Attached files
| file | filename |
|---|---|
| 8-K - ENDO PHARMACEUTICALS HOLDINGS INC. - FORM 8-K - ENDO HEALTH SOLUTIONS INC. | d8k.htm |
 grow.
collaborate. innovate. thrive.
ENDO PHARMACEUTICALS
Bank of America Merrill Lynch Specialty Pharmaceuticals Conference
August 12, 2010
Exhibit 99.1 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
FORWARD LOOKING STATEMENT
2
This presentation contains forward-looking statements regarding, among other things, the proposed
business combination between Endo and Penwest, Endo’s and Penwest's financial position,
results of operations, market position, product development and business strategy, as well as
estimates of Endo’s future total revenues, future expenses, future net income and future earnings per
share. Statements including words such as “believes,” “expects,”
“anticipates,” “intends,” “estimates,” “plan,” “will,” “may” “intend,”
“guidance” or similar expressions are forward-looking statements. Because these
statements reflect our current views, expectations and beliefs concerning future events, these
forward-looking statements involve risks and uncertainties. Investors should note that many
factors could affect the proposed business combination of the companies, future financial results and could cause actual
results to differ materially from those expressed in forward-looking statements contained in this
presentation. These factors include, but are not limited to: the risk that the tender offer and
merger will not close, the risk that Endo’s business and/or Penwest's business will be
adversely impacted during the pendency of the tender offer and merger, the risk that the operations of the two companies will
not be integrated successfully, Endo’s ability to successfully develop, commercialize and market
new products; timing and results of pre-clinical or clinical trials on new products;
Endo’s ability to obtain regulatory approval of any of Endo’s pipeline products;
competition for the business of Endo’s branded and generic products, and in connection with its
acquisition of rights to intellectual property assets; market acceptance of our future
products; government regulation of the pharmaceutical industry; Endo’s dependence on
a small number of products; Endo’s dependence on outside manufacturers for the manufacture of a majority of its
products; Endo’s dependence on third parties to supply raw materials and to provide
services for certain core aspects of its business; new regulatory action or lawsuits relating
to Endo’s use of narcotics in most of its core products; Endo’s exposure to product
liability claims and product recalls and the possibility that they may not be able to adequately insure themselves; the
successful efforts of manufacturers of branded pharmaceuticals to use litigation and legislative and
regulatory efforts to limit the use of generics and certain other products; Endo’s ability
to successfully implement its acquisition and in-licensing strategy; regulatory or other
limits on the availability of controlled substances that constitute the active ingredients of some of its products and products in
development; the availability of third-party reimbursement for Endo’s products; the outcome
of any pending or future litigation or claims by third parties or the government, and the
performance of indemnitors with respect to claims for which Endo has been indemnified;
Endo’s dependence on sales to a limited number of large pharmacy chains and wholesale drug distributors for a large
portion of its total revenues; a determination by a regulatory agency that Endo is engaging or has
engaged in inappropriate sales or marketing activities, including promoting the
“off-label” use of its products, the risk that demand for and acceptance of Endo’s and
Penwest's products or services may be reduced; the risk of changes in governmental regulations; the
impact of economic conditions; the impact of competition and pricing and other risks and
uncertainties, including those detailed from time to time in the companies’ periodic
reports filed with the Securities and Exchange Commission, including current reports on Form 8-K, quarterly reports on Form
10-Q and annual reports on Form 10-K, particularly the discussion under the caption “RISK
FACTORS" in their annual reports on Form 10-K for the year ended December 31, 2009,
which were filed with the Securities and Exchange Commission. The forward- looking
statements in this presentation are qualified by these risk factors. These are factors that, individually or in the aggregate,
could cause our actual results to differ materially from expected and historical results. The
companies’ assume no obligation to publicly update any forward-looking statements,
whether as a result of new information, future developments or otherwise. |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
FORWARD LOOKING STATEMENT CONTINUED
Additional Information
The
tender
offer
described
in
this
document
has
not
yet
commenced.
At
the
time
the
tender
offer
is
commenced,
we
will
file
with
the
SEC
and
mail
to
Penwest’s
stockholders
a
tender
offer
statement
on
Schedule
TO
and
Penwest
will
file
with
the
SEC
and
mail
to
its
stockholders a tender offer solicitation/recommendation statement on Schedule
14D-9 in connection with the transaction. Investors and
Penwest
shareholders are strongly advised to read the tender offer statement (including
the offer to purchase, letter of transmittal and related tender offer
documents) and the related solicitation/recommendation statement on Schedule 14D-9 when they are available
because
they
contain
important
information.
