Attached files
| file | filename |
|---|---|
| EX-23 - EXHIBIT 23 - MWI Veterinary Supply, Inc. | exhibit23.htm |
| EX-32 - EXHIBIT 32 - MWI Veterinary Supply, Inc. | exhibit32.htm |
| EX-31.1 - EXHIBIT 31.1 - MWI Veterinary Supply, Inc. | exhibit31_1.htm |
| EX-31.2 - EXHIBIT 31.2 - MWI Veterinary Supply, Inc. | exhibit31_2.htm |
| EX-21.1 - EXHIBIT 21.1 - MWI Veterinary Supply, Inc. | exhibit21_1.htm |
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
||
|
For the fiscal year ended September 30, 2010
Commission file number 000-51468
|
|||
|
OR
|
|||
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
||
|
For the transition period from ________ to ________
|
MWI VETERINARY SUPPLY, INC.
(Exact name of registrant as specified in its Charter)
|
Delaware
|
02-0620757
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
|
|
651 S. Stratford Drive, Suite 100
|
||
|
Meridian, ID
|
83642
|
|
|
(Address of Principal Executive Offices)
|
(Zip Code)
|
|
(800) 824-3703
|
||
|
(Registrant’s telephone number, including area code)
|
||
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE SECURITIES EXCHANGE ACT OF 1934:
|
Title of each class
|
Name of each exchange on which registered
|
|
Common Stock, $0.01 par value
|
The Nasdaq Stock Market, LLC
|
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended (the “Act”) during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large Accelerated Filer o
|
Accelerated Filer x
|
Non-Accelerated Filer o
|
Smaller Reporting Company o
|
|
|
(Do not check if a smaller reporting company)
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No x
As of March 31, 2010 (the last business day of the registrant’s most recently completed second fiscal quarter), the aggregate market value of the voting stock held by non-affiliates of the registrant was approximately $440,000,000. Shares of common stock held by each executive officer and director and by each person who owns 10% or more of our outstanding common stock have been excluded since such persons may be deemed affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of shares of the registrant’s common stock, $0.01 par value, outstanding as of November 12, 2010 was 12,453,053.
Documents Incorporated by Reference
Listed hereunder are the documents, any portions of which are incorporated by reference and the Parts of this Form 10-K into which such portions are incorporated:
|
1.
|
The registrant’s definitive proxy statement for use in connection with the Annual Meeting of Stockholders to be held on or about February 8, 2011 to be filed within 120 days after the registrant’s fiscal year ended September 30, 2010, portions of which are incorporated by reference into Part III of this Form 10-K.
|
MWI VETERINARY SUPPLY, INC.
FORM 10-K
TABLE OF CONTENTS
|
Item
|
Page
|
||||
|
PART I
|
|||||
|
PART II
|
|||||
|
PART III
|
|||||
|
PART IV
|
|||||
PART I
|
|
Cautionary Statement for Purposes of “Safe Harbor Provisions” of the Private Securities Litigation Reform Act of 1995
|
This annual report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. Statements that are not historical facts, including statements about our beliefs and expectations, are forward-looking statements. Forward-looking statements include statements preceded by, followed by or that include the words “may,” “could,” “would,” “should,” “believe,” “expect,” “anticipate,” “plan,” “estimate,” “target,” “project,” “intend” and similar expressions. These statements include, among others, statements regarding our expected business outlook, anticipated financial and operating results, our business strategy and means to implement our strategy, our objectives, the amount and timing of capital expenditures, the likelihood of our success in expanding our business, financing plans, budgets, working capital needs and sources of liquidity.
Forward-looking statements are only predictions and are not guarantees of performance. These statements are based on our management’s beliefs and assumptions, which in turn are based on currently available information. Important assumptions relating to the forward-looking statements include, among others, assumptions regarding demand for our products, the expansion of product offerings geographically or through new applications, the timing and cost of planned capital expenditures, competitive conditions and general economic conditions. These assumptions could prove inaccurate. Forward-looking statements also involve known and unknown risks and uncertainties, which could cause actual results to differ materially from those contained in any forward-looking statement. Many of these factors are beyond our ability to control or predict. Such factors include, but are not limited to, the following:
|
·
|
the impact of vendor consolidation on our business;
|
|
·
|
changes in or availability of vendor contracts or rebate programs;
|
|
·
|
vendor rebates based upon attaining certain growth goals;
|
|
·
|
changes in the way vendors introduce/deliver products to market;
|
|
·
|
exclusivity requirements with certain vendors that may prohibit us from distributing competing products manufactured by other vendors;
|
|
·
|
risks associated with our international operations;
|
|
·
|
transitional challenges associated with acquisitions, including the failure to achieve anticipated synergies;
|
|
·
|
financial risks associated with acquisitions;
|
|
·
|
the impact of general economic trends on our business;
|
|
·
|
the recall of a significant product by one of our vendors;
|
|
·
|
extended shortage or backorder of a significant product by one of our vendors;
|
|
·
|
seasonality;
|
|
·
|
the timing and effectiveness of marketing programs or price changes offered by our vendors;
|
|
·
|
the timing of the introduction of new products and services by our vendors;
|
|
·
|
the ability to borrow on our revolving credit facility, extend the terms of our revolving credit facility or obtain alternative financing on favorable terms or at all;
|
|
·
|
risks from potential increases in variable interest rates;
|
|
·
|
the impact of tightening credit standards and/or access to credit on behalf of our customers and suppliers;
|
|
·
|
unforeseen litigation;
|
|
·
|
a disruption caused by adverse weather or other natural conditions or disasters;
|
|
·
|
inability to ship products to the customer as a result of technological or shipping disruptions; and
|
|
·
|
competition.
|
Except as required by applicable law, including the securities laws of the United States and the rules and regulations of the Securities and Exchange Commission (“SEC”), we are under no obligation to publicly update or revise any forward-looking statements, whether as a result of any new information, future events or otherwise. You should not place undue reliance on our forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results or performance.
Investors should also be aware that while we do, from time to time, communicate with securities analysts, it is against our policy to disclose any material non-public information or other confidential commercial information. Accordingly, stockholders should not assume that we agree with any statement or report issued by any analyst irrespective of the content of the statement or report. Furthermore, we have a policy against issuing or confirming financial forecasts or projections issued by others. Thus, to the extent that reports issued by securities analysts contain any projections, forecasts or opinions, such reports are not the responsibility of MWI Veterinary Supply, Inc.
|
Item 1.
|
|
|
General
|
Our business commenced operations as part of a veterinary practice in 1976 and was incorporated as MWI Drug Supply, Inc., an Idaho corporation, in 1980. MWI Drug Supply, Inc. was acquired by Agri Beef Co. in 1981. MWI Veterinary Supply Co. was incorporated as an independent subsidiary of Agri Beef Co. in September 1994. Effective June 18, 2002, MWI Holdings, Inc. was formed by Bruckmann, Rosser, Sherrill & Co. II, L.P. (“BRS”) for the sole purpose of acquiring all of the outstanding stock of MWI Veterinary Supply Co. from Agri Beef Co. (“Agri Beef”). As a result of this transaction, MWI Veterinary Supply Co. (“MWI Co.”) became a wholly-owned subsidiary of MWI Holdings, Inc. On April 21, 2005, we changed our name from MWI Holdings, Inc. to MWI Veterinary Supply, Inc. References in this report to “we,” “us,” “our,” and “MWI” refer to MWI Veterinary Supply, Inc. unless otherwise indicated. Unless otherwise indicated, all statistical information provided about our business in this report is as of September 30, 2010.
We are a leading distributor of animal health products to veterinarians across the United States and United Kingdom. On February 8, 2010, we acquired the outstanding share capital of Centaur Services Limited (“Centaur”). Based in Castle Cary, England, Centaur is a supplier of animal health products to veterinarians in the United Kingdom. In fiscal year 2008, we acquired substantially all of the assets of Tri V Services, Inc. ("Tri V"). Also in fiscal year 2008, we acquired substantially all of the assets of AAHA Services Corporation, operating as AAHA MARKETLink ("AAHA MARKETLink"). In fiscal year 2007, we acquired substantially all of the assets of Securos, Inc. and International Veterinary Distribution Network, Inc. (collectively, "Securos").
Products we sell include pharmaceuticals, vaccines, parasiticides, diagnostics, capital equipment, supplies, specialty products, veterinary pet food and nutritional products. We market these products to veterinarians in both the companion animal and production animal markets. As of September 30, 2010, we had a sales force of 376 people covering the United States and a sales force of 21 people in the United Kingdom. We also offer our customers a variety of value-added services, including e-commerce platform, pharmacy fulfillment, inventory management system, equipment procurement consultation, special order fulfillment, educational seminars and pet cremation, which we believe closely integrates us with our customers’ day-to-day operations and provides them with meaningful incentives to continue ordering from us.
Historically, we estimate that approximately two-thirds of our total revenues have been generated from sales to the companion animal market and one-third from sales to the production animal market. We estimate that Centaur has historically had a similar mix of sales in the United Kingdom.
Centaur is a wholesaler of animal health products to veterinarians in the United Kingdom. It serves approximately 1,500 customers in a market of approximately 3,500 customers. Products distributed include pharmaceuticals, vaccines, diagnostics, parasiticides, pet foods and other consumable products supplied from approximately 400 vendors. Because of the small geographic size of the market, it is able to distribute the products with next-day service using primarily its own trucks.
While our business in the United Kingdom is very similar to our business in the United States, there are some differences between the operations of Centaur and the operations in the United States. The sales staff for Centaur does not actively promote products to the veterinarians, but rather sells products and maximizes the logistics services that Centaur provides. Veterinary practices typically purchase the majority of their animal health products from one wholesaler. Vendor rebates are not earned by the wholesaler but are typically offered directly to the veterinarians by the vendor.
Centaur's distribution center in Castle Cary, England is highly automated compared to our distribution centers in the United States which use more labor to prepare the products for shipment. Nearly 90% of Centaur's orders are received via some form of electronic communication, which includes an electronic order system via the internet, www.centaurweb.co.uk. There are also telesales representatives who take orders over the phone and assist in any other customer service issues that might arise.
While there are some differences as noted above, the business in the United Kingdom is otherwise similar to that in the United States. We ship either the same or similar products to a similar customer base. The description of our business which follows applies to our operations in the United States.
|
|
Geographic Information
|
Revenues and long-lived assets by geographic region are as follows:
|
|
2010
|
2009
|
2008
|
||||||||
|
Revenues for the fiscal years ended September 30
|
|
|
|
||||||||
|
United States
|
$ | 1,074,226 | $ | 941,332 | $ | 831,364 | |||||
|
International (United Kingdom)
|
155,116 | - | - | ||||||||
|
Total
|
$ | 1,229,342 | $ | 941,332 | $ | 831,364 | |||||
|
|
|||||||||||
|
Long-lived assets as of September 30
|
|||||||||||
|
United States
|
$ | 9,762 | $ | 9,313 | $ | 9,687 | |||||
|
International (United Kingdom)
|
5,476 | - | - | ||||||||
|
Total
|
$ | 15,238 | $ | 9,313 | $ | 9,687 | |||||
|
|
Industry Overview
|
According to the Animal Health Institute (“AHI”), an industry group representing manufacturers of animal health products, animal health product sales in the United States for calendar year 2008 (which is the most recent information available as of the filing date) grew to $6.7 billion, which represents a 6.9% compounded annual growth rate since calendar year 2002. The market for animal health products in the United States is split between products sold for companion and production animals. Companion animals include dogs, cats, horses and other pets, while production animals include cattle and other food-producing animals. AHI estimates that companion animal products accounted for 53% of the total market for animal health products in the United States in calendar year 2006.
We believe the companion animal health products market’s growth has slowed as a result of a decrease in consumer spending. Historically, the growth in the companion animal health market has been due to the increasing number of households with companion animals, an aging pet population, increased expenditures on animal health and preventative care, advancements in animal health products and extensive marketing programs sponsored by companion animal nutrition and pharmaceutical companies. Product sales in the production animal market have been negatively impacted by increasing volatility in commodity prices such as milk, corn, grain and feeder cattle, changes in weather patterns that allow cattle to graze for longer periods and changes in the general economy. We support production animal veterinarians with a broad range of products and value-added services. Historically, sales in this market have been largely driven by spending on animal health products to improve productivity, weight gain and disease prevention, as well as a growing focus on food safety. We believe that these growth factors have been mitigated by the downward influence of generic drugs on pricing.
Veterinarians are one of the primary purchasers of animal health products, particularly in the companion animal market. As of December 31, 2009, according to the American Veterinary Medical Association, or AVMA, there were more than 61,000 veterinarians in private practice nationwide. We believe that distributors play a vital role for veterinary practices by providing access to a broad selection of products through a single channel and helping them efficiently manage their inventory levels. Distributors also offer vendors substantial value by providing cost-effective access to a highly fragmented and geographically diverse customer base.
|
|
Competitive Strengths
|
We believe that our strengths include:
|
|
·
|
Leading Distributor to Veterinarians. While most of our products are available from several sources and our customers typically have relationships with several distributors, we have achieved this position primarily through internal growth. We believe that our broad product offering, competitive pricing, superior customer service, rapid product fulfillment and value-added services provide meaningful incentives for our customers to continue ordering from us.
|
|
|
·
|
Leading Sales and Marketing Franchise. Our sales representatives in the United States educate customers on new veterinary products, assist in product selection and purchasing, and offer inventory management solutions. We also publish detailed product catalogs and monthly magazines, which are often utilized by our customers as reference tools. While salespeople and printed materials are vital to our marketing strategy, we also provide on-line ordering, valuable business information and value-added services through our Internet site, www.mwivet.com.
|
|
|
·
|
Strong, Established Relationships with Veterinarians and Vendors. Our ability to serve as a single source for most of our customers’ animal health product needs has enabled us to develop strong and long-term customer relationships. For more than fourteen years we have maintained distribution arrangements with Banfield, The Pet Hospital (“Banfield”), the nation’s largest private veterinary practice, and with our non-controlled affiliate, Feeders’ Advantage, L.L.C. (“Feeders’ Advantage”), a related party and a buying group composed of several of the largest cattle feeders in the United States. Since we currently do not manufacture the vast majority of the products we sell, we are dependent on our vendors for the supply of our products. While our vendors often have relationships with multiple distributors, we have long-term relationships with many of our key vendors including Boehringer Ingelheim, IDEXX Laboratories, Intervet Schering-Plough, Merial, Pfizer and Vedco.
|
|
|
·
|
Recurring Revenue Product Base. Over 97% of our product sales were from consumable medicines and supplies commonly required by veterinarians in their practice. Historically, this aspect of our business has resulted in a recurring stream of revenues.
|
|
|
·
|
Sophisticated Technology and Information Systems. We continue to invest in our technology and information systems, which we believe has created a competitive advantage when delivering quality service to our customers. Our e-commerce platform offers enhanced online ordering capabilities for veterinarians. This platform provides many features for our customers with enhanced security.
|
|
|
·
|
Experienced Management Team. We have a strong and experienced senior management team with substantial animal health industry expertise. The members of our senior management team have been with us for an average of over sixteen years, and each member has demonstrated a commitment and capability to deliver growth in revenues and profitability.
|
|
|
Business Strategy
|
Our mission is to be the best resource to the veterinary profession by delivering superior value, efficiency and innovation. Our strategy to achieve our mission is outlined below.
Increase Sales to Existing Customers. We believe that veterinary practices typically purchase animal health products from multiple distributors. For our fiscal year ended September 30, 2010, our average annual product sales per veterinary practice served in the United States was approximately $39,000. We intend to increase our share of these purchases by utilizing our proprietary customer database to focus our marketing efforts, expanding our sales force, selectively adding products to our portfolio and increasing and expanding the value-added services we provide to our customers. By increasing the dollar value of purchases made by each customer as well as their average order size, we intend to increase our profitability. Competition for hiring sales representatives who have existing customer relationships is intense and we focus on retaining valued members of our staff who have demonstrated the skills needed to successfully market our products and services. If we fail to hire or retain a sufficient number of qualified sales professionals, it could adversely impact our business.
Expand Business Assistance Services for Veterinarians. We intend to enhance our customer relationships by expanding our business assistance services for veterinarians. These value-added services include among others our e-commerce platform, pharmacy fulfillment, inventory management system, equipment procurement consultation, special order fulfillment, educational seminars and pet cremation.
Increase the Total Number of Customers. We intend to add new customers by increasing the number and productivity of our sales representatives, selectively acquiring other animal health distributors and expanding
distribution centers. We believe the greatest opportunities to add new customers are in the Northeastern, Midwestern and Southeastern regions of the United States and abroad, areas where we do not hold the leading market position.
Continuously Seek to Improve Operations. We continuously evaluate opportunities to increase sales, lower costs and realize operating efficiencies. Current initiatives include investments in our technology and distribution center infrastructure. We also plan to pursue alternative product sourcing strategies and have a private label program on selected products to reduce our procurement costs and increase our profitability, while maintaining strong relationships with key vendors.
Make Selective Acquisitions. The United States market for animal health products distribution is highly fragmented, with numerous national, regional and local distributors. Many of these companies are small, privately-held businesses, some of which may represent attractive acquisition candidates. We also believe there are acquisition opportunities outside of the United States and we intend to seek out those companies that would be a strategic fit for us.
|
|
Products
|
During fiscal year 2010 in the United States, we sold more than 30,000 products, of which over 15,000 are stocked in our distribution centers, sourced from over 550 vendors. During fiscal year 2010 in the United Kingdom, we sold more than 19,000 products, of which nearly 10,000 are stocked in our distribution center, sourced from approximately 400 vendors. For our fiscal year ended September 30, 2010, our product revenues in the United States were comprised of approximately 40% pharmaceutical products, 18% vaccine products, 11% parasiticide products, 8% diagnostic products, 2% capital equipment products and 21% of other supplies. In addition, we sell approximately 600 products in the United States under agency agreements with our vendors. Under an agency agreement, we typically solicit orders and provide customer service for a commission, while the vendor stocks and ships the products. We also have available on special order approximately 7,000 products in the United States that we do not normally stock in our warehouses. We continually seek to update and improve the range of products we offer to address our customer requirements.
|
|
Pharmaceuticals, Vaccines and Parasiticides
|
We offer our customers a variety of pharmaceuticals, vaccines and parasiticides. Our pharmaceutical products typically include anesthetics, analgesics, antibiotics, ophthalmics and hormones. Our vaccine products are primarily comprised of small animal, equine and production animal biologicals. Our parasiticides are used for control of fleas, ticks, flies, mosquitoes and internal parasites.
|
|
Diagnostics, Capital Equipment and Supplies
|
We offer a wide range of diagnostics, capital equipment and supplies to veterinarians. Diagnostic sales typically include consumable in-clinic tests for detecting heartworm, lyme, feline leukemia and parvovirus, as well as consumable products for measuring blood chemistry, electrolyte balance and cell counts. Our capital equipment sales include anesthesia machines, surgical monitors, diagnostic equipment, dental machines, cages, lights and x-ray machines. We employ a team of capital equipment specialists to analyze the latest technologies and recommend equipment that meets our customers’ specific needs. Our sales of supplies include syringes, instruments, bandages, IV products, surgical consumables, grooming materials and other small equipment items used by veterinary practices.
|
|
Veterinary Pet Food and Nutritional Products
|
We offer our customers a broad selection of veterinary pet foods and nutritional products. We consider veterinary pet food to consist of two categories: foods for specialty diets and premium pet foods. Specialty diets are recommended by veterinarians to address specific medical and nutritional needs. Premium pet foods are recommended by veterinarians to promote optimal nutrition in healthy animals. Pet foods are typically sold under agency agreements. Nutritional products include dietary supplements, vitamins, dental chews and specialty treats which either help address specific medical conditions or are compatible with recommended nutritional guidelines.
|
|
Value-Added Services
|
We offer our customers a variety of value-added services, which we believe closely integrate us with our customers’ day-to-day operations and provide them with meaningful incentives to continue ordering from us. These services include the following:
|
Service
|
Description
|
|||
|
E-commerce platform
|
On-line ordering system that provides information to veterinary practices on products, vendor programs and purchasing history
|
|||
|
Pharmacy fulfillment
|
Shipment of prescription production and companion animal health products to end-users in the United States on behalf of veterinarians from our four licensed pharmacies located in Edwardsville, Kansas; Grand Prairie, Texas; Harrisburg, Pennsylvania and Nampa, Idaho and a licensed veterinary food-animal drug retailer in Visalia, California
|
|||
|
Inventory management system
|
Flexible system that facilitates counting, maintaining and ordering inventory in veterinary practices
|
|||
|
Equipment procurement consultation
|
Consultation and demonstrations provided by our dedicated capital equipment specialists in the United States
|
|||
|
Special order fulfillment
|
Procurement and shipment of approximately 7,000 unique products in the United States that we do not normally stock in our warehouses
|
|||
|
Educational seminars
|
Seminars for our customers covering business and medical topics, frequently sponsored in conjunction with our vendors
|
|||
|
Pet cremation
|
Business units presently operating with facilities in Idaho and Wisconsin that serve veterinary practices and their clients by providing cremation services
|
|||
|
|
Customers
|
We currently serve more than 22,000 veterinary practices located throughout the United States and approximately 1,500 veterinary practices in the United Kingdom. These veterinary practices are typically small, privately-held businesses that we believe place at least one order per week to avoid storing and managing large volumes of supplies. We believe that these veterinary practices usually purchase animal health products from multiple distributors. We seek to be the principal provider of animal health products to our customer base.
We maintain a diverse and stable customer base. Independent veterinary practices have historically accounted for approximately 85% of our product sales. Also, for more than fourteen years, we have maintained distribution arrangements with Banfield, the nation’s largest private veterinary practice with over 750 veterinary hospitals, and our non-controlled affiliate, Feeders’ Advantage, a buying group composed of several of the largest cattle feeders in the United States. We typically do not enter into long-term contracts with our independent veterinary customers.
We are a party to three written agreements with Banfield, a First Amendment to the Agreement for Product Purchases, a First Amendment to the Agreement for Logistics Services and an Agreement for Home Delivery Logistics Services. These contracts are effective from July 1, 2009 through June 30, 2012, and can be terminated by either party with or without cause upon 150 days prior written notice. These contracts provide that we will be the supplier of logistics to Banfield, and these agreements govern the pricing, shipping and other terms and conditions under which we sell our products and provide logistics to Banfield. Under the Agreement for Product Purchases, as amended, we provide a limited warranty with respect to all goods sold by us and paid for by Banfield that good title to the products is conveyed, that the products are delivered free of any security interest, that the products will conform to the description, grade and condition of the products invoiced and that all products will be free of any defects arising while the products are either in our possession or control or in the control of any carrier transporting the goods from us to Banfield. We are also required to maintain insurance in an amount equal to at least the replacement cost of all property that is purchased by Banfield from third parties and is held by us on their behalf.
|
|
Sales and Marketing
|
Our sales and marketing strategies are designed to establish and maintain strong customer relationships through personal visits by field sales representatives, frequent telesales contact and direct marketing, emphasizing our broad product lines, competitive pricing, efficient ordering capabilities, high levels of customer support and service and other value-added services. The key elements of our sales and marketing strategy are:
|
|
·
|
Field Sales Representatives: Our sales force is a key component of our value-added approach. Due to the fragmented nature of the animal health products market, we believe that a large sales force is vital to effectively support existing customers and target potential customers. As of September 30, 2010, we had 217 field sales personnel throughout the United States. Our field sales representatives educate customers on new veterinary products, assist in product selection and purchasing and offer inventory management solutions. Once a field sales representative has established a relationship with a customer, the field sales representative encourages the customer to use our telesales representatives and online ordering capabilities for day-to-day customer needs. Field sales representatives work under the supervision of regional sales managers within an assigned sales territory to ensure effective communication and timely sales calls with customers. Our field sales representatives complement our telesales and direct marketing efforts and enable us to better market, service and support the sale of our products and services. Our field sales representatives are employees of our Company and are compensated with a combination of salaries and commissions.
|
|
|
·
|
Telesales: We support our field sales representatives and direct marketing efforts with telesales representatives in nine call centers covering the United States. As of September 30, 2010, we had 159 telesales representatives in the United States. Telesales representatives work as partners with our field sales representatives, providing a dual coverage approach for individual customers. Telesales representatives process orders and generate new sales through frequent and direct contact with customers. Telesales representatives are responsible for assisting customers with ordering, purchasing decisions and general questions. Telesales representatives utilize our customized order entry system to process customer orders, access pricing, availability and promotional information about products, and research customer preferences and order history. Our nine call centers are connected by our telecommunications system which enables them to operate as one virtual call center.
|
|
|
·
|
Direct Marketing: We market to existing and potential customers by distributing product catalogs and monthly magazines. We publish a small animal catalog, livestock catalog and an equine catalog. These catalogs include detailed descriptions and specifications of our products and are often used as a reference tool by our customers and sales force. We also promote our products and services in our monthly magazine, the Messenger, which our field sales representatives use as a tool to educate customers on product and vendor programs. Additional marketing tools that we utilize include specialty catalogs, customer loyalty programs, specific product and vendor programs, flyers, faxes, order stuffers and other promotional materials. For the fiscal year ended September 30, 2010, we distributed nearly 700,000 pieces of direct marketing materials to existing and potential customers. We also participate in national and regional trade shows to extend our customer reach and enhance customer interaction.
|
|
|
·
|
E-Commerce Platform: We provide on-line ordering, valuable business information and value-added services to veterinarians via our primary Internet site, www.mwivet.com, and customized Internet sites that we maintain for two of our largest customers, Banfield and Feeders’ Advantage. Customers can use our Internet sites to order products, learn more about products and vendor programs, print forms needed for their veterinary practice, review their historical purchases and manage their inventory. For our fiscal years ended September 30, 2010, 2009 and 2008, approximately 34%, 31% and 27%, respectively, of our product sales in the United States were generated through orders placed over the Internet.
|
|
|
Product Sourcing
|
We currently do not manufacture the vast majority of our products and are dependent on vendors for our supply of products. We believe that effective purchasing is a key factor in maintaining our position as a leading provider of animal health care products. We regularly assess our purchasing needs and our vendors’ product offerings and prices to obtain products at favorable prices. While we purchase products from many vendors and there is generally more than one vendor for most animal health product categories, our concentration of aggregate purchases with key vendors is significant. In the United States, our ten largest vendors accounted for approximately 71%, 74% and 72% of our revenues for the fiscal years ended September 30, 2010, 2009 and 2008, respectively. Pfizer supplied products that accounted for approximately 25%, 24% and 23% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Of the Pfizer supplied products, production animal products under a livestock products agreement accounted for approximately 12%, 14% and 17% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Intervet-Schering, a
7
subsidiary of Schering Plough Corporation (“Schering Plough”), supplied products that accounted for approximately 10%, 11% and 9% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Boehringer Ingelheim (“Boehringer”) supplied products that accounted for approximately 10%, 4% and 3% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Merial Limited (“Merial”), a subsidiary of Sanofi-Aventis, supplies the majority of their products to us under an agency relationship. Commission revenue generated from Merial products accounted for approximately 49%, 56% and 60% of total commission revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively.
