Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - CHINA PEDIATRIC PHARMACEUTICALS, INC. | ex231.htm |
| EX-10.24 - EXHIBIT 10.24 - CHINA PEDIATRIC PHARMACEUTICALS, INC. | ex1024.htm |
As filed with the United States Securities and Exchange Commission on September 3, 2010
File No. 333-164562
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1/A
AMENDMENT NO. 5
TO
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CHINA PEDIATRIC PHARMACEUTICALS, INC.
Formerly Lid Hair Studios International, Inc.
(Exact name of registrant as specified in its charter)
|
Nevada
|
2834
|
20-271 8075
|
|
(State or other jurisdiction of
incorporation or organization)
|
(primary standard industrial
classification code number)
|
(I.R.S. Employer
Identification No.)
|
9th Floor, No. 29 Nanxin Street,
Xi’an, Shaanxi Province
P.R.C., 710004
86 29 8727 1818
(Address and telephone number of principal executive offices and principal place of business)
___________________
Copies to:
Marc J. Ross, Esq.
Jessica S. Yuan, Esq.
Sichenzia Ross Friedman Ference LLP
61 Broadway, 32 nd Floor
New York, New York 10006
Telephone: (212) 930-9700
Approximate date of commencement of proposed sale to the public : From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: þ
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer
|
¨
|
|
Accelerated Filer
|
¨
|
|
Non-Accelerated Filer
|
¨
|
Smaller Reporting Company
|
þ
|
1
Page 1
|
Title Of Each
Class of Securities
To be Registered
|
Amount To
Be Registered
|
Proposed
Maximum
Offering Price
Per Share(1)
|
Proposed
Maximum
Aggregate
Offering Price
|
Amount of
Registration Fee(3)
|
||||||||||||
|
Common stock, par value $0.001 per share (2)(3)
|
1,250,000
|
$
|
4.10
|
$
|
5,125,000
|
$
|
365.41
|
|||||||||
|
Common stock, par value $0.001 per share (2)(3)
|
1,250,000
|
$
|
4.10
|
$
|
5,125,000
|
$
|
365.41
|
|||||||||
|
Total
|
2,500,000
|
$
|
10,250,000
|
$
|
730.82
|
*
|
||||||||||
———————
(1) Estimated solely for purposes of calculating the registration fee. The registration fee is calculated pursuant to Rule 457(c). Our common stock is quoted under the symbol "CPDU.OB" on the Over-the-Counter Bulletin Board (“OTCBB”) administered by FINRA. As of January 28, 2010, the last reported high price was $4.10 per share and the last reported low price was $4.10 per share. The average of the high and low price was $4.10 per share. Accordingly, the registration fee is $730.82 based on $4.10 per share.
(2) An indeterminate number of additional shares of common stock shall be issuable pursuant to Rule 416 to prevent dilution resulting from stock splits, stock dividends or similar transactions and in such an event the number of shares registered shall automatically be increased to cover the additional shares in accordance with Rule 416 under the Securities Act.
(3) We are registering up to 2,500,000 shares of our common stock that we may issue upon the exercise of share purchase warrants.
*Previously paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the United States Securities and Exchange Commission, acting pursuant to said section 8(a), may determine.
2
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the United States Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 3, 2010
PRELIMINARY PROSPECTUS
CHINA PEDIATRIC PHARMACEUTICALS, INC.
2,500,000 shares of common stock
This prospectus relates to the resale of up to 2,500,000 shares of our common stock, $0.001 par value per share, by certain of our stockholders. These persons, together with their transferees, are referred to throughout this prospectus as “selling stockholders.”
We issued all of the shares described above in private placement transactions completed prior to the filing of this registration statement.
The selling stockholders may offer to sell the shares of common stock being offered in this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices or at negotiated prices. Our common stock is quoted on the OTC Bulletin Board under the symbol "CPDU". On September 1, 2010 , the closing bid price for one share of our common stock on the OTC Bulletin Board was $4.75.
You should consider carefully the risk factors beginning on page 4 of this prospectus.
Neither the United States Securities and Exchange Commission nor any state securities commission has approved of these securities or determined that this prospectus is accurate or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is: *, 2010
3
Table of Contents
|
|
|
|
About this Prospectus
|
5 |
|
Cautionary Note Regarding Forward-Looking Statements and Other Information Contained in this Prospectus
|
6
|
|
Prospectus Summary
|
7
|
|
The Company
|
7
|
|
The Offering
|
8
|
|
Plan of Distribution
|
9
|
|
Risk Factors
|
9 |
|
Use of Proceeds
|
23
|
|
Dilution
|
23
|
|
Market Price of and Dividends on the Registrant’s Common Equity and Related Stockholder Matters
|
23
|
|
Management’s Discussion and Analysis of Financial Conditions (MD&A)
|
25
|
|
Results of Operations
|
26
|
|
Critical Accounting Policies
|
30
|
|
Our Business
|
41
|
|
Corporate History
|
41
|
|
The Merger Transaction
|
41
|
|
Background of Shaanxi Jiali
|
41
|
|
Reorganization and Revised Structure
|
42
|
|
Our Business
|
44
|
|
Description of Property
|
56
|
|
Directors and Executive Officers , Promoters
|
57
|
|
Transactions with Related Persons, Promoters and Certain Control Persons
|
61
|
|
Executive Compensation
|
62
|
|
Security Ownership of Certain Beneficial Owners and Management
|
64
|
|
Selling Stockholders
|
65
|
|
Plan of Distribution
|
66
|
|
Description Of Securities
|
68
|
|
Legal Matters
|
70
|
|
Experts
|
70
|
|
Legal Proceedings
|
70
|
|
Changes in and Disagreements with Accountants
|
70
|
|
Disclosure of Commission Position of Indemnification for Securities Act Liabilities
|
70
|
|
Where You Can Find More Information
|
71
|
|
Financial Statements
|
72
|
4
You should rely only on the information contained in the prospectus. We have not authorized anyone to provide you with information or to make any representations about us, the selling stockholders, the securities or any matter discussed in this prospectus, other than that contained in the prospectus. If any other information or representation is given or made, such information or representations may not be relied upon as having been authorized by us or any selling stockholder. The selling stockholders are offering to sell and seeking offers to buy shares of our common stock only in jurisdictions where offers and sales are permitted. This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy the securities in any circumstances under which the offer or solicitation is unlawful. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our common stock. Neither the delivery of this prospectus nor any distribution of securities in accordance with this prospectus shall, under any circumstances, imply that there has been no change in our affairs since the date of this prospectus. The prospectus will be updated and updated prospectuses made available for delivery to the extent required by the federal securities laws.
When used in this prospectus, the terms:
|
●
|
“Asia Pharm,” refers to Asia Pharm Holdings, Inc., a British Virgin Islands Company
|
|
●
|
“China Pediatric,” “China Pediatric Pharmaceuticals,” “Lid Hair Studios International, Inc.,” “CPDU,” “LHSI,” the “Company,” “we,” “our” and “us” refers to China Pediatric Pharmaceuticals, Inc., formerly Lid Hair Studios International, Inc. a Nevada corporation.
|
|
●
|
“China Children Pharmaceuticals” refers to our wholly owned subsidiary China Children Pharmaceuticals Co., Ltd., a limited liability company organized under the laws of the Hong Kong.
|
|
|
●
|
“Xi’an Coova” refers to Coova Children Pharmaceuticals Co. Ltd., which is a “wholly foreign-owned enterprise” (“WFOE”) under the laws of the People’s Republic of China.
|
|
●
|
“Shaanxi Jiali” refers to our variable interest entity (“VIE”), Shaanxi Jiali Pharmaceutical Co., Ltd. a company organized under the laws of the PRC that has entered into a series of agreements with Xi’an Coova that (i) give Xi’an Coova control over the board of directors, officers, operations and finances of China Children Pharmaceuticals; (ii) permit China Children Pharmaceuticals to be treated as a subsidiary of Xi’an Coova under the laws of the People’s Republic of China; and (iii) allow us to consolidate its financial statements under GAAP.
|
|
●
|
“China Children Shareholders” refers to the shareholders of China Children Pharmaceuticals who are also some of the shareholders of China Pediatric Pharmaceuticals.
|
|
●
|
“Yuan” or “RMB” refer to the Chinese yuan (also known as the Renminbi). According to the currency website xe.com, as of December 31, 2009, $1 = 6.828 yuan.
|
|
●
|
“PRC” or “China” refers to the People’s Republic of China.
|
|
●
|
“SFDA” refers to the PRC State Food and Drug Administration.
|
|
●
|
“OTC Bulletin Board” or the “OTCBB” refers to the Over-the-Counter Bulletin Board, an electronic quotation system for equity securities overseen by the Financial Industry Regulatory Authority (formerly the National Association of Securities Dealers), which is accessible through its website at WWW.OTCBB.COM
|
5
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND OTHER INFORMATION CONTAINED IN THIS PROSPECTUS
This prospectus contains some forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties. Forward-looking statements include statements regarding, among other things, (a) projected sales, profitability, and cash flows, (b) growth strategies, (c) anticipated trends, (d) future financing plans and (e) anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipates,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend,” or the negative of these words or other variations on these words or comparable terminology. These statements may be found under “Management’s Discussion and Analysis of Financial Condition and Plan of Operation” and “Business,” as well as in this prospectus generally. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
Any or all of the forward-looking statements in this prospectus may turn out to be inaccurate. They can be affected by inaccurate assumptions we might make or by known or unknown risks or uncertainties. Consequently, no forward-looking statement can be guaranteed. Actual future results may vary materially as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in the prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this filing will in fact occur. You should not place undue reliance on these forward-looking statements.
6
This summary highlights selected information contained elsewhere in this prospectus. It is not complete and does not contain all of the information that you should consider before investing in our common stock. To understand this offering fully, you should read the entire prospectus carefully, including “Risk Factors” and the Consolidated Financial Statements and the Related Notes.
Business Overview
Through our operating company, Shaanxi Jiali, we are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops manufactures and distributes both prescription and over-the counter, including both conventional Western and Traditional Chinese Medicines (“TCMs”), for the treatment of some of the most common ailments and diseases, with pediatric medicine as its focus.
A TCM is understood in the PRC pharmaceutical industry to be a type of drug that is primarily composed of herbs and plants used in traditional Chinese medicinal preparations but, instead of being processed and applied using ancient traditional methods (such as a tea or decoction), is both produced and applied using modern methods and dosage forms. Unlike in conventional Western pharmaceuticals, where the active medicinal ingredients may be synthetically and chemically manufactured, the active substances in TCM products are usually derived from naturally-found plant and non-plant sources. TCMs are understood to be the modern form of traditional Chinese medicine whose remedies are based on Chinese instead of Western pharmacological principles. The PRC government recognizes and authorizes for production and sale two types of pharmaceuticals: Western-style chemically produced pharmaceuticals and TCMs. The former is denoted in the national pharmacological coding system with an “H,” and the latter is denoted with a “Z”.
Shaanxi Jiali has its own manufacturing facility located in Baoji, Shaanxi Province. Our manufacturing facility was issued a Good Manufacturing Practices (“GMP”) certification by the SFDA in 2004. We were awarded the National High-tech Enterprise Award by the Shaanxi Technology Administration in 2006. As a result of receiving the National High-tech Award, Shaanxi Jiali is entitled to the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy an exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax.
We distribute our high value, branded medicines, both prescription and OTC, through exclusive territory agents who sell our products directly to local pharmacies who in turn sell them to their retail customers.
Currently we are looking to increase production capacity and look to do so year by year primarily through acquisitions. The PRC government has recently pushed for consolidation of the pharmaceutical industry. We have a good relationship with our local government who will help us to find, acquire and merge with other factories, however our development may be limited by our ability to find suitable acquisition targets.
For more information about our business you should read the section entitled “OUR BUSINESS”.
Our Corporate Information
We maintain our corporate headquarters at 9 th Floor, No. 29 Nanxin Street, Xi’an, Shaanxi Province, People’s Republic of China. Our telephone number is (86) 29-8727-1818 and our facsimile number is (86) 29-8727-1818-8003.
Background
On September 30, 2009, we acquired control of Shaanxi Jiali from Asia-Pharm through a “reverse merger” transaction, by which we acquired 100% of the equity interest in China Children Pharmaceuticals for 7,000,000 shares of common stock to the shareholders of Asia-Pharm. In connection with the share exchange agreement, our former controlling shareholder submitted 5,000,000 post-split shares of common stock of the Company for cancellation and forgave the outstanding shareholder’s loan to the Company in the amount of $86,173 in exchange for 100% of the issued and outstanding shares of Lid Hair Studios’ wholly-owned subsidiary, Belford Enterprises B.C. Ltd., d/b/a Lid Hair Studio. Through the reverse merger we ceased to be a shell company as that term is defined in Rule 12b-2 under the Securities Exchange Act of 1934 (the “Exchange Act”) and are now in the business of developing, manufacturing and distributing conventional medicines and TCMs in China primarily for pediatric use.
Lid Hair Studios International, Inc. was established on April 20, 2005 in the state of Nevada. On June 27, 2008, Asia-Pharm formed China Children Pharmaceuticals, a Hong Kong company, as its wholly-owned subsidiary. On July 25, 2008, China Children Pharmaceuticals formed Xi’an Coova, a limited liability company organized under the laws of the PRC,
7
as its wholly-owned subsidiary and WFOE under PRC law. On August 4, 2008, Xi’an Coova signed a management entrustment agreement with Shaanxi Jiali ("Shaanxi Jiali"), a pediatric pharmaceutical developer, manufacturer and marketer formed on July 1, 1998 under the laws of the PRC, and the shareholders of Xi’an Coova. Pursuant to that agreement, Xi’an Coova acts as the management company for Shaanxi Jiali, and Shaanxi Jiali conducts the principal operations of the business.
In summary, we have no equity ownership interest in Shaanxi Jiali; however, through Xi’an Coova, we are entitled to receive all of the profits of Shaanxi Jiali, and we are obligated to pay all of its debts. As a result we are allowed to consolidate the financial statements of Shaanxi Jiali under GAAP. When we sell our equity or borrow funds we expect the proceeds will be forwarded to Shaanxi Jiali and accounted for as a loan to Shaanxi Jiali and eliminated during consolidation. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to certain consultants two year warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $5.00 per share (collectively, the “Warrants”). Pursuant to the terms of the Warrants, we agreed to register the shares of common stock issuable under the Warrants. We have filed the registration statement of which this prospectus forms a part in order to meet our obligations under those agreements. For more information about the reverse merger transaction and the issuance of the Warrants you should read the sections entitled “OUR BUSINESS – Corporate History,” “DESCRIPTION OF SECURITIES,” and “SELLING STOCKHOLDERS”.
|
Common stock outstanding
|
10,180,288 shares as of September 1, 2010.
|
|||
|
Common stock that may be offered by selling stockholders
|
Up to 2,500,000 shares.
|
|||
|
Total proceeds raised by offering
|
We will receive no proceeds from the resale of the common stock offered by the selling stockholders, but could receive up to $10,000,000 if all of the share purchase warrants held by the selling stockholders are exercised.
|
|||
|
Common Stock Outstanding after the offering
|
12,680,288
|
|||
|
Risk factors
|
There are significant risks involved in investing in our company. For a discussion of risk factors you should consider before buying our common stock, see “Risk Factors” beginning on page 5. These risk factors include:
·
● Various risks of doing business in China since all of our revenues are derived from operations of China Children Pharmaceuticals in China including changes in political and economics in China, changes in the laws of the People’s Republic of China, exchange rate issues, difficulty in establishing management, legal and financial controls;
·
● The risk of the loss of China Children Pharmaceuticals as our operating business;
|
|||
|
Risks associated with Shaanxi Jiali’s business, which include among other, competition, ability to manage growth, discovery and development of new products, need for additional capital to fund operations, dependence on key personnel, need for regulatory approval of products, need to obtain renewal of licenses to operate business, governmental price controls, products being replaced by newer products
Risk related to our common stock, which include but are not limited to the illiquidity of our stock price, need for internal controls, additional expenses due to regulatory requirements, penny stock regulations that will be applicable to our common stock.
|
||
|
Use of Proceeds
|
The shares of common stock offered hereby are being registered for the account of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the common stock will go to the selling stockholders and we will not receive any proceeds from the resale of the common stock by the selling stockholders, although we could receive proceeds of up to $10,000,000 if all of the share purchase warrants are exercised. We will, however, incur all costs associated with this registration statement and prospectus.
|
8
This offering is not being underwritten. The selling stockholders directly, through agents designated by them from time to time or through brokers or dealers also to be designated, may sell their shares from time to time, in or through privately negotiated transactions, or in one or more transactions, including block transactions, on the OTC Bulletin Board or on any stock exchange on which the shares may be listed in the future pursuant to and in accordance with the applicable rules of such exchange or otherwise. The selling price of the shares may be at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices after the shares are quoted on the OTC Bulletin Board. To the extent required, the specific shares to be sold, the names of the selling stockholders, the respective purchase prices and public offering prices, the names of any such agent, broker or dealer and any applicable commission or discounts with respect to a particular offer will be described in an accompanying prospectus. In addition, any securities covered by this prospectus which qualify for sale pursuant to Rule 144 may be sold under Rule 144 rather than pursuant to this prospectus. We will keep this prospectus current until the expiration dates of the convertible securities, even if the convertible securities which underlie certain shares of our common stock subject to this prospectus are out of the money.
The selling security holders and any other persons participating in the sale or distribution of the shares offered under this prospectus will be subject to applicable provisions of the Exchange Act, and the rules and regulations under that act, including Regulation M. These provisions may restrict activities of, and limit the timing of purchases and sales of any of the shares by, the selling security holders or any other person. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and other activities with respect to those securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. All of these limitations may affect the marketability of the shares.
We will not receive any proceeds from sales of shares by the selling stockholders. However, if any of the selling stockholders decide to exercise their Warrants, we will receive the net proceeds of the exercise of such security held by the selling stockholders. We intend to use any proceeds we receive from the exercise of the Warrants for working capital and other general corporate purposes. We cannot assure you that any of the Warrants will ever be exercised.
We will pay all expenses of registration incurred in connection with this offering (estimated to be $80,730), but the selling stockholders will pay all of the selling commissions, brokerage fees and related expenses.
The selling stockholders and any broker-dealers or agents that participate with the selling stockholders in the distribution of any of the shares may be deemed to be “underwriters” within the meaning of the Securities Act, and any commissions received by them and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act.
You should carefully consider the risks described below together with all of the other information included in this prospectus before making an investment decision with regard to our securities. The statements contained in or incorporated into this offering that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Business
Our operating history may not serve as an adequate basis to judge our future prospects and results of operations .
Shaanxi Jiali commenced its current line of business operations on July 1, 1998 and received its Good Manufacturing Practices (“GMP”) certifications in November 2004. These certifications must be renewed every five years for Shaanxi Jiali to stay in business. We have applied for renewal of all of our necessary GMP certifications, underwent routine review and inspection, and have received approval for these renewals in April 2010, with the renewed GMP certificate expected to be issued in early July 2010. Notwithstanding the above, Shaanxi Jiali’s operating history may not provide a meaningful basis on which to evaluate its business. We cannot assure you that Shaanxi Jiali will maintain GMP approval for its products, maintain its profitability, or assure you that we will not incur net losses in the future. Pursuant to SFDA regulation, if there is a gap at any time in the validity of a company’s GMP certificate, it must suspend production or faces stiff fines and the risk that its authorization for production may be permanently revoked. We expect that Shaanxi Jiali’s operating expenses will increase as it expands. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties frequently experienced by companies at a similar stage of development, including our potential failure to:
|
●
|
raise adequate capital for expansion and operations;
|
|
|
●
|
implement our business model and strategy and adapt and modify them as needed;
|
|
|
●
|
increase awareness of our brand name, protect our reputation and develop customer loyalty;
|
|
|
●
|
manage our expanding operations and service offerings, including the integration of any future acquisitions;
|
|
|
●
|
maintain adequate control of our expenses;
|
|
|
●
|
anticipate and adapt to changing conditions in the medical over the counter, pharmaceutical and nutritional supplement markets in which we operate as well as the impact of any changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics
|
If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
9
The loss of Shaanxi Jiali as our operating business would have a material adverse effect on our business and the price of our common stock.
We have no equity ownership interest in Shaanxi Jiali. Our ability to control Shaanxi Jiali and consolidate its financial results is through a series of contractual agreements between Shaanxi Jiali and our wholly-owned subsidiary Xi’an Coova. The management of Shaanxi Jiali is an affiliate of us and of Xi’an Coova and the stockholders of Shaanxi Jiali are also our shareholders. Thus the Management Entrustment Agreement was not entered into as a result of arms’ length negotiations because the parties to the agreement are under common control. Mr. Xia, our CEO and Chairman, holds approximately 35% of the shares of Shaanxi Jiali and approximately 26.82% of our common stock. The Management Entrustment Agreement may be terminated upon the termination of the business of Shaanxi Jiali or upon the date upon which Xi’an Coova completes the acquisition of Shaanxi Jiali. Any other termination would be a breach of the agreement. While the Company has been advised by its PRC counsel that the Management Entrustment Agreement is legal and enforceable under PRC law, these affiliates control the parties to the Management Entrustment Agreement and it could be possible for them to cause Shaanxi Jiali to breach the Management Entrustment Agreement and our unaffiliated investors would have little or no recourse because of the inherent difficulties in enforcing their rights since all our assets are located in the PRC. (See, Risk Factor “The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.”) In the event that management of Shaanxi Jiali decides to breach the Management Entrustment Agreement, the risk of loss of the affiliated shareholders of Shaanxi Jiali could be lower than unaffiliated investors and the interests of the management and shareholders of Shaanxi Jiali would be in conflict with the interest of our other stockholders.
Shaanxi Jiali’s failure to compete effectively may adversely affect our ability to generate revenue.
Shaanxi Jiali competes with other companies, many of whom are developing or can be expected to develop products similar to Shaanxi Jiali. Shaanxi Jiali’s market is a large market with many competitors. Many of its competitors are more established than Shaanxi Jiali is, and have significantly greater financial, technical, marketing and other resources than it presently possess. Some of Shaanxi Jiali’s competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure you that Shaanxi Jiali will be able to compete effectively with current or future competitors or that the competitive pressures it faces will not harm it business.
We may not be able to effectively control and manage the growth of Shaanxi Jiali.
If Shaanxi Jiali’s business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. An expansion would increase demands on existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect its operations and cause delay in production and delivery of its pharmaceutical prescription, over the counter and medical nutrient products as well as administrative inefficiencies.
We may require additional financing in the future and a failure to obtain such required financing will inhibit Shaanxi Jiali’s ability to grow.
The continued growth of Shaanxi Jiali’s business may require additional funding from time to time, which we expect to raise in private placements of our equity or debt securities with accredited investors or by offering our securities for sale pursuant to an effective registration statement on a market where our common stock is traded. The proceeds of these funding would be forwarded to Shaanxi Jiali and accounted for as a loan to Shaanxi Jiali and eliminated during consolidation. The proceeds would be used for general corporate purposes of Shaanxi Jiali, which could include acquisitions, investments, repayment of debt and capital expenditures among other things. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Obtaining additional funding would be subject to a number of factors including market conditions, operating performance and investor sentiment, many of which are outside of our control. These factors could make the timing, amount, terms and conditions of additional funding unattractive or unavailable to us. Our management believes that we currently have sufficient funds from working capital to meet our current operating costs over the next 12 months.
The terms of any future financing may adversely affect your interest as stockholders.
If we require additional financing in the future, we may be required to incur indebtedness or issue equity securities, the terms of which may adversely affect your interests in us. For example, the issuance of additional indebtedness may be senior in right of payment to your shares upon our liquidation. In addition, indebtedness may be under terms that make the operation of Shaanxi Jiali’s business more difficult because the lender's consent could be required before we take certain actions. Similarly the terms of any equity securities we issue may be senior in right of payment of dividends to your common stock and may contain superior rights and other rights as compared to your common stock. Further, any such issuance of equity securities may dilute your interest in us.
10
We, through our subsidiaries/affiliated companies China Pediatric, Xi’an Coova or Shaanxi Jiali, may engage in future acquisitions that could dilute the ownership interests of our stockholders, cause us to incur debt and assume contingent liabilities.
We, through our subsidiaries/affiliated companies China Children, Xi’an Coova or Shaanxi Jiali, may review acquisition and strategic investment prospects that we believe would complement the current product offerings of Shaanxi Jiali, augment its market coverage or enhance its technical capabilities, or otherwise offer growth opportunities. From time to time Shaanxi Jiali reviews investments in new businesses and we, through our subsidiaries/affiliated companies China Children, Xi’an Coova or Shaanxi Jiali, expect to make investments in, and to acquire, businesses, products, or technologies in the future. We expect that when we raise funds from investors for any of these purposes we will be either the issuer or the primary obligor while the proceeds will be forwarded to Shaanxi Jiali and accounted for as a loan to Shaanxi Jiali and eliminated during consolidation. In the event of any future acquisitions, we could:
|
●
|
issue equity securities which would dilute current stockholders’ percentage ownership;
|
|
|
●
|
incur substantial debt;
|
|
|
●
|
assume contingent liabilities; or
|
|
|
●
|
expend significant cash.
|
These actions could have a material adverse effect on our operating results or the price of our common stock. Moreover, even if through our subsidiaries/affiliated companies, China Children, Xi’an Coova, or Shaanxi Jiali, we do obtain benefits in the form of increased sales and earnings, there may be a lag between the time when the expenses associated with an acquisition are incurred and the time when we recognize such benefits. Acquisitions and investment activities also entail numerous risks, including:
|
●
|
difficulties in the assimilation of acquired operations, technologies and/or products;
|
|
|
●
|
unanticipated costs associated with the acquisition or investment transaction;
|
Standards for compliance with Section 404 of the Sarbanes-Oxley Act of 2002 are uncertain, and if we fail to comply in a timely manner, our business could be harmed and our stock price could decline.
We are constantly striving to improve our internal accounting controls. We expect to continue to improve our internal accounting control for budgeting, forecasting, managing and allocating our funds and to better account for them as we grow. There is no guarantee that such improvements will be adequate or successful or that such improvements will be carried out on a timely basis. If we do not have adequate internal accounting controls, we may not be able to appropriately budget, forecast and manage our funds, we may also be unable to prepare accurate accounts on a timely basis to meet our continuing financial reporting obligations and we may not be able to satisfy our obligations under US securities laws.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require annual assessment of U.S. public companies’ internal control over financial reporting, and attestation of this assessment by their independent registered public accountants. While the Dodd-Frank Wall Street Reform and Consumer Protection Act exempts smaller reporting companies with respect to the attestation by their independent registered public accountants as to our financial controls, this exception does not affect the requirement that we include a report of management on our intenal controls over financial reporting and will not affect the requirement to include the auditor's attestation if our public float exceeds $75 million and we cease to be smaller reporting company. Existing standards that must be met for management to assess the internal control over financial reporting as effective are new and complex, and require significant documentation, testing and possible remediation to meet the detailed standards. While there has not been any detected significant deficiency or material weakness in our internal control and with respect to the assessment of the internal control for the year ended December 31, 2009, we cannot guarantee the implementation of controls and procedures in future years to be without any significant deficiency or material weakness.
We are dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and pharmaceutical factory operational expertise of key personnel. Jun Xia, our Chief Executive Officer and Chairman of the Board perform key functions in the operation of our and Shaanxi Jiali’s business. There can be no assurance that Shaanxi Jiali will be able to retain Mr. Xia after the term of their employment contracts expire. The loss of Mr. Xia could have a material adverse effect upon our business, financial condition, and results of operations. Shaanxi Jiali must attract, recruit and retain a sizeable workforce of technically competent employees. We do not carry key man life insurance for any of our key personnel or personnel nor do we foresee purchasing such insurance to protect against a loss of key personnel and the key personnel.
11
We are dependent upon the services of Mr. Xia for the continued growth and operation of our company because of his experience in the industry and his personal and business contacts in the PRC. Although we have entered into two-year employment agreements with Mr. Xia and we have no reason to believe that he will discontinue their services with the Company or Shaanxi Jiali, the interruption or loss of his services would adversely affect our ability to effectively run our business and pursue our business strategy as well as our results of operations.
We may not be able to hire and retain qualified personnel to support its growth and if it is unable to retain or hire these personnel in the future, its ability to improve its products and implement its business objectives could be adversely affected.
Competition for senior management and senior personnel in the PRC is intense, the pool of qualified candidates in the PRC is very limited, and we may not be able to retain the services of our senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. This failure could materially and adversely affect our future growth and financial condition. We expect to hire additional sales and plant personnel throughout fiscal year 2010 in order to accommodate its growth.
If we fail to increase our brand recognition, we may face difficulty in obtaining new customers and business partners.
We believe that establishing, maintaining and enhancing our brand in a cost-effective manner is critical to achieving widespread acceptance of our current and future products and services and is an important element in our effort to increase our customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in our market develops. Some of our potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of our brand will depend largely on our ability to maintain a sizeable and active customer base, our marketing efforts and ability to provide reliable and useful products and services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses we will incur in building our brand. If we fail to successfully promote and maintain our brand, or if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, we may fail to attract enough new customers or retain our existing customers to the extent necessary to realize a sufficient return on our brand-building efforts, in which case our business, operating results and financial condition, would be materially adversely affected.
Our operating results may fluctuate as a result of factors beyond our control.
Our operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are beyond our control. These factors include:
|
●
|
the costs of pharmaceutical products and development;
|
|
|
●
|
the relative speed and success with which we can obtain and maintain customers, merchants and vendors for our products;
|
|
|
●
|
capital expenditure for equipment;
|
|
|
●
|
marketing and promotional activities and other costs;
|
|
|
●
|
changes in our pricing policies, suppliers and competitors;
|
|
|
●
|
the ability of our suppliers to provide products in a timely manner to their customers;
|
|
|
●
|
changes in operating expenses;
|
|
|
●
|
increased competition in the pharmaceutical markets; and
|
|
|
●
|
other general economic and seasonal factors.
|
We face risks related to product liability claims.
We presently do not maintain product liability insurance. We face the risk of loss because of adverse publicity associated with product liability lawsuits, whether or not such claims are valid. We may not be able to avoid such claims. Although product liability lawsuits in the PRC are rare, and we have not, to date, experienced significant failure of our products, there is no guarantee that we will not face such liability in the future. This liability could be substantial and the occurrence of such loss or liability may have a material adverse effect on our business, financial condition and prospects.
We face marketing risks.
Newly developed drugs and technology may not be compatible with market needs. Because markets for drugs differentiate geographically inside the PRC, we must develop and manufacture our products to accurately target specific markets to ensure product sales. We have spent $16,616, $53,273 and $63,594 on market research during the years ended December 31, 2009, 2008 and 2007, which account for 0.1%, 0.3% and 0.5% of net sales respectively. If we fail to invest in extensive market research to understand the health needs of consumers in different geographic areas, we may face limited market acceptance of our products, which could have material adverse effect on our sales and earnings.
12
We face risks relating to difficulty in defending intellectual property rights from infringement.
Our success depends on protection of our current and future technology and products and our ability to defend our intellectual property rights. Shaanxi Jiali currently holds 2 patents and 9 trademarks and has filed for trademark protection for additional names and brands of its products sold in the PRC. We also have certain limited administrative SFDA protection for our non-patented products. However, it is possible for its competitors to develop similar competitive products even though we have taken steps to protect our intellectual property. If we fail to protect Shaanxi Jiali’s intellectual property adequately, competitors may manufacture and market products similar to Shaanxi Jiali.
Presently, we sell our products mainly in PRC. To date, no trademark or patent filings have been made other than in PRC. To the extent that we market our products in other countries, we may have to take additional action to protect our intellectual property. The measures we take to protect our proprietary rights may be inadequate and we cannot give you any assurance that our competitors will not independently develop formulations and processes that are substantially equivalent or superior to our own or copy our products.
We expect to file additional patent applications seeking to protect newly developed technology and products in various countries, including the PRC. Some patent applications in the PRC are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of our discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for our products. Patents that are issued may be challenged, invalidated or circumvented by our competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
Currently, the SFDA does not automatically stay drug registration approval upon initiation of an infringement lawsuit by a third party. At present, we must wait until a copycat manufacturer has received marketing approval from SFDA before we can bring an infringement lawsuit. Furthermore, PRC courts have been hesitant to issue preliminary injunctions to suspend sales until a final judgment is issued in the lawsuit. Our sales could be lowered were a competitor to infringe our intellectual property rights by marketing one or more versions of SFDA-approved drugs proprietary to us until we can curtail such infringement through legal action. Pursuing infringement lawsuits would require us to devote financial and management resources that could impact the results of our operations.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
We face risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We are not aware of any infringement claims that have been filed against us by third parties. However, we may be required to commence or defend against charges relating to the infringement of patents or proprietary rights. Any such litigation could:
|
●
|
require us to incur substantial expense, even if covered by insurance or are successful in the litigation;
|
|
|
●
|
require us to divert significant time and effort of our technical and management personnel;
|
|
|
●
|
result in the loss of our rights to develop or make certain products; and
|
|
|
●
|
require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties.
|
Although intellectual property disputes within the pharmaceutical industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling some of our products or increase our costs to market these products.
In addition, when seeking regulatory approval for some of our products, we may be required to certify to regulatory authorities, including the SFDA, that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against us. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
13
Our launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to us. If we are found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of our operations and financial condition.
Shaanxi Jiali is currently entitled to a beneficial tax exemption for a five year period; however, such tax exemption may be interpreted to be not in compliance with PRC tax laws in the future causing us to set aside certain contingency funds for dealing with potential retrospective tax liabilities.
Shaanxi Jiali is entitled to enjoy the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy an exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax.
As to the tax treatment promised by local governments to purely domestic enterprises, i.e., Shaanxi Jiali, invested by non-local (but not foreign) investors under the so called preferential policy announced by local governments, our consultation with PRC certified public accountants and lawyers, is that the above policy is not compliant with the PRC laws. Even though such practice exists in many areas across the country, the policy faces the risk of being ruled illegal at any time for non-compliance with relevant laws. In this event, there is a risk that we might be assessed retrospective tax liabilities.
We face risks related to research and the ability to develop new drugs.
Our growth and survival depends on our ability to consistently discover, develop and commercialize new products and find new and improve on existing technology and platforms. During the year ended December 31, 2007, we spent $35,500 on research and development expenses, which amounted to 0.3% of our net sales. During the years ended December 31, 2009 and December 31, 2008, we have prepaid $955,900 and $808,800, respectively, of research and development expenses which amounted to 5.7% and 5.5%, respectively, of our net sales. We prepaid these expenses to Shaanxi Research Institution of Chinese Traditional Medicines (“SRICTM”), which develops certain drugs on our behalf. Should the applications for these drugs be rejected by the SFDA due to a technology-related error or any error of SRICTM, SRICTM is contractually obligated under Sections 5.3, 7.3 and 10.1 of the agreement to refund all fees already paid by the Company under the terms of the Medicine Research and Development Agreement and Supplemental Agreement of Medicine Research and Development Agreement. If we fail to make sufficient investments in research, be attentive to consumer needs or does not focus on the most advanced technology, our current and future products could be surpassed by more effective or advanced products of other companies.
Risk Related To the Pharmaceutical Industry
Our certificates, permits, and licenses related to our pharmaceutical operations are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
Shaanxi Jiali is subject to various PRC laws and regulations pertaining to the pharmaceutical industry. Shaanxi Jiali has attained certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC.
In 1998, the SFDA introduced the GMP Certificate in order to promote quality and safety of pharmaceutical production. The Good Manufacturing Practices were revised in July and October 2004. We and our competitors are required to meet GMP standards in order to continue manufacturing pharmaceutical products and health foods. For each new product, Shaanxi Jiali prepares documentation of pharmacological, toxicity, pharmacokinetics and drug metabolism studies in addition to providing samples of the drug. The documentation and samples are then submitted to provincial food and drug administration. This process typically takes approximately three months. After the documentation and samples have been approved by the provincial food and drug administration, the provincial administration submits the approved documentation and samples to the SFDA. The SFDA examines the documentation, tests the samples and presents the findings to the New Drug Examination Committee for approval. If the application is approved by the SFDA, the SFDA will issue a clinical trial license to the applicant for clinical trials. This clinical trial license approval typically takes one year, followed by approximately two years of trials, depending on the category and class of the new drug. The SFDA then examines the documentation from the trial and, if approved, issues the new drug license to the applicant. This process usually takes eight months. The entire process takes anywhere from three to four years.
Shaanxi Jiali initially obtained pharmaceutical products permits by submitting its manufacturing processes and product tests to the SFDA who verified that its production processes and products met the standards by onsite inspections, review of test results and a determination that the market was not saturated by its products. The production permits are permanent once issued as long as they are renewed by the expiration date. The GMP certificate is valid for a term of five years, and each must be renewed before its expiration, if applicable. Pursuant to SFDA regulation, if there is a gap at any time in the validity of a company’s GMP certificate, it must suspend production or face stiff fines and the risk that its authorization for production may be permanently revoked.
14
We manufacture and package our products at one factory in Baoji, Shaanxi Province, China. This facility is in compliance with Good Manufacturing Practice (GMP) standards and we received our initial GMP certificate on November 3, 2004. (Certificate No. Shaan F0076 for Baoji Production Base). The certificate remained valid until November 2, 2009. Our GMP certificate expired on November 2, 2009. It is not uncommon for companies in the pharmaceutical industry to suspend production while they are in application for a renewed permit in order to prepare for and accommodate GMP inspection. We applied to renew our GMP certificate prior to November 2, 2009, and as such we did not experience any penalties or delays in the processing of our application. Because, however, we experienced an increase in customer orders toward the end of the calendar year, we continued production until November 2, 2009 in order to meet all of our customers’ needs.
The SFDA generally takes about three to four months to review GMP renewal applications and as a result, we did not receive approval for the renewed certificate until April 2010. Between November 2009 and April 2010, while our application for renewal was being processed and our facilities underwent standard GMP review and inspection, we temporarily suspended production during the application period. During this time, we worked with our OEM partners to meet our manufacturing needs. We also experienced a lower demand for our products during this period due to China’s Spring Festival holiday in the month of February. As such, we continued to have sales between November 2009 and April 2010 even though we did not manufacture any products in-house and were able to use our OEM manufacturers to meet all of our customers’ needs. Since April 2010, when we were notified that the SFDA had approved our application, we have resumed regular production. We expect to officially receive our renewed certificate in early July 2010. However, we cannot guarantee that we will not experience interruptions or terminations in production in the future should our GMP certificate lapse.
According to Drug Administration Law of the PRC and its implementing rules, the SFDA approvals, including Pharmaceutical Manufacturing Permit and Drug Approval Numbers, may be suspended or revoked prior to the expiration date under circumstances that include:
|
●
|
producing counterfeit medicine;
|
|
|
●
|
producing inferior quality products;
|
|
|
●
|
failing to meet the drug GMP standards;
|
|
|
●
|
purchasing medical ingredients used in the production of products sources that do not have Pharmaceutical Manufacturing Permit or Pharmaceutical Trade Permit;
|
|
|
●
|
fraudulent reporting of results or product samples in application process;
|
|
|
●
|
failing to meet drug labeling and direction standards;
|
|
|
●
|
bribing doctors or hospital personnel to entice them to use products,
|
|
|
●
|
producing pharmaceuticals for use or resale by companies that are not approved by the SFDA, or
|
|
|
●
|
the approved drug has a serious side effect.
|
If our pharmaceutical products fail to receive regulatory approval or are severely limited in these products' scope of use, we may be unable to recoup considerable research and development expenditures.
Our research and development of pharmaceutical products is subject to the regulatory approval of the SFDA in the PRC. The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of resources not currently available; in such an event, it may be necessary for us to abandon our application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use. If approval of our product is denied, abandoned, or severely limited in terms of the scope of products use, it may result in the inability to recoup considerable research and development expenditures. If we do not receive timely approval for any of these drugs, then production will be delayed and sales of the products cannot be planned for.
Price control regulations may decrease our profitability.
