Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Ardea Biosciences, Inc./DE | a57780e8vk.htm |
| EX-99.2 - EX-99.2 - Ardea Biosciences, Inc./DE | a57780exv99w2.htm |
Exhibit 99.1

| Contact: | John Beck Ardea Biosciences, Inc. (858) 652-6523 jbeck@ardeabio.com |
Ardea Biosciences Announces Positive Results for RDEA594 in Combination with
Febuxostat or Allopurinol in Gout Patients
Febuxostat or Allopurinol in Gout Patients
100% Response Rate Achieved with All Doses of RDEA594 Combined with Febuxostat
or Allopurinol; Serum Uric Acid Levels Reduced by Up to 80%
or Allopurinol; Serum Uric Acid Levels Reduced by Up to 80%
Company to Host Webcast at 8:30 PM Eastern Time This Evening
SAN DIEGO, November 8, 2010 — Ardea Biosciences, Inc. (Nasdaq: RDEA) today announced positive
results from two clinical studies of RDEA594 in combination with currently marketed drugs for the
treatment of gout patients, febuxostat (Uloric®) and allopurinol. RDEA594, Ardea’s lead
product candidate for the chronic management of hyperuricemia and gout, is an orally administered
compound that inhibits the URAT1 transporter, a biological mechanism that is different from, but
complementary to, that of allopurinol and febuxostat. While allopurinol and febuxostat account for
greater than ninety percent of U.S. prescriptions for the chronic treatment of gout, approximately
one half of patients do not adequately respond to standard doses of these drugs, leaving a
significant portion of the gout population with limited options.
Combination of RDEA594 and Febuxostat (Study 111)
In this 21-patient, open-label, clinical pharmacology study, 100 percent of patients receiving the
combination of RDEA594 and febuxostat achieved serum urate (or “sUA”) levels below the clinically
important target of 6 mg/dL, compared to 67 percent and 56 percent for 40 mg and 80 mg,
respectively, of febuxostat alone. At the highest combination doses tested (600 mg of RDEA594 and
80 mg of febuxostat), 100 percent of patients also reached sUA levels below 4 mg/dL, with 58
percent achieving levels below 3 mg/dL. No patient achieved these reduced sUA levels on either
dose of febuxostat alone. The study included 2 cohorts of gout
patients with sUA greater than 8 mg/dL on
no urate-lowering therapy. All patients received colchicine beginning one week prior to baseline
and continuing for 5 weeks for flare prophylaxis. The first cohort of patients in the study
consisted of 12 gout patients with a median baseline sUA of 9.2 mg/dL who were administered 40 mg
febuxostat for the first week, 40 mg febuxostat in combination with 400 mg RDEA594 for the second
week, and then 40 mg febuxostat in combination with 600 mg RDEA594 for the third week. This
sequence was repeated with 80 mg febuxostat in a second cohort of 9 patients who had a median
baseline sUA of 10.4 mg/dL.
Cohort 1: Response Rates and Median Percent Changes from Baseline (Median Baseline
= 9.2 mg/dL) in Gout Patients for 40 mg Febuxostat Monotherapy and in Combination
with 400 mg or 600 mg RDEA594
= 9.2 mg/dL) in Gout Patients for 40 mg Febuxostat Monotherapy and in Combination
with 400 mg or 600 mg RDEA594
| Febuxostat | Combination with RDEA594 | |||||||||||
| 40 mg Alone | 400 mg | 600 mg | ||||||||||
Response Rate (< 6 mg/dL) |
67 | % | 100 | % | 100 | % | ||||||
Percent Patients < 4 mg/dL |
0 | % | 50 | %* | 64 | %** | ||||||
Percent sUA Change at Trough |
-35 | % | -56 | %*** | -61 | %*** | ||||||
Percent sUA Change Intraday |
-44 | % | -68 | %*** | -71 | %*** | ||||||
Cohort 2: Response Rates and Median Percent Changes from Baseline (Median Baseline
= 10.4 mg/dL) in Gout Patients for 80 mg Febuxostat Monotherapy and in Combination
with 400 mg or 600 mg RDEA594
= 10.4 mg/dL) in Gout Patients for 80 mg Febuxostat Monotherapy and in Combination
with 400 mg or 600 mg RDEA594
| Febuxostat | Combination with RDEA594 | |||||||||||
| 80 mg Alone | 400 mg | 600 mg | ||||||||||
Response Rate (< 6 mg/dL) |
56 | % | 100 | % * | 100 | %* | ||||||
Percent Patients < 4 mg/dL |
0 | % | 89 | %*** | 100 | %*** | ||||||
Percent sUA Change at Trough |
-47 | % | -65 | %*** | -73 | %*** | ||||||
Percent sUA Change Intraday |
-52 | % | -78 | %*** | -81 | %*** | ||||||
| * | P<0.05, **P<0.01, ***P<0.001 versus febuxostat alone |
The combination of RDEA594 and febuxostat was synergistic, with the addition of 600 mg RDEA594
producing additional 39 and 51 percent reductions compared to 40 mg and 80 mg febuxostat alone,
respectively. At the highest combination doses in cohorts one and two, patients achieved intraday
median sUA levels of 2.4 mg/dL and 2.0 mg/ dL, respectively. These levels of sUA reduction suggest
the combination of RDEA594 and febuxostat may be particularly useful in patients who have
accumulated large deposits of uric acid, or tophi. In these patients, the substantial reduction of
sUA, coupled with increased excretion of uric acid associated with RDEA594’s URAT1 mechanism, may
lead to improved resolution of these tophi.
No clinically relevant drug interactions were observed between RDEA594 and febuxostat. The
combination of RDEA594 and febuxostat was well tolerated, with no serious adverse events or
discontinuations due to adverse events. There were no Grade 2 or higher increases in serum
creatinine on RDEA594 alone or the combination with febuxostat, but one Grade 2 serum creatinine
increase occurred on colchicine alone. There was one Grade 3 increase in the liver enzyme, alanine
aminotransferase (ALT), on febuxostat 40 mg alone, which normalized during combination treatment
with RDEA594.
Combination of RDEA594 and Allopurinol (Study 110)
In this 20-patient, open-label clinical pharmacology study, 100 percent of patients receiving all
combinations of RDEA594 and allopurinol achieved sUA reductions to below the 6 mg/dL target. On
300 mg allopurinol alone, only 20 percent of patients achieved
target sUA levels below 6 mg/dL. On
600 mg RDEA594 alone, 67 percent of patients achieved sUA levels below 6 mg/dL, which was
significantly better than allopurinol alone (p <0.05). At the highest combination doses tested,
90 percent of patients also reached sUA levels below 5 mg/dL, and 50 percent reached levels below 4
mg/dL. Study 110’s design was similar, but not identical, to Study 111 and included 2 cohorts of
gout patients with sUA greater than 8 mg/dL on no urate-lowering therapy who began colchicine dosing one
week prior to baseline and continued it for 5 weeks. In this study, the first cohort of 10
patients with a median baseline sUA of 9.8 mg/dL received 300 mg allopurinol alone for the first
week, then 300 mg allopurinol plus 400 mg RDEA594 for the second week, followed by 400 mg RDEA594
alone for the third week. In the second cohort, 10 patients with a median baseline sUA of 9.1
mg/dL followed the same dosing scheme for the same time period at a dose of 300 mg allopurinol and
600 mg RDEA594.
