Attached files
| file | filename |
|---|---|
| 8-K - AUXILIUM PAHRMACEUTICALS, INC. - AUXILIUM PHARMACEUTICALS INC | d8k.htm |
 1
November 2010
(NASDAQ: AUXL)
Exhibit 99.1 |
 2
Safe Harbor Statement
We will make various remarks during this presentation that constitute “forward-looking
statements” for purposes of the safe harbor provisions under The Private Securities
Litigation Reform Act of 1995, including statements regarding: the pricing, time to market, size of
market, growth potential and therapeutic benefits of the Company’s products and product
candidates, including those for the treatment of Peyronie’s disease and Frozen Shoulder syndrome; the size of each of the Dupuytren’s market and the Peyronie’s
market in the U.S. and EU; the number of insured lives in the U.S. with access to XIAFLEX for the
treatment of their Dupuytren’s contracture; the potential for XIAFLEX to be a blockbuster
opportunity; the potential for XIAFLEX to be used in multiple indications; interpretation of market research data; competition within certain markets relevant to the
Company’s product candidates; interpretation of clinical results, including the efficacy and
tolerability of the Company’s products and product candidates, and recurrence rates; the
timing of the commencement and completion of clinical trials, the success of those trials and the timing of reporting of results therefrom; the effect of the identified leading
indicators on the success of the XIAFLEX launch and future net revenues; the ability to obtain
reimbursement in the U.S. for XIAFLEX for the treatment of Dupuytren’s; the timing of new
reimbursement codes for XIAFLEX and the effect of the reimbursement process on this success of the XIAFLEX launch; the average number of cords that
Dupuytren’s contracture patients will have treated with XIAFLEX and the average number of vials
used to treat each cord; the scope, timing, methodology, endpoints, safety, execution and
results of the global development plan and the phase III studies for XIAFLEX for the treatment of Peyronie’s disease; physicians and sites that are moving from
test drive to increasing usage; the generation of cash through licensing of XIAFLEX in other
territories and for new indications; the timing and likelihood of approval of the Marketing
Authorization Application for XIAFLEX for the treatment of Dupuytren’s contracture in the European Union; competitive developments affecting the Company’s
products and product candidates, including generic competition; the protection for XIAFLEX afforded by
U.S. Patent No. 7,811,560; the market exclusivity for XIAFLEX; the success of the
Company’s development activities; future Testim market share, prescriptions and sales growth and factors that may drive such growth; size and growth potential
of the testosterone replacement therapy market and the gel segment thereof and factors that may drive
such growth; the protection for Testim afforded by U.S. Patent No. 7,320,968, and those issued
on October 27, 2009 and their listing in the Orange Book, the value of extending patent protection for Testim through January 2025, the value and
likelihood that patents will be granted from the continuation applications filed by CPEX
Pharmaceuticals, Inc.; the impact of the filing by Upsher-Smith Laboratories, Inc. of an
ANDA for a testosterone gel; the likelihood of generic competition in the testosterone replacement
therapy gel market; the Company’s development and operational goals and strategic
priorities for fiscal 2010; the ability to fund future operations; the opportunities to build shareholder value; and the Company’s expected financial performance during
2010 and financial milestones that it may achieve for 2010, including 2010 Testim net revenues,
research and development spending, selling, general and administrative expenses,
stock-based compensation expenses and the range of XIAFLEX net revenues for the fourth quarter 2010. All remarks other than statements of historical facts made
during this presentation, including but not limited to, statements regarding future expectations,
plans and prospects for the Company, statements regarding forward-looking financial
information and other statements containing the words “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,”
“plan,” “expect,” and similar expressions, as they relate to the Company,
constitute forward-looking statements. Actual results may differ materially from those reflected in these forward-looking
statements due to various factors, including general financial, economic, regulatory and political
conditions affecting the biotechnology and pharmaceutical industries and those discussed in
Auxilium's Annual Report on Form 10-K for the year ended December 31, 2009 and in Auxilium’s Quarterly Report on Form 10-Q for the period ended September
30, 2010 under the heading “Risk Factors,” which are on file with the Securities and
Exchange Commission (the “SEC”) and may be accessed electronically by means of the
SEC’s home page on the Internet at http://www.sec.gov or by means of the Company’s home page
on the Internet at http://www.auxilium.com under the heading “For Investors - SEC
Filings.” There may be additional risks that the Company does not presently know or that the Company currently believes are immaterial which could also cause actual
results to differ from those contained in the forward-looking statements. Given these risks
and uncertainties, any or all of these forward-looking statements may prove to be
incorrect. Therefore, you should not rely on any such factors or forward-looking statements.
