Attached files
Table of Contents
As filed with the Securities and Exchange Commission on September 10, 2010
Registration No. 333-168439
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
COMPLETE GENOMICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 8731 | 20-3226545 | ||||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) | ||||
2071 Stierlin Court, Mountain View, CA 94043
(650) 943-2800
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Clifford A. Reid, Ph.D.
President and Chief Executive Officer
Complete Genomics, Inc.
2071 Stierlin Court
Mountain View, CA 94043
(650) 943-2800
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Alan C. Mendelson Gregory Chin Latham & Watkins LLP 140 Scott Drive Menlo Park, California 94025-1008 Telephone: (650) 328-4600 Facsimile: (650) 463-2600 |
Donald J. Murray Dewey & LeBoeuf LLP 1301 Avenue of the Americas New York, New York 10019-6092 Telephone: (212) 259-8000 Facsimile: (212) 259-6333 | |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ | |
| Non-accelerated filer x (Do not check if a smaller reporting company) | Smaller reporting company ¨ |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, September 10, 2010
Preliminary Prospectus
Shares

Common Stock
This is the initial public offering of our common stock. No public market currently exists for our common stock. We are offering all of the shares of our common stock offered by this prospectus. We expect the public offering price to be between $ and $ per share.
We have applied to list our common stock on The NASDAQ Global Market under the symbol “GNOM.”
Investing in our common stock involves a high degree of risk. Before buying any shares, you should carefully read the discussion of material risks of investing in our common stock in “Risk Factors” beginning on page 12 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share |
Total | |||||
| Public offering price |
$ | $ | ||||
| Underwriting discounts and commissions |
$ | $ | ||||
| Proceeds, before expenses, to us |
$ | $ | ||||
The underwriters have the option, exercisable on or before the thirtieth day after the date of this prospectus, to purchase up to an additional shares of our common stock at the public offering price, less the underwriting discounts and commissions payable by us, to cover over-allotments, if any. If the underwriters exercise this option in full, the total underwriting discounts and commissions will be $ , and our total proceeds, before expenses, will be $ .
The underwriters are offering the common stock as set forth under “Underwriting.” Delivery of the shares will be made on or about , 2010.
| UBS Investment Bank | Jefferies & Company | |
| Baird | Cowen and Company | |
The date of this prospectus is , 2010.
Table of Contents
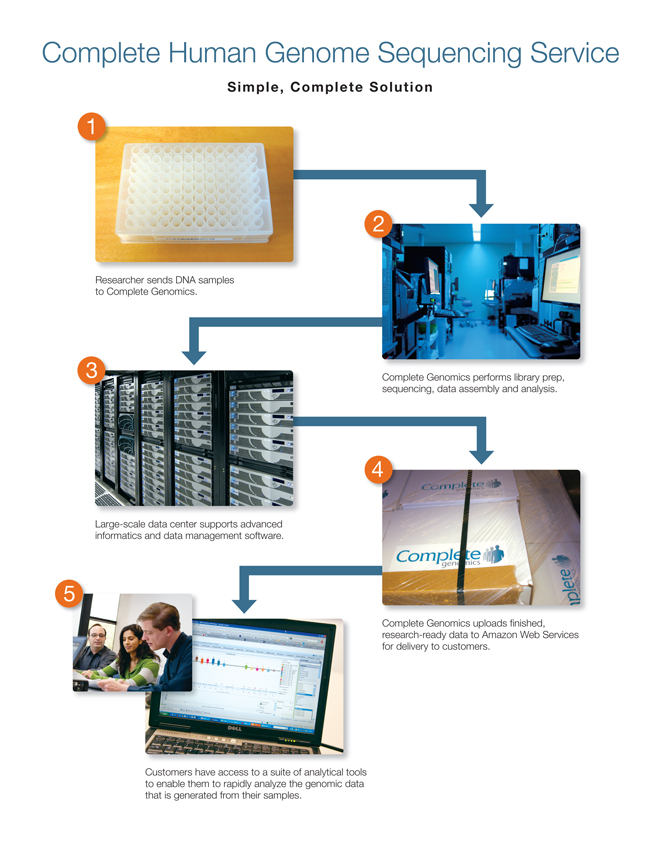
Complete Human Genome Sequencing Service
Simple, Complete Solution
1 Researcher sends DNA samples to complete Genomics.
2 Complete Genomics performs library prep, sequencing, data assembly and analysis.
3 Large-scale data center supports advanced informatics and data management software.
4 Complete Genomics uploads finished, research-ready data to Amazon Web Services for delivery to customers.
5 Customers have access to a suite of analytical tools to enable them to rapidly analyze the genomic data that is generated from their samples.
Table of Contents
You should rely only on the information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with additional information or information different from that contained in this prospectus. We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock.
| 1 | ||
| 12 | ||
| 34 | ||
| 35 | ||
| 36 | ||
| 37 | ||
| 40 | ||
| 44 | ||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
46 | |
| 68 | ||
| 86 | ||
| 97 | ||
| 114 | ||
| 121 | ||
| 125 | ||
| 131 | ||
| Material U.S. Federal Income Tax Consequences to Non-U.S. Holders |
134 | |
| 138 | ||
| 141 | ||
| 144 | ||
| 144 | ||
| 144 | ||
| F-1 |
Through and including , 2010 (25 days after the date of this prospectus), federal securities laws may require all dealers that effect transactions in our common stock, whether or not participating in this offering, to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Our logo, “Complete Genomics,” “Complete Genomics Analysis Platform,” “CGA Platform,” “cPAL” and “DNB” and other trademarks or service marks of Complete Genomics, Inc. appearing in this prospectus are the property of Complete Genomics, Inc. This prospectus contains additional trade names, trademarks and service marks of other companies. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply relationships with, or endorsement or sponsorship of us by, these other companies.
i
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider in making your investment decision. You should read this summary together with the more detailed information, including our financial statements and the related notes, elsewhere in this prospectus. You should carefully consider, among other things, the matters discussed in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” before making an investment decision. Unless otherwise indicated, “Complete Genomics, Inc.,” “Complete Genomics,” “the Company,” “we,” “us” and “our” refer to Complete Genomics, Inc.
Our Company
We are a life sciences company that has developed and commercialized an innovative DNA sequencing platform, and our goal is to become the preferred solution for complete human genome sequencing and analysis. Our Complete Genomics Analysis Platform, or CGA Platform, combines our proprietary human genome sequencing technology with our advanced informatics and data management software and our innovative, end-to-end, outsourced service model to provide our customers with data that is immediately ready to be used for genome-based research. We believe that our solution will provide academic and biopharmaceutical researchers with complete human genomic data and analysis at an unprecedented combination of quality, cost and scale without requiring them to invest in in-house sequencing instruments, high-performance computing resources and specialized personnel. By removing these constraints and broadly enabling researchers to conduct large-scale complete human genome studies, we believe that our solution has the potential to revolutionize medical research and expand understanding of the basis, treatment and prevention of complex diseases.
We believe that our complete human genome sequencing technology, which is based on our proprietary DNA arrays and ligation-based read technology, is superior to existing commercially available complete human genome sequencing methods in terms of quality, cost and scale. In the DNA sequencing industry, complete human genome sequencing is generally deemed to be coverage of at least 90% of the nucleotides in the genome. Because we have optimized our technology platform and our operations for the unique requirements of high-throughput complete human genome sequencing, we are able to achieve accuracy levels of 99.999% at a total cost that is significantly less than the total cost of purchasing and using commercially available DNA sequencing instruments. We believe that we will be able to further improve our accuracy levels and reduce the total cost of sequencing and analysis, enabling us to maintain significant competitive advantages over the next several years. Because our technology resides only in our centralized facilities, we can quickly and easily implement enhancements and provide their benefits to our entire customer base. Our goal is to be the first company to sequence and analyze high-quality complete human genomes, at scale, for a total cost of under $1,000 per genome.
While our competitors primarily sell DNA sequencing instruments and reagents that produce raw sequenced data, requiring their customers to invest significant additional resources to process that raw data into a form usable for research, we offer our customers an end-to-end, outsourced solution that delivers research-ready genomic data. As the cost of complete human genome sequencing declines, we believe the basis of competition in our industry will shift from the cost of sequencing to the value of the entire sequencing solution. We believe that our integrated, advanced informatics and data management services will emerge as a key competitive advantage as this shift occurs.
Our genome sequencing center, which began commercial operations in May 2010, combines a high-throughput sample preparation facility, a collection of our proprietary high-throughput sequencing instruments and a large- scale data center. Our customers ship us their samples via common carrier services such as Federal Express and
1
Table of Contents
United Parcel Service. We then sequence and analyze these samples and provide our customers with finished, research-ready data, enabling them to focus exclusively on their single highest priority, discovery.
As of March 31, 2010, there had been approximately 24 published and 200 unpublished complete human genomes sequenced worldwide, as reported in the April 2010 edition of Nature. As of July 20, 2010, we have sequenced over 200 complete human genomes year-to-date, including more than 100 in the first three weeks of July 2010, and have an order backlog of over 500 genomes. Our customers include some of the leading global academic and government research centers and biopharmaceutical companies. By the end of 2010, we expect our facility to have the capacity to sequence and analyze over 400 complete human genomes per month. We expect this capacity to increase between two- and three-fold in 2011 as we deploy additional sequencers and increase the throughput of our sequencing process through software refinements and component upgrades. In future years, we plan to construct additional genome centers in the United States and other strategic markets to accommodate an expected growing global demand for high-quality, low-cost complete human genome sequencing on a large scale.
Our Industry
Studying how genes differ between species and among individuals within a species, or genetic variations, helps scientists to determine their functions and roles in health and disease. Improving our understanding of the genome and its functions has driven and, we expect, will continue to drive advancements in medical research and diagnostics. Genetic analysis products comprise instruments and consumables, as well as associated hardware, software and services directly involved in the study of DNA and RNA. Scientia Advisors, a third-party research firm, estimated genomic revenue in 2009 to be approximately $5.8 billion and projects the market to grow to approximately $9.0 billion by 2014.
The primary genetic analysis methods traditionally used by genetic researchers fall into three categories: DNA sequencing, genotyping and gene expression analysis. DNA sequencing is the process of determining the exact order, or sequence, of the individual nucleotides in a DNA strand so that this information can be correlated to the genetic activity influenced by that segment of DNA. Genotyping is the process of examining certain known mutations or variations in the DNA sequence of genes to determine whether the particular variant can be associated with a specific disease susceptibility or drug response. Gene expression analysis is the process of examining the molecules that are produced when a gene is activated, or expressed, to determine whether a particular gene is expressed in a specific biological tissue.
The Importance of Complete Human Genome Sequencing and the Limitations of Existing Technologies
One of the most difficult challenges facing the genetic research and analysis industry is improving our understanding of how genes contribute to diseases that have a complex pattern of inheritance. For many diseases, multiple genes each make a subtle contribution to a person’s predisposition or susceptibility to a disease or response to a drug treatment protocol. Accordingly, we believe that unraveling this complex network will be critical to understanding human health and disease. We believe that sequencing complete human genomes is the most comprehensive and accurate method by which to achieve these objectives and improve our understanding of human disease.
Innovations in DNA sequencing have led to the development of high-throughput sequencing technologies, commonly referred to as next-generation or second-generation sequencing, which produce thousands to millions of sequences at once. Although second-generation sequencing technologies have led to dramatic reductions in cost and improvements in quality and throughput for complete human genome sequencing, they were designed as general-purpose instruments for sequencing the DNA or RNA of plants, animals, bacteria and viruses. We believe the key limitations of using second-generation technologies for sequencing large numbers of complete human genomes include the following:
| § | High Cost. Commercially available DNA sequencing instruments cannot sequence complete human genomes at a price low enough to make large-scale projects affordable to researchers. |
2
Table of Contents
| § | Insufficient Scale and Speed. Commercially available DNA sequencing instruments typically require weeks to sequence a complete human genome, which translates into months or years for large projects. |
| § | Difficulty of Data Management. Many users of commercially available DNA sequencing instruments lack the costly computing resources, storage capacity, network bandwidth and specialized personnel to process and analyze the massive data sets generated by sequencing complete human genomes. |
Our Solution
We have developed a novel approach to complete human genome sequencing. Our solution combines our proprietary sequencing technology, which achieves accuracy levels of 99.999%, with our advanced informatics and data management software and our innovative, end-to-end service model to deliver research-ready genomic data at a total cost that is significantly less than the total cost of purchasing and operating commercially available DNA sequencing instruments.
Proprietary Sequencing Technology
Our patterned DNA nanoball, or DNB, arrays, due to their small size and biochemical characteristics, enable us to pack DNA very efficiently on a silicon chip. In addition, we have developed a highly accurate combinatorial probe-anchor ligation, or cPAL, read technology, which enables us to read DNA fragments efficiently using small concentrations of low-cost reagents, while retaining extremely high single-read accuracy. We believe this unique combination of our proprietary DNB and cPAL technologies is superior in both quality and cost when compared to other commercially available approaches and provides us with significant competitive advantages. As reported in the January 2010 edition of Science, we sequenced a complete human genome at a consumables cost of approximately $1,800 and with a consensus error rate of approximately 1 error in 100,000 nucleotides. Our read accuracy was further validated by one of our customers, the Institute for Systems Biology, or ISB, as published in Science Express in March 2010.
Advanced Informatics and Data Management
Sequencing complete human genomes generates substantial amounts of data that must be managed, stored and analyzed. To address this potential need by our customers, we have built a genomics data processing facility with computing infrastructure for managing both small- and large-scale genomic sequencing projects. Our proprietary assembly software uses advanced data analysis algorithms and statistical modeling techniques to accurately reconstruct over 90% of the complete human genome from approximately two billion 70-base reads. After assembling the genomic data, we use our analysis software to identify and annotate key differences, or variants, in each genome.
By using our analytical tools and data management software, our customers can significantly reduce their investments in computing infrastructure. Our customers are provided with reliable access to assembled and annotated sequence data in multiple formats to ease data sharing and comparative analyses. In addition, our data storage options provide flexibility and allow customers to customize their data management strategy based on their particular business and scientific requirements. We have developed a suite of open source analytical tools, called CGATools, designed to enable our customers to rapidly analyze the data we generate from their samples. We are also developing additional analytical tools, such as a tumor-normal comparison tool designed to allow cancer researchers to compare a cancer genome to the normal genome from which it was derived. As the reagent cost of sequencing declines, we believe that the cost and complexity of data analysis and management will emerge as the primary limiting factor for conducting complete human genome analysis.
Innovative, End-to-End Outsourced Solution
While our competitors primarily sell DNA sequencing instruments and reagents that produce raw sequenced data, our end-to-end, outsourced solution enables our customers to offload to us the complex processes of sample preparation, sequencing, computing and data storage and management. We believe our services will expand the
3
Table of Contents
potential addressable market by enabling a broad base of researchers who may lack sufficient capital and the specialized personnel necessary to build and operate a sequencing laboratory, or who have historically been constrained by the high total cost of sequencing, to conduct large-scale complete human genome studies.
Our end-to-end solution provides the following advantages to our customers:
| § | High-Quality Data. Our technology delivers what we believe is the industry’s highest quality complete human genome data. |
| § | Cost-Savings. Our customers are not required to purchase expensive sequencing instruments and high-performance computing resources or hire the necessary specialized personnel to sequence and analyze large sets of complete human genome data. |
| § | Speed at Scale. Our customers can complete their large-scale projects more quickly by using our services than by using commercially available sequencing instruments. |
| § | Ease of Use. Our customers can avoid the difficulty and time-consuming process of purchasing and operating their own sequencing instruments and can outsource the entire process to us, from sample preparation to delivery of research-ready data. |
| § | Operational Flexibility. By outsourcing their large-scale complete human genome sequencing projects to us, our customers can free up the capacity of in-house instruments to run smaller or more targeted sequencing projects and applications. |
| § | Technological Flexibility. As DNA sequencing technology improves, our customers avoid the risk of their expensive instruments becoming technologically obsolete. |
| § | Enables Customers to Focus on Discovery. Outsourcing offloads the operational burdens of managing large-scale genome sequencing projects and enables our customers to focus their resources on research, which can reduce the time to discovery. |
We have more than 30 past and current customers, which include some of the leading global academic and government research centers and biopharmaceutical companies. In May 2010, we received an order from SAIC-Frederick, Inc., a prime contractor for the National Cancer Institute’s research and development facility in Frederick, Maryland, to sequence 50 tumor-normal pairs, or 100 complete human genomes, over a six-month period. This contract contains an option for SAIC-Frederick to engage us to sequence 564 additional cancer cases, or 1,128 complete human genomes, over an additional 18-month period. In addition, we sequenced complete genomes that enabled ISB to pinpoint the causal gene, and subsequently confirm that gene’s role, in Miller Syndrome. This work has led to a follow-on project with the ISB to sequence an additional 122 genomes. We also worked with Genentech, Inc. (a member of the Roche Group) on a non-small cell lung cancer study that was the first complete human genome sequence of a primary non-small cell lung tumor and matched normal tissue. The data we delivered allowed Genentech to measure the rate of smoking-induced mutations accumulated over time.
Applications for Our Sequencing Service
Potential applications for our complete human genome sequencing service include:
| § | Cancer Research. We believe understanding genetic mutations in cancer patients will guide development of new cancer therapeutics and diagnostics and ultimately enable doctors to select the best course of therapy based on the specific mutations found in a tumor. |
| § | Mendelian Disease Research. By sequencing the complete genomes of families affected with Mendelian diseases, which likely have a significant genetic component, we believe the genetic causes of these diseases can be discovered, which could lead to the development of novel diagnostics and therapeutics. |
4
Table of Contents
| § | Rare Variant Disease Research. Large-scale genomic studies of central nervous system disorders, cardiac disease and certain metabolic disorders may help to identify disrupted pathways and lead to the development of novel diagnostics and therapeutics. |
| § | Clinical Trial Optimization. We believe that selecting or stratifying patients on the basis of their genetic profiles could enable the preferential admission of high responders into clinical trials, lowering costs and resulting in faster clinical trials and drug commercialization. |
In addition to these research studies, we expect future clinical applications to include:
| § | Companion Diagnostics. We believe that therapeutics that are not first-line treatments for the general population may be elevated to first-line treatments or used in combination therapies for subsets of the population that share a common genetic profile. Complete human genome studies may unlock new market opportunities for these therapies or combination therapies. |
| § | Cancer Pathology. We believe that analyzing complex cancer genomes that involve large and unpredictable structural changes will be most reliably and economically implemented using complete human genome sequencing. |
| § | Universal Diagnostics. As medical records technology and public health policy advance, we believe that large numbers of people will have their complete human genomes sequenced and stored in their electronic medical records for use by their physicians in managing their health care decisions. |
Competitive Strengths
We believe that our competitive strengths are as follows:
| § | Proprietary Human Genome Sequencing Technology. Our proprietary sequencing technology achieves accuracy levels of 99.999% at a total cost that is significantly less than the total cost of purchasing and operating commercially available DNA sequencing instruments. |
| § | Fully Integrated Advanced Informatics and Data Management Software. Our solution enables our customers to manage and gain useful information from the massive data sets generated in complete human genome sequencing. |
| § | Highly Scalable and Capital-Efficient Business Model. Consolidating volume across our entire customer base enables us to sequence large numbers of genomes while avoiding the cost and complexity of employing a large field installation and support organization. By implementing a high degree of automation, we have reduced the possibility of human errors that could adversely affect quality and increase costs. |
| § | Unique Insight Into Customer Needs. We interact directly with our customers on their discovery projects, which enables us to develop and enhance our analysis software to meet our customers’ specific needs while expanding our understanding of variation in the human genome. |
| § | Fast and Efficient Deployment of Operational and Technological Enhancements. Because our sequencing operations and data center are centralized, we can rapidly upgrade our technology and deliver the benefits to our customers. In addition, our access to genomic data allows our software engineers to continually refine and improve our software with each genome we sequence. |
| § | Expanded Market Opportunity. We believe our outsourced model will expand the potential addressable market by providing academic and biopharmaceutical researchers who lack sufficient budgets or the specialized personnel necessary to build and operate a sequencing laboratory with access to high-quality, low-cost complete human genome data. |
5
Table of Contents
Our Strategy
We intend to become the leading complete human genome sequencing and analysis company and the preferred platform for human genome discovery by:
| § | continuing to deliver the highest quality genomic data and analysis at a low total cost; |
| § | maintaining and strengthening our technology; |
| § | capitalizing on our scalable model; |
| § | establishing ourselves as the leader in outsourced complete human genome sequencing; |
| § | expanding globally to increase capacity and reach new markets; and |
| § | expanding applications for the use of our technology. |
Risks Associated with our Business
Our business is subject to numerous risks, as discussed more fully in the section entitled “Risk Factors” immediately following this prospectus summary. These risks include the following, among others:
| § | We are an early, commercial-stage company and have a limited operating history. |
| § | We have a history of losses, and we may not achieve or sustain profitability in the future, on a quarterly or annual basis. |
| § | We may need substantial additional capital in the future in order to maintain and expand our business. |
| § | Our only source of revenue is our human genome sequencing service, which is a new business model in an emerging industry, and failure to achieve market acceptance will harm our business. |
| § | Potential customers may have significant reservations about allowing a third party to control the sequencing process or may want to sequence only portions of human genomes, which may prevent our complete human genome sequencing service from achieving market acceptance. |
| § | Our success depends on the growth of markets for analysis of genetic variation and biological function, and the shift of these markets to complete human genome sequencing. |
| § | We face significant competition from large, well-capitalized companies. The emergence of new competitive genome sequencing technologies may also harm our business. |
| § | We must significantly expand our capacity in order to meet projected demand. |
| § | If our Mountain View genome sequencing facility becomes inoperable, we will be unable to perform our genome sequencing services, and our business will be harmed. |
| § | If third parties assert that we have infringed their patents or other proprietary rights or challenge the validity of our patents or other proprietary rights, we may become involved in costly and time-consuming disputes and litigation that could affect our ability to sell our services. |
Corporate Information
We were incorporated in the state of Delaware on June 14, 2005. The address of our principal executive offices is 2071 Stierlin Court, Mountain View, California 94043, and our telephone number is (650) 943-2800. Our website address is www.completegenomics.com. We do not incorporate the information on, or that can be accessed through, our website into this prospectus, and you should not consider it part of this prospectus.
6
Table of Contents
The Offering
| Common stock offered by Complete Genomics |
shares (or shares if the underwriters exercise their over-allotment option in full). |
| Common stock to be outstanding after this offering |
shares (or shares if the underwriters exercise their over-allotment option in full). |
| Proposed NASDAQ Global Market symbol |
“GNOM” |
| Use of proceeds |
We currently intend to use the net proceeds of this offering for capital expenditures to expand the sequencing and computing capacity in our Mountain View and Santa Clara leased facilities, to finance the further development of our sequencing technology and services, for sales and marketing activities and for working capital and other general corporate purposes, including the costs associated with being a public company. Please see “Use of Proceeds.” |
| Risk factors |
See “Risk Factors” starting on page 12 of this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. |
The number of shares of common stock to be outstanding after this offering is based on shares outstanding as of September 1, 2010 and excludes:
| § | 2,687,100 shares of common stock issuable upon exercise of options outstanding as of September 1, 2010 with a weighted-average exercise price of $1.72 per share; |
| § | 771,900 shares of common stock reserved for future issuance under our 2006 Equity Incentive Plan as of September 1, 2010, which will become available for issuance under our 2010 Equity Incentive Award Plan after completion of this offering; |
| § | shares of common stock that will be reserved for future issuance under our 2010 Equity Incentive Award Plan, as well as any automatic increases in the number of shares of our common stock reserved for future issuance under this benefit plan, which will become effective immediately prior to the consummation of this offering; |
| § | up to 1,587,302 shares of our common stock that certain investors in our Series E financing will have the right to purchase from us if this offering is consummated before the second or third closing of that financing; |
| § | 1,965,479 shares of common stock subject to warrants outstanding as of September 1, 2010 that will not expire upon completion of this offering, with a weighted-average exercise price of $1.88 per share; and |
| § | 1,318,719 shares of common stock subject to warrants outstanding as of September 1, 2010 that will not expire upon completion of this offering and that are exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010. |
The number of shares of our common stock outstanding after this offering assumes:
| § | the conversion of all 13,094,629 shares of our convertible preferred stock outstanding on September 1, 2010 into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
7
Table of Contents
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
Except as otherwise indicated, all information in this prospectus reflects or assumes no exercise of the underwriters’ over-allotment option.
We refer to our Series A, Series B, Series C, Series D and Series E preferred stock collectively as “convertible preferred stock” for financial reporting purposes and in the financial tables included in this prospectus, as more fully explained in Note 6 to our financial statements. In other parts of this prospectus, we refer to our Series A, Series B, Series C, Series D and Series E preferred stock collectively as “preferred stock.”
8
Table of Contents
Summary Financial Data
The following tables set forth a summary of our historical financial data as of, and for the periods ended on, the dates indicated. You should read these tables together with our financial statements and the related notes, “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. The statement of operations data for the years ended December 31, 2007, 2008 and 2009 are derived from our audited financial statements included elsewhere in this prospectus. The statement of operations data for the six months ended June 30, 2009 and 2010 and for the cumulative period from June 14, 2005 (date of inception) to June 30, 2010, and the balance sheet data as of June 30, 2010 are derived from our unaudited financial statements included elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements and include, in the opinion of management, all adjustments, consisting only of normal recurring adjustments, necessary for the fair statement of the financial information in those statements. Our historical results are not necessarily indicative of the results to be expected in any future period, and the results for the six months ended June 30, 2010 are not necessarily indicative of the results to be expected for the year ending December 31, 2010.
The unaudited pro forma and pro forma as adjusted financial data is presented for informational purposes only and does not purport to represent what our results of operations or financial position actually would have been had the transactions reflected occurred on the dates indicated or to project our financial condition as of any future date or results of operations for any future period.
| Years ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 |
||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | 623 | $ | — | $ | 1,425 | $ | 2,048 | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Start-up production costs(1) |
— | — | 5,033 | 1,013 | 8,985 | 14,018 | ||||||||||||||||||
| Research and development(1) |
10,305 | 23,633 | 22,424 | 10,449 | 11,097 | 71,191 | ||||||||||||||||||
| General and administrative(1) |
1,896 | 3,179 | 4,953 | 2,120 | 4,862 | 15,841 | ||||||||||||||||||
| Sales and marketing(1) |
— | 1,045 | 1,798 | 620 | 2,539 | 5,382 | ||||||||||||||||||
| Total operating expenses(1) |
12,201 | 27,857 | 34,208 | 14,202 | 27,483 | 106,432 | ||||||||||||||||||
| Loss from operations |
(12,201 | ) | (27,857 | ) | (33,585 | ) | (14,202 | ) | (26,058 | ) | (104,384 | ) | ||||||||||||
| Interest expense |
(215 | ) | (974 | ) | (3,465 | ) | (2,051 | ) | (1,144 | ) | (5,809 | ) | ||||||||||||
| Interest and other income (expense), net |
163 | 437 | 1,101 | (70 | ) | 235 | 2,065 | |||||||||||||||||
| Net loss |
$ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,323 | ) | $ | (26,967 | ) | $ | (108,128 | ) | ||||||
| Net loss per share, basic and diluted |
$ | (211.00 | ) | $ | (369.36 | ) | $ | (386.56 | ) | $ | (177.96 | ) | $ | (45.09 | ) | |||||||||
| Weighted-average shares of common stock outstanding used in computing net loss per share, basic and diluted |
58,072 | 76,873 | 92,998 | 91,724 | 598,080 | |||||||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (6.59 | ) | $ | (2.53 | ) | ||||||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (37,037 | ) | $ | (27,202 | ) | ||||||||||||||||||
| Weighted-average shares of common stock outstanding used in computing the pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
5,619,600 | 10,763,515 | ||||||||||||||||||||||
(footnotes on following page)
9
Table of Contents
| (1) | Includes stock-based compensation expense as follows: |
| Years ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 | ||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||
| (in thousands) | ||||||||||||||||||
| Start-up production costs |
$ | — | $ | — | $ | 81 | $ | 15 | $ | 105 | $ | 186 | ||||||
| Research and development |
78 | 246 | 992 | 225 | 415 | 1,741 | ||||||||||||
| General and administrative |
22 | 90 | 262 | 78 | 301 | 677 | ||||||||||||
| Sales and marketing |
— | — | 75 | 25 | 68 | 143 | ||||||||||||
| Total stock-based compensation expense |
$ | 100 | $ | 336 | $ | 1,410 | $ | 343 | $ | 889 | $ | 2,747 | ||||||
| (2) | Net loss used in computing pro forma basic and diluted net loss per share of common stock, and the number of weighted-average common shares used in computing pro forma basic and diluted net loss per share of common stock, in the table above assume the conversion of all of our outstanding convertible preferred stock into common stock immediately prior to the consummation of this offering. See Note 2 to our financial statements for an explanation of the method used to compute pro forma basic and diluted net loss per share of common stock and the number of shares used in computing those per share amounts. |
The table below presents our balance sheet data as of June 30, 2010:
| § | on an actual basis; |
| § | on a pro forma basis to give effect to: |
| § | our issuance and sale, during August and September 2010, of an aggregate of 5,274,871 shares of our Series E preferred stock and warrants to purchase an aggregate of 1,318,719 shares of our common stock at an exercise price of $2.69 per share, which will become exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010; |
| § | the conversion of the convertible notes we issued and sold during April, May, June and August 2010 into shares of our Series E preferred stock, which occurred during August 2010 in connection with our Series E preferred stock financing; |
| § | the conversion of all shares of our convertible preferred stock outstanding on June 30, 2010, together with the shares of Series E preferred stock we issued during August and September 2010, including shares issued in connection with the related conversion of our convertible notes, into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
| § | the conversion of all of our warrants for convertible preferred stock into warrants for common stock immediately prior to the consummation of this offering, and the related reclassification of convertible preferred stock warrant liability to additional paid-in capital; |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public |
10
Table of Contents
| offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | on a pro forma as adjusted basis to give further effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| June 30, 2010 | |||||||||
| Actual | Pro forma | Pro forma as adjusted(1) | |||||||
| (in thousands) | |||||||||
| Balance Sheet Data: |
|||||||||
| Cash and cash equivalents |
$ | 7,972 | $ | 25,365 | |||||
| Working capital (deficit) |
(20,860 | ) | 13,834 | ||||||
| Total assets |
39,795 | 57,188 | |||||||
| Current and long-term notes payable |
23,179 | 5,878 | |||||||
| Convertible preferred stock warrant liability |
1,318 | — | |||||||
| Convertible preferred stock |
95,844 | — | |||||||
| Total stockholders’ equity (deficit) |
(96,451 | ) | 35,576 | ||||||
| (1) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital, total assets and stockholders’ equity by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of pro forma as adjusted cash and cash equivalents, working capital, total assets and stockholders’ equity by approximately $ million, assuming the initial public offering price per share, as set forth on the cover page of this prospectus, remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $ 1.00 increase in the assumed initial public offering price per share, would increase each of pro forma as adjusted cash and cash equivalents, working capital, total assets and stockholders’ equity by approximately $ million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price per share, would decrease each of pro forma as adjusted cash and cash equivalents, working capital, total assets and stockholders’ equity by approximately $ million. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price and other terms of this offering determined at pricing. |
11
Table of Contents
Investing in our common stock involves a high degree of risk. You should carefully consider the following risk factors, as well as the other information in this prospectus, before deciding whether to invest in shares of our common stock. The occurrence of any of the events described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the trading price of our common stock may decline and you may lose all or part of your investment.
Risks Related to Our Limited Operating History, Financial Condition and Capital Requirements
We are an early, commercial-stage company and have a limited operating history, which may make it difficult to evaluate our current business and predict our future performance.
We are an early, commercial-stage company and have a limited operating history. We were incorporated in Delaware in June 2005 and began operations in March 2006. From March 2006 until mid-2009, our operations focused on research and developing our DNA sequencing technology platform. In December 2009, we recognized our first revenue from the sale of our genome sequencing services. Our limited operating history, particularly in light of our novel, service-based business model in the rapidly evolving genome sequencing industry, may make it difficult to evaluate our current business and predict our future performance. Our lack of a long operating history, and especially our very short history as a revenue-generating company, make any assessment of our profitability or prediction about our future success or viability subject to significant uncertainty. We have encountered and will continue to encounter risks and difficulties frequently experienced by early, commercial-stage companies in rapidly evolving industries. If we do not address these risks successfully, our business will suffer.
Our quarterly operating results may fluctuate in the future. As a result, we may fail to meet or exceed the expectations of research analysts or investors, which could cause our stock price to decline.
Our financial condition and operating results may fluctuate from quarter to quarter and year to year in the future due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include the following, as well as other factors described elsewhere in this prospectus:
| § | our ability to achieve profitability; |
| § | the size and frequency of customer orders; |
| § | our ability to expand our sequencing operations; |
| § | our need for and ability to obtain capital necessary to operate and expand our business; |
| § | the cost of our sequencing services; |
| § | the demand for the sequencing of complete human genomes; |
| § | the existence and extent of government funding for research and development relating to genome sequencing; |
| § | the emergence of alternative genome sequencing technologies; |
| § | risks associated with expanding our business into international markets; |
| § | our ability to lower the average cost per genome that we sequence; |
| § | our dependence on single-source suppliers; |
| § | our ability to manage our growth; |
| § | our ability to successfully partner with other businesses in joint ventures or collaborations, or integrate any businesses we may acquire with our business; |
12
Table of Contents
| § | our dependence on, and the need to attract and retain, key management and qualified sales personnel; |
| § | our ability to obtain, protect and enforce our intellectual property rights and avoid infringing the intellectual property rights of others; |
| § | our ability to prevent the theft or misappropriation of our know-how or technologies; |
| § | lawsuits brought against us by third parties; |
| § | business interruptions, such as earthquakes and other natural disasters; |
| § | public concerns about the ethical, legal and social concerns related to the use of genetic information; |
| § | our ability to comply with current laws and regulations and new or expanded regulatory schemes; |
| § | our ability to properly handle and dispose of hazardous materials used in our business and biological waste; and |
| § | our ability to use our net operating loss carryforwards to offset future taxable income. |
Due to the various factors mentioned above, and others, the results of any prior quarterly or annual periods are not necessarily indicative of our future operating performance.
We have a history of losses, and we may not achieve or sustain profitability in the future, on a quarterly or annual basis.
We have not been profitable in any quarterly period since we were formed. We incurred net losses of $12.3 million, $28.4 million and $35.9 million for the years ended December 31, 2007, 2008 and 2009, respectively, and $27.0 million for the six months ended June 30, 2010. As of June 30, 2010, our deficit accumulated during the development stage was $108.1 million. Based on our current operating plans and assumptions, we do not expect to achieve profitability on an annual basis in the near future. In addition, we expect our cash expenditures to increase significantly in the near term, including significant expenditures for the expansion of our Mountain View, California sequencing facility and the development of additional sequencing centers, research and development, sales and marketing and general and administrative expenses. In order to continue operations, we must obtain additional debt or equity financing. We may encounter unforeseen difficulties, complications and delays in expanding our Mountain View sequencing facility or in establishing additional genome sequencing centers and other unforeseen factors that require additional expenditures. These costs, among other factors, have had and will continue to have an adverse effect on our working capital and stockholders’ equity. We will have to generate and sustain substantially increased revenue to achieve and maintain profitability, which we may never do. If we are unable to achieve and then maintain profitability, the market value of our common stock will decline.
We may need substantial additional capital in the future in order to maintain and expand our business.
Our future capital requirements may be substantial, particularly as we further develop our business, expand the sequencing and computing capacity in our Mountain View and Santa Clara, California leased facilities and establish additional genome sequencing centers. Historically, we have financed our operations through private placements of preferred stock and convertible debt and borrowings under our credit facility.
We believe that, based on our current level of operations and anticipated growth, the net proceeds from this offering, together with our cash and cash equivalent balances and interest income we earn on these balances, will be sufficient to meet our anticipated cash requirements through at least the next 12 months. However, we may need additional capital if our current plans and assumptions change. Our need for additional capital will depend on many factors, including:
| § | the financial success of our genome sequencing business; |
| § | our ability to increase the sequencing and computing capacity in our Mountain View and Santa Clara leased facilities; |
| § | the rate at which we establish additional genome sequencing centers and whether we can find suitable partners to establish such centers; |
13
Table of Contents
| § | whether we are successful in obtaining payments from customers; |
| § | whether we can enter into collaborations or establish a recurring customer base; |
| § | the progress and scope of our research and development projects; |
| § | the effect of any joint ventures or acquisitions of other businesses or technologies that we may enter into or make in the future; |
| § | the filing, prosecution and enforcement of patent claims; and |
| § | lawsuits brought against us by third parties. |
If our capital resources are insufficient to meet our capital requirements, and we are unable to enter into joint ventures or collaborations with partners able or willing to fund our development efforts or purchase our genome sequencing services, we will have to raise additional funds. If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we raise additional debt financing, we may be subject to restrictive covenants that limit our ability to conduct our business. We may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, further develop and enhance our technology or otherwise respond to competitive pressures could significantly suffer. If this happens, we may be forced to:
| § | slow or halt the expansion of our Mountain View facility and the establishment of additional genome sequencing centers; |
| § | slow the commercialization of our services; |
| § | delay or terminate research or development programs; |
| § | curtail or cease operations; or |
| § | seek to obtain funds through collaborative and licensing arrangements, which may require us to relinquish commercial rights or grant licenses on terms that are not favorable to us. |
The report of our independent registered public accounting firm has expressed substantial doubt as to our ability to continue as a going concern.
In its report accompanying our audited financial statements for the year ended December 31, 2009, our independent registered public accounting firm included an explanatory paragraph stating that our recurring losses from operations and lack of sufficient working capital raise substantial doubt as to our ability to continue as a going concern. A report with this type of explanatory paragraph could impair our ability to finance our operations through the sale of debt or equity securities or to obtain commercial bank loans. Our ability to continue as a going concern will depend, in large part, on our ability to generate positive cash flow from operations and obtain necessary financing, neither of which is certain. As a result, the report of our independent registered public accounting firm for the year ending December 31, 2010 may express substantial doubt about our ability to continue as a going concern. Accordingly, we may need to obtain sufficient financing in the short term, and the failure to do so may adversely affect the value of our common stock.
Risks Related to Our Business
Our only source of revenue is our human genome sequencing service, which is a new business model in an emerging industry, and failure to achieve market acceptance will harm our business.
Since our inception, all of our efforts have been focused on the creation of a technology platform for our human genome sequencing service, which we have only just recently commercialized. We expect to generate all of our revenue from our human genome sequencing service for the foreseeable future. As a result, market acceptance of our human genome sequencing service is critical to our future success.
14
Table of Contents
Providing genome sequencing as a service is a new and unproven business model in a relatively new and rapidly evolving industry. We are using proprietary technology, involving multiple scientific and engineering disciplines, and a novel service model to bring complete human genome sequencing to an unproven market. Historically, companies in this industry have sold sequencing instruments directly to customers, and the customer performs the sequencing itself. We do not know if the purchasers and users of sequencing instruments will adopt our service model. For example, many potential customers want to sequence human genomes for proprietary studies that may lead to discoveries which they would seek to exploit, either commercially or through the publication of scientific literature. Accordingly, these potential customers may have significant reservations about allowing a third party to control the sequencing processes for their proprietary studies. Alternatively, other potential customers may want to sequence only portions of human genomes, rather than complete human genomes. There are many reasons why our services might not become widely adopted, ranging from logistical or quality problems to a failure by our sales force to engage potential customers, and including the other reasons stated in this “Risk Factors” section. As a result, our genome sequencing service may not achieve sufficient market acceptance to allow us to become profitable.
Our success depends on the growth of markets for analysis of genetic variation and biological function, and the shift of these markets to complete human genome sequencing.
We are currently targeting customers for our genome sequencing service in academic and government research institutions and in the pharmaceutical and other life science industries. Our customers are using our service for large-scale human genome studies for a wide variety of diagnostic and discovery applications. These markets are new and emerging, and they may not develop as quickly as we anticipate, or reach their full potential. The development of the market for complete human genome sequencing and the success of our service depend in part on the following factors:
| § | demand by researchers for complete human genome sequencing; |
| § | the usefulness of genomic data in identifying or treating disease; |
| § | the ability of our customers to successfully analyze the genomic data we provide; |
| § | the ability of researchers to convert genomic data into medically valuable information; |
| § | the capacity and scalability of the hardware storage components necessary to store, manage, backup, retain and safeguard genomic data; and |
| § | the development of software tools to efficiently search, correlate and manage genomic data. |
For instance, demand for our genome sequencing service may decrease if researchers fail to find meaningful correlations between genetic variation and disease susceptibility through genome-wide association studies. In addition, factors affecting research and development spending generally, such as changes in the regulatory environment affecting pharmaceutical and other life science companies and changes in government programs that provide funding to companies and research institutions, could harm our business. If our target markets do not develop in a timely manner, demand for our service may grow at a slower rate than we expect, or may fall, and we may not achieve profitability.
To date, relatively few complete human genomes have been sequenced, in large part due to the high cost of large-scale sequencing. Our business plan assumes that the demand for sequencing complete human genomes will increase significantly as the cost of complete human genome sequencing decreases. This assumption may prove to be incorrect, or the increase in demand may take significantly more time than we anticipate. For example, potential customers may not think our cost reductions are sufficient to permit or justify large-scale sequencing. Moreover, some companies and institutions have focused on sequencing targeted areas of the genome that are believed to be primarily associated with disorders and diseases, as opposed to the entire genome. Demand for sequencing complete human genomes may not increase if these targeted sequencing strategies, such as exome sequencing, where selected regions containing key portions of genes are sequenced, prove to be more cost effective or are viewed as a more efficient method of genetic analysis than complete human genome sequencing.
15
Table of Contents
We face significant competition. Our failure to compete effectively could adversely affect our sales and results of operations.
We currently compete with companies that develop, manufacture and market genome sequencing instruments or provide genome sequencing services. We expect competition to increase as our competitors develop new, improved or cheaper instruments or expand their businesses to include sequencing services, and as new companies enter the market with innovative technologies.
The market for genome sequencing technology is highly competitive and is served by several large companies with significant market shares. For example, established companies such as Illumina, Inc., Life Technologies Corporation and Roche Diagnostics Corporation are marketing instruments for genetic sequencing that are directly competitive with our services, and these companies have significantly greater financial, technical, marketing and other resources than we do to invest in new technologies and have substantial intellectual property portfolios and substantial experience in product development and regulatory expertise. Also, there are many smaller companies, such as NABsys, Inc., Oxford Nanopore Technologies, Ltd., Pacific Biosciences, Inc. and Helicos Biosciences Corporation, that are developing sequencing technology that would compete with ours. Moreover, large established companies may acquire smaller companies, such as these, with emerging technologies and use their extensive resources to develop and commercialize such technologies or incorporate such technologies into their instruments and services. For example, Life Technologies recently announced that it entered into a definitive agreement to acquire Ion Torrent Systems, Inc., a chip-based sequencing technology startup.
In addition, there are many research, academic and other non-profit institutions that are pursuing new sequencing technologies. These institutions often have access to significant government and other funding. For example, BGI (formerly known as Beijing Genomics Institute) in the People’s Republic of China offers a service that is similar to ours and is funded by the government of China. In the United States, agencies such as the National Human Genome Research Institute provide funding to institutions to discover new sequencing technology. We may compete directly with these institutions, or these institutions may license their technologies to third parties with whom we would compete.
While many of our existing competitors primarily sell sequencing instruments, they may also begin to provide sequencing services like us. Since these competitors have already developed their own sequencing technology, they will not experience significant technological barriers to entry and can likely enter the sequencing services market fairly quickly and with little additional cost. For example, Illumina has recently announced that it will be providing individual genome sequencing services for as low as $9,500 per genome, and Life Technologies has recently announced a collaboration to build a genome sequencing facility. Illumina also announced that it is pursuing a global program designed to provide researchers with access to academic and commercial institutions that can perform large-scale whole human genome sequencing projects using Illumina’s technology. Furthermore, many of these instrumentation companies have already established a significant market presence and are trusted by customers in the industry. As established instrumentation companies enter the sequencing services market, many potential customers may purchase sequencing services from these companies instead of us, even if we offer superior technology and services.
For more information regarding our existing and potential competitors, please see “Business—Competition.”
The emergence of competitive genome sequencing technologies may harm our business.
The success of our genome sequencing services will depend, in part, on our ability to continue to enhance the performance and decrease the cost of our genome sequencing technology. A number of genome sequencing technologies exist, and new methods and improvement to existing methods are currently being developed, including technology platforms developed by companies that we expect will directly compete with us as providers of sequencing services or instruments. These new technologies may result in faster, more cost-effective and more accurate sequencing methods than ours. For example, our sequencing technology does not currently cover all of the nucleotides in the genome. If competitive technologies emerge that sequence portions of the genome that our technology does not, our business could suffer if those portions contain important genomic
16
Table of Contents
information. We expect to face competition from emerging companies, including NABsys, Oxford Nanopore Technologies and Pacific Biosciences. As a result of the emergence of these competitive sequencing technologies, demand for our service may decline or never develop sufficiently to sustain our operations.
Our industry is rapidly changing, with emerging and continually evolving technologies that increase the efficiency and reduce the cost of sequencing genomes. As new technologies emerge, we believe that the cost and error rates of, and the time required to, sequence human genomes will eventually decrease to a level where competition in the industry will shift to other factors, such as providing related services and analytical technologies. We may not be able to maintain any technological advantage over these new sequencing technologies, and if we fail to compete effectively on other factors relevant to our customers, our business will suffer.
Our order backlog may never be completed, and we may never earn revenue on backlogged contracts to sequence genomes.
In various sections of this prospectus, we have disclosed that, as of July 20, 2010, we have an order backlog for the sequencing of over 500 genomes. This figure represents the number of genomes for which we have executed purchase orders from our customers that we believe are firm and for which we have not yet recognized revenue. We have also disclosed that, as of June 30, 2010, our order backlog for which we believe we will sequence, bill and gain customer acceptance within twelve months was approximately $10.0 million. We may never sequence these genomes or receive revenue from these backlogged orders, and the order backlog we report may not be indicative of our future revenue.
Many events can cause a backlogged order not to be completed. Currently, many orders are considered backlog because we do not presently have sufficient capacity to immediately sequence all the genomes which we have agreed to sequence. If we delay fulfilling customer orders, those customers may seek to cancel their contracts with us or may turn to one of our competitors. For example, we have in the past had a customer cancel a contract with us due, in part, to a delay in sequencing their samples. If our backlogged orders do not result in sales, our operating results will suffer.
We must significantly increase our production capabilities in order to meet expected demand.
We have only just recently commercialized our complete human genome sequencing service, and we have very limited experience in running a commercial-scale production facility. We have only one sequencing facility, and we project that facility to have the capacity to sequence over 400 complete human genomes per month by the end of 2010. This capacity is significantly less than what would be required to achieve profitability, if demand for our sequencing services grows as anticipated. Our business plan assumes that we will be able to increase our capacity multiple fold.
We plan to increase the capacity of our sequencing facility by installing additional sequencing machines, improving our software and purchasing higher resolution cameras to image the DNA arrays. We also plan to construct additional genome sequencing centers in the United States and elsewhere. We may encounter difficulties in expanding our sequencing infrastructure, and we may not build and improve this infrastructure in time to meet the volume, quality or timing requirements necessary to be successful. Manufacturing and supply quality issues may arise, including due to third parties who provide the components of our technology platform. Implementing improvements to our sequencing technology may involve significant changes, which may result in delays, or may not achieve expected results. For example, we are experimenting with increasing the density of the silicon wafers that we use for our DNA arrays by reducing the grid size of those wafers and correspondingly reducing the diameter of our DNA nanoballs, or DNBs, and the sticky spots on those wafers. These experiments may be unsuccessful and may not lead to feasible technological improvements that increase the capacity or reduce the costs of our sequencing services. If capacity or cost limitations prevent us from meeting our customers’ expectations, we will lose revenue and our potential customers may take their business to our competitors.
17
Table of Contents
Our genome sequencing technology platform was developed for human DNA and is not currently optimized to sequence non-human DNA.
Our technology platform was developed and has been optimized for sequencing human DNA, and we do not intend to sequence non-human DNA. We face significant competition from established companies who sell genome sequencing instruments that can sequence both human and non-human DNA. Many of the academic and research institutions that are our target customers conduct studies on both human and non-human DNA. Prospective customers may choose to purchase sequencing instruments from a competitor because of their broader sequencing application. Our competitors may also choose to provide sequencing services for non-human DNA. As a result, there may not be sufficient demand for our human genome sequencing service, which will harm our business.
We depend on a limited number of suppliers, including single-source suppliers, of various critical components for our sequencing process. The loss of these suppliers, or their failure to supply us with the necessary components on a timely basis, could cause delays in the current and future capacity of our sequencing center and adversely affect our business.
We depend on a limited number of suppliers, including some single-source suppliers, of various critical components for our sequencing process. We do not have long-term contracts with our suppliers or service providers. Because we do not have long-term contracts, our suppliers generally are not required to provide us with any guaranteed minimum production levels. As a result, we may not be able to obtain sufficient quantities of critical components in the future.
Although alternative suppliers exist for each of the critical components of our sequencing process, that process has been designed around the functions, limitations, features and specifications of the components that we currently utilize. For example, the cameras in our sequencers are supplied by Hamamatsu Photonics and the optical equipment is supplied by Carl Zeiss, Inc. A failure by either or both of these companies to supply these components would require us to integrate alternative cameras and optical equipment, and potentially integrate other components, into future sequencing instruments. If we are required to integrate new components into future sequencers, we would experience a delay in the deployment of these sequencers, and, as a result, our efforts to expand our sequencing capacity would be delayed.
A delay or interruption by our suppliers may also harm our business. For example, the wafers that comprise the base of our sample slide are fabricated by SVTC Technologies, L.L.C. We have not yet qualified an alternative source for the supply of these wafers, which are critical to our sequencing process, and the custom manner in which these wafers are made may make it difficult to qualify other semiconductor suppliers to manufacture them for us. We recently experienced a significant delay in the delivery, from one of our suppliers, of certain components for our sequencing system, which delayed our planned expansion of our Mountain View sequencing facility. Similarly, an interruption of services by Amazon Web Services, on whom we rely to deliver finished genomic data to our customers, would result in our customers not receiving their data on time.
In addition, the lead time needed to establish a relationship with a new supplier can be lengthy, and we may experience delays in meeting demand in the event we must switch to a new supplier. The time and effort to qualify a new supplier could result in additional costs, diversion of resources or reduced manufacturing yields, any of which would negatively impact our operating results. Our dependence on single-source suppliers exposes us to numerous risks, including the following:
| § | our suppliers may cease or reduce production or deliveries, raise prices or renegotiate terms; |
| § | delays by our suppliers could significantly limit our ability to sequence customer data and delay our efforts to increase our sequencing capacity; |
| § | we may be unable to locate a suitable replacement on acceptable terms or on a timely basis, if at all; and |
| § | delays caused by supply issues may harm our reputation, frustrate our customers and cause them to turn to our competitors for future projects. |
18
Table of Contents
If our Mountain View genome sequencing facility becomes inoperable, we will be unable to perform our genome sequencing services and our business will be harmed.
We currently do not have redundant sequencing facilities on a scale that could support our business. We perform all of our commercial genome sequencing in our facility located in Mountain View, California. Mountain View is situated on or near earthquake fault lines. Our facility, the equipment we use to perform our sequencing services and our other business process systems are costly to replace and could require substantial time to repair or replace. The facility may be harmed or rendered inoperable by natural or man-made disasters, including earthquakes, wildfires, floods, acts of terrorism or other criminal activities, infectious disease outbreaks and power outages, which may render it difficult or impossible for us to sequence genomes for some period of time. In addition, these events may temporarily interrupt our ability to receive samples from our customers or materials from our suppliers and our access to our various systems necessary to operate our business. The inability to perform our sequencing service would result in the loss of customers and harm our reputation. While we currently maintain approximately $40.0 million in property insurance coverage, we do not currently have insurance coverage for damage arising from an earthquake. Our insurance covering damage to our property may not be sufficient to cover all of our potential losses and will not cover us in the event of an earthquake, and may not continue to be available to us on acceptable terms, or at all.
Failure to achieve expected sequencing process yields, or variability in our sequencing process yields, could harm our operating results and damage our reputation.
Our sequencing process, like any other commercial-scale production process, is not flawless. For example, our DNBs may not adhere to all of the “sticky” spots on the surface of the silicon wafers we use to sequence DNA, or parts of the wafers may be unreadable. We refer to the efficiency of our sequencing process as its yield. The sequencing process yields we achieve depend on the design and operation of our sequencing process, which uses a number of complex and sophisticated biochemical, informatics, optical and mechanical processes. An operational or technology failure in one of these complex processes may result in sequencing processing yields that are lower than we anticipate or that vary between sequencing runs. In addition, we are regularly evaluating and refining our sequencing process. These refinements may initially result in unanticipated issues that further reduce our sequencing process yields or increase the variability of our sequencing yields. Low sequencing yields, or higher than anticipated variability, increases total sequencing costs and reduces the number of genomes we can sequence in a given time period, which can cause variability in our operating results and damage our reputation.
We may have to resequence genomes due to contamination of DNA samples in the sequencing process.
In the past, we have had to resequence various genome samples as a result of contamination occurring in the sample preparation and library construction process. The sequencing process is highly sensitive, and the presence of any foreign substances during the preparation of the slide samples can corrupt the results of the sequencing process. Resequencing requires additional expense, time and capacity and delays the recognition of revenue from the service. Samples may be contaminated in the future, which may damage our reputation and decrease the demand for our service.
Mishandling or switching of DNA samples or genomic data may harm our reputation and result in litigation against us.
We may unintentionally mishandle DNA samples. For example, if customer samples or sequencing results are switched, our customers would receive the wrong sequencing data, which could have significant consequences, particularly if that data is used to diagnose or treat disease. Mishandling customer samples or data would harm our reputation and could result in litigation against us.
19
Table of Contents
If we are not successful in reducing the average cost of our sequencing service, demand for our services, and therefore our business, will suffer.
Our ability to expand our customer base depends highly on our ability to reduce the average cost of sequencing a human genome. For example, certain academic or government-sponsored research organizations may forgo or delay whole genome-wide studies based on the cost required to sequence complete human genomes, in favor of other less expensive studies. Additionally, certain of our target customers may decide it is more cost-effective to purchase sequencing instruments from a competitor than contract for our sequencing service. To compete effectively with competitors who sell and market sequencing instruments, our service must provide cost advantages, superior quality and time savings over the purchase of sequencing instruments. In addition, as new competitors enter the market or expand their business model to include sequencing services, we expect increased pricing pressure, which may force us to decrease the price of our genome sequencing service. Our gross profit and operating results will suffer if we are unable to offset any reductions in our prices by reducing our costs by developing new or enhanced technologies or methods, or increasing our sales volumes.
Reduction or delay in research and development budgets and government funding may adversely impact our sales.
We expect that for the foreseeable future, our revenue will be derived primarily from selling our genome sequencing service to a relatively small number of academic, governmental and other research institutions, as well as pharmaceutical and other life science companies. Our revenue may decline substantially due to reductions and delays in research and development expenditures by these customers, which depend, in part, on their budgets and the availability of government funding. Factors that could affect the spending levels of our customers include:
| § | weakness in the global economy and changing market conditions that affect our customers; |
| § | changes in the extent to which the pharmaceutical and life science industry may use genetic information and genetic testing as a methodology for drug discovery and development; |
| § | changes in government programs that provide funding to companies and research institutions; |
| § | changes in the regulatory environment affecting pharmaceutical and life science companies and research; |
| § | impact of consolidation within the pharmaceutical and life science industry; and |
| § | cost-reduction initiatives of customers. |
Also, government funding of research and development is subject to the political process, which is inherently unpredictable. Any reduction in the funding of life science research and development or delay surrounding the approval of government budget proposals may cause our customers to delay or forgo purchases of our services. A reduction or delay in demand for our service will adversely affect our ability to achieve profitability.
The timing and extent of funding provided by the American Recovery and Reinvestment Act of 2009 could adversely affect our business, financial condition or results of operations.
In February 2009, the U.S. government enacted the American Recovery and Reinvestment Act of 2009, which we refer to as the Recovery Act, to provide stimulus to the U.S. economy in the wake of the economic downturn. As part of the Recovery Act, over $10 billion in research funding was provided to the National Institutes of Health, or NIH, through September 2010 to support the advancement of scientific research. A portion of the stimulus funding supported the analysis of genetic variation and biological function and may have a significant positive long-term impact on our business and the industry generally. In the short-term, however, potential customers may delay or forgo their purchases of our services as they wait to learn whether, and to what extent, they will receive stimulus funding. If potential customers are unable to obtain stimulus money, they may reduce their research and development budgets, resulting in a decrease in demand for our service. In addition, even if potential customers receive these stimulus funds, they may not purchase our services, and we may not benefit from the Recovery Act.
20
Table of Contents
Ethical, legal and social concerns related to the use of genetic information could reduce demand for our genome sequencing services.
Our genome sequencing services are intended to facilitate large-scale human genome studies for a wide variety of diagnostic and discovery applications. However, genetic testing has raised ethical, legal and social issues regarding privacy and the appropriate uses of the resulting information. Governmental authorities could, for social or other purposes, limit or regulate the use of genetic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. Similarly, these concerns may lead individuals to refuse to use genetics tests even if permissible.
In addition, we do not control how our customers use the genomic data we provide. In most cases, we do not know the identity of the individuals whose DNA we sequence, the reason why their DNA is being sequenced or the intended use of the genomic data we provide. If our customers use our services or the resulting genomic data irresponsibly or in violation of legal restrictions, our reputation could be harmed and litigation may be brought against us.
Ethical and social concerns may also influence U.S. and foreign patent offices and courts with regard to patent protection for technology relevant to our business. These and other ethical, legal and social concerns may limit market acceptance of our technology for certain applications or reduce the potential markets for our technology, either of which could have an adverse effect on our business, financial condition or results of operations.
We use biological and hazardous materials that require considerable expertise and expense for handling, storage and disposal and may result in claims against us.
We work with materials, including chemicals, biological agents and compounds and DNA samples, that could be hazardous to human health and safety or the environment. Our operations also produce hazardous and biological waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental laws and regulations may restrict our operations. If we do not comply with applicable regulations, we may be subject to fines and penalties.
In addition, we cannot eliminate the risk of accidental injury or contamination from these materials or wastes. While our property insurance policy provides limited coverage in the event of contamination from hazardous and biological products and the resulting cleanup costs, we do not currently have any additional insurance coverage for legal liability for claims arising from the handling, storage or disposal of hazardous materials. Further, our general liability insurance and workers’ compensation insurance policies do not cover damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be liable for damages or penalized with fines in an amount exceeding our resources and our operations could be suspended or otherwise adversely affected.
We have limited selling and marketing resources and may be unable to successfully commercialize our human genome sequencing service.
We currently have a small sales and marketing team. To grow our business as planned, we must expand our sales, marketing and customer support capabilities. We may be unable to attract, retain and manage the specialized workforce necessary to gain market acceptance and successfully commercialize our services. In addition, developing these functions is time consuming and expensive.
The sale of genome sequencing services involves extensive knowledge about genomic research and sequencing technology, including the sequencing technology of our competitors. To be successful, our sales force and related personnel must be technically proficient in a variety of disciplines. For example, many of our existing salespersons have Ph.D. degrees in various scientific fields. There are relatively few people that have the necessary knowledge and qualifications to be successful salespersons or support personnel in our industry. We may not be able to recruit a sufficient number of these people, many of whom do not reside close to the locations of our existing and planned future sequencing centers.
21
Table of Contents
In certain regions or markets, we may seek to partner with others to assist us with sales, marketing and customer support functions. However, we may be unable to find appropriate third parties with whom to enter into these arrangements. Furthermore, if we do enter into these arrangements, these third parties may not perform as expected.
Our software may incorrectly analyze the raw genomic data produced by our sequencing equipment.
Our sequencing instruments generate raw genomic data from various segments of the genome being sequenced. This data must be arranged into the correct order to reconstruct the original genomic structure of the sample. We have developed software algorithms that facilitate this reconstruction. However, these algorithms rely on statistical models that provide only relative assurance, and not absolute assurance, that the original genomic structure has been reconstructed.
In addition, the genomic data we provide our customers includes a comparison of the sequenced genome against a reference genome to help identify possible mutations or variations. This reference genome is designed to approximate a “standard” human genome. However, this approximation may not be accurate.
If the algorithms we use to reconstruct genomic data incorrectly reconstruct the sequenced genome, or if our reference genome is significantly flawed, the genomic data we deliver could be inaccurate and of little or no use to our customers.
An inability to manage our planned growth or expansion of our operations could adversely affect our business, financial condition or results of operations.
Our business has grown rapidly, and we expect this growth to continue as we expand our sequencing capacity. For example, we had three employees at the end of 2005 and 163 employees as of September 1, 2010. The rapid expansion of our business and addition of new personnel may place a strain on our management and operational systems. To effectively manage our operations and growth, we must continue to expend funds to enhance our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. If we are unable to expand our genome sequencing capacity and implement improvements to our control systems efficiently and quickly, or if we encounter deficiencies in existing systems and controls, then we will not be able to successfully expand the commercialization of our services. In addition to enhancing our sequencing capacity, our future operating results will depend on our management’s ability to:
| § | implement and improve our sales, marketing and customer support programs and our research and development efforts; |
| § | enhance our operational and financial control systems; |
| § | expand, train and manage our employee base; |
| § | integrate acquired businesses, if applicable; and |
| § | effectively address new issues related to our growth as they arise. |
We may not manage our expansion successfully, which could adversely affect our business, financial condition or results of operations.
