Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MEDICINES CO /DE | y87914e8vk.htm |
Exhibit 99.1

| THE MEDICINES COMPANY Investor Update November 2010 |

| Safe harbor Forward looking statements Statements contained in these slides about The Medicines Company (the "Company"), the Company's projected revenues and financial results, the Company's products and product candidates, and the timing of clinical trial results, regulatory submissions, product or indication launches, including the future financial and operating results, and future opportunities for the Company, that are not purely historical, and all other statements that are not purely historical, may be deemed to be forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Without limiting the foregoing, the words "believes", "anticipates", "plans", "expects", "intends", "potential", "estimates" , "outlook" and similar expressions are intended to identify forward-looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward looking statements, including: the extent of the commercial success of Angiomax(r) (bivalirudin) and Cleviprex(r) (clevidipine butyrate); whether the Company's products will advance in the clinical trials process on a timely basis or at all; whether the clinical trial results will warrant submission of applications for regulatory approval; whether physicians and other key decision-makers will accept the clinical trial results; whether the Company's other product candidates will receive approvals from regulatory agencies; and such other factors as are set forth in the risk factors detailed from time to time in the Company's periodic reports filed with the Securities and Exchange Commission including, without limitation, the risk factors detailed in the Company's Quarterly Report on Form 10-Q filed with the SEC on November 9, 2010, which are incorporated herein by reference. The Company specifically disclaims any obligation to update these forward-looking statements The Medicines Company | 8 Sylvan Way | Parsippany | New Jersey 07054 Telephone: 973.290.6000 | Toll-free: 800.388.1183 www.themedicinescompany.com |

| Acute | intensive care hospital medicine Expanding acute episodes, concentration of care facilities, rising demand worldwide |

| Acute | intensive care hospital medicine is an attractive market Expanding global acute episodes, concentration of care facilities, rising demand Ischemic heart disease (#1), stroke (#2), and serious hospital infections (~#10) will remain among the leading causes of death, morbidity and hospital costs worldwide through at least 2030 (WHO 2010) Consolidation |networks 1200 institutions deliver ~80% Physician alignment Cost-efficiency | throughput United States ? DRG-switchover East European integration HTA leads choices Pricing pressures considerable Europe ? Growing high end care Capable clinician network Demand local investment Generic-level drug prices Brazil ? Explosive growth JV to hurdle regulation Hospital centralization Leapfrog to systems China ? Emerging acute care market Regionalized | variable service Physicians in charge Premium pricing - private pay Russia ? Attractive segment Academic focus Registration-price hurdle Slow generic incursion Japan ? Growing high end demand Capable clinician network Leapfrog to systems India ? |
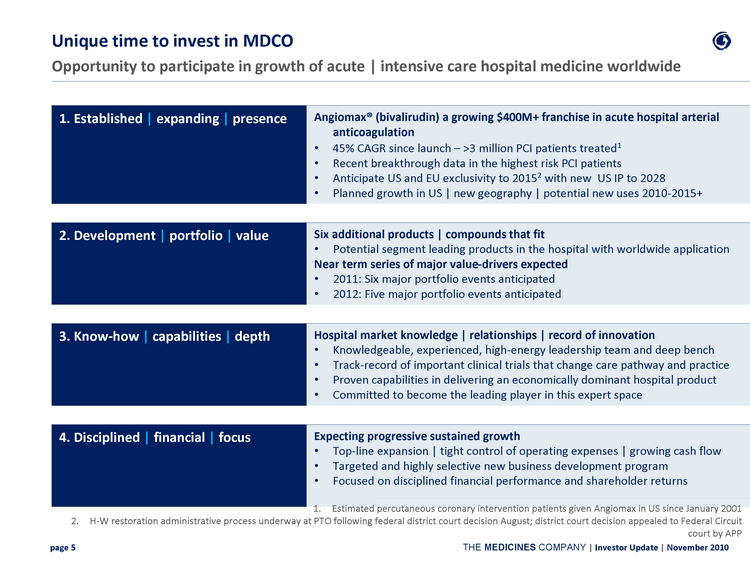
| Unique time to invest in MDCO Opportunity to participate in growth of acute | intensive care hospital medicine worldwide 1. Established | expanding | presence Angiomax(r) (bivalirudin) a growing $400M+ franchise in acute hospital arterial anticoagulation 45% CAGR since launch - >3 million PCI patients treated1 Recent breakthrough data in the highest risk PCI patients Anticipate US and EU exclusivity to 20152 with new US IP to 2028 Planned growth in US | new geography | potential new uses 2010-2015+ 2. Development | portfolio | value Six additional products | compounds that fit Potential segment leading products in the hospital with worldwide application Near term series of major value-drivers expected 2011: Six major portfolio events anticipated 2012: Five major portfolio events anticipated 3. Know-how | capabilities | depth Hospital market knowledge | relationships | record of innovation Knowledgeable, experienced, high-energy leadership team and deep bench Track-record of important clinical trials that change care pathway and practice Proven capabilities in delivering an economically dominant hospital product Committed to become the leading player in this expert space 4. Disciplined | financial | focus Expecting progressive sustained growth Top-line expansion | tight control of operating expenses | growing cash flow Targeted and highly selective new business development program Focused on disciplined financial performance and shareholder returns Estimated percutaneous coronary intervention patients given Angiomax in US since January 2001 H-W restoration administrative process underway at PTO following federal district court decision August; district court decision appealed to Federal Circuit court by APP |