These
documents
will
be
available
at
no
charge
on
the
SEC’s
website
at
www.sec.gov.
A
copy
of
the
solicitation/recommendation
statement
on
Schedule
14D-9
may
also
be
obtained
free
of
charge
from
Penwest's
website
at
www.penwest.com
or
by
directing
a
request
to
Penwest
at
2981
Route
22,
Patterson,
New
York
12563,
Attn:
Frank
Muscolo.
In
addition,
a
copy
of
the
offer
to
purchase,
letter
of
transmittal
and
certain
other
related
tender
offer
documents
may
be
obtained
free
of
charge from Endo’s website at www.endo.com
or by directing a request to Endo at www.endo.com, or Endo Pharmaceuticals, 100
Endo Boulevard, Chadds
Ford, PA 19317, Attn: Corporate Secretary’s Office.
3 |
 grow. collaborate. innovate. thrive.
ENDO PHARMACEUTICALS
I.
Our Business
II.
Executing Strategy for Growth
III.
Penwest
Acquisition
IV.
HealthTronics
Acquisition
V.
Financial Outlook
4 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
STRONG OPERATING PERFORMANCE
17% 3-YEAR CAGR FOR REVENUE*
5 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
STRONG CORE BUSINESS SUPPORTING GROWTH
6 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
STRONG CORE BUSINESS SUPPORTING GROWTH
7
LIDODERM®
key component of our core business
Strong source of operating cash flow
Stable TRx
trends
10-year Commercial launch anniversary in September 2009
Differentiated product profile provides unique offering for HCPs
and patients
suffering from PHN
Improved PHN physician targeting for more efficient utilization
of resources
Solid managed care contract positioning for 2010
Opportunities in second half with formulary position
|
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
LONG-ACTING OPIOID FRANCHISE
Opana®
ER
Settlement with generic companies provides for 2013 entry on primary
dosage forms.
28% TRx
growth YOY in Q2 2010
Gaining share; Growing source of cash flow.
Pending Acquisition of Penwest
for $5.00 per share
$0.05 accretive to adjusted diluted EPS in 2010
Advances our leadership and growth in pain management
NDA submission for new formulation of Oxymorphone
Long-acting Oxymorphone
designed to be crush-resistant
8 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
DEVELOPMENT PIPELINE
Phase I
Phase II
Phase III
NDA
Oxymorphone
Formulation designed to be crush-
resistant
FORTESTA™
2% Testosterone Gel
AVEED™
Long Acting Injectable
Testosterone
Urocidin™
Bladder Cancer
Octreotide
Implant
Acromegaly*
Axomadol
Moderate to moderately severe chronic pain
Octreotide
Implant
Carcinoid
Syndrome
Pending
Pending
* Granted orphan drug designation
9
Update Pending |
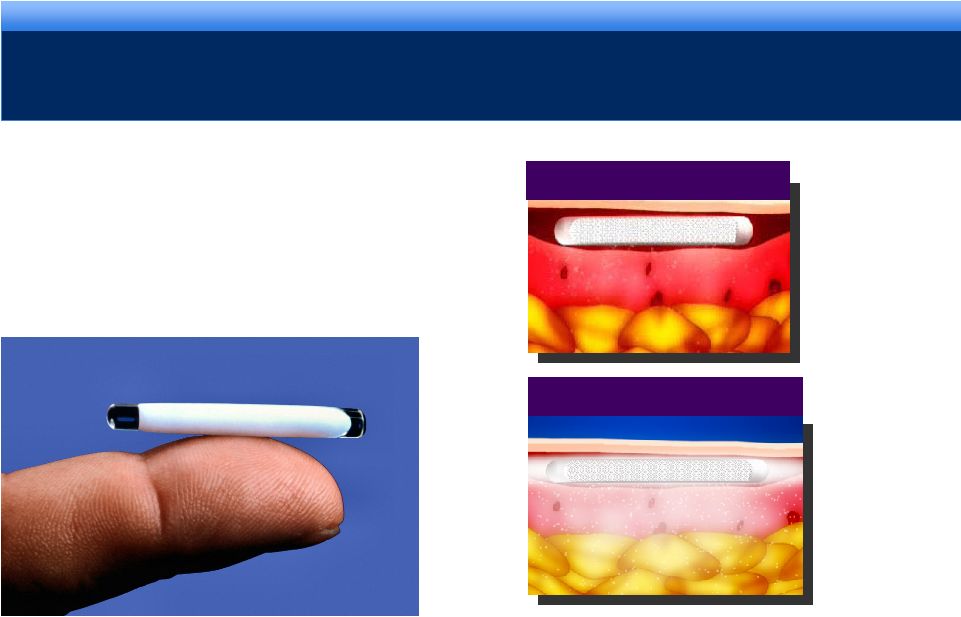 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
IMPLANT TECHNOLOGY
HYDRON®
DRUG DELIVERY TECHNOLOGY
10
IMPLANTATION
DISPERSION |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
SPECIALTY GENERICS DEVELOPMENT
11
~40 Projects
~40 Projects
25 Near-term*
25 Near-term*
12 Current
12 Current
ANDA
ANDA
reviews
reviews
*Near-term revenue 2010-2012
Approximately 40 projects under
development
14 Current ANDA submissions
12 with near-term launch targets
2 with long-term launch targets
Managing development for
sustainable growth
Potential efficiencies with branded
Rx business |
 grow.
