Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AIkido Pharma Inc. | a10-20561_18k.htm |
Exhibit 99.1
|
|
Spherix Incorporated (Nasdaq: SPEX) November 2010 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such statements, including statements relating to planned clinical study design, regulatory and business strategies, plans and objectives of management and growth opportunities for existing or proposed products, constitute forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those anticipated by the forward-looking statements. The risks and uncertainties include, without limitation, risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured, we may lack financial resources to complete development of D-tagatose, the FDA may interpret the results of studies differently than us, competing products may be more successful, demand for new pharmaceutical products may decrease, the biopharmaceutical industry may experience negative market trends, our continuing efforts to develop D-tagatose may be unsuccessful, our common stock could be delisted from the Nasdaq Capital Market, and other risks and challenges detailed in our filings with the U.S. Securities and Exchange Commission. You are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this presentation. We undertake no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this presentation or to reflect the occurrence of unanticipated events. |
|
|
Company Description Core expertise in scientific and technical aspects of food and drug development Operates two subsidiaries Biospherics: Pharmaceutical development of D-tagatose and pipeline products Spherix Consulting: Scientific consulting on food and drug approvals for clients worldwide Biospherics is developing D-tagatose for the treatment of diabetes and metabolic disorders Phase 3 trial as monotherapy in Type 2 Diabetes reported 10/10 Phase 2 dose-ranging study in Type 2 Diabetes to complete 12/10 Human dose-ranging studies in hypertriglyceridemia to start in 2011 |
|
|
