Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K RE INVESTOR PRESENTATION 07.27.10 - JUNIPER PHARMACEUTICALS INC | form8k.htm |

1
Corporate Presentation August 2010
Frank Condella, President and CEO
Larry Gyenes, CFO

2
This presentation contains forward-looking statements, which statements are indicated by the words
“may,” “will,” “plans,” “believes,” “expects,” “anticipates,” “potential,” and similar expressions. Such
forward-looking statements involve known and unknown risks, uncertainties, and other factors that may
cause actual results to differ materially from those projected in the forward-looking statements.
“may,” “will,” “plans,” “believes,” “expects,” “anticipates,” “potential,” and similar expressions. Such
forward-looking statements involve known and unknown risks, uncertainties, and other factors that may
cause actual results to differ materially from those projected in the forward-looking statements.
Factors that might cause future results to differ include, but are not limited to, the following: the
successful marketing of CRINONE® (progesterone gel) by Watson Pharmaceuticals, Inc., in the United
States; the successful marketing of CRINONE by Merck Serono outside the United States; the timely
and successful completion of the ongoing Phase III PREGNANT (PROCHIEVE® (progesterone gel)
Extending Gestation A New Therapy) Study of PROCHIEVE 8% to reduce the risk of preterm birth in
women with a short cervix at mid-pregnancy; successful development of a next-generation vaginal
progesterone product; success in obtaining acceptance and approval of new products and new
indications for current products by the United States Food and Drug Administration and international
regulatory agencies; the impact of competitive products and pricing; the timely and successful
negotiation of partnerships or other transactions; the strength of the United States dollar relative to
international currencies, particularly the euro; competitive economic and regulatory factors in the
pharmaceutical and healthcare industry; general economic conditions; and other risks and
uncertainties that may be detailed, from time-to-time, in Columbia’s reports filed with the SEC.
successful marketing of CRINONE® (progesterone gel) by Watson Pharmaceuticals, Inc., in the United
States; the successful marketing of CRINONE by Merck Serono outside the United States; the timely
and successful completion of the ongoing Phase III PREGNANT (PROCHIEVE® (progesterone gel)
Extending Gestation A New Therapy) Study of PROCHIEVE 8% to reduce the risk of preterm birth in
women with a short cervix at mid-pregnancy; successful development of a next-generation vaginal
progesterone product; success in obtaining acceptance and approval of new products and new
indications for current products by the United States Food and Drug Administration and international
regulatory agencies; the impact of competitive products and pricing; the timely and successful
negotiation of partnerships or other transactions; the strength of the United States dollar relative to
international currencies, particularly the euro; competitive economic and regulatory factors in the
pharmaceutical and healthcare industry; general economic conditions; and other risks and
uncertainties that may be detailed, from time-to-time, in Columbia’s reports filed with the SEC.
All forward-looking statements contained herein are neither promises nor guarantees. Columbia does
not undertake any responsibility to revise or update any forward-looking statements.
not undertake any responsibility to revise or update any forward-looking statements.

3
} Frank C. Condella, Jr. - President and CEO, Director
◦ Chairman of SkyePharma
◦ Formerly CEO of SkyePharma; European President of IVAX Corporation;
CEO of Faulding Pharmaceutical Co.; Vice President, Roche Laboratories
CEO of Faulding Pharmaceutical Co.; Vice President, Roche Laboratories
} Lawrence A. Gyenes - SVP, CFO & Treasurer
◦ Formerly CFO at Acusphere, Zila, Savient & Reliant; 15 years at Searle
} Michael McGrane - SVP, General Counsel and Secretary
◦ Formerly General Counsel, The Liposome Co.; Novartis Consumer Health
} George W. Creasy, MD - VP, Clinical Research & Development
• Fellow of the American College of Obstetricians and Gynecologists
• Formerly spent 16 years Johnson & Johnson

4
} Successful specialty pharmaceutical company
leveraging our bioadhesive drug delivery system and
clinical expertise to develop proprietary products
leveraging our bioadhesive drug delivery system and
clinical expertise to develop proprietary products
} Marketed products:
◦ CRINONE® 8% progesterone vaginal gel
– Sold worldwide for use in infertility treatments
– Marketed by Watson (US) and Merck Serono (RoW)
◦ STRIANT® testosterone buccal system
– Testosterone replacement for hypogonadal men
– Marketed by Columbia (US), The Urology Company (UK) &
Sandoz (Italy)
Sandoz (Italy)

5
} Concurrent transactions closed 7/2/2010
} Watson acquired:
◦ CRINONE/PROCHIEVE and related progesterone assets
◦ 11.2 million shares CBRX common stock
◦ Watson assumed responsibility for all US sales,
marketing & distribution activities
marketing & distribution activities
◦ Next generation development activities
} $56m debt retired
◦ $16m secured loan note
◦ $40m convertible notes

