Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - ThermoGenesis Holdings, Inc. | f56815exv32.htm |
| EX-23.1 - EX-23.1 - ThermoGenesis Holdings, Inc. | f56815exv23w1.htm |
| EX-31.2 - EX-31.2 - ThermoGenesis Holdings, Inc. | f56815exv31w2.htm |
| EX-31.1 - EX-31.1 - ThermoGenesis Holdings, Inc. | f56815exv31w1.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE
SECURITIES EXCHANGE ACT OF 1934
SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended: June 30, 2010
Commission File Number: 333-82900
ThermoGenesis Corp.
(Exact name of registrant as specified in its charter)
| Delaware (State of incorporation) |
94-3018487 (I.R.S. Employer Identification No.) |
2711 Citrus Road
Rancho Cordova, California 95742
(Address of principal executive offices) (Zip Code)
Rancho Cordova, California 95742
(Address of principal executive offices) (Zip Code)
(916) 858-5100
(Registrant’s telephone number, including area code)
(Registrant’s telephone number, including area code)
Securities Registered Pursuant to Section 12(b) of the Act: Common Stock, $0.001 par value
Nasdaq Stock Market, LLC Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of
the Securities Act. o Yes þ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or
Section 15(d) of the Act. o Yes þ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by
Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding twelve months (or
for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. þ Yes o No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K,
is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in
definitive proxy or information statements incorporated by reference in Part III of this Form 10-K
or any amendment of this Form 10-K. o
Indicate by check mark whether
the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data
File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12
months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer” and “small reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer* o *(Do not check if a smaller reporting company) |
Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
o Yes þ No
The aggregate market value of the common stock held by non-affiliates as of December 31, 2009 (the
last trading day of the second quarter) was $32,533,917, based on the closing sale price on such
day.
As of September 13, 2010, 14,023,240 shares of the registrant’s Common Stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: Portions of the registrant’s proxy statement for its 2010
Annual Meeting of Stockholders are incorporated by reference into Part III hereof.
TABLE OF CONTENTS
i
Table of Contents
PART I
| ITEM 1. | BUSINESS |
Business Overview
ThermoGenesis Corp. (“the Company”, “we”, “our”) mission is to design, develop and commercialize
medical products that enable the collection, processing and cryopreservation of stem cells and
other cellular tissues used in the practice of regenerative medicine. Regenerative medicine is an
emerging field which, among other things, aims to repair or restore lost or damaged tissue and cell
function using cell-based therapies. Our current products automate the volume reduction and
cryopreservation process of adult stem cell concentrates from cord blood and bone marrow for use in
laboratory and point of care settings. Our growth strategy is to expand our offerings in
regenerative medicine and partner with other pioneers in the stem cell arena to accelerate our
worldwide penetration in this potentially explosive market. We plan to have a product line that
encompasses all sources of stem cells, including cord blood, bone marrow, adipose, among others and
to leverage our technological investments into profitable adjacent markets, such as platelet rich
plasma (“PRP”). The Company was founded in 1986 and is located in Rancho Cordova, California.
Our business model is based on the sale of medical devices and the recurring revenues generated
from the companion single-use, sterile disposable products. We currently sell our products in 37
countries throughout the world to customers that include private and public cord blood banks,
surgeons, hospitals and research institutions. Our worldwide commercialization strategy relies
primarily on the utilization of distributors.
Based upon early clinical results, there is accumulating evidence that many of the stem cell
therapy trials and clinical trials underway may result in approved therapies in disease states and
tissue regeneration procedures affecting significant patient populations, leading to a revolution
in therapeutics involving stem cells. Although understanding the full potential of cell therapies
and their ultimate impact on the practice of medicine remains a longer term prospect, we believe
there are significant commercial opportunities in the market today for technologies supporting stem
cell research and cell-based treatments.
Our Solutions
We provide the tools necessary for the collection, separation, storage and delivery of stem cells
from adult tissue sources including cord blood and bone marrow, and potentially in the future,
adipose and placenta. These tools are being used by healthcare providers in both the laboratory
and point of care settings. Our competitive advantage is achieved through applying our advanced
research and engineering capabilities to develop a complete “tool box” for healthcare providers
advancing regenerative medicine. Our solutions enable our customers to automate their processes,
comply with quality regulations and achieve high stem cell yields. We believe our products
significantly enhance the safety and viability of stem cell and regenerative medical products and
will ultimately expand the use and success of those products in clinical treatment through their
ease of use and high cell recovery rates.
Key Events and Accomplishments
The following are key events and accomplishments that occurred in fiscal 2010:
| • | Launch of Res-Q System In July 2009, we launched the Res-Q™ 60 BMC (“Res-Q”) System, an automated cell processing medical device for the concentration of bone marrow-derived stem cells at the point of care. |
2
Table of Contents
| • | Established Res-Q Distribution We established global distribution for Res-Q, having signed agreements with distributors covering four continents: |
| • | GE Healthcare (“GEHC”): Non-exclusive, U.S. excluding orthopedics, Canada and 19 European countries; | ||
| • | Celling Technologies: Orthopedic applications in North America (exclusive) and worldwide (non-exclusive); | ||
| • | TotipotentSC: Exclusive for all fields of use (except orthopedics, non-exclusive) in India, Malaysia and Thailand; and | ||
| • | CEI: Exclusive for all fields of use (except orthopedics, non-exclusive) in Mexico, and Central and South American countries including Brazil, Chile, Columbia, Costa Rica, Panama, Peru, Uruguay and Venezuela. |
| • | Expanded Distribution of Existing Products In keeping with our strategy to extend the distribution reach of our existing products, we enhanced agreements in existing territories, added new territories and added new distributors: |
| • | GEHC — On February 4, 2010, we announced an improved distribution agreement with GEHC for the AutoXpress™ or AXP® Platform (“AXP”). GEHC will provide incremental funding for marketing support and market research beyond its previous commitments. | ||
| • | Fenwal — In March 2010, we signed a new distribution agreement with Fenwal, Inc. to market and distribute the AXP and BioArchive® systems in China, India and Japan. |
| • | Key Management Changes The following key management changes were made in fiscal 2010: |
| • | In August 2009, Harold Baker joined our Company and currently serves as Vice President of Commercial Operations. Mr. Baker has more than two decades of global sales and marketing experience in the healthcare sector, introducing new products, leading teams in the cord blood stem cell market, managing distributor relationships and building sales force organizations. | ||
| • | In October 2009, Jorge Artiles joined our Company as Vice President, Chief Quality and Regulatory Affairs Officer. Mr. Artiles has more than two decades of quality and regulatory affairs experience in the medical device industry, with expertise in quality system development and compliance, project management and process optimization. |
| • | Plan for CryoSeal® Fibrin Sealant System (“CryoSeal”) Product Line
Divestiture On June 16, 2010 we reached an agreement with Asahi Kasei Kuraray Medical Co., Ltd (“Asahi”) in which Asahi paid us $1 million to provide CryoSeal products and clinical support services until such time as Asahi assumes manufacturing of the product line in Japan or December 31, 2012 whichever comes first. As part of the $1 million payment, we granted Asahi an option to acquire the CryoSeal product line, which may be exercised over the next five years. |
3
Table of Contents
Recent significant event:
| • | Reverse Stock Split On August 11, 2010, we announced that our board of directors had approved a 1-for-4 reverse stock split of our common stock, pursuant to previously obtained stockholder authorization. The reverse stock split, which became effective at the close of business on August 26, 2010, reduced the number of shares of our common stock issued and outstanding from approximately 56.1 million to approximately 14 million. All share and per share amounts herein are presented on a post-reverse-split basis. |
Market Overview
The regenerative medicine market continues to experience meaningful advances in the clinical
efficacy, regulatory approval and product commercialization of cell based therapies. The vast
majority of this progress has been achieved through the application of adult stem cells, reflecting
a greater awareness and appreciation for the clinical value and safety of these cells in the
research and medical communities.
Examples of clinical efficacy and regulatory approvals include:
| • | Approval of the first autologous cellular immunotherapy for the treatment of prostrate cancer; | ||
| • | Positive results from a Phase II clinical trial to expand Mesenchymal Precursor Cells (“MPCs”) from cord blood to reduce graft-versus-host disease; | ||
| • | Expansion of treatments using cord blood beyond hematopoetic reconstitution; | ||
| • | Favorable outcomes from the first human clinical trial of adipose tissue-derived stem and regenerative cells for the treatment of heart attacks; and | ||
| • | Positive results from a Phase I clinical trial of an allogeneic cell therapy product, administered to individuals following acute myocardial infarction. |
Examples of commercialization include:
| • | Expansion of orthopedic procedures using stem cells and orthobiologics. | ||
| • | Growth of cord blood banking globally, especially in China. |
Increasing positive results from the application of adult stem cells have resulted in greater
government and private sector investment in research and development of new cell therapies as well
as the further advancement of existing treatments. For example, in October of 2009, the California
Institute of Regenerative Medicine awarded $250 million in research grants to 14 universities and
companies mainly for adult stem cell research.
The regenerative medicine market is comprised of companies that either harvest, process, purify,
cryopreserve, store or administer stem cells. Key success factors include, among other things:
| • | Stem cell recovery rates | ||
| • | Efficiency of cell processing | ||
| • | Cost of care | ||
| • | Product quality and efficacy | ||
| • | Purity, viability and potency of stem cells |
Cells are processed in the laboratory as well as in the operating room or point of care setting.
Point of care applications involve the processing of patient cells in conjunction with a surgical
procedure in an operating room or in an outpatient clinical setting. The laboratory market
requirements include, but are not limited to, Good Manufacturing Practices (“GMP”), objective
quality assurance and the ability to process multiple samples at one time. Requirements for the
point of care include sterile field packaging,
4
Table of Contents
portability, minimal processing steps and speed of processing. These market requirements must be
considered and translated into product features and benefits for successful market adoption.
The availability of stem cells at the point of care enables physicians to apply cells across an
array of applications. Physicians may also choose to study patient outcomes to understand the
benefit of stem cells under their own independently-sponsored and regulated studies. Such research
efforts are growing and already represent studies in diverse areas such as wound healing, radiation
injury, breast reconstruction and augmentation, cardiovascular applications, peripheral vascular
disease and liver disease among many others.
We expect the breadth of these applications will grow significantly as physicians continue to adopt
cell-based regenerative medicine into their treatment strategies based on the availability of safe,
clinical grade cells at the point of care.
Market Size
Market estimates for the regenerative medicine market include pathologies that affect vast numbers
of people of all age groups. A well known industry analyst, Robin Young, predicts the U.S.
regenerative medicine market will grow from $146.5 million in 2010 to over $8 billion by the year
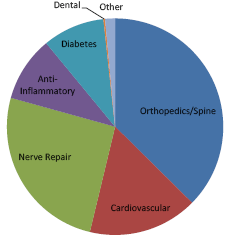
2020. The following chart highlights the disease states that are expected to be impacted by the
advent of stem cell therapies by the year 2020.
Industry Market Drivers
We expect a number of key market drivers to cause the practice of regenerative medicine to mature
over the next several years. As regenerative medicine matures, clinical studies and practice of
medicine will give way to broad clinical acceptance and substantial commercialization of cell based
therapies.
We expect the following key market drivers to be the primary forces in the near future to
positively impact the growth of regenerative medicine:
| • | Political actions | ||
| • | Government funding | ||
| • | Clinical outcomes | ||
| • | Corporate investment |
5
Table of Contents
| • | Awareness of availability | ||
| • | Growing endorsement by doctors | ||
| • | Increase in prevalence of conditions treated | ||
| • | New sources of stem cells |
Product Overview
We provide proprietary tools and technologies to enable highly effective separation and
cryopreservation of biological fluids including peripheral, bone marrow and umbilical cord blood at
a competitive cost.
| • | The AXP is a medical device with an accompanying disposable bag set that isolates and retrieves stem cells from umbilical cord blood. The AXP provides cord blood banks with an automated system to enrich adult stem cells combined with lower labor costs and a reduced risk of contamination under GMP conditions. The AXP product is an automated, closed, sterile system that volume-reduces cord blood to a user defined volume in 30 minutes while retaining over 97% of the mononuclear cells (“MNCs”). Self-powered and microprocessor-controlled, the AXP contains flow control optical sensors that achieve precise separation. | ||
| Our market for the AXP includes both private and public cord blood banks. In private banks, parents pay to preserve the cord blood cells from their offspring for family use, while a public bank owns cord blood stem cells donated by individuals, which are then available to the public for transplantation. | |||
| The AXP has been commercially available since 2006, marketed under a Master File with the U.S. Food and Drug Administration (“FDA”). In 2007, we received 510(k) clearance from the FDA for use in the processing of cord blood for cryopreservation. |
Worldwide Cord Blood Banks Growth Rate
(Data Gathered by ThermoGenesis)
(Data Gathered by ThermoGenesis)
| 2005 | 2010 | CAGR | ||||||||||
Public Banks |
118 | 176 | 8.3 | % | ||||||||
Private Banks |
91 | 222 | 19.5 | % | ||||||||
Total Banks |
209 | 398 | 13.7 | % | ||||||||
| • | The MarrowXpress™ or MXP™, an extension of the AXP, isolates and retrieves stem cells from bone marrow aspirate and its initial application is for the preparation of cells for regeneration of bone in spinal fusion procedures and tissues in cosmetic surgeries. The product is an automated, closed, sterile system that volume-reduces blood from bone marrow to a user-defined volume in 30 minutes, while retaining over 90% of the MNCs. Self-powered and microprocessor-controlled, the MXP contains flow control optical sensors that achieve precise separation. In June 2008, we received the CE-Mark, enabling commercial sales in Europe. In July 2008, we received authorization from the FDA to begin marketing the MXP in the U.S. for the preparation of cell concentrate from bone marrow. | ||
| • | The Res-Q product is also used for bone marrow stem cell processing. Launched in July 2009, the Res-Q can be used in a clinical laboratory or inter-operatively at the point of care. The technology is a next generation, centrifuge-based disposable device designed for the isolation and extraction of specific stem cell populations. Res-Q is a rapid, reliable, and easy-to-use product |
6
Table of Contents
| which achieves a higher recovery rate of stem cells from bone marrow. The key advantages of the Res-Q include (a) delivering a high number of target cells from a small sample of bone marrow, and (b) providing a disposable that is highly portable and packaged for the sterile field. These features allow the physician to process bone marrow and return the cells to the patient in as little as 15 minutes. As cell processing for regenerative medicine applications becomes more readily accepted, we believe the features and benefits of the Res-Q will position the product for broad-based adoption. | |||
| • | The BioArchive System is an automated cryogenic system used to cryopreserve and archive stem cells for future transplant and treatment. Launched in fiscal 1998, over 200 BioArchive Systems have been purchased by over 100 umbilical cord blood banks in over 33 countries to archive, cryopreserve and store stem cell preparations extracted from human placentas and umbilical cords for future use. | ||
| The BioArchive System is designed to store over 3,600 stem cell samples. It is the only fully-automated, commercially available system that integrates controlled-rate freezing, sample management and long term cryogenic storage in liquid nitrogen. The robotic storage and retrieval of these stem cell units improves cell viability, provides precise inventory management and minimizes the possibility of human error. | |||
| • | The ThermoLine™ product line includes the ultra-rapid plasma ThermoLine Freezer and ultra-rapid plasma ThermoLine Thawer. We offer two models of plasma freezers, which vary primarily by capacity and condenser type. The ThermoLine freezer optimizes plasma freezing through its unique liquid heat transfer and uniform freezing technologies that can freeze units of blood plasma in approximately 30 minutes. These products are suited for medium to large laboratories. | ||
| We also offer three models of blood component thawers which vary primarily by capacity. The product’s unique flexible membrane technology allows for a closed thawing system. These instruments can be used for rapid (less than 12 minute) homogeneous thawing of plasma and glycerolized frozen red blood cells. | |||
| • | The CryoSeal System is an automated system serving the wound market used to prepare an autologous hemostatic surgical sealant from a patient’s own blood or from a single donor in approximately one hour. We received a Premarket Approval (“PMA”) to market the CryoSeal in liver resection surgeries in July 2007. On June 16, 2010 we reached an agreement with Asahi in which Asahi paid us $1 million to provide CryoSeal products and clinical support services until such time as Asahi assumes manufacturing of the product line in Japan or December 31, 2012, whichever comes first. As part of the $1 million payment, we granted Asahi an option to acquire the CryoSeal product line, which may be exercised over the next five years. |
7
Table of Contents
Sales and Distribution Channels
During fiscal 2010, we significantly expanded our distribution channels to include more
geographies, products and indications targeted by our products. This expansion took the form of
new distributors, improvements to existing distribution agreements and further leveraging our
channels by granting rights to distribute multiple products. See Item 1. Business/Business
Overview.
The chart below outlines the distribution network for our products.
| Product | Distributor | Region | ||
AXP System
|
GEHC | U.S. and Canada, and approximately 30 countries throughout the world | ||
| CEI | 9 countries in Latin America | |||
| Fenwal, Inc. | China, India, Japan | |||
| Delrus | Russian Federation and CIS countries | |||
| ThermoGenesis | All other countries | |||
Res-Q System
|
Celling Technologies |
• U.S. orthopedic indications: Exclusive |
||
• Outside U.S. orthopedic indications: Non-exclusive |
||||
| GEHC | U.S. (except orthopedics), Canada and 19 European countries | |||
| CEI | 9 countries in Latin America for indications outside of orthopedics | |||
| TotipotentSC | India, Malaysia and Thailand | |||
BioArchive System
|
Fenwal, Inc. | China, India and Japan | ||
| CEI | 5 countries in Latin America | |||
| Additional Distribution Network | 10 Distributors covering over 30 countries | |||
| ThermoGenesis | All other countries including U.S., Canada and Australia | |||
MXP System
|
Celling Technologies |
• U.S. orthopedic indications: Exclusive |
||
• Outside U.S. orthopedic indications: Non-exclusive |
||||
| CEI | 9 countries in Latin America for indications outside of orthopedics | |||
| TotipotentSC | India, Malaysia and Thailand | |||
| ThermoGenesis | All other countries |
8
Table of Contents
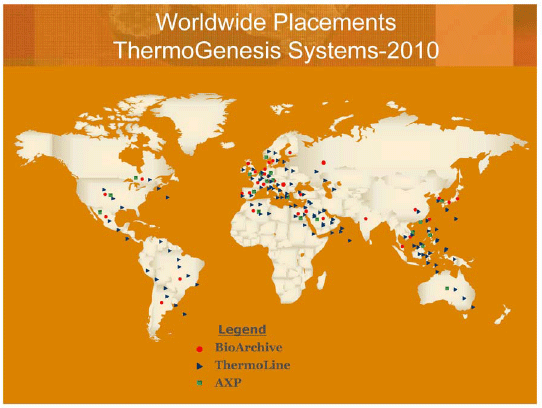
Our sales by geographic region are as follows for the years ended June 30:
| 2010 | 2009 | 2008 | ||||||||||
U.S. |
$ | 13,827,000 | $ | 11,489,000 | $ | 12,901,000 | ||||||
Asia |
4,303,000 | 3,544,000 | 2,125,000 | |||||||||
Europe |
3,117,000 | 2,510,000 | 5,565,000 | |||||||||
South America |
1,405,000 | 1,859,000 | 1,208,000 | |||||||||
Other |
436,000 | 397,000 | 147,000 | |||||||||
| $ | 23,088,000 | $ | 19,799,000 | $ | 21,946,000 | |||||||
9
Table of Contents
Competition
Following are our major competitors, listed by each of our major products and disclosing the
markets in which they currently distribute competing products.
| Competitors/Markets | Area of Focus | Geographic Distribution | ||
| AXP | ||||
BioSafe/Sepax
|
• Processing of cord blood
|
• Direct in Europe, Asia Pacific and
U.S. |
||
BioE/Prepacyte-cb
|
• Modified manual processing of cord
blood
|
• Worldwide via local distribution
networks |
||
| MXP | ||||
BioSafe/Sepax
|
• Laboratory processing of bone
marrow aspirate
|
• Direct in Europe, Asia Pacific and
U.S. |
||
COBE/Spectra
|
• Laboratory processing of bone
marrow aspirate
|
• Worldwide |
||
Ficoll/Paque
|
• Manual processing of bone marrow
aspirate
|
• Worldwide distribution through GEHC |
||
| Res-Q | ||||
Harvest/SmartPReP
|
• Point of care and laboratory
processing of bone marrow aspirate
|
• U.S distribution through Oteotech,
India distribution through LifeCell,
otherwise direct |
||
BioMet/MarrowStem
|
• Point of care processing of bone
marrow aspirate
|
• Direct |
||
| BioArchive | ||||
Chart
|
• Cryopreservation of cells and tissue
|
• Worldwide via local distribution networks |
||
• BioRepository (Storage of cell
lines, primarily in vials) |
||||
• BioPharma (Storage of drugs or
vaccines, primarily in vials) |
||||
• Cord blood banks |
||||
Taylor Wharton
|
• Cryopreservation of cells and
tissue.
|
• Worldwide via local distribution
networks |
||
• BioRepository |
||||
• BioPharma |
||||
• Cord blood banks |
||||
| Thawers | ||||
Helmer
|
• Blood banks, blood centers and
hospital blood banks
|
• Worldwide via Baxter in Europe.
