AS A RESULT OF SALES OF A LARGE NUMBER OF SHARES OF COMMON STOCK OF THE COMPANY OR PREFERRED STOCK OR SIMILAR SECURITIES IN THE MARKET, OR THE PERCEPTION THAT SUCH SALES COULD OCCUR IN THE FUTURE.
C-52
CHAPTER 5 COMPANY INFORMATION
| 5.1 | History and Development of the Company |
| 5.1.1 | The legal and commercial name of the Company |
The Company’s name is Cell Therapeutics, Inc.
| 5.1.2 | The place of registration of the Company and its registration number |
The registered and the principal administrative offices of the Company are located in the State of Washington at 501 Elliott Avenue West, Suite 400, Seattle, Washington 98119 (U.S.).
The Company is not registered with any companies’ register or any other registers having a legal relevance under the laws and regulations applicable to it.
| 5.1.3 | Date of incorporation and the length of life of the Company, except where indefinite |
The Company was incorporated on September 4, 1991 under the laws of the State of Washington (U.S.). Pursuant to its Amended and Restated Articles of Incorporation, the Company is to have perpetual existence.
| 5.1.4 | The domicile and legal form of the Company, the legislation under which the Company operates, its country of incorporation, and the address and telephone number of its registered office (or principal place of business if different from its registered office) |
As regards the domicile of the Company and its registered address, please see Chapter 5, Paragraph 5.1.2, “The place of registration of the Company and its registration number” of this Registration Document.
The Company (a U.S. corporation) is incorporated in the United States under, and governed by the laws of, the State of Washington (U.S.). In case of disputes, the courts of the State of Washington and the courts of the United States shall have jurisdiction thereon.
The telephone number of the Company’s registered offices is (206) 282-7100.
| 5.1.5 | Important events in the development of the Company’s business. |
The Company is a biopharmaceutical company incorporated in 1991 under the laws of the State of Washington (U.S.), which focuses on the development, acquisition and commercialization of novel treatments for cancer. One of the Company’s principal founders was James A. Bianco, who has been its Chief Executive Officer since February 1992 and one of its directors since September 1991. Dr. Bianco also served as the Company’s President from inception until August 2008.
A) Important events from the incorporation of the Company until the end of 2006
In January 2000, the Company acquired TRISENOX (arsenic trioxide) (“TRISENOX”) upon its acquisition of PolaRx, a single product company that owned the rights to TRISENOX.
In September 2000, the Company received approval of its NDA by the FDA for TRISENOX, and also completed a public offering of 90,000 shares of Common Stock at $1,520.00 per share, which generated net proceeds of $127.5 million.
In October 2000, the Company commenced sales of TRISENOX.
C-53
In September 2001, the Company entered into a supply agreement with Natural Pharmaceuticals, Inc. for paclitaxel, a key starting material for its OPAXIO drug candidate. For more information, see Chapter 6, Paragraph 6.4.3.
In March 2002, the Company received from the EMA approval to market TRISENOX in the EU and commenced the launch and sale of TRISENOX in the EU during the second quarter of 2002.
In the fourth quarter of 2002, the Company initiated three pivotal OPAXIO phase III clinical trials. These include one phase III trial for the second-line treatment of NSCLC, and two phase III trials of OPAXIO in the front-line treatment of PS2 patients with NSCLC.
In December 2002, the Company entered into a distribution agreement with Nippon Shinyaku Co. Ltd. for TRISENOX in Asia.
On June 16, 2003, the Company entered into a merger agreement with Novuspharma, and on October 23 2003, the shareholders of the Company approved the merger at a special meeting. The merger with Novuspharma was effective starting from January 1, 2004, and the Company’s shares were admitted to listing in Italy starting from January 2, 2004.
In April 2004, the Company and the Gynecologic Oncology Group (“GOG”), signed a clinical trials agreement for the GOG to sponsor and conduct a phase III clinical trial of OPAXIO in patients with ovarian cancer.
In March 2005, the GOG initiated the phase III clinical trial examining the ability of OPAXIO to maintain remission and prolong the survival of ovarian cancer patients.
In July 2005, the Company divested TRISENOX and certain proteasome assets to Cephalon, Inc. (“Cephalon”) for approximately $32.5 million net proceeds, including proceeds from transition services provided and after repayment of the PharmaBio agreement for TRISENOX. In December 2005, the Company launched a clinical trial, known as the PIONEER trial, in women of OPAXIO versus paclitaxel chemotherapy for the treatment of poor performance status (“PS2”) patients with chemotherapy-naïve advanced stage NSCLC.
In September 2006, the Company entered into an exclusive worldwide licensing agreement with Novartis International Pharmaceutical Ltd., for the development and commercialization of OPAXIO and pixantrone. Please refer to Chapter 22, Paragraph 22.1.1, “Agreements with Novartis” of this Registration Document for a detailed description of this transaction.
B) Important events occurred during the three-year period 2007-2009
In early 2007, the Company submitted two new protocols under a Special Protocol Assessment (“SPA”), to the FDA. These new trials, known as PGT306 and PGT307, focus exclusively on NSCLC in women with pre-menopausal estrogen levels, the subset of patients where OPAXIO demonstrated the greatest potential survival advantage in the STELLAR trials.
In May 2007, the Company formed Aequus Biopharma, Inc., and contributed a license to develop the Company’s Genetic Polymer™ technology in exchange for 70% of the equity ownership of Aequus Biopharma. The Genetic Polymer technology was created at the Company to speed the manufacture,
C-54
In July 2007, the Company acquired Systems Medicine, Inc., a privately held oncology company, through a stock-for-stock merger valued at $20 million.
In August 2007, the Company entered into an asset purchase agreement with Biogen Idec Inc. (“Biogen”), for the purchase of Zevalin (ibritumomab tiuxetan), a radiopharmaceutical product, for development, marketing and sale in the United States.
On January 30, 2008, the Company announced a plan to refocus its resources on late-stage and marketed products, which involved increasing sales of Zevalin in the United States and preparing the marketing applications for OPAXIO and pixantrone, while advancing the clinical development of brostallicin. This plan was intended to reduce operating expenses throughout the company by approximately 35% and reduce the company’s projected net cash operating expenses to a forecasted $77 million in 2008. As part of these refocusing efforts, approximately 30 of the Company’s U.S. employees were terminated.
On March 4, 2008, the Company submitted a Marketing Approval Application (a “MAA”) for OPAXIO to the EMA based on discussions with the EMA Scientific Advice Working Party. The MAA was accepted for review in April 2008.
On June 18, 2008, the Company announced an agreement with Bayer Schering Pharma to gain access to Bayer’s phase III Zevalin(R) (90Y-ibritumomab tiuxetan) First-line Indolent Trial, FIT data. The Company used the data from the trial to begin discussions with the FDA and to ultimately submit a supplemental Biologics License Application (a “sBLA”) in October 2008 for Zevalin based on Bayer’s trial results. The sBLA was accepted for review by the FDA in November 2008 and was granted priority review status with a target date of April 2, 2009 for an FDA decision regarding approval. As discussed below, in December 2008, the Company transferred all of its assets related to Zevalin including the sBLA to RIT Oncology, a joint venture formed by the Company and Spectrum, and in March 2009, the Company sold its entire interest in RIT Oncology to Spectrum.
On June 19, 2008, the Company held a Special Meeting in lieu of the Annual Meeting of Shareholders at its headquarters in Seattle. At the meeting, shareholders approved, among other things, a proposal to increase the number of shares of authorized stock (including an increase in the number of shares of authorized Common Stock), the reduction of quorum for future shareholder meetings to one-third of the outstanding shares entitled to vote and a proposal to allow the Board of Directors to implement a reverse stock split if and when the Board deemed such action appropriate.
On November 11, 2008, the Company announced that it achieved the primary efficacy endpoint of its phase III EXTEND (PIX301) trial of pixantrone for patients with advanced, relapsed aggressive non-Hodgkin’s lymphoma (NHL) based on a preliminary intent to treat efficacy analysis. The preliminary data indicated patients on the pixantrone arm of the study achieved a higher confirmed and unconfirmed complete remissions than those in the control group and had increased overall response rates. Complete safety information is not yet available for the study, however, the study was monitored on an ongoing basis by an independent Data Safety Monitoring Committee and no serious concerns were raised.
In November 2008, the Company entered into an agreement with Spectrum to form a 50/50 owned joint venture, RIT Oncology LLC, to commercialize and develop Zevalin in the United States. Upon closing the transaction in December 2008, assets related to Zevalin, including the sBLA, were transferred to RIT
C-55
Oncology. Please refer to subsequent Paragraph 22.1.7 of this Registration Document for more information about this transaction.
In January 2009, following a meeting with the FDA regarding the results of the phase III EXTEND (PIX 301) trial of pixantrone, the FDA provided a Pre-NDA communication on that trial that was the basis for supporting submission by the Company of an NDA for pixantrone.
On February 27, 2009, the Company announced that it has started a collective dismissal procedure pursuant to Law 223/1991 concerning all 62 employees at the branch in Bresso, and that if an appropriate buyer or partner for the Company’s European operations could not be found, the Company would proceed to shut down its Italian branch in order to save operating costs. With respect to the dismissal procedure, please also refer to the additional information provided hereinafter with respect to the date May 13, 2009.
Also in February 2009, the Company announced that it had entered into an agreement with IDIS to manage pixantrone as an investigational drug on a named patient basis in Europe. Under the agreement, pixantrone is supplied by IDIS to healthcare professionals for the treatment of individual patients with relapsing aggressive non-Hodgkin’s lymphoma. The program initiated in May 2009.
The Company exercised in March 2009 a put option under the agreement governing RIT Oncology, pursuant to which Spectrum purchased the Company’s 50% ownership of RIT Oncology for approximately $16.5 million. Please refer to subsequent Paragraph 22.1.7 of this Registration Document for more information about this transaction.
On March 24, 2009, the Company held a special meeting of the shareholders at its headquarters in Seattle. At the meeting, shareholders approved, among other things, an increase in the number of shares of authorized stock, including an increase in the number of shares of authorized Common Stock.
In addition, in a separate transaction, the Company entered into an Exchange Agreement dated April 8, 2009 with CD Investment Partners, Ltd. Pursuant to such Exchange Agreement, on April 17, 2009 the Company issued to CD Investment Partners, Ltd. 3,452,493 shares of newly-issued Common Stock in exchange for 1,000 shares of outstanding Series D 7% Convertible Preferred Stock and outstanding warrants to purchase 19,138 shares of Common Stock. The number of shares issued was derived by a formula, set forth in such Exchange Agreement, based on the average of the volume-weighted average prices of the Company’s Common Stock for the three trading days following the Company’s public announcement of an issuance of securities pursuant to its Form S-3 shelf registration statement as filed with the SEC, which resulted in using the trading prices of the Company’s Common Stock on April 14, 15 and 16, 2009.
On May 13, 2009, the Company entered into an agreement (the “Severance Agreement”) with the unions representing the employees of its Bresso, Italy operations in connection with the previously announced closure of the Company’s Bresso facilities. The Severance Agreement relates to a reduction in force of 56 positions at the Bresso facility. In addition, the Company has sent notices of termination to the six managers of the Bresso facility and will endeavor to enter into separate severance arrangements with these managers. The Bresso facility was used for pre-clinical research and was underutilized due to the Company’s focused business model on the development of late stage compounds and their commercialization. The Company completed the closure of the Bresso facility in September 2009.
C-56
On June 24, 2009, the Company announced that it has completed the submission of an NDA to the FDA for pixantrone to treat relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL). The beginning of the aforesaid submission was announced on April 14, 2009.
On September 28, 2009, the Company announced that it finalized the closing its Bresso, Italy operations and would have received approximately $1.1 million from the sale of assets. The Company expects the closure to save approximately $20 million in annual operating expenses in 2010 and beyond based on the exchange rates at the time of the closing. The Bresso facility was used for pre-clinical research and was underutilized due to the Company’s focused business model preparing for commercial launch of pixantrone and other phase III development stage products.
On September 29, 2009, the Company announced that it has applied to the European Medicines Agency (EMA) for orphan drug designation for pixantrone. Orphan drug designation is available in Europe for medical treatments and drugs intended to treat life-threatening or chronically debilitating conditions. Orphan drug designation can confer numerous benefits to companies developing such treatments, including regulatory assistance, reduced regulatory fees associated with applying for marketing approval, and assistance with clinical trial design.
On October 20, 2009, the Company’s Annual Meeting of Shareholders was held. At the meeting, shareholders elected Mr. Richard L. Love, Dr. Mary O. Mundinger, and Dr. Jack W. Singer to serve on the Company’s Board of Directors until the 2012 Annual Meeting. Shareholders approved the proposals to increase the number of shares available for issuance under its 2007 Equity Incentive Plan, increase the number of shares available for issuance under its 2007 Employee Purchase Plan and issue shares of Common Stock in lieu of future milestone payments related to the Company’s drug candidate brostallicin. Shareholders ratified the selection of Stonefield Josephson, Inc. as the Company’s independent auditors for the year ending December 31, 2009.
On December 17, 2009, the Company announced that on February 10, 2010, the U.S. Food and Drug Administration’s Oncologic Drugs Advisory Committee (ODAC) would have reviewed the New Drug Application (NDA) for pixantrone for the treatment of relapsed/refractory aggressive non-Hodgkin’s lymphoma (NHL). ODAC is an independent panel of experts that evaluates data concerning the efficacy and safety of marketed and investigational products for use in the treatment of cancer and makes appropriate recommendations to the FDA. The FDA regulations indicate that although the FDA considers the recommendation of the panel, the final decision regarding the approval of the a product is made by the FDA. At the ODAC meeting, committee members evaluate presentations of efficacy and safety data made by the pharmaceutical sponsor of the drug under review, FDA review staff, and occasionally third-party oncology experts in an open forum. Following the presentation, the committee members discuss questions posed by the Agency review staff and the meeting concludes with the committee voting on a recommendation to the FDA regarding approval.
On December 21, 2009, the Company announced that the EMA granted pixantrone orphan drug designation for the treatment of DLBCL which accounts for about 80% of aggressive non-Hodgkin’s lymphoma. Orphan drug designation is available in Europe for medical treatments and drugs intended to treat life-threatening or chronically debilitating conditions. As already mentioned, orphan drug
C-57
designation can confer numerous benefits to companies developing such treatments, including regulatory assistance, reduced regulatory fees associated with applying for marketing approval, and assistance with clinical trial design.
On December 24, 2009, CTI’s Board of Directors approved and adopted a Shareholder Rights Plan in which one preferred stock purchase right will be distributed for each common share held as of the close of business on January 7, 2010. Plesae refer to the subsequent Paragraph 21.2.6 for detailed information about such Shareholder Rights Plan.
C) Important events occurred in 2010
On January 13, 2010, the Company announced that it entered into an agreement to sell $30 million of shares of its Series 3 Preferred Stock and warrants to purchase shares of its common stock in a registered offering to two institutional investors. Please refer to subsequent Paragraph 21.1.4 for more information about shares of Series 3 Preferred Stock and connected warrants.
On March 4, 2010, the Company announced that it received a statement on March 1, 2010 from the Gynecologic Oncology Group (GOG) leadership that the phase III GOG-212 clinical trial of CTI’s OPAXIO(TM) used as maintenance therapy for ovarian cancer remains a high priority and enrollment will continue.
On March 8, 2010, the Company announced that the U.S. FDA has completed its inspection of the facility at NerPharMa (a pharmaceutical manufacturing company belonging to Nerviano Medical Sciences Srl, in Nerviano, Italy), which manufactures the CTI’s drug pixantrone and has found the site in compliance and acceptable for continued manufacturing of the drug product.
On March 22, 2010, the Company announced that U.S. FDA ODAC panel voted unanimously that clinical trial data was not adequate to support approval of pixantrone for patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma.
On March 31, 2010, the Company announced that it has entered into an agreement to sell $20 million of shares of its Series 4 Preferred Stock and warrants to purchase shares of its common stock in a registered offering to three institutional investors. Please refer to subsequent Paragraph 21.1.4 for more information about shares of Series 4 Preferred Stock and connected warrants.
On April 9, 2010, the Company announced the rejection by the FDA of the NDA for Pixantrone which was submitted on June 24, 2009. In particular, the Company announced that it had received a Complete Response Letter from the U.S. FDA regarding its NDA for Pixantrone (Pixuvri(TM) - pixantrone dimaleate) for relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL). The FDA cited as its primary reason for the action its concerns previously raised at the ODAC meeting on March 22, 2010 and recommended the Company conduct an additional trial to demonstrate the safety and effectiveness of its product. Based on the FDA’s ODAC presentation, which provided the Committee and the Company with alternative options to consider to make investigational drugs available to patients if drugs need to be studied further prior to approval, the Company has decided to pursue expanded access program for pixantrone while it conducts an additional study in aggressive NHL. The Company announced on September 14, 2010 that it intends to file an appeal of the FDA decision.
C-58
On April 15, 2010, the Company announced that it has conducted an immediate reduction in force of 36 employees. The Company expects the reduction in force and elimination of previously planned increase in commercial personnel along with a reduction in planned operating expenses to result in savings of approximately $16 million in 2010.
On April 19, 2010, the Company announced that it met with and received feedback from the rapporteurs and the European Medicines Agency’s (the “EMA”) medical reviewers regarding a proposed filing of a Marketing Authorization Application (“MAA”) for pixantrone in the European Union to treat relapsed/refractory aggressive non-Hodgkin’s lymphoma (NHL).
On April 21, 2010, the Company announced the European Medicines Agency (EMA) Pediatric Committee (the “PDCO”) recommended the Company submit an updated Pediatric Investigation Plan (“PIP”) for pixantrone following discussions with the Company about the preclinical and clinical pixantrone data, including PIX301, and the desire to explore the potential benefits pixantrone may offer to children with hematologic cancer.
On April 28, 2010, the Company announced that the North Central Cancer Treatment Group (“NCCTG”) plans to conduct a Phase II study of brostallicin in combination with cisplatin in patients with metastatic triple-negative breast (“mTNBC”) cancer, defined by tumors lacking expression of estrogen, progesterone receptors and without over-expression of HER2.
On May 13, 2010, the Company announced an agreement with the North Central Cancer Treatment Group (NCCTG) to conduct a phase II study of pixantrone in patients with HER2-negative metastatic breast cancer who have tumor progression after at least two, but not more than three, prior chemotherapy regimens. The trial will be conducted through the NCCTG, a national network of cancer specialists at community clinics, hospitals, and medical centers in the United States and Canada. The research base for NCCTG is located at Mayo Clinic in Rochester, MN.
On May 17, 2010, the Company announced that it entered into exchange agreements (the “Exchange Agreements”) with certain holders of some of the Company’s outstanding 4% Convertible Senior Subordinated Notes due 2010 (the “Notes”). Pursuant to one of the Exchange Agreements, the Company agreed to exchange an aggregate of 4,303,157 shares of the Company’s common stock for $1,848,000 aggregate outstanding principal amount of the Notes during the week ending May 21, 2010. The Company has since delivered a notice of termination of the Exchange Agreements to each of the holders party to the Exchange Agreements. The shares of common stock issued under the Exchange Agreements were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended, and/or Regulation D thereunder.
On May 24, 2010, the Company announced it has entered into an agreement to sell $21.0 million of shares of its Series 5 Preferred Stock in a registered offering to three institutional investors (collectively, the “Initial Purchasers”). The Initial Purchasers also received a warrant to purchase up to 1,250 shares of common stock for each share of Series 5 Preferred Stock purchased in the offering, for an aggregate of up to 26,250,000 shares of common stock issuable upon exercise of the warrants. Please refer to subsequent Paragraph 21.1.4 for more information about shares of Series 5 Preferred Stock and connected warrant.
C-59
On June 14, 2010, the Company announced that the Italian Medicines Agency (“AIFA”), the national authority responsible for drug regulation in Italy, has approved the facility at NerPharMa DS (a pharmaceutical manufacturing company belonging to Nerviano Medical Sciences Srl, in Nerviano, Italy) for the production of CTI’s drug candidate pixantrone.
On July 1, 2010, the Company announced that it has deposited $39.3 million in cash as trust funds with U.S. Bank National Association, as the trustee of the outstanding 4% convertible senior subordinated notes (the “Notes”), which is an amount sufficient to pay and discharge the entire amount due on the Notes, including accrued and unpaid interest.
On July 6, 2010, the Company announced that it has submitted an expanded PIP to the EMA, as part of the process for its submission for a MAA for pixantrone in the E.U. for the treatment of relapsed or refractory, aggressive non-Hodgkin’s lymphoma (NHL). CTI intends to file the MAA later this year. The pediatric program will study pixantrone in pediatric patients aged 6 months to 18 years with the goal of determining the comparative safety and effectiveness of pixantrone compared to doxorubicin in pediatric lymphoid cancers.
On July 14, 2010, the Company announced that it has signed a manufacturing agreement with NerPharMa DS (a pharmaceutical manufacturing company belonging to Nerviano Medical Sciences S.r.l. (“NerPharMa”), in Nerviano, Italy) for CTI’s drug candidate pixantrone. The five-year contract between CTI and NerPharMa provides for both the commercial and clinical supply of pixantrone.
On July 26, 2010, the Company announced that it has entered into an agreement to sell, subject to customary closing conditions, $4.06 million of securities to an accredited investor in a private offering pursuant to Section 4(2) of the Securities Act of 1933, as amended. The securities consist of 4,060 shares of Series 6 preferred stock, no par value per share, together with warrants to purchase an aggregate of 5.8 million shares of common stock. Please refer to subsequent Paragraph 21.1.4 for more information about shares of Series 6 Preferred Stock and connected warrants.
On August 4, 2010, the Company announced that it has filed for a Special Protocol Assessment (“SPA”) with the U.S. Food and Drug Administration (FDA) for the design of its new phase III trial of pixantrone for patients with relapsed or refractory aggressive B-Cell non-Hodgkin’s lymphoma (NHL).
On August 16, 2010, the Company announced that it has received notice that the European Medicines Agency (EMA) has validated the expanded Pediatric Investigation Plan (PIP) that CTI filed in July for pixantrone for the treatment of relapsed or refractory, aggressive non-Hodgkin’s lymphoma (NHL). Following the validation, the EMA Pediatric Committee (the PDCO) will review and comment or approve the content of pediatric plan. The pediatric program will study pixantrone in pediatric patients aged 6 months to 18 years with the goal of determining the comparative safety and effectiveness of pixantrone compared to doxorubicin in pediatric lymphoid cancers. As part of the PIP validation process which the validation of the PIP for pixantrone is part of the EMA reviews the PIP application and verifies that the PIP application is complete and the contents meet the EMA’s requirements for filing. The PIP approval process is in the early phase of filing for marketing approval typically prior to the submission of the marketing application.
C-60
On September 16, 2009 the Annual Meeting of shareholders of the Company approved:
| 1) | the election of John H. Bauer and Phillip M. Nudelman, MD as Class I directors to the Company’s Board of Directors, each to serve until the 2013 Annual Meeting; |
| 2) | an amendment to the Company’s amended and restated articles of incorporation to increase the total number of authorized shares from 810,000,000 to 1,210,000,000 and to increase the total number of authorized shares of common stock from 800,000,000 to 1,200,000,000; |
| 3) | an amendment to the Company’s 2007 Equity Incentive Plan, as amended (the “2007 Equity Plan”), to increase the number of shares available for issuance under the 2007 Equity Plan by 40,000,000 shares;(*) |
| 4) | the ratification of the selection of Stonefield Josephson, Inc. as the Company’s independent auditors for the year ending December 31, 2010. |
On October 19, 2010, the Company announced that the Pediatric Committee (the “PDCO”) of the European Medicines Agency (“EMA”) adopted an opinion agreeing to CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years. This positive opinion clears the way for CTI to submit its Marketing Authorization Application (“MAA”) in the E.U. within the fourth quarter of 2010 for pixantrone for the treatment of patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma (“NHL”).
On October 20, 2010, the Company entered into a securities purchase agreement, pursuant to which we agreed to issue in a registered offering an aggregate of 21,000 shares of its Series 7 preferred stock, initially convertible into approximately 56.8 million shares of our common stock and warrants to purchase up to approximately 22.7 million shares of our common stock for gross proceeds of $21.0 million. All 21,000 shares of the Series 7 preferred stock were converted into 56.8 million shares of our common stock upon closing of the transaction on October, 22 2010.
On November 1, 2010, the Company submitted a marketing authorization application (MAA) to the European Medicines Agency (EMA) for PIXUVRI (pixantrone dimaleate) as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive non-hodgkin’s lymphoma (NHL). The application submission follows a positive opinion from the EMA’s Pediatric Committee (PDCO), where PDCO agreed to CTI’s pediatric investigation plan (PIP) for PIXUVRI. As of the date of this Registration Document the Company can not anticipate the duration of such marketing authorization proceedings.
On November 2, 2010, the Company announced that it has been awarded $977,917 in grants under the Therapeutic Discovery Tax Credit program that was enacted under the U.S. government’s Patient Protection and Affordable Care Act. The program targets projects that show potential to produce new therapies, address unmet medical needs, reduce the long-term growth of health care costs and advance the goal of curing cancer. The Company was awarded the grants for the Company’s programs pixantrone, OPAXIO, brostallicin and bisplatinates which are all focused in the area of oncology.
On November 17, 2010, the EMA approved CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years.
C-61
D) Information about the conditions to maintain the listing of the Company’s common stock on the NASDAQ Capital Market
Effective with the opening of trading on January 8, 2009, the U.S. listing of the Company’s common stock was transferred to The NASDAQ Capital Market, subject to meeting a minimum market value of listed securities of $35 million. The NASDAQ Stock Market LLC’s (“NASDAQ”) Listing Qualifications Panel (the “Panel”) approved this transfer after the Company’s market capitalization did not comply with the minimum market capitalization required for companies listed on The NASDAQ Global Market, and the Company presented a plan to the Panel for regaining compliance with the NASDAQ Marketplace Rules. On January 23, 2009, the Company received an Additional Staff Determination Letter (the “Determination Letter”) from NASDAQ that stated the NASDAQ staff had concluded that the Company had violated Marketplace Rule 4350(i)(1)(c) (now NASDAQ Marketplace Rule 5635), which requires shareholder approval in connection with an acquisition if the issuance or potential issuance is greater than 20% of the pre-acquisition shares outstanding, and that the Company had at times not complied with Marketplace Rule 4310(c)(17) regarding submission of a “Listing of Additional Shares” form. On February 18, 2009, the Company updated the Panel on its plan for regaining compliance and requested an extension of the deadline to regain compliance with the minimum market capitalization requirement for the NASDAQ Capital Market. On March 6, 2009, the Company was notified by NASDAQ that the Panel had determined to continue the listing of its common stock on the NASDAQ Capital Market, subject to the condition that, on or before April 6, 2009, it demonstrates compliance with all applicable standards for continued listing on the NASDAQ Capital Market, including the $35 million minimum market capitalization requirement. The Panel also advised that the NASDAQ Marketplace Rules do not allow for an extension for compliance beyond April 6, 2009. In addition, the Panel issued a public reprimand for the Company’s prior failures to comply with the shareholder approval requirements and late filing of “Listing of Additional Shares” forms. On April 2, 2009, following the Company’s compliance for a minimum of ten consecutive trading days with the NASDAQ requirement that the market value of listed securities exceed $35 million, NASDAQ notified the Company that it had regained compliance with all applicable requirements for continued listing on the NASDAQ Capital Market.
NASDAQ reinstated the $1.00 minimum bid price requirement on August 3, 2009 and there can be no assurances that the Company’s stock price will be above $1.00. On May 3, 2010, the Company received a notice from NASDAQ indicating that for the last 30 consecutive business days the closing bid price of Company’s common stock was below the minimum $1.00 per share requirement for continued listing of the Company’s common stock on the NASDAQ Capital Market. This notification had no immediate effect on the listing of or the ability to trade the Company’s common stock on the NASDAQ Capital Market. In accordance with NASDAQ Marketplace Rule 5810(c)(3)(a), the Company had been provided a grace period of 180 calendar days, or until November 1, 2010, to regain compliance. On November 2, 2010, the Company received a notice from NASDAQ indicating that NASDAQ has granted the Company an additional 180 days to regain compliance with NASDAQ’s $1.00 minimum bid price rule under NASDAQ Marketplace Rule 5550(a)(2). The Company may achieve compliance during the 180-day period if the closing bid price of the Company’s common stock is at least a $1.00 per share for a minimum of 10 consecutive business days before May 2, 2011. In the event the common stock is delisted from the NASDAQ Capital Market, the Company currently expects that its common stock would be eligible to be listed on the OTC Bulletin Board or Pink Sheets.
C-62
| 5.2 | INVESTMENTS |
| 5.2.1 | Investments made by the Company for the nine months ended September 30, 2010 and the years ended December 31, 2009, 2008 and 2007 |
On December 15, 2008, the Company closed its transaction with Spectrum to form a 50/50 owned joint venture, RIT Oncology, to commercialize and develop Zevalin in the United States. The Company originally acquired the U.S. rights to develop, market and sell Zevalin from Biogen on December 21, 2007. At the closing of the joint venture transaction, the Company contributed to RIT Oncology all assets exclusively related to Zevalin in exchange for a 50% membership interest in RIT Oncology, an initial payment from RIT Oncology of $7.5 million upon closing of the transaction and an additional payment of $7.5 million in early January 2009. Also at closing, RIT Oncology issued to Spectrum a 50% membership interest in exchange for its capital contribution, a portion of which funded the purchase price paid to the Company by RIT Oncology, and the Company made an initial $1.8 million cash capital contribution.
Under the equity method of accounting, the Company recorded its initial investment in RIT Oncology at cost and adjusted for equity in earnings (loss) and cash contributions and distributions. As of December 31, 2008, the Company’s investment in its joint venture balance on the Company’s consolidated balance sheet was $5.8 million. In March 2009, the Company completed the sale of its 50% interest for $16.5 million and no longer had an investment in the joint venture.
At September 30, 2010 and December 31, 2009, 2008, and 2007 the Company recorded a goodwill equal to $17,064 thousands in connection with the acquisition in January 2000 of PolaRx Biopharmaceuticals, Inc. (PolaRx), a biopharmaceutical company that owned the rights to Trisenox (arsenic trioxide, ATO), an anti-cancer compound for which the Company submitted and received approval for a New Drug Application with the FDA. In accordance with FASB ASC 350, Intangibles-Goodwill and Other (formerly, Statement of Financial Accounting Standards or SFAS No. 142 - Goodwill and Other Intangible Assets), we test goodwill for impairment by comparing the fair value of our single reporting unit (i.e., the Company) to its carrying value. The Company’s estimate of fair value is based on our current market capitalization. If the implied fair value of goodwill is less than its carrying value, an impairment charge would be recorded. The Company reviews goodwill for impairment annually and whenever events or changes in circumstances indicate that the carrying value may not be recoverable. In practice, we have been reviewing goodwill quarterly (including as of September 30, 2010) to ensure that it is not impaired. No impairment of goodwill has been found to date.
The table below states the balance of the tangible and intangible assets at September 30, 2010 and December 31, 2009, 2008, and 2007.
| Fixed Assets |
September 30, 2010 (thousands $) (unaudited) |
% | December 31, 2009 (thousands $) |
% | December 31, 2008 (thousands $) |
% | December 31, 2007 (thousands $) |
% | ||||||||||||||||||||||||
| Intangible assets |
17,064 | 85 | % | 17,064 | 83 | % | 17,064 | 80 | % | 33,021 | 85 | % | ||||||||||||||||||||
| Tangible fixed assets |
3,114 | 15 | % | 3,430 | 17 | % | 4,324 | 20 | % | 6,025 | 15 | % | ||||||||||||||||||||
| Total fixed assets |
20,178 | 100 | % | 20,494 | 100 | % | 21,388 | 100 | % | 39,046 | 100 | % | ||||||||||||||||||||
C-63
In accordance with U.S. GAAP, the Company does not capitalize non-acquisition research and development costs related to its products and there are currently no capitalized investment costs related to its products. These costs are expensed as incurred as research and development expense as described further in our Research and Development discussion in Chapter 20.1.4.
Further details concerning investments, including additions to tangible and intangible fixed assets, are presented here below and in Chapter 20 of this Registration Document. For further information about the accounting items “Tangible fixed assets” and “Intangible assets”, please refer to the information disclosed in Chapter 20.
| 5.2.1.1 | Intangible assets |
The change in the value of the intangible assets, excluding the goodwill and including acquisitions of new assets, is as follows (in thousands of US dollars) and shows the changes (including the investments carried out, or additions to intangible assets) for each year:
| Developed and Core Technologies |
Manufacturing Intangible Asset |
Assembled Workforce |
||||||||||
| Balance as of December 31, 2006 |
$ | — | $ | — | $ | 1,663 | ||||||
| Increase due to acquisitions |
11,306 | 3,712 | 68 | |||||||||
| Amortization |
(28 | ) | (16 | ) | (869 | ) | ||||||
| Increase due to exchange rate |
— | — | 121 | |||||||||
| Balance as of December 31, 2007 |
11,278 | 3,696 | 983 | |||||||||
| Increase due to acquisition cost adjustments |
138 | 45 | — | |||||||||
| Amortization |
(111 | ) | (558 | ) | (927 | ) | ||||||
| Disposition of Zevalin to RIT Oncology |
(11,305 | ) | (3,183 | ) | — | |||||||
| Decrease due to exchange rate |
— | — | (56 | ) | ||||||||
| Balance as of December 31, 2008 |
$ | — | $ | — | $ | — | ||||||
As of December 31, 2009 and 2008, we had no intangible asset balance remaining.
PLEASE REFER TO CHAPTER 20 OF THIS REGISTRATION DOCUMENT FOR A DESCRIPTION OF INTANGIBLE ASSETS RELATIVE TO THE GOODWILL.
C-64
| 5.2.1.2 | Tangible fixed assets |
Investments carried out, or purchases, of tangible fixed assets (also shown as purchases of property and equipment in our consolidated statements of cash flows in Chapter 20) were $1.2 million, $1.5 million, $1.9 million and $1.8 million for the nine months ended September 30, 2010 and the years ended December 31, 2009, 2008 and 2007, respectively.
| 5.2.2 | Information on current investments as at the date of the Registration Document and related sources of financing |
Other than research and development activities, the Company has no investments that are in progress as of the date of the Registration Document.
Please see the section entitled “Research and development expenses” in Chapter 9, Paragraph 9.2.1 of this Registration Document for information regarding the Company’s investment in research and development activities for the nine months ended September 30, 2010.
| 5.2.3 | Information on major future investments as a part of commitment undertaken by the Company management |
The Company plans to make future investments in research, preclinical and clinical development of the following product candidates: pixantrone, OPAXIOTM (paclitaxel poliglumex CT-2103), brostallicin, and bisplatinates (CT-3610).
Pixantrone
The Company plans to conduct an additional clinical trial for pixantrone and is in the process of developing relationships with contract research organizations, statisticians, academic institutions, community groups and various other parties needed in the performance of such trial. Currently, the Company has not reached any formal agreements with these organizations for conducting this additional trial.
The Company entered into an agreement with the North Central Cancer Treatment Group (“NCCTG”) to conduct a phase II study of pixantrone in patients with HER2-negative metastatic breast cancer who have tumor progression after at least two, but not more than three, prior chemotherapy regimens.
OPAXIO
Currently, the Company has an agreement with the Gynecologic Oncology Group, or GOG, to conduct a study for OPAXIO and is expected to enroll 1,100 patients with more than 700 patients enrolled to date referred to as the GOG0212 trial.
Brostallicin
In the April 2010, the Company announced that the NCCTG plans to conduct a Phase II study of brostallicin in combination with cisplatin in patients with metastatic triple-negative breast cancer, defined by tumors lacking expression of estrogen, progesterone receptors and without over-expression of HER2. Subsequently, the NCCTG opened for enrollment for this study during the second quarter of 2010.
Bisplatinates
We are currently in the process of developing new analogues of the dinuclear-platinum complex, or CT-3610. We currently have engaged a third party to conduct the preclinical trial for CT-3610. At this time no clinical trial has been designed for CT-3610.
For information regarding the sources of funds for the Company’s future investments, please refer to Chapter 10, Paragraph 10.5 of this Registration Document.
C-65
CHAPTER 6 BUSINESS OVERVIEW
Introduction
The Company develops, acquires and commercializes novel treatments for cancer. The Company’s goal is to build a leading biopharmaceutical company with a diversified portfolio of proprietary oncology drugs. CTI’s research, development, acquisition and in-licensing activities concentrate on identifying and developing new, less toxic and more effective ways to treat cancer.
| 6.1 | Principal activities |
| 6.1.1 | Business Model: Description of main business and products of the Company |
The following table illustrates the Company’s development pipeline for pixantrone, brostallicin and OPAXIO, which are further described in the section below.
DEVELOPMENT PIPELINE
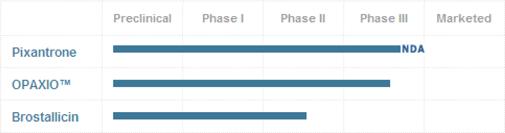
The information disclosed in the chart above must be read together with the information reported hereinafter and, in particular, with the information included in the following table about “CTI’s Ongoing Clinical Trials” here below.
CTI’s Ongoing Clinical Trials
The following table lists the Company’s active clinical trials (indicated by a status of “open”) and trials that have recently closed to enrollment.
| Product Candidate |
Indication/Intended Use |
Phase/Enrollment Status | ||
| Pixantrone |
Aggressive NHL, > 3 relapses, single-agent (PIX301) |
III / closed | ||
| Aggressive NHL, front-line, CPOP-R (PIX203) |
II / closed | |||
| OPAXIO (CT-2103) |
NSCLC, first-line, doublet therapy, PS0-2, females with pre menopausal estrogen levels (PGT307) |
III /open | ||
| Ovarian first-line maintenance (GOG0212) |
III / open | |||
C-66
| Product Candidate |
Indication/Intended Use |
Phase/Enrollment Status | ||
| Brostallicin |
Context of vulnerability (BRCA1 or BRCA2 Breast or Ovarian Cancer) (BRS201) |
II / open | ||
| Advanced or metastatic soft tissue sarcoma, first-line, single agent (EORTC 62061) |
II / closed | |||
| Myxoid liposarcoma with specific genomic translocations (BRS202) |
II / closed | |||
| Combination with other anti-cancer drugs (BRS101) |
I / closed |
Description of the development of the main products of the Comapny
Currently the Company is focusing its activities on pixantrone, OPAXIO, brostallicin and, as it will described below, also the Bisplatinates.
Pixantrone
| Date of the beginning of the research activity |
The Company acquired pixantrone and began the Company’s research activity related to pixantrone in connection with the Company’s merger with Novuspharma in January 2004. | |
| Development of the product | May 2007
CTI received fast track (1) designation from the FDA for pixantrone for the treatment of relapsed or refractory aggressive NHL.
April 14, 2009
CTI began a rolling submission (2) of a New Drug Application (NDA) to the FDA for pixantrone to treat relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL).
June 24, 2009
CTI completed the submission of the NDA to the FDA for pixantrone to treat relapsed or refractory, aggressive non-Hodgkin’s lymphoma (NHL).
August 24, 2009
The FDA accepted and filed for standard review (3) the Company’s NDA for pixantrone as treatment for relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL).
September 4, 2009
FDA notified CTI that a Prescription Drug User Fee Act (PDUFA) (4) action date of April 23, 2010 under standard review has been established regarding CTI’s NDA for pixantrone as potential treatment for relapsed or refractory aggressive non-Hodgkin’s | |
C-67
| lymphoma (NHL).
September 15, 2009
CTI submited a PIP to the EMA as part of the required filing process for approval of pixantrone for treating relapsed or refractory, aggressive non-Hodgkin’s Lymphoma in Europe, for the purposes of studying the drug in children.
September 29, 2009
CTI applied to the EMA for orphan drug designation for pixantrone.
December 21, 2009
EMA granted pixantrone orphan drug designation for the treatment of Diffuse Large B-Cell Lymphoma (DLBCL) which accounts for about 80% of aggressive non-Hodgkin’s lymphoma.
March 22, 2010
FDA ODAC panel voted unanimously that clinical trial data was not adequate to support approval of pixantrone for patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma.
April 9, 2010
The FDA informed CTI about the rejection of the NDA for the marketing of pixantrone in the USA and recommended that the Company conduct an additional trial to demonstrate the safety and effectiveness of pixantrone. The Company decided to pursue expanded access program for pixantrone while it conducts an additional study in aggressive NHL.
April 21, 2010
The EMA’s PDCO recommended the Company to submit an updated PIP for pixantrone.
July 6, 2010
CTI submited an expanded PIP to the EMA, as part of the process for its submission for a MAA for pixantrone in the E.U. for the treatment of relapsed or refractory, aggressive non-Hodgkin’s lymphoma. CTI intends to file the MAA during 2010 .
August 16, 2010
EMA validated the expanded PIP that CTI filed in July for pixantrone for the treatment |
C-68
| of relapsed or refractory, aggressive non-Hodgkin’s lymphoma (NHL).
October 18, 2010
The PDCO of the EMA adopted an opinion agreeing to CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years. This positive opinion clears the way for CTI to submit its MAA in the E.U. within the fourth quarter of 2010 for pixantrone for the treatment of patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL).
November 1, 2010
The Company announced that it submitted a Marketing Authorization Application (“MAA”) to the European Medicines Agency (“EMA”) for Pixuvri(R) (pixantrone dimaleate) as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s lymphoma (“NHL”).
November 17, 2010
The EMA approved CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years.
For further information please refer to the table “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph where in particular is reported that Phase III trials are closed. | ||
| Expect date of the final approval of the product | The Company cannot speculate as to the date of final approval of the product. | |
| Investments carried out with respect to the product and costs incurred since the beginning of the research activity | In accordance with US GAAP, the Company does not capitalize non-acquisition research and development costs related to its products and there are currently no capitalized investment costs related to its products. These costs are expensed as incurred as research and development costs as described further in the Company’s Research and Development discussion in Chapter 20.1.4. | |
| Most advanced development phase | Phase III
For further information please refer to the table “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph. | |
| (1) | The FDA’s fast track programs are intended to expedite the review of drugs that treat serious or life-threatening conditions and demonstrate the potential to address unmet medical needs. |
| (2) | The rolling submission process enables companies that have been granted fast track designation to submit sections of the NDA |
C-69
| to the FDA as they become available, allowing the review process to begin before the complete dossier has been submitted. |
| (3) | The standard review of a NDA establishes the undertaking by the FDA of taking a decision about the trading authorisation of a new drug within 10 months from the completion of the NDA submission to the FDA, therefore, with respect to pixantrone, within 10 months from June 24, 2009. The Company, when it began the submission of the NDA on April 14, 2009, requested to the FDA the priority review of such NDA that, if granted, would have lead to an approval decision about pixantrone by the FDA within 6 months from the completion of the submission of the relevant NDA. Nevertheless, it is point out that such priority review has not been granted by the FDA. |
| (4) | The Prescription Drug User Fee Act (PDUFA) is a law the U.S. Congress enacted by which drug companies that submit new drug applications must pay a fee to the food and drug administration (FDA) to review the entire application. |
| (5) | Orphan drug designation is available in Europe for medical treatments and drugs intended to treat life-threatening or chronically debilitating conditions. Orphan drug designation can confer numerous benefits to companies developing such treatments, including regulatory assistance, reduced regulatory fees associated with applying for marketing approval, and assistance with clinical trial design. |
The Company is developing pixantrone, a novel anthracycline derivative, for the treatment of non-Hodgkin’s lymphoma, or NHL, and various other hematologic malignancies, solid tumors and immunological disorders. Anthracyclines are one of the most potent classes of anti-cancer agents used in first-line treatment of aggressive NHL, leukemia, and breast cancer. For these diseases, anthracycline-containing regimens can often produce long-term cancer remissions and cures. However, the currently marketed anthracyclines can cause severe, permanent and life threatening cardiac toxicity when administered beyond widely recognized cumulative lifetime doses. This toxicity often prevents repeat use of anthracyclines in patients who relapse after first-line anthracycline treatment. In addition, the cardiac toxicity of anthracyclines prevents their use in combination with other drugs, such as trastuzumab, that also can cause cardiac toxicity. As a result, chemotherapy regimens that do not include anthracyclines often are used for the second-line treatment of relapsed NHL. There are no drugs approved in the United States for second- or third-line treatment for patients with relapsed aggressive NHL.
CTI believes a next-generation anthracycline with better ease of administration, greater anti-tumor activity and less cardiac toxicity could gain a significant share of the anthracycline market. CTI also believes that such a drug could allow repeat therapy in relapsed patients and could allow combination therapy with a broader range of chemotherapies. Pixantrone is being developed to improve the activity and safety in treating cancers usually treated with anthracycline family of anti-cancer agents. It is a novel DNA major groove binder with an aza-antracenedione molecular structure, differentiating it from anthracycline chemotherapy agents. Pixantrone has been studied in both indolent and aggressive NHL, and recent clinical results suggest the compound also may be synergistic with other agents commonly used in combination therapy.
Preclinical data and phase I and phase II clinical studies in approximately 410 patients indicate that pixantrone is easy to administer, may exhibit significantly lower potential for cardiac toxicity and may have more potent anti-tumor activity than marketed anthracyclines.
Pixantrone for relapsed or refractory aggressive non-Hodgkin’s lymphoma
CTI has initiated several clinical trials, including a pivotal phase III single-agent trial known as the EXTEND, or PIX301, trial of pixantrone (BBR2778) for the treatment of patients with relapsed aggressive NHL, a condition for which there are no chemotherapy drugs approved in the United States, who received two or more prior
C-70
therapies and who were sensitive to treatment with anthracyclines. This study is an international, randomized trial comparing pixantrone to a single agent of the treating physician’s choice. The primary endpoint of the study is complete response rate. The trial enrolled 140 patients and patients were randomized to receive either pixantrone or another single-agent drug currently used for the treatment of this patient population, as selected by the physician. CTI announced in November 2008 that it had achieved the primary efficacy endpoint of the PIX301 trial. Patients randomized to treatment with pixantrone achieved a high rate of confirmed and unconfirmed complete remissions compared to patients treated with standard chemotherapy (the control arm): in particular 14/70 (20.0%) for pixantrone arm compared to 4/70 (5.7%) for the standard chemotherapy arm, p=0.02). No patient (0%) in the standard chemotherapy arm achieved a confirmed complete remission compared to 8/70 (11%) of pixantrone recipients. Pixantrone treatment also significantly increased the overall response rate (which can be calculated by means of the formula CR/CRu+PR, where CR=Confirmed Complete Remission, Cru=Uncorfirmed Complete Remission and PR=Partial Remission) with 26/70 (37.1%) for pixantrone arm compared with 10/70 (14.3%) for the control arm, p=0.003. On an intent-to-treat analysis, pixantrone recipients who achieved a complete remission did so during the first 2 cycles of therapy, compared to 4 cycles among standard chemotherapy recipients (1.9 months vs. 3.6 months, pixantrone vs. standard chemotherapy). The duration of response in patients was similar in the 37% of pixantrone patients who had either a partial or complete response compared to the 14% of comparator patients with a major response. However, the overall progression-free survival (PFS) results that show patients treated with pixantrone experienced a statistically significant improvement in median progression-free survival, compared with other single-agent chemotherapeutic (4.7 months vs. 2.6 months, hazard tation =0.6; p=0.0074, pixantrone vs. standard chemotherapy) based on an intent-to-treat analysis. The most common (incidence greater than or equal to 10%) grade 3/4 adverse events reported for pixantrone-treated subjects across studies were neutropenia and leukopenia. Use of growth factor support was minimal. Other common adverse events (any grade) included infection, anemia, leukopenia, thrombocytopenia, asthenia, pyrexia and cough. Overall, the incidence of grade 3 or greater cardiac adverse events was 7% (5 patients) on the pixantrone arm and 2% (1 patient) on the comparator arm. There were an equal number of deaths due to an adverse event in both the pixantrone and comparator arm.
C-71
The Company also conducted the RAPID, or PIX203, phase II clinical trial study (CHOP-R vs. CPOP-R) in which pixantrone is substituted for doxorubicin in the CHOP-R regimen compared to the standard CHOP-R regimen in patients with aggressive NHL. An interim analysis of the RAPID trial, reported in July 2007, showed that to date, a majority of patients on both arms of the study achieved a major objective anti-tumor response (complete response or partial response). Patients on the pixantrone arm of the study had clinically significant less left ventricular ejection fraction (LVEF) drops, infections, and thrombocytopenia (a reduction in platelets in the blood), as well as significant reduction in febrile neutropenia. In early 2008, CTI closed enrollment on the RAPID trial because we had adequate sample size to demonstrate differences in cardiac events and other clinically relevant side effects between pixantrone and doxorubicin. The preliminary analysis of LVEF by Multigated Acquisition Scan, or MUGA, suggests that the patients in the pixantrone regimen (CPOP-R) experienced a lower incidence of >20% LVEF decline (2% vs. 13%) than patients in the doxorubicin control arm (CHOP-R). In addition, the preliminary analysis also suggests that grade 3/4 reductions in LVEF or symptomatic Congestive Heart Failure, or CHF, were lower in the pixantrone arm (CPOP-R) with no patients (0%) developing CHF in the pixantrone arm (CPOP-R) compared to 5% of the patients in the control arm (CHOP-R). Further the preliminary analysis suggests that grade 3/4 reductions in LVEF were also more frequent in the doxorubicin containing control arm (CHOP-R) at 10% compared to 2% in the pixantrone (CPOP-R) regimen. The Company expects to report results from the RAPID trial in the second half of 2010.
In February 2009, CTI entered into an agreement with IDIS Limited, or IDIS, to manage pixantrone as an investigational drug on a named patient basis in Europe. Pixantrone will be supplied by IDIS to healthcare professionals for the treatment of individual patients with relapsing aggressive non-Hodgkin’s lymphoma. The program was initiated in May 2009.
On April 14, 2009, the Company announced that, based on the outcome of the EXTEND trial and on the basis of pre-NDA communication the Company received from the FDA relating to this phase III trial, it began a rolling submission of an NDA to FDA for pixantrone to treat relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL). On June 24, 2009 the Company announced that it has completed the submission of the NDA to the FDA. On April 9, 2010 the FDA informed CTI about the rejection of the NDA for the marketing of pixantrone in the USA and recommended that the Company conduct an additional trial to demonstrate the safety and effectiveness of pixantrone.
In July 2009, the European Agency for Evaluation of Medicinal Products, or the EMA, notified to the Company that pixantrone was eligible to be submitted for a Marketing Authorization Application, or MAA, through the EMA’s centralized procedure. The centralized review process provides for a single coordinated review for approval of pharmaceutical products that has been conducted by the EMA on behalf of all European Union, or EU, member states. The EMA also designated pixantrone as a New Active Substance, or NAS; if approved, compounds designated as an NAS receive a 10-year market exclusivity period in EU member states. In September 2009, the Company applied to the EMA for orphan drug designation for pixantrone, which was granted in December 2009. In September 2009, CTI also submitted a Pediatric Investigation Plan, or PIP, to the EMA as part of the required filing process for approval of pixantrone for treating relapsed, refractory aggressive NHL in Europe. In April 2010, the EMA recommended that the Company has to submit an updated PIP for pixantrone following discussions with the Company about the preclinical and clinical pixantrone data, including EXTEND, and the desire to explore the potential benefits pixantrone may offer to children with hematologic cancer. The Company submitted an expanded PIP to the EMA in July 2010. CTI anticipates the formal MAA filing for pixantrone for the treatment of relapsed or refractory aggressive NHL in the second half of 2010.
The FDA completed its inspection of the facility at NerPharMa, S.r.l. (a pharmaceutical manufacturing company
C-72
belonging to Nerviano Medical Sciences S.r.l., in Nerviano, Italy), or NerPharMa, which has agreed to manufacture our drug, pixantrone, and found the site in compliance and acceptable for continued manufacturing of the drug product in early March 2010.
On March 22, 2010, the FDA’s Oncologic Drugs Advisory Committee, or ODAC, panel voted unanimously that the clinical trial data was not adequate to support approval of pixantrone for this patient population. On April 9, 2010, the Company announced the rejection by the FDA of the NDA for pixantrone that the Company submitted on June 24, 2009. In particular the Company received a Complete Response Letter from the FDA regarding our NDA for pixantrone and recommending that CTI designs and conducts an additional trial to demonstrate the safety and effectiveness of pixantrone. Based on the FDA’s March 22, 2010 ODAC presentation, which provided ODAC and the Company with alternative options to consider to make investigational drugs available to patients if drugs need to be studied further prior to approval, the Company will evaluate the establishment of an expanded access program for pixantrone. On August 3, 2010, CTI filed for a Special Protocol Assessment, or SPA, with the FDA for the design of CTI’s additional clinical study of pixantrone. Neverthless, the Company announced on September 14, 2010 that it intends to file an appeal of the FDA decision.
In June 2010, the Italian Medicines Agency, the national authority responsible for drug regulation in Italy, approved the facility at NerPharMa for the production of pixantrone. In July 2010, the Company signed a manufacturing agreement with NerPharMa for pixantrone. The five-year contract provides for both the commercial and clinical supply of pixantrone.
On August 16, 2010, the Company announced that it has received notice that the EMA has validated the expanded PIP that CTI filed in July for pixantrone for the treatment of relapsed or refractory, aggressive non-Hodgkin’s lymphoma (NHL).
On October 19, 2010, the Company announced that the PDCO of the EMA adopted an opinion agreeing to CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years. This positive opinion clears the way for CTI to submit its Marketing Authorization Application (“MAA”) in the E.U. within the fourth quarter of 2010 for pixantrone for the treatment of patients with relapsed or refractory aggressive non-Hodgkin’s lymphoma (NHL).
On November 1, 2010, the Company announced that it submitted a Marketing Authorization Application (“MAA”) to the European Medicines Agency (“EMA”) for Pixuvri(R) (pixantrone dimaleate) as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s lymphoma (“NHL”).
On November 17, 2010, the EMA approved CTI’s PIP (submitted by the Company in July 2010) concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years.
Pixantrone for other indications
Other clinical data suggest pixantrone may be useful in treating indolent NHL, a less rapidly progressive but ultimately fatal form of NHL. In November 2005, CTI presented results from a multi-center randomized trial, known as AZA302. This trial, evaluating pixantrone plus rituximab versus rituximab alone among patients with relapsed or refractory indolent NHL, was modified and reduced, as announced in CTI’s annual filing on Form 10-K in 2004, as a result of its strategy to conduct a pivotal phase III trial in aggressive NHL, which CTI believes provides the fastest route to registration for pixantrone. Of the 38 patients evaluable for response, patients
C-73
receiving the combination of rituximab and pixantrone had an 87% overall improvement in time to progression, or TTP, compared to rituximab alone. The median TTP estimate for the pixantrone/rituximab recipients was 13.2 months compared to 8.1 months for rituximab alone (hazard ratio 0.13, log rank p <0.001). The one- and two-year progression-free survival estimates were 66% and 44% for the pixantrone/rituximab recipients compared to 0% for the rituximab patients for both measurement intervals (p <0.001 and 0.003, respectively). The study also demonstrated a significant improvement in major objective responses (³ 50% shrinkage in tumor size). The pixantrone-rituximab combination produced a complete response (CR) in seven patients (35%), with eight patients (40%) experiencing a partial response (PR) and four patients (20%) with stable disease (SD). Rituximab monotherapy produced a CR in two patients (11%), PR in four patients (22%) with six patients having SD (33%). This corresponds to a major objective response rate of 75% in the combination therapy arm compared to 33% in the rituximab group (p=0.021). Side effects on pixantrone were generally mild to moderate (grade 1 or 2) with the exception of three cases of serious neutropenia associated with the pixantrone/rituximab arm. The median cumulative dose of pixantrone administered was 1014 mg/m²; no cases of treatment-related grade 3 or 4 cardiac toxicity were reported.
In May 2007, CTI received SPA approval for a new protocol designed to evaluate the combination of fludarabine, pixantrone, and rituximab versus fludarabine and rituximab in patients who have received at least one prior treatment for relapsed or refractory indolent NHL, and CTI received fast track designation from the FDA for pixantrone for the treatment of relapsed or refractory indolent NHL. The protocol, which became our phase III PIX303 trial, was launched in September 2007. However, CTI closed the trial in January 2008 based on, among other considerations, the plans to refocus the Company’s resources on obtaining pixantrone approval based on the EXTEND phase III trial before making additional substantive investments in alternative indications for pixantrone as well as the changing landscape in second line follicular NHL.
In the second quarter of 2010, the North Central Cancer Treatment Group, or NCCTG, opened for enrollment a phase II study of pixantrone in patients with HER2-negative metastatic breast cancer who have tumor progression after at least two, but not more than three, prior chemotherapy regimens.
OPAXIO (paclitaxel poliglumex)
| Date of the beginning of the research activity | The Company acquired OPAXIO and began the Company’s research activity related to OPAXIO in connection with the Company’s license agreement with PG-TXL Company L.P. in June 1998. | |
| Development of the product | March 2005
In March 2005, CTI announced that its OPAXIO phase III pivotal trial for the potential use in combination with platinum as front-line treatment of PS2 patients with NSCLC missed its primary endpoint of superior overall survival. However, in such trial, OPAXIO had a reduction in certain side effects, including hair loss, muscle and joint pain, and cardiac symptoms.
September 2005
In September 2005, CTI presented results from a phase II clinical trial in the first-line treatment of men and women with advanced NSCLC which demonstrated a survival advantage for women receiving OPAXIO as first-line therapy for NSCLC when compared to men. In this single arm study, the 35 women who received OPAXIO plus | |
C-74
| carboplatin had a 36 percent probability of living at least 1 year compared to 16 percent in the 39 men receiving the same regimen.
June 2009
In June 2009, the Company announced that, in a study released from Brown University at the 2009 American Society for Clinical Oncology Annual Meeting, patients with cancer of the lower esophagus had evidence of a high pathological complete response rate when given OPAXIO in addition to cisplatin and full-course radiotherapy. In this phase II clinical trial study, data suggests that OPAXIO may provide enhanced radiation sensitization as compared to standard therapy.
March 2010
On March 4, 2010 the Company announced that CTI received a statement on March 1, 2010 from the GOG leadership that the phase III GOG-212 clinical trial of CTI’s OPAXIO(TM) used as maintenance therapy for ovarian cancer remains a high priority and enrollment will continue.
For further information please refer to the table “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph where in particular is reported that Phase III trials are closed. | ||
| Expect date of the final approval of the product | The Company cannot speculate as to the date of final approval of the product. On September 21, 2009, the Company announced that that it has notified the EMA of its decision to withdraw its Marketing Authorization Application (MAA) for a non-inferiority indication in non-small cell lung cancer (NSCLC). | |
| Investments carried out with respect to the product and costs incurred since the beginning of the research activity | In accordance with US GAAP, the Company does not capitalize non-acquisition research and development costs related to its products and there are currently no capitalized investment costs related to its products. These costs are expensed as incurred as research and development costs as described further in the Company’s Research and Development discussion in Chapter 20.1.4. | |
| Most advanced development phase | Phase III
For further information please refer to the table “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph. | |
OPAXIO (paclitaxel poliglumex, CT-2103) is CTI’s novel biologically enhanced chemotherapeutic agent that links paclitaxel to a biodegradable polyglutamate polymer, resulting in a new chemical entity. CTI is developing OPAXIO for the potential treatment of NSCLC and ovarian and other cancers.
OPAXIO was designed to improve the delivery of paclitaxel to tumor tissue while protecting normal tissue from toxic side effects. Unlike vessels in healthy tissue, those in tumor tissue have openings that make them porous. Due to the larger size of OPAXIO compared to standard paclitaxel, OPAXIO leaks through the pores in tumor blood vessels and is preferentially trapped and distributed to the tumor tissue. Once in the tumor tissue, OPAXIO is taken up by the tumor cells through a cellular process called endocytosis. Because the bipolymer OPAXIO is made up of biodigestible amino acids, it is slowly metabolized by the lysosomal engines
C-75
(principallycathepsin B) inside the lysosome of the tumor cell This metabolism releases the active chemotherapy agent, paclitaxel. The activity of this enzyme and thus the rate of release of OPAXIO is increased in the presence of estrogen.
Because the polymer is water-soluble, OPAXIO can be administered without solvents or other routine pre-medications (such as steroids or antihistamines) generally used to prevent sever allergic reactions, and can be infused over a period of 10 to 20 minutes. Patients can drive themselves to and from their treatment centers. OPAXIO remains stable in the bloodstream for several days after administration; this prolonged circulation allows the passive accumulation of OPAXIO in tumor tissue.
Taxanes, including paclitaxel (Taxol®) and docetaxel (Taxotere®), currently are widely used for the treatment of various solid tumors, including non small-cell lung, ovarian, breast and prostate cancers. Paclitaxel is considered a standard of care in lung and ovarian cancers, where it is most widely used. Because taxanes are small, hydrophobic agents, their therapeutic potential is limited by unfavorable pharmokinetic properties. Solvents (such as Cremaphor) are needed for administration, and these solvents are often extremely irritating to blood vessels and requires surgical placement of a large catheter for administration and a minimum of three hours for infusion. They also can cause severe life threatening allergic reactions that typically require pre-medications with steroids and antihistamines. Patients usually require transportation patients to and from their treatment location. Taxanes exhibit high peak levels of drug immediately following the administration that expose normal tissues to toxic effects. Rapid elimination of the drug from blood limits tumor exposure.
The distribution and metabolism of OPAXIO to tumor tissue and subsequent release of active paclitaxel chemotherapy appears to be enhanced by estrogen allowing for superior effectiveness in women with pre-menopausal estrogen levels. This gender targeted benefit could also be exploited in post-menopausal women or men through estrogen supplementation. Preclinical data presented at the 2006 European Organization for Research and Treatment of Cancers, National Cancer Institute and American Association for Cancer Research or EORTC-NCA-AACR, meeting demonstrated that the efficacy of OPAXIO is enhanced in certain human tumors when mice are given additional estrogen. In subsequent clinical studies, more than 1,900 patients were treated in CTI’s four pivotal phase III trials of OPAXIO for the treatment of NSCLC. While the STELLAR 2, 3 and 4 trials completed in the first half of 2005 missed their primary endpoint of superior overall survival, women treated with OPAXIO for newly diagnosed advanced NSCLC in STELLAR 3 and 4 had a significant improvement in their overall survival compared to women or men treated with standard chemotherapy and the Company believes that the reduction in toxicities coupled with superior convenience and less supportive care demonstrated in the STELLAR 4 phase III clinical trial merits consideration for approval as single-agent therapy for patients with advanced NSCLC who have poor performance status, or PS2. In addition, with single-agent OPAXIO, the Company has observed a significant reduction in most of the severe toxic side effects associated with the standard chemotherapy agents studied in the STELLAR trials.
OPAXIO for non-small cell lung cancer and esophageal cancer
The cancer drug most commonly used to treat NSCLC in the United States is paclitaxel. The American Cancer Society, or ACS, estimated that 185,000 new cases of NSCLC would be diagnosed in the United States in 2008 and approximately 128,000 of these patients would be expected to receive chemotherapy. Of the estimated 128,000 NSCLC patients who would receive chemotherapy, approximately 32,000 are classified as PS2. These patients tolerate chemotherapy poorly and have a significantly shorter median survival than healthier patients.
In March 2005, CTI announced that its OPAXIO phase III pivotal trial, known as STELLAR 3, for the potential use in combination with platinum as front-line treatment of PS2 patients with NSCLC missed its primary
C-76
endpoint of superior overall survival. However, in the STELLER 3 trial, OPAXIO had a reduction in certain side effects, including hair loss, muscle and joint pain, and cardiac symptoms. In May 2005, CTI announced that both the STELLAR 2 and 4 clinical trials missed their primary endpoints of superior overall survival, but had significant reductions in certain severe side effects compared to the comparator agents. The STELLAR 2 pivotal trial was evaluating OPAXIO for potential use as second-line single agent treatment for patients with NSCLC, and the STELLAR 4 pivotal trial was evaluating OPAXIO for potential use as front-line single agent treatment for PS2 patients with NSCLC.
In July 2005, at the 11th World Conference on Lung Cancer, CTI announced that in a pooled analysis of its STELLAR 3 and 4 pivotal trials the 97 women who received OPAXIO had a significant increase in median and overall survival (9.5 months vs. 7.7 months, hazard ratio 0.70, log rank p=0.03) and in 1 year survival (40% vs. 25%, p=0.013) compared to 101 women who received comparator control agents. These results pooled data from all women randomized on the STELLAR 3 and 4 trials (a so-called “intent to treat” analysis). Individually, neither study reached statistical significance for overall survival for women, although a positive trend was observed in both trials, with a strong trend in the STELLAR 4 trial (p=0.069). While analysis of survival by gender was pre-specified in the analysis plans for the trials, a gender specific survival advantage for women over men was not a pre-specified endpoint in either trial.
In September 2005, CTI presented results from a phase II clinical trial, known as PGT202, of OPAXIO in the first-line treatment of men and women with advanced NSCLC which demonstrated a survival advantage for women receiving OPAXIO as first-line therapy for NSCLC when compared to men. In this single arm study, the 35 women who received OPAXIO plus carboplatin had a 36 percent probability of living at least 1 year compared to 16 percent in the 39 men receiving the same regimen. A pooled analysis of the 463 patients treated with OPAXIO in the STELLAR 3, STELLAR 4 and PGT202 trials demonstrated a statistically significant survival advantage for women treated when compared to men, with women having a 39 percent probability of surviving at least 1 year compared to 25 percent for men (hazard ratio 0.63, log rank p=0.014).
In December 2005, CTI initiated the PIONEER, or PGT305, study comparing OPAXIO to paclitaxel in the first-line treatment of PS2 women with advanced NSCLC. In addition, CTI initiated preclinical studies on the effect of gender/hormonal status on OPAXIO biodistribution, cellular uptake and metabolism to support the hypothesis for survival improvement in women.
In February 2006, CTI presented results that confirm the observation of enhanced efficacy in the presence of estrogen seen in the STELLAR first-line trials. In the three first-line trials of OPAXIO (PGT202, STELLAR 3 and STELLAR 4), women of pre-menompausal age or with normal estrogen levels had the strongest survival advantage over their counterparts. In an analysis of the 113 of 198 women in the pooled STELLAR 3 and 4 trial data who are of pre-menopausal age or have normal estrogen levels, women treated with OPAXIO had a highly significant prolongation in the 1-year and overall survival estimates compared to women treated with standard chemotherapy, with the OPAXIO patients having a 44% reduction in the overall risk of dying (log rank p=0.008) and a 42% 1-year survival estimate compared to 19% for women on standard chemotherapy (p=0.003). CTI believes these data indicate a potential favorable alternative to women with normal estrogen levels who have NSCLC.
In addition, CTI’s phase III trials demonstrated that, with the exception of neuropathy known to be associated with taxane therapy, single agent OPAXIO (175-210mg/m2) has a significantly reduced incidence of severe side effects, including a reduction in severe neutropenia, febrile neutropenia, infection and anemia when compared to patients receiving standard chemotherapy agents gemcitabine, vinrelbine or docetaxel. OPAXIO also resulted in less severe allergic reactions, less hair loss and significant reduction in the requirement for transfusions and the
C-77
use of hematopoietic growth factor support, such as Neuopgen®, Neulasta®, Aranesp® and/or Epogen® compared to patients receiving standard chemotherapy.
In November 2006, at the 18th Annual EORTC-NCI-AACR meeting, CTI scientists presented new preclinical data on the effect of circulating estrogen levels on tumor growth and levels of cathepsin B in tumor tissue. The study showed that when additional estrogen was given, it substantially increased the tumor growth rate in colon cancer (HT-29) and NSCLC (H460) models. In addition, cathepsin B activity in the tumors increased by 35 to 40 percent in the presence of estrogen. The study also found that in estradiol-supplemented female mice, OPAXIO demonstrated a nearly two-fold increase in anti-tumor activity compared to non-supplemented animals in the colon cancer tumor model. Studies are ongoing to evaluate the effect of estrogen on OPAXIO activity in the NSCLC tumor model.
In December 2006, CTI agreed with the recommendation of the Data Safety Monitoring Board to close the PIONEER lung cancer trial due, in part, to the diminishing utility of the PIONEER trial given its plans to submit a new protocol to the FDA. In January 2007, CTI submitted two new protocols under a SPA to the FDA. These new trials, known as PGT306 and PGT307, focus exclusively on NSCLC in women with pre-menopausal estrogen levels, the subset of patients where OPAXIO has demonstrated the greatest potential survival advantage in the STELLAR trials. CTI believes the lack of safe and effective treatment for women with advanced first-line NSCLC who have pre-menopausal estrogen levels represents an unmet medical need. CTI initiated enrollment on the PGT307 clinical trial in September 2007. Due to limited resources, we have discontinued enrollment in the PGT307 trial.
Currently there are no drugs approved for PS2 NSCLC patients. On March 4, 2008, the Company submitted an MAA to the EMA for first-line treatment of patients with advanced non-small cell lung cancer, or NSCLC, who are poor performance status, or PS2, based on a non-inferior survival and improved side effect profile which the Company believes was demonstrated in its previous clinical trials. The application was based on a positive opinion the Company received from the EMA’s Scientific Advice Working Party, or SAWP; the EMA agreed that switching the primary endpoint from superiority to non-inferiority is feasible if the retrospective justification provided in the marketing application is adequate. In September 2009, the Company notified the EMA of its decision to withdraw the MAA and the Company refocused its resources on the approval of OPAXIO for its potential superiority indication in maintenance therapy for ovarian cancer and as a radiation sensitizer in the treatment of esophageal cancer.
In June 2009, the Company announced that, in a study released from Brown University at the 2009 American Society for Clinical Oncology Annual Meeting, patients with cancer of the lower esophagus had evidence of a high pathological complete response rate when given OPAXIO in addition to cisplatin and full-course radiotherapy. In this phase II clinical trial study, data suggests that OPAXIO may provide enhanced radiation sensitization as compared to standard therapy. the Company plans to meet with the FDA following completion of the clinical study report to explore a potential phase III registration study utilizing OPAXIO as a radiation sensitizer in the treatment of esophageal cancer.
OPAXIO for ovarian cancer
CTI is also developing OPAXIO for women with pre-menopausal levels of estrogen, regardless of age, who have advanced NSCLC with normal or poor performance status. The ACS estimated that approximately 22,000 new cases of ovarian cancer would be diagnosed in the United States in 2008. The standard of care for first-line
C-78
treatment of ovarian cancer is paclitaxel and carboplatin. CTI believes that the lack of safe and effective treatment for women with advanced first-line NSCLC, who have pre-menopausal estrogen levels, represents an unmet medical need. In April 2004, CTI announced that it entered into a clinical trials agreement with the Gynecologic Oncology Group, or GOG, to perform a phase III trial of OPAXIO as maintenance therapy in patients with ovarian cancer. In July 2004, the GOG submitted an Investigational New Drug application, or IND, along with the protocol for a SPA to the FDA. The GOG reached agreement with the FDA regarding the SPA in December 2004 and initiated the phase III study in March 2005. This study is expected to enroll 1,100 patients by 2012. The primary endpoint of this trial is overall survival. Progression-free survival, safety and side effect profile are secondary endpoints.
On March 4, 2010 the Company announced that CTI received a statement on March 1, 2010 from the GOG leadership that the phase III GOG-212 clinical trial of CTI’s OPAXIO(TM) used as maintenance therapy for ovarian cancer remains a high priority and enrollment will continue. The GOG made the statement to clarify that the recent results of the GOG-218 clinical trial bevacizumab in maintenance therapy for ovarian cancer has not influenced the importance of completing the GOG-212 clinical trial.
Based on current enrollment and study duration, the interim analysis could be conducted as early as 2011. If successful, the Company could utilize those results to form the basis of its New Drug Application for OPAXIO.
Brostallicin
| Date of the beginning of the research activity | The Company acquired brostallicin and began the Company’s research activity related to brostallicin in connection with the Company’s acquisition of Systems Medicine LLC in July 2007. | |
| Development of the product | Please refer to the information disclosed below as well as to the table “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph where in particular is reported that Phase I and II are closed with respect to specific typologies of cancer and therapy. | |
| Expect date of the final approval of the product | The Company cannot speculate as to the date of final approval of the product. | |
| Investments carried out with respect to the product and costs incurred since the beginning of the research activity | In accordance with US GAAP, the Company does not capitalize non-acquisition research and development costs related to its products and there are currently no capitalized investment costs related to its products. These costs are expensed as incurred as research and development costs as described further in the Company’s Research and Development discussion in Chapter 20.1.4. | |
| Most advanced development phase | Phase II
For further information please refer to the table | |
| “CTI’s Ongoing Clinical Trial” disclosed at the beginning of this paragraph. | ||
C-79
CTI is developing brostallicin, which is a small molecule, anti-cancer drug with a novel, unique mechanism of action and composition of matter patent coverage. Data in more than 230 patients treated with brostallicin in phase I/II clinical trials reveal evidence of activity in patients with refractory cancer and patient/physician-friendly dosage and administration. A phase II study of brostallicin in relapsed/refractory soft tissue sarcoma met its pre-defined activity and safety hurdles and resulted in a first-line phase II study that is currently being conducted by the European Organization for Research and Treatment of Cancer (EORTC). Planned enrollment for this study was completed in August 2008 and the EORTC plans to conduct the first data analysis in 2009. Brostallicin also has demonstrated synergism with new targeted agents as well as established treatments in pre-clinical trials; consequently, we began a multi-arm combination study with brostallicin and other agents, including Avastin (bevacizumab) which was substantially completed in the fourth quarter of 2008.
CTI is developing brostallicin through CTI’s wholly-owned subsidiary Systems Medicine LLC, or SM, which holds worldwide rights to use, develop, import and export brostallicin, a synthetic DNA minor groove binding agent that has demonstrated anti-tumor activity and a favorable safety profile in clinical trials in which more than 230 patients have been treated to date. SM currently uses a genomic-based platform to guide development of brostallicin. CTI expect to use that platform to guide development of its licensed oncology products in the future. CTI also has a strategic affiliation with the Translational Genomics Research Institute, or TGen, and has the ability to use TGen’s extensive genomic platform and high throughput capabilities to target a cancer drug’s context-of-vulnerability, which is intended to guide clinical trials toward patient populations where the highest likelihood of success should be observed, thereby potentially lowering risk and shortening time to market.
In the second quarter of 2010, the NCCTG opened for enrollment a phase II study of brostallicin in combination with cisplatin in patients with metastatic triple-negative breast cancer, or mTNBC. mTNBC is defined by tumors lacking expression of estrogen, progesterone receptors and without over-expression of HER2. Women with mTNBC have very limited effective treatments and based on the novel mechanism of action of brostallicin and the recognized activity of cisplatin in this disease, the combination of the two agents will be explored by the NCCTG. In addition to standard clinical efficacy measures, biological endpoints will also be evaluated to assist in understanding the specific activity of brostallicin in this disease.
A phase II study of brostallicin in relapsed, refractory soft tissue sarcoma met its predefined activity and safety hurdles and resulted in a first-line phase II clinical trial study that was conducted by the European Organization for Research and Treatment of Cancer, or EORTC. Planned enrollment for this study was completed in August 2008 and the EORTC conducted final data analysis in 2009. The data was reported at the American Society of Clinical Oncology Annual Meeting in June 2010. The EORTC trial demonstrated, in this hard to treat patient group, a modest level of clinical activity with an acceptable level of toxicity. No further development is planned in this indication. A multi-arm phase I combination study with brostallicin and other agents, including Avastin (bevacizumab), was completed in the first quarter of 2009. Brostallicin also has demonstrated synergy with new targeted agents as well as established treatments in preclinical trials.
C-80
Bisplatinates
| Date of the beginning of the research activity | The Company acquired bisplatinates and began the | |
| Company’s research activity related to bisplatinates in connection with the Company’s merger with Novuspharma in January 2004. | ||
| Development of the product | Please refer to the information disclosed below in this paragraph | |
| Expect date of completion of the research activity | The Company cannot speculate as to the date of final approval of the product. | |
| Investments carried out with respect to the product and costs incurred since the beginning of the research activity | In accordance with US GAAP, the Company does not capitalize non-acquisition research and development costs related to its products and there are currently no capitalized investment costs related to its products. These costs are expensed as incurred as research and development costs as described further in the Company’s Research and Development discussion in Chapter 20.1.4. | |
Zevalin
Zevalin is a form of cancer therapy called radioimmunotherapy and is indicated for the treatment of patients with relapsed or refractory, low-grade or follicular B-cell NHL, including patients with rituximab refractory follicular NHL. It was approved by the FDA in February 2002 as the first radioimmunotherapeutic agent for the treatment of NHL.
CTI developed Zevalin from its acquisition of the U.S. rights to that product line in December 2007 until the transfer of CTI’s rights to Zevalin to RIT Oncology in December 2008; in March 2009, CTI sold its remaining 50% interest in RIT Oncology to Spectrum, thereby divesting its remaining interest in Zevalin.
Please see Chapter 9, Paragraph 9.2.1 of this Registration Document for information regarding the Company’s investment in research and development activities.
6.1.2 Development Process
CTI’s business strategy is to target development and registration/marketing approval of drug products in the United States and Europe that take advantage of the ability to accelerate approval, either because there is an unmet medical need or because our product profiles demonstrate significant improvement in the efficacy, toxicity or safety over competitive drugs. In addition, CTI actively explores opportunities to in-license or acquire complementary products, technologies or companies.
The following defines the general process for gaining marketing approval of oncology drugs both in the United States and in the European Union.
Overall, it generally takes between ten to twelve years to develop a product and gain marketing approval. The first step is to perform pre-clinical (laboratory and animal) studies which can take up to eight years to show safety and biological activity of the compound against the targeted disease. After completing pre-clinical testing, the Company must file an application to begin testing the drug in clinical (human) studies. For example in the
C-81
United States, this is an IND, or Investigational New Drug Application and if the FDA does not have any questions or does not disapprove the application, the IND becomes effective within 30 days of filing.
Generally, both in the United States and in the European Union clinical studies are conducted in three sequential phases. Phase I clinical studies can take up to three years are done to determine (i) how a drug is absorbed, distributed, metabolized and excreted, (ii) the duration of its action, and (iii) its short-term safety and safe dosage range. If possible, Phase I clinical studies are also used to get an early indication of the drug’s effectiveness. Phase II studies can take up to four years and are done in a limited number of patients who have the disease of interest. Phase II studies evaluate dosage tolerance and establish the appropriate dosage, identify adverse effects and safety risks, and also evaluate the effectiveness of the drug. Phase III clinical studies can take up to five years and further evaluate the safety and effectiveness of the drug in its final form and dosage in a larger number of patients who have the disease of interest. Clinical trial protocols are subject to review by the appropriate regulatory body, such as the FDA in the United States and the EMA in the European Union, and by Institutional Review Boards at hospitals or clinics in which the clinical trials are performed.
Specific progress reports on clinical trials must be periodically submitted to the competent authorities (FDA and/or EMA), and the Company is required to report to such authorities serious adverse events as they happen. Clinical trials may be suspended at any time during the conduct of the trial if trial participants are being exposed to unacceptable health risks.
If the clinical studies are completed and the results meet the pre-specified endpoints, CTI may submit a marketing approval request, which includes details on the preclinical and clinical studies as well as the manufacturing and quality processes, to the appropriate regulatory body.
In the United States, this application is known as an NDA, or New Drug Application. NDAs can be up to 100,000 pages or more. The FDA may take six to ten months to review an NDA. Once the FDA approves the NDA, the new drug can be marketed.
In the European Union, this application is known as a MAA, or Marketing Authorization Application. The regulatory approval process for a MAA can take up to ten months plus additional time if further review and additional data analysis is requested by the regulatory authorities. Once the EMA approves the MAA, the new drug can be marketed.
Both in the United States and in the European Union, the Company must continue to submit periodic reports to the regulatory authorities, including any manufacturing/quality records and reports of adverse reactions. In some cases, the regulatory authorities may require additional studies of the drug for additional evaluation. These are called Phase IV studies.
| 6.1.3 | Turnover resulting from product sales for the years 2007 and 2008 |
CTI considers its operations to be a single operating segment, focused in the development, acquisition and commercialization of novel treatments for cancer.
Product sales from Zevalin’s major customers were as follows:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Customer A |
77 | % | 67 | % | ||||
| Customer B |
5 | % | 33 | % | ||||
C-82
All sales of Zevalin during 2007 and 2008 were to customers North America.
Please also see Chapter 20, Paragraph 20.1.4 “Notes to the Statements of Operations”, point “A. Revenues”.
| 6.1.4 | Main Challenges, Opportunities and Weaknesses |
A. Challenges and Opportunities
Challenges (External Factors)
Competition
The biotechnology and pharmaceutical industries in which the Company operates are intensely competitive and are subject to regulatory changes/requirements as well as rapid and significant technological change. The Company’s competitors, including major, multinational pharmaceutical and chemical companies, specialized biotechnology firms and universities and other research institutions (many with greater financial and other resources, larger research and development staffs and more effective marketing and manufacturing organizations than the Company) may succeed in developing, licensing or marketing technologies and drugs that are more effective or less costly and obtain regulatory approvals for drug candidates before the company does. Specifically:
| • | Because pixantrone is intended to provide less toxic treatment to patients who have failed standard chemotherapy treatment, if pixantrone is brought to market, it is not expected to compete directly with many existing chemotherapies. However, pixantrone will face competition from currently marketed anthracyclines, such as Mitoxantrone (Novantrone®), and new anti-cancer drugs with reduced toxicity that may be developed and marketed. |
| • | If the Company is successful in bringing OPAXIO to market, it will face direct competition from oncology-focused multinational corporations. OPAXIO will compete with other Taxanes. Many oncology-focused multinational corporations currently market or are developing Taxanes, Epothilones, and other cytotoxic agents, which inhibit cancer cells by a mechanism similar to Taxanes, or similar products . Such corporation includes, among others, Bristol-Myers Squibb co., which markets Paclitaxel and other companies that manufacture generic forms of Paclitaxel; Sanofi Aventis, which markets Docetaxel; Genentech, Roche and OSI pharmaceuticals, which markets Tarceva™; Genentech and Roche, which market Avastin™, Eli Lilly, which markets Alimta®, and Abraxis, which markets Abraxane™. In addition, several companies such as Neopharm Inc. and Telik, Inc. Are also developing products which could compete with OPAXIO. |
| • | If the Company is successful in bringing Brostallicin to market, it will face direct competition from other minor groove binding agents including Yondelis®, which is currently developed by Pharmamar and has received authorization of commercialization from the European Commission for soft tissue sarcoma. |
Improvement of other therapies
The improvement of surgical therapy and/or radiotherapy (alternative or complementary therapies in the treatment of cancer), as a result of the continuous evolution of technology, could reduce the recourse to pharmaceutical therapy.