6
} Watson Transaction:
◦ $62m upfront for assets
◦ Royalties:
– 10% on sales up to $150m
– 15% on sales between $150m and $250m
– 20% on sales above $250m
◦ Pre-term Birth Study milestones:
– $6m or $8m on successful PREGNANT study outcome
– $5m on FDA acceptance of NDA
– $30m on U.S. commercial launch for preterm birth
– $2.5m on European regulatory submission and launch
◦ Watson will fund life-cycle management program

7
} $16m PharmaBio secured note:
◦ Paid with cash
} $40m 8% Notes Convertible @ $5.25 per share
◦ $26m of cash
◦ 7.4m shares of CBRX common stock issued @ $1.35*
◦ 7.75m warrants exercisable @ $1.35 per common share
*Repurchased 3.3m of 7.4m common shares on 8/9/10 @ $0.90
per share
per share

8
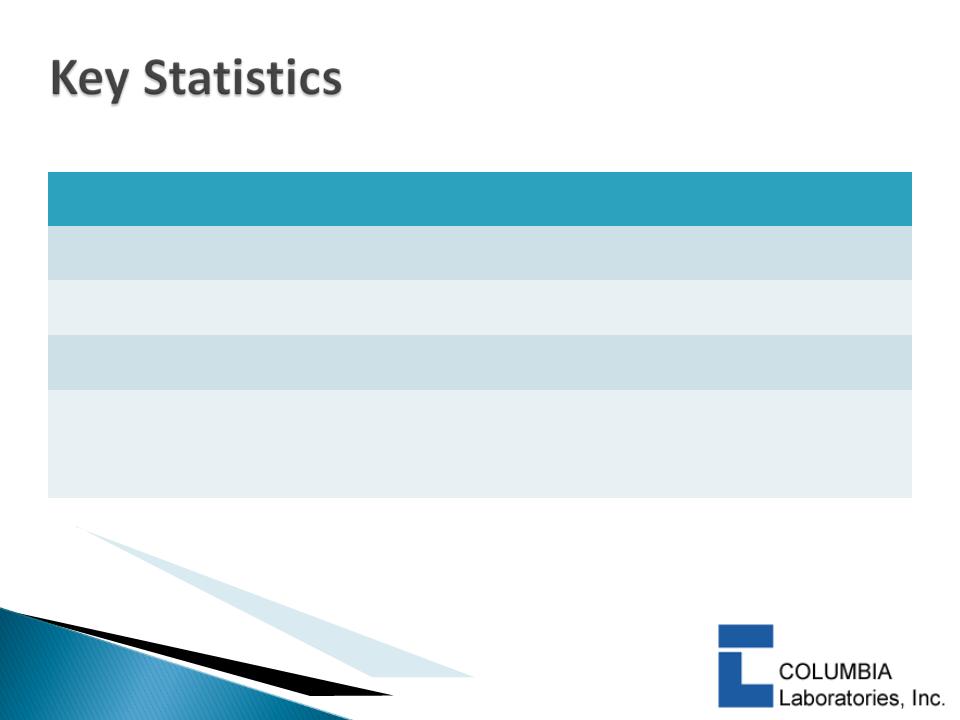
9
|
Nasdaq: CBRX
|
|
|
Recent market price (8/09/2010)
|
$1.06
|
|
Shares Outstanding
|
80.8 million
|
|
Market capitalization
|
$85.6 million
|
|
Cash and equivalents (8/09/2010)
|
$22.0+ million
|
|
Debt (8/09/2010)
|
$0.00
|
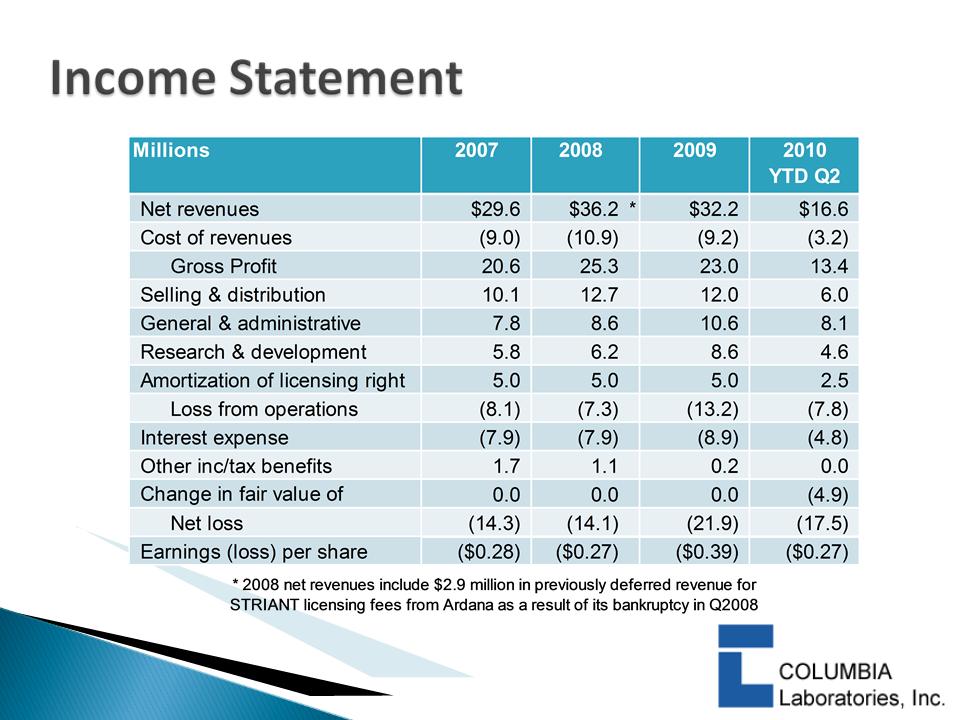
10
11
|
|
|
|
Revenues
|
$16.0
|
|
Cost of Revenues
|
5.6
|
|
Gross Profit
|
$10.4
|
|
S, G & A
|
7.6
|
|
R & D
|
2.8
|
|
Operating Inc
|
$0.0
|
|
Other Inc/Tax Benefits
|
0.4
|
|
Net Inc (Loss)
|
$0.5
|
|
|
|