Investment Highlights D-tagatose shows promise in multiple clinical applications Diabetes ($175 billion treatment market) Hypertriglyceridemia ($26 billion treatment market) Phase 3 study demonstrated statistically significant reductions in HbA1c in patients with mild Type 2 diabetes Pursuing partnership(s) for further clinical development Manufacturing supply agreement in place Preclinical and interim Phase 2 data showed lowering of triglycerides Much faster and less expensive development pathway vs. diabetes Clinical development program being finalized Positive cash flow from Spherix Consulting |
|
|
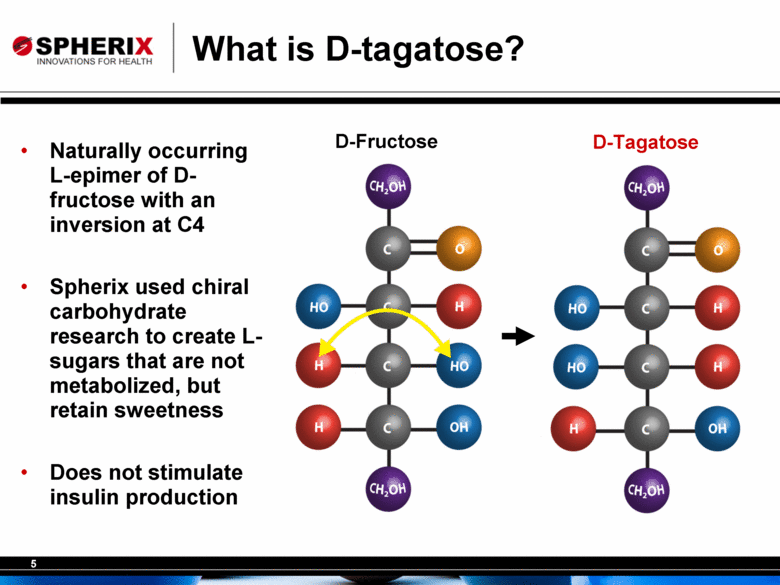
What is D-tagatose? D-Tagatose D-Fructose Naturally occurring L-epimer of D-fructose with an inversion at C4 Spherix used chiral carbohydrate research to create L-sugars that are not metabolized, but retain sweetness Does not stimulate insulin production |
|
|
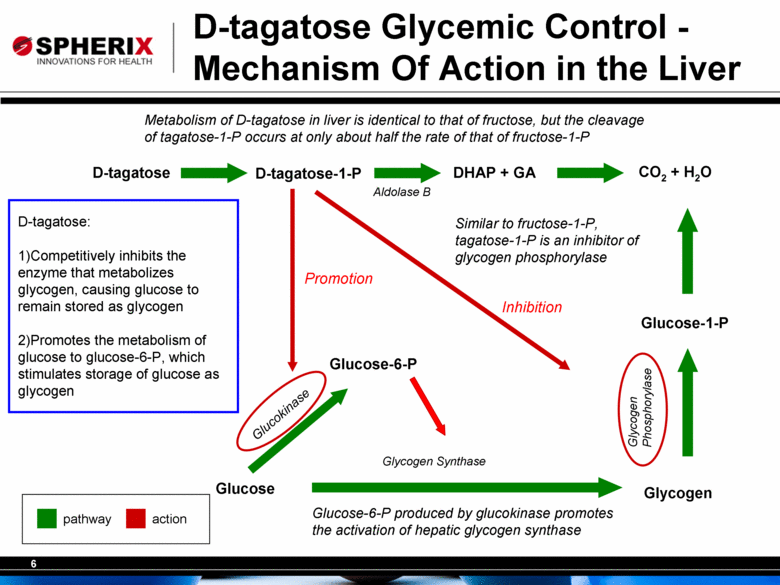
D-tagatose Glycemic Control - Mechanism Of Action in the Liver Metabolism of D-tagatose in liver is identical to that of fructose, but the cleavage of tagatose-1-P occurs at only about half the rate of that of fructose-1-P Similar to fructose-1-P, tagatose-1-P is an inhibitor of glycogen phosphorylase Glucose Glucose-6-P Glycogen Glucokinase Glycogen Synthase Glucose-6-P produced by glucokinase promotes the activation of hepatic glycogen synthase CO2 + H2O Glucose-1-P Glycogen Phosphorylase D-tagatose D-tagatose-1-P DHAP + GA Aldolase B pathway action D-tagatose: 1) Competitively inhibits the enzyme that metabolizes glycogen, causing glucose to remain stored as glycogen 2) Promotes the metabolism of glucose to glucose-6-P, which stimulates storage of glucose as glycogen Promotion Inhibition |
|
|
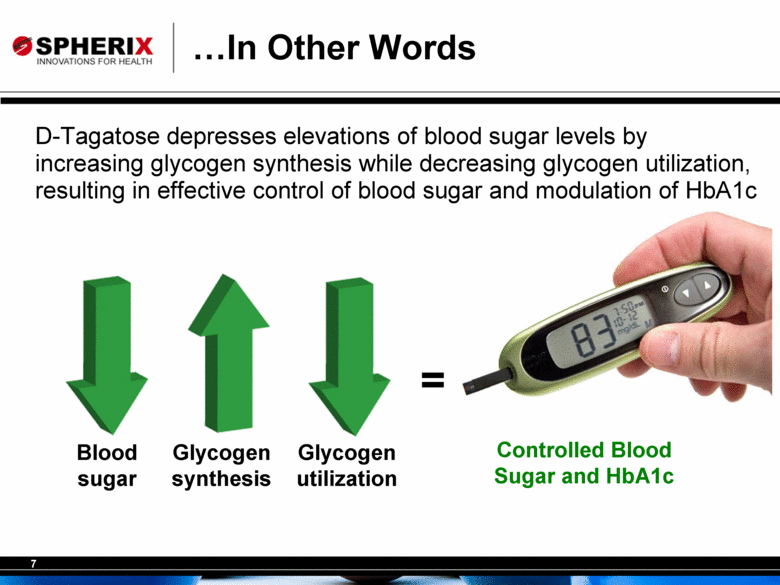
D-Tagatose depresses elevations of blood sugar levels by increasing glycogen synthesis while decreasing glycogen utilization, resulting in effective control of blood sugar and modulation of HbA1c Controlled Blood Sugar and HbA1c Blood sugar Glycogen synthesis Glycogen utilization = In Other Words |
|
|
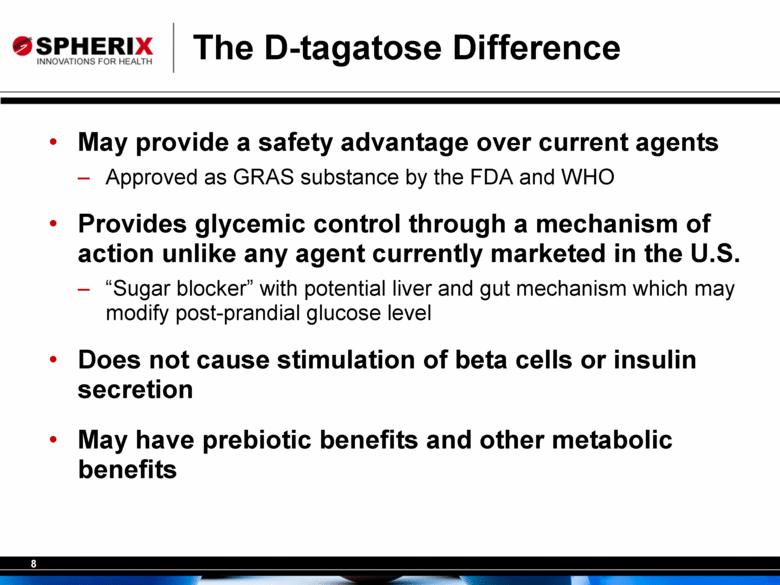