In addition, forward-looking statements provide the Company’s expectations, plans or
forecasts of future events and views as of the date of this presentation. The Company
anticipates that subsequent events and developments will cause the Company’s assessments to
change. However, while the Company may elect to update these forward- looking
statements at some point in the future, the Company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as
representing the Company’s assessments as of any date subsequent to the date of this
presentation. |
 3
Opportunities to Build Shareholder Value
Note: Seeking partners for Transmucosal film product candidates |
 4 |
 5
XIAFLEX –
Unique Blockbuster Opportunity
•
Potential to be the only effective non-surgical treatment for two high unmet
needs:
Approved
on
February
2,
2010
for
Dupuytren’s
contracture
in
U.S.
Peyronie’s
disease Phase III double-blind study enrollment anticipated to
complete in 1Q11 and top-line data expected in 1H12
•
Well-characterized mode of action
•
Worldwide rights support growth
Build company in North America
Partnered
with
Pfizer
in
EU
for
Dupuytren’s
and
Peyronie’s
Opportunity to add additional indications
Rights for other territories or indications could generate additional cash
•
We believe worldwide peak revenues for XIAFLEX could be in excess
of $1 Billion annually |
 6
•
Excessive collagen deposition in
fascia of hand
•
Nodules represent early, active form
•
Cords develop over time, are palpable,
and result in contractures
•
Quality of life and daily activities can
be significantly affected
•
Surgery has been the current standard of
care and may be reserved for advanced
disease due to unpredictable results,
complications, long recovery and
recurrence/additional surgeries
Dupuytren’s
Contracture is Debilitating for Patients |
 7
•
Surgery
•
Needle fasciotomy/
aponeurotomy
•
Amputation
Treatment Options Historically Limited to Invasive
Surgery with Significant Recuperation or Are
Unapproved and Ineffective
•
Non-surgical options
>
Splinting
>
Physical therapy
>
Corticosteroids |
 8
It Is About the Patient
Pre-XIAFLEX treatment
30 days post-single injection of XIAFLEX
Immediately post-operative
Intra-operative open fasciectomy
Pictures courtesy of Dr. Clayton Peimer |
 9
Early Intervention with XIAFLEX May Be the
Most Effective Treatment Approach
N=125
41%
9 |
 10
•
Most treatment-related adverse events were mild or moderate in
intensity and resolved without intervention within a median of 10
days across studies
•
Serious Adverse Events possibly related to treatment were limited in
number (10 in total; 0.39% of >2600 injections)
–
4 SAEs
were tendon and ligament damage
•
No reported deaths, clinically meaningful changes in grip strength,
arterial injuries or nerve injuries related to XIAFLEX
•
No clinically meaningful changes in laboratory values
•
No clinically meaningful systemic allergic reactions
XIAFLEX Was Well-Tolerated in Phase III Studies |
 11
1
3
Contracture Recurrence Affects Patients
(1) Anwar et al., The Journal of Hand Surgery, Vol. 32A No. 9 November 2007 ; 1423-1428 (2) Leclercq C. Epidemiology. In: Tubiana R, Leclercq C, Hurst LC,
Badalamente MA, Mackin EJ, eds, Dupuytren’s disease. Martin Dunitz Ltd, London,
2000;239-249. (3) A.L. Van Rijssen, 2010 International
Symposium on Dupuytren’s Disease
•
The earliest reports of recurrence for surgery were seen in 22% of
female and 19% of male Dupuytren’s contracture patients at a mean
of 12 months following fasciectomy
. Additionally, a rate of up to
34%
has
been
reported
within
the
first
2
years
following
surgery
2
.
•
Abstract
for
a
prospective
trial
comparing
needle
fasciotomy
to
open
fasciectomy
reported
a
recurrence
rate
for
needle
fasciotomy
of
85% at a mean of 2.3 years
.
