Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a10-19776_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a10-19776_1ex99d1.htm |
Exhibit 99.2
|
|
® ® TD-1211: Peripherally Restricted µ Opioid Receptor Antagonist Phase 1 and 2 Results October 21, 2010 Theravance, Theravance’s logo and Medicines That Make a Difference are registered trademarks of Theravance, Inc. © 2010 Theravance, Inc. |
|
|
Safe Harbor This presentation contains certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995. The words “may”, “will”, “should”, “could”, “would”, “plan”, “anticipate”, “believe”, “estimate”, “intend”, “goal,” “project”, “potential”, “expect”, “consistent”, “supportive”, “target” and “promising” and similar expressions are intended to identify such forward-looking statements. Examples of such statements include statements relating to the goals and timing of clinical studies and product commercialization, statements regarding the potential benefits and mechanisms of action of drug candidates, statements concerning the timing of seeking regulatory approval of our product candidates, statements concerning enabling capabilities of Theravance's approach to drug discovery and its proprietary insights, statements concerning expectations for product candidates through development and commercialization and projections of revenue, expenses and other financial items. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in its forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to delays or difficulties in commencing or completing clinical studies, risks related to the potential that results of clinical or preclinical studies indicate product candidates are unsafe or ineffective, our dependence on third parties in the conduct of our clinical studies, delays or failure to achieve regulatory approvals for product candidates, risks of relying on third-party manufacturers for the supply of our