On November 3, 2009, Merck and Schering-Plough completed their merger under which Merck acquired all of the outstanding stock of Schering-Plough. Merial was a joint venture between Merck and Sanofi-Aventis, and Sanofi-Aventis acquired Merck’s interest in Merial shortly before Merck and Schering-Plough completed their merger. On March 9, 2010, Sanofi-Aventis and Merck announced that Sanofi-Aventis exercised its option to combine Merial with Intervet/Schering-Plough, Merck’s animal health business, to form an animal health joint venture that would be equally owned by the new Merck and Sanofi-Aventis. The completion of the transaction is expected to occur in the next twelve months.
There are two major types of transactions that can affect the flow of our products from our vendors, through us, to our customers. The method of selling products to veterinarians is dictated by our vendors. Traditional “buy/sell” transactions, which account for the vast majority of our business, involve the direct purchase of products by us from vendors, which we manage and store in our warehouses. A customer then places an order with us, and the order is then picked, packed, shipped and invoiced by us to our customer, followed by payment from our customer to us.
We also sell certain product lines to our customers under agency agreements with some of our vendors. Under this model, when we receive orders for products from the customer, we transmit the order to the vendor who then picks, packs and ships the products. In some cases our vendor invoices and collects payment from our customer, while in other cases we invoice and collect payment from our customer. We receive a commission payment for soliciting the order and for providing other customer service activities. Our operating expenses associated with agency sales transactions are lower than in traditional “buy/sell” transactions.
We have written agreements with approximately 40 of our vendors. Our livestock products agreement with Pfizer provides that we shall supply selected customers in the livestock field with Pfizer products. In return, we are entitled to a fee for logistics. We are not entitled to any rebates under this contract. We are required to maintain sufficient inventory to meet our anticipated demand on a monthly basis and to store the Pfizer products in accordance with their respective label instructions. Pfizer also reserves the right to sell directly to our customers or any other party. The livestock products agreement has a one-year term that expires on December 31, 2010 and can be terminated by either party with or without cause upon 30 days written notice.
Our equine products marketing agreement with Pfizer provides that we shall distribute certain products to customers in the equine field. In return, we are entitled to receive certain service incentives and rebates. We are required to maintain sufficient staffing levels of sales representatives and store and handle inventory under appropriate conditions that will maintain the quality and integrity of the products. The agreement expires on December 31, 2010 and can be terminated by either party with or without cause upon 30 days prior written notice.
Our 2009 strategic brands distribution agreement with Pfizer provides that we shall distribute the Rimadyl, Clavamox and Simplicef products offered by Pfizer. In return, we are entitled to receive certain service incentives and rebates. We are required to maintain sufficient staffing levels of sales representatives and store and handle inventory under appropriate conditions that will maintain the quality and integrity of the products. The agreement expires on December 31, 2010 and can be terminated by Pfizer with or without cause upon 30 days written notice, and either party can terminate the agreement immediately upon written notice for material breach of the contract.
Our companion animal select brands distribution agreement with Pfizer provides that we shall distribute the Zeniquin, Temaril-P, Antirobe, Doxirobe, Medrol, Dexdomitor and Antisedan products offered by Pfizer. In return, we are entitled to receive a logistics fee and certain sales incentives. We are required to maintain sufficient staffing levels of sales representatives and store and handle inventory under appropriate conditions that will maintain the quality and integrity of the products. The agreement expires on December 31, 2010 and can be terminated by Pfizer with or without cause upon 30 days prior written notice, and either party can terminate the agreement immediately upon written notice for material breach of the contract.
Our companion animal AAHA MARKETLink management agreement with Pfizer provides that we are entitled to purchase designated companion animal products from Pfizer and resell them to selected veterinary hospitals serviced by MWI who are members of the American Animal Hospital Association. In connection with its resale of the Pfizer companion animal products, MWI will be paid a logistics fee and may receive certain sales incentive payments from Pfizer.
8
This agreement expires on December 31, 2010 and may be terminated by either party, with or without cause, upon 30 days written notice. Pfizer may also terminate the agreement upon 15 days written notice to MWI in the event it determines that MWI has failed to comply with certain terms and conditions of the agreement. Pfizer may also terminate the agreement upon 30 days written notice to MWI in the event that MWI fails to meet certain sales goals for two consecutive months.
Our independent sales agent agreement with Merial provides that we shall sell, market and provide services related to Merial’s companion animal products to the veterinary trade. In return, we are entitled to receive commissions. The agreement expires on December 31, 2011 and may be terminated by either party without cause and without penalty upon 120 days prior written notice. The agreement may also be terminated by either party without cause upon less than 120 days prior written notice; however, financial penalties will apply. Unlike the prior contract between MWI and Merial, the Agreement does not prohibit MWI from representing products that compete with certain of Merial’s products, particularly those which are used for the treatment and/or control and/or prevention of fleas, ticks or heartworms.
Animal health product vendors may implement sales promotions or price changes for products distributed to veterinarians that can affect the period in which we recognize revenues. In addition, at the time we negotiate vendor agreements for the upcoming year, our vendors typically establish sales growth goals for us to meet in order to receive performance rebates or may reduce or eliminate rebate opportunities. These growth goals may be based on quarterly, semi-annual or annual targets.
Product returns from our customers and to our vendors occur in the ordinary course of business. We extend our customers the same return of goods policies as are extended to us by our vendors. We do not believe that our operations will be adversely impacted due to the return of products.
|
|
Distribution
|
As of September 30, 2010, we distributed our products from twelve strategically located distribution centers throughout the United States and one in the United Kingdom. We are currently in the process of moving our distribution center in Visalia, California to a larger facility. We expect to have the move completed in December 2010. During our fiscal year 2009, we moved our distribution center from Holland, Michigan to a larger facility in Whitestown, Indiana. We also moved our distribution center in Grand Prairie, Texas to a larger facility, and consolidated our distribution center in San Antonio, Texas into our new Grand Prairie, Texas distribution center. During our fiscal year 2008, we opened our distribution center located in Edwardsville, Kansas.
We maintain inventory levels in our warehouses appropriate to satisfy customer demand for prompt delivery and fulfillment of their product orders. Inventory levels are managed on a daily basis through our information systems. In order to meet the rapid delivery requirements of our customers, we offer next-day delivery service on most of the products we stock in our warehouses. We estimate that as of September 30, 2010, we shipped same day from our warehouses approximately 98% of the dollar value of orders placed by our customers. We currently ship the majority of our orders in the United States through United Parcel Service, Inc. (“UPS”) with the balance of our orders being shipped by our own delivery trucks, regional carriers and other national carriers.
|
|
Competition
|
The distribution and manufacture of animal health products is highly competitive. We compete with numerous vendors and distributors based on customer relationships, service and delivery, product selection, price and e-commerce capabilities. Most of our products are available from several sources, including other distributors and vendors, and our customers tend to have relationships with several distributors. In addition, our competitors could obtain exclusive rights to distribute certain products, eliminating our ability to distribute those products. Consolidation in the veterinary distribution business could result in existing competitors increasing their market share, which could give them greater pricing power, decrease our revenues and profitability, and increase the competition for customers. Our primary competitors in the United States, excluding vendors, include the following:
|
|
·
|
Animal Health International, Inc. (formerly Walco International, Inc.);
|
|
|
·
|
Butler Schein Animal Health;
|
|
|
·
|
Lextron Animal Health, Inc.;
|
|
|
·
|
Webster Veterinary Supply, a division of Patterson Companies, Inc.; and
|
|
|
·
|
other national, regional, local and specialty distributors.
|
The role of the animal health product distributor has changed dramatically during the last decade. Successful distributors are increasingly providing value-added services in addition to the products they traditionally provided. We believe that to
9
remain competitive we must continue to add value to the distribution channel, while removing unnecessary costs associated with product movement.
Distribution of animal health products is characterized by either “ethical” or “over-the counter,” commonly referred to as OTC, channels of product movement. Ethical distribution is defined as those sales of goods to licensed veterinarians for use in their professional practice. Many of these products are prescription and must be sold or prescribed by a licensed professional. OTC distribution is the movement of goods to the animal owner and the end-user. Many of these products also are purchased by licensed veterinarians for professional use or for resale to their client. There are numerous ethical and OTC distribution companies operating throughout the United States and competition in the veterinary distribution industry is intense.
|
|
Seasonality in Operating Results
|
Our quarterly sales and operating results have varied significantly in the past, and will likely continue to do so in the future. Historically, our total revenues have typically been higher during the spring and fall months due to increased sales of production animal products. Product use cycles for production animal products are directly related to medical procedures performed by veterinarians on production animals during the spring and fall months. These buying patterns can also be affected by vendors’ and distributors’ marketing programs or price increase announcements, which can cause veterinarians to purchase production animal health products earlier than those products are needed. This kind of early purchasing may reduce our sales in the months these purchases would have otherwise been made. See “Risk Factors — Our quarterly operating results may fluctuate significantly, and these fluctuations may cause our stock price to fall.” Additionally, while we accrue rebates as they are earned, our rebates have historically been highest during the quarter ended December 31, since some of our vendors’ rebate programs were designed to include targets to be achieved near the end of the calendar year. This trend has slowed some in recent years due to certain changes in vendor contracts but it still remains our largest quarter for vendor rebates.
|
|
Trademarks
|
We have registered with the United States Patent and Trademark Office the marks “MWI,” “MWI Design,” “MWIVET.com,” “VETONE,” “PROXYRx” and “ANIMAL Rx PHARMACY.” We believe that the MWI mark is well recognized in the animal health products industry and by veterinarians and is therefore a valuable asset of ours.
|
|
Employees
|
As of September 30, 2010, we had 971 employees across the United States and 208 employees in the United Kingdom. We have not experienced a shortage of qualified personnel in the past, and believe that we will be able to attract such employees in the future. None of our employees are a party to a collective bargaining agreement, and we consider our relations with our employees to be good.
|
|
Website
|
Our website address is www.mwivet.com and can be used to access free of charge, through the investor relations category, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports, as soon as reasonably practicable after we electronically file such material with or furnish it to the SEC. The information on our website is not incorporated as a part of this annual report. The public can also obtain copies of these reports by visiting the SEC’s Public Reference Room at 100 F Street NE, Washington DC 20549, or by calling the SEC at 1-800-SEC-0330, or by accessing the SEC’s website at www.sec.gov.
|
|
Governmental Regulation
|
Certain of our businesses involve the distribution of pharmaceuticals, and in this regard we are subject to various local, state, federal and foreign governmental laws and regulations applicable to the distribution of pharmaceuticals. Our vendors of pharmaceuticals, vaccines, parasiticides and certain controlled substances are typically regulated by federal agencies, such as the Food and Drug Administration (“FDA”), the United States Department of Agriculture (“USDA”), the Environmental Protection Agency (“EPA”), and the Drug Enforcement Agency (“DEA”), as well as most similar state agencies. Therefore, we are subject, either directly or indirectly, to regulation by the same agencies. Most states and the DEA require us to be registered or otherwise keep a current permit or license to handle controlled substances. Manufacturers of vaccines are required by the USDA to comply with various storage and shipping criteria and requirements for vaccines. To the extent we distribute such products, we must comply with the same requirements, including, without limitation, the storage and shipping requirements for vaccines.
Most state boards of pharmacy require us to be licensed in their respective states for the sale of pharmaceutical products and medical devices within their jurisdictions. As a distributor of prescription pharmaceutical products, we are subject to the
10
Prescription Drug Marketing Act (“PDMA”). The PDMA provides governance and authority to the states to provide minimum standards, terms and conditions for the licensing by state licensing authorities of persons who “engage” in wholesale distribution (as defined by each state regulatory agency) in interstate commerce of prescription drugs. With this authority, states require site-specific registrations for the parties that engage in the selling and/or physical distribution of pharmaceutical products into their state in the form of out-of-state registrations. The federal pedigree regulations of the FDA under the PDMA require tracking human labeled prescription products through the entire distribution chain and are applicable to distributors that do not have a written agreement with the manufacturer granting the wholesale distributor status as an “Authorized Distributor of Record.” Selling and/or distributing products without the appropriate registrations may subject us to fines, penalties, misdemeanor or felony convictions, and/or seizure of the products involved. We have a Manager of Regulatory Compliance and have engaged outside consultants as needed to assist us in meeting and complying with the various state and federal licensure requirements to which we are subject.
In order to supply pharmaceutical products in the United Kingdom, we must comply with the requirements of the applicable regulatory bodies. The Veterinary Medicines Directorate regulates the use of veterinary products and the Medicines Healthcare Products Regulatory Authority regulates the use of human medicinal products within the United Kingdom.
Our pet cremation business is subject to state and local zoning laws, and we are required to maintain permits for the construction and operation of an animal incineration device. We are also required to have an air pollution permit in connection with the operation of our pet cremation business.
Some states (as well as certain cities and counties) require us to collect sales taxes/use taxes on certain types of animal products. We are also subject to laws governing our relationship with employees, including minimum wage requirements, overtime, working conditions and citizenship requirements. In addition, we are subject to additional regulations regarding our hiring practices because several federal, state and local governmental agencies are our customers.
|
|
Environmental Considerations
|
We do not currently manufacture or alter in any way the composition of the vast majority of the products that we distribute. All products are distributed in compliance with the relevant rules and regulations as approved by various state and federal agencies.
|
|
Executive Officers
|
The following table sets forth the names, ages and titles, as well as a brief account of the business experience, of each person who was an executive officer of the Company as of September 30, 2010:
|
Name
|
Age
|
Title
|
||||
|
James F. Cleary, Jr.
|
47
|
Board Director, President and Chief Executive Officer
|
||||
|
Mary Patricia B. Thompson
|
47
|
Senior Vice President of Finance and Administration, Chief Financial Officer
|
||||
|
James W. Culpepper
|
56
|
Vice President of Inventory Management
|
||||
|
Jeffrey J. Danielson
|
50
|
Vice President of Sales
|
||||
|
John J. Francis
|
57
|
Vice President and General Manager of the Specialty Resources Group
|
||||
|
James S. Hay
|
67
|
Vice President and Chief Information Officer
|
||||
|
Bryan P. Mooney
|
42
|
Vice President of Operations
|
||||
|
John R. Ryan
|
41
|
Vice President of Marketing
|
||||
James F. Cleary, Jr. has served as President since March 2000 and Chief Executive Officer since June 2002. Mr. Cleary has also been a member of the board of directors since June 2002. He joined MWI in January 1998 as Director of National Accounts and was promoted to Vice President of Demand Generation in 1999. Mr. Cleary was Vice President of Agri Beef Co., MWI’s former parent, from 1996 to 1998. From 1990 to 1996, Mr. Cleary was employed in management positions with Morrison Knudsen Corporation and its affiliate MK Rail Corporation. Mr. Cleary graduated from Dartmouth College in 1985 with a Bachelor of Arts in Economics and received his Masters of Business Administration from Harvard Business School in 1990. Mr. Cleary is also a member of the board of directors of Seroyal Holdings, L.P., and a manager of Feeders’ Advantage, L.L.C. Mr. Cleary is the brother-in-law of Mr. Robert Rebholtz, a member of our board directors.
Mary Patricia B. Thompson, CPA has served as Senior Vice President of Finance and Administration, Chief Financial Officer since August 2006, with oversight of finance, regulatory compliance, inventory management, information technology, and human resources. Prior to August 2006, Ms. Thompson had been the Vice President, Secretary and Chief Financial Officer since June 2002. Ms. Thompson joined Agri Beef Co. in 1989 as the Feedlot and Commodity Division Controller. In September 1991, Ms. Thompson was promoted to Controller of MWI Veterinary Supply Co., then a wholly-owned subsidiary of Agri Beef Co. Prior to joining Agri Beef Co., Ms. Thompson worked for Arthur Andersen LLP from 1985 to 1989, where she provided auditing and accounting services. Ms. Thompson graduated from the University of Idaho in 1985, summa cum laude, with a Bachelor of Science in Accounting. Ms. Thompson is a licensed Certified Public Accountant in the state of Idaho. Ms. Thompson is also a member of the board of directors and president of the American Veterinary Distributors Association.
James W. Culpepper has served as Vice President of Inventory Management since 1998. Mr. Culpepper joined MWI in 1997 as General Merchandise Manager. Prior to joining MWI, Mr. Culpepper worked for Payless Drug Stores for 20 years. During that period, Mr. Culpepper held various positions, including pharmacist, merchandiser and buyer of pharmaceutical supplies, and Inventory Control Manager. Mr. Culpepper graduated from Oregon State University’s School of Pharmacy in 1977.
Jeffrey J. Danielson has served as Vice President of Sales since 2001. Mr. Danielson joined MWI in 1985 as an Outside Sales Representative, serving the state of Washington. From 1989 to 1991, Mr. Danielson served as Assistant Sales Manager and from 1991 to 2001 served as National Sales Manager. Mr. Danielson graduated from Colorado State University in 1983 with a Bachelor of Science in Agricultural Business.
John J. Francis has served as Vice President and General Manager of the Specialty Resources Group since September 2006. Prior to joining MWI, Mr. Francis worked for Webster Veterinary Supply as the Vice President of Sales from April 2004 to September 2006. Mr. Francis was the Key Account Manager of Excel, a division of Cargill, Inc., from June 2002 to April 2004 and was the Vice President of Sales for Future Beef based in Parker, Colorado, from May 2000 to May 2002. From 1990 until 2000, Mr. Francis served as General Manager and then President of MWI Veterinary Supply Co. Mr. Francis graduated from Michigan State University in 1975 with a Bachelor of Science in Animal Husbandry and holds a Master of Science in Animal Science obtained in 1977 from the University of Illinois. Mr. Francis is a member of the board of directors and secretary of Vedco, Inc.
James S. Hay has served as Vice President and Chief Information Officer since September 2002. Mr. Hay joined Agri Beef Co. in 1996 as Vice President of Information Systems. Prior to joining Agri Beef Co., Mr. Hay served as Director of Management Information Systems at Natural Wonders Inc. from 1991 to 1995; Director of Management Information Systems at the Oakland Tribune from 1989 to 1991; Vice President and Chief Information Officer of Liquor Barn, Inc. from 1987 to 1989; Director of Information Services at Genstar Corporation from 1974 to 1987; management consultant at Price Waterhouse from 1968 to 1974; and as a systems engineer at IBM Corporation from 1965 to 1968. Mr. Hay graduated from the University of Manitoba in 1965 with a Bachelor of Science in Mathematics and Physics.
Bryan P. Mooney has served as Vice President of Operations since May 2005. Mr. Mooney joined MWI in January 1994 as the Operations Manager of our Denver, Colorado distribution operation and served in that capacity until May 1998. From May 1998 until February 2005, Mr. Mooney served as Manager of Transportation and Logistics and from January 2005 until May 2005 as the Western Regional Operations Manager. Mr. Mooney graduated from the University of Wyoming in 1991 with a Bachelor of Science in Agricultural Business.
John R. Ryan has served as Vice President of Marketing since 2000. Mr. Ryan joined MWI in June 1995 as an Outside Sales Representative and served in such capacity until June 2000. Prior to joining MWI, Mr. Ryan worked for the Virbac Corporation (a companion animal pharmaceutical company) as a Territory Manager from 1993 to 1995. Mr. Ryan graduated from the University of California, Davis in 1993 with a Bachelor of Science in Animal Physiology.
|
Item 1A.
|
Risk Factors.
|
In addition to the factors discussed elsewhere in this Form 10-K, the following are important factors which could cause actual results or events to differ materially from those contained in any forward-looking statements made by or on behalf of the Company.
|
|
Our operating results may fluctuate due to factors outside of management’s control.
|
Our future revenues and results of operations may significantly fluctuate due to a combination of factors, many of which are outside of management’s control. The most notable of these factors include:
|
·
|
the impact of vendor consolidation on our business;
|
|
·
|
changes in or availability of vendor contracts or rebate programs;
|
|
·
|
vendor rebates based upon attaining certain growth goals;
|
|
·
|
changes in the way vendors introduce/deliver products to market;
|
|
·
|
exclusivity requirements with certain vendors that may prohibit us from distributing competing products manufactured by other vendors;
|
|
·
|
risks associated with our international operations;
|
|
·
|
transitional challenges associated with acquisitions, including the failure to achieve anticipated synergies;
|
|
·
|
financial risks associated with acquisitions;
|
|
·
|
the impact of general economic trends on our business;
|
|
·
|
the recall of a significant product by one of our vendors;
|
|
·
|
extended shortage or backorder of a significant product by one of our vendors;
|
|
·
|
seasonality;
|
|
·
|
the timing and effectiveness of marketing programs or price changes offered by our vendors;
|
|
·
|
the timing of the introduction of new products and services by our vendors;
|
|
·
|
the ability to borrow on our revolving credit facility, extend the terms of our revolving credit facility or obtain alternative financing on favorable terms or at all;
|
|
·
|
risks from potential increases in variable interest rates;
|
|
·
|
the impact of tightening credit standards and/or access to credit on behalf of our customers and suppliers;
|
|
·
|
unforeseen litigation;
|
|
·
|
a disruption caused by adverse weather or other natural conditions;
|
|
·
|
inability to ship products to the customer as a result of technological or shipping disruptions; and
|
|
·
|
competition.
|
These factors could adversely impact our results of operations and financial condition. We may be unable to reduce operating expenses quickly enough to offset any unexpected shortfall in revenues or gross profit. If we have a shortfall in revenues or gross profit without a corresponding reduction to expenses, operating results may suffer. Our operating results for any particular fiscal year or quarter may not be indicative of future operating results. You should not rely on year-to-year or quarter-to-quarter comparisons of results of operations as an indication of our future performance.
Consolidation among our vendors could be harmful to our business.
On November 3, 2009, Merck and Schering-Plough completed their merger under which Merck acquired all of the outstanding stock of Schering-Plough. Merial was a joint venture between Merck and Sanofi-Aventis, and Sanofi-Aventis acquired Merck’s interest in Merial shortly before Merck and Schering-Plough completed their merger. On March 9, 2010, Sanofi-Aventis and Merck announced that Sanofi-Aventis exercised its option to combine Merial with Intervet/Schering-Plough, Merck’s animal health business, to form an animal health joint venture that would be equally owned by the new Merck and Sanofi-Aventis. The completion of the transaction is expected to occur in the next twelve months. Merial and
13
Intervet-Schering are also two of our largest vendors. We have contracts in place with both of these vendors that will remain in place through the end of calendar year 2010. We are currently negotiating new contracts with each of these companies for calendar year 2011.
The surviving companies from this transaction will have high market shares with respect to certain animal health products, and they could use their increased leverage in the channel to negotiate terms with distributors that are materially worse to the distributor than the terms that we have been able to negotiate with Merial and Intervet-Schering individually while they were competing with each other. There also remains uncertainty related to any changes to the terms that may be included in the vendor contracts we negotiate for the upcoming year as a result of this transaction. There is also a possibility of product disruption as these companies integrate their operations which could adversely impact our financial results. Further consolidation among animal health products vendors could result in our vendors further increasing their market share, which could give vendors greater pricing power and make it easier for such vendors to sell their products directly to animal health customers, both of which could decrease our net sales and profitability.
Our business, financial condition and results of operations depend upon maintaining our relationships with vendors.
We currently do not manufacture the vast majority of our products and are dependent on our vendors for our supply of products. Our ten largest vendors supplied products that accounted for approximately 71%, 74% and 72% of our revenues for the fiscal years ended September 30, 2010, 2009 and 2008, respectively. Pfizer supplied products that accounted for approximately 25%, 24% and 23% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Of the Pfizer supplied products, production animal products under a livestock agreement accounted for approximately 12%, 14% and 17% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Intervet-Schering, a subsidiary of Schering Plough, supplied products that accounted for approximately 10%, 11% and 9% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Boehringer supplied products that accounted for approximately 10%, 4% and 3% of our revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Merial, a subsidiary of Sanofi-Aventis, supplies the majority of their products under an agency relationship. Commission revenue generated from Merial products accounted for approximately 49%, 56% and 60% of total commission revenues for our fiscal years ended September 30, 2010, 2009 and 2008, respectively.