The D-tagatose Difference May provide a safety advantage over current agents Approved as GRAS substance by the FDA and WHO Provides glycemic control through a mechanism of action unlike any agent currently marketed in the U.S. “Sugar blocker” with potential liver and gut mechanism which may modify post-prandial glucose level Does not cause stimulation of beta cells or insulin secretion May have prebiotic benefits and other metabolic benefits |
|
|
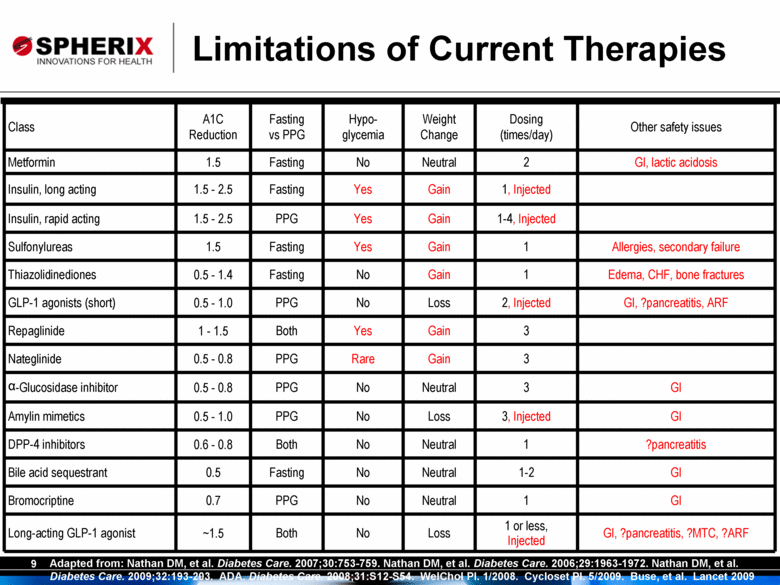
Class A1C Reduction Fasting vs PPG Hypo- glycemia Weight Change Dosing (times/day) Other safety issues Metformin 1.5 Fasting No Neutral 2 GI, lactic acidosis Insulin, long acting 1.5 - 2.5 Fasting Yes Gain 1, Injected Insulin, rapid acting 1.5 - 2.5 PPG Yes Gain 1-4, Injected Sulfonylureas 1.5 Fasting Yes Gain 1 Allergies, secondary failure Thiazolidinediones 0.5 - 1.4 Fasting No Gain 1 Edema, CHF, bone fractures GLP-1 agonists (short) 0.5 - 1.0 PPG No Loss 2, Injected GI, ?pancreatitis, ARF Repaglinide 1 - 1.5 Both Yes Gain 3 Nateglinide 0.5 - 0.8 PPG Rare Gain 3 -Glucosidase inhibitor 0.5 - 0.8 PPG No Neutral 3 GI Amylin mimetics 0.5 - 1.0 PPG No Loss 3, Injected GI DPP-4 inhibitors 0.6 - 0.8 Both No Neutral 1 ?pancreatitis Bile acid sequestrant 0.5 Fasting No Neutral 1-2 GI Bromocriptine 0.7 PPG No Neutral 1 GI Long-acting GLP-1 agonist ~1.5 Both No Loss 1 or less, Injected GI, ?pancreatitis, ?MTC, ?ARF Adapted from: Nathan DM, et al. Diabetes Care. 2007;30:753-759. Nathan DM, et al. Diabetes Care. 2006;29:1963-1972. Nathan DM, et al. Diabetes Care. 2009;32:193-203. ADA. Diabetes Care. 2008;31:S12-S54. WelChol PI. 1/2008. Cycloset PI. 5/2009. Buse, et al. Lancet 2009 Limitations of Current Therapies |
|
|
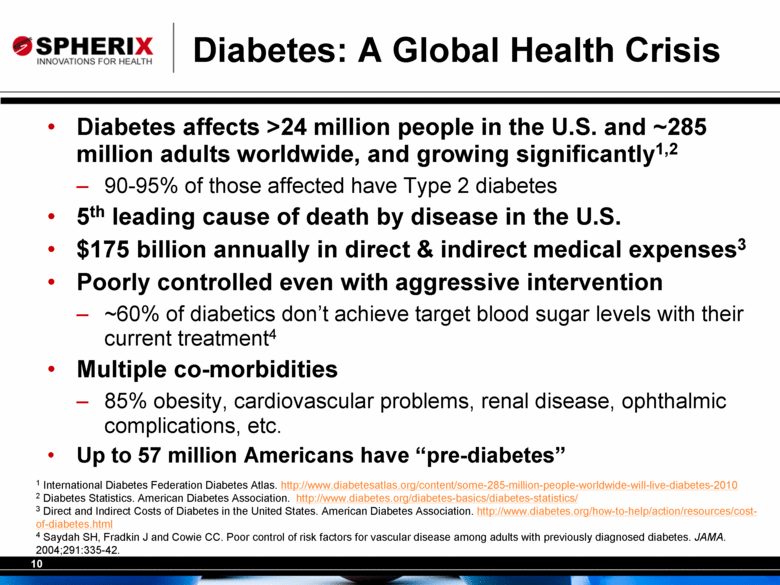
Diabetes: A Global Health Crisis Diabetes affects >24 million people in the U.S. and ~285 million adults worldwide, and growing significantly1,2 90-95% of those affected have Type 2 diabetes 5th leading cause of death by disease in the U.S. $175 billion annually in direct & indirect medical expenses3 Poorly controlled even with aggressive intervention ~60% of diabetics don’t achieve target blood sugar levels with their current treatment4 Multiple co-morbidities 85% obesity, cardiovascular problems, renal disease, ophthalmic complications, etc. Up to 57 million Americans have “pre-diabetes” 1 International Diabetes Federation Diabetes