product candidates and risks of collaborating with third parties to develop and commercialize products. These and other risks are described in greater detail under the heading "Risk Factors" contained in Theravance’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 4, 2010 and the risks discussed in our other period filings with SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements. 2 |
|
|
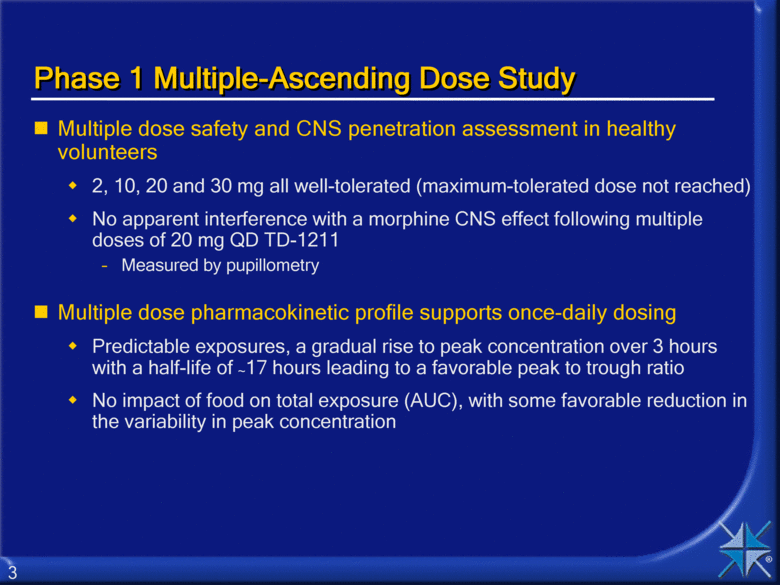
Phase 1 Multiple-Ascending Dose Study Multiple dose safety and CNS penetration assessment in healthy volunteers 2, 10, 20 and 30 mg all well-tolerated (maximum-tolerated dose not reached) No apparent interference with a morphine CNS effect following multiple doses of 20 mg QD TD-1211 Measured by pupillometry Multiple dose pharmacokinetic profile supports once-daily dosing Predictable exposures, a gradual rise to peak concentration over 3 hours with a half-life of ~17 hours leading to a favorable peak to trough ratio No impact of food on total exposure (AUC), with some favorable reduction in the variability in peak concentration 3 |
|
|
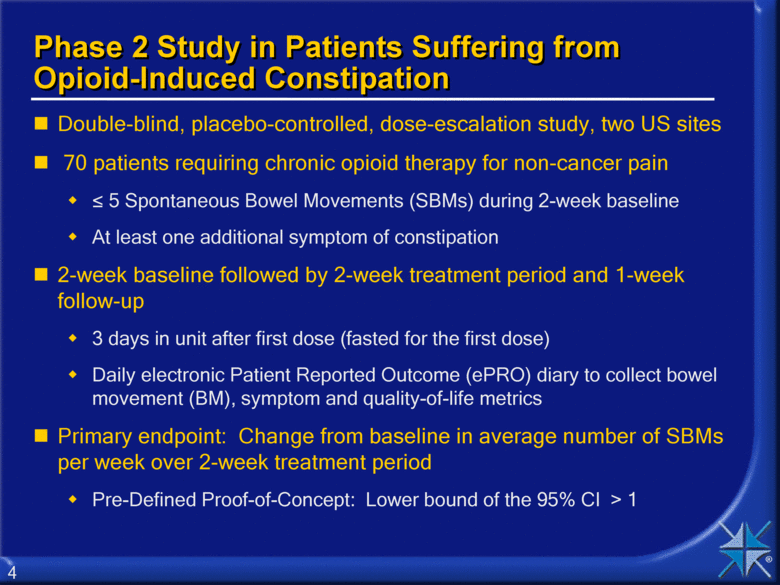
Phase 2 Study in Patients Suffering from Opioid-Induced Constipation Double-blind, placebo-controlled, dose-escalation study, two US sites 70 patients requiring chronic opioid therapy for non-cancer pain < 5 Spontaneous Bowel Movements (SBMs) during 2-week baseline At least one additional symptom of constipation 2-week baseline followed by 2-week treatment period and 1-week follow-up 3 days in unit after first dose (fasted for the first dose) Daily electronic Patient Reported Outcome (ePRO) diary to collect bowel movement (BM), symptom and quality-of-life metrics Primary endpoint: Change from baseline in average number of SBMs per week over 2-week treatment period Pre-Defined Proof-of-Concept: Lower bound of the 95% CI > 1 4 |
|
|
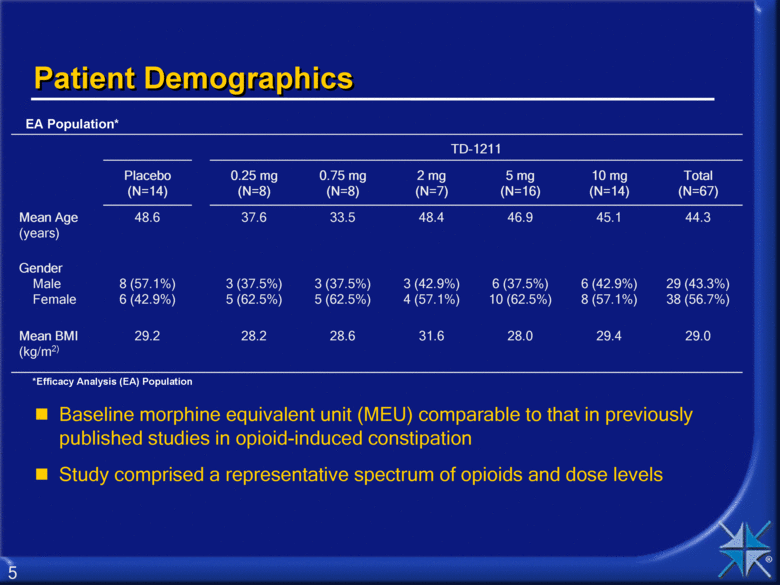