12
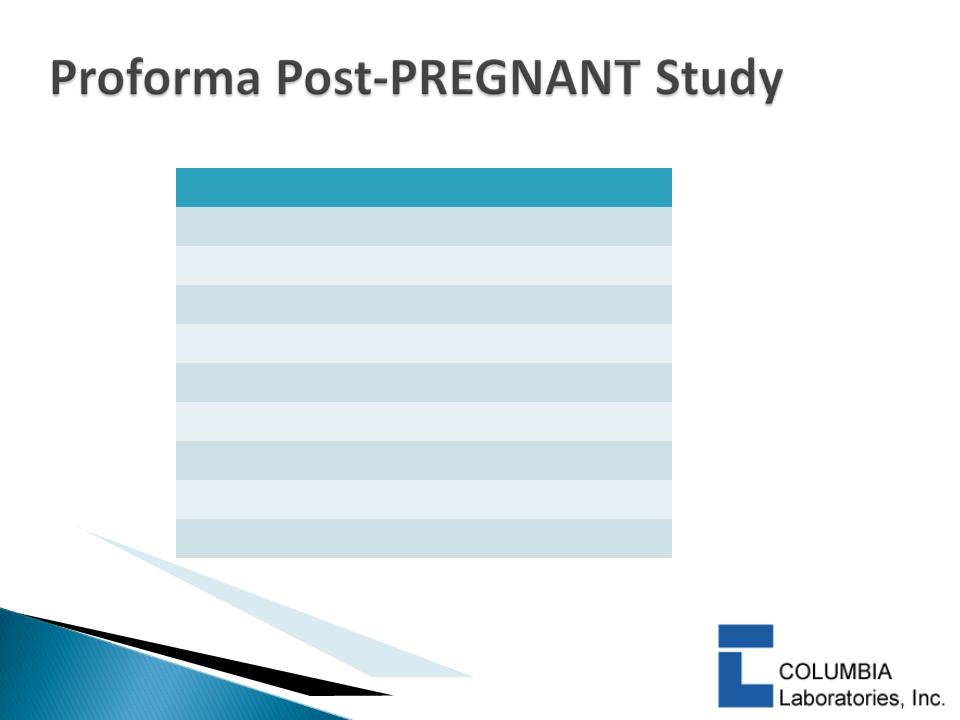
Cash Milestones:
Ø$6m or $8m on successful PREGNANT study outcome
Ø$5m on FDA acceptance of NDA
Ø$30m on commercial launch for preterm birth
Ø$2.5 for European regulatory submission and launch
|
|
$50M
|
$100M
|
$250M
|
$500M
|
|
Royalties
@ Different Sales Levels |
$5M
|
$10M
|
$30M
|
$80M
|