Atlas. http://www.diabetesatlas.org/content/some-285-million-people-worldwide-will-live-diabetes-2010 2 Diabetes Statistics. American Diabetes Association. http://www.diabetes.org/diabetes-basics/diabetes-statistics/ 3 Direct and Indirect Costs of Diabetes in the United States. American Diabetes Association. http://www.diabetes.org/how-to-help/action/resources/cost-of-diabetes.html 4 Saydah SH, Fradkin J and Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335-42. |
|
|
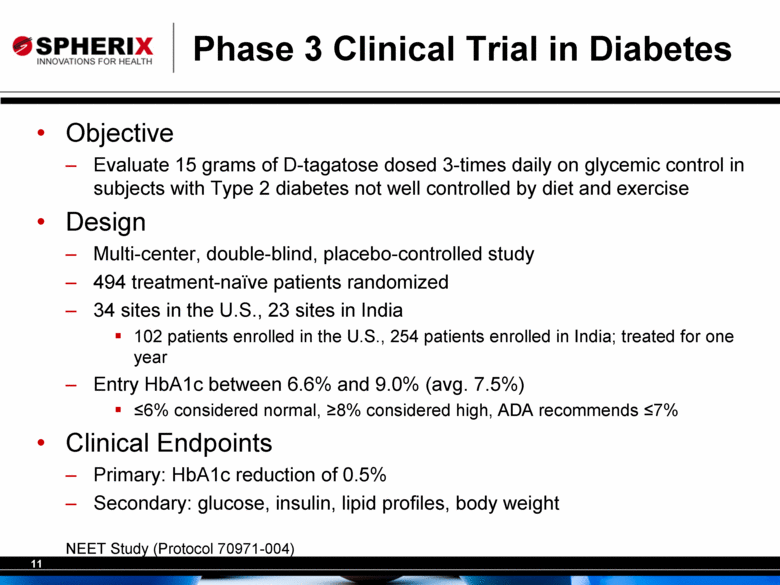
Phase 3 Clinical Trial in Diabetes Objective Evaluate 15 grams of D-tagatose dosed 3-times daily on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, double-blind, placebo-controlled study 494 treatment-naïve patients randomized 34 sites in the U.S., 23 sites in India 102 patients enrolled in the U.S., 254 patients enrolled in India; treated for one year Entry HbA1c between 6.6% and 9.0% (avg. 7.5%) <6% considered normal, >8% considered high, ADA recommends <7% Clinical Endpoints Primary: HbA1c reduction of 0.5% Secondary: glucose, insulin, lipid profiles, body weight NEET Study (Protocol 70971-004) |
|
|
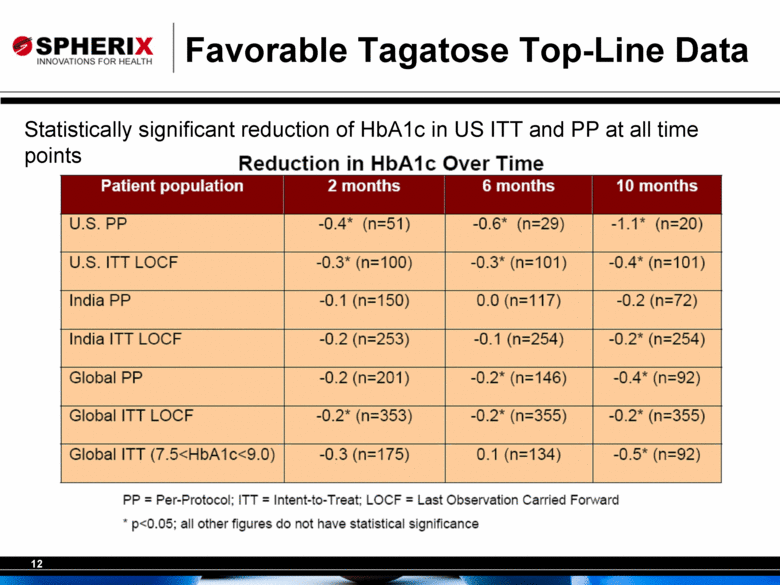
Favorable Tagatose Top-Line Data Statistically significant reduction of HbA1c in US ITT and PP at all time points Reduction in HbAlc Over Time Patient population 2 months 6 months 10 months U.S. PP -0.4* (n=51) -0.6* (n=29) -1.1* (n=20) U.S. ITT LOCF -0.3* (n=100) -0.3* (n=101) -0-4* (n=101) India PP -0.1 (n=150) 0.0 (n=117) -0.2 (n=72) India ITT LOCF -0.2 (n=253) -0.1 (n=254) -0.2* (n=254) Global PP -0.2 (n=201) -0.2* (n=146) -0.4* (n=92) Global ITT LOCF -0.2* (n=353) -0.2* (n=355) -0.2* (n355) Global ITT (7.5<HbAlc<9.0) -0.3 (n=175) 0.1 (n=134) -0.5* (n=92) PP = Per-Protocol; ITT = Intent-to-Treat; LOCF = Last Observation Carried Forward * p<0.05; all other figures do not have statistical significance |
|
|
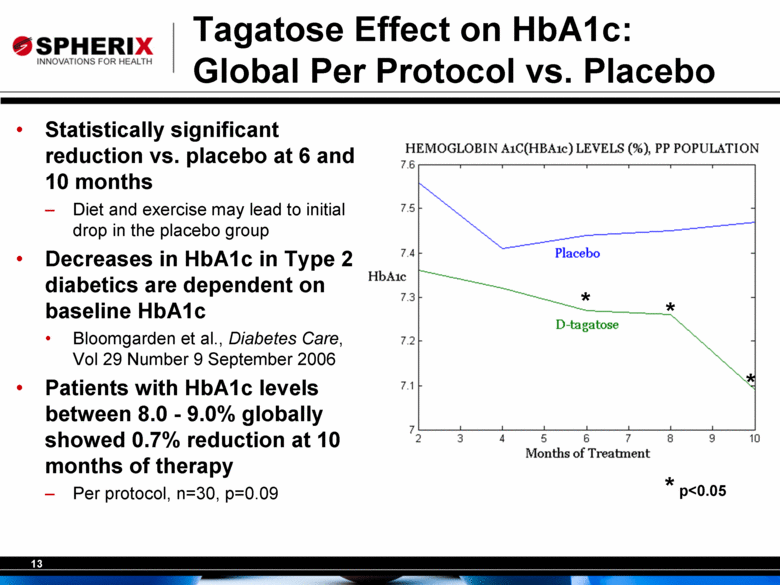
Tagatose Effect on HbA1c: Global Per Protocol vs. Placebo Statistically significant reduction vs. placebo at 6 and 10 months Diet and exercise may lead to initial drop in the placebo group Decreases in HbA1c in Type 2 diabetics are dependent on baseline HbA1c Bloomgarden et al., Diabetes Care, Vol 29 Number 9 September 2006 Patients with HbA1c levels between 8.0 - 9.0% globally showed 0.7% reduction at 10 months of therapy Per protocol, n=30, p=0.09 * * * p<0.05 * |