Cohort 1: Response Rates and Median Percent Changes from Baseline (Median Baseline
= 9.8 mg/dL) in Gout Patients for 300 mg Allopurinol Monotherapy, Allopurinol
Combination with 400 mg RDEA594, and 400 mg RDEA594 Monotherapy
= 9.8 mg/dL) in Gout Patients for 300 mg Allopurinol Monotherapy, Allopurinol
Combination with 400 mg RDEA594, and 400 mg RDEA594 Monotherapy
| Allopurinol | Combination with | RDEA594 | ||||||||||
| 300 mg Alone | RDEA594 400 mg | 400 mg Alone | ||||||||||
Response Rate (< 6 mg/dL) |
10 | % | 100 | %*** | 20 | % | ||||||
Percent Patients < 5 mg/dL |
5 | % | 50 | %** | 0 | % | ||||||
Percent sUA Change at Trough |
-31 | % | -45 | %*** | -28 | % | ||||||
Percent sUA Change Intraday |
-38 | % | -62 | %*** | -44 | %* | ||||||
Cohort 2: Response Rates and Median Percent Changes from Baseline (Median Baseline
= 9.1 mg/dL) in Gout Patients for 300 mg Allopurinol Monotherapy, Allopurinol
Combination with 600 mg RDEA594, and 600 mg RDEA594 Monotherapy
= 9.1 mg/dL) in Gout Patients for 300 mg Allopurinol Monotherapy, Allopurinol
Combination with 600 mg RDEA594, and 600 mg RDEA594 Monotherapy
| Allopurinol | Combination with | RDEA594 | ||||||||||
| 300 mg Alone | RDEA594 600 mg | 600 mg Alone | ||||||||||
Response Rate (< 6 mg/dL) |
30 | % | 100 | %*** | 67 | %* | ||||||
Percent Patients < 5 mg/dL |
5 | % | 90 | %*** | 33 | % | ||||||
Percent sUA Change at Trough |
-27 | % | -55 | %*** | -39 | %** | ||||||
Percent sUA Change Intraday |
-35 | % | -70 | %*** | -54 | %*** | ||||||
| * P<0.05, **P<0.01, ***P<0.001 versus allopurinol alone | ||
At the highest combination doses tested, intraday median sUA levels below 3 mg/dL were
achieved. No clinically relevant drug interactions were observed between RDEA594 and allopurinol
in this study; however, consistent with prior experience, plasma levels of oxypurinol, an active
metabolite of allopurinol, were decreased approximately 25-35 percent. Despite this small
decrease, the combination of 600 mg RDEA594 and allopurinol demonstrated a completely additive
response.
The combination of RDEA594 and allopurinol was well tolerated. There were no serious adverse
events or discontinuations that were considered possibly related to RDEA594 or the combination.
There were no clinically relevant increases in serum creatinine or ALT in this study. Two patients
receiving RDEA594 and colchicine had Grade 4 increases in creatine kinase (CK); one of these,
although asymptomatic, was considered to be rhabdomyolysis by the investigator. Both cases were
considered possibly related to colchicine and not related to RDEA594. Elevations in CK and
rhabdomyolysis are known side effects of colchicine. One of these patients was also receiving a
statin, which is also known to cause CK elevations, particularly when combined with colchicine.
Clinically significant elevations in CK have not been observed in more than 400 patients exposed to
RDEA594 who were not also receiving colchicine. Furthermore, in a previously reported, randomized,
monotherapy, placebo-controlled trial (Study 202), the rate of Grade 4 CK increases was lower in
patients receiving RDEA594 plus colchicine (2 percent) than those
receiving colchicine alone (4 percent).
“We are very pleased with these results, which demonstrate the ability of RDEA594 in combination
with current standard of care to reduce uric acid levels in gout patients to an extent generally
only achieved with intravenous therapy,” commented Barry D. Quart, PharmD, Ardea’s president and
chief executive officer. “Based on these results, we are well-positioned to conduct further
studies of these well-tolerated oral combinations in patients with the most severe forms of gout.”
Webcast – Monday, November 8, 2010 at 8:30 p.m.
Ardea will host a live webcast on Monday, November 8, 2010 from 8:30 p.m. to 9:30 p.m. Eastern
Standard Time. To access the webcast, please log onto Ardea’s website at www.ardeabio.com and click
on the Investors Relations section approximately 10 minutes prior to the start of the webcast to
allow for any software downloads that may be necessary. An archived recording of the event will be
available on Ardea’s website for one month.
About Hyperuricemia and Gout
Gout is a painful, debilitating and progressive disease caused by abnormally elevated levels of
uric acid in the blood stream. This leads to the deposition of painful, needle-like uric acid
crystals
in and around the connective tissue of the joints and in the kidneys, resulting in
inflammation, the formation of disfiguring nodules (tophi), intermittent attacks of severe pain
(acute flares) and
kidney damage (nephropathy). In addition, evidence suggests that the chronic elevation of uric
acid associated with gout, known as hyperuricemia, may also have systemic consequences, including
an increased risk for kidney dysfunction and cardiovascular disease.
In 2008, approximately 8.3 million patients in the U.S., 6.4 million patients in the European Union
and 2.9 million patients in Japan were diagnosed with gout. Gout is the most common form of
inflammatory arthritis in men over the age of 40 and represents a significant unmet medical need
with limited treatment options.
About RDEA594
Our most advanced product candidate for the treatment of hyperuricemia and gout, RDEA594, is an
oral, once-daily inhibitor of URAT1, a transporter in the kidney that regulates uric acid excretion
from the body. Approximately 90% of gout patients are considered to be under-excretors of uric
acid, and recent studies have shown that defects in renal transporters have been genetically linked
to gout. Consequently, increasing renal excretion of uric acid by moderating URAT1 transporter
activity may provide the most physiologically appropriate treatment for gout. In addition, because
increasing the excretion of serum uric acid is additive to the effects of drugs such as allopurinol
that decrease the production of uric acid, RDEA594 in combination with such drugs has the potential
to treat the significant portion of the gout population that is not adequately treated with
existing therapies.
RDEA594 is in Phase 2 development as a single agent and in combination with the approved xanthine
oxidase inhibitor, allopurinol. Over 500 people have received RDEA594 in Phase 1 and 2 clinical
trials.
About Ardea Biosciences, Inc.
Ardea Biosciences, Inc., of San Diego, California, is a biotechnology company focused on the
development of small-molecule therapeutics for the treatment of serious diseases. RDEA594, our
lead product candidate for the treatment of hyperuricemia and gout, is a selective URAT1
transporter inhibitor in Phase 2 clinical development. Our next-generation URAT1 inhibitor program
is currently in preclinical development. BAY 86-9766, formerly known as RDEA119, is a potent and
specific inhibitor of mitogen-activated ERK kinase (MEK) for the treatment of cancer being
developed under a global license agreement with Bayer HealthCare AG.
BAY 86-9766 has been evaluated in advanced cancer patients with different tumor types as a single agent in a Phase
1 study and is currently being evaluated in combination with sorafenib (Nexavar®; Bayer HealthCare, Onyx
Pharmaceuticals) in a Phase 1/2 study. Our two product candidates for the treatment of HIV,
RDEA806 and RDEA427, are non-nucleoside reverse transcriptase inhibitors (NNRTIs), which have
successfully completed a Phase 2a study in HIV patients and a human micro-dose pharmacokinetic
study in healthy volunteers, respectively.
Statements contained in this press release regarding matters that are not historical facts are
“forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of
1995. Because such statements are subject to risks and uncertainties, actual results may differ
materially from those expressed or implied by such forward-looking statements. Such statements
include, but are not limited to, statements regarding our plans and goals, the expected properties
and benefits of RDEA594, BAY 86-9766 (RDEA119), RDEA806, RDEA427 and our other compounds and the
timing and results of our preclinical, clinical and other studies. Risks that contribute to the
uncertain nature of the forward-looking statements include risks related to the outcome of
preclinical and clinical studies, risks related to regulatory approvals, delays in commencement of
preclinical and clinical studies, costs associated with our drug discovery and
development
programs, and risks related to the outcome of our business development activities, including
collaboration or license agreements. These and other risks and uncertainties are
described more fully in our most recently filed SEC documents, including our Annual Report on Form
10-K and our Quarterly Reports on Form 10-Q, under the headings “Risk Factors.” All
forward-looking statements contained in this press release speak only as of the date on which they
were made. We undertake no obligation to update such statements to reflect events that occur or
circumstances that exist after the date on which they were made.