13
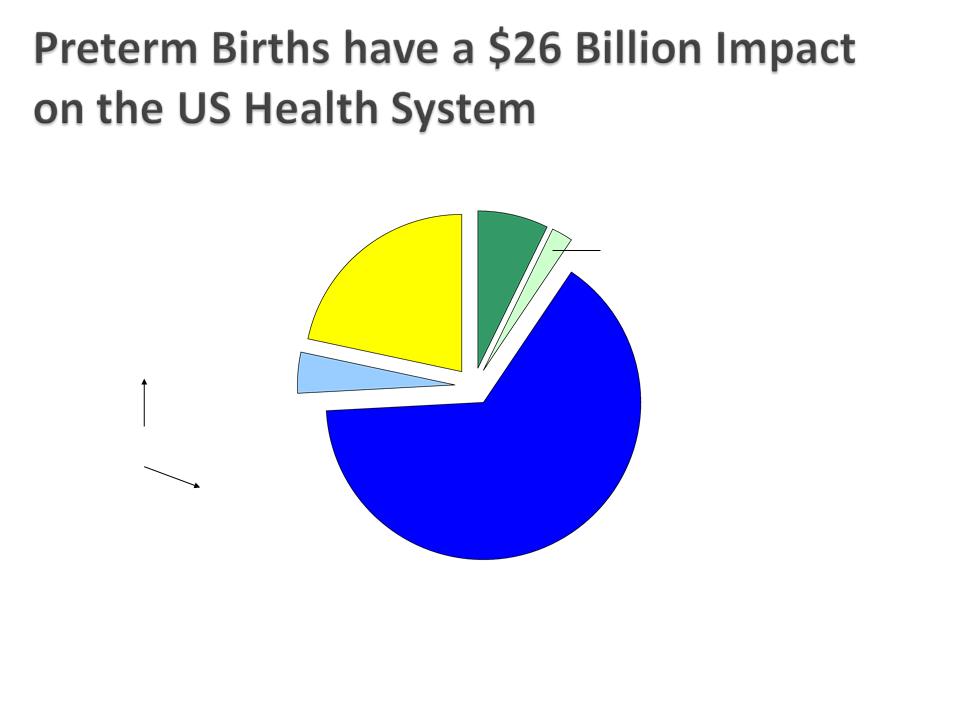
Behrman RE et al. in: Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences,
and Prevention. Washington, DC: The National Academies Press; 2006:329-354.
and Prevention. Washington, DC: The National Academies Press; 2006:329-354.
Lost household and
market productivity
market productivity
$5.7 billion
Maternal delivery costs
$1.9 billion
Children’s early intervention
services
services
$611 million
Infant Costs
Special education services
$1.1 billion
Medical care services
$16.9 billion
~$51,600 per Preterm Infant
15
} Studies have shown that “short cervix” is the most
powerful predictor of preterm birth
powerful predictor of preterm birth
◦ The risk of spontaneous preterm delivery increases as
cervical length (CL) decreases
cervical length (CL) decreases
} To assess risk, cervical length measurements must
be taken at mid-pregnancy (18-22 weeks)
be taken at mid-pregnancy (18-22 weeks)
◦ Transvaginal ultrasound
} A cervical length of <3.0cm is considered “short”
} The shorter the cervix at mid-pregnancy, the higher
the risk of PTB
the risk of PTB
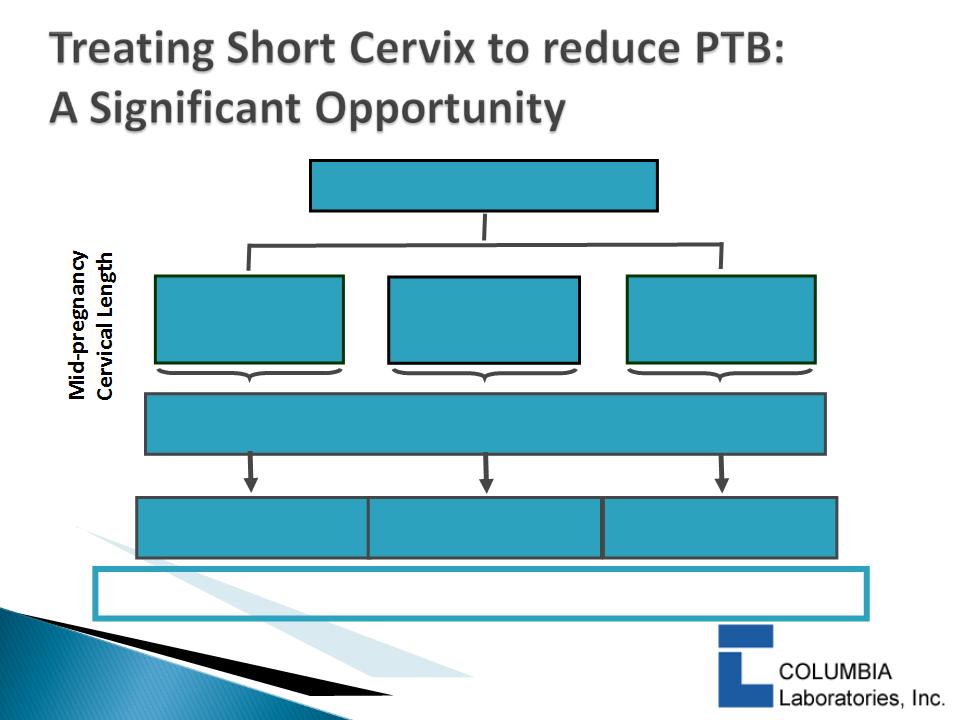
16
$1.7+ Billion Total US Market Opportunity
$225+ million market
potential
potential
4.3 Million Births Annually
>2.5 - 3.0 cm
(20%)
(20%)
$1.1+ billion
market potential
> 2.0 - 2.5 cm
(6%)
(6%)
1.0 - 2.0 cm
(4%)
(4%)
$340+ million market
potential
potential
16 weeks X $83.31 week =
$1,333 per patient (at current price levels)