The laws of the PRC provide for the government to fix and adjust prices. The prices of certain medicines we distribute, including those listed in the Chinese government's catalogue of medications that are reimbursable under the PRC’s social insurance program, or the Insurance Catalogue, are subject to control by the relevant state or provincial price administration authorities. The PRC establishes price levels for products based on market conditions, average industry cost, supply and demand and social responsibility. In practice, price control with respect to these medicines sets a ceiling on their retail price. The actual price of such medicines set by manufacturers, wholesalers and retailers cannot historically exceed the price ceiling imposed by applicable government price control regulations. Although, as a general matter, government price control regulations have resulted in drug prices tending to decline over time, there has been no predictable pattern for such decreases.
For the years ended December 31, 2009 and December 31, 2008, we have not had any products subject to specific pricing control and production and trading of none of our pharmaceutical products constitutes a monopoly. However, it is possible that our products may be subject to price control in the future. To the extent that our products are subject to price control, our revenue, gross profit, gross margin and net income will be affected since the revenue we derive from our sales will be limited and we may face no limitation on our costs. Further, if price controls affect both our revenue and costs, our ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
15
If the medicines we produce are replaced by other medicines or are removed from the PRC's insurance catalogue in the future, our revenue may suffer.
Under PRC regulations, patients purchasing medicine listed by the PRC's state and/or provincial governments in the Insurance Catalogue may be reimbursed, in part or in whole, by a social medicine fund. Accordingly, pharmaceutical distributors prefer to engage in the distribution of medicine listed in the Insurance Catalogue. Currently, our main prescription products are listed in the Insurance Catalogue. The content of the Insurance Catalogue is subject to change by the PRC Ministry of Labor and Social Security, and new medicine may be added to the Insurance Catalogue by provincial level authorities as part of their limited ability to change certain medicines listed in the Insurance Catalogue. If the medicine we produce are replaced by other medicines or removed from the Insurance Catalogue in the future, our revenue may suffer.
Adverse publicity associated with our products, ingredients or network marketing program, or those of similar companies, could harm our financial condition and operating results.
The results of our operations may be significantly affected by the public's perception of our product and similar companies. This perception is dependent upon opinions concerning:
|
●
|
the safety and quality of our products and ingredients;
|
|
|
●
|
the safety and quality of similar products and ingredients distributed by other companies; and
|
|
|
●
|
our sales force.
|
Adverse publicity concerning any actual or purported failure to comply with applicable laws and regulations regarding product claims and advertising, good manufacturing practices, or other aspects of our business, whether or not resulting in enforcement actions or the imposition of penalties, could have an adverse affect on our goodwill and could negatively affect our sales and ability to generate revenue.
In addition, our consumers' perception of the safety and quality of products and ingredients as well as similar products and ingredients distributed by other companies can be significantly influenced by media attention, publicized scientific research or findings, widespread product liability claims and other publicity concerning our products or ingredients or similar products and ingredients distributed by other companies. Adverse publicity, whether or not accurate or resulting from consumers' use or misuse of our products, that associates consumption of our products or ingredients or any similar products or ingredients with illness or other adverse effects, questions the benefits of our or similar products or claims that any such products are ineffective, inappropriately labeled or have inaccurate instructions as to their use, could negatively impact our reputation or the market demand for our products.
If we fail to develop new products with high profit margins, and our high profit margin products are substituted by competitor's products, our gross and net profit margins will be adversely affected.
There is no assurance that we will be able to sustain our profit margins in the future. The pharmaceutical industry is very competitive, and there may be pressure to reduce sale prices of products without a corresponding decrease in the price of raw materials. In addition, the medical industry in the PRC is highly competitive and new products are constantly being introduced to the market. In order to increase our sales and expand our market share, we may be forced to reduce prices in the future, leading to a decrease in gross profit margin. The research and development of new products and technology is costly and time consuming, and there are no assurances that our research and development of new products will either be successful or completed within the anticipated timeframe, if ever at all. There is no assurance that our competitors' new products, technology, and processes will not render our existing products obsolete or non-competitive. To the extent that we fail to develop new products with high profit margins and our high profit margin products are substituted by competitors' products, our gross profit margins will be adversely affected.
The commercial success of our products depends upon the degree of market acceptance among the medical community and failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
The commercial success of our products depends upon the degree of market acceptance among the medical community, such as hospitals and physicians. Even if our products are approved by the SFDA, there is no assurance that physicians will prescribe or recommend our products to patients. Furthermore, a product's prevalence and use at hospitals may be contingent upon its relationship with the medical community, particularly products that are only available by medical prescription. The acceptance of our products among the medical community may depend upon several factors, including but not limited to, the product's acceptance by physicians and patients as a safe and effective treatment, cost effectiveness, potential advantages over alternative treatments, and the prevalence and severity of side effects. Failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
16
We enjoy certain preferential tax concessions and loss of these preferential tax concessions will cause its tax liabilities to increase and its profitability to decline.
Shaanxi Jiali enjoys preferential tax concessions in the PRC as a high-tech enterprise because of the research and development methodologies it employs to develop new products. Pursuant to the State Council's Regulations on Encouraging Investment in and Development, Shaanxi Jiali was granted a reduction in its income tax rate under which it paid no income taxes from January 1, 2006 to December 31, 2007 and had had an income tax rate of 15% since January 1, 2008 which is a 50% reduction on the current effective income tax rate. This favorable 50% tax exemption treatment will expire on December 31, 2010. There is no assurance that the preferential tax treatment in the PRC will remain unchanged and effective. Its tax liabilities will increase and its profits may accordingly decline if its reduced income tax rate is no longer applicable and/or the tax relief on investment in PRC is no longer available. Additionally, the PRC Enterprise Income Tax Law (the "EIT Law") was enacted on March 16, 2007. Under the EIT Law, effective January 1, 2008, the PRC will adopt a uniform tax rate of 25.0% for all enterprises (including foreign-invested enterprises) and cancel several tax incentives enjoyed by foreign-invested enterprises. However, for foreign-invested enterprises established before the promulgation of the EIT Law, a five-year transition period is provided during which reduced rates will apply but gradually be phased out. Since the PRC government has not announced implementation measures for the transitional policy with regards to such preferential tax rates, we cannot reasonably estimate the financial impact of the new tax law to Shaanxi Jiali at this time. Further, any future increase in the enterprise income tax rate applicable to it or other adverse tax treatments, such as the discontinuation of preferential tax treatments for high and new technology enterprises, would have a material adverse effect on its results of operations and financial condition.
Risks Related to Doing Business in the PRC
Changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business .
Our business operations may be adversely affected by the current and future political environment in the PRC. The PRC has operated as a socialist state since the mid-1900s and is controlled by the PRC’s Communist Party. The Chinese government exerts substantial influence and control over the manner in which we and it must conduct our business activities. The PRC has only permitted provincial and local economic autonomy and private economic activities since 1988. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy, particularly the pharmaceutical industry, through regulation and state ownership. Our ability to operate in the PRC may be adversely affected by changes in Chinese laws and regulations, including those relating to taxation, import and export tariffs, raw materials, environmental regulations, land use rights, property and other matters. Under current leadership, the government of the PRC has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the government of the PRC will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
The PRC's economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case.
A change in policies by the PRC government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the PRC government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC's political, economic and social life.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.
The PRC laws and regulations governing our current business operations are sometimes vague and uncertain. The PRC’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to the laws and regulations governing our business, or the enforcement and performance of our arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are
17
considered a foreign persons or foreign funded enterprises under PRC laws, and as a result, we are required to comply with PRC laws and regulations. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on its businesses. If the relevant authorities find that we are in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
|
●
|
levying fines;
|
|
|
●
|
revoking Shaanxi Jiali’sbusiness and other licenses;
|
|
|
●
|
requiring that we restructure our ownership or operations; and
|
|
|
●
|
requiring that we discontinue any portion or all of our business.
|
Among the material laws that we are subject to are the Medicine Management Law, governing the management of pharmaceutical companies, medicine production procedure, packaging, prices, Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements from State Council, governing rules on advertising, Standardization of the Management on the Quality of Medicine Production issued by SFDA, providing standards for staff, plants, equipment, materials, environment, production management, products laws, and the Price Law of The People’s Republic of China, Measurement Law of The People’s Republic of China, Tax Law, Environmental Protection Law, Contract Law, Patent Law, Accounting Laws and Labor Law.
A slowdown, inflation or other adverse developments in the PRC economy may harm our customers and the demand for our services and products.
All of our operations are conducted in the PRC and all of our revenue is generated from sales in the PRC. Although the PRC economy has grown significantly in recent years, we cannot assure you that this growth will continue. A slowdown in overall economic growth, an economic downturn, a recession or other adverse economic developments in the PRC could significantly reduce the demand for our products and harm our business.
While the PRC economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth could lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may harm our profitability. In order to control inflation in the past, the PRC government has imposed controls on bank credit, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In October 2004, the People's Bank of China, the PRC's central bank, raised interest rates for the first time in nearly a decade and indicated in a statement that the measure was prompted by inflationary concerns in the Chinese economy. Repeated rises in interest rates by the central bank would likely slow economic activity in the PRC which could, in turn, materially increase its costs and also reduce demand for its products.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. We receive substantially all of our revenue in Renminbi, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of the PRC to pay capital expenses such as the repayment of bank loans denominated in foreign currencies.
The PRC government may also in the future restrict access to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
The fluctuation of the Renminbi may harm your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC's political and economic conditions. According to the currency website www.xe.com, as of December 31, 2009, $1 = 6.828 Renminbi. As we rely entirely on revenue earned in the PRC, any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenue and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from an offering of our securities into Renminbi for Shaanxi Jiali’s operations, appreciation of the Renminbi against the U.S. dollar would diminish the value of the proceeds of the offering and this could harm Shaanxi Jiali’s business, financial condition and results of operations because it would reduce the proceeds available to us for capital investment in proportion to the appreciation of the Renminbi. Thus if we raise 1,000,000 dollars and the Renminbi appreciates against the U.S. dollar by 15%, then the proceeds will be worth only RMB 5,803,800 as opposed to RMB 6,828,000 prior to the appreciation. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our common shares or for other business purposes and the U.S. dollar appreciates against the Renminbi; the U.S. dollar
18
equivalent of the Renminbi we convert would be reduced in proportion to the amount the U.S. dollar appreciates. In addition, the depreciation of significant RMB denominated assets could result in a charge to our income statement and a reduction in the dollar value of these assets. Thus if Shaanxi Jiali has RMB 1,000,000 in assets and Renminbi is depreciated against the U.S. dollar by 15%, then the assets will be valued at $124,487 as opposed to $146,456 prior to the depreciation.
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. This change in policy has resulted in an approximately 17.5% appreciation of the Renminbi against the U.S. dollar as of December 31, 2009. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar.
PRC state administration of foreign exchange ("SAFE") regulations regarding offshore financing activities by PRC residents which may increase the administrative burden we face. The failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
SAFE issued a public notice ("SAFE #75") effective from November 1, 2005, which requires registration with SAFE by the PRC resident shareholders of any foreign holding company of a PRC entity. Without registration, the PRC entity cannot remit any of its profits out of the PRC as dividends or otherwise.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of the PRC, referred to as an "offshore special purpose company," for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in the PRC. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
It is unclear whether our PRC resident shareholders must make disclosure to SAFE. While our PRC counsel has advised us that only PRC resident shareholders who receive ownership of the foreign holding company in exchange for ownership in the PRC operating company are subject to SAFE #75, there can be no assurance that SAFE will not require our other PRC resident shareholders to register and make the applicable disclosure. In addition, SAFE #75 requires that any monies remitted to PRC residents outside of the PRC be returned within 180 days; however, there is no indication of what the penalty will be for failure to comply or if shareholder non-compliance will be considered to be a violation of SAFE #75 by us or otherwise affect us.
In the event that the proper procedures are not followed under SAFE #75, Xi’an Coova could lose the ability to remit monies outside of the PRC and would therefore be unable to pay dividends or make other distributions. Our PRC resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the PRC Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
The PRC's legal and judicial system may not adequately protect our business and operations and the rights of foreign investors.
The PRC legal and judicial system may negatively impact foreign investors. In 1982, the National People's Congress amended the Constitution of the PRC to authorize foreign investment and guarantee the "lawful rights and interests" of foreign investors in the PRC. However, the PRC's system of laws is not yet comprehensive. The legal and judicial systems in the PRC are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the PRC lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the PRC judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The PRC's legal system is based on the civil law regime, that is, it is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
19
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in Chinese enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the PRC's political, economic or social life, will not affect the PRC government's ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on our business and prospects.
The practical effect of the PRC legal system on our business operations in the PRC can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the Foreign Invested Enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate Articles and contracts to Foreign Invested Enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are qualitatively different from the general corporation laws of the United States. Similarly, the PRC accounting laws mandate accounting practices, which are not consistent with U.S. generally accepted accounting principles. PRC's accounting laws require that an annual "statutory audit" be performed in accordance with PRC accounting standards and that the books of account of Foreign Invested Enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People's Republic of China Wholly Foreign-Owned Enterprise Law requires a wholly foreign-owned enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. While the enforcement of substantive rights may appear less clear than United States procedures, the Foreign Invested Enterprises and Wholly Foreign-Owned Enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution. Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the Chinese legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of Foreign Invested Enterprises.
Any recurrence of severe acute respiratory syndrome, or SARS, or another widespread public health problem, could harm our operations.
A renewed outbreak of SARS or another widespread public health problem (such as bird flu) in the PRC, where all of our revenue is derived, could significantly harm our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some of our offices that would adversely disrupt our operations. Any of the foregoing events or other unforeseen consequences of public health problems could significantly harm our operations.
Because our principal assets are located outside of the United States and most of our directors and all of our officers reside outside of the United States, it may be difficult for you to enforce your rights based on U.S. Federal Securities Laws against us and our officers or to enforce U.S. Court Judgments against us or them in the PRC.
Most of our directors and all of our officers reside outside of the United States. In addition, our operating company is located in the PRC and substantially all of our assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the U.S. Federal securities laws against us in the courts of either the U.S. or the PRC and, even if civil judgments are obtained in U.S. courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the U.S. Federal securities laws or otherwise.
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
Risks Related to our Common Stock
Our officers and directors control us through their positions and stock ownership and their interests may differ from other stockholders.
As of September 1, 2010 , there were 10,180,288 shares of our common stock issued and outstanding. Our officers and directors own approximately 26.85% of our common stock. As a result, they are able to influence the outcome of stockholder votes on various matters, including the election of directors and extraordinary corporate transactions including business combinations. Yet our officers’ and directors’ interests may differ from those of other stockholders, particularly as may relate to the election of directors or management. Our officers and directors may also delay, deter or prevent a change in our control or other business combinations that might otherwise be
20
beneficial to our stockholders. Furthermore, ownership of 26.85% of our common stock by our officers and directors reduces the public float and liquidity, and may affect the market price, of our common stock when and if our common stock as traded on the OTCBB.
We are not likely to pay cash dividends in the foreseeable future.
We intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate. Should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions.
There is a limited market for our common stock, which may make it difficult for you to sell your stock.
Our common stock trades on the OTCBB under the symbol “CPDU.OB.” There is a limited trading market for our common stock. Accordingly, there can be no assurance as to the liquidity of any markets that may develop for our common stock, the ability of holders of our common stock to sell our common stock, or the prices at which holders may be able to sell our common stock.
Our shares are subject to the U.S. “Penny Stock” Rules and investors who purchase our shares may have difficulty re-selling their shares as the liquidity of the market for our shares may be adversely affected by the impact of the “Penny Stock” Rules.
Our stock is subject to U.S. "Penny Stock" rules, which may make the stock more difficult to trade on the open market. Our common shares currently trade on the OTCBB. A "penny stock" is generally defined by regulations of the U.S. Securities and Exchange Commission ("SEC") as an equity security with a market price of less than US$5.00 per share. However, an equity security with a market price under US$5.00 will not be considered a penny stock if it fits within any of the following exceptions:
|
(i)
|
the equity security is listed on AMEX or a national securities exchange;
|
|
|
(ii)
|
the issuer of the equity security has been in continuous operation for less than three years, and either has (a) net tangible assets of at least US$5,000,000, or (b) average annual revenue of at least US$6,000,000; or
|
|
|
(iii)
|
the issuer of the equity security has been in continuous operation for more than three years, and has net tangible assets of at least US$2,000,000.
|
Our common stock does not currently fit into any of the above exceptions.
If an investor buys or sells a penny stock, SEC regulations require that the investor receive, prior to the transaction, a disclosure explaining the penny stock market and associated risks. Furthermore, trading in our common stock is currently subject to Rule 15g-9 of the Securities Exchange Act of 1934 (the “Exchange Act”), which relates to non-NASDAQ and non-exchange listed securities. Under this rule, broker/dealers who recommend our securities to persons other than established customers and accredited investors must make a special written suitability determination for the purchaser and receive the purchaser's written agreement to a transaction prior to sale. Securities are exempt from this rule if their market price is at least $5.00 per share.
Since our common stock is currently deemed a penny stock, it will be subject to penny stock regulations, which may reduce market liquidity of our common stock, because they limit the broker/dealers' ability to trade, and a purchaser's ability to sell, the stock in the secondary market.
The low price of our common stock has a negative effect on the amount and percentage of transaction costs paid by individual shareholders. The low price of our common stock also limits our ability to raise additional capital by issuing additional shares. There are several reasons for these effects. First, the internal policies of certain institutional investors prohibit the purchase of low-priced stocks. Second, many brokerage houses do not permit low-priced stocks to be used as collateral for margin accounts or to be purchased on margin. Third, some brokerage house policies and practices tend to discourage individual brokers from dealing in low-priced stocks. Finally, broker's commissions on low-priced stocks usually represent a higher percentage of the stock price than commissions on higher priced stocks. As a result, our shareholders may pay transaction costs that are a higher percentage of their total share value than if our share price were substantially higher.
As an issuer of “penny stock” the protection provided by the federal securities laws relating to forward-looking statements does not apply to us and as a result we could be subject to legal action.
Forward-looking statements are based on current expectations, estimates, forecasts and projections about the Company, us, our future performance, our beliefs and our Management’s assumptions. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties. Forward-looking statements include statements regarding, among other things, (a) projected sales, profitability, and cash flows, (b) growth strategies, (c) anticipated trends, (d) future financing plans and (e) anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,”
21
“should,” “anticipates,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend,” or the negative of these words or other variations on these words or comparable terminology. These statements may be found under “Management’s Discussion and Analysis of Financial Condition and Plan of Operation” and “Business,” as well as in this prospectus generally. In particular, these include statements relating to future actions, prospective products or product approvals, future performance or results of current and anticipated products, sales efforts, expenses, the outcome of contingencies such as legal proceedings, and financial results.
These forward-looking statements are not guarantees of future performance and involve risks, uncertainties and assumptions that we cannot predict. Any assumptions, upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business. However, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested in this prospectus. We assume no obligation to update forward-looking statements, except as otherwise required under the applicable federal securities laws.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stock. As a result, if we are a penny stock, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
For more information about penny stocks, contact the Office of Filings, Information and Consumer Services of the U.S. Securities and Exchange Commission, 100 F Street, N.E., Washington, D.C. 20549, or by telephone at 1-800-732-0330.
A large number of shares will be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them.
Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
Investors may experience immediate and substantial dilution in net tangible book value per share of our common stock if they elect to exercise the warrants.
Investors may experience immediate and substantial dilution in net tangible book value per share of our common stock if they elect to exercise the Warrants. The exercise price of the Warrants may be substantially higher than the net tangible book value per share. Accordingly, if the selling stockholders exercise the Warrants, they will likely experience immediate and substantial dilution in the net tangible book value per share and further dilution if we issue shares of our common stock in the future. As a result of this dilution, in the event of our subsequent liquidation, the selling stockholders exercising their Warrants may receive significantly less than the full exercise price that they paid for the shares of our common stock.
Risks Related to the Offering
We will have broad discretion in applying the net proceeds of this offering and may not use these proceeds in ways that will enhance the market value of our common stock.
We have broad discretion in applying the net proceeds we will receive in this offering. Such proceeds may be used to fund product development, corporate growth and for other general corporate purposes. For more information, see “Use of Proceeds.” As part of your investment decision, you will not be able to assess or direct how we apply these net proceeds.
If the China Securities Regulatory Commission, or CSRC, or another Chinese regulatory agency, determines that CSRC approval is required in connection with this offering or our contractual arrangement with Shaanxi Jiali and its shareholders,, this offering may be delayed or cancelled, or we may become subject to penalties.
On August 8, 2006, six Chinese regulatory agencies, including the Chinese Securities Regulatory Commission, or CSRC, promulgated the M&A Regulation, which became effective on September 8, 2006. This regulation, among other things, has certain provisions that require offshore special purpose vehicles formed for the purpose of acquiring Chinese domestic companies and directly or indirectly established or controlled by Chinese entities or individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock market. On September 21, 2006, the CSRC published on its official website a notice specifying the documents and materials that are required to be submitted for obtaining CSRC approval. It is not clear how the provisions in the regulation regarding the offshore listing and trading of the securities of a special purpose vehicle apply to us. We believe that CSRC approval is not required for this offering or our contractual arrangement with Shaanxi Jiali and its shareholders. There remains some uncertainty as to how this regulation will be interpreted or implemented. If the
22
CSRC or another Chinese regulatory agency subsequently determines that the CSRC’s approval is required for this offering or our contractual arrangement with Shaanxi Jiali and its shareholders, we may face sanctions by the CSRC or another Chinese regulatory agency. If this happens, these regulatory agencies may impose fines and penalties on our operations in China, limit our operating privileges in China, delay or restrict the repatriation of our net proceeds from this offering into China, restrict or prohibit payment or remittance of dividends to us or take other actions that could have a material adverse effect on our business, financial condition, results of operations, reputation and prospects, as well as the trading price of our shares of common stock. The CSRC or other Chinese regulatory agencies may also take actions requiring us, or making it advisable for us, to delay or cancel this offering before settlement and delivery of the shares being offered by us.
There may be future sales or other dilution of our equity, which may adversely affect the market price of our common stock.
We are not restricted from issuing additional common stock, including any securities that are convertible into or exchangeable for, or that represent the right to receive, common stock. The issuance of any additional shares of common stock or securities convertible into, exchangeable for or that represent the right to receive common stock or the exercise of such securities could be substantially dilutive to holders of our common stock. Holders of shares of our common stock have no preemptive rights that entitle holders to purchase their pro rata share of any offering of shares of any class or series. The market price of our common stock could decline as a result of sales of shares of our common stock made after this offering or the perception that such sales could occur. Because our decision to issue securities in any future offering will depend on market conditions and other factors beyond our control, we cannot predict or estimate the amount, timing or nature of our future offerings. Thus, our shareholders bear the risk of our future offerings reducing the market price of our common stock and diluting their interests in us.
The shares of common stock offered hereby are being registered for the account of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the common stock will go to the selling stockholders and we will not receive any proceeds from the resale of the common stock by the selling stockholders, although we could receive proceeds of up to $10,000,000 if all of the share purchase warrants are exercised. We intend to use the proceeds from the exercise of the Warrants, if any, for working capital and other general corporate purposes. We cannot assure you that any of the Warrants will ever be exercised, if at all. We will, however, incur all costs associated with this registration statement and prospectus.
The common stock to be sold by the selling shareholders is common stock that will be issued to our shareholders upon exercise of certain Warrants. Accordingly, there will be no dilution to our existing shareholders.
Our common stock, having $.001 par value per share, is traded on the Over-The-Counter Bulletin Board ("OTCBB") under the symbol "CPDU". Prior to December 14, 2009, the Company traded under the symbol “LHSI.” Although our stock is traded on the OTCBB, the market for our stock, particularly prior to September 30, 2009, has been relatively inactive.
The following table sets forth, for the periods indicated, the reported high and low closing bid quotations for CPDU’s common stock as reported on the OTCBB. The bid prices reflect inter-dealer quotations, do not include retail mark-ups, markdowns or commissions and do not necessarily reflect actual transactions.
Common Stock
|
Period Ended:
|
High
|
Low
|
||||||
|
April 1, 2010 to June 30, 2010
|
5.00
|
1.90
|
||||||
|
January 1, 2010 to March 31, 2010
|
4.20
|
3.00
|
||||||
|
October 1, 2009 to December 31, 2009
|
4.20
|
0.06
|
||||||
|
July 1, 2009 to September 30, 2009
|
0.06
|
0.06
|
||||||
|
April 1, 2009 to June 30, 2009
|
0.06
|
0.06
|
||||||
|
January 1, 2009 to March 31, 2009
|
0.06
|
0.06
|
||||||
|
October 1, 2008 to December 31, 2008
|
1.00
|
0.06
|
||||||
|
July 1, 2008 to September 30, 2008
|
1.01
|
1.00
|
||||||
|
April 1, 2008 to June 30, 2008
|
1.25
|
1.01
|
||||||
|
January 1, 2008 to March 31, 2008
|
2.50
|
1.25
|
||||||
Holders
As of September 1, 2010 , we had approximately 397 shareholders of record of our common stock, excluding the shares held in street name by brokerage firms.
23
Penny Stock Regulations
The SEC has adopted regulations which generally define "penny stock" to be an equity security that has a market price of less than $5.00 per share. Our common stock, falls within the definition of penny stock and subject to rules that impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000, or annual incomes exceeding $200,000 or $300,000, together with their spouse).
For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of such securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks. Consequently, the "penny stock" rules may restrict the ability of broker-dealers to sell our common stock and may affect the ability of investors to sell their common stock in the secondary market.
Dividends
Any future determination as to the declaration and payment of dividends on shares of our common stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of common stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from China Children Pharmaceutical, Xi’an Coova and Shaanxi Jiali. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
Determination of Market Price
Our shares of common stock are very thinly traded, and the price if traded may not reflect our value. The selling stockholders may offer to sell the shares of common stock being offered in this prospectus at fixed prices, at prevailing market prices at the time of sale, at varying prices or at negotiated prices. Our common stock is quoted on the OTC Bulletin Board under the symbol "CPDU". On September 1, 2010 , the closing bid price for one share of our common stock on the OTC Bulletin Board was $ 4.75.
The fixed exercise prices of $3.00 and $5.00 per warrant was determined after the Company entered into the Management Agreement and related agreements with Shaanxi Jiali, based upon advice of investment bankers after taking into account our prospects, revenue anticipated to be generated by China Children Pharmaceuticals and by using the overall valuation of our Company. No assurance, however, can be given that any warrants will be exercised at the $3.00 and $5.00 price. The public offering price does not necessarily bear any relation to our asset value, earnings, net financial condition or other established criteria of value applicable to us and should not be regarded as the actual value or future market price of our common stock. Such prices are subject to change as a result of market conditions and other factors and no assurance can be given that the shares can be resold at the public offering price.
Securities Authorized for Issuance Under Equity Compensation Plans
As of the date of this registration statement, we do not have any securities authorized for issuance under any equity compensation plans and we do not have any equity compensation plans.
Shares Eligible for Future Sale
Although our common stock is traded on the OTCBB, there is no established trading market for our common stock and the market for our stock has been relatively inactive. Future sales of substantial amounts of our common stock in the trading market could adversely affect market prices.
As of September 1, 2010, there were issued and outstanding (i) 10,180,288 shares of common stock, (ii) Warrants to purchase 2,500,000 shares of common stock; and (iv) no outstanding options. Assuming exercise of all of the Warrants, there will be 12,680,288 shares of common stock outstanding. All of the shares underlying the Warrants are being registered for resale in this prospectus. As of September 1, 2010, none of the 10,180,288 shares of common stock are currently eligible for resale under Rule 144.
24
Registration Rights
Other than the registration rights set forth in the Warrants, we have no other obligation to register under the Securities Act any of our shares of common stock.
Rule 144 Shares
After February 15, 2008, a person who has beneficially owned shares of a company’s common stock for at least six months is entitled to sell within any three month period a number of shares that does not exceed 1% of the number of shares of our common stock then outstanding which, in our case, would equal approximately 86,803 shares of our common stock as of the date of this prospectus.
Sales under Rule 144 are also subject to manner of sale provisions and notice requirements and to the availability of current public information about the company. Under Rule 144(b), a person who is not one of the company’s affiliates at any time during the three months preceding a sale, and who has beneficially owned the shares proposed to be sold for at least one year, is entitled to sell shares without complying with the manner of sale, public information, volume limitation or notice provisions of Rule 144.
Notwithstanding the allowances for resale described above, Rule 144(i) imposes additional restrictions on companies that have at any time previously qualified as a “shell” company (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended) . Under Rule 144(i), the shares of a former shell company are not available for resale unless (i) the company is subject to the reporting requirements of section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months (or for such shorter period that the company was required to file such reports and materials), and (ii) one year has elapsed from the time that the company has filed current Form 10 information with the SEC reporting that it has ceased to be a shell company.
As a result of Rule 144(i) and given that we filed Form 10 information reporting that we had ceased to be shell company under Item 5.06 of the 8-K filed with the SEC on October 7, 2009, none of our shares of common stock are currently available for resale to the public and in accordance with the volume and trading limitations of Rule 144 of the Act.
Reports
We are subject to certain filing requirements and furnish annual financial reports to our stockholders, certified by our independent accountant, and un-audited quarterly financial reports in our quarterly reports filed electronically with the SEC. All reports and information filed by us can be found at the SEC website, www.sec.gov .
The following discussion of the financial condition and results of operation of the Company for the years ended December 31, 2009, and 2008, should be read in conjunction with the consolidated financial statements and the notes to those statements that are included elsewhere in this registration statement.
Some of the information contained in this discussion and analysis or set forth elsewhere in this prospectus, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this prospectus for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Cautionary Note Regarding Forward-Looking Statements
This prospectus contains forward-looking statements. For this purpose, any statements contained in this prospectus that are not statements of historical fact may be deemed to be forward-looking statements, including any statements relating to future actions and outcomes including but not limited to prospective products, future performance or results of current or anticipated products, sales and marketing efforts, costs and expense trends, future interest rates, outcome of contingencies and their future impact on financial condition, results of operations, liquidity, business strategies, cost savings, objectives of management, and other matters. You can identify forward-looking statements by those that are not historical in nature, particularly those that use terminology such as “expects,” “anticipates,” “targets,” “goals,” “projects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “may,” “will,” “should,” “contemplates,” “predicts,” “potential,” or “continue” or variations and/or the negative of these similar terms. Forward-looking statements are based on current expectations, estimates, forecasts and projections about the Company, us, our future performance, our beliefs and our Management’s assumptions. All forward-looking statements made in this prospectus, including any future written or oral statements made by us or on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
25
These forward-looking statements are not guarantees of future performance and involve risks, uncertainties and assumptions that we cannot predict. Any assumptions, upon which they are based, are made in good faith and reflect our current judgment regarding the direction of our business, actual results will almost always vary, sometimes materially, from any estimates, predictions, projections, assumptions or other future performance suggested in this prospectus. Therefore, actual outcomes and results may differ materially from what is expressed or forecast in such forward-looking statements. In evaluating these forward-looking statements, you should consider various factors, including the following: (a) those risks and uncertainties related to general economic conditions, (b) whether we are able to manage our planned growth efficiently and operate profitable operations, (c) whether we are able to generate sufficient revenues or obtain financing to sustain and grow our operations, (d) whether we are able to successfully fulfill our primary requirements for cash. Please refer to the section “Liquidity and Capital Resources” and the risks outlined under “Risk Factors” contained in this prospectus for additional discussion. Please also refer to our other filings with the Securities and Exchange Commission. We assume no obligation to update forward-looking statements, except as otherwise required under the applicable federal securities laws.
COMPARISON OF THE THREE AND SIX MONTHS ENDED JUNE 30, 2010 AND 2009
Net Sales
|
Three Months Ended June 30,
|
% of
|
|||||||||||||||||||
|
2010
|
2009
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
397,215
|
6.10
|
%
|
$
|
436,327
|
12.30
|
%
|
(9
|
) %
|
||||||||||
|
"Cooer" Series
|
5,563,885
|
85.48
|
%
|
2,360,221
|
66.54
|
%
|
136
|
%
|
||||||||||||
|
"Qingsongling" Series
|
547,669
|
8.41
|
%
|
750,576
|
21.16
|
%
|
(27
|
) %
|
||||||||||||
|
Others
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||
|
Total net sales
|
$
|
6,508,769
|
100
|
%
|
$
|
3,547,124
|
100
|
%
|
83
|
%
|
||||||||||
|
Six Months Ended June 30,
|
% of
|
|||||||||||||||||||
|
2010
|
2009
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
410,055
|
3.10
|
%
|
$
|
837,242
|
11.72
|
%
|
(51
|
) %
|
||||||||||
|
"Cooer" Series
|
11,249,083
|
85.11
|
%
|
5,115,568
|
71.62
|
%
|
119
|
%
|
||||||||||||
|
"Qingsongling" Series
|
575,629
|
4.35
|
%
|
1,189,964
|
16.66
|
%
|
(52
|
) %
|
||||||||||||
|
Others
|
982,894
|
7.44
|
-
|
-
|
-
|
|||||||||||||||
|
Total net sales
|
$
|
13,217,661
|
100
|
%
|
$
|
7,142,774
|
100
|
%
|
85
|
%
|
||||||||||
During the three and six months ended June 30, 2010, total net sales increased by approximately $3 million or 83% and $6.1 million or 85%, respectively, compared to the same periods of 2009.
This was primarily due to the sales increases for "Cooer" Series by $3.2 million or 136% and $6.1 million or 119% in the three and six months ended June 30, 2010, respectively. The increase in “Cooer” Series sales was mainly due to the increase in sales volume as we expanded our customer base and as a result of the increase in number of customers from 8 to 11 in the three months ended June 30, 2010 and from 8 to 18 in the six months ended June 30, 2010. These were also as a result of our intensive promotion of the series in 2010.
26
Cost of Sales
|
Three Months Ended June 30,
|
% of
|
|||||||||||||||||||
|
2010
|
2009
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
45,495
|
1.75
|
%
|
$
|
47,715
|
3.58
|
%
|
(5
|
) %
|
||||||||||
|
"Cooer" Series
|
2,293,042
|
88.20
|
%
|
928,845
|
69.74
|
%
|
147
|
%
|
||||||||||||
|
"Qingsongling" Series
|
261,156
|
10.05
|
%
|
355,275
|
26.68
|
%
|
(26
|
) %
|
||||||||||||
|
Others
|
-
|
-
|
-
|
-
|
||||||||||||||||
|
Total cost of sales
|
$
|
2,599,693
|
100
|
%
|
$
|
1,331,835
|
100
|
%
|
95
|
%
|
||||||||||
|
Six Months Ended June 30,
|
% of
|
|||||||||||||||||||
|
2010
|
2009
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
46,910
|
0.85
|
%
|
$
|
149,264
|
5.36
|
%
|
(69
|
) %
|
||||||||||
|
"Cooer" Series
|
4,543,970
|
82.48
|
%
|
1,969,938
|
70.69
|
%
|
130
|
%
|
||||||||||||
|
"Qingsongling" Series
|
272,670
|
4.95
|
%
|
667,485
|
23.95
|
%
|
(59
|
) %
|
||||||||||||
|
Others
|
645,681
|
11.72
|
%
|
-
|
-
|
-
|
||||||||||||||
|
Total cost of sales
|
$
|
5,509,231
|
100
|
%
|
$
|
2,786,687
|
100
|
%
|
98
|
%
|
||||||||||
Compared to the same periods of 2009, the total cost of sales increased about $1.3 million or 95% and $2.7 million or 98%, in the three and six months ended June 30, 2010, respectively. This was primarily due to increase in cost of sales for "Cooer" Series which is in line with the increase in these products' net sales. The slight decrease in cost of sales for "Xianzhi" Series and "Qingsongling" Series were mainly due to decrease in these products' net sales. The increase in total cost of sales was primarily due to the increase in average unit costs. The increase in average unit costs was mainly caused by the increase in wages of workers due to the increase in statutory minimum wages in the PRC.
Gross Profit
|
Three Months Ended June 30,
|
||||||||||||||||||||
|
2010
|
2009
|
|||||||||||||||||||
|
gross profit
|
gross profit
|
% of
|
||||||||||||||||||
|
margin
|
margin
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
351,720
|
89
|
%
|
$
|
388,612
|
89
|
%
|
(9
|
) %
|
||||||||||
|
"Cooer" Series
|
3,270,843
|
59
|
%
|
1,431,376
|
61
|
%
|
129
|
%
|
||||||||||||
|
"Qingsongling" Series
|
286,513
|
52
|
%
|
395,301
|
53
|
%
|
(28
|
) %
|
||||||||||||
|
Others
|
-
|
-
|
-
|
-
|
-
|
|||||||||||||||
|
Total
|
$
|
3,909,076
|
60
|
%
|
$
|
2,215,289
|
62
|
%
|
76
|
%
|
||||||||||
|
Six Months Ended June 30,
|
||||||||||||||||||||
|
2010
|
2009
|
|||||||||||||||||||
|
gross profit
|
gross profit
|
% of
|
||||||||||||||||||
|
margin
|
margin
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
363,145
|
89
|
%
|
$
|
687,978
|
82
|
%
|
(47
|
) %
|
||||||||||
|
"Cooer" Series
|
6,705,113
|
60
|
%
|
3,145,630
|
61
|
%
|
113
|
%
|
||||||||||||
|
"Qingsongling" Series
|
302,959
|
53
|
%
|
522,479
|
44
|
%
|
(42
|
) %
|
||||||||||||
|
Others
|
337,213
|
34
|
%
|
-
|
-
|
-
|
||||||||||||||
|
Total
|
$
|
7,708,430
|
58
|
%
|
$
|
4,356,087
|
61
|
%
|
77
|
%
|
||||||||||
Gross profit increased about $1.7 million or 76% and $3.4 million or 77%, respectively, in the three and six months ended June 30, 2010 compared to the same periods of 2009. The increase in gross profits was due primarily to the increase in net sales of "Cooer" Series achieved through afore-mentioned expansion of our customer base.
The overall gross profit margin decreased 2% and 3% points in the three and six months ended June 30, 2010, respectively, compared to the same periods of 2009.
27
For the three months ended June 30, 2010, the slight decrease in overall gross profit margin was primarily due to the increase in cost of sales while the sales price of finished goods remained constant. The increase in cost of sales was primarily due to the increase in average unit cost of finished goods as direct labour costs increased following the increase in statutory minimum wages in PRC. The sales prices of finished goods were kept constant in order to maintain market share among competitors.
As a result of GMP inspection, production was temporarily suspended in the first quarter of 2010 and as a result we experienced a surplus in raw materials on hand for production. The Company therefore sold all the surplus raw materials with carrying value amounting to US$645,682 (i.e. at cost US$1,136,898 net of impairment brought forward US$491,216) included in “Others” back to its suppliers at US$982,894 (i.e. at a discount around 86% of the original costs US$1,136,898). Consequently, the overall gross profit ratio slightly decreased in the six months ended June 30, 2010 compared with the same period in 2009.
Selling, General and Administrative Expenses
|
Three Months Ended June 30,
|
||||||||||||||||||||
|
2010
|
2009
|
|||||||||||||||||||
|
% of total
|
% of total
|
% of
|
||||||||||||||||||
|
Selling, general and
|
net sales
|
net sales
|
change
|
|||||||||||||||||
|
administrative expenses
|
$
|
2,384,925
|
37
|
%
|
$
|
1,172,882
|
33
|
%
|
103
|
%
|
||||||||||
|
Six Months Ended June 30,
|
||||||||||||||||||||
|
2010
|
2009
|
|||||||||||||||||||
|
% of total
|
% of total
|
% of
|
||||||||||||||||||
|
net sales
|
net sales
|
change
|
||||||||||||||||||
|
Selling, general and
|
||||||||||||||||||||
|
administrative expenses
|
$
|
4,528,127
|
34
|
%
|
$
|
2,394,384
|
34
|
%
|
89
|
%
|
||||||||||
The increases in dollar amount and as a percentage of total net sales for the three and six months ended June 30, 2010 were mainly due to the increase in promotional and advertising expenditures of around $220,000 and $439,000, respectively, increase in sales commissions expenses of around $173,000 and 254,000, respectively, increase in sales rebate to customers of around $347,000 and $508,000, respectively, all resulting from the ongoing marketing expansion, and also due to increase in sales related tax levy of around $30,000 and $38,000, respectively, as a result of increased net sales. Other items contributed to the increase in selling, general and administrative expenses for the three and six months ended June 30, 2010 included increase in operating expenses in the nature of IPO expenses of around $214,000 and $401,000, respectively and stock-based compensation costs of around $240,000 and $480,000 , respectively.