If we expand our operations outside of the United States, we will face risks that may increase our operating costs.
We plan to expand our operations to include additional genome sequencing centers outside of the United States. Because the laws of certain countries currently prohibit the export of DNA, we will have to establish local facilities to access those markets and establish a presence in other markets. To date, we have not expanded our operations outside the United States. Operating in international markets requires significant resources and management attention and will subject us to regulatory, economic and political risks that are different from those
22
Table of Contents
in the United States. Because of our limited experience with international operations, our international expansion efforts may be unsuccessful. In addition, we will face risks in doing business internationally that could increase our operating costs, including the following:
| § | economic conditions in various parts of the world; |
| § | unexpected and more restrictive laws and regulations, including those laws governing ownership of intellectual property, collection and use of personal information and other privacy considerations, hazardous materials and other activities important to our business; |
| § | new and different sources of competition; |
| § | multiple, conflicting and changing tax laws and regulations that may affect both our international and domestic tax liabilities and result in increased complexity and costs; |
| § | the difficulty of managing and staffing additional genome sequencing centers and the increased travel, infrastructure and legal compliance costs associated with multiple international locations; |
| § | difficulties in enforcing contracts and collecting accounts receivable, especially in developing countries; |
| § | fluctuations in exchange rates; and |
| § | tariffs and trade barriers, import/export controls and other regulatory or contractual limitations on our ability to sell or develop our services in certain foreign markets. |
The success of the expansion of our business internationally will depend, in part, on our ability to anticipate and effectively manage these and other risks associated with international operations. Our failure to manage any of these risks successfully could increase our operating costs.
Certain of our potential customers may require that we become certified under the Clinical Laboratory Improvement Amendments of 1988.
Although we are not currently subject to the Clinical Laboratory Improvement Amendment of 1988, or CLIA, we may in the future be required by certain customers to obtain a CLIA certification. CLIA, which extends federal oversight over clinical laboratories by requiring that they be certified by the federal government or by a federally approved accreditation agency, is designed to ensure the quality and reliability of clinical laboratories by mandating specific standards in the areas of personnel qualifications, administration and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. If our customers require a CLIA certification, we will have to continually expend time, money and effort to ensure that we meet the applicable quality and safety requirements, which may divert the attention of management and disrupt our core business operations.
Because the market for genome sequencing is relatively new and rapidly evolving, we may become subject to additional future governmental regulation, which may place additional cost and time burdens on our operations.
We are subject, both directly and indirectly, to the adverse impact of existing and potential future government regulation of our operations and markets. The life sciences and pharmaceutical industries, which are significant target markets for our services, have historically been heavily regulated. There are comprehensive federal and state laws regarding matters such as the privacy of patient information and research in genetic engineering. For example, if we inadvertently disclose private patient information in the course of providing our sequencing services, we could be prosecuted for violations of federal law.
Legislative bodies or regulatory authorities may adopt additional regulation that adversely affects our market opportunities. They could also extend existing regulations to cover our services. For example, medical diagnostic products may, depending on their intended use, be regulated as medical devices by the Food and Drug Administration, or FDA, if they are:
| § | used in the diagnosis of disease or other conditions; |
23
Table of Contents
| § | used in the cure, mitigation, treatment or prevention of disease; or |
| § | intended to affect the structure or any function of the body. |
Medical devices generally cannot be marketed without first receiving clearance or approval (depending on the regulatory pathway) from the FDA. We do not believe that our sequencing services are currently subject to the FDA’s medical device requirements because we do not intend our services to be used for the diagnosis of disease. However, we cannot control how the genomic information we provide will be used by our customers.
In addition, the FDA is focusing on our market, which has created uncertainty regarding the regulatory landscape. The FDA has recently taken actions suggesting that it interprets the applicable regulations expansively to cover certain genomic devices and services, particularly those sold directly to consumers. Since June 2010, the FDA has sent numerous letters to certain companies in this market, including 23andMe, Inc., deCODE Genetics, Knome, Inc., Navigenics, Inc. and Pathway Genomics. In these letters, the FDA noted that it considers genetic tests marketed by these companies to be subject to FDA regulation and, accordingly, unapproved medical devices. The FDA may extend this position to services such as ours. In addition, the FDA may implement new regulations that may be broad enough to cover our operations. Changes to the current regulatory framework, including the imposition of new regulations, could arise anytime, and we may be unable to obtain or maintain FDA or comparable regulatory approval or clearance for our services, if required. For example, the FDA may impose restrictions on the types of customers to which we can market and sell our services and the types of persons whose DNA we may sequence. Also, future legislation may require that patients provide specific consent to have their DNA sequenced. This could require our customers to obtain new consents before they can submit DNA samples to us for sequencing.
In any event, if we expand our business to include sequencing services intended to be used for the diagnosis of disease, we will likely become subject to regulation by the FDA or other comparable agencies of other countries, which may require us to obtain regulatory approval or clearance before we can market those services.
These regulatory approval processes may be expensive, time-consuming and uncertain, and our failure to obtain or comply with these approvals or clearances could harm our business, financial condition or operating results.
Disruption to or failure of our data center or other technical systems may disrupt our business and harm our operating results.
We rely on our network infrastructure, data centers, enterprise applications and technology systems for the development and support of our sequencing service, including the preparation, analysis and transmission of data from our sequencing center, as well as for the internal operation of our business. These systems are susceptible to disruption or failure in the event of natural disasters such as a major earthquake, fire, flood, cyber-attack, terrorist attack, telecommunications failure, power outage or other catastrophic event. Further, our data center and our sequencing facility, which houses certain of our technology systems, are located near major earthquake faults. Disruptions to or the failure of our data center or any of these technology systems, including the network connection between our Mountain View facility and our data center, and the resulting loss of critical data, could cause delays in the transmission and analysis of the sequencing data, prevent us from fulfilling our customers’ orders and severely affect our ability to conduct normal business operations.
Our credit facility contains restrictions that limit our flexibility in operating our business.
In July 2008, we entered into a loan and security agreement, which we refer to as our credit facility. Our credit facility contains various covenants that limit our ability to engage in specified types of transactions. These covenants limit our ability to, among other things:
| § | sell, transfer, lease or dispose of our assets; |
| § | create, incur or assume additional indebtedness; |
| § | encumber or permit liens on certain of our assets; |
24
Table of Contents
| § | make restricted payments, including paying dividends on, repurchasing or making distributions with respect to our common stock; |
| § | make specified investments (including loans and advances); |
| § | consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; and |
| § | enter into certain transactions with our affiliates. |
A breach of any of these covenants could result in a default under our credit facility. Upon the occurrence of an event of default under our credit facility, our lenders could elect to declare all amounts outstanding under our credit facility to be immediately due and payable and terminate all commitments to extend further credit. If we were unable to repay those amounts, the lenders under our credit facility could proceed against the collateral granted to them to secure such indebtedness. We have pledged substantially all of our assets, other than our intellectual property, as collateral under our credit facility.
If we fail to retain the services of our key executives or if we are unable to attract and retain skilled personnel, our ability to grow our business and our competitive position would be impaired.
We believe our future success will depend in large part upon our ability to attract, retain and motivate highly skilled personnel. In particular, we depend highly on the contributions of Clifford A. Reid, Ph.D., our President and Chief Executive Officer, and Radoje Drmanac, Ph.D., our Chief Scientific Officer. The loss of either of these executives could make it more difficult to manage our operations and research and development activities, reduce our employee retention and revenue and impair our ability to compete. If either of these key executives were to leave us unexpectedly, we could face substantial difficulty in hiring qualified successors and could experience a loss in productivity, both during the search for, and integration of, any such successor.
Our research and development, operations and sales and marketing personnel represent a significant asset and serve as the source of our business strategy, scientific and technological innovations and sales and marketing initiatives. As a result, our success substantially depends on our ability to attract additional personnel for all areas of our organization, particularly in our research and development department. Competition for qualified personnel is intense, and we may not be successful in attracting and retaining qualified personnel on a timely basis or on competitive terms, if at all. In addition, many qualified personnel are located outside of Northern California, where we are located, and some qualified personnel that we may recruit may not be interested in relocating. If we are unable to attract and retain the necessary personnel on a cost-effective basis, our ability to grow our business and our competitive position would be impaired.
We may engage in joint ventures or acquisitions that could disrupt our business, cause dilution to our stockholders, reduce our financial resources and result in increased expenses.
In the future, we may enter into joint ventures or acquire other businesses, products or technologies. Because we have not entered into any joint ventures or made any acquisitions to date, our ability to do so successfully is unproven. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all, or successfully integrate any acquired business, products or technologies into our operations. If we do enter into any joint ventures or complete acquisitions, we may not strengthen our competitive position or achieve our goals, or these transactions may be viewed negatively by customers or investors. In addition, we may have difficulty integrating and motivating personnel, technologies and operations from acquired businesses and retaining and motivating key personnel from those businesses. Joint ventures and acquisitions may disrupt our ongoing operations, divert management from day-to-day responsibilities and increase our expenses. Future acquisitions may reduce our cash available for operations and other uses, and could result in an increase in amortization expense related to identifiable intangible assets acquired, potentially dilutive issuances of equity securities or the incurrence of debt. We cannot predict the number, timing or size of future joint ventures or acquisitions, or the effect that any such transactions might have on our operating results.
25
Table of Contents
We will incur significant costs as a result of operating as a public company, and our management will devote substantial time to new compliance initiatives. We may fail to comply with the rules that apply to public companies, including section 404 of the Sarbanes-Oxley Act of 2002.
We will incur significant legal, accounting and other expenses as a public company, including costs resulting from public company reporting obligations under the Securities Exchange Act of 1934, as amended, and regulations regarding corporate governance practices. The listing requirements of The NASDAQ Global Market require that we satisfy certain corporate governance requirements relating to director independence, distributing annual and interim reports, stockholder meetings, approvals and voting, soliciting proxies, conflicts of interest and a code of conduct. Our management and other personnel will need to devote a substantial amount of time to all of these requirements. Moreover, the reporting requirements, rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. These reporting requirements, rules and regulations, coupled with the increase in potential litigation exposure associated with being a public company, could make it more difficult for us to attract and retain qualified persons to serve on our board of directors or board committees or to serve as executive officers.
After this offering, we will be subject to section 404 of The Sarbanes-Oxley Act of 2002 and the related rules of the Securities and Exchange Commission, which generally require our management and independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting. We expect that our management and independent registered public accounting firm will have to provide the first of such reports with our annual report for the fiscal year ending December 31, 2011. To date, we have never conducted a review of our internal control for the purpose of providing the reports required by these rules. During the course of our review and testing, we may identify deficiencies and be unable to remediate them before we must provide the required reports. We or our independent registered public accounting firm may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting, which could harm our operating results, cause investors to lose confidence in our reported financial information and cause the trading price of our stock to fall.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
In general, under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to use its pre-change net operating loss carryforwards, or NOLs, to offset future taxable income. If the Internal Revenue Service challenges our analysis that our existing NOLs will not expire before utilization due to previous ownership changes, or if we undergo an ownership change in connection with or after this public offering, our ability to use our NOLs could be limited by Section 382 of the Code. Future changes in our stock ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Code. Furthermore, our ability to use NOLs of companies that we may acquire in the future may be subject to limitations. For these reasons, we may not be able to use a material portion of the NOLs reflected on our balance sheet, even if we attain profitability.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the recent global financial crisis, could result in a variety of risks to our business, including, reductions or delays in planned research and development and other expenditures by our customers or decreased funding of genomic research by governmental entities. A weak or declining economy could also put strain on our suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our services. Any of the foregoing could harm our business.
26
Table of Contents
Risks Related to Intellectual Property
We currently are, and could in the future be, subject to litigation regarding patent and other proprietary rights that could harm our business.
Our commercial success depends in part on not infringing patents and proprietary rights of third parties. On August 3, 2010, Illumina, Inc. and Solexa, Inc. (an entity acquired by Illumina) filed a complaint in the U.S. District Court in Delaware alleging patent infringement by us. The complaint alleges that our Complete Genomics Analysis Platform, and in particular our combinatorial probe anchor ligation technology, infringes upon three patents held by Illumina and Solexa. The complaint seeks, among other things, a preliminary and permanent injunction against us from infringing these patents and unspecified monetary damages. We may incur substantial time and expense in defending against this complaint. See “Business—Legal Proceedings.”
As we enter our markets, it is possible other competitors will claim that our services infringe their intellectual property rights as part of a business strategy to impede our successful entry into those markets. Such competitors and other third parties may have obtained and may in the future obtain patents covering products or processes that are similar to or may include steps or processes used in our sequencing technology, allowing them to claim that the use of our technologies infringes these patents. In particular, we are aware of issued U.S. patents owned by competitors and other third parties, including Illumina, to which we do not have licenses that may relate to our sequencing technology and which pertain to, among other things:
| § | sample preparation techniques; |
| § | processes for making nucleic acid templates (“library construction”); |
| § | processes for making DNBs from nucleic acid templates; |
| § | nucleic acid arrays; |
| § | methods of making arrays of DNBs; |
| § | sequencing methods, including those involving ligation; |
| § | identifying genomic sequences on nucleic acid arrays; |
| § | devices and apparatus used in nucleic acid detection systems, including optical systems; and |
| § | information processing systems including software for base calling, sequence mapping and assembly. |
Some of the third parties that own these patents, including Illumina, have strong economic incentives, and substantial financial resources, to claim that we are infringing their patent rights. In a patent infringement claim against us, we may assert, as a defense, that we do not infringe the relevant patent claims, that the patent is invalid or both. The strength of our defenses will depend on the patents asserted, the interpretation of these patents, our ability to identify invalidating “prior art” (that is, publication of the patent holder’s invention or technology prior to the stated invention date) in order to invalidate the asserted patent and on other factors. However, we could be unsuccessful in advancing non-infringement and/or invalidity arguments in our defense. In the United States, issued patents enjoy a presumption of validity, and the party challenging the validity of a patent claim must present clear and convincing evidence of invalidity, which is a high burden of proof. Conversely, the patent owner need only prove infringement by a preponderance of the evidence, which is a lower burden of proof.
If we were found by a court to have infringed a valid patent claim, we could be prevented from using the patented technology or be required to pay the owner of the patent rights for the rights to use that technology. If we decide to pursue a license to one or more of these patents, we may not be able to obtain such a license on commercially reasonable terms, if at all, or the license we obtain may require us to pay substantial royalties or grant cross licenses to our patent rights. For example, if the relevant patent is owned by a competitor, that competitor may choose not to license patent rights to us, as it would be under no obligation to do so. If we decide to develop alternative technology, we may not be able to do so on a timely or cost-effective manner, if at all.
27
Table of Contents
In addition, because patent applications can take years to issue and are often afforded confidentiality for some period of time, there may currently be pending applications, unknown to us, which later result in issued patents that processes in our sequencing technology infringe. Processes in our sequencing technology may also infringe existing issued patents of which we are currently unaware. Even though we own or have other rights to patents, these patents do not provide us with the freedom to offer our sequencing services unimpeded by the patent rights of others. For example, we may be required to pursue or defend a patent infringement action in order to protect our intellectual property rights or practice our sequencing technology.
It is possible that, in addition to our current litigation, we may in the future receive, particularly as a public company, communications from competitors and other companies alleging that we may be infringing their patents, trade secrets or other intellectual property rights or offering licenses to such intellectual property or threatening litigation. In addition to patent infringement claims, third parties may assert copyright, trademark or other proprietary rights against us. We may not be able to successfully defend against the claims asserted by Illumina, or future claims, and our business may suffer if we are found to have infringed upon the patents held by Illumina, or if future claims are brought against us.
We may not be able to protect our patent rights or other intellectual property which could impair our ability to compete effectively.
We depend on proprietary technology for our success and ability to compete. If others are able to reproduce our technology, our business will suffer significantly unless we can prevent them from competing with us. To protect our proprietary technology, we rely on patents and other intellectual property laws, as well as nondisclosure agreements, licensing arrangements and confidentiality provisions. U.S. patent, copyright and trade secret laws afford us only limited protection, and the laws of some foreign countries do not protect proprietary rights to the same extent.
We have licensed, from Callida Genomics, Inc., U.S. and international patents and patent applications relating to our business. Because the issuance of a patent is not conclusive of its validity or enforceability, our existing patent rights, and rights we may obtain in the future, may not provide us with meaningful protection. The patent rights on which we rely may be challenged and invalidated or may be interpreted not to be broad enough to cover the critical components of our technology. Our pending patent applications may have their claims limited or may not result in issued patents. Moreover, our patent rights become more limited as owned or licensed patents begin to expire in 2014. We will be able to protect our technologies from unauthorized use by third parties only to the extent that valid and enforceable patents or other proprietary rights cover them. Even if we have valid and enforceable patents or other proprietary rights, competitors may be able to design alternative methods or devices that avoid infringement of those patents or rights.
Our key patent rights are licensed from Callida, which is owned by our chief scientific officer and his spouse. If we breach the terms of these licenses, or if our relationship with Callida or its owners deteriorates, Callida may seek to terminate the licenses. If we lose our rights to use these patents, we may be forced to re-design our sequencing technology, which would be expensive and may not be possible.
The patent positions of biotechnology companies, including us, can be highly uncertain and involve complex and evolving legal and factual questions. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date in the United States. Legal developments may preclude or limit the patent protection available for our sequencing technology.
Despite our efforts to protect our proprietary rights, attempts may be made to copy or reverse engineer aspects of our sequencing technology or to obtain and use information that we regard as proprietary. Accordingly, we may be unable to protect our proprietary rights against unauthorized third-party copying or use. Furthermore, policing the unauthorized use of our intellectual property is difficult. Litigation may be necessary in the future to enforce our intellectual property rights, to protect our trade secrets or to determine the validity and scope of the proprietary rights of others. Litigation could result in substantial costs and diversion of resources and could harm our business.
28
Table of Contents
We may incur substantial costs as a result of our current, or future, litigation or other proceedings relating to patent and other proprietary rights.
The genomic sequencing industry includes several large companies that have rights to many broad issued patents and pending patent applications. Competitors in this industry have fiercely litigated their patent positions and alleged infringements by others. For example, Illumina and Affymetrix were recently involved in long and expensive patent litigation relating to DNA sequencing technology. This litigation resulted in a settlement involving the payment of $90 million by one party to the other.
Our involvement in intellectual property litigation, including our current litigation with Illumina, or administrative proceedings could result in significant expense. Some of our competitors, including Illumina, Life Technologies and Affymetrix, have considerable resources available to them. We, on the other hand, are an early-stage commercial company with comparatively few resources available to us to engage in costly and protracted litigation. Intellectual property infringement claims asserted against us, whether with or without merit, could be costly to defend and could limit our ability to use some technologies in the future. They will be time consuming, will divert our management’s and scientific personnels’ attention and may result in liability for substantial damages. In addition, our standard customer contract requires us to indemnify our customers for claims alleging that any of our products misappropriate or violate any third party patent, copyright, trade secret or other intellectual property or proprietary rights.
If third parties file patent applications or are issued patents claiming technology also claimed by us in pending applications, we may be required to participate in interference proceedings with the U.S. Patent Office or in other proceedings outside the United States, including oppositions, to determine priority of invention or patentability. Even if we are successful in these proceedings, we may incur substantial costs, and the time and attention of our management and scientific personnel will be diverted in pursuit of these proceedings.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the U.S. and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
Confidentiality agreements with employees and others may not adequately prevent disclosures of our trade secrets and other proprietary information.
We rely in part on trade secret protection to protect our confidential and proprietary information and processes. However, trade secrets are difficult to protect. We have taken measures to protect our trade secrets and proprietary information, but these measures may not be effective. We require new employees and consultants to execute confidentiality agreements upon the commencement of an employment or consulting arrangement with us. These agreements generally require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. These agreements also generally provide that inventions conceived by the individual in the course of rendering services to us will be our exclusive property. Despite these measures, our proprietary information may be disclosed, third parties could reverse engineer our sequencing technologies and
29
Table of Contents
others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Risks Related to This Offering and Ownership of Our Common Stock
An active, liquid and orderly market for our common stock may not develop, our stock price may be volatile and you may not be able to resell shares of our common stock at or above the price you paid.
Prior to this offering, there has been no public market for shares of our common stock, and an active public market for our shares may not develop or be sustained after this offering. We and the representatives of the underwriters will determine the initial public offering price of our common stock through negotiation. This price will not necessarily reflect the price at which investors in the market will be willing to buy and sell our shares following this offering. In addition, the trading price of our common stock following this offering could be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include those discussed in this “Risk Factors” section of this prospectus and others such as:
| § | quarterly variations in our results of operations or those of our competitors; |
| § | changes in earnings estimates or recommendations by securities analysts; |
| § | announcements by us or our competitors of new products or services, significant contracts, commercial relationships, acquisitions or capital commitments; |
| § | developments with respect to intellectual property rights; |
| § | our commencement of, or involvement in, litigation; |
| § | changes in financial estimates or guidance, including our ability to meet our future revenue and operating profit or loss estimates or guidance; |
| § | any major changes in our board of directors or management; |
| § | changes in governmental regulations; and |
| § | a decrease in government funding of research and development or a slowdown in the general economy. |
In recent years, the stock market in general, and the market for technology/life science companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our stock shortly following this offering. In addition, in the past, following periods of volatility in the overall market and the market price of a particular company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and divert our management’s attention and resources.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, business model, technology or stock performance, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
30
Table of Contents
Our directors, executive officers and principal stockholders and their respective affiliates will continue to have substantial influence over us after this offering and could delay or prevent a change in corporate control.
After this offering, our directors, executive officers and holders of more than 5% of our common stock, together with their affiliates, will beneficially own, in the aggregate, approximately % of our outstanding common stock, assuming no exercise of the underwriters’ option to purchase additional shares of our common stock in this offering. As a result, these stockholders, acting together, would have significant influence over the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these stockholders, acting together, would have significant influence over our management and affairs. Accordingly, this concentration of ownership might harm the market price of our common stock by:
| § | delaying, deferring or preventing a change in control; |
| § | impeding a merger, consolidation, takeover or other business combination involving us; or |
| § | discouraging a potential acquiror from making a tender offer or otherwise attempting to obtain control of us. |
Future sales of shares by existing stockholders could cause our stock price to decline.
If our existing stockholders sell, or if the market believes our existing stockholders will sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline significantly. Based on shares outstanding as of September 1, 2010, upon the completion of this offering, we will have outstanding shares of common stock, assuming the automatic conversion, upon the completion of this offering, of our preferred stock outstanding as of that date into shares of our common stock. Of these shares, shares of common stock, plus any shares sold pursuant to the underwriters’ option to purchase additional shares, will be immediately freely tradable, without restriction, in the public market. UBS Investment Bank and Jefferies & Company may, in their sole discretion, permit our officers, directors, employees and current stockholders to sell shares prior to the expiration of the lock-up agreements.
After the lock-up agreements pertaining to this offering expire and based on shares outstanding as of September 1, 2010, an additional shares will be eligible for sale in the public market. In addition, the following shares may become eligible for sale in the public market in the future, subject to certain legal and contractual limitations, as of September 1, 2010:
| § | 2,687,100 shares subject to outstanding options under our 2006 Equity Incentive Plan; |
| § | shares reserved for future issuance under our 2010 Equity Incentive Award Plan, as well as any automatic increases in the number of shares of our common stock reserved for future issuance under this benefit plan, which will become effective immediately prior to the consummation of this offering; and |
| § | 771,900 shares remaining available for issuance under our 2006 Equity Incentive Plan, which will become available for issuance under our 2010 Equity Incentive Award Plan after completion of this offering. |
If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the price of our common stock could decline substantially.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The initial public offering price of our common stock is substantially higher than the pro forma net tangible book value per share of our common stock before giving effect to this offering. Accordingly, if you purchase our common stock in this offering, you will incur an immediate substantial dilution of approximately $ per share, based on an assumed public offering price of $ per share and our pro forma net tangible book value as of June 30, 2010. In addition, following this offering, and assuming the sale by us of shares of our common stock in this offering at an initial public offering price of $ per share, purchasers in this offering will have contributed approximately % of the total gross consideration paid by stockholders to us to purchase shares of our
31
Table of Contents
common stock, through June 30, 2010, but will own only approximately % of the shares of common stock outstanding immediately after this offering. Furthermore, if the underwriters exercise their over-allotment option, or outstanding options and warrants are exercised, you could experience further dilution. For a further description of the dilution that you will experience immediately after this offering, see the section titled “Dilution.”
We have broad discretion to determine how to use the funds raised in this offering, and may use them in ways that may not enhance our operating results or the price of our common stock.
Our management will have broad discretion over the use of proceeds from this offering, and we could spend the proceeds from this offering in ways our stockholders may not agree with or that do not yield a favorable return, if at all. We currently intend to use the net proceeds of this offering for capital expenditures to expand the sequencing and computing capacity in our Mountain View and Santa Clara leased facilities, to finance the further development of our sequencing technology and services, for sales and marketing activities and for working capital and other general corporate purposes, including the costs associated with being a public company. If we do not invest or apply the proceeds of this offering in ways that improve our operating results, we may fail to achieve expected financial results, which could cause our stock price to decline.
Provisions in our charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management.
Our amended and restated certificate of incorporation and amended and restated bylaws that will be in effect immediately prior to the consummation of this offering will contain provisions that could delay or prevent changes in control or changes in our management without the consent of our board of directors. These provisions will include the following:
| § | a classified board of directors with three-year staggered terms, which may delay the ability of stockholders to change the membership of a majority of our board of directors; |
| § | no cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates; |
| § | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director, which prevents stockholders from being able to fill vacancies on our board of directors; |
| § | the ability of our board of directors to authorize the issuance of shares of preferred stock and to determine the price and other terms of those shares, including preferences and voting rights, without stockholder approval, which could be used to significantly dilute the ownership of a hostile acquiror; |
| § | the ability of our board of directors to alter our bylaws without obtaining stockholder approval; |
| § | the required approval of at least 66 2 /3% of the shares entitled to vote at an election of directors to adopt, amend or repeal our bylaws or repeal the provisions of our amended and restated certificate of incorporation regarding the election and removal of directors; |
| § | a prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders; |
| § | the requirement that a special meeting of stockholders may be called only by the chairman of the board of directors, the chief executive officer, the president or the board of directors, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors; and |
| § | advance notice procedures that stockholders must comply with in order to nominate candidates to our board of directors or to propose matters to be acted upon at a stockholders’ meeting, which may discourage or deter a potential acquiror from conducting a solicitation of proxies to elect the acquiror’s own slate of directors or otherwise attempting to obtain control of us. |
32
Table of Contents
We are also subject to the anti-takeover provisions contained in Section 203 of the Delaware General Corporation Law. Under Section 203, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other exceptions, the board of directors has approved the transaction. For a description of our capital stock, see the section titled “Description of Capital Stock.”
We do not currently intend to pay dividends on our common stock, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We do not currently intend to pay any cash dividends on our common stock for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth. Additionally, the terms of our credit facility restrict our ability to pay dividends. Therefore, you are not likely to receive any dividends on your common stock for the foreseeable future.
33
Table of Contents
Special Note Regarding Forward-Looking Statements
This prospectus contains forward-looking statements that involve risks and uncertainties. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.” These statements relate to future events or our future financial or operational performance and involve known and unknown risks, uncertainties and other factors that could cause our actual results, levels of activity, performance or achievement to differ materially from those expressed or implied by these forward-looking statements. These risks and uncertainties are contained principally in the section entitled “Risk Factors.”
Forward-looking statements include all statements that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “intend,” “could,” “would,” “continue,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “potential” or the negative of those terms, and similar expressions and comparable terminology intended to identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus, and, except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this prospectus.
This prospectus also contains estimates and other information concerning our current and target markets that are based on industry publications, surveys and forecasts, including those generated by Scientia Advisors. These estimates and information involve a number of assumptions and limitations, and you are cautioned not to give undue weight to these estimates and information. These industry publications, surveys and forecasts generally indicate that their information has been obtained from sources believed to be reliable. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause actual results to differ materially from those expressed in these publications, surveys and forecasts.
34
Table of Contents
We estimate that we will receive net proceeds of approximately $ million from the sale of shares of common stock offered in this offering, or approximately $ million if the underwriters exercise their over-allotment option in full, based on an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million, assuming the initial public offering price stays the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed initial public offering price per share, would increase the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price per share, would decrease the net proceeds to us from this offering, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, by approximately $ million. We do not expect that a change in the offering price or the number of shares by these amounts would have a material effect on our intended uses of the net proceeds from this offering, although it may impact the amount of time prior to which we may need to seek additional capital.
We currently intend to use approximately $20.0 million to $25.0 million of the net proceeds of this offering for capital expenditures to expand the sequencing and computing capacity in our Mountain View and Santa Clara leased facilities. We intend to use the remainder of the net proceeds of this offering to finance the further development of our sequencing technology and services, for sales and marketing activities and for working capital and other general corporate purposes, including the costs associated with being a public company.
The expected use of net proceeds of this offering represents our current intentions based upon our present plan and business conditions. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. Accordingly, we will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the proceeds of this offering.
Until we use the net proceeds of this offering, we intend to invest the net proceeds in short-term, interest-bearing, investment-grade securities. We cannot predict whether the proceeds invested will yield a favorable return, if any.
35
Table of Contents
We have never declared or paid cash dividends on our common stock and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. In addition, the terms of our credit facility currently prohibit us from paying cash dividends on our common stock. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. The payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, requirements under the Delaware General Corporation Law, restrictions and covenants pursuant to any other credit facilities we may enter into, our overall financial condition and any other factors deemed relevant by our board of directors.
36
Table of Contents
The following table sets forth our capitalization as of June 30, 2010:
| § | on an actual basis; |
| § | on a pro forma basis to give effect to: |
| § | our issuance and sale, during August and September 2010, of an aggregate of 5,274,871 shares of our Series E preferred stock and warrants to purchase an aggregate of 1,318,719 shares of our common stock at an exercise price of $2.69 per share, which will become exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010; |
| § | the conversion of the convertible notes we issued and sold during April, May, June and August 2010 into shares of our Series E preferred stock, which occurred during August 2010 in connection with our Series E preferred stock financing; |
| § | the conversion of all shares of our convertible preferred stock outstanding on June 30, 2010, together with the shares of Series E preferred stock we issued during August and September 2010, including shares issued in connection with the related conversion of our convertible notes, into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
| § | the conversion of all of our warrants for convertible preferred stock into warrants for common stock immediately prior to the consummation of this offering and the related reclassification of convertible preferred stock warrant liability to additional paid-in capital; |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the filing and effectiveness of our amended and restated certificate of incorporation, which will occur immediately prior to the consummation of this offering; and |
| § | on a pro forma as adjusted basis to give further effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
37
Table of Contents
You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
| As of June 30, 2010 | ||||||||||
| Actual | Pro forma | Pro forma as adjusted | ||||||||
| (unaudited) | ||||||||||
| (in thousands, except share and per share amounts) | ||||||||||
| Notes payable, net of current portion |
$ | 1,192 | $ | 1,192 | $ | |||||
| Convertible Notes |
17,301 | — | — | |||||||
| Convertible preferred stock warrant liability |
1,318 | — | — | |||||||
| Convertible preferred stock, $0.001 par value per share; 9,288,222 shares authorized, 7,819,758 shares issued and outstanding, actual; no shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
95,844 | — | — | |||||||
| Stockholders’ equity (deficit): |
||||||||||
| Preferred stock, $0.001 par value per share; no shares authorized, issued and outstanding, actual; shares authorized, no shares issued and outstanding, pro forma and pro forma as adjusted |
— | — | — | |||||||
| Common stock, $0.001 par value per share; 17,293,925 shares authorized, 950,493 shares issued and outstanding, actual; shares authorized, shares issued and outstanding, pro forma; shares authorized, shares issued and outstanding, pro forma as adjusted |
1 | |||||||||
| Additional paid-in capital |
11,676 | |||||||||
| Deficit accumulated during the development stage |
(108,128 | ) | ||||||||
| Total stockholders’ equity (deficit) |
(96,451 | ) | 35,576 | |||||||
| Total capitalization |
$ | 19,204 | $ | 36,768 | $ | |||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million, assuming the initial public offering price per share, as set forth on the cover page of this prospectus, remains the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed initial public offering price per share, would increase each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price per share, would decrease each of pro forma as adjusted additional paid-in capital, stockholders’ equity and total capitalization by approximately $ million. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price and other terms of this offering determined at pricing.
The outstanding share information in the table above excludes:
| § | 2,251,093 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2010, with a weighted-average exercise price of $1.50 per share; |
| § | 1,215,728 shares of common stock reserved for future issuance under our 2006 Equity Incentive Plan as of June 30, 2010, which will become available for issuance under our 2010 Equity Incentive Award Plan after completion of this offering; |
| § | shares of common stock that will be reserved for future issuance under our 2010 Equity Incentive Award Plan, as well as any automatic increases in the number of shares of our common stock reserved for |
38
Table of Contents
| future issuance under this benefit plan, which will become effective immediately prior to the consummation of this offering; |
| § | up to 1,587,302 shares of our common stock that certain investors in our Series E financing will have the right to purchase from us if this offering is consummated before the second or third closing of that financing; |
| § | 116,630 shares of common stock subject to warrants outstanding as of June 30, 2010 that will not expire upon completion of this offering, with a weighted-average exercise price of $7.97 per share; |
| § | 1,848,849 shares of common stock subject to warrants issued during April, May, June and August 2010 that will not expire upon completion of this offering, with a weighted-average exercise price of $1.50 per share; and |
| § | 1,318,719 shares of common stock subject to warrants outstanding as of September 1, 2010 that will not expire upon completion of this offering and that are exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010. |
39
Table of Contents
If you invest in our common stock, you will experience dilution to the extent of the difference between the public offering price per share of our common stock you pay in this offering and the net tangible book value per share of our common stock after this offering.
As of June 30, 2010, our net tangible book value was $(96.5) million, or $(101.47) per share of our common stock. Our net tangible book value represents total tangible assets less total liabilities and convertible preferred stock, all divided by the number of shares of common stock outstanding on June 30, 2010. Our pro forma net tangible book value at June 30, 2010, before giving effect to this offering, was $ , or $ per share of our common stock. Pro forma net tangible book value, before the issuance and sale of shares in this offering, gives effect to:
| § | our issuance and sale, during August and September 2010, of an aggregate of 5,274,871 shares of our Series E preferred stock and warrants to purchase an aggregate of 1,318,719 shares of our common stock at an exercise price of $2.69 per share, which will become exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010; |
| § | the conversion of the convertible notes we issued and sold during April, May, June and August 2010 into shares of our Series E preferred stock, which occurred during August 2010 in connection with our Series E preferred stock financing; |
| § | the conversion of all shares of our convertible preferred stock outstanding on June 30, 2010, together with the shares of Series E preferred stock we issued during August and September 2010, including shares issued in connection with the related conversion of our convertible notes, into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
| § | the conversion of all of our warrants for convertible preferred stock into warrants for common stock immediately prior to the consummation of this offering and the related reclassification of convertible preferred stock warrant liability to additional paid-in capital; |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the exercise, on a net issuance basis, of warrants outstanding as of June 30, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
40
Table of Contents
After giving effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) and after deducting the estimated underwriting discounts and commissions and estimated offering expenses, our pro forma as adjusted net tangible book value at June 30, 2010 would have been approximately $ million, or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors. The following table illustrates this per share dilution:
| Assumed initial public offering price per share |
$ | |||||||
| Historical net tangible book value per share as of June 30, 2010 |
$ | (101.47 | ) | |||||
| Increase attributable to the pro forma adjustments described above |
||||||||
| Pro forma net tangible book value per share as of June 30, 2010, before the issuance and sale of shares in this offering |
||||||||
| Increase per share attributable to the issuance and sale of shares in this offering |
||||||||
| Pro forma as adjusted net tangible book value per share after this offering |
||||||||
| Dilution per share to investors in this offering |
$ | |||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) our pro forma as adjusted net tangible book value as of June 30, 2010 after this offering by approximately $ million, or approximately $ per share, and would decrease (increase) dilution to investors in this offering by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) our pro forma as adjusted net tangible book value as of June 30, 2010 after this offering by approximately $ million, or approximately $ per share, and would decrease (increase) dilution to investors in this offering by approximately $ per share, assuming the initial public offering price per share remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed initial public offering price per share, would increase our pro forma as adjusted net tangible book value as of June 30, 2010 after this offering by approximately $ million, or approximately $ per share, and would decrease dilution to investors in this offering by approximately $ per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price per share, would decrease our pro forma as adjusted net tangible book value as of June 30, 2010 after this offering by approximately $ million, or approximately $ per share, and would increase dilution to investors in this offering by approximately $ per share, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price and other terms of this offering determined at pricing.
If the underwriters fully exercise their over-allotment option, pro forma as adjusted net tangible book value after this offering would increase to approximately $ per share, and there would be an immediate dilution of approximately $ per share to new investors.
To the extent that outstanding options or warrants with an exercise price per share that is less than the pro forma as adjusted net tangible book value per share, before giving effect to the issuance and sale of shares in this offering, are exercised, you will experience further dilution. If all of our outstanding options and warrants described above were exercised, our pro forma net tangible book value as of June 30, 2010, before giving effect to the issuance and sale of shares in this offering, would have been approximately $ million, or approximately $ per share, and our pro forma as adjusted net tangible book value as of June 30, 2010
41
Table of Contents
after this offering would have been approximately $ million, or approximately $ per share, causing dilution to new investors of approximately $ per share.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The following table shows, as of June 30, 2010, on a pro forma as adjusted basis, after giving effect to the pro forma adjustments described above, the number of shares of common stock purchased from us, the total consideration paid to us and the average price paid per share by existing stockholders and by new investors purchasing common stock in this offering at an assumed initial public offering price of $ per share, before deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
| Shares purchased from us |
Total consideration to us |
Average price per share | ||||||||||||
| Number | Percent | Amount | Percent | |||||||||||
| Existing stockholders |
% | $ | % | $ | ||||||||||
| New investors |
||||||||||||||
| Total |
100.0 | % | $ | 100.0 | % | $ | ||||||||
The table above, and the information below, assume that our existing stockholders do not purchase any shares in this offering.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus) would increase (decrease) total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders by $ , $ and $ , respectively, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders by $ , $ and $ , respectively, assuming the initial public offering price stays the same. An increase of 1,000,000 in the number of shares we are offering, together with a $1.00 increase in the assumed initial public offering price per share, would increase total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders by $ , $ and $ , respectively. A decrease of 1,000,000 in the number of shares we are offering, together with a $1.00 decrease in the assumed initial public offering price per share, would decrease total consideration paid by new investors, total consideration paid by all stockholders and the average price per share paid by all stockholders by $ , $ and $ , respectively. The pro forma as adjusted information is illustrative only, and we will adjust this information based on the actual initial public offering price and other terms of this offering determined at pricing.
The information and tables in this section are based on 950,493 shares of common stock issued and outstanding as of June 30, 2010 and exclude:
| § | 2,251,093 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2010 with a weighted-average exercise price of $1.50 per share; |
| § | 1,215,728 shares of common stock reserved for future issuance under our 2006 Equity Incentive Plan as of June 30, 2010, which will become available for issuance under our 2010 Equity Incentive Award Plan after completion of this offering; |
| § | shares of common stock that will be reserved for future issuance under our 2010 Equity Incentive Award Plan, as well as any automatic increases in the number of shares of our common stock reserved for future issuance under this benefit plan, which will become effective immediately prior to the consummation of this offering; |
42
Table of Contents
| § | up to 1,587,302 shares of our common stock that certain investors in our Series E financing will have the right to purchase from us if this offering is consummated before the second or third closing of that financing; |
| § | 116,630 shares of common stock subject to warrants outstanding as of June 30, 2010 that will not expire upon completion of this offering, with a weighted-average exercise price of $7.97 per share; |
| § | 1,848,849 shares of common stock subject to warrants issued during April, May, June and August 2010 that will not expire upon completion of this offering, with a weighted-average exercise price of $1.50 per share; and |
| § | 1,318,719 shares of common stock subject to warrants outstanding as of September 1, 2010 that will not expire upon completion of this offering and that are exercisable after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010. |
If the underwriters exercise their over-allotment option in full, the following will occur:
| § | the pro forma as adjusted number of shares of our common stock held by existing stockholders would decrease to approximately % of the pro forma as adjusted total number of shares of our common stock outstanding after this offering; and |
| § | the pro forma as adjusted number of shares of our common stock held by new investors would increase to approximately % of the pro forma as adjusted total number of shares of our common stock outstanding after this offering. |
43
Table of Contents
The following selected financial data should be read together with our financial statements and accompanying notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus. The selected financial data in this section is not intended to replace our financial statements and the accompanying notes.
We derived the statement of operations data for the years ended December 31, 2007, 2008 and 2009 and the balance sheet data as of December 31, 2008 and 2009 from our audited financial statements appearing elsewhere in this prospectus. The statement of operations data for the cumulative period from June 14, 2005 (date of inception) to December 31, 2005 and the year ended December 31, 2006 and the balance sheet data as of December 31, 2005, 2006 and 2007 have been derived from our audited financial statements not included in this prospectus. The statement of operations data for the six months ended June 30, 2009 and 2010 and for the cumulative period from June 14, 2005 (date of inception) to June 30, 2010 and the balance sheet data as of June 30, 2010 are derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on a basis consistent with our audited financial statements and include, in the opinion of management, all adjustments, consisting only of normal recurring adjustments, necessary for the fair statement of the financial information in those statements. Our historical results are not necessarily indicative of the results to be expected in any future period, and the results for the six months ended June 30, 2010 are not necessarily indicative of the results to be expected for the year ending December 31, 2010.
| Cumulative period from June 14, 2005 (date of inception) to December 31, 2005 |
Year ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 |
||||||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||||||||||||
| (in thousands, except share and per share amounts) | |||||||||||||||||||||||||||||||
| Statement of Operations Data: |
|||||||||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | 623 | $ | — | $ | 1,425 | $ | 2,048 | |||||||||||||||
| Operating expenses: |
|||||||||||||||||||||||||||||||
| Start-up production costs(1) |
— | — | — | — | 5,033 | 1,013 | 8,985 | 14,018 | |||||||||||||||||||||||
| Research and development(1) |
— | 3,732 | 10,305 | 23,633 | 22,424 | 10,449 | 11,097 | 71,191 | |||||||||||||||||||||||
| General and administrative(1) |
— | 951 | 1,896 | 3,179 | 4,953 | 2,120 | 4,862 | 15,841 | |||||||||||||||||||||||
| Sales and marketing(1) |
— | — | — | 1,045 | 1,798 | 620 | 2,539 | 5,382 | |||||||||||||||||||||||
| Total operating expenses(1) |
— | 4,683 | 12,201 | 27,857 | 34,208 | 14,202 | 27,483 | 106,432 | |||||||||||||||||||||||
| Loss from operations |
— | (4,683 | ) | (12,201 | ) | (27,857 | ) | (33,585 | ) | (14,202 | ) | (26,058 | ) | (104,384 | ) | ||||||||||||||||
| Interest expense |
— | (11 | ) | (215 | ) | (974 | ) | (3,465 | ) | (2,051 | ) | (1,144 | ) | (5,809 | ) | ||||||||||||||||
| Interest and other income (expense), net |
— | 129 | 163 | 437 | 1,101 | (70 | ) | 235 | 2,065 | ||||||||||||||||||||||
| Net loss |
$ | — | $ | (4,565 | ) | $ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,323 | ) | $ | (26,967 | ) | $ | (108,128 | ) | ||||||||
| Net loss per share, basic and diluted |
$ | (123.58 | ) | $ | (211.00 | ) | $ | (369.36 | ) | $ | (386.56 | ) | $ | (177.96 | ) | $ | (45.09 | ) | |||||||||||||
| Weighted-average shares outstanding used in computing net loss per share, basic and diluted |
36,941 | 58,072 | 76,873 | 92,998 | 91,724 | 598,080 | |||||||||||||||||||||||||
| Pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (6.59 | ) | $ | (2.53 | ) | |||||||||||||||||||||||||
44
Table of Contents
| Cumulative period from June 14, 2005 (date of inception) to December 31, 2005 |
Year ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 | |||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2009 | 2010 | |||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||||||
| Net loss used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
$ | (37,037 | ) | $ | (27,202 | ) | ||||||||||||||
| Weighted-average shares of common stock outstanding used in computing pro forma net loss per share of common stock, basic and diluted (unaudited)(2) |
5,619,600 | 10,763,515 | ||||||||||||||||||
| (1) | Includes stock-based compensation expense as follows: |
| Period
from June 14, 2005 (date of inception) to December 31, 2005 |
Year ended December 31, |
Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 | |||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2009 | 2010 | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Start-up production costs |
$ | — | $ | — | $ | — | $ | — | $ | 81 | $ | 15 | $ | 105 | $ | 186 | ||||||||
| Research and development |
— | 10 | 78 | 246 | 992 | 225 | 415 | 1,741 | ||||||||||||||||
| General and administrative |
— | 2 | 22 | 90 | 262 | 78 | 301 | 677 | ||||||||||||||||
| Sales and marketing |
— | — | — | — | 75 | 25 | 68 | 143 | ||||||||||||||||
| Total stock-based compensation expense |
$ | — | $ | 12 | $ | 100 | $ | 336 | $ | 1,410 | $ | 343 | $ | 889 | $ | 2,747 | ||||||||
| (2) | Net loss used in computing pro forma basic and diluted net loss per share of common stock, and the number of weighted-average common shares used in computing pro forma basic and diluted net loss per share of common stock, in the table above assume the conversion of all of our outstanding convertible preferred stock into common stock immediately prior to the consummation of this offering. See Note 2 to our financial statements for an explanation of the method used to compute pro forma basic and diluted net loss per share of common stock and the number of shares used in computing those per share amounts. |
| December 31, | June
30, 2010 |
||||||||||||||||||||||
| 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||
| Balance Sheet Data: |
|||||||||||||||||||||||
| Cash and cash equivalents |
$ | 2 | $ | 1,632 | $ | 4,260 | $ | 6,186 | $ | 7,765 | $ | 7,972 | |||||||||||
| Working capital (deficit) |
2 | 1,047 | 1,845 | 741 | 2,964 | (20,860 | ) | ||||||||||||||||
| Total assets |
2 | 2,748 | 8,762 | 15,754 | 30,278 | 39,795 | |||||||||||||||||
| Current and long-term notes payable |
— | 1,000 | 3,473 | 11,697 | 7,950 | 23,179 | |||||||||||||||||
| Convertible preferred stock warrant liability |
— | 39 | 386 | 1,100 | 1,553 | 1,318 | |||||||||||||||||
| Convertible preferred stock |
— | 6,236 | 20,223 | 45,622 | 85,833 | 95,844 | |||||||||||||||||
| Total stockholders’ equity (deficit) |
2 | (4,852 | ) | (17,121 | ) | (45,154 | ) | (77,690 | ) | (96,451 | ) | ||||||||||||
45
Table of Contents
Management’s Discussion and Analysis of
Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this prospectus. In addition to historical financial information, the following discussion contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly under the caption “Risk Factors.”
Overview
We are a life sciences company that has developed and commercialized an innovative DNA sequencing platform, and our goal is to become the preferred solution for complete human genome sequencing and analysis. Our Complete Genomics Analysis Platform, or CGA Platform, combines our proprietary human genome sequencing technology with our advanced informatics and data management software and our innovative, end-to-end, outsourced service model to provide our customers with data that is immediately ready to be used for genome-based research. We believe that our solution will provide academic and biopharmaceutical researchers with complete human genomic data and analysis at an unprecedented combination of quality, cost and scale without requiring them to invest in in-house sequencing instruments, high-performance computing resources and specialized personnel. By removing these constraints and broadly enabling researchers to conduct large-scale complete human genome studies, we believe that our solution has the potential to revolutionize medical research and expand understanding of the basis, treatment and prevention of complex diseases.
We were incorporated in Delaware in June 2005 and began operations in March 2006. From March 2006 through mid-2009, our operations focused on research and developing our sequencing technology platform. We recognized our first revenue from the sale of our genome sequencing services in the fourth quarter of 2009. As of June 30, 2010, we were considered to be a development stage enterprise.
We have targeted our complete human genome sequencing service at academic, governmental and other research institutions, as well as pharmaceutical and other life science companies. We perform our sequencing service at our Mountain View, California headquarters facility, which began commercial operation in May 2010. In the near term, we expect to make significant expenditures related to the expansion of our Mountain View sequencing facility and our research and development initiatives, as well as to increase our sales and marketing and general and administrative expenses to support our commercial operations and anticipated growth. In future years, we plan to construct additional genome centers in the United States and in other strategic markets to accommodate an expected growing global demand for high-quality, low-cost complete human genome sequencing on a large scale.
We have not been profitable in any quarterly period since we were formed. We incurred net losses of $12.3 million, $28.4 million and $35.9 million for the years ended December 31, 2007, 2008 and 2009, respectively, and $27.0 million for the six months ended June 30, 2010. For the six months ended June 30, 2010, we recognized revenue of $1.4 million, and for the year ended December 31, 2009, we recognized revenue of $0.6 million. As of June 30, 2010, our deficit accumulated during the development stage was $108.1 million. Although we do not anticipate any material seasonal effects, given our limited operating history as a revenue generating company, our sales cycle is uncertain. Seven customers accounted for the revenue we recognized for the year ended December 31, 2009. University of Texas Southwestern Medical Center accounted for 17% of our revenue, four customers, Pfizer Inc., The Broad Institute, the Flanders Institute for Biotechnology and Johns Hopkins University, each accounted for 16% of our revenue, and the Institute for Systems Biology accounted for 13% of our revenue. Thirteen customers accounted for the revenue we recognized for the six months ended June 30, 2010, and Pfizer, Sichuan University and The International Mesothelioma Program at Brigham and Women’s Hospital accounted for 20%, 15% and 11%, respectively, of our revenue. If demand for our services expands as expected, we do not anticipate that the loss of any of the customers named above would have a material adverse effect on our future results of operations.
46
Table of Contents
Key Financial Measures
Revenue
Our revenue is derived from selling our human genome sequencing service. We sell our service to our customers through a direct field sales and support organization. Our customers enter into purchase orders, and, in some cases, genome service contracts with us. For most arrangements, we recognize revenue upon shipment of genomic data to the customer. The per genome price of our sequencing service is based on the number of genomes to be sequenced in the arrangement. We anticipate periodically reducing the price per genome for our service as the costs of sequencing go down and competitive conditions change. We expect that our primary source of revenue for the foreseeable future will be derived from our human genome sequencing service.
Operating Expenses
Start-up Production Costs
Start-up production costs primarily consist of costs related to the acceptance testing of customer genomic samples, sample sequencing preparation, sample sequencing, the processing of data generated by the prototype sequencing instruments, continued validation of the production process and optimization of instrument performance. These costs primarily include personnel-related expenses and stock-based compensation, chemical reagents and engineering materials and supplies, consultant fees, depreciation of equipment and facilities-related costs. Prior to 2009, we were primarily involved in developing our sequencing technology platform, and, as a result, most of these expenses were accounted for as research and development. In 2009, we began providing our genome sequencing service to customers and accounted for these costs as start-up production costs.
Research and Development Expenses
Research and development expenses consist of costs associated with scientific research activities, software and hardware engineering development efforts and process automation. These costs primarily include personnel-related expenses, including stock-based compensation, chemical reagents and engineering materials and supplies, depreciation of equipment, consulting fees and facilities-related costs.
In 2007, we were awarded a grant from the National Institute of Science and Technology, or NIST, through which we are eligible to receive reimbursement of a portion of our research and development expenses and certain administrative expenses. In each of 2008 and 2009, we recognized a $0.8 million reduction in research and development expenses for activities funded by NIST.
General and Administrative Expenses
General and administrative expenses consist principally of personnel-related expenses, including stock-based compensation for our finance, human resources and certain executive personnel, professional services fees, such as consulting, audit, tax and legal fees, general corporate costs and facilities-related costs. We are eligible to receive reimbursement for a portion of our administrative expenses pursuant to our NIST grant. In each of 2008 and 2009, we recognized a $0.2 million reduction in general and administrative expenses for activities funded by NIST.
Sales and Marketing Expenses
Sales and marketing expenses consist principally of personnel-related expenses, including stock-based compensation for our sales and marketing personal, costs related to sales and marketing activities, marketing research and facilities-related costs.
Interest Expense
Interest expense consists of interest on our notes payable and issuance costs associated with our borrowings.
47
Table of Contents
Interest and Other Income (Expense), Net
Interest and other income (expense), net, consists of interest earned on our cash and cash equivalents and changes in the fair value of our convertible preferred stock warrant liability.
Income Taxes
As of December 31, 2009, we had federal and state net operating loss carryforwards of approximately $59.7 million each and federal and state research and development tax credit carryforwards of approximately $1.9 million and $2.0 million, respectively. Our federal net operating loss and research and development tax credit carryforwards begin expiring in 2026 unless used prior to that date. Our state net operating loss carryforwards will begin to expire in 2016, if not used, and our state research and development tax credit carryforwards do not expire. Our ability to use net operating loss and tax credit carryforwards is subject to ownership change rules as provided under the Internal Revenue Code and similar state provisions. We have performed an analysis to determine whether an ownership change has occurred from inception to December 31, 2009. Our analysis determined that two ownership changes have occurred during that period. Due to these ownership changes, the use of these net operating losses and research and development credits are subject to annual limitation. However, we concluded that as of December 31, 2009, no net operating losses or research and development credits will expire before utilization due to these ownership changes. In the event we have a subsequent change in ownership, net operating loss and research and development credit carryovers could be further limited and may expire unutilized.
Critical Accounting Policies and Estimates
Our financial statements have been prepared in conformity with generally accepted accounting principles in the United States, or GAAP. The preparation of our financial statements requires our management to make estimates, assumptions and judgments that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the applicable periods. Management bases its estimates, assumptions and judgments on historical experience and on various other factors that it believes to be reasonable under the circumstances. Different assumptions and judgments would change the estimates used in the preparation of our financial statements, which, in turn, could materially change the results from those reported. Our management evaluates its estimates, assumptions and judgments on an ongoing basis. Historically, our critical accounting estimates have not differed materially from actual results. However, actual results may differ from these estimates under different conditions. If actual results differ from these estimates and other considerations used in estimating amounts reflected in the financial statements, the resulting changes could have a material adverse effect on our statements of operations, liquidity and financial condition.
The estimates described below represent our critical accounting estimates. For more information regarding our critical accounting policies and estimates, please see Note 2 of the notes to our audited financial statements included elsewhere in this prospectus.
Revenue Recognition
We generate revenue from selling our human genome sequencing services under purchase orders or contracts. Revenues are recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, title has transferred, the price is fixed or determinable and collectability is reasonably assured. Upon completion of the sequencing process, we ship the research-ready genomic data to the customer. We use shipping documents and third-party evidence to verify shipment of the data. In order to determine whether collectability is reasonably assured, we assess a number of factors, including past transaction history with the customer and the creditworthiness of the customer. If we determine that collectability is not reasonably assured, we defer the recognition of revenue until collectability becomes reasonably assured. We also receive down payments from customers prior to the commencement of the genome sequencing process.
48
Table of Contents
For revenue generated under purchase orders, we have established standard terms and conditions that are specified for all orders. We use the purchase order to establish persuasive evidence of an arrangement and whether there is a fixed and determinable price for the order. Revenue is recognized based upon the shipment of genomic data to customers and satisfaction of all terms and conditions contained in the purchase order. Any down payments received are recorded as deferred revenue until we meet all revenue recognition criteria.
For revenue generated under contracts, we consider each contract’s terms and conditions to determine our obligations associated with the contract. We will defer revenue until all significant obligations, as defined in the contract, have been met. Any down payments received are recorded as deferred revenue, and revenue is recognized upon shipment of genomic data and satisfaction of all remaining obligations.
Allowance for Doubtful Accounts
We currently do not have any significant accounts receivable balances, and receipt of payment on our existing receivables has been reasonably assured such that we do not believe that an allowance for doubtful accounts is currently required. If our revenue increases as expected and our accounts receivable balance grows, we intend to perform regular evaluations of our customers’ creditworthiness and continuously monitor collections and payments to estimate an allowance for doubtful accounts that is based on the aging of the underlying receivables and our experiences with regard to specific collection issues.
Estimated Useful Lives of Property and Equipment
We depreciate our property and equipment using a straight line method over its estimated useful lives. Our property primarily consists of lease improvements, and our equipment primarily consists of our sequencing instruments and computer equipment used in the sequencing process. While we use our best judgment to determine the useful lives of our sequencing instruments and computer equipment, a significant change in technology or the emergence of an advanced technology could result in a shorter useful life than we initially anticipated. Our equipment represents the largest asset on our balance sheet, and a subsequent reduction in the useful lives of equipment could have a material impact on our statement of operations.
Convertible Preferred Stock Warrants
Freestanding warrants to purchase shares of our convertible preferred stock are classified as liabilities on our balance sheets at fair value because the warrants may conditionally obligate us to redeem the underlying convertible preferred stock at some point in the future. The warrants are subject to remeasurement at each balance sheet date, and any change in fair value is recognized as a component of interest and other income (expense), net, in the statements of operations. We estimated the fair value of these warrants at the respective balance sheet dates using the Black-Scholes option pricing model. We use a number of assumptions to estimate the fair value including the remaining contractual terms of the warrants, risk-free interest rates, expected dividend yield and expected volatility of the price of the underlying common stock. These assumptions are highly judgmental and could differ significantly in the future.
For the years ended December 31, 2007, 2008 and 2009, we recorded a charge of $0.2 million and gains of $0.1 million and $1.1 million to interest and other income (expense), net, respectively, to reflect the change in the fair value of the warrants. During the six months ended June 30, 2009 and 2010, we recorded a charge of $75,000 and a gain of $0.2 million, respectively, to interest and other income (expense), net, respectively, to reflect the change in fair value of the warrants.
We will continue to record adjustments to the fair value of the warrants until they are either exercised, converted into warrants to purchase common stock or expire, at which time the warrants will no longer be remeasured at each balance sheet date. Immediately prior to the consummation of this offering and the conversion of the underlying preferred stock to common stock, our outstanding preferred stock warrants will automatically convert into warrants to purchase shares of our common stock. The then-current aggregate fair value of these warrants will be reclassified from liabilities to additional paid-in capital, and we will cease to record any related periodic fair value adjustments.
49
Table of Contents
Inventory
Inventory consists of the raw materials we use in our sequencing process, work in process and finished goods. Inventories are stated at the lower of cost or market value. Cost is determined using standard costs, which approximate actual costs, on a first-in, first-out basis. Market value is determined as the lower of replacement cost or net realizable value. We regularly review inventory quantities on hand for excess and obsolete inventories, giving consideration to potential obsolescence, our product life cycle and development plans, product expiration and quality issues. To date, these factors have not been significant as our inventory amounts have been immaterial. However, we anticipate that these estimates will become more significant if our sequencing volumes increase as expected.
Valuation of Long-Lived Assets
We assess our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset may not be recoverable. These indicators may include, but are not limited to, significant decreases in the market value of an asset and significant changes in the extent or manner in which an asset is used. If these or other indicators are present, we test for recoverability by measuring the carrying amount of the assets against future net cash flows which the assets are expected to generate. If these assets are considered to be impaired, the impairment recognized is measured by the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows arising from the assets. We make estimates and judgments about future undiscounted cash flows and fair values. Although our cash flow forecasts are based on assumptions that are consistent with our plans, there is significant exercise of judgment involved in determining the cash flow attributable to a long-lived asset over its estimated remaining useful life. Our estimates of anticipated cash flows could be reduced significantly in the future. As a result, the carrying amounts of our long-lived assets could be reduced through impairment charges in the future. There have been no such impairments of long-lived assets to date. However, we anticipate that a future impairment could have a material impact on our financial statements in light of the dollar significance of the long-lived assets carried on our balance sheet.
Stock-Based Compensation
We recognize compensation expense related to the awarding of employee stock options, based on the estimated fair value of the awards granted using the Black-Scholes option-pricing model. We also use the Black-Scholes option-pricing model to determine the fair value of non-employee stock option grants. In accordance with authoritative guidance, the fair value of non-employee stock option grants is re-measured as they vest, and the resulting increase in value, if any, is recognized as expense during the period the related services are rendered.