| MDCO | established | expanding | presence in acute | intensive hospital medicine Focused business plan | Angiomax $400M+ franchise | continued growth anticipated 1. |

| MDCO | established | expanding Our purpose Vision | where we are going We provide leading solutions for leading hospitals Biopharmaceuticals | knowledge | hospital customers | world-class medical | economic | results | acute | intensive care Mission | how we plan to get there Required capabilities Focused expertise: Heart and vascular disease | stroke| hospital infections Global reach: 2,548 hospitals | 26 countries | ~80% of the total world market Innovation What jobs do customers do? Can we improve their performance - medical and economic? How do we drive positive change in the hospital process of care? Goals | how we measure progress Strategic goals Competitive: Economic dominance | leading market share | #1 depth of portfolio Human: #1 operating profit per employee per year | top 10% engagement metrics Financial growth: Peer leader for net revenue | net operating profit | EPS growth |

| MDCO | established | expanding Angiomax is the growing US market leader in percutaneous coronary intervention (PCI) Net revenue: 45% CAGR 2001-9 Volume has driven recent revenue growth Extensive clinical program in PCI Four major randomized trials from SA to STEMI Comparative effectiveness studies Established the link between bleeding & mortality Key findings from the clinical program Reduction in major hemorrhage Reduction in cardiac mortality in STEMI Reduction in all cause mortality (pooled data) Direct hospital cost savings $400 - $900+ per patient Class-I recommendations in US and EU guidelines Angiomax clinical and economic performance Percent share of US inpatient PCI procedures SA UA N-STEMI STEMI Angiomax boxes sold by MDCO (10 vials) Unaudited data |

| MDCO | established | expanding Continuing to build the Angiomax franchise worldwide Potential sources of growth: US volume & pricing EU volume growth 40% + p.a. Expanding geography Eastern Europe, Turkey, Russia India Brazil Exploratory work in China Immediate drivers Potential sources of growth: Stable angina PCI moving to outpatient procedures High risk PCI driven by HORIZONS 3- year and heparin switch data US and EU guidelines education Use of more than 1 vial per pt. Value proposition drives pricing Next 12+ months Potential sources of growth: Structural heart disease Peripheral angioplasty Carotid angioplasty Cardiac and vascular surgery HIT-HITTS New dosage form(s) Next 5+ years Doing more with less in US US frontline reduced ~90 FTE Eliminated 3 layers of managers Annual saving ~$25MM Productivity x2, continued growth Remain highly focused on EU growth France | Italy Germany | Switzerland | Austria UK | Scandinavia | Benelux Russia | Poland | Czech | Turkey Focus on productivity Working out of recession US RAC audits for PCI Premier Perspective data analyses Rest of world HTA in UK | Italy | Germany for STEMI Value proposition in E. Europe, Turkey, Russia, India Focus on customer value Standard of care in high risk cath lab procedures worldwide Expansion in India and China via joint venture partners. Competition in generic environment. Rigorous protection of 2028 patents in US Focus on global franchise |