Provision for Income Taxes
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
June 30,
|
June 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Provision for income taxes
|
$
|
254,793
|
$
|
137,072
|
$
|
443,579
|
$
|
267,981
|
||||||||
|
Effective tax rate
|
15
|
%
|
15
|
%
|
$
|
15
|
%
|
15
|
%
|
|||||||
The amount of Enterprise Income Tax payable is computed on the basis of the taxable income and by applying the tax rate of 25%. The Company enjoyed a reduced rate to 15% from the Enterprises Income Tax in 2010 and 2009 because the Company is considered to be a "High Technology Business" under PRC income tax laws.
Increase in the amount of provision for income taxes for the three and six months ended June 30, 2010 are mainly due to the increase in assessable income compared to the same period of 2009.
Net Income
As a result of the above, in the three and six months ended June 30, 2010, the net income was $1,257,809 and $2,725,257 compared to the net income of $797,400 and $1,585,799 for the three and six months ended June 30, 2009, respectively.
28
Liquidity and Capital Resources
As of June 30, 2010, we had cash and cash equivalents of approximately $6.6 million. We believe our existing cash and cash equivalents will be sufficient to maintain our operations at present level for at lease the next twelve months.
Net cash provided by operating activities for the six months ended June 30, 2010 was $1,154,458. This was primarily due to the net income of $2,725,257, adjusted by non-cash related expenses including depreciation and amortization of $209,682 and stock-based compensation costs of $483,138 and a net increase in working capital items of $2,263,619. The net increase in working capital items was mainly due to increase in accounts payable, accrued expenses and other payables and income tax payable. The net increase in working capital items was partially offset by the increase in accounts receivable, increase in other receivables and prepayments, and increase in inventories to prepare for the ongoing sales promotion, and decrease in VAT tax payable.
Net cash provided by operating activities for the six months ended June 30, 2009 was $14,499. This was primarily due to the net income of $1,585,799, adjusted by non-cash related expenses including depreciation and amortization of $223,582 and impairment loss on accounts and other receivables of $108,180 and offset by a net decrease in working capital items of $1,903,062. The net decrease in working capital items was mainly due to an increase in accounts receivable, which was a result of the significant increase in revenue during the first six months of 2009, an increase in prepayments, ab increase in inventories, and a decrease in VAT and income tax payable. The net decrease in working capital items was partially offset by the increase in accounts payable, accrued expenses and other payables.
Net cash flow provided by financing activities for the six months ended June 30, 2010 was $4,500,000. The increase of net cash inflow from financing activities for the six months ended June 30, 2010 was due to the proceeds from issuance of common stock of 1,875,000 shares.
Inflation
Inflation in recent years have affected the business results of the Company. First of all, on the global economic expansion, supply has been restricted and coupled with the fact that USA has adopted a relaxed currency policy etc., these increase inflation risks. Secondly, GNP increases in China also elevates consumption ability and production cost, prices increase as a natural tendency. Finally, as a result of the macro economic trend, prices increase, the Company’s procurement prices are also affected resulting in increase in cost of sales.
The Company operates in China and as such, the Company’s business activities, financial position and operational results will be affected by PRC politics, economic and legal environments and also affected by the overall economic situation of China. The business of the Company may be affected by the relevant laws, regulations, anti-inflation measures, currency conversion and overseas remittance and exchange rates issues, etc. that are related to PRC politics.
Off-Balance Sheet Commitments and Arrangements
The Company does not have any off balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity or capital expenditures or capital resources that is material to an investor in our securities.
Short-Term Loans
As of June 30, 2010, the Company had short-term debt as follows:
|
Amount
|
Interest rate
|
Due
|
|||||
|
Short term bank loan
|
$
|
440,619
|
0.85845% /per month
|
June 25, 2010, extended
|
|||
|
until June 25, 2011
|
|||||||
The Company uses these loans for working capital purposes. The loan is secured by the factory building of Shaanxi Jiali's Baoji production base.
Our anticipated needs for the future are to be negotiated in accordance with manufacturing and operation needs, and market conditions of next year.
29
Critical Accounting Policies and Estimates
Our financial statements and related public financial information are based on the application of accounting principles generally accepted in the United States ("US GAAP"). US GAAP requires the use of estimates; assumptions, judgments and subjective interpretations of accounting principles that have an impact on the assets, liabilities, revenues and expenses amounts reported. These estimates can also affect supplemental information contained in our external disclosures including information regarding contingencies, risk and financial condition. We believe our use of estimates and underlying accounting assumptions adhere to GAAP and are consistently and conservatively applied. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates under different assumptions or conditions. We continue to monitor significant estimates made during the preparation of our financial statements.
We believe the following is among the most critical accounting policies that impact our consolidated financial statements. We suggest that our significant accounting policies, as described in our condensed consolidated financial statements in the Summary of Significant Accounting Policies, be read in conjunction with this Management's Discussion and Analysis of Financial Condition and Results of Operations. See also Note 2 to our consolidated financial statements for further discussion of our accounting policies.
Revenue recognition
The Company’s revenue recognition policies are in compliance with Staff Accounting Bulletin (“SAB”) 104, included in the Codification as ASC 605, Revenue Recognition.
We recognize revenue at the date of shipment to customers as the sales price is either fixed or determinable at the date of shipment to customers, and the management deems no other significant obligations such as warranties or product returns of the Company exist. The Company signs general Annual Sales Cooperation Agreements with its distributors each year and also signs specific purchase orders for each sale. All sales proceeds can be collected reasonably.
Allowance for Doubtful Accounts
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves are recorded primarily on a specific identification basis.
The following table sets out the aging of our accounts receivable for each of the two balance sheet periods presented.
|
Accounts
|
1-30
|
31-60
|
61-90
|
91-180
|
181-365
|
> 365
|
||||||||||||||||||||||
|
Receivable Aging
|
Total
|
days
|
days
|
days
|
days
|
days
|
days
|
|||||||||||||||||||||
|
As of June 30, 2010
|
$
|
6,430,750
|
$
|
2,601,607
|
$
|
2,430,795
|
$
|
1,180,299
|
$
|
214,886
|
$
|
446
|
$
|
2,717
|
||||||||||||||
|
As of December 31, 2009
|
$
|
5,666,307
|
$
|
2,320,203
|
$
|
1,635,568
|
$
|
956,329
|
$
|
414,810
|
$
|
336,691
|
$
|
2,706
|
||||||||||||||
The following presents the days sales outstanding calculated based on sales and accounts receivables in RMB term for the three and six months ended June 30, 2010 and 2009.
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
June 30,
|
June 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
Days sales outstanding
|
89
|
130
|
88
|
129
|
||||||||||||
Prepaid expenses
A table of the outstanding prepaid expenses as of June 30, 2010, March 31, 2010, and December 31, 2009 are as follows:
|
Payee
|
Nature
|
6/30/2010
|
3/31/2010
|
12/31/2009
|
||||||||||||
|
Advance payment to R&D organization
|
Shaanxi Research Institution of Chinese Traditional Medicine
|
Advanced payment to Shaanxi Research Institution of Chinese Traditional Medicine for five new pediatric cold medicines
|
$
|
1,762,477
|
$
|
1,755,387
|
$
|
1,755,104
|
||||||||
|
Advance payment to suppliers
|
Suppliers
|
Advance payments to suppliers for goods purchased
|
2,938,561
|
2,926,740
|
2,487,493
|
|||||||||||
|
Total
|
$
|
4,701,038
|
4,682,127
|
$
|
4,242,597
|
|||||||||||
Prepayments to suppliers did not change from December 31, 2009 to June 30, 2010 due to the following reasons:
Prepayments to suppliers are primarily comprised of two types of expenses: (i) an advertising fee (the term of service which is generally one year), which will be expensed in the third and fourth quarters of 2010; and (ii)prepaid deposits for purchasing raw materials and packaging materials required for our manufacturing and operations, which is typically expensed with three months. Although a portion of prepayments for the raw materials and packaging materials have been expensed, due to the purchasing behavior of our customers in 2010 which has caused a slightly higher demand for raw materials and packaging materials, as of June 30, 2010, such prepayments have not reduced but have in fact increased. In the third and fourth quarters of 2010, when our advertising fees are expensed, our prepayment will be reduced.
Inventory
Management compares the cost of inventories with the market value. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand, future pricing and market conditions.
Property, plant and Equipment
Property, plant and equipment are stated at historical cost less accumulated depreciation and amortization. Expenditures for maintenance and repairs are charged to earnings as incurred, additions, renewals and betterments are capitalised. When property and equipment are retired or otherwise disposal of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss in included in operations. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Judgment is required to determine the estimated useful lives of assets, especially for plant and equipment and transportation equipment, including determining how long existing equipment can function and when new technologies will be introduced at cost-effective price points to replace existing equipment. Changes in these estimates and assumptions could materially impact the financial position and results of operations.
30
Accounting for Stock-Based Compensation
The account for stock-based compensation based on the provisions of Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees", as amended by the Financial Accounting Standards Board Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation." Accounting Principles Board Opinion No. 25 and Financial Accounting Standards Board Interpretation No. 44 state that no compensation expense is recorded for stock options or other stock-based awards to employees that are granted with an exercise price equal to or above the estimated fair value per share of the company’s common stock on the grant date. We adopted the disclosure requirements of Statement of Financial Accounting Standards No. 123, "Accounting for Stock-Based Compensation" which requires compensation expense to be disclosed based on the fair value of the options granted at the date of the grant.
In December 2004, the FASB issued FASB Statement No. 123R, "Share-Based Payment, an Amendment of FASB Statement No. 123" ("FAS No. 123R"). FAS No. 123R requires companies to recognize in the statement of operations the grant date fair value of stock options and other equity-based compensation issued to employees. FAS No. 123R is effective beginning in the first quarter of fiscal 2006.
We did not issue any stock options to employees during the three months ended June 30, 2010, therefore pro forma disclosures are not required.
Accounting for Defined Benefit Pensions and Other Postretirement Plans
In September 2006, FASB issued SFAS No. 158, “Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). SFAS No. 158 improves financial reporting by requiring an employer to recognize the over funded or under funded status of a defined benefit postretirement plan (other than a multiemployer plan) as an asset or liability in its statement of financial position and to recognize changes in that funded statues in the year in which the changes occur through comprehensive income of a business entity or changes in unrestricted net assets of a not-for-profit organization. This Statement also improves financial reporting by requiring an employer to measure the funded status of a plan as of the date of its year-end statement of financial position, with limited exceptions. An employer with publicly traded equity securities is required to initially recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the end of the fiscal year ending after
December 15, 2006. An employer without publicly traded equity securities is required to recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the end of the fiscal year ending after June 15, 2007. However, an employer without publicly traded equity securities is required to disclose the following information in the notes to financial statements for a fiscal year ending after December 15, 2006, but before June 16, 2007, unless it has applied the recognition provisions of this Statement in preparing those financial statements.
a.A brief description of the provisions of this Statement
b.The date that adoption is required
c.The date the employer plans to adopt the recognition provisions of this Statement, if earlier.
The requirement to measure plan assets and benefit obligations as of the date of the employer’s fiscal year-end statement of financial position is effective for fiscal years ending after December 15, 2008.The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Valuation of Intangibles
From time to time, we acquire intangible assets that are beneficial to our product development processes. Management periodically evaluates the carrying value of intangibles, including the related amortization periods. In evaluating acquired intangible assets, management determines whether there has been impairment by comparing the anticipated undiscounted cash flows from the operation and eventual disposition of the product line with its carrying value. If the undiscounted cash flows are less than the carrying value, the amount of the impairment, if any, will be determined by comparing the carrying value of each intangible asset with its fair value. Fair value is generally based on either a discounted cash flows analysis or market analysis. Future operating income is based on various assumptions, including regulatory approvals, patents being granted, and the type and nature of competing products. If regulatory approvals or patents are not obtained or are substantially delayed, or other competing technologies are developed and obtain general market acceptance or market conditions otherwise change, our intangibles may have a substantially reduced value, which could be material.
31
In April 2008, the FASB issued FASB Staff Position (FSP) FAS 142-3, “Determination of the Useful Life of Intangible Assets,” which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, “Goodwill and Other Intangible Assets.” This Staff Position is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of this new FSP did not have a material impact on the Company’s financial position, results of operations or cash flows.
The Fair Value Option for Financial Assets and Financial Liabilities
In February, 2007, FASB issued SFAS 159, ‘The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115.” This Statement permits entities to choose to measure many financial instruments and certain other items at fair value. This Statement is expected to expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. This Statement is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Income Taxes
The Company utilizes SFAS No. 109, "Accounting for Income Taxes," which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), "Accounting for uncertainty in Income Taxes." FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, "Accounting for Contingencies." FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company's financial statements.
Pursuant to the PRC income tax laws , the Enterprise Income Tax ("EIT") through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high technology business and under PRC Income Tax Laws, it is entitled to a two-year tax exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a reduced rate of 15% to the Company.
Foreign Currency
Our functional currency is the U.S. dollar and our subsidiary and our operating company in China use their respective local currencies as their functional currencies, i.e. the Chinese Yuan Renminbi (CNY). An entity's functional currency is the currency of the primary economic environment in which the entity operates. Management must use judgment in determining an entity's functional currency, assessing economic factors including cash flow, sales price, sales market, expense, financing and inter-company transactions and arrangements. Impact from exchange rate changes related to transactions denominated in currencies other than the functional currency is recorded as a gain and loss in the statements of operations, while impact from exchange rate changes related to translating a foreign entity's financial statements from the functional currency to its reporting currency, the U.S. dollar, is disclosed and accumulated in a separate component under the equity section of the balance sheets. Different judgments or assumptions resulting in a change of functional currency may materially impact our financial position and results of operations.
Statutory Reserve
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of June 30, 2010 and December 31, 2009, the Company had allocated $810,540 to these non-distributable reserve funds.
32
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
|
● FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”).
|
|
● FASB Staff Positions FAS No. 115-2, FAS 124-2, and EITF No. 99-20-2, Recognition and Presentation of Other-Than-Temporary Impairments, (“FSP FAS No. 115-2, FAS No. 124-2, and EITF No. 99-20-2”) provides additional guidance to provide greater clarity about the credit and noncredit component of an other-than-temporary impairment event and to more effectively communicate when an other-than-temporary impairment event has occurred. This FSP applies to debt securities. FSP FAS No. 115-2 and FAS No. 124-2 were superseded by the Investments-Debt and Equity Securities Topic of the FASB Accounting Standards Codification (“ASC 320”).
|
|
● FASB Staff Position FAS No. 107-1 and APB No. 28-1, Interim Disclosures about Fair Value of Financial Instruments, (“FSP FAS No. 107-1 and APB No. 28-1”) amends FASB Statement No. 107, Disclosures about Fair Value ofFinancial Instruments, to require disclosures about fair value of financial instruments in interim as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting, to require those disclosures in all interim financial statements. FSP FAS No. 107-1 and APB No. 28-1were superseded by the Financial Instruments Topic of the FASB Accounting Standards Codification (“ASC 825”).
|
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued ASU No. 2009-12, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), that amends ASC 820 to provide guidance on measuring the fair value of certain alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force, that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. ASU No. 2009-13 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
33
In October 2009, the FASB issued ASU No. 2009-14, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force, that reduces the types of transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product or service. As a result of the amendments included in ASU No. 2009-14, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the ASU, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components and non-software components function together to deliver the product’s essential functionality, and undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). ASU No. 2009-14 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
For information regarding these and other recent accounting pronouncements and their expected impact on our future financial condition or results of operations, see Note 2 to our consolidated financial statements .
COMPARISON OF THE YEAR ENDED DECEMBER 31, 2009 AND 2008
Net Sales
|
Years Ended December 31,
|
% of
|
|||||||||||||||||||
|
2009
|
2008
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
1,568,251
|
9
|
%
|
$
|
2,345,365
|
16
|
%
|
(33
|
)%
|
||||||||||
|
"Cooer" Series
|
12,124,760
|
73
|
%
|
7,710,927
|
53
|
%
|
57
|
%
|
||||||||||||
|
"Qingsongling" Series
|
3,045,448
|
18
|
%
|
4,612,587
|
31
|
%
|
(34
|
)%
|
||||||||||||
|
Total net sales
|
$
|
16,738,459
|
100
|
%
|
$
|
14,668,879
|
100
|
%
|
14
|
%
|
||||||||||
Total net sales for the fiscal year ended December 31, 2009 increased by $2,069,580 or approximately 14% compared to the same period of 2008. This was mainly due to increases in sales of "Cooer" Series by 57% in 2009, as a result of the intensive promotion in 2008.
Sales of our Xianzhi series decreased to 794,431 boxes in 2009 from 1,229,174 boxes in fiscal 2008, representing a decrease of 35%. Average unit price of these products increased .to RMB13.50 in 2009 from RMB 13.28, or approximately a increase of 1.66% Sales of our Cooer series increased to 15,657,758 boxes from 9,979,800 boxes in 2008, representing an increase of 57%. The average unit price of our Cooer series of products increased to RMB 5.37 in 2009 from RMB 5.30 in 2008, or approximately a increase of 1.32%. Sales of our Qingsongling series of products decreased to 4,577,985 boxes from 6,937,306 boxes in fiscal 2008, representing a decrease of 34%. Average unit prices for this series did not change.
We recognize the development potential of pediatric pharmaceuticals market in China and made a long term strategy “committing to the field of children health products, In our goal to become the No.1 Brand of Chinese Pediatric Medicines”, in 2009 we shifted our the market positioning from Qing Songling and Xianzhi series as the main brands to the Cooer series as the main brand. Due to funding constraints and because we have focused on developing our Cooer brand, funds used for promotional and advertising expenditures of our Xianzhi series and Qingsongling series decreased from fiscal year 2008 to 2009, and as a result, sales of the Xianzhi and Qingsongling series also experienced a decrease during this period. However, we expect that this trend will not be permanent, since we anticipate that the development of Cooer brand products will drive the popularity and sales of our other products.
Cost of Sales
|
Years Ended December 31,
|
% of
|
|||||||||||||||||||
|
2009
|
2008
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$
|
188,920
|
3
|
%
|
$
|
218,620
|
4
|
%
|
(14
|
)%
|
||||||||||
|
"Cooer" Series
|
5,269,261
|
75
|
%
|
2,675,101
|
53
|
%
|
97
|
%
|
||||||||||||
|
"Qingsongling" Series
|
1,540,971
|
22
|
%
|
2,189,831
|
43
|
%
|
(30
|
)%
|
||||||||||||
|
Total cost of sales
|
$
|
6,999,152
|
100
|
%
|
$
|
5,083,552
|
100
|
%
|
38
|
%
|
||||||||||
Compared to the fiscal year ended December 31, 2008, cost of sales increased $1,915,800 or approximately 38% in the year ended December 31, 2009. This was primarily resulting from the increase in product net sales. The increase in average unit costs was mainly caused by the general increase in costs of raw material in China in the year 2009 and increase in wages of workers due to statutory minimum wage increases.
Gross Profit
|
Years Ended December 31,
|
||||||||||||||||||||
|
2009
|
2008
|
|||||||||||||||||||
|
gross profit
|
gross profit
|
% of
|
||||||||||||||||||
|
margin
|
margin
|
change
|
||||||||||||||||||
|
"Xianzhi" Series
|
$ | 1,379,330 | 88 | % | $ | 2,126,745 | 91 | % | (35 | )% | ||||||||||
|
"Cooer" Series
|
6,855,499 | 57 | % | 5,035,825 | 65 | % | 36 | % | ||||||||||||
|
"Qingsongling" Series
|
1,504,478 | 49 | % | 2,422,757 | 53 | % | (38 | )% | ||||||||||||
|
Total
|
$ | 9,739,307 | 58 | % | $ | 9,585,327 | 65 | % | 2 | % | ||||||||||
Gross profit ratio decreased slightly compared with the same period in 2008 due to the increase in cost of sales in the year 2009, while the sales price for finished goods remained constant. The Company keeps the sales price of finished goods constant in order to maintain market share among competitors.
Selling, General and Administrative Expenses
|
Years Ended December 31,
|
||||||||||||||
|
2009
|
2008
|
|||||||||||||
|
% of total
|
% of total
|
% of
|
||||||||||||
|
net sales
|
net sales
|
change
|
||||||||||||
|
Selling, general and administrative expenses
|
$
|
6,290,264
|
37
|
%
|
$
|
6,593,341
|
45
|
%
|
(5)
|
%
|
||||
34
In 2009, selling, general and administrative expenses decreased in absolute dollars by $303,077 or about 4% and as a percentage of total net sales by about 8% . These decreases were primarily due to the decrease in promotional and advertising expenditures of approximately $1.2 million. Other items contributing to the decrease in selling, general and administrative expenses for the year ended 2009 included a decrease in operating expenses like office supplies and telecommunication expenses. Under this circumstance, the selling, general and administrative expenses decreased approximately 5%.
Provision for Income Taxes
|
Years Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Provision for income taxes
|
$
|
616,693
|
$
|
484,024
|
||||
|
Effective tax rate
|
15
|
%
|
15
|
%
|
||||
The amount of Enterprise Income Tax payable is computed on the basis of the taxable income and by applying the tax rate of 25%. The Company enjoyed a reduced rate of 15% from the Enterprises Income Tax in 2008 and 2009 because the Company qualifies as a "High Technology Business" under PRC Income Tax Laws.
In 2008, the income tax calculation was based on the Company's unaudited profit. But, the audited profit before tax was lower than the amount of tax charged based on unaudited figures so that the amount of tax overcharged was deducted to offset part of the 2009's income tax. However, the selling and administrative expenses for the year ended December 31, 2009 was decreased by approximately $0.3 million, therefore the assessable income was increased significantly.
Net Income
As a result of the above, in the year ended December 31, 2009, the net income was $2,740,853 compared to a net income of $2,542,575 for the year ended December 31, 2008.
Liquidity and Capital Resources
As of December 31, 2009, we had cash and cash equivalents of approximately $0.9 million. We believe our existing cash and cash equivalents will be sufficient to maintain our operations at present level for at least the next twelve months. We plan to review acquisition opportunities as a strategy for further growth.
Net cash used in operating activities for the year ended December 31, 2009 was $364,616. This was primarily due to the net income of $2,740,853, adjusted by non-cash related expenses including depreciation and amortization of $435,724 and stock based compensation cost of $396,152 offset by a net decrease in working capital items of $3,937,285. The net decrease in working capital items was mainly due to increase in accounts receivable which resulted from the significant increase in revenues during the years ended December 31, 2009 and the longer credit term provided to customers as part of sales promotion, increase in prepayments to suppliers, and increase in inventories to prepare for the ongoing sales promotion, decrease in accounts payable, decrease in income tax payable. The net decrease in working capital items was partially offset by the increase in accrued expenses.
Net cash generated in operating activities for the year ended December 31, 2008, was $157,437. This was primarily due to the net income of $2,542,575, adjusted by non-cash related expenses including depreciation and amortization of $438,867, offset by a net decrease in working capital items of $2,789,178. The net decrease in working capital items was mainly due to increase in inventory, increase in prepayments to suppliers, a decrease in accrued expenses and decrease in VAT tax payable. The net decrease in working capital items was partially offset by increase in accounts receivable and the increase in accounts payable and income tax payable.
Net cash generated from investing activities for the year ended December 31, 2009 and 2008 was $16,107 and $23,865.
Net cash generated in financing activities for the years ended December 31, 2009 was $438,553. This was due to the inception of new bank loans of $438,540 and subscription received of $13. There was no cash used in financing activities for the year ended December 31, 2008.
Off-Balance Sheet Arrangements
We do not have any off balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity or capital expenditures or capital resources that is material to an investor in our securities.
35
Short-Term Loans
As of December 31, 2009, the Company had short-term debt as follows:
|
Amount
|
Interest rate
|
Due
|
||||
|
Short term bank loan
|
$
|
438,776
|
0.85845% /Per month
|
June 25, 2010
|
||
The Company uses these loans for working capital purposes. The loan is secured by the land use rights of Shaanxi Jiali's Baoji production base.
Our anticipated needs for the future are to be negotiated in accordance with manufacturing and operation needs, and market conditions of next year.
Our financial statements and related public financial information are based on the application of accounting principles generally accepted in the United States ("US GAAP"). US GAAP requires the use of estimates; assumptions, judgments and subjective interpretations of accounting principles that have an impact on the assets, liabilities, revenues and expenses amounts reported. These estimates can also affect supplemental information contained in our external disclosures including information regarding contingencies, risk and financial condition. We believe our use of estimates and underlying accounting assumptions adhere to GAAP and are consistently and conservatively applied. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ materially from these estimates under different assumptions or conditions. We continue to monitor significant estimates made during the preparation of our financial statements.
We believe the following is among the most critical accounting policies that impact our consolidated financial statements. We suggest that our significant accounting policies, as described in our condensed consolidated financial statements in the Summary of Significant Accounting Policies, be read in conjunction with this Management's Discussion and Analysis of Financial Condition and Results of Operations. See also Note 2 to our consolidated financial statements for further discussion of our accounting policies.
Revenue recognition
The Company’s revenue recognition policies are in compliance with Staff Accounting Bulletin (“SAB”) 104, included in the Codification as ASC 605, Revenue Recognition .
We recognize revenue at the date of shipment to customers as the sales price is either fixed or determinable at the date of shipment to customers, and the management deems no other significant obligations such as warranties or product returns of the Company exist. The Company signs general Annual Sales Cooperation Agreements with its distributors each year and also signs specific purchase orders for each sale. All sales proceeds can be collected reasonably.
Allowance for Doubtful Accounts
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves are recorded primarily on a specific identification basis.
36
The following table sets out the aging of our accounts receivable for each balance sheet periods presented.
|
Accounts Receivable Aging
|
Total
|
1-30 days
|
31-60 days
|
61-90 days
|
91-180 days
|
181-365 days
|
> 365 days
|
|||||||||||||||||||||
|
As of December 31, 2009
|
$
|
5,666,307
|
$
|
2,320,203
|
$
|
1,635,568
|
$
|
956,329
|
$
|
414,810
|
$
|
336,691
|
$
|
2,706
|
||||||||||||||
|
As of December 31, 2008
|
$
|
3,286,863
|
$
|
1,792,090
|
$
|
1,308,980
|
$
|
132,918
|
$
|
-
|
$
|
-
|
$
|
52,875
|
||||||||||||||
The following presents the days sales outstanding calculated based on sales and accounts receivables in RMB term for each income statement periods presented.
|
Year Ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Days sales outstanding
|
122
|
80
|
||||||
Inventory
Management compares the cost of inventories with the market value. We write down our inventory for estimated obsolescence or unmarketable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand, future pricing and market conditions.
Property, Plant and Equipment
Property, plant and equipment are stated at historical cost less accumulated depreciation and amortization. Expenditures for maintenance and repairs are charged to earnings as incurred, additions, renewals and betterments are capitalised. When property and equipment are retired or otherwise disposal of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss in included in operations. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Judgment is required to determine the estimated useful lives of assets, especially for plant and equipment and transportation equipment, including determining how long existing equipment can function and when new technologies will be introduced at cost-effective price points to replace existing equipment. Changes in these estimates and assumptions could materially impact the financial position and results of operations.
Accounting for Stock-Based Compensation
The account for stock-based compensation based on the provisions of Accounting Principles Board Opinion No. 25, "Accounting for Stock Issued to Employees", as amended by the Financial Accounting Standards Board Interpretation No. 44, "Accounting for Certain Transactions Involving Stock Compensation." Accounting Principles Board Opinion No. 25 and Financial Accounting Standards Board Interpretation No. 44 state that no compensation expense is recorded for stock options or other stock-based awards to employees that are granted with an exercise price equal to or above the estimated fair value per share of the company’s common stock on the grant date. We adopted the disclosure requirements of Statement of Financial Accounting Standards No. 123, "Accounting for Stock-Based Compensation" which requires compensation expense to be disclosed based on the fair value of the options granted at the date of the grant.
In December 2004, the FASB issued FASB Statement No. 123R, "Share-Based Payment, an Amendment of FASB Statement No. 123" ("FAS No. 123R"). FAS No. 123R requires companies to recognize in the statement of operations the grant date fair value of stock options and other equity-based compensation issued to employees. FAS No. 123R is effective beginning in the first quarter of fiscal 2006.
We did not issue any stock options to employees during the year ended December 31, 2007, therefore pro forma disclosures are not required.
Accounting for Defined Benefit Pensions and Other Postretirement Plans
In September 2006, FASB issued SFAS No. 158, “Employers’ Accounting for Defined Benefit Pension and Other Postretirement Plans – an amendment of FASB Statements No. 87, 88, 106, and 132(R)” (“SFAS No. 158”). SFAS No. 158 improves financial reporting by requiring an employer to recognize the over funded or under funded status of a defined benefit postretirement plan (other than a multiemployer plan) as an asset or liability in its statement of financial position and to recognize changes in that funded statues in the year in which the changes occur through comprehensive income of a business entity or changes in unrestricted net assets of a not-for-profit organization. This Statement also improves financial reporting by requiring an employer to measure the funded status of a plan as of the date of its year-end statement of financial position, with limited exceptions. An employer with
37
publicly traded equity securities is required to initially recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the end of the fiscal year ending after December 15, 2006. An employer without publicly traded equity securities is required to recognize the funded status of a defined benefit postretirement plan and to provide the required disclosures as of the end of the fiscal year ending after June 15, 2007. However, an employer without publicly traded equity securities is required to disclose the following information in the notes to financial statements for a fiscal year ending after December 15, 2006, but before June 16, 2007, unless it has applied the recognition provisions of this Statement in preparing those financial statements.
|
a.
|
A brief description of the provisions of this Statement
|
|
b.
|
The date that adoption is required
|
|
c.
|
The date the employer plans to adopt the recognition provisions of this Statement, if earlier.
|
The requirement to measure plan assets and benefit obligations as of the date of the employer’s fiscal year-end statement of financial position is effective for fiscal years ending after December 15, 2008.The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Valuation of Intangibles
From time to time, we acquire intangible assets that are beneficial to our product development processes. Management periodically evaluates the carrying value of intangibles, including the related amortization periods. In evaluating acquired intangible assets, management determines whether there has been impairment by comparing the anticipated undiscounted cash flows from the operation and eventual disposition of the product line with its carrying value. If the undiscounted cash flows are less than the carrying value, the amount of the impairment, if any, will be determined by comparing the carrying value of each intangible asset with its fair value. Fair value is generally based on either a discounted cash flows analysis or market analysis. Future operating income is based on various assumptions, including regulatory approvals, patents being granted, and the type and nature of competing products. If regulatory approvals or patents are not obtained or are substantially delayed, or other competing technologies are developed and obtain general market acceptance or market conditions otherwise change, our intangibles may have a substantially reduced value, which could be material.
In April 2008, the FASB issued FASB Staff Position (FSP) FAS 142-3, “Determination of the Useful Life of Intangible Assets,” which amends the factors that should be considered in developing renewal or extension assumptions used to determine the useful life of a recognized intangible asset under FASB Statement No. 142, “Goodwill and Other Intangible Assets.” This Staff Position is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. Early adoption is prohibited. The adoption of this new FSP did not have a material impact on the Company’s financial position, results of operations or cash flows.
The Fair Value Option for Financial Assets and Financial Liabilities
In February, 2007, FASB issued SFAS 159, ‘The Fair Value Option for Financial Assets and Financial Liabilities – Including an Amendment of FASB Statement No. 115.” This Statement permits entities to choose to measure many financial instruments and certain other items at fair value. This Statement is expected to expand the use of fair value measurement, which is consistent with the Board’s long-term measurement objectives for accounting for financial instruments. This Statement is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The adoption of this new standard did not have a material impact on the Company’s financial position, results of operations or cash flows.
Income Taxes
The Company utilizes SFAS No. 109, "Accounting for Income Taxes," which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), "Accounting for uncertainty in Income Taxes." FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, "Accounting for Contingencies." FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company's financial statements.
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax ("EIT") through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high technology business and under PRC Income Tax Laws, it is entitled to a two-year tax exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a reduced rate of 15% to the Company.
38
Foreign Currency
Our functional currency is the U.S. dollar and our subsidiary and our operating company in China use their respective local currencies as their functional currencies, i.e. the Chinese Yuan Renminbi (CNY). An entity's functional currency is the currency of the primary economic environment in which the entity operates. Management must use judgment in determining an entity's functional currency, assessing economic factors including cash flow, sales price, sales market, expense, financing and inter-company transactions and arrangements. Impact from exchange rate changes related to transactions denominated in currencies other than the functional currency is recorded as a gain and loss in the statements of operations, while impact from exchange rate changes related to translating a foreign entity's financial statements from the functional currency to its reporting currency, the U.S. dollar, is disclosed and accumulated in a separate component under the equity section of the balance sheets. Different judgments or assumptions resulting in a change of functional currency may materially impact our financial position and results of operations.
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
|
●
|
FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”).
|
|
●
|
FASB Staff Positions FAS No. 115-2, FAS 124-2, and EITF No. 99-20-2, Recognition and Presentation of Other-Than-Temporary Impairments , (“FSP FAS No. 115-2, FAS No. 124-2, and EITF No. 99-20-2”) provides additional guidance to provide greater clarity about the credit and noncredit component of an other-than-temporary impairment event and to more effectively communicate when an other-than-temporary impairment event has occurred. This FSP applies to debt securities. FSP FAS No. 115-2 and FAS No. 124-2 were superseded by the Investments-Debt and Equity Securities Topic of the FASB Accounting Standards Codification (“ASC 320”).
|
|
●
|
FASB Staff Position FAS No. 107-1 and APB No. 28-1, Interim Disclosures about Fair Value of Financial Instruments , (“FSP FAS No. 107-1 and APB No. 28-1”) amends FASB Statement No. 107, Disclosures about Fair Value of Financial Instruments , to require disclosures about fair value of financial instruments in interim as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting , to require those disclosures in all interim financial statements. FSP FAS No. 107-1 and APB No. 28-1were superseded by the Financial Instruments Topic of the FASB Accounting Standards Codification (“ASC 825”).
|
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value , which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar
39
liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued ASU No. 2009-12, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent) , that amends ASC 820 to provide guidance on measuring the fair value of certain alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force , that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. ASU No. 2009-13 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
In October 2009, the FASB issued ASU No. 2009-14, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force , that reduces the types of transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product or service. As a result of the amendments included in ASU No. 2009-14, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the ASU, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components and non-software components function together to deliver the product’s essential functionality, and undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). ASU No. 2009-14 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
For information regarding these and other recent accounting pronouncements and their expected impact on our future financial condition or results of operations, see Note 2 to our consolidated financial statements.
Statutory Reserve
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of September 30, 2009 and September 30, 2008, the Company had allocated $810,540 and $721,553, respectively, to these non-distributable reserve funds.
40
Lid Hair Studios International, Inc. was established on April 20, 2005 in the state of Nevada. The company was originally incorporated under the name Belford Enterprises, Inc. and changed its name to Lid Hair Studios International Inc. on August 15, 2005. Our operating subsidiary Belford Enterprises B.C. Ltd., doing business as Lid Hair Studio, was established June 7, 2005, in the City of Vancouver, British Columbia, Canada. There are no bankruptcies, receivership, or similar proceedings against the parent or operating subsidiary.
On June 18, 2005, the company purchased hair salon equipment and the rights to an office lease from Farideh Jafari in the amount of approximately $39,150US ($45,000CDN).
The Merger Transaction
On September 30, 2009, we acquired China Children Pharmaceuticals, a Hong Kong based pharmaceutical manufacturer and distributor company in accordance with a Share Exchange Agreement (“Exchange Agreement”) entered into by and among the Company, Eric Anderson, the Company’s then-principal shareholder, and Asia-Pharm, a company incorporated in the British Virgin Islands, the sole shareholder of China Children Pharmaceuticals.
In exchange for the 100% equity interest in China Children Pharmaceuticals, the Company issued 7,000,000 shares of common stock to the shareholders of Asia-Pharm. As a result of the share exchange transaction, Shaanxi Jiali became our operating entity and our operations have been headquartered in Xi’an, Shaanxi Province, China.
In connection with the share exchange agreement of September 30, 2009, Eric Anderson transferred 5,000,000 shares of common stock of Lid Hair Studios to Lid Hair Studios for cancellation and forgave the outstanding shareholder’s loan to the company in the amount of $86,173 in exchange for 100% of the issued and outstanding shares of Lid Hair Studios’ wholly-owned subsidiary, Belford Enterprises B.C. Ltd., d/b/a Lid Hair Studio. Lid Hair Studios cancelled the shares of common stock received from Eric Anderson prior to affecting the reverse stock split which reduced the amount of common stock then issued and outstanding.
Effective October 9, 2009, the Company changed its name from Lid Hair Studios International Inc. to China Pediatric Pharmaceuticals, Inc. (the “Name Change Transaction”). The name change was effected through a parent/subsidiary merger of our wholly-owned subsidiary, China Pediatric Pharmaceuticals, Inc., with and into the Company, with the Company as the surviving corporation. To effectuate the merger, the Company filed its Articles of Merger with the Nevada Secretary of State and the merger became effective on October 9, 2009. The Company’s board of directors approved the merger which resulted in the name change on October 9, 2009. In accordance with Section 92A.180 of the Nevada Revised Statutes , shareholder approval of the merger was not required.
Background of Shaanxi Jiali
Shaanxi Jiali, our operating entity, was incorporated in the PRC under the name Shaanxi Jiali Technologies Co, Ltd. on July 1, 1998. Shaanxi Jiali is our operating company and is in the business of manufacturing, marketing and sales of pharmaceuticals in China with pediatric medicine as its focus. In October 2000, Shaanxi Jiali Technologies changed its name to Shaanxi Jiali Pharmaceutical Co. Ltd.
Asia-Pharm was incorporated in British Virgin Islands on June 20, 2008. As described below, on August 4, 2008, Asia-Pharm, through its wholly owned subsidiary China Children Pharmaceuticals, became the indirect holding company for Shaanxi Jiali, a medical and pharmaceutical developer, manufacturer and marketer in the PRC.
China Children Pharmaceuticals was incorporated in Hong Kong on June 27, 2008. China Children is a wholly owned subsidiary of Asia-Pharm.
Xi’an Coova is a “wholly owned foreign enterprise” incorporated in the PRC on July 25, 2008. Xi’an Coova is a wholly owned subsidiary of China Children Pharmaceuticals.
As described below, on August 4, 2008, an Management Entrustment Agreement (was entered into between Xi’an Coova and Shaanxi Jiali, which effectively gives China Children Pharmaceuticals, by way of Xi’an Coova, control over the operations and business of Shaanxi Jiali through Xi’an Coova. Pursuant to the Entrustment Management Agreement, China Children Pharmaceuticals shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi’an Coova.
Xi’an Coova entered into a Management Entrustment Agreement with Shaanxi Jiali and the shareholders of Shaanxi Jiali, in which Shaanxi Jiali and its shareholders agreed to transfer control, or entrust, the operations and management of its business to Xi’an Coova. Under the agreement, Xi’an Coova manages the operations and assets of Shaanxi Jiali, controls all of the cash flows of Shaanxi Jiali through a bank account controlled by Xi’an Coova, is
41
entitled to 100% of earnings before tax of Shaanxi Jiali, a management fee, and is obligated to pay all payables and loan payments of Shaanxi Jiali. In addition, under the terms of the Management Entrustment Agreement, Xi’an Coova has been granted certain rights which include, in part, the right to appoint and terminate members of Shaanxi Jiali’s Board of Directors, hire management and administrative personnel and control decisions relating to entering and performing customer contracts and other instruments. We anticipate that Shaanxi Jiali will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Xi’an Coova exercises its option. The agreement does not terminate unless the business of Shaanxi Jiali is terminated or Xi’an Coova exercises its option to acquire all of the assets or equity of Shaanxi Jiali under the terms of the Exclusive Option Agreement as more fully described below and completes the acquisition of Shaanxi Jiali.
