Attached files
| file | filename |
|---|---|
| EX-32 - HUIHENG MEDICAL, INC. | v194766_ex32.htm |
| EX-31.1 - HUIHENG MEDICAL, INC. | v194766_ex31-1.htm |
| EX-31.2 - HUIHENG MEDICAL, INC. | v194766_ex31-2.htm |
UNITED
STATES SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-Q
(Mark
One)
x QUARTERLY REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
Quarterly Period Ended June 30, 2010
¨ TRANSITION REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
transition period from _____ to _____.
Commission File No.
333-132056
HUIHENG MEDICAL,
INC.
(Exact
Name of Registrant as Specified in Its Charter)
|
Nevada
|
20-4078899
|
|
|
(State
or Other Jurisdiction
Of
Incorporation or Organization)
|
(I.R.S.
Employer Identification
Number)
|
|
|
Huiheng Building, Gaoxin 7 Street
South,
Keyuannan Road, Nanshan
District,
Shenzhen Guangdong, P.R. China
518057
|
N/A
|
|
|
(Address
of Principal Executive Offices)
|
(Zip
Code)
|
Registrant’s
telephone number, including area code 86-755-25331366
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports); and (2) has been subject to such filing requirements for
the past 90 days.
|
Yes
¨ No
x
|
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
|
Yes
¨ No
¨
|
Indicate
by checkmark whether the registrant is a large accelerated filer, an accelerated
filer, a non-accelerated filer or a smaller reporting company. See
the definitions of “large accelerated filer,” “accelerated filer” and “smaller
reporting company” in Rule 12b-3 of the Exchange Act.
|
Large
accelerated filer
|
¨
|
Accelerated
filer
|
¨
|
|
Non-accelerated
filer
|
¨
|
Smaller
reporting company
|
x
|
|
(Do
not check if a smaller reporting company)
|
|||
Indicate
by check mark whether the registrant is a shell company (as defined in Rule
12b-2 of the Exchange Act).
|
Yes
¨ No
x
|
As of
August 2, 2010, there were 13,935,290 shares of the issuer’s $0.001 par value
common stock issued and outstanding.
HUIHENG
MEDICAL, INC.
FORM
10-Q INDEX
|
Page
|
||
|
PART
I – FINANCIAL INFORMATION
|
||
|
Item
1. Financial Statements
|
||
|
Consolidated
Balance Sheets at June 30, 2010 (Unaudited) and December 31,
2009
|
1
|
|
|
Consolidated
Statements of Income for the Six Months Ended June 30, 2010 and 2009
(Unaudited)
|
2
|
|
|
Consolidated
Statements of Changes in Stockholders’ Equity and Other Comprehensive
Income for the Six Months Ended June 30, 2010 (Unaudited)
|
3
|
|
|
Consolidated
Statements of Cash Flows for the Six Months Ended June 30, 2010 and 2009
(Unaudited)
|
4
|
|
|
Notes
to the Consolidated Financial Statements (Unaudited)
|
5
|
|
|
Item
2. Management’s Discussion and Analysis of Financial Condition
and
Results of Operations
|
22
|
|
|
Item
3. Quantitative and Qualitative Disclosures About Market
Risk
|
29
|
|
|
Item
4T. Controls and Procedures
|
29
|
|
|
PART
II – OTHER INFORMATION
|
||
|
Item
1. Legal Proceedings
|
30
|
|
|
Item
1A. Risk Factors
|
30
|
|
|
Item
2. Unregistered Sales of Equity Securities and Use of
Proceeds
|
30
|
|
|
Item
3. Defaults Upon Senior Securities
|
30
|
|
|
Item
4. [Removed and Reserved]
|
30
|
|
|
Item
5. Other Information
|
30
|
|
|
Item
6. Exhibits
|
30
|
|
|
Signature
Page
|
|
32
|
In
this Quarterly Report on Form 10-Q, references to “dollars” and “$” are to
United States dollars and, unless the context otherwise requires, references to
“we,” “us”, “our” and the Company refer to Huiheng Medical, Inc. and its
consolidated subsidiaries.
This
Quarterly Report contains certain forward-looking statements. When
used in this Quarterly Report, statements which are not historical in nature,
including the words “anticipate,” “estimate,” “should,” “expect,” “believe,”
“intend,” “may,” “project,” “plan” or “continue,” and similar expressions are
intended to identify forward-looking statements. They also include
statements containing anticipated business developments, a projection of
revenues, earnings or losses, capital expenditures, dividends, capital structure
or other financial terms.
The
forward-looking statements in this Quarterly Report are based upon management’s
beliefs, assumptions and expectations of our future operations and economic
performance, taking into account the information currently available to
them. These statements are not statements of historical
fact. Forward-looking statements involve risks and uncertainties,
some of which are not currently known to us that may cause our actual
results, performance or financial condition to be materially different from the
expectations of future results, performance or financial condition we express or
imply in any forward-looking statements. These forward-looking
statements are based on our current plans and expectations and are subject
to a number of uncertainties and risks that could significantly affect current
plans and expectations and our future financial condition and
results.
We
undertake no obligation to publicly update or revise any forward-looking
statements, whether as a result of new information, future events or
otherwise. In light of these risks, uncertainties and assumptions,
the forward-looking events discussed in this filing might not
occur. We qualify any and all of our forward-looking statements
entirely by these cautionary factors. As a consequence, current
plans, anticipated actions and future financial conditions and results may
differ from those expressed in any forward-looking statements made by or on our
behalf. You are cautioned not to unduly rely on such forward-looking
statements when evaluating the information presented
herein.
PART
I – FINANCIAL INFORMATION
Item
1. Financial Statements
HUIHENG
MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED
BALANCE SHEETS
( IN US
DOLLARS)
|
June 30, 2010
(Unaudited)
|
December 31,
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT
ASSETS:
|
||||||||
|
Cash
|
$ | 123,287 | $ | 84,962 | ||||
|
Accounts
receivable, net of allowance for doubtful accounts of $903,353 and
$897,319, as of June 30, 2010 and December 31, 2009,
respectively
|
17,177,023 | 16,499,819 | ||||||
|
Prepaid
expenses
|
3,347,364 | 3,058,465 | ||||||
|
Other
receivables, net of allowance for doubtful accounts of $737,311 and
$732,386 as of June 30, 2010 and December 31, 2009,
respectively
|
296,976 | 248,790 | ||||||
|
Inventories
|
1,900,727 | 1,359,900 | ||||||
|
Total
Current Assets
|
22,845,377 | 21,251,936 | ||||||
|
INVESTMENT
IN AFFILIATE
|
88,190 | 43,152 | ||||||
|
PROPERTY,
PLANT AND EQUIPMENT, net
|
2,450,569 | 2,520,787 | ||||||
|
LAND
USE RIGHT, NET
|
936,241 | 939,575 | ||||||
|
INTANGIBLE
ASSETS, NET
|
990,772 | 777,086 | ||||||
|
OTHER
RECEIVABLES, net of current portion
|
1,451,168 | 1,582,093 | ||||||
|
Total
Assets
|
$ | 28,762,317 | $ | 27,114,629 | ||||
|
LIABILITIES
AND STOCKHOLDERS’ EQUITY
|
||||||||
|
CURRENT
LIABILITIES:
|
||||||||
|
Accounts
payable
|
$ | 788,446 | $ | 844,539 | ||||
|
Amount
due to related parties
|
374,726 | 24,560 | ||||||
|
Income
tax payable
|
639,832 | 389,632 | ||||||
|
Accrued
liabilities and other payables
|
1,624,559 | 1,676,639 | ||||||
|
Total
Current Liabilities
|
3,427,563 | 2,935,370 | ||||||
|
STOCKHOLDERS’
EQUITY:
|
||||||||
|
Preferred
stock, $0.001 par value; 1,000,000 shares authorized; Designated 300,000
shares of Series A convertible preferred stock; 220,467 shares issued and
outstanding with liquidation preference of $8,267,513 at June 30,
2010 and December 31, 2009
|
220 | 220 | ||||||
|
Common
stock, $0.001 par value; 74,000,000 shares authorized; 23,635,290 shares
issued, 13,935,290 shares outstanding
|
23,635 | 23,635 | ||||||
|
Treasury
stock, 9,700,000 common shares, at cost
|
(9,700 | ) | (9,700 | ) | ||||
|
Additional
paid-in capital
|
7,498,086 | 7,498,086 | ||||||
|
Retained
earnings
|
14,937,078 | 13,799,481 | ||||||
|
Accumulated
other comprehensive income Foreign currency translation
gain
|
1,922,100 | 1,756,510 | ||||||
|
Non-controlling
interests
|
963,335 | 1,111,027 | ||||||
|
Total
Stockholders’ Equity
|
25,334,754 | 24,179,259 | ||||||
|
Total
Liabilities and Stockholders’ Equity
|
$ | 28,762,317 | $ | 27,114,629 | ||||
See
accompanying notes to the consolidated financial statements.