###

| Ardea Biosciences Company Update November 8, 2010 |
| Statements contained in this presentation regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward- looking statements. Such statements include, but are not limited to, statements regarding: Ardea's goals, its preclinical and clinical trial plans, timelines and milestones, its expectations about the size of its markets and commercial potential of its compounds, expected results of future clinical trials, expected properties of compounds under development, financial position, cash usage, licensing and partnering opportunities, liquidity and anticipated milestones. Risks that contribute to the uncertain nature of the forward-looking statements include: risks related to the outcomes of preclinical and clinical trials, risks related to regulatory approvals, delays in commencement of preclinical and clinical tests, costs associated with internal development, and the outcome of our business development activities, including collaboration or licensing agreements. These and other risks and uncertainties are described more fully in Ardea's most recently filed SEC documents, including its Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, under the headings "Risk Factors." All forward-looking statements contained in this presentation speak only as of the date of this presentation, and Ardea undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof or otherwise. 2 |
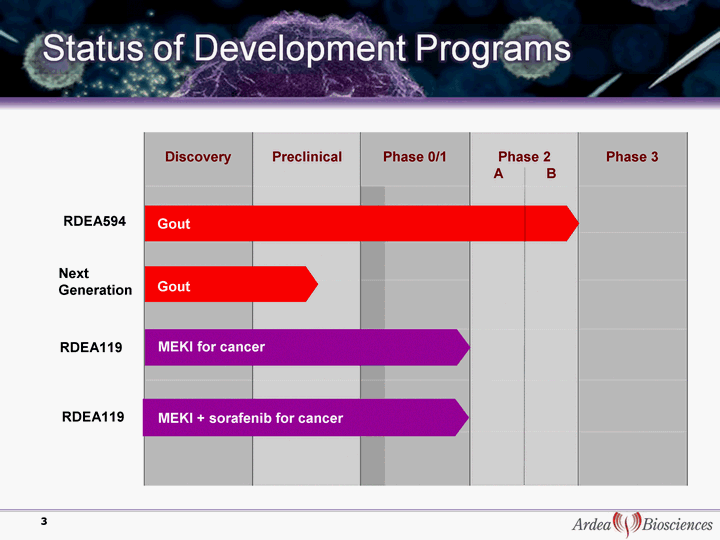
| Discovery Preclinical Phase 0/1 Phase 2 A B Phase 3 MEKI for cancer 3 Gout RDEA594 Next Generation MEKI + sorafenib for cancer RDEA119 RDEA119 Gout |
| HYPERURICEMIA/GOU T 4 |
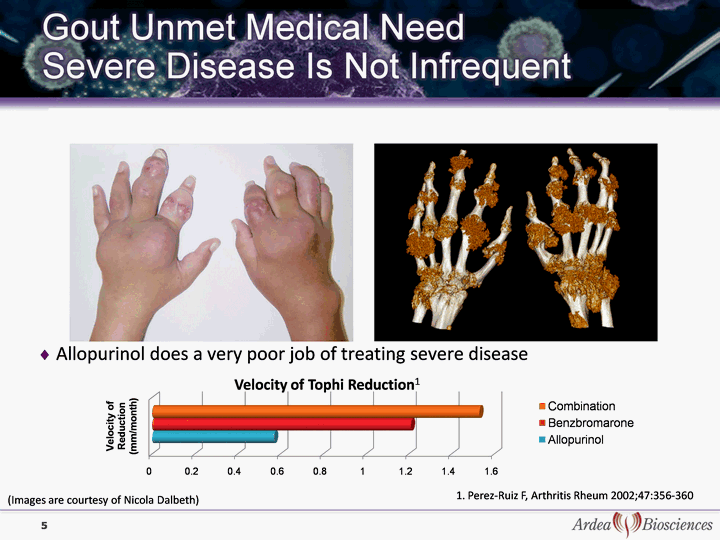
| 5 1. Perez-Ruiz F, Arthritis Rheum 2002;47:356-360 Allopurinol does a very poor job of treating severe disease (Images are courtesy of Nicola Dalbeth) Velocity of Tophi Reduction1 |
| Gout is caused by abnormally elevated levels of serum uric acid (>6.8 mg/dL) Increasing incidence and severity in US: approximately 8 million patients ~ 90% of patients are "under-excretors" of uric acid RDEA594 increases urinary excretion of uric acid back to normal levels Allopurinol and febuxostat reduce production, which reduces excretion further The combination of RDEA594 and xanthine oxidase inhibitors may produce greater reductions in uric acid and speed up clearance of tophi and body burden of uric acid Hyperuricemia linked to elevated hypertension in adults1 and children2, increased mortality in Chronic Kidney Disease3 and possibly other cardiovascular risk factors4,5, including elevated C-Reactive Protein. 6 1. Int Urol Nephrol 2007;39:1227-33 ; 2. D Feig, B Soletsky, R Johnson. JAMA. 2008;300(8):924-932; 3. Am J Kidney Dis 2009;53:796-803; 4. JAMA. 2008;300(8):924-932; 5. Chen Abstract 2088 ACR2010 |
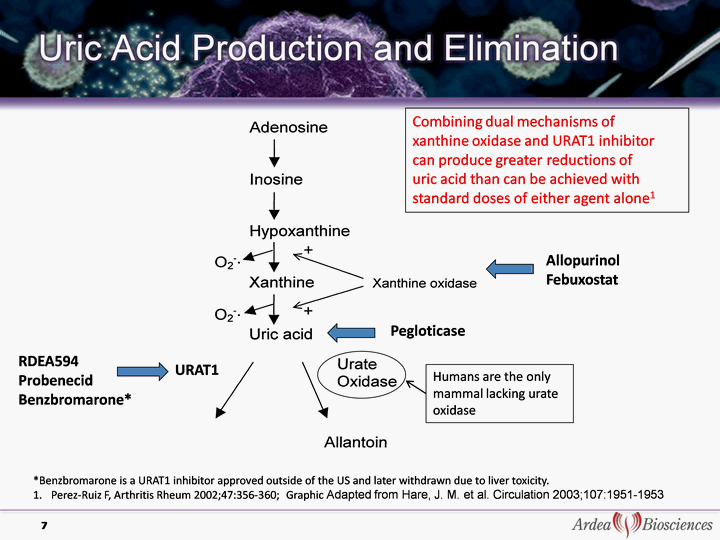