Regulations
The continuous changes in the regulations governing the registration and marketing of drugs and the differences among different countries might cause delays in the approvals, including approvals from the FDA and/or the
C-83
EMA and/or considerable increases in development costs. In addition, uncertainty regarding third-party reimbursement and health care cost containment initiatives may limit the Company’s returns.
Opportunities (External Factors)
Oncological drug market
People’s aging in industrialized countries will entail an ever higher incidence of cancer. This will determine a considerable growth of the oncological drug market aimed at finding increasingly effective pharmaceutical solutions to fight cancer or at least reduce its effects, allowing the patient to live longer and improving life conditions.
Improvement of diagnostic systems
The improvement of diagnostic systems will allow the extension of both the number of patients who may be treated with anti-cancer pharmaceutical agents and techniques used in order for patients to be treated.
B. Main Weaknesses (internal factors)
Uncertainties connected with the development of products
The Company’s capability to generate revenues will mainly depend on its ability to discover, develop and market products. Many of CTI’s drug candidates are still in research and pre-clinical development, which means that they have not yet been tested on humans. Clinical trials of drug candidates involve the testing of potential therapeutic agents, or effective treatments, in humans in three phases to determine the safety and efficacy of the drug candidates necessary for an approved drug. Many drugs in human clinical trials fail to demonstrate the desired safety and efficacy characteristics. Even if the Company’s drugs progress successfully through initial human testing, they may fail in later stages of development. In addition, data obtained from clinical trials are susceptible to varying interpretations. Government regulators and the Company’s collaborators may not agree with the Company’s interpretation of its future clinical trial results. The Company will need to commit significant time and resources to develop these and additional product candidates. There cannot be any assurance that:
| • | the Company’s product candidates will be developed to a stage that will enable it to commercialize them or sell related marketing rights to pharmaceutical companies; |
| • | the Company will be able to commercialize product candidates in clinical development or sell the marketing rights to third parties; or |
| • | product candidates, if developed, will be approved. |
C-84
Additional funding
As of September 30, 2010, the Company had approximately $17.3 million in cash and cash equivalents. This does not include (i) $21 million received in gross proceeds from the issuance of 21,000 shares of our Series 7 preferred stock and warrants to purchase up to 56.8 million shares of our common stock, and (ii) $977,917 in a grant awarded to the Company under the Therapeutic Discovery Tax Credit program. Even with this additional financing, the Company will need to raise additional capital this year and is currently exploring alternatives to do so, which may include potential partnerships or joint ventures, public or private equity financings, debt financings or restructurings, dispositions of assets or through other means in order to fund its continued operations.
Uncertainty in connection with intellectual property
Development and protection of the Company’s intellectual property are critical to its business. If the Company does not adequately protect its intellectual property, competitors may be able to practice the Company’s technologies. The Company’s success depends in part on its ability to obtain patent protection for its products and processes in the U.S., Italy and other countries, protect its trade secrets and prevent others from infringing on its proprietary rights. Further, the patent position of biopharmaceutical firms generally is highly uncertain and involves complex legal and factual questions. Patents issued to third parties may cover the Company’s products as ultimately developed, causing the Company to seek licenses, and third parties may challenge the patents that have been issued or licensed to the Company resulting in potential liability for infringement.
Dependence on insurance and risk of product liability
The Company’s business exposes it to potential product liability risks inherent in the testing, manufacturing and marketing of human pharmaceutical products, and it may not be able to avoid significant product liability exposure. While the Company intends to maintain insurance covering product use in its clinical trials, it is possible that the Company will not be able to maintain such insurance on acceptable terms or that any insurance obtained will not provide adequate coverage against potential liabilities. The Company’s inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or limit the commercialization of any products the Company develops. If a product liability claim were successful against CTI and the judgment against CTI was in excess of CTI’s insurance coverage against the claim, such result may harm CTI’s financial statements and results of operations
C. Main strength (Internal Factors)
Projects and products of the Company
The Company has a considerable and innovative range of research projects and products already in clinical trials.
Experienced management team
The Company’s executive officers have strong backgrounds and experience in the Company’s market.
Relationships with other companies
The Company has partnered with other companies, such as Novartis, to promote the development and commercialization of its products. The Company will continue to evaluate other strategic relationships to promote the development and commercialization of its products.
C-85
| 6.1.5 | Suppliers |
In regards to the production of OPAXIO, CTI purchases the raw materials paclitaxel and polyglutamic acid key intermediate from a single source. Should the purchase of these raw materials prove to be insufficient in quantity or quality, should the supplier fail to deliver in a timely fashion or at all, or should this relationship terminate, the Company may not be able to obtain a sufficient supply from alternate sources on acceptable terms, or at all. The Company has an agreement in place for clinical supply packaging with a sole supplier.
Additionally, the manufacturing of the OPAXIO drug substance and product is also conducted by a single source.
The active pharmaceutical ingredient and finished product for pixantrone are both manufactured by a single vendor. Should the purchase of these raw materials prove to be insufficient in quantity or quality, should the supplier fail to deliver in a timely fashion or at all, or should this relationship terminate, the Company may not be able to obtain a sufficient supply from alternate sources on acceptable terms, or at all. The Company has an agreement with a finished package manufacturer for the supply of commercial requirements and a separate agreement with a different vendor for clinical supply packaging of pixantrone.
The Company pays 100% of its costs for the raw materials and manufacture of OPAXIO and pixantrone to the suppliers described above.
The Company is dependent on third-party manufacturers, which means that the Company does not always have direct control over the manufacture, testing or distribution of its products. The Company does not currently have internal analytical laboratory or manufacturing facilities to allow the testing or production and distribution of drug products in compliance with cGMPs. Because the Company does not directly control its suppliers, these vendors may not be able to provide the Company with finished product when the Company needs it.
The Company will be dependent upon these third parties to supply us in a timely manner with products manufactured in compliance with cGMPs or similar manufacturing standards imposed by United States and/or foreign regulatory authorities where the Company’s products will be tested and/or marketed. While the FDA and other regulatory authorities maintain oversight for cGMP compliance of drug manufacturers, contract manufacturers and contract service providers may at times violate cGMPs. The FDA and other regulatory authorities may take action against a contract manufacturer who violates cGMPs. In October 2009, the FDA inspected the Company’s contract manufacturing facility located in Milan, Italy and, based on its inspection, made observations regarding the manufacturing process and controls over the Company’s lead compound, pixantrone. The Company’s contract manufacturer addressed and responded to the FDA’s observations in November 2009. Neither the Company’s contract manufacturer nor the Company have received any further response from the FDA regarding the Company’s contract manufacturer’s planned action as of February 22, 2010. Failure to comply with FDA, EMA or other applicable regulations may cause us to curtail or stop the manufacture of such products until we obtain regulatory compliance.
| 6.1.6 | Legal Regulatory Framework |
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of CTI’s products are extensively regulated by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations. Failure to comply with applicable U.S. requirements may subject CTI to administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution.
Drug Approval Process. None of CTI’s drugs may be marketed in the United States until the drug has received FDA approval. The steps required before a drug may be marketed in the United States include:
C-86
| • | preclinical laboratory tests, animal studies and formulation studies; |
| • | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug for each indication; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with cGMPs; and |
| • | FDA review and approval of the NDA. |
Preclinical tests include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about issues such as the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. CTI cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin.
Clinical trials involve the administration of the investigational drug to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. The study protocol and informed consent information for study subjects in clinical trials must also be approved by an Institutional Review Board for each institution where the trials will be conducted. Study subjects must sign an informed consent form before participating in a clinical trial. Phase I usually involves the initial introduction of the investigational drug into people to evaluate its short-term safety, dosage tolerance, metabolism, pharmacokinetics and pharmacologic actions, and, if possible, to gain an early indication of its effectiveness. Phase II usually involves trials in a limited patient population to (i) evaluate dosage tolerance and appropriate dosage, (ii) identify possible adverse effects and safety risks, and (iii) evaluate preliminarily the efficacy of the drug for specific indications. Phase III trials usually further evaluate clinical efficacy and test further for safety by using the drug in its final form in an expanded patient population. There can be no assurance that phase I, phase II or phase III testing will be completed successfully within any specified period of time, if at all. Furthermore, CTI or the FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
The FDCA permits the FDA and IND sponsor to agree in writing on the design and size of clinical studies intended to form the primary basis of an effectiveness claim in an NDA application. This process is known as special protocol assessment, or SPA. These agreements may not be changed after the clinical studies begin, except in limited circumstances. The existence of an SPA, however, does not assure approval of a product candidate.
C-87
Assuming successful completion of the required clinical testing, the results of the preclinical studies and of the clinical studies, together with other detailed information, including information on the manufacture and composition of the drug, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. The testing and approval process requires substantial time, effort and financial resources. The FDA will review the application and may deem it to be inadequate to support the registration, and we cannot be sure that any approval will be granted on a timely basis, if at all. The FDA may also seek the advice of an advisory committee, typically a panel of clinicians practicing in the field for which the drug is intended, for review, evaluation and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendations of the advisory committee.
The FDA has various programs, including fast track, priority review and accelerated approval, that are intended to expedite or simplify the process for reviewing drugs and/or provide for approval on the basis of surrogate endpoints. Generally, drugs that may be eligible for one or more of these programs are those for serious or life threatening conditions, those with the potential to address unmet medical needs and those that provide meaningful benefit over existing treatments. CTI cannot be sure that any of its drugs will qualify for any of these programs, or that, if a drug does qualify, that the review time will be reduced or that the product will be approved.
Before approving an NDA, the FDA usually will inspect the facility or the facilities where the drug is manufactured and will not approve the product unless cGMP compliance is satisfactory. If the FDA evaluates the NDA and the manufacturing facilities as acceptable, the FDA may issue an approval letter, or in some cases, an approvable letter. An approvable letter contains a number of conditions that must be met in order to secure final approval of the NDA. When and if those conditions have been met to the FDA’s satisfaction, the FDA will issue an approval letter. The approval letter authorizes commercial marketing of the drug for specific indications. As a condition of NDA approval, the FDA may require post marketing testing and surveillance to monitor the drug’s safety or efficacy, or impose other conditions.
After approval, certain changes to the approved product, such as adding new indications, making certain manufacturing changes or making certain additional labeling claims, are subject to further FDA review and approval. Obtaining approval for a new indication generally requires that additional clinical studies be conducted.
Post-Approval Requirements. Holders of an approved NDA are required to: (i) report certain adverse reactions to the FDA, (ii) comply with certain requirements concerning advertising and promotional labeling for their products, and (iii) continue to have quality control and manufacturing procedures conform to cGMP after approval. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing facilities; this latter effort includes assessment of compliance with cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance. We use and will continue to use third-party manufacturers to produce our products in clinical and commercial quantities, and future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal of the product from the market.
Marketing of prescription drugs is also subject to significant regulation through federal and state agencies tasked with consumer protection and prevention of medical fraud, waste and abuse. If we, or our future collaborators, are able to obtain FDA approval to market any of our product candidates, we must comply with restrictions on off-label use promotion, anti-kickback, ongoing clinical trial registration, and limitations on gifts and payments
C-88
to physicians. In addition, we have entered into a corporate integrity agreement, or CIA, with the Office of the Inspector General, Health and Human Services, or OIG-HHS, as part of our settlement agreement with the United States Attorney’s Office, or USAO, for the Western District of Washington arising out of their investigation into certain of our prior marketing practices relating to TRISENOX, which was divested to Cephalon Inc. in July 2005. The CIA, which became effective in December 2007 upon our acquisition of a commercially marketed drug, Zevalin, requires us to establish a compliance committee and compliance program and adopt a formal code of conduct.
Non-U.S. Regulation. Before CTI’s products can be marketed outside of the United States, they are subject to regulatory approval similar to that required in the United States, although the requirements governing the conduct of clinical trials, including additional clinical trials that may be required, product licensing, pricing and reimbursement vary widely from country to country. No action can be taken to market any product in a country until an appropriate application has been approved by the regulatory authorities in that country. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. In certain countries, the sales price of a product must also be approved. The pricing review period often begins after market approval is granted. Even if a product is approved by a regulatory authority, satisfactory prices may not be approved for such product.
In Europe, marketing authorizations may be submitted at a centralized, a decentralized or national level. The centralized procedure is mandatory for the approval of biotechnology products and provides for the grant of a single marketing authorization that is valid in all European Union members’ states. As of January 1995, a mutual recognition procedure is available at the request of the applicant for all medicinal products that are not subject to the centralized procedure. There can be no assurance that the chosen regulatory strategy will secure regulatory approvals on a timely basis or at all.
Environmental Regulation
In connection with CTI’s research and development activities, the Company is subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although CTI believes that it has complied with these laws, regulations and policies in all material respects and has not been required to take any significant action to correct any noncompliance, CTI may be required to incur significant costs to comply with environmental and health and safety regulations in the future. CTI’s research and development involves the controlled use of hazardous materials, including, but not limited to, certain hazardous chemicals and radioactive materials. Although CTI believes that its safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident, CTI could be held liable for any damages that result and any such liability could exceed our resources.
For information on U.S. corporate governance requirements, please see Chapter 16, Paragraph 16.4, “Compliance by the Issuer with the corporate governance regime to which it is subject” of this Registration Document.
C-89
Regulations applicable to the Company’s activities in Italy
The Company’s research and development activities, facilities and equipment and the production and marketing of its pharmaceutical products are subject to numerous laws and regulations issued by government authorities in the EU and Italy.
The Company’s research and development activities involve the use of dangerous materials. The Company will be subject to national and regional laws, regulations and procedures which govern the use, production, storage, containment conditions, manipulation and elimination of toxic and harmful substances.
The principal regulatory areas which concern the Company’s activities and products in the EU and Italy are the following:
(a) Italian and foreign laws and regulations governing the research, experimentation, production and marketing of new pharmaceutical products.
In particular Legislative Decree No. 219 dated 24 April 2006, implementing EU Directive 2001/83 (as subsequently modified) and EU Directive 2003/94, governs the registration, production and marketing of medicinal products for human application. At the Italian level, the authorizations necessary for manufacturing and marketing medicinal products are granted by AIFA (Agenzia Italiana del Farmaco), an entity responsible for the regulation of drugs in Italy and operating under the surveillance of the Italian Ministry of Health. This authorization, which upon certain circumstances may be limited or revoked, shall be valid for 5 years and may be renewed on the basis of a new evaluation of the risk benefit balance carried out by the AIFA. Medicinal products may also be registered in Italy pursuant to a passporting procedure whereby the relevant product has already been authorized in another EU member State. In such regard please note that the Company announced on June 14, 2010, that the AIFA has approved the facility at NerPharMa DS (a pharmaceutical manufacturing company belonging to Nerviano Medical Sciences Srl, in Nerviano, Italy) for the production of CTI’s drug candidate pixantrone.
At the EU level, the regulatory authority is the EMA. Based in London, EMA is responsible for coordinating scientific resources in EU countries and evaluates and supervises the manufacturing and marketing of medicinal products for use across the EU. The European Commission may authorize the marketing of new products pursuant to a centralized procedure.
As far as the research activity is concerned, Ministerial Decree of July 15, 1997 sets forth guidelines for clinical experimentation and Legislative Decree No. 211 dated 24 June 2003 implements EU Directive dated 2001/20/EC relating to the fair practices of the clinical trials of drugs. Moreover Legislative Decree No. 200 dated 6 November 2007 has implemented the EU Directive 28/2005 setting forth principles and detailed guidelines for a fair clinical practice with respect to drugs under experimentation for human use, as well as the requirements to obtain the authorization to manufacture or import such drugs. The Ministerial Decree of August 5, 1999, provides also for the inspection and verification of good laboratory practices. Relevant are also the laws and regulations of other countries where the Company’s potential products are intended to be produced or marketed.
Italy and the EU have adopted high standards of review for new pharmaceutical products, which are typically reviewed at each of the following stages:
| • | underlying research; |
| • | preclinical studies; |
| • | clinical trials; |
| • | registration of the product; |
C-90
| • | production of the product; and |
| • | marketing of the product. |
Consequently, the entire approval process for new pharmaceutical and /or medicinal products is typically lengthy.
(b) Italian and foreign laws and regulations governing intellectual property rights.
There are a series of national and international regulations that are relevant here. In Italy, these include the Italian Code of Industrial Property Rights (Legislative Decree No. 30/2005), which has repealed previous national laws on patents and inventions, designs, trademarks, plant breeders’ rights and semiconductor topographies, most of which dated back to the years between 1939 and 1942. At the EU level, the relevant provisions are the European Patent Convention of October 1973, as modified by subsequent decisions of the Administrative Council of the European Patent Organization and Council Regulation (EEC) No. 1768/92 of 18 June 1992 concerning the creation of a supplementary protection certificate for medicinal products. At the international level, the Paris Convention on the Protection of Intellectual Property and the Patent Cooperation Treaty, as supplemented and amended, are relevant.
(c) Italian and foreign laws and regulations governing reimbursement for the purchase of pharmaceutical products.
Pursuant to Italian laws and regulations, some medical products in Italy are subject to reimbursement from the Italian National Health System. In particular, pursuant also to Law No. 388 dated 23 December 2000 and Law No. 405 dated 16 November 2001, drugs bought by end users may fall either under (i) Class A which are reimbursed by the Italian National Health System (although, local entities may provide for a ticket to be paid by end-users) and (ii) Class C and C-bis whose costs are borne exclusively by end-users. Increase of the price on medicinal of Class C are subject to given restrictions pursuant to Law No. 149 dated 26 July 2005.
The medicinal products of Class A are determined by the Italian Ministry of Health in a list prepared by the Italian Ministry of Health which is updated on a regular basis and the validity of which refers to the whole national territory of Italy. Pursuant to the applicable law, AIFA shall also publish a so-called “transparency list” which is a list including medicinal products equivalent to Class A drugs and relevant reference price. Reference prices represent the maximum amount that can be reimbursed by the Italian National Health System.
(d) Italian regulations concerning safety and hygiene in the workplace and environmental protection.
Legislative Decree No. 81/2008 sets forth provisions on safety and hygiene in the workplace. Also relevant are Legislative Decree No. 206/2001, concerning the use of genetically modified microorganisms, Legislative Decree No. 230/95, as subsequently amended, concerning ionized radiation, Legislative Decree No. 152/2006 concerning, among others, the waste management and Legislative Decree No. 334/1999, as subsequently amended, which implements the 96/82/CE directive regarding the control of dangers related to specific dangerous materials.
(e) Italian regulations on products liability
To the extent that the Group carries out manufacturing activity, the same could be exposed to product liability. Product liability in Italy is governed by Section 102 and subsequent sections of Legislative Decree No. 206 dated 6 September 2005 (“Consumer Code”). Pursuant to such provisions, inter alia, the producer is liable for the damages caused by the defects of his products; if the producer cannot be singled out, the distributor is subject to the same liability unless it disclose the identity and address of producer/supplier within three month from the consumer’s request. A product is defective when it does not provide the safety which a person is entitled to
C-91
expect, taking all circumstances into account, including: (i) the modalities with which is put into circulation, the presentation of the product, its manifest features, the instructions and the warning; (ii) the use to which it could reasonably be expected that the product would be put; (iii) the time when the product was put into circulation. The right to damages is subject to forfeiture after three years from the consumer knowledge (or potential knowledge) of the damage, defects and identity of the responsible. Moreover, rights to damages expire after 10 years from the date on which the producer or the distributor has put the relevant product into circulation.
For the application of Italian disclosure requirements for listed companies, please make reference to Chapter 16, Section 16.4, “Compliance by the Issuer with the corporate governance regime to which it is subject” of this Registration Document.
| 6.1.7 | New products and services |
Although CTI does not currently have any products approved for sale in the market, CTI is working on a number of drug targets in discovery research. Among these programs are bisplatinum agents, HIF-1/p300 inhibitors, and proteasome inhibitors with indirect inhibition properties. In early 2008, CTI initiated a cost savings program, including the closure of CTI’s facilities in Bresso, Italy, in an effort to refocus corporate resources on drug candidates that were later stage and closer to entering the approval process. As a result, CTI is focusing on late stage drug approvals and commercialization and is no longer expending significant resources on developing new products and services. CTI continues to conduct research in the United States as part of CTI’s acquisition of Systems Medicine. In addition to discovery research, preclinical activities are focused on product lifecycle management, including the development of alternative dosage forms and routes of administration for existing products in the development pipeline. The continuous changes in the regulations governing the registration and marketing of drugs and the differences among different countries might cause delays in the approvals, including approvals from the FDA and/or the EMA and/or considerable increases in development costs and CTI’s future marketing costs are uncertain.
| 6.2 | Principal markets |
| 6.2.1 | The Oncology Market |
Overview
According to the American Cancer Society, or ACS, cancer is the second leading cause of death in the United States, resulting in close to 560,000 deaths annually, or more than 1,500 people per day. The National Cancer Institute estimates that approximately 11.1 million people in the United States with a history of cancer were alive in January 2005, and it is estimated that slightly more than one in three American women, and slightly less than one in two American men will develop cancer in their lifetime. Approximately 1.5 million new cases of cancer were expected to be diagnosed in 2009 in the United States. The most commonly used methods for treating patients with cancer are surgery, radiation and chemotherapy. Patients usually receive a combination of these treatments depending upon the type and extent of their disease.
Despite recent advances in sequencing the human genome and the introduction of new biologic therapies for the treatment of cancer, almost all patients with advanced cancer will receive chemotherapy at some point during the treatment of their disease. The cornerstone classes of chemotherapy agents include anthracyclines, camptothecins, platinates and taxanes. Unfortunately, there are significant limitations and complications
C-92
associated with these agents that result in a high rate of treatment failure. The principal limitations of chemotherapy include:
| • | treatment-related toxicities, |
| • | inability to selectively target tumor tissue, and |
| • | the development of resistance to the cancer-killing effects of chemotherapy. |
Treatment-related toxicities
The majority of current chemotherapy agents kill cancer cells by disrupting the cell division and replication process. Although this mechanism often works in cancer cells, which grow rapidly through cell division, non-cancerous cells are also killed because they too undergo routine cell division. This is especially true for cells that line the mouth, stomach and intestines, hair follicles, blood cells and reproductive cells (sperm and ovum). Because the mechanism by which conventional cancer drugs work is not limited to cancer cells, their use is often accompanied by toxicities. These toxicities limit the effectiveness of cancer drugs and seriously impact the patient’s quality of life.
Inability to selectively target tumor tissue
When administered, chemotherapy circulates through the bloodstream, reaching both tumor and normal tissues. Normally dividing tissues are generally as sensitive as tumor cells to the killing effects of chemotherapy and toxic side effects limit the treatment doses that can be given to patients with cancer.
Chemotherapy resistance
Resistance to the cancer killing effects of conventional chemotherapy is a major impediment to continued effective treatment of cancer. Many cancer patients undergoing chemotherapy ultimately develop resistance to one or more chemotherapy agents and eventually die from their disease. Because many chemotherapies share similar properties, when a tumor develops resistance to a single drug, it may become resistant to many other drugs as well. Drugs that work differently from existing chemotherapies and are less susceptible to the same mechanisms of resistance have consequently begun to play an important role in treating resistant tumors.
CTI believes developing agents which improve on the cornerstone chemotherapy classes, in addition to novel drugs designed to treat specific types of cancer and cancer patients, fills a significant unmet need for cancer patients. CTI cancer drug development pipeline includes a modified anthracycline, a taxane and a DNA minor groove binding agent, each of which has the potential to treat a variety of cancer types.
| 6.2.2 | Barriers to Entry |
The oncology market is competitive and difficult for companies to penetrate. The market is highly regulated by the FDA and international regulatory agencies, and dominated by large pharmaceutical and biotechnology companies with vast resources, as described in the section entitled “Competition” below. These large companies can also provide funding and resources to smaller companies. Companies in the oncology market that produce products that have already obtained regulatory approval or have been through a significant amount of clinical testing have an advantage over newer companies with fewer financial, technical, clinical and human resources.
C-93
| 6.2.3 | Competition |
Competition in the pharmaceutical and biotechnology industries is intense. The Company faces competition from a variety of companies focused on developing oncology drugs. The Company competes with large pharmaceutical companies and with other specialized biotechnology companies, including but not limited to: Bristol-Myers Squibb Company, Sanofi-Aventis, Wyeth, Roche Group, Genentech, Inc., OSI Pharmaceuticals, Inc., Eli Lilly and Company, Abraxis, Neopharm Inc., Telik, Inc., TEVA Pharmaceuticals Industries Ltd. and PharmaMar. Many of existing or potential competitors of the Company have substantially greater financial, technical and human resources than us and may be better equipped to develop, manufacture and market products. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biotechnology companies. Many of these competitors have products that have been approved or are in development and operate large, well-funded research and development programs.
The Company expects to encounter significant competition for the principal pharmaceutical products the Company plans to develop. Companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of their products before the Company may achieve a significant competitive advantage if their products work through a similar mechanism as products of the Company and if the approved indications are similar. The Company does not believe competition is as intense among products that treat cancer through novel delivery or therapeutic mechanisms where these mechanisms translate into a clinical advantage in safety and/or efficacy. A number of biotechnology and pharmaceutical companies are developing new products for the treatment of the same diseases being targeted by us. In some instances, such products have already entered late-stage clinical trials or received FDA approval. However, cancer drugs with distinctly different mechanisms of action are often used together in combination for treating cancer, allowing several different products to target the same cancer indication or disease type. Such combination therapy is typically supported by clinical trials that demonstrate the advantage of combination therapy over that of a single-agent treatment.
The Company believes that its ability to compete successfully will be based on its ability to create and maintain scientifically advanced technology, develop proprietary products, attract and retain scientific personnel, obtain patent or other protection for its products, obtain required regulatory approvals and manufacture and successfully market its products, either alone or through outside parties. The Company will continue to seek licenses with respect to technology related to its field of interest and may face competition with respect to such efforts.
| 6.2.4 | Customer and Geographic Concentrations |
All product sales for the years ended December 31, 2008 and 2007 related to Zevalin. There were no products sales for the year ended December 31, 2009 or the nine months ended September 30, 2010 as Zevalin was acquired in December 2007 and sold to CTI’s 50/50 owned joint venture, RIT Oncology, in December 2008.
Product sales from Zevalin’s major customers as a percentage of total product sales were as follows:
C-94
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Customer A |
77 | % | 67 | % | ||||
| Customer B |
5 | % | 33 | % | ||||
All sales of Zevalin during 2008 and 2007 were to North America. The following table depicts long-lived assets based on the following geographic locations (in thousands):
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| United States |
$ | 22,966 | $ | 39,777 | ||||
| Europe |
7,286 | 11,099 | ||||||
| $ | 30,252 | $ | 50,876 | |||||
| 6.3 | Exceptional factors |
Except as described under Chapter 5, Paragraph 5.1.5, “Important events in the development of the Issuer’s business” no information pursuant to Chapter 6, Paragraph 6.1, “Principal activities” or 6.2, “Development Process” has been influenced by exceptional factors or occurrences.
| 6.4 | Business profitability dependence |
| 6.4.1 | Patents |
Development of CTI’s intellectual property is critical to its business. CTI’s success depends in part on its ability to:
| • | obtain patent protection for its products or processes both in the united states and other countries, |
| • | protect trade secrets, and |
| • | prevent others from infringing on its proprietary rights. |
Please refer to subsequent Paragraph 11.1 of this Registration Document for detailed information about the patents of the Company (both currently owned and under application) and the relevant future perspectives.
| 6.4.2 | Collaboration, Licensing and Milestone Agreements |
6.4.2.1 PG-TXL Company, L.P. The Company has an agreement with PG-TXL Company, L.P, or PG-TXL, which grants the Company an exclusive worldwide license for the rights to OPAXIO and to all potential uses of PG-TXL’s polymer technology. Under the terms of the agreement, the Company acquired the rights to the research, development, manufacture, marketing and sale of anti-cancer drugs developed using this polymer technology. the Company is obligated to make payments to PG-TXL upon the achievement of certain development and regulatory milestones and the Company could be obligated to make payments of up to $14.4 million in the future if additional milestones are met.
6.4.2.2 Gynecologic Oncology Group. The Company has an agreement with the Gynecologic Oncology Group, or GOG, related to the GOG0212 trial which the GOG is conducting. Under this agreement the Company is required to pay up to $5.1 million in additional milestone payments related to the trial.
C-95
6.4.2.3 Acquisition of Systems Medicine, Inc. In connection with the acquisition of the Company of Systems Medicine, Inc., or SMI, the Company was required to pay to SMI stockholders a maximum of $15.0 million in additional consideration (payable in cash or stock at our election, subject to certain NASDAQ limitations on the issuance of stock) upon the achievement of certain FDA regulatory milestones for brostallicin. In August 2009, the Company entered into an amended agreement under which these milestone payments were replaced by an immediate substitute payment of $6.0 million payable in shares of CTI common stock subject to certain conditions, including required shareholder approval. If the conditions were not satisfied, the Company would have been required to pay SMI stockholders $5.0 million cash in lieu of the $6.0 million shares of CTI common stock. In October 2009, CTI shareholders approved the issuance of $6.0 million shares of our common stock and we issued 5.6 million shares of common stock to the SMI stockholders.
6.4.2.4 Brostallicin. Under a license agreement entered into for brostallicin, the Company may be required to pay up to $80.0 million in milestone payments, based on the achievement of certain product development results. Due to the early stage of development that brostallicin is in, the Company is not able to determine whether the clinical trials will be successful and therefore cannot make a determination that the milestone payments are reasonably likely to occur at this time.
6.4.2.5 Cephalon. Pursuant to an acquisition agreement entered into with Cephalon, Inc. in June 2005, the Company may receive up to $100 million in payments upon achievement by Cephalon of specified sales and development milestones related to TRISENOX. However, the achievement of any such milestones is uncertain at this time.
6.4.2.6 Novartis International Pharmaceutical Ltd. In September 2006, the Company entered into an exclusive worldwide licensing agreement with Novartis International Pharmaceutical Ltd., or Novartis, for the development and commercialization of OPAXIO. If Novartis elects to participate in the commercialization and development of OPAXIO, total product registration and sales milestones due from Novartis for OPAXIO under the agreement could reach up to $270 million. The agreement also provides Novartis with an option to develop and commercialize pixantrone based on agreed terms. If Novartis exercises its option on pixantrone under certain conditions and the Company is to negotiate a definitive agreement with Novartis, Novartis would pay CTI a $7.5 million license fee, up to $104 million in registration and sales related milestones and a royalty on pixantrone worldwide net sales as well as reimbursement for certain expenses. As of September 30, 2010, the Company has not received any milestone payments and the Company will not receive any milestone payments unless Novartis elects to participate in the development and commercialization of pixantrone or OPAXIO.
| 6.4.3 | Suppliers |
The Company currently uses, and expects to continue to be dependent upon, contract manufacturers to manufacture each of its product candidates. The Company has established a quality control and quality assurance program, including a set of standard operating procedures and specifications, designed to ensure that its products and product candidates are manufactured in accordance with current Good Manufacturing Practices, or cGMPs, and other applicable domestic and European regulations. The Company will need to invest in additional manufacturing development, manufacturing and supply chain resources, and may seek to enter into additional collaborative arrangements with other parties that have established manufacturing capabilities. It is likely that the Company will continue to rely on third-party manufacturers for its development and commercial products on a contract basis. Currently, the Company has agreements with third-party vendors to produce, test, and distribute pixantrone, OPAXIO and brostallicin drug supply for clinical studies. The Company will be dependent upon these third-party vendors to supply CTI in a timely manner with products manufactured in
C-96
compliance with cGMPs or similar standards imposed by U.S. and/or foreign regulatory authorities where our products are being developed, tested, and/or marketed.
The Company has a purchase agreement with Natural Pharmaceuticals, Inc., or NPI, which was assumed by Phyton Biotech, LLC, or Phyton, upon their purchase of NPI in 2009. Under this purchase agreement, Phyton currently must supply us with either 2.5 kilograms of paclitaxel or the cash equivalent of $0.5 million.
In October 2009, the FDA inspected the contract manufacturing facility of the Company located in Milan, Italy and, based on its inspection, made observations regarding the manufacturing process and controls over lead compound, pixantrone of the Company. The contract manufacturer of the Company addressed and responded to the FDA’s observations in November 2009. Neither the contract manufacturer of the Company nor the Company have received any further response from the FDA regarding contract manufacturer’s of the Company planned action as of February 22, 2010.
| 6.5 | Basis for the Company statements with regard to its competitive position |
The Company’s statements regarding competition above are based on senior management’s knowledge of the oncology industry.
C-97
CHAPTER 7 ORGANIZATIONAL STRUCTURE
| 7.1 | Description of the Company and its subsidiaries (the “Group”) |
As of the date of this Registration Document the Group includes the following wholly owned subsidiaries: Systems Medicine LLC, CTI Commercial LLC (from the date of formation in July 2008) and CTI Life Sciences Limited (from the date of formation in March 2009). CTI Technologies was liquidated in the fourth quarter of 2007, Cell Therapeutics (Ireland) Holding Limited was liquidated in the fourth quarter of 2006 and CTI Corporate Development, Inc. was liquidated in the fourth quarter of 2009. The financial statements of these companies are included in the consolidated financial statements of the Issuer as of September 30, 2010.
As of the date of this Registration Document, the Company also had a 69% ownership and voting interest in its majority owned subsidiary, Aequus Biopharma, Inc. In accordance with our fiscal 2010 adoption of Statement of Financial Accounting Standards No. 160, Noncontrolling Interests in Consolidated Statements, an amendment of ARB No. 51, noncontrolling interest in Aequus (previously shown as minority interest) is now reported as noncontrolling interest.
CTI is not a controlled company of any wider group.
All intercompany transactions and balances are eliminated in consolidation.
| 7.2 | Subsidiaries |
The following chart sets forth the structure of the Group as of the date of this Registration Document.
| Cell Therapeutics, Inc.
| ||||||||||||||
|
Systems Medicine, LLC |
CTI Life Sciences Limited
|
CTI Commercial, LLC | Aequus Biopharma, Inc. | |||||||||||
The Group headed by the Company is made up of the following wholly owned subsidiaries:
| • | Systems Medicine, LLC, or SM, is a Delaware (U.S.) limited liability company that was acquired in July 2007. SM develops brostallicin, which is a small molecule, anti-cancer drug with a novel, unique mechanism of action and composition of matter patent coverage. Share capital is $20,499,204 issued and fully paid. |
| • | CTI Life Sciences Limited was incorporated in March 2009 the United Kingdom and was formed to assist the Company with the EMA filing for OPAXIO. Share capital is $28,034 issued and fully paid. |
| • | CTI Commercial LLC is a Nevada (U.S.) limited liability company that was formed in July 2008 to facilitate the Company’s insurance reimbursements. There is no share capital as of the date of this Registration Document. |
In addition, with regard to CTI’s majority-owned significant subsidiary:
C-98
| • | Aequus Biopharma, or Aequus, is a Washington (U.S.) corporation that was formed in May 2007 to develop our Genetic Polymer™ technology which may speed the manufacture, development and commercialization of follow-on and novel protein-based therapeutics. As of the date of this Registration Document, the Company holds a 69% ownership in Aequus which has share capital of $549,019 issued and fully paid shares outstanding. |
In addition, please note that:
| • | Cell Therapeutics Inc.- Sede Secondaria, or Cell Therapeutics Europe S.r.l., was merged into Cell Therapeutics, Inc. in November 2007 and operated as an Italian branch of the Company. With respect to the closing of the Italian branch of the Company, please refer to Chapter 5, Paragraph 5.1.5 (dates: February 2009 and May 13, 2009) for further information. Even though Cell Therapeutics Inc. – Sede Secondaria or CTI (Europe) is still a branch of the Company, however all the core operations relating to this branch were ceased in September 2009. |
C-99
CHAPTER 8 PROPERTY, PLANT AND EQUIPMENT
| 8.1 | Property, plant and equipment |
| 8.1.1 | Property owned |
The Company does not own any real estate. Property, plant and equipment are composed of the following (in thousands of US dollars) as of September 30, 2010 and December 31, 2009, 2008 and 2007:
| September 30, | December 31, | |||||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| (unaudited) | ||||||||||||||||
| Leasehold improvements |
$ | 3,174 | $ | 3,277 | $ | 6,512 | $ | 11,644 | ||||||||
| Lab equipment |
470 | 560 | 7,240 | 7,452 | ||||||||||||
| Furniture and office equipment |
13,104 | 11,970 | 19,252 | 18,300 | ||||||||||||
| 16,748 | 15,807 | 33,004 | 37,396 | |||||||||||||
| Less: accumulated depreciation and amortization |
(13,634 | ) | (12,377 | ) | (28,680 | ) | (31,371 | ) | ||||||||
| $ | 3,114 | $ | 3,430 | $ | 4,324 | $ | 6,025 | |||||||||
We had one capital lease agreement related to our European branch to finance lab equipment which had a rate of 6.0% and terminated in May 2010. Additionally, a second capital lease terminated in February 2008. Currently, the Company is not a party of any capital lease.
| 8.1.2 | Property used |
CTI currently leases approximately 77,000 square feet of space at 501 Elliott Avenue West in Seattle, Washington under an amended lease for its executive offices and administrative operations, which expires in July 2012. The wholly owned subsidiary CTI Life Sciences Limited also leases approximately 2,700 square feet in Milan, Via Amedei 8, Italy with a lease expiration date of December 2015. In addition, the Company’s wholly owned subsidiary Systems Medicine LLC, acquired in July 2007, leased approximately 2,000 square feet of office and laboratory space in Scottsdale, Arizona which was terminated in January 2010. CTI believes its existing and planned facilities are adequate to meet its present requirements. The Company anticipates that additional space will be available, when needed, on commercially reasonable terms.
| 8.1.3 | Guarantees |
The Company has no guarantees or liens on any of the tangible assets described under Paragraphs 8.1.1 and 8.1.2 above.
| 8.2 | Environmental issues that may affect the Company’s use of the tangible fixed assets |
In connection with CTI’s research and development activities, CTI is subject to international, federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage,
C-100
air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although CTI believes that it has complied with these laws, regulations and policies in all material respects and has not been required to take any significant action to correct any noncompliance, CTI may be required to incur significant costs to comply with environmental and health and safety regulations in the future. CTI’s research and development involves the controlled use of hazardous materials, including, but not limited to, certain hazardous chemicals and radioactive materials. Although CTI believes that its safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of such an accident, CTI could be held liable for any damages that result and any such liability could exceed CTI’s resources.
C-101
CHAPTER 9 OPERATING AND FINANCIAL REVIEW
| 9.1 | Financial situation |
The following table provides a description of the issuer’s financial condition as of September 30, 2010 and December 31, 2009, 2008 and 2007. The amounts, in thousands of US Dollars, are derived from the Company’s consolidated balance sheet for the respective period. Please also refer to Chapter 10, “Capital Resources” of this Registration Document.
| September 30, | December 31, | |||||||||||||||
| Net Financial Standing |
2010 | 2009 | 2008 | 2007 | ||||||||||||
| Cash and cash equivalents |
(17,268 | ) | (37,811 | ) | (10,072 | ) | (15,798 | ) | ||||||||
| Restricted cash |
— | — | (6,640 | ) | — | |||||||||||
| Securities available-for-sale |
— | — | (599 | ) | (2,548 | ) | ||||||||||
| Long term obligations, current portion |
812 | 1,312 | 757 | 1,020 | ||||||||||||
| Current portion of derivative liability |
— | — | — | — | ||||||||||||
| Convertible senior subordinated notes, current portion |
— | 40,363 | — | 16,907 | ||||||||||||
| Convertible subordinated notes, current portion |
— | — | — | 2,910 | ||||||||||||
| Convertible senior notes, current portion |
10,187 | — | — | — | ||||||||||||
| Net Financial Standing, current portion |
(6,269 | ) | 3,864 | (16,554 | ) | 2,491 | ||||||||||
| Convertible senior subordinated notes |
— | — | 55,150 | 55,150 | ||||||||||||
| Convertible subordinated notes |
— | — | — | — | ||||||||||||
| Long term obligations, less current portion |
1,010 | 1,861 | 2,907 | 9,879 | ||||||||||||
| Convertible senior notes, less current portion |
11,985 | 21,779 | 87,223 | 62,429 | ||||||||||||
| Net Financial Standing, less current portion |
12,995 | 23,640 | 145,280 | 127,458 | ||||||||||||
| Net Financial Indebtedness |
6,726 | 27,504 | 128,726 | 129,949 | ||||||||||||
C-102
As shown in the above table, the net financial indebtedness, which is calculated as current assets less long term obligations, decreased as of September 30, 2010 as compared to December 31, 2009 due to a net increase in cash and cash equivalents resulting from $75.1 million in gross proceeds received from the issuance of preferred stock, offset by cash used in operations during the period. In addition, net financial indebtedness decreased due to exchanges of $1.8 million aggregate principal amount of the Company’s 4% convertible senior subordinated notes for common stock in May 2010.
Net financial indebtedness decreased as of December 31, 2009 as compared to December 31, 2008 primarily due to a decrease in convertible senior notes relating to conversions of $23.3 million of the Company’s 9% notes and 10% notes due 2011 and exchanges of approximately $57.4 million aggregate principal amount of convertible notes completed in June 2009, offset by a decrease in the Company’s restricted cash held for make-whole payments on certain of its convertible notes.
Net financial indebtedness decreased as of December 31, 2008 as compared to December 31, 2007 primarily due to an increase in convertible senior notes relating to issuances of the Company’s 9% and 10% notes due 2011, offset in part by an increase in restricted cash as well as repayment of current portion of long term obligation, convertible senior subordinated and convertible subordinated notes.
| 9.2 | Operating Results |
| 9.2.1 | Key factors for the years ended December 31, 2009, 2008 and 2007 |
CELL THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands of US dollars, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Revenues: |
||||||||||||
| Product sales |
$ | — | $ | 11,352 | $ | 47 | ||||||
| License and contract revenue |
80 | 80 | 80 | |||||||||
| Total revenues |
80 | 11,432 | 127 | |||||||||
| Operating expenses, net: |
||||||||||||
| Cost of product sold |
— | 3,244 | 49 | |||||||||
| Research and development |
30,179 | 51,614 | 72,019 | |||||||||
| Selling, general and administrative |
57,725 | 41,607 | 35,517 | |||||||||
| Amortization of purchased intangibles |
— | 1,658 | 913 | |||||||||
| Restructuring charges and related gain on sale of assets, net |
3,979 | — | — | |||||||||
| Gain on sale of Zevalin |
— | (9,444 | ) | — | ||||||||
| Gain on sale of investment in joint venture |
(10,244 | ) | — | — | ||||||||
| Acquired in-process research and development |
— | 36 | 24,615 | |||||||||
| Total operating expenses, net |
81,639 | 88,715 | 133,113 | |||||||||
| Loss from operations |
(81,559 | ) | (77,283 | ) | (132,986 | ) | ||||||
| Other income (expense): |
||||||||||||
C-103
| Investment and other income, net |
133 | 549 | 2,430 | |||||||||
| Interest expense |
(4,806 | ) | (8,559 | ) | (8,237 | ) | ||||||
| Amortization of debt discount and issuance costs |
(5,788 | ) | (66,530 | ) | (4,280 | ) | ||||||
| Foreign exchange gain |
33 | 3,637 | 4,657 | |||||||||
| Make-whole interest expense |
(6,345 | ) | (70,243 | ) | (2,310 | ) | ||||||
| Gain on derivative liabilities, net |
7,218 | 69,739 | 3,672 | |||||||||
| Gain (loss) on exchange of convertible notes |
7,381 | (25,103 | ) | (972 | ) | |||||||
| Equity loss from investment in joint venture |
(1,204 | ) | (123 | ) | — | |||||||
| Milestone modification expense |
(6,000 | ) | — | — | ||||||||
| Settlement expense |
(4,710 | ) | (3,393 | ) | (160 | ) | ||||||
| Write-off of financing arrangement costs |
— | (2,846 | ) | — | ||||||||
| Other expense, net |
(14,088 | ) | (102,872 | ) | (5,200 | ) | ||||||
| Net loss before noncontrolling interest |
(95,647 | ) | (180,155 | ) | (138,186 | ) | ||||||
| Noncontrolling interest |
252 | 126 | 78 | |||||||||
| Net loss attributable to CTI |
(95,395 | ) | (180,029 | ) | (138,108 | ) | ||||||
| Gain on restructuring of preferred stock |
2,116 | — | — | |||||||||
| Preferred stock dividends |
(24 | ) | (662 | ) | (648 | ) | ||||||
| Deemed dividends on preferred stock |
(23,460 | ) | (22,216 | ) | (9,549 | ) | ||||||
| Net loss attributable to common shareholders |
$ | (116,763 | ) | $ | (202,907 | ) | $ | (148,305 | ) | |||
| Basic and diluted net loss per common share |
$ | (0.25 | ) | $ | (7.00 | ) | $ | (32.75 | ) | |||
| Shares used in calculation of basic and diluted net loss per common share |
458,356 | 28,967 | 4,529 | |||||||||
HEREIN BELOW SPECIFIC INFORMATION ABOUT THE SIGNIFICANT FACTORS THAT HAVE MATERIALLY AFFECTED THE INCOME FOR THE YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007 IS REPORTED. FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.1.4 OF THIS REGISTRATION DOCUMENT.
Research and development expenses.
Our research and development expenses for 2009, 2008, and 2007 are primarily related to pixantrone, OPAXIO, brostallicin and Zevalin.
Research and development expenses decreased to $30.2 million for the year ended December 31, 2009, from $51.6 million for the year ended December 31, 2008. Pixantrone costs decreased primarily due to a decrease in clinical development activity mainly related to the cessation of patient enrollment during 2008 in our RAPID and EXTEND trials. Costs for our OPAXIO program decreased primarily due to a decrease in regulatory and quality activities as well as investigator-sponsored trial costs mainly due to patient enrollment. Costs for brostallicin decreased primarily due to a decrease in clinical development activities related to phase I and phase II studies. Zevalin costs decreased primarily due to the contribution of the product to RIT Oncology, the joint venture we formed with Spectrum on December 15, 2008, which assumed all related Zevalin expenses subsequent to that date.
C-104
Research and development expenses decreased to approximately $51.6 million for the year ended December 31, 2008, from approximately $72.0 million for the year ended December 31, 2007. Pixantrone costs decreased primarily due to a decrease in clinical development activity mainly related to the closure of our PIX303 clinical trial in January 2008 as well as a discontinuance of patient enrollment during 2008 in our RAPID and EXTEND trials. Costs for Zevalin increased due to our acquisition of the product in December 2007 and primarily relate to clinical development activity including $2.0 million in expense related to our payment to Bayer Schering for access to the data from the FIT trial. Costs for our OPAXIO program decreased primarily due to a decrease in clinical development activity related to our PGT307 trial, which was reduced in scope to U.S. sites only in early 2008, reduced costs associated with our PIONEER trial which was suspended and closed in the fourth quarter of 2006 and incurred certain wrap-up costs in the first half of 2007 and a decrease in the GOG0212 study related to the amendment to our contract with the GOG. Manufacturing activity for OPAXIO also decreased as we extended activities into 2009 in an effort to conserve cash in 2008. Costs for brostallicin decreased primarily due to a non-recurring license payment during 2007 related to a development agreement.
Selling, general and administrative expenses.
The changes in selling, general and administrative expenses for the years ended 31 December 2009, 2008 and 2007 are primarily due to changes in compensation and benefits, legal and consulting fees as well as sales and marketing expenses due to acquisition of Zevalin in December 2007 and subsequent expansion of our sales force.
In particular, selling, general and administrative expenses increased to $57.7 million for the year ended December 31, 2009, from $41.6 million for the year ended December 31, 2008. This is primarily due to an $18.9 million increase in non-cash stock-based compensation mainly related to restricted stock granted and vested during 2009.
Selling, general and administrative expenses increased to approximately $41.6 million for the year ended December 31, 2008, from approximately $35.5 million for the year ended December 31, 2007. This is primarily attributed to a $4.8 million increase in sales and marketing expenses due to the acquisition of Zevalin in December 2007 and subsequent expansion of our sales force. In addition, we incurred approximately $1.2 million in legal and consulting fees associated with the potential spin-off, asset divestment, or creation of a joint venture with regard to certain of our operations and assets. We also had an increase in our stock-based compensation expense of approximately $1.8 million as well as an increase in our legal expenses of approximately $0.9 million primarily due to our claim against the Lash Group, Inc. and Documedics Acquisition Co., Inc. Compensation and benefits also increased approximately $0.6 million in part due to key executive personnel hired in 2008.
Amortization of debt discount and issuance costs.
Amortization of debt discount and issuance costs decreased to $5.8 million for the year ended December 31, 2009 as compared to $66.5 million for the year ended December 31, 2008. This was primarily due to the accelerated amortization of issuance costs and debt discount related to conversions and exchanges of our 18.33%, 15.5%, 15%, 13.5%, 10% (due 2012), 9.66% and 9% convertible senior notes during 2008. For the year ended December 31, 2009 as compared to the same period in 2008, the decrease in the amortization of the debt discount related to these notes was $55.2 million and the decrease in the amortization of debt issuance costs was $5.4 million.
C-105
Amortization of debt discount and issuance costs increased to $66.5 million for the year ended December 31, 2008 as compared to $4.3 million for the year ended December 31, 2007. This increase was primarily due to the accelerated amortization of debt discount and issuance costs related to conversions of certain of our convertible notes issued in 2008. For the year ended December 31, 2008, amortization of the debt discount related to our 13.5% convertible senior notes, 9% convertible senior notes, 15.5% convertible senior notes, 18.33% convertible senior notes, 10% convertible senior notes due 2012, 10% convertible senior notes due 2011 and 9.66% convertible senior notes was approximately $23.4 million, $13.2 million, $8.6 million, $5.6 million, $3.4 million, $2.2 million and $1.8 million, respectively, and the amortization of debt issuance costs was approximately $2.0 million, $1.9 million, $0.3 million, $0.5 million, $0.4 million, $0.2 million and $0.3 million, respectively. This amortization was primarily due to conversions of these notes during the year ended December 31, 2008.
Make-whole interest expense.
Make-whole interest expense relates to payments made upon conversions of our 7.5%, 9%, 9.66%, 10% (due 2011), 10% (due 2012), 13.5%, 18.33%, 15.5% convertible senior notes.
In particular, make-whole interest expense of $6.3 million for the year ended December 31, 2009 is related to $5.4 million in payments made upon the conversion of $18.0 million of our 10% convertible senior notes due 2011 and $0.9 million in payments made upon the conversion of $5.3 million of our 9% convertible senior notes.
The amount of $70.2 million for the year ended December 31, 2008 is related to $22.4 million in payments made upon the conversion of $27.6 million of our 13.5% convertible senior notes, $15.5 million in payments made upon conversion of $28.3 million of our 18.33% convertible senior notes, $11.0 million in payments made upon conversion of $40.8 million of our 9% convertible senior notes, $8.8 million in payments made upon conversion of $14.2 million of our 15.5% convertible senior notes, $4.5 million in payments made upon conversion of $15.7 million of our 9.66% convertible senior notes, $4.4 million in payments made upon conversion of $14.7 million of our 10% convertible senior notes due 2011 and $3.6 million in payments made upon conversion of $9.0 million of our 10% convertible senior notes due 2012. Make-whole interest expense of $2.3 million for the year ended December 31, 2007 is due to payments made related to the conversion of $13.6 million of our 7.5% notes.
Gain on derivative liabilities.
The gain on derivative liabilities primarilty relate to change in the estimated fair values of the derivative liabilities related to our 7.5%, Series B Unit Warrant, 13.5%, 9%, 15.5%, 18.33%, 15%, 10% (due 2011), 10% (due 2012) and 9.66% convertible senior notes.
In particular, the gain on derivative liabilities of $7.2 million for the year ended December 31, 2009 is primarily due to a gain of $4.4 million resulting from the change in the estimated fair value of the derivative liability related to the embedded conversion option on our 10% convertible senior notes due 2011 as well as a gain of $2.8 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant that was issued in connection with our 13.5% convertible senior notes and Series E preferred stock financing and modified in July 2008 in connection with the issuance of our 18.33% convertible senior notes. The Series B Unit Warrant expired in the second quarter of 2009.
The gain of $69.7 million for the year ended December 31, 2008 is primarily due to gains of $22.3 million, $12.0 million, $8.6 million, $6.9 million, $4.6 million, $3.4 million, $2.4 million and $2.2 million resulting from the change in the estimated fair value of the derivative liabilities related to the embedded conversion options on our 13.5%, 9%, 15.5%, 18.33%, 15%, 10% (due 2012), 9.66% and 10% (due 2011) convertible senior notes,
C-106
respectively. There was also a gain of $7.3 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant.
The gain on derivative liabilities of $3.7 million for the year ended December 31, 2007 represents the change in the estimated fair value of the derivative liabilities related to the interest make-whole provisions on our 7.5% convertible senior notes.
Gain (loss) on exchange of convertible notes.
The Gain (loss) on exchange of convertible notes are due to exchanges of certain of our convertible notes in exchange for new convertible notes.
In particular, the $7.4 million gain on exchange of convertible notes for the year ended December 31, 2009 is primarily related to $7.2 million due to the exchange of $52.9 million principal amount of portions of our 9%, 7.5%, 6.75% and 5.75% convertible senior notes and 4% convertible senior subordinated notes for $7.1 million in cash and 24.2 million shares of our common stock, net of related transaction costs.
The loss on exchange of convertible notes of $25.1 million for the year ended December 31, 2008 is due to the repurchase of certain of our convertible notes in exchange for new convertible notes or common stock.
We recorded a loss of approximately $1.0 million during the year ended December 31, 2007 due to the extinguishment of approximately $36.1 million aggregate principal amount of our 5.75% convertible senior subordinated and convertible subordinated notes in exchange for approximately $23.3 million aggregate principal amount of our 5.75% convertible senior notes and approximately 5.5 million shares of our common stock in the fourth quarter of 2007.
| 9.2.2 | Key factor for the six months ended June 30, 2010 and 2009 |
This paragraph incorporates by reference specific pieces of information reported in the following documents available to the public on the Company’s website: http://investors.celltherapeutics.com (such specific pieces of information are identified with the pages of the aforesaid documents where they are reported).
Quarterly report of the Issuer for the six months ended June 30, 2010 reviewed by the independent registered public accounting firm
| • | Condensed consolidated statements of operations: page 4 |
| • | Notes to statements of operations: pages 23-25 |
Quarterly report of the Issuer for the six months ended June 30, 2009 reviewed by the independent registered public accounting firm
| • | Condensed consolidated statements of operations: page 4 |
| • | Notes to statements of operations: pages 26-28 |
C-107
| 9.2.3 | Key factor for the nine months ended September 30, 2010 and 2009 |
CELL THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
(unaudited)
| Nine Months Ended | ||||||||
| September 30, | ||||||||
| 2010 | 2009 | |||||||
| Revenues: |
||||||||
| License and contract revenue |
$ | 319 | $ | 60 | ||||
| Total revenues |
319 | 60 | ||||||
| Operating expenses, net: |
||||||||
| Research and development |
19,375 | 22,878 | ||||||
| Selling, general and administrative |
39,378 | 38,997 | ||||||
| Restructuring charges and related gain on sale of assets, net |
— | 3,766 | ||||||
| Gain on sale of investment in joint venture |
— | (10,244 | ) | |||||
| Total operating expenses, net |
58,753 | 55,397 | ||||||
| Loss from operations |
(58,434 | ) | (55,337 | ) | ||||
| Other income (expense): |
||||||||
| Investment and other income (expense), net |
240 | 97 | ||||||
| Interest expense |
(1,948 | ) | (4,026 | ) | ||||
| Amortization of debt discount and issuance costs |
(600 | ) | (5,575 | ) | ||||
| Foreign exchange gain (loss) |
(300 | ) | 278 | |||||
| Debt conversion expense |
(2,031 | ) | — | |||||
| Make-whole interest expense |
— | (6,345 | ) | |||||
| Gain on derivative liabilities, net |
— | 7,218 | ||||||
| Gain on exchange of convertible notes |
— | 7,381 | ||||||
| Equity loss from investment in joint venture |
— | (1,204 | ) | |||||
| Milestone modification expense |
— | (6,000 | ) | |||||
| Settlement expense, net |
— | (4,710 | ) | |||||
| Other income (expense), net |
(4,639 | ) | (12,886 | ) | ||||
| Net loss before noncontrolling interest |
(63,073 | ) | (68,223 | ) | ||||
| Noncontrolling interest |
149 | 205 | ||||||
| Net loss attributable to CTI |
(62,924 | ) | (68,018 | ) | ||||
| Gain on restructuring of preferred stock |
— | 2,116 | ||||||
| Preferred stock dividends |
— | (24 | ) | |||||
| Deemed dividends on preferred stock |
(50,519 | ) | (23,460 | ) | ||||
| Net loss attributable to CTI common shareholders |
$ | (113,443 | ) | $ | (89,386 | ) | ||
| Basic and diluted net loss per common share |
$ | (0.17 | ) | $ | (0.21 | ) | ||
| Shares used in calculation of basic and diluted net loss per common share |
659,244 | 420,520 | ||||||
C-108
HEREINBELOW SPECIFIC INFORMATION ABOUT THE SIGNIFICANT FACTORS THAT HAVE MATERIALLY AFFECTED THE INCOME FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2010, and 2009 IS REPORTED. FOR MORE INFORMATION, PLEASE REFER TO CHAPTER 20, PARAGRAPH 20.6.2 OF THIS REGISTRATION DOCUMENT
Research and development expenses.
Our research and development expenses for the nine months ended September 30, 2010 are primarily related to general operating expenses ($11.6 million), pixantrone ($5.2 milion) and OPAXIO ($1.8 milion).
It is noted that research and development expenses decreased to approximately $19.4 million for the nine months ended September 30, 2010 from approximately $22.9 million for the nine months ended September 30, 2009. In particular, pixantrone costs increased primarily due to an increase in clinical development activity mainly related to costs associated with our RAPID trial as we continued to incur costs during the study wind-down. In addition, there was an increase in activities associated with investigator-sponsored trials, advisory board meetings and consulting services associated with the startup of the additional clinical study of pixantrone, PIX306. These increases were partially offset by decreases in regulatory costs and manufacturing activity. Regulatory costs decreased due to the non-recurring expense associated with the filing fee for the NDA submission to the FDA, which was incurred in the second quarter of 2009. Manufacturing expenses decreased due to the reduction of pre-commercialization activities related to pixantrone. Costs for our OPAXIO program decreased primarily due to a decrease in regulatory, clinical development and quality activities. Costs for brostallicin decreased primarily due to a decrease in clinical development activities related to phase I and phase II studies. Zevalin costs decreased due to the contribution of the product to RIT Oncology, the joint venture we formed with Spectrum on December 15, 2008 which assumed all related Zevalin expenses subsequent to that date. Our operating expenses decreased primarily due to a reduction in headcount between periods, partially offset by an increase in non-cash stock-based compensation expense. Discovery research expense relates to costs incurred in preclinical activities.
Selling, general and administrative expenses.
Selling, general and administrative expenses increased to approximately $39.4 million for the nine months ended September 30, 2010 from approximately $39.0 million for the nine months ended September 30, 2009. This is primarily due to a $2.3 million increase in non-cash stock-based compensation expense and an increase in expenses related to sales personnel for the expected pixantrone launch in the first quarter of 2010. These increases were primarily offset by a decrease in overhead costs associated with our Bresso, Italy operations due to office closure, as well as decreases in discretionary bonus expense and professional services.
C-109
Gain on sale of investment in joint venture.
During the nine months ended September 30, 2009, we recorded a $10.2 million one-time gain on the sale of our 50% interest in RIT Oncology in March 2009. This amount was based on the difference between $16.5 million in gross proceeds and the approximately $4.6 million book value of our investment in RIT Oncology at the time of sale, net of approximately $1.6 million in transaction costs.
Gain on derivative liabilities, net.
The gain on derivative liabilities of $7.2 million for the nine months ended September 30, 2009 is primarily due to a gain of $4.4 million resulting from the change in the estimated fair value of the derivative liability related to the embedded conversion option on our 10% convertible senior notes as well as a gain of $2.8 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant that was issued in connection with our 13.5% convertible senior notes and Series E preferred stock financing in April 2008 and modified in July 2008 in connection with the issuance of our 18.33% convertible senior notes.
Gain on exchange of convertible notes.
The $7.4 million gain on exchange of convertible notes for the nine months ended September 30, 2009 is primarily related to the exchange of $52.9 million principal amount of portions of our 9%, 7.5%, 6.75%, 5.75% convertible senior and 4% Notes for $7.1 million in cash and approximately 24.2 million shares of common stock, net of related transaction costs.
Deemed dividends on preferred stock.
During the nine month period ending September 30, 2010, we recorded $50.5 million in deemed dividends on preferred stock upon issuances of our Series 3, 4, 5 and 6 preferred stock. During the nine month period ending September 30, 2009, we recorded $23.5 million in deemed dividends on preferred stock primarily due to issuances of our Series 1 and 2 preferred stock. Deemed Dividends on preferred stock primarily relate to amortized discount on preferred stock which arises from allocation of proceeds to the warrants and beneficial conversion features of the preferred stock. Since these are treated as deemed dividends pursuant to FASB ASC 260-10, like any other dividends, the amounts are recorded as negative values.
| 9.2.4 | Material changes in net sales or revenues. |
All product sales for the years ended December 31, 2008 and 2007 are due to sales of Zevalin. There were no product sales subsequent to the divestiture of Zevalin to RIT Oncology in December 2008. The Company subsequently sold its 50% interest in RIT Oncology in March 2009.
| 9.2.5 | Information regarding any governmental, economic, fiscal, monetary or political policies or factors that have materially affected, or could materially affect, directly or indirectly, the issuer’s operations. |
The Company carries out its activities around the world and such activities may be subject to different risks, including inflation, political, social and economic instability, changes in the applicable legal framework as well as changes in the license or tax regime, barriers to entry or foreign exchange controls. Each of these changes may affect the operations and results of the Company. These macroeconomic and regulatory aspects may also affect the decision to carry out investments in the field in which the Company operates and therefore may also affect the operations and results of the Company.
C-110
For further information related to governmental, economic, fiscal, monetary or political policies or factors that have materially affected, or could materially affect, directly or indirectly, the Company’s operations, refer to Chapter 4, Risk Factors of this Registration Document.
C-111
CHAPTER 10 CAPITAL RESOURCES
| 10.1 | Information concerning the Company’s capital resources |
The condensed consolidated financial statements reported in this Registration Document have been prepared assuming that the Company will continue as a going concern, which contemplates realization of assets and the satisfaction of liabilities in the normal course of business for the twelve month period following the date of these financial statements. However, CTI has incurred losses since inception and expect to generate losses for the next few years primarily due to research and development costs for pixantrone, OPAXIO and brostallicin. The Company’s available cash and cash equivalents are $17.3 million as of September 30, 2010 and subsequent to period end, in October 2010, CTI raised $21 million in gross proceeds from the issuance of 21,000 shares of its Series 7 preferred stock and warrants to purchase up to 22.7 million shares of its common stock.
CTI does not expect that its existing cash and cash equivalents, including the cash received from the issuance of its Series 7 preferred stock and warrants, will be sufficient to fund its presently anticipated operations beyond the first quarter of 2011. This raises substantial doubt about its ability to continue as a going concern.
The Company has commenced cost saving initiatives to reduce operating expenses, including the reduction of employees related to planned commercial pixantrone operations and it continues to seek additional areas for cost reductions. However, CTI will need to raise additional funds and is currently exploring alternative sources of equity or debt financing. The Company may seek to raise such capital through public or private equity financings, partnerships, joint ventures, disposition of assets, debt financings or restructurings, bank borrowings or other sources of financing.
On the occasion of the annual meeting of shareholders held on September 16, 2010 CTI shareholders approved the increase of the Company’s authorized shares of common and preferred stock from 810,000,000 to 1,210,000,000 shares. As a result of this increase, the Board of Directors now has the option to issue such shares depending on its financial needs and the market opportunities if deemed to be in the best interest of shareholders. However, additional funding may not be available on favorable terms or at all. If additional funds are raised by issuing equity securities, substantial dilution to existing shareholders may result. If the Company fails to obtain additional capital when needed, it may be required to delay, scale back, or eliminate some or all of its research and development programs and may be forced to cease operations, liquidate its assets and possibly seek bankruptcy protection. The condensed consolidated financial statements reported in this Registration Document do not include any adjustments that may result from the outcome of this uncertainty.
Moreover, the Company has applied for the new Qualifying Therapeutic Discovery Project Credit related to life science companies, which may allow CTI to receive grants in lieu of tax credits for years 2009 and 2010.
Please also refer to Chapter 9, Paragraph 9.1, “Financial Situation” of this Registration Document.
C-112
| 10.2 | Sources, amounts and description of the Company’s cash flows |
| 10.2.1. | Sources, amounts and description of the Company’s cash flows for the years ended December 31, 2007, 2008 and 2009 |
As detailed in the subsequent cash flow table below which sets forth the Company’s operating, investing and financing activities, the primary sources of the Company’s cash proceeds are as follows for the years ended December 31, 2009, 2008 and 2007:
| • | net cash used in operating activities totaled $88.2 million in 2009, compared to $80.2 million in 2008 and $103.6 million in 2007. The increase in net cash used in operating activities for the year ended December 31, 2009 as compared to 2008 was primarily due to an increase in cash payments used to decrease our accounts payable and accrued expenses for the year ended December 31, 2009 as compared to an increase in these liability amounts during the comparable period in 2008. During 2009, we also had a decrease in cash received from sales of Zevalin as well as increased cash payments due to settlement expenses and restructuring charges. These were offset by decreased selling, general and administrative and research and development expense, excluding the allocation of non-cash stock based compensation expense to these activities as well as a decrease in cash paid for interest expense. The decrease in net cash used in operating activities for the year ended December 31, 2008 as compared to 2007 was primarily due to a decrease in our selling, general and administrative and research and development expenses as well as an increase in cash collected from our sales of Zevalin; |
| • | net cash provided by investing activities totaled $21.8 million in 2009 as compared to $4.4 million in 2008 and $21.5 million in 2007. Net cash provided by investing activities during the year ended December 31, 2009 was primarily due to $6.8 million in net proceeds from Spectrum in January 2009 related to the initial formation of RIT Oncology in December 2008 and $15.0 million in net proceeds from Spectrum related to the sale of our 50% interest in RIT Oncology in 2009. Net cash provided by investing activities during the year ended December 31, 2008 was primarily due to $6.8 million in net cash received in December 2008 in connection with our disposition of Zevalin to RIT Oncology in exchange for a 50% interest in RIT Oncology as well as proceeds from sales and maturities of securities available-for-sale, offset by purchases of securities available-for-sale, purchases of property and equipment and cash paid for acquisition costs related to our purchase of Zevalin in December 2007. Net cash provided by investing activities during the year ended December 31, 2007 was primarily due to the net amount of cash received from sales, maturities and purchases of securities available-for-sale offset by cash paid for the acquisition of Zevalin; |
| • | net cash provided by financing activities totaled $94.8 million in 2009, $73.7 million in 2008 and $84.7 million in 2007. Net cash provided by financing activities for year ended December 31, 2009 was primarily due to $40.3 million in net proceeds from the issuance of 33.7 million shares of our common stock and warrants to purchase up to 8.4 million shares of our common stock in a public offering in July 2009 as well as $18.9 million in net proceeds from the issuance of 16.0 million shares of our common stock and warrants to purchase 4.8 million shares of our common stock May 2009. We also received $28.4 million in net proceeds from the issuance of 30,000 |
C-113
| shares of our Series 2 preferred stock and warrants to purchase up to 4.7 million shares of our common stock in August 2009. In addition, in May 2009, we received $18.7 million in net proceeds from the issuance of 20,000 shares of our Series 1 preferred stock and related Class A and Class B warrants as well as $3.8 million and $4.3 million upon the exercise of the Class A and Class B warrants in May and October 2009, respectively. These proceeds were offset by $10.0 million in cash paid, net of transaction costs and in addition to 24.2 million shares of our common stock, for the exchange of $52.9 million principal amount of our convertible notes. We also repurchased $6.4 million shares of our common stock for cash in connection with the vesting of employee share awards based on taxes owed by employees due to the vesting of the awards. In addition, we made a $3.0 million deemed dividend payment in connection with our settlement with Tang Capital Partners LP for full release of all claims against us in connection with our alleged breach of contract related to Tang’s Series B preferred stock. This amount was accrued as of December 31, 2008 and paid in January 2009; |
| • | net cash provided by financing activities for the year ended December 31, 2008 was primarily due to issuances of our convertible senior notes. Proceeds from the issuance of our 9% convertible senior notes were $35.4 million, net of issuance costs and restricted cash placed in escrow to fund make-whole payments. We also made a deemed dividend payment of $16.2 million to induce existing holders of our Series A, B, C and D convertible preferred stock to convert their shares of preferred stock into common stock in connection with this issuance. Proceeds from the issuance of our 13.5% convertible senior notes and Series E preferred stock were $19.6 million, net of issuance costs, restricted cash placed in escrow to fund make-whole payments and the cancellation of $5.3 million of our 9% convertible senior notes. Upon cancellation of these notes, $1.4 million was released to us from the amount placed in escrow to fund make-whole payments. Proceeds from the issuance of our 15% convertible senior notes were $11.4 million, net of issuance costs and restricted cash placed in escrow to fund make-whole payments. We received $1.8 million in proceeds from the issuance of our 18.33% convertible senior notes, net of issuance costs, restricted cash placed in escrow to fund make-whole payments and the repurchase of $17.5 million of our 13.5% convertible senior notes and warrants. Upon cancellation of the 13.5% convertible senior notes and warrants, $6.5 million was released to us from the amount placed in escrow to fund make-whole payments. We received proceeds of $10.1 million from the issuance of our 10% convertible senior notes (due 2012) and 15.5% convertible senior notes, net of issuance costs and restricted cash placed in escrow to fund make-whole payments. In connection with these issuances, we made another deemed dividend payment of $2.0 million to induce an existing holder of our Series C preferred stock to convert its shares of preferred stock into common stock. We made a net payment of $1.1 million for the issuance of our 9.66% convertible senior notes and the cancellation of $18.2 million of our 15% convertible senior notes, net of issuance costs and a net payment of $6.5 million for the issuance of our 10% convertible senior notes (due 2011) and the cancellation of $16.3 million of our 18.33% convertible senior notes, $9.0 million of our 9.66% convertible senior notes and $4.8 million of our 15% convertible senior notes, net of issuance costs. In connection with the cancellations of these notes, $20.8 million was released to us from amounts placed in escrow to fund make-whole payments. We also received $5.1 million in net proceeds from the sale of our common stock under equity financing agreements. Cash received from these financings were offset by the repayment of the outstanding $10.7 million principal balance on our 5.75% convertible subordinated and senior subordinated notes upon their maturity in June 2008; |
C-114
| • | net cash provided by financing activities for the year ended December 31, 2007 was primarily due to net proceeds of $18.6 million received from the sale of 20,000 shares of our Series A 3% convertible preferred stock and common stock warrants in February 2007, net proceeds of $34.8 million received from the sale of 37,200 shares of our Series B 3% convertible preferred stock and common stock warrants in April 2007, net proceeds of $18.9 million received from the sale of 20,250 shares of our Series C 3% convertible preferred stock and common stock warrants in July 2007, net proceeds of $6.1 million received from the sale of 6,500 shares of our Series D 7% convertible preferred stock and common stock warrants in December 2007 and net proceeds of $7.0 million received from the sale of our common stock and common stock warrants in December 2007. |
CELL THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands of US dollars)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (95,395 | ) | $ | (180,029 | ) | $ | (138,108 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Non-cash interest expense |
5.788 | 66,530 | 4,280 | |||||||||
| Non-cash gain on derivative liabilities |
(7,218 | ) | (69,739 | ) | (3,672 | ) | ||||||
| Non-cash milestone modification expense |
6,000 | — | — | |||||||||
| Gain on disposition of Zevalin to the JV |
— | (9,444 | ) | — | ||||||||
| Gain on sale of equity investment in joint venture |
(10,244 | ) | — | — | ||||||||
| (Gain) loss on exchange of convertible notes |
(7,381 | ) | 25,103 | 972 | ||||||||
| Acquired in-process research and development |
— | 36 | 24,615 | |||||||||
| Depreciation and amortization |
1,771 | 5,228 | 4,955 | |||||||||
| Equity-based compensation expense |
24,937 | 3,995 | 1,588 | |||||||||
| Equity loss from investment in joint venture |
1,204 | 123 | — | |||||||||
| Noncontrolling interest |
(252 | ) | — | — | ||||||||
| Other |
(487 | ) | (229 | ) | (512 | ) | ||||||
C-115
| Changes in operating assets and liabilities: |
||||||||||||
| Restricted cash |
6,640 | 71,608 | — | |||||||||
| Interest receivable |
9 | 37 | 524 | |||||||||
| Accounts receivable, net |
982 | (932 | ) | (51 | ) | |||||||
| Inventory |
— | 291 | (290 | ) | ||||||||
| Prepaid expenses and other current assets |
(2,649 | ) | 1,438 | 6,431 | ||||||||
| Other assets |
519 | 2,801 | (1,216 | ) | ||||||||
| Accounts payable |
(1,484 | ) | 2,786 | 4,297 | ||||||||
| Accrued expenses |
(10,750 | ) | 779 | (4,961 | ) | |||||||
| Other liabilities |
(176 | ) | (589 | ) | (2,470 | ) | ||||||
| Total adjustments |
7,209 | 99,822 | 34,490 | |||||||||
| Net cash used in operating activities |
(88,186 | ) | (80,207 | ) | (103,618 | ) | ||||||
| Investing activities |
||||||||||||
| Cash received for disposition of Zevalin to joint venture, net |
6,844 | 6,754 | — | |||||||||
| Proceeds received from sale of investment in joint venture, net |
14,987 | — | — | |||||||||
| Cash paid for acquisition of Zevalin |
— | (542 | ) | (11,735 | ) | |||||||
| Cash acquired in acquisition of Systems Medicine, Inc., net |
— | — | 555 | |||||||||
| Purchases of securities available-for-sale |
— | (10,721 | ) | (36,463 | ) | |||||||
| Proceeds from sales of securities available-for-sale |
— | 11,550 | 48,431 | |||||||||
| Proceeds from maturities of securities available-for-sale |
600 | 1,074 | 22,442 | |||||||||
| Investment in joint venture |
— | (1,800 | ) | — | ||||||||
| Purchases of property and equipment |
(1,478 | ) | (1,907 | ) | (1,753 | ) | ||||||
| Proceeds from sales of property and equipment |
887 | — | — | |||||||||
| Net cash provided by investing activities |
21,840 | 4,408 | 21,477 | |||||||||
| Financing activities |
||||||||||||
| Proceeds from issuance of Series 1 preferred stock, net of issuance costs |
18,745 | — | — | |||||||||
| Proceeds from issuance of Series 2 preferred stock, net of issuance costs |
28,430 | — | — | |||||||||
C-116
| Proceeds from issuance of common stock and warrants, net of issuance costs |
59,233 | 5,080 | 7,007 | |||||||||||
| Proceeds from exercise of Class A warrants |
3,765 | — | — | |||||||||||
| Proceeds from exercise of Class B warrants |
4,255 | — | — | |||||||||||
| Cash paid for the exchange of convertible notes, net of transaction costs |
(9,965 | ) | — | — | ||||||||||
| Cash paid for the repurchase of shares in connection with taxes on restricted stock vesting |
(6,394 | ) | ||||||||||||
| Payment of deemed dividends on conversion of preferred stock |
(3,000 | ) | (18,149 | ) | — | |||||||||
| Proceeds from issuance of 13.5% convertible senior notes and Series E preferred stock, net of exchange of 9% convertible senior notes and issuance costs |
— | 56,069 | — | |||||||||||
| Restricted cash from issuance of 13.5% convertible senior notes |
— | (36,456 | ) | — | ||||||||||
| Proceeds from issuance of 9% convertible senior notes, net of issuance costs |
— | 49,317 | — | |||||||||||
| Restricted cash from issuance of 9% convertible senior notes |
— | (13,947 | ) | — | ||||||||||
| Release of restricted cash in connection with exchange of 9% convertible senior notes |
— | 1,420 | — | |||||||||||
| Proceeds from issuance of 15% convertible senior notes, net of issuance costs |
— | 21,794 | — | |||||||||||
| Restricted cash form issuance of 15% convertible senior notes |
— | (10,350 | ) | — | ||||||||||
| Proceeds from issuance of 18.33% convertible senior notes, net of repurchase of 13.5% convertible senior note and issuance costs |
— | 26,226 | — | |||||||||||
| Restricted cash from issuance of 18.33% convertible senior notes |
— | (24,471 | ) | — | ||||||||||
| Release of restricted cash in connection with repurchase of 13.5% convertible senior notes |
— | 6,525 | — | |||||||||||
| Proceeds from issuance of 10% convertible senior note due 2012, net of issuance costs |
— | 8,635 | — | |||||||||||
| Restricted cash from issuance of 10% convertible senior notes due 2012 |
— | (3,600 | ) | — | ||||||||||
| Proceeds from issuance of 15.5% convertible senior note, net of issuance costs |
— | 13,863 | — | |||||||||||
| Restricted cash from issuance of 15.5% convertible senior notes |
— | (8,811 | ) | — | ||||||||||
| Proceeds from issuance of 9.66% convertible senior notes, net of repurchase of 15% convertible senior note and issuance costs |
— | 6,053 | — | |||||||||||
| Restricted cash from issuance of 9.66% convertible senior notes |
— | (7,158 | ) | — | ||||||||||
| Proceeds from issuance of 10% convertible senior notes due 2011, net of repurchase of 9.66%, 15% and 18.33% convertible senior note and issuance costs |
— | 3,252 | — | |||||||||||
| Restricted cash from issuance of 10% convertible senior notes due 2011 |
— | (9,795 | ) | — | ||||||||||
| Release of restricted cash in connection with repurchase of 9.66% convertible senior notes |
— | 2,553 | — | |||||||||||
| Release of restricted cash in connection with repurchase of 15% convertible senior notes |
— | 10,043 | — | |||||||||||
| Release of restricted cash in connection with repurchase of 18.33% convertible senior notes |
— | 8,224 | — | |||||||||||
C-117
| Repayment of 5.75% convertible subordinated and senior subordinated notes |
— | (10,724 | ) | — | ||||||||
| Transaction costs related to exchange of convertible subordinated and senior subordinated notes |
— | (304 | ) | — | ||||||||
| Proceeds from issuance of Series A 3% convertible preferred stock and warrants, net |
— | — | 18,607 | |||||||||
| Proceeds from issuance of Series B 3% convertible preferred stock and warrants, net |
— | — | 34,836 | |||||||||
| Proceeds from issuance of Series C 3% convertible preferred stock and warrants, net |
— | — | 18,938 | |||||||||
| Proceeds from issuance of Series D 7% convertible preferred stock and warrants, net |
— | — | 6,073 | |||||||||
| Payment of additional offering costs related to December 2007 issuance of common stock and warrants |
— | (473 | ) | — | ||||||||
| Payment of dividends on preferred stock |
(111 | ) | (708 | ) | (395 | ) | ||||||
| Repayment of long-term obligations |
(154 | ) | (343 | ) | (429 | ) | ||||||
| Other |
(29 | ) | (39 | ) | 63 | |||||||
| Net cash provided by financing activities |
94,775 | 73,726 | 84,700 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(690 | ) | (3,653 | ) | (3,890 | ) | ||||||
| Net decrease in cash and cash equivalents |
27,739 | (5,726 | ) | (1,331 | ) | |||||||
| Cash and cash equivalents at beginning of year |
10,072 | 15,798 | 17,129 | |||||||||
| Cash and cash equivalents at end of year |
$ | 37,811 | $ | 10,072 | $ | 15,798 | ||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid during the period for interest |
$ | 12,047 | $ | 77,499 | $ | 10,759 | ||||||
| Cash paid for taxes |
$ | — | $ | — | $ | — | ||||||
| Supplemental disclosure of noncash financing and investing activities |
||||||||||||
| Exchange of Series A 3% convertible preferred stock for Series F preferred stock |
$ | 151 | $ | — | $ | — | ||||||
| Exchange of Series B 3% convertible preferred stock for Series F preferred stock |
$ | 1,713 | $ | — | $ | — | ||||||
| Exchange of Series C 3% convertible preferred stock for Series F preferred stock |
$ | 3,221 | $ | — | $ | — | ||||||
| Issuance of Series F preferred stock for Series A, B and C convertible preferred stock |
$ | 3,931 | $ | — | $ | — | ||||||
| Conversion of Series F preferred stock to common stock |
$ | 3,866 | $ | — | $ | — | ||||||
| Conversion of Series 1 preferred stock to common stock |
$ | 18,537 | $ | — | $ | — | ||||||
C-118
| Conversion of Series 2 preferred stock to common stock |
$ | 27,796 | $ | — | $ | — | ||||||||
| Issuance of common stock in exchange for convertible notes |
$ | 35,193 | $ | — | $ | — | ||||||||
| Issuance of common stock in exchange for milestone modification |
$ | 6,000 | $ | — | $ | — | ||||||||
| Conversion of series A 3% convertible preferred stock to common stock |
$ | — | $ | 4,771 | $ | 9,959 | ||||||||
| Conversion of series B 3% convertible preferred stock to common stock |
$ | 2,317 | $ | 7,850 | $ | 16,855 | ||||||||
| Conversion of series C 3% convertible preferred stock to common stock |
$ | — | $ | 3,008 | $ | 8,998 | ||||||||
| Conversion of series D 7% convertible preferred stock to common stock |
$ | — | $ | 2,203 | $ | 1,836 | ||||||||
| Conversion of series E 13.5% convertible preferred stock to 13.5% convertible senior notes |
$ | — | $ | 9,118 | $ | — | ||||||||
| Issuance of common stock in exchange for Series A 3% convertible preferred stock |
$ | 688 | $ | — | $ | — | ||||||||
| Issuance of common stock in exchange for Series D 7% convertible preferred stock |
$ | 1,793 | $ | — | $ | — | ||||||||
| Conversion of 9% convertible senior notes to common stock |
$ | 5,250 | $ | 40,820 | $ | — | ||||||||
| Conversion of 18.33% convertible senior notes to common stock |
$ | — | $ | 28,250 | $ | — | ||||||||
| Conversion of 15.5% convertible senior notes to common stock |
$ | — | $ | 14,211 | $ | — | ||||||||
| Conversion of 13.5% convertible senior notes to common stock |
$ | — | $ | 27,600 | $ | — | ||||||||
| Conversion of 10% convertible senior notes due 2012 to common stock |
$ | — | $ | 9,000 | $ | — | ||||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
$ | 18,000 | $ | 14,651 | $ | — | ||||||||
| Conversion of 9.66% convertible senior notes to common stock |
$ | — | $ | 15,700 | $ | — | ||||||||
| Conversion of 7.5% convertible senior notes to common stock |
$ | — | $ | — | $ | 15,294 | ||||||||
| Conversion of 5.75% convertible senior notes to common stock |
$ | — | $ | 250 | $ | — | ||||||||
| Issuance of common stock for acquisition of Systems Medicine, Inc. |
$ | — | $ | — | $ | 19,872 | ||||||||
| Extinguishment of 5.75% convertible senior subordinated notes in exchange for common stock |
$ | — | $ | 8,943 | $ | — | ||||||||
| Extinguishment of 5.75% convertible subordinated notes in exchange for common stock |
$ | — | $ | 150 | $ | — | ||||||||
| Issuance of common stock in exchange for 5.75% convertible senior subordinated and convertible subordinated notes |
$ | — | $ | 11,437 | $ | 13,704 | ||||||||
| Extinguishment of 5.75% convertible senior subordinated notes in |
||||||||||||||
| exchange for 5.75% convertible senior notes and common stock |
$ | — | $ | — | $ | 10,500 | ||||||||
| Extinguishment of 5.75% convertible subordinated notes in exchange for 5.75% convertible senior notes and common stock |
$ | — | $ | — | $ | 25,580 | ||||||||
| Issuance of 5.75% convertible senior notes in exchange for 5.75% convertible senior subordinated and convertible subordinated notes |
$ | — | $ | — | $ | 23,250 | ||||||||
C-119
| 10.2.2 | Sources, amounts and description of the Company’s cash flows for the six months ended June 30, 2009 and 2010 |
This paragraph incorporates by reference specific pieces of information reported in the following documents available to the public on the Company’s website: http://investors.celltherapeutics.com (such specific pieces of information are identified with the pages of the aforesaid documents where they are reported).