17
} To & Nicolaides 2006: Short cervix is the most important single
predictor of PTB
predictor of PTB
} Fonseca & Nicolaides 2007: Women with a short cervix
responded to vaginal progesterone therapy
responded to vaginal progesterone therapy
} DeFranco 2007:
◦ PROCHIEVE 8% use was associated with a statistically significant
reduction in PTB in women with CL ≤ 3.0 cm & < 2.8 cm
reduction in PTB in women with CL ≤ 3.0 cm & < 2.8 cm
◦ PROCHIEVE 8% improved infant outcomes1 in women with
baseline CL < 2.8 cm
baseline CL < 2.8 cm
– Statistically significant reduction in Neonatal Intensive Care Unit
(NICU) admissions
(NICU) admissions
– Statistically significant reduction in average NICU days
1 First study to show this
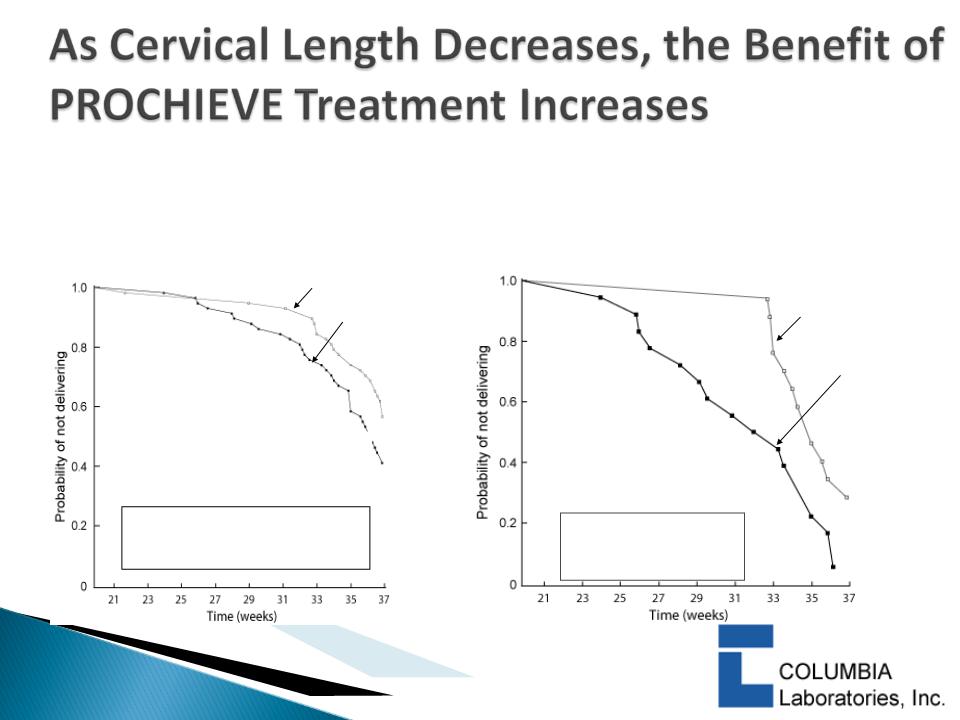
18
progesterone
placebo
33 investigators