•
XIAFLEX
Long
term
extension
study
of
phase
III
trials
will
follow
recurrence rates up to 5 years. Two year data now available.
|
 12
Two Year Recurrence Rate of 19.3% for
XIAFLEX in Dupuytren’s
Contracture
All Joints
MP Joints
PIP Joints
Patients from All Phase III Studies (n=950)
1,568
920
648
Patients Enrolled in Extension Study (n=634)
1,065
641
424
Patients Successfully Treated and Enrolled in Extension
Study (n=474)
619*
449*
170
Joints with Recurrence (n/%)
(119/618)
19.3%
(61/448)
13.6%
(58/170)
34.1%
Note: No patients at Year 2 had been retreated with commercial XIAFLEX
* One patient had unrelated hand surgery and post-operative bandaging prevented an
accurate assessment of recurrence at 2 years |
 13
Launch Update Through
November 2010 |
 14
•
Position XIAFLEX as a paradigm-changing non-surgical
treatment alternative for physicians, payers and patients
•
Achieve high awareness among targeted physicians and
diagnosed patients
•
Get
targeted
physicians
through
the
sales
cycle
–
from
interest
to
injection to reimbursement
•
Educate and assist physician offices with unfamiliar
reimbursement processes at each step of the way
Key Objectives for U.S. XIAFLEX Launch in 2010 |
 15
Targeting the Eligible Patient Population
Should Drive Development of the U.S. Market
to Its Full Potential |
 16
XIAFLEX Launch Strategy is Tailored to
Customer Needs
•
~ 7,000 target physicians
–
Hand surgeons, plastic surgeons, orthopedic surgeons, general
surgeons, rheumatologists
•
~ 100 field based personnel
–
Sales reps, sales managers, and reimbursement specialists
•
Medical science liaisons
–
Key opinion leader and regional opinion leader support
|
 17
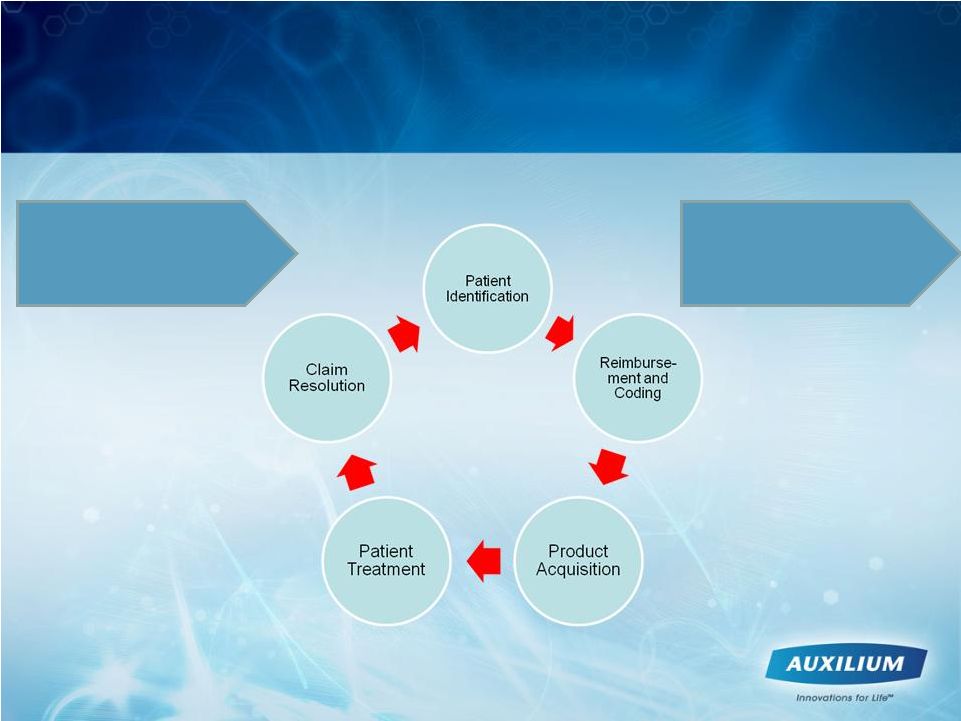
Unique Sales Cycle for Targeted Physicians
Physician Interest,
Training & Enrollment
Successful XIAFLEX
XPERIENCE |
 18
Changing Treatment Paradigm and Reimbursement
Cycle May Drive Physician Adoption Rate
Prior to treatment decision Physician must go through:
•Awareness
•Interest
•Training / Enrollment into XIAFLEX XPERIENCE program
•Reimbursement education / comfort
•Patient Identification / counseling
11 –
21 weeks from treatment decision through receipt of payment
possible |
 19
•