Our ability to sustain our gross profits has been, and will continue to be, dependent in part upon our ability to obtain favorable terms and access to new and existing products from our vendors. These terms may be subject to changes from time to time by vendors, such as changing from a “buy/sell” to an agency relationship, or from an agency to a “buy/sell” relationship which could adversely affect our revenues and operating income. The loss of one or more of our large vendors, a material reduction in their supply of products to us or material changes in the terms we obtain from them could have a material adverse effect on our business, financial condition and results of operations.
Vendors may also choose to change the method in which products are taken to market. For example, a vendor may change our relationship from a complete distribution provider, including logistics and sales support, to only a logistics provider or only a sales support provider. Only doing one of these services would reduce our margin on any sale. A change in the method in which products are taken to market caused by any vendor could have a material adverse effect on our business.
Some of our current and future vendors may decide to compete with us in the future by pursuing or increasing their efforts in direct marketing and sales of their products. These vendors could sell their products at lower prices and maintain a higher gross margin on their product sales than we can. In this event, veterinarians or animal owners may elect to purchase animal health products directly from these vendors. Increased competition from any vendor of animal health products could significantly reduce our market share and adversely impact our financial results.
An adverse change in vendor rebates could negatively affect our business.
The terms on which we purchase or sell products from many vendors of animal health products may entitle us to receive a rebate based on the attainment of certain growth goals. Vendors may adversely change the terms of or eliminate some or all of these rebate programs at any time without notice. Because the amount of rebates we earn is directly related to the attainment of pre-determined sales growth goals, and because the nature of the rebate programs and the amount of rebates available are determined by the vendors, there can be no assurance as to the amount of rebates that we will earn in any given year. Changes to or elimination of any rebate program initiated by our vendors may have a material effect on our gross profit and our operating results in any given quarter or year. Vendors may reduce or eliminate rebates offered under their programs, interpret the terms of their rebate programs in a way that is adverse to us or increase the growth goals or other conditions we must meet to earn rebates to levels that we cannot achieve. Additionally, factors outside of our control, such as customer preferences or vendor supply issues, can have a material impact on our ability to achieve the growth goals established by our vendors, which may reduce the amount of rebates we receive. The occurrence of any of these events could have an adverse
Our quarterly operating results may fluctuate significantly, and these fluctuations may cause our stock price to fall.
Our quarterly revenues and operating results have varied significantly in the past, and may continue to do so in the future. While we accrue rebates from vendors as they are earned, our rebates have historically been highest during the quarter ended December 31, since most of our vendors’ rebate programs were designed to include targets to be achieved during the calendar year. Historically, our revenues have been seasonal, with peak sales in the spring and fall months. The seasonal nature of our business is directly tied to the buying patterns of veterinarians for production animal health products used for certain medical procedures performed on production animals during the spring and fall months. These buying patterns can also be affected by vendors’ and distributors’ marketing programs or price increase announcements, which can cause veterinarians to purchase production animal health products earlier than those products are used. This kind of early purchasing may reduce our sales in the months these purchases would have otherwise been made. Additionally, the production animal market can be affected by volatility in commodity prices such as milk, corn, grain and feeder cattle, changes in weather patterns that allow cattle to graze for longer periods or a change in the general economy, which can shift the timing of when animals are treated and to what extent they are treated. This shift in treatment may have a significant impact on the timing of purchases. While companion animal products tend to have a different product use cycle than production animal health products, and approximately two-thirds of our revenues have been generated from sales to the companion animal market, we cannot assure you that our revenues and operating results will not continue to fluctuate on a quarter-to-quarter basis. We believe period-to-period comparisons of our results of operations are not necessarily meaningful as our future revenue and results of operations may vary substantially. It is also possible that in future quarters our results of operations will be below the expectations of securities analysts and investors. In either case, the price of our common stock could decline, possibly materially.
Our market is highly competitive. Failure to compete successfully could have a material adverse effect on our business, financial condition and results of operations.
The market for veterinary distribution services is highly competitive, continually evolving and subject to technological change. We compete with numerous vendors and distributors based on customer relationships, service and delivery, product selection, price and e-commerce capabilities. Our primary competitors, excluding vendors, include the following:
|
|
·
|
Animal Health International, Inc. (formerly Walco International, Inc.);
|
|
|
·
|
Butler Schein Animal Health;
|
|
|
·
|
Lextron Animal Health, Inc.;
|
|
|
·
|
Webster Veterinary Supply, a division of Patterson Companies, Inc.; and
|
|
|
·
|
other national, regional, local and specialty distributors.
|
Some of our competitors may have more customers, stronger brand recognition or greater financial and other resources than we do. Most of our products are available from several sources, including other distributors and vendors, and our customers typically have relationships with several distributors and vendors. Many of our competitors have comparable product lines, technical expertise or distribution strategies that directly compete with us. Our competitors could obtain exclusive rights to distribute certain products, eliminating our ability to distribute those products. The entry of new or additional distributors in the industry could also have a material adverse effect on our ability to compete. Additionally, some of our vendors may decide to compete with us by selling their products directly to our customers. If we do not compete successfully against these organizations, it could have a material and adverse effect on our business, financial condition and results of operations.
Consolidation in the veterinary distribution business and veterinary practices may decrease our revenues and profitability.
Consolidation in the veterinary distribution business could result in existing competitors increasing their market share, which could give them greater pricing power, decrease our revenues and profitability, and increase the competition for customers. Consolidation of the many small, privately-held veterinary practices would result in an increasing number of larger veterinary practices, which could have increased purchasing leverage and the ability to negotiate lower product costs. This could reduce our operating margins and negatively impact our revenues and profitability. Any of these developments could result in increased marketing expenses and have a material adverse effect on our business, financial condition and results of operations.
Exclusivity requirements and failures to continue relationships with vendors may cause us to lose access to certain products and erode our market share.
We may not be able to establish or maintain relationships with key vendors in the animal health industry if we have established relationships with competitors of these key vendors. We have written agreements with approximately 40 of our vendors. Some of our agreements with vendors are for one-year periods. Upon expiration, we may not be able to renew our existing agreements on favorable terms, or at all.
In addition, vendors may require us to distribute their products on an exclusive basis, which would cause us to forego distributing competing products that may also be profitable, including generic products that may gain increased market share. In this situation we are often forced to project future sales of competing products so that we can elect to distribute the product that we believe will be more profitable. Our projections may not be correct, and we may be contractually prohibited from distributing products that gain market share, including generic products, at the expense of the products that we distribute. Competitors of ours could also obtain exclusive rights to market particular products, which we would be unable to market. If we lose the right to distribute products under such agreements, or are required to exclusively distribute certain products at the expense of others that may be more profitable, we may lose access to certain products and lose a competitive advantage. Potential competitors could sell products from vendors that we fail to continue with and erode our market share.
Risks associated with our international operations.
On February 8, 2010, we acquired the outstanding share capital of Centaur. Centaur is based in the United Kingdom and uses the British pound as its functional currency. Prior to this acquisition, we had very limited exposure to international risks. International operations are subject to risks that may materially adversely affect our business, results of operations and financial condition. The risks that our international operations are subject to include, among other things:
|
·
|
difficulties and costs relating to staffing and managing foreign operations;
|
|
·
|
difficulties in establishing channels of distribution;
|
|
·
|
fluctuations in the value of foreign currencies;
|
|
·
|
repatriation of cash from our foreign operations to the United States;
|
|
·
|
regulatory requirements;
|
|
·
|
foreign countries may impose additional withholding taxes or otherwise tax our foreign income;
|
|
·
|
separate operating and financial systems;
|
|
·
|
disaster recovery;
|
|
·
|
unexpected difficulties in importing or exporting our products;
|
|
·
|
imposition of import/export duties, quotas, sanctions or penalties;
|
|
·
|
liability related to the defined benefit plan; and
|
|
·
|
unexpected regulatory, economic and political changes in foreign markets.
|
Our financial results could be adversely affected by foreign exchange fluctuations.
We operate in both the United States and the United Kingdom, but report revenues, costs and earnings in U.S. dollars. Exchange rates between the U.S. dollar and the British pound sterling are likely to fluctuate from period to period. Because our financial results are reported in U.S. dollars, we are subject to the risk of translation losses for reporting purposes. If we continue to expand our international operations, we will conduct more transactions in currencies other than the U.S. Dollar. To the extent that foreign revenue and expense transactions are not denominated in the local currency, we are also subject to the risk of transaction losses. Given the volatility of exchange rates, there is no assurance that we will be able to effectively manage currency transaction and/or translation risks. We have not entered into derivative instruments to offset the impact of foreign exchange fluctuations. Fluctuations in foreign currency exchange rates could have a material adverse affect on our results of operations and financial condition.
We only have one distribution center in the United Kingdom and any catastrophic event could materially impact our results.
We conduct all of our fulfillment operations in the United Kingdom from our distribution center in Castle Cary, England. This facility contains all of our product inventory. A natural disaster or other catastrophic event, such as an
16
earthquake, fire, flood, severe storm, break-in, server or systems failure, terrorist attack, or other comparable event at this facility would cause interruptions or delays in our business and loss of inventory and could render us unable to process or fulfill customer orders in a timely manner, or at all. Further, we have a limited disaster recovery plan, and our business interruption insurance may not adequately compensate us for losses that may occur, including with respect to any lost profits for any period in which the business is not able to operate. In the event that a significant part of this facility was destroyed or our operations were interrupted for any extended period of time, our business, financial condition, and operating results would be harmed.
An economic downturn could materially adversely affect our business.
Our business may be materially adversely affected by negative trends in the general economy that could reduce consumer discretionary spending on animal health products and reduce or eliminate sources of credit available to our customers. Levels of consumer spending have recently deteriorated significantly in the United States and may remain depressed for the foreseeable future. Some of the factors that could influence the levels of consumer spending include continuing increases in fuel and other energy costs, conditions in the residential real estate and mortgage markets, unemployment levels, healthcare costs, access to credit, consumer confidence and other macroeconomic factors affecting consumer spending behavior. In addition, volatility in commodity prices such as milk, corn, grain and feeder cattle, changes in weather patterns causing cattle to graze for longer periods and changes in the general economy may adversely impact the amount spent by animal owners in the production animal market. Such volatility and/or tightening of credit available to our customers could further deteriorate the financial condition of our customers, and may ultimately lead our customers to reduce their working capital available to purchase our products.
Our business ultimately depends on the ability and willingness of animal owners to pay for our products. This dependence could make us more vulnerable to any reduction in consumer confidence or disposable income than companies in other industries that are less reliant on consumer spending, such as the human health care industry, in which a large portion of payments are made by insurance programs. Additionally, any cost-cutting measures taken to offset the effects of an economic downturn could materially impact our ability to generate future revenue growth. For all of these reasons, an economic downturn could materially affect our business.
Any inability of our customers to pay us for our products and services due to their deteriorating financial condition or otherwise may adversely affect our results of operations and financial condition.
We generally extend some level of credit to our customers without requiring collateral, which exposes us to credit risk. For example, milk price declines in the dairy market could have a significant impact on dairy farmers, which would create cash-flow challenges for these farmers and, in turn, impact the time it takes for us to collect on outstanding accounts receivable from these customers. If customers’ cash flow or operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain other sources of credit, they may not be able to pay or may delay payment to us, or in some cases may return products to us. The financial difficulties of a customer could cause us to curtail business with that customer or the customer to reduce its business with us and cancel orders. Any inability of current and/or potential customers to pay us for our products and/or services due to their deteriorating financial condition or otherwise may adversely affect our results of operations and financial condition.
We rely substantially on third-party vendors, and the loss of products or delays in product availability from one or more third-party vendors could substantially harm our business.
We must contract for the supply of current and future products of appropriate quantity, quality and cost. These products must be available on a timely basis and be in compliance with any regulatory requirements. Failure to do so could substantially harm our business.
We often purchase products from our vendors under agreements that typically have a term of one year and can be terminated on a periodic basis. There can be no assurance, however, that our vendors will be able to meet their obligations under these agreements or that we will be able to compel them to do so. Risks of relying on vendors include:
|
·
|
If an existing agreement expires or a certain product line is discontinued or recalled, then we would not be able to continue to offer our customers the same breadth of products and our sales and operating results would likely suffer unless we are able to find an alternate supply of a similar product.
|
|
·
|
If market demand for our products increases suddenly, our current vendors might not be able to fulfill our commercial needs, which would require us to seek new manufacturing arrangements or find new sources of supply, and may result in substantial delays in meeting market demand. If we consistently generate more demand for a product than a given vendor is capable of handling, it could lead to large backorders and potentially lost sales to competitive products that are more readily available.
|
|
·
|
We may not be able to control or adequately monitor the quality of products we receive from our vendors. Poor quality products could damage our reputation with our customers.
|
|
·
|
Some of our third party vendors are subject to ongoing periodic unannounced inspection by regulatory authorities, including the FDA, the USDA, the EPA, the DEA and the PDMA, as well as other federal and state agencies for compliance with strictly enforced regulations. We do not have control over our vendors’ compliance with these regulations and standards. Violations could potentially lead to interruptions in supply that could lead to lost sales to competitive products that are more readily available.
|
|
·
|
If a vendor is unable to obtain the necessary credit to manage their business, they may not be able to deliver their products to us.
|
Potential problems with vendors such as those discussed above could substantially decrease sales of our products, lead to higher costs and damage our reputation with our customers.
We rely upon third parties to ship products to our customers and interruptions in their operations could harm our business, financial condition and results of operations.
We use UPS as our primary delivery service for our air and ground domestic shipments of products to our customers. If there were any significant service interruptions, there can be no assurance that we could engage alternative service providers to deliver these products in either a timely or cost-efficient manner, particularly in rural areas where many of our customers are located. Any labor disputes, slowdowns, transportation disruptions or other adverse conditions in the transportation industry experienced by UPS could impair or disrupt our ability to deliver our products to our customers on a timely basis, and could have a material adverse effect upon our customer relationships, business, financial condition and results of operations. In addition, rising fuel costs may result in continued increases in shipping costs charged by UPS, or other delivery service providers, and could have an adverse effect on our financial condition and results of operations.
The loss of one or more significant customers could adversely affect our profitability.
Our two largest customers, Banfield and Feeders’ Advantage (a related party), accounted for approximately 9% and 4%, respectively, of our product sales for our fiscal year ended September 30, 2010, 11% and 5%, respectively, of our product sales for our fiscal years ended September 30, 2009 and 10% and 5%, respectively, of our product sales for our fiscal years ended September 30, 2008. Our ten largest customers, excluding Banfield and Feeders’ Advantage, accounted for approximately 5%, 5% and 4% of our product sales for our fiscal years ended September 30, 2010, 2009 and 2008, respectively. Our business and results of operations could be adversely affected if the business of these customers was lost. We cannot guarantee that we will maintain or improve our relationships with these customers or that we will continue to supply these customers at current levels. Banfield, Feeders’ Advantage and other customers may seek to purchase some of the products that we currently sell directly from the vendors or from one or more of our competitors. Furthermore, our customers are not required to purchase any minimum amount of products from us. The loss of Banfield or Feeders’ Advantage or deterioration in our relations with either of them could significantly affect our financial condition and results of operations. Additionally, deterioration in the financial condition of one or more of our customers could have a material adverse effect on our results of operations.
Difficulties with the integration of acquisitions or the improvement of the performance of acquired companies may impose substantial costs and delays and cause other unanticipated problems for us.
Acquisitions involve a number of risks relating to our ability to integrate an acquired business into our existing operations. The process of integrating or improving the performance of the operations of an acquired business, particularly its personnel, could cause interruptions to our business. Some of the risks we face include:
|
|
·
|
the need to spend substantial operational, financial and management resources in integrating new businesses, technologies and products, and difficulties maagement may encounter in integrating or improving the performance of the operations, personnel or systems of the acquired business;
|
|
|
·
|
retention of key personnel, customers and vendors of the acquired business;
|
|
|
·
|
the occurrence of a material adverse effect on our existing business relationships with customers or vendors, or both, resulting from future acquisitions or business combinations could lead to a termination of or otherwise affect our relationships with such customers or vendors;
|
|
|
·
|
impairments of goodwill and other intangible assets; and
|
|
|
·
|
contingent and latent risks associated with the past operations of, and other unanticipated costs and problems arising in, an acquired business.
|
If we are unable to successfully integrate the operations of an acquired business into our operations, we could be required to undertake unanticipated changes. These changes could have a material adverse effect on our business.
In addition, it may be difficult to manage rapid growth from our acquired companies in the future, and the future success of our acquisitions depends on our ability to implement and/or maintain:
|
|
·
|
sales and marketing programs;
|
|
|
·
|
customer service levels;
|
|
|
·
|
current and new product and service lines and vendor relationships;
|
|
|
·
|
technological support which equals or exceeds our competitors;
|
|
|
·
|
recruitment and training of new personnel; and
|
|
|
·
|
operational and financial control systems.
|
If we are not able to manage our rapid growth from our acquisitions, there is a risk our customer service quality could deteriorate which may in turn lead to decreased sales or profitability. Also, due to acquisitions the cost of our operations could increase faster than growth in our revenues, negatively impacting our profitability.
|
Increases in over-the-counter sales of animal health products could adversely affect our business.
|
We rely, and will continue to rely, on animal owners who purchase their animal health products directly from veterinarians, which we refer to as the ethical channel. There can be no assurance that animal owners will continue to use the ethical channel with the same frequency as they have in the past, and will not increasingly purchase animal health products from sources other than veterinarians, such as the Internet and other over-the-counter channels. Increased competition from any distributor of animal health products making use of an over-the-counter channel could significantly reduce our market share and adversely impact our financial results.
If we fail to comply with or become subject to more onerous government regulations, our business could be adversely affected.
The veterinary distribution industry is subject to changing political and regulatory influences. Both state and federal government agencies regulate the distribution of certain animal health products and we are subject to regulation, either directly or indirectly, by the FDA, the USDA, the EPA, the DEA and comparable state agencies. As a distributor of prescription pharmaceutical products, we are also subject to the PDMA, which provides for minimum standards, terms and conditions to be maintained for licensing as a distributor. The regulatory stance these agencies take could change. Our vendors are subject to regulation by the FDA, the USDA, the EPA, the DEA, and the PDMA, as well as other federal and state agencies, and material changes to the applicable regulations could affect our vendors’ ability to manufacture certain products, which could adversely impact our product supply. In addition, some of our customers may rely, in part, on farm and agricultural subsidy programs. Changes in the regulatory positions that impact the availability of funding for such programs could have an adverse impact on our customers’ financial positions, which could lead to decreased sales.
We strive to maintain compliance with these and all other applicable laws and regulations. We retain a Manager of Regulatory Compliance and have engaged outside consultants as needed to assist us in meeting and complying with the various state licensure requirements to which we are subject. If we are unable to maintain or achieve compliance with these laws and regulations, however, we could be subject to substantial fines or other restrictions on our ability to provide competitive distribution services, which could have an adverse impact on our financial condition.
We cannot assure you that existing laws and regulations will not be revised or that new, more restrictive laws will not be adopted or become applicable to us or the products that we distribute or dispense. The federal pedigree regulations of the FDA under the PDMA require tracking human labeled prescription products through the entire distribution chain and are enforceable for distributors that do not have a written agreement with the manufacturer granting the wholesale distributor status as an “Authorized Distributor of Record.” We cannot assure you that the vendors of products that may become subject to more stringent laws will not try to recover any or all increased costs of compliance from us by increasing the prices at which we purchase products from them, or, that we will be able to recover any such increased prices from our customers. We also cannot assure you that our business and financial condition will not be materially and adversely affected by future changes in applicable laws and regulations.
|
Loss of key management or sales representatives could harm our business.
|
Our future success depends to a significant extent on the skills, experience and efforts of management, including our President and Chief Executive Officer, Mr. James F. Cleary, Jr. While we have not experienced problems in the past attracting and maintaining members of our management team, the loss of any or all of these individuals could adversely impact our business. We do not carry key-man life insurance on any member of management. In addition we do not have employment agreements with key members of our senior management team. We must continue to develop and retain a core group of individuals if we are to realize our goal of continued expansion and growth. We cannot assure you that we will be able to do so in the future.
Also, due to the specialized nature of our products and services, generally only highly qualified and trained sales representatives have the necessary skills to market our products and provide our services. These individuals develop relationships with our customers that could be damaged if these employees are not retained. We face intense competition for the hiring of these professionals, and many professionals in the field that may otherwise be attractive candidates for us to hire may be bound by non-competition agreements with our competitors. Any failure on our part to hire, train and retain a sufficient number of qualified professionals would damage our business. We do not generally enter into agreements that contain non-competition provisions with our employees, other than with members of our senior management team, former owners of acquired companies and certain other employees.
|
Failure of, or security problems with, our information systems could damage our business.
|
Our information systems are dependent on third party software, global communications providers, telephone systems and other aspects of technology and Internet infrastructure that are susceptible to failure. Though we have implemented security measures and some redundant systems, our customer satisfaction and our business could be harmed if we or our vendors experience any system delays, failures, loss of data, power outages, computer viruses, break-ins, unauthorized access by competitors or similar disruptions. We currently process all customer transactions and data at our facilities in Meridian, Idaho. Although we have safeguards for emergencies, including, without limitation, sophisticated back-up systems, the occurrence of a major catastrophic event or other system failure at any of our distribution facilities could interrupt data processing or result in the loss of stored data. This may result in the loss of customers or a reduction in demand for our services. Only some of our systems are fully redundant and although we do carry business interruption insurance, it may not be sufficient to compensate us for losses that may occur as a result of system failures. If a disruption occurs, our profitability and results of operations may suffer.
The outbreak of an infectious disease within either the production animal or companion animal population could have a significant adverse effect on our business and our results of operations.
An outbreak of disease affecting animals, such as foot-and-mouth disease, various forms of influenza or bovine spongiform encephalopathy, commonly referred to as “mad cow disease,” could result in the widespread destruction of affected animals and consequently result in a reduction in demand for animal health products. In addition, outbreaks of these or other diseases or concerns of such diseases could create adverse publicity that may have a material adverse effect on consumer demand for meat, dairy and poultry products, and, as a result, on our customers’ demand for the products we distribute. The outbreak of a disease among the companion animal population which could cause a reduction in the demand for companion animals could also adversely affect our business. Although we have not been adversely impacted by the outbreak of a disease in the past, there can be no assurance that a future outbreak of an infectious disease will not have an adverse effect on our business.
|
We may be subject to product liability and other claims in the ordinary course of business.
|
Our business involves a risk of product liability and other claims in the ordinary course of business, for example arising from shipping mislabeled or outdated product, product recalls or disputes among competing vendors. We maintain general liability insurance with policy limits of $1.0 million per incident and $2.0 million in the aggregate, and in many cases we have indemnification rights against such claims from the manufacturers of the products we distribute. We do not maintain a separate product liability insurance policy because we do not currently manufacture the vast majority of the products that we sell. Our ability to recover under insurance or indemnification arrangements is subject to the financial viability of the insurers and manufacturers. We cannot assure you that our insurance coverage or the manufacturers’ indemnity will be available or sufficient in any future cases brought against us.
|
We may not be able to raise needed capital in the future on favorable terms or at all.
|
We expect that our existing sources of cash, together with any funds generated from operations, will be sufficient to meet our anticipated capital needs for at least the next twelve months. However, we may require additional capital to finance our growth strategies or other activities in the future. Our capital requirements will depend on many factors, including the
costs associated with our growth and expansion. Additional financing may not be available when needed and, if such financing is available, it may not be available on terms favorable to us. Our failure to raise capital when needed could have an adverse effect on our business, financial condition and results of operations.
Our variable rate indebtedness subjects us to interest rate risk, which could cause our debt service obligations to increase significantly.
Any borrowings on the revolving credit facility will be at variable rates of interest and expose us to interest rate risk based on market rates. If interest rates increase, our debt service obligations on any future variable rate indebtedness that we may incur on our revolving credit facility would increase even if the amount borrowed remained the same, and our net income and cash available for servicing our indebtedness would decrease. Our variable rate debt as of September 30, 2010 was $10.1 million (comprised of revolving credit facilities). Our interest expense for fiscal year 2010 was approximately $0.5 million and was approximately $0.2 million for fiscal year 2009. A 1% increase in the average interest rate would not have a material impact on our operations assuming our current level of debt. However, if we had to borrow additional funds to operate our business, the change in interest rates could affect our operations. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources.”
Our lenders may have suffered losses related to the weakening economy and may not be able to fund our borrowings.