Biotechnology Medical Services in the Middle
East, local distributors and direct |
||
Thermo Fisher Scientific/Cyto Therm |
• Blood banks, blood centers and
hospital blood banks
|
• Worldwide |
||
| Freezers | ||||
Harris
|
• Source plasma companies and
recovered plasma suppliers
|
• Worldwide via local distribution
networks |
||
Jewett
|
• Source plasma companies and
recovered plasma suppliers
|
• Worldwide via local distribution
networks |
||
10
Table of Contents
Scientific Overview
Stem Cells
Stem cells have the remarkable potential to develop into many different cell types and serve as a
repair system for the body. They can theoretically divide without limit to replenish other cells
as long as the person or animal is alive. When a stem cell divides, each new cell has the
potential to either remain a stem cell or become another type of cell with a more specialized
function, such as a muscle, a red blood, or a brain cell.
There are two main types of stem cells: embryonic and adult. An embryonic stem cell is a primitive
cell derived from a 5-day pre-implantation embryo that has the potential to become a cell from a
wide variety of specialized cell types. Adult stem cells are found in human tissue and can renew
and differentiate themselves to yield the major specialized cell types of that tissue. Adult stem
cells are thought to reside in a specific area of each tissue where they may remain non-dividing
for many years until they are activated by disease or tissue injury. Our current products address
therapies with adult stem cells only. Initially, researchers’ greatest hope was for stem cells
derived from embryos. However, while embryonic stem cell therapies may offer great promise, it is
unlikely that embryonic stem cell treatments will be marketed for serious conditions for many
years.
It is reported that stem cells can be found in umbilical cord blood, bone marrow, brain, peripheral
blood, fat, blood vessels, amniotic fluid, skeletal muscle, skin, placenta, menstrual blood and
liver. Our products have been used in cord blood and bone marrow applications. The technologies
we are using for bone marrow also have application in PRP procedures. We are currently evaluating
opportunities for our technology in the adipose (fat tissue) stem cell market.
Stem Cell Therapy
Adult stem cell research is primarily focused on the isolation, characterization, purity,
plasticity and clinical uses of adult-derived, pluripotent stem cells from a variety of human
tissues. There are two principal types of adult stem cells being investigated today for medical
application: hematopoietic stem cell and mesenchymal stem cells. Hematopoietic adult stem cells
are capable of restoring the ability of a patient’s bone marrow to produce healthy blood cells and
are routinely used in the treatment of cancer. Mesenchymal stem cells are being intensively
investigated for their ability to promote healing of tissues by modulation of inflammatory and
immunologic responses and by promoting the growth of blood vessels in ischemic (blood restricted)
tissues.
Stem cell therapies include:
| • | Regenerating of bone marrow damaged by high-dose chemotherapy or radiation therapy used to treat patients with a variety of cancers such as leukemia and lymphoma; | ||
| • | Providing genetically healthy and functioning bone marrow to treat patients with more than 60 life threatening genetic diseases such as sickle cell anemia and immunodeficiency; and | ||
| • | Regenerating and repairing of tissue including the treatment of myocardial infarction, peripheral limb ischemia and non-union bone fractures. |
Perhaps the most important potential application of human stem cells is the generation of cells and
tissues that could be used for new regenerative medicine cell-based therapies. Today, donated
organs and tissues are often used to replace ailing or destroyed tissue, but the need for
transplantable tissues and organs far outweighs the available supply. Directed to differentiate
into specific cell types, stem cells offer the
11
Table of Contents
possibility of a renewable source of replacement cells and tissues that could possibly treat
conditions including spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis,
and rheumatoid arthritis.
Stem cell processing techniques are also capable of processing other cells including PRP for use in
orthopedics, cardio, dental and sports-related therapies.
Clinical Evaluations
We have the following Clinical Evaluations underway:
| Product | Indication | Purpose | Status | |||||
Celling Technologies/ Spine Smith |
Res-Q | Spinal Fusion | Full outcome, prospective 30 patient study, one year follow-up. Purpose of study is comparing the efficacy of three different concentrations of MNCs in spinal fusion | The study is ready to begin | ||||
TotipotentSC
|
Res-Q | Critical Limb Ischemia (“CLI”) /Peripheral Artery Disorder (“PAD”) | Pilot 15 patient limb salvage study, one year follow-up. Purpose is to establish Res-Q safety/efficacy for CLI | Study to start September 2010 | ||||
UC Davis
|
Res-Q | Non-Union Fractures | 30 patient study, one year follow-up, to establish efficacy for Non-union fractures | Study to start September 2010 | ||||
Naples
|
MXP | CLI | 30 patient study, one year follow-up to establish efficacy of bone marrow treatment for CLI | 15 patients enrolled. 9 patients completed 2-injection treatment. 4 patients had six month evaluation. |
Research and Development
Our research and development activities are focused principally on the development of new products
that serve the regenerative medicine market and on significant upgrades to our existing products.
Specific activities in fiscal 2010 included launching our Res-Q product for bone marrow
applications and development of enhanced versions of our AXP device and companion disposable bag
set. Activities planned for fiscal 2011 include evaluation of our current technologies for PRP,
further completion of AXP second generation product enhancements and the development of a new
BioArchive control box to avoid obsolescence issues. Research and development expense reflects the
cost of these activities, as well as the costs to obtain regulatory approvals of new products and
processes and to maintain the highest quality standards with respect to existing products. We have
no customer-sponsored research and development expense.
12
Table of Contents
Manufacturing
Our long-term manufacturing strategy is to utilize high quality, low cost contract manufacturers to
provide the routine production of our products. The Company outsources the manufacture of the
majority of our disposable products. During fiscal 2010, we added an in-house pilot manufacturing
capability to ensure that initial production scale-up batches meet product requirements and
manufacturability standards. Also, we completed the manufacturing transfer of all ThermoLine
products to our contract manufacturer. Once the transfer was complete, we initiated cost reduction
efforts in coordination with the contract manufacturer. We continue to manufacture or assemble all
of our other instruments and medical devices. It is our intent to transition substantially all our
device manufacturing to contract manufacturers over the next few years. In conjunction with our
outsourcing efforts and the eventual divestiture of our surgical wound care business, we expect to
streamline our supply chain and improve our order fulfillment process through vendor consolidation
and third party logistics providers.
The majority of the raw materials used to produce the Company’s products are readily available from
a variety of sources and, as such, the Company does not anticipate any shortage of supply. In the
event it becomes necessary to obtain raw materials from a new supplier, we would first be required
to qualify the quality systems and product of that alternative supplier.
Quality Strategy
Our quality strategy is now based on five key tenets:
| • | Doing things right the first time; | ||
| • | Meeting customer expectations; | ||
| • | Continuous improvement; | ||
| • | Maintaining supplier controls; and | ||
| • | Ensuring quality by design. |
We embrace the notion that quality must be a key component of everything we do. Our management and
employees are measured and evaluated based on our quality performance. The foundation of our
quality strategy is our “quality system.”
Our quality system is based on a process approach to quality management. Any activity that
receives inputs and converts them to outputs is considered a quality process. We have identified
and currently manage numerous linked processes. Often the output from one process directly forms
the input to the next. Our quality system defines the parameters under which we conduct our
business. The quality system embeds our quality policy and describes how we will consistently
provide our customers with products and services that meet their expectations. Our quality system,
and the obligations defined within it, is applicable to all our suppliers, operating facilities,
and functions. The principles embodied in our quality system are viewed worldwide as a means of
ensuring our products are produced in an acceptable manner.
Our quality system has been created to be harmonized with domestic and international standards and
is focused to ensure it is appropriate for the specific devices we manufacture. Our corporate
quality policies govern the methods used in, and the facilities and controls used for, the design,
manufacture, packaging, labeling, storage, installation, and servicing of all finished devices
intended for human use. These requirements are intended to ensure that finished devices will be
safe and effective and otherwise in compliance with the Federal Food, Drug, and Cosmetic Act
administered by the FDA and the applicable rules of other governmental agencies.
13
Table of Contents
We, as well as any contract manufacturers of our products, are subject to inspections by the FDA
and other regulatory agencies for compliance with applicable regulations, codified in the Quality
System Regulations (21 CFR 820) (“QSR”) which include requirements relating to manufacturing
processes, extensive testing, control documentation and other quality assurance procedures. Our
facilities have undergone International Organization of Standards (“ISO”) 13485:2003 and European
Union Medical Device Directive (93/42/EEC) (“MDD”) inspections and we have obtained approval to
CE-Mark our products. UL/CSA approval has also been obtained for our CryoSeal, BioArchive, MXP and
AXP products. We have obtained the CE-Mark for the Res-Q product line. Failure to obtain or
maintain necessary regulatory approvals to market our products would have a material adverse impact
on our business.
Over the last year, we improved our design process to better satisfy customer requirements and
bolster validation and verification of design outputs. We enhanced how we select and monitor our
supplier base to ensure components and products meet all relevant specifications.
Regulatory Strategy
Our regulatory strategy is to be involved in selective clinical programs that can generate data
that will help fuel adoption of our product offerings. We have a quality and regulatory compliance
management system that complies with the requirements of the ISO 13485: 2003 standard, the FDA’s
QSR, the European Union MDD, the Canadian Medical Device Regulations (“SOR 98-282”), and other
applicable local, state, national and international regulations.
Our medical devices are subject to regulation by numerous government agencies, including the FDA
and comparable foreign agencies. To varying degrees, each of these agencies requires us to comply
with laws and regulations governing the development, testing, manufacturing, labeling, marketing,
distribution, installation and servicing of our research, investigational, and
commercially-distributed medical devices. These international, national, state, and local agencies
set the legal requirements for ensuring our products are safe and effective. Virtually every
activity associated with the manufacture and sale of our products and services are scrutinized on a
defined basis and failure to implement and maintain a Quality Management System could subject the
Company to civil and criminal penalties.
Class III Devices
Before certain medical devices may be marketed in the U.S., they must be approved by the FDA. FDA
approval depends on the classification of the device. If the product is a Class III device, such
as the CryoSeal System, the FDA approval process includes the following:
| • | Extensive pre-clinical laboratory and animal testing; | |
| • | Submission and approval of an Investigational Device Exemption (“IDE”) application; | |
| • | Human clinical trials (Phase III) to establish the safety and efficacy of the medical device for the intended indication; and | |
| • | Submission and approval of a PMA to the FDA. |
Pre-clinical trials include laboratory evaluation, through in vitro and in vivo animal studies, to
obtain safety and dosage information about the product to justify future clinical trials in human
subjects. Safety testing is performed to demonstrate the biocompatibility of the device,
particularly if the device is intended to come into contact with blood or other body tissues.
Pre-clinical studies must be performed by laboratories which comply with the FDA’s Good Laboratory
Practices regulations. The results of the pre-clinical studies are submitted to the FDA as part of
an IDE application and are reviewed by the FDA before human clinical trials can begin.
14
Table of Contents
Clinical trials involve the application of the medical device or biologic produced by the medical
device to patients by a qualified medical investigator, after approval from an Institutional Review
Board (“IRB”). Clinical trials are conducted in accordance with FDA Good Clinical Practice
regulations, standards developed by the International Conference on Harmonization (“ICH”), and an
approved study protocol that details the objectives of the study, the parameters to be used to
monitor participant safety and effectiveness of the product, or other criteria to be evaluated.
Each protocol is submitted to the FDA as part of the IDE and each clinical study is conducted only
after the approval of the IRB. The IRB considers, among other things, ethical factors, the
potential risks to subjects participating in the trial, and the possible liability of the
institution. The IRB also approves the consent form signed by the study participants.
Medical device clinical trials are typically conducted as a Phase III clinical trial. A Phase II
safety pilot trial may be performed prior to initiating the Phase III clinical trial to determine
the safety of the product for specific targeted indications or dosage optimization studies. The
FDA, the clinical trial sponsor, the investigators or the IRB may suspend clinical trials at any
time if any one of them believes that study participants are being exposed to an unacceptable
health risk.
The combined results of product development, pre-clinical studies, and Phase III clinical studies
are submitted to the FDA as a PMA for approval of the marketing and commercialization of the
medical device in the U.S. The FDA may deny the approval of a PMA if applicable regulatory
criteria are not satisfied or it may require additional clinical testing. Even if the appropriate
data is submitted, the FDA may ultimately decide the PMA does not satisfy the criteria for
approval. Product approvals, once obtained, may be withdrawn if compliance with regulatory
standards is not maintained or if safety concerns arise after the product reaches the market. The
FDA may require post-marketing testing and surveillance programs to monitor the effect of the
medical devices that have been commercialized and has the power to prevent or limit future
marketing of the product based on the results of such programs.
Class II Devices
Several of our medical devices, such as the BioArchive and AXP are categorized as Class II. These
devices have a lower potential safety risk to the patient, user, or caregiver. A PMA submission is
not a requirement for these devices. A similar (but simpler and shorter) process of premarket
notification, known as a 510(k) submission, is required to demonstrate that the device is as safe
and effective as a substantially equivalent medical device that has been legally marketed in the
U.S. prior to May 29, 1976. Once the FDA has notified the Company that the product file has been
cleared, the medical device may be marketed and distributed in the U.S.
Class I Devices
Some of our products, such as MXP and Res-Q that have minimal risk to the intended user are deemed
by the FDA as being exempt from FDA approval or clearance processes. While submissions to the
Agency are not a requirement for these Class I (low risk) devices, compliance with the QSR is still
mandated.
Other U.S. Regulatory Information
Failure to comply with applicable FDA requirements can result in fines, injunctions, civil
penalties, recall or seizure of products, total or partial suspension of production or loss of
distribution rights. It may also include the refusal of the FDA to grant approval of a PMA or
clearance of a 510(k). Actions by the FDA may also include withdrawal of marketing clearances and
possibly criminal prosecution. Such actions, if taken by the FDA, could have a material adverse
effect on the Company’s business, financial condition, and results of operation.
In February of 2008, the Company initiated a voluntary recall for its AXP processing bag sets. The
product was recalled as a result of the omission of endotoxin testing during lot release testing.
The
15
Table of Contents
Company developed a process, with the approval of the FDA and other regulatory agencies, to assess
each manufactured lot and perform the appropriate testing. To date, all but one lot have been
“reconditioned” through the testing process and samples remain to be obtained for the final lot
before the recall can be completed and closed. Although this recall remains open a recall closure
request has been submitted to the FDA. FDA response to our recall closure request is pending.
Each manufacturing establishment must be registered with the FDA and is subject to a biennial
inspection for compliance with the Federal Food, Drug, and Cosmetic Act and the QSRs. In addition,
each manufacturing establishment in California must be registered with the California State Food
and Drug Branch of the California Department of Public Health and be subject to an annual
inspection by the State of California for compliance with the applicable state regulations.
Companies are also subject to various environmental laws and regulations, both within and outside
the U.S. Our operations involve the use of substances regulated under environmental laws,
primarily manufacturing and sterilization processes. Workplace safety, hazardous material, and
controlled substances regulations also govern our activities. The Company has a California
Environmental Protection Agency Identification number for the disposal of biohazardous waste from
its research and development biological lab.
International Regulatory Requirements
Internationally, we are required to comply with a multitude of other regulatory requirements. To
legally market our medical devices in Canada, for example, we fall under the auspices of Health
Canada and the Canadian Medical Device Regulations. Health Canada reviews medical devices to
assess their safety, effectiveness, and quality before allowing them to be authorized for sale in
Canada. The Therapeutic Products Directorate (“TPD”) undertakes a variety of activities, including
the promulgation of policies and regulations to support its role as the federal regulatory
authority for the sale of medical devices in Canada. In Canada, manufacturers must receive a
medical device license for certain health products defined as a “device” under the Canadian Food
and Drugs Act before they can be sold on the Canadian market. To determine which devices need a
license, medical devices are categorized based on the risks associated with their use. Prior to
selling a device in Canada, manufacturers of Class II, III and IV devices must obtain a medical
device license. Although Class I devices do not require a license, manufacturers, distributors,
and importers are required to obtain an establishment license. Health Canada requires medical
device manufacturers to use a quality system certificate as evidence of compliance to the
appropriate regulatory quality system requirement and Health Canada will only accept quality system
certificates that have been issued by special third party recognized auditing organizations
(registrars) under the Canadian Medical Devices Conformity Assessment System (“CMDCAS”). The
Medical Devices Regulations require class II, III and IV medical devices to be designed and/or
manufactured under ISO 13485:2003.
In the European Union, a single regulatory approval process has been created and approval is
represented by the CE-Mark. To be able to affix the CE-Mark to our medical devices and distribute
them in the European Union, we must meet minimum standards for safety and quality (known as the
essential requirements) and comply with one or more conformity rules. A Notified Body assesses our
quality management system and compliance to the MDD.
To be sold in Japan, most medical devices must undergo thorough safety examinations and demonstrate
medical efficacy before they can be granted approval (known as “shonin”). The Japanese government,
through the Ministry of Health, Labor, and Welfare (“MHLW”) regulates medical devices under the
Pharmaceutical Affairs Law (“PAL”).
16
Table of Contents
Patents and Proprietary Rights
The Company believes that patent protection is important for products and potential segments of its
current and proposed business. In the U.S., the Company currently holds 22 patents, and has three
patents pending to protect the designs of products which the Company intends to market. There can
be no assurance, however, as to the breadth or degree of protection afforded to the Company or the
competitive advantage derived by the Company from current patents and future patents, if any.
Although the Company believes that its patents and the Company’s existing and proposed products do
not infringe upon patents of other parties, it is possible that the Company’s existing patent
rights may be challenged and found invalid or found to violate proprietary rights of others. In
the event any of the Company’s products are challenged as infringing, the Company would be required
to modify the design of its product, obtain a license or litigate the issue. There is no assurance
that the Company would be able to finance costly patent litigation, or that it would be able to
obtain licenses or modify its products in a timely manner. Failure to defend a patent infringement
action or to obtain a license or implementation of modifications would have a material adverse
effect on the Company’s continued operations.
While patents have been issued or are pending, the Company realizes, (a) that the Company will
benefit from patents issued only if it is able to market its products in sufficient quantities of
which there is no assurance; (b) that substitutes for these patented items, if not already in
existence, may be developed; (c) that the granting of a patent is not a determination of the
validity of a patent, such validity can be attacked in litigation or the Company or owner of the
patent may be forced to institute legal proceedings to enforce validity; and (d) that the costs of
such litigation, if any, could be substantial and could adversely affect the Company.
Licenses and Distribution Rights
Asahi
In June 2010, the Company and Asahi entered into an amendment (the “Amendment”) of their
Distribution and License Agreement, originally effective March 28, 2005. Under the terms of the
Amendment, Asahi will obtain exclusive rights to distribute the CryoSeal System (“Products”) in
South Korea, North Korea, Taiwan, the Peoples Republic of China, the Philippines, Thailand,
Singapore, India and Malaysia. These rights shall include the exclusive right to market, distribute
and sell the processing disposables and Thrombin Reagent for production of thrombin in a stand
alone product. The Company will provide support to Asahi in the form of maintaining manufacturing
capabilities of the CryoSeal System products until the earlier of when Asahi receives regulatory
approval from the MHLW or December 31, 2012, upon which the Company shall have no further
obligation to manufacture the Products. Asahi shall continue to have the right to manufacture such
Products in Japan and shall additionally have a non-exclusive right to manufacture such Products
outside of Japan and would make royalty payments to the Company for Products it manufactures and
sells. The Amendment extends the agreement eight years with automatic one year renewals. Asahi
paid us a $1,000,000 license fee, of which $400,000 is refundable if the Company fails to provide
technical support or maintain manufacturing capabilities as specified in the Amendment.
In connection with the above-described Amendment, the Company and Asahi also entered into an Option
Agreement (“Option Agreement”). Under the terms of the Option Agreement, the Company granted Asahi
an option to purchase certain intellectual property rights of the Company related to the CryoSeal
System, including, but not limited to, patents and patent applications, FDA-PMA ownership relating
to the products and certain related contracts and contractual relationships. Asahi may exercise the
Option Agreement at any time after the effective date of the Amendment, but no later than the
earlier of the fifth anniversary of the Amendment or 90 days after receiving regulatory approval
from the MHLW.