50
Table of Contents
The Black-Scholes option-pricing model requires the input of our expected stock-price volatility, the expected life of the options, risk-free interest rate and expected dividends. Determining these assumptions requires significant judgment. We determined the expected life and volatility rate of the employee stock-option grants based on that of publicly traded companies in the DNA sequencing, diagnostics or personalized medicine industries. When selecting the public companies in these industries to be used in the volatility calculation, we selected companies with comparable characteristics to us, including enterprise value and financial leverage, and removed companies with significantly higher enterprise values, lower risk profiles or established positions within their applicable industry. We also selected companies with historical share price volatility information sufficient to meet the expected life of our stock options. The historical volatility data was computed using the daily closing prices for the selected companies’ shares during the equivalent period of the calculated expected term of our stock options. We will continue to analyze the historical expected term and stock price volatility assumptions as more historical data for our own options and common stock, respectively, becomes available. The expected life of the non-employee option grants was based on their remaining contractual life at the measurement date. The risk-free interest rate assumption was based on U.S. Treasury instruments whose terms were consistent with the option’s expected life. The expected dividend assumption was based on our history and expectation of dividend payouts. The following table sets forth these assumptions for our employee stock options for the periods provided:
| Year ended December 31, | Six months ended June 30, | |||||
| 2008 | 2009 | 2010 | ||||
| (unaudited) | ||||||
| Expected volatility |
82.05 – 84.79% | 74.03 – 92.44% | 80.54 – 80.79% | |||
| Expected life (in years) |
6.08 | 5.32 – 6.10 | 5.50 | |||
| Risk-free interest rate |
2.79 – 3.62% | 1.80 – 2.72% | 2.57 – 2.80% | |||
| Expected dividend yield |
0.00% | 0.00% | 0.00% | |||
In accordance with the authoritative guidance on stock compensation, we only record stock-based compensation expense for awards that are expected to vest. As a result, judgment is also required in estimating the amount of stock-based awards that are expected to be forfeited. Although we estimate forfeitures based on historical experience, actual forfeitures may differ. If actual results differ significantly from these estimates, stock-based compensation expense and our statements of operations could be materially impacted when we record a true-up for the difference in the period that the awards vest.
The following table summarizes the options granted from May 2, 2008 through June 30, 2010 with their exercise prices, the fair value of the underlying common stock and the intrinsic value per share, if any. We did not grant any options from January 1, 2008 through May 1, 2008.
| Grant date | Number of options granted |
Exercise price per share(1) |
Fair value of common stock per share |
Intrinsic value per share | |||||||||
| May 2, 2008 |
13,377 | $ | 35.40 | $ | 35.40 | $ | — | ||||||
| July 23, 2008 |
2,863 | 35.40 | 35.40 | — | |||||||||
| November 11, 2008 |
21,850 | 76.20 | 76.20 | — | |||||||||
| January 21, 2009 |
4,006 | 76.20 | 76.20 | — | |||||||||
| March 13, 2009 |
6,548 | 76.20 | 76.20 | — | |||||||||
| November 13, 2009 |
1,235,570 | 1.50 | 1.82 | (2) | 0.32 | ||||||||
| November 19, 2009 |
17,499 | 1.50 | 1.85 | (2) | 0.35 | ||||||||
| December 28, 2009 |
199,998 | 1.50 | 2.02 | (2) | 0.52 | ||||||||
| January 28, 2010 |
104,895 | 1.50 | 2.16 | (2) | 0.66 | ||||||||
| February 24, 2010 |
619,169 | 1.50 | 2.28 | (2) | 0.78 | ||||||||
| March 19, 2010 |
63,125 | 1.50 | 2.38 | (2) | 0.88 | ||||||||
| March 22, 2010 |
128,000 | 1.50 | 2.39 | (2) | 0.89 | ||||||||
| April 8, 2010 |
20,000 | 2.43 | (3) | 2.45 | (2) | 0.02 | |||||||
51
Table of Contents
| (1) | In January 2010, we approved a stock option modification, pursuant to which all outstanding unexercised options that were granted prior to January 28, 2010 under our 2006 Equity Incentive Plan and had an exercise price greater than $1.50 per share were modified to decrease the exercise price of these stock options to $1.50 per share. For more information see “—Stock Option Modification” below. |
| (2) | We reassessed the fair value of our common stock subsequent to the grant date of these options. |
| (3) | The exercise price of these grants was amended by our board of directors to reflect the fair value at the date of grant following our board’s contemporaneous valuation of our common stock as of March 31, 2010. |
All options granted by our board of directors on the dates noted above were intended to be exercisable at the fair value of our stock at the time of grant, based on information known at that time. To determine the fair value of our common stock, our board of directors considered input from management based on several factors, as described below. For the purposes of recording stock-based compensation expense, we have reassessed the fair value of our common stock for the options granted from November 13, 2009 through June 30, 2010. Because no single event or factor caused a change in the fair value of our common stock during this period, we reassessed the fair value of our common stock for each grant date based on the differences between the original fair value determinations and subsequent increases to the fair value of our common stock as determined by our board of directors. The intrinsic value of options to purchase our common stock on these grant dates represents the difference between the original fair value determination made by our board of directors on the date of option grant and the fair value applied retrospectively for accounting purposes, in light of subsequent fair value determinations by our board of directors.
The fair values of the common stock underlying our stock options were estimated by our board of directors with input from management based on several factors, including progress and milestones attained in our business, projected sales and earnings for multiple future periods and estimated probabilities of various financing and liquidation events. In the absence of a public trading market for our common stock, our board of directors has determined the fair value of the common stock using methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Practice Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Practice Guide. In addition, our board of directors considered numerous objective and subjective factors, including, among others, the following factors:
| § | the prices of preferred stock issued by us to sophisticated investors in arms-length transactions; |
| § | the rights, preferences and privileges of our preferred stock issued in financings relative to our common stock; |
| § | the execution of customer contracts and receipt of customer purchase orders; |
| § | the hiring of key personnel; |
| § | the status of development and implementation of our sequencing process; |
| § | our stage of development and business strategy; |
| § | the lack of an active public market for our common and preferred stock; |
| § | trends in the genome sequencing industry and performance of specific competitors; and |
| § | the likelihood of achieving a liquidity event for the shares of common stock underlying these stock options, such as an initial public offering or sale of the company, in light of the then-prevailing market conditions. |
For our contemporaneous valuations, our board of directors used the option pricing model, or OPM, if the valuation date approximated the close of recent financings by third parties and, in other situations, the probability-weighted expected return method, or PWERM. To ensure the reasonableness of the results of either method used, the results of the OPM allocation method were reconciled to the results of the PWERM allocation method, and the PWERM results were reconciled to the OPM results, if appropriate.
Under the PWERM method, the value of the common stock is estimated based on an analysis of future values for the company assuming various future outcomes. Stock value is based on the probability-weighted present value
52
Table of Contents
of expected future investment returns, considering each of the possible future outcomes available, as well as the rights of each class of stock. The future outcomes we considered were:
| § | initial public offering; |
| § | merger or sale; |
| § | liquidation; and |
| § | continuing operations as a viable private company. |
The OPM model treats the rights of the holders of preferred and common stock as equivalent to that of call options on any value of the enterprise above certain break points of value based on the liquidation preferences of the holders of preferred stock, as well as their rights to participation and conversion. Accordingly, the value of the common stock is determined by estimating the value of its portion of each of these call option rights. In order to determine the break points, we made estimates of the anticipated timing of a potential liquidity event and estimates of the volatility of our equity securities. The anticipated timing was based on our plans toward the liquidity event and on our board of directors’ judgment. Estimating the volatility of the stock price of a privately held company is complex because there is no readily available market for the shares. We estimated the volatility of our stock based on available information on volatility of stocks of publicly traded companies in the industry.
Discussion of Specific Valuation Factors from February 2008 through June 2010
We granted stock options in 2008, 2009 and 2010 with exercise prices between $1.50 and $76.20 per share. No single event caused the valuation of our common stock to increase or decrease from May 2008 to June 2010. Rather it has been a combination of the following factors that led to the changes in the fair value of the underlying common stock.
February 28, 2008 to July 23, 2008. Our board of directors determined the fair value of our common stock to be $35.40 per share as of each grant date between February 28, 2008 and July 23, 2008, primarily based on the OPM allocation method. The OPM allocation method assumed an enterprise value of $65 million, which was based on the recent closing of our Series C preferred stock in February 2008 at a price of $159.30 to several venture capital and private equity firms. Based on this enterprise value, we determined the minimum and maximum ranges of values at which our debt and preferred and common equity holders would receive value, or the break points. We applied the Black-Scholes option model with option strike prices equal to the break points and the current stock price equal to our enterprise value. The expected term of the debt and preferred and common equity was defined as the average expected holding period of the investors in the security until a liquidity event and was determined to be 2.1 years. The volatility was based on the average volatility over the expected term for our peer companies and was determined to be 53%. The risk-free interest rate was 1.89%, based on U.S. Treasury Securities matching the expected term. Based on this information, we determined the total value of each security. We applied a discount of 25.2% for lack of marketability to the value of the common stock. This valuation indicated a fair value of $35.40 per share for our common stock. Between February 28, 2008 and July 23, 2008, there were no factors that changed, or events that occurred to lead to a change in, the fair value of our common stock.
July 24, 2008 to September 29, 2008. No options were granted, and our board of directors did not make any determination of the fair value of our common stock.
September 30, 2008 to March 13, 2009. Our board of directors determined the fair value of our common stock to be $76.20 per share as of each grant date between September 30, 2008 and March 13, 2009, primarily based on the PWERM allocation method. The common stock valuation was performed following the successful sequencing of our first genome in September 2008. The PWERM allocation method used a risk-adjusted discount of 35%, a marketability discount of 29.9% and a weighted-average estimated time to an initial public offering or a strategic merger or sale of 2.6 years. The expected outcomes were weighted toward the respective liquidity events as follows:
| § | 5% toward an initial public offering in 2010; |
53
Table of Contents
| § | 10% toward a strategic merger or sale in 2009; |
| § | 20% toward a strategic merger or sale in 2010; |
| § | 30% toward a dissolution of us with no value to common stock holders; and |
| § | 35% toward remaining a private company. |
This valuation indicated a fair value of $76.20 per share for our common stock. After September 2008 and through March 13, 2009, there was a significant negative change in the financial capital markets and a macro-economic downturn. However, neither the extent of the change nor the duration of the economic downturn was known to us. Accordingly, we continued to grant stock options in the first quarter of 2009 at an exercise price of $76.20 per share.
March 14, 2009 to August 30, 2009. No options were granted, and our board of directors did not make any determination of the fair value of our common stock.
August 31, 2009 to April 8, 2010. Our board of directors determined the fair value of our common stock to be $1.50 per share as of each grant date between August 31, 2009 and April 8, 2010, based on the OPM valuation method. Prior to August 31, 2009, as a result of the severe macro-economic downturn we temporarily reduced spending by reducing employee compensation and delaying any start-up and pre-production related expenditures. This delay in spending slowed the completion of our sequencing production facilities. As a result, our sales efforts and related revenue projections were also delayed. The board of directors did not consider the PWERM allocation method for this valuation as the method seemed inappropriate given our stage of development and the uncertainty surrounding the probable liquidity exits for us. The OPM allocation method assumed an enterprise value of $67 million, which was based on the recent closing of our Series D preferred stock in August 2009 at a price of $7.56 per share to several venture capital and private equity firms, which represented only a small increase in our enterprise value from July 2008. Based on this enterprise value, we determined the minimum and maximum ranges of values at which our debt and preferred and common equity holders would receive value. We applied the Black-Scholes option model with option strike prices equal to these break points and the current stock price equal to our enterprise value. The expected term until liquidity was determined to be 1.6 years. The volatility was based on the average volatility over the expected term for our peer companies and was determined to be 85%. The risk-free interest rate was 0.74%, based on U.S. Treasury Securities matching the expected term. Based on this information, we determined the total value of each security. We applied a discount of 21.2% for lack of marketability to the value of the common stock. This valuation indicated a fair value of $1.50 per share for our common stock. Between August 31, 2009 and April 8, 2010, there was no single factor that changed, or single event that occurred to lead to a change in, the fair value of our common stock.
March 31, 2010. Our board of directors determined the fair value of our common stock to be $2.43 per share as of March 31, 2010, primarily based on the PWERM allocation method. The common stock valuation was performed following an additional closing of our Series D preferred stock financing at a price of $7.56 per share to several venture capital and private equity firms in March 2010. The PWERM allocation method used a risk-adjusted discount of 25%, a marketability discount of 15% and a weighted-average estimated time to an initial public offering or a strategic merger or sale of 1.9 years. The expected outcomes were weighted toward the respective liquidity events as follows:
| § | 17.5% toward an initial public offering in 2010; |
| § | 17.5% toward an initial public offering in 2011; |
| § | 5% toward a strategic merger or sale in 2011; |
| § | 5% toward a strategic merger or sale in 2012; |
| § | 35% toward a dissolution of us with no value to common stock holders; and |
| § | 20% toward a dissolution of us in which creditors are repaid and preferred investors will receive up to 0.5x of their original investment from the liquidation of assets and no value will be available to common stock holders. |
54
Table of Contents
The increase in the probability of a liquidity event from prior valuations was primarily related to the commencement of commercial operations and the achievement of revenue in the fourth quarter of 2009 and first quarter of 2010. However, substantial uncertainty still existed regarding our ability to sequence genomes on a cost-effective basis. This valuation indicated a fair value of $2.43 per share for our common stock. Following its fair value valuation, our board of directors modified the option grants approved on April 8, 2010 to adjust the exercise price to $2.43 per share, which it retrospectively determined to be the fair value of our common stock as of the date of grant.
April 9, 2010 to June 29, 2010. No options were granted, and our board of directors did not make any determination of the fair value of our common stock.
June 30, 2010. Our board of directors determined the fair value of our common stock to be $2.69 per share as of June 30, 2010, primarily based on the PWERM allocation method. The common stock valuation was performed in anticipation of the closing of our Series E preferred stock financing and in light of the executed non-binding term sheet for that financing. The initial closing of our Series E preferred stock financing subsequently occurred in August 2010 at a price of $7.56 per share to several venture capital and private equity firms. The PWERM allocation method used a risk-adjusted discount of 25%, a marketability discount of 15% and a weighted-average estimated time to an initial public offering or a strategic merger or sale of 1.6 years. The expected outcomes were weighted toward the respective liquidity events as follows:
| § | 20% toward an initial public offering in 2010; |
| § | 20% toward an initial public offering in 2011; |
| § | 5% toward a strategic merger or sale in 2011; |
| § | 5% toward a strategic merger or sale in 2012; |
| § | 30% toward a dissolution of us with no value to common stockholders; and |
| § | 20% toward a dissolution of us in which creditors are repaid and preferred investors will receive up to 0.5x of their original investment from the liquidation of assets and no value will be available to common stockholders. |
The increase in the probability of a liquidity event from prior valuations was primarily related to the commencement of efforts related to the filing of a registration statement with the Securities and Exchange Commission for our initial public offering. Specifically, on May 10, 2010, we held an organizational meeting with investment bankers to underwrite our initial public offering, and preparation of our registration statement commenced after that time. This increase in the probability of a liquidity event led to an increased valuation of $2.69 per share for our common stock.
Stock Option Modification
In January 2010, our board of directors approved a modification of outstanding unexercised stock options held by then-current employees and consultants to decrease the exercise price of these stock options to $1.50 per share. Other than a reduced exercise price, the terms and conditions of the stock options remained the same. All 85,477 unexercised options that were granted under our 2006 Equity Incentive Plan on or before January 28, 2010 and which had an exercise price greater than $1.50 per share were modified. The incremental stock-based compensation expense due to the modification was immaterial to our financial statements.
Income Taxes
We are subject to income taxes in the United States, and we use estimates in determining our provision for income taxes. We use the asset and liability method of accounting for income taxes. Under this method, deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect taxable income.
55
Table of Contents
Recognition of deferred tax assets is appropriate when realization of such assets is more likely than not. We recognize a valuation allowance against our net deferred tax assets if it is more likely than not that some portion of the deferred tax assets will not be fully realizable. This assessment requires judgment as to the likelihood and amounts of future taxable income by tax jurisdiction. At December 31, 2009, we had a full valuation allowance against all of our deferred tax assets.
Effective January 1, 2007, we adopted the new authoritative guidance to account for uncertain tax positions. None of our currently unrecognized tax benefits would affect our effective income tax rate if recognized, due to the valuation allowance that currently offsets our deferred tax assets. We do not anticipate the total amount of unrecognized income tax benefits relating to tax positions existing at December 31, 2009 will significantly increase or decrease in the next 12 months.
We assess all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position’s sustainability and is measured at the largest amount of benefit that is greater than 50% likely to be realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether:
| § | the factors underlying the sustainability assertion have changed; and |
| § | the amount of the recognized tax benefit is still appropriate. |
The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
As of December 31, 2009, we had federal and state net operating loss carryforwards in each case of $59.7 million. The federal net operating loss carryforwards will begin to expire in 2026, and the state net operating loss carryforwards will begin to expire in 2016. In addition, as of December 31, 2009, we had federal and state research and development tax credit carryforwards of $1.9 million and $2.0 million, respectively. The federal research and development tax credit carryforwards will expire in 2026, if not used, and the state research and development tax credit carryforwards do not expire. Because of the net operating loss and credit carryforwards, all of our tax years, dating to inception in 2005, remain open to federal tax examinations. Most state tax jurisdictions have four open tax years at any point in time.
Under federal and similar state tax statutes, substantial changes in our ownership, including as a result of this offering, may limit our ability to use our available net operating loss and tax credit carryforwards. The annual limitation, as a result of a change-in-control, may result in the expiration of net operating losses and credits before utilization. We conducted an analysis through December 31, 2009 to determine whether ownership changes occurred. We concluded that two ownership changes had occurred. However, as of December 31, 2009, no net operating losses or tax credits will expire unused as a result of these changes.
Results of Operations
During the year ended December 31, 2009, our results of operations were impacted by the following events, which should be considered when reading the discussion of our results of operations comparing the years ended December 31, 2008 and 2009 and the six months ended June 30, 2009 and 2010:
| § | In 2009, we initiated start-up production activities using resources from our research and development organization. Using these research and development resources for production activities decreased research and development expenses during 2009 by approximately $4.7 million. |
| § | In the second quarter of 2009, we implemented temporary cost-reduction initiatives to conserve cash in light of macro-economic conditions. The temporary cost-reduction initiatives included a salary reduction for all company employees that averaged approximately 50%. The impact of the temporary cost-reduction initiatives on our 2009 operating results was approximately $3.4 million, including reductions in employee salaries and benefits of approximately $2.0 million, consulting and outside engineering services by approximately $0.6 million and prototype equipment expenses by approximately $0.4 million. In the |
56
Table of Contents
| second quarter of 2009, as a result of these cost-reduction initiatives, research and development, general and administrative and sales and marketing expenses decreased $2.7 million, $0.5 million and $0.2 million, respectively, compared to the first quarter of 2009. |
| § | During the fourth quarter of 2009, we reevaluated the expected useful lives of our equipment and determined that for certain of our equipment, the useful life should be shortened. Accordingly, we accelerated depreciation of this equipment, resulting in an additional $1.0 million in depreciation expense in the fourth quarter of 2009. Of this $1.0 million charge, approximately $0.5 million was recorded as start-up production costs and approximately $0.5 million was recorded as research and development expense. |
Comparison of Six Months Ended June 30, 2009 and 2010
The following table shows the amounts of the listed items from our statements of operations for the periods presented, showing period-over-period changes (in thousands, except for percentages).
| Six months ended June 30, |
2010 vs. 2009 | ||||||||||||||
| 2009 | 2010 | $ Change | % Change | ||||||||||||
| Revenue |
$ | — | $ | 1,425 | $ | 1,425 | * | % | |||||||
| Operating expenses: |
|||||||||||||||
| Start-up production costs |
1,013 | 8,985 | 7,972 | 787 | % | ||||||||||
| Research and development |
10,449 | 11,097 | 648 | 6 | % | ||||||||||
| General and administrative |
2,120 | 4,862 | 2,742 | 129 | % | ||||||||||
| Sales and marketing |
620 | 2,539 | 1,919 | 310 | % | ||||||||||
| Total operating expenses |
14,202 | 27,483 | 13,281 | 94 | % | ||||||||||
| Loss from operations |
(14,202 | ) | (26,058 | ) | (11,856 | ) | 83 | % | |||||||
| Interest expense |
(2,051 | ) | (1,144 | ) | 907 | (44 | )% | ||||||||
| Interest and other income (expense), net |
(70 | ) | 235 | 305 | (436 | )% | |||||||||
| Net loss |
$ | (16,323 | ) | $ | (26,967 | ) | $ | (10,644 | ) | 65 | % | ||||
| * | Percentage not meaningful. |
Revenue
We recognized revenue of $1.4 million in the first six months of 2010, which represented sales to thirteen customers. We did not recognize revenue during the first six months of 2009.
Start-up Production Costs
During the first six months of 2010, we incurred $9.0 million of start-up production costs to support our genome sequencing service, compared to $1.0 million in the first six months of 2009. These activities include the acceptance testing of customer genomic samples, sample sequencing preparation, sample sequencing, the processing of data generated by our prototype sequencing instruments, continued validation of the production process and optimization of instrument performance. The increase in start-up production costs was primarily due to employee salaries and benefits and stock-based compensation expenses of $2.7 million and $0.1 million, respectively, depreciation expense of $1.5 million, facilities and maintenance costs of $1.3 million, data communication charges of $0.8 million, reagents, materials and supplies expenses of $0.8 million and consulting expenses of $0.3 million. As in 2009, we continued to incur considerable start-up costs in excess of revenue during the first six months of 2010 to initiate and bring our human genome sequencing production process to commercial-scale. We continued to commit significant personnel and equipment resources to our production process in advance of our achieving full commercial production volume. As only a portion of our production expenses varies with our revenue, our production costs will be greater than our revenue until we achieve significant product volume and revenues.
57
Table of Contents
We anticipate that our start-up production costs will decrease as we continue to improve and automate our human genome sequencing processes and increase the throughput of our sequencing technology. Conversely, we anticipate that our costs of providing sequencing services will increase if we sequence additional genomes and our revenue grows as we anticipate.
Research and Development
Research and development expenses were $11.1 million during the six months ended June 30, 2010, compared to $10.4 million during the six months ended June 30, 2009, representing an increase of $0.6 million, or 6%. The increase in research and development expenses was principally due to an increase in salaries and benefits expense and stock-based compensation expense of $1.4 million and $0.2 million, respectively, and an increase in facilities and maintenance costs of $0.6 million associated with the expansion of our facilities in 2009. The increase in salaries and benefits expense was primarily due to the temporary cost-reduction initiatives implemented during the second quarter of 2009, which caused a lower overall salaries and benefits expense for the six months ended June 30, 2009, offset by a charge associated with an equity grant to one of our founders. The overall increase in research and development expenses was offset by a reduction in depreciation expense of $1.1 million, a reduction in communication expenses of $0.3 million and a decrease in consulting expense and outside engineering services of $0.1 million.
We expect to continue to invest in research and development activities as we seek to enhance our sequencing processes, components and systems to improve the yield and throughputs and reduce the cost of our sequencing service. We believe that in the near future, our research and development expenses will increase.
General and Administrative
General and administrative expenses were $4.9 million for the six months ended June 30, 2010 compared to $2.1 million for the six months ended June 30, 2009, representing an increase of $2.7 million, or 129%. The increase in general and administrative expenses was due to increases in employee salaries and benefits and stock-based compensation expense of $2.0 million and $0.2 million, respectively. The increase in salaries and benefits expense was primarily due to the temporary cost-reduction initiatives implemented during the second quarter of 2009, which caused a lower overall salaries and benefits expense for the six months ended June 30, 2009, partially offset by charges associated with equity grants to two of our founders. In addition, consulting expense increased by $0.4 million, and outside services expenses for legal and accounting support increased by $0.3 million. The overall increase in general and administrative expenses was offset by a decrease in facilities and maintenance costs of $0.3 million.
We expect that general and administrative expenses will increase in 2010 and beyond as we increase the headcount of our finance and administrative staff as we operate as a public company. We anticipate that we will incur increased expenses related to audit, legal, regulatory and tax-related services associated with maintaining compliance with exchange listing and Securities and Exchange Commission requirements, directors’ and officers’ insurance and investor relation programs.
Sales and Marketing
Sales and marketing expenses were $2.5 million during the six months ended June 30, 2010 compared to $0.6 million during the six months ended June 30, 2009, representing an increase of $1.9 million, or 310%. The increase in sales and marketing expenses is due primarily to an increase in employee salaries and benefits of $1.1 million associated with the growth of our sales and marketing organization, an increase in marketing research and public relations expenses of $0.3 million and an increase in facilities and maintenance costs of $0.2 million as a result of an increase in headcount.
We expect that sales and marketing expenses will increase significantly in 2010 as we increase our headcount for sales, marketing and service personnel to support our expected growth in revenue and expansion of our customer base.
58
Table of Contents
Interest Expense
During the first six months of 2010, we incurred interest expense of $1.1 million compared to $2.1 million during the first six months of 2009. The decrease in interest expense was a result of a lower amortization of the debt discount related to the initial value of our Series D preferred stock warrants versus our common stock warrants. The Series D preferred stock warrants were issued in the first quarter of 2009 while the common stock warrants were issued during the second quarter of 2010, resulting in a shorter period of amortization in 2010.
Interest and Other Income (Expense), Net
The increase in interest and other income (expense), net, of $0.3 million between the first six months of 2010 and the first six months of 2009 was due primarily to the change in the valuation of our convertible preferred stock warrants.
Comparison of Years Ended December 31, 2007, 2008 and 2009
The following table shows the amounts of the listed items from our statements of operations for the periods presented, showing period-over-period changes (in thousands, except percentages).
| Years ended December 31, | 2008 vs. 2007 | 2009 vs. 2008 | ||||||||||||||||||||||||
| 2007 | 2008 | 2009 | $ Change | % Change | $ Change | % Change | ||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | 623 | $ | — | — | % | $ | 623 | * | % | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||
| Start-up production costs |
— | — | 5,033 | — | — | % | 5,033 | * | % | |||||||||||||||||
| Research and development |
10,305 | 23,633 | 22,424 | 13,328 | 129 | % | (1,209 | ) | (5 | )% | ||||||||||||||||
| General and administrative |
1,896 | 3,179 | 4,953 | 1,283 | 68 | % | 1,774 | 56 | % | |||||||||||||||||
| Sales and marketing |
— | 1,045 | 1,798 | 1,045 | * | % | 753 | 72 | % | |||||||||||||||||
| Total operating expenses |
12,201 | 27,857 | 34,208 | 15,656 | 128 | % | 6,351 | 23 | % | |||||||||||||||||
| Loss from operations |
(12,201 | ) | (27,857 | ) | (33,585 | ) | (15,656 | ) | 128 | % | (5,728 | ) | 21 | % | ||||||||||||
| Interest expense |
(215 | ) | (974 | ) | (3,465 | ) | (759 | ) | 353 | % | (2,491 | ) | 256 | % | ||||||||||||
| Interest and other income (expense), net |
163 | 437 | 1,101 | 274 | 168 | % | 664 | 152 | % | |||||||||||||||||
| Net loss |
$ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,141 | ) | 132 | % | $ | (7,555 | ) | 27 | % | |||||||
| * | Percentage not meaningful. |
Revenue
We recognized our first revenue of $0.6 million in the fourth quarter of 2009, which represented sales to seven customers.
Start-up Production Costs
During 2009, we incurred $5.0 million of start-up production costs to support our genome sequencing service. These activities include the acceptance testing of customer genomic samples, sample sequencing preparation, sample sequencing, the processing of data generated by our prototype sequencing instruments, continued validation of the production process and optimization of instrument performance. These costs primarily consisted of employee salaries and benefits and stock-based compensation expense of $1.7 million and $0.1 million, respectively, depreciation expense of $1.5 million, facilities and maintenance costs of $0.6 million, reagents, materials and supplies expense of $0.3 million, data communication charges of $0.2 million, equipment expense of $0.2 million and consulting expenses of $0.2 million. We incurred considerable start-up production costs in excess of revenue during 2009 to initiate and bring our human genome sequencing production process to commercial scale. We committed significant personnel and equipment resources to our production process in advance of our achieving full commercial production volume. Only a portion of our production expenses varies with our service revenue. Accordingly, unless we approach significant production volume and revenue, our production costs will be greater than our revenue.
59
Table of Contents
Research and Development
Research and development expenses decreased $1.2 million, or 5%, from 2008 to 2009 as we used resources from our research organization to initiate commercial operations and implemented temporary cost-reduction initiatives in the second quarter of 2009. This decrease reflects a reduction in equipment, reagents, materials and supplies and prototype equipment expense of $1.5 million. The reduction in equipment, reagents, materials and supplies expenses primarily reflects their usage in start-up production activities. The reduction in prototype equipment expense reflects our transition to developing production equipment as well as cost-reduction measures in the second quarter of 2009. In addition, the decrease in research and development expense reflects a reduction in licensing arrangements expense of $1.2 million, of which, $1.0 million represents a one-time payment to Callida Genomics, Inc. in 2008 pursuant to our intellectual property license arrangement. The decrease in research and development expense also reflects a reduction in consulting expense and outside engineering services of $0.9 million and the disposal of obsolete equipment in 2008 of $0.5 million. These decreases in research and development expense were offset by increased equipment depreciation expense of $0.9 million related to investments in computing and other equipment and software and acceleration of depreciation expense for certain equipment, increased employee salaries and benefits of $0.6 million related to increased headcount. The decrease in research and development expense was further offset by increased stock-based compensation expense of $0.7 million, increased facilities and maintenance costs of $0.6 million associated with the expansion of our facilities in 2009 and increased data communication costs of $0.5 million related to the expansion of our offsite data center.
Research and development expenses increased $13.3 million, or 129%, from 2007 to 2008 reflecting increased salaries and benefits of $4.7 million relating to increased headcount, increased employee recruiting fees of $0.6 million and increased stock-based compensation expense of $0.2 million. In addition, the increase in research and development expenses reflects increased consumption of reagents, materials and supplies and prototype equipment of $2.1 million, increased depreciation expense of $2.0 million primarily related to investments in computing and storage equipment, increased facilities and maintenance costs of $1.2 million related to occupying a larger facility beginning in September 2007, increased license fees of $1.0 million related to our intellectual property license arrangement with Callida Genomics, increased consulting expense and outside engineering services of $0.7 million and the disposal of obsolete equipment of $0.5 million.
General and Administrative
General and administrative expenses increased $1.8 million, or 56%, from 2008 to 2009 primarily reflecting increased facilities and maintenance costs of $1.1 million. During 2009, significant portions of our facility were renovated to accommodate our genome sequencing center. During the period of renovation, the space being renovated was unoccupied, and associated costs were recorded as general and administrative expense. In addition, general and administrative expenses reflect increased employee salaries and benefits and stock-based compensation expense of $0.1 million and $0.2 million, respectively, legal expenses of $0.2 million, primarily related to intellectual property matters and patent prosecution, increased consulting expenses of $0.1 million related to financial and human resources consultants and increased payroll and benefits servicing fees of $0.1 million.
General and administrative expenses increased $1.3 million, or 68%, from 2007 to 2008 reflecting increased employee salaries and benefits and stock-based compensation expense of $0.5 million and $0.1 million, respectively, related to increased headcount to support our growing research and development organization, legal expenses of $0.4 million primarily related to intellectual property matters and patent prosecution, increased facilities and maintenance expenses of $0.1 million, increased financial and human resources consultants expenses of $0.1 million and increased payroll and benefits servicing fees of $0.1 million.
Sales and Marketing
Sales and marketing expenses increased $0.8 million, or 72%, from 2008 to 2009 reflecting increased employee salaries and benefits and stock-based compensation expense of $0.7 million and $0.1 million, respectively,
60
Table of Contents
related to increased headcount, increased facilities and maintenance costs of $0.1 million and increased travel expenses $0.1 million. The increase in sales and marketing expenses was partially offset by decreased marketing research and public relations expenses of $0.3 million. Our sales and marketing expenses in 2008 reflects our initial investment in marketing activities and primarily consists of marketing research and public relations activities. We did not incur any sales and marketing expenses in 2007.
Interest Expense
Interest expense increased by $2.5 million, or 256%, from 2008 to 2009 primarily due to $1.0 million of interest expense on higher loan balances under our new credit facility and $1.5 million of interest charges related borrowings under convertible notes issued in 2009.
Interest expense increased $0.8 million, or 353%, from 2007 to 2008. During 2007, we had two credit facilities under which we had outstanding borrowings of $4.0 million. In 2008, we entered into a new credit facility for $13.0 million, a portion of which was used to repay in full the then-outstanding balances on the credit facilities. The increase in interest expense in 2008 is primarily a result of the larger loan balance and the costs associated with the prepayment of the two credit facilities.
Interest and Other Income (Expense), Net
The increase in interest and other income (expense), net, of $0.7 million from 2008 to 2009 was primarily due to the change in valuation of our convertible preferred stock warrants in 2009, offset by lower interest income due to lower cash and cash equivalents balances, as well as lower effective rates of interest earned on our cash equivalents balance.
The increase in interest and other income (expense), net, of $0.3 million from 2007 to 2008 was primarily due to the change in the valuation of our convertible preferred stock warrants.
Selected Quarterly Results of Operations
The following table presents our unaudited quarterly results of operations for the eight fiscal quarters ended June 30, 2010. This unaudited quarterly information has been prepared on the same basis as our audited financial statements and includes all adjustments, consisting of only normal recurring adjustments, necessary for the fair statement of the information for the quarters presented. You should read this table in conjunction with our financial statements and the related notes thereto included in this prospectus. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period.
| Fiscal 2008, quarter ended | Fiscal 2009, quarter ended | Fiscal 2010, quarter ended | ||||||||||||||||||||||||||||||
| Sept. 30 | Dec. 31 | Mar. 31 | Jun. 30 | Sept. 30 | Dec. 31 | Mar. 31 | Jun. 30 | |||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||
| Statements of Operations Data: |
||||||||||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | 623 | $ | 336 | $ | 1,089 | ||||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||||||
| Start-up production costs(1) |
— | — | 326 | 687 | 1,258 | 2,762 | 4,077 | 4,908 | ||||||||||||||||||||||||
| Research and development(1) |
5,440 | 8,446 | 6,746 | 3,703 | 5,638 | 6,337 | 6,169 | 4,928 | ||||||||||||||||||||||||
| General and administrative(1) |
704 | 1,037 | 1,298 | 822 | 1,352 | 1,481 | 3,099 | 1,763 | ||||||||||||||||||||||||
| Sales and marketing(1) |
511 | 300 | 427 | 193 | 366 | 812 | 1,226 | 1,313 | ||||||||||||||||||||||||
| Total operating expenses(1) |
6,655 | 9,783 | 8,797 | 5,405 | 8,614 | 11,392 | 14,571 | 12,912 | ||||||||||||||||||||||||
| Loss from operations |
(6,655 | ) | (9,783 | ) | (8,797 | ) | (5,405 | ) | (8,614 | ) | (10,769 | ) | (14,235 | ) | (11,823 | ) | ||||||||||||||||
| Interest expense |
(325 | ) | (392 | ) | (679 | ) | (1,372 | ) | (1,073 | ) | (341 | ) | (311 | ) | (833 | ) | ||||||||||||||||
| Interest and other income (expense), net |
31 | 62 | (22 | ) | (48 | ) | 439 | 732 | 210 | 25 | ||||||||||||||||||||||
| Net loss |
$ | (6,949 | ) | $ | (10,113 | ) | $ | (9,498 | ) | $ | (6,825 | ) | $ | (9,248 | ) | $ | (10,378 | ) | $ | (14,336 | ) | $ | (12,631 | ) | ||||||||
61
Table of Contents
| (1) | Includes stock-based compensation expense as follows: |
| Fiscal 2008, quarter ended | Fiscal 2009, quarter ended | Fiscal 2010, quarter ended | ||||||||||||||||||||||
| Sept. 30 | Dec. 31 | Mar. 31 | Jun. 30 | Sept. 30 | Dec. 31 | Mar. 31 | Jun. 30 | |||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Start-up production costs |
$ | — | $ | — | $ | 4 | $ | 11 | $ | 12 | $ | 54 | $ | 61 | $ | 44 | ||||||||
| Research and development |
54 | 101 | 116 | 109 | 100 | 667 | 250 | 165 | ||||||||||||||||
| General and administrative |
15 | 53 | 39 | 39 | 42 | 142 | 200 | 101 | ||||||||||||||||
| Sales and marketing |
— | — | 14 | 11 | 12 | 38 | 29 | 39 | ||||||||||||||||
| Total stock-based compensation expense |
$ | 69 | $ | 154 | $ | 173 | $ | 170 | $ | 166 | $ | 901 | $ | 540 | $ | 349 | ||||||||
Liquidity and Capital Resources
Since our inception, we have generated operating losses in every quarter, resulting in a deficit accumulated during the development stage of $108.1 million as of June 30, 2010. We have financed our operations to date primarily through private placements of preferred stock and convertible debt and borrowings under our credit facilities. Through June 30, 2010, we have received net proceeds of $95.4 million from the issuance of preferred stock and convertible debt. As of June 30, 2010, we had negative working capital of $(20.9) million, consisting of $12.4 million in current assets and $33.2 million in current liabilities. As of December 31, 2009, working capital was $3.0 million, consisting of $15.0 million in current assets and $12.1 million in current liabilities. Our cash is invested primarily in money market funds. Cash in excess of immediate operating requirements is invested in accordance with our investment policy, primarily with the goals of capital preservation and liquidity maintenance.
Summary Statement of Cash Flows
The following table summarizes our cash flows for the years ended December 31, 2007, 2008 and 2009 and the six months ended June 30, 2009 and 2010.
| Years ended December 31, | Six months ended June 30, |
|||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Net cash used in operating activities |
$ | (9,984 | ) | $ | (24,303 | ) | $ | (26,662 | ) | $ | (11,375 | ) | $ | (14,172 | ) | |||||
| Net cash used in investing activities |
(3,729 | ) | (7,419 | ) | (9,654 | ) | (1,607 | ) | (15,700 | ) | ||||||||||
| Net cash provided by financing activities |
16,341 | 33,648 | 37,895 | 10,136 | 30,079 | |||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 2,628 | $ | 1,926 | $ | 1,579 | $ | (2,846 | ) | $ | 207 | |||||||||
Cash Flows for the Six Months Ended June 30, 2009 and 2010
Operating Activities
Net cash used in operating activities was $14.2 million during the six months ended June 30, 2010 and consisted of a net loss of $27.0 million, offset by noncash items of $6.3 million and a net increase in operating assets and liabilities of $6.5 million. Noncash items for the six months ended June 30, 2010 consisted primarily of depreciation and amortization expense of $3.0 million, compensation expense for common stock issued to our founders of $1.8 million, stock-based compensation expense of $0.9 million and noncash interest expense related to our convertible notes and notes payable of $0.6 million. The significant items in the changes in operating assets and liabilities include a decrease in prepaid expenses of $4.6 million and increases in deferred revenue, inventory, accounts payable and accrued liabilities of $2.2 million, $1.9 million, $1.2 million and $0.5 million, respectively. The decrease in prepaid expenses was due to the use of fully refundable short-term deposits to order components used in the construction of sequencers whose specifications were validated during the six months ended June 30, 2010. The increase in deferred revenue was due to advance billing arrangements during the first six months of 2010. The increase in inventory was due to inventory purchases and work-in process in the first six
62
Table of Contents
months of 2010 to support customer orders. The increase in accounts payable was a result of inventory purchases to support an increase in production. The change in accrued liabilities was primarily due to accrued incentive compensation as a result of sales in 2010.
Net cash used in operating activities was $11.4 million during the six months ended June 30, 2009 and consisted of a net loss of $16.3 million, offset by noncash items of $3.9 million and a net increase in operating assets and liabilities of $1.1 million. Noncash items for the six months ended June 30, 2009 consisted primarily of depreciation and amortization expense of $2.0 million, noncash interest expense related to our convertible notes and notes payable of $1.3 million and stock-based compensation expense of $0.3 million. The significant changes in operating assets and liabilities include increases in deferred rent of $1.0 million and accounts payable of $0.3 million, offset by increases in accounts receivable and other assets of $0.2 million each.
Investing Activities
Net cash used in investing activities was $15.7 million and $1.6 million for the six months ended June 30, 2010 and 2009, respectively. The amounts related entirely to purchases of property and equipment. The purchases of property and equipment during the first six months of 2010 were primarily for sequencing equipment used in production, while the purchases of property and equipment during the first six months of 2009 were for equipment used in our research and development activities and leasehold improvements related to our facilities.
Financing Activities
Net cash provided by financing activities during the six months ended June 30, 2010 of $30.1 million consisted primarily of net proceeds from the issuance and sale of promissory notes in a bridge financing and net proceeds from the issuance and sale of Series D convertible preferred stock of $22.1 million and $10.0 million, respectively, offset by repayment of equipment loan borrowings of $2.2 million. Net cash provided by financing activities during the six months ended June 30, 2009 of $10.1 million consisted primarily of proceeds from the issuance and sale of promissory notes of $12.1 million, offset by repayment of notes payable of $2.0 million.
Cash Flows for the Years Ended December 31, 2007, 2008 and 2009
Operating Activities
Net cash used in operating activities was $26.7 million for the year ended December 31, 2009 and consisted of a net loss of $35.9 million, offset by noncash items of $8.0 million and a net increase in operating assets and liabilities of $1.3 million. Noncash items for the year ended December 31, 2009 consisted primarily of depreciation and amortization expense of $5.2 million, noncash interest expense related to our convertible notes and notes payable of $2.1 million and stock-based compensation expense of $1.4 million, offset by the change in fair value of our convertible preferred stock warrant liability of $1.1 million. The significant items in the changes in operating assets and liabilities include increases in deferred rent, deferred revenue and accounts payable of $5.0 million, $1.3 million and $1.1 million, respectively, offset by increases in prepaid expenses and accounts receivable of $4.7 million and $1.3 million, respectively. The significant change in deferred rent is due to the difference between rent amounts paid and amounts expensed during 2009 on our facility, while the significant increase in prepaid expenses is due to making fully refundable short-term deposits for components used in the construction of sequencers whose specifications were undetermined. The increase in accounts payable during 2009 is due to increased purchases associated with the start-up of our production facilities during the fourth quarter of 2009. The increases in accounts receivable and deferred revenue were due to revenue recognized in the fourth quarter of 2009 and advance billing arrangements with customers.
Net cash used in operating activities was $24.3 million for the year ended December 31, 2008 and consisted of a net loss of $28.4 million, offset by noncash items of $3.8 million and net increases in operating assets and liabilities of $0.3 million. Noncash items for the year ended December 31, 2008 consisted mainly of depreciation and amortization expense of $2.8 million, a loss on the disposal of property and equipment of $0.5 million and stock-based compensation expense of $0.3 million. Changes in operating assets and liabilities consisted of an increase in accrued liabilities of $0.7 million, offset by increases in prepaid expenses of $0.2 million and other current assets of $0.2 million.
63
Table of Contents
Net cash used in operating activities was $10.0 million for the year ended December 31, 2007 and consisted of a net loss of $12.3 million, offset by noncash charges of $1.1 million and a net increase in operating assets and liabilities of $1.1 million. Noncash items for the year ended December 31, 2007 consist of depreciation and amortization expense of $0.8 million, a change in the fair value of our convertible preferred stock warrant liability of $0.2 million and stock-based compensation expense of $0.1 million. The net increase in the changes in operating assets and liabilities was primarily due to increases in accrued liabilities and accounts payable of $0.8 million and $0.6 million, respectively, as a result of our increased research and development activities during the year ended December 31, 2007, offset by increases in prepaid expenses and other assets of $0.2 million and $0.1 million, respectively.
Investing Activities
Net cash used in investing activities were $9.7 million, $7.4 million and $3.7 million for the years ended December 31, 2009, 2008 and 2007, respectively, relating entirely to purchases of property and equipment. Purchases of property and equipment during 2009 consisted primarily leasehold improvements made to our facility in Mountain View, California and equipment to support the start-up of our production facilities. Purchases of property and equipment during 2008 and 2007 were primarily for equipment to support our research and development activities.
Financing Activities
Net cash provided by financing activities during the year ended December 31, 2009 of $37.9 million consisted primarily of net proceeds from the issuance of Series D preferred stock in August 2009 of $27.2 million and net proceeds from the issuance of convertible promissory notes of $14.7 million, offset by repayment of equipment loan borrowings of $4.0 million.
Net cash provided by financing activities during the year ended December 31, 2008 of $33.6 million consisted primarily of net proceeds from the issuance of Series C preferred stock in February 2008 of $25.4 million and proceeds from equipment loan borrowings of $13.2 million, offset by repayment of equipment loan borrowings of $5.0 million.
Net cash provided by financing activities during the year ended December 31, 2007 of $16.3 million consisted primarily of net proceeds from the issuance of Series B preferred stock in March 2007 of $12.9 million, proceeds from equipment loan borrowings of $2.8 million and proceeds from the issuance of convertible promissory notes of $1.0 million, offset by repayment of equipment borrowings of $0.3 million.
Operating and Capital Expenditure Requirements
To date, we have not achieved profitability on a quarterly or annual basis. We expect our cash expenditures to increase significantly in the near term, including significant expenditures for the expansion of our Mountain View sequencing facility and the possible development of additional sequencing centers, research and development, sales and marketing and general and administrative expenses. Specifically, we intend to expand our current sequencing and supporting computing capacity in our Mountain View and Santa Clara leased facilities in 2011 at an estimated capital expenditure of between $20.0 million and $25.0 million. As a public company, we will also incur significant legal, accounting and other expenses that we did not incur as a private company. We anticipate that we will continue to incur net losses for the foreseeable future as we continue to expand our business and build our infrastructure.
We believe that, based on our current level of operations and anticipated growth, net proceeds from this offering, together with our cash and cash equivalent balances and interest income we earn on these balances, will be sufficient to meet our anticipated cash requirements through at least the next 12 months. If our available cash balances and net proceeds from this offering are insufficient to satisfy our liquidity requirements, we may seek to sell additional equity or convertible debt securities or enter into another credit facility. The sale of additional equity or convertible debt securities may result in dilution to our stockholders. If we raise additional funds through the issuance of convertible debt securities, these securities could have rights senior to those of our
64
Table of Contents
common stock and could contain covenants that restrict our operations. We may require additional capital beyond our currently forecasted amounts and additional capital may not be available on reasonable terms, if at all.
Our forecast of the period of time through which our financial resources will be adequate to support our operations and the costs to support our general and administrative, sales and marketing and research and development activities are forward-looking statements and involve risks and uncertainties. Actual results could vary materially and negatively as a result of a number of factors, including the factors discussed under the caption “Risk Factors.” We have based these estimates on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect.
Our future capital requirements will depend on many factors, including, but not limited to, the following:
| § | the financial success of our genome sequencing business; |
| § | our ability to increase the genome sequencing capacity in our Mountain View facility; |
| § | whether we are successful in obtaining payments from customers; |
| § | whether we can enter into collaborations and establish a recurring customer base; |
| § | the progress and scope of our research and development projects; |
| § | the filing, prosecution and enforcement of patent claims; |
| § | the rate at which we establish satellite genome sequencing centers and whether we can find suitable partners to establish those centers; |
| § | the effect of any joint ventures or acquisitions of other businesses or technologies that we may enter into or make in the future; and |
| § | lawsuits brought against us by third parties. |
Contractual Obligations and Commitments
The following summarizes the future commitments arising from our contractual obligations at December 31, 2009 (in thousands):
| Contractual obligations
|
Payment due by period | ||||||||||||||
| Total | Less than 1 year |
1-3 years | 3-5 years | More than 5 years | |||||||||||
| Debt obligations(1) |
$ | 7,707 | $ | 4,440 | $ | 3,267 | $ | — | $ | — | |||||
| Interest expense payments(2) |
1,276 | 617 | 659 | — | — | ||||||||||
| Operating lease obligations(3) |
17,886 | 2,610 | 5,098 | 5,415 | 4,763 | ||||||||||
| Purchase obligations(4) |
6,972 | 6,133 | 428 | 411 | — | ||||||||||
| Total |
$ | 33,841 | $ | 13,800 | $ | 9,452 | $ | 5,826 | $ | 4,763 | |||||
| (1) | Represents our outstanding debt under our credit facility as of December 31, 2009. |
| (2) | Represents interest payments on our outstanding debt under our credit facility as of December 31, 2009 and termination payments related to our credit facility due on the maturity date of the notes. |
| (3) | Consists of contractual obligations under non-cancellable office space operating leases. |
| (4) | Consists of purchase obligations related to our data center and related connectivity and non-cancellable orders for sequencing components. |
Credit Facility
In July 2008, we entered into loan and security agreement with various financial institutions to provide for a term loan of $8.0 million and an equipment credit line of up to $5.0 million. Our borrowings under this credit facility have been secured by substantially all of our assets, other than our intellectual property. Payments of accrued interest and principal are due on the first day of each month, and one final payment of the remaining unpaid balance of principal and accrued interest is due on the maturity of each of the credit extensions. Outstanding
65
Table of Contents
borrowings, including the accreted portion of the termination payment, under the credit facility were $8.0 million and $5.9 million as of December 31, 2009 and June 30, 2010, respectively, and no further borrowings under our credit facility are available. Interest accrues at an annual rate between 10.50% and 11.04%, as determined at the time of the draw-down.
The credit facility includes various covenants. We were in compliance with all required covenants in our credit facility as of December 31, 2009 and June 30, 2010.
Indemnifications
In the normal course of business, we enter into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. Our exposure under these agreements is unknown because it involves claims that may be made against us in the future, but have not yet been made. To date, we have not paid any claims or been required to defend any action related to our indemnification obligations. However, we may record charges in the future as a result of these indemnification obligations.
In accordance with our certificate of incorporation and bylaws, we have indemnification obligations to our officers and directors for specified events or occurrences, subject to some limits, while they are serving our request in such capacities. There have been no claims to date, and we have director and officer insurance that may enable us to recover a portion of any amounts paid for future potential claims.
Off-Balance Sheet Arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board, or FASB, issued a new accounting standard that changes the accounting for revenue arrangements with multiple deliverables. Specifically, the new accounting standard requires an entity to allocate arrangement consideration at the inception of an arrangement to all of its deliverables based on their relative selling prices. In addition, the new standard eliminates the use of the residual method of allocation and requires the relative-selling-price method in all circumstances in which an entity recognizes revenue for an arrangement with multiple deliverables. In October 2009, the FASB also issued a new accounting standard that changes revenue recognition for tangible products containing software and hardware elements. Specifically, if certain requirements are met, revenue arrangements that contain tangible products with software elements that are essential to the functionality of the products are scoped out of the existing software revenue recognition accounting guidance and will be accounted for under these new accounting standards. Both standards will be effective for us in the first quarter of 2011. Early adoption is permitted. We are currently assessing the impact that the adoption of these standards will have on our financial statements.
In January 2010, the FASB issued an amendment to an accounting standard which requires new disclosures for fair value measures and provides clarification for existing disclosure requirements. Specifically, this amendment requires an entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfer. This amendment also requires an entity to disclose separately information about purchases, sales, issuances and settlements in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3 inputs. This amendment clarifies existing disclosure requirements for the level of disaggregation used for classes of assets and liabilities measured at fair value and requires disclosure about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements using Level 2 and Level 3 inputs. This guidance is effective for interim and annual reporting periods beginning after December 15, 2009, except for certain Level 3 activity disclosure requirements that will be effective for reporting periods after December 15, 2010. Accordingly, we adopted this amendment on January 1, 2010, except for the additional Level 3 requirements, which will be adopted in 2011. Other than requiring additional disclosures, adoption of this new guidance did not have a material impact on our financial statements.
66
Table of Contents
Quantitative and Qualitative Disclosures About Market Risk
Our exposure to market risk is principally limited to exposure to interest rate risk related to our cash and cash equivalent balances. Due to the fixed interest rates of the borrowing under our credit facility, we do not currently have any exposure to changes in our interest expense as a result of changes in interest rates. In addition, we are not exposed to material foreign exchange risk because sales to customers located outside of the United States are denominated in U.S. dollars.
Cash and Cash Equivalents
The primary objectives of our investment activity are to preserve principal, provide liquidity and maximize income without significantly increasing risk. By policy, we do not enter into investments for trading or speculative purposes. Some of the securities in which we invest, however, may be subject to interest rate risk. This means that a change in prevailing interest rates may cause the principal or stated amount of the investment to fluctuate. To minimize this risk, we invest primarily in money market funds. Due to the nature of these investments, we believe that we do not currently have any material exposure to changes in the fair value of our investment portfolio as a result of changes in interest rates.
67
Table of Contents
Overview
We are a life sciences company that has developed and commercialized an innovative DNA sequencing platform, and our goal is to become the preferred solution for complete human genome sequencing and analysis. Our Complete Genomics Analysis Platform, or CGA Platform, combines our proprietary human genome sequencing technology with our advanced informatics and data management software and our innovative, end-to-end, outsourced service model to provide our customers with data that is immediately ready to be used for genome-based research. We believe that our solution will provide academic and biopharmaceutical researchers with complete human genomic data and analysis at an unprecedented combination of quality, cost and scale without requiring them to invest in in-house sequencing instruments, high-performance computing resources and specialized personnel. By removing these constraints and broadly enabling researchers to conduct large-scale complete human genome studies, we believe that our solution has the potential to revolutionize medical research and expand understanding of the basis, treatment and prevention of complex diseases.
We believe that our complete human genome sequencing technology, which is based on our proprietary DNA arrays and ligation-based read technology, is superior to existing commercially available complete human genome sequencing methods in terms of quality, cost and scale. In the DNA sequencing industry, complete human genome sequencing is generally deemed to be coverage of at least 90% of the nucleotides in the genome. Because we have optimized our technology platform and our operations for the unique requirements of high-throughput complete human genome sequencing, we are able to achieve accuracy levels of 99.999% at a total cost that is significantly less than the total cost of purchasing and using commercially available DNA sequencing instruments. We believe that we will be able to further improve our accuracy levels and reduce the total cost of sequencing and analysis, enabling us to maintain significant competitive advantages over the next several years. Because our technology resides only in our centralized facilities, we can quickly and easily implement enhancements and provide their benefits to our entire customer base. Our goal is to be the first company to sequence and analyze high-quality complete human genomes, at scale, for a total cost of under $1,000 per genome.
While our competitors primarily sell DNA sequencing instruments and reagents that produce raw sequenced data, requiring their customers to invest significant additional resources to process that raw data into a form usable for research, we offer our customers an end-to-end, outsourced solution that delivers research-ready genomic data. As the cost of complete human genome sequencing declines, we believe the basis of competition in our industry will shift from the cost of sequencing to the value of the entire sequencing solution. We believe that our integrated advanced informatics and data management services will emerge as a key competitive advantage as this shift occurs.
Our genome sequencing center, which began commercial operations in May 2010, combines a high-throughput sample preparation facility, a collection of our proprietary high-throughput sequencing instruments and a large-scale data center. Our customers ship us their samples via common carrier services such as Federal Express and United Parcel Service. We then sequence and analyze these samples and provide our customers with finished, research-ready data, enabling them to focus exclusively on their single highest priority, discovery.
As of March 31, 2010, there had been approximately 24 published and 200 unpublished complete human genomes sequenced worldwide, as reported in the April 2010 edition of Nature. As of July 20, 2010, we have sequenced over 200 complete human genomes year-to-date, including more than 100 in the first three weeks of July 2010, and have an order backlog of over 500 genomes. Our customers include some of the leading global academic and government research centers and biopharmaceutical companies. By the end of 2010, we expect our facility to have the capacity to sequence and analyze over 400 complete human genomes per month. We expect this capacity to increase between two- and three-fold in 2011 as we deploy additional sequencers and increase the throughput of our sequencing process through software refinements and component upgrades. In future years, we plan to construct additional genome centers in the United States and other strategic markets to accommodate an expected growing global demand for high-quality, low-cost complete human genome sequencing on a large scale.
68
Table of Contents
Market Overview
Background
Every organism has a genome that contains the full set of biological instructions required to build and maintain a living example of that organism. The information contained in a genome is stored, or encoded, in deoxyribonucleic acid, or DNA, a nucleic acid that is found in each cell of the organism. DNA is divided into discrete units called genes, which carry specific information necessary to perform a particular biological function, such as instructions for making proteins. The chemical building blocks that make up each gene are the molecules adenine, cytosine, guanine and thymine, labeled as A, C, G and T, respectively, which are known as nucleotide bases. Human DNA has approximately three billion nucleotide bases, and their precise order is commonly known as the DNA or genetic sequence.
Studying how genes and proteins differ between species and among individuals within a species, or genetic variations, helps scientists to determine their functions and roles in health and disease. These genetic variations can have important medical consequences. Genetic variations may, for example, cause an individual to have a predisposition to certain diseases or to respond differently to certain drug treatments. Accordingly, improving our understanding of the genome and its functions has driven and, we expect, will continue to drive advancements in medical research and diagnostics.
Genetic Analysis Market
Genetic analysis products comprise instruments and consumables, as well as associated hardware, software and services directly involved in the study of DNA and RNA. Scientia Advisors, a third-party research firm, estimated genomic revenue in 2009 to be approximately $5.8 billion and projects the market to grow to approximately $9.0 billion by 2014. The medical research market consists of laboratories generally associated with universities, medical research centers and government institutions, as well as biotechnology and pharmaceutical companies. In the longer term, we believe genetic analysis tools will likely play a critical role in molecular diagnostics. By detecting small, individual genetic differences, we believe molecular diagnostic tests could be used to identify predisposition to or the presence of a disease, to select appropriate medication and dosage and to monitor disease progression and response to treatment.
Genetic Analysis Technologies
Since the development of the first genetic engineering techniques in the 1970s, there has been an ongoing evolution toward more accurate, faster and less expensive methods to conduct genetic analysis. The primary analysis methods traditionally used by genetic researchers fall into three categories:
| § | DNA Sequencing. DNA sequencing is the process of determining the exact order, or sequence, of the individual nucleotides in a DNA strand so that this information can be correlated to the genetic activity influenced by that segment of DNA. Complete human genome sequencing is currently the most comprehensive form of genetic analysis, in which every base is compared to a reference genome to determine possible mutations or variations. |
| § | Genotyping. Genotyping is the process of examining certain known mutations or variations in the DNA sequence of genes to determine whether the particular variant can be associated with a specific disease susceptibility or drug response. |
| § | Gene Expression Analysis. Gene expression analysis is the process of examining the molecules that are produced when a gene is activated, or expressed, to determine whether a particular gene is expressed in a specific biological tissue. |
The first major breakthrough in genetic technology was the development of the automated DNA sequencer in 1986. Subsequent versions of commercial first-generation DNA sequencers improved on speed and throughput, eventually becoming powerful enough to enable the first mapping of the human genome through the Human Genome Project, which was completed in 2003 at an overall cost of over $3 billion. The prohibitive cost of first
69
Table of Contents
generation sequencing technologies forced scientists to use the other primary genetic analysis technologies, genotyping and gene expression analysis. While these targeted genetic analysis technologies address the cost limitations of DNA sequencing, they generally provide only limited information to the user.
More recently, innovations in DNA sequencing have led to the development of high-throughput sequencing technologies, commonly referred to as next-generation or second-generation sequencing, which produce thousands to millions of sequences at once. These high-throughput sequencing technologies have led to a significant reduction in the time and cost required for DNA sequencing. Next-generation sequencing technologies are supplanting not only the older sequencing methods but also less comprehensive methods for assessing gene expression, protein binding and other biological information. Scientia Advisors estimated that the next-generation sequencing companies had sales approaching $600 million for 2009, with an installed base in the neighborhood of 1,300 to 1,500 instruments. Scientia Advisors expects these suppliers to generate more than $1.5 billion in sales in 2014.
The Importance of Complete Human Genome Sequencing and the Limitations of General Purpose Sequencing Technologies
One of the most difficult challenges facing the genetic research and analysis industry is improving our understanding of how genes contribute to diseases that have a complex pattern of inheritance. For many diseases, multiple genes each make a subtle contribution to a person’s predisposition or susceptibility to a disease or response to a drug treatment protocol. Accordingly, we believe that unraveling this complex network will be critical to understanding human health and disease. We believe that sequencing complete human genomes is the most comprehensive and accurate method by which to achieve these objectives and improve our understanding of human disease. However, the cost and complexity associated with complete human genome sequencing have been prohibitively high for researchers and have slowed our progress in understanding the genetic underpinnings of disease.
Although second-generation sequencing technologies have led to dramatic reductions in cost and improvements in quality and throughput for complete human genome sequencing, they were designed as general-purpose instruments for sequencing the DNA or RNA of plants, animals, bacteria and viruses. In particular, these second generation sequencing technologies were not designed for sequencing large numbers of complete human genomes. We believe the key limitations of using second-generation technologies for sequencing large numbers of complete human genomes include the following:
| § | High Cost. Commercially available DNA sequencing instruments cannot sequence complete human genomes at a price low enough to make large-scale complete human genome sequencing projects affordable to researchers. |
| § | Insufficient Scale and Speed. Commercially available DNA sequencing instruments typically require weeks to sequence a complete human genome, which translates into months or years to sequence all of the genomes for large projects. While the timeline can be accelerated by purchasing and operating multiple DNA sequencing instruments in parallel, the capital cost is prohibitive for all but the largest research centers. Many researchers are unable to generate the complete human genome data they need from in-house instruments in an acceptable period of time. |
| § | Difficulty of Data Management. Sequencing a large number of complete human genomes generates a substantial amount of data that must be managed, stored and analyzed. Many users of commercially available DNA sequencing instruments lack the costly computing resources, storage capacity, network bandwidth and specialized personnel to process and analyze these massive data sets. |
Due to these limitations, many researchers have been using an alternative approach in which a small portion of the genome, referred to as the exome, is targeted, enriched and sequenced, which requires less than 5% of the sequencing compared to sequencing of a complete genome. However, important areas of the genome lie outside of the exome, such as the promoter regions that control gene expression and other conserved regions of the genome that are believed to perform regulatory functions. Moreover, current exome selection technologies are inefficient, typically enabling sequencing of less than 80% of the exome, compared to complete human genome sequencing technologies that can capture over 90% of the exome. Within the next several years, we believe that
70
Table of Contents
the cost of sequencing complete human genomes will be low enough and the process simple enough that the added cost of selecting exomes will make complete human genomes sequencing less expensive overall and more widely used.