Our lenders, including the lenders participating in our revolving credit facility, may have suffered losses related to their lending and other financial relationships, especially because of the general weakening of the national economy and increased financial instability of many borrowers. As a result, lenders may become insolvent or tighten their lending standards, which could make it more difficult for us to borrow under our revolving credit facility or to obtain other financing on favorable terms or at all. Our financial condition and results of operations would be adversely affected if we were unable to draw funds under our revolving credit facility because of a lender default or to obtain other cost-effective financing.
|
Item 1B.
|
Unresolved Staff Comments.
|
None.
|
Item 2.
|
The table below provides a summary of the Company’s principal facilities as of September 30, 2010:
|
Location
|
Total Square Feet
|
(1) |
Own or Lease
|
Principal Function
|
|||
|
Meridian, Idaho
|
34,000 | (2) |
Lease
|
Headquarters and call center
|
|||
|
Edwardsville, Kansas
|
105,000 |
Lease
|
Distribution center and pharmacy
|
||||
|
Castle Cary, England
|
84,000 |
Own
|
Centaur office, call center and distribution center
|
||||
|
Denver, Colorado
|
75,000 |
Lease
|
Distribution center and call center
|
||||
|
Grand Prairie, Texas
|
70,000 |
Lease
|
Distribution center, call center and pharmacy
|
||||
|
Visalia, California
|
52,000 | (3) |
Lease
|
Distribution center and veterinary food-animal drug retailer
|
|||
|
Atlanta, Georgia
|
41,000 |
Lease
|
Distribution center
|
||||
|
Harrisburg, Pennsylvania
|
40,000 |
Lease
|
Distribution center and pharmacy
|
||||
|
Whitestown, Indiana
|
40,000 |
Lease
|
Distribution center
|
||||
|
Nampa, Idaho
|
36,000 |
Lease
|
Distribution center and pharmacy
|
||||
|
Fife, Washington
|
30,000 |
Lease
|
Distribution center
|
||||
|
Orlando, Florida
|
30,000 |
Lease
|
Distribution center
|
||||
|
Clear Lake, Wisconsin
|
25,000 |
Lease
|
Distribution center and call center
|
||||
|
Glendale, Arizona
|
20,000 |
Lease
|
Distribution center
|
||||
|
Sturbridge, Massachusetts
|
15,000 |
Lease
|
Securos office and call center
|
||||
|
Letchworth, England
|
9,000 |
Lease
|
Centaur shipping depot
|
||||
|
Stoke, England
|
8,000 |
Lease
|
Centaur shipping depot
|
||||
|
Holland, Michigan
|
5,000 |
Lease
|
Call center
|
||||
|
Omaha, Nebraska
|
4,000 |
Lease
|
Call center
|
||||
|
Rochester Hills, Michigan
|
3,000 |
Lease
|
Call center
|
||||
|
Sioux City, Iowa
|
3,000 |
Lease
|
Call center
|
||||
|
Ivybridge, England
|
2,000 |
Lease
|
Centaur shipping depot
|
||||
|
(1) Rounded to the nearest thousand square feet.
|
|
||||||
|
(2) In October 2010, we entered into a purchase and sales agreement to buy an office building for our headquarters in Boise, Idaho approximately 10 miles away from our existing headquarters. The building is approximately 60,000 square feet and the purchase price is $5,175. The closing is subject to a 45 day due diligence period.
|
|||||||
|
(3) We are in the process of moving our distribution center which has been leased in Visalia, California to a larger facility with 81,000 square feet. We expect the transition to be completed in December 2010.
|
|||||||
|
Item 3.
|
Legal Proceedings.
|
From time to time, in the normal course of business, we may become a party to legal proceedings that may have an adverse effect on our financial position, results of operations and cash flows. Management presently believes that the ultimate outcome of these proceedings will not have a material adverse effect on our financial position, cash flows or overall trends in results of operations. However, litigation is subject to inherent uncertainties, and unfavorable outcomes could occur. An unfavorable outcome could include the payment of monetary damages or, in cases for which injunctive relief is sought, an injunction prohibiting us from selling one or more products or taking certain other actions. Were an unfavorable outcome to occur, our business or results of operations could be materially harmed.
|
Item 4.
|
PART II
|
Item 5.
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
Our common stock has been quoted on the Nasdaq under the symbol “MWIV” since August 3, 2005. Prior to that date there was no public market for our common stock. The following table sets forth, for the two most recent fiscal years, the high and low sales prices of our common stock, reported by the Nasdaq Global Select Market.
|
|
Common Stock Price
|
||||||
|
|
High
|
Low
|
|||||
|
Fiscal Year Ended September 30, 2009
|
|
|
|||||
|
First Quarter
|
$ | 41.60 | $ | 20.16 | |||
|
Second Quarter
|
$ | 31.00 | $ | 20.37 | |||
|
Third Quarter
|
$ | 36.39 | $ | 27.14 | |||
|
Fourth Quarter
|
$ | 42.21 | $ | 29.68 | |||
|
Fiscal Year Ended September 30, 2010
|
|||||||
|
First Quarter
|
$ | 41.30 | $ | 34.06 | |||
|
Second Quarter
|
$ | 45.00 | $ | 36.21 | |||
|
Third Quarter
|
$ | 51.99 | $ | 40.02 | |||
|
Fourth Quarter
|
$ | 58.74 | $ | 46.04 | |||
At the close of business on November 12, 2010, we had 12,453,053 shares of common stock issued and outstanding. As of that date, there were 235 registered holders of record. This does not reflect beneficial stockholders who hold their stock in nominee or “street” name through brokerage firms.
We have not paid or declared any dividends on our common stock and do not anticipate paying any dividends on our common stock in the foreseeable future. We intend to retain future earnings to finance the ongoing operations and growth of our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors and will depend on conditions at that time, including our financial condition, results of operations, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
The table below provides information concerning our repurchase of shares of our common stock during the quarter ended September 30, 2010.
|
Issuer Purchases of Equity Securities
|
||||||||||||||
|
|
|
|
|
|
||||||||||
|
|
|
|
Total Number of
|
Maximum Number (or
|
||||||||||
|
|
|
|
Shares Purchased
|
Approximate Dollar
|
||||||||||
|
|
Total Number
|
Average
|
as Part of Publicly
|
Value) of Shares that May
|
||||||||||
|
|
of Shares
|
Price Paid
|
Announced Plans
|
Yet Be Purchased Under
|
||||||||||
|
Period
|
Purchased
|
per Share
|
or Programs
|
the Plans or Programs
|
||||||||||
|
July 1 to July 31, 2010
|
— | — | — | — | ||||||||||
|
August 1 to August 31, 2010
|
331 | (1) | $ | 52.82 | — | — | ||||||||
|
September 1 to September 30, 2010
|
1,160 | (1) | $ | 57.37 | — | — | ||||||||
|
Total
|
1,491 | $ | 56.36 | — | — | |||||||||
|
(1) These shares were withheld upon the vesting of employee stock grants in connection with payment |
||||||||||||||
|
of required withholding taxes.
|
||||||||||||||
The graph below compares the cumulative total stockholder return on $100 invested at the market close on September 30, 2005 through and including September 30, 2010, the last trading day before the end of our most recently completed fiscal year, with the cumulative total return of the same time period on the same amount invested in the Russell 2000 Index, the Standard and Poor’s SmallCap 600 Index and a Peer Group Index, consisting of 11 companies that compete or operate in a comparable industry as the Company. The chart below the graph sets forth the actual numbers depicted on the graph.
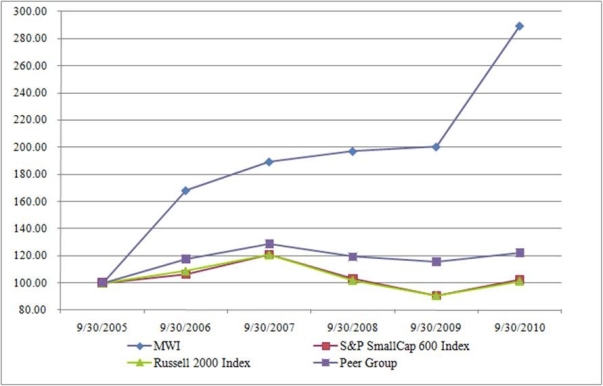 |
|
9/30/2005
|
9/30/2006
|
9/30/2007
|
9/30/2008
|
9/30/2009
|
9/30/2010
|
||||||||||||||||||
|
MWI Veterinary Supply, Inc.
|
$ | 100.00 | $ | 168.07 | $ | 189.22 | $ | 196.94 | $ | 200.25 | $ | 289.32 | |||||||||||
|
S&P SmallCap 600 Index
|
100.00 | 106.16 | 120.91 | 102.99 | 90.64 | 102.38 | |||||||||||||||||
|
Russell 2000 Index
|
100.00 | 108.65 | 120.61 | 101.76 | 90.49 | 101.25 | |||||||||||||||||
|
Peer Group(1)
|
100.00 | 117.58 | 128.73 | 119.27 | 115.47 | 122.09 | |||||||||||||||||
|
|
(1) |
Peer Group consists of Amerisourcebergen Corp. (ABC), Animal Health International Inc. (AHII), Cardinal Health Inc. (CAH), Henry Schein Inc. (HSIC), IDEXX Laboratories Inc. (IDXX), Owens & Minor Inc. (OMI), McKesson Corp. (MCK), Patterson Companies Inc. (PDCO), PetMed Express Inc. (PETS), PSS World Medical Inc. (PSSI) and VCA Antech Inc. (WOOF).
|
|
Item 6.
|
Selected Financial Data.
|
The selected consolidated financial data below represent portions of our financial statements and are not complete. You should read this information together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes to these statements included in this annual report. Historical results are not necessarily indicative of future performance.
|
|
Year Ended September 30,
|
|||||||||||||||||||
|
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
|
|
(In thousands, except per share data)
|
|||||||||||||||||||
|
Revenues:
|
|
|
|
|
|
|||||||||||||||
|
Product sales
|
$ | 1,169,545 | $ | 880,703 | $ | 778,953 | $ | 664,351 | $ | 564,289 | ||||||||||
|
Product sales to related party
|
43,017 | 46,406 | 39,452 | 36,122 | 34,123 | |||||||||||||||
|
Commissions
|
16,780 | 14,223 | 12,959 | 9,632 | 7,782 | |||||||||||||||
|
Total revenues
|
1,229,342 | 941,332 | 831,364 | 710,105 | 606,194 | |||||||||||||||
|
|
||||||||||||||||||||
|
Cost of product sales
|
1,064,339 | 806,677 | 711,812 | 607,756 | 518,068 | |||||||||||||||
|
Gross profit
|
165,003 | 134,655 | 119,552 | 102,349 | 88,126 | |||||||||||||||
|
|
||||||||||||||||||||
|
Selling, general and administrative expenses
|
105,793 | 90,827 | 84,123 | 73,215 | 62,470 | |||||||||||||||
|
Depreciation and amortization
|
4,992 | 3,365 | 3,078 | 2,424 | 1,964 | |||||||||||||||
|
Operating income
|
54,218 | 40,463 | 32,351 | 26,710 | 23,692 | |||||||||||||||
|
|
||||||||||||||||||||
|
Other income (expense):
|
||||||||||||||||||||
|
Interest expense
|
(539 | ) | (242 | ) | (265 | ) | (545 | ) | (1,959 | ) | ||||||||||
|
Earnings of equity method investees
|
220 | 230 | 179 | 169 | 161 | |||||||||||||||
|
Other
|
427 | 540 | 642 | 756 | 338 | |||||||||||||||
|
Total other income (expense)
|
108 | 528 | 556 | 380 | (1,460 | ) | ||||||||||||||
|
|
||||||||||||||||||||
|
Income before taxes
|
54,326 | 40,991 | 32,907 | 27,090 | 22,232 | |||||||||||||||
|
Income tax expense
|
(20,886 | ) | (16,086 | ) | (12,990 | ) | (10,215 | ) | (8,396 | ) | ||||||||||
|
Net income
|
$ | 33,440 | $ | 24,905 | $ | 19,917 | $ | 16,875 | $ | 13,836 | ||||||||||
|
|
||||||||||||||||||||
|
Earnings per common share:
|
||||||||||||||||||||
|
Basic
|
$ | 2.73 | $ | 2.06 | $ | 1.65 | $ | 1.43 | $ | 1.28 | ||||||||||
|
Diluted
|
$ | 2.70 | $ | 2.02 | $ | 1.62 | $ | 1.40 | $ | 1.25 | ||||||||||
|
|
||||||||||||||||||||
|
Weighted average common shares outstanding:
|
||||||||||||||||||||
|
Basic
|
12,241 | 12,088 | 12,053 | 11,764 | 10,773 | |||||||||||||||
|
Diluted
|
12,395 | 12,306 | 12,301 | 12,044 | 11,072 | |||||||||||||||
|
|
As of September 30,
|
|||||||||||||||||||
|
|
2010
|
2009
|
2008
|
2007
|
2006
|
|||||||||||||||
|
|
(In thousands)
|
|||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
|
Cash
|
$ | 911 | $ | 14,302 | $ | 3,419 | $ | 8,599 | $ | 37 | ||||||||||
|
Total assets
|
467,932 | 337,919 | 314,805 | 267,194 | 230,559 | |||||||||||||||
|
Total debt
|
14,724 | 97 | 194 | 292 | 10,948 | |||||||||||||||
|
Total stockholders’ equity
|
246,787 | 207,927 | 181,003 | 160,011 | 129,626 | |||||||||||||||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
All dollar and sterling pound amounts are presented in thousands, except for per share amounts.
|
|
Overview
|
We are a leading distributor of animal health products to veterinarians in the United States and the United Kingdom. We sell our products to veterinarians in both the companion and production animal markets. Our growth has primarily been from internal growth initiatives and, to a lesser extent, selective acquisitions.
Historically, we estimate that approximately two-thirds of our total revenues have been generated from sales to the companion animal market and one-third from sales to the production animal market. The state of the overall economy in both the United States and United Kingdom and consumer spending have impacted both the companion animal and production animal markets, with tightening credit markets, volatile commodity prices in milk, grain, corn and feeder cattle, and changes in weather patterns also affecting demand in the production animal market. Both the companion animal and production animal markets have been integral to our financial results and we intend to continue supporting both markets.
We believe that the companion animal market in both the United States and United Kingdom has slowed as a result of a decrease in consumer spending. Historically, growth in the companion animal market has been due to the increasing number of households with companion animals, increased expenditures on animal health and preventative care, an aging pet population, advancements in pharmaceuticals and diagnostic testing and extensive marketing programs sponsored by companion animal nutrition and pharmaceutical companies. While the average order size for companion animal health products is often smaller than production animal health products, companion animal health products typically have higher margins. We intend to continue to penetrate this market through internal growth initiatives and selective acquisitions. We believe that some of our customers in this market have experienced liquidity issues as a result of the tightening credit markets.
Product sales in the production animal market in both the United States and United Kingdom are impacted by volatility in commodity prices such as milk, corn, grain and feeder cattle, changes in weather patterns that allow cattle to graze for longer periods and changes in the general economy. Milk price declines in the dairy market have a significant impact on dairy farmers. This creates cash-flow challenges for these farmers and in turn, could impact the time it takes for us to collect our outstanding accounts receivable from these customers as well as affect the overall collectability of these accounts. However, we still believe that it is important to our business to service this market and we intend to continue to support production animal veterinarians with a broad range of products and value-added services. Historically, sales in this market have been largely driven by spending on animal health products to improve productivity, weight gain and disease prevention, as well as a growing focus on food safety.
We generally extend some level of credit to our customers without requiring collateral, which exposes us to credit risk. If customers’ cash flow or operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain other sources of credit, they may not be able to pay or may delay payment to us, or in some cases may return products to us. We continually assess our customers’ ability to pay us and adjust our allowance for doubtful accounts, as necessary.
We sell products that we source from our vendors to our customers through either a “buy/sell” transaction or an agency relationship with our vendors. In a “buy/sell” transaction, we purchase or take inventory of products from our vendors. When a customer places an order with us, we pick, pack, ship and invoice the customer for the order. We record sales from “buy/sell” transactions, which account for the vast majority of our business, as revenues in conformity with generally accepted accounting principles in the United States. In an agency relationship, we generally do not purchase and take inventory of products from our vendors. When we receive an order from our customer, we transmit the order to our vendor, who picks, packs and ships the order to our customer. In some cases, our vendor invoices and collects payment from our customer, while in other cases we invoice and collect payment from our customer on behalf of our vendor. We receive a commission payment for soliciting the order from our customer and for providing other customer service activities. The aggregate revenues we receive in agency transactions constitute the “commissions” line item on our consolidated statements of income and are recorded in conformity with accounting principles generally accepted in the United States. Our vendors determine the method we use to sell our products. Historically, vendors have occasionally switched between the “buy/sell” and agency models for particular products in response to market conditions related to that particular product. A switch between models can impact our revenues and our operating income. We cannot know in advance when a vendor will switch between the “buy/sell” and agency models or what impact, if any, such a change may have. A switch can occur even with vendors with whom we have written agreements, because most of our agreements with vendors have relatively short terms and are terminable with or without cause on short notice, normally 30 to 90 days. The impact of any individual change from a
26
“buy/sell” to an agency model depends on the costs and expenses associated with a particular product, and can have either a positive or a negative effect on our profitability.
We typically renegotiate vendor contracts annually. These vendor contracts may include terms defining margins, rebates, commissions, exclusivity requirements and the manner in which we go to market. For example, vendors could require us to distribute their products on an exclusive basis, which could cause us to forego distributing competing products which may also be profitable. Conversely, competitors could obtain exclusive rights to market particular products, which we would be unable to market. If we lose the right to distribute products under such exclusive agreements, we may lose access to certain products and lose a competitive advantage. Exclusivity agreements could allow potential competitors to sell products that we cannot offer and erode our market share. In addition, vendors have the ability to expand the distributors that they use which could have a material adverse effect on our business.
Many of our vendors’ rebate programs are based on a calendar year. Historically, the three months ended December 31 has been our most significant quarter for recognition of rebates. Vendor rebates based on sales are classified in our accompanying consolidated statements of income as a reduction to cost of product sales at the time the sales performance measures are achieved. Purchase rebates are measured against inventory purchases from the vendors and are a reduction of inventory until the product is sold. When the inventory is sold, purchase rebates are recognized as a reduction to cost of product sales.
Total Revenues. Our total revenues increased from $606,194 for our fiscal year ended September 30, 2006 to $1,229,342 for our fiscal year ended September 30, 2010. Our revenue growth has been driven by our acquisitions and our ability to offer a broad product selection at competitive prices with high levels of customer service and support and an expansion in the number of veterinary practices to which we distribute products. We have continually added new vendor relationships to expand our product offering and field sales representatives to increase our customer reach, principally in the Southeast, Northeast and Midwest regions of the United States.
Operating Expenses. Our selling, general and administrative expenses increased from $62,470 for our fiscal year ended September 30, 2006 to $105,793 for our fiscal year ended September 30, 2010. Selling, general and administrative expenses consist mainly of compensation and benefits, warehouse operating supplies, occupancy and location expenses and other general corporate expenses. Our selling, general and administrative expenses as a percentage of total revenues were 10.3% for our fiscal year ended September 30, 2006, compared to 8.6% for the same period in 2010.
Acquisitions. In fiscal year 2008, we acquired substantially all of the assets of Tri V. Based near Detroit, Michigan, Tri V was a distributor with an 11-year history of providing animal health products to approximately 850 veterinary practices, with a particular focus on emergency clinics and ophthalmology specialists.
Also in fiscal year 2008, we acquired substantially all of the assets of AAHA MARKETLink. Based near Denver, Colorado, AAHA MARKETLink was a distributor of animal health products to members of American Animal Hospital Association.
In February 2010, we acquired all of the share capital of Centaur. Based in Castle Cary, England, Centaur is a distributor of animal health products to veterinarians in the United Kingdom.
Fair values of the assets acquired and liabilities assumed as a result of these acquisitions are discussed in Note 3 “Business Acquisitions” to the consolidated financial statements.
|
|
Results of Operations
|
The following tables summarize our historical results of operations for our fiscal years ended September 30, 2010, 2009 and 2008.
|
|
Year Ended September 30,
|
|||||||||||||||||||||||
|
|
2010
|
%
|
2009
|
%
|
2008
|
%
|
||||||||||||||||||
|
|
(In thousands, except per share data)
|
|||||||||||||||||||||||
|
Revenues:
|
|
|
|
|
|
|
||||||||||||||||||
|
Product sales
|
$ | 1,169,545 | 95.1 | % | $ | 880,703 | 93.6 | % | $ | 778,953 | 93.7 | % | ||||||||||||
|
Product sales to related party
|
43,017 | 3.5 | % | 46,406 | 4.9 | % | 39,452 | 4.7 | % | |||||||||||||||
|
Commissions
|
16,780 | 1.4 | % | 14,223 | 1.5 | % | 12,959 | 1.6 | % | |||||||||||||||
|
Total revenues
|
1,229,342 | 100.0 | % | 941,332 | 100.0 | % | 831,364 | 100.0 | % | |||||||||||||||
|
|
||||||||||||||||||||||||
|
Cost of product sales
|
1,064,339 | 86.6 | % | 806,677 | 85.7 | % | 711,812 | 85.6 | % | |||||||||||||||
|
Gross profit
|
165,003 | 13.4 | % | 134,655 | 14.3 | % | 119,552 | 14.4 | % | |||||||||||||||
|
|
||||||||||||||||||||||||
|
Selling, general and administrative expenses
|
105,793 | 8.6 | % | 90,827 | 9.6 | % | 84,123 | 10.1 | % | |||||||||||||||
|
Depreciation and amortization
|
4,992 | 0.4 | % | 3,365 | 0.4 | % | 3,078 | 0.4 | % | |||||||||||||||
|
Operating income
|
54,218 | 4.4 | % | 40,463 | 4.3 | % | 32,351 | 3.9 | % | |||||||||||||||
|
|
||||||||||||||||||||||||
|
Other income (expense):
|
||||||||||||||||||||||||
|
Interest expense
|
(539 | ) | 0.0 | % | (242 | ) | 0.0 | % | (265 | ) | 0.0 | % | ||||||||||||
|
Earnings of equity method investees
|
220 | 0.0 | % | 230 | 0.0 | % | 179 | 0.0 | % | |||||||||||||||
|
Other
|
427 | 0.0 | % | 540 | 0.1 | % | 642 | 0.1 | % | |||||||||||||||
|
Total other income (expense)
|
108 | 0.0 | % | 528 | 0.1 | % | 556 | 0.1 | % | |||||||||||||||
|
|
||||||||||||||||||||||||
|
Income before taxes
|
54,326 | 4.4 | % | 40,991 | 4.4 | % | 32,907 | 4.0 | % | |||||||||||||||
|
Income tax expense
|
(20,886 | ) | -1.7 | % | (16,086 | ) | -1.7 | % | (12,990 | ) | -1.6 | % | ||||||||||||
|
Net income
|
$ | 33,440 | 2.7 | % | $ | 24,905 | 2.7 | % | $ | 19,917 | 2.4 | % | ||||||||||||
|
|
||||||||||||||||||||||||
|
Earnings per common share:
|
||||||||||||||||||||||||
|
Basic
|
$ | 2.73 | $ | 2.06 | $ | 1.65 | ||||||||||||||||||
|
Diluted
|
$ | 2.70 | $ | 2.02 | $ | 1.62 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Weighted average common shares
|
||||||||||||||||||||||||
|
outstanding:
|
||||||||||||||||||||||||
|
Basic
|
12,241 | 12,088 | 12,053 | |||||||||||||||||||||
|
Diluted
|
12,395 | 12,306 | 12,301 | |||||||||||||||||||||
|
|
Fiscal 2010 Compared to Fiscal 2009
|
Total Revenues. Total revenues increased $288,010, or 30.6%, to $ 1,229,342 for the fiscal year ended September 30, 2010 from $941,332 for the fiscal year ended September 30, 2009. Of the 30.6% increase in revenues, 14.1% was due to organic growth in the United States and 16.5%, or $155,116 was from the acquisition of Centaur. The organic growth was partially due to the increase in revenues, starting in our second fiscal quarter, from the sale of flea, tick and heartworm products that we did not sell in the same period of the prior fiscal year due to our previously exclusive arrangement with Merial. Certain new flea, tick and heartworm products that we now distribute are sold under “buy/sell” arrangements while most flea, tick and heartworm products in the past were sold under an agency agreement. The product sales under a “buy-sell” arrangement results in greater revenue than product sales under an agency arrangement because we recognize product sales under a “buy-sell” arrangement as total sales net of estimated product returns and sales tax, whereas we only recognize commission revenue in product sales under an agency relationship. The organic growth was also due to the fact that, starting in the latter part of our third fiscal quarter, certain of our existing customers as well as new customers placed additional orders with us when their primary supplier was no longer able to meet their needs. Organic revenues attributable to new customers represented approximately 62% of the growth in total revenues during the fiscal year ended September 30, 2010.
28
Organic revenues attributable to existing customers represented approximately 38% of the growth in total revenues during the fiscal year ended September 30, 2010. For the purpose of calculating growth rates of new and existing customer revenue, we have defined a new customer as a customer that did not purchase product from us in the corresponding fiscal quarter of the prior year, with the remaining customer base being considered an existing customer. Revenues from new customers for each fiscal quarter are summed to arrive at the estimated year-to-date revenue for new customers.
Commission revenues increased $2,557, or 18.0%, to $16,780 for the fiscal year ended September 30, 2010 from $14,223 for the fiscal year ended September 30, 2009. The increase of commission revenues was due to the increase in gross agency billings of $58,996, or 21.9%, to $328,053 for the fiscal year ended September 30, 2010 from $269,057 for the fiscal year ended September 30, 2009. The increase was partially due to the additional flea, tick and heartworm contracts that we entered into after the exclusive arrangement with Merial ended in January 2010.
Gross Profit. Gross profit increased $30,348, or 22.5%, to $165,003 for the fiscal year ended September 30, 2010 from $134,655 for the fiscal year ended September 30, 2009. The change in gross profit is primarily a result of increased total revenues as discussed above including the additional gross profit from Centaur starting from the acquisition date. Gross profit as a percentage of total revenues was 13.4% and 14.3% for the fiscal years ended September 30, 2010 and 2009, respectively. Gross profit as a percentage of total revenues decreased partially due to the addition of Centaur because Centaur's gross profit as a percentage of total revenues is generally lower than MWI's, which serves to reduce the overall gross margin of the consolidated Company when compared to our results for the same period in the prior year. Vendor rebates decreased by $684 for the fiscal year ended September 30, 2010 as compared to the fiscal year ended September 30, 2009. This decrease in vendor rebates was primarily due to the elimination of the Pfizer livestock rebate opportunity in the quarter ended December 31, 2009 as well as a rebate earned during the quarter ended September 30, 2009 from a vendor that is no longer in business. These decreases were partially offset by increases in rebates from other vendors due to the revenue growth previously discussed.