|
|
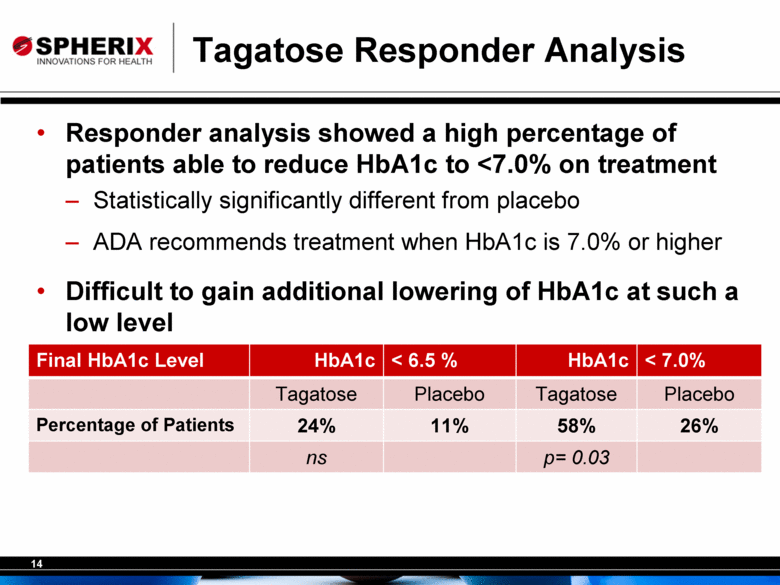
Tagatose Responder Analysis Responder analysis showed a high percentage of patients able to reduce HbA1c to <7.0% on treatment Statistically significantly different from placebo ADA recommends treatment when HbA1c is 7.0% or higher Difficult to gain additional lowering of HbA1c at such a low level Final HbA1c Level HbA1c < 6.5 % HbA1c < 7.0% Tagatose Placebo Tagatose Placebo Percentage of Patients 24% 11% 58% 26% ns p= 0.03 |
|
|
Other Diabetes Clinical Findings Patients with >1 treatment-emergent adverse events in the active group (163) was comparable to the placebo group (166) No serious adverse event deemed treatment related No episodes of hypoglycemia or pancreatitis were reported among any trial subjects The study was not powered for significance in secondary endpoints (e.g., triglycerides). |
|
|
The Metabolic Diseases Crisis-Hypertriglyceridemia In the US alone, >100 million people have elevated triglycerides (> 150 mg/dl) Associated with other lipid abnormalities and the metabolic syndrome abdominal obesity, insulin resistance, low high-density lipoprotein (HDL), and hypertension, which are linked to coronary artery disease Preclinical work and interim Phase 2 D-tagatose data support pursuit of this indication for development Good commercial opportunity! Large, but poorly-served market due to side effects of current products, with few competitors and low promotional intensity |
|
|
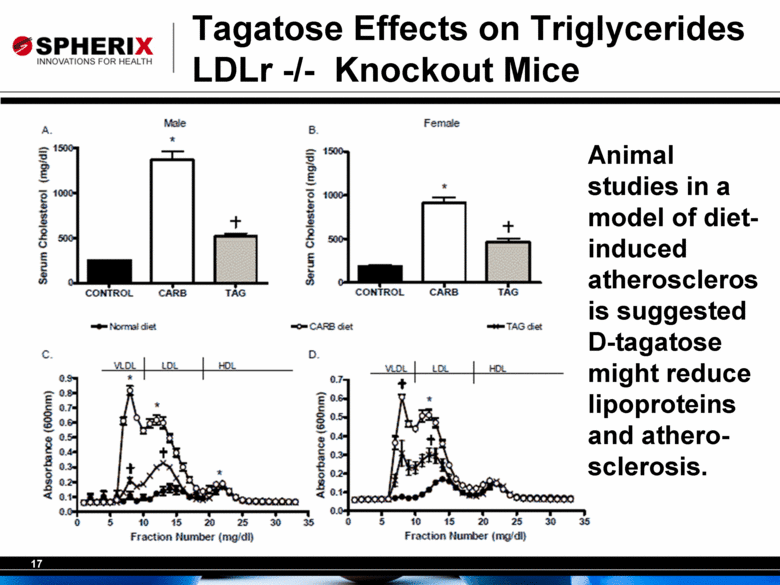
Tagatose Effects on Triglycerides LDLr -/- Knockout Mice Animal studies in a model of diet-induced atherosclerosis suggested D-tagatose might reduce lipoproteins and atherosclerosis. |
|
|
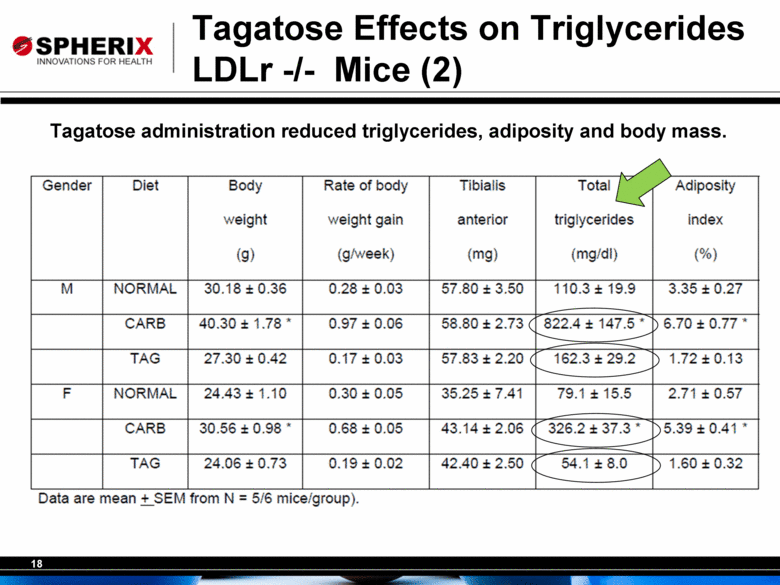
Tagatose Effects on Triglycerides LDLr -/- Mice (2) Tagatose administration reduced triglycerides, adiposity and body mass. |
|
|