Quarterly report of the Issuer for the six months ended June 30, 2009 reviewed by the independent registered public accounting firm
| • | Condensed consolidated statements of cash flow: page 5 |
| • | Paragraph “Liquidity and capital resources” included in the “Management’s discussion and analysis of financial condition and results of operations”: pages 28-31 |
Quarterly report of the Issuer for the six months ended June 30, 2010 reviewed by the independent registered public accounting firm
| • | Condensed consolidated statements of cash flow: page 5 |
| • | Paragraph “Liquidity and capital resources” included in the “Management’s discussion and analysis of financial condition and results of operations”: pages 25-28 |
| 10.2.3 | Sources, amounts and description of the Company’s cash flows for the nine months ended September 30, 2009 and 2010 |
As detailed in the subsequent cash flow table below which sets forth the Company’s operating, investing and financing activities, the primary sources the Company’s cash proceeds are as follows for the nine months ended September 30, 2009 and 2010:
| • | net cash used in operating activities decreased to approximately $50.4 million during the nine months ended September 30, 2010 compared to approximately $70.6 million for the same period during 2009 primarily due to a reduction in interest payments on convertible notes and a decrease in operating expenses, including research and development expense and selling, general and administrative expense, excluding the allocation of non-cash stock-based compensation. The decrease is also attributable to non-recurring cash payments made in connection with settlement of legal matters during the nine months ended September 30, 2009; |
C-120
| • | net cash used in investing activities of approximately $1.2 million for the nine months ended September 30, 2010 was primarily due to purchases of property and equipment. Net cash provided by investing activities of approximately $22.2 million for the nine months ended September 30, 2009 was primarily due to $6.8 million in net proceeds received from Spectrum in January 2009 related to the initial formation of RIT Oncology in December 2008 and $15.1 million in net proceeds received from Spectrum related to the subsequent sale of our 50% interest in RIT Oncology in 2009; |
| • | net cash provided by financing activities of approximately $30.6 million for the nine months ended September 30, 2010 was primarily due to the issuances of our Series 3, 4, 5 and 6 preferred stock. We received $28.0 million in net proceeds from the issuance of 30,000 shares of our Series 3 preferred stock and warrants to purchase approximately 8.6 million shares of our common stock in January 2010. We also received $18.6 million in net proceeds from the issuance of 20,000 shares of our Series 4 preferred stock and warrants to purchase 20.0 million shares of our common stock in April 2010. In addition, we received $19.7 million in net proceeds from the issuance of 21,000 shares of Series 5 preferred stock and warrants to purchase approximately 26.3 million contingently issuable shares of our common stock in May 2010. In July 2010, we received $3.6 million in net proceeds from the issuance of 4,060 shares of our Series 6 preferred stock and warrants to purchase 5.8 million shares of our common stock. These proceeds were offset by a $38.5 million payment to retire down the outstanding principal balance on our 4% Notes in July 2010 in addition to $0.7 million cash paid for the repurchase of shares in connection with satisfying tax withholding obligations on the vesting of restricted stock awards to employees. Net cash provided by financing activities of approximately $94.2 million for the nine months ended September 30, 2009 was primarily due to $40.6 million in net proceeds from the issuance of 33.7 million shares of our common stock and warrants to purchase up to 8.4 million shares of our common stock in a public offering in July 2009 as well as $18.9 million in net proceeds from the issuance of 16.0 million shares of our common stock and warrants to purchase 4.8 million shares of our common stock in May 2009. In addition, we received $18.7 million in net proceeds from the issuance of 20,000 shares of our Series 1 preferred stock and related Class A and Class B warrants in April 2009, as well as $3.8 million upon the exercise of the Class A warrants in May 2009. We also received $28.5 million in net proceeds from the issuance of 30,000 shares of our Series 2 preferred stock and warrants to purchase up to 4.7 million shares of our common stock in August 2009. These proceeds were offset by $9.9 million in cash paid, net of transaction costs and in addition to 24.2 million shares of our common stock, for the exchange of $52.9 million principal amount of our convertible notes. We also made a $3.0 million deemed dividend payment in connection with our settlement with Tang Capital Partners LP for full release of all claims against us in connection with our alleged breach of contract related to Tang’s Series B preferred stock. This amount was accrued as of December 31, 2008 and paid in January 2009. |
C-121
CELL THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(unaudited)
| Nine Months Ended September 30, |
||||||||
| 2010 | 2009 | |||||||
| Operating activities |
||||||||
| Net loss |
$ | (62,924 | ) | $ | (68,018 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Equity-based compensation expense |
16,260 | 13,283 | ||||||
| Gain on sale of investment in joint venture |
— | (10,244 | ) | |||||
| Non-cash interest expense |
600 | 5,575 | ||||||
| Depreciation and amortization |
1,413 | 1,424 | ||||||
| Debt conversion expense |
2,031 | — | ||||||
| Non-cash gain on derivative liabilities, net |
— | (7,218 | ) | |||||
| Gain on exchange of convertible notes |
— | (7,381 | ) | |||||
| Equity loss from investment in joint venture |
— | 1,204 | ||||||
| Noncontrolling interest |
(149 | ) | (205 | ) | ||||
| Other |
(217 | ) | (457 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Restricted cash |
— | 6,640 | ||||||
| Accounts receivable, net |
— | 991 | ||||||
| Prepaid expenses and other current assets |
1,472 | (1,843 | ) | |||||
| Other assets |
68 | (367 | ) | |||||
| Accounts payable |
(2,888 | ) | (4,436 | ) | ||||
| Accrued expenses |
(4,944 | ) | 327 | |||||
| Long-term obligations |
(532 | ) | 347 | |||||
| Other liabilities |
(546 | ) | (266 | ) | ||||
| Total adjustments |
12,568 | (2,626 | ) | |||||
| Net cash used in operating activities |
(50,356 | ) | (70,644 | ) | ||||
| Investing activities |
||||||||
| Purchases of property and equipment |
(1,232 | ) | (343 | ) | ||||
| Proceeds from the sales of property and equipment |
82 | — | ||||||
| Proceeds received from disposition of Zevalin to joint venture, net |
— | 6,844 | ||||||
| Proceeds received from sale of investment in joint venture, net |
— | 15,075 | ||||||
| Proceeds from maturities of securities available-for-sale |
— | 600 | ||||||
| Net cash provided by (used in) investing activities |
(1,150 | ) | 22,176 | |||||
| Financing activities |
||||||||
| Proceeds from issuance of Series 1 preferred stock and warrants, net of issuance costs |
— | 18,745 | ||||||
| Proceeds from issuance of Series 2 preferred stock and warrants, net of issuance costs |
— | 28,516 | ||||||
| Proceeds from issuance of Series 3 preferred stock and warrants, net of issuance costs |
27,951 | — | ||||||
| Proceeds from issuance of Series 4 preferred stock and warrants, net of issuance costs |
18,621 | — | ||||||
| Proceeds from issuance of Series 5 preferred stock and warrants, net of issuance costs |
19,704 | — | ||||||
| Proceeds from issuance of Series 6 preferred stock and warrants, net of issuance costs |
3,603 | — | ||||||
| Proceeds from issuance of common stock and warrants, net of issuance costs |
— | 59,519 | ||||||
| Proceeds from exercise of Class A warrants |
— | 3,765 | ||||||
| Repayment of 4% convertible senior subordinated notes |
(38,515 | ) | — | |||||
| Cash paid for the exchange of convertible notes, net of transaction costs |
— | (9,949 | ) | |||||
| Cash paid for repurchase of shares in connection with taxes on restricted stock vesting |
(748 | ) | (3,148 | ) | ||||
| Payment of deemed dividends on conversion of preferred stock |
— | (3,000 | ) | |||||
| Payment of dividends on preferred stock |
— | (111 | ) | |||||
| Other |
— | (135 | ) | |||||
| Net cash provided by financing activities |
30,616 | 94,202 | ||||||
C-122
| Effect of exchange rate changes on cash and cash equivalents |
347 | (814 | ) | |||||
| Net increase (decrease) in cash and cash equivalents |
(20,543 | ) | 44,920 | |||||
| Cash and cash equivalents at beginning of period |
37,811 | 10,072 | ||||||
| Cash and cash equivalents at end of period |
$ | 17,268 | $ | 54,992 | ||||
| Supplemental disclosure of cash flow information |
||||||||
| Cash paid during the period for interest |
$ | 2,401 | $ | 11,357 | ||||
| Cash paid for taxes |
$ | — | $ | — | ||||
| Supplemental disclosure of noncash financing and investing activities |
||||||||
| Conversion of Series 1 preferred stock to common stock |
$ | — | $ | 18,537 | ||||
| Conversion of Series 2 preferred stock to common stock |
$ | — | $ | 27,796 | ||||
| Conversion of Series 3 preferred stock to common stock |
$ | 27,761 | $ | — | ||||
| Conversion of Series 4 preferred stock to common stock |
$ | 18,621 | $ | — | ||||
| Conversion of Series 5 preferred stock to common stock |
$ | 19,464 | $ | — | ||||
| Conversion of Series 6 preferred stock to common stock |
$ | 2,970 | $ | — | ||||
| Conversion of Series B 3% convertible preferred stock to common stock |
$ | — | $ | 2,317 | ||||
| Conversion of Series F preferred stock to common stock |
$ | — | $ | 3,866 | ||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
$ | — | $ | 18,000 | ||||
| Conversion of 9% convertible senior notes to common stock |
$ | — | $ | 5,250 | ||||
| Exchange of 4% convertible senior subordinated notes for common stock |
$ | 1,848 | $ | — | ||||
| Exchange of Series A 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 151 | ||||
| Exchange of Series B 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 1,713 | ||||
| Exchange of Series C 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 3,221 | ||||
| Issuance of Series F preferred stock for Series A, B and C convertible preferred stock |
$ | — | $ | 3,931 | ||||
| Issuance of common stock in exchange for convertible notes |
$ | — | $ | 35,193 | ||||
| Issuance of common stock in exchange for Series A 3% convertible preferred stock |
$ | — | $ | 688 | ||||
| Issuance of common stock in exchange for Series D 7% convertible preferred stock |
$ | — | $ | 1,793 | ||||
| 10.3 | Information on the borrowing requirements and funding structure of the issuer |
In order to complete the development of pixantrone, OPAXIO and the Company’s other product candidates, the Company will need to raise additional capital to fund clinical trials, perform research and development activities and eventually market and sell the products. Because the time to complete clinical trials and the resources necessary to perform the requisite research and development is dependent on many factors beyond the Company’s control, the Company cannot predict how much additional funding will be necessary to complete development of its products, or if the development will ever be completed.
The Company estimates its average cash burn-rate for the remainder of 2010 to be about $5 million per month. Nevertheless, the company is reviewing alternative options to reduce its burn rate.
Moreover, the Company has a substantial amount of debt outstanding. The aggregate principal balance of the Company’s convertible notes as of September 30, 2010 is approximately $21 million in convertible
C-123
notes with interest rates ranging from 5.75% to 7.5%. Such amount does not include about $38.5 million in 4% convertible notes that were fully retired on July 1, 2010.
Please also refer to Paragraph 9.1, “Financial Situation” and Paragraph 5.2.3 “Information on major future investments as a part of commitment undertaken by the Company management” of this Registration Document.
| 10.3.1 | Net financial indebtedness |
Please refer to the table reporting the estimated and unaudited net debt financial indebtedness of the Company as of September 30, 2010, disclosed under previous Paragraph 9.1
| 10.3.2 | Information relating to the Company’s contractual obligations |
The following table includes information relating to our contractual obligations as of September 30, 2010 (in thousands):
| Contractual Obligations |
Payments Due by Period | |||||||||||||||||||
| Total | 1 Year | 2-3 Years | 4-5 Years | After 5 Years |
||||||||||||||||
| 7.5% convertible senior notes (1) |
$ | 10,250 | $ | 10,250 | $ | — | $ | — | $ | — | ||||||||||
| 5.75% convertible senior notes (2) |
10,913 | — | 10,913 | — | — | |||||||||||||||
| Interest on convertible notes |
1,205 | 1,074 | 131 | — | — | |||||||||||||||
| Operating leases: |
||||||||||||||||||||
| Facilities |
8,331 | 4,357 | 3,846 | 128 | — | |||||||||||||||
| Long-term obligations (3) |
1,196 | 457 | 726 | 13 | — | |||||||||||||||
| $ | 31,895 | $ | 16,138 | $ | 15,616 | $ | 141 | $ | — | |||||||||||
| (1) | The 7.5% convertible senior notes are convertible into shares of our common stock at a conversion rate of 11.96298 shares of our common stock per $1,000 principal amount of the notes, which is equivalent to a conversion price of approximately $83.59 per share. |
| (2) | The 5.75% convertible senior notes are convertible into shares of our common stock at a conversion rate of 33.33333 shares of our common stock per $1,000 principal amount of the notes, which is equivalent to a conversion price of approximately $30.00 per share. |
| (3) | Long-term obligations do not include $0.6 million related to excess facilities charges. |
| 10.3.3 | Convertible notes |
The following is a summarizing schedule of the characteristic elements of the convertible notes as of the date of the Registration Document.
C-124
| Balance as of date of the Registration Document (in thousands) |
Maturity Date |
Interest Rate |
Payment Timing (semi-annual) |
Conversion Price |
Number of potential shares of common stock which could result from the conversion of all the notes |
Presence of a make- whole clause |
Make- whole (per $1,000 principal) |
Make-whole amount held in escrow at 12/31/09 (in thousands) (3) |
||||||||||||||||||||||||||||
| 7.5% convertible senior notes |
10,250 | 30-Apr-11 | 7.5 | % | Apr/Oct | $ | 83.59 | 122,620 | Yes | $ | 225.00 | (1) | — | |||||||||||||||||||||||
| 5.75% convertible senior notes |
10,913 | 15-Dec-11 | 5.75 | % | Jun/Dec | $ | 30.00 | 363,766 | Yes | $ | 115.00 | (2) | — | |||||||||||||||||||||||
| Total |
$ | 21,163 | 486,386 | $ | — | |||||||||||||||||||||||||||||||
(1) This make-whole payment would only be due upon automatic conversion of the notes (the notes automatically convert if the closing price per share of Common Stock has exceeded 125% of the conversion price then in effect for at least 20 trading days within any 30-consecutive trading day period) or if the holder exercises their right to require the Company to repurchase the notes in connection with a non-stock change of control.
(2) This make whole-payment would only be due upon any automatic conversion of the 5.75% notes, or if the holders exercise their right to require the Company to repurchase the notes upon a change of control, or if the Company elects to redeem the notes, it shall be required to make a make-whole payment equal to $115 per $1,000 principal amount of the notes so converted, redeemed or repurchased, less any interest paid on such notes prior to the conversion date. Amount due would be less any interest paid prior to the conversion date. (3) This amount corresponds to the potential monetary disbursement by the Company in the event of conversion of the notes.
The following is a summary of the fair values of the Company’s convertible senior notes, convertible senior subordinated notes and convertible subordinated notes as of the date of this Registration Document and December 31, 2009, 2008 and 2007 (in thousands of US dollars). Please note that the September 30, 2010 amounts below are estimates and have not been reviewed or audited by the Company’s independent auditors as such disclosure in not required in the Company’s Quarterly Report on Form 10-Q filed with the SEC:
| September 30, | December 31, | |||||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| 10% convertible senior notes due 2011 |
$ | — | — | $ | 21,810 | — | ||||||||||
| 9% convertible senior notes |
$ | — | — | $ | 4,580 | — | ||||||||||
| 7.5% convertible senior notes |
$ | 10,187 | * | $ | 9,138 | $ | 27,308 | $ | 29,756 | |||||||
| 5.75% convertible senior notes |
$ | 11,985 | * | $ | 8,777 | $ | 16,728 | $ | 26,650 | |||||||
| 6.75% convertible senior notes |
$ | — | — | $ | 5,875 | $ | 6,100 | |||||||||
| 5.75% convertible senior subordinated notes |
$ | — | — | — | $ | 16,907 | ||||||||||
| 4.0% convertible senior subordinated notes |
$ | — | $ | 38,512 | $ | 46,375 | $ | 45,403 | ||||||||
| 5.75% convertible subordinated notes |
$ | — | — | — | $ | 2,910 | ||||||||||
| * | Such carrying value approximates the relative fair value as of September 30, 2010 |
C-125
Debt Covenants
The following are all of the debt covenants for each of the convertible notes described above:
| Debt Covenant |
5.75% Convertible Senior Notes |
5.75% Convertible Subordinated Notes |
5.75% Convertible Sr. Subordinated Notes |
4% Convertible Sr. Subordinated Notes |
6.75% Convertible Senior Notes |
7.5% Convertible Senior Notes |
10% Convertible Senior Notes Due 2011 |
9% Convertible Senior Notes | ||||||||
| Payment of Principal, Premium and Interest |
X | X | X | X | X | X | X | X | ||||||||
| Maintenance of Offices or Agencies |
X | X | X | X | X | X | X | X | ||||||||
| Money for Security Payments to Be Held in Trust |
X | X | X | X | X | X | X | X | ||||||||
| Existence |
X | X | X | X | X | X | X | X | ||||||||
| Statement by Officers as to Default* |
X | X | X | X | X | X | X | X | ||||||||
| Delivery of Certain Information |
X | X | X | X | X | X | X | X | ||||||||
| Payment in registered common stock |
X | X | X | |||||||||||||
| Issuance of additional relevant securities |
X | X | ||||||||||||||
| Incurrence of Indebtedness |
X | X | X | X | X | |||||||||||
| Liquidated damages |
X | X | X | |||||||||||||
| *Statement | due within 120 days after the end of the fiscal year. |
C-126
The covenants above are discussed in greater detail in each respective Indenture Agreement filed with the Securities and Exchange Commission in connection with the related convertible debt offering.
A roll-forward of the principle balances for the Company’s convertible debt is as follows for the years ended December 31, 2009, 2008 and 2007 and as of the date of this Registration Document:
| Balance at January 1, 2007 |
Issued | Converted | Exchanged | Balance at December 31, 2007 |
||||||||||||||||
| 7.5% convertible senior notes |
$ | 48,752 | $ | — | $ | (15,294 | ) | $ | — | $ | 33,458 | |||||||||
| 6.75% convertible senior notes |
7,000 | — | — | — | 7,000 | |||||||||||||||
| 5.75% convertible senior notes |
— | 23,250 | — | — | 23,250 | |||||||||||||||
| 5.75% convertible senior subordinated notes |
27,407 | — | — | (10,500 | ) | 16,907 | ||||||||||||||
| 5.75% convertible subordinated notes |
28,490 | — | — | (25,580 | ) | 2,910 | ||||||||||||||
| 4.0% convertible senior subordinated notes |
55,150 | — | — | — | 55,150 | |||||||||||||||
| Total |
$ | 166,799 | $ | 23,250 | $ | (15,294 | ) | $ | (36,080 | ) | $ | 138,675 | ||||||||
| Balance at January 1, 2008 |
Issued | Converted | Extinguished | Matured | Balance at December 31, 2008 |
|||||||||||||||||||
| 18.33% convertible senior notes |
$ | — | $ | 44,500 | $ | (28,250 | ) | $ | (16,250 | ) | $ | — | $ | — | ||||||||||
| 15.5% convertible senior notes |
— | 14,211 | (14,211 | ) | — | — | — | |||||||||||||||||
| 15% convertible senior notes |
— | 23,000 | — | (23,000 | ) | — | — | |||||||||||||||||
| 13.5% convertible senior notes |
— | 45,118 | (27,600 | ) | (17,518 | ) | — | — | ||||||||||||||||
| 10% convertible senior notes due 2011 |
— | 32,651 | (14,651 | ) | — | — | 18,000 | |||||||||||||||||
| 10% convertible senior notes due 2012 |
— | 9,000 | (9,000 | ) | ||||||||||||||||||||
| 9.66% convertible senior notes |
— | 24,700 | (15,700 | ) | (9,000 | ) | — | — | ||||||||||||||||
| 9% convertible senior notes |
— | 51,655 | (40,820 | ) | (5,250 | ) | — | 5,585 | ||||||||||||||||
| 7.5% convertible senior notes |
33,458 | — | — | — | — | 33,458 | ||||||||||||||||||
| 6.75% convertible senior notes |
7,000 | — | — | — | — | 7,000 | ||||||||||||||||||
| 5.75% convertible senior notes |
23,250 | — | (250 | ) | — | — | 23,000 | |||||||||||||||||
| 5.75% convertible senior subordinated notes |
16,907 | — | — | (8,943 | ) | (7,964 | ) | — | ||||||||||||||||
| 5.75% convertible subordinated notes |
2,910 | — | — | (150 | ) | (2,760 | ) | — | ||||||||||||||||
| 4.0% convertible senior subordinated notes |
55,150 | — | — | — | — | 55,150 | ||||||||||||||||||
| Total |
$ | 138,675 | $ | 244,835 | $ | (150,482 | ) | $ | (80,111 | ) | $ | (10,724 | ) | $ | 142,193 | |||||||||
C-127
| Balance at January 1, 2009 |
Converted | Exchanged, Extinguished or Repurchased |
Balance at December 31, 2009 |
|||||||||||||
| 10% convertible senior notes due 2011 |
$ | 18,000 | $ | (18,000 | ) | $ | — | $ | — | |||||||
| 9% convertible senior notes |
5,585 | (5,250 | ) | (335 | ) | — | ||||||||||
| 7.5% convertible senior notes |
33,458 | — | (23,208 | ) | 10,250 | |||||||||||
| 6.75% convertible senior notes |
7,000 | — | (7,000 | ) | — | |||||||||||
| 5.75% convertible senior notes |
23,000 | — | (12,087 | ) | 10,913 | |||||||||||
| 4.0% convertible senior subordinated notes |
55,150 | — | (14,787 | ) | 40,363 | |||||||||||
| Total |
$ | 142,193 | $ | (23,250 | ) | $ | (57,417 | ) | $ | 61,526 | ||||||
| Balance at January 1, 2010 |
Issued | Exchanged* | Converted | Extinguished | Matured | Balance at the date of this Registration Document |
||||||||||||||||||||||
| 7.5% convertible senior notes |
$ | 10,250 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 10,250 | ||||||||||||||
| 5.75% convertible senior notes |
10,913 | — | — | — | — | — | 10,913 | |||||||||||||||||||||
| 4.0% convertible senior subordinated notes |
40,363 | — | 1,848 | — | — | 38,515 | — | |||||||||||||||||||||
| Total |
$ | 61,526 | $ | — | $ | 1,848 | $ | — | $ | — | $ | 38,515 | $ | 21,163 | ||||||||||||||
| * | Following the exchanges in May 2010 |
Embedded Features
As reported in the “summarizing schedule of the characteristic elements of the convertible notes as of December 31, 2009” at the beginning of this paragraph 10.3.3, the following categories of convertible notes include a make-whole clause: 1) 7.5% Convertible senior notes due 2011; 2) 5.75% Convertible senior notes due 2011.
With respect to the nature and the mechanics of the make-whole provision, it is clarified that in general terms:
| • | the interest-make-whole provisions entitle the holders of the notes to receive all interest payable through scheduled maturity, less any interest paid before conversion; |
| • | for the convertible senior notes issued in 2008, the whole amount of such interest is held in escrow until conversion of the notes or until the shorter period established by the relevant debenture agreement. Such operation (that is the moving of the relevant cash amount to an escrow account) has a financial evidence and the relative amount is reported as “restricted cash” in the Assets of the Company condensed consolidated balance sheet; |
| • | the Company did not execute derivative agreements for hedging the inner risk of the make-whole interest clause, that is the risk of the payment of a quota of interests (to be differently calculated with respect to each series of notes and as better described hereinafter) upon early conversion of the notes into shares; |
| • | the total amount of the make-whole interests paid by the Company in 2008 is $70,243,170.64. |
For a description of the effects of the make-whole clause for the aforesaid 7.5% Convertible senior notes due 2011 and the 5.75% Convertible senior notes due 2011, please refer to the following paragraph.
C-128
Description of the Convertible Notes outstanding as of December 31, 2009 (with indication of the Convertible Notes still outstanding as of the date of this Registration Document)
7.5% Convertible Senior Notes due 2011
In April 2006, we issued approximately $66.3 million aggregate principal amount of our 7.5% convertible senior notes, or 7.5% notes, approximately $33.2 million of which was issued in a registered offering for cash with net proceeds of approximately $31.2 million, after deducting expenses and the initial purchaser’s discounts and commissions. Approximately $33.2 million was issued in a private exchange for approximately $39.5 million aggregate principal amount of our 5.75% convertible senior subordinated notes and approximately $1.2 million aggregate principal amount of our 5.75% convertible subordinated notes. We recognized a net gain of $8.0 million on the early extinguishment and exchange of these notes which is based on the carrying value of the exchanged notes less the fair value of the new notes, net of issuance costs of $0.4 million and accrued interest of $0.9 million attributable to the exchanged notes.
The notes are due April 30, 2011 with interest payable semi-annually in April and October. The notes are convertible, at the option of the holder, into shares of our common stock at any time prior to maturity, redemption or repurchase at a conversion rate of 11.963 shares of common stock per $1,000 principal amount of the notes, which is subject to adjustments in certain circumstances. This conversion rate is equivalent to a conversion price of approximately $83.59 per share. On or after April 30, 2009, we have the option to redeem all of the notes for cash at any time at a redemption price equal to par plus accrued and unpaid interest up to but not including the redemption date. Subject to certain conditions, the notes will automatically convert if, at any time after June 26, 2006 and prior to maturity, the closing price per share of our common stock has exceeded 125% of the conversion price then in effect for at least 20 trading days within any 30-consecutive trading day period. In addition, upon certain non-stock changes in control, the holder can require us to repurchase the notes at 100% of their principal amount, plus accrued and unpaid interest to, but not including, the repurchase date. Upon any automatic conversion of the notes, or if the holder exercises their right to require us to repurchase notes in connection with a non-stock change of control, we will pay the holder of the notes a make-whole interest payment equal to $225 per $1,000 principal amount of the notes so converted, less any interest paid on such notes prior to the conversion date.
The interest make-whole provision, along with the conversion option of the 7.5% notes, represents an embedded derivative which is required to be accounted for separate from the underlying notes and was recorded as a derivative liability and a discount to the carrying value of the notes. The resulting discount to the notes is being accreted over the life of the notes as additional interest expense using the effective interest method. Accordingly, we recorded interest expense of $0.4 million, $2.9 million and $1.4 million for the years ended December 31, 2008, 2007 and 2006, respectively, the majority of which represents accelerated accretion due to note conversions. The estimated fair value of the derivative liability is adjusted quarterly for changes in the estimated market value. The change in the estimated fair value for the years ended December 31, 2007 and 2006 was $3.6 million and $1.9 million, respectively, and is included in gain on derivative liabilities, net. As of December 31, 2008 and 2007, no value was assigned to the fair value of the derivative liability, therefore, there was no change in the estimated fair value for the year ended December 31, 2008.
For the years ended December 31, 2007 and 2006, $15.3 million and $17.6 million of our 7.5% notes were converted into 0.2 million and 0.2 million shares of common stock, respectively. There were no conversions during the year ended December 31, 2008. During the nine months ended September 30, 2009, approximately
C-129
$23.2 million of these notes were extinguished in connection with the Company’s Exchange Offers. In connection with the conversion of $13.6 million of these notes in 2007, we made discretionary interest make-whole payments of approximately $2.3 million which is included in make-whole interest expense for the year ended December 31, 2007. In connection with the conversion of $7.4 million of these notes in May 2006, we made a discretionary interest make-whole payment of approximately $1.7 million which is included in make-whole interest expense for the year ended December 31, 2006. As of the date of this Registration Document, 2010, approximately $10.3 million aggregate principal amount of our 7.5% notes due 2011 remained outstanding.
5.75% Convertible Senior Notes due 2011
In December 2007, we issued approximately $23.3 million aggregate principal amount of our 5.75% convertible senior notes, or 5.75% senior notes, and approximately 0.5 million shares of our common stock in exchange for $10.5 million of our 5.75% convertible senior subordinated notes and $25.6 million of our 5.75% convertible subordinated notes. The exchange resulted in a loss of approximately $1.0 million including a write-off of $0.1 million of unamortized issuance costs attributed to the extinguished notes. The resulting discount from the exchange is being accreted over the life of the notes as additional interest expense using the effective interest method. Accordingly, we recorded interest expense of approximately $0.8 million and $37,000 for the years ended December 31, 2008 and 2007, respectively.
The notes are due December 15, 2011 with interest payable semi-annually in June and December. The notes are convertible, at the option of the holder, into shares of our common stock at any time prior to maturity, redemption or repurchase at a conversion rate of 33.3333 shares of common stock per $1,000 principal amount of the notes, which is subject to adjustments in certain circumstances. This conversion rate is equivalent to a conversion price of approximately $30.00 per share. On or after December 15, 2009, we have the option to redeem all of the notes for cash at any time at a redemption price equal to par plus accrued and unpaid interest up to but not including the redemption date. Subject to certain conditions, the notes will automatically convert if, at any time after December 15, 2009 and prior to maturity, the closing price per share of our common stock has exceeded 140% of the conversion price then in effect for at least 20 trading days within any 30-consecutive trading day period. Upon a change in control, the holder can require us to repurchase the notes at 100% of their principal amount, plus accrued and unpaid interest and any other amounts due up to, but not including, the repurchase date. In addition, upon any of these occurrences (redemption, automatic conversion, or repurchase) we will pay the holder of the notes a make-whole interest payment equal to $115 per $1,000 principal amount of the notes so converted, less any interest paid on such notes prior to the conversion date. During the nine months ended September 30, 2009, approximately $12.1 million of these notes were extinguished in connection with the Company’s Exchange Offers. As of the date of this Registration Document, approximately $10.9 million aggregate principal amount of our 5.75% notes due 2011 remained outstanding.
4% Convertible Senior Subordinated Notes due 2010
Our 4% convertible senior subordinated notes, or 4% notes, are due July 1, 2010 with interest payable semi-annually in January and July. The 4% notes were convertible, at the option of the holder, into shares of our common stock at any time prior to maturity, redemption or repurchase at an initial conversion rate of 1.85185 shares of common stock per $1,000 principal amount of notes, which is subject to adjustment in certain circumstances. This conversion rate was equivalent to a conversion price of approximately $540.00 per share. Prior to maturity, we may redeem the notes upon certain conditions, the most significant of which is that the closing price of our common stock must exceed 150% of the conversion price for at least 20 trading days within a
C-130
period of 30 consecutive trading days. Upon such redemption, we would make an additional payment of $280.00 per $1,000 note, less any interest previously paid on the notes. The holder may elect to convert their notes prior to any such redemption. During the nine months ended September 30, 2009, approximately $14.8 million of these notes were extinguished in connection with the Company’s Exchange Offers. On July 1, 2010, the Company retired all the then outstanding 4% notes for $39.3 million, which was an amount sufficient to pay and discharge the entire amount due on the 4% notes, including accrued and unpaid interest. The assets/liabilities effects deriving from such reimbursement are represented by the decrease of the Company’s cash resources and, subsequently of its capacity to carry out new investments. As refers to the financial effects, the retirement of the 4% notes has entailed an amelioration of the net financial standing of the Company deriving from its lower indebtedness. It is worth pointing out that the amount of $39.3 million included $ 0.8 million for the payment of the relevant interests. As of the date of this Registration Document approximately no 4% notes due 2010 remained outstanding.
* * *
As of the date of this Registration Document, maturities of the Company’s outstanding convertible notes, including the 5.75% and 7.5% convertible senior notes, are as follows (in thousands):
| Years Ending December 31, |
||||
| 2010 |
$ | — | ||
| 2011 |
21,163 | |||
| 2012 |
— | |||
| 2013 |
— | |||
| 2014 |
— | |||
| Thereafter |
— | |||
| $ | 21,163 | |||
| 10.3.4 | Costs of the financing obtained by means of the issuance of convertible notes |
In 2008, the Company issued approximately $244.8 million in convertible senior notes and received a $19.5 million premium for warrants issued in connection with one of the notes. All the notes were issued without an issue disagio. The notes had coupon rates ranging from 9% to 18.33% and interest-make-whole provisions which entitled the holders of the notes to receive the coupon interest upon conversion of the notes.
The gross proceeds deriving from the issuance by the company of convertible senior notes in 2008 were equal to $264.3 million and the relevant net proceeds were equal to $80.4 million, for a total cost of $183.9 million (which, includes, nevertheless the amount regarding the repurchase of part of the convertible bonds) corresponding indicatively to an average impact of the financial resources raised through the above issuance of about 70%.
| 10.3.5 | Corporate interest for the recent issuance of notes |
The Company believes the use of coupon make-whole convertible notes was the only viable source of funding for the Company in 2008 given the global credit and capital market crisis. The interest make-
C-131
whole provisions of such convertible notes entitle the holders of the notes to receive all interest payable through scheduled maturity, less any interest paid before conversion. The whole amount of such interest is held in escrow until conversion of the notes. While these convertible notes came at an expensive price with a 30% to 45% coupon make whole provision, the ultimate cost to the Company’s shareholders was inferior to the alternatives the Company was facing. The Company believes that the alternative to the issuance of the aforementioned convertible notes was filing for insolvency under u.s. federal bankruptcy laws (chapter 11) which would have had a more deleterious impact on shareholder equity.
| 10.3.6 | Exchange offer for outstanding notes. |
Between May and June 2009 the Company performed tender offers to exchange a considerable portion of its outstanding notes.
Following the completion of the aforesaid exchange offers, hereinafter is a table disclosing the amounts of convertible notes outstanding as of September 30, 2010.
| Description |
Maturity / Redemption Date |
Principal/ Aggregated Stated Value Outstanding as of September 30, 2010 |
Number of Common Stock Reserve as of September 30, 2010 |
|||||||||
| 7.5% Convertible Senior Notes |
30-Apr-11 | $ | 10,250,000 | 122,620 | ||||||||
| 5.75% Convertible Senior Notes |
15-Dec-11 | 10,913,000 | 363,766 | |||||||||
| Totals |
$ | 21,163,000 | 486,386 | |||||||||
For detailed information on the mechanics of the Exchange Offer, also about the mechanics of a “Modified Dutch Auction” procedure, please refer to Chapter 21, Paragraph 21.1.4 “The amount of any convertible securities, exchangeable securities or securities with warrants, with an indication of the conditions governing and the procedures for conversion, exchange or subscription” of this Registration Document.
| 10.4 | Information regarding any restrictions on the use of capital resources that have materially affected, or could materially affect, directly or indirectly, the issuer’s operations |
Currently, there are no restrictions on the use of the Company’s capital resources that have materially affected or could materially affect, directly or indirectly, the Company’s operations.
C-132
| 10.5 | Information regarding the sources of funds which are planned to be required in order to fulfil the commitments referred to under items 5.2.3 and 8.1 |
In regards to items 5.2.3 and 8.1, the Company plans to continue to access the capital markets in order to raise funds required for operations. As previously demonstrated in the past, such funding may include, but not limited to, common stock offerings, convertible preferred stock issuances or acquisitions of equity lines of credit.
C-133
CHAPTER 11 RESEARCH AND DEVELOPMENT, PATENTS AND LICENSES
| 11.1 | Patents and licenses |
The Company dedicates significant resources to protecting its intellectual property, which is important to its business. The Company has exclusive rights to 12 issued U.S. patents and 129 pending or issued U.S. and foreign patent applications relating to our polymer drug delivery technology, of which seven issued U.S. patents and 83 pending or issued U.S. and foreign patent applications are directed to OPAXIO. The Company has three issued U.S. patents and another 19 pending or issued U.S. and foreign patent applications that are directed to CT-2106. Additionally, the Company has four issued U.S. patents and 76 pending or issued U.S. and foreign issued patents directed to pixantrone and has licensed five granted U.S. patents and 394 pending and issued U.S. and foreign patent applications directed to brostallicin.
The Company transferred licenses to 45 pending and issued U.S. patents applications directed to Zevalin to RIT Oncology in December 2008 in connection with the transfer of Zevalin-specific assets to that entity.
The Company intends to file additional patent applications when appropriate, with respect to improvements in its core technology and to specific products and processes that it develops. Patents may not issue from any present or future applications or, if patents do issue, such patents may not be issued on a timely basis or claims allowed on issued patents may not be sufficient to protect the Company’s technology. In addition, the patents issued to the Company may be challenged, invalidated or circumvented or the rights granted there under may not provide proprietary protection or commercial advantage to the Company. With respect to such issued U.S. patents or any patents that may issue in the future, they may not effectively protect the technology involved, foreclose the development of competitive products by others or otherwise be commercially valuable.
The Company has sought and intends to aggressively seek patent protection in the United States, Canada, Mexico, Europe and Japan to protect any products that it may develop. The Company also intends to seek patent protection or rely upon trade secrets to protect certain of its enabling technologies that will be used in discovering and evaluating new drugs that could become marketable products. However, such steps may not effectively protect the technology involved. To protect any such trade secrets and other proprietary information, the Company relies on confidentiality and material transfer agreements with its corporate partners, employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These agreements may be breached, the Company may not have adequate remedies for breach or its trade secrets may otherwise become known or independently discovered by competitors. The Company also has its clinical advisors, its consultants and, in most cases, its employees enter into agreements requiring disclosure to it of ideas, developments, discoveries or inventions conceived during employment or consulting and assignment to the Company of proprietary rights to such matters related to the Company’s business and technology.
C-134
| 11.2 | Research and development activities |
The Company’s laboratories were originally located at its Italian subsidiary in Bresso, Italy, which was closed in September 2009. Please refer to Chapter 5, Paragraph 5.1.5, “Important events in the development of the Company’s business” of this Registration Document for a description of the closures of the Bresso facilities.
Please refer to Chapter 6, Paragraph 6.1.1, “Business Model: Description of main business of the Company” for a description of the Company’s products included in research and development activities.
Research and development expenses include related salaries and benefits, clinical trial and related manufacturing costs, contract and other outside service fees, and facilities and overhead costs related to the Company’s research and development efforts. Research and development expenses also consist of costs incurred for proprietary and collaboration research and development and include activities such as product registries and investigator-sponsored trials. In accordance with the applicable US regulation, research and development costs are expensed as incurred.
Research and development expenses for the years ended December 31, 2009, 2008 and 2007 were $30.2 million, $51.6 million and $72.0 million, respectively. Research and development expenses for the nine months ended September 30, 2010 and 2009 were $19.4 million and $22.9 million, respectively.
See a further discussion of the Company’s research and development expenses for the years ended December 31, 2009, 2008 and 2007 and the nine months ended September 30, 2010 and 2009 in Chapter 20, Paragraph 20.1.4., “Notes to the Statements of Operations” of this Registration Document.
C-135
CHAPTER 12 TREND INFORMATION
| 12.1 | The most significant recent trends in production, sales and inventory, and costs and selling prices since the end of the last financial year to the date of the registration document |
Following the transfer to Spectrum Pharmaceuticals of its interest in the joint venture RIT Oncology related to the drug pharmaceutical product Zevalin executed on March 15, 2009, starting from such date the Company does not have any product approved for sale in the market and therefore relevant trends did not occur in production, sales and inventory, as well as in costs and selling prices, capable to affect, positively or negatively, the activities of the Company.
More in general with respect to the oncology market, as already pointed out in Paragraph 6.1.4 of this Registration Document, people’s aging in industrialized countries will entail an ever higher incidence of cancer. This will therefore determine a considerable growth of the oncological drug market (with a likely increase in the aggregate production of such drugs, as well as in the relative sales and inventory), aimed at finding increasingly effective pharmaceutical solutions to fight cancer or at least reduce its effects, allowing the patient to live longer and improving life conditions. Nevertheless, presently it is not possible to determine how this evolution will affect the trend of costs and selling prices in the oncology market.
| 12.2 | Information on any known trends, uncertainties, demands, commitments or events that are likely to have a material effect on the issuer’s prospects for the current financial year |
Following is a description of the main events and uncertainties that are likely to have a material effect on the issuer’s prospects for the current financial year. Apart from them the Company is not aware of any trends, demands or commitments that could have a material effect on the issuer’s prospects for the current financial year.
a) Product candidates
The Company’s prospects depend in large part on the results of its clinical trials. Negative or inconclusive results or adverse medical events during a clinical trial could delay, limit or prevent regulatory approval. The Company is unable to provide the nature, timing, and estimated costs of the efforts necessary to complete the development of its product candidates because, among other reasons, the Company cannot predict with any certainty the pace of enrollment of its clinical trials and/or clinical trials being conducted by third parties. Even after a clinical trial is enrolled, preclinical and clinical data can be interpreted in different ways, which could delay, limit or preclude regulatory approval and advancement of the Company’s product candidates through the development process. For these reasons, among others, the Company cannot estimate the date on which clinical development of the Company’s product candidates will be completed or when the Company will be able to begin commercializing its product candidates to generate material net cash inflows.
The Company’s prospects also depend in large part on receiving regulatory approval for its product candidates. Obtaining regulatory approval to market drugs to treat cancer is expensive, difficult and risky. Although the Company does not currently have any products approved for sale in the market, the Company is working on a number of drug targets. The continuous changes in the regulations governing
C-136
the registration and marketing of drugs and the differences among different countries may cause delays in the approvals, including approvals from the FDA and/or the EMA and/or considerable increases in development costs and the Company’s future marketing costs are uncertain.
As mentioned in previous paragraph 6.1.1 of this Registration Document relative to pixantrone, in early April 2010, the Company received a Complete Response Letter from the FDA regarding the NDA for pixantrone rejecting it and recommending that the Company designs and conducts an additional trial to demonstrate the safety and effectiveness of pixantrone. The Company intends to appeal the FDA’s decision regarding the NDA for pixantrone; however, the Company cannot guarantee that its appeal will be successful. For further information about the principal steps of the process for the trading authorization of pixantrone managed with the FDA, please refer to the table reported at the end of the subsection headed “Pixantrone for relapsed or refractory aggressive non-Hodgkin’s lymphoma” included in the aforesaid section of the paragraph 6.1.1 of this Registration Document relative to pixantrone.
Again with respect to pixantrone, as already mentioned in previous paragraph 6.1.1 of this Registration Document, on November 1, 2010, the Company announced that it submitted a Marketing Authorization Application (“MAA”) to the European Medicines Agency (“EMA”) for Pixuvri(R) (pixantrone dimaleate) as monotherapy for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s lymphoma (“NHL”) and on November 17, 2010 the EMA approved CTI’s pediatric investigation plan (PIP), submitted by the Company in July 2010, concerning pixantrone for the treatment of lymphoid malignancies and solid tumors in children between ages of 6 months and 18 years.
b) Source of fundings
The Company has incurred losses since inception and expects to generate losses for the next few years primarily due to research and development costs for pixantrone, OPAXIO and brostallicin. The net loss attributable to CTI common shareholders for the nine months ended September 30, 2010 amounts to approximately $113.4 million. The Company’s available cash and cash equivalents are $17.3 million as of September 30, 2010. As of September 30, 2010, approximately $10.3 million aggregate principal amount of the Company’s 7.5% convertible senior notes due 2011 are due and outstanding and approximately $10.9 million aggregate principal amount of the Company’s 5.75% convertible senior notes due 2011 are due and outstanding.
During the nine month period ended September 30, 2010, in particular in July 2010, the Company raised $4.06 million in gross proceeds from the issuance of 4,060 shares of its Series 6 preferred stock and warrants to purchase up to 5.8 million shares of common stock. The Company’s Series 6 preferred stock was converted to 11.6 million shares of common stock upon closing of the transaction.
Subsequent to the aforesaid period of nine month, in October 2010, the Company raised $21.0 million in gross proceeds from the issuance of 21,000 shares of its Series 7 preferred stock and warrants to purchase up to 22.7 million shares of common stock. The Company’s Series 7 preferred stock was converted to 56.8 million shares of common stock upon closing of the transaction.
The total costs born by the Company for the issuance of shares of preferred stock of Series 6 and Series 7 amount respectively to approximately $1.1 million (corresponding to approximately 27% of the total
C-137
raised proceeds through such issuance) and $1.7 million (corresponding to approximately 8% of the total raised proceeds through such issuance).
The Company does not expect that its existing cash and cash equivalents, including the cash received from the issuance of its Series 7 preferred stock and warrants, will be sufficient to fund its presently anticipated operations beyond the first quarter of 2011. This raises substantial doubt about the Company’s ability to continue as a going concern.
Since the second quarter of 2010, the Company has implemented cost saving initiatives to reduce operating expenses, including the reduction of employees related to planned commercial pixantrone operations. However, the Company will need to raise additional funds and are currently exploring alternative sources of equity or debt financing. The Company may need to seek to raise such capital through public or private equity financings, partnerships, joint ventures, disposition of assets, debt financings or restructurings, bank borrowings or other sources of financing. On September 16, 2010, the Company’s shareholders approved an amendment to its amended and restated articles of incorporation to increase its authorized shares of common and preferred stock from 810,000,000 shares to 1,210,000,000 shares and to increase the total number of its authorized shares of common stock from 800,000,000 shares of common stock to 1,200,000,000 shares of common stock. As such, the Company’s board of directors has the option to issue such shares depending on its financial needs and market opportunities, if deemed to be in the best interest of the shareholders. However, additional funding may not be available on favorable terms or at all. If additional funds are raised by issuing equity securities, substantial dilution to existing shareholders may result. If the Company fails to obtain additional capital when needed, it may be required to delay, scale back, or eliminate some or all of the Company’s research and development programs and may be forced to cease operations, liquidate its assets and possibly seek bankruptcy protection.
c) Legal proceedings
The Company and certain of its officers and directors are named as defendants in purported securities class action and shareholder derivative lawsuits filed in the U.S. District Court for the Western District of Washington. These securities class action lawsuits are brought on behalf of a putative class of purchasers of the Company’s securities from May 5, 2009 through March 19, 2010, and seek unspecified damages. The Company may have to incur substantial expenses in connection with these lawsuits. In the event of an adverse outcome, the Company’s business could be materially harmed. Please refer to subsequent paragraph 20.8 of this Registration Document for more information about these securities class action lawsuits.
The Company may owe additional amounts for value added taxes, or VAT, related to its operations in Europe. The Company’s European operations are subject to VAT, which is usually applied to all goods and services purchased and sold throughout Europe. On April 14, 2009 and December 21, 2009, the Italian Tax Authority, or ITA, issued notices of assessment to CTI (Europe) based on the ITA’s audit of CTI (Europe)’s VAT returns for the years 2003 and 2005. On June 25, 2010, the ITA issued notices of assessment to CTI (Europe) for the years 2006 and 2007 based on similar findings of the 2003 and 2005 assessments. The ITA audits concluded that CTI (Europe) did not collect and remit VAT on certain invoices issued to non-Italian clients for services performed by CTI (Europe). The assessments, including interest and penalties, for the years 2003, 2005, 2006 and 2007 are €0.5 million, €5.5 million, €2.5 million and €0.8 million, as of June 30, 2010, respectively. Please refer to the subsequent paragraph
C-138
20.8 of this Registation Document for a description of the relevant legal proceedings. In any case, if the Company is unable to defend itself against the assessments and if the Company receives an assessment for subsequent years, it may harm its results of operations and financial condition.
C-139
CHAPTER 13 PROFIT FORECASTS OR ESTIMATES
The Company does not intend to provide any forecast or estimate regarding its profits, if any.
C-140
CHAPTER 14 ADMINISTRATIVE, MANAGEMENT, AND SUPERVISORY BODIES AND SENIOR MANAGEMENT
| 14.1 | Names, addresses and role of the member of the Issuer’s Board of Directors, Audit Committee and Executive Officers |
| 14.1.1 | Board of Directors, Audit Committee and Executive Officers |
Board of Directors
Pursuant to the current Articles of Incorporation and Bylaws, the board of directors of the Company shall carry out the management of all the affairs, property and interests of the Company. In particular, the Board of Directors of the Company may, among other things:
| (a) | fix from time to time the Issuer’s registered office within the State of Washington; |
| (b) | call at any time a shareholders’ special meeting; |
| (c) | authorize the issuance of any shares, specifying the number of shares to be issued, the consideration to be received and a statement regarding the adequacy of the consideration; |
| (d) | authorize the issue of any of the Company’s classes or series of shares without certificates; |
| (e) | resolve at any time upon the change of the number of directors; |
| (f) | by resolution adopted by a majority of the full board of directors, create one or more committees of directors which may exercise the authority of the same board of directors (with the exception of some powers as expressly indicated); |
| (g) | appoint annually and remove at any time the Company’s officers; |
| (h) | authorize the distributions of dividends to the shareholders in certain circumstances; |
| (i) | amend or repeal the bylaws of, or adopt new bylaws for, the Company; |
| (j) | adopt emergency bylaws, subject to repeal or change by action of the shareholders, which shall operative during any emergency in the conduct of the business of the Company resulting from an attack on the U.S., any state of emergency declared by the federal government or any subdivision thereof, or any other catastrophic event; |
| (k) | fix the designations and powers, preferences and rights, if any, and qualifications, limitations or other restrictions, including, without limitation, the dividend rate (and whether dividends are cumulative), conversion rights, if any, voting rights, rights and terms of redemption (including sinking fund provisions, if any), redemption price and liquidation preferences of any wholly unissued series of preferred stock and the number of shares constituting any such series and the designation thereof, or any of them; and |
| (l) | increase or decrease the number of shares of any series subsequent to the issue of shares of that series, not below the number of shares of such series then outstanding. |
C-141
The Company’s Board of Directors (please see below for the names of the members) approved the issuance and eventual buy-back of the Company’s convertible notes and equity offerings, including the Company’s issuances of shares of preferred stock and Common Stock. The Company was advised by external legal counsel in each of these transactions. The Audit Committee (please see below for the names of the members) of the Board of Directors approves the Company’s financial statements. The Company’s Chief Executive Officer (James A. Bianco, M.D.) and Chief Financial Officer (Mr. Louis A. Bianco) also certify that the information contained in the Company’s Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, including the Company’s financial statements prepared by the Company’s Finance Department, fairly present in all material respects the financial condition and results of operations of the Company as required by Sections 302 and 906of the Sarbanes-Oxley Act of 2002, as required by U.S. federal securities laws.
Under the Company’s amended and restated bylaws currently in force, the number of directors constituting the entire board of directors may be decreased or increased by majority action of either the board of directors or the shareholders. Unless a director resigns or is removed for cause, no decrease in the number of directors may have the effect of shortening the term of any incumbent director. In the event of a vacancy on the board of directors, the Company’s bylaws permit a majority of the remaining directors in office to fill the vacancy, and the director then chosen will hold office until the next shareholders’ meeting at which directors are elected. At such meeting, the director will stand for election until the later of the term elected or until his or her successor is elected and qualified.
Currently, there are eight members of the board of directors.
The following table lists the Company’s members of its Board of directors as of September 30, 2010, including the year that they were appointed and their term expiration:
| POSITION |
DIRECTOR SINCE |
TERM OF DURATION |
FIRST AND LAST NAME |
PLACE AND DATE
OF | ||||||
| CHAIRMAN OF THE BOARD | 1994 | ANNUAL MEETING TO BE HELD IN 2013 | PHILLIP M. NUDELMAN, PH.D. (1)(2)(3) | OREGON, 12-13-35 | ||||||
| DIRECTOR | 1991 | ANNUAL MEETING TO BE HELD IN 2011 | JAMES A. BIANCO, M.D. | NEW YORK, 7-26-56 | ||||||
| DIRECTOR | 2005 | ANNUAL MEETING TO BE HELD IN 2013 | JOHN H. BAUER (2) | INDIANA, 12-23-40 | ||||||
| DIRECTOR | 2001 | ANNUAL MEETING TO BE HELD IN 2011 | VARTAN GREGORIAN, PH. D. (2)(3) | IRAN, 4-8-34 | ||||||
| DIRECTOR | 2007 | ANNUAL MEETING TO BE HELD IN 2012 | RICHARD L. LOVE (1)* | VIRGINIA, 7-29-43 | ||||||
| DIRECTOR | 1997 | ANNUAL MEETING TO BE HELD IN 2012 | MARY O. MUNDINGER, DRPH (3) | NEW YORK, 4-26-37 | ||||||
| DIRECTOR | 1991 | ANNUAL MEETING TO BE HELD IN 2012 | JACK W. SINGER, M.D. | NEW YORK, 11-9-42 | ||||||
| DIRECTOR | 2006 | ANNUAL MEETING TO BE HELD IN 2011 | FREDERICK W. TELLING, PH.D. (1)(2) | PENNSYLVANIA, 10-21-51 | ||||||
C-142
| (1) | Member of the compensation committee. |
| (2) | Member of the audit committee. |
| (3) | Member of the nominating and governance committee. |
All the members of the Board of Directors are domiciled for their office at the Company’s registered offices. As provided by the Company’s Amended and Restated Bylaws, directors may serve on the Board of Directors for any number of consecutive terms. Unless a director dies, resigns or is removed, he or she shall hold office for the term elected or until his or her successor is elected and qualified, whichever is later.
Set forth below is a brief account of the business experience and education of the above directors as of December 31, 2009.
Dr. Bianco is the Company’s principal founder and served as the Company’s president and chief executive officer and director from February 1992 to July 2008. With the addition of Craig W. Philips as President in August 2008, Dr. Bianco now serves as the Company’s chief executive officer and director. Prior to founding the Company, Dr. Bianco was an assistant professor of medicine at the University of Washington, Seattle, and an assistant member in the clinical research division of the Fred Hutchinson Cancer Research Center. From 1990 to 1992, Dr. Bianco was the director of the Bone Marrow Transplant Program at the Veterans Administration Medical Center in Seattle. Dr. Bianco currently serves on the board of directors of Seattle Police Foundation and Marsha Rivkin Center for Ovarian Cancer Research. Dr. Bianco received his B.S. degree in biology and physics from New York University and his M.D. from Mount Sinai School of Medicine. Dr. Bianco is the brother of Louis A. Bianco, our Executive Vice President, Finance and Administration.
Mr. Bauer was appointed to the Board in October 2005. Mr. Bauer serves as an executive advisor and Chief Financial Officer at DigiPen Institute of Technology. He was formerly Executive Vice President for Nintendo of America Inc. from 1994 to 2004. While at Nintendo of America Inc., he had direct responsibility for all administrative and finance functions, and since 2004, he has also served as a consultant to Nintendo of America Inc. From 1963 to 1994, he worked for Coopers & Lybrand, including serving as the business assurance (audit) practice Partner. He was also a member of Coopers & Lybrand’s Firm Council, the senior policy making and governing board for the firm. Mr. Bauer is also a member of the board of directors of Caliber Data, Inc., RIPL Corporation and Zones, Inc. Mr. Bauer received his B.S. degree in accounting from St Edward’s University.
Mr. Love has been one of the Company’s directors since September 2007. Mr. Love is presently the managing director of Translational Accelerators, LLC. Mr. Love is also a director of Parexel International and ImaRx Therapeutics, and, prior to its acquisition by the Company in July 2007, served as Chairman of the Board of Systems Medicine, Inc. He started two biopharmaceutical companies, Triton Biosciences Inc. and ILEX Oncology Inc; he served as CEO for Triton Biosciences from 1983 to 1991, and as CEO for ILEX Oncology 1994 to 2001. In addition, Mr. Love has served in executive positions at not-for-profit organizations, including the Cancer Therapy and Research Center, The San Antonio Technology Accelerator Initiative and the Translational Genomics Research Institute. Mr. Love received his B.S. and M.S. degrees in chemical engineering from Virginia Polytechnic Institute.
C-143
Dr. Gregorian has been one of the Company’s directors since December 2001. He is the twelfth president of Carnegie Corporation of New York, a grant-making institution founded by Andrew Carnegie in 1911. Prior to his current position, which he assumed in June 1997, Dr. Gregorian served for eight years as Brown University’s sixteenth president. He was awarded a Ph.D. in history and humanities from Stanford University. A Phi Beta Kappa and a Ford Foundation Foreign Area Training Fellow, he is a recipient of numerous fellowships, including those from the John Simon Guggenheim Foundation, the American Council of Learned Societies, the Social Science Research Council, and the American Philosophical Society.
Dr. Mundinger has been one of the Company’s directors since April 1997. Since 1986, she has been a dean and professor at the Columbia University School of Nursing, and an associate dean on the faculty of medicine at Columbia University. Dr. Mundinger currently serves on the board of directors of Gentiva Health Services. Dr. Mundinger received her doctorate in public health from Columbia’s School of Public Health.
Dr. Nudelman has been one of the Company’s directors since March 1994. From 2000 to 2007, he served as the President and Chief Executive Officer of The Hope Heart Institute and is currently a member of the board of directors for Hope Heart Institute. From 1998 to 2000, he was the Chairman of the board of Kaiser/Group Health, retiring in 2000 as Chief Executive Officer Emeritus. From 1990 to 2000, Dr. Nudelman was the President and Chief Executive Officer of Group Health Cooperative of Puget Sound, a health maintenance organization. He also currently serves on the board of directors of OptiStor Technologies, Inc. and Zynchros, Inc. Dr. Nudelman served on the White House Task Force for Health Care Reform from 1992 to 1994 and the President’s advisory Commission on Consumer Protection and Quality in Health Care from 1996 to 1998. He has also served on the Pew Health Professions Commission and the AMA Task Force on Ethics, the Woodstock Ethics Commission, and currently serves as Chairman of the American Association of Health Plans. Dr. Nudelman received his B.S. degree in microbiology, zoology and pharmacy from the University of Washington, and holds an M.B.A. and a Ph.D. in health systems management from Pacific Western University.
Dr. Singer is one of the Company’s founders and directors and currently serves as our Executive Vice President, Chief Medical Officer. Dr. Singer has been one of the Company’s directors since its inception in September 1991. From July 1995 to January 2004, Dr. Singer was the Company’s Executive Vice President, Research Program Chairman and from April 1992 to July 1995, he served as the Company’s Executive Vice President, Research and Development. He also serves on the board of directors of DiaKine Therapeutics, Inc. Prior to joining the Company, Dr. Singer was a professor of medicine at the University of Washington and a full member of the Fred Hutchinson Cancer Research Center. From 1975 to 1992, Dr. Singer was the Chief of Medical Oncology at the Veterans Administration Medical Center in Seattle. Dr. Singer received his M.D. from State University of New York, Downstate Medical College.
Dr. Telling has been one of the Company’s directors since December 2006. Prior to his retirement in 2007, Dr. Telling was a corporate officer of Pfizer, most recently as Vice President of Corporate Policy and Strategic Management since 1994. He joined Pfizer in 1977 and was responsible for strategic planning and policy development throughout the majority of his career. He currently serves on the board of directors of Eisai N.A., Medex, and Aequus. Dr. Telling is also a member of the Committee for Economic Development, IBM’s Healthcare & Life Sciences Advisory Council, the March of Dimes National Foundation Board, ORBIS, the EAA, and the United Hospital Fund. Dr. Telling received his BA
C-144
from Hamilton College and his Masters of Industrial and Labor Relations and Ph.D. in Economics and Public Policy from Cornell University.
Audit Committee
The Audit Committee’s role is to oversee the accounting and financial reporting processes of the Company and audits of the financial statements of the Company, assist the board of directors in oversight and monitoring of (a) the integrity of the Company’s financial statements, (b) the Company’s compliance with legal and regulatory requirements, (c) the independent auditors’ qualifications and independence, (d) the performance of the Company’s internal audit function and independent auditors, and (e) the Company’s internal accounting and financial controls, prepare any report that the rules of the SEC require be included in the Company’s annual proxy statement, provide the Company’s board of directors with the results of its monitoring and recommendations derived therefrom, provide the board of directors with such additional information and materials as it may deem necessary to make the board of directors aware of significant financial matters that require its attention and undertake any other duties and responsibilities as the board of directors may from time to time prescribe.
The following table lists the members of the Company’s Audit Committee, including the year that they were appointed and their term expiration:
| POSITION |
DIRECTOR SINCE |
TERM OF DURATION |
FIRST AND LAST NAME |
PLACE AND DATE
OF | ||||
| MEMBER | 2005 | ANNUAL MEETING TO BE HELD IN 2013 | JOHN H. BAUER | INDIANA, 12-23-40 | ||||
| MEMBER | 2001 | ANNUAL MEETING TO BE HELD IN 2011 | VARTAN GREGORIAN, PH. D. |
IRAN, 4-8-34 | ||||
| MEMBER | 1994 | ANNUAL MEETING TO BE HELD IN 2013 | PHILLIP M. NUDELMAN, PH.D. | OREGON, 12-13-35 | ||||
| MEMBER | 2006 | ANNUAL MEETING TO BE HELD IN 2011 | FREDERICK W. TELLING, PH.D. | PENNSYLVANIA, 10-21-51 | ||||
All the members of the Audit Committee will be domiciled for their office at the Company’s registered offices.
C-145
Chief Executive Officer and principal executive officers
At the date of this Registration Document, the principal executive officers of the Company are as follows:
| TITLE |
FIRST AND LAST NAME |
PLACE AND DATE OF BIRTH |
SENIORITY | |||
| Chief Executive Officer of the Company | James A. Bianco, M.D. | New York, 7-26-56 | 1992 | |||
| President of the Company | Craig W. Phillips | Ohio, 3-31-60 | 2008 | |||
| Executive Vice President of the Company, Finance and Administration | Louis A. Bianco | New York, 10-24-52 | 1992 | |||
| Executive Vice President of the Company, Corporate Communications | Dan Eramian | Massachusetts, 7-30-48 | 2006 | |||
| Executive Vice President of the Company, Chief Medical Officer | Jack W. Singer, M.D. | New York, 11-9-42 | 1992 | |||
As provided by the Company’s amended and restated bylaws, the Company’s President reports to the Company’s Chief Executive Officer and the President of the Company has such powers and discharge such duties as are incident to the office of President and such duties as may be assigned from time to time by the Board of Directors. Further, each Vice President has the powers and duties as may be assigned from time to time by the Board of Directors.
Set forth below is a brief account of the business experience and education of the persons named above who will serve as the executive officers of the Company indicated above.
Mr. Philips assumed his role as the Company’s president in August 2008. In that role, he manages the company’s day-to-day drug development and commercial operations. Mr. Philips provided services to the Company as a consultant from April 2008 until he assumed the position of president. Prior to joining the Company, Mr. Philips was Vice President and General Manager of Bayer Healthcare Oncology from December 2006 to April 2008. Prior to Bayer Healthcare, Mr. Philips was Vice President and General Manager of Berlex Oncology from October 2004 to December 2006. He was also with Schering Plough from 1989 to 2003 in a variety of commercial and general management positions in the U.S., Canada, Southeast Asia and Australia. From 1984 to 1989 he was with Bristol Myers in a variety of commercial roles. Mr. Philips has also served as a member and a chair of the alliance executive committees, which included Onyx, Novartis, Genzyme, and Favrille. Mr. Philips received his B.Sc. in marketing and M.B.A. from Ohio State University.
Mr. Bianco is one of the Company’s founders and has been the Company’s Executive Vice President, Finance and Administration since February 1, 1992. He was also a director from the Company’s inception in September 1991 to April 1992 and from April 1993 to April 1995. He currently serves on the board of directors of Hallock-Ryno Investments, Inc. From January 1989 through January 1992, Mr. Bianco was a Vice President at Deutsche Bank Capital Corporation in charge of risk management. Mr. Bianco is a Certified Public Accountant and received his M.B.A. from New York University. Mr. Bianco and Dr. Bianco are brothers.
Mr. Eramian joined the Company as the Company’s Executive Vice President, Corporate Communications in March 2006. Prior to joining the Company, Mr. Eramian was Vice President of Communications at BIO, an
C-146
industry organization representing more than 1,200 biotechnology companies, academic institutions, state biotechnology centers and related organizations. Prior to that, he was Assistant Administrator of Communications at the Small Business Administration and Director of Public Affairs at the Department of Justice and Chief Spokesman for the Attorney General of the United States of America.
With reference to James A. Bianco and Jack W. Singer, please make reference to the information above.
All the aforementioned officers shall be domiciled for purposes of their office at the Company’s registered office.
| 14.1.2 | Family relationships among the persons indicated under Paragraph 14.1.1 above |
None of the directors, members of the Audit Committee or principal executive officers has any family relationship with the others, except that James A. Bianco, M.D. is the brother of Louis A. Bianco.
| 14.1.3 | Description of the main, pertinent achievements of the persons indicated under Paragraph 14.1.1 outside the Issuer in the last five years |
The following table sets forth the principal activities carried out by the members of the Board of Directors and of the Audit Committee, as well as by the Chief Executive Officer and the other principal executive officers, outside the Company:
| FIRST AND
LAST |
ACTIVITIES | |
| John H. Bauer | Consultant for Nintendo of America Inc., Executive Advisor and Chief Financial Officer of DigiPen Institute of Technology, and member of the board of directors of: Arts Fund, Caliber Data, Inc., Junior Achievement of Washington, RiPL Corporation and St. Edwards University and Zones Inc. | |
| James A. Bianco, M.D. | Member of the board of directors of: Aequus, Arts Fund, DiaKine Therapeutics, Inc., Fred Hutchinson Business Alliance, Jose Carreras International Leukemia Foundation, Marsha Rivkin Center for Ovarian Cancer, Nakea, LLC, and Seattle Police Foundation. | |
| Louis A. Bianco | Member of the board of directors of Hallock-Ryno Investment Company and member of the board of trustees of the Greater Washington Chapter of the National Multiple Sclerosis Society. | |
| Dan Eramian | Vice President of Communications of BIO, Assistant Administrator of Communications at the Small Business Administration, Director of Public Affairs at the U.S. Department of Justice, and Chief Spokesman for the Attorney General of the United States of America. | |
| Vartan Gregorian, Ph.D. | President of Carnegie Corporation of New York, and member of the board of directors of: The American Academy in Berlin, Brandeis University, Central European University, Human Rights Watch, The Hunter Foundation, Institute for Advanced Study, Princeton, Museum of Modern Art, National September 11 Memorial Musem at that World Trade Center, and the Qatar Foundation. | |
C-147
| FIRST AND
LAST |
ACTIVITIES | |
| Richard L. Love | Manager of Translational Accelerators, LLC and member of the board of directors of: Applied Microarrays Inc., Ascalon, Cancer Therapy and Research Center, ImaRX Therapeutics Inc., MedTrust OnLine, LLC., PARAXEL International, SalutarisMD Inc., Systems Medicine, Inc., The San Antonio Technology Accelerator Initiative, Translational Accelerators, LLC, and the Translational Genomics Research Insitute. | |
| Mary O. Mundinger, DrPH | Dean and professor at the Columbia University School of Nursing and member of the board of directors of: Gentiva Health Services and United Health Group. | |
| Phillip M. Nudelman, Ph.D. | President and chief executive officer of The Hope Heart Institute and member of the board of directors of: AMA Task Force on Ethics, American Association of Health Plans, OptiStor Technologies, Inc., The Hope Heart Institute, Pew Health Professions Commission, Village Theater, the Woodstock Ethics Commission, and Zynchros, Inc. | |
| Craig W. Philips | Vice President and General Manager of Bayer Healthcare Oncology, Vice President and General Manager of Berlex Oncology member of the board of directors of the Hope Heart Institute, and member or a chair of the alliance executive committees, which included Favrille, Genzyme, Novartis and Onyx. | |
| Jack W. Singer, M.D. | Member of the board of directors of Aequus and DiaKine Therapeutics, Inc. | |
| Frederick W. Telling, Ph.D. | Vice President of Corporate Policy and Strategic Management for Pfizer, Inc., member of the board of directors of: Aequus, Eisai N.A., Inc., Medex, Inc.. and member of the Committee for Economic Development, the EAA, IBM’s Healthcare & Life Sciences Advisory Council, the March of Dimes National Foundation Board, ORBIS, and the United Hospital Fund. | |
| 14.1.4 | Description of the qualified shareholdings in publicly traded companies of the persons indicated under Paragraph 14.1.1 |
None of the Company’s officers or directors indicated under Paragraph 14.1.4 has held a greater than 5% interest in any publicly held company in the United States for the past five years.
The Company’s officers and directors indicated under Paragraph 14.1.4 currently hold and have held the following interests in private companies for the past five years:
| Name |
Director |
Officer |
Ownership in U.S. private Companies | |||
| John H. Bauer | x | The current following ownership in real estate partnerships (none are related to the Company):
1. Pleasant Creek Corners Associates L. P. Ownership of 1% acquired on May, 2006
2. NAP/Springman Fund XII (Woodbridge) L. P. Ownership of 2.45% acquired on December 2000 | ||||
C-148
| 3. Sherron Associates Loan Fund XX (Kendall) LLC Ownership of 1.60% acquired on October 2006
4. Oates Creek Equity Fund LLC Ownership of 1.60% acquired on January 2006
5. Cleveland Street Square Ownership: 1.50% acquired on February 2006
6. Oak Glen (Sherron Associates) LLC Ownership of 1.0% acquired September 2005
7. Carousel Information Management Solutions, Inc. Ownership of 1.00% acquired on November 2006
8. RiPL Corporation Ownership of less than 1.0% acquired in October 2008
9. Caliber Data, Inc. Ownership of less than 1.0% acquired in March 2009 | ||||||
| James A. Bianco, M.D. | x | x | Acquired 1 million shares of restricted stock of Aequus on May 10, 2007 (i.e., 5% of Aequus outstanding common stock). | |||
| Vartan Gregorian, Ph.D | x | None | ||||
| Richard L. Love | x | 1. Systems Medicine, Inc. (“SMI”), acquired 969,064 shares (approximately 11%) of SMI in November 2005 and April 2007. Shares were exchanged for Common Stock of the Company in July 2007 in connection with the acquisition of SMI by the Company.
2. MedTrust-Online, LLC, acquired in September 2007, current ownership of 3.9%.
3. Translational Accerator LLC, acquired in September 2007, current ownership of 4.0%.
4. CerRX Inc., acquired in February 2010, current ownership of 2.8%. | ||||
| Mary O. Mundinger, Dr. PH | x | None | ||||
| Phillip M. Nudelman, PH | x | None | ||||
C-149
| Jack W. Singer, M.D. | x | x | Acquired 1 million shares of restricted stock of Aequus on May 10, 2007 (i.e., 5% of Aequus outstanding common stock). | |||
| Frederick W. Telling, Ph.D | x | Acquired 200,000 shares of restricted stock of Aequus on August 29, 2007. | ||||
| Louis A. Bianco | x | Owns approximately 1% of a private company called Hallock-Ryno Investment Company (not related to the Company). The investment was made for $100,000 in 2006. Mr. Bianco also sits on the board of this company. | ||||
| Dan Eramian | x | None | ||||
| Craig W. Philips | x | Quayle Associates LLC, ownership of 50%. | ||||
“Restricted Stock” as used in this Registration Document means shares of stock that has been granted to an Employee of the Company that is nontransferable, which may be subject to forfeiture under certain conditions and a vesting schedule set forth in the restricted stock plan such shares were granted pursuant to.
Additionally, material information regarding the securities holdings of the Company’s directors and executive officers is also included in Chapter 19 of this Registration Document, as such information discloses all relevant related party transactions.
| 14.1.5 | Description of certain convictions, bankruptcy proceedings or incriminations possibly related to the persons indicated under Paragraph 14.1.1 |
During the last five years none of the members of the Board of Directors or of the Audit Committee nor any of the Issuer’s main executive officers indicated under Paragraph 14.1.1 has been:
| a) | convicted in relation to fraudulent offences; |
| b) | associated with any bankruptcies, receiverships or liquidations, while acting as director, auditor or executive officer of the company concerned; or |
| c) | officially incriminated and/or sanctioned by statutory or regulatory authorities (including designated professional bodies) or disqualified by a court from acting as a member of the administrative, management or supervisory bodies or from acting in the management or conduct of the affairs of any issuer. |
| 14.1.6 | Relationships between directors |
Pursuant to the Company’s Code of Business Conduct and Ethics and its Amended and Restated Charter for the Audit Committee of the Board of Directors, any potential related party transaction must be fully disclosed to the Company’s Executive Vice President, Finance and Administration (the “Chief Financial Officer”). Upon review, if the Chief Financial Officer determines that the transaction is material to the Company, then the Company’s Audit Committee must review and approve in writing in advance such related party transaction. The Company is required to disclose in its proxy statement any transaction involving more than $120,000 in which the Company is a participant and in which any related person has or will have a direct or indirect material
C-150
interest. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons. Other than the information disclosed under “Related Party Transactions” in Chapter 19 of this Registration Document, there are no relationships or related party transactions to which the Company is a party.
| 14.2 | Possible conflict of interest among the Company and members of the Board of Directors, the Audit Committee and senior management |
There is currently no conflict of interest among the Company and members of the Board of Directors, the Audit Committee and senior management. However, as the members of the Board of Directors, including the members of the Audit Committee, the Chief Executive Officer and the principal executive officers are appointed in companies that are not affiliated with the Company, as indicated in Paragraph 14.1.3 above, it is possible that the Company may transact business with such unaffiliated companies out of the ordinary course of business or in a transaction which may materially affect the operating or financial results of the Company. Any such transaction may give rise to a possible conflict of interest. As used in the Registration Document, the term “affiliate” of, or individual or entity “affiliated” with, a specified individual or entity means an individual or entity that directly, or indirectly through one or more intermediaries, controls or is controlled by, or is under common control with, the individual or entity specified.
| 14.2.1 | Arrangement or understanding pursuant to which any person referred to in Paragraph 14.1 was selected as a member of the administrative, management or supervisory bodies or member of senior management |
The members of the Board of Directors have been elected by the Company’s shareholders. There are no agreements among the Company’s shareholders, with clients or other third parties regarding such election or appointment.
The members of the Audit Committee have been appointed or their appointment has been renewed by the Board of Directors. The members of the Audit Committee have been appointed in the absence of an agreement among the Company’s shareholders, with clients or other third parties.
| 14.2.2 | Restrictions on the disposal of any holding in the issuer’s capital undertaken by any person referred to in Paragraph 14.1 for a certain period of time |
Pursuant to the Company’s Insider Trading Policy, all directors, officers and employees of the Company may engage in transactions in the Company’s securities only during authorized periods upon written notification from the Company’s Chief Financial Officer following the publication of the Company’s quarterly and annual financial results, unless pursuant to an approved 10b(5)-1 trading plan. A 10b(5)-1 trading plan is a contract, instruction or written plan for the purchase or sale of the Company’s Securities that meets the requirements of SEC Rule 10b(5)-1 and is approved by the Company’s Chief Financial Officer. An “approved Rule 10b(5)-1 trading plan” is a plan that has been approved by the Corporation in writing.
The Company is not aware of any existing lock-up agreement entered into by any directors or executive officers in connection with their holdings in the company.
C-151
CHAPTER 15 REMUNERATION AND BENEFIT
| 15.1 | The amount of remuneration paid (including any contingent or deferred compensation), and benefits in kind granted in the period ended December 31, 2009 to the persons referred to under Chapter 14, Paragraph 14.1 by the Company and its subsidiaries for the services rendered to them under any role |
The Company’s Compensation Committee is comprised of three independent directors and the Compensation Committee oversees the Company’s Board of Directors responsibilities relating to the compensation of the Company’s Chief Executive Officer and all other executive officers of the Company with a title of Executive Vice President and above or who otherwise report directly to our CEO (referred to herein as our “executive officers”). In discharging this responsibility, the Compensation Committee evaluates and approves our compensation plans, policies and programs as they affect executive officers.
Compensation Process
As part of its process for determining the compensation for the named executive officers, the Compensation Committee considers competitive market data. As authorized by its charter, the Compensation Committee has engaged Milliman, Inc. (“Milliman”), an independent executive compensation consultant, to review the Company’s compensation plans, policies and programs that affect executive officers and to provide advice and recommendations on competitive market practices and specific compensation decisions. Milliman has worked directly with the Compensation Committee to assist the Compensation Committee in satisfying its responsibilities and will undertake no projects for management except at the request of the Compensation Committee chair and in the capacity of the Compensation Committee’s agent. To date, Milliman has not undertaken any projects for management or provided any services to the Company other than its services to the Compensation Committee.
In order to assess competitive market data for executive compensation, the Compensation Committee works with its compensation consultant to develop a peer group of companies with which the Company competes for executive talent (which may or may not be the same organizations that the company competes with directly on a business level). In early 2009, Milliman assisted the Compensation Committee in reviewing the peer group identified for 2008, focusing most closely on industry type and organization size/complexity, with the best indicators of organization size in the Company’s industry being number of employees and enterprise value, although each company’s revenue and net income were also considered. Following this process, the Compensation Committee selected the following peer group for fiscal 2009 compensation decisions, all of which are biotechnology organizations with an oncology focus and at a stage of company development that is comparable to the Company in the current or near-term stage: Arena Pharmaceuticals, Inc., Ariad Pharmaceuticals, Inc., Array BioPharma, Inc., Cougar Biotechnology, Inc., Dendreon Corp., IDM Pharma, Inc., Intermune, Inc., Medviation, Inc., Progenics Pharmaceuticals Inc., Rigel Pharmaceutical, Inc., Seattle Genetics, Inc., Spectrum Pharmaceuticals, Inc. and ZymoGenetics, Inc.
C-152
Once the peer group is established, the Compensation Committee then reviews the base salaries, annual cash-incentive compensation, long-term equity incentive compensation and total compensation for the Company’s executive officers as compared to the compensation paid by the companies within the Company’s peer group, comparing each executive officer to their counterparts in similar positions with the peer group companies. However, the Compensation Committee does not base its decisions on targeting compensation levels to specific benchmarks against the peer group. Instead, the Compensation Committee refers to the peer group compensation data as background information regarding competitive pay levels and also considers the other factors identified below in making its decisions.
In addition to consideration of the peer group data, the Compensation Committee also considers the value of each item of compensation, both separately and in the aggregate, in light of Company performance, each executive officer’s position within the Company, the executive officer’s performance history and potential for future advancement, and, with respect to long-term equity incentive compensation, the value of existing vested and unvested outstanding equity awards. The Compensation Committee also considers the recommendations of the Company’s chief executive officer with respect to the compensation for each executive other than himself (the Company’s chief executive officer does not provide recommendations on his compensation). In setting compensation, the Compensation Committee also considers, among other factors, the possible tax consequences to the Company and its executive officers, the accounting consequences and the impact on shareholder dilution. The relative weight given to each of these factors varies among individual executives at the Compensation Committee’s discretion and none of these factors by itself will compel a particular compensation decision.
Summary Compensation Information
The following table presents information regarding the compensation paid for fiscal 2009 to members of the Board of Directors who are not also employees of the Company. The compensation paid for the year 2009 to Dr. Bianco and Dr. Singer, who are also employed by the Company, for fiscal 2009 is presented below. Dr. Bianco and Dr. Singer are generally not entitled to receive additional compensation for their services as directors.
C-153
| Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($)(1) |
Option Awards ($)(1) |
Total ($) | ||||||||||||
| John H. Bauer |
120,500 | 1,194,175 | 16,758 | 1,331,433 | ||||||||||||
| Vartan Gregorian |
104,250 | 1,194,175 | 16,758 | 1,315,183 | ||||||||||||
| Richard L. Love |
119,750 | 1,194,175 | 16,758 | 1,330,683 | ||||||||||||
| Mary O. Mundinger |
102,500 | 1,194,175 | 16,758 | 1,313,433 | ||||||||||||
| Phillip M. Nudelman |
166,000 | 1,780,562 | 16,758 | 1,963,320 | ||||||||||||
| Frederick W. Telling |
138,750 | 1,194,175 | 16,758 | 1,349,683 | ||||||||||||
| (1) | The amounts reported in the “Stock Awards” and “Option Awards” columns of the table above reflect the fair value on the grant date of the stock awards and option awards, respectively, granted to the Company’s non-employee directors during fiscal 2009 as determined under generally accepted accounting principles used to calculate the value of equity awards for purposes of the Company’s financial statements. |
The following table sets forth information concerning compensation earned for services rendered to the Company by the Chief Executive Officer (the “CEO”), the Chief Financial Officer (the “CFO”) and the Company’s next three most highly compensated executive officers for fiscal year 2009. Collectively, these are the “Named Executive Officers”.