| MDCO | established | expanding Cleviprex is being set up for re-launch in US and approval in Germany | UK | Australia Strong formulary adoption during 1st launch year Cleviprex was approved by FDA in 3Q 2008 with a broad label for the treatment of blood pressure when oral agents are not feasible or desirable After US launch Cleviprex was added to >400 US hospital formularies during the first year In early 2010, Cleviprex was voluntarily withdrawn from the US market due to stainless steel contamination introduced at the Hospira manufacturing plant Ongoing ex-US regulatory review was put on hold Most 1st year use was in cardiovascular surgery pts Greatest value probably in neurocritical care Addressable populations in US 30 ICH patients treated in ACCELERATE trial 30 ICH patients treated in ACCELERATE trial 30 ICH patients treated in ACCELERATE trial Time to SBP 140-160 mmHg (min) 6.5 95% CI 3-10 Patients at target in 30 min 30 100% Mean infusion rate, mg/h 7.5 SD 5.2 Mean infusion duration, hrs 30 SD 26 Patients in neuro-critical care usually require prolonged infusions. Typically 8-12 vials of Cleviprex are used. In cardiac surgery patients with hypertension, typically 1-2 vials of Cleviprex are used. Patients in neuro-critical care usually require prolonged infusions. Typically 8-12 vials of Cleviprex are used. In cardiac surgery patients with hypertension, typically 1-2 vials of Cleviprex are used. Patients in neuro-critical care usually require prolonged infusions. Typically 8-12 vials of Cleviprex are used. In cardiac surgery patients with hypertension, typically 1-2 vials of Cleviprex are used. 3.4 million patients given iv anti-hypertensives per year US data (SDI hospital audit - thousand discharges) US data (SDI hospital audit - thousand discharges) 2008 Growth Medical Total 2,300 12% Neurocritical care 221 15% Cardiac 288 7% Renal 923 15% NPO 540 19% Other 589 5% Surgical Total 1,100 7% Neurocritical care 37 4% Cardiac 297 7% High risk surgery 530 5% Vascular surgery 180 4% ECT 57 38% |

| MDCO | established | expanding Re-launch Cleviprex in US, launch in rest of world Secure high quality supply chain Hospira currently making validation batches for US release Anticipate drug availability 1H 2011 Resume ex-US regulatory process Immediate drivers Refocus brand on neurocritical care Intracranial bleeding (intracerebral and subarachnoid hemorrhage) Thrombotic stroke requiring blood pressure control prior to lysis Support cardiovascular users Next 12+ months Establish as the drug of choice in neuro-hypertensive emergencies 8-12 vials per patient Create new dosage form(s) to facilitate efficiency of care Develop value proposition for other indications Next 5+ years Ensure robust chain for US | RoW Soft launch to established users Revisit price considerations Develop improved formulation Focus on quality of drug supply The value of time and precision Management of intracranial bleeding (ICH | SAH | thrombolysis-induced bleeds) Secure recommendation in neuro- critical care guidelines Develop ready-to-use syringe for CV uses and complete PRONTO in hypertensive CCF Focus on customer value Launch in 24 major markets 2011- 2014 Exclusivity in US and EU until 2020 Opportunities for lifecycle management beyond Focus on global franchise |

| Development | portfolio | value Six products | compounds in addition to Angiomax - a series of major value-drivers expected 2. |

| Development | portfolio | value Six products | compounds in addition to Angiomax - a series of major value-drivers expected Acute | intensive care products and compounds Anticipated major milestones 2010-2013 Start of CHAMPION PHOENIX phase III trial Start of SOLO phase III trials Start of MDCO-2010 phase II trial 2011 Angiomax HORIZONS heparin switch data Cleviprex (re)launch in US Argatroban launch in US Cangrelor BRIDGE trial results MDCO-2010 phase II trial results MDCO-216 - phase I clinical trials start Oritavancin in C. difficile - phase I Cleviprex launch in EU SOLO phase III trial results MDCO-216 phase I-II results MDCO-2010 phase II/III Oritavancin NDA 2013 MDCO-2010 phase II/III data CHAMPION PHOENIX phase III complete MDCO-216 phase III trials Phase 0 I I II II III III III NDA NDA Market Market Angio(ma)x IV thrombin inhibitor Cleviprex IV Ca++ channel blocker Argatroban IV thrombin inhibitor US only US only US only Cangrelor IV platelet P2Y12 inhibitor Oritavancin IV MRSA antibiotic MDCO-2010 IV antifibrinolytic MDCO-216 IV HDL therapy Serine protease inhibitors |