Phase 2 Clinical Trial in Diabetes Objective Evaluate dose-response effect of minimal doses of D-tagatose (2.5, 5.0 or 7.5 grams dosed 3-times daily) on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, single-blind, randomized, parallel group clinical study; 6 months duration Clinical Endpoints Primary: HbA1c Secondary: glucose, insulin, lipid profiles, body weight Interim data showed 172 patients receiving 7.5 grams reduced triglycerides by 38 mg/dl Mean triglyceride level was 180 at start of trial Trial expected to lock in 4Q2010 Protocol 70971-005 |
|
|
D-tagatose Development Plans Secure development & commercialization agreement or outright sale for diabetes indication Additional clinical trials costing hundreds of millions of $ required to secure registration Progress development for triglyceride indication Conduct selective animal studies for efficacy and dose-range setting Conduct human proof-of-concept study in 2011 Conduct in vitro cell studies to further define mechanism of action in lipid metabolism Contemplate partnerships following additional animal studies |
|
|
Outsourced Manufacturing Inalco S.p.A. of Italy DMF 22715 and LoA One metric ton batches Supplied product for Phase 2 and 3 trials Has capacity for full scale commercial supply |
|
|
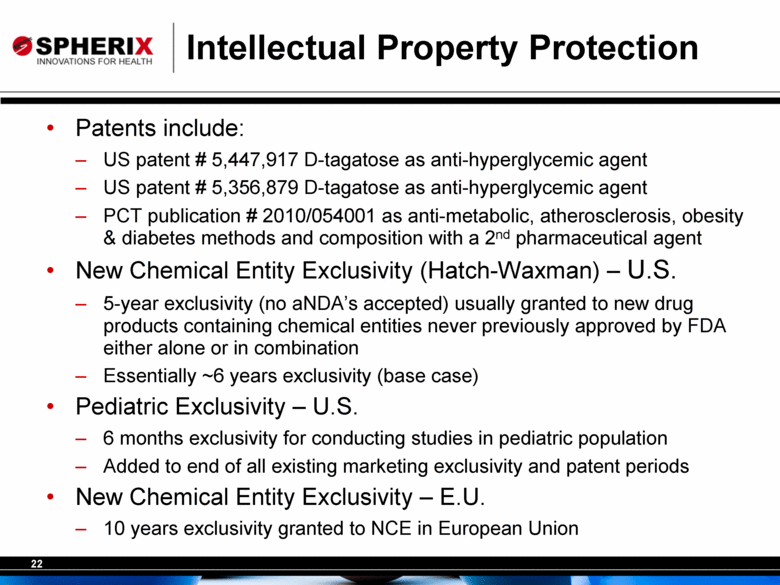
Intellectual Property Protection Patents include: US patent # 5,447,917 D-tagatose as anti-hyperglycemic agent US patent # 5,356,879 D-tagatose as anti-hyperglycemic agent PCT publication # 2010/054001 as anti-metabolic, atherosclerosis, obesity & diabetes methods and composition with a 2nd pharmaceutical agent New Chemical Entity Exclusivity (Hatch-Waxman) – U.S. 5-year exclusivity (no aNDA’s accepted) usually granted to new drug products containing chemical entities never previously approved by FDA either alone or in combination Essentially ~6 years exclusivity (base case) Pediatric Exclusivity – U.S. 6 months exclusivity for conducting studies in pediatric population Added to end of all existing marketing exclusivity and patent periods New Chemical Entity Exclusivity – E.U. 10 years exclusivity granted to NCE in European Union |
|
|