1
HUIHENG
MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF INCOME (UNAUDITED)
(IN US
DOLLARS)
|
For
The Three Months Ended
|
For
The Six Months Ended
|
|||||||||||||||
|
June 30,
|
June 30,
|
|||||||||||||||
|
2010
|
2009
|
2010
|
2009
|
|||||||||||||
|
REVENUES
|
$ | 1,436,905 | $ | 2,364,189 | $ | 2,837,758 | $ | 3,762,808 | ||||||||
|
COST
OF REVENUES
|
201,494 | 191,566 | 297,926 | 333,912 | ||||||||||||
|
GROSS
PROFIT
|
1,235,411 | 2,172,623 | 2,539,832 | 3,428,896 | ||||||||||||
|
OPERATING
EXPENSES:
|
||||||||||||||||
|
Sales
and marketing expenses
|
60,098 | 78,158 | 122,221 | 143,619 | ||||||||||||
|
General
and administrative expenses
|
654,069 | 566,197 | 1,149,817 | 1,434,853 | ||||||||||||
|
Research
and development costs
|
21,498 | 118,485 | 36,838 | 207,889 | ||||||||||||
|
Total
Operating Expenses
|
735,665 | 762,840 | 1,308,876 | 1,786,361 | ||||||||||||
|
OPERATING
INCOME
|
499,746 | 1,409,783 | 1,230,956 | 1,642,535 | ||||||||||||
|
OTHER
INCOME / (EXPENSES)
|
||||||||||||||||
|
Interest
income
|
61 | 59 | 104 | 175 | ||||||||||||
|
Gain
on business acquisition
|
21,508 | - | 21,508 | - | ||||||||||||
|
Equity
in income / (loss) of affiliate
|
55,042 | (10,851 | ) | 44,502 | 16,579 | |||||||||||
|
Total
Other Income / (Expenses)
|
76,611 | (10,792 | ) | 66,114 | 16,754 | |||||||||||
|
NET
INCOME BEFORE INCOME TAXES
|
576,357 | 1,398,991 | 1,297,070 | 1,659,289 | ||||||||||||
|
INCOME
TAXES
|
186,355 | 212,010 | 313,782 | 345,687 | ||||||||||||
|
NET
INCOME
|
390,002 | 1,186,981 | 983,288 | 1,313,602 | ||||||||||||
|
NET
LOSS ATTRIBUTABLE TO NON-CONTROLLING INTERESTS
|
91,172 | 42,177 | 154,309 | 117,812 | ||||||||||||
|
NET
INCOME ATTRIBUTABLE TO COMMON SHAREHOLDERS
|
$ | 481,174 | $ | 1,229,158 | $ | 1,137,597 | $ | 1,431,414 | ||||||||
|
EARNINGS
PER SHARE
|
||||||||||||||||
|
-
Basic
|
$ | 0.03 | $ | 0.09 | $ | 0.08 | $ | 0.10 | ||||||||
|
-
Diluted
|
$ | 0.03 | $ | 0.08 | $ | 0.07 | $ | 0.09 | ||||||||
|
Weighted
Common Shares Outstanding
|
||||||||||||||||
|
-
Basic
|
13,935,290 | 13,914,282 | 13,935,290 | 13,910,800 | ||||||||||||
|
-
Diluted
|
16,251,113 | 16,251,113 | 16,251,113 | 16,251,113 | ||||||||||||
See
accompanying notes to the consolidated financial statements.
2
HUIHENG
MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY AND OTHER COMPREHENSIVE
INCOME
FOR THE
SIX MONTHS ENDED JUNE 30, 2010 (UNAUDITED)
(IN US
DOLLARS)
|
Series
A Preferred Stock
|
Common
Stock
|
Accumulated
|
||||||||||||||||||||||||||||||||||
|
Number
of
Shares
|
Amount
|
Number
of
Shares
|
Amount
|
Additional
Paid-in
Capital
|
Retained
Earnings
|
Other
Comprehensive
Income
|
Non-controlling
interests
|
Total
Stockholders’
Equity
|
||||||||||||||||||||||||||||
|
Balance,
December 31, 2009
|
220,467 | $ | 220 | 13,935,290 | $ | 13,935 | $ | 7,498,086 | $ | 13,799,481 | $ | 1,756,510 | $ | 1,111,027 | $ | 24,179,259 | ||||||||||||||||||||
|
Comprehensive
income:
|
||||||||||||||||||||||||||||||||||||
|
Net
income / (loss)
|
- | - | - | - | - | 1,137,597 | - | (154,309 | ) | 983,288 | ||||||||||||||||||||||||||
|
Foreign
currency translation gain
|
- | - | - | - | - | - | 165,590 | 6,617 | 172,207 | |||||||||||||||||||||||||||
|
Total
comprehensive income
|
- | - | - | - | - | - | - | - | 1,155,495 | |||||||||||||||||||||||||||
|
Balance,
June 30, 2010
|
220,467 | $ | 220 | 13,935,290 | $ | 13,935 | $ | 7,498,086 | $ | 14,937,078 | $ | 1,922,100 | $ | 963,335 | $ | 25,334,754 | ||||||||||||||||||||
See
accompanying notes to the consolidated financial
statements.
3
HUIHENG
MEDICAL, INC. AND SUBSIDIARIES
CONSOLIDATED
STATEMENTS OF CASH FLOWS (UNAUDITED)
(IN US
DOLLARS)
|
For
The Six Months Ended
|
||||||||
|
June
30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Cash
flows from operating activities:
|
||||||||
|
Net
income
|
$ | 983,288 | $ | 1,313,602 | ||||
|
Adjustments
to reconcile net income to net cash used in operating
activities:
|
||||||||
|
Depreciation
of property, plant and equipment
|
121,526 | 122,540 | ||||||
|
Recovery
of bad debts
|
(731,989 | ) | ||||||
|
Amortization
of land use rights
|
9,599 | 7,984 | ||||||
|
Amortization
of intangible assets
|
41,310 | 41,230 | ||||||
|
Write
off of deferred offering costs
|
- | 460,209 | ||||||
|
Equity
in income of affiliate
|
(44,502 | ) | (16,579 | ) | ||||
|
Gain
on acquisition of subsidiary
|
(21,508 | ) | - | |||||
|
Changes
in assets and liabilities:
|
||||||||
|
Accounts
receivable
|
(677,204 | ) | (1,072,961 | ) | ||||
|
Prepaid
expenses
|
(288,899 | ) | (292,498 | ) | ||||
|
Other
receivables
|
82,739 | 265,603 | ||||||
|
Inventories
|
(540,827 | ) | (246,759 | ) | ||||
|
Accounts
payable
|
(56,093 | ) | (152,256 | ) | ||||
|
Income
tax payable
|
250,200 | 345,737 | ||||||
|
Accrued
liabilities and other payables
|
(52,080 | ) | (137,357 | ) | ||||
|
Net
cash used in operating activities
|
(192,451 | ) | (93,494 | ) | ||||
|
Cash
flows from investing activities:
|
||||||||
|
Capital
expenditures on addition of property, plant and equipment
|
(3,522 | ) | - | |||||
|
Advance
from related party
|
90,000 | |||||||
|
Repayment
of advances to related party
|
731,989 | |||||||
|
Payment
for land use right
|
- | (956,226 | ) | |||||
|
Net
cash provided by / (used in) investing activities
|
86,478 | (224,237 | ) | |||||
|
Net
decrease in cash
|
(105,973 | ) | (317,731 | ) | ||||
|
Effect
on change of exchange rates
|
144,298 | (22,216 | ) | |||||
|
Cash
as of January 1
|
84,962 | 1,019,176 | ||||||
|
Cash
as of June 30
|
$ | 123,287 | $ | 679,229 | ||||
|
Supplemental
disclosures of cash flow information:
|
||||||||
|
Cash
paid during the period for:
|
||||||||
|
Interest
paid
|
$ | - | $ | - | ||||
|
Income
tax paid
|
$ | 67,562 | $ | - | ||||
Acquisition
of subsidiary:
During
the period the group acquired subsidiary, Portola Medical, Inc., the fair value
of assets acquired and liabilities assumed were as follows:
|
Plant
and equipment
|
$ | 31,508 | ||
|
Intangible
assets
|
250,000 | |||
| 281,508 | ||||
|
Less:
Cash consideration paid
|
(260,000 | ) | ||
|
Gain
on acquisition of Portola Medical, Inc.
|
$ | 21,508 |
See
accompanying notes to the consolidated financial statements.
4
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
1 - ORGANIZATION AND OPERATIONS
Huiheng
Medical, Inc. (“the Company” or “Huiheng”), formerly known as Mill Basin
Technologies, Limited (“Mill Basin”), is a China-based medical device company
that, through its subsidiaries, designs, develops and markets radiation therapy
systems used for the treatment of cancer. The Company is a Nevada
holding company and conducts all of its business through operating subsidiaries
in China.
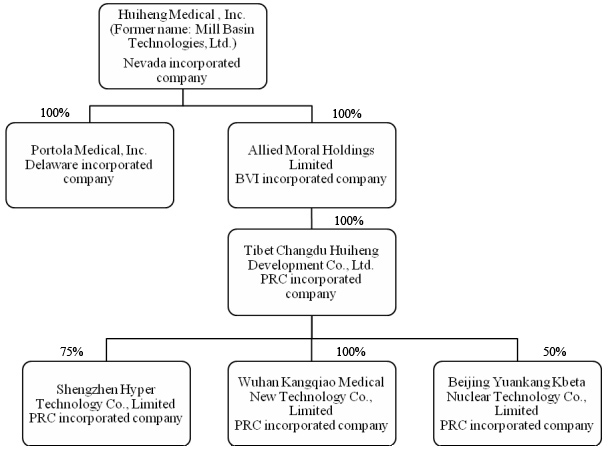
Acquisition
of New Subsidiary
On June
7, 2010, the Company acquired a new wholly-owned subsidiary, Portola Medical,
Inc. with authorized 100 common shares with par value $0.01 per share which
registered capital of $1 in Delaware, USA, for the purpose of expanding the
product line.
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING
POLICIES
Basis
of Presentation
The
consolidated financial statements include all accounts of the Company and its
wholly-owned and majority-owned subsidiaries. All material
inter-company balances and transactions have been eliminated in
consolidation.
The
accompanying unaudited consolidated financial statements were prepared in
accordance with U.S. generally accepted accounting principles (“GAAP”) for
interim financial information and with the instructions to Form 10-Q and Article
10 of Regulation S-X. Accordingly, they may not include all of the information
and footnotes required by GAAP for complete consolidated financial statements.