In order to induce Shaanxi Jiali to enter into the Management Entrustment Agreement, Xi’an Coova, through its parent company Asia-Pharm, issued an aggregate of 12,000,000 shares of Asia-Pharm’s common stock to the shareholders of Shaanxi Jiali which was allocated based on their respective pro rata ownership of Shaanxi Jiali.
In order to give Xi’an Coova further control over Shaanxi Jiali, the Shaanxi Jiali shareholders and Xi’an Coova, a wholly owned subsidiary of China Children in the PRC, entered into a shareholders’ Voting Proxy Agreement (the “Voting Proxy Agreement”) whereby the Shaanxi Jiali shareholders irrevocably and exclusively appointed the members of Xi’an Coova’s board of directors, as their proxies to vote on all matters that require Shaanxi Jiali shareholder approval, including, without limitation, the right to appoint members of the board of directors of Shaanxi Jiali. The agreement further provides that Shaanxi Jiali will appoint all of the board of directors of Xi’an Coova as its board of directors and if Xi’an Coova’s board of directors undergoes any changes, Shaanxi Jiali must remove and appoint the same members to its board. The agreement terminates upon the exercise of the option by Xi’an Coova to purchase the shares of Shaanxi Jiali as described below and is governed by the laws of the PRC.
In order to permit Shaanxi Jiali to become an indirectly wholly owned subsidiary of China Children when permitted under PRC law, Xi’an Coova, Shaanxi Jiali and the Shaanxi Jiali shareholders entered into an exclusive option agreement (the “Exclusive Option Agreement”) whereby the Shaanxi Jiali shareholders granted Xi’an Coova an irrevocable and exclusive purchase option (the “Option”) to acquire Shaanxi Jiali’s equity and/or remaining assets, but only to the extent that the acquisition does not violate limitations imposed by PRC law on such transactions. Current PRC law does not specifically provide for the equity of a non-PRC entity to be used as consideration for the purchase of a PRC entity’s assets or equity unless the value of the shares are equal to or greater than the value of the enterprise acquired. In addition, there is a lengthy appraisal process which must be approved by the provincial PRC government entities. The consideration for the exercise of the Option is to be determined by the parties and memorialized in future definitive agreements setting forth the kind and value of such consideration. Accordingly, we will consider exercising the Option under such circumstances we believe will be in our best interests and our shareholders and the Exclusive Option Agreement has been drafted to give us such flexibility. In considering whether or not we will exercise the Option we may consider such factors as (1) if the exercise price can be lower than the appraised value under current PRC law (2) availability of funds, (3) any relevant tax considerations at the time, (4) any other relevant PRC laws that may exist at the time, (5) the value of our shares that were previously paid to shareholders of Shaanxi Jiali, and (6) whether or not the exercise of the Option will provide any other additional benefits to us or our shareholders. Upon exercise of the Option, the parties will prepare transfer documents to be submitted for governmental approval and work together to obtain all approvals and permits. The agreement may be terminated by agreement of all parties or by with 30 days notice and is governed by the laws of the PRC.
In order to further solidify China Children’s rights, benefits and control of Shaanxi Jiali through its ownership of Xi’an Coova under the Entrusted Management Agreement, Voting Proxy Agreement and Exclusive Option Agreement, Xi’an Coova and the Shaanxi Jiali shareholders entered into a share pledge agreement (the “Share Pledge Agreement”) whereby the Shaanxi Jiali shareholders pledged all of their equity interests in Shaanxi Jiali, including the proceeds thereof, to guarantee the performance by the shareholders of all of the agreements they entered into with Xi’an Coova. Upon breach by any of the shareholders of any of the Management Entrustment Agreement, Voting Proxy Agreement, the Exclusive Option Agreement or the Share Pledge Agreement, Xi’an Coova is entitled by operation of law to become the beneficial owner of the shares of Shaanxi Jiali. Prior to termination of the Share Pledge Agreement, the pledged equity interests of Shaanxi Jiali cannot be transferred without Xi’an Coova’s prior written consent. The provisions of the Share Pledge Agreement and the Voting Proxy Agreement work together to give the board of directors of Xi’an Coova control over transfers by the shareholders of Shaanxi Jiali . The agreement will not terminate until agreed to by all of the parties in writing and is governed by the laws of the PRC.
Reorganization and Revised Structure
The organization and ownership structure of the Company subsequent to the consummation of the August 2008 Management Entrustment Agreement and related agreements as summarized in the paragraphs above was as follows:
42
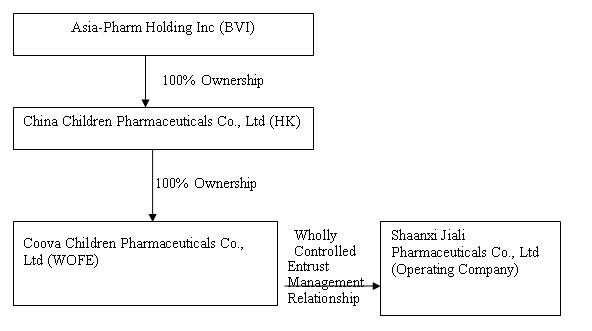
The following diagram sets forth the current corporate structure of China Pediatric Pharmaceuticals, Inc., giving effect to the merger transaction consummated on September 30, 2009 and the Name Change Transaction consummated on October 9, 2009:
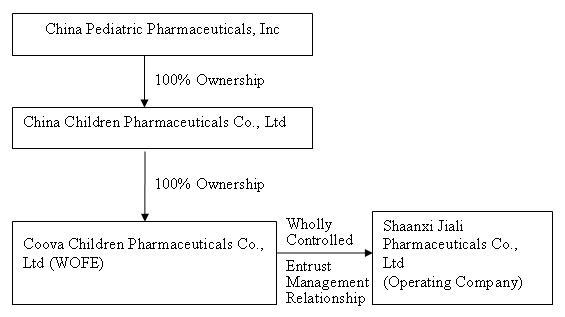
Subsidiaries
As a result of the Merger Transaction, China Children and Xi’an Coova are our wholly-owned subsidiaries. Shaanxi Jiali, the entity through which we operate our business, currently has no subsidiaries, either wholly-owned or partially-owned.
43
Our Business
Our operations are headquartered in Xi’an, Shaanxi Province, China. Through our operating entity, Shaanxi Jiali, we are a profitable, mid-sized Chinese pharmaceutical company that identifies, discovers, develops manufactures and distributes both prescription and over-the counter drugs (including conventional Western medicines as well asTCMs) primarily for the treatment of some of the most common children’s ailments and diseases. Approximately 72% of our products are intended for children’s use and approximately 28% of our products are intended for adult use.
Our operating company, Shaanxi Jiali, has its own manufacturing facility located in Baoji, Shaanxi Province. Our manufacturing facility was issued a Good Manufacturing Practices (“GMP”) certification by the State Food and Drug Administration (“SFDA”) in 2004, which has been approved for renewal as of April 2010. The Chinese government agency, SFDA, is analogous to the Food and Drug Administration (“FDA”) in the United States. Unlike the FDA, however, the SFDA provides intellectual property and competitive protection to certain classes of approved drugs.
GMP is an internationally-recognized standard for pharmaceutical plant design and construction. GMP has been defined as “that part of quality assurance which ensures that products are consistently produced and controlled to the quality standards appropriate for their intended use and as required by the marketing authorization” (World Health Organization). GMP covers all aspects of the manufacturing process: defined manufacturing process; validated critical manufacturing steps; suitable premises, storage, transport; qualified and trained production and quality control personnel; adequate laboratory facilities; approved written procedures and instructions; records to show all steps of defined procedures taken; full traceability of a product through batch processing records and distribution records; and systems for recall and investigation of complaints.
We were awarded the National High-tech Enterprise Award by the Shaanxi Technology Administration in 2006. As a result of receiving the National High-tech Award Shaanxi Jiali is entitled to the “Two exemption Three half” tax holiday based on the local government’s policy to encourage outside investment into the locality. According to PRC tax laws, “Two exemption Three half” policy means foreign investment enterprises including Shaanxi Jiali may enjoy an exemption from corporate income tax for 2 years starting from its first profitable year, followed by 3 years at a rate that is one half of the regular rate for corporate income tax. The Company expects to enjoy for this benefit through fiscal year 2010.
We distribute our high value, branded medicines, both prescription and OTC, through exclusive territory agents who sell our products directly to local pharmacies who in turn sell them to their retail customers.
Principal Products
In the fiscal year ended December 31, 2009, our revenues were principally derived from sales of 26 products listed below, of which 7 products are adult Western medicine products and 19 are finished pediatric TCM products. We have SFDA approval for all medicines that we market. Detailed information is provided in the table of products below.
We currently have 26 products that are manufactured by both our in-house production facility and through third-party manufacturers (“OEM Companies”). Of these products, 13 are produced in-house at our production plant:
Table of Products Manufactured by Shaanxi Jiali
|
Manufacturer
|
Brand Name &Product
|
Type: Prescription/
OTC Conventional
OTC TCM
|
Primary Function
|
|||
|
Shaanxi Jiali
|
Cooer Xiaoer Radix lsatidis Granule (5g)
|
OTC TCM
|
Treatment for inflammation and pain.
|
|||
|
Shaanxi Jiali
|
Qingsongling Radix lsatidis Granule (10g)
|
OTC TCM
|
Treatment for inflammation and pain.
|
|||
|
Shaanxi Jiali
|
Cooer Xiaoer Da shanzha Granule
|
OTC TCM
|
Treatment for indigestion.
|
|||
|
Shaanxi Jiali
|
Cooer Xiaoer Paracetamol, Artificial Cow-bezoar and Chlorphenamine Maleale Granule
|
OTC Conventional
|
Treatment for inflammation, pain and fever.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Erythromycin Estolate Granule
|
OTC Conventional
|
Treatment for indigestion.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Compound Danshen Tablet
|
OTC TCM
|
Treatment for heart and blood conditions.
|
44
|
Shaanxi Jiali
|
Qingsongling Series
Ganmao Tuire Granule
|
OTC TCM
|
Treatment for inflammatory and pain.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Qingrejiedu Tablet
|
OTC TCM
|
Treatment for respiratory tract inflammation.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Erythromycin Ethylsuccinate Tablets (0.125 g)
|
OTC Conventional
|
Treatment for indigestion.
|
|
Shaanxi Jiali
|
Qingsongling Series
Yuanhu Pain killer
|
OTC TCM
|
Treatment for pain.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Vitamin C Tablets (50mg)
|
OTC Conventional
|
Vitamin
|
|||
|
Shaanxi Jiali
|
Xianzhi Series
Kechuanqing Tablet
|
Prescription
|
Treatment for bronchitis and asthma.
|
|||
|
Shaanxi Jiali
|
Qingsongling Series
Paracetamol,Caffein,Atificial Cow-bezoar and chlorphenamine Maleate Capsules
|
OTC Conventional
|
Treatment for common colds.
|
The table below represents our 13 of our medicines that are produced by OEM Companies.
Table of Products Manufactured by OEM Companies
|
Manufacturer
|
Brand Name &Product
|
Type: Prescription/
OTC Conventional
OTC TCM
|
Primary Function
|
|||
|
OEM: Hubei Kanger Jian Bioengineering Co. Ltd.
|
Cooer Jin 21 Multivitamin Chewing Tablets
|
OTC Conventional
|
Vitamin.
|
|||
|
OEM: Hubei Kanger Jian Bioengineering Co. Ltd.
|
Cooer Lacidophilin Granules
|
OTC Conventional
|
Treatment for indigestion and diarrhea.
|
|||
|
OEM Partner: Taiji Group Sichuan Nanchong Pharmaceutical Factory
|
Cooer Xiaochaihu Granules
|
OTC TCM
|
Treatment for indigestion.
|
|||
|
OEM Partner: Taiji Group Sichuan Nanchong Pharmaceutical Factory
|
Cooer Xiaoer Expectorant Granules
|
OTC TCM
|
Treatment for coughs.
|
|||
|
OEM: Taiji Group Sichuan Nanchong Pharmaceutical Factory
|
Cooer Xiaoer Throat-cleaning Granules
|
OTC TCM
|
Treatment for throat pain.
|
|||
|
OEM : Taiji Group Sichuan Nanchong Pharmaceutical Factory
|
Cooer Xiaoer Cough-curing Syrup
|
OTC TCM
|
Treatment for coughs.
|
|||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd.
|
Cooer Xiaoer Xishi Granule
|
OTC TCM
|
Treatment for indigestion and diarrhea.
|
|||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd.
|
Cooer Xiaoer Kechuanling Granule
|
OTC TCM
|
Treatment for respiratory tract infection and coughs.
|
|||
|
OEM: Xi’an KeLi Pharmaceuticals Co. Ltd.
|
Cooer I+1 Suit
|
OTC Conventional
|
Treatment for fever.
|
|||
|
OEM : Fosham City Yichuang Biochemistry Science and Technology Co. Ltd.
|
Cooer Fever Release Paste
|
OTC TCM
|
Treatment for fever.
|
45
|
OEM: Jiyuan City, Taiji Medical Supplies Co. Ltd.
|
Cooer Paste for Pediatric Diarrhea
|
OTC TCM
|
Treatment for diarrhea.
|
|||
|
OEM Partner: Jiyuan City, Taiji Medical Supplies Co. Ltd.
|
Cooer Paste for Pediatric Cough & Asthma
|
OTC TCM
|
Treatment for asthma and coughs.
|
|||
|
OEM: Jiyuan City, Taiji Medical Supplies Co. Ltd.
|
Cooer Paste for Pediatric Cold
|
OTC TCM
|
Treatment for the common cold.
|
We currently market our products under three brands:
Cooers Brand Products
The Cooers Brand product line is focused on the pediatric medicine market. The product line includes both prescription and over–the-counter pharmaceutical products, including both conventional and traditional Chinese medicines. The Cooers product line has 15 products that address numerous ailments including, but not limited, to pain relief, anti-inflammatory, anti-bacteria, infection, digestion, respiratory tract infections, gangrene, diarrhea and fever, cold and cough remedies and vitamins.
Qingsongling Brand Products
The Qingsongling product line is focused on the adult market. This product line includes both prescription and over –the-counter pharmaceutical products including both conventional and traditional Chinese medicines. The Qingsongling product line has 8 products for treatment of numerous ailments, including but not limited to, treatments for heart disease and angina, respiratory tract infection, influenza, gangrene, anti-inflammatory pain relief, cold and fever remedies and vitamins.
Xianzhi Brand Product
The Xianzhi product line is focused on the adult medicine market. Xianzhi series includes one product which is prescription medicine (TCM). The Xianzhi product line has one product for treatment for chronic bronchitis and asthma.
Marketing and Sales
Shaanxi Jiali’s high value branded prescription and OTC medicines are distributed through the following distribution channel: Pharmaceutical Manufacturer (Shaanxi Jiali) - Exclusive Agents - Pharmacies – Patients.
Shaanxi Jiali currently sells its products through 15 exclusive territory agents in 7 provinces throughout China. The exclusive territory agents sell the Company’s products directly to local pharmacies in their assigned territory. This method of distribution minimizes the Company’s need to maintain a large direct sales force.
Exclusive Territory Agents
Shaanxi Jiali has entered into contracts with Exclusive Territory Agents for the sale and distribution of our products to local pharmacies in the agent’s respective exclusive territory, whereby the agent receives approximately 10% commission as consideration for serving as the Company’s exclusive agent in that territory. In 2008, we signed six Exclusive Territory Agent contracts, and as of December 31, 2009 we have increased our number of contracts to a total of fifteen. These contracts are typically for a period of one year. Neither party may transfer their rights under the agreement without the prior consent of the other party, or else the breaching party will incur damages of RMB50,000. As the Company expands into new markets and gains more access to additional capital, we expect to increase the number of contracts with Exclusive Territory Agents. Due to the favorable pricing terms we offer, we have not experienced any difficulties with finding new Exclusive Territory Agents should we terminate our relationships with existing agents.
Special Marketing Initiatives
Cooers Brand Initiative. Shaanxi Jiali intends to further develop the series of products based on the Cooers Brand and designed to target the pediatric medicine market. These products will continue be sold through exclusive territory agents who in turn distribute to local pharmacies in their assigned territories.
Regional Expansion Initiative. Shaanxi Jiali intends to further expand its distribution channels by increasing the number of exclusive territory agents. These additional exclusive territory agents will be contracted to distribute Shaanxi Jiali products to local pharmacies in several additional provinces or regions throughout China.
Status of Publicly Announced New Products
We expect that the following products in our development pipeline will generate growth in our revenue in the next few years. We expect to launch commercial production for the five products for which we have applied for SFDA approval as soon as possible.
46
Development Status of Key Products in our Pipeline as of September 1, 2010 :
|
Proposed
Manufacturer
|
Name of
Brand and
Product name
|
Type:
Prescription/
OTC Conventional/
OTC TCM
|
Primary
Function
|
Application
No.
|
SFDA
Application
Date
|
SFDA
Application
Status
|
||||||
|
Shaanxi Jiali
|
Cooer Xiaoer Fuxiening Granules
|
OTC TCM
|
Treatment for common cold symptoms
|
CYZS0800023
|
Nov.28, 2007
|
Received first round of comments from SFDA in June 2010. Approval is not expected before 2012.
|
||||||
|
Shaanxi Jiali
|
Cooer Xiaoer Kechuanling Granule
|
OTC TCM
|
Treatment for common cold symptoms
|
CYXS0700240
|
Apr.29, 2008
|
Received first round of comments from SFDA in June 2010. Approval is not expected before 2012.
|
||||||
|
Shaanxi Jiali
|
Cooer Xiaoer Kangganjiedu Granules
|
OTC TCM
|
Treatment for common cold symptoms
|
CYZS0700239
|
Nov.28, 2007
|
Received first round of comments from SFDA in June 2010. Approval is not expected before 2012.
|
||||||
|
Shaanxi Jiali
|
Cooer Xiaoer Digesting Tablet
|
OTC TCM
|
Treatment for indigestion and strengthening the stomach
|
CYZS0700241
|
Nov.28, 2007
|
Received first round of comments from SFDA in June 2010. Approval is not expected before 2012.
|
||||||
|
Shaanxi Jiali
|
Cooer Xiaoer Jianpibuxue Granules
|
OTC TCM
|
Treatment for iron-deficiency anemia strengthening the spleen and enriching the blood
|
CYZS0700312
|
Nov.28, 2007
|
Received first round of comments from SFDA in June 2010. Approval is not expected before 2012.
|
During the six months ended June 30, 2010, we spent approximately $1,512,553 on marketing. During the years ended December 31, 2009 and 2008, we spent approximately $4.96 million and $5.62 million, respectively, on marketing.
Quality Control
Our production facilities are designed and maintained with a view towards conforming with good practice standards. To comply with GMP operational requirements, we have implemented a quality assurance plan setting forth our quality assurance procedures. Our Quality Control department is responsible for maintaining quality standards throughout the production process. Quality Control executes the following functions:
|
●
|
setting internal controls and regulations for semi-finished and finished products;
|
|
|
●
|
implementing sampling systems and sample files;
|
|
|
●
|
maintaining quality of equipment, instruments, reagents, test solutions, volumetric solutions, culture media and laboratory animals;
|
|
|
●
|
auditing production records to ensure delivery of quality products;
|
47
|
●
|
monitoring the number of dust particles and microbes in the clean areas;
|
|
|
●
|
evaluating stability of raw materials, semi-finished products and finished products in order to generate accurate statistics on storage duration and shelf life;
|
|
|
●
|
articulating the responsibilities of Quality Control staff; and
|
|
|
●
|
on-site evaluation of supplier quality control systems.
|
Competition
Company research indicates that there are primarily two competitors in the pediatric pharmaceutical market that are comparable in R&D, manufacturing scale and brand awareness. They are Sunstone (Tangshan) Pharmaceutical Co., Ltd. (“Sunstone (Tangshan) Pharmaceuticals”), which manufactures pediatric medicine under the brand “Good Baby”, and the general pharmaceutical company Ha’erbin Pediatric Pharmaceuticals (“Ha’erbin Pediatric Pharmaceuticals”). A comparison of these two companies to Shaanxi Jiali are provided below:
Comparison of Primary Competitors
|
Shaanxi Jiali
|
Sunstone (Tangshan) Pharmaceuticals
|
Ha’erbin Pediatric
Pharmaceuticals
|
||||
|
Market Position
|
Both develops and manufactures pediatric medicines. With 9 OEM factories and 15 strategic partners, our network has extended to community clinics. Sales were approximately $ 14.67 million in 2009.
|
Both develops and manufactures women and children medicines. There are 35 offices in prefecture-level cities. Sales of its Good Baby series of products were $114,997,644 in 2009.
|
Both develops and manufactures pediatric medicine. Sales were approximately $9 million in 2009.
|
|||
|
Brand Awareness
|
We have our own Pediatric medicine brand “Cooer”, but expand our brand awareness primarily through marketing and customer service and less through direct advertising. We are planning to launch further advertising efforts.
|
Pediatric medicine brand “Good Baby” has been designated a Chinese famous brand. Sunstone expands its brand awareness through substantial advertising and marketing.
|
As an OEM manufacturer, this company does not carry its own brand. However it has been recognized for its high standards as a pediatric medicine manufacturer due to its 15-year presence in the industry.
|
|||
|
Manufacturing Scale
|
Production capacity of approximately $13 million. Actual production totaled about $8.6 million in 2009. Company has 9 OEM cooperating factories, including Haerbin Pediatric Pharmaceuticals.
|
Production capacity of approximately $183.585 million. Actual production totaled about $146.868 million in 2009, of which $114,997,644 represented sales from pediatric medicine.
|
Production capacity of approximately $15 million. Actual production totaled about $9 million in 2009.
|
|||
|
Products
|
Nearly 30 medicine products, including pediatric medicines for common use and pediatric nutritional supplements.
|
Nearly 20 pediatric medicine products, including pediatric medicines for common use and pediatric nutritional supplements.
|
13 pediatric medicine products.
|
|||
|
Customer Service
|
Professional customer service center in Xi’an that provides both pre-sales and after-sales consultations to distributors and consumers.
|
Provides after-sales service only.
|
No after-sales service.
|
48
|
Price
|
Average price is $ 0.80 per box.
|
Average price is $ 1.20 per box.
|
Average price is $ 0.50 per box.
|
|||
|
R&D
|
Cooperation with Shaanxi Research Institution of Chinese Traditional Medicines, Xi’an Jiaotong University and the Fourth Military Medical University. Invested $955,900 in R&D in 2009, 5 pediatric medicines have been submitted to SFDA for approval.
|
Invested $1,906,000 in R&D in 2009, including the development of 13 pediatric medicines.
|
Maintains a sole proprietorship R&D company with obvious R&D technical superiority. Currently 13 pediatric medicines are under development.
|
*Data with respect to Sunstone (Tangshan) Pharmaceuticals and Ha’erbin Pediatric Pharmaceuticals is derived from such companies’ corporate websites and, with respect to Sunstone (Tangshan) Pharmaceuticals, the company’s public filings with the SEC.
Notwithstanding the above, the Company has been actively developing its business to build the following competitive advantages:
1. Positioning in the pediatric medicines market: Shaanxi Jiali has positioned itself as an early entrant in the developing pediatric segment of the pharmaceutical market, focusing on the research and development, manufacture and sales of pediatric medicine.
Despite the heavy requirements it places on its participants, the PRC pediatric medicine market and is growing at a steady rate. According to data from the Southern Medicine Economic Institute, a PRC research institute which is affiliated with the SFDA, among the 7145 pharmaceutical preparations in 2008 that were authorized and marketed in the PRC (including generic names and formulations), there were only 500 pediatric drugs (including 320 TCMs), or approximately 7%, that provided clearly specified dosages and usage applications for children/infants. The pediatric market provides a greater opportunity for growth than the adult pharmaceutical market and, according to the Southern Medicine Economic Institute, it has kept a steady 11.64% average annual growth rate over the past 6 years.
Based on Shaanxi Jiali’s as well as general industry experience, we estimate that a drug (be it for adult or pediatric use) generally takes at least five years to from product development to commercial launch, as the drug must be developed, undergo clinical trials, and clear the SFDA approval process before it is ready to enter the market. Due to the special considerations of the child consumer, the manufacture and sale of pediatric pharmaceuticals are more heavily regulated due to greater concerns about safety and dosage effectiveness in children/infants than adults. Additionally, these products must first be tested before they are marketed, and it is more difficult to find child/infant test subjects to conduct clinical trials. According to the data from the Southern Medicine Economic Institute, which is affiliated with the SFDA, a new drug generally takes at least five years to research and develop and obtain the SFDA approval. As such, due to the substantial resources and time inputs that are required to develop new drugs and the current size of the pediatric market, established manufacturers who have enjoyed their market share in the larger adult pharmaceutical industry may not feel the need to expand into the more limited pediatric market and will require professional research teams with long-term expertise to do so.
2. Diversity of products: After long-term efforts, the company's competitive line of “Cooer” products includes a total of 26 products spanning six product categories (as grouped by treatment type, such as Anticold, Antidiarrheal, Nutritional supplements, Digestion, Antibiotics, Antitussive (cough suppressants)), and the clinical effects of our products have been recognized by our customers and have been well-received at pharmaceutical sales conferences and exhibitions. The distinguishing feature of our Cooer products is the comprehensive coverage that our products span the entire conventional pediatric medicine market in China. Although our competitors also produce a number of pediatric drugs, their product range is not as diverse and do not span all six product categories described above. We believe that we dominate the pediatric medicine industry in terms of our marketing capability, concentration and expertise in the pediatric sector. Our other competitors either act as OEM manufacturers for other companies or produce but do not specialize in the pediatric field of pharmaceuticals.
In the short term, we believe that our competitive advantages will be difficult to be surpassed: unlike our competitors, we concentrate the majority of our R&D, sales, and manufacturing efforts on the pediatric pharmaceutical market. What’s more, as a managing director of Shaanxi Pharmaceutical Association in China, we can make full use of the R&D resources of Shaanxi province and have done so by teaming with reputable R&D partners, such as the Shaanxi Research Institution of Chinese Traditional Medicines. We believe that we have strong manufacturing capabilities, both through our in-house production and utilization of OEM partners to fulfill our manufacturing requirements. We have also established strategic partnerships with 15 exclusive distribution agents in 2009, which we believe have given our products a competitive edge in the pediatric pharmaceuticals market.
Our Future Goals and Plans for Expansion
There are certain opportunities for growth that we intend to seize by employing the following growth strategies:
49
Rapidly growing Chinese pharmaceutical market. With approximately one-fifth of the world’s population and a fast-growing gross domestic product, China represents a significant potential market for the pharmaceutical industry. We believe the significant expected growth of the pharmaceutical market in China is due to factors such as robust economic growth and increased pharmaceutical expenditure, aging population and increased lifestyle-related diseases, government support of the pharmaceutical industry, the relatively low research and development and clinical trial costs in China as compared to developed countries, as well as the increased availability of funding for medical insurance and industry consolidation in China.
Currently China has about 3,500 drug companies, falling from more than 5,000 in 2004, according to the National Development & Reform Commission of China. The number is expected to drop further. The domestic companies compete in the $10 billion market (according to the National Development & Reform Commission of China) without a dominant leader. Most often cited adverse factors include a lack of protection of intellectual property rights, a lack of visibility for drug approval procedures, a lack of effective governmental incentives, poor corporate support for drug research and differences in the treatment in China accorded to local and foreign firms. The profile of the pharmaceutical industry in China remains very low. According to the National Development & Reform Commission of China, China accounts for approximately 20% of the world’s population but only 1.5% of the global drug market. In addition, according to the National Development & Reform Commission of China, as of 2008, China was the world's eighth largest drug market.
New Product Development. We will continue to evaluate and develop additional product candidates, both through our in-house research and development department and working with our research and development partners, to expand our pipeline where we perceive an unmet need and commercial potential. We will face the risk that in developing new products we may spend substantial sums of money and the new products developed may not effectively meet the perceived need or may not be successfully commercialized.
Focus on brand development. With intense price competition among many similar or identical products in the industry, we believe that building brand equity is the primary means to generate and sustain profitable growth in the future. The company intends to focus its brand development efforts on building the Cooers brand name with the intent on it becoming a leading pediatric medicine brand in China.
Marketing and Sales Function. We intend to grow our internal marketing and sales function and increase our relationships with other exclusive territory distributors to expand the distribution and presence of our pharmaceutical products. In expanding market share of our products, we intend to take advantage of our large manufacturing scale and reasonable cost control mechanisms, and our strong sales network. In addition, our goal is to establish our products as a preferred choice for children’s medicines in local pharmacies. We hope to add other pharmaceutical products into this channel over the next few years. We may face risks in obtaining adequate quantities of raw materials at reasonable prices in order to meet increased demand for our products that result from any growth. In seeking additional employees, sales representatives, and exclusive territory agents, we will compete with many other established pharmaceutical manufacturers that may have greater resources than we do.
Low cost producer. We benefit from focusing on a specialized division of the pharmaceuticals market, i.e., by focusing on pediatric medicine, and investing efforts into research and development of new pediatric drugs. We also plan to build about our specialty and early market entry advantage by building better facilities and equipment to increasingly automate our manufacturing process. We believe that these efforts will lower our production costs in the following respects:
1. Research and Development. As an early market entrant, we have accumulated a large amount of confidential data from our experiences in the industry and our prior research and development efforts that we believe (it is a matter of opinion and not of fact) enables to have first access and lead-time advantages to the pediatric market. Pediatric medicine has a long R&D term with high costs and risks. Challenges to effective R&D include the difficulty of employing accurate methods to monitor trial dosages and finding suitable child test subjects who adhere to testing requirements. Such challenges have served as a deterrent for many companies who have eyed the pediatric pharmaceutical market. Since Shaanxi Jiali focused on the pediatric field early on, it has already passed the difficult initial stage and no longer incurs the large up-front costs that a new entrant would require.
2. Manufacturing. We are continually optimizing and technologically upgrading production lines to make them suit the nature of pediatric medicine, including improvements to our granule packaging line in order to improve the safety of our products.
Standard packing bags for granules have three layers: (1) the first packing layer (the outermost layer); (2) the second protective layer (the middle layer, which is the thickest layer and mainly plays a protective role in providing structure and resisting moisture, pressure and gas leakage; and (3) the innermost layer which is in direct contact with the medicine. Currently pediatric pharmaceutical manufacturers generally make the first and third layers of the same materials and most manufacturers make the second layer out of the blend of aluminum and plastic.
In order to meet and improve upon the more stringent safety requirements of pediatric medicine, the second layer of our granule bags are made out of pure aluminum, which enhances the airtightness of the package and improves the safety of the medicine. To do so, we purchased a DXDB80 granule bag packing machine in 2007 to deal with the deficiency that our older machines could not package our granule products with the pure aluminum packaging materials. We also required our OEM factories, Xi’an KeLi Pharmaceuticals Co. Ltd, and Sichuan Nanchong Pharmaceutical Factory, to purchase the same granule bag packing machines, so that the production lines of all our OEM factories can meet our internal quality control standards.
Given the special considerations involved in manufacturing pediatric medicine, it is difficult for larger, more established pharmaceutical manufacturers to produce pediatric medicine on a general product line, while up-and-coming companies encounter high cost barriers in recruiting the talent and expertise need to construct product lines suitable for pediatric pharmaceutical products. We also currently have partially automated production lines and plan to advance our technology towards full automation, which will help in the long term to lower our production costs.
3. Acquisitions. We are considering expanding our production capacity by acquiring existing pharmaceutical facilities or manufacturing entities. We believe this will be a quick and efficient way of increasing and diversifying our capabilities.
4. Human Resources. We are continually striving to reduce human resources costs by improving our management oversight and training of our manufacturing department employees to enhance the capability and productivity of our workers.
50
We plan to enhance the quality of our products to ensure that we remain in compliance with the PRC’s higher thresholds and intend to broaden our national market share of our Cooer series of products. We may also decide to acquire other small and medium enterprises that process certain competitive advantages to assist us in our goal of becoming the No. 1 brand for Chinese pediatric pharmaceuticals.
We also intend to capitalize on the opportunities granted from any integration of the distribution of pharmaceuticals by establishing strategic and cooperative relationships with more distributors that will secure reliable agents, extend the reach of our distribution networks, and improve turnover of goods.
Customers
Shaanxi Jiali has entered into contracts with Exclusive Territory Agents for the sale and distribution of our products to local pharmacies in the agent’s respective exclusive territory, whereby the agent receives approximately 10% commission as consideration for serving as the Company’s exclusive agent in that territory. These agents serve as our primary customers for our products. In 2008, we signed six Exclusive Territory Agent contracts and as of December 31, 2009 we have increased our number of contracts to a total of fifteen. These contracts are typically for a period of one year.
The five largest customers of Shaanxi Jiali in the fiscal year ended December 31, 2009 are as follows:
|
Customer Name
|
% of Sales
|
||||||
|
1
|
Xi’an Zaolutang Kushidai
|
11.61
|
%
|
||||
|
2
|
Jiangsu Yabang Pharmaceuticals Distribution Center Co., Ltd.
|
10.90
|
%
|
||||
|
3
|
Hunan Jiuwang Pharmaceuticals Co., Ltd.
|
8.86
|
%
|
||||
|
4
|
Hangzhou Zhencheng Pharmaceuticals Co., Ltd.
|
8.77
|
%
|
||||
|
5
|
Shandong Yaoshan Pharmaceuticals Co., Ltd
|
8.59
|
%
|
||||
The five largest customers of Shaanxi Jiali in the fiscal year ended December 31, 2008 are as follows:
|
Customer Name
|
% of Sales
|
||||||
|
1
|
Zhengzhou Tianhe Pharmaceuticals Inc.
|
23.38
|
%
|
||||
|
2
|
Xi’an Zaolutang Kushidai Medicine Sales Department
|
21.22
|
%
|
||||
|
3
|
Hangzhou Zhencheng Pharmaceuticals Co., Ltd.
|
14.41
|
%
|
||||
|
4
|
Jiangsu Yabang Pharmaceuticals Distribution Center Co., Ltd.
|
14.37
|
%
|
||||
|
5
|
Hunan Jiuwang Pharmaceuticals Co., Ltd.
|
13.64
|
%
|
||||
Raw Materials and Principal Suppliers
The primary raw materials used to manufacture our products include various medicinal herbs, which we obtain from numerous qualified suppliers with national qualifications. We also enter into supply agreements for many other materials used to manufacture and package our products. Shaanxi Jiali has written agreements with substantially all of its suppliers. These agreements typically take the form of one-time purchase orders with requirements for one-time delivery and one-time payments. The agreements give the Company the right to inspect the materials purchased for quality and quantity compliance.
The five largest suppliers of Shaanxi Jiali in the fiscal year ended December 31, 2009 are as follows:
|
Supplier
|
Type of Raw Material Purchased
|
% of Raw Material Costs
|
|||||||
|
1
|
Shaanxi Dayang Mark Co.,Ltd.
|
Packaging materials
|
13.14
|
%
|
|||||
|
2
|
Xianyang Wenhui Printing Co., Ltd.
|
Packaging materials
|
12.17
|
%
|
|||||
|
3
|
Baoji Meixian Lvyuan Chinese Herbal Medicine Planting and R&D Co., Ltd
|
Active TCM ingredients
|
11.12
|
%
|
|||||
|
4
|
Baoji Weixin Tangye Wholesale Shop
|
Sugar
|
10.20
|
%
|
|||||
|
5
|
Shaanxi Aokesen Laboratory Co., Ltd
|
Excipients/nonactive ingredients
|
7.75
|
%
|
|||||
The five largest suppliers of Shaanxi Jiali in the fiscal year ended December 31, 2008 are as follows:
51
|
Supplier
|
Type of Raw Material Purchased
|
% of Raw Material Costs
|
|||||||
|
1
|
Shaanxi Aokesen Laboratory Co., Ltd
|
Ingredients
|
21
|
||||||
|
2
|
Baoji Weixin Tangye Wholesale Shop
|
Sugar
|
16
|
||||||
|
3
|
Baoji Meixian lvyuan Chinese Herbal Medicine Planting and R&D Co., Ltd
|
Active TCM ingredients
|
13
|
||||||
|
4
|
Baoji Chencang Qu JieLi Carton Factory
|
Packaging materials
|
11
|
||||||
|
5
|
Shaanxi Daxin Suye Co.Ltd
|
Packaging materials
|
10
|
||||||
Intellectual Property
We regard our service marks, trademarks, trade secrets, patents and similar intellectual property as critical factors to our success. We rely on patent, trademark and trade secret law, as well as confidentiality and license agreements with certain of our employees, customers and others to protect our proprietary rights.
Patents
Because the concept and implementation of intellectual property protection is somewhat new in the PRC, the PRC government offers two types of IP protection for manufacturers of pharmaceutical products: the medicine production technique patent and medicine invention formula patent. A company can obtain “legal” protection for the manufacture of its proprietary products by applying for a patent with the State Patent Bureau. This patent will apply not only to the particular formulation for the medicine, but also the production technique and/or dosage form or type for such medicine. A state-issued patent is afforded a period of 20 years of protection.
Separately, certain over-the-counter TCM products which have received SFDA approval are afforded certain intellectual property rights. A company can apply for “administrative” IP protection for the production technique of its SFDA-approved product with the SFDA. The SFDA administrative protection lasts for 8 years and only covers the production technique for the particular dosage type. In PRC, illegal infringements of production technique patents are widespread. A tiny modification to a filed medicine production technique might be construed as a new one and argued as not violating the patent laws. Invention formula patents are as easily imitated as production technique patents. However, since only one SFDA production certificate can be issued on new branded medicine based on the major pharmaceutical ingredients used, a minor modification to the formula would not warrant a new SFDA production certificate. This means that, even if another manufacturer gets to know a patented formula, it would not be able to produce it in the PRC without the proper SFDA production certificate.
Once the SFDA protection has expired, it cannot be renewed, except under special circumstances as to be determined at the SFDA’s discretion. Once the SFDA protection period has expired, a company may apply for patent protection.
To a large extent, we rely on SFDA protection regulation to protect our intellectual property rights with respect to our products. Except for the two patents that we have received and described further below, all other products are protected under the SFDA.
Two types of medicine-related patents exist in PRC: the medicine production technique patent and medicine invention formula patent.
Shaanxi Jiali currently holds two patents: ZL03 1 08011.1 for the treatment of chronic bronchitis and ZL 03 1 08012.X and for the treatment of dermatosis. Only one patented medicine, ZL03 1 08011.1, is currently being produced.
|
Patent No.
|
Published date
|
Expiration Date
|
Product Brand
|
|||||
|
A Chinese Traditional Medicine for the treatment of chronic bronchitis disease and the pharmacy
|
ZL03 1 08011.1
|
Sept. 14, 2005
|
Sept. 13, 2025
|
Xianzhi
|
||||
|
A Chinese Traditional Medicine for the treatment of dermatosis and gynecology disease and the pharmacy
|
ZL 03 1 08012.X
|
Sept. 14, 2005
|
Sept. 13, 2025
|
Not under production.
|
Trademarks
Shaanxi Jiali currently holds nine registered trade names for the following medicine names and logos: Sanseqi (logo), Cooer, Tailaishi, Qingsongling, Beishiting, Zhiben, Xianzhi, Chugan, Yunlaitongjingbao and has filed trademark applications, the approvals of which are pending, for 3 other medicine names or general trademarks.
52
The Sanseqi logo for which we have obtained a trademark appears below:

|
Brand Name
|
Certificate No.
|
Type of
Products
|
Validity Period
|
|||
|
Sanseqi
|
1795539
|
5
|
2002/6/28 to 2012/06/27
|
|||
|
Cooer
|
3180187
|
5
|
2003/11/21to 2013/ 11/ 20
|
|||
|
Tailaishi
|
3180186
|
5
|
2003/11/21 to 2013/11/20
|
|||
|
Qingsongling
|
3180188
|
5
|
2003/11/21 to 2013/11/20
|
|||
|
Beishiting
|
3241933
|
5
|
2004/01/07 to 2014/ 01/06
|
|||
|
Zhiben
|
3389585
|
5
|
2004/07/21 to 2014/07/20
|
|||
|
Xianzhi
|
3898392
|
5
|
2006/06/28 to 2016/06/27
|
|||
|
Chugan
|
4203833
|
5
|
2009/02/28 to 2019/02/27
|
|||
|
Yunlaitongjingbao
|
4506025
|
5
|
2008/07/07 to 2018/07/06
|
Research and Development
We develop new products through an arrangement with the Shaanxi Research Institution of Chinese Traditional Medicines, in Xi’an. We expect to continue to develop additional new drugs under this method.