Selling, General and Administrative Expenses (“SG&A”). SG&A increased $14,966, or 16.5%, to $105,793 for the fiscal year ended September 30, 2010 from $90,827 for the fiscal year ended September 30, 2009. This increase was primarily due to the addition of Centaur’s operating expenses starting from the acquisition date and increased compensation and benefits costs, partially offset by a decrease in our bad debt expense as a result of payments made by certain customers. Also included in the increase in SG&A expenses are the direct acquisition-related expenses of $1,100 incurred in connection with the acquisition of Centaur. SG&A as a percentage of revenue was 8.6% for the fiscal year ended September 30, 2010 and 9.6% for the fiscal year ended September 30, 2009. SG&A expenses as a percentage of total revenues decreased due, in part, to the addition of Centaur because Centaur’s SG&A expenses as a percentage of total revenues are generally lower than MWI’s, which serves to reduce the overall SG&A expenses as a percentage of total revenues when compared to our results for the same period in the prior year.
Depreciation and Amortization. Depreciation and amortization expense increased $1,627, or 48.4%, to $4,992 for the fiscal year ended September 30, 2010 from $3,365 for the fiscal year ended September 30, 2009. The increase was primarily due to the acquisition of Centaur.
Other Income/Expenses. Other income decreased $420, or 79.5%, to $108 for the fiscal year ended September 30, 2010 from $528 for the fiscal year ended September 30, 2009. The decrease in other income was primarily due to an increase in interest expense during the fiscal year ended September 30, 2010 as compared to the same period in the prior year. This was primarily due to a higher average outstanding balance during the fiscal year ended September 30, 2010 on our revolving credit facility compared to the fiscal year ended September 30, 2009 due to borrowings incurred to finance the acquisition of Centaur.
Income Tax Expense. Our effective tax rate was 38.4% for the fiscal year ended September 30, 2010 and 39.2% for the fiscal year ended September 30, 2009. This decrease was primarily due to the impact of the Centaur acquisition that contributed additional earnings at a lower effective tax rate. Additionally, there was a lower international enacted tax rate change during the September quarter.
|
|
Fiscal 2009 Compared to Fiscal 2008
|
Total Revenues. Total revenues increased $109,968, or 13.2%, to $941,332 for the fiscal year ended September 30, 2009 from $831,364 for the fiscal year ended September 30, 2008. This increase was attributable to an increase in product sales volumes of a wide variety of products to both existing and new customers. Revenues attributable to new customers represented approximately 65% of the growth in total revenues during the fiscal year ended September 30, 2009. Revenues attributable to existing customers represented approximately 35% of the growth in total revenues during the fiscal year ended September 30, 2009. Included in the new customer growth were approximately $8,937 of revenues attributable to new customers acquired as a result of the acquisition of substantially all of the assets of AAHA MARKETLink as of July 1, 2008.
29
Additionally, we had $17,628 of incremental revenues as a result of the acquisition of substantially all of the assets of AAHA MARKETLink from customers who had previously been purchasing from both MWI and AAHA MARKETLink.
Commission revenues increased $1,264, or 9.8%, to $14,223 for the fiscal year ended September 30, 2009 from $12,959 for the fiscal year ended September 30, 2008. The increase of commission revenues was due to the increase in gross agency billings of $24,012, or 9.8%, to $269,057 for the fiscal year ended September 30, 2009 from $245,045 for the fiscal year ended September 30, 2008.
Gross Profit. Gross profit increased $15,103, or 12.6%, to $134,655 for the fiscal year ended September 30, 2009 from $119,552 for the fiscal year ended September 30, 2008. The change in gross profit is primarily a result of increased total revenues as discussed above. Vendor rebates decreased by approximately $1,600 for the fiscal year ended September 30, 2009 as compared to the fiscal year ended September 30, 2008. This decrease in vendor rebates was primarily due to the elimination of the Pfizer livestock rebate opportunity, partially offset by increases in rebates from other vendors, including a strong performance on a significant vendor rebate program offered from a certain vendor during the quarter ended September 30, 2009. The decrease in vendor rebates was partially offset by an improvement in freight costs as a percentage of total revenues compared to the fiscal year ended September 30, 2008. Gross profit as a percentage of total revenues was 14.3% and 14.4% for the fiscal years ended September 30, 2009 and 2008, respectively.
Selling, General and Administrative Expenses. SG&A increased $6,704, or 8.0%, to $90,827 for the fiscal year ended September 30, 2009 from $84,123 for the fiscal year ended September 30, 2008. This increase was primarily due to increased compensation and benefits costs and an increase in our allowance for doubtful accounts, partially offset by a decrease in travel expenses. SG&A as a percentage of revenue was 9.6% for the fiscal year ended September 30, 2009 and 10.1% for the fiscal year ended September 30, 2008. This improvement was primarily due to improvements in compensation costs, outside fees and services and travel expenses.
Depreciation and Amortization. Depreciation and amortization expense increased $287, or 9.3%, to $3,365 for the fiscal year ended September 30, 2009 from $3,078 for the fiscal year ended September 30, 2008. The move of the Grand Prairie, Texas distribution center and the implementation of new technologies contributed to the increase in depreciation expense. In addition, amortization expense increased as a result of intangible assets acquired in the purchase of substantially all of the assets of AAHA MARKETLink.
Other Income/Expenses. Other income decreased $28, or 5.0%, to $528 for the fiscal year ended September 30, 2009 from $556 for the fiscal year ended September 30, 2008. The decrease in other income was primarily due to a reduction in interest revenue of during the fiscal year ended September 30, 2009 as compared to the same period in the prior year. This was a result of lower interest rates during fiscal year 2009 as compared to fiscal year 2008. This decrease in interest revenue was partially offset by an increase in earnings from equity method investees.
Income Tax Expense. Our effective tax rate was 39.2% for the fiscal year ended September 30, 2009 and 39.5% for the fiscal year ended September 30, 2008.
|
|
Seasonality in Operating Results
|
Our quarterly sales and operating results have varied significantly in the past, and will likely continue to do so in the future. Historically, our total revenues have typically been higher during the spring and fall months due to increased sales of production animal products. Product use cycles for production animal products are directly related to medical procedures performed by veterinarians on production animals during the spring and fall months. These buying patterns can also be affected by vendors’ and distributors’ marketing programs or price increase announcements, which can cause veterinarians to purchase production animal health products earlier than those products are needed. This kind of early purchasing may reduce our sales in the months these purchases would have otherwise been made. See “Risk Factors — Our quarterly operating results may fluctuate significantly, and these fluctuations may cause our stock price to fall.” Additionally, while we accrue rebates as they are earned, our rebates have historically been highest during the quarter ended December 31, since some of our vendors’ rebate programs were designed to include targets to be achieved near the end of the calendar year.
Our companion animal products tend to have a different product use cycle that minimally overlaps with that of production animal products. In the companion animal market, sales of flea, tick and mosquito products are highest during the spring and summer months. The differing product use cycles of companion animal products partially offsets the seasonality we typically experience due to our sales of production animal products.
For the reasons and factors discussed above our quarterly operating results may fluctuate significantly. Accordingly, results for any one quarter are not necessarily indicative of results to be expected for any other quarter or for any year and our sales for any particular future period may decrease. In the future, operating results may fall below the expectations of securities analysts and investors. If this occurs, the price of our stock would likely decrease.
|
|
Liquidity and Capital Resources
|
Our principal sources of liquidity are cash flows generated from operations and borrowings on our credit facilities. We use capital primarily to fund day-to-day operations and to maintain sufficient inventory levels in order to promptly fulfill customer orders and to expand our operations and sales growth. We believe our capital resources, including our ability to borrow funds from our credit facilities, will be sufficient to meet our anticipated cash needs for at least the next twelve months.
Our lenders may have suffered losses related to their lending and other financial relationships, especially because of the general weakening of the national economy and increased financial instability of many borrowers. As a result, the lenders may become insolvent or tighten their lending standards, which could make it more difficult for us to borrow under our revolving credit facility, extend the terms of our revolving credit facility or obtain alternative financing on favorable terms or at all. Our financial condition and results of operations could be adversely affected if we were unable to draw funds under our revolving credit facility because of a lender default or if we fail to obtain other cost-effective financing.
We generally extend some level of credit to our customers without requiring collateral, which exposes us to credit risk. If customers’ cash flow or operating and financial performance deteriorates, or if they are unable to make scheduled payments or obtain other sources of credit, they may not be able to pay or may delay payment to us, or in some cases may return products to us. Any inability of current and/or potential customers to pay us for our products and/or services due to their deteriorating financial condition or otherwise may adversely affect our results of operations and financial condition. Volatility in commodity prices, such as milk, corn, grain and feeder cattle, and deteriorating economic conditions can have a significant impact on the financial results of our customers. We continually assess our customers’ ability to pay us and adjust our allowance for doubtful accounts, as necessary.
Capital Resources. On February 8, 2010 and August 10, 2010, MWI Co., our wholly-owned subsidiary as borrower, entered into a First Amendment (the “First Amendment”) and Second Amendment (the “Second Amendment”), respectively, to its Credit Agreement with us and Memorial Pet Care, Inc., as guarantors, and Bank of America, N.A. and Wells Fargo Bank, N.A., (collectively, the “lenders”) amending the Credit Agreement dated December 13, 2006 among MWI Co., MWI Veterinary Supply, Inc., Memorial Pet Care, Inc. and the lenders a party thereto (the “facility”). The First Amendment increased the aggregate revolving commitment of the lenders under the facility from $70,000 to $100,000. The First Amendment also extended the maturity date of the loans under the facility from December 1, 2011 to March 1, 2013. The variable interest rate is now equal to the Daily LIBOR Floating Rate or the LIBOR 1-month fixed rate (at MWI Co.’s option) plus a margin ranging from 1.50% to 2.25%, which was previously 1.75% to 2.50% under the First Amendment, and 0.7% to 1.25% under the facility. The lenders also receive an unused line fee and letter of credit fee which is now equal to 0.2% of the unused amount of the facility, which was previously 0.125%. The Second Amendment increased the amount of permitted unsecured indebtedness from $20 million to $30 million. The facility contains financial covenants, including a fixed charge ratio and a funded debt to EBITDA calculation. We were in compliance with both of these covenants as of September 30, 2010. Our outstanding balance on the revolving credit facility at September 30, 2010 was $9,600, and the interest rate was 1.73% as of September 30, 2010.
As of September 30, 2010, Centaur operated with a credit facility (“Fortis facility”) with Fortis Bank as the lender. The Centaur facility allowed for borrowings in the aggregate of £12,000. The Fortis facility had a variable interest rate equal to a base rate of 0.50% plus GBP one-month LIBOR plus a margin of 0.85%. The outstanding balance at September 30, 2010 on the Fortis facility was £342, or $540 using the exchange rate on September 30, 2010. The interest rate for the Fortis facility was 1.90% as of September 30, 2010.
On November 5, 2010, Centaur entered into a £12,500 unsecured revolving line of credit facility (the “sterling revolving credit facility”) with Wells Fargo Bank, N.A. London Branch (“Wells Fargo”). The sterling revolving credit facility is for a three year term with interest paid at the end of the applicable one month, three month or six month interest period. Interest is based on LIBOR for the applicable interest period plus an applicable margin of 1.05% to 1.90%. The facility contains financial covenants requiring Centaur to maintain a minimum tangible net worth of £3,000.
Operating Activities. For fiscal year ended September 30, 2010, cash provided by operating activities was $16,866, and was primarily attributable to net income of $33,440 and an increase in accounts payable of $39,781. This amount was partially offset by an increase in receivables of $14,154 and an increase in inventory of $41,106. The increase in net income is a result of the factors discussed above in “Results of Operations.” The increase in receivables was primarily due to the addition of the Centaur balances from the acquisition. The increases in inventories and accounts payable were primarily due to the inventory purchased during the quarter to accommodate the growth from our customers who placed additional orders with us when their primary supplier was no longer able to meet their needs.
For fiscal year ended September 30, 2009, cash provided by operating activities was $11,939, and was primarily attributable to net income of $24,905, a decrease in inventory of $2,072 and an increase in accrued expenses of $2,091. This amount was partially offset by an increase in receivables of $14,019 and a decrease in accounts payable of $4,712. The increase in net income is a result of the factors discussed above in “Results of Operations.” The increase in accounts receivable is primarily related to the increase in sales as well as an increase in receivables with extended payment terms due to a vendor promotion offered during the fourth quarter of fiscal year 2009. We provide financing terms to our customers that we believe are customary for our industry. There are occasions when we offer extended terms based on market conditions and customer demand. Our inventory levels decreased in the current year due to focused inventory management. Our accounts payable decreased primarily due to the decrease in inventory and the timing of payments for inventory.
For fiscal year ended September 30, 2008, cash provided by operating activities was $10,622, and was primarily attributable to net income of $19,917 and an increase in accounts payable and accrued expenses of $23,163. This amount was partially offset by an increase in receivables of $11,180 and inventories of $23,543. The increase in net income is a result of the factors discussed above in “Results of Operations.” The increase in accounts receivable is primarily related to the increase in sales. Additionally, we opened a new distribution center in Edwardsville, Kansas and we increased our inventory levels at that facility during the year. We also increased our inventory to accommodate the growth in sales to AAHA MARKETLink customers. Our accounts payable is primarily used to offset the increases in inventory.
Investing Activities. For fiscal year ended September 30, 2010, net cash used in investing activities was $44,090. Significant transactions impacting cash used in investing activities include the acquisition of Centaur of $39,645, capital expenditures of $4,388 primarily related to the equipment purchased in preparation for the move to a larger distribution center in Visalia, California as well as other facility and technology infrastructure improvements.
For fiscal year ended September 30, 2009, net cash used in investing activities was $2,520. Significant transactions impacting cash used in investing activities include capital expenditures of $2,662 primarily related to the relocation of our distribution center in Grand Prairie, Texas and the opening of our distribution center in Whitestown, Indiana. Additionally, we continued to improve our information technology infrastructure.
For fiscal year ended September 30, 2008, net cash used in investing activities was $16,198. Significant transactions impacting cash used in investing activities include the acquisition of substantially all of the assets of AAHA MARKETLink for $8,676, net of cash received of $1,320, the acquisition of substantially all of the assets of Tri V for $4,606, capital expenditures of $3,246 primarily related to the relocation of our distribution center in Grand Prairie, Texas and expansion and improvements made to our existing distribution centers. Additionally, we continued to improve our information technology infrastructure and had multiple projects related to improving our technology.
Financing Activities. For fiscal year ended September 30, 2010, net cash provided by financing activities was $13,785, and was primarily attributable to the borrowings on our revolving credit facilities of $10,116 and the tax benefit of $3,611 from the exercise of common stock options.
For fiscal year ended September 30, 2009, net cash provided by financing activities was $1,464, and was primarily attributable to the tax benefit of $1,178 from the exercise of common stock options. We had proceeds of $143 from the stock option exercises and $240 related to the sale of stock under our employee stock purchase plan.
For fiscal year ended September 30, 2008, net cash provided by financing activities was $396, and was primarily attributable to the proceeds of $218 from stock option exercises. We also had a tax benefit from the stock option exercises of $216.
|
|
Off Balance Sheet Arrangements
|
At September 30, 2010, we had no significant investments that were accounted for under the equity method in accordance with accounting principles generally accepted in the United States except for Feeders Advantage. Feeders Advantage has no liabilities associated with them that were guaranteed by or that would be considered material to us. Accordingly, we do not have any off balance sheet arrangements.
|
|
Contractual Obligations
|
|
|
Contractual Obligations
|
Our contractual obligations at September 30, 2010 mature as follows (in thousands):
|
Payments Due by Period
|
||||||||||||||||||||
|
Total
|
1 Year or less
|
2-3 Years
|
4-5 Years
|
More than 5 Years
|
||||||||||||||||
|
Revolving credit facility(1)
|
$ | 10,140 | $ | 10,140 | $ | — | $ | — | $ | — | ||||||||||
|
Operating lease commitments
|
15,539 | 3,048 | 4,990 | 2,837 | 4,664 | |||||||||||||||
|
Capital lease commitments
|
1,811 | 858 | 914 | 39 | — | |||||||||||||||
|
Long-term debt obligations (including current portion)
|
2,773 | 2,773 | — | — | — | |||||||||||||||
|
Interest on long-term debt
and revolving credit facility(2)
|
678 | 395 | 283 | — | — | |||||||||||||||
|
Other long-term obligations(3)
|
1,897 | 1,659 | 145 | 47 | 46 | |||||||||||||||
|
Total contractual
obligations
|
$ | 32,838 | $ | 18,873 | $ | 6,332 | $ | 2,923 | $ | 4,710 | ||||||||||
|
(1)
|
The balance on our revolving credit facilities at September 30, 2010 was $10,140. The terms of the revolving credit facilities do not require payment until the maturity date which is in three years. However, we classify this as a current liability as it is our intent to pay this amount in the next twelve months.
|
|
(2)
|
For debt instruments with variable interest rates and unused commitment fees, interest has been calculated for all future periods using the rates in effect at September 30, 2010.
|
|
(3)
|
Other long-term obligations include contracts related to information technology services, telecommunications and financial services. This also includes $222, which is an estimate of certain tax positions that are expected to be settled within the next fiscal year.
|
|
|
Guarantees
|
We provide guarantees, indemnifications and assurances to others in the ordinary course of our business. We have evaluated our agreements that contain guarantees and indemnification clauses in accordance with the guidance of financial accounting rules.
We enter into a wide range of indemnification arrangements in the ordinary course of business. These include tort indemnities, tax indemnities, indemnities against third party claims arising out of arrangements to provide services to us, indemnities in merger and acquisition agreements and indemnities in agreements related to the sale of our securities. Also, our governance documents and the governance documents of all of our subsidiaries provide for the indemnification of individuals made party to any suit or proceeding by reason of the fact that the individual was acting as an officer, director or agent of the relevant company or as a fiduciary of a company-sponsored welfare benefit plan. We also provide guarantees and indemnifications for the benefit of our wholly-owned subsidiaries for the satisfaction of performance obligations, including certain lease obligations. We are not aware of any material liabilities arising from these indemnification arrangements.
|
|
Inflation
|
Most of our operating expenses are inflation-sensitive, with inflation generally producing increased costs of operations. During the past three years, the most significant effects of inflation have been on employee wages, costs of products and fuel-intensive costs including freight, packing supplies and travel. We manage the effects of inflation by controlling increases in compensation expense, renegotiating freight carrier contracts and utilizing a central source for warehouse shipping supplies.
|
|
Critical Accounting Policies
|
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to sales returns, allowance for doubtful accounts, customer incentives, vendor rebates, inventories, goodwill and intangible assets, income taxes, impairment of long-lived assets, depreciation and amortization, employee benefits and contingencies. We base our estimates on historical experience and on various other assumptions and factors that are believed to be reasonable under the circumstances, the results of which form
33
the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We, based on our ongoing review, will make adjustments to our judgments and estimates where facts and circumstances dictate. Historically, actual results have not significantly deviated from those determined using the estimates described above.
We believe the following critical accounting policies are important to understand our financial condition and results of operations and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain.
|
|
Revenue Recognition
|
We sell products that we source from vendors to our customers through either a “buy/sell” transaction or an agency relationship with our vendors. In a “buy/sell” transaction, we purchase and take inventory of products from the vendor. When a customer places an order with us, we pick, pack, ship and invoice the customer for the order. We recognize revenue from “buy/sell” transactions as product sales when the product is delivered to the customer. We accept product returns from our customers. We estimate sales returns based on historical experience, and returns are recognized as a reduction of product sales. Product returns have not been significant to our financial statements. In an agency relationship, we generally do not purchase and take inventory of products from our vendors. We receive an order from a customer, then transmit the order to the vendor, who picks, packs and ships the order to the customer. In some cases, the vendor invoices and collects payment from the customer, while in other cases we invoice and collect payment from the customer on behalf of the vendor. We receive a commission payment for soliciting the order from the customer and for providing other customer service activities. Commissions are recognized when the services upon which the commissions are based are complete.
|
|
Vendor Rebates
|
Vendor rebates are recorded based on the terms of the contracts with each vendor. We may receive quarterly, semi-annual and annual performance-based rebates from third-party vendors based upon attainment of certain sales and/or purchase goals. Sales rebates are classified in our accompanying consolidated statements of income as a reduction to cost of product sales at the time the sales performance measures are achieved. Purchase rebates are measured against inventory purchases from the vendors and are a reduction of inventory until the product is sold. When the inventory is sold, purchase rebates are recognized as a reduction to cost of product sales.
Historically, actual results have not significantly deviated from those determined using the estimates described above. We expect that our estimates in the future will continue to be reasonable as our rebates are based on specific vendor program goals and are principally recorded upon achievement of sales or purchase performance measures. Vendors may change or eliminate rebate programs from year to year.
|
|
Customer Incentives
|
Customer incentives are accrued based on the terms of the contracts with each customer. These incentive programs provide that the customer receives an incentive based on their product purchases or attainment of performance goals. Incentives are estimated based on the specific terms in each agreement, historical experience and product growth rates. The incentives are recognized as a reduction of product sales.
|
|
Goodwill and Intangible Assets
|
We assess the potential impairment of goodwill and non-amortizing intangible assets annually and on an interim basis whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Factors that we consider important and may trigger an interim impairment review include:
|
|
·
|
significant underperformance relative to expected historical or projected future operating results;
|
|
|
·
|
significant changes in the manner of our use of acquired assets or the strategy of our overall business; and
|
|
|
·
|
significant negative industry or economic trends.
|
If we determine through the impairment review process that goodwill or non-amortizing intangible assets are impaired, an impairment charge is recognized in our consolidated statement of income.
Goodwill and non-amortizing intangible assets were evaluated for impairment in our fourth quarter of 2010 and we determined that the recorded amount was not impaired. The fair value calculations used for these tests require us to make assumptions about items that are inherently uncertain. Assumptions related to future market demand, market prices and product costs could vary from actual results, and the impact of such variations could be material. Factors that could affect the assumptions include changes in economic conditions, changes in government regulations, success in marketing products and
34
competitive conditions in our industry. The factors that most significantly affect the fair value calculation are market multiples and estimates of future cash flows. Fair value was determined using the discounted cash flow method.
|
|
Recently Issued and New Accounting Pronouncements
|
In June 2009, the Financial Accounting Standards Board issued authoritative guidance that amends the consolidation guidance applicable to variable interest entities and requires additional disclosures concerning an enterprise’s continuing involvement with variable interest entities. The guidance is effective for our fiscal year beginning October 1, 2010. We do not believe that this will have a material impact on our consolidated financial statements.
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
We are exposed to market risks primarily from changes in interest rates, in particular, the Daily LIBOR Floating Rate, and foreign currency translation risk. We do not engage in financial transactions for trading or speculative purposes. We do not hedge the translation of foreign currency profits into U.S. dollars. We continually evaluate our foreign currency exchange rate risk and the different options available for managing such risk.
We are now exposed to foreign currency risk due to the acquisition of Centaur. Prior to this acquisition, we had very limited foreign currency risk exposure. A hypothetical 10% change in the value of the U.S. dollar in relation to the British Pound, which is the Company’s most significant foreign currency exposure, would have changed fiscal 2010 net sales by approximately $16 million. This amount is not indicative of the hypothetical net earnings impact due to the partially offsetting impact of the currency exchange movements on cost of sales and operating expenses.
The interest payable on the facility is based on variable interest rates and is affected by changes in market interest rates. The outstanding balance on the revolving credit facility as of September 30, 2010 was $9,600. Therefore, there was limited exposure to market risks as of this date. If there had been a balance on the facility of $100,000, which is the maximum available amount on the facility, a change of 10% from the interest rate as of September 30, 2010, which was approximately 1.73% (Daily LIBOR Floating Rate plus a margin of 1.5%), would have changed interest by $173 for fiscal year 2010.
|
Item 8.
|
Financial Statements and Supplementary Data.
|
MWI Veterinary Supply, Inc.
Index to Consolidated Financial Statements
|
Page
Number
|
||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
MWI Veterinary Supply, Inc.
Meridian, Idaho
We have audited the accompanying consolidated balance sheets of MWI Veterinary Supply, Inc. and subsidiaries (the "Company") as of September 30, 2010 and 2009, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended September 30, 2010. Our audits also included the consolidated financial statement schedule listed in the Index at Item 15(a)2. These consolidated financial statements and consolidated financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on the consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of September 30, 2010 and 2009, and the results of their operations and their cash flows for each of the three years in the period ended September 30, 2010, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such consolidated financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note 10 to the consolidated financial statements, on October 1, 2007 the Company adopted the provisions of Accounting Standards Codification 740 (formerly Financial Accounting Standards Board Interpretation No. 48, Accounting for Uncertainty in Income Taxes – an interpretation of FASB Statement No. 109).
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company's internal control over financial reporting as of September 30, 2010, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated November 23, 2010, expressed an unqualified opinion on the Company's internal control over financial reporting.
/s/ DELOITTE & TOUCHE LLP
Boise, Idaho
November 23, 2010
|
|
|
|
|
|||||||||
|
MWI VETERINARY SUPPLY, INC.