Patient Demographics Baseline morphine equivalent unit (MEU) comparable to that in previously published studies in opioid-induced constipation Study comprised a representative spectrum of opioids and dose levels TD-1211 Placebo (N=14) 0.25 mg (N=8) 0.75 mg (N=8) 2 mg (N=7) 5 mg (N=16) 10 mg (N=14) Total (N=67) Mean Age (years) 48.6 37.6 33.5 48.4 46.9 45.1 44.3 Gender Male Female 8 (57.1%) 6 (42.9%) 3 (37.5%) 5 (62.5%) 3 (37.5%) 5 (62.5%) 3 (42.9%) 4 (57.1%) 6 (37.5%) 10 (62.5%) 6 (42.9%) 8 (57.1%) 29 (43.3%) 38 (56.7%) Mean BMI (kg/m2) 29.2 28.2 28.6 31.6 28.0 29.4 29.0 EA Population* *Efficacy Analysis (EA) Population 5 |
|
|
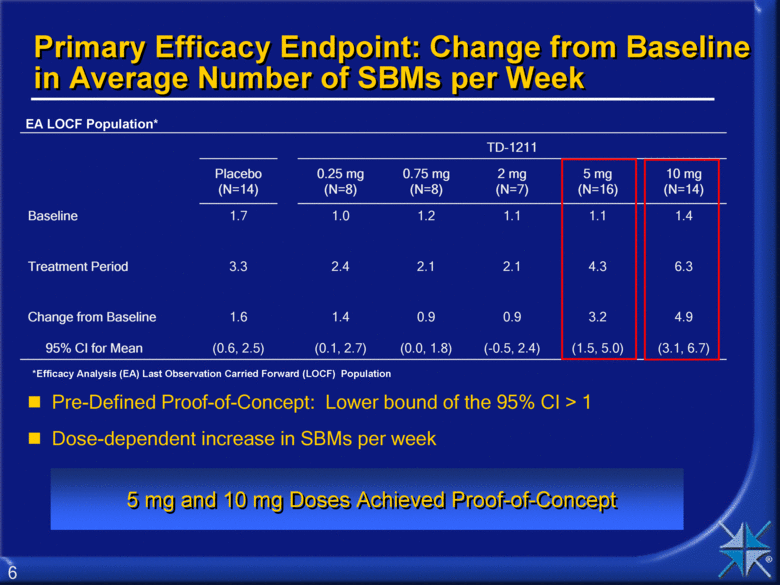
Primary Efficacy Endpoint: Change from Baseline in Average Number of SBMs per Week TD-1211 Placebo (N=14) 0.25 mg (N=8) 0.75 mg (N=8) 2 mg (N=7) 5 mg (N=16) 10 mg (N=14) Baseline 1.7 1.0 1.2 1.1 1.1 1.4 Treatment Period 3.3 2.4 2.1 2.1 4.3 6.3 Change from Baseline 95% CI for Mean 1.6 (0.6, 2.5) 1.4 (0.1, 2.7) 0.9 (0.0, 1.8) 0.9 (-0.5, 2.4) 3.2 (1.5, 5.0) 4.9 (3.1, 6.7) Pre-Defined Proof-of-Concept: Lower bound of the 95% CI > 1 Dose-dependent increase in SBMs per week 5 mg and 10 mg Doses Achieved Proof-of-Concept EA LOCF Population* *Efficacy Analysis (EA) Last Observation Carried Forward (LOCF) Population 6 |
|
|
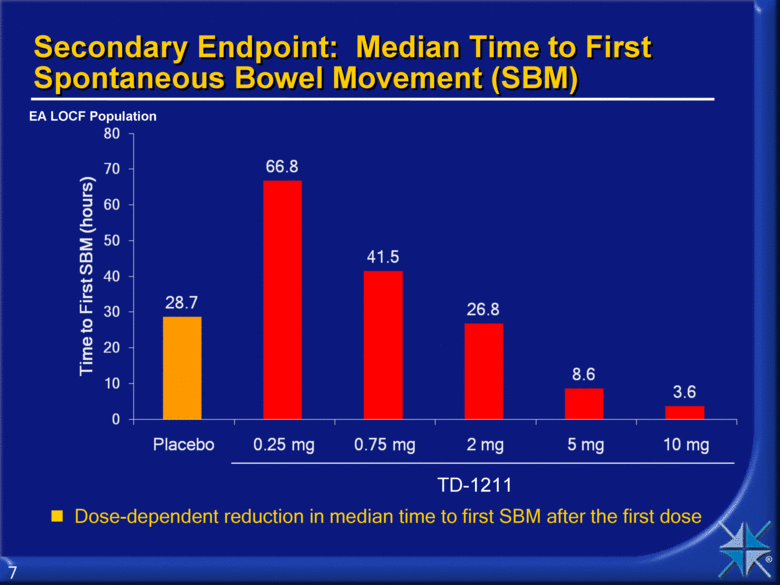
Secondary Endpoint: Median Time to First Spontaneous Bowel Movement (SBM) Dose-dependent reduction in median time to first SBM after the first dose TD-1211 EA LOCF Population 80 28.7 70 66.8 60 41.5 50 26.8 40 8.6 30 3.6 20 0.25 mg 10 0.75 mg 0 2 mg 5 mg 10 mg 7 |
|
|
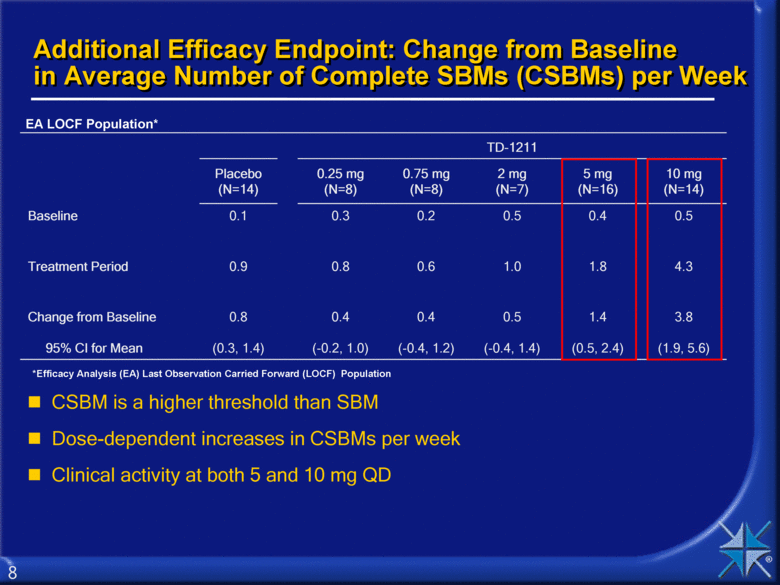