All adjustments that are, in the opinion of management, of a normal recurring
nature and are necessary for a fair presentation of the consolidated financial
statements have been included. Nevertheless, these consolidated financial
statements should be read in conjunction with the Company’s audited consolidated
financial statements contained in its Annual Report on Form 10-K for the year
ended December 31, 2009 as filed with the Securities and Exchange Commission on
April 15, 2010. The results of operation for the six months ended June 30, 2010,
are not necessarily indicative of the results that may be expected for the
entire fiscal year or any other interim period.
The
Company’s common stock is listed on the Over-the-counter Bulletin Board
(“OTCBB”) market and traded under the symbol “HHGM”.
5
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies
Estimates
The
preparation of the financial statements in accordance with US GAAP requires
management of the Company to make a number of estimates and assumptions relating
to the reported amounts of assets and liabilities and the disclosure of
contingent assets and liabilities at the date of the financial statements and
the reported amounts of revenues and expenses during the years. Significant
items subject to such estimates and assumptions include the recoverability of
the carrying amount and the estimated useful lives of long-lived assets;
valuation allowances for receivables and realizable values for inventories.
Actual results could differ from those estimates in consolidation.
Accounts
receivable
Accounts
receivable are recorded at the invoiced amount, net of allowances for doubtful
accounts, sales returns, trade discounts and value added tax. The allowance for
doubtful accounts is the Company’s best estimate of the amount of probable
credit losses in the Company’s existing accounts
receivable. The Company performs ongoing credit evaluations of
its customers’ financial conditions. The Company provided an allowance of
$903,353 and $897,319 for doubtful accounts respectively as of June 30, 2010 and
December 31, 2009.
Outstanding
account balances are reviewed individually for collectability. Account balances
are charged off against the allowance after all means of collection have been
exhausted and the potential for recovery is considered remote.
Inventories
The
Company values inventories, consisting of work in process and raw materials, at
the lower of cost or market. Cost of material is determined on the
weighted average cost method. Cost of work in progress includes direct
materials, direct production cost and an allocated portion of production
overhead.
The final
steps of assembly of our products, including installation of radioactive service
materials, are completed at customer locations. Accordingly, the Company
generally does not carry finished goods (inventory held for sale in the ordinary
course of business) inventory.
Property,
plant and equipment
Property,
plant and equipment are recorded at cost less accumulated
depreciation. Expenditures for major additions and betterments are
capitalized. Maintenance and repairs are charged to general and
administrative expenses as incurred. Depreciation of property, plant and
equipment is computed by the straight-line method (after taking into account
their respective estimated residual values) over the assets estimated useful
lives ranging from three to twenty years. Building improvements are amortized on
a straight-line basis over the estimated useful life. Depreciation of property,
plant and equipment are stated at cost less accumulated
depreciation. Upon sale or retirement of property, plant and
equipment, the related cost and accumulated depreciation are removed from the
accounts and any gain or loss is reflected in operations. The estimated useful
lives of the assets are as follows:
|
Estimated Life
|
||
|
Building
improvements
|
3 to 5
|
|
|
Buildings
|
20
|
|
|
Production
equipment
|
3 to 5
|
|
|
Furniture
fixtures and office equipment
|
3 to 5
|
|
|
Motor
vehicles
|
|
5 to 10
|
6
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Land
use right
Land use
rights represent the prepayments for the use of the parcels of land in PRC where
the Company charged to expense over their respective lease periods of 50
years. According to the laws of the PRC, the government owns all of
the land in the PRC. Companies or individuals are authorized to possess and use
the land only through land use rights granted by the PRC government for a
certain period usually 50 years.
Intangible
assets
The
Company’s intangible assets include patent and pending patents
applications. The Company accounts for its intangible assets pursuant
to FASB ASC Subtopic 350-30, “General Intangibles Other Than Goodwill”. Under
ASC 350-30-35, intangibles with definite lives are amortized on a straight-line
basis over the lesser of their estimated useful lives or contractual terms.
Accordingly, the Company amortizes the patent over their remaining legal term of
20 years, on a straight-line basis.
Effective
January 1, 2010, the Company adopted the provisions in ASU 2010-06, “Fair Value
Measurements and Disclosures (ASC Topic 820): Improving Disclosures about Fair
Value Measurements, which requires new disclosures related to transfers in and
out of levels 1 and 2 and activity in level 3 fair value measurements, as well
as amends existing disclosure requirements on level of disaggregation and inputs
and valuation techniques. The adoption of the provisions in ASU 2010-06 did not
have a material impact on the Company’s consolidated financial
statements.
|
Estimated Life
|
||
|
Patented
technology
|
20
|
Investment
in affiliate
The
Company owns a 50% equity interest of Beijing Yuankang Kbeta Nuclear Technology
Company, Ltd (“Beijing Kbeta”) and accounts for the investment using the equity
method of accounting. The equity method is utilized as the Company has the
ability to exercise significant influence over the investee, but does not have a
controlling financial interest.
If
circumstances indicate that the carrying value of the Company’s investment in
Beijing Kbeta may not be recoverable, the Company would recognize an impairment
loss by writing down its investment to its estimated net realizable value if
management concludes such impairment is other than
temporary.
7
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Impairment
of long-lived assets
Long-lived
assets, which include tangible assets and intangible assets, are reviewed for
impairment whenever events or changes in circumstances indicate that the
carrying amount of an asset may not be recoverable.
Recoverability
of long-lived assets to be held and used is measured by a comparison of the
carrying amount of the asset to the estimated undiscounted future cash flows
expected to be generated by the asset. If the carrying amount of an asset
exceeds its estimated undiscounted future cash flows, an impairment charge is
recognized by the amount by which the carrying amount of the asset exceeds the
fair value of the asset. Assets to be disposed of, if any, are separately
presented in the balance sheet and reported at the lower of the carrying amount
or fair value less costs to sell, and are no longer depreciated. At
June 30, 2010 and 2009, the Company determined that there was no impairment of
value.
Fair
value of financial instruments
The fair
value of a financial instrument is the amount at which the instrument could be
exchanged in a current transaction between willing parties. The carrying amounts
of financial assets and liabilities, such as cash, accounts receivable, current
income tax assets, prepaid expenses and other current assets, accounts payable,
income taxes payable, accrued expenses and other current liabilities,
approximate their fair values because of the short maturity of these instruments
and market rates of interest.
Revenue
recognition
The
Company generates revenue primarily from sales of medical equipment and the sale
of maintenance and support services. Revenue is recognized as
follows:
|
(i)
|
Sales
of medical equipment
|
The
Company recognizes revenue when products are delivered and the customer takes
ownership and assumes risk of loss, collection of the relevant receivable is
probable, persuasive evidence of an arrangement exists and the sales price is
fixed or determinable. The sales price of the medical equipment includes the
training services, which generally take about 1 month. These services are
ancillary to the purchase of medical equipment by customers and are normally
considered by the customers to be an integral part of the acquired equipment. As
training services do not have separately determinable fair values, the Company
recognizes revenue for the entire arrangement upon customer acceptance, which
occurs after delivery and installation.
In the
PRC, value added tax (“VAT”) of 17% on invoiced amounts is collected in respect
of the sales of goods on behalf of tax authorities. The VAT collected is not
revenue of the Company; instead, the amount is recorded as a liability on the
balance sheet until such VAT is paid to the authorities.
Pursuant
to the laws and regulations of the PRC, Shenzhen Hyper is entitled to a refund
of VAT on the sales of self-developed software embedded in medical equipment.
The VAT refund represents the amount of VAT collected from customers and paid to
the authorities in excess of 3% of relevant sales. The amount of VAT refund is
calculated on a monthly basis. As the refund relates directly to the sale of
self-developed software that is embedded in the Company’s products, the Company
recognizes the VAT refund at the time the product is sold. The amount is
included in the line item “Revenues” in the consolidated statements of income
and is recorded on an accrual basis.
8
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Revenue
recognition (…/Cont’d)
The
medical equipment sold by the Company has embedded self-developed software which
can also be sold on a standalone basis.
|
(ii)
|
Provision
of maintenance and support services
|
The
Company also provides comprehensive post-sales services to certain distributors
for medical equipment used by hospitals. These contracts are negotiated and
signed independently and separately from the sales of medical equipments. In
accordance with the agreements, the Company provides comprehensive services
including replace of cobalt, additional training to users of the medical
equipment, maintenance of medical equipment, software upgrades and consulting.
Fees for these services are recognized over the life of the contract on a
monthly basis.
Government
subsidies
Pursuant
to the confirmation of tax position of Changdu Huiheng dated December 16, 2004
with No.173 issued by Tibet Finance Bureau, the profits tax payment of Changdu
Huiheng in excess of RMB 900,000 for a year will be refundable by Tibet Finance
Bureau. The 31% of business tax payment will be refundable by Tibet Finance
Bureau provided that the business tax payment exceeds RMB 1.0 million for a
year. The 38.75% of value added tax payment will be refundable by Tibet Finance
Bureau provided that the value added tax payment exceeds RMB 1.5 million for a
year. All tax incentive policies will be valid for five (5) years from the year
of commencement of tax refund, starting from September 2006.
Warranty
The
Company provides a product warranty to its customers to repair any product
defects that occur generally within twelve months from the date of sale. The
Company’s purchase contracts generally allow the customer to withhold up to 10%
of the total purchase price for the duration of the warranty period and included
in Accounts receivable. Based on the limited number of actual warranty claims
and the historically low cost of such repairs, the Company has not recognized a
liability for warranty claims, but rather recognizes such cost when product
repairs are made.
Research
and development costs
Research
and development costs are charged to expense as incurred. Research and
development costs mainly consist of remuneration for the research and
development staff and material costs for research and development. The Company
incurred $36,838 and $207,889 for the three months ended June 30, 2010 and 2009,
respectively.