| RDEA594 Probenecid Benzbromarone* 7 *Benzbromarone is a URAT1 inhibitor approved outside of the US and later withdrawn due to liver toxicity. Perez-Ruiz F, Arthritis Rheum 2002;47:356-360; Graphic Adapted from Hare, J. M. et al. Circulation 2003;107:1951- 1953 Pegloticase URAT1 Combining dual mechanisms of xanthine oxidase and URAT1 inhibitor can produce greater reductions of uric acid than can be achieved with standard doses of either agent alone1 Allopurinol Febuxostat Humans are the only mammal lacking urate oxidase |
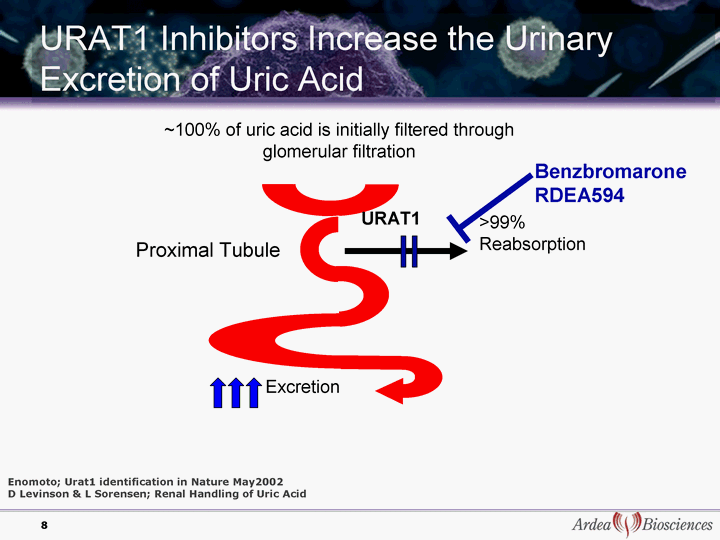
| >99% Reabsorption Excretion ~100% of uric acid is initially filtered through glomerular filtration URAT1 Inhibitors Increase the Urinary Excretion of Uric Acid URAT1 Enomoto; Urat1 identification in Nature May2002 D Levinson & L Sorensen; Renal Handling of Uric Acid Proximal Tubule Benzbromarone RDEA594 8 |
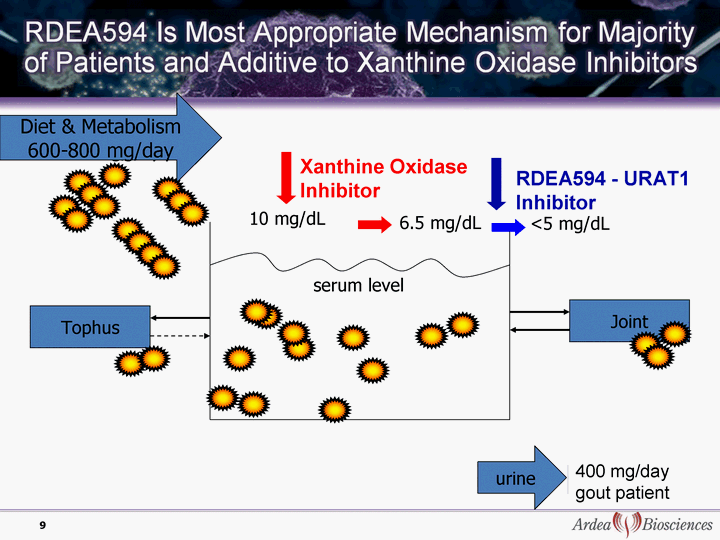
| Diet & Metabolism <400 mg/day Diet & Metabolism 600-800 mg/day Joint Tophus 10 mg/dL 6.5 mg/dL serum level Xanthine Oxidase Inhibitor RDEA594 - URAT1 Inhibitor <5 mg/dL <5 mg/dL urine urine 200 mg/day 9 600 mg/day 400 mg/day gout patient |
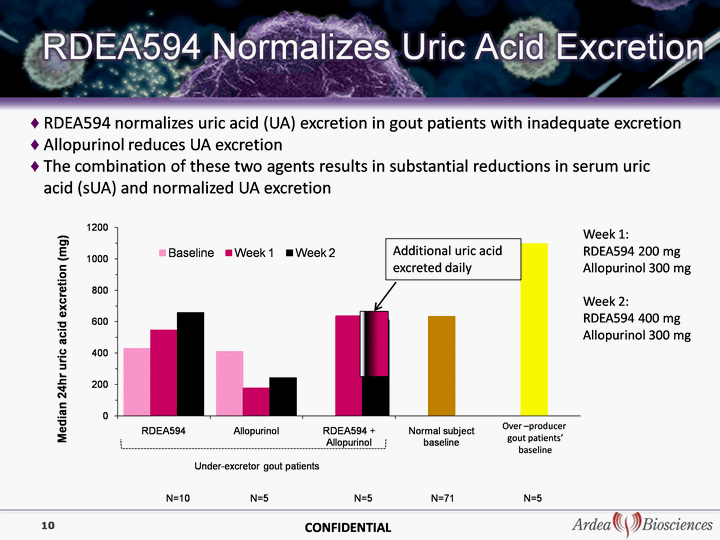
| RDEA594 normalizes uric acid (UA) excretion in gout patients with inadequate excretion Allopurinol reduces UA excretion The combination of these two agents results in substantial reductions in serum uric acid (sUA) and normalized UA excretion uric acid (sUA) and normalized UA excretion uric acid (sUA) and normalized UA excretion uric acid (sUA) and normalized UA excretion uric acid (sUA) and normalized UA excretion uric acid (sUA) and normalized UA excretion 10 Additional uric acid excreted daily Over -producer gout patients' baseline Week 1: RDEA594 200 mg Allopurinol 300 mg Week 2: RDEA594 400 mg Allopurinol 300 mg CONFIDENTIAL |
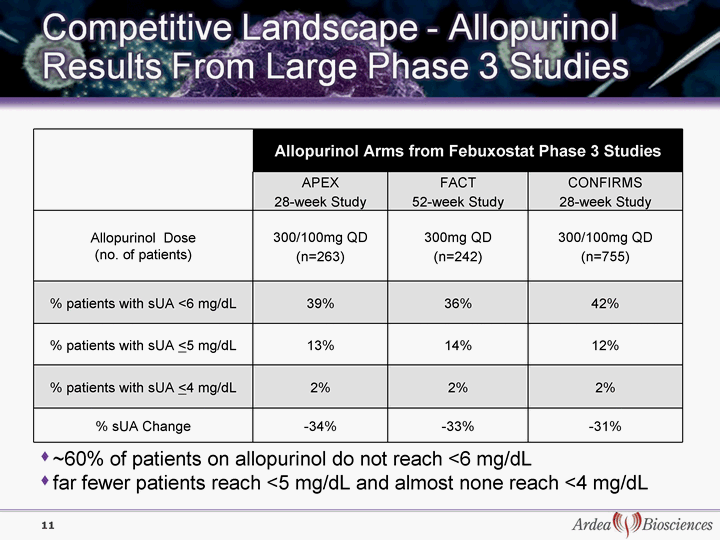
| 11 Allopurinol Arms from Febuxostat Phase 3 Studies Allopurinol Arms from Febuxostat Phase 3 Studies Allopurinol Arms from Febuxostat Phase 3 Studies APEX 28-week Study FACT 52-week Study CONFIRMS 28-week Study Allopurinol Dose (no. of patients) 300/100mg QD (n=263) 300mg QD (n=242) 300/100mg QD (n=755) % patients with sUA <6 mg/dL 39% 36% 42% % patients with sUA <5 mg/dL 13% 14% 12% % patients with sUA <4 mg/dL 2% 2% 2% % sUA Change -34% -33% -31% ~60% of patients on allopurinol do not reach <6 mg/dL far fewer patients reach <5 mg/dL and almost none reach <4 mg/dL |
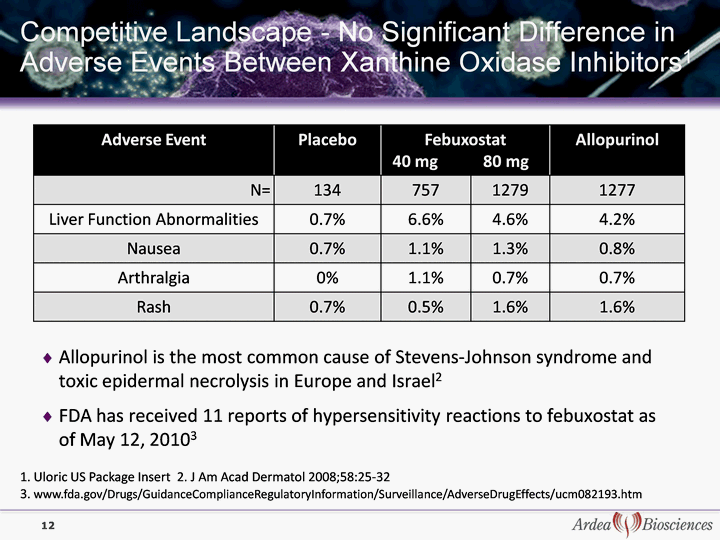
| Competitive Landscape - No Significant Difference in Adverse Events Between Xanthine Oxidase Inhibitors1 Adverse Event Placebo Febuxostat 40 mg 80 mg Febuxostat 40 mg 80 mg Allopurinol N= 134 757 1279 1277 Liver Function Abnormalities 0.7% 6.6% 4.6% 4.2% Nausea 0.7% 1.1% 1.3% 0.8% Arthralgia 0% 1.1% 0.7% 0.7% Rash 0.7% 0.5% 1.6% 1.6% Allopurinol is the most common cause of Stevens-Johnson syndrome and toxic epidermal necrolysis in Europe and Israel2 FDA has received 11 reports of hypersensitivity reactions to febuxostat as of May 12, 20103 12 1. Uloric US Package Insert 2. J Am Acad Dermatol 2008;58:25-32 3. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm |
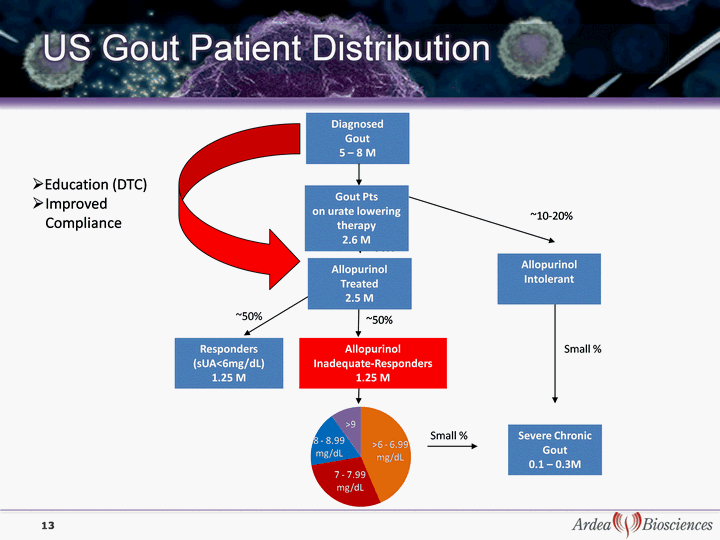