Accounts need customized support every step of the way
•
Miscellaneous and component CPT codes being used and
accepted
•
C-code for institutional use effective as of July 1, 2010
•
CMS has announced that J0775 will be the J-code for
XIAFLEX effective as of January 1, 2011
•
CPT code specific to XIAFLEX expected to come in 2012
Reimbursement Processes Are New to Target
Physicians |
 20
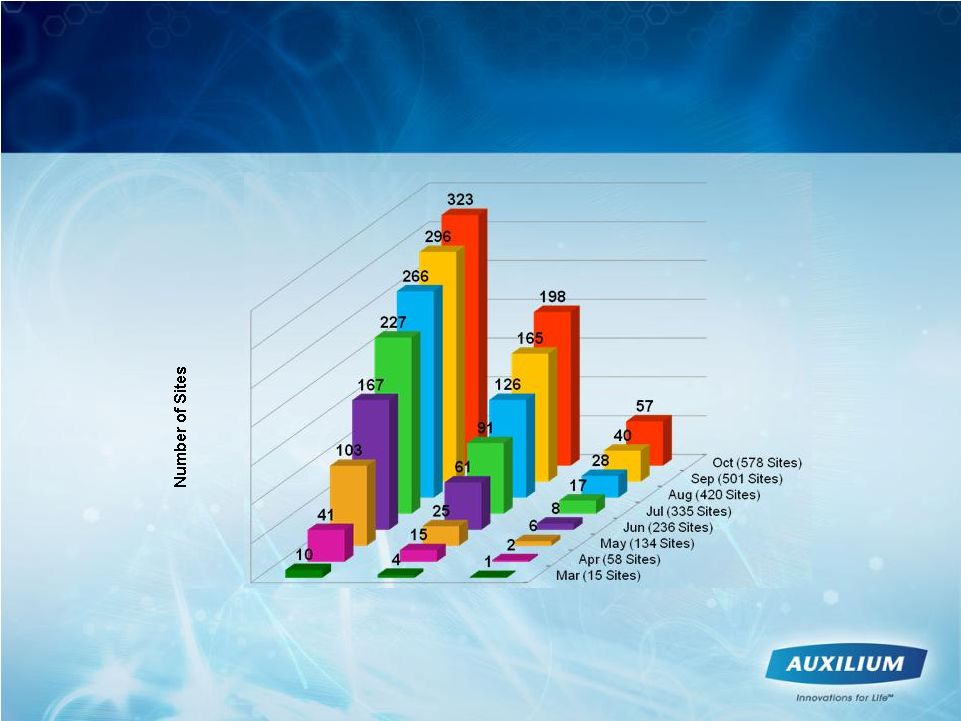
Leading Indicators Trending Positively
-
Unique sites that have ordered XIAFLEX
597
1,131
1,419
1,627
1,746
1,853
1,993
2,093
393
752
954
1,114
1,213
1,304
1,427
1,516
15
58
134
236
335
420
501
578
0
500
1000
1500
2000
2500
Mar
Apr
May
Jun
Jul
Aug
Sep
Oct
PHYSICIAN ENROLLMENTS
SITES ENROLLED
UNIQUE SITES ORDERING |
 21
Cumulative Call Center Volume |
 22
Sites Continue to Move from Test Drive to Increasing
Usage
Injections per Site
0
50
100
150
200
250
300
350
1-2
Injections
3-10
Injections
11 +
injections |
 ~91% of insured lives have access
23 |
 24
Out of Pocket Costs In Line With Expectations
A significant portion of Medicare patients with a
20% liability have secondary insurance, which
may cover a portion of this cost
% Of Treatment Cost Paid by Patient
55%
4%
8%
8%
23%
2%
0%
10%
20%
30%
40%
50%
60%
0%
Up to 2%
2.1-9.9%
10-19.9%
20%
>20% |
 25
Distribution Network is Operating Smoothly
Unit Sales by Channel
12%
44%
44%
Speciality
Pharmacy
Speciality
Distributor
Wholesaler |
 26
Direct to Patient Program Launching |
 27
Upcoming Peer to Peer Events
•
National webcasts on November 8 and
December 11 with Larry Hurst performing
injection and manipulation
•
Peer to peer meetings on a territory basis |
 28
We Believe Launch Strategy Is Sound:
•
Product efficacy and safety are consistent with clinical
data
•
Access to targeted physicians is good
•
Usage is characterized by “test driving”, which is typical
for paradigm-changing treatment
•
Payers willing to provide access to drug and procedures
•
All distribution channels active and operating as
expected
•
Leading indicators trending positively |
 29
Sustainable Blockbuster Market for
Dupuytren’s
Contracture Anticipated
•
High disease prevalence
Based
on
literature,
~
6M
to
11M
patients
in
U.S.