9
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Income
taxes
The
Company accounts for income taxes under ASC 740 “Income Taxes”. Deferred income
tax assets and liabilities are determined based upon differences between the
financial reporting and tax bases of assets and liabilities and are measured
using the enacted tax rates and laws that will be in effect when the differences
are expected to reverse. Deferred tax assets are reduced by a valuation
allowance to the extent management concludes it is more likely than not that the
assets will not be realized. Deferred tax assets and liabilities are measured
using enacted tax rates expected to apply to taxable income in the years in
which those temporary differences are expected to be recovered or settled. The
effect on deferred tax assets and liabilities of a change in tax rates is
recognized in the statements of operations in the period that includes the
enactment date.
During
2008, the Company adopted ASC740 “Income Taxes”, which prescribes a
more-likely-than-not threshold for financial statement recognition and
measurement of a tax position taken in the tax return. This interpretation also
provides guidance on de-recognition of income tax assets and liabilities,
classification of current and deferred income tax assets and liabilities,
accounting for interest and penalties associated with tax positions, accounting
for income taxes in interim periods and income tax disclosures.
Foreign
currency translation
Assets
and liabilities of foreign subsidiaries are translated at the rate of exchange
in effect on the balance sheet date; income and expenses are translated at the
average rate of exchange prevailing during the period. The related transaction
adjustments are reflected in “Accumulated other comprehensive income / (loss)”
in the equity section of our consolidated balance sheet.
The
average monthly exchange rates for period ended June 30, 2010 and the closing
rate as of June 30, 2010 were RMB 6.8189 and RMB 6.7814 to one USD,
respectively. The average monthly exchange rates for period ended June 30, 2009
and the closing rate as of June 30, 2009 were RMB 6.8322 and RMB 6.8307 to one
USD, respectively.
Stock
Option Plan
During
2009, the Company adopted a stock option plan (the “Plan”) for selected
employees, directors, consultants to promote the success of the Company’s
business by offering these individuals an opportunity to acquire a proprietary
interest in the Company. The Plan provides both for direct awards of
shares and for the granting of options to purchase shares as determined by the
Administrator at the time of the grant. The Plan replaces the
Company’s 2007 Share Plan which was never approved by the Company’s
shareholders. Under the Plan, 1,566,666 shares have been reserved for awards.
The number of shares reserved under the Plan is the same number that was
reserved under the Company’s 2007 Stock Option Plan.
Awards
under the Plan will be accounted for in accordance with ASC 718 “Stock
Compensation”.
10
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Other
comprehensive income
The
Company has adopted ASC 220 “Comprehensive Income”. This statement establishes
rules for the reporting of other comprehensive income and its
components. Other comprehensive income consists of net income and
foreign currency translation adjustments and is presented in the Consolidated
Statements of Income and the Consolidated Statement of Changes in Stockholders’
Equity.
Earnings
per share
The value
of basic earnings per share is computed on the basis of the weighted-average
number of shares of our common stock outstanding during the period. Diluted
earnings per share is computed on the basis of the weighted-average number of
shares of our common stock plus the effect of dilutive potential common shares
outstanding during the period using the if-converted method. Dilutive potential
common shares include Series A Convertible Preferred Stock.
The
following table sets forth the computation of basic and diluted net income per
common share:
|
For
the Six Months Ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Net
income per common share
|
$ | 1,137,597 | $ | 1,431,414 | ||||
|
Weighted
average outstanding shares of common stock
|
13,935,290 | 13,910,800 | ||||||
|
Dilutive
effect of Convertible Preferred Stock
|
2,315,823 | 2,340,313 | ||||||
|
Diluted
weighted average outstanding shares
|
16,251,113 | 16,251,113 | ||||||
|
Earnings
per common share:
|
||||||||
|
Basic
|
$ | 0.08 | $ | 0.10 | ||||
|
Diluted
|
$ | 0.07 | $ | 0.09 | ||||
For the
six months ended June 30, 2010 options to purchase 30,000 common shares were not
included in diluted earnings per share because the effect would be
anti-dilutive.
Commitments
and contingencies
Liabilities
for loss contingencies arising from claims, assessments, litigation, fines and
penalties and other sources are recorded when it is probable that a liability
has been incurred and the amount of the assessment can be reasonably
estimated.
Segment
reporting
ASC 280
“Segment Reporting”, establishes standards for reporting information on
operating segments in interim and annual financial statements. The
Company operates in two segments (i) selling the medical equipment and, (ii)
providing the consultancy, repairs and maintenance services for the
customers. The chief operating decision-makers review the Company’s
operation results on an aggregate basis and manage the operations as two
operating segments as disclosed in note 13.
11
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Summary
of significant accounting policies (…/Cont’d)
Financial
instruments with characteristics of both liabilities and equity
The
Company accounts for its Series A Preferred Stock in accordance with ASC 480
“Distinguishing Liabilities from Equity” and ASC 815 “Derivatives and
Hedging”. We
have determined that our Series A Preferred Stock is not mandatorily redeemable.
Accordingly, the Company accounts for the Preferred stock as permanent
equity.
Non-controlling
interest in consolidated financial statements
In
December 2007, the FASB issued authoritative guidance related to non-controlling
interests in consolidated financial statements, which was an amendment of ARB
No. 51. This guidance is set forth in Topic 810 in the Accounting Standards
Codification (ASC 810). ASC 810 establishes accounting and reporting standards
for the non-controlling interest in a subsidiary and for the deconsolidation of
a subsidiary. This accounting standard is effective for fiscal years beginning
after December 15, 2008. The Company adopted the presentation and disclosure
requirements of ASC 810 retrospectively to the December 31, 2008 financial
statements.
Fair
value measurements
ASC Topic
820, Fair Value Measurement and Disclosures, defines fair value as the exchange
price that would be received for an asset or paid to transfer a liability (an
exit price) in the principal or most advantageous market for the asset or
liability in an orderly transaction between market participants on the
measurement date. This topic also establishes a fair value hierarchy which
requires classification based on observable and unobservable inputs when
measuring fair value. There are three levels of inputs that may be used to
measure fair value:
|
Level
1 -
|
Quoted
prices in active markets for identical assets or
liabilities.
|
|
Level
2 -
|
Observable
inputs other than Level 1 prices such as quoted prices for similar assets
or liabilities; quoted prices in markets that are not active; or other
inputs that are observable or can be corroborated by observable market
data for substantially the full term of the assets or
liabilities.
|
|
Level
3 -
|
Unobservable
inputs that are supported by little or no market activity and that are
significant to the fair value of the assets or
liabilities.
|
Determining
which category an asset or liability falls within the hierarchy requires
significant judgment. The Company evaluates its hierarchy disclosures each
quarter.
The
carrying values of cash and cash equivalents, account receivable, and account
payable, approximate fair values due to their short maturities.
There was
no asset or liability measured at fair value on a non-recurring basis as of June
30, 2010 or 2009.
12
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(…/Cont’d)
Impact
of new accounting standards
We
describe below recent pronouncements that have had or may have a significant
effect on our financial statements. We do not discuss recent pronouncements that
are not anticipated to have an impact on or are unrelated to our financial
condition, results of operations, or disclosures.
In June
2009, the FASB issued Updates No. 2009-01, which establishes the FASB Accounting
Standards Codification TM (the Codification) as the source of authoritative
accounting principles recognized by the FASB to be applied by nongovernmental
entities in the preparation of financial statements in conformity with generally
accepted accounting principles (GAAP). The Codification is effective for interim
and annual periods ending after September 15, 2009. We adopted the Codification
when referring to GAAP in this quarterly report on Form 10-Q for the fiscal
period ending June 30, 2010. The adoption of the Codification did not have an
impact on our consolidated results.
The FASB
issued authoritative guidance related to subsequent events in May 2009, which
establishes general standards of accounting for and disclosure of events that
occur after the balance sheet date but before the financial statements are
issued or are available to be issued. This guidance is set forth in Topic 855 in
the Accounting Standards Codification (ASC 855). ASC 855 provides guidance on
the period after the balance sheet date during which management of a reporting
entity should evaluate events or transactions that may occur for potential
recognition or disclosure in the financial statements, the circumstances under
which an entity should recognize events or transactions occurring after the
balance sheet date in its financial statements and the disclosures that an
entity should make about events or transactions that occurred after the balance
sheet date. We adopted ASC 855 and its application had no impact on our
consolidated financial statements.
In
October 2009, the FASB issued authoritative guidance that amends existing
guidance for identifying separate deliverables in a revenue-generating
transaction where multiple deliverables exist, and provides guidance for
allocating and recognizing revenue based on those separate deliverables. The
guidance is expected to result in more multiple-deliverable arrangements being
separable than under current guidance and is required to be applied
prospectively to new or significantly modified revenue arrangements. This
guidance, for which the Company is currently assessing the impact on its
financial condition and results of operations, will become effective for the
Company on January 1, 2011.
In
January 2010, the FASB issued authoritative guidance intended to improve
disclosures about fair value measurements. The guidance requires entities to
disclose significant transfers in and out of fair value hierarchy levels and the
reasons for the transfers and to present information about purchases, sales,
issuances and settlements separately in the reconciliation of fair value
measurements using significant unobservable inputs (Level 3). Additionally, the
guidance clarifies that a reporting entity should provide fair value
measurements for each class of assets and liabilities and disclose the inputs
and valuation techniques used for fair value measurements using significant
other observable inputs (Level 2) and significant unobservable inputs (Level 3).
This guidance is effective for interim and annual periods beginning after
December 15, 2009 except for the disclosures about purchases, sales, issuances
and settlements in the Level 3 reconciliation, which will be effective for
interim and annual periods beginning after December 15, 2010. As this guidance
provides only disclosure requirements, the adoption of this guidance will not
impact the Company’s financial condition or results of operations.
In April
2010, the FASB issued guidance on defining a milestone and determining when it
may be appropriate to apply the milestone method of revenue recognition for
research or development transactions. The guidance is effective on a prospective
basis for milestones achieved in fiscal years, and interim periods within those
years, beginning on or after June 15, 2010, with early adoption permitted. The
Company does not expect a material impact on its consolidated financial
statements upon the adoption of the new accounting standard.
