Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Emergent BioSolutions Inc. | w79613e8vk.htm |
Exhibit 99.1

| Corporate Overview All Employee Meeting at Trubion's Corporate HQ PLEASE NOTE: ALL TITLE SLIDES BASED ON A TITLE MASTER IN AUTO LAYOUT DATE AUTOMATIC TO TURN OFF: [View>Header and Footer...] On Slide tab, uncheck Date and Time, then click Apply |

| This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, including any potential future securities offering, our expected revenue growth and net earnings for 2010, and any other statements containing the words "believes", "expects", "anticipates", "plans", "estimates" and similar expressions, are forward-looking statements. There are a number of important factors that could cause the company's actual results to differ materially from those indicated by such forward-looking statements, including appropriations for BioThrax(r) procurement; our ability to obtain new BioThrax(r) sales contracts; our plans to pursue label expansions and improvements for BioThrax(r); our ability to win a development award with the U.S. government for our recombinant protective antigen anthrax vaccine candidate; our plans to expand our manufacturing facilities and capabilities; the rate and degree of market acceptance and clinical utility of our products; the success of our ongoing and planned development programs, preclinical studies and clinical trials; our ability to identify and acquire or in license products and product candidates that satisfy our selection criteria; the potential benefits of our existing collaboration agreements and our ability to enter into selective additional collaboration arrangements; the timing of and our ability to obtain and maintain regulatory approvals for our other product candidates; our commercialization, marketing and manufacturing capabilities and strategy; our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; and other factors identified in the company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2010 and subsequent reports filed with the SEC. The company disclaims any intention or obligation to update any forward-looking statements as a result of developments occurring after the date of this presentation. The guidance in this presentation is only effective as of the date given, August 5, 2010, and will not be updated or affirmed unless and until the Company publicly announces updated or affirmed guidance. Emergent BioSolutions Forward-Looking Statements / Note on Guidance |

| Market Focus Infectious disease market Unmet medical needs & underserved global markets Product Focus Immune-related biologics (e.g., vaccines & antibody therapies) Disease Focus Anthrax Tuberculosis Typhoid Universal Flu Chlamydia Customer Focus Government Private Sector Corporate Overview Clear Business Focus |

| Microscience Ltd (UK) acquired Antex Biologics (US) acquired Michigan Biologics Products Institute assets acquired Lansing facility renovation approved by FDA ViVacs GmbH (Germany) acquired 1998 IPO and NYSE listing completed 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 Joint venture with University of Oxford formed rPA candidate acquired Anthrax monoclonal candidate acquired Baltimore facility acquired 4-year expiry dating for BioThrax(r) granted by FDA Corporate Overview Successful Track Record of Acquisitions and Delivering Results |
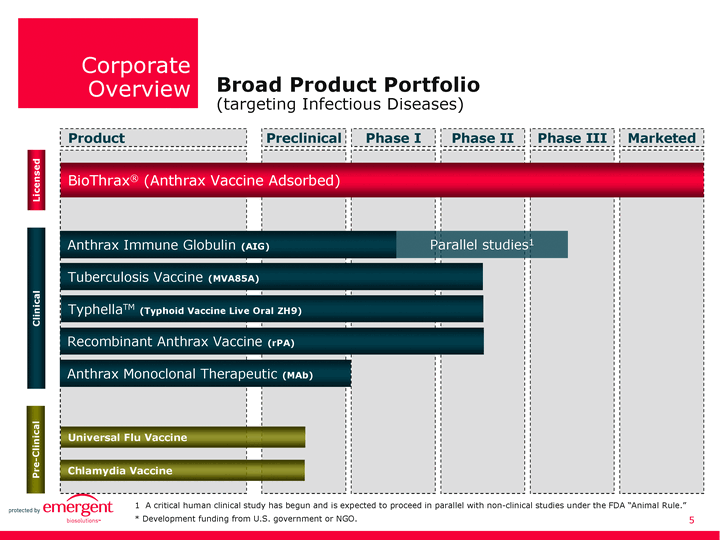
| Product Preclinical Phase I Phase II Phase III Marketed BioThrax(r) (Anthrax Vaccine Adsorbed) Tuberculosis Vaccine (MVA85A) Clinical Anthrax Immune Globulin (AIG) Parallel studies1 Licensed TyphellaTM (Typhoid Vaccine Live Oral ZH9) Recombinant Anthrax Vaccine (rPA) Universal Flu Vaccine 1 A critical human clinical study has begun and is expected to proceed in parallel with non-clinical studies under the FDA "Animal Rule." * Development funding from U.S. government or NGO. Chlamydia Vaccine Pre-Clinical Anthrax Monoclonal Therapeutic (MAb) Corporate Overview Broad Product Portfolio (targeting Infectious Diseases) |