have
a
Dupuytren’s
pit,
nodule or cord, but only ~300,000 new patients annually
diagnosed in U.S. •
Sustainable patient pool
New patients within an aging population
Average of 1.5 cords expected to be treated and average 1.1 injections per
cord
~ 50% of patients have bilateral disease
Disease progression to additional joints
High recurrence rate for surgery
1,2
•
No nonsurgical competition
•
Market development represents upside
1
Mikkelsen
1976 Tubiana
2006
2 |
 30
XIAFLEX Intellectual Property Position
•
Highly purified collagenase
product and method of manufacturing patent
U.S. Pat. No. 7,811,560 issued in October 2010; expected expiry in 2028
Patent applications are under review outside of U.S.
•
Market Exclusivity expected in U.S. for 12 years post-approval, per 2010
Patient Protection and Affordable Care Act
•
U.S. Orphan Drug designation granted on May 23,1996 provides
exclusivity through February 2017
•
Market Exclusivity expected in EU for 10 years post-approval
Additional year granted for second indication
Data protection granted for 8 years
•
Method
of
Use
Patents
in
U.S.
through
2014
for
Dupuytren’s
contracture
and 2019 for Peyronie’s
disease |
 31
1
Smith
BH.
Am
J
Clin
Pathol.
1966;45:670-678.
2
Somers KD, Dawson DM. J Urol. 1997;157:311-315.
•
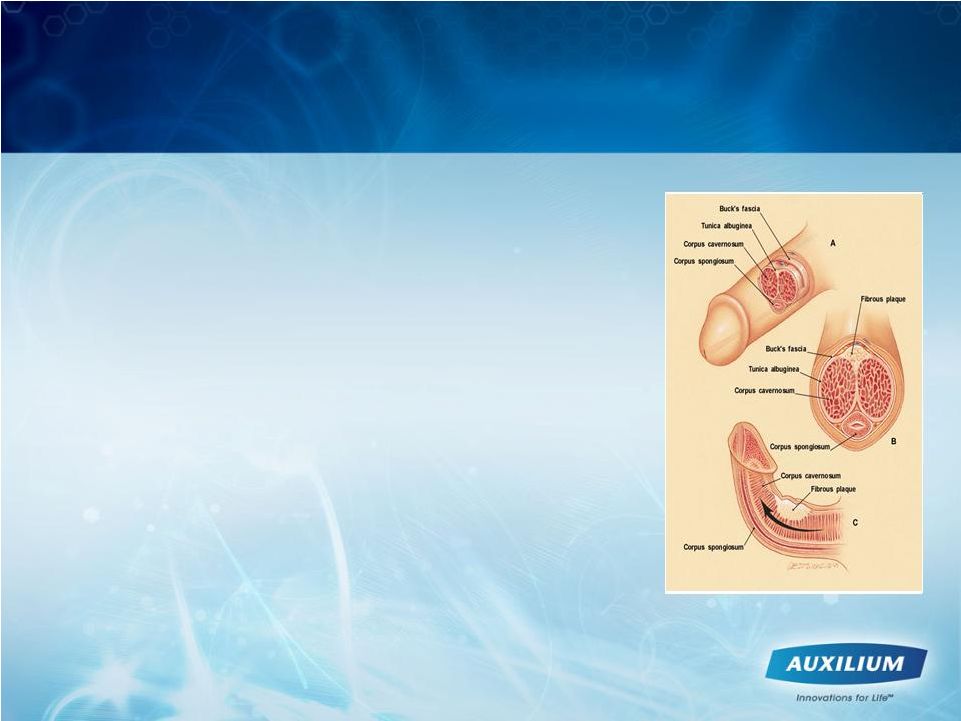
Scarring phenomenon affecting the
tunica
albuginea
1
•
Plaques
show
excessive
collagen
deposition
2
•
Potential Symptoms
>
Pain with erection, penile curvature/
deviation, penile shortening, indentations,
and/or erectile dysfunction
>
May experience difficulty with sexual
intercourse, loss of self-esteem,
and depression
•
There are no approved therapies for the
treatment of Peyronie’s
disease
Peyronie’s
Disease is a Devastating Disorder |
 32
1
Bella A. Peyronie’s Disease J Sex Med 2007;4:1527–1538
2
Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med
2004;1:6–23. 3
Mulhall
JP,
et
al.