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF INCOME
|
||||||||||||
|
For the Years Ended September 30, 2010, 2009 and 2008
|
||||||||||||
|
Dollars and shares in thousands, except per share amounts
|
||||||||||||
|
|
|
|
|
|||||||||
|
|
2010
|
2009
|
2008
|
|||||||||
|
Revenues:
|
|
|
|
|||||||||
|
Product sales
|
$ | 1,169,545 | $ | 880,703 | $ | 778,953 | ||||||
|
Product sales to related party
|
43,017 | 46,406 | 39,452 | |||||||||
|
Commissions
|
16,780 | 14,223 | 12,959 | |||||||||
|
Total revenues
|
1,229,342 | 941,332 | 831,364 | |||||||||
|
Cost of product sales
|
1,064,339 | 806,677 | 711,812 | |||||||||
|
Gross profit
|
165,003 | 134,655 | 119,552 | |||||||||
|
Selling, general and administrative expenses
|
105,793 | 90,827 | 84,123 | |||||||||
|
Depreciation and amortization
|
4,992 | 3,365 | 3,078 | |||||||||
|
Operating income
|
54,218 | 40,463 | 32,351 | |||||||||
|
|
||||||||||||
|
Other income (expense):
|
||||||||||||
|
Interest expense
|
(539 | ) | (242 | ) | (265 | ) | ||||||
|
Earnings of equity method investees
|
220 | 230 | 179 | |||||||||
|
Other
|
427 | 540 | 642 | |||||||||
|
Total other income (expense), net
|
108 | 528 | 556 | |||||||||
|
Income before taxes
|
54,326 | 40,991 | 32,907 | |||||||||
|
Income tax expense
|
(20,886 | ) | (16,086 | ) | (12,990 | ) | ||||||
|
Net income
|
$ | 33,440 | $ | 24,905 | $ | 19,917 | ||||||
|
|
||||||||||||
|
Earnings per common share:
|
||||||||||||
|
Basic
|
$ | 2.73 | $ | 2.06 | $ | 1.65 | ||||||
|
Diluted
|
$ | 2.70 | $ | 2.02 | $ | 1.62 | ||||||
|
|
||||||||||||
|
Weighted average common shares outstanding:
|
||||||||||||
|
Basic
|
12,241 | 12,088 | 12,053 | |||||||||
|
Diluted
|
12,395 | 12,306 | 12,301 | |||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
See notes to consolidated financial statements
|
||||||||||||
|
MWI VETERINARY SUPPLY, INC.
|
||||||||
|
CONSOLIDATED BALANCE SHEETS
|
||||||||
|
As of September 30, 2010 and 2009
|
||||||||
|
Dollars and shares in thousands, except per share amounts
|
||||||||
|
|
|
|
||||||
|
|
|
|
||||||
|
|
|
|
||||||
|
|
2010
|
2009
|
||||||
|
Assets
|
|
|
||||||
|
|
|
|
||||||
|
Current Assets:
|
|
|
||||||
|
Cash and cash equivalents
|
$ | 911 | $ | 14,302 | ||||
|
Receivables, net
|
189,428 | 142,485 | ||||||
|
Inventories
|
175,292 | 116,119 | ||||||
|
Prepaid expenses and other current assets
|
8,729 | 3,946 | ||||||
|
Deferred income taxes
|
1,556 | 1,517 | ||||||
|
Total current assets
|
375,916 | 278,369 | ||||||
|
|
||||||||
|
Property and equipment, net
|
15,238 | 9,313 | ||||||
|
Goodwill
|
47,330 | 37,610 | ||||||
|
Intangibles, net
|
26,710 | 10,194 | ||||||
|
Other assets, net
|
2,738 | 2,433 | ||||||
|
Total assets
|
$ | 467,932 | $ | 337,919 | ||||
|
|
||||||||
|
Liabilities And Stockholders’ Equity
|
||||||||
|
|
||||||||
|
Current Liabilities:
|
||||||||
|
Credit facilities
|
$ | 10,140 | $ | - | ||||
|
Accounts payable
|
183,604 | 117,830 | ||||||
|
Accrued expenses
|
15,118 | 10,767 | ||||||
|
Note payable
|
2,000 | - | ||||||
|
Current maturities of long-term debt and capital lease obligations
|
1,631 | 97 | ||||||
|
Total current liabilities
|
212,493 | 128,694 | ||||||
|
|
||||||||
|
Deferred income taxes
|
5,310 | 1,298 | ||||||
|
|
||||||||
|
Long-term debt and capital lease obligations
|
953 | - | ||||||
|
|
||||||||
|
Other long-term liabilities
|
2,389 | - | ||||||
|
|
||||||||
|
Commitments and contingencies (See Note 12)
|
||||||||
|
|
||||||||
|
Stockholders’ Equity
|
||||||||
|
Common stock $0.01 par value, 40,000 authorized; 12,457 and 12,196 shares issued and outstanding, respectively
|
125 | 122 | ||||||
|
Additional paid in capital
|
129,675 | 124,337 | ||||||
|
Retained earnings
|
116,908 | 83,468 | ||||||
|
Accumulated other comprehensive income
|
79 | - | ||||||
|
Total stockholders’ equity
|
246,787 | 207,927 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 467,932 | $ | 337,919 | ||||
|
|
||||||||
|
|
||||||||
|
|
||||||||
|
See notes to consolidated financial statements
|
||||||||
|
MWI VETERINARY SUPPLY, INC.
|
||||||||||||||||||||||||
|
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
|
||||||||||||||||||||||||
|
For the Years Ended September 30, 2010, 2009 and 2008
|
||||||||||||||||||||||||
|
Dollars and shares in thousands
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
|
||||||||||||||||||
|
|
Shares of
|
|
Additional
|
Accum.
|
|
|
||||||||||||||||||
|
|
Common
|
Common
|
Paid In
|
Other Comp.
|
Retained
|
|
||||||||||||||||||
|
|
Stock
|
Stock
|
Capital
|
Inc/(Loss)
|
Earnings
|
Total
|
||||||||||||||||||
|
Balance at October 1, 2007
|
12,024 | $ | 120 | $ | 120,988 | $ | — | $ | 38,903 | $ | 160,011 | |||||||||||||
|
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 19,917 | 19,917 | ||||||||||||||||||
|
Cumulative effect of change in accounting principle
|
||||||||||||||||||||||||
|
principle
|
— | — | — | — | (257 | ) | (257 | ) | ||||||||||||||||
|
Issuance of common stock
|
2 | — | 59 | — | — | 59 | ||||||||||||||||||
|
Issuance of restricted stock for
|
||||||||||||||||||||||||
|
purchase of Tri V
|
13 | — | 505 | — | — | 505 | ||||||||||||||||||
|
Exercises of common stock options
|
19 | — | 218 | — | — | 218 | ||||||||||||||||||
|
Tax benefit of common stock
|
— | — | 216 | — | — | 216 | ||||||||||||||||||
|
exercises
|
||||||||||||||||||||||||
|
Issuance of stock awards, net of forfeitures
|
40 | 1 | 333 | — | — | 334 | ||||||||||||||||||
|
Balance at September 30, 2008
|
12,098 | 121 | 122,319 | — | 58,563 | 181,003 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 24,905 | 24,905 | ||||||||||||||||||
|
Issuance of common stock
|
9 | — | 240 | — | — | 240 | ||||||||||||||||||
|
Exercises of common stock options
|
81 | 1 | 142 | — | — | 143 | ||||||||||||||||||
|
Tax benefit of common stock
|
||||||||||||||||||||||||
|
exercises
|
— | — | 1,178 | — | — | 1,178 | ||||||||||||||||||
|
Issuance of stock awards, net of forfeitures
|
8 | — | 458 | — | — | 458 | ||||||||||||||||||
|
Balance at September 30, 2009
|
12,196 | 122 | 124,337 | — | 83,468 | 207,927 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
|
Net income
|
— | — | — | — | 33,440 | 33,440 | ||||||||||||||||||
|
Issuance of common stock
|
6 | — | 248 | — | — | 248 | ||||||||||||||||||
|
Exercises of common stock options
|
199 | 2 | 401 | — | — | 403 | ||||||||||||||||||
|
Tax benefit of common stock
|
||||||||||||||||||||||||
|
exercises
|
— | — | 3,611 | — | — | 3,611 | ||||||||||||||||||
|
Issuance of stock awards, net of forfeitures
|
56 | 1 | 1,078 | — | 1,079 | |||||||||||||||||||
|
Foreign currency translation
|
— | — | — | 536 | — | 536 | ||||||||||||||||||
|
Other
|
— | — | — | (457 | ) | — | (457 | ) | ||||||||||||||||
|
Balance at September 30, 2010
|
12,457 | $ | 125 | $ | 129,675 | $ | 79 | $ | 116,908 | $ | 246,787 | |||||||||||||
|
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
|
||||||||||||||||||||||||
|
See notes to consolidated financial statements
|
||||||||||||||||||||||||
|
MWI VETERINARY SUPPLY, INC.
|
||||||||||||
|
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
||||||||||||
|
For the Years Ended September 30, 2010, 2009 and 2008
|
||||||||||||
|
Dollars in thousands
|
||||||||||||
|
|
|
|
|
|||||||||
|
|
2010
|
2009
|
2008
|
|||||||||
|
Cash Flows From Operating Activities:
|
|
|
|
|||||||||
|
Net income
|
$ | 33,440 | $ | 24,905 | $ | 19,917 | ||||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||||||
|
Depreciation and amortization
|
5,004 | 3,377 | 3,089 | |||||||||
|
Amortization of debt issuance costs
|
58 | 43 | 43 | |||||||||
|
Stock-based compensation
|
1,088 | 482 | 334 | |||||||||
|
Deferred income taxes
|
249 | (161 | ) | (14 | ) | |||||||
|
Earnings of equity method investees
|
(220 | ) | (230 | ) | (179 | ) | ||||||
|
Excess tax benefit of exercise of common stock options
|
(3,611 | ) | (1,178 | ) | (216 | ) | ||||||
|
(Gain)/loss on disposal of property and equipment
|
(11 | ) | 29 | 2 | ||||||||
|
Pension payment
|
(2,047 | ) | - | - | ||||||||
|
Other
|
97 | - | - | |||||||||
|
Changes in operating assets and liabilities (net of effects of business acquisitions):
|
||||||||||||
|
Receivables
|
(14,154 | ) | (14,019 | ) | (11,180 | ) | ||||||
|
Inventories
|
(41,106 | ) | 2,072 | (23,543 | ) | |||||||
|
Prepaid expenses and other current assets
|
(2,419 | ) | (760 | ) | (794 | ) | ||||||
|
Accounts payable
|
39,781 | (4,712 | ) | 22,203 | ||||||||
|
Accrued expenses
|
717 | 2,091 | 960 | |||||||||
|
Net cash provided by operating activities
|
16,866 | 11,939 | 10,622 | |||||||||
|
Cash Flows From Investing Activities:
|
||||||||||||
|
Business acquisitions, net of cash acquired in 2010 of $674
|
(39,645 | ) | 117 | (13,224 | ) | |||||||
|
Purchases of property and equipment
|
(4,388 | ) | (2,662 | ) | (3,246 | ) | ||||||
|
Other
|
(57 | ) | 25 | 272 | ||||||||
|
Net cash used in investing activities
|
(44,090 | ) | (2,520 | ) | (16,198 | ) | ||||||
|
Cash Flows From Financing Activities:
|
||||||||||||
|
Borrowings on line-of-credit
|
151,616 | 183,300 | 113,040 | |||||||||
|
Payments on line-of-credit
|
(141,500 | ) | (183,300 | ) | (113,040 | ) | ||||||
|
Proceeds from issuance of common stock
|
248 | 240 | 59 | |||||||||
|
Proceeds from exercise of common stock options
|
403 | 143 | 218 | |||||||||
|
Tax benefit of exercise of common stock options
|
3,611 | 1,178 | 216 | |||||||||
|
Payment of debt issuance costs
|
(116 | ) | - | - | ||||||||
|
Payment on long-term debt and capital lease obligations
|
(477 | ) | (97 | ) | (97 | ) | ||||||
|
Net cash provided by financing activities
|
13,785 | 1,464 | 396 | |||||||||
|
|
||||||||||||
|
Effect of exchange rate on Cash and Cash Equivalents
|
48 | - | - | |||||||||
|
|
||||||||||||
|
Net Increase/(Decrease) in Cash and Cash Equivalents
|
(13,391 | ) | 10,883 | (5,180 | ) | |||||||
|
Cash and Cash Equivalents at Beginning of Period
|
14,302 | 3,419 | 8,599 | |||||||||
|
Cash and Cash Equivalents at End of Period
|
$ | 911 | $ | 14,302 | $ | 3,419 | ||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
See notes to consolidated financial statements
|
||||||||||||
|
|
MWI VETERINARY SUPPLY, INC.
|
|
|
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
Dollars and sterling pounds in thousands, except share and per share data
|
|
|
1. Business Description and Basis of Presentation
|
MWI Veterinary Supply, Inc. is a leading distributor of animal health products to veterinarians in the United States and United Kingdom. We sell our products to veterinarians in both the companion and production animal markets. We operate twelve distribution centers located across the United States and one distribution center in the United Kingdom.
|
|
2. Summary of Significant Accounting Policies
|
Principles of Consolidation — The accompanying consolidated financial statements consist of MWI Veterinary Supply, Inc. and its wholly-owned subsidiaries, collectively referred to herein as “MWI.” All intercompany transactions have been eliminated. We use the equity method of accounting for our investments in entities in which we have significant influence; generally this represents an ownership interest between 20% and 50%. Our share of income or loss from these investments is reported as increases or decreases in the respective investment with a corresponding amount reported as other income.
Basis of Accounting and Use of Estimates — The accompanying consolidated financial statements have been prepared on the accrual basis of accounting using accounting principles generally accepted in the United States. In preparing financial information, we use certain estimates and assumptions that may affect the reported amounts and disclosures. Some of these estimates require difficult, subjective and complex judgments about matters that are inherently uncertain. As a result, actual results could differ from these estimates. Estimates are used when accounting for sales returns, allowance for doubtful accounts, customer incentives, vendor rebates, inventories, goodwill and intangible assets, income taxes, impairment of long-lived assets, depreciation and amortization, employee benefits, and contingencies. The estimates of fair value of assets and liabilities and the disclosure of contingent assets and liabilities as of the balance sheet date and reported amounts of revenue and expenses for the periods are based on assumptions that we believe to be reasonable.
Segment Information — We are a distributor of animal health products to veterinarians. Our financial results are disclosed as one reportable segment. We identified two operating segments based on geographic areas but aggregate based on applicable accounting standards. We determined that the two operating segments have similar operating margins and are expected to maintain this similarity into the future. Additionally, our products, customers, operations, delivery to market and regulatory environments are all similar in nature.
Foreign Currency Translation — For our international operations, local currencies have been determined to be the functional currencies. We translate functional currency assets and liabilities to their U.S. dollar equivalents at rates in effect at the balance sheet date and record these translation adjustments in Stockholders’ equity – Accumulated other comprehensive income/(loss). We translate functional currency statement of income amounts to their U.S. dollar equivalents at average rates for the period.
Other Comprehensive Income — Comprehensive income includes cumulative foreign currency translation adjustments and actuarial loss on pension valuation. Comprehensive income has been reflected in the consolidated statements of stockholders’ equity and in Note 15 – Other Comprehensive Income.
Revenue Recognition — We sell products we source from vendors to our customers through either a “buy/sell” transaction or an agency relationship with our vendors. In a “buy/sell” transaction, we purchase or take inventory of products from the vendor. When a customer places an order with us, we pick, pack, ship and invoice the customer for the order. We recognize revenue from “buy/sell” transactions as product sales when the product is delivered to the customer. We accept product returns from our customers. We estimate returns based on historical experience and recognize these estimated returns as a reduction of product sales. Product returns have not been significant to our financial statements. We record revenues net of sales tax. In an agency relationship, we generally do not purchase and take inventory of products from vendors. We receive an order from a customer, then transmit the order to the vendor, who picks, packs and ships the order to the customer. In some cases, the vendor invoices and collects payment from the customer, while in other cases we invoice and collect payment from the customer on behalf of the vendor. We receive a commission payment for soliciting the order from the customer and for providing other customer service activities. Commissions are recognized when the services upon which the commissions are based are complete. Gross billings from agency contracts were $328,053, $269,057 and $245,045 for the
42
years ended September 30, 2010, 2009 and 2008, respectively, and generated commission revenue of $16,780, $14,223 and $12,959 for the years ended September 30, 2010, 2009 and 2008, respectively.
Cost of Product Sales and Vendor Rebates — Cost of product sales consist of our inventory product cost, including shipping costs to and from our distribution centers. Vendor rebates are recorded based on the terms of the contracts or programs with each vendor. We receive quarterly, semi-annual and annual performance-based rebates from third-party vendors based upon attainment of certain sales and/or purchase goals. Sales rebates are classified in the accompanying consolidated statements of income as a reduction to cost of product sales at the time the sales performance measures are achieved. Purchase rebates are measured against inventory purchases from the vendors and are classified as a reduction of inventory until the product is sold. When the inventory is sold and purchase measures are achieved, purchase rebates are recognized as a reduction to cost of product sales.
Historically, actual results have not significantly deviated from those determined using the estimates described above. We expect that our estimates in the future will continue to be reasonable as our rebates are based on specific vendor program goals and are principally recorded upon achievement of sales or purchase performance measures. Vendors may change or eliminate rebate programs from year to year.
Customer Incentives — Customer incentives are accrued based on the terms of the contracts with each customer. These incentive programs provide that the customer receive an incentive based on their product purchases or attainment of performance goals. Incentives are estimated based on the specific terms in each agreement, historical experience and product growth rates. Incentives are recognized as a reduction to product sales.
Cash and Cash Equivalents — Cash equivalents consist of highly liquid investments with a maturity of three months or less from the date of purchase. Our banking arrangements allow us to fund outstanding checks when presented to the financial institution for payment.
Inventories — Inventories, consisting of pharmaceuticals, vaccines, parasiticides, diagnostics, capital equipment, supplies and nutritional products, are stated at the lower of cost (on a moving-average basis) or market.
Property and Equipment — Property and equipment are stated at cost and depreciation is computed using the straight-line method over the estimated useful lives, which includes the shorter of useful life or lease term for leasehold improvements, of the related assets as follows:
|
Building
|
25 years
|
||
|
Machinery, furniture and equipment
|
3 to 7 years
|
||
|
Computer hardware and software
|
3 to 7 years
|
||
|
Leasehold improvements
|
1 to 10 years
|
The cost and accumulated depreciation of items sold or retired are removed from the property accounts and any resulting gain or loss is reflected in net income. Repairs and maintenance are expensed as incurred and renewals and improvements are capitalized.
We periodically review long-lived assets for impairment when events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. No impairments were identified during the fiscal years ended September 30, 2010 and 2009.
Goodwill and Intangible Assets — We recognize the excess purchase price over the fair value of net assets acquired and liabilities assumed in a business combination as goodwill on the consolidated balance sheet. Goodwill and non-amortizing intangible assets, consisting of trade names and patents, are tested for impairment at least annually. We perform an annual impairment test as of September 30th each year. We calculate the fair value of each reporting unit using a discounted cash flow analysis and compare the fair value to its book value. There were no indicators during this evaluation that would require additional testing. We have concluded that there was no impairment during the fiscal years ended September 30, 2010, 2009 and 2008. These impairment tests will continue to be performed at least annually and more frequently if circumstances indicate a possible impairment. Amortizing intangible assets primarily include customer relationships and covenants not to compete and are amortized over their useful lives or contractual term which range from 1-20 years.
We review identifiable intangible assets at least annually, excluding goodwill and non-amortizing intangible assets for impairment when events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. No impairments were identified during the fiscal years ended September 30, 2010, 2009 and 2008.
Other Assets — Included in other assets are our equity method investments and investments in entities accounted for under the cost method of accounting. Other assets also consist of debt issuance costs that are being amortized over the life of the related debt.
Earnings Per Common Share — Basic earnings per common share is calculated based on the weighted-average number of outstanding common shares during the applicable period. Diluted earnings per common share is based on the weighted-average number of outstanding common shares plus the weighted-average number of potential outstanding common shares. Potential common shares that would increase earnings per share amounts or decrease loss per share amounts are antidilutive and are, therefore, excluded from the earnings per common share computations. Earnings per common share is computed separately for each period presented.
Income Taxes — Income taxes are accounted for under the asset and liability method. Deferred income taxes are recognized to provide for temporary differences between the financial reporting and tax basis of assets and liabilities. Deferred taxes are measured using enacted tax rates in effect during the years in which the temporary differences are expected to reverse.
Concentrations of Risk — Our financial instruments that are exposed to concentrations of credit risk consist primarily of our receivables. Our customers are geographically dispersed throughout the United States and United Kingdom. We routinely assess the financial strength of our customers and review their credit history before extending credit. In addition, we establish an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information.
Product sales to Banfield were approximately 9% of our total product sales in fiscal year 2010, 11% in fiscal year 2009 and 10% in fiscal year 2008. Product sales to Feeder’s Advantage, a related party (See Note 13), were approximately 4% of our total product sales in fiscal year 2010 and 5% of our total product sales in fiscal years 2009 and 2008.
Advertising — Advertising costs are expensed when incurred and are included as part of selling, general and administrative expenses. Advertising costs were $717, $783 and $563 in fiscal years 2010, 2009 and 2008, respectively.
Recently Issued and New Accounting Pronouncements — In June 2009, the Financial Accounting Standards Board issued authoritative guidance that amends the consolidation guidance applicable to variable interest entities and requires additional disclosures concerning an enterprise’s continuing involvement with variable interest entities. The guidance is effective for our fiscal year beginning October 1, 2010. We do not believe that this will impact our consolidated financial statements.
|
|
3. Business Acquisitions
|
In October 2007, we acquired substantially all of the assets of Tri V Services, Inc. (“Tri V”) for approximately $5,111, consisting of $4,606 in cash (including direct acquisition costs of approximately $106) and 12,692 shares of restricted common stock valued at the time of the issuance at approximately $505. The purchase price was reduced by $8 as a result of a post closing working capital adjustment. Based near Detroit, Michigan, Tri V was a distributor with an 11-year history of providing animal health products to approximately 850 veterinary practices, with a particular focus on emergency clinics and ophthalmology specialists. The amount recorded in goodwill is expected to be deductible for tax purposes over 15 years.
In July 2008, we acquired substantially all of the assets of AAHA Services Corporation, operating as AAHA MARKETLink (“AAHA MARKETLink”) for approximately $8,676, net of cash acquired of $1,320 (including direct acquisition costs of approximately $212). The purchase price was reduced by $117 as a result of a post closing working capital adjustment. Based near Denver, Colorado, AAHA MARKETLink was a distributor of animal health products to members of the American Animal Hospital Association. The amount recorded in goodwill is expected to be deductible for tax purposes over 15 years.
On February 8, 2010, MWI Veterinary Supply Co. (“MWI Co.”) purchased all of the outstanding share capital of Centaur Services Limited (“Centaur”), based in the United Kingdom for an initial purchase price of $44,053, consisting of $42,053 in cash and $2,000 in a note payable due in one year. Subsequent to the acquisition of Centaur, we funded $2,047 to the pension plan as required by the terms of the share purchase agreement. The purchase price was reduced subsequent to the acquisition date by $1,868 as a result of a post-closing working capital and debt adjustment. Centaur is a supplier of animal health products to veterinarians in the United Kingdom. Centaur sells products to both the companion animal market and production animal market. The acquisition of Centaur has allowed us to expand into the international markets. We incurred $1,100 of direct acquisition-related expenses. The intangible assets acquired in the acquisition have estimated useful lives between 1 and 20 years, which include customer relationships, trade names and other intangible assets. The amount recorded in goodwill will not be deductible for tax purposes.