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($)(1)(2) |
Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($) |
All Other Compensation ($) (3) |
Total ($) | ||||||||||||||||||||||||
| James A. Bianco, M.D. Chief Executive |
2009 | 650,000 | 585,000 | 11,275,903 | — | — | 81,127 | 12,592,030 | ||||||||||||||||||||||||
| 2008 | 650,000 | 362,793 | 57,000 | — | 216,645 | 219,718 | 1,506,156 | |||||||||||||||||||||||||
| 2007 | 650,000 | 487,500 | 531,657 | 373,766 | — | 154,881 | 2,197,804 | |||||||||||||||||||||||||
| Louis A. Bianco Executive Vice President, |
2009 | 330,000 | 204,600 | 4,512,112 | — | — | 13,249 | 5,059,961 | ||||||||||||||||||||||||
| 2008 | 330,000 | 99,000 | 28,500 | — | 66,000 | 16,472 | 539,972 | |||||||||||||||||||||||||
| 2007 | 330,000 | 148,500 | 167,038 | 95,656 | — | 16,622 | 757,816 | |||||||||||||||||||||||||
| Daniel G. Eramian Executive Vice |
2009 | 315,000 | 181,125 | 3,382,770 | — | — | 315 | 3,879,210 | ||||||||||||||||||||||||
| 2008 | 315,000 | 78,750 | 28,500 | — | 63,000 | 518 | 485,768 | |||||||||||||||||||||||||
| 2007 | 315,000 | 141,750 | 151,805 | 86,147 | — | 3,091 | 697,793 | |||||||||||||||||||||||||
C-154
| Craig W. Philips President |
2009 | 402,000 | 241,200 | 6,765,543 | — | — | 14,775 | 7,423,518 | ||||||||||||||||||||||||
| 2008 | 167,500 | 22,344 | 147,500 | 23,147 | 44,656 | — | 405,147 | |||||||||||||||||||||||||
| Jack W. Singer, M.D. Executive Vice |
2009 | 340,000 | 119,000 | 4,512,112 | — | — | 40,490 | 5,011,602 | ||||||||||||||||||||||||
| 2008 | 340,000 | 85,000 | 28,500 | — | 68,000 | 46,748 | 568,248 | |||||||||||||||||||||||||
| 2007 | 340,000 | 153,000 | 167,038 | 95,656 | — | 55,369 | 811,063 |
| (1) | In accordance with recent changes in the SEC’s disclosure rules, the amounts reported in the “Stock Awards” and “Option Awards” columns of the table above for fiscal 2009 reflect the fair value on the grant date of the stock awards (including restricted stock, stock bonuses and the December 2009 Performance Awards) and option awards, respectively, granted to the Company’s Named Executive Officers during fiscal 2009. These values have been determined under generally accepted accounting principles used to calculate the value of equity awards for purposes of the Company’s financial statements. |
| (2) | The amounts reported in the “Stock Awards” column of the table above for fiscal 2009 and fiscal 2007 include the grant date fair value of performance-based stock awards (including the December 2009 Performance Awards) granted to the named executive officers in each of these years based on the probable outcome (as of the grant date) of the performance-based conditions applicable to the awards, as determined under generally accepted accounting principles. |
| (3) | The following table provides detail on the amounts reported in the “All Other Compensation” column of the table above for each named executive officer: |
|
Name |
Tax Gross-ups ($) |
Insurance Premiums ($) |
401(k) Match ($) |
Other Personal Benefits ($)(7) |
Total ($) |
|||||||||||||||
| James A. Bianco, M.D. |
4,912 | (1) | 50,759 | — | 25,456 | (5) | 81,127 | |||||||||||||
| Louis A. Bianco |
3,490 | (2) | 6,084 | 3,675 | — | 13,249 | ||||||||||||||
| Daniel G. Eramian |
315 | (3) | — | — | — | 315 | ||||||||||||||
| Craig W. Philips |
— | — | 3,675 | 11,100 | (6) | 14,775 | ||||||||||||||
| Jack W. Singer, M.D. |
10,265 | (4) | 26,550 | 3,675 | — | 40,490 | ||||||||||||||
C-155
| (1) | This amount represents tax reimbursements for taxable compensation related to health and disability premiums. These tax reimbursements were terminated in fiscal 2009. |
| (2) | This amount represents tax reimbursements for taxable compensation related to disability and life insurance premiums. These tax reimbursements were terminated in fiscal 2009. |
| (3) | This amount represents tax reimbursements for taxable compensation related to tax preparation fees. These tax reimbursements were terminated in fiscal 2009. |
| (4) | This amount represents tax reimbursements for taxable compensation related to tax preparation fees and health and disability insurance premiums. These tax reimbursements were terminated in fiscal 2009. |
| (5) | This amount includes $20,735 for family member’s travel on commercial aircraft and $4,721 for health club dues. |
| (6) | This amount includes $9,000 for automobile allowance and $2,100 for tax preparation fees. |
| (7) | Certain named executive officers were accompanied by spouses or other family members on trips using chartered aircraft where the use of the chartered aircraft was primarily for business purposes. In those cases, there was no incremental cost to the Company of having additional passengers on the chartered aircraft, and as a result, no amount is reflected in this table with respect to this benefit. |
Principal Elements of Compensation
The principal elements of compensation for the Company’s executive officers are composed of base salary, annual cash incentive compensation, and long-term equity incentive compensation. The Company also provides other compensation, including certain perquisites and other benefits. The Compensation Committee generally reviews, considers and approves each element of compensation, as well as all combined elements of compensation.
Base Salaries. Base salaries, including merit-based salary increases, for the named executive officers are established based on the scope of their respective responsibilities, competitive market salaries and general levels of market increases in salaries, individual performance, achievement of the Company’s corporate and strategic goals and changes in job duties and responsibilities.
In January 2009, the Compensation Committee reviewed the base salaries of the named executive officers and determined that they are generally competitive with the market when compared to the Company’s peer group despite the fact that the Company has not raised the base salaries of most of its executive officers in recent years. Given this continued competitiveness of the Company’s base salaries combined with its current business situation and the current economic climate, and consistent with the Company’s philosophy of providing reduced or flat levels of cash compensation while increasing equity awards during this challenging time, the Compensation Committee again determined that base salaries should not be raised in 2009.
Annual Cash Incentive Compensation. Annual cash incentives for the Company’s executive officers are designed to reward performance for achieving key corporate goals, which the Company believes in turn should increase shareholder value.
C-156
In March 2009, the Compensation Committee established the 2009 cash incentive program for the Company’s named executive officers, including target and maximum bonus opportunities for each executive as well as performance goals that would need to be achieved in order for the executive to receive such bonuses. Both target and maximum bonus opportunities under the program are determined by reference to a percentage of the executive officer’s base salary. For fiscal 2009 performance, the target bonus opportunities are 50% for Dr. Bianco, 40% for Mr. Philips, and 30% of each of Mr. Bianco, Dr. Singer and Mr. Eramian, and the maximum bonus opportunities are 125% for Dr. Bianco, 100% for Mr. Philips, and 75% for each of Mr. Bianco, Dr. Singer and Mr. Eramian. These target and maximum bonus levels were determined by the Compensation Committee, after consulting with Milliman, to be appropriate based on its subjective assessment of the executive’s position and ability to directly impact and responsibility for the Company’s performance, and its subjective assessment of general compensation practices in place at companies in the Company peer group identified above. Bonuses under the 2009 cash incentive program will be paid out in March 2010 only if the executive officer is employed by the Company on the payment date.
There are three core elements to the 2009 cash incentive program, which together comprise each executive’s cash incentive opportunity: financial performance, drug development and individual performance. As indicated in the table below, a portion of each executive’s bonus opportunity was allocated to each of these elements, with the percentage of the total bonus opportunity allocated to a particular element based on the executive’s position and ability to affect the outcome for that particular goal. With the exception of the individual performance element, each element is composed of sub-elements as identified below. As indicated in the table below, the individual performance element constitutes little or none of each executive’s target bonus. Any bonus awarded under this element will be determined by in the sole discretion of the Compensation Committee based on its subjective assessment of the executive’s performance during the fiscal year and any other factors it deems appropriate.
For the financial performance element, performance for fiscal 2009 is measured based on the Company’s operating capital raised and the percentage of the Company’s then-outstanding notes due in 2010-2011 that were tendered in the Company’s publicly-registered tender offers described at the following Paragraph 21.1.4 (the “Percentage of Tendered Notes”) compared with goals established by the Compensation Committee. The executive would be entitled to receive the target bonus for the operating capital sub-element if the Company’s operating capital raised for fiscal 2009 is $50 million. The executive would be entitled to receive the maximum bonus if the Company’s operating capital for fiscal 2009 is $100 million (or if the Company’s operating capital for fiscal 2009 is $75 million and more than 35% of the capital is raised through means other than selling or committing stock). For the Percentage of Tendered Notes, the executive would be entitled to receive the target bonus for this sub-element if the Percentage of Tendered Notes for fiscal 2009 is 50%, with the maximum bonus for this sub-element being payable if Percentage of Tendered Notes for fiscal 2009 is 75%.
For the drug development element, the performance goals established by the Compensation Committee for fiscal 2009 related to pixantrone. The executive would be entitled to payment of his target bonus for this element if, during fiscal 2009, the Company entered into a pixantrone license
C-157
agreement and completed its new drug application (“NDA”) submission for pixantrone (with a portion of the target bonus being payable if only one of these goals was achieved). The executive would be entitled to payment of an additional bonus for this element if the Company received approval from the U.S. Food and Drug Administration (the “FDA”) of pixantrone during fiscal 2009 (so that the executive would receive his maximum bonus for this element only if all three of these sub-elements were achieved).
The following table presents the relative weightings between sub-elements of each executive’s target and maximum cash incentive opportunity for fiscal 2009 (with the incentive opportunity for each sub-element being expressed as a percentage of the executive’s base salary). The relative weightings are intended as guidelines, with the Compensation Committee having final authority to determine weightings and the appropriate final bonus amounts.
| Financial | Drug Development | Individual Performance |
||||||||||||||||||||||||||||||||||
| Operating Capital | Company Debt | Pix License Agreement |
Pix NDA Submission |
Pix FDA Approval |
Target | Maximum | ||||||||||||||||||||||||||||||
| Name |
Target | Maximum | Target | Maximum | ||||||||||||||||||||||||||||||||
| James A. Bianco, M.D. |
15 | % | 45 | % | 5 | % | 10 | % | 10 | % | 15 | % | 25 | % | 5 | % | 20 | % | ||||||||||||||||||
| Craig W. Philips |
10 | % | 35 | % | 5 | % | 10 | % | 10 | % | 5 | % | 30 | % | 5 | % | 10 | % | ||||||||||||||||||
| Louis A. Bianco |
18 | % | 35 | % | 2 | % | 5 | % | 5 | % | 5 | % | 10 | % | 0 | % | 15 | % | ||||||||||||||||||
| Jack W. Singer, M.D. |
2.5 | % | 10 | % | 5 | % | 10 | % | 10 | % | 10 | % | 25 | % | 0 | % | 5 | % | ||||||||||||||||||
| Daniel G. Eramian |
10 | % | 25 | % | 5 | % | 5 | % | 7.5 | % | 7.5 | % | 15 | % | 0 | % | 15 | % | ||||||||||||||||||
In March 2010, the Compensation Committee determined that the Company had achieved the maximum performance goal established under the 2009 cash incentive program for operating capital raised in 2009 (based on gross and net operating capital raised during the fiscal year of approximately $121.9 million and $114.4 million, respectively, from the Company’s sale of equity securities) and determined to pay the target performance goal for the Percentage of Tendered Notes (based on 56.7% of the Company’s then-outstanding notes due in 2010-2011 having been tendered in the Company’s publicly-registered tender offers for those notes or having been converted into shares of common stock). In addition, the Compensation Committee noted that the Company had completed its new drug application submission for pixantrone in 2009. The Compensation Committee also determined that each executive should, based upon the Compensation Committee’s subjective assessment of each executive’s individual contributions
C-158
during the year, receive the maximum incentive opportunity that corresponded to individual performance plus an additional 5% of the executive’s base salary. The additional bonus was within the Compensation Committee’s discretion as the relative weightings set forth in the table above were only guidelines. While the Compensation Committee’s determination of these amounts was inherently subjective, the key factors in the Compensation Committee’s determination were the executives’ successful presentation and defense of a plan to regain compliance with NASDAQ listing standards, the executives’ work in the Company’s efforts seeking FDA approval of pixantrone, and the Compensation Committee’s subjective assessment that these bonuses were appropriate to help continue to retain the executive team.
Based on the maximum bonus opportunity related to the Company’s operating capital achievement, the target bonus opportunity related to the Percentage of Tendered Notes, the bonus opportunity related to the pixantrone NDA submission, and the maximum individual bonus opportunity plus the additional 5% bonus in the Compensation Committee’s discretion during fiscal 2009, the Compensation Committee determined to award cash incentives to each of the named executive officers in the following amounts (expressed as a percentage of such executive’s base salary): James A. Bianco, M.D., 90%; Craig W. Philips, 60%; Louis A. Bianco, 62%; Daniel G. Eramian, 57.5%; and Jack W. Singer, M.D., 35%. These amounts are reflected in the “Bonus” column of the Summary Compensation Table above.
Long-Term Equity Incentive Compensation. As discussed above, in light of the business environment and existing challenges facing it, the Compensation Committee has generally been reducing or keeping unchanged annual cash compensation while increasing equity compensation. In implementing this part of the compensation policy, the Compensation Committee is cognizant of the key compensation goals for the Company, including (i) recognizing that the next one to three years will be extremely critical to the Company’s future and shareholder value, (ii) taking into consideration present and projected trials, (iii) considering pipeline products and their status, (iv) the need for a retention plan for critical executives and for the chief executive officer, and (v) supplying a mechanism for motivating the chief executive officer and the executive team during the upcoming critical time period.
In determining the size of the Company’s long-term equity incentive awards, the Compensation Committee reviews competitive market data for similar positions in the Company’s peer companies, the executive officer’s performance history and/or potential for future responsibility and promotion, the chief executive officer’s recommendations (with respect to executives other than himself) and the value of existing vested and unvested outstanding equity awards. The relative weight given to each of these factors will vary from individual to individual at the Compensation Committee’s discretion and adjustments may be made as the Compensation Committee deems reasonable to attract candidates in the competitive environment for highly qualified employees in which the Company operates.
Equity Awards Approved in Fiscal 2009.
The first step in the Compensation Committee’s approach to the fiscal 2009 equity awards was the grant, in March 2009, of retention restricted stock awards to each of the named executive officers.
C-159
These grants are scheduled to vest over a two-year period, subject to the executive’s continued employment with the Company through the vesting date. The time-based vesting schedule (as opposed to a performance-based vesting schedule) for these grants was believed to be appropriate to help ensure retention, but since the ultimate value of the awards is linked to stock price the grants also continue to link executives’ interests with those of shareholders.
The Compensation Committee determined that it was critical to focus management on the goal of restoring shareholder value and, as the second step in the Compensation Committee’s approach to the fiscal 2009 equity awards, it communicated to management that bonuses of fully-vested stock would be considered if the Company achieved certain regulatory approvals or if the Company achieved certain values for its common stock. The share appreciation goals were based on 500% and 1,000% increases in the value of a share of the Company’s common stock over the per-share closing price of a share of Company common stock of $0.14 on March 23, 2009. In June 2009, the 30-day moving average of the Company’s stock price reached $1.54, an increase of more than 1,000% over the March 23 level. Accordingly, on July 31, 2009 and again on November 10, 2009 the Compensation Committee approved bonuses to the named executive officers in the form of fully vested shares of Company common stock in connection with the attainment of these prices for the Company’s stock. The numbers of shares awarded (on a pre-tax basis) to each of the executive officers is reflected in the Grants of Plan-Based Awards Table–Fiscal 2009 on the lines corresponding to these two particular grant dates. The actual number of shares delivered to the executive officers on payment of these bonuses was reduced by the number of shares (valued at their then current value) required to satisfy applicable tax withholding obligations. (The regulatory goals noted in this paragraph are consistent with the goals that were ultimately formally adopted by the Compensation Committee in December 2009 and are discussed below).
Finally, in December 2009, the Compensation Committee decided to grant restricted stock units that will be payable in fully vested shares of the Company’s common stock upon the achievement of a particular performance goal, subject to the goal’s being achieved before December 31, 2011 and the individual’s continued employment or service with the Company. (The Company refers to these awards as the “December 2009 Performance Awards”). The Compensation Committee believed these awards at the grant levels identified below would provide an appropriate level of incentive to executives to help achieve the performance goals noted below, to help maximize and restore shareholder value, and to help provide enhanced retention incentives.
The performance goals under the December 2009 Performance Awards are as follows:
| (a) | OPAXIO marketing authorization application (“MAA”) approval (“OPAXIO MAA Approval”); |
| (b) | OPAXIO NDA approval (“OPAXIO NDA Approval”); |
| (c) | achievement by the Company of fiscal year sales equal to or greater than $50,000,000 (the “$50M Sales Goal”); |
C-160
| (d) | achievement by the Company of fiscal year sales equal to or greater than $100,000,000 (the “$100M Sales Goal”); |
| (e) | pixantrone NDA Approval (“Pix NDA Approval”); |
| (f) | achievement by the Company of break-even cash flow in the fourth quarter of Fiscal Year 2010 (the “Fiscal 2010 4th Quarter Break Even”); |
| (g) | achievement by the Company of earnings per share results in any fiscal year equal to or greater than $0.05 per share of Company common stock (the “EPS Goal”); and |
| (h) | achievement of a price per share of Company common stock equal to $2.94 (the “Share Appreciation Goal”). |
If one or more of the performance goals are timely achieved, an award recipient will be entitled to receive a number of shares of Company common stock (subject to the applicable share limits of the Company’s equity incentive plan) determined by multiplying (1) the award percentage corresponding to that particular performance goal by (2) the total number of outstanding shares of Company common stock, determined on a non-fully diluted basis, as of the date the Compensation Committee certifies that the particular performance goal has been achieved. The award percentages corresponding to the various performance goals for each of the named executive officers are set forth in the following table:
| Performance Goals and Applicable Award Percentages | ||||||||||||||||||||||||||||||||
| Name |
OPAXIO MAA Approval |
OPAXIO NDA Approval |
$50M Sales Goal |
$100M Sales Goal |
Pix NDA Approval |
Fiscal 2010 4th Quarter Break Even |
EPS Goal |
Share Appreciation Goal |
||||||||||||||||||||||||
| James A. Bianco, M.D. |
0.15 | % | 0.2 | % | 0.3 | % | 0.6 | % | 0.45 | % | 0.3 | % | 0.7 | % | 0.75 | % | ||||||||||||||||
| Louis A. Bianco |
0.061 | % | 0.081 | % | 0.122 | % | 0.243 | % | 0.182 | % | 0.122 | % | 0.284 | % | 0.305 | % | ||||||||||||||||
| Daniel G. Eramian |
0.045 | % | 0.06 | % | 0.09 | % | 0.18 | % | 0.135 | % | 0.09 | % | 0.21 | % | 0.225 | % | ||||||||||||||||
| Craig W. Philips |
0.09 | % | 0.12 | % | 0.18 | % | 0.36 | % | 0.27 | % | 0.18 | % | 0.42 | % | 0.45 | % | ||||||||||||||||
| Jack W. Singer, M.D. |
0.061 | % | 0.081 | % | 0.122 | % | 0.243 | % | 0.182 | % | 0.122 | % | 0.284 | % | 0.305 | % | ||||||||||||||||
A performance goal will not be considered achieved unless and until the date on which the Compensation Committee certifies that is has been achieved.
C-161
In June 2010, the Compensation Committee modified the Fiscal 2010 4th Quarter Break Even performance goal applicable to each of the December 2009 Performance Awards granted to the named executive officers such that the break-even cash flow component of the performance goals will be satisfied if the Company achieves a cash-flow break-even in any fiscal quarter during the term of the awards. In addition, in July 2010, the Compensation Committee approved an amendment to each of the December 2009 Performance Awards granted to the named executive officers that converted a portion of the award to a grant of restricted shares. The Compensation Committee believed, particularly in light of the current economic environment, that the link between executives’ interests and shareholders’ interests would be further enhanced if the executives held restricted shares (whereas the December 2009 Performance Awards had provided for delivery of shares only upon the vesting of the awards). The restricted shares issued to the executives pursuant to this conversion are subject to the same performance-based vesting requirements as the December 2009 Performance Award to which they relate and will be forfeited back to the Company should these vesting requirements not be satisfied. Any restricted shares that vest in connection with the achievement of a performance goal on or before December 31, 2011 will reduce on a share-for-share basis the number of shares that would have been delivered under the original December 2009 Performance Award upon achievement of that performance goal (and, if the number of shares that would have been delivered under the original December 2009 Performance Award on achievement of a performance goal is less than the number of restricted shares that vest on achievement of that performance goal under the July 2010 award, a number of such restricted shares equal to the difference will be forfeited to the Company so that the executive retains no more shares related to that particular performance goal than the number of shares that would have otherwise been deliverable with respect to that goal under the original December 2009 Performance Award).
Also, in 2010, the Company’s shareholders and Board of Directors approved an amendment to the Company’s 2007 Equity Incentive Plan that increased the number of shares of the Company’s common stock that may be delivered pursuant to awards granted under the 2007 Equity Plan by an additional 40,000,000 shares.
| 15.2 | The total amounts set aside or accrued by the issuer or its subsidiaries in the period ended December 31, 2009 to provide pension, retirement or similar benefits |
In connection with CTI’s merger with Novuspharma, on January 1, 2004, CTI assumed a defined benefit plan and related obligation for benefits owed to its Italian employees who, pursuant to Italian law, were entitled to a payment upon separation from the Company. Related costs are accrued over the employees’ service periods based on compensation and years of service. In accordance with EITF 88-1, Determination of Vested Benefit Obligation for a Defined Benefit Pension Plan, CTI has elected to carry the obligation under the plan at the amount of the vested benefit obligation which is defined as the actuarial present value of the vested benefit to which the employee is entitled if the employee separates immediately. Benefits of approximately $0.6 million, $0.5 million, $0.3 million and $0.8 million were paid to employees who separated from the Company during 2009, 2008, 2007 and 2006, respectively.
As of December 31, 2009, 2008, 2007 and 2006, the vested benefit obligation was approximately $0.6 million, $0.9 million, $1.0 million and $0.9 million, respectively.
C-162
CHAPTER 16 PRACTICES OF THE BOARD OF DIRECTORS
| 16.1 | In relation to the persons referred to in Paragraph 14.1 above, date of expiration of the current term of office, if applicable, and the period during which the person has served in that office |
Please see Chapter 14, Paragraph 14.1 above.
| 16.2 | Information about agreements entered into by and between a) any director or member of the Audit Committee and b) the issuer or any of its subsidiaries providing for benefits upon termination of the employment |
Employment Agreement with Dr. Bianco
In December 2008, the Company entered into an employment agreement with Dr. James A. Bianco, the Company’s Chief Executive Officer and a member of its Board of Directors, that replaced his original employment agreement entered into in 2005. The employment agreement has a two-year term. The agreement provides that Dr. Bianco will receive an initial annualized base salary of $650,000, subject to review by the Compensation Committee of the Company’s Board of Directors. Based on its review, the Compensation Committee may increase (but not reduce) the base salary level. The agreement also provides for annual bonuses for Dr. Bianco with a target annual bonus of at least 50% of his base salary and for an additional bonus to be paid if certain “stretch” performance goals established by the Compensation Committee for the applicable year are achieved. The agreement also provides for Dr. Bianco to participate in the Company’s usual benefit programs for senior executives, payment by the Company of premiums for universal life insurance with a coverage amount of not less than $5,000,000 (up to an annual limit of $41,500, subject to adjustment) and certain other personal benefits set forth in the agreement. Provisions of Dr. Bianco’s agreement relating to post-termination of employment benefits are discussed below.
Severance Arrangements
The following section describes the benefits that may become payable to the Company’s executive officers in connection with a termination of their employment with the Company.
James A. Bianco, M.D. Pursuant to his employment agreement described above, if Dr. Bianco’s employment is terminated by the Company without cause or if he resigns for good reason (as the terms “cause” and “good reason” are defined in the agreement), he will receive the following severance benefits: (i) cash severance equal to two years of his base salary, (ii) reimbursement for up to two years by the Company for COBRA premiums to continue his medical coverage and that of his eligible dependents pursuant to the Consolidated Omnibus Budget Reconciliation Act (“COBRA”), (iii) continued payment for up to two years by the Company of premiums to maintain life insurance paid for by the Company at the time of his termination, and (iv) a cash payment for the value of his accrued and unpaid vacation. In addition, Dr. Bianco would be entitled to accelerated vesting of all of his then-outstanding and unvested stock-based compensation, and his outstanding stock options would remain exercisable for a period of two years following the severance date. In the event of a change of control of the Company, if Dr. Bianco
C-163
is terminated without cause or resigns for good reason, he will receive cash severance in the form of a lump sum payment equal to two years of his base salary, plus an amount equal to the greater of the average of his three prior years’ bonuses or thirty percent of his base salary, as well as the benefits described in clauses (ii) through (iv) above. Dr. Bianco’s right to receive these severance benefits is conditioned upon his executing a release of claims in favor of the Company and complying with certain restrictive covenants set forth in the agreement. Further, if the Company is required to restate financials due to its material noncompliance with any financial reporting requirement under the U.S. securities laws during any period for which Dr. Bianco was chief executive officer of the Company or Dr. Bianco acts in a manner that would have constituted cause for his termination had he been employed at the time of such act, Dr. Bianco will not be entitled to any severance benefits that have not been paid, and will be required to repay any portion of the severance to the Company that has already been paid. The agreement further provides that if there is a change of control of the Company during Dr. Bianco’s employment with the Company, all of his then-outstanding and unvested stock-based compensation will fully vest and all outstanding stock options will remain exercisable for a period of two years following Dr. Bianco’s severance date. In addition, in the event that Dr. Bianco’s benefits under the agreement are subject to the excise tax imposed under Section 280G of the U.S. Internal Revenue Code (“Section 280G”), the Company will make an additional payment to him so that the net amount of such payment (after taxes) he receives is sufficient to pay the excise tax due.
Craig W. Philips. Pursuant to his employment agreement with the Company, if Mr. Philips’ employment is terminated by the Company without cause or if he resigns for good cause (as the terms “cause” and “good cause” are defined in the agreement), he will receive the following severance benefits: (i) cash severance equal to 18 months of his base salary, (ii) reimbursement for up to 18 months by the Company for COBRA premiums to continue his health coverage and that of his eligible dependents, and (iii) a cash payment for the value of his accrued and unpaid vacation. In addition, Mr. Philips would be entitled to accelerated vesting of any portion of his then-outstanding and unvested stock-based compensation that was scheduled to vest within one year following the date of his termination. If a change in control of the Company occurs and, within 12 months following the change in control, Mr. Philips’ employment is terminated by the Company without cause or Mr. Philips voluntarily resigns for any reason, he would be entitled to accelerated vesting of all of his then-outstanding and unvested stock-based compensation in addition to the benefits described in clauses (i) through (iii) above. Mr. Philips’ right to receive these severance benefits is conditioned upon his executing a release of claims in favor of the Company and complying with certain restrictive covenants set forth in the agreement.
If Mr. Philips’ employment is terminated on account of disability, in addition to any short-term or long-term disability benefits he may be entitled to under any Company group disability plans, the Company will pay Mr. Philips a pro rata share of his target bonus for the year in which his termination occurs, and the Company will also pay Mr. Philips’ COBRA premiums for the period of time he is eligible for COBRA.
Other Executive Officers. The Company has entered into severance agreements with each of Mr. Bianco, Dr. Singer and Mr. Eramian. These agreements provide that in the event the executive is discharged from employment by the Company without cause or resigns for good reason (as each such term is defined in the agreements), he will receive the following severance benefits: (i) cash severance equal to 18 months of his base salary, plus an amount equal to the greater of the average of his three prior years’ bonuses or thirty percent of his base salary, (ii) reimbursement for up to 18 months by the Company for COBRA premiums to continue his medical coverage and that of his eligible dependents, (iii) continued payment for up to 18 months by the Company of premiums to maintain life insurance paid for by the Company at
C-164
the time of his termination, and (iv) a cash payment for the value of his accrued and unpaid vacation. In addition, the executive would be entitled to accelerated vesting of all of his then-outstanding and unvested stock-based compensation, and his outstanding stock options would remain exercisable for a period of 21 months following the severance date. In addition, in the event that the executive’s benefits under the agreement are subject to the excise tax imposed under Section 280G, the Company will make an additional payment to him so that the net amount of such payment (after taxes) he receives is sufficient to pay the excise tax due. The executive’s right to receive these severance benefits is conditioned upon his executing a release of claims in favor of the Company and not breaching his inventions and proprietary information agreement with the Company.
With the exception of the above mentioned arrangements, the Company has not entered into any employment or other service agreements or any agreements providing for benefits upon a termination of employment with any of its directors or executive officers.
| 16.3 | Information about the issuer’s Audit Committee and Compensation Committee, including the names of the members and a summary of the mandate under which the committees operate |
The Board of Directors has three standing committees, each of which will have certain delegated powers, as described below. Each committee customarily reports all of its material actions to the full Board at the next Board meeting. Each committee has a charter that describes its purpose, membership and responsibilities. Each committee is authorized to exercise only those powers that have been delegated to it by the Board.
| 16.3.1 | Audit Committee. |
With regard to the Audit Committee, please see information provided in Chapters 14 and 15 above.
| 16.3.2 | Compensation committee |
At the date of this Registration Document, the Compensation Committee of the Company is composed as follows:
| POSITION |
DIRECTOR SINCE |
TERM OF DURATION |
FIRST AND LAST NAME |
PLACE AND DATE OF BIRTH | ||||
| MEMBER | 1994 | ANNUAL MEETING TO BE HELD IN 2013 | PHILLIP M. NUDELMAN, PH.D. | OREGON, 12-13-35 | ||||
| MEMBER | 2006 | ANNUAL MEETING TO BE HELD IN 2011 | FREDERICK W. TELLING, PH.D. | PENNSYLVANIA, 10-21-51 | ||||
| MEMBER | 2007 | ANNUAL MEETING TO BE HELD IN 2012 | RICHARD L. LOVE | VIRGINIA, 7-29-43 | ||||
All the members of the Compensation Committee are domiciled for their office at the Company’s registered offices.
Pursuant to the Compensation Committee Charter, all of the members of the Compensation Committee must be “independent” within the meaning of the NASDAQ rules, must be non-employee directors pursuant to the rules and regulations of the SEC and must be “outside directors” as defined in the U.S. Internal Revenue Code of 1986, as amended. The Compensation Committee discharges the Board’s
C-165
responsibilities relating to compensation of the Issuer’s executive officers, oversees all compensation programs involving the use of the Issuer’s stock and produces an annual report on executive compensation for inclusion in the Issuer’s annual proxy statement. CTI’s board has delegated to the Compensation Committee the power to review and approve all compensation and bonuses for the Chief Executive Officer and other executive officers. The Board has also delegated to the Compensation Committee the non-exclusive power to oversee compensation programs involving the use of CTI’s stock (including the 2007 Equity Incentive Plan, the 1994 Equity Incentive Plan, the Novuspharma S.p.A. Stock Option Plan, and the 2007 Employee Stock Purchase Plan), including the power to determine stock option grants for employees, consultants, directors and other individuals. (These powers are also retained by the full Board.) In practice, both the Compensation Committee and the full Board approve grants of stock options. The Compensation Committee produces an annual report on executive compensation for inclusion in the Company’s proxy statement for its annual meeting of shareholders.
| 16.3.3 | The Nominating and Governance Committee |
At the date of this Registration Document, the Nominating and Governance Committee of the Company is composed as follows:
| POSITION |
DIRECTOR SINCE |
TERM OF DURATION |
FIRST AND LAST NAME |
PLACE AND DATE OF BIRTH | ||||
| MEMBER | 2001 | ANNUAL MEETING TO BE HELD IN 2011 | VARTAN GREGORIAN, PH. D. | IRAN, 4-8-34 | ||||
| MEMBER | 1997 | ANNUAL MEETING TO BE HELD IN 2012 | MARY O. MUNDINGER, DRPH | NEW YORK, 4-26-37 | ||||
| MEMBER | 1994 | ANNUAL MEETING TO BE HELD IN 2013 | PHILLIP M. NUDELMAN, PH.D. | OREGON, 12-13-35 | ||||
All the members of the Nominating and Governance Committee are domiciled for their office at the Company’s registered offices.
The role of the Nominating and Governance Committee is to ensure that the board of directors of the Company is properly constituted to meet its fiduciary obligations to shareholders and the Company and that the Company has and follows appropriate governance standards. To carry out these objectives, the Nominating and Governance Committee: (a) assists the board of directors by identifying prospective director nominees and recommends to the board of directors the director nominees for the each annual meeting of shareholders, (b) develops and recommends to the board of directors the governance principles applicable to the Company, (c) oversees the evaluation of the board of directors and management of the Company, and (d) recommends to the board of directors nominees for each committee of the board of directors.
| 16.4 | Compliance by the Issuer with the corporate governance regime to which it is subject |
| 16.4.1 | U.S. corporate governance requirements |
The Company is subject to the corporate governance requirements of the state of Washington, as well as the regulatory standards of NASDAQ and the SEC. These requirements primarily include, but are not limited to, the following:
C-166
| • | a majority of independent directors on the Company’s board; |
| • | regular meetings (at least twice a year) of the independent directors of the Company’s board; |
| • | the adoption of a code of ethics applicable to all directors, officers and employees; |
| • | an audit committee of the Company’s board consisting of all independent directors, including one member who meets the definition of an “audit committee financial expert”, and with a charter that specifies the scope of the audit committee’s responsibilities and the means by which it carries out those responsibilities, the outside auditor’s accountability to the audit committee and the audit committee’s responsibility to ensure the independence of the outside auditor as required under the NASDAQ and SEC rules; |
| • | a nominating/corporate governance committee consisting of all independent directors with a charter which establishes the nominating/corporate governance committee’s purpose and scope of responsibilities as required under the NASDAQ and SEC rules; and |
| • | a compensation committee consisting of all independent directors with a charter which establishes the compensation committee’s purpose and scope of responsibilities s required under the NASDAQ and SEC rules. |
The Company believes it is compliant with all material requirements relating to these and all other corporate governance provisions.
For additional information regarding the compliance with law requirements, please refer to Annex C to this Registration Document “Section 12.5 of the 2003 Prospectus”, as amended in the light of the amended applicable law.
| 16.4.2 | Italian disclosure requirements |
As the Company has its common stock listed on an Italian regulated market, the same is subject in particular to the following disclosure requirements:
| • | Disclosure requirements set forth under Section 114 and Section 115 of the TUF, including disclosure to the public of “privileged information” and disclosure to CONSOB and the public of “internal dealing” transactions carried out, inter alia, by the Company’s directors, executive officers and qualified shareholders. |
| • | Pursuant to Section 116, par. 1, of CONSOB Regulation in force at the time of the admission to listing, CONSOB is enabled to determine, upon the listing of a foreign issuer on a regulated market other than “borsa”, the information and documents to be circulated and the language to be adopted for the purposes thereof, having regard to the rules existing in the issuer’s country of origin. On the basis of such provision, at the time of the listing of the Company on the Nuovo Mercato in 2003, CONSOB established that CTI would also be subject to Sections 66, 67, 68, 69 and 84 (to be complied with in the Italian language), of Regulation 11971/1999 in force at the time of the admission to listing, to the extent that information and documents provided for thereby exist in the case of the Company. Following subsequent resolutions of Borsa Italiana S.p.A., Nuovo Mercato was first named MTAX and therefore merged with the MTA market as of 3 March 2008, therefore falling under the definition of “borsa” according to TUF and CONSOB Regulation. |
The Company is also subject to further obligations regarding continuing information, information on extraordinary transactions, as well as information concerning the exercise of rights by shareholders. In
C-167
this respect, in the 2003 Listing Prospectus, CTI generally undertook to make available to the public any additional information supplied to the public in the U.S. if this is relevant for the purposes of evaluating the financial instruments listed on the Italian market in compliance with the principle of equivalence of information set forth by article 88 of the CONSOB Regulation currently in force at the time of the admission to listing,. CTI will make this information public by filing it with the SEC; upon filing, it will be available on the SEC’s website over the Internet. In particular, CTI undertook a number of obligations which were published in Chapter 12.6 of the 2003 Listing Prospectus, attached to this Registration Document as Appendix B.
C-168
CHAPTER 17 EMPLOYEES
| 17.1 | Organization chart of the issuer |
The following table sets forth the number of employees of the Group registered as of December 31, 2009, 2008 and 2007 and as of September 30, 2010, including a breakdown of persons employed by main category of activity and geographic location.
| September 30, | December 31, | |||||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| United States |
||||||||||||||||
| Research and development |
49 | 51 | 62 | 91 | ||||||||||||
| Selling, general and administrative |
40 | 53 | 65 | 68 | ||||||||||||
| Total United States |
89 | 104 | 127 | 159 | ||||||||||||
| Europe |
||||||||||||||||
| Research and development |
— | — | 56 | 60 | ||||||||||||
| Selling, general and administrative |
1 | 3 | 11 | 11 | ||||||||||||
| Total Europe |
1 | 3 | 67 | 71 | ||||||||||||
The reduction of the number of persons employed in Europe is due to the the closing of the Company’s operations in Bresso, Italy, occurred in September 2009.
In addition the Company has seven temporary employees and 46 consultants as of the date of this Registration Document.
As of the date of this Registration Document no matierial variation in the number of CTI employees has occurred with respect to September 30, 2010.
| 17.2 | Shareholdings and stock options |
The following table provides certain information regarding beneficial ownership of common stock as of October 22, 2010, by (1) each shareholder known by us to be the beneficial owner of more than 5% of our outstanding shares of common stock, (2) each of our directors, and (3) each of our principle executive officer (PEO), principal financial officer (PFO) and the three most highly compensated executive officers other than the PEO and PFO who were still serving as executive officers as of October 22, 2010:
| FIRST AND LAST NAME |
NUMBER OF SHARES BENEFICIALLY OWNED (1) |
SHARES SUBJECT TO OPTIONS(2) |
PERCENTAGE OF SHARES BENEFICIALLY OWNED AGAINST THE TOTAL SHARE CAPITAL (1) |
|||||||||
| John H. Bauer (4) |
1,840,208 | 35,400 | * | |||||||||
| James A. Bianco, M.D. (3) |
13,069,529 | 46,869 | 1.6 | % | ||||||||
C-169
| FIRST AND LAST NAME |
NUMBER OF SHARES BENEFICIALLY OWNED (1) |
SHARES SUBJECT TO OPTIONS(2) |
PERCENTAGE OF SHARES BENEFICIALLY OWNED AGAINST THE TOTAL SHARE CAPITAl (1) | |||||||
| Louis A. Bianco (5) |
6,087,302 | 17,935 | * | |||||||
| Daniel G. Eramian (8) |
4,683,124 | 8,225 | * | |||||||
| Vartan Gregorian, Ph.D. (4) |
1,946,458 | 36,525 | * | |||||||
| Richard. L. Love (4) |
2,413,173 | 35,400 | * | |||||||
| Mary O. Mundinger, DrPH (4) |
1,906,724 | 36,875 | * | |||||||
| Phillip M. Nudelman, Ph.D. (6) |
2,471,590 | 36,725 | * | |||||||
| Craig W. Philips (7) |
9,318,206 | 10,000 | 1.1% | |||||||
| Jack W. Singer, M.D. (5) |
6,097,944 | 21,117 | * | |||||||
| Frederick W. Telling, Ph.D. (4) |
1,839,023 | 35,100 | * | |||||||
| * | Less than 1% |
| (1) | Beneficial ownership generally includes voting or investment power with respect to securities and is calculated based on 814,781,932 shares of our common stock outstanding as of October 22, 2010. This table is based upon information supplied by officers, directors and other investors including information from Schedules 13D, 13G and 13F and Forms 3 and 4 filed with the SEC. Shares of common stock subject to options, warrants or other securities convertible into common stock that are currently exercisable or convertible, or exercisable or convertible within 60 days of October 22, 2010, are deemed outstanding for computing the percentage of the person holding the option, warrant or convertible security, but are not deemed outstanding for computing the percentage of any other person. Except as indicated in the footnotes to this table and pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of stock beneficially owned. |
| (2) | Shares subject to convertible securities included in this column reflect all options, warrants and convertible debt held by the holder exercisable within 60 days after October 22, 2010. These shares are also included in the column titled “Number of Shares Beneficially Owned”. |
| (3) | The share holdings of Dr. Bianco have increased by 13,000,204 shares from January 1, 2006 through October 22, 2010 as a result of the following transactions: |
| • | sale of 2,841,435 shares; of this amount, 319,924 shares were sold pursuant to a pre-established 10b5-1 plan; |
| • | gift of 26,375 shares; |
| • | forfeiture of 28,287 restricted shares resulting from contingencies on award not being met; |
| • | forfeiture of 2,247,267 shares to satisfy tax withholding obligations upon vesting of restricted stock awards; |
| • | restricted stock grants totaling 28,225 shares from grants that were approved in December 2006, but were not effectively granted until September 25, 2007, the date CTI’s shareholders approved, among other things, an increase in the number of shares of common stock available for issuance under the |
C-170
| 2007 plan; |
| • | additional restricted stock grants totaling 18,098,993 shares, of which 10,959,129 are unvested as of October 22, 2010, including 9,990,610 shares that have contingent vesting terms. Of these contingent shares, 12,000 shares will vest if the Company obtains FDA approval of OPAXIO prior to December 31, 2010 and 12,000 shares will not vest due to the divestiture of Zevalin (such shares would have vested if the Company had obtained a specific annual net sales threshold for Zevalin prior to December 31, 2010). In addition, the remaining contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 1,426,963 shares upon OPAXIO NDA Approval; 3,210,667 shares upon Pix NDA Approval; 2,140,444 shares upon achievement of $50M Sales Goal; 1,048,092 shares upon achievement of $100M Sales Goal; and 2,140,444 shares upon achievement of break-even cash flow in any quarter; and purchase of 16,350 shares; and |
| • | purchase of 16,350 shares. |
Number of shares beneficially owned by Dr. Bianco includes 20 shares held by Dr. Bianco’s wife and two shares held by Dr. Bianco’s wife as custodian.
| (4) | Number of shares beneficially owned includes 1,016,661 shares of unvested restricted stock, 996,661 of which have contingent vesting terms. The contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 142,697 shares upon OPAXIO NDA Approval; 321,067 shares upon Pix NDA Approval; 214,044 shares upon achievement of $50M Sales Goal; 104,809 shares upon achievement of $100M Sales Goal; and 214,044 shares upon achievement of break-even cash flow in any quarter. |
| (5) | Number of shares beneficially owned includes 4,339,482 shares of unvested restricted stock, 4,049,827 of which have contingent vesting terms. Of these contingent shares, 4,000 shares will vest if the Company obtains FDA approval of OPAXIO prior to December 31, 2010 and 4,000 will not vest due to the divestiture of Zevalin (such shares would have vested if the Company had obtained a specific annual net sales threshold for Zevalin prior to December 31, 2010). In addition, the remaining contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 577,920 shares upon OPAXIO NDA Approval; 1,298,536 shares upon Pix NDA Approval; 870,447 shares upon achievement of $50M Sales Goal; 424,477 shares upon achievement of $100M Sales Goal; and 870,447 shares upon achievement of break-even cash flow in any quarter. Includes 1,118 shares held by Mr. Bianco in trust for his children. |
| (6) | Number of shares beneficially owned includes 1,518,559 shares of unvested restricted stock, 1,498,559 of which have contingent vesting terms. The contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 214,044 shares upon OPAXIO NDA Approval; 485,167 shares upon Pix NDA Approval; 321,067 shares upon achievement of $50M Sales Goal; 157,214 shares upon achievement of $100M Sales Goal; and 321,067 shares upon achievement of break-even cash flow in any quarter. |
| (7) | Number of shares beneficially owned includes 6,567,611 shares of unvested restricted stock, 5,979,967 of which have contingent vesting terms. The contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 856,178 shares upon OPAXIO NDA Approval; 1,926,400 shares upon Pix NDA Approval; 1,284,267 shares upon achievement of $50M Sales Goal; 628,855 shares upon achievement of $100M Sales Goal; and 1,284,267 shares upon achievement of break-even cash flow in any quarter. |
| (8) | Number of shares beneficially owned includes 3,287,638 shares of unvested restricted stock, 2,997,983 of which have contigent vesting terms. Of these contingent shares, 4,000 shares will vest if the Company obtains FDA approval of OPAXIO prior to December 31, 2010 and 4,000 will not vest due to the divestiture of Zevalin (such shares would have vested if the Company had obtained a specific annual net sales threshold |
C-171
| for Zevalin prior to December 31, 2010). In addition, the remaining contingent shares will vest if any of the following conditions are met prior to December 31, 2011: 428,089 shares upon OPAXIO NDA Approval; 963,200 shares upon Pix NDA Approval; 642,133 shares upon achievement of $50M Sales Goal; 314,428 shares upon achievement of $100M Sales Goal; and 642,133 shares upon achievement of break-even cash flow in any quarter. |
Please see Chapter 15 for information regarding the value of option grants to CTI’s directors and principal executive officers for the year ended December 31, 2009.
| 17.3 | Description of any equity incentive plan devoted to the Issuer’s employees |
| 17.3.1 | Equity Incentive Plans |
During 2003, shareholders approved the 2003 Equity Incentive Plan, which replaced the 1994 Equity Incentive Plan, or 1994 Plan. The 1994 Plan has since been terminated, except with respect to outstanding awards previously granted thereunder. The 2003 Equity Incentive Plan was amended and restated, renamed as the 2007 Equity Incentive Plan (the “Plan”). Pursuant to the Plan the Company may grant the following types of incentive awards: (1) stock options, including incentive stock options and nonqualified stock options, (2) stock appreciation rights, (3) restricted stock, (4) restricted stock units and (5) cash awards. The Plan is administered by the Compensation Committee of the Board of Directors which has the discretion to determine which employees, consultants and directors shall be granted incentive awards. Options are typically exercisable ratably over a four-year period commencing one year from the date of grant, and expire not more than 10 years from the date of grant. As of September 30, 2010, approximately 39.9 million shares of common stock were available for future grants under the Plan.
| 17.3.2 | Employee Stock Purchase Plan |
CTI maintains an Employee Stock Purchase Plan, or the Purchase Plan, that was approved by the Company’s shareholders and adopted in September 2007 and amended and restated in August 2009. Under the Purchase Plan eligible employees may purchase a limited number of shares of our common stock at 85% of the lower of the subscription date fair market value and the purchase date fair market value. There are two six-month offerings per year. According to the Purchase Plan, CTI issued approximately 42,000 and 8,000 shares to employees in 2009 and 2008, respectively. The Company did not issue any shares under a purchase plan during 2007. There are 1,525,000 shares of common stock authorized under the Purchase Plan and 1,457,357 are reserved for future purchases as of September 30, 2010.
C-172
CHAPTER 18 MAJOR SHAREHOLDERS
| 18.1 | Major Shareholders |
As of the date of submission of this Registration Document, based on information publicly available, the Company is not aware of the any shareholders owning a percentage of shares of common stock (including shares of common stock issuable on conversion or exercise of derivative securities) in the Company’s capital higher than 2% thereof. This declaration takes into account also the selling of shares of Series 7 Preferred Stock and their full conversion into shares of common stock on October 22, 2010.
| 18.2 | Special voting rights |
Not applicable.
| 18.3 | Controlling entities |
As of the date of the Registration Document, there is no shareholder who controls CTI pursuant to art. 93 of Legislative Decree no. 58 of February 24, 1998.
| 18.4 | Arrangements, if any, which may result in a change in the control of the issuer after the publication of the Registration Document |
There are currently no arrangements of which the Issuer is aware that may result in a change in control of CTI after the publication of the Registration Document.
C-173
CHAPTER 19 RELATED PARTY TRANSACTIONS
This chapter contains the information required by International Accounting Standards No. 24, Related Party Disclosures, as well as Item 404 of Regulation S-K under the United States Securities Act of 1933. Information required by Item 404 of Regulation S-K under the Securities Act of 1933, includes descriptions of any transactions since the beginning of the last fiscal year in which the Company was or is to be a participant where the amount involved exceeds $120,000, and in which any related person had or will have a direct or indirect material interest. For purposes of this disclosure, related person is defined as an executive officer, director, 5% or greater shareholder, any nominee for director and any family members of any such persons.
The chart below sets forth the structure of the Company and its subsidiaries as of the date of this Registration Document.
| Cell Therapeutics, Inc.
| ||||||||||||||
|
Systems Medicine LLC
100%
|
CTI Life Sciences Limited
100%
|
CTI Commercial LLC
100%
|
Aequus Biopharma, Inc.
69%
| |||||||||||
All intercompany transactions between the Company and its subsidiaries are eliminated in the Company’s consolidated financial statements and other amounts included in this document and are publicly available in the Company’s public filings. None of the Company’s subsidiaries produce financial statements that are publicly available.
| A. | In the case of termination, the Company has severance agreements with our executive officers that provide benefits for eighteen to twenty-four months. See Chapter 15, Paragraph 15.1, “The amount of remuneration paid (including any contingent or deferred compensation) and benefits in kind granted in the period ended December 31, 2009 to the persons referred to under Chapter 14, Paragraph 14.1 by the Company and its subsidiaries for the services rendered to them under any role” for further disclosure on remuneration of key management personnel. |
| B. | See Chapter 15, Paragraph 15.2, “The total amounts set aside or accrued by the issuer or its subsidiaries in the period ended December 31, 2009 to provide pension, retirement or similar benefits” for discussion on the Company’s post-employment benefit plan for the benefit of the Company’s employees. |
| C. | Mr. Love served in previous years in an executive position and was a consultant in the first quarter of 2008 at Translational Genomics Research Institute (TGen), a non-profit biomedical research institute, and was a consultant in the first quarter of 2008. The Company made payments to TGen in 2008 and 2009 for services related to clinical trials for brostallicin, however the amounts fall within NASDAQ prescribed limits. |
| D. | Dr. Nudelman serves on the Board of Directors of the Hope Heart Institute and Dr. Nudelman’s son, Mark Nudelman, serves as its President and Chief Executive Officer. The Company made a charitable donation to |
C-174
the Hope Heart Institute in 2007, 2008 and 2009, however the amounts fall within NASDAQ prescribed limits.
| E. | Phillip M. Nudelman serves on the Board of Directors of OptiStor Technologies, Inc. (OptiStor). The Company made payments of $0.8 million to OptiStor for hardware and software in 2009 and further payments in 2008 that falled within NASDAQ prescribed limits. |
| F. | The Company owns 4.5% of the equity of DiaKine Therapeutics, Inc., or DiaKine. Louis A. Bianco currently serves on the Board of Directors of DiaKine and Jack W. Singer, M.D. recently resigned from the Board of Directors of DiaKine. In 2005, the Company entered into a license agreement with DiaKine for the exclusive license of Lisofylline material to DiaKine. In connection with the license agreement, the Company also entered into a joint representation letter with DiaKine and a law firm for legal services provided by the law firm with respect to the Lisofylline material. Pursuant to the license agreement, DiaKine agreed to pay all fees of legal services provided by the law firm with respect to the Lisofylline material. Pursuant to the joint representation letter, the Company agreed to be jointly responsible to the law firm with DiaKine for the payment of such fees to the law firm. In 2009, DiaKine failed to pay certain amounts payable to the law firm pursuant to the joint representation letter. In February 2010, the Company severed the joint representation letter with DiaKine and paid the outstanding third-party payables owed to the law firm in the amount of $206,000. In connection, DiaKine issued to the Company an unregistered convertible subordinated note due February 2013 in the amount of $206,000. The note is convertible into equity of DiaKine upon the occurrence of certain events, including certain financings of DiaKine and a sale of DiaKine. |
| G. | In May 2007, the Company formed Aequus Biopharma, Inc., or Aequus, a majority owned subsidiary of which CTI ownership was approximately 69% as of December 31, 2009. The Company entered into a license agreement with Aequus whereby Aequus gained rights to our Genetic Polymer™ technology which Aequus will continue to develop. The Genetic Polymer technology may speed the manufacture, development, and commercialization of follow-on and novel protein-based therapeutics. In May 2007, the Company also entered into an agreement to fund Aequus in exchange for a convertible promissory note that becomes due and payable in five years and earns interest at a rate of 6% per annum. The note can be converted into equity at any time prior to its maturity upon CTI’s demand, or upon other triggering events. The number of shares of Aequus equity securities to be issued upon conversion of this note is equal to the quotient obtained by dividing (i) the outstanding balance of the note by (ii) 100% of the price per share of the equity securities. The Company funded Aequus $0.6 million, $0.3 million and $0.5 million during the years ended December 31, 2009, 2008 and 2007, respectively. In addition, the Company entered into a services agreement to provide certain administrative and research and development services to Aequus. The amounts charged for these services, if unpaid by Aequus within 30 days, will be considered additional principal advanced under the promissory note. CTI’s President and Chief Executive Officer, James A. Bianco, M.D. and our Executive Vice President, Chief Medical Officer, Jack W. Singer, M.D. are both minority shareholders of Aequus, each owning approximately 4.9% of the equity in the company. Additionally, both Dr. Bianco and Dr. Singer are members of Aequus’ board of directors and each have entered into a consulting agreement with Aequus. Additionally, Frederick W. Telling, Ph.D., a member of our board of directors, owns approximately 1% of Aequus and is also a member of Aequus’ board of directors. |
| H. | In July 2007, the Company acquired Systems Medicine, Inc., or SMI, a privately-held oncology company. SMI continues to operate as our wholly-owned subsidiary. Richard L. Love previously owned shares of SMI. His shares were exchanged in July 2007 for shares of our common stock and a contingent right to receive future earn out payments in connection with our acquisition of SMI. The contingent right to future earn out |
C-175
| payments was satisfied by immediate payment to Mr. Love of shares of our common stock in November 2009 and the Company registered those shares and voting agreement. |
| I. | Corey Masten-Legge, a stepson of James A. Bianco, M.D., is employed as a corporate attorney in CTI’s legal department. Mr. Masten-Legge earned approximately $68,000 in base salary and bonus in 2008 and $150,000 in base salary and bonus in 2009. |
Except for the transactions described in this Chapter 19, during the period covered by the historical financial information and up to the date of this Registration Document, the Company has not entered into any other related party transactions subject to public disclosure pursuant to Item 404 of Regulation S-K under the United States Securities Act of 1933, as amended, or according to International Accounting Standards No. 24, Related Party Disclosures.
The cross-reference index at the end of this Chapter 19 discloses, for the related party transactions under letters C), D), E), F), G) and H) above, in which of the following documents (all publicly available at http://investors.celltherapeutics.com) and in which relative pages the information on the relevant transaction is available:
| (i) | Form 10-K filed on March 26, 2008, which includes the audited financial statements for the year ended December 31, 2007 (hereinafter in this Chapter “Financial Statements 2007”). |
| (ii) | Form 10-K filed on March 16, 2009, which includes the audited financial statements for the year ended December 31, 2008 (hereinafter in this Chapter “Financial Statements 2008”). |
| (iii) | Form 10-K filed on February 26, 2010, which includes the audited financial statements for the year ended December 31, 2009 (hereinafter in this Chapter “Financial Statements 2009”). |
| Financial Statements 2007 |
Financial Statements 2008 | Financial Statements 2009 | ||||
| Transactions with Tgen (letter C above) |
p. 149 | p. 153 | ||||
| Transactions with the Hope Heart Institute (letter D above) |
p. 112 | pp. 126 and 149 | p. 153 | |||
| Transactions with OptiStor (letter E above) |
pp. 126 and 149 | p. 153 | ||||
| Licensing agreement with DiaKine Therapeutics, Inc. (letter F above) |
p. 112 | p. 152 | ||||
| Aequus Biopharma project (letter G above) |
p. 69 p. 111 |
p. 71 p. 119 |
p. 152 | |||
| Acquisition of Systems Medicine, Inc. (letter H above) |
p. 69 pp. 83-84 |
p. 71 p. 107 |
p. 152 and 153 | |||
| Employment of Mr. Masten-Legge |
p. 149 | p. 153 | ||||
C-176
CHAPTER 20
FINANCIAL INFORMATION CONCERNING THE ISSUER’S ASSETS AND LIABILITIES, FINANCIAL POSITION AND PROFITS AND LOSSES
Introduction
This chapter is prepared according to Regulation 809/2004 CE and further amended as provided for by Regulation 1787/2006 CE December 4, 2006.
This chapter includes consolidated financial information for the Company for the years ended December 31, 2009, 2008 and 2007 and for the nine month periods ended September 30, 2010 and 2009. The financial information was prepared according to accounting principles generally accepted in the United States (US GAAP).
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. The Company has sustained loss from operations over 2009, incurred an accumulated deficit, and has substantial monetary liabilities in excess of monetary assets as of December 31, 2009. Accordingly the report of the independent auditors of the Company notes that CTI’s ability to satisfy these obligations upon maturity raises substantial doubt about the Company’s ability to continue as a going concern.
On April 15, 2007, the Company affected a one-for-four reverse stock split of its common stock and on August 31, 2008, the Company affected a one-for-ten reverse stock split of its outstanding common stock. All impacted amounts included in the consolidated financial statements and notes thereto have been retroactively adjusted for the stock splits. Impacted amounts include shares of common stock authorized and outstanding, share issuances, shares underlying stock options and warrants, shares reserved and loss per share.
Summary of contents
The following financial statements are present in this chapter 20:
| 1. | Consolidated Balance Sheets as of December 31, 2009, 2008 and 2007 and prepared according to accounting principles generally accepted in the United States (US GAAP) (par.20.1); |
| 2. | Consolidated Statements of Operations for the periods ended December 31, 2009, 2008 and 2007; |
| 3. | Consolidated Statements of Shareholders’ Deficit and Other Comprehensive Loss for the same periods represented in point 1 (par 20.1); |
| 4. | Consolidated Statements of Cash Flows for the periods ended December 31, 2009, 2008 and 2007 (par 20.1); |
| 5. | Consolidated Balance Sheet as of September 30, 2010 prepared according to accounting principles generally accepted in the United States (US GAAP) (par. 20.6.2); |
C-177
| 6. | Consolidated Statements of Operations for the period ended September 30, 2010 and 2009 (par. 20.6.2); and |
| 7. | Consolidated Statements of Cash Flows for the periods ended September 30, 2010 and 2009 (par. 20.6.2). |
| 20.1 | Historical financial information |
Introduction
All financial information presented in this Paragraph has been prepared according to Regulation (EC) No 1606/2002, or if not applicable to a Member State national accounting standards for issuers from the Community.
For third country issuers (such as CTI), such financial information has been prepared according to the international accounting standards adopted pursuant to the procedure of Article 3 of Regulation (EC) No 1606/2002 or to a third country’s national accounting standards equivalent to these standards.
The last three years audited historical financial information had been presented and prepared in a form consistent with that which will be adopted in the issuer’s next published annual financial statements having regard to accounting standards and policies and legislation applicable to such annual financial statements.
The external auditor opinion for the financial statements for the year ended December 31, 2009 is included on the Company’s Annual Report on Form 10-K filed on February 26, 2010, the external auditor opinions for the financial statements for the years ended December 31, 2008 is included in the Company’s Annual Report on Form 10-K, as amended, filed on March 16, 2009 and the external auditor opinions for the financial statements for the years ended December 31, 2007 is included in the Company’s Annual Report on Form 10-K, as amended, filed on March 26, 2008, all of which can be found on the Company’s website at http://investors.celltherapeutics.com.
The external auditor opinions for the Company’s 2009, 2008 and 2007 financial statements and the external auditor limited review for the financial information regarding the six-month period ended June 30, 2010 are also attached to this Registration Document as Appendix A.
| 20.1.1 | Description of the consolidated entity and of Significant Accounting Policies |
Consolidated Entity
The chart below represents the consolidated entity and its wholly owned subsidiaries for the periods presented (in thousands of US dollars):
| December 31, | ||||||||||||||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||||||||||||||
| Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
||||||||||||||||||||||||||||
| Holding |
||||||||||||||||||||||||||||||||||||
| Cell Therapeutics, Inc. |
1,418,382 | 23,772 | 1,187,625 | (87,802 | ) | 979,123 | 37,279 | |||||||||||||||||||||||||||||
| Cell Therapeutics Europe S.r.l (1) |
— | (6,767 | ) | 100 | % | — | 2,232 | 100 | % | 142,406 | 38,791 | 100 | % | |||||||||||||||||||||||
C-178
| December 31, | ||||||||||||||||||||||||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||||||||||||||||||||||
| Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
Capital Stock |
Share- holders’ Equity (Deficit) |
Owned % |
||||||||||||||||||||||||||||
| Subsidiaries |
||||||||||||||||||||||||||||||||||||
| Systems Medicine, LLC (2) |
20,499 | (12,876 | ) | 100 | % | 20,499 | (10,913 | ) | 100 | % | 20,499 | (6,440 | ) | 100 | % | |||||||||||||||||||||
| CTI Corporate Development, Inc. (3) |
— | — | N/A | — | (13,823 | ) | 100 | % | — | (13,823 | ) | 100 | % | |||||||||||||||||||||||
| CTI Commercial LLC (4) |
— | (80 | ) | 100 | % | — | — | 100 | % | |||||||||||||||||||||||||||
| Aequus Biopharma, Inc. (5) |
549 | (1,705 | ) | 69 | % | 650 | (1,256 | ) | 69 | % | 250 | (793 | ) | 69 | % | |||||||||||||||||||||
| CTI Technologies, Inc.(6) |
— | — | N/A | — | — | N/A | — | — | N/A | |||||||||||||||||||||||||||
| Cell Therapeutics Ireland Holding Limited (7) |
— | — | N/A | — | — | N/A | — | — | N/A | |||||||||||||||||||||||||||
| CTI Life Sciences Limited (8) |
28 | (381 | ) | 100 | % | — | — | N/A | — | — | N/A | |||||||||||||||||||||||||
| (1) | Branch of Cell Therapeutics, Inc. |
| (2) | Acquired in July 2007 |
| (3) | Dissolved in 2009 |
| (4) | Formed in July 2008 |
| (5) | Formed in May 2007 |
| (6) | Dissolved in 2007 |
| (7) | Dissolved in 2006 |
| (8) | Acquired in March 2009 |
The registered and the principal administrative offices of the Issuer and its subsidiaries are located as follows:
Cell Therapeutics, Inc.: 501 Elliott Avenue West Ste 400, Seattle, WA 98119
Cell Therapeutics Europe (branch of Cell Therapeutics, Inc.): Via Valentino Mazzola n. 18, 00142 Rome, Italy
Systems Medicine, LLC: 4320 N. Campbell Ave., Ste. 226, Tucson, AZ 85718
Aequus Biopharma, Inc.: 11042 Forest Lane NE, Bainbridge Island, WA 98110
CTI Commercial, LLC: 3370 Alpine Lily, Las Vegas, NV 89141
CTI Technologies, Inc.: 101 Convention Center Drive Ste 850, Las Vegas, NV 89109
Cell Therapeutics Ireland Holding Limited (prior to dissolution): 38 Main Street, Swords, Co. Dublin, Ireland
CTI Life Sciences Limited: BioPark, Broadwater Road, Welwyn Garden City, Hertfordshire, AL7 3AX, United Kingdom
CTI Corporate Development (prior to liquidation): 501 Elliott Avenue West Ste 400, Seattle, WA 98119
C-179
Principles of Consolidation and Significant Accounting Policies
Please refer to Paragraph 20.3 of this Chapter 20 for relevant information.
| 20.1.2 | CONSOLIDATED BALANCE SHEETS, CONSOLIDATED STATEMENTS OF OPERATIONS, CONSOLIDATED STATEMENTS OF CASH FLOWS AND CONSOLIDATED STATEMENTS OF SHAREHOLDERS EQUITY (DEFICIT) AND OTHER COMPREHENSIVE LOSS AS OF DECEMBER 31, 2009, 2008 AND 2007. |
CELL THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands of US dollars, except share amounts)
| December 31, | ||||||||||||||||
| Notes | 2009 | 2008 | 2007 | |||||||||||||
| ASSETS |
A | |||||||||||||||
| Current assets: |
||||||||||||||||
| Cash and cash equivalents |
1 | $ | 37,811 | $ | 10,072 | $ | 15,798 | |||||||||
| Restricted cash |
2 | — | 6,640 | — | ||||||||||||
| Securities available—for—sale |
3 | — | 599 | 2,548 | ||||||||||||
| Interest receivable |
— | 9 | 46 | |||||||||||||
| Accounts receivable, net |
4 | — | 982 | 51 | ||||||||||||
| Inventory |
5 | — | — | 290 | ||||||||||||
| Note receivable from joint venture |
6 | — | 7,500 | |||||||||||||
| Prepaid expenses and other current assets |
7 | 4,354 | 2,359 | 3,904 | ||||||||||||
| Total current assets |
42,165 | 28,161 | 22,637 | |||||||||||||
| Property and equipment, net |
8 | 3,430 | 4,324 | 6,025 | ||||||||||||
| Goodwill |
9 | 17,064 | 17,064 | 17,064 | ||||||||||||
| Other intangibles, net |
9 | — | — | 15,957 | ||||||||||||
| Investment in joint venture |
10 | — | 5,830 | |||||||||||||
| Other assets |
11 | 6,936 | 8,864 | 11,830 | ||||||||||||
| Total assets |
$ | 69,595 | $ | 64,243 | $ | 73,513 | ||||||||||
| LIABILITIES AND SHAREHOLDERS’ DEFICIT |
B | |||||||||||||||
| Current liabilities: |
||||||||||||||||
| Accounts payable |
1 | 7,297 | 9,327 | 6,595 | ||||||||||||
| Accrued expenses |
2 | 14,807 | 29,308 | 26,034 | ||||||||||||
| Warrant liability |
— | 2,830 | — | |||||||||||||
| Current portion of deferred revenue |
3 | 80 | 80 | 80 | ||||||||||||
| Current portion of long-term obligations |
4 | 1,312 | 757 | 1,020 | ||||||||||||
| Current portion of derivative liability |
5 | — | — | — | ||||||||||||
| Current portion of convertible senior subordinated notes |
5 | — | — | 16,907 | ||||||||||||
| Current portion of convertible subordinated notes |
5 | — | — | 2,910 | ||||||||||||
| 4% convertible senior subordinated notes |
40,363 | — | — | |||||||||||||
| Total current liabilities |
63,859 | 42,302 | 53,546 | |||||||||||||
| Deferred revenue, less current portion |
3 | 239 | 319 | 398 | ||||||||||||
C-180
| December 31, | ||||||||||||||||
| Notes | 2009 | 2008 | 2007 | |||||||||||||
| Long-term obligations, less current portion |
4 | 1,861 | 2,907 | 9,879 | ||||||||||||
| 10% convertible senior notes due 2011 |
5 | — | 19,784 | — | ||||||||||||
| 9% convertible senior notes |
5 | — | 4,104 | — | ||||||||||||
| 7.5% convertible senior notes |
5 | 10,102 | 32,601 | 32,220 | ||||||||||||
| 6.75% convertible senior notes |
5 | — | 6,926 | 6,922 | ||||||||||||
| 5.75% convertible senior notes |
5 | 11,677 | 23,808 | 23,287 | ||||||||||||
| Convertible senior subordinated notes |
5 | — | 55,150 | 55,150 | ||||||||||||
| Convertible subordinated notes |
5 | — | — | — | ||||||||||||
| Total liabilities |
87,738 | 187,901 | 181,402 | |||||||||||||
| Commitments and contingencies |
||||||||||||||||
| Minority interest in subsidiary |
— | — | — | |||||||||||||
| Preferred stock, no par value: |
6 | |||||||||||||||
| Authorized shares—10,000,000 |
||||||||||||||||
| Series A 3% Convertible Preferred Stock, $1,000 stated value, 20,000 shares designated; 0, 550, and 6,850 shares issued and outstanding at December 31, 2009, 2008 and 2007, respectively |
— | 417 | 5,188 | |||||||||||||
| Series B 3% Convertible Preferred Stock, $1,000 stated value, 37,200 shares designated; 0, 5,218 and 15,380 and 0 shares issued and outstanding at December 31, 2009, 2008 and 2007, respectively |
— | 4,031 | 11,881 | |||||||||||||
| Series C 3% Convertible Preferred Stock, $1,000 stated value, 20,250 shares designated; 0, 4,284 and 8,284 shares issued and outstanding at December31, 2009, 2008 and 2007, respectively |
— | 3,221 | 6,229 | |||||||||||||
| Series D 7% Convertible Preferred Stock, $1,000 stated value, 6,500 shares designated; 0, 1,000 and 4,000 shares issued and outstanding at December, 2009, 2008 and 2007, respectively |
— | 734 | 2,938 | |||||||||||||
| Common stock purchase warrants |
626 | — | — | |||||||||||||
| Shareholders’ deficit: |
7 | |||||||||||||||
| Common Stock, NO PAR VALUE: |
||||||||||||||||
| Authorized shares – 800,000,000 |
||||||||||||||||
| Issued and outstanding shares – 590,282,575, 186,411,922 and 6,244,423 at December 31, 2009, 2008 and 2007, respectively |
1,418,931 | 1,188,071 | 979,295 | |||||||||||||
| Deferred stock-based compensation |
— | — | — | |||||||||||||
| Accumulated other comprehensive income (loss) |
(8,412 | ) | (7,812 | ) | (4,007 | ) | ||||||||||
| Accumulated deficit |
(1,429,083 | ) | (1,312,320 | ) | (1,109,413 | ) | ||||||||||
| Total CTI shareholders’ deficit |
(18,564 | ) | (132,061 | ) | (134,125 | ) | ||||||||||
| Noncontrolling interest |
(205 | ) | — | — | ||||||||||||
| Total shareholders’ deficit |
(18,769 | ) | (132,061 | ) | (134,125 | ) | ||||||||||
| Total liabilities and shareholders’ deficit |
$ | 69,595 | $ | 64,243 | $ | 73,513 | ||||||||||
C-181
CELL THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands of US dollars, except per share amounts)
| Year Ended December 31, | ||||||||||||||||
| Notes | 2009 | 2008 | 2007 | |||||||||||||
| (In thousands of US dollars) | ||||||||||||||||
| Revenues: |
A | |||||||||||||||
| Product sales |
1 | $ | — | $ | 11,352 | $ | 47 | |||||||||
| License and contract revenue |
2 | 80 | 80 | 80 | ||||||||||||
| Total revenues |
80 | 11,432 | 127 | |||||||||||||
| Operating expenses: |
B | |||||||||||||||
| Cost of product sold |
3 | — | 3,244 | 49 | ||||||||||||
| Research and development |
4 | 30,179 | 51,614 | 72,019 | ||||||||||||
| Selling, general and administrative |
5 | 57,725 | 41,607 | 35,517 | ||||||||||||
| Amortization of purchased intangibles |
6 | — | 1,658 | 913 | ||||||||||||
| Restructuring charges and related gain on sale of assets, net |
3,979 | — | — | |||||||||||||
| Gain on sale of Zevalin |
7 | — | (9,444 | ) | — | |||||||||||
| Gain on sale of investment in joint venture |
(10,244 | ) | — | — | ||||||||||||
| Acquired in-process research and development |
8 | — | 36 | 24,615 | ||||||||||||
| Total operating expenses |
81,639 | 88,715 | 133,113 | |||||||||||||
| Loss from operations |
(81,559 | ) | (77,283 | ) | (132,986 | ) | ||||||||||
| Other income (expense): |
C | |||||||||||||||
| Investment and other income |
9 | 133 | 549 | 2,430 | ||||||||||||
| Interest expense |
10 | (4,806 | ) | (8,559 | ) | (8,237 | ) | |||||||||
| Amortization of debt discount and issuance costs |
11 | (5,788 | ) | (66,530 | ) | (4,280 | ) | |||||||||
| Foreign exchange gain |
12 | 33 | 3,637 | 4,657 | ||||||||||||
| Make-whole interest expense |
13 | (6,345 | ) | (70,243 | ) | (2,310 | ) | |||||||||
| Gain on derivative liabilities |
14 | 7,218 | 69,739 | 3,672 | ||||||||||||
| Gain (loss) on exchange of convertible notes |
15 | 7,381 | (25,103 | ) | (972 | ) | ||||||||||
| Write-off of financing arrangement costs |
16 | — | (2,846 | ) | — | |||||||||||
| Equity loss from investment in joint venture |
17 | (1,204 | ) | (123 | ) | — | ||||||||||
| Miestone Modification expense |
(6,000 | ) | — | — | ||||||||||||
| Settlement expense |
18 | (4,710 | ) | (3,393 | ) | (160 | ) | |||||||||
| Other expense, net |
(14,088 | ) | (102,872 | ) | (5,200 | ) | ||||||||||
| Loss before minority interest |
(95,647 | ) | (180,155 | ) | (138,186 | ) | ||||||||||
| Minority interest in net loss of subsidiary |
D | 252 | 126 | 78 | ||||||||||||
| Net loss |
(95,395 | ) | (180,029 | ) | (138,108 | ) | ||||||||||
| Gain on restructuring of preferred stock |
2,116 | — | — | |||||||||||||
| Preferred stock dividends |
(24 | ) | (662 | ) | (648 | ) | ||||||||||
C-182
| Year Ended December 31, | ||||||||||||||||
| Notes | 2009 | 2008 | 2007 | |||||||||||||
| (In thousands of US dollars) | ||||||||||||||||
| Deemed dividends on preferred stock |
(23,460 | ) | (22,216 | ) | (9,549 | ) | ||||||||||
| Net loss attributable to common shareholders |
$ | (116,763 | ) | $ | (202,907 | ) | $ | (148,305 | ) | |||||||
| Basic and diluted net loss per share |
$ | (0.25 | ) | $ | (7.00 | ) | $ | (32.75 | ) | |||||||
| Shares used in calculation and diluted net loss per common shares |
458,356 | 28,967 | 4,529 | |||||||||||||
CELL THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ DEFICIT AND OTHER COMPREHENSIVE LOSS
(In thousands)
| Accumulated Other |
Total | |||||||||||||||||||||||
| Common Stock | Accumulated Deficit |
Comprehensive Income/(Loss) |
Noncontrolling Interest |
Shareholders’ (Deficit) |
||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||
| Balance at December 31, 2006 |
3,639 | $ | 860,691 | $ | (961,108 | ) | $ | (1,187 | ) | $ | — | $ | (101,604 | ) | ||||||||||
| Conversion of convertible preferred stock to common stock |
924 | 37,648 | — | — | — | 37,648 | ||||||||||||||||||
| Proceeds from issuance of warrants in connection with issuance of convertible preferred stock, net |
— | 14,526 | — | — | — | 14,526 | ||||||||||||||||||
| Value of beneficial conversion feature of preferred stock |
— | 9,549 | — | — | — | 9,549 | ||||||||||||||||||
| Conversion of 7.5% convertible senior notes to common stock |
183 | 15,294 | — | — | — | 15,294 | ||||||||||||||||||
| Issuance of common stock in connection with SMI acquisition |
421 | 19,872 | — | — | — | 19,872 | ||||||||||||||||||
| Issuance of common stock in connection with exchange of 5.75% senior subordinated and subordinated notes |
||||||||||||||||||||||||
| 546 | 13,704 | — | — | — | 13,704 | |||||||||||||||||||
| Proceeds from issuance of common stock and warrants, net |
||||||||||||||||||||||||
| 347 | 6,537 | — | — | — | 6,537 | |||||||||||||||||||
| Equity-based compensation |
185 | 1,588 | — | — | — | 1,588 | ||||||||||||||||||
C-183
| Other |
(1 | ) | (114 | ) | — | — | — | (114 | ) | |||||||||||||||
| Dividends on preferred stock |
— | — | (648 | ) | — | — | (648 | ) | ||||||||||||||||
| Deemed dividends on preferred stock |
— | — | (9,549 | ) | — | — | (9,549 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | (2,807 | ) | — | (2,807 | ) | ||||||||||||||||
| Unrealized losses on securities available-for-sale |
— | — | — | (13 | ) | — | (13 | ) | ||||||||||||||||
| Net loss for the year ended December 31, 2007 |
— | — | (138,108 | ) | — | — | (138,108 | ) | ||||||||||||||||
| Comprehensive loss |
(140,928 | ) | ||||||||||||||||||||||
| Balance at December 31, 2007 |
6,244 | $ | 979,295 | $ | (1,109,413 | ) | $ | (4,007 | ) | $ | — | $ | (134,125 | ) | ||||||||||
| Conversion of convertible preferred stock to common stock |
||||||||||||||||||||||||
| 463 | 17,832 | — | — | — | 17,832 | |||||||||||||||||||
| Conversion of 18.33% convertible senior notes to common stock |
3,576 | 28,250 | — | — | — | 28,250 | ||||||||||||||||||
| Conversion of 15.5% convertible senior notes to common stock |
11,189 | 14,210 | — | — | — | 14,210 | ||||||||||||||||||
| Conversion of 13.5% convertible senior notes to common stock |
3,494 | 27,600 | — | — | — | 27,600 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2012 to common stock |
7,087 | 9,000 | — | — | — | 9,000 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
106,944 | 14,651 | — | — | — | 14,651 | ||||||||||||||||||
| Conversion of 9.66% convertible senior notes to common stock |
41,316 | 15,700 | — | — | — | 15,700 | ||||||||||||||||||
| Conversion of 9% convertible senior notes to common stock |
2,895 | 40,820 | — | — | — | 40,820 | ||||||||||||||||||
| Conversion of 5.75% convertible senior notes to common stock |
8 | 250 | — | — | — | 250 | ||||||||||||||||||
| Issuance of common stock in connection with with exchange of 5.75% convertible subordinated and senior subordinated notes |
685 | 11,133 | — | — | — | 11,133 | ||||||||||||||||||
| Issuance of common |
C-184
| stock in connection with financing agreement |
80 | 1,183 | — | — | — | 1,183 | ||||||||||||||||||
| Issuance of common stock under the Midsummer Equity Line |
1,545 | 4,351 | — | — | — | 4,351 | ||||||||||||||||||
| Premium on 15% convertible senior notes due to exercise of Series B warrant |
— | 11,158 | — | — | — | 11,158 | ||||||||||||||||||
| Issuance of warrants in connection with the 9% convertible senior notes |
— | 3,358 | — | — | — | 3,358 | ||||||||||||||||||
| Issuance of warrants in connection with the 13.5%, 15% notes to common stock and 18.33% convertible senior notes |
— | 7,491 | — | — | — | 7,491 | ||||||||||||||||||
| Repurchase of warrants in connection with the issuance of notes to common stock 13.5% and 18.33% notes |
— | (2,042 | ) | — | — | — | (2,042 | ) | ||||||||||||||||
| Equity-based compensation |
878 | 3,995 | — | — | — | 3,995 | ||||||||||||||||||
| Noncontrolling interest |
— | (126 | ) | — | — | — | (126 | ) | ||||||||||||||||
| Other |
8 | (38 | ) | — | — | — | (38 | ) | ||||||||||||||||
| Dividends on preferred stock |
— | — | (662 | ) | — | — | (662 | ) | ||||||||||||||||
| Deemed dividends on preferred stock |
— | — | (22,216 | ) | — | — | (22,216 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | (3,801 | ) | — | (3,801 | ) | ||||||||||||||||
| Unrealized losses on securities available-for-sale |
— | — | — | (4 | ) | — | (4 | ) | ||||||||||||||||
| Net loss for the year ended December 31, 2008 |
— | — | (180,029 | ) | — | — | (180,029 | ) | ||||||||||||||||
| Comprehensive loss |
(183,834 | ) | ||||||||||||||||||||||
| Balance at December 31, 2008 |
186,412 | $ | 1,188,071 | $ | (1,312,320 | ) | $ | (7,812 | ) | $ | — | $ | (132,061 | ) | ||||||||||
| Issuance of common stock and warrants |
49,732 | 59,233 | — | — | — | 59,233 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
131,387 | 18,000 | — | — | — | 18,000 | ||||||||||||||||||
| Conversion of 9% convertible senior notes to common stock |
372 | 5,250 | — | — | — | 5,250 | ||||||||||||||||||
| Conversion of Series F |
47,871 | 3,866 | — | — | — | 3,866 | ||||||||||||||||||
C-185
| preferred stock to common stock |
||||||||||||||||||||||||
| Conversion of Series 1 preferred stock to common stock |
66,667 | 18,537 | — | — | — | 18,537 | ||||||||||||||||||
| Conversion of Series 2 preferred stock to common stock |
18,853 | 27,796 | — | — | — | 27,796 | ||||||||||||||||||
| Value of beneficial conversion features related to Series 1 and 2 preferred stock |
— | 13,194 | — | — | — | 13,194 | ||||||||||||||||||
| Issuance of warrants in connection with Series 2 preferred stock |
— | 6,138 | — | — | — | 6,138 | ||||||||||||||||||
| Exercise of Class A warrants |
9,184 | 5,222 | — | — | — | 5,222 | ||||||||||||||||||
| Exercise of Class B warrants |
10,378 | 5,732 | — | — | — | 5,732 | ||||||||||||||||||
| Issuance of common stock in exchange for convertible notes |
27,535 | 39,523 | — | — | — | 39,523 | ||||||||||||||||||
| Issuance of common stock in connection with Series A preferred stock settlement |
4,000 | 509 | — | — | — | 509 | ||||||||||||||||||
| Issuance of common stock in exchange for milestone modification |
5,607 | 6,000 | — | — | — | 6,000 | ||||||||||||||||||
| Conversion or exchange of Series A, B and D convertible preferred stock to common stock |
3,786 | 4,288 | — | — | — | 4,288 | ||||||||||||||||||
| Reacquisition of BCF in connection with exchange of Series A, B and C convertible preferred stock for Series F preferred stock |
— | (961 | ) | — | — | — | (961 | ) | ||||||||||||||||
| Equity-based compensation |
33,821 | 24,937 | — | — | — | 24,937 | ||||||||||||||||||
| Repurchase of shares in connection with taxes on restricted stock vesting |
(5,364 | ) | (6,394 | ) | — | — | — | (6,394 | ) | |||||||||||||||
| Employee stock purchase plan |
42 | 36 | — | — | — | 36 | ||||||||||||||||||
| Noncontrolling interest |
— | (47 | ) | — | — | (205 | ) | (252 | ) | |||||||||||||||
| Dividends on preferred stock |
— | 1 | (24 | ) | — | — | (23 | ) | ||||||||||||||||
| Gain on restructuring of preferred stock |
2,116 | — | — | 2,116 | ||||||||||||||||||||
| Deemed dividends on preferred stock |
— | — | (23,460 | ) | — | — | (23,460 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | (601 | ) | — | (601 | ) | ||||||||||||||||
C-186
| Unrealized gains on securities available-for-sale |
— | — | — | 1 | — | 1 | ||||||||||||||||||
| Net loss for the year ended December 31, 2009 |
— | — | (95,395 | ) | — | — | (95,395 | ) | ||||||||||||||||
| Comprehensive loss |
(95,995 | ) | ||||||||||||||||||||||
| Balance at December 31, 2009 |
590,283 | $ | 1,418,931 | $ | (1,429,083 | ) | $ | (8,412 | ) | $ | (205 | ) | $ | (18,769 | ) | |||||||||
CELL THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands of US dollars)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (95,395 | ) | $ | (180,029 | ) | $ | (138,108 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Non-cash interest expense |
5,788 | 66,530 | 4,280 | |||||||||
| Non-cash gain on derivative liabilities |
(7,218 | ) | (69,739 | ) | (3,672 | ) | ||||||
| Non-cash milestone modification expense |
6.000 | — | — | |||||||||
| Gain on disposition of Zevalin to the JV |
— | (9,444 | ) | — | ||||||||
| Gain on sale of equity investment in joint venture |
(10,244 | ) | — | — | ||||||||
| (Gain) loss on exchange of convertible notes |
(7,381 | ) | 25,103 | 972 | ||||||||
| Acquired in-process research and development |
— | 36 | 24,615 | |||||||||
| Depreciation and amortization |
1,771 | 5,228 | 4,955 | |||||||||
| Equity-based compensation expense |
24,937 | 3,995 | 1,588 | |||||||||
| Equity loss from investment in joint venture |
1,204 | 123 | — | |||||||||
| Noncontrolling interest |
(252 | ) | — | — | ||||||||
| Other |
(487 | ) | (229 | ) | (512 | ) | ||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Restricted cash |
6,640 | 71,608 | — | |||||||||
| Interest receivable |
9 | 37 | 524 | |||||||||
| Accounts receivable, net |
982 | (932 | ) | (51 | ) | |||||||
| Inventory |
— | 291 | (290 | ) | ||||||||
| Prepaid expenses and other current assets |
(2,649 | ) | 1,438 | 6,431 | ||||||||
C-187
| Other assets |
519 | 2,801 | (1,216 | ) | ||||||||
| Accounts payable |
(1,484 | ) | 2,786 | 4,297 | ||||||||
| Accrued expenses |
(10,750 | ) | 779 | (4,961 | ) | |||||||
| Other liabilities |
(176 | ) | (589 | ) | (2,470 | ) | ||||||
| Total adjustments |
7,209 | 99,822 | 34,490 | |||||||||
| Net cash used in operating activities |
(88,186 | ) | (80,207 | ) | (103,618 | ) | ||||||
| Investing activities |
||||||||||||
| Cash received for disposition of Zevalin to joint venture, net |
6,844 | 6,754 | — | |||||||||
| Proceeds received from sale of investment in joint venture, net |
14,987 | — | — | |||||||||
| Cash paid for acquisition of Zevalin |
— | (542 | ) | (11,735 | ) | |||||||
| Cash acquired in acquisition of Systems Medicine, Inc., net |
— | — | 555 | |||||||||
| Purchases of securities available-for-sale |
— | (10,721 | ) | (36,463 | ) | |||||||
| Proceeds from sales of securities available-for-sale |
— | 11,550 | 48,431 | |||||||||
| Proceeds from maturities of securities available-for-sale |
600 | 1,074 | 22,442 | |||||||||
| Investment in joint venture |
— | (1,800 | ) | — | ||||||||
| Purchases of property and equipment |
(1,478 | ) | (1,907 | ) | (1,753 | ) | ||||||
| Proceeds from sales of property and equipment |
887 | — | — | |||||||||
| Net cash provided by investing activities |
21,840 | 4,408 | 21,477 | |||||||||
| Financing activities |
||||||||||||
| Proceeds from issuance of Series 1 preferred stock, net of issuance costs |
18,745 | — | — | |||||||||
| Proceeds from issuance of Series 2 preferred stock, net of issuance costs |
28,430 | — | — | |||||||||
| Proceeds from issuance of common stock and warrants, net of issuance costs |
59,233 | 5,080 | 7,007 | |||||||||
| Proceeds from exercise of Class A warrants |
3,765 | — | — | |||||||||
| Proceeds from exercise of Class B warrants |
4,255 | — | — | |||||||||
| Cash paid for the exchange of convertible notes, net of transaction costs |
(9,965 | ) | — | — | ||||||||
| Cash paid for the repurchase of shares in connection with taxes on restricted stock vesting |
(6,394 | ) | ||||||||||
| Payment of deemed dividends on conversion of preferred stock |
(3,000 | ) | (18,149 | ) | — | |||||||
| Proceeds from issuance of 13.5% convertible senior notes and Series E preferred stock, net of exchange of 9% convertible senior notes and issuance costs |
— | 56,069 | — | |||||||||
| Restricted cash from issuance of 13.5% convertible senior notes |
— | (36,456 | ) | — | ||||||||
| Proceeds from issuance of 9% convertible senior notes, net of issuance costs |
— | 49,317 | — | |||||||||
| Restricted cash from issuance of 9% convertible senior notes |
— | (13,947 | ) | — | ||||||||
| Release of restricted cash in connection with exchange of 9% convertible senior notes |
— | 1,420 | — | |||||||||
| Proceeds from issuance of 15% convertible senior notes, net of issuance costs |
— | 21,794 | — | |||||||||
| Restricted cash form issuance of 15% convertible senior notes |
— | (10,350 | ) | — | ||||||||
| Proceeds from issuance of 18.33% convertible senior notes, net of repurchase of 13.5% convertible senior note and issuance costs |
— | 26,226 | — | |||||||||
| Restricted cash from issuance of 18.33% convertible senior notes |
— | (24,471 | ) | — | ||||||||
| Release of restricted cash in connection with repurchase of 13.5% convertible senior notes |
— | 6,525 | — | |||||||||
C-188
| Proceeds from issuance of 10% convertible senior note due 2012, net of issuance costs |
— | 8,635 | — | |||||||||
| Restricted cash from issuance of 10% convertible senior notes due 2012 |
— | (3,600 | ) | — | ||||||||
| Proceeds from issuance of 15.5% convertible senior note, net of issuance costs |
— | 13,863 | — | |||||||||
| Restricted cash from issuance of 15.5% convertible senior notes |
— | (8,811 | ) | — | ||||||||
| Proceeds from issuance of 9.66% convertible senior notes, net of repurchase of 15% convertible senior note and issuance costs |
— | 6,053 | — | |||||||||
| Restricted cash from issuance of 9.66% convertible senior notes |
— | (7,158 | ) | — | ||||||||
| Proceeds from issuance of 10% convertible senior notes due 2011, net of repurchase of 9.66%, 15% and 18.33% convertible senior note and issuance costs |
— | 3,252 | — | |||||||||
| Restricted cash from issuance of 10% convertible senior notes due 2011 |
— | (9,795 | ) | — | ||||||||
| Release of restricted cash in connection with repurchase of 9.66% convertible senior notes |
— | 2,553 | — | |||||||||
| Release of restricted cash in connection with repurchase of 15% convertible senior notes |
— | 10,043 | — | |||||||||
| Release of restricted cash in connection with repurchase of 18.33% convertible senior notes |
— | 8,224 | — | |||||||||
| Repayment of 5.75% convertible subordinated and senior subordinated notes |
— | (10,724 | ) | — | ||||||||
| Transaction costs related to exchange of convertible subordinated and senior subordinated notes |
— | (304 | ) | — | ||||||||
| Proceeds from issuance of Series A 3% convertible preferred stock and warrants, net |
— | — | 18,607 | |||||||||
| Proceeds from issuance of Series B 3% convertible preferred stock and warrants, net |
— | — | 34,836 | |||||||||
| Proceeds from issuance of Series C 3% convertible preferred stock and warrants, net |
— | — | 18,938 | |||||||||
| Proceeds from issuance of Series D 7% convertible preferred stock and warrants, net |
— | — | 6,073 | |||||||||
| Payment of additional offering costs related to December 2007 issuance of common stock and warrants |
— | (473 | ) | — | ||||||||
| Payment of dividends on preferred stock |
(111 | ) | (708 | ) | (395 | ) | ||||||
| Repayment of long-term obligations |
(154 | ) | (343 | ) | (429 | ) | ||||||
| Other |
(29 | ) | (39 | ) | 63 | |||||||
| Net cash provided by financing activities |
94,775 | 73,726 | 84,700 | |||||||||
| Effect of exchange rate changes on cash and cash equivalents |
(690 | ) | (3,653 | ) | (3,890 | ) | ||||||
| Net decrease in cash and cash equivalents |
27,739 | (5,726 | ) | (1,331 | ) | |||||||
| Cash and cash equivalents at beginning of year |
10,072 | 15,798 | 17,129 | |||||||||
| Cash and cash equivalents at end of year |
$ | 37,811 | $ | 10,072 | $ | 15,798 | ||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid during the period for interest |
$ | 12,047 | $ | 77,499 | $ | 10,759 | ||||||
| Cash paid for taxes |
$ | — | $ | — | $ | — | ||||||
| Supplemental disclosure of noncash financing and investing activities |
||||||||||||
| Exchange of Series A 3% convertible preferred stock for Series F preferred stock |
$ | 151 | $ | — | $ | — | ||||||
| Exchange of Series B 3% convertible preferred stock for Series F preferred stock |
$ | 1,713 | $ | — | $ | — | ||||||
| Exchange of Series C 3% convertible preferred stock for Series F preferred stock |
$ | 3,221 | $ | — | $ | — | ||||||
| Issuance of Series F preferred stock for Series A, B and C convertible preferred stock |
$ | 3,931 | $ | — | $ | — | ||||||
C-189
| Conversion of Series F preferred stock to common stock |
$ | 3,866 | $ | — | $ | — | ||||||
| Conversion of Series 1 preferred stock to common stock |
$ | 18,537 | $ | — | $ | — | ||||||
| Conversion of Series 2 preferred stock to common stock |
$ | 27,796 | $ | — | $ | — | ||||||
| Issuance of common stock in exchange for convertible notes |
$ | 35,193 | $ | — | $ | — | ||||||
| Issuance of common stock in exchange for milestone modification |
$ | 6,000 | $ | — | $ | — | ||||||
| Conversion of series A 3% convertible preferred stock to common stock |
$ | — | $ | 4,771 | $ | 9,959 | ||||||
| Conversion of series B 3% convertible preferred stock to common stock |
$ | 2,317 | $ | 7,850 | $ | 16,855 | ||||||
| Conversion of series C 3% convertible preferred stock to common stock |
$ | — | $ | 3,008 | $ | 8,998 | ||||||
| Conversion of series D 7% convertible preferred stock to common stock |
$ | — | $ | 2,203 | $ | 1,836 | ||||||
| Conversion of series E 13.5% convertible preferred stock to 13.5% convertible senior notes |
$ | — | $ | 9,118 | $ | — | ||||||
| Issuance of common stock in exchange for Series A 3% convertible preferred stock |
$ | 688 | $ | — | $ | — | ||||||
| Issuance of common stock in exchange for Series D 7% convertible preferred stock |
$ | 1,793 | $ | — | $ | — | ||||||
| Conversion of 9% convertible senior notes to common stock |
$ | 5,250 | $ | 40,820 | $ | — | ||||||
| Conversion of 18.33% convertible senior notes to common stock |
$ | — | $ | 28,250 | $ | — | ||||||
| Conversion of 15.5% convertible senior notes to common stock |
$ | — | $ | 14,211 | $ | — | ||||||
| Conversion of 13.5% convertible senior notes to common stock |
$ | — | $ | 27,600 | $ | — | ||||||
| Conversion of 10% convertible senior notes due 2012 to common stock |
$ | — | $ | 9,000 | $ | — | ||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
$ | 18,000 | $ | 14,651 | $ | — | ||||||
| Conversion of 9.66% convertible senior notes to common stock |
$ | — | $ | 15,700 | $ | — | ||||||
| Conversion of 7.5% convertible senior notes to common stock |
$ | — | $ | — | $ | 15,294 | ||||||
| Conversion of 5.75% convertible senior notes to common stock |
$ | — | $ | 250 | $ | — | ||||||
| Issuance of common stock for acquisition of Systems Medicine, Inc. |
$ | — | $ | — | $ | 19,872 | ||||||
| Extinguishment of 5.75% convertible senior subordinated notes in exchange for common stock |
$ | — | $ | 8,943 | $ | — | ||||||
| Extinguishment of 5.75% convertible subordinated notes in exchange for common stock |
$ | — | $ | 150 | $ | — | ||||||
| Issuance of common stock in exchange for 5.75% convertible senior subordinated and convertible subordinated notes |
$ | — | $ | 11,437 | $ | 13,704 | ||||||
| Extinguishment of 5.75% convertible senior subordinated notes in exchange for 5.75% convertible senior notes and common stock |
$ | — | $ | — | $ | 10,500 | ||||||
| Extinguishment of 5.75% convertible subordinated notes in exchange for 5.75% convertible senior notes and common stock |
$ | — | $ | — | $ | 25,580 | ||||||
| Issuance of 5.75% convertible senior notes in exchange for 5.75% convertible senior subordinated and convertible subordinated notes |
$ | — | $ | — | $ | 23,250 | ||||||
| 20.1.3 | NOTES TO CONSOLIDATED FINANCIAL STATEMENTS |
| A. | ASSETS |
| 1. | Cash and cash equivalents |
The balance consists of the following as of December 31 (in thousands of US dollars):
C-190
| Cash and cash equivalents (in thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | 37,407 | $ | 9,626 | $ | 9,677 | ||||||
| Subsidiaries |
404 | 446 | 6,121 | |||||||||
| Total cash and cash equivalents |
$ | 37,811 | $ | 10,072 | $ | 15,798 | ||||||
Please refer to the paragraph 10.2.1 “Sources, amounts and description of the Company’s cash flows for the years ended December 31, 2007, 2008 and 2009”of this Registration Document for more information about the evolution of the item cash and cash equivalents.