17
Table of Contents
Cord Blood Registry Systems, Inc. (“CBR”)
In June 2010, the Company and CBR entered into a License and Escrow Agreement as a method to
provide assurances to CBR of continuity of product delivery and manufacturing for CBR’s business,
and to alleviate concerns about long term supply risk. We are the sole provider for CBR of devices
and disposables used in the processing of cord blood samples in CBR’s operations. Under the
agreement, the Company granted CBR a non-exclusive, royalty-free license to certain intellectual
property necessary for the potential manufacture and supply of AXP devices and certain AXP
disposables. The license is for the sole and limited purpose of manufacturing and supplying the
AXP and related disposables for use by CBR. The licensed intellectual property will be maintained
in escrow and will be released to and used by CBR if and only if the Company defaults under the
Agreement. Default occurs if the Company (1) fails to meet certain positive cash flow metrics for
each rolling quarterly measurement period beginning December 31, 2010, except where the following
two measures are met, (2) failure to meet cash balance and short-term investments of at least $6
million at the end of any given month, or (3) failure to meet a quick ratio of 2 to 1 at the end of
any given month.
GEHC
In May 2010, the Company and GEHC signed a non-exclusive distribution agreement for the Res-Q
System. Under the agreement, GEHC has the right to distribute the Res-Q in the U.S. excluding
orthopedic indications, Canada and 19 European countries. The agreement has a two and a half year
term, with automatic one year renewals, unless terminated by either party with six months advance
notice. The Agreement provides for a price reduction mechanism should the Company fail to meet
certain product quality and delivery metrics.
In January 2010, the Company and GEHC also signed an amendment (the “Amendment”) of their Amended
and Restated International Distribution Agreement, effective February 1, 2010. Under the terms of
the Amendment, the initial term runs through July 31, 2012, GEHC will continue to distribute the
AXP product line in the United States, Canada and approximately 30 countries throughout the world,
excluding certain countries in Latin America, Asia, CIS, Eastern Europe and the Middle East. The
parties will implement a joint operating committee to oversee, review and coordinate marketing and
sales activities and performance of the parties. GEHC will provide incremental funding for
marketing support and market research beyond its previous commitments. The Amendment provides
incentives for both parties related to sales success, product quality and delivery. The Amendment
will automatically renew for one year terms unless terminated at least six months prior to the end
of the then current term. Under the original agreement, signed October 13, 2005, the Company
received fees for the rights granted under the agreement. The amounts received are being
recognized as revenue on the straight-line method over the initial five year term of the contract.
Fenwal
In March 2010, the Company and Fenwal, Inc. signed a five year distribution agreement. Under the
agreement, Fenwal will have exclusive rights to market and distribute the AXP System and BioArchive
System for use in cord blood processing and storage in China, India and Japan.
Celling Technology
In September 2008, the Company and Celling Technology signed a distribution agreement for the
Company’s MXP and Res-Q product lines. The distribution rights are for the field of use in
orthopedic intraoperative or point of care applications. The five year agreement provides Celling
with an initial two year period of exclusive distribution rights in the U.S. and non-exclusive
distribution rights throughout the rest of the world, excluding Central and South America, Russia
and certain Eastern European countries. The exclusivity period and field of use may be extended
under certain circumstances. The parties amended the agreement on July 29, 2009 to provide shared
funding for clinical studies to demonstrate the clinical effectiveness of the products in
orthopedic applications.
18
Table of Contents
CBR
On August 22, 2006, the Company announced that GEHC and CBR, the world’s largest family cord blood
bank, signed a multi-year contract to supply CBR with the Company’s AXP Platform and disposables.
In conjunction with this agreement, the Company signed a Product Development and Supply Assurance
Agreement with CBR which assures the supply of AXP products for a 15 year period. This agreement
also initiates the development of an advanced cord blood stem cell container.
Biomet
In July 2006, the Company entered into a Product Development and Supply Agreement with Biomet.
Under the development phase of this agreement, Biomet paid the Company $1.1 million in milestone
payments to develop a fibrinogen concentration kit. The Company will grant intellectual property
license rights to Biomet and its affiliates to manufacture, use and sell the product for use in
surgical hemostats, graft delivery systems and surgeries. The Company has the right of first offer
to manufacture the product; and if the Company does not manufacture the product, Biomet will pay a
royalty. The agreement has a term of five years.
New York Blood Center (“NYBC”)/Pall Medical
In March 1997, the Company and NYBC, as licensors, entered into a license agreement with Pall
Medical, a subsidiary of Pall Corporation, as a Licensee through which Pall Medical became the
exclusive worldwide manufacturer (excluding Japan) for a system of sterile, disposable containers
developed by the Company and NYBC for the processing of hematopoietic stem cells sourced from
placental cord blood (“PCB”). The system is designed to simplify and streamline the harvesting of
stem cells from umbilical cord blood and the manual concentration, cryopreservation (freezing) and
transfusion of the PCB stem cells while maintaining the highest stem cell population and viability
from each PCB donation. In May 1999, the Company and Pall Medical amended the original agreement,
and the Company regained the rights to distribute the bag sets outside North America and Europe
under the Company’s name, and in May 2000, the Company negotiated rights to directly co-market the
bag sets in Europe in exchange for an additional royalty fee, while continuing to utilize Pall
Europe’s distribution centers.
Backlog
Our backlog was $600,000 and $500,000 as of June 30, 2010 and 2009, respectively. Our backlog
consists of product orders for which a customer purchase order has been received and is scheduled
for shipment within the next twelve months. Orders are subject to cancellation or rescheduling by
the customer, sometimes with a cancellation charge. Due to timing of order placement, product lead
times, changes in product delivery schedules and cancellations, and because sales will often
reflect orders shipped in the same quarter received, our backlog at any particular date is not
necessarily indicative of sales for any succeeding period.
Employees
As of June 30, 2010, the Company had 76 employees, 31 of whom were engaged in manufacturing and
quality control, 19 in research and new product development, regulatory affairs, clinical and
scientific affairs, 12 in sales, marketing and customer service, and 14 in administration. The
Company also utilizes temporary employees throughout the year to address business needs and
significant fluctuations in orders and product manufacturing. None of our employees are
represented by a collective bargaining agreement, nor have we experienced any work stoppage.
19
Table of Contents
FOREIGN SALES AND OPERATIONS
For fiscal year 2010, foreign sales were $9,261,000 or 40% of net revenues. For fiscal year 2009,
foreign sales were $8,310,000 or 42% of net revenues. For fiscal year 2008, foreign sales were
$9,045,000 or 41% of net revenues.
Our second-source bag set supplier manufactures the AXP bag sets in Costa Rica.
WHERE YOU CAN FIND MORE INFORMATION
The Company is required to file annual reports on Form 10-K, quarterly reports on Form 10-Q,
current reports on Form 8-K and other information with the Securities and Exchange Commission
(“SEC”). The public can obtain copies of these materials by visiting the SEC’s Public Reference
Room at 100 F Street, NE, Room 1580, Washington, DC 20549, by calling the SEC at 1-800-732-0330, or
by accessing the SEC’s website at http://www.sec.gov. In addition, as soon as reasonably
practicable after these materials are filed with or furnished to the SEC, the Company will make
copies available to the public free of charge through its website, www.thermogenesis.com. The
information on the Company’s website is not incorporated into, and is not part of, this annual
report.
ITEM 1A. RISK FACTORS
An investment in ThermoGenesis’ common stock is subject to risks inherent to our business. The
material risks and uncertainties that management believes affect us are described below. Before
making an investment decision, you should carefully consider the risks and uncertainties described
below together with all of the other information included or incorporated by reference in this
report. The risks and uncertainties described below are not the only ones facing ThermoGenesis.
Additional risks and uncertainties that management is not aware of or focused on or that management
currently deems immaterial may also impair ThermoGenesis’ business operations. This report is
qualified in its entirety by these risk factors.
If any of the following risks actually occur, our financial condition and results of operations
could be materially and adversely affected. If this were to happen, the value of our common stock
could decline significantly, and you could lose all or part of your investment.
Risks Related to Our Business
Our New Products Are at Initial Market Introduction, and We Are Not Sure the Market Will Accept
Them. The market acceptance of our new products will depend upon the medical community and
third-party payers accepting the products as clinically useful, reliable, accurate, and cost
effective compared to existing and future products or procedures. Market acceptance will also
depend on our ability to adequately train technicians on how to use the MXP and Res-Q Systems and
future products. Even if our new products are released for sale, their use may not be recommended
by the medical profession or hospitals unless acceptable reimbursement from healthcare and third
party payers is available. Failure of these new products to achieve significant market share could
have material adverse effects on our long term business, financial condition, and results of
operation.
A Significant Portion of our Revenue is Derived from Customers in Foreign Countries. We may Lose
Revenues, Market Share, and Profits due to Exchange Rate Fluctuations, Political and Economic
Changes related to our Foreign Business. In the year ended June 30, 2010, sales to customers in
foreign countries comprised approximately 40% of our revenues. This compares to 42% in fiscal
2009. Our foreign business is subject to economic, political and regulatory uncertainties and
risks that are unique to each area of the world. Fluctuations in exchange rates may also affect
the prices that our foreign customers are willing to pay, and may put us at a price disadvantage
compared to other competitors. Potentially volatile shifts in exchange rates may negatively affect
our financial position and results.
20
Table of Contents
Outcomes of Pending or Future Clinical Trials May be Negative and the Regenerative Medicine Market
May not Expand, or May Not Expand in the Areas Targeted by our Products. The marketing and sales
of new products may depend on successful clinical trial outcomes in the regenerative medicine areas
targeted by our products and the approval of regulators. Clinical trials also represent a
significant expenditure of resources. Negative clinical trial results in connection with our
products or in the areas targeted by the Company could negatively impact regulatory approval or
market acceptance of our products. Failure to attain successful clinical trials, to obtain
regulatory approval, or to target areas with successful clinical trials could have material adverse
effects on our long term business, financial condition, and results of operation
Risks Related to Our Operations
Our Inability to Protect Our Patents, Trademarks, Trade Secrets and Other Proprietary Rights could
Adversely Impact Our Competitive Position. We believe that our patents, trademarks, trade secrets
and other proprietary rights are important to our success and our competitive position.
Accordingly, we devote substantial resources to the establishment and protection of our patents,
trademarks, trade secrets and proprietary rights. We use various methods, including
confidentiality agreements with employees, vendors, and customers, to protect our trade secrets and
proprietary know-how for our products. We currently hold patents for products, and have patents
pending for additional products that we market or intend to market. However, our actions to
establish and protect our patents, trademarks, and other proprietary rights may be inadequate to
prevent imitation of our products by others or to prevent others from claiming violations of their
trademarks and proprietary rights by us. If our products are challenged as infringing upon patents
of other parties, we may be required to modify the design of the product, obtain a license, or
litigate the issues, all of which may have an adverse business effect on us.
Any Failure to Achieve and Maintain the High Design and Manufacturing Standards that our Products
Require may Seriously Harm our Business. Our products require precise, high-quality manufacturing.
Achieving precision and quality control requires skill and diligence by our personnel as well as
our vendors. Our failure to achieve and maintain these high manufacturing standards, including the
incidence of manufacturing errors, design defects or component failures, could result in patient
injury or death, product recalls or withdrawals, delays or failures in product testing or delivery,
cost overruns or other problems that could seriously hurt our business. Additionally, the large
amount of AXP disposable inventory certain distributors and end-users maintain may delay the
identification of a manufacturing error and expand the financial impact. A manufacturing error or
defect, or previously undetected design defect, or uncorrected impurity or variation in a raw
material component, either unknown or undetected, could affect the product. Despite our very high
manufacturing standards, we cannot completely eliminate the risk of errors, defects or failures. If
we or our vendors are unable to manufacture our products in accordance with necessary quality
standards, our business and results of operations may be negatively affected.
We are Dependent on our Suppliers and Manufacturers to Meet Existing Regulations. Certain of our
suppliers and manufacturers are subject to heavy government regulations, including FDA QSR
compliance, in the operation of their facilities, products and manufacturing processes. Any
adverse action by the FDA against our suppliers or manufacturers could delay supply or manufacture
of component products required to be integrated or sold with our products. There are no assurances
we will be successful in locating an alternative supplier or manufacturer to meet product shipment
or launch deadlines. As a result, our sales, contractual commitments and financial forecasts may
be significantly affected by any such delays.
21
Table of Contents
Dependence on Suppliers for Disposable Products and Custom Components May Impact the Production
Schedule. The Company obtains certain disposable products and custom components from a limited
number of suppliers. If the supplier raises the price or discontinues production, the Company may
have to find another qualified supplier to provide the item or re-engineer the item. In the event
that it becomes necessary for us to find another supplier, we would first be required to qualify
the quality assurance systems and product quality of that alternative supplier. Any operational
issues with re-engineering or the alternative qualified supplier may impact the production
schedule, therefore delaying revenues, and this may cause the cost of disposables or key components
to increase.
Our Products May Be Subject to Product Recalls which May Harm Our Reputation and Divert Our
Managerial and Financial Resources. The FDA and similar governmental authorities in other
countries have the authority to order the mandatory recall of our products or order their removal
from the market if the governmental entity finds our products might cause adverse health
consequences or death. The FDA may also seize product or prevent further distribution. A
government-mandated or voluntary recall by us could occur as a result of component failures,
manufacturing errors or design defects (including labeling defects). In the past we have initiated
voluntary recalls of some of our products and we could do so in the future. Any recall of our
products may harm our reputation with customers, divert managerial and financial resources and
negatively impact our profitability.
Quality Problems with our Products or Processes could Harm our Reputation for Producing High
Quality Products and Decrease our Future Revenues. Quality is extremely important to us and to our
customers due to the consequences of product failure. Our quality certifications and product
performance during evaluations and validations are critical to the marketing success of our
products. If we fail to meet our customer’s quality standards our reputation could be damaged. We
could lose current and potential customers and our future revenues could decline as a result.
All of our Operations are Conducted at a Single Location. Any Disruption at our Facility could
Delay Revenues or Increase our Expenses. All of our operations are conducted at a single location
although we contract the manufacturing of certain devices, disposables and components. We take
precautions to safeguard our facility, through insurance, health and safety protocols, and off-site
storage of computer data. However, a natural disaster, such as a fire, flood or earthquake, could
cause substantial delays in our operations, damage or destroy our manufacturing equipment or
inventory, and cause us to incur additional expenses. The insurance we maintain against fires,
floods, and other natural disasters may not be adequate to cover our losses in any particular case.
We are Heavily Reliant on a Single Distributor to Market and Sell our AXP Products. GEHC is the
primary distributor of the AXP Platform. We have limited control over their sales and marketing
efforts for these products. Although we have added distributors in other territories, we must
manage our distribution network effectively. Since the AXP Platform products are a significant
portion of our revenues and projected revenue growth, a delay or failure by our distributor to
successfully market these products may decrease our future revenues and competitive advantage.
We are Heavily Reliant on a Single Distributor to Market and Sell our MXP and Res-Q Products.
Currently, Celling Technologies is the primary distributor of our MXP and Res-Q products. For
orthopedic applications, Celling Technologies has exclusive distribution rights in the U.S. and
non-exclusive rights in the rest of the world. Although we have added distributors in other
territories and for other indications, we must manage our distribution network effectively to gain
additional revenue and gross profit. We have limited control over our distributor’s sales and
marketing efforts for these products. A delay or failure by our distributors to successfully
market these products may decrease our revenues and competitive advantage.
22
Table of Contents
Our Business is Indirectly Subject to Customer and Distributor Inventory Requirements and
Continuity of Inventory Purchasing. Our end user customers may have separate agreements with our
distributors that
require them to hold a certain level of inventory. Similarly, other customers have historically
purchased ahead of their utilization to insure growth within their business, particularly for the
processing of stem cells. Given the tightening of credit and other financial constraints,
including possible downturns in collection and processing for cord blood, our customers could
reduce the amount of inventory levels our distributors hold, or which they hold internally in lieu
of new purchases. If that were to occur, sales of our products could decline significantly, which
would have a material adverse effect on our financial performance in any period where such events
occur.
Failure to Meet the Quality and Delivery Metrics specified in the GEHC Distribution Agreements
could Decrease our Revenues. Under the AXP and Res-Q distribution agreements with GEHC, if we fail
to meet certain quality and delivery metrics, the price paid by GEHC will be reduced in the
following quarter. If this were to occur, our revenues would be negatively impacted.
Failure to Meet Certain Financial Covenants could Decrease our AXP Revenues. Under the CBR license
and escrow agreement, if we fail to meet certain financial covenants, CBR may take possession of
the escrowed intellectual property and initiate manufacturing of the AXP device and disposables for
their own use. If this were to occur, our revenues would be negatively impacted.
Failure to Retain or Hire Key Personnel May Adversely Affect Our Ability to Sustain or Grow Our
Business. Our ability to operate successfully and manage our potential future growth depends
significantly upon retaining key research, technical, clinical, regulatory, sales, marketing and
managerial personnel and attracting and retaining highly qualified personnel in these areas. Our
future success partially depends upon the continued services of key technical and senior management
personnel. Our future success also depends on our continuing ability to attract, retain and
motivate highly qualified managerial and technical personnel. The inability to retain or attract
qualified personnel could have a significant negative effect upon our efforts and thereby
materially harm our business and future financial condition.
Risks Related to Operating Results and Financial Markets
We Have Incurred Net Losses since Our Inception and Losses May Continue. Except for net income of
$11,000 for fiscal 1994, we have not been profitable since our inception. For the fiscal year
ended June 30, 2010, we had a net loss of $5,193,000 and an accumulated deficit at June 30, 2010,
of $103,552,000. We will continue to incur significant costs as we develop and market our current
products and related applications. Although we are executing on our business plan to develop and
market launch new products, continuing losses may impair our ability to fully meet our objectives
for new product sales.
We May Need to Raise Additional Capital in the Future to Fund Our Operations. We May be Unable to
Raise Funds When Needed or on Acceptable Terms. During the year ended June 30, 2010, our operating
activities used cash of $4,428,000. As of June 30, 2010, we had a cash balance of $10,731,000.
Based on our cash balance, historical trends, planned cost reductions and future revenue
projections, we believe our current funds are sufficient to provide for our projected needs to
maintain operations and working capital requirements for at least the next 12 months. However, if
actual sales do not meet expectations, or product development, marketing and production costs
increase significantly, we may need to seek additional financing beyond the next 12 months. We may
also raise money for strategic initiatives which may be dilutive. Any additional equity financings
may be dilutive to our existing stockholders.
The Continuing Crisis in the U.S. and World Financial and Securities Markets Could Have a Material
Adverse Effect on our Customers’ Business and Effect our Operations and Revenues. Our products are
purchased by cord blood banks and hospitals. We believe these entities have been negatively
affected by
23
Table of Contents
the deterioration in the U.S. and global economies in several ways. For instance, cord
blood banks and hospitals are facing increased pressure from reduction in donations or in
government funding that support
their operations. The current economic crisis heightens the risk that our customers may lack the
funding or credit facilities that they may have previously used for acquiring our products. Such
credit or funding restrictions could delay or lower our future revenues.
The Preparation of our Consolidated Financial Statements in Accordance with U.S. Generally Accepted
Accounting Principles Requires Us to Make Estimates, Judgments, and Assumptions that may Ultimately
Prove to be Incorrect. The accounting estimates and judgments that management must make in the
ordinary course of business affect the reported amounts of assets and liabilities at the date of
the consolidated financial statements and the reported amounts of revenue and expenses during the
periods presented. If the underlying estimates are ultimately proven to be incorrect, subsequent
adjustments could have a material adverse effect on our operating results for the period or periods
in which the change is identified. Additionally, subsequent adjustments could require us to
restate our consolidated financial statements. Restating consolidated financial statements could
result in a material decline in the price of our stock.
We and our Customers are Subject to Various Political, Economic and Regulatory Changes in the
Healthcare Industry that Could Force us to Modify how we Develop and Price our Components,
Manufacturing Capabilities and Services, and could Harm our Business. The healthcare industry is
highly regulated and is influenced by changing political, economic and regulatory factors. Federal
and state legislatures have periodically considered programs to reform or amend the U.S. healthcare
system at both the federal and state levels. Regulations affecting the healthcare industry in
general, and the medical device industry in particular, are complex, change frequently and have
tended to become more stringent over time. In addition, these regulations may contain proposals to
increase governmental involvement in healthcare, lower reimbursement rates or otherwise change the
environment in which healthcare industry participants, including medical device companies, operate.