Complete Genomics’ Solution
We have developed a novel approach to complete human genome sequencing. We combine our proprietary human genome sequencing technology, which achieves accuracy levels of 99.999%, with our advanced informatics and data management software and our innovative, end-to-end service model, to deliver research-ready genomic data at a total cost that is significantly less than the total cost of purchasing and using commercially available DNA sequencing instruments. We believe this novel outsourced solution overcomes the key limitations of other sequencing technologies and addresses the unmet needs of the complete human genome research market.
Proprietary Sequencing Technology
There are two primary components of our proprietary human genome sequencing technology: DNA nanoball, or DNB, arrays and combinatorial probe-anchor ligation, or cPAL, reads. Our patterned DNB arrays, due to their small size and biochemical characteristics, enable us to pack DNA very efficiently on a silicon chip. We have developed a proprietary process that causes the DNA to adhere to desired spots on the chip, while conversely preventing the DNA from adhering to the area between these spots. This enables us to affix individual particles of DNA to over 90% of these spots, leading to increased efficiency in nanoarray assembly. In addition, we have developed a highly accurate cPAL read technology, which enables us to read the DNA fragments efficiently using small concentrations of low-cost reagents while retaining extremely high single-read accuracy.
We believe this unique combination of our proprietary DNB and cPAL technologies is superior in both quality and cost to other commercially available approaches and provides us with significant competitive advantages. As reported in the January 2010 edition of Science, we sequenced a complete human genome at a consumables cost of approximately $1,800 and with a consensus error rate of approximately 1 error in 100,000 nucleotides. Our read accuracy was further validated by one of our customers, the Institute for Systems Biology, or ISB, as published in Science Express in March 2010. We have identified and are developing additional performance enhancements to our core technologies that we believe will enable us to maintain significant competitive advantages over the next several years. As we implement these technological enhancements, our goal is to be the first company to sequence and analyze high-quality complete human genomes, at scale, for a total cost of under $1,000 per genome.
Advanced Informatics and Data Management Software
Sequencing complete human genomes generates substantial amounts of data that must be managed, stored and analyzed. While many users of instrument-based sequencing systems have historically conducted their own in-house data analysis on a limited number of genomes, many of these users lack the computing, storage and network bandwidth necessary to manage the massive data sets generated by larger scale complete human genome studies. In response to this need by our customers, we have built a genomic data processing facility with computing infrastructure for managing both small- and large-scale genomic sequencing projects.
There are two major components of our complete data management solution: assembly software and analysis software. Assembly is the process of using computers to organize all of the overlapping 70-base nucleotide sequences to reconstruct the complete human genome. Our proprietary assembly software uses advanced data analysis algorithms and statistical modeling techniques to accurately reconstruct over 90% of the complete human genome from approximately two billion 70-base reads. After assembling the genomic data, we use our analysis software to identify and annotate key differences, or variants, in each genome.
By using our analytical tools and data management software, our customers can significantly reduce their investments in computing infrastructure. Our customers are provided with reliable access to assembled and annotated sequence data in multiple formats to ease data sharing and comparative analyses. In addition, our data
71
Table of Contents
storage options provide flexibility and allow customers to customize their data management strategy based on their particular business and scientific requirements. We have also developed a suite of open source analytical tools, called CGATools, designed to enable our customers to rapidly analyze the data we generate from their samples. As the reagent cost of sequencing declines, we believe that the cost and complexity of data analysis and management will emerge as the primary limiting factor for conducting complete human genome analysis.
Innovative, End-to-End, Outsourced Solution
While our competitors primarily sell DNA sequencing instruments and reagents that produce raw sequenced data, requiring their customers to invest significant additional resources to process that raw data into a form usable for research, we offer our customers an end-to-end, outsourced solution that delivers research-ready genomic data. Our genome sequencing center combines a high-throughput sample preparation facility, a collection of our proprietary high-throughput sequencing instruments and a large-scale data center. Our customers ship us their samples via common carrier services such as Federal Express and United Parcel Service. We then sequence and analyze these samples and provide our customers with finished, research-ready genomic data, enabling them to focus exclusively on their single highest priority, discovery.
Our customers are not required to purchase expensive sequencing instruments and high-performance computing resources to sequence and analyze large sets of complete human genomes. Our outsourced service model enables our customers to offload to us the complex processes of sample preparation, sequencing, computing and data storage and management. We believe our services will expand the potential addressable market by enabling a broad base of researchers who may lack sufficient capital and the specialized personnel necessary to build and operate a sequencing laboratory, or who have historically been constrained by the high total cost of sequencing, to conduct large-scale complete human genome studies.
Customer Benefits
Our end-to-end solution provides the following advantages to our customers:
| § | High-Quality Data. Our technology delivers what we believe is the industry’s highest quality complete human genome data. |
| § | Cost-Savings. Our customers are not required to purchase expensive sequencing instruments and high-performance computing resources or hire the necessary specialized personnel to sequence and analyze large sets of complete human genome data. |
| § | Speed at Scale. Our customers can complete their large-scale projects more quickly by using our services than by using commercially available sequencing instruments. |
| § | Ease of Use. Our customers can avoid the difficulty and time-consuming process of purchasing and operating their own sequencing instruments and can outsource the entire process to us, from sample preparation to delivery of research-ready data. |
| § | Operational Flexibility. By outsourcing their large-scale complete human genome sequencing projects to us, our customers can free up the capacity of in-house instruments to run smaller or more targeted sequencing projects and applications. |
| § | Technological Flexibility. As DNA sequencing technology improves, our customers avoid the risk of their expensive instruments becoming technologically obsolete. |
| § | Enables Customers to Focus on Discovery. Outsourcing offloads the operational burdens of managing large-scale genome sequencing projects and enables our customers to focus their resources on research, which can reduce the time to discovery. |
Competitive Strengths
We believe that our competitive strengths are as follows:
| § | Proprietary Human Genome Sequencing Technology. Our proprietary sequencing technology achieves accuracy levels of 99.999% at a total cost that is significantly less than the total cost of purchasing and using commercially available DNA sequencing instruments. We believe that our quality improvement and |
72
Table of Contents
| cost-reduction initiatives will allow us to maintain our quality and cost advantages over competing sequencing technologies for the next several years. |
| § | Fully Integrated Advanced Informatics and Data Management Software. Our solution incorporates powerful informatics, analysis and data management software that enable our customers to manage and gain useful information from the massive data sets generated in complete human genome sequencing. Our informatics software allows us to provide research-ready data from the billions of nucleotide sequences we identify and is optimized to reflect the characteristics of the genomic data our sequencers generate. Unlike software solutions offered by instrument manufacturers or third-party providers, we are able to continuously refine our informatics and data management software because of our significant experience in sequencing and analyzing large numbers of complete human genomes for our customers. As the reagent cost of sequencing declines, we believe that the cost and complexity of in-house data analysis and management will emerge as the primary limiting factor for researchers conducting complete human genome analysis. |
| § | Highly Scalable and Capital-Efficient Business Model. Consolidating volume across our entire customer base enables us to run a large number of genomes through our sequencing process while avoiding the cost and complexity of employing a large field installation and support organization. By implementing a high degree of automation, we have reduced the possibility of human errors that could adversely affect quality and increase costs. Our service-based model allows us to reduce cost and improve quality as volume increases. |
| § | Unique Insight into Customer Needs. Because of our unique, end-to-end service model, we interact directly with our customers on their discovery projects. This interaction enables us to develop and enhance our analysis software to meet our customers’ specific needs while expanding our understanding of variation in the human genome. |
| § | Fast and Efficient Deployment of Operational and Technological Enhancements. Because our sequencing operations and data center are centralized, we can rapidly upgrade our technology and deliver the benefits to our customers. In addition, our access to genomic data allows our software engineers to continually refine and improve our software with each genome we sequence. |
| § | Expanded Market Opportunity. We believe our outsourced model will expand the potential addressable market by providing academic and biopharmaceutical researchers who lack sufficient budgets or the specialized personnel necessary to build and operate a sequencing laboratory with access to high-quality, low-cost complete human genome data. In addition, we believe we will be able to service customers who require rapid sequencing of large numbers of complete human genomes. |
Customers and Applications
Customers
As of July 20, 2010, we have sequenced over 200 complete human genomes year-to-date, including more than 100 in the first three weeks of July 2010, and have an order backlog of over 500 genomes. We have more than 30 past and current customers, including the following:
| § Academic Medical Center University of Amsterdam § Brigham & Women’s Hospital § Broad Institute of MIT and Harvard § Children’s Hospital of Philadelphia § Eli Lilly and Company § Erasmus Medical Centre in Rotterdam, the Netherlands |
§ Flanders Institute for Biotechnology § Genentech, Inc. § HudsonAlpha Institute for Biotechnology § Institute of Cancer Research United Kingdom § Institute of Molecular Medicine at the University of Texas Health Science Center at Houston |
§ Institute for Systems Biology § Ontario Institute for Cancer Research § Pfizer Inc. § SAIC-Frederick, Inc., National Cancer Institute § University of North Carolina § University of Texas Southwestern Medical Center |
73
Table of Contents
Selected Customer Examples
SAIC-Frederick, Inc., National Cancer Institute — Pediatric Cancer Study
Our project with SAIC-Frederick, Inc., the prime contractor for the National Cancer Institute’s research and development facility in Frederick, Maryland, involves sequencing and analyzing 50 tumor-normal pairs, or 100 complete human genomes, over a six-month period, to identify patterns relating to the genesis of cancerous tumors. This study may potentially lead to improved diagnosis and treatment of pediatric cancers. This project forms part of the National Cancer Institute’s Therapeutically Applicable Research to Generate Effective Treatments, or TARGET, Initiative. TARGET seeks to use genomic technologies to rapidly identify valid therapeutic targets in childhood cancers so that new, more effective treatments can be developed. It is currently focusing on five childhood cancers: acute lymphoblastic leukemia, acute myeloid leukemia, neuroblastoma, osteosarcoma and Wilms tumor. Our contract with SAIC-Frederick contains an option for SAIC-Frederick to engage us to sequence 564 additional cancer cases, or 1,128 complete human genomes, over an additional 18-month period.
Institute for Systems Biology — Miller Syndrome Study
Our project with Dr. Leroy Hood of the ISB involved sequencing the complete genomes of a four-member nuclear family, including two healthy parents and their two children who suffer from two genetic disorders: Miller Syndrome and primary ciliary dyskinesia. The data we provided allowed ISB researchers to pinpoint the causal gene and subsequently confirm that gene’s role in Miller Syndrome, a disease in which the genetic basis had evaded detection. The results were published in Science Express in March 2010 and have led to a follow-on project with the ISB to sequence an additional 122 genomes.
Genentech, Inc. — Non-Small Cell Lung Cancer Study
Our project with Genentech, Inc. (a member of the Roche Group) compared the complete human genome sequences of a primary non-small cell lung tumor with nearby non-tumor tissue taken from the lung of a long-term smoker. This project was the first complete human genome sequence of a primary non-small cell lung tumor and matched normal tissue. Comparison of these sequences revealed both known (KRAS G12C) and novel mutations in numerous oncogenes and led to the discovery of numerous somatic mutations. The data we delivered allowed Genentech to measure the rate of smoking-induced mutations accumulated over time and resulted in a publication in Nature in May 2010.
University of Texas Southwestern Medical Center — Hypercholesterolemia Study
Our project with Dr. Jonathan Cohen of University of Texas Southwestern involved sequencing the complete genome of an 11 month old breast-fed girl with cholesterol-laden deposits and very high blood cholesterol levels. Doctors ruled out a diagnosis of a disease called sitosterolemia, based on certain test results. However, sequencing of the girl’s genome revealed a mutation in a relevant gene. This finding indicated that the infant definitely has sitosterolemia, despite the prior contradictory test results. The major finding of this study is that complete genome sequencing identified the culprit mutations and provided a definitive diagnosis in a patient who did not have the classical hallmark features of the disease in question. This finding demonstrates that complete genome sequencing can be a valuable aid to diagnose and treat genetic diseases, even in individual patients. These results were published by the Oxford University Press in August 2010.
Applications
Potential applications for our complete human genome sequencing service include:
| § | Cancer Research. Researchers are sequencing cancer genomes and comparing them to normal genomes, which are referred to as tumor-normal pairs, to identify the mutations in cancer genomes. We believe understanding these mutations will guide development of new cancer therapeutics and diagnostics and ultimately enable doctors to select the best course of therapy based on the specific mutations found in a tumor. |
74
Table of Contents
| § | Mendelian Disease Research. There are thousands of Mendelian diseases, or diseases that have been found to run in families, and are accordingly likely to have a significant genetic component. However, the genetic cause of most of these diseases is currently unknown. By sequencing the complete genomes of the affected families, we believe the genetic causes of these Mendelian diseases can be discovered, which could lead to the development of novel diagnostics and therapeutics. |
| § | Rare Variant Disease Research. Diseases such as central nervous system disorders, cardiac disease and certain metabolic disorders that appear broadly in the population are thought to be caused by rare variants. Large-scale studies of affected individuals may help to identify the disrupted pathways and lead to the development of novel diagnostics and therapeutics. |
| § | Clinical Trial Optimization. We believe that selecting or stratifying patients on the basis of their genetic profiles could enable the preferential admission of high responders into a clinical trial. This stratification could enable the trial to reach its conclusion with fewer patients and lower costs and result in faster clinical trials and drug commercialization. |
In addition to these research studies, we expect future clinical applications to include:
| § | Companion Diagnostics. We believe that therapeutics that are not first-line treatments for the general population may be elevated to first-line treatments or used in combination therapies for subsets of the population that share a common genetic profile. Complete human genome studies may unlock new market opportunities for these therapies or combination therapies. |
| § | Cancer Pathology. We believe that analyzing complex cancer genomes that involve large and unpredictable structural changes will be most reliably and economically implemented using complete human genome sequencing. According to the National Cancer Institute SEER Cancer Statistics, there are approximately 1.5 million new cases of cancer diagnosed each year in the United States. |
| § | Universal Diagnostics. As medical records technology and public health policy advance, we believe that large numbers of people will have their complete human genomes sequenced and stored in their electronic medical records for use by their physicians in managing their health care decisions. |
Our Strategy
We intend to become the leading complete human genome sequencing and analysis company and the preferred platform for human genome discovery by:
| § | Continuing to Deliver the Highest Quality Genomic Data and Analysis at a Low Total Cost. By continuing to deliver the highest quality research-ready data and by enabling our customers to avoid the cost, complexity and risks associated with purchasing and operating the instruments and computing resources required to undertake complete human genome sequencing, our goal is to become the preferred solution for our customers. |
| § | Maintaining and Strengthening our Technology. We plan to continue to conduct research and product development activities to further improve quality, reduce costs, increase throughput and reduce our turnaround time. We plan to further develop the biochemistry, informatics, instrumentation and software that we believe together make up the industry’s most robust solution. We will also seek to continually improve our operational processes and analysis software. |
| § | Capitalizing on our Scalable Model. Due to the highly scalable nature of our service model, we believe we are well positioned to serve customers looking to sequence a small number of genomes as well as customers who are looking to rapidly sequence a very large number of genomes. |
| § | Establishing Ourselves as the Leader in Outsourced Complete Human Genome Sequencing. We intend to continue to focus exclusively on complete human genome sequencing. We believe that this focus will put us in a strong position to become the preferred platform for complete human genome sequencing. |
75
Table of Contents
| § | Expanding Globally to Increase Capacity and Reach New Markets. We expect to enter into partnership agreements with domestic and international organizations to build additional genome sequencing centers around the world. These genome sequencing centers will increase our sequencing capacity, provide us with improved access to global markets and expand our revenue opportunities. |
| § | Expanding Applications for the Use of our Technology. While our current focus is on providing complete human genome solutions primarily to academic and biopharmaceutical researchers, we believe that as we sequence and deliver more complete human genomes to our customers, our growing understanding of the genetic basis of human disease may lead to future applications in areas such as cancer pathology. |
Our Human Genome Sequencing Platform Technology
Our proprietary human genome sequencing platform consists of three major technologies: our proprietary human genome sequencing technology, our high-throughput process automation technology and our complete data management solution.
Proprietary Sequencing Technology
There are two primary components of our proprietary human genome sequencing technology: DNB arrays and cPAL reads.
DNB Arrays
We have developed a novel approach to preparing fragmented DNA for reading on our sequencing instruments. Using a biochemical process for copying DNA, we reproduce each DNA fragment in a manner that connects all of the copies together in a head-to-tail configuration, forming a long single molecule of connected nucleotides. We have developed proprietary techniques for causing each long single molecule to consolidate, or ball up, into a small particle of DNA that we call a DNB. The DNBs are approximately 200-300 nanometers in average diameter. Each DNB contains hundreds of copies of the 70 bases of DNA we are seeking to read in each fragment.
The small size and biochemical characteristics of our DNBs enable us to pack them together very tightly on a silicon chip. We use established photolithography processes developed in the semiconductor industry to create a silicon chip that has a grid pattern of small spots. The small spots are approximately 300 nanometers in diameter, and the center of each spot is separated by approximately 700 nanometers from neighboring spots. Each silicon chip has approximately 2.8 billion spots in an area 25 millimeters wide and 75 millimeters long. We have developed a proprietary process that cause the DNA to adhere to these spots, which we refer to as “sticky spots,” while conversely preventing the DNA from adhering to the area between the sticky spots. When a solution of DNBs is spread across the chip, the DNBs adhere to the sticky spots, with one DNB per spot. We have also developed proprietary techniques to fill over 90% of the sticky spots with exactly one DNB. We refer to the silicon chip filled with DNA as a DNA nanoball array. Each finished DNA nanoball array contains up to 180 billion bases of genomic DNA prepared for imaging.
cPAL Read
To read the sequence of nucleotides in each DNB, we have developed a highly accurate proprietary ligase-based DNA reading technology called cPAL. Our cPAL technology uses the naturally occurring ligase enzyme, which accurately distinguishes between the A, C, T and G nucleotides, to attach fluorescent molecules that light up with a different color for each of the four nucleotides. By imaging the color lights of a DNB array and decoding the color images, we can determine the sequence of nucleotides in each DNB. A key characteristic of our cPAL technology is its high accuracy of reading very short five-base sequences of DNA. We have developed a proprietary technique for preparing the DNA fragments so that we can read seven five-base segments from each of the two ends of the DNA fragment for a total of 70 bases from each fragment. We have also developed proprietary software that generally reconstructs over 90% of the complete human genomes from these 70 base reads from each fragment.
76
Table of Contents
Advantages of our DNB arrays and our cPAL technology over other commercially available DNA sequencing technologies include:
| § | High Accuracy. Our cPAL technology has very high single-read accuracy due to the intrinsic nature (high accuracy) of the ligase enzyme. By reading each nucleotide multiple times, we achieve a consensus error rate equal to approximately 1 error in 100,000 nucleotides. |
| § | No Accumulation of Errors. Many other DNA sequencing methods employ sequential processes that cause errors to accumulate as each successive nucleotide is read, which results in a higher potential error rate for each successive nucleotide. Our cPAL technology reads each nucleotide independently, and as a result there is no accumulation of errors, which enables us to read successive bases without increasing our error rate. |
| § | Low Reagent Cost. Our cPAL technology uses low concentrations of low-cost commodity reagents. Our DNB arrays achieve a very high density of DNA on each array, which reduces the quantity, or volume, of reagents we use compared to other DNA array approaches. The combination of low concentration of low-cost reagents and smaller quantities results in lower reagent costs compared to other commercially available DNA sequencing methods. As reported in Science published in January 2010, we sequenced a complete human genome at a consumables cost of approximately $1,800. |
High Throughput Process Automation
There are five major components of our high-throughput process automation technology: high-throughput sample preparation, high-throughput sequencing instruments, high-performance computing infrastructure, workflow automation software and service delivery technology.
High-Throughput Sample Preparation
Our high-throughput sample preparation technology consists of step-by-step protocols for preparing DNA for sequencing and pipetting robots that automatically execute these protocols. We prepare genome samples in batches of 88 and load the samples into a 96-well plate (the other eight wells in the plate contain known, or reference, DNA that we use to monitor the quality of the sample preparation process). A sample preparation run processes four 88-sample plates for a total of 352 genomes per run. We are building the instruments and staffing capacity to perform two runs in parallel for a total of 704 genomes prepared for sequencing. The result of a sample preparation run is up to 352 genomes loaded onto flow slides, ready to be loaded on sequencing instruments. Our sample preparation capacity can be scaled by adding additional sample preparation instruments and staff as needed.
High-Throughput Sequencing Instruments
Our sequencing instruments consist of a fluidics robot that pipettes multiple types of chemical reagents (including fluorescent molecules) onto the flow slides and an imaging system that records images of the fluorescent molecules attached to the DNA. Each sequencing instrument processes 18 flow slides at a time. The 18 flow slides are robotically moved back-and-forth from the fluidics robot to the imaging system. While one flow slide is being imaged, the other 17 flow slides are prepared with reagents or waiting for the imager to become available. A sequencing run takes approximately 12 days. Currently, our sequencing instruments can generate between 50 and 70 gigabases of usable data from each flow slide in a 12-day run. To sequence a complete human genome at an average redundancy of 40 times requires 120 gigabases of usable data. We expect to make continued enhancements in our technology to further increase the amount of usable data we get from each flow slide.
High-Performance Computing Infrastructure
We have built a genomic data processing facility that consists of approximately 5,000 core processors and 1,750 terabytes (a terabyte is one thousand gigabytes) of high-speed disk storage. Our sequencing instruments are connected to our data center by a network connection that transfers data at a rate of 30 gigabits per second. Our data center has the capacity to perform all of the required computation, starting with the images generated by the
77
Table of Contents
sequencing instruments, and ending with sequencing complete human genomes, for several hundred genomes per month. We plan to expand our data center as needed, and we expect to make continued enhancements to our software to further increase the efficiency of our data center.
Workflow Automation Software
Our workflow automation software tracks each sample from arrival at our facility to delivery of research-ready data to the customer. Sample tracking is accomplished through bar codes. Each 96-well plate of samples has a bar code, and each flow slide has a bar code. The instruments that process plates and flow slides have bar code readers attached to them. User interfaces to our workflow automation software allow us to track the progress of each sample throughout sample preparation, sequencing and computing. We are also developing a web-based customer portal to enable customers to track their projects real-time throughout the sequencing process.
Service Delivery Technology
Our cloud-based data delivery system is based on our vendor relationship with Amazon Web Services, or AWS. We upload our customers’ finished genomic data to AWS, who copies the data to hard disks and ships the hard disks to our customers. Our customers also can pay AWS to store their data on an ongoing basis. As we develop additional analytical tools, we plan to host them on AWS so that customers can rapidly analyze their genomic data as soon as it is available.
Complete Data Management Solution
There are two major components of our complete data management solution: assembly software and analysis software.
Assembly Software
Assembly is the process of using computing methods to organize the overlapping 70-base nucleotide sequences to reconstruct the complete genome. We have developed a proprietary approach to assembly that uses a combination of advanced data analysis algorithms and statistical modeling techniques to reconstruct over 90% of the complete human genome from approximately two billion 70-base reads. We have designed our assembly software to run in parallel across our large network of Linux computers.
As reported in Science published in January 2010, we generated high-quality base calls in as much as 95% of the genomes sequenced, identifying between 3.2 million and 4.5 million sequence variants per genome processed. Detailed validation of one genome dataset demonstrated a consensus error rate of approximately 1 error in 100,000 nucleotides.
Analysis Software
After assembling the genomic data, we use our analysis software to identify key variants in each genome and automatically annotate the genomic data. We have developed a suite of open source analytical tools, called CGATools, designed to enable our customers to rapidly analyze the data we generate from their samples. For example, we offer a tool facilitating the comparison of two genomes, enabling the quick determination of where the genomes differ. We are also developing additional analytical tools, such as a tumor-normal comparison tool designed to allow cancer researchers to compare a cancer genome to the normal genome from which it was derived, a family analysis tool designed to enable researchers to compare parental genomes with the genomes of their children and a large-scale genome browser designed to allow researchers to compare the hundreds of genomes sequenced in a large-scale study.
78
Table of Contents
Technology Strategy
We plan to continue to advance our complete human genome sequencing and analysis technology in four major areas:
| § | Array Density. Our unique grid patterned arrays currently consist of a 700 nanometer grid. We may reduce the grid size to 250 nanometers and correspondingly reduce the diameter of the sticky spots and DNA nanoballs. If successful, this improvement will increase the density of the DNA on an array by a factor of eight, which will decrease the reagent cost of sequencing a given amount of DNA by a factor of eight. |
| § | Instrument Speed. Our unique grid patterned arrays enable us to align the grid pattern of the DNA on the array with the grid pattern of the pixels in the detector, allowing us to image our arrays with very short exposure times. We may increase the speed of our instruments by acquiring and deploying new cameras that take images and transfer data at approximately six times the speed of our existing cameras. We may also increase the number of cameras per sequencing instrument from two to four. |
| § | Process Automation. As our instruments get faster, we intend to improve our sample preparation, process automation and data management technologies to process and deliver an increasing number of genomes to our customers with reduced turnaround time. |
| § | Analytic Software. We continue to improve and extend our analytic capabilities through the development of software designed to decrease genome assembly time and address specific application requirements of our customers. For instance, we are developing software that identifies and assembles the complex structural variants found in cancer genomes. |
As we implement a combination of these technology enhancements, our goal is to be the first company to sequence and analyze high-quality complete human genomes, at scale, for a total cost of under $1,000 per genome.
Sales, Marketing and Customer Support
We sell our complete human genome sequencing service through our direct field sales and support organizations. Our sales process with each new customer typically involves undertaking a small project, or pilot program, which enables the customer to become familiar with our outsourced solution and research-ready data. We then work with our customers to expand the relationship to larger projects.
Sales
We have assembled a highly experienced and technically qualified sales team, with most sales managers holding a Ph.D. in a relevant scientific field. Each of these sales managers brings a network of extensive contacts in our targeted customer segments. The sales group develops business opportunities and obtains orders for our complete human genome sequencing service by proactively identifying, qualifying and visiting well-funded prospects at major companies, institutions and universities.
Marketing
Our marketing group has developed and maintains the Complete Genomics brand, increases market awareness and generates demand for our solution through a variety of methods. First, we have created and continue to maintain a clear media presence via our website, press releases, interviews and articles that reinforce our market presence and scientific credibility. Second, we generate demand by promoting the company via marketing programs and by attending and exhibiting at relevant tradeshows and conferences. Third, we continue to evolve our marketing strategy by tracking market trends, understanding customer needs and developing appropriate products and programs. The marketing group also fulfills traditional product management requirements, such as defining our service and application strategy and roadmap, including partnering strategies, and developing sales tools, training materials and competitive analyses for the sales group.
79
Table of Contents
Customer Support
We are committed to supporting our customers through a network of scientific applications staff based in both Mountain View and locations near our most concentrated customer bases. This team currently consists mostly of Ph.D.-level scientists with extensive bioinformatics experience. Our scientific applications team works with customers to address technical questions related to our service offering and provide detailed training and support.
Most of the training and support efforts are focused on helping customers understand and use the large amounts of data that are delivered as part of multi-human genome sequencing projects. In late 2010, we intend to supplement these efforts with a new team of Mountain View-based bioinformatics support specialists that we expect to expand quickly as the number of sequencing projects increases.
Research and Development
Our research and development team brings together a variety of technical disciplines required for the development of a high-throughput sequencing system for commercial human genome sequencing services and includes DNA engineers, biochemists, molecular biologists, chemists, mathematicians, statisticians and electrical, mechanical, optical and software engineers. As of June 30, 2010, we had approximately 64 employees engaged in research and development, including 29 scientists and engineers with Ph.D.s. These professionals apply their skills in disciplines including:
| § | biochemistry (sample preparation, DNA array preparation, DNA sequencing assay); |
| § | hardware (optics, fluidics, mechanical design, flow slides); |
| § | software (algorithms, instrument software, genome sequencing software, bioinformatics); |
| § | information technology (high-performance data center management); |
| § | semiconductors (mask design, surface chemistry); and |
| § | process automation. |
The research and development teams are engaged in optimization and automation of our human genome sequencing processes, components and systems to improve the performance of our human genome sequencing technologies, including increasing accuracy and completeness, reducing cost and increasing throughput. Notable research and development accomplishments in this area include:
| § | DNA engineering of four directional adapters inserted in genomic DNA segments, providing DNA substrates for unchained DNA sequencing of 35+35 mate-pair bases; |
| § | clonal DNA replication in solution to create concatamers with 300-500 DNA copies folded in DNBs of approximately 200-300 nanometers in diameter; |
| § | high density (700 nanometer pitch) patterned DNB arrays on a 25mm x 75mm silicon slide with over 2.8 billion spots and a single DNB on over 90% of the spots; |
| § | reaction flow slide suitable for gravity loading sequencing chemistry by direct pipetting without using pressure or tubing that enables the processing of up to 18 slides per run on one instrument; |
| § | unchained DNA sequencing chemistry using cPAL technology that allows reading 10 bases from each adapter end, or up to 80 bases per DNA spot, using a 4-adapter library; |
| § | high-throughput, two-camera DNA sequencing instrument that processes 18 flow slides in parallel by robotic transfer of slides from biochemistry station to imaging station that employs TDI imaging at 30 frames per second; and |
| § | probability-based primary DNA base calling, read mapping and genome sequence and sequence variants determination algorithms and software for fast and accurate sequence analysis of a large number of human genomes using up to 200 gigabases of unchained base reads per genome. |
80
Table of Contents
In addition, our research and development teams are engaged in developing new applications for our technologies, including:
| § | Cancer Genomes. We are developing new methods for sequencing the complex structural variations found in cancer genomes, such as duplications, deletions and translocations in tumor genomes. We believe these methods will enable the research community to better understand the genetic basis for cancer. |
| § | Diploid Sequencing. We have invented and are developing a method for independently sequencing the maternal and paternal chromosomes. We believe this independent chromosome sequencing will be required for many molecular diagnostics, because multiple variants within a gene may or may not affect both copies of the gene. |
| § | Human Methylomes. We are researching possible methods of sequencing the human methylome, which we believe will be important in understanding cancer genomes. |
| § | Human Transcriptomes. We are researching possible methods of sequencing the human transcriptome, which, when combined with complete human genomes, we believe will shed light on the genetic basis of human disease and drug response. |
In the years ended December 31, 2007, 2008 and 2009, we spent $10.3 million, $23.6 million and $22.4 million, respectively, on company-sponsored research and development activities.
Intellectual Property
Our success depends in part upon our ability to obtain and maintain intellectual property rights with respect to our products, technology and know-how, to prevent others from infringing these intellectual property rights and to operate without infringing the proprietary rights of others. Our policy is to seek to protect our proprietary position by, among other methods, filing patent applications related to our proprietary technology, inventions and improvements that are important to the development and conduct of our business. We also rely on trade secrets and know-how to develop and maintain our proprietary position.
Our patent strategy is to seek broad patent protection on new developments in genome sequencing technology, and also file patent applications covering new implementations of our technology. Additionally, we file new patent applications directed at equipment and software that are used in conjunction with our genome sequencing technology.
Our core genome sequencing technology originated at Callida Genomics, Inc., or Callida, in the laboratory of Radoje (Rade) Drmanac, Ph.D., our Chief Scientific Officer and one of our co-founders. Dr. Drmanac played an important role in high-throughput sequencing of whole genomes using ligation-based sequencing reactions performed on microarrays. In March 2006, we entered into a license agreement with Callida pursuant to which we exclusively licensed from Callida the relevant patent filings relating to the use of the technology in random arrays, or arrays of genomic DNA fragments wherein the position of any specific fragment on the array is not predetermined, and probe anchor ligation, which we utilize in our commercial sequencing technology. Under this license agreement, we also obtained a nonexclusive license under additional patent filings owned by Callida that permits us to use the random array technology without infringing such additional patents. In exchange for the licenses, we issued to Callida 13,333 shares of our common stock, paid $1.0 million in cash for repayment of certain promissory notes held by Callida and agreed to pay $250,000 each year until the earlier of (a) March 28, 2012 and (b) such time as our common stock is traded on a national exchange and the shares issued to Callida are freely tradeable and have a minimum fair market value of $2.0 million. The license agreement remains in effect until each of the licensed patents has either expired or has been abandoned or ruled invalid. Either party may terminate the agreement for a material breach upon 120 days’ notice (or 30 days’ notice if the breach is due to failure to make a payment under the license agreement). Pursuant to the license agreement, Callida retains the rights to use the exclusively licensed technology for research purposes only.
81
Table of Contents
As of July 17, 2010, we have licensed from Callida six issued U.S. patents and six issued international patents that will expire between 2014 and 2027. We own or have licensed 97 pending patent applications, including 54 in the United States, 30 international applications and 13 applications filed under the Patent Cooperation Treaty.
In addition to pursuing patents on our technology, we have taken steps to protect our intellectual property and proprietary technology by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors.
The patent positions of companies like ours are generally uncertain, and the validity and breadth of claims in DNA sequencing technology patents may involve complex factual and legal issues for which no consistent policy exists. Our patents and licenses may not enable us to obtain or keep any competitive advantage. Our pending U.S. and foreign patent applications may not issue as patents or may not issue in a form that will be advantageous to us. Patents we have obtained or do obtain in the future may be challenged by re-examination, opposition or other administrative proceeding, or may be challenged in litigation, and such challenges could result in a determination that the patent is invalid.
Our licensed patents may be successfully circumvented by competitors. In addition, the patent laws of foreign countries differ from those in the United States, and the degree of protection afforded by foreign patents may be different from the protection offered by U.S. patents.
Our commercial success depends in part on our non-infringement of the patents or proprietary rights of third parties. For a description of the risks we face relating to intellectual property, please see “Risk Factors—Risks Related to Intellectual Property.”
Competition
Competition among organizations developing or commercializing sequencing instruments and services is intense. The sequencing industry is dominated by large, established companies that provide instruments and reagents, mostly for expression analysis and genotyping. These companies include Illumina, Inc., Life Technologies Corporation and Roche Diagnostics Corporation. These competitors are large and well-established, and each maintains a significant market share. Although historically these companies have sold instruments and reagents, some of these competitors have made recent forays into the sequencing services market. For example, Illumina recently announced that it will be providing individual genome sequencing services for as low as $9,500 per genome, and Life Technologies recently announced a collaboration to build a genome sequencing facility. Illumina also announced that it is pursuing a global program designed to provide researchers with access to academic and commercial institutions that can perform large-scale whole human genome sequencing projects using Illumina’s technology. Additionally, new competitors may enter the whole genome sequencing market, either by providing sequencing services like we do or by selling less expensive and more powerful sequencing instruments. We expect to face more intense competition if our service-based model is successful. In addition, future competitors may include smaller companies, like NABsys, Inc., Oxford Nanopore Technologies, Ltd., Pacific Biosciences, Inc. and Helicos Biosciences Corporation, which have developed or are developing sequencing technologies that may compete with ours in the future. Large, established companies may acquire smaller companies, such as these, with emerging technologies and use their extensive resources to develop and commercialize or incorporate these technologies into their instruments and services. For example, Life Technologies recently announced that it entered into a definitive agreement to acquire Ion Torrent Systems, Inc., a chip-based sequencing technology startup.
A number of these and other organizations are developing methods for DNA sequencing using single molecule or long-read sequencing technologies. To date, the developers of single molecule technologies have not demonstrated that single molecule sequencing can achieve the quality required for complete human genome discovery projects at a reasonable cost. While long-reads are critically important for de novo sequencing, or sequencing organisms which have not previously been sequenced, they are not required for sequencing high quality complete human genomes.
82
Table of Contents
In addition to commercial companies, there are large, government-funded or research-sponsored organizations, such as the Broad Institute of MIT and Harvard, the Genome Center at Washington University, the Baylor College of Medicine Human Genome Sequencing Center, the Wellcome Trust Sanger Institute and BGI (formerly known as Beijing Genome Institute), that purchase commercial DNA sequencing instruments and offer DNA sequencing services to academic and commercial customers.
For a description of the risks we face related to competition, please see “Risk Factors—Risks Related to Our Business—We face significant competition. Our failure to compete effectively could adversely affect our sales and results of operations” and “—The emergence of competitive genome sequencing technologies may impact our business.”
Operations
We have established laboratory operations for our first genome center in Mountain View, California, and we have additional laboratory operations in Sunnyvale, California. Our Tier III certified data center is located in Santa Clara, California. We expect to deploy additional sequencing centers in the future, and it is likely that these will be located in other regions, including outside of the United States.
Genomic samples arrive at our facilities by common carrier, such as FedEx or UPS, and are tested for a number of pre-defined quality acceptance criteria. Samples that pass acceptance testing are prepared for sequencing, loaded on a flow slide and sequenced on a sequencing instrument. After the sequencing process, data generated by the sequencing instrument is processed in our high performance computing center to generate the final customer data deliverable. We upload our customers’ finished genomic data to AWS, who copies the data to hard disks and ships the hard disks to our customers. Our customers also can pay AWS to store their data on an ongoing basis.
The time to deliver, from sample receipt to data shipment, typically takes 90 to 120 days. Our genome sequencing center has a finite capacity, and time to delivery will increase if demand exceeds our capacity. In the future, we expect to reduce time to delivery though technical and procedural improvements. Our sequencing capacity is primarily determined by the equipment, automation and personnel we deploy.
Facilities
We lease approximately 67,000 square feet of office and laboratory space at our headquarters in Mountain View, California, under a lease that expires in August 2016. We lease approximately an additional 11,000 square feet of office and laboratory space in Sunnyvale, California, under a lease that expires in December 2010. These facilities contain laboratory and non-laboratory personnel. We believe that our current facilities are adequate for our first genome center over the next two years. We may need to acquire additional space as we deploy additional genome centers, either in Mountain View or in other locales, and we may need to expand our Mountain View non-laboratory space for other uses. We believe additional non-laboratory expansion space near our Mountain View facility is readily available.
We also lease approximately 2,200 square feet of data center space in Santa Clara, California, under a contract that expires in May 2011. Our computing capabilities are principally located in this facility, which is sufficient for our current operations. As our operations expand, we will need to acquire additional data center space, either at this location, or in other regional facilities.
Manufacturing and Supply
We have adopted a manufacturing strategy of purchasing most components we use to conduct our sequencing services, including silicon wafers, optical microscopes and various other imaging components, sophisticated cameras and chemicals and reagents, from third party suppliers. This allows us to maintain a more flexible infrastructure while focusing our expertise on deploying these components and supplies to provide high-quality, lower-cost whole genome sequencing services on a large scale.
83
Table of Contents
We currently rely on single-source suppliers for certain key materials used in our sequencing process. In particular, we rely on SVTC Technologies L.L.C. to provide us with the silicon chips that are the base of the flow slide used in our sequencing process. We also rely on Hamamatsu Corporation for the cameras used in our sequencing instruments. We are in the process of identifying and qualifying additional suppliers, although we cannot predict how long that qualification process will last, and the time needed to establish a relationship can be lengthy.
Delays, quality issues or interruptions by our suppliers may harm our business, and we may be forced to establish relationships with new suppliers. However, because the lead time needed to engage a new supplier can be lengthy, we may experience delays in meeting demand if we must switch to a new supplier. For more information regarding risks related to our supply chain, please see “Risk Factors—Risks Related to Our Business—We depend on a limited number of suppliers, including single-source suppliers, of various critical components for our sequencing process. The loss of these suppliers, or their failure to supply us with the necessary components on a timely basis, could cause delays in the current and future capacity of our sequencing center and adversely affect our business.”
Government Regulations
We believe our sequencing service is not currently subject to FDA regulation, clearance or approval. However, if we expand our service to encompass products that are intended to be used for the diagnosis of disease, such as molecular diagnostic products, regulation by governmental authorities in the United States and other countries will be a significant factor in the development, testing, production, and marketing of such products.
Our laboratory is subject to federal, state, regional and local regulations relating to the handling and disposal of hazardous materials and biohazardous waste, including chemicals, biological agents and compounds and blood and other human tissue. We utilize qualified third party vendors for waste disposal and handling, and these vendors are contractually obligated to comply with any applicable regulations. Our cost of waste disposal has historically not been material, and we expect this to be true in the future.
Due to the nature of our current operations, we have not sought accreditation for our facilities under the Clinical Laboratory Improvement Amendments, or CLIA. In addition, because the genomic data we provide generally neither identifies nor provides a reasonable basis to identify an individual, we are not currently subject to the Health Insurance Portability and Accountability Act of 1996, or HIPAA. However, once provided to certain of our customers, the genomic data and the activities of those customers may be regulated under both HIPAA and the Genetic Information Nondiscrimination Act of 2008.
Given the evolving nature of this industry, legislative bodies or regulatory authorities may adopt additional regulation or expand existing regulation to include our service. For example, in the future, our service could be subject to FDA regulation or we may be required to seek CLIA accreditation for our facilities. Changes to the current regulatory framework, including the imposition of additional or new regulations, could arise at any time, and we may be unable to obtain or maintain FDA or comparable regulatory approval or clearance of our service, if required. These regulations and restrictions may materially and adversely affect our business, financial condition and results of operations. For more information regarding the risks of future government regulation, please see “Risk Factors—Risks Related to Our Business—Because the market for genome sequencing is relatively new and rapidly evolving, we may become subject to additional future governmental regulation, which may place additional cost and time burdens on our operations.”
Backlog
In this prospectus, we refer to order backlog, which is the number of genomes for which we have executed purchase orders from our customers that we believe are firm and for which we have not yet recognized revenue. Estimating the dollar value of backlog that will be fulfilled within the current fiscal year requires significant judgments and estimates, as the mix of customer orders and pricing terms varies between arrangements depending on the number of genomes covered by the arrangement. It also requires estimating the timing of the receipt of qualified samples and the timing of the sequencing of the genomes. In addition, given the revenue
84
Table of Contents
variance resulting from this mix and the rapidly evolving nature of the sequencing industry, we do not believe that backlog as of any particular date is necessarily indicative of future results. However, we do believe that order backlog is an indication of our customers’ willingness to utilize our solution. As of June 30, 2010, our order backlog for which we believe we will sequence, bill and gain customer acceptance within twelve months was approximately $10.0 million. We did not have any commercial operations as of June 30, 2009, and, as a result, we did not have any backlog. See “Risk Factors—Risk Related to Our Business—Our order backlog may never be completed, and we may never earn revenue on backlogged contracts to sequence genomes.”
Segment and Geographical Information
We operate in one reportable business segment, and we have derived a majority of our revenue from customers located in the United States. As of June 30, 2010, we have derived approximately 28% of our revenue since inception from customers located in Asia, Canada and Europe. Sales to customers located outside of the United States are denominated in U.S. dollars. We expect that sales to international customers will be an important and growing source of revenue, particularly as we construct additional genome sequencing centers outside of the United States. All of our long-lived assets are located in the United States.
Employees
As of September 1, 2010, we had a total of 163 employees, 45 of whom hold Ph.D. degrees and 64 of whom are engaged in full-time research and development activities. We plan to expand our production, our sales and marketing and our research and development programs, and we plan to hire additional staff as these initiatives are implemented. None of our employees is represented by a labor union, and we consider our employee relations to be in good standing.
Legal Proceedings
On August 3, 2010, a patent infringement lawsuit was filed by Illumina, Inc. and Solexa, Inc. (an entity acquired by Illumina), or the plaintiffs, against us in the U.S District Court in Delaware. The case caption is Illumina, Inc. and Solexa, Inc. v. Complete Genomics, Inc., Civil Action No. 10-649. The complaint alleges that our Complete Genomics Analysis Platform, and in particular our combinatorial probe anchor ligation technology, infringes upon three patents held by Illumina and Solexa. The plaintiffs seek unspecified monetary damages and injunctive relief. If an injunction is granted, it could force us to stop or alter certain of our business activities. We believe that we have substantial and meritorious defenses to these claims and intend to vigorously defend our position. However, a negative outcome in this matter could have a material adverse effect on our financial position, results of operations, cash flows and business. For more information regarding the risk of this litigation and future litigation, please see “Risk Factors—We currently are, and could in the future be, subject to litigation regarding patent and other proprietary rights that could harm our business” and “—We may incur substantial costs as a result of our current, or future, litigation or other proceedings relating to patent and other proprietary rights.” We are not currently able to estimate the potential loss, if any, that may result from this litigation.
From time to time, we may become involved in other legal proceedings and claims arising in the ordinary course of our business. Other than as described above, we are not currently a party to any legal proceedings the outcome of which, if determined adversely to us, we believe would individually or in the aggregate have a material adverse effect on our business, operating results, financial condition or cash flows.
85
Table of Contents
Executive Officers, Key Employees and Directors
The following table sets forth certain information about our executive officers, key employees and directors, as of September 1, 2010.
| Name | Age | Position | ||
| Executive Officers |
||||
| Clifford A. Reid, Ph.D. |
51 | President, Chief Executive Officer and Director | ||
| Ajay Bansal |
48 | Chief Financial Officer | ||
| Radoje Drmanac, Ph.D. |
52 | Chief Scientific Officer | ||
| Bruce Martin |
45 | Senior Vice President of Product Development | ||
| Mark J. Sutherland |
53 | Senior Vice President of Business Development | ||
| Key Employees |
||||
| Dennis Ballinger, Ph.D. |
55 | Vice President of Genomics | ||
| William Banyai, Ph.D. |
55 | Vice President of Hardware | ||
| Robert J. Curson |
67 | Vice President of Financial Operations | ||
| Alan Dow, Ph.D. |
55 | Vice President of Intellectual Property and Legal Affairs | ||
| Ken Prokuski |
60 | Vice President of Operations | ||
| Aaron Solomon |
45 | Vice President of Sales | ||
| Jennifer Turcotte |
40 | Vice President of Marketing | ||
| Directors |
||||
| Clifford A. Reid, Ph.D. |
51 | President, Chief Executive Officer and Director | ||
| Alexander E. Barkas, Ph.D.(1) |
63 | Director | ||
| C. Thomas Caskey, M.D. |
71 | Director | ||
| Carl L. Gordon, Ph.D., CFA(2) |
45 | Director | ||
| Michael P. Rubin, M.D. |
35 | Director | ||
| Andrew E. Senyei, M.D.(2) |
60 | Director | ||
| Lewis J. Shuster(2) |
55 | Director | ||
| Charles P. Waite, Jr.(1) |
55 | Director | ||
| (1) | Member of the Compensation Committee. |
| (2) | Member of the Audit Committee. |
Executive Officers and Key Employees
Clifford A. Reid, Ph.D., is our co-founder and has served as our President and Chief Executive Officer since July 2005 and as a member of our board of directors since July 2005. From March 2003 to September 2005, Dr. Reid was Vice President of Collaborative Solutions at Open Text Corporation, a software company. In 1995, Dr. Reid co-founded Eloquent, Inc., a digital video communications company, and served as its Chief Executive Officer until 1999 and as its Chairman until 2003, when it was acquired by Open Text. In 1988, Dr. Reid co-founded Verity, Inc., an enterprise text search engine company, and served as its Vice President of Engineering from 1988 to 1992 and as its Executive Vice President from 1992 to 1993. Dr. Reid received a B.S. in Physics from the Massachusetts Institute of Technology, an M.B.A. from Harvard University and a Ph.D. in Management Science and Engineering from Stanford University. As our President, Chief Executive Officer and co-founder, Dr. Reid brings expertise and knowledge regarding our business and operations to our board of directors. He also brings to our board of directors an extensive background in the technology industry and in leadership roles, providing both strategic and operational vision and guidance.
Ajay Bansal has served as our Chief Financial Officer since May 2010. From June 2009 to January 2010, Mr. Bansal served as Chief Financial Officer and Executive Vice President of Business Development at Lexicon Pharmaceuticals, Inc., a biopharmaceutical company. From December 2007 to October 2008, Mr. Bansal served as Chief Financial Officer and Executive Vice President of Finance of Tercica, Inc., a biopharmaceutical
86
Table of Contents
company acquired by the Ipsen Group in October 2008. He also served as Chief Financial Officer and Senior Vice President of Finance of Tercica from March 2006 until December 2007. From February 2003 to January 2006, Mr. Bansal served as Vice Present of Finance and Administration and Chief Financial Officer of Nektar Therapeutics, a biopharmaceutical company. From July 2002 to February 2003, Mr. Bansal served as Director of Operations Analysis at Capital One Financial, a bank holding company. From August 1998 to June 2002, Mr. Bansal was at Mehta Partners LLC, a financial advisory firm, where he was named partner in January 2000. Prior to joining Mehta Partners, Mr. Bansal spent more than ten years in management roles at Novartis, a pharmaceuticals company, and in consulting at Arthur D. Little, Inc., McKinsey & Company, Inc. and ZS Associates. Mr. Bansal received a B.S. in Mechanical Engineering from the Indian Institute of Technology (Delhi) and an M.S. in Operations Management and an M.B.A. from Northwestern University.
Radoje Drmanac, Ph.D., is our co-founder and has served as our Chief Scientific Officer since July 2005. In 2001, Dr. Drmanac co-founded Callida Genomics, Inc., a DNA sequencing company, and served as Callida’s Chief Scientific Officer from 2001 to 2004 and has served as its President since 2004. In 1994, Dr. Drmanac co-founded Hyseq, Inc., a DNA array technology company that became Hyseq Pharmaceuticals, Inc. and later merged with Variagenics, Inc. to become Nuvelo, Inc., and served as its Senior Vice President of Research from 1994 to 1998 and as its Chief Scientific Officer from 1998 to 2001. Prior to that, Dr. Drmanac served as a group leader at Argonne National Laboratory. Dr. Drmanac received a B.S., M.S. and Ph.D. in Molecular Biology from the University of Belgrade.
Bruce Martin has served as our Senior Vice President of Product Development since March 2010. From May 2007 to March 2010, Mr. Martin was our Vice President of Product Development. From 2005 to May 2007, Mr. Martin served as Vice President of Product Strategy at PSS Systems, Inc., an internet software company. From 2002 to 2003, Mr. Martin served as Chief Technical Officer of Openwave Systems Inc., a software company. Mr. Martin received a B.S. in Computer Science and Electrical Engineering from the University of California, Davis.
Mark J. Sutherland has served as our Senior Vice President of Business Development since March 2010. From October 2008 to November 2009, Mr. Sutherland served as Senior Vice President of Business Development at GenVault Corporation, a DNA storage company. From November 2005 to September 2007, Mr. Sutherland served as Chief Business Officer for Flashpoint Technology, Inc., a digital content management company. Beginning in August 1988, Mr. Sutherland served for 17 years in roles of increasing responsibility at Molecular Dynamics, a manufacturer of molecular biology and genetic engineering equipment, and its successor companies, Amersham Biosciences and GE Healthcare. Mr. Sutherland served as Vice President of Genomics at Amersham from 1998 to 2001 and as VP, Strategic Alliances, at Amersham and for the Discovery Systems business of GE Healthcare from 2001 to 2005. Mr. Sutherland received a B.S. in Chemistry with Honors from Stanford University.
Dennis Ballinger, Ph.D., has served as our Vice President of Genomics since October 2008. From 2002 to 2008, Dr. Ballinger served as Vice President of Genomics at Perlegen Sciences, Inc., a developer of genetic tests. From 1999 to 2001, he served as Director of Product Development at Ingenuity Systems, Inc., a software company. From 1998 to 1999, he served as Senior Director of Functional Genomics at Hyseq, Inc. From 1994 to 1998, he served as Director of Cardiovascular Disease Research at Myriad Genetics, a developer of molecular diagnostic products. From 1989 to 1994, Dr. Ballinger was an Assistant Professor of molecular biology at the Sloan-Kettering Institute and Cornell University Graduate School. Dr. Ballinger received a B.S. in molecular cell biology from University of Colorado, Boulder and a Ph.D. in cellular and developmental biology from the Massachusetts Institute of Technology and completed his postdoctoral training at the California Institute of Technology.
William Banyai, Ph.D., has served as our Vice President of Hardware since July 2006. From March 2000 to February 2006, Dr. Banyai was a founder and the Chief Technical Officer of Glimmerglass Networks, an optical networking company. From February 1996 to February 2000, Dr. Banyai served as a staff physicist at the Lawrence Livermore National Lab. From November 1994 to November 1995, Dr. Banyai served as the Chief Technologist of Silicon Light Machines, a high-resolution display company. From September 1992 to October
87
Table of Contents
1994, Dr. Banyai served as a research engineer at Stanford University’s Ginzton Laboratory. From January 1991 to August 1992, Dr. Banyai served as a research scientist at the Sandia National Lab’s Compound Semiconductor Research Facility. Dr. Banyai received a B.S. in physics and an M.S. in electrical science from the University of Michigan, an Engineer of Electrical Engineering from the University of Southern California and a Ph.D. in optical sciences from the University of Arizona.
Robert J. Curson is our co-founder and has served as our Vice President of Financial Operations since May 2010. From July 2005 to May 2010, he served as our Chief Financial Officer. From April 2003 to July 2005, Mr. Curson was a partner of Neon Partners, an investment partnership focusing on special turn-around situations. From July 1999 until its acquisition by Open Text Corporation in April 2003, Mr. Curson served as Chief Financial Officer of Eloquent, Inc., a digital video communications company. From July 1993 to July 1999, Mr. Curson served as Chief Financial Officer of RasterOps Inc., a digital imaging and peripherals company which, during Mr. Curson’s tenure, was re-incorporated as Truevision Inc., a digital video company. Mr. Curson received a B.S. in Mechanical Engineering and an M.A. in Operations Research and Economics from the University of Leeds, U.K., and an M.B.A. from the University of California, Los Angeles.
Alan Dow, Ph.D., has served as our Vice President of Intellectual Property and Legal Affairs since September 2008. From September 2004 to September 2008, Dr. Dow worked as an attorney in private practice at BioTechnology Law Group in San Diego, California, specializing in patent prosecution and intellectual property-related transactions. From June 2001 to July 2004, Dr. Dow served as Vice President and General Counsel of Vical Incorporated, a biopharmaceutical company. Dr. Dow received a B.S. in Chemistry from the University of Maine at Orono, a Ph.D. in Genetics from Harvard University and a J.D. from Stanford Law School.
Ken Prokuski has served as our Vice President of Operations since January 2010. From August 2009 to January 2010, Mr. Prokuski served as an independent consultant to us. From October 1993 to January 2009, Mr. Prokuski served as Senior Director of Manufacturing Operations at Applied Biosystems, a life sciences company and a division of Life Technologies. From September 1992 to September 1993, Mr. Prokuski served as Material Manager at Arico Coating Technology, a thin film deposition equipment manufacturer. From September 1991 to September 1992, Mr. Prokuski served as Director of Materials at Toshiba America, Inc., an electronics company. From January 1990 to August 1991, Mr. Prokuski served as Operations Manager at Millipore Corporation, a life sciences company. Mr. Prokuski received a B.A. in Psychology from Northeastern Illinois University and an Executive M.B.A. from Golden Gate University.
Aaron Solomon, has served as our Vice President of Sales since March 2009. From April 2008 to March 2009, Mr. Solomon served as Vice President of Business Development at Tethys Bioscience, a developer of diagnostic tests. From October 2005 to April 2008, Mr. Solomon served as Director of Western Region Industrial Sales and later as Vice President of Industrial Sales at Affymetrix, a leading DNA microarray company. From February 2004 until its acquisition by Affymetrix in October 2005, Mr. Solomon served as Vice President of Business Development at ParAllele, a targeted genotyping company. Mr. Solomon received a B.S. in Biological Sciences from the University of California, Irvine.
Jennifer Turcotte has served as our Vice President of Marketing since September 2008. From November 2007 to August 2008, she served as our Senior Director of Product Marketing. From June 2007 to October 2007, Ms. Turcotte served as Senior Director, Marketing Solutions, at SAP, Inc., a business management software company. From March 2004 to May 2007, Ms. Turcotte served as Senior Director of Product Marketing at Siperian, Inc., a software company acquired by Informatica in March 2010. Prior to joining Siperian, Ms. Turcotte held senior product marketing positions at Rearden Commerce, technology-based personal assistance company, Ariba, Inc., a software and information technology company, Nuance Communications, a software company, and BEA Systems, Inc., a software company that was acquired by Oracle Corporation. Ms. Turcotte received a B.Eng. in mechanical engineering from Carleton University in Ottawa, Canada.
88
Table of Contents
Directors
Alexander E. Barkas, Ph.D., has served as a member of our board of directors since March 2007. In 1997, Dr. Barkas co-founded Prospect Venture Partners, a venture capital firm, and currently serves as a Managing Director at that firm. From 1991 to 1997, Dr. Barkas was a Partner at Kleiner Perkins Caufield & Byers, a venture capital firm. Prior to that, Dr. Barkas was a Founder and Chief Executive Officer of BioBridge Associates, a healthcare industry consulting firm. Dr. Barkas has served as Chairman of Geron Corporation, a biopharmaceutical company, since 1993 and as a member of the board of directors since 1992. Dr. Barkas has also served as a director of Amicus Therapeutics, Inc., a biotechnology company, since 2004. From May 2002 to October 2008, Dr. Barkas served as a director of Tercica, and as its Chairman from August 2003 to October 2008. Dr. Barkas received a Ph.D. in Biology from New York University and a B.A. in Biology from Brandeis University, where he currently is Chairman of the University Science Advisory Council and serves on the Board of Trustees. As a venture capitalist, Dr. Barkas brings to our board of directors broad experience in advising and managing life science and biotechnology companies from early-stage to mature companies, including expertise in capital raising, strategic transactions, financial budgeting and reporting, strategy and operations, recruiting and compensation. In addition, Dr. Barkas brings insight on compensation-related matters to the compensation committee based on his breadth of exposure to life science companies.
C. Thomas Caskey, M.D., has served as a member of our board of directors since August 2009. Since January 2006, Dr. Caskey has served as Director and Chief Executive Officer of the Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, part of the University of Texas Health Science Center at Houston. Since 2003, Dr. Caskey has served as an Adjunct Partner at Essex Woodlands Health Ventures, a venture capital firm. Dr. Caskey served as Managing Director of Cogene Biotech Ventures, Ltd., a venture capital firm, from March 2005 to October 2005 and as President and Chief Executive Officer from April 2000 to March 2005. He served as Senior Vice President, Research, at Merck Research Laboratories, a division of Merck & Co., Inc., a pharmaceutical company, from 1995 to 2000 and as President of the Merck Genome Research Institute from 1996 to 2000. Before joining Merck, Dr. Caskey served 25 years at Baylor College of Medicine in a series of senior positions, including Chairman, Department of Human and Molecular Genetics, and Director, Human Genome Center. He is a member of the National Academy of Sciences. Dr. Caskey served as a director of Lexicon Genetics Incorporated, a biopharmaceutical company, from April 2000 to April 2006 and as Chairman from April 2000 to March 2005. Dr. Caskey served as a director of Luminex Corporation, a developer of biological testing technologies, from January 2001 to May 2005. Dr. Caskey received a B.A. from the University of South Carolina and an M.D. from Duke University. Dr. Caskey brings to the board an extensive scientific and operational background gained as a research scientist, executive and venture capital advisor focused on life science and pharmaceutical companies.
Carl L. Gordon, Ph.D., CFA, has served as a member of our board of directors since August 2009. In 1998, Dr. Gordon co-founded OrbiMed Advisors LLC, an asset management firm, and has served as a General Partner since that time. From 1995 to 1997, Dr. Gordon served as a Senior Biotechnology Analyst at Mehta & Isaly, the predecessor firm to OrbiMed. From 1993 to 1995, Dr. Gordon served as a Fellow at The Rockefeller University. Since May 2008, Dr. Gordon has served as a director of Amarin Corporation, a biopharmaceutical company. From March 2004 to May 2007, Dr. Gordon served as a director of Biocryst Pharmaceuticals, Inc., a pharmaceutical company. Dr. Gordon received an A.B. from Harvard University and a Ph.D. in molecular biology from the Massachusetts Institute of Technology. As a venture capitalist focused on life science companies who sits on numerous boards, Dr. Gordon provides financial and operational expertise regarding our industry. In addition, Dr. Gordon provides substantial expertise in the particularly relevant scientific field of molecular biology.
Michael P. Rubin, M.D., has served as a member of our board of directors since August 2010. Since January 2010, Dr. Rubin has been a financial analyst at Sands Capital Management, an asset management firm. Prior to beginning his employment with Sands Capital Management, Dr. Rubin was a graduate business student at the University of Massachusetts, Amherst. From July 2006 through September 2007, Dr. Rubin completed a fellowship at Harvard Medical School and Massachusetts Eye and Ear Infirmary, where he conducted genetic research in macular degeneration. From July 2003 through June 2006, Dr. Rubin was a resident physician in
89
Table of Contents
ophthalmology at the University of Chicago. From June 2002 through June 2003, Dr. Rubin completed an internship in internal medicine at Harvard Medical School and Mount Auburn Hospital. Dr. Rubin received a B.S. in electrical engineering from the University of California, Los Angeles, an M.B.A. from the University of Massachusetts, Amherst, and an M.D. from the University of Chicago Pritzker School of Medicine. Dr. Rubin’s experience as a financial analyst provides our board with substantive knowledge in financial markets and, coupled with his background in genetic research and medical training, valuable insight into the life science industry.