| 2. | Restricted Cash |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Restricted Cash (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | 6,640 | $ | — | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total restricted cash |
$ | — | 6,640 | $ | — | |||||||
The balance of restricted cash at December 31, 2008 represents $6.6 million of cash held in escrow to fund the potential make-whole payments on certain of our convertible senior notes.
| 3. | Securities Available-for-Sale |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Securities Available-for-Sale (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | 599 | $ | 2,548 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total securities available-for-sale |
$ | — | $ | 599 | $ | 2,548 | ||||||
We had no securities available-for-sale outstanding as of December 31, 2009.
The decrease in the balance of securities available-for-sale as of December 31, 2008 as compared to December 31, 2007 is primarily due to $12.6 million in sales and maturities of securities available-for-sale, offset by $10.7 million in purchases of securities available-for-sale during the year ended December 31, 2008.
Securities available-for-sale consist of the following debt securities as of December 31 (in thousands of US dollars):
C-191
| 2008 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||||||
| Corporate obligations |
$ | 600 | $ | — | $ | (1 | ) | $ | 599 | |||||||
| $ | 600 | $ | — | $ | (1 | ) | $ | 599 | ||||||||
| 2007 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | |||||||||||||
| Corporate obligations |
$ | 1,001 | $ | — | $ | (1 | ) | $ | 1,000 | |||||||
| Municipal obligations |
$ | 799 | $ | 3 | $ | — | $ | 802 | ||||||||
| U.S. government obligations |
$ | 745 | $ | 1 | $ | — | $ | 746 | ||||||||
| $ | 2,545 | $ | 4 | $ | (1 | ) | $ | 2,548 | ||||||||
As of December 31, 2008 and 2007, all securities available-for-sale had contractual maturities of less than one year. Gross realized gains and losses to date have not been material.
| 4. | Accounts Receivable, net |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Accounts Receivable (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | 982 | $ | 51 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total accounts receivable |
$ | — | $ | 982 | $ | 51 | ||||||
CTI’s accounts receivable balance as of December 31, 2008 and 2007 consists of trade receivables related to Zevalin which was acquired in December 2007 and contributed to RIT Oncology, our 50/50 joint venture with Spectrum in December 2008. The amount as of December 31, 2008 relates to receivables remaining on sales prior to the divestiture. Allowances for uncollectible accounts receivable and product returns, which are offset against the accounts receivable balance, totalled approximately $93,000 and $2,000 as of December 31, 2008 and 2007.
| 5. | Inventory |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Inventory (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | — | $ | 290 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total inventory |
$ | — | $ | — | $ | 290 | ||||||
All inventory as of December 31, 2007 consists of finished goods inventory for Zevalin which was acquired in December 2007. All inventory was sold to RIT Oncology subsequent to its formation in December 2008.
C-192
| 6. | Note receivable from joint venture |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Note receivable from joint venture (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | 7,500 | $ | — | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total note receivable from joint venture |
$ | — | $ | 7,500 | $ | — | ||||||
Total note receivable from joint venture as of December 31, 2008 relates to the initial payment to us from RIT Oncology upon the formation of the joint venture, RIT Oncology and was in exchange for our contribution of the Zevalin assets to the joint venture. This amount was funded by Spectrum and was the second of two $7.5 million payments and was received in January 2009.
| 7. | Prepaid expenses and other current assets |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Prepaid expenses and other current assets (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | 3,315 | $ | 1,991 | $ | 2,522 | ||||||
| Subsidiaries |
1,039 | 368 | 1,382 | |||||||||
| Total prepaid expenses and other current assets |
$ | 4,354 | $ | 2,359 | $ | 3,904 | ||||||
The increase in prepaid expenses and other current assets as of December 31, 2009 as compared to December 31, 2008 is primarily due to prepayments for pixantrone clinical study, legal retainer, an increase in our VAT Receivable and tax credits due to CTI.
| 8. | Property and Equipment, net |
Property and equipment are carried at cost, less accumulated depreciation and amortization. Depreciation commences at the time assets are placed in service. It is calculated using the straight-line method over the estimated useful lives of the assets ranging from three to five years for assets other than leasehold improvements, which are amortized over the lesser of their useful life of 10 years or the applicable lease using the straight-line method.
Property and equipment are composed of the following as of December 31 (in thousands of US dollars):
C-193
| 2009 | 2008 | 2007 | ||||||||||
| Leasehold improvements |
$ | 3,277 | $ | 6,512 | $ | 11,644 | ||||||
| Lab equipment |
560 | 7,240 | 7,452 | |||||||||
| Furniture and office equipment |
11,970 | 19,252 | 18,300 | |||||||||
| 15,807 | 33,004 | 37,396 | ||||||||||
| Less: accumulated depreciation and amortization |
(12,377 | ) | (28,680 | ) | (31,371 | ) | ||||||
| $ | 3,430 | $ | 4,324 | $ | 6,025 | |||||||
Depreciation expense of $1.8 million, $3.5 million and $4.1 million was recognized during 2009, 2008, and 2007, respectively.
All sales of Zevalin during 2008 and 2007 were to customers in North America.
The following table depicts long-lived assets based on the following geographic locations (in thousands of US dollars)
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| United States |
$ | 21,501 | $ | 22,966 | $ | 39,777 | ||||||
| Europe |
5,929 | 7,286 | 11,099 | |||||||||
| $ | 27,430 | $ | 30,252 | $ | 50,876 | |||||||
| 9. | Goodwill and Other Intangible Assets |
Goodwill is not amortized but is tested for impairment at least annually or more frequently if indicators of impairment are present. If goodwill is impaired it is written down; however, no impairment of goodwill has been found to date.
There were no changes in the net carrying amount of goodwill during the years ended December 31, 2009, 2008 and 2007.
Other intangible assets consist of acquisition-related intangible assets. These other intangible assets have finite lives and are carried at cost less accumulated amortization.
As of December 31, 2009 and 2008, we had no intangible asset balance remaining. Other intangible assets are composed of the following as of December 31 2007 (in thousands):
| 2007 | ||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||
| Developed and core technologies |
$ | 11,306 | $ | (28 | ) | $ | 11,278 | |||||
| Manufacturing intangible asset |
3,712 | (16 | ) | 3,696 | ||||||||
| Assembled workforce |
5,699 | (4,716 | ) | 983 | ||||||||
| Other intangibles assets |
$ | 20,717 | $ | (4,760 | ) | $ | 15,957 | |||||
C-194
The change in the value of other intangible assets is as follows (in thousands):
| Developed and Core Technologies |
Manufacturing Intangible Asset |
Assembled Workforce |
||||||||||
| Balance as of December 31, 2007 |
$ | 11,278 | $ | 3,696 | $ | 983 | ||||||
| Increase due to acquisition cost adjustments |
138 | 45 | — | |||||||||
| Amortization |
(111 | ) | (558 | ) | (927 | ) | ||||||
| Disposition of Zevalin to RIT Oncology |
(11,305 | ) | (3,183 | ) | — | |||||||
| Decrease due to exchange rate |
— | — | (56 | ) | ||||||||
| Balance as of December 31, 2008 |
$ | — | $ | — | $ | — | ||||||
Amortization of the assembled workforce intangible asset is computed using the straight-line method over the estimated useful life of the assembled workforce asset, which is approximately 5 years. As of December 31, 2008 all workforce intangibles had been fully amortized.
In 2007, we recorded certain intangible assets in connection with the acquisition of Zevalin. Developed and core technologies were amortized over the terms of the patents related to such technologies of approximately 11.2 years based on a method of amortization that reflected the pattern in which the economic benefit of the intangible assets were consumed in accordance with SFAS No. 142, Goodwill and Other Intangible Assets. The manufacturing intangible asset was amortized straight-line over the term of the supply agreement, which was approximately 6.5 years. In connection with the formation of RIT Oncology in December 2008, our intangible asset balances related to Zevalin were included in the disposition of Zevalin to RIT Oncology.
| 10. | Investment in joint venture |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Investment in joint venture (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | — | $ | 5,830 | $ | — | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total investment in joint venture |
$ | — | $ | 5,830 | $ | — | ||||||
The balance of investment in joint venture as of December 31, 2008 equals our initial capital contribution in the joint venture offset by our equity loss in the joint venture
| 11. | Other assets |
The balance consists of the following as of December 31 (in thousands of US dollars):
C-195
| Other assets (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | 1,005 | $ | 2,539 | $ | 5,149 | ||||||
| Subsidiaries |
5,931 | 6,325 | 6,681 | |||||||||
| Total other assets and deferred charges |
$ | 6,936 | $ | 8,864 | $ | 11,830 | ||||||
The decrease in the balance of other assets as of December 31, 2009 as compared to December 31, 2008 is primarily due to a decrease in net Debt Issuance Costs attributable to conversions and tender offer in 2009 and a decrease in the long term portion of our VAT receivable.
Value Added Tax Receivable
CTI’s European operations are subject to Value Added Tax, or VAT, which is usually applied to all goods and services purchased and sold throughout Europe. The VAT receivable is approximately $6.3 million, $6.3 million and $7.2 million as of December 31, 2009, December 31, 2008 and December 31, 2007, respectively, of which $5.9 million, $6.2 million and $6.5 million is included in other assets and $0.4 million, $0.1 million and $0.7 million is included in prepaid expenses and other current assets as of December 31, 2009, December 31, 2008 and December 31, 2007, respectively. This receivable balance relates to CTI’s Italian operations and typically has a three year collection period. CTI reviews its VAT receivable balance for impairment whenever events or changes in circumstances indicate the carrying amount might not be recoverable.
| B. | LIABILITIES |
| 1. | Accounts Payable |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Accounts Payable (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | 6,570 | $ | 7,095 | $ | 5,544 | ||||||
| Subsidiaries |
727 | 2,232 | 1,051 | |||||||||
| Total accounts payable |
$ | 7,297 | $ | 9,327 | $ | 6,595 | ||||||
The change in accounts payable as between December 31, 2009, 2008 and 2007 is due to fluctuation in the timing of expenses and payments.
| 2. | Accrued Expenses |
Accrued expenses consisted of the following as of December 31 (in thousands of US dollars):
C-196
| 2009 | 2008 | 2007 | ||||||||||
| USAO litigation claim |
$ | — | $ | — | $ | — | ||||||
| Clinical development and regulatory expense |
5,560 | 8,293 | 11,936 | |||||||||
| Employee compensation and related expenses |
4,113 | 5,920 | 4,738 | |||||||||
| Deemed dividend on conversion of preferred stock |
— | 3,000 | — | |||||||||
| Manufacturing expense |
651 | 2,662 | 2,319 | |||||||||
| Settlement expenses |
— | 2,595 | — | |||||||||
| Insurance financing and accrued interest expense |
1,031 | 2,032 | 689 | |||||||||
| Royalties and rebates |
9 | 1,549 | 12 | |||||||||
| Corporate development and sales and marketing expense |
— | — | 1,924 | |||||||||
| Legal Expense |
805 | 1,048 | — | |||||||||
| Other research and development expenses |
— | — | 464 | |||||||||
| Other |
2,638 | 2,209 | 3,952 | |||||||||
| $ | 14,807 | $ | 29,308 | $ | 26,034 | |||||||
Accrued expenses decreased as of December 31, 2009 as compared to December 31, 2008 primarily due to Oracle system implementation account payable efficiencies, a reduction in royalties and rebates, decrease in deemed dividend on conversion of preferred stock, Tang Settlement, and decreases in clinical accrued expenses.
| 3. | Deferred revenue |
The balance consists of the following as of December 31 (in thousands of US dollars):
| Deferred revenue (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Current portion of deferred revenue |
$ | 80 | $ | 80 | $ | 80 | ||||||
| Deferred revenue, less current portion |
239 | 319 | 398 | |||||||||
| Total deferred revenue |
$ | 319 | $ | 399 | $ | 478 | ||||||
The slight decrease in deferred revenue as of December 31, 2009 as compared to December 31, 2008 and December 31, 2007 is due to recognition of deferred revenue related to DiaKine Licensing Agreement of Lysofilline.
| 4. | Long term Obligations |
Other long-term obligations
Other long-term obligations consist of the following as of December 31 (in thousands of US dollars):
C-197
| 2009 | 2008 | 2007 | ||||||||||
| Capital lease equipment financing agreement, due May 2010, monthly payments of $1, including interest at 6.0% |
— | 26 | 44 | |||||||||
| Capital lease equipment financing agreement, due February 2008, monthly payments of $7, including interest at 5.1% |
— | — | 54 | |||||||||
| Excess facilities liability |
854 | 1,128 | 1,547 | |||||||||
| Accrued rent |
1,165 | 1,415 | 1,567 | |||||||||
| Employee defined benefit plan |
583 | 899 | 1,034 | |||||||||
| Italian Regional Production tax |
528 | — | — | |||||||||
| European public loans |
— | 116 | 241 | |||||||||
| Other long-term obligations |
43 | 80 | 6,412 | |||||||||
| 3,173 | 3,664 | 10,899 | ||||||||||
| Less current portion |
(1,312 | ) | (757 | ) | (1,020 | ) | ||||||
| $ | 1,861 | $ | 2,907 | $ | 9,879 | |||||||
| 5. | Convertible notes |
Based on their respective trading prices, the fair values of CTI’s convertible senior notes, convertible senior subordinated notes and convertible subordinated notes are as follows as of December 31 (in thousands of US dollars):
| 2009 | 2008 | 2007 | ||||||||||
| 10% convertible senior notes due 2011 |
— | $ | 21,810 | — | ||||||||
| 9% convertible senior notes |
— | $ | 4,580 | |||||||||
| 7.5% convertible senior notes |
$ | 9,138 | $ | 27,308 | $ | 29,756 | ||||||
| 6.75% convertible senior notes |
— | $ | 5,875 | $ | 6,100 | |||||||
| 5.75% convertible senior notes |
$ | 8,777 | $ | 16,728 | $ | 26,650 | ||||||
| 4.0% convertible senior subordinated notes |
$ | 38,512 | $ | 46,375 | $ | 45,403 | ||||||
| 5.75% convertible senior subordinated notes |
— | — | $ | 16,907 | ||||||||
| 5.75% convertible subordinated notes |
— | — | $ | 2,910 | ||||||||
The following table summarizes the changes in the principal balances of CTI’s convertible notes during the years ended December 31, 2009 and 2008 (in thousands):
| Balance at January 1, 2009 |
Converted | Exchanged, Extinguished or Repurchased |
Balance at December 31, 2009 |
|||||||||||||
| 10% convertible senior notes due 2011 |
$ | 18,000 | $ | (18,000 | ) | $ | — | $ | — | |||||||
| 9% convertible senior notes |
5,585 | (5,250 | ) | (335 | ) | — | ||||||||||
| 7.5% convertible senior notes |
33,458 | — | (23,208 | ) | 10,250 | |||||||||||
| 6.75% convertible senior notes |
7,000 | — | (7,000 | ) | — | |||||||||||
| 5.75% convertible senior notes |
23,000 | — | (12,087 | ) | 10,913 | |||||||||||
| 4.0% convertible senior subordinated notes |
55,150 | — | (14,787 | ) | 40,363 | |||||||||||
| Total |
$ | 142,193 | $ | (23,250 | ) | $ | (57,417 | ) | $ | 61,526 | ||||||
C-198
| Balance at January 1, 2008 |
Issued | Converted | Extinguished | Matured | Balance at December 31, 2008 |
|||||||||||||||||||
| 18.33% convertible senior notes |
$ | — | $ | 44,500 | $ | (28,250 | ) | $ | (16,250 | ) | $ | — | $ | — | ||||||||||
| 15.5% convertible senior notes |
— | 14,211 | (14,211 | ) | — | — | — | |||||||||||||||||
| 15% convertible senior notes |
— | 23,000 | — | (23,000 | ) | — | — | |||||||||||||||||
| 13.5% convertible senior notes |
— | 45,118 | (27,600 | ) | (17,518 | ) | — | — | ||||||||||||||||
| 10% convertible senior notes due 2011 |
— | 32,651 | (14,651 | ) | — | — | 18,000 | |||||||||||||||||
| 10% convertible senior notes due 2012 |
— | 9,000 | (9,000 | ) | — | — | — | |||||||||||||||||
| 9.66% convertible senior notes |
— | 24,700 | (15,700 | ) | (9,000 | ) | — | — | ||||||||||||||||
| 9% convertible senior notes |
— | 51,655 | (40,820 | ) | (5,250 | ) | — | 5,585 | ||||||||||||||||
| 7.5% convertible senior notes |
33,458 | — | — | — | — | 33,458 | ||||||||||||||||||
| 6.75% convertible senior notes |
7,000 | — | — | — | — | 7,000 | ||||||||||||||||||
| 5.75% convertible senior notes |
23,250 | — | (250 | ) | — | — | 23,000 | |||||||||||||||||
| 5.75% convertible senior subordinated notes |
16,907 | — | — | (8,943 | ) | (7,964 | ) | — | ||||||||||||||||
| 5.75% convertible subordinated notes |
2,910 | — | — | (150 | ) | (2,760 | ) | — | ||||||||||||||||
| 4.0% convertible senior subordinated notes |
55,150 | — | — | — | — | 55,150 | ||||||||||||||||||
| Total |
$ | 138,675 | $ | 244,835 | $ | (150,482 | ) | $ | (80,111 | ) | $ | (10,724 | ) | $ | 142,193 | |||||||||
For a description of the Company’s notes and terms of the notes, including a description of those series of notes which have been completely converted as of the date of this Registration Document, please refer to Chapter 21, Paragraph 21.1.4 of this Registration Document. Pursuant to the indentures governing each series of notes, the Company is not obligated to keep a record of the individual holders of the notes, which are held in the form of a global note by The Depositary Trust Company.
| 6. | Convertible Preferred Stock |
| Convertible Preferred Stock (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Series A 3% Convertible Preferred Stock |
$ | — | $ | 417 | $ | 5,188 | ||||||
| Series B 3% Convertible Preferred Stock |
— | 4,031 | 11,881 | |||||||||
| Series C 3% Convertible Preferred Stock |
— | 3,221 | 6,229 | |||||||||
| Series D 7% Convertible Preferred Stock |
— | 734 | 2,938 | |||||||||
| Total Convertible Preferred Stock |
$ | — | $ | 8,403 | $ | 26,236 | ||||||
As of the date of this Registration Document, the Company had no shares of preferred stock outstanding.
For a description of the aforesaid Company’s convertible preferred stock please refer to subsequent Chapter 21, Paragraph 21.1.4, Subparagraph “Preferred Stock no longer outstanding but that were outstanding for a certain time (as better specified below) included in the period from January 1, 2007 to the date of this Registration Document”.
C-199
| 7. | Shareholders’ Deficit |
| a. | Analysis of shareholders’ deficit variation |
| Shareholders’ deficit (In thousands of US dollars) | December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Common stock, no par value |
$ | 1,418,931 | $ | 1,188,071 | $ | 979,295 | ||||||
| Accumulated other comprehensive income (loss) |
(8,412 | ) | (7,812 | ) | (4,007 | ) | ||||||
| Accumulated deficit |
(1,429,083 | ) | (1,312,320 | ) | (1,109,413 | ) | ||||||
| Total shareholders’ deficit |
$ | (18,564 | ) | $ | (132,061 | ) | $ | (134,125 | ) | |||
Common Stock
For net common stock (deficit), please see Paragraph 20.1.5 of this Chapter 20.
The increase in common stock as of December 31, 2009 as compared to December 31, 2008 is primarily due to $62.8 million related to the conversion and exchanges of convertible notes to common stock, $59.2 million related to the issuance of common stock and warrants, $54.5 million related to conversions of convertible preferred stock to common stock, $24.9 million related to equity-based compensation, $13.2 million related to the value of the beneficial conversion feature of the convertible preferred stock issued and converted, $11.0 million related to the issuance of common stock upon exercise of common stock purchase warrants, and $6.1 million related to the issuance of warrants in connection with the issuance of convertible preferred stock.
The increase in common stock as of December 31, 2008 as compared to December 31, 2007 is primarily due to $17.8 million in conversions of convertible preferred stock to common stock, $150.5 million in conversions of convertible senior notes to common stock, $16.7 million in issuance of common stock, $10.9 million in issuance of warrants, and an $11.2 million premium on 15% convertible senior notes due to exercise of Series B warrant.
The increase in common stock as of December 31, 2007 as compared to December 31, 2006 is primarily due to $37.6 million in conversions of convertible preferred stock to common stock, $19.9 million of common stock issued in connection the acquisition of SM, $15.3 million related to the conversion of 7.5% convertible senior notes to common stock, $14.5 million related to the issuance of warrants in connection with the issuance of CTI’s convertible preferred stock, $13.7 million related to the issuance of common stock in connection with the exchange of CTI’s 5.75% senior subordinated and subordinated notes, $9.5 million related to the value of the beneficial conversion feature of the convertible preferred stock and $6.5 million due to the issuance of common stock and warrants.
Accumulated Other Comprehensive Loss
The change in accumulated other comprehensive loss as of December 31, 2009 as compared to December 31, 2008 is primarily due to a foreign currency translation loss of approximately $0.6 million.
The change in accumulated other comprehensive loss as of December 31, 2008 as compared to December 31, 2007 is primarily due to a foreign currency translation loss of approximately $3.8 million.
C-200
The change in accumulated other comprehensive loss as of December 31, 2007 as compared to December 31, 2006 is primarily due to a foreign currency translation loss of approximately $2.8 million.
Accumulated Deficit
The increase in accumulated deficit from December 31, 2007 to December 31, 2008 and from December 31, 2008 to December 31, 2009 is due to CTI’s net losses.
| b. | Capital Stock and Warrants |
Common Stock
For the description of the variations occurred with respect to the number of shares of common stock outstanding during the financial years covered by the financial statements disclosed in this Chapter 20, please refer to the information reported under subsequent Paragraph 21.1.7.
Warrants
For the description of the “warrants still outstanding on December 31, 2009 and September 30, 2010” please refer to the relevant section under subsequent Paragraph 21.1.7.
Moreover, hereinbelow is a short description of the further warrants outstanding during the financial years covered by the financial statements disclosed in this Chapter 20.In particular, in connection with our November 2005 6.75% convertible senior notes offering, we issued warrants to purchase approximately 9,000 shares of common stock within five years at an exercise price of $140.00 per share to the initial purchaser of these notes. The estimated fair value of the warrants of approximately $0.6 million was capitalized as a debt issuance cost and is being amortized over the life of the convertible senior notes of five years. No warrants have been exercised as of December 31, 2009.
Moreover, in 1998 we issued contingently exercisable warrants to purchase approximately 9,000 shares of our common stock in connection with a license agreement with PG-TXL Company, L.P. at a per share exercise price of $800.00. No warrants were exercised and they expired in November 2008.
Common Stock Reserved
A summary of common stock reserved for issuance is as follows as of December 31, 2009:
| Convertible senior notes |
486,386 | |||
| Convertible senior subordinated notes |
74,746 | |||
| Equity incentive plans |
36,700,675 | |||
| Common stock warrants |
24,793,070 | |||
| Employee stock purchase plan |
1,474,591 | |||
| Restricted share rights |
391 | |||
| 63,529,859 | ||||
C-201
| c. | Stock-Based Compensation |
Stock-Based Compensation Expense
Stock-based compensation expense for all stock-based payment awards made to employees and directors is measured based on the grant-date fair value estimated in accordance with generally accepted accounting principles for stock-based compensation. We recognized stock-based compensation using the straight-line single-award method based on the value of the portion of stock-based payment awards that is ultimately expected to vest during the period. Stock-based compensation is reduced for estimated forfeitures at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. For performance-based awards that do not include market-based conditions, we record stock-based compensation expense only when the performance-based milestone is deemed probable of achievement. We utilize both quantitative and qualitative criteria to judge whether milestones are probable of achievement. For awards with market-based performance conditions, we recognize the grant-date fair value of the award over the derived service period regardless of whether the underlying performance condition is met.
Stock-based compensation expense recognized for the years ended December 31, 2009, 2008 and 2007 was $24.9 million, $4.0 million and $1.6 million, which consisted of $23.2 million, $3.3 million and $0.7 million of stock-based compensation expense related to restricted stock and $0.4 million, $0.7 million and $0.9 million of stock-based compensation expense related to employee stock options and employee stock purchases, respectively. Stock-based compensation expense for the year ended December 31, 2009 also consisted of $1.3 million related to our December 2009 performance awards; no such expense was recorded for the years ended December 31, 2008 and 2007.
The following table summarizes stock-based compensation expense for the years ended December 31, 2009, 2008 and 2007, which was allocated as follows (in thousands):
| 2009 | 2008 | 2007 | ||||||||||
| Research and development |
$ | 3,281 | $ | 1,249 | $ | 772 | ||||||
| Selling, general and administrative |
21,656 | 2,746 | 816 | |||||||||
| Stock-based compensation expense included in operating expenses |
$ | 24,937 | $ | 3,995 | $ | 1,588 | ||||||
Employee stock-based compensation had a $24.9 million, $4.0 million and $1.6 million effect on our net loss attributable to common shareholders and a $(0.05), $(0.14) and $(0.35) effect on basic and diluted net loss per common share for the years ended December 31, 2009, 2008 and 2007, respectively. It had no effect on cash flows from operations or financing activities for the periods presented; however, during 2009 we repurchased shares of our common stock totaling $6.4 million for cash in connection with the vesting of employee restricted stock awards based on taxes owed by employees due to the vesting of the awards.
As of December 31, 2009, the total remaining unrecognized compensation cost related unvested stock options and restricted stock amounted to $3.5 million, which will be recognized over the weighted-average remaining requisite service period of 1.35 years. In addition, we have unrecognized compensation cost related to our December 2009 performance awards as discussed further below. The unrecognized compensation cost related to
C-202
unvested options and restricted stock does not include the cost related 3.2 million performance-based restricted stock awards granted in December 2009 with a grant-date fair value of $3.8 million which, as of December 31, 2009, had not been deemed probable of achievement. This amount also excludes 48,000 shares of performance-based restricted stock awards granted in December 2007 with a grant date fair value of $0.9 million which have been deemed improbable of achievement. As of December 31, 2009, we have not recognized any expense related to either of these performance-based award grants. In addition, unvested stock-based compensation expense excludes the fair value of 275,000 restricted stock awards and 152,000 options granted to external consultants as the fair value is periodically remeasured as discussed below.
For the years ended December 31, 2009, 2008 and 2007, no tax benefits were attributed to the stock-based compensation expense because a valuation allowance was maintained for substantially all net deferred tax assets.
Stock Plan
Pursuant to our 2007 Equity Incentive Plan, as amended and restated in August 2009, or the Plan, we may grant the following types of incentive awards: (1) stock options, including incentive stock options and nonqualified stock options, (2) stock appreciation rights, (3) restricted stock, (4) restricted stock units and (5) cash awards. The Plan is administered by the Compensation Committee of the Board of Directors which has the discretion to determine which employees, consultants and directors shall be granted incentive awards. Options are typically exercisable ratably over a four-year period commencing one year from the date of grant, and expire not more than 10 years from the date of grant. As of December 31, 2009, 36.1 million shares of common stock were available for future grants under the Plan. However, assuming the performance goals underlying the December 2009 performance awards (as discussed below) had been achieved as of December 31, 2009, there would have been no shares of common stock remaining for future grants under the Plan.
Stock Options
Fair value for employee stock options was estimated at the date of grant using the Black-Scholes pricing model, with the following weighted average assumptions:
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Risk-free interest rates |
1.4 | % | 2.8 | % | 3.9 | % | ||||||
| Expected dividend yield |
None | None | None | |||||||||
| Expected life (in years) |
2.8 | 2.7 | 3.0 | |||||||||
| Volatility |
88 | % | 79 | % | 76 | % | ||||||
The risk-free interest rate used in the Black-Scholes valuation method is based on the implied yield currently available in U.S. Treasury securities at maturity with an equivalent term. We have not declared or paid any dividends on our common stock and do not currently expect to do so in the future. The expected term of options represents the period that our stock-based awards are expected to be outstanding and was determined based on historical weighted average holding periods and projected holding periods for the remaining unexercised shares. Consideration was given to the contractual terms of our stock-based awards, vesting schedules and expectations of future employee behavior. Expected volatility is based on the annualized daily historical volatility, including
C-203
consideration of the implied volatility and market prices of traded options for comparable entities within our industry.
Our stock price volatility and option lives involve management’s best estimates, both of which impact the fair value of the option calculated under the Black-Scholes methodology and, ultimately, the expense that will be recognized over the life of the option. As we also recognize compensation expense for only the portion of options expected to vest, we apply estimated forfeiture rates that we derive from historical employee termination behavior. If the actual number of forfeitures differs from our estimates, additional adjustments to compensation expense may be required in future periods.
The following table summarized stock option activity for all of the stock option plans is as follows:
| Options | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value (Thousands) |
|||||||||||||
| Outstanding January 1, 2007 (118,000 exercisable) |
155,000 | $ | 392.30 | |||||||||||||
| Granted |
96,000 | $ | 39.20 | |||||||||||||
| Exercised |
— | $ | — | |||||||||||||
| Forfeited |
(7,000 | ) | $ | 134.70 | ||||||||||||
| Cancelled and expired |
(20,000 | ) | $ | 284.30 | ||||||||||||
| Outstanding December 31, 2007 (127,000 exercisable) |
224,000 | $ | 258.60 | |||||||||||||
| Granted |
122,000 | $ | 4.90 | |||||||||||||
| Exercised |
— | $ | — | |||||||||||||
| Forfeited |
(18,000 | ) | $ | 45.30 | ||||||||||||
| Cancelled and expired |
(30,000 | ) | $ | 159.70 | ||||||||||||
| Outstanding December 31, 2008 (147,000 exercisable) |
298,000 | $ | 177.40 | |||||||||||||
| Granted |
404,000 | $ | 1.26 | |||||||||||||
| Exercised |
— | $ | — | |||||||||||||
| Forfeited |
(56,000 | ) | $ | 6.41 | ||||||||||||
| Cancelled and expired |
(24,000 | ) | $ | 132.37 | ||||||||||||
| Outstanding December 31, 2009 |
622,000 | $ | 80.17 | 8.4 | $ | 43 | ||||||||||
| Vested or expected to vest at December 31, 2009 |
585,000 | $ | 84.91 | 8.4 | $ | 36 | ||||||||||
| Exercisable at December 31, 2009 |
202,000 | $ | 241.81 | 6.3 | $ | 15 | ||||||||||
The weighted average exercise price of shares exercisable at December 31, 2008 and 2007 was $345.40 and $420.10, respectively. The weighted average fair value of options granted was $0.52, $2.00 and $19.30 during 2009, 2008 and 2007, respectively.
C-204
The following table summarizes information about common stock options outstanding at December 31, 2009:
| Options Outstanding | Exercisable Options Outstanding (Without Restriction) |
|||||||||||||||||||
| Range of Exercise Prices |
Number Outstanding |
Weighted Average Remaining Contractual Life |
Weighted Average Exercise Price |
Number Exercisable |
Weighted Average Exercise Price |
|||||||||||||||
| $0.08 – $1.07 |
217,000 | 9.7 Years | $ | 0.94 | 17,000 | $ | 0.25 | |||||||||||||
| $1.08 – $1.76 |
184,000 | 9.5 Years | $ | 1.66 | — | $ | — | |||||||||||||
| $1.77 – $8.30 |
64,000 | 8.4 Years | $ | 5.59 | 38,000 | $ | 5.43 | |||||||||||||
| $8.31 – $99.20 |
96,000 | 7.2 Years | $ | 47.44 | 86,000 | $ | 47.53 | |||||||||||||
| $99.21 – $1,721.30 |
61,000 | 2.8 Years | $ | 733.38 | 61,000 | $ | 733.38 | |||||||||||||
| $ 0.08 – $1,721.30 |
622,000 | 8.4 Years | $ | 80.17 | 202,000 | $ | 241.81 | |||||||||||||
Restricted Stock
We issued 34.1 million, 1.0 million and 0.2 million shares of restricted common stock in 2009, 2008 and 2007, respectively. Additionally, 322,000, 26,000 and 12,000 shares of restricted stock were cancelled during 2009, 2008 and 2007, respectively. The weighted average fair value of restricted shares issued during 2009, 2008 and 2007 was $0.87, $1.70 and $18.60, respectively.
A summary of the status of nonvested restricted stock awards as of December 31, 2009 and changes during the period then ended, is presented below:
| Nonvested Shares |
Weighted Average Grant Date Fair Value Per Share |
|||||||
| Nonvested at December 31, 2008 |
942,000 | $ | 1.90 | |||||
| Granted |
34,143,000 | $ | 0.87 | |||||
| Vested |
(23,269,000 | ) | $ | 0.98 | ||||
| Forfeited |
(322,000 | ) | $ | 0.63 | ||||
| Nonvested at December 31, 2009 |
11,494,000 | $ | 0.75 | |||||
The total fair value of restricted stock awards vested during the year ended December 31, 2009, 2008 and 2007 was $26.0 million, $0.4 million and $0.4 million, respectively.
December 2009 Performance Awards
In December 2009, we granted restricted stock units (which we refer to as our December 2009 performance awards) to our executive officers and directors which vest upon milestone-based performance conditions. If one or more of the eight underlying performance-based conditions are timely achieved, the award recipient will be entitled to receive a number of shares of our common stock (subject to share limits of the Plan), determined by
C-205
multiplying (1) the award percentage corresponding to that particular performance goal by (2) the total number of outstanding shares of our common stock as of the date that the particular performance goal is achieved. The total award percentages related to all eight performance goals are 9.36% and 2.63% of shares outstanding at the time a performance goal is achieved for executive officers and directors, respectively.
The fair value of the December 2009 performance awards was estimated based on the average present value of the awards to be issued upon achievement of the performance conditions. The average present value is calculated based upon the expected date the shares of common stock underlying the performance rights will vest, or the event date, the expected stock price on the event date, and the expected shares outstanding as of the event date. The event date, stock price and the shares outstanding are estimated using a Monte Carlo simulation model which is based on assumptions by management, including the likelihood of achieving the milestones and potential future financings. The total fair value of the December 2009 performance awards based on this calculation was $49.8 million. As of December 31, 2009, we have not deemed the December 2009 performance awards probable of achievement and no expense has been recognized except for the awards with an underlying market-based performance condition.
We determined that the December 2009 performance awards with the market-based performance condition have a fair value of $15.2 million, of which we have recognized $1.3 million in stock-based compensation expense for the year ended December 31, 2009 as discussed above. As of December 31, 2009, the remaining unrecognized compensation expense related to the market-based December 2009 performance awards is $14.0 million, which will be recognized over the weighted-average remaining requisite service period of 0.48 years.
Non-Employee Stock-Based Compensation
Stock compensation expense for awards granted to non-employees is determined using the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measured. The fair value of options and restricted stock awards granted to non-employees is periodically remeasured as the underlying options or awards vest. The value of the instrument is amortized to expense over the vesting period with final valuation measured on the vesting date. At December 31, 2009, 2008 and 2007, unvested non-employee options to acquire approximately 152,000, 16,000 and 12,000 shares of common were outstanding, respectively. Additionally, unvested non-employee restricted stock awards totaled 275,000 as of December 31, 2009. No such awards were outstanding as of December 31, 2008 and 2007. We recorded compensation expense of $157,000 and $4,000 in 2009 and 2007, respectively, and reversed previously recorded compensation expense of $5,000 in 2008 related to non-employee stock options.
Employee Stock Purchase Plan
Under our 2007 Employee Stock Purchase Plan, as amended and restated in August 2009, or Purchase Plan, eligible employees may purchase a limited number of shares of our common stock at 85% of the lower of the subscription date fair market value and the purchase date fair market value. There are two six-month offerings per year. Under the Purchase Plan, we issued approximately 42,000 and 8,000 shares to employees in 2009 and 2008, respectively. We did not issue any shares under a purchase plan during 2007. There are 1,525,000 shares of common stock authorized under the Purchase Plan and 1,475,000 are reserved for future purchases as of December 31, 2009.
C-206
| d. | Net Loss per Share |
Basic and diluted net loss per share is calculated using the weighted average number of shares outstanding as follows (in thousands, except per share amounts):
| Year Ended December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Net loss attributable to common shareholders |
$ | (116,763 | ) | $ | (202,907 | ) | $ | (148,305 | ) | |||
| Basic and diluted: |
||||||||||||
| Weighted average shares outstanding |
466,352 | 29,383 | 4,564 | |||||||||
| Less weighted-average restricted shares outstanding |
(7,996 | ) | (416 | ) | (35 | ) | ||||||
| Shares used in calculation of basic and diluted net loss per common share |
458,356 | 28,967 | 4,529 | |||||||||
| Net loss per common share: Basic and diluted |
$ | (0.25 | ) | $ | (7.00 | ) | $ | (32.75 | ) | |||
As of December 31, 2009, 2008 and 2007, options, warrants, unvested restricted share awards and rights, convertible debt, and convertible preferred stock aggregating 34.1 million, 136.1 million and 3.7 million common equivalent shares, respectively, prior to the application of the treasury stock method for options and warrants, were not included in the calculation of diluted net loss per share as their effects on the calculation are anti-dilutive. These amounts do not include performance or market-based awards, including options, restricted share awards and December 2009 performance awards.
| e. | Equity Compensation Plan Information |
The following table gives information about the Company’s common stock that may be issued upon the exercise of options, warrants and rights under all of the Company’s existing compensation plans as of December 31, 2009, including the 2007 Equity Plan, 1994 Equity Incentive Plan and the ESPP.
| Plan Category |
(a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
(b) Weighted Average Exercise Price of Outstanding Options, Warrants, and Rights |
(c) Number
of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) |
|||||||||
| Plans Approved by Shareholders(1) |
622,250 | $ | 80.17 | 1,474,591 | ||||||||
| Plan Not Approved by Shareholders |
— | — | — | |||||||||
| Totals |
622,250 | $ | 80.17 | 1,474,591 | ||||||||
C-207
All of the shares reported in Column (c) were available for issuance under the ESPP. As described above, the Compensation Committee approved the December 2009 Performance Awards under the 2007 Equity Plan that would be payable in shares of the Company’s common stock upon satisfaction of the performance and other requirements imposed on the award. Columns (a) and (b) of this table are presented without giving effect to the December 2009 Performance Awards as the number of shares that would be issuable in payment of these awards depends on the Company’s total issued and outstanding shares at the time of payment and was therefore not determinable as of December 31, 2009. Column (c) is presented after giving effect to the December 2009 Performance Awards (assuming the performance goals applicable to these awards were achieved). As of December 31, 2009, 36,078,425 shares of the Company’s common stock were available for award grant purposes under the 2007 Equity Plan (before giving effect to the December 2009 Performance Awards) and all of these shares would have been used to pay the December 2009 Performance Awards if the performance goals applicable to these awards had been achieved. If the December 2009 Performance Awards become payable and sufficient shares are not available under the 2007 Equity Plan (after reserving sufficient shares to cover the other awards then outstanding under the 2007 Equity Plan), the number of shares payable with respect to the December 2009 Performance Awards will be proportionately reduced such that the share limits of the 2007 Equity Plan will not be exceeded.
Of these shares, 582,496 were subject to options then outstanding under the 2007 Equity Plan, and 39,754 were subject to options then outstanding under the 1994 Equity Incentive Plan. The Company’s authority to grant new awards under the 1994 Equity Incentive Plan has terminated.
| f. | Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities |
Our common stock is currently traded on the NASDAQ Capital Market under the symbol “CTIC” and the MTA (formerly known as the MTAX and, prior to that, as the Nuovo Mercato) in Italy, also under the ticker symbol “CTIC”. Prior to January 8, 2009, our common stock was traded on the NASDAQ Global Market. The following table sets forth, for the periods indicated, the high and low reported sales prices per share of the common stock as reported on the NASDAQ Global or Capital Market, our principal trading market (as adjusted to reflect the one-for-ten reverse stock split effective August 31, 2008).
| High | Low | |||||||
| 2008 |
||||||||
| First Quarter |
19.90 | 4.70 | ||||||
| Second Quarter |
9.60 | 4.60 | ||||||
| Third Quarter |
4.90 | 0.58 | ||||||
| Fourth Quarter |
0.89 | 0.12 | ||||||
C-208
| 2009 |
||||||||
| First Quarter |
0.97 | 0.05 | ||||||
| Second Quarter |
2.23 | 0.27 | ||||||
| Third Quarter |
1.83 | 1.10 | ||||||
| Fourth Quarter |
1.30 | 0.86 |
On February 22, 2009, the last reported sale price of our common stock on the NASDAQ Capital Market was $0.71 per share. As of February 22, 2009, there were approximately 234 shareholders of record of our common stock.
Dividend Policy
We have never declared or paid any cash dividends on our common stock and do not currently anticipate declaring or paying cash dividends on our common stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance operations. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and other factors that our board of directors may deem relevant.
Sales of Unregistered Securities
Not applicable.
Stock Repurchases in the Fourth Quarter
Not applicable.
8. Employee Benefit Plans
CTI’s U.S. employees participate in the Cell Therapeutics, Inc. 401(k) Plan whereby eligible employees may defer up to 80% of their compensation, up to the annual maximum allowed by the Internal Revenue Service. We may make discretionary matching contributions based on certain plan provisions. We made contributions of $0.1 million during each of the years ended December 31, 2009, 2008 and 2007.
In connection with our merger with Novuspharma, on January 1, 2004, we assumed a defined benefit plan and related obligation for benefits owed to our Italian employees who, pursuant to Italian law, are entitled to a lump sum payment upon separation from the Company. Related costs are accrued over the employees’ service periods based on compensation and years of service. In accordance with ASC 715, Compensation-Retirement Benefits, we elected to carry the obligation under the plan at the amount of the vested benefit obligation which is defined as the actuarial present value of the vested benefit to which the employee is entitled if the employee separates immediately. Benefits of $0.6 million, $0.5 million and $0.3 million were paid to employees who separated from the Company during 2009, 2008 and 2007, respectively. As of December 31, 2009 and 2008, the vested benefit obligation was $0.6 million and $0.9 million and was included in current portion of long-term obligations, and long-term obligations, less current portion, respectively. We expect that the remaining vested benefit obligation will be paid by mid-2010 in connection with the reduction in force of our Italian employees related to our 2009 restructuring activities.
C-209
9. Contractual Arrangements and Commitments
Lease Agreements
Facilities
We lease our office and laboratory space under operating leases. Leases for our corporate office space contain an annual escalation clause of approximately 3% and the related rent expense is recognized on a straight-line basis over the term of the respective lease. In connection with a lease agreement, we have a $0.7 million irrevocable, unconditional standby letter of credit which is secured by a certificate of deposit classified in our consolidated balance sheet in other assets as of December 31, 2009 and 2008. Rent expense amounted to $3.4 million, $4.6 million and $4.0 million for the years ended December 31, 2009, 2008 and 2007, respectively. Rent expense is net of sublease income and amounts offset to excess facilities charges (see Note 6, Restructuring Activities).
We entered into sublease agreements to sublet a portion of our facilities considered to be in excess of current requirements. These subleases expired in 2008 along with the related original lease. Total sublease rental income for fiscal years 2008 and 2007 was $0.1 million and $1.0 million, respectively, and was recorded as an offset to lease expense.
Future Minimum Lease Payments
Future minimum lease commitments for noncancelable operating leases at December 31, 2009 are as follows (in thousands):
| Operating Leases |
||||
| 2010 |
$ | 4,470 | ||
| 2011 |
4,426 | |||
| 2012 |
2,673 | |||
| 2013 |
179 | |||
| 2014 |
90 | |||
| Thereafter |
— | |||
| Total minimum lease commitments |
$ | 11,838 | ||
As of December 31, 2009 and 2008, we had a liability of $0.9 million and $1.1 million, respectively, in charges for excess facilities under our current operating leases in accordance with ASC 420, Exit or Disposal Obligations, or ASC 420 (see Note 6, Restructuring Activities).
10. Other Significant Events
a. Formation of Joint Venture
For a description of the transaction please refer to the subsequent Paragraph 22.1.7.
C-210
b. Restructuring Activities
Italian Operations
In September 2009, we closed our Bresso, Italy operations. These operations were used primarily for pre-clinical research and were underutilized due to our current focused business model on the development of late-stage compounds and their commercialization. We have recorded restructuring charges related to this closure as discussed further below in accordance with ASC 420.
Due to the restructuring of CTI and the need to focus on late stage development and commercialization, in May 2009, we entered into a severance agreement with the employee union of the Italian branch of CTI that worked in the area of preclinical research and early development. This severance agreement relates to a reduction in force of 56 positions and the closure of our Bresso, Italy facility. Employee separation costs associated with the reduction in force primarily relate to severance payments that we are paying over 42 months, with the majority of these payments made through the first 15 months. In addition, we have entered into separate severance/termination agreements with our Bresso-based scientific directors and which have been accrued for as of December 31, 2009 For the year ended December 31, 2009, we recorded $2.6 million in employee termination benefits related to these Bresso employees and directors of which $1.5 million was unpaid and included in accrued expenses as of December 31, 2009. We may have adjustments to our employee termination benefit expense related to our estimate of amounts due under Italian labor laws. While we cannot predict additional amounts, if any, we do not expect to have material adjustments to this expense.
In connection with the closure of the Bresso operations, we had certain contract termination and clean-up charges related to the Bresso facility’s laboratories. For the year ended December 31, 2009 we recorded $1.5 million for these charges which was paid during 2009. We completed closure of the Bresso facility in September 2009.
We also had certain laboratory equipment related to the Bresso facility that we sold in connection with the closure of the facility. We recognized a $0.3 million gain on the sale of these assets which is included in restructuring charges and related gain on sale of assets, net for the year ended December 31, 2009.
Zevalin Operations
In connection with the sale of our 50% interest in RIT Oncology to Spectrum, we terminated certain employees directly and indirectly involved in the operations of Zevalin. During the first half of 2009, we terminated 24 Zevalin-related employees. We recorded employee separation costs of $0.1 million in accordance with ASC 420 for the year ended December 31, 2009 which is included in restructuring charges and related gain on sale of assets, net. All amounts have been paid as of December 31, 2009 and we do not expect to incur additional restructuring charges related to this transaction.
2005 Restructuring
During 2005, we reduced our workforce in the United States and Europe. In conjunction with this, we vacated a portion of our laboratory and office facilities and recorded excess facilities charges. Charges for excess facilities relate to our lease obligation for excess laboratory and office space in the United States that we vacated as a
C-211
result of the restructuring plan. We recorded these restructuring charges when we ceased using this space. As of December 31, 2009 we had $0.9 million accrued related to excess facilities charges, of which $0.4 million was included in current portion of long-term obligations and $0.5 million of which was included in long-term obligations, less current portion. We will periodically evaluate our existing needs, the current and estimated future values of our subleases, and other future commitments to determine whether we should record additional excess facilities charges or adjustments to such charges.
The following table summarizes the changes in the liability for our 2005 restructuring activities during the years ended December 31, 2009 and 2008 (in thousands):
| Excess Facilities Liability |
Employee Separation Liability |
|||||||
| Balance at January 1, 2008 |
$ | 1,548 | $ | 9 | ||||
| Adjustments |
161 | 1 | ||||||
| Payments |
(581 | ) | (10 | ) | ||||
| Balance at December 31, 2008 |
1,128 | — | ||||||
| Adjustments |
96 | — | ||||||
| Payments |
(370 | ) | — | |||||
| Balance at December 31, 2009 |
$ | 854 | $ | — | ||||
| 12. | Related Party Transactions |
Please refer to the information disclosed under Chapter 19 of this Registration Document
| 20.1.4 | NOTES TO THE STATEMENTS OF OPERATIONS |
A. Revenues
Revenues consist of the following as of December 31 (in thousands of US dollars):
| December 31, | ||||||||||||||||
| Revenues (In thousands of US dollars) |
Notes | 2009 | 2008 | 2007 | ||||||||||||
| Product sales |
1 | $ | — | $ | 11,352 | $ | 47 | |||||||||
| License and contract revenue |
2 | 80 | 80 | 80 | ||||||||||||
| Total revenues |
$ | 80 | $ | 11,432 | $ | 127 | ||||||||||
| 1. | Product Sales |
Product sales consist of the following as of December 31 (in thousands of US dollars):
| December 31, | ||||||||||||
| Product Sales (In thousands of US dollars) |
2009 | 2008 | 2007 | |||||||||
| CTI |
$ | — | $ | 11,352 | $ | 47 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total product sales |
$ | — | $ | 11,352 | $ | 47 | ||||||
C-212
Product sales for the year ended December 31, 2008 and 2007 relate to Zevalin and increased due to the fact that we did not acquire Zevalin from Biogen until December 2007.
Customer and Geographic Concentrations
We consider our operations to be a single operating segment focused on the development, acquisition and commercialization of novel treatments for cancer. Financial results of this reportable segment are presented in the accompanying consolidated financial statements.
Product sales from Zevalin’s major customers as a percentage of total product sales were as follows:
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| Customer A |
77 | % | 67 | % | ||||
| Customer B |
5 | % | 33 | % | ||||
All sales of Zevalin during 2008 and 2007 were to North America. The following table depicts long-lived assets based on the following geographic locations (in thousands):
| Year Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
| United States |
$ | 22,966 | $ | 39,777 | ||||
| Europe |
7,286 | 11,099 | ||||||
| $ | 30,252 | $ | 50,876 | |||||
| 2. | License and Contract Revenue |
License and contract revenue consists of the following as of December 31 (in thousands of US dollars):
| License and contract revenue | December 31, | |||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||
| CTI |
$ | 80 | $ | 80 | $ | 80 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total license and contract revenue |
$ | 80 | $ | 80 | $ | 80 | ||||||
We may generate revenue from technology licenses, collaborative research and development arrangements, cost reimbursement contracts and research grants. Revenue under technology licenses and collaborative agreements typically consists of nonrefundable and/or guaranteed technology license fees, collaborative research funding, and various milestone and future product royalty or profit-sharing payments.
Revenue associated with up-front license fees and research and development funding payments under collaborative agreements is recognized ratably over the relevant periods specified in the agreement, generally the
C-213
research and development period. If the time period is not defined in the agreement, we calculate the revenue recognition period based on our current estimate of the research and development period considering experience with similar projects, level of effort and the stage of development. Should there be a change in our estimate of the research and development period, we will revise the term over which the initial payment is recognized. Revenue from substantive at-risk milestones and future product royalties is recognized as earned based on the completion of the milestones and product sales, as defined in the respective agreements. Revenue under cost reimbursement contracts and research grants is recognized as the related costs are incurred. Payments received in advance of recognition as revenue are recorded as deferred revenue.
For multiple element arrangements that had continuing performance obligations, we recognized contract, milestone or license fees together with any up-front payments over the term of the arrangement as we completed our performance obligation, unless the delivered technology had stand alone value to the customer and there was objective, reliable evidence of fair value of the undelivered element in the arrangement. Additionally, unless evidence suggests otherwise, revenue from consideration received was recognized on a straight-line basis over the expected term of the arrangement.
License and contract revenue for the year ended December 31, 2009, 2008 and 2007 represents recognition of deferred revenue from the sale of Lisofylline material to Diakine.
B. Operating expenses
Operating expenses consist of the following as of December 31 (in thousands of US dollars):
| Operating expenses | Notes | December 31, | ||||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||||
| Cost of product sold |
3 | $ | — | $ | 3,244 | $ | 49 | |||||||
| Research and development |
4 | 30,179 | 51,614 | 72,019 | ||||||||||
| Selling, general and administrative |
5 | 57,725 | 41,607 | 35,517 | ||||||||||
| Amortization of purchased intangibles |
6 | — | 1,658 | 913 | ||||||||||
| Restructuring charges and related gain on sale of assets, net |
3,979 | — | — | |||||||||||
| Gain on sale of Zevalin |
7 | — | (9,444 | ) | — | |||||||||
| Gain on sale of investment in joint venture |
(10,244 | ) | — | — | ||||||||||
| Acquired in-process research and development |
8 | — | 36 | 24,615 | ||||||||||
| Total operating expenses |
$ | 81,639 | $ | 88,715 | $ | 133,113 | ||||||||
| 3. | Cost of Product Sold |
Cost of product sold consists of the following as of December 31 (in thousands of US dollars):
| Cost of product sold | December 31, | |||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||
| CTI |
$ | — | $ | 3,244 | $ | 49 | ||||||
| Subsidiaries |
— | — | — | |||||||||
| Total cost of product sold |
$ | — | $ | 3,244 | $ | 49 | ||||||
C-214
Cost of product sold for the years ended December 31, 2008 and 2007 relates to sales of Zevalin and consists primarily of contractual royalties on product sales in addition to cost of product sold to customers. The increase in cost of product sold is consistent with the increase in product sales.
| 4. | Research and Development Expenses |
Our research and development expenses for compounds under development and discovery research are as follows (in thousands):
| 2009 | 2008 | |||||||
| Compounds under development: |
||||||||
| Pixantrone |
$ | 6,256 | $ | 8,238 | ||||
| OPAXIO |
3,365 | 4,145 | ||||||
| Brostallicin |
1,096 | 3,860 | ||||||
| Zevalin |
987 | 5,271 | ||||||
| Other compounds |
137 | 391 | ||||||
| Operating expenses |
17,920 | 27,878 | ||||||
| Discovery research |
418 | 1,831 | ||||||
| Total research and development expenses |
$ | 30,179 | $ | 51,614 | ||||
Costs for compounds under development include external direct expenses such as principal investigator fees, clinical research organization charges and contract manufacturing fees incurred for preclinical, clinical, manufacturing and regulatory activities associated with preparing the compounds for submissions of NDAs or similar regulatory filings to the FDA, EMA or other regulatory agencies outside the United States and Europe. Operating costs include our personnel and occupancy expenses associated with developing these compounds. Discovery research costs include primarily personnel, occupancy and laboratory expenses associated with the discovery and identification of new drug targets and lead compounds. We do not allocate operating costs to the individual compounds under development as our accounting system does not track these costs by individual compound. As a result, we are not able to capture the total cost of each compound. Direct external costs incurred to date for pixantrone, OPAXIO and brostallicin are $55.1 million, $220.6 million, and $9.2 million, respectively. Costs for pixantrone prior to our merger with Novuspharma S.p.A, a public pharmaceutical company located in Italy, in January 2004 are excluded from this amount. Costs for brostallicin prior to our acquisition of SM in July 2007 are also excluded from this amount.
Research and development expenses decreased to $30.2 million for the year ended December 31, 2009, from $51.6 million for the year ended December 31, 2008. Pixantrone costs decreased primarily due to a decrease in clinical development activity mainly related to the cessation of patient enrollment during 2008 in our RAPID and EXTEND trials. In early 2008, we closed enrollment on the RAPID trial based on adequate sample size to demonstrate differences in cardiac events and other clinically relevant side effects between pixantrone and doxorubicin. Additionally, we closed enrollment on the EXTEND trial during 2008 as we believed that the current accrual rate would not contribute substantially to the trial’s chance of success. Manufacturing activity for pixantrone decreased during the period. These decreases were partially offset by an increase in clinical activity due to a change in estimate of costs associated with our PIX303 trial, which was closed in early 2008 based on, among other considerations, our plans to refocus our resources on obtaining pixantrone approval based on the EXTEND trial before making additional substantive investments in alternative indications. In addition,
C-215
regulatory activities increased primarily due to consulting costs and the filing fee for the NDA submission to the FDA. Costs for our OPAXIO program decreased primarily due to a decrease in regulatory and quality activities as well as investigator-sponsored trial costs mainly due to patient enrollment. These decreases were partially offset by an increase in clinical development activity related to our PGT307 trial as well as an increase in the GOG0212 study related to the August 2008 amendment to our contract with the GOG, which resulted in a reduction in scope of the GOG0212 study and, accordingly, a reversal of accrued expenses during that period. Costs for brostallicin decreased primarily due to a decrease in clinical development activities related to phase I and phase II studies. Zevalin costs decreased primarily due to the contribution of the product to RIT Oncology, the joint venture we formed with Spectrum on December 15, 2008, which assumed all related Zevalin expenses subsequent to that date. The decrease related to the divestiture of the Zevalin product was partially offset by a change in estimate of our costs associated with clinical studies prior to the divestiture of Zevalin. Our operating expenses decreased primarily due to a reduction in personnel and overhead costs associated with the closure of our Bresso, Italy facility as well as external consulting costs, partially offset by an increase in stock-based compensation costs associated with restricted stock awards. Discovery research also decreased due to the closure of the Bresso, Italy operations as we shift focus to other products closer to commercialization.
Research and development expenses decreased to approximately $51.6 million for the year ended December 31, 2008, from approximately $72.0 million for the year ended December 31, 2007. Pixantrone costs decreased primarily due to a decrease in clinical development activity mainly related to the closure of our PIX303 clinical trial in the fourth quarter of 2007 as well as a discontinuance of patient enrollment during 2008 in our RAPID and EXTEND trials. We closed the PIX303 trial based on, among other considerations, our plans to refocus the Company’s resources on obtaining pixantrone approval based on the EXTEND phase III trial before making additional substantial investments in alternative indications for pixantrone as well as the changing competitive landscape in second line follicular NHL. In early 2008, we closed enrollment on the RAPID trial based on adequate sample size to demonstrate differences in cardiac events and other clinically relevant side effects between pixantrone and doxorubicin. Additionally, we closed enrollment on the EXTEND trial during 2008 as we believed that the current accrual rate would not contribute substantially to the trial’s chance of success. These decreases were partially offset by an increase in manufacturing activity for pixantrone. Costs for Zevalin increased due to our acquisition of the product in December 2007 and primarily relate to clinical development activity including $2.0 million in expense related to our payment to Bayer Schering for access to the data from the FIT trial. Our Zevalin product was contributed to RIT Oncology, a joint venture we formed with Spectrum, on December 15, 2008 and all related expenses subsequent to this date have been assumed by the joint venture. In addition, as of March 9, 2009, we are engaged in the process of selling our interest in the joint venture to Spectrum. Costs for our OPAXIO program decreased primarily due to a decrease in clinical development activity related to our PGT307 trial, which was reduced in scope to U.S. sites only in early 2008, reduced costs associated with our PIONEER trial which was suspended and closed in the fourth quarter of 2006 and incurred certain wrap-up costs in the first half of 2007 and a decrease in the GOG0212 study related to the amendment to our contract with the GOG. Manufacturing activity for OPAXIO also decreased as we extended activities into 2009 in an effort to conserve cash in 2008. Costs for brostallicin decreased primarily due to a non-recurring license payment during 2007 related to a development agreement, partially offset by an increase in clinical development activities related to phase I and phase II studies. Our operating expenses remained fairly consistent in both years, while our discovery research decreased slightly due to a shift in focus to our commercial product Zevalin, which was transferred to the joint venture, as well as other products closer to commercialization.
C-216
| 5. | Selling, General and administrative expenses |
Selling, general and administrative expenses consist of the following as of 31 December (in thousands of US dollars):
| Selling, general and administrative expenses (In thousands of US dollars) |
December 31, | |||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| CTI |
$ | 54,829 | $ | 156,343 | $ | 28,893 | ||||||
| Subsidiaries |
2,896 | (114,736 | ) | 6,624 | ||||||||
| Total selling, general and administrative |
$ | 57,725 | $ | 41,607 | $ | 35,517 | ||||||
Selling, general and administrative expenses increased to $57.7 million for the year ended December 31, 2009, from $41.6 million for the year ended December 31, 2008. This is primarily due to an $18.9 million increase in non-cash stock-based compensation mainly related to restricted stock granted and vested during 2009. This was offset, in part by a decrease in compensation and benefits due to a reduction in headcount primarily related to our restructuring activities and our sale of Zevalin. If we receive FDA approval for pixantrone, we expect selling, general and administrative expenses to increase in 2010 as compared to 2009 due to increased sales and marketing expenses for pixantrone, including increased compensation expense for our pixantrone sales force.
Selling, general and administrative expenses increased to approximately $41.6 million for the year ended December 31, 2008, from approximately $35.5 million for the year ended December 31, 2007. This is primarily attributed to a $4.8 million increase in sales and marketing expenses due to the acquisition of Zevalin in December 2007 and subsequent expansion of our sales force. In addition, we incurred approximately $1.2 million in legal and consulting fees associated with the potential spin-off, asset divestment, or creation of a joint venture with regard to certain of our operations and assets. We also had an increase in our stock-based compensation expense of approximately $1.8 million as well as an increase in our legal expenses of approximately $0.9 million primarily due to our claim against the Lash Group, Inc. and Documedics Acquisition Co., Inc. Compensation and benefits also increased approximately $0.6 million in part due to key executive personnel hired in 2008. These increases were offset by a $1.3 million decrease in finance and administration and human resources expenses in our Italian operations due to a reduced level of activities. In addition, corporate development expenses decreased approximately $0.8 million primarily related to a reduction in travel costs. Finance and administration expenses also decreased approximately $0.8 million primarily due to a decrease in expenses associated with our shareholder meetings as well as a decrease in certain taxes and insurance premiums. The large fluctuation from December 31, 2007 and December 31, 2008 between CTI standalone and its subsidiaries (from about 28.9 USD/mln. to about 156.3 USD/mln. for CTI and from about 6.6 USD/mln. to about 114.7 USD/mln.) is primarily due to an intercompany transaction to record (for accounting purposes) the change of CTEU’s legal status as it relates to the parent company, CTI. In fact in November 2007 CTEU was merged into the Company and the relevant accounting effects were recorded in 2008. From an exclusive accounting point of view this change of CTEU from a subsidiary of CTI to a branch of CTI resulted in a loss of approximately $119.7 million on CTI’s books due to the write-off of CTI’s investment in CTEU (in particular, in order to write–off this investment that was reported in
C-217
the “Assets” of CTI’s balance sheets, the account “Selling, general and administrative expenses” included in CTI’s statements of operations has been debited) and a corresponding gain of approximately $119.7 on CTEU’s books (in particular, in order to write–off the corporate capital of CTEU that was reported in the “Liabilities and shareholders’ deficit” of CTEU’s balance sheets, the account “Selling, general and administrative expenses” included in CTEU’s statements of operations has been credited). This intercompany transaction did not have any impact on CTI’s cash flow and the relevant amounts, recorded only for accounting purposes, eliminate on a consolidated level and, as such, are not reflected in CTI’s consolidated statement of operations for the periods.
| 6. | Amortization of purchased intangibles |
Amortization of purchased intangibles consists of the following as of 31 December (in thousands of US dollars):
| Amortization of purchased intangibles | December 31, | |||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||
| CTI |
$ | — | $ | 669 | $ | 43 | ||||||
| Subsidiaries |
— | 989 | 870 | |||||||||
| Total amortization of purchased intangibles |
$ | — | $ | 1,658 | $ | 913 | ||||||
Amortization for the year ended December 31, 2008 was due to amortization of our workforce intangible related to our Italian operations, which became fully amortized during 2008, and amortization of intangible assets acquired in connection with our acquisition of Zevalin in December 2007, which were contributed to RIT Oncology in December 2008.
Amortization for the year ended December 31, 2007 is primarily related to the amortization of our assembled workforce asset in our European branch.
| 7. | Gain on sale of Zevalin |
The gain on sale of Zevalin for the year ended December 31, 2008 is related to the gain recognized, net of transaction costs, on the sale of Zevalin to RIT Oncology, the 50/50 joint venture we formed with Spectrum. Due to the fact that we received cash for assets contributed, we recorded a gain based on the difference between the book value of the assets contributed and the fair value of these assets as recorded under the joint venture.
| 8. | Acquired in-process research and development |
Acquired in-process research and development for the year ended December 31, 2008 relates to adjustments to our one-time charge recorded in connection with our acquisition of Zevalin in
C-218
December 2007. These adjustments resulted from changes in the estimated acquisition costs used in determining the total estimated purchase price of the acquisition.
Acquired in-process research and development for the year ended December 31, 2007 relates to one-time charges of $21.4 million and $3.2 million recorded in connection with our acquisitions of SM and Zevalin, respectively.
C. Other income (expense)
Other income (expense) consists of the following as of December 31 (in thousands of US dollars):
| Other income (expense) | Notes | December 31, | ||||||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||||||
| Investment and other income |
9 | $ | 133 | $ | 549 | $ | 2,430 | |||||||||
| Interest expense |
10 | (4,806 | ) | (8,559 | ) | (8,237 | ) | |||||||||
| Amortization of debt discount and issuance costs |
11 | (5,788 | ) | (66,530 | ) | (4,280 | ) | |||||||||
| Foreign exchange gain |
12 | 33 | 3,637 | 4,657 | ||||||||||||
| Make-whole interest expense |
13 | (6,345 | ) | (70,243 | ) | (2,310 | ) | |||||||||
| Gain on derivative liabilities |
14 | 7,218 | 69,739 | 3,672 | ||||||||||||
| Gain (loss) on exchange of convertible notes |
15 | 7,381 | (25,103 | ) | (972 | ) | ||||||||||
| Write-off of financing arrangement costs |
16 | — | (2,846 | ) | — | |||||||||||
| Equity loss from investment in joint venture |
17 | (1,204 | ) | (123 | ) | — | ||||||||||
| Milestone Modification expense |
(6,000 | ) | — | — | ||||||||||||
| Settlement expense |
18 | (4,710 | ) | (3,393 | ) | (160 | ) | |||||||||
| Total other expense, net |
$ | (14,088 | ) | $ | (102,872 | ) | $ | (5,200 | ) | |||||||
| 9. | Investment and other income, net |
Investment and other income for the year ended December 31, 2009 decreased to $0.1 million as compared to $0.5 million for the year ended December 31, 2008 primarily due to a lower average securities available-for-sale balance.
Investment and other income for the year ended December 31, 2008 decreased to approximately $0.5 million as compared to $2.4 million for the year ended December 31, 2007 primarily due to a lower average securities available-for-sale balance.
| 10. | Interest expense |
Interest expense decreased to $4.8 million for the year ended December 31, 2009 from $8.6 million for the year ended December 31, 2008. This was due to a decrease of $2.4 million in interest expense on our 10% (due 2012), 9%, 7.5%, 6.75% and 5.75% convertible senior notes and our 4% convertible senior subordinated notes due to conversions and exchanges of these notes during 2009. There was also a
C-219
decrease of $1.1 million related to our 18.33%, 15% and 9.66% convertible senior notes, which were issued in and were entirely converted or exchanged by the end of 2008. In addition, interest expense related to our 5.75% convertible subordinated and senior subordinated notes decreased by $0.3 million due to their maturity in June 2008.
Interest expense increased to approximately $8.6 million for the year ended December 31, 2008 from approximately $8.2 million for the year ended December 31, 2007. This was primarily due to increases of approximately $3.0 million related to interest on our 5.75% convertible senior notes issued in December 2007 as well as interest on our 9% notes, 15% notes, 18.33% notes, 9.66% notes and 10% notes due 2012 which were all issued during 2008. These increases were offset by a decrease of $2.8 million in interest expense on our 5.75% convertible subordinated and senior subordinated notes due to the exchange of approximately $36.1 million of these notes for our 5.75% senior notes in December 2007, the cancellation of $9.1 million of these notes in exchange for shares of our common stock in February 2008 and repayment of the remaining amount upon maturity in June 2008.
| 11. | Amortization of debt discount and issuance costs |
Amortization of debt discount and issuance costs decreased to $5.8 million for the year ended December 31, 2009 as compared to $66.5 million for the year ended December 31, 2008. This was primarily due to the accelerated amortization of issuance costs and debt discount related to conversions and exchanges of our 18.33%, 15.5%, 15%, 13.5%, 10% (due 2012), 9.66% and 9% convertible senior notes during 2008. For the year ended December 31, 2009 as compared to the same period in 2008, the decrease in the amortization of the debt discount related to these notes was $55.2 million and the decrease in the amortization of debt issuance costs was $5.4 million.
Amortization of debt discount and issuance costs increased to $66.5 million for the year ended December 31, 2008 as compared to $4.3 million for the year ended December 31, 2007. This increase was primarily due to the accelerated amortization of debt discount and issuance costs related to conversions of certain of our convertible notes issued in 2008. For the year ended December 31, 2008, amortization of the debt discount related to our 13.5% notes, 9% notes, 15.5% notes, 18.33% notes, 10% notes due 2012, 10% notes due 2011 and 9.66% notes was approximately $23.4 million, $13.2 million, $8.6 million, $5.6 million, $3.4 million, $2.2 million and $1.8 million, respectively, and the amortization of debt issuance costs was approximately $2.0 million, $1.9 million, $0.3 million, $0.5 million, $0.4 million, $0.2 million and $0.3 million, respectively. This amortization was primarily due to conversions of these notes during the year ended December 31, 2008. These increases were offset by a decrease of $2.9 million in amortization of debt discount and issuance costs on our 7.5% notes primarily related to conversions of these notes during the year ended December 31, 2007.
C-220
| 12. | Foreign exchange gain |
Foreign exchange gains for the years ended December 31, 2009, 2008 and 2007 are due to fluctuations in foreign currency exchange rates, primarily related to payables and receivables in our European branch denominated in foreign currencies.
| 13. | Make-whole interest expense |
Make-whole interest expense of $6.3 million for the year ended December 31, 2009 is related to $5.4 million in payments made upon the conversion of $18.0 million of our 10% convertible senior notes due 2011 and $0.9 million in payments made upon the conversion of $5.3 million of our 9% convertible senior notes.