| 13 Diagnosed Gout 5 - 8 M ~90% ~50% ~50% Allopurinol Treated 2.5 M Responders (sUA<6mg/dL) 1.25 M Severe Chronic Gout 0.1 - 0.3M Allopurinol Inadequate- Responders 1.25 M ~10-20% Small % Allopurinol Intolerant Gout Pts on urate lowering therapy 2.6 M Small % Education (DTC) Improved Compliance |
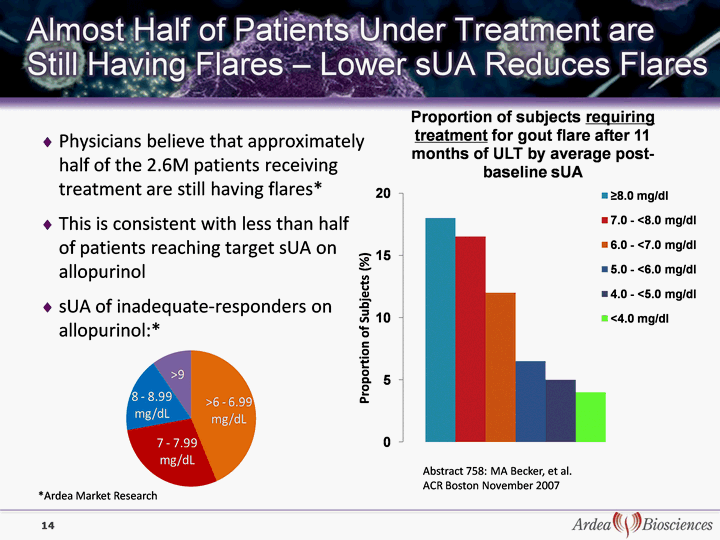
| Physicians believe that approximately half of the 2.6M patients receiving treatment are still having flares* This is consistent with less than half of patients reaching target sUA on allopurinol sUA of inadequate-responders on allopurinol:* 14 14 Proportion of Subjects (%) Abstract 758: MA Becker, et al. ACR Boston November 2007 *Ardea Market Research |

| Chronic therapy: The combination of RDEA594 (URAT1 inhibitor) and xanthine oxidase inhibitors may produce greater reductions in sUA than either mechanism alone, with many more patients reaching levels below 6 mg/dL, and even below 5 mg/dL Reduction of sUA from 6-7mg/dL to less than 6 mg/dL reduces the flare rate by approximately one-half Treatment initiation: Faster renal clearance of urate could lead to faster reduction in urate deposits (tophi, crystals, etc.), reducing the risk of flares 15 |
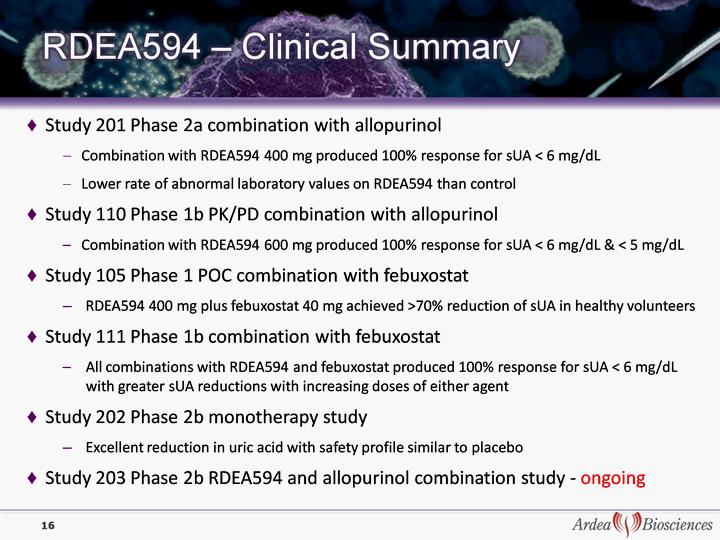
| 16 Study 201 Phase 2a combination with allopurinol Combination with RDEA594 400 mg produced 100% response for sUA < 6 mg/dL Lower rate of abnormal laboratory values on RDEA594 than control Study 110 Phase 1b PK/PD combination with allopurinol Combination with RDEA594 600 mg produced 100% response for sUA < 6 mg/dL & < 5 mg/dL Study 105 Phase 1 POC combination with febuxostat RDEA594 400 mg plus febuxostat 40 mg achieved >70% reduction of sUA in healthy volunteers Study 111 Phase 1b combination with febuxostat All combinations with RDEA594 and febuxostat produced 100% response for sUA < 6 mg/dL with greater sUA reductions with increasing doses of either agent Study 202 Phase 2b monotherapy study Excellent reduction in uric acid with safety profile similar to placebo Study 203 Phase 2b RDEA594 and allopurinol combination study - ongoing |

| Population: 21 gout patients with hyperuricemia (sUA ^ 8 mg/dL) febuxostat 40 mg panel: 12 patients with median sUA of 9.2 mg/dL febuxostat 80 mg panel: 9 patients with median sUA of 10.4 mg/dL Objective: Plasma PK and urinary excretion of RDEA594 in combination with febuxostat; PK of colchicine alone and in combination with febuxostat or both febuxostat and RDEA594. Effect of febuxostat alone and in combination with RDEA594 on serum urate concentrations and urinary urate excretion. RDEA594 Study 111 - Phase 1b Febuxostat and RDEA594 Dose Titration Study in Gout Patients febuxostat 80mg QD 7 days 7 days 7 days Screening Period -21 day to day 0 Colchicine Treatment febuxostat 80mg QD + 400mg RDEA594 febuxostat 80mg QD + 600mg RDEA594 febuxostat 40mg QD febuxostat 40mg QD + 400mg RDEA594 febuxostat 40mg QD + 600mg RDEA594 17 |
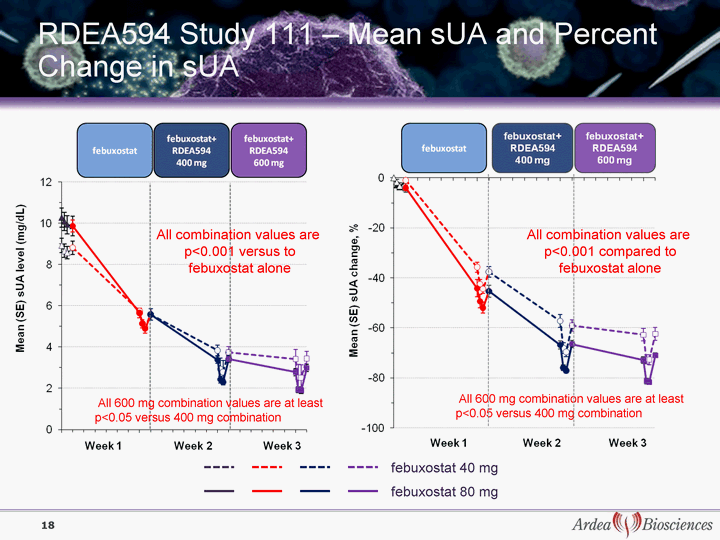