Andrew E. Senyei, M.D., has served as a member of our board of directors since March 2006. Since 1987, Dr. Senyei has served as a Managing Director and a General Partner of Enterprise Partners, a venture capital firm. In 1989, Dr. Senyei co-founded Molecular Biosystems, Inc., a biotechnology company acquired by Alliance Pharmaceutical Corp. in 2000. Prior to joining Enterprise Partners, Dr. Senyei served as a practicing clinician and Adjunct Associate Professor of Obstetrics and Gynecology at the University of California, Irvine. Dr. Senyei has served as Chairman of Genoptix, Inc., a specialized medical laboratory service provider, since April 2000. Dr. Senyei served as a director of Adeza Biomedical Corporation, a healthcare products company, from 1987 until Adeza was acquired by Cytyc Corporation in April 2007. Dr. Senyei has served on the Board of Trustees of Northwestern University since 2005 and on the Advisory Council of the Jacobs School of Engineering at the University of California, San Diego, since 2002. Dr. Senyei received a B.S. from Occidental College and an M.D. from Northwestern University Medical School, and he completed his residency training at the University of California, Irvine, Medical Center. Dr. Senyei’s medical and business expertise, including his experience as a venture capitalist building and serving on the board of directors of more than 25 public and private emerging life sciences and healthcare companies, combined with his extensive prior experience as an inventor and practicing clinician, give him valuable insight into our industry. His experience provides the seasoned business judgment and broad strategic vision which enables him to serve as an effective and valuable director and to have the qualifications and leadership and other skills to serve on our board of directors.
Lewis J. Shuster has served as a member of our board of directors since April 2010. In 2002, Mr. Shuster founded Shuster Capital, a strategic and operating advisor to and angel investor in life science companies, and has served as its Chief Executive Officer since that time. From June 2003 to November 2007, Mr. Shuster served as Chief Executive Officer of Kemia, Inc., a drug discovery and development company. From February 2000 to December 2001, Mr. Shuster held various operating executive positions at Invitrogen Corporation, a biotechnology company that merged with Applied Biosystems Inc. and became Life Technologies Corporation. From 1994 to 1999, Mr. Shuster served as Chief Financial Officer of Pharmacopeia, Inc., a drug discovery product and service company, and later as Chief Operating Officer of Pharmacopeia Labs, a division of Pharmacopeia, Inc. Mr. Shuster joined Human Genome Sciences as its first employee in September 1992 and as Executive Vice President of Operations and Finance and helped guide the development and operation of what was the world’s leading gene sequencing operation in 1994. Mr. Shuster served as a director of Epitomics, Inc., a private monoclonal antibody firm, from 2002 until May 2010, and a director of Sorrento Therapeutics, Inc., a biopharmaceutical company, from September 2009 to February 2010 and has served as a director of Retrotope, a privately held biotechnology company, since April 2008. Mr. Shuster received a B.A. in Economics from Swarthmore College and an M.B.A. from Stanford University. Mr. Shuster provides the board with an extensive background in financial and strategic planning, auditing and accounting and financial leadership expertise. As Chairman of the Audit Committee, Mr. Shuster also keeps the board abreast of current audit issues and collaborates with our independent registered public accounting firm and senior management team.
Charles P. Waite, Jr., has served as a member of our board of directors since March 2006. Mr. Waite has been a General Partner of OVP Venture Partners II and a Vice President of Northwest Venture Services Corp. since 1987, a General Partner of OVP Venture Partners III since 1994, a General Partner of OVP Venture Partners IV since 1997, a General Partner of OVP Venture Partners V since 2000 and a General Partner of OVP Venture Partners VI since 2001, all of which are venture capital firms. He currently serves on the board of directors of eight private companies. Mr. Waite received an A.B. in History from Kenyon College and an M.B.A. from Harvard University. Mr. Waite brings to the board significant operational and leadership experience as a venture capital investor who sits on a number of boards. Mr. Waite’s investment focus on life science companies also provides substantial expertise in our industry.
90
Table of Contents
Scientific Advisory Board
We maintain a scientific advisory board consisting of members with experience and expertise in the field of genomics and genetics who provide us with consulting services. Our scientific advisory board consists of the following members:
Mark Chee, Ph.D., is an internationally recognized expert in genomics. He presently serves as Chief Executive Officer and Chief Scientific Officer of Prognosys Biosciences, Inc. Previously, he co-founded Illumina, Inc., and was Director of Genetics Research at Affymetrix, Inc. He has published scientific papers on microarray technology and applications and is an inventor on over 40 issued patents. He also serves on the External Scientific Committee of The Cancer Genome Atlas project. Dr. Chee received his B.Sc. in Biochemistry from the University of New South Wales and his Ph.D. in Molecular Biology from the University of Cambridge.
George Church, Ph.D., is Professor of Genetics at Harvard Medical School and Director of the Center for Computational Genetics. With degrees from Duke University in Chemistry and Zoology, he co-authored research on 3D-software & RNA structure with Sung-Hou Kim. His 1984 Ph.D. from Harvard in Biochemistry & Molecular Biology with Wally Gilbert included the first direct genomic sequencing method. He co-initiated the Human Genome Project a few months later as a Research Scientist at newly formed Biogen Inc. and was a Monsanto Life Sciences Research Fellow at UCSF. He invented the broadly applied concepts of molecular multiplexing and tags, homologous recombination methods and array DNA synthesizers. Technology transfer of automated sequencing and software to Genome Therapeutics Corp. resulted in the first commercial genome sequence (the human pathogen, H. pylori, 1994). He has served in advisory roles for 12 journals, five granting agencies and 22 biotechnology companies. His current research focuses on integrating biosystems-modeling with personal genomics and synthetic biology.
Leroy Hood, M.D., Ph.D., is currently President of the Institute for Systems Biology. His research has focused on the study of molecular immunology, biotechnology and genomics. His professional career began at the California Institute of Technology where he and his colleagues pioneered four instruments, the DNA gene sequencer and synthesizer and the protein synthesizer and sequencer, which comprise the technological foundation for contemporary molecular biology. In particular, the DNA sequencer has revolutionized genomics by allowing the rapid, automated sequencing of DNA, which played a crucial role in contributing to the successful mapping of the human genome during the 1990s. In 1992, he moved to the University of Washington as founder and Chairman of the cross-disciplinary Department of Molecular Biotechnology. In 2000, he co-founded the Institute for Systems Biology in Seattle, Washington to pioneer systems approaches to biology and medicine. He is a member of the National Academy of Sciences, the American Philosophical Society, the American Association of Arts and Sciences and the Institute of Medicine. He has also played a role in founding numerous biotechnology companies, including Amgen, Inc., Applied Biosystems, Inc., Systemix Institute, Darwin Molecular Corp. and Rosetta Inpharmatics LLC. Dr. Hood received an M.D. from Johns Hopkins School of Medicine and a Ph.D. in Biochemistry from the California Institute of Technology. He has published more than 600 peer-reviewed papers, is listed as an inventor on 14 issued patents and has co-authored textbooks in biochemistry, immunology, molecular biology and genetics.
Douglas A. Lauffenburger, Ph.D., is the Uncas & Helen Whitaker Professor of Bioengineering and Director of the Biological Engineering Division at the Massachusetts Institute of Technology and also holds appointments in the Department of Biology and the Department of Chemical Engineering. Dr. Lauffenburger received B.S. and Ph.D. degrees in chemical engineering from the University of Illinois in 1975 and the University of Minnesota in 1979, respectively. His major research interests are in cell engineering, the fusion of engineering with molecular cell biology. A central focus of his research program is in receptor-mediated cell communication and intracellular signal transduction, with emphasis on development of predictive computational models derived from quantitative experimental studies, on cell cue/signal/response relationships. These models have applications in drug discovery and development.
Larry Smarr, Ph.D., is the founding Director of the California Institute for Telecommunications and Information Technology, a partnership between the University of California, San Diego, and University of California, Irvine, created in 2000. Dr. Smarr also holds the Harry E. Gruber professorship in the Jacobs School’s Department of
91
Table of Contents
Computer Science and Engineering at the University of California, San Diego. Dr. Smarr was the founding director of the National Center for Supercomputing Applications, a position he held until 2000. From 1997 until 2005, Dr. Smarr served on the Advisory Committee to the Director of the National Institutes of Health. Previously, he was a professor in the Astronomy and Physics departments of the University of Illinois at Urbana-Champaign from 1979 until March 2000. Dr. Smarr received a B.A. and a M.S. in physics from the University of Missouri, a M.S. in physics from Stanford University and a Ph.D. in physics for the University of Texas, Austin. Dr. Smarr was a member of the Fisher Scientific Biotechnology Council from 1993 through 1998, has been a member of the InterWest MedTech Advisory Committee since 1999 and has been Advisor to the CEO, MedExpert International, Inc. since 2000. Dr. Smarr is a pioneer in the field of biomedical computing and has numerous publications in the field, including publications and quotations in Science, Nature, the New York Times, Wall Street Journal, Time, Newsweek, Wired, Fortune, Business Week, the Sydney Morning Herald, the Age and the Australian Broadcasting Company.
Leadership Structure of the Board
The board appointed as the lead independent director in to help reinforce the independence of the board as a whole. The lead independent director is empowered to, among other duties and responsibilities, provide general leadership of the affairs of the independent directors, preside over board meetings in the absence of the Chief Executive Officer and during independent director closed session portions of the meetings, preside over and establish the agendas for meetings of the independent directors and act as liaison between the Chief Executive Officer and the independent directors. The board believes that the lead independent director can help ensure the effective independent functioning of the board in its oversight responsibilities.
Board Composition
Independent Directors
Our board of directors consists of eight members. Our board of directors has determined that all of our directors, other than Dr. Reid, qualify as “independent” directors in accordance with the NASDAQ listing requirements. Dr. Reid is not considered independent because he is an employee of Complete Genomics. The NASDAQ independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management.
Classified Board of Directors
In accordance with our amended and restated certificate of incorporation to be in effect immediately prior to the consummation of this offering, our board of directors will be divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Effective upon the completion of this offering, our directors will be divided among the three classes as follows:
| § | the Class I directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2011; |
| § | the Class II directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2012; and |
| § | the Class III directors will be , and , and their terms will expire at the annual meeting of stockholders to be held in 2013. |
92
Table of Contents
Our amended and restated certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change-in-control of our company.
Voting Arrangements
The election of the members of our board of directors is governed by the amended and restated voting agreement that we entered into with certain holders of our common stock and certain holders of our convertible preferred stock and the related provisions of our amended and restated certificate of incorporation. Pursuant to the voting agreement and these provisions:
| § | the holders of our Series A Preferred Stock, voting separately as a single class, have the right to elect two directors to our board of directors, one of whom is designated by OVP Venture Partners VI, L.P. and its affiliates, for which Mr. Waite has been designated, and one of whom is designated by Enterprise Partners VI, L.P. and its affiliates, for which Dr. Senyei has been designated; |
| § | the holders of our Series B Preferred Stock, voting separately as a single class, have the right to elect one director to our board of directors, who is designated by Prospect Venture Partners III, L.P., for which Dr. Barkas has been designated; |
| § | the holders of our Series C Preferred Stock, voting separately as a single class, have the right to elect one director to our board of directors, who is designated by Highland Capital Management, L.P., for which Mr. Shuster has been designated; |
| § | the holders of our Series D Preferred Stock, voting separately as a single class, have the right to elect two directors to our board of directors, one of whom is designated by Essex Woodlands Health Ventures Fund VIII, L.P., for which Dr. Caskey has been designated, and one of whom is designated by OrbiMed Associates III, LP and its affiliates, for which Dr. Gordon has been designated; |
| § | the holders of our Series E Preferred Stock, voting separately as a single class, have the right to elect one director to our board of directors, who is designated by SCV-CG, LLC, for which Dr. Rubin has been designated; |
| § | the holders of our common stock, voting separately as a single class, have the right to elect one director to our board of directors, who is the then-current chief executive officer, currently Dr. Reid; and |
| § | the holders of our preferred stock and common stock, voting together as a single class, have the right to elect the remaining director, if any. |
The holders of our common stock and preferred stock who are parties to our voting agreement are obligated to vote for such designees indicated above. The provisions of this voting agreement will terminate upon the completion of this offering and our certificate of incorporation will be amended and restated, after which there will be no further contractual obligations or charter provisions regarding the election of our directors. Our directors hold office until their successors have been elected and qualified or appointed, or the earlier of their death, resignation or removal.
Board Diversity
Upon completion of our initial public offering, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individuals candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
| § | personal and professional integrity; |
93
Table of Contents
| § | ethics and values; |
| § | experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
| § | experience in the industries in which we compete; |
| § | experience as a board member of another publicly held company; |
| § | diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
| § | conflicts of interest; and |
| § | practical and mature business judgment. |
Currently, our board of directors evaluates, and following the completion of our initial public offering will evaluate, each individual in the context of the board of directors as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Board Committees
Our board of directors has an audit committee, a compensation committee and a nominating and corporate governance committee. Our board of directors may establish other committees to facilitate the management of our business. The composition and functions of each committee are described below.
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee:
| § | appoints our independent registered public accounting firm; |
| § | evaluates the independent registered public accounting firm’s qualifications, independence and performance; |
| § | determines the engagement of the independent registered public accounting firm; |
| § | reviews and approves the scope of the annual audit and the audit fee; |
| § | discusses with management and the independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements; |
| § | approves the retention of the independent registered public accounting firm to perform any proposed permissible non-audit services; |
| § | monitors the rotation of partners of the independent registered public accounting firm on our engagement team as required by law; |
| § | is responsible for reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| § | reviews our critical accounting policies and estimates; and |
| § | annually reviews the audit committee charter and the committee’s performance. |
The current members of our audit committee are Carl Gordon, Ph.D., CFA, Andrew E. Senyei, M. D. and Lewis J. Shuster. Mr. Shuster serves as the chairman of the committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Our board of directors has determined that Mr. Shuster is an audit committee financial expert as defined under the
94
Table of Contents
applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of The NASDAQ Stock Market. Under the rules of the SEC, members of the audit committee must also meet heightened independence standards. However, a minority of the members of the audit committee may be exempt from the heightened audit committee independence standards for one year from the date of effectiveness of the registration statement of which this prospectus forms a part. Our board has determined that each of Dr. Senyei and Mr. Shuster meet these heightened independence standards. Due to his relationship with OrbiMed Advisors LLC and its affiliated funds, which may be deemed to be an affiliate of us due to the size of its holdings in our securities, Dr. Gordon does not meet the heightened independence requirements under Rule 10A-3 of the Exchange Act. Our board intends to appoint a new director meeting these heightened independence standards to replace Dr. Gordon as a member of our audit committee in reliance on the phase-in exemption pursuant to Rule 10A-3(b)(1)(iv)(A)(2) under the Exchange Act. Upon expiration of this phase-in exemption one year from the effectiveness of the registration statement of which this prospectus is a part, each of our audit committee members must meet the heightened independence requirements under Rule 10A-3(b)(1) under the Exchange Act. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and approves corporate goals and objectives relevant to compensation of our Chief Executive Officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and sets the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our stock plans. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance by the compensation committee with its charter. The current members of our compensation committee are Alexander E. Barkas, Ph.D., and Charles P. Waite, Jr. Dr. Barkas serves as the chairman of the committee. Each of the members of our compensation committee is independent under the applicable rules and regulations of The NASDAQ Global Market, is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act and is an “outside director” as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or Section 162(m). The compensation committee operates under a written charter.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board of directors. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies and reporting and making recommendations to our board of directors concerning governance matters. The current members of our nominating and corporate governance committee are , and . serves as the chairman of the committee. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of The NASDAQ Global Market relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter.
There are no family relationships among any of our directors or executive officers.
Oversight of Risk Management
Our board of directors has an active role, as a whole and also at the committee level, in overseeing the management of our risks. The board of directors is responsible for general oversight of risks and regularly reviews information regarding our risks, including credit risks, liquidity risks and operational risks. Our compensation committee is responsible for overseeing the management of risks relating to our company’s executive compensation plans and arrangements. Our audit committee is responsible for overseeing the management of our risks relating to accounting matters and financial reporting and legal and regulatory
95
Table of Contents
compliance. Our nominating and corporate governance committee is responsible for overseeing the management of our risks associated with the independence of our board of directors and potential conflicts of interest. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire board of directors is regularly informed through committee reports about such risks. Our board of directors believes its administration of its risk oversight function has not affected the board of directors’ leadership structure.
Compensation Committee Interlocks and Insider Participation
During 2009, our compensation committee consisted of Alexander E. Barkas, Ph.D., Carl L. Gordon, Ph.D., CFA, and Charles P. Waite, Jr. Dr. Barkas served as Chairman of the Compensation Committee. None of the members of the compensation committee is currently, or has been at any time, one of our officers or employees. None of our executive officers currently serves, or has served during the last completed three fiscal years, as a member of the board of directors or compensation committee of any other entity that has or had one or more executive officers serving as a member of our board of directors or compensation committee. In July 2010, Dr. Gordon resigned from the compensation committee.
Code of Business Conduct and Ethics
We will adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. Following the completion of this offering, the code of business conduct and ethics will be available on our website at www.completegenomics.com. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website.
Director Compensation
Our directors do not currently receive any cash compensation for their services as members of our board of directors or any committee of our board of directors. Directors are reimbursed for reasonable travel, lodging and other expenses incurred in connection with their attendance at board of directors or committee meetings. In 2009, our non-employee directors, Drs. Barkas, Caskey, Gordon and Senyei and Messrs. Hukill and Waite, did not receive any compensation in connection with their service on our board of directors or any committee of our board of directors. Mr. Hukill resigned from our board of directors effective January 7, 2009.
None of our directors held any unvested restricted stock or stock options as of December 31, 2009.
In April 2010, our board of directors granted each of Dr. Caskey and Mr. Shuster an option to purchase 10,000 shares of our common stock for $1.50 per share, which our board of directors determined to be the fair market value of our common stock on the date of grant. In July 2010, the compensation committee of our board of directors amended each of the option grants to Dr. Caskey and Mr. Shuster to increase the exercise price of these stock options to $2.43 per share, which the compensation committee determined to be the fair market value of our common stock on the date of grant following a valuation of our common stock as of March 31, 2010. At a subsequent meeting of our board of directors in July 2010, our board granted each of Dr. Caskey and Mr. Shuster an option to purchase 2,500 shares of our common stock for $2.69 per share, which our board of directors determined to be the fair market value of our common stock on the date of grant. Each of the stock options vest in 12 equal monthly installments measured from the respective vesting commencement dates of August 12, 2009 for Dr. Caskey and April 8, 2010 for Mr. Shuster, which correspond to their dates of appointment to our board of directors.
In connection with our initial public offering, our compensation committee intends to evaluate our director compensation policy.
96
Table of Contents
Compensation Discussion and Analysis
This section discusses the principles underlying our policies and decisions with respect to the compensation of our executive officers who are named in the Summary Compensation Table that appears below and the most important factors relevant to an analysis of these policies and decisions. These officers, whom we refer to as our named executive officers, consist of Clifford A. Reid, Ph.D., President and Chief Executive Officer, Robert J. Curson, Chief Financial Officer, and Radoje Drmanac, Ph.D., Chief Science Officer. We did not have any executive officers other than the named executive officers during fiscal year 2009.
Objectives and Philosophy Regarding our Executive Compensation
We recognize that the ability of our business to excel depends on the integrity, knowledge, imagination, skill, diversity and teamwork of our employees. To this end, we strive to create an environment of mutual respect, encouragement and teamwork that rewards commitment and performance and that is responsive to the needs of our employees. The principles and objectives of our compensation and benefits programs for our named executive officers are to:
| § | attract, engage and retain individuals of superior ability, experience and managerial talent to lead our company; |
| § | align compensation decisions with our corporate strategies, business and financial objectives and the long-term interests of our stockholders; |
| § | motivate and reward executives whose knowledge, skills and performance are the foundation for our continued collective success; |
| § | ensure that the elements of compensation, individually and in the aggregate, do not encourage excessive risk-taking; and |
| § | ensure that total compensation is fair, reasonable and competitive. |
The compensation components described below are designed to simultaneously fulfill one or more of these principles and objectives.
Components of our Executive Compensation
The individual components of our executive compensation consist primarily of:
| § | base salary; |
| § | performance bonuses; |
| § | equity incentives; |
| § | post-termination benefits; and |
| § | various other employee benefits. |
We view each of these components as related but distinct, reviewing them each individually, as well as collectively, to ensure that the total compensation paid to our executive officers meets the objectives as set forth above. Not all elements are provided to all named executive officers. Instead, we determine the appropriate level for each compensation component based in part, but not exclusively, on our understanding of the market based on the experience of our President and Chief Executive Officer and members of our board of directors and consistent with our recruiting and retention goals, our view of internal equity and consistency, the length of service of our executives, our overall performance and other considerations we deem relevant.
97
Table of Contents
Historically, except as described below, we have not adopted any formal or informal policies or guidelines for allocating compensation between long-term and currently paid out compensation, between cash and noncash compensation or among different forms of noncash compensation. However, our philosophy is to make a greater percentage of our executive officer compensation tied to stockholder returns and to keep cash compensation to a nominally competitive level while providing the opportunity to be well-rewarded through equity if we perform well over time. To this end, we use stock options as a significant component of compensation because we believe that they best tie an individual’s compensation to the creation of stockholder value. While we offer competitive base salaries, we believe stock-based compensation is a significant motivator in attracting employees in our field.
Each of the primary elements of our executive compensation is discussed in more detail below. While we have identified particular compensation objectives that each element of executive compensation serves, our compensation programs are designed to be flexible and complementary and to collectively serve all of the executive compensation objectives described above. Accordingly, whether or not specifically mentioned below, we believe that, as a part of our overall executive compensation policy, each individual element, to a greater or lesser extent, serves each of our objectives.
Compensation Determination Process
Compensation for our named executive officers has historically been highly individualized, as compensation levels were often the result from arm’s-length negotiations and were based on a variety of informal factors including, in addition to the factors listed above, our financial condition and available resources, evaluation by our board of directors of the competitive market based on the experience of our directors with other companies and the compensation levels of our other executive officers, each as of the time of the applicable compensation decision.
Previously, our President and Chief Executive Officer, and, with respect to our President and Chief Executive Officer, our board of directors, has reviewed the performance of each named executive officer. This process generally occurred on an annual basis, though we do not set a predetermined time for such review. Historically, we have not used relevant market compensation data in setting compensation levels for our named executive officers. Instead, our President and Chief Executive Officer, based on his experience, and in light of the factors described above, made recommendations regarding our executive compensation packages for our named executive officers, other than for himself, to our board of directors for review. The factors we have relied upon in making these decisions include the company’s performance and an evaluation of each named executive officer’s performance during the year, leadership qualities, operational performance, business responsibilities, career with us, current compensation arrangements and long-term potential to enhance stockholder value. Following this analysis, our board of directors, based in part on the recommendations of our President and Chief Executive Officer, set the compensation levels for all of our named executive officers, including our President and Chief Executive Officer.
In March 2009, our board of directors approved a temporary reduction in annual base salaries in connection with a company-wide cost-reduction initiative, pursuant to which we reduced the base salaries of our named executive officers and other employees by approximately 50% and granted them additional stock options. This decision was designed to reduce the cash demands on our company in light of the then-existing macro-economic climate while continuing to provide our named executive officers with adequate incentives.
In September 2009, our compensation committee took on the responsibility of proposing compensation levels to our board of directors for adoption. The compensation committee began with a review of the primary aspects of our compensation programs for our named executive officers, including base salaries, performance bonuses and equity incentive targets. As part of this process, the compensation committee directly engaged Radford, a compensation consulting company, to provide compensation surveys and a general understanding of current compensation practices. The 2008 Radford Global Life Sciences Pre-IPO Survey compiled compensation data from global life sciences companies without publicly traded securities that have raised at least $80 million in invested capital, and the 2009 Radford Benchmark Survey compiled compensation data for technology companies with less than 200 employees and operations in the San Francisco Bay Area. The surveys provided by Radford
98
Table of Contents
reported statistics on the total compensation, position and responsibilities of executives. While our compensation committee reviewed the statistical compensation data and elements derived from this supplemental industry information, the surveys did not include, nor was our compensation committee aware of, the identity of any of the surveyed companies. As a result, our compensation committee did not benchmark executive compensation against any single company or an identifiable select group of companies. In November 2009, as a result of our compensation committee’s review, the compensation committee recommended, and our board of directors approved, adjustments to executive compensation.
In December 2009, our board of directors reviewed the equity holdings of our named executive officers in light of the significant dilutive effect of our Series D preferred stock financing. In order to offset our named executive officers for a portion of the dilutive effect of the financing and to continue to incentivize their efforts for our company, our board of directors approved a program to grant equity awards to our named executive officers, as well as the payment of cash bonuses to mitigate the tax effects of certain of those grants. See “— Long-Term Equity Incentives” below.
Following the completion of this offering, our compensation committee will take on the responsibility of determining and approving executive compensation for all of our named executive officers, other than our President and Chief Executive Officer, for whom our board of directors will retain primary authority regarding the determination and approval of compensation.
Base Salaries
In general, base salaries for our executive officers are initially established through arm’s-length negotiation at the time the executive is hired, taking into account such executive’s qualifications, experience and prior salary. Historically, base salaries of our named executive officers were approved and reviewed periodically by our President and Chief Executive Officer or, in the case of our President and Chief Executive Officer’s base salary, by our board of directors. In September 2009, our compensation committee took over responsibility for conducting reviews of the base salaries of our named executive officers and making adjustments based on the scope of the executive’s responsibilities, individual contribution, prior experience and sustained performance. Decisions regarding salary increases may take into account the named executive officer’s current salary and equity ownership. In making decisions regarding salary increases, we may also draw upon the experience of members of our board of directors or compensation committee with other companies and may also consider internal equity. Base salaries are also reviewed in the case of significant changes in responsibility. No formulaic base salary increases are provided to our named executive officers. This strategy is consistent with our intent of offering compensation that is cost-effective, competitive and linked to performance.
In April 2009, in response to the macro-economic climate and in order to preserve our cash resources, our board of directors temporarily reduced annual base salary compensation for Dr. Reid, Dr. Drmanac and Mr. Curson by over 50% from $240,000, $220,400 and $195,000 to $95,200, $89,908 and $83,050, respectively. Our named executive officers were granted additional stock options to partially offset the reduced salaries, as further described under “— Long-Term Equity Incentives” below.
In June 2009, our compensation committee determined, and the board of directors approved, the restoration of our named executive officers’ salaries to their previous levels in light of the anticipated improved cash position of the company from its Series D preferred stock financing and the outlook of the greater economy as a whole. In addition, the compensation committee reviewed a compensation survey provided by Radford which indicated that our named executive officers received base salaries at levels below the 25th percentile of executives in similar positions at the companies included in the surveys. In November 2009, upon recommendation of the compensation committee, our board of directors authorized increases to the annual base salary of Dr. Reid, Dr. Drmanac and Mr. Curson to $320,000, $250,000 and $230,000, respectively. After giving effect to these base salary increases, the base salaries of Dr. Reid and Mr. Curson remain below the 25th percentile of the companies that participate in the Radford compensation survey and Dr. Drmanac’s base salary is between the 25th and 50th percentile.
99
Table of Contents
Performance Bonuses
Historically, our named executive officers have not participated in a performance bonus program. Instead, we have relied on stock option grants to provide performance-based incentives to our named executive officers.
In November 2009, upon the recommendation of our compensation committee following its review of all aspects of our executive compensation program, our board of directors approved a target performance bonus amount for each of our named executive officers. For 2010, the annual target amount for Dr. Reid, Dr. Drmanac and Mr. Curson is $100,000, $70,000 and $70,000, respectively. Our compensation committee recommended the level of the target bonus amounts based on its review of the Radford compensation survey and based on the experience of the members of the compensation committee with similar bonus programs. In March 2010, our named executive officers each agreed to forgo these target bonus amounts and instead receive cash bonuses granted to offset taxes in connection with the stock grants described under “— Long-Term Equity Incentives” below.
Long-Term Equity Incentives
The goal of our long-term, equity-based incentive awards is to align the interests of our named executive officers with the interests of our stockholders by focusing our executives on long-term objectives. In addition, because vesting is based on continued employment, our equity-based incentives also encourage the retention of our named executive officers through the vesting period of the awards.
Our board of directors approves all equity grants to our employees, including our named executive officers. In determining the number of shares of our common stock to be subject to long-term equity incentives awarded to our named executive officers, our board of directors has taken into account a number of internal factors, such as the relative job scope, the value of existing long-term incentive awards, individual performance history, prior contributions to us and the size of prior grants. Historically, our board of directors has not referred to competitive market data in determining long-term equity incentive awards, although it has drawn upon the experience of its members with other companies. Based on these factors, our board of directors determines the size of the long-term equity incentives at levels it considers appropriate to create a meaningful opportunity for reward based on the creation of long-term stockholder value. Following the completion of this offering, we expect our compensation committee to oversee our long-term equity incentive program.
We use stock options to compensate our executive officers both in the form of initial grants in connection with the commencement of employment and periodic grants aimed at both rewarding exceptional performance and continuing to incentivize the executive officers. To date, there has been no set program for the award of these periodic grants, and our board of directors retains discretion to make stock option awards to employees at any time, including in connection with the promotion of an employee, to reward an employee, for retention purposes or in other circumstances. From time to time, our board of directors conducts a review of the stock option equity positions of those employees who may be nearing the end of the vesting periods of their outstanding options. As a part of this review, the board of directors will make grants based on considerations including, among other factors, the amount of vesting remaining for the particular employee’s stock options as well as any management recommendations regarding the employee’s performance or role in the company’s future operations.
The exercise price of each stock option grant is the fair market value of our common stock on the grant date, as determined by our board of directors. Initial stock options granted to our named executive officers typically vest over a four-year period as follows:
| § | 25% of the shares underlying the option vest on the first anniversary of the date of the vesting commencement date, which is typically the date of hire; and |
| § | the remainder of the shares underlying the option vest in equal monthly installments over the next 36 months. |
Our periodic grants will typically vest in equal monthly installments over four years from the vesting commencement date. We believe these vesting schedules appropriately encourage long-term employment with our company while allowing our executives to realize compensation in line with the value they have created for our stockholders.
100
Table of Contents
In November 2009, our board of directors made stock option grants to each of our named executive officers following the completion of our Series D preferred stock financing. Our board of directors granted Dr. Reid, Dr. Drmanac and Mr. Curson an option to purchase 3,090, 2,864 and 2,510 shares of our common stock, respectively. These option grants were intended to partially offset the reductions in the officers’ base salaries related to the company-wide cost reduction measures in April 2009. Our board of directors determined the number of shares for each of the named executive officers’ option grants by calculating the percentage reduction in base salary that named executive officer had experienced relative to all employees and applied that percentage to a predetermined option pool to derive the size of the option grant. These options had a per share exercise price equal to $1.50, which our board of directors determined equaled the per share fair market value of our common stock on the date of grant, and vest as to 1/48th of the total number of shares on each monthly anniversary of April 1, 2009.
In connection with our Series D preferred stock financing, Dr. Reid, Dr. Drmanac and Mr. Curson each experienced substantial dilution with respect to their common stock holdings and outstanding stock option grants. In December 2009, our board of directors implemented a plan to offset a portion of this dilution with a series of equity grants to our named executive officers. On December 28, 2009, our board of directors granted each of Dr. Reid, Dr. Drmanac and Mr. Curson an option to purchase 66,666 shares of our common stock. These options have a per share exercise price of $1.50, which our board of directors determined to be the per share fair market value of our common stock on the date of grant, are immediately exercisable and vest as to 1/48th of the total number of shares on each monthly anniversary of August 12, 2009, the completion date of our Series D preferred stock financing.
In February 2010, our board of directors approved additional equity grants to the executive officers to partially offset the dilutive effect of the Series D preferred stock financing. Dr. Reid, Dr. Drmanac and Mr. Curson were granted options to purchase 341,667, 218,501 and 59,001 shares of our common stock, respectively, for a per share exercise price equal to $1.50, which our board of directors determined to be the per share fair market value of our common stock on the date of grant. These stock options vest with respect to 1/48th of the total number of shares subject to the options on each monthly anniversary of August 12, 2009. In addition, in March 2010, Dr. Reid, Dr. Drmanac and Mr. Curson received 285,867, 395,333 and 105,333 fully vested shares of our common stock, respectively, to partially offset the dilutive effect of the Series D preferred stock financing. Our board of directors also approved cash bonuses to Dr. Reid, Dr. Drmanac and Mr. Curson in the amounts of $361,615, $500,087 and $133,244, respectively, to offset the incremental taxes incurred by our named executive officers in connection with the stock grants.
As a result of our board of directors’ assesment of the fair market value of our common stock following the Series D preferred stock financing, our board of directors approved a stock option modification in January 2010. The exercise price of all outstanding stock options granted before January 28, 2010 that were held by then-current employees and consultants and had a per share exercise price greater than $1.50 per share were modified to decrease the exercise price to $1.50 per share, which our board of directors determined to be the fair market value of our common stock on January 28, 2010. Other than a reduced exercise price, the terms and conditions of the stock options remained the same. Our board of directors determined that the modification was appropriate in light of the significant decrease in the fair market value of our common stock.
We do not have any formal security ownership requirements for our named executive officers. As a privately owned company, there has been no market for our common stock. Accordingly, in 2009, we had no program, plan or practice pertaining to the timing of stock option grants to executive officers coinciding with the release of material non-public information. We intend to adopt a formal policy regarding the timing of grants after the completion of this offering.
Severance Arrangements
Our board of directors has provided post-termination severance benefits to our named executive officers, as it determined that such benefits were necessary to retain our named executive officers. Our termination benefits are intended and designed to alleviate the financial impact of an involuntary termination and maintain a stable work
101
Table of Contents
environment. In determining the severance benefits to be payable pursuant to the agreements entered into with our named executive officers, we relied on the experience of the members of our board of directors to determine the level of severance benefits sufficient to retain our named executive officers. In connection with certain terminations of employment, our named executive officers may be entitled to receive severance payments and benefits pursuant to their respective severance agreements, as further described in “— Severance Arrangements” below.
We have routinely granted and will continue to grant our named executive officers stock options under our equity incentive plans. For a description of the change-in-control provisions in our equity incentive plans applicable to these stock options, see “— Employee Benefit and Stock Plans — 2010 Equity Incentive Award Plan” and “— 2006 Equity Incentive Plan” below. The estimated value of these benefits, along with the benefits payable upon the termination of employment of certain named executive officers, is set forth below in the section entitled “Potential Payments Upon Termination or Change-in-Control.”
Employee Benefits
We provide standard employee benefits to our full-time employees in the United States, including our named executive officers, including health, disability and life insurance and a 401(k) plan, as a means of attracting and retaining our employees.
Tax Considerations
Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, generally disallows a tax deduction for compensation in excess of $1.0 million paid to our Chief Executive Officer and our Chief Scientific Officer. Qualifying performance-based compensation is not subject to the deduction limitation if specified requirements are met. We generally intend to structure the performance-based portion of our executive compensation, when feasible, to comply with exemptions in Section 162(m) so that the compensation remains tax deductible to us. However, our board of directors may, in its judgment, authorize compensation payments that do not comply with the exemptions in Section 162(m) when it believes that such payments are appropriate to attract and retain executive talent.
2009 Summary Compensation Table
The following table summarizes the compensation that we paid to our Chief Executive Officer, Chief Financial Officer and Chief Scientific Officer, who were our only executive officers during the year ended December 31, 2009. We refer to these officers in this prospectus as our named executive officers.
| Name and principal position | Year | Salary ($) |
Option ($)(1) |
All other ($) |
Total ($) | |||||||||
| Clifford A. Reid, Ph.D. |
2009 | $ | 215,783 | $ | 96,711 | $ | 5,400 | $ | 317,894 | |||||
| President and Chief Executive Officer |
||||||||||||||
| Robert J. Curson(2) |
2009 | 171,496 | 96,005 | 5,400 | 272,901 | |||||||||
| Chief Financial Officer |
||||||||||||||
| Radoje Drmanac, Ph.D. |
2009 | 191,360 | 96,436 | 5,400 | 293,196 | |||||||||
| Chief Scientific Officer |
||||||||||||||
| (1) | Amounts represent the grant date fair value for stock options granted in fiscal 2009 calculated in accordance with ASC Topic 718 and exclude the impact of estimated forfeitures related to service-based vesting conditions. See Note 10 to our financial statements included in this prospectus for a discussion of assumptions made in determining the grant date fair value. |
| (2) | Mr. Curson served as our Chief Financial Officer until May 2010. |
Grants of Plan-based Awards in 2009
All options granted to our named executive officers in 2009 were incentive stock options, to the extent permissible under the Code. The exercise price per share of each option granted to our named executive officers
102
Table of Contents
in 2009 was determined by our board of directors to be equal to the fair market value of our common stock on the date of the grant. All options identified below were granted under our 2006 Equity Incentive Plan as described in the section entitled “Employee Benefit and Stock Plans — 2006 Equity Incentive Plan.”
The following table shows information regarding grants of equity awards during the year ended December 31, 2009 to each of our named executive officers.
| Name | Grant date | Board approval date |
All other option (#) |
Exercise or base price of option awards ($/share) |
Grant date fair value of stock and option awards ($) | |||||||
| Clifford A. Reid, Ph.D. |
11/13/2009 | 11/6/2009 | 3,090 | $ | 1.50 | $ | 3,151 | |||||
| 12/28/2009 | 12/28/2009 | 66,666 | 1.50 | 78,073 | ||||||||
| Robert J. Curson |
11/13/2009 | 11/6/2009 | 2,510 | 1.50 | 2,550 | |||||||
| 12/28/2009 | 12/28/2009 | 66,666 | 1.50 | 78,073 | ||||||||
| Radoje Drmanac, Ph.D. |
11/13/2009 | 11/6/2009 | 2,864 | 1.50 | 2,915 | |||||||
| 12/28/2009 | 12/28/2009 | 66,666 | 1.50 | 78,073 | ||||||||
Narrative to Summary Compensation Table and Grants of Plan-based Award Table
Employment Agreements, Offer Letters and Arrangements
We do not currently have employment agreements or offer letter agreements with any of our named executive officers.
Equity Compensation
As described under “— Compensation Discussion and Analysis” above, the compensation committee approved equity compensation awards in the form of stock options to each of our named executive officers in November and December 2009. For more information regarding the equity compensation awards and our equity award practices, please see the section titled “— Compensation Discussion and Analysis — Long-Term Equity Incentives.” In addition, the named executive officers’ equity compensation awards may, under certain circumstances, be subject to accelerated vesting in the event that a named executive officer is terminated or in the event of a change-in-control. For more information regarding the accelerated vesting provisions and treatment of the equity compensation awards in the event a named executive officer is terminated or a change-in-control of the company, see the sections titled “— Severance Arrangements,” “— Potential Payments upon Termination or Change-in-Control” and “— Employee Benefit and Stock Plans” below.
Other Benefits
For a description of the other elements of our executive compensation program, see the section titled “— Compensation Discussion and Analysis — Employee Benefits.”
103
Table of Contents
Outstanding Equity Awards at 2009 Fiscal Year-End
The following table shows grants of stock options outstanding on December 31, 2009, the last day of last completed fiscal year, to each of our named executive officers. None of our named executive officers have received grants of unvested stock awards.
| Option Awards(1) | ||||||||||
| Name | Number of securities underlying unexercised options (#) exercisable |
Number of securities underlying unexercised options (#) unexercisable |
Option exercise price ($) |
Option expiration date | ||||||
| Clifford A. Reid, Ph.D. |
515 | (2) | 2,575 | $ | 1.50 | 11/12/2019 | ||||
| 66,666 | (3) | — | 1.50 | 12/27/2019 | ||||||
| Robert J. Curson |
418 | (2) | 2,092 | 1.50 | 11/12/2019 | |||||
| 66,666 | (3) | — | 1.50 | 12/27/2019 | ||||||
| Radoje Drmanac, Ph.D. |
477 | (2) | 2,387 | 1.50 | 11/12/2019 | |||||
| 66,666 | (3) | — | 1.50 | 12/27/2019 | ||||||
| (1) | Each stock option was granted pursuant to our 2006 Equity Incentive Plan. The vesting schedule and exercisability of each stock option is described in the footnotes below. |
| (2) | This award vests over a four-year period as to 1/48th of the shares subject to the stock option on a monthly basis following the vesting commencement date, April 1, 2009. |
| (3) | This award vests over a four-year period as to 1/48th of the shares subject to the stock option on a monthly basis following the vesting commencement date, August 12, 2009. This award contains an early exercise provision. |
Option Exercises and Stock Vested in 2009
None of our named executive officers exercised stock options during 2009 and none of our named executive officers hold stock awards.
Pension Benefits
We do not maintain any defined benefit pension plans.
Nonqualified Deferred Compensation
We do not maintain any nonqualified deferred compensation plans.
Severance Arrangements
In March 2006, we entered into severance arrangements with each of our named executive officers. These arrangements set forth the terms of each named executive officer’s severance in the event of his termination of employment under specified circumstances. Under the arrangements, if a named executive officer’s employment is terminated at any time without cause or he experiences a constructive termination (as each term is defined in his severance agreement), the company will provide continuation of his base salary for six months following the termination date, as well as six months’ vesting acceleration for stock options and restricted stock. In addition, severance benefits would include reimbursement for group health continuation coverage premiums for each named executive officer and his eligible dependents under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended for six months.
However, if the termination occurs within 12 months following a change-in-control, all stock options, restricted stock and other equity awards outstanding automatically vest in full and become fully exercisable. A named executive officer must provide a general release of claims against our company in order to be eligible for any severance payments.
104
Table of Contents
Potential Payments Upon Termination or Change-in-Control
The following table illustrates the potential payments to each of our named executive officers in connection with:
| § | his termination without cause outside the context of a change-in-control of us, assuming such termination occurred on December 31, 2009; |
| § | his termination without cause, or constructive termination, within 12 months following a change-in-control of us, assuming such termination in connection with a change-in-control of us occurred on December 31, 2009 and his options were assumed or substituted by the acquiring entity; and |
| § | the acceleration of his equity awards in connection with a change-in-control of us, where the potential acquiring entity does not assume or substitute the options held by him and his employment is continued with us and/or the acquiring entity, assuming such change-in-control occurred on December 31, 2009. |
| Potential payments in connection with: | ||||||||||||||
| Name | Type of benefit | Termination without cause |
Termination without cause or within 12 months following a change-in-control |
Equity awards are not assumed or substituted in connection with a change-in-control |
||||||||||
| Clifford A. Reid, Ph.D. |
Salary | $ | 160,000 | (1) | $ | 160,000 | (1) | $ | — | |||||
| Benefits | 8,791 | (2) | 8,791 | (2) | — | |||||||||
| Equity Award Acceleration | (3) | (4) | (4) | |||||||||||
| Total |
||||||||||||||
| Robert J. Curson |
Salary | 115,000 | (1) | 115,000 | (1) | — | ||||||||
| Benefits | 971 | (2) | 971 | (2) | — | |||||||||
| Equity Award Acceleration | (3) | (4) | (4) | |||||||||||
| Total |
||||||||||||||
| Radoje Drmanac, Ph.D. |
Salary | 125,000 | (1) | 125,000 | (1) | — | ||||||||
| Benefits | 7,932 | (2) | 7,932 | (2) | — | |||||||||
| Equity Award Acceleration | (3) | (4) | (4) | |||||||||||
| Total |
||||||||||||||
| (1) | Represents six months’ of base salary. See discussion under “— Severance Arrangements.” |
| (2) | Represents six months’ of health care benefits. See discussion under “— Severance Arrangements.” |
| (3) | Represents the value of six months of vesting acceleration of the unvested option awards subject to acceleration, calculated assuming a price per share of $ (the midpoint of the price range set forth on the cover page of this prospectus) minus the exercise price. |
| (4) | Represents the value of the vesting acceleration of 100% of the then-unvested option awards subject to acceleration, calculated assuming a price per share of $ (the midpoint of the price range set forth on the cover page of this prospectus) minus the exercise price. |
Risk Analysis of Our Compensation Plans
The compensation committee has reviewed our compensation policies generally applicable to our employees and believes that our policies do not encourage excessive and unnecessary risk-taking, and that the level of risk that they do encourage is not reasonably likely to have a material adverse effect on us. Our compensation policies and programs are designed to encourage our employees to remain focused on both the short- and long-term goals of us. For example, while our cash bonus plans are intended to measure performance on an annual basis, our equity awards typically vest over a number of years, which we believe encourages our employees to focus on sustained stockholder appreciation and limits the potential value of excessive risk-taking. The committee believes that the mix of long-term equity incentive, short-term cash incentive bonus and base salary appropriately balances both the short-and long-term performance goals of us without encouraging excessive risk-related behavior. While the compensation committee regularly evaluates its compensation programs, the committee believes that its current balance of incentives both adequately compensates its employees and does not promote excessive risk taking.
Confidentiality Information, Secrecy and Invention Agreements
Each of our named executive officers has entered into a standard form agreement with respect to confidential information, secrecy and inventions. Among other things, these agreements obligate each named executive
105
Table of Contents
officer to refrain from disclosing any of our proprietary information received during the course of employment and, with some exceptions, to assign to us any inventions conceived or developed during the course of employment.
Employee Benefit and Stock Plans
2010 Equity Incentive Award Plan
We intend to adopt a 2010 Equity Incentive Award Plan, or the 2010 Plan, which will become effective immediately prior to the consummation of this offering. The principal purpose of the 2010 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The 2010 Plan is also designed to permit us to make cash-based awards and equity-based awards intended to qualify as “performance-based compensation” under Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code. The principal features of the 2010 Plan are summarized below. This summary is qualified in its entirety by reference to the text of the 2010 Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Share Reserve. Under the 2010 Plan, shares of our common stock will be initially reserved for issuance pursuant to a variety of stock-based compensation awards, including stock options, stock appreciation rights, or SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, dividend equivalent awards, stock payment awards and performance awards and other stock-based awards, plus the number of shares remaining available for future awards under our 2006 Equity Incentive Plan as of the completion of this offering. The number of shares initially reserved for issuance or transfer pursuant to awards under the 2010 Plan will be increased by (i) the number of shares represented by awards outstanding under our 2006 Equity Incentive Plan that are forfeited or lapse unexercised and which, following the effective date of the 2010 Plan, are not issued under the 2006 Equity Incentive Plan and (ii) an annual increase on the first day of each fiscal year, beginning in 2011 and ending in 2020, equal to the least of:
| § | shares; |
| § | % of the shares of our common stock outstanding (on an as-converted basis) on the last day of the immediately preceding fiscal year; and |
| § | such smaller number of shares of stock as determined by our board of directors. |
However, no more than shares of stock may be issued upon the exercise of incentive stock options.
The following counting provisions will be in effect for the share reserve under the 2010 Plan:
| § | to the extent that an award terminates, expires or lapses for any reason or an award is settled in cash without the delivery of shares, any shares subject to the award at such time will be available for future grants under the 2010 Plan; |
| § | to the extent shares are tendered or withheld to satisfy the grant, exercise price or tax withholding obligation with respect to any award under the 2010 Plan, such tendered or withheld shares will be available for future grants under the 2010 Plan; |
| § | to the extent that shares of our common stock granted under the 2010 Plan are repurchased by, and returned to, us prior to vesting, such shares will be available for future grants under the 2010 Plan; |
| § | the payment of dividend equivalents in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2010 Plan; and |
| § | to the extent permitted by applicable law or any exchange rule, shares issued in assumption of, or in substitution for, any outstanding awards of any entity acquired in any form of combination by us or any of our subsidiaries will not be counted against the shares available for issuance under the 2010 Plan. |
Administration. The compensation committee of our board of directors will administer the 2010 Plan unless our board of directors assumes authority for administration. The compensation committee must consist of at least two
106
Table of Contents
members of our board of directors, each of whom is intended to qualify as an “outside director,” within the meaning of Section 162(m) of the Code, a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act and an “independent director” within the meaning of the rules of The NASDAQ Global Market, or other principal securities market on which shares of our common stock are traded. The 2010 Plan provides that the compensation committee may delegate its authority to grant awards to employees other than executive officers and certain senior executives of the company to a committee consisting of one or more members of our board of directors or one or more of our officers. However, our compensation committee charter prohibits such delegation in the case of awards to employees at or above the level of vice president, and the equity awards policy we adopted in 2010 calls for the compensation committee to approve all equity awards, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2010 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2010 Plan. The administrator is also authorized to adopt, amend or rescind rules relating to administration of the 2010 Plan. Our board of directors may at any time remove the compensation committee as the administrator and revest in itself the authority to administer the 2010 Plan. The full board of directors will administer the 2010 Plan with respect to awards to non-employee directors.
Eligibility. Options, SARs, restricted stock and all other stock-based and cash-based awards under the 2010 Plan may be granted to individuals who are then our officers, employees or consultants or are the officers, employees or consultants of certain of our subsidiaries. Such awards also may be granted to our directors. Only employees of us or certain of our subsidiaries may be granted incentive stock options, or ISOs.
Awards. The 2010 Plan provides that the administrator may grant or issue stock options, SARs, restricted stock, restricted stock units, deferred stock, dividend equivalents, performance awards, stock payments and other stock-based and cash-based awards, or any combination thereof. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| § | Nonqualified Stock Options, or NQSOs, will provide for the right to purchase shares of our common stock at a specified price, which may not be less than fair market value on the date of grant, and usually will become exercisable (at the discretion of the administrator) in one or more installments after the grant date, subject to the participant’s continued employment or service with us and/or subject to the satisfaction of corporate performance targets and individual performance targets established by the administrator. NQSOs may be granted for any term specified by the administrator that does not exceed ten years. |
| § | Incentive Stock Options, or ISOs, will be designed in a manner intended to comply with the provisions of Section 422 of the Code and will be subject to specified restrictions contained in the Code. Among such restrictions, ISOs must have an exercise price of not less than the fair market value per share of common stock on the date of grant, may only be granted to employees and must not be exercisable after a period of ten years measured from the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our capital stock, the 2010 Plan provides that the exercise price must be at least 110% of the fair market value per share of common stock on the date of grant and the ISO must not be exercisable after a period of five years measured from the date of grant. |
| § | Restricted Stock may be granted to any eligible individual and made subject to such restrictions as may be determined by the administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until the restrictions are removed or expire. Purchasers of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse. However, extraordinary dividends will generally be placed in escrow, and will not be released until the restrictions are removed or expire. |
107
Table of Contents
| § | Restricted Stock Units may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| § | Deferred Stock Awards represent the right to receive shares of our common stock on a future date. Deferred stock may not be sold or otherwise hypothecated or transferred until issued. Deferred stock will not be issued until the deferred stock award has vested, and recipients of deferred stock generally will have no voting or dividend rights prior to the time when the vesting conditions are satisfied and the shares are issued. Deferred stock awards generally will be forfeited, and the underlying shares of deferred stock will not be issued, if the applicable vesting conditions and other restrictions are not met. |
| § | Stock Appreciation Rights, or SARs, may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our common stock over a set exercise price. The exercise price of any SAR granted under the 2010 Plan must be at least 100% of the fair market value per share of our common stock on the date of grant. Except as required by Section 162(m) of the Code with respect to a SAR intended to qualify as performance-based compensation under Section 162(m) of the Code, there are no restrictions specified in the 2010 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements. SARs under the 2010 Plan will be settled in cash or shares of our common stock, or in a combination of both, at the election of the administrator. |
| § | Dividend Equivalents represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by the award. Dividend equivalents may be settled in cash or shares and at such times as determined by the compensation committee or board of directors, as applicable. |
| § | Performance Awards may be granted by the administrator on an individual or group basis. Generally, these awards will be based upon specific performance targets and may be paid in cash or in common stock or in a combination of both. Performance awards may include “phantom” stock awards that provide for payments based upon the value of our common stock. Performance awards may also include bonuses that may be granted by the administrator on an individual or group basis and which may be payable in cash or in common stock or in a combination of both. |
| § | Stock Payments may be authorized by the administrator in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation or other arrangement in lieu of all or any part of compensation, including bonuses, that would otherwise be payable in cash to the employee, consultant or non-employee director. |
Change-in-Control. If a change-in-control occurs where the acquiror does not assume or replace awards granted under the 2010 Plan prior to the consummation of such transaction, then such awards will be subject to accelerated vesting such that 100% of such awards will become vested and exercisable or payable, as applicable. In addition, the administrator will also have complete discretion to structure one or more awards under the 2010 Plan to provide that such awards will become vested and exercisable or payable on an accelerated basis if such awards are assumed or replaced with equivalent awards but the individual’s service with us or the acquiring entity is subsequently terminated within a designated period following the change in control event. The administrator may also make appropriate adjustments to awards under the 2010 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. Under the 2010 Plan, a change in control is generally defined as:
| § | the transfer or exchange, in a single or series of related transactions, by our stockholders of more than 50% of our voting stock to a person or group; |
108
Table of Contents
| § | a change in the composition of our board of directors over a two-year period such that 50% or more of the members of the board were elected through one or more contested elections; |
| § | a merger, consolidation, reorganization or business combination in which we are involved, directly or indirectly, other than a merger, consolidation, reorganization or business combination which results in our outstanding voting securities immediately before the transaction continuing to represent a majority of the voting power of the acquiring company’s outstanding voting securities and after which no person or group beneficially owns 50% or more of the outstanding voting securities of the surviving entity immediately after the transaction; |
| § | the sale, exchange, or transfer of all or substantially all of our assets; or |
| § | stockholder approval of our liquidation or dissolution. |
Adjustments of Awards. In the event of any stock dividend, stock split, combination or exchange of shares, merger, consolidation, spin-off, recapitalization, distribution of our assets to stockholders (other than normal cash dividends) or any other corporate event affecting the number of outstanding shares of our common stock or the share price of our common stock that would require adjustments to the 2010 Plan or any awards under the 2010 Plan in order to prevent the dilution or enlargement of the potential benefits intended to be made available thereunder, the administrator will make appropriate, proportionate adjustments to:
| § | the aggregate number and type of shares subject to the 2010 Plan; |
| § | the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); and |
| § | the grant or exercise price per share of any outstanding awards under the 2010 Plan. |
Amendment and Termination. Our board of directors or the committee (with board approval) may terminate, amend or modify the 2010 Plan at any time and from time to time. However, we must generally obtain stockholder approval to:
| § | increase the number of shares available under the 2010 Plan (other than in connection with certain corporate events, as described above); |
| § | grant options with an exercise price that is below 100% of the fair market value per share of our common stock on the grant date; |
| § | extend the exercise period for an option beyond ten years from the date of grant; or |
| § | the extent required by applicable law, rule or regulation (including any applicable stock exchange rule). |
Notwithstanding the foregoing, an option may be amended to reduce the per share exercise price below the per share exercise price of such option on the grant date, and options may be granted in exchange for, or in connection with, the cancellation or surrender of options having a higher per share exercise price, without receiving additional stockholder approval.
Expiration Date. The 2010 Plan will expire on, and no option or other award may be granted pursuant to the 2010 Plan after, the tenth anniversary of the effective date of the 2010 Plan. Any award that is outstanding on the expiration date of the 2010 Plan will remain in force according to the terms of the 2010 Plan and the applicable award agreement.
Securities Laws. The 2010 Plan is intended to conform to certain provisions of the Securities Act and the Exchange Act and related regulations and rules promulgated by the SEC thereunder, including without limitation, Rule 16b-3. The 2010 Plan will be administered, and options will be granted and may be exercised, only in such a manner as to conform to such laws, rules and regulations.
Section 409A of the Code. Certain awards under the 2010 Plan may be considered “nonqualified deferred compensation” for purposes of Section 409A of the Code, which imposes certain additional requirements
109
Table of Contents
regarding the payment of deferred compensation. Generally, if at any time during a taxable year a nonqualified deferred compensation plan fails to meet the requirements of Section 409A, or is not operated in accordance with those requirements, all amounts deferred under the 2010 Plan and all other equity incentive plans for the taxable year and all preceding taxable years by any participant with respect to whom the failure relates are includible in gross income for the taxable year to the extent not subject to a substantial risk of forfeiture and not previously included in gross income. If a deferred amount is required to be included in income under Section 409A, the amount also is subject to interest and an additional income tax. The interest imposed is equal to the interest at the underpayment rate plus one percentage point imposed on the underpayments that would have occurred had the compensation been includible in income for the taxable year when first deferred, or, if later, when not subject to a substantial risk of forfeiture. The additional U.S. federal income tax is equal to 20% of the compensation required to be included in gross income. In addition, certain states, including California, have laws similar to Section 409A, which impose additional state penalty taxes on such compensation.
Section 162(m) of the Code. In general, under Section 162(m) of the Code, income tax deductions of publicly held corporations may be limited to the extent total compensation (including, but not limited to, base salary, annual bonus and income attributable to stock option exercises and other nonqualified benefits) for certain executive officers exceeds $1,000,000 (less the amount of any “excess parachute payments” as defined in Section 280G of the Code) in any taxable year of the corporation. However, under Section 162(m), the deduction limit does not apply to certain “performance-based compensation” established by an independent compensation committee that is adequately disclosed to and approved by stockholders. In particular, stock options and SARs will satisfy the “performance-based compensation” exception if the awards are made by a qualifying compensation committee, the 2010 Plan sets the maximum number of shares that can be granted to any person within a specified period, and the compensation is based solely on an increase in the stock price after the grant date. Specifically, the option exercise price must be equal to or greater than the fair market value of the stock subject to the award on the grant date. Under a Section 162(m) transition rule for compensation plans of corporations which are privately held and which become publicly held in an initial public offering, the 2010 Plan will not be subject to Section 162(m) until the earlier of:
| § | a material modification of the 2010 Plan; |
| § | the issuance of all of the shares of our common stock reserved for issuance under the 2010 Plan; |
| § | the expiration of the 2010 Plan; and |
| § | the first meeting of our stockholders at which members of our board of directors are to be elected that occurs after the close of the third calendar year following the calendar year in which our initial public offering occurs. |
After the transition date, rights or awards granted under the 2010 Plan, other than options and SARs, will not qualify as “performance-based compensation” for purposes of Section 162(m) unless such rights or awards are granted or vest upon pre-established objective performance goals, the material terms of which are disclosed to and approved by our stockholders. Thus, after the transition date, we expect that such other rights or awards under the plan will not constitute performance-based compensation for purposes of Section 162(m).
We intend to file with the SEC a registration statement on Form S-8 covering the issuance of shares of our common stock under the 2010 Plan.
2006 Equity Incentive Plan
Our board of directors adopted, and our stockholders approved, the 2006 Equity Incentive Plan in March 2006. The 2006 Equity Incentive Plan provides for the grant of ISOs, NQSOs and stock purchase rights. As of June 30, 2010, options to purchase 2,251,093 shares of our common stock at a weighted average exercise price per share of $1.50 remained outstanding under the 2006 Equity Incentive Plan. No stock purchase rights have been granted under the 2006 Equity Incentive Plan. As of June 30, 2010, options to purchase 1,215,728 shares of our common stock remained available for future issuance pursuant to awards granted under the 2006 Equity Incentive Plan. Following the completion of this offering, no further awards will be granted under the 2006 Equity Incentive Plan. However, all outstanding awards will continue to be governed by their existing terms.
110
Table of Contents
Administration. Our board of directors, or a committee thereof appointed by our board of directors, has the authority to administer the 2006 Equity Incentive Plan and the awards granted under it.
Stock Options. The 2006 Equity Incentive Plan provides for the grant of ISOs under the federal tax laws or NQSOs. ISOs may be granted only to employees. NQSOs and stock purchase rights may be granted to employees, directors or consultants. The exercise price of ISOs granted to employees who at the time of grant own stock representing more than 10% of the voting power of all classes of our common stock may not be less than 110% of the fair market value per share of our common stock on the date of grant, and the exercise price of ISOs granted to any other employees may not be less than 100% of the fair market value per share of our common stock on the date of grant. The exercise price of NQSOs to employees, directors or consultants who at the time of grant own stock representing more than 10% of the voting power of all classes of our common stock may not be less than 110% of the fair market value per share of our common stock on the date of grant, and the exercise price of nonstatutory stock options to all other employees, directors or consultants may not be less than 100% of the fair market value per share of our common stock on the date of grant. Shares subject to options under the 2006 Equity Incentive Plan generally vest in a series of installments over an optionee’s period of service, with a minimum vesting rate of at least 20% per year over five years from the date of grant, except with respect to options granted to officers, directors and consultants.
In general, the maximum term of options granted is ten years. The maximum term of options granted to an optionee who owns stock representing more than 10% of the voting power of all classes of our common stock is five years. If an optionee’s service relationship with us terminates other than by disability or death, the optionee may exercise the vested portion of any option in such period of time as specified in the optionee’s option agreement, but in no event will such period be less than 30 days following the termination of service. If an optionee’s service relationship with us terminates by disability or death, the optionee, or the optionee’s designated beneficiary, as applicable, may exercise the vested portion of any option in such period of time as specified in the optionee’s option agreement, but in no event will such period be less than 12 months following the termination of service. Shares of common stock representing any unvested portion underlying the option on the date of termination will immediately cease to be issuable and will become available for future issuance under the 2006 Equity Incentive Plan. If, after termination, the optionee does not exercise the option within the time period specified, the option will terminate and the shares of common stock covered by such option will become available for future issuance under the 2006 Equity Incentive Plan.
Stock Purchase Rights. The 2006 Equity Incentive Plan provides that we may issue stock purchase rights alone, in addition to or in tandem with options granted under the 2006 Equity Incentive Plan and/or cash awards made outside of the 2006 Equity Incentive Plan. Each stock purchase right will be governed by a restricted stock purchase agreement. We will have the right to repurchase shares of common stock acquired by the purchaser upon exercise of a stock purchase right upon the termination of the purchaser’s status as an employee, director or consultant for any reason. The repurchase price for shares acquired by the purchaser upon exercise of a stock purchase right will be the original price paid by the purchaser. Except with respect to shares purchased by officers, directors and consultants, the repurchase option lapses at a rate of at least 20% per year over five years from the date of purchase. However, this lapsing does not apply to stock purchase rights granted to individuals who are tax residents of Germany. Once the stock purchase right is exercised, the purchaser will have rights equivalent to those of our other stockholders.