n=116 (58 Prochieve, 58 Placebo)
Baseline Cervical Length ≤ 3.0 cm
Baseline Cervical Length < 2.8 cm
progesterone
placebo
22 Investigators
N=46 (19 Prochieve, 27 Placebo)
Fishers Exact Test
at <32 weeks*:
at <32 weeks*:
(p = 0.014)
At ≤ 32 Weeks no Preterm Births were seen with PROCHIEVE® vs. 29.6% on Placebo
The Kaplan-Meier method was
used for calculation; (Wilcoxon
P = 0.043).
used for calculation; (Wilcoxon
P = 0.043).
DeFranco et al, Ultrasound Obstet
Gynecol. 2007; 30: 697-705.
Gynecol. 2007; 30: 697-705.
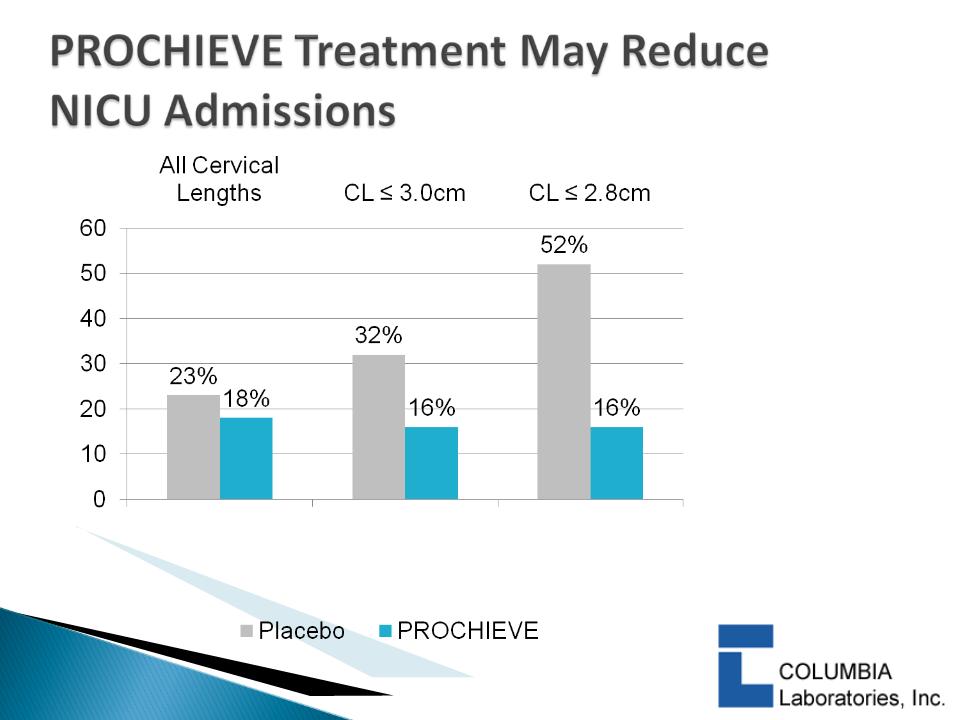
19
p=0.016
p=0.077
p=0.16
DeFranco et al, Ultrasound Obstet
Gynecol. 2007; 30: 697-705.
Gynecol. 2007; 30: 697-705.
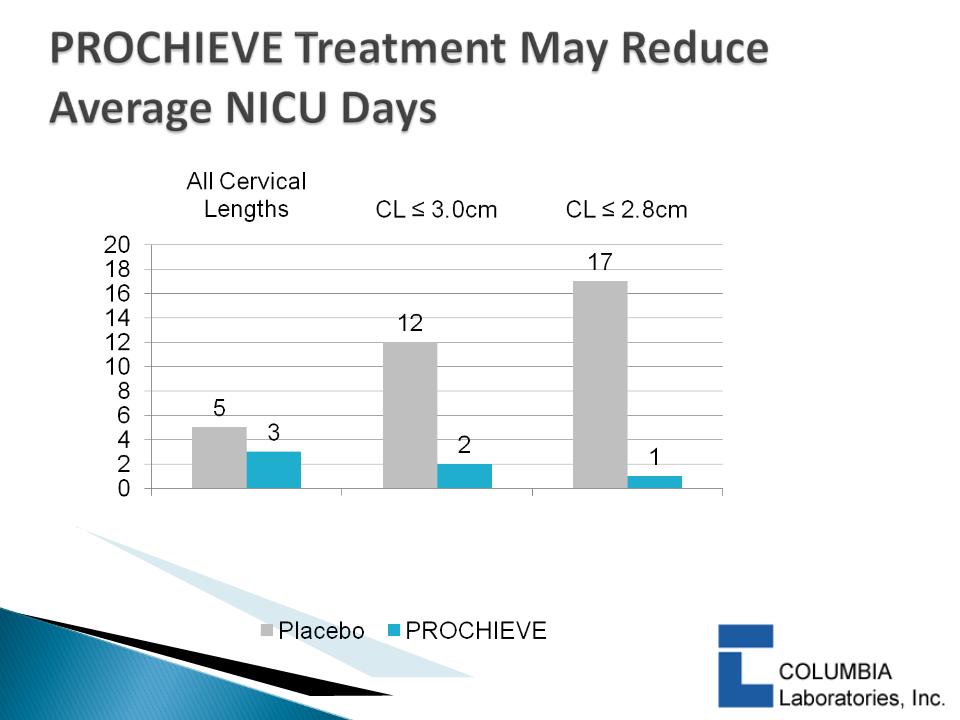
20
p=0.013
p=0.026
p=0.05
DeFranco et al, Ultrasound Obstet
Gynecol. 2007; 30: 697-705.
Gynecol. 2007; 30: 697-705.