While we are not aware of any legislation or regulations specifically targeting the medical device
industry that are currently pending, any such regulations could impair our ability to operate
profitably. In addition, any failure by us to comply with applicable government regulations could
also result in the cessation of portions or all of our operations, impositions of fines and
restrictions on our ability to continue or expand our operations.
Risks Related to Our Industry
Our Business is Heavily Regulated, Resulting in Increased Costs of Operations and Delays in Product
Sales. Many of our products require FDA approval or clearance to sell in the U.S. and will require
approvals from comparable agencies to sell in foreign countries. These authorizations may limit
the U.S. or foreign markets in which our products may be sold. Although the majority of our
products related to freezing blood components are currently exempt from the requirement to file a
510(k) or PMA, that situation may change in the future if the FDA moves to regulate cell therapy
products. In anticipation of possible future regulation by the FDA, the Company has filed, and is
maintaining, a Master File on the BioArchive System and the AXP Platform. However, currently the
BioArchive, AXP, and the ThermoLine products are being marketed and sold worldwide. Further, our
products must be manufactured under principles of our quality system for continued CE-Marking so
they can continue to be marketed and sold in Europe. These principles are similar to the QSR of
both the FDA and California Department of Public Health. Failure to comply with or inappropriately
interpret these quality system requirements and regulations may subject the Company to delays in
production while it corrects deficiencies found by the FDA, the State of California, or the
Company’s Notifying Body as a result of any audit of our quality system. If we are found to be out
of compliance, we could receive a Warning Letter or an untitled letter from the FDA or even be
temporarily shut down in manufacturing while the non-conformances are rectified. The FDA may also
invalidate our PMA if appropriate regulations
24
Table of Contents
relative to the PMA product are not met. The
Notified Bodies may elect to not renew CE-Mark certification. Any of these events would negatively
impact our revenues and costs of operations.
Future Regulatory Changes May Affect Our Business. On August 3, 2010, the FDA released for public
comment two internal working group reports with numerous recommendations (1) to improve the 510(k)
process, and (2) to utilize science in regulatory decision making in ways that encourage innovation
yet maintain predictability. Comments are due in 60 days and the FDA is targeting the
implementation of or setting timelines for the implementation of “non-controversial”
recommendations by the end of the year. At the same time, the FDA acknowledges that the
recommendations are preliminary and no decisions have been made on specific changes to pursue.
Nevertheless, we anticipate significant changes will result in the way 510(k) programs will operate
and the data requirements, including clinical data, to obtain 510(k) clearance or PMA approval. We
cannot predict what effect these reforms will have on our ability to obtain 510(k) clearances or
PMA approvals in a timely manner or the effect on our business.
Competition in Our Industry is Intense and Will Likely Involve Companies with Greater Resources
than We Have. We hope to develop a competitive advantage in the medical applications of our
products, but there are many competitors that are substantially larger and possess greater
financial resources and more personnel than we do. Our current principal market is cord blood
banks, and with regards to the BioArchive System and AXP Platform, numerous larger and
better-financed medical device manufacturers may choose to enter this market as it develops.
Influence By the Government and Insurance Companies May Adversely Impact Sales of Our Products.
Our business may be materially affected by continuing efforts by government, third party payers
such as Medicare, Medicaid, and private health insurance plans, to reduce the costs of healthcare.
For example, in certain foreign markets the pricing and profit margins of certain healthcare
products are subject to government controls. In addition, increasing emphasis on managed care in
the U.S. will continue to place pressure on the pricing of healthcare products. As a result,
continuing efforts to contain healthcare costs may result in reduced sales or price reductions for
our products. To date, we are not aware of any direct impact on our pricing or product sales due
to such efforts by governments to contain healthcare costs, and we do not anticipate any impact in
the near future.
Product Liability and Uninsured Risks May Adversely Affect the Continuing Operations. We operate
in an industry susceptible to significant product liability claims. We may be liable if any of our
products cause injury, illness, or death. These claims may be brought by individuals seeking
relief or by groups seeking to represent a class. We also may be required to recall certain of our
products should they become damaged or if they are defective. We are not aware of any material
product liability claims against us. However, product liability claims may be asserted against us
in the future based on events we are not aware of at the present time. We maintain a general
liability policy that includes product liability coverage of $1,000,000 per occurrence and
$2,000,000 per year in the aggregate. However, a product liability claim against us could have a
material adverse effect on our business or future financial condition.
25
Table of Contents
Risks Related to Our Common Stock
Trading Prices for our Common Stock Have Been, and May Continue To Be, Volatile. The trading price
of our common stock has been subject to wide fluctuations and may continue to be volatile in the
future. Trading price fluctuations can be caused by a variety of factors, many of which are beyond
our control, including, among other things:
| • | Variations in operating results, | ||
| • | Regulatory actions, such as product recalls, | ||
| • | Governmental regulatory acts, | ||
| • | Biological or medical discoveries, | ||
| • | Changes in earnings estimates by securities analysts, and | ||
| • | Market conditions in our industry and the economy as a whole. |
If our revenues or operating results fall below the expectations of securities analysts and
investors, the price of our common stock would likely decline. In the last few years, the stock
market experienced extreme price and volume fluctuations due to the unprecedented turmoil and
upheaval of the credit markets and the financial services industry, which have particularly
affected the market prices for emerging biotechnology and medical device companies, and has
adversely affected the market price of our common stock.
If the Price of our Common Stock Does Not Meet the Requirements of the NASDAQ Capital Market Stock
Exchange (“NASDAQ”), Our Shares may be Delisted. Our Ability to Publicly or Privately Sell Equity
Securities and the Liquidity of Our Common Stock Could be Adversely Affected if We Are Delisted.
One of the requirements for continued listing on NASDAQ requires a company’s minimum bid price to
be above $1.00 per share. On August 9, 2010, our Board of Directors declared a one for four
reverse stock split effective at the close of business on August 26, 2010 which resulted in a $2.08
per share stock price. If our share price falls below $1.00 for 30 days, our shares may be
delisted or the Company may have to take other action to avoid delisting. Delisting from NASDAQ
could adversely affect our ability to raise additional financing through the public or private sale
of equity securities, would significantly affect the ability of investors to trade our securities
and would negatively affect the value and liquidity of our common stock. Delisting could also have
other negative results, including the potential loss of confidence by employees, the loss of
institutional investor interest and fewer business development opportunities.
We Do Not Pay Cash Dividends. We have never paid any cash dividends on our common stock and may
not pay cash dividends in the future. Instead, we intend to apply earnings to the expansion and
development of our business. Thus, the liquidity of your investment is dependent upon your ability
to sell stock at an acceptable price. The price can go down as well as up and may limit your
ability to realize any value from your investment, including the initial purchase price.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
The Company leases a facility with approximately 28,000 square feet of space located in Rancho
Cordova, California. Approximately 50% of the facility is devoted to warehouse space and
manufacturing of products, including 500 square feet for a clean room. The other 50% is comprised
of office space, a biologics lab and a Research and Development lab. Under the current amendment,
the lease expires in October 2016.
26
Table of Contents
The Company leases a second facility with approximately 14,000 square feet. The two facilities are
located in the same commercial complex. Approximately 30% of the second facility is devoted to
warehouse space. The remaining 70% is comprised of office space. The lease expires in March 2012.
Under the terms of the lease, on July 22, 2010 the Company gave a “Notice to Vacate” in 180 days or
January 21, 2011.
At fiscal year end, the Company did not own or lease any other facilities.
ITEM 3. LEGAL PROCEEDINGS
The Company and its property are not a party to any pending legal proceedings. In the normal course
of operations, the Company may have disagreements or disputes with employees, vendors or customers.
These disputes are seen by the Company’s management as a normal part of business, and there are no
currently pending actions or threatened actions that management believes would have a significant
material impact on the Company’s financial position, results of operations or cash flows.
ITEM
4. [Removed and Reserved]
27
Table of Contents
PART II
| ITEM 5. | MARKET FOR THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
The Company’s common stock, $0.001 par value, is traded on NASDAQ under the symbol KOOL. At the
close of business on August 26, 2010, we effected a 1-for-4 reverse split of our common stock. The
following table sets forth the range of high and low bid prices for the Company’s common stock for
the past two fiscal years as reported by NASDAQ. All amounts in the table have been adjusted to
give effect to the reverse stock split.
| Fiscal 2010 | High | Low | ||||||
|
|
||||||||
|
First Quarter (Sep.
30)
|
$ | 2.92 | $ | 2.16 | ||||
|
Second Quarter (Dec.
31)
|
$ | 2.80 | $ | 2.24 | ||||
|
Third Quarter (Mar.
31)
|
$ | 3.08 | $ | 2.04 | ||||
|
Fourth Quarter (June 30)
|
$ | 3.20 | $ | 1.84 | ||||
| Fiscal 2009 | High | Low | ||||||
| First Quarter (Sep. 30) | $ | 7.36 | $ | 4.68 | ||||
| Second Quarter (Dec. 31) | $ | 5.12 | $ | 1.24 | ||||
| Third Quarter (Mar. 31) | $ | 3.36 | $ | 1.60 | ||||
| Fourth Quarter (June 30) | $ | 3.08 | $ | 2.04 | ||||
The Company has not paid cash dividends on its common stock and does not
intend to pay a cash dividend in the foreseeable future. There were approximately
271 stockholders of record on June 30, 2010 (not including
street name holders).
28
Table of Contents
The following graph compares the performance of the Company’s common stock during the period June
30, 2005 to June 30, 2010, with the NASDAQ Stock Market Index and the Company’s peer group of
NASDAQ stocks:
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among ThermoGenesis Corp., the NASDAQ Composite Index
and a Peer Group
Among ThermoGenesis Corp., the NASDAQ Composite Index
and a Peer Group
| 6/05 | 6/06 | 6/07 | 6/08 | 6/09 | 6/10 | |||||||||||||||||||
ThermoGenesis Corp. |
100.00 | 94.71 | 63.45 | 32.18 | 14.48 | 11.19 | ||||||||||||||||||
NASDAQ Composite |
100.00 | 107.08 | 130.99 | 114.02 | 90.79 | 105.54 | ||||||||||||||||||
Peer Group |
100.00 | 120.33 | 146.76 | 149.27 | 105.83 | 134.27 | ||||||||||||||||||
29
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
ThermoGenesis Corp.
Five-Year Review of Selected Financial Data
Five-Year Review of Selected Financial Data
| Year Ended June 30, | ||||||||||||||||||||
| Summary of Operations | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||
Net revenues |
$ | 23,088,000 | $ | 19,799,000 | $ | 21,946,000 | $ | 16,751,000 | $ | 12,048,000 | ||||||||||
Cost of revenues |
(15,643,000 | ) | (14,106,000 | ) | (14,976,000 | ) | (11,554,000 | ) | (7,705,000 | ) | ||||||||||
Gross profit |
7,445,000 | 5,693,000 | 6,970,000 | 5,197,000 | 4,343,000 | |||||||||||||||
Selling, general and
administration |
(7,686,000 | ) | (9,249,000 | ) | (10,165,000 | ) | (9,630,000 | ) | (7,156,000 | ) | ||||||||||
Research and development |
(5,013,000 | ) | (5,222,000 | ) | (7,172,000 | ) | (4,108,000 | ) | (4,157,000 | ) | ||||||||||
Interest and other income, net |
61,000 | 228,000 | 1,186,000 | 1,765,000 | 828,000 | |||||||||||||||
Net loss |
($5,193,000 | ) | ($8,550,000 | ) | ($9,181,000 | ) | ($6,776,000 | ) | ($6,142,000 | ) | ||||||||||
Per share data: |
||||||||||||||||||||
Basic and diluted net loss per
common share |
($0.37 | ) | ($0.61 | ) | ($0.66 | ) | ($0.49 | ) | ($0.50 | ) | ||||||||||
| Balance Sheet Data | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||
Cash, cash equivalents and
short term investments |
$ | 10,731,000 | $ | 15,631,000 | $ | 25,287,000 | $ | 33,379,000 | $ | 38,999,000 | ||||||||||
Working capital |
$ | 16,587,000 | $ | 20,923,000 | $ | 29,978,000 | $ | 37,759,000 | $ | 42,342,000 | ||||||||||
Total assets |
$ | 24,030,000 | $ | 27,655,000 | $ | 38,282,000 | $ | 43,790,000 | $ | 47,603,000 | ||||||||||
Total liabilities |
$ | 6,251,000 | $ | 5,201,000 | $ | 7,757,000 | $ | 5,978,000 | $ | 5,631,000 | ||||||||||
Total stockholders’ equity |
$ | 17,779,000 | $ | 22,454,000 | $ | 30,525,000 | $ | 37,812,000 | $ | 41,972,000 | ||||||||||
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
CERTAIN STATEMENTS CONTAINED IN THIS SECTION AND OTHER PARTS OF THIS REPORT ON FORM 10-K WHICH ARE
NOT HISTORICAL FACTS ARE FORWARD-LOOKING STATEMENTS AND ARE SUBJECT TO CERTAIN RISKS AND
UNCERTAINTIES. THE COMPANY’S ACTUAL RESULTS MAY DIFFER SIGNIFICANTLY FROM THE PROJECTED RESULTS
DISCUSSED IN THE FORWARD-LOOKING STATEMENTS. FACTORS THAT MIGHT AFFECT ACTUAL RESULTS INCLUDE, BUT
ARE NOT LIMITED TO, THOSE DISCUSSED IN ITEM 1A “RISK FACTORS” AND OTHER FACTORS IDENTIFIED FROM
TIME TO TIME IN THE COMPANY’S REPORTS FILED WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION.
The following discussion should be read in conjunction with the Company’s consolidated financial
statements contained in this report.
30
Table of Contents
(a) Overview
ThermoGenesis designs, develops, and sells medical products that enable the practice of
regenerative medicine. The Company was founded in 1986 and is located in Rancho Cordova,
California. Our products automate the volume reduction and cryopreservation process of adult stem
cell concentrates from cord blood and bone marrow for use in laboratory and point of care settings.
Our growth strategy is to expand our offerings in regenerative medicine and partner with other
pioneers in the stem cell arena to accelerate our worldwide penetration in this potentially
explosive market.
Recent Significant Event
On August 11, 2010, we announced that our board of directors had approved a 1-for-4 reverse stock
split of our common stock, pursuant to previously obtained stockholder authorization. The reverse
stock split, which became effective at the close of business on August 26, 2010, reduced the number
of shares of our common stock issued and outstanding from approximately 56.1 million to
approximately 14 million. All share and per share amounts herein are presented on a
post-reverse-split basis.
Critical Accounting Policies:
The Company’s discussion and analysis of its financial condition and results of operations are
based upon the Company’s consolidated financial statements, which have been prepared in accordance
with accounting principles generally accepted in the U.S. The preparation of these consolidated
financial statements requires the Company to make estimates and judgments that affect the reported
amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets
and liabilities. On an on-going basis, the Company evaluates its estimates, including those
related to stock-based compensation, bad debts, inventories, warranties, contingencies and
litigation. The Company bases its estimates on historical experience and on various other
assumptions that are believed to be reasonable under the circumstances, the results of which form
the basis for making judgments about the carrying values of assets and liabilities that are not
readily apparent from other sources. Actual results may differ from these estimates under
different assumptions or conditions.
The Company believes the following critical accounting policies affect its more significant
judgments and estimates used in the preparation of its consolidated financial statements.
Stock-Based Compensation:
The Company calculates stock-based compensation on the date of the grant using the Black
Scholes-Merton option-pricing formula. The compensation expense is then amortized over the vesting
period. The Company uses the Black-Scholes-Merton option-pricing formula in determining the fair
value of the Company’s options at the grant date and applies judgment in estimating the key
assumptions that are critical to the model such as the expected term, volatility and forfeiture
rate of an option. The Company’s estimate of these key assumptions is based on historical
information and judgment regarding market factors and trends. If actual results are not consistent
with the Company’s assumptions and judgments used in estimating the key assumptions, the Company
may be required to record additional compensation or income tax expense, which could have a
material impact on the Company’s financial position and results of operations.
Revenue Recognition:
Revenues from the sale of the Company’s products are recognized when persuasive evidence of an
arrangement exists, delivery has occurred (or services have been rendered), the price is fixed or
determinable, and collectability is reasonably assured. The Company generally ships products
F.O.B. shipping point. There is no conditional evaluation on any product sold and recognized as
revenue. All foreign sales are denominated in U.S. dollars. Amounts billed in excess of revenue
recognized are recorded as deferred revenue on the balance sheet.
31
Table of Contents
The Company’s sales are generally through distributors. There is no right of return provided for
distributors. For sales of products made to distributors, the Company considers a number of
factors in determining whether revenue is recognized upon transfer of title to the distributor, or
when payment is received. These factors include, but are not limited to, whether the payment terms
offered to the distributor are considered to be non-standard, the distributor history of adhering
to the terms of its contractual arrangements with the Company, the level of inventories maintained
by the distributor, whether the Company has a pattern of granting concessions for the benefit of
the distributor, and whether there are other conditions that may indicate that the sale to the
distributor is not substantive. The Company currently recognizes revenue primarily on the sell-in
method with its distributors.
Revenue arrangements with multiple elements are divided into separate units of accounting if
certain criteria are met, including whether the delivered item has value to the customer on a
stand-alone basis and whether there is objective and reliable evidence of the fair value of the
undelivered items. Revenue is recognized as specific elements indicated in sales contracts are
executed. If an element is essential to the functionality of an arrangement, the entire
arrangement’s revenue is deferred until that essential element is delivered. The fair value of
each undelivered element that is not essential to the functionality of the system is deferred until
performance or delivery occurs. The fair value of an undelivered element is based on vendor
specific objective evidence or third party evidence of fair value as appropriate. Costs associated
with inconsequential or perfunctory elements in multiple element arrangements are accrued at the
time of revenue recognition. The Company accounts for training and installation as a separate
element of a multiple element arrangement. The Company therefore recognizes the fair value of
training and installation services upon their completion when the Company is obligated to perform
such services.
Service revenue generated from contracts for providing maintenance of equipment is amortized over
the life of the agreement. All other service revenue is recognized at the time the service is
completed.
Milestone payments the Company receives under research and development arrangements are recognized
as revenue upon achievement of the milestone events, which represent the culmination of the
earnings process, and when collectability is reasonably assured. Milestone payments are triggered
by the results of the Company’s development efforts. Accordingly, the milestone payments are
substantially at risk at the inception of the contract, and the amounts of the payments assigned
thereto are commensurate with the milestone achieved. Upon the achievement of a milestone event,
which may include acceptance by the counterparty, the Company has no future performance obligations
related to that milestone as the milestone payments received by the Company are nonrefundable.
For licensing agreements pursuant to which the Company receives up-front licensing fees for
products or technologies that will be provided by the Company over the term of the arrangements,
the Company defers the up-front fees and recognizes the fees as revenue on a straight-line method
over the term of the respective license. For license agreements that require no continuing
performance on the Company’s part, license fee revenue is recognized immediately upon grant of the
license.
Shipping and handling fees billed to customers are included in product and other revenues, while
the related costs are included in cost of product and other revenues.
Warranty:
The Company provides for the estimated cost of product warranties at the time revenue is
recognized. While the Company engages in extensive product quality programs and processes,
including actively monitoring and evaluating the quality of its component suppliers, the Company’s
warranty obligation is affected by product failure rates, material usage and service delivery costs
incurred in correcting a product failure. Should actual product failure rates, material usage or
service delivery costs differ from the
32
Table of Contents
Company’s estimates, revisions to the estimated warranty liability could have a material impact on
the Company’s financial position, cash flows or results of operations.
Inventory Reserve:
The Company states inventories at lower of cost or market value determined on a first-in, first-out
basis. The Company provides inventory allowances when conditions indicate that the selling price
could be less than cost due to physical deterioration, obsolescence, changes in price levels, or
other causes, which it includes as a component of cost of product and other revenues.
Additionally, the Company provides reserves for excess and slow-moving inventory on hand that are
not expected to be sold to reduce the carrying amount of slow-moving inventory to its estimated net
realizable value. The reserves are based upon estimates about future demand from our customers and
distributors and market conditions. Because some of the Company’s products are highly dependent on
government and third-party funding, current customer use and validation, and completion of
regulatory and field trials, there is a risk that we will forecast incorrectly and purchase or
produce excess inventories. As a result, actual demand may differ from forecasts and the Company
may be required to record additional inventory reserves that could adversely impact our gross
margins. Conversely, favorable changes in demand could result in higher gross margins when
products previously reserved are sold.
(b) Results of Operations
The following is Management’s discussion and analysis of certain significant factors which have
affected the Company’s financial condition and results of operations during the periods included in
the accompanying consolidated financial statements.