Corporate Transactions. In the event of a proposed dissolution or liquidation, the administrator of the 2006 Equity Incentive Plan has the discretion to take one or more of the following actions:
| § | provide that any option or stock purchase right be made exercisable until ten days prior to such transaction; and |
| § | provide that our option to repurchase any shares purchased upon exercise of an option or stock purchase right will lapse as to all such shares. |
To the extent options and stock purchase rights have not been previously exercised, all such options and stock purchase rights will terminate immediately prior to the consummation of the proposed transaction.
111
Table of Contents
In the event of certain corporate transactions, the administrator of the 2006 Equity Incentive Plan will adjust the number of shares of common stock that may be delivered under the 2006 Equity Incentive Plan and/or the number, class and price of shares of common stock covered by each outstanding option or stock purchase right.
Change-in-Control. If we undergo a change-in-control, and any surviving corporation does not assume options or stock purchase rights under the 2006 Equity Incentive Plan, or substitute an equivalent option of the successor corporation or a parent or subsidiary of the successor corporation, then the vesting of options or stock purchase rights held by participants in the 2006 Equity Incentive Plan will be accelerated and the options or stock purchase rights will become fully exercisable during the 15-day period specified below. The holder of such options or stock purchase rights not assumed or substituted will be notified by the 2006 Equity Incentive Plan administrator that the option or stock purchase right is fully exercisable for a period of 15 days from the date of such notice and will be terminated if not exercised within such 15 day period.
401(k) Plan
We maintain a defined contribution employee retirement plan, or 401(k) Plan, for our employees. Our executive officers are also eligible to participate in the 401(k) Plan on the same basis as our other employees. The 401(k) Plan provides that each participant may contribute up to the statutory limit, which is $16,500 for calendar year 2010. Participants that are 50 years or older can also make “catch-up” contributions, which in calendar year 2010 may be up to an additional $5,500 above the statutory limit. In 2009, we did not make any contributions to the 401(k) Plan on behalf of eligible employees. The 401(k) Plan is intended to qualify under Section 401 of the Code so that contributions by employees to the 401(k) Plan, and income earned on the 401(k) Plan contributions, are not taxable to employees until withdrawn from the 401(k) Plan. The trustees under the 401(k) Plan may, at the direction of each participant, invest the 401(k) Plan employee salary deferrals in selected investment options.
Limitation on Liability and Indemnification Matters
Our amended and restated certificate of incorporation, which will become effective immediately prior to the consummation of this offering, contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| § | any breach of the director’s duty of loyalty to us or our stockholders; |
| § | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| § | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| § | any transaction from which the director derived an improper personal benefit. |
Our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective immediately prior to the consummation of this offering provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our amended and restated bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under Delaware law. We have entered and expect to continue to enter into agreements to indemnify our directors, executive officers and other employees as determined by our board of directors. With specified exceptions, these agreements provide for indemnification for related expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. We also maintain directors’ and officers’ liability insurance.
112
Table of Contents
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and our stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or officer. The director or officer may amend or terminate the plan in limited circumstances. Our directors and executive officers may also buy or sell additional shares of our common stock outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
113
Table of Contents
Certain Relationships and Related Party Transactions
We describe below each transaction, since January 1, 2007, to which we were a party or will be a party, in which:
| § | the amounts involved exceeded or will exceed $120,000; and |
| § | a director, executive officer, holder or group of holders known to us to beneficially own more than 5% of any class of our voting securities or any member of their immediate family had or will have a direct or indirect material interest in the transaction. |
The share numbers and purchase prices disclosed below have been adjusted to reflect the 30:1 reverse stock split that occurred on November 13, 2009.
Preferred Stock Issuances
2010 Bridge Financing
In April, May, June and August 2010, we sold convertible promissory notes, or the 2010 Notes, to certain of our existing investors for an aggregate purchase price of $22.2 million. The 2010 Notes accrued interest at a rate of 8% per annum and had a maturity date of the earliest of (i) a corporate reorganization as defined in the 2010 Notes, (ii) the closing of the initial public offering of our stock, (iii) an event of default pursuant to the terms of the 2010 Notes or (iv) April 12, 2011. In August 2010, in connection with our Series E preferred stock financing described below, the full principal amount of the 2010 Notes, along with accrued but unpaid interest thereon of $364,266, were automatically converted into an aggregate of 2,990,355 shares of our Series E preferred stock at a conversion price of $7.56 per share. In addition, each investor who purchased 2010 Notes also received warrants to purchase a number of shares of our common stock equal to (a) the product of (i) 5% of the principal amount of 2010 Notes purchased by such investor and (ii) the number of months between the date of issuance of the warrant and the date of our next financing (up to five months), divided by (b) $1.50, resulting in the issuance of warrants to purchase an aggregate of 1,848,849 shares of common stock.
The table below sets forth the participation in the sale of the 2010 Notes by our directors, executive officers and 5% stockholders and their affiliates.
| Name | Principal amount of 2010 Notes purchased |
Number of shares of common stock underlying warrants received in connection with purchase of 2010 Notes | |||
| Caduceus Private Investments III, LP(1)(2) |
$ | 3,291,190 | 294,379 | ||
| Essex Woodlands Health Ventures VIII, L.P.(3)(4) |
$ | 3,291,190 | 294,380 | ||
| C. Thomas Caskey, M.D.(4) |
$ | 4,786 | 252 | ||
| Enterprise Partners VI, L.P.(5)(6) |
$ | 2,144,834 | 191,844 | ||
| Highland Crusader Offshore Partners, L.P.(7) |
$ | 2,049,630 | 176,496 | ||
| OVP Venture Partners VI, L.P.(8)(9) |
$ | 2,144,820 | 191,841 | ||
| Prospect Venture Partners III, L.P.(10) |
$ | 2,078,348 | 185,897 | ||
| SCV-CG, LLC |
$ | 7,000,000 | 501,667 | ||
| (1) | Includes $31,050 in 2010 Notes and warrants to purchase 2,777 shares of common stock purchased by OrbiMed Associates III, LP, an affiliate of Caduceus Private Investments III, LP. |
| (2) | Carl L. Gordon, Ph.D., is one of our directors and is a partner of OrbiMed Advisors, LLC. |
| (3) | Includes $215,050 in 2010 Notes and warrants to purchase 19,235 shares of common stock purchased by Essex Woodlands Health Ventures Fund VIII – A, L.P. and $93,500 in 2010 Notes and warrants to purchase 8,364 shares of common stock purchased by Essex Woodlands Health Ventures Fund—B, L.P., each of which is an affiliate of Essex Woodlands Health Ventures VIII, L.P. |
| (4) | C. Thomas Caskey, M.D., is one of our directors and is a partner in Essex Woodlands Health Ventures. |
| (5) | Includes $74,417 in 2010 Notes and warrants to purchase 7,856 shares of common stock purchased by Enterprise Partners Management, LLC and $690,167 in 2010 Notes and warrants to purchase 55,808 shares of common stock purchased by Enterprise Partners V, L.P., each of which is an affiliate of Enterprise Partners VI, L.P. |
| (6) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (7) | Includes $600,672 in 2010 Notes and warrants to purchase 51,725 shares of common stock purchased by Highland Credit Opportunities CDO, L.P., an affiliate of Highland Crusader Offshore Partners, L.P. Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
114
Table of Contents
| (8) | Includes $35,418 in 2010 Notes and warrants to purchase 3,167 shares of common stock purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (9) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (10) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
Issuance of Series E Preferred Stock and Warrants to Purchase Common Stock
In August and September 2010, we issued 5,274,871 shares of Series E preferred stock at a price of $7.56 per share for gross proceeds of approximately $39.9 million. The table below sets forth the number of shares of Series E preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates, and includes the shares of Series E preferred stock issued in connection with the conversion of the 2010 Notes, including the consideration paid for such shares by cancellation of the 2010 Notes as disclosed above.
| Name | Number of Shares of Series E Preferred Stock |
Aggregate Purchase | |||
| Caduceus Private Investments III, LP(1)(2) |
936,412 | $ | 7,079,275 | ||
| Essex Woodlands Health Ventures VIII, L.P.(3)(4) |
936,411 | $ | 7,079,267 | ||
| C. Thomas Caskey, M.D.(4) |
1,345 | $ | 10,168 | ||
| Enterprise Partners VI, L.P.(5)(6) |
610,250 | $ | 4,613,490 | ||
| Highland Crusader Offshore Partners, L.P.(7) |
582,983 | $ | 4,407,351 | ||
| OVP Venture Partners VI, L.P.(8)(9) |
610,246 | $ | 4,613,460 | ||
| Prospect Venture Partners III, L.P.(10) |
591,333 | $ | 4,470,477 | ||
| SCV-CG, LLC |
938,957 | $ | 7,098,515 | ||
| (1) | Includes 8,834 shares held by OrbiMed Associates III, LP, an affiliate of Caduceus Private Investments III, LP. |
| (2) | Carl L. Gordon, Ph.D., is one of our directors and is a partner of OrbiMed Advisors, LLC. |
| (3) | Includes 61,186 shares purchased by Essex Woodlands Health Ventures Fund VIII—A, L.P. and 26,602 shares purchased by Essex Woodlands Health Ventures Fund—B, L.P., each of which is an affiliate of Essex Woodlands Health Ventures VIII, L.P. |
| (4) | C. Thomas Caskey, M.D., is one of our directors and is a partner of Essex Woodlands Health Ventures. |
| (5) | Includes 15,339 shares purchased by Enterprise Partners Management, LLC and 193,320 shares purchased by Enterprise Partners V, L.P., each of which is an affiliate of Enterprise Partners VI, L.P. |
| (6) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (7) | Includes 170,851 shares purchased by Highland Credit Opportunities CDO, L.P., an affiliate of Highland Crusader Offshore Partners, L.P. Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
| (8) | Includes 4,767 shares purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (9) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (10) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
2009 Bridge Financing
In February 2009, April 2009, June 2009 and August 2009, we sold convertible promissory notes, or the 2009 Notes, to certain of our existing investors for an aggregate purchase price of $14.7 million. The 2009 Notes accrued interest at a rate of 8% per annum and had a maturity date of the earliest of (i) a change of control as defined in our certificate of incorporation, (ii) an event of default pursuant to the terms of the 2009 Notes or (iii) on or after December 31, 2009, within five business days after our receipt of written notice from the investors holding at least 51% of the principal amount of the 2009 Notes. In August 2009, in connection with our Series D preferred stock financing described below, the full principal amount of the 2009 Notes, along with accrued but unpaid interest thereon of $329,393, were automatically converted into an aggregate of 1,991,325 shares of our Series D preferred stock at a conversion price of $7.56 per share. In addition, investors who purchased 2009 Notes also received warrants to purchase up to an aggregate of 278,165 shares of our Series D preferred stock at an exercise price of $7.56 per share.
115
Table of Contents
The table below sets forth the participation in the sale of the 2009 Notes by our directors, executive officers and 5% stockholders and their affiliates.
| Name | Principal amount of 2009 Notes purchased |
Number of shares of Series D preferred stock issued upon conversion of the 2009 Notes |
Number of shares of Series D preferred stock underlying warrants received in connection with purchase of 2009 Notes | ||||
| Enterprise Partners VI, L.P.(1)(2) |
$ | 4,000,458 | 541,720 | 79,318 | |||
| Highland Crusader Offshore Partners, L.P. |
$ | 3,027,535 | 407,297 | 46,057 | |||
| OVP Venture Partners VI, L.P.(3)(4) |
$ | 4,000,458 | 541,720 | 79,318 | |||
| Prospect Venture Partners III, L.P.(5) |
$ | 3,621,549 | 490,422 | 71,872 | |||
| (1) | Includes $1,234,762 in 2009 Notes purchased by Enterprise Partners V, L.P., an affiliate of Enterprise Partners VI, L.P., which converted into 166,700 shares of Series D preferred stock. In connection with its purchase of the 2009 Notes, Enterprise Partners V, L.P. received warrants to purchase up to 25,639 shares of Series D preferred stock at an exercise price of $7.56 per share. |
| (2) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (3) | Includes $59,811 in 2009 Notes purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P., which converted into 8,141 shares of Series D preferred stock. In connection with its purchase of the 2009 Notes, OVP VI Entrepreneurs Fund, L.P. received warrants to purchase up to 1,423 shares of Series D preferred stock at an exercise price of $7.56 per share. |
| (4) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (5) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
Issuance of Series D Preferred Stock
Between August 2009 and March 2010, we sold 7,310,816 shares of Series D preferred stock at a price of $7.56 per share for gross proceeds of approximately $55.3 million. The table below sets forth the number of shares of Series D preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates and includes the shares of Series D preferred stock issued in connection with the conversion of the 2009 Notes, including the consideration paid for such shares by cancellation of the 2009 Notes as disclosed above.
| Name | Number of shares of |
Aggregate price | |||
| Caduceus Private Investments III, LP(1)(2) |
2,274,354 | $ | 17,194,116 | ||
| Essex Woodlands Health Ventures VIII, L.P.(3)(4) |
2,274,354 | $ | 17,194,116 | ||
| C. Thomas Caskey, M.D.(4) |
3,307 | $ | 25,001 | ||
| Enterprise Partners VI, L.P.(5)(6) |
731,664 | $ | 5,531,380 | ||
| Highland Crusader Offshore Partners, L.P.(7) |
588,665 | $ | 4,450,307 | ||
| OVP Venture Partners VI, L.P.(8)(9) |
731,662 | $ | 5,531,365 | ||
| Prospect Venture Partners III, L.P.(10) |
674,426 | $ | 5,098,661 | ||
| (1) | Includes 21,456 shares held by OrbiMed Associates III, LP, an affiliate of Caduceus Private Investments III, LP. |
| (2) | Carl L. Gordon, Ph.D., is one of our directors and is a partner of OrbiMed Advisors, LLC. |
| (3) | Includes 148,608 shares purchased by Essex Woodlands Health Ventures Fund VIII—A, L.P. and 64,612 shares purchased by Essex Woodlands Health Ventures Fund—B, L.P., each of which is an affiliate of Essex Woodlands Health Ventures VIII, L.P. |
| (4) | C. Thomas Caskey, M.D., is one of our directors and is a partner of Essex Woodlands Health Ventures. |
| (5) | Includes 66,138 shares purchased by Enterprise Partners Management, LLC and 176,737 shares purchased by Enterprise Partners V, L.P., each of which is an affiliate of Enterprise Partners VI, L.P. |
| (6) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (7) | Includes 52,969 shares purchased by Highland Credit Opportunities CDO, L.P., an affiliate of Highland Crusader Offshore Partners, L.P. Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
| (8) | Includes 9,477 shares purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (9) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (10) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
116
Table of Contents
Issuance of Common Stock Warrants
In August and September 2010, we issued warrants to purchase up to 1,318,719 shares of our common stock at an exercise price of $2.69 per share to certain existing investors who purchased shares in our Series E preferred stock financing. These warrants are exercisable by the holders after September 30, 2010 in the event that we do not ship genomic data for at least 369 genomes between May 1, 2010 and September 30, 2010. The table below sets forth the number of shares of common stock issuable upon the exercise of these warrants issued to our directors, executive officers and 5% stockholders and their affiliates.
| Name | Number of Shares of Common Stock Issuable Upon Exercise of Warrants | |
| Caduceus Private Investments III, LP(1)(2) |
234,104 | |
| Essex Woodlands Health Ventures VIII, L.P.(3)(4) |
234,104 | |
| C. Thomas Caskey, M.D.(4) |
336 | |
| Enterprise Partners VI, L.P.(5)(6) |
152,563 | |
| Highland Crusader Offshore Partners, L.P.(7) |
145,746 | |
| OVP Venture Partners VI, L.P.(8)(9) |
152,562 | |
| Prospect Venture Partners III, L.P.(10) |
147,833 | |
| SCV-CG, LLC |
234,739 |
| (1) | Includes warrants to purchase 2,209 shares of common stock held by OrbiMed Associates III, LP, an affiliate of Caduceus Private Investments III, LP. |
| (2) | Carl L. Gordon, Ph.D., is one of our directors and is a partner of OrbiMed Advisors, LLC. |
| (3) | Includes warrants to purchase 15,297 shares of common stock held by Essex Woodlands Health Ventures Fund VIII—A, L.P. and warrants to purchase 6,651 shares of common stock held by Essex Woodlands Health Ventures Fund—B, L.P., each of which is an affiliate of Essex Woodlands Health Ventures VIII, L.P. |
| (4) | C. Thomas Caskey, M.D., is one of our directors and is a partner of Essex Woodlands Health Ventures. |
| (5) | Includes warrants to purchase 3,835 shares of common stock held by Enterprise Partners Management, LLC and warrants to purchase 48,330 shares of common stock held by Enterprise Partners V, L.P., each of which is an affiliate of Enterprise Partners VI, L.P. |
| (6) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (7) | Includes warrants to purchase 42,713 shares of common stock held by Highland Credit Opportunities CDO, L.P., an affilaite of Highland Crusader Offshore Partners, L.P. Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
| (8) | Includes warrants to purchase 1,192 shares of common stock purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (9) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (10) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
In August 2009, we issued warrants to purchase up to an aggregate of 1,630,629 shares of our common stock at an exercise price of $1.50 per share to certain existing investors who purchased at least $1.0 million more than their pro rata portion of our Series D preferred stock financing. The table below sets forth the number of shares of common stock issuable upon the exercise of warrants issued to our directors, executive officers and 5% stockholders and their affiliates.
| Name | Number of shares of | |
| Enterprise Partners VI, L.P.(1)(2) |
447,724 | |
| Highland Crusader Offshore Partners, L.P.(3) |
329,638 | |
| OVP Venture Partners VI, L.P.(4)(5) |
447,725 | |
| Prospect Venture Partners III, L.P.(6) |
405,542 |
| (1) | Includes warrants to purchase 308,930 shares of common stock held by Enterprise Partners V, L.P., an affiliate of Enterprise Partners VI, L.P. |
117
Table of Contents
| (2) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (3) | Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
| (4) | Includes warrants to purchase 3,134 shares of common stock purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (5) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (6) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
Issuance of Series C Preferred Stock
In February 2008, we sold 166,346 shares of Series C preferred stock at a price of $159.30 per share for gross proceeds of approximately $26.5 million. In March 2008, we issued 1,004 shares of Series C preferred stock valued at approximately $160,000 to a professional consulting services firm in exchange for their services. The table below sets forth the number of shares of Series C preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name | Number of shares of |
Aggregate purchase price | |||
| Enterprise Partners VI, L.P.(1) |
21,488 | $ | 3,423,038 | ||
| Highland Crusader Offshore Partners, L.P.(2) |
100,438 | $ | 15,999,773 | ||
| OVP Venture Partners VI, L.P.(3)(4) |
21,488 | $ | 3,423,038 | ||
| Prospect Venture Partners III, L.P.(5) |
19,482 | $ | 3,103,483 | ||
| (1) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and general partner of Enterprise Partners. |
| (2) | Includes 43,941 shares held by Highland Credit Opportunities CDO, L.P., an affiliate of Highland Crusader Offshore Partners, L.P. Highland Crusader Offshore Partners, L.P. beneficially owns more than 5% of our outstanding common stock. |
| (3) | Includes 429 shares held by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (4) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (5) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
2007 Bridge Financing
In February 2007 and March 2007, we sold convertible promissory notes, or the 2007 Notes, to certain of our existing investors for an aggregate purchase price of $1.0 million. The 2007 Notes accrued interest at a rate of 6% per annum and had a maturity date of May 31, 2007. In March 2007, in connection with our Series B preferred stock financing described below, the full principal amount of the 2007 Notes, along with accrued but unpaid interest thereon of $2,712, were automatically converted into an aggregate of 14,492 shares of our Series B preferred stock at a conversion price of $69.00 per share. In addition, investors who purchased 2007 Notes also received warrants to purchase up to an aggregate of 393 shares of our Series B preferred stock at an exercise price of $69.00 per share.
The table below sets forth the participation in the sale of the 2007 Notes by our directors, executive officers and 5% stockholders and their affiliates.
| Name | Principal amount of 2007 Notes purchased |
Number of shares of Series B preferred stock issued upon conversion of the 2007 Notes |
Series B preferred stock warrants received in connection with purchase of 2007 Notes | ||||
| Enterprise Partners VI, L.P.(1) |
$ | 500,000 | 7,246 | 197 | |||
| OVP Venture Partners VI, L.P.(2)(3) |
$ | 500,000 | 7,246 | 196 | |||
| (1) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (2) | Includes $10,000 in 2007 Notes purchased by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P., which converted into 145 shares of Series B preferred stock. In connection with its purchase of the 2007 Notes, OVP VI Entrepreneurs Fund, L.P. received warrants to purchase up to three shares of Series B preferred stock at an exercise price of $69.00 per share. |
| (3) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
118
Table of Contents
Issuance of Series B Preferred Stock
In March and September 2007, we sold 203,620 shares of Series B preferred stock at a price of $69.00 per share for gross proceeds of approximately $14.0 million. The table below sets forth the number of shares of Series B preferred stock sold to our directors, executive officers and 5% stockholders and their affiliates.
| Name | Number of shares of |
Aggregate purchase | |||
| Enterprise Partners VI, L.P.(1) |
43,478 | $ | 2,999,982 | ||
| OVP Venture Partners VI, L.P.(2)(3) |
43,477 | $ | 2,999,913 | ||
| Prospect Venture Partners III, L.P.(4) |
101,449 | $ | 6,999,981 | ||
| (1) | Andrew E. Senyei, M.D., is one of our directors and is a managing director and a general partner of Enterprise Partners. |
| (2) | Includes 869 shares held by OVP VI Entrepreneurs Fund, L.P., an affiliate of OVP Venture Partners VI, L.P. |
| (3) | Charles P. Waite, Jr., is one of our directors and is a general partner of OVP Venture Partners. |
| (4) | Alexander E. Barkas, Ph.D., is one of our directors and is a managing member of Prospect Management Co. III, LLC, the general partner of Prospect Venture Partners III, L.P. |
Investor Rights Agreement
We have entered into an investors’ rights agreement with the purchasers of our outstanding preferred stock and certain holders of common stock and warrants to purchase our common stock and preferred stock, including entities with which certain of our directors are affiliated. As of June 30, 2010, the holders of 15.3 million shares of our common stock, including the shares of common stock issuable upon the conversion of our preferred stock and shares of common stock issued upon exercise of warrants, are entitled to rights with respect to the registration of their shares under the Securities Act. For a more detailed description of these registration rights, see “Description of Capital Stock—Registration Rights.” The investor rights agreement also provides for rights of first refusal and a requirement to obtain the consent of certain stockholders before we may incur indebtedness exceeding $500,000 that is not already included in a board-approved budget, other than trade credit incurred in the ordinary course of business. We have obtained waivers so that the rights of first refusal will not apply to, and will terminate upon completion of, this offering.
Voting Agreement
We have entered into an amended and restated voting agreement with certain holders of our common stock and holders of our convertible preferred stock. Upon the closing of this offering, the amended and restated voting agreement will terminate. For a description of the amended and restated voting agreement, see the section titled “Management—Board composition—Voting Arrangements.”
Right of First Refusal and Co-sale Agreement
We have entered into an amended and restated right of first refusal and co-sale agreement with certain holders of our common stock and holders of our preferred stock. This agreement provides for rights of first refusal and co-sale relating to the shares of our common stock and common stock issuable upon conversion of the shares of preferred stock held by the parties thereto. Upon the closing of this offering, the amended and restate right of first refusal and co-sale agreement will terminate.
Indemnification Agreements
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
119
Table of Contents
Stock Options and Stock Option Modification
We have granted stock options to our executive officers. For more information regarding these stock options, see the section titled “Executive Compensation — Compensation Discussion and Analysis.”
In January 2010, our board of directors approved a stock option modification. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Stock Option Modification” for a description of our 2010 option exchange program.
Other Transactions
In March 2006, we entered into a licensing agreement with Callida Genomics, Inc., or Callida, for use of certain patents, patent applications, know-how and other intellectual property relating to sequencing by hybridization. See “Business—Intellectual property.” As partial consideration for the rights acquired pursuant to the license agreement, we issued 13,333 shares of our Common Stock to Callida. Callida is owned by Radoje Drmanac, Ph.D., our Chief Scientific Officer, and his wife.
We have granted stock options to our executive officers and certain of our directors. For a description of these options, see “Executive Compensation — Compensation Discussion and Analysis — Long-Term Equity Incentives.” In addition, in March 2010, we issued:
| § | 285,867 shares of common stock to our President, Chief Executive Officer and director, Clifford A. Reid, Ph.D., at a purchase price of $1.50 per share; |
| § | 105,333 shares of common stock to Robert J. Curson, who was our Chief Financial Officer at the time of grant and is now our Vice President of Financial Operations, at a purchase price of $1.50 per share; and |
| § | 395,333 shares of common stock to our Chief Scientific Officer, Radoje Drmanac, Ph.D., at a purchase price of $1.50 per share. |
Dr. Reid, Mr. Curson and Dr. Drmanac received bonuses of $361,615, $133,244 and $500,087, respectively, in connection with these grants. See “Executive Compensation — Compensation Discussion and Analysis — Long-Term Equity Incentives.”
We have entered into indemnification agreements with each of our directors and will enter into new indemnification agreements with each of our current directors, officers, and certain employees before the completion of this offering. See “Management — Limitation on Liability and Indemnification Matters.”
Policies and Procedures for Related Party Transactions
Our board of directors intends to adopt a written related person transaction policy to set forth the policies and procedures for the review and approval or ratification of related person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person.
120
Table of Contents
The following table sets forth information known to us about the beneficial ownership of our common stock at September 1, 2010, as adjusted to reflect the sale of the shares of common stock in this offering, by:
| § | each person or group of affiliated persons known to us to be the beneficial owner of more than 5% of our common stock; |
| § | each named executive officer and each director; and |
| § | all of our executive officers and directors as a group. |
The information in the table below is calculated based on shares of common stock outstanding before this offering and shares of common stock outstanding after this offering. The number of shares outstanding before and after this offering is based on the number of shares of common stock outstanding on September 1, 2010 as adjusted to give effect to:
| § | the conversion of all 13,094,629 shares of our convertible preferred stock outstanding on September 1, 2010 into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
In computing the number of shares of common stock beneficially owned by a person, entity or group and the corresponding percentage ownership of that person, entity or group, shares of common stock underlying common stock or preferred stock options and warrants that are held by that person, entity or group and that are currently exercisable or exercisable within 60 days of September 1, 2010 are considered to be outstanding. We did not deem these shares to be outstanding, however, for the purpose of computing the percentage ownership of any other person, entity or group.
121
Table of Contents
Unless otherwise noted below, the address of each beneficial owner listed on the table is c/o Complete Genomics, Inc., 2071 Stierlin Court, Mountain View, CA 94043. We have determined beneficial ownership in accordance with the rules of the SEC. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the tables below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws where applicable.
| Beneficial ownership prior to this offering | Beneficial ownership after this offering | |||||||||||
| Common stock |
Number of shares exercisable within 60 days |
Number of shares beneficially owned |
Percentage of beneficial ownership |
Number of shares beneficially owned |
Percentage of beneficial ownership | |||||||
| 5% Stockholders |
||||||||||||
| Entities affiliated with OrbiMed Advisors LLC(1) |
3,210,766 | 294,379 | 3,505,145 | |||||||||
| Entities affiliated with Essex Woodlands Health Ventures(2) |
3,210,765 | 294,380 | 3,505,145 | |||||||||
| Entities affiliated with Enterprise Partners(3) |
2,092,422 | 720,054 | 2,812,476 | |||||||||
| Entities affiliated with OVP Venture Partners(4) |
2,092,409 | 720,046 | 2,812,455 | |||||||||
| Prospect Venture Partners III, L.P.(5) |
2,027,561 | 663,311 | 2,690,872 | |||||||||
| Entities affiliated with Highland Capital Management, L.P.(6) |
1,999,365 | 552,191 | 2,551,556 | |||||||||
| SCV-CG, LLC(7) |
938,957 | 501,667 | 1,440,624 | |||||||||
| Named Executive Officers and Directors |
||||||||||||
| Clifford A. Reid, Ph.D.(8) |
319,200 | 167,476 | 486,676 | |||||||||
| Robert J. Curson(9) |
116,999 | 84,815 | 201,814 | |||||||||
| Radoje Drmanac, Ph.D.(10) |
441,999 | 131,469 | 573,468 | |||||||||
| Alexander E. Barkas, Ph.D.(11) |
2,027,561 | 663,311 | 2,690,872 | |||||||||
| C. Thomas Caskey, M.D.(12) |
4,652 | 12,752 | 17,404 | |||||||||
| Carl L. Gordon, Ph.D., CFA(13) |
3,210,766 | 294,379 | 3,505,145 | |||||||||
| Michael P. Rubin, M.D. |
— | — | — | |||||||||
| Lewis J. Shuster(14) |
— | 6,250 | 6,250 | |||||||||
| Andrew E. Senyei, M.D.(15) |
2,092,422 | 720,054 | 2,812,476 | |||||||||
| Charles P. Waite, Jr.(16) |
2,092,409 | 720,046 | 2,812,455 | |||||||||
| All Executive Officers and directors as a group (12 persons)(17) |
10,222,210 | 2,731,994 | 12,954,204 | |||||||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | Includes: (i) 3,180,476 shares held, and 291,602 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Caduceus Private Investments III, LP (“Caduceus”) and (ii) 30,290 shares held, and 2,777 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by OrbiMed Associates III, LP (“OrbiMed Associates”). Excludes warrants to purchase 231,895 shares held by Caduceus and warrants to purchase 2,209 shares held by OrbiMed Associates, whose exercisability will not be determined until September 30, 2010. OrbiMed Capital GP III, LP is the general partner of Caduceus, and Samuel D. Isaly is the general partner of OrbiMed Capital GP III, LP. OrbiMed Advisors LLC (“OrbiMed Advisors”) is the general partner of OrbiMed Associates, and Mr. Isaly is the managing member of OrbiMed Advisors, and Carl L. Gordon, Ph.D., a member of our board of directors, is a member of OrbiMed Advisors. Mr. Isaly is deemed to have voting and dispositive power over the shares held by Caduceus and OrbiMed Associates. The address of OrbiMed Advisors LLC is 767 Third Avenue, 30th Floor, New York, New York 10017. |
| (2) | Includes: (i) 2,909,757 shares held, and 266,781 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Essex Woodlands Health Ventures Fund VIII, L.P. (“Essex Woodlands Ventures Fund”); (ii) 209,794 shares held, and 19,235 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Essex Woodlands Health Ventures Fund VIII—A, L.P. (“Essex Woodlands Fund A”) and (iii) 91,214 shares held, and 8,364 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Essex Woodlands Health Ventures Fund VIII—B, L.P. (“Essex Woodlands Fund B”). Excludes warrants to purchase 212,156 shares held by Essex Woodlands Venture Fund, warrants to purchase 15,297 shares held by Essex Woodlands Fund A and warrants to purchase 6,651 shares held by Essex Woodlands Fund B, whose exercisability will not be determined until September 30, 2010. Essex Woodlands Health Ventures VIII, L.P. (“Essex Ventures L.P.”) is the general partner of each of Essex Woodlands Ventures Fund, Essex Woodlands Fund A and Essex Woodlands Fund B. Essex Woodlands Health Ventures VIII, LLC (“Essex Ventures LLC”) is the general partner of Essex Ventures L.P. James Currie, Ron Eastman, Jeff Himawan, Ph.D., Guido Neels, Martin Sutter, Immanuel Thangaraj, Petri Yaino, M.D., Ph.D., and Steve Wiggans are managing directors of Essex Ventures LLC and are deemed to have shared voting and dispositive power over the shares held by Essex Woodlands Ventures Fund, Essex Woodlands Fund A and Essex Woodlands Fund B. Each of the managing directors disclaims beneficial ownership of the shares held by these entities, except to the extent of any pecuniary interest therein. The address of each of the entities affiliated with Essex Woodlands Health Ventures is 335 Bryant Street, Palo Alto, California 94301. |
122
Table of Contents
| (3) | Includes: (i) 1,640,888 shares held, and 491,957 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Enterprise Partners VI, LP (“Enterprise VI”); (ii) 370,057 shares held, and 220,241 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Enterprise Partners V, LP (“Enterprise V”) and (iii) 81,477 shares held, and 7,856 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Enterprise Partners Management, LLC (“Enterprise LLC”). Excludes warrants to purchase 100,398 shares held by Enterprise VI, warrants to purchase 48,330 shares held by Enterprise V and warrant to purchase 3,835 held by Enterprise LLC, whose exercisability will not be determined until September 30, 2010. Andrew E. Senyei, M.D., a member of our board of directors, is a managing director of Enterprise Management Partners VI, LLC, the general partner of Enterprise VI, a managing director of Enterprise Management Partners V, LLC, the general partner of Enterprise V and a managing director of Enterprise LLC. Dr. Senyei, together with Carl Eibl, J.D., are deemed to have shared voting and dispositive power over the shares held by each of Enterprise VI, Enterprise V and Enterprise LLC. Each of Dr. Senyei and Mr. Eibl disclaims beneficial ownership of these shares, except to the extent of his pecuniary interest therein. The address for each of the entities affiliated with Enterprise Partners is 2223 Avenida de la Playa, Suite 300, La Jolla, California 92037. |
| (4) | Includes: (i) 2,063,167 shares held, and 712,304 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by OVP Venture Partners VI, L.P. (“OVP VI”) and (ii) 29,242 shares held, and 7,742 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by OVP VI Entrepreneurs Fund, L.P. (“OVP Entrepreneurs Fund”). Excludes warrants to purchase 151,370 shares held by OVP VI and warrants to purchase 1,192 shares held by OVP Entrepreneurs Fund, whose exercisability will not be determined until September 30, 2010. Charles P. Waite, Jr., a member of our board of directors, is a managing member of OVMC VI, LLC, the general partner of each of OVP VI and OVP Entrepreneurs Fund. Mr. Waite, together with the other managing members of OVMC VI, LLC are deemed to have shared voting and dispositive power over the shares held by OVP VI and OVP Entrepreneurs Fund. The address of each of the entities affiliated with OVP Venture Partners is 1010 Market Street, Kirkland, Washington 98033. |
| (5) | Includes 2,027,561 shares held and 663,311 shares that may be acquired pursuant to the exercise of warrants held prior to this offering. Excludes warrants to purchase 147,833 shares, whose exercisability will not be determined until September 30, 2010. Alexander E. Barkas, Ph.D., a member of our board of directors, is a managing member of Prospect Management Co. III, L.L.C., the general partner of Prospect Venture Partners III, L.P. The managing members of Prospect Management Co. III, L.L.C., are deemed to have shared voting and dispositive power over the shares held by Prospect Venture Partners III, L.P., and each disclaims beneficial ownership of these shares, except to the extent of his or her pecuniary interest therein. The address for Prospect Management Co. III, L.L.C. is 435 Tasso Street, Suite 200, Palo Alto, California 94301. |
| (6) | Includes: (i) 1,413,424 shares held, and 500,466 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Highland Crusader Offshore Partners, L.P. (“Highland Crusader”) and (ii) 585,941 shares held, and 51,725 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by Highland Credit Opportunities CDO, L.P. (“Credit Opportunities”). Excludes warrants to purchase 103,033 shares held by Highland Crusader and warrants to purchase 42,713 shares held by Credit Opportunities, whose exercisability will not be determined until September 30, 2010. Highland Capital Management, L.P. (“Highland”) acts as investment adviser to certain funds, including Highland Crusader and Credit Opportunities. Strand Advisors, Inc. (“Strand Advisors”) is the general partner of Highland. James Dondero is the President of Strand Advisors and Highland. Highland, Strand Advisors and Mr. Dondero may be deemed to have shared voting and dispositive power over the shares held by each of Highland Crusader and Credit Opportunities. The address for Highland, Strand Advisors and Mr. Dondero is Two Galleria Tower, 13455 Noel Road, Suite 800, Dallas, Texas 75240. |
| (7) | Includes 938,957 shares held and 501,667 shares that may be acquired pursuant to the exercise of warrants held prior to this offering. Excludes warrants to purchase an aggregate of 234,739 shares, whose exercisability will no be determined until September 30, 2010. Charles M. Preston III is the manager of SCV-CG, LLC and is deemed to have voting and dispositive power over the shares held by SCV-CG, LLC. The address for SCV-CG, LLC is 600 Congress Avenue, Suite 200, Austin, Texas 78701. |
| (8) | Consists of: (i) 319,200 shares held by the Clifford A. Reid Living Trust, dated September 3, 1997, of which Dr. Reid is trustee and (ii) 167,476 shares that may be acquired pursuant to the exercise of stock options within 60 days of September 1, 2010. |
| (9) | Consists of: (i) 116,999 shares held by The Curson Family Living Trust, dated July 30, 2001, of which Mr. Curson is trustee and (ii) 84,815 shares that may be acquired pursuant to the exercise of stock options within 60 days of September 1, 2010. |
| (10) | Consists of: (i) 428,666 shares held by the Drmanac Family Trust, dated June 21, 2000, of which Dr. Drmanac is trustee; (ii) 131,469 shares that may be acquired pursuant to the exercise of stock options within 60 days of September 1, 2010 and (iii) 13,333 shares held by Callida Genomics, Inc. |
| (11) | Consists of the shares described in Note (5) above. Dr. Barkas disclaims beneficial ownership of the shares held by Prospect Venture Partners III, L.P. as described in Note (5) above, except to the extent of his pecuniary interest therein. |
| (12) | Consists of: (i) 4,652 shares, (ii) 252 shares that may be acquired pursuant to the exercise of warrants held prior to this offering and (iii) 12,500 shares that may be acquired pursuant to the exercise of stock options within 60 days of September 1, 2010. Excludes warrants to purchase 336 shares, whose exercisability will not be determined until September 30, 2010. |
| (13) | Consists of the shares described in Note (1) above. Dr. Gordon disclaims beneficial ownership of the shares held by the entities affiliated with OrbiMed Advisors LLC as described in Note (1) above, except to the extent of his pecuniary interest therein. |
| (14) | Consists of 6,250 shares that may be acquired pursuant to the exercise of stock options within 60 days of September 1, 2010. |
| (15) | Consists of the shares described in Note (3) above. Dr. Senyei disclaims beneficial ownership of the shares held by the entities affiliated with Enterprise Partners as described in Note (3) above, except to the extent of his pecuniary interest therein. |
| (16) | Consists of the shares described in Note (4) above. Mr. Waite disclaims beneficial ownership of the shares held by the entities affiliated with OVP Venture Partners as described in Note (4) above, except to the extent of his pecuniary interest therein. |
123
Table of Contents
| (17) | Includes 9,418,506 shares held, and 2,397,538 shares that may be acquired pursuant to the exercise of warrants held prior to this offering, by entities affiliated with certain of our directors and 1,133,256 shares beneficially owned by our executive officers and directors, which includes 252 shares that many be acquired pursuant to the exercise of warrants held prior to this offering by a director and 333,952 shares may be acquired pursuant the exercise of stock options within 60 days of September 1, 2010. Excludes warrants to purchase 687,398 shares, whose exercisability will not be determined until September 30, 2010. |
124
Table of Contents
General
Upon the completion of this offering, we will have authorized under our amended and restated certificate of incorporation shares of common stock, $0.001 par value per share, and shares of preferred stock, $0.001 par value per share. As of September 1, 2010, there were outstanding:
| § | shares of our common stock held by approximately 33 stockholders of record; |
| § | shares of our common stock issuable upon exercise of outstanding warrants; and |
| § | 2,687,100 shares of our common stock issuable upon exercise of outstanding stock options. |
The number of shares of our common stock outstanding after this offering assumes:
| § | the filing and effectiveness of our amended and restated certificate of incorporation; |
| § | the conversion of all 13,094,629 shares of our convertible preferred stock outstanding on September 1, 2010 into an aggregate of 15,808,361 shares of our common stock, which will be effective immediately prior to the consummation of this offering; |
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our common stock, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus); and |
| § | the exercise, on a net issuance basis, of warrants outstanding as of September 1, 2010, which will expire upon completion of this offering if unexercised, to purchase shares of our convertible preferred stock, and the conversion of those shares of preferred stock immediately prior to the consummation of this offering, resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
The following description of our capital stock and provisions of our amended and restated certificate of incorporation and amended and restated bylaws to be in effect upon the completion of this offering are summaries. Copies of these documents have been filed with the SEC as exhibits to our registration statement of which this prospectus forms a part. The descriptions of the common stock and preferred stock reflect changes to our capital structure that will occur upon the closing of this offering. Currently, there is no established public trading market for our capital stock.
Common Stock
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Our stockholders do not have cumulative voting rights in the election of directors. Accordingly, holders of a majority of the voting shares are able to elect all of the directors.
Dividends
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. The terms of our credit facility currently prohibit us from paying cash dividends on our common stock.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and
125
Table of Contents
other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Other Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
Fully Paid and Nonassessable.
All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred Stock
Immediately prior to the consummation of this offering, all outstanding shares of our preferred stock will be converted into shares of our common stock. See Note 6 to notes to our audited financial statements for a description of our currently outstanding preferred stock. Immediately prior to the consummation of this offering, our amended and restated certificate of incorporation will be amended and restated to delete all references to such shares of preferred stock. Upon the completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control of our company or other corporate action. Immediately after completion of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Warrants
The following table sets forth information about outstanding warrants to purchase shares of our stock as of September 1, 2010. Immediately prior to the consummation of this offering, the warrants to purchase shares of our preferred stock will convert into warrants to purchase our common stock based on the applicable conversion ratio of the preferred stock. In addition, certain of the outstanding warrants contain a “net exercise” provision, which provides that the warrants will net exercise on the expiration date or upon consummation of this offering, if not exercised before that time.
| Class of stock underlying warrants | Number of shares |
Exercise price per share |
Expiration date | ||||
| Common Stock, par value $0.001 per share(1) |
1,630,629 | $ | 1.50 | 8/12/2016 | |||
| Common Stock, par value $0.001 per share |
1,848,849 | $ | 1.50 | Various dates between 4/12/2015 | |||
| and 6/28/2015 | |||||||
| Common Stock, par value $0.001 per share(2) |
1,318,719 | $ | 2.69 | Various dates between 8/6/2015 | |||
| and 9/1/2015 | |||||||
| Series A preferred stock, par value $0.001 per share(3) |
684 | $ | 43.85 | 9/6/2016 | |||
| Series B preferred stock, par value $0.001 per share(4) |
393 | $ | 69.00 | Various dates between 2/21/2012 | |||
| and 3/12/2012 | |||||||
| Series B preferred stock, par value $0.001 per share(5) |
1,738 | $ | 69.00 | 8/3/2017 | |||
| Series D preferred stock, par value $0.001 per share(6) |
278,165 | $ | 7.56 | Various dates between 2/13/2014 | |||
| and 8/5/2014 | |||||||
| Series D preferred stock, par value $0.001 per share(7) |
103,173 | $ | 7.56 | 7/30/2018 | |||
126
Table of Contents
| (1) | Pursuant to the terms of these warrants, immediately prior to the consummation of this offering, these warrants will automatically net exercise (if not exercised prior to that time), resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
| (2) | Pursuant to the terms of these warrants, these warrants will expire upon the earlier of (a) five years from the date of issuance, (b) the sale, conveyance or disposal of all or substantially all of our property or business or our merger with or into or consolidation with any other corporation or (c) on September 30, 2010 if we have shipped genomic data for at least 369 genomes during the period between May 1, 2010 and September 30, 2010. |
| (3) | Immediately prior to the consummation of this offering, these warrants to purchase Series A preferred stock will convert into warrants to purchase an aggregate of 3,156 shares of our common stock. |
| (4) | Pursuant to the terms of these warrants, immediately prior to the consummation of this offering, these warrants will automatically net exercise (if not exercised prior to that time). Assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), the exercise price of these warrants will be greater than the public offering price, resulting in the expiration of the warrants. |
| (5) | Immediately prior to the consummation of this offering, these warrants to purchase Series B preferred stock will convert into warrants to purchase an aggregate of 10,301 shares of our common stock. |
| (6) | Pursuant to the terms of these warrants, immediately prior to the consummation of this offering, these warrants will automatically net exercise (if not exercised prior to that time), resulting in the issuance of shares of common stock, assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus). |
| (7) | Immediately prior to the consummation of this offering, these warrants to purchase Series D preferred stock will convert into warrants to purchase an aggregate of 103,173 shares of our common stock. |
Registration Rights
Under our amended and restated investor rights agreement, following the closing of this offering, the holders of 15.3 million shares of common stock, including shares issuable upon exercise of warrants, or their transferees, have the right to require us to register their shares under the Securities Act so that those shares may be publicly resold, or to include their shares in any registration statement we file, in each case as described below.
Demand Registration Rights
Based on the number of shares outstanding as of June 30, 2010, after the completion of this offering, the holders of approximately 14.4 million shares of our common stock, including shares issuable upon exercise of warrants, or their transferees, will be entitled to certain demand registration rights. Beginning six months following the consummation of this offering, the holders of at least 40% of these shares can, on not more than two occasions, request that we register all or a portion of their shares. Such request for registration must cover a number of shares with an anticipated aggregate offering price, net of underwriting discounts and commissions, of at least $5.0 million. Additionally, we will not be required to affect a demand registration during the period beginning 60 days prior to the filing and 180 days following the effectiveness of a company-initiated registration statement relating to a public offering of our securities, provided that we have complied with certain notice requirements to the holders of these shares.
Piggyback Registration Rights
Based on the number of shares outstanding as of June 30, 2010, after the completion of this offering, in the event that we determine to register any of our securities under the Securities Act, either for our own account or for the account of other security holders, the holders of approximately 15.3 million shares of our common stock, including shares issuable upon exercise of warrants, or their transferees, will be entitled to certain “piggyback” registration rights allowing the holders to include their shares in such registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act, other than with respect to a registration related to employee benefit plans or corporate reorganizations, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration.
127
Table of Contents
Form S-3 Registration Rights
Based on the number of shares outstanding as of June 30, 2010, after the completion of this offering, the holders of approximately 14.4 million shares of our common stock, including shares issuable upon exercise of warrants, or their transferees, will be entitled to certain Form S-3 registration rights. The holders of at least 5% of these shares can make a written request that we register their shares on Form S-3 if we are eligible to file a registration statement on Form S-3 and if the aggregate price to the public of the shares offered is at least $5.0 million. These stockholders may make an unlimited number of requests for registration on Form S-3. However, we will not be required to affect a registration on Form S-3 if we have previously affected two such registrations in the 12-month period preceding the request for registration. Additionally, we will not be required to affect a registration on Form S-3 during the period beginning 60 days prior to the filing and 180 days following the effectiveness of a company-initiated registration statement relating to a public offering of our securities, provided that we have complied with certain notice requirements to the holders of these shares.
Expenses of Registration
We will pay the registration expenses of the holders of the shares registered pursuant to the demand, piggyback and Form S-3 registration rights described above. In an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions, to limit the number of shares such holders may include.
Expiration of Registration Rights
The demand, piggyback and Form S-3 registration rights described above will expire, with respect to any particular stockholder, five years after our initial public offering or when that stockholder can sell all of its shares under Rule 144 of the Securities Act. In any event, all such registration rights shall expire upon the earlier of five years after the consummation of this offering or the consummation of certain events, including the sale of all of our assets or a change in control of our company in which our stockholders receive cash or marketable securities.
Anti-Takeover Provisions
Certificate of Incorporation and Bylaws to be in Effect Upon the Completion of This Offering
Our amended and restated certificate of incorporation to be in effect upon the completion of this offering will provide for our board of directors to be divided into three classes, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the shares of common stock outstanding will be able to elect all of our directors. Our amended and restated certificate of incorporation and amended and restated bylaws to be effective upon the completion of this offering will provide that all stockholder action must be effected at a duly called meeting of stockholders and not by a written consent, and that only our board of directors, chairman of the board, chief executive officer or president (in the absence of a chief executive officer) may call a special meeting of stockholders.
Our amended and restated certificate of incorporation will require a 66 2/3% stockholder vote for the amendment, repeal or modification of certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws relating to:
| § | the classification of our board of directors; |
| § | the requirement that stockholder actions be effected at a duly called meeting; and |
| § | the designated parties entitled to call a special meeting of the stockholders. |
The combination of the classification of our board of directors, the lack of cumulative voting and the 66 2/3% stockholder voting requirements will make it more difficult for our existing stockholders to replace our board of directors as well as for another party to obtain control of us by replacing our board of directors. Because our
128
Table of Contents
board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management. In addition, the authorization of undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change our control.
These provisions may have the effect of deterring hostile takeovers or delaying changes in our control or management. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of us. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions also are intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares or otherwise attempting to control of us, and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in our management.
Section 203 of the Delaware General Corporation Law
Upon completion of this offering, we will be subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, unless:
| § | before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested holder; |
| § | upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned by: |
| § | persons who are directors and also officers; and |
| § | employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| § | on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder. |
In general, Section 203 defines “business combination” to include the following:
| § | any merger or consolidation involving the corporation and the interested stockholder; |
| § | any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation or its majority-owned subsidiary that involves the interested stockholder; |
| § | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| § | subject to certain exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
| § | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or is an affiliate or associate of the corporation and within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
129
Table of Contents
Limitations of Liability and Indemnification Matters
For a discussion of liability and indemnification, please see “Management—Limitation on Liability and Indemnification Matters.”
The NASDAQ Global Market Listing
We have applied to have our common stock listed on The NASDAQ Global Market under the symbol “GNOM.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is .
130
Table of Contents
Shares Eligible for Future Sale
Prior to this offering, there has been no public market for our common stock. Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices of our common stock prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price of our common stock and our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of September 1, 2010, upon completion of this offering, shares of common stock will be outstanding, assuming no exercise of the underwriters’ over-allotment option and no exercise of options or warrants. All of the shares sold by us in this offering will be freely tradable unless purchased by our affiliates. The remaining shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements as described below. Following the expiration of the lock-up period, all shares will be eligible for resale in compliance with Rule 144 or Rule 701 to the extent such shares have been released from any repurchase option that we may hold. “Restricted securities” as defined under Rule 144 were issued and sold by us in reliance on exemptions from the registration requirements of the Securities Act. These shares may be sold in the public market only if registered or pursuant to an exemption from registration, such as Rule 144 or Rule 701 under the Securities Act. Excluding the shares issued in this offering, the remaining shares of common stock outstanding after this offering will generally become available for sale in the public market as follows:
| Approximate number of shares |
Date | |
| Immediately. | ||
| Beginning 90 days after the effective date of the registration statement of which this prospectus forms a part, subject, in certain cases, to the holding period, public information, volume limitation, public notice and manner-of-sale requirements of Rule 144. | ||
| At various times after the effective date of the registration statement of which this prospectus forms a part. | ||
The above does not take into consideration the effect of the lock-up agreements described below.
Rule 144
Rule 144 provides an exemption from the registration and prospectus-delivery requirements of the Securities Act. This exemption is available to affiliates of ours that sell our restricted or non-restricted securities and also to non-affiliates that sell our restricted securities. Restricted securities include securities acquired from the issuer of those securities, or from an affiliate of the issuer, in a transaction or chain of transactions not involving any public offering. The shares we are selling in this offering are not restricted securities. However, all the shares we have issued before this offering are restricted securities, and they will continue to be restricted securities until they are resold pursuant to Rule 144 or pursuant to an effective registration statement.
In general, under Rule 144, as in effect on the date of this prospectus, a person who is, or at any time during the 90 days preceding the sale was, an affiliate of ours generally may sell, within any three-month period, a number of shares that does not exceed the greater of:
| § | 1% of the number of shares of common stock outstanding, which will equal approximately shares immediately after this offering (or shares if the underwriters’ over-allotment is exercised in full); or |
| § | the average weekly trading volume of our common stock on The NASDAQ Global Market during the four calendar weeks immediately preceding the date on which a notice of sale is filed with the SEC. |
In addition, sales by these persons must also satisfy requirements relating to the manner of sale, public notice, the availability of current public information about us and, in the case of restricted securities, a minimum holding
131
Table of Contents
period for those securities. All other persons may rely on Rule 144 to freely sell our restricted securities, so long as they satisfy both the minimum holding period requirement and, until a one-year holding period has elapsed, the current public information requirement.
Rule 144 does not supersede our security holders’ contractual obligations under the lock-up agreements described below.
Rule 701
Generally, an employee, officer, director or qualified consultant of ours who purchased shares of our common stock before the effective date of the registration statement relating to this prospectus, or who holds options as of that date, pursuant to a written compensatory plan or contract may rely on the resale provisions of Rule 701 under the Securities Act. Under Rule 701, of these persons:
| § | those who are not our affiliates may generally sell those securities, commencing 90 days after the effective date of the registration statement, without having to comply with the current public information and minimum holding period requirements of Rule 144; and |
| § | those who are our affiliates may generally sell those securities under Rule 701, commencing 90 days after the effective date of the registration statement, without having to comply with Rule 144’s minimum holding period restriction. |
Rule 701 does not supersede our security holders’ contractual obligations under the lock-up agreements described above.
Lock-Up Agreements
We, along with our directors, executive officers and substantially all of our other security holders, have generally agreed with the underwriters that for a period of 180 days following the date of this prospectus, we or they will not offer, sell, contract to sell, pledge, or otherwise dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to effect any of these transactions, subject to specified exceptions. UBS Investment Bank and Jefferies & Company may, in their sole discretion, at any time without prior notice, release all or any portion of the shares from the restrictions in any such agreement.
If:
| § | during the period that begins on the date that is 15 calendar days plus 3 business days before the last day of the 180-day lock-up period and ends on the last day of the 180-day lock-up period, |
| § | we issue an earnings release; or |
| § | material news or a material event relating to us occurs; or |
| § | prior to the expiration of the 180-day lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day lock-up period, |
then the 180-day lock-up period described in the preceding paragraph will be extended until the expiration of the date that is 15 calendar days plus 3 business days after the date on which the issuance of the earnings release or the material news or material event occurs.
Registration Rights
Based on the number of shares outstanding as of June 30, 2010, after the completion of this offering, the holders of 15.3 million shares of our common stock, including shares issuable upon exercise of warrants, or their transferees, will, subject to any lock-up agreements they have entered into, be entitled to certain rights with
132
Table of Contents
respect to the registration of the offer and sale of those shares under the Securities Act. For a description of these registration rights, please see the section titled “Description of Capital Stock—Registration Rights.” If the offer and sale of these shares are registered, they will be freely tradable without restriction under the Securities Act.
Stock Plans
As soon as practicable after the completion of this offering, we intend to file a Form S-8 registration statement under the Securities Act to register the offer and sale of shares of our common stock subject to options outstanding or reserved for issuance under our 2006 Equity Incentive Plan and our 2010 Equity Incentive Award Plan. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will then be eligible for sale in the public markets, subject limitations applicable to affiliates and any lock-up agreements. For a more complete discussion of our stock plans, see “Executive Compensation—Employee Benefit and Stock Plans.”
133
Table of Contents
Material U.S. Federal Income Tax Consequences to Non-U.S. Holders
The following is a summary of material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the acquisition, ownership and disposition of our common stock issued pursuant to this offering. This discussion is based on the Internal Revenue Code of 1986, as amended, or the Code, Treasury Regulations promulgated thereunder, judicial decisions and published rulings and administrative pronouncements of the Internal Revenue Service, or IRS, all as in effect as of the date of this prospectus. These authorities may change, possibly retroactively, resulting in U.S. federal income tax consequences different from those discussed below. This discussion is not a complete analysis of all of the potential U.S. federal income tax consequences relating thereto, nor does it address any estate and gift tax consequences or any tax consequences arising under any state, local or foreign tax laws or any other U.S. federal tax laws. No ruling has been or will be sought from the IRS with respect to the matters discussed below, and there can be no assurance that the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership or disposition of our common stock by a non-U.S. holder, or that any such contrary position would not be sustained by a court.
This discussion is limited to non-U.S. holders who purchase our common stock issued pursuant to this offering and who hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (property held for investment). This discussion does not address all of the U.S. federal income tax consequences that may be relevant to a particular holder in light of such holder’s particular circumstances. This discussion also does not consider any specific facts or circumstances that may be relevant to holders subject to special rules under the U.S. federal income tax laws, including, without limitation:
| § | U.S. expatriates or former long-term residents of the United States; |
| § | partnerships or other pass-through entities classified as a partnership for U.S. federal income tax purposes; |
| § | real estate investment trusts; |
| § | regulated investment companies; |
| § | “controlled foreign corporations,” “passive foreign investment companies” or corporations that accumulate earnings to avoid U.S. federal income tax; |
| § | banks, insurance companies or other financial institutions; |
| § | brokers, dealers or traders in securities, commodities or currencies; |
| § | tax-exempt organizations; |
| § | tax-qualified retirement plans; |
| § | persons subject to the alternative minimum tax; or |
| § | persons holding our common stock as part of a hedging or conversion transaction, straddle or a constructive sale or other risk-reduction strategy. |
PROSPECTIVE INVESTORS ARE URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL INCOME AND ESTATE TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING AND DISPOSING OF OUR COMMON STOCK, AS WELL AS ANY TAX CONSEQUENCES ARISING UNDER ANY STATE, LOCAL OR FOREIGN TAX LAWS AND ANY OTHER U.S. FEDERAL TAX LAWS AND TAX TREATIES.
Definition of Non-U.S. Holder
For purposes of this discussion, a non-U.S. holder is any beneficial owner of our common stock that is not a “U.S. person” or a partnership (or other entity treated as a partnership) for U.S. federal income tax purposes. A U.S. person is any of the following:
| § | an individual citizen or resident of the United States; |
134
Table of Contents
| § | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state therein or the District of Columbia; |
| § | an estate the income of which is subject to U.S. federal income tax regardless of its source; or |
| § | a trust (1) the administration of which is subject to the primary supervision of a U.S. court and all substantial decisions of which are controlled by one or more U.S. persons or (2) that has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
Distributions on Our Common Stock
If we make cash or other property distributions on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and will first be applied against and reduce a holder’s adjusted tax basis in the common stock, but not below zero. Distributions in excess of our current and accumulated earnings and profits and in excess of a non-U.S. holder’s tax basis in its shares will be treated as gain realized on the sale or other disposition of the common stock and will be treated as described under “— Gain on Disposition of Our Common Stock” below.
Dividends paid to a non-U.S. holder of our common stock that are not effectively connected with a U.S. trade or business conducted by such holder will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends, or such lower rate specified by an applicable income tax treaty. To receive the benefit of a reduced treaty rate, a non-U.S. holder must furnish to us or our paying agent a valid IRS Form W-8BEN (or applicable successor form) certifying such holder’s qualification for the reduced rate. This certification must be provided to us or our paying agent prior to the payment of dividends and must be updated periodically. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, who then will be required to provide certification to us or our paying agent, either directly or through other intermediaries. Non-U.S. holders that do not timely provide us or our paying agent with the required certification, but who qualify for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisors regarding possible entitlement to benefits under a tax treaty.
If a non-U.S. holder holds our common stock in connection with such holder’s conduct of a trade or business in the United States, and dividends paid on the common stock are effectively connected with such holder’s U.S. trade or business, and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the U.S., the non-U.S. holder will be exempt from U.S. federal withholding tax. To claim the exemption, the non-U.S. holder must furnish to us or our paying agent a properly executed IRS Form W-8ECI (or applicable successor form), certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States.
Any dividends paid on our common stock that are effectively connected with a non-U.S. holder’s U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States) will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates in the same manner as if such holder were a resident of the United States, unless an applicable income tax treaty provides otherwise. A non-U.S. holder that is a foreign corporation also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of a portion of its effectively connected earnings and profits for the taxable year, as adjusted for certain items.
Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty.
135
Table of Contents
Gain on Disposition of Our Common Stock
Subject to the discussion below regarding backup withholding, a non-U.S. holder generally will not be subject to U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
| § | the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business in the United States, and if required by an applicable income tax treaty, attributable to a permanent establishment maintained by the non-U.S. holder in the United States; |
| § | the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the sale or disposition, and certain other requirements are met; or |
| § | our common stock constitutes a “United States real property interest” by reason of our status as a United States real property holding corporation, or USRPHC, for U.S. federal income tax purposes during the relevant statutory period. |
Unless an applicable income tax treaty provides otherwise, gain described in the first bullet point above will be subject to U.S. federal income tax on a net income basis at the regular graduated U.S. federal income tax rates in the same manner as if such holder were a resident of the United States. Further, non-U.S. holders that are foreign corporations also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of a portion of their effectively connected earnings and profits for the taxable year, as adjusted for certain items. Non-U.S. holders are urged to consult their tax advisors regarding any applicable income tax treaties that may provide for different rules.