| RDEA594 Study 111 - Mean sUA and Percent Change in sUA febuxostat 40 mg febuxostat 80 mg All 600 mg combination values are at least p<0.05 versus 400 mg combination All combination values are p<0.001 versus to febuxostat alone 18 All combination values are p<0.001 compared to febuxostat alone All 600 mg combination values are at least p<0.05 versus 400 mg combination |
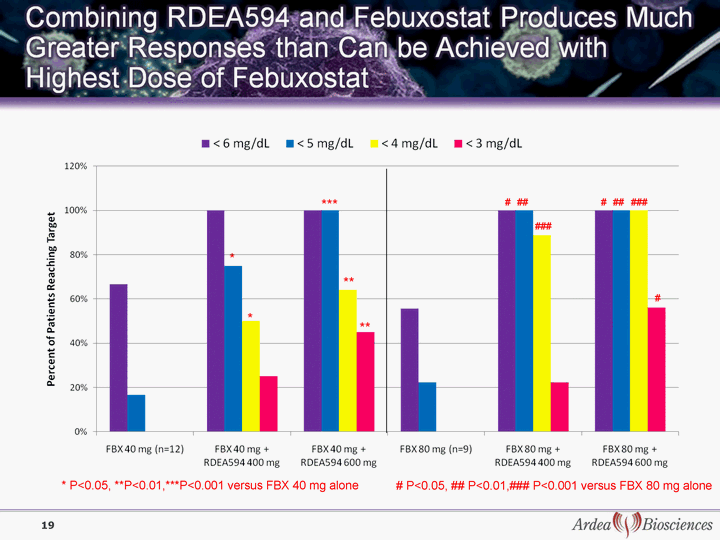
| * P<0.05, **P<0.01,***P<0.001 versus FBX 40 mg alone # P<0.05, ## P<0.01,### P<0.001 versus FBX 80 mg alone *** ** ** * * ### ## # # # ## ### 19 |
| Overall, the combination of RDEA594 and febuxostat was well tolerated, with no serious adverse events, no discontinuations due to adverse events, and no increase in side effects with the combination Renal safety: Grade 2 increase in serum creatinine (SCr) on colchicine alone, no Grade 2 or greater SCr increases on RDEA594 or the combination Grade 3 increase in ALT on febuxostat 40 mg alone, which normalized during combination treatment No difference in rate of adverse events across dosing periods, with lowest rate observed in the RDEA594 600 mg + febuxostat arms 20 |
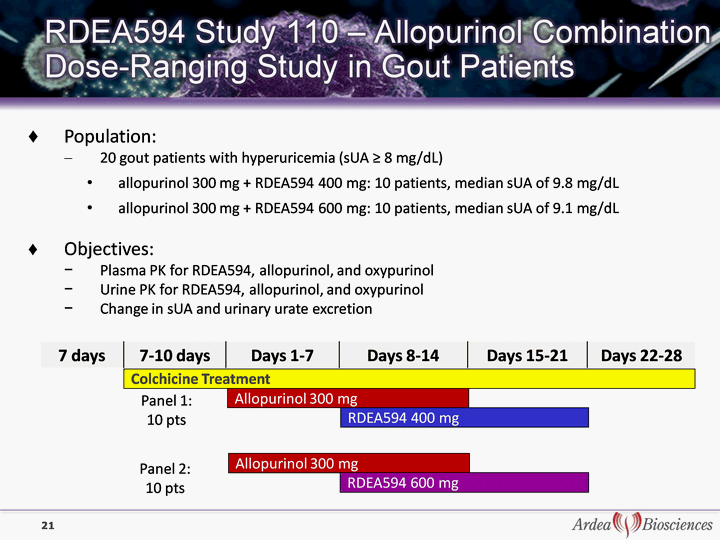
| 7 days 7-10 days Days 1-7 Days 8-14 Days 15-21 Days 22-28 Colchicine Treatment Allopurinol 300 mg RDEA594 400 mg Allopurinol 300 mg RDEA594 600 mg Panel 1: 10 pts Panel 2: 10 pts Population: 20 gout patients with hyperuricemia (sUA ^ 8 mg/dL) allopurinol 300 mg + RDEA594 400 mg: 10 patients, median sUA of 9.8 mg/dL allopurinol 300 mg + RDEA594 600 mg: 10 patients, median sUA of 9.1 mg/dL Objectives: Plasma PK for RDEA594, allopurinol, and oxypurinol Urine PK for RDEA594, allopurinol, and oxypurinol Change in sUA and urinary urate excretion 21 |
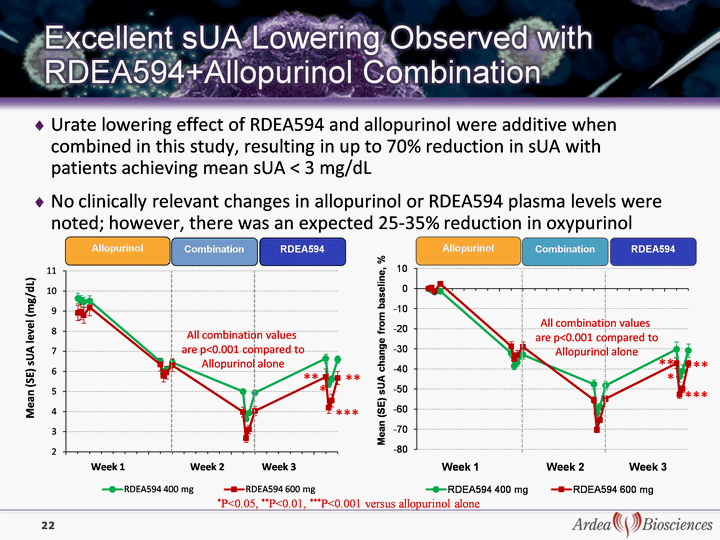
| Urate lowering effect of RDEA594 and allopurinol were additive when combined in this study, resulting in up to 70% reduction in sUA with patients achieving mean sUA < 3 mg/dL No clinically relevant changes in allopurinol or RDEA594 plasma levels were noted; however, there was an expected 25-35% reduction in oxypurinol *P<0.05, **P<0.01, ***P<0.001 versus allopurinol alone ** *** All combination values are p<0.001 compared to Allopurinol alone ** All combination values are p<0.001 compared to Allopurinol alone *** * * ** ** 22 |
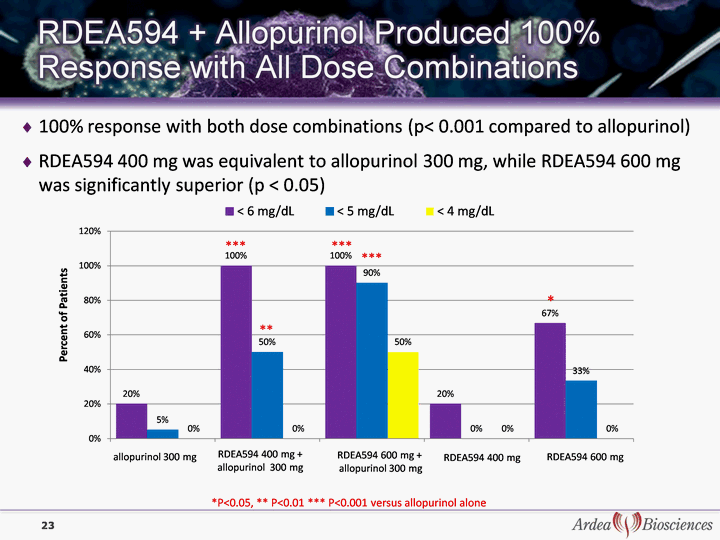
| 100% response with both dose combinations (p< 0.001 compared to allopurinol) RDEA594 400 mg was equivalent to allopurinol 300 mg, while RDEA594 600 mg was significantly superior (p < 0.05) *P<0.05, ** P<0.01 *** P<0.001 versus allopurinol alone ** *** *** *** * 23 |
| The combination of RDEA594 and allopurinol was well tolerated, with no serious adverse events, and no discontinuations due to adverse events considered possibly related to RDEA594 or the combination No clinically relevant changes in SCr or ALT were observed Two patients receiving RDEA594 + colchicine had Grade 4 increases in creatine kinase (CK or CPK) considered to be due to colchicine: One patient also taking a statin discontinued colchicine and RDEA594; his CK returned to normal over 10 days A second patient, who had finished dosing of RDEA594, discontinued colchicine due to asymptomatic Grade 4 CK, which was identified by the investigator as rhabdomyolysis; patient felt well and declined follow-up CK testing was added to our gout studies that include colchicine due to the warnings in Colcrys(r) label issued in September 2009 about elevated CK and myopathies, including rhabdomyolysis, with and without statins |