Shaanxi Jiali’s in-house Research & Development department coordinates and supervises the contract based outsourced R&D processes. Shaanxi Jiali’s Research & Development had a staff of five during the years ended December 31, 2008 and 2009.
Shaanxi Jiali entered into a Medicine Research and Development Agreement with the Shaanxi Research Institute of Chinese Traditional Medicines (“SRICTM”) on December 10, 2007. Under the terms of the Medicine Research and Development Agreement the Shaanxi Research Institute of Chinese Traditional Medicines was contracted for the research and development and for the application of productional approval of five new “pediatric cold treatment medicines”: Cooer Xiaoer Fuxiening Granules; Cooer Xiaoer Kechuanling Granule; Cooer Xiaoer Kangganjiedu Granules; Cooer Xiaoer Digesting Tablet, and Cooer Xiaoer Jianpibuxue Granules. Under the terms of the research and development agreement Shaanxi Jiali is committed to paying the Shaanxi Research Institute of Chinese Traditional Medicines $293,000 for each of the five new products contracted for development. Under the terms of the research and development agreement Shaanxi Jiali owns the Intellectual Property rights for the five new pharmaceutical products developed by the Shaanxi Research Institute of Chinese Traditional Medicines.
If approved by SFDA, we will be authorized to commence manufacturing of these new products. Management has estimated that all new products would enter the market and material net cash inflows from these projects would be expected to commence within 6 months of receiving approval.
Pursuant to the terms of Medicine Research and Development Agreement, at this stage of completion, we have prepaid all R&D expenses to SRICTM. Should the applications be rejected by the SFDA due to a technology-related error or any error of SRICTM, SCRICTM is contractually obligated under Sections 5.3, 7.3 and 10.1 of the agreement to refund all fees already paid under the terms of the Medicine Research and Development Agreement and Supplemental Agreement of Medicine Research and Development Agreement. Therefore, the Company recorded it as prepaid expense.
We did not obtain any certificates or approvals of drug production for any new drug batches during the fiscal year ended December 31, 2009. However, we expect to develop additional new drugs in 2010. During the last two fiscal years ended December 31, 2009 and 2008, we spent $808,800 and $955,900, respectively, to purchase the exclusive rights to certain new products from several research institutes and for ongoing research and development activities.
Government Regulation of Pharmaceuticals
The testing, approval, manufacturing, labeling, advertising and marketing, post-approval safety reporting, of our products or product candidates are extensively regulated by governmental authorities in the PRC. Our sole sales market is presently in China. We are subject to the Drug Administration Law of China, which governs the licensing, manufacturing, marketing and distribution of pharmaceutical products in China and sets penalties for violations of the law. Additionally, we are subject to various regulations and permit systems by the Chinese government. These regulations and their impact on the business of Shaanxi Jiali are set forth in more detail below.
53
|
●
|
Drug Administration Law of the PRC was promulgated by the Standing Committee of National People’s Congress on February 28, 2001 and effective as of December 1, 2001, and its implemental rules were promulgated by the State Council on August 4, 2004 and effective as of September 15, 2002. According to Drug Administration Law of the PRC and its implemental rules, a pharmaceutical manufacturer is to obtain a Pharmaceutical Manufacturing Permit and the Drug Approval Number for each manufactured medicine from relevant SFDA’s provincial branch, which are valid for five years and are renewable upon application before expiration. Shaanxi Jiali is required to file for these approvals for each of its medicines and renew them prior to expiration.
|
|
●
|
Administration Regulations for Drug Registration was promulgated by the SFDA on July 10, 2007, and was effective as of October 1, 2007. Administration Regulations for Drug Registration specifies the requirements and procedures of obtaining a Drug Approval Number for new drugs, including the requirements for clinical trial of new drugs, procedures of registering imported medicines and report and approval procedures of generic medicines . The Drug Approval Number is valid for five years and can be re-registered upon expiration. Shaanxi Jiali is required to obtain a Drug Approval Number for each of its new drugs and reapply prior to the expiration date.
|
|
●
|
Good Manufacturing Practices (GMP) for Pharmaceutical Products , as revised in 1998 was promulgated by the SFDA on June 18, 1999 and became effective as of August 1, 1999, and the Authentication Regulations for Drug GMP was promulgated by the SFDA on September 7, 2005 and became effective on of October1, 2005. A pharmaceutical manufacturer must meet the GMP standards and obtain the GMP Certificate with a five-year validity period from SFDA. Before the GMP Certification expires, the pharmaceutical manufacturer must apply again and complete the relevant procedures, which may take about 120 working days, to obtain a new GMP Certificate. On October 24, 2007, the SFDA issued the new guideline for authentication standards of GMP, effective as of January 1, 2008. The new guideline may result in a rise of cost for a pharmaceutical manufacturer to meet the new standards so as to maintain the GMP qualification. If a pharmaceutical manufacturer fails to obtain or maintain GMP Certification and still carry on its production, it will be fined and the Pharmaceutical Manufacturing Permit may be revoked under serious circumstances. Shaanxi Jiali received its first GMP certificate in November 2004 and has received approval for its renewal as of April 2010 after a temporary suspension of production between November 2009 and April 2010 while the renewal application was being processed.
Our GMP certificate expired on November 2, 2009. It is not uncommon for companies in the pharmaceutical industry to suspend production while they are in application for a renewed permit in order to prepare for and accommodate GMP inspection. We applied to renew our GMP certificate prior to November 2, 2009, and as such we did not experience any penalties or delays in the processing of our application. Because, however, we experienced an increase in customer orders toward the end of the calendar year, we continued production until November 2, 2009 in order to meet all of our customers’ needs.
The SFDA generally takes about three to four months to review GMP renewal applications and as a result, we did not receive preliminary approval for the renewed certificate until April 2010. Between November 2009 and April 2010, while our application for renewal was being processed and our facilities underwent standard GMP review and inspection, we temporarily suspended production during the application period. During this time, we worked with our OEM partners to meet our manufacturing needs. We also experienced a lower demand for our products during this period due to China’s Spring Festival holiday in the month of February. As such, we continued to have sales between November 2009 and April 2010 even though we did not manufacture any products in-house and were able to use our OEM manufacturers to meet all of our customers’ needs. Since April 2010, when we were notified that the SFDA had approved our application, we have resumed regular production.
|
|
●
|
Administration Regulations for Drug Call-back was promulgated by the SFDA on December 10, 2007 and effective on the same day. According to the Administration Regulations for Drug Call-back, the pharmaceutical manufacturer should establish a drug call-back system and collect information regarding the drug safety. If a manufacturer discovers any unreasonable danger of drug that threatens people’s safety and health, it should immediately stop the manufacturing and sale of such drug, notify the distributors and report to the branch of the SFDA. This regulation also stipulates the procedures of drug call-back and danger valuation standards established and maintain a drug call back system in conformance the regulations.
|
|
●
|
Administration Regulations for Drug Instructions and Labels was promulgated by the SFDA on March 15, 2006 and was effective as of June 1, 2006. According to Administration Regulations for Drug Instructions and Labels, the contents of instructions and labels of each drug must be approved by the SFDA, and the smallest packing unit of drug shall be attached with instructions. Shaanxi Jiali received approval and maintains drug labeling in conformance with the regulations for its existing products and must do so for new products.
|
54
|
●
|
Supervision Administration Regulations for Drug Distribution was promulgated by the SFDA on January 31, 2007 and effective as of May 1, 2007. According to Supervision Administration Regulations for Drug Distribution, a pharmaceutical manufacturer can only sell drugs produced by itself, and it shall not sell drugs produced by other manufacturers or produced by itself but for commissioning manufacturing purpose. Shaanxi Jiali does not resell any other pharmaceutical manufacturers drugs.
|
|
●
|
Regulations for Drug Advertisement Censoring was promulgated by the SFDA and State Administration for Industry and Commerce (the “ SAIC ”) on March 13, 2007 and effective as of May 1, 2007. Standards for Drug Advertisement Censoring and Publication promulgated by the SFDA and the SAIC on March 3, 2007 and effective as of May 1, 2007. According to Regulations for Drug Advertisement Censoring, a pharmaceutical manufacturer must obtain a Drug Advertisement Approval Number from the provincial branch of the SFDA which is valid period of one year if the drug advertisement describes the functions or benefits of a drug. However, if an over the counter drug advertisement in any media, or a prescription drug advertisement in professional medical magazine, only refers to the name of the drug, including the general name and commercial name, without any other addition promotional information, the advertisement does not need to be censored or approved. Shaanxi Jiali obtained a Drug Advertisement Approval Number and reviews all of its over the counter and prescription drug advertisements so that it is in conformance with the regulations relating to advertising its products.
The application and approval procedure in China for a newly developed drug product has numerous steps. New drug applicants prepare the documentation of pharmacological study, toxicity study and pharmacokinetics and drug metabolism (PKDM) study and new drug samples. Documentation and samples are then submitted to provincial food and drug administration (“provincial FDA”). The provincial FDA sends its officials to the applicant to check the applicant’s research and development facilities and to arrange new drug examination committee meeting for approval deliberations. This process usually takes three months. After the documentation and samples are approved by the provincial FDA, the provincial FDA will submit the approved documentation and samples to SFDA. SFDA examines the documentation and tests the samples and arranges new drug examination committee meeting for approval deliberations. If the application is approved by SFDA, SFDA will issue a clinical trial license to the applicant for clinical trials. The clinical trial license approval typically takes one year. The applicant completes the clinical trial process and prepares documentation and files that are submitted to SFDA for new drug approval. The clinical trial process usually takes one year or two depending on the category and class of the new drug. SFDA examines the documentation and gives final approval for the new drug and issues the new drug license to the applicant. This process usually takes eight months. The whole process for new drug approval usually takes three to four years.
|
|
|
The SFDA and China Traditional Medicine Administration Bureau regulate the process for new drug approval and licensing in China, which can involve many layers of authority, lacks transparency, and presents one of the greatest obstacles for companies in introducing new drugs into the market. One of the preliminary aspects of the application process involves a review of the Chinese market’s need for a particular drug. If the SFDA determines that the market niche for a particular drug is saturated, the drug will not receive further consideration and the licensing application will be denied. According to industry analysts, eighty-five percent of the applications for new drugs licensing are determined by SFDA to be in saturated markets and thus are not considered for approval. Only fifteen percent of new-to-market drug applications are considered for approval by the SFDA.
Shaanxi Jiali's receipt of the GMP certificate and approval by the SFDA of its prescription and OTC drugs represents a significant competitive advantage as these approvals present a significant barrier to entry by new companies. Any new products may not pass the clinical review and testing process which can negatively affect cash flow and income.
|
Compliance with Environmental Law
We comply with the Environmental Protection Law of PRC as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
Environmental Regulation
We comply with the Environmental Protection Law of PRC as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Penalties would be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
China Pediatric Pharmaceuticals’ operation and facilities are subject to environmental laws and regulations stipulated by the national and the local environment protection bureaus in China. Relevant laws and regulations include provisions governing air emissions, water discharges and the management and disposal of hazardous substances and wastes. The PRC regulatory authorities require pharmaceutical companies to carry out environmental impact studies before engaging in new construction projects to ensure that their production processes meet the required environmental standards.
55
China Pediatric Pharmaceuticals maintains controls at its production facilities to facilitate compliance with environmental rules and regulations. China Pediatric Pharmaceuticals is not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has it been subject to any action made by any environmental administration authorities of the PRC. To management’s knowledge, China Pediatric Pharmaceuticals’ operation meets or exceeds the existing requirements of the PRC.
Advertising Laws
Advertisement Law of the People’s Republic of China and Rules of Medicine Advertisements Management from State Admission for Industry and Commerce, Regulations on Control of Advertisements (tentative) from State Council provide guidelines for advertising prescription and OTC drugs and nutrients made by Shaanxi Jiali. The rules limit where advertisements may be place and govern the claims that may be made by the manufacturer.
Price Controls
The prices of approximately 1,500 pharmaceuticals are presently set by the Chinese government. These constitute approximately 10% of all distributing drugs. The prices for the other 90%, or 12,000 pharmaceuticals, are established by the market (by the companies themselves). Corporations typically establish these prices based on operating costs, and market supply and demand. The Supervision Department of the Chinese government will intervene only if there is a significant fluctuation in prices or a monopoly develops in a particular drug. We have not had any products subject to specific pricing control and production and trading of none of our pharmaceutical products constitutes a monopoly.
Employees
We have approximately 141 full-time employees. Our employee breakdown includes 21 in management and administration, 59 in sales, marketing and distribution, 56 in production and quality control, and 5 in research and development. Our employees are neither unionized nor represented by a labor group for purposes of collective bargaining. We have good employee relations with little turnover in staff.
Our properties are located in Baoji and Xi'an, which is the capital of the Shaanxi province in the People's Republic of China and a sub-provincial city. As one of the oldest cities in Chinese history, Xi'an is one of the Four Great Ancient Capitals of China because it has been the capital of some of the most important dynasties in Chinese history, including the Zhou, Qin, Han, the Sui, and Tang dynasties. Xi'an is the eastern terminus of the Silk Road and known as the site of the Terracotta Army, made during the Qin Dynasty. The city has more than 3,100 years of history, and was known as Chang'an before the Ming Dynasty. Since the 1990s, as part of the economic revival of interior China especially for the central and northwest regions, the city of Xi'an has re-emerged as an important cultural, industrial and educational center of the central-northwest region, with facilities for research and development, national security and China's space exploration program.
Headquarters and Administration Offices
Shaanxi Jiali leases approximately 2,200 square feet of premium office space at the 9 th Floor of the China Construction Bank Building, No. 29 Nanxin Street, Xi’an, Shaanxi, PRC, for the purposes of accommodating the company’s executive and administration staff. The annual lease expense is RMB 156,240.which equals approximately USD22, 900. Please refer to the Exhibit 10.4 Lease Agreement.
Production Plant
Shaanxi Jiali owns the land use rights to approximately 54,000 square meters of land in Shaanxi province. The location of our 28,500 square meter manufacturing facility is located at No. 48 Xibao Road, Weibin District, City of Baoji , Shaanxi Province, P.R.C. We have a Certificate of House Ownership and Appendix Permit Certificate of State-owned Land Usufruct.
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants landholders a "land use right" after a purchase price for such "land use right" is paid to the government. The "land use right" allows the holder the right to use the land for a specified long-term period of time and enjoys all the incidents of ownership of the land. Our Baoji production base covers an area of 54,000 square meters with building area of 28,500 square meters. The land use rights for the Baoji production base expires on June 18, 2050.
56
In connection with the Exchange Agreement, we appointed five new directors to our board and hired six new officers. Furthermore, concurrent with the closing of the Exchange Agreement, Mr. Eric Anderson, our former President, Treasurer, Secretary, and sole Director, resigned from these positions.
Set forth below is certain information regarding our current directors and executive officers. Our Board of Directors is comprised of five directors. Executive officers are elected annually by our Board of Directors. Each executive officer holds his office until he resigns, is removed by the Board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal.
|
NAME
|
AGE
|
POSITION
|
||
|
Jun Xia
|
37
|
Chairman, Chief Executive Officer, and President
|
||
|
Minggang Xiao
|
28
|
Director, Chief Financial Officer
|
||
|
Wanxiang Li
|
68
|
Independent Director
|
||
|
Nanjing Lin
|
71
|
Independent Director
|
||
|
Xaioying Zhang
|
30
|
Independent Director
|
Jun Xia, Chairman, Chief Executive Officer and President, became our Chairman, Chief Executive Officer and President upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Xia founded Shaanxi Jiali on July 1, 1998. Prior to founding Shaanxi Jiali, Mr. Xia founded Shaanxi Jiali Broadcasting Co., Ltd in the year 1996 which provided advertising marketing promotion and brand management strategies to clients that included Volkswagon AG and major drink manufacture Wahaha. Mr. Xia is the Standing Director of Shaanxi Pharmaceuticals Association and Vice Chairman of Biomedical Branch of Shaanxi Scientific and Research Association of Chinese Traditional Medicines. In 2001, Mr. Xia was named “Youngest Entrepreneur in Shaanxi Province” by Xi’an National Hi-Tech Industrial Development Zone. Mr. Jun Xia, graduated from Economy Management School of Northwest University, in 1995. Mr. Xia also attended the Fudan University in Shaanhai.
Minggang Xiao, Director & Chief Financial Officer, became our director and Chief Financial Officer upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Xiao has been with Shaanxi Jiali since 2006. Mr. Xiao manages the day-to-day operations of the Shaanxi Jiali’s Finance Department. Prior to joining Shaanxi Jiali, Mr. Xiao was the Finance
57
Manager at Shaanxi Xinchuang Management Co. Ltd. in Xi’an from 2005 to 2006. From 2004 to 2005, Mr. Xiao was an Accountant with Financial Bureau of Baoji City, Shaanxi. Mr. Xiao holds the professional designation of Accountant. Mr. Xiao graduated from Xi’an Industrial University with his Bachelor of Accounting Degree in 2004.
Wanxiang Li, Independent Director, became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Ms. Li has been a Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University in Xi'an since 1984. From 1969 to 1984, Ms. Li was an Accountant in the Finance Department of the Science and Technology Management Bureau of Government Shaanxi in Xi’an. Ms. Li holds the professional designation of Senior Accountant. Ms Li graduated from the Economy Management School of China Agricultural University in Xi’an with her Master’s Degree in Accounting in 1964.
Nanjing Lin, Independent Director, became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Mr. Lin has been Committee Chief of Government Consulting & Policies Management in Shaanxi province in Xi’an since 1993, after joining in 1986. From 1964 to 1986, Mr. Lin was Department Head, Finance, at Shayuan Farm of Agr-cultivation Bureau of Shaanxi province in Xi’an. Mr. Lin holds the professional designation of Senior Accountant. Mr. Lin graduated from the Economy Management School of Shaanxi Finance and Economy University where he earned his Bachelor’s degree of Accounting in the year 1964.
Xiaoying Zhang, Independent Director, became an Independent Director upon the closing of the Share Exchange transaction on September 30, 2009. Ms. Zhang has been Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd. in Xi’an since 2007. Prior to her employment with Shaanxia Deli Energy Ms. Zhang was employed as an Accountant with the Department of Finance of Xi’an Instrument Group Ltd. Co. in Xi’an from 2003 until 2007. Ms. Zhang holds the professional designation of Accountant. Ms. Zhang graduated from the Xi’an International University, in Xi’an in 2003 and earned her Bachelor’s Degree of Accounting.
Director Experience
The Board believes that each of the Company’s directors should possess the highest personal and professional ethics, integrity and values, and be committed to representing the long-term interests of the Company’s shareholders. When evaluating candidates for election to the Board, the Corporate Governance and Nominating Committee seeks candidates with certain qualities that it believes are important, including integrity, an objective perspective, good judgment, and leadership skills. The Board has also considered the fact that all of our directors have worked for, or served on the boards of directors of, a variety of companies in a range of industries. The Board believes that through their varying backgrounds, the Company’s directors bring a wealth of experiences, new ideas and solutions to the Board. Specifically, the Board has noted that our directors have the following skills and qualifications that, among others, have made them particularly suited to serve as a director of China Pharmaceuticals:
|
●
|
Mr. Jun Xia has significant business management experience, entrepreneurial vision, and extensive knowledge of the pharmaceuticals industry and has served in various leadership positions in the Shaanxi pharmaceutical community since 2007.
|
|
|
●
|
Mr. Minggang Xiao has significant financial experience, and in 2008 was awarded the designation of accountant by the PRC Department of Treasury.
|
|
|
●
|
Ms. Wanxiang Li processes an extensive knowledge of accounting principles, having served as both a practicing accountant and as a professor of accounting for each of 15 years, respectively.
|
|
|
●
|
Mr. Nanjing Lin is an “audit committee financial expert” with over 20 years of financial and accounting experience in the PRC.
|
|
|
●
|
Ms. Xiaoying Zhang has significant accounting experience and served as supervisor to the accounting operations of a PRC technology company since 2007.
|
Committees of the Board of Directors
We have appointed the follow committees of the Board of Directors with membership as follows:
Audit Committee and Audit Committee Financial Expert
Our board of directors established an audit committee on September 30, 2009. The audit committee is comprised of the following three independent board members:
|
●
|
Nanjing Lin, Chair, Committee Chief of Government Consulting & Policies Management in Shaanxi province
|
|
|
●
|
Wanxiang Li, Member, Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University
|
|
|
●
|
Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd.
|
The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
58
|
●
|
selecting our independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by our independent auditors;
|
|
|
●
|
reviewing with our independent auditors any audit problems or difficulties and management’s response;
|
|
|
●
|
reviewing and approving all proposed related-party transactions, as defined in Item 404 of Regulation S-K under the Securities Act;
|
|
|
●
|
discussing the annual audited financial statements with management and our independent auditors;
|
|
|
●
|
reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies;
|
|
|
●
|
annually reviewing and reassessing the adequacy of our audit committee charter;
|
|
|
●
|
such other matters that are specifically delegated to our audit committee by our board of directors from time to time;
|
|
|
●
|
meeting separately and periodically with management and our internal and independent auditors; and
|
|
|
●
|
reporting regularly to the full board of directors.
|
Our board of directors has determined that it has an "audit committee financial expert" as defined by Item 401(h) of Regulation S-K as promulgated by the Securities and Exchange Commission. Our audit committee financial expert is Nanjing Lin. The directors who serve on the audit committee are "independent" directors based on the definition of independence in the listing standards of the National Association of Securities Dealers. Our Board of Directors has adopted a written charter for the Audit Committee.
Compensation Committee
Our board of directors established a compensation committee on September 30, 2009.
The compensation committee of the board of directors is comprised of the following three independent board members:
|
●
|
Wanxiang Li, Chair, Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University
|
|
|
●
|
Nanjing Lin, Member, Committee Chief of Government Consulting & Policies Management in Shaanxi province
|
|
|
●
|
Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd.
|
Our compensation committee assists the board in reviewing and approving the compensation structure of our directors and executive officers, including all forms of compensation to be provided to our directors and executive officers. Members of the compensation committee are not prohibited from direct involvement in determining their own compensation. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
|
●
|
approving and overseeing the compensation package for our executive officers;
|
|
●
|
reviewing and making recommendations to the board with respect to the compensation of our directors;
|
|
●
|
reviewing and approving corporate goals and objectives relevant to the compensation of our chief executive officer, evaluating the performance of our chief executive officer in light of those goals and objectives, and setting the compensation level of our chief executive officer based on this evaluation; and
|
|
●
|
reviewing periodically and making recommendations to the board regarding any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans.
|
Our board of directors has adopted a written compensation committee charter. The directors who serve on the compensation committee are "independent" directors based on the definition of independence in the listing standards of the National Association of Securities Dealers.
Corporate Governance and Nominating Committee
Our board of directors established a corporate governance and nominating committee on September 30, 2009.
The Company’s corporate governance and nominating committee is comprised of the following three independent board members:
59
|
●
|
Wanxiang Li, Chair, Professor of Accounting at the School of Finance and Economy at Xi’an Jiaotong University
|
|
|
●
|
Nanjing Lin, Member, Committee Chief of Government Consulting & Policies Management in Shaanxi province
|
|
|
●
|
Xiaoying Zhang, Member, Accountant Supervisor with Shaanxi Deli Energy Science and Technology Co., Ltd.
|
Our corporate governance and nominating committee consists of our independent directors Ms. Lin, Mr. Li and Ms Zhang. The corporate governance and nominating committee assists the board of directors in identifying individuals qualified to become our directors and in determining the composition of the board and its committees. The corporate governance and nominating committee is responsible for, among other things:
|
●
|
identifying and recommending to the board nominees for election or re-election to the board, or for appointment to fill any vacancy;
|
|
●
|
reviewing annually with the board the current composition of the board in light of the characteristics of independence, age, skills, experience and availability of service to us;
|
|
●
|
identifying and recommending to the board the directors to serve as members of the board’s committees;
|
|
●
|
advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to the board on all matters of corporate governance and on any corrective action to be taken; and
|
|
●
|
monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance.
|
The directors who serve on the nominating committee are "independent" directors based on the definition of independence in the listing standards of the National Association of Securities Dealers. The nominating committee has a written charter.
At this time, no additional specific procedures to propose a candidate for consideration by the nominating committee, nor any minimum criteria for consideration of a proposed nomination to the board, have been adopted. The committee does not currently consider diversity in identifying nominees for our Board of Directors.
To the best of our knowledge, none of the following ever occurred to any of our directors and officers:
(1) Any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
(2) Any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
(4) Being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated.
Code of Ethics
On September 30, 2009, we adopted a code of ethics to apply to all of our executive officers, including our principal executive, financial and accounting officers, our directors, our financial managers and all employees are expected to adhere and promote regarding individual and peer responsibilities, and responsibilities to other employees, the Company, the public and other stakeholders.
Board Leadership and Risk Oversight
Our Chief Executive Officer also serves as Chairman of the Board. We have four other directors, three of whom are independent. Nanjing Lin, the chairman of the Audit Committee, is our lead independent director. The Board has three standing committees, each of which is comprised solely of independent directors with a committee chair. We believe that this leadership structure has served the Company well. The Board believes that the Company’s Chief Executive Officer is best situated to serve as Chairman of the Board because he is the director most familiar with our business and industry and the director most capable of identifying strategic priorities and executing our business strategy. In addition, having one person serve as both Chairman and Chief Executive Officer eliminates potential for confusion and demonstrates to our employees, suppliers, customers, shareholders and other stakeholders that China Pediatric Pharmaceuticals has clear leadership with a single person setting the tone and managing our operations. The Board has delegated responsibility for the oversight of specific risks to Board committees as follows:
60
|
●
|
appointing, retaining and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting;
|
|
|
●
|
approving all auditing and non-auditing services permitted to be performed by the independent auditors;
|
|
|
●
|
reviewing annually the independence and quality control procedures of the independent auditors;
|
|
|
●
|
Reviewing, approving, and overseeing risks arising from proposed related party transactions;
|
|
|
●
|
discussing the annual audited financial statements with the management;
|
|
|
●
|
meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter and other material written communications between the independent auditors and the management; and
|
|
|
●
|
monitoring the risks associated with management resources, structure, succession planning, development and selection processes, including evaluating the effect the compensation structure may have on risk decisions.
|
The board and its committees held the following number of meetings during the fiscal year of 2009:
|
Board of Directors
|
3
|
|||
|
Audit Committee
|
0
|
|||
|
Compensation Committee
|
0
|
|||
|
Corporate Governance and Nominating Committee
|
0
|
The meetings include meetings that were held by means of a conference telephone call, but do not include actions taken by unanimous written consent.
Each director attended at least 75% of the total number of meetings of the board and those committees on which he served during the year. Our non-management directors did not meet in executive session during 2009.
Conflicts of Interest
With respect to transactions involving real or apparent conflicts of interest, we have adopted policies and procedures which require that: (i) the fact of the relationship or interest giving rise to the potential conflict be disclosed or known to the directors who authorize or approve the transaction prior to such authorization or approval, (ii) the transaction be approved by a majority of our disinterested outside directors, and (iii) the transaction be fair and reasonable to us at the time it is authorized or approved by our directors.
Litigation
Neither we, nor any of our controlled affiliates, are involved in any lawsuit outside the ordinary course of business, the disposition of which would have a material effect upon either our results of operations, financial position, or cash flows.
On September 30, 2009, the Company entered into a Share Exchange Agreement with Eric Anderson and Asia-Pharm, pursuant to which the Company acquired all of the outstanding capital stock an ownership interest of China Children from Asia-Pharm. In exchange for the equity interest in China Children, the Company issued 7,000,000 (post-split) shares of common stock to the shareholders of Asia-Pharm.
Immediately after the closing of the Share Exchange Agreement, the Company had a total of 8,305,288 shares of common stock outstanding, with all of the Asia-Pharm shareholders owning approximately 84.28% of the Company’s outstanding common stock. In addition, pursuant to an agreement dated September 30, 2009, Eric Anderson transferred 1,428,571(post-split) shares of the Company’s common stock for cancellation and forgave his outstanding loan to the company in the amount of $86,173 in exchange for 100% of the issued and outstanding shares of Lid Hair Studios’ wholly-owned subsidiary, Belford Enterprises B.C. Ltd., d/b/a Lid Hair Studio.
Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to certain consultants two year warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $5.00 per share (collectively, the “Warrants”). Specifically, we issued to IFG Investments Services, Inc. a $3.00 warrant to purchase 600,000 shares of common stock and a $5.00 warrant to purchase 600,000 shares of common stock as consideration for certain professional consulting services rendered, including merger/acquisition
61
support, NASDAQ and/or Hong Kong listing support. We also issued to Kang Xiulan, an individual who assisted us in the share exchange transaction, a $3.00 warrant to purchase 250,000 shares of common stock and a $5.00 warrant to purchase 250,000 shares of common stock as consideration for certain “shell” company referral services rendered in connection with the Merger Transaction. The referral services rendered included an introduction by Kang Xiulan of the OTCBB shell company, Lid Hair Studios International, Inc., as a potential merger partner to Shaanxi Jiali Pharmaceutical Co. Ltd. for the purposes of a merger. On account of Mr. Kang’s involvement with the Company’s restructuring and reverse acquisition transaction, we determined him to be a related person.
The exercise price and number of shares of Common Stock to be received upon the exercise of the Warrants are subject to adjustment upon the occurrence of certain events, such as stock splits, stock dividends or our recapitalization. In the event of our liquidation, dissolution or winding up, the holders of the Warrants will not be entitled to participate in the distribution of our assets. If the Company shall make any dividend or other distribution of assets to holders of its common stock (a “Distribution”), then the Exercise Price shall be reduced by multiplying such Exercise Price by a fraction of which (i) the numerator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date minus the value of the Distribution applicable to one share of common stock, and (ii) the denominator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date. Additionally, the number of shares underlying the Warrant shall be increased to a number of shares of common stock obtainable immediately prior to the close of business on the record date fixed for determining holders of shares entitled to receive the Distribution multiplied by the reciprocal of the fraction set forth above. Holders of the Warrants will have no voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the Holders be entitled to receive dividends.
Other than as disclosed above, there have been no other transactions since the beginning of the fiscal year preceding our last fiscal year or any currently proposed transactions in which we are, or plan to be, a participant and the amount involved exceeds $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest.
Procedures for Approval of Related Party Transactions
Our Board of Directors and audit committee is charged with reviewing and approving all potential related party transactions. All such related party transactions must then be reported under applicable SEC rules. We have not adopted other procedures for review, or standards for approval, of such transactions, but instead review them on a case-by-case basis.
Director Independence
The Company currently has three independent directors, Wanxiang Li, Nanjing Lin, and Xiaoying Zhang, as that term is defined under the National Association of Securities Dealers Automated Quotation system.
The following summary compensation table indicates the cash and non-cash compensation earned during the years ended December 31, 2009 and 2008 by each person who served as chief executive officer and chief financial officer during 2009. No officer received compensation of $100,000 or more during 2008.
|
Change in
|
||||||||||||||||||||||||||||||||||
|
Pension
|
||||||||||||||||||||||||||||||||||
|
Value and
|
||||||||||||||||||||||||||||||||||
|
Nonqualified
|
||||||||||||||||||||||||||||||||||
|
Non-equity
|
Deferred
|
|||||||||||||||||||||||||||||||||
|
Name and
|
Stock
|
Option
|
Incentive Plan
|
Compensation
|
All Other
|
|||||||||||||||||||||||||||||
|
Principal
|
Fiscal
|
Salary
|
Bonus
|
Awards
|
Awards
|
Compensation
|
Earnings
|
Compensation
|
Total
|
|||||||||||||||||||||||||
|
Position
|
Year
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
($)
|
|||||||||||||||||||||||||
|
Eric Anderson, Former CEO
|
2009
|
1,173
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
1,173
|
|||||||||||||||||||||||||
|
and Director (1)
|
2008
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
|||||||||||||||||||||||||
|
Jun Xia,
|
2009
|
10,553
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
10,553
|
|||||||||||||||||||||||||
|
Chairman and CEO (2)
|
2008
|
8,824
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
8,824
|
|||||||||||||||||||||||||
|
Minggang Xiao,
|
2009
|
7,036
|
-0-
|
735
|
-0-
|
-0-
|
-0-
|
-0-
|
7,771
|
|||||||||||||||||||||||||
|
CFO (2)
|
2008
|
7,036
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
-0-
|
7,036
|
|||||||||||||||||||||||||
62
|
(1)
|
Eric Anderson resigned as our Chairman, Chief Executive Officer, President and Chief Financial Officer on September 30, 2009.
|
|
(2)
|
These amounts represent compensation paid by Shaanxi Jiali. We executed employment agreements with our CEO and CFO as of September 30, 2009. The employment agreement for Jun Xia is for a term of three years. The employment agreement for Minggang Xiao is for one year. The employment agreements will provide for annual salaries and annual bonuses in amounts not less than the amounts set forth in the table above. Additionally, on October 2, 2009. Minggang Xiao received 3,500 shares of common stock (valued at $735) as additional compensation for his services as CFO.
|
SHAANXI JIALI EXECUTIVE COMPENSATION SUMMARY
Summary Compensation Table
The following table sets forth all cash compensation paid by Shaanxi Jiali, for last fiscal year, specifically, for the year ending December 31, 2009. The table below sets for the positions for each person at Shaanxi Jiali. All amounts are in USD.
|
Name/Principal Position
|
Year
|
Salary (Cash & Non-Cash)
|
Bonus
|
||||||
|
Jun Xia, Chairman of the Board of Directors and Chief Executive Officer
|
2009
|
$
|
10,553
|
$
|
0
|
||||
|
Minggang Xiao, Director & Chief Financial Officer
|
2009
|
$
|
7,036
|
$
|
0
|
||||
|
Jing Fu, Senior Vice President
|
2009
|
$
|
8,794
|
$
|
0
|
||||
|
Qina Sun, Vice President, Sales & Marketing
|
2009
|
$
|
6,156
|
$
|
0
|
||||
|
Yanxin Li, Vice President of Operations
|
2009
|
$
|
5,277
|
$
|
0
|
||||
|
Lijuan Feng, Vice President Administration
|
2009
|
$
|
5,277
|
$
|
0
|
||||
|
Juan Wei, Vice President Corporate Affairs
|
2009
|
$
|
5,277
|
$
|
0
|
||||
Option Grants
We do not maintain any equity incentive or stock option plan. Accordingly, we did not grant options to purchase any equity interests to any employees or officers, and no stock options are issued or outstanding to any officers.
Employment Contracts
On September 30, 2009, we entered into a two year Employment Agreement with Jun Xia to serve as our Chief Executive Officer. The Agreement provides for an annual salary of USD$10,553 and an annual bonus of up to 50% of the executive’s annual salary.
On September 30, 2009, we entered into a one year Employment Agreement with Minggang Xiao to serve as our Chief Financial Officer. The Agreement provides for an annual salary of USD$7,036 and an annual bonus of up to 50% of the executive’s annual salary.
Compensation Discussion and Analysis
We strive to provide our named executive officers (as defined in Item 402 of Regulation S-K) with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in similar locations.
We plan to implement a more comprehensive compensation program, which takes into account other elements of compensation, including, without limitation, short and long term compensation, cash and non-cash, and other equity-based compensation such as stock options. We expect that this compensation program will be comparable to the programs of our peer companies and aimed to retain and attract talented individuals.
Our compensation committee oversees the compensation of our named executive officers.
63
Compensation of Directors
As of the date of the registration statement of which this prospectus is a part, our directors have received no compensation for their service on the board of directors. We plan to implement a compensation program for our independent directors, which we anticipate will include such elements as an annual retainer, meeting attendance fees and stock options. The details of that compensation program will be negotiated with each independent director.
The following table sets forth certain information with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than 5% of any class of voting securities, (ii) each director, (iii) our Chief Executive Officer, President and Chief Financial Officer, and (iv) all executive officers and directors as a group as of September 1, 2010 .
Amount and Nature of Beneficial Ownership
|
Title of Class
|
Name and Address (1)
of Beneficial Owner
|
Amount and Nature of Beneficial Ownership (2)
|
Percentage of
Common
Stock
|
|||||
|
Owners of More than 5% of Class
|
||||||||
|
Common Stock
|
Yangling Bodisen Biotech Development, Inc. (3)
North part of Xinqiao Road,Yangling Agricultural High-tech Industries Demonstration Zone,
Shaanxi 712100, P.R.C.
|
2,018,590
|
19.83
|
%
|
||||
|
Common Stock
|
IFG Investments Services, Inc. (4)
Ram's Business Complex
Stoney Grove, Box 822, Charlestown,
Nevis Federation of St Kitts & Nevis
|
1,200,000
|
10.54
|
%
|
||||
|
Common Stock
|
54 Veterans Boulevard
Strafford, CT
|
800,000
|
7.29
|
%
|
|
Directors and Executive Officers
|
||||||||
|
Common Stock
|
Jun Xia (6)
CEO and Chairman
|
2,730,000
|
26.82
|
%
|
||||
|
Common Stock
|
Minggang Xiao
Chief Financial Officer
|
3,500
|
*
|
%
|
||||
|
Common Stock
|
Wanxiang Li
Director
No.119 South Cuihua Road , Xi’an City,
Shaanxi Province
|
0
|
0
|
%
|
||||
|
Common Stock
|
Nanjing Lin
Director
Unit 1,Building17, Zhui hui Cheng Community, No.169 Ziwu Dadao,Chang'an District, Xi'an City,
Shaanxi Province, 710062
|
0
|
0
|
%
|
64
|
Xiaoying Zhang
Director
No. 17 Labor Road Lianhu District, Xi'an City,
Shaanxi Province
|
0
|
0
|
%
|
|||||
|
Common Stock
|
||||||||
|
All Directors and Executive Officers as a Group (6 persons)
|
2,733,500
|
26.85
|
%
|
|||||
|
*Represents less than 1% of the issued and outstanding shares of the Company’s common stock.
|
||||||||
|
(1) Except as otherwise indicated, the address of each beneficial owner is c/o China Pediatric Pharmaceuticals, Inc., 9th Floor, No. 29 Nanxin Street, Xi’an, Shaanxi Province P.R.C., 710004.
|
||||||||
|
(2) Information with respect to beneficial ownership is based upon information furnished by each shareholder or contained in filings made with the Securities and Exchange Commission. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. In determining the percent of common stock owned by a person or entity on September 1, 2010, (a) the numerator is the number of shares of the class beneficially owned by such person and includes shares which the beneficial owner may acquire within 60 days upon conversion or exercise of a derivative security, and (b) the denominator is the sum of (i) the shares of that class outstanding on September 1, 2010 (10,180,288 shares of Common Stock) and (ii) the total number of shares that the beneficial owner may acquire upon conversion or exercise of a derivative security within such 60 day period. Unless otherwise stated, each beneficial owner has sole power to vote and dispose of the shares.
(3) Bo Chen, the chief executive officer of Yangling Bodisen Biotech Development, Inc., has sole power to vote and dispose of the shares held by Yangling Bodisen Biotech Development, Inc.
(4) The shares deemed to be beneficially owned by IFG Investments Services, Inc. represent shares of common stock underlying a $3.00 warrant to purchase 600,000 shares of common stock and a $5.00 warrant to purchase 600,000 shares of common stock, each expiring September 30, 2012. Daniel MacMullin, the President and beneficial owner of IFG Investments Services, Inc., has sole power to vote and dispose of the shares held by IFG Investments Services, Inc.
(5) The shares deemed to be beneficially owned by China National Information Network Trust represent shares of common stock underlying a $3.00 warrant to purchase 400,000 shares of common stock and a $5.00 warrant to purchase 400,000 shares of common stock, each expiring September 30, 2012. Clare Donahue, the beneficial owner of China National Information Network Trust, has sole power to vote and dispose of the shares held by China National Information Network Trust.