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of each acquisition, as adjusted during the allocation period as defined in ASC 280. These purchase price allocations are based on a combination of valuations and other analyses.
|
|
2010
|
2009
|
2008
|
||||||
|
Cash
|
$ | 674 | $ | - | $ | - | |||
|
Receivables
|
32,527 | - | 5,705 | ||||||
|
Inventories
|
17,830 | - | 237 | ||||||
|
Other current assets
|
480 | - | 12 | ||||||
|
Property and equipment
|
5,275 | - | 5 | ||||||
|
Goodwill
|
9,483 | (117 | ) | 4,763 | |||||
|
Intangibles
|
17,658 | - | 6,569 | ||||||
|
Total assets acquired
|
83,927 | (117 | ) | 17,291 | |||||
|
|
|||||||||
|
Accounts payable
|
25,811 | - | 3,562 | ||||||
|
Accrued expenses
|
5,455 | - | - | ||||||
|
Other liabilities
|
10,476 | - | - | ||||||
|
Total liabilities assumed
|
41,742 | - | 3,562 | ||||||
|
|
|||||||||
|
Net assets acquired
|
$ | 42,185 | $ | (117 | ) | $ | 13,729 | ||
The following table presents information for Centaur that is included in our consolidated statements of income from the acquisition date of February 8, 2010 through the fiscal year ended September 30, 2010:
|
|
Centaur's operations included in MWI's results
|
||
|
|
Fiscal year ended September 30, 2010
|
||
|
Revenues
|
$ | 155,116 | |
|
Net Income
|
$ | 2,302 | |
The following table presents supplemental pro forma information for the Company as if the acquisition of Centaur had occurred on October 1, 2009 for the periods ended September 30, 2010 and on October 1, 2008 for the periods ended September 30, 2009 (unaudited):
|
|
Unaudited Pro Forma Consolidated Results
|
||||||
|
|
Fiscal year ended September 30,
|
||||||
|
|
2010
|
2009
|
|||||
|
Revenues
|
$ | 1,314,046 | $ | 1,154,761 | |||
|
Net Income
|
$ | 34,726 | $ | 27,213 | |||
For the pro forma calculation, we used an average foreign currency exchange rate for each of the periods presented and the annual net income as a percentage of revenues for purposes of determining the net income for interim periods. The unaudited pro forma consolidated results are not necessarily indicative of what our consolidated results of operations would have been had we completed the acquisition on October 1, 2009 and on October 1, 2008. Additionally, the unaudited pro forma consolidated results do not purport to project the future results of operations of the combined company.
|
|
4. Receivables
|
Receivables consist of the following at September 30:
|
|
2010
|
2009
|
||||||
|
Trade
|
$ | 177,317 | $ | 132,369 | ||||
|
Vendor rebates and programs
|
14,681 | 13,122 | ||||||
|
|
191,998 | 145,491 | ||||||
|
Allowance for doubtful accounts
|
(2,570 | ) | (3,006 | ) | ||||
|
|
$ | 189,428 | $ | 142,485 | ||||
Approximately 8% and 13% of our trade receivables resulted from transactions with a single customer as of September 30, 2010 and 2009, respectively.
|
|
5. Property and Equipment
|
Property and equipment consists of the following at September 30:
|
|
2010
|
2009
|
||||||
|
Land
|
$ | 261 | $ | 20 | ||||
|
Building and leasehold improvements
|
5,870 | 3,566 | ||||||
|
Machinery, furniture and equipment
|
17,495 | 12,792 | ||||||
|
Computer equipment
|
4,886 | 3,325 | ||||||
|
Construction in progress
|
1,626 | 1,140 | ||||||
|
|
30,138 | 20,843 | ||||||
|
Accumulated depreciation and amortization
|
(14,900 | ) | (11,530 | ) | ||||
|
|
$ | 15,238 | $ | 9,313 | ||||
We recorded depreciation expense of $3,692, $2,546 and $2,441 for the years ended September 30, 2010, 2009 and 2008, respectively.
|
|
6. Goodwill and Intangibles
|
The changes in the carrying value of goodwill for the fiscal years ended September 30, 2010 and 2009 are as follows:
|
2010
|
2009
|
|||||||
|
Goodwill - Beginning of year
|
$ | 37,610 | $ | 37,727 | ||||
|
Acquisition activity
|
9,483 | - | ||||||
|
Other (1)
|
237 | (117 | ) | |||||
|
Goodwill - End of year
|
$ | 47,330 | $ | 37,610 | ||||
|
|
||||||||
|
(1) In fiscal year 2009, relates to post-closing working capital adjustment for the AAHA Marketlink acquisition. In fiscal year 2010, relates to foreign exchange and contingent consideration payment to the former Securos shareholders for the achievement of defined financial targets.
|
||||||||
Intangible assets consist of the following as of September 30:
|
|
Useful Lives
|
2010
|
2009
|
||||||
|
Amortizing:
|
|
|
|
||||||
|
Customer relationships
|
9-20 years
|
$ | 25,027 | $ | 9,076 | ||||
|
Covenants not to compete
|
1-5 years
|
811 | 686 | ||||||
|
Other
|
3-5 years
|
458 | 257 | ||||||
|
|
|
26,296 | 10,019 | ||||||
|
Accumulated amortization
|
|
(3,361 | ) | (2,177 | ) | ||||
|
|
|
22,935 | 7,842 | ||||||
|
Non-Amortizing:
|
|
||||||||
|
Trade names and patents
|
|
3,775 | 2,352 | ||||||
|
|
|
$ | 26,710 | $ | 10,194 | ||||
We recorded amortization expense of $1,312, $831 and $648 for the years ended September 30, 2010, 2009 and 2008, respectively. Estimated amortization expense related to intangible assets as of September 30, 2010 is as follows:
|
|
Amount
|
|||
|
2011
|
$ | 1,626 | ||
|
2012
|
1,597 | |||
|
2013
|
1,513 | |||
|
2014
|
1,507 | |||
|
2015
|
1,809 | |||
|
Thereafter
|
14,883 | |||
|
|
$ | 22,935 | ||
|
|
7. Debt
|
Outstanding debt consists of the following as of September 30:
|
|
2010
|
2009
|
||||||
|
Revolving credit facility
|
$ | 9,600 | $ | - | ||||
|
Fortis credit facility (1)
|
540 | - | ||||||
|
Note payable to AHN (UK) Holdings Limited (2)
|
2,000 | - | ||||||
|
Capital lease obligations (3)
|
1,811 | - | ||||||
|
Term note (3)
|
773 | - | ||||||
|
Other long-term debt
|
- | 97 | ||||||
|
Total debt and capital lease obligations
|
14,724 | 97 | ||||||
|
Less: Long-term debt and capital lease obligations
|
(953 | ) | - | |||||
|
Total debt included in current liabilities
|
$ | 13,771 | $ | 97 | ||||
|
|
||||||||
|
(1) Fortis credit facility was retired on November 5, 2010, and Centaur, an indirect subsidiary of MWI, entered into a £12,500 unsecured revolving line of credit facility with Wells Fargo Bank, N.A. London Branch.
|
||||||||
|
(2) Note payable is related to the acquisition of Centaur and is due February 8, 2011.
|
||||||||
|
(3) Term note and capital lease obligations were assumed as a result of the acquisition of Centaur. The term note matures in March 2011 and the capital lease obligations have varying maturity dates.
|
||||||||
Revolving Credit Facility — On February 8, 2010 and August 10, 2010, MWI Co., our wholly-owned subsidiary as borrower, entered into a First Amendment (the “First Amendment”) and Second Amendment (the “Second Amendment”), respectively, to its Credit Agreement with us and Memorial Pet Care, Inc., as guarantors, and Bank of America, N.A. and Wells Fargo Bank, N.A., (collectively, the “lenders”) amending the Credit Agreement dated December 13, 2006 among MWI
47
Co., MWI Veterinary Supply, Inc., Memorial Pet Care, Inc. and the lenders a party thereto (the “facility”). The First Amendment increased the aggregate revolving commitment of the lenders under the facility from $70,000 to $100,000. The First Amendment also extended the maturity date of the loans under the facility from December 1, 2011 to March 1, 2013. The variable interest rate is now equal to the Daily LIBOR Floating Rate or the LIBOR 1-month fixed rate (at MWI Co.’s option) plus a margin ranging from 1.50% to 2.25%, which was previously 1.75% to 2.50% under the First Amendment, and 0.7% to 1.25% under the facility. The lenders also receive an unused line fee and letter of credit fee which is now equal to 0.2% of the unused amount of the facility, which was previously 0.125%. The Second Amendment increased the amount of permitted unsecured indebtedness from $20 million to $30 million. The facility contains financial covenants, including a fixed charge ratio and a funded debt to EBITDA calculation. We were in compliance with both of these covenants as of September 30, 2010. Our outstanding balance on the revolving credit facility at September 30, 2010 was $9,600, and the interest rate was 1.73% as of September 30, 2010.
Fortis credit facility— As of September 30, 2010, Centaur operated with a credit facility (“Fortis facility”) with Fortis Bank as the lender. The Centaur facility allowed for borrowings in the aggregate of £12,000. The Fortis facility had a variable interest rate equal to a base rate of 0.50% plus GBP one-month LIBOR plus a margin of 0.85%. The outstanding balance at September 30, 2010 on the Fortis facility was £342, or $540 using the exchange rate on September 30, 2010. The interest rate for the Fortis facility was 1.90% as of September 30, 2010.
On November 5, 2010, Centaur entered into a £12,500 unsecured revolving line of credit facility (the “sterling revolving credit facility”) with Wells Fargo Bank, N.A. London Branch (“Wells Fargo”). The sterling revolving credit facility is for a three year term with interest paid at the end of the applicable one month, three month or six month interest period. Interest is based on LIBOR for the applicable interest period plus an applicable margin of 1.05% to 1.90%. The facility contains financial covenants requiring Centaur to maintain a minimum tangible net worth of £3,000.
|
|
8. Common Stock and Stock-Based Awards
|
|
|
2002 Stock Plan
|
We have a 2002 Stock Plan (the “2002 Plan”) to provide our directors, executives and other key employees with additional incentives by allowing them to acquire an ownership interest in us and, as a result, encouraging them to contribute to our success. At September 30, 2010 and 2009, we had 164,788 and 344,220 shares, respectively, of our common stock available for issuance under the 2002 Plan. The options granted under the 2002 Plan are nonqualified stock options that have an exercise price per share equal to fair market value of the common stock at the time of grant. The term of each option is determined by our board of directors or by a designated committee of the board. The term of any option may not exceed ten years from the date of grant.
|
|
2005 Stock Plan
|
We have a 2005 Stock-Based Award and Incentive Compensation Plan (the “2005 Plan”), under which we may offer restricted shares of our common stock and grant options to purchase shares of our common stock to selected employees and non-employee directors. The purpose of the 2005 Plan is to promote our long-term financial success by attracting, retaining and rewarding eligible participants. At September 30, 2010 and 2009 we had 991,970 and 1,064,752 shares, respectively, of our common stock available for issuance under the 2005 Plan.
The 2005 Plan permits us to grant stock options (both incentive stock options and non-qualified stock options), restricted stock and deferred stock. The compensation committee will determine the number and type of stock-based awards to each participant, the exercise price of each award, the duration of the award (not to exceed ten years), vesting provisions and all other terms and conditions of such award in individual award agreements. The 2005 Plan provides that upon termination of employment with us, unless determined otherwise by the compensation committee at the time options are granted, the exercise period for vested awards will generally be limited, provided that vested awards will be canceled immediately upon a termination for cause or voluntary termination. The 2005 Plan provides for the cancellation of all unvested awards upon termination of employment with us, unless determined otherwise by the compensation committee at the time awards are granted.
|
A summary of common stock options activity under the 2002 and 2005 Plans are as follows:
|
|
||||||||||||||
|
|
2010
|
2009
|
2008
|
||||||||||||
|
|
|
|
Weighted
|
|
Weighted
|
|
Weighted
|
||||||||
|
|
|
|
average
|
|
average
|
|
average
|
||||||||
|
|
Number of
|
|
exercise
|
Number of
|
exercise
|
Number of
|
exercise
|
||||||||
|
|
Shares
|
|
price
|
Shares
|
price
|
Shares
|
price
|
||||||||
|
Outstanding at beginning of year
|
339,404
|
|
$
|
3.11
|
|
420,898
|
|
$
|
2.87
|
|
441,502
|
|
$
|
$3.27
|
|
|
Granted
|
—
|
|
|
—
|
|
—
|
|
|
—
|
|
—
|
|
|
—
|
|
|
Exercised
|
(199,670)
|
|
|
2.02
|
|
(81,004)
|
|
|
1.77
|
|
(19,618)
|
|
|
11.11
|
|
|
Cancelled or expired
|
(184)
|
|
|
17.00
|
|
(490)
|
|
|
17.57
|
|
(986)
|
|
|
17.00
|
|
|
Outstanding at end of year
|
139,550
|
|
$
|
4.65
|
|
339,404
|
|
$
|
3.11
|
|
420,898
|
|
$
|
$2.87
|
|
|
Exercisable at end of year
|
139,550
|
|
$
|
4.65
|
|
339,404
|
|
$
|
3.11
|
|
364,490
|
|
$
|
$3.28
|
|
|
Expected to vest
|
—
|
|
|
—
|
|
—
|
|
|
—
|
|
56,408
|
|
$ |
0.18
|
|
The intrinsic value of the shares outstanding and exercisable as of September 30, 2010 was $7,405.
|
|
Outstanding and exercisable options
|
||||||||
|
|
|
|
Weighted
|
|
|
|
|
||
|
|
|
|
average
|
|
|
|
|
||
|
|
|
|
remaining
|
|
Weighted
|
||||
|
|
|
|
contractual
|
|
average
|
||||
|
|
Number of
|
|
life
|
|
exercise
|
||||
|
Range of exercise prices
|
Shares
|
|
(in years)
|
|
price
|
||||
|
$ 0.18 - $16.99
|
104,348
|
|
|
1.7
|
|
|
$
|
0.18
|
|
|
$17.00 - $19.99
|
29,092
|
|
|
4.8
|
|
|
$
|
17.00
|
|
|
$20.00 - $23.06
|
6,110
|
|
|
5.0
|
|
|
$
|
22.25
|
|
During the fiscal years ended September 30, 2010 and 2009, we issued stock awards of 58,350 and 11,500 shares, respectively. During the fiscal years ended September 30, 2010 and 2009, there were 2,427 and 3,210 shares forfeited, respectively, related to the vesting of stock awards. During the fiscal years ended September 30, 2010 and 2009, we recognized $1,151 and $583, respectively, in compensation expense related to common stock awards.
|
|
2008 Employee Stock Purchase Plan
|
In February 2008, we approved the 2008 Employee Stock Purchase Plan (the “ESPP”). The plan allows substantially all employees to purchase shares of our common stock at 95% of the fair market value on the date of purchase. The purchase date is the last trading date of the purchase period, which begins in March, June, September and December. Employees accumulate amounts through payroll deductions during the purchase period of between 1% and 10% but no more than $20,000 annually. An employee is allowed to purchase a maximum of 200 shares per purchase period. We issued 5,852 and 8,998 shares of our common stock under the ESPP during fiscal years ended September 30, 2010 and 2009, respectively.
9. Computation Of Earnings Per Common Share (In thousands, except per share data)
|
|
2010
|
2009
|
2008
|
|||||||||||||||||||||
|
|
Basic
|
Diluted
|
Basic
|
Diluted
|
Basic
|
Diluted
|
||||||||||||||||||
|
Net income
|
$ | 33,440 | $ | 33,440 | $ | 24,905 | $ | 24,905 | $ | 19,917 | $ | 19,917 | ||||||||||||
|
Weighted average common
|
||||||||||||||||||||||||
|
shares outstanding
|
12,241 | 12,241 | 12,088 | 12,088 | 12,053 | 12,053 | ||||||||||||||||||
|
Effect of diluted securities
|
||||||||||||||||||||||||
|
Stock options and restricted stock
|
154 | 218 | 248 | |||||||||||||||||||||
|
Weighted average shares
|
||||||||||||||||||||||||
|
outstanding
|
12,395 | 12,306 | 12,301 | |||||||||||||||||||||
|
Earnings per share
|
$ | 2.73 | $ | 2.70 | $ | 2.06 | $ | 2.02 | $ | 1.65 | $ | 1.62 | ||||||||||||
|
Anti-dilutive shares
|
||||||||||||||||||||||||
|
excluded from calculation
|
- | - | - | |||||||||||||||||||||
10. Income Taxes
The components of income tax expense consist of the following:
|
|
2010
|
2009
|
2008
|
|||||||||
|
United States
|
|
|
|
|||||||||
|
Current payable
|
|
|
|
|||||||||
|
Federal
|
$ | 17,060 | $ | 13,868 | $ | 10,752 | ||||||
|
State
|
2,751 | 2,379 | 1,964 | |||||||||
|
Deferred
|
||||||||||||
|
Federal
|
538 | (136 | ) | 236 | ||||||||
|
State
|
95 | (25 | ) | 38 | ||||||||
|
Total U.S. tax expense
|
20,444 | 16,086 | 12,990 | |||||||||
|
International
|
||||||||||||
|
Current payable
|
826 | — | — | |||||||||
|
Deferred
|
(384 | ) | — | — | ||||||||
|
Total international tax expense
|
442 | — | — | |||||||||
|
Total income tax expense
|
$ | 20,886 | $ | 16,086 | $ | 12,990 | ||||||
Our deferred tax assets and liabilities consist of the following at September 30:
|
|
2010
|
2009
|
||||||
|
Deferred tax assets:
|
|
|
||||||
|
Allowance for doubtful accounts
|
$ | 996 | $ | 1,166 | ||||
|
Inventories
|
358 | 312 | ||||||
|
Revenue recognition
|
175 | 182 | ||||||
|
Lease expense
|
192 | 226 | ||||||
|
Employee benefits
|
1,012 | — | ||||||
|
Other
|
456 | 202 | ||||||
|
Total deferred tax assets
|
3,189 | 2,088 | ||||||
|
Deferred tax liabilities:
|
||||||||
|
Property and equipment
|
(6,481 | ) | (1,447 | ) | ||||
|
Prepaid expenses
|
(306 | ) | (284 | ) | ||||
|
Other
|
(156 | ) | (138 | ) | ||||
|
Total deferred tax liabilities
|
(6,943 | ) | (1,869 | ) | ||||
|
Net deferred tax assets/(liabilities)
|
$ | (3,754 | ) | $ | 219 | |||
We believe realization of these deferred assets is more likely than not. Accordingly, no valuation allowance has been recorded.
Income tax expense differed from income taxes at the U.S. federal statutory tax rate for all periods presented as follows:
|
|
|
2010
|
|
2009
|
|
2008
|
|||
|
|
Taxes computed at statutory rate
|
|
35.0%
|
|
|
35.0%
|
|
|
35.0%
|
|
|
State income taxes (net of federal income tax benefit)
|
|
3.5
|
|
|
3.8
|
|
|
3.8
|
|
|
Foreign
|
|
(1.1)
|
|
|
—
|
|
|
—
|
|
|
Other
|
|
1.0
|
|
|
0.4
|
|
|
0.7
|
|
|
|
|
38.4%
|
|
|
39.2%
|
|
|
39.5%
|
In general, it is the practice and intention of the Company to reinvest the earnings of its non-U.S. subsidiaries in those operations. As of September 30, 2010, the Company has not made a provision for U.S. or additional foreign withholding taxes on the excess of the amount for financial reporting over the tax basis of investments in foreign subsidiaries that are essentially permanent in duration. Generally, such amounts become subject to U.S. taxation upon the remittance of dividends and under certain other circumstances. It is not practicable to estimate the amount of deferred tax liability related to investments in foreign subsidiaries.
In 2006, the FASB issued a new accounting standard included in ASC 740, Income Taxes, which clarifies the accounting for uncertainty in tax positions. This standard requires that management evaluate its tax positions for all open tax years to determine if the position is more likely than not to be sustained upon examination by taxing authorities having full knowledge of all relevant information. We adopted the provisions of this standard as of October 1, 2007, the beginning of our 2008 fiscal year. As a result of the adoption of this standard, we reported a cumulative effect adjustment of $257 which reduced our retained earnings.
A reconciliation of the unrecognized tax benefits is as follows:
|
|
2010
|
2009
|
2008
|
|||||||||
|
Unrecognized tax benefits – Beginning of year
|
$ | 223 | $ | 523 | $ | 449 | ||||||
|
Gross increases related to prior period tax positions
|
— | — | — | |||||||||
|
Gross decreases related to prior period tax positions
|
— | (54 | ) | (8 | ) | |||||||
|
Gross increases related to current period tax positions
|
175 | 182 | 185 | |||||||||
|
Settlements
|
(200 | ) | (428 | ) | (103 | ) | ||||||
|
Unrecognized tax benefits – End of year
|
$ | 198 | $ | 223 | $ | 523 | ||||||
For the fiscal years ended September 30, 2010, 2009 and 2008, the amount included in our income tax expense for tax-related interest and penalties was not significant. Of the $198 unrecognized tax benefits as of September 30, 2010, $15 would impact our effective rate, if recognized. Of the $223 unrecognized tax benefits as of September 30, 2009, $27 would have impacted our effective rate, if recognized. Of the $523 unrecognized tax benefits as of September 30, 2008, $220 would have impacted our effective rate, if recognized. Our policy for classifying interest and penalties associated with unrecognized tax benefits is to include such items in income tax expense.
We filed Form 3115 Application of Change in Accounting Method with the Internal Revenue Service during the fiscal year ended September 30, 2008. We filed an advance consent request for a non-automatic account method change for tax purposes for which we had not received approval prior to our reporting period end. The method change will make revenue recognition for tax purposes the same as revenue recognized for book purposes. We expect resolution within the next twelve months which would decrease the liability for unrecognized tax benefits by approximately $175.
With few exceptions, we are no longer subject to income tax examination for years before 2005 in the U.S. and significant state and local jurisdictions. We are no longer subject to income tax examinations for years before 2008 in significant foreign jurisdictions.
|
|
11. Statements of Cash Flows — Supplemental and Noncash Disclosures
|
|
|
2010
|
2009
|
2008
|
|||||||||
|
Supplemental Disclosures
|
|
|
|
|||||||||
|
Cash paid for interest
|
$ | 340 | $ | 173 | $ | 189 | ||||||
|
Cash paid for income taxes
|
19,850 | 15,251 | 12,865 | |||||||||
|
Noncash Activities
|
||||||||||||
|
Issuance of restricted common stock for asset acquisition
|
- | - | 505 | |||||||||
|
Equipment acquisitions financed with accounts payable
|
23 | 119 | 580 | |||||||||
Restatement of Statements of Cash Flows — We have restated the presentation of borrowings on the credit facility for the fiscal year ended September 30, 2009 and 2008. Related amounts had previously been presented on a net basis, rather than on a gross basis in accordance with ASC 230. The correction had no effect on net cash used in financing activities.
|
|
12. Commitments and Contingencies
|
We have operating leases for office and distribution center space and equipment for varying periods. We also lease some of our vehicles in the United Kingdom under capital leases. Certain leases have renewal options and require contingent payments for increases, including executory costs, property taxes, insurance and certain other costs in excess of a base year amount. Total rent expense for the years ended September 30, 2010, 2009 and 2008 were $4,051, $3,968 and $3,629, respectively.
The aggregate future noncancelable minimum rental payments on operating leases and capital leases at September 30, 2010 are as follows:
|
|
Lease Obligations
|
|||||||
|
Fiscal Year
|
Operating Leases
|
Capital Leases
|
||||||
|
2011
|
$ | 3,048 | $ | 858 | ||||
|
2012
|
2,762 | 778 | ||||||
|
2013
|
2,228 | 136 | ||||||
|
2014
|
1,532 | 29 | ||||||
|
2015
|
1,305 | 10 | ||||||
|
Thereafter
|
4,664 | - | ||||||
|
Total future minimum obligations
|
$ | 15,539 | $ | 1,811 | ||||
|
|
13. Related Party Transactions
|
MWI Co., our subsidiary, holds a 50% membership interest in Feeders’ Advantage that is accounted for as an investment using the equity method. Sales of products to Feeders’ Advantage, which are at our cost, were $43,017, $46,406 and $39,443 for the fiscal years ended September 30, 2010, 2009 and 2008, respectively. MWI Co. charged Feeders’ Advantage for certain operating and administrative services of $794, $771 and $562 for the fiscal years ended September 30, 2010, 2009 and 2008, respectively. Our President and Chief Executive Officer and a member of our Board of Directors are each members of the board of managers of Feeders’ Advantage.
MWI Co. provides Feeders’ Advantage with a line-of-credit to finance its day-to-day operations. This line-of-credit bears interest at the prime rate. The interest due on the line-of-credit is calculated and charged to Feeders’ Advantage on the last day of each month. Conversely, to the extent MWI Co. has a payable balance due to Feeders’ Advantage, the payable balance accrues interest in favor of Feeders’ Advantage at the average federal funds rates in effect for that month. As of September 30, 2010 and 2009, MWI Co. had a payable balance to Feeders’ Advantage of $281 and $210, respectively.
|
|
14. Employee Benefit Plans
|
We have a multi-employer defined contribution profit sharing plan with a 401(k) arrangement for employees in the United States. To become eligible for the profit sharing portion of the plan, an employee must complete two years of service and attain the age of twenty-one. Participation is automatic beginning the following January or July. To become eligible for the 401(k) portion of the plan, the employee must complete three-months of service and attain the age of twenty-one.
Both portions of the plan allow for employer contributions. We are required to match 50% of the employee’s contribution to the 401(k) portion of the plan up to 6% of the employee’s salary. Our matching contributions for the 401(k) portion of the plan were $1,108, $975 and $911 for the fiscal years ended September 30, 2010, 2009 and 2008, respectively. Employee’s contributions are fully vested immediately while employer contributions vest over a five-year period.
Contributions to the profit sharing portion of the Plans are discretionary, ranging from 0% to 3%, and are approved by our Board of Directors. Total profit sharing expense for the fiscal years ended September 30, 2010, 2009 and 2008 were $1,244, $1,106 and $956, respectively. Employer contributions are fully vested immediately.
Centaur sponsors a defined contribution plan for all other staff not participating in the defined benefit plan described below. The contributions made by the employer over the period are detailed below. Contributions are currently payable at a minimum of 3% up to a maximum of 6% of eligible pay if matched by employee. The matching contribution for the plan was $207 for the fiscal year ended September 30, 2010.
Centaur operates a defined benefit pension plan which provides benefits based on pensionable pay and is closed to future benefit accrual. We recorded the liability at the estimated fair value at the date of the acquisition. Subsequent to the acquisition of Centaur, we funded $2,047 to the pension plan as required by the terms of the share purchase agreement.