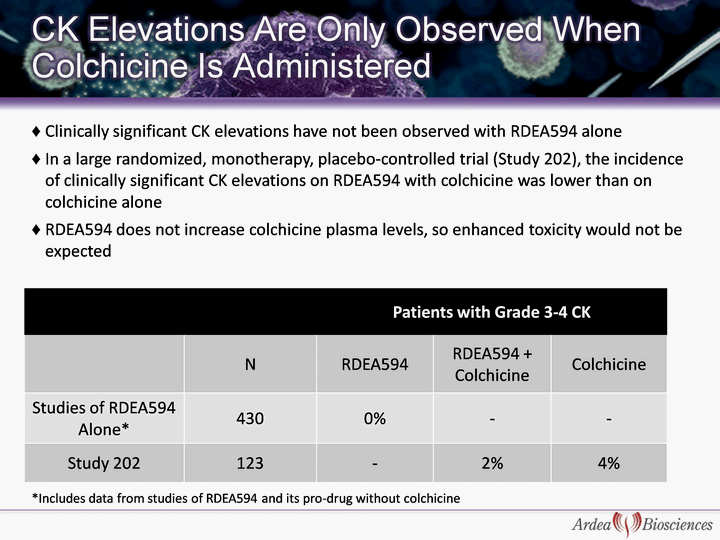
| Patients with Grade 3-4 CK Patients with Grade 3-4 CK Patients with Grade 3-4 CK N RDEA594 RDEA594 + Colchicine Colchicine Studies of RDEA594 Alone* 430 0% - - Study 202 123 - 2% 4% Clinically significant CK elevations have not been observed with RDEA594 alone In a large randomized, monotherapy, placebo-controlled trial (Study 202), the incidence of clinically significant CK elevations on RDEA594 with colchicine was lower than on colchicine alone RDEA594 does not increase colchicine plasma levels, so enhanced toxicity would not be expected *Includes data from studies of RDEA594 and its pro-drug without colchicine |

| Conclusions from Studies 110 & 111 sUA reduction observed with combination therapy is far greater than what can be achieved with xanthine oxidase inhibitors alone, including the highest approved dose of febuxostat 100% response rate (sUA < 6 mg/dL) achieved with either RDEA594 + allopurinol or RDEA594 + febuxostat Use of RDEA594 + xanthine oxidase inhibitors also provides a means for getting all patients to below 5 mg/dL, which may be a more appropriate target than 6 mg/dL Both combinations resulted in the majority of patients achieving sUA below 2-3 mg/dL, which should be sufficient to rapidly dissolve tophi The combination of RDEA594 + xanthine oxidase inhibitors was well tolerated 26 |
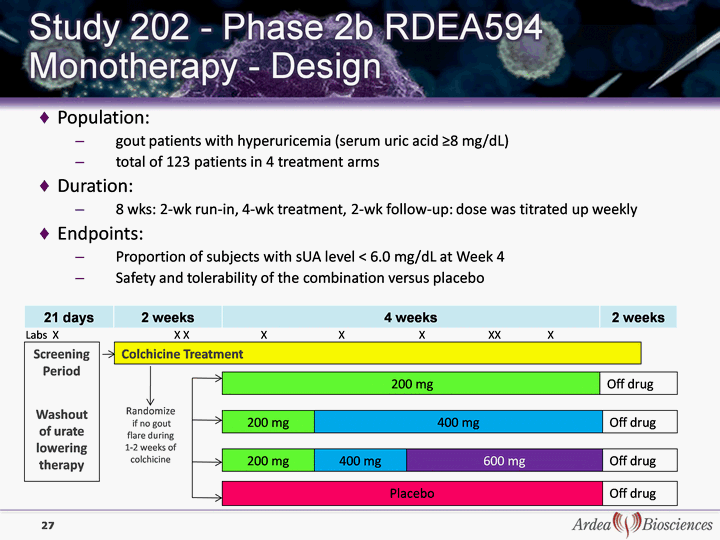
| 21 days 2 weeks 4 weeks 2 weeks Population: gout patients with hyperuricemia (serum uric acid ^8 mg/dL) total of 123 patients in 4 treatment arms Duration: 8 wks: 2-wk run-in, 4-wk treatment, 2-wk follow-up: dose was titrated up weekly Endpoints: Proportion of subjects with sUA level < 6.0 mg/dL at Week 4 Safety and tolerability of the combination versus placebo Screenin g Period Washout of urate lowering therapy Colchicine Treatment Randomize if no gout flare during 1-2 weeks of colchicine colchicine colchicine colchicine colchicine colchicine 27 200 mg Off drug Off drug Off drug 200 mg Placebo 400 mg 200 mg 400 mg 600 mg Off drug Labs X X X X X X XX X |
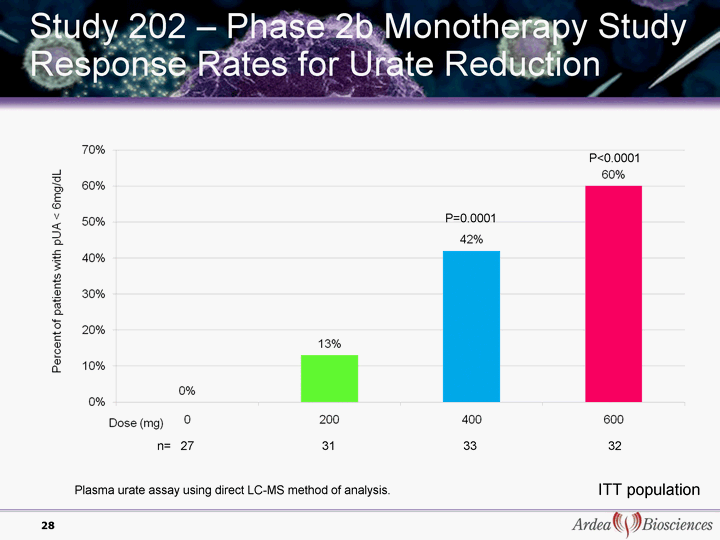
| Study 202 - Phase 2b Monotherapy Study Response Rates for Urate Reduction 28 ITT population n= 27 31 33 32 P=0.0001 P<0.0001 Plasma urate assay using direct LC-MS method of analysis. |
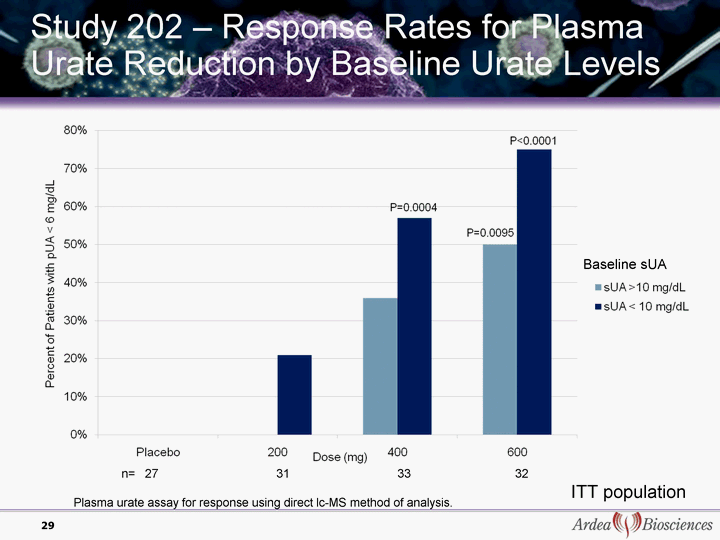
| Study 202 - Response Rates for Plasma Urate Reduction by Baseline Urate Levels Baseline sUA 29 ITT population n= 27 31 33 32 Plasma urate assay for response using direct lc-MS method of analysis. |
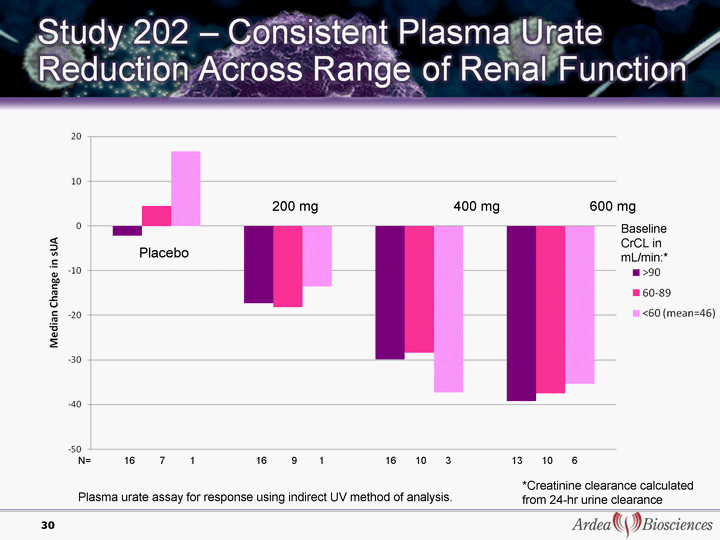