Additional Efficacy Endpoint: Change from Baseline in Average Number of Complete SBMs (CSBMs) per Week TD-1211 Placebo (N=14) 0.25 mg (N=8) 0.75 mg (N=8) 2 mg (N=7) 5 mg (N=16) 10 mg (N=14) Baseline 0.1 0.3 0.2 0.5 0.4 0.5 Treatment Period 0.9 0.8 0.6 1.0 1.8 4.3 Change from Baseline 95% CI for Mean 0.8 (0.3, 1.4) 0.4 (-0.2, 1.0) 0.4 (-0.4, 1.2) 0.5 (-0.4, 1.4) 1.4 (0.5, 2.4) 3.8 (1.9, 5.6) CSBM is a higher threshold than SBM Dose-dependent increases in CSBMs per week Clinical activity at both 5 and 10 mg QD EA LOCF Population* *Efficacy Analysis (EA) Last Observation Carried Forward (LOCF) Population 8 |
|
|
Phase 2 Study Safety Summary No Serious Adverse Events (SAEs) reported No evidence of CNS opioid withdrawal or analgesic interference Most adverse events (AEs) were mild/moderate Early onset (Day 1 or Day 2) Majority of GI-related AEs resolved within a few days No clinically significant changes in laboratory tests, ECGs, vital signs, physical exam 9 |
|
|
Most Common GI Adverse Events TD-1211 Placebo (N=14) 0.25 mg (N=8) 0.75 mg (N=8) 2 mg (N=8) 5 mg (N=16) 10 mg (N=16) No. of Patients with GI AEs 2 1 3 3 9 13 Abdominal Pain Mild Moderate Severe 1 0 0 0 0 0 3 0 0 1 1 0 5 3 0 7 2 3 Diarrhea Mild Moderate Severe 1 0 0 0 0 0 0 0 0 0 1 0 1 0 0 2 2 1 Nausea Mild Moderate Severe 0 0 0 1 0 0 2 0 0 0 2 0 2 1 0 5 1 2 Vomiting Mild Moderate Severe 0 0 0 0 0 0 0 0 0 2 1 0 3 1 0 0 2 1 Early onset (Day 1 or Day 2); majority of GI-related AEs resolved within a few days Moderate/severe abdominal pain generally coincided with bowel movements Headache was the most common non-GI AE and it occurred more frequently on TD-1211 than placebo Safety Population 10 |
|
|
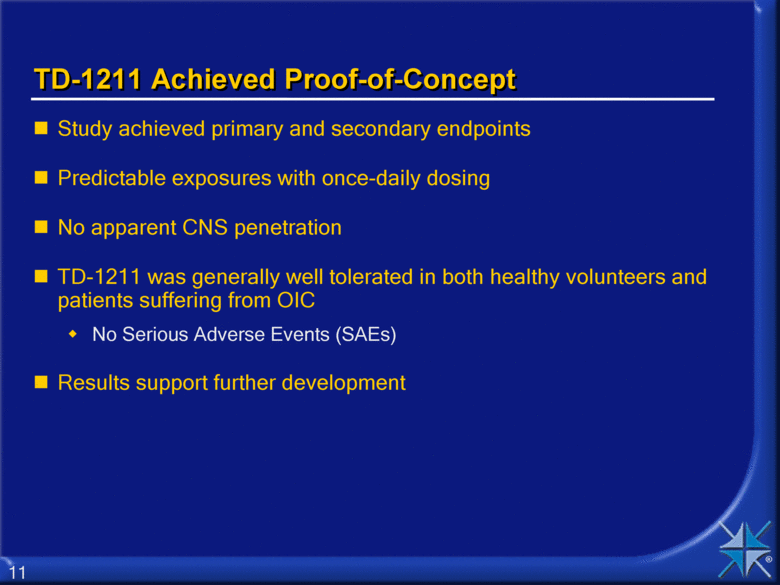
TD-1211 Achieved Proof-of-Concept Study achieved primary and secondary endpoints Predictable exposures with once-daily dosing No apparent CNS penetration TD-1211 was generally well tolerated in both healthy volunteers and patients suffering from OIC No Serious Adverse Events (SAEs) Results support further development 11 |
|
|
Theravance, Theravance’s logo and Medicines That Make a Difference are registered trademarks of Theravance, Inc. © 2010 Theravance, Inc. |