Spherix Consulting Provides scientific and regulatory consulting services Clients include food and pharmaceutical companies in US, Europe and Asia Provides technical support to the Company’s pharmaceutical development arm Cash flow positive business 1H 10 revenue of $659,000 1H 10 operating income of $155,000 |
|
|
Experienced Leadership Claire L. Kruger, Ph.D., Chief Executive Officer Toxicologist with 20 years of consulting experience; primary area of expertise is in pharmaceuticals, consumer products and foods, where she provides scientific, regulatory, and strategic support to clients in both the US and international regulatory arenas Robert A. Lodder, Ph.D., President Founder of InfraReDx, Inc. and Prescient Medical, Inc., Professor of Pharmaceutical Sciences at the College of Pharmacy, University of Kentucky Medical Center Robert L. Clayton, Chief Financial Officer 16 years of experience in finance and accounting, including 5 years in public accounting; previously served as Director of Finance and Controller for Spherix Ram Nimmagudda, Ph.D., Director of New Business Development 15 years of experience in business development, nutrition and nutraceutical industries, with particular expertise in novel food ingredients; previous positions of Director of New Business Development for South Asia at DSM Functional Foods, Director and Sr. Principal Technologist, External Technologies at Wm. Wrigley Jr. Co. and Vice President of Technology Growth at Char. Hansen, Inc. |
|
|
Contracted Staff Highly Experienced in Drug Development Leisa Dennehy, Commercial and Corporate Development Advisor With over 20 years in a variety of commercial roles including new product planning, product launches, and pharma partnering at Glaxo/GW, Protez and Affinium,Pharmaceuticals, Leisa’s guides development of target product profiles and commercial assessment for Spherix. Vicky Hines, Ph.D, Regulatory Affairs Advisor Formerly with small phama companies such as Chiron and Celtic Pharma, Dr Hines experience ranging from discovery research to commercialized products brings an investor-oriented mindset to help small companies navigate the complexities of Regulatory strategy with the FDA and EMA. Nick Livington, Ph.D. Pre-Clinical Advisor With decades of senior roles in pre-clinical development at Bayer and GlaxoSmithKline