| Type of Contract Funding Agency Description Term Expected Timing Potential Total Contract Amount Development BARDA Large-scale Manufacturing Process for BioThrax(r) (Building 55) Multi Year Announced 07/13/10 $107M Development BARDA rPA Development Multi Year End of 3Q >$200M Procurement CDC/HHS Doses of BioThrax(r) Multi Year (begin 3Q '11) Year End >$400M TOTAL TOTAL TOTAL TOTAL TOTAL >$700M Corporate Overview 2010 Key Corporate Milestones |

| Total Revenues -- $62.1M $108.9M Net Income -- $9.8M $12.3M EPS -- $0.32 $0.40 Cash Balance -- $102.9M* * Excluding accounts receivable of $45.8M Corporate Overview Recent Announcement - 2Q / 6 Month 2010 Financial Performance 2Q10 6M10 |

| 2005 2006 2007 2008 2009 2010P Total 131 153 182.915 178.718 234.786 275 25 $131 $153 $183 $234 $179 $300 to $275 Total Revenue ($ millions) 2005 2006 2007 2008 2009 2010P Total 16 23 23 21 31 40 10 $16 $23 $23 $31 $21 $50 to $40 Net Income ($ millions) Corporate Overview Continued Revenue Growth and Profitability |

| Signed Doses Contract Value Contract Term Delivery Status September 2004 5M $124M September 2004 to September 2007 Completed May 2005 5M $120M May 2005 to May 2006 Completed May 2006 5M $123M May 2006 to May 2007 Completed September 2007 18.75M $448M September 2007 to September 2010 Completed October 2008 14.5M $405M September 2009 to September 2011 Completion anticipated 2Q/3Q 2011 TOTAL 48.25M $1,220M Current contract YE 2009: Current SNS of ~20M doses of BioThrax(r) Continuing History of Delivery Under USG Contracts Corporate Overview |

| Product Partner $ (millions) BioThrax(r) dual adjuvant NIAID/BARDA 30 AIG NIAID 13 Anthrax MAb NIAID/BARDA 24 rPA NIAID/BARDA 1001 Typhoid Vaccine (TyphellaTM) Wellcome Trust (UK) 2 TB Vaccine (MVA85A) Wellcome Trust (UK) / AERAS (US) 16 TOTAL TOTAL $185 TB Chlamydia Flu Typhoid Anthrax 1 Reflects funding by NIAID prior to EBS acquisition of rPA candidate. Extensive USG and NGO Funding for R&D Pipeline Corporate Overview |

| 2010P 2011P 2012P 2013P 2014P Building 12 7 7.5 8 7 Building 55 0 0 0 18 25 7.0 7.5 25.0 Building 12 Building 551 1 Assumes one 1320L fermentation train; second fermentation train for surge requirement Total Capacity (millions) 8.0 25.0 Expanding Lansing Manufacturing Capacity Corporate Overview |

| Anthrax TB Typhoid Flu Chlamydia Baltimore Facility Baltimore Facility Baltimore Facility Facility Size 56,000 sq. ft. Including 11,000 sq. ft. for manufacturing 56,000 sq. ft. Including 11,000 sq. ft. for manufacturing Production Configuration Multiple segregated production suites Concurrent manufacturing Multiple segregated production suites Concurrent manufacturing Manufacturing Capabilities VIRAL NON-VIRAL Manufacturing Capabilities TB, Flu, Chlamydia rPA, MAb Manufacturing Capability for R&D Pipeline Corporate Overview |

| Near term growth from licensed vaccine BioThrax(r) Medium term growth from high value R&D pipeline Longstanding financial strength Corporate Overview Company Highlights |

| Corporate Overview All Employee Meeting at Trubion's Corporate HQ PLEASE NOTE: ALL TITLE SLIDES BASED ON A TITLE MASTER IN AUTO LAYOUT DATE AUTOMATIC TO TURN OFF: [View>Header and Footer...] On Slide tab, uncheck Date and Time, then click Apply |