| Development | portfolio | value Cangrelor: Phase III iv platelet P2Y12 inhibitor for PCI and ACS Acute anti-platelet profile Rapid and titrateable onset of action Complete platelet inhibition Recovery of platelet function in 1 hour Clinical effect and safety demonstrated Significant 45% reduction in death | QMI | IDR and stent thrombosis in large randomized trials versus clopidogrel 600 mg oral loading in PCI patients Exclusivity US-RoW: 2020 including pediatric exclusivity EU: 2025 or 10 years data exclusivity Important characteristics of the compound Worldwide PCI volume ~3M patients per year Potentially highest value in high risk patients Patients unable to take oral medications Patients requiring angiogram before decision Pricing opportunity comparable to GPIIb/IIIa inhibitors Additional potential in bridging patients to surgery Potentially complementary to oral ticagrelor and other oral platelet P2Y12 inhibitors Market opportunity Pharmacological profile relative to competitors Pharmacological profile relative to competitors Pharmacological profile relative to competitors Pharmacological profile relative to competitors Pharmacological profile relative to competitors Pharmacological profile relative to competitors ORAL ORAL ORAL IV/oral IV Clopidogrel Prasugrel Ticagrelor Elinogrel Cangrelor1 Effect onset 2 hour 1 hour 1 hour 20 min Seconds Half life Irreversible Irreversible 12 hours 12 hours 5 minutes Platelet recovery time 5 days 5-7 days 3 days 2-3 days 1 hour ADP inhibition Frequent non-response Better than clopidogrel Better than clopidogrel TBD 100% (1) Based on clinical trials (1) Based on clinical trials Phase III PHOENIX trial designed based on prior studies 48 hour death / QMI / IDR Odds ratio (95% CI) P-value PCI (n=5488 ) 0.57 ( 0.33, 0.98) 0.0430 PLATFORM (n= 5312) 0.55 ( 0.33, 0.93) 0.0243 Pooled (n=10800 ) 0.56 ( 0.38, 0.81) 0.0025 Cangrelor better Clopidogrel better Data from patients who were not pre-treated with clopidogrel |

| Development | portfolio | value Cangrelor: Phase III iv platelet P2Y12 inhibitor for PCI and ACS Development program considerations CHAMPION PHOENIX phase III in a PCI population Lessons from the large CHAMPION dataset Universal MI definition No P2Y12 inhibitor prior to randomization Add ARC-defined stent thrombosis to endpoints Include patients with no pre PCI-ischemia enzymes Information availability PCI-PLATFORM post-hoc analyses 1H 2011 PCI-PLATFORM 1 year data 1H 2011 BRIDGE Trial data 2H 2011 PHOENIX interim analysis 2H 2012 PK-PD with ticagrelor 2H 2012 2011 2012 2013 2014 2015 PHOENIX trial NDA preparation NDA review Launch BRIDGE trial p d d a Key events i d = data | p = advisory panel | a = approval d PK|PD d 10,800 PCI patients Stable angina ACS-NSTE ACS-STEMI P2Y12 naive GPI bailout only 0 h 1 h 2 h Primary endpoint 48 hour Death | MI | IDR | ST Cangrelor infusion PCI 300-600 mg clopidogrel per usual hospital practice CHAMPION PHOENIX design of trial Concentration (ng/mL) % Platelet Activity Time (minutes) Infusion 4 ug/kg/min Bolus 30 ug/kg |