Results of Operations for the Year Ended June 30, 2010 as Compared to the Year Ended June 30, 2009
Net Revenues:
Net revenues for the year ended June 30, 2010 were $23,088,000 compared to $19,799,000 for the year
ended June 30, 2009, an increase of $3,289,000 or 17%. Our increase in revenues is primarily due
to an increase in disposable revenues across all product lines of $3,713,000. This increase in
disposable revenue was partially offset by a decrease in ThermoLine revenues of $745,000.
Sales analysis for the year ending June 30:
| Percentage | Percentage | |||||||||||||||
| 2010 | of Revenues | 2009 | of Revenues | |||||||||||||
Disposable revenues: |
||||||||||||||||
AXP |
$ | 8,880,000 | $ | 6,387,000 | ||||||||||||
BioArchive |
4,152,000 | 3,766,000 | ||||||||||||||
MXP/Res-Q |
759,000 | 144,000 | ||||||||||||||
CryoSeal |
516,000 | 297,000 | ||||||||||||||
| 14,307,000 | 62 | % | 10,594,000 | 54 | % | |||||||||||
Non-disposable revenues: |
||||||||||||||||
BioArchive |
4,267,000 | 18 | % | 4,262,000 | 22 | % | ||||||||||
ThermoLine |
1,616,000 | 7 | % | 2,361,000 | 12 | % | ||||||||||
AXP/MXP/Res-Q |
743,000 | 4 | % | 722,000 | 4 | % | ||||||||||
CryoSeal |
67,000 | — | 37,000 | — | ||||||||||||
Milestone payments and
license fees |
687,000 | 3 | % | 676,000 | 3 | % | ||||||||||
Other |
1,401,000 | 6 | % | 1,147,000 | 5 | % | ||||||||||
Total Company revenues |
$ | 23,088,000 | 100 | % | $ | 19,799,000 | 100 | % | ||||||||
33
Table of Contents
The following represents the Company’s cumulative BioArchive System placements in the following
geographies:
| June 30, | ||||||||
| 2010 | 2009 | |||||||
Asia |
74 | 64 | ||||||
United States |
51 | 49 | ||||||
Europe |
57 | 51 | ||||||
Rest of World |
41 | 38 | ||||||
| 223 | 202 | |||||||
Gross Profit:
The Company’s gross profit was $7,445,000 or 32% of net revenues for the year ended June 30, 2010,
as compared to $5,693,000 or 29% for the year ended June 30, 2009. The higher gross profit was
primarily due to increases in inventory reserves and write-offs of obsolete inventory in fiscal
2009. There was a net release of inventory reserves in fiscal 2010 of $270,000 as CryoSeal device
and disposable sales were higher than previously estimated. This was offset by higher warranty
costs associated with the BioArchive device and AXP disposable.
Selling, General and Administrative Expenses:
Selling, general and administrative expenses were $7,686,000 for the year ended June 30, 2010,
compared to $9,249,000 for the year ended June 30, 2009, a decrease of $1,563,000 or 17%. The
decrease is primarily due to a decrease in severance expense of $550,000 which related to the
severance accruals in fiscal 2009 for the Company’s former Chief Executive Officer and vice
presidents of sales and marketing, lower salaries and benefits of $315,000, lower
labeling/translation costs of $300,000 and lower board of director and committee fees of $150,000.
Research and Development Expenses:
Research and development expenses for the year ended June 30, 2010 were $5,013,000 compared to
$5,222,000 for fiscal 2009, a decrease of $209,000 or 4%. The decrease is primarily due to a
reduction of costs associated with the Vantus subsidiary during the six months ended December 31,
2008 of $290,000.
Management believes that product development and refinement are essential to maintaining the
Company’s market position. Therefore, the Company considers these costs as continuing costs of
doing business. No assurances can be given that the products or markets recently developed or
under development will be successful.
Results of Operations for the Year Ended June 30, 2009 as Compared to the Year Ended June 30, 2008
Net Revenues:
Net revenues for the year ended June 30, 2009 were $19,799,000 compared to $21,946,000 for the year
ended June 30, 2008, a decrease of $2,147,000 or 10%. Our decrease in revenues is primarily a
result of the slowing global economy which has impacted the majority of our product lines. The
CryoSeal product line decreased $1,477,000 from the year ended June 30, 2008. Revenues from the
BioArchive product line decreased approximately $1,000,000 as there were six fewer devices sold in
fiscal 2009 as compared to fiscal 2008. The AXP product line decreased $560,000 primarily due to
the timing of bag set orders. Offsetting these decreases was an increase in revenues of $367,000
due to the launch of the MXP in fiscal 2009 and Freezer sales increased $370,000 primarily due to
the sale of ten freezers to two different customers.
34
Table of Contents
Sales analysis for the year ending June 30:
| 2009 | 2008 | |||||||||||||||
Disposable revenues: |
||||||||||||||||
AXP/MXP |
$ | 6,531,000 | $ | 6,828,000 | ||||||||||||
BioArchive |
3,766,000 | 3,757,000 | ||||||||||||||
CryoSeal |
297,000 | 1,143,000 | ||||||||||||||
| 10,594,000 | 54 | % | 11,728,000 | 53 | % | |||||||||||
Non-disposable revenues: |
||||||||||||||||
BioArchive |
4,262,000 | 22 | % | 5,564,000 | 25 | % | ||||||||||
ThermoLine |
2,361,000 | 12 | % | 2,058,000 | 9 | % | ||||||||||
AXP/MXP |
722,000 | 4 | % | 594,000 | 3 | % | ||||||||||
CryoSeal |
37,000 | — | 481,000 | 2 | % | |||||||||||
Milestone payments and license
fees |
676,000 | 3 | % | 866,000 | 5 | % | ||||||||||
Other |
1,147,000 | 5 | % | 655,000 | 3 | % | ||||||||||
Total Company revenues |
$ | 19,799,000 | 100 | % | $ | 21,946,000 | 100 | % | ||||||||
The following represents the Company’s cumulative BioArchive System placements in the following
geographies:
| June 30, | ||||||||
| 2009 | 2008 | |||||||
Asia |
64 | 58 | ||||||
United States |
49 | 46 | ||||||
Europe |
51 | 47 | ||||||
Rest of World |
38 | 30 | ||||||
| 202 | 181 | |||||||
Gross Profit:
The Company’s gross profit was $5,693,000 or 29% of net revenues for the year ended June 30, 2009,
as compared to $6,970,000 or 32% for the year ended June 30, 2008. The lower gross profit was due
to a lower volume of disposable products and additional inventory allowances and reserves for
obsolete inventory at AXP bag set suppliers, excess AXP device inventory given the planned
transition to an upgraded AXP device and excess CryoSeal inventory. These were offset by lower
warranty costs for the BioArchive and CryoSeal products and lower material costs on the AXP bag
sets.
Selling, General and Administrative Expenses:
Selling, general and administrative expenses were $9,249,000 for the year ended June 30, 2009,
compared to $10,165,000 for the year ended June 30, 2008, a decrease of $916,000 or 9%. The
decrease is primarily due to lower salaries and benefits of $580,000 as there were four management
positions open during the year, and lower legal fees of $325,000, as there was $300,000 of legal
fees incurred in fiscal 2008 associated with the GEHC distribution agreement negotiations and for
consultation during the voluntary recall effort. Recruiting costs decreased $275,000 in fiscal
2009 as there were expenses paid in fiscal 2008 in searches for new board members and executive
officers. Additionally, stock compensation expense decreased $220,000. These decreases were
offset by an increase in severance expense of $500,000 in fiscal 2009 primarily due to the
severance accruals for the Company’s former Chief Executive Officer and vice presidents of sales
and marketing.
35
Table of Contents
Research and Development Expenses:
Research and development expenses for the year ended June 30, 2009 were $5,222,000 compared to
$7,172,000 for fiscal 2008, a decrease of $1,950,000 or 27%. The decrease is primarily due to a
decrease in stock compensation expense of $1,230,000 as the restricted stock awarded to the
company’s former Chief Technology Architect (“CTA”) fully vested in April 2008, a decrease of
$330,000 in expenses associated with the Vantus subsidiary which was formed in February 2008 and a
reduction of $460,000 of expenses for new product development.
(c) Liquidity and Capital Resources
At June 30, 2010, the Company had a cash balance of
$10,731,000 and working capital of $16,587,000.
This compares to a cash, cash equivalents, and short-term investments balance of $15,631,000 and
working capital of $20,923,000 at June 30, 2009. The cash was used to fund operations and other
cash needs of the Company. In addition to product revenues, the Company has primarily financed
operations through the private and public placement of equity securities and has raised
approximately $108 million, net of expenses, through common and preferred stock financings and
option and warrant exercises.
Net cash used in operating activities for the year ended June 30, 2010 was $4,428,000, primarily
due to the net loss of $5,193,000, offset by depreciation and stock-based compensation expense of
$492,000 and $518,000, respectively. Accounts receivable used $1,797,000 in cash due to the growth
in revenues in the fourth quarter of fiscal 2010. Other liabilities
generated $1,156,000
in cash due to the $1,000,000 license payment from Asahi. Investing activities generated
$8,508,000 of cash primarily due to short-term investments maturing.
We believe our currently available cash and cash equivalents and cash generated from operations
will be sufficient to satisfy our operating and working capital requirements for at least the next
twelve months. We have reduced expenses without sacrificing development plans we consider
essential to our near-term revenue growth and do not anticipate we will have to seek additional
debt or equity capital. Our ability to fund our longer-term cash needs is subject to various
risks, many of which are beyond our control. Should we require additional funding, such as
additional capital investments, we may need to raise the required additional funds through bank
borrowings or public or private sales of debt or equity securities. We cannot assure that such
funding will be available in needed quantities or on terms favorable to us, if at all.
The Company generally does not require extensive capital equipment to produce or sell its current
products. In fiscal 2008, the Company spent $514,000 for development of the Company’s website,
laboratory equipment and manufacturing equipment. In fiscal 2009, the Company spent $1,008,000 for
quality system software, centrifuges to be placed at MXP customer sites, tooling for new products
or additional vendors and computer equipment. In fiscal 2010, the Company spent $470,000 for
centrifuges to be placed at MXP customer sites, tooling for new products or additional vendors and
quality system software.
During the fiscal year ended June 30, 2010, revenues from one significant customer, GEHC, totaled
$9,890,000 or 43% of net revenues. During the fiscal year ended June 30, 2009, revenues from one
significant customer, GEHC, totaled $7,735,000 or 39% of net revenues. During the fiscal year
ended June 30, 2008, revenues from one significant customer, GEHC, totaled $13,310,000 or 61% of
net revenues.
At June 30, 2010, the Company had one customer that individually accounted for 42% of accounts
receivable. At June 30, 2009, the Company had two customers that individually accounted for 43%
and 19% of accounts receivable.
36
Table of Contents
The Company manages the concentration of credit risk with these customers through a variety of
methods including, letters of credit with financial institutions, pre-shipment deposits, credit
reference checks and credit limits. Although management believes that these customers are sound
and creditworthy, a severe adverse impact on their business operations could have a corresponding
material effect on their ability to pay timely and therefore on our net revenues, cash flows and
financial condition.
Off Balance Sheet Arrangements:
The Company has no off-balance sheet arrangements.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Market risk represents the risk of changes in the value of market risk sensitive instruments caused
by fluctuations in interest rates, foreign exchange rates and commodity prices.
Our exposure to interest rate risk at June 30, 2010, is related to the investment of our excess
cash into highly liquid, short-term financial investments. We invest in money market funds in
accordance with our investment policy. The primary objectives of our investment policy are to
preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our
investment policy specifies credit quality standards for our investments. We do not hold
auction-rate or mortgage-backed securities. Due to the short-term nature of our investments, we
have assessed that there is no material exposure to interest rate risk arising from them.
All sales, including those involving foreign entities, are denominated in U.S. dollars and as
a result, we have experienced no significant foreign exchange gains and losses to date. We have
not engaged in foreign currency hedging activities to date, and have no intention of doing so. Our
future revenues may be negatively impacted in periods of a strengthening U.S. dollar. We have not
entered into any derivative financial instruments or derivative commodity instruments.
37
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
| Page | ||||
| Number | ||||
| 39 | ||||
| 40 | ||||
| 42 | ||||
| 43 | ||||
| 44 | ||||
| 45 | ||||
| 46 | ||||
38
Table of Contents
Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control
over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Under
the supervision and with the participation of the Company’s management, including the Company’s
Chief Executive Officer, and Executive Vice President, Chief Operating Officer and Chief Financial
Officer, the Company conducted an evaluation of the effectiveness of its internal control over
financial reporting based on criteria established in the framework in Internal Control —
Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway
Commission. Based on this evaluation, the Company’s management concluded that its internal control
over financial reporting was effective as of June 30, 2010.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Projections of any evaluation of effectiveness to future periods are subject
to the risk that controls may become inadequate because of changes in conditions, or that the
degree of compliance with the policies or procedures may deteriorate. All internal control
systems, no matter how well designed, have inherent limitations. Therefore, even those systems
determined to be effective can provide only reasonable assurance with respect to financial
statement preparation and presentation.
The Company’s independent registered public accounting firm has issued an attestation report on the
effectiveness of the Company’s internal control over financial reporting as of June 30, 2010, which
appears on the following page of this Annual Report on Form 10-K.
39
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of ThermoGenesis Corp.
We have audited ThermoGenesis Corp.’s internal control over financial reporting as of June 30,
2010, based on criteria established in Internal Control—Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria).
ThermoGenesis Corp.’s management is responsible for maintaining effective internal control over
financial reporting, and for its assessment of the effectiveness of internal control over financial
reporting included in the accompanying Management’s Report on Internal Control over Financial
Reporting. Our responsibility is to express an opinion on the company’s internal control over
financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether effective internal control over financial reporting was
maintained in all material respects. Our audit included obtaining an understanding of internal
control over financial reporting, assessing the risk that a material weakness exists, testing and
evaluating the design and operating effectiveness of internal control based on the assessed risk,
and performing such other procedures as we considered necessary in the circumstances. We believe
that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable
assurance regarding the reliability of financial reporting and the preparation of financial
statements for external purposes in accordance with generally accepted accounting principles. A
company’s internal control over financial reporting includes those policies and procedures that (1)
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the
transactions and dispositions of the assets of the company; (2) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance
with generally accepted accounting principles, and that receipts and expenditures of the company
are being made only in accordance with authorizations of management and directors of the company;
and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized
acquisition, use, or disposition of the company’s assets that could have a material effect on the
financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or
detect misstatements. Also, projections of any evaluation of effectiveness to future periods are
subject to the risk that controls may become inadequate because of changes in conditions, or that
the degree of compliance with the policies or procedures may deteriorate.
In our opinion, ThermoGenesis Corp. maintained, in all material respects, effective internal
control over financial reporting as of June 30, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), the consolidated balance sheets of ThermoGenesis Corp. as of June 30, 2010
and 2009, and the related consolidated statements of operations, stockholders’ equity, and cash
flows for each of the three years in the period ended June 30, 2010 and the financial statement
schedule listed in the Index of Item 15(a)(2) and our report dated September 14, 2010 expressed an
unqualified opinion thereon.
| /s/ Ernst & Young LLP | ||||
Sacramento, California
September 14, 2010
September 14, 2010
40
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of ThermoGenesis Corp.
We have audited the accompanying consolidated balance sheets of ThermoGenesis Corp. as of June 30,
2010 and 2009, and the related consolidated statements of operations, stockholders’ equity, and
cash flows for each of the three years in the period ended June 30, 2010. Our audits also included
the financial statement schedule listed in the Index at Item 15(a)(2). These financial statements
and schedule are the responsibility of the Company’s management. Our responsibility is to express
an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. An
audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles used and
significant estimates made by management, as well as evaluating the overall financial statement
presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material
respects, the consolidated financial position of ThermoGenesis Corp. at June 30, 2010 and 2009, and
the consolidated results of its operations and its cash flows for each of the three years in the
period ended June 30, 2010, in conformity with U.S. generally accepted accounting principles. Also,
in our opinion, the related financial statement schedule, when considered in relation to the basic
financial statements taken as a whole, presents fairly in all material respects the information set
forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight
Board (United States), ThermoGenesis Corp.’s internal control over financial reporting as of June
30, 2010, based on criteria established in Internal Control-Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission and our report dated September 14,
2010 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP | ||||
Sacramento, California
September 14, 2010
September 14, 2010
41
Table of Contents
| June 30, 2010 | June 30, 2009 | |||||||
ASSETS |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 10,731,000 | $ | 6,655,000 | ||||
Short-term investments |
— | 8,976,000 | ||||||
Accounts receivable, net of allowance for
doubtful accounts of $34,000 ($26,000 at June 30, 2009) |
6,095,000 | 4,235,000 | ||||||
Inventories |
5,034,000 | 5,233,000 | ||||||
Prepaid expenses and other current assets |
301,000 | 662,000 | ||||||
Total current assets |
22,161,000 | 25,761,000 | ||||||
Equipment at cost less accumulated depreciation of $3,241,000
($3,316,000 at June 30, 2009) |
1,701,000 | 1,784,000 | ||||||
Other assets |
168,000 | 110,000 | ||||||
| $ | 24,030,000 | $ | 27,655,000 | |||||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 2,383,000 | $ | 1,781,000 | ||||
Accrued payroll and related expenses |
309,000 | 881,000 | ||||||
Deferred revenue |
854,000 | 850,000 | ||||||
Other current liabilities |
2,028,000 | 1,326,000 | ||||||
Total current liabilities |
5,574,000 | 4,838,000 | ||||||
Deferred revenue |
227,000 | 363,000 | ||||||
Other non-current liabilities |
450,000 | — | ||||||
Commitments and contingencies (Footnote 6) |
||||||||
Stockholders’ equity: |
||||||||
Preferred stock, $0.001 par value; 2,000,000 shares authorized |
— | — | ||||||
Common stock, $0.001 par value; 80,000,000 shares
authorized; 14,023,240 issued and outstanding
(14,023,240 at June 30, 2009) |
14,000 | 14,000 | ||||||
Paid in capital in excess of par |
121,317,000 | 120,799,000 | ||||||
Accumulated deficit |
(103,552,000 | ) | (98,359,000 | ) | ||||
Total stockholders’ equity |
17,779,000 | 22,454,000 | ||||||
| $ | 24,030,000 | $ | 27,655,000 | |||||
See accompanying notes.
42
Table of Contents
| Years ended June 30, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Net revenues |
$ | 23,088,000 | $ | 19,799,000 | $ | 21,946,000 | ||||||
Cost of revenues |
15,643,000 | 14,106,000 | 14,976,000 | |||||||||
Gross profit |
7,445,000 | 5,693,000 | 6,970,000 | |||||||||
Expenses: |
||||||||||||
Selling, general and administrative |
7,686,000 | 9,249,000 | 10,165,000 | |||||||||
Research and development |
5,013,000 | 5,222,000 | 7,172,000 | |||||||||
Total expenses |
12,699,000 | 14,471,000 | 17,337,000 | |||||||||
Loss before interest and other income, net |
(5,254,000 | ) | (8,778,000 | ) | (10,367,000 | ) | ||||||
Interest and other income, net |
61,000 | 228,000 | 1,186,000 | |||||||||
Net loss |
($5,193,000 | ) | ($8,550,000 | ) | ($9,181,000 | ) | ||||||
Per share data: |
||||||||||||
Basic and diluted net loss per common share |
($0.37 | ) | ($0.61 | ) | ($0.66 | ) | ||||||
Shares used in computing per share data |
14,023,240 | 14,015,115 | 13,938,644 | |||||||||
See accompanying notes.
43
Table of Contents
| Paid in | Total | |||||||||||||||||||
| Common Stock | capital in | Accumulated | stockholders’ | |||||||||||||||||
| Shares | Amount | excess of par | deficit | equity | ||||||||||||||||
Balance at June 30, 2007 |
13,875,131 | $ | 14,000 | $ | 118,426,000 | ($80,628,000 | ) | $ | 37,812,000 | |||||||||||
Issuance of shares for
exercise of options |
50,163 | — | 266,000 | — | 266,000 | |||||||||||||||
Issuance of common shares and
compensation related to common
stock restricted awards, net of
stock surrenders |
81,696 | — | 1,138,000 | — | 1,138,000 | |||||||||||||||
Stock-based compensation expense |
— | — | 490,000 | — | 490,000 | |||||||||||||||
Net loss |
— | — | — | (9,181,000 | ) | (9,181,000 | ) | |||||||||||||
Balance at June 30, 2008 |
14,006,990 | 14,000 | 120,320,000 | (89,809,000 | ) | 30,525,000 | ||||||||||||||
Issuance of common shares and
compensation related to
unrestricted common stock
awards |
16,250 | — | 36,000 | — | 36,000 | |||||||||||||||
Stock-based compensation expense |
— | — | 443,000 | — | 443,000 | |||||||||||||||
Net loss |
— | — | — | (8,550,000 | ) | (8,550,000 | ) | |||||||||||||
Balance at June 30, 2009 |
14,023,240 | 14,000 | 120,799,000 | (98,359,000 | ) | 22,454,000 | ||||||||||||||
Stock-based compensation expense |
— | — | 518,000 | — | 518,000 | |||||||||||||||
Net loss |
— | — | — | (5,193,000 | ) | (5,193,000 | ) | |||||||||||||
Balance at June 30, 2010 |
14,023,240 | $ | 14,000 | $ | 121,317,000 | ($103,552,000 | ) | $ | 17,779,000 | |||||||||||
See accompanying notes.