(6) The shares deemed to be beneficially owned by Jun Xia, the Company’s chief executive officer, include 2,450,000 shares of common stock held by Mr. Xia and 280,000 shares of common stock held by Tianqiu Xia, Mr. Xia’s father.
|
||||||||
The table below sets forth the name of each person who is offering for resale shares of common stock covered by this prospectus, the number of shares of common stock beneficially owned by each person, the number of shares of common stock that may be sold in this offering, and the number of shares of common stock each person will own after the offering, assuming they sell all of the shares offered. To the extent that any successor(s) to the named selling stockholders wishes to sell under this prospectus, we will file a prospectus supplement identifying such successor(s) as selling stockholders. None of the selling stockholders are affiliates of broker-dealers.
The shares of common stock being offered in this prospectus (including shares issuable upon the conversion of convertible promissory notes) were issued in private placement transactions by us, each of which was exempt from the registration requirements of the Securities Act of 1933, as amended, pursuant to Section 4(2) of the Securities Act.
Because the selling stockholders may offer all, some, or none of their shares of our common stock, we cannot provide a definitive estimate of the number of shares that the selling stockholders will hold after this offering.
65
Except as set forth below or in the section “Recent Sales of Unregistered Securities,” none of the selling stockholders has at any time during the past three years acted as one of our employees, officers, or directors or otherwise had a material relationship with us.
Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to the selling stockholders two year warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $5.00 per share (collectively, the “Warrants”).
Specifically, we issued to IFG Investments Services, Inc. a $3.00 warrant to purchase 600,000 shares of common stock and a $5.00 warrant to purchase 600,000 shares of common stock as consideration for certain professional consulting services rendered, including merger/acquisition support, NASDAQ and/or Hong Kong listing support.
We issued to China National Information Network Trust a $3.00 warrant to purchase 400,000 shares of common stock and a $5.00 warrant to purchase 400,000 shares of common stock as consideration for certain investor relations services to be provided. We also issued to Kang Xiulan a $3.00 warrant to purchase 250,000 shares of common stock and a $5.00 warrant to purchase 250,000 shares of common stock as consideration for certain “shell” company referral services rendered in connection with the reverse merger transaction.
Under applicable SEC rules, a person is deemed to beneficially own securities which the person as the right to acquire within 60 days through the exercise of any option or warrant or through the conversion of a convertible security. Also under applicable SEC rules, a person is deemed to be the “beneficial owner” of a security with regard to which the person directly or indirectly, has or shares (a) voting power, which includes the power to vote or direct the voting of the security, or (b) investment power, which includes the power to dispose, or direct the disposition, of the security, in each case, irrespective of the person’s economic interest in the security. Each listed selling stockholder has the sole investment and voting power with respect to all shares of common stock shown as beneficially owned by such selling stockholder, except as otherwise indicated in the footnotes to the table.
As of September 1, 2010, there were 10,180,288 shares of our common stock issued and outstanding. In determining the percent of common stock beneficially owned by a selling stockholder on September 1, 2010, (a) the numerator is the number of shares of common stock beneficially owned by such selling stockholder (including shares that he has the right to acquire within 60 days of August 13 , 2010), and (b) the denominator is the sum of (i) 10,180,288 shares outstanding on September 1, 2010, and (ii) the number of shares of common stock which such selling stockholders has the right to acquire within 60 days of September 1, 2010.
|
Selling Stockholder
|
Shares beneficially
owned prior to the
offering
|
Number of
common shares
registered in
this prospectus
|
Shares beneficially
owned after the
offering
|
|||||||
|
Number
|
Percent
|
Number
|
Percent
|
|||||||
|
IFG Investments Service, Inc. (1)
|
1,200,000
|
10.54
|
1,200,000
|
0
|
0%
|
|||||
|
China National Information Network Trust (2)
|
800,000
|
7.29
|
800,000
|
0
|
0%
|
|||||
|
Kang Xiulan
|
500,000
|
4.68
|
500,000
|
0
|
0%
|
|||||
|
———————
|
|
|
(1)
|
Daniel MacMullin, the President and beneficial owner of IFG Investments Services, Inc., has sole power to vote and dispose of the shares held by IFG Investments Services, Inc.
|
|
(2)
|
Clare Donahue, the beneficial owner of China National Information Network Trust, has sole power to vote and dispose of the shares held by China National Information Network Trust.
|
The selling stockholders and any of their respective pledgees, donees, assignees, and other successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions.
We have agreed, subject to certain limits, to bear all costs, expenses, and fees of registration of the shares of our common stock offered by the selling stockholders for resale. However, any brokerage commissions, discounts, concessions, or other fees, if any, payable to broker-dealers in connection with any sale of shares of common stock will be borne by the selling stockholders selling those shares or by the purchasers of those shares.
66
On our being notified by a selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of shares through a block trade, special offering, exchange distribution, or secondary distribution, or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act, disclosing the following:
|
●
|
the name of each such selling stockholder and of any participating broker-dealer
|
|
|
●
|
the number of securities involved
|
|
|
●
|
the price at which such securities were sold
|
|
|
●
|
the commissions paid or discounts or concessions allowed to any broker-dealer, where applicable that any broker-dealer did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus
|
|
|
●
|
other facts material to the transaction.
|
|
|
●
|
the price at which such securities were sold
|
|
|
●
|
the commissions paid or discounts or concessions allowed to any broker-dealer, where applicable that any broker-dealer did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus
|
|
|
●
|
other facts material to the transaction.
|
The selling stockholders may use any one or more of the following methods when selling shares:
|
●
|
directly as principals
|
|
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers
|
|
|
●
|
block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account
|
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange
|
|
|
●
|
privately negotiated transactions
|
|
|
●
|
short sales that are in compliance with the applicable laws and regulations of any state or the United States
|
|
|
●
|
broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share
|
|
|
●
|
a combination of any such methods of sale
|
|
|
●
|
any other method permitted pursuant to applicable law
|
The selling stockholders may also sell shares under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Any sales of the shares may be effected through the OTC Bulletin Board, in private transactions or otherwise, and the shares may be sold at market prices prevailing at the time of sale, at prices related to prevailing market prices.
The selling stockholders may also engage in short sales against the box, puts and calls, and other transactions in our securities or derivatives of our securities and may sell or deliver shares in connection with these trades. The selling stockholders may pledge their shares to their brokers under the margin provisions of customer agreements. If a selling stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares. We believe that the selling stockholders have not entered into any agreements, understandings or arrangements with any underwriters or broker-dealers regarding sale of their shares other than ordinary course brokerage arrangements, nor is there an underwriter or coordinating broker acting in connection with the proposed sale of shares by the selling stockholders.
Broker-dealers engaged by the selling stockholders may arrange for other brokers-dealers to participate in sales. If the selling stockholders effect sales through underwriters, brokers, dealers or agents, such firms may receive compensation in the form of discounts, concessions or commissions from the selling stockholders or the purchasers of the shares for whom they may act as agent, principal or both in amounts to be negotiated. Those persons who
67
act as broker-dealers or underwriters in connection with the sale of the shares may be selected by the selling stockholders and may have other business relationships with, and perform services for, us. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
Any selling stockholder or broker-dealer who participates in the sale of the shares may be deemed to be an “underwriter” within the meaning of section 2(11) of the Securities Act. Any commissions received by any underwriter or broker-dealer and any profit on any sale of the shares as principal may be deemed to be underwriting discounts and commissions under the Securities Act.
The anti-manipulation provisions of Rules 101 through 104 of Regulation M promulgated under the Exchange Act may apply to purchases and sales of shares of common stock by the selling stockholders. In addition, there are restrictions on market-making activities by persons engaged in the distribution of the common stock.
Under the securities laws of certain states, the shares may be sold in those states only through registered or licensed brokers or dealers. In addition, in certain states the shares may not be able to be sold unless our common stock has been registered or qualified for sale in that state or an exemption from registration or qualification is available and is complied with.
We are required to pay expenses incident to the registration, offering, and sale of the shares under this offering. We estimate that our expenses will total approximately $50,000. We have agreed to indemnify certain selling stockholders and certain other persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments to which those selling stockholders or their respective pledgees, donees, transferees or other successors in interest may be required to make in respect thereof. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
As of September 30, 2009, our authorized capital stock consists of 75,000,000 shares of common stock, par value $0.001 per share. As of September 1, 2010 , an aggregate of 10,180,288 shares of common stock were outstanding, including shares issued pursuant to the Closing.
There are no shares of preferred stock outstanding.
Common Stock
Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of Common Stock are entitled to receive dividends out of assets legally available therefore at times and in amounts as our board of directors may determine. Each stockholder is entitled to one vote for each share of Common Stock held on all matters submitted to a vote of the stockholders. Cumulative voting is not provided for in our amended articles of incorporation, which means that the majority of the shares voted can elect all of the directors then standing for election. The Common Stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon the occurrence of a liquidation, dissolution or winding-up, the holders of shares of Common Stock are entitled to share rateably in all assets remaining after payment of liabilities and satisfaction of preferential rights of any outstanding preferred stock. There are no sinking fund provisions applicable to the Common Stock. The outstanding shares of Common Stock are, and the shares of Common Stock to be issued upon conversion of the Warrants will be, fully paid and non-assessable.
Voting Rights
Holders of our common stock have the right to cast one vote for each share of stock in their name on the books of our company, whether represented in person or by proxy, on all matters submitted to a vote of holders of common stock, including election of directors. There is no right to cumulative voting in the election of directors. Except where a greater requirement is provided by statute or by the articles of incorporation, or in the by-laws, the presence, in person or by proxy duly authorized, of the one or more holders of a majority of the outstanding shares of our common stock constitutes a quorum for the transaction of business. The vote by the holders of a majority of outstanding shares is required to effect certain fundamental corporate changes such as liquidation, merger, or amendment of our articles of incorporation.
Dividends
Any future determination as to the declaration and payment of dividends on shares of our Common Stock will be made at the discretion of our board of directors out of funds legally available for such purpose. We are under no contractual obligations or restrictions to declare or pay dividends on our shares of Common Stock. In addition, we currently have no plans to pay such dividends. However, even if we wish to pay dividends, because our cash flow is dependent on dividend distributions from our affiliated entities in PRC, we may be restricted from distributing dividends to our holders of shares of our common stock in the future if at the time we are unable to obtain sufficient dividend distributions from China Children Pharmaceutical, Xi’an Coova and Shaanxi Jiali. Our board of directors currently intends to retain all earnings for use in the business for the foreseeable future. See “Risk Factors.”
68
Preemptive Rights
Holders of our common stock are not entitled to preemptive rights, and no redemption or sinking fund provisions are applicable to our common stock. All outstanding shares of our common stock are, and the shares of common stock sold in the offering will when issued be fully paid and non-assessable.
Warrants
Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to certain consultants two year warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase an aggregate of 1,250,000 shares of our common stock at an exercise price of $5.00 per share (collectively, the “Warrants”). Specifically, we issued to IFG Investments Services, Inc. a $3.00 warrant to purchase 600,000 shares of common stock and a $5.00 warrant to purchase 600,000 shares of common stock as consideration for certain professional consulting services rendered, including merger/acquisition support, NASDAQ and/or Hong Kong listing support. We issued to China National Information Network Trust a $3.00 warrant to purchase 400,000 shares of common stock and a $5.00 warrant to purchase 400,000 shares of common stock as consideration for certain investor relations services to be provided. We also issued to Kang Xiulan a $3.00 warrant to purchase 250,000 shares of common stock and a $5.00 warrant to purchase 250,000 shares of common stock as consideration for certain “shell” company referral services rendered in connection with the Merger Transaction.
Warrants
Each Warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable, in whole or in part, at an exercise price equal to $3.00 per share. The Piggyback Warrants will be exercisable, in whole or in part, at an exercise price equal to $5.00 per share. The Warrants may be exercised at any time upon the election of the Investor, beginning on the date of issuance and ending of the second anniversary of the closing of the Share Exchange Agreement. The Piggy-back Warrants may be exercised at any time upon the election of the Investor, beginning on the date of issuance and ending of the third anniversary of the closing of the Share Exchange Agreement.
The Warrants will be detachable and separately transferable only during the Warrant exercise period; upon the expiration of the Warrant exercise period, the Warrants will expire and become void.
In order to exercise the Warrants, the Warrant must be surrendered at the office of the Warrant Agent prior to the expiration of the Warrant exercise period, with the form of exercise appearing with the Warrant completed and executed as indicated, accompanied by payment of the full exercise price for the number of Warrants being exercised. Payment shall be by certified funds or cashier's check payable to “China Pediatric Pharmaceuticals, Inc.” In the case of partial exercise, the Warrant Agent will issue a new warrant to the exercising warrant holder, or assigns, evidencing the Warrants which remain unexercised. In our discretion, the Warrant Agent may designate a location other than our office for surrender of Warrants in the case of transfer or exercise.
The exercise price and number of shares of common stock to be received upon the exercise of Warrants are subject to adjustment upon the occurrence of certain events, such as stock splits, stock dividends or our recapitalization. In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate in the distribution of our assets.
If we shall make any dividend or other distribution of assets to holders of its common stock (a “Distribution”), then the exercise price shall be reduced by multiplying such exercise price by a fraction of which (i) the numerator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date minus the value of the Distribution applicable to one share of common stock, and (ii) the denominator shall be the closing bid price of the shares of common stock on the trading day immediately preceding such record date. Additionally, the number of shares underlying the Warrant shall be increased to a number of shares of common stock obtainable immediately prior to the close of business on the record date fixed for determining holders of shares entitled to receive the Distribution multiplied by the reciprocal of the fraction set forth above. Holders of Warrants will have no voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the holders be entitled to receive dividends.
Under the terms of the Warrants, the Company has certain call rights, whereby the Company may, upon 60 days prior written notice to warrantholders or their transferees, call the Warrants at a redemption price equal to $0.01 per share of common stock then purchasable under the Warrants, provided that (i) the common stock underlying the Warrants have been registered on a registration statement for at least 60 days and (ii) the closing sales price of the Company’s common stock equals or exceeds 150% of the Warrant’s exercise price for at least five (5) consecutive trading days.
Registration Rights with Respect to Common Stock Underlying the Warrants
We have agreed to register an amount of stock equal to 100% of the shares of common stock issuable in connection with the Warrants on a registration statement to be filed by us. We shall pay the usual costs of such registration.
69
No holder of any of our currently outstanding securities has any registration rights with respect to the securities held by them. We shall not file any other registration statement for any of our securities (other than the shares of Common Stock underlying the warrants) until such time as the Registration Statement has been filed and declared effective; provided, however, we may, subject to stockholder approval, establish an equity performance or stock option plan for the benefit of our employees and directors for up to 5% of the outstanding shares of our common stock and file a registration statement to register such shares on Form S-8 or a comparable form for such purpose.
Our Transfer Agent
TranShare Corporation, 5105 DTC Parkway, Suite 325, Greenwood Village, CO 80111-2608 Tel: 303-662-1112.
W.L. MacDonald Law Corporation, located at Suite 1210, 777 Hornby Street, Vancouver, B.C. V6Z 1S4, Canada, is passing on the validity of the issuance of the common stock offered under this prospectus.
Our financial statements as of and for the years ended December 31, 2009 and 2008, included in this prospectus, have been audited by Acquavella, Chiarelli, Shuster, Berkower & Co. LLP, Certified Public Accountants and Advisors, our independent registered public accountants, as stated in their report appearing herein and are so included herein in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
Our financial statements as of and for the years ended May 31, 2009 and 2008, included in this registration statement, have been audited by Schumacher & Associates, Inc., our former independent registered public accountants, as stated in their report appearing herein and are so included herein in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
No expert or counsel named in this registration statement as having prepared or certified any part of this statement or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the common stock was employed on a contingency basis, or had, or will receive, in connection with the offering, a substantial interest, direct or indirect, in us. Nor was any such person connected with us as a promoter, managing or principal underwriter, voting trustee, director, officer, or employee.
We know of no material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
Not Applicable.
The General Corporation Law of Nevada provides that directors, officers, employees or agents of Nevada corporations are entitled, under certain circumstances, to be indemnified against expenses (including attorneys’ fees) and other liabilities actually and reasonably incurred by them in connection with any suit brought against them in their capacity as a director, officer, employee or agent, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. This statute provides that directors, officers, employees and agents may also be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by them in connection with a derivative suit brought against them in their capacity as a director, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification may be made without court approval if such person was adjudged liable to the corporation.
Our Articles of Incorporation and By-Laws provide that we will indemnify our directors and officers from all liabilities incurred by them in connection with any action, suit or proceeding in which they are involved by reason of their acting as our directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
70
In accordance with the Securities Act of 1933, we filed with the SEC a registration statement on Form S-1 covering the securities in this offering. As permitted by rules and regulations of the SEC, this prospectus does not contain all of the information in the registration statement. For further information regarding both our company and the securities in this offering, we refer you to the registration statement, including all exhibits and schedules, which you may inspect without charge at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549 on official business days during the hours of 10:00 a.m. to 3:00 p.m. You may obtain information on the operation of the Public Reference room by calling the Commission at 1-800-SEC-0330. The Commission maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Commission. The address of this Internet site is ( http://www.sec.gov ). We expect to be subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, and in accordance with the Securities Exchange Act of 1934, we expect to file annual, quarterly and special reports, and other information with the SEC. These periodic reports and other information will be available for inspection and copying at the public reference facilities and website of the SEC referred to above. You also may request a copy of the registration statement and these filings by writing or calling us at 9th Floor, No. 29 Nanxin Street, Xi’an, Shaanxi Province P.R.C., 710004, telephone: 86 29 8727 1818.
71
INDEX TO FINANCIAL STATEMENTS
|
Audited Financial Statements of China Pediatric Pharmaceuticals, Inc. for the Years ended December 31, 2009 and December 31, 2008
|
||
|
i.
|
Report of Independent Registered Public Accounting Firm
|
F-1
|
|
ii.
|
Balance Sheets as of December 31, 2009 and 2008
|
F-2
|
|
iii.
|
Statements of Income and Comprehensive Income for the Years ended December 31, 2009 and 2008
|
F-3
|
|
iv.
|
Statements of Cash Flows for the Years Ended December 31, 2009 and 2008
|
F-4
|
|
v.
|
Statements of Changes in Stockholders' Equity for the Years ended December 31, 2009 and 2008
|
F-5
|
|
vi.
|
Notes to Financial Statements
|
F-6
|
|
Pro Forma Financial Information
|
F-21
|
|
|
Unaudited Financial Statements of China Pediatric Pharmaceuticals, Inc. for the Three and Six Months Ended June 30, 2010 and June 30, 2009
|
||
|
i.
|
Balance Sheets as of June 30, 2010 (Unaudited) and December 31, 2009
|
F-23
|
|
ii.
|
Statements of Income and Comprehensive Income for the Three and Six Months Ended June 30, 2010 and 2009
|
F-24
|
|
iii.
|
Statements of Cash Flows for the Six Months Ended June 30, 2010 and 2009
|
F-25
|
|
iv.
|
Statements of Changes in Stockholders' Equity for the Six Months Ended June 30, 2010 and 2009
|
F-26
|
|
v.
|
Notes to Financial Statements
|
F-27
|
72
|
517 Route one
|
1 Penn Plaza
|
|
Iselin, New Jersey, 08830
|
36the Floor
|
|
732.855.9600
|
New York, NY 10119
|
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
China Pediatric Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheet of China Pediatric Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the related consolidated statement of income, stockholders’ equity and comprehensive income, and cash flow for the years ended December 31, 2009 and 2008. China Pediatric Pharmaceuticals, Inc.’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that out audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of China Pediatric Pharmaceuticals, Inc. as of December 31, 2009 and 2008, and the consolidated results of its operation and its cash flow for the year ended December 31, 2009 and 2008 in conformity with accounting principles generally accepted in the United States of America.
/s/ Acquavella, Chiarelli, Shuster, Berkower & Co., LLP
Certified Public Accountants
New York, N.Y.
March 30, 2010
F-1
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2009 AND 2008
|
12/31/2009
(Audited)
|
12/31/2008
(Audited)
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
905,874
|
$
|
813,252
|
||||
|
Accounts receivable, net
|
5,666,307
|
3,286,863
|
||||||
|
Other receivable
|
43,305
|
41,578
|
||||||
|
Inventory
|
886,082
|
543,879
|
||||||
|
Prepaid expenses
|
2,487,493
|
2,935,404
|
||||||
|
Total Current Assets
|
9,989,061
|
7,620,976
|
||||||
|
Long-term prepaid expense
|
1,755,104
|
-
|
||||||
|
Property & equipment, net
|
797,790
|
900,857
|
||||||
|
Goodwill
|
612,745
|
612,745
|
||||||
|
Intangible Assets
|
1,856,718
|
2,189,375
|
||||||
|
Total Assets
|
$
|
15,011,418
|
$
|
11,323,953
|
||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
$
|
254,097
|
$
|
451,182
|
||||
|
Accrued expenses and other payables
|
1,592,440
|
1,490,671
|
||||||
|
Trade deposit received
|
6,308
|
-
|
||||||
|
Short-term bank loan
|
438,776
|
5,936
|
||||||
|
VAT tax payable
|
321,521
|
147,612
|
||||||
|
Income tax payable
|
194,386
|
213,228
|
||||||
|
Total Current Liabilities
|
2,807,528
|
2,308,629
|
||||||
|
Stockholders' Equity
|
||||||||
|
Common stock, $ 0.001 per value, 75,000,000 shares authorized, 8,305,171 and 8,228,571 shares issued and outstanding at December 31, 2009 and 2008
|
8,305
|
8,229
|
||||||
|
Additional paid in capital
|
2,135,883
|
1,478,134
|
||||||
|
Statutory reserve
|
810,540
|
721,553
|
||||||
|
Other comprehensive income
|
328,567
|
313,429
|
||||||
|
Retained earnings
|
8,920,595
|
6,493,979
|
||||||
|
Total Stockholders' Equity
|
12,203,890
|
9,015,324
|
||||||
|
Total Liabilities and Stockholders' Equity
|
$
|
15,011,418
|
$
|
11,323,953
|
||||
The accompanying notes are an integral part of these financial statements.
F-2
|
Year Ended
12/31/2009
|
Year Ended
12/31/2008
|
|||||||
|
Sales, net
|
$
|
16,738,459
|
$
|
14,668,8799
|
||||
|
Cost of sales
|
6,999,152
|
5,083,552
|
||||||
|
Gross profit
|
9,739,307
|
9,585,327
|
||||||
|
Selling, general and administrative expense
|
6,515,641
|
6,544,265
|
||||||
|
Income from operations
|
3,223,666
|
3,041,062
|
||||||
|
Interest income
|
61
|
34,827
|
||||||
|
Interest expenses
|
(22,073
|
)
|
-
|
|||||
|
Other income
|
2,609
|
113
|
||||||
|
Other expense
|
(71,967
|
)
|
(208
|
)
|
||||
|
Total Other Income (Expense)
|
(91,370
|
)
|
34,732
|
|||||
|
Income before income taxes
|
3,132,296
|
3,075,794
|
||||||
|
Provision for income taxes
|
616,693
|
484,024
|
||||||
|
Net income
|
$
|
2,515,603
|
$
|
2,591,770
|
||||
|
Net income for common share
|
||||||||
|
Earnings per share – Basic
|
$
|
0.31
|
$
|
0.31
|
||||
|
Earnings per share – Diluted
|
$
|
0.29
|
$
|
0.31
|
||||
|
Weighted average common shares outstanding
|
||||||||
|
Basic
|
8,247,669
|
8,288,571
|
||||||
|
Diluted
|
8,583,034
|
8,288,571
|
||||||
|
Net income
|
2,531,663
|
2,591,770
|
||||||
|
Other comprehensive income (loss)
|
15,138
|
188,741
|
||||||
|
Comprehensive income (loss)
|
2,546,801
|
2,780,511
|
||||||
The accompanying notes are an integral part of these financial statements.
F-3
CONSOLDIATED STATEMENTS OF CASH FLOWS
(AUDITED)
|
Year Ended
|
Year Ended
|
|||||||
|
12/31/2009
|
12/31/2008
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net Income
|
$
|
2,515,603
|
$
|
2,591,770
|
||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
provided by operating activities:
|
||||||||
|
Depreciation
|
103,067
|
112,009
|
||||||
|
Amortization of intangible assets
|
332,657
|
326,858
|
||||||
|
Obsolescence reserve
|
-
|
161,440
|
||||||
|
Stock-based compensation
|
657,811
|
-
|
||||||
|
(Increase) / decrease in assets:
|
||||||||
|
Accounts receivables
|
(2,369,996
|
)
|
(628,589
|
)
|
||||
|
Inventory
|
(340,667
|
)
|
(282,510
|
)
|
||||
|
Prepaid expense
|
(1,299,196
|
)
|
(1,348,456
|
)
|
||||
|
Other receivable
|
(151,456
|
)
|
(24,208
|
)
|
||||
|
Increase / (decrease) in current liabilities:
|
||||||||
|
Accounts payable
|
(198,100
|
)
|
11,184
|
|||||
|
Accrued expenses and other payables
|
98,010
|
(731,702
|
)
|
|||||
|
Due to related party
|
-
|
(57,453
|
)
|
|||||
|
Trade deposits received
|
-
|
-
|
||||||
|
Value-added tax payable
|
173,448
|
(98,808
|
)
|
|||||
|
Income tax payable
|
(19,361
|
)
|
209,919
|
|||||
|
Net cash provided by operating activities
|
(498,180
|
)
|
241,454
|
|||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Acquisitions fixed assets
|
-
|
(10,376
|
)
|
|||||
|
Acquisitions of intangible assets
|
-
|
-
|
||||||
|
Net cash used by investing activities
|
-
|
(10,376
|
)
|
|||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Proceeds from bank loans
|
438,540
|
-
|
||||||
|
Capital contribution
|
13
|
-
|
||||||
|
Net cash provided by financing activities
|
438,553
|
-
|
||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
152,249
|
40,024
|
||||||
|
Net change in cash and cash equivalents
|
92,622
|
271,102
|
||||||
|
Cash and cash equivalents, beginning balance
|
813,252
|
542,150
|
||||||
|
Cash and cash equivalents, ending balance
|
$
|
905,874
|
$
|
813,252
|
||||
|
SUPPLEMENTAL DISCLOSURES:
|
||||||||
|
Cash paid during the year for:
|
||||||||
|
Income tax payments
|
$
|
636,054
|
$
|
274,106
|
||||
|
Interest payments
|
$
|
22,073
|
$
|
-
|
||||
The accompanying notes are an integral part of these financial statements.
F-4
CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
(AUDITED)
|
Additional
|
Other
|
Total
|
||||||||||||||||||||||||||
|
Capital Stock
|
Paid-in
|
Comprehensive
|
Statutory
|
Retained
|
Stockholders
|
|||||||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Income
|
Reserves
|
Earnings
|
Equity
|
||||||||||||||||||||||
|
Balance December 31, 2007
|
8,228,571
|
$
|
8,229
|
$
|
1,478,134
|
$
|
124,688
|
$
|
462,376
|
$
|
4,161,386
|
$
|
6,234,813
|
|||||||||||||||
|
-
|
||||||||||||||||||||||||||||
|
Foreign currency translation adjustments
|
188,741
|
188,741
|
||||||||||||||||||||||||||
|
Transferred to Statutory reserve
|
259,177
|
(259,177
|
)
|
-
|
||||||||||||||||||||||||
|
Income for the year ended December 31, 2008
|
2,591,770
|
2,591,770
|
||||||||||||||||||||||||||
|
Balance December 31, 2008
|
8,228,571
|
$
|
8,229
|
1,478,134
|
313,429
|
721,553
|
6,493,979
|
9,015,324
|
||||||||||||||||||||
|
Foreign currency translation adjustments
|
15,138
|
15,138
|
||||||||||||||||||||||||||
|
Transferred to Statutory reserve
|
88,987
|
(88,987
|
)
|
-
|
||||||||||||||||||||||||
|
Stock-based compensation
|
76,600
|
76
|
16,011
|
16,087
|
||||||||||||||||||||||||
|
Stock-based compensation - warrants
|
641,725
|
641,725
|
||||||||||||||||||||||||||
|
Recapitalization
|
13
|
13
|
||||||||||||||||||||||||||
|
Income for the year ended December 31, 2009
|
2,515,603
|
2,515,603
|
||||||||||||||||||||||||||
|
Balance December 31, 2009
|
8,305,171
|
$
|
8,305
|
$
|
2,135,883
|
$
|
328,567
|
$
|
810,540
|
$
|
8,920,595
|
$
|
12,203,890
|
|||||||||||||||
The accompanying notes are an integral part of these financial statements
F-5
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 1 – ORGANIZATION
China Pediatric Pharmaceuticals, Inc. was incorporated on April 20, 2005 in the state of Nevada. The Company was originally incorporated under the name Belford Enterprises, Inc. Asia-Pham holding Inc. (“Asia-Pham”) was incorporated in British Virgin Islands on June 20, 2008. China Children Pharmaceuticals Co., Ltd. (“China Children”), a wholly owned subsidiary of Asia-Pham Holding Inc. was formed on June 27, 2008 under the laws of Hong Kong. China Children formed Coova Children Pharmaceuticals Co., Ltd,)”Coova” or the “WOFE”) was formed on July 25, 2008. Cove is a wholly owned subsidiary of China Children Pharmaceuticals Co., Ltd.
On September 20, 2009, the Company acquired China Children in accordance with a Share Exchange Agreement. Pursuant to the terms of the Exchange Agreement, the Company acquired all of the outstanding Capital stock and ownership interest of China Children from Asia-Pham.
On August 4, 2008, Coova Children Pharmaceuticals Co., Ltd, entered into a Management Entrustment Agreement, with Shaanxi Jiali Pharmaceuticals Co., Ltd. ("Jiali") and its shareholders (the “Transaction”). Shaanxi Jiali Pharmaceuticals Co., Ltd. is a corporation formed under the laws of the PRC. According to this Agreement, Coova acquired management control of Jiali whereby Cove is entitled to all of the net profits of Jiali, as a management fee, and is obligated to fund Jiali operations and pay all of the debts.
The contractual arrangements completed on August 4, 2008 provide that Coova has controlling interest in Jiali as defined by FASB Interpretation No. 46R, “Consolidation of Variable Interest Entities” (“FIN 46R”), an Interpretation of Accounting Research Bulletin No. 51, which requires Coova to consolidate the financial statements of Jiali and ultimately consolidate with its parent company, China Children Pharmaceuticals Co., Ltd.
The Share Exchange was accounted for as a “reverse merger” since the stockholders of China Children own a majority of the outstanding shares of China Pediatric's common stock immediately following the Share Exchange. Under accounting principles generally accepted in the United States, the share exchange is considered to be a capital transaction in substance, rather than a business combination. Thus the share exchange is equivalent to the issuance of stock by China Children for the net monetary assets of China Pediatric, accompanied by a recapitalization, and is accounted for as a change in capital structure. Accordingly, the accounting for the share exchange was identical to that resulting from a reverse acquisition, except no goodwill was recorded. Under reverse takeover accounting, the post reverse acquisition comparative historical financial statements of the legal acquirer, China Pediatric, are those of the legal acquiree, China Children, which is considered to be the accounting acquirer, and thus represent a continuation of the financial statements of China Children. Share and per share amounts stated have been retroactively adjusted to reflect the merger. The accompanying financial statements present the historical financial condition, results of operations and cash flows of the operating company prior to the recapitalization.
The Company, through its subsidiary, and exclusive contractual arrangement with Shaanxi Jiali Pharmaceuticals Co., Ltd., is engaged in the business of manufacturing and marketing over-the-counter (“OTC”) and prescription pharmaceutical products for the Chinese marketplace as treatment for a variety of diseases and conditions.
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The Company's functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in United States Dollars.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted the Consolidation Topic of the FASB Accounting Standards Codification (“ASC 810”) which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
F-6
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
In determining Shaanxi Jiali Pharmaceutical Co. Ltd. is the VIE of Coova Children Pharmaceuticals Co., Ltd., the Company considered the following indicators, among others:
|
●
|
Coova has the full right to control and administrate the financial affairs and daily operation of Jiali and has the right to manage and control all assets of Jiali. The equity holders of Jiali as a group have no right to make any decision about Jiali’s activities without the consent of Coova.
|
|
●
|
Coova was assigned all voting rights of Jiali and has the right to appoint all directors and senior management personnel of Jiali. The equity holders of Jiali possess no substantive voting rights.
|
|
●
|
Coova was assigned all voting rights of Jiali and has the right to appoint all directors and senior management personnel of Jiali. The equity holders of Jiali possess no substantive voting rights.
|
|
●
|
Coova will provide financial support if Jiali requires additional funds to maintain its operations and to repay its debts.
|
|
●
|
Coova should be paid a management fee equal to Jiali’s earnings before taxes. If there are no earnings before taxes and other cash expenses, then no fee shall be paid. If Jiali sustains losses, they will be carried over to the next period and deducted from the next management fee. Coova should assume all operation risks of Jiali and bear all losses of Jiali. Therefore, Coova is the primary beneficiary of Jiali.
|
Translation Adjustment
As of December 31, 2009 and 2008, the accounts of the Company were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (“CNY”). Such financial statements were translated into U.S. Dollars (“USD”) in accordance with the Foreign Currency Matters Topic of the FASB Accounting Standards Codification (“ASC 830”), with the CNY as the functional currency. According to ASC 830, all assets and liabilities were translated at the current exchange rate, stockholders’ equity is translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”), as a component of shareholders’ equity. Transaction gains and losses are reflected in the income statement.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
F-7
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Risks and Uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC’s economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company is subject to substantial risks from, among other things, intense competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves are recorded primarily on a specific identification basis. These are no allowances for doubtful accounts as of December 31, 2009 and 2008.
Prepaid Expenses
Prepaid account is primarily comprised of two factors: advance payments suppliers for goods purchased, and advance payments to R&D organizations for new medicines to be developed and purchased.
In December 2007, the Company signed a Medicine Research and Development Agreement with Shaanxi Research Institution of Chinese Traditional Medicine (SRICTM). Pursuant to the terms of the agreement and Supplemental Agreement between the Company and SRICTM, SRICTM must successfully develop and obtain governmental approval for production of five new pediatric medicines, otherwise, the Company is entitled to a full refund of fees. Therefore, the costs paid in connection with these services were not classified as research and development costs, but rather as prepaid expenses. Such advance payments will not be expensed if we do not receive the desired results from SRICTM.
A table of the outstanding prepaid expenses for the fiscal year ended December 31, 2009 is as follows:
F-8
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED )
|
Payee
|
Nature
|
12/31/2009
|
|||||
|
Advance payment to R&D organization
|
Shaanxi Research Institution of Chinese Traditional Medicine
|
Advanced payment to Shanxi Research Institution of Chinese Traditional Medicine for five new pediatric cold medicines
|
$
|
1,755,104
|
|||
|
Advance payment to suppliers
|
Suppliers
|
Advance payments suppliers for goods purchased
|
2,487,493
|
||||
|
Total
|
$
|
4,242,597
|
|||||
Advance payments to suppliers include the following:
|
Classification
|
Payee
|
($)
Period Ended 31
March, 2010
|
Nature
|
|
Advertising fee
|
Xi’an Xinshengdao Chuangyi Chuanbo Co., Ltd
|
146,282.25
|
In order to increase sales, the Company requires the service of the advertising companies and in this respect has incurred certain prepaid costs. These costs will be consumed within the FY 2010 accounting cycle and recorded as an expense.
|
|
Xi’an Hanneng brand management Co., Ltd
|
146,282.25
|
||
|
Consigned processing fee
|
Xi’an KeLi Pharmaceuticals Co. Ltd.
|
146,282.25
|
These represent advance payments to OEM companies for the consigned processing of certain products that are currently solds. According to the the terms of our agreements with these parties, we are required to pay a certain amount of money when signing the consignment processing agreement to ensure performance of the agreement.
These costs will be consumed within the FY 2010 accounting cycle and recorded as an expense.
|
|
Jiyuan Taiji Medical Supplies Co. Ltd.
|
146,282.25
|
||
|
Hubei Kangerjian biotechnology Co. Ltd
|
146,282.25
|
||
|
Taiji Group Sichuan Nanchong Pharmaceutical Factory
|
117,025.80
|
||
|
Fosham City Yichuang Biochemistry Science and Technology Co. Ltd
|
133,116.85
|
||
|
Ha’erbin Pediatric Pharmaceuticals Co.Ltd
|
149,207.89
|
||
|
Purchasing traditional Chinese medicinal materials
|
Baoji Meixian lvyuan Chinese Herbal Medicine Planting and R&D Co., Ltd
|
182,852.81
|
These advance payments were made to purchase certain raw materials and packaging materials to be used in the manufacture of products currently sold. According to the terms of our agreements with these suppliers, we are required to pay a certain amount of the purchase price when signing the purchasing agreement and when the other party commences to provide raw materials and packaging materials. These costs will be consumed within the FY 2010 accounting cycle and recorded as an expense.
|
|
Xi’an Kangfu Yaocai Company
|
111,174.51
|
||
|
Baoji Second TCM wholesale Department
|
46,810.32
|
||
|
Shaanxi North medical purchase and supply station
|
77,529.59
|
||
|
Baoji White Wine Yinpian Factory
|
99,471.93
|
||
|
Baoji TCM service department processing
|
76,066.77
|
||
|
Purchasing conventional medicinal materials
|
Xi’an Acetar Bio-Tech Inc.
|
111,174.51
|
|
|
Xi’an Ruihua Pharmaceuticals, Co.,Ltd
|
51,198.79
|
||
|
Purchasing excipients/nonactive ingredients
|
Shaanxi Aokesen Laboratory Co., Ltd
|
138,968.14
|
|
|
Shaanxi Qiangli Keji Co., Ltd
|
95,083.46
|
||
|
Baoji Weixin Tangye Wholesale Shop
|
160,910.47
|
||
|
Baoji Daming Alcohol Co.,Ltd
|
114,100.15
|
||
|
Xi’an Baoli Meterial printing and packaging trade Co.,Ltd
|
21,942.34
|
||
|
Purchasing packaging materials
|
Xianyang Wenhui Printing Co., Ltd
|
117,025.80
|
|
|
Chengcang Qu Jieli carton Plant
|
62,901.37
|
||
|
Shaanci Huangyi Keji Printing Co.,Ltd
|
80,455.24
|
||
|
Xi’an Jiecheng Package Co.,Ltd
|
36,570.56
|
||
|
Xi’an Jiuxing Printing Package Co.,Ltd
|
89,232.17
|
||
|
Xi’an Qingsong Printing Co.,Ltd
|
92,157.82
|
||
|
Purchasing other materials
|
|
30351.47
|
|
|
|
Total:
|
2,926,740.01
|
|
F-9
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED )
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market. The Management compares the cost of inventories with the market value and allowance is made for writing down their inventories to market value, if lower. As of December 31, 2009 and 2008, inventories consist of the following:
|
12/31/2009
|
12/31/2008
|
|||||||
|
Raw materials
|
$
|
1,162,963
|
$
|
406,666
|
||||
|
Finished goods
|
214,335
|
301,199
|
||||||
|
Provision for impairment loss on inventory
|
(491,216
|
)
|
(163,986
|
)
|
||||
|
Total
|
$
|
886,082
|
$
|
543,879
|
||||
F-10
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED )
As of December 31, 2009 and 2008, $491,216 and $163,986, respectively, represent obsolete inventory.
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings
|
30 years
|
|
Plant and equipment
|
5-14 years
|
|
Transportation equipment
|
5-10 years
|
|
Office equipment
|
5-10 years
|
As of December 31, 2009 and 2008, Property, Plant & Equipment consist of the following:
|
12/31/2009
|
12/31/2008
|
|||||||
|
Buildings
|
$
|
445,845
|
$
|
445,845
|
||||
|
Plant and equipment
|
859,665
|
859,665
|
||||||
|
Transportation equipment
|
5,609
|
5,609
|
||||||
|
Office equipment
|
40,354
|
40,354
|
||||||
|
Total
|
1,351,473
|
1,351,473
|
||||||
|
Accumulated depreciation
|
(553,683
|
)
|
(450,616
|
)
|
||||
|
$
|
797,790
|
$ |
900,857
|
|||||
Depreciation expense for the years ended December 31, 2009 and 2008 were $103,067 and $112,009 respectively.