Management
does not believe that any other recently issued, but not yet effective
accounting pronouncements, if adopted, would have a material effect on the
accompanying financial statements.
13
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
3 – INVENTORIES
Inventories
consisted of the following:
|
June
30, 2010
|
December
31, 2009
|
|||||||
|
Raw
materials
|
$ | 455,358 | $ | 281,112 | ||||
|
Work-in-progress
|
1,445,369 | 1,078,788 | ||||||
| $ | 1,900,727 | $ | 1,359,900 | |||||
Final
assembly of our products, including installation of radioactive source
materials, is conducted on site at our customers’ locations. Our products are
not considered to be finished good (available for sale in the normal course of
business) until such time as the source material is installed in the
units.
NOTE
4 – OTHER RECEIVABLES
Other
receivables, net, consisted of the following:
|
June
30, 2010
|
December
31, 2009
|
|||||||
|
Other
receivables
|
||||||||
|
-
current portion
|
||||||||
|
-
construction in progress paid on behalf of landlord (a)
|
||||||||
|
current
portion
|
$ | 157,814 | $ | 156,759 | ||||
| - loan or advance to staff for business travelling | 86,705 | 43,072 | ||||||
| - utilities and rental deposits | 1,961 | 1,948 | ||||||
| - prepaid expenses made by director | 2,949 | 2,930 | ||||||
| - others (net of allowance for doubtful accounts | ||||||||
|
of
$737,311 and $732,386)
|
47,547 | 44,081 | ||||||
|
Other
receivables – current portion
|
$ | 296,976 | $ | 248,790 | ||||
|
Other
receivables - Non-current portion
|
$ | 1,451,168 | $ | 1,582,093 | ||||
|
(a)
|
Under
the agreement signed with the landlord, Shenzhen OUR Technology Co., Ltd.,
Shenzhen Hyper will make payment for the construction in progress of the
building in advance on behalf the landlord. Meanwhile, Shenzhen Hyper
signed a rental agreement with the landlord to rent the building for 20
years at about US $23,500 (RMB160,000) per month. The balance of the
amount due from the landlord will be used to set off with the rental
expenses incurred by Shenzhen
Hyper.
|
14
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
5 – PROPERTY, PLANT AND EQUIPMENT
Property,
plant and equipment consisted of the following:
|
June
30, 2010
|
December
31, 2009
|
|||||||
|
Building
improvements
|
$ | 173,390 | $ | 172,232 | ||||
|
Buildings
|
2,323,067 | 2,307,550 | ||||||
|
Production
equipment
|
662,092 | 656,423 | ||||||
|
Furniture,
fixture and office equipment
|
403,113 | 366,868 | ||||||
|
Motor
vehicles
|
188,568 | 187,308 | ||||||
| 3,750,230 | 3,690,381 | |||||||
|
Less:
Accumulated depreciation
|
(1,299,661 | ) | (1,169,594 | ) | ||||
| $ | 2,450,569 | $ | 2,520,787 | |||||
Depreciation
expense is included in the consolidated statements of income. For the six months
ended June 30, 2010 and 2009, depreciation expenses were $121,526 and $122,540,
respectively.
NOTE
6 - LAND USE RIGHT
Land use
right consisted of the following:
|
June
30, 2010
|
December
31, 2009
|
|||||||
|
Land
use right
|
$ | 936,241 | $ | 939,575 | ||||
Land use
right represents prepaid lease payments to the Local Government for land use
right held for a period of 50 years from January 20, 2009 to December 26, 2058
in Wuhan, People’s Republic of China.
Land use
right is amortized using the straight-line method over the lease term of 50
years. The amortization expense for the six months ended June 30,
2010 and 2009 were $9,599 and $7,984, respectively.
15
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
7 – INTANGIBLE ASSETS
Intangible
assets, net consisted of the following:
|
June 30, 2010
|
December 31, 2009
|
|||||||
|
Patent
technology in Shenzhen Hyper (A)
|
$ | 1,474,622 | $ | 1,464,772 | ||||
|
Patent
technology in Portola Medical, Inc. (B)
|
250,000 | - | ||||||
|
Less:
Accumulated amortization
|
(733,850 | ) | (687,686 | ) | ||||
| $ | 990,772 | $ | 777,086 | |||||
(A)
Patent represents a patent technology for the production of a component of the
radiation treatment system. The patent was applied prior to its injection to
Shenzhen Hyper as a capital contribution. Pursuant to the patent certificate,
the patent was valid for 20 years from the application date, May 1999. Therefore
it was amortized over the rest of the valid patent period, which is the
estimated remaining useful life.
(B)
Patent acquired in Portola Medical, Inc. No amortization expense have been
charged since the amount is immaterial.
Patent
technology is utilized in the production of medical equipment and is amortized
over its estimated useful life.
Amortization
expenses were $41,310 and $41,230 for the six months ended June 30, 2010 and
2009 ($4,794 and $3,191 for the three months ended March 31, 2010 and 2009). The
expected amortization for the next five years and thereafter is as
follows:
|
Patent
technology
in
|
Patent
technology
in
|
|||||||||||
|
Portola
|
Shenzhen
|
|||||||||||
|
Medical,
Inc.
|
Hyper
|
Total
|
||||||||||
|
For
the twelve months ended June 30
|
||||||||||||
|
2011
|
$ | 12,500 | $ | 83,076 | $ | 95,576 | ||||||
|
2012
|
12,500 | 83,076 | 95,576 | |||||||||
|
2013
|
12,500 | 83,076 | 95,576 | |||||||||
|
2014
|
12,500 | 83,076 | 95,576 | |||||||||
|
2015
|
12,500 | 83,076 | 95,576 | |||||||||
|
Thereafter
|
187,500 | 325,392 | 512,892 | |||||||||
|
TOTAL
|
$ | 250,000 | $ | 740,772 | $ | 990,772 | ||||||
16
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
8 – ACCRUED LIABILITIES AND OTHER PAYABLES
Accrued
liabilities and other payables consisted of the following:
|
June 30, 2010
|
December 31,
2009
|
|||||||
|
Accrued
expenses
|
$ | 430,352 | $ | 365,923 | ||||
|
Accrued
payroll and welfare
|
190,268 | 188,125 | ||||||
|
Value
added tax, other taxes payable and surcharges
|
991,687 | 1,121,406 | ||||||
|
Customer
deposits
|
12,252 | 1,185 | ||||||
| $ | 1,624,559 | $ | 1,676,639 | |||||
NOTE
9 – NON-CONTROLLING INTEREST
Non-controlling
interest included in the Company’s balance sheets as of June 30, 2010 represent
25% equity interest in Shenzhen Hyper.
NOTE
10 –AMOUNTS DUE TO RELATED PARTIES
A
summary of related party payables at June 30, 2010 (unaudited) and December 31,
2009 is as follows:
Amounts
due to related parties at June 30, 2010 and December 31, 2009 represents the
remaining balance due to Clear Honest International Limited which was former
shareholder of Allied Moral pursuant to the 2007 share redemption as well as
advances related to the acquisition of Changdu Huiheng.
In June
2010, cash consideration of $260,000 and relevant expenses of $30,000 for
acquiring the new subsidiary, Portola Medical, Inc., were paid in advance by the
Company’s Chairman, Hui Xiaobing.
NOTE 11 –STOCKHOLDERS’
EQUITY
(a) Capital
The
Company has authorized 74,000,000 shares of Common stock. As of June 30, 2010,
23,635,290 shares were issued and 13,935,290 shares were outstanding which is
net of 9,700,000 treasury shares contributed from Mill Basin’s
shareholders.
The
Company has authorized 1,000,000 shares of Preferred stock, with 300,000 shares
designated as Series A convertible preferred stock. As of June 30, 2010, 220,467
shares were issued and outstanding with a liquidation preference of
$8,267,513.
17
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
11 –STOCKHOLDERS’ EQUITY (…/Cont’d)
(b) Retained
Earnings
As of
June 30, 2010, the Company established and segregated in retained earnings an
aggregate amount for the Statutory Surplus Reserve and the Statutory Common
Welfare Fund of $1,310,516.
Statutory
surplus reserve
In
accordance with PRC Company Law, Changdu Huiheng is required to appropriate at
least 10% of the profit to the statutory surplus reserve. Appropriation to the
statutory surplus reserve by Changdu Huiheng is based on profits arrived at
under PRC accounting standards for business enterprises for each
year.
The
profit arrived at must be set off against any accumulated losses sustained by
Changdu Huiheng in prior years, before allocation is made to the statutory
surplus reserve. Appropriation to the statutory surplus reserve must be made
before distribution of dividends to owners. The appropriation is required until
the statutory surplus reserve reaches 50% of the equity. This statutory surplus
reserve is not distributable in the form of cash dividends.
NOTE
12 - PORTOLA MEDICAL, INC. ACQUISITION
The
Company entered into an Agreement to purchase all the common stock of Portola
Medical, Inc, dated May 7, 2010, from Three Arch Capital, L.P., TAC Associates,
L.P., Three Arch Partners IV, L.P., and Three Arch Associates IV,
L.P.
The
authorized capital stock of Portola Medical, Inc. consists of Common Stock, par
value $0.01 per share, of which 100 shares were issued and outstanding. Under
the terms of the Agreement, the Company acquired 100% of the common stock in
Portola Medical, Inc. at $2,600 per share and the total consideration is
$260,000.
The
following table presents the allocation of the purchase price to the assets
acquired and liabilities assumed, based on their fair values:
|
Property,
plant, and equipment
|
$ | 31,508 | ||
|
Intangible
assets
|
250,000 | |||
|
Total
asset acquired
|
281,508 | |||
|
Total
liabilities assumed
|
- | |||
|
Net
assets acquired
|
$ | 281,508 |
The net
assets acquired exceeded the purchase price by $21,508 which was recorded as a
gain on business acquisition. Included in the net assets acquired, $250,000
represents the cost of acquired intangible assets, which is made up of 8 patents
with 20-year useful life (Note 7).