| Development | portfolio | value Oritavancin: Phase III iv antibiotic for serious gram-positive infections including MRSA Microbiological profile Rapid bactericidal action Active against clostridium difficile including spores Potential single dose iv treatment to cure MRSA Potentially reduced hospital length of stay and costs No drug level or toxicity monitoring Potential safety advantages over vancomycin Exclusivity US-RoW: 2021 including pediatric exclusivity EU: 10 years data exclusivity; pat. pending to 2028 Important characteristics of the compound Gram positive infections including MRSA | VISA | VRE Acute bacterial skin and skin structure infections Clostridium difficiile Bacteremia | osteomyelitis | endocarditis Total MRSA market valued at $2.5B+ MRSA ~53% of ICU and 46% non-ICU infections SOLO-1 and SOLO-2 trials starting in 2010 Non-inferiority to vancomycin FDA (SPA) | EMEA concordance with study design Market opportunity Microbiological profile relative to competitors Microbiological profile relative to competitors Microbiological profile relative to competitors Microbiological profile relative to competitors Microbiological profile relative to competitors Microbiological profile relative to competitors Vancomycin Linezolid (Zyvox) Daptomycin (Cubicin) Telavancin (Vivativ) Oritavancin1 MSSA 1 2 0.5 0.5 0.12 MRSA 1 2 0.5 0.25 0.12 VISA 8 4 8 1 2 VRSA >64 2 0.5 2- 4 0.5 VRE (E. faecalis) >256 2 2 16 1 VRE (E. faecium) >256 2 4 8 0.25 (1) Based on clinical trials (1) Based on clinical trials Clinical profile relative to competitors Clinical profile relative to competitors Clinical profile relative to competitors Clinical profile relative to competitors Clinical profile relative to competitors Clinical profile relative to competitors Vancomycin Linezolid (Zyvox) Daptomycin (Cubicin) Telavancin (Vivativ) Oritavancin1 Course of tx 10-14 days 2x / day 10-14 days 2x / day 7-14 days 1x / day 7-14 days 1x / day 3 hours Once Hospital stay In-patent In-patient In-patient, out-patient In-patient Discharge possible Safety Nephrotoxicity Marrow tox. CPK elevation QTc interval None Monitoring Renal Blood count Weekly CPK Renal None (1) Based on clinical trials (1) Based on clinical trials (1) Based on clinical trials (1) Based on clinical trials (1) Based on clinical trials (1) Based on clinical trials |

| Development | portfolio | value Oritavancin: Phase III iv antibiotic for serious gram-positive infections including MRSA Multicenter, double-blind, randomized Single-dose iv oritavancin vs. vancomycin 7-10 days Non-inferiority for the primary efficacy outcome Cessation of spread or reduction in size of the baseline lesion Absence of fever No rescue antibiotic At 48 - 72 hours Gram-positive pathogens. ABSSSI includes wound infections, infective cellulitis, and major cutaneous abscesses (30% cap) SOLO-1 and SOLO-2 are identical ABSSSI trials 960 patients randomized in each study SOLO-1 and 2 70% (672 patients) expected clinically evaluable ~175 patients with MRSA (total 350) Males or females ^18 years old ABSSSI suspected or confirmed by gram+ bacteria Wound infection Cellulitis/erysipelas Major cutaneous abscess Pus and/or induration 75 cm2 Systemic inflammation symptoms (nodes | fever ^38.0°C, bandemia, increase WBC, CRP Entry criteria - serious ABSSSI infections only Primary success measure assumed to be 75% in both groups 480 patients per treatment group provides at least 90% power to demonstrate non-inferiority at the 1-sided alpha level of 0.025, using a non-inferiority margin of 10% Sample size also provides at least 90% power to demonstrate non- inferiority for clinical cure event rate of 65%, margin 10%. Enrollment will continue until 175 MRSA patients in each study. Planned pooling of SOLO-1 and SOLO-2 Statistical methods follow FDA guidelines 2011 2012 2013 2014 2015 NDA preparation NDA review Launch in ABSSSI ABSSSI: SOLO-1 | 2 I C. Diff II-III a d d d p Key events d = data | p = advisory panel | a = approval Estimated development timeline: Phase III results late 2012 |