F-11
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Goodwill
Goodwill represents the excess cost of a business acquisition over the fair value of the net assets acquired. In accordance with the Intangibles, Goodwill and other topic of the FASB Accounting Standard Codification (“ASC 350”), indefinite-life identifiable intangible assets and goodwill are not amortized. Under the provisions of ASC 350, we are required to perform an annual impairment test of our goodwill. Goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit, which we define as our business segments, with its net book value or carrying amount including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test compares the implied fair value of the reporting unit's goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit's goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. The fair value of the reporting unit is allocated to all of the assets and liabilities of that unit including any unrecognized intangible assets as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit. Goodwill as of December 31, 2009 and 2008 are $612,745 and $612,745.
Intangible Assets
The Company has four proprietary technologies: propriety technology for antioxidant technique, proprietary technology for “liren” capsule, patent-Chinese medicine and production method for skin and gyhecology disease and patent Chinese medicine and production method for tracheitis. Proprietary technology for antioxidant technique was contributed by a shareholder in exchange for shares of the Company’s common stock. Proprietary technology for “liren” capsule was purchased from third party at the price agreed by the Company and the third party. Two patents were purchased from the shareholders at the prices determined by an independent appraiser. These proprietary technologies were acquired for the future use of the Company. In accordance with ASC 730-10-25-2c, we capitalized these four proprietary technologiesas intangible assets as acquired.
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from ten to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No impairments of intangible assets have been identified during any of the periods presented. The land use rights will expire in 2056 and 2058. All of the Company’s intangible assets are subject to amortization with estimated lives of:
|
Land use right
|
50 years
|
|
Proprietary technologies
|
10 years
|
The components of finite-lived intangible assets are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2009
|
2008
|
|||||||
|
Land use right
|
$
|
264,625
|
$
|
264,625
|
||||
|
Proprietary technologies
|
2,966,355
|
2,966,355
|
||||||
|
3,230,980
|
3,230,980
|
|||||||
|
Less: Accumulated amortization
|
(1,374,262)
|
(1,041,605)
|
||||||
|
$
|
1,856,718
|
$
|
2,189,375
|
|||||
Amortization expense for the years ended December 31, 2009 and 2008 were $332,657 and $326,858, respectively.
F-12
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
The estimated future amortization expenses related to intangible asset as of December 31, 2009 are as follows:
|
2010
|
332,657
|
|||
|
2011
|
332,657
|
|||
|
2012
|
332,657
|
|||
|
2013
|
332,657
|
|||
|
2014
|
332,657
|
|||
|
Thereafter
|
$
|
193,433
|
Long-Lived Assets
Effective January 1, 2002, the Company adopted the Property, Plant and Equipment Topic of the FASB Accounting Standard Codification (“ASC 360”), which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of,” and the accounting and reporting provisions of Accounting Principles Board Opinion No. 30, “Reporting the Results of Operations for a Disposal of a Segment of a Business.” The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with ASC 360, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on its review, the Company believes that, as of December 31, 2009 and 2008, there were no significant impairments of its long-lived assets.
Fair Value of Financial Instruments
The Financial Instrument Topic of the FASB Accounting Standards Codification (“ASC 825”) requires that the Company disclose estimated fair values of financial instruments. The carrying amounts reported in the statements of financial position for current assets and current liabilities qualifying as financial instruments are a reasonable estimate of fair value.
Revenue Recognition
The Company’s revenue recognition policies are in compliance with the Revenue Recognition Topic of the FASB Accounting Standards Codification (“ASC 605”). We recognize revenue at the date of shipment to customers as the sales price is either fixed or determinable at the date of shipment to customers, and the management deems no other significant obligations such as warranties or product returns of the Company exist. The Company signs general Annual Sales Cooperation Agreements with its distributors each year and also signs specific purchase orders for each sale. All sales proceeds can be collected reasonably.
F-13
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Customer rebates
Rebates are paid to customers every quarter and we recorded customer rebates as customers earned. They are classified as a reduction of revenue according to ASC 605-55-64. As of December 31, 2009 and 2008, rebates the Company paid to customers were $1,951,584 and $1,690,461, respectively.
Advertising
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company expenses all advertising costs as incurred.
Income Taxes
The Company utilizes SFAS No. 109, “Accounting for Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In July 2006, the FASB issued Interpretation No. 48 (FIN 48), “Accounting for uncertainty in Income Taxes.” FIN 48 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, “Accounting for Contingencies. ” FIN 48 is effective for fiscal years beginning after December 15, 2006. As a result of implementing FIN 48, there have been no adjustments to the Company’s financial statements. SFAS No. 109 and FIN 48 were superseded by the Income Taxes Topic of the FASB Accounting Standards Codification (“ ASC 740”) in September 2009.
Statement of Cash Flows
In accordance with the Statement of Cash Flows Topic of the FASB Accounting Standards Codification (“ASC 230”), cash flows from the Company’s operations are based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited.
F-14
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
|
●
|
FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”).
|
|
●
|
FASB Staff Positions FAS No. 115-2, FAS 124-2, and EITF No. 99-20-2, Recognition and Presentation of Other-Than-Temporary Impairments , (“FSP FAS No. 115-2, FAS No. 124-2, and EITF No. 99-20-2”) provides additional guidance to provide greater clarity about the credit and noncredit component of an other-than-temporary impairment event and to more effectively communicate when an other-than-temporary impairment event has occurred. This FSP applies to debt securities. FSP FAS No. 115-2 and FAS No. 124-2 were superseded by the Investments-Debt and Equity Securities Topic of the FASB Accounting Standards Codification (“ASC 320”).
|
|
●
|
FASB Staff Position FAS No. 107-1 and APB No. 28-1, Interim Disclosures about Fair Value of Financial Instruments , (“FSP FAS No. 107-1 and APB No. 28-1”) amends FASB Statement No. 107, Disclosures about Fair Value of Financial Instruments , to require disclosures about fair value of financial instruments in interim as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting , to require those disclosures in all interim financial statements. FSP FAS No. 107-1 and APB No. 28-1were superseded by the Financial Instruments Topic of the FASB Accounting Standards Codification (“ASC 825”).
|
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The
F-15
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value , which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued ASU No. 2009-12, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent) , that amends ASC 820 to provide guidance on measuring the fair value of certain alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force , that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. ASU No. 2009-13 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
In October 2009, the FASB issued ASU No. 2009-14, Certain Revenue Arrangements That Include Software Elements—a consensus of the FASB Emerging Issues Task Force , that reduces the types of
F-16
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
transactions that fall within the current scope of software revenue recognition guidance. Existing software revenue recognition guidance requires that its provisions be applied to an entire arrangement when the sale of any products or services containing or utilizing software when the software is considered more than incidental to the product or service. As a result of the amendments included in ASU No. 2009-14, many tangible products and services that rely on software will be accounted for under the multiple-element arrangements revenue recognition guidance rather than under the software revenue recognition guidance. Under the ASU, the following components would be excluded from the scope of software revenue recognition guidance: the tangible element of the product, software products bundled with tangible products where the software components and non-software components function together to deliver the product’s essential functionality, and undelivered components that relate to software that is essential to the tangible product’s functionality. The ASU also provides guidance on how to allocate transaction consideration when an arrangement contains both deliverables within the scope of software revenue guidance (software deliverables) and deliverables not within the scope of that guidance (non-software deliverables). ASU No. 2009-14 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
Note 3 – OTHER RECEIVABLES
Other receivables mainly consist of cash advances to employees. As of December 31, 2009 and 2008, other receivables were $ 43,305 and $41,578, respectively.
Note 4 – COMPENSATED ABSENCES
Regulation 45 of the local labor law of the People’s Republic of China (“PRC”) entitles employees to annual vacation leave after 1 year of service. In general, all leave must be utilized annually, with proper notification. Any unutilized leave is cancelled.
Note 5 – SHORT-TERM BANK LOAN
As of December 31, 2009, the Company had debt as follows:
|
Amount
|
Interest rate
|
Due
|
||||
|
Short tem bank loan
|
$
|
438,776
|
0.85845% /Per month
|
June 25, 2010
|
||
The Company is using these loans for working capital purposes and secured by property right.
F-17
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 6 – INCOME TAXES
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax (“EIT”) through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high tech enterprise and under PRC Income Tax Laws, it is entitled to a two-year exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a discounted rate of 15%.
|
The following is a reconciliation of income tax expense:
|
||||||||
|
12/31/2009
|
International
|
Total
|
||||||
|
Current
|
$
|
616,693
|
$
|
616,693
|
||||
|
Deferred
|
-
|
-
|
||||||
|
Total
|
$
|
616,693
|
$
|
616,693
|
||||
|
12/31/2008
|
International
|
Total
|
||||||
|
Current
|
$
|
484,024
|
$
|
484,024
|
||||
|
Deferred
|
-
|
|||||||
|
Total
|
$
|
484,024
|
$
|
484,024
|
||||
Note 7 – COMMITMENTS & CONTINGENCIES
The Company leases facilities under operating leases that it pays for on an annual basis and accrues for throughout the year. For the years ended December 31, 2009 and 2008, rent expenses were $ 795 and $25,515, respectively.
The Company is also committed to pay $ 1,523,196 for advertising thru October 2010. The Company is also committed to pay a total of $2,925,174 under an agreement for services rendered in connection with production approval on 5 new products. Unpaid portion of commitment as of December 31, 2009 is $1,170,070.
Note 8 – COMMON STOCK, STOCK OPTION AND COMPENSATION
On October 2, 2009 the Company granted 76,600 shares of common stock to employees for services rendered having a fair market value on that date of $16,087 or $.21 per share, which was the closing price of our common stock on that date.
Warrants-- On August 15, 2009, the Company signed Warrant Agreements with consultants, investor relationship service provider and referral. Pursuant to the Warrant Agreements, the Company will issue warrants grant to purchase 1,250,000 shares of common stock at $ 3.00 exercise price with piggy-back warrants to purchase 1,250,000 shares of common stock at $ 5.00 exercise price. The warrants were issuable at the closing of the merger and are discussed in further detail below.
On August 15, 2009, Kang Xiulan recommended a public shell company(Lid Hair Studios International, Inc.)to China Children Pharmaceutical Inc. China Children Pharmaceutical Inc. came to be quoted on the OTCBB successfully through the reverse merger with Lid Hair Studios International, Inc on September 30, 2009 and changed the name into China Pediatric Pharmaceuticals Inc. As consideration for the services provided by Kang Xiulan (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and Kang Xiulan), The Company agreed to issue to Kang Xiulan warrants to acquire 250,000 shares of the Company’s common stock with an exercise price of $3, 00 with piggyback warrants to purchase 250,000 shares of the Company’s common stock with an exercise price of $5, 00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
During the above-mentioned reverse merger process, the Company obtained certain consulting services from IFG Investments Services, Inc., including advising on a merger/acquisition transaction and regulatory filings, and other services and support as requested from IFG. In consideration for the consulting services to be performed by IFG (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and IFG), The Company agreed to issue to IFG warrants to acquire 600,000 shares of the Company’s common stock with an exercise price of $3, 00 with piggyback warrants to purchase 600,000 shares of the Company’s comment stock with an exercise price of $5,00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
Company also obtained certain public company sector services, including certain investor relations advisory services. In consideration for the investor relation services provided by China National Information Network (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and China National Information Network), the Company agreed to issue to China National Information Network the warrants to acquire 400,000 shares of the Company’s common stock with an exercise price of $3,00 with piggyback warrants to purchase 400,000 shares of the Company’s comment stock with an exercise price of $5,00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
Based on the fair value method under ASC Topic 505, the fair value of each warrant granted is estimated on the date of the grant using the Black-Scholes option pricing model and is recognized as compensation expense over the service period of each warrants issued. The Black-Scholes option pricing model has assumptions for risk free interest rates, dividends, stock volatility and expected life of an option grant. The risk free interest rate is based upon market yields for US Treasury debt securities at a maturity near the term remaining on the warrant. Dividend rates are based on the Company’s dividend history. The stock volatility factor is based on the historical volatility of the similar company’s stock price. The fair value was estimated at the date of grant using the following range of assumptions: average risk-free interest rate – 1.61%; expected life –2-3 years; expected volatility – 105.1%; and expected dividends – nil. No estimate of forfeitures was made as the Company has a short history of granting warrants. The fair market value of warrants, $641,725, has been recorded as stock based compensation cost with a corresponding increase to additional paid-in capital (warrants) for the year ended December 31, 2009.
|
|
Total
|
Exercise Price
|
Remaining Life
|
Aggregate
Intrinsic Value
|
|||||||||
|
Outstanding, December 31, 2008
|
-
|
||||||||||||
|
Granted in 2009
|
2,500,000
|
$
|
3.00-5.00
|
1.75-2.75yrs
|
|||||||||
|
Outstanding, December 31,2009
|
2,500,000
|
|
|||||||||||
Stock options--- The option holder has no voting or dividend rights. The company records the expense of the stock options over the related vesting period. The options were valued using the Black-Scholes option pricing model with the following assumptions: average risk-free interest rate – 1.61%; expected life –2-3 years; expected volatility – 105.1%; and expected dividends – nil. The fair market value of warrants, $ 641,725, has been recorded as stock based compensation cost with a corresponding increase to additional paid-in capital (warrants).
F-18
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 9 – STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of December 31, 2009 and 2008, the Company had allocated $ 810,540 and $721,553, respectively, to these non-distributable reserve funds.
Note 10 – NET INCOME (LOSS) PER SHARE
In accordance with FASB ASC Topic 260-1-50, “Earnings per Share”, and SEC Staff Accounting Bulletin No. 98, basic net income or loss per common share is computed by dividing net income or loss for the period by the weighted - average number of common shares outstanding during the period. Under FASB ASC 260-10-50, diluted income or loss per share is computed by dividing net income or loss for the period by the weighted - average number of common and common equivalent shares, such as stock options, warrants and convertible securities outstanding during the period.
The following table sets forth the computation of basic and diluted earnings per share of common stock:
|
Year ended December 31,
|
||||||||
|
2009
|
2008
|
|||||||
|
Basic earnings from continuing operations per share:
|
||||||||
|
Numerator:
|
||||||||
|
Income from operations used in computing basic earnings per share
|
$
|
2,515,603
|
$
|
2,591,770
|
||||
|
Income from operations applicable to common shareholders
|
$
|
2,515,603
|
$
|
2,591,603
|
||||
|
Denominator:
|
||||||||
|
Weighted average common shares outstanding
|
8,247,669
|
8,228,571
|
||||||
|
Basic earnings per share from continuing operations
|
$
|
0.31
|
$
|
0.31
|
||||
|
Diluted earnings (losses) per share from operations:
|
||||||||
|
Numerator:
|
||||||||
|
Income from operations used in computing diluted earnings per share
|
$
|
2,515,603
|
$
|
2,591,770
|
||||
|
Income (loss) from operations applicable to common shareholders
|
$
|
2,515,603
|
$
|
2,591,770
|
||||
|
Denominator:
|
||||||||
|
Weighted average common shares outstanding
|
8,247,669
|
8,288,571
|
||||||
|
Weighted average effect of dilutive securities:
|
||||||||
|
Stock options and warrants
|
335,365
|
-
|
||||||
|
Shares used in computing diluted net income per share
|
8,583,034
|
8,288,571
|
||||||
|
Diluted earnings per share from operations
|
0.29
|
0.31
|
||||||
F-19
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2009
(AUDITED)
Note 11 – OTHER COMPREHENSIVE INCOME
Balances of related after-tax components comprising accumulated other comprehensive income, included in stockholders’ equity, at of December 31, 2009, are as follows:
|
Foreign Currency Translation Adjustment
|
Accumulated Other Comprehensive Income
|
|||||||
|
Balance at December 31, 2008
|
$
|
313,429
|
$
|
313,429
|
||||
|
Change for 2009
|
15,138
|
15,138
|
||||||
|
Balance at December 31, 2009
|
$
|
328,567
|
$
|
328,567
|
||||
Note 12 – CURRENT VULNERABILITY DUE TO CERTAIN RISK FACTORS
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC's economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Note 13 – MAJOR CUSTOMERS AND CREDIT RISK
Five customers accounted for more than 10% of accounts receivable at December 31, 2009 and 2008 totaling 61% and 91%, respectively; As of December 31, 2009 and 2008, three and five vendors were greater than 10% of accounts payable, totaling 63% and 68%, respectively.
Two and six customers accounted for 23% and 100% of sales for the year ended December 31, 2009 and 2008. Five vendors supplied 84% and 74% of purchases for the years ended December 31, 2009 and 2008, respectively.
F-20
Pro Forma Financial Information
LID HAIR STUDIOS INTERNATIONAL, INC. AND
CHINA CHILDREN PHARMACEUTICALS CO., LTD.
Statements of Operations
As of September 30, 2009
(unaudited)
|
Lid Hair
|
China Children
|
||||||||||||||||||||||||
|
Studios
|
Pharmaceutical
|
Pro Forma Adjustments
|
Pro Forma
|
||||||||||||||||||||||
|
International, Inc.
|
Co., Ltd.
|
Combined
|
Debit
|
Credit
|
Combined
|
||||||||||||||||||||
|
ASSETS
|
|||||||||||||||||||||||||
|
Current assets
|
|||||||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
5,720
|
$
|
2,821,187
|
$
|
2,826,907
|
(a)(c)
|
$
|
-
|
$
|
5,720
|
$
|
2,821,187
|
||||||||||||
|
Accounts receivable, net
|
-
|
3,908,552
|
3,908,552
|
-
|
-
|
3,908,552
|
|||||||||||||||||||
|
Other receivable
|
-
|
8,649
|
8,649
|
-
|
-
|
8,649
|
|||||||||||||||||||
|
Due from related parties
|
-
|
6,684
|
6,684
|
-
|
-
|
6,684
|
|||||||||||||||||||
|
Inventory
|
642
|
1,711,196
|
1,711,838
|
(a)
|
-
|
642
|
1,711,196
|
||||||||||||||||||
|
Prepaid expenses and deposits
|
1,374
|
3,336,038
|
3,337,412
|
(a)
|
-
|
1,374
|
3,336,038
|
||||||||||||||||||
|
-
|
|||||||||||||||||||||||||
|
7,736
|
11,792,306
|
11,800,042
|
-
|
7,736
|
11,792,306
|
||||||||||||||||||||
|
Property and equipment, net
|
18,148
|
819,466
|
837,614
|
(a)
|
-
|
18,148
|
819,466
|
||||||||||||||||||
|
Goodwill
|
-
|
612,745
|
612,745
|
-
|
-
|
612,745
|
|||||||||||||||||||
|
Intangible assets
|
9,092
|
1,939,942
|
1,949,034
|
(a)
|
-
|
9,092
|
1,939,942
|
||||||||||||||||||
|
-
|
|||||||||||||||||||||||||
|
$
|
34,976
|
$
|
15,164,459
|
$
|
15,199,435
|
$
|
-
|
$
|
34,976
|
$
|
15,164,459
|
||||||||||||||
|
Current liabilities
|
|||||||||||||||||||||||||
|
Accounts payable
|
$
|
21,157
|
$
|
431,134
|
$
|
452,291
|
(a)(c)
|
$
|
21,157
|
$
|
-
|
$
|
431,134
|
||||||||||||
|
Accrued expenses and other payables
|
6,250
|
2,381,301
|
2,387,551
|
(c)
|
6250
|
-
|
2,381,301
|
||||||||||||||||||
|
Due to related party
|
51,261
|
121,686
|
172,947
|
(c)
|
51,261
|
-
|
121,686
|
||||||||||||||||||
|
Trade deposit received
|
-
|
6,308
|
6,308
|
-
|
-
|
6,308
|
|||||||||||||||||||
|
Short-term loan
|
438,750
|
438,750
|
438,750
|
||||||||||||||||||||||
|
VAT tax payable
|
-
|
16,592
|
16,592
|
-
|
-
|
16,592
|
|||||||||||||||||||
|
Income tax payable
|
-
|
155,021
|
155,021
|
-
|
-
|
155,021
|
|||||||||||||||||||
|
78,668
|
3,550,792
|
3,629,460
|
78,668
|
-
|
3,550,792
|
||||||||||||||||||||
|
Stockholders’ equity
|
|||||||||||||||||||||||||
|
Capital stock
|
9,300
|
13
|
9,313
|
(a)(b) (c)
|
8,084
|
7000
|
8,229
|
||||||||||||||||||
|
Additional paid in capital
|
227,200
|
1,486,363
|
1,713,563
|
(a)(b) (c)(d)
|
235,416
|
396,152
|
1,874,299
|
||||||||||||||||||
|
Statutory reserve
|
-
|
880,133
|
880,133
|
-
|
-
|
880,133
|
|||||||||||||||||||
|
Other comprehensive income
|
1,225
|
328,368
|
329,593
|
(a)
|
1,225
|
328,368
|
|||||||||||||||||||
|
Retained earnings (deficit)
|
-281,417
|
8,918,790
|
8,637,373
|
(c)(d)
|
396,152
|
281,417
|
8,522,638
|
||||||||||||||||||
|
-43,692
|
11,613,667
|
11,569,975
|
640,877
|
684,569
|
11,613,667
|
||||||||||||||||||||
|
$
|
34,976
|
$
|
15,164,459
|
$
|
15,199,435
|
$
|
719,545
|
$
|
719,545
|
$
|
15,164,459
|
||||||||||||||
F-21
LID HAIR STUDIOS INTERNATIONAL, INC. AND
CHINA CHILDREN PHARMACEUTICALS CO., LTD.
Statements of Operations
For the nine months ended September 30, 2009
(unaudited)
|
Lid Hair International, Inc
|
China Pharmaceutic Co., Ltd.
|
Combined
|
Pro Forma Adjustments
|
Pro Forma
Combined
|
|||||||||||||||||
|
Sales, net
|
$
|
25,316
|
$
|
11,323,143
|
$
|
11,348,459
|
(a)
|
$
|
-25,316
|
$
|
11,323,143
|
||||||||||
|
Cost of sales
|
1,110
|
4,376,541
|
4,377,651
|
(a)
|
-1,110
|
4,376,541
|
|||||||||||||||
|
24,206
|
6,946,602
|
6,970,808
|
-24,206
|
6,946,602
|
|||||||||||||||||
|
General and administrative expenses
|
61,296
|
3,930,219
|
3,991,515
|
(a) (c)
|
-61,296
|
3,930,219
|
|||||||||||||||
|
Stock based compensation
|
(d)
|
396,152
|
396,152
|
||||||||||||||||||
|
Income (loss) from operations
|
-37,090
|
3,016,383
|
2,979,293
|
-359,062
|
2,620,231
|
||||||||||||||||
|
Other income(expense)
|
-
|
(10,276
|
)
|
(10,276
|
)
|
-
|
-10,276
|
||||||||||||||
|
Income (loss) before income taxes
|
-37,090
|
3,006,107
|
2,969,017
|
-359,062
|
2,609,955
|
||||||||||||||||
|
Provision for income taxes
|
-
|
422,716
|
422,716
|
-
|
422,716
|
||||||||||||||||
|
Net income (loss) for the period
|
-37,090
|
2,583,391
|
2,546,301
|
-359,062
|
2,187,239
|
||||||||||||||||
|
Foreign currency translation adjustment
|
11,451
|
14,939
|
26,390
|
-11,451
|
14,939
|
||||||||||||||||
|
Comprehensive income (loss) for the period
|
$
|
-25,639
|
$
|
2,598,330
|
$
|
2,572,691
|
$
|
-370,513
|
$
|
2,202,178
|
|||||||||||
|
(A) To reflect the spin-out of the Company’s wholly-owned subsidiary, Belford Enterprises B.C. Ltd., resulting in the re-purchase and cancellation of 5,000,000 shares of the Company’s common stock for all of the issued and outstanding shares of Belford
|
|
(B) To effectuate the 7 for 2 reverse stock split of the Company’s common stock.
|
|
(C) To recapitalize for the Share Exchange Transaction.
|
|
(D) To reflect the issuance of 1,250,000 warrants at an exercise price of $3.00 per share with 1,250,000 piggyback warrants at an exercise price of $5.00 per share to the consultants. The fair value of warrants was estimated using a Black-Scholes option pricing model with the following assumptions: average risk-free interest rate – 1.61%; expected life –2-3 years; expected volatility – 105.1%; and expected dividends – nil. The fair mark value of warrants, $396,152, has been recorded as stock based compensation cost with a corresponding increase to additional paid-in capital (warrants).
|
F-22
CHINA PEDIATRIC PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
AS OF JUNE 30, 2010 AND DECEMBER 31, 2009
|
6/30/2010 (Unaudited)
|
12/31/2009
(Audited)
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
$
|
6,585,350
|
$
|
905,874
|
||||
|
Accounts receivable, net
|
6,430,750
|
5,666,307
|
||||||
|
Other receivable
|
1,436,579
|
43,305
|
||||||
|
Inventory
|
2,111,576
|
886,082
|
||||||
|
Prepaid expenses
|
2,938,561
|
2,487,493
|
||||||
|
Total Current Assets
|
19,502,816
|
9,989,061
|
||||||
|
Long-term prepaid expenses
|
1,762,477
|
1,755,104
|
||||||
|
Property & equipment, net
|
754,586
|
797,790
|
||||||
|
Goodwill
|
612,745
|
612,745
|
||||||
|
Intangible Assets
|
1,690,240
|
1,856,718
|
||||||
|
Total Assets
|
$
|
24,322,864
|
$
|
15,011,418
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
Current Liabilities
|
||||||||
|
Accounts payable
|
$
|
731,604
|
$
|
254,097
|
||||
|
Accrued expenses and other payables
|
2,530,321
|
1,592,440
|
||||||
|
Trade deposit received
|
6,335
|
6,308
|
||||||
|
Short-term bank loan
|
440,619
|
438,776
|
||||||
|
VAT tax payable
|
71,720
|
321,521
|
||||||
|
Income tax payable
|
261,761
|
194,386
|
||||||
|
Total Current Liabilities
|
4,042,360
|
2,807,528
|
||||||
|
Stockholders' Equity
|
||||||||
|
Common stock, $ 0.001 per value, 75,000,000 share authorized,
|
||||||||
|
10,180,171 and 8,305,171 shares issued and outstanding at June 30, 2010 and December 31, 2009
|
10,180
|
8,305
|
||||||
|
Additional paid in capital
|
7,117,146
|
2,135,883
|
||||||
|
Statutory reserve
|
810,540
|
810,540
|
||||||
|
Other comprehensive income
|
696,786
|
328,567
|
||||||
|
Retained earnings
|
11,645,852
|
8,920,595
|
||||||
|
Total Stockholders' Equity
|
20,280,504
|
12,203,890
|
||||||
|
Total Liabilities and Stockholders' Equity
|
$
|
24,322,864
|
$
|
15,011,418
|
||||
The accompanying notes are an integral part of these financial statements.
F-23
CHINA PEDIATRIC PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF INCOME
(UNAUDITED)
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
6/30/2010
|
6/30/2009
|
6/30/2010
|
6/30/2009
|
|||||||||||||
|
Sales, net
|
$
|
6,508,769
|
$
|
3,547,124
|
$
|
13,217,661
|
$
|
7,142,774
|
||||||||
|
Cost of sales
|
2,599,693
|
1,331,835
|
5,509,231
|
2,786,687
|
||||||||||||
|
Gross profit
|
3,909,076
|
2,215,289
|
7,708,430
|
4,356,087
|
||||||||||||
|
Selling, general and administrative expense
|
2,384,925
|
1,172,882
|
4,528,127
|
2,394,384
|
||||||||||||
|
Income from operations
|
1,524,151
|
1,042,407
|
3,180,303
|
1,961,703
|
||||||||||||
|
Other income
|
514
|
250
|
825
|
288
|
||||||||||||
|
Other expense
|
(12,063
|
)
|
(108,185
|
)
|
(12,292
|
)
|
(108,211
|
)
|
||||||||
|
Total Other Income (Expense)
|
(11,549
|
)
|
(107,935
|
)
|
(11,467
|
)
|
(107,923
|
)
|
||||||||
|
Income before income taxes
|
1,512,602
|
934,472
|
3,168,836
|
1,853,780
|
||||||||||||
|
Provision for income taxes
|
254,793
|
137,072
|
443,579
|
267,981
|
||||||||||||
|
Net income
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Net income for common share
|
||||||||||||||||
|
Earnings per share - Basis
|
$
|
0.13
|
$
|
0.10
|
$
|
0.30
|
$
|
0.19
|
||||||||
|
Earnings per share - Diluted
|
$
|
0.13
|
$
|
0.10
|
$
|
0.29
|
$
|
0.19
|
||||||||
|
Weighted average common shares outstanding
|
||||||||||||||||
|
Basis
|
9,537,314
|
8,228,571
|
9,026,165
|
8,228,571
|
||||||||||||
|
Diluted
|
9,965,432
|
8,228,571
|
9,323,477
|
8,228,571
|
||||||||||||
|
Net income
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Other comprehensive income (loss)
|
369,626
|
79
|
368,219
|
6,882
|
||||||||||||
|
Comprehensive income (loss)
|
1,627,435
|
797,479
|
3,093,476
|
1,592,681
|
||||||||||||
The accompanying notes are an integral part of these financial statements.
F-24
CHINA PEDIATRIC PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
Six Months Ended
|
||||||||
|
Current Assets
|
6/30/2010
|
6/30/2009
|
||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Net Income
|
$
|
2,725,257
|
$
|
1,585,799
|
||||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
Provided by operating activities:
|
||||||||
|
Depreciation and Amortization
|
209,682
|
223,582
|
||||||
|
Stock-based compensation
|
483,138
|
-
|
||||||
|
Impairment loss on accounts and other receivables
|
-
|
108,180
|
||||||
|
(Increase) / decrease in assets:
|
||||||||
|
Accounts receivables
|
(737,866
|
)
|
(1,895,483
|
)
|
||||
|
Inventory
|
(1,217,041
|
)
|
(214,104
|
)
|
||||
|
Prepaid expense
|
(438,851
|
)
|
(444,397
|
)
|
||||
|
Other receivable
|
(1,388,227
|
)
|
988
|
|||||
|
Increase / (decrease) in current liabilities:
|
||||||||
|
Accounts payable
|
474,602
|
107,622
|
||||||
|
Accrued expenses and other payables
|
1,227,565
|
618,499
|
||||||
|
Trade deposit received
|
-
|
357
|
||||||
|
Value-added tax payable
|
(250,118
|
)
|
(23
|
)
|
||||
|
Income tax payable
|
66,317
|
(76,521
|
)
|
|||||
|
Net cash provided by operating activities
|
1,154,458
|
14,499
|
||||||
|
Net cash from financing activities
|
||||||||
|
Proceeds from issuance of common stock
|
4,500,000
|
-
|
||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
25,018
|
1,563
|
||||||
|
Net change in cash and cash equivalents
|
5,679,476
|
16,062
|
||||||
|
Cash and cash equivalents, beginning balance
|
905,874
|
813,252
|
||||||
|
Cash and cash equivalents, ending balance
|
$
|
6,585,350
|
$
|
829,314
|
||||
|
SUPPLEMENTAL DISCLOSURES:
|
||||||||
|
Cash paid during the year for:
|
||||||||
|
Income tax payments
|
$
|
377,262
|
$
|
344,502
|
||||
The accompanying notes are an integral part of these financial statements.
F-25
CHINA PEDIATRIC PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
|
Capital Stock
|
Additional
Paid-in
|
Other
Comprehensive
|
Statutory
|
Retained
|
Total
Stockholders
|
|||||||||||||||||||||||
|
Shares Amount
|
Capital
|
Income
|
Reserves
|
Earnings
|
Equity
|
|||||||||||||||||||||||
|
Balance December 31, 2009
|
8,305,171
|
$
|
8,305
|
$
|
2,135,883
|
$
|
328,567
|
$
|
810,540
|
$
|
8,920,595
|
$
|
12,203,890
|
|||||||||||||||
|
Foreign currency translation adjustments
|
368,219
|
368,219
|
||||||||||||||||||||||||||
|
Stock-based compensation
|
483,138
|
483,138
|
||||||||||||||||||||||||||
|
Issuance of common stock
|
1,875,000
|
1,875
|
4,498,125
|
4,500,000
|
||||||||||||||||||||||||
|
Income for the six months ended June 30, 2010
|
2,725,257
|
2,725,257
|
||||||||||||||||||||||||||
|
Balance June 30, 2010
|
10,180,171
|
$
|
10,180
|
$
|
7,117,146
|
$
|
696,786
|
$
|
810,540
|
$
|
11,645,852
|
$
|
20,280,504
|
|||||||||||||||
|
Balance December 31, 2008
|
8,228,571
|
$
|
8,229
|
$
|
1,478,134
|
$
|
313,429
|
$
|
721,553
|
$
|
6,493,979
|
$
|
9,015,324
|
|||||||||||||||
|
Foreign currency translation adjustments
|
15,138
|
15,138
|
||||||||||||||||||||||||||
|
Transferred to Statutory reserve
|
88,987
|
(88,987
|
)
|
-
|
||||||||||||||||||||||||
|
Stock-based compensation-warrants
|
641,725
|
641,725
|
||||||||||||||||||||||||||
|
Stock-based compensation
|
76,600
|
76
|
16,011
|
16,087
|
||||||||||||||||||||||||
|
Recapitalization
|
13
|
13
|
||||||||||||||||||||||||||
|
Income for the year ended December 31, 2009
|
2,515,,603
|
2,515,603
|
||||||||||||||||||||||||||
|
Balance December 31, 2009
|
8,305,171
|
$
|
8,305
|
$
|
2,135,883
|
$
|
328,567
|
$
|
810,540
|
$
|
8,920,595
|
$
|
12,203,890
|
|||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-26
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 1 – ORGANIZATION
China Pediatric Pharmaceuticals, Inc. ("the Company") was incorporated on April 20, 2005 in the state of Nevada. The Company was originally incorporated under the name Belford Enterprises, Inc. and changed its name to Lid Hair Studios International Inc. on August 15, 2--5. Asia-Pharm Holding Inc. ("Asia-Pharm") was incorporated in British Virgin Islands on June 20, 2008. China Children Pharmaceuticals Co. Limited ("China Children") a wholly owned subsidiary of Asia-Pharm Holdings Inc. was formed on June 27, 2008 under the laws of Hong Kong. Xi'an Coova Children Pharmaceuticals Co., Ltd. ("Xi'an Coova" or "WOFE") is a "wholly owned foreign enterprise" incorporated in People's Republic of China ("PRC"). Xi'an Coova is a wholly owned subsidiary of China Children.
On September 30, 2009 the Company completed its merger with China Children, a Hong Kong based pharmaceutical manufacturer company in accordance with the Share Exchange Agreement. The Share Exchange Transaction is being accounted for as a reverse acquisition. In accordance with the Accounting and Financial Reporting Interpretations and Guidance prepared by the staff of the U.S. Securities and Exchange Commission, the Company (the legal acquirer) is considered the accounting acquiree and China Children (the legal acquiree) is considered the accounting acquirer for accounting purposes. Subsequent to the Share Exchange Transaction, the financial statements of the combined entity will in substance be those of China Children. The assets, liabilities and historical operations prior to the share exchange transaction will be those of China Children. Subsequent to the date of Share Exchange Transaction, China Children is deemed to be a continuation of the business of the Company. Therefore post exchange financial statements will include the combined balance sheet of the Company and China Children, the historical operations of China Children and the operations of the Company and China Children from the closing date of the Share Exchange Transaction forward.
On August 4, 2008, an Entrustment Management Agreement was entered into between Xi'an Coova and Shaanxi Jiali Pharmaceuticals Co., Ltd. ("Shaanxi Jiali") to which China Children exercises control over the operations and business of Shaanxi Jiali through Xi'an Coova. Pursuant to the Entrustment Management Agreement, China Children shall receive all net profits and assume all operational losses of Shaanxi Jiali through Xi'an Coova.
Xi'an Coova entered into a Management Entrustment Agreement with Shaanxi Jiali and the shareholders of Shaanxi Jiali (the "Management Entrustment Agreement"), in which Shaanxi Jiali and its shareholders agreed to transfer control, or entrust, the operations and management of its business to Xi'an Coova. Under the agreement, Xi'an Coova manages the operations and assets of Shaanxi Jiali, controls all of the cash flows of Shaanxi Jiali through a bank account controlled by Xi'an Coova, is entitled to 100% of earnings before tax of Shaanxi Jiali, a management fee, and is obligated to pay all payables and loan payments of Shaanxi Jiali. In addition, under the terms of the Management Entrustment Agreement, Xi'an Coova has been granted certain rights which include, in part, the right to appoint and terminate members of Shaanxi Jiali's Board of Directors, hire management and administrative personnel and control decisions relating to entering and performing customer contracts and other instruments. We anticipate that Shaanxi Jiali will continue to be the contracting party under its customer contracts, bank loans and certain other instruments unless Xi'an Coova exercises its option. The agreement does not terminate unless the business of Shaanxi Jiali is terminated or Xi'an Coova exercises its option to acquire all of the assets or equity of Shaanxi Jiali under the terms of the Exclusive Option Agreement.
The contractual arrangements completed on August 4, 2008 provide that Xi'an Coova has controlling interest in Shaanxi Jiali as defined by FASB Interpretation No. 46R, "Consolidation of Variable Interest Entities" ("FIN 46R"), an Interpretation of Accounting Research Bulletin No. 51, which requires Xi'an Coova to consolidate the financial statements of Shaanxi Jiali and ultimately consolidate with its parent company, China Children.
The outstanding stock of the Company prior to the Share Exchange Transaction will be accounted for at their net book value and no goodwill will be recognized. Details of the shares outstanding upon completion of the Merger are as follows:
|
Pre-Exchange Transaction Common Shares Outstanding:
|
9,300,000
|
|||
|
Re-purchase of the Company's shares:
|
(5,000,000
|
)
|
||
|
Reverse stock split (7 pre-shares for 2 post-shares):
|
(3,071,429
|
)
|
||
|
Issuance of the Company's shares for all outstanding shares of China Children:
|
7,000,000
|
|||
|
Post-Exchange Transaction Common Shares Outstanding:
|
8,228,571
|
The Company, through its subsidiary, and exclusive contractual arrangement with Shaanxi Jiali, is engaged in the business of manufacturing and marketing over-the-counter ("OTC") and prescription pharmaceutical products for the Chinese marketplace as treatment for a variety of diseases and conditions.
F-27
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America.The Company's functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in United States Dollars.
Principles of Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiary and variable interest entity (“VIE”) for which the Company is the primary beneficiary. All inter-company accounts and transactions have been eliminated in consolidation. The Company has adopted the Consolidation Topic of the FASB Accounting Standards Codification (“ASC 810”) which requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns.
In determining Shaanxi Jiali is the VIE of Xi'an Coova, the Company considered the following indicators, among others:
|
|
Xi'an Coova has the full right to control and administrate the financial affairs and daily operation of Shaanxi Jiali and has the right to manage and control all assets of Shaanxi Jiali. The equity holders of Shaanxi Jiali as a group have no right to make any decision about Shaanxi Jiali’s activities without the consent of Xi'an Coova.
|
|
|
Xi'an Coova was assigned all voting rights of Shaanxi Jiali and has the right to appoint all directors and senior management personnel of Shaanxi Jiali. The equity holders of Shaanxi Jiali possess no substantive voting rights.
|
|
|
Xi'an Coova will provide financial support if Shaanxi Jiali requires additional funds to maintain its operations and to repay its debts.
|
|
|
Xi'an Coova should be paid a management fee equal to Shaanxi Jiali's earning before taxes. If there are no earnings before taxes and other cash expenses, then no fee shall be paid. If Shaanxi Jiali sustains losses, they will be carried over to the next period and deducted from the next management fee. Xi'an Coova should assume all operation risks of Shaanxi Jiali and bear all losses of Shaanxi Jiali. Therefore, Xi'an Coova is the primary beneficiary of Shaanxi Jiali.
|
Translation Adjustment
As of June 30, 2010 and December 31, 2009, the accounts of the Company were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (“CNY”). Such financial statements were translated into U.S. Dollars (“USD”) in accordance with the Foreign Currency Matters Topic of the FASB Accounting Standards Codification (“ASC 830”), with the CNY as the functional currency. According to ASC 830, all assets and liabilities were translated at the current exchange rate, stockholders’ equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”), as a component of shareholders’ equity. Transaction gains and losses are reflected in the income statement.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Comprehensive Income
The Company follows the Comprehensive Income Topic of the FASB Accounting Standards Codification (“ASC 220”). Comprehensive income is a more inclusive financial reporting methodology that includes disclosure of certain financial information that historically has not been recognized in the calculation of net income.