Subjective
and
objective
analysis
of
the
prevalence
of
Peyronie’s
disease
in
a
population
of
men
presenting for prostate cancer 4creening. J Urol 2004;171:2350–3.
4
Smith
BH.
Am
J
Clin
Pathol.
1966;45:670-678.
5
Lindsay MB, J
Urol. 1991;146:1007-1009.
6
Nyberg L, J Urol.128:
48, 1982 •
Prevalence of Peyronie’s disease is estimated to be approximately
5% in adult men
1,2,3
>
Actual prevalence may be higher, based on autopsies
4
•
Prevalence rate increases with age
>
The average age of disease onset is 53 years
5
•
High association with other diseases such as:
>
Diabetes, erectile dysfunction (ED), Dupuytren’s
contracture, plantar fascial
contracture,
tympanosclerosis, gout, and Paget’s disease
6
•
We believe Peyronie’s disease is under-diagnosed
and under-treated
Peyronie’s
Disease
-
an
Unmet
Medical
Need |
 33
•
The goal of surgery is simply to make the two sides of
the penis equal in size through reduction of the longer
side
•
Post-surgically, graft or prosthetic may be required
•
Cost of surgery in U.S. is ~$10-12K
•
Patients are highly motivated to attempt other
treatments first
Current Surgical Options Are the Treatment of Last
Resort |
 34
•
Verapamil
•
Vitamin E
•
Colchicine
•
Potassium aminobenzoate
(Potaba)
•
Tamoxifen
•
Interferon alpha-2a
•
Corticosteroids
•
Energy transfer treatment including extracorporeal
shock wave therapy (ESWT), laser and ultrasound
therapy, and orthovoltage
radiation
Unapproved Treatments for Peyronie’s
Disease
Have Been Used With Little Reported Success |
 35
54.4
50.6
38.2
45.1
0
10
20
30
40
50
60
Xiaflex
Placebo
Phase IIb
Overall Results–
Statistically Significant
Reduction of Penile Curvature and Improvement in
Peyronie’s
Disease Bother (PRO Domain) with XIAFLEX
P = 0.001
-29.7%
-11.0%
XIAFLEX N = 109
Placebo N = 36
8.1
8.1
5.5
7.4
0
4
8
12
16
20
Xiaflex
Placebo
P = 0.046
-32.1%
-8.6%
XIAFLEX N = 100
Placebo N = 34
Penile Curvature
Bother |
 36
Phase
IIb
with
Modeling
Results-
Statistically
Significant
Effect
in
Both
Penile
Curvature
and
Peyronie’s
Disease
Bother Endpoints with XIAFLEX
P < 0.001
-32.4%
2.5%
XIAFLEX N = 54
Placebo N = 20
8.6
7.7
5
7.5
0
4
8
12
16
20
Xiaflex
Placebo
P = 0.004
-41.8%
-2.6%
XIAFLEX N = 50
Placebo N = 18
Penile Curvature
Bother
54.7
51.9
37.2
52.5
0
10
20
30
40
50
60
Xiaflex
Placebo |
 37
Phase IIb
Safety Profile Consistent with Previous
XIAFLEX Studies in Peyronie’s
Disease
•
Well tolerated
•
Immunogenicity profile similar to Dupuytren’s
data
•
Injection
site
bruising,
edema,
pain
–
most
common
•
No drug related SAEs
•
No systemic immunologic events |
 38
Peyronie’s
Disease Phase III Development
Program
Study
Type
~ #
Subjects
Sites
XIAFLEX:
Placebo
Duration
AUX-CC-
803
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-
804
Double-blind,
placebo controlled
300
~ 30 US
5 AUS
2:1
52 Wks
AUX-CC-
802
Open Label
250
12 US
6 NZ
12 EU
N/A
36 Wks
AUX-CC-
805
Pharmacokinetics
16
1 US
N/A
4 Wks
XIAFLEX 0.58 mg
Two injections per treatment cycle, up to 4 cycles
24 to 72 hours between injections
Penile plaque modeling following each cycle |
 39
Phase IIb
vs. Phase III
Protocol Differences
Phase IIb
Double Blind
Placebo Controlled Trial
Phase III Double Blind
Placebo Controlled Trial
Up to 3 treatment cycles
Up to 4 treatment cycles
Modeling vs. No-modeling
Modeling
3 : 1
XIAFLEX : Placebo
2 : 1
XIAFLEX : Placebo
6 months of disease
12 months of disease
No specific AEs
monitoring
Specific AEs
monitoring
36 weeks
52 weeks |
 40
We Believe Phase III Double-blind Studies Are at Least
85% Powered to Achieve Co-primary Endpoints
•
Statistical significance requires greater
than 27% mean net improvement from
baseline in the Peyronie’s
disease bother
domain of the PDQ over placebo
•
Statistical significance requires greater
than 19% mean net improvement from
baseline in penile curvature over placebo |
 41
Extensive Market Research Performed
with Peyronie’s
Surgeons
•
Two rounds of quantitative primary research have been
commissioned by Auxilium
on XIAFLEX for Peyronie’s
disease
•
A combined 575 Urologists have been interviewed to
estimate market size (415 in U.S.; 160 in Europe)
and 383 have given feedback on potential usage of
XIAFLEX (223 U.S.; 160 Europe)
•
Second study (n=472 total & 333 in depth) was designed
to provide 95% confidence level and 7.5% margin of
error |
 42
Annually, >210,000 Peyronie’s Candidates
Could Exist between U.S. and Europe |
 43
Strategic Partnership with Pfizer for XIAFLEX
in EU
•
Strong working relationship with goal to obtain EMA approval
•
Auxilium
is primarily responsible for the global development of
XIAFLEX, including all clinical & commercial manufacturing and supply.