The amount of $70.2 million for the year ended December 31, 2008 is related to $22.4 million in payments made upon the conversion of $27.6 million of our 13.5% convertible senior notes, $15.5 million in payments made upon conversion of $28.3 million of our 18.33% convertible senior notes, $11.0 million in payments made upon conversion of $40.8 million of our 9% convertible senior notes, $8.8 million in payments made upon conversion of $14.2 million of our 15.5% convertible senior notes, $4.5 million in payments made upon conversion of $15.7 million of our 9.66% convertible senior notes, $4.4 million in payments made upon conversion of $14.7 million of our 10% convertible senior notes due 2011 and $3.6 million in payments made upon conversion of $9.0 million of our 10% convertible senior notes due 2012.
Make-whole interest expense of $2.3 million for the year ended December 31, 2007 is due to payments made related to the conversion of $13.6 million of our 7.5% notes.
| 14. | Gain on derivative liabilities |
The gain on derivative liabilities of $7.2 million for the year ended December 31, 2009 is primarily due to a gain of $4.4 million resulting from the change in the estimated fair value of the derivative liability related to the embedded conversion option on our 10% convertible senior notes due 2011 as well as a gain of $2.8 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant that was issued in connection with our 13.5% convertible senior notes and Series E preferred stock financing and modified in July 2008 in connection with the issuance of our 18.33% convertible senior notes. The Series B Unit Warrant expired in the second quarter of 2009. The gain of $69.7 million for the year ended December 31, 2008 is primarily due to gains of $22.3 million, $12.0 million, $8.6 million, $6.9 million, $4.6 million, $3.4 million, $2.4 million and $2.2 million resulting from the change in the estimated fair value of the derivative liabilities related to the embedded conversion options on our 13.5%, 9%, 15.5%, 18.33%, 15%, 10% (due 2012), 9.66% and 10% (due 2011) convertible senior notes, respectively. There was also a gain of $7.3 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant.
The gain on derivative liabilities of $69.7 million for the year ended December 31, 2008 is primarily due to gains of $22.3 million, $12.0 million, $8.6 million, $6.9 million, $4.6 million, $3.4 million, $2.4
C-221
million and $2.2 million resulting from the change in the estimated fair value of the derivative liabilities related to the embedded conversion options on our 13.5% notes, 9% notes, 15.5% notes, 18.33% notes, 15% notes, 10% notes due 2012, 9.66% notes and 10% notes due 2011, respectively. There was also a gain of $7.3 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant that was issued in connection with the issuance of our 13.5% notes and Series E preferred stock financing and modified in connection with the issuance of our 15% and 18.33% notes.
The gain on derivative liabilities of $3.7 million for the year ended December 31, 2007 represents the change in the estimated fair value of the derivative liabilities related to the interest make-whole provisions on our 7.5% and 6.75% notes of $3.6 million and $0.1 million, respectively.
A derivative liability relates to the value of an embedded conversion option of the Company’s notes, which also considers the effect of the make-whole provision. The embedded conversion option relates to a note holder’s ability to convert notes into shares of the Company’s common stock at the holder’s option. The embedded conversion option and the make-whole provision are interrelated because make-whole payments are generally triggered upon conversion of the notes.
There are no derivative contracts entered into or executed by the Company.
For a description of the Company’s senior convertible notes, please refer to Chapter 20, Paragraph 20.1.3.B.5 of this Registration Document.
| 15. | Gain (loss) on exchange of convertible notes |
The $7.4 million gain on exchange of convertible notes for the year ended December 31, 2009 is primarily related to $7.2 million due to the exchange of $52.9 million principal amount of portions of our 9%, 7.5%, 6.75% and 5.75% convertible senior notes and 4% convertible senior subordinated notes for $7.1 million in cash and 24.2 million shares of our common stock, net of related transaction costs. In addition, we recorded a $0.2 million gain related to the exchange of $3.0 million of our 4% convertible senior subordinated notes and $1.5 million of our 6.75% convertible senior notes as well as accrued and unpaid interest on these notes for 3.3 million shares of our common stock.
The loss on exchange of convertible notes of $25.1 million for the year ended December 31, 2008 is due to the repurchase of certain of our convertible notes in exchange for new convertible notes or common stock. In July and August 2008, we recorded a $10.3 million loss due to the repurchase of $17.5 million aggregate principal of our 13.5% convertible senior notes in connection with the issuance of our 18.33% convertible senior notes. A loss of $5.5 million was due to the repurchase of $18.2 million of our 15% convertible senior notes in connection with the issuance of our 9.66% convertible senior notes in October 2008. In addition, we repurchased the remaining $4.8 million of our 15% convertible senior notes, $16.3 million of our 18.33% convertible senior notes and $9.0 million of our 9.66% convertible senior notes in connection with the issuance of our 10% convertible senior notes due 2011 and recorded a $3.7 million loss. We also recorded a $3.3 million loss due to the exchange of $5.3 million of our 9% convertible senior notes for units of our 13.5% convertible senior notes, Series E preferred stock and related warrants issued in April 2008 and a loss of $2.3 million due to the extinguishment of $9.1 million aggregate principal amount of our 5.75% convertible senior subordinated and convertible subordinated notes in exchange for 0.7 million shares of our common stock in February 2008.
C-222
We recorded a loss of approximately $1.0 million during the year ended December 31, 2007 due to the extinguishment of approximately $36.1 million aggregate principal amount of our 5.75% convertible senior subordinated and convertible subordinated notes in exchange for approximately $23.3 million aggregate principal amount of our 5.75% convertible senior notes and approximately 0.5 million shares of our common stock in the fourth quarter of 2007.
| 16. | Write-off of financing arrangements costs |
The write-off of financing arrangement costs of $2.8 million for the year ended December 31, 2008 primarily relates to a $2.4 million write-off of offering costs associated with the Step-Up Equity Financing Agreement with Société Générale, including costs related to the Italian Listing Prospectus that was published in January 2008 as an Italian regulatory requirement to issue shares under this agreement. The write-off was primarily due to significant uncertainty regarding our ability to pursue further financings under this agreement which terminated in January 2009. In addition, we wrote-off $0.5 million in expenses associated with our equity line of credit with Midsummer Investment, Ltd., or Midsummer, based on our plans to terminate the agreement; that termination occurred in March 2009.
| 17. | Equity loss from investment in joint venture |
The equity loss from investment in joint venture for the years ended December 31, 2009 and 2008 relates to our 50% interest in RIT Oncology, prior to the sale of this interest in March 2009, which we accounted for using the equity method of accounting.
| 18. | Settlement expense |
Settlement expense of $4.7 million for the year ended December 31, 2009 was due to $3.2 million related to amounts paid to Spectrum for the settlement of the final installment payment related to our sale of our 50% interest in RIT Oncology based on the outcome of arbitration proceedings. This amount includes the $3.5 million escrow amount released to Spectrum, our $0.8 million payment to Spectrum based on arbitration proceedings and $0.9 million in receivables recognized in prior periods and owed to us by RIT Oncology. The settlement amount is also net of $2.0 million in payables assumed by Spectrum on our behalf. We also incurred $1.3 million in settlement expense related to the payment made in accordance with our settlement agreement and release with Ingenix Pharmaceutical Services, Inc., or Ingenix, whereby each party agreed to a full release of the other party from any and all claims related to our dispute with Ingenix. The settlement expense recorded is net of $0.3 million in payables to Ingenix that were relieved from our books.
Settlement expense of $3.4 million for the year ended December 31, 2008 was primarily related to $2.9 million in payments accrued or made to certain of our preferred stockholders for the release of all claims against us in connection with our alleged breach of contract related to their preferred stock held. In addition, we recorded expense of $0.5 million for the settlement of attorney’s fees and costs related to claims brought against us by a private party plaintiff in connection with our litigation with the United States Attorney’s Office, or USAO.
Settlement expense for the year ended December 31, 2007 relates to interest accrued on the $10.5 million payment to the USAO for release of all claims in connection with the investigation of our
C-223
marketing practices relating to TRISENOX and related matters. Interest was accrued from the date of reaching an agreement in principle with the USAO in the fourth quarter of 2006 and the payment was made in April 2007. Settlement expense for the year ended December 31, 2006 is due to $10.5 million accrued for the pending settlement of the USAO litigation and approximately $0.9 million related to the settlement of our dispute with Micromet AG in May 2006 and was net of payables previously due to Micromet.
D. Minority interest in net loss of subsidiary
Minority interest in net loss of subsidiary consists of the following as of December 31 (in thousands of US dollars):
| Minority interest in net loss of subsidiary | December 31, | |||||||||||
| (In thousands of US dollars) | 2009 | 2008 | 2007 | |||||||||
| Minority interest in net loss of subsidiary |
$ | 252 | $ | 126 | $ | 78 | ||||||
Minority interest in net loss of subsidiary was approximately $0.3 million, $0.1 million and $0.1 million for the years ended December 31, 2009, 2008 and 2007, respectively, and represents the minority owner’s pro rata allocation of the losses in Aequus Biopharma, Inc.
Income Taxes
We file income tax returns in the United States, Italy and the United Kingdom. Due to substantial book and tax losses from our global operations, we have reported no income tax provisions in jurisdictions in which we file returns. A substantial part of our operations takes place in the State of Washington, which does not impose an income tax as that term is defined in ASC 740, Income Taxes. As such, our state income tax expense or benefit, if recognized, would be immaterial to our operations. We are not currently under examination by an income tax authority, nor have we been notified that an examination is contemplated.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying values of assets and liabilities for financial reporting and income tax reporting in accordance with ASC 740. We have a valuation allowance equal to net deferred tax assets due to the uncertainty of realizing the benefits of the assets. Our valuation allowance increased $12.0 million, $17.8 million, and $34.6 million during 2009, 2008 and 2007, respectively.
C-224
The reconciliation between our effective tax rate and the income tax rate as of December 31 is as follows:
| 2009 | 2008 | 2007 | ||||||||||
| Federal income tax rate |
(34 | %) | (34 | %) | (34 | %) | ||||||
| Research and development tax credits |
— | — | (1 | ) | ||||||||
| Non-deductible debt/equity costs |
13 | 20 | 4 | |||||||||
| Non-deductible executive compensation |
5 | — | — | |||||||||
| In process research and development |
— | — | 5 | |||||||||
| Valuation allowance |
10 | 9 | 23 | |||||||||
| Expired tax attribute carryforwards |
6 | 4 | 2 | |||||||||
| Other |
— | 1 | 1 | |||||||||
| Net effective tax rate |
— | % | — | % | — | % | ||||||
Significant components of our deferred tax assets and liabilities as of December 31 are as follows (in thousands):
| 2009 | 2008 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 268,220 | $ | 243,616 | ||||
| Capitalized research and development |
59,766 | 68,486 | ||||||
| Research and development tax credit carryforwards |
20,434 | 19,954 | ||||||
| Stock based compensation |
4,069 | 4,485 | ||||||
| Intangible assets |
578 | 1,808 | ||||||
| Depreciation and amortization |
270 | 1,026 | ||||||
| Other deferred tax assets |
1,874 | 3,389 | ||||||
| Total deferred tax assets |
355,211 | 342,764 | ||||||
| Less valuation allowance |
(354,192 | ) | (342,233 | ) | ||||
| 1,019 | 531 | |||||||
| Deferred tax liabilities: |
||||||||
| GAAP adjustments on Novuspharma merger |
(208 | ) | (208 | ) | ||||
| Deductions for tax in excess of financial statements |
(811 | ) | (323 | ) | ||||
| Total deferred tax liabilities |
(1,019 | ) | (531 | ) | ||||
| Net deferred tax assets |
$ | — | $ | — | ||||
As of December 31, 2009, we had net operating loss carryforwards of approximately $788.9 million, of which $83.8 million relates to stock compensation deductions, and research credit carryforwards of approximately $20.0 million. The carryforwards began to expire in 2007.
Due to our equity financing transactions, and other owner shifts as defined in Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, we incurred “ownership changes” pursuant to the Code. Accordingly, our use of net operating loss carryforwards is limited. We are currently studying the impact of Section 382 on the future realization of our various tax attributes. In particular, Section 382 of the Internal Revenue Code imposes a limitation on the amount of income that a corporation may offset with Net Operating Loss and certain other tax attributes upon an “ownership change.” In general, an “ownership change” is an
C-225
aggregate increase of more than 50% of the ownership of a corporation over a prescribed period by the corporation’s “5% shareholders” from the lowest percentage of ownership of these shareholders in that period.
Effective January 1, 2007, we adopted the provisions of FASB Interpretation 48, Accounting for Uncertainty in Income Taxes, as codified in ASC 740-10, and we have analyzed filing positions in our tax returns for all open years. We are subject to United States federal and state, Italian and United Kingdom income taxes with varying statutes of limitations. Tax years from 1995 forward remain open to examination due to the carryover of net operating losses or tax credits. Our policy is to recognize interest related to unrecognized tax benefits as interest expense and penalties as operating expenses. As of December 31, 2009, we had no unrecognized tax benefits and therefore no accrued interest or penalties related to unrecognized tax benefits. We believe that our income tax filing positions and deductions will be sustained on audit and do not anticipate any adjustments that will result in a material change to our consolidated financial position, results of operations and cash flows. Therefore, no reserves for uncertain income tax positions have been recorded.
| 20.1.5 | NOTES TO THE STATEMENT OF SHAREHOLDERS’ DEFICIT AND OTHER COMPREHENSIVE LOSS |
CELL THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ DEFICIT AND OTHER COMPREHENSIVE LOSS
(In thousands)
| Common Stock | Deferred Stock- based |
Accumulated | Accumulated Other Comprehensive |
Total Share- holders’ Equity |
||||||||||||||||||||
| Shares | Amount | Compensation | Deficit | Income/(Loss) | (Deficit) | |||||||||||||||||||
| Balance at December 31, 2006 |
3,639 | 860,691 | — | (961,108 | ) | (1,187 | ) | (101,604 | ) | |||||||||||||||
| Conversion of convertible preferred stock to common stock |
924 | 37,648 | — | — | — | 37,648 | ||||||||||||||||||
| Proceeds from issuance of warrants in connection with issuance of convertible preferred stock, net |
— | 14,526 | — | — | — | 14,526 | ||||||||||||||||||
| Value of beneficial conversion feature of preferred stock |
— | 9,549 | — | — | — | 9,549 | ||||||||||||||||||
| Deemed dividends on preferred stock |
— | — | — | (9,549 | ) | — | (9,549 | ) | ||||||||||||||||
| Conversion of 7.5% convertible senior notes to common stock |
183 | 15,294 | — | — | — | 15,294 | ||||||||||||||||||
| Issuance of common stock in connection with SMI acquisition |
421 | 19,872 | — | — | — | 19,872 | ||||||||||||||||||
| Issuance of common stock in connection with exchange of 5.75% senior subordinated and subordinated notes |
546 | 13,704 | — | — | — | 13,704 | ||||||||||||||||||
| Proceeds from issuance of common stock and warrants, net |
347 | 6,537 | — | — | — | 6,537 | ||||||||||||||||||
| Equity-based compensation |
185 | 1,588 | — | — | — | 1,588 | ||||||||||||||||||
| Other |
(1 | ) | (114 | ) | — | — | — | (114 | ) | |||||||||||||||
| Dividends on preferred stock |
— | — | — | (648 | ) | — | (648 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation gain |
— | — | — | — | (2,807 | ) | (2,807 | ) | ||||||||||||||||
| Unrealized losses on securities available-for-sale |
— | — | — | — | (13 | ) | (13 | ) | ||||||||||||||||
| Net loss for the year ended December 31, 2007 |
— | — | — | (138,108 | ) | — | (138,108 | ) | ||||||||||||||||
C-226
| Common Stock | Deferred Stock- based |
Accumulated | Accumulated Other Comprehensive |
Total Share- holders’ Equity |
||||||||||||||||||||
| Shares | Amount | Compensation | Deficit | Income/(Loss) | (Deficit) | |||||||||||||||||||
| Comprehensive loss |
(140,928 | ) | ||||||||||||||||||||||
| Balance at December 31, 2007 |
6,244 | $ | 979,295 | $ | — | $ | (1,109,413 | ) | $ | (4,007 | ) | (134,125 | ) | |||||||||||
| Conversion of convertible preferred stock to common stock |
463 | 17,832 | — | — | — | 17,832 | ||||||||||||||||||
| Conversion of 18.33% convertible senior notes to common stock |
3,576 | 28,250 | — | — | — | 28,250 | ||||||||||||||||||
| Conversion of 15.5% convertible senior notes to common stock |
11,189 | 14,210 | — | — | — | 14,210 | ||||||||||||||||||
| Conversion of 13.5% convertible senior notes to common stock |
3,494 | 27,600 | — | — | — | 27,600 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2012 to common stock |
7,087 | 9,000 | — | — | — | 9,000 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
106,944 | 14,651 | — | — | — | 14,651 | ||||||||||||||||||
| Conversion of 9.66% convertible senior notes to common stock |
41,316 | 15,700 | — | — | — | 15,700 | ||||||||||||||||||
| Conversion of 9% convertible senior notes to common stock |
2,895 | 40,820 | — | — | — | 40,820 | ||||||||||||||||||
| Conversion of 5.75% convertible senior notes to common stock |
8 | 250 | — | — | — | 250 | ||||||||||||||||||
| Issuance of common stock in connection with exchange of 5.75% convertible subordinated and senior subordinated notes |
685 | 11,133 | — | — | — | 11,133 | ||||||||||||||||||
| Issuance of common stock in connection with financing agreement |
80 | 1,183 | — | — | — | 1,183 | ||||||||||||||||||
| Issuance of common stock under the Midsummer Equity Line |
1,545 | 4,351 | — | — | — | 4,351 | ||||||||||||||||||
| Premium on 15% convertible senior notes due to exercise of Series B warrant |
— | 11,158 | — | — | — | 11,158 | ||||||||||||||||||
| Issuance of warrants in connection with the 9% convertible senior notes |
— | 3,358 | — | — | — | 3,358 | ||||||||||||||||||
| Issuance of warrants in connection with the 13.5%, 15% and 18.33% convertible senior notes |
— | 7,491 | — | — | — | 7,491 | ||||||||||||||||||
| Repurchase of warrants in connection with the issuance of 13.5% and 18.33% notes |
— | (2,042 | ) | — | — | — | (2,042 | ) | ||||||||||||||||
| Equity-based compensation |
878 | 3,995 | — | — | — | 3,995 | ||||||||||||||||||
| Minority interest |
— | (126 | ) | — | — | — | (126 | ) | ||||||||||||||||
| Other |
8 | (38 | ) | — | — | — | (38 | ) | ||||||||||||||||
| Dividends on preferred stock |
— | — | — | (662 | ) | — | (662 | ) | ||||||||||||||||
| Preferred stock beneficial conversion feature |
— | — | — | (1,067 | ) | — | (1,067 | ) | ||||||||||||||||
| Deemed dividends on conversion of preferred stock |
— | — | — | (21,149 | ) | — | (21,149 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation gains |
— | — | — | — | (3,801 | ) | (3,801 | ) | ||||||||||||||||
| Unrealized losses on securities available-for-sale |
— | — | — | — | (4 | ) | (4 | ) | ||||||||||||||||
| Net loss for the year ended December 31, 2008 |
— | — | — | (180,029 | ) | — | (180,029 | ) | ||||||||||||||||
| Comprehensive loss |
(183,834 | ) | ||||||||||||||||||||||
| Balance at December 31, 2008 |
186,412 | 1,188,071 | — | (1,312,320 | ) | (7,812 | ) | (132,061 | ) | |||||||||||||||
| Issuance of common stock and warrants |
49,732 | 59,233 | — | — | — | 59,233 | ||||||||||||||||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
131,387 | 18,000 | — | — | — | 18,000 | ||||||||||||||||||
| Conversion of 9% convertible senior notes to common stock |
372 | 5,250 | — | — | — | 5,250 | ||||||||||||||||||
| Conversion of Series F preferred stock to common stock |
47,871 | 3,866 | — | — | — | 3,866 | ||||||||||||||||||
| Conversion of Series 1 preferred stock to common stock |
66,667 | 18,537 | — | — | — | 18,537 | ||||||||||||||||||
| Conversion of Series 2 preferred stock to common stock |
18,853 | 27,796 | — | — | — | 27,796 | ||||||||||||||||||
C-227
| Value of beneficial conversion features related to Series 1 and 2 preferred stock |
— | 13,194 | — | — | — | 13,194 | ||||||||||||||||||
| Issuance of warrants in connection with Series 2 preferred stock |
— | 6,138 | — | — | — | 6,138 | ||||||||||||||||||
| Exercise of Class A warrants |
9,184 | 5,222 | — | — | — | 5,222 | ||||||||||||||||||
| Exercise of Class B warrants |
10,378 | 5,732 | — | — | 5,732 | |||||||||||||||||||
| Issuance of common stock in exchange for convertible notes |
27,535 | 39,523 | — | — | — | 39,523 | ||||||||||||||||||
| Issuance of common stock in connection with Series A preferred stock settlement |
4,000 | 509 | — | — | — | 509 | ||||||||||||||||||
| Issuance of common stock in exchange for milestone modification |
5,607 | 6,000 | — | — | — | 6,000 | ||||||||||||||||||
| Conversion or exchange of Series A, B and D convertible preferred stock to common stock |
3,786 | 4,288 | — | — | — | 4,288 | ||||||||||||||||||
| Reacquisition of BCF in connection with exchange of Series A, B and C convertible preferred stock for Series F preferred stock |
— | (961 | ) | — | — | — | (961 | ) | ||||||||||||||||
| Equity-based compensation |
33,821 | 24,937 | — | — | — | 24,937 | ||||||||||||||||||
| Repurchase of shares in connection with taxes on restricted stock vesting |
(5,364 | ) | (6,394 | ) | — | — | — | (6,394 | ) | |||||||||||||||
| Employee stock purchase plan |
42 | 36 | — | — | — | 36 | ||||||||||||||||||
| Noncontrolling interest |
— | (47 | ) | — | — | (205 | ) | (252 | ) | |||||||||||||||
| Dividends on preferred stock |
— | 1 | (24 | ) | — | — | (23 | ) | ||||||||||||||||
| Gain on restructuring of preferred stock |
2,116 | — | — | 2,116 | ||||||||||||||||||||
| Deemed dividends on preferred stock |
— | — | (23,460 | ) | — | — | (23,460 | ) | ||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Foreign currency translation loss |
— | — | — | (601 | ) | — | (601 | ) | ||||||||||||||||
| Unrealized gains on securities available-for-sale |
— | — | — | 1 | — | 1 | ||||||||||||||||||
| Net loss for the year ended December 31, 2009 |
— | — | (95,395 | ) | — | — | (95,395 | ) | ||||||||||||||||
| Comprehensive loss |
(95,995 | ) | ||||||||||||||||||||||
| Balance at December 31, 2009 |
590,283 | $ | 1,418,931 | $ | (1,429,083 | ) | $ | (8,412 | ) | $ | (205 | ) | $ | (18,769 | ) | |||||||||
Common Stock
The increase in common stock as of December 31, 2009 as compared to December 31, 2008 is primarily due to $62.8 million related to the conversion and exchanges of convertible notes to common stock, $59.2 million related to the issuance of common stock and warrants, $54.5 million related to conversions of convertible
C-228
preferred stock to common stock, $24.9 million related to equity-based compensation, $13.2 million related to the value of the beneficial conversion feature of the convertible preferred stock issued and converted, $11.0 million related to the issuance of common stock upon exercise of common stock purchase warrants, and $6.1 million related to the issuance of warrants in connection with the issuance of convertible preferred stock.
The increase in common stock as of December 31, 2008 as compared to December 31, 2007 is primarily due to $17.8 million in conversions of convertible preferred stock to common stock, $150.5 million in conversions of convertible senior notes to common stock, $16.7 million in issuance of common stock, $10.9 million in issuance of warrants, and $11.2 million premium on 15% convertible senior notes due to exercise of Series B warrant.
The increase in common stock as of December 31, 2007 as compared to December 31, 2006 is primarily due to $37.6 million in conversions of convertible preferred stock to common stock, $19.9 million of common stock issued in connection the acquisition of SM, $15.3 million related to the conversion of 7.5% convertible senior notes to common stock, $14.5 million related to the issuance of warrants in connection with the issuance of CTI’s convertible preferred stock, $13.7 million related to the issuance of common stock in connection with the exchange of CTI’s 5.75% senior subordinated and subordinated notes, $9.5 million related to the value of the beneficial conversion feature of the convertible preferred stock and $6.5 million due to the issuance of common stock and warrants.
Deferred Stock-Based Compensation
In 2006 we reversed all remaining deferred stock-based compensation in connection with our implementation of SFAS 123R.
Accumulated Other Comprehensive Loss
The change in accumulated other comprehensive loss as of December 31, 2009 as compared to December 31, 2008 is primarily due to a foreign currency translation loss of approximately $0.6 million.
The change in accumulated other comprehensive loss as of December 31, 2008 as compared to December 31, 2007 is primarily due to a foreign currency translation loss of approximately $3.8 million.
The change in accumulated other comprehensive loss as of December 31, 2007 as compared to December 31, 2006 is primarily due to a foreign currency translation loss of approximately $2.8 million.
Accumulated Deficit
The increase in accumulated deficit for all periods shown is due to CTI’s net losses.
| 20.2 | PRO-FORMA FINANCIAL INFORMATION |
No pro-forma financial information is provided due to the lack of significant changes relative to one or more indicators of the size of the issuer’s business.
C-229
| 20.3 | Significant Accounting Policies |
Principles of Consolidation
The consolidated financial statements include the accounts of CTI and its wholly owned subsidiaries which include Systems Medicine LLC, or SM (from the date of acquisition on July 31, 2007), CTI Commercial LLC (from the date of formation in July 2008), CTI Life Sciences Limited (from the date of formation in March 2009) and Cell Therapeutics Inc. – Sede Secondaria, or CTI (Europe), which is a branch of the Company; however, the Company ceased operations related to this branch in September 2009. In addition, CTI Corporate Development, Inc. and CTI Technologies, Inc. were liquidated in the fourth quarter of 2009 and 2007, respectively.
As of December 31, 2009, we also had a 69% interest in our majority owned subsidiary, Aequus Biopharma, Inc. In accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, 810, Consolidation, noncontrolling interest in Aequus (previously shown as minority interest) is reported below net loss in noncontrolling interest in the consolidated income statement and shown as a component of equity in the consolidated balance sheet.
Additionally, we held a 50% interest in RIT Oncology from the date of its formation in December 2008 to the sale of our interest in March 2009 which we accounted for using the equity method of accounting.
All intercompany transactions and balances are eliminated in consolidation.
Reverse Stock-Split
We effected a one-for-ten and one-for-four reverse stock split of our common stock on August 31, 2008 and April 15, 2007, respectively. All impacted amounts included in the consolidated financial statements and notes thereto have been retroactively adjusted for the stock splits. Impacted amounts include shares of common stock authorized and outstanding, share issuances, shares underlying preferred stock, convertible notes, warrants and stock options, shares reserved and loss per share.
Liquidity
Our accompanying consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates realization of assets and the satisfaction of liabilities in the normal course of business for the twelve month period following the date of these financials. However, we have incurred losses since inception and unless we receive FDA approval for pixantrone, we expect to generate losses from operations for at least the next couple of years due to research and development costs for pixantrone, OPAXIO and brostallicin. Our available cash and cash equivalents are $37.8 million as of December 31, 2009 and we also received gross proceeds of $30.0 million in January 2009 for the issuance of 30,000 shares of our Series 3 preferred stock and related warrants. We have achieved cost saving initiatives to reduce operating expenses, including the reduction of employees related to Zevalin operations and the closure of our operations in Italy as discussed in Note 6, Restructuring Activities, and we continue to seek additional areas for cost reductions. However, we will also need to raise additional funds and are currently exploring alternative sources of equity or debt financing. We may seek to raise such capital through public or private equity financings, partnerships, joint ventures, disposition of assets, debt financings or restructurings, bank borrowings or other sources. However, additional funding may not be available on favorable terms or at all. If additional funds are raised by issuing equity securities, substantial dilution to existing shareholders may result. If we fail to obtain additional capital when needed, we may be required to delay, scale back, or eliminate some or all of our research and development
C-230
programs. The accompanying consolidated financial statements do not include any adjustments that may result from the outcome of this uncertainty.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. For example, estimates include assumptions used in calculating stock-based compensation expense, our allocation of purchase price to acquired assets and liabilities, our liability for excess facilities, the useful lives of fixed assets, the fair value of our derivatives, calculating our tax provision and related valuation allowance, and determining potential impairment of goodwill and other intangible assets. Actual results could differ from those estimates.
Cash and Cash Equivalents
We consider all highly liquid debt instruments with maturities of three months or less at the time acquired to be cash equivalents. Cash equivalents represent short-term investments consisting of investment-grade corporate and government obligations, carried at cost, which approximates market value.
Certain Risks and Concentrations
We are exposed to risks associated with foreign currency transactions to use U.S. dollars to make contract payments denominated in euros or vice versa. As the net positions of our unhedged foreign currency transactions fluctuate, our earnings might be negatively affected. In addition, the reported carrying value of our euro-denominated assets and liabilities that remain in our Bresso branch will be affected by fluctuation in the value of the U.S. dollar as compared to the euro. We currently do not utilize forward exchange contracts or any type of hedging instruments to hedge foreign exchange risk as we believe our overall exposure is relatively limited.
If we are unable to obtain sufficient quantities of needed starting materials for the manufacture of our products in development from existing suppliers, or if we were unable to source these materials and services from other suppliers and manufacturers, certain research and development and sales activities may be delayed.
Additionally, see the Paragraph named “Customer and Geographic Concentrations”, for further concentration disclosure.
Product Sales
We recognize revenue from product sales when there is persuasive evidence that an arrangement exists, title has passed and delivery has occurred, the price is fixed and determinable, and collectability is reasonably assured. Product sales are generally recorded upon shipment net of an allowance for estimated product returns and rebates. We analyze historical return patterns for our products in determining an appropriate estimate for returns allowance. We may need to adjust our estimates if actual results vary which could have an impact on our earnings in the period of adjustment. If customers have product acceptance rights or product return rights and we are unable to reasonably estimate returns related to that customer or market, we defer revenue recognition until such rights have expired. All product sales in 2008 and 2007 consisted of sales of Zevalin prior to the disposition of Zevalin to RIT Oncology in December 2008. Following the transfer of Zevalin, we no longer have a direct ownership in any commercial products generating product sales revenue.
C-231
License and Contract Revenues
We may generate revenue from technology licenses, collaborative research and development arrangements, cost reimbursement contracts and research grants. Revenue under technology licenses and collaborative agreements typically consists of nonrefundable and/or guaranteed technology license fees, collaborative research funding, and various milestone and future product royalty or profit-sharing payments.
Revenue associated with up-front license fees and research and development funding payments under collaborative agreements is recognized ratably over the relevant periods specified in the agreement, generally the research and development period. If the time period is not defined in the agreement, we calculate the revenue recognition period based on our current estimate of the research and development period considering experience with similar projects, level of effort and the stage of development. Should there be a change in our estimate of the research and development period, we will revise the term over which the initial payment is recognized. Revenue from substantive at-risk milestones and future product royalties is recognized as earned based on the completion of the milestones and product sales, as defined in the respective agreements. Revenue under cost reimbursement contracts and research grants is recognized as the related costs are incurred. Payments received in advance of recognition as revenue are recorded as deferred revenue.
For multiple element arrangements that had continuing performance obligations, we recognized contract, milestone or license fees together with any up-front payments over the term of the arrangement as we completed our performance obligation, unless the delivered technology had stand alone value to the customer and there was objective, reliable evidence of fair value of the undelivered element in the arrangement. Additionally, unless evidence suggests otherwise, revenue from consideration received was recognized on a straight-line basis over the expected term of the arrangement.
Cost of Product Sold
Cost of product sold consists of the cost of the product sold to our customers, including any necessary allowances for excess inventory that may expire and become unsaleable. Prior to the transfer of Zevalin assets to RIT Oncology in December 2008, we purchased Zevalin from Biogen Idec Inc., or Biogen, pursuant to a supply agreement entered into in connection with the acquisition of this product. Contractual royalties based on product sales are also included in cost of product sold.
Inventory
Inventory is stated at the lower of cost or market. We determine cost based on the specific identification method. If the cost of the inventory exceeds the expected market value, we record a provision for the difference between the cost and the net realizable value. When required, an allowance for excess inventory that may expire and become unsaleable is recorded. All inventory was sold to RIT Oncology subsequent to its formation in December 2008.
Accounts Receivable
We analyze historical returns patterns for our products in determining an appropriate estimate for our returns allowance. This estimate is evaluated periodically and adjusted, if necessary. Actual returns are written off against the existing allowance. The allowance for doubtful accounts is based on estimates of losses related to customer receivable balances. We estimate the allowance based upon the age of the outstanding receivables and
C-232
our historical experience of collections, adjusting for risk of loss for specific customer accounts. We periodically review the estimation process and made changes to the estimates as necessary. When it is deemed probable that a customer account is uncollectible, that balance is written off against the existing allowance.
Our accounts receivable balance as of December 31, 2008 included trade receivables related to sales of Zevalin prior to the disposition of Zevalin to RIT Oncology in December 2008. This balance is net of an allowance for product returns totaling $0.1 million and, as customer payments had generally been made in a timely manner, no allowance for doubtful accounts related to our remaining accounts receivable balance was deemed necessary.
Research and Development Expenses
Research and development expenses include related salaries and benefits, clinical trial and related manufacturing costs, contract and other outside service fees, and facilities and overhead costs related to our research and development efforts. Research and development expenses also consist of costs incurred for proprietary and collaboration research and development and include activities such as product registries and investigator-sponsored trials. Research and development costs are expensed as incurred. In instances where we enter into agreements with third parties for research and development activities we may prepay fees for services at the initiation of the contract. We record the prepayment as a prepaid asset and amortize the asset into research and development expense over the period of time the contracted research and development services are performed. Other types of arrangements with third parties may be fixed fee or fee for service, and may include monthly payments or payments upon the completion of milestones or receipt of deliverables.
Acquired in-process research and development
For our transactions that occurred prior to December 31, 2008, based on accounting guidance then in effect for business combinations, costs to acquire in-process research and development, or IPRD, projects and technologies which have no alternative future use and which have not reached technological feasibility as of acquisition date were expensed as incurred. We have not had any business combination transactions subsequent to December 31, 2008.
Value Added Tax Receivable
Our European operations were subject to Value Added Tax, or VAT, which is usually applied to all goods and services purchased and sold throughout Europe. The VAT receivable is $6.3 million as of December 31, 2009 and 2008, of which $5.9 million and $6.2 million is included in other assets and $0.4 million and $0.1 million is included in prepaid expenses and other current assets as of December 31, 2009 and 2008, respectively. This receivable balance relates to our Italian operations and typically has a three year collection period. We review our VAT receivable balance for impairment whenever events or changes in circumstances indicate the carrying amount might not be recoverable. On April 14, 2009 and December 21, 2009, the Italian Tax Authority, or ITA, issued notices of assessment to CTI (Europe) based on the ITA’s audit of CTI (Europe)’s VAT returns for the years 2003 and 2005, respectively. The ITA audits concluded that CTI (Europe) did not collect and remit VAT on certain invoices issued to non-Italian clients for services performed by CTI (Europe). The assessment for the year 2003 is €0.5 million, or approximately $0.8 million as of December 31, 2009, including interest and penalties. The assessment for the year 2005 is €5.5 million, or approximately $7.7 million as of December 31, 2009, including interest and penalties. We believe that the services were non-VAT taxable consultancy services and that the VAT returns are correct as originally filed. As such, we have not booked an impairment to the carrying
C-233
amount of our VAT receivable and we intend to vigorously defend ourselves against the assessment and have requested a dismissal on procedural grounds and merits of the case.
Property and Equipment
Property and equipment are carried at cost, less accumulated depreciation and amortization. Depreciation commences at the time assets are placed in service. It is calculated using the straight-line method over the estimated useful lives of the assets ranging from three to five years for assets other than leasehold improvements which are amortized over the lesser of their useful life of 10 years or the term of the applicable lease using the straight-line method.
Impairment of Long-lived Assets
We review our long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Each impairment test is based on a comparison of the undiscounted future cash flows to the recorded value of the asset. If an impairment is indicated, the asset is written down to its estimated fair value based on quoted fair market values.
Goodwill and Other Intangible Assets
Goodwill represents the excess, at the date of acquisition, of the purchase price of a business acquired over the fair value of the net tangible and intangible assets acquired. Goodwill is not amortized but is tested for impairment at least annually using a fair-value based, two-step test. An impairment analysis is done more frequently if certain events or circumstances arise that would indicate a change in fair value of the non-financial asset occurred (i.e. an impairment indicator). If goodwill is impaired it is written down; however, no impairment of goodwill has been found to date.
We conducted our annual impairment test and concluded that the fair value of our single reporting unit exceeded the carrying value of our net assets (i.e. step one of the impairment test) for the years ended December 31, 2009, 2008 and 2007.
Other intangible assets consisted of acquisition-related intangible assets. These intangible assets had finite lives and were carried at cost less accumulated amortization.
Amortization of our assembled workforce intangible was computed using the straight-line method over the estimated useful life of the assembled workforce asset, which was approximately 5 years. As of December 31, 2008 all workforce intangibles had been fully amortized.
In 2007, we recorded certain intangible assets in connection with the acquisition of Zevalin. Developed and core technologies were being amortized over the terms of the patents related to such technologies of approximately 11.2 years based on a method of amortization that reflected the pattern in which the estimated economic benefit of the intangible assets were consumed. The manufacturing intangible asset was being amortized straight-line over the term of the supply agreement, which was approximately 6.5 years. In connection with the formation of RIT Oncology in December 2008, our intangible asset balances related to Zevalin were included in the disposition of Zevalin to RIT Oncology.
C-234
As of December 31, 2009 and 2008, we had no intangible asset balance remaining. The change in the value of other intangible assets for the years ended December 31, 2008 and 2007 is as follows (in thousands):
| Developed and Core Technologies |
Manufacturing Intangible Asset |
Assembled Workforce |
||||||||||
| Balance as of December 31, 2006 |
$ | — | $ | — | $ | 1,663 | ||||||
| Increase due to acquisitions |
11,306 | 3,712 | 68 | |||||||||
| Amortization |
(28 | ) | (16 | ) | (869 | ) | ||||||
| Increase due to exchange rate |
— | — | 121 | |||||||||
| Balance as of December 31, 2007 |
11,278 | 3,696 | 983 | |||||||||
| Increase due to acquisition cost adjustments |
138 | 45 | — | |||||||||
| Amortization |
(111 | ) | (558 | ) | (927 | ) | ||||||
| Disposition of Zevalin to RIT Oncology |
(11,305 | ) | (3,183 | ) | — | |||||||
| Decrease due to exchange rate |
— | — | (56 | ) | ||||||||
| Balance as of December 31, 2008 |
$ | — | $ | — | $ | — | ||||||
Advertising Costs
The costs of advertising are expensed as incurred. We incurred advertising costs of $1.0 million, $0.8 million and $0.6 million in 2009, 2008, and 2007 respectively.
Net Loss per Share
Basic net loss per share is calculated based on the net loss attributable to common shareholders divided by the weighted average number of shares outstanding for the period excluding any dilutive effects of options, warrants, unvested share awards and convertible securities. Diluted net loss per common share assumes the conversion of all dilutive convertible securities, such as convertible subordinated debt and convertible preferred stock using the if-converted method, and assumes the exercise or vesting of other dilutive securities, such as options, warrants and restricted stock using the treasury stock method.
Derivatives Embedded in Certain Debt Securities
Derivative instruments are recorded at fair value with changes in value recognized in the statement of operations in the period of change.
Certain of our convertible senior notes include a feature that calls for make-whole payments upon conversion of these notes. These make-whole features along with the conversion options on the notes represent embedded derivatives that must be accounted for separately from the related debt securities except where our convertible senior notes are recorded entirely at fair value.
We have calculated the fair value of the derivatives related to our convertible notes using either a Monte Carlo simulation model or a discounted cash flow model. Changes in the estimated fair value of the derivative liabilities related to the convertible senior notes are included in gain on derivative liabilities, net and will be remeasured at the end of each reporting period until the relevant feature expires or all of the relevant notes are converted or repurchased.
C-235
Other Financial Instruments
At December 31, 2009 and 2008, the carrying value of financial instruments such as receivables and payables approximated their fair values based on the short-term maturities of these instruments. The carrying value of other long-term liabilities approximated fair values because the underlying interest rates approximate market rates at the balance sheet dates.
The estimated fair values of our convertible notes are determined using a discounted cash flow modeling technique. The carrying values of our convertible notes are net of accretion of debt discount and changes in the fair value of derivative liabilities, if any. The carrying values of our convertible preferred stock were net of issuance costs and the proceeds which were allocated to stock warrants based on a relative market value approach.
The following is a summary of the estimated fair value of our convertible notes as of December 31, 2009 and 2008 (in thousands):
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| 10% convertible senior notes due 2011 |
$ | — | $ | 21,810 | ||||
| 9% convertible senior notes |
$ | — | $ | 4,580 | ||||
| 7.5% convertible senior notes |
$ | 9,138 | $ | 27,308 | ||||
| 6.75% convertible senior notes |
$ | — | $ | 5,875 | ||||
| 5.75% convertible senior notes |
$ | 8,777 | $ | 16,728 | ||||
| 4.0% convertible senior subordinated notes |
$ | 38,512 | $ | 46,375 | ||||
We had no preferred stock outstanding as of December 31, 2009. The estimated fair value of our convertible preferred stock as of December 31, 2008 is as follows (in thousands):
| Series A 3% convertible preferred stock |
$ | 544 | ||
| Series B 3% convertible preferred stock |
$ | 5,024 | ||
| Series C 3% convertible preferred stock |
$ | 3,957 | ||
| Series D 7% convertible preferred stock |
$ | 919 |
Foreign Currency Translation and Transaction Gains and Losses
We record foreign currency translation adjustments and transaction gains and losses in accordance with ASC 830, Foreign Currency Matters. For our operations that have a functional currency other than the U.S. dollar, gains and losses resulting from the translation of the functional currency into U.S. dollars for financial statement presentation are not included in determining net loss but are accumulated in the cumulative foreign currency translation adjustment account as a separate component of shareholders’ deficit. The Company and its subsidiaries also have transactions in foreign currencies other than the functional currency. We record transaction gains and losses in our consolidated statements of income related to the recurring measurement and settlement of such transactions.
Income Taxes
We record a tax provision for the anticipated tax consequences of our reported results of operations. The provision for income taxes is computed using the asset and liability method, under which deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial reporting and tax bases of assets and liabilities, and for operating losses and tax credit carryforwards. Deferred tax assets and liabilities are measured using the currently enacted tax rates that apply to taxable income in effect
C-236
for the years in which those tax assets are expected to be realized or settled. We record a valuation allowance to reduce deferred tax assets to the amount that is believed more likely than not to be realized.
Comprehensive Loss
Comprehensive loss is comprised of net loss and other comprehensive income or loss. Our other comprehensive income or loss includes unrealized gains and losses on our securities available-for-sale and net exchange gains or losses resulting from the translation of assets and liabilities of foreign subsidiaries. Total comprehensive loss consisted of the following (in thousands):
| 2009 | 2008 | 2007 | ||||||||||
| Net loss before noncontrolling interest |
$ | (95,647 | ) | $ | (180,155 | ) | $ | (138,186 | ) | |||
| Foreign currency translation loss |
(601 | ) | (3,801 | ) | (2,807 | ) | ||||||
| Net unrealized gain (loss) on securities available-for-sale |
1 | (4 | ) | (13 | ) | |||||||
| Comprehensive loss before noncontrolling interest |
(96,247 | ) | (183,960 | ) | (141,006 | ) | ||||||
| Noncontrolling interest |
252 | 126 | 78 | |||||||||
| Comprehensive loss attributable to CTI |
$ | (95,995 | ) | $ | (183,834 | ) | $ | (140,928 | ) | |||
Information regarding the components of accumulated other comprehensive loss is as follows (in thousands):
| 2009 | 2008 | |||||||
| Foreign currency translation adjustment |
$ | (8,412 | ) | $ | (7,811 | ) | ||
| Net unrealized gain (loss) on securities available-for-sale |
— | (1 | ) | |||||
| Total accumulated other comprehensive loss |
$ | (8,412 | ) | $ | (7,812 | ) | ||
New Accounting Standards
In June 2009, the FASB, issued the FASB Accounting Standards Codification, or Codification. All existing accounting standard documents were superseded by the Codification and the Codification became the source of all authoritative generally accepted accounting principles, or GAAP, except for rules and interpretive releases from the SEC, which are still sources of authoritative GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. All other non-grandfathered, non-SEC accounting literature not included in the Codification has become nonauthoritative. The Codification is effective for interim or annual periods ending after September 15, 2009, and we are using the new guidelines and numbering systems prescribed by the Codification when referring to GAAP in these financial statements for the year ended December 31, 2009. As the Codification was not intended to change or alter existing GAAP, it did not have any impact on our financial position or results of operations.
In May 2009, the FASB issued a new accounting standard that established general standards of accounting for and disclosure of events that occur after the balance sheet date but before the financial statements are issued or are available to be issued. As codified in ASC 855, this standard requires the disclosure of the date through which an entity has evaluated subsequent events and whether that date represents the date the financial statements were issued or were available to be issued. This standard is effective for annual and interim periods ending after
C-237
June 15, 2009 and should be applied prospectively. We have evaluated subsequent events through February 26, 2010, the issuance date of our financial statements.
On April 1, 2009, the Financial Accounting Standards Board, or FASB, issued Staff Position FAS 141(R)-1, Accounting for Assets Acquired and Liabilities Assumed in a Business Combination that Arises from Contingencies, as codified in ASC 805, Business Combinations, which is effective January 1, 2009 and amends the guidance in SFAS No. 141(R), also codified as ASC 805, to require that assets and liabilities assumed in a business combination that arise from contingencies be recognized at fair value if fair value can be reasonably estimated. The adoption of this provision did not have a material impact on our financial statements.
Reclassifications
Certain prior year items have been reclassified to conform to current year presentation.
| 20.4 | INDICATION OF THE OTHER AUDITED INFORMATION CONTAINED IN THE REGISTRATION DOCUMENT |
| 20.4.1 | Statement attesting that the historical financial information has been audited |
All December 31, 2009, 2008 and 2007 financial information has been audited by CTI’s external auditors.
| December 31, 2007 |
December 31, 2008 | December 31, 2009 | ||||
| Auditor | Stonefield Josephson | Stonefield Josephson | Stonefield Josephson | |||
| Work | Audit | Audit | Audit | |||
| Opinion | Unqualified modified opinion (1) |
Unqualified modified opinion (1) |
Unqualified modified opinion (1) | |||
| Internal Control Opinion | Unqualified opinion | Unqualified opinion | Unqualified opinion | |||
| (1) | Due to substantial doubt about the Company’s ability to continue as a going concern. |
The external auditor opinions for the Company’s 2009, 2008 and 2007 financial statements and the external auditor limited review for the financial information regarding the six-month period ended June 30, 2010 are attached to this Registration Document as Appendix A.
We have also included below the translation into Italian of the relations of our external auditors accompanying our audited financial statements for the years ended December 31, 2007, 2008 and 2009 that, even if they are defined as “Unqualified opinions”, express doubts of the auditors about the Company’s ability to continue to perform its activities as a going concern.
C-238
Stonefield Josephson Opinion Accompanying Audited Financial Statements for the Year Ended December 31, 2009 (Financial Statements Opinion issued on February 26, 2010)
We have audited the accompanying consolidated balance sheets of Cell Therapeutics, Inc. (the “Company”) as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ deficit and other comprehensive loss, and cash flows for each of the years in the three-year period ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cell Therapeutics, Inc. as of December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2009, in conformity with accounting principles generally accepted in the United States of America. The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has sustained loss from operations over the audit periods, incurred an accumulated deficit, and has substantial monetary liabilities in excess of monetary assets as of December 31, 2009. Given these factors and the Company’s inability to demonstrate its ability to satisfy the monetary liabilities raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are described in Note 1 to the consolidated financial statements. These consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated February 26, 2010 expressed an unqualified opinion.
C-239
Stonefield Josephson Opinion Accompanying Audited Financial Statements for the Year Ended December 31, 2008 (Financial Statements Opinion issued on March 16, 2009)
We have audited the accompanying balance sheets of Cell Therapeutics, Inc. (the “Company”) as of December 31, 2008 and 2007, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2008. Our audits also included the financial statement schedule listed in the index at Item 15. These financial statements and the schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cell Therapeutics, Inc. as of December 31, 2008 and 2007, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2008, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has sustained loss from operations over the audit periods, incurred an accumulated deficit, and has substantial monetary liabilities in excess of monetary assets as of December 31, 2008. Given the above factors and the Company’s inability to demonstrate its ability to satisfy the monetary liabilities raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are described in Note 1. These consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2008, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 16, 2009 expressed an unqualified opinion.
C-240
Stonefield Josephson Opinion Accompanying Audited Financial Statements for the Year Ended December 31, 2007 (Financial Statements Opinion issued on March 26, 2008)
We have audited the accompanying balance sheets of Cell Therapeutics, Inc. as of December 31, 2007 and 2006, and the related statements of operations, shareholders’ deficit and other comprehensive loss, and cash flows for each of the years in the three-year period ended December 31, 2007. Our audits also included the consolidated financial statement schedule listed in the index at Item 15(a)(ii) as of and for the years ended December 31, 2007 and 2006. Cell Therapeutics Inc.’s management is responsible for these financial statements and schedule. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cell Therapeutics, Inc. as of December 31, 2007 and 2006, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2007 in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has substantial monetary liabilities in excess of monetary assets as of December 31, 2007, including approximately nineteen million, eight hundred thousand dollars of convertible subordinated notes and senior subordinated notes which mature in June 2008. The Company’s ability to satisfy these obligations upon maturity raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans concerning these matters are described in Note 1. These consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that might be necessary in the event the Company cannot continue in existence.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cell Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2007, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 26, 2008 expressed an unqualified opinion.
It is pointed out that the above relations have been signed by the external auditors exclusively in the English version and that the above translation has been prepared for informative purposes only. Therefore investors are invited to refer exclusively to the original copies of the declarations in English disclosed under Annex A to this Registration Document, since no reliance could be placed on such translation.
| 20.4.2 | Indication of other information in the Registration Document which has been audited by the auditors. |
This Registration Document does not include any other audited information except the financial statements as of December 31, 2009, 2008 and 2007.
| 20.4.3 | Source of data and statement that are not audited |
All financial information has been audited or is derived from audited information.
C-241
| 20.5 | DATE OF THE LAST FINANCIAL INFORMATION |
The latest financial information presented by the Company in this Registration Document is as of September 30, 2010. This unaudited nine-month financial data is examined in the following Paragraph.
| 20.6 | INTERIM AND OTHER FINANCIAL INFORMATION |
| 20.6.1 | SIX-MONTH FINANCIAL INFORMATION AS OF JUNE 30, 2010 AND 2009 |
This paragraph incorporates by reference the following documents available to the public on the Company’s website: http://investors.celltherapeutics.com.
Quarterly report of the Issuer for the six months ended June 30, 2010 reviewed by the independent registered public accounting firm
| • | Condensed consolidated balance sheets: page 3 |
| • | Condensed consolidated statement of operations: page 4 |
| • | Condensed consolidated statement of cash flows: page 5 |
| • | Notes to condensed consolidates financial statements: pages 6-16 |
| • | Management’s discussion and analysis of financial condition and results of operations: pages 17-28 |
| • | Report of independent registered public accounting firm: page 30 |
Quarterly report of the Issuer for the six months ended June 30, 2009 reviewed by the independent registered public accounting firm
| • | Condensed consolidated balance sheets: page 3 |
| • | Condensed consolidated statement of operations: page 4 |
| • | Condensed consolidated statement of cash flows: page 5 |
| • | Notes to condensed consolidates financial statements: pages 6-17 |
| • | Management’s discussion and analysis of financial condition and results of operations: pages 18-31 |
| • | Report of independent registered public accounting firm: page 33 |
| 20.6.2 | NINE-MONTH FINANCIAL INFORMATION AS OF SEPTEMBER 30, 2010 and 2009 |
| 20.6.2.1 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
C-242
Basis of Presentation
The accompanying unaudited financial information of CTI as of September 30, 2010 and for the three and nine months ended September 30, 2010 and 2009 has been prepared in accordance with accounting principles generally accepted in the United States for interim financial information and with the instructions to Quarterly Report on Form 10-Q and Article 10 of Regulation S-X. In the opinion of management, such financial information includes all adjustments (consisting only of normal recurring adjustments) considered necessary for a fair presentation of our financial position at such date and the operating results and cash flows for such periods. Operating results for the three and nine month periods ended September 30, 2010 are not necessarily indicative of the results that may be expected for the entire year.
Certain information and footnote disclosure normally included in financial statements prepared in accordance with generally accepted accounting principles have been omitted pursuant to the rules of the U.S. Securities and Exchange Commission, or SEC. These unaudited financial statements and the related notes should be read in conjunction with our audited annual financial statements for the year ended December 31, 2009 included in our Annual Report on Form 10-K.
The condensed consolidated balance sheet at December 31, 2009 has been derived from the audited financial statements at that date, but does not include all of the information and footnotes required by generally accepted accounting principles in the United States for complete financial statements.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of CTI and its wholly-owned subsidiaries, which include CTI Corporate Development, Inc., Systems Medicine LLC, or SM, CTI Commercial LLC, and CTI Life Sciences Limited (from the date of formation in March 2009). CTI Life Sciences Limited opened a branch in Italy in December 2009. We also retain ownership of our branch, Cell Therapeutics Inc. – Sede Secondaria, or CTI (Europe); however, we ceased operations related to this branch in September 2009. In addition, CTI Corporate Development, Inc. was liquidated in the fourth quarter of 2009.
As of September 30, 2010, we also had a 69% interest in our majority-owned subsidiary, Aequus Biopharma, Inc., or Aequus. In accordance with the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, 810, Consolidation, the noncontrolling interest in Aequus (previously shown as minority interest) is reported below net loss in noncontrolling interest in the condensed consolidated statement of operations and shown as a component of equity in the condensed consolidated balance sheet.
Additionally, we held a 50% interest in RIT Oncology from the date of its formation in December 2008 to the sale of our interest in March 2009, which we accounted for using the equity method of accounting.
All intercompany transactions and balances are eliminated in consolidation.
Liquidity
The accompanying condensed consolidated financial statements have been prepared assuming that we will continue as a going concern, which contemplates realization of assets and the satisfaction of liabilities in the normal course of business for the twelve month period following the date of these financial statements. However, we have incurred losses since inception and expect to generate losses for the next few years primarily due to research and development costs for pixantrone, OPAXIO and brostallicin. Our available cash and cash equivalents are $17.3 million as of September 30, 2010. Subsequent to period end, in October 2010, we raised $21.0 million in gross proceeds from the issuance of 21,000 shares of our Series 7 preferred stock and warrants to purchase up to 22.7 million shares of our common stock. Our Series 7 preferred stock was converted to 56.8 million shares of common stock upon closing of the transaction.
C-243
We do not expect that our existing cash and cash equivalents, including the cash received from the issuance of our Series 7 preferred stock and warrants, will be sufficient to fund our presently anticipated operations beyond the first quarter of 2011. This raises substantial doubt about our ability to continue as a going concern.
Since the second quarter of 2010, we have implemented cost saving initiatives to reduce operating expenses, including the reduction of employees related to planned commercial pixantrone operations. However, we will need to raise additional funds and are currently exploring alternative sources of equity or debt financing. We may seek to raise such capital through public or private equity financings, partnerships, joint ventures, disposition of assets, debt financings or restructurings, bank borrowings or other sources of financing.
On September 16, 2010, our shareholders approved an amendment to our amended and restated articles of incorporation to increase our authorized shares of common and preferred stock from 810,000,000 shares to 1,210,000,000 shares and to increase the total number of our authorized shares of common stock from 800,000,000 shares of common stock to 1,200,000,000 shares of common stock. As such, our board of directors has the option to issue such shares depending on our financial needs and market opportunities, if deemed to be in the best interest of the shareholders. However, additional funding may not be available on favorable terms or at all. If additional funds are raised by issuing equity securities, substantial dilution to existing shareholders may result. If we fail to obtain additional capital when needed, we may be required to delay, scale back, or eliminate some or all of our research and development programs and may be forced to cease operations, liquidate our assets and possibly seek bankruptcy protection. The accompanying condensed consolidated financial statements do not include any adjustments that may result from the outcome of this uncertainty.
Value Added Tax Receivable
Our European operations were subject to a value added tax, or VAT, which is usually applied to all goods and services purchased and sold throughout Europe. The VAT receivable is approximately $5.5 million and $6.3 million as of September 30, 2010 and December 31, 2009, respectively, of which $5.4 million and $5.9 million is included in other assets and $0.1 million and $0.4 million is included in prepaid expenses and other current assets as of September 30, 2010 and December 31, 2009, respectively. This receivable balance relates to our Italian operations and typically has a three year collection period. We review our VAT receivable balance for impairment whenever events or changes in circumstances indicate the carrying amount might not be recoverable.
Net Loss Per Share
Basic net loss per common share is calculated based on the net loss attributable to common shareholders divided by the weighted average number of shares outstanding for the period excluding any dilutive effects of options, warrants, unvested share awards and convertible securities. Diluted net loss per common share assumes the conversion of all dilutive convertible securities, such as convertible debt and convertible preferred stock using the if-converted method, and assumes the exercise or vesting of other dilutive securities, such as options, warrants and share awards using the treasury stock method. As of September 30, 2010 and 2009, options, warrants, unvested share awards and rights, convertible debt and convertible preferred stock aggregating 80.7 million and 45.4 million common share equivalents, respectively, prior to the application of the treasury stock method for options and warrants, are not included in the calculation of diluted net loss per share as they are anti-dilutive.
C-244
New Accounting Standards
In February 2010, the FASB issued amended guidance on subsequent events to alleviate potential conflicts between FASB guidance and SEC requirements. Under this amended guidance, SEC filers are no longer required to disclose the date through which subsequent events have been evaluated in originally issued and revised financial statements. This guidance was effective immediately and we adopted these new requirements during the first quarter of 2010. The adoption of this guidance did not have a material impact on our financial statements.
In April 2010, the FASB issued guidance on the milestone method for revenue recognition purposes. Previously, definitive guidance on when the use of the milestone method was appropriate did not exist. This guidance provides a framework of the criteria that should be met for determining whether the milestone method of revenue recognition is appropriate. This guidance was effective on a prospective basis for milestones achieved in fiscal years and interim periods within those years, beginning on or after June 15, 2010 with early adoption permitted. We do not anticipate the adoption of this guidance will have a material impact on our financial statements.
Reclassifications
Certain prior year items have been reclassified to conform to current year presentation.
| 20.6.2.2 | CONSOLIDATED BALANCE SHEET, CONSOLIDATED STATEMENT OF OPERATIONS, AND CONSOLIDATED STATEMENT OF CASH FLOWS AS OF SEPTEMBER 30, 2010 |
All information as of September 30, 2010 and for the nine months ending September 30, 2010 and 2009 is unaudited.
CELL THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands of US dollars, except share amounts)
| Note | September 30, 2010 (unaudited) |
December 31, 2009 |
||||||||
| ASSETS |
A | |||||||||
| Current assets: |
||||||||||
| Cash and cash equivalents |
1 | 17,268 | 37,811 | |||||||
| Prepaid expenses and other current assets |
2 | 2,813 | 4,354 | |||||||
| Total current assets |
20,081 | 42,165 | ||||||||
| Property and equipment, net |
3 | 3,114 | 3,430 | |||||||
| Goodwill |
17,064 | 17,064 | ||||||||
| Other assets |
4 | 6,354 | 6,936 | |||||||
| Total assets |
46,613 | 69,595 | ||||||||
C-245
| LIABILITIES AND SHAREHOLDERS’ DEFICIT |
B | |||||||||
| Current liabilities: |
||||||||||
| Accounts payable |
1 | 6,506 | 7,297 | |||||||
| Accrued expenses |
2 | 8,368 | 14,807 | |||||||
| Current portion of deferred revenue |
3 | — | 80 | |||||||
| Current portion of long-term obligations |
4 | 812 | 1,312 | |||||||
| 7.5% convertible senior notes |
10,187 | — | ||||||||
| 4% convertible senior subordinated notes |
— | 40,363 | ||||||||
| Total current liabilities |
25,873 | 63,859 | ||||||||
| Deferred revenue, less current portion |
3 | — | 239 | |||||||
| Long-term obligations, less current portion |
4 | 1,010 | 1,861 | |||||||
| 7.5% convertible senior notes |
5 | — | 10,102 | |||||||
| 5.75% convertible senior notes |
5 | 11,985 | 11,677 | |||||||
| Total liabilities |
38,868 | 87,738 | ||||||||
| Commitments and contingencies |
||||||||||
| Common stock purchase warrants |
6 | 13,461 | 626 | |||||||
| Shareholders’ deficit: |
||||||||||
| Common stock, no par value: |
7 | |||||||||
| Authorized shares - 1,200,000,000 and 800,000,000 at September 30, 2010 and December 31, 2009, respectively |
||||||||||
| Issued and outstanding shares - 758,453,915 (unaudited) and 590,282,575 at September 30, 2010 and December 31, 2009, respectively |
1,545,324 | 1,418,931 | ||||||||
| Accumulated other comprehensive loss |
7 | (8,159 | ) | (8,412 | ) | |||||
| Accumulated deficit |
7 | (1,542,526 | ) | (1,429,083 | ) | |||||
| Total CTI shareholders’ deficit |
(5,361 | ) | (18,564 | ) | ||||||
| Noncontrolling interest |
(355 | ) | (205 | ) | ||||||
| Total shareholders’ deficit |
(5,716 | ) | (18,769 | ) | ||||||
| Total liabilities and shareholders’ deficit |
46,613 | 69,595 | ||||||||
C-246
CELL THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| (unaudited) | ||||||||||||
| Note | Nine Months Ended September 30, |
|||||||||||
| 2010 | 2009 | |||||||||||
| Revenues: |
A | |||||||||||
| License and contract revenue |
1 | $ | 319 | $ | 60 | |||||||
| Total revenues |
319 | 60 | ||||||||||
| Operating expenses, net: |
B | |||||||||||
| Research and development |
2 | 19,375 | 22,878 | |||||||||
| Selling, general and administrative |
3 | 39,378 | 38,997 | |||||||||
| Restructuring charges and related gain on sale of assets, net |
4 | — | 3,766 | |||||||||
| Gain on sale of investment in joint venture |
5 | — | (10,244 | ) | ||||||||
| Total operating expenses, net |
58,753 | 55,397 | ||||||||||
| Loss from operations |
(58,434 | ) | (55,337 | ) | ||||||||
| Other income (expense): |
C | |||||||||||
| Investment and other income (expense), net |
240 | 97 | ||||||||||
| Interest expense |
(1,948 | ) | (4,026 | ) | ||||||||
| Amortization of debt discount and issuance costs |
(600 | ) | (5,575 | ) | ||||||||
| Foreign exchange gain (loss) |
(300 | ) | 278 | |||||||||
| Debt conversion expense |
(2,031 | ) | — | |||||||||
| Make-whole interest expense |
— | (6,345 | ) | |||||||||
| Gain on derivative liabilities, net |
— | 7,218 | ||||||||||
| Gain on exchange of convertible notes |
— | 7,381 | ||||||||||
| Equity loss from investment in joint venture |
— | (1,204 | ) | |||||||||
| Milestone modification expense |
— | (6,000 | ) | |||||||||
| Settlement expense, net |
— | (4,710 | ) | |||||||||
| Other income (expense), net |
(4,639 | ) | (12,886 | ) | ||||||||
| Net loss before noncontrolling interest |
(63,073 | ) | (68,223 | ) | ||||||||
| Noncontrolling interest |
149 | 205 | ||||||||||
| Net loss attributable to CTI |
(62,924 | ) | (68,018 | ) | ||||||||
| Gain on restructuring of preferred stock |
— | 2,116 | ||||||||||
| Preferred stock dividends |
— | (24 | ) | |||||||||
| Deemed dividends on preferred stock |
(50,519 | ) | (23,460 | ) | ||||||||
| Net loss attributable to CTI common shareholders |
$ | (113,443 | ) | $ | (89,386 | ) | ||||||
| Basic and diluted net loss per common share |
$ | (0.17 | ) | $ | (0.21 | ) | ||||||
| Shares used in calculation of basic and diluted net loss per common share |
659,244 | 420,520 | ||||||||||
C-247
CELL THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH
FLOWS
(In thousands)
(unaudited)
| Nine Months
Ended September 30, 2010 |
Nine Months
Ended September 30, 2009 |
|||||||
| Operating activities |
||||||||
| Net loss |
$ | (62,924 | ) | $ | (68,018 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Equity-based compensation expense |
16,260 | 13,283 | ||||||
| Gain on sale of investment in joint venture |
— | (10,244 | ) | |||||
| Non-cash interest expense |
600 | 5,575 | ||||||
| Depreciation and amortization |
1,413 | 1,424 | ||||||
| Debt conversion expense |
2,031 | — | ||||||
| Non-cash gain on derivative liabilities, net |
— | (7,218 | ) | |||||
| Gain on exchange of convertible notes |
— | (7,381 | ) | |||||
| Equity loss from investment in joint venture |
— | 1,204 | ||||||
| Noncontrolling interest |
(149 | ) | (205 | ) | ||||
| Other |
(217 | ) | (457 | ) | ||||
| Changes in operating assets and liabilities: |
||||||||
| Restricted cash |
— | 6,640 | ||||||
| Accounts receivable, net |
— | 991 | ||||||
| Prepaid expenses and other current assets |
1,472 | (1,843 | ) | |||||
| Other assets |
68 | (367 | ) | |||||
| Accounts payable |
(2,888 | ) | (4,436 | ) | ||||
| Accrued expenses |
(4,944 | ) | 327 | |||||
| Long-term obligations |
(532 | ) | 347 | |||||
| Other liabilities |
(546 | ) | (266 | ) | ||||
| Total adjustments |
12,568 | (2,626 | ) | |||||
| Net cash used in operating activities |
(50,356 | ) | (70,644 | ) | ||||
| Investing activities |
||||||||
| Purchases of property and equipment |
(1,232 | ) | (343 | ) | ||||
| Proceeds from the sales of property and equipment |
82 | — | ||||||
C-248
| Proceeds received from disposition of Zevalin to joint venture, net |
— | 6,844 | ||||||
| Proceeds received from sale of investment in joint venture, net |
— | 15,075 | ||||||
| Proceeds from maturities of securities available-for-sale |
— | 600 | ||||||
| Net cash provided by (used in) investing activities |
(1,150 | ) | 22,176 | |||||
| Financing activities |
||||||||
| Proceeds from issuance of Series 1 preferred stock and warrants, net of issuance costs |
— | 18,745 | ||||||
| Proceeds from issuance of Series 2 preferred stock and warrants, net of issuance costs |
— | 28,516 | ||||||
| Proceeds from issuance of Series 3 preferred stock and warrants, net of issuance costs |
27,951 | — | ||||||
| Proceeds from issuance of Series 4 preferred stock and warrants, net of issuance costs |
18,621 | — | ||||||
| Proceeds from issuance of Series 5 preferred stock and warrants, net of issuance costs |
19,704 | — | ||||||
| Proceeds from issuance of Series 6 preferred stock and warrants, net of issuance costs |
3,603 | — | ||||||
| Proceeds from issuance of common stock and warrants, net of issuance costs |
— | 59,519 | ||||||
| Proceeds from exercise of Class A warrants |
— | 3,765 | ||||||
| Repayment of 4% convertible senior subordinated notes |
(38,515 | ) | — | |||||
| Cash paid for the exchange of convertible notes, net of transaction costs |
— | (9,949 | ) | |||||
| Cash paid for repurchase of shares in connection with taxes on restricted stock vesting |
(748 | ) | (3,148 | ) | ||||
| Payment of deemed dividends on conversion of preferred stock |
— | (3,000 | ) | |||||
| Payment of dividends on preferred stock |
— | (111 | ) | |||||
| Other |
— | (135 | ) | |||||
| Net cash provided by financing activities |
30,616 | 94,202 | ||||||
| Effect of exchange rate changes on cash and cash equivalents |
347 | (814 | ) | |||||
| Net increase (decrease) in cash and cash equivalents |
(20,543 | ) | 44,920 | |||||
| Cash and cash equivalents at beginning of period |
37,811 | 10,072 | ||||||
| Cash and cash equivalents at end of period |
$ | 17,268 | $ | 54,992 | ||||
| Supplemental disclosure of cash flow information |
||||||||
| Cash paid during the period for interest |
$ | 2,401 | $ | 11,357 | ||||
| Cash paid for taxes |
$ | — | $ | — | ||||
| Supplemental disclosure of noncash financing and Investing activities |
||||||||
| Conversion of Series 1 preferred stock to common stock |
$ | — | $ | 18,537 | ||||
| Conversion of Series 2 preferred stock to common stock |
$ | — | $ | 27,796 | ||||
| Conversion of Series 3 preferred stock to common stock |
$ | 27,761 | $ | — | ||||
| Conversion of Series 4 preferred stock to common stock |
$ | 18,621 | $ | — | ||||
| Conversion of Series 5 preferred stock to common stock |
$ | 19,464 | $ | — | ||||
| Conversion of Series 6 preferred stock to common stock |
$ | 2,970 | $ | — | ||||
| Conversion of Series B 3% convertible preferred stock to common stock |
$ | — | $ | 2,317 | ||||
C-249
| Conversion of Series F preferred stock to common stock |
$ | — | $ | 3,866 | ||||
| Conversion of 10% convertible senior notes due 2011 to common stock |
$ | — | $ | 18,000 | ||||
| Conversion of 9% convertible senior notes to common stock |
$ | — | $ | 5,250 | ||||
| Exchange of 4% convertible senior subordinated notes for common stock |
$ | 1,848 | $ | — | ||||
| Exchange of Series A 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 151 | ||||
| Exchange of Series B 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 1,713 | ||||
| Exchange of Series C 3% convertible preferred stock for Series F preferred stock |
$ | — | $ | 3,221 | ||||
| Issuance of Series F preferred stock for Series A, B and C convertible preferred stock |
$ | — | $ | 3,931 | ||||
| Issuance of common stock in exchange for convertible notes |
$ | — | $ | 35,193 | ||||
| Issuance of common stock in exchange for Series A 3% convertible preferred stock |
$ | — | $ | 688 | ||||
| Issuance of common stock in exchange for Series D 7% convertible preferred stock |
$ | — | $ | 1,793 | ||||
| 20.6.2.3 | NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
| A. | ASSETS |
| 1. | Cash and Cash equivalents |
Cash and cash equivalents consists of the following (in thousands of US dollars):
| Cash and cash equivalents (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| CTI |
$ | 17,073 | $ | 37,407 | ||||
| Subsidiaries |
195 | 404 | ||||||
| Total cash and cash equivalents |
$ | 17,268 | $ | 37,811 | ||||
Please refer to the paragraph 10.2.3 “Sources, amounts and description of the Company’s cash flows for the nine months ended September 30, 2009 and 2010”of this Registration Document for more information about the evolution of the item cash and cash equivalents.
| 2. | Prepaid Expenses and Other Current Assets |
Prepaid expenses and other current assets consists of the following (in thousands of US dollars):
| Prepaid Expenses and Other Current Assets (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| CTI |
$ | 2,170 | $ | 3,315 | ||||
| Subsidiaries |
643 | 1,039 | ||||||
| Total prepaid expenses and other current assets |
$ | 2,813 | $ | 4,354 | ||||
C-250
Prepaid expenses and other current assets decreased as of September 30, 2010 as compared to December 31, 2009 primarily due to timing of prepayments and a decrease in our VAT Receivable.
| 3. | Property and Equipment, net |
Property and equipment, net consists of the following (in thousands of US dollars):
| September 30, 2010 |
December 31, 2009 |
|||||||
| Leasehold improvements |
$ | 3,174 | $ | 3,277 | ||||
| Lab equipment |
470 | 560 | ||||||
| Furniture and office equipment |
13,104 | 11,970 | ||||||
| 16,748 | 15,807 | |||||||
| Less: accumulated depreciation and amortization |
(13,634 | ) | (12,377 | ) | ||||
| $ | 3,114 | $ | 3,430 | |||||
Property and equipment, net decreased as of September 30, 2010 as compared to December 31, 2009 primarily due to closure of Systems Medicine, LLC during Q1 2010 .
| 4. | Other Assets |
Other assets consists of the following (in thousands of US dollars):
| Other Assets (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| CTI |
$ | 841 | $ | 1,005 | ||||
| Subsidiaries |
5,513 | 5,931 | ||||||
| Total other assets |
$ | 6,354 | $ | 6,936 | ||||
Other assets decreased as of September 30, 2010 as compared to December 31, 2009 primarily due to a decrease in the VAT Receivable.
| B. | LIABILITIES |
| 1. | Accounts Payable |
Accounts payable consists of the following (in thousands of US dollars):
| Accounts Payable (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| CTI |
$ | 6,187 | $ | 6,570 | ||||
| Subsidiaries |
319 | 727 | ||||||
| Total accounts payable |
$ | 6,506 | $ | 7,297 | ||||
The decrease in accounts payable as of September 30, 2010 as compared to December 31, 2009 is primarily related to the timing of expenses and payments.
C-251
| 2. | Accrued Expenses |
Accrued expenses consists of the following (in thousands of US dollars):
| Accrued Expenses (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| CTI |
$ | 8,048 | $ | 12,700 | ||||
| Subsidiaries |
320 | 2,107 | ||||||
| Total accrued expenses |
$ | 8,368 | $ | 14,807 | ||||
The decrease in accrued expenses as of September 30, 2010 as compared to December 31, 2009 is primarily related to the timing of expenses and payments and the payout of accrued expenses related to the closure of our Bresso Italy operations.
| 3. | Deferred Revenue |
Deferred revenue consists of the following (in thousands of US dollars):
| Deferred Revenue (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| Current portion of deferred revenue |
$ | — | $ | 80 | ||||
| Deferred revenue, less current portion |
— | 239 | ||||||
| Total deferred revenue |
$ | — | $ | 319 | ||||
The decrease in deferred revenue as of September 30, 2010 as compared to December 31, 2009 is due to the DiaKine Licensing Agreement revenue balance recognized in 2010 due to insolvency of DiaKine.
| 4. | Long-term Obligations |
Long-term obligations consists of the following (in thousands of US dollars):
| Long-Term Obligations (In thousands of US dollars) |
September 30, 2010 |
December 31, 2009 |
||||||
| Current portion of long-term obligations |
$ | 812 | $ | 1,312 | ||||
| Long-term obligations, less current portion |
1,010 | 1,861 | ||||||
| Total long-term obligations |
$ | 1,822 | $ | 3,173 | ||||
The decrease in other long-term obligations as of September 30, 2010 as compared to December 31, 2009 is due to a decrease in deposits and deferred tax liability of our European operations and a decrease in CTI’s accrual for excess facilities due to rent payments made and a decrease in rent benefit related to the straight-line of rent expense.
C-252
| 5. | Convertible Notes |
| Balance
at January 1, 2010 |
Converted | Exchanged | Paid | Balance
at September 30, 2010 |
||||||||||||||||
| 7.5% convertible senior notes |
$ | 10,250 | $ | — | $ | — | $ | — | $ | 10,250 | ||||||||||
| 5.75% convertible senior notes |
10,913 | — | — | — | 10,913 | |||||||||||||||
| 4.0% convertible senior subordinated notes |
40,363 | — | 1,848 | 38,515 | — | |||||||||||||||
| Total |
$ | 61,526 | $ | — | $ | 1,848 | $ | 38,515 | $ | 21,163 | ||||||||||
In May 2010, we entered into exchange agreements with certain holders of our 4% Notes pursuant to which we issued approximately 4.3 million shares of common stock, upon conversion of the 4% notes as defined in ASC 470-20, Debt with Conversion and Other Options, in exchange for $1.8 million aggregate outstanding principal amount of our 4% Notes. The transactions were accounted for as induced conversions since, for the purpose of ASC 470-20, the issuance of the common stock effectively resulted in the change to the conversion privileges provided in the terms of our 4% Notes at issuance. We recorded $2.0 million in debt conversion expense for the nine months ended September 30, 2010.
In May 2010, we delivered a notice of termination of the exchange agreements to each of the holders party to the exchange agreements.
In July 2010, the remaining outstanding amount of our 4% Notes reached maturity and we made a cash payment of $39.3 million to repay the outstanding balance, including accrued interest.
6. Preferred Stock and common stock purchase warrants
Issuance of Series 4 Preferred Stock
For the description of the transaction and of the subsequent effects with respects to the assets and income accounts, please refer to the relevant section reported under subsequent Paragraph 21.1.4.
Issuance of Series 5 Preferred Stock
For the description of the transaction and of the subsequent effects with respects to the assets and income accounts, please refer to the relevant section reported under subsequent Paragraph 21.1.4.
Issuance of Series 6 Preferred Stock
For the description of the transaction and of the subsequent effects with respects to the assets and income accounts, please refer to the relevant section reported under subsequent Paragraph 21.1.4.
C-253
7. Shareholder’s Deficit
| Common stock, no par value (in thousands): |
||||||||
| September 30, 2010 |
December 31, 2009 |
|||||||
| Issued and outstanding shares - 758,453,915 (unaudited) and 590,282,575 at September 30, 2010 and December 31, 2009, respectively |
$ | 1,545,324 | $ | 1,418,931 | ||||
| Accumulated other comprehensive loss |
(8,159 | ) | (8,412 | ) | ||||
| Accumulated deficit |
(1,542,526 | ) | (1,429,083 | ) | ||||
| Total CTI shareholders’ deficit |
(5,361 | ) | (18,564 | ) | ||||
| Noncontrolling interest |
(355 | ) | (205 | ) | ||||
| Total shareholders’ deficit |
(5,716 | ) | (18,769 | ) | ||||
| Total liabilities and shareholders’ deficit |
46,613 | 69,595 | ||||||
a. Analysis of Shareholders’ Deficit Variations
Common Stock
The increase in common stock as of September 30, 2010 as compared to December 31, 2009 is primarily due to the issuance of 128.8 million shares of common stock upon conversion of our convertible preferred stock, 4.3 million shares in exchange for $1.8 million principal balance of our 4% convertible senior subordinated notes and share-based compensation recorded in accordance with SFAS 123(R) during the nine month period ended September 30, 2010.
The increase in common stock as of September 30, 2009 as compared to December 31, 2008 is primarily due to the issuance of 131.4 shares of common stock of upon the conversion of our 10% notes due 2011, the issuance of 27.7 million shares of common stock in exchange for convertible notes as discussed above, the issuance of 133.4 million shares of common stock upon the conversion of our convertible preferred stock and the issuance of 49.7 million shares of common stock and warrants.
Accumulated Other Comprehensive Loss
The decrease in accumulated other comprehensive loss as of September 30, 2010 as compared to December 31, 2009 is primarily due to foreign currency translation losses.
Accumulated Deficit
The increase in accumulated deficit as of September 30, 2010 as compared to December 31, 2009 is due to CTI’s net losses for the period ended September 30, 2010.
Noncontrolling Interest
As of September 30, 2010, we had a 69% interest in our majority-owned subsidiary, Aequus Biopharma. In accordance with our fiscal 2009 adoption of Statement of Financial Accounting Standards No. 160, Noncontrolling Interest in Consolidated Statements, an Amendment of ARB No. 51 (codified as ASC 810), noncontrolling interest in Aequus (previously shown as minority interest) is shown as a component of equity in the consolidated balance sheet.
C-254
b. Stock-Based Compensation
The following table summarizes stock-based compensation expense for nine months ended September 30, 2010 and 2009, which was allocated as follows (in thousands):
| Nine Months Ended September 30, |
||||||||
| 2010 | 2009 | |||||||
| Research and development |
$ | 2,494 | $ | 1,808 | ||||
| Selling, general and administrative |
13,766 | 11,475 | ||||||
| Stock-based compensation expense included in operating expenses |
$ | 16,260 | $ | 13,283 | ||||
Events Subsequent to September 30, 2010
Issuance of Series 7 Preferred Stock
For the description of the transaction and of the subsequent effects with respects to the assets and income accounts, please refer to the relevant section reported under subsequent Paragraph 21.1.4.
20.6.2.4 NOTES TO THE STATEMENT OF OPERATIONS
A. Revenues
1. License and Contract Revenue
License and contract revenue consists of the following for the nine months ended September 30, 2010 and 2009 (in thousands of US dollars):
| License and Contract Revenue (In thousands of US dollars) | Nine Months
Ended September 30, |
|||||||
| 2010 | 2009 | |||||||
| CTI |
$ | 319 | $ | 60 | ||||
| Subsidiaries |
— | — | ||||||
| Total license and contract revenue |
$ | 319 | $ | 60 | ||||
License and contract revenue for the nine months ended September 30, 2010 and 2009 represents recognition of deferred revenue from the sale of Lisofylline material to DiaKine.
B. Operating Expenses
2. Research and development
Research and development expenses for compounds under development and discovery research are as follows (in thousands):
C-255
| Nine Months
Ended September 30, |
||||||||
| 2010 | 2009 | |||||||
| Compounds under development: |
||||||||
| Pixantrone |
$ | 5,195 | $ | 4,897 | ||||
| OPAXIO |
1,818 | 3,102 | ||||||
| Brostallicin |
223 | 844 | ||||||
| Zevalin |
— | 987 | ||||||
| Operating expenses |
11,589 | 12,677 | ||||||
| Discovery research |
550 | 371 | ||||||
| Total research and development expenses |
$ | 19,375 | $ | 22,878 | ||||
Research and development expenses decreased to approximately $19.4 million for the nine months ended September 30, 2010 from approximately $22.9 million for the nine months ended September 30, 2009. Pixantrone costs increased primarily due to an increase in clinical development activity mainly related to costs associated with our RAPID trial as we continued to incur costs during the study wind-down. In addition, there was an increase in activities associated with investigator-sponsored trials, advisory board meetings and consulting services associated with the startup of the additional clinical study of pixantrone, PIX306. These increases were partially offset by decreases in regulatory costs and manufacturing activity. Regulatory costs decreased due to the non-recurring expense associated with the filing fee for the NDA submission to the FDA, which was incurred in the second quarter of 2009. Manufacturing expenses decreased due to the reduction of pre-commercialization activities related to pixantrone. Costs for our OPAXIO program decreased primarily due to a decrease in regulatory, clinical development and quality activities. Costs for brostallicin decreased primarily due to a decrease in clinical development activities related to phase I and phase II studies. Zevalin costs decreased due to the contribution of the product to RIT Oncology, the joint venture we formed with Spectrum on December 15, 2008 which assumed all related Zevalin expenses subsequent to that date. Our operating expenses decreased primarily due to a reduction in headcount between periods, partially offset by an increase in non-cash stock-based compensation expense. Discovery research expense relates to costs incurred in preclinical activities.