The fair value of plan assets, benefit obligation and funded status of the defined benefit plan as of September 30 is as follows:
|
|
2010
|
|||
|
Fair value of plan assets
|
$ | 4,771 | ||
|
Benefit obligation
|
(7,160 | ) | ||
|
Unfunded pension liability
|
$ | (2,389 | ) | |
The unfunded pension liability is recognized as other long-term liabilities in the Consolidated Balance Sheets. Net periodic benefit expense for the fiscal year ended September 30 consisted of the following:
|
|
2010
|
|||
|
Interest cost
|
$ | 159 | ||
|
Expected return on plan assets
|
(139 | ) | ||
|
Net periodic benefit cost
|
20 | |||
|
|
||||
|
Other changes recognized in other comprehensive income
|
||||
|
Actuarial loss
|
591 | |||
|
Total recognized in net periodic benefit costs and other comprehensive income
|
$ | 611 | ||
|
|
||||
|
Total recognized in other comprehensive income, net of tax
|
$ | 457 | ||
The estimated net actuarial loss that will be amortized from accumulated other comprehensive losses into net periodic benefit costs during fiscal year 2011 is not significant. The following table provides the weighted-average actuarial assumptions:
|
|
2010
|
|||
|
Assumptions used to determine benefit obligations
|
|
|||
|
Discount rate
|
3.6 | % | ||
|
Rate of compensation increase (1)
|
N/A | |||
|
|
||||
|
Assumptions used to determine net periodic benefit cost
|
||||
|
Discount rate
|
4.2 | % | ||
|
Expected return on plan assets
|
5.2 | % | ||
|
Rate of compensation increase (1)
|
N/A | |||
|
|
||||
|
(1) There is no assumed rate of compensation increase as there have been no current active members since April 2006
|
||||
The assets of the plan are invested as follows as of September 30, 2010:
|
Asset Class
|
|
Market Value
|
|
Asset Allocation
|
|
|
Equities
|
|
$
|
836
|
|
17%
|
|
Corporate bonds
|
|
|
828
|
|
17%
|
|
UK Government bonds
|
|
|
417
|
|
9%
|
|
Property
|
|
|
175
|
|
4%
|
|
Cash
|
|
|
2,378
|
|
50%
|
|
Other assets
|
|
|
137
|
|
3%
|
|
Total
|
|
$
|
4,771
|
|
100%
|
Note 15 — Other Comprehensive Income
The components of comprehensive income (loss) were as follows:
|
|
2010
|
2009
|
2008
|
|||||||||
|
Net income
|
$ | 33,440 | $ | 24,905 | $ | 19,917 | ||||||
|
Other comprehensive income:
|
||||||||||||
|
Foreign currency translation
|
536 | - | - | |||||||||
|
Actuarial loss on pension valuation, net of deferred taxes
|
(457 | ) | - | - | ||||||||
|
Total comprehensive income
|
$ | 33,519 | $ | 24,905 | $ | 19,917 | ||||||
|
|
16. Fair Value of Financial Instruments
|
Current fair value accounting guidance includes a hierarchy that is intended to increase consistency and comparability in fair value measurements and disclosures. This hierarchy prioritizes inputs to valuation techniques based on observable and unobservable data. The guidance categorizes these inputs used in measuring fair value into three levels which include the following:
|
·
|
Level 1 – observable inputs such as quoted prices in active markets;
|
|
·
|
Level 2 – inputs, other than quoted prices, that are observable for the asset or liability, either directly or indirectly. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active; and
|
|
·
|
Level 3 – unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
|
Financial instruments include cash and cash equivalents, receivables and accounts payable, and the fair values approximate book values due to their short maturities. All pension assets are measured using level 1 inputs.
In August 2010, we amended our revolving credit facility in the United States. Because this amendment was done recently and includes interest rates based on current market conditions, we believe that the estimated fair value of our long-term debt (including current maturities) was materially the same as our carrying value.
We have recently refinanced our Fortis credit facility. Based on the terms and pricing received in the refinance, we believe that the estimated fair value for the Fortis credit facility was materially the same as our carrying value.
|
|
17. Geographic Information
|
Revenues and long-lived assets by geographic region are as follows:
|
|
2010
|
2009
|
2008
|
|||||||||
|
Revenues for the fiscal years ended September 30
|
|
|
|
|||||||||
|
United States
|
$ | 1,074,226 | $ | 941,332 | $ | 831,364 | ||||||
|
International
|
155,116 | - | - | |||||||||
|
Total
|
$ | 1,229,342 | $ | 941,332 | $ | 831,364 | ||||||
|
|
||||||||||||
|
Long-lived assets as of September 30
|
||||||||||||
|
United States
|
$ | 9,762 | $ | 9,313 | $ | 9,687 | ||||||
|
International
|
5,476 | - | - | |||||||||
|
Total
|
$ | 15,238 | $ | 9,313 | $ | 9,687 | ||||||
|
|
18. Quarterly Financial Data (Unaudited)
|
|
|
Three-Months Ended
|
|
||||||||||||||||||
|
|
Dec. 31,
|
Mar. 31,
|
June 30,
|
Sept. 30,
|
Year
|
|||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
|
|
(Dollars and shares in thousands, except per share data) (1)
|
|||||||||||||||||||
|
Fiscal Year 2010
|
|
|
|
|
|
|||||||||||||||
|
Total revenues
|
$ | 236,111 | $ | 286,597 | $ | 347,687 | $ | 358,947 | $ | 1,229,342 | ||||||||||
|
Gross profit
|
36,009 | 39,522 | 43,937 | 45,535 | 165,003 | |||||||||||||||
|
Operating income
|
12,733 | 12,664 | 15,064 | 13,757 | 54,218 | |||||||||||||||
|
Net income
|
7,834 | 7,671 | 9,137 | 8,798 | 33,440 | |||||||||||||||
|
Earnings per common share — basic
|
$ | 0.64 | $ | 0.63 | $ | 0.74 | $ | 0.71 | $ | 2.73 | ||||||||||
|
Earnings per common share — diluted
|
$ | 0.63 | $ | 0.62 | $ | 0.74 | $ | 0.71 | $ | 2.70 | ||||||||||
|
Weighted average common shares
|
||||||||||||||||||||
|
outstanding:
|
||||||||||||||||||||
|
Basic
|
12,173 | 12,207 | 12,265 | 12,318 | 12,241 | |||||||||||||||
|
Diluted
|
12,356 | 12,376 | 12,408 | 12,437 | 12,395 | |||||||||||||||
|
Fiscal Year 2009
|
||||||||||||||||||||
|
Total revenues
|
$ | 231,819 | $ | 214,512 | $ | 247,463 | $ | 247,538 | $ | 941,332 | ||||||||||
|
Gross profit
|
33,655 | 31,634 | 34,483 | 34,883 | 134,655 | |||||||||||||||
|
Operating income
|
9,687 | 9,269 | 10,891 | 10,616 | 40,463 | |||||||||||||||
|
Net income
|
5,949 | 5,787 | 6,616 | 6,553 | 24,905 | |||||||||||||||
|
Earnings per common share — basic
|
$ | 0.49 | $ | 0.48 | $ | 0.55 | $ | 0.54 | $ | 2.06 | ||||||||||
|
Earnings per common share — diluted
|
$ | 0.48 | $ | 0.47 | $ | 0.54 | $ | 0.53 | $ | 2.02 | ||||||||||
|
Weighted average common shares
|
||||||||||||||||||||
|
outstanding:
|
||||||||||||||||||||
|
Basic
|
12,071 | 12,076 | 12,079 | 12,125 | 12,088 | |||||||||||||||
|
Diluted
|
12,295 | 12,296 | 12,303 | 12,331 | 12,306 | |||||||||||||||
|
(1) The sums of the quarterly net income and earnings per share amounts do not agree to the year-to-date |
||||||||||||||||||||
|
earnings per common share amount as a result of rounding.
|
||||||||||||||||||||
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
|
Item 9A.
|
Controls and Procedures.
|
Our management, including the Chief Executive Officer and the Chief Financial Officer, have evaluated the effectiveness of the our disclosure controls and procedures (as defined in Rule 13a-15(e) of the Securities Exchange Act of 1934) as of September 30, 2010. Based on this evaluation, the Chief Executive Officer and the Chief Financial Officer concluded that our Company’s disclosure controls and procedures, including the accumulation and communication of disclosures to the Company’s Chief Executive Officer and Chief Financial Officer as appropriate to allow timely decisions regarding required disclosure, are effective to provide reasonable assurance that information required to be disclosed by the us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified by the Securities and Exchange Commission‘s rules and forms.
Management’s annual report on internal control over financial reporting and the attestation report of our independent registered public accounting firm are set forth below on this Annual Report on Form 10-K.
There were no changes in our internal controls over financial reporting that occurred during our most recently completed fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
The management of MWI Veterinary Supply, Inc. and subsidiaries (the “Company”) is responsible for establishing and maintaining effective internal control over financial reporting (as such term is defined in Exchange Act Rules 13a-15(f)).
There are inherent limitations in the effectiveness of any internal control, including the possibility of human error and the circumvention or overriding of controls. Accordingly, even effective internal control can provide only reasonable assurance with respect to financial statement preparation. Further, because of changes in conditions, the effectiveness of internal control may vary over time.
Management assessed the effectiveness of the Company’s internal control over financial reporting as of September 30, 2010. This assessment was based on criteria for effective internal control over financial reporting described in “Internal Control – Integrated Framework,” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this assessment, management believes that, as of September 30, 2010, internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with U.S. generally accepted accounting principles.
The effectiveness of the Company’s internal control over financial reporting has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, in accordance with the standards of the Public Company Accounting Oversight Board (United States). Their report is included herein.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
MWI Veterinary Supply, Inc.
Meridian, Idaho
We have audited the internal control over financial reporting of MWI Veterinary Supply, Inc. and subsidiaries (the "Company") as of September 30, 2010, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed by, or under the supervision of, the company's principal executive and principal financial officers, or persons performing similar functions, and effected by the company's board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2010, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and consolidated financial statement schedule as of and for the year ended September 30, 2010, of the Company, and our report dated November 23, 2010 expressed an unqualified opinion on those consolidated financial statements and consolidated financial statement schedule and included an explanatory paragraph regarding the Company’s adoption of Accounting Standards Codification 740 (formerly Financial Accounting Standards Board Interpretation No. 48, Accounting Uncertainty in Income Taxes – an interpretation of FASB Statement No. 109).
/s/ DELOITTE & TOUCHE LLP
Boise, Idaho
November 23, 2010
|
Item 9B.
|
Other Information.
|
None.
PART III
|
Item 10.
|
Directors, Executive Officers and Corporate Governance of the Registrant.
|
The information regarding directors and nominees for directors of the Company, including identification of the audit committee and audit committee financial expert, is presented under the headings “Corporate Governance—Committees of the Board of Directors,” and “Election of Directors—Nominees For Directors” in the Company’s definitive proxy statement for use in connection with the 2011 Annual Meeting of Stockholders (the “Proxy Statement”) to be filed within 120 days after the end of the Company’s fiscal year ended September 30, 2010. The information contained under these headings is incorporated herein by reference. Information regarding the executive officers of the Company is included in this Annual Report on Form 10-K under Item 1 of Part I as permitted by Instruction 3 to Item 401(b) of Regulation S-K.
The Company has adopted a code of conduct that applies to its Chief Executive Officer and Chief Financial Officer. This code of conduct is available on the Company’s Web site at www.mwivet.com. If the Company makes any amendments to this code other than technical, administrative or other non-substantive amendments, or grants any waivers, including implicit waivers, from a provision of this code to the Company’s Chief Executive Officer or Chief Financial Officer, the Company will disclose the nature of the amendment or waiver, its effective date and to whom it applies in a report on Form 8-K filed with the SEC.
|
Item 11.
|
Executive Compensation.
|
Information concerning executive compensation is presented under the headings “Executive Compensation” and “Compensation Committee Report” in the Proxy Statement. The information contained under these headings is incorporated herein by reference.
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
|
Information with respect to security ownership of certain beneficial owners and management is set forth under the heading “Security Ownership of Certain Beneficial Owners and Directors and Officers” in the Proxy Statement. The information contained under these headings is incorporated herein by reference.
|
|
Equity Compensation Plan Information
|
The following table provides information as of September 30, 2010 about the common stock that may be issued under all of our existing equity compensation plans, including the 2002 Stock Plan and 2005 Stock-Based Incentive Compensation Plans. Both of these plans have been approved by our stockholders.
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
(a)
|
Weighted-average exercise
price of outstanding options,
warrants and rights
(b)
|
Number of securities
remaining available for
future issuance under equity
compensation plans
(excluding securities reflected in
column (a))
(c)
|
||||||||||
|
Equity compensation plans approved by security holders
|
139,550
|
$ |
4.65
|
1,017,208
|
||||||||
|
Equity compensation plans not approved by security holders
|
—
|
—
|
—
|
|||||||||
|
Total
|
139,550
|
$ |
4.65
|
1,017,208
|
||||||||
|
Item 13.
|
Certain Relationships, Related Transactions and Director Independence.
|
Information concerning related transactions and director independence is presented under the heading “Certain Relationships, Related Transactions and Director Independence” in the Proxy Statement. The information contained under this heading is incorporated herein by reference.
|
Item 14.
|
Principal Accountant Fees and Services.
|
Information concerning principal accountant fees and services is presented under the heading “Ratification of Appointment of Independent Registered Public Accounting Firm” in the Proxy Statement. The information contained under this heading is incorporated herein by reference.
PART IV
|
Item 15.
|
Exhibits and Financial Statement Schedules.
|
|
(a)
|
The following documents are filed as part of this report:
|
|
|
1)
|
Consolidated Financial Statements: See Index to Consolidated Financial Statements at Item 8 on page 36 of this report.
|
|
|
2)
|
Financial Statement Schedule: Schedule II—Consolidated Valuation and Qualifying Accounts
|
|
|
3)
|
Exhibits are incorporated herein by reference or are filed with this report as set forth in the Index to Exhibits
|
MWI VETERINARY SUPPLY, INC.
SCHEDULE II—CONSOLIDATED VALUATION AND QUALIFYING ACCOUNTS
(Amounts in thousands)
|
Balance at
Beginning
of Period
|
Charged
(Credited) to
Costs and
Expenses
|
Deductions/
Write-offs
|
Balance at
End of Period
|
|||||||||||||
|
Allowance for Doubtful Accounts
|
||||||||||||||||
|
Year ended September 30, 2008
|
$ |
874
|
$ |
454
|
$ |
(450
|
)
|
$ |
878
|
|||||||
|
Year ended September 30, 2009
|
878
|
2,769
|
(641
|
)
|
3,006
|
|||||||||||
|
Year ended September 30, 2010
|
3,006
|
(134)
|
(302
|
)
|
2,570
|
|||||||||||
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
MWI Veterinary Supply, Inc.
|
|
|
By: /s/ Mary Patricia B. Thompson
|
|
|
Mary Patricia B. Thompson
|
|
|
(Senior Vice President of Finance and Administration, Chief Financial Officer)
|
|
|
Date: November 23, 2010
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated as of November 23, 2010.
|
/s/ James F. Cleary, Jr.
|
/s/ Mary Patricia B. Thompson
|
|
|
James F. Cleary, Jr.
Director, President and
Chief Executive Officer
(Principal Executive Officer)
|
Mary Patricia B. Thompson
Senior Vice President of Finance and Administration,
Chief Financial Officer
(Principal Financial Officer and Principal
Accounting Officer)
|
|
|
/s/ Keith E. Alessi
|
/s/ Bruce C. Bruckmann
|
|
|
Keith E. Alessi (Director)
|
Bruce C. Bruckmann (Director)
|
|
|
/s/ John F. McNamara
|
/s/ A. Craig Olson
|
|
|
John F. McNamara (Director)
|
A. Craig Olson (Director)
|
|
|
/s/ Robert N. Rebholtz
|
/s/ William J. Robison
|
|
|
Robert N. Rebholtz (Director)
|
William J. Robison (Director)
|
Index to Exhibits
Filed with the Annual Report
on Form 10-K for the
Year Ended September 30, 2010
|
Number
|
Description
|
|||
|
2.1
|
Asset Purchase Agreement among MWI Veterinary Supply Co., AAHA Services Corporation and American Animal Hospital Association effective July 1, 2008, incorporated herein by reference to Exhibit 2.1 of the Company’s Annual Report on Form 10-K, filed November 24, 2008.
|
|||
|
2.2
|
Agreement Relating to the Sale and Purchase of the Entire Issued Share Capital of Centaur Services Limited dated February 8, 2010 by and among MWI Veterinary Supply Co., MWI Supply (UK Acquisition) Limited, AHN (UK) Holdings Limited and AHN International, LLC, incorporated herein by reference to Exhibit 2.1 of the Company’s Current Report on Form 8-K filed February 12, 2010
|
|||
|
3.1
|
Form of Amended and Restated Certificate of Incorporation of MWI Veterinary Supply, Inc., incorporated herein by reference to Exhibit 3.1 of the Company’s Quarterly Report on Form 10-Q, filed August 1, 2007.
|
|||
|
3.2
|
Form of Amended and Restated Bylaws of MWI Veterinary Supply, Inc., incorporated herein by reference to Exhibit 3.2 of the Company’s Quarterly Report on Form 10-Q, filed August 1, 2007.
|
|||
|
4.1
|
Form of MWI Veterinary Supply, Inc. common stock certificate, incorporated herein by reference to Exhibit 4.1 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).
|
|||
|
4.2
|
Registration Rights Agreement dated as of June 18, 2002 by and between MWI Holdings, Inc., Bruckmann, Rosser, Sherrill & Co. II, L.P., Agri Beef Co. and the other parties thereto, incorporated herein by reference to Exhibit 4.3 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).
|
|||
|
4.3
|
Executive Stock Agreement dated as of June 18, 2002 by and among MWI Veterinary Supply Co., MWI Holdings, Inc. and James Cleary, incorporated herein by reference to Exhibit 4.4 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.4
|
Executive Stock Agreement dated as of June 18, 2002 by and among MWI Veterinary Supply Co., MWI Holdings, Inc. and Jeff Danielson, incorporated herein by reference to Exhibit 4.5 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.5
|
Executive Stock Agreement dated as of June 18, 2002 by and among MWI Veterinary Supply Co., MWI Holdings, Inc. and James Hay, incorporated herein by reference to Exhibit 4.6 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.7
|
Executive Stock Agreement dated as of June 18, 2002 by and among MWI Veterinary Supply Co., MWI Holdings, Inc. and Mary Pat Thompson, incorporated herein by reference to Exhibit 4.8 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.8
|
First Amendment to Executive Stock Agreement dated as of May 6, 2005 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co. and James F. Cleary, Jr., incorporated herein by reference to Exhibit 4.9 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.9
|
First Amendment to Executive Stock Agreement dated as of May 5, 2005 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co. and Jeffrey J. Danielson, incorporated herein by reference to Exhibit 4.10 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.10
|
First Amendment to Executive Stock Agreement dated as of May 6, 2005 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co. and James S. Hay, incorporated herein by reference to Exhibit 4.11 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
4.12
|
First Amendment to Executive Stock Agreement dated as of May 6, 2005 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co. and Mary Patricia B. Thompson, incorporated herein by reference to Exhibit 4.13 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
10.1
|
2002 Stock Option Plan, incorporated herein by reference to Exhibit 10.11 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).*
|
|||
|
10.2
|
Third Amendment to Office Lease Agreement dated February 28, 2008 between Rafanelli and Nahas and MWI Veterinary Supply Co., incorporated herein by reference to Exhibit 99.1 of the Company’s Quarterly Report on Form 10Q, filed February 4, 2009.
|
|||
|
10.3
|
MWI Veterinary Supply, Inc. 2005 Stock-Based Incentive Compensation Plan, adopted July 27, 2005, as Amended and Restated, effective July 24, 2006, incorporated herein by reference to Exhibit 10.21 of the Company’s Current Report on Form 8-K, filed February 8, 2007.*
|
|||
|
10.4
|
Form of Option Letter, incorporated herein by reference to Exhibit 10.22 of the Company’s Registration Statement on Form S-1 (Reg No. 333-124264).
|
|||
|
10.5
|
2010-2011 Merial Independent Sales Agent Agreement between MWI Veterinary Supply Co. and Merial Limited effective as of January 1, 2010, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010.†
|
|||
|
10.6
|
First Amendment to the Agreement for Product Purchases effective as of July 1, 2009 by and between MWI Veterinary Supply Co. and Medical Management International, Inc., dba Banfield, The Pet Hospital®, incorporated herein by reference to Exhibit 10.7 of the Company’s Annual Report on Form 10K, filed November 20, 2009.†
|
|||
|
10.7
|
First Amendment to the Agreement for Logistics Services effective as of July 1, 2009 by and between MWI Veterinary Supply Co. and Medical Management International, Inc., dba Banfield, The Pet Hospital®, incorporated herein by reference to Exhibit 10.8 of the Company’s Annual Report on Form 10K, filed November 20, 2009.†
|
|||
|
10.8
|
Agreement for Home Delivery Logistics Services effective as of July 1, 2009 by and between MWI Veterinary Supply Co. and Medical Management International, Inc., dba Banfield, the Pet Hospital®, incorporated herein by reference to Exhibit 10.9 of the Company’s Annual Report on Form 10K, filed November 20, 2009.†
|
|||
|
10.9
|
Credit Agreement dated as of December 13, 2006 by and between MWI Veterinary Supply Co., as Borrower, MWI Veterinary Supply, Inc. and Memorial Pet Care, Inc. as Guarantors, Bank of America, N.A. and Wells Fargo Bank, N.A., as Lenders, incorporated herein by reference to Exhibit 10.4 of the Company’s Quarterly Report on Form 10-Q, filed February 5, 2007.
|
|||
|
10.10
|
2010 Livestock Products Distribution Agreement between MWI Veterinary Supply, Inc. and Pfizer, Inc. effective as of January 1, 2010, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010.†
|
|||
|
10.11
|
2010 Pfizer Equine Products Marketing Agreement between MWI Veterinary Supply Co. and Pfizer, Inc. effective as of January 1, 2010, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed July 29, 2010.†
|
|||
|
10.12
|
2009 Strategic Brands Distribution Agreement effective as of January 1, 2009 by and between Pfizer Inc. and MWI Veterinary Supply Co., incorporated herein by reference to Exhibit 10.3 of the Company’s Quarterly Report on Form 10-Q, filed April 30, 2009.†
|
|||
|
10.13
|
Companion Animal Select Brands Distribution Agreement effective as of January 1, 2009 by and between Pfizer Inc. and MWI Veterinary Supply, Inc., incorporated herein by reference to Exhibit 10.4 of the Company’s Quarterly Report on Form 10-Q, filed April 30, 2009. †
|
|||
|
10.14
|
MWI Veterinary Supply, Inc. 2008 Employee Stock Purchase Plan, incorporated herein by reference to Exhibit 10.3 of the Company’s Quarterly Report on Form 10-Q, filed May 1, 2008.
|
|||
|
10.15
|
License Agreement dated and effective July 1, 2008 between MWI Veterinary Supply Co. and American Animal Hospital Association, incorporated herein by reference to Exhibit 10.14 of the Company’s Annual Report on Form 10-K, filed November 24, 2008. †
|
|||
|
10.16
|
Sponsorship Letter Agreement dated June 30, 2008 between American Animal Hospital Association and MWI Veterinary Supply Co, incorporated herein by reference to Exhibit 10.15 of the Company’s Annual Report on Form 10-K, filed November 24, 2008.
|
|||
|
10.17
|
First Amendment to Credit Agreement dated February 8, 2010 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co., Memorial Pet Care, Inc., Bank of America, N.A. and Wells Fargo Bank, N.A., incorporated herein by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed February 12, 2010.
|
|||
|
10.18
|
Second Amendment to Credit Agreement dated August 10, 2010 by and among MWI Veterinary Supply, Inc., MWI Veterinary Supply Co., Memorial Pet Care, Inc., Bank of America, N.A. and Wells Fargo Bank, N.A., incorporated herein by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed August 13, 2010.
|
|||
|
10.19
|
Sterling Revolving Credit Facility dated November 5, 2010 by and among Centaur Services Limited and Wells Fargo Bank, N.A., incorporated herein by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed November 10, 2010.
|
|||
|
10.20
|
Continuing Guaranty of MWI Veterinary Supply Co. dated November 5, 2010, incorporated herein by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed November 10, 2010.
|
|||
|
10.20
|
Distributor Agreement between MWI Veterinary Supply Co. and Intervet Inc., dba as Intervet/Schering Plough Animal Health effective as of December 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.21
|
Non-Disclosure and Non-Competition Agreement dated as of September 10, 2006 by and among MWI Veterinary Supply Co. and John Francis, incorporated herein by reference to Exhibit 4.1 of the Company’s Quarterly Report on Form 10Q, filed May 6, 2010.*
|
|||
|
10.22
|
2009 Livestock Products Distribution Agreement between MWI Veterinary Supply, Inc. and Pfizer, Inc. effective as of January 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.23
|
2009 Pfizer Equine Products Marketing Agreement between MWI Veterinary Supply Co. and Pfizer Inc. effective as of January 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.24
|
Companion Animal AAHA MARKETLink Management Agreement between MWI Veterinary Supply Co. and Pfizer Inc. effective as of March 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.25
|
2009 Strategic Brands Distribution Agreement between MWI Veterinary Supply, Inc. and Pfizer Inc. effective as of January 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.26
|
Companion Animal Select Brands Distribution Agreement between MWI Veterinary Supply, Inc. and Pfizer Inc. effective as of January 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
10.27
|
Companion Animal AAHA MARKETLink Management Agreement between MWI Veterinary Supply, Co. and Pfizer, Inc. effective as of September 1, 2009, incorporated herein by reference to Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q, filed May 6, 2010. †
|
|||
|
21.1
|
Subsidiaries of MWI Veterinary Supply, Inc.
|
|||
|
23
|
Consent of Deloitte & Touche LLP
|
|||
|
31.1
|
Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|||
|
31.2
|
Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
|||
|
32
|
Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|||
|
*
|
Identifies management contracts or compensatory plans or arrangements required to be filed as an exhibit hereto.
|
|
†
|
Certain portions of the exhibit have been omitted pursuant to a confidential treatment request submitted to and approved by the SEC.
|