| 30 *Creatinine clearance calculated from 24-hr urine clearance Plasma urate assay for response using indirect UV method of analysis. 200 mg 400 mg 600 mg Placebo N= 16 7 1 16 9 1 16 10 3 13 10 6 Baseline CrCL in mL/min:* |
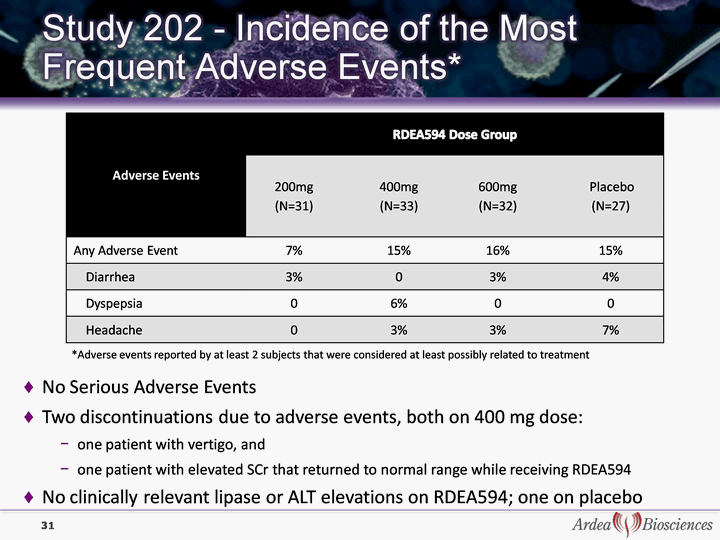
| 31 *Adverse events reported by at least 2 subjects that were considered at least possibly related to treatment No Serious Adverse Events Two discontinuations due to adverse events, both on 400 mg dose: one patient with vertigo, and one patient with elevated SCr that returned to normal range while receiving RDEA594 No clinically relevant lipase or ALT elevations on RDEA594; one on placebo |
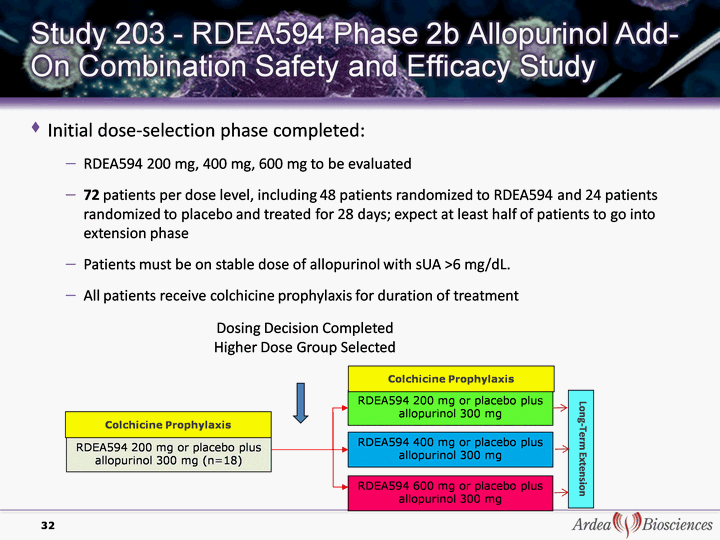
| Initial dose-selection phase completed: RDEA594 200 mg, 400 mg, 600 mg to be evaluated 72 patients per dose level, including 48 patients randomized to RDEA594 and 24 patients randomized to placebo and treated for 28 days; expect at least half of patients to go into extension phase Patients must be on stable dose of allopurinol with sUA >6 mg/dL. All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment All patients receive colchicine prophylaxis for duration of treatment 32 RDEA594 200 mg or placebo plus allopurinol 300 mg (n=18) RDEA594 200 mg or placebo plus allopurinol 300 mg RDEA594 400 mg or placebo plus allopurinol 300 mg Dosing Decision Completed Higher Dose Group Selected RDEA594 600 mg or placebo plus allopurinol 300 mg Colchicine Prophylaxis Long-Term Extension Colchicine Prophylaxis |
| CANCER 33 |
| MEK program licensed to Bayer HealthCare on April 28, 2009 $35 million upfront license fee Payments could total $407 million, excluding royalties Low double-digit royalties on worldwide sales Ardea responsible for completing two dose-finding studies MTD reached in both studies 34 |
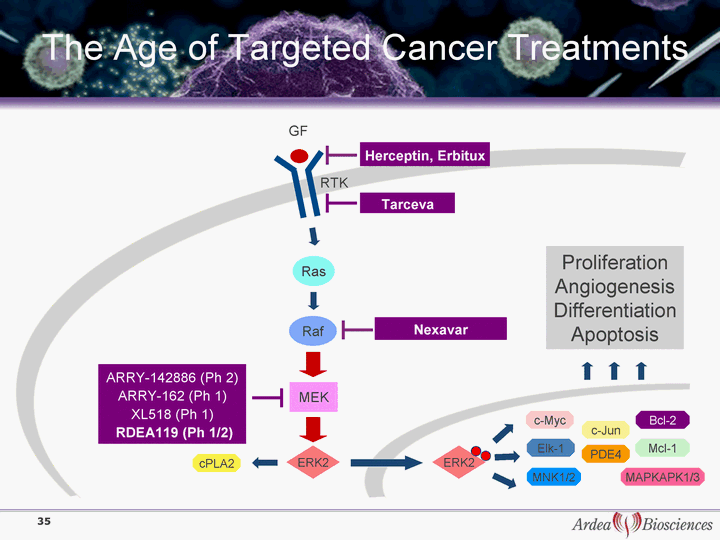
| The Age of Targeted Cancer Treatments Nexavar MEK Ras Raf c-Myc Herceptin, Erbitux Proliferation Angiogenesis Differentiation Apoptosis cPLA2 PDE4 MNK1/2 Elk-1 MAPKAPK1/3 ARRY-142886 (Ph 2) ARRY-162 (Ph 1) XL518 (Ph 1) RDEA119 (Ph 1/2) RTK Bcl-2 Mcl-1 c-Jun Tarceva GF ERK2 ERK2 35 |
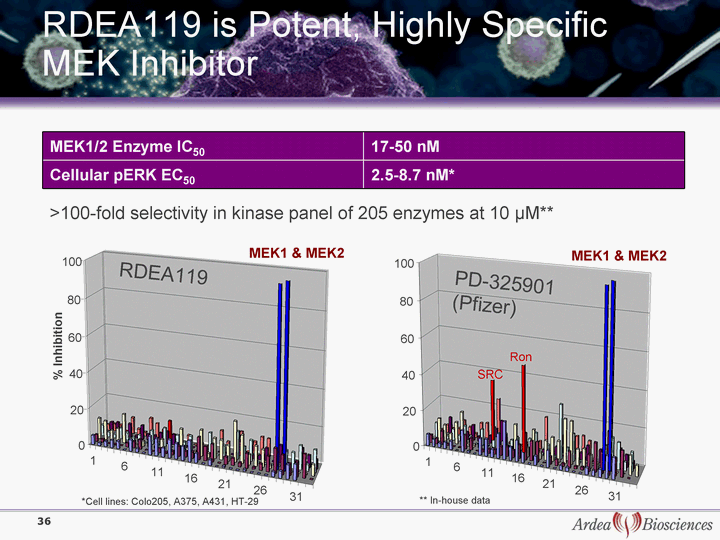
| RDEA119 is Potent, Highly Specific MEK Inhibitor MEK1/2 Enzyme IC50 17-50 nM Cellular pERK EC50 2.5-8.7 nM* >100-fold selectivity in kinase panel of 205 enzymes at 10 µM** *Cell lines: Colo205, A375, A431, HT-29 ** In-house data 1 6 11 16 21 26 31 0 20 40 60 80 100 1 6 11 16 21 26 31 MEK1 & MEK2 MEK1 & MEK2 0 20 40 60 80 100 Ron SRC RDEA119 PD-325901 (Pfizer) % Inhibition 36 |
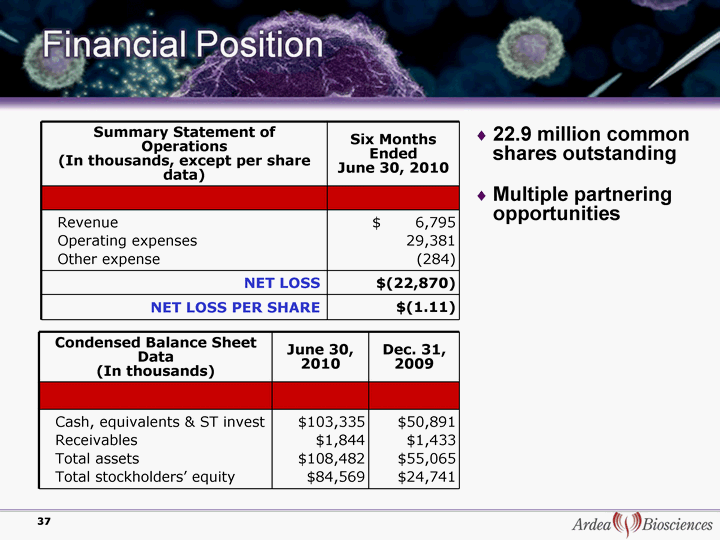
| 22.9 million common shares outstanding Multiple partnering opportunities Summary Statement of Operations (In thousands, except per share data) Six Months Ended June 30, 2010 Revenue Operating expenses Other expense $ 6,795 29,381 (284) NET LOSS $(22,870) NET LOSS PER SHARE $(1.11) Condensed Balance Sheet Data (In thousands) June 30, 2010 Dec. 31, 2009 Cash, equivalents & ST invest Receivables Total assets Total stockholders' equity $103,335 $1,844 $108,482 $84,569 $50,891 $1,433 $55,065 $24,741 37 |