44
Table of Contents
| Years ended June 30, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
Cash flows from operating activities: |
||||||||||||
Net loss |
($5,193,000 | ) | ($8,550,000 | ) | ($9,181,000 | ) | ||||||
Adjustments to reconcile net loss to net cash
used in operating activities: |
||||||||||||
Depreciation and amortization |
492,000 | 474,000 | 543,000 | |||||||||
Stock-based compensation expense |
518,000 | 479,000 | 1,921,000 | |||||||||
Accretion of discount on short-term investments |
(2,000 | ) | (161,000 | ) | (918,000 | ) | ||||||
Loss on sale/retirement of equipment |
— | — | 238,000 | |||||||||
Loss on impairment of equipment |
26,000 | 149,000 | — | |||||||||
Net changes in operating assets and liabilities: |
||||||||||||
Accounts receivable, net |
(1,797,000 | ) | 1,741,000 | (2,750,000 | ) | |||||||
Inventories |
34,000 | (102,000 | ) | (200,000 | ) | |||||||
Prepaid expenses and other current assets |
361,000 | (295,000 | ) | 48,000 | ||||||||
Other assets |
79,000 | 12,000 | 51,000 | |||||||||
Accounts payable |
602,000 | (2,405,000 | ) | 2,112,000 | ||||||||
Accrued payroll and related expenses |
(572,000 | ) | 317,000 | 39,000 | ||||||||
Deferred revenue |
(132,000 | ) | (562,000 | ) | (633,000 | ) | ||||||
Other liabilities |
1,156,000 | 107,000 | 279,000 | |||||||||
Net cash used in operating activities |
(4,428,000 | ) | (8,796,000 | ) | (8,451,000 | ) | ||||||
Cash flows from investing activities: |
||||||||||||
Purchase of short-term investments |
(6,741,000 | ) | (25,957,000 | ) | (44,336,000 | ) | ||||||
Maturities of investments |
15,719,000 | 38,045,000 | 52,000,000 | |||||||||
Capital expenditures |
(470,000 | ) | (1,008,000 | ) | (514,000 | ) | ||||||
Net cash provided by investing
activities |
8,508,000 | 11,080,000 | 7,150,000 | |||||||||
Cash flows from financing activities: |
||||||||||||
Exercise of stock options |
— | — | 266,000 | |||||||||
Repurchase of common stock |
— | — | (293,000 | ) | ||||||||
Payments on capital lease obligations and note
payable |
(4,000 | ) | (13,000 | ) | (18,000 | ) | ||||||
Net cash used in financing activities |
(4,000 | ) | (13,000 | ) | (45,000 | ) | ||||||
Net increase (decrease) in cash and cash equivalents |
4,076,000 | 2,271,000 | (1,346,000 | ) | ||||||||
Cash and cash equivalents at beginning of year |
6,655,000 | 4,384,000 | 5,730,000 | |||||||||
Cash and cash equivalents at end of year |
$ | 10,731,000 | $ | 6,655,000 | $ | 4,384,000 | ||||||
Supplemental non-cash financing and investing
information: |
||||||||||||
Transfer of inventories to equipment |
$ | 165,000 | — | $ | 157,000 | |||||||
Transfer of equipment to inventories |
— | — | $ | 42,000 | ||||||||
Transfer of equipment to receivables |
$ | 63,000 | — | — | ||||||||
Transfer of equipment to other assets |
$ | 137,000 | $ | 51,000 | — | |||||||
See accompanying notes.
45
Table of Contents
1. Summary of Significant Accounting Policies
Organization and Basis of Presentation
The Company was incorporated in Delaware in July 1986. The Company designs, manufactures and
markets automated and semi-automated devices and single-use processing disposables that enable
hospitals and blood banks to manufacture a therapeutic dose of stem cells. Initially, the Company
developed medical devices for ultra rapid freezing and thawing of blood components, which the
Company manufactures and distributes to blood banks and hospitals.
On August 11, 2010, we announced that our board of directors had approved a 1-for-4 reverse stock
split of our common stock, pursuant to previously obtained stockholder authorization. The reverse
stock split, which became effective at the close of business on August 26, 2010, reduced the number
of shares of our common stock issued and outstanding from approximately 56.1 million to
approximately 14 million. All share and per share amounts herein are presented on a
post-reverse-split basis.
Events subsequent to the balance sheet date have been evaluated for inclusion in the accompanying
consolidated financial statements through the date of issuance.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the parent company,
ThermoGenesis Corp., and its wholly-owned subsidiary, Vantus. All significant intercompany
balances and transactions have been eliminated in consolidation.
Use of Estimates
Preparation of financial statements in conformity with U.S. generally accepted accounting
principles and pursuant to the rules and regulations of the SEC requires management to make
estimates and assumptions that affect the reported amounts of assets and liabilities at the date of
the consolidated financial statements and the reported amounts of revenues and expenses during the
reporting period. Estimates are used for, but not limited to, the allowance for doubtful accounts,
slow-moving inventory reserves, depreciation, warranty costs, certain accruals and contingencies.
Actual results could materially differ from the estimates and assumptions used in the preparation
of our consolidated financial statements. Events subsequent to the balance sheet date have been
evaluated for inclusion in the accompanying consolidated financial statements through the date of
issuance.
46
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1.
Summary of Significant Accounting Policies (Continued)
Revenue Recognition
Revenues from the sale of the Company’s products are recognized when persuasive evidence of an
arrangement exists, delivery has occurred (or services have been rendered), the price is fixed or
determinable, and collectability is reasonably assured. The Company generally ships products
F.O.B. shipping point. There is no conditional evaluation on any product sold and recognized as
revenue. All foreign sales are denominated in U.S. dollars. Amounts billed in excess of revenue
recognized are recorded as deferred revenue on the balance sheet.
The Company’s sales are generally through distributors. There is no right of return provided for
distributors. For sales of products made to distributors, the Company considers a number of
factors in determining whether revenue is recognized upon transfer of title to the distributor, or
when payment is received. These factors include, but are not limited to, whether the payment terms
offered to the distributor are considered to be non-standard, the distributor history of adhering
to the terms of its contractual arrangements with the Company, the level of inventories maintained
by the distributor, whether the Company has a pattern of granting concessions for the benefit of
the distributor, and whether there are other conditions that may indicate that the sale to the
distributor is not substantive. The Company currently recognizes revenue primarily on the sell-in
method with its distributors.
Revenue arrangements with multiple elements are divided into separate units of accounting if
certain criteria are met, including whether the delivered item has value to the customer on a
stand-alone basis and whether there is objective and reliable evidence of the fair value of the
undelivered items. Revenue is recognized as specific elements indicated in sales contracts are
executed. If an element is essential to the functionality of an arrangement, the entire
arrangement’s revenue is deferred until that essential element is delivered. The fair value of
each undelivered element that is not essential to the functionality of the system is deferred until
performance or delivery occurs. The fair value of an undelivered element is based on vendor
specific objective evidence or third party evidence of fair value as appropriate. Costs associated
with inconsequential or perfunctory elements in multiple element arrangements are accrued at the
time of revenue recognition. The Company accounts for training and installation as a separate
element of a multiple element arrangement. The Company therefore recognizes the fair value of
training and installation services upon their completion when the Company is obligated to perform
such services.
Service revenue generated from contracts for providing maintenance of equipment is amortized over
the life of the agreement. All other service revenue is recognized at the time the service is
completed.
Milestone payments the Company receives under research and development arrangements are recognized
as revenue upon achievement of the milestone events, which represent the culmination of the
earnings process, and when collectability is reasonably assured. Milestone payments are triggered
by the results of the Company’s development efforts. Accordingly, the milestone payments are
substantially at risk at the inception of the contract, and the amounts of the payments assigned
thereto are commensurate with the milestone achieved. Upon the achievement of a milestone event,
which may include acceptance by the counterparty, the Company has no future performance obligations
related to that milestone as the milestone payments received by the Company are nonrefundable.
47
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Revenue Recognition (Continued)
For licensing agreements pursuant to which the Company receives up-front licensing fees for
products or technologies that will be provided by the Company over the term of the arrangements,
the Company defers the up-front fees and recognizes the fees as revenue on a straight-line method
over the term of the respective license. For license agreements that require no continuing
performance on the Company’s part, license fee revenue is recognized immediately upon grant of the
license.
Shipping and handling fees billed to customers are included in product and other revenues, while
the related costs are included in cost of product and other revenues.
Cash, Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments with a maturity of three months or less at the
time of purchase to be cash equivalents. Short-term investments are comprised of certificates of
deposit and marketable debt securities which are classified as held-to-maturity and have maturities
greater than 90 days, but not exceeding one year.
Management determines the appropriate classification of debt securities at the time of purchase and
reevaluates such designation as of each balance sheet date. Debt securities are classified as
held-to-maturity when the Company has the positive intent and ability to hold the securities to
maturity. Held-to-maturity securities are stated at acquisition cost, adjusted for amortization of
premiums and accretion of discounts to maturity computed under the effective interest method. Such
amortization and accretion is included in interest income. The cost of securities sold is based on
the specific identification method. The fair value of debt securities are determined by quoted
market prices.
Fair Value of Financial Instruments
The carrying values of cash and cash equivalents, accounts receivable, accounts payable and accrued
liabilities, approximate fair value due to their short duration.
In accordance with ASC 820 “Fair Values Measurements and Disclosures” (“ASC 820”), we measure our
cash equivalents (money market funds and certificates of deposit) and short-term investments
(certificates of deposit) at fair value. ASC 820 clarifies that fair value is an exit price,
representing the amount that would be received to sell an asset or paid to transfer a liability in
an orderly transaction between market participants. As such, fair value is a market-based
measurement that should be determined based on assumptions that market participants would use in
pricing an asset or liability.
ASC 820 establishes a valuation hierarchy for disclosure of the inputs to valuation used to measure
fair value. This hierarchy prioritizes the inputs into three broad levels as follows. Level 1
inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities. Level
2 inputs are quoted prices for similar assets and liabilities in active markets or inputs that are
observable for the asset or liability, either directly or indirectly through market corroboration,
for substantially the full term of the financial instrument. Level 3 inputs are unobservable inputs
based on management’s own assumptions used to measure assets and liabilities at fair value. A
financial asset or liability’s classification within the hierarchy is determined based on the
lowest level input that is significant to the fair value measurement.
48
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Fair Value of Financial Instruments (Continued)
Assets measured at fair value on a recurring basis include the following as of June 30, 2010:
| Significant | ||||||||||||||||
| Quoted Prices | Other | Significant | ||||||||||||||
| in Active | Observable | Unobservable | Total Fair | |||||||||||||
| Markets | Inputs | Inputs | Value as of | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | June 30, 2010 | |||||||||||||
Cash equivalents |
||||||||||||||||
Money market funds |
$ | 1,059,000 | — | — | $ | 1,059,000 | ||||||||||
Assets measured at fair value on a recurring basis include the following as of June 30, 2009:
| Significant | ||||||||||||||||
| Quoted Prices | Other | Significant | ||||||||||||||
| in Active | Observable | Unobservable | Total Fair | |||||||||||||
| Markets | Inputs | Inputs | Value as of | |||||||||||||
| (Level 1) | (Level 2) | (Level 3) | June 30, 2009 | |||||||||||||
Cash equivalents |
||||||||||||||||
Money market funds |
$ | 1,059,000 | $ | — | $ | — | $ | 1,059,000 | ||||||||
Certificates of deposit |
$ | — | $ | 3,096,000 | $ | — | $ | 3,096,000 | ||||||||
Short-term investments |
||||||||||||||||
Certificates of deposit |
$ | — | $ | 8,976,000 | $ | — | $ | 8,976,000 | ||||||||
Accounts Receivable and Allowance for Doubtful Accounts
The Company’s receivables are recorded when billed and represent claims against third parties that
will be settled in cash. The carrying value of the Company’s receivables, net of the allowance for
doubtful accounts, represents their estimated net realizable value. The Company estimates its
allowance for doubtful accounts based on historical collection trends, age of outstanding
receivables and existing economic conditions. If events or changes in circumstances indicate that
a specific receivable balance may be impaired, further consideration is given to the collectability
of those balances and the allowance is adjusted accordingly. A customer’s receivable balance is
considered past-due based on its contractual terms. Past-due receivable balances are written-off
when the Company’s internal collection efforts have been unsuccessful in collecting the amount due.
Inventories
Inventories are stated at the lower of cost or market and include the cost of material, labor and
manufacturing overhead. Cost is determined on the first-in, first-out basis.
Equipment
Equipment is recorded at cost. Repairs and maintenance costs are expensed as incurred.
Depreciation for office, computer, machinery and equipment is computed under the straight-line
method over the estimated useful lives. Leasehold improvements are depreciated under the straight
line method over their estimated useful lives or the remaining lease period, whichever is shorter.
49
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Warranty
The Company provides for the estimated cost of product warranties at the time revenue is
recognized. While the Company engages in extensive product quality programs and processes,
including actively monitoring and evaluating the quality of its component suppliers, the Company’s
warranty obligation is affected by product failure rates, material usage and service delivery costs
incurred in correcting a product failure. Should actual product failure rates, material usage or
service delivery costs differ from the Company’s estimates, revisions to the estimated warranty
liability could have a material impact on the Company’s consolidated financial position, cash flows
or results of operations.
Stock-Based Compensation
The Company has four stock-based compensation plans, which are described more fully in Note 7.
The Company recognizes the fair value method of all share-based payment awards granted after
January 1, 2006, in our statements of operations over the requisite vesting period of each award.
Valuation and Amortization Method — The Company estimates the fair value of stock options granted
using the Black-Scholes-Merton option-pricing formula. This fair value is then amortized on a
straight-line basis over the requisite service periods of the awards, which is generally the
vesting period.
Expected Term — For options which the Company has limited available data, the expected term of the
option is based on the simplified method. This simplified method averages an award’s vesting term
and its contractual term. For all other options, the Company’s expected term represents the period
that the Company’s stock-based awards are expected to be outstanding and was determined based on
historical experience of similar awards, giving consideration to the contractual terms of the
stock-based awards, vesting schedules and expectations of future employee behavior.
Expected Volatility — The Company uses the trading history of its common stock in determining an
estimated volatility factor when using the Black-Scholes-Merton option-pricing formula to determine
the fair value of options granted.
Expected Dividend — The Company has not declared dividends and we do not anticipate declaring any
dividends in the foreseeable future. Therefore, the Company uses a zero value for the expected
dividend value factor when using the Black-Scholes-Merton option-pricing formula to determine the
fair value of options granted.
Risk-Free Interest Rate — The Company bases the risk-free interest rate used in the
Black-Scholes-Merton valuation method on the implied yield currently available on U.S. Treasury
zero-coupon issues with the same or substantially equivalent remaining term.
Estimated Forfeitures — When estimating forfeitures, the Company considers voluntary and
involuntary termination behavior as well as analysis of actual option forfeitures.
50
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Stock-Based Compensation (Continued)
The fair value of the Company’s stock options granted to employees for the years ended June 30,
2010, 2009 and 2008 was estimated using the following weighted-average assumptions:
| 2010 | 2009 | 2008 | ||||||||||
Expected life (years) |
3.4 | 3.0 | 3.1 | |||||||||
Risk-free interest rate |
1.6 | % | 1.4 | % | 3.9 | % | ||||||
Expected volatility |
87 | % | 83 | % | 57 | % | ||||||
Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
The weighted average grant date fair value of options granted during the years ended June 30, 2010,
2009 and 2008 was $1.48, $1.68 and $3.28, respectively.
Research and Development
Research and development costs, consisting of salaries and benefits, costs of consumables, facility
costs, contracted services and stock-based compensation that are useful in developing new products,
services, processes or techniques, as well as expenses for activities that may significantly
improve existing products or processes are expensed as incurred. Costs to acquire technologies
that are utilized in research and development and that have no future benefit are expensed when
incurred.
Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk
consist of cash, cash equivalents and investments. The Company places its cash in checking
accounts, money market funds and certificate of deposits with reputable financial institutions,
which are within the Federal Deposit Insurance Corporation insurable limits. The Company has not
experienced any realized losses on its deposits of cash, cash equivalents and investments.
The Company manufactures and sells thermodynamic devices principally to the blood component
processing industry and performs ongoing evaluations of the credit worthiness of its customers.
The Company believes that adequate provisions for uncollectible accounts have been made in the
accompanying consolidated financial statements. To date, we have not experienced significant
credit related losses.
Segment Reporting
The Company operates in a single segment providing medical devices and disposables to hospitals and
blood banks throughout the world which utilize the equipment to process blood components.
51
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Income Taxes
Effective July 1, 2007, we adopted the provisions of ASC topic 740 “Income Taxes”. There was no
impact on our financial statements upon adoption. Because of our historical significant net
operating losses, we have not been subject to income tax since inception. The tax years 1993-2009
remain open to examination by the major taxing jurisdictions to which we are subject. The
Company’s policy is to recognize interest and penalties related to the underpayment of income taxes
as a component of income tax expense. To date, there have been no interest or penalties charged to
the Company in relation to the underpayment of income taxes. There were no unrecognized tax
benefits during all the periods presented.
The Company accounts for income taxes using the liability method. Under this method, deferred tax
assets are based on differences between the carrying amounts of assets and liabilities for
financial reporting purposes and the amounts used for income tax purposes and are measured using
the enacted tax rates and laws that are expected to be in effect when the differences are expected
to reverse. These deferred tax assets include net operating loss carryforwards, research credits
and deferred revenue. The net deferred tax asset has been fully offset by a valuation allowance
because of our history of losses. Utilization of operating losses and credits may be subject to
annual limitation due to ownership change provisions of the Internal Revenue Code of 1986 and
similar state provisions. The annual limitation may result in the expiration of net operating
losses and credits before utilization.
Net Loss per Share
Net loss per share is computed by dividing the net loss to common stockholders by the weighted
average number of common shares outstanding. The calculation of the basic and diluted earnings per
share is the same for all periods presented, as the effect of the potential common stock
equivalents is anti-dilutive due to the Company’s net loss position for all periods presented.
Anti-dilutive securities, which consist of stock options and common stock restricted awards, that
were not included in diluted net loss per common share, were 1,230,830, 774,785 and 753,609 as of
June 30, 2010, 2009 and 2008, respectively.
Reclassifications
Certain amounts in the prior year’s financial statements have been reclassified to conform with the
2010 presentation. These reclassifications had no effect on previously reported total assets, net
loss or stockholders’ equity.
52
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Recently Adopted Accounting Pronouncements
In February 2007, the FASB issued ASC topic 825-10 (“ASC 825”). ASC 825 allows entities to
voluntarily choose to measure many financial assets and financial liabilities at fair value. The
Company adopted ASC 825 effective July 1, 2008 and has not elected the fair value option for its
financial instruments. The adoption of ASC 825 did not have an impact on the Company’s
consolidated results of operations or financial condition.
In June 2007, the FASB ratified a consensus opinion reached by the Emerging Issues Task Force
(“EITF”) on EITF Issue 07-3, Accounting for Nonrefundable Advance Payments for Goods or Services
Received for Use in Future Research and Development Activities (“EITF 07-3”) which is now included
in ASC topic 730 (“ASC 730”). The guidance in ASC 730 requires the Company to defer and capitalize
nonrefundable advance payments made for goods or services to be used in research and development
activities until the goods have been delivered or the related services have been performed. If the
goods are no longer expected to be delivered nor the services expected to be performed, the Company
would be required to expense the related capitalized advance payments. The consensus in ASC 730
was effective for fiscal years, and interim periods within those fiscal years, beginning after
December 15, 2007 and is applied prospectively to new contracts entered into on or after December
15, 2007. The Company adopted ASC 730 effective July 1, 2008. The adoption of ASC 730 did not
have a material impact on the Company’s results of operations or financial condition.