| Development | portfolio | value MDCO-2010: Phase II iv antifibrinolytic to reduce surgical blood loss Targets fibrinolysis and coagulation Primary: plasmin | plasma kallikrein Secondary: factor X | factor XI | aPC Small molecule Liquid ready-to-use formulation Preclinical data, potential to overcome aprotinin limitations Rapid elimination Low potential for renal dysfunction Reduced risk of thrombotic complications Low anaphylaxis risk Important characteristics of the compound Worldwide ~200K high risk cardiac | vascular surgical procedures per year Coronary bypass and valve patients Repeat coronary bypass procedures Patients at high risk of bleeding Trasylol proved economically dominant in this group of patients. Trasylol worldwide sales were >$350M pa and growing rapidly at the time of market withdrawal Near perfect fit with MDCO frontline team Market opportunity Canine cardiopulmonary bypass model Blood loss in mL % change in ventricular performance 2011 2012 2013 2014 2015 Phase III high risk CTS NDA preparation NDA review Launch Phase II p d a Key events d d = data | p = advisory panel | a = approval Estimated development timeline |

| Development | portfolio | value MDCO-216: Phase 0-I genetic variant reverse cholesterol transport agent HDL-C is a potent inverse CV risk factor HDL apo A-I is anti-atherosclerotic in animals Human carriers of variant apo A-I Milano Have low levels of HDL-C (0.25-0.78 mmol/L), yet Enjoy less atherosclerosis and greater longevity Apo A-I Milano protein and HDL particles Cysteine substitute for arginine 173 - dimer forms MDCO-216 is recombinant Apo-A1 Milano + lipid Important characteristics of the compound Worldwide epidemic of cardiovascular risk factors Low HDL | diabetes | family history | smoking >6 million patients present for angiography each year 3 million PCI alone 2 million ACS in PCI labs High risk factor subsets of SA and ACS Populations with cerebral and peripheral disease MDCO-201 has the potential to modify risk in patients with advanced, vulnerable atheroma in all sites Market opportunity Proof of concept in clinical trial in 47 ACS patients % change in carotid plaque area after 5 doses given every 4 days Dose-dependent effects in rabbit model Treatment group No. pts Change from baseline, mm3 Change from baseline, mm3 Change from baseline, mm3 Mean (SD) p-value Placebo 11 -2.9 (23.3) 0.97 MDCO-216 15 mg/kg 21 -15.1 (50.6) 0.02 45 mg/kg 15 -12.6 (15.3) 0.007 Combined 36 -14.1 (39.5) <0.001 Nissen et al. JAMA 2003;290:2292-2300 Parolini C. et al. J. Am. Coll. Cardio.. 2008;51:1098-1103 |

| Development | portfolio | value MDCO-216: Phase 0-I genetic variant reverse cholesterol transport agent CSL-112 (Reconstituted HDL) - CSL ApoA-I Milano/Phospholipid Complex - SemBioSys ATI-5261 - Artery Therapeutics / Roche 5-Ala - NIH / KineMed Cerenis HDL - Cerenis Therapeutics Trimeric A-I - Borean / Roche Recent news from AHA - Merck 11-17-2010 Anacetrapib CETP inhibitor found safe DEFINE trial in 1623 CHD risk patients 138% increase in HDL | 40% decrease in LDL Renewed interest in HDL Phase II imaging studies of natural history PROSPECT trial of IVUS in CAD in 697patients The 3-year cumulative MACE rate 20.4% Culprit lesions 12.9% patients, non-culprit 11.6%. Predictors of non-culprit MACE Insulin-requiring diabetes (HR 3.32). Plaque burden ^70% (5.03) Minimal lumen area ^4.0mm2 (3.21) Thin-cap fibroatheromas (3.35). Imaging can predict risk and may be a revealing tool in Phase II studies Focus on high risk patients and imaging PoC Lesion classification according to radiofrequency IVUS 2011 2012 2013 2014 2015 Phase II (imaging) Phase III (outcomes) Manufacturing dev. Phase I d Key events d d d = data | p = advisory panel | a = approval NDA preparation d Estimated development timeline: Early proof of concept |

| Leadership | development know-how | commercial focus Hospital market knowledge | relationships | record of innovation 3. |