collaborate. innovate. thrive. ©2010 Endo Pharmaceuticals Inc.
12 |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
Enhance
Revenue
Growth
Through
Diversification
Sustainable, long-term growth
Diversified revenue stream
Enhanced product offerings in urology
Expand Urology Business
Elevates Endo’s leadership in urology
Expands Endo’s reach and relationships with key urology practices
Increase Shareholder Value
Accretive to adjusted earnings in 2010
Diversified revenue stream beyond pharmaceuticals
Enhanced offerings in urology
13
HEALTHTRONICS –
TRANSACTION RATIONALE |
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
Leading provider of urology services
Leader in lithotripsy, BPH laser and cryosurgery
Emerging urologic businesses in:
Anatomic pathology
Radiation therapy
Unique business relationship with 1/3 of urologists in U.S.
Total solution for the urology marketplace
Improve patient care
Enhance practice economics
14
HEALTHTRONICS –
CORPORATE OVERVIEW |
   grow.
collaborate. innovate. thrive. ©2010 Endo Pharmaceuticals Inc.
ENDO’S CARE PATHWAY
15
Diagnostics
Drugs
Devices
Services
Pain
Bladder
Cancer
BPH
Prostate
Cancer
Endo with HealthTronics
will offer a full suite of products
and services as well as a direct channel to urologists
|
 grow. collaborate. innovate. thrive.
©2010 Endo Pharmaceuticals Inc.
OUTLOOK
2010 Financial Guidance
Revenue range of $1.63B -
$1.68B
Adjusted diluted EPS range of $3.30 -
$3.35
Reported (GAAP) diluted EPS range of $1.89 -
$1.97
Flexible cost structure
Expect to generate $350 -
$400M in annual Operating Cash Flow this
year
16 |
 grow. collaborate. innovate. thrive.
Endo Pharmaceuticals |
 grow. collaborate. innovate. thrive.
RECONCILIATION OF NON-GAAP MEASURES
For an explanation of Endo’s reasons for using non-GAAP measures, see
Endo’s Current Report on Form 8-K filed today with the Securities
and Exchange Commission Reconciliation of Projected GAAP Diluted Earnings Per
Share to Adjusted Diluted Earnings Per Share Guidance for the Year Ending
December 31, 2010 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$1.89
$1.97
Upfront and milestone-related payments to partners
$0.38
$0.33
Amortization of commercial intangible assets
$0.59
$0.59
Costs incurred in connection with continued efforts to enhance the cost
structure of the Company
$0.08
$0.08
Indevus
related costs and change in fair value of contingent
consideration
$0.01
$0.01
Impairment of indefinite-lived intangibles
$0.11
$0.11
Costs related to the acquisition of HealthTronics, Inc.
$0.30
$0.30
Costs related to the acquisition of Penwest
Pharmaceuticals Co.
$0.22
$0.22
Interest expense adjustment for ASC 470-20
and the amortization of
the premium on debt acquired from Indevus
$0.15
$0.15
Tax effect of pre-tax adjustments at the applicable tax rates
and
certain other expected cash tax savings as a result of the Indevus,
HealthTronics
and Penwest
acquisitions
($0.43)
($0.41)
Diluted adjusted income per common share guidance
$3.30
$3.35
The company's guidance is being issued based on certain assumptions
including: •Certain of the above amounts are based on estimates and
there can be no assurance that Endo will achieve these results
•Includes
all
completed
business
development
transactions
as
of
August
9,
2010
and
the
acquisition
of
Penwest
Pharmaceuticals
Co. |