•
Pfizer is primarily responsible for:
European
territory
regulatory
activities
for
Dupuytren’s
and
Peyronie’s
All
European
territory
commercialization
activities
for
Dupuytren’s
and
Peyronie’s
All
European
territory
phase
IV
clinical
development
for
Dupuytren’s
and
Peyronie’s
•
Compelling economics for Auxilium:
Up-front payment of $75 million
$150 million tied to regulatory milestones ($15M received for MAA acceptance in 1Q10)
$260 million based on sales milestones
•
First EU-only partnership for Pfizer
Significant increasing
double-digit tiered royalties based on sales of XIAFLEX in
Pfizer’s territories
|
 Testim
®
1% Testosterone Gel
44
Testim
®
1% Testosterone Gel |
 45
*Mulligan T. et al. Int J. Clin Pract
2006
Testim
®
Maintains Double-Digit Growth in
Testosterone Replacement Therapy Market
•
Proprietary, topical 1% testosterone gel
>Once-a-day application
>Favorable clinical and commercial profile
•
Recent study indicates 39% of U.S.
males over 45 yrs are hypogonadal*
>We estimate that <10% of affected
population receives treatment
•
We believe diagnosis is increasing
through education and awareness |
 46
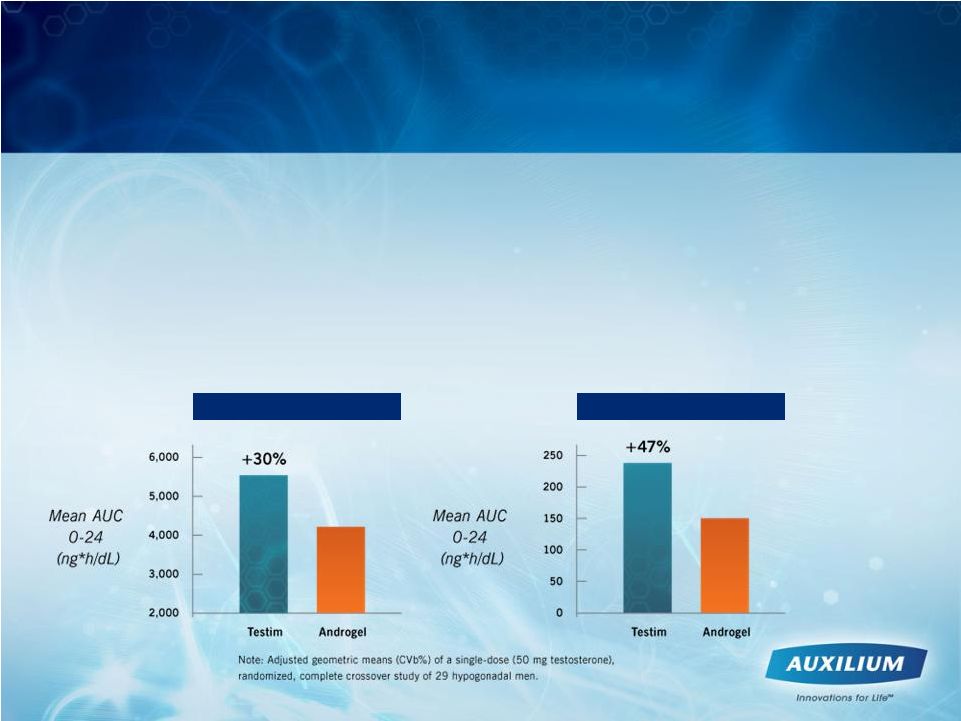
•
16 clinical studies involving approx. 1,800 patients
>
Largest placebo-controlled study ever conducted
•
Clinical trial of Testim
vs. AndroGel
®
>
Testim
provides 30% higher testosterone absorption (p<0.001)
Patient Results Were Proven in Clinical Studies
Free Testosterone
Total Testosterone |
 Source: IMS data
47
Gels Continue to Drive Significant Growth in
TRT Marketplace |
 48
Continuing Track Record of Consistent
Revenue Growth |
 49
*Note: The referenced Testim
patents are licensed from CPEX Pharmaceuticals, Inc.