3. Selling, general and administrative
Selling, general and administrative consists of the following for the nine months ended September 30, 2010 and 2009 (in thousands of US dollars):
| Selling, general and administrative (In thousands of US dollars) | Nine Months Ended September 30, |
|||||||
| 2010 | 2009 | |||||||
| CTI |
$ | 38,890 | $ | 35,649 | ||||
| Subsidiaries |
488 | 3,348 | ||||||
| Total selling, general and administrative |
$ | 39,378 | $ | 38,997 | ||||
Selling, general and administrative expenses increased to approximately $39.4 million for the nine months ended September 30, 2010 from approximately $39.0 million for the nine months ended September 30, 2009. This is primarily due to a $2.3 million increase in non-cash stock-based
C-256
compensation expense and an increase in expenses related to sales personnel for the expected pixantrone launch in the first quarter of 2010. These increases were primarily offset by a decrease in overhead costs associated with our Bresso, Italy operations due to office closure, as well as decreases in discretionary bonus expense and professional services.
4. Restructuring charges
Restructuring charges consists of the following for the nine months ended September 30, 2010 and 2009 (in thousands of US dollars):
| Restructuring Charges (In thousands of US dollars) | Nine Months
Ended September 30, |
|||||||
| 2010 | 2009 | |||||||
| CTI |
$ | — | $ | 124 | ||||
| Subsidiaries |
— | 3,642 | ||||||
| Total gain on sale of investment in joint venture |
$ | — | $ | 3,766 | ||||
Restructuring charges of $3.8 million for the nine months ended September 30, 2009 primarily relate to activities associated with the closure of our Bresso, Italy operations, including approximately $2.6 million in employee termination benefits and approximately $1.4 million in contract termination and clean-up charges related to the Bresso facilities. These amounts were offset by a gain of $0.3 million on the sale of assets related to the Bresso operations. In addition, we incurred $0.1 million in restructuring charges related to employee separation costs associated with the termination of Zevalin-related employees in connection with the sale of our 50% interest in RIT Oncology to Spectrum.
5. Gain on sale of investment in joint venture
Gain on sale of investment in joint venture consists of the following for the nine months ended September 30, 2010 and 2009 (in thousands of US dollars):
| Gain on Sale of Investment in Joint Venture (In thousands of US dollars) | Nine Months
Ended September 30, |
|||||||
| 2010 | 2009 | |||||||
| CTI |
$ | — | $ | 10,244 | ||||
| Subsidiaries |
— | — | ||||||
| Total gain on sale of investment in joint venture |
$ | — | $ | 10,244 | ||||
During the nine months ended September 30, 2009, we recorded a $10.2 million one-time gain on the sale of our 50% interest in RIT Oncology in March 2009. This amount was based on the difference between $16.5 million in gross proceeds and the approximately $4.6 million book value of our investment in RIT Oncology at the time of sale, net of approximately $1.6 million in transaction costs.
C) Other income (expense)
Other income(expense) consists of the following for the nine months ended September 30, 2010 and 2009 (in thousands):
C-257
| Other income (expense) (In thousands of US dollars) | Nine Months
Ended September 30, |
|||||||
| 2010 | 2009 | |||||||
| Investment and other income, net |
$ | 240 | $ | 97 | ||||
| Interest expense |
(1,948 | ) | (4,026 | ) | ||||
| Debt conversion expense |
(2,031 | ) | — | |||||
| Amortization of debt discount and issuance costs |
(600 | ) | (5,575 | ) | ||||
| Foreign exchange gain (loss) |
(300 | ) | 278 | |||||
| Make-whole interest expense |
— | (6,345 | ) | |||||
| Gain on derivative liabilities, net |
— | 7,218 | ||||||
| Gain (loss) on exchange of convertible notes |
— | 7,381 | ||||||
| Equity loss from investment in joint venture |
— | (1,204 | ) | |||||
| Milestone modification expense |
— | (6,000 | ) | |||||
| Settlement expense |
— | (4,710 | ) | |||||
| Other expense, net |
$ | (4,639 | ) | $ | (12,886 | ) | ||
Investment and Other Income. Investment and other income for the nine months ended September 30, 2010 increased to approximately $240,000 as compared to $97,000 for the nine months ended September 30, 2009 primarily due to the sale of equipment from the Bresso, Italy operations and US State tax refund interest received.
Interest expense. Interest expense decreased to approximately $1.9 million for the nine months ended September 30, 2010 from approximately $4.0 million for the nine months ended September 30, 2009. This decrease is primarily due to the exchanges of $42.3 million principal balance of our 5.75%, 6.75% and 7.5% convertible senior notes and $14.8 million of our 4% Notes in 2009. In addition, we fully repaid the $38.5 million outstanding principal balance of our 4% Notes in July 2010.
Amortization of debt discount and issuance costs. Amortization of debt discount and issuance costs decreased to approximately $0.6 million for the nine months ended September 30, 2010 from approximately $5.6 million for the nine months ended September 30, 2009. During the nine months ended September 30, 2009, conversions of our 9% and 10% convertible senior notes resulted in accelerated amortization of debt discount and issuance costs of $4.4 million. In addition, amortization of debt discount and issuance costs decreased by $0.5 million due to accelerated amortization of debt discount and amortization costs on our 5.75% and 7.5% convertible senior notes and 4% Notes as a result of exchanges and conversions in 2009 reducing the remaining cost basis and discount amount to be amortized over the remaining term of the respective convertible notes.
C-258
Foreign exchange gain (loss). The foreign exchange loss for the nine months ended September 30, 2010 and foreign exchange gain for the nine months ended September 30, 2009 are due to fluctuations in foreign currency exchange rates, primarily related to payables and receivables in our European branch denominated in foreign currencies.
Debt conversion expense. Debt conversion expense of $2.0 million for the nine months ended September 30, 2010 is related to the exchange of $1.8 million principal balance of our 4% Notes in May 2010 for approximately 4.3 million shares of our common stock.
Make-whole interest expense. Make-whole interest expense of $6.3 million for the nine months ended September 30, 2009 is related to $5.4 million in payments made upon the conversion of $18.0 million of our 10% convertible senior notes and $0.9 million in payments made upon the conversion of $5.3 million of our 9% convertible senior notes.
Gain on derivative liabilities, net. The gain on derivative liabilities of $7.2 million for the nine months ended September 30, 2009 is primarily due to a gain of $4.4 million resulting from the change in the estimated fair value of the derivative liability related to the embedded conversion option on our 10% convertible senior notes as well as a gain of $2.8 million due to the change in the estimated fair value of the derivative liability related to the Series B Unit Warrant that was issued in connection with our 13.5% convertible senior notes and Series E preferred stock financing in April 2008 and modified in July 2008 in connection with the issuance of our 18.33% convertible senior notes. The Series B Unit Warrant expired in the second quarter of 2009.
Gain on exchange of convertible notes. The $7.4 million gain on exchange of convertible notes for the nine months ended September 30, 2009 is primarily related to the exchange of $52.9 million principal amount of portions of our 9%, 7.5%, 6.75%, 5.75% convertible senior and 4% Notes for $7.1 million in cash and approximately 24.2 million shares of common stock, net of related transaction costs.
Equity loss from investment in joint venture. The loss of $1.2 million for the nine months ended September 30, 2009 relates to our 50% interest in RIT Oncology, prior to the sale of this interest in March 2009, which we accounted for using the equity method of accounting.
Milestone modification expense. Milestone modification expense for the nine months ended September 30, 2009 was due to the amendment of our acquisition agreement of Systems Medicine, Inc., or SMI, pursuant to which we acquired SMI in a stock-for-stock merger in July 2007. Under the amended agreement, we agreed to distribute a $6.0 million payment in shares of our common stock to the former SMI stockholders as consideration for the replacement of $15.0 million in potential milestone payments to the former SMI stockholders. The payment in shares of our common stock was subject to CTI shareholder approval, which was obtained at our annual shareholders meeting in October 2009. We issued the former SMI stockholders approximately 5.6 million shares of our common stock in connection with the substitute payment.
Settlement expense. Settlement expense of $4.7 million for the nine months ended September 30, 2009 is primarily due to $3.2 million related to amounts paid to Spectrum for the settlement of the final installment payment related to our sale of our 50% interest in RIT Oncology based on the outcome of arbitration proceedings. This amount includes the $3.5 million escrow amount released to Spectrum, our $0.8 million payment to Spectrum based on arbitration proceedings and approximately $0.9 million in receivables recognized in prior periods and owed to us by RIT Oncology. The settlement amount is also
C-259
net of approximately $2.0 million in payables assumed by Spectrum on our behalf. We also incurred $1.3 million in settlement expense related to the payment made in accordance with our settlement agreement with Ingenix whereby each party agreed to a full release of the other party from any and all claims related to our dispute with Ingenix.
| 20.6.2.5 | NOTES TO THE CONSOLIDATED STATEMENT OF CASH FLOWS |
Please refer to the information reported under paragraph 10.2.3 “Sources, amounts and description of the Company’s cash flows for the nine months ended September 30, 2009 and 2010” of this Registration Document.
| 20.7 | Dividend policy |
We have never declared or paid any cash dividends on our common stock and do not currently anticipate declaring or paying cash dividends on our common stock in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance operations. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on a number of factors, including future earnings, capital requirements, financial conditions, future prospects, contractual restrictions and other factors that our board of directors may deem relevant.
Under Section 23B.06.400 of the Revised Code of Washington, the Board of Directors is prohibited from authorizing the payment of dividends on any of our stock if following the payment of the dividend we would not be able to pay our liabilities as they become due in the usual course of business or if the total amount of our assets would be less than the sum of our total liabilities plus any amounts we would be required to pay to satisfy preferential rights of shareholders due on dissolution of the Company, if any, under our Articles of Incorporation at the time the dividend was paid. The statute allows the Board of Directors to base their determination that the assets are not less than liabilities plus preferential amounts due on dissolution on either the financial statements prepared on the basis of accounting practices and principles that are reasonable in the circumstances or on a fair valuation or other method that is reasonable in the circumstances.
Each outstanding share of Series A, Series B and Series C Convertible Preferred Stock was entitled to quarterly payments of a dividend of 3% per annum of that stock’s stated value, which was initially $1,000 per share. The Series D Convertible Preferred Stock wasentitled to quarterly payments of a dividend of 7% of that stock’s stated value, which was also initially $1,000 per share. In the event we were not legally able to make those dividend payments, the amount of such dividend would have accreted to and increased the stated value of the stock. In addition, the terms of Series A, Series B, Series C and Series D Preferred Shares restricted the payment of dividends on the Common Shares unless the Company had paid or set aside the cumulative dividends then owed on the Preferred Shares. As of September 30, 2009, there are no longer any shares of Preferred Stock outstanding. For more information on the specific dividend rights of the Preferred Stock, please refer to Chapter 21, Paragraph 21.2.3 below.
Dividends on our Preferred Stock were paid each January 1, April 1, July 1 and October 1 for the dividends accrued in the prior quarter. In addition, we paid all accrued but unpaid dividends upon conversion of a holder of that holder’s the Preferred Stock to common stock. There were a significant number of conversions of our Preferred Stock, therefore the amount we paid in dividends on each series of Preferred Stock declined over time. From 2007, when the Series A was issued, until the date of this Registration Document we paid the following dividends on our Preferred Stock:
C-260
| Q1 2007 | Q2 2007 | Q3 2007 | Q4 2007 | Q1 2008 | Q2 2008 | Q3 2008 | Q4 2008 | Q1 2009 | ||||||||||||||||||||||||||||
| Series A |
$ | 33,718 | $ | 51,375 | $ | 51,375 | $ | 51,375 | $ | 37,200 | $ | 4,125 | $ | 4,125 | $ | 4,125 | $ | 750 | ||||||||||||||||||
| Series B |
— | $ | 96,125 | $ | 115,350 | $ | 115,350 | $ | 92,485 | $ | 39,135 | $ | 39,135 | $ | 34,410 | — | ||||||||||||||||||||
| Series C |
— | — | $ | 47,508 | $ | 62,130 | $ | 57,630 | $ | 47,130 | $ | 45,880 | $ | 32,130 | — | |||||||||||||||||||||
| Series D |
— | — | — | $ | 23,139 | $ | 54,250 | $ | 17,500 | $ | 17,500 | $ | 17,500 | $ | 17,500 | |||||||||||||||||||||
Amounts for each quarter include quarterly dividends paid on preferred stock outstanding at the end of that quarter (in each case paid on the first business day following the end of such quarter), as well as any dividends paid on Preferred Stock converted to common stock during that quarter.
| 20.8 | Legal Proceedings |
On January 22, 2007, the Company filed a complaint in King County Washington Superior Court against The Lash Group, Inc. (the “Lash Group”) and Documedics Acquisition Co., Inc. (“Documedics”), the Company’s former third party reimbursement expert for TRISENOX, seeking recovery of damages, including losses incurred by the Company in connection with our investigation, defense and settlement of claims by the United States concerning Medicare reimbursement for TRISENOX. Documedics (later acquired by the Lash Group), advised doctors that they could prescribe TRISENOX for certain diseases and be reimbursed by the U.S. government. This advice to the doctors turned out to be faulty, and ultimately the Company reimbursed the U.S. government for the expenses it had incurred. The Company is seeking damages from Documedics and the Lash Group for having given this faulty advice and causing the Company to pay the U.S. government. On February 28, 2007, the Lash Group removed the case to federal court in the Western District of Washington. On June 19, 2008, the trial judge dismissed our claims and the Company filed a timely notice of appeal in the Ninth Circuit Court of Appeals. An appeal hearing was held on August 31, 2009, and on November 18, 2009, the Ninth Circuit Court of Appeals reversed the lower court and held that the False Claims Act did not preclude us from seeking recovery and bringing claims against The Lash Group, Inc. for their alleged violations. On December 1, 2009, the Lash Group, Inc. filed a petition for rehearing with the Ninth Circuit Court of Appeals, which was formally denied on January 6, 2010. The case has been remanded for trial in the District Court. On April 30, 2010, the District Court denied a motion by Lash to strike our supplemental damages disclosure, and granted our motion for leave to amend our complaint to more fully address our claims for supplemental and independent damages. On May 21, 2010, the Court issued a minute order setting trial and related dates. On May 24, 2010, Lash filed its answer to the amended complaint and asserted counterclaims for contractual indemnification, common law indemnification and contribution, and declaratory relief. On June 3, 2010, Lash filed a motion to bifurcate the trial to address in the first phase only its assertion that our claims are barred due to FCA liability. We opposed the motion, and on June 10, 2010, we filed our own motion to strike Lash’s affirmative defense based on its FCA liability claim. The case is currently scheduled for trial on September 6, 2011. There is no guarantee that we will prevail at trial.
On February 20, 2009, the Company notified Spectrum that the Company had exercised its option to sell to Spectrum all of the Comany’s membership interest in their 50/50 owned joint venture, RIT Oncology, and on March 2, 2009, Spectrum made the first payment totaling $6.5 million. The sale of the Company’s membership interest to Spectrum closed on March 15, 2009, and the remaining $10.0 million of the total $16.5 million purchase price was deposited into an escrow account to be paid to the Company in two additional installments. On April 3, 2009, $6.5 million was released to the Company from this escrow account and the final installment
C-261
of the $3.5 million, subject to an adjustment for certain liabilities and other obligations, was scheduled to be released to the Company on April 15, 2009. Spectrum disputed the amount of the adjustment and the final installment was not released to the Company. On April 10, 2009, the Company filed a demand for arbitration regarding Spectrum’s payment of the final installment. On April 22, 2009, Spectrum filed a cross-claim alleging that Spectrum was entitled to the entire amount held in escrow and that Spectrum was owed additional amounts by the Company. The arbitration was held on May 14, 2009. On May 21, 2009, the arbitrator ordered that the final installment of $3.5 million be released from the escrow account and distributed to Spectrum; additionally, the Company was ordered to pay $776,454 to Spectrum. Of these amounts, $3,203,671 was determined by the arbitrator to be outstanding “Excluded Liabilities.” Accordingly, Spectrum is responsible for paying certain liabilities incurred or to be incurred by the Company totaling $3,203,671, including an obligation payable to Bayer for a clinical trial. On May 26, 2009, the Company paid Spectrum $776,454.
On May 1, 2008 Ingenix Pharmaceutical Services, Inc., or Ingenix, a contract research organization, sent a letter claiming that the Company owed Ingenix $2.2 million pursuant to clinical support work. The Company had hired Ingenix to assist in a clinical trial. Ingenix incurred expenses and fees without documentation that these expenses were actually incurred (e.g., plane tickets, hotel bills, etc.) or that the services were actually provided to the Company. All of these charges have been previously invoiced to CTI, but the invoices are being evaluated for the association of the work being billed to the contract assignments, as well as the relationship of the pass-through costs to approvable work. The Company was seeking documentation that the expenses and fees were incurred and truly related to the Company’s clinical trial before making payment. Rather than provide documentation, on November 6, 2008, Ingenix filed a demand for arbitration of this dispute with the American Arbitration Association, seeking damages of $2.2 million. On September 28, 2009, we entered into a settlement agreement and release with Ingenix pursuant to which we paid Ingenix $1.6 million and each party agreed to a full release of the other party from any and all claims related to the dispute.
On August 3, 2009, Sicor Società Italiana Corticosteroidi S.r.l., or Sicor, filed a lawsuit in the Court of Milan to compel us to source pixantrone from Sicor according to the terms of a supply agreement executed between Sicor and NovusPharma on October 4, 2002. A hearing was held on January 21, 2010 to discuss preliminary matters and set a schedule for future filings and hearings. The parties filed the authorized pleadings and submitted to the Court their requests for evidence. The next hearing date regarding admission of evidence and testimony is scheduled for November 11, 2010. Sicor alleges that the agreement was not terminated according to its terms. We assert that the supply agreement in question was properly terminated and that we have no further obligation to comply with its terms. No estimate of a loss, if any, can be made at this time in the event that we do not prevail.
On April 14, 2009 and December 21, 2009, the Italian Tax Authority, or ITA, issued notices of assessment to CTI (Europe) based on the ITA’s audit of CTI (Europe)’s VAT returns for the years 2003 and 2005, respectively. On June 25, 2010, the ITA issued notices of assessment to CTI (Europe) for the years 2006 and 2007 based on similar findings of the 2003 and 2005 assessments. The ITA audits concluded that CTI (Europe) did not collect and remit VAT on certain invoices issued to non-Italian clients for services performed by CTI (Europe). The assessments, including interest and penalties, for the years 2003, 2005, 2006 and 2007 are €0.5 million, €5.5 million, €2.5 million and €0.8 million, or approximately $0.7 million, $7.5 million, $3.4 million and $1.2 million as of September 30, 2010, respectively. On July 14, 2010, the ITA issued a notice of deposit payment to CTI (Europe) based on the 2005 assessment including interest and collection fees for an amount of €0.9 million, or approximately $1.2 million, payable in the third quarter 2010. We successfully filed a petition with the Provincial Tax Court of Milan, or the Tax Court, for suspension of the 2005 notice of deposit payment. On September 28, 2010, the merits of the case for the 2005 assessment were discussed in a public hearing before the Tax Court, which has reserved its decision in order to carefully review the arguments, relevant documents and other
C-262
supporting evidence (including an appraisal from an independent expert) that our counsel filed and presented during the hearing. As of the date of this Registration Document the outcome of the aforesaid hearing of September 28, 2010 is still unknown. The Company believes that the services invoiced were non-VAT taxable consultancy services and that the VAT returns are correct as originally filed. The Company has been vigorously defending against the assessments and are confident that the Tax Court will duly take into account our arguments both on procedural grounds and the merits of the case. If the Tax Court’s decision is unfavorable, the Company plans to appeal to the higher courts in order to further defend our interests.
On December 23, 2008, CONSOB sent the Company a notice requesting that the Company issue (i) immediately, a press release providing, among other things, information about our debt restructuring plan, the current state of compliance with the relevant covenants regulating our debt and the equity line of credit agreement we entered into with Midsummer Investment Ltd. on July 29, 2008, and (ii) by the end of each month and starting from the month of December 2008, a press release providing certain information relating to our management and financial situation, updated to the previous month, or the Monthly CONSOB Press Release. On July 31, 2009, CONSOB sent the Company a notice asserting three violations of the provisions of Section 114, paragraph 5 of the Italian Legislative Decree no. 58/98, namely: (a) the non-disclosure without delay of the press release mentioned under previous point (i) and the subsequent incomplete disclosure of the relevant information through the press releases dated January 9 and 13, 2009; (b) the non-disclosure of the Monthly CONSOB Press Release in December 2008; and (c) the incomplete disclosure of the Monthly CONSOB Press Release in January 2009. The sanctions established by Section 193, paragraph 1 of the Italian Legislative Decree no. 58/1998 for such violations are pecuniary administrative sanctions amounting to between €5,000 and €500,000, applicable to each one of the three asserted violations. According to the applicable Italian legal provisions, CONSOB may impose such administrative sanctions by means of a decree stating the grounds of its decision only after evaluating our possible defenses that were submitted to CONSOB on August 28, 2009 (within 30 days of July 31, 2009, the notification date of the relevant charges, according to the applicable Italian rules). On May 5, 2010, CONSOB (x) notified the Company that it has begun the preliminary investigation for its decision on these administrative proceedings and (y) provided the Company with a preliminary investigation report in reply to our defenses submitted on August 28, 2009. On June 4, 2010 (within 30 days of May 5, 2010, the notification date of the beginning of the aforesaid preliminary investigation, according to the applicable Italian rules), we submitted further defenses that CONSOB will have to evaluate before imposing any possible administrative sanctions. Based on our assessment, the likelihood that these pecuniary administrative sanctions will be imposed on the Company is probable.
Separately, on December 10, 2009, CONSOB sent the Company a notice claiming two violations of the provisions of Section 114, paragraph 1 of the Italian Legislative Decree no. 58/98 due to the asserted late disclosure of certain information then reported, at CONSOB’s request, in the press release disseminated on December 19, 2008 and March 23, 2009. Such information concerned, respectively: (i) the conversion by BAM Opportunity Fund LP of 9.66% notes into shares of common stock that occurred between October 24 and November 19, 2008; and (ii) the contents of the opinion expressed, with respect to our 2008 financial statements, by the auditor Stonefield Josephson, Inc. The sanctions established by Section 193, paragraph 1 of the Italian Legislative Decree no. 58.1998 for such violations are pecuniary administrative sanctions amounting to between €5,000 and €500,000, applicable to each one of the two asserted violations. According to the applicable Italian legal provisions, CONSOB may impose such administrative sanctions by means of a decree stating the grounds of its decision only after evaluating our possible defenses that were submitted to CONSOB on January 8, 2010 (within 30 days of December 10, 2009, the notification date of the relevant charges, according to the applicable Italian rules). On July 12, 2010, CONSOB (a) notified the Company that it has begun the preliminary
C-263
investigation for its decision on these administrative proceedings and (b) provided the Company with a preliminary investigation report in reply to our defenses submitted on January 8, 2010. On August 12, 2010 (within 30 days of July 12, 2010, the notification date of the beginning of the aforesaid preliminary investigation, according to the applicable Italian rules), we submitted further defenses that CONSOB will have to evaluate before imposing any possible administrative sanctions. Based on our assessment, the likelihood that these pecuniary administrative sanctions will be imposed on the Company is probable.
On March 12, 2010, a purported securities class action complaint was filed in the United States District Court for the Western District of Washington against the Company and certain of our officers and directors, styled Cyril Sabbagh, individually and on behalf of all others similarly situated v. Cell Therapeutics, Inc., Dr. James A. Bianco, M.D., and Dr. Jack W. Singer (Case No. 2:10-sv-00414), or the Sabbagh action. On March 19, 2010, a substantially similar class action complaint was filed in the same court, styled Michael Laquidari, individually and on behalf of all others similarly situated v. Cell Therapeutics, Inc., Dr. James A. Bianco, M.D., and Dr. Jack W. Singer (Case No. 2:10-cv-00480), or the Laquidari action. On March 31, 2010, a third substantially similar class action complaint was filed in the same court, styled William Snyder, individually and on behalf of all others similarly situated v. Cell Therapeutics, Inc., James A. Bianco, Phillip M. Nudelman, Louis A. Bianco, John H. Bauer, Richard L. Love, Mary O. Mundinger, Jack W. Singer, Frederick W. Telling and Rodman & Renshaw, LLC (Case No. 2:10-cv-00559), or the Snyder action. The securities actions are pending before Judge Marsha Pechman in the Western District of Washington. The securities complaints allege that the defendants violated the federal securities laws by making certain alleged false and misleading statements. Specifically, plaintiffs claim that defendants misled the public about the validity of a SPA — a process by which the company gained the FDA’s agreement on clinical trial design in advance of initiating the trial — for a pixantrone Phase III clinical trial (“EXTEND Trial”). Plaintiffs claim that defendants knew or should have known that the SPA was invalidated when the trial closed early, and therefore defendants should have disclosed that the EXTEND trial was no longer operating under the SPA. Plaintiffs’ consolidated amended complaint alleges that beginning on March 25, 2008, defendants engaged in a fraudulent scheme to inflate the Company’s stock price by misrepresenting that the SPA was still valid after the Company stopped the extend trial enrollment early. The plaintiffs in the Sabbagh and Laquidari actions seek unspecified damages on behalf of a putative class of purchasers of our securities from May 5, 2009 through February 8, 2010. The plaintiffs in the Snyder action seek unspecified damages on behalf of a putative class of purchasers of our securities from May 5, 2009 through March 19, 2010, including purchasers of securities issued pursuant to or traceable to the Company’s July 22, 2009 public offering. On May 11, 2010, motions were filed to consolidate the securities actions and to appoint lead plaintiff and lead plaintiffs’ counsel. On August 2, 2010, the court consolidated the three securities actions, appointed lead plaintiffs, and approved lead plaintiffs’ counsel. On September 27, 2010, lead plaintiff filed an amended consolidated complaint. On October 27, 2010, the defendants filed a motion to dismiss. The lawsuits are at a preliminary stage in the proceedings. We believe that the securities actions are without merit and intend to defend them vigorously.
On April 1, 2010, a shareholder derivative complaint was filed in the United States District Court for the Western District of Washington, derivatively on behalf of the Company against the members of its Board of Directors, styled Shackleton v. John A. Bauer, James A. Bianco, Vartan Gregorian, Richard L. Love, Mary O’Neil Mundinger, Phillip M. Nudelman, Jack W. Singer, and Frederick W. Telling (Case No. 2:10-cv-564). On April 5, 2010, and April 13, 2010, substantially similar derivative actions were filed in the same court, styled, respectively, Marbury v. James A. Bianco, et al. (Case No. 2:10-cv-00578) and Cyrek v. John H. Bauer, et al. (Case No. 2:10-cv-00625). The derivative complaints allege that the defendants breached their fiduciary duties to the Company under Washington law by making or failing to prevent the disclosure of certain alleged false and
C-264
misleading statements. The allegations in the derivative actions are substantially similar to those in the securities actions. On May 10, 2010, pursuant to the parties’ stipulation, the Court consolidated these three shareholder derivative actions and appointed the law firms Robbins Umeda LLP and Federman & Sherwood as co-lead counsel for derivative plaintiffs.
On June 1, 2010, a fourth related shareholder derivative action was filed in the Western District of Washington, styled Souda v. John H. Bauer et. al. (Case No 2:10-cv-00905). These four shareholders derivative actions filed against our board of directors have been consolidated and are pending before Judge Marsha Pechman. Plaintiff Souda has filed a motion to reconsider the portion of the Court’s Order dated May 10, 2010, appointing Robbins Umeda and Federman & Sherwood as co-lead derivative counsel. Souda’s motion is currently pending.
On July 27, 2010, a fifth related shareholder derivative action, styled Bohland v. John H. Bauer et al. (Case No. 2:10-cv-1213), was filed in the Western District of Washington and assigned to Judge John C. Coughenour and was subsequently transferred to Judge Pechman. On October 4, 2010, a sixth shareholder derivative action was filed in the Superior Court of Washington, County of King, styled Lawrence J. Alexander, derivatively on behalf of Cell Therapeutics, Inc. v. James A. Bianco, et al. (Case No. 10-2-34849-2-SEA), or the Alexander action. On October 5, 2010, the Alexander action was removed to the United States District Court for the Western District of Washington and assigned to Judge Pechman. The Bohland and Alexander actions are pending consolidation with the other four derivative actions. Also pending are motions concerning lead plaintiff appointment.
For the shareholder derivative complaints, no estimate of a loss, if any, can be made at this time in the event that we do not prevail.
On July 28, 2010, the former General Manager of our Italian Branch office, CTI (Europe), initiated a Court proceeding against the Company to challenge the former General Manager’s dismissal which occurred in 2009. The former General Manager’s claims are based on the alleged unlawfulness and lack of justifications of his dismissal. The former General Manager alleges that he has suffered and requests compensation for damages ranging up to approximately €0.7 million, plus the costs of the proceedings. The first hearing is scheduled for December 9, 2010. CTI is entitled to file a brief regarding its defences any time prior to 10 days before the hearing. Management believes that the allegations in the claim are without merit and intend to defend the claim vigorously. At this time, we are not able to make a determination whether the likelihood of an unfavorable outcome is probable or remote.
In addition to the litigation discussed above, the Company is from time to time subject to legal proceedings and claims arising in the ordinary course of business, some of which may be covered in whole or in part by insurance.
Besides the ones described above, currently there are no pending claims which may have relevant impacts on the economic and financial situation of the Company.
In accordance with ASC 450, the Company has accrued €110,000 as of September 30, 2010 for those matters that management has assessed the likelihood of an unfavorable outcome as probable.
| 20.9 | Significant change in the issuer’s financial or commercial position |
Please see Chapter 3 of this Registration Document for the changes in the Company’s financial position during the year ended December 31, 2009. There have been no significant change in the Company’s commercial position during the the nine months ended September 30, 2010.
C-265
CHAPTER 21 ADDITIONAL INFORMATION
| 21.1 | Share Capital |
Below is a table detailing the number of authorized common shares and authorized preferred shares as of the date of this Registration Document (as of December 31, 2009 the number of authorized common shares and authorized preferred shares was, respectively 800,000,000 and 10,000,000):
| Authorized Common Shares |
Authorized Preferred Shares | |
| 1,200,000,000 |
10,000,000 |
The number of shares of CTI common stock outstanding as of October 22, 2010 amounts to 814,781,932.
As of October 22, 2010 there is not any series of preferred stock outstanding.
CTI does not have any shares paid in kind. All shares outstanding are issued and fully paid and have no par value. For a reconciliation of the number of shares common stock outstanding from December 31, 2006 to October 22, 2010, please see Paragraph 21.1.7 of this Chapter 21 below.
| 21.1.2 | If there are shares not representing capital, state the number and main characteristics of such shares |
CTI has not issued shares not representing the share capital.
| 21.1.3 | Treasury stock |
There is no treasury stock of CTI outstanding.
| 21.1.4 | The amount of any convertible securities, exchangeable securities or securities with warrants, with an indication of the conditions governing and the procedures for conversion, exchange or subscription |
Convertible Notes
For all the relevant information concerning the Convertible Notes please refer to the previous paragraph 10.3.3 of this Registration Document.
B) Tender offer for outstanding notes
On December 5, 2008, the Company announced that the Board of Directors had authorized a modified Dutch Auction (as described below) tender offer to repurchase or exchange up to $124 million of the Company’s outstanding (i) 4% convertible senior notes due 2010, (ii) 5.75% convertible senior notes due 2011, (iii) 6.75% convertible senior notes due 2010, (iv) 7.5% convertible senior notes due 2011 and (v) 9% convertible senior notes due 2012. The intention of the offer was to reduce the principal amount of the Company’s outstanding indebtedness and reduce its substantial interest payments on its outstanding debt while offering liquidity to the holders of its convertible debt that they might not otherwise have had. Pursuant to the terms and conditions of
C-266
the authorized tender offer, the Company would have offere to purchase or exchange a portion or all of the outstanding principal amount of the tendered notes at a price expected to be no greater than $80 or less than $50 per $1,000 principal amount of the tendered notes, plus accrued and unpaid interest. It expected to use up to $10 million for the tender of these notes, with such funds to come from cash currently on hand, cash received or expected to be received in connection with the closing of the proposed joint venture with Spectrum and the subsequent sale of the Company’s 50% interest in RIT Oncology to Spectrum, and/or cash to be received in connection with the sale of certain convertible notes that could be issued to an institutional investor under a put option granted to the Company in connection with the offering of the Company’s 10% convertible senior notes due 2011 issued in December 2008. As of December 5, 2008, there were an aggregate of $124 million of Notes outstanding.
Pursuant to the aforesaid authorization dated December 5, 2008, by the Board of Directors, on May 12, 2009 the Company announced that it had commenced a the tender offer to exchange shares of Common Stock and cash for an aggregate of up to $89.2 million principal amount representing 75% of the outstanding Notes (the “Exchange Offer”). The Exchange Offerwas conditioned on a minimum of $83.2 million aggregate principal amount of Notes being validly tendered and not withdrawn. For each $1,000 principal amount of Notes, holders were to receive consideration with a value not greater than $300 nor less than $250 (the “Exchange Consideration”), with such value determined by a “Modified Dutch Auction” procedure, plus accrued and unpaid interest to, but excluding, the settlement date payable in shares of Common Stock. A “Modified Dutch Auction” tender offer allows holders of the Notes to indicate the principal amount of Notes that such holders desire to tender and the consideration within the specified range at which they wish to tender such Notes. The mix of Exchange Consideration was to consist of (i) $200 in cash, and (ii) a number of shares of Common Stock with a value equal to the Exchange Consideration minus $200 (the “Common Stock Portion”, and such number of shares of our Common Stock being the “Common Stock Consideration”). The number of shares of Common Stock received by holders as part of the Exchange Consideration was equal the quotient obtained by dividing (x) the Common Stock Portion by (y) the average of the daily volume weighted average price of the Common Stock on the national securities exchange on which the Common Stock is listed or quoted for trading as reported by Bloomberg L.P. (based on a trading day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)) for the 10 trading days from and including May 19, 2009 to and including June 2, 2009 (the “10-day VWAP”). Accrued and unpaid interest on Notes validly tendered and not withdrawn, up to, but not including, the settlement date were to be exchanged for that number of shares of Common Stock determined by dividing (1) the amount of accrued and unpaid interest on a Note, by (2) the 10-day VWAP. However, in no event could the aggregate number of shares of Common Stock Consideration plus the aggregate number of shares of Common Stock to be exchanged for accrued and unpaid interest exceed a maximum number of shares of the Company’s Common Stock (the “Common Stock Limit”) disclosed in the Schedule TO (including the Offer to Exchange and related Letter of Transmittal) that was filed by the Company with the SEC on May 12, 2009. In the event the aggregate number of shares of Common Stock Consideration plus the aggregate number of shares of Common Stock to be exchanged for accrued and unpaid interest exceeded the Common Stock Limit, the Company would have terminate the Exchange Offer.
On May 18, 2009, the Company amended the Exchange Offer and announced that the Exchange Consideration has been amended to increase the range of Exchange Consideration to a value not greater than $600 nor less than $550 for each $1,000 principal amount of Notes validly tendered and not withdrawn, with such value determined by a “Modified Dutch Auction” procedure. Originally, the minimum value had been $250 per $1,000 principal amount and the maximum value had been $300 per $1,000 principal amount. The cash portion of the
C-267
Exchange Consideration continued to be $200. Accrued and unpaid interest to, but excluding, the settlement date continued to be exchanged for shares of Common Stock. As a result of the increase in the portion of the Exchange Consideration consisting of shares of Common Stock, the Company has increased the maximum number of shares of Common Stock to be issued upon exchange of the Notes and to pay accrued and unpaid interest to 30.63 million shares of Common Stock.
On June 2, 2009, the Company announced that it has amended and restated its Exchange Offer to a fixed price tender offer for any and all of the outstanding principal amount of the five series of its convertible notes that were the object of the Tender offer. In particular the Company offered to exchange, in separate concurrent offers as set forth hereinafter, shares of Common Stock and cash for any and all of its: (i) $55,150,000 aggregate outstanding principal amount of 4% Convertible Senior Subordinated Notes due 2010; (ii) $23,000,000 aggregate outstanding principal amount of 5.75% Convertible Senior Notes due 2011; (iii) $7,000,000 aggregate outstanding principal amount of 6.75% Convertible Senior Notes due 2010; (iv) $33,458,000 aggregate outstanding principal amount of 7.5% Convertible Senior Notes due 2011; and (v) $335,000 aggregate outstanding principal amount of 9.0% Convertible Senior Notes due 2012 (hereinafter, the “Notes”, that jointly represented all the outstanding notes of the Company). Under the Exchange Offers, CTI was offering to exchange (i) $134.50 cash, and (ii) 458 shares of its Common Stock for each $1,000 principal amount of Notes validly tendered and not withdrawn in each Exchange Offer (the “Exchange Consideration”). The Exchange Consideration was the same for each Exchange Offer. Accrued and unpaid interest to, but excluding, the settlement date on Notes validly tendered and not withdrawn in the Exchange Offers were to be paid in cash. The Exchange Offers were conditioned upon the satisfaction of certain terms and conditions described in the Company’s Amended and Restated Offer to Exchange, dated June 2, 2009, the related Amended and Restated Letter of Transmittal, and other related offer materials.
On June 19, 2009, the Company announced that in accordance with the terms and conditions of fix priced Exchange Offers (as described above with respect of the date of June 2, 2009) for any and all of the approximately $118.9 million outstanding principal amount of the Notes. The Exchange Offers expired on June 16, 2009. In accordance with the terms and conditions of the Exchange Offers, and based on the final count by U.S. Bank National Association, the depositary for the Exchange Offers, the Company has accepted for exchange approximately $52.9 million aggregate principal amount of the Notes for the previously announced exchange consideration of (i) $134.50 cash, and (ii) 458 shares of common stock per $1,000 principal amount of Notes validly tendered and not withdrawn in each Exchange Offer, for a total amount of exchange consideration (excluding interest, fees and other expenses in connection with the Exchange Offers) of approximately $7.1 million cash and approximately 24.2 million shares of common stock. The $1.9 million reduction in the final aggregate principal amount of Notes accepted for exchange compared to the preliminary aggregate principal amount of Notes tendered for exchange announced by the Company on June 17, 2009 is due to the depositary’s receipt of separate notices of guaranteed delivery from two different brokers for the same Notes. As a result of this transaction, the Company eliminated approximately $52.9 million of debt, reduced its annual interest expense by approximately $3.3 million, and increased its shareholder’s equity by approximately $43.7 million. In addition, the Company expects to book an estimated gain on the exchange of approximately $7.9 million. In particular, the Company has accepted for exchange the following approximate principal amount of each series of Notes: (i) $11,787,000, or 21.4%, of the $55,150,000 aggregate outstanding principal amount of 4% Convertible Senior Subordinated Notes due 2010; (ii) $12,087,000, or 52.6%, of the $23,000,000 aggregate outstanding principal amount of 5.75% Convertible Senior Notes due 2011; (iii) $5,500,000, or 78.6%, of the $7,000,000 aggregate outstanding principal amount of 6.75% Convertible Senior Notes due 2010; (iv) $23,208,000, or 69.4%, of the $33,458,000 aggregate outstanding principal amount of 7.5% Convertible Senior Notes due 2011; and (v) $335,000, or 100.0%, of the $335,000 aggregate outstanding principal amount of 9.0% Convertible
C-268
Senior Notes due 2012. The aggregate principal amount of Notes that the Company has accepted for exchange in the Exchange Offers represents approximately 44.5% of the outstanding principal amount of Notes as of the date of June 16, 2009. The Company settled the Exchange Offers on June 22, 2009.
C) Warrants still Outstanding at December 31, 2009 (with indication of the warrants still outstanding at September 30, 2010)
In connection with CTI’s November 2005 6.75% convertible senior notes offering, CTI issued warrants to purchase 8,750 shares of common stock within five years at an exercise price of $140.00 per share to the initial purchaser of these notes. None of these warrants have been exercised as of September 30, 2010.
In July 2007, CTI issued warrants to purchase 259,614 shares of common stock at an exercise price of $45.30 per share in connection with the issuance of its Series C convertible preferred stock. The warrants became exercisable on January 27, 2008 and terminate two years after this date. These warrants expired on January 27, 2010, and none of these warrants remain outstanding as of September 30, 2010.
In December 2007, CTI issued warrants to purchase 124,401 shares of common stock at an exercise price of $25.50 per share in connection with the issuance of its Series D convertible preferred stock. The warrants became exercisable on June 3, 2008 and terminate two years after this date. In December 2008, warrants to purchase 57,416 shares of common stock were cancelled in connection with the issuance of CTI’s 10% convertible senior notes due 2011. In April 2009, warrants to purchase 19,138 shares of common stock were cancelled in connection with our exchange of Series D convertible preferred stock. These warrants expired on June 3, 2010, and none of these warrants remained outstanding as of September 30, 2010.
In December 2007, CTI issued warrants to purchase 346,999 shares of common at an exercise price of $20.20 per share in connection with its direct offering of common stock. In December 2008, warrants to purchase 222,990 shares of common stock were cancelled in connection with the issuance of CTI’s 10% convertible senior notes due 2011. The warrants became exercisable on June 20, 2008 and will terminate three years after this date. None of these warrants have been exercised and 124,009 warrants are still outstanding as of September 30, 2010.
In March 2008, CTI issued warrants to purchase 732,696 shares of common stock at an exercise price of $14.10 per share in connection with the issuance of its 9% convertible senior notes. The warrants became exercisable on July 4, 2008 and expire three years after this date. In connection with the issuance of CTI’s 13.5% notes in April 2008, 74,468 of these warrants were cancelled. As of September 30, 2010, none of these warrants have been exercised and 658,226 warrants are still outstanding.
In April 2008, CTI issued warrants to purchase 2,848,100 share of common stock at an exercise price of $9.50 per share (which was subsequently amended to $7.90 per share) in connection with the issuance of its 13.5% convertible senior notes and other financial instruments. In July, August, and December 2008, all of these warrants were repurchased in connection with the issuances of CTI’s 18.33% convertible senior notes and 10% convertible senior notes due 2011 . As of September 30, 2010, none of these warrants remain outstanding.
In July and August 2008, CTI issued warrants to purchase 2,816,455 shares of common stock at an exercise price of $7.90 per share in connection with its issuance of 18.33% convertible senior notes. The warrants were exercisable immediately and expire on June 19, 2013. In December 2008, all of these warrants were repurchased in connection with the issuances of CTI’s 10% convertible senior notes due 2011. As of September 30, 2010, none of these warrants remain outstanding.
C-269
In April 2009, CTI issued warrants to purchase 9,183,562 shares of common stock (“Class A Warrant”) and 13,316,438 shares of common stock (“Class B Warrant”) at an exercise price of $0.41 per share, respectively, in connection with its issuance of Series 1 preferred stock. The Class A Warrant was exercisable immediately, and 9,183,562 shares of common stock were issued upon exercise. The Class B Warrant is exercisable on and after October 14, 2009 and will expire on October 14, 2014. In October 2009, the holder of the Class B warrants elected to exercise 10,377,625 warrants for CTI common stock. As of September 30, 2010, 2,938,813 Class B warrants are still outstanding.
In May 2009, CTI issued warrants to purchase 4,800,000 shares of common stock at an exercise price of $1.40 per share in connection with its offering of common stock and warrants. The warrants were exercisable immediately and will expire on May 11, 2014. None of the warrants have been exercised and 4,800,000 warrants are still outstanding as of September 30, 2010.
In July 2009, CTI issued warrants to purchase 8,432,981 shares of common stock at an exercise price of $1.70 per share in connection with its offering of common stock and warrants. The warrants were exercisable immediately and expire nine months after the issuance date. These warrants expired on April 28, 2010 and none of these warrants remain outstanding as of September 30, 2010.
In August 2009, CTI issued warrants to purchase 4,713,276 shares of common stock at an exercise price of $1.70 per share in connection with its issuance of Series 2 preferred stock. The warrants were exercisable immediately and expire nine months after the issuance date. These warrants expired on May 19, 2010 and none of these warrants remain outstanding as of September 30, 2010.
In January 2010, the Company issued warrants to purchase 8,640,000 shares of common stock at an exercise price of $1.18 per share in connection with its issuance of Series 3 preferred stock. The warrants were exercisable immediately and will expire on January 20, 2011. In July 2010, we exchanged existing warrants to purchase 4,320,000 shares of common stock at an exercise price of $1.18 per share for new warrants to purchase the same number of shares of common stock at an exercise price of $0.42 per share. The terms of the new warrants issued upon exchange are substantially similar to the warrants issued in the Series 6 preferred stock transaction. None of the warrants have been exercised and 4,320,000 of the warrants issued in connection with the Series 3 preferred stock and 4,320,000 of the new warrants are still outstanding as of September 30, 2010.
In April 2010, CTI issued warrants to purchase 20,000,000 shares of common stock at an exercise price of $0.60 per share in connection with its issuance of Series 4 preferred stock. The warrants are exercisable on October 7, 2010 and expire on April 7, 2014. As of September 30, 2010, all 20,000,000 warrants are still outstanding.
In May 2010, CTI issued warrants to purchase 26,250,000 shares of common stock at an exercise price of $0.50 per share in connection with its issuance of Series 5 preferred stock. The warrants are exercisable on November 28, 2010 and contingent upon CTI obtaining shareholder approval to increase shares authorized for issuance by 400 million shares or notification from CTI that the shares underlying the warrants have been reserved. Shareholders approved the 400 million increase in authorized shares at the annual shareholders’ meeting held on September 16, 2010. The warrants expire on May 29, 2014. As of September 30, 2010, all 26,250,000 warrants are still outstanding.
In July 2010, CTI issued warrants to purchase 5,800,000 shares of common stock at an exercise price of $0.42 per share in connection with its issuance of Series 6 preferred stock. The warrants are exercisable on January 28, 2010 and contingent upon CTI obtaining shareholder approval to increase shares authorized for issuance by 400 million shares or notification from CTI that the shares underlying the warrants have been reserved. Shareholders
C-270
approved the 400 million increase in authorized shares at the annual shareholders’ meeting held on September 16, 2010. The warrants expire on January 28, 2015. As of September 30, 2010, all 5,800,000 warrants are still outstanding.
D) Preferred Stock outstanding as of December 31, 2009 or issued after such date
Series 3
In January 2010, the Company entered into an agreement to sell $30 million of shares of its Series 3 Preferred Stock and warrants to purchase shares of its common stock in a registered offering to two institutional investors. Each share of Series 3 Preferred Stock was convertible at the option of the holder, at any time during its existence, into approximately 823 shares of common stock at a conversion price of $1.21375 per share of common stock, for a total of approximately 24,690,000 common shares. Shares of the Series 3 Preferred Stock would have been entitled to receive dividends in the same amount as any dividends declared and paid on shares of common stock and had no voting rights on general corporate matters. The closing of the offering occurred on January 19, 2010, date on which the Company received the cash proceeds and delivered the securities. The Company cannot disclose to third parties the names of the initial investors due to the confidential nature of this information.
Series 4
In March 2010, we entered into a securities purchase agreement for the issuance of 20,000 shares of our Series 4 preferred stock, which was convertible into 40.0 million shares of our common stock, and warrants to purchase up to 20.0 million shares of our common stock for gross proceeds of $20.0 million. Issuance costs related to this transaction were $1.4 million. In April 2010 at the date of closing, all 20,000 shares of our Series 4 preferred stock were converted into 40.0 million shares of our common stock.
Each share of our Series 4 preferred stock was entitled to a liquidation preference equal to the stated value of such share of our Series 4 preferred stock plus any accrued and unpaid dividends. Our Series 4 preferred stock was not entitled to dividends except to share in any dividends actually paid on our common stock or any pari passu or junior securities. It was convertible into our common stock, at the option of the holder, at a conversion price of $0.50 per share, subject to a 4.99% blocker provision. A holder of our Series 4 preferred stock could elect to increase the blocker provision to 9.99% by providing the Company with 61 days’ prior notice. Our Series 4 preferred stock did not have voting rights except for limited protective provisions and except as is otherwise required by law.
The warrants have an exercise price of $0.6029 per share of our common stock, are exercisable six months and one day after the date of issuance and expire four years and one day after the date of issuance. As the warrants include a redemption feature that may be triggered upon a certain liquidation event that is outside of our control, we classified these warrants as mezzanine equity. We estimated the $5.6 million fair value of the warrants using the Black-Scholes pricing model.
Upon conversion of our Series 4 preferred stock, we recognized $15.5 million in deemed dividends on preferred
C-271
stock related to the transaction, including $5.6 million resulting from the allocation of net proceeds to the warrants and $9.9 million related to the beneficial conversion feature on the 20,000 shares of our Series 4 preferred stock as the stock was converted immediately.
Series 5
In May 2010, we entered into a securities purchase agreement for the issuance of 21,000 shares of our Series 5 preferred stock, which was convertible into 52.5 million shares of our common stock, and warrants to purchase up to 26.3 million shares of our common stock for gross proceeds of $21.0 million. Issuance costs related to this transaction were $1.5 million, including $0.2 million related to the placement agent warrants as discussed below. In May 2010 at the date of closing, all 21,000 shares of our Series 5 preferred stock were converted into 52.5 million shares of our common stock.
Each share of our Series 5 preferred stock was entitled to a liquidation preference equal to the stated value of such share of our Series 5 preferred stock plus any accrued and unpaid dividends. Our Series 5 preferred stock was not entitled to dividends except to share in any dividends actually paid on our common stock or any pari passu or junior securities. It was convertible into our common stock, at the option of the holder, at a conversion price of $0.40 per share, subject to a 4.99% blocker provision. A holder of Series 5 preferred stock could elect to increase the blocker provision to 9.99% by providing the Company with 61 days’ prior notice. In addition, if 1,000 or less shares of Series 5 preferred stock are outstanding, all outstanding shares of Series 5 preferred stock automatically convert into shares of common stock. Our Series 5 preferred stock did not have voting rights except for limited protective provisions and except as otherwise required by law.
The warrants have an exercise price of $0.50 per share of our common stock and are exercisable six months and one day after the date of issuance and expire four years, six months and one day after the date of issuance, provided that the exercisability of the warrants is subject to, and conditioned upon our receipt of shareholder approval after the date of the prospectus supplement relating to the offering of an amendment to its amended and restated articles of incorporation to increase the authorized shares of common stock available for issuance thereunder by 400 million shares or our notification to holders of the warrants that shares of common stock have become available and are reserved for issuance upon exercise of the warrants. The amendment to increase the Company’s authorized shares available for issuance was approved by shareholders at annual meeting of shareholders held on September 16, 2010. As the warrants include a redemption feature that may be triggered upon a certain liquidation event that is outside of our control, we classified these warrants as mezzanine equity. We estimated the $6.0 million fair value of the warrants using the Black-Scholes pricing model.
Upon conversion of the Company’s Series 5 preferred stock, we recognized $14.6 million in deemed dividends on preferred stock related to the transaction, including $6.0 million resulting from the allocation of net proceeds to the warrants and $8.6 million related to the beneficial conversion feature on the 21,000 shares of our Series 5 preferred stock as the stock was converted immediately.
C-272
In connection with the offering of our Series 5 preferred stock, we also issued warrants to purchase 1.1 million shares of our common stock to the placement agent which are classified in mezzanine equity due to the same redemption feature described above. The warrants were estimated to have a fair value of $0.2 million using the Black-Scholes pricing model. These warrants have an exercise price of $0.50 per share and are exercisable after six months and one day after the date of issuance and expire five years after the date of issuance, provided that the exercisability of the warrants is subject to, and conditioned upon, our receipt of the shareholder approval or notification described above.
Series 6
In July 2010, we entered into a securities purchase agreement, pursuant to which we agreed to issue in a private offering an aggregate of 4,060 shares of our Series 6 preferred stock, initially convertible into 11.6 million shares of our common stock and warrants to purchase up to 5.8 million shares of our common stock for gross proceeds of $4.06 million. The warrants have an exercise price of $0.42 per share of our common stock. The warrants are exercisable at any time on or after the six month and one day anniversary of the date of initial issuance and on or before the four year, six month and one day anniversary of the date of the initial issuance, provided that the warrants will not be exercisable unless and until (i) we amend our amended and restated articles of incorporation to increase the authorized shares of common stock available for issuance thereunder by 400 million shares after receiving shareholder approval of such amendment or (ii) we notify the holders of the warrants that shares of common stock have otherwise become available and are reserved for issuance upon exercise of the warrants. In the event that shares of common stock otherwise become available for reservation following the initial issuance of the warrants, we will reserve all or a portion of such shares for issuance upon exercise of the warrants, provided that (a) the foregoing obligation does not apply to shares of common stock reserved pursuant to our equity incentive plans, (b) if shares of common stock must be reserved pursuant to the terms of any outstanding warrants to purchase common stock issued on or about the closing date of our Series 5 preferred stock financing, shares must be reserved for issuance upon the exercise of those warrants in priority to the reservation of shares for issuance upon the exercise of the warrants issued pursuant to the Series 6 preferred stock financing and (c) any such shares that become available shall be reserved pro rata among all warrants originally issued on or about the closing date of our Series 6 preferred stock financing (or in exchange or substitution thereof) and any other warrants that otherwise have a substantially similar reservation provision. The amendment to increase the Company’s authorized shares available for issuance was approved by shareholders at the Company’s annual meeting of shareholders held on September 16, 2010. As the warrants include a redemption feature that may be triggered upon a certain liquidation event that is outside of the Company’s control, we classified these warrants as mezzanine equity. The Company estimated the $1.1 million fair value of the warrants using the Black-Scholes pricing model.
All 4,060 shares of the Series 6 preferred stock were converted into 11.6 million shares of our common stock upon closing of the transaction in July 2010.
Each share of Series 6 preferred stock is entitled to a liquidation preference equal to the stated value plus any accrued and unpaid dividends before the holders of our common stock or any other junior securities receive any payments upon such liquidation. The Series 6 preferred stock is not entitled to dividends except to share in any dividends actually paid on our common stock or any pari passu or junior securities. The Series 6 preferred stock is convertible into common stock, at the option of the holder, at an initial conversion price of $0.35 per share, subject to a 4.99% blocker provision. A holder of Series 6 preferred stock may elect to increase the blocker
C-273
provision to 9.99% by providing 61 days’ prior notice. The Series 6 preferred stock will vote with the common stock on an as-converted basis.
In connection with the offering of our Series 6 preferred stock, we also issued warrants to purchase 0.3 million shares of our common stock to the placement agent which are classified in mezzanine equity due to the same redemption feature described above. The warrants were estimated to have a fair value of $0.1 million using the Black-Scholes pricing model. These warrants have an exercise price of $0.42 per share and are exercisable after six months and one day after the date of issuance and expire four years, six months and one day after the date of issuance. The exercisability of the warrants was also subject to, and conditioned upon, our receipt of the shareholder approval described above.
Series 7
In October 2010, we entered into a securities purchase agreement, pursuant to which we agreed to issue in a registered offering an aggregate of 21,000 shares of our Series 7 preferred stock, initially convertible into approximately 56.8 million shares of our common stock and warrants to purchase up to approximately 22.7 million shares of our common stock for gross proceeds of $21.0 million. All 21,000 shares of the Series 7 preferred stock were converted into 56.8 million shares of our common stock upon closing of the transaction on October, 22 2010.
The warrants have an exercise price of $0.45 per share of our common stock. The warrants are exercisable at any time on or after the six month and one day anniversary of the date of initial issuance and on or before the five year and one day anniversary of the date of the initial issuance.
Each share of Series 7 preferred stock is entitled to a liquidation preference equal to the stated value plus any accrued and unpaid dividends before the holders of our common stock or any other junior securities receive any payments upon such liquidation. The Series 7 preferred stock is not entitled to dividends except to share in any dividends actually paid on our common stock or any pari passu or junior securities. The Series 7 preferred stock is convertible into common stock, at the option of the holder, at an initial conversion price of $0.37 per share, subject to a 4.99% blocker provision. A holder of Series 7 preferred stock may elect to increase the blocker provision to 9.99% by providing 61 days’ prior notice, and the maximum percentage will automatically increase to 19.99% in the event of an automatic conversion. The Series 7 preferred stock has no voting rights.
| 21.1.5 | Information about and terms of any acquisition rights and or obligations over authorized but unissued capital or an undertaking to increase the capital |
See discussion of the Company’s stock option plans in Chapter 17, Paragraph 17.3.1 and convertible securities in Chapter 21, Paragraph 21.1.4 of this Registration Document.
| 21.1.6 | Information about any capital of a member of the group which is under option or agreed conditionally or unconditionally to be put under option and details of such options including those persons to whom such options relate |
Not applicable.
C-274
| 21.1.7 | A history of the changes in the Issuer’s share capital, highlighting information about any changes for the period going from December 31, 2006 to October 22, 2010 |
CTI’s share capital increased from 3,639,723 Common Shares as of December 31, 2006 to 6,244,423 Common Shares as of December 31, 2007 as a result of the following transactions:
| • | the issuance of 196,562 Common Shares upon conversion of 13,150 shares of Series A 3% Convertible Preferred Stock; |
| • | the issuance of 324,219 Common Shares upon conversion of 21,820 shares of Series B 3% Convertible Preferred Stock; |
| • | the issuance of 306,820 Common Shares upon conversion of 11,966 shares of Series C 3% Convertible Preferred Stock; |
| • | the issuance of 95,694 Common Shares upon conversion of 2,500 shares of Series D 7% Convertible Preferred Stock; |
| • | the issuance of 182,961 Common Shares due to the conversion of 7.5% convertible senior notes; |
| • | the issuance of 421,185 Common Shares in connection with the acquisition of Systems Medicine, Inc. on July 31, 2007; |
| • | the issuance of 545,957 Common Shares to retire a portion of CTI’s 5.75% convertible subordinated and senior subordinated notes; |
| • | the issuance of 347,000 Common Shares in a registered offering in December 2007; and |
| • | the issuance of 184,302 Common Shares due to 197,125 shares of restricted stock granted to employees, offset by 12,823 shares of restricted stock cancelled. |
CTI’s share capital increased from 6,244,423 Common Shares as of December 31, 2007 to 186,411,922 Common Shares as of December 31, 2008 as a result of the following transactions:
| • | the issuance of 94,170 Common Shares upon conversion of 6,300 shares of Series A 3% Convertible Preferred Stock; |
| • | the issuance of 150,994 Common Shares upon conversion of 10,162 shares of Series B 3% Convertible Preferred Stock; |
| • | the issuance of 102,562 Common Shares upon conversion of 4,000 shares of Series C 3% Convertible Preferred Stock; |
| • | the issuance of 114,832 Common Shares upon conversion of 3,000 shares of Series D 7% Convertible Preferred Stock; |
| • | the issuance of 80,000 Common Shares in January 2008 under the Step-Up Equity Financing Agreement with Société Générale; |
| • | the issuance of 684,868 Common Shares in exchange for the cancellation of $150,000 of 5.75% convertible subordinated notes and approximately $8.9 million of 5.75% convertible senior subordinated notes; |
| • | the issuance of 1,544,948 Common Share under the Equity Line of Credit with Midsummer Investment, Ltd; |
C-275
| • | the issuance of 3,575,947 Common Shares due to conversions of 18.33% convertible senior notes; |
| • | the issuance of 2,895,035 Common Shares due to conversions of 9% convertible senior notes; |
| • | the issuance of 3,493,670 Common Shares due to conversions of 13.5% convertible senior notes; |
| • | the issuance of 7,086,614 Common Shares due to conversions of 10% convertible senior notes due 2012; |
| • | the issuance of 8,333 Common Shares due to conversions of 5.75% convertible senior notes; |
| • | the issuance of 11,189,390 Common Shares due to conversions of 15% convertible senior notes; |
| • | the issuance of 41,315,779 Common Shares due to conversions of 9.66% convertible senior notes; |
| • | the issuance of 106,943,947 Common Shares due to conversions of 10% convertible senior notes due 2011; |
| • | the issuance of 8,400 Common Shares for options exercised and stock sold via the employee stock purchase plan; and |
| • | the issuance of 878,010 Common Shares due to 956,720 shares of restricted stock granted to employees, offset by 78,710 shares of restricted stock cancelled. |
CTI’s share capital increased from 186,411,922 Common Shares as of December 31, 2008 to 590,282,575 Common Shares as of December 31, 2009 as a result of the following transactions:
| • | the issuance of 4,000,000 Common Shares upon conversion of 100 shares of Series A 3% Convertible Preferred Stock in connection with our litigation settlement with RHP Master Fund, Ltd.; |
| • | the issuance of 44,576 Common Shares upon conversion of 3,000 shares of Series B 3% Convertible Preferred Stock in connection with our litigation settlement with Tang Capital Partners, LP. |
| • | the issuance of 131,386,860 Common Shares due to conversions of 10% convertible senior notes due 2011; |
| • | the issuance of 372,340 Common Shares due to conversions of 9% convertible senior notes; |
| • | the issuance of 288,517 Common Shares in connection with our Series A 3% Convertible Preferred Stock exchange; |
| • | the issuance of 3,452,493 Common Shares in connection with our Series D 7% Convertible Preferred Stock exchange; |
| • | the issuance of 47,871,425 Common Shares due to conversions of Series F Convertible Preferred Stock; and |
| • | the issuance of 66,666,667 Common Shares due to conversions of Series 1 Preferred Stock; |
| • | the issuance of 9,183,562 Common Shares due to exercise of Class A Warrant; |
| • | the issuance of 16,000,000 Common Shares in connection with our offering of Common Stock and Warrants; |
| • | the issuance of 24,235,986 Common Shares in connection with the settlement of the Exchange Offers; |
| • | the issuance of 33,731,923 Common Shares in connection with a registered offering in July 2010; |
| • | the issuance of 18,853,103 Common Shares due to the conversion of Series 2 Preferred Stock; |
C-276
| • | the issuance of 3,299,310 Common Shares in connection with exchanges of our 6.75% convertible senior notes and 4% convertible senior subordinated notes; |
| • | the issuance of 10,377,625 Common Shares due to exercise of Class B Warrant; |
| • | the issuance of 5,607,468 Common Shares pursuant to the Second Amendment to the Acquisition Agreement of Systems Medicine, Inc. |
| • | the issuance of 42,009 Common Shares for options exercised and stock sold via the employee stock purchase plan; and |
| • | the issuance of 28,456,789 Common Shares due to 34,144,204 shares of restricted stock granted to employees, offset by 5,687,415 shares of restricted stock cancelled. |
CTI’s share capital increased from 590,282,575 Common Shares as of December 31, 2009 to 814,781,932 Common Shares as of October 22, 2010 as a result of the following transactions:
| • | the issuance of 24,690,000 Common Shares due to conversions of Series 3 Preferred Stock; |
| • | the issuance of 40,000,000 Common Shares due to conversions of Series 4 Preferred Stock; |
| • | the issuance of 52,500,000 Common Shares due to conversions of Series 5 Preferred Stock; |
| • | the issuance of 11,600,000 Common Shares due to conversion of Series 6 Preferred Stock; |
| • | the issuances of 56,756,756 Common Shares due to conversions of Series 7 Preferred Stock; |
| • | the issuance of 507,656 Common Shares in connection with the exercise of common stock purchase warrants; |
| • | the issuance of 4,303,157 Common Shares in connection with exchanges of our 4% convertible senior subordinated notes; |
| • | the issuance of 17,234 Common Shares for options exercised and stock sold via the employee stock purchase plan; and |
| • | the issuance of 34,124,554 Common Shares due to 37,923,278 shares of restricted stock granted to employees, offset by 3,798,724 shares of restricted stock cancelled. |
| 21.2 | MEMORANDUM AND ARTICLES OF ASSOCIATION |
| 21.2.1 | A description of the issuer’s objectives and purposes and where they can be found in the memorandum and articles of association |
The Company’s Amended and Restated Articles of Incorporation currently in force do not address the Company’s objective and purposes. Pursuant to Washington law, the corporate purpose of the Company is to engage in any lawful business under the laws and regulations of the State of Washington.
| 21.2.2 | A summary of any provisions of the issuer’s articles of association, statutes, charter or bylaws with respect to the members of the administrative, management and supervisory bodies |
Washington law provides that a company must have at least one director. CTI’s bylaws sets the size of the board to be twelve, but the number of directors may be increased or decreased by resolution of either
C-277
the shareholders or the directors at any annual, regular or special meeting. There are currently eight directors on the board.
The shareholders elect directors. Each director shall hold office until such director’s successor is elected and qualified or until such director’s earlier death, resignation or removal. Any director may resign at any time by giving written notice to the company. Washington law provides that the shareholders may remove any or all directors with or without cause, unless the articles of incorporation provide that directors may be removed only for cause. If one or more series or classes of shares elect the directors, only the holders of those shares may remove them. CTI’s amended and restated articles of incorporation currently in force provide that directors may be removed only for cause, and only if the number of votes cast to remove the director by holders of shares then entitled to vote in an election of directors exceeds the number of votes cast not to remove the director.
Vacancies on the board of directors and newly created directorships may be filled by a vote of the directors remaining in office, unless the articles of incorporation or bylaws provide otherwise.
Washington law provides that a company’s articles of incorporation may provide for the board of directors to be divided into up to three classes with staggered terms. CTI’s amended and restated articles of incorporation currently in force provide that the directors shall be divided into three approximately equal classes with staggered terms of three years for each class. The board of directors will remain classified in three classes with staggered terms.
With regard to the authority of the Board of Directors, the Audit Committee, the Compensation Committee and the Nominating and Governance Committee, please see Chapter 14 of this Registration Document.
CTI does not currently maintain and has no present intention to establish an Executive Committee of the board of directors.
| 21.2.3 | A description of the rights, preferences and restrictions attaching to each class of the existing shares |
Common Shares
Pursuant to Article V, Section 2 of the Articles of Incorporation, each holder of Common Shares is entitled to one vote for each share held on all matters to be voted upon by the shareholders and there are no cumulative voting rights. Subject to preferences that may be applicable to any outstanding preferred stock, holders of Common Shares are entitled to receive ratably the dividends, if any, that are declared from time to time by the board of directors out of funds legally available for that purpose. In the event of a liquidation, dissolution or winding up of the Company, the holders of Common Shares are entitled to share in our assets remaining after the payment of liabilities and the satisfaction of any liquidation preference granted to the holders of any outstanding shares of preferred stock. Holders of Common Shares have no pre-emptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the Common Shares. All outstanding Common Shares are fully paid and nonassessable. The rights, preferences and privileges of the holders of Common Shares are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that CTI may designate in the future.
Preferred Shares
As of the date of this Registration Document, there were no Preferred Stock outstanding.
C-278
| 21.2.4 | A description of what action is necessary to change the rights of holders of the shares, indicating where the conditions are more significant than is required by law |
The rights of holders of common stock of the Company cannot be changed without an affirmative vote of the holders of a majority of the outstanding shares of the Company. No separate meetings of the holders of Preferred Shares are required by the Company’s charter documents.
| 21.2.5 | A description of the conditions governing the manner in which annual general meetings and special meetings of shareholders are called including the conditions of admission |
The modalities in which CTI’s annual general meetings and extraordinary general meetings of shareholders are called, are governed by U.S. law and CTI’s amended and restated bylaws. Moreover, at the time of its listing on the Nuovo Mercato (now MTA), CTI has undertaken certain obligations for the purposes of providing adequate disclosure to those shareholders who hold CTI shares vis-à-vis Monte Titoli S.p.A. (the “Italian Shareholders”), as described in the 2003 Listing Prospectus (the “2003 Listing Prospectus”).
Generally, all shareholders who hold shares of CTI stock on the record date set for the particular meeting have the right to vote at such meeting.
Generally, there are two ways that shareholders can hold shares in a U.S. public company: as record holders (these are the shareholders registered in the Company’s books), or through a nominee which is generally a broker, where they continue to remain as beneficial holders. The transfer agent (the Company’s transfer agent is Computershare at the date of this Registration Document) can provide to the Company, at its request, a list of record holders. In the U.S., the single largest record holder for most public companies is CEDE & Co., the nominee name for the Depository Trust Company (“DTC”).
As described in more detail below, the Company does not have direct access to information pertaining to all of the beneficial holders who hold shares through intermediaries, such as brokers or banks. Those beneficial holders are represented in the books of CTI under CEDE & Co. DTC is the depository that holds shares on behalf of underlying custodian participants, including brokerages and banks. Monte Titoli S.p.A., the central securities depository in Italy (“Monte Titoli”), is also a custodian participant under DTC. These custodian participants hold shares on behalf of the beneficial holders. These beneficial holders, or those that are not record holders, include individuals and Italian intermediary banks. Although the Company does not list the beneficial holders as record holders, they have the right to vote at each meeting, and DTC grants voting authority to the custodian participants who then pass this authority along to the beneficial holders. In the case of Monte Titoli, DTC provides an omnibus proxy as described under point B) below.
The following Paragraphs provide a description of A) U.S. laws and regulations governing shareholders’ meetings, B) disclosure requirements under Italian law regarding the exercise of voting rights in the context of the shareholders’ meeting and C) difficulties faced by CTI in conducting its shareholders’ meetings and prospective solutions going forward.
| A) | U.S. laws and regulations |
Calling of CTI’s shareholders’ meetings
Article II, Section 5 of CTI’s amended and restated bylaws currently in force provide that, for every shareholders’ meeting, a written notice of the time and place must be mailed to each shareholder entitled
C-279
to vote at the meeting not more than sixty and not less than ten days before the meeting, unless such notice is waived by the shareholder pursuant to Article II, Section 13 of CTI’s amended and restated bylaws in writing or by the shareholder’s presence at the meeting.
In compliance with Washington state law and with Article II, Section 5 of its amended and restated bylaws currently in force, CTI shall call:
| • | the annual shareholders’ meeting with a notice given not more than 60 days before the meeting and not less than 10 days before a meeting; |
| • | extraordinary shareholders’ meetings concerning a plan of merger or share exchange, a proposed sale of assets other than in the regular course of business or the dissolution of the corporation with a notice given not more than 60 days and not less than 20 days before the meeting date; and |
| • | any other extraordinary shareholders’ meetings with a notice given not more than 60 days before the meeting and not less than 10 days before a meeting. |
For the purposes of the meeting call, the CTI Board of Directors shall determine: (a) the meeting date and (b) the “record date” that is the reference date on which it is determined who are the shareholders entitled to vote at the meeting; as a matter of practice, the record date is usually fixed at the discretion of the Board of Directors approximately one month in advance of the meeting date, however, pursuant to Article II, Section 6 of CTI’s amended and restated bylaws, the record date may be any date not more than 70 days before the meeting and not less than ten days before the meeting.
CTI shall give notice of the meeting date in the U.S. in compliance with the U.S. applicable law by mailing to each shareholder a meeting call notice, as described above. A complete list of shareholders entitled to notice of, and to vote at, the meeting will be open to examination by the shareholders beginning ten days prior to the meeting for any purpose germane to the meeting during normal business hours at the office of the Secretary of the Company in Seattle.
Shareholder proposals (including nominations of directors of CTI) may be considered at an annual meeting only if CTI receives written notice in a timely manner. In order to be timely, a shareholder’s notice must be delivered to the Secretary of CTI not later than ninety days prior to the anniversary date of the immediately preceding annual meeting of shareholders. Under the rules of the SEC, if CTI changed the date of an annual meeting by more than thirty days from the date of the previous year’s meeting, the notice must be received by CTI a reasonable time before it begins to print and mail its proxy materials. A shareholder’s notice must set forth:
| • | the name and address of the shareholder who intends to make the proposal; |
| • | a representation that the shareholder is a holder of record of CTI common stock entitled to vote at such meeting and intends to appear in person or by proxy at the meeting to vote for the proposal; and |
| • | such other information as would be required to be included in a proxy statement filed pursuant to the proxy rules of the SEC. |
In addition, a nomination for a director of CTI must include:
| • | a description of all arrangements or understandings between the shareholder and each nominee and any other person pursuant to which the nomination is to be made by the shareholder; and |
C-280
| • | the consent of the nominee to serve as a director of CTI if so elected. |
Washington law provides that the holders of at least 10% of all the votes entitled to be cast on any issue proposed to be considered at the special meeting may call a special meeting if they deliver to the secretary an executed dated record for the meeting describing the purpose for which the meeting is to be held. The articles of incorporation or bylaws may require a higher percentage (not in excess of 25%), and the articles of incorporation may limit or deny this right.
Article II, Section 4 of CTI’s amended and restated bylaws currently in force provide that special meetings of shareholders may be held only upon notice given by or at the direction of the president, the board of directors or the holders of at least 10% of all the votes entitled to be cast on any issue proposed to be considered at the special meeting. Such notice must state the time, place and purposes of the special meeting. In order to call a special meeting, the holders of at least 10% of the votes entitled to be cast on any issue proposed to be considered at the proposed special meeting must execute one or more demands to the secretary of CTI set forth in an executed and dated record describing the purpose for which it is to be held. CTI’s amended and restated articles of incorporation currently in force do not limit or deny the right of holders of at least 10% of the votes entitled to be cast on a matter to call a special meeting as provided by Washington law.
CTI has typically held its shareholders’ meetings in Seattle, Washington, however, shareholders’ meetings are not required to be held in Seattle, Washington, and from time to time have been held in Italy such as the annual meeting which was held at the Borsa Italiana in Milan on September 25, 2007.
Brief description of the mechanics concerning the annual shareholders meeting
The modalities of call and the voting procedures of CTI’s annual meeting of shareholders is described in the section above entitled “Calling of CTI’s shareholders’ meetings”. For the purposes of the annual meeting quorum, CTI is subject to the corporate laws under which it was incorporated. At the Company’s annual meeting held on June 19, 2008, the Company’s shareholders approved a proposal to reduce the quorum required for shareholder meetings from a majority to requiring that one-third (1/3) of outstanding shares entitled to vote must be represented at an annual meeting before the Company can take action on proposed items.
Proxy materials
CTI must file with the SEC and mail a proxy statement to shareholders at least ten days in advance of its annual shareholders’ meeting (or twenty business days if information is being incorporated by reference to other documents filed with the SEC, as permitted in certain circumstances) each year, which proxy statement includes material information (including the recommendation of the board of directors) about the matters to be considered at the annual shareholders’ meeting. The proxy statement must also include detailed disclosure about executive compensation.
SEC Requirements and Procedures
CTI must file with the SEC and mail a proxy statement to shareholders in advance of any special (extraordinary) shareholders meeting (final version is due to be filed with the SEC no later than twenty days in advance of the meeting), which proxy statement includes material information (including the
C-281
recommendation of the board of directors) about the matters to be considered at the meeting. The proxy statement must supply all information required by Section 14 of the Exchange Act, which sets forth detailed requirements depending upon the type of proposals being considered at the meeting, including the recommendation of the board of directors on each proposal.
Pursuant to the above Section 14 of the Exchange Act, a proxy statement relating to an annual meeting may generally include:
| • | information about the annual meeting (e.g., date, time and place and matters to be voted on); |
| • | information about each class of CTI’s voting securities (e.g., number of shares outstanding and number of votes per share); |
| • | any limitations or restrictions imposed on shareholders’ abilities to revoke proxies following the granting of the same to representatives of CTI; |
| • | information about any legal proceedings to which CTI’s directors and executive officers are a party; |
| • | information about material transactions between CTI and its directors and officers; |
| • | descriptions of the committees of CTI’s board of directors, including a list of each committee member, the number of committee meetings held by each committee during the last fiscal year and the functions performed by such committees; |
| • | additional information about CTI’s audit committee, including a report of the audit committee on CTI’s financial statements, a copy of the audit committee’s charter, if any, and disclosure as to whether the members of CTI’s audit committee are “independent” for purposes of NASDAQ’s listing standards; |
| • | the number of meetings of the board of directors held during the last full fiscal year and information on the attendance of the board members; |
| • | certain information about directors who resign or decline to stand for re-election to the board due to a disagreement with CTI on any matter related to CTI’s operations, policies or practices; and |
| • | information about the auditors selected or being recommended to shareholders for election, approval or ratification for the current year. |
For annual meetings where the only proposals for shareholder action are routine proposals, such as election of directors, approval of equity compensation or employee stock purchase plans and ratification of the Company’s independent auditors, the Company will generally not need to file a preliminary proxy statement with the SEC and can file the final or “definitive” proxy statement with the SEC no later than the date that it mails such proxy statement to its shareholders. However, if the Company includes proposals in the proxy statement on extraordinary matters that require the filing of a preliminary proxy statement, such as an amendment to the articles of incorporation or a merger or other significant transaction, the Company must first file a preliminary proxy statement with the SEC and allow the SEC ten days to determine whether or not it wishes to review such proxy statement. If the SEC decides not to review or not to comment on the proxy statement, the Company may, not less than 10 calendar days after the filing of the preliminary proxy, file a definitive proxy statement and mail such definitive proxy statement to the shareholders. If the SEC chooses to comment on a preliminary proxy statement, then the comment process can take anywhere from approximately 30-90 days (or sometimes even longer), depending on the extent of the SEC comments and the amendments required in the proxy (as well as
C-282
other public filings of the Company) as well as the subject matter of the special meeting (for example, a proxy statement to approve a merger will generally undergo a substantial greater review than a proposal to amend the charter to increase the authorized shares). Typically, a public company in the United States would not include the date of the meeting in the proxy statement until it had completed the SEC review process and filed its definitive proxy statement. However, because CTI will provide meeting information to its Italian shareholders in connection with the custody transfer process described below, CTI may need to set the meeting date prior to filing its definitive proxy statement. In the event that the SEC has comments that delays the filing of the definitive proxy statement, CTI may have to postpone the meeting. The SEC comment process is the means by which the SEC oversees the shareholder meeting process in advance of a meeting, however in certain circumstances the SEC may review a proxy statement subsequent to a meeting with respect to disclosure about executive compensation or otherwise with the general outcome that it will request that further information be provided on a future basis in the following year’s proxy statement. The decision whether or not to review a preliminary proxy statement and whether or not to provide comments on a preliminary proxy statement is solely in the discretion of the Securities and Exchange Commission.
If the shareholder listed in CTI’s records is a clearing agency such as DTC, which is the main centralized clearing system in the U.S., Rule 14a-13 of the Exchange Act promulgated by the SEC requires a company to make inquiry to the clearing agency at least twenty business days in advance of the record date of a meeting (or, if twenty business days is impracticable in the case of a special meeting, as soon as practicable in advance of the special meeting) in order to learn the identity of the brokerages (i.e., intermediaries) that hold shares on DTC’s records (and are obligated to provide to the shareholders’ the company’s materials as explained below). CTI must then arrange for a sufficient number of proxy statements to be provided to those brokerages (intermediaries) so that they can re-deliver the materials (through Broadridge) to the ultimate beneficial owners of the shares (as described in greater detail below). SEC rules (Exchange Act Rules 14b-1 and 14b-2) require U.S. intermediaries to forward the company’s materials to the persons shown on their records as holding the shares. The procedures described in this Paragraph are the modalities by which shareholders who are not registered in the Company’s books receive the shareholders meeting call because such shareholders would be listed in the Company’s records via the listing of the clearing agency, such as DTC.
The Company shall make available the proxy statement relating to the meeting on the both the Company’s and SEC’s Internet website which the Company does by filing the proxy statement electronically with the SEC at http://www.sec.gov/.
Beginning on July 1, 2007, public companies in the U.S. were able to begin using new proxy solicitation rules recently promulgated by the SEC, referred to as the “notice and access” model, under which proxy solicitation may be satisfied by sending notice to shareholders of how to access an electronic version of the required proxy materials rather than mailing out the full set of proxy materials. As part of the “notice and access” requirements, companies will need to post proxy materials on a website that does not track any information regarding visitors to that site (other than to log numeric IP addresses) and may not use any email addresses collected solely for the purpose of providing electronic copies of the proxy statement for any other reason (with the intent of such restrictions to maintain the privacy of those U.S. holders who hold their shares with intermediaries or brokerage firms and hold less than 5% of the outstanding shares of the Company). Beginning on January 1, 2008, certain large public companies in the U.S. were required to use the “notice and access” model, and beginning on January 1, 2009, all companies, including CTI, will be required to use the “notice and access” model, however, any company may
C-283
voluntarily use the new rules at any time prior to that date. Under these new rules, the Company may either alternatively (a) mail written notice of the meeting, the proposals to be voted on at the meeting and instructions for accessing the proxy materials, along with information for shareholders on how to request printed copies of the proxy materials to be sent to them by first-class mail or electronic copies to be sent to them via electronic mail or (b) mail out the full set of proxy materials to shareholders as has been done in traditional mailings and additionally provide a website where the proxy material can be accessed electronically. In addition, companies that do not send a full set of soliciting material may choose to provide for proxies to be executed via the internet, a telephone number or on proxy cards that can be downloaded, printed and mailed to the company or the agent soliciting proxies on behalf of the company.
Distribution of proxy materials and exercise of voting rights
Pursuant to Washington State Law (the law to which CTI is subject), the individuals who are entitled to exercise their voting rights at the shareholders’ meetings are (i) those who at a given date (record date) are recorded in the shareholders’ books of CTI (including those shareholders who are clearing agencies, such as DTC) as CTI shareholders and (ii) those third parties to whom these shareholders have conferred an appropriate power of attorney, which includes, but is not limited to a proxy (through a U.S. intermediary holding the shares). A power of attorney is a legal instrument that is used to delegate legal authority to another. The power of attorney gives legal authority to another person to make property, financial and other legal decisions for the person who grants the power of attorney. The power of attorney can be granted in a number of ways, and as discussed above, would include the granting of a proxy.