| Know-how | capabilities | depth MDCO senior leadership MDCO has considerable strength, depth and breadth of leadership and continues to build professional competences All five of the founders of the firm has either stayed with MDCO or have returned after periods working in other entities The group has a unique blend of large pharmaceutical, small biotech, financial institution and deal-making experience The firm has ~410 employees |
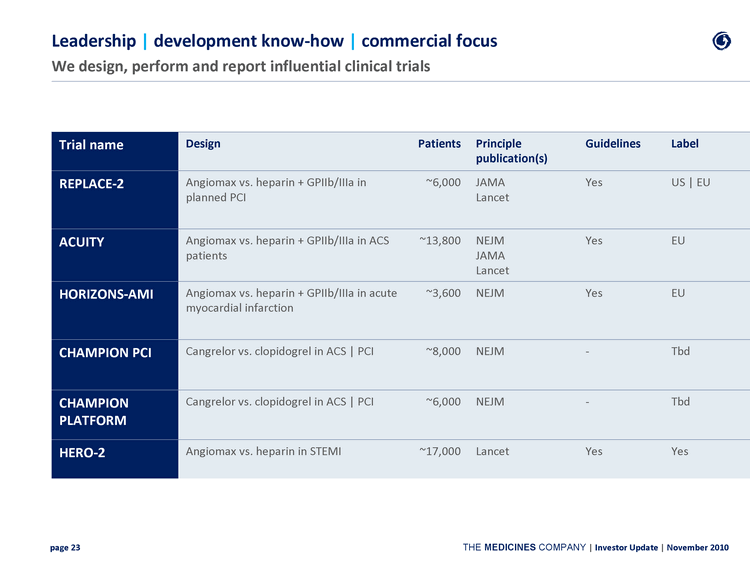
| Leadership | development know-how | commercial focus We design, perform and report influential clinical trials Trial name Design Patients Principle publication(s) Guidelines Label REPLACE-2 Angiomax vs. heparin + GPIIb/IIIa in planned PCI ~6,000 JAMA Lancet Yes US | EU ACUITY Angiomax vs. heparin + GPIIb/IIIa in ACS patients ~13,800 NEJM JAMA Lancet Yes EU HORIZONS-AMI Angiomax vs. heparin + GPIIb/IIIa in acute myocardial infarction ~3,600 NEJM Yes EU CHAMPION PCI Cangrelor vs. clopidogrel in ACS | PCI ~8,000 NEJM - Tbd CHAMPION PLATFORM Cangrelor vs. clopidogrel in ACS | PCI ~6,000 NEJM - Tbd HERO-2 Angiomax vs. heparin in STEMI ~17,000 Lancet Yes Yes |

| Leadership | development know-how | commercial focus We concentrate on <3,000 hospitals worldwide More than 80% of acute cardiac care, including PCI, is performed in 2,548 leading hospitals in 26 countries across 14 language zones. These institutions represent our call list. We have mapped their whereabouts, their business and their clinical capacity. We prioritize these institutions in our global business development and organizational roll-out. 175 367 103 212 41 Number of institutions 450 USA Germany France Italy UK Netherlands Switzerland Spain Poland Belgium Czech Republic China India Brazil Canada Australia Austria Sweden Norway Denmark Finland Turkey Hungary Greece UAE Total Japan |

| Disciplined | financial | focus Strong foundations and planned sustainable growth 4. |
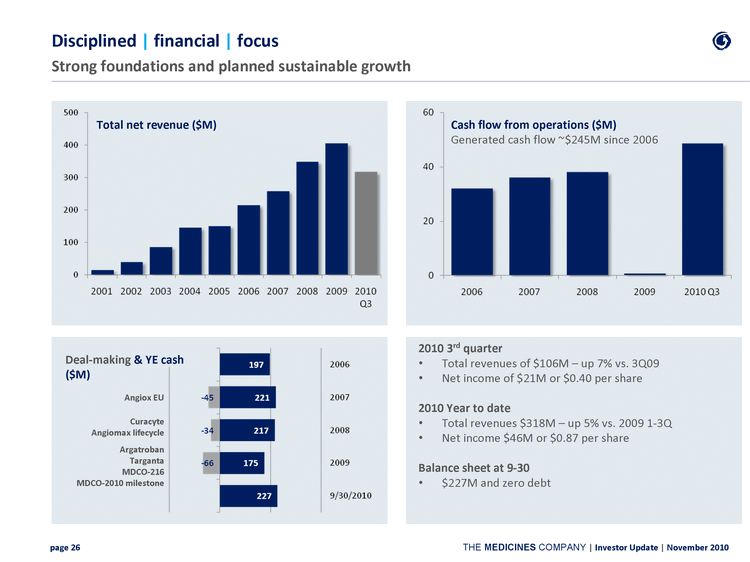
| Disciplined | financial | focus Strong foundations and planned sustainable growth Total net revenue ($M) Cash flow from operations ($M) Generated cash flow ~$245M since 2006 Deal-making & YE cash ($M) Angiox EU Curacyte Angiomax lifecycle Argatroban Targanta MDCO-216 MDCO-2010 milestone 2010 3rd quarter Total revenues of $106M - up 7% vs. 3Q09 Net income of $21M or $0.40 per share 2010 Year to date Total revenues $318M - up 5% vs. 2009 1-3Q Net income $46M or $0.87 per share Balance sheet at 9-30 $227M and zero debt |