18
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
13 - SEGMENT REPORTING
The Group
has two reportable segments: products and services.
The
following table presents information about the Company’s operating segments for
the six months ended June 30, 2010 and 2009:
|
June 30, 2010
|
June 30, 2009
|
|||||||
|
Revenues:
|
||||||||
|
Products
|
$ | - | $ | - | ||||
|
Services
|
2,808,195 | 3,030,253 | ||||||
|
Other
|
29,563 | 732,555 | ||||||
| $ | 2,837,758 | $ | 3,762,808 | |||||
|
June 30, 2010
|
June 30, 2009
|
|||||||
|
Operating
(loss) income:
|
||||||||
|
Products
|
$ | (59,784 | ) | $ | (104,768 | ) | ||
|
Services
|
2,514,768 | 2,750,627 | ||||||
|
Other
|
29,563 | 732,555 | ||||||
| 2,484,547 | 3,378,414 | |||||||
|
Corporate
expenses
|
(1,253,591 | ) | (1,735,879 | ) | ||||
|
Operating
income
|
$ | 1,230,956 | $ | 1,642,535 | ||||
Other
revenue consists principally of government financial subsidies to Changdu
Huiheng.
NOTE
14 – INCOME TAXES
Huiheng
Medical, Inc. is a non-operating holding company. All of the Company’s income
before income taxes and related tax expenses are from PRC sources. The Company’s
PRC subsidiaries file income tax returns under the Income Tax Law of the
People’s Republic of China concerning Foreign Investment Enterprises and Foreign
Enterprises and local income tax laws.
Income
tax expense for the three months ended June 30, 2010 and 2009 was $313,782 and
$345,687, respectively.
As
Changdu Huiheng, Huiheng’s subsidiary, is located in the western area in the PRC
and is within the industry specified by relevant laws and regulations of the
PRC, the tax rate applicable to Changdu Huiheng is 15% (2009: 12%).
Wuhan
Kangqiao is a high-tech enterprise with operations in an economic-technological
development area in the PRC. Therefore, the applicable tax rate is
25%.
Shenzhen
Hyper is a high-tech manufacturing company located in the Shenzhen special
economic region. Therefore, the applicable tax rate is also 15% (2009:18%).
According to local tax regulation, Shenzhen Hyper is entitled to a tax-free
period for the first two years, commencing from the first profit-making year and
a 50% reduction in state income tax rate for the next six years.
19
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
14 – INCOME TAXES (…/Cont’d)
A
reconciliation of the expected income tax expense to the actual income tax
expense for the period ended June 30, 2010 and 2009 are as follows:
|
Six Months Ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
Income
before income taxes
|
$ | 1,297,070 | $ | 1,659,289 | ||||
|
Expected
PRC income tax expense at statutory tax rate of 25% (2009:
25%)
|
324,268 | 414,822 | ||||||
|
Non-deductible
expenses
|
1,434 | 39,891 | ||||||
|
Others
|
147,596 | 206,158 | ||||||
|
Tax
rate differences
|
(154,139 | ) | (315,184 | ) | ||||
|
Actual
income tax expense
|
$ | 319,159 | $ | 345,687 | ||||
The PRC
tax system is subject to substantial uncertainties and has been subject to
recently enacted changes. The interpretation and enforcement of which are also
uncertain. The Company remains open to examination by the major jurisdictions to
which the Company is subject to, in this case, the PRC tax
authorities.
No
deferred tax liability has been provided as the amount involved is
immaterial.
NOTE
15 – CONCENTRATION OF CREDIT RISK
Customers’
concentrations
Customers
accounting for 10% or more of the Group’s net revenue as follows:
|
Six months ended June 30,
|
||||||||
|
2010
|
2009
|
|||||||
|
%
|
%
|
|||||||
|
Customer
A
|
53 | % | 53 | % | ||||
|
Customer
B
|
35 | % | 33 | % | ||||
|
Customer
C
|
11 | % | - | |||||
Three
customers accounted for 99% and three customers accounted for 93% of revenue for
the six months ended June 30, 2010 and 2009, respectively. These customers also
accounted for 84% and 77% of accounts receivable as of June 30, 2010 and 2009,
respectively. As a result, a termination in relationship with or a reduction in
orders from any of these customers could have a material impact on the Company’s
results of operations and financial condition.
Except as
disclosed above, no other single customer accounted for 10% or more of the
Group’s net revenue for the six months ended June 30, 2010 and
2009.
20
HUIHENG
MEDICAL, INC.
NOTES TO
THE CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
(IN US
DOLLARS)
NOTE
15 – CONCENTRATION OF CREDIT RISK (…/Cont’d)
Other
credit risks
As of
June 30, 2010, all of the Company’s cash and cash equivalents were held by major
financial institutions located in the PRC, none of which were insured or
collateralized. However, management believes those financial institutions are of
high credit quality and has assessed the loss arising from the non-insured cash
and cash equivalents from those financial institutions to be immaterial to the
consolidated financial statements. Therefore, no loss in respect of the cash and
cash equivalent were recognized as of June 30, 2010.
NOTE
16 - FOREIGN OPERATIONS
Operations
All of
the Company’s operations are carried out and substantially all of its assets are
located in the PRC. Accordingly, the Company’s business, financial condition and
results of operations may be influenced by the political, economic and legal
environments in the PRC. The Company’s business may be influenced by changes in
governmental policies with respect to laws and regulations, anti-inflationary
measures, currency fluctuation and remittances and methods of taxation, among
other things.
Dividends
and reserves
Under
laws of the PRC, net income after taxation can only be distributed as dividends
after appropriation has been made for the following: (i) cumulative prior years’
losses, if any; (ii) allocations to the “Statutory Surplus Reserve” of at least
10% of net income after tax, as determined under PRC accounting rules and
regulations, until the fund amounts to 50% of the Company’s equity; (iii)
allocations of 5-10% of income after tax, as determined under PRC accounting
rules and regulations, to the Company’s “Statutory Common Welfare Fund”, which
is established for the purpose of providing employee facilities and other
collective benefits to employees in China; and (iv) allocations to any
discretionary surplus reserve, if approved by equity owners.
NOTE
17 - OPERATING LEASE COMMITMENTS
As of
June 30, 2010, the total future minimum lease payments under non-cancellable
operating leases in respect of premises are $4.91 million (RMB33.28 million),
which was based on the closing rate as of June 30, 2010. The amounts payable are
as follows:
|
For
the twelve months ended June 30
|
||||
|
2011
|
$ | 275,189 | ||
|
2012
|
275,189 | |||
|
2013
|
275,189 | |||
|
2014
|
275,189 | |||
|
2015
|
275,189 | |||
|
Thereafter
|
3,531,596 | |||
|
TOTAL
|
$ | 4,907,541 | ||
21
Item
2. Management’s Discussion and Analysis of Financial Condition and Results of
Operations
The
following discussion and analysis of our financial condition and results of
operations should be read in conjunction with our unaudited consolidated
financial statements and related notes appearing elsewhere in this Quarterly
Report. In addition to historical financial information, the
following discussion contains forward-looking statements that reflect our plans,
estimates and beliefs. Our actual results could differ materially
from those discussed in the forward-looking statements. Factors that
could cause or contribute to these differences include those discussed below and
elsewhere in this Quarterly Report. See also Risk Factors contained in our Form
10-K for the year ended December 31, 2009.
OVERVIEW
We
develop, design and sell precision radiotherapy equipment used for the treatment
of cancerous tumours. In addition to providing radiotherapy equipment, we also
offer our customers comprehensive post-sales services for our products as well
as products manufactured by others. These services include radioactive cobalt
source replacement and disposal, medical expert training, clinical trial
analysis, patient tumour treatment analysis, software upgrades and patient care
consulting. We currently have five products: the Super Gamma System (“SGS”), the
Body Gamma Treatment System (“BGTS”), OPEN Stereotactic Gamma-ray Radiotherapy
System, the Head Gamma Treatment System (“HGTS”) and a multileaf collimator
device (“MLC”) used in conjunction with a linear accelerator.
We
currently sell our products primarily to a small number of hospital equipment
investors in the People’s Republic of China (“PRC” or “China”), who install our
systems in hospitals or clinics. We also offer comprehensive
post-sales services for our medical equipment to our customers. The
service contracts are negotiated and signed independently and separately from
the sales of medical equipment. Our post-sales services include
radioactive cobalt source replacement and disposal, medical expert training,
clinical trial analysis, patient tumor treatment analysis, product maintenance,
software upgrades, and consulting.
Further,
we have sought to expand our product offering. Accordingly, we successfully
acquired Portola Medical, Inc., whose primary asset consists of its rights to
develop, manufacture and sell an adjustable Multi-Catheter Source Applicator
which is intended to provide brachytherapy when a physician chooses to deliver
intracavitary radiation to the surgical margins following lumpectomy of breast
cancer. We plan to market and sell this product in the United States
and in Asia.
In
addition, our research and development team is focused on developing and
producing technologically advanced radiotherapy and gamma treatment systems
(“GTS”) products. Currently, the focus of our research and
development efforts is on five main projects. The first project is
the development of next-generation SGS unit that will incorporate advanced
radiotherapy technologies through the addition of an Image Guided System
(“IGS”), which improves the targeting of the radiation beam through use of
computer-generated images, and a Respiration Tracking System (“RTS”), which
automatically adjusts the targeting of the radiation to compensate for the
patient’s breathing. The other major projects include the development
of an integrated linear accelerator (“LINAC”) and multileaf collimator unit,
another type of radiotherapy device that is used in less demanding applications,
an advanced magnetic resonance imaging (“MRI”) device and an industrial LINAC
unit used for, among other things, preserving food through
irradiation. These projects are in various stages of
development.