In December 2007, the FASB issued ASC topic 808 “Collaborative Arrangements” (“ASC 808”). ASC 808
defines collaborative arrangements and establishes reporting requirements for transactions between
participants in a collaborative arrangement and between participants in the arrangement and third
parties. ASC 808 also establishes the appropriate income statement presentation and classification
for joint operating activities and payments between participants, as well as the sufficiency of the
disclosures related to these arrangements. ASC 808 is effective for fiscal years beginning after
December 15, 2008. ASC 808 shall be applied retrospectively to all prior periods presented for all
collaborative arrangements existing as of the effective date. The adoption of ASC 808 did not have
a material impact on the Company’s results of operations or financial condition.
53
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Recently Adopted Accounting Pronouncements (Continued)
In December 2007, the FASB issued ASC topic 805 “Business Combinations” (“ASC 805”). The statement
retains the purchase method of accounting for acquisitions, but requires a number of changes,
including changes in the way assets and liabilities are recognized in the purchase accounting. It
also changes the recognition of assets acquired and liabilities assumed arising from contingencies,
requires the capitalization of in-process research and development at fair value and requires the
expensing of acquisition-related costs as incurred. ASC 805 is effective for business combinations
for which the acquisition date is on or after the beginning of the first annual reporting period
beginning on or after December 15, 2008. The Company will assess the potential impact of the
adoption of ASC 805 if and when a future acquisition occurs.
In April 2009, the FASB issued ASC subtopic 825-10-65-1 (“ASC 825-10-65-1”). ASC 825-10-65-1
requires disclosures about fair values of financial instruments for interim periods of publicly
traded companies. These disclosures include fair value methods and significant assumptions used.
The adoption of ASC 825-10-65-1 did not have a material impact on the Company’s results of
operations or financial condition.
In June 2009, the FASB issued ASC topic 105, “Generally Accepted Accounting Principles” (“ASC
105”). The statement confirmed that the FASB Accounting Standards Codification (the
“Codification”) will become the single official source of authoritative U.S. GAAP (other than
guidance issued by the SEC, superseding existing FASB, American Institute of Certified Public
Accountants, EITF, and related literature. After that date, only one level of authoritative U.S.
GAAP will exist. All other literature will be considered non-authoritative. The Codification does
not change U.S. GAAP; instead, it introduces a new structure that is organized in an easily
accessible, user-friendly online research system. The Codification, which changes the referencing
of financial standards, becomes effective for interim and annual periods ending on or after
September 15, 2009. The adoption of ASC 105 did not have a material impact on the Company’s results
of operations or financial condition.
In January 2010, the FASB issued Accounting Standards Update (“ASU”) No. 2010-06, “Fair Value
Measurements and Disclosures (Topic 820) — Improving Disclosures about Fair Value Measurements”
(“ASU 2010-06”). ASU 2010-06 amends ASC Topic 820, “Fair Value Measurements and Disclosures” (“ASC
820”) to require additional disclosures regarding fair value measurements. Specifically, ASU
2010-06 requires entities to disclose additional information regarding (i) the reconciliation of
recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis,
(ii) the amounts of significant transfers between Level 1 and Level 2 of the fair value hierarchy
and the reasons for these transfers and (iii) the reasons for any transfers in or out of Level 3.
In addition to these new disclosure requirements, ASU 2010-06 also amends ASC 820 to further
clarify existing guidance pertaining to the level of disaggregation at which fair value disclosures
should be made and the requirements to disclose information about the valuation techniques and
inputs used in estimating Level 2 and Level 3 fair value measurements. Our adoption of the
requirements of this guidance on January 1, 2010, except for the requirement to separately disclose
information about purchases, sales, issuances, and settlements in the reconciliation of recurring
Level 3 measurements on a gross basis which becomes effective for fiscal years (and interim periods
within those fiscal years) beginning after December 15, 2010, did not have a material impact on the
Company’s results of operations or financial condition.
54
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
1. Summary of Significant Accounting Policies (Continued)
Recently Adopted Accounting Pronouncements (Continued)
In February 2010, the FASB issued ASU No. 2010-09, “Subsequent Events (Topic 855) — Amendments to
Certain Recognition and Disclosure Requirements” (“ASU 2010-09”). ASU 2010-09 amends ASC Topic 855
to remove the requirement for an SEC filer to disclose the date through which subsequent events
have been evaluated both in issued and revised financial statements. ASU 2010-09 was effective
immediately. The adoption of ASU 2010-09 did not have a material impact on the Company’s results
of operations or financial condition.
Recently Issued Accounting Pronouncements
In September 2009, the FASB issued ASU No. 2009-14, “Certain Revenue Arrangements that Include
Software Elements-A Consensus of the FASB Emerging Issues Task Force” which amends ASC 985-605,
“Software Revenue Recognition” to exclude tangible products that include software and non-software
components that function together to deliver the product’s essential functionality. This Issue
shall be applied on a prospective basis for revenue arrangements entered into or materially
modified in fiscal years beginning on or after June 15, 2010. Early adoption is permitted,
provided that the guidance is retroactively applied at the beginning of the year of adoption. The
Company is currently evaluating the potential impact of ASU 985 on the Company’s results of
operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, “Revenue Recognition (Topic 605):
Multiple-Deliverable Revenue Arrangements (“ASU 2009-13”). ASU 2009-13 addresses the accounting
for multiple-deliverable arrangements to enable vendors to account for products or services
separately rather than as a combined unit and modifies the manner in which the transaction
consideration is allocated across the separately identified deliverables. ASU 2009-13
significantly expands the disclosure requirements for multiple-deliverable revenue arrangements.
ASU 2009-13 will be effective for the first annual reporting period beginning on or after June 15,
2010, and may be applied retrospectively for all periods presented or prospectively to arrangements
entered into or materially modified after the adoption date. Early adoption is permitted, provided
that the guidance is retroactively applied to the beginning of the year of adoption. The Company
is currently evaluating the potential impact of ASU 2009-13 on the Company’s results of operations
or financial condition.
55
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
2. Short-Term Investments
The following is a summary of held-to-maturity securities:
| Gross | Gross | |||||||||||||||
| Amortized | Unrealized | Unrealized | Estimated | |||||||||||||
| Cost | Gains | Losses | Fair Value | |||||||||||||
June 30, 2009 |
||||||||||||||||
Certificate of deposit |
$ | 8,976,000 | — | — | $ | 8,976,000 | ||||||||||
3. Inventories
Inventories consisted of the following at June 30:
| 2010 | 2009 | |||||||
Raw materials |
$ | 1,496,000 | $ | 1,116,000 | ||||
Work in process |
1,690,000 | 1,871,000 | ||||||
Finished goods |
1,848,000 | 2,246,000 | ||||||
| $ | 5,034,000 | $ | 5,233,000 | |||||
4. Equipment
Equipment consisted of the following at June 30:
| 2010 | 2009 | Estimated Useful Life | ||||||||||
Machinery and equipment |
$ | 3,113,000 | $ | 2,916,000 | 3-10 years or lease term | |||||||
Computer and software |
904,000 | 1,171,000 | 2-5 years | |||||||||
Office equipment |
639,000 | 706,000 | 5-10 years | |||||||||
Leasehold improvements |
286,000 | 307,000 | 5 years or lease term | |||||||||
| 4,942,000 | 5,100,000 | |||||||||||
Less accumulated depreciation and
amortization |
(3,241,000 | ) | (3,316,000 | ) | ||||||||
| $ | 1,701,000 | $ | 1,784,000 | |||||||||
56
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
5. Liabilities
License and Option Agreements
In June 2010, the Company and Asahi entered into an amendment (the “Amendment”) of their
Distribution and License Agreement, originally effective March 28, 2005. Under the terms of the
Amendment, Asahi will obtain exclusive rights to distribute the CryoSeal System (“Products”) in
South Korea, North Korea, Taiwan, the Peoples Republic of China, the Philippines, Thailand,
Singapore, India and Malaysia. These rights shall include the exclusive right to market, distribute
and sell the processing disposables and Thrombin Reagent for production of thrombin in a stand
alone product. The Company will provide support to Asahi in the form of maintaining manufacturing
capabilities of the CryoSeal System products until the earlier of when Asahi receives regulatory
approval from the MHLW or December 31, 2012, upon which the Company shall have no further
obligation to manufacture the Products. Asahi shall continue to have the right to manufacture such
Products in Japan and shall additionally have a non-exclusive right to manufacture such Products
outside of Japan and would make royalty payments to the Company for Products it manufactures and
sells. The Amendment extends the agreement eight years with automatic one year renewals. Asahi
paid $1,000,000, of which $400,000 is refundable if the Company fails to provide technical support
or maintain manufacturing capabilities as specified in the Amendment.
In connection with the above-described Amendment, the Company and Asahi also entered into an Option
Agreement (“Option Agreement”). Under the terms of the Option Agreement, the Company granted Asahi
an option to purchase certain intellectual property rights of the Company related to the CryoSeal
System, including, but not limited to, patents and patent applications, FDA-PMA ownership relating
to the products and certain related contracts and contractual relationships. Asahi may exercise the
Option Agreement at any time after the effective date of the Amendment, but no later than the
earlier of the fifth anniversary of the Amendment or 90 days after receiving regulatory approval
from the MHLW.
We allocated $250,000 of the $1,000,000 to the additional license rights granted under the
Amendment based on the fair value of those rights. The $250,000 is being amortized on the straight
line basis over the term of the Amendment, or 8 years. Accordingly, at June 30, 2010, $219,000 is
included in long-term deferred revenue.
The remaining $750,000 has been allocated to offset future expenses we expect to
incur in fulfilling our obligations under the Amendment, of which $350,000 will be released as the
expenses are incurred and the remainder will be released upon completion of the refundable
activities. At June 30, 2010, $71,000 has been released, $229,000 is expected to be released in
the next year and $450,000 has been classified as other non-current liabilities
Other current liabilities consisted of the following at June 30:
| 2010 | 2009 | |||||||
Accrued warranty reserves |
$ | 1,113,000 | $ | 529,000 | ||||
Asahi prepayment |
229,000 | — | ||||||
Accrued professional fees |
353,000 | 274,000 | ||||||
Other accrued liabilities |
333,000 | 523,000 | ||||||
| $ | 2,028,000 | $ | 1,326,000 | |||||
57
Table of Contents
6. Commitments and Contingencies
Operating Leases
The Company leases its facilities pursuant to two operating leases, which contain scheduled rent
increases. One facility lease expires in 2016, has a cancellation option beginning November 1,
2014 and has a renewal option of five years. The other facility lease expires in 2012, is
cancelable with a six month notice and does not have an option to renew. The Company recognizes
rent expense on a straight-line basis over the terms of the respective facility lease. The annual
future minimum lease payments for the non-cancelable operating lease are as follows:
2011 |
$ | 353,000 | ||
2012 |
363,000 | |||
2013 |
373,000 | |||
2014 |
387,000 | |||
2015 |
397,000 | |||
Thereafter |
550,000 | |||
Total |
$ | 2,423,000 | ||
Rent expense was $760,000, $751,000 and $697,000 for the years ended June 30, 2010, 2009 and 2008,
respectively.
Contingencies
In the normal course of operations, the Company may have disagreements or disputes with customers,
employees or vendors. These disputes are seen by the Company’s management as a normal part of
business, and there are no pending actions currently or no threatened actions that management
believes would have a significant material impact on the Company’s financial position, results of
operations or cash flow.
A product manufacturing supplier made purchases of raw materials based on company provided
forecasts, which the Company may be required to pay for as part of normal manufacturing processes,
including scrap and obsolete parts that result from the Company’s product design changes, and or
discontinuation of manufacturing by a particular vendor. These are normal and standard
manufacturing terms, and the company recorded an estimated loss contingency of $137,000 as
management considers it probable that the payment will be made.
Vendor Purchase Commitments
The Company has initiated discussions with a product manufacturing supplier (Supplier) regarding
various manufacturing and quality issues. The Supplier was instructed to suspend production, but
has incurred some costs under existing purchase orders. The Company
recorded an estimated loss contingency of $58,000 during the quarter ended December 31, 2009 as
management considers it probable that the payment will be made.
58
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
6. Commitments and Contingencies (Continued)
Warranty
The Company offers a one year warranty on all of its products. The Company warrants disposable
products through their expiration date. The Company periodically assesses the adequacy of its
recorded warranty liabilities and adjusts the amounts as necessary.
Changes in the Company’s product liability which is included in accrued liabilities during the
period are as follows:
| For years ended June 30, | ||||||||
| 2010 | 2009 | |||||||
Beginning balance |
$ | 529,000 | $ | 507,000 | ||||
Warranties issued during the period |
303,000 | 267,000 | ||||||
Settlements made during the period |
(232,000 | ) | (667,000 | ) | ||||
Changes in liability for pre-existing
warranties during
the period, including expirations |
513,000 | 422,000 | ||||||
Ending balance |
$ | 1,113,000 | $ | 529,000 | ||||
As a result of various quality issues experienced by high usage customers of the AXP disposable bag
sets, the Company made revisions to its estimated warranty liability for the three month period
ended September 30, 2009. The Company recorded a change in estimate, which increased the Company’s
cost of revenues and net loss by $190,000 and net loss per share of $0.01. There were no changes
to the estimated warranty liability during the quarters ended December 31, 2009, March 31, 2010 and
June 30, 2010.
In the prior fiscal year, as a result of the voluntary recall of certain lots of the AXP disposable
bag sets, the Company made revisions to its estimated warranty liability. These changes in
estimates increased the Company’s cost of revenues and net loss by $520,000 and net loss per share
of $0.04 for the quarter ended September 30, 2008 and decreased the Company’s cost of revenues and
net loss by $115,000 and net loss per share by $0.01 for the quarter ended December 31, 2008.
There were no changes to the estimated warranty liability for the voluntary recall during the
quarters ended March 31, 2009 or June 30, 2009.
59
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
7. Stockholders’ Equity
Common Stock
As of June 30, 2010, the Company had 1,560,433 shares of common stock reserved for future issuance.
On August 11, 2010, we announced that our board of directors had approved a 1-for-4 reverse stock
split of our common stock, pursuant to previously obtained stockholder authorization. The reverse
stock split, which became effective at the close of business on August 26, 2010, reduced the number
of shares of our common stock issued and outstanding from approximately 56.1 million to
approximately 14 million. All share and per share amounts herein are presented on a
post-reverse-split basis.
Stock Options
The Amended 1994 Stock Option Plan (“1994 Plan”) permits the grant of stock or options to
employees, directors and consultants. A total of 362,500 shares were approved by the stockholders
for issuance under the 1994 Plan. Options are granted at prices that are equal to 100% of the fair
market value on the date of grant, and expire over a term not to exceed ten years. Options
generally vest ratably over a five-year period, unless otherwise determined by the Board of
Directors. The 1994 Plan, but not the options granted, expired in October 2004.
The Amended 1998 Stock Option Plan (“1998 Plan”) permits the grant of stock or options to
employees, directors and consultants. A total of 949,500 shares were approved by the stockholders
for issuance under the 1998 Plan. Options are granted at prices that are equal to 100% of the fair
market value on the date of grant, and expire over a term not to exceed ten years. Options
generally vest ratably over three to five years, unless otherwise determined by the Board of
Directors. The 1998 Plan, but not the options granted, expired in February 2008.
The 2002 Independent Directors Equity Incentive Plan (“2002 Plan”) permits the grant of stock or
options to independent directors. A total of 87,500 shares were approved by the stockholders for
issuance under the 2002 Plan. Options are granted at prices which are equal to 100% of the fair
market value on the date of grant, and expire over a term not to exceed ten years. Options
generally vest immediately, unless otherwise determined by the Board of Directors.
The 2006 Equity Incentive Plan (“2006 Plan”) permits the grant of options, restricted stock, stock
bonuses and stock appreciation rights to employees, directors and consultants. Under the 2006
Plan, the number of shares of common stock equal to 6% of the number of outstanding shares of the
Company are authorized to be issued. The number of shares available to grant for awards adjusts at
the beginning of each fiscal year if additional options to purchase shares of common stock were
issued in the preceding fiscal year. As of June 30, 2010 there were 841,395 shares approved under
the Plan for issuance.
60
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
7. Stockholders’ Equity (Continued)
Stock Compensation Expense
At June 30, 2010, the total compensation cost related to unvested stock-based awards granted to
employees under the Company’s stock option plans but not yet recognized was $942,000, net of
estimated forfeitures of $146,000. This cost will be amortized on a straight-line basis over a
weighted-average period of approximately two years and will be adjusted for subsequent changes in
estimated forfeitures. The total fair value of options vested during the years ended June 30,
2010, 2009 and 2008 was $525,000, $342,000 and $377,000.
The Company issues new shares of common stock upon exercise of stock options. The following is a
summary of option activity for the Company’s stock option plans:
| Weighted- | Weighted- | |||||||||||||||
| Average | Average | Aggregate | ||||||||||||||
| Number of | Exercise | Remaining | Intrinsic | |||||||||||||
| Shares | Price | Contractual Life | Value | |||||||||||||
Outstanding at June 30, 2009 |
769,910 | $ | 6.60 | |||||||||||||
Granted |
616,250 | $ | 2.44 | |||||||||||||
Forfeited or Expired |
(160,205 | ) | $ | 7.64 | ||||||||||||
Exercised |
— | — | ||||||||||||||
Outstanding at June 30, 2010 |
1,225,955 | $ | 4.36 | 3.2 | — | |||||||||||
Vested and Expected to Vest
at June 30, 2010 |
1,052,818 | $ | 4.64 | 2.8 | — | |||||||||||
Exercisable at June 30, 2010 |
331,367 | $ | 8.40 | 2.1 | — | |||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the
underlying awards and the quoted price of the Company’s common stock. There were no options that
were exercised during the years ended June 30, 2010 and 2009. During the year ended June 30, 2008,
the aggregate intrinsic value of options exercised under the Company’s stock option plans was
$248,000, determined as of the date of option exercise.
The following table summarizes information about stock options outstanding at June 30, 2010:
| Weighted- | ||||||||||||||||||||
| Average | Weighted- | Weighted- | ||||||||||||||||||
| Range of | Remaining | Average | Average | |||||||||||||||||
| Exercise | Number | Contractual | Exercise | Number | Exercise | |||||||||||||||
| Prices | Outstanding | Life | Price | Exercisable | Price | |||||||||||||||
$2.24-$3.20 |
957,250 | 3.6 | $ | 2.52 | 125,162 | $ | 2.68 | |||||||||||||
$5.36-$7.32 |
72,500 | 2.2 | $ | 6.24 | 38,333 | $ | 6.20 | |||||||||||||
$9.24-$11.52 |
112,814 | 1.1 | $ | 9.52 | 86,731 | $ | 9.60 | |||||||||||||
$14.32-$20.04 |
83,391 | 2.2 | $ | 17.00 | 81,141 | $ | 16.92 | |||||||||||||
| 1,225,955 | 331,367 | |||||||||||||||||||
61
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
7. Stockholders’ Equity (Continued)
Common Stock Restricted Awards
On April 26, 2007, the Company’s Chief Executive Officer (“incumbent CEO”) was granted 125,000
shares of restricted common stock with three year vesting. The grant had a value of $1,700,000
based on the fair market value of the Company’s stock on the grant date. The vesting is subject to
acceleration upon certain conditions: (1) entry into the Employment Agreement for a term of three
years, (2) Company’s engagement of a new Chief Executive Officer (“new CEO”) and confirmation by
the Board of Directors, and (3) development and Board approval of a transition plan for the new CEO
and transition of the incumbent CEO to the position of CTA. However, in accordance with the 2006
Plan, performance based stock option awards must have a minimum vesting period of at least one
year. The performance conditions were all satisfied by May 2008, therefore, the compensation
expense of $1,700,000 was amortized over one year of which $1,417,000 and $283,000 has been
included in the accompanying consolidated statement of operations in fiscal 2008 and 2007,
respectively. In connection with the vesting of the restricted stock, the election was made by the
CTA to satisfy the applicable federal income tax withholding obligation by a net share settlement,
pursuant to which the Company withheld 44,554 shares and used the deemed proceeds from those shares
to pay the income tax withholding. The net share settlement is deemed to be a repurchase by the
Company of its common stock.
During fiscal 2007, the Company’s Compensation Committee granted 2,500 shares of restricted common
stock to an officer, one half vesting immediately and one half on the first anniversary of the
grant date. The shares had a fair market value of $13.60 per share on the date of grant.
On August 9, 2004, the Company’s Compensation Committee approved the grant of 12,729 shares of
restricted common stock to selected members of management and key employees, excluding its
executive officers, which had a fair market value of $14.32 per share on the date of grant. These
common stock restricted awards vest in three equal installments, on the date of grant and the first
and second anniversary of the grant date. One third vested immediately on the grant date and the
remaining value was amortized on a straight-line basis over the remaining two year service period.