U.S.
•
U.S. Patent No. 7,320,968* covering method of use claims for
Testim
issued January 22, 2008; expires 2025
•
6 additional U.S. Patents (# 7,608,605 through 610*) covering
method of use claims for Testim
issued October 27, 2009; expire 2023
•
Upsher-Smith Laboratories, Inc. filed an ANDA with paragraph
IV certification referring to the ’968 patent; AUXL filed lawsuit
under Hatch-Waxman on Dec. 4, 2008; 30 month stay intact
ROW
•
Patent issued in Canada; expires 2023
•
Patent issued in Europe; expires 2023
•
Patents granted and applications pending in numerous countries worldwide
Testim
Patent Coverage |
 50
•
Auxilium’s
Citizen’s Petition was filed in February 2009 and FDA response
was received in August 2009.
•
FDA has agreed with some of the statements we made in our Citizen's Petition
regarding the testing required for generic versions of Testim
and disagreed with
other statements.
•
Although not commenting upon any filing in particular, the FDA did state that
"The practical effect of this determination is that any application for
a testosterone gel product that has different penetration enhancers than the
reference listed drug cannot be submitted as an ANDA and, instead, will have
to be submitted as an NDA under section 505(b) of the Act.“
FDA Has Granted Our Citizen's Petition In
Part and Denied It In Part |
 51
FDA Recently Granted Abbott’s Citizen’s Petition in Part and
Denied in Part
•
Although not commenting upon any filing in particular, the
FDA agreed that:
–
Applications for testosterone gel products with different
penetration enhancers must be submitted as a 505(b)(2)
NDA rather than an ANDA.
–
A subsequent 505(b)(2) application cannot rely on or
reference the patent certifications and notifications
submitted with an earlier ANDA application.
–
The patent certifications and any required notifications for
the NDA application would be “clocked”
for regulatory
purposes from the time the NDA patent certification was
provided. |
 52
2010
Q3 ’10 9M ’10
Guidance Testim
Revenues
$47.9
$139.6 $185-195
XIAFLEX Revenues
$5.7
$10.0
N/A
R&D Expense*
$14.4
$34.1
$50-60
SG&A Expense*
$40.3
$117.7 $160-170
Net Loss
($12.8)
($34.8) N/A
Stock –
Based
Comp Expense (* included)
$3.5
$11.9 $22-25
Cash & Cash Equivalents
$142.4
2010 Financial Results and 2010 Guidance
($ Millions)
Currently, approximately 47.7 million shares of common stock outstanding,
plus 6.2 million outstanding options to purchase shares of our common
stock |
 53
Auxilium’s
Plan to Provide Visibility on
XIAFLEX Launch
•
For 4Q 2010, we anticipate that XIAFLEX net
revenues will be in the range of $7.0 million to
$7.5 million, including approximately $1.1 million
in revenue recognized from milestones
previously received under the Pfizer contract.
•
We will continue to provide quarterly revenue
guidance through 2010. |
 54
Strategic Priorities in 2010
•
Execute the launch in the U.S. for XIAFLEX in Dupuytren’s
contracture;
•
Support Pfizer in the ongoing regulatory review of the EU
Dupuytren’s
submission and prepare for their eventual
launch;
•
Rapidly enroll Peyronie’s
disease phase III clinical program
•
Projected double-blind study enrollment to be completed in 1Q11
•
Phase III studies top-line results anticipated in 1H12
•
Advance XIAFLEX new indication(s); and
•
Continue to maximize Testim
revenues while vigorously
defending our intellectual property. |