| Disciplined | financial | focus New business ventures activities We will continue to focus on acute | intensive care business development opportunities for leading hospitals Cardiovascular | stroke | serious infection Innovative products Low upfront payments Late stage - ideally with low R&D requirements Strong fit with current competence | capacity We have no plans or appetite for 'transformative deals' Cath. lab. Pharmacy Blood bank E.P. lab. ED Card surg. CCU ICU Anesthesia Heart trsplnt Neuro surg. Stroke unit Trauma Obstetrics Burn unit NICU Pain mgt Lung trsplnt H/L trsplnt Renal trsplnt Liver trsplnt Renal dialys Pancr trsplnt Hospital lines of service (simplified version) |

| Disciplined | financial | focus Our strategic approach to growth Net sales development We believe that Angiomax will continue to grow in US with extended exclusivity through mid 20151 as the results of a combination of volume expansion and price increases linked to the value our health and economic outcomes data We will defend our 2028 IP rigorously Angiomax is expected to grow in Europe and RoW as we drive adoption and expand our reach to key hospitals We plan to reintroduce Cleviprex to US in 2011 and expect to launch the product in key markets of EU and RoW in 2011-2012 Cost of sales Expected to continue to be around 30% Research and development We will manage R&D expenditures with discipline and expect to spend 17-20% of net revenue annually in order to optimize the pace and content of our development programs over the coming three years Sales, general and administration Our current level of SG&A expense allows us to pursue our commercial goals and we expect to remain at around this level of resources and expenses, optimizing frontline efforts worldwide and managing administrative expenses tightly Cash flow Based on these assumptions, the business should generate considerable cash. We will use this to improve our profitability and may undertake highly selective business development activities. We do not expect to chase so-called 'transformative deals'. H-W restoration administrative process underway at PTO following federal district court decision August; district court decision appealed to Federal Circuit court by APP |

| Unique time to invest in MDCO Opportunity to participate in growth of acute | intensive care hospital medicine worldwide 1. Established | expanding | presence Angiomax(r) (bivalirudin) a growing $400M+ franchise in acute hospital arterial anticoagulation 45% CAGR since launch - >3 million PCI patients treated1 Recent breakthrough data in the highest risk PCI patients Anticipate US and EU exclusivity to 2015 with new US IP to 20282 Planned growth in US | new geography | potential new uses 2010-2015+ 2. Development | portfolio | value Six additional products | compounds that fit Potential segment leading products in the hospital with worldwide application Near term series of major value-drivers expected 2011: Six major portfolio events anticipated 2012: Five major portfolio events anticipated 3. Know-how | capabilities | depth Hospital market knowledge | relationships | record of innovation Knowledgeable, experienced, high-energy leadership team and deep bench Track-record of important clinical trials that change care pathway and practice Proven capabilities in delivering an economically dominant hospital product Committed to become the leading player in this expert space 4. Disciplined | financial | focus Expecting progressive sustained growth Top-line expansion | tight control of operating expenses | growing cash flow Targeted and highly selective new business development program Focused on disciplined financial performance and shareholder returns Estimated percutaneous coronary intervention patients given Angiomax in US since January 2001 H-W restoration administrative process underway at PTO following federal district court decision August; district court decision appealed to Federal Circuit court by APP |