for both diabetes and metabolic products, Dr Livingston is ideally suited to guide Spherix in developing robust proof of principle and MoA studies in animals and cell lines which meet pharma companies diligence requirements John Amatruda, M.D., Clinical Advisor Previously head of Clinical for Januvia and Janumet at Merck, and Precose at Bayer , Dr Amatruda brings more than 20 years of experience in strategizing and conducting clinical studies in Type 2 Diabetes. Dr Amatruda brings the experience few consultants have in diabetes drug development. Jonathan Tobert, M.D. Ph.D, Clinical Advisor Dr Tobert has more experience in lipid drug development than almost anyone, with 30 years of clinical experience at Merck developing statins. Dr Tobert support s the clinical work on triglycerides. |
|
|
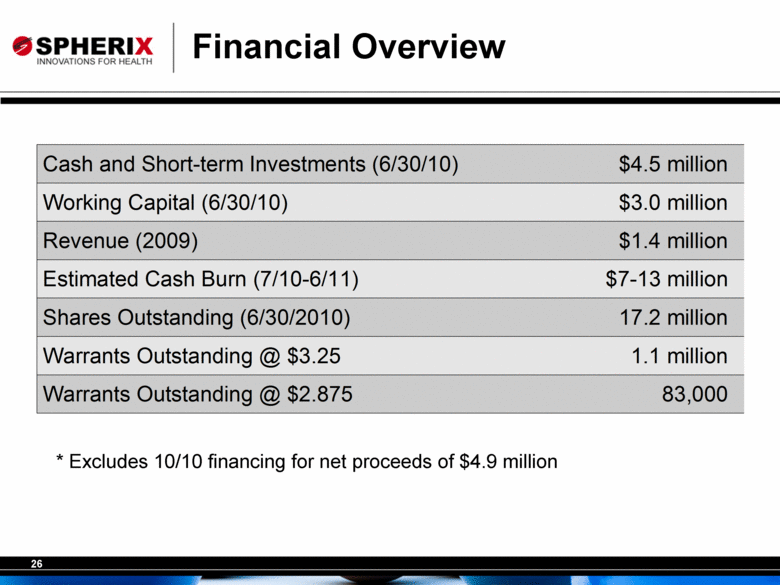
Financial Overview * Excludes 10/10 financing for net proceeds of $4.9 million Cash and Short-term Investments (6/30/10) $4.5 million Working Capital (6/30/10) $3.0 million Revenue (2009) $1.4 million Estimated Cash Burn (7/10-6/11) $7-13 million Shares Outstanding (6/30/2010) 17.2 million Warrants Outstanding @ $3.25 1.1 million Warrants Outstanding @ $2.875 83,000 |
|
|
Near-Term Milestones Report Phase 2 dose-finding data on diabetes, hypertriglyceridemia, body mass index 4Q10 Engage partner for D-tagatose in diabetes to continue development Ongoing Perform additional in vitro and animal studies on hypertriglyceridemia mechanism of action 1Q11 Begin human study with D-Tagatose in hyper-triglyceridemia 1Q11 |
|
|
Investment Highlights D-tagatose shows promise in multiple clinical applications Diabetes ($175 billion treatment market) Hypertriglyceridemia ($26 billion treatment market) Phase 3 study demonstrated statistically significant reductions in HbA1c in patients with mild Type 2 diabetes Pursuing partnership(s) for further clinical development Manufacturing supply agreement in place Preclinical and interim Phase 2 data demonstrated ability to lower triglycerides Much faster and less expensive development pathway vs. diabetes Clinical development program being finalized Positive cash flow from Spherix Consulting |