A gain described in the second bullet point above will be subject to U.S. federal income tax at a flat 30% rate (or such lower rate specified by an applicable income tax treaty), but may be offset by U.S. source capital losses (even though the individual is not considered a resident of the United States), provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we are not currently and do not anticipate becoming a USRPHC for U.S. federal income tax purposes. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property interests relative to the fair market value of our other trade or business assets and our non-U.S. real property interests, there can be no assurance that we are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, a gain arising from the sale or other taxable disposition by a non-U.S. holder of our common stock will not be subject to tax if such class of stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such non-U.S. holder owned, actually or constructively, 5% or less of such class of our stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition of the stock or the non-U.S. holder’s holding period for such stock. We expect our common stock to be “regularly traded” on an established securities market, although we cannot guarantee it will be so traded. If a gain on the sale or other taxable disposition of our stock were subject to taxation under the third bullet point above, the non-U.S. holder would be subject to U.S. federal income tax with respect to such gain in the same manner as a U.S. person (subject to any applicable alternative minimum tax and a special alternative minimum tax in the case of nonresident alien individuals).
Information Reporting and Backup Withholding
We must report annually to the IRS and to each non-U.S. holder the amount of distributions on our common stock paid to such holder and the amount of any tax withheld with respect to those distributions. These information reporting requirements apply even if no withholding was required because the distributions were effectively connected with the holder’s conduct of a U.S. trade or business, or if withholding was reduced or eliminated by an applicable income tax treaty. This information also may be made available under a specific treaty or agreement with the tax authorities in the country in which the non-U.S. holder resides or is established. Backup withholding, currently at a 28% rate, will not apply to distribution payments to a non-U.S. holder of our common stock provided the non-U.S. holder furnishes to us or our paying agent the required certification as to its non-U.S. status, by providing a valid IRS Form W-8BEN or IRS Form W-8ECI, as applicable, and satisfying
136
Table of Contents
certain other requirements. Notwithstanding the foregoing, backup withholding may apply if either we have or our paying agent has actual knowledge, or reason to know, that the holder is a U.S. person that is not an exempt recipient.
Unless a non-U.S. holder complies with certification procedures to establish that it is not a U.S. person, information returns may be filed with the IRS in connection with, and the non-U.S. holder may be subject to backup withholding on the proceeds from, a sale or other disposition of our common stock. The certification procedures described in the above paragraph will satisfy these certification requirements as well.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS. Backup withholding and information reporting rules are complex. Non-U.S. holders are urged to consult their tax advisors regarding the application of these rules to them.
New Legislation Relating to Foreign Accounts
Newly enacted legislation may impose withholding taxes on certain types of payments made to “foreign financial institutions” (as specially defined under these rules) and certain other non-U.S. entities. Under this legislation, the failure to comply with additional certification, information reporting and other specified requirements could result in withholding tax being imposed on payments of dividends and sales proceeds to foreign intermediaries and certain non-U.S. holders. The legislation imposes a 30% withholding tax on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a foreign financial institution or to a foreign non-financial entity, unless (i) the foreign financial institution undertakes certain diligence and reporting obligations or (ii) the foreign non-financial entity either certifies it does not have any substantial U.S. owners or furnishes identifying information regarding each substantial U.S. owner. If the payee is a foreign financial institution, it must enter into an agreement with the U.S. Treasury requiring, among other things, that it undertake to identify accounts held by certain U.S. persons or U.S.-owned foreign entities, annually report certain information about such accounts, and withhold 30% on payments to account holders whose actions prevent it from complying with these reporting and other requirements. The legislation would apply to payments made after December 31, 2012. Prospective investors should consult their tax advisors regarding this legislation.
The foregoing discussion of U.S. federal income tax considerations is for general information purposes only and is not tax or legal advice. Accordingly, you should consult your own tax advisor as to the particular tax consequences to you of purchasing, owning and disposing of our common stock, including the applicability and effect of any U.S. federal, state or local or non-U.S. tax laws, and of any changes or proposed changes in applicable law.
137
Table of Contents
We are offering the shares of our common stock described in this prospectus through the underwriters named below. UBS Securities LLC and Jefferies & Company, Inc. are the book-running managers of this offering and the representatives of the underwriters. We have entered into an underwriting agreement with the representatives. Subject to the terms and conditions of the underwriting agreement, each of the underwriters has severally agreed to purchase the number of shares of common stock listed next to its name in the following table.
| Underwriter | Number of shares | |
| UBS Securities LLC |
||
| Jefferies & Company, Inc. |
||
| Robert W. Baird & Co. Incorporated |
||
| Cowen and Company, LLC |
||
| Total |
||
The underwriting agreement provides that the underwriters must buy all of the shares if they buy any of them. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Our common stock is offered subject to a number of conditions, including:
| § | receipt and acceptance of our common stock by the underwriters; and |
| § | the underwriters’ right to reject orders in whole or in part. |
In connection with this offering, certain of the underwriters or securities dealers may distribute prospectuses electronically.
Over-Allotment Option
We have granted the underwriters an option to buy up to an aggregate of additional shares of our common stock. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. The underwriters have 30 days from the date of this prospectus to exercise this option. If the underwriters exercise this option, they will each purchase additional shares approximately in proportion to the amounts specified in the table above.
Commissions and Discounts
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount of up to $ per share from the initial public offering price. Any of these securities dealers may resell any shares purchased from the underwriters to other brokers or dealers at a discount of up to $ per share from the public offering price.
Sales of shares made outside the United States may be made by affiliates of the underwriters. If all the shares are not sold at the initial public offering price, the representatives may change the offering price and the other selling terms. Upon execution of the underwriting agreement, the underwriters will be obligated to purchase the shares at the prices and upon the terms stated therein. The representatives of the underwriters have informed us that they do not expect to sell more than an aggregate of shares of common stock to accounts over which such representatives exercise discretionary authority.
138
Table of Contents
The following table shows the per share and total underwriting discounts and commissions we will pay to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares of our common stock.
| No exercise | Full exercise | |||||
| Per share |
$ | $ | ||||
| Total |
$ | $ | ||||
We estimate that the total expenses of this offering payable by us, not including the underwriting discounts and commissions, will be approximately $ million.
No Sales of Similar Securities
We, our executive officers and directors and holders of shares our common stock and options and warrants to purchase shares of our common stock have entered into lock-up agreements with the underwriters. Under these agreements, subject to certain exceptions, we and each of these persons may not, without the prior written approval of UBS Securities LLC and Jefferies & Company, Inc., offer, sell, contract to sell or otherwise dispose of, directly or indirectly, or hedge our common stock or securities convertible into or exchangeable or exercisable for our common stock. These restrictions will be in effect for a period of 180 days after the date of this prospectus. At any time and without public notice, UBS Securities LLC and Jefferies & Company, Inc. may, in their sole discretion, release some or all of the securities from these lock-up agreements.
If:
| § | during the period that begins on the date that is 15 calendar days plus 3 business days before the last day of the 180-day lock-up period and ends on the last day of the 180-day lock-up period, |
| § | we issue an earnings release; or |
| § | material news or a material event relating to us occurs; or |
| § | prior to the expiration of the 180-day lock-up period, we announce that we will release earnings results during the 16-day period beginning on the last day of the 180-day lock-up period, |
then the 180-day lock-up period will be extended until the expiration of the date that is 15 calendar days plus 3 business days after the date on which the issuance of the earnings release or the material news or material event occurs.
Indemnification
We have agreed to indemnify the underwriters and their controlling persons against certain liabilities, including certain liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters may be required to make in respect of those liabilities.
NASDAQ Global Market Listing
We have applied to have our common stock listed on The NASDAQ Global Market under the trading symbol “GNOM.”
Price Stabilization, Short Positions
In connection with this offering, the underwriters may engage in activities that stabilize, maintain or otherwise affect the price of our common stock, including:
| § | stabilizing transactions; |
| § | short sales; |
| § | purchases to cover positions created by short sales; |
| § | imposition of penalty bids; and |
| § | syndicate covering transactions. |
139
Table of Contents
Stabilizing transactions consist of bids or purchases made for the purpose of preventing or retarding a decline in the market price of our common stock while this offering is in progress. These transactions may also include making short sales of our common stock, which involve the sale by the underwriters of a greater number of shares of common stock than they are required to purchase in this offering, and purchasing shares of common stock on the open market to cover positions created by short sales. Short sales may be “covered short sales,” which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked short sales,” which are short positions in excess of that amount.
The underwriters may close out any covered short position either by exercising their over-allotment option, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option.
Naked short sales are short sales made in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market that could adversely affect investors who purchased in this offering.
The underwriters also may impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of that underwriter in stabilizing or short covering transactions.
As a result of these activities, the price of our common stock may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they may be discontinued by the underwriters at any time. The underwriters may carry out these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
Determination of Offering Price
Prior to this offering, there was no public market for our common stock. The initial public offering price will be determined by negotiation by us and the representatives of the underwriters. The principal factors to be considered in determining the initial public offering price include:
| § | the information set forth in this prospectus and otherwise available to representatives; |
| § | our history and prospects and the history of and prospects for the industry in which we compete; |
| § | our past and present financial performance and an assessment of our management; |
| § | our prospects for future earnings and the present state of our development; |
| § | the general condition of the securities markets at the time of this offering; |
| § | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| § | other factors deemed relevant by the underwriters and us. |
Affiliations
Certain of the underwriters and their affiliates may from time to time in the future provide certain commercial banking, financial advisory, investment banking and other services for us for which they will be entitled to receive separate fees. The underwriters and their affiliates may from time to time in the future engage in transactions with us and perform services for us in the ordinary course of their respective businesses.
140
Table of Contents
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area, or EEA, which has implemented the Prospectus Directive (each, a “Relevant Member State”), with effect from, and including, the date on which the Prospectus Directive is implemented in that Relevant Member State (the “Relevant Implementation Date”), an offer to the public of our securities which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State, except that, with effect from, and including, the Relevant Implementation Date, an offer to the public in that Relevant Member State of our securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
a) to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in our securities;
b) to any legal entity which has two or more of: (1) an average of at least 250 employees during the last (or, in Sweden, the last two) financial year(s); (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last (or, in Sweden, its last two) annual or consolidated accounts;
c) to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the representatives for any such offer; or
d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of our securities shall result in a requirement for the publication by us or any underwriter or agent of a prospectus pursuant to Article 3 of the Prospectus Directive.
As used above, the expression “offered to the public” in relation to any of our securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and our securities to be offered so as to enable an investor to decide to purchase or subscribe for our securities, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State; and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
The EEA selling restrictions are in addition to any other selling restrictions set forth in this prospectus.
Notice to Prospective Investors in the United Kingdom
This prospectus is being distributed only to and is only directed at: (1) persons who are outside the United Kingdom; (2) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”) or (3) high net worth companies, and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons falling within (1) through (3) together being referred to as “relevant persons”). The shares are available only to, and any invitation, offer or agreement to subscribe, purchase or otherwise acquire such shares will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this prospectus or any of its contents.
Notice to Prospective Investors in Switzerland
This prospectus does not constitute an issue prospectus pursuant to Article 652a or Article 1156 of the Swiss Code of Obligations (“CO”), and the shares will not be listed on the SIX Swiss Exchange. Therefore, this prospectus may not comply with the disclosure standards of the CO and/or the listing rules (including any prospectus schemes) of the SIX Swiss Exchange. Accordingly, the shares may not be offered to the public in or from Switzerland, but only to a selected and limited circle of investors, which do not subscribe to the shares with a view to distribution.
141
Table of Contents
Notice to Prospective Investors in Australia
This prospectus is not a formal disclosure document and has not been, nor will be, lodged with the Australian Securities and Investments Commission. It does not purport to contain all information that an investor or his, her or its professional advisers would expect to find in a prospectus or other disclosure document (as defined in the Corporations Act 2001 (Australia)) for the purposes of Part 6D.2 of the Corporations Act 2001 (Australia) or in a product disclosure statement for the purposes of Part 7.9 of the Corporations Act 2001 (Australia), in either case, in relation to the securities.
The securities are not being offered in Australia to “retail clients” as defined in sections 761G and 761GA of the Corporations Act 2001 (Australia). This offering is being made in Australia solely to “wholesale clients” for the purposes of section 761G of the Corporations Act 2001 (Australia), and, as such, no prospectus, product disclosure statement or other disclosure document in relation to the securities has been, or will be, prepared.
This prospectus does not constitute an offer in Australia other than to wholesale clients. By submitting an application for our securities, you represent and warrant to us that you are a wholesale client for the purposes of section 761G of the Corporations Act 2001 (Australia). If any recipient of this prospectus is not a wholesale client, no offer of, or invitation to apply for, our securities shall be deemed to be made to such recipient and no applications for our securities will be accepted from such recipient. Any offer to a recipient in Australia, and any agreement arising from acceptance of such offer, is personal and may only be accepted by the recipient. In addition, by applying for our securities, you undertake to us that, for a period of 12 months from the date of issue of the securities, you will not transfer any interest in the securities to any person in Australia other than to a wholesale client.
Notice to Prospective Investors in Hong Kong
Our securities may not be offered or sold in Hong Kong by means of this prospectus or any document other than (1) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, (2) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong) or (3) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong). No advertisement, invitation or document relating to our securities may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere) which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to the securities which are, or are intended to be, disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Japan
Our securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the “Financial Instruments and Exchange Law”), and our securities will not be offered or sold, directly or indirectly, in Japan, or to, or for the benefit of, any resident of Japan (which term, as used herein, means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan, or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
Notice to Prospective Investors in Singapore
This document has not been registered as a prospectus with the Monetary Authority of Singapore, and, in Singapore, the offer and sale of our securities is made pursuant to exemptions provided in sections 274 and 275 of the Securities and Futures Act, Chapter 289 of Singapore (“SFA”). Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of our
142
Table of Contents
securities may not be circulated or distributed, nor may our securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore, other than (1) to an institutional investor as defined in Section 4A of the SFA pursuant to Section 274 of the SFA; (2) to a relevant person as defined in section 275(2) of the SFA pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (3) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with the conditions (if any) set forth in the SFA. Moreover, this document is not a prospectus as defined in the SFA. Accordingly, statutory liability under the SFA in relation to the content of prospectuses would not apply. Prospective investors in Singapore should consider carefully whether an investment in our securities is suitable for them.
Where our securities are subscribed for or purchased under Section 275 of the SFA by a relevant person, which is:
(a) a corporation (which is not an accredited investor as defined in Section 4A of the SFA) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
(b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, shares of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for six months after that corporation or that trust has acquired the shares under Section 275 of the SFA, except:
(1) to an institutional investor (for corporations under Section 274 of the SFA) or to a relevant person defined in Section 275(2) of the SFA, or to any person pursuant to an offer that is made on terms that such shares of that corporation or such rights and interest in that trust are acquired at a consideration of not less than 200,000 Singapore dollars (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid in cash or by exchange of securities or other assets, and further for corporations, in accordance with the conditions, specified in Section 275 of the SFA;
(2) where no consideration is given for the transfer; or
(3) where the transfer is by operation of law.
In addition, investors in Singapore should note that securities acquired by them are subject to resale and transfer restrictions specified under Section 276 of the SFA, and they, therefore, should seek their own legal advice before effecting any resale or transfer of their securities.
143
Table of Contents
The validity of our common stock offered by this prospectus will be passed upon for us by Latham & Watkins LLP, Menlo Park, California. Certain attorneys and investment funds affiliated with the firm own shares of our preferred stock, which will convert into an aggregate of 33,144 shares of our common stock, warrants to purchase convertible preferred stock, which will net exercise into shares of our common stock assuming an initial public offering price of $ per share (the midpoint of the price range set forth on the cover page of this prospectus), in each case, immediately prior to the consummation of this offering, and warrants to purchase an aggregate of 1,800 shares of common stock. Dewey & LeBoeuf LLP, New York, New York, is counsel for the underwriters in connection with this offering.
The financial statements as of December 31, 2008 and 2009 and for each of the three years in the period ended December 31, 2009, included in this prospectus, have been so included in reliance on the report (which contains an explanatory paragraph relating to the Company’s ability to continue as a going concern as described in Note 1 to the financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of such firm as experts in auditing and accounting.
Where You Can Find Additional Information
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act relating to the shares of our common stock we are offering. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules to the registration statement. Some items are omitted in accordance with the rules and regulations of the SEC. For further information with respect to us and the common stock we are offering, we refer you to the registration statement and its exhibits and schedules. Statements contained in this prospectus as to the contents of any contract, agreement or other document are summaries of the material terms of that contract, agreement or other document, and we refer you to a copy of the entire contract, agreement or other document that we have filed as an exhibit to the registration statement. A copy of the registration statement, and its exhibits and schedules, may be inspected without charge at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of these materials may be obtained by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facility. The SEC maintains a web site that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the SEC’s website is http://www.sec.gov.
Upon completion of this offering, we will become subject to the information and periodic reporting requirements of the Exchange Act and will file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information will be available for inspection and copying at the public reference facility and web site of the SEC referred to above. We maintain a website at www.completegenomics.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements on Schedule 14A and amendments to those reports free of charge at our website as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The reference to our website address does not constitute incorporation by reference of the information contained in, or that can be accessed through, our website.
144
Table of Contents
(A development stage enterprise)
Financial Statements Index
| Page | ||
| F-2 | ||
| Financial Statements |
||
| F-3 | ||
| F-4 | ||
| Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit) |
F-5 | |
| F-7 | ||
| F-9 | ||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Complete Genomics, Inc.
(A development stage enterprise)
In our opinion, the accompanying balance sheets and the related statements of operations, of convertible preferred stock and stockholders’ equity (deficit) and of cash flows present fairly, in all material respects, the financial position of Complete Genomics, Inc. (the “Company”) (a development stage enterprise) at December 31, 2008 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to December 31, 2009 (not separately presented) in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred losses from operations since its inception and negative cash flow from operations that raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. These financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ PricewaterhouseCoopers LLP
San Jose, California
July 30, 2010
F-2
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
| December 31, | June 30, 2010 |
Pro forma stockholders’ equity as of June 30, 2010 |
||||||||||||||
| 2008 | 2009 | |||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||
| Assets |
||||||||||||||||
| Current assets |
||||||||||||||||
| Cash and cash equivalents |
$ | 6,186 | $ | 7,765 | $ | 7,972 | ||||||||||
| Accounts receivable |
— | 1,288 | 1,154 | |||||||||||||
| Inventory |
— | 354 | 2,239 | |||||||||||||
| Prepaid expenses |
468 | 5,156 | 579 | |||||||||||||
| Other current assets |
566 | 456 | 415 | |||||||||||||
| Total current assets |
7,220 | 15,019 | 12,359 | |||||||||||||
| Property and equipment, net |
8,023 | 14,864 | 26,557 | |||||||||||||
| Other assets |
511 | 395 | 879 | |||||||||||||
| Total assets |
$ | 15,754 | $ | 30,278 | $ | 39,795 | ||||||||||
| Liabilities, Convertible Preferred Stock and Stockholders’ Equity (Deficit) |
||||||||||||||||
| Current liabilities |
||||||||||||||||
| Accounts payable |
$ | 745 | $ | 4,281 | $ | 5,121 | ||||||||||
| Accrued liabilities |
1,744 | 2,032 | 2,575 | |||||||||||||
| Notes payable, current |
3,990 | 4,440 | 21,987 | |||||||||||||
| Deferred revenue |
— | 1,302 | 3,536 | |||||||||||||
| Total current liabilities |
6,479 | 12,055 | 33,219 | |||||||||||||
| Notes payable, net of current |
7,707 | 3,510 | 1,192 | |||||||||||||
| Deferred rent, net of current |
— | 5,017 | 4,673 | |||||||||||||
| Convertible preferred stock warrant liability |
1,100 | 1,553 | 1,318 | $ | — | |||||||||||
| Total liabilities |
15,286 | 22,135 | 40,402 | |||||||||||||
| Commitments and contingencies (Note 5) |
||||||||||||||||
| Convertible preferred stock, par value $0.001—6,933,332 shares authorized; 508,942 and 6,472,996 shares issued and outstanding at December 31, 2008 and 2009, respectively; 9,288,222 shares authorized and 7,819,758 shares issued and outstanding at June 30, 2010 (unaudited); no shares issued and outstanding pro forma (unaudited) (liquidation value—$127,721 and $142,994 (unaudited) at December 31, 2009 and June 30, 2010, respectively) |
45,622 | 85,833 | 95,844 | — | ||||||||||||
| Stockholders’ equity (deficit) |
||||||||||||||||
| Common stock, $0.001 par value—13,333,334 shares authorized; 93,825 and 94,281 shares issued and outstanding at December 31, 2008 and 2009, respectively; 17,293,925 shares authorized; 950,493 shares issued and outstanding at June 30, 2010 (unaudited); shares issued and outstanding, pro forma (unaudited) |
— | — | 1 | 12 | ||||||||||||
| Additional paid-in capital |
58 | 3,471 | 11,676 | 108,827 | ||||||||||||
| Deficit accumulated during the development stage |
(45,212 | ) | (81,161 | ) | (108,128 | ) | (108,128 | ) | ||||||||
| Total stockholders’ equity (deficit) |
(45,154 | ) | (77,690 | ) | (96,451 | ) | $ | 711 | ||||||||
| Total liabilities, convertible preferred stock and stockholders’ deficit |
$ | 15,754 | $ | 30,278 | $ | 39,795 | ||||||||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
| Years ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 |
||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||||||||||
| Revenue |
$ | — | $ | — | $ | 623 | $ | — | $ | 1,425 | $ | 2,048 | ||||||||||||
| Operating expenses: |
||||||||||||||||||||||||
| Start-up production costs |
— | — | 5,033 | 1,013 | 8,985 | 14,018 | ||||||||||||||||||
| Research and development |
10,305 | 23,633 | 22,424 | 10,449 | 11,097 | 71,191 | ||||||||||||||||||
| General and administrative |
1,896 | 3,179 | 4,953 | 2,120 | 4,862 | 15,841 | ||||||||||||||||||
| Sales and marketing |
— | 1,045 | 1,798 | 620 | 2,539 | 5,382 | ||||||||||||||||||
| Total operating expenses |
12,201 | 27,857 | 34,208 | 14,202 | 27,483 | 106,432 | ||||||||||||||||||
| Loss from operations |
(12,201 | ) | (27,857 | ) | (33,585 | ) | (14,202 | ) | (26,058 | ) | (104,384 | ) | ||||||||||||
| Interest expense |
(215 | ) | (974 | ) | (3,465 | ) | (2,051 | ) | (1,144 | ) | (5,809 | ) | ||||||||||||
| Interest and other income (expense), net |
163 | 437 | 1,101 | (70 | ) | 235 | 2,065 | |||||||||||||||||
| Net loss |
$ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,323 | ) | $ | (26,967 | ) | $ | (108,128 | ) | ||||||
| Net loss per share—basic and diluted |
$ | (211.00 | ) | $ | (369.36 | ) | $ | (386.56 | ) | $ | (177.96 | ) | $ | (45.09 | ) | |||||||||
| Weighted-average shares of common stock outstanding used in computing net loss per share—basic and diluted |
58,072 | 76,873 | 92,998 | 91,724 | 598,080 | |||||||||||||||||||
| Pro forma net loss per share of common stock—basic and diluted (unaudited) |
$ | (6.59 | ) | $ | (2.53 | ) | ||||||||||||||||||
| Weighted-average shares of common stock outstanding used in computing the pro forma net loss per share of common stock—basic and diluted (unaudited) |
5,619,600 | 10,763,515 | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Statement of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
| Convertible preferred stock |
Common stock | Additional paid-in capital |
Deficit accumulated during the development stage |
Total stockholders’ equity (deficit) |
||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||
| (in thousands, except share and per share amounts) |
(in thousands, except share and per share amounts) |
|||||||||||||||||||||||
| Beginning Balance |
— | $ | — | — | $ | — | $ | — | $ | — | $ | — | ||||||||||||
| Issuance of common stock to founders for cash in June 2005 at $0.001 per share |
— | — | 78,332 | — | 2 | — | 2 | |||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | |||||||||||||||||
| Balances at December 31, 2005 |
— | — | 78,332 | — | 2 | — | 2 | |||||||||||||||||
| Issuance of Series A redeemable convertible preferred stock for cash in March 2006 at $43.85 per share, net of issuance costs of $184 |
137,972 | 5,866 | — | — | — | — | — | |||||||||||||||||
| Issuance of common stock in connection with an intellectual property agreement in March 2006 at $5.10 per share |
— | — | 13,333 | — | 69 | — | 69 | |||||||||||||||||
| Employee stock based compensation |
— | — | — | — | 11 | — | 11 | |||||||||||||||||
| Nonemployee stock based compensation |
— | — | — | — | 1 | — | 1 | |||||||||||||||||
| Accretion of interest due on Series A redeemable convertible preferred stock |
— | 370 | — | — | (370 | ) | — | (370 | ) | |||||||||||||||
| Net loss |
— | — | — | — | — | (4,565 | ) | (4,565 | ) | |||||||||||||||
| Balances at December 31, 2006 |
137,972 | 6,236 | 91,665 | — | (287 | ) | (4,565 | ) | (4,852 | ) | ||||||||||||||
| Issuance of Series B convertible preferred stock for cash in March 2007 at $69.00 per share, net of issuance costs of $179 |
189,128 | 12,868 | — | — | — | — | — | |||||||||||||||||
| Issuance of Series B convertible preferred stock upon conversion of promissory notes and accrued interest in March 2007 at $69.00 per share |
14,492 | 1,003 | — | — | — | — | — | |||||||||||||||||
| Employee stock based compensation |
— | — | — | — | 88 | — | 88 | |||||||||||||||||
| Nonemployee stock based compensation |
— | — | — | — | 12 | — | 12 | |||||||||||||||||
| Accretion of interest due on Series A redeemable convertible preferred stock |
— | 116 | — | — | (116 | ) | — | (116 | ) | |||||||||||||||
| Net loss |
— | — | — | — | — | (12,253 | ) | (12,253 | ) | |||||||||||||||
| Balances at December 31, 2007 |
341,592 | 20,223 | 91,665 | — | (303 | ) | (16,818 | ) | (17,121 | ) | ||||||||||||||
F-5
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Statement of Convertible Preferred Stock and Stockholders’ Equity (Deficit)—(Continued)
| Convertible preferred stock |
Common stock | Additional paid-in capital |
Deficit accumulated during the development stage |
Total stockholders’ equity (deficit) |
|||||||||||||||||||||
| Shares | Amount | Shares | Amount | ||||||||||||||||||||||
| (in thousands, except share and per share amounts) |
(in thousands, except share and per share amounts) |
||||||||||||||||||||||||
| Balances at December 31, 2007 |
341,592 | 20,223 | 91,665 | — | (303 | ) | (16,818 | ) | (17,121 | ) | |||||||||||||||
| Issuance of Series C convertible preferred stock for cash in February 2008 at $159.30 per share, net of issuance costs of $1,101 |
166,346 | 25,399 | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series C convertible preferred stock as issuance costs in February 2008 at $159.30 per share |
1,004 | — | — | — | — | — | — | ||||||||||||||||||
| Employee stock based compensation |
— | — | — | — | 284 | — | 284 | ||||||||||||||||||
| Nonemployee stock based compensation |
— | — | — | — | 52 | — | 52 | ||||||||||||||||||
| Exercise of stock options |
— | — | 2,160 | — | 25 | — | 25 | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (28,394 | ) | (28,394 | ) | ||||||||||||||||
| Balances at December 31, 2008 |
508,942 | 45,622 | 93,825 | — | 58 | (45,212 | ) | (45,154 | ) | ||||||||||||||||
| Issuance of Series D convertible preferred stock for cash in August 2009 at $7.56 per share, net of issuance costs of $2,879 |
3,972,729 | 27,156 | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series D convertible preferred stock upon conversion of promissory notes and accrued interest in August 2009 |
1,991,325 | 15,054 | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock warrants in connection with Series D convertible preferred stock |
— | (1,999 | ) | — | — | 1,999 | — | 1,999 | |||||||||||||||||
| Employee stock based compensation |
— | — | — | — | 1,142 | — | 1,142 | ||||||||||||||||||
| Nonemployee stock based compensation |
— | — | — | — | 268 | — | 268 | ||||||||||||||||||
| Exercise of stock options |
— | — | 456 | — | 4 | — | 4 | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (35,949 | ) | (35,949 | ) | ||||||||||||||||
| Balances at December 31, 2009 |
6,472,996 | 85,833 | 94,281 | — | 3,471 | (81,161 | ) | (77,690 | ) | ||||||||||||||||
| Issuance of Series D convertible preferred stock for cash in February and March 2010 at $7.56 per share, net of issuance costs of $171 (unaudited) |
1,346,762 | 10,011 | — | — | — | — | — | ||||||||||||||||||
| Issuance of common stock to founders in March 2010 at $2.34 per share for services rendered (unaudited) |
— | — | 786,533 | 1 | 1,839 | — | 1,840 | ||||||||||||||||||
| Issuance of common stock warrants in connection with promissory notes (unaudited) |
— | — | — | — | 5,372 | — | 5,372 | ||||||||||||||||||
| Employee stock based compensation (unaudited) |
— | — | — | — | 819 | — | 819 | ||||||||||||||||||
| Nonemployee stock based compensation (unaudited) |
— | — | — | — | 70 | — | 70 | ||||||||||||||||||
| Exercise of stock options (unaudited) |
— | — | 69,679 | — | 105 | — | 105 | ||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | — | (26,967 | ) | (26,967 | ) | ||||||||||||||||
| Balances at June 30, 2010 (unaudited) |
7,819,758 | $ | 95,844 | 950,493 | $ | 1 | $ | 11,676 | $ | (108,128 | ) | $ | (96,451 | ) | |||||||||||
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
| Years ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 |
||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Cash flows from operating activities |
||||||||||||||||||||||||
| Net loss |
$ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,323 | ) | $ | (26,967 | ) | $ | (108,128 | ) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||||||||||||||
| Depreciation and amortization |
788 | 2,795 | 5,240 | 2,034 | 3,033 | 11,994 | ||||||||||||||||||
| Amortization of debt issuance costs |
78 | 226 | 259 | 130 | 130 | 693 | ||||||||||||||||||
| Issuance of common stock in exchange for intellectual property |
— | — | — | — | — | 69 | ||||||||||||||||||
| Issuance of common stock to founders |
— | — | — | — | 1,840 | 1,840 | ||||||||||||||||||
| Change in fair value of convertible preferred stock warrant liability |
175 | (65 | ) | (1,088 | ) | 75 | (235 | ) | (1,210 | ) | ||||||||||||||
| Stock-based compensation |
100 | 336 | 1,410 | 343 | 889 | 2,747 | ||||||||||||||||||
| Noncash interest expense related to the promissory notes and notes payable |
3 | — | 2,113 | 1,322 | 638 | 2,754 | ||||||||||||||||||
| (Gain) loss on the disposal of property and equipment |
— | 506 | 31 | (42 | ) | 36 | 573 | |||||||||||||||||
| Changes in assets and liabilities |
||||||||||||||||||||||||
| Accounts receivable |
— | — | (1,288 | ) | (230 | ) | 134 | (1,154 | ) | |||||||||||||||
| Inventory |
— | — | (354 | ) | (88 | ) | (1,885 | ) | (2,239 | ) | ||||||||||||||
| Prepaid expenses |
(161 | ) | (231 | ) | (4,688 | ) | (157 | ) | 4,577 | (567 | ) | |||||||||||||
| Other current assets |
(128 | ) | (190 | ) | 110 | 184 | 41 | (167 | ) | |||||||||||||||
| Other assets |
(61 | ) | 27 | (143 | ) | (171 | ) | (62 | ) | (293 | ) | |||||||||||||
| Accounts payable |
633 | (30 | ) | 1,078 | 270 | 1,226 | 3,049 | |||||||||||||||||
| Accrued liabilities |
842 | 717 | 288 | 35 | 543 | 2,575 | ||||||||||||||||||
| Deferred revenue |
— | — | 1,302 | 250 | 2,234 | 3,536 | ||||||||||||||||||
| Deferred rent |
— | — | 5,017 | 993 | (344 | ) | 4,673 | |||||||||||||||||
| Net cash used in operating activities |
(9,984 | ) | (24,303 | ) | (26,662 | ) | (11,375 | ) | (14,172 | ) | (79,255 | ) | ||||||||||||
| Cash flows from investing activities |
||||||||||||||||||||||||
| Purchase of property and equipment |
(3,729 | ) | (7,419 | ) | (9,654 | ) | (1,607 | ) | (15,700 | ) | (37,604 | ) | ||||||||||||
| Net cash used in investing activities |
(3,729 | ) | (7,419 | ) | (9,654 | ) | (1,607 | ) | (15,700 | ) | (37,604 | ) | ||||||||||||
F-7
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Statements of Cash Flows—(Continued)
| Years ended December 31, | Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 |
||||||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Cash flows from financing activities |
||||||||||||||||||||||||
| Proceeds from promissory notes |
1,000 | — | 14,725 | 12,075 | 22,121 | 37,846 | ||||||||||||||||||
| Proceeds from notes payable |
2,806 | 13,194 | — | — | — | 17,000 | ||||||||||||||||||
| Repayment of notes payable |
(333 | ) | (4,970 | ) | (3,990 | ) | (1,943 | ) | (2,158 | ) | (11,451 | ) | ||||||||||||
| Proceeds from issuance of convertible preferred stock, net of issuance costs |
12,868 | 25,399 | 27,156 | — | 10,011 | 81,300 | ||||||||||||||||||
| Proceeds from issuance of common stock |
— | — | — | — | — | 2 | ||||||||||||||||||
| Exercise of stock options |
— | 25 | 4 | 4 | 105 | 134 | ||||||||||||||||||
| Net cash provided by financing activities |
16,341 | 33,648 | 37,895 | 10,136 | 30,079 | 124,831 | ||||||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
2,628 | 1,926 | 1,579 | (2,846 | ) | 207 | 7,972 | |||||||||||||||||
| Cash and cash equivalents at beginning of period |
1,632 | 4,260 | 6,186 | 6,186 | 7,765 | — | ||||||||||||||||||
| Cash and cash equivalents at end of period |
$ | 4,260 | $ | 6,186 | $ | 7,765 | $ | 3,340 | $ | 7,972 | $ | 7,972 | ||||||||||||
| Supplemental disclosure of cash flow information |
||||||||||||||||||||||||
| Cash paid for interest |
$ | 94 | $ | 349 | $ | 1,068 | $ | 586 | $ | 369 | $ | 1,888 | ||||||||||||
| Supplemental disclosure of noncash investing and financing activities |
||||||||||||||||||||||||
| Accretion of interest due on Series A redeemable convertible preferred stock |
$ | 116 | $ | — | $ | — | $ | — | $ | — | $ | 486 | ||||||||||||
| Issuance of warrants for convertible preferred stock in connection with promissory notes and notes payable |
172 | 779 | 1,541 | 1,517 | — | 2,528 | ||||||||||||||||||
| Issuance of warrants for common stock in connection with Series D convertible preferred stock financing |
— | — | 1,999 | — | — | 1,999 | ||||||||||||||||||
| Issuance of common stock in connection with intellectual property |
— | — | — | — | — | 69 | ||||||||||||||||||
| Conversion of promissory notes and interest into convertible preferred stock |
1,003 | — | 15,054 | — | — | 16,057 | ||||||||||||||||||
| Issuance of convertible preferred stock as payment for costs associated with the issuance of Series C preferred convertible stock |
— | 160 | — | — | — | 160 | ||||||||||||||||||
| Acquisition of property and equipment under accounts payable |
— | — | 2,458 | 1,558 | (938 | ) | 1,520 | |||||||||||||||||
| Issuance of warrants for common stock in connection with promissory notes |
— | — | — | — | 5,372 | 5,372 | ||||||||||||||||||
| Accrued deferred offering costs |
— | — | — | — | 552 | 552 | ||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-8
Table of Contents
(A development stage enterprise)
Notes to Financial Statements
| 1. | Nature of Operations |
Complete Genomics, Inc., (the “Company”) is a life sciences company that has developed and commercialized a DNA sequencing platform for complete human genome sequencing and analysis. The Company’s Complete Genomics Analysis Platform (“CGA Platform”) combines its proprietary human sequencing technology with its advanced informatics and data management software and its end-to-end outsourced service model to provide customers with data that is immediately ready to be used for genome-based research. The Company’s solution provides academic and biopharmaceutical researchers with complete human genomic data and analysis without requiring them to invest in in-house sequencing instruments, high-performance computing resources and specialized personnel. The Company was incorporated in Delaware on June 14, 2005 and began operations in March 2006. The Company is in the development stage and since inception has been engaged in developing its complete human genome sequencing technology, raising capital and recruiting personnel.
These financial statements are prepared on a going concern basis that contemplates the realization of assets and discharge of liabilities in their normal course of business. The Company has incurred net operating losses and negative cash flows from operations during every year since inception. At December 31, 2009 and June 30, 2010, the Company had a deficit accumulated during the development stage of $81,161,000 and $108,128,000 (unaudited), respectively, and currently does not have financing sufficient for continued operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. In order to continue its operations, the Company must achieve profitable operations and/or obtain additional debt or equity financing. Management is currently pursuing financing alternatives. However, there can be no assurance that such financing will be successfully completed or completed on terms acceptable to the Company. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation and Use of Estimates
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and include all adjustments necessary for the fair presentation of the Company’s financial position, results of operations and cash flows for the periods presented. In preparing the financial statements, management must make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Unaudited Interim Financial Information
The accompanying balance sheet as of June 30, 2010, the statements of operations and of cash flows for the six months ended June 30, 2009 and 2010, and for the cumulative period from June 14, 2005 (date of inception) to June 30, 2010, and the statements of convertible preferred stock and stockholders’ equity (deficit) for the six months ended June 30, 2010 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all normal recurring adjustments necessary to state fairly the Company’s financial position as of June 30, 2010 and results of operations and cash flows for the six months ended June 30, 2009 and 2010, and for the cumulative period from June 14, 2005 (date of inception) to June 30, 2010. The financial data and other information disclosed in these notes to the financial statements related to the six month periods are unaudited. The results for the six months ended June 30, 2010 are not necessarily indicative of the results to be expected for the year ending December 31, 2010 or for any other interim periods or for any future year.
F-9
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Unaudited Pro Forma Information
The June 30, 2010 unaudited pro forma stockholders’ equity has been prepared assuming that immediately prior to the consummation of an initial public offering, (i) all of the Company’s convertible preferred stock outstanding will convert into shares of common stock, (ii) 105,595 of the Company’s convertible preferred stock warrants will become warrants for common stock and (iii) 278,558 of the Company’s convertible preferred stock warrants will net exercise into shares of the Company’s common stock. The June 30, 2010 unaudited pro forma stockholders’ equity reflects the conversion of all 7,819,758 outstanding shares of preferred stock into 10,533,490 shares of common stock and the reclassification of the convertible preferred stock warrant liability to common stock.
Stock Split
The Company initiated a 30-for-1 reverse stock split effective November 2009. All share and per share amounts in these financial statements have been retroactively adjusted to give effect to the reverse stock split.
Segment Information
The Company operates in one segment. Management uses one measure of profitability and does not segment its business for internal reporting. All of the Company’s assets are located in the United States.
Revenue Recognition
The Company generates revenue from selling its human genome sequencing services under purchase orders or contracts. Revenues are recognized when all of the following criteria are met: persuasive evidence of an arrangement exists, title has transferred, the price is fixed or determinable and collectability is reasonably assured. Upon completion of the sequencing process, the Company ships the research-ready genomic data to the customer. The Company uses shipping documents and third-party evidence to verify shipment of the data. In order to determine whether collectability is reasonably assured, the Company assesses a number of factors, including past transaction history with the customer and the creditworthiness of the customer. If the Company determines that collectability is not reasonably assured, the Company defers the recognition of revenue until collectability becomes reasonably assured. The Company also receives down payments from customers prior to the commencement of the genome sequencing process.
For revenue generated under purchase orders, the Company has established standard terms and conditions that are specified for all orders. The Company uses the purchase order to establish persuasive evidence of an arrangement and whether there is a fixed and determinable price for the order. Revenue is recognized based upon the shipment of genomic data to customers and satisfaction of all terms and conditions contained in the purchase order. Any down payments received are recorded as deferred revenue until the Company meets all revenue recognition criteria.
For revenue generated under contracts, the Company considers each contract’s terms and conditions to determine its obligations associated with the contract. The Company will defer revenue until all significant obligations, as defined in the contract, have been met. Any down payments received are recorded as deferred revenue, and revenue is recognized upon shipment of genomic data and satisfaction of all remaining obligations.
Concentration of Credit Risk and Other Risks and Uncertainties
The Company’s accounts receivable are derived from direct sales and amounts contractually due, but not received, under contracts. The Company reviews its exposure to accounts receivable and generally requires no collateral for any of its accounts receivable. The allowance for doubtful accounts is the Company’s best estimate
F-10
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
of the amount of expected credit losses existing in accounts receivable and is based upon specific customer issues that have been identified. As of December 31, 2009 and June 30, 2010 (unaudited), the Company has not recorded any allowance for doubtful accounts.
The Company is subject to all of the risks inherent in an early-stage company developing a new approach to DNA sequencing. These risks include, but are not limited to, limited management resources, intense competition, dependence upon consumer acceptance of the products in development and the changing nature of the DNA sequencing industry. The Company’s operating results may be materially affected by the foregoing factors.
The Company depends on a limited number of suppliers, including single-source suppliers, of various critical components in the sequencing process. The loss of these suppliers, or their failure to supply the Company with the necessary components on a timely basis, could cause delays in the sequencing process and adversely affect the Company.
As of December 31, 2009 and June 30, 2010, customers representing greater than 10% of accounts receivable were as follows:
| Customer | December 31, 2009 |
June 30, 2010 |
||||
| (unaudited) | ||||||
| Customer A |
43 | % | * | |||
| Customer B |
20 | % | * | |||
| Customer C |
* | 32 | % | |||
| Customer D |
* | 12 | % | |||
| * | Less than 10% |
For the year ended December 31, 2009 and the six months ended June 30, 2010, customers representing greater than 10% of revenue were as follows:
| Customer | December 31, 2009 |
June 30, 2010 |
||||
| (unaudited) | ||||||
| Customer A |
16 | % | 20 | % | ||
| Customer E |
16 | % | * | |||
| Customer F |
16 | % | * | |||
| Customer G |
17 | % | * | |||
| Customer H |
16 | % | * | |||
| Customer I |
13 | % | * | |||
| Customer J |
* | 15 | % | |||
| Customer K |
* | 11 | % | |||
| * | Less than 10% |
For the year ended December 31, 2009 and the six months ended June 30, 2010, countries representing greater than 10% of revenue were as follows:
| Country | December 31, 2009 |
June 30, 2010 |
||||
| (unaudited) | ||||||
| Belgium |
16 | % | * | |||
| China |
* | 15 | % | |||
| United States |
84 | % | 67 | % | ||
| * | Less than 10% |
F-11
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Fair Value of Financial Instruments
Carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued liabilities, approximate fair value due to short maturities. Based on borrowing rates currently available to the Company for promissory notes and notes payable with similar terms, the carrying value of promissory notes and notes payable approximate their fair value.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less to be cash equivalents. Cash and cash equivalents are deposited in a demand account at one financial institution and a money market fund. The use of one financial institution potentially subjects the Company to a significant concentration of credit risk. At times, such deposits may be in excess of federally insured limits.
Property and Equipment, Net
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives as follows:
| Computer equipment |
3 years | |
| Computer software |
3 years | |
| Furniture and fixtures |
5 years | |
| Machinery and equipment |
3 years |
Leasehold improvements are amortized over the shorter of the useful life or the remaining term of the lease. Upon retirement or sale, the cost and related accumulated depreciation are removed from the balance sheet and the resulting gain or loss is reflected in operations. Maintenance and repairs are charged to operations as incurred.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparison of the carrying amount to the future net cash flows which the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows arising from the asset. There have been no such impairments of long-lived assets to date.
Inventories
Inventories consist of chemical reagents and sequencing supplies that are stated at the lower of cost or market value. Cost is determined using standard costs, which approximate actual costs on a first-in, first-out basis. Market value is determined as the lower of replacement cost or net realizable value.
Start-up Production Costs
Start-up production costs are incurred during the period of development and validation of the production process and include the costs associated with commercialization of the sequencing process. The Company’s start-up production costs primarily consist of costs related to the acceptance testing of customer genomic samples, sample
F-12
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
sequencing preparation, sample sequencing, the processing of data generated by the prototype sequencing instruments, continued validation of the production process and optimization of instrument performance. These costs primarily include salaries and benefits and stock-based compensation expenses, chemical reagents and engineering materials and supplies, consultant fees, depreciation of equipment and facilities-related costs.
Research and Development
Research and development costs are charged to operations as incurred. Research and development costs include, but are not limited to, salaries and benefits and stock-based compensation expenses, laboratory supplies, consulting costs and other overhead expenses.
Government Grant
The Company accounts for its government grant as a reduction of expense related to either research and development or general and administrative expense. The allocation is based on the grant agreement and the related expense reimbursed. Total reimbursement of expenses by the government grant is as follows:
| Years ended December 31, |
Six months ended June 30, |
Cumulative period from June 14, 2005 (date of inception) to June 30, 2010 | ||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||||
| (in thousands) | ||||||||||||||||||
| Research and development expense |
$ | 22 | $ | 773 | $ | 800 | $ | 377 | $ | 386 | $ | 1,981 | ||||||
| General and administrative expense |
8 | 250 | 213 | 107 | 97 | 568 | ||||||||||||
| $ | 30 | $ | 1,023 | $ | 1,013 | $ | 484 | $ | 483 | $ | 2,549 | |||||||
Stock-Based Compensation
The Company accounts for stock-based compensation arrangements with employees using a fair value method which requires the recognition of compensation expense for costs related to all stock-based payments, including stock options. The fair value method requires the Company to estimate the fair value of stock-based payment awards on the date of grant using an option pricing model. The Company uses the Black-Scholes pricing model to estimate the fair value of options granted, which is expensed on a straight-line basis over the vesting period.
The Company accounts for stock options issued to nonemployees based on the estimated fair value of the awards using the Black-Scholes option pricing model. The measurement of stock-based compensation is subject to periodic adjustments as the underlying equity instruments vest, and the resulting change in value, if any, is recognized in the Company’s statements of operations during the period the related services are rendered.
Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires, among other things, that deferred income taxes be provided for temporary differences between the tax basis of the Company’s assets and liabilities and their financial statement reported amounts. In addition, deferred tax assets are recorded for the future benefit of utilizing net operating losses and research and development credit carryforwards. A valuation allowance is provided against deferred tax assets unless it is more likely than not that they will be realized.
Effective January 1, 2007, the Company adopted the accounting guidance for uncertainties in income taxes, which prescribes a recognition threshold and measurement process for recording uncertain tax positions taken, or
F-13
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
expected to be taken, in a tax return in the financial statements. Additionally, the guidance also prescribes new treatment for the derecognition, classification, accounting in interim periods and disclosure requirements for uncertain tax positions. The Company accrues for the estimated amount of taxes for uncertain tax positions if it is more likely than not that the Company would be required to pay such additional taxes. An uncertain tax position will not be recognized if it has a less than 50% likelihood of being sustained.
Convertible Preferred Stock Warrants
The Company accounts for its freestanding warrants for shares of the Company’s convertible preferred stock that are contingently redeemable as liabilities at fair value on the balance sheets. The warrants are subject to re-measurement at each balance sheet date, and the change in fair value, if any, is recognized as interest and other income (expense), net. The Company will continue to adjust the liability for changes in fair value until the earlier of (i) exercise of the warrants, (ii) conversion into warrants to purchase common stock or (iii) expiration of the warrants. Upon conversion, the convertible preferred stock warrant liability will be reclassified to additional paid-in capital.
Convertible Preferred Stock
The holders of the Company’s outstanding convertible preferred stock, voting or consenting together as a separate class, control the vote of the Company’s stockholders. As a result, the holders of all series of the Company’s convertible preferred stock can force a change-in-control that would trigger liquidation. As redemption of the convertible preferred stock through liquidation is outside the control of the Company, all shares of convertible preferred stock have been presented outside of stockholders’ equity (deficit) in the Company’s balance sheets. All series of convertible preferred stock are collectively referred to in the financial statements as convertible preferred stock.
Net Loss Per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding shares subject to repurchase. The Company’s potential dilutive shares, which include outstanding common stock options, unvested common shares subject to repurchase, convertible preferred stock and warrants, have not been included in the computation of diluted net loss per share for all the periods as the result would be anti-dilutive. Such potentially dilutive shares are excluded when the effect would be to reduce the net loss per share.
Pro Forma Net Loss Per Share
The unaudited pro forma basic and diluted net loss per share for the year ended December 31, 2009 and the six months ended June 30, 2010 (unaudited) reflects the conversion of all outstanding shares of convertible preferred stock to common stock. The unaudited pro forma stockholders’ equity and pro forma basic and diluted net loss per share do not give effect to the issuance of shares from the planned initial public offering nor do they give effect to potential dilutive securities where the impact would be anti-dilutive. Also, the numerator in the pro forma basic and diluted net loss per share calculation has been adjusted to remove gains and losses resulting from re-measurements of the outstanding convertible preferred stock warrant liability through June 30, 2010 as it is assumed that these warrants will either be converted to potentially dilutive shares or be net exercised prior to an initial public offering and will no longer require periodic revaluation.
F-14
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
A reconciliation of the numerator and denominator used in the calculation of basic and diluted net loss per share follows:
| Years ended December 31, |
Six months ended June 30, |
|||||||||||||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||||||||||||
| (unaudited) | ||||||||||||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||||||||||
| Historical net loss per share: |
||||||||||||||||||||
| Numerator |
||||||||||||||||||||
| Net loss attributed to common stockholders |
$ | (12,253 | ) | $ | (28,394 | ) | $ | (35,949 | ) | $ | (16,323 | ) | $ | (26,967 | ) | |||||
| Denominator |
||||||||||||||||||||
| Weighted-average common shares outstanding |
91,665 | 92,549 | 94,242 | 94,212 | 598,080 | |||||||||||||||
| Less: Weighted-average shares subject to repurchase |
(33,593 | ) | (15,676 | ) | (1,244 | ) | (2,488 | ) | — | |||||||||||
| Denominator for basic and diluted net loss per share |
58,072 | 76,873 | 92,998 | 91,724 | 598,080 | |||||||||||||||
| Basic and diluted net loss per share |
$ | (211.00 | ) | $ | (369.36 | ) | $ | (386.56 | ) | $ | (177.96 | ) | $ | (45.09 | ) | |||||
| Pro forma net loss per share: |
||||||||||||||||||||
| Net loss attributed to common stockholders |
$ | (35,949 | ) | $ | (26,967 | ) | ||||||||||||||
| Pro forma adjustment to reverse the mark-to-market adjustment attributable to the convertible preferred stock warrants (unaudited) |
(1,088 | ) | (235 | ) | ||||||||||||||||
| Net loss used to compute pro forma net loss per share (unaudited) |
$ | (37,037 | ) | $ | (27,202 | ) | ||||||||||||||
| Denominator |
||||||||||||||||||||
| Weighted-average shares of common stock outstanding used in computing net loss per share of common stock, basic and diluted |
92,998 | 598,080 | ||||||||||||||||||
| Pro forma adjustments to reflect assumed weighted-average effect of conversion of convertible preferred stock (unaudited) |
5,526,602 | 10,165,435 | ||||||||||||||||||
| Denominator for pro forma basic and diluted net loss per share (unaudited) |
5,619,600 | 10,763,515 | ||||||||||||||||||
| Pro forma basic and diluted net loss per share (unaudited) |
$ | (6.59 | ) | $ | (2.53 | ) | ||||||||||||||
The following outstanding shares of potentially dilutive securities were excluded from the computation of diluted net loss per share for the periods presented because including them would have had an anti-dilutive effect:
| December 31, | June 30, | |||||||||
| 2007 | 2008 | 2009 | 2009 | 2010 | ||||||
| (unaudited) | ||||||||||
| Options to purchase common stock |
76,943 | 105,920 | 1,535,469 | 110,474 | 2,251,093 | |||||
| Common stock subject to repurchase |
25,381 | 7,465 | — | — | — | |||||
| Warrants to purchase convertible preferred stock |
2,815 | 7,710 | 384,153 | 281,788 | 384,153 | |||||
| Warrants to purchase common stock |
— | — | 1,630,629 | — | 2,581,005 | |||||
| Convertible preferred stock (on an as-if converted basis) |
1,843,530 | 3,222,674 | 9,186,728 | 3,222,674 | 10,533,490 | |||||
| Total |
1,948,669 | 3,343,769 | 12,736,979 | 3,614,936 | 15,749,741 | |||||
F-15
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Recent Accounting Pronouncements
In October 2009, the Financial Accounting Standards Board (FASB) issued a new accounting standard that changes the accounting for revenue arrangements with multiple deliverables. Specifically, the new accounting standard requires an entity to allocate arrangement consideration at the inception of an arrangement to all of its deliverables based on their relative selling prices. In addition, the new standard eliminates the use of the residual method of allocation and requires the relative-selling-price method in all circumstances in which an entity recognizes revenue for an arrangement with multiple deliverables. In October 2009, the FASB also issued a new accounting standard that changes revenue recognition for tangible products containing software and hardware elements. Specifically, if certain requirements are met, revenue arrangements that contain tangible products with software elements that are essential to the functionality of the products are scoped out of the existing software revenue recognition accounting guidance and will be accounted for under these new accounting standards. Both standards will be effective for the Company in the first quarter of 2011. Early adoption is permitted. The Company is currently assessing the impact that the adoption of these standards will have on its financial statements.
In January 2010, the FASB issued an amendment to an accounting standard which requires new disclosures for fair value measures and provides clarification for existing disclosure requirements. Specifically, this amendment requires an entity to disclose separately the amounts of significant transfers in and out of Level 1 and Level 2 fair value measurements and to describe the reasons for the transfers; and to disclose separately information about purchases, sales, issuances and settlements in the reconciliation for fair value measurements using significant unobservable inputs, or Level 3 inputs. This amendment clarifies existing disclosure requirements for the level of disaggregation used for classes of assets and liabilities measured at fair value and requires disclosure about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements using Level 2 and Level 3 inputs. This guidance is effective for interim and annual reporting periods beginning after December 15, 2009, except for certain Level 3 activity disclosure requirements that will be effective for reporting periods after December 15, 2010. Accordingly the Company adopted this amendment on January 1, 2010, except for the additional Level 3 requirements, which will be adopted in 2011. Other than requiring additional disclosures, adoption of this new guidance did not have a material impact on the Company’s financial statements.
| 3. | Fair Value Measurement |
Assets and liabilities recorded at fair value in the financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. Hierarchical levels which are directly related to the amount of subjectivity associated with the inputs to the valuation of these assets or liabilities are as follows:
Level 1—Observable inputs, such as quoted prices in active markets for identical assets or liabilities.
Level 2—Observable inputs, other than Level 1 prices, such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The following table sets forth the Company’s financial instruments that were measured at fair value on a recurring basis as of December 31, 2008 and 2009 and June 30, 2010 by level within the fair value hierarchy. Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability.
F-16
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
As of December 31, 2008, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows:
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| (in thousands) | ||||||||||||
| Assets |
||||||||||||
| Money market fund (included in Cash and cash equivalents) |
$ | 6,186 | $ | — | $ | — | $ | 6,186 | ||||
| Liabilities |
||||||||||||
| Convertible preferred stock warrant liability |
$ | — | $ | — | $ | 1,100 | $ | 1,100 | ||||
As of December 31, 2009, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows:
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| (in thousands) | ||||||||||||
| Assets |
||||||||||||
| Money market fund (included in Cash and cash equivalents) |
$ | 6,120 | $ | — | $ | — | $ | 6,120 | ||||
| Liabilities |
||||||||||||
| Convertible preferred stock warrant liability |
$ | — | $ | — | $ | 1,553 | $ | 1,553 | ||||
As of June 30, 2010, the Company’s fair value hierarchy for its financial assets and financial liabilities that are carried at fair value was as follows (unaudited):
| Level 1 | Level 2 | Level 3 | Total | |||||||||
| (in thousands) | ||||||||||||
| Assets |
||||||||||||
| Money market fund (included in Cash and cash equivalents) |
$ | 621 | $ | — | $ | — | $ | 621 | ||||
| Liabilities |
||||||||||||
| Convertible preferred stock warrant liability |
$ | — | $ | — | $ | 1,318 | $ | 1,318 | ||||
The Company values its convertible preferred stock warrant liability (Note 7) using the Black-Scholes option pricing model. The expected term for these warrants is based on the remaining contractual life of these warrants. The expected volatility assumption was determined by examining the historical volatility for industry peers, as the Company does not have a trading history for its common stock. The risk-free interest rate assumption is based on U.S. Treasury investments whose term is consistent with the expected term of the warrants. The expected dividend assumption is based on the Company’s history and expectation of dividend payouts.
The change in the fair value of the convertible preferred warrant liability is summarized below:
| (in thousands) | ||||
| Fair value at December 31, 2007 |
$ | 386 | ||
| Decrease in the fair value recorded in interest and other income (expense), net |
(65 | ) | ||
| Issuance of convertible preferred stock warrants |
779 | |||
| Fair value at December 31, 2008 |
1,100 | |||
| Decrease in the fair value recorded in interest and other income (expense), net |
(1,088 | ) | ||
| Issuance of convertible preferred stock warrants |
1,541 | |||
| Fair value at December 31, 2009 |
1,553 | |||
| Decrease in the fair value recorded in interest and other income (expense), net (unaudited) |
(235 | ) | ||
| Fair value at June 30, 2010 (unaudited) |
$ | 1,318 | ||
F-17
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
| 4. | Balance Sheet Components |
Inventory
Inventory consists of the following:
| December 31, 2009 |
June 30, 2010 | |||||
| (unaudited) | ||||||
| (in thousands) | ||||||
| Raw materials |
$ | 237 | $ | 1,290 | ||
| Work-in-progress |
38 | 665 | ||||
| Finished goods |
79 | 284 | ||||
| Total |
$ | 354 | $ | 2,239 | ||
The Company did not have any inventory as of December 31, 2008.
Property and Equipment, Net
Property and equipment, net, consist of the following:
| December 31, | June 30, | |||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| (unaudited) | ||||||||||||
| (in thousands) | ||||||||||||
| Computer equipment |
$ | 3,323 | $ | 5,107 | $ | 6,934 | ||||||
| Computer software |
1,038 | 1,390 | 1,801 | |||||||||
| Furniture and fixtures |
267 | 341 | 351 | |||||||||
| Machinery and equipment |
6,140 | 7,612 | 13,592 | |||||||||
| Leasehold Improvements |
337 | 6,064 | 6,567 | |||||||||
| Equipment under construction |
— | 1,451 | 4,663 | |||||||||
| 11,105 | 21,965 | 33,908 | ||||||||||
| Less: Accumulated depreciation and amortization |
(3,082 | ) | (7,101 | ) | (7,351 | ) | ||||||
| $ | 8,023 | $ | 14,864 | $ | 26,557 | |||||||
Depreciation and amortization expense for the years ended December 31, 2007, 2008 and 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010 was $788,000, $2,795,000, $5,240,000 and $11,994,000 (unaudited), respectively. Depreciation and amortization expense for the six months ended June 30, 2009 and 2010 was $2,034,000 (unaudited) and $3,033,000 (unaudited), respectively.
F-18
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Accrued Liabilities
Accrued liabilities consist of the following:
| December 31, | June 30, | ||||||||
| 2008 | 2009 | 2010 | |||||||
| (unaudited) | |||||||||
| (in thousands) | |||||||||
| Accrued professional fees |
$ | 295 | $ | 70 | $ | — | |||
| Accrued vacation expense |
653 | 923 | 1,182 | ||||||
| Accrued 401(k) expense |
27 | 64 | 54 | ||||||
| Outside services |
349 | 135 | 41 | ||||||
| Accrued interest |
102 | 69 | 50 | ||||||
| Accrued compensation |
— | — | 437 | ||||||
| Deferred rent, current |
— | 341 | 667 | ||||||
| Other |
318 | 430 | 144 | ||||||
| $ | 1,744 | $ | 2,032 | $ | 2,575 | ||||
| 5. | Commitments and Contingencies |
Operating Lease Obligations
In October 2007, the Company entered into an agreement for office facilities consisting of approximately 10,560 square feet under an operating lease, which began on January 1, 2008 and expires on December 31, 2010.
In October 2008, the Company entered into an agreement for office facilities consisting of approximately 66,096 square feet under an operating lease, which began on March 1, 2009 and expires on August 31, 2016.
The Company recognizes rent expense on a straight-line basis over the non-cancellable lease term and records the difference between cash rent payments and the recognition of rent expense as a deferred rent liability. Where leases contain escalation clauses, rent abatements and/or concessions, such as rent holidays and landlord or tenant incentives or allowances, the Company applies them in the determination of straight-line rent expense over the lease term. Rent expense for the years ended December 31, 2007, 2008 and 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010 was $438,000, $850,000, $1,914,000 and $4,312,000 (unaudited), respectively. Rent expense for the six months ended June 30, 2009 and 2010 was $924,000 (unaudited) and $988,000 (unaudited), respectively.
Future minimum lease payments under these non-cancellable operating leases as of December 31, 2009 are as follows:
| (in thousands) | |||
| Years Ending December 31, |
|||
| 2010 |
$ | 2,610 | |
| 2011 |
2,510 | ||
| 2012 |
2,588 | ||
| 2013 |
2,668 | ||
| 2014 |
2,747 | ||
| Thereafter |
4,763 | ||
| Total future minimum lease payments |
$ | 17,886 | |
F-19
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Secured Equipment Loan Agreements
In September 2006, the Company entered into a secured equipment loan agreement with Silicon Valley Bank (“SVB”). The loan was for $1,000,000 and had an interest rate of the greater of 8.5% or prime plus 0.5% as determined at the time of the draw down. Repayment of the amount outstanding began on January 1, 2007 and was payable in 36 equal monthly installments. In connection with the loan, the Company issued warrants to purchase the Company’s Series A convertible preferred stock as discussed in Note 7. The loan was repaid in full as part of the July 2008 loan agreement.