22
RESULTS
OF OPERATIONS
Comparison of three months
ended June 30, 2010 and 2009
The
following table sets forth certain information regarding our results of
operation.
|
Three Months Ended June 30
|
||||||||
|
2010
|
2009
|
|||||||
|
Statements
of Operations Data
|
||||||||
|
Revenues
|
$ | 1,436,905 | $ | 2,364,189 | ||||
|
Cost
of Revenues
|
201,494 | 191,566 | ||||||
|
Gross
Profit
|
1,235,411 | 2,172,623 | ||||||
|
Operating
Expenses
|
||||||||
|
Sales
and marketing expenses
|
60,098 | 78,158 | ||||||
|
General
and administrative expenses
|
654,069 | 566,197 | ||||||
|
Research
and development costs
|
21,498 | 118,485 | ||||||
|
Other
income
|
76,611 | (10,792 | ) | |||||
|
Income
from operation before income tax expenses
|
576,357 | 1,398,991 | ||||||
|
Income
tax expenses
|
186,355 | 212,010 | ||||||
|
Net
income
|
390,002 | 1,186,981 | ||||||
Operating
Revenues
For the
three months ended June 30, 2010, revenues amounted to $1,436,905, a decrease of
$927,284, or 39%, compared to $2,364,189 for the same period of the prior year.
This decrease was primarily due to less revenue from services for the three
months ended June 30, 2010 compared to the three months ended June 30, 2009. We
did not recognize any revenue from product sales.
Revenues
from product sales, services and tax refunds and subsidies are broken down
below.
There was
no revenue from product sales for the three months ended June 30, 2010,
representing no change from the amount of product sales from the same period of
the prior year. No units were installed in the three months ended June 30, 2010
nor were any units installed in the three months ended June 30, 2009. This lack
of any unit installation was due primarily to the following factors: (i) the
first two quarters of the year are typically the lowest season for our business
(due partially to the occurrence of the Chinese New Year); and (ii) the unit
sales process took longer than expected.
Revenues
from services were $1.41 million for the three months ended June 30, 2010,
representing a decrease of $0.22 million, or 13.5%, compared to $1.63 million in
revenue from services from the same period of the prior year. This decrease was
mainly due to the Company’s provision of occasional and extra repair service to
a customer in the same period last year that did not occur in the three months
ended June 30, 2010.
Other
revenue consists principally of government financial subsidies to Changdu
Huiheng for the three months ended June 30, 2010, and totalled $29,563,
representing a decrease of $702,992, or 96.0%, compared to $732,555 for the same
period of the prior year. The Company did not receive any tax refunds or
subsidies for the three months ended June 30, 2009. For the three months ended
June 30, 2009, the Company received an outstanding amount of $0.73 million which
was previously written off in prior years. The Company did not receive a similar
payment for the three months ended June 30, 2010.
23
Cost
of Revenues
For the
three months ended June 30, 2010, the total cost of revenues amounted to
$201,494, an increase of $9,928, or 5.2%, compared to $191,566 for the same
period of the prior year. This increase was due to increased travel expenses for
the service engineers.
Gross
Margin
As a
percentage of total revenues, the overall gross margin decreased to 86% for the
three months ended June 30, 2010 as compared with 91.90% for the same period in
the prior year. This decrease was due to the decrease of revenue from service in
the three months ended June 30, 2010 and the reversal of allowance of the prior
year’s doubtful account in this same period of 2009.
Operating
Expenses
Sales and
marketing expenses
Sales and
marketing expenses mainly consist of salaries and related expenses for personnel
engaged in sales, marketing and customer support functions and costs associated
with advertising and other marketing activities. Sales and marketing expenses
were $60,098 for the three months ended June 30, 2010, a decrease of $18,060, or
23.1%, compared with $78,158 for the same period of the prior year. This
decrease in sales and marketing expenses resulted from less marketing activities
in this period, compared with the same period of the prior year.
General
and administrative expenses
General
and administrative expenses amounted to $654,069 for the three months ended June
30, 2010, representing an increase of $87,872, or 15.5%, compared to $566,197
for the same period of the prior year. The increase in general and
administrative expenses resulted from an increase of consulting fees for legal
services.
Research
and development expenses
Research
and development expenses are comprised primarily of employee compensation,
materials consumed and experiment expenses for specific new product research and
development, and any expenses incurred for basic research for advanced
technologies. Research and development expenses were $21,498 for the three
months ended June 30, 2010, a decrease of $96,987, or 81.9%, compared to
$118,485 in the same period of the prior year. This decrease was mainly due to
the Company incurring fewer expenses related to research and development in the
applicable period for this quarterly report.
Income
Tax Provision
For the
three months ended June 30, 2010, the Company’s income tax provision was
$186,355, compared to $212,010 for the same period of the prior year,
representing a decrease of $25,655, or 12.1%. The decrease in income tax was due
to the decrease of income in the three months ended June 30, 2010, compared to
the same period of the prior year.
24
Net
Income
For the
three months ended June 30, 2010, the Company’s net income amounted to $390,002,
a decrease of $796,979, or 32.9%, compared to $1,186,981 for the same period of
the prior year. This decrease was attributable to (i) the significant decrease
of revenues from service in the three months ended June 30, 2010 and (ii) the
Company’s reversal of the 33.9% allowance provided for the whole year’s doubtful
accounts for the same period of the prior year. This reversal did not occur in
the three months ended June 30, 2010.
Comparison of six months
ended June 30, 2010 and 2009
The
following table sets forth certain information regarding our results of
operation.
|
Six Months Ended June 30
|
||||||||
|
2010
|
2009
|
|||||||
|
Statements
of Operations Data
|
||||||||
|
Revenues
|
$ | 2,837,758 | $ | 3,762,808 | ||||
|
Cost
of Revenues
|
297,926 | 333,912 | ||||||
|
Gross
Profit
|
2,539,832 | 3,428,896 | ||||||
|
Operating
Expenses
|
||||||||
|
Sales
and marketing expenses
|
122,221 | 143,619 | ||||||
|
General
and administrative expenses
|
1,149,817 | 1,434,853 | ||||||
|
Research
and development costs
|
36,838 | 207,889 | ||||||
|
Other
income
|
66,114 | 16,754 | ||||||
|
Income
from operation before income tax expenses
|
1,297,070 | 1,659,289 | ||||||
|
Income
tax expenses
|
313,782 | 345,687 | ||||||
|
Net
income
|
983,288 | 1,313,602 | ||||||
Operating
Revenues
For the
six months ended June 30, 2010, revenues amounted to $2,837,758, a decrease of
$925,050, or 24.6%, compared to $3,762,808 for the same period of the prior
year. This decrease was primarily due to less revenue from services for the six
months ended June 30, 2010 compared to the six months ended June 30, 2009. We
did not recognize any revenue from product sales.
Revenues
from product sales, services and tax refunds and subsidies are broken down
below.
There was
no revenue from product sales for the six months ended June 30, 2010,
representing no change from the amount of product sales from the same period of
the prior year. No units were installed in the six months ended June 30, 2010
nor were any units installed in the six months ended June 30, 2009. This lack of
any unit installation was due primarily to the following factors: (i) the first
two quarters of the year is typically the lowest season for our business (due
partially to the occurrence of the Chinese New Year); and (ii) the unit sales
process took longer than expected.
Revenues
from services were $2,808,195 for the six months ended June 30, 2010,
representing a decrease of $222,058, or 7.3% compared to $3,030,253 in revenues
from services from the same period of the prior year. This decrease was mainly
due to the Company’s provision of occasional and extra repair service to a
customer in the same period last year that did not occur in the six months ended
June 30, 2010.
Other
revenue consists principally of government financial subsidies to Changdu
Huiheng for the six months ended June 30, 2010, and totalled $29,563,
representing a decrease of $702,992, or 96.0%, compared to $732,555 for the same
period of the prior year. The Company did not receive any tax refunds or
subsidies for the six months ended June 30, 2009. For the six months ended June
30, 2009, the Company received an outstanding amount of $0.73 million which was
previously written off in prior years. The Company did not receive a similar
payment for the six months ended June 30, 2010.
25
Revenue
Backlog
Revenue
backlog represents the total amount of unrecognized revenue associated with
existing purchase orders for our products. Any deferral of revenue recognition
is reflected in an increase in backlog as of the end of the applicable period.
The backlog as of June 30, 2010 amounted to $11.82 million, representing a
decrease of $0.15 million, or 1.3%, compared to $11.97 million as of June 30,
2009. The backlog has remained steady due to regulatory approval delays
concerning client facility preparation needed to install our products, which
caused some of the units to stay in our backlog longer than we
expected.
The
purchase orders are not cancellable and are subject to certain conditions,
including but not limited to, customers obtaining regulatory approval from their
local government for our products. Notwithstanding, we expect the purchase
orders comprising the revenue backlog to be installed in the second half of 2010
and 2011.
Cost
of Revenues
For the
six months ended June 30, 2010, the total cost of revenues amounted to $297,926,
a decrease of $35,986, or 10.7%, compared to $333,912 for the same period of the
prior year. This decrease was due to decreased travel expenses for the service
engineers.
Gross
Margin
As a
percentage of total revenues, the overall gross margin decreased to 90% for the
six months ended June 30, 2010 as compared with 91% for the same period in the
prior year. This decrease was due to the decrease of revenue from service in
the six months ended June 30, 2010 and the reversal of allowance of the
prior year’s doubtful account in this same period of 2009.
Operating
Expenses
Sales and
marketing expenses
Sales and
marketing expenses mainly consist of salaries and related expenses for personnel
engaged in sales, marketing and customer support functions and costs associated
with advertising and other marketing activities. Sales and marketing expenses
were $122,221 for the six months ended June 30, 2010, a decrease of $21,398, or
14.9%, compared with $143,619 for the same period of the prior year. This
decrease in sales and marketing expenses resulted from less marketing activities
in this period, compared with the same period of the prior year.