21
} Phase III clinical trial with PROCHIEVE 8%
◦ Double-blind, placebo controlled
◦ Enrolled 465 pregnant women with cervical length 1.0-2.0cm
◦ 40+ centers (US & abroad)
◦ Primary endpoint: a reduction in preterm births at ≤32 6/7
weeks vs. placebo
weeks vs. placebo
◦ Improved infant outcomes important secondary endpoint
} NIH co-sponsor
◦ Validates science and design of trial

22
} Enrollment complete June 2010
} Last infant is born Q4 2010
} Report study outcomes Around Y/E 2010
} File with FDA* 2011
} FDA decision* 2011/2012**
*Assuming positive outcome
**PDUFA limits review time to 10 months;
could shorten to 6 months if granted
priority review
could shorten to 6 months if granted
priority review

23

24
} Solid track record in development of women’s health
products:
products:
◦ Bioadhesive products
◦ Vaginal delivery systems
} Unique expertise in clinical development in PTB
} Proven ability to design and successfully manage
complex global development projects, including
collaboration with NIH
complex global development projects, including
collaboration with NIH
} Expertise in utilizing technology platform in multiple
therapeutic areas
therapeutic areas

25
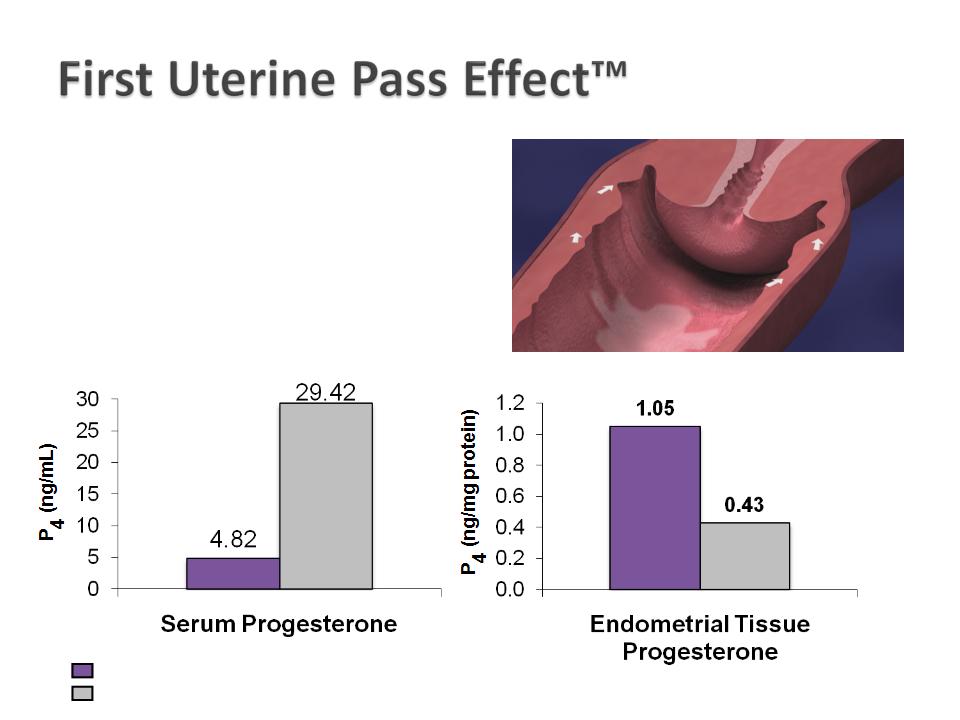
} Progesterone delivery (2013)*
} Progressive hydration for extended release (2019)
} First Uterine Pass Effect (2018)
} pH buffering to prevent preterm labor (2018)
} Treatment of endometriosis/infertility with β-adrenergic agonists (2020)
} Carbamide peroxide for bacterial vaginosis (2022)
} Local anesthetic for chronic pelvic pain (2022) pending
} Extended release ionic treating agents (2023) pending
} Progesterone for treatment of preterm (2028) pending*
*Progesterone-specific patents were transferred to Watson
following the close of the Watson Transactions
following the close of the Watson Transactions
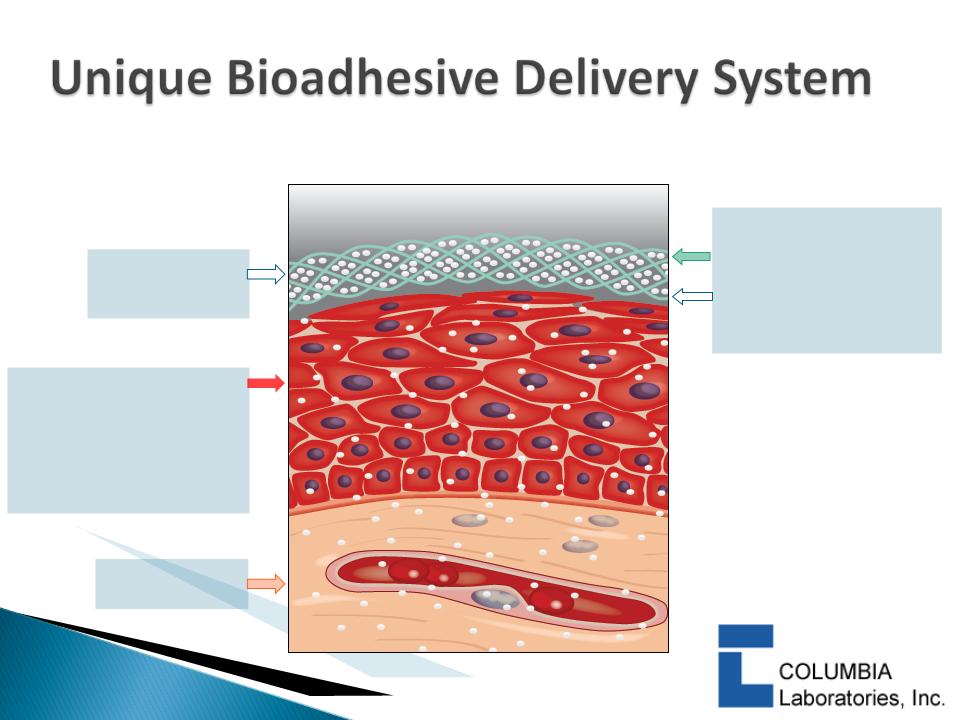
26
Polycarbophil:
hydrogen bonding
with the cell surface
hydrogen bonding
with the cell surface
Active entrapped in
the cross-linked polymer
polycarbophil
Bioadhesive gel,
tablet or system
with active
tablet or system
with active
Adheres to mucosal surface
Discharged upon normal cell
turnover:
turnover:
Oral mucosa up to 24
hours
hours
Vaginal epithelium 72+
hours
hours
Subcutaneous
tissue
tissue
Results in drug delivery
directly to the endometrium
with minimal systemic levels
directly to the endometrium
with minimal systemic levels
CRINONE® 8% (progesterone gel) (90 mg)
IM progesterone (50 mg)
Cicinelli E et al. Obstet Gynecol. 2000; 95: 403-406.