F-28
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) Risks and Uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC’s economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
The Company is subject to substantial risks from, among other things, intense competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the financial statements are issued, which may result in a loss to the Company but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed.
Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash and cash equivalents include cash in hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
Prepaid Expenses
Prepaid account is primarily comprised of two factors: advance payments suppliers for goods purchased, and advance payments to R&D organizations for new medicines to be developed and purchased.
In December 2007, the Company signed a Medicine Research and Development Agreement with Shaanxi Research Institution of Chinese Traditional Medicine (SRICTM). Pursuant to the terms of the agreement and Supplemental Agreement between the Company and SRICTM, SRICTM must successfully develop and obtain governmental approval for production of five new pediatric medicines, otherwise, the Company is entitled to a full refund of fees. Therefore, the costs paid in connection with these services were not classified as research and development costs, but rather as prepaid expenses. Such advance payments will not be expensed if we do not receive the desired results from SRICTM.
F-29
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
A table of the outstanding prepaid expenses as of June 30, 2010, March 31, 2010, and December 31, 2009 are as follows:
|
Payee
|
Nature
|
6/30/2010
|
3/31/2010
|
12/31/2009
|
||||||||||
|
Advance payment to R&D organization
|
Shaanxi Research Institution of Chinese Traditional Medicine
|
Advanced payment to Shaanxi Research Institution of Chinese Traditional Medicine for five new pediatric cold medicines
|
$
|
1,762,477
|
$
|
1,755,387
|
$
|
1,755,104
|
||||||
|
Advance payment to suppliers
|
Suppliers
|
Advance payments to suppliers for goods purchased
|
2,938,561
|
2,926,740
|
2,487,493
|
|||||||||
|
Total
|
$
|
4,701,038
|
4,682,127
|
$
|
4,242,597
|
|||||||||
Accounts Receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. Reserves are recorded primarily on a specific identification basis. These are no allowances for doubtful accounts as of June 30, 2010 and December 31, 2009.
Inventories
Inventories are valued at the lower of cost (determined on a weighted average basis) or market. The Management compares the cost of inventories with the market value and allowance is made for writing down their inventories to market value, if lower. As of June 30, 2010 and December 31, 2009, inventories consist of the following:
|
6/30/2010
|
12/31/2009
|
|||||||
|
Raw materials
|
$
|
1,248,401
|
$
|
1,163,589
|
||||
|
Finished goods
|
863,175
|
214,450
|
||||||
|
Provision for obsolescence
|
-
|
(491,957
|
)
|
|||||
|
Total
|
$
|
2,111,576
|
$
|
886,082
|
||||
Property, Plant & Equipment
Property and equipment are stated at cost. Expenditures for maintenance and repairs are charged to earnings as incurred; additions, renewals and betterments are capitalized. When property and equipment are retired or otherwise disposed of, the related cost and accumulated depreciation are removed from the respective accounts, and any gain or loss is included in operations. Depreciation of property and equipment is provided using the straight-line method for substantially all assets with estimated lives of:
|
Buildings
|
30 years
|
|
Plant and equipment
|
5-14 years
|
|
Transportation equipment
|
5-10 years
|
|
Office equipment
|
5-10 years
|
F-30
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
As of June 30, 2010 and December 31, 2009, Property, Plant & Equipment consist of the following:
|
6/30/2010
|
12/31/2009
|
|||||||
|
Buildings
|
$
|
445,845
|
$
|
445,845
|
||||
|
Plant and equipment
|
859,665
|
859,665
|
||||||
|
Transportation equipment
|
5,609
|
5,609
|
||||||
|
Office equipment
|
40,354
|
40,354
|
||||||
|
Total
|
1,351,473
|
1,351,473
|
||||||
|
Accumulated depreciation
|
(596,887
|
)
|
(553,683
|
)
|
||||
|
$
|
754,586
|
$
|
797,790
|
|||||
Depreciation expense for the three months ended June 30, 2010 and 2009 were $21,599 and $28,622, respectively. Depreciation expense for the six months ended June 30, 2010 and 2009 were $43,204 and $57,311, respectively.
Goodwill
Goodwill represents the excess cost of a business acquisition over the fair value of the net assets acquired. In accordance with the Intangibles, Goodwill and other topic of the FASB Accounting Standard Codification (“ASC 350”), indefinite-life identifiable intangible assets and goodwill are not amortized. Under the provisions of ASC 350, we are required to perform an annual impairment test of our goodwill. Goodwill impairment is determined using a two-step process. The first step of the goodwill impairment test is used to identify potential impairment by comparing the fair value of a reporting unit, which we define as our business segments, with its net book value or carrying amount including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test compares the implied fair value of the reporting unit's goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit's goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The implied fair value of goodwill is determined in the same manner as the amount of goodwill recognized in a business combination. The fair value of the reporting unit is allocated to all of the assets and liabilities of that unit including any unrecognized intangible assets as if the reporting unit had been acquired in a business combination and the fair value of the reporting unit was the purchase price paid to acquire the reporting unit. Goodwill as of June 30, 2010 and December 31, 2009 is $612,745.
Intangible Assets
The Company has four proprietary technologies: propriety technology for antioxidant technique, proprietary technology for “liren” capsule, patent-Chinese medicine and production method for skin and gyhecology disease and patent: Chinese medicine and production method for tracheitis. Propriety technology for antioxidant technique was contributed by a shareholder in exchange for shares of the Company’s common stock. Proprietary technology for “liren” capsule was purchased from third party at the price agreed by the Company and the third party. Two patents were purchase from the shareholders at the prices determined by an independent appraiser. These proprietary technologies were acquired for the future use of the Company. We capitalized them as intangible assets as acquired.
Intangible assets are amortized using the straight-line method over their estimated period of benefit, ranging from ten to fifty years. Management evaluate the recoverability of intangible assets periodically and take into account events or circumstances that warrant revised estimates of useful lives or that indicate that impairment exists. No impairments of intangible assets have been identified during any of the periods presented. The land use rights will expire in 2056 and 2058. All of the Company’s intangible assets are subject to amortization with estimated lives of:
Land use right 50 years
Proprietary technologies 10 years
F-31
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
The components of finite-lived intangible assets are as follows:
|
||||||||
|
June 30
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Land use right
|
$
|
264,625
|
$
|
264,625
|
||||
|
Proprietary technologies
|
2,966,355
|
2,966,355
|
||||||
|
Total
|
3,230,980
|
3,230,980
|
||||||
|
Less: Accumulated amortization
|
(1,540,740
|
)
|
(1,374,262
|
)
|
||||
|
$
|
1,690,240
|
$
|
1,856,718
|
|||||
Amortization expense for the three months ended June 30, 2010 and 2009 were $83,255 and $83,176, respectively. Amortization expense for the six months ended June 30, 2010 and 2009 were $166,478 and $166,271, respectively. The estimated future amortization expenses related to intangible asset as of June 30, 2010 are as follows:
|
2010
|
332,892
|
|||
|
2011
|
333,018
|
|||
|
2012
|
333,018
|
|||
|
2013
|
333,018
|
|||
|
2014
|
333,018
|
|||
|
Thereafter
|
$
|
25,276
|
Long-Lived Assets
Effective January 1, 2002, the Company adopted the Property, Plant and Equipment Topic of the FASB Accounting Standard Codification (“ASC 360”), which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of,” and the accounting and reporting provisions of Accounting Principles Board Opinion No. 30, “Reporting the Results of Operations for a Disposal of a Segment of a Business.” The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with ASC 360, which requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal. Based on its review, the Company believes that, as of June 30, 2010 and December 31, 2009, there were no significant impairments of its long-lived assets.
Fair Value of Financial Instruments
The Financial Instrument Topic of the FASB Accounting Standards Codification (“ASC 825”) requires that the Company disclose estimated fair values of financial instruments. The carrying amounts reported in the statements of financial position for current assets and current liabilities qualifying as financial instruments are a reasonable estimate of fair value.
Revenue Recognition
The Company’s revenue recognition policies are in compliance with the Revenue Recognition Topic of the FASB Accounting Standards Codification (“ASC 605”). Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist and collectibility is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue.
Advertising
Advertising expenses consist primarily of costs of promotion for corporate image and product marketing and costs of direct advertising. The Company expenses all advertising costs as incurred.
F-32
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED) Customer rebates
Rebates are paid to customers every quarter and we recorded customer rebates as customers earned. They are classified as a reduction of revenue according to ASC 605-55-64. The Company paid rebates to customers for the three months ended June 30, 2010 and 2009 were $761,526 and $415,014 respectively. The Company paid rebates to customers for the six months ended June 30, 2010 and 2009 were $1,343,541 and $835,558 respectively.
Income Taxes
The Company utilizes the Income Taxes Topic of the FASB Accounting Standards Codification (“ASC 740”) which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized .
In September 2009, the FASB issued Income Taxes Topic of the FASB Accounting Standards Codification (“ASC 740”). ASC 740 clarifies the accounting for Income Taxes by prescribing the minimum recognition threshold a tax position is required to meet before being recognized in the financial statements. It also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition and clearly scopes income taxes out of SFAS 5, “Accounting for Contingencies”.
Statement of Cash Flows
In accordance with the Statement of Cash Flows Topic of the FASB Accounting Standards Codification (“ASC 230”), cash flows from the Company’s operations are based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the statement of financial position.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, accounts receivable and other receivables arising from its normal business activities. The Company places its cash in what it believes to be credit-worthy financial institutions. The Company has a diversified customer base, most of which are in China. The Company controls credit risk related to accounts receivable through credit approvals, credit limits and monitoring procedures. The Company routinely assesses the financial strength of its customers and, based upon factors surrounding the credit risk, establishes an allowance, if required, for uncollectible accounts and, as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is limited.
Recent Accounting Pronouncements
In April 2009, the Financial Accounting Standards Board (“FASB”) issued the following new accounting standards:
|
● FASB Staff Position FAS No. 157-4, Determining Whether a Market Is Not Active and a Transaction Is Not Distressed, (“FSP FAS No. 157-4”) provides guidelines for making fair value measurements more consistent with the principles presented in SFAS No. 157. FSP FAS No. 157-4 provides additional authoritative guidance in determining whether a market is active or inactive and whether a transaction is distressed. It is applicable to all assets and liabilities (i.e., financial and non-financial) and will require enhanced disclosures. FSP FAS No. 157-4 was superseded by the Fair Value Measurements and Disclosures Topic of the FASB Accounting Standards Codification (“ASC 820”).
|
|
● FASB Staff Positions FAS No. 115-2, FAS 124-2, and EITF No. 99-20-2, Recognition and Presentation of Other-Than-Temporary Impairments, (“FSP FAS No. 115-2, FAS No. 124-2, and EITF No. 99-20-2”) provides additional guidance to provide greater clarity about the credit and noncredit component of an other-than-temporary impairment event and to more effectively communicate when an other-than-temporary impairment event has occurred. This FSP applies to debt securities. FSP FAS No. 115-2 and FAS No. 124-2 were superseded by the Investments-Debt and Equity Securities Topic of the FASB Accounting Standards Codification (“ASC 320”).
|
F-33
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
|
● FASB Staff Position FAS No. 107-1 and APB No. 28-1, Interim Disclosures about Fair Value of Financial Instruments, (“FSP FAS No. 107-1 and APB No. 28-1”) amends FASB Statement No. 107, Disclosures about Fair Value of Financial Instruments, to require disclosures about fair value of financial instruments in interim as well as in annual financial statements. This FSP also amends APB Opinion No. 28, Interim Financial Reporting, to require those disclosures in all interim financial statements. FSP FAS No. 107-1 and APB No. 28-1were superseded by the Financial Instruments Topic of the FASB Accounting Standards Codification (“ASC 825”).
|
In May 2009, the FASB issued SFAS No. 165, “Subsequent Events”, which is included in ASC Topic 855, Subsequent Events. ASC Topic 855 established principles and requirements for evaluating and reporting subsequent events and distinguishes which subsequent events should be recognized in the financial statements versus which subsequent events should be disclosed in the financial statements. ASC Topic 855 also required disclosure of the date through which subsequent events are evaluated by management. ASC Topic 855 was effective for interim periods ending after June 15, 2009 and applies prospectively. Because ASC Topic 855 impacted the disclosure requirements, and not the accounting treatment for subsequent events, the adoption of ASC Topic 855 did not impact our results of operations or financial condition.
Effective July 1, 2009, the Company adopted the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 105-10, Generally Accepted Accounting Principles – Overall (“ASC 105-10”). ASC 105-10 establishes the FASB Accounting Standards Codification (the “Codification”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with U.S. GAAP. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative U.S. GAAP for SEC registrants. All guidance contained in the Codification carries an equal level of authority. The Codification superseded all existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification is non-authoritative. The FASB will not issue new standards in the form of Statements, FASB Staff Positions or Emerging Issues Task Force Abstracts. Instead, it will issue Accounting Standards Updates (“ASUs”). The FASB will not consider ASUs as authoritative in their own right. ASUs will serve only to update the Codification, provide background information about the guidance and provide the bases for conclusions on the change(s) in the Codification. References made to FASB guidance throughout this document have been updated for the Codification.
In August 2009, the FASB issued ASU No. 2009-05, Measuring Liabilities at Fair Value, which provides additional guidance on how companies should measure liabilities at fair value under ASC 820. The ASU clarifies that the quoted price for an identical liability should be used. However, if such information is not available, an entity may use, the quoted price of an identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities traded as assets, or another valuation technique (such as the market or income approach). The ASU also indicates that the fair value of a liability is not adjusted to reflect the impact of contractual restrictions that prevent its transfer and indicates circumstances in which quoted prices for an identical liability or quoted price for an identical liability traded as an asset may be considered level 1 fair value measurements. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In September 2009, the FASB issued ASU No. 2009-12, Investments in Certain Entities That Calculate Net Asset Value per Share (or Its Equivalent), that amends ASC 820 to provide guidance on measuring the fair value of certain alternative investments such as hedge funds, private equity funds and venture capital funds. The ASU indicates that, under certain circumstance, the fair value of such investments may be determined using net asset value (NAV) as a practical expedient, unless it is probable the investment will be sold at something other than NAV. In those situations, the practical expedient cannot be used and disclosure of the remaining actions necessary to complete the sale is required. The ASU also requires additional disclosures of the attributes of all investments within the scope of the new guidance, regardless of whether an entity used the practical expedient to measure the fair value of any of its investments. This ASU is effective October 1, 2009. The Company is currently evaluating the impact of this standard, but would not expect it to have a material impact on the Company’s consolidated results of operations or financial condition.
In October 2009, the FASB issued ASU No. 2009-13, Multiple-Deliverable Revenue Arrangements—a consensus of the FASB Emerging Issues Task Force, that provides amendments to the criteria for separating consideration in multiple-deliverable arrangements. As a result of these amendments, multiple-deliverable revenue arrangements will be separated in more circumstances than under existing U.S. GAAP. The ASU does this by establishing a selling price hierarchy for determining the selling price of a deliverable. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available, or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. A vendor will be required to determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a
F-34
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONTINUED)
standalone basis. This ASU also eliminates the residual method of allocation and will require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method, which allocates any discount in the overall arrangement proportionally to each deliverable based on its relative selling price. Expanded disclosures of qualitative and quantitative information regarding application of the multiple-deliverable revenue arrangement guidance are also required under the ASU. The ASU does not apply to arrangements for which industry specific allocation and measurement guidance exists, such as long-term construction contracts and software transactions. ASU No. 2009-13 is effective beginning January 1, 2011. The Company is currently evaluating the impact of this standard on its consolidated results of operations and financial condition.
Note 3 – OTHER RECEIVABLES
Other receivables mainly consist of cash advances to employees. As of June 30, 2010 and December 31, 2009, other receivables were $1,436,579 and $43,305, respectively.
Note 4 – COMPENSATED ABSENCES
Regulation 45 of the local labour law of the People’s Republic of China (“PRC”) entitles employees to annual vacation leave after 1 year of service. In general, all leave must be utilized annually, with proper notification. Any unutilized leave is cancelled.
Note 5 –SHORT-TERM BANK LOAN
As of June 30, 2010, the Company had debt as follows :
|
Amount
|
Interest rate
|
Due
|
||||
|
Short term bank loan
|
$
|
440,619
|
0.85845% /per month
|
June 25, 2010
|
||
The Company is using these loans for working capital purposes and secured by property right.
Note 6 –INCOME TAXES
Pursuant to the PRC Income Tax Laws, the Enterprise Income Tax (“EIT”) through December 31, 2007 is at a statutory rate of 33%, which is comprised of 30% national income tax and 3% local income tax. As of January 1, 2008, the EIT is at a statutory rate of 25%. The Company is a high tech enterprise and under PRC Income Tax Laws, it is entitled to a two-year exemption for 2006 through 2007. Starting from January 1, 2008, the EIT is at a discounted rate of 15%.
The following is a reconciliation of income tax expense:
|
For the six months ended June 30, 2010
|
International
|
Total
|
||||||
|
Current
|
$
|
443,579
|
$
|
443,579
|
||||
|
Deferred
|
-
|
-
|
||||||
|
Total
|
$
|
443,579
|
$
|
443,579
|
||||
|
For the six months ended June 30, 2009
|
International
|
Total
|
||||||
|
Current
|
$
|
267,981
|
$
|
267,981
|
||||
|
Deferred
|
-
|
-
|
||||||
|
Total
|
$
|
267,981
|
$
|
267,981
|
||||
Note 7 – COMMITMENTS & CONTINGENCIES
The Company is committed to pay $649,179, for advertising through November 2010.
F-35
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 8 – COMMON STOCK, STOCK OPTION AND COMPENSATION
On October 2, 2009 the Company granted 76,600 shares of common stock to employees for services rendered having a fair market value on that date of $16,087 or $.21 per share, which was the closing price of our common stock on that date.
On August 15, 2009, the Company signed Warrant Agreements with consultants, investor relationship service provider and referral. Pursuant to the Warrant Agreements, the Company will issue warrants grant to purchase 1,250,000 shares of common stock at $3.00 exercise price with piggy-back warrants to purchase 1,250,000 shares of common stock at $5.00 exercise price. The warrants will be issuable at the closing of the merger.
On February 11, 2010, the Company closed a private placement issued 375,000 shares of common stock at $4 per share to several investors for total cash consideration of $1,500,000.
On April 29, 2010, the Company issued 1,200,000 shares common stock at $2 per share to several investors for total cash consideration of $2,400,000.
On June 23, 2010, the Company issued 300,000 shares common stock at $2 per share to several investors for total cash consideration of $600,000.
Warrants
On August 15, 2009, Kang Xiulan referred a public shell company(Lid Hair Studios International, Inc.)to China Children Pharmaceutical Inc. China Children Pharmaceutical Inc. came to be quoted on the OTCBB successfully through the reverse merger with Lid Hair Studios International, Inc. on September 30, 2009 and changed its name into China Pediatric Pharmaceuticals, Inc. As consideration for the services provided by Kang Xiulan (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and Kang Xiulan), The Company agreed to issue to Kang Xiulan warrants to acquire 250,000 shares of the Company’s common stock with an exercise price of $3, 00 with piggyback warrants to purchase 250,000 shares of the Company’s common stock with an exercise price of $5, 00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
During the above-mentioned reverse merger process, the Company obtained certain consulting services from IFG Investments Services, Inc., including advising on a merger/acquisition transaction and regulatory filings, and other services and support as requested from IFG. In consideration for the consulting services to be performed by IFG (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and IFG), The Company agreed to issue to IFG warrants to acquire 600,000 shares of the Company’s common stock with an exercise price of $3, 00 with piggyback warrants to purchase 600,000 shares of the Company’s comment stock with an exercise price of $5,00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
The Company also obtained certain public company sector services, including investor relations advisory services. In consideration for the investor relation services provided by China National Information Network (and in accordance with a warrant placement agreement dated September 30, 2009 between the Company and China National Information Network), the Company agreed to issue to China National Information Network the warrants to acquire 400,000 shares of the Company’s common stock with an exercise price of $3,00 with piggyback warrants to purchase 400,000 shares of the Company’s comment stock with an exercise price of $5,00. The warrants will expire on September 30, 2011 and September 30, 2012, respectively.
Based on the fair value method under ASC Topic 505, the fair value of each warrant granted is estimated on the date of the grant using the Black-Scholes option pricing model and is recognized as compensation expense over the service period of each warrants issued. The Black-Scholes option pricing model has assumptions for risk free interest rates, dividends, stock volatility and expected life of an option grant. The risk free interest rate is based upon market yields for US Treasury debt securities at a maturity near the term remaining on the warrant. Dividend rates are based on the Company’s dividend history. The stock volatility factor is based on the historical volatility of the similar company’s stock price. The fair value was estimated at the date of grant using the following range of assumptions: average risk-free interest rate – 1.61%; expected life –2-3 years; expected volatility – 105.1%; and expected dividends – nil. No estimate of forfeitures was made as the Company has a short history of granting warrants. The fair market value of warrants, $483,138, has been recorded as stock based compensation cost with a corresponding increase to additional paid-in capital (warrants) for the six months ended June 30, 2010.
|
|
Total
|
Exercise Price
|
Remaining Life
|
Aggregate
Intrinsic Value
|
|||||||||
|
Outstanding, December 31, 2009
|
2,500,000
|
$
|
3.00-5.00
|
1.25-2.25yrs
|
-
|
||||||||
|
Outstanding, June 30, 2010
|
2,500,000
|
|
|||||||||||
The option holder has no voting or dividend rights. The company records the expense of the stock options over the related vesting period. The options were valued using the Black-Scholes option pricing model with the following assumptions: average risk-free interest rate – 1.61%; expected life –1--1.33 years; expected volatility – 105.1%; and expected dividends – nil.
Note 9 – STATUTORY RESERVE
In accordance with the laws and regulations of the PRC, a wholly-owned Foreign Invested Enterprises income, after the payment of the PRC income taxes, shall be allocated to the statutory surplus reserves and statutory public welfare fund. Prior to January 1, 2006 the proportion of allocation for reserve was 10 percent of the profit after tax to the surplus reserve fund and additional 5-10 percent to the public affair fund. The public welfare fund reserve was limited to 50 percent of the registered capital. Effective January 1, 2006, there is now only one fund requirement. The reserve is 10 percent of income after tax, not to exceed 50 percent of registered capital.
Statutory Reserve funds are restricted for set off against losses, expansion of production and operation or increase in register capital of the respective company. Statutory public welfare fund is restricted to the capital expenditures for the collective welfare of employees. These reserves are not transferable to the Company in the form of cash dividends, loans or advances. These reserves are therefore not available for distribution except in liquidation. As of June 30, 2010 and December 31, 2009, the Company had allocated $810,541 to these non-distributable reserve funds.
F-36
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010 (UNAUDITED)
Note 10 – NET INCOME (LOSS) PER SHARE
In accordance with FASB ASC Topic 260-1-50, “Earnings per Share”, and SEC Staff Accounting Bulletin No. 98, basic net income or loss per common share is computed by dividing net income or loss for the period by the weighted - average number of common shares outstanding during the period. Under FASB ASC 260-10-50, diluted income or loss per share is computed by dividing net income or loss for the period by the weighted - average number of common and common equivalent shares, such as stock options, warrants and convertible securities outstanding during the period.
The following table sets forth the computation of basic and diluted earnings per share of common stock:
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
6/30/2010
|
6/30/2009
|
6/30/2010
|
6/30/2009
|
|||||||||||||
|
Basic earnings from continuing operations per share:
|
||||||||||||||||
|
Numerator:
|
||||||||||||||||
|
Income from operations used in computing basic earnings per share
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Income from operations applicable to common shareholders
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Denominator:
|
||||||||||||||||
|
Weighted average common shares outstanding
|
9,537,314
|
8,228,571
|
9,026,165
|
8,228,571
|
||||||||||||
|
Basic earnings per share from continuing operations
|
$
|
0.13
|
$
|
0.10
|
$
|
0.30
|
$
|
0.19
|
||||||||
|
Diluted earnings (losses) per share from operations:
|
||||||||||||||||
|
Numerator:
|
||||||||||||||||
|
Income from operations used in computing diluted earnings per share
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Income (loss) from operations applicable to common shareholders
|
$
|
1,257,809
|
$
|
797,400
|
$
|
2,725,257
|
$
|
1,585,799
|
||||||||
|
Denominator:
|
||||||||||||||||
|
Weighted average common shares outstanding
|
9,537,314
|
8,228,571
|
9,026,165
|
8,228,571
|
||||||||||||
|
Weighted average effect of dilutive securities:
|
||||||||||||||||
|
Stock options and warrants
|
428,118
|
-
|
297,312
|
-
|
||||||||||||
|
Shares used in computing diluted net income per share
|
9,965,432
|
8,228,571
|
9,323,477
|
8,228,571
|
||||||||||||
|
Diluted earnings per share from operations
|
$
|
0.13
|
$
|
0.10
|
$
|
0.29
|
$
|
0.19
|
||||||||
Note 11 – OTHER COMPREHENSIVE INCOME
Balances of related after-tax components comprising accumulated other comprehensive income, included in stockholders’ equity, at of June 30, 2010, are as follows:
|
Foreign
|
Accumulated
|
|||||||
|
Currency
|
Other
|
|||||||
|
Translation
|
Comprehensive
|
|||||||
|
Adjustment
|
Income
|
|||||||
|
Balance at December 31, 2009
|
$
|
328,567
|
$
|
328,567
|
||||
|
Change for 2010
|
368,219
|
368,219
|
||||||
|
Balance at June 30, 2010
|
$
|
696,786
|
$
|
696,786
|
||||
F-37
CHINA PEDIATRIC PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
(UNAUDITED)
Note 12 – CURRENT VULNERABILITY DUE TO CERTAIN RISK FACTORS
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, by the general state of the PRC’s economy. The Company’s business may be influenced by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Note 13–MAJOR CUSTOMERS AND CREDIT RISK
Six and Three customers accounted for more than 10% of accounts receivable at June 30, 2010 and December 31, 2009 totalling 66% and 33%, respectively; As of June 30, 2010 and December 31, 2009, four vendors were greater than 10% of accounts payable, totalling 64% and 71%, respectively.
Two and six customers accounted for 21% and 97% of sales for the six months ended June 30, 2010 and 2009, respectively. Two and five vendors accounted for 85% and 86% of purchases for the six months ended June 30, 2010 and 2009, respectively.
Note 14–SUBSEQUENT EVENT
For the six months ended June 30, 2010, the Company has evaluated subsequent events for potential recognition and disclosure. No significant events occurred subsequent to the end of the reporting period but prior to the filing of this report that would have a material impact on our consolidated financial statements.
F-38
PART II
Information Not Required in Prospectus
Item 13. Other Expense of Issuance and Distribution
We will pay all expenses in connection with the registration and sale of the common stock by the selling stockholders. The estimated expenses of issuance and distribution are set forth below:
|
Registration fees
|
$
|
731.00
|
||
|
Legal fees
|
$
|
55,000.00
|
||
|
Accounting fees
|
$
|
20,000.00
|
||
|
Miscellaneous
|
$
|
5,000.00
|
||
|
Total estimated costs of offering
|
$
|
80,731.00
|
||
Item 14. Indemnification of Directors and Officers
The General Corporation Law of Nevada provides that directors, officers, employees or agents of Nevada corporations are entitled, under certain circumstances, to be indemnified against expenses (including attorneys’ fees) and other liabilities actually and reasonably incurred by them in connection with any suit brought against them in their capacity as a director, officer, employee or agent, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, and with respect to any criminal action or proceeding, if they had no reasonable cause to believe their conduct was unlawful. This statute provides that directors, officers, employees and agents may also be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by them in connection with a derivative suit brought against them in their capacity as a director, if they acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification may be made without court approval if such person was adjudged liable to the corporation.
Our Articles of Incorporation and By-Laws provide that we will indemnify our directors and officers from all liabilities incurred by them in connection with any action, suit or proceeding in which they are involved by reason of their acting as our directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Item 15. Recent Sales of Unregistered Securities
Pursuant to the Exchange Agreement, on September 30, 2009, we issued 7,000,000 shares of our Common Stock to Asia-Pharm in exchange for 100% of the outstanding shares of China Children Holdings Limited. Such securities were not registered under the Securities Act of 1933 (the “Securities Act”). The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Act and in part pursuant to the Securities Act. We made this determination based on the representations of China Children which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Securities Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that Asia-Pharm understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Concurrent with the Share Exchange Agreement on September 30, 2009, we issued to the consultants two year warrants to purchase 1,250,000 shares of our common stock at an exercise price of $3.00 per share with three year piggyback warrants to purchase 1,250,000 shares of our common stock at an exercise price of $5.00 per share. Such securities were not registered under the Securities Act. The issuance of these securities was exempt from registration under Section 4(2) of the Securities Act. We made this determination based on the representations of the Placement Agent, which included, in pertinent part, that such Placement Agent was an "accredited investors" within the meaning of Rule 501 of Regulation D promulgated
73
under the Securities Act and that the Placement Agent was acquiring our common stock for investment purposes for its own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the Placement Agent understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On October 2, 2009, we issued an aggregate of 76,600 shares of our common stock to employees of the Company as part of their 2009 compensation packages. Such securities were not registered under the Securities Act of 1933. The issuance of these shares was exempt from registration, in part pursuant to Regulation S and Regulation D under the Securities Act and in part pursuant to Section 4(2) of the Securities Act. We made this determination based on the representations of employees which included, in pertinent part, that such shareholders were either (a) "accredited investors" within the meaning of Rule 501 of Regulation D promulgated under the Securities Act, or (b) not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Securities Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the employees understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On February 10, 2010, we issued an aggregate of 375,000 shares of our common stock to Ji Wu, Jianming Yue, Xiang Cheng, and YMC Holdings Company Ltd., at a price of $4.00 per share, for an aggregate purchase price of $1.5 million. Such securities were not registered under the Securities Act. The issuance of these shares was exempt from registration, in part pursuant to Regulation S under the Securities Act and in part pursuant to Section 4(2) of the Securities Act. We made this determination based on the representations of employees which included, in pertinent part, that such shareholders were not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Securities Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the employees understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On April 29, 2010, the Company issued an aggregate of 1,200,000 shares of common stock at $2.00 per share that were sold to several investors in March and April 2010 for total cash consideration of $2,400,000. The issuances of these shares were exempt from registration, in part pursuant to Regulation S under the Securities Act and in part pursuant to Section 4(2) of the Securities Act. We made this determination based on the representations of the investors which included, in pertinent part, that such shareholders were not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Securities Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the investors understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
On June 23, 2010, the Company issued 300,000 shares common stock at $2.00 per share to three investors for a total cash consideration of $600,000. The issuances of these shares were exempt from registration, in part pursuant to Regulation S under the Securities Act and in part pursuant to Section 4(2) of the Securities Act. We made this determination based on the representations of the investors which included, in pertinent part, that such shareholders were not a "U.S. person" as that term is defined in Rule 902(k) of Regulation S under the Securities Act, and that such shareholders were acquiring our common stock, for investment purposes for their own respective accounts and not as nominees or agents, and not with a view to the resale or distribution thereof, and that the investors understood that the shares of our common stock may not be sold or otherwise disposed of without registration under the Securities Act or an applicable exemption therefrom.
Item 16. Exhibits
|
Description
|
||
|
2.1 (1)
|
Share Exchange Agreement by and among Lid Hair Studios International, Inc., Eric Anderson, and Asia-Pharm Holdings, Inc. dated September 30, 2009
|
|
|
2.2 (2)
|
Share Exchange Agreement between Lid Hair Studios International, Inc. and Eric Anderson dated September 30, 2009
|
|
|
3.1 (2)
|
Charter and Articles of Incorporation of Lid Hair Studios International, Inc., a Nevada corporation. (April 2005)
|
|
|
3.2 (2)
|
Amended Articles of Incorporation of Lid Hair Studios International, Inc., a Nevada Corporation (August 2005)
|
|
|
3.3 (2)
|
Amended Articles – Name Change to China Pediatric Pharmaceuticals, Inc. a Nevada Corporation (October 2009) and Plan of Merger Agreement
|
|
|
5.1 (4)
|
Legal Opinion of W. L. MacDonald Law Corporation
|
|
|
10.1 (1)
|
Agreement with Exclusive Territory Agents - Sample Agreement
|
|
|
10.2 (1)
|
Medicine Research and Development Agreement dated December 10, 2007, by and between Shaanxi Jiali Pharmaceutical Co., Ltd. and Shaanxi Research Institute of Chinese Traditional Medicines
|
|
|
10.3 (1)
|
Form of Common Stock Purchase Warrant
|
|
|
10.4 (1)
|
Lease Agreement
|
|
|
10.5 (1)
|
Employment Agreement with Jun Xia, President and CEO
|
|
|
10.6 (1)
|
Employment Agreement with Minggang Xiao, CFO
|
|
|
10.7 (1)
|
Warrant Agreement – IFG Investments Services, Inc.
|
|
|
10.8 (1)
|
Warrant Agreement – China National Information Network Trust Amended October 8, 2009
|
|
|
10.9 (1)
|
Warrant Agreement – Kang Xiulan
|
|
|
10.10 (1)
|
Warrant Terms and Conditions General
|
|
|
10.11 (1)
|
Consulting Agreement - IFG Investment Services, Inc.
|
|
|
10.12 (1)
|
Investor Relations Agreement – China National Information Network Trust (f/k/a Donahue & Associates)
|
|
|
10.13 (1)
|
Referral Agreement - Kang Xiulan
|
|
|
10.14 (3)
|
Agreement on Entrustment for Operation and Management, dated August 4, 2008, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd., the Shareholders of Shaanxi Jiali Pharmaceuticals Co., Ltd., and Xi’an Coova Children Pharmaceuticals Co., Ltd.
|
|
|
10.15 (3)
|
Exclusive Option Agreement, dated August 4, 2008, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd., the Shareholders of Shaanxi Jiali Pharmaceuticals Co., Ltd., and Xi’an Coova Children Pharmaceuticals Co., Ltd.
|
74
|
10.16 (3)
|
Agreement on Share Pledge, dated August 4, 2008, by and between the Shareholders of Shaanxi Jiali Pharmaceuticals Co., Ltd. and Xi’an Coova Children Pharmaceuticals Co., Ltd.
|
|
|
10.17 (3)
|
Shareholders’ Voting Proxy Agreement, dated August 4, 2008, by and between the Shareholders of Shaanxi Jiali Pharmaceuticals Co., Ltd. and Xi’an Coova Children Pharmaceuticals Co., Ltd.
|
|
|
10.18 (3)
|
Supplementary Agreement, dated August 4, 2008, by and between Asia-Pharm Holding, Inc., Weizhong Zhang. and Xi’an Coova Children Pharmaceuticals Co., Ltd.
|
|
|
10.19 (6)
|
Annual Sales Cooperation Agreement, dated December 21, 2009, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd. and Shandong Yaoshan Pharmaceuticals, Co., Ltd.
|
|
|
10.20 (6)
|
Annual Sales Cooperation Agreement, dated December 25, 2009, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd. and Hangzhou Zhencheng Pharmaceuticals, Co., Ltd.
|
|
|
10.21 (6)
|
Annual Sales Cooperation Agreement, dated December 26, 2009, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd. and Hunan Jiuwang Pharmaceuticals, Co., Ltd.
|
|
|
10.22 (6)
|
Annual Sales Cooperation Agreement, dated December 19, 2009, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd. and Xi’an Zaolutang Kushidai.
|
|
|
10.23 (6)
|
Annual Sales Cooperation Agreement, dated December 17, 2009, by and between Shaanxi Jiali Pharmaceuticals Co., Ltd. and Jiangsu YaBang Pharmaceuticals Logistics Center Co., Ltd.
|
|
|
10.24*
|
Supplementary Medicine Research and Development Agreement, dated April 3, 2010, by and between Shaanxi Jiali Pharmaceutical Co., Ltd. and Shaanxi Research Institution of Chinese Traditional Medicines
|
|
|
21.1 (3)
|
List of Subsidiaries
|
|
|
23.1*
|
Consent of Acquavella, Chiarelli, Shuster, Berkower & Co. LLP, for the use of their report.
|
|
|
23.2
|
Consent of Counsel to the use of the opinion annexed as Exhibit 5.1 (contained in the opinion annexed as Exhibit 5.1)
|
|
|
99.2 (1)
|
Patents
|
|
|
99.3 (1)
|
Trademarks
|
|
|
99.4 (5)
|
Audited Financial Statements and Management’s Discussion and Analysis of Lid Hair Studios International, Inc. for the fiscal years ended May 31, 2009 and 2008.
|
|
|
99.5 (5)
|
Interim Unaudited Financial Statements and Management’s Discussion and Analysis of Lid Hair Studios International, Inc. for the period ended August 31, 2009.
|
*Filed Herewith.
|
(1)
|
Incorporated by reference to the exhibit to our Current Report on Form 8-K filed with the SEC on October 6, 2009.
|
|
(2)
|
Incorporated by reference to the exhibit to Amendment No. 1 to our Current Report on Form 8-K/A filed with the SEC on October 14, 2009.
|
|
(3)
|
Incorporated by reference to the exhibit to our Annual Report on Form 10-K filed with the SEC on March 31, 2010.
|
|
(4)
|
Incorporated by reference to the exhibit to our Registration Statement on Form S-1 (File No. 333-164562) filed with the SEC on January 28, 2010.
|
|
(5)
|
Incorporated by reference to the exhibit to our Registration Statement on Form S-1/A (File No. 333-164562) filed with the SEC on April 12, 2010.
|
|
Incorporated by reference to the exhibit to our Registration Statement on Form S-1/A (File No. 333-164562) filed with the SEC on July 12, 2010.
|
Item 17. Undertakings
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the "Calculation of Registration Fee" table in the effective registration statement.
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
75
(4) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(5) Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
i. Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
ii. Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
iii. The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
iv. Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
76
Signatures
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements of filing on Form S-1/A and authorized this registration statement to be signed on its behalf by the undersigned, in Xi’an City, People’s Republic of China on September 3, 2010 .
|
CHINA PEDIATRIC PHARMACEUTICALS, INC.
|
|||||
|
By:
|
/s/ Jun Xia
|
||||
|
Jun Xia
|
|||||
|
Chairman
|
|||||
|
(principal executive officer)
|
|||||
|
By:
|
/s/ Minggang Xiao
|
||||
|
Minggang Xiao
|
|||||
|
Chief Financial Officer
|
|||||
|
(principal financial officer and principal accounting officer)
|
|||||
In accordance with the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Date:
|
September 3, 2010
|
By:
|
/s/ Jun Xia
|
||
|
Jun Xia
|
|||||
|
Chairman
|
|||||
|
(principal executive officer)
|
|||||
|
Date:
|
By:
|
/s/ Minggang Xiao*
|
|||
|
Minggang Xiao
|
|||||
|
Chief Financial Officer
|
|||||
|
(principal financial officer and principal accounting officer )
|
|
Date:
|
By:
|
/s/ Wanxiang Li*
|
|||
|
Wanxiang Li
|
|||||
|
Director
|
|||||
|
Date:
|
By:
|
/s/ Nanjing Lin*
|
|||
|
Nanjing Lin
|
|||||
|
Director
|
|||||
|
Date:
|
By:
|
/s/ Xiaoying Zhang*
|
|||
|
Xiaoying Zhang
|
|||||
|
Director
|
|||||
|
Date:
|
September 3, 2010
|
*
|
By:
|
/s/ Jun Xia
|
|
|
Jun Xia
|
|||||
|
Attorney-in-Fact
|
78