General
and administrative expenses
General
and administrative expenses amounted to $1,149,817 for the six months ended June
30, 2010, representing a decrease of $285,036, or 20%, compared to $1,434,853
for the same period of the prior year. The decrease in general and
administrative expenses resulted from a decrease in fees paid to our legal
counsel and auditors.
Research
and development expenses
Research
and development expenses are comprised primarily of employee compensation,
materials consumed and experiment expenses for specific new product research and
development, and any expenses incurred for basic research on advanced
technologies. Research and development expenses were $36,838 for the six months
ended June 30, 2010, a decrease of $171,051, or 82.3%, compared to $207,889 in
the same period of the prior year. This decrease was mainly due to the Company
expending fewer costs related to research and development in the applicable
period for this quarterly report.
26
Income
Tax Provision
For the
six months ended June 30, 2010, the Company’s income tax provision was $313,782,
compared to $345,687 for the same period of the prior year, representing a
decrease of $31,905, or 9.2%. The decrease in income tax was due to the decrease
of income in the six months ended June 30, 2010, compared to the same period of
the prior year.
Net
Income
For the
six months ended June 30, 2010, the Company’s net income amounted to $983,288, a
decrease of $330,314, or 25.1%, compared to $1,313,602 for the same period of
the prior year. This decrease was attributable to (i) the significant decrease
of revenues from service in the three months ended June 30, 2010 and (ii) the
Company’s reversal of the 33.9% allowance provided for the whole year’s doubtful
accounts for the period of the prior year. This reversal did not occur in the
six months ended June 30, 2010.
Comprehensive
Income
For the
six months ended June 30, 2010, the Company’s comprehensive income, which
reflects the change in foreign currency translations on net income, amounted to
approximately $1.0 million, a decrease of approximately $0.3 million, or 23.1%,
compared to $1.3 million for the same period of the prior year. The foreign
currency gain for the six month period ended June 30, 2010 was $165,590. The
major decrease of this comprehensive income resulted from not recognizing any
revenues from product sales over the period.
LIQUIDITY
AND CAPITAL RESOURCES
To date,
we have financed our operations primarily through the issuance of equity and
cash flows from operations. We currently do not have any outstanding short-term
or long-term bank debt. Our relatively high margins have historically provided
us with sufficient cash to purchase various raw materials, meet our component
inventory needs and pay our vendors. In addition, there are very few direct
costs associated with our service business which further enhances our margins.
We have longstanding, positive relationships with our vendors and have
maintained favourable payment terms and believe that we will continue to
maintain such favourable payment terms. Also, we believe that we can defer
certain tax payments, if we choose to do so. We plan to raise additional equity
capital that would help to finance a number of expansion initiatives including
new product development.
As of
June 30, 2010, the company had total assets of $28,762,317. Our cash was
$123,287, accounts receivable were $17,177,023, prepayment and other receivables
were $3,644,340 and inventories were $1,900,727. Working capital was
approximately $19,417,814. The current ratio was approximately 6.67. The quick
ratio was approximately 6.11.
Net cash
used in operating activities totalled $192,451 for the six months ended June 30,
2010, an increase of $98,957 from $93,494 for the prior year. This increase
resulted primarily from the following changes in the operating assets and
liabilities:
|
|
•
|
$677,204
increase in accounts
receivables;
|
27
|
|
•
|
$540,827
increase in inventories;
|
|
|
•
|
$206,160
increase in prepayments and other
receivables;
|
|
|
•
|
$56,093
decrease in accounts payable;
|
|
|
•
|
$250,200
increase in tax payable; and
|
|
|
•
|
$52,080
decrease in accrued expenses and other current
liabilities;
|
The
increase in accounts receivable was due to a combination of
factors:
First,
the majority of our product sales and installations in 2009 occurred during the
last quarter of the year. As a result of the short amount of time between the
time of installation and the end of the period, our cash collection in the six
months ended June 30, 2010 was lower than our cash collected over the same
period of the prior year.
Second,
due to our strong, long-standing relationships with our customers, we have
extended their payment terms and are confident that we will collect all the
money owed by these customers.
The
increase in prepayments and other receivables was attributed to an increase in
payments made to our manufacturing suppliers for parts and services needed to
manufacture the radiotherapy units that comprise our backlog.
Net cash
provided by/(used in) investing activities was $86,478 and ($224,237) for the
six months ended June 30, 2010 and 2009, respectively. The cash provided by
investing activities was from a related party advance. The cash used in
investing activities was primarily used for the acquisition of a subsidiary
entity this year, compared to land usage rights for our new manufacturing
facility in Wuhan last year.
Cash
flows from financing activities both amounted to nil for the three months ended
June 30, 2010 and 2009.
On June
7, 2010, we completed the acquisition of Portola Medical, Inc. (see Note 1 to
the Consolidated Financial Statements). Under the terms of the agreement, we
acquired all of the outstanding shares of Portola Medical for $2,600 per share
in cash (without interest) for a total purchase price of $260,000. The purchase
price and related costs were funded by Mr. Hui Xiaobing, our chairman and chief
executive officer, pursuant to an unsecured promissory note carrying interest at
the short term Applicable Federal Rate and with a maturity date one year from
its effective date, unless sooner accelerated upon an event of
default.
Off-Balance
Sheet Arrangements
We do not
have any off-balance sheet arrangements.
Critical
Accounting Policies and Estimates
Our
significant accounting policies are summarized in Note 2 of the Consolidated
Financial Statements.
28
New
Accounting Standards
See Note
2 of the Consolidated Financial Statements for information regarding other new
accounting standards that could affect us.
Contractual
Obligations
Through
our subsidiary, Shenzhen Hyper Technology Company, Ltd., we lease an office
building from Shenzhen OUR Technology Co., Ltd. (“Shenzhen OUR”). We own 75% and
Shenzhen OUR owns 25% of Shenzhen Hyper. The lease agreement is for a twenty
year period at RMB160,000 (approximately USD $23,530) per month. Under the lease
agreement, Shenzhen Hyper will make payments, on behalf of Shenzhen OUR, in
connection with further construction of the office building. The balance of the
amount due from the landlord will be used to set off the rental expenses
incurred by Shenzhen Hyper.
Item
3. Quantitative and Qualitative Disclosures About Market Risk
Not
Applicable.
Item
4T. Controls and Procedures
Evaluation
of Disclosure Controls and Procedures
Under the
supervision and with the participation of our management, including our
principal executive officer and principal financial officer, we conducted an
evaluation of our disclosure controls and procedures, as such term is defined
under Rules 13a-15(e) and Rule 15d-15(e) promulgated under the Securities
Exchange Act of 1934, as amended (the “Exchange Act”). Based on this evaluation,
our principal executive officer and our principal financial officer concluded
that our disclosure controls and procedures were effective as of the end of the
period covered by this quarterly report.
Changes
in Internal Controls Over Financial Reporting
There
have been no changes in our internal controls over financial reporting that
occurred during the period covered by this quarterly report, that have
materially affected, or are reasonably likely to materially affect, our internal
control over financial reporting.
29
PART
II - OTHER INFORMATION
Item
1. Legal Proceedings
To the
best of management’s knowledge, there are no material legal proceedings pending
against the Company.
Item
1A. Risk Factors
Not
Applicable.
Item 2.
Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item
3. Defaults Upon Senior Securities
None.
Item
4. [REMOVED AND RESERVED]
Item
5. Other Information
None.
Item
6. Exhibits
|
Exhibit
No
|
Description
|
|
|
3.1
|
Articles
of Incorporation, as revised, of Huiheng Medical, Inc. (1)
|
|
|
3.2
|
Amended
and Restated Bylaws of Huiheng Medical, Inc. (2)
|
|
|
4.1
|
Amended
and Restated Certificate of Designations of Rights and Preferences of the
Series A 7% Convertible Preferred Stock (3)
|
|
|
10.1
|
Huiheng
Medical, Inc. 2009 Share Plan (4)
|
|
|
10.2
|
Common
Stock Purchase Agreement for Portola Medical, Inc. (5)
|
|
|
31.1
|
Certification
by the Chief Executive Officer pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002*
|
|
|
31.2
|
Certification
by the Chief Financial Officer pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002*
|
|
|
32.1
|
Certification
by the Chief Executive Officer and Chief Financial Officer pursuant to
Section 906 of the Sarbanes-Oxley Act of
2002*
|
30
|
*
|
Filed
herewith
|
|
(1)
|
Incorporated
by reference to the exhibit to the registrant’s annual report on Form
10-KSB filed with the SEC on April 10,
2008.
|
|
(2)
|
Incorporated
by reference to the exhibit to the registrant’s annual report on Form
10-KSB filed with the SEC on April 10,
2008.
|
|
(3)
|
Incorporated
by reference to the exhibit to the registrant’s current report on Form 8-K
filed with the SEC on January 16,
2008.
|
|
(4)
|
Incorporated
by reference to the exhibit to the registrant’s current report on Form 8-K
filed with the SEC on June 10,
2009.
|
|
(5)
|
Incorporated
by reference to the exhibit to the registrant’s current report on Form 8-K
filed with the SEC on May 13, 2010.
|
31
SIGNATURES
Pursuant to the requirements of the
Securities Exchange Act of 1934, the registrant has duly caused this report to
be signed on its behalf by the undersigned thereunto duly
authorized.
|
HUIHENG
MEDICAL, INC.
|
||
|
Date: August
23, 2010
|
By:
|
/s/
Hui Xiaobing
|
|
Hui
Xiaobing
|
||
|
Chief
Executive Officer
|
||
|
(Principal
Executive Officer)
|
||
|
Date: August
23, 2010
|
By:
|
/s/
Richard Shen
|
|
Richard
Shen
|
||
|
Chief
Financial Officer
|
||
|
(Principal
Accounting and Financial
Officer)
|
||
32