28
|
Product
|
Indication
|
Clinical Stage
|
Market
Opportunity |
|
Crinone/Prochieve 8%
Vaginal Gel
|
Reduce risk of preterm birth in
women with a short cervix in mid -pregnancy |
Pivotal Phase III
|
$1.7 billion (US)
|
|
Crinone/Prochieve 8%
Next generation
|
Lifecycle management program:
ART (Infertility) |
Phase I underway
|
$300+ million
(US) |
|
Crinone/Prochieve 8%
Next generation
|
Lifecycle management program:
Preterm Birth |
Phase I underway
|
$1.7 billion (US)
|
|
COL-2401
Vaginal Tablet
|
Treatment and prevention of
bacterial vaginosis (BV) |
Preclinical work
in-process |
$200+ million
(US) |
|
COL-1777
Vaginal delivery |
Fibroid reduction
|
Phase I work
underway |
$1.2 billion (US)
|
|
COL-1077
Vaginal Gel
|
Treatment of chronic pelvic pain
(CPP) |
Pilot clinical work
complete |
$1.5 billion (US)
|

29
} Most common bacterial infection for women of
childbearing years
childbearing years
◦ 7.4 million new cases annually (US est.)
} Involves an imbalance in the vaginal bacterial ecosystem,
such that natural flora are diminished
such that natural flora are diminished
} Current treatments: antibiotics given orally or intravaginally
◦ Significant side effects from oral forms
◦ Patients report greater satisfaction with intravaginal forms
} Risk factor for preterm birth
} High recurrence rate

30
} Facilitates restoration of normal, protective bacteria
} Maintains normal pH to prevent growth of harmful
bacteria
bacteria
} Vaginally administered
} Natural bacteriostatic agent
} $200+ million US market opportunity
} Patent protection to 2022

31
} Symptoms include excessive vaginal bleeding, pelvic
pressure and anemia
pressure and anemia
◦ Increased risk of infertility and pregnancy complications
} Affects 30 million women (up to 20%) in US
} Most common indication for hysterectomy
} Health care costs >$2 billion in 1997 for surgical
management of uterine fibroids
management of uterine fibroids
} $1.2 billion market opportunity

32
} Current treatment options
◦ Medications such as GnRH agonists
– Side effects: menopausal symptoms & bone loss
– Short-term therapy only
◦ Surgical hysterectomy or myomectomy
– Hysterectomy not an option for women who want to preserve
childbearing capacity
childbearing capacity
} COL-1777 could
◦ Avoid menopausal side effects caused by GnRH agonists
◦ Avoid costs and side effects of surgery
◦ Preserve childbearing ability
} Patent protection to 2019

33
} Strong balance sheet; healthy cash position; debt free
} Ongoing royalty revenues from Watson & Merck
Serono
Serono
} STRIANT sales
} Potential milestone payments
} Significantly lower operating expenses
} Cash burn rate ~ $1 million/quarter through YE 2010
} With successful PREGNANT Study outcome CBRX
will be cash flow & earnings positive on an annual
basis from that point forward
will be cash flow & earnings positive on an annual
basis from that point forward

34
|
Investor Relations Contacts
|
|
|
Larry Gyenes
Chief Financial Officer,
Columbia Laboratories, Inc.
|
Seth Lewis
Vice President,
The Trout Group
|
|
T: (973) 486-8860
|
T: (646) 378-2952
|
|
lgyenes@columbialabs.com
|
slewis@troutgroup.com
|
|
www.columbialabs.com
|
www.columbialabs.com
|