In August 2007, the Company entered into a secured equipment loan agreement with SVB and Gold Hill Venture Lending (“Gold Hill”). The loan was for $3,000,000 and had an interest rate of the greater of 9.25% or prime plus 1% as determined at the time of the draw down. Repayment on the amount outstanding began three months after the initial draw and was payable in 36 equal monthly installments. In connection with the loan, the Company issued warrants to purchase the Company’s Series B convertible preferred stock as discussed in Note 7. The Company drew down $194,000 in 2008. The loan was repaid as part of the July 2008 loan agreement.
In July 2008, the Company entered into a secured equipment loan agreement with SVB, Leader Equity LLC and Oxford Finance Corporation. The loan is for $13,000,000 and was drawn in four tranches between July and December 2008. The interest rate for each tranche was set at an interest rate of the greater of 10.50% or prime plus 8.03%. The interest rates on the tranches of the loans range between 10.50% to 11.04% as determined at the time of the draw down. The loans require a termination payment be made with the final loan payment. The termination payment is calculated based upon 4% of each of the draw amounts and causes the loans to have an effective interest rate of 12.81% to 13.34%. The loan required that the proceeds be first used to repay the balances outstanding on the August 2007 and September 2006 loan agreements noted above. Repayment of the July 2008 loan began one month after the first draw down under the loan and continues for 36 equal monthly installments. In connection with the loan, the Company issued warrants to purchase the Company’s Series C convertible preferred stock, which were later converted into warrants to purchase Series D convertible preferred stock, as discussed in Note 7. The Company has pledged as collateral all property and equipment purchased pursuant to the secured equipment loan agreements. The Company drew down the full amount during the year ended December 31, 2008. There are no financial covenants in the secured equipment loan agreement.
Future loan repayments under the July 2008 loan agreement as of December 31, 2009 are as follows:
| (in thousands) | ||||
| Years Ending December 31, |
||||
| 2010 |
$ | 5,057 | ||
| 2011 |
3,926 | |||
| Total payments |
8,983 | |||
| Less: |
||||
| Interest expense |
(756 | ) | ||
| Unaccreted termination payment |
(277 | ) | ||
| Total principal payments |
7,950 | |||
| Less: Notes payable, current |
4,440 | |||
| Notes payable, net of current |
$ | 3,510 | ||
Promissory Notes
In April, May and June 2010, the Company issued promissory notes to certain of its existing investors for an aggregate principal amount of $22,121,452. The principal amount of the promissory notes accrues interest at an
F-20
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
annual rate of 8%. The promissory notes mature at the earliest of a corporate reorganization as defined in the promissory notes, the consummation of an initial public offering of the Company’s common stock, an event of default pursuant to the terms of the promissory notes or April 12, 2011. In the event that the Company issues shares of a new series of preferred stock with aggregate gross cash proceeds in excess of $17,000,000, the outstanding principal and interest of the promissory notes will automatically convert into that series of preferred stock at the lowest price paid by an investor in the financing (not to exceed $7.56 per share). In August 2010, the promissory notes converted into Series E preferred stock, as discussed in Note 14. In connection with the promissory notes, the Company issued warrants to purchase the Company’s common stock, as discussed in Note 9.
Intellectual Property Agreement
In March 2006, the Company entered into an intellectual property agreement (Note 11) with Callida Genomics, Inc. for licenses related to the Company’s core technology. Under the agreement, the Company will make annual payments of $250,000 for a duration of six years starting in March 2006. The payments are recorded as research and development expense.
Purchase Commitments
As of December 31, 2009, the Company had outstanding purchase commitments related to its data center and related connectivity and non-cancellable orders for sequencing components of $7.0 million.
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company’s management does not believe that any such matters, individually or in the aggregate, will have a material adverse effect on the Company’s business, financial condition, results of operations or cash flows.
Indemnification
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the future, but that have not yet been made. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
In accordance with its Certificate of Incorporation and bylaws, the Company has indemnification obligations to its officers and directors for certain events or occurrences, subject to certain limits, while they are serving at the Company’s request in such capacity. There have been no claims to date, and the Company has director and officer insurance that enables it to recover a portion of any amounts paid for future potential claims.
F-21
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
| 6. | Convertible Preferred Stock |
The Company’s Certificate of Incorporation, as amended, authorizes the Company to issue 9,288,222 shares of $0.001 par value preferred stock.
Convertible preferred stock (“Preferred Stock”) at December 31, 2008 consists of the following:
| Series | Shares authorized |
Shares issued and outstanding |
Liquidation amount |
Proceeds, net of issuance costs | ||||||
| (in thousands, except share amounts) | ||||||||||
| A |
138,658 | 137,972 | $ | 6,050 | $ | 5,866 | ||||
| A-1 |
138,658 | — | — | — | ||||||
| B |
206,000 | 203,620 | 14,050 | 13,871 | ||||||
| B-1 |
206,000 | — | — | — | ||||||
| C |
175,000 | 167,350 | 39,989 | 25,399 | ||||||
| C-1 |
175,000 | — | — | — | ||||||
| 1,039,316 | 508,942 | $ | 60,089 | $ | 45,136 | |||||
Preferred Stock at December 31, 2009 consists of the following:
| Series | Shares authorized |
Shares issued and outstanding |
Liquidation amount |
Proceeds, net of issuance costs | ||||||
| (in thousands, except share amounts) | ||||||||||
| A |
138,658 | 137,972 | $ | 6,050 | $ | 5,866 | ||||
| B |
205,758 | 203,620 | 14,050 | 13,871 | ||||||
| C |
167,357 | 167,350 | 39,989 | 25,399 | ||||||
| D |
6,421,559 | 5,964,054 | 67,632 | 40,211 | ||||||
| 6,933,332 | 6,472,996 | $ | 127,721 | $ | 85,347 | |||||
Preferred Stock at June 30, 2010 consists of the following (unaudited):
| Series | Shares authorized |
Shares issued and outstanding |
Liquidation amount |
Proceeds, net of issuance costs | ||||||
| (in thousands, except share amounts) | ||||||||||
| A |
138,658 | 137,972 | $ | 6,050 | $ | 5,866 | ||||
| B |
205,758 | 203,620 | 14,050 | 13,871 | ||||||
| C |
167,357 | 167,350 | 39,989 | 25,399 | ||||||
| D |
8,776,449 | 7,310,816 | 82,905 | 50,222 | ||||||
| 9,288,222 | 7,819,758 | $ | 142,994 | $ | 95,358 | |||||
The rights and preferences of holders of Preferred Stock are as follows:
Dividends
Holder of Series A, B, C and D convertible preferred stock are entitled to receive non-cumulative dividends at the per annum rate of $3.51, $5.52, $12.74 and $0.60 per share, respectively, (as adjusted for stock splits, combinations reorganizations and the like) out of any assets at the time legally available therefor, when, as and if declared by the Board of Directors. Such dividends are payable in preference to any dividends on common stock. There have been no dividends declared to date.
F-22
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Conversion Rights
Each share of Preferred Stock is convertible, at the option of the holder, at any time after the date of issuance of such share. Each share of Series A, B, C and D convertible preferred stock shall be convertible into that number of fully paid and nonassessable shares of common stock that is equal to $43.85, $69.00, $159.30 and $159.30, respectively, divided by the conversion price of $9.50, $11.64, $19.33 and $159.30, respectively, (as adjusted for stock splits, combinations, reorganizations and the like). The current conversion terms with respect to Series A, B, C and D convertible preferred stock are 1:4.6 shares, 1:5.9 shares, 1:8.2 shares and 1:1 share, respectively.
Each share of Preferred Stock, automatically converts into the number of shares of common stock into which such shares are convertible at the then effective conversion price immediately upon (1) the affirmative vote of the holders of more than 60% of the outstanding Preferred Stock, (2) the consummation of a firmly underwritten public offering pursuant to the Securities Act of 1933, as amended, on Form S-1 or any successor form, provided, however, that (i) the per share price to the public is at least $238.95 (as adjusted for stock splits, combinations, reorganizations and the like) and (ii) the aggregate gross proceeds to the Company are not less than $40,000,000.
Special Mandatory Conversion
If the Company issues preferred stock at a price per share less than $159.30, (as adjusted for stock splits, combinations, reorganizations and the like) with aggregate proceeds of over $2,000,000 from an institutional or other professional investor and a preferred stockholder does not purchase its pro rata shares in the offering, its shares shall automatically convert into shares of common stock at the applicable conversion rate at the time of the preferred stock issuance.
Liquidation Rights
In the event of liquidation, dissolution or winding up of the Company, the holders of Series A, B, C and D convertible preferred stock shall be entitled to receive, in preference to the distribution of any assets of the Company to holders of common stock, an amount equal to $43.85, $69.00, $238.95 and $11.34 per share, respectively, plus any declared but unpaid dividends. If upon the occurrence of such event, the amounts available for distribution among holders of Preferred Stock are insufficient to pay the aforementioned preferential amounts, the entire assets of the Company legally available for distribution shall be distributed among the holders of Series D convertible preferred stock, then among the holders of Series C convertible preferred stock and then ratably among the holders of Series A and B convertible preferred stock together in proportion to the preferential amount each holder is otherwise entitled to receive.
After completion of the distribution to holders of Preferred Stock, any remaining assets of the Company shall be distributed with equal priority, pro rata among the holders of the Series A, B and D convertible preferred stock and the holders of common stock, treating each share of Series A, B and D convertible preferred stock as if it had been converted into common stock at the then applicable conversion rate up to 300%, 300% and 225%, respectively, of the liquidation preference for such shares of convertible preferred stock. Any remaining funds will then be distributed to common stockholders.
Voting Rights
The holder of each share of Preferred Stock is entitled to one vote for each share of common stock into which shares of such Preferred Stock could then be converted. The holder of each share of Preferred Stock votes together with common stockholders and not as a separate class.
F-23
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Redemption
Prior to the issuance of Series B convertible preferred stock in March 2007, Series A convertible preferred stock was redeemable at the election of 75% of the then outstanding shares of Series A convertible preferred stock on or after March 24, 2013. The redemption would have required the Company to pay a per share sum equal to the liquidation preference of the Series A redeemable convertible preferred stock plus an amount per share calculated at a rate of 8% per annum compounded quarterly, and unpaid but declared dividends. The shares were to be redeemed in three equal annual payments after the notice to redeem was issued. The first payment would occur no later than 40 days after such written notice. Upon the issuance of Series B convertible preferred stock in March 2007, Series A became nonredeemable and the cumulative dividend stated above was cancelled. Since the shares of Series A convertible preferred stock were mandatorily redeemable through March 2007, the Company has accreted interest costs of $486,000 as of December 31, 2009 and June 30, 2010 (unaudited).
| 7. | Warrants for Convertible Preferred Stock |
The Company had the following unexercised convertible preferred stock warrants:
| Exercise price per share |
Shares as of December 31, |
Fair value as
of December 31, | |||||||||||
| Underlying Stock | 2008 | 2009 | 2008 | 2009 | |||||||||
| (in thousands, except share and per share amounts) | |||||||||||||
| Series A |
$ | 43.85 | 684 | 684 | $ | 61 | $ | 5 | |||||
| Series B |
$ | 69.00 | 2,131 | 2,131 | 198 | 21 | |||||||
| Series C |
$ | 159.30 | 4,895 | — | 841 | — | |||||||
| Series D |
$ | 7.56 | — | 381,338 | — | 1,527 | |||||||
| Total |
7,710 | 384,153 | $ | 1,100 | $ | 1,553 | |||||||
| Underlying Stock | Exercise price per share |
Shares as of 2010 |
Fair value as of 2010 | |||||
| (unaudited) | ||||||||
| (in thousands, except share and per share amounts) | ||||||||
| Series A |
$ | 43.85 | 684 | $ | 6 | |||
| Series B |
$ | 69.00 | 2,131 | 23 | ||||
| Series D |
$ | 7.56 | 381,338 | 1,289 | ||||
| Total |
384,153 | $ | 1,318 | |||||
Series A
In 2006, the Company issued warrants to purchase 684 shares of Series A convertible preferred stock at an exercise price of $43.85 per share. These warrants were issued in connection with the secured equipment loan agreement. The initial fair value of the warrants of $36,000 was recorded as a credit to warrant liability and a discount to the carrying value of the debt. The discount was fully amortized in 2008. The warrants expire on the tenth anniversary after their issuance date. Immediately prior to the consummation of an initial public offering as defined in the warrants, the preferred stock warrants will convert to common stock warrants.
Series B
In 2007, the Company issued warrants to purchase 393 shares of Series B convertible preferred stock at an exercise price of $69.00 per share. These warrants were issued in connection with promissory notes. The initial fair value of the warrants of $25,000 was recorded as a credit to warrant liability and a discount to the carrying
F-24
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
value of the debt. The discount on the debt was fully amortized to interest expense upon conversion of the debt in 2007. These warrants expire upon the earlier of the fifth anniversary after their issuance date or the closing of an initial public offering as defined in the warrant. If these warrants are not exercised prior to an initial public offering and have not yet expired, these warrants will automatically net exercise into shares of common stock at the initial public offering price of the common stock immediately prior to the consummation of the offering, assuming the initial public offering price is greater than the exercise price of the warrants.
In 2007, the Company issued warrants to purchase 1,738 shares of Series B convertible preferred stock at an exercise price of $69.00 per share. These warrants were issued in connection with the August 2007 secured equipment loan. The initial fair value of the warrants of $172,000 was recorded as a credit to warrant liability and an issuance cost of the debt. The issuance cost of the debt was fully amortized in 2008. The warrants expire on the tenth anniversary after their issuance date. Immediately prior to the consummation of an initial public offering, the preferred stock warrants will convert to common stock warrants.
Series C converted into Series D
In 2008, the Company issued warrants to purchase 4,895 shares of Series C convertible preferred stock at an exercise price of $159.30 per share. These warrants were issued in connection with the July 2008 secured equipment loan. The exercise price and number of warrants were subject to change upon the closing of a Series D preferred stock financing agreement. In conjunction with the Series D convertible preferred stock financing in August 2009, the warrants became exercisable for Series D convertible preferred stock. The conversion resulted in warrants to purchase 103,173 shares of Series D convertible preferred stock with an exercise price of $7.56. The initial fair value of the warrants of $779,000 was recorded as a credit to warrant liability and an issuance cost of the debt. The issuance cost of the debt is being amortized to interest expense over the life of the debt. In 2008 and 2009, the Company had amortized debt issuance costs of $108,000 and $259,000, respectively. The Company had amortized debt issuance costs of $130,000 (unaudited) for both the six months ended June 30, 2009 and 2010. The warrants expire on the tenth anniversary after their issuance date. Immediately prior to the consummation of an initial public offering, the preferred stock warrants will convert to common stock warrants.
Series D
In 2009, the Company issued warrants to purchase 278,165 shares of Series D convertible preferred stock at an exercise price of $7.56 per share. These warrants were issued in connection with promissory notes. The initial fair value of the warrants of $1,541,000 was recorded as a credit to warrant liability and a discount to the carrying value of the promissory note. The discount on the promissory notes was amortized to interest expense over the life of the debt in 2009. In August 2009, the discount was fully amortized upon the conversion of the promissory notes. These warrants expire upon the earlier of the fifth anniversary after their issuance date or upon consummation of an initial public offering. If these warrants are not exercised prior to an initial public offering and have not yet expired, these warrants will automatically net exercise into shares of common stock at the initial public offering price of the common stock immediately prior to the consummation of the offering, assuming the initial public offering price is greater than the exercise price of the warrants.
F-25
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
The assumptions used in the initial valuation of all convertible preferred stock warrants using the Black-Scholes model are as follows:
| 2006 Series A |
2007 Series B |
2008 Series C |
2009 Series D | |||||
| Contractual term (years) |
10 | 5-10 | 10 | 5 | ||||
| Volatility |
98.56% | 100.48 - 102.01% | 99.97% | 91.39 - 106.57% | ||||
| Dividend |
0% | 0% | 0% | 0% | ||||
| Risk-free interest rate |
4.69% | 4.56 - 4.72% | 4.01% | 1.82 - 2.71% |
The assumptions used to revalue all convertible preferred stock warrants using the Black-Scholes model are as follows:
| December 31, |
June 30, | |||||||
| 2008 | 2009 | 2009 | 2010 | |||||
| (unaudited) | ||||||||
| Remaining contractual term (years) |
3.15 - 9.58 | 2.15 - 8.58 | 2.65 - 9.08 | 1.65 - 8.08 | ||||
| Volatility |
91.27 - 102.65% | 71.98 - 91.55% | 101.58 - 124.65% | 76.71 - 85.53% | ||||
| Dividend |
0% | 0% | 0% | 0% | ||||
| Risk-free rate |
1.07 - 2.42% | 1.38 - 3.33% | 1.76 - 3.55% | 1.17 - 3.20% | ||||
The Company adjusts the fair value of the convertible preferred warrants at the end of each reporting period. The fair value of the warrants as of December 31, 2009 and June 30, 2010 was $1,553,000 and $1,318,000 (unaudited), respectively. The change in the fair value for the years ended December 31, 2007, 2008 and 2009 and cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010, resulted in an increase of $175,000, and a decrease of $65,000, $1,088,000 and $1,210,000 (unaudited), respectively, which was reflected in interest and other income (expense), net. The change in fair value for the six months ended June 30, 2009 and 2010 resulted in an increase of $75,000 (unaudited) and a decrease of $235,000 (unaudited), respectively, which was reflected in interest and other income (expense), net.
| 8. | Common Stock |
The Company’s Certificate of Incorporation, as amended, authorizes the Company to issue 17,293,925 shares of $0.001 par value common stock. Common stockholders are entitled to dividends, subject to preferred stock dividends, when and if declared by the Board of Directors. There have been no dividends declared to date. The holder of each common share is entitled to one vote.
On June 14, 2005, 78,332 shares of restricted common stock were issued at $0.001 per share to the Company’s founders under a restricted stock purchase agreement. The shares vested over a four-year period. The unvested shares of common stock are subject to repurchase by the Company in the event of termination of the employment relationship. Included in common stock outstanding as of December 31, 2008 and 2009 are 7,465 and 0 shares, respectively, which are subject to the Company’s right to repurchase relating to these agreements.
On March 28, 2006, the Company issued 13,333 shares of common stock to Callida Genomics, Inc. in consideration for entering into an intellectual property license agreement. The Company recorded the common stock’s fair value of $69,000 as research and development expense.
On March 10, 2010 (unaudited), the Company granted 786,533 shares of common stock to its founders. The Company recorded the common stock’s fair value of $1,840,000 as an expense, of which $915,000 was recorded in general and administrative expense and $925,000 in research and development expense.
F-26
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
| 9. | Warrants for Common Stock |
On August 12, 2009, the Company issued warrants to purchase 1,630,629 shares of common stock at an exercise price of $1.50 per share. These warrants were issued in connection with the Company’s Series D convertible preferred stock offering. The initial fair value of the warrants was calculated using the Black-Scholes option pricing model with the following assumptions: seven year expected term; 105.56% volatility; 0% dividend; and a risk-free interest rate of 3.21%. The fair value of $1,999,000 was allocated to the warrants and recorded as a credit to additional paid-in capital and as a reduction of the proceeds from Series D convertible preferred stock. These warrants expire upon the earlier of the seventh anniversary after their issuance date or upon consummation of an initial public offering. If these warrants are not exercised prior to an initial public offering and have not yet expired, these warrants will automatically net exercise into shares of common stock at the initial public offering price of the common stock immediately prior to the consummation of the offering, assuming the initial public offering price is greater than the exercise price of the warrants.
In April, May and June 2010, the Company issued to the holders of promissory notes a warrant to purchase a number of shares of common stock equal to the product of 5% of the principal amount of the promissory notes and the number of months between the date of issuance of the warrant and the date of the next financing (up to five months), divided by $1.50. Warrants to purchase an aggregate of 3,686,893 (unaudited) shares of common stock were outstanding as of June 30, 2010, of which the contingency for warrants to purchase 950,376 (unaudited) shares of common stock was satisfied as of June 30, 2010. The warrants have an exercise price of $1.50 per share and expire upon the fifth anniversary of their issuance date which is the same date as the issue date of the relevant promissory notes. The initial value of the warrants was calculated using the Black-Scholes option pricing model with the following assumptions: five year expected term; volatility ranging from 80.94% to 81.43% (unaudited); 0% dividend (unaudited); and a risk-free interest rate ranging from 2.0% to 2.58% (unaudited). The fair value of $5,372,000 (unaudited) was allocated to the warrants and recorded as a credit to additional paid-in capital and as a reduction of the proceeds of the promissory notes. The fair value of the warrants is being amortized to interest expense using the effective interest rate method over the term of the notes.
| 10. | Stock Option Plan |
In 2006, the Company adopted the 2006 Equity Incentive Plan (the “Plan”) which provides for the granting of stock options to employees, directors and consultants of the Company. Options granted under the Plan may be either incentive stock options (“ISOs”) or nonqualified stock options (“NSOs”). The Company has reserved 3,539,116 shares of common stock for issuance under the Plan.
Options to purchase the Company’s common stock may be granted at a price not less than the fair market value (“FMV”) in the case of both NSO and ISO, except for an employee or nonemployee who owns more than 10% of the voting power of all classes of stock of the Company, in which case the exercise price shall be no less than 110% of the FMV per share on the grant date. Fair market value is determined by the Board of Directors. Options are exercisable as determined by the Board of Directors, but must become exercisable at a rate of not less than 20% per annum over five years from the vesting date, except in the case of options granted to officers, directors and consultants. The Company has historically granted options that vest over a four year period. Options expire as determined by the Board of Directors, but not more than ten years after the date of grant.
F-27
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Activity under the Plan is as follows:
| Shares available for grant |
Outstanding options | Aggregate intrinsic value | ||||||||||
| Number of shares |
Weighted average exercise price |
|||||||||||
| (in thousands, except share and per share amounts) | ||||||||||||
| Shares reserved at 2006 Equity Incentive Plan inception |
45,166 | — | $ | — | ||||||||
| Options granted |
(33,243 | ) | 33,243 | 5.10 | ||||||||
| Options cancelled |
666 | (666 | ) | 5.10 | ||||||||
| Balance, December 31, 2006 |
12,589 | 32,577 | 5.10 | $ | — | |||||||
| Additional shares reserved |
42,933 | — | — | |||||||||
| Options granted |
(47,531 | ) | 47,531 | 15.22 | ||||||||
| Options cancelled |
3,165 | (3,165 | ) | 5.10 | ||||||||
| Balance, December 31, 2007 |
11,156 | 76,943 | 11.35 | 396 | ||||||||
| Additional shares reserved |
21,261 | — | — | |||||||||
| Options granted |
(38,090 | ) | 38,090 | 58.80 | ||||||||
| Options exercised |
— | (2,160 | ) | 11.52 | 80 | |||||||
| Options cancelled |
6,953 | (6,953 | ) | 17.13 | ||||||||
| Balance, December 31, 2008 |
1,280 | 105,920 | 28.03 | 5,101 | ||||||||
| Additional shares reserved |
1,824,054 | — | — | |||||||||
| Options granted |
(1,463,621 | ) | 1,463,621 | 2.04 | ||||||||
| Options exercised |
— | (456 | ) | 8.30 | 30 | |||||||
| Options cancelled |
33,616 | (33,616 | ) | 18.26 | ||||||||
| Balance, December 31, 2009 |
395,329 | 1,535,469 | 3.47 | 754 | ||||||||
| Additional shares reserved (unaudited) |
1,605,702 | — | ||||||||||
| Options granted (unaudited) |
(935,189 | ) | 935,189 | 1.50 | ||||||||
| Options exercised (unaudited) |
— | (69,679 | ) | 1.50 | 65 | |||||||
| Options cancelled (unaudited) |
149,886 | (149,886 | ) | 1.53 | ||||||||
| Balance, June 30, 2010 (unaudited) |
1,215,728 | 2,251,093 | $ | 1.50 | $ | 2,679 | ||||||
The following table summarizes information about stock options outstanding at December 31, 2009:
| Number of options |
Weighted average price |
Weighted average contractual | |||||
| As of December 31, 2009 |
|||||||
| Options outstanding |
1,535,469 | $ | 3.47 | 9.78 | |||
| Options vested and expected to vest |
1,351,432 | $ | 3.38 | 9.78 | |||
| Options vested and exercisable |
787,630 | $ | 2.73 | 9.77 | |||
F-28
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
The following table summarizes information about stock options outstanding at June 30, 2010 (unaudited):
| Number of options |
Weighted average exercise price |
Weighted average remaining contractual life (years) | |||||
| As of June 30, 2010 |
|||||||
| Options outstanding |
2,251,093 | $ | 1.50 | 9.43 | |||
| Options vested and expected to vest |
1,944,792 | $ | 1.50 | 9.43 | |||
| Options vested and exercisable |
746,235 | $ | 1.50 | 9.28 | |||
Stock Option Modification
In January 2010, the Company modified stock options to purchase 85,477 (unaudited) shares of the Company’s common stock held by 106 employees and consultants. The modification did not change any of the other terms or conditions of the options, and it did not have a significant impact on the compensation expense recognized in the statement of operations for the six months ended June 30, 2010.
Stock-Based Compensation Associated with Awards to Employees
During the years ended December 31, 2008 and 2009, and during the six months ended June 30, 2010, the Company granted stock options to employees to purchase 38,090, 1,307,910 and 935,189 (unaudited), respectively, shares of common stock with a weighted-average grant date fair value of $1.42, $1.70 and $1.70 per share, respectively. Stock-based compensation expense recognized during the years ended December 31, 2007, 2008 and 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010 for stock-based awards granted to employees amounts to $88,000, $284,000, $1,142,000 and $2,344,000 (unaudited), respectively. Stock-based compensation expense recognized during the six months ended June 30, 2009 and 2010 for stock-based awards granted to employees amounts to $297,000 (unaudited) and $819,000 (unaudited), respectively. As of December 31, 2009 and June 30, 2010, there were total unrecognized compensation costs of $2,653,000 and $2,249,000 (unaudited), respectively, related to these stock options. These costs are expected to be recognized over a period of approximately 2.5 and 2.7 years (unaudited), respectively.
The Company estimated the fair value of stock options using the Black-Scholes option valuation model. The fair value of employee stock options is being amortized on a straight-line basis over the requisite service period of the awards. The fair value of employee stock options was estimated using the following assumptions:
| Years ended December 31, | Six months ended June 30, | |||||||
| 2008 | 2009 | 2009 | 2010 | |||||
| (unaudited) | ||||||||
| Expected term |
6.08 years | 5.32 - 6.10 years | 5.82 - 6.10 years | 5.50 years | ||||
| Expected volatility |
82.05 - 84.79% | 74.03 - 92.44% | 87.27 - 92.44% | 80.54 - 80.79% | ||||
| Expected dividends |
0.00% | 0.00% | 0.00% | 0.00% | ||||
| Risk-free interest rate |
2.79 - 3.62% | 1.80 - 2.72% | 1.80 - 2.19% | 2.57 - 2.80% | ||||
Risk-Free Interest Rate: The Company determined the risk-free interest rate by using a weighted average assumption equivalent to the expected term based on the U.S. Treasury constant maturity rate as of the date of grant.
Expected Volatility: The Company used an average historical stock price volatility of comparable companies to be representative of future stock price volatility, as the Company did not have any trading history for its common stock.
F-29
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Expected Term: Expected term represents the period that the Company’s stock-based awards are expected to be outstanding. The Company’s assumptions about the expected term were based on companies that have similar industry, life cycle, revenue and market capitalization.
Expected Dividend: The Company has not paid and does not anticipate paying any dividends in the near future.
The total fair value of employee stock options vested during the years ended December 31, 2007, 2008 and 2009 was $40,000, $201,000 and $898,000, respectively. The total fair value of employee shares vested during the six months ended June 30, 2009 and 2010 was $179,000 (unaudited) and $355,000 (unaudited), respectively.
In December 2009, the Company modified the vesting schedule of 731,944 options originally granted in November 2009 to approximately 100 employees. The modification did not change the number of options granted, and it did not have a significant impact on the compensation expense recognized in the statement of operations for the year ended December 31, 2009.
Options Granted to Nonemployees
Since inception, the Company has granted 161,043 stock options to nonemployees. Stock-based compensation expense related to stock options granted to nonemployees is recognized as the stock option is earned. The Company believes that the estimated fair value of the stock options is more readily measurable than the fair value of the services rendered. The fair value of the stock options granted to nonemployees is calculated at each reporting date using the Black-Scholes options pricing model using the following assumptions:
| December 31, |
June 30, | |||||||
| 2008 | 2009 | 2009 | 2010 | |||||
| (unaudited) | ||||||||
| Remaining contractual term |
6.02 years | 6.5 - 10 years | 6.03 - 7.00 years | 6.04 - 9.57 years | ||||
| Expected volatility |
84.79% | 104.2 - 111.5% | 98.55 - 98.84% | 77.58 - 79.13% | ||||
| Expected dividends |
0% | 0% | 0% | 0% | ||||
| Risk-free interest rate |
2.59% | 2.83 - 3.40% | 2.59 - 2.82% | 2.86 - 3.68% | ||||
Compensation expense related to these options for the years ended December 31, 2007, 2008 and 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010 was $12,000, $52,000, $268,000 and $403,000 (unaudited), respectively. Compensation expense related to those options for the six months ended June 30, 2009 and 2010 was $47,000 (unaudited) and $70,000 (unaudited), respectively.
| 11. | Related Party Transactions |
On March 28, 2006, the Company entered into an intellectual property license agreement (“the Agreement”) with Callida Genomics Inc. (“Callida”), a company owned by the Company’s Chief Scientific Officer, for use of certain patents, patent applications, know-how and other intellectual property relating to the Company’s core technology. As consideration for the rights and licenses granted in this agreement, the Company issued 13,333 shares of common stock to Callida on March 28, 2006, which was recorded at its fair market value as research and development expense. The Company agreed to pay Callida a cash payment of $250,000 on a yearly basis until the earlier of: (i) six years from the date of the agreement of March 28, 2006, (ii) a sale, or other similar liquidity event, of Callida, for consideration equal to, or exceeding, $2,000,000, (iii) the registration, listing and trading of the Company’s securities on an established stock exchange or national market system or (iv) the fair market value of the common shares issued to Callida becoming equal to, or exceeding, $2,000,000. The agreement also required the Company to pay Callida a cash payment of $1,000,000, which was paid in November 2008 and was recorded as a research and development expense. Additionally, the Company reimbursed Callida $50,000 for the cost of patent prosecution incurred through the date of the agreement.
F-30
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
The intellectual property license agreement with Callida includes several termination provisions, including: any time after 15 months from the contract date until the expiration of the contract term the Company may terminate the agreement for any reason, or no reason, by giving Callida written notice of termination. Upon termination of this Agreement, the Company’s obligation to make any further payments, including those referred to in the paragraph above, and the Company’s right to use any items covered in the license agreement, are both cancelled.
During the years ended December 31, 2007, 2008 and 2009 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010, the aggregate expenses under the license agreement were $391,000, $1,333,000, $250,000 and $2,307,000 (unaudited), respectively. During the six months ended June 30, 2009 and 2010, the aggregate expense under the license agreement was $125,000 (unaudited) for both periods.
On March 28, 2006, the Company entered into a service agreement with Callida for use of Callida’s equipment, location and staff. The agreement was effective through June 30, 2006. In July 2006, the agreement was modified and extended to August 2007. During the year ended December 31, 2007 and, cumulatively, for the period from June 14, 2005 (date of inception) to June 30, 2010, the aggregate expenses under this agreement were $266,000 and $1,030,000 (unaudited), respectively.
On February 21, 2007, the Company entered into promissory notes with entities affiliated with OVP Partners and Enterprise Partners (existing investors). The borrowing was for an aggregate principal amount of up to $2,000,000, plus simple interest on the outstanding principal amount, at the annual rate of 6%. The Company drew down on the notes for a total of $1,000,000 during the year ended December 31, 2007. The outstanding balance plus interest on the balance was converted into Series B convertible preferred stock during 2007. In connection with the notes, the Company issued warrants to purchase shares of the Company’s Series B convertible preferred stock as discussed in Note 7.
On February 13, 2009, the Company entered into promissory notes with various holders of the Company’s preferred stock. The borrowings were for an aggregate principal amount of up to $14,725,000, plus simple interest on the outstanding principal amount, at the annual rate of 8%. The Company drew down on the notes for the full principal amount during 2009. The outstanding balance plus interest on the balance was converted into Series D convertible preferred stock during 2009. In connection with the notes, the Company issued warrants to purchase shares of the Company’s Series D convertible preferred stock as discussed in Note 7.
F-31
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
| 12. | Income Taxes |
The Company has not recorded any income tax expense for the years ended December 31, 2007, 2008 and 2009 due to its history of operating losses. The tax effects of temporary differences that give rise to significant portions of the deferred tax assets are as follows:
| December 31, | ||||||||||||
| 2007 | 2008 | 2009 | ||||||||||
| (in thousands) | ||||||||||||
| Deferred tax assets: |
||||||||||||
| Net operating loss carryforwards |
$ | 4,686 | $ | 13,370 | $ | 23,797 | ||||||
| Research and development credits |
660 | 1,484 | 2,406 | |||||||||
| Capitalized start-up costs |
1,953 | 4,071 | 4,119 | |||||||||
| Accruals and reserves |
89 | 221 | 2,347 | |||||||||
| Fixed assets and depreciation |
— | 143 | 261 | |||||||||
| Deferred tax assets |
7,388 | 19,289 | 32,930 | |||||||||
| Deferred tax liabilities: |
||||||||||||
| Fixed assets and depreciation |
(186 | ) | — | — | ||||||||
| Net deferred tax assets prior to valuation allowance |
7,202 | 19,289 | 32,930 | |||||||||
| Valuation allowance |
(7,202 | ) | (19,289 | ) | (32,930 | ) | ||||||
| Net deferred tax assets |
$ | — | $ | — | $ | — | ||||||
The Company has established a full valuation allowance against its deferred tax assets due to the uncertainty surrounding realization of these assets. The valuation allowance increased $5,171,000, $12,087,000 and $13,641,000 during the years ended December 31, 2007, 2008 and 2009, respectively.
As of December 31, 2009, the Company had net operating loss carryforwards of approximately $59,747,000 and $59,745,000 available to reduce future taxable income, if any, for federal and California state income tax purposes, respectively. The federal net operating loss carryforward begins expiring in 2026, if not utilized, and the state net operating loss carryforward begins expiring in 2016, if not utilized. The net operating loss related deferred tax assets do not include excess tax benefits from employee stock option exercises.
As of December 31, 2009, the Company had research and development credit carryforwards of approximately $1,889,000 and $1,987,000 available to reduce taxable income, if any, for federal and California state income tax purposes, respectively. The federal credit carryforwards begin expiring in 2026, and the state credit carryforwards do not expire. Because of net operating loss and credit carryforwards, all of the Company’s tax years, dating to inception in 2005 remain open to federal tax examinations. Most state jurisdictions have four open tax years at any point in time.
Utilization of net operating loss and tax credit carryforwards is subject to ownership change rules as provided under the Internal Revenue Code and similar state provisions. The Company has performed an analysis to determine whether an ownership change has occurred from inception to December 31, 2009. The analysis has determined that two ownership changes have occurred during that period. Due to the ownership changes, the utilization of these net operating losses and research credits are subject to annual limitation. However, the Company concluded that as of December 31, 2009 no net operating losses or research and development credits will expire before utilization due to these ownership changes. In the event the Company has a subsequent change in ownership, net operating loss and research and development credit carryovers could be further limited and may expire unutilized.
F-32
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
Effective January 1, 2007, the Company adopted the provisions of the FASB’s guidance on accounting for uncertainty in income taxes. These provisions provide a comprehensive model for the recognition, measurement and disclosure in financial statements of uncertain income tax position that a company has taken or expects to take on a tax return. Under these provisions, a company can recognize the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit can be recognized. Assessing an uncertain tax position begins with the initial determination of the sustainability if the position and is measured at the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed. Additionally, companies are required to accrue interest and related penalties, if applicable, on all tax exposures for which reserves have been established consistent with jurisdictional tax laws. The cumulative effect of this adoption is recorded as an adjustment to the opening balance of its deficit accumulated during the development stage on the adoption date.
As a result of the implementation of these provisions, the Company recognized a $40,000 increase in its unrecognized tax benefits. There was no increase in the January 1, 2007 balance of deficit accumulated during the development stage since the benefit relates to attribute carryovers for which the related deferred tax asset was subject to a full valuation allowance. At the adoption date of January 1, 2007 and as of December 31, 2009, the Company had no accrued interest or penalties related to the tax contingencies. Since the unrecognized tax benefit relates to attribute carryovers for which the related deferred tax asset was subject to a full valuation allowance, the recognition of the unrecognized tax benefits will not affect the Company’s effective tax rate. The Company has elected to include interest and penalties as a component of tax expense. The Company does not anticipate that the amount of unrecognized tax benefits relating to tax positions existing at December 31, 2009 will significantly increase or decrease within the next 12 months.
The aggregate changes in the balance of gross unrecognized tax benefits were as follows:
| (in thousands) | |||
| January 1, 2007 |
$ | 40 | |
| Increases in balances related to tax positions taken during the current period |
164 | ||
| December 31, 2007 |
204 | ||
| Increases in balances related to tax positions taken during the current period |
395 | ||
| December 31, 2008 |
599 | ||
| Increases in balances related to tax positions taken during the current period |
370 | ||
| December 31, 2009 |
$ | 969 | |
| 13. | Subsequent Events |
On July 30, 2010, the Board of Directors of the Company approved the filing of a registration statement on Form S-1 with the Securities and Exchange Commission for an initial public offering of the Company’s common stock.
In April, May and June 2010, the Company issued convertible promissory notes to certain of its existing investors for an aggregate principal amount of $22,121,452. The principal amount of the convertible promissory notes accrues interest at an annual rate of 8%. The convertible promissory notes mature at the earliest of a corporate reorganization as defined in the convertible promissory notes, the consummation of an initial public offering of the Company’s common stock, an event of default pursuant to the terms of the convertible promissory notes or April 12, 2011. In the event that the Company issues shares of a new series of preferred stock with aggregate gross cash proceeds in excess of $17,000,000, the outstanding principal and interest of the convertible promissory notes will automatically convert into that series of preferred stock at the lowest price paid by an
F-33
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
investor in the financing (not to exceed $7.56 per share). In addition, each investor who was issued a convertible promissory note also received a warrant to purchase a number of shares of common stock equal to the product of 5% of the principal amount of the convertible promissory notes and the number of months between the date of issuance of the warrant and the date of the next financing (up to five months), divided by $1.50. These warrants expire upon the fifth anniversary of their issuance date.
The Company has evaluated subsequent events through July 30, 2010, the date on which the financial statements were issued for inclusion in the Company’s registration statement on Form S-1.
| 14. | Subsequent Events (unaudited) |
On August 4, 2010, the Company issued additional convertible promissory notes in the amount of $121,000 and related contingent common stock warrants for 20,237 shares with an initial fair value of $20,000 calculated using the Black-Scholes option pricing model.
On August 5, 2010, the Company amended its Certificate of Incorporation to increase its authorized share capital to 28,067,001 shares of common stock and 15,134,722 shares of preferred stock. The shares of preferred stock may be issued in five series: 138,658 shares of Series A preferred stock; 205,758 shares of Series B preferred stock; 167,357 shares of Series C preferred stock; 7,692,154 shares of Series D preferred stock and 6,930,795 shares of Series E preferred stock. None of the terms of the existing series of preferred stock were amended, except that the minimum per share price (as adjusted for stock splits, combinations, reorganizations and the like) to the public in a firmly underwritten public offering pursuant to the Securities Act of 1933, as amended, on Form S-1 that would trigger an automatic conversion of all series of preferred stock was reduced from $238.95 to $11.34. The Series E preferred stock shares have a liquidation preference of $11.34 per share and will receive a distribution prior to Series D preferred stock shares in a liquidation event.
In August and September 2010, the Company sold 2,284,516 shares of Series E preferred stock at $7.56 per share to existing investors for proceeds for $17,271,000. In addition, in conjunction with the Series E financing, the $22,607,000 of the principal amount and accrued interest on the Company’s convertible promissory notes converted into 2,990,355 shares of Series E preferred stock. The total gross proceeds from the Series E financing, including the conversion of the convertible promissory notes, was $39,878,000. As a result of the conversion of the convertible promissory notes, the remaining value of the debt discount recorded upon issuance of the common stock warrants of $4,544,000 was charged to interest expense in August 2010. In connection with the conversion of the promissory notes, the Company determined the actual number of shares of common stock underlying warrants previously contingently issued to be 1,848,849 shares of common stock. The terms of these warrants are discussed in Note 9. Certain of the investors in the Series E preferred stock financing have the right to purchase up to 1,587,302 shares of common stock if the Company’s initial public offering is consummated before the second or third closing of that financing.
Each investor in the Series E preferred stock financing received common stock warrants to purchase 25% of the number of shares of Series E preferred stock purchased. Warrants for 1,318,719 shares of common stock were issued. The common stock warrants are exercisable if the Company fails to ship at least 369 genomes between May 1, 2010 and September 30, 2010. The warrants have an exercise price of $2.69 per share and expire upon the fifth anniversary of their issuance date, which is the applicable date of the purchase of Series E preferred stock.
On August 3, 2010, a patent infringement lawsuit was filed against the Company by Illumina, Inc. and Solexa, Inc. (an entity acquired by Illumina), or the Plaintiffs, in the U.S District Court in Delaware. The case caption is Illumina, Inc. and Solexa, Inc. v. Complete Genomics, Inc., Civil Action No. 10-649. The complaint alleges that the Company’s Complete Genomics Analysis Platform, and in particular its combinatorial probe anchor ligation technology, infringes upon three patents held by Illumina and Solexa. The Plaintiffs seek unspecified monetary damages and injunctive relief. If an injunction is granted, it could force the Company to stop or alter certain of its
F-34
Table of Contents
Complete Genomics, Inc.
(A development stage enterprise)
Notes to Financial Statements—(Continued)
business activities. The Company believes it has substantial and meritorious defenses to these claims and intends to vigorously defend its position; however, a negative outcome in this matter could have a material adverse effect on the Company’s financial position, results of operations, cash flows and business. The Company is not currently able to estimate the potential loss, if any, that may result from this claim.
In connection with the issuance of the interim financial statements for the six-month period ended June 30, 2010, the Company has evaluated subsequent events through September 10, 2010, the date the interim financial statements were issued.
F-35
Table of Contents

Through and including , 2010 (25 days after the date of this prospectus) federal securities laws may require all dealers that effect transactions in our common stock, whether or not participating in this offering, to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of Contents
Part II
Information not required in prospectus
Item 13. Other Expenses of Issuance and Distribution
The expenses relating to the registration of the shares of common stock being offered hereby, other than underwriting discounts and commissions, will be borne by us. Such expenses are estimated to be as follows:
| Amount to be Paid | |||
| Securities and Exchange Commission registration fee |
$ | 6,150 | |
| FINRA filing fee |
9,125 | ||
| NASDAQ Global Market listing fee |
* | ||
| Blue Sky fees and expenses |
* | ||
| Printing and engraving expenses |
* | ||
| Legal fees and expenses |
* | ||
| Accounting fees and expenses |
* | ||
| Transfer Agent and Registrar fees |
* | ||
| Miscellaneous expenses |
* | ||
| Total |
$ | * | |
| * | to be filed by amendment. |
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify its directors and officers from certain expenses in connection with legal proceedings and permits a corporation to include in its charter documents, and in agreements between the corporation and its directors and officers, provisions expanding the scope of indemnification beyond that specifically provided by this section.
The Registrant’s amended and restated certificate of incorporation provides for the indemnification of directors to the fullest extent permissible under Delaware law.
The Registrant’s amended and restated bylaws provide for the indemnification of officers, directors and third parties acting on the Registrant’s behalf if such persons act in good faith and in a manner reasonably believed to be in and not opposed to the Registrant’s best interest, and, with respect to any criminal action or proceeding, such indemnified party had no reason to believe his or her conduct was unlawful.
The Registrant is entering into indemnification agreements with each of its directors and executive officers, in addition to the indemnification provisions provided for in its charter documents, and the Registrant intends to enter into indemnification agreements with any new directors and executive officers in the future.
The underwriting agreement (to be filed as Exhibit 1.1 hereto) will provide for indemnification by the underwriters of the Registrant and the Registrant’s executive officers and directors, and indemnification of the underwriters by the Registrant, for certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, in connection with matters specifically provided in writing by the underwriters for inclusion in the registration statement.
The Registrant intends to purchase and maintain insurance on behalf of any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
II-1
Table of Contents
Item 15. Recent Sales of Unregistered Securities
The following information sets forth information regarding all unregistered securities sold by the Registrant since January 1, 2007 and give effect to the 1:30 reverse stock split effected in November 2009:
| 1. | In February 2007 and March 2007, the Registrant issued warrants to purchase an aggregate of 393 shares of its Series B preferred stock at an exercise price of $69.00 per share to three accredited investors in connection with a bridge loan financing. The warrants may be exercised at any time prior to their termination dates, which are five years from the date of issuance. |
| 2. | In March 2007 and September 2007, in a series of closings, the Registrant issued and sold an aggregate of 203,620 shares of its Series B preferred stock at a price of $69.00 per share for a combination of cash and the conversion of $1.0 million in convertible debt, for aggregate gross consideration of $14.05 million, to seven accredited investors. |
| 3. | In August 2007, the Registrant issued warrants to purchase an aggregate of 1,738 shares of its Series B preferred stock at an exercise price of $69.00 per share to financial institution lenders in connection with the entry into a credit facility. The warrants may be exercised at any time prior to their termination dates, which are ten years from the date of issuance. |
| 4. | In February 2008 and March 2008, in a series of closings, the Registrant issued and sold an aggregate of 167,350 shares of its Series C preferred stock at a price of $159.30 per share, for aggregate gross consideration of $26.7 million, to eleven accredited investors. |
| 5. | In July 2008, the Registrant issued warrants to purchase an aggregate of 4,895 shares of Series C preferred stock at an exercise price of $159.30 per share, which were subsequently converted in August 2009 into warrants to purchase an aggregate of 103,173 shares of Series D preferred stock at an exercise price of $7.56 per share, to financial institution lenders in connection with the entry into a credit facility. The warrants may be exercised at any time prior to their termination dates, which are ten years from the date of issuance. |
| 7. | In February 2009 through August 2009, in a series of closings, the Registrant issued warrants to purchase an aggregate of 278,165 shares of its Series D preferred stock at an exercise price of $7.56 per share to nine accredited investors in connection with a bridge loan financing. The warrants may be exercised at any time prior to their terminate dates, which are five years from the date of issuance. |
| 8. | In August 2009, the Registrant issued and sold an aggregate of 5,964,054 shares of its Series D preferred stock at a price of $7.56 per share for a combination of cash and conversion of $15.05 million in convertible debt, for aggregate gross consideration of $45.1 million, to fourteen accredited investors, and issued warrants to purchase an aggregate of 1,630,629 shares of its common stock at an exercise price of $1.50 per share to six accredited investors each of which also purchased Series D preferred stock. |
| 9. | In February 2010 and March 2010, in a series of closings, the Registrant issued and sold an aggregate of 1,346,762 shares of its Series D preferred stock at price of $7.56 per share, for aggregate gross consideration of $10.2 million, to nineteen accredited investors. |
| 10. | In March 2010, the Registrant issued and sold an aggregate of 786,533 shares of its common stock at a price of $1.50 per share, for aggregate gross consideration of $1.18 million, to three of its executive officers: Robert J. Curson, Radoje Drmanac, Ph.D. and Clifford A. Reid, Ph.D. Each of the executives purchased with and holds their respective shares in the name of a trust. |
| 11. | From April 2010 through August 2010, in a series of closings, the Registrant issued and sold an aggregate of $22,242,892 in principal amount of convertible notes and warrants to purchase an aggregate of 1,848,849 shares of its common stock at an exercise price of $1.50 per share to 20 accredited investors. The warrants may be exercised at any time prior to their termination dates, which are five years from the date of issuance. |
| 12. | In August and September 2010, in a series of closings, the Registrant issued and sold an aggregate of 5,274,871 shares of its Series E preferred stock at a price of $7.56 per share for a combination of cash and the conversion of $22.6 million in convertible debt and accrued interest, for aggregate gross consideration of $39.9 million, to 20 accredited investors, and issued warrants to purchase an aggregate of 1,318,719 shares of its common stock at an exercise price of $2.69. |
II-2
Table of Contents
| 13. | Since January 1, 2007, the Registrant has granted stock options to purchase 2,958,687 shares of its common stock at exercise prices ranging from $1.50 to $76.20 per share to a total of 211 employees, consultants and directors under its 2006 Equity Incentive Plan. |
| 14. | The Registrant has issued and sold an aggregate of 80,116 shares of its common stock to employees and consultants at prices ranging from $1.50 to $35.40 per share pursuant to the exercise of options granted under its 2006 Equity Incentive Plan. |
The issuance of securities described above in Item 15 paragraphs (1) – (12) were exempt from registration under the Securities Act of 1933, as amended, in reliance on Section 4(2) of the Securities Act of 1933, as amended, and Regulation D promulgated thereunder, as transactions by an issuer not involving any public offering. The purchasers of the securities in these transactions represented that they were accredited investors and that they were acquiring the securities for investment only and not with a view toward the public sale or distribution thereof. Such purchasers received written disclosures that the securities had not been registered under the Securities Act of 1933, as amended, and that any resale must be made pursuant to a registration statement or an available exemption from registration. All purchasers either received adequate financial statement or non-financial statement information about the Registrant or had adequate access, through their relationship with the Registrant, to financial statement or non-financial statement information about the Registrant. The sale of these securities was made without general solicitation or advertising.
The issuance of securities described above in Item 15 paragraphs (13) and (14) were exempt from registration under the Securities Act of 1933, as amended, in reliance on either (1) Rule 701 or Regulation S under the Securities Act of 1933, as amended, pursuant to compensatory benefit plans or agreements approved by the Registrant’s board of directors or (2) Section 4(2) of the Securities Act of 1933, as amended, and Regulation D promulgated thereunder, as transactions by an issuer not involving any public offering. The recipients of securities in each of these transactions represented their intention to acquire the securities for investment only and not with view to or for sale in connection with any distribution thereof, and appropriate legends were affixed to the share certificates and instruments issued in such transactions. All recipients had adequate access, through their relationships with us, to information about us.
All certificates representing the securities issued in these transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
| Exhibit No. | Description of Exhibits | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1 | Seventh Restated Certificate of Incorporation of Complete Genomics, Inc., currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of Complete Genomics, Inc., to be in effect immediately prior to the consummation of this offering. | |
| 3.3+ | Bylaws of Complete Genomics, Inc., as amended, currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws of Complete Genomics, Inc., to be in effect immediately prior to the consummation of this offering. | |
| 4.1 | Reference is made to exhibits 3.1 through 3.4. | |
| 4.2* | Specimen Common Stock Certificate. | |
| 4.3+ | Form of Warrant to purchase shares of Common Stock issued in connection with a 2009 convertible bridge loan financing transaction. | |
| 4.4+ | Form of Warrant to purchase shares of Common Stock issued in connection with the 2010 convertible bridge loan financing transaction. | |
II-3
Table of Contents
| Exhibit No. | Description of Exhibits | |
| 4.5+ | Form of Warrant to purchase shares of Series A preferred stock issued in connection with the Loan and Security Agreement, dated September 21, 2006. | |
| 4.6+ | Form of Warrant to purchase shares of Series B preferred stock issued in connection with the 2007 convertible bridge loan financing transaction. | |
| 4.7+ | Form of Warrant to purchase shares of Series B preferred stock issued in connection with the Loan and Security Agreement, dated August 3, 2007. | |
| 4.8+ | Form of Warrant to purchase shares of Series D preferred stock issued in connection with a 2009 convertible bridge loan financing transaction. | |
| 4.9+ | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Loan and Security Agreement, dated July 30, 2008. | |
| 4.10 | Form of Warrant to purchase shares of common stock issued in connection with the Series E preferred stock financing. | |
| 5.1* | Opinion of Latham & Watkins LLP. | |
| 10.1 | Fourth Amended and Restated Investor Rights Agreement, dated August 6, 2010, between Complete Genomics, Inc. and certain of its stockholders. | |
| 10.2* | Form of Indemnity Agreement for directors and officers. | |
| 10.3a†+ | Intellectual Property License Agreement by and between Callida Genomics, Inc. and Complete Genomics, Inc. effective as of March 28, 2006. | |
| 10.3b+ | Amendment to the Intellectual Property License Agreement, effective as of December 17, 2008, by and between Callida Genomics, Inc. and Complete Genomics, Inc. | |
| 10.4+ | Loan and Security Agreement, dated July 30, 2008, by and among Silicon Valley Bank, Oxford Finance Corporation, Leader Lending LLC—Series A, Leader Lending LLC—Series B and Complete Genomics, Inc. | |
| 10.5+ | Lease Agreement by and between Britania Hacienda VIII, LLC and Complete Genomics, Inc., dated October 31, 2008. | |
| 10.6a+ | Complete Genomics, Inc. 2006 Equity Incentive Plan, as amended. | |
| 10.6b+ | Form of Stock Option Agreement under the 2006 Equity Incentive Plan. | |
| 10.7a* | Complete Genomics, Inc. 2010 Equity Incentive Award Plan. | |
| 10.7b* | Form of Stock Option Grant Notice and Stock Option Agreement under the 2010 Equity Incentive Award Plan. | |
| 10.8+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Bruce Martin, dated March 26, 2010. | |
| 10.9+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Mark J. Sutherland, dated March 11, 2010. | |
| 10.10+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Ajay Bansal, dated May 7, 2010. | |
| 10.11a+ | Severance Agreement, by and between Complete Genomics, Inc. and Clifford A. Reid, Ph.D., dated March 28, 2006. | |
| 10.11b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Clifford A. Reid, Ph.D., dated December 31, 2008. | |
| 10.12a+ | Severance Agreement, by and between Complete Genomics, Inc. and Robert J. Curson, dated March 28, 2006. | |
| 10.12b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Robert J. Curson, dated December 31, 2008. | |
| 10.13a+ | Severance Agreement, by and between Complete Genomics, Inc. and Radoje Drmanac, Ph.D., dated March 28, 2006. | |
| 10.13b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Radoje Drmanac, Ph.D., dated December 31, 2008. | |
| 23.1 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | |
II-4
Table of Contents
| Exhibit No. | Description of Exhibits | |
| 23.2* | Consent of Latham & Watkins LLP (included in Exhibit 5.1). | |
| 24.1+ | Power of Attorney. | |
| † | Certain portions have been omitted pursuant to a confidential treatment request. Omitted information has been filed separately with the SEC. |
| * | To be filed by amendment. |
| + | Previously filed. |
(b) Financial Statement Schedules
Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
The undersigned registrant hereby undertakes to provide to the underwriters at the closing as specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
II-5
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 1 to the Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Mountain View, State of California, on the 10th day of September, 2010.
| COMPLETE GENOMICS, INC. | ||
| By: | /S/ CLIFFORD A. REID | |
| Clifford A. Reid, Ph.D. President and Chief Executive Officer | ||
Signatures and Power of Attorney
Each person whose individual signature appears below hereby authorizes and appoints Clifford A. Reid, Ph.D. and Ajay Bansal, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of such person, individually and in each capacity stated below, and to file any and all amendments to this Registration Statement, including any and all post-effective amendments and amendments thereto, and any registration statement relating to the same offering as this Registration Statement that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Amendment No. 1 to the Registration Statement has been signed by the following persons in the capacities indicated below on the dates indicated.
| Signature | Title | Date | ||
| /S/ CLIFFORD A. REID Clifford A. Reid, Ph.D. |
President, Chief Executive Officer and Director (Principal Executive Officer) | September 10, 2010 | ||
| /S/ AJAY BANSAL Ajay Bansal |
Chief Financial Officer (Principal Financial and Accounting Officer) | September 10, 2010 | ||
| * Alexander E. Barkas, Ph.D. |
Director | September 10, 2010 | ||
| * C. Thomas Caskey, M.D. |
Director | September 10, 2010 | ||
| * Carl L. Gordon, Ph.D., CFA |
Director | September 10, 2010 | ||
|
Michael P. Rubin, M.D. |
Director | |||
| * Andrew E. Senyei, M.D. |
Director | September 10, 2010 | ||
II-6
Table of Contents
| Signature | Title | Date | ||
| * Lewis J. Shuster |
Director | September 10, 2010 | ||
| * Charles P. Waite, Jr. |
Director | September 10, 2010 | ||
| *By: |
/S/ CLIFFORD A. REID Clifford A. Reid, Ph.D. Attorney-in-Fact |
September 10, 2010 | ||||
II-7
Table of Contents
Exhibit Index
| Exhibit No. | Description of Exhibits | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1 | Seventh Restated Certificate of Incorporation of Complete Genomics, Inc., currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation of Complete Genomics, Inc., to be in effect immediately prior to the consummation of this offering. | |
| 3.3+ | Bylaws of Complete Genomics, Inc., as amended, currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws of Complete Genomics, Inc., to be in effect immediately prior to the consummation of this offering. | |
| 4.1 | Reference is made to exhibits 3.1 through 3.4. | |
| 4.2* | Specimen Common Stock Certificate. | |
| 4.3+ | Form of Warrant to purchase shares of Common Stock issued in connection with a 2009 convertible bridge loan financing transaction. | |
| 4.4+ | Form of Warrant to purchase shares of Common Stock issued in connection with the 2010 convertible bridge loan financing transaction. | |
| 4.5+ | Form of Warrant to purchase shares of Series A preferred stock issued in connection with the Loan and Security Agreement, dated September 21, 2006. | |
| 4.6+ | Form of Warrant to purchase shares of Series B preferred stock issued in connection with the 2007 convertible bridge loan financing transaction. | |
| 4.7+ | Form of Warrant to purchase shares of Series B preferred stock issued in connection with the Loan and Security Agreement, dated August 3, 2007. | |
| 4.8+ | Form of Warrant to purchase shares of Series D preferred stock issued in connection with a 2009 convertible bridge loan financing transaction. | |
| 4.9+ | Form of Warrant to purchase shares of Series D preferred stock issued in connection with the Loan and Security Agreement, dated July 30, 2008. | |
| 4.10 | Form of Warrant to purchase shares of common stock issued in connection with the Series E preferred stock financing. | |
| 5.1* | Opinion of Latham & Watkins LLP. | |
| 10.1 | Fourth Amended and Restated Investor Rights Agreement, dated August 6, 2010, between Complete Genomics, Inc. and certain of its stockholders. | |
| 10.2* | Form of Indemnity Agreement for directors and officers. | |
| 10.3a†+ | Intellectual Property License Agreement by and between Callida Genomics, Inc. and Complete Genomics, Inc. effective as of March 28, 2006. | |
| 10.3b+ | Amendment to the Intellectual Property License Agreement, effective as of December 17, 2008, by and between Callida Genomics, Inc. and Complete Genomics, Inc. | |
| 10.4+ | Loan and Security Agreement, dated July 30, 2008, by and among Silicon Valley Bank, Oxford Finance Corporation, Leader Lending LLC—Series A, Leader Lending LLC—Series B and Complete Genomics, Inc. | |
| 10.5+ | Lease Agreement by and between Britania Hacienda VIII, LLC and Complete Genomics, Inc., dated October 31, 2008. | |
| 10.6a+ | Complete Genomics, Inc. 2006 Equity Incentive Plan, as amended. | |
| 10.6b+ | Form of Stock Option Agreement under the 2006 Equity Incentive Plan. | |
| 10.7a* | Complete Genomics, Inc. 2010 Equity Incentive Award Plan. | |
| 10.7b* | Form of Stock Option Grant Notice and Stock Option Agreement under the 2010 Equity Incentive Award Plan. | |
Table of Contents
| Exhibit No. | Description of Exhibits | |
| 10.8+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Bruce Martin, dated March 26, 2010. | |
| 10.9+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Mark J. Sutherland, dated March 11, 2010. | |
| 10.10+ | Offer letter employment agreement, by and between Complete Genomics, Inc. and Ajay Bansal, dated May 7, 2010. | |
| 10.11a+ | Severance Agreement, by and between Complete Genomics, Inc. and Clifford A. Reid, Ph.D., dated March 28, 2006. | |
| 10.11b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Clifford A. Reid, Ph.D., dated December 31, 2008. | |
| 10.12a+ | Severance Agreement, by and between Complete Genomics, Inc. and Robert J. Curson, dated March 28, 2006. | |
| 10.12b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Robert J. Curson, dated December 31, 2008. | |
| 10.13a+ | Severance Agreement, by and between Complete Genomics, Inc. and Radoje Drmanac, Ph.D., dated March 28, 2006. | |
| 10.13b+ | Amendment to Severance Agreement, by and between Complete Genomics, Inc. and Radoje Drmanac, Ph.D., dated December 31, 2008. | |
| 23.1 | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Latham & Watkins LLP (included in Exhibit 5.1). | |
| 24.1+ | Power of Attorney. | |
| † | Certain portions have been omitted pursuant to a confidential treatment request. Omitted information has been filed separately with the SEC. |
| * | To be filed by amendment. |
| + | Previously filed. |