When the Company holds a shareholder meeting, an entity acting as agent for banks and U.S. brokers who is legally obliged to distribute proxy materials to its customers (currently the Company utilizes Broadridge, an independent third party who provides, on a professional basis, shareholder meeting services to issuers) will (i) collect from U.S. brokers the personal data of CTI shareholders holding their interests with the same U.S. brokers, following the determination of the record date and of the meeting date (which data would include the numbers of shares held by such person as of the record date as well as their mailing address or email address if they have elected to receive communications electronically), and (ii) assign a security code (“security code”) to each shareholder for the purposes described below. The Company is responsible for paying Broadridge’s fee, which is in accordance with SEC rules. The Company also instructs its transfer agent to provide to Broadridge information concerning its registered shareholders (the registered holders are the record holders). This information is obtained for purposes of distributing material for the meeting. Once the material for the meeting is finalized, the Company provides the material to Broadridge, who works with the brokers to send the material to the beneficial holders; the information is directly sent to the record holders by either a third party agent such as Broadridge or by the transfer agent.
CTI shareholders are entitled to vote in person, by naming a substitute proxy (other than the proxy designated by CTI), by any other means permitted by Washington law and CTI’s bylaws, by the proxy designated by CTI, according to its amended and restated bylaws currently in force, by sending via mail to the Company a proxy card contained in the proxy statement (In Italy, an Italian shareholder must obtain a Certification - as defined below - from its Italian intermediary bank and present it along with a copy of the proxy card, in order to vote) or by voting on the Internet or by telephone.
C-284
Under the latter circumstance (voting on the Internet or by telephone), the security code (note that this number is a unique number for each shareholder – and changes with each meeting) assigned by Broadridge and included on the proxy statement which is sent by mail (or e-mail to those shareholders who elect to receive the proxy statements via e-mail) ad personam to each shareholder as included in Broadridge’s list, will allow such shareholder to vote on the Internet or by telephone, stating his/her security code.
The proxy statement sent to the shareholders generally includes a proxy card which permits each shareholder to designate in writing on the proxy card specified representatives of the company (which may be also members of the Board of Directors) as proxies and instructs the proxies to vote on the shareholder’s behalf for, against or abstaining on the proposals to be voted on at the meeting, in the manner directed by the shareholder in the proxy card. The proxy card may specify that if the relevant shareholder sends the proxy card without any specific voting instruction, the proxy may be voted in favor of the relevant proposal. CTI’s proxy cards always contain a designated proxy. Under U.S. federal law and Washington state law, a shareholder may designate anyone as a proxy; however, as a matter of practice, companies generally designate individuals affiliated with the company as the proxy.
Also, as an alternative modality of voting by proxy, the shareholders may name as a substitute proxy by any other means permitted by Washington law and our bylaws, any other person at their own discretion. Under Washington law, a shareholder or its agent or attorney-in-fact may name a substitute proxy by (i) executing a signed writing naming such proxy, (ii) affixing the shareholders signature to a proxy (including any manual, conformed, facsimile, or electronic signature), or (iii) transmitting or authorizing the transmission of a recorded telephone call, voice mail or other electronic transmission to the person or agent for the person who will be the holder of the proxy provided that such transmission either sets forth or is submitted with information, including any security or validation controls used, from which it can be reasonably determined that the transmission came from the shareholder. An appointment of proxy is revocable by the shareholder unless the proxy states that it is irrevocable and the proxy is coupled with an interest. CTI’s bylaws do not impose any additional requirements on naming a substitute proxy, however, pursuant to the bylaws such proxy is effective on receipt by the person authorized to tabulate votes and such proxy, if not revoked, will be deemed to be valid for eleven months unless a longer period is expressly provided for in the appointment form.
The tally procedures in connection with the voting are supervised by an inspector of elections, who is appointed by the Company. The Board of Directors can appoint the inspector for the meeting or can delegate the appointment of the inspector to one or more officers of the Company. The appointment of an inspector of elections is required under Washington law for a public company such as CTI. The inspector of elections is generally a third party, such as a representative from Broadridge or the transfer agent, having sufficient experience to carry out such role (although no specific professional requirements are required by law and the inspector may be an employee or officer of the Company). The inspector passes on the validity of proxies submitted for tally and certifies as to the accuracy of the tally (and the resulting quorum) and is granted the ability to do this by its appointment by the Board of the Company (including appointment by an officer to whom that duty has been delegated). In carrying out such activity, the Inspector of Elections will generally rely on the accuracy of the information received from Broadridge and the transfer agent and will attend the shareholders’ meeting to record the voting expressed therein.
C-285
| B) | Disclosure obligations relating to the exercise of the voting rights on the part of the Italian Shareholders |
Agreement between the Company and Monte Titoli
With reference to the shares held with Monte Titoli, in light of the fact that only Monte Titoli has a position in DTC register as holding such shares and in order to enable CTI shareholders to exercise their voting rights, at the time of the admission of CTI’s shares to listing on the MTA (previously, MTAX and prior to that, New Market), CTI and Monte Titoli set up an internal operational procedure aimed at providing rules for the compliance with the following obligations:
| 1) | CTI shall notify Monte Titoli of the date and of the time of each CTI shareholders’ meeting within the periods mentioned under Paragraph A “Calling of CTI Shareholders Meetings” above; |
| 2) | DTC shall grant an Omnibus Proxy (i.e., a general proxy representing all CTI’s shares held by Monte Titoli vis-à-vis DTC) to Monte Titoli; |
| 3) | Monte Titoli, in its turn, shall execute an Omnibus Proxy delegating the voting rights to the CTI beneficial shareholders who are named in the certification for attendance at the shareholder meetings issued by the intermediaries participating in Monte Titoli’s system upon CTI’s beneficial shareholders request (the “Certification”). This Certification certifies that the person named in the Certification is a CTI beneficial shareholder at the record date and, therefore, its production at the meeting entitles the persons named in the Certification or his/her proxy, on the basis of the Omnibus Proxy mechanism mentioned above, to vote at the meeting. |
| 4) | Furthermore, Monte Titoli will transmit to CTI the executed Omnibus Proxy and the list of the intermediaries adhering to the centralized management System and holding CTI shares; |
| 5) | Monte Titoli shall provide information to the brokers participating in the Monte Titoli System in relation to the meeting call and the grant to the intermediaries or to those named in the Certifications of the Omnibus Proxy sent to CTI; and |
| 6) | CTI will send directly to the intermediaries adhering to the centralized management System, holding CTI shares and indicated by Monte Titoli (as above mentioned at point 4), the documentation (proxy statement included) in relation to the meeting that has been called and the relevant matter concerning the exercise of the voting rights. The intermediaries shall make such documentation and information available to CTI shareholders named in the Certifications upon request by the latter, except as described below. |
In order to call a special meeting as described under Paragraph A) “Calling of CTI’s Shareholders Meeting” above, the holders of at least 10% of the votes entitled to be cast on any issue proposed to be considered at the proposed special meeting, who are Italian holders, shall also send to CTI ‘s secretary adequate evidence of their ownership interests in the Company (including a certification of their ownership of CTI shares which may be provided by their Depository Bank), in addition to the other documentation required as described above under Paragraph A). While there is no specific statutory requirement to provide such a certification under Washington law and no requirement to do so under the Company’s bylaws, the Company would need to confirm that the shareholders seeking to call the meeting are actually shareholders of the Company. A certification from the Depository Bank would serve this purpose, or alternatively, the shareholders could provide an affidavit of ownership. However, the certification would still be required to vote.
C-286
Publication and availability of meeting documentation to Italian shareholders
On the basis of the procedure mentioned above and as indicated in the 2003 Listing Prospectus, in Italy, the meeting call notice is sent by CTI, together with a copy of the proxy statement in English, within the terms mentioned under Paragraph A “Calling of CTI Shareholders Meetings” (i.e., at least 20 days or 10 days in advance, according to whether the meeting which is being called is an annual or a special one), to Borsa Italiana S.p.A. and (through the centralized management system Monte Titoli as far as the meeting call notice only is concerned), to the authorized intermediaries adhering to the said system.
CTI will also publish the meeting call notice in an Italian newspaper. Please note that the publication deadline will be the same as its mailing commencement deadline (i.e., at least 10 or 20 days in advance of the meeting). The publication will state where the proxy statement is available and will instruct shareholders who wish to vote to contact their Depository Banks (as defined below) to receive the meeting documentation, including the proxy card, and instruct them regarding how to vote their shares.
For the purposes of facilitating the Internet/Phone Voting Procedure (as defined below), CTI will also publish (pursuant to the same modalities as those used for the publication of the meeting call notice) a preliminary notice in an Italian newspaper indicating CTI’s intention to call a shareholders’ meeting. Such preliminary notice will have to be published at least ten days before the relevant record date. This preliminary notice will facilitate the Internet/Phone Voting Procedure as well as remind Italian shareholders the possibility for the same to be registered in the Company’s shareholders books.
The Italian Shareholders may obtain a copy of the meeting notice and of the proxy statement in the following manner:
| (i) | at the office of Borsa Italiana S.p.A.; |
| (ii) | at the office of the intermediaries having CTI shares on their accounts (the “Depository Banks”). The Depository Banks shall make such documentation and information available to CTI shareholders named in the Certifications upon request by the latter. Whether the Italian Shareholder is entitled to directly obtain the relevant meeting documentation from the Depository Bank, on an on-going basis without any specific request by the former, depends upon the specific terms and conditions governing the contractual relationship between the relevant shareholder and its Depository Bank; |
| (iii) | on the SEC’s website (www.sec.gov); |
| (iv) | on the Company’s website (www.celltherapeutics.com); |
the meeting call notice is also published on an Italian newspaper as mentioned above.
The proxy statement will be available to Italian shareholders at least 20 days or 10 days in advance of the meeting, depending on which meeting is being called (i.e., annual or special).
Voting modalities
Italian Shareholders, after having requested and received the relevant Certification by their Depository Banks, may vote in the following manner:
| 1. | vote in person, presenting his/her Certification to the inspector of elections at the meeting together with an ID card; |
C-287
| 2. | name another person as a substitute proxy by any other means permitted by Washington law and the Company’s bylaws (as described under A) above). Such person must provide at the meeting the relevant shareholder’s Certification (or a complete copy thereof) together with the relevant proxy. In connection with the substitute proxy, please also see Paragraph A “Distribution of Proxy Materials and Exercise of Voting Rights” above; |
| 3. | by submitting the proxy card included with the proxy statement (as described under section A) above), as duly filled in and executed, to the Company (at the address shown on the proxy card), together with the Certification, via mail or via fax. |
The voting modalities under item 1, 2 and 3 above are available to Italian shareholders without the need for the latter to set up a custody transfer to U.S. accounts.
Internet and telephone voting
With reference to the setting up of the voting modalities over the Internet and over the phone (“Internet/Phone Voting Procedure”), in the 2003 Listing Prospectus, the Company stated that voting modalities over the Internet and over the phone were available to Italian shareholders as well. The Company has been trying to facilitate the voting by its Italian shareholders in this manner, but Italian laws prevent the Company from knowing the names of all its shareholders (save in case and until such shareholders express their intention to participate to the meeting). Also, for the details of the shareholders to be accepted and taken into account by Broadridge, the same would need to be provided to Broadridge by an intermediary having a contractual relationship with Broadridge itself. As the identification of each shareholder and the existence of a contractual relationship between Broadridge and the intermediary providing the information are conditions to allow Broadridge to assign a security code (as a matter of fact, for U.S. shareholders Broadridge collects the personal data of CTI shareholders from U.S. brokers who are allowed to disclose such information to Broadridge and have a contractual relationship with Broadridge but such information is not made available to CTI preserving such U.S. holders privacy) and, therefore, to vote over the Internet or by phone, the Company has not been in a position to provide such facility to its Italian shareholders.
Nevertheless, Italian Shareholders who would like to vote over the Internet or by phone shall be allowed to vote so if they follow the procedure as described below.
After the publication of the preliminary notice of call (which will be published in Italy prior to the setting of the record date and in time for the shareholders to exercise their rights) and before the record date set for each meeting, any Italian Shareholder wishing to have the ability to vote by Internet or phone should give instructions to its Italian Depository Bank to immediately transfer to its U.S. correspondent bank the shares that he wishes to vote via Internet or by phone (i.e., to make book entry of the shares to the Cede Participant account of their U.S. broker-dealer affiliate) on a separate customer account in the name and for the account of the Italian Shareholder, to be maintained at the U.S. broker-dealer affiliate of that U.S. correspondent bank, and held in custody by the broker-dealer affiliate through the close of business on the record date. The instructions to the Italian Depository Bank should be given early enough for the Italian Depository Bank to make the custody transfer in time for the shares of the Italian Shareholder to be held in custody by the broker-dealer affiliate by the record date.
Once the shares of the Italian Shareholder are held in custody by the broker-dealer affiliate, the latter shall be in a position to provide the Italian Shareholder’s details to Broadridge so that the shareholder information compiled by Broadridge (for the purposes of allowing shareholders to vote via Internet or by
C-288
phone) can include the details of the Italian Shareholder. A computer-generated control number is then included on the proxy card (which is mailed with the proxy statement) and forwarded to each shareholder (including the Italian Shareholder) by mail (or e-mail to those shareholders who elect to receive the proxy statements via e-mail), allowing the shareholder to vote by logging on to proxyvote.com or calling the number provided on the voting card (as this control number identifies him as a shareholder of CTI having the right to vote on the proposals). Italian Shareholders willing to use the Internet/Phone Voting Procedure will need to inquire of their banks as to the time required for the transfer of the shares (which will have to precede the record date and the custody transfer, the latter being generally carried out the day before the record date). Please note that the costs deriving from the transfer, including those debited or claimed by the U.S. broker for the management of the account in the U.S., may be charged to the Italian shareholder requesting the transfer of its shares.
The setting up of the aforesaid procedure was and is aimed at enabling CTI shareholders resident in Italy to validly exercise their voting rights at CTI’s meetings via internet and telephone voting.
Registration in the Company’s shareholders book
In the alternative, Italian or U.S. Shareholders may also request to be registered in the Company’s shareholders book at any time as a record holder by submitting the relevant request to be conveyed (directly or through the Depository Banks or broker or other intermediary holder of record of such shares) to the Company’s transfer agent (whose details are available on the Company website at its address set forth on the website). The Shareholder or its bank shall attach to the relevant registration request a certification of their ownership to be requested to their Depository Banks or broker or other intermediary holder of record of such shares.
For an Italian Shareholder, in order to submit a request this way, the Italian Shareholder would first have to contact his/her Italian Depository Bank in which his/her shares are held, and then the Italian Depository Bank would have to arrange with Monte Titoli and DTC the completion of the registration procedure in order to remove those shares from the Monte Titoli account and transfer them to the transfer agent in the United States in the name of the shareholder directly. Once the shareholder’s holdings have been put into the direct registration system of the transfer agent via book entry, the transfer agent will provide the shareholder with either a statement of his/her shares or, on request of such shareholder, a paper stock certificate for such shares.
As a consequence of the procedure mentioned above, such shares will be maintained by the transfer agent in the United States in the name of the relevant shareholders who have requested to become a CTI “record holder”. Upon registration, the transfer agent requires to be provided with certain information (for example, the shareholder’s address, and a Form W-8 for non-U.S. holders).
Once an Italian Shareholder is registered with CTI’s shareholders’ book as a record holder, such shareholder will receive the shareholders’ materials at the address indicated upon registration. Such shareholder will also show as a “registered shareholder” or record holder (as described above) at the Record Date.
In order to sell the shares subsequent to the registration, the shareholder would then need to either transfer the securities (direct registration) to the broker or bank of the shareholders’ choice, such that the shares are sold through such broker or bank, or would need to request sale be made by the transfer agent directly, which may limit the shareholder’s ability to control the price at which such shares are sold as the transfer agent is not able to use order limits or other mechanisms that may limit the price at which
C-289
stock can be sold but must instead sell the stock at prevailing market price within a certain period of time after the request to sell is made.
Payments made to the shareholders in connection with the shares (which would be limited to proceeds from the sale of shares since the Company does not pay dividends on its common stock), are generally made by check for proceeds of the sale after certain transaction fees are withheld. If the shareholder prefers, proceeds may be paid via wire transfer (though wire fees may apply) if the shareholder provides account information to the transfer agent.
The relevant shareholder is cancelled as record holder from the Company’s books when he/she requests to re-register his/her holdings with a broker or a bank or when he/she disposes of them.
Timetable
The following tables summarize the main steps and the indicative timing prior to annual and special shareholders meeting for U.S. and Italian shareholders (“X” is the date of the shareholders’ meeting):
| 1. | Annual shareholders meeting |
| U.S. Shareholders |
Italian Shareholders* | |||||
| Action |
Date |
Action |
Date | |||
| Request to DTC of list of intermediaries holding shares on DTC’s records | Record Date – 20 | Request to Monte Titoli of list of intermediaries holding shares on Monte Titoli’s records. | Because the process is not statutory, there are no specific timing requirements for this action, but the Company historically has sent this request prior to the record date. | |||
| _ | - | Publication of preliminary notice in Italian newspaper and on the Company’s website in Italian. | Record date – 10 | |||
| _ | - | Deadline for transfer of CTI shares to U.S. accounts, upon instructions of the shareholders to its Depository Bank, for the purposes of the Internet/Telephone Voting Procedure (allowing voting by phone or Internet). | Record date -1 | |||
| Record date | Between X-70 to X-10** | Record Date | Between X-70 to X-10** | |||
| Deadline for filing with SEC, publication on the Company’s website and mailing to each shareholders of the meeting call notice and proxy statement. | X-10*** | Deadline for making available to Borsa Italiana S.p.A. and to Italian intermediaries of meeting call notice and proxy statement, and publication on the Company’s website. | X-10 | |||
C-290
| _ | _ | Deadline for publication of the meeting call notice on Italian newspaper. | X-10 | |||
| Availability at CTI’s premises of list of shareholders as at the record date. | X-10 | Availability at CTI’s premises of list of shareholders as at the record date. | X-10 | |||
| Ultimate deadline for shareholders to vote using their proxy card, over the internet or telephone.**** | X | Ultimate deadline for shareholders to vote using their proxy card, over the internet or telephone.**** | X | |||
| Shareholders’ meeting. | X | Shareholders’ meeting. | X | |||
| * | Please note that Italian Shareholders may request to be registered in the Company’s shareholders books at any time. For the purposes of being considered as a registered shareholder as at a given record date in connection with a given shareholders meeting, the Italian Shareholder shall ensure to instruct its Depository Banks for the purposes of the registration procedure and submit the relevant request in time prior to such record date. |
| ** | Article II, Section 6 of CTI’s bylaws provides that the record date is to be not more than seventy days and not less than ten days prior to the date of the shareholders meeting. Typically, the record date is approximately thirty days prior to the meeting, but the date can be set at any point between ten and seventy days prior to the meeting. |
| *** | If the action to be taken at the annual meeting of the shareholders includes a vote to amend the articles of incorporation or a vote on a plan of merger or share exchange, a proposed sale of assets other than in the regular course of business or the dissolution of the corporation, then pursuant to Article II, Section 5 of CTI’s bylaws, notice of the meeting shall be sent to the shareholders not more than 60 days and not less than 20 days prior to such meeting. |
| **** | In general, votes received prior to the closing of the polls at the shareholder meeting will be counted toward the final tally of votes, however, in some instances intermediaries who provide telephone and internet voting services to beneficial owners require votes to be submitted via those means prior to midnight on the day before the shareholder meeting. |
2. Special shareholders meeting
| U.S. Shareholders |
Italian Shareholders* | |||||
| Action |
Date |
Action |
Date | |||
| Request to DTC of list of intermediaries holding shares on DTC’s records | Record Date – 20 | Request to DTC of list of intermediaries holding shares on DTC’s records. | Record Date - 20 | |||
| _ | Publication of preliminary notice on Italian newspaper and on the Company’s website in Italian. | Record Date – 10 | ||||
| _ | - | Deadline for transfer of CTI shares to U.S. accounts, upon instructions of the shareholders to its Depository Bank, for the purposes of the Alternative Voting Procedure (allowing voting by phone or Internet). | Record Date -1 | |||
C-291
| Record date | Between X-70 to X-10** | Record Date | Between X-70 to X-10** | |||
| Deadline for filing with SEC, publication on the Company’s website and mailing to each shareholders of the meeting call notice and the proxy statement. | X-10 or X-20, depending on the purpose of the meeting | Deadline for making available to Borsa Italiana S.p.A. and to Italian intermediaries of meeting call notice and proxy statement and publication on the Company’s website. | X-10 or X-20, depending on the purpose of the meeting | |||
| _ | Publication of the meeting call notice on Italian newspaper | X-10 | ||||
| X-10 | Availability at CTI’s premises of list of shareholders as at the record date. | X-10 | ||||
| Ultimate deadline for shareholders to vote using their proxy card, over the internet or telephone.*** | X | Ultimate deadline for shareholders to vote using their proxy card, over the internet or telephone.*** | X | |||
| Shareholders’ meeting | X | Shareholders’ meeting | X | |||
| * | Please note that Italian Shareholders may request to be registered in the Company’s shareholders books at any time. For the purposes of being considered as a registered shareholder as at a given record date in connection with a given shareholders meeting, the Italian Shareholder shall ensure to instruct its Depository Banks for the purposes of the registration procedure and submit the relevant request in time prior to such record date. |
| ** | Article II, Section 6 of CTI’s bylaws provide that the record date is to be not more than seventy days and not less than ten days prior to the date of the shareholders meeting, provided, however, that if the action to be taken at the meeting includes a vote to amend the articles of incorporation or a vote on a plan of merger or share exchange, a proposed sale of assets other than in the regular course of business or the dissolution of the corporation, then pursuant to Article II, Section 5 of CTI’s bylaws, notice of the meeting shall be sent to the shareholders not more than 60 days and not less than 20 days prior to such meeting. |
| *** | In general, votes received prior to the closing of the polls at the shareholder meeting will be counted toward the final tally of votes, however, in some instances intermediaries who provide telephone and internet voting services to beneficial owners require votes to be submitted via those means prior to midnight on the day before the shareholder meeting. |
| C) | Difficulties have arisen over the time in connection with the Italian Shareholders’ exercise of voting rights and custody transfer to U.S. accounts. |
| C.1 | Issues |
For the purposes of the annual meeting quorum, CTI is subject to the corporate laws under which it was incorporated. In particular, under Washington state law, unless a corporation’s articles of incorporation provide otherwise an absolute majority (greater than 50 percent of the outstanding shares) of Company shareholders’ votes must be represented at an annual meeting before the Company can take action on
C-292
proxy items. At CTI’s Special Meeting in Lieu of Annual Meeting of the Shareholders on June 19, 2008, the Company’s shareholders approved a resolution to amend CTI’s articles of incorporation to decrease the quorum requirement to one-third of the outstanding shares entitled to vote at a meeting. Therefore, for future meetings, the Company will be able to take action on proxy items so long as one-third of the outstanding shares entitled to vote at the meeting are represented at that meeting. However, under the laws of the state of Washington, some actions, such as amendments to the articles of incorporation, require the affirmative vote of at least an absolute majority to pass. Therefore, even though quorum may be met, there may not be enough shares represented at the meeting to take action on certain agenda items if a majority of shares entitled to vote is not represented.
During the first two years after the admission to listing on the MTA (formerly MTAX and prior to that, Nuovo Mercato), the composition of the Company’s shareholdings remained fairly balanced between the NASDAQ and the Italian exchange, the U.S. component being however slightly prevalent over the Italian one. The annual shareholders’ meeting voting quorum at that time of an absolute majority (i.e., the minimum number of shareholders which are required to be present in person or by proxy at a meeting in order to validly take action at the meeting) was easily reached in both years because most of shareholders holding CTI’s shares in their accounts in the U.S. (the “U.S. Shareholders”) attended personally or provided voting proxies valid for the purposes of the quorum as it is customary in the U.S., while – with regard to shares held on accounts in Italy – three funds, holding substantial shareholdings in the Company, ensured an adequate representation of CTI’s common stock held on Italian accounts.
Between 2005 and 2006 the above three funds, however, substantially reduced their shareholdings in the Company so that, based on the information provided by Italian Depository Banks, as of today most of CTI’s shares held on Italian accounts result to be owned by retail investors.
At the beginning of 2006, following the survey made upon the instructions of the Company, as it is customary in the U.S., for the purposes of the annual shareholders’ meeting, it came to light that many CTI shares were held on Italian accounts managed by the Italian depository banks and opened with Monte Titoli; in the light of the particular quorum provided for by the Washington Business Corporation Act with respect to shareholders’ meetings having among the items in their agenda the adoption of certain resolutions, the above change in the Company’s shareholdings composition made the vote of the Italian Shareholders an essential condition for the purposes of validly adopting the above resolutions; these resolutions were among the items in the agenda of the impending 2006 annual shareholders’ meeting.
The Company is required to hold an annual meeting of its shareholders by the laws of the State of Washington, United States, where it is domiciled, as well as the listing requirements of NASDAQ where its shares are traded in the United States. The Company scheduled two annual meetings in 2006, one in June and one in November held at the Borsa Italiana, however, CTI was unable to reach a quorum to conduct business and thus no action could be taken. This is because the majority of CTI’s outstanding shares are held by the Italian Shareholders and only about 7% of these shares were actually voted.
Notwithstanding the small number of votes given by the Italian Shareholders, CTI could not contact directly such shareholders since – as anticipated above - the Company cannot have access to any information relating to its shareholders, unlike the U.S. For the same reason, the delivery of the documentation relating to each shareholders meeting is left completely with the Depository Banks which generally believe that they are not under any obligation to transmit such documentation unless so
C-293
provided by the terms and conditions governing their relationship with their clients (while in the U.S. brokerages are under the obligation to transmit voting materials to shareholders).
| C.2 | Description of the custody transfer to U.S. accounts carried out in connection with the Special Meeting held in April 2007 |
On April 10, 2007, following publication of the meeting call notice on an Italian newspaper on March 2, 2007 and the setting of a record date on March 12, 2007, the Company was eventually able to hold a special meeting of its shareholders for the purposes of increasing its authorized common stock with the assistance of certain Italian Depository Banks which agreed - subject to the relevant shareholder not taking any action - to make book entry transfer of their share positions, held in the name and on behalf of their clients, to their U.S. correspondent banks, which on their turn transferred the shares to an account at its U.S. affiliate broker-dealer (each correspondent bank may have a U.S. affiliate broker-dealer different from those of other correspondent banks) on the record date set for that meeting. This permitted such broker-dealers under the securities laws of the United States and the rules of the New York Stock Exchange to consider these shares for the quorum and to vote them at the Special Meeting, to the extent that the Italian Shareholders did not instruct their brokers, to vote the shares pursuant to the modalities provided in the proxy statement (as described under Section B above) or did not instruct the U.S. affiliate broker-dealer, through their brokers, to abstain from taking any action in relation to the shares, including the attendance to the meeting and the exercise of voting rights.
Please note that, following the custody transfer with the U.S. affiliate broker-dealer on the record date, Italian Shareholders have maintained the right to vote at the meeting with the modalities described under Section B above (except for the Internet/Telephone Voting Procedure, as better specified below) or to provide instruction to U.S. affiliate broker-dealer, through their Depository Banks to abstain from taking any action in relation to the shares, including the exercise of voting rights on their behalf. It has to be observed that after the record date, the Italian shareholders having their shares at such date deposited in an account opened in the Italian bank’s name at an Affiliate Broker-Dealer, pursuant to the procedure just described, would have not been entitled to vote by internet/telephone, since such possibility was recognized only to shareholders having their shares, at the record date, deposited in a bank account opened in their own name at the same or other Affiliate Broker-Dealer (please refer to what has already been reported in the section “Internet and telephone voting”, letter B), of this Paragraph 21.1.5, where reference is made to a “separate customer account in the name and for the account of the Italian Shareholder).Under such circumstance, the Company gave in the second half of February 2007 to the Depository Banks that accepted to participate to the aforesaid transfer (a) an operational memorandum including the necessary instructions to carry out the above mentioned custody transfer and (b) a letter from the CEO of the Company to its shareholders for the purposes of (i) mainly inviting the shareholders to vote participating in person to the shareholders’ meeting or by the delivery of the proxy card and (ii) describing the timetable and the consequences resulting from the custody transfer to the Affiliate Broker-Dealerin case of failure to take any action on the part of the relevant shareholder. The Company undertook to reimburse the Depository Banks and certain Affiliate Brokers Dealer of any cost borne by the same for the purposes of the delivery of the abovementioned letter to the shareholders.The special shareholders’ meeting was held on April 10, 2007 and approved the amendment of the Company’s Articles of Incorporation in order to increase the number of authorised shares from 210,000,000 to 410,000,000 (with respect to just the shares of common stock from 200,000,000m to 400,000,000). After the reverse stock split on April 15, 2007, the corporate capital resulted in 100,000,000 authorised shares, of which 100,000,000 were shares of common stock.
C-294
| C.3 | Description of the custody transfer to U.S. accounts carried out in connection with the Annual Meeting held in September 2007 |
The Company convened the Annual Shareholders Meeting at the offices of Borsa Italiana on September 25, 2007 with a record date set on July 31, 2007 and publication of the meeting call notice on an Italian newspaper on July 19, 2007. The Company used the same process adopted at the special meeting held in April (see Section C.2. above) for the proposals in connection with which was possible to apply the custody transfer.
In light of the above, the Company contacted all the Depository Banks which were registered with Monte Titoli as holding CTI shares on the basis of a list provided by Monte Titoli for the purposes of obtaining, on an individual negotiation basis, their necessary collaboration. Only part of the contacted Depository Banks agreed to the custody transfer to the U.S. accounts.
The Company gave in the second half of June 2007 to the Depository Banks which agreed to the custody transfer (i) an operational memorandum including the necessary instructions to carry out the above mentioned custody transfer and (ii) a letter from the CEO of the Company to CTI’s shareholders. The letter of the CEO, James Bianco, mainly encouraged the shareholders to attend in person the meeting or to vote by sending a proxy card. Such letter also summarized the custody transfer procedure, describing the timetable and the consequences resulting from the custody transfer in case of failure to take any action on the part of the relevant shareholder (stating that, if the shareholder consented to the custody transfer and did not provide voting instructions, the U.S. broker/dealer could exercise its discretionary voting authority in connection with certain subject matters in the agenda). Most of the Banks confirmed that they had sent the letter mentioned above to their clients/shareholders prior to the record date. The letter did not include a description of the access modalities to the meeting documentation as, at the time of the delivery of the letter to the Depository Banks, the relevant proxy statement was not in a definitive form, had not been filed with the SEC and therefore was not accessible. The Company undertook again to reimburse the Depository Banks and certain Affiliate Broker Dealers of any cost borne by the same for the purposes of the delivery of the abovementioned letter to the shareholders.
On the basis of the procedure described in the memorandum above, each Monte Titoli participating Depository Bank which agreed to the custody transfer made a book entry transfer of that bank’s customer holdings to the Cede participant account of their U.S. correspondent Bank. Instructions were also given by the Italian Depository Bank to their U.S. correspondent Bank to immediately transfer the same shares to a separate customer account for the Italian Bank, to be maintained and held in custody by the broker-dealer affiliate through the close of business on the record date. In accordance with NYSE Rule 452, in the absence of the receipt of separate voting instructions from the Italian customers of such Depository Bank or the Italian Depository Bank (according to the contractual arrangements with the client), the Italian Bank’s entire account position, as of record date, was voted by Broadridge, on matters for which brokers had discretionary authority, as proxy agent for the U.S. broker-dealer affiliate. The broker-dealer affiliates had legal discretion to vote as they chose, although in most cases they followed the recommendations of the Board of Directors.
Therefore, in accordance with NYSE Rule 452 (which also applies to NASDAQ-listed companies), with regard to those Italian Shareholders whose Depository Banks agreed to the custody transfer, after having received the relevant documentation mentioned above (i.e., the operational memorandum and the letter
C-295
from the CEO of CTI), (i) if they did not instruct their brokers to vote the shares pursuant to the modalities provided in the proxy statement (as described under Section B above) or (ii) did not instruct the U.S. affiliate broker-dealer, through their brokers, to abstain from taking any action in relation to the shares, including the attendance to the meeting or the exercise of voting rights, the relevant shares were taken into account for the purposes of calculating the achievement of the quorum and the affiliate broker-dealers were permitted to vote these shares at the Annual Meeting for the proposals in connection with which it was possible to apply the custody transfer on the basis of the discretionary authority provided to them by U.S. law. Please note that Italian Shareholders consented to the custody transfer procedure by a mechanism of “silent-consent”.
Please note that, following the custody transfer with the U.S. affiliate broker-dealer on the record date, Italian Shareholders maintained the right to vote at the meeting with the voting modalities described under Section B above (i.e. in person, by proxy or by means of the proxy card) or to provide instruction to the U.S. affiliate broker-dealer, through their Depository Banks, to abstain from taking any action in relation to the shares, including the attendance to the meeting or the exercise of voting rights. Please note that if a shareholder provided instructions to abstain from voting, those shares still counted towards the establishment of a quorum.
Once the custody transfer had been activated, the U.S. affiliate broker was allowed to receive specific voting instructions to be voted at the meeting by a Depository Bank (acting on behalf of a client, upon his/her specific voting instructions). Such voting instructions can be provided at any time up until the time of meeting even if initial instructions have already been provided.
The transfer procedure mentioned above does not imply any additional charge/burden on the part of, nor does it limit the right of disposal of the shares by the Italian shareholders whose Depository Bank agreed to such transfer.
The following table summarizes the activities that Italian shareholders may carry out in connection with the custody transfer procedure and the indicative deadlines:
| Activity |
Deadline | |
| Instructions to the relevant Depository Bank not to carry out the custody transfer | Prior to the record date as indicated by Depository Banks on a case-by-case basis | |
| Provide the relevant Affiliate Broker-Dealer (through the Depository Bank if that is the case) with instructions to abstain from taking any initiative (including voting) | On or prior to the Shareholders’ Meeting as indicated by Depository Banks on a case-by-case basis | |
| Providing voting instructions (also through the Depository Bank * | Shareholders’ Meeting | |
| * | Note that whether or not the Italian shareholder has asked the Depository Bank to hold its shares out of the custody transfer, the Italian shareholder still retains the right to vote his or her own shares by providing instructions to the Company regarding voting (or abstaining from voting). In the event the Company receives directions on voting shares directly from an Italian shareholder, the inspector of elections must determine whether or not such shares have already been included in the calculations regarding shares voted by the U.S. Affiliate Broker/Dealer on behalf of the bank through which such Italian shareholder holds his or her shares and, if such shares have been voted by such U.S. Affiliate |
C-296
| Broker/Dealer, to remove the relevant number of votes from those cast by the U.S. Affiliate Broker/Dealer on behalf of the Italian bank. |
Conditions to the exercise of discretionary authority under NYSE Rule 452
NYSE Rule 452 describes how U.S. brokers are permitted to vote on matters when given proxies. Brokers are required to vote as instructed on proxies, and if no instructions are provided may exercise discretion in voting, except in cases where they are prohibited from exercising discretionary authority. Pursuant to NYSE Rule 452, generally speaking, brokers may not exercise discretionary authority if a matter: (i)is not submitted to stockholders by means of a proxy statement comparable to that specified in Schedule 14-A of the Securities and Exchange Commission; (ii) is the subject of a counter-solicitation, or is part of a proposal made by a stockholder which is being opposed by management (i.e., a contest); (iii) relates to a merger or consolidation (except when the company’s proposal is to merge with its own wholly owned subsidiary, provided its shareholders dissenting thereto do not have rights of appraisal); (iv) involves right of appraisal; (v) authorizes mortgaging of property; (vi) authorizes or creates indebtedness or increases the authorized amount of indebtedness; (vii) authorizes or creates a preferred stock or increases the authorized amount of an existing preferred stock; (viii) alters the terms or conditions of existing stock or indebtedness; (ix) involves waiver or modification of pre-emptive rights; (x) changes existing quorum requirements with respect to stockholder meetings; (xi) alters voting provisions or the proportionate voting power of a stock, or the number of its votes per share; (xii) authorizes the implementation of any equity compensation plan, or any material revision to the terms of any existing equity compensation plan; (xiii) authorizes a new profit-sharing or special remuneration plan, or a new retirement plan, the annual cost of which will amount to more than 10% of average annual income before taxes for the preceding five years, or the amendment of an existing plan which would bring its cost above 10% of such average annual income before taxes; (xiv) changes the purposes or powers of a company to an extent which would permit it to change to a materially different line of business and it is the company’s stated intention to make such a change; (xv) authorizes the acquisition of property, assets, or a company, where the consideration to be given has a fair value approximating 20% or more of the market value of the previously outstanding shares; (xvi) authorizes the sale or other disposition of assets or earning power approximating 20% or more of those existing prior to the transaction; (xvii) authorizes a transaction not in the ordinary course of business in which an officer, director or substantial security holder has a direct or indirect interest; or (ix) reduces earned surplus by 51% or more, or reduces earned surplus to an amount less than the aggregate of three years’ common stock dividends computed at the current dividend rate.
The requirement under the first bullet is satisfied by including the item (on which the discretionary authority is to be exercised) in a proxy statement that meets the SEC’s requirements regarding the content of such proxy statement.
As mentioned above the U.S. broker-dealers are permitted to vote on certain matters on a discretionary basis pursuant to Rule 452, which means that the broker-dealers may vote as they choose on such matters and they are not required to vote in any particular way. The pre-requisite of such authority is that the U.S. broker-dealers hold in custody securities for the account of their clients in the context of the provision of broker-dealing services. On July 1, 2009, NYSE Rule 452 was amended to eliminate broker discretionary voting in director elections. Pursuant to this amendment, beginning with CTI’s shareholder meetings held on or after January 1, 2010, a broker will no longer be permitted to vote a customer’s shares on the uncontested election of directors (i.e., an election where the only nominees are
C-297
those recommended by the Board of Directors, as opposed to a “contested election” where some nominees are proposed by shareholders and are not recommended by the Board of Directors) at a meeting of shareholders except in accordance with voting instructions received from the customer. This amendment expands the current NYSE Rule 452 to prohibit brokers from voting uninstructed customer shares in all director elections whether contested or uncontested (previously such prohibition applied only to contested election of directors).
In exercising their discretionary authority, U.S. broker-dealers will generally vote as recommended by the board of directors. Broker-dealers are, however, completely free to vote as they choose and do not have any liability vis-à-vis their clients and how they decide to vote.
Broker-dealers in the United States are not compensated or reimbursed by their clients or any other parties for voting on behalf of their clients pursuant to Rule 452. They are compensated from their clients for their services as broker-dealers only.
| C.4 | Description of the custody transfer to U.S. accounts carried out in connection with the Special Meeting held in January 2008 |
The Company convened a further Special Shareholders Meeting on January 28, 2008 with a record date set on December 12, 2007 and publication of a meeting call notice on an Italian newspaper on December 21, 2007.
The Company used the same process adopted at the special meeting held in 2007 (see Section C.2. and C.3. above) for the proposals in connection with which was possible to apply the custody transfer (except that it implemented all the improvements described under C.9 below).
| C.5 | Description of the custody transfer to U.S. accounts carried out in connection with the Special Meeting held in June 2008 |
The Company convened a further Special Shareholders Meeting in lieu of its Annual Meeting on June 19, 2008 with a record date set on May 14, 2008 and publication of a meeting call notice on an Italian newspaper on May 19 2008.
The Company used the same process adopted at the meetings held in 2007 and 2008 (see Section C.2., C.3. and C.4. above) for the proposals in connection with which was possible to apply the custody transfer (except that it implemented all the improvements described under C.9 below).
At the shareholders meeting held on June 19, 2008, the shareholders approved, among other things, an amendment to the Company’s amended and restated articles of incorporation to reduce quorum requirements for future shareholder meetings to one-third of the outstanding shares entitled to vote at the meeting.
| C.6 | Description of the custody transfer to U.S. accounts carried out in connection with the Special Meeting in March 2009 |
The Company convened another Special Shareholders Meeting on March 24, 2009 with a record date set on February 4, 2009 and publication of a meeting call notice on an Italian newspaper on February 27, 2009. The purposes of the special meeting included, among other things, approval of amendments to the
C-298
Company’s articles of incorporation in order to increase the authorized shares of the Company and effect a reverse stock split and approval of amendments to the Company’s equity incentive plan and employee stock purchase plan. The special meeting was held in accordance with the provisions of the Company’s amended and restated bylaws, the SEC requirements and the policies and procedures described in Paragraph A above. Paragraph A above also described the differences in policies and procedures that apply to annual shareholders’ meeting and extraordinary shareholders’ meeting (i.e., special meetings). The Company used the same process adopted at the special meetings held in 2007 and 2008 (see Section C.2., C.3., C.4. and C.5. above) for the proposals in connection with which was possible to apply the custody transfer (except that it implemented all the improvements described under C.9 below).
At the shareholders meeting held on March 24, 2009, the shareholders approved, among other things, an amendment to the Company’s amended and restated articles of incorporation to authorize an additional number of shares but did not approve a proposal to authorize a reverse stock split in the discretion of the board of directors. Although the required quorum is one-third of the shares entitled to vote being present or represented at the meeting, under Washington law, both of these proposals required approval by a majority of the shares outstanding and entitled to vote.
| C.7 | Description of the custody transfer to U.S. accounts carried out in connection with the Annual Meeting in October 2009 |
The Company convened an Annual Shareholders Meeting on October 20, 2009 with a record date set on February 4, 2009 and publication of a meeting call notice on an Italian newspaper on August 28, 2009.
The Company used the same process adopted at the meetings held in 2007, 2008 and 2009 (see Section C.2., C.3., C.4., C.5. and C.6. above) for the proposals in connection with which was possible to apply the custody transfer.
At the shareholders meeting held on October 20, 2009, the shareholders (i) elected Mr. Richard L. Love, Dr. Mary O. Mundinger, and Dr. Jack W. Singer to serve on the Company’s Board of Directors until the 2012 Annual Meeting; (ii) approved the proposals to increase the number of shares available for issuance under its 2007 Equity Incentive Plan, increase the number of shares available for issuance under its 2007 Employee Purchase Plan and issue shares of common stock in lieu of future milestone payments related to the Company’s drug candidate brostallicin; and (iii) Shareholders ratified the selection of Stonefield Josephson, Inc. as the Company’s independent auditors for the year ending December 31, 2009.
| C.8 | Description of the custody transfer to U.S. accounts carried out in connection with the Annual Meeting in September 2010 |
The Company convened an Annual Shareholders Meeting on September 16, 2010 with a record date set on July 27, 2010 and publication of a meeting call notice on an Italian newspaper on August 26, 2010.
The Company used the same process adopted at the meetings held in 2007, 2008 and 2009 (see Section C.2., C.3., C.4., C.5., C.6. e C.7. above) for the proposals in connection with which was possible to apply the custody transfer. Because the vast majority of the Company’s shares are held by Italian Shareholders, the Company expects that it will still need to implement the custody transfer process described in this section for future meetings of the Company’s shareholders in order to obtain quorum for this and other meetings.
C-299
At the shareholders meeting held on September 16, 2010, the shareholders approved (i) the election of John H. Bauer and Phillip M. Nudelman, MD as Class I directors to the Company’s Board of Directors, each to serve until the 2013 Annual Meeting; (ii) an amendment to the Company’s amended and restated articles of incorporation to increase the total number of authorized shares from 810,000,000 to 1,210,000,000 and to increase the total number of authorized shares of common stock from 800,000,000 to 1,200,000,000; (iii) an amendment to the Company’s 2007 Equity Incentive Plan, as amended (the “2007 Equity Plan”), to increase the number of shares available for issuance under the 2007 Equity Plan by 40,000,000 shares; and (iv) the ratification of the selection of Stonefield Josephson, Inc. as the Company’s independent auditors for the year ending December 31, 2010.
| C.9 | Outlook and further undertakings by the Company |
The Company currently expects that action to be taken at a subsequent special meeting or annual meeting would be substantially similar to the procedures set forth above so long as the vast majority of the Company’s shares remain held by Italian shareholders, subject to the cooperation of the banks holding CTI shares vis-à-vis Monte Titoli. However, the Company is also currently exploring alternative solutions to further facilitate the participation to shareholders’ meeting (including the exercise of voting rights) by Italian Shareholders and the reaching of quorums to validly conduct business at its shareholders’ meetings. For the time being and failing the finding of any alternative solution, the Company does not exclude to use the same process adopted in connection with the meetings held in 2007, 2008, 2009 and 2010 subject to the cooperation of the banks holding CTI shares vis-à-vis Monte Titoli. With respect to the custody transfer (the “Custody Transfer Procedure”), the Company has recently reviewed – as requested by CONSOB- the modalities thereof for the purposes of providing even more information to Italian shareholders and facilitate their participation and voting in the context of shareholders’ meeting. In particular, in connection with the last two shareholders’ meetings, the Company:
1) has amended the CEO’s letter to shareholders (which is delivered to the Depository Banks at least 20 days prior to the record date and distributed by the Depository Bank to their clients at least 10 days prior to the record date) so that this informs Italian shareholders in advance of the meeting:
- of how to have access to the proxy materials which will be available at least 20 or 10 days prior to the shareholders meeting, according to the meeting (i.e., by accessing the SEC or the Company’s website, by requesting their Depository Bank, by requesting the Company’s Italian offices directly or by requesting Borsa Italiana);
- of how to exercise their voting rights, including how to resort to the Internet/Telephone Voting procedure (for the purposes of voting by phone or by Internet);
- that the Company will request the Depository Banks to carry out the custody transfer for the purposes of reaching the quorum;
- of how to prevent their Banks from carrying out the custody transfer and of how to prevent the U.S. broker-dealer from exercising its discretionary authority once the custody transfer has been carried out;
- of how to have their shares registered in the Company’s shareholders book;
- that the U.S. broker-dealers are permitted to vote on certain matters on a discretionary basis pursuant to Rule 452; and
C-300
- that the transfer procedure does not imply any additional charge/burden on the part of the Italian shareholders whose Depository Bank agreed to such transfer.
2) has amended the operational memorandum so that this:
- expressly requests the Depository Banks to provide the Company CEO’s letter to their clients within at least 10 days prior to the record date and to warn the latter that, failing any explicit prohibition to the custody transfer, their Banks will proceed with transferring their shares on the U.S. accounts;
- expressly refers to this Registration Document and to the sections hereof which describe, in particular, the custody transfer, the Internet/Telephone Voting Procedure and the modalities for registration of the shares with the Company’s shareholders book;
- expressly requests the Depository Banks to make the proxy materials available to their clients upon request by the latter.
3) has requested the Depository Banks to provide their clients after the record date with a second notice informing them of where they can have access to the proxy materials (meeting call notice and proxy statements), including at the Depository Banks themselves.
The Company will continue to explore that possibility of requesting the Depository Banks to enter into framework agreements for the purposes of governing the technical modalities and the terms and conditions of any future custody transfer the Company may decide to use.
* * * * *
With respect to the registration of Italian shareholders in the Company’s shareholders book as well as U.S. record holders, the Company will contract with a third party, such as Broadridge or the Company’s transfer agent, for all future special and annual meetings so that shareholders registered in the Company shareholders book held by the transfer agent will be permitted to avail themselves of the voting modalities over the internet and over the phone.
| 21.2.6 | A brief description of any provision of the issuer’s articles of association, statutes, charter or bylaws that would have an effect of delaying, deferring or preventing a change in the control of the issuer |
Washington law contains certain provisions that may have the effect of delaying, deterring or preventing a change in control of the company. Chapter 23B.17 of the Washington Business Corporation Act (the WBCA) prohibits, subject to certain exceptions, a merger, sale of assets or liquidation of the company involving an “interested shareholder” (defined as a person or group of affiliated persons who own beneficially 20% or more of the company’s voting securities) unless the transaction is determined to be at a “fair price” or otherwise approved by a majority of the company’s disinterested directors or is approved by holders of two–thirds of the company’s outstanding voting securities, other than those held by the interested shareholder. A Washington corporation may, in its articles of incorporation, exempt itself from coverage of this provision, but the company has not done so. In addition, Chapter 23B.19 of the WBCA prohibits the company, with certain exceptions, from engaging in certain significant business transactions with an “acquiring person” (defined as a person or group of persons who acquire 10% or more of the company’s voting securities without the prior approval of the company’s board of directors)
C-301
for a period of five years following the acquiring person’s share acquisition date. The prohibited transactions include, among others, a merger or consolidation with, disposition of assets to, or issuance or redemption of stock to or from, the acquiring person, or otherwise allowing the acquiring person to receive any disproportionate benefit as a shareholder. The company may not exempt itself from coverage of this statute. These statutory provisions may have the effect of delaying, deterring or preventing a change in control of the company.
CTI’s board of directors is divided into three approximately equal classes of directors serving staggered three-year terms. In addition, CTI’s Amended and Restated Articles of Incorporation currently in force provide that directors may be removed from office only at a meeting of shareholders called expressly for that purpose and only for cause. CTI’s Amended and Restated Articles of Incorporation currently in force limit “cause” to willful misfeasance having a material adverse effect on the company or conviction of a felony, provided that any action by a director shall not constitute “cause” if, in good faith, the director believed the action to be in or not opposed to the best interests of the company or if the director is entitled to be indemnified with respect to such action under applicable law, CTI’s Amended and Restated Articles of Incorporation currently in force or Amended and Restated Bylaws currently in force, or a contract with the company. Further, CTI’s Amended and Restated Bylaws currently in force require a shareholder to provide notice to the company of such shareholder’s intent to nominate a person or persons for election as directors not later than 90 days prior to the first anniversary of the previous year’s annual meeting of shareholders or, in the case of an election to be held at a special meeting of shareholders for the election of directors, the close of business on the tenth day following the date on which notice of such meeting is first given to shareholders. A shareholder must also provide CTI with notice of such shareholder’s intent to make any proposal at an annual meeting of shareholders not later than 90 days prior to the first anniversary of the previous year’s annual meeting of shareholders. These provisions may have the effect of deterring hostile takeovers or delaying change in control or management of the Company.
It is lastly noted that on December 24, 2009, CTI’s Board of Directors approved and adopted a Shareholder Rights Plan (the “Rights Plan”) in which one preferred stock purchase right will be distributed for each common share held as of the close of business on January 7, 2010. Initially, the rights are not exercisable, and are attached to and trade with, all of the shares of CTI’s common stock outstanding as of, and issued subsequent to, the record date. The Rights Plan is designed to deter coercive takeover tactics, and to prevent an acquirer from gaining control of CTI without offering a fair price to all of CTI’s shareholders. The Rights Plan will not prevent a takeover, but should encourage anyone seeking to acquire CTI to negotiate with the Board of Directors prior to attempting a takeover. Each right, if and when it becomes exercisable, will entitle the holder to purchase one tenthousandth of a share of a new series of junior participating cumulative preferred stock for $6.00, subject to standard adjustment in the Rights Plan. The rights will become exercisable for CTI preferred stock if a person or group acquires 20% or more of CTI’s common stock. Upon acquisition of 20% or more of CTI’s common stock, the Board of Directors could decide that each right (except those held by a 20% shareholder, which become null and void) would become exercisable entitling the holder to receive upon exercise, in lieu of a number of units of preferred stock, that number of shares of CTI common stock having a market value of two times the exercise price of the right, in effect doubling the value of the right to the shareholder. In certain circumstances, including if there are insufficient shares of CTI’s common stock to permit the exercise in full of the rights, the holder may receive units of preferred stock, other securities, cash or property, or any combination of the foregoing. If CTI is acquired in a merger or other business combination transaction
C-302
after any such event, each holder of a right, except those held by a 20% shareholder, which become null and void, would then have the right to receive, upon exercise, common stock of the acquiring company having a market value equal to two times the exercise price of the right. CTI’s Board of Directors may redeem the rights for $0.0001 per right or terminate the Rights Plan at any time prior to an acquisition by a person or group holding 20% or more of CTI’s common stock. The Rights Plan will expire on January 7, 2013.
| 21.2.7 | An indication of the articles of association, statutes, charter or bylaw provisions, if any, governing the ownership threshold above which shareholder ownership must be disclosed |
Directors and officers of the Company and any 10% or greater beneficial owner of shares of Company stock are required to file reports with the U.S. Securities and Exchange Commission to reflect any change in ownership of such shares.
| 21.2.8 | A description of the conditions imposed by the memorandum and articles of association statutes, charter or bylaw governing changes in the capital, where such conditions are more stringent than is required by law |
An amendment to the articles of incorporation of CTI, including an amendment of its authorized share capital, requires the approval of CTI shareholders by not less than a majority of outstanding shares entitled to vote on the proposal as of the relevant record date, which would be obtained at an annual or special meeting. CTI shall disclose the recommendation of the board regarding shareholder proposals in an English version of the proxy statement at least 10 days before the shareholders’ meeting. The proxy statement will be filed with the SEC and available to the public also on the SEC’s website over the Internet. The Company will file with its registered offices in Italy and with Borsa Italiana the proxy statement, which shall include the new articles of incorporation to be approved by the shareholders.
C-303
CHAPTER 22 MATERIAL CONTRACTS
| 22.1 | Relevant contracts other than those entered into in the normal course of business |
| 22.1.1 | Collaboration agreements |
| 22.1.1.1 | Co-Development Agreement with Novartis |
In September 2006, CTI entered into an exclusive worldwide licensing agreement with Novartis International Pharmaceutical Ltd., or Novartis, for the development and commercialization of OPAXIO. This agreement granted Novartis the right, in its sole discretion, to exercise certain rights with respect to OPAXIO and the option, in its sole discretion, to exercise certain rights with respect to pixantrone, each as further described below.
Under this agreement, Novartis has the right to elect to participate and control the future development and commercialization of OPAXIO (the “OPAXIO Election”) at any time until 30 days after the approval of OPAXIO in the U.S. or Europe which satisfies certain conditions. If Novartis makes the OPAXIO Election, then it would be obligated to pay 100% of development and commercialization costs incurred after the election; provided that CTI would reimburse Novartis for 20% of development costs associated with label expansion or regulatory required post-approval trials. Additionally, if Novartis makes the OPAXIO Election, then subject to certain conditions, CTI would (i) have the right to devote up to 35 full-time employees to OPAXIO commercialization efforts, (ii) receive a royalty on OPAXIO worldwide net sales, and (iii) be eligible to receive up to $270 million of milestone payments if all milestones are achieved. Milestone payments are based on a mix of targets regarding sales levels (dollar thresholds) and regulatory approvals for various indications.
The table of the milestones targets in connection with the OPAXIO Election is set forth under Section 8.2 (a) of the Novartis Co-Development Agreement (which is available to the public as indicated below) and reported below (please refer to the Agreement for the definitions used in the table):
MILESTONE
Product Approvals in the U.S.:
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in the U.S. for First Line treatment of NSCLC or Second Line treatment of NSCLC |
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in the U.S. for the treatment of Ovarian cancer |
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of the first Product Approval for the Product with an Acceptable Label in the U.S. for the treatment of any one of the following cancers: Breast, Prostate, Head and Neck or Radiosensitization |
Product Approvals in Europe:
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in Europe for First Line treatment of NSCLC or Second Line treatment of NSCLC |
C-304
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in Europe for the treatment of Ovarian cancer |
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of the first Product Approval for the Product with an Acceptable Label in Europe for the treatment of any one of the following cancers: Breast, Prostate, Head and Neck or Radiosensitization |
Product Approvals in Japan:
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in Japan for First Line treatment of NSCLC or Second Line treatment of NSCLC |
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of Product Approval for the Product with an Acceptable Label in Japan for the treatment of Ovarian cancer |
| • | Receipt by Novartis or receipt by Licensor and transfer to Novartis of the first Product Approval for the Product with an Acceptable Label in Japan for the treatment of any one of the following cancers: Breast, Prostate, Head and Neck or Radiosensitization |
Sales Milestones:
| • | The first achievement of Net Sales of Product in the Territory in any Calendar Year of U.S.$1 billion |
| • | The first achievement of Net Sales of Product in the Territory in any Calendar Year of U.S.$1.5 billion |
| • | The first achievement of Net Sales of Product in the Territory in any Calendar Year of U.S.$2 billion |
In addition, Novartis has an option to enter into an exclusive worldwide license to develop and commercialize pixantrone (the “Pixantrone Option”) at any time until the later to occur of (i) three hundred sixty-five (365) days after the database on CTI’s ongoing phase III study of pixantrone is locked or (ii) thirty (30) days after the expiration of the OPAXIO Participation Period. If Novartis exercises its Pixantrone Option, then CTI and Novartis are obligated to use commercially reasonable efforts to negotiate in good faith the details of a definitive agreement, based on certain agreed terms. These agreed financial terms vary depending on whether Novartis has also made the OPAXIO Election. In general, if Novartis has decided not to make the OPAXIO Election, then CTI would be eligible to receive (i) a customary royalty on pixantrone worldwide sales and (ii) up to $71 million of milestone payments (if all milestones were achieved). If Novartis decides to exercise its Pixantrone Option and at such time has exercised, or has not given up, its rights with respect to OPAXIO, then CTI would be eligible to receive (i) up to $104 million of milestone payments if all milestones were achieved, (ii) a $7.5 million license fee, (iii) a customary royalty on pixantrone worldwide net sales and (iv) expense reimbursement and future expense sharing on terms similar to those related to the OPAXIO Election. Milestone payments under the Pixantrone Option are also based on a mix of targets regarding sales levels and regulatory approvals.
The additional milestone payments that will be due to CTI on election of the Pixantrone Option if Novartis has decided not to make the OPAXIO Election range from $3 million to $18 million based on regulatory approvals in each of the U.S. and Europe for two different designated therapeutics areas as well as additional milestones ranging from $10 million to $25 million based on the achievement of two specific net sales thresholds in the U.S. The additional milestone payments that will be due to CTI on election of the Pixantrone Option if Novartis has made the OPAXIO Election (or has not given up its rights with respect to OPAXIO) range from $3 million to $18 million based on regulatory approvals in each of the U.S. and Europe for five different designated therapeutics areas as well as additional milestones ranging from $5 million to $20 million based on the achievement of three specific net sales thresholds in the U.S.
C-305
As of the date of this Registration Document no election or exercise of any option under the Novartis Co-Development Agreement has yet occurred, and therefore no payments have yet been made under the agreement. As of September 30, 2010, the Company has not received any milestone payments and the Company will not receive any milestone payments unless Novartis elects to participate in the development and commercialization of pixantrone or OPAXIO.
The Novartis Co-Development Agreement is available (save for the omitted confidential information as agreed with the SEC) on Form 8-K filed with the SEC on September 18, 2007, on the Company’s website www.celltherapeutics.com and on the SEC’s website www.sec.gov.
| 22.1.1.2 | Supply Agreement with Biogen |
On December 21, 2007, in connection with the closing of CTI’s purchase of the Zevalin, CTI and Biogen entered into a Supply Agreement (“Supply Agreement”), pursuant to which CTI agreed to purchase from Biogen, and Biogen agreed to provide to CTI, kits to make single doses (a “Kit”), as part of one treatment to a patient, of either (i) Indium-111 Ibritumomab Tiuxetan (In-111 Zevalin) or (ii) Yttrium-90 Ibritumomab Tiuxetan (Y-90 Zevalin) either as single Kits or in packages containing one dose of each of In-111 Zevalin and Y-90 Zevalin (a “Package”), each for sale to end-users in the United States at a “cost plus” manufacturing price. From the effective date of the Supply Agreement through June 9, 2014 (unless earlier terminated), CTI agreed to purchase such Kits and/or Packages solely from Biogen unless and until the parties agreed to the establishment of a replacement manufacturing source in accordance with the terms and conditions of the Supply Agreement. Each party has agreed to indemnify the other party from and against certain third-party claims related to the manufacture, sale, distribution or use of the goods, as the case may be. A copy of the Supply Agreement is attached as an exhibit to CTI’s Current Report on Form 8-K filed with the SEC on December 31, 2007. The Supply Agreement was assumed by RIT Oncology in connection with the transfer of Zevalin assets to RIT Oncology in December 2008. In March 2009, CTI sold its interest in RIT Oncology to Spectrum, thereby transferring its remaining interest in Zevalin. In the event CTI were to be required to pay any obligations related to Zevalin under this Agreement, for instance in the event RIT Oncology defaults on those obligations, Spectrum would be required to reimburse CTI for those obligations.
| 22.1.1.3 | MDS Nordion Agreement |
Also in December 2007, in connection with our acquisition of Zevalin, CTI assumed from Biogen a manufacturing and supply agreement with MDS (Canada) Inc., MDS Nordion Division, or MDS (Canada), pursuant to which MDS (Canada) supplies CTI with yttrium-90, a radioisotope used in connection with the administration of Zevalin. Under the terms of the agreement, CTI was required to purchase, and MDS (Canada) is required to manufacture and supply, all of CTI’s yttrium-90 requirements for commercial uses of Zevalin. The agreement expires under its current terms in February 2010 and may be terminated by MDS (Canada) at any time without cause on 24 months written notice or by CTI at any time without cause on 6 months written notice. The Supply Agreement with MDS (Canada) was assumed by RIT Oncology in connection with the transfer of Zevalin assets to RIT Oncology in December 2008. In March 2009, CTI sold its interest in RIT Oncology to Spectrum, thereby transferring its remaining interest in Zevalin
C-306
| 22.1.1.4 | Joint Venture with Spectrum Pharmaceuticals, Inc. and subsequent Agreements Related to Sale of Interest in RIT Oncology |
In November 2008, CTI entered into an agreement with Spectrum to form a 50/50 owned joint venture, RIT Oncology LLC, to commercialize and develop Zevalin in the United States. Upon closing the transaction in December 2008, assets related to Zevalin were transferred to RIT Oncology and under the terms of the agreement, Spectrum paid CTI an initial payment of $7.5 million at the closing and an additional $7.5 million on January 5, 2009. At the closing, CTI also made an initial capital contribution of $1.8 million. Under the terms of the initial agreement governing the joint venture, Spectrum would be responsible for 50% of expenses related to the development and commercialization of Zevalin and CTI and Spectrum would share equally in net profits from the sales of Zevalin in the United States. In February 2009, CTI exercised a put option granted to the Company in the initial agreement governing the joint venture pursuant to which Spectrum purchased all of CTI’s interest in RIT Oncology for approximately $16.5 million, subject to certain adjustments based on among other things payables owing between CTI and RIT Oncology. The transaction closed in March 2009, thereby effectively transferring all of CTI’s remaining interest in RIT Oncology.
On March 15, 2009, CTI completed the sale of its 50% membership interest in RIT Oncology to Spectrum and fully divested its ownership of Zevalin in exchange for approximately $16.5 million, pursuant to the terms and conditions of a Limited Liability Company Interest Assignment Agreement dated March 15, 2009 between CTI and Spectrum.
In consideration for the interest, on March 2, 2009, CTI received a cash payment of $6.5 million (less the amount of a consent fee paid to Biogen Idec Inc.), and following the closing, on March 16, 2009, Spectrum funded into escrow $10 million. On April 3, 2009, $6.5 million was released to the Company from this escrow account and the final installment of the $3.5 million, subject to an adjustment for certain liabilities and other obligations, was scheduled to be released to the Company on April 15, 2009. Spectrum disputed the amount of the adjustment and the final installment was not released to the Company. On April 10, 2009, the Company filed a demand for arbitration regarding Spectrum’s payment of the final installment. For further information regarding the arbitration please refer to Chapter 20, Paragraph 20.8 of this Registration Document. As part of the transaction, CTI agreed to forego the right to receive up to $15 million in product sales milestone payments provided to CTI in connection with the original transaction establishing RIT Oncology.
As part of the closing, CTI assigned to Spectrum an amended and restated security agreement and guarantee granted in favor of Biogen Idec Inc. to secure the performance of RIT Oncology’s obligations with respect to Zevalin that were assumed from CTI on formation of RIT Oncology, and Spectrum has agreed to reimburse CTI for any liability incurred based upon claims made by Biogen under such contracts or any other contracts associated with the Zevalin business to which CTI was previously a party. In addition, CTI extended the terms of the existing master services agreement with RIT Oncology and has agreed to perform transition services for the benefit of the Zevalin business until May 31, 2009. As of the date of this registration document no relevant new facts occurred with respect to this agreement.
| 22.1.1.5 | Agreement with PG-TXL Company, L.P. |
PG-TXL Company, L.P. We have an amended agreement with PG-TXL Company, L.P which grants us an exclusive worldwide license for the rights to OPAXIO and to all potential uses of PG-TXL Company, L.P.’s polymer technology. Under the terms of the agreement, we acquired the rights to the research, development,
C-307
manufacture, marketing and sale of anti-cancer drugs developed using this polymer technology. We are obligated to make payments to PG-TXL Company upon the achievement of certain development and regulatory milestones. To date we have made $6.1 million in milestone payments, including a $0.5 million payment that became due upon the acceptance of our MAA for review by the EMA in March 2008. In addition, we could be obligated to make additional payments of up to $14.4 million in the future if additional milestones are met. The timing of the remaining milestone payments under the amended agreement is based on trial commencements and completions, as well as regulatory and marketing approval with the FDA, EMA or equivalent in another major market country. Additionally, we are required to make royalty payments to PG-TXL based on the net sales. Our royalty payments range from low-single digits to mid-single digits as a percentage of the net sales. Unless otherwise terminated, the term of the PG-TXL Agreement continues until no royalties are payable to PG-TXL. We may terminate the PG-TXL Agreement, among others, upon advance written notice to PG-TXL in the event issues regarding the safety of the products licensed pursuant to the PG-TXL Agreement arise during development or clinical data obtained reveal a materially adverse tolerability profile for the licensed product in humans. In addition, either party may terminate the PG-TXL Agreement, among others in the event of an uncured material breach of the respective material obligations and conditions of the PG-TXL Agreement or in the event of liquidation or bankruptcy of a party.
| 22.1.1.6 | Agreement with the Gynecologic Oncology Group |
Gynecologic Oncology Group. We have an agreement with the Gynecologic Oncology Group, or GOG, related to the GOG0212 trial which the GOG is conducting. We recorded a $1.6 million payment due to the GOG based on the 650 patient enrollment milestone achieved in the first quarter of 2010. As of September 30, 2010, this amount is included in accounts payable. Under this agreement we are required to pay up to $3.5 million in additional milestone payments related to the trial of which $1.7 million may become due in the next four to six months based on current planned patient enrolment and $ 0.5 million may become due in 2011.
| 22.1.1.7 | License Agreement for Brostallicin |
Brostallicin. We acquired our rights to brostallicin through our acquisition of Systems Medicine Inc., a privately held oncology company, completed in July 2007. Under the license agreement we entered into for brostallicin, we may be required to pay up to $80.0 million in milestone payments, based on the achievement of certain product development results. Due to the early stage of development that brostallicin is in, we are not able to determine whether the clinical trials will be successful and therefore cannot make a determination that the milestone payments are reasonably likely to occur at this time.
| 22.1.1.8 | Agreement with IDIS Limited |
In line with Company’s values, CTI has made pixantrone available on a compassionate use basis. Accordingly, in May 2009 the Company entered into an agreement with IDIS, Limited, or IDIS, to manage pixantrone as an investigational drug on a named-patient basis in Europe. Pixantrone will be supplied by IDIS to healthcare professionals for the treatment of individual patients with relapsing aggressive non-Hodgkin’s lymphoma.
| 22.1.1.9 | Manufacturing Supply Agreement |
In July 2010, the Company entered into a Drug Product Manufacturing Supply Agreement with NerPharMa. Pursuant to the terms of the agreement, NerPharMa has agreed to manufacture and supply to us, the bulk unlabeled vials of drug product for our drug candidate pixantrone, which is BBR 2778 (pixantrone dimaleate) from the effective date of the agreement through the fifth anniversary of the date that the first government or regulatory approval has been obtained for pixantrone in the United States or Europe, whichever is earlier, unless
C-308
earlier terminated. The NerPharMa Agreement may be terminated for an uncured material breach, insolvency or the filing of bankruptcy, or by mutual agreement. We may also terminate the NerPharMa Agreement (i) upon prior written notice in the event of failure of three or more of seven consecutive lots of product or (ii) in the event NerPharMa is acquired or a substantial portion of NerPharMa’s assets related to the NerPharMa Agreement are sold to another entity. The Company agreed to purchase supply of pixantrone from NerPharMa on the basis of a rolling commercial forecast.
| 22.1.1.10 | License agreement |
Under a license agreement entered into with Nerviano Medical Sciences, S.r.l. for Brostallicin, we may be required to pay up to $80.0 million in milestone payments based on the achievement of certain product development results. Due to the early stage of development that brostallicin is in, we are not able to determine whether the clinical trials will be successful and therefore cannot make a determination that the milestone payments are reasonably likely to occur at this time.
| 22.1.1.11 | Divestiture of TRISENOX and Certain Proteasome Assets |
On July 18, 2005, CTI completed the divestiture of TRISENOX® (arsenic trioxide), an anti-cancer compound, and certain proteasome assets to Cephalon Inc., or Cephalon. Proceeds from the divestiture, net of broker fees, were approximately $71.9 million which includes proceeds received from transition services provided. In addition, in the future CTI may potentially receive up to an additional $100 million if Cephalon is successful in achieving certain sales and development milestones, although achievement of such milestones is uncertain.
| 22.1.2 | Other agreements |
| 22.1.2.1 | Securities Purchase Agreement |
In connection with the co-development agreement entered into with Novartis reported in paragraph 22.1.1.1 above, CTI also entered into a securities purchase agreement with Novartis, under which CTI agreed to sell and Novartis agreed to purchase an aggregate of 216,763 shares of our common stock for a total purchase price of $15 million.
In October 2006, both the co-development and securities purchase agreements became effective upon the receipt of antitrust regulatory clearance, and accordingly, CTI closed the sale of the shares of common stock to Novartis. Expenses related to this sale were approximately $0.2 million and were recorded in common stock as an offset to proceeds received.
| 22.1.2.2 | Registration Rights Agreement |
In connection with the sale of its common stock mentioned in paragraph 22.1.2.1 above, CTI entered into a registration rights agreement with Novartis, under which CTI agreed to prepare, file and have declared effective a shelf registration statement with the Securities and Exchange Commission, or SEC, covering the resale of this common stock and to maintain the effectiveness of such registration statement. If CTI fails to achieve either of these obligations CTI is required to make certain payments to Novartis of up to 1% of the purchase price of the common stock per month for each that CTI is not in compliance with these requirements. The registration statement was declared effective in November 2006.
C-309
CTI analyzed the default payment requirement related to the continuing registration effectiveness obligation in accordance with FSP EITF 00-19-2 which specifies that the contingent obligation to make future payments under a registration payment arrangement should be separately recognized and accounted for under SFAS 5. As CTI has never had a registration rights agreement lose effectiveness after its initial effectiveness has been declared, it was determined that the probability of payment under the registration rights agreement is remote under SFAS 5 guidance. As such, CTI did not recognize a liability related to the registration payment arrangement.
| 22.1.2.3 | Acquisition of Systems Medicine, Inc. |
On July 24, 2007, the Company announced it had entered into an Acquisition Agreement (the “Acquisition Agreement”) by and among the Company, Cactus Acquisition Corp., a Delaware corporation and direct, wholly-owned subsidiary of the Company (“Cactus Sub”), Saguaro Acquisition Company LLC, a Delaware limited liability company and direct, wholly owned subsidiary of the Company (“Saguaro Sub”), Systems Medicine, Inc., a Delaware corporation (“Systems Medicine”), and Tom Hornaday and Lon Smith as Stockholder Representatives (as that term is defined in the Acquisition Agreement), pursuant to which (1) Cactus Sub would merge with and into Systems Medicine in a statutory reverse triangular merger (the “First Merger”) with Systems Medicine as the surviving corporation of the First Merger, and (2) immediately following the effectiveness of the First Merger, Systems Medicine would merge with and into Saguaro Sub in a statutory forward triangular merger (the “Second Merger” and collectively or in seriatim with the First Merger, as appropriate, the “Merger”) with Saguaro Sub as the surviving entity as a direct, wholly owned subsidiary of the Company.
The purchase price under the Acquisition Agreement was $20 million, paid almost entirely in shares of common stock of the Company, no par value. The Merger was approved by the boards of directors of both the Company and Systems Medicine and by a sufficient vote of the stockholders of Systems Medicine. Approval by the shareholders of the Company was not required for this transaction. The Acquisition Agreement included customary closing conditions, including waivers by additional stockholders of Systems Medicine of statutory appraisal rights. Under the terms of the agreement, Systems Medicine will continue to operate as a wholly-owned subsidiary of the Company, utilizing its genomic-based platform to guide development of the Company’s oncology products, including Brostallicin. The Acquisition Agreement is available on the Current Report on Form 8-K filed with the SEC on July 27, 2007, on the Company’s website www.celltherapeutics.com and on the SEC’s website www.sec.gov.
On July 31, 2007, the Company completed the acquisition, pursuant to which the Company issued to Systems Medicine stockholders an aggregate of 421,185 shares of Common Stock in exchange for outstanding Systems Medicine common stock. Of the total shares issued, 42,118 were initially placed in an escrow account subject to any claim for indemnification made by the Company. Upon the twelve month anniversary of the closing date, per the acquisition agreement, all remaining escrowed shares were released to the former shareholders of Systems Medicine. The shares were issued pursuant to the exemptions from registration afforded by Section 4(2) and Regulation D of the Securities Act of 1933, as amended. Under the agreement, Systems Medicine, Inc. became Systems Medicine, LLC, or SM, and now operates as a wholly owned subsidiary of the Company.
System Medicine’s stockholders as of the date of acquisition could also receive a maximum of $15 million in additional consideration (payable in cash or stock at our election, subject to certain Nasdaq limitations on issuance of stock) upon the achievement of certain FDA regulatory milestones in the development of Brostallicin. A $5 million payment is due upon receipt of written acknowledgement by Systems Medicine or the Company or any of their affiliates from the U.S. Food and Drug Administration regarding the acceptability of pivotal trial(s) for NDA approvability of Brostallicin (such NDA to be based on “context of vulnerability” studies focused on
C-310
indications such as, but not limited to, fibrosarcoma (t12/16), tumors with DNA mismatched repair defects or GSH/.GST over-expressing tumors), and a $10 million payment is due upon receipt by Systems Medicine or the Company of notice from the FDA of approval of a new drug application for brostallicin. At this time, it is not possible to predict whether these milestones will be achieved. . In August 2009, the Company entered into an amended agreement under which these milestone payments were replaced by an immediate substitute payment of $6.0 million payable in shares of CTI’s common stock subject to certain conditions, including required shareholder approval. If the conditions were not satisfied, the Company would have been required to pay the SMI stockholders $5.0 million cash in lieu of the $6.0 million shares of CTI’s common stock. In October 2009, Company’s shareholders approved the issuance of $6.0 million shares of our common stock and we issued approximately 5.6 million shares to SMI stockholders.
| 22.1.2.4 | Asset Purchase Agreement with Biogen |
On August 15, 2007, CTI entered into an Asset Purchase Agreement with Biogen pursuant to which CTI would acquire Biogen’s radiopharmaceutical product Zevalin (ibritumomab tiuxetan) for development, marketing and sale of that product in the United States, along with certain assets of Biogen related to Zevalin for an initial purchase price of $10 million. The parties closed this transaction per the terms of the agreement on December 21, 2007. Pursuant to the Purchase Agreement, CTI had a contingent obligation to make up to two additional future payments of $10 million each due upon reaching certain FDA approval milestones and a further obligation to make royalty payments to Biogen based on net sales related to Zevalin from December 21, 2007 until the latest of (a) the expiration date of the last to expire of any patents related to Zevalin, (b) the first date on which any third person lawfully sells a biosimilar product in the United States or (c) December 31, 2015. A copy of the Purchase Agreement was attached as an exhibit to CTI’s Current Report on Form 8-K filed with the SEC on August 21, 2007. The Purchase Agreement was amended in December 2008 in connection with the transfer of Zevalin to RIT Oncology LLC, a joint venture formed by CTI and Spectrum. In connection with this amendment, RIT Oncology made a payment of $2 million to Biogen and assumed responsibility for the milestone and royalty payments. In March 2009, CTI sold its interest in RIT Oncology to Spectrum, thereby transferring its remaining interest in Zevalin. In the event CTI were required to pay any obligations related to Zevalin arising under these agreements, for instance in the event RIT Oncology were to default on payment of those obligations, Spectrum would be required to reimburse CTI for such obligations.
The Purchase Agreement contains representations, warranties and covenants of both CTI and Biogen. The representations, warranties and covenants contained in the Purchase Agreement were made only for purposes of such agreement and as of specific dates, were solely for the benefit of the parties to such agreement, and may be subject to limitations agreed by the contracting parties, including being qualified by disclosures exchanged between the parties in connection with the execution of the Purchase Agreement. The representations and warranties may have been made for the purposes of allocating contractual risk between the parties to the Purchase Agreement instead of establishing these matters as facts, and may be subject to standards of materiality applicable to the contracting parties that differ from those applicable to investors. Investors are not third-party beneficiaries under the Purchase Agreement and should not rely on the representations, warranties and covenants or any descriptions thereof as characterizations of the actual state of facts or conditions of CTI or Biogen or any of their respective assets.
C-311
| 22.1.2.5 | Security Agreement |
On December 21, 2007, in connection with the closing of CTI’s purchase of the Zevalin, CTI and Biogen entered into a Security Agreement (the “Security Agreement”) relating to certain security interests granted by CTI to Biogen. Pursuant to the Security Agreement, CTI granted a first priority security interest to Biogen in all of CTI’s right, title and interest (a) in and to certain assets purchased by CTI pursuant to the Asset Purchase Agreement discussed above, together with any other assets or rights related to any of such assets or otherwise used in the development, manufacture or commercialization of Zevalin and (b) under certain license, sublicense and supply agreements entered into pursuant to the Asset Purchase Agreement (the “Collateral”). Upon the occurrence of an ongoing event of default including, without limitation, CTI’s failure to pay or perform its obligations under the Security Agreement, the Asset Purchase Agreement or the related sublicense and service agreements (collectively, the “Secured Obligations”), a breach by CTI of its representations and warranties under the Security Agreement, CTI’s application for, or consent to, the appointment of a receiver, trustee or liquidator of all or a substantial portion of CTI’s assets, the transfer by CTI of its assets as part of a general assignment or other arrangement for the benefit of creditors, CTI’s insolvency, the filing of a voluntary or involuntary petition filed under the provisions of the Unites States Bankruptcy Code, or the attachment or execution upon, or seizure of, all or substantially all of CTI’s assets, under the Security Agreement Biogen could take any action with respect to the Collateral that it deems necessary or advisable to accomplish the purposes of the Security Agreement. The Security Agreement creates a continuing security interest in the Collateral to remain in full force and effect until the payment in full of any Secured Obligation and performance of all other obligations secured by the Security Agreement. A copy of the Security Agreement is attached as an exhibit to CTI’s Current Report on Form 8-K filed with the SEC on December 31, 2007.
In December 2008, in connection with the transfer of Zevalin to RIT Oncology, the Security Agreement was amended to reflect the transfer of the Collateral and the obligations secured by the collateral to RIT Oncology. In addition, CTI entered into a Guarantee Agreement with Biogen providing that CTI would remain responsible for certain obligations related to Zevalin in the event RIT Oncology were to default on this payment of those obligations. Following the sale of CTI’s interest in RIT Oncology to Spectrum, in the event CTI was required to pay any amount to Biogen under this agreement, Spectrum would be required to reimburse CTI for the amount of such obligation.
| 22.1.2.6 | Put Option Related to Tender Offer |
On December 5, 2008, in connection with the issuance to a single institutional investor of $32.6 million in principal amount of the Company’s 10% Convertible Senior Notes due 2011, and pursuant to a Purchase Agreement, the institutional investor also granted the Company a conditional put option right to issue and sell to the investor an additional $3 million of Series C 10% Convertible Senior Notes of the Company (the “C Notes”) if the Company makes a convertible notes repurchase tender offer and receives tenders (which are not withdrawn) of at least $62 million principal amount of convertible notes, or to issue and sell to the Investor an additional $6 million of C Notes if the Company makes a convertible notes repurchase tender offer and receives tenders (which are not withdrawn) of at least $93 million principal amount of convertible notes. The C Notes would have substantially the same terms as the Notes. The put option right expired on March 31, 2009. As of the date of this registration document no relevant new facts occurred with respect to this agreement.
C-312
| 22.2 | Other relevant contracts involving obligation or rights for the member of the CTI group that could significantly affect the Issuer’s ability to meet its obligations vis-a-vis the shareholders |
Not applicable.
C-313
CHAPTER 23 THIRD PARTY INFORMATION AND STATEMENT BY EXPERTS AND
DECLARATIONS OF ANY INTEREST
| 23.1 | Opinions and reports of experts |
This Registration Document contains no opinion or report of third parties acting as experts.
| 23.2 | Faithful reproduction of information concerning the Issuer provided by third parties |
Third-party information contained in this Registration Document has been faithfully reported and, as far as the Issuer knows or could know based on the information published by the aforesaid third parties, no fact or part of this information, which would make the information reproduced inaccurate or deceitful have been omitted. The relevant source is indicated every time that the aforesaid information from third parties is cited in this Registration Document.
C-314
CHAPTER 24 DOCUMENTS AVAILABLE TO THE PUBLIC
The following documents are available to the public during the life of this Registration Document:
| 1. | All financial information publicly filed, including CTI’s audited consolidated annual financial statements for the fiscal years ended December 31, 2007, 2008 and 2009 in its annual reports can be found on the Company’s website at http://investors.celltherapeutics.com. |
| 2. | CTI’s Second Amended and Restated Bylaws currently in force can be found as an exhibit to the Company’s Current Report on Form 8-K filed on February 22, 2010, which can be found on the Company’s website at http://investors.celltherapeutics.com. |
| 3. | CTI’s amended and restated articles of incorporation currently in force, as amended can be found in an exhibit to the Registrant’s Registration Statement on Form S-3 (File No. 333-153358), filed on September 5, 2008 (with amendments to the articles in exhibits to the Registrant’s Current Report on Form 8-K filed on February 9, 2009, March 27, 2009, April 13, 2009, August 21, 2009, December 28, 2009, January 19, 2010, April 5, 2010, May 27, 2010, July 27, 2010, September 17, 2010, October 22, 2010), which can be found on the Company’s website at |
http://investors.celltherapeutics.com/phoenix.zhtml?c=92775&p=irol-govHighlights.
C-315
CHAPTER 25 INFORMATION ON HOLDING
Since the only undertakings in which the Issuer holds a proportion of the capitaly to have a significant effect on the assessment of its own assets and liabilities, financial position or profits and losses are exactly the controlled companies, please refer to the information reported in previous Paragraph 7.2 of this Registration Document.
C-316
APPENDICES
| A. | Auditor’s opinions for the 2007, 2008 and 2009 Consolidated Financial Statements and limited review report for the financial information regarding the six-month period ended June 30, 2010 |
| B. | Chapter 12.6 of the 2003 Listing Prospectus |
| C. | Chapter 12.5 of the 2003 Listing Prospectus |
C-317