62
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
8. Concentrations
At June 30, 2010, the Company had one distributor that accounted for 42% of accounts receivable. At
June 30, 2009, the Company had two distributors that individually accounted for 43% and 19% of
accounts receivable.
Revenues from one significant distributor totaled $9,890,000 or 43% of net revenues, $7,735,000 or
39% of net revenues and $13,310,000 or 61% of net revenues during the years ended June 30, 2010,
2009 and 2008, respectively. For the year ended June 30, 2010, approximately 50% of the
significant distributor’s revenue came from one customer.
The following is a summary of product revenues as a percentage of total net revenues for the
Company’s principal product lines:
| 2010 | 2009 | 2008 | ||||||||||
AXP |
40 | % | 35 | % | 34 | % | ||||||
BioArchive |
36 | % | 41 | % | 42 | % | ||||||
MXP/Res-Q |
5 | % | 2 | % | — | |||||||
ThermoLine |
7 | % | 12 | % | 9 | % | ||||||
CryoSeal |
3 | % | 2 | % | 7 | % | ||||||
The Company had sales to customers as follows for the years ended June 30:
| 2010 | 2009 | 2008 | ||||||||||
United States |
$ | 13,827,000 | $ | 11,489,000 | $ | 12,901,000 | ||||||
Asia |
4,303,000 | 3,544,000 | 2,125,000 | |||||||||
Europe |
3,117,000 | 2,510,000 | 5,565,000 | |||||||||
South America |
1,405,000 | 1,859,000 | 1,208,000 | |||||||||
Other |
436,000 | 397,000 | 147,000 | |||||||||
| $ | 23,088,000 | $ | 19,799,000 | $ | 21,946,000 | |||||||
63
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
9. Income Taxes
The reconciliation of federal income tax attributable to operations computed at the federal
statutory tax rate of 34% to income tax expense (benefit) is as follows for the years ended June
30:
| 2010 | 2009 | 2008 | ||||||||||
Statutory federal income tax benefit |
($1,766,000 | ) | ($2,907,000 | ) | ($3,122,000 | ) | ||||||
Net operating loss with no tax benefit |
1,766,000 | 2,907,000 | 3,122,000 | |||||||||
Total federal income tax |
$ | — | $ | — | $ | — | ||||||
At June 30, 2010, the Company had net
operating loss carryforwards for federal and state income tax
purposes of approximately $86,650,000 and $55,587,000 respectively, that are available to offset future
income. The federal and state loss carryforwards expire in various
years between 2011 and 2030,
and 2013 and 2020, respectively.
At June 30, 2010, the Company has research and experimentation credit carryforwards of
approximately $1,158,000 for federal tax purposes that expire in various years between 2011 and 2030,
and $1,266,000 for state income tax purposes that do not have an expiration date.
Significant components of the Company’s deferred tax assets and liabilities for federal and state
income taxes are as follows:
| June 30, 2010 | June 30, 2009 | |||||||
Deferred tax assets: |
||||||||
Net operating loss carryforwards |
$ | 32,530,000 | $ | 30,862,000 | ||||
Income tax credits |
2,012,000 | 1,976,000 | ||||||
Other |
2,773,000 | 2,527,000 | ||||||
Total deferred taxes |
37,315,000 | 35,365,000 | ||||||
Valuation allowance |
(37,315,000 | ) | (35,365,000 | ) | ||||
Net deferred taxes |
$ | — | $ | — | ||||
The valuation allowance increased by approximately $1,950,000, $3,595,000 and $3,041,000 in 2010,
2009 and 2008, respectively. As of June 30, 2010, the Company has a benefit of approximately
$1,858,000 related to stock option deductions, which will be credited to paid-in capital when
realized, of which $1,624,000 is included in the valuation allowance.
Because of the “change of ownership” provisions of the Tax Reform Act of 1986, a portion of the
Company’s federal net operating loss and credit carryovers may be subject to an annual limitation
regarding their utilization against taxable income in future periods.
64
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
10. Employee Retirement Plan
The Company sponsors an Employee Retirement Plan, generally available to all employees, in
accordance with Section 401(k) of the Internal Revenue Code. Employees may elect to contribute up
to the Internal Revenue Service annual contribution limit. Under this Plan, at the discretion of
the Board of Directors, the Company may match a portion of the employees’ contributions. We made
no discretionary or matching contributions to the Plan for the years ended June 30, 2010, 2009 and
2008.
11. Subsequent Events
On August 11, 2010, we announced that our board of directors had approved a 1-for-4 reverse stock
split of our common stock, pursuant to previously obtained stockholder authorization. The reverse
stock split, which became effective at the close of business on August 26, 2010, reduced the number
of shares of our common stock issued and outstanding from approximately 56.1 million to
approximately 14 million. All share and per share amounts herein are presented on a
post-reverse-split basis.
On July 22, 2010, the Company gave a “Notice to Vacate” in 180 days or January 21, 2011 on one
facility with an original expiration of March 2012. Under the terms of the lease, the Company will
pay $111,000 as the early termination fee.
12. Unaudited Quarterly Financial Data
The following tables provide quarterly data for fiscal years ended June 30, 2010 and 2009.
| First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||||
| Ended | Ended | Ended | Ended | |||||||||||||
| September 30, | December 31, | March 31, | June 30, | |||||||||||||
| 2009 | 2009 | 2010 | 2010 | |||||||||||||
Net revenues |
$ | 5,193,000 | $ | 5,955,000 | $ | 4,764,000 | $ | 7,176,000 | ||||||||
Gross Profit |
$ | 1,557,000 | $ | 2,011,000 | $ | 1,401,000 | $ | 2,476,000 | ||||||||
Net loss |
($2,189,000 | ) | ($1,468,000 | ) | ($1,365,000 | ) | ($171,000 | ) | ||||||||
Per share data: |
||||||||||||||||
Basic and diluted net loss per
common share |
($0.16 | ) | ($0.10 | ) | ($0.10 | ) | ($0.01 | ) | ||||||||
Shares used in computing per
share data |
14,023,240 | 14,023,240 | 14,023,240 | 14,023,240 | ||||||||||||
65
Table of Contents
THERMOGENESIS CORP.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (CONTINUED)
12. Unaudited Quarterly Financial Data (Continued)
| First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||||
| Ended | Ended | Ended | Ended | |||||||||||||
| September 30, | December 31, | March 31, | June 30, | |||||||||||||
| 2008 | 2008 | 2009 | 2009(1) | |||||||||||||
Net revenues |
$ | 4,502,000 | $ | 6,126,000 | $ | 5,148,000 | $ | 4,023,000 | ||||||||
Gross Profit |
$ | 1,280,000 | $ | 2,213,000 | $ | 1,794,000 | $ | 406,000 | ||||||||
Net loss |
($2,679,000 | ) | ($1,695,000 | ) | ($1,092,000 | ) | ($3,084,000 | ) | ||||||||
Per share data: |
||||||||||||||||
Basic and diluted net loss per
common share |
($0.19 | ) | ($0.12 | ) | ($0.08 | ) | ($0.22 | ) | ||||||||
Shares used in computing per
share data |
14,006,990 | 14,006,990 | 14,023,240 | 14,023,240 | ||||||||||||
| (1) | During the fourth quarter of 2009, the gross margin was impacted by increases in inventory reserves and write-offs of obsolete inventory of $1,006,000. Selling, general and administrative expenses were impacted by a severance accrual of $175,000 and a loss on impairment of equipment of $150,000. |
66
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
The Company carried out an evaluation, under the supervision and with the participation of the
Company’s management, including the Company’s Principal Executive Officer along with the Company’s
Principal Financial Officer, of the effectiveness of the design of the Company’s disclosure
controls and procedures (as defined by Exchange Act Rule 13a-15(e) and 15a-15(e)) as of the end of
the Company’s fiscal year pursuant to Exchange Act Rule 13a-15. Based upon that evaluation, the
Company’s Principal Executive officer along with the Company’s Principal Financial Officer
concluded that the Company’s disclosure controls and procedures are effective.
Management’s Report on Internal Control over Financial Reporting
The report of management required under 9A is considered in Item 8 Part II of this Annual Report on
Form 10-K under the heading “Management’s Report on Internal Control over Financial Reporting.”
Attestation Report of Independent Registered Public Accounting Firm
The attestation report required under this Item 9A is contained in Item 8 of Part II of this Annual
Report on Form 10-K under the heading “Report of Independent Registered Public Accounting Firm on
Internal Control over Financial Reporting.”
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal controls over financial reporting that occurred
during the fiscal quarter ended June 30, 2010, that have materially affected, or are reasonably
likely to materially affect its internal controls over financial reporting. The Company believes
that a control system, no matter how well designed and operated, cannot provide absolute assurance
that the objectives of the control system are met, and no evaluation of controls can provide
absolute assurance that all control issues and instances of fraud, if any, within any company have
been detected.
| ITEM 9B. | OTHER INFORMATION |
None.
67
Table of Contents
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this Item will be included in and is hereby incorporated by reference
from our Proxy Statement for the 2010 Annual Meeting of Stockholders. We have adopted a Code of
Ethics applicable to all employees including our CEO and CFO. A copy of the Code of Ethics is
available at www.thermogenesis.com.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this Item will be included in and is hereby incorporated by reference
from our Proxy Statement for the 2010 Annual Meeting of Stockholders.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this Item will be included in and is hereby incorporated by reference
from our Proxy Statement for the 2010 Annual Meeting of Stockholders.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this Item will be included in and is hereby incorporated by reference
from our Proxy Statement for the 2010 Annual Meeting of Stockholders.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this Item will be included in and is hereby incorporated by reference
from our Proxy Statement for the 2010 Annual Meeting of Stockholders.
68
Table of Contents
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
The following documents are filed as a part of this report on Form 10-K.
| Page Number | ||||
(a) (1) Financial Statements |
||||
| 41 | ||||
| 42 | ||||
| 43 | ||||
| 44 | ||||
| 45 | ||||
| 46 | ||||
Management’s Report on Internal Control over Financial Reporting is contained as part of this
report under Item 9A “Controls and Procedures.” |
||||
(a) (2) Financial Statement Schedules |
||||
| 74 | ||||
All other financial statement schedules have been omitted because they are not required or
not applicable. |
||||
(b) Exhibits |
||||
Exhibits required by Item 601 of Regulation S-K are listed in the Exhibit Index on the next
page, which are incorporated herein by this reference. |
||||
69
Table of Contents
Exhibit Description
3.1
|
(a) | Amended and Restated Certificate of Incorporation (1) | ||
| (b) | Revised Bylaws (2) | |||
10.1
|
(a) | License Agreement with Pall/Medsep Corporation (3) | ||
| (b) | Securities Purchase Agreement dated March 10, 2004 (form) (4) | |||
| (c) | Amended 2002 Independent Directors Equity Incentive Plan (5) | |||
| (d) | Product Development and Supply Agreement with Biomet Biologics (6) | |||
| (e) | First Amendment License Agreement (Clotalyst) (7) | |||
| (f) | Amended & Restated International Distribution Agreement with GEHC (8) | |||
| (g) | Employment Agreement with J. Melville Engle (9) | |||
| (h) | Employment Agreement for Matthew Plavan (10) | |||
| (i) | License and Escrow Agreement with CBR Systems, Inc. (11) | |||
| (j) | Amendment to Amended and Restated International Distribution Agreement with GEHC (12) | |||
| (k) | Amended Distribution and License Agreement with Asahi Kasei Kuraray Medical Co., Ltd. (13) |
| 14 | Amended and Restated Code of Ethics (14) | |
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | |
| 31.1 | Rule 13(a) — 14(a)/15(d) — 14(a) Certification (Principal Executive Officer) | |
| 31.2 | Rule 13(a) — 14(a)/15(d) — 14(a) Certification (Principal Financial Officer) | |
| 32 | Section 1350 Certifications |
Footnotes to Exhibit Index
| (1) | Incorporated by reference to ThermoGenesis’ proxy statement for the Special Meeting hold on December 5, 2005. | |
| (2) | Incorporated by reference to Form 10-KSB for the year ended June 30, 1994. | |
| (3) | Incorporated by reference to Form 8-K dated April 14, 1997. | |
| (4) | Incorporated by reference to Form 8-K dated March 10, 2004. | |
| (5) | Incorporated by reference to Form 8-K dated December 15, 2004. | |
| (6) | Incorporated by reference to Form 8-K dated August 3, 2006. | |
| (7) | Incorporated by reference to Form 10-Q for quarter ended March 31, 2007. | |
| (8) | Incorporated by reference to Form 8-K dated May 7, 2008. | |
| (9) | Incorporated by reference to Form 8-K dated April 15, 2009. | |
| (10) | Incorporated by reference to Form 10-K for the year ended June 30, 2008. | |
| (11) | Incorporated by reference to Form 8-K dated June 18, 2010. | |
| (12) | Incorporated by reference to Form 8-K dated February 4, 2010. | |
| (13) | Incorporated by reference to Form 8-K dated June 16, 2010. | |
| (14) | Incorporated by reference to ThermoGenesis’ proxy statement for the Annual Meeting held on October 28, 2005. |
70
Table of Contents
GLOSSARY OF CERTAIN TECHNICAL TERMS
510(k): Formal notification to FDA to obtain clearance to market the medical device. The device
must be substantially equivalent to devices manufactured prior to 1976, or which have been found
substantially equivalent after that date.
ADIPOSE: Tissue in which fat is stored and which has the cells swollen by droplets of fat.
ADULT STEM CELLS: All non-embryonic stem cells.
ALLOGENEIC CELL THERAPY: Allogeneic cell therapy is a cell therapy that uses a donor’s cells to
treat a patient’s (recipient) disease. These cells could be stem cells, peripheral blood cells or
any other type of cells.
AMNIOTIC FLUID: The watery fluid within the amnion that surrounds the fetus.
AUTOLOGOUS: Autogenous; related to self; originating within an organism itself, as obtaining blood
from the patient for use in the same patient.
BOND MARROW ASPIRATE: When a small amount of bone marrow is removed and tested.
CELLULAR IMMUNOTHERAPY: Immunotherapy is an innovative new treatment approach that empowers the
human immune system to fight off cancer and other debilitating diseases. Cellular immunotherapy
usually starts with harvesting a patient’s own immune cells such as lymphocytes by leukapheresis
procedure. Then the immune cells are exposed to a specific cancer antigen so that the immune cells
can recognize and destroy the cancer cells. After the immune cells have been “re-educated”, the
cells are infused back to the patient to fight the cancer.
CRYOPRECIPITATE: Any precipitate (substance that is separated out of a solution of plasma) that
results from cooling, as cryoglobulin or antihemophilic factor. When used in the context of the
CryoSeal FS System, cryoprecipitate means a “fibrinogen-rich” cryoprecipitate.
CRYOPRESERVATION: Maintaining the life of excised tissue or organs by freezing and storing at very
low temperatures.
CRYOSEAL: System for harvesting fibrinogen-rich cryoprecipitate from a donor’s blood plasma, a
blood component that is currently licensed by the FDA for the treatment of clotting protein
deficient patients.
DEWAR: Container that keeps its contents at a constant and generally low temperature by means of
two external walls between which a vacuum is maintained.
EMBRYONIC STEM CELL: Cells obtained from an embryo when they are still only a few days old.
Because they have only begun to differentiate, these cells have the capability of developing into
any cell in the human body, a fact which makes them potentially important in medicine.
FIBRINOGEN: A blood protein that is converted to fibrin in the clotting of blood.
GLYCEROLIZED: A term used to describe the protection of tissues and in particular red blood cells
from the harmful effects of freezing by the addition of a molecule called glycerol.
71
Table of Contents
GLOSSARY OF CERTAIN TECHNICAL TERMS (CONTINUED)
HEMATOPOIETIC: The formation of blood.
HEMOSTATIC: (1) Checking the flow of blood; (2) an agent that stops the flow of blood.
HOMOGENEOUS: Uniform in structure or composition throughout.
ISCHEMIA: Deficient supply of blood to a body part.
MESENCHYMAL PRECURSOR CELLS (“MPCs”): Mesenchymal precursor cells, or MPCs, are multipotent
precursor cells that can differentiate into a variety of cell types, including: osteoblasts (bone
cells), chondrocytes (cartilage cells) and adipocytes (fat cells). This has been shown in ex vivo
cultures and in vitro or in vivo. Mesenchymal precursor cells are very similar to Mesenchymal stem
cells (MSC). Some believe they are a sub-population of MSC.
MESENCHYMAL STEM CELLS: Multipotent stem cells that can differentiate into a variety of cell
types.
MONONUCLEAR CELLS: A term used to refer to blood cells that under a microscope can be seen to have
a large round shaped nucleus. These cells include monocytes and lymphocytes which are involved in
fighting infections in the body and also stem cells which have the potential to replicate and to
generate new tissues as part of the body’s healing process.
PERIPHERAL BLOOD: A term used to describe the blood that is contained in the body’s circulatory
system. It can be collected by a health care professional by inserting a needle into a vein.
PLURIPOTENT STEM CELLS: A term used to describe stem cells that have the ability to produce more
than one type of body tissue but not all of the different types of body tissues.
REGENERATIVE MEDICINE: The process of creating living, functional tissues to repair or replace
tissue or organ function lost due to age, disease, damage, or congenital defects.
STEM CELLS: Undifferentiated, primitive cells in the bone marrow with the ability both to multiply
and to differentiate into specific blood cells.
THERMOLINE PRODUCTS: (1) Device for the ultra-rapid freezing of human blood plasma; (2) Portable
device for the ultra-rapid freezing of human blood plasma; (3) Device for the rapid thawing of
frozen plasma for hospital patient care.
THROMBIN: Generated in blood clotting that acts on fibrinogen to produce fibrin.
72
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the
registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| ThermoGenesis Corp. |
||||
| Date: September 14, 2010 | By: | /s/ J. MELVILLE ENGLE | ||
| J. Melville Engle, Chief Executive | ||||
| Officer & Director | ||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed
below by the following persons on behalf of the registrant and in the capacities and on the dates
indicated.
| By: | /s/ J. MELVILLE ENGLE | Date: September 14, 2010 | ||
| J. Melville Engle, Chief Executive | ||||
| Officer & Director (Principal Executive Officer) |
||||
| By: | /s/ MATTHEW T. PLAVAN | Dated: September 14, 2010 | ||
| Matthew T. Plavan, CFO & EVP, | ||||
| Business Development (Principal Financial and Accounting Officer) |
||||
| By: | /s/ HUBERT E. HUCKEL | Dated: September 14, 2010 | ||
| Hubert E. Huckel, M.D., Chairman | ||||
| of the Board | ||||
| By: | /s/ DAVID W. CARTER | Dated: September 14, 2010 | ||
| David W. Carter, Director | ||||
| By: | /s/ PATRICK J. MCENANY | Dated: September 14, 2010 | ||
| Patrick J. McEnany, Director | ||||
| By: | /s/ CRAIG W. MOORE | Dated: September 14, 2010 | ||
| Craig W. Moore, Director | ||||
| By: | /s/ MAHENDRA S. RAO | Dated: September 14, 2010 | ||
| Mahendra S. Rao, M.D., Ph.D., | ||||
| Director | ||||
73
Table of Contents
SCHEDULE II
THERMOGENESIS CORP.
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
| Balance at | Charged to | Charged to | Balance at | |||||||||||||||||
| beginning | costs and | other | end of | |||||||||||||||||
| Description | of period | expenses | accounts | Deductions | period | |||||||||||||||
For the year ended June 30, 2010 |
||||||||||||||||||||
Allowance for doubtful accounts: |
$ | 26,000 | $ | 30,000 | $ | 22,000 | $ | 34,000 | ||||||||||||
Reserve for slow moving inventory: |
$ | 1,362,000 | $ | 549,000 | $ | 700,000 | $ | 1,211,000 | ||||||||||||
For the year ended June 30, 2009 |
||||||||||||||||||||
Allowance for doubtful accounts: |
$ | 31,000 | $ | 22,000 | — | $ | 27,000 | $ | 26,000 | |||||||||||
Reserve for slow moving inventory: |
$ | 697,000 | $ | 905,000 | — | $ | 240,000 | $ | 1,362,000 | |||||||||||
For the year ended June 30, 2008 |
||||||||||||||||||||
Allowance for doubtful accounts: |
$ | 50,000 | — | — | $ | 19,000 | $ | 31,000 | ||||||||||||
Reserve for slow moving inventory: |
$ | 915,000 | $ | 53,000 | — | $ | 271,000 | $ | 697,000 | |||||||||||
74
